Chapter 11 Gas-Vapor Mixtures and Air Conditioning11章气体混合物的蒸气与空气调节-文档资料-PPT课件
Vacon nXP和nXc AC驱动器:纯粹的控制力2说明书

vacon nxp & nxc ac drives delivering pure power3nxp wall-mounted range vacon nxp/nxcnxp drive modulesnxc drive cabinetswhat’s in it for youcount on a smooth rideVacon has partnered with global elevator manufacturers to provide drives solutions for both IM and PM motors used in high, mid and low rise buildings. Vacon drives are appreciated for their exceptionally smooth performance and approval ratings on harmonics, safety, and technology.4option boardsour nXP control provides exceptional modularity by offering five (a, B, c, D and E) plug-in extension slots. Fieldbus boards, encoder boards as well as wide range of Io boards can simply be plugged-in at any time without the need to remove any other components.a listing of all options boards is provided on pg. 21multiple optionsethernet connectivityVacon nXP is the smart drive of choice, as there is no need to purchase additional communication tools. Ethernet connectivity allows remote drive access for monitoring, configuring and troubleshooting. Vacon’s Ethernet protocols such as Profinet Io, Ethernet IP and Modbus/TcP are available for all nXP drives. new Ethernet protocols are being continuously developed.Modbus/TCP • Profinet IO • Ethernet I/Pfieldbus optionsYour Vacon nXP is easily integrated within a plant’s automation system by using plug-in fieldbus option boards including Profibus DP, Modbus RTU, Devicenet and canopen. Fieldbus technology ensures increased control and monitoring of the process equipment with reduced cabling - ideal for industries where the need to ensure that products are produced under the right conditions is of paramount importance. an external +24 V supply option enables communication with the control unit even if the main supply is switched off. Fast drive-to-drive communication is possible using Vacon’s fast SystemBus fiber optic communication.Profibus DP • DeviceNet • Modbus RTU • CANopenvacon nxp controlVacon nXP offers a high-performance control platform for all demanding drive applications. The micro controller provides both exceptional prosessing and calculation power. The Vacon nXP supports both induction and permanent magnet motors in open and closed loop control modes. The Vacon nXP features built-in PLc functionality without the need for any additional hardware. Vacon nc61131-3 Engineering can be used to improve performance and create cost savings by integrating customer-specific functionality into the drive. The same control board is used in all nXP drives, allowing the maximum utilization of nXP control features over a wide powerand voltage range.Engineering, HMIControllerFieldbusIntelligentField Device5functional safetydc cooling fansVacon nXP high-performance air-cooled products are equipped with Dc fans. This significantly increases the reliability and lifetime of the fan also fulfilling the ERP2015 directive on decreasing fan losses. Likewise, the Dc-Dc supply board component ratings fulfill industrial requirement levels.conformal coatingTo increase performance and durability, conformally coated circuit boards (also known as varnished boards) are provided as standard for power modules (FR7 - FR14).The upgraded boards offer reliable protection against dustand moisture and extend the lifetime of the drive and criticalcomponents.safe torque off, safe stop 1Safe Torque Off (STO) is available for all nXP drives. It prevents the drive from generating torque on the motor shaft and prevents unintentional start-ups. The function also corresponds to an uncontrolled stop in accordance with stop category 0, En60204-1. Safe Stop 1 (SS1) initiates the motor deceleration and initiates the STo function after an application specific time delay. The function also corresponds to a controlled stop in accordance with stop category 1, En 60204-1.The advantage of the integrated STo and SS1 safety options compared to standard safety technology using electromechanical switchgear is the elimination of separate components and the effort required to wire and service them, while still maintaining the required level of safety at work.atex certified thermistor inputVacon has developed an aTEX approved thermistor input, as an integrated option. certified and compliant with the European aTEX directive 94/9/Ec, the integrated thermistor input is specially designed for the temperature supervision of motors that are placed in areas in which potentially explosive gas, vapor, mist or air mixtures are present and areas with combustible dust. Typical industries requiring such supervision include chemical, petrochemical, marine, metal, mechanical, mining, and oil drilling.If over-heating is detected, the drive immediately stops feeding energy to the motor. as no external components are needed, the cabling is minimized, improving reliability and saving on both space and costs.ConventionalATEX Thermistor InputEx areaThermistor Relay ContactorEx areaSafe Torque Off Conventional STO switchSafety switchSupply disconnectingswitchSupply disconnectingswitchMechanical maintenanceMechanicalmaintenance6commissioning made easydocumentation wizardMake use of our Vacon Documentation Wizard and achieve dramatic savings in engineering time. The Documentation Wizard is a technical documentation tool, which creates a complete set of drawings for each nXc configuration. Just enter the product information, i.e. a type code, required variations and extra equipment (plus codes) into the user interface field, and the tool will automatically generate the documentation in any of the following formats: DWG (AutoCAD) drawings, DXF (autocaD) drawings, PDF (adobe reader), and E-planproject (prj).all-in-one application packageVacon’s handy all-in-one application package has seven built-in software applications, which can be selected with one parameter.In addition to the all-in-one package, Vacon offers several segment specific and advanced applications such as System Interface, Marine, Lift and Shaft Synchronisation for more demanding uses.VACON NXP applications can be downloaded from All-in-One ApplicationsStandard•Basic •Pump & Fan control•Multi-purpose•PID control•Multi-step speed•Local/remotevacon ncdriveVacon ncD rive is used for setting, copying, storing, printing, monitoring and controlling parameters. The Vacon ncDrive communicates with the drive via the following interfaces: RS-232, Ethernet TcP/IP, can (fast multiple drive monitoring), can@net (remote monitoring).Vacon ncD rive also includes a handy D atalogger function, which offers you the possibilty to track failure modes and perform root cause analysis.Vacon PC-tools can be downloaded from user-friendly keypadVacon has ensured that the user interface is intuitive to use. You will enjoy the keypad’s well-structured menu system that allows for fast commissioning and trouble-free operation.• Removable panel with plug-in connection• Graphical and text keypad with multiple language support • Text display multi-monitoring function• Parameter backup and copy function with the panel’s internal memory • Vacon’s Startup Wizard ensures a hassle-free set up. Choose the language, application type and main parameters during the first power-up.7high power and improved redundancyHigh power ac drives up to 5 MW can be built using standard drive components and have the following benefits:• The system is modular and easy to extend• High total power can be obtained by combining smaller drives• System redundancy is higher than in a conventional drive because each unit can run independently • Individual drive is easy to maintain and service• Identical units reduce the required amount of spare parts thus reducing overall costs• No special skills are required for the engineering, installation, commissioning and maintenance ofhigh-power drives as they are comprised of standard modules• It is possible to run multiple winding motors with a phase shift between the windingsVacon DriveSynch is an innovative control concept for running standard drives in parallel to control high-power ac motors or increase the redundancy of a system. This concept suits high power single or multiple winding motors typically above 1 MW.LoadabilityMotor shaft power Low (+40°C)High (+40°C)690 V supply AC drive typeRated continu-ous current I L (a)10%overload current (a)Rated continuous current I H (a)50%overload current (a)Maximum current I S(a)10%overload P (kW)50%overload P (kW)Frame sizeDimensions and weight W x H x D (mm)/ kg2 x nXc 09206 a 2 L 0 SSF1748192015002337267917101520 2 x FR131406 x 2275 x 605/12502 x nXc 1030 6 a 2 L 0 SSF 18102000150023372679171015202 x nXc 1180 6 a 2 L 0 SSF *19502140163025003335190016103 x nXc 09206 a 2 L 0 SSF 2622288423373490401925002200 3 x FR131406 x 2275 x 605/12503 x nXc 1030 6 a 2 L 0 SSF 27063000233734904019250022003 x nXc 1180 6 a 2 L 0 SSF *2910321025003735500228002410* max. ambient temperature of +35°c.values are given at switching frequency 2.0 kHz.typical vacon drivesynch examples using nxp/nxc drivesvalues are given at switching frequency 2.0 kHz.Example of the DriveSynch configuration.F i b e r o p t i c l i n kF i b e r o p t i c l i n kF i b e r o p t i c l i n kLoadabilityMotor shaft power Low (+40°C)High (+40°C)400 V supply AC drive typeRated continuous current I L (a)10%overload current (a)Rated continuous current I H (a)50%overload current (a)Maximum current I S(a)10%overload P (kW)50%overload P (kW)Frame sizeDimensions and weight W x H x D (mm)/ kg2 x nXc 1150 5 a 2 L 0 SSF 2150236519402910349212001100 2 x FR131606 x 2275 x 605/13502 x nXc 1300 5 a 2 L 0 SSF 24702717218532783933135011002 x nXc 1450 5 a 2 L 0 SSF 27553031247037054446150013503 x nXc 1150 5 a 2 L 0 SSF 3278360529364403528418001500 3 x FR131606 x 2275 x 605/13503 x nXc 1300 5 a 2 L 0 SSF 37054076327849165900200018003 x nXc 1450 5 a 2 L 0 SSF 4133454637055558666922502000Mains voltage380-500 V 50/60 HzMains voltage525-690 V 50/60 Hzvacon nxp (fr8)vacon nxp (fr7) 8ratings and dimensionsAC drive typeLoadability Motor shaft powerFramesizeDimensionsand weightW x H x D (mm)/ kg Low (+40°C)High (+50°C)230 V / 400 V / 690 VRatedcontinuous10%overloadRatedcontinuous50%overloadMaximumcurrent I SMainsvoltage10%overload50% overloadvacon nxp drive module10ratings and dimensionsMainsvoltage AC drive typeLoadability Motor shaft powerFramesizeModule Chokes Low (+40°C)High(+40°C)400 V / 690 VRated10%Rated50%Maximum10%50%12vacon nxp standalone (fr11)1314benefits• Trouble free installation and operation• Adapts to your needs w/o engineering• Easy to fit into small spaces• Global enclosure availability, easy to extend• Fast service, easy maintenancevacon nxc (fr10)15typical applications• Pumps & fans • Extruders• Main propulsion & bow thrusters • Wood handling machines• Conveyors & crushers • Feeders & mixers • Test benches • Water treatment• Winches• Compressors• Static power supply • Industrial elevators16hardware configurations, 12-pulse supplyS = Standard O = optional 1) (W: +400) = contact factory * nXc07305 and nXc05906, H: +170 mm* max. ambient temperature of +35˚chardware configurations, 6-pulse supplyS = Standard O = optional 1) (W: +400) = contact factory * nXc07305 and nXc05906, H: +170 mmpure performanceRising energy prices, environmental legislation and process improvement are key issues when designing water handling systems. Use of Vacon ac drives for flow and pressure control instead of dampers or valves gives substantial energy savings resulting in short payback time of the initial investment.1718vacon nxc low-harmonic (af10)19* contact factory S = Standard O = optional*with oPT-aF board 20RS232 adapter card (galvanically decoupled), used mainly for application engineering to connect another keypadcan-bus adapter (galvanically decoupled)the vacon nxp/nxc product range21Hospital Residential Area Commercial Light Industry Area Heavy Industry MarineoR oThe product family standard En 61800-3 sets limits for both emissions and immunity to radio frequency disturbances. The environment has been divided into the first and second environments; in practice, public and industrial networks, respectively.Radio Frequency Interference (RFI) filters are typically required to meet the En 61800-3 standard. These filters are integrated in the Vacon nXP as standard.The 208–240 V and 380–500 V ranges of the Vacon nXP (FR4-FR9) meet the requirements of the first and second environments (H level: En 61800-3(2004), category c2). no additional RFI filters or cabinets are required. The FR10-FR14 and the 500-690 V ranges of the Vacon nXP meet the requirements of the second environment (L-level: En 61800-3(2004), category c3).The units in the frame sizes FR4, FR5 and FR6 (with a voltage range from 380 to 500 V) are also available with extremely low-emission integrated EMc filters (c level: En 61800-3 (2004), category c1). This is sometimes required in very sensitive locations, such as hospitals.emc selection table * Included as standard in low-harmonic drives22type code keynotes23vacon – truly globalmanufacturingand R&D on 3 continentsvacon salesand services in 27 countriesservice centersin 52 countries (including partners)Vacon partnerSubject to changes without prior notice.Vacon is driven by a passion to develop, manufacture and sell the best ac drives and inverters in the world — and to provide efficient life-cycle services for its customers. our ac drives offer optimum process control and energy efficiency for electric motors. Vacon inverters are a key component in producing energy from renewable sources. We have R&D and production units in Finland, the USa, china and Italy, and sales & service offices in 27 countries. In 2011, Vacon had revenues of EUR 380.9 million and globally employed 1,500 people. The shares of Vacon Plc (Vac1V) are quoted on the main list of the Helsinki stock exchange.vacon at your serviceB C 00167GDistributore Italia ELLEUNO srl Via Bari 24 20143 MILANOTel +39 028131848 - Fax: 02.89.19.0444 *****************************。
Very Good-Design review of gas absorbers

Design Review of Absorbers Used for Gaseous PollutantsGoalTo familiarize you with the factors to be considered when reviewing absorber design plans for the permit process.ObjectivesAt the end of this lesson, you will be able to do the following:1. Explain the importance of the following factors in absorber design:•Exhaust gas characteristics•Liquid flow•Pressure drop•pH•Removal of entrained liquids2. Estimate the liquid flow rate, the diameter, and the packing height of a packed towerusing appropriate tables and equations3. Estimate the number of plates and the height of a plate tower using appropriate tables andequationsIntroductionGas absorbers are most often used to remove soluble inorganic contaminants from an airstream. The design of an absorber used to reduce gaseous pollutants from process exhauststreams involves many factors including the pollutant collection efficiency, pollutantsolubility in the absorbing liquid, liquid-to-gas ratio, exhaust flow rate, pressure drop, andmany construction details of the absorbers such as packing, plates, liquid distributors,entrainment separators, and corrosion-resistant materials. These have been discussed in detailin the previous lessons.Lesson 11___________________________________________________________________________________ The same three basic review approaches discussed for particle removal are applicable for gasabsorber evaluation:1. Empirical relationships based on historical data2. Theoretical principles based on gas chemistry and physics3. Pilot scale test dataThe theoretical relationships for gas absorption have been well defined over the many yearsthat gas absorption has been studied; however, they can be very complex and are dependenton the mechanical design of the scrubber. As with particulate scrubbers, empiricalrelationships and general rules of thumb are often used to evaluate absorber designs and thereis no one easy set of equations to evaluate the design of all absorbers.All wet scrubbing systems are able to collect both particulate and gaseous pollutants emittedfrom process exhaust streams. However, spray towers, plate towers, packed towers, andmoving-bed scrubbers are most often used for gaseous pollutant removal. This lesson willfocus on equations used to estimate liquid flow rate, the diameter and the height of a packedtower, and the diameter and number of plates used in a plate tower to achieve a specifiedpollutant removal efficiency.In evaluating an absorption system, the reviewer can use the equations in this lesson toestimate critical operating parameters or component sizes, then supplement this informationwith operating information on the particular scrubber type from previous lessons to completethe review process.Review of Design CriteriaThe principal design criteria are the exhaust flow rate to the absorber, measured in units ofm3/min (ft3/min, or acfm), and the gaseous pollutant concentration, measured in units of partsper million (ppm). The exhaust volume and pollutant concentration are set by the processexhaust conditions. Once these criteria are known, the vendor can begin to design theabsorber for the specific application. A thorough review of the design plans should considerthe factors presented below.Exhaust gas characteristics - average and maximum flow rates to the absorber, andchemical properties such as dew point, corrosiveness, pH, and solubility of the pollutant to beremoved should be measured or accurately estimated.Liquid flow - the type of scrubbing liquid and the rate at which the liquid is supplied to theabsorber. If the scrubbing liquid is to be recirculated, the pH and amount of suspended solids(if any) should be monitored to ensure continuous reliability of the absorbing system.Pressure drop - the pressure drop (gas-side) at which the absorber will operate; the absorberdesign should also include a means for monitoring the pressure drop across the system,usually by manometers.pH - the pH at which the absorber will operate; the pH of the absorber should be monitoredso that the acidity or alkalinity of the absorbing liquor can be properly adjusted.Design Review of Absorbers Used for Gaseous Pollutants ___________________________________________________________________________________ Removal of entrained liquid - mists and liquid droplets that become entrained in the"scrubbed" exhaust stream should be removed before exiting the stack. Some type ofentrainment separator, or mist eliminator, should be included in the design.Emission requirements - collection efficiency in terms of parts per million to meet the airpollution regulations; collection efficiency can be high (90 to 99%) if the absorber is properlydesigned. The agency review engineer can use the equations listed in this lesson to estimatethe absorber removal efficiency, liquid flow rate, tower diameter, and packing height.However, these equations can only estimate these values, and they should not be used as thebasis to either accept or reject the design plans submitted for the permit process.AbsorptionAbsorption is a process that refers to the transfer of a gaseous pollutant from a gas phase to aliquid phase. More specifically, in air pollution control, absorption involves the removal ofobjectionable gaseous pollutants from a process stream by dissolving them in a liquid.The absorption process can be categorized as physical or chemical. Physical absorptionoccurs when the absorbed compound dissolves in the liquid; chemical absorption occurswhen the absorbed compound and the liquid (or a reagent in the liquid) react. Liquidscommonly used as solvents include water, mineral oils, nonvolatile hydrocarbon oils, andaqueous solutions.Some common terms used when discussing the absorption process follow:Absorbent - the liquid, usually water, into which the pollutant is absorbed.Solute, or absorbate - the gaseous pollutant being absorbed, such as SO2, H2S, etc.Carrier gas - the inert portion of the gas stream, usually air, from which the pollutant isbeing removed.Interface - the area where the gas phase and the absorbent contact each other.Solubility - the capability of a particular gas to be dissolved in a given liquid.Absorption is a mass-transfer operation. In absorption, mass transfer of the gaseous pollutantinto the liquid occurs as a result of a concentration difference (of the pollutant) between theliquid and gas phases. Absorption continues as long as a concentration difference existswhere the gaseous pollutant and liquid are not in equilibrium with each other. Theconcentration difference depends on the solubility of the gaseous pollutant in the liquid.Lesson 11___________________________________________________________________________________ Absorbers remove gaseous pollutants by dissolving them into a liquid called the absorbent. Indesigning absorbers, optimum absorption efficiency can be achieved by doing the following:•Providing a large interfacial contact area•Providing for good mixing between the gas and liquid phases•Allowing sufficient residence, or contact, time between the phases•Choosing a liquid in which the gaseous pollutant is very solubleSolubilitySolubility is a very important factor affecting the amount of a pollutant, or solute, that can beabsorbed. Solubility is a function of both the temperature and, to a lesser extent, the pressureof the system. As temperature increases, the amount of gas that can be absorbed by a liquiddecreases. From the ideal gas law: as temperature increases, the volume of a gas alsoincreases; therefore, at the higher temperatures, less gas is absorbed due its larger volume.Pressure affects the solubility of a gas in the opposite manner. By increasing the pressure of asystem, the amount of gas absorbed generally increases.The solubility of a specific gas in a given liquid is defined at a designated temperature andpressure. Table 11-1 presents data on the solubility of SO2 gas in water at 101 kPa, or 1 atm,and various temperatures. In determining solubility data, the partial pressure (in mm Hg) ismeasured with the concentration (in grams of solute per 100 grams of liquid) of the solute inthe liquid. The data in Table 11-1 were taken from The International Critical Tables, a goodsource of information concerning gas-liquid systems.Table 11-1. Partial pressure of SO2 in aqueous solution,mm HgGrams ofSO2 per100g H2O 10°C 20°C 30°C 40°C 50°C 60°C 70°C0.0 - - - - - - -0.5 21 29 42 60 83 111 1441.0 42 59 85 120 164 217 2811.5 64 90 129 181 247 328 4262.0 86 123 176 245 333 444 5812.5 108 157 224 311 421 562 7393.0 130 191 273 378 511 682 8973.5 153 227 324 447 603 804 -4.0 176 264 376 518 698 - -4.5 199 300 428 588 793 - -5.0 223 338 482 661 - - -Design Review of Absorbers Used for Gaseous Pollutants ___________________________________________________________________________________Solubility data are obtained at equilibrium conditions. This involves putting measuredamounts of a gas and a liquid into a closed vessel and allowing it to sit for a period of time.Eventually, the amount of gas absorbed into the liquid will equal the amount coming out ofthe solution. At this point, there is no net transfer of mass to either phase, and theconcentration of the gas in both the gaseous and liquid phases remains constant. The gas-liquid system is at equilibrium.Equilibrium conditions are important in operating an absorption tower. If equilibrium were tobe reached in the actual operation of an absorption tower, the collection efficiency would fallto zero at that point since no net mass transfer could occur. The equilibrium concentration,therefore, limits the amount of solute that can be removed by absorption. The most commonmethod of analyzing solubility data is to use an equilibrium diagram. An equilibriumdiagram is a plot of the mole fraction of solute in the liquid phase, denoted as x, versus themole fraction of solute in the gas phase, denoted as y. (See Appendix A for a brief refresheron mole fractions.) Equilibrium lines for the SO 2 and water system given in Table 11-1 areplotted in Figure 11-1. Figure 11-1 also illustrates the temperature dependence of theabsorption process. At a constant mole fraction of solute in the gas (y), the mole fraction ofSO 2 that can be absorbed in the liquid (x) increases as the temperature decreases.Figure 11-1. Equilibrium lines for SO 2 - H 2O systems atvarious temperaturesUnder certain conditions, Henry's law may also be used to express equilibrium solubility ofgas-liquid systems. Henry's law is expressed as:p = Hx (11-1)Where:p = partial pressure of solute at equilibrium, Pax = mole fraction of solute in the liquid H =Henry's law constant, Pa/mole fractionLesson 11___________________________________________________________________________________ From Equation 11-1, you can see that H has the units of pressure per concentration. Henry'slaw can be written in a more useful form by dividing both sides of Equation 11-1 by the totalpressure, P T, of the system. The left side of the equation becomes the partial pressure dividedby the total pressure, which equals the mole fraction in the gas phase, y. Equation 11-1 nowbecomes:y = H'x (11-2)Where: y = mole fraction of gas in equilibrium with liquidH' = Henry's law constant, mole fraction in vapor per molefraction in liquidx = mole fraction of the solute in equilibriumNote: H' now depends on the total pressure.Equation 11-2 is the equation of a straight line, where the slope (m) is equal to H'. Henry'slaw can be used to predict solubility only when the equilibrium line is straight. Equilibriumlines are usually straight when the solute concentrations are very dilute. In air pollutioncontrol applications, this is usually the case. For example, an exhaust stream that contains a1,000-ppm SO2 concentration corresponds to a mole fraction of SO2 in the gas phase of only0.001. Figure 11-2 demonstrates that the equilibrium lines are still straight at this lowconcentration of SO2.Figure 11-2. Equilibrium diagram for SO2 - H2O systemfor the data given in Example 11-1Another restriction on using Henry's law is that it does not hold true for gases that react ordissociate upon dissolution. If this happens, the gas no longer exists as a simple molecule. Forexample, scrubbing HF or HCl gases with water causes both compounds to dissociate insolution. In these cases, the equilibrium lines are curved rather than straight. Data on systemsthat exhibit curved equilibrium lines must be obtained from experiments.Design Review of Absorbers Used for Gaseous Pollutants ___________________________________________________________________________________ Henry's law constants for the solubility of several gases in water are listed in Table 11-2. Theunits of Henry's law constants are atmospheres per mole fraction. The smaller the constant,the more soluble the gas. Table 11-2 demonstrates that SO2 is approximately 100 times moresoluble in water than CO2 is.Table 11-2. Henry's law constants forgases in H2O1Gas 20°C 30°CN2 80.4 92.4CO 53.6 62.0H2S 48.3 60.9O2 40.1 47.5NO 26.4 31.0CO2 1.42 1.86SO2 0.014 0.0161. Expressed in H × 10-5, atm/mole fraction.The following example illustrates how to develop an equilibrium diagram from solubilitydata.Example 11-1Given the data in Table 11-3 for the solubility of SO2 in pure water at 303°K (30°C) and101.3 kPa (760 mm Hg), calculate y and x, plot the equilibrium diagram, and determine ifHenry's law applies.Table 11-3. Equilibrium datac so2(g of SO2 per 100g of H2O)p so2(partial pressure ofSO2)y(mole fraction ofSO2 in gasphase)x(mole fraction of SO2 inliquid phase)0.5 6 kPa (42 mm Hg)1.0 11.6 kPa (85 mmHg)1.5 18.3 kPa (129 mmHg)2.0 24.3 kPa (176 mmHg)2.5 30.0 kPa (224 mmHg)3.0 36.4 kPa (273 mmHg)Lesson 11 ___________________________________________________________________________________ Solution In steps 1 and 2, convert the data for the concentration of SO 2 in water and the partial pressure of SO 2 in air into mole fraction units. 1. Calculate the mole fraction of SO 2 in the gas phase, y , by dividing the partial pressure of SO 2 by the total pressure of the system.y p P y so T ===26006 kPa 101.3 kPa . The mole fractions of SO 2 in the gas phase (y) are tabulated in Table 11-4. 2. Calculate the mole fraction of the solute (SO 2) in the liquid phase, x , by dividing the moles of SO 2 dissolved into the solution by the total moles of liquid.x O =moles of SO in solution moles of SO in solution +moles of H 222 Where: moles of SO 2 in solution = c SO 2/64 g SO 2 per mole moles of H 2O = 100 g of H 2O/18 g H 2O per mole = 5.55 molesx =c so 2//.....646455505640564555000142c so +=+= The mole fractions of the solute in the liquid phase are tabulated in Table 11-4.Design Review of Absorbers Used for Gaseous Pollutants ___________________________________________________________________________________3. Plot the mole fraction of SO 2 in air, (y), against the mole fraction of SO 2dissolved in water, (x).Figure 11-2. (repeated) Equilibrium diagram for SO 2 - H 2Osystem for the data given in Example 11-1The plot in Figure 11-2 is a straight line; therefore, Henry's law applies.7.420042.00056.0180.0239.0x y Slope ≈−−== The slope of the line (Δy/Δx), Henry's law constant (H'), is approximately equalto 42.7.To test your knowledge of the preceding section, answer the questions in Part 1 of theReview Exercise.Lesson 11___________________________________________________________________________________ Absorber DesignTheoryThe first step in designing an air pollution control device is to develop a mathematicalexpression describing the observed phenomenon. A valid mathematical expressiondescribing absorber performance makes it possible to determine the proper absorber sizefor a given set of conditions, and predict how a change in operating conditions affectsabsorber performance. A number of theories, or models, attempt to analytically describethe absorption mechanism. However, in practice, none of these analytical expressionscan solely be used for design calculations. Experimental or empirical data must also beused to obtain reliable results.The most widely used model for describing the absorption process is the two-film, ordouble-resistance, theory, which was first proposed by Whitman in 1923. The modelstarts with the three-step mechanism of absorption previously discussed in Lesson 2.From this mechanism, the rate of mass transfer was shown to depend on the rate ofmigration of a molecule in either the gas or liquid phase. The two-film model starts byassuming that the gas and liquid phases are in turbulent contact with each other, separatedby an interface area where they meet. This assumption may be correct, but nomathematical expressions adequately describe the transport of a molecule through bothphases in turbulent motion. Therefore, the model proposes that a mass-transfer zoneexists to include a small portion (film) of the gas and liquid phases on either side of theinterface. The mass-transfer zone is comprised of two films, a gas film and a liquid filmon their respective sides of the interface. These films are assumed to flow in a laminar, orstreamline, motion. In laminar flow, molecular motion occurs by diffusion, and can becategorized by mathematical expressions. This concept of the two-film theory isillustrated in Figure 11-3.Figure 11-3. Visualization of two-film theoryAccording to the two-film theory, for a molecule of substance A to be absorbed, it must proceed through a series of five steps. The molecule must:1. Migrate from the bulk-gas phase to the gas film2. Diffuse through the gas film3. Diffuse across the interface4. Diffuse through the liquid film5. Mix into the bulk liquidThe theory assumes that complete mixing takes place in both gas and liquid bulk phases and that the interface is at equilibrium with respect to pollutant molecules transferring in or out of the interface. This implies that all resistance to movement occurs when the molecule is diffusing through the gas and liquid films to get to the interface area, hence the name double-resistance theory. The partial pressure (concentration) in the gas phase changes from p AG in the bulk gas to p AI at the interface.A gas concentration is expressed by its partial pressure. Similarly, the concentration in the liquid changes from c AI at the interface to c AL in the bulk liquid phase as mass transfer occurs. The rate of mass transfer from one phase to the other then equals the amount of molecule A transferred multiplied by the resistance molecule A encounters in diffusing through the films.N A = kg (pAG− pAI) (11-3)N A= kl (cAI− cAL) (11-4)Where: N A= rate of transfer of component A, g-mol/h•m2(lb-mole/hr•ft2)k g= mass-transfer coefficient for gas film, g-mol/h•m2•Pa(lb-mole/hr•ft2•atm)k l= mass-transfer coefficient for liquid film,g-mol/h•m2•Pa (lb-mole/hr•ft2•atm)p AG = partial pressure of solute A in the gasp AI= partial pressure of solute A at the interfacec AI= concentration of solute A at the interfacec AL = concentration of solute A in the liquidThe mass-transfer coefficients, k g and k l , represent the flow resistance the soluteencounters in diffusing through each film respectively (Figure 11-4). As you can seefrom the above equations, as the value for a mass transfer coefficient increases, theamount of pollutant transferred (per unit of time) from the gas to the liquid increases. Ananalogy is the resistance electricity encounters as it flows through a circuit.Figure 11-4. Resistance to motion encountered by amolecule being absorbedEquations 11-3 and 11-4 define the general case of absorption and are applicable to bothcurved and straight equilibrium lines. In practice, Equations 11-3 and 11-4 are difficult touse, since it is impossible to measure the interface concentrations, p AI and c AI . Theinterface is a fictitious state used in the model to represent an observed phenomenon.Using the interface concentrations in calculations can be avoided by defining the mass-transfer system at equilibrium conditions and combining the individual film resistancesinto an overall resistance from gas to liquid and vice versa. If the equilibrium line isstraight, the rate of absorption is given by the equations below:()N K p p A OG AG A=−*(11-5) ()N K c c A OL A AL =−*(11-6)Where: N A = rate of transfer of component, A, g-mol/h•m 2(lb-mole/hr•ft 2)p A *= equilibrium partial pressure of solute A at operatingconditionsc A *= equilibrium concentration of solute A at operatingconditionsK OG = overall mass-transfer coefficient based on gasphase, g-mol/h•m 2•Pa (lb-mole/hr•ft 2•atm)K OL = overall mass-transfer coefficient based on liquidphase, g-mol/h•m 2•Pa (lb-mole/hr•ft 2•atm)p AG = partial pressure of solute A in the gasc AL = concentration of solute A in the liquidAn important fact concerning Equations 11-5 and 11-6 is that they impose an upper limiton the amount of solute that can be absorbed. The rate of mass transfer depends on theconcentration departure from equilibrium in either the gas (p AG - p A *) or liquid(c A *- c AL ) phase. The larger these concentration differences are, the greater the rate ofmass transfer becomes. If equilibrium is ever reached (p AG = p a *and c AL = c A *) absorptionstops and no net transfer occurs. Thus, the equilibrium concentrations determine themaximum amount of solute that is absorbed.At equilibrium, the overall mass-transfer coefficients are related to the individual mass-transfer coefficients by the equations below. 11K k H k OG g l=+′ (11-7) 111K k H OL l g=+′ k (11-8) H' is Henry's law constant (the slope of the equilibrium). Equations 11-7 and 11-8 areuseful in determining which phase controls the rate of absorption. From Equation 11-7, ifH' is very small (which means the gas is very soluble in the liquid), then K OG ≈ k g , andabsorption is said to be gas-film controlled . The major resistance to mass transfer is inthe gas phase. Conversely, if a gas has limited solubility, H' is large, and Equation 11-8reduces to K OL ≈ k l . The mass-transfer rate is liquid-film controlled and depends on thesolute's dispersion rate in the liquid phase. Most systems in the air pollution control fieldare gas-phase controlled since the liquid is chosen so that the solute will have a highdegree of solubility.The discussion so far has been based on the two-film theory of absorption. Other theoriesoffer different descriptions of gas molecule movement from the gas to the liquid phase.Some of the significant mass-transfer models follow. For these theories, the mass-transferrate equation does not differ from that of the two-film method. The difference lies in theway they predict the mass-transfer coefficient. It has been shown that the rate of masstransfer depends on a concentration difference multiplied by a resistance factor. Like most theories describing how something functions, absorption theories provide a basic understanding of the process, but due to the complexities of "real life" operations, it is difficult to apply them directly. Concentrations can easily be determined from operating (c and p) and equilibrium (c A*and p a* ) data of the system. Mass-transfer coefficients are very difficult to determine from theory. Theoretically predicted values of the individual mass-transfer coefficients (k g and k l) based on the two-film theory, do not correlate well with observed values. Overall mass-transfer coefficients are more easily determined from experimental or operational data. However, the overall coefficients apply only when the equilibrium line is straight.Mass-Transfer ModelsThe following discussion on mass-transfer models is taken from Diab and Maddox (1982).Film Theory (Whitman 1923) - First, and probably the simplest theory proposed for mass transfer across a fluid. Details of this model are discussed in the text because it is the most widely used.Penetration Theory (Higbie 1935) - Assumes that the liquid surface in contact with the gas consists of small fluid elements. After contact with the gas phase, the fluid elements return to the bulk of the liquid and are replaced by another element from the bulk-liquid phase. The time each element spends at the surface is assumed to be the same.Surface-Renewal Theory (Danckwerts 1951) - Improves on the penetration theory by suggesting that the constant exposure time be replaced by an assumed timedistribution.Film-Penetration Theory (Toor and Marchello 1958) - Combination of the film and penetration theories. Assumes that a laminar film exists at the fluid interface (as in the film theory), but further assumes that mass transfer is a nonsteady-state process.Mass-transfer coefficients are often expressed by the symbols K OG a, k l a, etc., where "a" represents the surface area available for absorption per unit volume of thecolumn. This allows for easy determination of the column area required toaccomplish the desired separation. These mass-transfer coefficients are developed from experimental data and are usually reported in one of two ways: as an empirical relationship based on a function of the liquid flow, gas flow, or slope of theequilibrium line; or correlated in terms of a dimensionless number, usually either the Reynolds or Schmidt Number.Figure 11-5 provides an example comparing the effect of two types of packingmaterials on the mass-transfer coefficient for SO2 in water (Perry 1973). Packing A consists of one-inch rings and packing B consists of three-inch spiral tiles. As can be seen from this example, packing A has the higher transfer coefficient and wouldprovide a better service in this application. Note that G'is the gas mass flow rate per cross-sectional area of tower (i.e. ft2). Similar figures are used extensively to comparedifferent absorbers or similar absorbers with varying operating conditions. It shouldbe noted that these estimated mass-transfer coefficients are system and packing-typedependent and, therefore, do not have widespread applicability. The ChemicalEngineers' Handbook gives a comprehensive listing of empirically derivedcoefficients. In addition, manufacturers of packed and plate towers have graphs intheir literature similar to the one in Figure 11-5.Figure 11-5. Comparison of overall absorption coefficient forSO2 in waterSource: Perry 1973.Although the science of absorption is considerably developed, much of the work inpractical design situations is empirical in nature. The following sections will applythe principles discussed to the design of gas absorption equipment. Emphasis hasbeen placed on presenting information that can be used to estimate absorber size and liquid flow rate.To test your knowledge of the preceding section, answer the questions in Part 2 of the Review Exercise.ProceduresThe effectiveness of an absorption system depends on the solubility of the gaseouscontaminant. For very soluble gases, almost any type of absorber will give adequateremoval. However, for most gases, only absorbers that provide a high degree of turbulent contact and a long residence time are capable of achieving high absorption efficiencies.The two most common high-efficiency absorbers are plate and packed towers. Both of these devices are used extensively to control gaseous pollutants. Absorber designcalculations presented in this lesson will focus on these two devices.Numerous procedures are used to design an absorption system. These procedures range in difficulty and cost from short-cut "rules of thumb" equations to in-depth designprocedures based on pilot plant data. Procedures presented here will be based on the。
专业英语带译文

数字拉丁或希腊前缀烷烃烷基烯烃醇醛中文译名从左至右alkane alkyl alkene alcohol aldehyde 烷烃、烷基、烯烃、醇、醛one mono- methane methyl —methanol methyl aldehyde 甲烷、甲基、甲醇、甲醛two di-bi- ethane ethyl ethene,ethyleneethanol ethyl aldehyde,ethanal 乙烷、乙基、乙烯、乙醇、乙醛three tri- propane propyl propene propanol propylaldehyde 丙four tetra-Quadri-butane butyl butene butanol butyladehyde 丁five pent(a)- pentane pentyl pentene pentanol pentanal 戊six hex(a)- hexane hexyl hexene hexanol hexanal 己seven hept(a)- heptane heptyl heptene heptanol heptanal,heptylaldehyde庚eight oct(a)- octane octyl octene octanol octyl aldehyde 辛nine non(a)- nonane nonyl nonene nonanol nonyl aldehyde 壬ten dec(a)- decane decyl decene decanol decylaldehyde,decanal癸它们能与其它有机化合物自由地混合并能溶于多种有机溶剂中。
They will mix freely with other organic compounds and are often soluble in organic solvents.氧的两对电子可以与两个不直接相连的碳共用而形成单键。
科技英语翻译课后题答案课后习题答案

The power plant is the heart of a ship. 动力装置是船舶的心脏。
The power unit for driving the machines is a 50-hp induction motor.驱动这些机器的动力装置是一台50马力的感应电动机。
Semiconductor devices, called transistors, are replacing tubes in many applications.半导体装置也称为晶体管,在许多场合替代电子管。
Cramped conditions means that passengers’legs cannot move around freely.空间狭窄,旅客的两腿就不能自由活动。
All bodies are known to possess weight and occupy space. 我们知道,所有的物体都有重量并占据空间。
The removal of minerals from water is called softening. 去除水中的矿物质叫做软化。
A typical foliage leaf of a plant belonging to the dicotyledons is composed of two principal parts: blade and petiole.Einstein’s relativity theory is the only one which can explain such phenomena.All four (outer planets) probably have cores of metals, silicates, and water.The designer must have access to stock lists of the materials he employs.设计师必须备有所使用材料的储备表。
空气的热力学性质

ρ kg m–3 957.6 0.1421 956.5 0.1504 948.2 0.2318 939.9 0.3460 931.5 0.5018 923.0 0.7089 914.4 0.9785 905.7 1.322 897.0 1.753 888.1 2.285 879.1 2.933 870.0 3.711 860.7 4.635 851.3 5.724 841.7 6.993 832.0 8.464
ρ kg m–3 822.0 10.16 811.8 12.09 801.4 14.29 790.7 16.78 779.7 19.60 768.4 22.76 756.7 26.32 744.6 30.31 732.1 34.78 719.1 39.79 705.5 45.41 691.2 51.73 676.2 58.84 660.3 66.88 643.4 76.04 625.1 86.55 605.3 98.76 583.3 113.2 558.3 130.6 528.3 152.6 488.3 182.7 411.2 235.4 302.6
Cv kJ kg–1 K–1
1.174 0.7184 1.173 0.7186 1.157 0.7198 1.143 0.7212 1.129 0.7230 1.115 0.7252 1.102 0.7277 1.090 0.7305 1.078 0.7338 1.067 0.7375 1.056 0.7416 1.045 0.7460 1.035 0.7510 1.025 0.7563 1.016 0.7620 1.007 0.7682
fied by the high densities in the liquid and the low densities in the vapor. Additional calculations at state points not listed below can be obtained by using the NIST program REFPROP (http://www. /srd/nist23.htm).
《物理化学》的中英文翻译

《物理化学》的中英文翻译第一篇:《物理化学》的中英文翻译复习《物理化学》过程中,顺便整理了专业名词的翻译,大家凑合着,依我看,简单的会考汉译英,复杂的会考英译汉。
不管怎么样,中文英文背过最好。
如果有错误,赶紧的,说。
1多相系统 heterogeneous system2自由度degree of freedom3相律 phase rule4独立组分数 number of independent component5凝聚系统 condensed system6三相点 triple point7超临界流体 supercritical fluid8超临界流体萃取supercritical fluid extraction9超临界流体色谱supercritical fluid chromatography10泡点 bubbling point11露点dew point12杠杆规则 level rule13连结线 tie line14部分蒸馏(分馏)fractional distillation15缔合分子 associated molecule16最低恒沸点 minimum azeotropic point17最低恒沸混合物low-boiling azeotrope18无水乙醇(绝对乙醇)absolute ethyl alcohol19最高恒沸点maximum azeotropic point20会溶点 consolute point21共轭层 conjugate layer22烟碱 nicotine23蒸汽蒸馏 steam distillation24步冷曲线 cooling curve25热分析法 thermal analysis26低共熔点 eutectic point27低共熔混合物eutectic mixture28异成分熔点 incongruent melting point29转熔温度 peritectic tempreture30固溶体 solid solution31退火 annealing32淬火 quenching33区域熔炼 zone melting34分凝系数 fractional coagulation coefficient35褶点 plait point36等温会溶点 isothermal consolute point37双节点溶解度曲线 binodal solubility cueve38一(二)级相变first(second)order phase transition39超流体 super fluid40顺磁体 paramagnetic substance41铁磁体 ferromagnetic substance第二篇:中英文翻译蓄电池 battery 充电 converter 转换器 charger开关电器Switch electric 按钮开关Button to switch 电源电器Power electric 插头插座 Plug sockets第三篇:中英文翻译Fundamentals This chapter describes the fundamentals of today’s wireless communications.First a detailed description of the radio channel and its modeling are presented, followed by the introduction of the principle of OFDM multi-carrier transmission.In addition, a general overview of the spread spectrum technique, especially DS-CDMA, is given and examples of potential applications for OFDM and DS-CDMA areanalyzed.This introduction is essential for a better understanding of the idea behind the combination of OFDM with the spread spectrum technique, which is briefly introduced in the last part of this chapter.1.1 Radio Channel Characteristics Understanding the characteristics of the communications medium is crucial for the appropriate selection of transmission system architecture, dimensioning of its components, and optimizing system parameters, especially since mobile radio channels are considered to be the most difficult channels, since they suffer from many imperfections like multipath fading, interference, Doppler shift, and shadowing.The choice of system components is totally different if, for instance, multipath propagation with long echoes dominates the radio propagation.Therefore, an accurate channel model describing the behavior of radio wave propagation in different environments such as mobile/fixed and indoor/outdoor is needed.This may allow one, through simulations, to estimate and validate the performance of a given transmission scheme in its several design phases.1.1.1 Understanding Radio Channels In mobile radio channels(see Figure 1-1), the transmitted signal suffers from different effects, which are characterized as follows: Multipath propagation occurs as a consequence of reflections, scattering, and diffraction of the transmitted electromagnetic wave at natural and man-made objects.Thus, at the receiver antenna, a multitude of waves arrives from many different directions with different delays, attenuations, and phases.The superposition of these waves results in amplitude and phase variations of the composite received signal.Doppler spread is caused by moving objects in the mobile radio channel.Changes in the phases and amplitudes of the arriving waves occur which lead to time-variant multipathpropagation.Even small movements on the order of the wavelength may result in a totally different wave superposition.The varying signal strength due to time-variant multipath propagation is referred to as fast fading.Shadowing is caused by obstruction of the transmitted waves by, e.g., hills, buildings, walls, and trees, which results in more or less strong attenuation of the signal pared to fast fading, longer distances have to be covered to significantly change the shadowing constellation.The varying signal strength due to shadowing is called slow fading and can be described by a log-normal distribution [36].Path loss indicates how the mean signal power decays with distance between transmitter and receiver.In free space, the mean signal power decreases with the square of the distance between base station(BS)and terminal station(TS).In a mobile radio channel, where often no line of sight(LOS)path exists, signal power decreases with a power higher than two and is typically in the order of three to five.Variations of the received power due to shadowing and path loss can be efficiently counteracted by power control.In the following, the mobile radio channel is described with respect to its fast fading characteristic.1.1.2 Channel Modeling The mobile radio channel can be characterized by the time-variant channel impulse response h(τ , t)or by the time-variant channel transfer function H(f, t), which is the Fourier transform of h(τ, t).The channel impulse response represents the response of the channel at time t due to an impulse applied at time t −τ.The mobile radio channel is assumed to be a wide-sense stationary random process, i.e., the channel has a fading statistic that remains constant over short periods of time or small spatial distances.In environments with multipath propagation, the channel impulseresponse is composed of a large number of scattered impulses received over Np different paths,Whereand ap, fD,p, ϕp, and τp are the amplitude, the Doppler frequency, the phase, and the propagation delay, respectively, associated with path p, p = 0,..., Np −1.The assigned channel transfer function isThe delays are measured relative to the first detectable path at the receiver.The Doppler Frequencydepends on the velocity v of the terminal station, the speed of light c, the carrier frequency fc, and the angle of incidence αp of a wave assigned to path p.A channel impulse response with corresponding channel transfer function is illustrated in Figure 1-2.The delay power density spectrum ρ(τ)that characterizes the frequency selectivity of the mobile radio channel gives the average power of the channel output as a function of the delay τ.The mean delay τ , the root mean square(RMS)de lay spread τRMS and the maximum delay τmax are characteristic parameters of the delay power density spectrum.The mean delay isWhereFigure 1-2 Time-variant channel impulse response and channel transfer function with frequency-selective fading is the power of path p.The RMS delay spread is defined as Similarly, the Doppler power density spectrum S(fD)can be defined that characterizes the time variance of the mobile radio channel and gives the average power of the channel output as a function of the Doppler frequency fD.The frequency dispersive properties of multipath channels are most commonly quantified by the maximum occurring Doppler frequency fDmax and the Doppler spread fDspread.The Doppler spread is the bandwidth of theDoppler power density spectrum and can take on values up to two times |fDmax|, i.e.,1.1.3Channel Fade Statistics The statistics of the fading process characterize the channel and are of importance for channel model parameter specifications.A simple and often used approach is obtained from the assumption that there is a large number of scatterers in the channel that contribute to the signal at the receiver side.The application of the central limit theorem leads to a complex-valued Gaussian process for the channel impulse response.In the absence of line of sight(LOS)or a dominant component, the process is zero-mean.The magnitude of the corresponding channel transfer functionis a random variable, for brevity denoted by a, with a Rayleigh distribution given byWhereis the average power.The phase is uniformly distributed in the interval [0, 2π].In the case that the multipath channel contains a LOS or dominant component in addition to the randomly moving scatterers, the channel impulse response can no longer be modeled as zero-mean.Under the assumption of a complex-valued Gaussian process for the channel impulse response, the magnitude a of the channel transfer function has a Rice distribution given byThe Rice factor KRice is determined by the ratio of the power of the dominant path to thepower of the scattered paths.I0 is the zero-order modified Bessel function of first kind.The phase is uniformly distributed in the interval [0, 2π].1.1.4Inter-Symbol(ISI)and Inter-Channel Interference(ICI)The delay spread can cause inter-symbol interference(ISI)when adjacent data symbols overlap and interfere with each other due to differentdelays on different propagation paths.The number of interfering symbols in a single-carrier modulated system is given by For high data rate applications with very short symbol duration Td < τmax, the effect of ISI and, with that, the receiver complexity can increase significantly.The effect of ISI can be counteracted by different measures such as time or frequency domain equalization.In spread spectrum systems, rake receivers with several arms are used to reduce the effect of ISI by exploiting the multipath diversity such that individual arms are adapted to different propagation paths.If the duration of the transmitted symbol is significantly larger than the maximum delay Td τmax, the channel produces a negligible amount of ISI.This effect is exploited with multi-carrier transmission where the duration per transmitted symbol increases with the number of sub-carriers Nc and, hence, the amount of ISI decreases.The number of interfering symbols in a multi-carrier modulated system is given byResidual ISI can be eliminated by the use of a guard interval(see Section 1.2).The maximum Doppler spread in mobile radio applications using single-carrier modulation is typically much less than the distance between adjacent channels, such that the effect of interference on adjacent channels due to Doppler spread is not a problem for single-carrier modulated systems.For multi-carrier modulated systems, the sub-channel spacing Fs can become quite small, such that Doppler effects can cause significant ICI.As long as all sub-carriers are affected by a common Doppler shift fD, this Doppler shift can be compensated for in the receiver and ICI can be avoided.However, if Doppler spread in the order of several percent of the sub-carrier spacing occurs, ICI may degrade the system performance significantly.T oavoid performance degradations due to ICI or more complex receivers with ICI equalization, the sub-carrier spacing Fs should be chosen assuch that the effects due to Doppler spread can be neglected(see Chapter 4).This approach corresponds with the philosophy of OFDM described in Section 1.2 and is followed in current OFDM-based wireless standards.Nevertheless, if a multi-carrier system design is chosen such that the Doppler spread is in the order of the sub-carrier spacing or higher, a rake receiver in the frequency domain can be used [22].With the frequency domain rake receiver each branch of the rake resolves a different Doppler frequency.1.1.5Examples of Discrete Multipath Channel Models Various discrete multipath channel models for indoor and outdoor cellular systems with different cell sizes have been specified.These channel models define the statistics of the 5 discrete propagation paths.An overview of widely used discrete multipath channel models is given in the following.COST 207 [8]: The COST 207 channel models specify four outdoor macro cell propagation scenarios by continuous, exponentially decreasing delay power density spectra.Implementations of these power density spectra by discrete taps are given by using up to 12 taps.Examples for settings with 6 taps are listed in Table 1-1.In this table for several propagation environments the corresponding path delay and power profiles are given.Hilly terrain causes the longest echoes.The classical Doppler spectrum with uniformly distributed angles of arrival of the paths can be used for all taps for simplicity.Optionally, different Doppler spectra are defined for the individual taps in [8].The COST 207 channel models are based on channel measurements with a bandwidth of 8–10 MHz in the 900-MHz band used for 2Gsystems such as GSM.COST 231 [9] and COST 259 [10]: These COST actions which are the continuation of COST 207 extend the channel characterization to DCS 1800, DECT, HIPERLAN and UMTS channels, taking into account macro, micro, and pico cell scenarios.Channel models with spatial resolution have been defined in COST 259.The spatial component is introduced by the definition of several clusters with local scatterers, which are located in a circle around the base station.Three types of channel models are defined.The macro cell type has cell sizes from 500 m up to 5000 m and a carrier frequency of 900 MHz or 1.8 GHz.The micro cell type is defined for cell sizes of about 300 m and a carrier frequency of 1.2 GHz or 5 GHz.The pico cell type represents an indoor channel model with cell sizes smaller than 100 m in industrial buildings and in the order of 10 m in an office.The carrier frequency is 2.5 GHz or 24 GHz.COST 273: The COST 273 action additionally takes multi-antenna channel models into account, which are not covered by the previous COST actions.CODIT [7]: These channel models define typical outdoor and indoor propagation scenarios for macro, micro, and pico cells.The fading characteristics of the various propagation environments are specified by the parameters of the Nakagami-m distribution.Every environment is defined in terms of a number of scatterers which can take on values up to 20.Some channel models consider also the angular distribution of the scatterers.They have been developed for the investigation of 3G system proposals.Macro cell channel type models have been developed for carrier frequencies around 900 MHz with 7 MHz bandwidth.The micro and pico cell channel type models have been developed for carrier frequencies between 1.8 GHz and 2 GHz.The bandwidths of the measurements are in the range of 10–100 MHz for macro cells and around 100 MHz for pico cells.JTC [28]: The JTC channel models define indoor and outdoor scenarios by specifying 3 to 10 discrete taps per scenario.The channel models are designed to be applicable for wideband digital mobile radio systems anticipated as candidates for the PCS(Personal Communications Systems)common air interface at carrier frequencies of about 2 GHz.UMTS/UTRA [18][44]: Test propagation scenarios have been defined for UMTS and UTRA system proposals which are developed for frequencies around 2 GHz.The modeling of the multipath propagation corresponds to that used by the COST 207 channel models.HIPERLAN/2 [33]: Five typical indoor propagation scenarios for wireless LANs in the 5 GHz frequency band have been defined.Each scenario is described by 18discrete taps of the delay power density spectrum.The time variance of the channel(Doppler spread)is modeled by a classical Jake’s spectrum with a maximum terminal speed of 3 m/h.Further channel models exist which are, for instance, given in [16].1.1.6Multi-Carrier Channel Modeling Multi-carrier systems can either be simulated in the time domain or, more computationally efficient, in the frequency domain.Preconditions for the frequency domain implementation are the absence of ISI and ICI, the frequency nonselective fading per sub-carrier, and the time-invariance during one OFDM symbol.A proper system design approximately fulfills these preconditions.The discrete channel transfer function adapted to multi-carrier signals results inwhere the continuous channel transfer function H(f, t)is sampled in time at OFDM symbol rate s and in frequency at sub-carrier spacing Fs.The durations is the total OFDM symbol duration including the guardinterval.Finally, a symbol transmitted onsub-channel n of the OFDM symbol i is multiplied by the resulting fading amplitude an,i and rotated by a random phase ϕn,i.The advantage of the frequency domain channel model is that the IFFT and FFT operation for OFDM and inverse OFDM can be avoided and the fading operation results in one complex-valued multiplication per sub-carrier.The discrete multipath channel models introduced in Section 1.1.5 can directly be applied to(1.16).A further simplification of the channel modeling for multi-carrier systems is given by using the so-called uncorrelated fading channel models.1.1.6.1Uncorrelated Fading Channel Models for Multi-Carrier Systems These channel models are based on the assumption that the fading on adjacent data symbols after inverse OFDM and de-interleaving can be considered as uncorrelated [29].This assumption holds when, e.g., a frequency and time interleaver with sufficient interleaving depth is applied.The fading amplitude an,i is chosen from a distribution p(a)according to the considered cell type and the random phase ϕn,I is uniformly distributed in the interval [0,2π].The resulting complex-valued channel fading coefficient is thus generated independently for each sub-carrier and OFDM symbol.For a propagation scenario in a macro cell without LOS, the fading amplitude an,i is generated by a Rayleigh distribution and the channel model is referred to as an uncorrelated Rayleigh fading channel.For smaller cells where often a dominant propagation component occurs, the fading amplitude is chosen from a Rice distribution.The advantages of the uncorrelated fading channel models for multi-carrier systems are their simple implementation in the frequency domain and the simple reproducibility of the simulation results.1.1.7Diversity The coherence bandwidth of amobile radio channel is the bandwidth over which the signal propagation characteristics are correlated and it can be approximated byThe channel is frequency-selective if the signal bandwidth B is larger than the coherence bandwidth.On the other hand, if B is smaller than , the channel is frequency nonselective or flat.The coherence bandwidth of the channel is of importance for evaluating the performance of spreading and frequency interleaving techniques that try to exploit the inherent frequency diversity Df of the mobile radio channel.In the case of multi-carrier transmission, frequency diversity is exploited if the separation of sub-carriers transmitting the same information exceeds the coherence bandwidth.The maximum achievable frequency diversity Df is given by the ratio between the signal bandwidth B and the coherence bandwidth,The coherence time of the channel is the duration over which the channel characteristics can be considered as time-invariant and can be approximated byIf the duration of the transmitted symbol is larger than the coherence time, the channel is time-selective.On the other hand, if the symbol duration is smaller than , the channel is time nonselective during one symbol duration.The coherence time of the channel is of importance for evaluating the performance of coding and interleaving techniques that try to exploit the inherent time diversity DO of the mobile radio channel.Time diversity can be exploited if the separation between time slots carrying the same information exceeds the coherence time.A number of Ns successive time slots create a time frame of duration Tfr.The maximum time diversity Dt achievable in one time frame is given by the ratio between the duration of a timeframe and the coherence time, A system exploiting frequency and time diversity can achieve the overall diversityThe system design should allow one to optimally exploit the available diversity DO.For instance, in systems with multi-carrier transmission the same information should be transmitted on different sub-carriers and in different time slots, achieving uncorrelated faded replicas of the information in both dimensions.Uncoded multi-carrier systems with flat fading per sub-channel and time-invariance during one symbol cannot exploit diversity and have a poor performance in time and frequency selective fading channels.Additional methods have to be applied to exploit diversity.One approach is the use of data spreading where each data symbol is spread by a spreading code of length L.This, in combination with interleaving, can achieve performance results which are given forby the closed-form solution for the BER for diversity reception in Rayleigh fading channels according to [40] Whererepresents the combinatory function,and σ2 is the variance of the noise.As soon as the interleaving is not perfect or the diversity offered by the channel is smaller than the spreading code length L, or MCCDMA with multiple access interference is applied,(1.22)is a lower bound.For L = 1, the performance of an OFDM system without forward error correction(FEC)is obtained, 9which cannot exploit any diversity.The BER according to(1.22)of an OFDM(OFDMA, MC-TDMA)system and a multi-carrier spread spectrum(MC-SS)system with different spreading code lengths L is shown in Figure 1-3.No other diversity techniques are applied.QPSK modulation is used for symbol mapping.The mobile radio channel is modeled as uncorrelatedRayleigh fading channel(see Section 1.1.6).As these curves show, for large values of L, the performance of MC-SS systems approaches that of an AWGN channel.Another form of achieving diversity in OFDM systems is channel coding by FEC, where the information of each data bit is spread over several code bits.Additional to the diversity gain in fading channels, a coding gain can be obtained due to the selection of appropriate coding and decoding algorithms.中文翻译 1基本原理这章描述今日的基本面的无线通信。
Gas-vapor separating and gas purifying apparatus

专利名称:Gas-vapor separating and gas purifying apparatus发明人:Charles L. Lamoreaux申请号:US05/589387申请日:19750623公开号:US03961919A公开日:19760608专利内容由知识产权出版社提供摘要:Apparatus for separating vapor from pressurized gas and for then purifying the gas comprises a cylindrical pressure tank having a lower opening closed by a removable base member and having disposed within the tank a gas purifying element. An annular vapor separating chamber is formed in the base member and an annular passageway communicating therewith is formed between the outer wall of the purifying element and the inner wall of the tank. A formed tube connected to an inlet passageway in the base member directs a pressurized gas-vapor mixture tangentially into the annular chamber and in a plane generally at right angles to the axis thereof, thereby causing vortexing of the mixture around the chamber and upwardly, around the annular passageway between the purifying element and tank, to a gas permeable upper region of the purifying element. Vapor is centrifugally separated from the vortexing gas-vapor mixture, the condensate being gravity collected in the bottom of the annular chamber. The purifying element, which may contain several different purifying media, removes residual vapor and chemical and particulate impurities from the gas as the gas passes downwardly therethrough. Purified gas exits the tank through an outlet passageway in the base member. A drain valve, by which condensate is drained from the annular chamber, and aburst diaphragm, which prevents over- pressurization of the tank, are provided in the base member.申请人:LAMOREAUX; CHARLES L.代理人:Henry M. Bissell更多信息请下载全文后查看。
Appliance for the production and administration of

专利名称:Appliance for the production andadministration of gas vapor mixtures 发明人:EDMONDSON WILLIAM,JONES WILFRED 申请号:US6213448申请日:19481126公开号:US2499734A公开日:19500307专利内容由知识产权出版社提供摘要:628,709. Anaesthetic apparatus. EDMONDSON, W., and JONES, W. Oct. 6, 1947, No. 26774. [Class 81 (ii)] An analgesia appliance comprises a container A fitted with concentric tubes E, J having holes E2, J1 and surrounded by baffles F and a cylindrical wick H. The lower part of the container is filled with the liquid to be vaporised and this is soaked up into the wick. The lower end of the tube E is closed by a plug E1 and, when the patient breathes in, a non-return valve T opens. and air passes through passages P2, P1 down to the perforations in the tube J; it is deflected outwards into the space surrounded by the wick and then passes inwards to tube E and up to the face mask attached to the outlet R2. An expiratory valve U is fitted in the upper wall of the top chamber R. The plate P may be rotated about the central axis so that, by co-operation with apertures in the fixed plates O, D, the relative proportions of air passing directly to the face mask and through the anµsthetic container may be varied.申请人:WILLIAM EDMONDSON,WILFRED JONES更多信息请下载全文后查看。
MixturesandSolutions

Copyright © 2010 Pearson Education, Inc.
Chapter Nine
11
► Weight/Volume Percent Concentration [(w/v)%]
► Mathematically, (w/v)% concentration is found by taking the number of grams of solute per milliliters of solution and multiplying by 100.
Chapter Nine
2
-The Solutions Process
A good rule of thumb for predicting solubility is that “like dissolves like”. Substances with similar intermolecular forces form solutions and substances with different intermolecular forces do not.
Mass of solute (g)
(w/v)% concentration =
x 100
Volume of solution (mL)
Copyright © 2010 Pearson Education, Inc.
Chapter Nine
The Properties of Gases and Their Mixtures

The Properties of Gases and TheirMixtures气体及其混合物的性质气体是无定形的、可以通过升高温度、增加压强和减小体积而变得无限扩散的物质。
气体在自然界中广泛存在,例如空气、水蒸气和二氧化碳等。
在化学和工业生产中,气体的性质和行为是非常重要的,因此对气体及其混合物的研究一直是自然科学中的核心内容之一。
1. 气体压力和分子运动气体存在于容器中时,它们对容器壁的压力是由分子撞击壁面造成的。
气体的分子随时随地以不断碰撞的方式相互作用,这种作用导致了气体的体积和压力。
气体分子的速度和平均动能取决于气体的温度。
通过控制温度和压力,我们可以改变气体的状态和性质。
2. 气体的扩散和弥散气体的无限扩散,是由于气体的分子没有固定的位置,而是以不同的速度和方向运动。
在高压下,气体分子密集,扩散速度较慢,因此气体的弥散速度和压强成反比例关系。
根据弥散规律,气体分子的平均自由程是气体分子间距离的一半,可以用来对气体的弥散速度进行预测。
3. 理想气体定律理想气体定律是描述体积、压力、温度和摩尔数之间关系的基本规律。
根据理想气体定律,气体的压力P、体积V和温度T之间存在关系:P V/T = 常数。
这个常数被称为理想气体常数R,可以被表示为R=kN,其中k是玻尔兹曼常数,N是气体的分子数。
理想气体定律可以用来计算气体的状态和行为,但是它只适用于低密度气体,因为当气体分子间距离非常接近时,分子间的相互作用就变得非常重要。
4. 叶克斯定律和气体混合物当两种或多种气体混合在一起时,它们的行为是复杂的,并且通常需要使用一些特殊的定律进行描述。
叶克斯定律描述了气体混合物的行为和活度系数的关系,其中活度系数是描述混合物中每种成分的效应的轻便方法。
当气体混合物从一个容器流入另一个容器时,每种成分的分压力都会根据相应的摩尔分数分配在容器中。
摩尔分数是指每种成分占混合物中总摩尔数的比例,因此它对混合物中每种成分的压力和体积的贡献都不同。
燃烧理论

FACE9 – Theory of Combustion
- 3 components of internal energy: translational, vibrational & rotational;
- Diatomic molecule: not only translational but also vibrational & rotational contributions.
·
Chemical equilibrium
▫ ▫ ▫ Second law of thermodynamics Chemical equilibrium principle Full equilibrium products of combustion
3
·
Ideal-gas behavior
◦ Combustion: ”Rapid oxidation generating heat, or both light and heat; also slow oxidation accompanied by relatively little heat and no light” ”Ideal gas is the system where interaction is absent (i.e., zero potential energy)”. High temperatures associated with combustion generally result in sufficiently low densities for the Ideal Gas Behaviour to hold.
FACE9 – Theory of Combustion
Yi = xi MWi / MW mix
Chapter 11 Gas-Vapor Mixtures and Air Conditioning11章气体混合物的蒸气与空气调节

t2, ω2, 2100% hf
Conservation of mass
m a1m a2m a m v1m f m v2
m f m v2m v1
Conservation of energy
m a 1 h a 1 m v 1 h v 1 m fh f m a 2 h a 2 m v 2 h v 2 m a 1 h a 1 m v 1 h v 1 ( m v 2 m v 1 ) h f m a 2 h a 2 m v 2 h v 2
Rv Pa
Pa
P Pv
11-2-2 Relative humidity of air
The comfort level depends more on the amount
of moisture the air holds(mv) relative to the
maximum amount of moisture the air can hold at
h= 1.005t + ω( 2501 + 1.863t) kJ/kg.℃
11-3 Adiabatic Saturation and Wet-Bulb Temperature
11-3-1 Adiabatic saturation
Unsaturated air
Saturated air
t1, ω1, 1
The dry air and vapor of atmospheric air in air conditioning application range (temperature changes from -10 ℃ to 50 ℃) can be treated as ideal gas
Chapter 11 Gas-Vapor Mixtures and Air Conditioning11章气体混合物的蒸气与空气调节

0.622 Pv
PPv
0.622 Pg P Pg
11-2-3 Enthalpy of air
HHaHv mahamvhv
Specific enthalpy:
h
ha
mv ma
hv
ha
hv
1.863kJ/kg. ℃ is the
In the field of air-conditioning, the reafveerreangceespecific heat
m a 1 h a 1 m v 1 h v 1 ( m v 2 m v 1 ) h f m a 2 h a 2 m v 2 h v 2
Dividing by m am a1m a2 gives:
h a 1 1 h v 1 (2 1 ) h f h a 2 2 h v 2
temperature is 0℃. Then:
of dry air
ha= 1.005t kJ/kg.℃ hv= 2501 + 1.863t kJ/kg.℃
2501kJ/kg means the enthalpy at 0 ℃
1.863kJ/kg. ℃ is the averagБайду номын сангаас specific heat of vapor
t2, ω2, 2100% hf
Conservation of mass
m a1m a2m a m v1m f m v2
m f m v2m v1
Conservation of energy
m a 1 h a 1 m v 1 h v 1 m fh f m a 2 h a 2 m v 2 h v 2 m a 1 h a 1 m v 1 h v 1 ( m v 2 m v 1 ) h f m a 2 h a 2 m v 2 h v 2
Quick Guide Hazardous Materials说明书
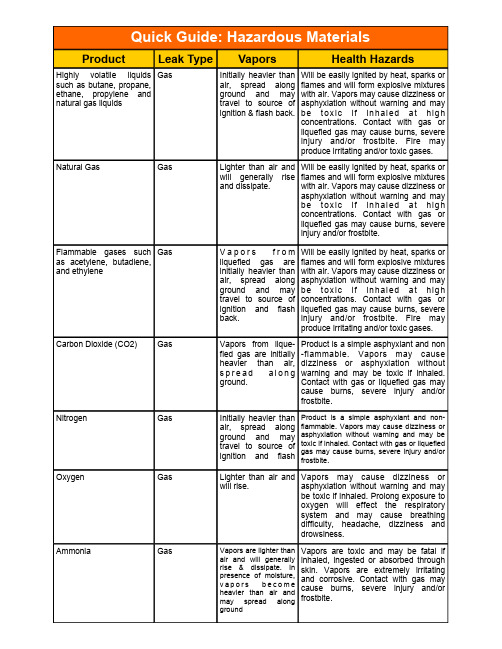
Highly volatile liquids such as butane, propane, ethane, propylene and natural gas liquidsGas Initially heavier than air, spread along ground and may travel to source of ignition & flash back. Will be easily ignited by heat, sparks or flames and will form explosive mixtures with air. Vapors may cause dizziness or asphyxiation without warning and may b e t o x i c i f i n h a l e d a t h ig hconcentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating and/or toxic gases. Natural Gas GasLighter than air and will generally rise and dissipate. Will be easily ignited by heat, sparks or flames and will form explosive mixtures with air. Vapors may cause dizziness orasphyxiation without warning and may b e t o x i c i f i n h a l e d a t h ig h concentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Flammable gases such as acetylene, butadiene, and ethyleneGas V a p o r s f r o m liquefied gas are initially heavier than air, spread along ground and may travel to source of ignition and flash back. Will be easily ignited by heat, sparks or flames and will form explosive mixtures with air. Vapors may cause dizziness or asphyxiation without warning and may b e t o x i c i f i n h a l e d a t h ig h concentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire mayproduce irritating and/or toxic gases. Carbon Dioxide (CO2) GasVapors from lique-fied gas are initially heavier than air, s p r e a d a l o n g ground. Product is a simple asphyxiant and non -flammable. Vapors may cause dizziness or asphyxiation without warning and may be toxic if inhaled. Contact with gas or liquefied gas maycause burns, severe injury and/or frostbite. Nitrogen GasInitially heavier than air, spread alongground and maytravel to source of ignition and flash Product is a simple asphyxiant and non-flammable. Vapors may cause dizziness or asphyxiation without warning and may be toxic if inhaled. Contact with gas or liquefiedgas may cause burns, severe injury and/orfrostbite. Oxygen GasLighter than air and will rise. Vapors may cause dizziness or asphyxiation without warning and maybe toxic if inhaled. Prolong exposure to oxygen will effect the respiratory system and may cause breathing difficulty, headache, dizziness and drowsiness.Ammonia GasVapors are lighter than air and will generally rise & dissipate. In presence of moisture, v a p o r s b e c o m eheavier than air andmay spread alonggroundVapors are toxic and may be fatal if inhaled, ingested or absorbed through skin. Vapors are extremely irritating and corrosive. Contact with gas may cause burns, severe injury and/or frostbite.Hazardous liquids such as crude oil, diesel fuel, jet fuel, gasoline, and other refined productsGas Initially heavier than air and spread along ground and collect in low and confined ar-eas. Vapors maytravel to source ofignition and flash back. Explosion hazard in-doors, outdoors and sewers.Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. Methanol GasV a p o r s f r o mliquefied gas areinitially heavier than air, spread alongground and may travel to source of ignition and flash back. Toxic, may be fatal if inhaled, ingested or absorbed through skin. Inhalation or contact will irritate or burn skin and eyes. Fire willproduce irritating, corrosive and/or toxic gases. Vapors may cause dizziness orsuffocation. Runoff from fire control or dilution water may cause pollution. Runoff to sewer may create fire or explosion hazard. Carbon Monoxide GasV a p o r s f r o mliquefied gas areinitially heavier than air, spread alongground. Vapors may travel to source of ignition and flashToxic, may be fatal if inhaled, ingested or absorbed through skin. Contact may cause burns, severe injury and/or frostbite. Firewill produce irritating, corrosive and/or toxic gases. Flammable may be ignited by heat,sparks or flames. Runoff from fire control or dilution water may cause pollution.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
t1, ω1, 1
hf
t2, ω2, 2100%
Conservation of mass
m m m a 1 a 2 a v f m m m 1 v 2
f v2 v m m m 1
Conservation of energy
The ratio of these two quantities is called the relative humidity The saturated
mv pv R vT p gV pg mg R vT
p vV
pressure under the temperature of atmospheric air
m h m h m h m h m h a 1 a 1 v 1 v 1 f f a 2 a 2 v 2 v 2
m h m h ( m m ) h m h m h a 1 a 1 v 1 v 1 v 2 v 1 f a 2 a 2 v 2 v 2
1.863kJ/kg. ℃ is the average specific heat of vapor
h= 1.005t + ω( 2501 + 1.863t) kJ/kg.℃
11-3 Adiabatic Saturation and Wet-Bulb Temperature
11-3-1 Adiabatic saturation
11-1-2 The pressure of atmospheric air
pp a p v
The total pressure of atmospheric air The partial pressure of dry air
The partial pressure of vapor, increases with the amount of vapor in air
Since Vv=Va and Tv=Ta
p vV v
Ra= 287.1 J/kg.K
Rv= 461.4 J/kg.K
Pv P R a Pv 0 .622 0.622 v Pa P P Rv Pa v
11-2-2 Relative humidity of air The comfort level depends more on the amount of moisture the air holds(mv) relative to the maximum amount of moisture the air can hold at the same temperature(mg).
P P v g 0.622 0.622 PP P P v g
11-2-3 Enthalpy of air
ቤተ መጻሕፍቲ ባይዱ
m h m h HH H a a v v a v
Specific enthalpy:
mv h ha hv h h a v ma
1.863kJ/kg. ℃ is the In the field of air-conditioning, the reference average specific heat temperature is 0℃. Then: of dry air ha= 1.005t kJ/kg.℃ hv= 2501 + 1.863t kJ/kg.℃ 2501kJ/kg means the enthalpy at 0 ℃
Chapter 11 Gas-Vapor Mixtures and Air Conditioning
11-1 Dry and Atmospheric Air
11-1-1 Atmospheric air The air in the atmosphere normally contains some water vapor and is referred to atmospheric air. Air that contains no water is called dry air. Although the amount of water vapor in the air is small, it plays a major role in human comfort. Therefore it is an important consideration in air conditioning applications The dry air and vapor of atmospheric air in air conditioning application range (temperature changes from -10 ℃ to 50 ℃) can be treated as ideal gas
11-2 The properties of air
11-2-1 Specific humidity of air The ratio of the mass of water vapor present in a unit mass of dry air, denoted by ω
R vT v mv p aV a ma R aT a
T
pmax
p2
p1 T1
s
11-1-3 Dew-point Temperature T p1 1 T1 As to vapor state 1, decrease the temperature , then pressure of vapor won’t change
Dew- point
The dew-point Tdp is defined as the temperature at which condensation begins if the air is cooled at constant pressure
