CL-82198_SDS_MedChemExpress
朗道多项生化定值质控

0843PAGE 1 OF 24LIQUID ASSAYED CHEMISTRY CONTROL PREMIUM PLUS - LEVEL 1 (LIQ CHEM ASY PREMIUM PLUS 1)Cat. No . LAL 4213 Lot No . 153UL Size : 12 x 5 ml Expiry : 2015-02INTENDED USEThis product is intended for in vitro diagnostic use, in the quality control of diagnostic assays. The Liquid Assayed Chemistry Control Premium Plus is for the control of accuracy.DEVICE DESCRIPTIONThe Liquid Assayed Chemistry Control Premium Plus is supplied at 3 levels, level 1, 2 and 3. Target values and ranges are supplied for the analytes listed in the values section at all three levels.SAFETY PRECAUTIONS AND WARNINGSFor in vitro diagnostic use only. Do not pipette by mouth. Exercise the normal precautions required for handling laboratory reagents.Human source material from which this product has been derived has been tested at donor level for the Human Immunodeficiency Virus (HIV 1, HIV 2) antibody, Hepatitis B Surface Antigen (HbsAg), and Hepatitis C Virus (HCV) antibody and found to be NON-REACTIVE. FDA approved methods have been used to conduct these tests.However, since no method can offer complete assurance as to the absence of infectious agents, this material and all patient samples should be handled as though capable of transmitting infectious diseases and disposed of accordingly.Health and Safety Data Sheets are available on request.STORAGE AND STABILITY OPENED: Store refrigerated (+2ºC to + 8ºC). Thawed serum is stable for 7 days at +2ºC to +8ºC, with the followingexceptions: Troponin T is stable for 3 days at +2ºC to +8ºC. Only the required amount of product should be removed. After use, any residual product should NOT BE RETURNED to the original vial.UNOPENED: Store frozen at -20ºC to -70ºC. Stable to expiration date printed on individual vials (see Limitations).LIMITATIONSFor Total Acid Phosphatase, the material should be stabilised by adding 1 drop (25 µl – 30 µl) of 0.7M Acetic acid solution to 1ml of the serum after thawing. After stabilisation, Total Acid Phosphatase is stable for 7 days at +2ºC to +8ºC. Bilirubin in the serum is light sensitive and it is recommended that the serum is stored in the dark.ALT, Total Acid Phosphatase, Alkaline Phosphatase, Total and Direct Bilirubin values may gradually decrease during the products shelf life.Bacterial contamination of the thawed serum will cause reductions in the stability of many components. The control should not be used as a calibration material.PREPARATION1. Allow the frozen control to thaw at room temperature (+15ºC to +25ºC) until completely thawed. Swirl the contents to ensurehomogeneity.2. Refer to the Control section of the individual analyser application.3. Refrigerate any unused material. Prior to reuse, mix contents thoroughly.MATERIALS PROVIDEDLiquid Assayed Chemistry Control Premium Plus - Level 1 12 x 5 mlMATERIALS REQUIRED BUT NOT PROVIDED NoneASSIGNED VALUESEach lot of serum is submitted to a number of external laboratories. Values are assigned from a consensus of results obtained by these laboratories and internal testing conducted at Randox Laboratories Ltd. With each batch, a control range is provided for individual parameters and each parameter method.If an instrument specific value is not available, refer to the Mean of all Instruments section. If necessary, contact Randox Laboratories – Customer Technical Services, Northern Ireland, Tel: +44 (0) 28 9445 1070 or email Technical.Services@29 Nov 13 rwPage 2 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 3 of 24 29/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 4 of 24 29/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 5 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 6 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 7 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 8 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 9 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 10 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 11 of 2429/11/2013___________________________________________________________________________________________________Page 12 of 2429/11/2013___________________________________________________________________________________________________Page 13 of 2429/11/2013___________________________________________________________________________________________________Page 14 of 2429/11/2013___________________________________________________________________________________________________Page 15 of 2429/11/2013___________________________________________________________________________________________________Page 16 of 2429/11/2013___________________________________________________________________________________________________Page 17 of 2429/11/2013___________________________________________________________________________________________________Page 18 of 2429/11/2013___________________________________________________________________________________________________Page 19 of 2429/11/2013___________________________________________________________________________________________________Page 20 of 2429/11/2013___________________________________________________________________________________________________Page 21 of 2429/11/2013___________________________________________________________________________________________________Page 22 of 2429/11/2013___________________________________________________________________________________________________Page 23 of 2429/11/2013___________________________________________________________________________________________________Page 24 of 2429/11/2013___________________________________________________________________________________________________。
L-半胱氨酸盐酸盐一水物AJI92标准
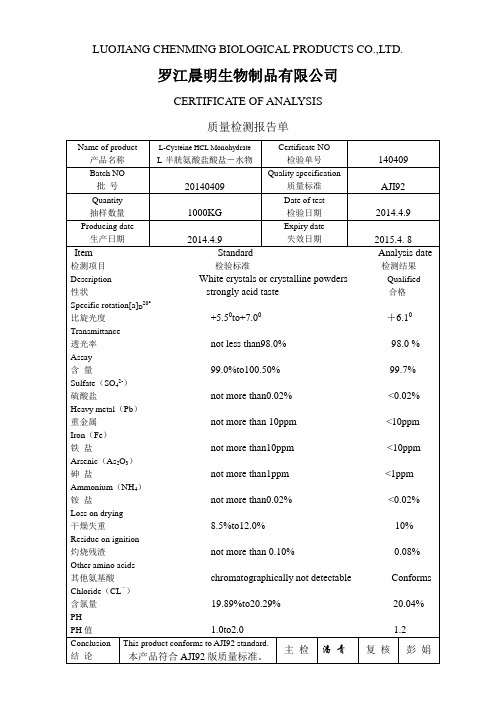
透光率not less than98.0% 98.0 %
Assay
含量99.0%to100.50% 99.7%
Sulfate(SO42-)
硫酸盐not more than0.02%<0.02%
Heavy metal(Pb)
重金属not more than 10ppm <10ppm
8itemstandardanalysisdatenameofproduct产品名称lcysteinehclmonohydratecertificateno检验单号检测项目检验标准检测结果descriptionwhitecrystalsorcrystallinepowdersqualified性状stronglyacidtaste合格specificrotationad20比旋光度55to7061transmittance000透光率notlessthan980980assay含量990to10050997sulfateso42硫酸盐notmorethan002002heavymetalpb重金属notmorethan10ppm10ppmironfe铁盐notmorethan10ppm10ppmarsenicas2o3砷盐notmorethan1ppm1ppmammoniumnh4铵盐notmorethan002002lossondrying干燥失重85to12010residueonignition灼烧残渣notmorethan010008otheraminoacids其他氨基酸chromatographicallynotdetectableconformschloridecl含氯量1989to20292004phph值10to2012conclusionthisproductconformstoaji92standard
SDSE所有详细试剂配方

SDSE所有详细试剂配方SDSE(Sodium dodecyl sulfate-electrolyte system)是一种常用的聚丙烯酰胺凝胶电泳缓冲液,主要用于蛋白质电泳分离。
SDSE的制备是根据一定的配方进行的,下面是SDSE的详细试剂配方:1.氯化钠(NaCl):18.3g溶解在1L去离子水中2.磷酸二氢钠(NaH2PO4):3.5g溶解在1L去离子水中3. 溴酚蓝(Bromophenol Blue):0.25g溶解在10mL水中4. SDS(Sodium Dodecyl Sulfate):10g溶解在1L去离子水中5. 甘油(Glycerol):10mL溶解在100mL去离子水中以上是SDSE的基础配方,下面是其制备步骤:1.将NaCl和NaH2PO4溶解在分别容量为1L的去离子水中,用磁力搅拌器搅拌均匀。
2.分别将溶解好的NaCl和NaH2PO4溶液倒入大容量烧杯中,混合均匀。
3.在pH计的帮助下调节缓冲液的pH值,使其在6.8-7.0之间。
4.加入溴酚蓝溶液,此时溴酚蓝的浓度为0.0025%,用磁力搅拌器搅拌均匀。
5.加入SDS溶液,用磁力搅拌器搅拌均匀。
注意,加入SDS时要小心,避免溅出。
6.最后加入甘油溶液,搅拌均匀。
甘油的添加主要是为了增加样品的密度,使其在凝胶中下沉更快。
SDSE的配方比较简单,主要成分包括NaCl、NaH2PO4、溴酚蓝、SDS和甘油。
其中,NaCl和NaH2PO4是缓冲液的主要组成部分,用于维持适当的离子强度和pH值;溴酚蓝用于着色样品,方便观察电泳结果;SDS主要起到断开蛋白质的二级结构和线性化作用;甘油则用于增加样品密度,使其在凝胶中下沉更快。
通过恰当的配方和制备步骤,可以获得高质量的SDSE缓冲液,用于蛋白质电泳分离和分析。
但需要注意的是,不同实验目的和要求可能需要微调配方中一些成分的浓度,因此在具体实验过程中,可以根据需要进行相应的调整。
超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量

超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量田晔;江骥;胡蓓;薛金萍;王洪允【摘要】建立了超高效液相色谱-串联质谱(UPLC-MS/MS)法同时测定使用艾普拉唑后人血浆中二甲基精氨酸(ADMA)、对称二甲基精氨酸(SDMA)、单甲基精氨酸(NMMA)、瓜氨酸(Cit)和L-精氨酸(L-Arg)的浓度.采用HILIC亲水相互作用色谱和非衍生化的蛋白沉淀法进行分离分析,色谱柱选取Waters Atlantic HILIC柱(2.1 mm×50 mm×3μm),流动相由乙腈(含0.5%乙酸和0.025%三氟乙酸)-水(含0.5%乙酸和0.025%三氟乙酸)(85:15,v/V)组成,流速0.25 mL/min.采用多反应离子监测(MRM)模式,以电喷雾离子源(ESI)正离子方式检测.结果显示,ADMA、SDMA、NMMA、L-Arg和Cit的线性关系良好,相关系数r均大于0.994 0;ADMA、SDMA和NMMA的线性范围为0.1~5 mmol/L,L-Arg和Cit的线性范围为10~250 mmol/L;5种氨基酸的日内、日间精密度均小于15%,准确度在85%~115%之间.该方法快速、简便、灵敏,可为相关疾病的临床诊断提供一种高效的检测手段.【期刊名称】《质谱学报》【年(卷),期】2016(037)005【总页数】7页(P446-452)【关键词】超高效液相色谱-串联质谱(UPLC-MS/MS);艾普拉唑;蛋白沉淀法;亲水性色谱【作者】田晔;江骥;胡蓓;薛金萍;王洪允【作者单位】福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730【正文语种】中文【中图分类】O657.63一氧化氮是人体重要的信使分子,L-精氨酸(L-Arg)在一氧化氮全酶(NOS)的催化下,产生一氧化氮(NO)和瓜氨酸(Cit)[1-2]。
制备格式试剂

Copyright © 2018 Elsevier Life Sciences IP Limited except certain content provi‐ ded by third parties. Reaxys is a trademark of Elsevier Life Sciences IP Limited.
ห้องสมุดไป่ตู้
1/3
2018-10-09 05:45:20
Br
Cl
Mg
Br
Mg
Cl
Rx-ID: 23053998 View in Reaxys 1/1 Yield Conditions & References 4-Chlorophenyl-5-[(3,4-isopropylidine)-2-methylpyridine]-methanol hydrochloride; 4b To a refluxing (.similar.70°C) suspension of magnesium metal turning (18.23 g, 1.25 eq) in 500 ml of THF was added 4-bromochlorobenzene (14.45 g, 10percent of total quantity) and a crystal of iodine. After the initiation of the reac‐ tion, the remaining quantity of 4-bromochlorobenzene (130.05 g in 200 ml of THF) was added over a period of 60 minutes at reflux temperature. 30 minutes after the comple
盐酸利多卡因注射剂遗传毒性杂质研究_NormalPdf

Journal of China Pharmaceutical University2020,51(4):466-471学报盐酸利多卡因注射剂遗传毒性杂质研究冼芷然1,孙春萌2,骆雪芳1*,钟文英1**(1中国药科大学理学院药物质量研究中心,南京211198;2中国药科大学药学院,南京211198)摘要确定2,6-二甲基苯胺为盐酸利多卡因注射液中遗传毒性杂质,N-氯乙酰-2,6-二甲基苯胺为潜在遗传毒性杂质,建立LC-MS/MS方法,用色谱柱Agilent ZORBAX Eclipse Plus C18(4.6mm×250mm,5μm)对原料、自制制剂及原研制剂进行遗传毒性杂质研究。
研究结果表明自制制剂中杂质2,6-二甲基苯胺与N-氯乙酰-2,6-二甲基苯胺除由原料引入外,可能分别由氧化条件或碱性条件下降解引入,为盐酸利多卡因注射液的遗传毒性风险评估和工艺优化提供参考与指导。
关键词盐酸利多卡因注射液;遗传毒性杂质;LC-MS/MS中图分类号R917文献标志码A文章编号1000-5048(2020)04-0466-06doi:10.11665/j.issn.1000-5048.20200412引用本文冼芷然,孙春萌,骆雪芳,等.盐酸利多卡因注射剂遗传毒性杂质研究[J].中国药科大学学报,2020,51(4):466–471.Cite this article as:XIAN Zhiran,SUN Chunmeng,LUO Xuefang,et al.Profiling of genotoxic impurities in a lidocaine hydrochloride injec‐tion[J].J China Pharm Univ,2020,51(4):466–471.Profiling of genotoxic impurities in a lidocaine hydrochloride injection XIAN Zhiran1,SUN Chunmeng2,LUO Xuefang1*,ZHONG Wenying1**1Drug Quality Research Center,College of Science,China Pharmaceutical University;2School of Pharmacy,China Pharmaceutical University,ChinaAbstract2,6-dimethylbenzenamine was determined as a genotoxic impurity in lidocaine hydrochloride injec‐tion,and2-chloro-N-(2,6-dimethylphenyl)acetamide was determined as potential genotoxic impurity.An LC-MS/ MS method was established to research the profiling of genotoxic impurities in active pharmaceutical ingredients (API),homemade preparation and reference preparation on column Agilent ZORBAX Eclipse Plus C18(4.6mm×250mm,5μm).The results show that in the homemade preparation the2,6-dimethylbenzenamine and the 2-chloro-N-(2,6-dimethylphenyl)acetamide may be degraded under oxidation condition and alkaline condition in addition to the introduction from API preparation process.This study provides guidance for genotoxic risk assess‐ment and prescription process optimization of lidocaine hydrochloride.Key words lidocaine hydrochloride injection;genotoxic impurities;LC-MS/MS盐酸利多卡因(lidocaine hydrochloride)为临床上常制成盐酸利多卡因注射剂应用于局部麻醉药[1]和抗心律失常药物等[2-3]。
BeyoLytic

BeyoLytic™细菌活性蛋白抽提试剂产品简介:碧云天生产的BeyoLytic™细菌活性蛋白抽提试剂(BeyoLytic™ Bacterial Active Protein Extraction Reagent),也称BeyoLytic™ Bacterial Native Protein Extraction Reagent ,是一种无需超声或高压破碎,能快速、高质量、高产量、高活性地直接裂解并抽提大肠杆菌表达的重组蛋白以及大肠杆菌自身表达的可溶性蛋白的细菌活性蛋白提取试剂。
本产品非常适合快速高通量的蛋白表达和筛选。
本产品不仅可以用于抽提可溶性蛋白,也可以用于洗涤去除粘附在包涵体表面的细胞碎片以获得高纯度的包涵体蛋白。
需要注意的是,本产品不能溶解包涵体。
本产品与Sigma 的CelLytic™ B (B7435)、CelLytic™ B Plus Kit (CB0500/CB0050),Thermo 的B-PER ® Bacterial Protein Extraction Reagent (78243/78248/90084)、B-PER ® II Bacterial Protein Extraction Reagent (78260)、B-PER ® Direct Bacterial Protein Extraction Kit (90080/90081)、B-PER™ with Enzymes Bacterial Protein Extraction Kit (90078/90079)等非常相近,使用效果和用途也非常相近,很多时候可以考虑相互替代。
本产品经过优化,适用于BL21菌,也同样适用于DH5ɑ、JM109以及其它类似的细菌。
本产品是一种含有特定的经过精心筛选以确保蛋白抽提效果良好的比较温和的非变性去垢剂(detergent)的缓冲液(含40mM Tris, pH8.0)。
放射免疫沉淀法裂解缓冲液

放射免疫沉淀法裂解缓冲液
放射免疫沉淀法(RIPA)裂解缓冲液是一种用于裂解细胞膜并释放细胞内蛋白质的缓冲液。
这种缓冲液通常包含以下成分,盐类(如氯化钠)、表面活性剂(如聚氧乙烯醚硫酸酯类)、缓冲剂(如Tris-HCl)、蛋白酶抑制剂(如苯甲烷磺酰氟化物)和其他辅助成分(如甘油)。
这些成分的配比和浓度可以根据具体实验的要求进行调整。
RIPA裂解缓冲液的作用是破坏细胞膜结构,释放细胞内的蛋白质,使其可以被进一步分析和检测。
在放射免疫沉淀法中,这种缓冲液可以用于提取细胞内的蛋白质,以便进行免疫沉淀和后续的放射性测定。
从化学角度来看,RIPA裂解缓冲液中的盐类和表面活性剂可以破坏细胞膜的脂质双层结构,使细胞膜失去完整性,从而释放细胞内的蛋白质。
而缓冲剂则可以维持溶液的pH值,保持蛋白质的稳定性。
蛋白酶抑制剂的作用是防止蛋白质在裂解过程中被降解。
这些成分共同作用,使RIPA裂解缓冲液成为一种有效的细胞裂解工具。
在实验操作中,选择合适的RIPA裂解缓冲液对于成功提取目标
蛋白质至关重要。
不同类型的细胞或组织可能需要不同配方的RIPA 裂解缓冲液,因此在实验前需要根据具体情况进行优化配比。
总的来说,RIPA裂解缓冲液在放射免疫沉淀法中扮演着至关重要的角色,通过破坏细胞膜并释放细胞内蛋白质,为后续的实验操作提供了必要的基础。
760-78-1_DL-正缬氨酸_MED11074技术资料_上海_Medbio脉铂
1g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11127
Fmoc-L-丝氨酸
Fmoc-Ser-OH
73724-45-5
100g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
CAS
包装
纯度
MedBio
MED11025
N-羟基琥珀酰亚胺
HOSu
6066-82-6
100g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11040
丝氨酸苄酯盐酸盐
H-Ser-OBzl.HCl
1738-72-3
100g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11086
5g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11059
N-Fmoc-N'-Boc-L-2,3-二氨基丙酸
Fmoc-Dap(Boc)-OH
162558-25-0
1g
≥98%
纯度
MedBio
MED11049
Fmoc-N-三苯甲基-L-天冬酰胺
Fmoc-Asn(Trt)-OH
132388-59-1
100g
≥98%
品牌
货号
中文名称
爱必信WB彩色凝胶试剂盒上新啦!让您的WB实验多姿多彩

爱必信WB彩色凝胶试剂盒上新啦!
让您的WB实验多姿多彩
蛋白免疫印迹实验(Western Blot)实验大家肯定都不陌生,要想做出好看的条带,从制胶开始就不容半点马虎。
WB制胶可谓实验室新手的必修课了,想想小编我在当初实验室的时候,就没少帮师兄师姐配置WB凝胶,泛黄的配方表小编还记忆犹新。
WB凝胶配
方表
说起WB凝胶配置,小编可有一肚子苦水要倒,第一个让人头疼的一点就是多种溶液都需要计算量取,每次调移液器就要调半天。
还有各种试剂的保存条件也不一样,配胶之前还需要从冰箱,室温柜子里分别拿出不同的溶液。
这还不算,最容易出问题的促凝剂APS,需要新鲜配置,4度保存不能超过一周,关键是粉末还特容易吸水,打开后需要密封保存。
小编之前就踩过一次坑,APS粉末吸水结块,称量配置后的APS含量不够,凝胶凝结不好,整个WB实验条带跑的七扭八歪。
蛋白质印迹(Western Blot)实验中产品推荐:。
加速溶剂萃取-超高效液相色谱-串联质谱法测定大枣中3种五环三萜酸

食品与药品Food and Drug2021年第23卷第1期17加速溶剂萃取-超高效液相色谱-串联质谱法测定大枣中3种五环三祜酸张萍,何婷,王颖,胡克特,顾丁,陈荣祥**(遵义医科大学基础医学院,贵州遵义563000)摘要:目的建立加速溶剂萃取-超高效液相色谱-串联质谱法(ASE-UPLC-MS/MS)同时测定大枣中桦木酸、齐墩果酸和熊果酸的方法。
方法样品釆用ASE提取,优化提取条件,并与超声辅助提取法进行比较。
优化 后的提取条件为:以80%甲醇为提取溶剂,提取温度100-C,静态萃取时间15min,萃取1次。
提取液釆用Waters ACQUITY BEH C18色谱柱分离,以乙ffi-15mmol/L乙酸钱(pH9.3)为流动相,梯度洗脱,经UPLC-MSZMS仪,釆用电喷雾电离源,负离子模式下多反应监测模式检测。
结果桦木酸、齐墩果酸、熊果酸在0.5~10 mg/L范围内,浓度与峰面积线性关系良好,相关系数大于0.9900。
不同浓度3种化合物的加标回收率93.6%〜101.7%,相对标准偏差在1.18%~6.83%之间。
结论此法简单快速、准确稳定、重复性好,可用于大枣中桦木酸、齐墩果酸、熊果酸的含量测定。
关键词:加速溶剂萃取;超高效液相色谱;串联质谱法;大枣中图分类号:R284.1文献标识码:A文章编号:1672-979X(2021)01-0017-06DOI:10.3969/j.issn.l672-979X.2021.01.004Simultaneous Determination of Three Pentacyclic Triterpenic Acids in Jujubae Fructus byAccelerated Solvent Extraction-UPLC-MS/MSZHANG Ping,HE Ting,WANG Ying,HU Ke-te,GU Ding,CHEN Rong-xiang(School of B asic Medical Sciences,Zunyi Medical University,Zu^yi563000,China)Abstract:Objective To establish a method for the simultaneous determination of betulinic acid,oleanolic acid and ursolic acid in Jujubae fructus by ultra-high performance liquid chromatography-tandem mass spectrometry(UPLC-MS/MS)with accelerated solvent extraction(ASE).Methods The extract parameters of A SE were optimized and the efficiency was compared with the ultrasound-assisted extraction method.The optimum extraction conditions were as follows:80%methanol was selected as extraction solvent,oven temperature was100°C,the static extraction time was 15min and one extraction cycle was adopted.A waters ACQUITY BEH C18(2.1mmx]00mn,1.7“m)column was used as the stationary phase,acetonitrile and ammonium acetate solution(15mmol/L,pH9.3)was used as the mobile phase.Mass detection was conducted by electrospray ionization in negative ion multiple reaction monitoring mode. Results The calibration curves were linear over a concentration range of0.5-10mg/L for betulinic acid,oleanolic acid and ursolic acid.The correlation coefficients were greater than0.9900.The recoveries of different spiked levels were between93.6%and101.7%,with RSDs between1.18%and6.83%.Conclusion The method is simple,rapid,收稿日期:2020-09-04基金项目:国家自然科学基金(31660131,81760652);贵州省联合基金(黔科合J字LKZ[2013]17号);遵义医学院博士启动基金(F-568)作者简介:张萍,硕士研究生,研究方向:药用植物开发与利用E-mail:******************通讯作者:陈荣祥,教授,博士,研究方向:药用植物开发与利用E-mail:*************************18食品与药品Food and Drug2021年第23卷第1期accurate,stable and reproducible.It can be used for the determination of betulinic acid,oleanolic acid and ursolic acid in Jujubae f ructus.Key Words:accelerated solvent extraction;ultra-high performance liquid chromatography;tandem mass spectrometry; Jujubae f ructus.大枣为鼠李科植物枣树(Ziziphus jujuba Mill.)的干燥成熟果实,不仅作为食物,还是传统中医药中的常用药材。
液相色谱-质谱联用法测定血浆中氯吡格雷浓度及其人体药动学研究

附件12017年度申报专业技术职务任职资格评审答辩论文题目:液相色谱-质谱联用法测定血浆中氯吡格雷浓度及其人体药动学研究作者姓名:周文佳单位:核工业总医院申报职称:副主任药师专业:临床药理二○一七年七月二十六日液相色谱-质谱联用法测定血浆中氯吡格雷浓度及其人体药动学研究周文佳(核工业总医院药物临床试验机构江苏苏州)摘要:目的:建立液相色谱-质谱联用(HPLC-MS/MS)法测定人血浆中氯吡格雷的血药浓度,研究健康受试者空腹及高脂高热量饮食情况下口服硫酸氢氯吡格雷片的药动学特征,考察高脂高热量饮食对硫酸氢氯吡格雷片药动学的影响。
方法:采用随机、开放、双周期交叉试验设计。
60名男性健康志愿者在两个试验周期分别空腹或餐后口服硫酸氢氯吡格雷片75 mg。
采用液相色谱质谱联用法测定人血浆中氯吡格雷的浓度。
用DAS3.2.3药动学软件计算药动学参数,并用SPSS17.0软件对主要参数进行统计分析。
结果:空腹与进食后的主要药动学参数如下:C max分别为(1440 ± 2397)ng·L-1和(4155 ± 2117)ng·L-1,AUC0-36 h分别为(2268 ± 3887)ng·L-1·h和(8691 ± 3628)ng·L-1·h,AUC0-∞分别为(2324 ± 3899)ng·L-1·h和(8816 ± 3668)ng·L-1·h,t1/2分别为(5.7 ± 4.7)h和(8.8 ± 3.8)h,t max分别为(0.7 ± 0.5)h和(1.7 ± 0.7)h, V d分别为(520115 ± 471187)L和(118826 ± 59077)L,Cl分别为(82365 ± 70072)L·h-1和(9949 ± 4017)L·h-1,MRT0-36 h分别为(3.0 ± 1.8)h和(3.6 ± 0.9)h。
VE-821_1232410-49-9_MSDS_MedChemExpress

MSDS1 Composition7 Accident Release MeasureProduct Name:VE-821Chemical Name:PROCEDURE(S) OF PERSONAL PRECAUTION(S)-Wear respirator, chemical safety goggles, rubber boots, and heavyrubber gloves.METHODS FOR CLEANING UP-Sweep up, place in a bag and hold for waste disposal. Avoid raising dust. Ventilate area andwash spill site after material pickup is complete.2-Pyrazinecarboxamide, 3-amino-6-[4-(methylsulfonyl)phenyl]-N-phenyl-CAS No.:1232410-49-98 Accident Release MeasureAppearance:Light green to green(solid)Formula:C18H16N4O3S 9 Toxicological InformationSolubility:To the best of our knowledge, the chemical, physical, andtoxicological properties have not been thoroughly investigated.No data available.p p p p DMSO2 Handling and Storage10 Regulary Information3 Stability and Reactivity11Disposal ConsiderationsCLASSIFICATION- Substance not yet fully tested.SAFETY PHASES- 26-36 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Wear suitable protective clothing.) 36/37/38 (Irritating to eyes,respiratory system and skin.)STABILITY- Stable under normal handling conditions.HANDLING- Do not breathe dust. Avoid contact with eyes,skin,and clothing.Avoid prolonged or repeated exposure.STORAGE- Store in a properly sealed container store at -20℃,shelflife is 2 years.11 Disposal Considerations 4 Hazards Identification12 Transport Information5First Aid RID/ADR- Non-hazardous for road transport. IMDG- Non-hazardous for sea transport.IATA - Non-hazardous for air transport.As specific country, federal, state and local environmentalregulations vary and change frequently we suggest you contact a local, authorized waste disposal contractor for adequate disposal.Special indication of hazards to humans and the environment.Irritating to eyes, respiratory system and skin.MATERIALS TO AVOID- Strong oxidizing agents.REACTIVITY- May emit toxic gasses like Carbon monoxide,Carbon dioxide, Nitrogen oxides upon thermal decomposition.5 First Aid13 Other InformationThe above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Medchemexpress LLC shall not be held liable for any damage resulting from h dli f t t ith th b d tINHALATION- If inhaled, remove to fresh air. If not breathing give, artificial respiration. If breathing is difficult, give oxygen.SKIN CONTACT- In case of contact, immediately wash skin withsoap and copious amounts of water.EYE CONTACT- In case of contact, immediately flush eyes withcopious amounts of water for at least 15 minutes.INGESTION- If swallowed, wash out mouth with water provided person is conscious. Call a physician.6 Fire Fighting Measureshandling or from contact with the above product.EXTINGUISHING MEDIA Water spray- Carbon dioxide, dry chemical powder, or appropriate foam.SPECIAL RISKS Specific Hazard(s)- Emits toxic fumes under fire conditions. SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS Wear self-contained breathing apparatus and protective clothing Caution: Not fully tested. For research purposes onlyMedchemexpress LLCto prevent contact with skin and eyes.18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
柠檬酸裂解酶ELISA试剂盒使用说明书
柠檬酸裂解酶(CL)ELISA试剂盒使用说明书南京森贝伽现货供应,质量保证,价格优惠,试剂盒首选森贝伽。
本试剂仅供研究使用目的:本试剂盒用于测定微生物样本中柠檬酸裂解酶(CL)的活性。
实验原理:本试剂盒应用双抗体夹心法测定标本中柠檬酸裂解酶(CL)水平。
用纯化的柠檬酸裂解酶(CL)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入柠檬酸裂解酶(CL),再与HRP标记的柠檬酸裂解酶(CL )抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB显色。
TMB在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的柠檬酸裂解酶(CL)呈正相关。
用酶标仪在450nm波长下测定吸光度(0D值),通过标准曲线计算样品中柠檬酸裂解酶(CL)活性浓度。
b5E2RGbCAP样本处理及要求:1•血清:室温血液自然凝固10-20分钟,离心20分钟左右(2000-3000转份)。
仔细收集上清,保存过程中如出现沉淀,应再次离心。
p1EanqFDPw2. 血浆:应根据标本的要求选择EDTA或柠檬酸钠作为抗凝剂,混合10-20分钟后,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如有沉淀形成,应该再次离心。
DXDiTa9E3d 3. 尿液:用无菌管收集,离心20分钟左右(2000-3000转份)。
仔细收集上清,保存过程中如有沉淀形成,应再次离心。
胸腹水、脑脊液参照实行。
RTCrpUDGiT4. 细胞培养上清:检测分泌性的成份时,用无菌管收集。
离心20分钟左右(2000-3000转/分)。
仔细收集上清。
检测细胞内的成份时,用PBS(PH7.2-7.4 )稀释细胞悬液,细胞浓度达到100万/ml左右。
通过反复冻融,以使细胞破坏并放出细胞内成份。
离心20分钟左右(2000-3000 转/分)。
仔细收集上清。
保存过程中如有沉淀形成,应再次离心。
5PCzVD7HxA 5. 组织标本:切割标本后,称取重量。
医用耗材中标结果2012z
R2:
3 试剂
谷氨酰基转 豫食药监械 河南博研 临床化 γ -谷氨酰基转移酶 动力学 谷氨酰基 塑料 R1:1*80ml 盒 移酶测定试 (准)字2010 生物工程股 国产 学试剂 (GGT) 法 转移酶 瓶 1*20ml 剂盒 第2400242号 份有限公司 肌氨酸 肌酐测定试 氧化酶 盒 肌酐 剂盒 法 双缩脲 总蛋白测定 盒 总蛋白 终点法 试剂盒 豫食药监械 河南博研 塑料 R1:1*60ml (准)字2010 生物工程股 国产 瓶 1*20ml 第2400229号 份有限公司 豫食药监械 河南博研 塑料 (准)字2010 生物工程股 国产 100ml 瓶 第2400230号 份有限公司 豫食药监械 河南博研 塑料 R1:1*80ml (准)字2010 生物工程股 国产 瓶 1*20ml 第2400231号 份有限公司
32 试剂
33 试剂
微生物 轮状病毒检测 试剂
免疫学 法
34 试剂
微生物 淋球菌鉴定药敏试 注明具 试剂 剂盒 体规格
国 浙食药监械 艾康生物技 产,F 纸质 (准)字2011 术(杭州) DA/C 100条/筒 包装 第2400208号 有限公司 E认 证 国 结核分枝杆 结核分枝 国食药监械 艾康生物技 产,F 菌抗体检测 杆菌抗体 盒 准字2010第 术(杭州) DA/C 铝箔 40人/盒 试剂盒(胶 检测试剂 3400381号 有限公司 E认 体金法) 盒 证 国 轮状病毒抗 轮状病毒 国食药监械准 艾康生物技 产,F 原检测试剂 盒 抗原检测 字2010第 术(杭州) DA/C 铝箔 25人/盒 盒(胶体金 试剂盒 3400517号 有限公司 E认 法) 证 淋球菌检测 国食药监械 艾康生物技 淋球菌检 盒 试剂(胶体 (准)字2010 术(杭州) 国产 铝箔 20人份/盒 测试剂 金法) 第3400389号 有限公司 国 国食药监械 艾康生物技 产,F 乙肝DNA 纸质 (准)字2009 术(杭州) DA/C 32人份/盒 定量 包装 第3400360号 有限公司 E认 证 国 国食药监械 艾康生物技 产,F 丙肝DNA 纸质 (准)字2008 术(杭州) DA/C 32人份/盒 定量 包装 第3401095号 有限公司 E认 证 国 丙型肝炎 国食药监械 艾康生物技 产,F 病毒HCV 准字2010第 术(杭州) DA/C 铝箔 50人/盒 抗体检测 3400511号 有限公司 E认 试剂 证
等电点沉淀法实例

讨论
等电点现象是蛋白质、氨基酸的特点之一。蛋 白质由多个氨基酸分子以肽键连接而成,具有 两性离解性质, 其离解情况复杂, 总离解可用下 式表示(其中P 代表蛋白质) :
参考文献 [1 ] 杭州商学院教材, 乳与乳制品工艺学, 1992 年。 [2 ] 郑集编, 普通生物化学, 高等教育出 版社, 1989 年。
乳清粉不含酪蛋白, 只含乳清蛋白, 而常规的 全脂奶粉、脱脂奶粉以及其他调制奶粉等均含 有酪蛋白。实验中, 以酸调节待测样品水溶液 pH 值至4.6 附近,奶粉溶液有白色絮状凝块 从溶液中沉淀出来, 乳清粉溶液,则无此沉淀反 应, 利用这个现象立即(1+ 19) : 量取10m l 盐酸, 加水稀释至200m l。 精密pH 试纸(pH318- 514)。 鉴定方法及结果判断 取约015g 样品置烧杯中, 加入10m l 水, 搅拌溶解成 均匀溶液, 用吸管逐滴滴入盐酸(1+19) , 边滴边摇匀,并 用试纸测其pH 值, 当pH 值在416 或其附近(如418411) 时, 观察有无白色絮状凝块生成。若有, 即为奶粉, 若溶液仍呈乳浊状, 无凝块形成, 即为乳清粉。
实验步骤
缓冲液的配制 预分离血清 Tricine - SDS - PAGE电泳分离检测 Brandford法测定不同pH缓冲溶液沉淀后上清液的 蛋白质浓度。
结果
Tricine - SDS - PAGE电泳检测分析
Brandford法检测蛋白含量
[参考文献]
[ 1 ] Riley LW, Remis RS, Helgeron SD, et al1Hemorhagic colitis associatedwith a rare Escherichia coli serotype[ J ]1N Engl JMed, 1983, 308: 681- 6851 [ 2 ] Lijima Y, Honda T1Characteristics and molecular biology of verotoxinp roduced by enterohemorrhagic Escherichia coli [ J ] 1Nippon Rinsho,1997, 55: 646 - 6501 [ 3 ] 顾宝柯,徐学斌,金汇明,等1上海地区家禽、家畜及肉制品 大肠埃希菌O157: H7监测[ J ]1疾病监测, 2003, 18 (1) : 5 - 71 [ 4 ] 王炜,刘爱红1衢州市首次检出O157: H7大肠埃希菌[ J ] 1 疾病控制杂志, 2003, 7 (1) : 791 [ 5 ] 李景学,崔树玉,刘家齐1肠出血性大肠埃希菌O157: H7 的 实验室诊断[ J ]1国外医学微生物分册, 1993, 16 (2) : 78 8016511
蛋白胶条鉴定

百泰派克生物科技
蛋白胶条鉴定
蛋白胶条鉴定是对SDS- PAGE胶中切下来的蛋白胶条进行鉴定。
胶条中的蛋白可以是已知蛋白也可以是未知蛋白。
百泰派克生物科技提供基于质谱的蛋白胶条鉴定服务。
蛋白胶条鉴定简介
谈到蛋白胶条,首先要介绍一下SDS-PAGE。
SDS-PAGE,中文名称为十二烷基硫酸
钠-聚丙烯酰胺凝胶电泳,是一种不连续的电泳系统,由Ulrich K. Laemmli开发,通常用作分离分子量在5至250 kDa之间的蛋白质。
十二烷基硫酸钠(SDS,也称
为月桂基硫酸钠)和聚丙烯酰胺凝胶(PAGE)的组合使用,可以消除结构和电荷的影响,仅根据分子量的差异对蛋白质进行分离。
SDS-PAGE实验中,蛋白质样品会
与SDS形成复合物,该复合物表现为SDS的带电特性(负电荷),在电场力的作用下,蛋白质-SDS复合物在PAGE胶上电泳迁移,蛋白质样品会按照样品分子量的大
小分离成条带,从而实现蛋白质的分离。
每一条蛋白条带中的蛋白可能是一种蛋白质也可能是多种蛋白质,可能是已知蛋白也可能是未知蛋白,可以对切割后的胶条做进一步的鉴定。
这种对切割后所得的蛋白胶条中的蛋白质进行鉴定的方法,被称为蛋白胶条鉴定。
蛋白胶条鉴定的方法
蛋白胶条鉴定可以采用液相与质谱联用的技术进行,基于鸟枪法原理先将蛋白条带进行酶切,然后将肽段通过高效液相色谱(HPLC)法进行分离。
分离后的肽段直接进入质谱仪进行分析。
SDS-PAGE与质谱技术结合,是一种对低复杂度蛋白质进行分
离与鉴定的有效的方法。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CL-82198Catalog No. :HY-100359CAS No. :307002-71-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:CL82198; CL 82198Formula:C17H22N2O3Molecular Weight:302.37CAS No. :307002-71-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
