美国药典-溶出方法
溶出度检查法美国药典USP-711

<711> DISSOLUTION溶出度(USP39-NF34 Page 540) General chapter Dissolution <711> is being harmonized with the corresponding texts of the European Pharmacopoeia and/or the Japanese Pharmacopoeia. These pharmacopeias have undertaken to not make any unilateral change to this harmonized chapter.通则<711>溶出度与欧盟药典和日本药典中的相应部分相统一。
这三部药典承诺不做单方面的修改。
Portions of the present general chapter text that are national USP text, and therefore not part of the harmonized text, are marked with symbols to specify this fact.本章中的部分文字为本国USP内容,并没有与其他药典统一。
此部分以()标注。
This test is provided to determine compliance with the dissolution requirements where stated in the individual monograph for dosage forms administered orally. In this general chapter, a dosage unit is defined as 1 tablet or 1 capsule or the amount specified. Of the types of apparatus designs described herein, use the one specified in the individual monograph. Where the label states that an article is enteric coated and a dissolution or disintegration test does not specifically state that it is to be applied to delayed-release articles and is included in the individual monograph, the procedure and interpretation given for Delayed-Release Dosage Forms are applied, unless otherwise specified in the individual monograph.本测试用于检测药品口服制剂的溶出度是否符合各论中的规定。
溶出度检查法美国药典USP-711

溶出度检查法美国药典USP-711<711> DISSOLUTION溶出度(USP39-NF34 Page 540) General chapter Dissolution <711> is being harmonized with the corresponding texts of the European Pharmacopoeia and/or the Japanese Pharmacopoeia. These pharmacopeias have undertaken to not make any unilateral change to this harmonized chapter.通则<711>溶出度与欧盟药典和日本药典中的相应部分相统一。
这三部药典承诺不做单方面的修改。
Portions of the present general chapter text that are national USP text, and therefore not part of the harmonized text, are marked with symbols to specify this fact.本章中的部分文字为本国USP内容,并没有与其他药典统一。
此部分以()标注。
This test is provided to determine compliance with the dissolution requirements where stated in the individual monograph for dosage forms administered orally. In this general chapter, a dosage unit is defined as 1 tablet or 1 capsule or the amount specified. Of the types of apparatus designs described herein, use the one specified in the individual monograph. Where the label states that an article is enteric coated and a dissolution or disintegration test does not specifically state that it is to be applied to delayed-release articles and is included in the individual monograph, the procedure and interpretation given for Delayed-Release Dosage Forms are applied, unless otherwise specified in the individual monograph.本测试用于检测药品口服制剂的溶出度是否符合各论中的规定。
美国药典溶出介质缓冲液的配制

美国药典溶出介质缓冲液的配制
美国药典配制法
1、在标准溶液配制标准指导下配制:0.2mol/L 盐酸溶液;0.2mol/L 氢氧化钠溶液
2、0.2mol/L邻苯二甲酸氢钾溶液:在水中溶解40.85g邻苯二甲酸氢钾,稀释制1000ml
3 、0.2mol/L磷酸二氢钾溶液:在水中溶解27.22g磷酸二氢钾,稀释制1000ml
4 、0.2mol/L硼酸氯化钾溶液:在水中溶解12.37g硼酸和14.91g氯化钾,稀释至1000ml
5、0.2mol/L氯化钾溶液:在水中溶解14.91g氯化钾,稀释至1000ml
6 、2N(当量浓度)的乙酸:在标准溶液配制标准指导下配制
标准缓冲液
盐酸缓冲液:将50ml氯化钾溶液置于200ml容量瓶中,加入一定量的盐酸溶液,用水定容至刻度。
酸性邻苯二甲酸缓冲液:将50ml邻苯二甲酸氢钾溶液置于200ml容量瓶中,加入一定量的盐酸溶液,用水定容至刻度。
中性邻苯二甲酸缓冲液:将50ml邻苯二甲酸氢钾溶液置于200ml容量瓶中,加入一定量的氢氧化钠溶液,用水定容至刻度。
磷酸二氢钾缓冲液:将50ml磷酸二氢钾溶液置于200ml容量瓶中,加入一定量的氢氧化钠溶液,用水定容至刻度。
碱性硼酸缓冲液:将50ml硼酸氯化钾溶液置于200ml容量瓶中,加入一定量的氢氧化钠溶液,用水定容至刻度。
醋酸缓冲液:将规定量的三水乙酸纳置于1000ml容量瓶中,加入规定量的乙酸溶液,用水定容至刻度,混匀。
美国药典溶出度试验方法的建立与验证指导原则的解读

小结
➢注重实操,关注细节。 ➢认真验证,数据为优。 ➢逻辑缜密,有理有节。 ➢宽容有度,得心应手。
重点关注
1、溶出的目的 2、造成溶出差异的因素
➢ 样品(是我们想知道,想找出的) ➢ 试验(是不想要的,应降到最低)
3、目测检视 4、方法学验证
谢谢!
溶出度与释放度的方法学验证
准确度
溶液稳 定性
专属性
验证 项目
耐用性
线性范 围
精密度
测定方法与验证-方法学验证
专属性
胶囊
辅料与其他 活性成分
沉降篮
干扰来自何方?如何除去干扰?
测定方法与验证-方法学验证
专属性应注意的问题
• 空白包括:其他活性成分(复方制剂中)、 辅料、包衣、油墨、沉降篮、胶囊壳、装 置(桨、杆、篮)等。
自动取 样
对比验证 内容
试验设计
取样应注意的问题
➢ 手动取样与自动取样要进行比较验证 ➢ 自动取样应注意日常性能检查和维护 ➢ 自动取样的装置要注意对溶出杯中流体力学的
干扰。 ➢ 自动取样的验证还包括:
残留药物的扣减 药物的吸附 洗涤或循环洗涤
试验设计
滤膜吸附及验证
滤过与离心
滤膜吸附的考察
滤膜吸附的验证
美国药典溶出度试验 方法的建立与验证指
导原则的解读
2017.6.30
涵盖内容
▪ 总体评价 ▪ 溶出介质 ▪ 溶出仪器 ▪ 实验设计 ▪ 测定方法与验证
总体评价
限度 范围
区分 力
总体 评价
稳定 性
变异 范围
总体评价-限度
▪ 限度范围应考虑的问题
1、多批次的考量 2、具有代表性 3、具有针对性(针对重点药品) 4、考虑样品的稳定性
[精品]对乙酰氨基酚片溶出度测定
![[精品]对乙酰氨基酚片溶出度测定](https://img.taocdn.com/s3/m/8619601c443610661ed9ad51f01dc281e53a563c.png)
[精品]对乙酰氨基酚片溶出度测定对乙酰氨基酚片是一种非处方药,用于缓解疼痛、退烧、减轻关节炎和牙痛等。
在药品质量控制方面,溶出度测定是测定药品释放活性成分的关键方法之一,可用于评估药品的有效性和一致性。
本实验旨在采用美国药典(USP)提供的方法,测定对乙酰氨基酚片的溶出度。
1.实验原理溶出度是指药品中活性成分从药片或胶囊中释放出来的比例。
药品的溶出度对药效产生影响,因此,需要对它进行检测以确保药品质量达到标准。
常用的溶出度测定方法包括旋转篮法和流动池法。
旋转篮法适用于药片和胶囊,有利于模拟胃肠道的运动。
而流动池法适用于注射剂和眼药水等液体制剂。
本实验采用的是旋转篮法,将对乙酰氨基酚片放入篮中,放到模拟胃肠道的容器中,并在旋转的条件下采集样品,测定其对乙酰氨基酚溶出度。
2.实验步骤2.1 准备a.先将篮子和箔纸放在烘箱中烘干,预热至50℃左右。
b.称取一定量的对乙酰氨基酚片粉末,将其压制成药片。
c.提取篮子和箔纸,稍稍冷却,然后将篮子置于箔纸上,放回烘箱中预热至50℃。
d.取一定量的模拟胃液,将其预热至37℃。
2.2 实验操作a.取一个已称好药片,将其放入篮子中,然后盖上篮子盖子,确保药片合适地固定在篮子中。
b.用钢丝将篮子悬挂在模拟胃液的容器中,保证篮子不接触容器底部。
c.启动旋转器,设置旋转速度为50rpm,开始测定。
d.每个时间点收集一个样品。
将采样器插入模拟胃液中,确保其到达药片所在的位置(通常为时间的1/3、2/3和3/3),并将样品收集到样品瓶中,以供后续分析。
e.持续测定至90分钟,并在每个时间点记录剩余的药片和溶出度数据。
f.一旦测定完成,将篮子和样品在干燥的环境下过夜。
2.3数据处理a.逐一记录采集的样品的时间和对乙酰氨基酚溶出量。
b.将数据绘制成输药时间与对乙酰氨基酚溶出量的关系图。
c.计算药物的平均溶出度,这是从剩余药物的总质量中计算出来的。
3.实验注意事项a.对乙酰氨基酚片的制备需准确称取药材,不得有误差。
美国药典溶出介质缓冲液的配制
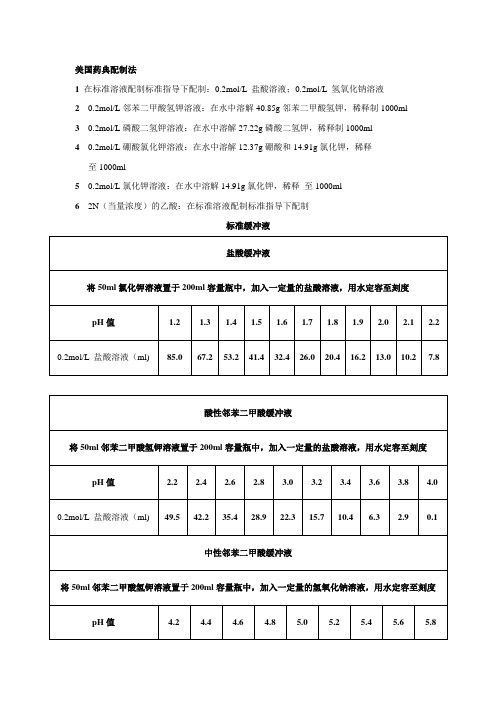
2.1
2.2
0.2mol/L盐酸溶液(ml)
85.0
67.2
53.2
41.4
32.4
26.0
20.4
16.2
13.0
10.2
7.8
酸性邻苯二甲酸缓冲液
将50ml邻苯二甲酸氢钾溶液置于200ml容量瓶中,加入一定量的盐酸溶液,用水定容至刻度
pH值
2.2
2.4
2.6
2.8
3.0
3.2
3.4
3.6
3.8
4.0
0.2mol/L盐酸溶液(ml)
49.5
42.2
35.4
28.9
22.3
15.7
10.4
6.3
2.9
0.1
中性邻苯二甲酸缓冲液
将50ml邻苯二甲酸氢钾溶液置于200ml容量瓶中,加入一定量的氢氧化钠溶液,用水定容至刻度
pH值
4.2
4.4
4.6
4.8
5.0
5.2
5.4
5.6
5.8
0.2mol/L NaOH溶液(ml)
8.1
11.6
16.4
22.4
29.1
34.7
39.1
42.4
44.5
46.1
碱性硼酸缓冲液
将50ml硼酸氯化钾溶液置于200ml容量瓶中,加入一定量的氢氧化钠溶液,用水定容至刻度
pH值
8.0
8.2
8.4
8.6
8.8
9.0
9.2
9.4
9.6
9.8
10.0
0.2mol/L NaOH溶液(ml)
3.9
6.0
5.30
中_美_英_日四国药典溶出度研究方法比较(1)

为 900 ml 。小杯法尚未有介质体积的规定 ,一般选
择 100~250 ml 。虽然国际上并不建议使用有机溶
媒 ,中国药典上仍有个别药物 ,如吲达帕胺片以稀乙
四个国家药典中 ,唯有我国药典桨法中存在转 速过高的问题 ,有 4 个药品转速大于 100 r/ min ,国 内文献中一些药品的溶出度试验也存在同样的问 题 ,尤其是儿童和老年人服用的制剂 ,应考虑到其特 殊的生理状况 ,采用低转速 。建议不应为了符合药 典标准而选用不适宜的转速 ,最好从制剂的制备工 艺入手来提高溶出度 。四个国家药典转速选择情况 见表 2 。
试验次数 试验片 (个) 数
判断标准
《美国药典》
试验片 (个) 数
判断标准
每片 (个) ≥Q ;6 片
1
6
(个) 平 均 值 ≥Q , 不小于 Q - 10 % ,
6
每片 ( 个 ) ≥Q + 5%
片 (个) 数 ≤2
对照品 ,可消除样品与对照品组分不同而产生的误 差 ,可减少辅料和杂质的干扰 。目前 ,对于将该法应
本草天工科技有限公司 南昌 330000)
摘要 :文章对中国 、美国 、英国和日本药典中溶出度的研究方法进行了比较和讨论 ,为药物溶出度检查合理的选择测定条件 ,
更好地发挥溶出度在药品质量控制中的作用提供参考 。
关键词 :溶出度 ;药典 ;中国 ;美国 ;英国 ;日本
中图分类号 : R 921 文献标识码 :A
在溶出方法方面 ,各国越来越趋于统一 ,流室法
典》15 版) 中口服固体制剂的溶出度检测方法进行 已与篮法 、桨法一样 ,为日本和一些欧洲国家所接
比较 。
受 。《美国药典》在此方面较为领先和创新 ,不但大
阿齐沙坦片体外溶出方法

阿齐沙坦片体外溶出方法阿齐沙坦片是一种常用的降压药物,它属于类似素拮抗剂,通过抑制血管紧张素Ⅱ对血管的收缩作用,从而达到降压的效果。
在临床上,对药物的体外溶出性能进行研究是非常重要的,下面将介绍一种常用的阿齐沙坦片体外溶出方法。
一、研究目的研究阿齐沙坦片的体外溶出性能,确定药物的释放速率和溶出度。
二、实验仪器和试剂1. 仪器:UV/VIS分光光度计、振荡器、离心机。
2. 试剂:阿齐沙坦片标准品、甲醇。
三、实验步骤1. 准备样品溶液:取一定量的阿齐沙坦片标准品,用甲醇溶解并稀释至合适浓度的溶液。
2. 准备模拟体液溶液:按照美国药典(USP)推荐的生理缓冲液pH 6.8来配制模拟体液溶液。
3. 准备试剂:将模拟体液溶液和甲醇按照适当比例混合,得到合适的模拟体液溶液。
4. 液体驱动:将准备好的模拟体液溶液注入振荡器中,并进行液体驱动,设置转速和温度,使其与人体模拟体液相近。
5. 开始测试:将阿齐沙坦片标准品制备的样品溶液加入试剂中,开始测试。
6. 反应时间:根据实验要求,确定每次测试的反应时间。
7. 收集样品:分别在规定的时间点(如0.5小时、1小时、2小时、4小时等)离心收集样品。
8. 检测:用UV/VIS分光光度计测定样品的吸光度,并据此计算阿齐沙坦片的溶出度。
四、结果评价根据实验数据,计算出不同时间点的吸光度值,并转化为溶出度百分数。
根据溶出度与时间的关系,得出阿齐沙坦片的体外溶出曲线。
根据美国药典(USP)提供的溶出度标准,判断阿齐沙坦片的溶出速率是否符合要求。
五、实验注意事项1. 实验过程中,要保持模拟体液溶液的温度和转速恒定。
2. 收集样品时,要注意严格按照规定的时间收集,避免误差的产生。
3. 在测定样品吸光度时,要选择适当的波长,确保准确性。
4. 实验结束后,要及时清洗仪器,并妥善保存实验数据。
六、结论通过上述实验方法,可以明确阿齐沙坦片的体外溶出性能,得出药物的溶出速率和溶出度。
这有助于判断药物的释放特性,从而指导临床用药的安全性和有效性。
美国药典25版收载的溶出度品种概况

美国药典25版收载的溶出度品种概况溶出度系指药物从片剂或胶囊剂等固体制剂在规定溶剂中溶出的速度和程度,溶出限度以标示量的百分数表示,是一种模拟口服固体制剂在胃肠道中崩解和溶出体外试验,是控制药物制剂质量的检测方法,是研究固体及半固体制剂所含主药的晶型、粒度、处方组成、辅料品种和性质、生产工艺等对制剂质量统一性的新方法。
因此溶出度已迅速发展成为广泛应用于质量标准中的检验方法。
美、英、日、中国药典已把溶出度列为保证药物制剂安全有效的重要检测项目。
现将美国药典25版收载溶出度品种内容概述如下:1、美国药典25版共收载的片剂和胶囊有698个品种,其中进行溶出度品种有535个,约占77%。
缓释制剂一般测定释放度,如碳酸锂延迟释放片、盐酸多西环素延迟释放胶囊、吲哚美辛持续释放片、阿斯匹林持续释放片。
阴道片和溶液片一般采用崩解时限,如克霉唑阴道片、盐酸可卡因溶液片。
无机盐类制剂如硅镁铝片、氧化镁片、碳酸钙镁鍶复方片,溶出量测定方法繁琐,需用效价测定或溶出量难以测定的品种,如维生素D2片剂或胶囊、酯化雌激素片、已烯雌酚片、颠茄提取片、洋地黄片、依托红霉素胶囊及片、三乙酰竹桃霉素胶囊、胡箩卜素片等均采用崩解时限。
2、采用仪器装置有1法(转篮法)211个品种,2法(桨法)约332个品种,采用释放度装置3法(往复瓶法)3个品种,如卡马西平片、盐酸羟嗪片、碘甲腺氨酸钠片。
有的品种设2~4种测定法按标签中规定选择测定。
3、转速 I法转速100rpm 185个品种II法转速50rpm 228个品种(也有采用转速35rpm、75rpm、120rpm、150rpm)。
4、溶出介质以水(245个品种),0.01mol/L~0.1mol/L盐酸溶液(约146个品种)为主,其他介质有:醋酸盐缓冲液(pH4.5),磷酸盐缓冲液(pH4.0~8.6),三羟基甲基氨基甲烷溶液(pH7.2~9.0),不含酶的人工胃液或人工肠液等。
由于某些药物在上述溶液中溶解度很少,需加其他有机溶剂如乙醇、异丙醇或表面活性剂如十二烷基硫酸钠溶液(0.01%~5%)、聚山梨酯20或80,或混合溶出介质如磷酸盐缓冲液(pH8.0)-正丙醇(3∶2),0.1N盐酸-正丙醇(3∶2)等。
溶出度检查法美国药典USP

溶出度检查法美国药典USP溶出度检查法USP711中英文对照711 DISSOLUTION溶出度(USP39-NF34 Page 540) General chapter Dissolution 711 is being harmonized with the corresponding texts of the European Pharmacopoeia and/or the Japanese Pharmacopoeia. These pharmacopeias have undertaken to not make any unilateral change to this harmonized chapter.通则 711 溶出度与欧盟药典和日本药典中的相应部分相统一。
这三部药典承诺不做单方面的修改。
Portions of the present general chapter text that are national USP text, and therefore not part of the harmonized text, are marked with symbols to specify this fact.本章中的部分文字为本国USP内容,并没有与其他药典统一。
此部分以()标注。
This test is provided to determine compliance with the dissolution requirements where stated in the individual monograph for dosage forms administered orally. In this general chapter, a dosage unit is defined as 1 tablet or 1 capsule or the amount specified. Of the types of apparatus designs described herein, use the one specified in the individual monograph. Where the label states that an article is enteric coated and a dissolution or disintegration test does not specifically state that it is to be applied to delayed-release articles and is included in the individual monograph, the procedure and interpretation given for Delayed-Release Dosage Forms are applied, unless otherwise specified in the individual monograph.本测试用于检测药品口服制剂的溶出度是否符合各论中的规定。
美国药典溶出度4 法联合高效液相色谱分析低释放眼用植入制剂

美国药典溶出度4法联合高效液相色谱分析低释放眼用植入制剂David C. Browne and Shawn Kieselmann*Intertek, 291路东22号,塞勒姆工业园,白宫,美国新泽西州08888目的建立美国药典溶出度4法联合高效液相色谱分析低剂量缓释眼用植入制剂的方法背景眼部植入制剂是一种体积小,以聚合物为基底的圆盘或者圆柱形的药用制剂,可改善药物进入眼睛后的治疗释放(1)。
植入剂一些常见的类型包括巩膜外层植入剂,放置在眼睛的中纬线上;和玻璃体内植入剂,经外科手术放置在眼睛(2)玻璃体内。
图1展示了眼睛的解剖图和各种植入剂的放置位置。
眼植入物用于治疗各种疾病,如糖尿病视网膜病变和黄斑变性(1)。
选择USP4法来测试该类产品的溶出度是由于该法采用层流的方式与体内条件具有良好的相关性,并且开环的配置方式提供的漏槽条件有利于难溶药物的溶出。
实现目标该方法的建立主要有三个目标。
第一,由于眼部植入制剂药物释放浓度低,需要优化色谱条件以获得定量限≤1ng/ml。
其次,确定美国药典溶出度4法在测试过程中产生的干扰或吸附。
最后,用美国药典溶出度4法联合高效液相色谱法获取溶出曲线。
方法学实验初期,选取Sotax CE 7 smart溶出仪(开环),pH 7.4的磷酸盐缓冲液作为溶出介质进行实验。
泵流速设置为最低流速(1.5ml/min),用以模拟体内条件,因为人体眼部玻璃体的流速大约为2μl/min(3)。
一个剂量单位的药物置于铺有玻璃珠的22.6mm的流通池内。
溶出液直接在线注入高效液相色谱仪进行分析,几天内每小时采样一次。
不推荐使用收集器和自动进样器,因为待分析物与一些塑料会发生化学反应。
图1 眼部解剖图和局部给药的主要方法为使溶出液直接注入高效液相色谱仪,溶出仪出口管路经缩小接头与一PEEK管相连,然后进入进样阀。
在进样位置,溶出液流经阀门,进入废液接口。
在载样位置,溶出液充满进样环。
阀开关由电子控制。
溶出度测试方法桨法篮法

桨法/篮法AstraZeneca、Johnson & Johnson、GSK、MSD、Pfizer、Bayer 等公司和大学的多位科学家,分别为Edmund S. Kostewicz, Bertil Abrahamsson, Marcus Brewster, Joachim Brouwers, JamesButler, Sara Carlert, Paul A. Dickinson, Jennifer Dressman, RenéHolm, Sandra Klein, James Mann, Mark McAllister, Mans Minekus, Uwe Muenster, Anette Müllertz, Miriam Verwei, Maria Vertzoni, Werner Weitschies, Patrick Augustijns小编将其中关于篮法和桨法应用时应关注的事项进行了翻译,分享给研发人员,希望大家多了解一些“为什么”。
文章来源:European Journal of Pharmaceutical Sciences 57(2014)342-3662.2桨法/篮法桨法(方法2)和篮法(方法1)见图1,是第一批引入药典的溶出度测试方法,广泛应用在USP专论中。
这些USP专论中规定的溶出测试主要是针对药品的质量控制(QC)的,在这些情况下,QC方法也实现了QbD的目标,也可以用于药品的研发中。
QC方法用于药品研发的例子包括含BCS I类或III类药物的速释制剂(IR)和调释制剂(MR)的研发(Grundy and Foster, 1996; Sandberg et al., 1991)。
对于其他剂型,把药典推荐方法转换成研发方法的可能性很低。
在20世纪90年代出现了生物药剂学分类系统(BCS),认为溶解性和渗透性是影响药物体内性能的关键因素,这对速释制剂(IR)处方的开发具有重要意义。
(参考资料)(USP1092)溶出度试验的开发和验证中文

前言目的:溶出度试验的开发和验证(1092)目的是为溶出度的测定提供了全面的开发和验证的方法以及相应的分析技术。
本指导原则贯穿溶出度测定的全部过程,并对方法验证提供了指导和验证标准。
同时它还涉及对普通制剂和缓释制剂产生的数据和接受标准进行说明。
范围:本指导原则讨论了溶出度试验的开发和验证,重点是固体口服剂型。
所提出的概念也可能适用于其他剂型和给药途径。
对于一些不同于USP章节中的设备和程序均已给出合适的解释。
本指导原则的基本框架如下:1.前期评估(对产品开发以及溶出度方法开发的前期研究评估)1.1滤膜相容性研究(Performing Filter Compatibility)1.2原料药在不同溶媒中溶解度和稳定性的测定1.3选择溶出介质和体积1.4选择溶出设备(桨法和篮法以及其他方法)2.方法开发2.1脱气2.2沉降2.3搅拌2.4研究设计2.4.1取样时间点2.4.2观察2.4.3取样2.4.4清洗2.5数据处理2.6溶出度试验的评估3.分析整理3.1样品的处理3.2过滤3.3离心3.4分析过程3.5光谱分析3.6HPLC分析4.程序化4.1溶出介质的准备4.2样品的选择和取样时间的设计4.3取样和过滤4.4清洗4.5使用软件和计算机处理结果4.6找出需要验证的存在偏差的过程5.验证5.1专属性/安慰剂的干扰5.2线性和范围5.3准确度/回收率5.4精密度试验5.4.1重复性试验5.4.2中间精密度试验5.4.3重现性试验5.5耐用性试验5.6对照品和供试品的稳定性试验5.7程序化验证6.接受标准6.1普通速释制剂6.2缓释制剂6.3控释制剂6.4多重溶出度试验6.5溶出度结果的解释6.5.1普通速释制剂6.5.2缓释制剂6.5.3控释制剂7.参考文献1. 前期评估(对产品发展以及溶出度方法开发的前期研究评估)在方法开发之前,对用以评价剂型的溶出行为的滤膜、溶出介质、介质体积和溶出设备进行筛选是非常重要的。
药物溶出度试验方法的验证

药物溶出度试验方法的验证概述药物溶出度试验是一项重要的药物质量控制方法,用于评估药物在特定溶媒中的溶出速度和程度。
本文将探讨药物溶出度试验方法的验证,确保试验结果准确可靠。
一、试验方法选择药物溶出度试验方法的选择应根据药物特性、制剂类型和产品要求等因素综合考虑。
常见的试验方法包括美国药典(USP)溶出度试验、欧洲药典(EP)溶出度试验和中国药典(CP)溶出度试验等。
二、验证参数的确定验证药物溶出度试验方法时,需明确以下参数:1. 单一或多个试验条件:例如,使用不同pH值、温度和搅拌速度等条件进行试验;2. 药物释放曲线的判定:通过选定特定时间点或选定时间段内累积药物释放量的百分比等方法进行判定;3. 溶出度试验仪器的准确性和重复性:对于采用自动化仪器进行试验的情况,需验证仪器的准确性和重复性,并进行适当的校准或调整。
三、实验操作步骤药物溶出度试验的实验操作步骤包括以下几个方面:1. 试剂准备:准备适当的溶媒,并根据试验方法要求进行调整;2. 试验器具准备:根据试验方法选用合适的试验仪器,并进行适当的校准和清洁;3. 试验条件设定:根据验证参数确定的试验条件进行设定,包括温度、搅拌速度等;4. 样品准备:制备样品溶液,确保样品溶液的浓度适当;5. 试验进行:将样品溶液加入试验器具中,开始试验,并记录试验时间;6. 结果分析:根据试验方法要求,进行药物释放曲线的分析和数据处理。
四、数据处理与结果分析药物溶出度试验的数据处理和结果分析要根据试验方法的要求进行。
一般来说,需要计算溶出度曲线的均值、标准差、变异系数等参数,并与设定的试验条件进行比较。
此外,还需对实验结果进行统计学分析,如方差分析等,以判断试验方法是否可靠。
五、验证报告撰写药物溶出度试验方法的验证应编写验证报告,报告内容一般包括以下几个方面:1. 验证目的和背景:明确验证的目的和背景;2. 验证参数和试验条件:列出已确定的验证参数和试验条件;3. 样品准备和试验操作:详细描述样品准备和试验操作步骤;4. 数据处理和结果分析:对试验数据进行处理和分析,并阐述结果的可靠性和合理性;5. 结论和建议:根据验证结果,给出验证结论和相关建议;6. 附录:附上试验记录和相关数据等。
中美英三国药典溶出度、释放度检查方法比较 共32页PPT资料

于规定限度(Q);除另有规定外,Q应为标示量的70%
2
6
6片(粒)中仅有1~2片(粒)低于Q,但不低于Q-10%,且
其平均释放量不低于Q
3
12
6片(粒)中如有1~2片(粒)低于Q,其中仅有1片(粒)
低于Q-10%,但不低于Q-20%,且其平均释放量不低于Q时,应另
取6片(粒)复试;初复试的12片(粒)中有1~3片(粒)低于
1990年版(第22版)增加了测定透皮贴剂的三种装 置桨碟法:(paddle over disk)、筒 法(cylinder)、往复碟法 (reciprocating disk)
2019年版(第23版)仪器增至7种 2000年版(第24版)进一步增加和完善设备
. . . . .
2019年版(第30版) 7种仪器设备 篮法 basket 桨法 paddle 往复筒法 reciprocating cylinder 流通池(流室)法 flow-through cell 桨碟法 paddle over disk 筒法 cylinder 往复架法 peciprocating holder
2.装置四(流通池法)主要用于缓释、难溶剂型的 测试。
3.装置五(桨碟法)和装置六(筒法)专门用于透 皮吸收贴剂的测试。
• 英国药典
1973年版规定了地高辛片的溶出度和释放度检查 1988年版引入溶出度检查法,篮法、桨法 1993年版增加流通池法装置,未规定药物释放度
检查法 2019年版增加透皮每个点测得的释放量,如有1~2片(粒)超出 规定范围,其中仅有1片(粒)超出规定范围的10%,但未超过规 定范围的20%,且其平均释放量未超出规定范围,应另取6片(粒) 复试;初复试的12片(粒)中,在每个时间点测得的释放量,如 有1~3片(粒)超出规定范围,其中仅有1片(粒)超出规定范 围的10%,但未超出规定范围的20%,且其平均释放量未超出规定 范围
美国药典溶出度4 法联合高效液相色谱分析低释放眼用植入制剂

美国药典溶出度4法联合高效液相色谱分析低释放眼用植入制剂David C. Browne and Shawn Kieselmann*Intertek, 291路东22号,塞勒姆工业园,白宫,美国新泽西州08888目的建立美国药典溶出度4法联合高效液相色谱分析低剂量缓释眼用植入制剂的方法背景眼部植入制剂是一种体积小,以聚合物为基底的圆盘或者圆柱形的药用制剂,可改善药物进入眼睛后的治疗释放(1)。
植入剂一些常见的类型包括巩膜外层植入剂,放置在眼睛的中纬线上;和玻璃体内植入剂,经外科手术放置在眼睛(2)玻璃体内。
图1展示了眼睛的解剖图和各种植入剂的放置位置。
眼植入物用于治疗各种疾病,如糖尿病视网膜病变和黄斑变性(1)。
选择USP4法来测试该类产品的溶出度是由于该法采用层流的方式与体内条件具有良好的相关性,并且开环的配置方式提供的漏槽条件有利于难溶药物的溶出。
实现目标该方法的建立主要有三个目标。
第一,由于眼部植入制剂药物释放浓度低,需要优化色谱条件以获得定量限≤1ng/ml。
其次,确定美国药典溶出度4法在测试过程中产生的干扰或吸附。
最后,用美国药典溶出度4法联合高效液相色谱法获取溶出曲线。
方法学实验初期,选取Sotax CE 7 smart溶出仪(开环),pH 7.4的磷酸盐缓冲液作为溶出介质进行实验。
泵流速设置为最低流速(1.5ml/min),用以模拟体内条件,因为人体眼部玻璃体的流速大约为2μl/min(3)。
一个剂量单位的药物置于铺有玻璃珠的22.6mm的流通池内。
溶出液直接在线注入高效液相色谱仪进行分析,几天内每小时采样一次。
不推荐使用收集器和自动进样器,因为待分析物与一些塑料会发生化学反应。
图1 眼部解剖图和局部给药的主要方法为使溶出液直接注入高效液相色谱仪,溶出仪出口管路经缩小接头与一PEEK管相连,然后进入进样阀。
在进样位置,溶出液流经阀门,进入废液接口。
在载样位置,溶出液充满进样环。
阀开关由电子控制。
最新USP-1092-溶出度方法的开发和验证(中英文对照版)第一部分(1)

最新USP <1092>:溶出度方法的开发与验证(中英文对照)-开发部分2015-2020 USP通则-制剂专家委员会修订了USP 41溶出度方法的开发与验证<1092>通则,全文内容发表在PF46(6),最后的评论限期为2019年1月31日。
主要修改部分如下:1.1.2节中溶解度测试和原料药在不同介质中的稳定性分为了两部分:1.2.1溶解度和1.2.2稳定性,这样可以讨论地更加清楚。
在修订部分给到了溶出方法数据库的参考文献,供使用者参考。
同时修订内容中提到了参考文献:溶解度测试通则<1236>,该通则在PF44(5)中被提出,是溶解度测试的指南。
2.1.3节中介质和体积的选择包含了USP对于漏槽条件新的描述。
修订部分中更加清楚的阐述了与体积的关系。
3.2.4.1节更新了“取样时间点部分”,以呼应最近的FDA指南更新的“取样时间点部分”。
4.2.5数据处理章节引用了一篇新的参考文献。
该文献中提到了一种情形:低溶解度的口服溶液在展示生物利用度时也许不合适。
文献提到了一种方法即用体外溶出曲线的方法初步评估体内溶出。
5.3.4分析方法章节加入了一个新图,用来描述在溶出样品分析时遇到的情形:溶出成分有全部或者部分进行了衍生化者降解。
6.5.3准确性和回收率章节中加入了延迟缓释制剂中原料药成分在酸性阶段测试时释放至酸性介质中,溶出后成分有降解发生的情况。
这种情形在验证时必须考虑到,需要提示的是,该情况在溶出<711>接受标准表3中没有被认识到。
7.在6.5.2延迟制剂章节更新部分,讨论了耐酸的延迟制剂在酸性阶段的测试结果,不仅要考虑溶出的原料药成分,降解物也需要考虑到。
限于译者水平有限,有不足的地方或者不正确的地方还请读者包涵,谢谢批评与指正。
<1092>THE DISSOLUTION PROCEDURE: DEVELOPMENT ANDVALIDATION<1092>溶出度试验的开发和验证INTRODUCTION前言Purpose目的The chapter provides a comprehensive approach covering items to consider for developing and validating dissolution procedures and the accompanying analytical procedures. It addresses the use of automation throughout the test and provides guidance and criteria for validation. It also addresses the treatment of the data generated and the interpretation of acceptance criteria for immediate- andmodified-release solid oral dosage forms.该通则提供了在溶出度方法开发和验证过程中,以及采用相应分析方法时需要考虑的因素。
阿齐沙坦片体外溶出方法

阿齐沙坦片体外溶出方法
阿齐沙坦片是一种常用的抗高血压药物,其主要成分是阿齐沙坦。
阿齐沙坦片的体外
溶出方法是评价药物在体外释放速度和溶出特性的方法之一,对于评估药物的溶出性和释
放行为非常重要。
阿齐沙坦片的体外溶出方法通常采用美国药典(USP)提供的体外溶出试验仪进行实验。
具体步骤如下:
1. 准备样品:将一定数量的阿齐沙坦片称量,并记录质量。
2. 准备介质:根据USP的要求,使用适当的介质,例如模拟胃液或模拟肠液,将体外溶出试验仪的溶出槽中加入适量的介质。
3. 实验条件设置:根据USP的要求,设置适当的条件,例如温度、搅拌速度等。
4. 开始实验:将阿齐沙坦片放入溶出槽中,开始实验。
在一定时间间隔内,取出一
定量的样品。
5. 分析样品:将取出的样品进行分析,通常可以采用高效液相色谱法(HPLC)或紫外分光光度法等方法。
6. 计算溶出度:根据分析结果,计算出不同时间点上的溶出度,并绘制溶出度-时间
曲线。
对于阿齐沙坦片的体外溶出方法,有一些注意事项需要注意:
通过上述步骤,可以评估阿齐沙坦片在体外的溶出速度和溶出特性,为药物开发和质
量控制提供重要的参考。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
711 DISSOLUTIONThis general chapter is harmonized with the corresponding texts of the European Pharmacopoeia and/or the Japanese Pharmacopoeia. These pharmacopeias have undertaken not to make any unilateral change to this harmonized chapter.Portions of the present general chapter text that are national USP text, and therefore not part of the harmonized text, are marked with symbols () to specify this fact.This test is provided to determine compliance with the dissolution requirements where stated in the individual monograph for dosage forms administered orally. In this general chapter, a dosage unit is defined as 1 tablet or 1 capsule or the amount specified. Of the types of apparatus described herein, use the one specified in the individual monograph. Where the label states that an article is enteric-coated, and where a dissolution or disintegration test that does not specifically state that it is to be applied to delayed-release articles is included in the individual monograph, the procedure and interpretation given for Delayed-Release Dosage Forms is applied unless otherwise specified in the individual monograph. For hard or soft gelatin capsules and gelatin-coated tablets that do not conform to the Dissolution specification, repeat the test as follows. Where water or a medium with a pH of less than 6.8 is specified as the Medium in the individual monograph, the same Medium specified may be used with the addition of purified pepsin that results in an activity of 750,000 Units or less per 1000 mL. For media with a pH of 6.8 or greater, pancreatin can be added to produce not more than 1750 USP Units of protease activity per 1000 mL.USP R EFERENCE S TANDARDS11—USP Chlorpheniramine Maleate Extended-Release Tablets RS. USP Prednisone Tablets RS.APPARATUSApparatus 1 (Basket Apparatus)The assembly consists of the following: a vessel, which may be covered, made of glass or other inert, transparent material1; a motor; a metallic drive shaft; and a cylindrical basket. The vessel is partially immersed in a suitable water bath of any convenient size or heated by a suitable device such as a heating jacket. The water bath or heating device permits holding the temperature inside the vessel at 37 ± 0.5 during the test and keeping the bath fluid in constant, smooth motion. No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation, or vibration beyond that due tothe smoothly rotating stirring element. An apparatus that permits observation of the specimen and stirring element during the test is preferable. The vessel is cylindrical, with ahemispherical bottom and with one of the following dimensions and capacities: for a nominal capacity of 1 L, the height is 160 mm to 210 mm and its inside diameter is 98 mm to 106 mm; for a nominal capacity of 2 L, the height is 280 mm to 300 mm and its inside diameter is 98 mm to 106 mm; and for a nominal capacity of 4 L, the height is 280 mm to 300 mm and its inside diameter is 145 mm to 155 mm. Its sides are flanged at the top. Afitted cover may be used to retard evaporation.2 The shaft is positioned so that its axis is not more than 2 mm at any point from the vertical axis of the vessel and rotates smoothly and without significant wobble that could affect the results. A speed-regulating device is used thatallows the shaft rotation speed to be selected and maintained at the specified rate given in the individual monograph, within ±4%.Shaft and basket components of the stirring element are fabricated of stainless steel, type 316, or other inert material, to the specifications shown in Figure 1. A basket having a gold coating of about 0.0001 inch (2.5 µm) thick may be used. A dosage unit is placed in a dry basket at the beginning of each test. The distance between the inside bottom of the vessel and the bottom of the basket is maintained at 25 ± 2 mm during the test.Figure 1. Basket Stirring ElementApparatus 2 (Paddle Apparatus)Use the assembly from Apparatus 1, except that a paddle formed from a blade and a shaft is used as the stirring element. The shaft is positioned so that its axis is not more than 2 mm from the vertical axis of the vessel at any point and rotates smoothly without significant wobble that could affect the results. The vertical center line of the blade passes through the axis of the shaft so that the bottom of the blade is flush with the bottom of the shaft. The paddle conforms to the specifications shown in Figure 2. The distance of 25 ± 2 mm between the bottom of the blade and the inside bottom of the vessel is maintained during the test. The metallic or suitably inert, rigid blade and shaft comprise a single entity. A suitable two-part detachable design may be used provided the assembly remains firmly engaged during the test. The paddle blade and shaft may be coated with a suitable coating so as to make them inert. The dosage unit is allowed to sink to the bottom of the vessel before rotation of the blade is started. A small, loose piece of nonreactive material, such as not more than a few turns of wire helix, may be attached to dosage units that would otherwise float. An alternative sinker device is shown in Figure 2a. Other validated sinker devices may be used.Figure 2. Paddle Stirring ElementFigure 2a. Alternative sinker. All dimensions are expressed in mm.Apparatus 3 (Reciprocating Cylinder)NOT ACCEPTED BY THE JAPANESE PHARMACOPOEIAThe assembly consists of a set of cylindrical, flat-bottomed glass vessels; a set of glassreciprocating cylinders; inert fittings (stainless steel type 316 or other suitable material), and screens that are made of suitable nonsorbing and nonreactive material and that are designed to fit the tops and bottoms of the reciprocating cylinders; and a motor and drive assembly to reciprocate the cylinders vertically inside the vessels and, if desired, index the reciprocating cylinders horizontally to a different row of vessels. The vessels are partially immersed in a suitable water bath of any convenient size that permits holding the temperature at 37 ± 0.5 during the test. No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation, or vibration beyond that due to the smooth, vertically reciprocating cylinder. A device is used that allows thereciprocation rate to be selected and maintained at the specified dip rate given in the individual monograph within ±5%. An apparatus that permits observation of the specimens and reciprocating cylinders is preferable. The vessels are provided with an evaporation cap that remains in place for the duration of the test. The components conform to the dimensionsshown in Figure 3 unless otherwise specified in the individual monograph.Figure 3. Apparatus 3 (reciprocating cylinder)Apparatus 4 (Flow-Through Cell)The assembly consists of a reservoir and a pump for the Dissolution Medium; a flow-through cell; and a water bath that maintains the Dissolution Medium at 37 ± 0.5. Use the specified cell size as given in the individual monograph.The pump forces the Dissolution Medium upwards through the flow-through cell. The pump has a delivery range between 240 and 960 mL per hour, with standard flow rates of 4, 8, and 16 mL per minute. It must deliver a constant flow (±5% of the nominal flow rate); the flow profile is sinusoidal with a pulsation of 120 ± 10 pulses per minute. A pump without pulsation may also be used. Dissolution test procedures using a flow-through cell must be characterized with respect to rate and any pulsation.The flow-through cell (see Figures 4 and 5), of transparent and inert material, is mounted vertically with a filter system (specified in the individual monograph) that prevents escape of undissolved particles from the top of the cell; standard cell diameters are 12 and 22.6 mm; the bottom cone is usually filled with small glass beads of about 1-mm diameter with one bead of about 5 mm positioned at the apex to protect the fluid entry tube; and a tablet holder (see Figures 4 and 5) is available for positioning of special dosage forms, for example, inlay tablets. The cell is immersed in a water bath, and the temperature is maintained at 37 ± 0.5.Figure 4. Apparatus 4, large cell for tablets and capsules (top), tablet holder for the large cell (bottom). (All measurements are expressed in mm unless noted otherwise.)Figure 5. Apparatus 4, small cell for tablets and capsules (top), tablet holder for the small cell (bottom). (All measurements are expressed in mm unless noted otherwise.)The apparatus uses a clamp mechanism and two O-rings to assemble the cell. The pump is separated from the dissolution unit in order to shield the latter against any vibrations originating from the pump. The position of the pump should not be on a level higher than the reservoir flasks. Tube connections are as short as possible. Use suitably inert tubing, such as polytef, with about 1.6-mm inner diameter and chemically inert flanged-end connections.APPARATUS SUITABILITYThe determination of suitability of a test assembly to perform dissolution testing must include conformance to the dimensions and tolerances of the apparatus as given above. In addition, critical test parameters that have to be monitored periodically during use include volume and temperature of the Dissolution Medium, rotation speed (Apparatus 1 and Apparatus 2), dip rate (Apparatus 3), and flow rate of medium (Apparatus 4).Determine the acceptable performance of the dissolution test assembly periodically.The suitability for the individual apparatus is demonstrated by the Performance Verification Test. Performance Verification Test, Apparatus 1 and 2— Test USP Prednisone Tablets RS according to the operating conditions specified. The apparatus is suitable if the results obtained are within the acceptable range stated in the technical data sheet specific to the lot used and the apparatus tested.Performance Verification Test, Apparatus 3— Test USP Chlorpheniramine Maleate Extended-Release Tablets RS according to the operating conditions specified. The apparatus is suitable if the results obtained are within the acceptable range stated in the technical data sheet specific to the lot used.Performance Verification Test, Apparatus 4— [To come.]PROCEDUREApparatus 1 and Apparatus 2IMMEDIATE-RELEASE DOSAGE FORMSPlace the stated volume of the Dissolution Medium (±1%) in the vessel of the specified apparatus given in the individual monograph, assemble the apparatus, equilibrate the Dissolution Medium to 37 ± 0.5, and remove the thermometer. Place 1 dosage unit in the apparatus, taking care to exclude air bubbles from the surface of the dosage unit, andimmediately operate the apparatus at the specified rate given in the individual monograph.Within the time interval specified, or at each of the times stated, withdraw a specimen from a zone midway between the surface of the Dissolution Medium and the top of the rotating basket or blade, not less than 1 cm from the vessel wall. [NOTE—Where multiple sampling times are specified, replace the aliquots withdrawn for analysis with equal volumes of fresh Dissolution Medium at 37 or, where it can be shown that replacement of the medium is not necessary, correct for the volume change in the calculation. Keep the vessel covered for the duration of the test, and verify the temperature of the mixture under test at suitable times. ]Perform the analysis as directed in the individual monograph using a suitable assaymethod.3 Repeat the test with additional dosage form units.If automated equipment is used for sampling or the apparatus is otherwise modified, verification that the modified apparatus will produce results equivalent to those obtained with the standard apparatus described in this general chapter is necessary.Dissolution Medium— A suitable dissolution medium is used. Use the solvent specified in the individual monograph. The volume specified refers to measurements made between 20and 25. If the Dissolution Medium is a buffered solution, adjust the solution so that its pH is within 0.05 unit of the specified pH given in the individual monograph. [NOTE—Dissolvedgases can cause bubbles to form, which may change the results of the test. If dissolved gases influence the dissolution results, dissolved gases should be removed prior to testing.4 ] Time— Where a single time specification is given, the test may be concluded in a shorter period if the requirement for minimum amount dissolved is met. Specimens are to be withdrawn only at the stated times within a tolerance of ±2%.Procedure for a Pooled Sample for Immediate-Release Dosage Forms—Use this procedure where Procedure for a Pooled Sample is specified in the individual monograph. Proceed as directed in Procedure for Apparatus 1 and Apparatus 2 in Immediate-Release Dosage Forms. Combine equal volumes of the filtered solutions of the six or twelve individual specimens withdrawn, and use the pooled sample as the test specimen. Determine the average amount of the active ingredient dissolved in the pooled sample.EXTENDED-RELEASE DOSAGE FORMSProceed as directed for Immediate-Release Dosage Forms.Dissolution Medium— Proceed as directed for Immediate-Release Dosage Forms.Time— The test-time points, generally three, are expressed in hours.DELAYED-RELEASE DOSAGE FORMS NOT ACCEPTED BY THE JAPANESE PHARMACOPOEIAUse Method A or Method B and the apparatus specified in the individual monograph. All test times stated are to be observed within a tolerance of ±2%, unless otherwise specified. Method A—Procedure (unless otherwise directed in the individual monograph)—ACID STAGE— Place 750 mL of 0.1 N hydrochloric acid in the vessel, and assemble the apparatus. Allow the medium to equilibrate to a temperature of 37 ± 0.5. Place 1 dosage unitin the apparatus, cover the vessel, and operate the apparatus at the specified rate given in the monograph.After 2 hours of operation in 0.1 N hydrochloric acid, withdraw an aliquot of the fluid, and proceed immediately as directed under Buffer Stage.Perform an analysis of the aliquot using a suitable assay method. The procedure is specified in the individual monograph.BUFFER STAGE— [NOTE—Complete the operations of adding the buffer and adjusting the pH within 5 minutes. ]With the apparatus operating at the rate specified in the monograph, add to the fluid in the vessel 250 mL of 0.20 M tribasic sodium phosphate that has been equilibrated to 37 ± 0.5. Adjust, if necessary, with 2 N hydrochloric acid or 2 N sodium hydroxide to a pH of 6.8 ±0.05. Continue to operate the apparatus for 45 minutes, or for the specified time given in the individual monograph. At the end of the time period, withdraw an aliquot of the fluid, and perform the analysis using a suitable assay method. The procedure is specified in the individual monograph. The test may be concluded in a shorter time period than that specified for the Buffer Stage if the requirement for the minimum amount dissolved is met at an earlier time.Method B—Procedure (unless otherwise directed in the individual monograph)—ACID STAGE— Place 1000 mL of 0.1 N hydrochloric acid in the vessel, and assemble theapparatus. Allow the medium to equilibrate to a temperature of 37 ± 0.5. Place 1 dosage unit in the apparatus, cover the vessel, and operate the apparatus at the rate specified in the monograph. After 2 hours of operation in 0.1 N hydrochloric acid, withdraw an aliquot of thefluid, and proceed immediately as directed under Buffer Stage.Perform an analysis of the aliquot using a suitable assay method. The procedure is specified in the individual monograph.BUFFER STAGE— [NOTE—For this stage of the procedure, use buffer that previously has been equilibrated to a temperature of 37 ± 0.5. ] Drain the acid from the vessel, and add to the vessel 1000 mL of pH 6.8 phosphate buffer, prepared by mixing 0.1 N hydrochloric acid with 0.20 M tribasic sodium phosphate (3:1) and adjusting, if necessary, with 2 N hydrochloric acid or 2 N sodium hydroxide to a pH of 6.8 ± 0.05. [NOTE—This may also be accomplished by removing from the apparatus the vessel containing the acid and replacing it with another vessel containing the buffer and transferring the dosage unit to the vessel containing the buffer. ]Continue to operate the apparatus for 45 minutes, or for the specified time given in the individual monograph. At the end of the time period, withdraw an aliquot of the fluid, and perform the analysis using a suitable assay method. The procedure is specified in the individual monograph. The test may be concluded in a shorter time period than that specified for the Buffer Stage if the requirement for minimum amount dissolved is met at an earlier time.Apparatus 3 (Reciprocating Cylinder)NOT ACCEPTED BY THE JAPANESE PHARMACOPOEIA IMMEDIATE-RELEASE DOSAGE FORMS Place the stated volume of the Dissolution Medium in each vessel of the apparatus, assemble the apparatus, equilibrate the Dissolution Medium to 37 ± 0.5, and remove the thermometer. Place 1 dosage-form unit in each of the six reciprocating cylinders, taking care to exclude air bubbles from the surface of each dosage unit, and immediately operate the apparatus asspecified in the individual monograph. During the upward and downward stroke, thereciprocating cylinder moves through a total distance of 9.9 to 10.1 cm. Within the time interval specified, or at each of the times stated, raise the reciprocating cylinders and withdraw a portion of the solution under test from a zone midway between the surface of theDissolution Medium and the bottom of each vessel. Perform the analysis as directed in the individual monograph. If necessary, repeat the test with additional dosage-form units.Dissolution Medium—Proceed as directed for Immediate-Release Dosage Forms under Apparatus 1 and Apparatus 2.Time—Proceed as directed for Immediate-Release Dosage Forms under Apparatus 1 and Apparatus 2.EXTENDED-RELEASE DOSAGE FORMSProceed as directed for Immediate-Release Dosage Forms under Apparatus 3.Dissolution Medium—Proceed as directed for Extended-Release Dosage Forms under Apparatus 1 and Apparatus 2.Time—Proceed as directed for Extended-Release Dosage Forms under Apparatus 1 and Apparatus 2.DELAYED-RELEASE DOSAGE FORMSProceed as described for Delayed-Release Dosage Forms, Method B under Apparatus 1 and Apparatus 2 using one row of vessels for the acid stage media and the following row of vessels for the buffer stage media and using the volume of medium specified (usually 300 mL).Time—Proceed as directed for Immediate-Release Dosage Forms under Apparatus 1 and Apparatus 2.Apparatus 4 (Flow-Through Cell)IMMEDIATE-RELEASE DOSAGE FORMSPlace the glass beads into the cell specified in the monograph. Place 1 dosage unit on top of the beads or, if specified in the monograph, on a wire carrier. Assemble the filter head,and fix the parts together by means of a suitable clamping device. Introduce by the pump the Dissolution Medium warmed to 37 ± 0.5 through the bottom of the cell to obtain the flow rate specified in the individual monograph and measured with an accuracy of 5%. Collect theeluate by fractions at each of the times stated. Perform the analysis as directed in the individual monograph. Repeat the test with additional dosage-form units.Dissolution Medium—Proceed as directed for Immediate-Release Dosage Forms under Apparatus 1 and Apparatus 2.Time—Proceed as directed for Immediate-Release Dosage Forms under Apparatus 1 and Apparatus 2.EXTENDED -RELEASE DOSAGE FORMSProceed as directed for Immediate-Release Dosage Forms under Apparatus 4.Dissolution Medium —Proceed as directed for Immediate-Release Dosage Forms under Apparatus 4.Time —Proceed as directed for Immediate-Release Dosage Forms under Apparatus 4.DELAYED -RELEASE DOSAGE FORMSProceed as directed for Delayed-Release Dosage Forms under Apparatus 1 and Apparatus 2, using the specified media.Time —Proceed as directed for Delayed-Release Dosage Forms under Apparatus 1 and Apparatus 2.INTERPRETATIONImmediate-Release Dosage FormsUnless otherwise specified in the individual monograph , the requirements are met if the quantities of active ingredient dissolved from the dosage units tested conform to Acceptance Table 1. Continue testing through the three stages unless the results conform at either S 1 or S 2. The quantity, Q, is the amount of dissolved active ingredient specified in the individual monograph , expressed as a percentage of the labeled content of the dosage unit; the 5%, 15%, and 25% values in Acceptance Table 1 are percentages of the labeled content so that these values and Q are in the same terms. Acceptance Table 1Immediate-Release Dosage Forms Pooled Sample — Unless otherwise specified in the individual monograph, the requirements are met if the quantities of active ingredient dissolved from the pooled sample conform to the accompanying Acceptance Table for a Pooled Sample.Stage NumberTested Acceptance CriteriaS 16Each unit is not less than Q + 5%.S 26Average of 12 units (S 1 + S 2) is equal to or greater than Q, and no unit isless thanQ 15%.S 312Average of 24 units (S 1 + S 2 +S 3) is equal to or greater than Q , not morethan 2 units are less than Q 15%, and no unit is less than Q 25%.Continue testing through the three stages unless the results conform at either S 1 or S 2. The quantity, Q is the amount of dissolved active ingredient specified in the individual monograph, expressed as a percentage of the labeled content.Acceptance Table for a Pooled SampleExtended-Release Dosage FormsUnless otherwise specified in the individual monograph , the requirements are met if the quantities of active ingredient dissolved from the dosage units tested conform to Acceptance Table 2. Continue testing through the three levels unless the results conform at either L 1 or L 2. Limits on the amounts of active ingredient dissolved are expressed in terms of the percentage of labeled content. The limits embrace each value of Q i , the amount dissolved at each specified fractional dosing interval. Where more than one range is specified in the individual monograph , the acceptance criteria apply individually to each range. Acceptance Table 2Stage Number Tested Acceptance Criteria S 16Average amount dissolved is not less thanQ + 10%.S 26Average amount dissolved (S 1 + S 2) is equal to or greater than Q + 5%.S 312Average amount dissolved (S 1 + S 2 + S 3) is equal to or greater than Q.Level NumberTested CriteriaL 16No individual value lies outside each of the stated ranges and no individualvalue is less than the stated amount at the final test time.L 26The average value of the 12 units (L 1 + L 2) lies within each of the statedranges and is not less than the stated amount at the final test time; none ismore than 10% of labeled content outside each of the stated ranges; andnone is more than 10% of labeled content below the stated amount at thefinal test time.L 312The average value of the 24 units (L 1 + L 2 + L 3) lies within each of the statedranges, and is not less than the stated amount at the final test time; not morethan 2 of the 24 units are more than 10% of labeled content outside each ofthe stated ranges; not more than 2 of the 24 units are more than 10% oflabeled content below the stated amount at the final test time; and none ofthe units is more than 20% of labeled content outside each of the statedranges or more than 20% of labeled content below the stated amount at thefinal test time.Delayed-Release Dosage FormsNOT ACCEPTED BY THE JAPANESE PHARMACOPOEIA .Acid Stage — Unless otherwise specified in the individual monograph , the requirements of this portion of the test are met if the quantities, based on the percentage of the labeledcontent, of active ingredient dissolved from the units tested conform to Acceptance Table 3. Continue testing through all levels unless the results of both acid and buffer stages conform at an earlier level. Acceptance Table 3Buffer Stage — Unless otherwise specified in the individual monograph , the requirements are met if the quantities of active ingredient dissolved from the units tested conform to Acceptance Table 4. Continue testing through the three levels unless the results of both stages conform at an earlier level. The value of Q in Acceptance Table 4 is 75% dissolved unless otherwise specified in the individual monograph . The quantity, Q specified in the individual monograph is the total amount of active ingredient dissolved in both the Acid and Buffer Stages , expressed as a percentage of the labeled content. The 5%, 15%, and 25% values in Acceptance Table 4 are percentages of the labeled content so that these values and Q are in the same terms. Acceptance Table 4Level NumberTested CriteriaA 16No individual value exceeds 10% dissolved.A 26Average of the 12 units (A 1 +A 2) is not more than 10% dissolved, and no individual unit is greater than 25% dissolved.A 312Average of the 24 units (A 1 + A 2 +A 3) is not more than 10% dissolved, andno individual unit is greater than 25% dissolved.Level NumberTested CriteriaB 16Each unit is not less than Q + 5%.B 26Average of 12 units (B 1 + B 2) is equal to or greater than Q, and no unit isless thanQ – 15%.B 312Average of 24 units (B 1 + B 2 + B 3) is equal to or greater than Q, not morethan 2 units are less than Q – 15%, and no unit is less than Q – 25%.1The materials should not sorb, react, or interfere with the specimen being tested.2If a cover is used, it provides sufficient openings to allow ready insertion of the thermometer and withdrawal of specimens.3Test specimens are filtered immediately upon sampling unless filtration is demonstrated to be unnecessary. Use an inert filter that does not cause adsorption of the active ingredient or contain extractable substances that would interfere with the analysis.4One method of deaeration is as follows: Heat the medium, while stirring gently, to about 41, immediately filter under vacuum using a filter having a porosity of 0.45 µm or less, with vigorous stirring, and continue stirring under vacuum for about 5 minutes. Other validated deaeration techniques for removal of dissolved gases may be used.Auxiliary Information—Please check for your question in the FAQs before contacting USP. Array USP34–NF29 Page 278Pharmacopeial Forum: Volume No. 35(3) Page 719。
