Optimized_Protocol_Human Stem Cell
诱导性多功能干细胞——产生,发展,应用及展望

诱导性多功能干细胞——产生,发展,应用及展望张博文,杨星九,李玖一,白末*摘要:在胚胎干细胞研究因伦理道德和免疫排斥问题而受阻的时候,诱导性多功能干细胞(induced pluripotent stem cell,以下简称iPS细胞)的横空出世为干细胞研究指明了一条新的方向。
近几年来iPS细胞研究取得了许多突破性的进展,其广泛的应用前景更向人们昭示着一个新的时代的到来。
本文主要从iPS细胞的发展历程入手,综述了iPS细胞的理论及应用研究的关键进展,并对之后的研究进行了展望。
关键词:诱导性多功能干细胞,胚胎干细胞,病毒,癌变,细胞治疗Abstract:When the embryonic stem cell research was blocked by ethical issues and immune rejection, induced pluripotent stem cell (hereinafter referred to as iPS cells), turned out for stem cell research indicated a new direction. iPS cells’ research in recent years has made many breakthroughs, prospects for its wide application to remind people of a new era. This article summarizes the theory and application of iPS cells, and the key to progress in the study, from the iPS cells to start the development process, and discussed the study in the future.Key words:induced pluripotent stem cell, embryonic stem cell, virus, Canceration, cell therapyIPS细胞是通过向体细胞中导入诱导基因,使体细胞重编程获得具有胚胎干细胞样特性的多能干细胞。
stem cell
Mouse embryonic fibroblasts (MEF medium) Human embryonic stem cell (hESC medium)
MEF Medium DMEM Heat-inactivated FBS Non-essential amino acids L-Glutamine hESC Medium DMEM/F12 Knockout Serum Replacer Non-essential amino acids L-Glutamine β-mercaptoethanol Invitrogen 11330-032 Invitrogen 10828-028 Invitrogen 11140-050 Invitrogen 25030-081 Sigma 7522 400ml 100ml 5ml 2.5ml 3.5μl Invitrogen 11965-092 Invitrogen 16000-044 Invitrogen 11140-050 Invitrogen 25030-081 450ml 50ml 5ml 5ml
To passage hESC, cells are washed once or twice with PBS and incubated with filtersterilized 1mg/ml collagenase IV (Invitrogen Cat #17104-019) in DMEM/F12 for 10 to 30 minutes. Plates should be agitated every 10 minutes until colonies begin to detach. When moderate tapping of the plate causes the colonies to dislodge, they are collected and the wells washed with hESC medium to collect any remaining hESC. Alternatively, colonies may be removed using a cell scraper and collected. Colonies are allowed to sediment for 10 minutes. The supernatant, containing residual MEFs, is aspirated, and the colonies are washed with 5ml hESC medium and allowed to sediment again. This is repeated once more. After the final sedimentation, the colonies are resuspended in 1ml of hES medium and triturated gently to break up the colonies to approximately 100-cell size. Generally, cell lines are passaged at a ratio of 1:3 every four to seven days.
人类胚胎干细胞和诱导多能干细胞的研究

人类胚胎干细胞和诱导多能干细胞的研究人类胚胎干细胞(human embryonic stem cells,hESCs)和诱导多能干细胞(induced pluripotent stem cells, iPSCs)是当今生物医学研究的重要热点之一。
这两种干细胞都具有自我更新和分化为多种不同类型细胞的能力,因此在组织再生、疾病治疗、药物筛选等领域有着广泛的应用前景。
本文将综述人类胚胎干细胞和诱导多能干细胞的研究现状和前景。
一、人类胚胎干细胞1. 发现和特点人类胚胎干细胞是在1998年,由美国犹他大学埃文斯实验室发现的。
它们是从人类胚胎的内细胞团(inner cell mass,ICM)中分离出来的非常原始的细胞,具有自我更新和无限增殖的能力,并可以分化为身体的所有不同类型的细胞,包括神经元、心肌细胞、肝细胞等。
这些特点使得人类胚胎干细胞成为组织工程和再生医学领域的重要研究材料,有着广泛的用途。
2. 研究进展和问题尽管人类胚胎干细胞潜力巨大,但是在研究和应用过程中依旧受到很多限制和问题。
首先,人类胚胎干细胞的获取受到伦理和法律的限制。
在许多国家和地区,胚胎干细胞研究和应用仍然是禁止或受严格限制的。
即使是在开放的国家,也需遵循伦理标准和规定的程序,获得胚胎干细胞。
其次,人类胚胎干细胞的使用也存在一些问题。
首先,人类胚胎干细胞具有致癌性和免疫排异等风险,不当的使用会导致一些不良后果。
其次,人类胚胎干细胞分化过程中的影响因素、机制以及调控方法还不完全清楚,因此在分化过程中的控制更为困难。
此外,用于分化人类胚胎干细胞的培养基和因子组合等方法,也在不断的优化和改进中。
二、诱导多能干细胞1. 发现和特点在人类胚胎干细胞受到法律和伦理限制的背景下,2006年,日本的山中伸弥等一众科学家发现了诱导多能干细胞(induced pluripotent stem cells,iPSCs),这是人类成体细胞被诱导再生为早期胚胎干细胞状态的一种细胞,可以用于组织工程、疾病治疗、药物筛选等领域。
提名人简介

提名人简介仇子龙博士,男,1976年12月出生。
2009年回国后担任中国科学院上海生命科学研究院神经科学研究所研究员至今,主要从事自闭症、瑞特综合征等神经发育疾病的生物学研究,研究成果阐述了神经发育疾病的遗传、分子与神经环路机制,并建立了自闭症的非人灵长类动物模型。
在Nature, Developmental Cell, Molecular Psychiatry, Current Opinion in Neurobiology等国际生物学权威期刊上发表研究论文与应邀综述十余篇,引用逾两千余次。
自闭症的非人灵长类动物模型工作入选科技部2016年“中国科学十大进展”,中国科协2016年“中国生命科学十大进展”。
仇子龙研究员的工作围绕MECP2基因,从非人灵长类动物模型到分子细胞机制,获得了一系列原创性成果,代表性工作包括:1、自闭症相关基因MeCP2调控microRNA核内剪切加工与神经系统发育仇子龙研究员的工作发现MeCP2蛋白直接参与小RNA (microRNA)的核内剪切加工过程,而与其传统的转录调控功能无关。
此工作为自闭症相关蛋白MeCP2的功能研究提供了崭新的角度,进而提出神经发育性疾病的致病机理很可能与大脑中microRNA表达失调密切相关,为DNA甲基化与microRNA两种表观遗传学调控建立联系的同时,也为开展转化医学研究提供了理论依据。
2、自闭症的非人灵长类动物模型仇子龙研究员与神经所非人灵长类转基因平台合作,开展了自闭症的非人灵长类动物模型构建工作。
通过构建携带人类自闭症基因MECP2的转基因猴及对转基因猴进行分子遗传学与行为学分析,历时5年的工作发现MECP2转基因猴表现出类人类自闭症病人的重复运动模式、焦虑水平上升、刻板行为与社交障碍等行为表型。
研究团队还通过精巢异体移植与体外受精等方法,成功的得到了携带人类MECP2基因的第二代转基因猴,且发现其在社交行为方面也表现出了严重障碍。
改善重组人干细胞因子包涵体复性与同时纯化放大过程的回收率

Improve on recovery of the recombinant human stem cell factor inclusion body in refolding with simultaneous purificationprocess1Wang Lili, Wang Chaozhan, Liu Jiangfeng, Geng XinduInstitute of Modern Separation Science, Shaanxi Key Laboratory of Modern Separation Science, Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education,Northwest University, Xi’an, China (710069)E-mail: llwang@AbstractRecombinant expressed human stem cell factor (rhSCF) as cytoplasmic inclusion bodies (IB) are reported. In present work, to increase mass recovery of rhSCF production in large scale, the factors affecting about efficiency of rhSCF IB were recovered and solubilised in urea solution, and refolding with simultaneous purification process using protein folding liquid chromatography (PFLC) were investigated, including normal chromatographic column, the unit for the simultaneous renaturation and purification of proteins (USRPP). Finally, by combining the optimized buffer and USRPP, we were able to obtain22 mg rhSCF with >95% purity.The mass recovery is 24 % for dilution, 38 % for the normal chromatographic column, 49 % for the USRPP. An average specific bioactivity is 4.27 ×105 IU/mg, 6.9×105 IU/mg, and 1.28×106 IU/mg, respectively. These protocol dates and new refolding with purification method -USRPP provide a cost effective and an efficient way to produce quantities of high purity rhSCF in large-scale.Keywords: recombinant human stem cell factor, inclusion body, protein folding liquid chromatography1. INTRODUCTIONStem cell factor (SCF) is a cytokine produced by multiple types of cells including stromal cells andfibroblasts [1]. The soluble form of SCF has 165 amino acids, and exists as a non-covalently associated homodimer [2, 3]; with each SCF monomer containing two intra-chain disulfide bridges, Cys4–Cys89 and Cys43–Cys138 [4]. The human soluble SCF (hSCF) shows multi-lineage hematopoiesis-stimulating activities, and therefore has been considered as a potential therapeutic for various diseases [5]. So far, the recombinant hSCF (rhSCF) has been produced in genetically engineered E. coli [6, 7, 8 ]. Because rhSCF is mostly in the inclusion body fraction of bacterial lysates, a refolding process is necessary to produce soluble rhSCF possessing the same bioactivity as its native state. Evidence has demonstrated that the oxidative refolding of rhSCF produced in E. coli might produce at least five intermediate forms, I-1 to I-5, detectable by their differences in hydrophobicity using reverse-phase high performance liquid chromatography [9], suggesting that oxidative refolding is not efficient enough to refold all rhSCF in solubilized inclusion bodies. Thus, in order to obtain pure rhSCF with high a bioactivity, new refolding and purification procedures are required to provide correctly folded rhSCF with high yields.Protein folding liquid chromatography (PFLC) technology is a new method developed at present [10]. Their characteristics are easier to achieve scale preparation, such as high performance hydrophobic interaction chromatography (HPHIC) [ 11,12],Size exclusion chromatography (SEC) [13]and ion exchange chromatography (IEC) [14,15]. Especially, HPHIC process, solubilized inclusion body proteins interact with the hydrophobic medium tightly preventing not only aggregation of unfolded proteins, but also dominates the formation of steric structures inproteins and thus assists in the refolding of the denatured proteins, the refolded proteins can be simultaneously purified during HPHIC the1 This work was supported by grants from the National Natural Science Foundation of China (No.20475042) , the Foundation of the Key Laboratory for Modern Separation Science in Shaanxi Province (No.05JS60) and Specialized Research Fund for the Doctoral Program of Higher Education (No.20040697002).process[16]. A specially designed unit, with diameter much larger than its length, was designed and employed for both laboratory and preparative scales of the unit for the simultaneous renaturation and purification of proteins (USRPP)[17].In recent years, the USRPP has been used for a scale manufacturing of recombinant protein [17, 18, 19]. We have been reported previously to use HPHIC for the refolding and simultaneous purification of rhSCF expressed in E coli [20]. In this work, to increase of rhSCF production in large scale, we discusses the renaturation buffer, and the optimization of factors affecting the efficiency of refolding and purification of rhSCF with HPHIC column, USRPP.The efficient procedure of refolding and purification may be useful for the mass production of rhSCF proteins.2. EXPERIMENTAL2.1 ApparatusHPHIC was carried out using an LC–10ATvp high-performance liquid chromatograph (Shimadzu, Kyoto, Japan) consisting of two pumps (LC–10A), a variable–wavelength UV–Vis detector (SPD–10A V), and a system controller (SCL–10B). The HPHIC column (150mm×4.6mm i.d.), and the USRPP (10mm×20mm i.d.) were bought from Shaanxi Xida Keli Gene-Pharmcy Co.Ltd (Xi’an, China), and was packed materials with PEG-200, PEG-400, PEG-600 and furfural,2.2 ChemicalsAcrylamide,bis-acrylamide, BSA, reduced glutathione(GSH), oxidized glutathione(GSSG)and the referents of standard molecular weight were obtained from Sigma. Tris, glycine and SDS were obtained from Amresco; Coomassie Brilliant Blue G-250 (Fluka, MO, USA). All other chemicals were of analytical grade.2.3 Preparation of rhSCF extractThe strain used was recombinant E. coli DH5α harboring the plasmid pBV220 [8,21].The bacteria were produced with a 5 L fermenter (B. Brawn Co, Germany). E. coli cells containing rhSCF were disrupted by sonication in a buffer containing 20 mmol/L phosphate buffer solution (PBS), pH 7.4, 1 mmol/L EDTA, and 0.20 mg/ml lysozyme, were collection of rhSCF from E.coli DH5α was performed using the procedure described by Wang [20] .The cleaned inclusion bodies were were recovered by cen-trifugation at 16000g, 4°C for 20 min and solubilized in 50 mmol/L Tris-HCl, pH 8.0, 8 mol/L urea, 10 mmol/L dithiothreitiol, 1 mmol/L EDTA and were centrifuged at for 20 min. The protein concentration of the supernatant was was adjusted by Bradford method to a final concentration using 8 mmol/L urea solubilizing solution.2.4 Chromatographic procedureA chromatography run was carried at room temperature. The packed HPHIC column, and URSPP was equilibrated with 100% mobile phase A (3.0 mol/L ammonium sulfate [(NH4)2SO4], 50 mmol/L potassium dihydrogen phosphate (KH2PO4), pH 7.0) at a selected flow rate for concentration gradient elution depended on the size of column. 8 mol/L urea of crude rhSCF solution was directly injected into the column through the sample valve, respectively. All chromatograms were detected using UV absorbance at 280 nm.,Gradient elution (linear and nonlinear) was used during the purification of rhSCF and fractions containing target protein were collected for the measurements of the recoveries of bioactivity and mass of the rhSCF.2.5 Analytical proceduresThe column-refolding fraction was incubated for 30 min at 30°C, followed by dialysis against 20 mmol/L PBS, pH 7.4 at 4°C. The dialysis solution was collected and lyophilized (stored frozen until further tests). The total protein concentration of the product in the purification fractions of rhSCF was determined using the Bradford method, and evaluated mass recovery by Wang [20] .The purity of rhSCF was analyzed by SDS-PAGE with 15% acrylamide, and the density of each band after staining with Coomassie bright blue (Uppsala, Sweden) was quantified by scanning the gel using a thin-layer gel scanner (Cs-930, Shimadzu, and Kyoto). Monomers and aggregate forms of rhSCF were analyzed by gel chromatography using Sephadex G-75(Amersham Pharmacia,200mm×16mm i.d.). The molecular weight of rhSCF was evaluated by MALDI-TOF-MS (Axima CFR plus, Kratos, Shimazu, Japan).2.6 Assay for bioactivity of hSCFThe bioactivity of rhSCF was measured using the hSCF-dependent cell line UT-7 [22,23]. Briefly, cells were cultured in RPMI-1640 (from Sigma) medium supplemented with 10% fetal calf serum (v/v) and maintained in the presence of erythropoietin (from the National Institute for the Control of Pharmaceutical and Biological Products of China). Cells were washed with the culture medium and cultured in the presence of the purified rhSCF at different concentrations. Proliferation of the cells was determined using the MTT method.3. RESULTS AND DISCUSION3.1 Optimal buffer composition for refolding of rhSCFAt present, mostly presence problem of refolding and purification recombinant human proteins are still lost of activity, scaling up, low recovery. Some literature date have provided information aimed at enhancing the refolding yield of inclusion body proteins by reducing the causes of aggregation and misfolded configurations, respectively[24,25]. It has been reported that certain Gu.HCl, urea (1-2 mol/L), and L-arginine (0.3-1 mol/L) can inhibit protein aggregating [26,27]. Valente et al reported that an optimized protocol could significantly increase the yield [28].To obtain complete refolding of the solubilized inclusion bodies, we optimized the pH and components of the refolding buffers in dilution process, such as the urea, arginine components, which are usually used in the refolding of recombinant proteins, by monitoring protein recovery in the soluble fraction after refolding (Fig.1). Usually, aggregation decreases when the pH of the medium is far away from the protein’s isoeletric point [29]. The most favorable pH value varies from protein to protein, it effect directly aggregation of protein and the formation of disulfide bonds. A proper pH has a cooperative effect on enhancing the renaturation yield of rhSCF and on the formation of disulfide bonds. Fig.1A shows how refolding of rhSCF in buffers of different pH with dilution. The refolding of the rhSCF had the highest mass recovery in pH 8.2-8.5.Low concentration of denaturant such as urea has been included in various renaturing buffers as an efficient inhibitor of protein aggregation, which can assist spontaneous protein refolding in solution and increase the output of correctly folded proteins,as shown in Fig.1B. It is has been reported arginine to efficiently inhibit inclusion body aggregation of recombinant proteins [30]. Fig.1C shows the turbidity and protein recovery of rhSCF refolded in the presence of different concentrations of arginine. The turbidity was measured by transparency at A450. The result indicated that the most suitable concentration of arginine is between 0.5-0.6 mol/L.Each SCF monomer contains two intra-chain disulfide bridges, Cys4–Cys89 and Cys43–Cys138 [1]. We further examined the effect of the redox agent glutathione (GSSG/GSH) on the folding property of rhSCF. In the absence of GSSG /GSH (0.25 mmol/L) does not promote the folding of rhSCF. Theinfluence of the ratio of GSH/GSSG on the refolding of rhSCF as shown in Fig.1D, the results indicate that refolding of rhSCF is very favorable in 5:1 with GSH/GSSG .From the above results, Fig.1A-1D showed the solubilized rhSCF using the optimal of a refolding buffer 50 mmol/L Tris-HCl, pH 8.2, 1 mmol/L EDTA, 1 mmol/L oxidized glutathione, 0.2 mmol/L reduced glutathione, 2.5 mol/L urea, 0.5 mol/L arginine at 4°C with slight agitation, and mass recovery of rhSCF was improved from 10 % to.24 % .These dates will follow as a result using chromatography processing.Figure 1: Optimal buffer composition for refolding of rhSCF1A: The influence pH on the recovery of refolded rhSCF in denaturing buffer 1B: The influence urea on the recovery of refolded rhSCF in denaturing buffer 1C: The influence of the ratio of GSH/GSSG on the refolding of rhSCF1D: Effect of arginine concerntration on aggregation and on the recovery of refolded rhSCF in denaturing buffer3.2 Selection ligand structure of STHICSilica-based packing material was reported to have bi-functions of purification and renaturation for proteins [18]. The hydrophobicity and structure of the ligand used in the stationary phase of hydrophobic interaction chromatography (STHIC) were found to be the most important factors affecting mass recovery [31,32].Urea concentration (mol L Mass recovery ( %)P r o t e i n c o n c e n t r a t i o n (m g m l -1)-◆-Tris-urea; -☆-PBS-urea buffer;-■-Tris buffer;-▲-PBS buffer0 2 4 6 7 8-1)Activity recovery (%)P r o t e i n c o n c e n t r a t i o n (m g m L -1)4 5 6 7 8 9 10 11 12pH-▲-Protein concentration -■-Activity recoveryRecovery of protein (%)-◇-Protein concentration -■-Activity recovery0 1 2 3 4 5 6Arginine concentration (mol l -1)T u r b i d i t y (450 n m )ABCTable 1 The mass recoveries of refolded rhSCF and retention on different ligands aLigands Chromatography column (150×4.6mm i.d.)USRPP(10×20mm i.d.)Retention time (min) The massRecovery (%)Retention time (min) The massRecovery (%) -(CH 2-CH 2-O)400 33.0811 36 31.701 49 -(CH 2-CH 2-O)600 34.556 34 31.709 37 -(CH 2-CH 2-O)800 34.954 30 31.854 32 -O-CH 2-O-phenyl 36.152 2831.925 29aThe 100µL solutions of 8.0 mol/L urea solution were directly injected into the four kinds of HIC columns,respectively.We firstly determine which type of STHIC media is suitable for the specific protein we wish to refold. Four kinds of ligands with different hydrophobicities and molecular structures were tested, and the order of four hydrophobic ligands were furfural>PEG600>PEG400>PEG200 [17,32].8.0 mol/L urea dissolved inclusion body was directly loaded onto the four kinds of ligands chromatographic columns, and listed in Table 1 show result. From this Table 1,although the obtained rhSCF peaks shows the almost same retention time in USRPP, the mass recoveries of the collected rhSCF from the four STHIC are significantly with each other and PEG 400 is best one, in other words, the ligand PEG-400 is more favorable for rhSCF refolding..3.3 Optimal buffer composition of mobile phasesAs descried above “ptimization of buffer composition”, when the refolding buffer contains 2.5 mol/L urea, 0.5 mol / L arginine and the pH value is maintained at 8.2 using refolding buffer of dilution, the highest mass recovery and refolding efficiency of the rhSCF is achieved. This indicates the net environmental contribution of irrespective of stationary phase. In practice; it is predictable that the composition of the mobile phase should also play an important role in rhSCF refolding by HPHIC also. The continuously changing environment of the mobile phase during gradient elution provides a broad concentration range of salt for its refolding. Especially, it is the disulfide bridges of protein molecules.As shown in Table 2, buffers 1 and buffer 2 were used for comparing the contribution of the mobile phase to rhSCF refolding. In terms of mass recovery and the specific bioactivity of the refolded rhSCF, buffer 2 performs better than buffer 1. Apparent, mobile phase composition is one of the most important factors affecting the efficiency of protein renaturation in chromatographic procedure. The optimization of mobile phase composition protein renaturation with simultaneous purification is thus much more important than that in usual liquid chromatography [32].Table 2 Effects of eluting buffer on the renaturation of rhSCF**Eluting buffer Mass recovery a Specific bioactivity Purify(% ) (×106 IUmg -1) (%)bBuffer 1 36.5 0.73 >95 cBuffer 2 47.9 1.14 >95**Column (150×4.6mm i.d.);aThe ratio of the mass of recovered in collected fractions and total mass of injection after 8mol /L urea dissolved ; bBuffer 1: Mobile phase A, 3.0 mol/L (NH 4)2SO 4 ,50 m mol/L KH 2PO 4, pH 7.0 ; Mobile phase B, 50 m mol/L KH 2PO 4 pH 7.0 . cBuffer 2: Mobile A,3.0 mol/L (NH 4)2SO 4 ,50 mmol/L KH 2PO 4, 2.0 mol/L urea, 1.0 mmol/L GSH , 0.20 mmol/L GSSG , and 0.5 mol/L arginine, pH 7.0;Mobile phase B, 50 m mol/L KH 2PO 4 ,2.0 mol/L urea, 1.0 m mol/L GSH , 0.20 m mol/L GSSG , 0.5 mol/L arginine, pH 7.0.3.4 Gradient elution modeThe advantage of USRPP is that protein refolding and purification can be achieved simultaneously in one chromatographic run [18]. This means, in addition to the efficiency of protein folding, good resolution is an indicator of the purity of the refolded rhSCF also. We tried to optimize the gradient elution modes for the refolding of rhSCF using linear and nonlinear gradient elution. Fig.2 shows the comparison between the chromatograms for linear gradient (Fig.2A) and nonlinear gradient (Fig.2B), respectively. As can be seen from figure 2, the resolution using nonlinear gradients is better than using a linear gradient when all other conditions are the same.Figure 2 Chromatogram of rhSCF by using a USRPP on different gradient modes linear gradient elutionFigure 2A: 40min linear gradient elution; Figure2B: 40min non- linear gradient.Columns (10×20mm i.d.),100% solution A, 3.0 M ammonium sulphate – 50mM potassium dihydrogen phosphate (pH 7.0) to 100% solution B, 50mM potassium dihydrogen phosphate (pH 7.0) in 30 min with 10 min delay. Flow rate: 2.0 mlmin-1.3.5 Refolding with simultaneous purification of rhSCF by USRPPDuo to one of the advantages of USRPP is much bigger diameter than its length, the flow rate of the mobile phase is very high which decreases the time the sample is in the sample loop thereby; reducing formation of precipitates [17,18]. In addition, if some precipitates form on the surface area of the filter frit, the column backpressure can still remain at a low level, because the precipitates only block a very small fraction of the total surface area. Thus, unlike normal chromatographic columns, which can be blocked by precipitates, USRPP can maintain a normal chromatographic run. One-step refold with simultaneous purification process in a USRPP (PEG400, 10mm ×20 mm i.d.), allowed us to run sampleA b s o r b a n c e (280 n m )A b s o r b a n c e (280 n m C o n c e n t r a t i o n o f b u f f e r 10 20 30 4010 20 30 400 50 100 C o n c e n t r a t i o n o f b u f f e r B0 50 100t / min t / min ABsolution of 1 ml rhSCF was loaded onto the USRPP, then a nonlinear gradient was performed for 40 min. 50ml fractions were collected, and we checked the purity of rhSCF by Coomassie blue stained SDS-15% PAGE (Fig.3, lane 4), the purity is ≥ 95%.Figure 3 SDS-15%PAGE analysis of rhSCF step -by-step purificationLane1: Molecular weight marker (14,400Da; 20,000 Da; 24,000Da; 29,000Da; 36,000 Da, 45,000 Da, 66,200 Da); Lane 2: 8mol/L urea dissolved inclusion body solution; Lane 3: Refolded by dilution; Lane 4: Collected fraction refolded and purification of rhSCF by USRPP (10×20mm i.d.); Lane 5: Collected fraction refolded and purification of rhSCF bycolumn (150×4.6mm i.d.); Lane 6: The final bulk after semi-permeation (freeze-drying)3.6 Analysis characteristic in the final bulkThe recovery of the bioactivity and mass of the rhSCF obtained from the dilution-refolded to the column-refolded methods is shown in Table 3. From Table 3, the 8 mol/L urea-dissolved rhSCF could be efficiently refolded with simultaneous purification in one-step using USRPP, and obtain over 22 mg of hSCF from per liter of M9 media culture.SEC was employed for the analysis of the purified rhSCF under native conditions for monomeric rhSCF.The biological activities of production provide valuable information for refoding of rhSCF protein; this is a very important characteristic for large-scale renaturation. As shown in Fig 4, the renaturated and purified rhSCF was examined by UT-7(human megakaryoblastic leukemia cell) dependent cell line [22]. According to dose-response curve of SCF on UT-7 cell proliferation, the refolded rhSCF also possesses a higher bioactivity in supporting the growth of an SCF-dependent cell line UT-7; the purified rhSCF protein has comparable activity as natural product. Compared to the dilution-refolded rhSCF (4.27 ×105 IU/mg), the column-refolded rhSCF had an average specific bioactivity of 6.9×105 IU/mg and 1.28×106 IU/mg, respectively. MALDI-TOF analysis demonstrated the molecular weight of 18,573 Da (18,589 Da for natural hSCF).1 2 3 4 5 666.045.036.029.024.020.014.2Figure 4 The dose-response curve of rhSCF for the proliferation of UT-7 cells1: Production of renatured of rhSCF by dilution; 2: Standard of the rhSCF (from sigma, Std.). 3: Production of renaturedwith simultaneous purification of rhSCF by USRPPTable 3 The comparison of the refolding and purification of rhSCF with different protocols aSteps Total proteins (mg )Purity (%)Yield ofrhSCF(mg) Recovery of rhSCF(%)Cell lysate 213 32 45 1008M urea dissolved IB b 71 81 38 84 Dilution refolding 19 85 11 24HPHIC column d31 >95 17 38 USRPP d 37>95 22 49 From 1L of E. coli culture; after optimal cleaning buffer; The 200µL solutions of 8.0 mol/L urea solution weredirectly injected into different columns, respectively.3.7 ConclusionComparisons of rhSCF produced with different methods, show a comparable mass recovery of rhSCF, refolded the rhSCF simultaneously using USRPP possesses a higher specific bioactivity: i.e. 3 times that produced by dilution. Experiments indicated that the STHIC having a weaker interaction than affinity, ion-exchange or reversed-phase chromatography, the structural damage to the proteins is assumed to be minimized, and the biological activity of the proteins is maintained. However the advantage of STHIC is that the chromatographic condition is much closed to the physiological condition, such as neutral pH, aqueous salt solution, being favorable to remain protein bioactivity [33,34,35].Our results show that (1) Refolding and purification of recombinant proteins occurred more efficiently using USRPP rather than usually chromatography column.(2)it possible that some specific methods and strategies have made to enhance high yields of biologically active proteins by taking into account process parameters, include refolding buffer of additive, pH, redox conditions ionic strength, and generation of correct disulphide bonds.(3)Due to a combination of adsorption on STHIC and a gradient elution, with mobile phase containing additive, the mass and bioactivity recoveries of rhSCF should markedly increase.(4) It is expected that this procedure will be useful in the large-scale manufacture of rhSCF for therapeutic purposes. This data provides new evidence that PFLC is a reliable tool for the refolding with simultaneous purification of recombinant proteins.0.2 0.4 0.6 0.8 1.0A 570 n m0 0.5 5 50 500SCF(ngmL -1)123References1.Broudy V.C., Stem Cell Factor and Hematopoiesis, Blood 1997; 90:1345-13642.Martin F.H., Suggs S.V., Langley K.E., Lu H.S., Ting J., Okino K.H., Morris C.F., et al. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell 1990; 63:203-2113.Zsebo K.M., Williams D.A., Geissler E.N., et al, Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 1990; 63:213-2244.Anderson D.M., Lyman S.D., Baird A., et al, Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms.Cell 1990;63:235-2435.McNiece I.K., Langley K.E., Zsebo K.M., Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and Epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Exp. Hematol.1991; 19: 226-231ngley K.E., Mendiaz E.A., Liu N., Narhi L.O., Zeni L., Parseghian C.M., Clogston C.L., Leslie I., Pope J.A., LuH.S., et al, Properties of variant, forms of human stem cell factor recombinantly expressed in Escherichia coli, Arch Biochem.Biophys. 1994; 311:55-617.Chen W.D., Di X., Li J., Song F., Chen S.S., cDNA cloning of human stem cell factor and its high level expression in E.coli. Acta Bioch Bioph Sinica 1997; 19:29-348.Wang L.L., Geng X.D., Han H.,Cloning,expression,renaturation and purification of soluble hSCF. Chin.J.Cell.MolImmunol. 2004; 20:402-4059.Jones M.D., NarhiL.O., Chang W.C., Lu H.S., Oxidative folding of recombinant human stem cell factor (rhSCF) produced in Escherichia coli, J Biol- Chem. 1996;271:1301-1130810.Geng X.D., Wang C.Z., Protein folding liquid chromatography and its recent developments. J.Chromatogr.B 2007; 849: 69-8011.Geng X D, Chang X Q. High performance hydrophobic interaction chromatography as a tool for protein refolding. J Chromatogr, 1992; 599:185 -19412.Wang C.Z., Geng X.D., Wang D.W., Tian B., Purification of recombinant bovine normal prion protein PrP(104-242) by HPHIC, Journal of chromatogr. B 2004; 806:185-19013.Lu H.Q., Zang Y.H., Ze Y.G., Zhu J., Chen T., Han J.H., Qin J.H., Expression, refolding, and characterization of a novel recombinant dual human stem cell factor. Protein Expr.Purif.2005; 43:126–13214.Wang C.Z., Wang L.L., Geng X.D., Renaturation of recombinant human granulocyte colony-stimulating factor produced from Escherichia coli using size exclusion chromatography, J Liquid Chromatogr. & Related Tech. 29(2006)203-21715.Wang C.Z., Wang L.L., Geng X.D., Renaturation with simultaneous purification of rhG-CSF from Escherichia coli by ion exchange chromatography, Biomedical Chromatography, 2007;21: 1291-129616.Geng X.D., Bai Q., Mechanism of simultaneously refolding and purification of proteins by hydrophobic interaction chromatographic unit and applications. Sci. Chin. (Ser. B) 2002; 45: 655-66917.Geng X.D., Bai Q., Zhang Y.J., Li X., Wu D., Refolding and purification of interferon-gamma in industry by hydrophobic interaction chromatography. J Biotechnol. 2004; 113: 137-149.18.Geng X.D., Zhang Y.J., Caky chromatography column and the method for producing it and its applications. United States Patent 7, 208,085 B2, 200719.Wang C.Z., Wang L.L., Geng X.D., Refolding recombinant human granulocyte colony-stimulating factor expressed by E.coli, BioProcess international, 2006;5:48-5220.Wang L.L., Wang C.Z., Geng X.D., Refolding with simultaneous purification of recombinant human stem cell factor expressed in Escherichia coli by high performance hydrophobic interaction chromatography, Biotechnol. Lett. 2006; 28: 993-99721.Zhang Z.Q., Yao L.H., Hou Y.D., Construction and application of a high level expression vector containing P R P L promator, Chin.J.Vir. 6(1994), pp.111-118.22.Liesveld J.L., Harbol A.W., Abboud C.N., Stem cell factor and stromal cell co-culture prevent apoptosis in a subculture of the megakaryoblastic cell line, UT-7. Leuk Res.1996; 20:591-60023.Wang J.Z., Zhao Y., Chen G.Q., Rao C.M., Quality control for bioassay of recombinant human stem cell factor (rhSCF),Chin J Cancer Biother. 2001; 8:294-29624.Clark E.D.,Protein refolding for industrial processes. Curr Opin Biotechnol. 2001; 12:202-20725.Tsumoto K., Ejima D., Kumagai I., et al. Practical considerations in refolding proteins from inclusion bodies. Protein Expr Purif. 2003; 28:1-826.Arakawa T., Tsumoto K., The effects of arginine on refolding of aggregated proteins: not facilitate refolding, but suppress aggregation, Biochem Biophys Res Commun. 2003; 304:148-15227.Swietnicki W., Folding aggregated proteins into functionally active forms, Curr.Opin.Biotech. 2006; 17: 367-37228.Valente C.A., Monteiro G.A., Cabral J.M.S., Fevereiro M. and Prazeres D.M.F., Optimization of the primary recovery of human interferon-α2b from Escherichia coli inclusion bodies. Protein Expr Purif. 2006; 45:226-23429.Clark D.B., Hevehan D., Szela S., and Maachupalli-Reddy J., Oxidative Renaturation of Hen Egg-White Lysozyme. Folding vs Aggregation. Biotechnol. Prog. 1998; 14:47-5430.Tsumoto K., Ejima D., Nagase K., Arakawa T., Arginine improves protein elution in hydrophobic interaction chromatography: The cases of human interleukin-6 and activin-A. J. Chromatogr. A 2007; 1154:81-8631.Gong B.L., Wang L.L., Wang C.Z., Geng X.D., Preparation of hydrophobic interaction chromatographic packings based on monodisperse poly (glycidylmethacrylate-co- ethylenedimethacrylate) beads and their application. J.Chromatogr.A 2004; 1022:33-3932.Wu D., Wang C.Z., Geng X.D., An approach for increasing the mass recovery of proteins derived from inclusion bodies in biotechnology. Biotechnol.Progr. 2007:23:407-413。
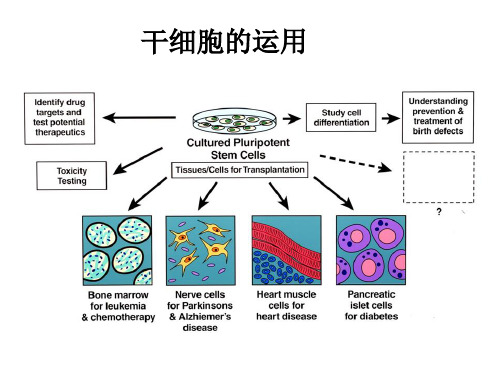
细胞与材料存在多种相互作用关系
Ⅰ Materials as Protector
Gelatin was used to mimic the Extracellular Matrix (ECM) that provided a biocompatible environment for encapsulated cells
FIG.2 GB & Preparation scheme of cationic gelatin (GA).
The sucrose solution, which was 37 μm in diameter in air, spread out once it landed on the glass surface to a final diameter of 250 μm.
FIG.7 3T3 fibroblasts printed in 8.5% sucrose, 0.3% dextrose in DI water on a glass substrate. White field and fluorescent image (cell tracker die) as well as the overlap of these two images demonstrated that the captured entity is a cell
The focused beam causes the thermal and/or photomechanical volatilization of a small amount of solution near the interface, resulting in ejection of a small number of cells.
Ⅰ Laser assisted bioprinting of human osteosarcoma cells
诱导多能干细胞名词解释

诱导多能干细胞名词解释概述诱导多能干细胞(induced pluripotent stem cells,简称iPSCs)是一种在实验室中通过重新编程成熟细胞而获得的一类多能干细胞。
与胚胎干细胞相比,iPSCs具有相似的多能性和自我更新能力,但不涉及胚胎的形成过程,从而避免了伦理和法律争议。
iPSCs的发现被认为是一项重大突破,为疾病治疗、组织再生和药物筛选等领域带来了新的可能。
历史背景iPSCs的发现可以追溯到2006年,由日本科学家山中伸弥和他的团队首次成功地通过将成熟的人体皮肤细胞转录因子的表达方式,将其重新编程成具有胚胎干细胞样特征的细胞。
这项研究是在老鼠细胞中进行的,但随后的研究证实了在人体细胞中也可以实现这一转化。
山中伸弥因此成为首位获得诺贝尔生理学或医学奖的日本科学家。
iPSCs的制备方法iPSCs的制备方法通常包括以下几个关键步骤:1.选择原始细胞:可以使用多种类型的成熟细胞作为起始细胞,如皮肤细胞、血液细胞等。
起始细胞的选择在一定程度上会影响后续iPSCs的性质和应用。
2.重编程方法:通过引入转录因子或使用其他技术,将起始细胞中特定转录因子的表达重新激活,使其回到未分化状态。
这些转录因子通常包括Oct4、Sox2、Klf4和c-Myc等。
3.细胞培养和扩增:将重编程后的细胞进行培养和扩增,使其成为一个细胞系。
这个细胞系中的细胞具有类似于胚胎干细胞的特征,包括多能性和自我更新能力。
4.鉴定和纯化:通过特定的标志物或性状,对iPSCs进行鉴定和纯化。
这些标志物包括Oct4、Nanog、Sox2等。
纯化后的iPSCs可以用于进一步的应用研究。
iPSCs的特性iPSCs具有以下几个重要的特性:1.多能性:iPSCs具有向三个胚胎发层(内胚层、中胚层和外胚层)分化的潜能,从而有能力分化成各种类型的成熟细胞,如神经细胞、心肌细胞、肝细胞等。
这种多能性使得iPSCs在疾病治疗和组织再生方面具有巨大潜力。
化学干细胞学综述

A
DOI: 10.1021/jm500838d J. Med. Chem. XXXX, XXX, XXX−XXX
Journal of Medicinal Chemistry Particular attention will be paid to testing protocols involving phenotypic screening and model organisms. These enable the discovery of active molecules both from lead structures with defined pharmacology and by high-throughput screening of large compound libraries. The modification of these active compounds by conventional medicinal chemistry techniques to maximize the potency and selectivity of candidate compounds will be discussed and, where appropriate, the leading “proof-ofprinciple” compounds presented. While there have been many examples of drug approvals from phenotypic screening campaigns, there can be challenges in translating candidate compounds to the clinic where the molecular target, or off-target effects, may be unknown. It is clear from the multiple studies described that the detailed investigation of the mechanisms of action of these compounds will produce valuable information on the critical signaling pathways in the cells and present further targets for drug discovery; examples of these will be discussed. More detailed investigations of the pharmacological actions of significant compounds in a wide range of cell and tissue types may be used to give a detailed picture of their overall biological activity, tissue specificity, and safety profile before the undertaking of clinical studies. Thus, through the use of key examples, this perspective will pay particular attention to: (a) documenting what is already known about molecules acting on these cell systems; (b) reviewing the state of the art for initial screening protocols which have the potential to be used to discover active compounds by both traditional and high-throughput assays; (c) describing the more detailed studies necessary on key compounds before human use could be envisaged; (d) describing such compounds which have been used in the clinic. The concept of stimulating stem cells in situ is an emerging approach in regenerative medicine, and a few examples of candidate drugs operating through this mechanism have been reported and reviewed.4,5 Recently Hoffman and Metternich have used the term “molecular organ repair”6 to describe the processes whereby small molecules are able to trigger cellular self-renewal mechanisms or celomatic stem cells. Further, Poss has discussed tissue regeneration and pointed out that many mammalian tissues, including cardiac muscle, spinal cord, and the major appendages, appear to have very little regenerative capacity.7 He also pointed out that stem cells are not always involved even in the well-established regenerative processes. Indeed, the example par excellence of limb regeneration, namely the salamander, appears to use predominantly a process of dedifferentiation rather than stem cell differentiation. Further, in the mammalian heart, multiple potential candidate cell types have been identified. However, there appears to be more promise in targeting resident progenitor cells of developmental origin which contribute multiple cell types to the developing heart for cardiac regeneration as opposed to the rare adult-only resident cardiac “stem cells”.8 While the objective of these studies is obviously to find in vivo active molecules and several pioneering examples have been described in recent years, the majority of the work disclosed to date has come from in vitro studies. Nonetheless, these systems represent useful in vitro models and selected examples will be discussed in this context. The comparatively
他汀类药物治疗心脑血管疾病的研究进展

他汀类药物治疗心脑血管疾病研究进展他汀类药物(statins)为一大类英文词尾为statin(他汀)的药物的统称,是羟甲基戊二酰辅酶A(HMG-CoA)还原酶抑制剂。
大量的临床试验及流行病学研究证实他汀类药物具有降血脂作用及对冠心病的预防作用。
在国际医药市场上畅销的形形色色的降血脂药物中,他汀类居于领先地位,成为20世纪90年代开发最成功的医药产品之一。
他汀类药物分为天然化合物(如洛伐他丁、辛伐他汀、普伐他汀、美伐他汀)和完全人工合成化合物(如氟伐他汀、阿托伐他汀、西立伐他汀、罗伐他汀、pitavastatin)是最为经典和有效的降脂药物,广泛应用于心脑血管疾病的治疗。
他汀类药物除了在治疗高血脂症方面有良好的疗效外,其在心脑血管疾病的预防治疗中也起到了很好的作用[1、2]。
本文就其在治疗治疗心脑血管疾病方面的进展作一综述。
1 他汀类药物抑制血小板聚集近些年,相关研究结果显示[3],内皮衍生的NO除了调节血压、增加局部血流外,还能减轻白细胞的激活和抑制血小板聚集。
而他汀类则间接通过增加内皮NO的产生和生物利用来抑制血小板的聚集,不依赖于血浆胆固醉水平。
Pedrono E等[4]报道,阿托伐他汀治疗14d后急性脑梗死小鼠的PF4和β2TG的水平显著下降,但是停药2d后两者的水平分别上调2.9和3.1倍,同时阿伐他汀治疗使主动脉和脑血管的eNOS上调2.3和1.7倍,但同样仅在停约2d以后,上述二处的eNOS分别下调严重,由此说明急性撤退他汀类药物,2d后即丧失对小鼠脑缺血和血栓形成的保护功能。
他汀类除了抗血小板的作用以外还通过影响疑血系统发挥着抗血栓形成的功能。
2 他汀类药物治疗急性冠脉综合征粥样硬化为急性冠脉综合征(acute coronarysyndrome,ACS)的基础原因,是一种可隐匿进展许多年的慢性疾病[5]。
ACS急性期在抗凝、抗栓、抗心肌缺血治疗的同时积极进行调脂治疗,并通过他汀类药物的“多效性”,改善血管内皮功能、减少脂质过氧化,增加高密度胆固醇(HDL-C)水平,抑制其炎症反应,使粥样硬化斑块稳定,并通过抑制血小板聚集,减少凝血因子,增加纤溶抗血栓形成,可有效降低ACS患者急性期病死率和改善心肌缺血症状,明显改善ACS患者预后[6]。
《人诱导多能干细胞》团体标准

《人诱导多能干细胞》团体标准人诱导多能干细胞是一种重要的细胞工程技术,它可以将成体细胞重新编程为多能干细胞,具有巨大的临床应用前景。
本文将从人诱导多能干细胞的定义、发现历程、应用前景以及团体标准等方面进行详细介绍。
人诱导多能干细胞(induced pluripotent stem cells,iPSCs)是指通过基因转导等手段,将成体细胞重新编程为类似于胚胎干细胞的多能干细胞。
与传统的胚胎干细胞相比,人诱导多能干细胞无需侵入性手术获取,避免了伦理道德问题,具有更广泛的来源和更好的应用前景。
人诱导多能干细胞的发现历程可以追溯到2006年,当时日本科学家山中伦也等人通过转导4种基因,成功将小鼠成纤维细胞转化为多能干细胞,并命名为iPS细胞。
这一突破性发现引起了全球科学界的广泛关注和研究热潮。
随后,研究人员又成功将这一技术应用于人类细胞,并在2007年取得了重要突破。
人诱导多能干细胞具有广泛的临床应用前景。
首先,它可以解决传统胚胎干细胞获取过程中的伦理道德问题,为干细胞研究提供了新的方向。
其次,人诱导多能干细胞可以作为疾病模型进行研究,帮助科学家深入了解疾病发生机制,并开发新的治疗方法。
此外,它还可以用于药物筛选、组织工程和再生医学等领域,为临床医学带来革命性的变革。
为了规范和推动人诱导多能干细胞的研究和应用,国际科学界制定了一系列团体标准。
首先,对于iPSCs的制备过程,要求严格遵循操作规范和实验室安全要求,确保实验结果的准确性和可重复性。
其次,对于iPSCs的鉴定和鉴别,要求使用标准化的检测方法,确保其真实性和稳定性。
此外,在iPSCs的应用过程中,还要遵循伦理原则和法律法规,保护受试者的权益和安全。
此外,为了促进国内外学术界和产业界在人诱导多能干细胞领域的交流与合作,各国科学家还建立了多个国际合作组织和学术会议。
这些组织和会议不仅提供了一个交流平台,还推动了技术的进一步创新和应用。
总之,人诱导多能干细胞作为一种重要的细胞工程技术,在医学和生物科学领域具有巨大的潜力。
TGF-β1诱导的BMSCs外泌体促进软骨修复的研究

TGF-β1诱导的BMSCs外泌体促进软骨修复的研究TGF-β1诱导的BMSCs外泌体促进软骨修复的研究摘要:本研究主要探究了通过TGF-β1诱导的BMSCs外泌体在软骨修复方面的作用。
我们将培养的大鼠骨髓间充质干细胞(BMSCs)分为TGF-β1诱导组和非诱导组,分别获得了TGF-β1诱导的BMSCs和非诱导的BMSCs外泌物,并用于软骨细胞的培养和实验组。
结果表明,TGF-β1诱导后的BMSCs外泌物中含有大量的生长因子,如TGF-β1、TIMP-1等,可促进软骨细胞的增殖。
使用TGF-β1诱导的BMSCs外泌物治疗软骨损伤模型,发现在TGF-β1诱导组中软骨修复效果更好,同时修复后的组织中蛋白多样性较大。
本研究证实,TGF-β1诱导的BMSCs外泌物含有促进软骨细胞增殖的生长因子,具有促进软骨修复的作用。
关键词: TGF-β1诱导、BMSCs外泌体、软骨修复、生长因子Abstract:This study aimed to explore the role of TGF-β1-induced BMSCs exosomes in cartilage repair. Rat bone marrow mesenchymal stem cells (BMSCs) were culturedand divided into TGF-β1 induction group and non-induction group, respectively obtaining TGF-β1-induced BMSCs exosomes and non-induced BMSCs exosomes for cartilage cell culture and experimental group. The results showed that TGF-β1-induced BMSCs exosomes contained a large amount of growth factors, such as TGF-β1 and TIMP-1, which could promote proliferation of cartilage cells. Using TGF-β1-induced BMSCs exosomes to treat cartilage injury model, we foundthat the repair effects were better in TGF-β1 induction group, and the protein diversity in the repaired tissues was greater. This study proved that TGF-β1-induced BMSCs exosomes contained growthfactors that promoted proliferation of cartilage cells and had the ability to promote cartilage repair.Keywords: TGF-β1 i nduction, BMSCs exosomes, cartilage repair, growth factors。
细胞培养小室资料

Membrane material
I stimulation and maintenance of cell function and differentiation,
I cultivation of embryonic stem cells on feeder cells, I applications in reproductive medicine (e.g. autologous
The following protocol presents technical details for co-culture and may be easily adapted to match individual requirements and research interests other than paracrine growth regulation.
Features
stable housing made of highly transparent polystyrene
hanging geometry
sealed PET capillary pore membrane
improved cell adhesion due to physical surface treatment
452,4
0.4
1 x 108
translucent
452,4
0.4
Applications and formulations of optimized, modifi

专利名称:Applications and formulations of optimized,modified human embryonic fertility culturemedia with biguanides and/or functionalequivalents发明人:Jan Zak,Ami Mezezi,Julian Pino,Nildi Pino申请号:US15929863申请日:20200526公开号:US11065188B2公开日:20210720专利内容由知识产权出版社提供专利附图:摘要:A dermatological preparation comprises an optimized, modified humanembryonic fertility culture media with biguanides and/or functional equivalents and the use of said preparation for skin care, hair care, or body care, or dental procedures, or for regenerative medicine such as promoting wound or bone healing. The preparation is formulated to target the hypoxic microenvironment niches of the skin tissue in combination with optimal concentration of nutrients, ions and minerals; to stimulate existing stem cells to trigger the capacity of stem cells to divide and renew and differentiate into specialized cells; and to stimulate molecular and physiological processes, e.g., autophagy, to replenish the substrate pool through the recycling of organelles and the recycling of old damaged proteins and countering free-radical damage to promote anti-aging.申请人:AV Laboratories LLC地址:N Redington Beach FL US国籍:US代理机构:Halloran Sage LLP更多信息请下载全文后查看。
泛素连接酶LRSAM1对人神经胶质母细胞瘤细胞生物学行为的影响及其可能机制

• 456•中华实用诊断与治疗杂志2021年5月第35卷第5期J Chin Pract Diagn Ther,May 2021,Vol. 35,No. 5•论著•泛素连接酶LR SA M1对人神经胶质母细胞瘤细胞生物学行为的影响及其可能机制梁博,王新军,王建业,周少龙,杨卓,刘荣俊郑州大学第五附属医院神经外科,河南郑州450052摘要:目的探讨泛素连接酶LR SA M1在CD166降解中的作用及其对人神经胶质母细胞瘤细胞增殖、侵袭和迁移的影响。
方法人神经胶质母细胞瘤U-87 M G细胞,采用免疫共沉淀法检测LR SA M1与CD166是否存在结合;将对数生 长期 U-87 MG 细胞分为pcDNA3. 1-LRSAM1 组、pcDNA3. 1-NC 组和空白对照组,pcDNA3. 1-LRSAM1 组和pcDNA3. 1-NC组细胞分别转染pcDNA3. 1-LRSAM1质粒载体和PcDNA3. 1空质粒载体,空白对照组细胞正常培养,采 用实时荧光定量P C R法检测3组细胞LRSAM1 m R N A相对表达量,采用Western blot法检测LRSA M1蛋白相对表达 量,采用免疫荧光染色法检测LR SA M1与CD166表达情况,采用细胞克隆形成实验检测细胞集落形成数目,采用 Armexin V-FIT C/P I染色检测细胞凋亡率,采用细胞划痕试验检测细胞划痕愈合率,采用Tnm swell小室实验检测侵袭细胞数。
结果U-87 M G细胞中LR SA M1与CD166存在结合。
pcDNA3. 1-LRSAM1组细胞LRSAM1 m R N A和蛋白 相对表达量(3.26士0. 28、0. 72±0.06)均髙于空白对照组(1.00±0. 03、0. 18±0. 0.1)和 pcDNA3. 1-NC组(0•99士0. 02、0. 19±0. 02)(P<0. 05),转染成功。
ipsc重编程阶段、克隆筛选阶段、纯化扩增阶段。

IPSC重编程阶段1.概述IPSC(induced pluripotent stem cells)是一种由成年体细胞重新编程而来的多潜能干细胞,具有类似胚胎干细胞的多能性。
IPSC的重编程过程包括细胞采集、重编程因子的导入和多能性干细胞的诱导三个主要阶段。
2.细胞采集在IPSC重编程过程中,首先需要从成年体中采集细胞,主要包括皮肤细胞、血细胞等。
一般来说,细胞的采集需要经过严格的无菌操作和个体信息保护,以确保细胞的纯度和安全性。
3.重编程因子导入将采集到的细胞导入实验室中,通过转染或病毒导入等方法,将重编程因子(如Oct4、Sox2、Klf4、c-Myc等)导入细胞内,重新激活细胞中的干细胞相关基因,从而使细胞发生重编程并获得多能性。
4.多能性干细胞诱导经过重编程因子的导入后,细胞将逐渐转变成多能性干细胞,拥有分化成各种细胞类型的潜能。
这一过程需要经过严格的培养和筛选,以确保所得到的IPSC具有多能性和稳定性。
克隆筛选阶段1.概述在IPSC的克隆筛选阶段,研究人员需要对获得的多能性干细胞进行克隆和筛选,以得到稳定的IPSC细胞系。
这一阶段包括细胞克隆、单细胞分离和细胞鉴定等步骤。
2.细胞克隆将获得的多能性干细胞进行克隆,得到同源性较高的细胞裙。
通常采用限稀稀释法或单细胞培养法,以保证每个细胞的同质性和纯度。
3.单细胞分离对克隆后的细胞裙进行单细胞分离,以得到单个细胞。
这一步骤需要经过精细的操作和严格的观察,确保每个细胞的完整性和活力。
4.细胞鉴定对分离得到的单个细胞进行鉴定,包括细胞形态观察、遗传学检测和表观遗传学分析等,以确认细胞的同源性和干细胞的多能性,保证所得到的IPSC细胞系达到研究要求。
纯化扩增阶段1.概述在IPSC的纯化扩增阶段,需要对经过克隆筛选的IPSC细胞系进行纯化和扩增,以得到足够的细胞数量和纯度,用于后续的研究和应用。
2.细胞纯化通过细胞培养、培养基优化和细胞分选等方法,对经过筛选的IPSC细胞系进行纯化,去除杂质细胞和非干细胞成分,提高细胞的同质性和纯度。
诱导多能干细胞分化的心肌细胞在心脏毒性评价中的应用

诱导多能干细胞分化的心肌细胞在心脏毒性评价中的应用*陈高建1,2,黄芝瑛2,王三龙1**,王雪1**(1.中国食品药品检定研究院国家药物安全评价监测中心、药物非临床安全评价研究北京市重点实验室,北京100176;2.中山大学药学院,广州510006)摘要:药物研发失败的重要原因之一是由于其心脏毒性,其中许多原因归因于临床前动物模型未能预见人体中的心脏毒性作用,这是制药工业面临的重大问题。
自从2006年诱导性多能干细胞(iPSCs)技术取得突破性的发现以来,对药物研发领域产生了巨大的影响。
iPSCs在心肌损伤修复、心血管疾病相关模型的建立和治疗药物筛选等方面表现出明显的优势和应用前景。
本文综述了iPSCs技术在心脏毒性评价中的一些应用并讨论了未来的方向和面临的挑战。
关键词:诱导多能干细胞分化;心肌细胞;心脏毒性;重编程;心率失常;临床应用中图分类号:R 917 文献标识码:A 文章编号:0254-1793(2018)05-0758-07doi:10.16155/j.0254-1793.2018.05.03Applications of induced pluripotent stem cells derived cardiomyocytesin the cardiac toxicity evaluation*CHEN Gao-jian1,2,HUANG Zhi-ying2,WANG San-long1**,WANG Xue1**(1. Beijing Key Laboratory,National Center for Safety Evaluation of Drugs,National Institutes for Food and Drug Control,Beijing 100176,China ;2. School of Pharmaceutical Science,Sun Yat-sen University,Guangzhou 510006,China)Abstract:One of the most important reasons for the failure of drug development is its cardiac toxicity,many of which attributed to preclinical animal models incapable of foreseeing cardiotoxic side effects of the drugs being′tested in humans.The emergence of induced pluripotent stem cells(iPSCs) technology has had a great impact on the field of medicine ever since the ground-breaking discovery in 2006.iPSCs has certainly advantages and application prospects in myocardial injury repair,establishing cardiovascular disease-related model and drug screening.The application of iPSCs technology in cardiac toxicity evaluation has been reviewed in this paper,and the future directions and ongoing challenges ahead in this exciting field has also been discussed. Keywords:human induced pluripotent stem cell derived;cardiomyocyte;cardiomyocytes;cardiac toxicity evaluation reprogramming;arrhythmia;clinical application * 中检院2017年度“学科带头人培养基金”(课题编号:2017X6) ** 通信作者 王三龙 Tel:(010) 67872233-8002;E-mail: wangsanlong@ 王 雪 Tel:(010)67872233-8203; E-mail: xue_wang@ 第一作者 Tel: 132********;E-mail: chengj67@心脏毒性是药物临床使用过程中退市的主要原因,所占比例高达33%,其中大约一半是心律失常[1],包括QT间期延长和致命的尖端扭转型室性心动过速(Torsade de Pointes,TdP),为此,FDA和制药行业在新药上市之前都制定了强制性的药物心脏毒性筛选指南。
【高中生物】Stem Cell Rep:自身干细胞可以治疗痴呆症

【高中生物】Stem Cell Rep:自身干细胞可以治疗痴呆症【高中生物】stemcellrep:自身干细胞可以治疗痴呆症近日,来自鲁汶大学的研究人员通过研究开发出了治疗遗传性痴呆症的新型疗法,文章中研究人员将病人机体的干细胞转化成为受痴呆影响的神经元细胞,在携带易致痴呆突变的病人机体的干细胞中,研究者发现了一种可以抑制正常神经发育的靶向性缺失,而当这种缺失被修正后,这些干细胞就会回归正常状态,相关研究刊登于国际杂志stemcellreports上。
研究者catherineverfaillie则表示,利用诱导多能性干细胞技术,我们就可以生产出来演示痴呆症的模型以供研究,额颞障碍就是由大脑中额叶和颞叶的神经元损失而引起的一种疾病,其通常可以引致患者行为障碍或语言障碍及情感障碍等;而一种名叫颗粒蛋白前体(grn)的基因的变异往往和额颞痴呆(frontotemporaldementia)的发作轻易有关,但小鼠机体中grn变异并不能整体表现出来和人类疾病全然相近的症状,从而就影响了化疗人类疾病疗法的研发。
研究者表示,如今我们就可以利用诱导多能性干细胞来理解痴呆症的发病机制,尤其是揭示额颞痴呆的发病机理,这项研究中研究人员从三名病人机体中构建了携带grn突变的诱导多能性干细胞,这些不成熟的细胞在转化为成熟的过程中发生了损伤,而在额颞痴呆中往往是一种名为皮层神经元的细胞会受到损伤。
在研究人员构筑的诱导多能性干细胞中一种缺位的通路就为wnt信号通路,该信号路径在神经发育过程中扮演着关键角色,然而遗传修正或药物疗法就可以协助恢复正常诱导多能性干细胞向皮层神经元的能力,本文研究表明,grn变异可以通过发生改变wnt信号通路的方式去引起皮层神经元构成的缺位。
最后研究者指出,神经发育过程中一系列的信号事件或许在神经发育中扮演重要的角色,而靶向这些信号通路,比如wnt信号路径或许就可以帮助开发治疗额颞痴呆的新型靶向,下一步研究人员计划进行深入研究来理解grn突变细胞为何会引发疾病,同时也可以帮助鉴别出新型的药物靶点。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2 x 0.2 ml
pmaxGFP® Vector (0.5 µg/µl in 10 mM Tris pH 8.0)
30 µg
Certified cuvettes
20
Plastic pipettes
20
Storage and stability
Store Nucleofector® Solution, Supplement and pmaxGFP® Vector at 4°C. For long-term storage,
Collagenase, Dispase or another enzymes for this purpose
2
Optimized Protocol for Human Stem Cells
Detachment of stem cells
A. Harvest of stem cells cultured on feeder cells There are three possibilities to remove feeder cells from your stem cell culture prior to Nucleofection®: ––If your stem cells are usually cultured on feeder cells, passage them once to Matrigel® coated plates to remove the feeder cells (described in reference 2). Then proceed to step B –– Cultivate the cells on feeder cells until the day of the experiment. Detach the stem cells with Collagenase. Dissociate the clumps with Accutase into a single cell suspension –– Cultivate the cells on feeder cells until the day of the experiment. Detach all cells with Accutase. Incubate the cells on an uncoated cell culture flask for 1 hour in a humidified 37°C/5% CO2 incubator. The feeder cells will attach and the stem cells will stay in suspension. Harvest the cells in suspension
Cell culture recommendations
1.1 Replace media every day 1.2 Cells should be passaged 1 – 2 times per week with a sub cultivation ratio of 1 : 3 to 1 : 10. You may use
––The use of apoptosis inhibitors like ROCK inhibitor (reference 5) and neurotrophins (reference 6) have been reported to increase viability of hES cells. Depending on hESC culture conditions it might be advantageous to use ROCK inhibitor or neurotrophins to obtain higher viabilities
Product Description
Cat. No.
VPH-5002
Size (reactions)
20
Human Stem Cell Nucleofector® Solution 1
0.9 ml
Human Stem Cell Nucleofector® Solution 2
0.9 ml
Supplement 1
pmaxGFP® Vector is ideally stored at -20°C. The expiration date is printed on the solution box. Once the
Nucleofector® Supplement is added to the Nucleofector® Solution it is stable for three months at 4°C.
EDTA solution ––Medium for culture with feeder cells: DMEM F-12 [Lonza, Cat.No. 12-719F] supplemented with 15 –
20% Knockout™ serum replacement [Invitrogen, Cat. No. 10828-028], 1 – 2% nonessential amino acids [Lonza, Cat. No. 13-114E], 1 – 4 mM L-glutamine [Lonza, Cat. No. 17-605C], 0.1 mM 2-Mercaptoethanol and 4 – 8 ng / ml fibroblast growth factor-2 [Milipore, Cat. No. GF003AF-MG] ––Plates for culture with feeder cells: Prepare a 24-well plate coated with gelatine and inactivated feeder cells (one well per sample) 24 hours before Nucleofection® ––Medium for feeder-free culture: mTesSR™ 1 medium [StemCell Technologies, Cat. No. 05850] ––Plates for feeder-free culture:Preparea24-wellcultureplatecoatedwithBDMatrigel™[BDBiosciences, Cat. No. 354277] ––Prewarm appropriate volume of culture medium to 37°C (1 ml per sample) ––Appropriate number of cells (8 x 105 cells per sample; lower or higher cell numbers may influence transfection results)
Optimized Protocol for Human Stem Cells
Required Material
Note Please make sure that the entire supplement is added to the Nucleofector® Solutions.
––Nucleofector® Device ––Supplemented Nucleofector® Solutions at room temperature ––Supplied certified cuvettes –––Supplied pmaxGFP® Vector ––Substrate of interest, highly purified, preferably by using endotoxin free Kits; A260 : A280 ratio should
Amaxa® Human Stem Cell Nucleofector® Starter Kit
For Human Stem Cells
e.g. BG01V, H1, H7, H9.2, HSF6, RH1 and RH6.
Note This Starter Kit is based upon feedback collected from researchers. You can determine the optimal combination of program and Nucleofector® Solution (Nucleofection® Condition) for your stem cell using this Human Stem Cell Nucleofector® Starter Kit [Cat. No. VPH-5002]. If the Human Stem Cell Nucleofector® Solution 1 yields the best results, simply use the Human Stem Cell Nucleofector® Kit 1 [Cat. No. VPH-5012] or the Human Stem Cell Nucleofector® Kit 2 [Cat. No. VPH-5022] if Human Stem Cell Nucleofector® Solution 2 yields the best results.
be at least 1.8 ––Culture dishes of your choice ––For detaching cells: Accutase solution [PAA Laboratories, Cat. No. L11-007] or 0.05 or 0.25% Trypsin/
Optimization Guidelines
The initial optimization experiment is comprised of 12 reactions: 5 different Nucleofector® Programs are tested with 2 different Nucleofector® Solutions (HSC 1 and HSC 2) plus 1 control (no program). The Nucleofection® condition which turns out to be the most appropriate should be used for all subsequent transfections. A further fine tuning of the Nucleofection® Condition can be performed with the help of our Scientific Support Team.
