Journal of Alloys and Compounds 492 (2010) 496–499
热膨胀系数低的铝合金

热膨胀系数低的铝合金热膨胀系数是描述材料在温度变化下长度变化的性质,它是一个衡量材料在热胀冷缩过程中相对于温度变化的敏感程度的物理量。
在实际应用中,我们通常希望材料的热膨胀系数尽可能小,因为这样可以减少材料在温度变化下的尺寸变化,提高材料的工作稳定性和精确度。
铝合金是一种广泛应用的轻质合金材料,具有良好的机械性能、导热性能和加工性能等特点。
下面将介绍一些热膨胀系数低的铝合金及其相关参考内容:1. 铝-硅合金:铝-硅合金是一种常用的铝合金,在电子、航天等领域有广泛应用。
相比于纯铝,铝-硅合金的热膨胀系数较小。
相关参考内容可以参考以下文献:- Kruger M., et al. "Thermal expansion behavior of in-situ reinforced Al–Si–C composites." Journal of Materials Science, 2011, 46(14): 4980-4986.- Yoo W.S. "The linear coefficient of thermal expansion of some alloys." Journal of Physical and Chemical Reference Data, 1976,5(2): 895-902.2. 铝-镁合金:铝-镁合金也是一种常见的铝合金,具有轻质、高强度等特点,广泛用于汽车和航空航天领域。
该合金的热膨胀系数相对较小,相关参考内容如下:- Schopf U., et al. "Rapid expansion ofaluminum−magnesium−sulfate hydrates: A simplified model for concrete cracking." Journal of the American Chemical Society, 2007, 129(32): 10078-10079.- Choe H.C., et al. "Effects of magnesium on thermal expansion behavior of aluminum alloys." Journal of Materials Science, 1985, 20(3): 1015-1020.3. 铝-锆合金:铝-锆合金是一种耐高温、耐腐蚀的合金材料,广泛应用于航空航天、化工等领域。
北大考研-工学院研究生导师简介-夏定国

dationinDMFC:PtBi/XC-72withPtSolid-SolutionStructure,J.Electrochem.Soc.,2010,Volume157,Is
sue4,PagesB580-584.
6.JiongLi,HaimingLi,Xianqing,Liang,ShuoZhang,TingZhao,DingguoXia*,ZiyuWu,FirstPrinciples
InvestigationofElectronicConductivityandOccupancySitesofMoDopedintoLiFePO4byabInitioCalc
ulationandX-rayAbsorptionSpectroscopy , JournalofPhysicalChemistryC , 2008 ,
爱考机构 中国高端考研第一品牌(保过 保录 限额)
aniawithDifferentPolymorphsatRoom-temperature,Adv.Mater.,2010,22,1258-1262.
3.TaoYang,FanLi,DingguoXia*,AuBICUVOX10CompositeCathodeforNovelStructБайду номын сангаасreLow-Tempe
StudyontheDiffusionofAlkali-MetalIonsontheArmchairSingle-WallNanotubes,JournalofPhysicalC
hemistryA,2009,Volume113,Issue5,Pages791-796.
7.ShaoruiSun,DingguoXia*,ThetheoreticalstudyofthecationicconductivityofAgBr,SolidStateIonics
冶金工业类核心期刊表
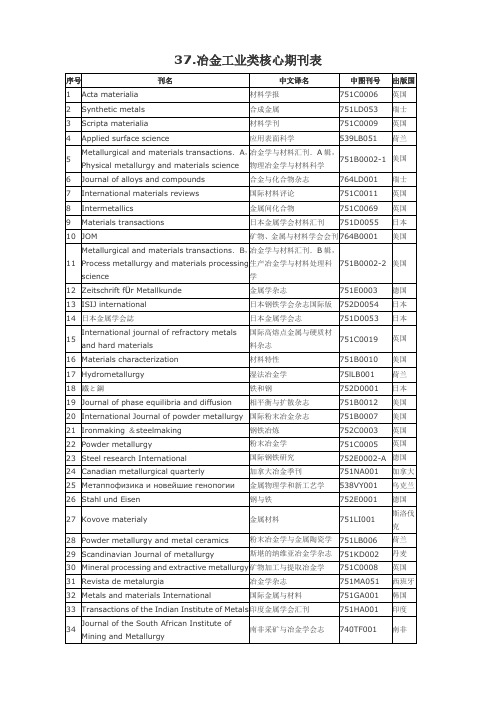
37.冶金工业类核心期刊表38.冶金工业类扩展区期刊表(按刊名顺序排列)(32种)steel research internationalISIJ international冶金会刊也就是Mettallurgical and Materials Transactions A也不错Journal of Iron and Steel Research(International) 是钢铁研究学报的英文版吧?好像算不上行业内顶级期刊。
Mettallurgical and Materials Transactions A在冶金行业内知名度比较高,但IF都很低,不知还有没有更高些的?Ironmaking & SteelmakingJOM冶金只能算门技术,还谈不上科学,从业人员有限,IF无法与材料、生物相比,IF》0.5的就基本上可以算顶尖的了对于过程冶金而言,美国的Metallurgical and Materials Transaction B 是国际公认的最高水平的刊物,侧重理论研究。
影响因子不高,仅0.6多一点。
不仅刊登钢铁冶金、还有有色冶金。
日本的ISIJ 影响因子0.7多些,是过程冶金领域最高的,主要涉及钢铁冶金,与transaction B 相比,侧重工业过程的内容多一些。
如果算是顶级刊物的话,冶金领域只有这两个算得上了。
其他该领域的SCI期刊,德国的steel research还可以,IF〉0.4,英国的ironmaking and steelmaking, IF>0.4,但是最近几年,都有下降得趋势,尤其是ironmaking steelmaking,文章质量和水平越来越低。
加拿大的Canadian Metallugical Quartly 以前水平很高,现在稿源严重下降,影响因子只有0.2左右,但是文章水平还不算很差(可见北美对学术要求的严谨)。
中国的钢铁研究学报英文版,客观地讲水平很低,影响因子也是0.2左右,但文章比CMQ差得多,很多在中文的冶金Ei级的期刊(东大学报、北科大学报、中南学报)都难以发表的,可以翻译成英文在该杂志上发表,并且该杂志基本上被国内几家冶金研究单位和高校控制,其它学校发表还不太容易。
材料类容易中的SCI期刊

速度快并且容易中的材料类SCI期刊(更新中)推荐:1. Journal of alloy and compounds 影响因子IF 1点多,1个月给消息,容易中,现在几乎成为中国人的专刊了,哈哈;2. applied surface science 影响因子IF 1点多,发表容易,3. Materials Letter 1.7 速度快,快报一般都要求有新意(当然,新意太高可以投APL了)4. Materials & Design 影响因子不到1,很快,快点一个月就接受的!适合特别想要文章毕业或者评奖学金的。
5. Physica B 影响因子不到1,很快,我一个同学已经在上面发了2篇了,最快不到一个月就接受了,还是容易中的,最好是工作全面细致些。
6. Materials science and engineering B 影响因子1点多,从投稿到接受一般3-4个月,相对容易中。
7. Optoelectronics and Advanced Materials-Rapid Communications, 罗马尼亚期刊,影响因子0.2,很快,一个月可以搞定,适合灌水和急需文章。
8. Optical materials 发光材料期刊,影响因子1点多,相对容易中,速度也快。
9. Journal of Luminescence 发光方面专业期刊,老牌杂志,虽然影响因子只有1点多,但很多发光方面的经典文章出自此期刊,相对容易中,速度也可以。
10. Journal of Physics D: Applied physics 偏物理材料方面,影响因子2 左右,速度快,也不难中,中国人投稿还比较多。
黑名单:1. Thin solid films 影响因子1点多,但审稿巨慢,不推荐;2. Materials Characterization 影响因子不高,容易中,但速度慢,如果不急着要文章,也可以投的;3. Materials Chemistry and Physics 影响因子1点多,速度巨慢,我一个同学投稿半年还没消息,现在1年过去了还没查到这篇文章,估计没戏了吧。
Ni-Co-W合金电沉积行为及成核机理

Ni-Co-W合金电沉积行为及成核机理温林洁,张丽楠,周宗熠,李运刚,杨海丽*(华北理工大学冶金与能源学院,现代冶金技术教育部重点实验室,河北唐山063210)摘要:采用循环伏安、阴极极化曲线、电化学阻抗谱、计时电流等方法对Ni-Co-W合金的电沉积行为及成核机理进行研究。
结果表明,Ni-Co-W合金的电沉积是一个存在成核行为的不可逆过程。
Ni-Co-W合金的成核机制为瞬时成核,合金的电沉积由动力学和扩散过程混合控制,主要受动力学控制。
关键词:Ni-Co-W合金;电沉积行为;成核机理;速率控制步骤中图分类号:TQ153.2文献标识码:AElectrodeposition Behavior and Nucleation Mechanism of Ni-Co-W AlloyWEN Linjie,ZHANG Linan,ZHOU Zongyi,LI Yungang,YANG Haili*(College of Metallurgy and Energy,Key Laboratory of the Ministry of Education for Modern Metallur‐gy Technology,North China University of Science and Technology,Tangshan063210,China)Abstract:The electrodeposition behavior and nucleation mechanism of Ni-Co-W alloy were studied by cyclic voltammetry,cathodic polarization curve,electrochemical impedance spectroscopy and chrono‐amperometry.The results show that electrodeposition of Ni-Co-W alloy is an irreversible process with nucleation behavior.The nucleation mechanism of Ni-Co-W alloy is instantaneous nucleation.The elec‐trodeposition of alloys is controlled by both kinetics and diffusion processes,and is mainly controlled by kinetics.Keywords:Ni-Co-W alloy;electrodeposition behavior;nucleation mechanism;rate-determining stepNi-Co合金具有良好的延展性、软磁性,被广泛应用于各种磁性器件领域[1]。
全球SCI收录材料期刊影响因子排名

全球SCI收录材料期刊影响因子排名Nature自然31。
434Science科学28。
103Nature Material自然(材料)23。
132Nature Nanotechnology自然(纳米技术)20。
571Progress in Materials Science材料科学进展18。
132Nature Physics自然(物理)16.821Progress in Polymer Science聚合物科学进展16.819Surface Science Reports表面科学报告12。
808Materials Science & Engineering R—reports材料科学与工程报告12。
619 Angewandte Chemie—International Edition应用化学国际版10.879Nano Letters纳米快报10。
371Advanced Materials先进材料8。
191Journal of the American Chemical Society美国化学会志8.091Annual Review of Materials Research材料研究年度评论7。
947Physical Review Letters物理评论快报7。
180Advanced Functional Materials先进功能材料6.808Advances in Polymer Science聚合物科学发展6。
802Biomaterials生物材料6.646Small微观?6.525Progress in Surface Science表面科学进展5。
429Chemical Communications化学通信5。
34MRS Bulletin材料研究学会(美国)公告5.290Chemistry of Materials材料化学5。
046Advances in Catalysis先进催化4.812Journal of Materials Chemistry材料化学杂志4。
内氧化法制备MgO弥散强化铁基材料

内氧化法制备MgO弥散强化铁基材料徐延龙;罗骥;郭志猛;杨薇薇;于海华【摘要】The MgO dispersion strengthening iron powder was prepared by mechanical alloying, surface oxidation at low temperature, internal oxidation at high temperature and reduction treatment. Then the MgO dispersion strengthening ferrous material was fabricated by spark plasma sintering (SPS). The analysis of SEM and EDS on the microstructure and fracture shows that the MgO particle size is 200 nm~1μm and the MgO is uniformly distributed in the matrix which can refine the grain. The dimple fractures become smaller after the addition of MgO. The mechanical properties at room temperature of the Fe+1.0%MgO are that, the tensile strength is 342.6 MPa, the yield strength is 276.3 MPa, the hardness is 61 HRB, which compared with pure iron are increased by 20.5%, 54.2% and 84.8% respectively.%采用机械合金化—低温表面氧化—高温内氧化—还原处理制备MgO弥散强化铁粉后再经放电等离子(SPS)烧结制备MgO弥散强化铁基材料,并通过SEM和EDS对材料的组织和断口进行分析。
材料类的国外期刊以及投稿经验

英文材料期刊简介Journal of Alloys and Compounds《合金与化合物杂志》瑞士ISSN:0925-8388,1959年创刊,全年36期,Elsevier Science出版社出版,SCI收录期刊,SCI2003年影响因子1.080。
国际性材料科学和固体化学与固体物理学杂志。
刊载稀有金属及其化合物、合金的实验和理论研究论文、会议报告、简讯与书评。
文章多用英文发表。
Journal of Composites for Construction《建筑复合材料杂志》美国ISSN:1090-0268,1997年创刊,每年4期,American Society of Civil Engineers,USA出版。
刊载有关建筑用合成纤维增强复合材料的研究论文。
SCI、EI收录期刊,SCI2003年影响因子1.234,被引频次249、即年指标0.125、年载文量40。
2003年EI收录91篇。
EI收录期刊,EI2001年收录25篇。
Journal of Materials in Civil Engineering《土木工程材料杂志》美国ISSN:0899-1561,1989年创刊,每年6期,American Society of Civil Engineers,USA出版。
刊载土建材料的开发、加工与现场生产、特性评价、应用和性能等方面的研究论文。
SCI、EI收录期刊,2002年SCI影响因子0.346、被引频次193、即年指标0.015、年载文量66、被引半衰期4.9。
EI2002年收录66篇。
Journal of the European Ceramic Society《欧洲陶瓷学会志》英国ISSN:0955-2219,1985年创刊,全年16期,Elsevier Science出版社出版,SCI、EI收录期刊,SCI2003年影响因子1.248,2003年EI收录396篇。
主要发表研究陶瓷材料结构、特性和加工的原始论文。
锂离子电池硅碳复合负极材料的研究

锂离子电池硅碳复合负极材料的研究王英;孙文;唐仁衡;肖方明;黄玲【摘要】以商品化纳米硅粉和沥青为原料,采用喷雾干燥热解法制得Si@C复合物.将Si@C复合物和人造石墨混合,制得Si@C/G硅碳复合材料作为锂离子电池的负极材料.借助X射线衍射(XRD)、扫描电镜(SEM)、透射电镜(TEM)和电化学测试等方法,对Si@C复合物和Si@C/G复合材料的结构、形貌和电化学性能进行表征.结果表明,当硅碳复合材料中Si@C复合物和石墨的质量比为15∶85时,在100 mA/g的恒电流下,首次放电比容量为695.4 mAh/g,首次库仑效率为86.1%,循环80周后容量仍有596.6mAh/g.【期刊名称】《材料研究与应用》【年(卷),期】2018(012)003【总页数】6页(P161-166)【关键词】锂离子电池;硅碳复合负极材料;纳米硅;人造石墨;碳包覆【作者】王英;孙文;唐仁衡;肖方明;黄玲【作者单位】广东省稀有金属研究所,广东省稀土开发及应用重点实验室,广东广州510650;广东省稀有金属研究所,广东省稀土开发及应用重点实验室,广东广州510650;华南理工大学材料科学与工程学院,广东广州510641;广东省稀有金属研究所,广东省稀土开发及应用重点实验室,广东广州510650;广东省稀有金属研究所,广东省稀土开发及应用重点实验室,广东广州510650;广东省稀有金属研究所,广东省稀土开发及应用重点实验室,广东广州510650【正文语种】中文【中图分类】TM912 9;TM531为了不断提升新能源汽车的续航里程,近年来对锂离子电池的能量密度要求越来越高.到2020年,我国对锂离子电池电芯能量密度的期望值将达到350 Wh/kg.由于现有的商用负极材料石墨难以满足上述要求,因此,开发新型高容量负极材料成为研究热点.硅的理论嵌锂容量高达4200 mAh/g,且具有脱锂电位低、资源丰富、成本低和环境友好等优势,成为综合性能最具发展潜力的新型负极材料[1-5].硅材料虽然储锂容量较大,但锂离子在嵌入硅过程中会引起体积膨胀(300%),易造成材料结构的崩塌和活性物质的脱落,使循环稳定性大大下降.同时,这种体积效应也使电极表面难以形成稳定的固体电解质界面膜(SEI膜),导致不断有硅裸露到电解液中.针对硅负极材料循环稳定性的问题,近年来,研究人员将硅进行纳米化处理,即硅单质材料体系的改性.通过制备各种纳米硅材料来缓解硅嵌锂产生的体积膨胀.研究表明[6-7],当硅颗粒尺寸小于单个硅纳米颗粒嵌锂过程中的破碎临界值,纳米硅颗粒在参与电化学反应过程所产生的应力能不足以使得电极表面生成裂纹,从而避免颗粒的破碎粉化.但是,纳米硅的高活性表面则会使电极发生较多的副反应,造成较高的不可逆容量损失.因此,除了硅纳米化改性技术外,还应通过硅与碳材料的二元或多元复合来制备复合材料,即建立硅复合材料体系[8-12].基本原理是利用第二相的机械性能和导电性来抑制硅的体积效应和增强硅的导电性,减少电极副反应的发生,并防止嵌脱锂过程中纳米颗粒的团聚.李纯莉[13]先采用酸浸蚀方法从铝硅合金得到纳米硅,然后将纳米硅与石墨烯进行复合制得石墨烯/多孔硅复合负极材料.复合结构中的石墨烯片或均匀分散在多孔纳米硅颗粒间,或包裹着小尺寸的纳米硅颗粒,有效改善了纳米硅的导电性和减缓多孔硅结构的衰变.用复合材料制成的电极在循环120周后,其放电比容量仍可达1843 mAh/g.Julien[14]利用激光化学沉积热解法(LCVP)制备出包覆1 nm厚度碳层的纳米非晶硅复合材料,经充放电循环后,极片厚度从循环前的12.6 μm到嵌脱锂300周后的14.9 μm,体积膨胀率仅18%,表现出良好的循环性能,所设计的核壳结构保持了材料结构和电极的稳定性.Zhuang[15]以纳米氧化镁为造孔剂,将纳米硅嵌入多孔碳中,制备的复合材料在循环40周后仍有1172 mAh/g的可逆容量,主要归功于多孔碳支架为纳米硅提供充足的空间以缓冲硅的体积变化.综上所述,采用硅纳米化和复合化相结合的方法制备电化学性能优异的硅碳复合材料是切实可行的.本文以纳米硅粉和沥青为原料,通过喷雾干燥热解法在纳米硅颗粒表面包覆一层无定形碳层制得Si@C复合物,将Si@C复合物和人造石墨颗粒混合可制得用于锂离子动力电池的Si@C/G复合负极材料.1 试验部分1.1 硅碳材料的制备以平均粒径80 nm硅粉、沥青为原料,按硅粉和沥青质量比为1∶1混合均匀,然后依次加入无水乙醇和去离子水搅拌,搅拌均匀后得到浆料,再经喷雾干燥制得Si@C前驱物(喷雾干燥设备进口温度180 ℃,出口温度110 ℃).将前驱物放入充有高纯氩气保护的管式炉内在1050 ℃保温3 h,然后冷却至室温,再研磨筛分,获得Si@C复合物.将Si@C复合物和人造石墨分别按质量比10∶90,15∶85,20∶80混合,制得硅碳复合负极材料Si@C/G,分别标记为样品a、样品b和样品c.1.2 硅碳材料的性能表征将活性物质(Si@C或Si@C/G)、导电乙炔黑和粘结剂(羧甲基纤维素钠CMC和丁苯橡胶SBR混合物,质量比3∶5)按质量比8∶1∶1混合,以去离子水为溶剂混合成浆料,然后将浆料均匀涂敷于铜箔基体上,充分干燥后制成正极.以金属锂片为负极,Celgard 2500型聚丙烯多孔膜为隔膜,1 mol/L的LiPF6溶于碳酸乙烯酯(EC)、碳酸甲基乙基酯(EMC)和碳酸二甲酯(DMC)(体积比1∶1∶1)为电解液,在真空手套箱中组装成2032型扣式电池.采用蓝电CT2001A二次电池性能检测装置对电池进行充放电性能测试,测试电流密度为100 mA/g,电压范围为0.01~1.5 V.采用荷兰Philips X'pert MPD diffractometer XRD衍射仪(20 kV,40 mA,Cu Kα)分析样品结构,扫描角度为10°~90°,步长为0.02°/s;用德国蔡司公司Zeiss supra 40扫描电镜(SEM)和日本精工JOEL JSM-2100F透射电镜(TEM)观察复合材料的微观形貌.2 试验结果与讨论2.1 Si@C复合物的性能图1为纳米硅和Si@C复合物的XRD谱图.由图1可知,Si和Si@C均在位于2θ为28.43°,47.29°,56.13°,69.13°,76.45°,88.07°左右处出现Si峰,分别对应硅的晶面(111),(220),(311),(400),(331),(422).包覆碳前后硅特征峰的位置基本一致.图谱中2θ为25°左右处有一个宽化的弥散峰,没有观察到其他明显的特征峰,表明沥青热解生成的碳为无定形态.图1 材料的XRD图Fig.1 XRD patterns of the materials图2为 Si@C复合物的SEM和TEM及Si材料SEM图.由图2(a~e)给出的Si@C 复合物的SEM和TEM图可以清晰地看出,纳米硅颗粒表面包覆着一层稳定致密的碳层,硅颗粒通过包覆碳层连接成的导电性骨架形成良好的电接触.多个这样的一次小颗粒组成较大的二次颗粒,如图2(b)、2(c)和2(e)所示.Si@C二次颗粒尺寸大小均匀,分散性较好.图2(f)为纳米硅的SEM图,与图2(c)相比,发现通过喷雾干燥热解可以有效地在纳米硅表面包覆碳膜.图2 Si@C复合物的SEM和TEM图及Si材料SEM图(a),(b),(c)Si@C复合物的SEM;(d),(e) Si@C复合物的TEM;(f) Si材料的SEMFig.2SEM(a,b,c) ,TEM(d,e) images of Si@C composites and image of SEM(f) of Si 图3 Si和Si@C复合物的电化学性能 (a) 首次充放电曲线;(b)循环性能曲线Fig.3 The electrochemical performance of Si@C composites and Si (a) the first charge/discharge curves;(b) the cycling performance curves将Si和Si@C复合物分别组装模拟电池进行充放电循环测试,其电化学性能如图3所示.图3(a)为电池的首次充放电曲线.由图3(a)可知,两种硅材料在首次放电曲线0.9 V左右处均出现倾斜下降的一个小平台,对应电解液浸润活性物质时,在活性物质颗粒表面形成SEI膜的过程.包覆Si@C复合物的平台电压略低于未包覆Si 材料,说明包碳可以促进电极表面SEI膜的生成.首次放电曲线上较长的电压平台是典型的晶体硅嵌锂电压平台.与Si材料的嵌锂平台电压相比,Si@C复合物的嵌锂平台低,主要原因是碳包覆层增强了Si@C复合物的表面电性,降低了电极表面极化.图3(b)为电池的循环曲线.由图3(b)可知,Si@C的首次循环放电比容量为1706.4 mAh/g,首次库仑效率为86.5%.循环80周后,容量仍有731.2 mAh/g,容量保持率达到42.9%;纳米硅的首次放电比容量为2915.8 mAh/g,首次库伦效率为79.4%.经80周循环后,放电比容量仅有66.6 mAh/g.与纯硅材料相比,Si@C复合物的库仑效率和循环性能明显提高.将硅颗粒均匀分散于碳基体获得具有包覆型的Si@C复合物,热解碳在硅颗粒表面形成的一层无定形碳膜具有缓冲硅体积效应和增强复合材料电子导电率的作用,可避免内部硅颗粒与电解液直接接触,形成完整的SEI膜,在一定程度上改善了复合材料电极的充放电性能.2.2 Si@C/G复合材料的性能将Si@C复合物直接应用于锂离子动力电池,循环稳定性仍然难以达到使用要求.基于石墨的高导电性,在牺牲一定放电容量的前提下,将Si@C复合物和石墨混合后制得Si@C/G复合材料,可进一步提升负极材料的充放电性能.图4(a)为Si@C/G复合材料样品a,b,c的首次充放电曲线.由图4(a)可知,首次放电曲线在0~0.2 V之间的一个明显的放电平台与锂离子嵌入活性物质硅和石墨的过程相对应,由于两种物质的嵌锂电位较相近,曲线上仅显示出一个平台.首次充电曲线上位于0.15 V,0.45V左右的两个电压平台则分别对应着锂离子从石墨、硅中脱出的过程.随着样品a,b,c中Si@C复合物含量的增加,充电平台延长,复合材料的比容量增大.图4 Si@C/G复合材料的电化学性能(a)首次充放电曲线;(b)循环性能曲线Fig.4 The electrochemical performance of Si@C/G composites (a) the first charge/discharge curves;(b) the cycling performance curves图4(b)为Si@C/G复合材料a,b,c三种样品的循环性能曲线.由图4(b)可知,三种复合材料首次放电比容量分别为559.5 mAh/g,695.4 mAh/g和779 mAh/g,首次库仑效率分别为86.8%,86.1%,86.2%.循环80周后,放电比容量分别为497 mAh/g,596.6 mAh/g和627.1 mAh/g,容量保持率分别为88.8%,85.8%和80.5%,平均每周容量衰减率分别仅为0.14%,0.18%和0.24%.三种复合材料表现出良好的循环稳定性,主要是由于纳米硅颗粒的表面包覆碳层和石墨有效缓解了硅材料在锂化过程中的体积膨胀.特别是石墨基体在硅颗粒膨胀时能够承受较大的弹性形变,使嵌锂过程中的残余应力较小.同时,石墨的良好导电性和容量特性也显著改善了Si@C复合物的综合电化学性能.从平衡放电容量、首次库仑效率和循环稳定性的角度来看,Si@C复合物和石墨的质量比为15∶85(样品b)的硅碳复合材料的电化学性能稍优.该复合材料的XRD图如图5所示.图5 复合材料样品b的XRD图Fig.5 XRD patterns of sample b从图5可以看出,在2θ为26.56°,44.39°和54.54°处出现石墨特征峰.复合材料的Si@C复合物颗粒均匀地附着在石墨表面,分散性较好,见图6.图6 复合材料样品b不同放大倍数的SEM图Fig.6 SEM images of sample b3 结论通过喷雾干燥热解的方法制备核壳型Si@C复合物,将Si@C复合物和石墨混合制得Si@C/G复合材料,可作为锂离子动力电池的负极材料.当Si@C/G复合材料中Si@C复合物和石墨的质量比为15∶85时,在100 mA/g的恒电流下,首次放电比容量为695.4 mAh/g,首次库仑效率为86.1%.循环80周后容量仍有596.6 mAh/g,容量保持率达到85.8%.【相关文献】[1] 王静,陈志柠,郭玉忠,等.有序介孔硅/碳复合结构负极材料的制备与电化学性能研究[J].无机材料学报,2018,33(3):313-319.[2] 罗金华,倪伟.三维纳米硅/多孔碳的储锂性能[J].电池,2017,47(6):328-331.[3] 白雪君,刘婵,侯敏,等.锂离子电池硅/碳纳米管/石墨烯自支撑负极材料研究[J].无机材料学报,2017,32(7):705-712.[4] PAIREAU C,JOUANNEAU S,AMMAR M R,et al. Si/C composites prepared by spary drying from cross-linked polyvinyl alcohol as Li-ion batteries anodes[J]. Electrochimica Acta,2015,174:361-368[5] LAI Jun,GUO Hua-jun,LI Xiang-qun,et al.Silicon/flake graphite/carbon anode materials prepared with different dispersants by spray-drying method for lithium ion batteries[J].Trans Nonferrous Met Soc China,2013,23:1413-1420.[6] LIU Xiaohua,LI Zhong,SHAN Huang,et al.Size-dependent fracture of silicon nanoparticle during lithiation [J].ACS Nano,2012,6(2):1522-1531.[7] LI Hong,HUANG Xuejie,CHEN Liquan,et al.A high capacity nano-Si composite anode material for lithium rechargeable batteries[J].Electrochemical and solid-state letters,1999,2(11):547-549.[8] ZHOU Yu,GUO Huajun,WANG Zhixing,et al.Improved electrochemical performance of Si/C material based on the interface stability[J].Journal of Alloys and Compounds,2017,725:1304-1312.[9] CHEN Hedong,WANG Zhoulu,HOU Xianhua,et al.Mass-producible method for preparation of a carbon-coated graphite@plasma nano-silicon@carbon composite with enhanced performance as lithium ion battery anode[J].Electrochimica Acta,2017,249:113-121.[10] LI Xiaotian,YANG Dandan,HOU Xiaocun,et al.Scalable preparation of mesoporous silicon@C/graphite hybrid as stable anodes for lithium-ion batteries[J].Journal of Alloys and Compounds,2017,728:1-9.[11] YUN Qinbai,QIN Xianying,HE Yanbing,et al.Micron-sized spherical Si/C hybrids assembled via Water/Oil system for high-performance lithium ionbattery[J].Electrochimica Acta,2016,211:982-988.[12] 杨昱霖,高铭,梁静爽,等.硅纳米粒子聚苯胺包覆改性及其嵌/脱锂电化学性能[J].无机化学学报,2017,33(12):2262-2270.[13] 李纯莉,杨广,张平,等.石墨烯/多孔纳米硅负极的电化学性能[J].电化学,2015,21(6):572-576.[14] SOURIC J,BORDE A,BOULINEAU A,et al.Core-shell amorphous silicon-carbon nanoparticles for high performance anodes in lithium ion batteries[J].Journal of Power Sources,2016,328:527-535.[15] ZHUANG Xiangyang,ZHANG Yao,HE Lingxiao,et al.Scalable synthesis of nano-Si embedded in porous C and its enhanced performance as anode of Li-ionbatteries[J].Electrochimica Acta,2017,249:166-172.。
不同晶粒尺寸材料的霍尔佩奇关系

不同晶粒尺寸材料中的H-P关系细化晶粒一直是改善多晶体材料强度的一种有效手段。
根据位错理论,晶界是位错运动的障碍,在外力作用下,为了在相邻晶粒产生切变变形,晶界处必须产生足够大的应力集中,细化晶粒可以产生更多的晶界,如果晶界结构未发生变化,则需施加更大的外力才能产生位错塞积, 从而使材料强化。
Hall-Petch 关系就是在位错塞积模型基础上导出的。
H-P关系的历史20世纪50年代初,人们开始研究晶粒尺寸与材料强度的关系,1951年当时还在谢菲尔德大学读书的E. O. Hall在64册装订的《物理学进程表》上发表了三篇文章。
在第三篇文章中,他指出了滑动带的长度或裂纹尺寸与晶粒尺寸成正比,即,式子中的第一项代表了材料的强度,k是常数。
由于技术条件的限制,Hall只能推出成正比的关系,但是x的取值没有具体给出。
当时Hall选取的研究对象是锌但是他发现这个关系应用于低碳钢同样成立。
英国利兹大学的N. J. Petch根据自己在1946-1949年的实验研究和Hall的理论基础发表了一篇论文,这篇论文着重讲述了有关脆性断裂方面的知识,通过测量在低温条件下不同晶粒尺寸的解理强度,Petch把Hall提出的数学关系进行了精确地完善,这个重要的数学关系就以他们的名字命名为霍尔佩奇关系。
即σy代表了材料的屈服极限,是材料发生0.2%变形时的屈服应力σ0.2通常可以用显微硬度Hv来表示σ0表示移动单个位错时产生的晶格摩擦阻力Ky一个常数与材料的种类性质以及晶粒尺寸有关d 平均晶粒直径Hall-Petch关系图由于Hall和Petch所处的年代技术的落后他们能研究的晶粒尺寸还是很大的,所以早期的H-P关系是不完善的,只有图中前半部分。
后半部分是随着科技的进步,逐渐完善的。
近几十年来, 材料的细晶强化研究大量开展。
在一般晶粒尺寸范围内, 材料的强度随晶粒尺寸的变化是符合Hall-Petch 关系的, 但在纳米晶体材料中出现了偏离甚至反Hall-Petch 关系的现象, 因此Hall-Petch 关系的使用具有一定的局限性。
Journal of Alloys and Compounds 448(2008)73-76 The

Journal of Alloys and Compounds448(2008)73–76The magnetic entropy change in CoMnSballoys with different crystal sizesShandong Li a,b,∗a Department of Physics,Fujian Normal University,Fuzhou350007,Chinab National Laboratory of Solid State Microstructure and Department of Physics,Nanjing University,Nanjing210093,ChinaReceived30November2006;received in revised form11March2007;accepted12March2007Available online16March2007AbstractThe magnetocaloric effect(MCE)in CoMnSb has been investigated by comparing two samples with different crystal size.One sample is the ingot with crystal size of120nm,referred as sample A.The other with average crystal size of30nm has been fabricated by rapid solidification method,referred as sample B.It has been found that crystal size dramatically affects the magnetic properties and MCE for CoMnSb alloy.For example,in comparison with sample A,sample B exhibits a lower magnetization and Curie temperature,but an enhanced refrigerant capacity and broader working temperature range.These facts indicate that sample B is superior to the ingot in practical application.The above results are explained in terms of the effect of crystal size and atomic disorder on the intrinsic magnetic properties and magnetic entropy change.©2007Elsevier B.V.All rights reserved.Keywords:Nanostructured materials;Magnetocaloric;Transition metals alloys and compounds1.IntroductionIn recent years,the magnetic refrigerants have drawn an increasing attention because they are more protective towards our living environment than the conventional vapor-cycle refrig-erant[1,2].In comparison with gas refrigerators,magnetic refrigerators have a number of advantages,such as high effi-ciency,small volume and ecological cleanliness.The magnetic refrigeration makes use of the cycles of magnetization and demagnetization of a magnetic material,so that the develop-ment of new materials with a giant MCE is strongly desired. The research for materials with large magnetocaloric effect is being continued since the discovery of MCE in iron by Warburg about100years ago[1].Some magnetic materials with afirst-order or second-order transition have attracted much attention, since they have large MCE[2–6].∗Correspondence address:Department of Physics,Fujian Normal University, Fuzhou350007,China.Tel.:+8659183486160;fax:+86-591-83465313.E-mail address:dylsd007@.It is known that above15K,Ericsson cycle is used in magneticrefrigeration in order to remove the effect of the lattice entropy[7].Thermodynamic analysis shows that efficient operation ofan ideal Ericsson cycle requires a constant-induced magneticentropy change as a function of temperature over the requiredoperating range[8].If a magnetic working material has a largemagnetic entropy change(| S M|)peak at the transition tem-perature,but falls off rapidly on either side,it is not suitablefor use in devices utilizing the Ericsson cycle[3].Therefore,it is significant to explore a magnetocaloric material with highMCE and wide operating temperature span and/or to widen theoperating temperature span for the high MCE material by useof some novel methods.It was reported that nanoparticles fab-ricated by rapid solidification or chemical method,may haverelatively wider working temperature span[9].In our previous work,the MCE of CoMnSb alloy has beenreported as an exploration for useful MCE materials[10].Dueto the large Mn magnetic moments,this kind of rare-earth-freealloy may be a potential candidate of large MCE materi-als.In order to extend the operating temperature span and toenhance the refrigerant capacity of CoMnSb alloy,the nanocrys-talline CoMnSb alloy has been fabricated by rapid solidification0925-8388/$–see front matter©2007Elsevier B.V.All rights reserved. doi:10.1016/j.jallcom.2007.03.05274S.Li/Journal of Alloys and Compounds448(2008)73–76 method.In this paper,the effect of crystal size and atomic dis-order on the MCE of CoMnSb alloy have been investigated bycomparing the MCE of two samples with different crystal sizes.2.Experimental procedureTwo types of CoMnSb alloys with different crystal sizes have been preparedby an induction-melting and a melt-spinning method,respectively.The highpurity metals of Co,Mn and Sb were melted in an induction melting furnacefor three times under Ar atmosphere.Then,the ingot was sealed in a quartztube under vacuum atmosphere(less than3×10−3Pa).The sealed ingot wasannealed at873K for30h for eliminating inner stress.The annealed samplewith large crystal size was referred to as sample A,while the other sample withsmall crystal size,fabricated by melt-spun part of the ingot at a circumferencespeed of30m/s in vacuum,was referred to as sample B.The magnetic properties of the samples were measured by using vibratingsample magnetometer(VSM)and superconducting quantum interference device(SQUID)magnetometer.The microstructure of the materials was characterizedby an X-ray diffractometer(XRD)with Cu K␣radiation.3.Results and discussionFig.1shows the XRD curves for the samples A and B.Theindexes of CoMnSb facets were signed in Fig.1.As illustrated,both samples are composed of a single phase of CoMnSb.It canalso be seen that the diffraction peaks of sample A are greatsharper than those of sample B,indicating that the crystal sizeof sample A is great coarser than that of sample B.The crystalsizes of samples A and B,calculated by Scherrer equation,areabout120and30nm,respectively.The temperature dependence of magnetization for both sam-ples was measured by VSM in the magneticfield of0.2T.Fig.2shows the M–T relationship curves for samples A and B.It can beseen that:(1)the transition temperature of sample B is slightlylower than that of sample A.The Curie temperatures are471and468K for samples A and B,respectively,and(2)the mag-netization of sample B is slightly lower than that of sample A attemperature range less than T C.Fig.3shows a series of magnetization isotherms measured atdifferent temperatures in the vicinity of Curie temperature,T C,with the maximum appliedfield of0.9T.The magnetic entropychange,| S M|,was determined as a function of temperatureandFig.1.The XRD traces for samples A andB.Fig.2.The temperature dependence of magnetization for samples A and B.magneticfield from isothermal magnetization curves by use ofMaxwell equation:S M=H2H1∂M(H,T)∂THd H(1)Fig.4shows the plots of| S M|versus temperature of sam-ples A and B for the magneticfield changing from0to0.9T,respectively.Although,the maximum value of| S M|for sampleB(1.32J/kg K)is smaller than that for sample A(2.06J/kg K),a broader peak for sample B is observed in the| S M|–T curves,indicating that sample B may be superior to the bulk materialfor practical application in Ericsson cycle.In practice,how much heat can be transferred between the cold and hot sinks in one ideal refrigeration cycle is characterizedby the refrigerant capacity[11].The refrigerant capacity,q,isdefined asq=ThotT coldS(T,P, H)P, H d T(2)Fig.3.The magnetization isotherms measured at different temperatures near T Cfor samples A and B.S.Li/Journal of Alloys and Compounds448(2008)73–7675Fig.4.The plots of| S M|vs.temperature for samples A and B with H=0.9T. where T cold and T hot are the temperature of the cold and hotsinks,respectively.Therefore,when two different materials areused in the same refrigeration device,the material with higherrefrigerant capacity is expected to perform better,since it willsupport transport of greater amounts of heat in a real cycle,provided all parameters of a magnetic refrigerator remain thesame.From Fig.4,it can be seen that the optimum operatingtemperature range of sample B is wider than that of sample A.Inorder to accurately evaluate the refrigerant capacity for materialswith different peak site and operating temperature span,we takethe temperature range between the full-width at half maximumas the calculating temperature span in Eq.(2).The temperaturespans for samples A and B are466–475and452.3–477.4K,respectively.For the magneticfield changing from0to0.9T,therefrigerant capacities for samples A and B are15.3and27.3J/kg,respectively,according to Eq.(2).In addition,even taking thesame temperature span of433–483K,the refrigerant capacityof sample B(42.83J/kg)is slightly larger than that of sampleA(41.28J/kg).Consequently,sample B is superior to sample Ain practical paring with sample A,the smoothpeak of magnetic entropy change and larger refrigerant capacityof sample B indicate that sample B is a preferential choice ratherthan sample A.It is believed that magnetic properties of CoMnSb alloy withcrystal size of120nm can be considered as the bulk ones.The saturation magnetization(M s)of sample A was measuredby SQUID at3T and2K.If,approximately,taking the M sof94.2533emu/g at2K as the real saturation magnetizationM s(0)of CoMnSb alloy,the calculated saturation magnetiza-tion is3.978B/f.u.for sample A.This value is very close tothe theoretical and experimental result of M s∼4.0B/f.u.for CoMnSb alloy[12].While the crystal size is decreasing to smallsize(e.g.30nm),the magnetic exchange interaction and mag-netic anisotropy deviate from the bulk material,giving rise to aslight reduction of the magnetization and T C[13,14].This phe-nomenon was widely observed in nanocrystalline ferromagneticsystems[15,16].Moreover,the effect of crystal size distributionon the inner magnetic properties(e.g.the saturation magnetiza-tion,T C)for nanocrystallite materials is great larger than that forbulk one[17].With raising temperature,the nanocrystallite fer-romagnetic material transforms to the paramagnetic state priorto the bulk one.As a result,a relatively lower transition tem-perature in M–T curve for nanocrystalline materials than thatfor bulk ones is expected.In addition to that,the distributionof crystal size for the nano-ferromagnetic materials also givesrise to afluctuation of T C accordingly.Therefore,the broaden-ing of| S M|peak and enhancement of refrigerant capacity in sample B in comparison with sample A can be,at least partially,attributed to the broad T C distribution induced by small size andits distribution.Atomic disorder generally occurs in half-Heusler alloys,suchas CoMnSb and NiMnSb[18,19].In our previous work[20],theatomic disorder of CoMnSb alloys was reduced by annealing thesample at1323K for50h.A superstructure with low atomic dis-order was formed for the sample annealed at high temperature.The broadening of operating temperature span and the enhance-ment of refrigerant capacity can be attributed to the formationof the superstructure.Ref.[20]implies that the atomic disorderin CoMnSb alloy deteriorates the MCE.In this study,sampleA was annealed at873K for30h for the aims of eliminatinginner stress and reducing the atomic disorder.Sample B was notannealed for avoiding the grain growth.It is well known that theatomic disorder is stronger for the sample prepared by quench-ing than the ingot.This can also be demonstrated by the XRDresults.The diffraction peaks of sample B are slightly shiftedto left side in comparison with those of sample A,suggestinga relatively larger atomic disorder in quenched sample B thanin sample A.Therefore,the measured refrigerant capacity islower than the real value due to the stronger atomic disorder insample B.In other words,the effect of crystal size on MCE ispartially reduced by atomic disorder for the quenched sample.The improvement of MCE in sample B is dominated by crystalsize effect.4.ConclusionThe magnetic properties and magnetocaloric effect ofCoMnSb alloys with different crystal sizes have been investi-paring to the sample with crystal size larger than100nm,the refrigerant capacity is enhanced and the operatingtemperature span is extended for the sample with crystal size assmall as several tens of nanometer.These results suggest thatCoMnSb alloy with small crystal size is superior to the largerone in practical application.AcknowledgementsThis work wasfinancially supported by National ScienceFoundation of China(NSFC)for Young Scientists(Grant No.:10504010),Key Project of Fujian Provincial Department of Sci-ence&Technology(2006H0018)and Science Foundation ofFujian Province of China(2006J0152and2005J023). References[1]E.Warburg,Ann.Phys.(Leipzig)13(1881)141.[2]V.K.Pecharsky,K.A.Gschneidner Jr.,Phys.Rev.Lett.78(1997)4494.76S.Li/Journal of Alloys and Compounds448(2008)73–76[3]B.J.Korte,V.K.Pecharsky,K.A.Gschneidner Jr.,J.Appl.Phys.10(1998)5677.[4]F.W.Wang,X.X.Zhang,F.X.Hu,Appl.Phys.Lett.77(2000)1360.[5]O.Tegus,E.B¨u rck,K.H.J.Buschow,F.R.de Boer,Nature415(2002)150.[6]S.D.Li,M.M.Liu,Z.R.Yuan,L.Y.L¨u,Z.C.Zhang,Y.B.Lin,Y.W.Du,J.Alloys Compd.427(2007)15.[7]T.Hashimoto,T.Kuzuhara,M.Sahashi,K.Inomata,A.Tomokiyo,H.Yayama,J.Appl.Phys.62(1987)3873.[8]A.Smaili,R.Chahine,J.Appl.Phys.81(1997)824.[9]D.H.Wang,S.L.Tang,H.D.Liu,S.D.Li,J.R.Zhang,Y.W.Du,Jpn.J.Appl.Phys.40(2001)6815.[10]S.D.Li,M.M.Liu,Z.G.Huang,F.Xu,W.Q.Zou,F.M.Zhang,Y.W.Du,J.Appl.Phys.99(2006)063901.[11]V.P.Pecharsky,K.A.Gschneidner Jr.,J.Appl.Phys.90(2001)4614.[12]V.Ksenofontov,G.Melnyk,M.Wojcik,S.Wurmehl,K.Kroth,S.Reiman,P.Blaha,C.Felser,Phys.Rev.B74(2006)134426.[13]R.H.Kodama,S.H.Makhlouf,A.E.Berkowitz,Phys.Rev.Lett.79(1997)1393.[14]J.M.D.Coey,Phys.Rev.Lett.27(1971)1140.[15]T.Sato,T.Iijima,M.Seki,N.Inagaki,J.Magn.Magn.Mater.65(1987)252.[16]J.F.L¨o ffler,J.P.Meier,B.Doudin,J.P.Ansermet,W.Wagner,Phys.Rev.B57(1998)2915.[17]R.H.Kodama,J.Magn.Magn.Mater.200(1999)359.[18]C.PalmstrØm,MRS Bull.(October)(2003)725.[19]K.Kaczmarska,J.Pierre,J.Tobola,R.V.Skolozdra,Phys.Rev.B60(1999)373.[20]S.D.Li,Z.R.Yuan,L.Y.L¨u,M.M.Liu,Z.G.Huang,F.M.Zhang,Y.W.Du,Mater.Sci.Eng.A428(2006)332.。
英文SCI期刊-金属材料

1. nature IF:36.28 (2011)2. science IF:31.201 (2011)3. composites science and technology IF:3.14 (2011)杂志简介/稿件收录要求:Composite materials offer lively new solutions to important engineering problems and exciting challenges to the designer who seeks to exploit their potential. In the competition with conventional engineering materials, the development of safe and economic applications for composites requires scientific understanding of their behaviour, rigorous modelling of complex stress analytical problems, and innovative approaches to manufacturing. The journal publishes refereed original articles, occasional review papers, and letters, on all aspects of the fundamental and applied science of engineering composites. It deals with fibre and particulate composites, with reinforced metals, plastics and ceramics, including cementitious materials, and with natural composites like wood and bone. The editors welcome theoretical and experimental papers dealing with rheological, mechanical, chemical and physical properties, fatigue, fracture toughness, creep, durability and environmental effects, and papers on manufacture and design for specific purposes.The journal particularly encourages an interdisciplinary approach to the study of composites, with a balanced experimental and analytical view of their behaviour. Composites Online This free service is a combination of discussion forum and awareness service. There are also features such as a Calender of events and Jobs Board, and will be of interest to all composites researchers. Visit Composites Online now and submit your comments.4. journal of materials science IF:2.01 (2011)杂志简介/稿件收录要求:The Journal of Materials Science and its companion journal Journal of Materials Science Letters are now firmly established as the leading sources of primary communication for scientists investigating the structure and properties of all engineering materials. The Journal of Materials Science publishes reviews and full-length papers recording original research results on or techniques for studying the relationship between structure properties and uses of materials. Journal of Materials Science Letters is concerned with timely short communications on materials science (less that 1500 words). The subject in both journals is seen from international and interdisciplinary perspectives covering areas including metals ceramics glasses polymers electrical materials composite materials fibres biological and biomedical materials.5. materials letters IF:2.31 (2011)6. materials & design IF:2.2 (2011) 实验室有投杂志简介/稿件收录要求:Mechanisms, machines and structures have to meet increasingly stringent requirements during operation. The economic and human costs of failure during service impose a great responsibility on those who develop materials and those who select and integrate materials in a final engineering design. A critical feature of successful development is the selection of the best material based on an awareness of the capabilities and opportunities afforded by all candidate materials, coupled with a design which takes full advantage of those capabilities. Materials & Design, as its title implies, publishes papers, articles and reports which describe the properties of a material which influence or control any practical design. All types of materials are covered, and all scales of application from micromachinery to large structural components. The technical level is postgraduate but not specialist: development rather than theory is stressed. Papers should be understandable and offer information useful to professionals working in fields outside the immediate subject of the paper. The aim is to promote a greater understanding of the attributes and capabilities of all types of modern engineering materials.7. rare metal materials and engineering IF:0.164 (2011) 实验室有投8 journal of materials engineering and performance IF:0.855 (2011)杂志简介/稿件收录要求:The Journal of Materials Engineering and Performance (JMEP) offers articles that assist in solving day-to-day engineering challenges, especially those involving components for larger systems. Coverage includes all aspects of materials selection, design, processing characterization and evaluation. Topics include improvement of materials properties through processes and process control of casting, forming, heat treating, surface modification and coating, and fabrication. Testing and characterization are demonstrated through mechanical and physical tests, NDE, metallography, failure analysis, corrosion resistance, chemical analysis, surface characterization, and microanalysis of surfaces, features and fractures. The Journal of Materials Engineering and Performance publishes contributions on all aspects of materials selection, design, processing, characterization, and evaluation. The scope includes all materials used in engineering applications, especially those that typically result in components for larger systems. This is a publication of ASM International, The Materials Information Society.9. materials and manufacturing processes IF:1.058 (2011)杂志简介/稿件收录要求:Materials and Manufacturing Processes deals with issues that result in better utilization of raw materials and energy, integration of design and manufacturing activities requiring the invention of suitable new manufacturing processes and techniques, unmanned production dependent on efficient and reliable control of various processes including intelligent processing, introduction of new materials in industrial production necessitating new manufacturing process technology, and more. Information is offered in various formats, including research articles, letter reports, review articles, conference papers, applied research, book and conference reviews, patent reports, and entire issues devoted to symposia.10. materials science and engineering a-structural materials properties microstructure and processing IF:2.003 (2011) 实验室有投11. journal of alloys and compounds IF:2.003 (2011) 实验室有投杂志简介/稿件收录要求:The aim of the Journal of Alloys and Compounds is identical to the journal's aim under its previous title: Journal of the Less-Common Metals. The journal was originally intended to serve as an international medium for the publication of work on the physical sciences of usually called less-common-metals, their compounds and their alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics. The interdisciplinary nature of the journal is evident in many subject areas. Experimental and theoretical approaches to materials problems require an active interplay between a variety of traditional and novel scientific disciplines. In much of the work published in the journal, synthetic and structural studies are combined with investigations of chemical and physical properties of alloys and compounds, contributing to the development of areas of current scientific interest. The Journal of Alloys and Compounds provides a unique international forum where materials scientists, chemists and physicists can present their results both to workers in their own fields and to others active in related areas.12. acta materialia IF:3.755 (2011)13. Scripta Materialia IF:2.699 (2011)杂志简介/稿件收录要求:Scripta Materialia, the companion journal to Acta Materialia, is an International journal for the Science of Materials. Its purpose is to provide a medium for the rapid publication of cutting edge short papers which advance the understanding of structural, nanostructured, and functional materials, both crystalline and amorphous, across all materials classes. Emphasis is placed on those aspects of the science of materials that address:(i) the relationship between the microstructure of materials and their properties, including mechanical(from both the defect and continuum viewpoints), electrical, magnetic and chemical properties; (ii) the relationship between the microstructure of materials and the thermodynamics, kinetics and mechanisms of processes occurring within solids; (iii) the synthesis and processing of materials, with emphasis on microstructural mechanisms and control. (iv) advances in the characterization of the microstructure and properties of materials. Scripta Materialia encourages the submission of letters of opinion, and of comments, particularly concerning work published in Acta Materialia and Scripta Materialia. The journal also publishes Viewpoint Sets, which are a series of short articles focused on topics of current interest within the scope of the journal and co-ordinated by invited editors.±14. journal of materials processing technology IF:(1.783)The journal covers the processing techniques of metals and other traditional and advanced materials. The articles published focus mainly on the developments in, and the analysis of, equipment and processes. Through this approach the journal aims to contribute to increased production efficiency and improved component performance. Materials and techniques covered in the journal include: Materials: Metals, ceramics, fibre reinforced materials, composites and polymers. Processing Techniques: Sheet forming: blanking, piercing, pressing, deep drawing, spinning and flow turning, stretch forming, fluid and rubber forming, high-rate forming processes, welding, etc. Bulk forming: hot, warm and cold forging, rolling and extrusion, rotary forging, ring rolling, hydrostatic extrusion, conforming, net-shape manufacturing, etc. Powder forming: compaction, sintering, forging, extrusion, etc. Forming in the melt or near-melt condition: die casting, mushy-state forging, net-shape manufacturing, etc. Material-removal processes: cutting, grinding, ECM, EDM, etc. Non-traditional processes: shot peening and other impact-related processes, die-manufacturing processes, and laser processing, etc. Surface engineering: deposition, treatment and characterisation technologies. Simulation: Analytical and numerical methods applied to any of the above processing techniques. Relevant Areas of Materials Technology and Metallurgy: Thermomechanical treatments, annealing schedules, superplastic materials, etc. Including computer applications in all relevant areas, such as process modeling, computer-aided design of equipment, computer-integrated manufacturing, and the development of expert systems for materials processing. Environmental Issues: In addition to the above, environmental issues, in so far as they affect the choice of material or the selection or development of the processes employed: - the minimisation of sound pollution in the case of the forging industry; - health aspects in casting and heat treatment; - energy conservation in primary ingot production, bulk forging, extrusion, rolling, heat treatment and near net-shape manufacturing; - materials conservation. A special feature of the journal is the Industrial Summary wherein each article provides a summary of the industrial significance of the work and the industrial implications arising from the results of the work.15. metallurgical and materials transactions a IF:(1.545)杂志简介/稿件收录要求This journal emphasizes the relationships among processing, structure and properties of materials. It contains only critically reviewed and original research: the results of extensive field, plant, laboratory or theoretical investigation, or new interpretations of existingproblems. Main topics are mechanical behavior, alloy phases and structure, transformations, environmental interactions, physical chemistry and transport phenomena.。
复合材料方面的核心期刊和杂志

国内复合材料权威杂志和期刊:复合材料学报高分子学报玻璃钢高等学校化学学报无机材料学报功能材料材料导报材料研究学报材料科学与工程学报师材料工程复合材料新型炭材料国外复合材料权威杂志和期刊:Composites Business AnalystComposite Structures《复合材料结构》英国ISSN:0263-8223,1983年创刊,全年16期,Elsevier Science出版社,SCI、EI收录期刊,2000年SCI影响因子0.359,被引频次786、年载文量95。
EI 2001年收录117篇。
刊载工程结构中应用复合材料的论文,包括设计、制造技术、开发、实验研究、理论分析等方面。
Composites Part A: Applied Science and Manufacturing《复合材料A:实用科学与制造》英国ISSN:1359-835X,1969年创刊,全年12期,Elsevier Science出版社,SCI、EI收录期刊,2000年SCI影响因子0.723,被引频次354、年载文量145。
EI 2001年收录180篇。
刊载塑料、水泥、金属、陶瓷等基质与其它物质合成强化材料的化学与技术论文和评论,涉及强化材料制造、研究、生产、规划和发展。
兼载会议报告、文摘与书评。
Composites Part B: Engineering《复合材料B:工程》英国ISSN:1359-8368,1991年创刊,全年8期,Elsevier Science出版社,SCI、EI收录期刊,2000年SCI影响因子0.436,被引频次131、年载文量72。
EI 2001年收录58篇。
刊载复合材料与工程结构方面的研究论文,涉及新型材料和新型结构在各个领域,特别是在航空、机械和海洋工程领域的应用,包括设计与分析方法的研究。
Composites Science and Technology《复合材料科学与技术》英国ISSN:0266-3538,1968年创刊,全年16期,Elsevier Science出版社,SCI、EI收录期刊,2000年SCI影响因子0.680,被引频次1628、年载文量218。
journal of alloys and compounds是几区

journal of alloys and compounds 是几区
Journal of Alloys and Compounds是SCI期刊,属于2区。
Journal of Alloys and Compounds(JALCOM)是由Elsevier出版社发行的一份学术性期刊,其主要关注研究合金合制备、物理性能、力学性能、用途以及表面、相变等性质等领域。
JALCOM发行如微电子、磁性、超导、燃料电池和玻璃等各种特殊合金的研究内容,在内容上十分广泛,所以受到国际学术界的很高关注。
JALCOM旨在提高国际学术界对合金性能的研究水平,以支持合金设计和应用的发展。
其主要的研究方向是合金离子掺杂、多元合金、低维纳米结构、加工力学行为等。
JALCOM推出有关合金分析、合金组成和结构、合金物理性质和热学性质、合金强度与塑性、合金组织与腐蚀、合金表面以及合金加工等研究文章。
同时,JALCOM定期发行合金研究的国际性会议,以克服科技的界限,支持国际研究者之间的技术交流,使不同领域的学者都能访问JALCOM的研究内容。
JALCOM在学术界已经具有很高的知名度,同时也是国际科技界首要的合金研究平台,是国际合金研究者读者必备的学术研究期刊之一。
常温搅拌和回流搅拌的简写

常温搅拌和回流搅拌的简写常温搅拌和回流搅拌是制备高质量材料和合金的常见方法。
本文将探讨这两种搅拌技术的定义、原理、应用和优点,并分享个人的观点和理解。
一、常温搅拌(RTM)常温搅拌,全称为常温搅拌铸造(Room Temperature Stir Casting),是一种常用的金属合金制备方法。
它通过将金属或合金预先熔化,然后在室温下进行搅拌,使冷却而凝固的材料具有良好的均匀性和机械性能。
1.1 原理常温搅拌的原理是将粉末朝向搅拌区域引入,搅拌区域有一个高速旋转的搅拌器。
粉末在搅拌器的作用下与熔融金属进行混合,并形成均匀的合金熔液。
合金熔液会冷却凝固,形成常温搅拌材料。
1.2 应用常温搅拌广泛应用于制备各种金属合金材料,如铜铝合金、铝镍硅合金等。
它可以用于制备各种金属合金坯料、复合材料、铸造材料等。
1.3 优点常温搅拌的优点在于制备过程简单、成本低、能耗小,并且可以获得高纯度和均匀性较好的材料。
搅拌金属和粉末的过程中,还可以添加一些增强相,提高材料的力学性能。
个人观点和理解:对于常温搅拌,我认为它是一种简便而有效的常用技术。
它不仅可以制备高质量的金属合金材料,还可以应用于各种领域,如航空航天、汽车制造和电子工业等。
常温搅拌的广泛应用促进了材料科学的发展,并对实现可持续发展目标有重要意义。
二、回流搅拌(RSM)回流搅拌,全称为回流搅拌铸造(Reflux Stir Casting),是一种新兴的金属合金制备方法。
它通过在高温条件下将金属熔融,然后在特定搅拌条件下进行搅拌,使合金具有更好的均匀性和机械性能。
2.1 原理回流搅拌的原理是将金属或合金熔液注入搅拌容器,然后在特定温度和搅拌条件下进行搅拌。
搅拌过程中,材料中的不均匀相会逐渐熔化并与其他相混合,在搅拌器的作用下,形成均匀的合金结构。
合金冷却凝固形成回流搅拌材料。
2.2 应用回流搅拌逐渐在各个领域得到应用,如航空航天、电子器件、汽车制造和电力工业等。
稀有金属 影响因子

稀有金属影响因子
稀有金属是指地壳中含量较低的金属元素,具有珍贵性和战略性,具有广泛的应用前景。
稀有金属在现代科技领域中发挥着重要的作用,如磁性材料、光电材料、电子材料、催化剂等方面。
稀有金属的研究和开发是当前材料科学与工程领域的热点之一,也是全球资源分配和能源安全问题的重要组成部分。
稀有金属的研究涉及到多个学科,如材料科学、化学、物理、地球科学等。
稀有金属材料的制备、表征和应用等方面的研究也在不断发展。
由于其重要性和特殊性,稀有金属材料的研究一直备受关注,相关研究论文也在国际上广泛发表。
稀有金属材料的研究与发展已经成为国际上的热点话题。
影响因子是衡量学术期刊影响力的指标之一,也是衡量学者学术成就的重要标志。
稀有金属领域的影响因子反映了该领域内不同期刊的学术质量和影响力。
目前,影响因子较高的稀有金属期刊包括Journal of Alloys and Compounds、Materials Science and Engineering: B、Journal of Rare Earths等。
稀有金属的研究和开发有着广阔的前景,也面临着一系列的挑战。
未来,稀有金属材料的研究将继续推动新材料、新技术的发展,为人类的发展和进步做出更多的贡献。
- 1 -。
等离子体电解技术及其应用

等离⼦体电解技术及其应⽤等离⼦体电解作为⼀种独特的等离⼦体应⽤技术,是指在电极和溶液之间加以⼀定的电压,击穿电极周围由于焦⽿热形成的溶液蒸汽层,产⽣放电等离⼦体,进⽽对溶液或对放电电极表⾯进⾏处理的过程。
等离⼦体电解实际上是把电解溶液作为⼀个电极,将放电等离⼦体维持在电极和电极周围的电解液之间,包括常规电解、在电极附近由焦⽿热引起的溶剂汽化、伴随电极上形成的蒸汽层⽽产⽣的流体不稳定性以及蒸汽层中的放电三个过程。
等离⼦体电解兼具等离⼦体化学和电化学技术的优点。
等离⼦体电解过程中,放电等离⼦体层内产⽣⼤量⾼能活性粒⼦从⽽引发各种等离⼦体化学反应。
等离⼦体电解是⼀种⾮常有前景的技术, 在等离⼦体电解沉积、等离⼦体电解渗透、⾼分⼦材料改性等⽅⾯显⽰出独特的优点。
如在纳⽶材料(⾦刚⽯、⽯墨烯等)、光电材料(ZrN、GaN等)和催化材料(CuO、ZnO等)的改性与制备等⽅⾯显⽰出了较好的应⽤前景。
低温等离⼦体化学应⽤技术:(1)等离⼦体电解制氢;(2)等离⼦体电解低碳醇能源化⼯品转化;(3)等离⼦体电解氧化⾦属表⾯制陶瓷膜;(4)等离⼦体废⽔处理;(5)等离⼦体空⽓净化。
代表性论⽂:1. Hydrogen generation by GDPE of methanol solutions. International Journal of Hydrogen Energy. 2009,34, 48~55. (3.452)2. Effect of potassium fluoride on structure and corrosion resistance of plasma electrolytic oxidation films formed on AZ31 magnesium alloy. Journal of Alloys and Compounds. 2009, 480:469~474. (1.510)发明专利:1. ⼀种等离⼦体重整制备富氢⽓的⽅法及其装置,发明专利2. ⼀种辉光放电电解⼄醇溶液制备⼄醛的⽅法,发明专利3. ⼀种辉光放电电解甲醇溶液制备甲醛的⽅法,发明专利。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Journal of Alloys and Compounds 492 (2010) 496–499Contents lists available at ScienceDirectJournal of Alloys andCompoundsj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /j a l l c omMixing of iron and molybdenum and photo-doping effect on Sr 2FeMoO 6Y.C.Hu a ,Q.Ji a ,J.J.Ge a ,R.B.Xie a ,Z.S.Jiang a ,X.S.Wu a ,∗,G.F.Cheng b ,Hairui Liu c ,Qingfeng Lu caNanjing National Laboratory of Microstructures,Key Lab of Solid State Microstructures,Department of Physics,Nanjing University,Hankou Road,Nanjing 210093,China bShanghai Institute of Ceramics,CAS,1295Dingxi Road,Shanghai 200050,China cDepartment of Physics,Henan Normal University,Xinxiang 453007,Henan,Chinaa r t i c l e i n f o Article history:Received 17August 2009Received in revised form 19November 2009Accepted 20November 2009Available online 26 November 2009Keywords:Magnetically ordered materials X-ray diffraction Crystal structurePositron spectroscopya b s t r a c tThe structure and photo-doping effect on Sr 2FeMoO 6are investigated using the X-ray powder diffraction and positrons annihilation technique,respectively.Anti-site defect is about 8.8%,can be estimated directly from the XRD pattern,which is well consistent with that of obtained from the XRD refinements.The integral intensity ratio of the reflection (101)and the reflections (200)and (112)may used to estimate the concentration of the anti-site defect.The positron annihilation lifetime in Sr 2FeMoO 6is sensitive to photo-doping.The average lifetime and the electron density n e vary with photo-doping.© 2009 Elsevier B.V. All rights reserved.1.IntroductionThe double perovskite compound Sr 2FeMoO 6(SFMO),with a high magnetic transition temperature (T c ≈410K)[1]and a remarkable magneto-resistance around room temperature,has become one of the most promising candidates in magnetic storage materials [2].SFMO is a typical ordered double perovskite structure A 2BB O 6,where A is the alkali-earth ion,B and B are the transition metals.As reported in [3,4],the crystal structure of SFMO is cubic or tetragonal,where the alternating FeO 6and MoO 6octahedral are arranged regularly in a rock salt superlattice with the voluminous Sr cation occupying the voids among the octahedral.The existence of anti-site defects (Mo on Fe site and vice versa)has great influence on the magnetic and transport properties of Sr 2FeMoO 6[5,6].Defects are important structural information in understanding the properties of material [7].Positron annihilation technique (PAT)can provide unique information about defects and has many advan-tages in structural characteristic.It is a nondestructive and effective method to detect the information of defects [8],whereas there are few studies about SFMO using this technique recently.Photo-doping,which remains the component and structure of material unchanged,is an effective way to improve the property [9,10].It has been extensively used in semiconductor material [11,12],but∗Corresponding author.Tel.:+862583594402;fax:+862583595535.E-mail address:xswu@ (X.S.Wu).seldom studies are performed on magnetic materials.In order to understand the photo-doping effect on defects in SFMO,we synthe-size the compounds using standard solid-state reaction.The crystal structure and photo-doping effect on defects have been studied by means of X-ray powder diffraction and the positrons annihilation technique,respectively.2.ExperimentalSample of Sr 2FeMoO 6is prepared by standard solid-state reaction.Stoichiomet-ric powders of SrCO 3,Fe 2O 3and MoO 3are mixed,ground and heated at 900◦C for 10h in air.The pre-reacted mixture is then finely ground,pressed into pellets and sintered at 1280◦C in a stream of 5%H 2/Ar gas for 15h with several intermediate grindings.The samples are heated and cooled at a rate of 5◦C/min under the same atmosphere.Structure of the sample is examined by X-ray powder diffraction (XRD)using a Rigaku D/max 2500diffractometer with CuK ␣radiation (50kV 250mA)and a graphite monochromator.The XRD pattern shows that the sample crystallizes in sin-gle phase.The XRD data are analyzed by means of the Rietveld refinement program GSAS [13].Sample is illuminated by halogen lamp (60mW/cm 2)with varying illumina-tion time from 0min to 40min in a space of 10min.Power of the lamp is 100W and distance between sample and lamp is 7.9cm.The sample temperature values reached at 28±1◦C after 40min irradiation.The heating rate of the samples is about 2±1◦C/10min through the illumination.We use pieces of the samples at the same time.After each illumination,the positron lifetime measurements are carried out using a conventional fast–fast coincidence ORTEC-100U system with a prompt time resolution of 228ps (FWHM,full width at half maximum)and 10Ci 22Na source in sandwich geometry with the pellets.The total count for spectrum is 1million with the counting rate of 1000cps.Lifetime spectrum is analyzed by the PATFIT computer program with necessary source corrections.The measurements of positron lifetime are all performed at room temperature (20±1◦C).0925-8388/$–see front matter © 2009 Elsevier B.V. All rights reserved.doi:10.1016/j.jallcom.2009.11.148Y.C.Hu et al./Journal of Alloys and Compounds492 (2010) 496–499497Fig. 1.Observed(circles)and calculated(continuous line)XRD pattern for Sr2FeMoO6.The lowest curve is the difference between the observed and the calcu-lated XRD patterns.The vertical bars at the bottom indicate the Bragg reflection positions.(101)is the Bragg reflection at2Â≈19◦and(200)+(112)is that at 2Â≈32◦.3.Results and discussionFig.1presents the XRD pattern for Sr2FeMoO6.The excellent crystalline quality of sample used in this work can be appreci-ated.The XRD data can be used to refine the structure of the sample and abundance of information could be revealed due to precise counting statistics.Rietveld refinements of XRD diffraction pattern are carried out using the I4/m and the corresponding Wyck-off positions:Sr,4d(1/2,0,1/4);Fe1,2a(0,0,0);Fe2,2b(0,0,1/2); Mo1,2b(0,0,1/2);Mo2,2a(0,0,0);O1,8h(x,y,0);O2,4e(0,0,z). The refinement is performed according to the following group order[14,15]:(1)scale factor;background;zero point shift/sample displacement,transparency coefficient;(2)cell parameters;(3) peak shape;half width;asymmetry parameter and preferred ori-entation;(4)atom position parameter;(5)site occupancies;(6) overall thermal parameters;(7)isotropic thermal parameters.The Gaussian function is considered to refine the profile.No absorp-tion correction is taken into account.The wavelengths of CuK␣1, CuK␣2and the intensity ratio arefixed as1.5406A,1.5444A,and 0.497A,respectively.Full occupancy at every site in the unit cell is assumed during the refinements,i.e.the occupancies of Fe and Mo at2a and2b in total are1,respectively.The preferred orien-tations are very small and the temperature factors B Sr,B Fe1,B Fe2,B Mo1,B Mo2,B O1,and B O2are0.01304Å2,0.00611Å2,0.00611Å2,0.00392Å2,0.00392Å2,0.02301Å2,and0.01271Å2,respectively. The refinement process is smooth and leads to good quality fac-tors Rwp=10.00%and Rp=7.26%.All reflections can be indexed and a=b=5.58235(1)Å,c=7.87965(4)Å.The occupancy of Fe on Mo site (which is the occupancy of Fe2,equivalent in value to that of Mo on Fe site)is8.8%,which means the order concentrationÁis82.4% due toÁ=1−2x,where x is fraction of Fe on Mo site.Superstructure reflection(101)at2Â≈19◦can be seen clearly,which may indicate the existence of cation order-ing between Fe/Mo.Fig.2is the sketch of the unit cell of Sr2FeMoO6,in which Fe and Mo are completely ordered.Each unit cell is composed of10ions and the coordinates ofthe Fig.2.The unit cell of Sr2FeMoO6,in which Fe and Mo is completely ordered. ions are as following:Sr(1/2,1/2,1/4),(1/2,1/2,3/4),Fe(0,0,1/2), Mo(0,0,0),and O(1/2,0,0),(0,1/2,0),(0,0,1/4),(0,0,3/4),(1/2,0,1/2), (0,1/2,1/2).Based on the structure factor formula F(h k l)= nj=1f j e2 i(hx j+ky j+lz j),we can calculate the F(h k l)of Sr2FeMoO6as:F(h k l)=f Sre2 i(h/2+k/2+l/4)+e2 i(h/2+k/2+(3/4)l)+f Oe2 i(h/2)+e2 i(k/2)+e2 i(l/2)+e2 i(3/4)l+e2 i(h/2+l/2)+e2 i(k/2+l/2)+f Fe e2 i(l/2)+f Mo e2 i0when(h k l)=(101),F(101)=f Sr(e(3/2) i+e(5/2) i)+f O(e i+e0i+e(1/2) i+e(3/2) i+e2 i+e i)+f Mo−f Fe =f Mo−f FeSo we can obtain:I101∝P101|F(101)|2=P101|f Mo−f Fe|2,whereP101=(1+cos22Â)/(4sin2ÂcosÂ)is the Lorentz polarization fac-tor.The reflection of(101)is indeed observed at2Â≈19◦.Infact,as reported in Ref.[16],there is a fraction of Mo atoms onthe B site,equivalent in value to that of Fe atoms on the B site,which is defined as the anti-site defect.Suppose there are x Moions on Fe sites in the unit cell.The F(101)can be modified as:F(101)=(1−x)f Mo+xf Fe−(1−x)f Fe−xf Mo=(1−2x)f Mo−(1−2x)f Fe=(1−2x)(f Mo−f Fe)Four interesting results can be seen from above consequences.Firstly,the intensity of(101)reflection decreases with the increaseof the anti-site defect.Secondly,the integral intensity ratio betweenthe reflection peak of about2Â≈19◦and the reflection peak ofabout2Â≈32◦,I19◦/I32◦,which is used to indicate the order of Fe/Mo[17],can be interpreted as following:I32◦=I200+I112I200∝P200F2002,I112∝P112F1122,Thus,I32◦∝P200F2002+P112F1122Due to F(200)=(f Fe+f Mo)+2(f Sr+3f O),F(112)=(f Fe+f Mo)−2(f Sr+3f O).So I32◦∝P200|(f Fe+f Mo)+2(f Sr+3f O)|2+P112|(f Fe+f Mo)−2(f Sr+3f O)|2,which has no relation to Fe/Mo order.I19◦/I32◦can498Y.C.Hu et al./Journal of Alloys and Compounds 492 (2010) 496–499indicate the Fe/Mo order concentration as I 19◦is induced by Fe/Mo order.The larger I 19◦/I 32◦,the higher order of Fe/Mo.Thirdly,we define R is the integral intensity ratio of I 19◦/I 32◦for ideal sample without anti-site defect and R e as that for our sample which is prepared by standard solid-state reaction.R =I 19◦I 32◦∝P 101 F (101)2P 200F 2002+P112F1122∝P 101 f Mo −f Fe2P 200 (f Fe +f Mo )+2(f Sr +3f O ) 2+P 112 (f Fe +f Mo )−2(f Sr +f O )2When consider the fraction of anti-site defect x ,R e ∝(1−2x )2P 101 f Mo −f Fe2P 200(f Fe +f Mo )+2(f Sr +3f O ) 2+P 112(f Fe +f Mo )−2(f Sr +f O )2,soR e /R =1−2x .Due to Á=1−2x ,thus Á=R e /R .R e =I 19◦/I 32◦=2.56%from our experiment and we cancalculate R =3.76%[18,19]as sin Â(101)/ =0.11and sin Â(200)/ ≈sin Â(112)/ =0.18.ThusR e /R =82.5%,which is also similar with the order concentration Áfrom Rietveldrefinement.So we think Á=R e /R is a convenient method to calculate the order concentration approximately.Fourthly,based on the third result,we can define the disorder concentration as:=⎧⎨⎩2x,0<x ≤12(1−x )+(1−x ),12≤x <1.When x =1/2,F (101)=0, =100%.This means that the peak at 19◦will disappear as Fe/Mo complete disorder.When x =0or 1,the Fe/Mo is perfect ordered.Analysis on the positron lifetime spectra usually tells us three meaningful components of 1, 2and 3in the ratio I 1+I 2+I 3=100%.The second lifetime component 2with the typ-ical value of 278–280ps and a relative intensity of 1.5–2%,is due to the partial trapping of positrons at residual extrinsic vacancy-type defects. 2suggests that photo-doping has little effect on vacancy-type defect,according to our experiments [20],we here do not consider for further discussion.Fig.3(a)left shows the short lifetime component 1against different illumination time in the Sr 2FeMoO 6.The short lifetime component 1,having a value of 180–200ps,is due to the free annihilation of positrons.Anti-site defect is not open volume defect and contributes to 1.Electrons in the sample are activated by photo-doping and the concentration of electrons at the site of free annihilation increases,which improves the annihilation rate 1. 1decreases because of 1=1/ 1.The increase of 1is due to the decrease of the electron concentration at the site of free annihilation when t ≥30min.The longest lifetime component 3against different illumina-tion time in SFMO is shown in Fig.3(a)right.The longest component 3,with the typical value >0.5ns,results from positronium formed and annihilated through the ‘pick-off’process.The three main mod-els that describe the positronium formation in condensed matter are the Ore model (OM)[21],the spur model (SM)[22]and the free volume model (FVM)[23].Our work can be better explained according to FVM.Illumination may make electrons transit into the free volume and improve the annihilation rate 3. 3decreases because of 3=1/ 3. 3continually decreases because more elec-trons might locate the free volume.This is similar with Ref.[24],which suggests that two or more electrons can locate one vacancy.The concentration of electron at the free volume is saturated when t =30min due to Coulomb compel potential betweenelectrons.Fig.3.Positron lifetime parameters (a)left 1,right 3;(b)left I 1,right I 3;and (c)left AV ,right n e against different illumination time in the Sr 2FeMoO 6.The solid lines are guides for the eyes.Fig.3(b)shows I 1(left)and I 3(right)against different illu-mination time in the Sr 2FeMoO 6.I 1is found first to increase (0min ≤t ≤30min)and then decrease (t >30min)with a relative intensity of 85–88%,which is the main part of the positron lifetime spectra.This can be explained in light of the following scenario:electrons at the site of free annihilation is easier to active by illu-mination than at the free volume.When t =30min,electrons at the site of free annihilation is completely activated.When t >30min,electrons at the free volume is continually activated but at the site of free annihilation has already saturated,thus the intensity of 1decreases.Fig. 4.Observed (circles)and calculated (continuous line)XRD pattern for Sr 2FeMoO 6after 30min illumination,which is the representative XRD pattern for illuminated samples.The lowest curve is the difference between the observed and the calculated XRD patterns.The vertical bars at the bottom indicate the Bragg reflection positions.Y.C.Hu et al./Journal of Alloys and Compounds 492 (2010) 496–499499Table 1Unit cell parameters for Sr 2FeMoO 6after irradiation obtained from Rietveld refinement of XRD data.0min10min 20min 30min 40mina 5.58235(1) 5.58246(9) 5.58236(5) 5.58250(5) 5.58261(9)c7.87965(4)7.87968(8)7.87982(4)7.87985(6)7.87990(6)v245.55084(2)245.56228(3)245.55737(1)245.57068(5)245.58227(3)Rwp (%)10.0010.4110.289.9210.12Rp (%)7.267.596.866.767.41We have calculated the average positron lifetime AV ,defined as AV =I i i ,where I i is the relative intensity of the i th lifetime com-ponent. AV is better to describe the mean size of the defects in the samples.As shown in Fig.3(c)left, AV of the photo-doped is smaller than that of photo-free.According to Navarro et al.[25],Sr 2FeMoO 6may be unstable when heated,and that may have important oxida-tion effects if exposure it to air for several days.We measured the structure of the samples after PALS measurements.Fig.4shows the XRD pattern for Sr 2FeMoO 6after 30min illumination,which is the representative XRD pattern for illuminated samples.There is no trace of SrMoO 4is detected,due to that 40min might be a very short time for oxidation procedure.Structure parameters after irra-diation obtained from Rietveld refinement are shown in Table 1.The increase of unit cell makes the size of defects small.This might be important reason for decrease of average lifetime.Ions may absorb photons and occupy part of defects,thus also reduce the mean size of defects.We calculated the electron density n e ,defined asn e =1/(¯ r 20c ),where c is the speed of light and r 0is classical elec-tron radius.The variation of n e against illumination time is shown in Fig.3(c)right.The minimum value of AV suggests the maximum of electrons density when t =30min.4.ConclusionTo investigate the structure and photo-doping effect on defect in SFMO,we performed X-ray powder diffraction and the positrons annihilation technique.The result of X-ray powder diffraction sug-gests that the superstructure reflection (101)appearing due to the Fe/Mo order on the B/B site can index the concentration of anti-defect.Positron lifetime measurements after illumination suggest that the positron annihilation lifetime is sensitive to photo-doping.The minimum of defects size and the maximum of electrons den-sity when t =30min indicate that photo-doping may be an effective way to improve the property of the material.AcknowledgementsThis work is supported by NNSFC (10774065,10523001)and NKPBRC (2006CB921802,2010CB923404).Professor QFL thanks for the financial support from Henan Education foundation (No.2007140008).References[1]K.-I.Kobayashi,T.Kimura,H.Sawada,K.Terakura,Y.Tokura,Nature 395(1998)677.[2]A.Gary,Prinz,J.Magn.Magn.Mater.200(1999)57.[3]F.K.Patterson,C.W.Moeller,R.Ward,Inorg.Chem.2(1963)196.[4]F.S.Galasso,F.C.Douglas,R.J.Kasper,J.Chem.Phys.44(1966)1672.[5]D.Stoeffler,S.Colis,Mater.Sci.Eng.B 126(2006)133.[6]M.F.LÜ,J.P.Wang,J.F.Liu,W.Song,X.F.Hao,D.F.Zhou,X.J.Liu,Z.J.Wu,J.Meng,J.Alloy Compd.428(2007)214.[7]Z.Bajnok,Zs.Simon,Nucl.Phys.B 802(2008)307.[8]X.J.Hu,J.S.Ye,H.J.Liu,S.Mariazzi,R.S.Brusa,Thin Solid Films 516(2008)1699.[9]J.H.Hao,W.D.Si,X.X.Xi,Appl.Phys.Lett.76(2000)3100.[10]J.H.Hao,X.T.Zeng,H.K.Wong,J.Appl.Phys.79(1996)1810.[11]M.Idrish Miah,Mater.Chem.Phys.111(2008)249.[12]O.V.Prezhdo,Chem.Phys.Lett.460(2008)1.[13]B.H.Toby,J.Appl.Crystallogr.34(2001)210.[14]X.S.Wu,W.M.Chen,X.Jin,S.S.Jiang,Physica C 273(1996)99–106.[15]X.S.Wu,S.S.Jiang,N.Xu,F.M.Pan,X.R.Huang,W.Ji,Z.Q.Mao,G.J.Xu,Y.H.Zhang,Physica C 266(1996)296–302.[16]M.T.Anderson,K.B.Greenwood,G.A.Taylor,K.R.Poppelmeier,Prog.Solid StateChem.22(1993)197.[17]Ll.Balcells,J.Navarro,M.Bibes,A.Roig,B.Martínez,J.Fontcuberta,Appl.Phys.Lett.78(2001)781.[18]/xray/comp/scatfac.htm .[19]A.Guinier,X-ray Diffraction,W.H.Freeman and Company,San Francisco,1963.[20]Y.C.Hu,P.F.Wang,B.Lv,Q.Ji,X.S.Wu,Q.F.Lu,J.Appl.Phys.105(2009)07D726.[21]A.Ore,Univ.Bergen Arbok,Naturvet Rekke,1949,p.9.[22]O.E.Mogensen,J.Chem.Phys.60(1974)998.[23]W.Brandt,S.Berko,W.W.Walker,Phys.Rev.120(1960)1289.[24]P.Hautojarvt,Positrons in Solid,Springer-Verlag,Berlin,Heidelberg/New York,1979.[25]J.Navarro,C.Frontera,D.Rubi,N.Mestres,J.Fontcuberta,Mater.Res.Bull.38(2003)1477–1486.。
