咪唑二肽Imidazole dipeptide
日本首批声称抗疲劳功能食品_咪唑二肽_

中国食品学报2015年第8期made the strain R23in a high density and provide material base for the preparation of high -activity bacteria agent.Method :Cell biomass was used as the main index ,the culture conditions and media were optimized by orthogonal ex -periment.Result :The proper culture conditions for Lactobacillus plantarum R23were :30℃for temperature ,5.5for pH value ,6%for inoculum percent ;the suitable medium ingredient were :0.74%for yeast extract , 1.0%for beef extract ,0.5%for peptone , 2.0%for sucrose ,0.2%for ammonium citrate ,0.036%for MgSO 4,0.005%for MnSO 4,0.1%for Tween-80,2%for sodium malate ,5%for tomato juice ,5%for mushroom juice ,and 0.2NNa 2HPO 4-0.2NKH 2PO 4buffer salt was diluted four times ;Conclusion :Lactobacillus plantarum R23shorten the time to achieve logarithmic growth phase for 6hours ,and the highest biomass could be reached 1.27×1010cfu/mL ,which was 2.1times compared with the basic mediumKeywords Lactobacillus plantarum R23;culture media ;culture condition ;optimization ;biomass日本首批声称抗疲劳功能食品“咪唑二肽”据日媒报道,8月5日,日本预防医药有限公司把“咪唑二肽”重新打造成具有抗疲劳作用的功能食品,通过网络和部分店铺开始销售。
咪唑啉结构范文

咪唑啉结构范文咪唑啉(imidazoline)是一类化学化合物,具有咪唑环和啉环的结构。
咪唑啉化合物可分为两种结构:一种是含有一个咪唑环和一个啉环的联环结构,另一种是含有两个咪唑环和一个啉环的联环结构。
1.单咪唑啉结构:咪唑啉化合物的单咪唑啉结构含有一个咪唑环和一个啉环。
咪唑环是由两个氮原子和三个碳原子构成的五元环,三个碳原子上连着一个氢原子或取代基。
啉环由一个氮原子和四个碳原子构成的五元环,四个碳原子分别与两个氢原子或取代基相连。
这种结构的一个典型代表是咪唑胺(imidamine),其化学式为C3H5N3咪唑胺的分子式表明它是含有一个咪唑环和一个啉环的化合物。
咪唑环中的两个氮原子上分别连接着一个氢原子和一个氮原子取代基,啉环上的氮原子连接着两个碳原子和一个氢原子。
咪唑胺是一种具有强碱性的化合物,可作为配体与过渡金属形成配位化合物。
2.双咪唑啉结构:双咪唑啉结构是含有两个咪唑环和一个啉环的化合物。
双咪唑啉结构与单咪唑啉结构相似,只是其中一个碳原子上带有两个氢原子或取代基,而另一个碳原子上带有一个氢原子或取代基。
这种结构的一个代表是咪唑双胺(imidazolidine),其化学式为C3H6N4咪唑双胺的分子式表明它是含有两个咪唑环和一个啉环的化合物。
咪唑环中的两个氮原子上分别连接着一个氢原子和一个氮原子取代基,啉环上的氮原子连接着两个碳原子和两个氢原子。
咪唑双胺是一种具有稳定性的化合物,可用于制备其他咪唑啉类化合物。
咪唑啉化合物具有多种应用。
在医药领域,咪唑啉类化合物可用作抗高血压药物、降血糖药物、抗抑郁药物等。
在农业领域,咪唑啉类化合物可用作植物生长调节剂、杀虫剂等。
此外,咪唑啉类化合物还可用作染料、催化剂、表面活性剂等。
总结起来,咪唑啉是一类具有咪唑环和啉环的化合物,可分为单咪唑啉结构和双咪唑啉结构。
咪唑啉化合物广泛应用于医药、农业、化工等领域,并具有多种功能和应用。
288-32-4,咪唑,技术规格说明书(SDS)

产品技术规格说明书由上海创赛科技有限公司收集整理,仅作参考使用。
英文名称:
Glyoxaline
英文别名:
1H-Imidazole;IMZ;Glyoxaline;Imidazole Glyoxaline;Imidazole, ULTROL Grade;1,3-diazole;Imidazole [for Buffer];Imidazole Zone Refined (number of passes:30);Imidazole [for Fluorimetric Analysis];Imidazole;1,3-diaza-2,4-cyclopentadiene;1,3-Diaza-2,4-cyclopentadiene,Glyoxaline;1,3-imidazole;1-imidazole;glioksal;GLYOXALIN;IMD;Imidazoie;Imidazol;Imidazole buffer;IMINAZOLE;Imutex;LMIDAZOLE;Miazole;Miazole ;Pyrro[b]Monazole;1,3-Diazole
288-32-4|咪唑,技术规格说明书(SDS)
简介:
咪唑,Imidazole是一种平面五元环, 是一种高极性化合物, 已被广泛用作腐蚀抑制剂。
咪唑物理化学性质:
密度
1.1±0.1 g/cm3
沸点
257.0±9.0 °C at 760 mmHg
熔点
88-91 °C(lit.)
分子式
C3H4N2
分子量
测定:≥99% 生物技术级
tirzepatide分子量

tirzepatide分子量Tirzepatide是一种药物分子,具有非常重要的药物活性和治疗潜力。
它的分子式为C400H610N116O124S9,分子量约为9468.17克/摩尔。
Tirzepatide是一种人源胰岛素类似物(GLP-1和胰岛素双激素)。
它可以通过增加胰岛素分泌和抑制胰高血糖素分泌,帮助降低血糖水平,从而有效控制糖尿病。
此外,它还可以延缓胃肠道的排空速度,降低食欲,促进体重减轻。
Tirzepatide的研究始于2018年,开展的结果非常令人振奋。
根据多个临床试验结果显示,该药物在降低HbA1c(糖化血红蛋白)水平的有效性方面表现出色。
目前已经有多项研究证明,与其他药物相比,Tirzepatide在控制血糖、降低体重和改善心血管风险因素方面具有更好的效果。
一个重要的临床试验是SURPASS-4试验,该试验旨在评估Tirzepatide对2型糖尿病患者的治疗效果。
研究结果显示,与参比药物相比,Tirzepatide在降低HbA1c水平和体重方面表现出更显著的效果。
此外,Tirzepatide还显示出降低心血管风险和改善胰岛素抵抗等其他积极效果。
这些研究结果都证实了Tirzepatide的良好治疗潜力,对于那些需要更好控制血糖水平和减轻体重的糖尿病患者,这是一个积极的消息。
另外,Tirzepatide还在SURPASS-5, 6和7等多个研究中进行评估。
这些研究试图进一步探索Tirzepatide的治疗潜力,并评估其对特定人群,如肥胖症和心血管风险患者的效果。
虽然这些研究仍在进行中,但预期的结果将对Tirzepatide的临床应用和潜在的市场前景产生积极的影响。
总而言之,Tirzepatide是一种具有非常重要药物活性和治疗潜力的分子。
通过其双激素作用机制,Tirzepatide可以在糖尿病治疗中起到双重作用,降低血糖水平和体重,并改善心血管风险因素。
临床试验结果显示,Tirzepatide在降低HbA1c水平和体重方面表现出优良的效果。
咪唑二肽对
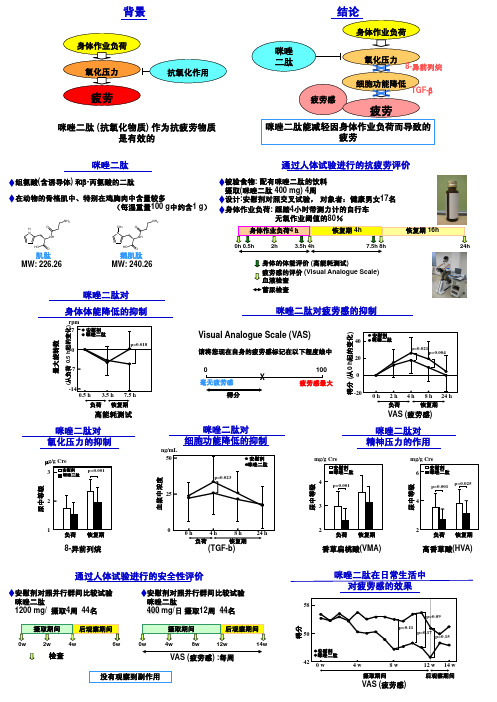
0
X
毫无疲劳感
得分
100 疲劳感最大
安慰剂
40 咪唑二肽
20
p=0.021 p=0.004
0
-20 0 h 2 h 4 h 8 h 24 h
负荷
恢复期
VAS (疲劳感)
ng/mL 50
咪唑二肽对 细胞功能降低的抑制
安慰剂 咪唑二肽
p=0.023
25
咪唑二肽对 精神压力的作用
mg/g Cre
安慰剂 咪唑二肽
rpm 7 安慰剂
咪唑二肽
p=0.018
0
-7
-14 0.5 h 3.5 h 7.5 h 负荷 恢复期
高能耗测试
咪唑二肽对 氧化压力的抑制
µg/g Cre
3
安慰剂 咪唑二肽
p=0.001
2
咪唑二肽对疲劳感的抑制
得分 (从0 h起的变化)
Visual Analogue Scale (VAS)
请将您现在自身的疲劳感标记在以下程度线中
摄取(咪唑二肽 400 mg) 4周
在动物的骨格肌中、特别在鸡胸肉中含量较多
设计:安慰剂对照交叉试验, 对象者:健康男女17名
(每湿重量100 g中约含1 g) 身体作业负荷: 踩踏4小时带测力计的自行车
NH2
O H N
NH
NH2
CH3
O
N NH
无氧作业阈值的80%
身体作业负荷4h
恢复期 4h
恢复期 16h
4 p<0.001
3
mg/g Cre
安慰剂
6 咪唑二肽
p=0.025 p=0.001
4
尿中等级 尿中等级
尿中等级 血浆中浓度
咪唑

咪唑 更多供应商 288-32-4 咪唑 价格性状咪唑纯品为白色结晶,工业品为微黄色晶体,m.p.88~89℃,b.p.255~256℃,易溶于水、乙醚和乙醇,呈强碱性。
所属类别农药中间体: 杀菌剂中间体: 三唑类杀菌剂 用途与作用咪唑是农药抑霉唑、咪鲜胺等杀菌剂的中间体,也是医药抗真菌药双氯苯咪唑、益康唑、酮康唑、克霉唑的中间体。
合成工艺与制法其制备方法是将乙二醛、甲醛、硫酸铵投入反应锅,搅拌加热至85~88℃,保温4h ,冷至50~60℃,用石灰水中和至pH =10以上,加热至85~90℃,排氨1h 以上,稍冷,过滤,滤饼用热水洗涤,合并洗滤液,减压浓缩至无水蒸出时,继续蒸馏至低沸物全部蒸完,收集105~160℃/133~266Pa 馏分得咪唑。
也可用邻苯二胺为原料,加入到甲酸中搅拌加热,在95~98℃保温2h ,降温到50~60℃,用10%NaOH 调节至pH =10,降至室温,过滤水洗,干燥得苯并咪唑。
在搅拌下将苯并咪唑投入浓硫酸,升温至100℃,慢慢滴入H 2O 2。
加毕,在140~150℃搅拌反应1h ,降温至40℃,加水稀释,析出结晶,过滤,水洗,干燥,得4,5-二羧基咪唑。
将4,5-二羧基咪唑与氧化铜混合,加热至100~280℃,放出大量二氧化碳气体,收集馏出液,即得白色块状物粗品,用苯重结晶得精品咪唑。
咪唑价格(试剂级)更新日期产品编号品牌产品信息CAS 号MDL 号规格与纯度 包装价格 库存信息操作2011/08/2330104916咪唑;间二氮茂;1,3-二氮杂茂 288-32-4AR (沪试) 100g60元详细2011/08/23K5675001咪唑;间二氮茂;1,3-二氮杂茂288-32-4Fluka Puriss100g 33元详细 2011/04/16I0001咪唑 Imidazole288-32-4MFCD00005183>98.0%(T) 25G 160元4 详细2011/04/16I0001咪唑 Imidazole288-32-4MFCD00005183>98.0%(T) 500G 1100元8 详细2011/04/16I0288咪唑[用于缓冲溶液]Imidazole [for Buffer] 288-32-4MFCD00005183>99.0%(GC )(T)25G405元1 详细2011/04/16I0288咪唑[用于缓冲溶液]Imidazole [for Buffer] 288-32-4MFCD00005183>99.0%(GC )(T)500G 5340元1 详细2011/04/16I0290咪唑[用于荧光法分析]Imidazole [for Fluorimetr ic Analysis] 288-32-4MFCD00005183>99.0%(GC )(T)25G810元1 详细2011/04/16I0014咪唑(区域精制法精制,熔段数:30)Imidazole Zone Refined (number ofpasses:30) 288-32-4MFCD00005183>99.8%(GC )1SAM PLE1280元1 详细2010/06/21122020020咪唑 Imidazole 99% 288-32-42 KG 2168元详细 2010/06/21122020050咪唑 Imidazole 99% 288-32-45 GR 134元详细2010/06/21396740025咪唑Imidazole , for analysis288-32-42.5 KG3027元详细ACS2010/06/21301872500咪唑Imidazole 99+%, crystallin e 288-32-4250 GR626元详细2010/06/21122021000咪唑 Imidazole 99% 288-32-4100 GR294元详细 2010/06/21122025000咪唑 Imidazole 99% 288-32-4500 GR977元详细2010/06/21396745000咪唑Imidazole , for analysis ACS 288-32-4500 GR766元详细2010/06/21396741000咪唑Imidazole , for analysis ACS 288-32-4100 GR270元详细2010/06/21301870025咪唑Imidazole 99+%, crystallin e 288-32-42.5 KG2634元详细2010/06/21301870010咪唑Imidazole 99+%, crystallin e288-32-41 KG 1772元详细2010/05/25A10221咪唑, 99%Imidazole, 99% 288-32-4MFCD00005183100g 265元详细 2010/05/25A10221咪唑, 99%Imidazole, 99%288-32-4MFCD000051832.5k g2772元详细2010/0 5/25A10221咪唑, 99%Imidazole,99%288-32-4MFCD00005183500g769元详细咪唑相关信息中文名称咪唑英文名称Imidazole中文别名1,3-二氮唑;间二氮茂;甘恶啉;IMZ;1,3-二氨杂环戊二烯;1,3-二氮杂环戊二烯CAS RN 288-32-4EINECS号206-019-2分子式C3H4N2分子量68.0773危险品标志C:Corrosive风险术语R22;R34;R63;安全术语S22;S26;S36/37/39;S45;物化性质性状无色棱形结晶,呈弱碱性。
咪唑啉结构范文

咪唑啉结构范文咪唑啉(imidazoline)是一种含有五元环的有机化合物,其分子式为C3H6N2、它是一种官能团,可以存在于化合物的结构中。
咪唑啉分子由两个氮原子和一个碳原子组成的五元环,其中一个氮原子与一个氢原子连接,另一个氮原子与一个碳氢基团连接。
咪唑啉结构如下所示:HH—C—HN#N咪唑啉是一种重要的药物分子结构单元,被广泛应用于药物和农药的合成中。
它具有较高的生物活性和化学稳定性,可以和其他官能团结合形成多样化的化合物。
咪唑啉结构还可以与金属离子形成配合物,形成一系列具有特定功能的金属配合物。
以下是咪唑啉结构的一些重要应用和化合物:1.α2-肾上腺素受体激动剂:咪唑啉类药物(例如克尼地尔、东蒙莫旦)是一类可以激活α2-肾上腺素受体的药物,具有镇静、降压和抗焦虑作用,可用于治疗高血压、焦虑和睡眠障碍等疾病。
2.除草剂:咪唑啉类化合物(例如草甘膦、恩奈吉)是一类有效的除草剂,可以抑制植物体内的氨基酰磷酸合酶,从而抑制植物生长和发育,达到除草的目的。
3.金属配合物:咪唑啉结构可以与各种金属离子形成配合物,形成稳定的金属配合物。
这些金属配合物具有不同的功能,如催化剂、生物活性剂和光敏剂等。
例如,咪唑啉和镍离子形成的配合物具有催化硫杂环化反应的活性,咪唑啉和铜离子形成的配合物具有抗菌和抗病毒活性。
4. 核酸化学:咪唑啉结构中的两个氮原子可以作为缺电子的底物参与与氧、环状双酯的偶氮烯反应,形成咪唑啉类似物(imidazole motif)。
这些咪唑啉类似物在核酸化学中具有重要的应用,可以用于合成核酸荧光探针、发光标记剂和DNA交联剂等。
总之,咪唑啉结构是一种重要的有机化合物结构单元,具有广泛的应用前景。
通过结合其他官能团和金属离子,可以生成多样化的化合物,并展现不同的物理和化学性质。
咪唑啉结构在药物、农药、金属配合物和核酸化学等领域都有重要的应用,对于促进科学研究和提高生物活性具有重要意义。
药物化学丨抗寄生虫药

药物化学丨抗寄生虫药结构分为五类(五个大标题)一、哌嗪类二、咪唑类(考纲要求)三、嘧啶类四、苯咪类五、三萜类和酚类1.盐酸左旋咪唑化学名:S-(-)6-苯基-2,3,5,6- 四氢咪唑并[2,1-b]噻唑盐酸盐S构型左旋体,作用高于外消旋体,毒副作用低性质:1.水溶液与氢氧化钠共沸,噻唑开坏,产生巯基,与亚硝酰生成红色2.叔氮原子可与氯化汞试液等生物沉淀剂反应广谱驱虫药和免疫调节作用合成前体毒性高2.阿苯达唑结构:苯并咪唑、含S侧链广谱高效的驱肠虫,致畸,孕妇小儿禁用3.甲苯咪唑三种晶型,A型无效结构:同阿苯达唑、苯甲酰侧链抗血吸虫病药及抗丝虫病药一、抗血吸虫病药临床用消旋体,左旋体疗效高化学名:2-(环己基甲酰基)-1,2,3,6,7,11b-六氢-4H-吡嗪并[2,1-a]异喹啉-4-酮广谱,对三种血吸虫均有效二、抗丝虫病药物化学名:4-甲基-N,N二乙基-1- 哌嗪甲酰胺枸橼酸二氢盐游离的乙胺嗪与钼酸胺加热有蓝色沉淀除抗丝虫、也可用于哮喘抗疟药抗疟药的结构分类(4个标题)一、喹啉醇类二、氨基喹啉类三、2,4-二氨基嘧啶类四、青蒿素类一、喹啉醇类1.二盐酸奎宁(1)喹啉和喹核碱环相连(2)四个手性碳,活性各不相同奎宁(3R, 4S, 8S, 9R)抗疟药(3)奎尼丁(3R, 4S, 8R, 9S)又是钠通道拮抗剂(4)大剂量,有金鸡钠反应2.本芴醇(新)化学名:α-(二正丁氨基甲基)-2,7-二氯-9-(对氯苯亚甲基)-4-芴甲醇恶性疟疾二、氨基喹啉类1.磷酸氯喹化学名N’,N’-二乙基-N4-(7-氯-4- 喹啉基)-1,4-戊二胺二磷酸盐(1)作用机制:插入疟原虫DNA双螺旋之间,形成复合物,影响DNA复制(2)一个手性碳,异构体的活性相同,但d-构型毒性低,临床用外消旋体(3)代谢物为N-去乙基氯喹,有活性(4)抗疟,还用于阿米巴、风湿关节炎、红斑狼疮2.磷酸伯氨喹化学名 N4-(6-甲氧基-8- 喹啉基)-1,4-戊二胺二磷酸盐(1)8-氨基喹啉衍生物(2)防止疟疾复发和传播(3)注射引起低血压,只能口服三、2,4-二氨基嘧啶类化学名:6-乙基-5-(4-氯苯基)-2,4-嘧啶二胺1.具弱碱性2.与碳酸钠炽灼后,水溶液显氯离子反应3.二氢叶酸还原酶抑制剂,用于预防疟疾和该酶抑制剂磺胺多辛复合制剂,双重作用4.作用持久,维持一周四、青蒿素类(天然产物来源)(一)青蒿素1.结构特点:(1)含倍半萜内酯结构(2)有含过氧键的七元环(3)10位羰基2.代谢产物:双氢青蒿素抗疟活性强3.构效关系:(1)内过氧化物对活性是必需的,且活性归于内过氧化物-缩酮-乙缩醛-内酯的结构(2)疏水基团的存在和过氧化桥的位置对活性重要(二)蒿甲醚。
咪唑啉结构范文

咪唑啉结构范文咪唑啉(imidazoline)是一类含有咪唑环和嗜水氨基基团(imidazolidine)的化合物。
它是一种重要的有机化合物,具有广泛的应用领域,包括药物、农药、染料和配体等。
下面将介绍咪唑啉的结构及其在不同领域的应用。
咪唑啉的结构式为C3H6N2,它由两个咪唑环(imidazole ring)和嗜水氨基基团组成。
咪唑环是一种五元环,由两个氮原子和三个碳原子组成。
嗜水氨基基团则由一个取代了一氢的五元环(imidazolidine ring)组成,该五元环由一个氮原子和四个碳原子组成。
咪唑啉的分子式为C7H12N2咪唑啉具有许多重要的生理活性。
例如,它可以作为药物分子的骨架结构,具有抗高血压、抗精神病、降血糖等作用。
咪唑啉类化合物还可以用作染料中的配体,可以通过配位键与金属离子结合,形成稳定的配合物。
此外,咪唑啉还可以用作有机合成中的中间体,用于合成其他有机化合物。
在药物领域,咪唑啉类化合物被广泛应用于抗高血压和降血糖药物的合成。
例如,咪唑啉衍生物克唑呋新(clonidine)和洛唑雅(lofexidine)是一类常用的降压药物,它们通过刺激咪唑啉受体,降低中枢神经系统的兴奋性,从而降低血压。
另外,咪唑啉类化合物也在抗精神病药物的研发中发挥重要作用。
例如,氟哌唑烷(fluparoxan)是一种抗精神病药物,它是一种咪唑啉类化合物,可以通过调节多巴胺和5-羟色胺系统的功能来治疗精神疾病。
在农药领域,咪唑啉类化合物也被用作杀虫剂和除草剂的原料。
例如,拉莫多汀(lamododine)是一种广谱杀虫剂,它是一种含有咪唑啉结构的化合物,可以通过调节昆虫神经系统的功能来杀死害虫。
此外,咪唑啉类化合物还可以用作有机合成中的催化剂,促进化学反应的进行。
例如,咪唑啉配合物可以被用作金属催化剂,催化碳-碳偶联反应、氧化反应或还原反应等重要的有机合成反应。
另外,咪唑啉类化合物还被广泛应用于染料和涂料领域。
咪唑的工作原理范文

咪唑的工作原理范文咪唑(Imidazole)是一种有机化合物,化学式为C3H4N2,在许多领域中都有广泛应用。
咪唑的工作原理涉及其分子结构和性质,以及其在生物化学、医药领域等的应用。
首先,咪唑是一种含有五元环的芳香化合物。
它由两个氮原子和三个碳原子组成,其中一个氮原子与两个邻近的碳原子形成共轭双键,从而使得咪唑呈现出共轭体系。
这种共轭体系使得咪唑具有良好的电子传递性能,利于其在化学反应中的活性。
同时,咪唑的杂环结构还赋予其很强的酸碱性。
其次,咪唑的酸碱性是其工作原理的重要基础。
咪唑分子中的氮原子有一个孤对电子,可以很容易地与电子亲和性较强的质子结合形成咪唑盐酸盐。
这种酸性可以在一定程度上改变化学反应的速率和产物的性质。
同时,咪唑也可以作为碱,在适当条件下接受质子形成中性咪唑分子,或作为酸碱催化剂参与反应。
咪唑在生物化学中的工作原理主要涉及其作为缓冲剂和金属离子配位剂的作用。
由于咪唑的酸碱性能和化学惰性,它被广泛应用于生物体系中作为缓冲剂。
当溶液的pH发生变化时,咪唑可以快速接受或释放质子,以维持溶液的稳定性。
此外,咪唑还具有与金属离子形成配位化合物的能力。
由于咪唑分子中的氮原子具有孤对电子,可以与金属原子上的空轨道发生配位键形成咪唑金属络合物。
这种络合物在生物体系中广泛参与酶的催化过程,影响酶的催化效率和特异性。
在医药领域,咪唑的工作原理体现在其作为药物的作用机制。
咪唑及其衍生物被广泛应用于抗真菌、抗肿瘤、抗病毒等药物的开发。
这些药物通过与靶分子发生特异性相互作用,从而调节靶分子的功能。
例如,抗真菌药物克霉唑通过与真菌细胞膜中的酵素系统相互作用,抑制其正常功能,从而达到抑制真菌生长的效果。
抗肿瘤药物乙撑咪唑则通过与肿瘤细胞中的DNA分子相互作用,干扰其复制和修复的过程,从而诱导肿瘤细胞死亡。
抗病毒药物阿昔洛韦则通过与病毒DNA链的合成酶相互作用,抑制病毒复制和扩增,达到抗病毒的效果。
综上所述,咪唑的工作原理主要包括其分子结构和性质以及在生物化学、医药领域中的应用。
咪唑化学结构式范文

咪唑化学结构式范文咪唑(Imidazole)是一种含有两个氮原子的五元杂环化合物,化学式为C3H4N2、咪唑具有许多重要的化学性质和广泛的应用,特别是在药物和农药合成中。
本文将详细介绍咪唑的化学结构式及其相关特性。
咪唑的化学结构式如下所示:H/H-N=N-C-N-H\H在咪唑的结构中,两个氮原子通过一个共轭的双键(N=N)连接起来。
咪唑分子中的氢原子可以与其他原子或官能团进行取代反应,形成各种各样的衍生物。
咪唑的分子式为C3H4N2,相对分子质量为68.075 g/mol。
咪唑是一种无色晶体,可在水中溶解,并具有特殊的气味。
咪唑是一种亲电性较强的环状五元杂环化合物。
它可以通过将氮原子上的一个质子转移给一个亲核试剂来表现出亲电性。
咪唑的环结构也使它可以轻易地参与共轭体系,形成共轭双键或共轭环。
这使得咪唑在许多有机合成反应中大显身手。
咪唑具有多种化学性质和反应,最常见的是质子化和去质子化反应。
当咪唑暴露在酸性条件下时,它会吸收质子形成质子化的咪唑离子(ImH+)。
通常情况下,质子会与较较接近的氮原子结合,而离子中的两个氮原子之间的共轭结构保持不变。
咪唑去质子化反应是质子化反应的逆过程。
当咪唑离子处于碱性环境中时,反应中的质子将被移除,还原为中性的咪唑分子。
咪唑还可以通过在氮原子上引入不同的取代基来形成多种衍生物。
对于咪唑衍生物,取代基的位置和类型将会影响其性质和反应性。
一些常见的咪唑衍生物包括甲基咪唑(methylimidazole),乙基咪唑(ethylimidazole)和丙基咪唑(propylimidazole)。
此外,咪唑还可以与许多有机分子发生配位反应,形成配位化合物。
这些配位化合物在许多药物和催化剂的合成中起着重要的作用。
总结起来,咪唑是一种具有重要化学性质和广泛应用的五元杂环化合物。
它的化学结构式为C3H4N2,由两个氮原子和一个共轭双键组成。
咪唑可以进行质子化和去质子化反应,并且可以通过在氮原子上引入不同的取代基形成各种衍生物。
咪唑

咪唑-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------【中文名称】咪唑【中文别称】甘恶啉;间二氮茂;咪唑;1,3-二氨杂环戊二烯;1,3-二氮杂茂;1,3-二氮杂-2,4-环戊二烯【英文名称】Imidazole【分子式】C3H4N2【相对分子量或原子量】68.08CAS号:288-32-4华越洋生物咪唑用途:用于抗新陈代谢,抗组织胺;用于配制缓冲液,pH值应在6.2-7.8之间。
外观:白色或浅黄色结晶纯度(%):>99.0熔点:88-92℃pH (5%,水)25℃:9.5-10.5溶解性:易溶于水、乙醇、乙醚和氯仿,微溶于苯。
储存:室温干燥保存性质与稳定性:呈弱碱性。
有毒,生产设备要密封,防止跑、冒、滴、漏。
操作人员应穿戴防护用具,避免直接接触本品。
有毒,对小鼠经口LD5018.80mg/kg。
注射LD50610mg/kg,其毒性及防护方法与乙二胺相似。
合成方法:由乙二醛经环合;中和而得。
将乙二醛、甲醛、硫酸铵投入反应锅,搅拌加热至85-88℃,保温4h。
冷至50-60℃,用石灰水中和至pH 为10以上。
加热至85-90℃,排氨1h以上,稍冷,过滤,滤饼用热水洗涤,合并洗;滤液,减压浓缩至无水蒸出时,继续减压蒸馏至低沸物全部蒸完,收集105-160℃(0.133-0.267kPa)馏分,得咪唑。
收率约45%。
另一种制法是使邻苯二胺与甲酸环合生成苯骈咪唑,再经双氧水反应开环为4,5-二羟基咪唑,最后脱羧制得咪唑。
4,5-二羟基咪唑也可由d-酒石酸经硝化、环合而得。
4,5-二羧基咪唑的脱羧制取咪唑的工艺过程如下:将4,5-二羟基咪唑与氧化铜混合,加热至100-280℃,放出大量二氧化碳气体,收集馏出液即得粗品,用苯重结晶得成品,收率76%。
咪唑二肽对

安慰剂对照并行群间比较试验 咪唑二肽 400 mg/日 摄取12周 44名
摄取期间
后观察期间
摄取期间
后观察期间
0w
2w
4w
检查
6w
0w
4w
8w
12w
14w
VAS (疲劳感) :毎周
没有观察到副作用
得分
咪唑二肽在日常生活中 对疲劳感的效果
58
50
安慰剂 咪唑二肽
42 0 w
p=0.09
p=0.11 p=0.17 p=0.15
4 p<0.001
3
mg/g Cre
安慰剂
6 咪唑二肽
p=0.025 p=0.001
4
尿中等级 尿中等级
尿中等级 血浆中浓度
1 负荷 恢复期
8-异前列烷
0
0h
4h
8h
24 h
负荷
恢复期
(TGF-b)
2 负荷 恢复期
香草扁桃酸(VMA)
2 负荷 恢复期
高香草酸(HVA)
通过人体试验进行的安全性评价
安慰剂对照并行群间比较试验 咪唑二肽 1200 mg/ 摄取4周 44名
背景
身体作业负荷 氧化压力
疲劳
抗氧化作用
咪唑二肽 (抗氧化物质) 作为抗疲劳物质 是有效的
结论
身体作业负荷
咪唑 二肽
氧化压力
8-异前列烷
疲劳感
细胞功能降低
TGF-β
疲劳
咪唑二肽能减轻因身体作业负荷而导致的 疲劳
咪唑二肽
通过人体试验进行的抗疲劳评价
组氨酸(含诱导体) 和β-丙氨酸的二肽
被验食物: 配有咪唑二肽的饮料
三苯基膦咪唑碘体系碘代反应

三苯基膦咪唑碘体系碘代反应三苯基膦、咪唑、碘体系进⾏碘代反应,三者⼀般1eq-1.5eq。
溶剂⽤的有:⼆氯甲烷,甲苯,四氢呋喃,⼄腈和⼄醚,反应的时候最好避避光,反应要求⽆⽔。
通常情况下反应温度低,反应时间短,产率⾼,另外有些底物是需要加热才能反应。
反应机理反应实例To dry dichloromethane (4 mL) was added in order: triphenylphosphine (1.2~1.5 mmol), imidazole (1.2~1.5 mmol) and iodine (1.2~1.5mmol). A solution of the alcohol (1.0mmol) in dry dichloromethane (1 mL) was added and the mixture was stirred at room temperature under argon for 0.5~3.0 h. The disappearance of the alcohol and formation of the alkyl iodide wasfollowed by TLC. When the reaction wascomplete, most of the solvent was removed in vacuo and the product waspurified by passing it through a column of silica gel with pentane as solvent.【Synthetic. Communications, 1990, 20, 10, 1473-1479】Iodine (2.72 g, 10.7 mmol) was added in portions to a mixture of the alcohol(1.00 g, 5.14 mmol, finelypowdered), triphenylphosphine (3.02 g, 11.5 mmol) and imidazole (1.59 g, 23.3 mmol) in toluene-acetonitrile (2:1 60 mL)stirred at 90o C. After 2h themixture was cooled to room temperature. Water (50 mL) and toluene (20 mL) were added to the mixture which wasthen shaken vigorously and transferred to a separating funnel. The organic phase was extracted with wateruntil only triphenylphosphine and triphenylphosphine oxide remained in theorganic phase. The combined aqueousphase was washed with a small amount of toluene and then concentrated. The residue was acetylated with aceticanhydride and pyridine at room temperature. When the acetylation was complete, the reaction mixture was concentratedand the residue dissolved in toluene. The toluene solution was extracted with water in water in order toremove imidazole impurities, dried and concentrated. The product was purified bychromatography ona short silica gel column to yield compound (1.41 g, 63%).【J. C.S., Perkin Trans. 1, 1982, 681-683】。
2024年咪唑市场发展现状

2024年咪唑市场发展现状简介咪唑(Imidazole)是一种含氮杂环化合物,具有广泛的应用领域,包括药物、农药、电子材料等。
本文将对咪唑市场的发展现状进行分析和总结。
咪唑的应用领域咪唑作为一种重要的中间体化合物,具有广泛的应用领域。
- 药物领域:咪唑及其衍生物具有抗菌、抗炎、抗肿瘤等活性,常被用于合成抗生素、抗真菌药物等。
- 农药领域:咪唑类化合物对多种害虫和杂草具有良好的防治效果,被广泛应用于化学农药的制备。
- 电子材料领域:咪唑类化合物可用于制备离子液体电解质、有机光电材料等,具有广阔的发展前景。
咪唑市场现状全球咪唑市场规模据市场研究机构统计数据显示,全球咪唑市场规模不断增长。
目前,全球咪唑市场年销售额超过10亿美元。
预计未来几年内,市场规模将进一步扩大。
咪唑市场主要地区北美地区北美地区是全球咪唑市场的主要消费地区之一。
该地区的制药和化工行业相对发达,对咪唑等中间体化合物的需求量较大。
欧洲地区欧洲地区是全球咪唑市场的另一个重要市场。
该地区的农药和电子材料制造业发达,对咪唑的需求量较高。
同时,欧洲对环境保护的重视也促进了咪唑替代传统化学农药的发展。
亚太地区亚太地区的经济发展迅速,对咪唑的需求量也在不断增加。
该地区的制药、农药和电子材料领域对咪唑的需求量较大。
咪唑市场发展趋势技术创新随着科学技术的进步,新合成方法和新催化剂的引入,咪唑的合成方法不断改进,合成效率和产率也得到了提高。
技术创新将进一步推动咪唑市场的发展。
环保需求近年来,环境问题引起了全球的关注,对环保型农药和电子材料的需求日益增长。
咪唑类化合物由于其较低的毒性和良好的生物可降解性,被视为一种环保替代品,将受到更多市场青睐。
新兴市场潜力发展中国家对于咪唑的需求也在不断增加。
由于这些国家的经济增长和科技进步,咪唑在医药、农业等领域的应用将会得到进一步拓展。
结论咪唑市场在全球范围内呈现出良好的发展势头。
随着技术创新和环保需求的推动,咪唑在药物、农药、电子材料等领域的应用前景广阔。
咪唑衍生物及其应用
咪唑衍生物及其应用摘要:本文介绍咪唑衍生物的分类、性能特点及其作为环氧树脂固化剂/促进剂的应用。
该研究为选择使用咪唑衍生物提供了一定的依据。
关键词:咪唑;衍生物;固化剂/促进剂;覆铜箔层压板(CCL);EMC一基础咪唑材料咪唑是一种五元杂环化合物(N NH),其结构特征为:在氮(杂)环戊二烯结构的间位上含有两个氮原子。
咪唑易于生成衍生物,被广泛用于环氧树脂固化(促进)剂、药剂、尿烷触媒、铜的防锈、炸药控制剂以及电解质等。
与同类的脂肪胺、芳香胺固化剂相比,咪唑具有毒性低、刺激性小的特点,是重要的基础化工材料。
咪唑的化学合成有腈类合成法和乙二醛合成法两条路线。
(1)腈类合成法H2NCH2CH2NH2 +R-CN N NH N NH (1)脱氢R(咪唑啉)(2)乙二醛合成法OHC-CHO+NH3+RCHO N N N NH (2)R式(1)(2)中,R为H或烷基及其它基团。
二咪唑衍生物咪唑易于生成衍生物,生成的途径有氰乙化反应、烷基取代反应、季胺化反应、卤代反应、羟甲基化反应和盐化作用等。
常用的咪唑衍生物是烷基取代作用后的生成物。
另外,还有氰乙化系列,氰乙化产物盐系列,氮杂苯系列,三聚异氰酸系列,羟甲基咪唑系列,咪唑啉系列等。
下面介绍一些咪唑衍生物的一般性能特点。
1 普通咪唑衍生物普通咪唑衍生物有二甲基咪唑(2MI)、二乙基四甲级咪唑(2E4MI)及二苯基咪唑(2PI )等。
该系列常在CCL、EMC和粉末涂料制造中用作固化(促进)剂,其特点有:固化速度快,固化温度低,分子量小,低量高效且价格便宜等。
具体性能见表.1。
2 氰乙化系列氰乙化系列(简写为CN)是咪唑氰乙化作用后的产物,适合于做酸酐促进剂,其熔点比较低,但要注意有释放丙烯腈的危险。
具体性能见表.2。
3 氰乙化产物盐系列氰乙化产物盐系列(简写为CNS)是氰乙化产物的二羧基苯羧酸盐。
该系列衍生物的特点有:室温下适用期长,其羧基能和环氧树脂反应,环境污染少等,被广泛用于粉末涂料。
笛柏试剂网:咪唑类

在 许 多 重 要 的 生 物 分 子 中 含 有 咪 唑 官 能 团 。最 常 见 的 是 含 有 咪 唑 侧 链 的 组 氨 酸 。组 氨 酸 出 现 在 许 多蛋白质和酶中, 也 是血红蛋白的重要 结 构组成,对其配位 能 力具有重要意义。 蛋 白质中的 His 残 基 可 以 扮 演 酸 碱 两 性 基 团 , 因 此 常 是 酶 中 发 生 酸 碱 催 化 机 理 的 部 分 ( 如 碳 酸 酐 酶 )。 组 氨 酸 可 发生脱羧生成组胺,这也是一种常见的生物分子,具有降低血压,收缩子宫等功能。细胞释放的 组胺常是导致荨麻疹的原因之一。 组氨酸生成组胺的反应如下所示:
目录价 ¥263.00 ¥737.00 ¥176.00 ¥493.00 ¥263.00 ¥737.00 ¥189.00 ¥529.00 ¥256.00 ¥717.00 ¥386.00 ¥1,351.00 ¥156.00 ¥468.00 ¥68.00 ¥238.00 ¥126.00 ¥441.00 ¥289.00 ¥1,012.00 ¥138.00 ¥483.00 ¥151.00 ¥423.00 ¥267.00 ¥534.00 ¥102.00 ¥204.00 ¥336.00 ¥1,176.00 ¥398.00 ¥1,393.00丙基咪唑 K204020
2-Isopropylimidazole
CAS 22884-10-2
530-62-1 153433-26-2
1122-28-7 121482
50257-40-4 101385-69-7 50257-39-1
693-98-1 443-48-1 822-36-6 1072-63-5 583-39-1 83857-96-9 3034-38-6 1739-84-0 36947-68-9
咪唑的性质和溶液配制知识讲解

咪唑的性质和溶液配制咪唑的性质名称:Imidazole; 1,3-diazacyclopenta-2,4-dieneCAS号:288-32-4Compound ID:795分子式: C3H4N2分子量:68.07726 [g/mol]H-Bond Donor: 1H-Bond Acceptor: 2pKa: 7.1熔点: 90-91℃密度:1.23 g/cm 3外观:白色固体,或淡黄色固体咪唑与不同二价阳离子结合力强弱:(括弧内是结合常数对数,该值越大结合力越强)Ca 2+ (0.1); Mn 2+ (1.6); Fe 2+ (3.3); Co 2+ (2.4); Ni 2+ (2.9); Cu 2+ (4.2); Zn 2+ (2.0)溶液配制:咪唑的水溶性很好,可达500mg/ml,溶液澄清。
可以用咪唑来配制缓冲液,其缓冲范围是pH6.2-7.8(25℃)。
稳定性及保存:咪唑溶液很稳定,可以高温灭菌处理。
2-8℃可保存两年。
注意,应当避光保存。
注意:咪唑本身在紫外波段下有光吸收!蛋白结晶用的缓冲液中是否应该除去咪唑?问:过完Ni亲和层析柱子之后,洗脱液(elute)中250mM(或500mM甚至1M)的咪唑是否应该出去?答:一般情况下,单纯过一个Ni柱是不够的,即便是样品足够纯,最好还是走一个凝胶过滤层析(分子筛)。
走分子筛的过程当中可以很方便将咪唑除去。
如果咪唑对于你样品蛋白的稳定性和均一性很重要,则可以考虑保留一定浓度的咪唑,一般来说20mM - 200mM是比较合理的浓度范围。
一些情况下,如果有咪唑存在,蛋白便不能结晶,这时就需要除去咪唑。
咪唑与蛋白之间的关系不能一概而论,不能说一定是什么样的,要看是什么蛋白。
例如,前面提到咪唑的合理浓度范围是20mM - 200mM,但Artem G. Evdokimov有一个晶体只能在0.6M咪唑和乙酸缓冲液中长出来,晶体解析出来以后发现每个蛋白分子上结合了两个咪唑分子。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Purification and characterization of a novel imidazole dipeptide synthasefrom the muscle of the Japanese eel Anguilla japonicaShiori Tsubone,Naoko Yoshikawa,Shigeru Okada,Hiroki Abe ⁎Department of Aquatic Bioscience,Graduate School of Agricultural and Life Sciences,The University of Tokyo,Bunkyo,Tokyo 113-8657,JapanReceived 14August 2006;received in revised form 5December 2006;accepted 6December 2006Available online 12December 2006AbstractWe have purified a novel enzyme from eel white muscle which catalyzes the syntheses of imidazole dipeptides,such as carnosine (β-alanyl-L -histidine),anserine (β-alanyl-π-methyl-L -histidine),and balenine (ophidine;β-alanyl-τ-methyl-L -histidine),directly from their precursors.The enzyme was purified 1130-fold from eel muscle by a series of column chromatographies.Although eel muscle contains a large amount of carnosine and only trace amounts of anserine and balenine,the anserine synthesizing activity was by far the highest.From gel permeation chromatography,the molecular mass of the enzyme was calculated to be 275kDa.SDS-PAGE of the purified enzyme represented a band around 43kDa,suggesting that the native enzyme is a hexamer or heptamer.The optimal pH and temperature were around 9.5and 60°C,respectively.K m values for β-alanine and π-methyl-L -histidine were 44and 89mM,respectively.The enzyme was greatly activated by Zn 2+and inhibited by EDTA.The N-terminal amino acid sequence of 25residues of the purified enzyme showed 52%amino acid identity to 38–62residues of zebrafish haptoglobin precursor.The purified enzyme also exhibited hydrolytic activity against these imidazole dipeptides.©2007Elsevier Inc.All rights reserved.Keywords:Imidazole dipeptides;Carnosine;Anserine;Balenine;Imidazole dipeptide synthase;Japanese eel;Muscle;Anguilla japonica1.IntroductionImidazole dipeptides in vertebrates,i.e.,carnosine (β-alanyl-L -histidine),anserine (β-alanyl-π-methyl-L -histidine),and bale-nine (ophidine;β-alanyl-τ-methyl-L -histidine),have long been known to exist in excitable tissues such as skeletal muscle and brain,and are proposed to have versatile physiological func-tions.Of these dipeptides,carnosine received special attention as a research target for functional analyses because of the availability of the reagent.Carnosine has been proven to work as a proton buffering constituent in fast-twitch white muscle (Abe et al.,1985;Abe and Okuma,1991;Abe,2000),as an antioxidant and scavenger for reactive oxygen species (Kohen et al.,1988;Boldyrev et al.,1989,2004;Boldyrev and Abe,1999),as a neurotransmitter or modulator (Margolis,1974;Bonfanti et al.,1999),as a neuroprotector (Hipkiss et al.,1997;Boldyrev et al.,2004),and as an anti-aging agent (Hipkiss,2000;Hipkiss et al.,2001).A large amount of carnosine is found in human muscle (10mM)and in sprinting mammals such as horses (34mM)and oxen (20mM)(Abe,1995).Although the distribution of carnosine in fish muscle is rather restricted,a high concentration of carnosine is resident in eel muscle (20mM).Anserine,on the other hand,is abundant in chicken pectoral muscle (43mM)and the white muscle of fast swimming fish such as tunas (27mM),skipjack tuna (51mM),and blue marlin (105mM).Copious concentrations of balenine is found only in whales (78mM)and snakes (13mM),although balenine has been found in trace amounts of all vertebrate muscles thus far examined (Abe,1995).Apparently,the distribution of these imidazole dipep-tides in vertebrate muscle is highly species specific and does not have any phylogenetic relationship.Although many researchers have focused on the physiolog-ical functions,information on the biosynthetic pathway and the constituent enzymes that catalyze formation of these dipeptides has remained deficient.Carnosine is known to be synthesized from β-alanine and L -histidine by carnosine synthetase (EC 6.3.2.11)in the presence of ATP,which has been characterized from chick pectoral muscle (Kalyankar and Meister,1959;Comparative Biochemistry and Physiology,Part B 146(2007)560–567/locate/cbpb⁎Corresponding author.Tel.:+81358415296;fax:+81358418166.E-mail address:aabe@mail.ecc.u-tokyo.ac.jp (H.Abe).1096-4959/$-see front matter ©2007Elsevier Inc.All rights reserved.doi:10.1016/j.cbpb.2006.12.002Winnick and Winnick,1959,1960;Stenesh and Winnick,1960; Bauer and Schulz,1994),mouse olfactory system(Harding and Margolis,1976),and rat and mouse brains(Skaper et al.,1973; Bauer et al.,1979,1982).No information,however,has been published on the primary structure of carnosine synthetase.Anserine,on the other hand,has been proposed to be syn-thesized by two different pathways.The first is the direct meth-ylation of carnosine catalyzed by carnosine-N-methyltransferase (EC2.1.1.22)in the presence of S-adenosyl-L-methionine,which was confirmed in chick pectoral muscle(McManus,1962;Bauer and Schulz,1994).The second pathway is the A TP-dependent condensation of the precursors,β-alanine andπ-methyl-L-histidine,by carnosine synthetase or some closely related enzyme (Winnick and Winnick,1959,1960;Stenesh and Winnick,1960).In contrast to the synthesis of imidazole dipeptides,their hydrolysis has been elucidated in more detail.Carnosine is hydrolyzed by carnosinases(Rosenberg,1960;Harding and Margolis,1976;Lenney,1976)which are classified into two groups,tissue carnosinase(cytosolic non-specific dipeptidase; EC3.4.13.18)and serum carnosinase(EC3.4.13.20),both of which belong to a large family of metalloproteases. Recently,cDNAs of human brain carnosinase and cytosolic non-specific dipeptidase were cloned and characterized(Teufel et al.,2003).Moreover,an anserine hydrolyzing enzyme, anserinase(EC3.4.13.5),was purified from the brain of Nile tilapia Oreochromis niloticus and its nucleotide and amino acid sequences were identified(Yamada et al.,2005).For these enigmatic imidazole dipeptides,their physiological functions and synthetic pathways,as well as the reasons for their species specific distribution remain unsolved.In this report,we describe the purification and characterization of a novel imidazole dipeptide synthase from the muscle of the Japanese eel Anguilla japonica,which catalyzes both the synthesis and hydrolysis of these three dipeptides.2.Materials and methods2.1.MaterialsLive Japanese eel(Anguilla japonica)were obtained from the Tokyo Metropolitan Central Wholesale Market(Tokyo, Japan).β-Alanine was purchased from Wako Pure Chemical Industries(Osaka,Japan).L-Histidine,π-methyl-L-histidine(1-methyl-L-histidine),carnosine,andγ-aminobutyric acid were from Sigma-Aldrich(St.Louis,MO,USA).τ-Methyl-L-histidine(3-methyl-L-histidine)was obtained from Bachem (Bubendorf,Switzerland).Anserine and balenine were prepared from the skeletal muscle of big-eye tuna Thunnus obesus and minke whale Balaenoptera acutorostrata,respectively(Abe and Okuma,1991).TSK-gel DEAE-Toyopearl650M and Ether-Toyoperl650M were obtained from Tosoh(Tokyo, Japan).His-Trap HP,MonoQ HR5/5,and Superdex20010/ 300were obtained from Amersham Biosciences(Uppsala, Sweden).Macro-prep Ceramic Hydroxyapatite Type1(CHT-1) column was from Bio-Rad Laboratories(Hercules,CA,USA). All other reagents were of analytical grade and purchased either from Sigma-Aldrich or Wako unless otherwise stated.2.2.Assay of imidazole dipeptide synthetic activitiesDipeptide synthetic activities were assayed by measuring the formation of products by high performance liquid chromatography(HPLC)according to the method of Togashi et al.(1998)with some modifications.The standard incubation mixture for the assay of anserine synthesis consisted of160mM π-methyl-L-histidine,250mMβ-alanine,and2mM ZnCl2in 50mM Tris–HCl,pH7.5.For carnosine or balenine synthesis,L-histidine orτ-methyl-L-histidine was used,respectively,instead ofπ-methyl-L-histidine.γ-Aminobutyric acid and L-histidine were used as substrates for homocarnosine synthesis.The enzyme preparation was added to the reagent mixture prepared on ice and incubated for1h at37°C unless otherwise described.The reaction was stopped by placing the tubes in a boiling water bath for3min followed by centrifugation at2300×g for3min.The supernatant was filtered with Ultrafree-MC(Millipore;Bedford,MA,USA) and used for HPLC analysis.For a time course experiment, aliquots of reaction mixture were taken out at appropriate time intervals(0to24h)and treated as above.The HPLC determination of dipeptides was carried out on a Jasco HPLC system(Jasco;Tokyo,Japan)with a Superiorex ODS column(φ4.6×250mm,Shiseido;Tokyo,Japan).Elution was performed isocratically with50mM KH2PO4,pH 3.4, containing6mM1-heptanesulfonic acid and2%acetonitrile at a flow rate of1mL/min at50°C and monitored at210nm.Protein concentration was measured with a protein assay dye solution(Bio-Rad Laboratories)using bovine serum albumin as a standard.During enzyme purification,protein elution was monitored by the absorbance at280nm using a micro plate reader,SpectraMax M2(Molecular Devices;Sunnyvale,CA, USA).2.3.Purification of imidazole dipeptide synthaseDuring the purification,the enzyme activity was checked for anserine synthesis.All purification procedures were performed at4°C.The white muscle of Japanese eel,weighing about 400g,was homogenized with5vol of50mM Tris–HCl,pH7.5, and the homogenate was centrifuged at12,000×g for15min.Ammonium sulfate was added to the supernatant up to50% saturation.After being stirred for2h,the suspension was centrifuged as above and the supernatant was brought to75% saturation with ammonium sulfate.The resultant precipitate (50–75%fraction)was dialyzed overnight against10mM Tris–HCl,pH8.0.After centrifugation as above,the supernatant was applied onto a DEAE-Toyopearl column(3×50cm)previously equilibrated with the same buffer,and the column was washed with500mL of the same buffer.The enzyme was eluted with a linear gradient of0−250mM NaCl at a flow rate of2mL/min. The active fractions were collected and ammonium sulfate was added to the solution at a final concentration of2M.This pooled solution was loaded onto an Ether-Toyopearl column(3×15cm)equilibrated with the same buffer containing 2M ammonium sulfate,and the column was washed with the same buffer.The enzyme was eluted with a linear gradient of2–0M ammonium sulfate in the same buffer at a flow rate of2mL/min.561S.Tsubone et al./Comparative Biochemistry and Physiology,Part B146(2007)560–567The pooled active fractions were desalted and concentrated by ultrafiltration using a Vivaspin concentrator (Vivascience;Stone-house,UK).The enzyme solution was subjected to a His-Trap 5mL column (1.6×2.5cm)previously equilibrated with 20mM phosphate buffer,pH 7.4,containing 500mM NaCl.From this step,purification was carried out on an automated liquid chromatography system,ÄKTA purifier 10/100(Amersham Bioscience).The protein was eluted with 10mM imidazole in the above phosphate buffer.The active fractions were applied onto a CHT-1column (7×52mm)equilibrated with 5mM phosphate buffer,pH 7.0,and then eluted with a linear gradient of 5–175mM phosphate buffer.The active fractions obtained were applied to a MonoQ HR5/5column (5×50mm)previously equilibrated with 10mM phosphate buffer,pH 8.0,and the protein was then eluted with a linear gradient of 0–300mM NaCl.The pooled active fractions were concentrated by ultrafiltration using Ultrafree-MC to approximately 100μL.Finally,this fraction was loaded onto a Superdex 20010/300column (1×30cm)and the enzyme was eluted with 50mM phosphate buffer,pH 7.2,containing 500mM NaCl at a flow rate of 0.4mL/min.The active fractions were collected and stored frozen as a final preparation.2.4.ElectrophoresisSodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)was carried out according to the method of Laemmli (1970),using 4.75%acrylamide in the stacking gel and 10%in the separating gel.Protein bands were visualized by staining with Coomassie Brilliant Blue R-250.2.5.N-terminal amino acid analysisThe purified enzyme was subjected to SDS-PAGE under reducing conditions,and the protein band on the gel was elec-trophoretically blotted onto a polyvinylidene difluoride (PVDF)membrane with a transfer buffer (20mM Tris and 190mM glycine with 20%methanol).The protein band was applied to a protein sequencer,Procise (Applied Biosystems;Foster,CA,USA).2.6.Determination of enzymatic properties of the purified imidazole dipeptide synthaseThe effect of pH on the enzyme activity for anserine synthesis was investigated over the pH range of 5–12at 37°C.The optimal temperature of the purified enzyme was determined by incubating the enzyme –substrate mixture in 50mM Tris –HCl,pH 7.5,for 1h on the heat block at various temperatures from 20to 80°C.The apparent K m and V max values for anserine synthesis against β-alanine and π-methyl-L -histidine were determined by Lineweaver –Burk plots using the substrate concentrations from 10to 250mM.Effects of metal ions on anserine synthetic activity were determined by incubating the reaction mixture containing β-alanine,π-methyl-L -histidine,and the purified enzyme solu-tion with 2to 10mM of metal ions or 1mM of EDTA.2.7.Enzymatic hydrolysis of imidazole dipeptides by the purified enzymeAs substrates,0.8mM carnosine,anserine,or balenine were individually incubated at 37°C with the purified enzyme preparation in 50mM Tris –HCl,pH 7.5,and the amount of remaining dipeptide was measured at each sampling time (0to 60min)by the above described HPLC method.Inhibitory effects on anserine hydrolyzing activity were determined by the incubation of 4mM anserine with the purified enzyme preparation and 2mM inhibitors including phenyl-methylsulfonyl fluoride (PMSF),p -chloromercuribenzoic acidTable 1Purification of imidazole dipeptide synthase from eel muscle PurificationProtein (mg)Total activity (μmol/min)Yield (%)Specific activity (nmol/min mg protein)Purification(fold)50–75%(NH 4)2SO 4558832.3100 5.831DEAE-Toyopearl 49316.751.533.8 5.80Ether-Toyopearl 145 2.537.8517.53.02His-Trap 8.5 1.92 5.9522739.2Hydroxyapatite 3.85 1.35 4.1835060.6MonoQ 0.94 1.43 4.451535265Superdex 2000.0780.521.5965671126Purification was performed based on the anserine syntheticactivity.Fig.1.SDS-PAGE patterns of each purification step of imidazole dipeptide synthase.M,molecular weight marker;lane 1,50–75%(NH 4)2SO 4fraction;lane 2,DEAE-Toyopearl;lane 3,Ether-Toyopearl;lane 4,His-Trap;lane 5,Hydroxyapatite;lane 6,MonoQ;lane 7,Superdex 200.562S.Tsubone et al./Comparative Biochemistry and Physiology,Part B 146(2007)560–567(PCMB),1,10-o -phenanthroline monohydrate (o -phenanthro-line),bestatin,and 10mM EDTA.3.Results3.1.Purification of imidazole dipeptide synthaseThe activity of imidazole dipeptide synthase could hardly be detected in the crude enzyme solution of eel white muscle butclearly detected in 50–75%ammonium sulfate fraction.Carno-sine was synthesized by the enzyme in this fraction from its constituents,β-alanine and L -histidine,with a specific activity of 0.52nmol/min d mg protein.Anserine and balenine were also synthesized from β-alanine and either π-methyl-L -histidine or τ-methyl-L -histidine,respectively.Specific activities for anse-rine and balenine syntheses were 5.8and 0.78nmol/min d mg protein,respectively.ATP was not required in these reactions.The purification of imidazole dipeptide synthase is summa-rized in Table 1.The enzyme was finally purified about 1130-fold with 1.6%yield from ammonium sulfate fraction by six steps of column chromatography.The specific activity for the anserine synthesis of the final preparation was 6.5μmol/min d mg protein.The specific activities for carnosine and balenine syntheses were 0.25and 0.53μmol/min .mg protein,respectively.The native molecular mass of the enzyme was estimated to be approximately 275kDa by the final Superdex gel permeation chromatography.The highest activity peak coincided with the largest protein peak.The major protein band of the purified enzyme preparation on SDS-PAGE gel showed an estimated molecular mass of approximately 43kDa (Fig.1).Although a minor smear band was also found on the SDS-PAGE gel,the final preparation was subjected to subsequent analyses without further purification.3.2.N-terminal amino acid sequenceThe amino acid sequence of the N-terminal region was determined on the main protein band of purified imidazole dipeptide synthase.The N-terminal amino acid sequence of 25residues was estimated to be LVGGSLTRNVPWQVLLQFSD-SVLYG.A homology search using the BLAST algorithm gave 52%amino acid identity to residues 38–62of the haptoglobin precursor from the zebrafish Danio rerio .The N-terminal amino acid sequence of 16residues of the smear band in Fig.1gave high homologies to some proteins which were not related to the metabolism of thedipeptides.Fig.2.Time course of the dipeptide syntheses by the purified imidazole dipeptide synthase.Amounts (mmol)synthesized per mg of protein for anserine (•),carnosine (▴),and balenine (○)were shown.Mean±SE for triplicatedeterminations.Fig.3.Effects of pH and temperature on anserine synthetic activity of imidazole dipeptide synthase.(A)Optimal pH.The activity was assayed in 50mM acetate (▪),50mM MES (•),50mM Tris –HCl (▴),and 500mM CAPS (□)buffers.(B)Optimal temperature.The reaction time is 1h at each temperature.Mean±SE for triplicate determinations.563S.Tsubone et al./Comparative Biochemistry and Physiology,Part B 146(2007)560–5673.3.Characteristics of purified imidazole dipeptide synthaseTime course analyses of the purified enzyme for carnosine, anserine,and balenine syntheses are depicted in Fig.2.The enzyme clearly shows time-dependent syntheses of these three imidazole dipeptides.After24h of incubation,carnosine, anserine,and balenine synthesized by the enzyme amounted to 0.2,2.4,and0.35mmol/mg protein,respectively.Anserine synthetic activity was by far the highest at each incubation time.The effects of pH on anserine synthetic activity of the purified enzyme are shown in Fig.3A.The optimal pH for anserine synthesis is around9.5.The activity decreases sharply below pH6and above pH9.5.The enzyme shows the highest activity of anserine synthesis at60°C(Fig.3B).A significant decrease of the activity was observed above 70°C.The apparent K m and V max values of the purified enzyme for anserine synthesis were determined by Lineweaver–Burk plots (Fig.4A and B).The apparent K m values againstβ-alanine and π-methyl-L-histidine were calculated to be44and89mM,respec-tively.The V max values forβ-alanine andπ-methyl-L-histidine were7.4and11μmol/min d mg protein,respectively.The effects of metal ions and EDTA are presented in Table2. Anserine synthesis is greatly activated by2mM Zn2+and Co2+, more than5-and4-fold,respectively.However,10mM of both metal ions largely reduced the activating effects on the enzyme. Mg2+and Mn2+ions gave a slight activating effect on the enzyme at each concentration.The activity was strongly inhibited to58%of the control by1mMEDTA.Fig.4.Lineweaver–Burk plots of imidazole dipeptide synthase againstβ-alanine(A)andπ-methyl-L-histidine(B).Mean±SE for triplicate determinations.Table2Effects of metal ions and EDTA on anserine synthetic activities of imidazole dipeptide synthaseCompound Concentration(mM)Relative activity(%) Control100±3.76Zn2+2525±23.410119±2.19Mg2+2112±2.7710123±5.72Mn2+2140±11.610140±31.4Co2+2423±7.4510196±13.7EDTA158.3±0.78Mean±SE for triplicatedeterminations.Fig.5.Time course of hydrolyses of dipeptides by the purified imidazole dipeptide synthase.As substrates,0.8mM each of anserine(•),carnosine(▴), and balenine(○)was used.Mean±SE for triplicate determinations.564S.Tsubone et al./Comparative Biochemistry and Physiology,Part B146(2007)560–5673.4.Enzymatic hydrolysis of imidazole dipeptides by the purified enzyme preparationCarnosine,anserine,and balenine were hydrolyzed to their constituents,β-alanine and L-histidine or its derivatives by the purified imidazole dipeptide synthase(Fig.5).The increase of the hydrolytic reaction products,L-histidine and its methyl derivatives,were confirmed on the same HPLC chromatograms (data not shown).Anserine was the most susceptible to hydrolysis and hydrolyzed completely within10-min incuba-tion,with a specific activity of1.95μmol/min d mg protein. Carnosine and balenine were also hydrolyzed although at a slower rate than anserine.Specific activities for carnosine and balenine hydrolysis were0.86and0.43μmol/min d mg protein, respectively.The inhibition of anserine hydrolytic activity by several protease inhibitors are shown in Table3.A metal chelator,o-phenanthroline,completely inhibited the anserine hydrolyzing activity at2mM,whereas the inhibitory effect of EDTA was not clear.The serine protease inhibitor,PMSF,gave a low inhibitory effect on the activity.Anserine hydrolytic activity was inhibited about70%by bestatin,a specific inhibitor of various amino-and di-peptidases including serum carnosinase.The cysteine protease inhibitor,PCMB,inhibited the anserine hydrolytic activity about50%.4.DiscussionThe enzyme purified from eel muscle in the present study catalyzed the synthesis of carnosine,anserine,and balenine in the absence of ATP.Watanabe and Konosu(1979)have demonstrated the incorporation of14C-histidine into carnosine in eel muscle24h after intramuscular injection of labeled histidine,but no enzymatic evidence for the dipeptide syntheses in fish has been obtained hitherto.Since the purified enzyme did not require ATP for dipeptide synthesis,the enzyme found in this study might be distinct from carnosine synthetase and may be a new type synthase.Unexpectedly,anserine synthetic activity was the highest of all activities for these dipeptides syntheses,although eel contains a large amount of carnosine and only trace amounts of anserine and balenine.Thus,the enzyme is thought to prefer π-methyl-L-histidine to L-histidine orτ-methyl-L-histidine.In addition to these three dipeptides,a weak activity for homo-carnosine(γ-aminobutyryl-L-histidine)synthesis was also detected (data not shown).The substrate affinity forγ-aminobutyric acid was supposed to be extremely lower than that forβ-alanine.From these data,it is possible that the enzyme catalyzes the syntheses of any of the three dipeptides depending on the availability of substrate,histidine or its methyl derivatives.Anserine synthetic activity was also found in50–75% ammonium sulfate fraction of the white muscle of big-eye tuna Thunnus obesus which contains a high-level of anserine(data not shown).We also detected balenine synthetic activity in the same fraction of the skeletal muscle of minke whale Balaenoptera acutorostrata which contains high concentrations of balenine, although the activity was very weak(data also not shown). Evidently,the imidazole dipeptide synthase distributes in many vertebrates having large amounts of the dipeptides in muscle.The molecular mass of the native enzyme was estimated to be approximately275kDa and that of subunit was to be43kDa (Fig.1),suggesting that the native enzyme is a hexamer or heptamer.Wood and Johnson(1981)reported that the purified carnosine synthetase from chick pectoral muscle was a homodimer composed of119kDa monomer.There is a clear difference between the chick carnosine synthetase and eel dipeptide synthase based upon their molecular size.As seen in Table2,anserine synthetic activity was greatly elevated by2mM Zn2+and inhibited by a metal chelator, EDTA.Therefore,the enzyme is believed to be divalent cation-dependent or a metalloenzyme.Carnosine synthetase in chick muscle is reported to require2mM Mg2+or Mn2+for optimal activity whereas higher concentrations are inhibitory(Stenesh and Winnick,1960).For rat brain enzyme,the maximal activity is attained with4to6mM Mg2+with equimolar amounts of ATP(Skaper et al.,1973).The purified enzyme showed a maximal pH at about9.5 (Fig.3A)which was different from that for carnosine synthetase, at pH7.5(Winnick and Winnick,1959).Surprisingly,the enzyme displays an optimal activity at a temperature of60°C which is higher than expected for fish enzymes.For anserine synthesis,K m values againstβ-alanine andπ-methyl-L-histidine was43.8and 88.5mM,respectively,suggesting the very low substrate affinity of the enzyme.π-Methyl-L-histidine exists only in trace amounts in eel muscle whereas L-histidine is much higher,around1mM (Abe,1983).Thus,it remains unclear whether these dipeptides are synthesized by this enzyme in eel muscle under low substrate concentrations and high K m values.However,the rate of incorporation of labeled L-histidine into carnosine and anserine has been reported to be very low in rainbow trout(Abe and Hochachka,1987)and skipjack tuna(Abe et al.,1986).The N-terminal amino acid sequence of the purified imidazole dipeptide synthase showed52%amino acid identity to the partial sequence of haptoglobin precursor of zebrafish. Haptoglobin is a plasma glycoprotein known to have an ability to bind hemoglobin released on hemolysis and protect the tissues from oxidative damage(Wicher and Fries,2004).Human haptoglobin is composed of two short and two long polypep-tides,α-andβ-chains,respectively,which are synthesized as a single precursor polypeptide and processed post-translationally (Raugei et al.,1983;Yang et al.,1983).Theβ-chain is related toTable3Effects of inhibitors on anserine hydrolytic activity by the purified enzyme preparationCompound Concentration(mM)Relative activity(%) Control100±0.1EDTA1089.4±14.7o-Phenanthroline20±1.58PMSF281.2±0.69PCMB247.9±1.58 Bestatin229.7±3.24Mean±SE for triplicate determinations.o-Phenanthroline,1,10-o-phenanthro-line;PMSF,phenylmethylsulfonyl fluoride;PCMB,p-chloromercuribenzoic acid.565S.Tsubone et al./Comparative Biochemistry and Physiology,Part B146(2007)560–567trypsine-like serine protease but does not possess the essential catalytic amino acid residues(Kurosky et al.,1980).It is suggested that haptoglobin is evolutionarily related to serine proteases(Barnett et al.,1970,1972;Kurosky et al.,1980).Thus,we predicted that imidazole dipeptide synthase purified in the present study was a kind of protease and could hydrolyze dipeptides.To solve the question,the hydrolytic activity of purified enzyme against these three dipeptides was examined. These dipeptides were all hydrolyzed by the purified preparation (Fig.5).Anserine was hydrolyzed markedly,and carnosine and balenine were also hydrolyzed with a slower rate than anserine, as was seen in the order of synthetic activity.Thus,we propose that one enzyme catalyzes both the synthesis and hydrolysis of imidazole dipeptides.However,the N-terminal amino acid sequence showed no similarity to human carnosinase,cytosolic non-specific dipeptidase(Teufel et al.,2003),or Nile tilapia anserinase(Yamada et al.,2005).The mechanisms of dipeptide synthesis without ATP remain unclear.However,one possibility is that proteases are known to catalyze the reverse condensation reaction from amino acids leading to peptides which are called plastein(Watanabe and Arai,1992).More detailed research is required to unambiguously ascertain the mechanisms of dipeptide synthesis and hydrolysis.As seen in Table3,the hydrolytic activity was inhibited completely by a metal chelator,o-phenanthroline,and about 70%by bestatin,a specific inhibitor of various amino-and di-peptidases,and50%by PCMB for cysteine protease.From these data,the enzyme was thought to be at least a metalloprotease-like enzyme.It is not clear how the enzyme regulates the synthesis and hydrolysis of imidazole dipeptides in eel muscle. One possibility is that dipeptide synthase is located in some subcellular organelle other than cytosol where dipeptides exist in large amount.Due to the scarce amount of the purified enzyme obtained,further experiments could not be performed on the properties of hydrolyzing activity.In conclusion,we purified for the first time a novel enzyme from eel white muscle,which synthesizes and hydrolyzes imidazole dipeptides.Further studies are needed to clarify the enzyme kinetics for both synthesis and hydrolysis of dipeptides, as well as the regulatory and structural characteristics of the enzyme.Additional research is also required to explain the distribution of the enzyme in various vertebrates containing these dipeptides in large amount.These lines of research may pave the way for understanding the reasons for the species specific distribution of these imidazole dipeptides in vertebrates. ReferencesAbe,H.,1983.Distribution of free L-histidine and related dipeptides in the muscle of fresh-water p.Biochem.Physiol.B76,35–39. Abe,H.,1995.Histidine-related dipeptides:distribution,metabolism,and physiological function.In:Hochachka,P.W.,Mommsen,T.P.(Eds.), Biochemistry and Molecular Biology of Fishes.Metabolic Biochemistry, vol.4.Elsevier,Amsterdam,pp.309–333.Abe,H.,2000.Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle.Biochemistry(Mosc.)65,757–765. Abe,H.,Hochachka,P.W.,1987.Turnover of14C-labelled L-histidine and its incorporation into carnosine and anserine in rainbow trout.Nippon Suisan Gakkaishi53,1089–1094.Abe,H.,Okuma,E.,1991.Effect of temperature on the buffering capacities of histidine-related compounds and fish skeletal muscle.Nippon Suisan Gakkaishi57,2101–2107.Abe,H.,Dobson,G.P.,Hoeger,U.,Parkhouse,W.S.,1985.Role of histidine-related compounds to intracellular buffering in fish skeletal muscle.Am.J.Physiol.249,R449–R454.Abe,H.,Brill,R.W.,Hochachka,P.W.,1986.Metabolism of L-histidine, carnosine,and anserine in skipjack tuna.Physiol.Zool.59,439–450. Barnett,D.R.,Lee,T.-H.,Bowman,B.H.,1970.Amino-acid sequence of the carboxyl terminal octapeptide of human haptoglobinβchain.Nature225, 938–939.Barnett,D.R.,Lee,T.-H.,Bowman,B.H.,1972.Amino acid sequence of the human haptoglobinβ-chain.1.Amino-and carboxyl-terminal sequences.Biochemistry11,1189–1194.Bauer,K.,Schulz,M.,1994.Biosynthesis of carnosine and related peptides by skeletal muscle cells in primary culture.Eur.J.Biochem.219,43–47. Bauer,K.,Salnikow,J.,de Vitry,F.,Tixier-Vidal,A.,Kleinkauf,H.,1979.Characterization and biosynthesis ofω-aminoacyl amino acids from rat brain and the C-6glioma cell line.J.Biol.Chem.254,6402–6407. Bauer,K.,Hallermayer,K.,Salnikow,J.,Kleinkauf,H.,Hamprecht,B.,1982.Biosynthesis of carnosine and related peptides by glial cells in primary culture.J.Biol.Chem.257,3593–3597.Boldyrev, A.,Abe,H.,1999.Metabolic transformation of neuropeptide carnosine modifies its biological activity.Cell.Mol.Neurobiol.19, 163–175.Boldyrev,A.A.,Dupin,A.M.,Batrukova,M.A.,Bavykina,N.I.,Korshunova,G.A.,Shvachkin,Y.P.,1989.A comparative study of synthetic carnosineanalogs as p.Biochem.Physiol.B94,237–240. Boldyrev,A.,Bulygina,E.,Leinsoo,T.,Petrushanko,I.,Tsubone,S.,Abe,H., 2004.Protection of neuronal cells against reactive oxygen species by carnosine and related p.Biochem.Physiol.B137,81–88. Bonfanti,L.,Peretto,P.,De Marchis,S.,Fasolo,A.,1999.Carnosine-related dipeptides in the mammalian brain.Prog.Neurobiol.59,333–353. Harding,J.,Margolis,F.L.,1976.Denervation in the primary olfactory pathway of mice.3.Effect on enzymes of carnosine metabolism.Brain Res.110, 351–360.Hipkiss, A.R.,2000.Carnosine and protein carbonyl groups:a possible relationship.Biochemistry(Mosc.)65,771–778.Hipkiss,A.R.,Preston,J.E.,Himswoth,D.T.M.,Worthington,V.C.,Abbot,N.J., 1997.Protective effects of carnosine against malondialdehyde-induced toxicity towards cultured rat brain endothelial cells.Neurosci.Lett.238, 135–138.Hipkiss,A.R.,Brownson,C.,Carrier,M.J.,2001.Carnosine,the anti-ageing, anti-oxidant dipeptide,may react with protein carbonyl groups.Mech.Ageing Dev.122,1431–1445.Kalyankar,G.D.,Meister,A.,1959.Enzymatic synthesis of carnosine and relatedβ-alanyl andγ-aminobutyryl peptides.J.Biol.Chem.234, 3210–3218.Kohen,R.,Yamamoto,Y.,Cundy,K.C.,Ames,B.N.,1988.Antioxidant activity of carnosine,homocarnosine,and anserine present in muscle and brain.Proc.Natl.Acad.Sci.U.S.A.85,3175–3179.Kurosky,A.,Barnett,D.R.,Lee,T.-H.,Touchstone,B.,Hay,R.E.,Arnott,M.S., Bowman,B.H,Fitch,W.M.,1980.Covalent structure of human haptoglobin:a serine protease homolog.Proc.Natl.Acad.Sci.U.S.A.77,3388–3392. Laemmli,U.K.,1970.Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature227,680–685.Lenney,J.F.,1976.Specificity and distribution of mammalian carnosinase.Biochim.Biophys.Acta429,214–219.Margolis,F.L.,1974.Carnosine in the primary olfactory pathway.Science184, 909–911.McManus,I.R.,1962.Enzymatic synthesis of anserine in skeletal muscle by N-methylation of carnosine.J.Biol.Chem.237,1207–1211.Raugei,G.,Bensi,G.,Colantuoni,V.,Romano,V.,Santoro,C.,Costanzo,F., Cortese,R.,1983.Sequence of human haptoglobin cDNA:evidence that the αandβsubunits are coded by the same mRNA.Nucleic Acids Res.11, 5811–5819.Rosenberg,A.,1960.Purification and some properties of carnosinase of swine kidney.Arch.Biochem.Biophys.88,83–93.566S.Tsubone et al./Comparative Biochemistry and Physiology,Part B146(2007)560–567。
