Electrochemical behaviour of a Mg-rich primer in the protection of Al alloys
二氧化碳电催化英文文献

二氧化碳电催化英文文献1.In situ Probing the Electrochemical Performance of Nanoscale Amorphous Ni-Fe Oxides for CO2 ReductionAbstractCO2 electroreduction into liquid chemical products is a carbon-neutral technology that converts a non-renewable carbon resource into renewable chemicals, offering promising opportunities for chemical production and for sustainability. Materials that could efficiently catalyze the electrochemical conversion of CO2 are highly attractive, yet few studies have investigated the intrinsic behaviour of catalysts during electrolysis with in situ methods. Here, we use a multitechnique approach (in situ spectroelectrochemistry, impedance spectroscopy,X-ray photoelectron spectroscopy, and optical-grade cryofixation) to track the electrochemical behaviour of amorphous Ni-Fe oxides down to nanometer scale during CO2 electrolysis. The results unambiguously confirm that under CO2 electrolysis the electrochemical performance of amorphous Ni-Fe oxides, down to 6 nm crystallite size, does not appreciably depend on the particle morphology or on the crystallite size. Such findings are a must for the development of CO2 electrolysisreactors that require nanostructured materials with improved performance.2.Catalytic oxidation of formaldehyde to CO2 and H2O oxidation on Iridium oxide-based thin filmsAbstractThe catalytic oxidation of formaldehyde to CO2 and H2O on thin films of mainly Iridium oxide-based catalysts with composition IrOx/CeO2 and reinforcements of TiO2 and La2O3 is studied. Samples with Ir/Ce molar ratios equal to 1, 0.75, and 0.5 are studied. The films were synthesised by pulsed laser deposition on aluminosilicate substrates. The FBRM particle size distributions obtained by laser diffraction showed particles between 10 and 250 nm, in all the films. The BET surface area measured were 22, 26 and 36 m2 /g for Ir 1, Ir 0.75 and Ir 0.5, respectively. The XRD showed that the films were only composed by amorphous around Iridium, for the different molar ratios. The catalytic performance was evaluated in a fixed-bed flow reactor and carbon dioxide and water vapour selectivitiesobtained with Ir 0.75 as high as 100%. Temporal stability tests showed fast and monotonic deterioration in the activity of the Ir 1, while the Ir 0.75 showed the highest stability on 60 h of operation. These results are promising results for the development of thin film-catalysts for the fine chemicals industry.。
有机化学方面的专业英语(改过)
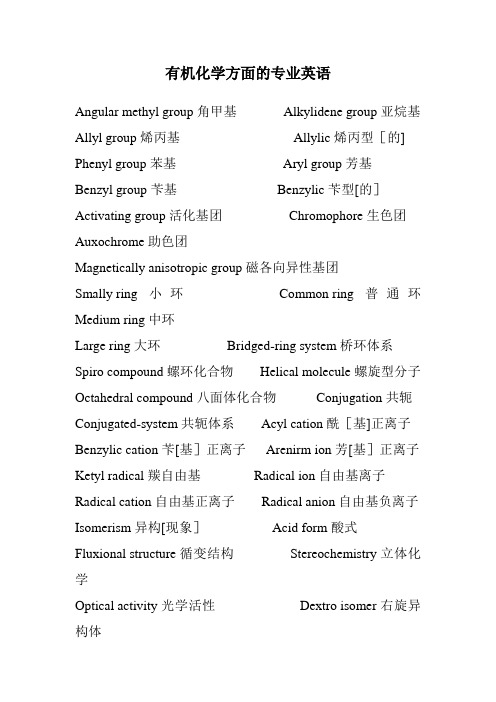
有机化学方面的专业英语Angular methyl group 角甲基Alkylidene group 亚烷基Allyl group 烯丙基Allylic 烯丙型[的] Phenyl group 苯基Aryl group 芳基Benzyl group 苄基Benzylic 苄型[的]Activating group 活化基团Chromophore 生色团Auxochrome 助色团Magnetically anisotropic group 磁各向异性基团Smally ring 小环Common ring 普通环Medium ring 中环Large ring 大环Bridged-ring system 桥环体系Spiro compound 螺环化合物Helical molecule 螺旋型分子Octahedral compound 八面体化合物Conjugation 共轭Conjugated-system 共轭体系Acyl cation 酰[基]正离子Benzylic cation 苄[基]正离子Arenirm ion 芳[基]正离子Ketyl radical 羰自由基Radical ion 自由基离子Radical cation 自由基正离子Radical anion 自由基负离子Isomerism 异构[现象]Acid form 酸式Fluxional structure 循变结构Stereochemistry 立体化学Optical activity 光学活性Dextro isomer 右旋异构体Laevo isomer 左旋异构体Tetrahedral configuration 四面体构型Stereoisomerism 立体异构[现象] Asymmetric atom 不对称原子Asymmetric carbon 不对称碳Pseudoasymmetric carbon 假不对称碳Phantom atom 虚拟原子Homotopic 等位[的] Heterotopic 异位[的]Enantiotopic 对映异位[腯Diastereotopic 非对映异位[的] Configuration 构型Absolute configuration 绝对构型Chiral 手性[的]Chiral center 手性中心Chiral molecule 手性分子Achiral 非手性[的]Fischer projection 费歇尔投影式Neoman projection 纽曼投影式D—L system of nomenclature D—L命名体系R—S syytem of nomenclature R-S命名体系Cahn-Ingold-Prelon sequence 顺序规则Symmetry factor 对称因素Plane of symmetry 对称面Mirror symmetry 镜面对称Enantiomer 对映[异构]体Diastereomer 非对映[异构]体Epimer 差向异构体Anomer 端基[差向]异构体Erythro configuration 赤型构型Erythro isomer 赤型异构体Threo configuration 苏型构型Threo isomer 苏型异构体Trigonal carbon 三角型碳Cis—trans isomerism 顺反异构 E isomer E异构体Z isomer Z异构体Endo isomer 内型异构体Exo isomer 外型异构体Prochirality 前手性Pro-R group 前R基团Pro—S proup 前S基团Re face Re面Si face Si面Racemic mixture 外消旋混合物Racemic compound 外消旋化合物Racemic solid solution 外消旋固体溶液Meso compound 内消旋化合物Quasi racemate 准外消旋体Conformation 构象Conformational 构象分析Torsion angle 扭转角Rotamer 旋转异构体Anti conformation 反式构象Bisecting conformation 等分构象Eclipsed conformation 重叠构象Gauche conformation, skew con—formation 邻位交叉构象Staggered conformation 对位交叉构象Steric effect 空间效应Steric hindrance 位阻Atropisomer 阻转异构体Puckered ring 折叠环Conformational inversion 构象反转Chair conformation 椅型构象Boat conformation 船型构象Twist conformation 扭型构象Skew boat conformation 扭船型构象Half—chair conformation 半椅型构象Pseudorotation 假旋转Envelope conformation 信封[型]构象Axial bond 直[立]键Equatorial bond 平[伏]键Cisoid conformation 顺向构象Transoid conformation 反向构象Retention of configuration 构型保持Regioselectivity 区域选择性Regiospecificity 区域专一性Stereoselectivity 立体选择性Stereospecificty 立体专一性Conformer 构象异构体Conformational effect 构象效应Stereochemical orientation 立体[化学]取向Conformational transmission 构象传递Homolog 同系物Ipso position 本位Ortho position 邻位Meta position 间位Para position 对位Trigonal hybridization 三角杂化Molecular orbiral method 分子轨道法Valence bond method 价键法Delocalezed bond 离域键Cross conjugation 交叉共轭Vinylog 插烯物Mesomeric effect 中介效应Resonance 共振Resonance effect 共振效应Hyperconjugation 超共轭Isovalent hyperconjugation 等价超共轭No—bond resonance 无键共振Aromaticity 芳香性Aromatic sexter 芳香六隅Huckel’rule休克尔规则Paramagnetic ring current 顺磁环电流Diamagnetic ring cruuent 抗磁环电流Homoaromaticity 同芳香性Antiaromaticity 反芳香性Alternant hydrocarbon 交替烃Non—alternant hydrocarbon 非交替烷Pericyclic reaction 周环反应Electrocyclic rearrangement 电环[化]重排Conrotatory 顺旋Disroatatory 对旋Cycloaddition 环加成Symmetry forbidden-reaction 对称禁阻反应Synfacial reaction 同面反应Antarafacial reaction 异面反应Lewis structure 路易斯结构Coordinate-covalent bond 配位共价键Banana bond 香蕉键Pauling electronegativity scale 鲍林电负性标度Polarizability 可极化性Inductive effect 诱导效应Field effect 场效应Electrical effect 电场效应tautomerism 互变异构Tautomerization 互变异构化Keto-enol tautomerism 酮-烯醇互变异构Phenol-keto tautomerism 酚—酮互变异构Imine—enamine atutomerism 亚胺-烯胺互变异构Ring—chain tautomerism 环-链互变异构Valence tautomerism 价互变异构Solvent effect 溶剂Ambident 两可[的]效应Acid—base catalyzed reaction 酸—碱催化反应Basic solvent 碱性溶剂Dielectric constant 介电常数Solvated electron 溶剂化电子Acid—base catalyzed reaction 酸碱催化反应Conjugated acid 共轭酸Conjugated base 共轭碱Thermodynamic acidity 热力学酸度Kinetic acidity 动力学酸度Electron donator—acceptor complex,EDA complex 电子给[体]受体络合物Host 主体Guest 客体Primary isotope effect 一级同位素效应Secondary isotope effect 二级同位数效应Inverse isotope effect 逆同位素效应Kinetic control 动力学控制Thermodynamic control 热力学控制Substrate 底物Intermediate 中间体Reactive intermediate 活泼中间体Microscopic reversibility 微观可逆性Hammond postulate 哈蒙德假说Linear free energy 线性自由能Non-bonded interaction 非键相互作用Torsional effect 扭转效应Restricted rotation 阻碍旋转Eclipsing effect 重叠效应Eclipsing strain 重叠张力Small-angle strain 小角张力Large angle strain 大角张力Transannular interaction 跨环相互作用Transannular strain 跨环张力Anomeric effect 端基异构效应Walden inversion 瓦尔登反转Racemization 外消旋化Isoinversion 等反转Isoracemization 等消旋Homochiral 纯手性[的]Mechanism 机理Unimolecular nucleophilic substitution单分子亲核取代Bimolecular nucleophilic substitution 双分子亲核取代Bimolecular nucleophilic substitution(with allylic rearrangement ) 双分子亲核取代(含烯丙型重排)Internal nucleophilic substitution 分子内亲核取代Aromatic nucleophilic substitution 芳香亲核取代Unimolecular electrophilic substitution 单分子亲电取代Bimolecular electrophilic substitution 双分子亲电取代Nucleophile—assisted unimolecular electrophilic substitution 亲核体协助单分子亲电取代Unimolecular elimination 单分子消除Bimolecular elimination 双分子消除Unimolecular elimination through the conjugated base 单分子共轭碱消除Bimolecular elimination through the conjugated base 双分子共轭碱消除Bimolecular elimination with formation of a carbonyl group 双分子消除形成羰基Unimolecular acid—catalyzed acyl-oxygen cleavage 单分子酸催化酰氧断裂Bimolecular base—catalyzed acyl-oxygen cleavage 双分子碱催化酰氧断裂Unimolecular acid—catalyzed alkyl-oxygen cleavage 单分子酸催化烷氧断裂Bimolecular base—catalyzed alkyl-oxygen cleavage 双分子碱催化烷氧断裂π-allyl complex mechanism π烯丙型络合机理Borderline mechanism 边理机理Homolysis 均裂Heterolysis 异裂Heterolytic mechanism 异裂机理Counter ion 反荷离子Ion pair 离子对Carbocation 碳正离子Nonclassical carbocation 非经典碳正离子Carbanion 碳负离子Masked carbanion 掩蔽碳负离子Carbenoid 卡宾体Carbene 卡宾Nitrene 氮宾Carbine 碳炔Electrophilic addition 亲电加成Electrophile 亲电体Diaxial addition 双直键加成Ma rkovnikov’s rule 马尔科夫尼科规则Anti—Markovnikov addition 反马氏加成Michael addition 迈克尔加成Substitution 取代Electrophilic substitution 亲电取代Addition-elimination mechanism 加成消除机理Electrophilic aromatic substitution 亲电芳香取代Electron transfer 电子转移Electron-donating group 给电子基团Electron—withdrawing group 吸电子基团Deactivating group 钝化基团Orinentation 取向Ortho—para directing group 邻对位定位基Meta directing group 间位定位基Ortho effect 邻位效应Partial rate factor 分速度系数Nucleophilic reaction 亲核反应Internal return 内返Nucleophilicitor亲核体Nucleophilicity 亲核性α-effect α—效应Backside attack 背面进攻Inversion 反转Umbrella effect 伞效应Push-pull effect 推拉效应Leaving group 离去基团Electrofuge 离电体Nucleofuge 离核体Phase-transfer catalysis 相转移催化Neighboring group participation 邻基参与Neighboring group assistance, anchimeric assistance 邻助作用Neighboring group effect 邻基效应Apofacial reaction 反面反应Bridgehead displacement 桥头取代Aryl cation 芳正离子Benzyne 苯炔Zaitsev rule 札依采夫规则Anti—Zaitsev orientation 反札依采夫定向Hofmann’s rule 霍夫曼规则Bredt rule 布雷特规则Initiation 引发Anionic cleavage 负离子裂解Partial bond fixation 键[的]部分固定化02.3有机化学反应Alkylation 烷基化C- alkylation C—烷基化O- alkylation O—烷基化N-alkylation N-烷基化Silylation 硅烷[基]化Exhaustive methylation 彻底甲基化Seco alkylation 断裂烷基化Demethylation 脱甲基化Ethylation 乙基化Arylation 芳基化Acylation 酰化Formylation 甲酰化Carbalkoxylation 烷氧羰基化Carboamidation 氨羰基化Carboxylation 羧基化Amination 氨基化Bisamination 双氨基化Cine substitution 移位取代Transamination 氨基交换Hydroxylation 羟基化acyloxyation 酰氧基化Decarboxylative halogenation 脱羧卤化Allylic halogenation 烯丙型卤化Dehalogenation 脱卤Nitration 硝化Decarboxylative nitration 脱羧硝化Nitrosation 亚硝化Sulfonation 磺化Chlorosulfonation 氯磺酰化Desulfonation 脱磺酸基Sulfenylation 亚磺酰化Sulfonylation 磺酰化Chlorosulfenation 氯亚磺酰化Chlorocarbonylation 氯羰基化Diazotization 重氮化Diazo transfer 重氮基转移Coupling reaction 偶联反应Diazonium coupling 重氮偶联Cross—coupling reaction 交叉偶联反应1,4—addition 1,4—加成Conjugate addition 共轭加成Dimerization 二聚Trimefization 三聚Additive dimerization 加成二聚sulfurization 硫化Selenylation 硒化Hydroboration 硼氢化Oxyamination 羟氨基化Insertion 插入carbonylation 羧基化Hydroformylation 加氢甲酰基化Hydroacylation 加氢酰化Oxo process 羰基合成Decarbonylation 脱羰Hydrocarboxylation 氢羧基化Homologization 同系化Cyanoethylation 氰乙基化Decyanoethylation 脱氰乙基Ring clsure 环合Diene synthesis 双烯合成Dienophile 亲双烯体Endo addition 内型加成Exo addition 外型加成Diels-Alder reaction 第尔斯—尔德反应Retro Diels-Alder reaction 逆第尔斯—阿尔德反应Ene synthesis 单烯合成Anionic cycloaddition 负离子环加成Dipolar addition 偶极加成— elimination -消除- elimination —消除— elimination -消除—elimination —消除Dehydrohalogenation 脱卤化氢Deamination 脱氨基Pyrolytic elimination 热解消除Elimination-addition 消除-加成Decarboxylation 脱羧Decarboxamidation 脱酰胺Decyanation 脱氰基Alkylolysis,alkyl cleavage 烷基裂解Acylolysis,acyl cleavage 酰基裂解Flash pyrolysis 闪热裂Fragmentation 碎裂Chiletropic reaction 螯键反应Chelation 螯环化Esterification 酯化Transesterification 酯交换Saponification 皂化Alcoholysis 醇解Ethanolysis 乙醇解Cyanomethylation 氰甲基化Aminomethylation 氨甲基化Hydroxymethylation 羟甲基化Hydroxyalkylation 羟烷基化Cholromethylation 氯甲基化Haloalkylation 卤烷基化Transacetalation 缩醛交换Enolization 烯醇化Haloform reaction 卤仿反应Condensation 缩合Aldol condensation 羟醛缩合Cross aldol condensation 交叉羟醛缩合Retrograde aldol condensation 逆羟醛缩合Acyloin condensation 偶姻缩合Cyclization 环化Annulation,annelation 增环反应Spiroannulation 螺增环Autoxidation 自氧化Allylic hydroperoxylation 烯丙型氢过氧化Epoxidation 环氧化Oxonolysis 臭氧解Electrochemical oxidation 电化学氧化Oxidative decarboxylation 氧化脱羧Aromatization 芳构化Catalytic hydrogenation 催化氢化Heterogeneous hydrogenation 多相氢化Homogeneous hydrogenation 均相氢化Catalytic dehydrogenation 催化脱氢Transfer hydrogenation 转移氢化Hydrogenolysis 氢解Dissolving metal reduction 溶解金属还原Internal abstraction 内夺取[反应]Rearrangement 重排Prototropic rearrangement 质了转移重排Double bond migration 双键移位Allylic migration 烯丙型重排Allylic migration 烯丙型迁移Ring contraction 环缩小[反应] Ring expansion,ring enlargement 扩环[反应]—ketol rearrangement —酮醇重排Pinacol rearrangement 频哪醇重排Retropinacol rearrangement 逆频哪醇重排Semipinacol rearrangement 半频哪醇重排Benzilic rearrangement 二苯乙醇酸重排Acyl rearrangement 酰基重排Migratory aptitude 迁移倾向Transannular insertion 跨环插入Transannular rearrangement 跨环重排Migration 迁移Prototropy 质子转移Cationotropic rearrangement 正离子转移重排Anionotropy 负离子转移Anionotropic rearrangement 负离子转移重排Sigmatropic rearrangement —迁移重排Homosigmatropic rearrangement 同迁移重排Electrophilic rearrangement 亲电重排Photosensitization 光敏化Forbidden transition 禁阻跃迁photooxidation 光氧化Photoisomerization 光异构化Photochemical rearrangement 光化学重排2.4 有机化合物类名Aliphatic compound 脂肪族化合物Hydrocarbon 碳氢化合物Alkane 烷Wax 蜡Paraffin wax 石蜡Alkene 烯Alkyne 炔Acetylide炔化物Active hydrogen compounds 活泼氢化合物Carbon acid 碳氢酸Super acid 超酸Diene 双烯Triene 三烯Allene 丙二烯Cumulene 累积多烯Enyne 烯炔Diyne 二炔Alkyl halide 卤代烷Alcohol 醇Homoallylic alcohol 高烯丙醇Ether 醚Epoxide 环氧化物Cellosolve 溶纤剂Crown ether 冠醚Nitro compound 硝基化合物Amine 胺Quaternary ammonium compound 季铵化合物Amine oxide 氧化胺Diazoalkane 重氮烷Mercaptan 硫醇Sulfonic acid 磺酸Sulfoxide 亚砜Sulfone 砜Aldehyde 醛ketone 酮Aldehyde hydrate 醛水合物Ketone hydrate 酮水合物Hemiacetal 半缩醛Acetal 缩醛acetal[化]乙缩醛,乙缩醛二乙醇Ketal 缩酮Dithiane 二噻烷Aminal 缩醛胺imine 亚胺Aldimine 醛亚胺Oxime 肟Aldimine 醛肟Oxime 亚硝基化合物aldoxime 硝酮Hydrazone 腙Azine 嗪Semicarbazone 缩氯基脲Cyanohydrin 羟腈Pinacol 频哪醇Enol 烯醇Enol ether 烯醇醚Enol ester 烯醇酯Enamine 烯胺Ynamine 炔胺Mannich base 曼尼希碱Carboxylic acid 羧酸Ester 酯orthoester 原酸酯Acyl halide 酰卤Acyl fluoride 酰氟Acyl chloride 酰氯Acyl bromide 酰溴Acyl iodide 酰碘Carbobenzoxy chloride 苄氧甲酰氯Acyl tosylate 酰基对甲苯磺酸酐Ketene 乙烯酮Peracid 过酸Perester 过酸酯Acyl peroxide 酰基过氧化物Nitrile 腈Nitrile oxide 氧化腈Isonitrile 异腈Amide 酰胺Imide 二酰亚胺N—bromo compound N-溴化物Hydrazide 酰肼Acyl azide 酰叠氮Amidine 脒Keto ester 酮酸酯Acyl cyanide 酰腈Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸。
泵类英汉翻译

发一份泵类汉英对照表,请进入!汉语术语英文凹槽 groove饱和压力 Saturation Pressure保持环 retaining ring保护层的形成 Protective Layer Formation保护形式 Types of Protection保证 Guarantee背靠背叶轮泵 Back-to-back Impeller Pump背叶片 Back Vane泵 pump泵测试效率 pump test efficiency泵出力 pump delivery泵的基础 Pump Foundation泵的类型 Pump Types泵的排出口 Pump Discharge Nozzle泵的使用范围 Application Fields for Pumps泵的输出功率 Pump Output泵的旋转方向 Direction of Pump Rotation泵房 pump house(room)泵功率 Pump Power泵和输送装置噪音 Noises in Pumps and Pumping Installations 泵壳 Pump Casings泵内沉积物 Deposits in Pumps泵试验台 Pump Test Bed泵输出功率的降低 Reduction in Pump Output泵输入功率 pump input power泵体 pump casing泵吸入槽 Pump Sump泵吸入管吸上高度 pump lift泵吸入口 Pump Suction Nozzle泵效率 pump efficiency泵效率 Pump Efficiency泵芯包 pump cartridge泵性能曲线 pump performance curve泵站 pumping house泵轴 Pump Shaft比例泵 proportioning pump比输送功 Specific Delivery Work比转数 Specific Speed边界层 Boundary Layer变矩桨叶 Variable Pitch Blade标准泵 Standard Pump标准化工泵 Standard Chemical Pump标准孔板 Standard Orifice Plate标准喷嘴 Standard Nozzle标准温度 Standard Temperature标准文丘里管 Standard Venturi Nozzle标准压力 Standard Pressure标准状态 Standard Conditions表面保护 Surface Protection表压 Manometric Pressure并联运转 Parallel Operation伯努利方程 BERNOULLI Equation补偿器 Compensator补给水泵 make-up water pump部分负荷运转 Part-load Operation残油输出装置 Residual Pump-out Device槽 slot测量的不精确性 Uncertainty in Measurement 测量技术 Measuring Technique测量孔板 Measuring Orifice Plate测量偏差 Measuring Tolerance测量误差 Error of Measurement测量仪器 Measuring Device测试转速 test speed层流 Laminar Flow齿轮泵 gear pump齿轮传动泵 Geared Pump齿轮箱 Gearbox冲击冷凝 Shock Condensation冲击压力 Impact Pressure冲角 Angle of Incidence抽出体积 Extraction Volume抽气(吸)泵;真空泵 suction pump出口侧盖板(大端盖) discharge cover出口截面 Outlet Cross-section出口截面宽度 Outlet Width出口弯管 Outlet Elbow出口压力 discharge pressure初始汽蚀 Incipient Cavitation传输损失 tansmission loss传送器 Transmitter船头推进舵 Bow Thruster Rudder船坞泵 Dock Pump船用泵 Marine Pump大气压 Barometric Pressure大气压力 Atmospheric Pressure带式过滤器 Band Sereen单吸离心泵 single-suctoin centrifugal单相交流电 Single-phase Alternating Current单叶片叶轮 Single Vane Impeller导向叶片 Guide Vane导向装置 Guide Arrangement低温泵 Crygenic Pump低压泵 Low Pressure Pump底料泵 Sump Pump点蚀 Pitting电感应式流量测量 Inductive Flow Measurement电功率 Electric Power电化学腐蚀 Electrochemical Corrosion电机 Electric Motor电机电耗 Current Consumption of Electric Motors 电机温升 Temperature Rise in Electric Motors电机转矩曲线 Torque Curve of Electric Motors电解腐蚀 Electrolytic Corrosion电缆密封压盖 Cable Gland电力驱动 Electric Drive电流 current电路 Electrical Circuits电气开关设备 Electrical Switchgear电位序 Electrochemical Series电压 voltage电压降 Voltage Drop顶点 Apex定位螺栓 fitted bolt动力液流 Motive Water Flow动力粘度 Dynamic Viscosity动量矩定理 Theorem of Momentum动平衡 Dynamic Balancing动下室排水泵 Cellar Drainage Pump动压力 Dynamic Pressure对开的润滑油密封 split oil seal对轮 the coupling对轮防护罩 the coupling guard对轮螺栓 coupling nut多级泵 Multistage Pump多流式泵 Multiflow Pump恩氏粘度 Degrees Engler阀门和管件 Valves and Fittings法兰结构 Flange Construction反向流动 Reversal of Flow反应堆泵 Reactor Pump反转转速 Reverse Rotational Speed防爆 Explosion Protection防反转装置 Reverse Rotation Locking Device 防腐 Corrosion Protection防护装置 guard放射 Emission非堵塞式叶轮 Non-clogging Impeller非扰动管道长度 Undisturbed Length of Piping 非稳定流 Non-steady Flow非稳定扬程曲线 Unstable Throttling Curve费鲁德准数 FROUDE Number费用 Costs复算 Re-evaluation干式安装 Dry Installation干运转 Dry Running高度 Height高速离心泵 High Speed Centrifugal Pump高压泵 High Pressure Pump隔膜泵 diaphragm pump隔膜式计量泵 diaphragm type metering pump 给水泵 feed water pump给水泵 Feed Pump工业水泵 industrial water pump工作特征 Operating Characteristics公称尺寸 Nominal Size公称压力 Nominal Pressure功 work功率 power功率 power功率测量 power measurement功率调节 power control功率损失 power loss功率因数 power factor cosφ供水泵 Water Supply Pump固体颗粒的输送 Conveying of Solids过流翼型 Flow Profile过滤器 Filter海水 Sea Water海水泵 Sea Water Pump海水淡化装置 Sea Water Desalination Plant 海水箱 Sea Chest耗水量 Water Consumption合适的流量 corrected flowrate恒定油位油杯 Constant Level Oiler虹吸流动 Syphon Flow虹吸装置 Syphoning Installation化工泵 Chemical Pump化工稳定性表 Chemical Stability Table环境保护 Environmental Protection环形泵 Ring-section Pump环形壳 Annular casing回流导向叶片 Return Guide Vane回路试验 Loop Test混流叶轮 Mixed-flow Impeller活塞传送器 Piston Transmitter货船油泵 Cargo Oil Pump机械传动 Mechnical Drive机械密封 Mechanical Seal机械效率 Mechanical Efficiency基板 Baseplate基本方程 Fundation基础 Foundation基建投资 Capital Investment级 Stage级间导叶 interstage diffuser极数 Number of Poles继电器 Relay家用供水装置 Domestic Water Supply Plant间隙宽度 Clearance Gap Width间隙密封 Clearance Gap Seal间隙汽蚀 Clearance Gap Cavitation间隙压力 Clearance Gap Pressure监测装置 Monitoring Device键 key键槽 keyway交流电 Alternating Current角速度 Angular Velocity接触器 Contactor接通压力 Switching-on Pressure节流调节 throttling control节流系数 throttling coefficient进液室 Intake Chamber进液条件 Intake Conditions进液弯管 Intake Elbow浸蚀 Erosion经济可行性计算 Economic Viability Calcuation 净正吸入压头 NPSH(Net Positive Suction Head)径向力 Radial Force径向剖分壳体 Radially Split Casing径向推力 Radial Thrust径向叶轮 Radial Impeller径向轴承 journal bearing绝对速度 Absolute Velocity卡诺冲击损失 Carnot Shock Loss卡普兰弯管 Kaplan Elbow铠装泵 Armoured Pump抗腐蚀性 Corrosion Resistance壳体 Casing可抽出性 withdrawability可调叶片 adjustable vane空气泵 Air Pump空气提升泵 Air-Lift Pump空心旋涡 Hollow Vortex孔板 Oriffice Plate孔径比 Apertuer Ratio扩压管(圆锥管) Diffuser(Conical Duct)扩压器(导轮) Diffuser(Guide Wheel)冷凝液泵 Condensate Pump冷却剂泵 Coolant Pump冷却水泵 Cooling Water Pump离心泵 centrifugal pump离心泵 Centrifugal Pump离心泵的安装 Installation of Centrifugal Pump离心泵的操作条件 Operating Conditions of Centrifugal Pumps离心泵的操作性能 Operating Behaviour of Centrifugal Pumps离心泵的冲击损失 Shock Loss in Centrifugal Pumps离心泵的间隙损失 Clearance Gap Loss in Centrifugal Pumps离心泵的平稳运行 Smoop and Quiet Running of Centrifugal Pumps离心泵的验收试验规范 Acceptance Test Codes for Centrifugal Pumps 离心泵的制造材料 Construction Material for Centrifugal Pumps离心泵的注水 Priming of Centrifugal Pumps离心泵调节 Control of Centrifugal Pumps离心泵和驱动设备保养 Care of Centrifugal Pumps and Drives离心泵失衡 Out-of-Balance of Centrifugal Pumps离心泵装置 Centrifugal Pump Plant离心式鼓风机 centrifugal blower离心式滤油机 centrifugal oil filter离心式压缩机 centrifugal compressor离心水泵 centrifugal water pump立式泵 vertical pump立式屏蔽泵(筒形泵) Vertical Can-type Pump立轴式井泵 Vertical Spindle Well Pump利息支付 Interest Payment连续性方程 Continuity Equation两相流动 Two-phase Flow临界转速 Critical Speed of Rotation流程泵 Process Pump流程型结构 Process Type Construction流道涡流 Channel Vortex流动分离 Flow Separation流动功 Flow Output流量 flowrate流量 Flow流量(体积流量) Capacity流量测定 Flow Measurement流量测量 Capacity Measurement流量调节器 Flow Controller流量系数 Flow Coefficient流量指示器、流量观察窗 flow indicators流速 Flow Velocity流体 Fluid流体动力学 Fluid Dynamics流体机械 Fluid Flow Machine流线 Flow Line轮毂比 Hub Ratio螺杆泵 screw pump(spiral pump)螺纹紧固件 threaded fastener马达输出功率 motor output power马达输入功率 motor input power马达效率 motor efficiency迷宫密封 labyrinth gland迷宫密封箱体 labyrinth gland housing迷宫式密封 Labyrinth Seal密封衬套 shaft sleeve内效率 Internal Efficiency那维尔-斯托克斯方程 NAVIER-STOKES Equation耐酸泵 Acid Pump焾 Enthalpy能 Energy能量级 Energy Level逆时针旋转叶轮 Counterclockwise Rotating Impeller 凝泵 condensate extraction pump凝结水泵 condensate transfer pump凝汽器抽气泵 condenser air pump牛顿流体 NEWTONian Fluid扭力杆 Torsion Rod扭力计 Torsion Dynamometer欧拉方程 EULER Equation排出段 Discharge Casing排出管线 Discharge Line排出管嘴 Discharge Nozzle排出损失 Discharge Loss排灌站水泵 Drainage Station Punp排气 Venting排气阀 Vent Valve排水泵 Dewatering Pump排水量 Water Yield排污泵 blowdown pump(sewage pump) 排污泵 Faeces Pump旁路 By-Pass皮带传动 Belt Drive皮托管 Pitot Tube频率 frequency平垫片 flat gasket平衡鼓 balance drum平衡鼓螺母 balance drum nut平衡孔 balance'hole平衡液体流量 balance water flow平衡装置 balancing device平衡状态 equilibrium condition平皮带传动 flat belt drive屏蔽泵 Canned Motor Pump起动 Start Up起动过程 Starting Process起动时间 Starting Time起动转矩 Starting Torgue气囊的形成 Formation of Air Pockets 气体分离 Gas Separation汽蚀 Cavitation汽蚀磨损 Cavitation Wear汽蚀噪音 Cavitation Noise强制循环泵 forced-circulation pump 切断压力 Switching-off Pressure切换频率 Switching Frequency氢指数 Hydrogen Exponent清水泵 Clean Water Pump驱动 Drive热 Heat热泵 Heat Pump热含量 Heat Content热虹吸 Thermosyphon热量 Quantity of Heat热载体泵 Heat Transfer Media Pump热障 Thermal Barrier容积泵 Positive Displacement Pump入口导叶 suction guide入口管嘴 Entry Nozzle入口截面 Inlet Cross-section入口压力 suction pressure入口锥管 Entry Cone润滑油泵 Lubricating Oil Pump三相电机 Three-phase Motor三相电流 Three-Phase Current三相制 Three-phase System设计工作点 Design Duty Point声学 Acoustics时间 Time视在功率 Apparent Power试验台 Test Bed手动泵 Hand Pump首级内泵壳 first stage ring section输送黏性液体黏性泵 centrifugal pump handling viscous liquids 甩油环,抛油环 oil thrower双缸泵组 Twin Pumping Set双流道泵 two-channel impeller pump双流道叶轮 two-passage impeller双蜗壳 Double Volute水厂泵 waterworks pumps水锤 water hammer水的硬度 hardness of water水力效率 hydraulic efficiency水流量计 water meter水泥壳泵 concrete casing pump水喷射 water jet水喷射泵 water jet pump水温 water temperature水下泵 underwater pump水下电机 underwater motor水下电机泵 underwater motor pump水银泵 mercury pump水硬度 water hardness速度 Velocity速度测定 Measuring of Speeds速度测量 Velocity Measurement速度三角形 Velocity Triangle损失系数 Loss Coefficient锁紧垫圈、防松垫圈 lock-washer探头 Probe特性 Characteristic特性曲线 Characteristic Curve特性因数 Characteristic Factor体积流量 Volume Flow停转时间 Run-down Time通量线 Flux Line同步转速 Synchronous Speed铜导体 Copper Conductor投资评价 Capital Servicing透平驱动泵 Turbine Driven Pump涂层 Coat of Paint推力盘 thrust collar推力瓦 thrust pad推力轴承撑板 thrust carrier ring 推力轴承室 thrust bearing housing 托轮 Jockey Pulley弯管 Elbow弯管壳体泵 Elbow Casing Pump卧式泵 Horizontal Pump污泥泵 Sludge Pump污水泵 Sewage Pump无冲击流入 Shock-free Entry无泄漏 Leak-tightness吸入口 Suction Nozzle吸入室 Suction Chamber吸入性能 Suction Behaviour吸入压头 Suction Head吸入叶轮 Sution Impeller纤维素 Cellulose纤维性物料 Fibrous Material相对速度 Relative Velocity相合定律 Congruence Law相似条件 Similarity Conditions相位移 Phase Displacement消防泵 Fire-fighting Pump消声测量 Noise Abatement Measures 销钉、定位销 dowel pin小舌片 tab效率复算 Efficiency Re-evaluation斜度 Steepness斜流叶轮 Diagonal Impelier斜盘式泵 Swash Plate Pump泄漏出的密封水隔离门(相当于密封水卸荷阀) leak-off isolating valve 泄漏损失 Leakage Loss星形轮 Star Wheel星形-三角形起动器电路 Star-delta Starter Circuit性能图 Performance Chart许可公差 Warranty Tolerance许可区域 Warranty Zone旋流 Swirl Flow旋塞阀 Cock旋涡泵 Peripheral Pump旋涡式叶轮 Peripheral Impeller旋转方向 Direction of Rotation旋转速度 Rotational Speed压差 Differential Pressure压降 Pressure Drop压力 Pressure压力波 Pressure Wave压力波动 Pressure Surge压力测量 Pressure Fluctuation压力测量 Pressure Measurement压力等级 Pressure Categories压力给水装置 Hydrophor Plant压力计 Manometer压力脉动 Pressure Fluctuation压力容器 Pressure Vessel压力损失 Pressure Loss压力损失 Head Loss压力系数 Pressure Coefficient压头 Pressure Head验收试验 Acceptance Test扬程 head扬程曲线 Throttling Curve叶轮 impeller叶轮 ipeller叶轮 impeller叶轮侧面摩擦 Impeller Side Friction叶轮的修整 Trimming of Impellers叶轮叶片 Impeller Vane叶轮叶片节距调节 Impeller Blade Pitch Adjustment叶片叶片 vane叶片角 Blade Angle叶片节距调节机构 Blade Pitch Adjustment Gear叶片节距调节装置 Blade Pitch Adjustment Device叶梢背部切削 Cutting Back of Impeller Vane Tips叶栅 vane cascade叶栅流动 Cascade Flow液化气泵 Liquefied Gas Pump液环泵 Liquid Ring Pump异步电机 Asynchronous Motor阴极保护 Cathodic Protection音量级 Sound Volume Level音速 Sound Velociyt引水级 Priming Stage应变测定技术 Strain Measurement Technology应力腐蚀(裂纹) Stress Corrosion (Cracking)永久磁铁联轴器 Permanent Magnet Coupling用入口导向叶片控制涡流 Rotation Swirl Control by Inlet Guide Vanes 油泵 Oil Pump油挡 oil guard有势流动 Potential Flow有效功率 Acitive Power有效汽蚀余量 n.p.s.h.r有用功率输出 Useful Output右旋叶轮 right-handed impeller诱导轮 Inducer圆弧形叶片 Circular Arc Vane圆周速度 Peripheral Speed(Circumferential Velocity)运动粘度 Kinematic Viscosity运行时数 Number or Running Hours再生泵 Regenerative Pump脏水泵 Dirty Water Pump折旧 Amortization振动 Vibration正吸入压头 positive suction head直径系数 Diameter Coefficient直联泵组 Close-coupled Pumping Set直流电 Direct Current直流电机 Direct Current Motor直流平板式起动器 Direct Current Face Plate Starter止动销 stop pin纸浆泵 Pulp Pump纸浆浓度 Pulp Density纸浆输送 Conveying of Pulp制造公差 Manufacturing Tolerance质量 Mass质量惯性矩 Mass Moment of Inertia质量流量 Mass Flow中点线 Mid-point Conductor中性线 Neutral Conductor中压泵 Medium Pressure Pump重力加速度 Gravitational Constant重量 Weight轴轴承 Bearing轴承支架 bearing housing轴承支架盖 bearing housing cover轴封 Shaft Seal轴封环 Shaft Sealing Ring轴功率 Shaft Power轴颈 shaft journal轴流泵 axial pump轴流泵 Axial Pump轴流叶轮 Axial Impeller轴套、衬套 gland bush轴推力 Shaft Thrust轴向力 Axial Force轴向剖分壳体 Longitudinally Split Casing 轴向推力 Axial Thrust主循环泵 Primary Circulating Pump柱塞泵 Plunger Pump转动惯量 Moment of Gyration转矩 Torque转矩的测定 Torque Measurement转速传感器 Rotational Speed Transmitter自动调节 Self-regulation自动断路器 Self-acting Circuit Breaker自动开关 Automatic Switches自耦变压器式起动器 Autotransformer Starter 自吸泵 Self-priming Pump自由流动泵 Free-flow Pump总测量偏差 Overall Measuring Tolerance总静压头 Total Static Head总偏差 Overall Tolerance总效率 Overall Efficiency总压力 Tital Pressure总扬程 Total Headmeasured flowratevelocity headgauge/lever correctiongenerated headseal sleeve nuts短心轴 stub axle轴 axes轴 axis轴 axle(U.S.A)轴承衬套 bearing bushing(U.S.A)轴肩挡圈;防护罩 protecting collar巴氏合金;白合金;轴承合金 white metal半贯流式轴流泵;弯管轴流泵 angle-type axial flow pump半可调式轴流泵 axial flow pump with blades adjustable when stationary 泵轴 Pump Shaft泵轴承支架 pump bearing bracket长轴深井泵 borehole shaft driven(centrifugal) pump长轴深井泵(美) multistage vertical turbine pump衬套;轴套 bushing齿形联轴器 gear-type coupling出口轴面速度系数;流量系数 capacity constant磁力联轴节齿轮泵 magnetically coupled gear pump从动螺杆;从动心轴 idler spindle从动轴 driven shaft从动轴 idler shaft弹性盘联轴器 flexible disc coupling弹性圆柱销联轴器 flexible pin coupling电磁联轴器 electromagneic coupling电磁联轴器 magneto coupling电磁联轴器 magneto-coupling调节轴 adjusting spindle副传动轴 countershaft spindle副轴;中间轴;从轴 auxiliary shaft刚性联轴器 solid coupling刚性联轴器;刚性联接 rigid coupling功率曲线;轴功率曲线;制动功率曲线 brake horsepower curve贯流泵;直管轴流泵 rubular type axial flow pump滚动轴承 anti-friction bearing滚动轴承 ball/rolling bearing滚动轴承 rolling contact bearing滚针轴承 needle bearing恒定轴向间隙齿轮泵 fixed axial clearance gear pump滑动轴承 plain friction bearing滑动轴承 sleeve bearing滑动轴承 sliding bearing活塞销;轴头销 gudgeon pin架;轴承衬套(美);泵支架(美) frame径向滚柱轴承 radial roller bearing径向球轴承 radial ball bearing径向轴封 radial shaft seal可调式轴流泵 axial flow pump with adjustable(or variable)pitch blades 可调式轴流泵 propeller pump with adjustable or variable pitch blades 可逆叶片轴流泵 axial flow pump with reversible blades可逆叶片轴流泵 propeller pump with reversible blades空心轴 hollow shaft shaft冷冻装置用无轴封泵 glandless pump for refrigerating installation立轴式井泵 Vertical Spindle Well Pump联轴器 coupling笼形轴承托架 bearing bracket lantern笼形轴承托架 bearing housing lantern螺纹联轴器 screwed coupling米切尔型推力轴承 Michell type thrust bearing内轴承泵 pump with internal bearing(s)挠性法兰联轴器 flexible flange coupling挠性联轴器 elastic coupling挠性联轴器 flexible coupling挠性轴 flexible shaft配流盘式轴向活塞泵 flat valve axial piston pump配流盘式轴向活塞泵 port plate axial piston pump配流盘式轴向活塞泵 valve plate axial piston pump喷水推进轴流泵 axial flow pump for water jet propulsion喷水推进轴流泵 water jet propulsion axial flow pump偏心轴 eccentric shaft强制润滑轴承;压力润滑轴承 forced oil lubricated bearing球面推力轴承 spherically mounted thrust bearing曲轴 crankshaft曲轴防护罩 crankguard曲轴防护罩 crankshaft guard曲轴箱支座 crankcase pedestal曲轴销 crank pin驱动轴;传动轴 drive shaft全可调式轴流泵 axial flow pump with blades adjustable in operation全可调式轴流泵 axial flow pump with variable piteh blades水润滑轴承 water lubricating bearing套筒联轴器 sleeve coupling推力滚柱轴承 thrust roller bearing推力球轴承 ball thrust bearing推力轴承 thrust bearing推力轴承;扇形块 thrust bearing segment推力轴承扇形块 thrust bearing pad外轴承泵 pump with external bearing(s)万向轴 cardan shaft无轴封泵 glandless pump无轴封计量泵 glandless metering pump橡胶轴承 rubber bearing斜垫轴向推力轴承 tilting pad axial thurst bearing斜缸型轴向柱塞泵 tilting cylinder block type axial plunger pump 斜盘式轴向活塞泵 cam plate type axial piston pump斜盘式轴向活塞泵 swash plate axial piston pump斜盘式轴向活塞泵 wobble plate axial piston pump斜置轴流泵 inclined axial flow pump斜轴式轴向活塞泵 angle-type axial piston pump斜轴式轴向活塞泵 bent axis axial piston pump旋涡轴线 eddy axis旋涡轴线 vortex axis压入式轴承盖 pressed-in type bearing cover叶轮轴面形状;工作轮图 profile of the impeller液体动力轴承 hydrodynamic bearing永久磁铁联轴器 Permanent Magnet Coupling油环轴承 ring lubricating bearing油环轴承 ring oiling bearing油脂润滑轴承 grease lubricated bearing支轴销;支点销 fulcrum pin支座;轴架 pedestal中间心轴 idler axle中间心轴 intermediate axle中间轴;副轴;从轴 counter shaft中间轴;副轴;从轴 intermediate shaft轴 shaft轴;心轴;锭子 spindle轴承 bearing轴承 Bearing轴承衬(套) bearing bush轴承衬套 bearing cartridge轴承衬套 bearing insert轴承端盖 bearing end cover轴承盖 bearing cover轴承合金 bearing alloy轴承架 bearing bracket轴承架 bearing pedestal轴承架 bearing spider轴承架;泵托架 headstock轴承架固定式泵 pump with bearing bracket轴承浸泡试验;轴承泡胀试验 bearing swelling test 轴承冷却室 bearing cooling chamber轴承螺母 bearing nut轴承套 bearing sleeve轴承体;轴承箱 bearing box轴承托架箱 bearing bracket housing轴承瓦;轴承衬垫;轴承衬套 bearing line轴承吸水试验 bearing absorption test轴承箱;轴承体 bearing housing轴承座 bearing carrier轴对称 axial symmetry轴对称;旋转对称 rotational symmetry轴对称流 axisymmetrical flow轴封 shaft seal轴封 Shaft Seal轴封环 Shaft Sealing Ring轴功率 Shaft Power轴功率;制动功率;制动马力 brake horsepower轴护套 Shaft Protecting Sleeve轴护套 shaft tunnel tube轴护套(美) shaft enclosing tube轴肩挡圈 loose(shaft) collar轴肩挡圈 shaft collar轴肩挡圈 shoulder ring轴颈 neck journal轴颈 shaft neck轴颈 axle journal轴颈;期刊 journal轴颈套;填料衬套 neck bush轴流泵 Axial Pump轴流泵;螺桨泵 axial flow pump轴流泵叶轮室 propeller bowl轴流式涡轮机 axial-flow turbine轴流式叶轮 axial flow impeller轴流叶轮 Axial Impeller轴流增压器 axial flow booster轴马力;轴功率 shaft horsepower轴面流线 meridian streamline轴面速度 meridional velocity轴面投影图 elevation view轴配流径向活塞泵 pintle valve radial peston pump 轴配流径向活塞泵 valve spindle radial piston pump 轴套 shaft wearing sleeve轴套 Axis Guide轴套 shaft sleeve轴套;套(筒) sleeve轴套拆卸器 sleeve puller轴推力 Shaft Thrust轴瓦;轴承箱;轴承体 bearing sheet轴位指示器 shaft position indicator轴吸泵 axial inlet pump轴向单吸液环泵 axial single entry liquid ring pump轴向导叶 axial diffuser轴向滑移 aixal slip轴向活塞泵 axial piston pump轴向加速度 axial acceleration轴向间隙 axial clearance轴向间隙压力补偿齿轮泵 gear pump with pressure-dependent axial clearance 轴向间隙压力补偿齿轮泵 gear pump with pressurized side plate轴向力 axial force轴向力 Axial Force轴向流动 axial flow轴向磨损指示器 axial wear indicator轴向剖分壳体 Longitudinally Split Casing轴向双吸液环泵 axial double entry liquid ring pump轴向速度 axial velocity轴向推力 axial thrust轴向推力 Axial Thrust轴向吸入泵 axial suction pump轴向压力 axial pressure轴向柱塞泵 axial plunger pump主动螺杆;驱动心轴 driving spindle主动轴 driving shaft柱销联轴器 pin coupling爪形联轴器 claw coupling爪形联轴器 dog coupling爪形联轴器 jaw coupling锥形联轴器 cone(type) coupling锥形联轴器 taper coupling子午面;轴面 meridian plane自锁轴套 self-locking sleeve半贯流式轴流泵;弯管轴流泵 angle-type axial flow pump半可调式轴流泵 axial flow pump with blades adjustable when stationary泵 pump泵房 pump house泵盖 casing cover泵缸 pump barrel泵缸 pump cylinder泵缸套 pump barrel line泵缸套 pump cylinder line泵缸体 pump cylinder block泵工况 pump operating condition泵工况 pump operating duty泵体 pump body泵体衬套 pump body insert泵体衬套 pump casing insert泵托架 pump bracket泵站设计 design of pump station泵罩 outer pump mantle泵支架 pump bearing lantern泵轴承支架 pump bearing bracket泵装置 pumping device泵装置 pumping machine泵组 integral pump group泵座 pump frame闭式叶轮泵 pump with enclosed impellers波纹管泵 bellows pump仓底泵 bilge pump侧流道泵壳;旋涡泵泵体 casing with transfer passages 侧流道泵壳;旋涡泵泵体 annular casing长轴深井泵 borehole shaft driven(centrifugal) pump 衬青铜泵 bronze fitted pump齿轮比例泵 abjustable discharge gear pump冲击波泵(振荡器) shock wave oscillator导流壳式多级泵 pump with bowl-type vaned casing低温泵 cryopump底料泵;塔底液泵 bottoms pump底吸泵 bottom inlet pump底吸泵 bottom suction pump电磁驱动泵 pump with cyclic electromagnetic drive风动活塞泵 air-powered piston pump辅泵;辅助泵 auxiliary pump辅扫仓泵;副清仓泵 auxiliary stripping pump附属泵 attached pump工程用泵 building site pump固定缸体泵 pump with nonrotating cylinder固体输送泵 pump for water-borne solids管网泵 pump for pipe system灌泵 priming of pump锅炉给水泵 BFW锅炉给水泵 boiler feed pump锅炉回水循环泵 boiler return circulating pump锅炉酸洗泵 pump for acid washing of boiler航空用泵 aviation pump合金泵 alloy pump合金钢泵 alloy steel pump环壳泵 annular casing pump灰渣泵 ash pump回水泵 back water pump活性污泥泵 activated sludge pump活性污泥泵 biological sludge pump机械密封泵 pump with mechanical seal基本负荷泵 base load pump加硼泵 boron addition pump甲板泵 board pump架;轴承衬套(美);泵支架(美) frame减摇泵 anti-roll pump铰链滑片泵 articulated vane pump接力泵 boost pump铠装泵 armoured pump可调隔膜泵 adjustable diaphragm pump可调式轴流泵 axial flow pump with adjustable(or variable)pitch blades 可逆叶片轴流泵 axial flow pump with reversible blades沥青泵 bitumen pump螺旋泵 Archimedean screw pump密闭式螺杆泵 airtight screw pump内轴承泵 pump with internal bearing(s)耐磨泵 abrasion resisting pump耐酸泵 acid pump逆洗泵 back wash pump农用喷药泵 agricultural spray pump for chemicals盘状活塞泵 bucket piston pump喷灌泵 agricultural spray pump喷射泵;喷射器 ejector喷水推进轴流泵 axial flow pump for water jet propulsion喷雾器-泵组 atomizer-pump皮带传动泵 belt driven pump啤酒泵 beer pump平衡转子式滑片泵 balanced rotor vane pump气抽液喷射泵 gas jet liquid ejector气动泥浆泵 air-pressure actuated slurry pump气泡泵;曼木特泵;气举泵 air lift pump气体喷射泵 gas ejector汽车用泵 automobile pump汽抽气喷射泵 steam jet air ejector汽抽水喷射泵 steam jet water ejector前置泵;增压泵 booster pump潜水深井泵 borehole submerged pump球形活塞泵 ball piston pump全可调式轴流泵 axial flow pump with blades adjustable in operation 全可调式轴流泵 axial flow pump with variable piteh blades全青铜泵 all bronze pump熔盐泵 pump for liquid salts pump上壳(井泵);吐出段 delivery casing上壳(井泵);吐出段 discharge casing深井泵 borehole pump食品泵 pump for edible fluids双壳泵 barrel insert pump双吸泵 balanced suction pump水抽气喷射泵 water jet air ejector水锤泵;振动机 pulsator水喷射泵 water ejector饲槽自动泵;家畜自动饮水泵 animal self-operated drinking water pump 饲槽自动泵;家畜自动饮水泵 automatic trough pump碎纸浆泵 broke pump甜菜泵 beet pump甜菜根泵 beet tails pump筒袋式泵 barrel pump筒袋式油泵 barrel oil pump外轴承泵 pump with external bearing(s)往复式深井泵 borehole reciprocating pump微型泵 micro-pump无叶片泵 bladeless pump吸附真空泵 absorption vacuum pump吸收泵 absorption pump相对叶轮泵;背靠背叶轮泵 pump with opposed impellers箱桶抽空泵 barrel emptying pump斜轴式轴向活塞泵 angle-type axial piston pump斜轴式轴向活塞泵 bent axis axial piston pump悬臂叶轮泵 pump with overhung impeller压缩空气驱动泵 air operated pump压载泵 ballast pump盐水泵 brine pump叶轮串并联泵 pump with series or parallel connection impellers液化天然气用低温泵 cryopump for liquefied natural gas液态金属泵 pump for liquid metals液体喷射泵 liquid ejector增压泵 boosting pump增压泵;压送泵;气筒 inflator增压给水泵 booster-feed pump增压扩散泵 booster diffusion pump蔗渣泵 bagnsse pump蒸汽机泵 steam pumping engine蒸汽脉冲泵;脉冲泵 steam pulsator蒸汽脉冲泵;脉冲泵 pulsometer蒸汽喷射泵 seam ejector中段泵壳;中段 stage casing中开泵 axially split pump轴承架;泵托架 headstock轴承架固定式泵 pump with bearing bracket轴流泵;螺桨泵 axial flow pump轴流泵叶轮室 propeller bowl轴吸泵 axial inlet pump轴向单吸液环泵 axial single entry liquid ring pump轴向活塞泵 axial piston pump轴向双吸液环泵 axial double entry liquid ring pump轴向吸入泵 axial suction pump轴向柱塞泵 axial plunger pump铸铁泵 all iron pump转子可抽出式泵 pump with withdrawable rotor assembly钻井冲洗泵 borehole flushing pump摆线螺杆泵 cycloidal screw pump半开式叶轮离心泵 centrifugal pump with semien closed impeller pump 闭式旋涡泵 closed peripheral pump闭式叶轮离心泵 centrifugal pump with shrouded impeller不堵塞泵 chokeless pump舱壁泵 bulkhead mounted pump除氧化皮泵 descaling pump除氧器供给泵 deaerator lift pump除氧器循环泵 deaerator recirculating pump传导泵 conduction pump地下室排水泵 cellardrainage pump定压泵 constant pressure pump非接触齿轮泵 contactless gear pump伏辊断头纸桨泵 couch break pump给料泵 charge pump恒速泵 constant speed pump化工用泵 chemical pump化学纸浆泵 chemical pulp pump化学纸浆泵 chemical stock pump环壳泵 circular casing pump混料泵 contactor pump混凝土泵 cement grout pump混凝土泵 cementation pump混凝土泵 concrete pump混凝土涡壳泵 concrete volute pump货油泵 cargo oil pump夹装泵 bung mounted pump甲板泵 deck pump甲板冲洗泵 deck wash pump开式叶轮离心泵 centrifugal pump with open impeller矿井工作面泵 coal face pump冷剂泵 collant pump冷剂泵 coolant pump冷剂泵 cooling medium pump冷凝泵 condensate pump冷凝泵 condensate extraction pump冷却水泵 cooling water pump离心泵 centrifugal pump离心机出料泵 centrifugal run-off pump离心喷射泵 centrifugal-jet pump离心-旋涡泵 centrifugal-peripheral pump离心-旋涡泵 centrifugal-turbine pump链传动泵 chain drive pump链式泵 chain pump裂化装置泵 cracking pump流道式叶轮泵 channel impeller pump滤清污水泵 decanted sewage pump氯乙烯塑料泵 chlorovinyl plastic pump煤泥泵 coal slurry pump煤水泵 coal pump内啮合齿轮泵 crescent gear pump内啮合齿轮泵 crescent seal gear pump内配流径向活塞泵 centrally ported radial piston pump内循环形屏蔽泵 canned motor pump with inner recirculation 耐蚀泵 corrosion free pump耐蚀泵 corrosion resisting pump凝水回收泵 condensate recovery pump凝水回收泵 condensate return pump凝水-增压泵 condensate-booster pump凝水-增压-给水泵 condensate-booster-feed pump屏蔽泵 canned motor pump汽车冲洗泵 car wash pump巧克力输送泵 chocolate pump切削冷却乳剂泵 cutting oil pump清水泵 clean water pump曲柄泵 crank pump曲柄飞轮泵 crank and flywheel pump深井泵 deepwell pump石灰汁泵;清汁泵 clarified juice pump手压泵;手动泵 cottage pump水泥桨泵 cement slurry pump陶瓷泵 ceramic pump陶瓷酸泵 ceramic acid pump甜菜丝泵 cossette pump凸轮泵 cam pump凸轮转子式刮片泵 cam rotor vane pump凸轮转子式刮片泵 cam-vane pump外循环形屏蔽泵 canned motor pump with outer recirculation 无曲柄泵 crankless pump无曲柄多缸泵 crank-less multicylinder pump无曲柄污水泵 crankless sewage pump无曲柄消防泵 crankless fire pump洗舱泵 butterworth pump洗煤用泵 coal washing pump向心泵 centripetal pump斜盘式轴向活塞泵 cam plate type axial piston pump循环泵 circulating pump压缩空气驱动泵 compressed air pump液化天然气用低温泵 cryo pump for LNG诱导轮离心泵 centrifugal pump with inducent原生污泥泵 crude sludge pump原生污水泵 crude sewage pump原油泵 crude oil pump圆筒感应泵 cylindric induction pump粘浆泵 cellulose pulp pump直联泵;单体泵 close coupled pump中央密封式活塞泵 centre-packed type piston pump铸钢泵 cast steel pump装入式泵 built-in pump(主机)半负荷泵 half load pump(主机)全负荷泵 full load pump比例泵 dosing pump玻璃泵 glass pump差动活塞泵 differential piston pump柴油机驱动泵 diesel pump长喉管喷射泵 jet pump with long Venturi tube衬硬胶泵 ebonite pump衬硬胶泵 hard rubber lined pump。
电化学除钙 创新点

电化学除钙创新点(中英文实用版)英文文档:Electrochemical Calcium Removal: Innovative PointsCalcium ions (Ca) can be effectively removed from water and other liquids using electrochemical methods.This innovative approach offers several advantages over traditional techniques, such as ease of operation, lower maintenance requirements, and higher efficiency.The key innovative points of electrochemical calcium removal include:1.Non-chemical purification: Unlike traditional water softening methods that involve adding chemicals like sodium chloride, electrochemical calcium removal does not require the addition of chemicals.This makes it a more environmentally friendly and healthier option.2.Reusable electrode materials: The electrodes used in electrochemical calcium removal systems can be made from sustainable and reusable materials, such as graphite or carbon paste.This reduces waste and lowers the overall cost of the purification process.3.Energy-efficient operation: Electrochemical calcium removal systems require minimal energy input, making them an eco-friendly and cost-effective choice.The energy efficiency of these systems is attributed to the use of direct current (DC) and the absence of chemical reactions.4.Simplified operation and maintenance: Electrochemical calcium removal systems are relatively easy to operate and maintain.The electrodes require periodic cleaning and replacement, but this can be easily done by the user or a professional technician.5.Wide applicability: Electrochemical calcium removal techniques can be used for various applications, including drinking water purification, industrial process water treatment, and the removal of calcium-containing impurities from beverages and other liquids.6.Customizable design: Electrochemical calcium removal systems can be designed to meet the specific needs of different applications.The size, shape, and material of the electrodes can be customized, allowing for optimal performance and efficiency.In conclusion, electrochemical calcium removal offers a innovative and effective solution for the removal of calcium ions from water and other liquids.Its non-chemical nature, reusable electrode materials, energy efficiency, simplicity, wide applicability, and customizable design make it a promising technique for purification and water treatment applications.中文文档:电化学除钙:创新点电化学方法可以有效地去除水和其他液体中的钙离子(Ca)。
心理学专业英语单词

专业英语1psychology n.心理学mind n.心理;心灵;精神 soul n.灵魂behaviour n.行为 The scientific study of behaviour and mental processes 行为与心理过程的科学研究psychologist n.心理学家philosophy n.哲学 philosopher n.哲学家Empiricism n.经验主义,源于英国哲学家洛克,认为知识源于后天学习经验。
行为主义坚持这一观点,强调必须通过观察与实验来研究客观事实为对象的心理现象,例如外显行为。
Positivism n.实证主义,源于法国哲学家孔德,认为科学只研究可以观察到或经验到的事实,实证即只承认能确证的事实。
biology n.生物学 evolution n.进化 genetics n.遗传学physiology 生理学endocrine n.内分泌;激素physics n.物理学physicist n.物理学家 psychophysics n.心理物理学separate scientific discipline 独立的科学学科Principles of psychology 心理学原理structuralism 结构主义 conscious a.有意识的 introspection n.内省image n.意象;心象 sensation n.感觉,知觉feeling n.触觉,知觉,感觉;感情,情感 functionalism n.功能主义thought n.思想 psychoanalysis n.心理分析 therapy n.治疗,疗法The interpretation of dreams 梦的解析 unconscious mind 无(潜)意识心理Behaviourism行为主义 experimental psychology 实验心理学cognitive a.认知的 humanistic a.人本主义的cognitive psychology 认知心理学专业英语2variables 变量 aggression 攻击;侵犯 intelligence 智力operationalisation 操作化abstract concepts 抽象概念observable behaviour 可观察行为puzzle 测验智力的问题(或玩具);难题reification (抽象概念等)具体化;观念与现象混淆 observations 观察法case studies 个案研究法 surveys 调查法 correlations 相关性experiments 实验法 independent variable 自变量dependent variable 因变量extraneous variables 外扰变量;无关变量controls 控制 confounding variables 混杂变量random 随机 constant 恒定 hypotheses 假设2-tailed hypotheses 双极假设 1-tailed hypotheses 单极假设operationalised variables 操作性的变量 statistically singnificant 统计学意义上的显着null hypotheses 零假设 significant effect 显着性效果manipulation of the independent variable 自变量的操纵laboratory 实验室 deliberately manipulates 仔细操纵strict control 严格控制 subject 被试natural environment 自然环境 quasi experiment 准实验专业英语3perception 知觉 sense 感觉;感官 visual perception 视觉,视知觉two-dimensional 二维的 retina 视网膜 three-dimensional 三维的viewpoint 观察点,注视点 shape constancy 形状恒常性size constancy 大小恒常性 luminescence 发光brightness constancy 明度恒常性 luminescence 发光brightness constancy 明度恒常性 illusions 错觉Necker cube 尼克尔立方体 Gestalt 格式塔emergent properties 突变特性 phi phenomenon 似动现象Law of Pragnanz 完形倾向性定律 proximity 邻近性similarity 相似性 continuity 连续性closure 闭合 figure-ground 图形-背景common fate 共同命运,以相同方向运动的物体会被组织在一起专业英语4attention 注意 sensory stimuli 感觉刺激focused or selective attention 集中或选择注意divided attention 分配注意 vision 视觉 hearing 听觉visual field 视野 target 目标;靶专业英语5encode 编码memory 记忆photon 光子;见光度(等于通过一平方厘米大的瞳孔看到每平方米一支蜡烛的照明度)represent 描述;代表;象征 imagery memory 形象记忆representation 表征 iconic (visual) 映象的;形象的echoic (auditory) 回声的;声象的 recall 回忆 tune 声调working memory 工作记忆 the central executive 中央执行器Visuospatial scratchpad 视觉空间模板 phonological loop 语音回路photographic (eidetic) memory 映象记忆 procedural memory 程序记忆implicit memory 内隐记忆enactive mode 动作性模式,指人们用“动作”来表达他们关于世界的知识和经验。
电催化反应的英文

电催化反应的英文Electrochemical Catalysis: Unlocking the Potential of Energy Conversion and StorageElectrochemical catalysis is a rapidly evolving field that has garnered significant attention in recent years due to its pivotal role in addressing the global energy and environmental challenges. This transformative technology harnesses the power of chemical reactions driven by electrical energy, enabling the efficient conversion and storage of various forms of energy, from renewable sources to fossil fuels.At the heart of electrochemical catalysis lies the concept of using specialized catalysts to facilitate and accelerate electrochemical reactions. These catalysts, often made of precious metals or advanced materials, play a crucial role in enhancing the kinetics and selectivity of the desired reactions, ultimately improving the overall efficiency and performance of electrochemical systems.One of the primary applications of electrochemical catalysis is in the field of energy conversion. Fuel cells, for instance, rely on electrochemical catalysts to facilitate the oxidation of fuels, such ashydrogen or methanol, and the reduction of oxygen, generating electricity in a clean and efficient manner. The development of highly active and durable electrocatalysts has been a driving force behind the advancement of fuel cell technology, enabling the widespread adoption of these clean energy devices in various sectors, including transportation, stationary power generation, and portable electronics.Similarly, electrochemical catalysis plays a pivotal role in the storage and conversion of energy from renewable sources. In the case of water electrolysis, catalysts are employed to split water molecules into hydrogen and oxygen, allowing for the storage of energy in the form of hydrogen, which can then be used as a clean fuel or converted back into electricity through fuel cells. This process is particularly important for the integration of renewable energy sources, such as solar and wind, into the energy grid, as it provides a means to store excess energy generated during periods of high production.Moreover, electrochemical catalysis is essential in the developmentof advanced energy storage technologies, such as rechargeable batteries and metal-air batteries. Catalysts are used to enhance the efficiency and durability of the electrochemical reactions that occur during charging and discharging, enabling the storage and retrieval of energy with improved performance and safety.Beyond energy applications, electrochemical catalysis has also found important uses in the fields of environmental remediation and chemical synthesis. In the former, catalysts are employed to facilitate the electrochemical treatment of wastewater, enabling the removal of harmful pollutants and the recovery of valuable resources. In the latter, electrochemical catalysis is used to drive selective chemical transformations, opening up new pathways for the production of various chemicals and pharmaceuticals.The success of electrochemical catalysis is heavily dependent on the development of advanced catalytic materials and the optimization of the catalytic processes. Researchers in academia and industry are continuously exploring new strategies to design and synthesize highly active, selective, and durable catalysts, drawing inspiration from fields such as materials science, nanotechnology, and computational chemistry.One promising approach is the use of nanostructured materials, which offer a large surface area-to-volume ratio and the ability to fine-tune the electronic and structural properties of the catalysts. The incorporation of transition metals, noble metals, and their alloys into these nanostructured materials has led to significant improvements in catalytic performance, with researchers exploring innovative synthesis methods and novel catalyst architectures to further enhance activity and stability.Another area of active research is the development of non-precious metal-based catalysts, which aim to reduce the reliance on scarce and expensive precious metals, such as platinum and iridium. The exploration of earth-abundant elements, including iron, nickel, and cobalt, has yielded promising results, with researchers investigating ways to improve the catalytic activity and durability of these alternative materials.Computational modeling and simulation have also played a crucial role in the advancement of electrochemical catalysis. By coupling advanced computational techniques with experimental data, researchers can gain deeper insights into the underlying mechanisms of electrochemical reactions, enabling the rational design of more efficient and selective catalysts.As the world continues to grapple with the pressing challenges of energy security, environmental sustainability, and resource scarcity, the importance of electrochemical catalysis cannot be overstated. This transformative technology holds the potential to revolutionize the way we produce, store, and utilize energy, while also contributing to the development of more sustainable chemical processes and environmental remediation strategies.Through continued research, innovation, and collaboration amongscientists, engineers, and policymakers, the field of electrochemical catalysis is poised to play a pivotal role in shaping a more sustainable and prosperous future for our planet.。
发一份泵类汉英对照表

发一份泵类汉英对照表,请进入!汉语术语英文凹槽groove饱和压力Saturation Pressure保持环retaining ring保护层的形成Protective Layer Formation保护形式Types of Protection保证Guarantee背靠背叶轮泵Back-to-back Impeller Pump背叶片Back Vane泵pump泵测试效率pump test efficiency泵出力pump delivery泵的基础Pump Foundation泵的类型Pump Types泵的排出口Pump Discharge Nozzle泵的使用范围Application Fields for Pumps泵的输出功率Pump Output泵的旋转方向Direction of Pump Rotation泵房pump house(room)泵功率Pump Power泵和输送装置噪音Noises in Pumps and Pumping Installations 泵壳Pump Casings泵内沉积物Deposits in Pumps泵试验台Pump Test Bed泵输出功率的降低Reduction in Pump Output泵输入功率pump input power泵体pump casing泵吸入槽Pump Sump泵吸入管吸上高度pump lift泵吸入口Pump Suction Nozzle泵效率pump efficiency泵效率Pump Efficiency泵芯包pump cartridge泵性能曲线pump performance curve泵站pumping house泵轴Pump Shaft比例泵proportioning pump比输送功Specific Delivery Work比转数Specific Speed边界层Boundary Layer变矩桨叶Variable Pitch Blade标准泵Standard Pump标准化工泵Standard Chemical Pump标准孔板Standard Orifice Plate标准喷嘴Standard Nozzle标准温度Standard Temperature标准文丘里管Standard Venturi Nozzle标准压力Standard Pressure标准状态Standard Conditions表面保护Surface Protection表压Manometric Pressure并联运转Parallel Operation伯努利方程BERNOULLI Equation补偿器Compensator补给水泵make-up water pump部分负荷运转Part-load Operation残油输出装置Residual Pump-out Device槽slot测量的不精确性Uncertainty in Measurement 测量技术Measuring Technique测量孔板Measuring Orifice Plate测量偏差Measuring Tolerance测量误差Error of Measurement测量仪器Measuring Device测试转速test speed层流Laminar Flow齿轮泵gear pump齿轮传动泵Geared Pump齿轮箱Gearbox冲击冷凝Shock Condensation冲击压力Impact Pressure冲角Angle of Incidence抽出体积Extraction Volume抽气(吸)泵;真空泵suction pump出口侧盖板(大端盖)discharge cover出口截面Outlet Cross-section出口截面宽度Outlet Width出口弯管Outlet Elbow出口压力discharge pressure初始汽蚀Incipient Cavitation传输损失tansmission loss传送器Transmitter船头推进舵Bow Thruster Rudder船坞泵Dock Pump船用泵Marine Pump大气压Barometric Pressure大气压力Atmospheric Pressure带式过滤器Band Sereen单吸离心泵single-suctoin centrifugal单相交流电Single-phase Alternating Current 单叶片叶轮Single Vane Impeller导向叶片Guide Vane导向装置Guide Arrangement低温泵Crygenic Pump低压泵Low Pressure Pump底料泵Sump Pump点蚀Pitting电感应式流量测量Inductive Flow Measurement电功率Electric Power电化学腐蚀Electrochemical Corrosion电机Electric Motor电机电耗Current Consumption of Electric Motors 电机温升Temperature Rise in Electric Motors电机转矩曲线Torque Curve of Electric Motors电解腐蚀Electrolytic Corrosion电缆密封压盖Cable Gland电力驱动Electric Drive电流current电路Electrical Circuits电气开关设备Electrical Switchgear电位序Electrochemical Series电压voltage电压降Voltage Drop顶点Apex定位螺栓fitted bolt动力液流Motive Water Flow动力粘度Dynamic Viscosity动量矩定理Theorem of Momentum动平衡Dynamic Balancing动下室排水泵Cellar Drainage Pump动压力Dynamic Pressure对开的润滑油密封split oil seal对轮the coupling对轮防护罩the coupling guard对轮螺栓coupling nut多级泵Multistage Pump多流式泵Multiflow Pump恩氏粘度Degrees Engler阀门和管件Valves and Fittings法兰结构Flange Construction反向流动Reversal of Flow反应堆泵Reactor Pump反转转速Reverse Rotational Speed防爆Explosion Protection防反转装置Reverse Rotation Locking Device 防腐Corrosion Protection防护装置guard放射Emission非堵塞式叶轮Non-clogging Impeller非扰动管道长度Undisturbed Length of Piping 非稳定流Non-steady Flow非稳定扬程曲线Unstable Throttling Curve费鲁德准数FROUDE Number费用Costs复算Re-evaluation干式安装Dry Installation干运转Dry Running高度Height高速离心泵High Speed Centrifugal Pump高压泵High Pressure Pump隔膜泵diaphragm pump隔膜式计量泵diaphragm type metering pump 给水泵feed water pump给水泵Feed Pump工业水泵industrial water pump工作特征Operating Characteristics公称尺寸Nominal Size公称压力Nominal Pressure功work功率power功率power功率测量power measurement功率调节power control功率损失power loss功率因数power factor cosφ供水泵Water Supply Pump固体颗粒的输送Conveying of Solids过流翼型Flow Profile过滤器Filter海水Sea Water海水泵Sea Water Pump海水淡化装置Sea Water Desalination Plant 海水箱Sea Chest耗水量Water Consumption合适的流量corrected flowrate恒定油位油杯Constant Level Oiler虹吸流动Syphon Flow虹吸装置Syphoning Installation化工泵Chemical Pump化工稳定性表Chemical Stability Table环境保护Environmental Protection环形泵Ring-section Pump环形壳Annular casing回流导向叶片Return Guide Vane回路试验Loop Test混流叶轮Mixed-flow Impeller活塞传送器Piston Transmitter货船油泵Cargo Oil Pump机械传动Mechnical Drive机械密封Mechanical Seal机械效率Mechanical Efficiency基板Baseplate基本方程Fundation基础Foundation基建投资Capital Investment级Stage级间导叶interstage diffuser极数Number of Poles继电器Relay家用供水装置Domestic Water Supply Plant间隙宽度Clearance Gap Width间隙密封Clearance Gap Seal间隙汽蚀Clearance Gap Cavitation间隙压力Clearance Gap Pressure监测装置Monitoring Device键key键槽keyway交流电Alternating Current角速度Angular Velocity接触器Contactor接通压力Switching-on Pressure节流调节throttling control节流系数throttling coefficient进液室Intake Chamber进液条件Intake Conditions进液弯管Intake Elbow浸蚀Erosion经济可行性计算Economic Viability Calcuation净正吸入压头NPSH(Net Positive Suction Head) 径流泵Radial Pump径向力Radial Force径向剖分壳体Radially Split Casing径向推力Radial Thrust径向叶轮Radial Impeller径向轴承journal bearing绝对速度Absolute Velocity卡诺冲击损失Carnot Shock Loss卡普兰弯管Kaplan Elbow铠装泵Armoured Pump抗腐蚀性Corrosion Resistance壳体Casing可抽出性withdrawability可调叶片adjustable vane空气泵Air Pump空气提升泵Air-Lift Pump空心旋涡Hollow Vortex孔板Oriffice Plate孔径比Apertuer Ratio扩压管(圆锥管)Diffuser(Conical Duct)扩压器(导轮)Diffuser(Guide Wheel)冷凝液泵Condensate Pump冷却剂泵Coolant Pump冷却水泵Cooling Water Pump离心泵centrifugal pump离心泵Centrifugal Pump离心泵的安装Installation of Centrifugal Pump离心泵的操作条件Operating Conditions of Centrifugal Pumps离心泵的操作性能Operating Behaviour of Centrifugal Pumps离心泵的冲击损失Shock Loss in Centrifugal Pumps离心泵的间隙损失Clearance Gap Loss in Centrifugal Pumps离心泵的平稳运行Smoop and Quiet Running of Centrifugal Pumps 离心泵的验收试验规范Acceptance Test Codes for Centrifugal Pumps 离心泵的制造材料Construction Material for Centrifugal Pumps离心泵的注水Priming of Centrifugal Pumps离心泵调节Control of Centrifugal Pumps离心泵和驱动设备保养Care of Centrifugal Pumps and Drives 离心泵失衡Out-of-Balance of Centrifugal Pumps离心泵装置Centrifugal Pump Plant离心式鼓风机centrifugal blower离心式滤油机centrifugal oil filter离心式压缩机centrifugal compressor离心水泵centrifugal water pump立式泵vertical pump立式泵Vertical Pump立式屏蔽泵(筒形泵)Vertical Can-type Pump立轴式井泵Vertical Spindle Well Pump利息支付Interest Payment连续性方程Continuity Equation两相流动Two-phase Flow临界转速Critical Speed of Rotation流程泵Process Pump流程型结构Process Type Construction流道涡流Channel Vortex流动分离Flow Separation流动功Flow Output流量flowrate流量Flow流量(体积流量)Capacity流量测定Flow Measurement流量测量Capacity Measurement流量调节器Flow Controller流量系数Flow Coefficient流量指示器、流量观察窗flow indicators流速Flow Velocity流体Fluid流体动力学Fluid Dynamics流体机械Fluid Flow Machine流线Flow Line轮毂比Hub Ratio螺杆泵screw pump(spiral pump)螺纹紧固件threaded fastener马达输出功率motor output power马达输入功率motor input power马达效率motor efficiency迷宫密封labyrinth gland迷宫密封箱体labyrinth gland housing迷宫式密封Labyrinth Seal密封衬套shaft sleeve内效率Internal Efficiency那维尔-斯托克斯方程NAVIER-STOKES Equation耐酸泵Acid Pump焾Enthalpy能Energy能量级Energy Level逆时针旋转叶轮Counterclockwise Rotating Impeller 凝泵condensate extraction pump凝结水泵condensate transfer pump凝汽器抽气泵condenser air pump牛顿流体NEWTONian Fluid扭力杆Torsion Rod扭力计Torsion Dynamometer欧拉方程EULER Equation排出段Discharge Casing排出管线Discharge Line排出管嘴Discharge Nozzle排出损失Discharge Loss排灌站水泵Drainage Station Punp排气Venting排气阀Vent Valve排水泵Dewatering Pump排水量Water Yield排污泵blowdown pump(sewage pump) 排污泵Faeces Pump旁路By-Pass皮带传动Belt Drive皮托管Pitot Tube频率frequency平垫片flat gasket平衡鼓balance drum平衡鼓螺母balance drum nut平衡孔balance'hole平衡液体流量balance water flow平衡装置balancing device平衡状态equilibrium condition平皮带传动flat belt drive屏蔽泵Canned Motor Pump起动Start Up起动过程Starting Process起动时间Starting Time起动转矩Starting Torgue气囊的形成Formation of Air Pockets气体分离Gas Separation汽蚀Cavitation汽蚀磨损Cavitation Wear汽蚀噪音Cavitation Noise强制循环泵forced-circulation pump切断压力Switching-off Pressure切换频率Switching Frequency氢指数Hydrogen Exponent清水泵Clean Water Pump驱动Drive热Heat热泵Heat Pump热含量Heat Content热虹吸Thermosyphon热量Quantity of Heat热载体泵Heat Transfer Media Pump热障Thermal Barrier容积泵Positive Displacement Pump入口导叶suction guide入口管嘴Entry Nozzle入口截面Inlet Cross-section入口压力suction pressure入口锥管Entry Cone润滑油泵Lubricating Oil Pump三相电机Three-phase Motor三相电流Three-Phase Current三相制Three-phase System设计工作点Design Duty Point声学Acoustics时间Time视在功率Apparent Power试验台Test Bed手动泵Hand Pump首级内泵壳first stage ring section输送黏性液体黏性泵centrifugal pump handling viscous liquids 甩油环,抛油环oil thrower双缸泵组Twin Pumping Set双流道泵two-channel impeller pump双流道叶轮two-passage impeller双蜗壳Double Volute水厂泵waterworks pumps水锤water hammer水的硬度hardness of water水力效率hydraulic efficiency水流量计water meter水泥壳泵concrete casing pump水喷射water jet水喷射泵water jet pump水温water temperature水下泵underwater pump水下电机underwater motor水下电机泵underwater motor pump 水银泵mercury pump水硬度water hardness速度Velocity速度测定Measuring of Speeds速度测量Velocity Measurement速度三角形Velocity Triangle损失系数Loss Coefficient锁紧垫圈、防松垫圈lock-washer探头Probe特性Characteristic特性曲线Characteristic Curve特性因数Characteristic Factor体积流量Volume Flow停转时间Run-down Time通量线Flux Line同步转速Synchronous Speed铜导体Copper Conductor投资评价Capital Servicing透平驱动泵Turbine Driven Pump涂层Coat of Paint推力盘thrust collar推力瓦thrust pad推力轴承撑板thrust carrier ring推力轴承室thrust bearing housing托轮Jockey Pulley弯管Elbow弯管壳体泵Elbow Casing Pump卧式泵Horizontal Pump污泥泵Sludge Pump污水泵Sewage Pump无冲击流入Shock-free Entry无泄漏Leak-tightness吸入口Suction Nozzle吸入室Suction Chamber吸入性能Suction Behaviour吸入压头Suction Head吸入叶轮Sution Impeller纤维素Cellulose纤维性物料Fibrous Material相对速度Relative Velocity相合定律Congruence Law相似条件Similarity Conditions相位移Phase Displacement消防泵Fire-fighting Pump消声测量Noise Abatement Measures销钉、定位销dowel pin小舌片tab效率复算Efficiency Re-evaluation斜度Steepness斜流叶轮Diagonal Impelier斜盘式泵Swash Plate Pump泄漏出的密封水隔离门(相当于密封水卸荷阀)leak-off isolating valve 泄漏损失Leakage Loss星形轮Star Wheel星形-三角形起动器电路Star-delta Starter Circuit 性能图Performance Chart许可公差Warranty Tolerance许可区域Warranty Zone旋流Swirl Flow旋塞阀Cock旋涡泵Peripheral Pump旋涡式叶轮Peripheral Impeller旋转方向Direction of Rotation旋转速度Rotational Speed压差Differential Pressure压降Pressure Drop压力Pressure压力波Pressure Wave压力波动Pressure Surge压力测量Pressure Fluctuation压力测量Pressure Measurement压力等级Pressure Categories压力给水装置Hydrophor Plant压力计Manometer压力脉动Pressure Fluctuation压力容器Pressure Vessel压力损失Pressure Loss压力损失Head Loss压力系数Pressure Coefficient压头Pressure Head验收试验Acceptance Test扬程head扬程曲线Throttling Curve叶轮impeller叶轮ipeller叶轮impeller叶轮侧面摩擦Impeller Side Friction叶轮的修整Trimming of Impellers叶轮叶片Impeller Vane叶轮叶片节距调节Impeller Blade Pitch Adjustment叶片叶片vane叶片角Blade Angle叶片节距调节机构Blade Pitch Adjustment Gear叶片节距调节装置Blade Pitch Adjustment Device叶梢背部切削Cutting Back of Impeller Vane Tips叶栅vane cascade叶栅流动Cascade Flow液化气泵Liquefied Gas Pump液环泵Liquid Ring Pump异步电机Asynchronous Motor阴极保护Cathodic Protection音量级Sound Volume Level音速Sound Velociyt引水级Priming Stage应变测定技术Strain Measurement Technology应力腐蚀(裂纹)Stress Corrosion (Cracking)永久磁铁联轴器Permanent Magnet Coupling用入口导向叶片控制涡流Rotation Swirl Control by Inlet Guide Vanes 油泵Oil Pump油挡oil guard有势流动Potential Flow有效功率Acitive Power有效汽蚀余量n.p.s.h.r有用功率输出Useful Output右旋叶轮right-handed impeller诱导轮Inducer圆弧形叶片Circular Arc Vane圆周速度Peripheral Speed(Circumferential Velocity) 运动粘度Kinematic Viscosity运行时数Number or Running Hours再生泵Regenerative Pump脏水泵Dirty Water Pump折旧Amortization振动Vibration正吸入压头positive suction head直径系数Diameter Coefficient直联泵组Close-coupled Pumping Set直流电Direct Current直流电机Direct Current Motor直流平板式起动器Direct Current Face Plate Starter 止动销stop pin纸浆泵Pulp Pump纸浆浓度Pulp Density纸浆输送Conveying of Pulp制造公差Manufacturing Tolerance质量Mass质量惯性矩Mass Moment of Inertia质量流量Mass Flow中点线Mid-point Conductor中性线Neutral Conductor中压泵Medium Pressure Pump重力加速度Gravitational Constant重量Weight轴轴承Bearing轴承支架bearing housing轴承支架盖bearing housing cover轴封Shaft Seal轴封环Shaft Sealing Ring轴功率Shaft Power轴颈shaft journal轴流泵axial pump轴流泵Axial Pump轴流叶轮Axial Impeller轴套、衬套gland bush轴推力Shaft Thrust轴向力Axial Force轴向剖分壳体Longitudinally Split Casing轴向推力Axial Thrust主循环泵Primary Circulating Pump柱塞泵Plunger Pump转动惯量Moment of Gyration转矩Torque转矩的测定Torque Measurement转速传感器Rotational Speed Transmitter自动调节Self-regulation自动断路器Self-acting Circuit Breaker自动开关Automatic Switches自耦变压器式起动器Autotransformer Starter 自吸泵Self-priming Pump自由流动泵Free-flow Pump总测量偏差Overall Measuring Tolerance总静压头Total Static Head总偏差Overall Tolerance总效率Overall Efficiency总压力Tital Pressure总扬程Total Headmeasured flowratevelocity headgauge/lever correctiongenerated headseal sleeve nuts短心轴stub axle轴axes轴axis轴axle(U.S.A)轴承衬套bearing bushing(U.S.A)轴肩挡圈;防护罩protecting collar巴氏合金;白合金;轴承合金white metal半贯流式轴流泵;弯管轴流泵angle-type axial flow pump半可调式轴流泵axial flow pump with blades adjustable when stationary 泵轴Pump Shaft泵轴承支架pump bearing bracket长轴深井泵borehole shaft driven(centrifugal) pump长轴深井泵(美)multistage vertical turbine pump衬套;轴套bushing齿形联轴器gear-type coupling出口轴面速度系数;流量系数capacity constant磁力联轴节齿轮泵magnetically coupled gear pump从动螺杆;从动心轴idler spindle从动轴driven shaft从动轴idler shaft弹性盘联轴器flexible disc coupling弹性圆柱销联轴器flexible pin coupling电磁联轴器electromagneic coupling电磁联轴器magneto coupling电磁联轴器magneto-coupling调节轴adjusting spindle副传动轴countershaft spindle副轴;中间轴;从轴auxiliary shaft刚性联轴器solid coupling刚性联轴器;刚性联接rigid coupling功率曲线;轴功率曲线;制动功率曲线brake horsepower curve贯流泵;直管轴流泵rubular type axial flow pump滚动轴承anti-friction bearing滚动轴承ball/rolling bearing滚动轴承rolling contact bearing滚针轴承needle bearing恒定轴向间隙齿轮泵fixed axial clearance gear pump滑动轴承plain friction bearing滑动轴承sleeve bearing滑动轴承sliding bearing活塞销;轴头销gudgeon pin架;轴承衬套(美);泵支架(美) frame径向滚柱轴承radial roller bearing径向球轴承radial ball bearing径向轴封radial shaft seal可调式轴流泵axial flow pump with adjustable(or variable)pitch blades 可调式轴流泵propeller pump with adjustable or variable pitch blades 可逆叶片轴流泵axial flow pump with reversible blades可逆叶片轴流泵propeller pump with reversible blades空心轴hollow shaft shaft冷冻装置用无轴封泵glandless pump for refrigerating installation立轴式井泵Vertical Spindle Well Pump联轴器coupling笼形轴承托架bearing bracket lantern笼形轴承托架bearing housing lantern螺纹联轴器screwed coupling米切尔型推力轴承Michell type thrust bearing内轴承泵pump with internal bearing(s)挠性法兰联轴器flexible flange coupling挠性联轴器elastic coupling挠性联轴器flexible coupling挠性轴flexible shaft配流盘式轴向活塞泵flat valve axial piston pump配流盘式轴向活塞泵port plate axial piston pump配流盘式轴向活塞泵valve plate axial piston pump喷水推进轴流泵axial flow pump for water jet propulsion喷水推进轴流泵water jet propulsion axial flow pump偏心轴eccentric shaft强制润滑轴承;压力润滑轴承forced oil lubricated bearing球面推力轴承spherically mounted thrust bearing曲轴crankshaft曲轴防护罩crankguard曲轴防护罩crankshaft guard曲轴箱支座crankcase pedestal曲轴销crank pin驱动轴;传动轴drive shaft全可调式轴流泵axial flow pump with blades adjustable in operation 全可调式轴流泵axial flow pump with variable piteh blades水润滑轴承water lubricating bearing套筒联轴器sleeve coupling推力滚柱轴承thrust roller bearing推力球轴承ball thrust bearing推力轴承thrust bearing推力轴承;扇形块thrust bearing segment推力轴承扇形块thrust bearing pad外轴承泵pump with external bearing(s)万向轴cardan shaft无轴封泵glandless pump无轴封计量泵glandless metering pump橡胶轴承rubber bearing斜垫轴向推力轴承tilting pad axial thurst bearing斜缸型轴向柱塞泵tilting cylinder block type axial plunger pump斜盘式轴向活塞泵cam plate type axial piston pump斜盘式轴向活塞泵swash plate axial piston pump 斜盘式轴向活塞泵wobble plate axial piston pump 斜置轴流泵inclined axial flow pump斜轴式轴向活塞泵angle-type axial piston pump斜轴式轴向活塞泵bent axis axial piston pump旋涡轴线eddy axis旋涡轴线vortex axis压入式轴承盖pressed-in type bearing cover叶轮轴面形状;工作轮图profile of the impeller液体动力轴承hydrodynamic bearing永久磁铁联轴器Permanent Magnet Coupling油环轴承ring lubricating bearing油环轴承ring oiling bearing油脂润滑轴承grease lubricated bearing支轴销;支点销fulcrum pin支座;轴架pedestal中间心轴idler axle中间心轴intermediate axle中间轴;副轴;从轴counter shaft中间轴;副轴;从轴intermediate shaft轴shaft轴;心轴;锭子spindle轴承bearing轴承Bearing轴承衬(套)bearing bush轴承衬套bearing cartridge轴承衬套bearing insert轴承端盖bearing end cover轴承盖bearing cover轴承合金bearing alloy轴承架bearing bracket轴承架bearing pedestal轴承架bearing spider轴承架;泵托架headstock轴承架固定式泵pump with bearing bracket轴承浸泡试验;轴承泡胀试验bearing swelling test 轴承冷却室bearing cooling chamber轴承螺母bearing nut轴承套bearing sleeve轴承体;轴承箱bearing box轴承托架箱bearing bracket housing轴承瓦;轴承衬垫;轴承衬套bearing line轴承吸水试验bearing absorption test轴承箱;轴承体bearing housing轴承座bearing carrier轴对称axial symmetry轴对称;旋转对称rotational symmetry轴对称流axisymmetrical flow轴封shaft seal轴封Shaft Seal轴封环Shaft Sealing Ring轴功率Shaft Power轴功率;制动功率;制动马力brake horsepower 轴护套Shaft Protecting Sleeve轴护套shaft tunnel tube轴护套(美)shaft enclosing tube轴肩挡圈loose(shaft) collar轴肩挡圈shaft collar轴肩挡圈shoulder ring轴颈neck journal轴颈shaft neck轴颈axle journal轴颈;期刊journal轴颈套;填料衬套neck bush轴流泵;螺桨泵axial flow pump轴流泵叶轮室propeller bowl轴流式涡轮机axial-flow turbine轴流式叶轮axial flow impeller轴流叶轮Axial Impeller轴流增压器axial flow booster轴马力;轴功率shaft horsepower轴面流线meridian streamline轴面速度meridional velocity轴面投影图elevation view轴配流径向活塞泵pintle valve radial peston pump轴配流径向活塞泵valve spindle radial piston pump轴套shaft wearing sleeve轴套Axis Guide轴套shaft sleeve轴套;套(筒)sleeve轴套拆卸器sleeve puller轴推力Shaft Thrust轴瓦;轴承箱;轴承体bearing sheet轴位指示器shaft position indicator轴吸泵axial inlet pump轴向单吸液环泵axial single entry liquid ring pump轴向导叶axial diffuser轴向滑移aixal slip轴向活塞泵axial piston pump轴向加速度axial acceleration轴向间隙axial clearance轴向间隙压力补偿齿轮泵gear pump with pressure-dependent axial clearance 轴向间隙压力补偿齿轮泵gear pump with pressurized side plate轴向力axial force轴向力Axial Force轴向磨损指示器axial wear indicator轴向剖分壳体Longitudinally Split Casing轴向双吸液环泵axial double entry liquid ring pump轴向速度axial velocity轴向推力axial thrust轴向推力Axial Thrust轴向吸入泵axial suction pump轴向压力axial pressure轴向柱塞泵axial plunger pump主动螺杆;驱动心轴driving spindle主动轴driving shaft柱销联轴器pin coupling爪形联轴器claw coupling爪形联轴器dog coupling爪形联轴器jaw coupling锥形联轴器cone(type) coupling锥形联轴器taper coupling子午面;轴面meridian plane自锁轴套self-locking sleeve半贯流式轴流泵;弯管轴流泵angle-type axial flow pump半可调式轴流泵axial flow pump with blades adjustable when stationary 泵pump泵房pump house泵盖casing cover泵缸pump barrel泵缸pump cylinder泵缸套pump barrel line泵缸套pump cylinder line泵缸体pump cylinder block泵工况pump operating condition泵工况pump operating duty泵体pump body泵体衬套pump body insert泵体衬套pump casing insert泵托架pump bracket泵站设计design of pump station泵罩outer pump mantle泵支架pump bearing lantern泵轴承支架pump bearing bracket泵装置pumping device泵装置pumping machine泵组integral pump group泵座pump frame闭式叶轮泵pump with enclosed impellers波纹管泵bellows pump仓底泵bilge pump侧流道泵壳;旋涡泵泵体casing with transfer passages 侧流道泵壳;旋涡泵泵体annular casing长轴深井泵borehole shaft driven(centrifugal) pump 衬青铜泵bronze fitted pump齿轮比例泵abjustable discharge gear pump冲击波泵(振荡器)shock wave oscillator导流壳式多级泵pump with bowl-type vaned casing低温泵cryopump底料泵;塔底液泵bottoms pump底吸泵bottom inlet pump底吸泵bottom suction pump电磁驱动泵pump with cyclic electromagnetic drive风动活塞泵air-powered piston pump辅泵;辅助泵auxiliary pump辅扫仓泵;副清仓泵auxiliary stripping pump附属泵attached pump工程用泵building site pump固定缸体泵pump with nonrotating cylinder固体输送泵pump for water-borne solids管网泵pump for pipe system灌泵priming of pump锅炉给水泵BFW锅炉给水泵boiler feed pump锅炉回水循环泵boiler return circulating pump锅炉酸洗泵pump for acid washing of boiler航空用泵aviation pump合金泵alloy pump合金钢泵alloy steel pump环壳泵annular casing pump灰渣泵ash pump回水泵back water pump活性污泥泵activated sludge pump活性污泥泵biological sludge pump机械密封泵pump with mechanical seal基本负荷泵base load pump加硼泵boron addition pump甲板泵board pump架;轴承衬套(美);泵支架(美) frame减摇泵anti-roll pump铰链滑片泵articulated vane pump接力泵boost pump铠装泵armoured pump可调隔膜泵adjustable diaphragm pump可调式轴流泵axial flow pump with adjustable(or variable)pitch blades 可逆叶片轴流泵axial flow pump with reversible blades沥青泵bitumen pump螺旋泵Archimedean screw pump密闭式螺杆泵airtight screw pump内轴承泵pump with internal bearing(s)耐磨泵abrasion resisting pump耐酸泵acid pump逆洗泵back wash pump农用喷药泵agricultural spray pump for chemicals盘状活塞泵bucket piston pump喷灌泵agricultural spray pump喷射泵;喷射器ejector喷水推进轴流泵axial flow pump for water jet propulsion喷雾器-泵组atomizer-pump皮带传动泵belt driven pump啤酒泵beer pump平衡转子式滑片泵balanced rotor vane pump气抽液喷射泵gas jet liquid ejector气动泥浆泵air-pressure actuated slurry pump气泡泵;曼木特泵;气举泵air lift pump气体喷射泵gas ejector汽车用泵automobile pump汽抽气喷射泵steam jet air ejector汽抽水喷射泵steam jet water ejector前置泵;增压泵booster pump潜水深井泵borehole submerged pump球形活塞泵ball piston pump全可调式轴流泵axial flow pump with blades adjustable in operation 全可调式轴流泵axial flow pump with variable piteh blades全青铜泵all bronze pump熔盐泵pump for liquid salts pump上壳(井泵);吐出段delivery casing上壳(井泵);吐出段discharge casing深井泵borehole pump食品泵pump for edible fluids双壳泵barrel insert pump双吸泵balanced suction pump水抽气喷射泵water jet air ejector水锤泵;振动机pulsator水喷射泵water ejector饲槽自动泵;家畜自动饮水泵animal self-operated drinking water pump 饲槽自动泵;家畜自动饮水泵automatic trough pump碎纸浆泵broke pump甜菜泵beet pump甜菜根泵beet tails pump筒袋式泵barrel pump筒袋式油泵barrel oil pump外轴承泵pump with external bearing(s)往复式深井泵borehole reciprocating pump微型泵micro-pump无叶片泵bladeless pump吸附真空泵absorption vacuum pump吸收泵absorption pump相对叶轮泵;背靠背叶轮泵pump with opposed impellers箱桶抽空泵barrel emptying pump斜轴式轴向活塞泵angle-type axial piston pump斜轴式轴向活塞泵bent axis axial piston pump悬臂叶轮泵pump with overhung impeller压缩空气驱动泵air operated pump压载泵ballast pump盐水泵brine pump叶轮串并联泵pump with series or parallel connection impellers液化天然气用低温泵cryopump for liquefied natural gas液态金属泵pump for liquid metals液体喷射泵liquid ejector增压泵boosting pump增压泵;压送泵;气筒inflator增压给水泵booster-feed pump增压扩散泵booster diffusion pump蔗渣泵bagnsse pump蒸汽机泵steam pumping engine蒸汽脉冲泵;脉冲泵steam pulsator蒸汽脉冲泵;脉冲泵pulsometer蒸汽喷射泵seam ejector中段泵壳;中段stage casing中开泵axially split pump轴承架;泵托架headstock轴承架固定式泵pump with bearing bracket轴流泵;螺桨泵axial flow pump轴流泵叶轮室propeller bowl轴吸泵axial inlet pump轴向单吸液环泵axial single entry liquid ring pump轴向活塞泵axial piston pump轴向双吸液环泵axial double entry liquid ring pump轴向吸入泵axial suction pump轴向柱塞泵axial plunger pump铸铁泵all iron pump转子可抽出式泵pump with withdrawable rotor assembly钻井冲洗泵borehole flushing pump摆线螺杆泵cycloidal screw pump半开式叶轮离心泵centrifugal pump with semien closed impeller pump 闭式旋涡泵closed peripheral pump闭式叶轮离心泵centrifugal pump with shrouded impeller不堵塞泵chokeless pump舱壁泵bulkhead mounted pump除氧化皮泵descaling pump除氧器供给泵deaerator lift pump除氧器循环泵deaerator recirculating pump传导泵conduction pump地下室排水泵cellardrainage pump定压泵constant pressure pump非接触齿轮泵contactless gear pump伏辊断头纸桨泵couch break pump给料泵charge pump恒速泵constant speed pump化工用泵chemical pump化学纸浆泵chemical pulp pump化学纸浆泵chemical stock pump环壳泵circular casing pump混料泵contactor pump混凝土泵cement grout pump混凝土泵cementation pump混凝土泵concrete pump混凝土涡壳泵concrete volute pump货油泵cargo oil pump夹装泵bung mounted pump甲板泵deck pump甲板冲洗泵deck wash pump开式叶轮离心泵centrifugal pump with open impeller 矿井工作面泵coal face pump冷剂泵collant pump冷剂泵coolant pump冷剂泵cooling medium pump冷凝泵condensate pump冷凝泵condensate extraction pump冷却水泵cooling water pump离心泵centrifugal pump离心机出料泵centrifugal run-off pump离心喷射泵centrifugal-jet pump离心-旋涡泵centrifugal-peripheral pump离心-旋涡泵centrifugal-turbine pump链传动泵chain drive pump裂化装置泵cracking pump流道式叶轮泵channel impeller pump滤清污水泵decanted sewage pump氯乙烯塑料泵chlorovinyl plastic pump煤泥泵coal slurry pump煤水泵coal pump内啮合齿轮泵crescent gear pump内啮合齿轮泵crescent seal gear pump内配流径向活塞泵centrally ported radial piston pump内循环形屏蔽泵canned motor pump with inner recirculation 耐蚀泵corrosion free pump耐蚀泵corrosion resisting pump凝水回收泵condensate recovery pump凝水回收泵condensate return pump凝水-增压泵condensate-booster pump凝水-增压-给水泵condensate-booster-feed pump屏蔽泵canned motor pump汽车冲洗泵car wash pump巧克力输送泵chocolate pump切削冷却乳剂泵cutting oil pump清水泵clean water pump清水泵clear water pump曲柄泵crank pump曲柄飞轮泵crank and flywheel pump深井泵deepwell pump石灰汁泵;清汁泵clarified juice pump手压泵;手动泵cottage pump水泥桨泵cement slurry pump陶瓷泵ceramic pump陶瓷酸泵ceramic acid pump甜菜丝泵cossette pump凸轮转子式刮片泵cam rotor vane pump凸轮转子式刮片泵cam-vane pump外循环形屏蔽泵canned motor pump with outer recirculation 无曲柄泵crankless pump无曲柄多缸泵crank-less multicylinder pump无曲柄污水泵crankless sewage pump无曲柄消防泵crankless fire pump洗舱泵butterworth pump洗煤用泵coal washing pump向心泵centripetal pump斜盘式轴向活塞泵cam plate type axial piston pump循环泵circulating pump压缩空气驱动泵compressed air pump液化天然气用低温泵cryo pump for LNG诱导轮离心泵centrifugal pump with inducent原生污泥泵crude sludge pump原生污水泵crude sewage pump原油泵crude oil pump圆筒感应泵cylindric induction pump粘浆泵cellulose pulp pump直联泵;单体泵close coupled pump中央密封式活塞泵centre-packed type piston pump铸钢泵cast steel pump装入式泵built-in pump(主机)半负荷泵half load pump(主机)全负荷泵full load pump比例泵dosing pump玻璃泵glass pump差动活塞泵differential piston pump柴油机驱动泵diesel pump长喉管喷射泵jet pump with long Venturi tube衬硬胶泵ebonite pump衬硬胶泵hard rubber lined pump成品泵discharge pump成品泵final product pump齿轮泵gear pump齿轮比例泵gear dosing pump齿轮传动泵geared pump冲洗泵flushing pump船坞泵dock pump带隔片的偏心转子泵eccentric rotary pump with dividing plate 带柔性环的偏心滚柱泵eccentric rotary pump with elastic ring 单列活塞泵in-line piston pump单螺杆泵helical rotor pump弹性刮片泵flexible vane pump淡水泵fresh water pump导叶泵diffuser pump灯笼架固定泵lantern mounted pump灯笼架支承泵lantern based pump底脚固定泵foot mounted pump地区取热泵;分区供暖泵district heating pump地下水排水泵groundwater drainage pump地下水排水泵;排水泵de-watering pump电磁泵electromagnetic pump电磁隔膜泵electromagnetic diaphragm pump电磁计量泵electromagnetic metering pump电磁偶合泵electromagnetically coupled pump电磁喷射泵electro-magnetic jet pump电磁式比例泵electromagnetic proportioning pump电动泵electrically driven pump电动给水泵electrically driven feed pump电动潜水泵electro-submersible pump电动潜水排水泵electro-submersible drainage pump。
镁锂合金的腐蚀机理及表面防护方法研究进展

2017年第36卷第9期 CHEMICAL INDUSTRY AND ENGINEERING PROGRESS·3373·化 工 进展镁锂合金的腐蚀机理及表面防护方法研究进展高晓辉1,2,李玉峰3,祝晶晶2,景晓燕1(1哈尔滨工程大学材料科学与化学工程学院,黑龙江 哈尔滨 150001;2齐齐哈尔大学化学与化学工程学院,黑龙江 齐齐哈尔 161006;3齐齐哈尔大学材料科学与工程学院,黑龙江 齐齐哈尔 161006)摘要:镁锂(Mg-Li )合金具有质量超轻、强度高、延展性好和成形性好等优点,在汽车、航空航天、军事及核工业等领域具有广阔的应用前景。
但是高化学活性的锂使该合金易在使用环境中发生腐蚀且难以防护,限制了其广泛应用。
因此,研究Mg-Li 合金的腐蚀机理并发展有效的防腐蚀技术极为重要。
基于近年来国内外的研究进展,本文综述了Mg-Li 合金在大气、中性及碱性NaCl 溶液和模拟人体体液中的腐蚀机理,介绍了Mg-Li 合金在不同环境中的腐蚀过程。
同时对Mg-Li 合金的表面防护方法作了系统总结,包括阳极氧化、电镀与化学镀、化学转化、涂层及其他表面防腐蚀方法,分析了各种表面防护方法的特点、优势与不足。
并对Mg-Li 合金表面防护的未来发展进行了展望,提出通过涂层的复合化、功能化及自修复化可以提高涂层对Mg-Li 合金的防腐蚀性能和实用性。
关键词:镁锂合金;腐蚀;表面;阳极氧化;化学镀;化学转化膜;涂层中图分类号:TB31 文献标志码:A 文章编号:1000–6613(2017)09–3373–07 DOI :10.16085/j.issn.1000-6613.2017-0198Corrosion mechanism and surface protection method formagnesium-lithium alloyGAO Xiaohui 1,2,LI Yufeng 3,ZHU Jingjing 2,JING Xiaoyan 1(1College of Material Science and Chemical Engineering ,Harbin Engineering University ,Harbin 150001,Heilongjiang ,China ;2College of Chemistry and Chemical Engineering ,Qiqihar University , Qiqihar 161006,Heilongjiang ,China ;3College of Materials Science and Engineering ,Qiqihar University ,Qiqihar 161006,Heilongjiang ,China )Abstract :Magnesium-lithium (Mg-Li )alloy have attracted considerable interest in automobiles ,aerospace ,military and nuclear industries because of their super lightweight ,high strength ,high ductility and good formability. However ,the high chemical activity of lithium means the Mg-Li alloys are susceptible to corrode in applied environment and difficult to protect ,which limit their widespread practical application. Therefore ,it is important to investigate the corrosion mechanism of Mg-Li alloys and develop efficiently anticorrosion technology. In this paper ,a review was provided on the current status of corrosion mechanism of Mg-Li alloys in atmosphere ,neutral and alkaline NaCl solution and Hank’s solution based on recent progress at home and abroad. The corrosion behavior of Mg-Li alloys in different environment was introduced. The research progress of surface protection technologies for Mg-Li alloys were systematically summarized ,including anodic oxidation ,electroplating and electroless plating ,chemical conversion coatings ,coating and other surface anticorrosion methods. The第一作者:高晓辉(1972—),女,副教授,从事金属的腐蚀与防护方面的研究。
2A12铝合金微弧氧化膜电化学腐蚀性能研究

2A12铝合金微弧氧化膜电化学腐蚀性能研究刘 竝(中南大学 材料科学与工程学院,长沙 410083)[摘 要]采用微弧氧化技术对粉末冶金制备的2A12铝合金进行表面改性,表征改性层微观结构和相组成,评价改性后2A12铝合金的电化学腐蚀性能。
研究发现:微弧氧化处理之后,2A12铝合金表面生成厚度约为80 μm 的均匀氧化膜,其主要由α-Al 2O 3、γ-Al 2O 3及非晶组织构成;采用微弧氧化对2A12铝合金表面改性之后,改性层极大程度地抑制合金表面阳极反应和阴极反应的进行,自腐蚀电流密度明显下降,腐蚀速率显著降低,合金电化学腐蚀性能明显提高。
[关键词]粉末冶金; 微弧氧化; 2A12铝合金; 表面改性; 电化学腐蚀[中图分类号] TG178 [文献标志码] A doi :10.3969/j.issn.1673-6214.2020.05.007[文章编号] 1673-6214(2020)05-0305-07Electrochemical Corrosion Behaviour of Microarc Oxidized Filmon 2A12 Aluminium AlloyLIU Bing(School of Materials Science and Engineering, Central South University, Changsha 410083, China)Abstract: The surface of 2A12 aluminium alloy fabricated by powder metallurgy has been modified by microarc oxidation. The microstructure and phase composition of the modified layer have been characterized, and the electrochemical corrosion performance of 2A12 aluminium alloy after surface modification has been evaluated. It is found that a uniform oxidized film with the depth of about 80 μm is formed on 2A12 aluminium alloy, which consists of α-Al 2O 3, γ-Al 2O 3 and amorphous structure. After surface modification by microarc oxidation, the modified layer on 2A12 aluminium alloy greatly inhibits the anodic and cathodic reactions of the alloy surface, and the corrosion current density and corrosion rate significantly decrease, suggesting the electrochemical corrosion performance of the alloy is obviously improved.Key words: powder metallurgy; microarc oxidation; 2A12 aluminium alloy; surface modification; electrochemical corrosion0 引言铝合金粉末冶金的研究始于20世纪50年代[1]。
第2章电化学分析法导论仪器分析ppt课件

式中EL为液体接界电位 。
铜锌原电池由于右边铜电极的电位比锌电极高,
故E电池为正值,表示电池反应能自发地进行;
铜锌电解池右边锌电极的电位比铜电极低,则其
E电池为负值,表示电池反应不能自发地进行,必须
外加一个大于该电池电动势的外加电压,才能使电 池反应进行。
15
二、电极电位(Electrode Potential)
ቤተ መጻሕፍቲ ባይዱ原电池
电解池
13
正确区分阴、阳极,正、负极
( ) 右 左
E为正时,为自发电池,为负时,是电解池。
原电池(Galvanic Cell) : 阳极—负极(左-,氧化反应,失电子) 阴极—正极(右+,还原反应,得电子)
电解池(Electrolytic Cell) : 阴极—负极(右-,与电源负极相连,得电子) 阳极—正极(左+,与电源正极相连,失电子)
30
以阴极还原过程为例,在电流密度较大的情 况下,单位时间内供给电极的电荷数量相当多, 如果电极反应速度很快,则可在维持平衡电位不 变的条件下,使金属离子被还原。
相反,如果电极反应速度有限,离子来不及 与电极表面上过剩的电子结合,就将使电子在电 极表面上积聚起来,从而使阴极电位变负。对于 阳极来说,则将使阳极电位变正。
可用于常量组分、微量组分和痕量组分的测定;
选择性高,应用范围广等。
3
2010年7月28日,吉林省永吉县境内发生特大洪水,永吉县经济
开发区新亚强化工厂一批装有三甲基一氯硅烷的原料桶被冲入松花江中。最新
统计称,流入松花江的化工物料桶达7000只左右,其中4000只左右为空桶,
3000只左右为原辅料桶。
氯化镁-DMF体系类离子液体物理性质的测定及意义

氯化镁-DMF体系类离子液体物理性质的测定及意义姜小萍;汪继辉【摘要】离子液体是指全部由离子组成的液体,如高温下呈液体状态的KCl、KOH 等,它有着优异的物理化学性质,如高电导率、低黏度、宽的电化学窗口、无毒和低蒸汽压等,受到了广泛的关注[1-4].利用DMF和六水合氯化镁固体,在常温、混合搅拌的条件下合成了一种无色、透明、均一性良好的类离子液体.该类离子液体有着与离子液体相似的物化性质.在密封良好的情况下,采用卡尔费体电解法测定类离子液体中的微量水含量,并进行了水含量的理论值与实际值的比较,得出了去除氯化镁中结晶水的方法.利用电导率仪测定了该类离子液体的电导率,并得出了其电导率随温度和组成的变化关系.【期刊名称】《山西化工》【年(卷),期】2014(034)001【总页数】4页(P31-33,47)【关键词】类离子液体;电导率;水含量;六水合氯化镁;DMF【作者】姜小萍;汪继辉【作者单位】青海民族大学化学与生命科学学院,青海西宁810007;青海民族大学化学与生命科学学院,青海西宁810007【正文语种】中文【中图分类】O646在电化学研究中,常常会以离子液体作电解液应用于制造新型高性能电池、太阳能电池以及电容器等。
作为一种绿色环保的溶剂,同时对反应还起到催化作用,常会以离子液体作为溶剂来达到合成的目的。
而在最近几年,Abbott等[5-7]发现了一类含有氯化胆碱的低共熔溶剂,这类低共熔溶剂具有与离子液体相似的物化性质,被称为类离子液体。
该类离子液体因其具有低黏度、高电导率等优点而主要应用于金属及金属合金的电沉积研究。
目前,对于含有镁元素的类离子液体的报道很少,非氯化胆碱体系中含镁的类离子液体则更少。
本文利用六水氯化镁和DMF溶剂作为反应物,在常温搅拌的条件下合成氯化镁-DMF体系的类离子液体,并对合成物众多的物理化学性质进行测定。
其中,主要为电导率和含水量的测定。
1 实验部分1.1 试剂和仪器氯化镁,分析纯,天津市科密欧化学试剂有限公司;DMF,国药集团化学试剂有限公司;卡尔费休试剂,国药集团化学试剂有限公司。
扫描振动电极(SVET)方法表征涂层的防腐蚀性能

SVET method for characterizing anti-corrosion performance of metal-rich coatingsMaocheng Yan *,Victoria J.Gelling **,Brian R.Hinderliter,Dante Battocchi,Dennis E.Tallman,Gordon P.BierwagenDepartment of Coatings and Polymeric Materials,North Dakota State University,Fargo,ND 58108,USAa r t i c l e i n f o Article history:Received 17January 2010Accepted 12April 2010Available online 28April 2010Keywords:A.Metal-rich coating A.SteelB.SVETB.Microelectrode techniqueC.Galvanic interactiona b s t r a c tThe galvanic interaction between a metal-rich coating and the underlying metal substrate was character-ized by a new analysis method based on the scanning vibrating electrode technique (SVET).The total ano-dic current at various immersion periods was evaluated by integrating the anodic current density on SVET maps.Zinc-rich paints (ZRPs)coated on a steel panel were used to demonstrate the experimental approach.The anti-corrosion performance of the ZRP was analyzed based on the integrated anodic cur-rent and the experimental E OC –i Int diagram.Closely correlative behaviour was found between the inte-grated anodic current and the open-circuit potential.Published by Elsevier Ltd.1.IntroductionAs one of the most cost effective methods for corrosion protec-tion of metallic objects,organic coatings provide corrosion protec-tion mainly by four ways:a barrier effect,sacrificial cathodic protection,corrosion inhibitor release,and anodic protection.Metal-rich coatings (MRCs)[1,2]are a class of corrosion protection coatings containing sacrificial metal pigments that are more elec-trochemically reactive than the underlying metal substrate,which inhibit corrosion by providing sacrificial/cathodic protection to the metal substrate.MRCs are generally designed with high volume fraction of metal pigment (near critical pigment volume concentra-tion,CPVC)dispersed in non-conductive polymer or inorganic matrix.The most effective and commonly used MRCs for steels are Zn-rich primer (ZRP)coatings [3–5].Most recently,Mg-rich coatings have been developed and found to provide similar protec-tion to aerospace Al alloys [6–8].Various electrochemical methods have been employed to assess the anti-corrosion performance of metal-rich coatings,such as cor-rosion potential measurements,electrochemical impedance spec-troscopy (EIS)[3,9–12],electrochemical noise methods (ENM)and galvanic coupling measurement [3,13].Murray [9,14]reviewed electrochemical methods used for evaluating organic anti-corrosion coatings.Sekine [15]gave a review on characteristics of various electrochemical measurement methods and even their correlations.The development of microelectrode techniques and scanning elec-trode techniques has made it possible to measure electrochemical processes on a local scale,which has yielded new types of informa-tion relevant to the local electrochemical processes on corroding surfaces and advanced the investigations of localized corrosion.Among all the techniques are the Scanning Vibrating Electrode Tech-nique (SVET),the Scanning Reference Electrode Technique (SRET),Local Electrochemical Impedance Spectroscopy (LEIS),and the Scan-ning Kelvin Probe (SKP).SVET was originally devised for detecting the extra-cellular cur-rent near living cells in the 1970s [16].It was firstly developed to study localized corrosion processes by Isaacs in the 1980s [17,18].The electrochemical process of corrosion contains an ionic current flow in the electrolyte balanced by the electron flow through the metal.The ionic current flow causes a potential gradi-ent to exist in the solution at the electrochemically active site.SVET was designed to detect the potential gradient via a movable vibrating microelectrode.The electrode potential difference between the two extreme points of its vibration,r /,is recorded at the extremes of the vibration amplitude,generating a sinusoidal AC signal.The AC signal is then converted to the ion current density (i )by a calibration procedure [10,19].The local current is related to r /and the electrolyte conductivity k by Ohm’s law [20]i ¼Àj r /ð1ÞSVET systems are designed to oscillate the probe in a Lissajous mode so that both parallel component i x and perpendicular compo-nent i z of the current can be obtained by partial differentiation of (1)with respect to x or z .0010-938X/$-see front matter Published by Elsevier Ltd.doi:10.1016/j.corsci.2010.04.012*Corresponding author.Tel.:+17012318027;fax:+17012318439.**Corresponding author.E-mail addresses:Maocheng.Yan@ (M.Yan),V.J.Gelling@ (V.J.Gelling).Corrosion Science 52(2010)2636–2642Contents lists available at ScienceDirectCorrosion Sciencej o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /c o r s ci1.1.SVET for characterizing anti-corrosion performance of coatingsSVET has enjoyed wide acceptance as a powerful electrochemi-cal technique for evaluation of corrosion inhibitor,detection of cor-rosion activity and quantification of corrosion defects in coatings. SVET has been used in the research of various types of corrosion, such as pitting[21],cut-edge corrosion[22–24],galvanic corrosion [18],microbiologically influenced corrosion(MIC)[25],weld corro-sion,and stress corrosion cracking(SCC)[26].In the case of corro-sion of a coated metal,SVET is able to give detailed insights into the electrochemical interactions between a coating and its sub-strate at a defect,which has been provided valuable information on the anti-corrosion mechanism by a coating,including the gener-ation and development of defects,and the influence of pigments/ inhibitors on corrosion of substrate at a defect[27,28].In previous studies from this laboratory,series of coatings for Al alloy(AA)2024-T3has been characterized by SVET.To monitor both the corrosion activity of the substrate and the possible gal-vanic interaction between the coating and the substrate,the mea-surement was conducted in the vicinity of a scratch exposing the underlying substrate.The SVET results for polypyrrole deposited on AA2024-T3showed that a large anodic current occurred at the defect due to anodic dissolution of the alloy and that the catho-dic current was rather uniformly distributed over the polymer sur-face,which implies that a p-doped CP would promote active dissolution of the AA2024-T3substrate at the defect if the passiv-ation could not be obtained[29,30].Most recently,interesting interactions between neutral or n-doped poly(2,3-dihexylthieno-[3,4-b]pyrazine)and AA2024-T3has been demonstrated by SVET [31].The n-doped conjugated polymer exhibited the ability to sac-rificially protect the exposed Al alloy in a defect.For a redox inactive barrier coating,such as a plain epoxy coat-ing on steel or AA2024-T3,the SVET showed that both anodic cur-rent and cathodic current were located at the scribe[32].Due to the high impedance/low-conductivity of the intact barrier coating, a complete corrosion cell,if any,would be established within the defect,and no current was distributed on the coating.In the case of metal-rich coatings,such as Mg-rich primer coated on Al alloys, the SVET results exhibited a well-defined cathodic current peak above the scratch.The anodic current(related to the anodic disso-lution of the sacrificial pigment)distributed on the primer,which demonstrates that the sacrificial pigment functions by the cathodic protection mechanism[1,33,34].In this work,a SVET method is provided for further understand-ing the galvanic interaction between a metal-rich coating and the metal substrate,and evaluating the anti-corrosion performance of the metal-rich coating.A zinc-rich primer(ZRP)coated on steel was examined to demonstrate the efficacy of the method.Several characteristic indexes are obtained from the SVET current density map to characterize the galvanic interaction between the ZRP and its substrate.The total anodic current(and hence the corrosion rate)over the scan area is evaluated by integration of the overall anodic current density on the SVET maps.The variations of the to-tal anodic current and that of open-circuit potential(E OC)were analyzed as a function of the immersion time.Additionally,the SVET current indexes were compared with the galvanic current ob-tained by zero resistance amperometry(ZRA).2.Experimental2.1.Materials and electrode preparationAn epoxy resin(Epon828,from Hexion)and a modified poly-amide(Epikure3175,from Hexion)curing agent were mixed in a 1:1.1stoichiometric ratio.This ratio results in the optimal barrier properties and hardness of the primer by giving near to the max-imum amount of crosslinking.Methyl isobutyl ketone(MIBK) was used as solvent.Then,zinc powder was added to the solu-tion and was stirred to form a thick mortar-like mixture.A steel panel(R-35,from Q-Panel)was pretreated by grinding with400-and600-grit SiC sandpaper,followed by degreasing with hexane. The coatings were applied using a drawn down bar at a wet thickness of200l m.The coated panels were placed in a convec-tion oven at70°C for24h afterflashing off for approximately 30min.For the primers of pigment volume concentration(PVC) lower than25%,the zinc pigment wasfirstly dispersed in MIBK for full dispersion.The dryfilm thicknesses were in the range of140–190l m.2.2.SVET measurement and data analysisThe current distribution over the interface of solution/ZRP(67% PVC,subsequently referred to as ZRP67)was measured using a SVET system from Applicable Electronics(USA).The Pt–Ir microelectrode (Microprobe Inc.)with a10l m diameter tip which was platinized to a20l m diameter sphere.The microprobe was vibrated$200l m above the samples with the amplitude20l m along the X and Y directions.A pair of platinized platinum wires was used as both the reference and bath ground electrodes.The probe made20Â20 measurements in each scan($600s),generating a400-point mesh across the surface.Scans were initiated5min after immersion and repeated every60min.The ZRP67sample(1Â1cm2)was masked by a polyester tape,exposing an open area of3Â3mm2as the scan-ning area.An artificial scratch was introduced in the center of the scanning area.For comparison,the same SVET measurement was also conducted on an unscratched ZRP67.All SVET measurements were performed at the free-corrosion condition in a cell containing $5mL3.5wt.%NaCl aqueous solution.The SVET current density mapping and the statistical analysis of the data were performed with Origin software.The current densi-ties were displayed in three-dimensional(3D)maps,showing the spatial distribution of the current density as a function of the(x, y)position in the scan region on ZRP.The current values in the SVET map are positive for anodic currents and negative for catho-dic currents.The contour map of the current densities is at the bot-tom of the3D map.The SVET current density vector images superimposing the measured current vector onto an optical image of the sample showed images of the sample surface as well as the locations of anodic/cathodic area.Based on the SVET current density map,the anodic current den-sity peak(i A,max),the cathodic current density peak(i C,max),the average current density(i Ave)and the integrated anodic current (I Int)were used to characterize the anti-corrosion properties of the coating.I Int was evaluated by integration of the overall anodic current(I A)on a SVET current density map,which is theoretically equal to the total cathodic current(I C)over the ZRP surface.Split-ting the scan area(S,3Â3mm2)into20Â20small squares,we calculate the anodic or cathodic current on each square,and sum all the resulting currents to obtain I Int(l A)on the scan area,as shown byI Int¼SPi An¼ÀSPi Cnð2Þwhere i A is the anodic current density(i A P0),i C the cathodic cur-rent density(i C<0)and n the number of measurement points in each scan(n=400).2.3.Galvanic coupling measurementGalvanic coupling(both the mixed potential,E Mix,and the cou-pling current)between the coated metal and the bare substrateM.Yan et al./Corrosion Science52(2010)2636–26422637was measured using a Gamry PC4/300potentiostat in a zero resis-tance ammeter(ZRA)mode.The experiments were carried out in a two-compartment enclosed cell described in our previous work [29].The two-compartment cell permitted careful control of the atmospheric conditions in each compartment.The working elec-trode in one compartment was ZRP67-coated on the steel(subse-quently referred to as the ZRP-compartment)and the working electrode in the other compartment was the bare steel(subse-quently referred to as the steel-compartment).The exposed area of ZRP67was 1.0cm2and the bare steel was in a pinhole of 0.04cm2(simulating a coating defect),yielding an area ratio (ZRP67to steel)of ca.25.To simulate the condition for a top-coated sample,where a topcoat would protect the primer from di-rect oxygen access,the solution in the coating-compartment was purged with N2,while the solution in the alloy-compartment was purged with air.3.Results and discussion3.1.SVET current density mapsThe SVET current density maps for the bare steel in3.5%NaCl solution,as presented in Fig.1,showed several anodic current peaks that appear after several minutes,due to possible pitting nucleation.After30min,these anodic current peaks combined into one broad anodic peak,where dark corrosion products began to ap-pear on the steel surface.Fig.2displays SVET current density maps above the defect on the scratched ZRP67(67%PVC)after various immersion periods in3.5%NaCl solution.The cathodic current was mainly located at the scratch where a well-defined cathodic peak existed throughout the5-day immersion period.The anodic area appeared at different sites on ZRP67during the immersion.At the beginning of the immersion from0.1to4h,anodic areas were found to initiate only at the corners of the scratch,as shown in Fig.2a and b.After5h of the immersion,anodic areas were scattered around the scratch (Fig.2c).After10h of the immersion,significant changes occurred both in current distribution and in value,as presented in Fig.2d. The anodic activity moved from one area to another near the scratch;the cathodic area included to almost all the scratch and a well-defined cathodic peak was observed.After20h of the immersion(Fig.2e and f),the cathodic current decreased with time,and the anodic current was evenly distributed over the sur-face of the primer.The variations of the anodic current peak(i A,max),the cathodic current peak(i C,max)and the average current density(i Ave)in SVET maps are shown in Fig.3.The i A,max decreased over the immersion time on the whole,except for two peaks appearing at3and10h. The SVET experiment was conducted under free-corrosion condi-tion(without external polarization applied)where the anodic cur-rents and cathodic currents above the ZRP67are balanced and the net current should be zero.It should be note that,in a scanning plane above the free-corro-sion surface,the integrated anodic current I A should be theoreti-cally equal to the integrated cathodic current I C in the absolute value and hence the average current density(i Ave)should be zero. But deviations between I A and I C are usually obtained by SVET, which causes i Ave to deviate from zero.The deviations may be attributed to the fact that the current density on a SVET map was not taken at the same time.The corrosion behaviour and current distribution on the scan area are changing during scanning(one scan takes$10min).3.2.Integrated anodic current of the ZRP obtained by SVETThe ZRP paints under study here are heterogeneous systems with pores and zinc particles distributed randomly in the binder. The probable reactions on the primer in an electrolyte are as fol-lows:Zinc dissolution to the oxide(ZnO)/polymeric-binderfilm, electrochemical dissolution of the active zinc particles,and oxygen reduction on zinc particles or on the substrate through pores[35].A key aspect of the above mentioned mechanisms is the galvanic interaction between ZRP and the metal substrate.The galvanic interaction between ZRP and substrate may be influenced by any or all of the following factors:the electrochemical state of zinc par-ticles at ZRP/solution interface,reactive Zn/Fe area ratio(S Zn/Fe) interface,as well as the diffusion process through the coating and the deposit of Zn corrosion products[3,12].The electrochem-ical behaviour and cathodic protection performance of ZRPs have been well studied by corrosion potential monitoring[3],EIS [3,12,36],conductive atomic force microscopy(AFM)[2],as well as the scanning electron microscopy(SEM)[4,12].In this work, the electrochemical and corrosion performance of the ZRP was characterized by the SVET method.The integrated anodic current I Int above the ZRP67obtained by the SVET method is presented in Fig.4as a function of the immer-sion time,together with E OC measured under the same conditions. For the scratched ZRP67,closely correlative behaviour was found between E OC and the total anodic current.Most obviously,two sig-nificant anodic current peaks appeared during the immersion ex-actly before and after the E OC peak occurred.For comparison,I Int and E OC for the unscratched ZRP67are also presented in Fig.4. The trend of I Int of the unscratched ZRP67was similar to that of the scratched ZRP67but with much lower amplitude.The most2638M.Yan et al./Corrosion Science52(2010)2636–2642positive potential of ZRPs(unscratched)reached at$3h immersion and this potential was observed to depend significantly on the PVC, as shown in Fig.5.The most positive potentials for ZRPs with PVC5%,15%,45%and67.5%were0.166,0.00,À0.60andÀ0.91V,respec-tively.The following several stages were clearly recognized from both I Int and E OC shown in Fig.4for the scratched ZRP67.distributions over an artificial line scratch on ZRP(67%superimposed with current vectors(right of a and f)M.Yan et al./Corrosion Science52(2010)2636–264226393.2.1.The activating stageThe E OC(ca.À0.9V initially)shifted gradually towards negative direction during thefirst1h immersion as a result of the activation of Zn particles through dissolving ZnOfilm or soaking the binder film,approximatingÀ1.0V(E OC of the zinc particles)at$1h.In the following3h,the E OC shifted in the positive direction,implying that the steel substrate began to be wetted by the electrolyte through pores(decreasing the ratio of S Zn/Fe).The I Int($1.2Â10À2l A initially)slightly decreased in thefirst 1h immersion.Then,it gradually increased as a result of the disso-lution of ZnO/zinc particles at the ZRP surface.From3h immer-sion,I Int decreased sharply accompanying with the rapid increase of E OC due to the wetting process of the steel substrate until E OC reaching a peak(À0.71V)at5h,where the steel substrate was ex-pected to be totally wetted.A current valley(7.3l A)exactly corre-sponded to the E OC peak at5h.Once the substrate was wetted, galvanic interaction between zinc particles and steel substrate would be expected to occur.The period of the beginning$5h immersion can be referred to as the activating stage,where the main process was the dissolution of the ZnOfilm and then the zinc particles reaction with the electrolyte.In the activating stage,the electrochemical reaction process mainly occurred on the ZRP/solu-tion interface.3.2.2.The sacrificial protection stageEven after the steel surface became completely wetted at$5h in Fig.4,some zinc particles were still covered by thick oxides or by binderfilms.Zinc particles continued to be activated by the dis-solution of the ZnOfilm and/or galvanically coupling to the sub-strate.At the beginning of the galvanic stage,corrosion product was formed in pores in the primer,which tended to seal the pores, reducing the number and size of pores[3].This process shifted E OC toward negative directions and increased I Int sharply until attain-ing2.3Â10À2l A at$11h.Thefluctuation of E OC in the range ofÀ0.78toÀ0.87V in the vicinity of9h and the significant decrease in I Int at$10h might be attributed to the accumulation of Zn corrosion product which improved the barrier property of the coating.For the unscratched ZRP67,the E OCfluctuation at$8h immersion disappeared,which further implied that the E OCfluctuation might be related to the de-posit of the zinc corrosion product in the defect and its adhesion to the surface.3.2.3.The barrier stageThe E OC continued to decay,with somefluctuations,beginning from$11h(Fig.4).I Int decayed to a low value of1.2Â10À2l A, where it remained for the following duration of the experiment. The distinct decrease in I Int may be attributed to the zinc depleting and/or the improved barrier property of the primer.The barrier properties of the primer would improve by deposit of the corrosion product on the primer.The zinc corrosion product also trends to improve barrier effect by sealing pores in the primer,as has been proposed and observed by other authors[3,4,36].3.2.4.E OC–i Int diagramThe evolution of the E OC–i Int results for the scribed ZRP67is shown in Fig.6,with the numbers indicating the immersion time (in hour)and the dashed lines showing the shifting direction of the E OC–i Int points.Several stages(the wetting stage,the cathodic protection stage and the barrier stage)were demonstrated in the E OC–i Int evolution for the scratched ZRP67during the immersion period.Almost all the conventional electrochemical measurements re-quire an externally imposed polarization which would possibly have an unfavorable effect on the specimen.By contrast,SVET al-lows to measure integrated corrosion current at the free-corrosion condition(without external polarization applied)which is benefi-cial,especially for the highly active metal-rich coatings.2640M.Yan et al./Corrosion Science52(2010)2636–26423.3.Galvanic coupling between ZRP and the bare steelThe coupling current and mixed potential for ZRP67(1.0cm2,N2 purged)coupled with bare steel(0.04cm2,air purged)in3.5%NaCl solution are shown in Fig.7.The coupling current started at 4Â10À3l A and rapidly increased to12Â10À3l A at$25min.After immersion,the zinc particles were gradually activated through dis-solution of the oxide(ZnO)surface and water penetration through polymeric-binderfilm.The zinc particles were fully activated at the end of1h,where the mixed potential attained the lowest value and the coupling current was highest.Then the mixed potential in-creased and the coupling current declined steadily.At$26h,the E Mix attainedÀ0.85V,and the coupling current was6.9Â10À3l A.In the case of the galvanic coupling measurement in the two-compartment cell,the bare steel pinhole(simulating coating de-fect)is separated in another cell,where the bare steel was not af-fected by the deposit of the zinc corrosion product in the defect. The depletion of the zinc particles and the lack of the corrosion product deposit may lead to the steady decrease of the coupling current,which was a fundamental difference from the case of the SVET measurement,where the barrier stage was clearly observed as the result of a barrier effect of the Zn products deposit.4.ConclusionsBy integrating the overall anodic current density measured by scanning vibrating electrode technique(SVET),the total anodic current(and therefore the corrosion rate)over the coating surface was obtained and used to evaluate the galvanic interaction be-tween coatings and substrates.This SVET method allows the mea-surement of corrosion current at the free-corrosion condition (without external polarization applied),which is highly beneficial, especially for active metal-rich coatings.For the zinc-rich primer, several stages during immersion were distinctly recognized by the SVET method:the activating stage,the sacrificial protection stage and the barrier stage.Closely correlative behaviour was found between the total anodic current and the open-circuit poten-tial.This SVET analysis method may provide a new insight for the corrosion protection performance and even the service life of me-tal-rich coatings.AcknowledgmentThe authors would like to thank the US Army Research Labora-tory(Contract#W911NF-04-2-0029)for sponsoring this research. References[1]G.Bierwagen,D.Battocchi,A.Simoes,A.Stamness,D.Tallman,The use ofmultiple electrochemical techniques to characterize Mg-rich primers for A1 alloys,.Coat.59(2007)172–178.[2]G.Bierwagen,K.Allahar,B.Hinderliter,H.Jung,Zn-rich coatings revisited,in:Tri-Service Corrosion Conference,Denver,Co.,2007.[3]C.M.Abreu,M.Izquierdo,M.Keddam,X.R.Novoa,H.Takenouti,Electrochemical behaviour of zinc-rich epoxy paints in3%NaCl solution, Electrochim.Acta41(1996)2405–2415.[4]M.Morcillo,R.Barajas,S.Feliu,J.M.Bastidas,A SEM study on the galvanicprotection of zinc-rich paints,J.Mater.Sci.25(1990)2441–2446.[5]C.Hare,Corrosion control of steel by organic coatings,in:R.W.Revie(Ed.),Uhlig’s Corrosion Handbook,John Wiley&Sons,New York,2000,pp.1023–1038.[6]M.E.Nanna,G.P.Bierwagen,Mg-rich coatings:a new paradigm for Cr-freecorrosion protection of Al aerospace alloys,JCT Res.1(2004)69–80.[7]D.Battocchi, A.M.Simoes, D.E.Tallman,G.P.Bierwagen,Electrochemicalbehaviour of a Mg-rich primer in the protection of Al alloys,Corros.Sci.48 (2006)1292–1306.[8]G.Bierwagen,R.Brown,D.Battocchi,S.Hayes,Observations on the Testing ofMg-rich Primers for Totally Chromate-free Corrosion Protection of Aerospace Alloys,Department of Defense Corrosion Conference,Gaylord National, Washington DC,2009.[9]J.N.Murray,Electrochemical test methods for evaluating organic coatings onmetals:an update.Part III:multiple test parameter measurements,.Coat.31(1997)375–391.[10]G.Grundmeier,W.Schmidt,M.Stratmann,Corrosion protection by organiccoatings:electrochemical mechanism and novel methods of investigation, Electrochim.Acta45(2000)2515–2533.[11]F.Mansfeld,Use of electrochemical impedance spectroscopy for the study ofcorrosion protection by polymer-coatings,J.Appl.Electrochem.25(1995) 187–202.[12]J.R.Vilche,E.C.Bucharsky,C.A.Giudice,Application of EIS and SEM to evaluatethe influence of pigment shape and content in ZRP formulations on the corrosion prevention of naval steel,Corros.Sci.44(2002)1287–1309. [13]S.E.Faidi,J.D.Scantlebury,P.Bullivant,N.T.Whittle,R.Savin,Anelectrochemical study of zinc-containing epoxy coatings on mild steel, Corros.Sci.35(1993)1319–1328.[14]J.N.Murray,Electrochemical test methods for evaluating organic coatings onmetals:an update.Part II:single test parameter measurements,.Coat.31(1997)255–264.[15]I.Sekine,Recent evaluation of corrosion protective paintfilms byelectrochemical methods,.Coat.31(1997)73–80.[16]L.F.Jaffe,R.Nuccitelli,An ultrasensitive vibrating probe for measuring steadyextracellular currents,J.Cell Biol.63(1974)614–628.[17]H.S.Isaacs,Initiation of stress corrosion cracking of sensitized type304stainless steel in dilute thiosulfate solution,J.Electrochem.Soc.135(1988) 2180–2183.[18]H.S.Isaacs,The measurement of the galvanic corrosion of soldered copperusing the scanning vibrating electrode technique,Corros.Sci.28(1988)547–558.[19]J.Elvins,J.H.Sullivan,J.A.Spittle,D.A.Worsley,Short term predictive testingfor cut edge corrosion resistance in zinc–aluminium alloy galvanised steels, Corros.Eng.Sci.Tech.40(2005)43–50.[20]J.He,Applications of the Scanning Vibrating Electrode Technique to the Studyof Corrosion Protection by Conductive Polymers,Ph.D.Thesis,North Dakota State University,2002.[21]H.Krawiec,V.Vignal,R.Oltra,Use of the electrochemical microcell techniqueand the SVET for monitoring pitting corrosion at MnS inclusions,Electrochem.Commun.6(2004)655–660.M.Yan et al./Corrosion Science52(2010)2636–26422641。
EleChemr电化学反应模拟软件说明书

Package‘EleChemr’October12,2022Title Electrochemical Reactions SimulationVersion1.2.0Description Digital simulation of electrochemical processes.Each function allows for implicit and explicit solution of the differential equation using meth-ods like Euler,Backwards implicit,Runge Kutta4,Crank Nicholson and Backward differentia-tion formula as well as different number of points for derivative approximation.Several electro-chemical processes can be simulated such as:Chronoamperometry,Potential Step,Lin-ear Sweep,Cyclic V oltammetry,Cyclic V oltammetry with electrochemical reaction fol-lowed by chemical reaction(EC mechanism)and CV with two following electrochemical reac-tion(EE mechanism).In update1.1.0has been added a general purpose CV function that al-low to simulate up to4EE mechanism combined with chemical reac-tion for each species.Update1.2.0improved the accuracy of the measurements and allow person-alized data resolution for simulation.Bibliography regarding this methods can be found in the following texts.Dieter Britz,Jorg Strutwolf(2016)<ISBN:978-3-319-30292-8>.Allen J.Bard,Larry R.Faulkner(2000)<ISBN:978-0-471-04372-0>.License GPL-3Encoding UTF-8LazyData trueImports ggplot2RoxygenNote7.1.1NeedsCompilation noAuthor Federico Maria Vivaldi[aut,cre]Maintainer Federico Maria Vivaldi<****************************>Repository CRANDate/Publication2021-02-0914:20:03UTCR topics documented:ChronAmp (2)CottrCheck (3)CV (4)12ChronAmp CVEC (5)CVEE (6)Derv (8)Gen_CV (9)invMat (11)LinSwp (12)OneMat (13)ParCall (14)PotStep (16)ZeroMat (17)Index18 ChronAmp Chrono amperometry digital simulationDescriptionReturn a graph I vs t of the electrochemical processUsageChronAmp(Co=0.001,exptime=1,Dx=1e-05,Dm=0.45,Temp=298.15,n=1,Area=1,DerApprox=2,l=100,errCheck=FALSE,Method="Euler")ArgumentsCo bulk concentration expressed in Molarexptime experimental time to be simulated expressed in secondsDx diffusion coefficient expressed in cm^2/sDm simulation parameter,maximum0.5for explicit methodsTemp temperature in kelvinn number of electrons involved in the processArea area of the electrode expressed in cm^2DerApprox number of point for the approximation of thefirst derivativeCottrCheck3l number of time steps of the simulationerrCheck if true the function returns a list with parameters for CottrCheck functionMethod method to be used for the simulation="Euler""BI""RK4""CN""BDF"Valueif errCheck==F a graph I vs t,if errCheck==T a listExamplesChronAmp(Co=0.001,exptime=1,DerApprox=2,Dm=0.45,errCheck=FALSE,Method="Euler") CottrCheck Cottrel current check for the Chronoamperometric simulationDescriptionReturn a graph G/Gcot vs t of the electrochemical processUsageCottrCheck(Elefun)ArgumentsElefun the function to be checked=ChronAmp,PotStepValueA graph G/Gcot vs t for the simulation data selectedExamplesCottrCheck(ChronAmp(errCheck=TRUE,Method="BI"))4CV CV Cyclic voltammetry digitial simulationDescriptionReturn a graph I vs E of the electrochemical processUsageCV(Co=0.001,Dx=1e-05,Eo=0,Dm=0.45,Vi=0.3,Vf=-0.3,Vs=0.001,ko=0.01,alpha=0.5,Temp=298.15,n=1,Area=1,l=100,DerApprox=2,errCheck=FALSE,Method="Euler")ArgumentsCo bulk concentration expressed in MolarDx diffusion coefficient expressed in cm^2/sEo reduction potential of the species expressed in V oltsDm simulation parameter,maximum0.5for explicit methodsVi initial potential of the sweep expressed in V oltsVffinal potential of the sweepexpressed in V oltsVs potential scan rate of the simulation expressed in V/sko heterogeneous electron transfer rate constant expressed in m/salpha charge transfer coefficientTemp temperature in kelvinn number of electrons involved in the processArea area of the electrode expressed in cm^2l number of time steps of the simulationCVEC5DerApprox number of point for the approximation of thefirst derivativeerrCheck if true the function returns a list with parameters for CottrCheck functionMethod method to be used for the simulation="Euler""BI""RK4""CN""BDF"Valueif errCheck==F a graph I vs E,if errCheck==T a listExamplesCV(Co=0.001,DerApprox=2,Dm=0.45,errCheck=FALSE,Method="Euler")CVEC EC behaviour cyclic voltammetry simulatorDescriptionReturn a graph I vs E of the electrochemical processUsageCVEC(Co=0.001,Dx=1e-05,Eo=0,Dm=0.45,Vi=0.3,Vf=-0.3,Vs=0.001,ko=0.01,kc=0.001,l=100,alpha=0.5,Temp=298.15,n=1,Area=1,DerApprox=2,errCheck=FALSE,Method="Euler")6CVEE ArgumentsCo bulk concentration expressed in MolarDx diffusion coefficient expressed in cm^2/sEo reduction potential of the species expressed in V oltDm simulation parameter,maximum0.5for explicit methodsVi initial potential of the sweep expressed in V oltVffinal potential of the sweep expressed in V oltVs potential scan rate of the simulation expressed in V/sko heterogeneous electron transfer rate constant expressed in m/skc rate constant of the reaction Red->C expressed in s^-1l number of time steps of the simulationalpha charge transfer coefficientTemp temperature in kelvinn number of electrons involved in the processArea area of the electrode expressed in cm^2DerApprox number of point for the approximation of thefirst derivativeerrCheck if true the function returns a list with parameters for CottrCheck functionMethod method to be used for the simulation="Euler""BI""RK4""CN"BDF"Valueif errCheck==F a graph I vs E,if errCheck==T a listExamplesCVEC(Co=0.001,DerApprox=2,Dm=0.45,kc=0.00001,errCheck=FALSE,Method="Euler") CVEE EE behaviour cyclic voltammetry simulatorDescriptionReturn a graph I vs E of the electrochemical processCVEE7UsageCVEE(Co=0.001,Dx1=1e-05,Eo1=0,Vi=0.3,Vf=-0.3,Vs=0.001,ko1=0.01,alpha1=0.5,Dred=1e-05,Dred2=1e-05,Eo2=0,ko2=0.01,alpha2=0.5,Dm=0.45,l=100,Temp=298.15,n=1,Area=1,DerApprox=2,errCheck=FALSE,Method="Euler")ArgumentsCo bulk concentration expressed in MolarDx1diffusion coefficient of the oxidized species expressed in cm^2/sEo1reduction potential of thefirst electrochemical reaction expressed in V oltVi initial potential of the sweep expressed in V oltVffinal potential of the sweep expressed in V oltVs potential scan rate of the simulation expressed in V/sko1heterogeneous electron transfer rate constant of thefirst electrochemical reaction expressed in m/salpha1charge transfer coefficient of thefirst electrochemical reactionDred diffusion coefficient of thefirst reduced species expressed in cm^2/sDred2diffusion coefficient of the second reduced species expressed in cm^2/sEo2reduction potential of the second electrochemical reaction expressed in V olt ko2heterogeneous electron transfer rate constant of the second electrochemical re-action expressed in m/salpha2charge transfer coefficient of the second electrochemical reactionDm simulation parameter,maximum0.5for explicit methodsl number of time steps of the simulation8DervTemp temperature in kelvinn number of electrons involved in the processArea area of the electrode expressed in cm^2DerApprox number of point for the approximation of thefirst derivativeerrCheck if true the function returns a list with parameters for CottrCheck functionMethod method to be used for the simulation="Euler""BI""RK4""CN"BDF"Valueif errCheck==F a graph I vs E,if errCheck==T a listExamplesCVEE(Co=0.001,DerApprox=2,Dm=0.45,errCheck=FALSE,Method="Euler")CVEE(Co=0.001,Eo2=-0.15,Dm=0.45)Derv Derivative calculation of concentration profileDescriptionReturn a the derivative of the concentration profile simulatedUsageDerv(npoints=2,h,Ox,mode="Forward",Derivative="First",CoefMat=FALSE)Argumentsnpoints number of points to be used for the derivativeh space for thefinite differenceOx data upon the derivative is calculatedmode"Forward"or"Backward"the derivative will be calculated for the npointsDerivative"First"or"Second"derivative to calculateCoefMat if T return the derivative coefficient matrix for selected derivativeValuea vector with the derivative requested or the coefficient of such derivativeExamplesDerv(npoints=2,h=0.13,Ox=matrix(c(1,2),nrow=1),mode="Forward",Derivative="First") Gen_CV General Purpose CV simulationDescriptionReturn a graph I vs E of the electrochemical process,up to4EE mechanisms and CE mechanisms can be simulatedUsageGen_CV(Co=0.001,Cred=0,kco=0,Dx1=1e-05,Eo1=0,kc1=0,Vi=0.3,Vf=-0.3,Vs=0.001,ko1=0.01,alpha1=0.5,Dred=1e-05,Dred2=1e-05,Eo2=0,kc2=0,ko2=0,alpha2=0.5,Dm=0.45,Dred3=1e-05,Eo3=0,kc3=0,ko3=0,alpha3=0.5,Dred4=1e-05,Eo4=0,kc4=0,ko4=0,alpha4=0.5,Temp=298.15,n=1,Area=1,l=100,DerApprox=2,errCheck=FALSE,Method="Euler")ArgumentsCo bulk concentration oxidated speciesexpressed in MolarCred bulk concentration of reduced species expressed in Molarkco Chemical rate constant for Ox Species expressed in s^-1Dx1diffusion coefficient of the oxidized species expressed in cm^2/sEo1reduction potential of thefirst electrochemical reaction expressed in V oltkc1Chemical rate constant for Red Species expressed in s^-1Vi initial potential of the sweep expressed in V oltVffinal potential of the sweep expressed in V oltVs potential scan rate of the simulation expressed in V/sko1heterogeneous electron transfer rate constant of thefirst electrochemical reaction expressed in m/salpha1charge transfer coefficient of thefirst electrochemical reactionDred diffusion coefficient of thefirst reduced species expressed in cm^2/SDred2diffusion coefficient of the second reduced species expressed in cm^2/sEo2reduction potential of the second electrochemical reaction expressed in V olt kc2Chemical rate constant for second Red Species expressed in s^-1ko2heterogeneous electron transfer rate constant of the second electrochemical re-action expressed in m/salpha2charge transfer coefficient of the second electrochemical reactionDm simulation parameter,maximum0.5for explicit methodsDred3diffusion coefficient of the third reduced species expressed in cm^2/sEo3reduction potential of the third electrochemical reaction expressed in V oltkc3Chemical rate constant for third Red Species expressed in s^-1ko3heterogeneous electron transfer rate constant of the third electrochemical reac-tion expressed in m/salpha3charge transfer coefficient of the third electrochemical reactionDred4diffusion coefficient of the fourth reduced species cm^2/sEo4reduction potential of the fourth electrochemical reaction expressed in V olt kc4Chemical rate constant for fourth Red Species expressed in s^-1invMat11ko4heterogeneous electron transfer rate constant of the fourth electrochemical reac-tion expressed in m/salpha4charge transfer coefficient of the fourth electrochemical reactionTemp temperature in kelvinn number of electrons involved in the processArea area of the electrode expressed in cm^2l number of time steps of the simulationDerApprox number of point for the approximation of thefirst derivativeerrCheck if true the function returns a list with parameters for CottrCheck functionMethod method to be used for the simulation="Euler""BI""RK4""CN"BDF"Valueif errCheck==F a graph I vs E,if errCheck==T a listExamplesGen_CV(Co=0.001,DerApprox=2,Dm=0.45,errCheck=FALSE,Method="Euler")Gen_CV(Co=0.001,Eo2=-0.15,Dm=0.45,kc1=0.0001)invMat Inverse matrixDescriptionReturns the inverse matrix of the selected oneUsageinvMat(A)ArgumentsA matrix to be invertedValueinverse matrix of the selectedExamplesinvMat(A=matrix(c(1,2,6,14),nrow=2))12LinSwp LinSwp Linear Sweep digitial simulationDescriptionReturn a graph I vs E of the electrochemical processUsageLinSwp(Co=0.001,Dx=1e-05,Eo=0,Dm=0.45,Vi=0.3,Vf=-0.3,Vs=0.001,ko=0.01,alpha=0.5,Temp=298.15,n=1,Area=1,l=100,DerApprox=2,errCheck=FALSE,Method="Euler")ArgumentsCo bulk concentration expressed in MolarDx diffusion coefficient expressed in cm^2/sEo reduction potential of the species expressed in V oltDm simulation parameter,maximum0.5for explicit methodsVi initial potential of the sweep expressed in V oltVffinal potential of the sweep expressed in V oltVs potential scan rate of the simulation expressed in V/sko heterogeneous electron transfer rate constant expressed in m/salpha charge transfer coefficientTemp temperature in kelvinn number of electrons involved in the processArea area of the electrode expressed in cm^2l number of time steps of the simulationOneMat13DerApprox number of point for the approximation of thefirst derivativeerrCheck if true the function returns a list with parameters for CottrCheck functionMethod method to be used for the simulation="Euler""BI""RK4""CN""BDF"Valueif errCheck==F a graph I vs E,if errCheck==T a listExamplesLinSwp(Co=0.001,Dm=0.45,DerApprox=2,errCheck=FALSE,Method="Euler")OneMat Starting Matrix of oxidazed speciesDescriptionReturn a matrix ixjfilled with1valueUsageOneMat(i,j=i)Argumentsi number of rowsj number of columnsValuea matrix of dimention ixjfilled with1valueExamplesOneMat(2,2)ParCall Parameters callDescriptionReturns a list with the parameters necessary for the simulationUsageParCall(Fun,n.,Temp.,Dx1.,eta.,exptime.,Eo1.,ko1.,ko2.,kc.,Dm.,Vf.,Vi.,Vs.,alpha1.,Eo2.,Dred1.,Dred2.,alpha2.,Dred3.,Dred4.,ko3.,ko4.,kco.,kc1.,kc2.,kc3.,kc4.,alpha3.,alpha4.,Eo3.,Eo4.,l.)ArgumentsFun Name of the function this function is called to.Must be a string.n.Number of electronsTemp.Temperature for the simulationDx1.Diffusion coefficient of species Oneeta.OverPotential for potential stepexptime.experimental time for the simulationEo1.reduction potential of thefirst electrochemical reactionko1.heterogeneous electron transfer rate constant of thefirst electrochemical reaction ko2.heterogeneous electron transfer rate constant of the second electrochemical re-actionkc.Chemical rate constant forfirst Ox Species,used in simulation with just one speciesDm.Simulation parameter,maximum0.5for explicit methodsVf.Final potential of the sweepVi.Initial potential of the sweepVs.Scan rate of the simulationalpha1.charge transfer coefficient of thefirst electrochemical reactionEo2.reduction potential of the second electrochemical reactionDred1.diffusion coefficient of thefirst reduced speciesDred2.diffusion coefficient of the second reduced speciesalpha2.charge transfer coefficient of the second electrochemical reactionDred3.diffusion coefficient of the third reduced speciesDred4.diffusion coefficient of the fourth reduced speciesko3.heterogeneous electron transfer rate constant of the third electrochemical reac-tionko4.heterogeneous electron transfer rate constant of the fourth electrochemical reac-tionkco.Chemical rate constant forfirst Ox Specieskc1.Chemical rate constant forfirst Red Specieskc2.Chemical rate constant for second Red Specieskc3.Chemical rate constant for third Red Specieskc4.Chemical rate constant for fourth Red Speciesalpha3.charge transfer coefficient of the third electrochemical reactionalpha4.charge transfer coefficient of the fourth electrochemical reactionEo3.reduction potential of the third electrochemical reactionEo4.reduction potential of the fourth electrochemical reactionl.numer of time stepsValueinverse matrix of the selected16PotStepExamplesParCall("ChronAmp",n.=1,Temp.=298,Dx1.=0.0001,exptime.=1,Dm.=0.45,l.=100) PotStep Chrono amperometry with afinite step digital simulationDescriptionReturn a graph I vs t of the electrochemical processUsagePotStep(Co=0.001,exptime=1,Dx=1e-05,Dm=0.45,eta=0,Temp=298.15,n=1,Area=1,l=100,DerApprox=2,errCheck=FALSE,Method="Euler")ArgumentsCo bulk concentration expressed in Molarexptime experimental time to be simulated expressed in secondsDx diffusion coefficient expressed in cm^2/sDm simulation parameter,maximum0.5for explicit methodseta overpotential of the step expressed in V oltTemp temperature in kelvinn number of electrons involved in the processArea area of the electrode expressed in cm^2l number of time steps of the simulationDerApprox number of point for the approximation of thefirst derivativeerrCheck if true the function returns a list with parameters for CottrCheck functionMethod method to be used for the simulation="Euler""BI""RK4""CN""BDF"ZeroMat17Valueif errCheck==F a graph I vs t,if errCheck==T a listExamplesPotStep(Co=0.001,exptime=1,Dm=0.45,DerApprox=2,errCheck=FALSE,Method="Euler") ZeroMat Starting Matrix of reduces species andfluxesDescriptionReturn a matrix ixjfilled with0valueUsageZeroMat(i,j=i)Argumentsi number of rowsj number of columnsValuea matrix of dimention ixjfilled with1valueExamplesZeroMat(2,2)IndexChronAmp,2CottrCheck,3CV,4CVEC,5CVEE,6Derv,8Gen_CV,9invMat,11LinSwp,12OneMat,13ParCall,14PotStep,16ZeroMat,1718。
电化学脱合金的英文

电化学脱合金的英文Electrochemical Dealloying: Principles, Applications, and Challenges.Introduction.Electrochemical dealloying is a process that involves the selective removal of one or more constituent metalsfrom a multicomponent metallic alloy by electrochemical means. This process, often referred to as "dealuminization" in the context of aluminum-based alloys, has found widespread applications in materials science, nanotechnology, and energy conversion and storage systems. The primary advantage of electrochemical dealloying lies in its ability to create nanostructured materials with unique physical and chemical properties, such as high surface area, porosity, and conductivity.Principles of Electrochemical Dealloying.The electrochemical dealloying process occurs when an alloy is immersed in an electrolyte solution and apotential is applied between the alloy and a counter-electrode. The applied potential drives the electrochemical reactions at the alloy surface, resulting in thedissolution of one or more constituent metals. The dissolution rate of each metal depends on its electrochemical properties, such as the redox potential and electrochemical activity in the given electrolyte.During the dealloying process, the alloy is typically the anode, and the counter-electrode is the cathode. The anode is connected to the positive terminal of the power source, while the cathode is connected to the negative terminal. When the potential is applied, the alloy begins to dissolve, and the dissolved metal ions migrate towards the cathode. At the cathode, the metal ions are reduced and deposited on the surface, forming a new metal layer.The rate of metal dissolution during electrochemical dealloying is controlled by several factors, including the electrolyte composition, applied potential, temperature,and alloy composition. By optimizing these parameters, researchers can precisely control the morphology, porosity, and composition of the resulting nanostructured materials.Applications of Electrochemical Dealloying.Electrochemical dealloying has found numerous applications in materials science and engineering. Some of the key applications are discussed below:1. Nanoporous Metals: Electrochemical dealloying is widely used to create nanoporous metals with high surface area and porosity. These materials exhibit unique physical and chemical properties that are beneficial in various applications, such as catalysis, sensors, and energy storage.2. Battery Materials: Nanoporous metals produced by electrochemical dealloying have been explored as anode materials for lithium-ion batteries. The high porosity and surface area of these materials enhance the lithium storage capacity and improve the battery's performance.3. Fuel Cells: Electrochemical dealloying has also been used to create nanostructured catalysts for fuel cells. These catalysts exhibit enhanced activity and durability, which are crucial for efficient fuel cell operation.4. Biomedical Applications: Nanoporous metals produced by electrochemical dealloying have potential applicationsin biomedicine, such as drug delivery, tissue engineering, and implant materials. The porous structure of these materials allows for controlled drug release and improved cell adhesion and growth.Challenges and Future Directions.Despite the significant progress made inelectrochemical dealloying, several challenges remain to be addressed. One of the primary challenges is the control of the dealloying process at the nanoscale, as it is crucialfor achieving the desired material properties. Additionally, the development of new electrolytes and optimization of dealloying parameters are ongoing research efforts.Future research in electrochemical dealloying could focus on exploring new alloy systems, optimizing the dealloying process for specific applications, and understanding the fundamental mechanisms underlying metal dissolution and nanostructure formation. Furthermore, the integration of electrochemical dealloying with other nanotechnology approaches, such as lithography and templating, could lead to the development of even more advanced materials with tailored properties.Conclusion.Electrochemical dealloying is a powerful technique for creating nanostructured materials with unique physical and chemical properties. Its applications span multiple fields, including materials science, energy conversion and storage, and biomedicine. While significant progress has been madein this field, there are still numerous challenges and opportunities for further research and development. With the advancement of nanotechnology and materials science, electrochemical dealloying holds promise for enabling thecreation of next-generation materials with improved performance and functionality.。
大环多胺

New1H-Pyrazole-Containing Polyamine Receptors Able ToComplex L-Glutamate in Water at Physiological pH ValuesCarlos Miranda,†Francisco Escartı´,‡Laurent Lamarque,†Marı´a J.R.Yunta,§Pilar Navarro,*,†Enrique Garcı´a-Espan˜a,*,‡and M.Luisa Jimeno†Contribution from the Instituto de Quı´mica Me´dica,Centro de Quı´mica Orga´nica Manuel Lora Tamayo,CSIC,C/Juan de la Cier V a3,28006Madrid,Spain,Departamento de Quı´mica Inorga´nica,Facultad de Quı´mica,Uni V ersidad de Valencia,c/Doctor Moliner50, 46100Burjassot(Valencia),Spain,and Departamento de Quı´mica Orga´nica,Facultad deQuı´mica,Uni V ersidad Complutense de Madrid,A V plutense s/n,28040Madrid,SpainReceived April16,2003;E-mail:enrique.garcia-es@uv.esAbstract:The interaction of the pyrazole-containing macrocyclic receptors3,6,9,12,13,16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene1[L1],13,26-dibenzyl-3,6,9,12,13,16,-19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene2[L2],3,9,12,13,16,22,-25,26-octaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene3[L3],6,19-dibenzyl-3,6,9,12,13,-16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene4[L4],6,19-diphenethyl-3,6,9,12,13,16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene5[L5],and 6,19-dioctyl-3,6,9,12,13,16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetra-ene6[L6]with L-glutamate in aqueous solution has been studied by potentiometric techniques.The synthesis of receptors3-6[L3-L6]is described for the first time.The potentiometric results show that4[L4]containing benzyl groups in the central nitrogens of the polyamine side chains is the receptor displaying the larger interaction at pH7.4(K eff)2.04×104).The presence of phenethyl5[L5]or octyl groups6[L6]instead of benzyl groups4[L4]in the central nitrogens of the chains produces a drastic decrease in the stability[K eff )3.51×102(5),K eff)3.64×102(6)].The studies show the relevance of the central polyaminic nitrogen in the interaction with glutamate.1[L1]and2[L2]with secondary nitrogens in this position present significantly larger interactions than3[L3],which lacks an amino group in the center of the chains.The NMR and modeling studies suggest the important contribution of hydrogen bonding andπ-cation interaction to adduct formation.IntroductionThe search for the L-glutamate receptor field has been andcontinues to be in a state of almost explosive development.1 L-Glutamate(Glu)is thought to be the predominant excitatory transmitter in the central nervous system(CNS)acting at a rangeof excitatory amino acid receptors.It is well-known that it playsa vital role mediating a great part of the synaptic transmission.2However,there is an increasing amount of experimentalevidence that metabolic defects and glutamatergic abnormalitiescan exacerbate or induce glutamate-mediated excitotoxic damageand consequently neurological disorders.3,4Overactivation ofionotropic(NMDA,AMPA,and Kainate)receptors(iGluRs)by Glu yields an excessive Ca2+influx that produces irreversible loss of neurons of specific areas of the brain.5There is much evidence that these processes induce,at least in part,neuro-degenerative illnesses such as Parkinson,Alzheimer,Huntington, AIDS,dementia,and amyotrophic lateral sclerosis(ALS).6In particular,ALS is one of the neurodegenerative disorders for which there is more evidence that excitotoxicity due to an increase in Glu concentration may contribute to the pathology of the disease.7Memantine,a drug able to antagonize the pathological effects of sustained,but relatively small,increases in extracellular glutamate concentration,has been recently received for the treatment of Alzheimer disease.8However,there is not an effective treatment for ALS.Therefore,the preparation of adequately functionalized synthetic receptors for L-glutamate seems to be an important target in finding new routes for controlling abnormal excitatory processes.However,effective recognition in water of aminocarboxylic acids is not an easy task due to its zwitterionic character at physiological pH values and to the strong competition that it finds in its own solvent.9†Centro de Quı´mica Orga´nica Manuel Lora Tamayo.‡Universidad de Valencia.§Universidad Complutense de Madrid.(1)Jane,D.E.In Medicinal Chemistry into the Millenium;Campbell,M.M.,Blagbrough,I.S.,Eds.;Royal Society of Chemistry:Cambridge,2001;pp67-84.(2)(a)Standaert,D.G.;Young,A.B.In The Pharmacological Basis ofTherapeutics;Hardman,J.G.,Goodman Gilman,A.,Limbird,L.E.,Eds.;McGraw-Hill:New York,1996;Chapter22,p503.(b)Fletcher,E.J.;Loge,D.In An Introduction to Neurotransmission in Health and Disease;Riederer,P.,Kopp,N.,Pearson,J.,Eds.;Oxford University Press:New York,1990;Chapter7,p79.(3)Michaelis,E.K.Prog.Neurobiol.1998,54,369-415.(4)Olney,J.W.Science1969,164,719-721.(5)Green,J.G.;Greenamyre,J.T.Prog.Neurobiol.1996,48,613-63.(6)Bra¨un-Osborne,H.;Egebjerg,J.;Nielsen,E.O.;Madsen,U.;Krogsgaard-Larsen,P.J.Med.Chem.2000,43,2609-2645and references therein.(7)(a)Shaw,P.J.;Ince,P.G.J.Neurol.1997,244(Suppl2),S3-S14.(b)Plaitakis,A.;Fesdjian,C.O.;Shashidharan,S Drugs1996,5,437-456.(8)Frantz,A.;Smith,A.Nat.Re V.Drug Dico V ery2003,2,9.Published on Web12/30/200310.1021/ja035671m CCC:$27.50©2004American Chemical Society J.AM.CHEM.SOC.2004,126,823-8339823There are many types of receptors able to interact with carboxylic acids and amino acids in organic solvents,10-13yielding selective complexation in some instances.However,the number of reported receptors of glutamate in aqueous solution is very scarce.In this sense,one of the few reports concerns an optical sensor based on a Zn(II)complex of a 2,2′:6′,2′′-terpyridine derivative in which L -aspartate and L -glutamate were efficiently bound as axial ligands (K s )104-105M -1)in 50/50water/methanol mixtures.14Among the receptors employed for carboxylic acid recogni-tion,the polyamine macrocycles I -IV in Chart 1are of particular relevance to this work.In a seminal paper,Lehn et al.15showed that saturated polyamines I and II could exert chain-length discrimination between different R ,ω-dicarboxylic acids as a function of the number of methylene groups between the two triamine units of the receptor.Such compounds were also able to interact with a glutamic acid derivative which has the ammonium group protected with an acyl moiety.15,16Compounds III and IV reported by Gotor and Lehn interact in their protonated forms in aqueous solution with protected N -acetyl-L -glutamate and N -acetyl-D -glutamate,showing a higher stability for the interaction with the D -isomer.17In both reports,the interaction with protected N -acetyl-L -glutamate at physiological pH yields constants of ca.3logarithmic units.Recently,we have shown that 1H -pyrazole-containing mac-rocycles present desirable properties for the binding of dopam-ine.18These polyaza macrocycles,apart from having a highpositive charge at neutral pH values,can form hydrogen bonds not only through the ammonium or amine groups but also through the pyrazole nitrogens that can behave as hydrogen bond donors or acceptors.In fact,Elguero et al.19have recently shown the ability of the pyrazole rings to form hydrogen bonds with carboxylic and carboxylate functions.These features can be used to recognize the functionalities of glutamic acid,the carboxylic and/or carboxylate functions and the ammonium group.Apart from this,the introduction of aromatic donor groups appropriately arranged within the macrocyclic framework or appended to it through arms of adequate length may contribute to the recognition event through π-cation interactions with the ammonium group of L -glutamate.π-Cation interactions are a key feature in many enzymatic centers,a classical example being acetylcholine esterase.20The role of such an interaction in abiotic systems was very well illustrated several years ago in a seminal work carried out by Dougherty and Stauffer.21Since then,many other examples have been reported both in biotic and in abiotic systems.22Taking into account all of these considerations,here we report on the ability of receptors 1[L 1]-6[L 6](Chart 2)to interact with L -glutamic acid.These receptors display structures which differ from one another in only one feature,which helps to obtain clear-cut relations between structure and interaction(9)Rebek,J.,Jr.;Askew,B.;Nemeth,D.;Parris,K.J.Am.Chem.Soc.1987,109,2432-2434.(10)Seel,C.;de Mendoza,J.In Comprehensi V e Supramolecular Chemistry ;Vogtle,F.,Ed.;Elsevier Science:New York,1996;Vol.2,p 519.(11)(a)Sessler,J.L.;Sanson,P.I.;Andrievesky,A.;Kral,V.In SupramolecularChemistry of Anions ;Bianchi,A.,Bowman-James,K.,Garcı´a-Espan ˜a,E.,Eds.;John Wiley &Sons:New York,1997;Chapter 10,pp 369-375.(b)Sessler,J.L.;Andrievsky,A.;Kra ´l,V.;Lynch,V.J.Am.Chem.Soc.1997,119,9385-9392.(12)Fitzmaurice,R.J.;Kyne,G.M.;Douheret,D.;Kilburn,J.D.J.Chem.Soc.,Perkin Trans.12002,7,841-864and references therein.(13)Rossi,S.;Kyne,G.M.;Turner,D.L.;Wells,N.J.;Kilburn,J.D.Angew.Chem.,Int.Ed.2002,41,4233-4236.(14)Aı¨t-Haddou,H.;Wiskur,S.L.;Lynch,V.M.;Anslyn,E.V.J.Am.Chem.Soc.2001,123,11296-11297.(15)Hosseini,M.W.;Lehn,J.-M.J.Am.Chem.Soc.1982,104,3525-3527.(16)(a)Hosseini,M.W.;Lehn,J.-M.Hel V .Chim.Acta 1986,69,587-603.(b)Heyer,D.;Lehn,J.-M.Tetrahedron Lett.1986,27,5869-5872.(17)(a)Alfonso,I.;Dietrich,B.;Rebolledo,F.;Gotor,V.;Lehn,J.-M.Hel V .Chim.Acta 2001,84,280-295.(b)Alfonso,I.;Rebolledo,F.;Gotor,V.Chem.-Eur.J.2000,6,3331-3338.(18)Lamarque,L.;Navarro,P.;Miranda,C.;Ara ´n,V.J.;Ochoa,C.;Escartı´,F.;Garcı´a-Espan ˜a,E.;Latorre,J.;Luis,S.V.;Miravet,J.F.J.Am.Chem.Soc .2001,123,10560-10570.(19)Foces-Foces,C.;Echevarria,A.;Jagerovic,N.;Alkorta,I.;Elguero,J.;Langer,U.;Klein,O.;Minguet-Bonvehı´,H.-H.J.Am.Chem.Soc.2001,123,7898-7906.(20)Sussman,J.L.;Harel,M.;Frolow,F.;Oefner,C.;Goldman,A.;Toker,L.;Silman,I.Science 1991,253,872-879.(21)Dougherty,D.A.;Stauffer,D.A.Science 1990,250,1558-1560.(22)(a)Sutcliffe,M.J.;Smeeton,A.H.;Wo,Z.G.;Oswald,R.E.FaradayDiscuss.1998,111,259-272.(b)Kearney,P.C.;Mizoue,L.S.;Kumpf,R.A.;Forman,J.E.;McCurdy,A.;Dougherty,D.A.J.Am.Chem.Soc.1993,115,9907-9919.(c)Bra ¨uner-Osborne,H.;Egebjerg,J.;Nielsen,E.;Madsen,U.;Krogsgaard-Larsen,P.J.Med.Chem.2000,43,2609-2645.(d)Zacharias,N.;Dougherty,D.A.Trends Pharmacol.Sci.2002,23,281-287.(e)Hu,J.;Barbour,L.J.;Gokel,G.W.J.Am.Chem.Soc.2002,124,10940-10941.Chart 1.Some Receptors Employed for Dicarboxylic Acid and N -AcetylglutamateRecognitionChart 2.New 1H -Pyrazole-Containing Polyamine Receptors Able To Complex L -Glutamate inWaterA R T I C L E SMiranda et al.824J.AM.CHEM.SOC.9VOL.126,NO.3,2004strengths.1[L1]and2[L2]differ in the N-benzylation of the pyrazole moiety,and1[L1]and3[L3]differ in the presence in the center of the polyamine side chains of an amino group or of a methylene group.The receptors4[L4]and5[L5]present the central nitrogens of the chain N-functionalized with benzyl or phenethyl groups,and6[L6]has large hydrophobic octyl groups.Results and DiscussionSynthesis of3-6.Macrocycles3-6have been obtained following the procedure previously reported for the preparation of1and2.23The method includes a first dipodal(2+2) condensation of the1H-pyrazol-3,5-dicarbaldehyde7with the corresponding R,ω-diamine,followed by hydrogenation of the resulting Schiff base imine bonds.In the case of receptor3,the Schiff base formed by condensation with1,5-pentanediamine is a stable solid(8,mp208-210°C)which precipitated in68% yield from the reaction mixture.Further reduction with NaBH4 in absolute ethanol gave the expected tetraazamacrocycle3, which after crystallization from toluene was isolated as a pure compound(mp184-186°C).In the cases of receptors4-6, the precursor R,ω-diamines(11a-11c)(Scheme1B)were obtained,by using a procedure previously described for11a.24 This procedure is based on the previous protection of the primary amino groups of1,5-diamino-3-azapentane by treatment with phthalic anhydride,followed by alkylation of the secondary amino group of1,5-diphthalimido-3-azapentane9with benzyl, phenethyl,or octyl bromide.Finally,the phthalimido groups of the N-alkyl substituted intermediates10a-10c were removed by treatment with hydrazine to afford the desired amines11a-11c,which were obtained in moderate yield(54-63%).In contrast with the behavior previously observed in the synthesis of3,in the(2+2)dipodal condensations of7with 3-benzyl-,3-phenethyl-,and3-octyl-substituted3-aza-1,5-pentanediamine11a,11b,and11c,respectively,there was not precipitation of the expected Schiff bases(Scheme1A). Consequently,the reaction mixtures were directly reduced in situ with NaBH4to obtain the desired hexaamines4-6,which after being carefully purified by chromatography afforded purecolorless oils in51%,63%,and31%yield,respectively.The structures of all of these new cyclic polyamines have been established from the analytical and spectroscopic data(MS(ES+), 1H and13C NMR)of both the free ligands3-6and their corresponding hydrochloride salts[3‚4HCl,4‚6HCl,5‚6HCl, and6‚6HCl],which were obtained as stable solids following the same procedure previously reported18for1‚6HCl and2‚6HCl.As usually occurs for3,5-disubstituted1H-pyrazole deriva-tives,either the free ligands3-6or their hydrochlorides show very simple1H and13C NMR spectra,in which signals indicate that,because of the prototropic equilibrium of the pyrazole ring, all of these compounds present average4-fold symmetry on the NMR scale.The quaternary C3and C5carbons appear together,and the pairs of methylene carbons C6,C7,and C8are magnetically equivalent(see Experimental Section).In the13C NMR spectra registered in CDCl3solution, significant differences can be observed between ligand3,without an amino group in the center of the side chain,and the N-substituted ligands4-6.In3,the C3,5signal appears as a broad singlet.However,in4-6,it almost disappears within the baseline of the spectra,and the methylene carbon atoms C6and C8experience a significant broadening.Additionally,a remark-able line-broadening is also observed in the C1′carbon signals belonging to the phenethyl and octyl groups of L5and L6, respectively.All of these data suggest that as the N-substituents located in the middle of the side chains of4-6are larger,the dynamic exchange rate of the pyrazole prototropic equilibrium is gradually lower,probably due to a relation between proto-tropic and conformational equilibria.Acid-Base Behavior.To follow the complexation of L-glutamate(hereafter abbreviated as Glu2-)and its protonated forms(HGlu-,H2Glu,and H3Glu+)by the receptors L1-L6, the acid-base behavior of L-glutamate has to be revisited under the experimental conditions of this work,298K and0.15mol dm-3.The protonation constants obtained,included in the first column of Table1,agree with the literature25and show that the zwitterionic HGlu-species is the only species present in aqueous solution at physiological pH values(Scheme2and Figure S1of Supporting Information).Therefore,receptors for(23)Ara´n,V.J.;Kumar,M.;Molina,J.;Lamarque,L.;Navarro,P.;Garcı´a-Espan˜a,E.;Ramı´rez,J.A.;Luis,S.V.;Escuder,.Chem.1999, 64,6137-6146.(24)(a)Yuen Ng,C.;Motekaitis,R.J.;Martell,A.E.Inorg.Chem.1979,18,2982-2986.(b)Anelli,P.L.;Lunazzi,L.;Montanari,F.;Quici,.Chem.1984,49,4197-4203.Scheme1.Synthesis of the Pyrazole-Containing MacrocyclicReceptorsNew1H-Pyrazole-Containing Polyamine Receptors A R T I C L E SJ.AM.CHEM.SOC.9VOL.126,NO.3,2004825glutamate recognition able to address both the negative charges of the carboxylate groups and the positive charge of ammonium are highly relevant.The protonation constants of L 3-L 6are included in Table 1,together with those we have previously reported for receptors L 1and L 2.23A comparison of the constants of L 4-L 6with those of the nonfunctionalized receptor L 1shows a reduced basicity of the receptors L 4-L 6with tertiary nitrogens at the middle of the polyamine bridges.Such a reduction in basicity prevented the potentiometric detection of the last protonation for these ligands in aqueous solution.A similar reduction in basicity was previously reported for the macrocycle with the N -benzylated pyrazole spacers (L 2).23These diminished basicities are related to the lower probability of the tertiary nitrogens for stabilizing the positive charges through hydrogen bond formation either with adjacent nonprotonated amino groups of the molecule or with water molecules.Also,the increase in the hydrophobicity of these molecules will contribute to their lower basicity.The stepwise basicity constants are relatively high for the first four protonation steps,which is attributable to the fact that these protons can bind to the nitrogen atoms adjacent to the pyrazole groups leaving the central nitrogen free,the electrostatic repulsions between them being therefore of little significance.The remaining protonation steps will occur in the central nitrogen atom,which will produce an important increase in the electrostatic repulsion in the molecule and therefore a reduction in basicity.As stated above,the tertiary nitrogen atoms present in L 4-L 6will also contribute to this diminished basicity.To analyze the interaction with glutamic acid,it is important to know the protonation degree of the ligands at physiological pH values.In Table 2,we have calculated the percentages ofthe different protonated species existing in solution at pH 7.4for receptors L 1-L 6.As can be seen,except for the receptor with the pentamethylenic chains L 3in which the tetraprotonated species prevails,all of the other systems show that the di-and triprotonated species prevail,although to different extents.Interaction with Glutamate.The stepwise constants for the interaction of the receptors L 1-L 6with glutamate are shown in Table 3,and selected distribution diagrams are plotted in Figure 1A -C.All of the studied receptors interact with glutamate forming adduct species with protonation degrees (j )which vary between 8and 0depending on the system (see Table 3).The stepwise constants have been derived from the overall association constants (L +Glu 2-+j H +)H j LGlu (j -2)+,log j )provided by the fitting of the pH-metric titration curves.This takes into account the basicities of the receptors and glutamate (vide supra)and the pH range in which a given species prevails in solution.In this respect,except below pH ca.4and above pH 9,HGlu -can be chosen as the protonated form of glutamate involved in the formation of the different adducts.Below pH 4,the participation of H 2Glu in the equilibria has also to be considered (entries 9and 10in Table 3).For instance,the formation of the H 6LGlu 4+species can proceed through the equilibria HGlu -+H 5L 5+)H 6LGlu 4+(entry 8,Table 3),and H 2Glu +H 4L 4+)H 6LGlu 4(entry 9Table 3),with percentages of participation that depend on pH.One of the effects of the interaction is to render somewhat more basic the receptor,and somewhat more acidic glutamic acid,facilitating the attraction between op-positely charged partners.A first inspection of Table 3and of the diagrams A,B,and C in Figure 1shows that the interaction strengths differ markedly from one system to another depending on the structural features of the receptors involved.L 4is the receptor that presents the highest capacity for interacting with glutamate throughout all of the pH range explored.It must also be remarked that there are not clear-cut trends in the values of the stepwise constants as a function of the protonation degree of the receptors.This suggests that charge -charge attractions do not play the most(25)(a)Martell,E.;Smith,R.M.Critical Stability Constants ;Plenum:NewYork,1975.(b)Motekaitis,R.J.NIST Critically Selected Stability Constants of Metal Complexes Database ;NIST Standard Reference Database,version 4,1997.Table 1.Protonation Constants of Glutamic Acid and Receptors L 1-L 6Determined in NaCl 0.15mol dm -3at 298.1KreactionGluL 1aL 2aL 3bL 4L 5L 6L +H )L H c 9.574(2)d 9.74(2)8.90(3)9.56(1)9.25(3)9.49(4)9.34(5)L H +H )L H 2 4.165(3)8.86(2)8.27(2)8.939(7)8.38(3)8.11(5)8.13(5)L H 2+H )L H 3 2.18(2)7.96(2) 6.62(3)8.02(1) 6.89(5)7.17(6)7.46(7)L H 3+H )L H 4 6.83(2) 5.85(4)7.63(1) 6.32(5) 6.35(6) 5.97(8)L H 4+H )L H 5 4.57(3) 3.37(4) 2.72(8) 2.84(9) 3.23(9)L H 5+H )L H 6 3.18(3) 2.27(6)∑log K H n L41.135.334.233.634.034.1aTaken from ref 23.b These data were previously cited in a short communication (ref 26).c Charges omitted for clarity.d Values in parentheses are the standard deviations in the last significant figure.Scheme 2.L -Glutamate Acid -BaseBehaviorTable 2.Percentages of the Different Protonated Species at pH 7.4H 1L aH 2LH 3LH 4LL 11186417L 21077130L 3083458L 4083458L 51154323L 6842482aCharges omitted for clarity.A R T I C L E SMiranda et al.826J.AM.CHEM.SOC.9VOL.126,NO.3,2004outstanding role and that other forces contribute very importantly to these processes.26However,in systems such as these,which present overlapping equilibria,it is convenient to use conditional constants because they provide a clearer picture of the selectivity trends.27These constants are defined as the quotient between the overall amounts of complexed species and those of free receptor and substrate at a given pH[eq1].In Figure2are presented the logarithms of the effective constants versus pH for all of the studied systems.Receptors L1and L2with a nonfunctionalized secondary amino group in the side chains display opposite trend from all other receptors. While the stability of the L1and L2adducts tends to increase with pH,the other ligands show a decreasing interaction. Additionally,L1and L2present a close interaction over the entire pH range under study.The tetraaminic macrocycle L3is a better(26)Escartı´,F.;Miranda,C.;Lamarque,L.;Latorre,J.;Garcı´a-Espan˜a,E.;Kumar,M.;Ara´n,V.J.;Navarro,mun.2002,9,936-937.(27)(a)Bianchi,A.;Garcı´a-Espan˜a,c.1999,12,1725-1732.(b)Aguilar,J.A.;Celda,B.;Garcı´a-Espan˜a,E.;Luis,S.V.;Martı´nez,M.;Ramı´rez,J.A.;Soriano,C.;Tejero,B.J.Chem.Soc.,Perkin Trans.22000, 7,1323-1328.Table3.Stability Constants for the Interaction of L1-L6with the Different Protonated Forms of Glutamate(Glu) entry reaction a L1L2L3L4L5L6 1Glu+L)Glu L 3.30(2)b 4.11(1)2HGlu+L)HGlu L 3.65(2) 4.11(1) 3.68(2) 3.38(4) 3Glu+H L)HGlu L 3.89(2) 4.48(1) 3.96(2) 3.57(4) 4HGlu+H L)H2Glu L 3.49(2) 3.89(1) 2.37(4) 3.71(2)5HGlu+H2L)H3Glu L 3.44(2) 3.73(1) 2.34(3) 4.14(2) 2.46(4) 2.61(7) 6HGlu+H3L)H4Glu L 3.33(2) 3.56(2) 2.66(3) 4.65(2) 2.74(3) 2.55(7) 7HGlu+H4L)H5Glu L 3.02(2) 3.26(2) 2.58(3) 4.77(2) 2.87(3) 2.91(5) 8HGlu+H5L)H6Glu L 3.11(3) 3.54(2) 6.76(3) 4.96(3) 4.47(3) 9H2Glu+H4L)H6Glu L 2.54(3) 3.05(2) 3.88(2) 5.35(3) 3.66(4) 3.56(3) 10H2Glu+H5L)H7Glu L 2.61(6) 2.73(4) 5.51(3) 3.57(4) 3.22(8) 11H3Glu+H4L)H7Glu L 4.82(2) 4.12(9)a Charges omitted for clarity.b Values in parentheses are standard deviations in the last significantfigure.Figure1.Distribution diagrams for the systems(A)L1-glutamic acid, (B)L4-glutamic acid,and(C)L5-glutamicacid.Figure2.Representation of the variation of K cond(M-1)for the interaction of glutamic acid with(A)L1and L3,(B)L2,L4,L5,and L6.Initial concentrations of glutamate and receptors are10-3mol dm-3.Kcond)∑[(H i L)‚(H j Glu)]/{∑[H i L]∑[H j Glu]}(1)New1H-Pyrazole-Containing Polyamine Receptors A R T I C L E SJ.AM.CHEM.SOC.9VOL.126,NO.3,2004827receptor at acidic pH,but its interaction markedly decreases on raising the pH.These results strongly suggest the implication of the central nitrogens of the lateral polyamine chains in the stabilization of the adducts.Among the N-functionalized receptors,L4presents the largest interaction with glutamate.Interestingly enough,L5,which differs from L4only in having a phenethyl group instead of a benzyl one,presents much lower stability of its adducts.Since the basicity and thereby the protonation states that L4and L5 present with pH are very close,the reason for the larger stability of the L4adducts could reside on a better spatial disposition for formingπ-cation interactions with the ammonium group of the amino acid.In addition,as already pointed out,L4presents the highest affinity for glutamic acid in a wide pH range,being overcome only by L1and L2at pH values over9.This observation again supports the contribution ofπ-cation inter-actions in the system L4-glutamic because at these pH values the ammonium functionality will start to deprotonate(see Scheme2and Figure1B).Table4gathers the percentages of the species existing in equilibria at pH7.4together with the values of the conditional constant at this pH.In correspondence with Figure1A,1C and Figure S2(Supporting Information),it can be seen that for L1, L2,L5,and L6the prevailing species are[H2L‚HGlu]+and[H3L‚HGlu]2+(protonation degrees3and4,respectively),while for L3the main species are[H3L‚HGlu]+and[H4L‚HGlu]2+ (protonation degrees4and5,respectively).The most effective receptor at this pH would be L4which joins hydrogen bonding, charge-charge,andπ-cation contributions for the stabilization of the adducts.To check the selectivity of this receptor,we have also studied its interaction with L-aspartate,which is a competitor of L-glutamate in the biologic receptors.The conditional constant at pH7.4has a value of3.1logarithmic units for the system Asp-L4.Therefore,the selectivity of L4 for glutamate over aspartate(K cond(L4-glu)/K cond(L4-asp))will be of ca.15.It is interesting to remark that the affinity of L4 for zwiterionic L-glutamate at pH7.4is even larger than that displayed by receptors III and IV(Chart1)with the protected dianion N-acetyl-L-glutamate lacking the zwitterionic charac-teristics.Applying eq1and the stability constants reported in ref17,conditional constants at pH7.4of 3.24and 2.96 logarithmic units can be derived for the systems III-L-Glu and IV-L-Glu,respectively.Molecular Modeling Studies.Molecular mechanics-based methods involving docking studies have been used to study the binding orientations and affinities for the complexation of glutamate by L1-L6receptors.The quality of a computer simulation depends on two factors:accuracy of the force field that describes intra-and intermolecular interactions,and an adequate sampling of the conformational and configuration space of the system.28The additive AMBER force field is appropriate for describing the complexation processes of our compounds,as it is one of the best methods29in reproducing H-bonding and stacking stabiliza-tion energies.The experimental data show that at pH7.4,L1-L6exist in different protonation states.So,a theoretical study of the protonation of these ligands was done,including all of the species shown in5%or more abundance in the potentiometric measurements(Table4).In each case,the more favored positions of protons were calculated for mono-,di-,tri-,and tetraprotonated species.Molecular dynamics studies were performed to find the minimum energy conformations with simulated solvent effects.Molecular modeling studies were carried out using the AMBER30method implemented in the Hyperchem6.0pack-age,31modified by the inclusion of appropriate parameters. Where available,the parameters came from analogous ones used in the literature.32All others were developed following Koll-man33and Hopfinger34procedures.The equilibrium bond length and angle values came from experimental values of reasonable reference compounds.All of the compounds were constructed using standard geometry and standard bond lengths.To develop suitable parameters for NH‚‚‚N hydrogen bonding,ab initio calculations at the STO-3G level35were used to calculate atomic charges compatible with the AMBER force field charges,as they gave excellent results,and,at the same time,this method allows the study of aryl-amine interactions.In all cases,full geometry optimizations with the Polak-Ribiere algorithm were carried out,with no restraints.Ions are separated far away and well solvated in water due to the fact that water has a high dielectric constant and hydrogen bond network.Consequently,there is no need to use counteri-ons36in the modelization studies.In the absence of explicit solvent molecules,a distance-dependent dielectric factor quali-tatively simulates the presence of water,as it takes into account the fact that the intermolecular electrostatic interactions should vanish more rapidly with distance than in the gas phase.The same results can be obtained using a constant dielectric factor greater than1.We have chosen to use a distance-dependent dielectric constant( )4R ij)as this was the method used by Weiner et al.37to develop the AMBER force field.Table8 shows the theoretical differences in protonation energy(∆E p) of mono-,bi-,and triprotonated hexaamine ligands,for the (28)Urban,J.J.;Cronin,C.W.;Roberts,R.R.;Famini,G.R.J.Am.Chem.Soc.1997,119,12292-12299.(29)Hobza,P.;Kabelac,M.;Sponer,J.;Mejzlik,P.;Vondrasek,put.Chem.1997,18,1136-1150.(30)Cornell,W.D.;Cieplak,P.;Bayly,C.I.;Gould,I.R.;Merz,K.M.,Jr.;Ferguson,D.M.;Spelmeyer,D.C.;Fox,T.;Caldwell,J.W.;Kollman,P.A.J.Am.Chem.Soc.1995,117,5179-5197.(31)Hyperchem6.0(Hypercube Inc.).(32)(a)Fox,T.;Scanlan,T.S.;Kollman,P.A.J.Am.Chem.Soc.1997,119,11571-11577.(b)Grootenhuis,P.D.;Kollman,P.A.J.Am.Chem.Soc.1989,111,2152-2158.(c)Moyna,G.;Hernandez,G.;Williams,H.J.;Nachman,R.J.;Scott,put.Sci.1997,37,951-956.(d)Boden,C.D.J.;Patenden,put.-Aided Mol.Des.1999, 13,153-166.(33)/amber.(34)Hopfinger,A.J.;Pearlstein,put.Chem.1984,5,486-499.(35)Glennon,T.M.;Zheng,Y.-J.;Le Grand,S.M.;Shutzberg,B.A.;Merz,K.M.,put.Chem.1994,15,1019-1040.(36)Wang,J.;Kollman,P.A.J.Am.Chem.Soc.1998,120,11106-11114.Table4.Percentages of the Different Protonated Adducts[HGlu‚H j L](j-1)+,Overall Percentages of Complexation,andConditional Constants(K Cond)at pH7.4for the Interaction ofGlutamate(HGlu-)with Receptors L1-L6at Physiological pH[H n L‚HGlu]an)1n)2n)3n)4∑{[H n L‚HGlu]}K cond(M-1)L13272353 2.44×103L2947763 4.12×103L31101324 3.99×102L423737581 2.04×104L51010222 3.51×102L6121224 3.64×102a Charges omitted for clarity.A R T I C L E S Miranda et al. 828J.AM.CHEM.SOC.9VOL.126,NO.3,2004。
乙醇电氧化催化的英文

乙醇电氧化催化的英文英文回答:Ethanol electro-oxidation catalysis (EEOC) is a promising technology for a variety of applications, including fuel cells and sensors. The electro-oxidation of ethanol involves the transfer of electrons from the ethanol molecule to an electrode, which results in the formation of acetaldehyde and protons. The reaction can be represented as follows:C2H5OH + H2O → CH3CHO + 2H+ + 2e-。
The efficiency of EEOC is influenced by a number of factors, including the nature of the electrode material, the electrolyte, and the operating conditions. The most commonly used electrode materials for EEOC are platinum and platinum-based alloys. These materials are highly activefor the electro-oxidation of ethanol, but they are also expensive.The electrolyte used in EEOC typically consists of an aqueous solution of a strong acid, such as sulfuric acid or phosphoric acid. The acid provides the protons that are required for the reaction. The operating conditions for EEOC can vary, but the most common temperature range is 50-80 °C.EEOC is a complex process that involves a number of different steps. The first step is the adsorption of ethanol onto the electrode surface. This is followed by the dehydrogenation of ethanol to form acetaldehyde. The acetaldehyde is then further oxidized to form carbon dioxide and water.The development of efficient and cost-effective EEOC catalysts is an important area of research. The success of this research will lead to the development of new fuelcells and sensors that can be used for a variety of applications.中文回答:乙醇电氧化催化 (EEOC) 是一项应用于燃料电池和传感器等多种领域的颇具前景的技术。
循环伏安法合成导电聚苯胺导电性及热稳定性

循环伏安法合成导电聚苯胺导电性及热稳定性周婉秋;王宇玲;赵玉明;孙秋菊;辛士刚;张洪波【摘要】聚苯胺近年来在金属防腐领域备受关注,在质子交换膜燃料电池(PEMFC)不锈钢双极板表面电化学沉积聚苯胺薄膜,能够进一步提高双极板的耐腐蚀性能,有望替代传统的石墨双极板在PEMFC中得到应用.电池工作条件下要求双极板具有导电性,工作温度在70~100℃,因此研究聚苯胺薄膜的导电性和热稳定性十分必要.采用循环伏安法,在0.2 mol/L H2 SO4和0.1 mol/L苯胺组成的水溶液体系中,在316 L不锈钢表面制备了聚苯胺(PANI)薄膜;采用红外光谱研究PANI薄膜的化学结构;采用四探针技术测量PANI的电导率;采用热重分析技术研究了PANI的热稳定性;采用扫描电镜观察表面形貌.结果表明:聚苯胺薄膜的电导率最大值达到8.96 S/cm,分解温度为352.7℃,表面呈现纤维状簇集状态,颗粒分布比较均匀.【期刊名称】《沈阳师范大学学报(自然科学版)》【年(卷),期】2017(035)001【总页数】5页(P29-33)【关键词】导电聚苯胺;循环伏安法;热稳定性;导电性;双极板【作者】周婉秋;王宇玲;赵玉明;孙秋菊;辛士刚;张洪波【作者单位】沈阳师范大学化学化工学院,沈阳110034;沈阳师范大学化学化工学院,沈阳110034;沈阳师范大学化学化工学院,沈阳110034;沈阳师范大学化学化工学院,沈阳110034;沈阳师范大学化学化工学院,沈阳110034;沈阳师范大学化学化工学院,沈阳110034【正文语种】中文【中图分类】TB333聚苯胺(PANI)是一种导电聚合物,因其合成方法简单,原料廉价,易掺杂改性等优点,被认为是最具有应用前景的导电高分子材料之一,目前,聚苯胺在抗静电[1]、二次电源[2]、电磁波屏蔽[3]、金属防腐[4-6]、吸波材料[7]等领域取得了重要研究进展,在金属防腐领域的应用备受关注。
219413425_LiCl-KCl-MgCl2熔盐体系中Li-Mg_共沉积机理研究

第14卷第3期2023年6月有色金属科学与工程Nonferrous Metals Science and EngineeringVol.14,No.3Jun. 2023LiCl-KCl-MgCl 2熔盐体系中Li-Mg 共沉积机理研究张远景1,2, 刘兆庭1,2, 朱实贵3, 路贵民*1,2(1.华东理工大学盐湖资源综合利用国家工程研究中心,上海 200237; 2.钾锂战略资源国际联合实验室,上海 200237;3.宜春赣锋锂业有限公司,江西 宜春 336000)摘要:Li-Mg 合金作为锂电池负极材料在新能源领域中具有广阔的应用前景, 熔盐电解法制备Li-Mg 合金极具优势。
本文采用三电极体系研究了Mg 2+在LiCl-KCl-MgCl 2熔体中钨电极上的电化学行为及Li-Mg 共沉积机理,探究了MgCl 2浓度对电解共沉积Li-Mg 的影响。
方波伏安法与计时电流法实验结果表明:Mg 2+在钨电极上一步两电子还原为金属Mg ,属于瞬时成核过程,不受温度的影响。
计时电位法实验结果表明:随着MgCl 2浓度的增加,LiCl-KCl-MgCl 2熔体电解共沉积Li-Mg 所需的阴极电流密度逐渐增大。
当LiCl-KCl-MgCl 2熔体中MgCl 2浓度为5%时,实现Li-Mg 共沉积的最小阴极电流密度为0.287 A/cm 2。
恒电流电解结果表明:当MgCl 2浓度≤5%时,Li-Mg 产品中金属Mg 含量随着熔体中MgCl 2浓度的增加而增大,当MgCl 2浓度达到10%时,电解仅得到金属Mg 。
关键词:熔盐电解;Li-Mg 共沉积;电化学;MgCl 2浓度中图分类号:TF822 文献标志码:AStudy on Li-Mg co-deposition mechanism in LiCl-KCl-MgCl 2 meltZHANG Yuanjing 1, 2, LIU Zhaoting 1, 2, ZHU Shigui 3, LU Guimin *1, 2(1. National Engineering Research Center for Integrated Utilization of Salt Lake Resources , East China University of Science andTechnology , Shanghai 200237, China ; 2. Joint International Laboratory for Potassium and Lithium Strategic Resources , Shanghai200237, China ; 3. Fengxin Ganfeng Lithium Industry Co., Ltd., Yichun 336000, Jiangxi , China )Abstract: Li-Mg alloys , as cathode materials for lithium batteries , have broad application prospects in the field of new energy , and the preparation of Li-Mg alloys by molten salt electrolysis has great advantages. The electrochemical behavior of Mg 2+ on a tungsten electrode in LiCl-KCl-MgCl 2 melt and the Li-Mg co-deposition process were studied by a three-electrode system , respectively. The effect of MgCl 2 concentration on electrolytic co-deposition of Li-Mg was also investigated. The experimental results of square wave voltammetry and timing current method show that the one-step two electrons reduction of Mg 2+ to metallic Mg on the tungsten electrode is an instantaneous nucleation process , which is not affected by temperature. The results of the timing potentiometric experiment show that with the increasing concentration of MgCl 2, the cathodic current density required for the electrolytic co-deposition of Li-Mg from LiCl-KCl-MgCl 2 melt is gradually increased. When the MgCl 2 concentration in the LiCl-KCl-MgCl 2 melt is 5%, the minimum cathodic current density to achieve Li-Mg co-deposition is 0.287 A/cm 2. The galvanostatic electrolysis results show that when the MgCl 2 concentration is less than or equal to 5%, the metal Mg content in the Li-Mg product increases with MgCl 2 concentration in the melt.收稿日期:2022-05-26;修回日期:2022-06-14基金项目:国家自然科学基金资助项目(U20A20147)通信作者:路贵民(1965— ),教授,主要从事熔盐化学与技术、新能源材料方面的研究。
AZ91D镁合金在几种典型介质中的腐蚀性能研究

AZ91D镁合金在几种典型介质中的腐蚀性能研究王建;周婉秋;武士威;赵强【摘要】采用电化学方法,在0.1M NaCl和0.1M Na2SO4及0.1M Na2CO3等几种典型介质中研究了AZ91D镁合金的腐蚀行为.极化曲线结果表明:在NaCl和Na2SO4中,阳极极化曲线为活性溶解,在Na2CO3中阳极极化曲线呈现钝化特征.在3种介质中的耐腐蚀性顺序为:NaCl<Na2SO4<Na2CO3.EIS结果显示:AZ91D 镁合金在几种介质中的电化学阻抗谱均由一个高频容抗弧和一个低频容抗弧组成.形貌观察表明:AZ91D镁合金的腐蚀均只发生在α相,β相不发生腐蚀.在NaCl中腐蚀最为严重,腐蚀的面积较大;在Na2SO4中腐蚀面积较小,在Na2CO3中腐蚀程度最轻,且腐蚀得比较均匀.%Corrosion behavior of AZ91D magnesium alloy in several typical media including 0.1 M NaCl, 0.1M Na2SO4 and 0.1 MNa2CO3 were investigated by electrochemical methods.Polarization curve tests showed that anodic polarization curves presented in active dissolution characteristic in 0.1 M NaCl and 0.1 M Na2SO4, while, the anodic branch of polarization curve in 0.1 M Na2CO3 presented in passivation characteristic.The order of anti-corrosion performance in the three media was as follows: NaCl< Na2SO4 < Na2CO3.EIS measurements illustrated that the Nyquist plots included a high frequency capacitive impedance arc and a low frequency capacitive impedancearc.Morphologies observation indicated that the corrosion on AZ91D magnesium alloy surface only occurred in α phase interior, andβ phase did not be corroded.For AZ91D magnesium alloy, the worst corrosion occurred in the NaCl medium, and the area of attack was largest, the district ofcorrosion was less in Na2SO4 media, and the region of corrosion inNa2CO3 was the mildest and showed in uniform feature.【期刊名称】《沈阳师范大学学报(自然科学版)》【年(卷),期】2011(029)001【总页数】5页(P95-99)【关键词】镁合金;腐蚀行为;极化曲线;电化学阻抗谱【作者】王建;周婉秋;武士威;赵强【作者单位】沈阳师范大学,化学与生命科学学院,沈阳,110034;沈阳师范大学,化学与生命科学学院,沈阳,110034;沈阳师范大学,化学与生命科学学院,沈阳,110034;沈阳师范大学,化学与生命科学学院,沈阳,110034【正文语种】中文【中图分类】TQ0509+1镁合金的密度小,质量轻、比强度高,是最轻的金属结构材料[1],并以其广泛的分布和优异的物理及力学性能而成为一种非常理想的现代工业结构材料,在汽车工业[2]、航空航天、电子产品等领域有广阔的应用前景[3]。
循环伏安法英文
循环伏安法英文Cyclic voltammetry (CV) is an electrochemical technique used to study the redox behavior of chemical species at a solid-liquid interface. It involves the application of a time-varying potential between working and reference electrodes in the presence of an electrolyte solution. The potential is swept back and forth across a range of values, allowing the measurement of current as a function of electrode potential. The resulting voltammogram provides information on the thermodynamics and kinetics of the electrochemical process. In this article, we will discuss the principles and applications of cyclic voltammetry.Principles of CV:CV involves the application of a potential that is swept back and forth between two extreme values (anodic and cathodic limits) while a current is recorded. The direction of the current flow is dependent on the nature of the redox process and the polarity of the applied potential. If the redox couple undergoes oxidation, an anodic current is obtained, while a cathodic current is obtained during reduction. By sweeping the potential between these limits, the reaction proceeds in the forward and reverse directions, allowing the measurement of the redox potential (E0) and reaction kinetics.Applications of CV:The cyclic voltammetry technique has proved useful in many fields, including electrochemistry, analytical chemistry, physical chemistry, and materials science. Below are some examples of itsapplications:1. Electrochemical detection of biomolecules and drugs:Cyclic voltammetry has been used for the detection and quantification of various biomolecules such as glucose, cholesterol, and DNA. The technique involves the modification of electrodes with specific biomolecules, which interact with the target analyteto produce a redox signal. This method is highly sensitive and can be used for rapid analysis of complex biological samples.2. Corrosion analysis:CV is employed in the study of corrosion processes of metals and alloys. The technique helps to identify the oxidation and reduction reactions involved in corrosion and evaluate the effectiveness of corrosion inhibitors.3. Battery research:CV is used to study the electrochemical behavior of battery materials, such as anodes, cathodes, and electrolytes, and to evaluate their electrochemical performance. This information aids the development of efficient and long-lasting batteries.4. Catalysis studies:CV is used to investigate the electrocatalytic activity of catalysts. The technique involves the application of a potential to the catalyst, which results in the generation of redox-active intermediates andthe measurement of the catalytic current.Conclusion:Cyclic voltammetry is a powerful electrochemical technique used to study the redox behavior of chemical species at a solid-liquid interface. It has many applications in various fields, including electrochemistry, analytical chemistry, physical chemistry, and materials science. Its versatility, sensitivity, and speed make it a popular method of analysis. By obtaining the voltammogram, CV enables the elucidation of the electrochemical properties of the redox system under study, including the redox potential and the kinetics of electron transfer. Cyclic voltammetry continues to be an important tool for the study of electrochemical systems and for the advancement of various technologies.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Electrochemical behaviour of a Mg-rich primerin the protection of Al alloysD.Battocchia,*,A.M.Simo ˜es b ,D.E.Tallmana,c ,G.P.Bierwagen a a NDSU,Department of Coatings and Polymeric Materials,1735NDSU Research Park Drive,Fargo,ND 58105-5376,USAb IST,Chemical Engineering Department,Av.Rovisco Pais,1049-001Lisboa,Portugalc NDSU,Department of Chemistry,Fargo,ND 58105,USAReceived 28January 2005;accepted 5April 2005Available online 15August 2005AbstractThe electrochemical behaviour of Mg-rich primer on Al Alloys,AA2024and AA7075,has been studied via Electrochemical Impedance Spectroscopy (EIS),Open Circuit Potential (OCP)and potentiodynamic polarization.Results showed that the Mg-rich primer provides sacrificial protection to the Al substrate by a two-stage mechanism.In a first stage,corrosion of aluminium is prevented by cathodic polarization,whereas at a later stage the precipitation of a porous barrier layer of magnesium oxide was observed.Ó2005Elsevier Ltd.All rights reserved.Keywords:Mg-rich primer;AA2024;AA7075;EIS;OCP;Potentiodynamic polarization;Sacrificial protection0010-938X/$-see front matter Ó2005Elsevier Ltd.All rights reserved.doi:10.1016/j.corsci.2005.04.008*Corresponding author.Tel.:+17012316219;fax:+17012318439.E-mail address:dante.battocchi@ (D.Battocchi).Corrosion Science 48(2006)1292–1306D.Battocchi et al./Corrosion Science48(2006)1292–130612931.IntroductionWhen two metals exposed to a corrosive environment are in put contact,the more active metal will corrode preferentially,providing sacrificial protection to the nobler one.This concept has been applied for many years to the protection of steel by the use of zinc-rich primers(ZRPs)[1,2]and trials using other compositions[3,4]and metals,including manganese[5],have also been made.For aluminium,however, such sacrificial protection has not been applied.Aluminium is very close to the bot-tom of the galvanic series,below zinc,and therefore ZRPs would not be effective. Corrosion protection of aluminium surfaces is usually achieved by using chromate pre-treatments[6]or primers[7].The severe restrictions to Cr use,dictated by its toxicity,require the development of environmentally less aggressive alternatives. The potential of Mg is positive of almost all the other structural materials[8],includ-ing aluminium,and its use as a pigment presents therefore a possible alternative for the sacrificial protection of aluminium alloys.This new class of metal-rich primer for the protection of aluminium alloys can thus be formulated,in analogy to the formu-lation of Zn-rich primer for the protection of steel.To provide sacrificial protection, the Mg metal particles in the primer have to be in electrical contact with the substrate and also with each other.To achieve this requirement,the primer has to be formu-lated near or above the Critical Pigment Volume Concentration(CPVC)for the coating[9].A Mg-based system for the protection of aluminium structures,based on this concept,has been developed and tested by Nanna and Bierwagen[10], who have observed an excellent performance of aluminium panels sprayed with this Mg-rich primer in Prohesion testing.Aluminium alloys AA2024-T3and AA7075-T6are widely used in the aircraft industry for their high strength and low density.Their high strength is achieved by heat treatments that affect the microstructure of the alloys and lead to the forma-tion of intermetallic precipitates.These precipitates,mostly rich in copper,are responsible for the generation of potential differences along the surface that make the alloys prone to localized corrosion[11,12].The mode of protection of these alloys by Mg-rich primers is the concern of this work.Electrochemical characterization of the system was obtained using electrochemi-cal impedance spectroscopy(EIS),measurement of the open circuit potential(OCP) and potentiodynamic polarization.EIS is a technique that can simultaneously pro-vide information on the corrosion mechanisms and quantitatively assesses corrosion protection provided by a coating to a metal substrate.For barrier coatings the impedance values should be above106X cm2,in order to decrease the corrosion rate, whereas for sacrificial coatings low impedance is required to ensure conductivity be-tween the cathodic metal substrate and the anodic pigment[13].When used together, potential monitoring and potentiodynamic polarization can also provide valuable information about the status of the sacrificial protection provided by the primer, namely in what concerns the rate of Mg oxidation and the time evolution of the system.These two techniques were used and complemented by scanning electron microscopy(SEM).1294 D.Battocchi et al./Corrosion Science48(2006)1292–13062.ExperimentalAluminium panels of AA2024-T3and AA7075-T6,with dimensions150·75·2mm3,supplied by Q Panel Lab products(Cleveland,OH)were used in the tests. The samples for the tests of the bare alloys were polished to grit600and then washed with distilled water and ethanol,whereas those coated with the Mg-rich primer were brushed with a wire brush and washed in distilled water and hexane.The magnesium-rich primer was made using a stabilized Mg particulate,of30–40l m in average size,manufactured by Non Ferrum-Metallpulver GmbH,Salzburg, Austria.This particulate consists of Mg covered with a thin layer of MgO,intended to control the reactivity of magnesium[14]and thus prevent further oxidation under dry conditions.Dispersion was made using a silane-modified multi-layer/ IPN polymer matrix[10].In order to ensure electronic conduction,the Mg-rich primer was formulated at50%PVC(approximately the CPVC of the system).The primer was applied through an air spray gun to a thickness of approximately 70l m.A period of three days was allowed for complete drying before measurements were started.A magnesium electrode was made by pressing the pigmentary magnesium parti-cles using an International Crystal Laboratories(ICL)press at20MPa,which pro-duced a pellet with surface area of$1cm2.For ease of handling,this pellet was glued onto an inert glass substrate using epoxy resin.Electric contact was made by a Pt wire embedded in the resin.For both the bare and the coated aluminium,the electrochemical cell consisted of a glass cylinder reservoir clamped on the surface.Leaking was avoided by using an o-ring.The cylinder wasfilled with the electrolytic solution for the dura-tion of the experiment.The exposed area of the working electrode was7.06cm2.A saturated calomel electrode(SCE)was used as the reference electrode(RE) and a Pt mesh with approximately1cm2area was used as counter electrode. Most of the electrochemical tests were made in0.1wt.%NaCl in distilled water. One of the experiments was conducted in Dilute HarrisonÕs Solution,which emu-lates acid rain and consists of0.35wt%(NH4)2SO4and0.05wt%NaCl in distilled water.A Gamry PC4/300potentiostat/galvanostat with dedicated EIS300software (both from Gamry Instruments Inc.)was used to collect the electrochemical data. Impedance spectra were collected at the open circuit potential,using the frequency range of50kHz to0.1/0.01Hz.The signal amplitude was5mV for the magnesium electrode and10mV for all the other systems.All the currents presented are normal-ized to1cm2.Potentiodynamic plots were obtained at a scanning rate of5mV/s, starting from the open circuit potential.For SEM investigations,samples were mounted on aluminium mounts and coated with gold using a Technics Hummer II sputter coater.Images were obtained using a JEOL JSM-6300Scanning Electron Microscope.EDAX information was obtained via a Thermo EDS detector using a VANTAGE Digital Acquisition Engine at 15keV.D.Battocchi et al./Corrosion Science48(2006)1292–130612953.Results and discussion3.1.Coating structureMacroscopically,the dry coating is matte to the sight and rough to the touch,as a result of the optically large metal particles used in its formulation.SEM inspection of the cross-section shows that the coating consists essentially of magnesium particles covered by a thin layer of binder.The distance between neighbour particles typically does not exceed1l m—Fig.1.3.2.OCPThe solution pH was approximately5.At this pH,Al becomes passive,as indi-cated in the Pourbaix diagram.The presence of inclusions and precipitates,however, can induce instability of the passivefilm and lead to localized corrosion,and so can the presence of aggressive ions,such as chlorides.For the bare alloys the potential was stable from thefirst minutes of immersion, with the AA2024potential higher than that of the AA7075alloy by$0.15V—Fig.2. Magnesium had a very negative potential,À1.6V,and underwent fast corrosion, with visible bubbling on the surface due to the reduction of protons: 2Hþþ2eÀ!H2ð1Þwhich is the counter-reaction for the oxidation of Mg,which can occur either with the formation[15]of Mg+or by the direct oxidation to Mg2+:Mg!Mg2þþ2eÀð2ÞThe high rate of the cathodic reaction was assessed by measuring the pH on the Mg surface.With that purpose,the electrode was removed from the solution andpH1296 D.Battocchi et al./Corrosion Science48(2006)1292–1306coloured indicator paper was immediately put in contact with the surface.The pH measured in this way was approximately11.When the aluminium alloy was coated with the Mg-rich coating,the potentials achieved at steady-state were intermediate between those of the bare substrate and of the magnesium.They corresponded to the potential of the galvanic couple,in which magnesium polarized cathodically both of the Al alloys by approximately 0.35V.This potential took approximately1h to be achieved.At the beginning it started from a more anodic potential and thenfluctuated until a stable value was at-tained.This initial phase was probably due to the activation of the sacrificial protec-tion,which requires both the penetration of electrolyte to the surface of the Mg particles and the dissolution of the MgO from the particle surface,leaving the Mg exposed for oxidation.3.3.DC potentiodynamic plotsThe DC potentiodynamic plot for Mg shows an approximately symmetrical curve around the corrosion potential,revealing that the metal is in its active state—Fig.3. The corrosion rate obtained by extrapolation of the Tafel lines is approximately 3–5·10À4A cmÀ2.For the bare Al alloys,the cathodic curves have a plateau corre-sponding to the diffusion-limited reduction of dissolved oxygen,followed by a loga-rithmic increase of the current,probably due to reduction of protons.Both alloys are very susceptible to localized corrosion at their open circuit potential,since just a small anodic polarization leads to a current burst of several orders of magnitude, corresponding to the quick growth of pits.For the aluminium substrates coated with Mg-rich primer,the cathodic branches of the plots are practically parallel,with a Tafel slope of0.4V/decade,corresponding to the simultaneous reduction of oxygen and H+.The anodic branch can be divided in two regions,corresponding to the behaviour of each of the materials. Below the pitting potential of the Al alloys the Tafel plot has a very high slope,D.Battocchi et al./Corrosion Science48(2006)1292–13061297ca.0.8V/decade,and reaches a limiting current with the coated2024alloy.Near the corrosion potential of the bare alloys,the slope of the curve decreases abruptly, probably revealing pitting of the substrate in parallel with the Mg oxidation.The slope of thefirst part of the anodic curve,i.e.,the portion related with the oxidation of Mg,is considerably higher than that of bare magnesium.The relative values of the cathodic and anodic slopes on the bare Mg electrode show that magnesium dissolu-tion proceeds under mixed cathodic–anodic control,whereas anodic control occurs when the magnesium particles are embedded in the polymer matrix.Thus,although in a normal situation of galvanic coupling the dissolution rate of the more active metal is accelerated,the use of resin decreases the kinetics of magnesium oxidation, consequently prolonging its lifetime.Furthermore,pitting on either of these alloys is prevented by coupling to magnesium because of the cathodic polarization away from the pitting potential.3.4.Electrochemical impedance spectroscopyCorrosion of the Al alloys in0.1%NaCl resulted in the formation of small pits. This was revealed in the impedance spectrum by a double layer capacitor in parallel with a resistor of several thousand ohm—Fig.4.Near the low frequency end of the spectrum another time constant,probably due to mass transfer control at the pits, was observed.This low frequency part of the spectrum was unstable at the beginning of exposure and became better defined with time.Apart from that,the shape of the spectrum was practically undisturbed during the immersion period,revealing only a rise of the total impedance.Magnesium exhibited very low impedance with visible bubbling on the surface, corresponding to a high corrosion rate.The spectrum reveals only a simple Randles equivalent circuit—Fig.5—corresponding to active corrosion and the system seemed to be at approximately steady state.However,due to the rapid dissolution,the1298 D.Battocchi et al./Corrosion Science48(2006)1292–1306experiment was restricted to short times,and for that reason the spectrum presented refers to1h of immersion.The impedance of magnesium was smaller than that of the bare aluminium substrate by approximately one order of magnitude—Fig.6,a conclusion that is in reasonably good agreement with the results from potentiody-namic polarization.When the Mg-rich coating was applied,the total impedance of the system increased significantly—Fig.6—again in good agreement with the poten-tiodynamic observations and showing that the sacrificial protection was manifested at a comparatively low Mg oxidation rate.Unlike the bare magnesium and the bare aluminium alloy,the coated system impedance decreased with time—Fig.7.On the high impedance part of the spectra,there is an ill-defined capacitance,that goes out of the working frequency window and that is followed by a resistance at$104Hz. This resistance exhibits values that are considered too high to be due to the solution resistance and consequently both the resistor and the capacitor at the high frequency portion of the spectrum can be interpreted as being due to the polymer phase in the coating,a possibility that is corroborated by their change with time,i.e.,due to water penetration.The capacitive region following in the spectrum,at intermediate fre-quencies,probably corresponds to the double layer at the magnesium surface.Mag-nesium particles are initially not only coated with the polymer,but also covered by a layer of magnesium oxide.As water penetrates across the coating and the oxide be-comes dissolved,the number of particles exposed to water,and thus the total active area,will increase,leading to a higher capacitance.This increase in the active area is also revealed in a charge transfer resistance drop,also observed in Fig.7in the de-crease of the plateau at$1Hz.Fitting of the spectra requires a three time constant equivalent circuit as seen in Fig.8.For the polymer layer,i.e.,at the high frequencypart of the spectrum,the capacitance can be taken as 31nF cm À2,a value that is slightly high for a typical polymer film according to the equation for the capacitance of a dielectric layer:C ¼ee 0dA in which e is the dielectric constant of the polymer,e 0is the permittivity of vacuum,d is the thickness and A is the area.This capacitance can be increased by the increase of the real area A of the electrode with respect to the geometrical area,i.e.,the roughness,which is high in this system.Further,the SEM inspection of the sample (Fig.1)has shown that the thickness of polymer above magnesium particles ca be less than 1l m.These two factors account for the high capacitance measured.The physical meaning of the low frequency pro-cess is not totally clear.It can be due to the cathodic reaction proceeding on theD.Battocchi et al./Corrosion Science 48(2006)1292–130612991300 D.Battocchi et al./Corrosion Science48(2006)1292–1306D.Battocchi et al./Corrosion Science48(2006)1292–13061301aluminium exposed at the areas where the coating had voids and therefore water pen-etrated easily.If this is the case,it can be related to a mass diffusion process of charged species,possibly of H+or of OHÀ.Abreu et al.[16]obtained similar spectra with ZRP applied on steel substrates.They interpreted the EIS spectra of ZRP and concluded that the zinc oxide layer could lead to a capacitive loop in the spectrum.The evolution of the coating parameters can be better observed in Fig.9.The drop of the coating resistance with time is consistent with a process of water pene-tration,whereas the charge transfer resistance decrease and the double layer capac-itance increase reveal an increasing exposed area or corrosion rate of the magnesium particles.This is confirmed by SEM and EDAX inspection of the surface of exposed and non-exposed areas,presented in Fig.10.The left half of sample seen in theFig.10.(a)SEM micrograph of primer at the edge of the exposed area,the exposed area being on the right part of the micrograph;(b)ED line scan made at the same area,showing the difference in Mg content.micrograph was kept dry,whereas the right side corresponds to an area that was ex-posed for three weeks.The separation between the two areas consisted of the limit of the cup clamped on the surface.The micrograph reveals a significant difference in the structure of the surface,but the most striking feature consists of the line scan ana-lysis,that reveals practically total depletion of magnesium at the exposed area.3.5.Mechanism of action at defective areasIn order to assess the efficiency and protection mechanism at defective areas of the primer,a large scribe was induced with a knife on a coated sample,exposing an area of approximately1cm2prior to immersion in Dilute HarrisonÕs Solution.Theimpedance of this sample was comparatively small in thefirst hour of immersion,but increased with time,becoming practically only capacitive in the range of frequencies of the spectrum after10days of immersion—Fig.11.Inspection of the scribed area at the end of the experiment by SEM and EDAX revealed the aluminium surface was covered by a precipitate of magnesium oxides.This precipitate was porous and had severalflaws at which the substrate was exposed,as in Fig.12.Theseflaws,however, did not correspond to sites of pitting of aluminium.Fig.12.SEM micrograph(a)and EDAX elemental mapping of precipitates formed on a scratch on AA7075:(b)Mg;(c)Cl;(d)O and(e)Al.4.DiscussionOne major requirement for the effectiveness of sacrificial metal-pigmented primers is that the metal particles be in electrical contact among themselves and also with the substrate.If this were not the case,then each particle would behave as an isolated cell,with the cathodic and the anodic reactions occurring on its surface,and no pro-tection would be provided to the substrate.Our results have now shown that sacri-ficial protection is effective.In fact,the isolated electrochemical activity occurring at each particle would not be measured by the electrochemical techniques used in the work.Further,if the corrosion process measured by impedance were due only to the corrosion of the substrate,then also the potential would have to be due to the substrate only.The OCP measurements,however,have shown that the potential of the galvanic couple was lower than that of the bare alloys,and this has to be a result of cathodic protection.It is not possible to determine from our results what fraction of magnesium is actually used for the protection of the aluminium.Knowl-edge of that fraction would allow the determination of the coating lifetime,in terms of cathodic protection.The oxidation rate of the sacrificial anode is another key factor in the viability of the protective system.Magnesium is at the bottom of the electrochemical series, which provides a high electromotive force for its oxidation by H+from the solution. Further,it does not undergo passivation at neutral pH.Unlike zinc,whose hydrox-ides precipitate at neutral pH,magnesium has a vast pH range over which it remains active[17].This range includes not only the regions of stability of Al3+and Al2O3, but also overlaps the region of alkaline corrosion of aluminium,up to pH$11. Magnesium thus becomes oxidised at a high rate,which could lead to exhaustion of the coating after a relatively short exposure period.Mixing with the polymer, however,significantly decreased its corrosion rate,due to the barrier effect of the polymer.This will certainly extend the lifetime of the coating,for a period that is still uncertain at this point of the research.For zinc-rich primers,it has been observed that zinc corrosion products precipi-tate inside the coating,around the zinc particles that originated them,blocking the pores of the coating and therefore increasing its barrier resistance[18].Magnesium acts in a somewhat different way.Because at the near-neutral pH of the solution the magnesium ions are soluble,they actually diffuse out of the primer layer.Fur-ther,because of the high rate of the electrochemical reactions,the pH can become quite alkaline at the cathodic sites,particularly if there is a relatively small defect in the primer.When these ions reach the cathodic areas,they will then precipitate as Mg(OH)2:Mg2þþ2OHÀ!MgðOHÞð3Þ2$MgOþH2Oð4ÞMgðOHÞ2The mixture of the two oxides is thought to be the main composition of the layer that was observed on the scratched surface.The layer thus formed had a thickness of1–2l m and was highly porous.The oxide layer may therefore not be totally protective, but it can nevertheless provide some degree of barrier protection.5.ConclusionsThe magnesium-rich coating used in this work has the capability of protecting al-loys AA2024and AA7075against corrosion.The effect of magnesium is based upon two different mechanisms,each one associated with one stage.In afirst stage,mag-nesium polarizes aluminium cathodically,shifting its potential below the pitting cor-rosion potential.The consequence of this polarization can be either the prevention of pit nucleation at the exposed aluminium areas,or the inhibition of pit growth for the nucleated pits.During this stage,any defects on the surface will become cathodic, whereas the magnesium particles will be anodic.At the cathodic areas,reduction of hydrogen and possibly dissolved oxygen increases the pH above the threshold for the precipitation of magnesium oxide.This precipitation leads to the formation of a porous layer that further inhibits corrosion by a barrier mechanism.The typically high dissolution rate of magnesium is significantly decreased by its incorporation in the polymer.AcknowledgementsThe authors are grateful to AFOSR(Grant#49620-02-1-0398,Program Officer Major Jennifer Gresham)for the funding provided and to Dr.Scott Payne(USDA/ NDSU)for the assistance in the SEM study.The sabbatical scholarship granted by the Portuguese Foundation for Science and Technology to A.M.Simo˜es is gratefully acknowledged.References[1]S.Feliu Jr.,M.Morcillo,S.Feliu,Corrosion57(2001)591.[2]J.E.O.Mayne,Brit.Corros.J.5(1970)106–111.[3]H.Marchebois,S.Touzain,S.Joiret,J.Bernard,C.Savall,.Coat.45(2002)415–421.[4]W.-B.Chen,P.Chen,H.Y.Chen,W.-T.Tsai,Appl.Surf.Sci.187(2002)154–164.[5]M.Selvaraj,S.Guruviah,.Coat.28(1996)271–277.[6]Y.Liu,A.M.Arenas,S.G.Garcia-Vergara,P.Skelton,G.E.Thompson,K.Shimizu,H.Habazaki,Corros.Sci.47(2005)145–150.[7]J.He,V.J.Gelling,D.E.Tallman,G.P.Bierwagen,J.Electrochem.Soc.147(2000)3661–3666.[8]ASM Handbook,Corrosion:Fundamentals,Testing,and Protection,vol.13A,ASM International,Materials Park,OH,2003.[9]C.Hare,J.Kurnas,J.Coat.Technol.72(2000)21–27.[10]M.E.Nanna,G.P.Bierwagen,J.Coat.Technol.Res.1(2004)69–80.[11]J.R.Davis(Ed.),Corrosion of Aluminum and Aluminum Alloys,ASM,OH,1999.[12]F.Andreatta,H.Terryn,J.H.W.de Wit,Electrochim.Acta49(2004)2851–2862.[13]J.R.Vilche,E.C.Bucharsky,C.A.Giu´dice,Corros.Sci.44(2002)1287–1309.[14]P.F.George,J.J.Newport,J.L.Nichols,Corrosion12(1956)627t–633t.[15]M.G.Lo´pez-Buisa´n Natta,Corrosion57(2001)712–720.[16]C.M.Abreu,M.Izquierdo,M.Keddam,X.R.Novoa,H.Takenouti,Electrochim.Acta41(1996)2405.[17]M.Pourbaix,Atlas of Electrochemical Equilibria in Aqueous Solutions,Pergamon Press,Oxford,1966.[18]S.Feliu Jr.,R.Barajas,J.M.Bastidas,M.Morcillo,S.Feliu,Study of protection mechanisms of zinc-rich paints by electrochemical impedance spectroscopy,in:J.R.Scully, D.C.Silverman,M.W.Kendig(Eds.),Electrochemical Impedance:Analysis and Interpretation,ASTM STP1188,American Society for Testing and Materials,Philadelphia,1993,pp.438–449.。
