特耐凯纷说明书解读150430
特耐凯纷说明书解读150430

吸收
峰浓度 血浆蛋白结合率 分布容积 代谢部位与代谢酶
分布 代谢
55L 肝脏代谢;细胞色素P450(CYP)3A4 与CYP2C9 同工酶代谢以及磺胺葡萄 糖醛酸化 无介绍
半衰期
8h
6L/h
5.8h 无介绍
消除
血浆清除率
解读
1. 注射用帕瑞昔布钠半衰期8h,而氟比洛芬酯注射液为5.8h,注射用帕瑞昔布钠更耐 久镇痛 2. 氟比洛芬酯注射液说明书很多信息未涉及到
儿童
尚未确定18岁以下儿童使用的安全性和疗效, 儿童使用的安全性尚未确定,因 故不推荐使用。 此儿童不宜使用。 不必对轻度至中度肾功能损伤(肌酐清除率: 30~80ml/min)的患者进行剂量调整。对于 重度肾功能损伤(肌酐清除率:<30ml/min) 或有液体潴留倾向的患者,应选择最低推荐 剂量开始治疗并密切监测肾功能。 轻度肝功能损伤的患者(Child-Pugh 评分:56)不必进行剂量调整。中度肝功能损伤的患 者(Child-Pugh 评分:7-9)应慎用帕瑞昔布, 剂量应减至常规推荐剂量的一半且每日最高 剂量降至40mg。目前尚无严重肝功能损伤患 者(Child-Pugh 评分:≥10)的临床用药经验, 因此禁止在此类患者中使用帕瑞昔布。 严重肾功能不全者禁用; 肾功能不全或有既往史的患者 慎用
全球首个和唯一的注射用
选择性COX-2抑制剂
• 用于手术后疼痛的短期治疗 • 20mg, 40mg(以帕瑞昔布计)
Stichtenoth DO, Frölich JC. The second generation of COX-2 inhibitors: what advantages do the newest offer? Drug. 2003; 63(1): 33-45 仅供辉瑞内部使用
特洛凯中文说明书

特罗凯说明书【药品名称】商品名:特罗凯通用名:盐酸厄洛替尼片英文商品名:Tavceva英文通用名:Erlotinib HCL Tablets【成份】每片内含150mg厄洛替尼(以盐酸厄洛替尼形式存在)【性状】圆形、双凸、白色包衣片,一面印有棕色"T"和"150",另一面空白。
【作用机制】Tarceva的抗肿瘤作用机制主要为抑制表皮生长因子(EGFR)酪氨酸激酶胞内磷酸化。
【药代动力学】Tarceva口服后60%吸收,半衰期约36小时,主要由CYP3A4代谢清除。
口服Tarceva150mg的生物利用度约60%,4小时后达血浆峰浓度。
对591例接受单药Tarceva治疗的药代动力学分析显示,达到稳定血药浓度需7-8 天,患者的年龄、体重、性别与药物的清除速率无显著关系,吸烟可使药物清除率增加24%。
【适应症】Tarceva用于两个或两个以上化疗方案失败的局部晚期或转移的非小细胞肺癌的三线治疗。
【禁忌症】对本品及成份过敏者禁用。
【不良反应】最常见的不良反应是皮疹和腹泻,3/4度皮疹和腹泻的发生率分别为9%和6%,皮疹的中位出现时间是8天,腹泻中位出现时间为12天。
发生率大于10%的不良反应有:皮疹、腹泻、食欲减低、疲劳、呼吸困难、咳嗽、恶心、感染、呕吐、口腔炎、瘙痒、皮肤干燥、结膜炎、角膜结膜炎、腹痛。
肺毒性:有较少的报道提示在接受Tarceva治疗的NSCLC患者或其他实体瘤患者中可出现严重的间质性肺病(ILD),甚至导致死亡。
在随机对照研究中,ILD的发生率是0.8%,并且这一发生率在Tarceva治疗组和安慰剂组是相同的。
报道的ILD包括:肺炎、间质性肺炎、间质性肺病、闭塞性细支气管炎、肺纤维化、急性呼吸应激综合征和肺渗出。
症状发生于治疗后5天~超过9个月,中位发生时间为47天。
多数患者常有混杂因素导致ILD发生,如:之前有化疗/放疗、原有实质性肺疾病、肺转移或肺部感染。
特耐凯纷说明书解读

药物相互作用 —药效学相互作用
注射用帕瑞昔布钠
增加发生出血并发症的风险,尤其在治疗开始 后数天内。应密切监测同时服用抗凝血药物患 者的凝血酶原时间国际标准化比(INR),特别是 在开始使用帕瑞昔布或对帕瑞昔布进行剂量调 整后数日内 对阿司匹林抑制血小板聚集的作用或出血时间 没有影响。帕瑞昔布可以与低剂量(≤325mg)阿 司匹林合用
药代动力学参数
转化
注射用帕瑞昔布钠
帕瑞昔布在静注或肌注后经肝脏酶水 解,迅速转化为有药理学活性的物 质——伐地昔布 帕瑞昔布钠单次静注或肌注20mg, 伐地昔布分别于注射后约30 分钟或1 小时达到峰浓度 98%(最高推荐剂量80mg/天)
氟比洛芬酯注射液
健康男子静脉内单次给予氟比洛芬酯 注射液5ml(50mg),在5分钟内全部 水解为氟比洛芬 静脉内单次给予氟比洛芬酯注射液 5ml(50mg),6-7分钟后氟比洛芬血 中浓度达到最高(8.9μg/ml) 无介绍
慎与其合用
锂剂
注射用帕瑞昔布 慎与其合用 钠可与喹诺酮类 抗生素合用,而 氟比洛芬酯注射 液禁止与其合用
未提及
格列本脲
1. 注射用帕瑞昔布钠说明书 2. 氟比洛芬酯注射液说明书 仅供辉瑞内部使用
吸收
峰浓度 血浆蛋白结合率 分布容积 代谢部位与代谢酶
分布 代谢
55L 肝脏代谢;细胞色素P450(CYP)3A4 与CYP2C9 同工酶代谢以及磺胺葡萄5.8h 无介绍
消除
血浆清除率
解读
1. 注射用帕瑞昔布钠半衰期8h,而氟比洛芬酯注射液为5.8h,注射用帕瑞昔布钠更耐 久镇痛 2. 氟比洛芬酯注射液说明书很多信息未涉及到
注射用帕瑞昔布钠 VS 氟比洛芬酯注射液
仅供辉瑞内部使用
Omega CN-TOT-A Series 蜂窝式热敏电导温度控制器说明书

Immediate hazards which WILL result in severe personal injury or death
Hazards or unsafe practices which COULD result in severe personal injury or death
Hazards or unsafe practices which COULD result in minor personal injury or property damage.
SAVE THESE INSTRUCTIONS!
Additional copies of this manual are available upon request.
End User Must Comply to the Following:
• Must be mounted vertically for outdoor use • Only qualified personnel are allowed to connect
electrical wiring. • All electrical wiring must follow local electrical
codes and highly recommend following NEC Article 427. • Final installation / wiring is to be inspected by the authority who has jurisdication in the area that the heater and temperature controller is installed. • The end-user is responsible for providing a suitable disconnecting device. • The end-user is responsible for providing suitable electrical protection device. It is highly recommended that a ground fault circuit breaker is used. Failure to observe these warnings may result in personal injury or damage to the controller.
凯纷PPT课件

全国最大的靶向药物研制基地
2
核心竞争力—五大技术平台
3
பைடு நூலகம்
凯纷 — 靶向镇痛药物
未受受损损血血管管
4
NSAIDs是围术期镇痛的基础用药
5
NSAIDs使用时机直接影响患者术后康复
6
凯纷—NSAIDs与靶向制剂的完美结合
镇痛效果强:静脉给予50mg凯纷,其疗效媲美4.4mg吗啡
7
徐国柱,李晓玲,段砺瑕,等.氟比洛芬酯注射液治疗中度术后疼痛的临床试验研究.中国新药杂志.2004,13(9);846-8. 安峥 ,谭元菊.氟比洛芬酯脂微球载体制剂与注射用酮洛芬对照治疗术后及癌性疼痛.中国新药杂志.2004,13(9);848-51.
李倩,等.实用医学杂志.2011,27:1967-1970
23
舒适镇痛的最佳选择
胃肠道副反应大大减少 ,安全系数是传统剂型
11
氟比洛芬酯持续静脉给药的术后镇痛效用.JSPA. 1996; 9(1):19-22.
凯纷复合芬太尼用于围手术期
--随机、多中心疗效及安全性研究
用药
A
B
术毕凯纷
100mg
-----
C 100mg
术后芬太尼 1mg
0.6mg
0.6mg
术后凯纷
-----
200mg
200mg
12
研究单位(47家医院)
16
凯纷与液体混合72小时性质稳定
国外 :凯纷配液72小时 药效稳定实验 17
凯纷与阿片类药物混合48小时性质稳定
国外:氟比洛芬酯(Flurbiprofen axetil) 注射液与阿片类药物配合变化试验结果 2009、4 18
围术期镇痛要选择安全性更高NSAIDs
STEINEL电子热气栓产品说明书

Products > Heat Guns Heat Guns Glue Guns Exterior Lights Sensors Voltage Testers ELECTRONIC HEAT GUNSSTEINEL Electronic Heat Guns are ideal for applications requiring precisetemperature control such as shrink tubing or welding plastics. Electronic control makes itpossible to restrict airflow through pin-point reduction nozzles used for soldering sink wireconnectors, de-soldering circuit boards, shrinking small diameter tubes. Also can be used toweld with narrow-slit nozzles.Electronic thermo couple control is what sets these heat guns apart. This feature continuousmonitors and adjusts output to maintain a consistant temperature and avoid overheating.You can count on STEINELheat guns for precision, durability and long life.Roofing Welding PlasticMaterialsButt-weldingPVCSoldering &De-solderingShaping Plastic Sealing VinylFlooringApplying ShTubesHG 3002 LCDElectronically monitored heat gun with variable airflow and digital temperaturedisplay for precise control.Temperature Continuously variable 120°F – 1100°F.Airflow Continuously variable up to 17.6 cfm with selectablecool air stage.LCD Displays temperature output in 10°F increments.Double Insulated Power Cord Rubber construction for increased safety.Output1,500 watts.Voltage120 VAC / 60 Hz.Certifications UL / CSA.Full One Year Warranty Refer to owner's manual for details.AccessoriesAccepts a full range of nozzles and accessories.HG 2002 LEElectronically monitored heat gun with 3-stage airflow and LED temperature rangeindicator.Temperature Continuously variable 120°F – 1100°F.3-Stage Airflow Stage 1: cool air at 17.6 cfmStage 2: 9.5 cfmStage 3: 17.6 cfmLED Display Diodes indicate temperature range selected. Double Insulated Power Cord Rubber construction for increased saftey.Output1,500 watts.Voltage120 VAC / 60 Hz.Certifications UL / CSA.Full One Year Warranty Refer to owner's manual for details. AccessoriesAccepts a full range of nozzles and accessories.HG 2000 EElectronically controlled heat gun with constant air flow.Lightweight , ergonomically designed.Temperature Continuously variable 120°F – 1100°F.Constant Airflow14.8 cfm.Ergonomic Design Lightweight (21.7 oz.) and easy to use, even in tightquarters.Double Insulated Power Cord Rubber construction for increased saftey.Output1,500 watts.Voltage120 VAC / 60 Hz.Certifications UL / CSA.Full One Year Warranty Refer to owner's manual for details. AccessoriesAccepts a full range of nozzles and accessories.HG 1802 EElectronically monitored heat gun with variable temperature and 3 stage airflow. Temperature Continuously variable 120°F – 1100°F.3 Stage Airflow Stage 1: cool air at 17.6 cfmStage 2: 9.5 cfmStage 3: 17.6 cfmDouble Insulated Power Cord Rubber construction for increased safety.Output1,500 watts.Voltage120 VAC / 60 Hz.Certifications UL / CSA.Full One Year Warranty Refer to owner's manual for details. AccessoriesAccepts a full range of nozzles and accessories.。
Stikatak 1000粘合剂安全数据说明书

SAFETY DATA SHEETSTIKATAK 1000Page:1Compilation date:25/01/2017Revision No:1Product name:STIKATAK 1000Use of substance / mixture:PC1: Adhesives, sealants.Company name:Interfloor LtdBroadwayHaslingdenRossendaleLancashireBB4 4LSUnited KingdomTel:01706 213131Fax:01706 224915Email:Emergency tel:01706 213131Classification under CLP:Aquatic Acute 1: H400; Aquatic Chronic 1: H410; Aquatic Chronic 2: H411; Eye Irrit. 2:H319; Repr. 2: H361fd; Skin Irrit. 2: H315; Skin Sens. 1: H317Most important adverse effects:Causes skin irritation. May cause an allergic skin reaction. Causes serious eye irritation.Suspected of damaging fertility. Suspected of damaging the unborn child. Very toxic toaquatic life. Very toxic to aquatic life with long lasting effects. Toxic to aquatic life with longlasting effects.Label elements:Hazard statements:H315: Causes skin irritation.H317: May cause an allergic skin reaction.STIKATAK 1000Page:2 H319: Causes serious eye irritation.H361fd: Suspected of damaging fertility. Suspected of damaging the unborn child.H400: Very toxic to aquatic life.H410: Very toxic to aquatic life with long lasting effects.H411: Toxic to aquatic life with long lasting effects.Hazard pictograms:GHS07: Exclamation markGHS08: Health hazardGHS09: EnvironmentalPrecautionary statements:P201: Obtain special instructions before use.P280: Wear protective gloves/protective clothing/eye protection/face protection.P302+352: IF ON SKIN: Wash with plenty of water/.P305+351+338: IF IN EYES: Rinse cautiously with water for several minutes. Removecontact lenses, if present and easy to do. Continue rinsing.P308+313: IF exposed or concerned: Get medical attention.P321: Specific treatment (see instructions on this label).This product is not identified as a PBT/vPvB substance.Hazardous ingredients:EPOXY RESINEPOXY HARDENERimmediately with plenty of soap and water.STIKATAK 1000Page:3 Eye contact:Bathe the eye with running water for 15 minutes. Consult a doctor.Ingestion:Wash out mouth with water. Consult a doctor.Inhalation:Remove casualty from exposure ensuring one's own safety whilst doing so. Consult adoctor.Skin contact:There may be irritation and redness at the site of contact.Eye contact:There may be irritation and redness. The eyes may water profusely.Ingestion:There may be soreness and redness of the mouth and throat.Inhalation:There may be irritation of the throat with a feeling of tightness in the chest. Exposure maycause coughing or wheezing.Delayed / immediate effects:Immediate effects can be expected after short-term exposure.Immediate / special treatment:Eye bathing equipment should be available on the premises.Extinguishing media:Suitable extinguishing media for the surrounding fire should be used. Use water sprayto cool containers.Exposure hazards:In combustion emits toxic fumes.Advice for fire-fighters:Wear self-contained breathing apparatus. Wear protective clothing to prevent contactwith skin and eyes.Personal precautions:Refer to section 8 of SDS for personal protection details. If outside do not approach fromdownwind. If outside keep bystanders upwind and away from danger point. Mark out thecontaminated area with signs and prevent access to unauthorised personnel. Turnleaking containers leak-side up to prevent the escape of liquid.Environmental precautions:Do not discharge into drains or rivers. Contain the spillage using bunding.Clean-up procedures:Absorb into dry earth or sand. Transfer to a closable, labelled salvage container fordisposal by an appropriate method.STIKATAK 1000Page:4Reference to other sections:Refer to section 8 of SDS.Handling requirements:Avoid direct contact with the substance. Ensure there is sufficient ventilation of the area.Do not handle in a confined space. Avoid the formation or spread of mists in the air.Storage conditions:Store in a cool, well ventilated area. Keep container tightly closed. The floor of thestorage room must be impermeable to prevent the escape of liquids.Specific end use(s):No data available.Workplace exposure limits:No data available.Hazardous ingredients:EPOXY RESINEPOXY HARDENERSTIKATAK 1000Page:5Engineering measures:Ensure there is sufficient ventilation of the area. The floor of the storage room must beimpermeable to prevent the escape of liquids.Respiratory protection:Self-contained breathing apparatus must be available in case of emergency.Hand protection:Protective gloves.Eye protection:Safety glasses. Ensure eye bath is to hand.Skin protection:Protective clothing.State:LiquidColour:BlueEvaporation rate:SlowOxidising:Not applicable.Solubility in water:Not applicable.Viscosity:ViscousBoiling point/range°C:>35Melting point/range°C:Not applicable. Flammability limits %: lower:Not applicable.upper:Not applicable.Flash point°C:>93Part.coeff. n-octanol/water:Not applicable.Autoflammability°C:Not applicable.Vapour pressure:Not applicable.Relative density:Not applicable.pH:Approx. 7VOC g/l:Not applicable.Other information:No data available.Reactivity:Stable under recommended transport or storage conditions.Chemical stability:Stable under normal conditions.Hazardous reactions:Hazardous reactions will not occur under normal transport or storage conditions.Decomposition may occur on exposure to conditions or materials listed below.STIKATAK 1000Page:6Conditions to avoid:Heat.Materials to avoid:Strong oxidising agents. Strong acids.Haz. decomp. products:In combustion emits toxic fumes.Hazardous ingredients:EPOXY RESINEPOXY HARDENERRelevant hazards for product:Skin contact:There may be irritation and redness at the site of contact.Eye contact:There may be irritation and redness. The eyes may water profusely.Ingestion:There may be soreness and redness of the mouth and throat.Inhalation:There may be irritation of the throat with a feeling of tightness in the chest. Exposure maycause coughing or wheezing.Delayed / immediate effects:Immediate effects can be expected after short-term exposure.STIKATAK 1000Page:7 Hazardous ingredients:EPOXY RESINEPOXY HARDENERPersistence and degradability:Not biodegradable.Bioaccumulative potential:Bioaccumulation potential.Mobility:Readily absorbed into soil.PBT identification:This product is not identified as a PBT/vPvB substance.Other adverse effects:Toxic to aquatic organisms. Toxic to soil organisms.Disposal operations:Transfer to a suitable container and arrange for collection by specialised disposalcompany.NB:The user's attention is drawn to the possible existence of regional or nationalregulations regarding disposal.UN number:UN3082Shipping name:ENVIRONMENTALLY HAZARDOUS SUBSTANCE, LIQUID, N.O.S.Transport class:9STIKATAK 1000Page:8Packing group:IIIEnvironmentally hazardous:Yes Marine pollutant:NoSpecial precautions:No special precautions.Tunnel code:ETransport category:3Specific regulations:Not applicable.Chemical safety assessment: A chemical safety assessment has not been carried out for the substance or the mixtureby the supplier.Other information:This safety data sheet is prepared in accordance with Commission Regulation (EU) No2015/830.* indicates text in the SDS which has changed since the last revision.Phrases used in s.2 and s.3:H314: Causes severe skin burns and eye damage.H315: Causes skin irritation.H317: May cause an allergic skin reaction.H318: Causes serious eye damage.H319: Causes serious eye irritation.H332: Harmful if inhaled.H361: Suspected of damaging fertility or the unborn child <state specific effect if known><state route of exposure if it is conclusively proven that no other routes of exposurecause the hazard>.H361fd: Suspected of damaging fertility. Suspected of damaging the unborn child.H400: Very toxic to aquatic life.H410: Very toxic to aquatic life with long lasting effects.H411: Toxic to aquatic life with long lasting effects.Legal disclaimer:The above information is believed to be correct but does not purport to be all inclusiveand shall be used only as a guide. This company shall not be held liable for anydamage resulting from handling or from contact with the above product.。
Treofan产品说明书
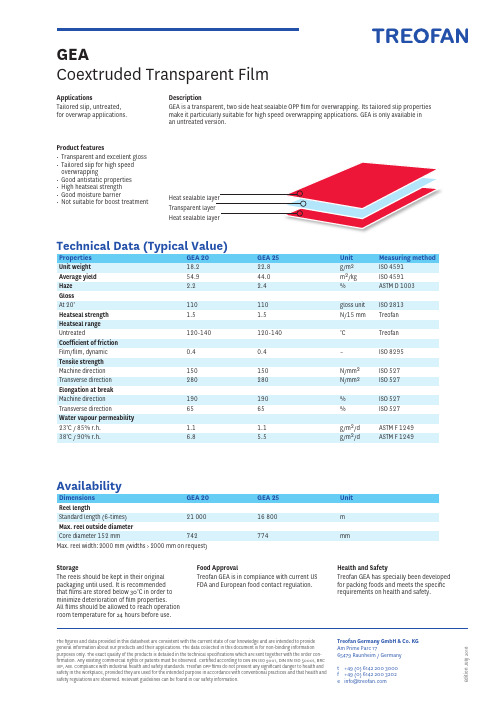
E d i t i o n J u l y 2018The figures and data provided in this datasheet are consistent with the current state of our knowledge and are intended to provideg eneral information about our products and their applications. The data collected in this document is for non-binding information purposes only. The exact quality of the products is detailed in the technical specifications which are sent together with the order con-firmation. Any existing commercial rights or patents must be observed. Certified according to DIN EN ISO 9001, DIN EN ISO 50001, BRC IoP, AIB. Compliance with industrial health and safety standards. Treofan OPP films do not present any significant danger to health and safety in the workplace, provided they are used for the i ntended purpose in accordance with conventional practices and that health and safety regulations are observed. Relevant g uidelines can be found in our safety information.Treofan Germany GmbH & Co. KGAm Prime Parc 1765479 Raunheim / Germanyt +49 (0) 6142 200 3000f +49 (0) 6142 200 3202*****************GEACoextruded Transparent FilmApplicationsTailored slip, untreated, for overwrap applications.Technical Data (Typical Value)AvailabilityStorageThe reels should be kept in their originalp ackaging until used. It is recommendedt hat films are stored below 30°C in order tom inimize deterioration of film properties. All films should be allowed to reach operation room temperature for 24 hours before use.Food ApprovalTreofan GEA is in compliance with current US FDA and European food contact regulation.Health and SafetyTreofan GEA has specially been developed for packing foods and meets the specificr equirements on health and safety.Properties GEA 20 GEA 25 Unit Measuring methodUnit weight 18.2 22.8g/m 2 ISO 4591Average yield 54.9 44.0 m 2/kg ISO 4591Haze 2.2 2.4 %ASTM D 1003Gloss At 20°110 110 gloss unit ISO 2813Heatseal strength 1.51.5N/15 mmTreofanHeatseal rangeUntreated 120-140 120-140 °C Treofan Coefficient of friction Film/film, dynamic 0.4 0.4 – ISO 8295Tensile strength Machine direction 150 150 N/mm 2 ISO 527Transverse direction 280 280 N/mm 2 ISO 527Elongation at break Machine direction 190 190 % ISO 527Transverse direction65 65 % ISO 527Water vapour permeability 23°C / 85% r.h. 1.1 1.1 g/m 2/d ASTM F 124938°C / 90% r.h.6.85.5g/m 2/dASTM F 1249Dimensions GEA 20 GEA 25 UnitReel lengthStandard length (6-times) 21 000 16 800 m Max. reel outside diameter Core diameter 152 mm 742 774mmMax. reel width: 2000 mm (widths > 2000 mm on request)DescriptionGEA is a transparent, two side heat sealable OPP film for overwrapping. Its tailored slip properties make it particularly suitable for high speed overwrapping applications. GEA is only available in an untreated version.Product features• Transparent and excellent gloss • Tailored slip for high speed overwrapping• Good antistatic properties • High heatseal strength • Good moisture barrier• Not suitable for boost treatment。
Tet-On 3G Вector Set (with ZsGreen1) 商品说明书

Certificate of Analysis Takara Bio USA, Inc.1290 Terra Bella Avenue, Mountain View, CA 94043, USA U.S. Technical Support: ********************United States/Canada 800.662.2566 Asia Pacific+1.650.919.7300Europe+33.(0)1.3904.6880Japan+81.(0)77.565.6999Page 1 of 6Tet-On® 3G Vector Set (with ZsGreen1)Table of ContentsDescription (1)pCMV-Tet3G Vector Information (2)pTRE3G-ZsGreen1 Vector and pTRE3G-Luc Control Vector Information (4)Quality Control Data (6)Catalog No. Lot Number631159 (Not sold separately) Specified on product label.DescriptionThe Tet-On 3G Vector Set (with ZsGreen1) is used to create tightly regulated and highly responsive tetracycline (Tet)-inducible mammalian expression systems that are turned on by the addition of doxycycline to the culture medium. The Tet-On 3G Vector Set (with ZsGreen1) allows the simultaneous expression of a gene of interest and a green fluorescent protein marker.Package Contents•20 μl pCMV-Tet3G Vector (500 ng/μl)•20 μl pTRE3G-ZsGreen1 Vector(500 ng/μl)•20 μl pTRE3G-Luc Control Vector(500 ng/μl)•40 μl Linear Hygromycin Marker (50 ng/μl)•40 μl Linear Puromycin Marker (50 ng/μl)Storage Conditions•Store plasmids at –20°C.•Spin briefly to recover contents.•Avoid repeated freeze/thaw cycles.Shelf Life• 1 year from date of receipt under proper storage conditions.Storage Buffer•10 mM Tris-HCl (pH 8.0), 1 mM EDTA (pH 8.0)Shipping Conditions• Dry ice (–70°C)Product DocumentsDocuments for our products are available for download at /manualsThe following documents apply to this product:•Tet-On 3G Expression Systems User Manual (PT5148-1)pCMV-Tet3G Vector InformationFigure 1. pCMV-Tet3G Vector Map.DescriptionThe pCMV-Tet3G Vector expresses Tet-On 3G, a tetracycline-controlled transactivator that exhibits high activity in the presence of the inducer doxycycline (Dox), and exceptionally low activity in its absence. Tet-On 3G results from the fusion of amino acids 1–207 of a mutant Tet repressor (TetR) to 39 amino acids that form three minimal "F"-type transcriptional activation domains from the herpes simplex virus VP16 protein. Tet-On 3G was derived from Tet-On Advanced (Zhou et al. 2006; Urlinger et al. 2000; Gossen and Bujard 1992; Gossen et al. 1995); as a result, it’s fully synthetic, lacks cryptic splice sites, and is codon-optimized for stable expression in mammalian cells. Compared to both of its predecessors, however, this 3rd generation Tet-On transactivator demonstrates increased sensitivity to Dox (Zhou et al. 2006). Constitutive expression of Tet-On 3G is driven by the human cytomegalovirus immediately early promoter (P CMV IE).Location of Features in pCMV-Tet3G•P CMV IE(human cytomegalovirus immediate early promoter): 2–688•Tet-On 3G (transactivator gene): 775–1521•SV40 polyA signal: 1536–1991•pUC origin of replication: 2342–2996•Amp r (ampicillin resistance gene; β-lactamase): 3144–4004 (complementary)•SV40 polyA signal: 4275–4809 (complementary)•Kan r/Neo r (kanamycin/neomycin resistance gene): 5417–6211 (complementary)•P SV40 e (SV40 early promoter): 6532–6891 (complementary)Additional InformationpCMV-Tet3G is used to develop stable Tet-On 3G cell lines, which are hosts for Tet-inducible gene expression systems. To create a Tet-inducible expression system, a vector containing a gene of interest under the control of the Tet-inducible TRE3G promoter(P TRE3G) is transfected into a Tet-On 3G cell line. The addition of Dox to the system causes Tet-On 3G to undergo a conformational change that allows it to bind to P TRE3G, activating transcription of the gene of interest in a highly dose-dependent manner. Additional information on TRE-containing vectors, and protocols describing the construction of Tet-On 3G cell lines can be found in the Tet-On 3G Expression Systems User Manual (PT5148-1).Propagation in E. coli•Suitable host strain: Stellar™ Competent Cells•Selectable marker: plasmid confers resistance to ampicillin (100 μg/ml) in E. coli hosts.• E. coli replication origin: pUCpTRE3G-ZsGreen1 Vector and pTRE3G-Luc Control Vector InformationFigure 2. pTRE3G-ZsGreen1 Vector and pTRE3G-Luc Control Vector Maps.Figure 3. pTRE3G-ZsGreen Vector Multiple Cloning Site. The internal start site (ATG) at the IRES2/MCS junction is indicated in bold.DescriptionpTRE3G-ZsGreen1is a Tet-inducible, mammalian expression vector designed to coexpress a gene of interest and the green fluorescent protein ZsGreen1 under the control of the Tet-responsive promoter P TRE3G. This promoter consists of a highly optimized Tet-responsive element (TRE) just upstream of a minimal CMV promoter. P TRE3G exhibits exceptionally low basal activity; it’s induced by the binding of Tet-On 3G but is virtually silent in its absence. The vector is designed to be used as part of our Tet-On 3G Inducible Expression System (Cat. No. 631164).ZsGreen1 is a human codon-optimized variant of the reef coral Zoanthus sp. green fluorescent protein (ZsGreen) that has been engineered for brighter fluorescence (excitation and emission maxima: 493 and 505 nm, respectively; Matz et al. 1999; Haas, Park, and Seed 1996). p TRE3G-ZsGreen allows Dox-inducible coexpression of ZsGreen1 and a gene of interest from a bicistronic mRNA transcript. An encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES2), positioned between ZsGreen1 and the gene of interest, facilitates cap-independent translation of the gene of interest from an internal start site at the IRES2/MCS junction (Jang et al. 1988). This ensures that a high percentage of ZsGreen1-expressing clones also express the gene of interest, allowing ZsGreen1 to be used as an indicator of inducibility and transfection efficiency, as well as a marker for selection by flow cytometry. The vector also contains a pUC origin of replication and an ampicillin resistance gene (Amp r) to allow for propagation and selection in E. coli.The pTRE3G-Luc is a Tet-inducible control vector that expresses firefly luciferase under the control of P TRE3G. When used with standard luciferase detection reagents, this vector can be used as a reporter of induction efficiency (see User Manual for protocol). pTRE3G-Luc is not intended to be used as a cloning vector.Location of Features in pTRE3G-ZsGreen1•P TRE3G (3rd generation Tet-responsive promoter): 7–382•ZsGreen1: 389–1084•IRES2 (encephalomyocarditis virus internal ribosome entry site): 1091–1673•MCS (multiple cloning site): 1686–1721•SV40 polyA signal: 1776–2573•pUC origin of replication: 2838–3481•Amp r (ampicillin resistance gene; β-lactamase): 3629–4489 (complementary)Location of Features in pTRE3G-Luc•P TRE3G (3rd generation Tet-responsive promoter): 7–382•Luciferase: 432–2084•SV40 polyA signal: 2151–2948•pUC origin of replication: 3213–3856•Amp r (ampicillin resistance gene; β-lactamase): 4004–4864 (complementary)Additional InformationpTRE3G-ZsGreen1 is a mammalian expression vector that allows tightly regulated, doxycycline-controlled coexpression of a gene of interest and ZsGreen1. The gene of interest must have both a start and a stop codon. The gene of interest should be cloned in-frame with the start codon at the IRES2/MCS junction (this codon is shown in bold in the MCS sequence in Figure 3, page 3; see the User Manual for details on how to use In-Fusion® to simplify your cloning). Cotransfection of pTRE3G-ZsGreen1 constructs with Linear Hygromycin or Puromycin Markers allows antibiotic selection of stable transfectants. In order to function, the system requires the presence of the Tet-On 3G transactivator protein, supplied by a stable Tet-On 3G cell line created with our Tet-On 3G Inducible Expression System (Cat. No. 631164).Propagation in E. coli•Suitable host strain: Stellar™ Competent Cells•Selectable marker: plasmid confers resistance to ampicillin (100 μg/ml) in E. coli hosts.• E. coli replication origin: pUCExcitation and Emission of pTRE3G-ZsGreen1•Excitation: 493 nm•Emission: 505 nmReferences•Gossen, M. et al. Transcriptional activation by tetracyclines in mammalian cells. Science268, 1766–9 (1995).•Gossen, M. & Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U. S. A.89, 5547–51 (1992).•Haas, J., Park, E. C. & Seed, B. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr.Biol.6, 315–24 (1996).•Jang, S. K. et al. A segment of the 5’ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol.62, 2636–43 (1988).•Matz, M. V et al. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol.17, 969–73 (1999).•Urlinger, S. et al. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. U. S. A.97, 7963–8 (2000).•Zhou, X., Vink, M., Klaver, B., Berkhout, B. & Das, A. T. Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther.13, 1382–90 (2006).Quality Control DataPlasmid Identity & Purity•Digestion with the indicated restriction enzymes produced fragments of the indicated sizes on a 0.8% agarose/EtBr gel:Vector Enzyme(s) Fragment(s)pCMV-Tet3G EcoRI 7.1 kbEcoRI & HindIII 1.2 & 5.9 kbpTRE3G-ZsGreen1 XhoI 4.7 kbEcoRV 1.2 & 3.5 kbpTRE3G-Luc XhoI 5.1 kbEcoRI & BamHI 2.1 & 3.0 kbLinear Hygromycin Marker HindIII & XbaI0.5, 0.6 & 1.1 kbLinear Puromycin Marker HindIII & XbaI0.45, 0.6, & 0.75 kb•Vector identity was confirmed by sequencing.•A260/A280: 1.8–2.0Functional Testing of Linear Markers•HEK 293 cells were transfected with 200 ng of either the Linear Hygromycin Marker or the Linear Puromycin Marker. After 5 hr at 37°C, the transfection solution was removed, and the cells were given fresh medium. 48 hr later, the cells were plated in two 10 cm plates. 48 hr after plating, medium containing either hygromycin orpuromycin (depending on the linear marker used to transfect the cells) was added to the plates. After 2–3 weeks, >20 clones were identified.It is certified that this product meets the above specifications, as reviewed and approved by the Quality Department.CATALOG NO.631159NOTICE TO PURCHASER:Our products are to be used for research purposes only. They may not be used for any other purpose, including, but not limited to, use in drugs, in vitro diagnostic purposes, therapeutics, or in humans. Our products may not betransferred to third parties, resold, modified for resale, or used to manufacture commercial products or to provide a service to third parties without prior written approval of Takara Bio USA, Inc.Your use of this product is also subject to compliance with the licensing requirements listed below and described on the product´s web page at . It is your responsibility to review, understand and adhere to any restrictions imposed by these statements.STATEMENT 24The RCFPs (including DsRedExpress, DsRedExpress2, and E2-Crimson) are covered by one or more of thefollowing U.S. Patent Nos. 7,166,444; 7,157,565; 7,217,789; 7,338,784; 7,338,783; 7,537,915; 6,969,597; 7,150,979;7,442,522 and 8,012,682.STATEMENT 72Living Colors Fluorescent Protein Products: Not-For-Profit Entities: Orders may be placed in the normal manner by contacting your local representative or Takara Bio USA, Inc. Customer Service. Any and all uses of this product will be subject to the terms and conditions of the Non-Commercial Use License Agreement (the “Non-Commercial License”), a copy of which can be found below. As a condition of sale of this product to you, and prior to using this product, you must agree to the terms and conditions of the Non-Commercial License. Under the Non-Commercial License, Takara Bio USA, Inc. grants Not-For-Profit Entities a non-exclusive, non-transferable, non-sublicensable and limited license to use this product for internal, non-commercial scientific research use only. Such licensespecifically excludes the right to sell or otherwise transfer this product, its components or derivatives thereof to third parties. No modifications to the product may be made without express written permission from Takara Bio USA, Inc.Any other use of this product requires a different license from Takara Bio USA, Inc. For license information, please ***************************************************************************************.For-Profit Entities wishing to use this product are required to obtain a license from Takara Bio USA, Inc. For license information, please contact a licensing representative by phone at 650.919.7320 or by e-mail at ***********************.STATEMENT 42Use of the Tetracycline controllable expression systems (the "Tet Technology") is covered by a series of patents including U.S. Patent # 7541446, # 8383364, # 9181556 , European patents EP # 1200607, # 1954811, #2352833Academic research institutions are granted an automatic license with the purchase of this product to use the Tet Technology only for internal, academic research purposes, which license specifically excludes the right to sell, or otherwise transfer, the Tet Technology or its component parts to third parties. Notwithstanding the above, academicand not-for profit research institutions whose research using the Tet Technology is sponsored by for profitorganizations, which shall receive ownership to any data and results stemming from the sponsored research, shall need a commercial license agreement from TET Systems in order to use the Tet Technology. In accepting this license, all users acknowledge that the Tet Technology is experimental in nature. TET Systems GmbH & Co. KG makes no warranties, express or implied or of any kind, and hereby disclaims any warranties, representations, or guarantees of any kind as to the Tet Technology, patents, or products. All others are invited to request a license from TET Systems GmbH & Co. KG prior to purchasing these reagents or using them for any purpose. Takara Bio USA, Inc. is required by its licensing agreement to submit a report of all purchasers of the Tet-controllable expression system to TET Systems.For license information, please contact:GSF/CEOTET Systems GmbH & Co. KG,Im Neuenheimer Feld 58269120 Heidelberg GermanyTel: +49 6221 5880400Fax: +49 6221 5880404email:*******************or use the electronic licensing request form via /ip-licensing/licensing/for-profit-research TRADEMARKS:© 2015 Takara Bio Inc. All Rights Reserved.All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be registered in all jurisdictions.。
卡丁健康 骨科解决方案产品介绍说明书

βCardinal Health™Orthopedic Solutions• Mini Fragment (Locking and Non-Locking)• Extremities (Hand and Foot)• Small Fragment (Locking and Non-Locking)• Cannulated Screws1Humerus • 3.5mm Locking Proximal Humerus Plate • 4.5mm Narrow and Broad Locking Plates Radius• 2.4mm Locking Distal Radius Plate• 3.5mm Locking Plate Fibula • 3.5mm Locking One-Third Tubular Plate Ulna• 3.5mm Locking PlateFemur• 6.5 and 7.3mm Cannulated Screws• 4.5mm Narrow and BroadLocking Plates • 4.5mm Locking Condylar Plate Lower extremity/foot and ankle• 2.4, 3.0, 3.5, 4.0, 4.5, 6.5 and 7.3mmCannulated Screws• 2.0, 2.4, 2.7 and 3.5mm Foot Specific Plates• Calcaneal Plate• Locking T, Y, Adaption and Straight Plates Pelvis• 6.5 and 7.3mmCannulated Screws• 3.5mm LockingReconstruction Plate Tibia• 4.0 and 4.5mm Cannulated Screws• 3.5mm Locking Proximal Tibia Plate (Standard and Low Bend)• 4.5mm Locking Proximal Tibia Plate• 3.5mm Locking Medial Distal Tibia Plate (Low Bend)• Large External FixatorClavicle• 3.5mm Locking Reconstruction PlateHip• 6.5 and 7.3mm Cannulated Screws• Trochanteric Nail (Short and Long) with 11.0mm Lag Screw*Upper extremity/hand • 2.4 and 3.0mm Cannulated Screws• 1.0, 1.3, 1.5, 2.0 and 2.4mm Hand Specific Plates*Pending 510(k) submission.2Cardinal Health™ PlatesOur plates come in two material options and meet the required industry standards to help ensure quality and consistency.• FDA premarket notification clearances: K133536, K133541, K122489, K131480and K133452Locking One-Third Tubular PlateEM: 241.351.EM | CH: P2AL435-053.5mm Locking Reconstruction PlateEM: 245.051.EM | CH: P2B535-0843.5mm Locking Proximal Humerus PlateEM: 241.901.EM | CH: P2H235-013.5mm Locking T-PlateEM: 241.152.EM | CH: P2B635-141.3mm Straight PlateEM: 221.306.EM | CH: P213-01EM = Emerge Medical Catalog NumberCH = Cardinal Health Catalog Number 316L Stainless SteelLow carbon, high strength medical implant-gradestainless steel• Meets ASTM F1381 and F1392Ti-6Al-4V ELI Titanium AlloyHigh strength, medical implant-gradetitanium alloy• Meets ASTM F13631.3mm Ti Straight PlateEM: 421.306.EM | CH: P413-01231.0mmüü1.3mm üü1.5mm üü2.0mm Locking ü2.0mm Non-Locking üü2.4mm Locking ü2.4mm Non-Locking üüMini Fragment / Extremities: Foot 2.0mm Lockingü2.0mm Non-Locking üü2.4mm Locking ü2.4mm Non-Locking üü2.7mm Locking ü2.7mm Non-Locking üSmall Fragment 3.5mm Straight Plate (Locking and Non-Locking)ü3.5mm Reconstruction Plate (Locking and Non-Locking)ü3.5mm T-Plate (Oblique and Right Angle) (Locking and Non-Locking)ü 3.5mm One-Third Tubular Plate (Locking and Non-Locking)ü3.5mm Locking Proximal Humerus Plate ü3.5 Locking Proximal Humerus Plate (EM: 241.901.EM | CH: P2H235-01) with 3.5mm Locking and 3.5mm Cortex Screws4Cardinal Health ™ ScrewsCardinal Health offers five different types of screws — cannulated, shaft, cortex, cancellous and locking screws.Designed for use with instruments commonly available to Orthopedic Surgeons Self-drilling and self-tapping Choice of multiple thread lengths and diameters Reverse cutting flutes to assist in removal Available in medical implant-grade stainless steel and titanium Cannulated screws are made in the USA Compliant with industry standards and requirements — FDA premarket notification clearances: K102343, K122489, K131480 and K133536All corresponding washers, guide wires, cannulated drill bits and instrumentation are available üüüüüüüü51.0mm Cortex Screw üüüüüCruciate1.3mm Cortex Screw üüüüüCruciate1.5mm Cortex Screw üüüüüCruciate2.0mm Locking Screw üüüüT62.0mm Cortex Screw üüüüüüT6 and Cruciate2.4mm Locking Screw üüüüüüT62.4mm Cortex Screw üüüüüüT6 and Cruciate Mini Fragment / Extremities: Foot2.0mm Locking Screw üüüüT62.0mm Cortex Screw üüüüüüT6 and Cruciate2.4mm Locking Screw üüüüT62.4mm Cortex Screw üüüüüüT6 and Cruciate2.7mm Locking Screw üüüüT82.7mm Cortex Screw üüüüüüT8 and Hex (2.5mm)Small Fragment2.7mm Cortex Screw üüüüüüT8 and Hex (2.5mm)3.5mm Cortex Screw üüüüüT15 and Hex (2.5mm)3.5mm Shaft Screw üüüüüHex (2.5mm)3.5mm Locking Screw üüüüT154.0mm Cancellous Screw üüFull thread Partial thread üüHex (2.5mm)Cannulated Screws2.4mm Cannulated Screw üüShort (1/4 thread) Long (1/2 thread)üüüüüT83mm Cannulated Screw üüShort (1/3 thread) Long (1/2 thread)üüüüüüCruciate3.5mm Cannulated Screw üüPartial thread Full thread üüüüüHex (2.5mm)4.0mm Cannulated Screw üüShort (1/3 thread) Long (1/2 thread)üüüüüüHex (2.5mm)4.5mm Cannulated Screw üüShort (1/3 thread) Full thread üüüüüüHex (3.5mm)6.5mm Cannulated Screw üü16mm thread 32mm thread üüüüüüHex (4.0mm)7.3mm Cannulated Screw üü16mm thread 32mm thread üüüüüüHex (4.0mm)Cardinal Health™ Drill BitsWe offer a broad portfolio of drill bits in a variety of lengths, diameters and cannulations. Our drill bits offer multiple drill quick-coupling connections — Mini/Dental, AO Standard/Large, J-Latch — and include color-coding and measurement calibrations.A variety of lengths, diameters and cannulations availableMade of surgical-grade 440A or 455 Stainless SteelIncludes color-coding and measurement calibrations 2-fluted and 3-fluted drill bits available Drill bits are made in the USA Compliant with industry standards and requirementsüüüüüüProduct features you want and need:68Cardinal Health™ InstrumentationDuring the manufacturingprocess, we have multiplequality control checkpointsto help ensure that ourproducts are manufacturedto Cardinal Health standards.üüüOur instrumentation isdesigned for use with drillbits, screws and platescommonly available toOrthopedic Surgeons.All Cardinal Health™Instruments aremanufactured in theUSA and Germany.9Biocompatability and materialsScrewsScrews are manufactured from 316L Stainless Steel(ASTM F1381) or Ti-6Al-4V ELI Titanium Alloy (ASTM F1363).ScrewdriversScrewdrivers are manufactured from surgical-grade 465 Stainless Steel (ASTM F8998) for strength and durability, which provides excellent tensile strength and reduction of twist during use.All components are made from surgical-grade materials and are proven to be biocompatible. Surface finishes have been applied to help prevent corrosion.9Products areengineered so that interfaces function together for consistent quality.© 2015 Cardinal Health. All rights reserved. CARDINAL HEALTH, the Cardinal Health LOGO and ESSENTIAL TO CARE are trademarks or registered trademarks of Cardinal Health. All other marks are the property of their respective owners. Lit. No. 2ORTHO14-25706 (01/2015)Cardinal HealthOrthopedic Solutions Waukegan, Illinois 60085/orthopedics To place an order, please contact your Cardinal Health Sales Representative or call customer service at 800.964.5227.1 A STM F138-13a: Standard Specification for Wrought 18Chromium – 14Nickel-2.5Molybdenum Stainless Steel Bar and Wire for Surgical Implants.2 A STM F139-12: Standard Specification for Wrought 18Chromium – 14Nickel-2.5Molybdenum Stainless Steel Sheet and Strip for Surgical Implants.3 A STM F136-13: Standard Specification for Wrought Titanium – 6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant Applications.4 T hird-party test data on file with Cardinal Health.5 A STM F382-99 (2003) – Standard Specification and Test Method for Metallic Bone Plates.6 A STM F543 – Standard Specification and Test Methods for Metallic Medical Bone Screws.7 ISO 1797-1 Dentistry – Shanks for rotary instruments – Part 1: Shanks made of metals.8 A STM F899 – 12b: Standard Specification for Wrought Stainless Steels for Surgical Instruments.9 S pecification drawings on file with Cardinal Health.Cardinal Health ™ Orthopedic SolutionsEfficient supply chainFrom the point of ordering to the point of care, and at every point in between, our supply chain expertise improves efficiency, increases productivity, and reduces costs. • No separate procurement process or additional labor • Next-day delivery*• Exceptional fill rates• RFID capabilities to drive greater efficiency in order and inventory controlHigh-quality productsBegin saving on high-quality, standard products based on industry-proven and accepted designs. Leveraging a new model and reducing overhead allows us to offer high-quality products for less.• A broad portfolio of products available that includes orthopedic trauma and surgical products and accessories• No change to clinical practice or technique • Manufactured in the United States, UK and Germany• Stringent quality-control procedures • Quality manufacturing process: -ISO Certified-Conforms to ASTM standards -Complies with FDA Good Manufacturing PracticesFlexible service optionsCustomizable service options, complete with analytics support, tailored to your facility’s needs.Clinical and supply chain solutions• Evaluation and implementation support • Ongoing on-site support • Product and process training • Par optimization• Periodic on-hand inventory checks and recommended replenishment orders Data analytics• Ongoing savings analyses and inventory optimization• Economic alignment, consulting and administration*Customers must place their order before 12 p.m. local time. Next-day delivery applies only to customers within the continental 48 states.。
Carter-Hoffmann HL1-HL4 和 HLM1 系列铝制加热保温柜与加热炉产品说明书

HL1, HL2, HL3, HL4 & HLM1 Series ALUMINUM HEATED HOLDINGCABINETS & HEATER PROOFERSOWNERS / OPERATORS MANUALPart Number: 18400-3130f Printed in The United States of AmericaRev: KBA042017TABLE OF CONTENTSSAFETY PRECAUTIONS......................................................... 2 FEATURES & SPECIFICATIONS............................................... 3-7 UNPACKING, INSPECTION & FREIGHT DAMAGE...................... 8 INSTALLATION & STARTUP .................................................. 9 DETAILS: CONTROLS AND WATER PANS................................ 10 NORMAL OPERATION: HL1, HL3 & HLM1 HEATED CABINETS..... 11 NORMAL OPERATION: HL2 & HL4 HEATER PROOFERS ........... 12 FOOD HOLDING GUIDE......................................................... 13 DAILY CLEANING PROCEDURES............................................ 14 CABINET MAINTENANCE....................................................... 15 WIRING DIAGRAMS................................................ ............... 16 REPLACEMENT PARTS (17)TROUBLESHOOTING GUIDE ................................................. 18 WARRANTY STATEMENT (19)MANUFACTURED BY:CARTER-HOFFMANN 1551 McCormick Avenue Mundelein, IL 60060 U.S.A.Phone: 847-362-5500 Fax: 847-367-8981 Toll Free: 800-323-9793Email:************************************HL2-18HL1 HEATED CABINETS (non-insulated)Models: HL1-5, HL1-8, HL1-14 &HL1-18Formerly models: HBU5A2GM HBU8A2GM HBU14A2GM HBU18A2GM HBC10A2GM HBC16A2GM HBC28A2GM HBC36A2GM HBF5A2GM HBF8A2GM HBF14A2GM HBF18A2GMHL2 HEATER/PROOFERS (non-insulated)Models: HL2-5, HL2-8, HL2-14 &HL2-18Formerly models: HWU5A2GM HWU8A2GM HWU14A2GM HWU18A2GM HWC10A2GM HWC16A2GM HWC28A2GM HWC36A2GM HWF5A2GM HWF8A2GM HWF14A2GM HWF18A2GMHL3 HEATED CABINETS (insulated)Models: HL3-5, HL3-8, HL3-14,HL3-18Formerly models: HBU5A1GM HBU8A1GM HBU14A1GM HBU18A1GM HBC10A1GM HBC16A1GM HBC28A1GM HBC36A1GM HBF5A1GM HBF8A1GM HBF14A1GM HBF18A1GMHL4 HEATER/PROOFERS (insulated)Models: HL4-5, HL4-8, HL4-14,HL4-18Formerly models: HWU5A1GM HWU8A1GM HWU14A1GM HWU18A1GM HWC10A1GM HWC16A1GM HWC28A1GM HWC36A1GM HWF5A1GM HWF8A1GM HWF14A1GM HWF18A1GMHLM1 HEATED CABINETS (non-insulated)Models: HLM1-14 , HLM1-183HL1 NON-INSULATED ALUMINUM HEATED CABINET FEATURES & SPECIFICATIONSModel NumberPan/Tray Capacity* 12”x20” 18”x26” Slide Pairs ProvidedOverall Dimensions Height Depth Width in (mm) Caster Diameter in (mm) Class 100 Shipping Weightlbs (kgs) HL1-5(under-counter)10 5 5 31-1/16 29-1/2 24-11/16(789) (749) (627) 5 (127) 99 (45) HL1-8(1/2 size)16 8 8 40-1/16 29-1/2 24-11/16 (1018) (749) (627) 5 (127) 137 (62) HL1-14(3/4 size)28 14 14 58-1/16 29-1/2 24-11/16 (1474) (749) (627) 5 (127) 155 (70) HL1-18(full size) 36 181870-1/16 29-1/2 24-11/16 (1780) (749) (627)5 (127)198 (90)* fixed spacing at 3”HL1 Features & Benefits∙ Precision-engineered bottom mount heater with superior heat-up and recovery time ∙ Analog heat control. Digital thermometer. Temperature range of 90°- 190°F∙ Four sizes, available with adjustable universal, fixed universal or adjustable universal slides ∙ All swivel heavy-duty casters, front casters fitted with brakes∙ Field reversible glass door in heavy-duty extruded aluminum frame is standard, black anodized solid aluminum door is optional∙Fixed wire universal slides for 18”x26” sheet pans and 12”x20”x2.5” steam table pansCabinets with Adjustable Universal, Fixed Universal or Angle SlidesInternational voltage: 220 volts, 1800 watts, 8.2 Amps, 50 Hz, single phase, plug cap per specific country require-ments / standardsAPPROVALS:3174974Conforms to UL Std 197 certified to CAN/CSA Std C22.2 No. 1093174974Conforms to NSF/ANSI Std 4Canadian Voltage: 120v, 1400 watts, 12Amps, NEMA 5-15P3HLM1 NON-INSULATED ALUMINUM MERCHANDISING CABINETSModelNumberCapacity*12”x20” 18”x26”Pizza SheetBoxes PansShelves Overall DimensionsHeight Depth Widthin (mm)CasterDiameterin (mm)Class 100ShippingWeightlbs (kgs) HLM1-14(3/4 size))28 14* 3 58-5/8 30-1/4 24-5/8(1489) (781) (625)5(127)155(70)HLM1-18(full size)36 18* 4 70-5/8 30-1/4 24-5/8(1780) (781) (625)5(127)198(90)* on optional wire racks with fixed spacing at 3”HL1 Features & Benefits∙ Precision-engineered bottom mount heater with superior heat-up andrecovery time∙ Analog heat control. Digital thermometer. Temperature range of 90°-190°F∙ 3/4 and full sized cabinets with adjustable racks and shelves to accom-modate a variety of items, including pizza boxes∙ All swivel heavy-duty casters, front casters fitted with brakes∙ Field reversible glass door in heavy-duty extruded aluminum frame isstandard, black anodized solid aluminum door is optional∙ Tempered glass doors and walls; pass-through designCabinets with Adjustable Racks and ShelvesInternational voltage:220 volts, 1800 watts,8.2 Amps, 50 Hz, singlephase, plug cap perspecific country require-ments / standardsAPPROVALS:3174974Conforms to UL Std 197certified to CAN/CSA StdC22.2 No. 1093174974Conforms toNSF/ANSIStd 4Canadian Voltage:120v, 1400 watts,12Amps, NEMA 5-15P5HL2 NON-INSULATED ALUMINUM HEATER/PROOFER FEATURES & SPECIFICATIONSModel NumberPan/Tray Capacity* 12”x20” 18”x26” Slide Pairs ProvidedOverall Dimensions Height Depth Width in (mm) Caster Diameter in (mm) Class 100 Shipping Weightlbs (kgs) HL2-5(under-counter)10 5 5 31-1/16 29-1/2 24-11/16(789) (749) (627) 5 (127) 99 (45) HL2-8(1/2 size)16 8 8 40-1/16 29-1/2 24-11/16 (1018) (749) (627) 5 (127) 137 (62) HL2-14(3/4 size)28 14 14 58-1/16 29-1/2 24-11/16 (1474) (749) (627) 5 (127) 155 (70) HL2-18(full size) 36 181870-1/16 29-1/2 24-11/16 (1780) (749) (627)5 (127)198 (90)* fixed spacing at 3”HL2 Features & Benefits∙ Precision-engineered bottom mount heater with superior heat-up and recovery time ∙ Analog heat and humidity levels. Digital thermometer. Temperature range of 90°- 190°F ∙ Can be operated with or without humidity. Capable of humidified holding as well as proofing ∙ Four sizes, available with adjustable universal, fixed universal or adjustable universal slides ∙ All swivel heavy-duty casters, front casters fitted with brakes∙ Field reversible glass door in heavy-duty extruded aluminum frame is standard, black anodized solid aluminum door is optional∙Fixed wire universal slides for 18”x26” sheet pans and 12”x20”x2.5” steam table pansCabinets with Adjustable Universal, Fixed Universal or Angle SlidesAPPROVALS:3174974Conforms to UL Std 197 certified to CAN/CSA Std C22.2 No. 1093174974Conforms to NSF/ANSI Std 4International voltage: 220 volts, 2100 watts, 9.5 Amps, 50 Hz, single phase, plug cap per specific country require-ments / standardsCanadian Voltage: 120v, 1400 watts, 12Amps, NEMA 5-15P6HL3 INSULATED ALUMINUM HEATED CABINET FEATURES & SPECIFICATIONSModel NumberPan/Tray Capacity* 12”x20” 18”x26” Slide Pairs ProvidedOverall Dimensions Height Depth Width in (mm) Caster Diameter in (mm) Class 100 Shipping Weightlbs (kgs) HL3-5(under-counter)10 5 5 31-5/8 31-1/2 26-1/2(803) (800) (673) 5 (127) 104 (47) HL3-8(1/2 size)16 8 8 40-5/8 31-1/2 26-1/2 (1032) (800) (673) 5 (127) 147 (67) HL3-14(3/4 size)28 14 14 58-5/8 31-1/2 26-1/2 (1489) (800) (673) 5 (127) 170 (77) HL3-18(full size) 36 181870-5/8 31-1/2 26-1/2 (1794) (800) (673)5 (127)218 (99)* fixed spacing at 3”HL3 Features & Benefits∙ Precision-engineered bottom mount heater with superior heat-up and recovery time ∙ Analog heat control. Digital thermometer. Temperature range of 90°- 190°F∙ Four sizes, available with adjustable universal, fixed universal or adjustable universal slides ∙ All swivel heavy-duty casters, front casters fitted with brakes∙ Field reversible glass door in heavy-duty extruded aluminum frame is standard, black anodized solid aluminum door is optional∙Fixed wire universal slides for 18”x26” sheet pans and 12”x20”x2.5” steam table pansCabinets with Adjustable Universal, Fixed Universal or Angle SlidesAPPROVALS:3174974Conforms to UL Std 197 certified to CAN/CSA Std C22.2 No. 1093174974Conforms to NSF/ANSI Std 4International voltage: 220 volts, 1800 watts, 8.2 Amps, 50 Hz, single phase, plug cap per specific country require-ments / standardsCanadian Voltage: 120v, 1400 watts, 12Amps, NEMA 5-15P7HL4 INSULATED ALUMINUM HEATER/PROOFER FEATURES & SPECIFICATIONSModel NumberPan/Tray Capacity* 12”x20” 18”x26” Slide Pairs ProvidedOverall Dimensions Height Depth Width in (mm) Caster Diameter in (mm) Class 100 Shipping Weightlbs (kgs) HL4-5(under-counter)10 5 5 31-5/8 31-1/2 26-1/2(803) (800) (673) 5 (127) 104 (47) HL4-8(1/2 size)16 8 8 40-5/8 31-1/2 26-1/2 (1032) (800) (673) 5 (127) 147 (67) HL4-14(3/4 size)28 14 14 58-5/8 31-1/2 26-1/2 (1489) (800) (673) 5 (127) 170 (77) HL4-18(full size) 36 181870-5/8 31-1/2 26-1/2 (1794) (800) (673)5 (127)218 (99)* fixed spacing at 3”HL4 Features & Benefits∙ Precision-engineered bottom mount heater with superior heat-up and recovery time ∙ Analog heat and humidity levels. Digital thermometer. Temperature range of 90°- 190°F ∙ Can be operated with or without humidity. Capable of humidified holding as well as proofing ∙ Four sizes, available with adjustable universal, fixed universal or adjustable universal slides ∙ All swivel heavy-duty casters, front casters fitted with brakes∙ Field reversible glass door in heavy-duty extruded aluminum frame is standard doors are standard, solid aluminum door is optional∙Fixed wire universal slides for 18”x26” sheet pans and 12”x20”x2.5” steam table pansCabinets with Adjustable Universal, Fixed Universal or Angle SlidesAPPROVALS:3174974Conforms to UL Std 197 certified to CAN/CSA Std C22.2 No. 1093174974Conforms to NSF/ANSI Std 4International voltage: 220 volts, 2100 watts, 9.5 Amps, 50 Hz, single phase, plug cap per specific country require-ments / standardsCanadian Voltage: 120v, 1400 watts, 12Amps, NEMA 5-15PHL1 & HL3 Models HL2 & HL4 ModelsHL2 & HL4 Series Heater/Proofer CabinetsON/OFF power switch and indicator lightHeat control knob. Settings from 1 (low heat) to 10 (high heat) Heating element cycle lightDigital temperature read-outON/OFF power switch and indicator light Heat control knob. Settings from 1 (low heat) to 10 (high heat) Heating element cycle light Digital temperature read-outHumidity control knob. Settings from 1 (low humidity) to 10 (high humidity) Humidity element cycle lightWATER PAN LOCATIONS140°F41°FDANGER ZONE: 41°F to 140°F (5Bacteria grow rapidlyCOOK TO AT LEAST 165°F (74tion of most bacteria165°F HOLD at 140°F (60higherdoors are open.2.3.HL1, HL3 & HLM1 HB SERIESStandard US Electric: 120 VOLT, 1800 WATT, 15AMP, 1 PH, 60 HZ International Electric: 220 VOLT, 1800 WATT, 8.2AMP, 1 PH, 50/60 HZHL2 & HL4 HW SERIESStandard US Electric: 120 VOLT, 2100 WATT, 17.5 AMP, 1 PH International Electric: 220 VOLT, 2100 WATT, 9.5AMP, 1 PH, 50/60 HZWIRING DIAGRAMSTROUBLESHOOTING GUIDESYMPTOM POSSIBLE CAUSE SUGGESTED REMEDYNo power on display Not plugged in or circuit breakertripped Check or reset circuit breaker Connect to proper receptaclePower cord damaged Check - replace if required Circuit breaker tripped Check circuit breaker Power switch damaged or defective Check - replace if requiredFood dries out too quickly Operation where product temp is toohigh; food pans should be coveredCheck product temps going intoholding cabinet; cover food pans Control incorrectly set or defective Check proper operation or calibrationof controlInternal wiring error Call service technicianElement hi-limit trip / defective Call service technicianTakes too long to get to temperature Improper voltage Call service technician to verifyincoming voltage matches cabinetspecifications.Unit is hotbut low or no airflow Internal wiring error Call service technician Circulation motor has quit Call service technicianImproper voltage Call service technician to verifyincoming voltage matches cabinetspecifications.Unit is on, motors are running but no heatNOTE:The technical content of this manual, including any wiring diagrams, schemat-ics, parts breakdown illustrations and / or adjustment procedures, is intended for use ONLY by qualified technical personnel.NOTE:For warranty service, call Carter-Hoffmann direct at 800-323-9793 for authorization,we will dispatch the nearest authorizedservice agency.18。
Stryker 膝关节导航系统 安钉针说明书

• Prior to surgery, this equipment should be checked with the Stryker Navigation System to ensure it is functioning properly.
3
Intended Use of the Navigation System
For diaphyseal location, select bicortical fixation.
• Prior to each use, this product should be operated and inspected for any loose components or damage. DO NOT use if these conditions exist. Contact your Stryker Navigation sales representative immediately in this case.
IMPORTANT INFORMATION: Read carefully
Instructions For Use
Anchoring Pins
Anchoring Pin 20 mm / 4 mm REF 6007-420-000 Anchoring Pin 25 mm / 4 mm REF 6007-425-000 Anchoring Pin 30 mm / 4 mm REF 6007-430-000 Anchoring Pin 35 mm / 4 mm REF 6007-435-000 Anchoring Pin 40 mm / 4 mm REF 6007-440-000 Anchoring Pin 45 mm / 4 mm REF 6007-445-000 Anchoring Pin 50 mm / 4 mm REF 6007-450-000 Anchoring Pin 55 mm / 4 mm REF 6007-455-000 Anchoring Pin 60 mm / 4 mm REF 6007-460-000 Anchoring Pin 30 mm / 5 mm REF 6007-530-000 Anchoring Pin 40 mm / 5 mm REF 6007-540-000 Anchoring Pin 50 mm / 5 mm REF 6007-550-000 Pelvic Pin for Knee Navigation REF 6007-551-000
Eaton PDG13G0045TFFJ产品说明说明书

Eaton PDG13G0045TFFJEaton Power Defense molded case circuit breaker, Globally Rated, Frame 1, Three Pole, 45A, 35kA/480V, T-M (Fxd-Fxd) TU, Standard Line and Load (PDG1X3T125)Eaton Power Defense molded case circuit breakerPDG13G0045TFFJ 78667931781576 mm 139.7 mm 76.2 mm 1.36 kg Eaton Selling Policy 25-000, one (1) year from the date of installation of theProduct or eighteen (18) months from thedate of shipment of the Product,whichever occurs first.RoHS Compliant IEC 60947-2CSACCC MarkedUL 489Product NameCatalog Number UPCProduct Length/Depth Product Height Product Width Product Weight WarrantyCompliancesCertifications45 AComplete breaker 1Three-polePD1 Global Class A T-M (Fxd-Fxd) TU600 Vac600 VStandard Line and Load35 kAIC at 480 Vac 35 kAIC @480V (UL)36 kAIC Icu/ 36 kAIC Ics/ 76 kAIC Icm @380-415V (IEC) 18 kAIC @600V (UL/CSA) 65 kAIC @240V (UL) 22 kAIC Icu @125 Vdc55 kAIC Icu/ 55 kAIC Ics/ 121 kAIC Icm @240V (IEC) 22 kAIC Icu @250 VdcEaton Power Defense MCCB PDG13G0045TFFJ 3D drawing Consulting application guide - molded case circuit breakers Power Defense technical selling bookletPower Defense molded case circuit breaker selection poster Power Defense brochurePower Defense molded case circuit breakers - Frame 1 product aidAmperage Rating Circuit breaker frame type Frame Number of poles Circuit breaker type Class Trip Type Voltage rating Voltage rating - max TerminalsInterrupt rating Interrupt rating range 3D CAD drawing packageApplication notesBrochuresCatalogsMolded case circuit breakers catalogCertification reportsEU Declaration of Conformity - Power Defense molded case circuit breakersPower Defense Declaration concerning California’s Proposition 65PDG1 CCC certificationPDG1 UL authorizationPDG1 CSA certificationInstallation instructionsPower Defense Frame 1 UL global screw terminal end cap kit metric 125A 3P - IL012171ENPower Defense Frame 1 UL global DIN rail adapter three or four pole - IL012186ENPower Defense Frame 1 UL global lock padlockable handle haspIL012180ENPower Defense Frame 1 UL global handle block padlockable off only - IL012179ENPower Defense Frame 1 UL global interphase barrier - IL012176EN Power Defense Frame 1 UL global handle block padlockable -IL012178ENPower Defense Frame 1 UL global handle block non padlockable -IL012177ENPower Defense Frame 1 UL global interphase barrier instructions -IL012313ENPower Defense padlockable handle lock hasp top off only installation instructions - IL012226ENPower Defense Frame 1 UL global screw terminal end cap kit 125A 3P - IL012163ENPower Defense Frame 1 UL global box terminal (steel) 125A 3P -IL012165EN H03Power Defense Frame 1 UL global DIN rail adapter 2, 3, 4-pole -IL012185ENPower Defense Frame 1 UL global Padlockable Handle Lock Hasp -IL012225ENPower Defense Frame 1-2-3-4 IP door barrier assembly instructions -IL012278ENPower Defense Frame 1 UL Global variable depth rotary handle mech installation instructions - IL012308ENPower Defense Frame 1 UL global tunnel terminal (aluminum) 125A 3P - IL012166EN H03Power Defense Frame 1 UL global DIN rail adapter metal three pole -Eaton Corporation plc Eaton House30 Pembroke Road Dublin 4, Ireland © 2023 Eaton. All Rights Reserved. Eaton is a registered trademark.All other trademarks areproperty of their respectiveowners./socialmediaIL012187ENPower Defense Frame 1 UL global terminal shield cover IP30 3P - IL012174ENPower Defense Frame 1 Instructions - IL012152EN Power Defense Frame 1 UL Global Aux, Alarm, ST and UVR Animated Instructions.rh Power Defense Frame 3 Variable Depth Rotary Handle Mechanism Installation How-To VideoEaton Power Defense for superior arc flash safetyPower Defense Frame 1 Aux, Alarm, and Shunt Trip How-To Video Power Defense Frame 5 Trip Unit How-To Video Power Defense molded case circuit breakersPower Defense Frame 2 Variable Depth Rotary Handle Mechanism Installation How-To Video Power Defense BreakersPower Defense Frame 6 Trip Unit How-To Video Eaton Specification Sheet - PDG13G0045TFFJ Power Defense time current curve Frame 1 - PDG1Single and double break MCCB performance revisited Safer by design: arc energy reduction techniques Molded case and low-voltage breaker healthInstallation videosMultimediaSpecifications and datasheetsTime/current curvesWhite papers。
1Tarceva产品简介及不良反应处理

酪氨酸激酶(jīméi)结构域改动 1–3
EGFR 失活
ATP
P
P
细胞内酪氨酸激酶结构域 磷酸化,下游信号通路 (tōnglù)激活,肿瘤细胞
发作增殖,迁移,黏附等
特罗凯 与ATP 竞争性结合在 酪氨酸酶结构域,抑制磷酸化, 从而阻断(zǔ duàn)细胞内信号
通路的传导
1.Cohen S, et al. J Biol Chem 1980;255:4834–42; 2.Soderquist AM, et al. Fed Proc 1983;42:2615–20
3.Chinkers M, et al. Nature 1981;290:516–9; 4.Carey et al. Cancer Res 2006;66:8163–71
5.Wells A. Int J Biochem Cell Biol 1999;31:637–43
第九页,共56页。
特罗凯 概略(gàilüè)
特罗凯 —更强的药代动力学特征(tèzhēng)
Cmax(ng/ml)
AUC0-24 (ng•hour/mL)
特罗凯1
( 150mg/d )
吉非替尼2 吉非替尼2 吉非替尼2
( 225mg/d ) ( 525mg/d ) ( 700mg/d )
2,120
307
903
2,146
38,420
5,041 14,727 36,077
药物抑制EGFR后可影响角质化细胞的增生,分化,迁移 以及黏附,这一实践有助于解释丘疹脓疱及单调病的构 Lacouture M. Nat Rev Cancer 2006;6:803–12 成
第十八页,共56页。
表皮生长因子受体抑制剂惹起的皮肤不良反响 (fǎnxiǎng)
Eaton Power Defense PDD32K0300TFAN 产品说明书

Eaton PDD32K0300TFANPower Defense UL/CSA 240V Max, Frame 3, Two Pole, 300A, 85kA/240V, T-M (Fxd-Adj) TU, No TerminalsEaton Power Defense molded case circuit breakerPDD32K0300TFAN 786679787496109.1 mm 257.1 mm 138.9 mm 5.2163 kg Eaton Selling Policy 25-000, one (1) year from the date of installation of theProduct or eighteen (18) months from thedate of shipment of the Product,whichever occurs first.RoHS Compliant UL 489CSAProduct NameCatalog Number UPCProduct Length/Depth Product Height Product Width Product Weight WarrantyCompliancesCertifications300 AComplete breaker 3Two-polePD3 UL/CSA Only Class A T-M (Fxd-Adj) TU240 V AC240 VNo Terminals85 kAIC at 240 Vac 85 kAIC @240V (UL) 10 kAIC Icu @250 VdcEaton Power Defense PDD32K0300TFAN 3D drawing Consulting application guide - molded case circuit breakers StrandAble terminals product aid Power Defense technical selling booklet Power Defense brochurePower Defense molded case circuit breaker selection poster Molded case circuit breakers catalog Amperage Rating Circuit breaker frame type Frame Number of poles Circuit breaker type Class Trip Type Voltage rating Voltage rating - max Terminals Interrupt rating Interrupt rating range 3D CAD drawing packageApplication notesBrochuresCatalogsCertification reportsEaton Corporation plc Eaton House30 Pembroke Road Dublin 4, Ireland © 2023 Eaton. All Rights Reserved. Eaton is a registered trademark.All other trademarks areproperty of their respectiveowners./socialmediaPower Defense Declaration concerning California’s Proposition 65PDG3 UL authorization 250-600a TMTU PDG3 UL authorization 100-400a Power Defense Frame 3 Breaker Instructions (IL012107EN).pdf Power Defense Frame 3 Variable Depth Rotary Handle Mechanism Installation How-To VideoEaton Power Defense for superior arc flash safety Power Defense Frame 5 Trip Unit How-To Video Power Defense Frame 6 Trip Unit How-To Video Power Defense BreakersPower Defense Frame 2 Variable Depth Rotary Handle Mechanism Installation How-To VideoPower Defense molded case circuit breakers Eaton Specification Sheet - PDD32K0300TFAN Power Defense time current curve Frame 3 - PD3Molded case and low-voltage breaker health Safer by design: arc energy reduction techniquesInstallation instructionsMultimediaSpecifications and datasheetsTime/current curvesWhite papers。
2022 Montesa Cota 4RT 301RR Race Replica 商品说明书

DUAL-MAP ECU Does your course feature sections with poor traction, followed by sections with better grip? No problem — the 4RT 301RR Race Replica features a handlebar-mounted mode switch that lets you choose between two maps/modes to change engine power delivery. And the bike even includes special ECU mapping that improves engine response in the low and middle rpm ranges.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
伐地昔布与锂剂合用可导致锂血清清除率及肾 脏清除率明显下降(分别为25%,30%),同时, 锂的血清暴露水平较单独使用锂剂时升高34%。 正在接受锂剂治疗的患者,在开始帕瑞昔布治 疗或者调整帕瑞昔布剂量时,应严密监测其血
清中的锂浓度
不影响格列本脲的药代动力学(暴露水平)及药 效学(血糖及胰岛素水平)特性
注射用帕瑞昔布钠 VS
氟比洛芬酯注射液
仅供辉瑞内部使用
内容摘要
• 说明书解读篇 • 推广信息疗效篇 • 推广信息安全篇
2 仅供辉瑞内部使用
商品名 通用名 剂型 规格
基本信息
特耐® 注射用帕瑞昔布钠1 白色或类白色冻干块状物 20mg/40mg(以帕瑞昔布计)
凯纷® 氟比洛芬酯注射液 2 白色乳液略带粘性
司匹林合用
ACE抑制剂或利尿剂
帕瑞昔布钠与ACE 抑制剂或利尿药合用时,将 增加发生急性肾功能不全的风险
氟比洛芬酯注射液
慎与其合用 未提及
慎与合用
环孢霉素或他克莫司
帕瑞昔布钠与此类药物合用时,应监测肾功能
阿片类药物
1. 注射用帕瑞昔布钠说明书 2. 氟比洛芬酯注射液说明书
帕瑞昔布可以和阿片类止痛药合用。在临床研 究中,当与帕瑞昔布联合用药时,可以显著减
健康男子静脉内单次给予氟比洛芬酯 注射液5ml(50mg),在5分钟内全部
水解为氟比洛芬
静脉内单次给予氟比洛芬酯注射液 5ml(50mg),6-7分钟后氟比洛芬血
中浓度达到最高(8.9μg/ml)
98%(最高推荐剂量80mg/天) 55L
无介绍
肝脏代谢;细胞色素P450(CYP)3A4 与CYP2C9 同工酶代谢以及磺胺葡萄
5ml:50mg
化学结构式
作用机制
1. 注射用帕瑞昔布钠®说明书 2. 氟比洛芬酯注射液®说明书
C19H17N2O4SNa
选择性COX-2抑制剂
C19H19FO4
非选择性NSAIDs
仅供辉瑞内部使用
适应症与用法用量对比
注射用帕瑞昔布钠
氟比洛芬酯注射液
日本氟比洛芬酯注 射液说明书
适应症 手术后疼痛的短期治疗
药物相互作用 —药效学相互作用
抗凝血药物 阿司匹林
注射用帕瑞昔布钠
增加发生出血并发症的风险,尤其在治疗开始 后数天内。应密切监测同时服用抗凝血药物患 者的凝血酶原时间国际标准化比(INR),特别是 在开始使用帕瑞昔布或对帕瑞昔布进行剂量调
整后数日内
对阿司匹林抑制血小板聚集的作用或出血时间 没有影响。帕瑞昔布可以与低剂量(≤325mg)阿
糖醛酸化
无介绍
半衰期
8h
消除
血浆清除率
6L/h
5.8h 无介绍
解读
1. 注射用帕瑞昔布钠半衰期8h,而氟比洛芬酯注射液为5.8h,注射用帕瑞昔布钠更耐 久镇痛
2. 氟比洛芬酯注射液说明书很多信息未涉及到
1. 注射用帕瑞昔布钠说明书 2. 氟比洛芬酯注射液说明书
仅供辉瑞内部使用
特殊人群用药
注射用帕瑞昔布钠
慎用
肝功能不全的患者
轻度肝功能损伤的患者(Child-Pugh 评分:56)不必进行剂量调整。中度肝功能损伤的患 者(Child-Pugh 评分:7-9)应慎用帕瑞昔布, 剂量应减至常规推荐剂量的一半且每日最高 剂量降至40mg。目前尚无严重肝功能损伤患 者(Child-Pugh 评分:≥10)的临床用药经验,
仅供辉瑞内部使用
药代动力学参数对比
药代动力学参数
吸收
转化 峰浓度
分布 代谢
血浆蛋白结合率 分布容积
代谢部位与代谢酶
注射用帕瑞昔布钠
氟比洛芬酯注射液
帕瑞昔布在静注或肌注后经肝脏酶水 解,迅速转化为有药理学活性的物
质——伐地昔布
帕瑞昔布钠单次静注或肌注20mg, 伐地昔布分别于注射后约30 分钟或1
小时达到峰浓度
少按需给药的阿片类药物的每日需求量
未提及 未提及 仅供辉瑞内部使用
药物相互作用 —药代动力学相互作用
抗生素 甲氨蝶呤
锂剂 格列本脲
注射用帕瑞昔布钠
氟比洛芬酯注射液
无影响
禁止与喹诺酮类抗生素(洛美沙星、诺 氟沙星、依诺沙星)合用,合用有导致
抽搐发生的可能
口服伐地昔布(每次40mg,一日两次)对甲氨蝶 呤的血浆浓度不会产生临床显著影响。然而, 当上述两种药物合用时,仍应对甲氨蝶呤相关
慎与其合用
注射用帕瑞昔布 钠可慎与与其喹合诺用酮类 抗生素合用,而 氟比洛芬酯注射 液禁止与其合用
未提及
1. 注射用帕瑞昔布钠说明书 2. 氟比洛芬酯注射液说明书
仅供辉瑞内部使用
禁忌症对比
术后镇痛 癌症镇痛
用于下列疾病及状态 时的镇痛。
术后、各种癌症
用法 用量
快速静脉推注 通过已有静脉通路给药 肌肉注射应选择深部肌肉
缓慢推注
推荐剂量为40mg ,IV / IM,随后视需要间隔6~
12 小时给予20mg 或 40mg,每天总剂量不超
过80mg。
一般采用静脉给药,尽 可能缓慢给药(1分钟以
故不推荐使用。
此儿童不宜使用。
肾功能不全的患者
不必对轻度至中度肾功能损伤(肌酐清除率: 30~80ml/min)的患者进行剂量调整。对于 重度肾功能损伤(肌酐清除率:<30ml/min) 或有液体潴留倾向的患者,应选择最低推荐
剂量开始治疗并密切监测肾功能。
严重肾功能不全者禁用; 肾功能不全或有既往史的患者
氟比洛芬酯注射液
老年患者
老年患者(≥65 岁)应用帕瑞昔布一般不需进行 剂量调整。对于体重低于50kg 的老年患者, 初始剂量应减至常规推荐剂量的一半且每日
最高剂量应减至40mg。
要特别当心老年患者出现不良反 应,要从小剂量开始慎重给药。
儿童
尚未确定18岁以下儿童使用的安全性和疗效, 儿童使用的安全性尚未确定,因
因此禁止在此类患者中使用帕瑞昔布。
严重肝功能不全者禁用; 肝功能不全或有既往史的患者
慎用
氟比洛芬约50%经肾排出,而特耐经肝代谢消除,仅5%以原型经肾排泄,肾功能不 全不会造成体内药物蓄积。出血倾向及血液系统异常患者慎用氟比洛芬。
1. 注射用帕瑞昔布钠说明书 2. 氟比洛芬酯注射液说明书
仅供辉瑞内部使用
上) 必要时可采用镇痛泵
通常成人每次静脉给予 50mg,根据年龄、症状
适当增减剂量
尽量缓慢(超过1分钟 的时间)静脉注射
通常成人的用量为每 次静脉注射50mg,应 根据年龄、症状进行
适当的剂量增减。
氟比洛芬酯注射液不能肌注,特耐可以静注或肌注,使用更方便
1. 注射用帕瑞昔布钠说明书 2. 氟比洛芬酯注射液说明书
