2017年民间固定资产投资增长6.0%
2022款福特 Bronco 两门和四门车型规格说明书

* H orsepower and torque ratings based on premium fuel per SAE J1349 standard. Horsepower and torque are independent attributes and may not be achieved simultaneously.For editorial use only. Information correct at time of publication. Check for updates.BODYVehicle type Midsize two-door and four-door rugged 4x4 SUVTrim series Base, Big Bend ™, Black Diamond ™, Everglades ™, Outer Banks ™, Badlands ™, Wildtrak ™, Raptor ™ (see specs on separate page)Option packages Mid, High, Lux, Sasquatch ™ Package Final assembly locationMichigan Assembly Plant, Wayne, MichiganENGINES2.3-liter EcoBoost ® 2.7-liter EcoBoost Configuration Ti-VCT GTDI I-4Ti-VCT GTDI 60-degree V6Displacement 138 cu. in. (2,264 cc)164 cu. in. (2,694 cc)Bore x stroke 3.45 in. x 3.7 in. (87.55 mm x 94 mm) 3.27 in. x 3.27 in. (83 mm x 83 mm)Compression ratio 10.0:110.0:1Fuel deliveryDirect injectionPort fuel/direct injectionEngine control system Chassis integrated powertrain control module Chassis integrated powertrain control module Engine block material High-pressure die cast aluminum alloyCompacted graphite ironPistonsLightweight, high-strength cast aluminum with low-friction skirt coating and steel ring Lightweight, high-strength cast aluminum with low-friction skirt coating and steel ring Connecting rods Forged steel Forged steel Crankshaft Forged steelForged steelValve diameter/lift Intake, 32.5 mm/8.9 mm Exhaust, 30 mm/7.8 mm Intake, 32.50 mm/10 mm Exhaust, 28.30 mm/9.0 mmValve lifters Direct acting mechanical bucket Roller finger follower, hydraulic compensating lash adjusters Intake Composite, split plenum Composite Ignition Coil on plugCoil on plugInduction Twin-scroll turbocharger with electrically actuated wastegate Dual mono-scroll turbochargers with electrically actuated wastegate ExhaustCast ironIntegrated cylinder head casting Recommended fuel Regular unleaded 87 octane minimum Regular unleaded 87 octane minimum Oil capacity 6.2 quarts 7.0 quarts Coolant capacity 11.1 liters11.7 litersExhaust system Single catalyst with resonator and single muffler Double catalyst with resonator and single muffler Alternator Standard: Single 240A Standard: Single 240A Battery groupH7 AGM 80 AH 800 CCA H7 AGM 80 AH 800 CCA SAE horsepower (regular fuel)275 @ 5,700 rpm 315 @ 5,500 rpm SAE torque (regular fuel)315 @ 3,400 rpm 410 @ 3,250 rpm SAE horsepower (premium fuel)*300 @ 5,700 rpm 330 @ 5,250 rpm SAE torque (premium fuel)*325 @ 3,400 rpm 415 @ 3,100 rpm Build locationCleveland Engine PlantLima Engine Plant* M aximum ratios with high-capability option packages.For editorial use only. Information correct at time of publication. Check for updates.DRIVETRAINLayoutLongitudinally mounted front engine with center-mounted transfer case, independent front axles/solid rear axlesTRANSMISSIONSStandard 7-speed (6+1 crawler gear) Getrag manual (offered on 2.3-liter engine only)Optional10-speed automatic (available on both 2.3- and 2.7-liter engines)Gear Ratios7-Speed Manual 10-Speed Automatic Crawler 6.588:1First 4.714:1First 4.283:1Second 2.997:1Second 2.365:1Third 2.149:1Third1.453:1Fourth 1.769:1Fourth 1.000:1Fifth 1.521:1Fifth 0.776:1Sixth 1.275:1Sixth 0.646:1Seventh 1.000:1Reverse-5.625:1Eight 0.853:1Ninth 0.689:1Tenth 0.636:1Reverse-4.885:1Transfer Cases StandardOptional4x4 with part-time engagement electronic shift-on-the-fly, 2.72:1 low ratioAdvanced 4x4 with 4A mode automatic on-demand engagement, 3.06:1 low ratioMaximum crawl ratios*79.92:1 – 7-speed manual with standard ESOF57.19:1 – 10-speed speed automatic with standard ESOF 94.75:1 – 7-speed manual with optional Advanced 4x467.80:1 – 10-speed automatic with optional Advanced 4x4Flat-tow capability Yes Yes Axles FrontRearStandard Dana ™ AdvanTEK ® M190 independent front suspension Dana 44™ AdvanTEK M220 solid rear differential OptionalDana ™ AdvanTEK ® M210 independent front suspension with Spicer ® Performa-TraK electronic lockerDana 44™ AdvanTEK M220 solid rear differential with Spicer ®Performa-TraK electronic lockerCHASSISTypeBody-on-frame; fully boxed high-strength steel frame with seven cross members, High-Performance Off-Road Stability Suspension (HOSS) System with low-mass independent front suspension, five-link solid rear axle with Panhard rod and outboard coilover springs Suspensions FrontRearStandard Twin forged alloy A-arms with long-travel coil-over springs, HOSS-tuned heavy-duty dampers 220 mm solid rear axle with long-travel, variable rate coil-over springs, HOSS-tuned heavy-duty dampers OptionalBilstein ® position-sensitive dampers with Bilstein position-sensitive dampers with* R equires heavy-duty modular front bumper.For editorial use only. Information correct at time of publication. Check for updates.BODYTwo-door Four-door Tops standard(varies by trim series)Removable hardtop Removable soft-top Removable hardtopRoof panel weight (pounds)14.0 front left, 15.4 front right 13.6 front left, 14.5 front right, 28.1 mid panel Doors/storage Two removeable frameless doors Four removable frameless doors Available rear storage for four doors Door weight (pounds)Front 62Front 55/Rear 44Modular section Doors, roof panels, front fenders, grille, fender flares, rear quarter panels, bumpersRock rail Standard on Black Diamond, Everglades and Badlands series dealer installed accessory on all other Bronco series Tube stepsStandard on Outer Banks, available on Big Bend and WildtrakUnderbody protectionBlack Diamond, Everglades and Widltrak (optional): Five steel underbody shields including engine shield, transfer case shield, fuel tank shield, shin guards and heavy-duty front bash plate*Badlands: Six steel underbody shields including engine shield, transfer case shield, fuel tank shield, shin guards, stabilizer-bar shield and heavy-duty front bash plate*Modular bumpers Heavy-duty powder-coated modular steel front bumper with heavy-duty bash plate standard on Everglades, available on all other seriesWashout vinyl flooring with drain plugsStandard on Black Diamond, Everglades and Badlands series Trail sights with 150-pound tie-down capacityStandard on all Bronco seriesBRAKESFront RearType Four-wheel power disc brakes with four-sensor, four-channel antilock braking system and electronic stability control Boost2.3-liter engine, vacuum brake boost, 2.7-liter engine, electronic brake boost Brake configuration Vented discs, twin-piston floating caliper Discs with single-piston floating caliper, integral electronic parking brake Rotor diameter/thickness 311 mm x 34 mm 308 mm x 24 mm Pad material Non-asbestos organic Non-asbestos organic Caliper type Twin 51-mm floating Single 54-mm floating Pad swept area413 cm 2373 cm2TypeThree-mode rack-and-pinion steering with standard, comfort and sport modes controlled via Terrain Management System ™ with G.O.A.T. Modes ™ (Goes Over Any Type of Terrain)Turning radius (curb-to-curb)35.53 feet (10.83 m)12.13 m (Badlands)/12.15 m (Sasquatch)BANG & OLUFSEN ©2021 and B&O ©2021. BANG & OLUFSEN ™ and B&O ™ are registered trademarks of Bang & Olufsen Group. Licensed by Harman Becker Automotive Systems Manufacturing Kft. All rights reserved.For editorial use only. Information correct at time of publication. Check for updates.TECHNOLOGYTerrain Management with G.O.A.T. ModesStandard modes, Base (5): Normal, Eco, Sport, Slippery and SandBig Bend, Outer Banks (6): Normal, Eco, Sport, Slippery, Mud/Ruts and SandBlack Diamond and Everglades (7): Normal, Eco, Sport, Slippery, Mud/Ruts, Sand and Rock Crawl Badlands, First Edition (7): Normal, Eco, Slippery, Mud/Ruts, Baja, Sand and Rock Crawl Wildtrak (7): Normal, Eco, Sport, Slippery, Baja, Mud/Ruts and Sand Trail control Standard on all Bronco series with 10-speed automatic transmission Trail turn assist Standard on all Bronco series with 10-speed automatic transmission Trail one-pedal driveStandard on 2.7-liter V6 models with 10-speed automatic transmissionBaseBig Bend Black Diamond Everglades Outer Banks Badlands Wildtrak HMI/audio system, SYNC ® 4 8-inch8-inch 8-inch 12-inch 8-inch 8-inch 8-inchABS/stability control electric power-assisted steering, and ABSAirbagsFirst-row driver and passenger dual-stage front and seat side airbagsAll rows: Safety Canopy side-curtain airbags with rollover sensors for all rows*D enotes Sasquatch ™ PackageFor editorial use only. Information correct at time of publication. Check for updates.WHEELSSeries StandardBase 16-inch bright polished silver-painted steel Standard Big Bend 17-inch Carbonized Gray-painted aluminum Standard Black Diamond 17-inch black gloss-painted black steel Optional Black Diamond 17-inch black mid-gloss-painted aluminum Standard Everglades 17-inch Carbonized Gray-painted alloy wheelsStandard Outer Banks 18-inch bright machined black high-gloss-painted aluminum Standard Badlands 17-inch machined Carbonized Gray-painted aluminumOptional Badlands 17-inch black high-gloss-painted aluminum with Carbonized Gray beauty ring, beadlock-capable StandardWildtrak*17-inch black high-gloss-painted aluminum with black beauty ring, beadlock-capableSasquatch Package SeriesOptionalBase, Big Bend, Black Diamond, Badlands17-inch black high-gloss-painted aluminum with warm alloy beauty ring, beadlock-capableLIGHTING/CONVENIENCEBaseBig Bend Black Diamond Everglades Outer Banks Badlands Wildtrak Headlamps Auto on/off LED Auto high-beamS S S S S S S Bronco signature LED lighting N/A O N/A N/A S S S LED fog lampsN/A S S S S S S Mirror LED approach lamps and LED spotlightsN/A N/A N/A N/A O O O Overhead upfitter switches with six-pre-wired user-defined electrical connectionsO O S S O S S Dual-control HVAC automatic temperature control N/A O O S S O S Heated front seatsN/A O O S S O S Central locking system with keyless entry and push-button start S S S S S S S Push button startS S S S S S S Intelligent access (lock/unlock)N/A O O S S O S Remote start system with FordPass Connect (automatic transmission models only)S S S S S S S Power window SSSSSSSAccessory ElectricalOne 110V AC 400 watt power outlet (Mid Package and up; rear of center console)Power points (12V) – one (1) center floor console, one (1) cargo areaStandard: Smart Charging Multimedia USB Ports, First Row – One (1) USB-A and one (1) USB-C; Smart Charging USB Ports, Second Row – One (1) USB-A and one (1) USB-C in the back side of the center floor consoleOptional: Lux package – Smart Charging USB Ports, dash board – One (1) USB-A and one (1) USB-CDIMENSIONS/CAPACITIES (INCHES)Two-door Four-doorWheelbaseAll series100.4116.1LengthBase173.7189.4Big Bend173.7189.4Wildtrak173.7189.5Badlands174.8190.5Everglades N/A198.9HeightBase71.973.0 (soft-top)Big Bend72.972.9Wildtrak75.275.3Badlands73.873.9( W hen available roof racks are installed, add 3.6 inches to two-doormodels, 3.4 inches to four-door models for maximum height)Everglades N/A78.7 (includes standard roof rack) Width (with mirrors folded)Base/Big Bend75.975.9Wildtrak79.379.3Badlands76.376.3Everglades N/A79.4 (includes mirrors)Track width, front/rearBase/Big Bend/Badlands65.0/65.065.0/65.0Wildtrak/Everglades66.9/66.966.9/66.9Seating capacity Four FivePassenger volume, hardtop (cu. ft.)99103.7Passenger volume, soft-top (cu. ft.)N/A108.2Behind first row, hardtop (cu. ft.)52.377.6Behind first row, soft-top (cu. ft.)N/A83.0Behind second row, hardtop (cu. ft.)22.435.6Behind second row, soft-top (cu. ft.)N/A38.3HeadroomFirst row, hardtop41.040.8First row, soft-top N/A43.3Second row, hardtop39.840.1Second row, soft-top N/A41.1LegroomFirst row43.143.1Second row35.736.3Hip roomFirst row56.355.9Second row43.354.8Shoulder roomFirst row57.157.1Second row51.856.5Fuel capacity (gallons)16.920.8For editorial use only. Information correct at time of publication. Check for updates.* R emote start on automatic transmission models only. **Trailer tow capability determined using SAE J2807 standard. † Requires optional Ford Accessories hitch. †Additional options may decrease payload.For editorial use only. Information correct at time of publication. Check for updates.OPTION GROUP HIGHLIGHTSMid Package equipment group highlightsSYNC 4 with 8-inch LCD touch screen/audio system, connected navigation, dual-zone HVAC, front-rowheated seats, two-door intelligent access, remote start system,* Ford Co-Pilot360, reverse sensing system, rear passenger power outletHigh Package equipment group highlightsIncludes all Mid Package content plus:SYNC 4 with 12-inch LCD touch screen/audio system and information on-demand panel, 360-degree camera, forward sensing system, additional sound deadening, mirror-mounted LED approach lamps and spotlights Lux Package equipment group highlightsIncludes all Mid and High Package content plus:10-speaker B&O Sound System by Bang & Olufsen, adaptive cruise control and evasive steering assist, heated steering wheel, connected built-in navigation with three years of service, wireless charging padand dash-mounted USB-A and USB-C smart charging portsBase 2.3-liter EcoBoost I-47-speed manual 2,3384,2941,2465,5402,9003,0003,500†8,780Big Bend 2.3-literEcoBoost I-47-speed manual 2,3444,3041,2365,5402,9003,0003,5008,780Black Diamond 2.3-liter EcoBoost I-47-speed manual 2,4904,5871,0535,6402,9003,0003,5008,780Badlands 2.3-liter EcoBoost I-47-speed manual 2,5764,6991,0215,7202,9003,0003,5008,780Base 2.3-liter EcoBoost I-410-speed SelectShift automatic 2,3534,3141,2265,5402,9003,0003,500†8,480Big Bend 2.3-liter EcoBoost I-410-speed SelectShift automatic 2,3594,3241,2165,5402,9003,0003,5008,780Black Diamond 2.3-liter EcoBoost I-410-speed SelectShift automatic 2,5044,6051,0345,6402,9003,0003,5008,480Outer Banks 2.3-liter EcoBoost I-410-speed SelectShift automatic 2,4024,4131,1275,5402,9003,0003,5008,480Badlands 2.3-liter EcoBoost I-410-speed SelectShift automatic 2,5914,7191,0015,7203,0003,0003,5008,780Base 2.7-liter EcoBoost V610-speed SelectShift automatic 2,4934,4661,2345,7003,0003,0003,500†8,740Big Bend 2.7-liter EcoBoost V610-speed SelectShift automatic 2,4994,4761,2245,7003,0003,0003,5008,740Black Diamond 2.7-liter EcoBoost V610-speed SelectShift automatic 2,6444,7571,0225,7803,0003,0003,5008,840Outer Banks 2.7-liter EcoBoost V610-speed SelectShift automatic 2,5424,5651,1355,7003,0003,0003,5008,740Badlands 2.7-liter EcoBoost V610-speed SelectShift automatic 2,7314,8719895,8603,0003,0003,5008,840Wildtrak2.7-liter EcoBoost V610-speed SelectShift automatic2,5444,5741,0695,7403,0003,0003,5008,740†Requires optional Ford Accessories hitch.For editorial use only. Information correct at time of publication. Check for updates.WARRANTYBumper-to-bumper Three years/36,000 miles Powertrain Five years/60,000 miles CorrosionFive years/unlimited miles Roadside assistance24-hour/day (3 years/36 miles)。
TUNEL细胞凋亡检测试剂盒(显色法)说明书

TUNEL 细胞凋亡检测试剂盒(显色法)产品编号 产品名称包装 C1098TUNEL 细胞凋亡检测试剂盒(显色法)50次产品简介:碧云天生产的显色法TUNEL 细胞凋亡检测试剂盒(Colorimetric TUNEL Apoptosis Assay Kit)为您提供了一种高灵敏度又快速简便的细胞凋亡检测方法。
对于待检测的细胞或组织样品,经过生物素标记和后续的DAB 显色等步骤,即可在普通光学显微镜下观察到凋亡细胞。
细胞在发生凋亡时,会激活一些DNA 内切酶,这些内切酶会切断核小体间的基因组DNA 。
细胞凋亡时抽提DNA 进行电泳检测,可以发现180-200bp 的DNA ladder 。
基因组DNA 断裂时,暴露的3'-OH 可以在末端脱氧核苷酸转移酶(Terminal Deoxynucleotidyl Transferase, TdT)的催化下加上生物素(Biotin)标记的dUTP(Biotin-dUTP),随后和辣根过氧化物酶(HRP)标记的Streptavidin (Streptavidin-HRP)结合,最后在HRP 的催化下通过DAB 显色来显示凋亡细胞,从而可以通过普通光学显微镜检测到凋亡的细胞,这就是TUNEL(TdT-mediated dUTP Nick-End Labeling)法检测细胞凋亡的原理。
本试剂盒有如下优点。
(1) 高灵敏度:背景染色极低,阳性染色强,可以在单细胞水平检测到细胞凋亡,同时由于凋亡早期就有DNA 断裂,可以检测到早期的细胞凋亡。
(2) 特异性好:TUNEL 检测时通常更容易标记凋亡细胞,而不容易标记坏死细胞。
(3) 快速:仅需约2-3个小时即可完成。
(4) 应用范围广:可以用于检测冷冻或石蜡切片中的细胞凋亡情况,也可以检测培养的贴壁细胞或悬浮细胞的凋亡情况。
(5) 实测效果好:参考图1。
图1. 本试剂盒的检测效果图。
A. HeLa 细胞未处理或用DNase I 室温孵育10分钟后的检测效果图。
白灵酊质量标准

白灵酊质量标准
白灵酊是一种常见的药物,主要用于治疗感冒、咳嗽等疾病。
在使用白灵酊之前,我们需要了解它的质量标准,以确保药物的安全有效。
首先,白灵酊的外观应为无色或淡黄色液体,无杂质。
其气味应为清香或芳香,不应有刺激性气味。
其次,白灵酊的密度应在0.980~1.030g/mL之间。
PH值应在4.0~6.0之间。
这些参数的测定可以通过仪器进行。
另外,白灵酊中化学成分的含量也是需要检测的。
其中,氯化铵的含量应在3.5%~4.5%之间,甘草酸二钾的含量应在
0.35%~0.45%之间,苯甲酸钠的含量应在0.10%~0.20%之间,氯化钠的含量应在0.15%~0.25%之间。
这些参数的测定可以通过药典中规定的方法进行。
此外,白灵酊还需要进行微生物检测。
其细菌总数应不超过1000CFU/mL,霉菌和酵母菌总数应不超过100CFU/mL,大肠杆菌和金黄色葡萄球菌应为阴性。
这些检测可以通过微生物实验室进行。
最后,白灵酊还需要进行稳定性测试。
在常温下保存6个月后,药物的外观、PH值、化学成分含量等参数应与新制药物相比
变化不大。
综上所述,白灵酊的质量标准包括外观、密度、PH值、化学
成分含量、微生物检测和稳定性测试等多个方面。
在购买和使用白灵酊时,我们需要注意这些标准,并选择正规厂家生产的产品,以确保药物的安全有效。
核苷类药物知识

核苷类药物知识核苷类药物的综述,免费下载的,大家给好评吧!O(∩_∩)O~1. 前言核苷和脱氧核苷是由核苷碱基分别和核糖或脱氧核糖以苷键形式而构成的,它们是组成核糖核酸(RNA)和脱氧核糖核酸(DNA)的基本元件,是遗传基因的基础。
核苷和脱氧核苷系列衍生物具有多种生物活性物质,可以直接或间接地作为药物使用,在治疗多种重大的疾病方面起到极其重要的作用,国外已经研究开发出系列化药物并商品化,国内研究与开发较晚,发展前景非常广阔.1。
1 核苷类药物的合成与生产从20世纪40年代末期,国外就开始核苷及其系列药物的合成与开发。
目前世界排名前25位制药大公司都有自己的核苷衍生物生产或加工厂,并且均有持有专利的核苷类药物上市,并且从20世纪90年代起投入巨资用于基因药物的研究。
据国外有关资料预计,2003年基因药物的市场价值将超过30亿美元.在亚洲,日本是最早开发核苷类药物和基因药物的国家,如武田、住友、味之素等公司均有相关的中间体开发机构和生产基地。
另外韩国、印度在20世纪90年代初开始投入这类产品的开发与生产.中国在核苷及其衍生物方面的开发研究与生产始于20世纪90年代末期,但是核苷及其中间体品种少,部分原料依赖进口,与目前快速发展的生命科学及相关药物研究不相适应。
1。
2 核苷类药物的应用核苷与脱氧核苷系列化合物主要用于医药领域,用途广泛,而且新产品层出不穷,应用范围不断扩大.(一)抗病毒药物。
核苷类抗病毒药物品种繁多,结构多样,主要以破坏病毒转录,干扰或终止病毒核酸的合成为目的,用于抗疱疹病毒、HIV、HBV、以及流感和呼吸系统病毒等DNA和RNA病毒。
目前在这方面应用最多,而且新出现的药物主要集中于治疗上述疾病。
(二)抗肿瘤药物。
目前用于临床和正在研究的核苷类抗肿瘤药物有数十种,它们的主要作用是干扰肿瘤的DNA合成,或者影响核酸的转录过程,抑制蛋白质的合成,从而达到治疗肿瘤的效果。
(三)抗真菌类药物.具有这方面作用的核苷类化合物已经有多种用于临床应用,其中有部分产品对多种真菌具有抑制作用,而且对哺乳动物几乎无毒性。
technical_guide_for_the_elaboration_of_monographs_7th_edition_2015

Technical guide for the ELABORATION OF MONOGRAPHSEuropean Pharmacopoeia7th Edition2015European Directorate for the Quality of Medicines & HealthCareEnglish version2015 Making copies of this file for commercial purposes or posting it on a websitefor which access is charged is strictly prohibited. Re-use of the file, in whole or in part, requires that the sourcebe clearly cited and the EDQM(@edqm.eu)be informed.European Directorate for the Qualityof Medicines & HealthCare (EDQM)Council of Europe7, allée KastnerCS 30026F-67081 STRASBOURGFRANCE Cover image: © EDQM - Council of Europe Director of the Publication: Dr S. KeitelPage layout: EDQMwww.edqm.eu© Council of Europe, 2015TECHNICAL GUIDE FOR THE ELABORATION OF MONOGRAPHS7th Edition – 2015TABLE OF CONTENTSI.INTRODUCTION (1)I.1.PURPOSE OF THE GUIDE (1)I.2.TEST PROCEDURES (1)I.3.EQUIPMENT (2)I.4.QUANTITIES (2)I.5.REAGENTS (4)MERCIAL NAMES (4)I.7.REFERENCE STANDARDS (4)II.MONOGRAPH ON A SUBSTANCE FOR PHARMACEUTICAL U SE (5)II.1.TITLE (5)II.2.DEFINITION (6)binations (7)II.2.2.Content (7)II.3.CHARACTERS (8)II.3.1.Appearance (8)II.3.2.Taste (9)II.3.3.Odour (9)II.3.4.Solubility (9)II.3.5.Stability factors (10)II.3.6.Hygroscopicity (10)II.3.7.Solid-state properties (10)II.3.8.Other characteristics (10)II.3.9.Behaviour in solution (11)II.4.IDENTIFICATION (11)II.4.1.General (11)II.4.2.Second Identification series (12)II.4.3.Infrared absorption spectrophotometry (13)II.4.4.Ultraviolet and visible absorption spectrophotometry (13)II.4.5.Melting point, freezing point and boiling point (14)II.4.6.Specific optical rotation (15)II.4.7.Thin-layer chromatography (15)II.4.8.Gas chromatography and liquid chromatography (16)II.4.9.Chemical reactions (16)II.5.TESTS (16)II.5.1.General (16)II.5.2.Title of tests (17)II.5.3.Solution S (18)II.5.4.Appearance of solution (19)II.5.4.1. Clarity and degree of opalescence (2.2.1.) (19)II.5.4.2. Degree of coloration (2.2.2.) (19)II.5.5.pH and Acidity or alkalinity (20)II.5.6.Optical rotation (2.2.7.) (21)II.5.7.Absorption spectrophotometry (ultraviolet and visible) (2.2.25.) (22)II.5.8.Related substances (23)II.5.8.1. Thin-layer chromatography (TLC) (2.2.27.) (27)II.5.8.2. Liquid chromatography (LC) (2.2.29.) (28)II.5.8.3. Gas-liquid chromatography (GC) (2.2.28.) (33)II.5.8.4. Capillary electrophoresis (CE) (2.2.31.) (33)II.5.9.Readily carbonisable substances (34)II.5.10.Foreign anions and/or cations (35)II.5.11.Heavy metals – Elemental Impurities (35)II.5.12.Loss on drying (2.2.32.) (36)II.5.13.Thermogravimetry (2.2.34.) (36)II.5.14.Semi-micro determination of water (2.5.12.) – (volumetric Karl Fischer) (37)II.5.15.Micro determination of water (2.5.32.) – (coulometric Karl Fischer) (37)II.5.16.Gas chromatographic determination of water (37)II.5.17.Determination of water by distillation (2.2.13.) (38)II.5.18.Sulfated ash (2.4.14.) (38)II.5.19.Residue on evaporation (38)II.5.20.Residual solvents (2.4.24.) (38)II.5.21.Bacterial endotoxins (38)II.6.ASSAY (39)II.6.1.Ultraviolet and visible spectrophotometry (2.2.25.) (40)II.6.1.1. Direct measurement (40)II.6.1.2. Measurement after a colour reaction (40)II.6.2.Volumetric analysis (40)II.6.3.Chromatography (41)II.6.4.Determination of nitrogen by sulfuric acid digestion (2.5.9.) (41)II.7.STORAGE (41)BELLING (42)II.9.IMPURITIES (42)II.10.FUNCTIONALITY-RELATED CHARACTERISTICS (43)III.ANALYTICAL VALIDATION (44)III.1.DEFINITIONS AND TERMINOLOGY (44)III.1.1.Introduction (44)III.1.2.Types of analytical procedures to be validated (44)III.1.3.Validation characteristics and requirements (45)III.1.4.Glossary (46)III.2.METHODOLOGY (47)III.2.1.Introduction (47)III.2.2.Specificity (48)III.2.2.1. Identification (48)III.2.2.2. Assays and impurity tests (49)III.2.3.Linearity (49)III.2.4.Range (50)III.2.5.Accuracy (51)III.2.5.1. Assay (51)III.2.5.2. Impurities (quantification) (51)III.2.5.3. Recommended data (51)III.2.6.Precision (51)III.2.6.1. Repeatability (52)III.2.6.2. Intermediate precision (52)III.2.6.3. Reproducibility (52)III.2.6.4. Recommended data (52)III.2.7.Detection limit (52)III.2.7.1. Based on visual evaluation (52)III.2.7.2. Based on signal-to-noise ratio (52)III.2.7.3. Based on the standard deviation of the response and the slope (53)III.2.7.4. Recommended data (53)III.2.8.Quantitation limit (53)III.2.8.1. Based on visual evaluation (53)III.2.8.2. Based on signal-to-noise ratio (53)III.2.8.3. Based on the standard deviation of the response and the slope (54)III.2.8.4. Recommended data (54)III.2.9.Robustness (54)III.2.10.System suitability testing (55)III.3.SPECIFIC APPLICATION TO METHODS USED IN THE PH. EUR. (55)III.3.1.Optical rotation (2.2.7.) (55)III.3.1.1. Introduction (55)III.3.1.2. Identification (55)III.3.1.3. Tests (55)III.3.2.Ultraviolet spectrophotometry (2.2.25.) (56)III.3.2.1. Identification (56)III.3.2.2. Limit test (56)III.3.2.3. Assay (56)III.3.3.Non-instrumental limit tests (57)III.3.3.1. Appearance of solution (2.2.1. and 2.2.2.) (57)III.3.3.2. Acidity or alkalinity (57)III.3.3.3. Limit tests for anions/cations (2.4.) (57)III.3.4.Atomic absorption spectrometry (2.2.23.) (58)III.3.4.1. Specificity (58)III.3.4.2. Calibration (58)III.3.4.3. Matrix effects (59)III.3.4.4. Detection and quantification limit (based on the standard deviation of the blank) (59)III.3.5.Separation techniques (59)III.3.5.1. Thin-layer chromatography (2.2.27.) (59)III.3.5.2. Liquid chromatography (2.2.29.) (60)III.3.5.3. Gas chromatography (2.2.28.) (62)III.3.6.Semi-micro determination of water (2.5.12.) (63)III.3.7.Volumetric titrations (2.5.11. - 2.2.19. - 2.2.20.) (64)III.3.8.Peptide identification by nuclear magnetic resonance spectrometry (2.2.64.) (66)I.INTRODUCTIONI.1.PURPOSE OF THE GUIDEThis document is a guide for the authors of monographs and also a means of communicating the principles for the elaboration of monographs to the users of the European Pharmacopoeia (Ph. Eur.), especially industry, licensing authorities and official medicines control laboratories. Since the principles applied and guidance given for the elaboration of monographs should be the same as those applied by licensing authorities, the Technical Guide may also serve as a guideline in the elaboration of specifications intended for inclusion in licensing applications.It is necessary to bear in mind that a monograph will be a mandatory standard and must be applicable in licensing procedures in all Member States of the Convention on the Elaboration of a European Pharmacopoeia.I.2.TEST PROCEDURESThe methods chosen for the identification tests, purity tests and assay(s) constituting the bulk of a pharmacopoeial monograph are preferably those already described and utilised in the Ph. Eur.. In this context, the author of a monograph is referred not only to the General Methods of the Ph. Eur.. but also to published monographs on similar materials. The above considerations aim at ensuring a reasonable degree of harmonisation within the Ph. Eur. and they only apply in cases where the methods are found to be adequate for the specific purposes. However, due attention is also to be paid to the development of new methods that offer significant improvements in terms of sensitivity, precision, accuracy or discriminating power (selectivity).Methods included in monographs must be validated as described in the chapter on analytical validation and other relevant specific chapters of this guide. Validation reports are provided to the EDQM but are not published or otherwise provided to users.The test procedures included in a monograph should be verified in 2 or more laboratories and the laboratory reports on this verification should be provided to the EDQM to ensure future traceability.The instructions describing any method of analysis cover all factors that can influence the results and that are deemed essential to enable an experienced analyst working according to acknowledged laboratory practices, yet without necessarily having any prior knowledge of the investigation in question, to perform the analysis. Variations in the description of similar methods are to be avoided.If an analytical procedure is expected to be used generally or if it requires a lengthy description and is used more than once, it may be proposed for inclusion in the general chapters of the Ph. Eur., to be referred to in the individual monographs. The methods are prescribed on the scale conventionally applied in the Ph. Eur. except in cases where for reasons of availability of the material to be analysed, or because of its toxicity or its cost, work on a small scale would be advantageous.I.3.EQUIPMENTIf the equipment utilised for a method of analysis is not generally available in the States party to the European Pharmacopoeia Convention, it must be possible to have it constructed according to its description in the Ph. Eur.I.4.QUANTITIESIn prescribing the quantities, i.e. masses and volumes, of substances, reagents, and solvents to be taken for identifications, tests and assays, it is the practice of the Ph. Eur. to indicate the accuracy with which they are to be measured (see General Notices). It is therefore necessary to take this aspect into consideration when drafting pharmacopoeial texts.As guidance to minimise errors in the preparation of analytical solutions, Table 1, giving estimations of the relative uncertainty, is to be consulted.In order to avoid either the use of extremely low amounts or an unnecessarily large expenditure of solvents, a dilution series will often have to be prescribed for the preparation of dilute solutions used particularly for spectrophotometric measurement. In this context not all combinations of (usually 2 or 3) dilution steps will contribute equally to the random error of the dilution procedure. If critical for the purpose, the optimal dilution is prescribed in consideration of the relative errors (capacity tolerance divided by nominal volume) associated with the various sizes of volumetric pipettes and volumetric flasks commonly used for these operations (taking the usual formula: square root of the sum of the squares of individual relative errors, to estimate the relative dilution error).Tables giving the optimal number and nature of dilution steps needed to achieve a given dilution ratio, based upon given specifications for the capacity tolerances of volumetric glassware, are available in the literature. For guidance see Table 2 (it is to be noted that these factors do not include reading errors).Table 1 – Relative uncertainties in the preparation of analytical solutionsConcentration to be prepared Preparation of solution Percentage relative uncertainty Mass Volume Total10 g/1000 mL 10 g/1000 mL1 g/100 mL0.5 g/50 mL0.25 g/25 mL0.1 g/10 mL< 0.010.020.040.080.020.050.120.170.230.500.050.120.170.240.541g/1000 mL1 g/1000 mL0.5 g/500 mL0.25 g/25 mL100 mg/100 mL50 mg/50 mL10 mg/10 mL0.020.040.080.20.42.00.050.070.230.120.170.500.050.080.240.230.432.060.1 g/1000 mL 100 mg/1000 mL50 mg/500 mL25 mg/250 mL10 mg/100 mL5 mg/50 mL1 mg/10 mL0.20.40.82.04.020.00.050.070.080.120.170.500.210.410.802.04.020.00.01 g/1000 mL 10 mg/1000 mL5 mg/500 mL1 mg/100 mL2.04.020.00.050.070.122.04.020.0An uncertainty of 0.2 mg for the weighing procedure has been assumed for the calculations of the percentage relative uncertainties.Table 2 – Relative errors for dilution with analytical glassware (pipettes P/flasks F)Concentration ratio No. of steps Step 1 Step 2 Relative errorP F P F1/2 1 25 50 0.161/2.5 1 20 50 0.181/5 1 20 100 0.171/10 1 25 250 0.131/12.5 1 20 250 0.161/30 1 15 500 0.201/50 1 20 1000 0.151/100 1 25 250 25 250 0.181/125 2 20 250 25 250 0.201/160 2 25 1000 25 100 0.191/200 2 25 500 25 100 0.181/250 2 20 250 25 500 0.201/400 2 25 250 25 1000 0.181/500 2 20 500 25 500 0.201/1000 2 20 1000 25 500 0.20Adapted from R.B. Lam and T.L. Isenhour, Minimizing relative error in preparation of standard solutions by judicious choice of volumetric glassware, Analytical Chemistry, 1980, 53, 1158-1161.I.5.REAGENTSWhen the quality of a reagent substance in one or more respects is critical for its intended use, it must be carefully defined, when necessary by prescribing appropriate tests to demonstrate its suitability. Normally, analytical grade reagents are employed in which case it is sufficient to give the name of the reagent, the CAS number and its formula.Whenever possible, the reagent substances, reagent solutions, volumetric solutions and standard solutions for limit tests already described in the chapter 4.Reagents of the Ph. Eur. are to be employed. Simple solutions of reagent substances or solutions that are prepared for use on a single occasion are to be described in the monograph itself.The use of reagents that are acknowledged to be extremely toxic or otherwise hazardous (e.g. carcinogenic), is to be avoided, especially in circumstances where their dangerous properties are difficult to control, e.g. when handled as fine powders or in spray reagents. The use of those substances that are prohibited or restricted in one or more of the States party to the European Pharmacopoeia Convention is also to be avoided (e.g. mercury containing reagents, REACH regulation annex XIV).MERCIAL NAMESCommercial names are given systematically as footnotes in draft monographs for chromatography columns/plates and based on usefulness for the analysts in other cases (e.g. test kits, reagents that are available from a single supplier, etc.). Commercial names are not included in the text published in the Ph. Eur. but are transferred to the EDQM Knowledge Database after adoption of the monograph.I.7.REFERENCE STANDARDSThe policy and procedures regarding reference standards are described for information in general chapter 5.12.Reference standards. Procurement, establishment, storage and monitoring of reference standards are the responsibility of the EDQM. Many reference standards, notably impurity standards, are available only in limited quantities, and the amount prescribed for preparation of solutions must be kept to a minimum. Before publication of a monograph in Pharmeuropa, the required quantities of reference standards should be supplied to the EDQM, who will advise on the best strategy for optimising the use of substances that are available in limited quantities (for example, preparation of a spiked substance rather than supply of the single impurity). The aim of the EDQM is to present the reference standards for adoption at the same time as the monograph or, failing that, by the time of publication at the very latest.For IR identification, preference is given to chemical reference substances over reference spectra, except in special cases, for example where provision of a reference substance entails practical difficulties.II.MONOGRAPH ON A SUBSTANCE FOR PHARMACEUTICAL USE Monographs are based on the specifications for substances used in medicinal products approved in Member States. When a monograph is added to the work programme, enquiries are made by the EDQM to identify manufacturers of such substances and all data received is taken into account for preparation of the monograph. Interested parties should be invited to participate in the elaboration of the monograph before publication in Pharmeuropa, since the 3-month public period will often be too short for all interested parties to check the draft monograph.Prior to the preparation of any monograph, it is essential to gather as much information as possible on the substance in question.In particular it is necessary to ascertain:•whether the substance is of natural, synthetic or semi-synthetic origin;•whether the substance is a mixture or a single entity;•the method(s) of preparation in detail;•whether there are different crystalline forms, since the properties of the substance may vary in accordance with this parameter;•whether both an enantiomer as well as the racemate or other mixtures of enantiomers are available;•whether different hydrates are available;•whether different entities (acid, base, salt, etc) are available.The Ph. Eur. and other relevant documents on the state of work must be consulted to see if monographs on similar substances exist or are being elaborated. If this is the case, it is important to ensure that similar monographs follow the same approach unless there are good reasons to deviate, e.g. developments in analytical techniques.When a substance exists both in a water-free form and in the form of (a) hydrate(s) with different water contents, and if all these forms are used, they are normally treated as individual substances requiring separate monographs. The same rule applies for other solvates.Substances that are to be described in a monograph may be members of a group of very similar substances (family). This holds true especially for excipients such as macrogols. A master monograph (family monograph) is to be drafted clearly stating the attributes common to all members of the family and that can be used to identify single members of the family.All active substances and excipients described in the Ph. Eur. are subject to the provisions of the general monograph Substances for pharmaceutical use (2034).II.1.TITLEThe International Nonproprietary Name (INN) established by the World Health Organization should be used wherever it is available; it is supplemented as appropriate by the name of the anion or cation and by the degree of hydration. Anions and cations are indicated as “mono-, di-, tri-, etc.”, as appropriate.The following rules apply for the degree of hydration.•In the case of a well-defined hydrate, “mono-, di-, tri-, etc…hydrate” is added to the title whereas if the monograph covers more than one degree of hydration, the general term “hydrate” is used. In the latter case, a sentence is added to the DEFINTION section (see chapter II.2). For monographs published prior to the 9th edition of the Ph. Eur., retrospective introduction of the degree of hydration in titles would only be made after careful consideration.•As of the 9th edition of the Ph. Eur, monographs referring to “anhydrous” substances will no longer specify this in their title with the exception of a few monographs where the information has a recognised added value and/or is used in common scientific language(e.g. Ethanol, anhydrous).•For monographs covering substances that can be either water-free or with, a defined or variable, degree of hydration, no mention will be added to the title. This information will be supplemented in the DEFINITION section of the monographs (see part II.2.).Where a substance is used in approved medicinal products for veterinary use only in Member States, “for veterinary use” is included in the title.II.2.DEFINITIONThe chemical structure must be ascertained with the greatest possible care in order to establish the exact:•graphic formula;•empirical formula and relative molecular mass. The latter is calculated as follows: first, the relative atomic masses, or multiples thereof, are added together using all thefigures of the International Table of Relative Atomic Masses; the total is then roundedoff to 4 significant figures if the initial digit is 1, 2, 3, 4 or 5, or to 3 significant figures ifthe initial digit is 6, 7, 8 or 9; the last figure is increased by 1 when the part rejectedexceeds one half-unit. When the part rejected is equal to or less than one half-unit, thelast figure taken is not modified;•chemical name. This involves investigating in particular:o the possible existence of isomers so as to be able to specify which isomer is used or, otherwise, to state that the product is a mixture of isomers;o in the case of an optical isomer, it is insufficient to take into account only the direction of the optical rotation. The absolute configuration is given by the R/Ssystem at the asymmetrical centre(s) or any other appropriate system (e.g., forcarbohydrates and amino acids);o ascertaining the state of hydration so as to distinguish clearly between the well-defined hydrates (mono-, di-, tri-, etc… hydrate) and the products that containvariable quantities of water. In the latter case, the term “x-hydrate” is introduced inthe chemical name.If the substance contains a variable quantity of water, or refers to both water-free and hydrate form, a sentence is added to the DEFINITION section to explain the exact scope of the monograph.Some chemical substances, particularly those obtained from raw materials of natural origin and substances produced by fermentation may not be easily separated from certain related substances (for instance, quinine salts). These may be treated as:• a chemical product when obtained in a very pure state and when they can be assayed by a physico-chemical method;• a substance accompanied by a certain proportion of related substances, giving an exact definition of the main component only (e.g. neomycin);• a mixture of several components, sometimes difficult to define, where an overall description may suffice (e.g. nystatin).Where applicable, the origin of the substance must be specified (name and strain of the organism from which the substance is derived). Where applicable, the monograph indicates that the substance is semi-synthetic and derived from a fermentation product [to clarify application of the general monograph Substances for pharmaceutical use (2034)].binationsIn therapeutics, more or less well-defined chemical combinations (for instance, theophylline- ethylenediamine) or even mixtures are sometimes used. In such cases, it is necessary to specify precisely each component of the combination or mixture, with its chemical structure and the proportion in which it is present.II.2.2.ContentThe substance described by a monograph is never a wholly pure substance but contains a limited proportion of impurities. The content is therefore an important part of the definition. Assay limits are specified between which the content must fall.In setting these limits for the active substance content, account is taken of:•the method of preparation, which determines the degree of purity that may be reasonably required;•the reproducibility and accuracy of the analytical method;•current batch data of about 10 production batches at release;•the evaluation of batch stability data;• a sufficient number of experimental results obtained on several batches (at least 3), if possible, of different origins and ages.For a non-specific assay by titrimetry the limits are set according to the table provided in part III.3.7 i.e. usually 99.0-101.0 %. Some monographs still include an assay by UV-Vis spectrophotometry usually bearing wider limits.For a specific assay using a separation technique (for example, liquid or gas chromatography), the upper assay limit is normally 102.0 %; the lower assay limit will take any necessary account of the impurities present based on the available batch data and approved specifications. It may therefore be lower than 98.0 %.assay, or when it contains only a very low proportion of impurities interfering with the assay, the results of the assay can be used directly. It will then be stated that: “[the substance] contains not less than x per cent and not more than the equivalent of y per cent (at least 100.5 %, but often a little more) of [chemical definition of the pure product]”. The content of the substance is usually expressed with reference to the anhydrous or dried substance. According to the general monograph Substances for pharmaceutical use (2034) the content of residual solvent is taken into account for calculation of the assay content of the substance, therefore no reference in the DEFINITION section of the individual monograph is made.In cases where the water content is high (e.g. in the case of disodium phosphate dodecahydrate), limits of content may be expressed with reference to the hydrate form of the substance, taking into account the molecular mass of the hydrate form (only for well-defined hydrates) or with reference to the substance on the anhydrous/dried basis in combination with determination of water content/loss on drying.When the substance to be examined contains a relatively large proportion (a few %) of impurities, which are determined at the same time as the active ingredient, an appropriate wording is to be used (for instance, in the case of quinine salts: “x per cent of total alkaloid salts, expressed as quinine salts”).Exceptionally reference is made to only a part of the molecule or to an element (for example assay of magnesium oxide in light magnesium carbonate or assay of magnesium in magnesium stearate).In the case of antibiotics determined by microbiological assays, the active ingredient content is expressed in International Units, where these exist, and only a minimum value is given.See also under part II.6.II.3.CHARACTERSAs defined in the General Notices, statements under the heading CHARACTERS are not to be interpreted in a strict sense and are not regarded as analytical requirements. The principal items that may be referred to under this heading are the following.II.3.1.AppearanceThis description will normally embrace colour and physical form. The term “white” is not used without qualification since, if viewed against a standard white material, very few pharmaceutical materials will appear truly white. It is, of course, not intended that such a comparison be made but experience shows that certain users of the Ph. Eur. may insist on doing so as part of a purchasing contract. The term “white or almost white” is used instead. Where positive colours are to be described, this is done in terms of primary colours or combinations of primary colours.Colour: the following descriptive terms are used: black, orange, blue, pink, brown, red, colourless, violet, green, white/almost white, grey, yellowCompound terms may be used:English Frenchgreenish-blue bleu-vertbluish-green vert-bleuviolet-red rouge-violetreddish-violet violet-rougebrownish-red rouge-brunreddish-brown brun-rougeIt can be noted that in English, the dominant is placed second, whereas in French, it is placed first. Expressions such as lemon-yellow, buff, salmon-pink are to be avoided; standard dictionaries give equivalents for such terms as spectral colours with suitable qualifiers (for example, buff is described as “dull yellow”). The following adjectives are also used; light, slight, fluorescent, intense, pale, dull, deep, dark.It is to be noted that the allowed colours and colour combinations also apply to the description of the colour changes of indicators when used in acid/alkalinity tests or in titrimetric assay procedures.II.3.2.TasteThe taste is not to be taken into consideration.II.3.3.OdourIn general, no reference is made to odour. In particular no reference to odour is made for those materials that would constitute a hazard if inhaled. Mention of odour in other cases must be justified.II.3.4.SolubilityA method recommended for the estimation of solubility is given in general chapter5.11.Characters section in monographs. All solubilities are quoted in the general terms defined in the General Notices. Solvents quoted are normally confined to water, an alcohol and a lipophilic solvent (e.g. water, ethanol, heptane). Solubilities in chloroform and ether are not mentioned and the use of hexane is discouraged. In special cases the solubility of different samples of a material may vary rather considerably even though their composition is still within the limits set by the monograph. The solubilities in the solvents thereby affected are then given to cover more than one solubility class, e.g. “sparingly soluble to soluble in...”. The solubilities or miscibilities in other solvents with which the material is often combined in practice such as fatty oils, etc., may also be mentioned. In some cases it may be useful to specify solubility in alkalis or acids and, particularly in cases of materials that are very insoluble in the above-mentioned solvents, a special solvent may be indicated, e.g. dimethylformamide or dimethyl sulfoxide. It is not necessary to specify the solubility in every solvent that is used in performing the tests of the。
总局关于发布第一批2017年45号文20170317

1-40
佐匹克隆片
Zopiclone Tablets/Imovane
is France
原研进口
1-41
佐匹克隆片
Zopiclone Tablets/Imovane
3.75mg
片剂
Sanofi-Aventis France
欧盟上市(产地:法国 ;上市国家:法国)
片剂
GlaxoSmithKline
美国橙皮书
1-5
头孢呋辛酯片
Cefuroxime Axetil Tablets/Zinacef
按头孢呋辛(C16H16N4O8S)计0.25g
片剂
Glaxo Wellcome UK Limited
原研进口
1-6
辛伐他汀片
Simvastatin Tablets/Zocor
原研进口
1-17
醋酸甲羟孕酮片
Medroxyprogesterone Acetate Tablets/Provera
0.1g
片剂
Pfizer SA/NV
原研进口
1-18
醋酸甲羟孕酮片
Medroxyprogesterone Acetate Tablets/Provera
0.25g
片剂
Pfizer Italia s.r.l
10mg
片剂
Merck Sharp & Dohme (Australia) Pty. Ltd.
原研进口
1-7
辛伐他汀片
Simvastatin Tablets/Zocor
20mg
片剂
Merck Sharp & Dohme B.V.
原研进口
1-8
盐酸氨溴索片
特碳17-40B-PD 产品数据单据说明书

Product Data SheetTrigonox 17-40B-PD Butyl 4,4-di(tert-butylperoxy) valerateTrigonox® 17-40B-PD is a 40% formulation on an inert carrier system in powder form.CAS number995-33-5EINECS/ELINCS No.213-626-6TSCA statuslisted on inventoryMolecular weight334.5Active oxygen contentperoxide9.57%Concentration3.73-3.92%SpecificationsAppearance Off-white powderAssay39.0-41.0 %ApplicationsTrigonox® 17-40B-PD is a bifunctional peroxide which is used for the crosslinking of natural rubber and synthetic rubbers, as well as polyolefins. Safe processing temperature: 125°C (rheometer ts2 > 20 min.). Typical crosslinking temperature: 160°C (rheometer t90 about 12 min.).Thermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT60°CMethod The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts Max.30°CNote When stored under the recommended storage conditions, Trigonox® 17-40B-PDwill remain within the Nouryon specifications for a period of at least 6 months afterdelivery.Packaging and transportThe standard packaging is a cardboard box for 25 kg peroxide formulation. Both packaging and transport meet the international regulations. For the availability of other packed quantities consult your Nouryon representative. Trigonox®17-40B-PD is classified as Organic peroxide type E; solid, Division 5. 2; UN 3108.Safety and handlingKeep containers tightly closed. Store and handle Trigonox® 17-40B-PD in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalines and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Trigonox® 17-40B-PD. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsMethane, Carbon dioxide, Acetone, tert-Butanol, n-Butyl propionate,All information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® is a registered trademark of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-6-30© 2022Polymer crosslinking Trigonox 17-40B-PD。
2010年禁用清单(国际标准)

附件32010年禁用清单 (国际标准)2010年禁用清单 (国际标准)本清单自2010年1月1日起施行。
禁用清单的正式文本由世界反兴奋剂机构保存,并以英文和法文发布。
如果英文本与法文本存在不一致的地方,应以英文本为准。
2010年禁用清单世界反兴奋剂条例2010年1月1日起生效除禁用物质S1、S2.1-S2.5、S4.4、S6.a以及禁用方法M1、M2、M3以外,所有禁用物质均视为“特定物质”禁用物质S1. 蛋白同化制剂蛋白同化制剂禁用。
1.蛋白同化雄性激素类固醇(AAS)a.外源性*蛋白同化雄性类固醇包括:1-androstendiol (5α-androst-1-ene-3β,17β-diol )雄-1-烯二醇(5α-雄-1-烯-3β,17β-二醇)1-androstendione (5α-androst-1-ene-3,17-dione)雄-1-烯-二酮(5α-雄-1-烯-3,17-二酮)bolandiol (19-norandrostenediol)勃雄二醇(19-去甲雄烯二醇)bolasterone勃拉睾酮(双甲睾酮)boldenone勃地酮(宝丹酮)boldione (androsta-1,4-diene-3,17-dione)1,4-雄二烯-3,17-二酮calusterone卡芦睾酮(7β,17α-双甲睾酮)clostebol氯司替勃(氯斯太宝)danazol (17α-ethynyl-17β-hydroxyandrost-4-eno[2,3-d]isoxazole) 达那唑dehydrochlormethyltestosterone (4-chloro-17β-hydroxy-17α-methylandrosta-1,4-dien-3-one)脱氢氯甲基睾酮desoxymethyltestosterone (17α-methyl-5α-androst-2-en-17β-ol)去氧甲基睾酮drostanolone屈他雄酮(羟甲雄酮)ethylestrenol (19-nor-17α-pregn-4-en-17-ol)乙雌烯醇fluoxymesterone氟甲睾酮formebolone甲酰勃龙(醛甲宝龙)furazabol (17β-hydroxy-17α-methyl-5α-androstano[2,3-c]-furazan) 夫拉扎勃(呋咱甲氢龙)gestrinone孕三烯酮4-hydroxytestosterone (4,17β-dihydroxyandrost-4-en-3-one)4-羟基睾酮mestanolone美雄诺龙mesterolone美睾酮metenolone美替诺龙methandienone (17β-hydroxy-17α-methylandrosta-1,4-dien-3-one)美雄酮methandriol美雄醇methasterone (2α, 17α-dimethyl-5α-androstane-3-one-17β-ol)2α,17α-二甲基-5α-雄烷-3-酮-17β-醇methyldienolone (17β-hydroxy-17α-methylestra-4,9-dien-3-one)17α-甲基-17β-羟基雌-4, 9-二烯-3-酮methyl-1-testosterone (17β-hydroxy-17α-methyl-5α-androst-1-en-3-one)甲基-1-睾酮methylnortestosterone (17β-hydroxy-17α-methylestr-4-en-3-one)甲基去甲睾酮methyltestosterone甲睾酮metribolone (methyltrienolone, 17β-hydroxy-17α-methylestra-4,9,11-trien-3-one) 17α-甲基-17β-羟基雌-4,9,11-三烯-3-酮mibolerone米勃龙nandrolone诺龙19-norandrostenedione (estr-4-ene-3,17-dione)19-去甲雄烯二酮norboletone诺勃酮(双乙基诺龙)norclostebol诺司替勃(去甲氯司替勃)norethandrolone诺乙雄龙(乙基诺龙)oxabolone环戊丙羟勃龙(羟勃龙、氧宝龙)oxandrolone氧雄龙(氧甲氢龙)oxymesterone羟甲睾酮oxymetholone羟甲烯龙prostanozol (17β-hydroxy-5α-androstano[3,2-c] pyrazole)17 -羟基-5α-雄烷[3,2-c]吡唑quinbolone奎勃龙stanozolol司坦唑醇stenbolone司腾勃龙1-testosterone (17β-hydroxy-5α-androst-1-en-3-one)1-睾酮tetrahydrogestrinone (18a-homo-pregna-4,9,11-trien-17β-ol-3-one) 四氢孕三烯酮trenbolone群勃龙(追宝龙)以及其他具有相似化学结构或相似生物作用的物质。
Deore XT PD-T8000 使用说明书
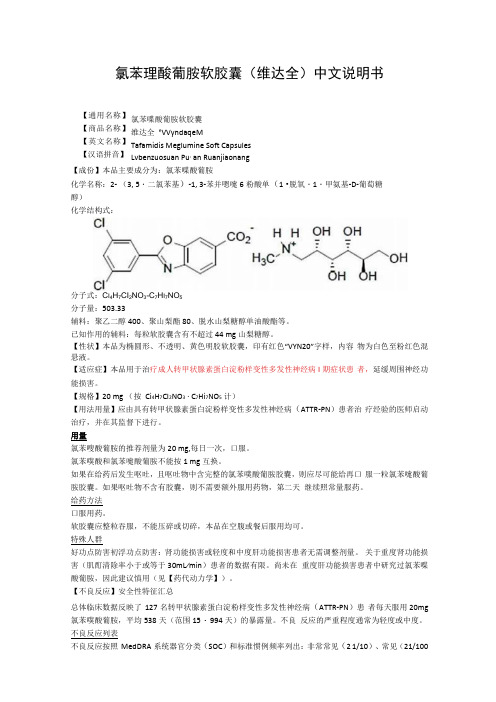
(English)DM-TRPD001-02Dealer's ManualDEORE XTPD-T8000CONTENTSIMPORTANT NOTICE (3)TO ENSURE SAFETY (4)LIST OF TOOLS TO BE USED (7)INSTALLATION (9)Cleat types (9)Attaching the cleats (9)Adjusting cleat position (10)Waterproof seal (11)Mounting the pedals on the crank arms (11)ADJUSTMENT (13)Adjusting the gripping force (13)Adjusting the spring tension of the pedals (14)MAINTENANCE (16)Axle unit (16)Replacement of the body cover (18)•This dealer’s manual is intended primarily for use by professional bicycle mechanics.Users who are not professionally trained for bicycle assembly should not attempt to install the components themselves using the dealer’s manuals.If any part of the information on the manual is unclear to you, do not proceed with the installation. Instead, contact your place of purchase or a local bicycle dealer for their assistance.•Make sure to read all instruction manuals included with the product.•Do not disassemble or modify the product other than as stated in the information contained in this dealer’s manual.•All dealer’s manuals and instruction manuals can be viewed on-line on our website ().•Please observe the appropriate rules and regulations of the country, state or region in which you conduct your business as a dealer.•For child safety, make sure the child uses this product correctly by following the instructions below. Both guardians and children should gain an adequate understanding of the content of this manual. Failure to follow the provided instructions may lead to serious injury.•Be sure to follow the instructions provided in the manuals when installing the product.It is recommended to use genuine Shimano parts only. If parts such as bolts and nuts become loose or damaged, the bicycle may suddenly fall over, which may cause serious injury.In addition, if adjustments are not carried out correctly, problems may occur, and the bicycle may suddenly fall over, which may cause serious injury.•Be sure to wear safety glasses or goggles to protect your eyes while performing maintenance tasks such as replacing parts.•After reading the dealer's manual thoroughly, keep it in a safe place for later reference.Be sure to also inform users of the following:If the warnings below are not followed, your shoes may not come out of the pedals when you intend or they may come out unexpectedly or accidentally, and severe injury may result.Descriptions regarding SPD pedals•SPD pedals are designed to be released only when intended. They are not designed to be released automatically when you have fallen off the bicycle.•Before attempting to ride with these pedals and shoes, make sure you understand the operation of the engagement/release mechanism for the pedals and cleats (shoes).•Before you attempt to ride with these pedals and shoes, apply the brakes, then place one foot on the ground and practice engaging and releasing each shoe from its pedal until you can do so naturally and with minimal effort.•Ride on level ground first until you become accustomed to engaging and releasing your shoes from the pedals.•Before riding, adjust the spring tension of the pedals to your liking. If the spring tension of the pedals is low, the cleats may become accidentally released and you may lose balance and fall off the bicycle. If the spring tension of the pedals is high, the cleats cannot be easily released.•When riding at low speed or when there is a possibility that you might need to stop riding, (for example, when doing a U-turn, nearing an intersection, riding uphill or turning a blind curve), release your shoes from the pedal beforehand so that you can quickly put your feet onto the ground at any time.•Use a lighter spring tension for attaching the pedal cleats when riding in adverse conditions.•Keep cleats and bindings out of dirt and debris to ensure proper engagement and release.•Remember to check the cleats periodically for wear. When the cleats are worn, replace them, and always check the spring tension before riding and after replacing the pedal cleats.•Use only SPD shoes with this product. Other types of shoe may not release from the pedals, or may release unexpectedly.•Use only Shimano cleats (SM-SH51/SM-SH56) and make sure that the mounting bolts are tightened securely to the shoes.Descriptions regarding flat pedals•If the gripping force between the shoes and the pedals (the force which stops the shoes from slipping sideways) is insufficient use long pins to increase the gripping force. This will increase the gripping force. If the gripping force on the shoes is increased, you will not be able to disengage your feet from the pedals by sliding them sideways unless you first raise your feet from the pedals. To avoid falling off the bicycle and suffering serious injury, practice engaging and disengaging one foot from the pedal with the other foot firmly on the ground until you become used to the operation. If you cannot get used to the operation, use short pins instead.•Because the pins are long, they may cause injury if they come into direct contact with your skin. Be sure to wear clothing and protective gear which is suitable for the way in which the bicycle is to be used.•Do not continue riding the bicycle if the reflectors are dirty or damaged, otherwise it becomes more difficult for oncoming vehicles to see you.Be sure to also inform users of the following:•Check that there is no looseness in any joints or connections before riding the bicycle.•Check that there is no looseness in the cleats before riding the bicycle.•If pedaling performance does not feel normal, check the bicycle once more.•If you experience any trouble with the rotating parts of the pedal, the pedal may require adjustment. Consult a dealer or an agency.•Be sure to retighten the crank arms and pedals at periodic intervals at the place of purchase or a bicycle dealer.•If you are unsure of how to replace the short and long pins on the pedals, consult a dealer or an agency.•Products are not guaranteed against natural wear and deterioration from normal use and aging.•For maximum performance we highly recommend Shimano lubricants and maintenance products.The actual product may differ from the illustration because this manual is intended mainly to explain the procedures for using the product.The following tools are needed for installation, adjustment, and maintenance purposes.Cleat typesAttaching the cleatsSet the cleat on the bottom of each shoe as shown in the illustration, and then tighten the cleat mounting bolts, temporarily.(A) Rubber cover for cleat mounting holes(B) SPD shoeThis step may not be necessary depending on the type of shoe.(A) Cleat nut(B) SocklinerThis step may not be necessary depending onthe type of shoe.(A) 4mm hexagon wrench(B) Cleat mounting bolt(C) Cleat adapter(D)CleatWaterproof seal(A) Waterproof seal(B)Socklinershoes that require this step to be carried out.Mounting the pedals on the crank arms(A) 8mm hexagon wrenchAdjusting the gripping forceLong pins and shorts pins are included with this product. Adjust the gripping force between the pedal and the shoe sole by using either short pins or long pins in all locations.(A) Short pin (B) Long pin(C) 2mm hexagon wrench•If the gripping force between the shoesand the pedals (the force which stops the shoes from slipping sideways) is insufficient use long pins to increase the gripping force. This will increase the gripping force. If the gripping force on the shoes is increased, you will not be able todisengage your feet from the pedals by sliding them sideways unless you first raise your feet from the pedals. To avoid falling off the bicycle and suffering serious injury, practice engaging and disengaging one foot from the pedal with the other foot firmly on the ground until you become used to the operation. If you cannot get used to the operation, use short pins instead.•Because the pins are long, they may causeinjury if they come into direct contact with your skin. Be sure to wear clothing and protective gear which is suitable for the way in which the bicycle is to be used.Adjusting the spring tension of the pedals•The spring tension of the pedals can be adjusted by turning the adjustment bolt. •Clicking the adjustment bolt changes the tension one step. There are four clicks per turn. •The adjustment bolt is located at the rear of each binding, resulting in two positions in total.•Adjust the spring force to the optimal cleat holding force as needed when releasing the cleats from the bindings.•Equalize the cleat holding forces at all positions by checking the adjustment plate position and counting the number of turns of the adjustment bolts. •Turning the adjustment bolt clockwise increases the spring tension, and turning it counterclockwise decreases it.(w) Decrease(x) Increase (y) Weakest position (z) Strongest position (A) Adjustment bolt (B) Adjustment plate (C) 3mm hexagon wrench•In order to prevent accidental shoe releaseand ensure that release is possible when needed, make sure all spring tensions are properly adjusted.•If the cleats are not adjusted equally, it cancause the rider difficulty in engaging or releasing the pedals.The spring tensions for the right and left pedals should be adjusted so they are equal.•If the adjustment plate is at the strongestor the weakest position, do not turn the adjustment bolt any further.Axle unitAdjustment is required if the rotating parts are not functioning properly. Follow the procedure shown below.(A)Lock bush (B) Cone (C) Lock nutThe lock bush of the right pedal has a left-hand thread; the lock bush of the left pedal has a right-hand thread.•Right-hand thread: Black-colored (withoutslit)If the fitted lock nut is black-colored (without slit), the cone and the lock nuthave a right-hand thread.•Left-hand thread: Black-colored (with slit)If the fitted lock nut is black-colored (with slit), the cone and the lock nut have aleft-hand thread.•Adjust the cone so as to achieve a smoothrotation without looseness when the axleunit is set into the pedal.The rotating parts are fastened when the axle unit is set into the pedal. Adjust them slightlyloosely before setup.Apply grease to the extent that it does not flow out when the axle is set into the pedal (about 1.5g).(A) Rubber seal (B) Lock bush (C) Body cupReplacement of the body coverTighten the three screws equally.Please note: specifications are subject to change for improvement without notice. (English) © Aug. 2016 by Shimano Inc. HTR。
氯苯唑酸葡胺软胶囊维达全中文说明书
氯苯理酸葡胺软胶囊(维达全)中文说明书氯苯喋酸葡胺软胶囊维达全 e VVyndaqeMTafamidis Meglumine Soft CapsulesLvbenzuosuan Pu , an Ruanjiaonang【成份】本品主要成分为:氯苯喋酸葡胺化学名称:2- (3, 5・二氯苯基)-1, 3-苯并嗯嚏6粉酸单(1 •脱氧・1・甲氨基-D-葡萄糖醇)化学结构式:分子式:C I 4H 7CI 2NO 3-C 7H I 7NO 5分子量:503.33辅料:聚乙二醇400、聚山梨酯80、脱水山梨糖醇单油酸酯等。
已知作用的辅料:每粒软胶囊含有不超过44 mg 山梨糖醇。
【性状】本品为椭圆形、不透明、黄色明胶软胶囊,印有红色“VYN20”字样,内容 物为白色至粉红色混悬液。
【适应症】本品用于治疗成人转甲状腺素蛋白淀粉样变性多发性神经病I 期症状患 者,延缓周围神经功能损害。
【规格】20 mg (按 Ci 4H 7CI 2NO 3 ∙ C 7Hi 7NO 5 计)【用法用量】应由具有转甲状腺素蛋白淀粉样变性多发性神经病(ATTR-PN )患者治 疗经验的医师启动治疗,并在其监督下进行。
用量 氯苯嗖酸葡胺的推荐剂量为20 mg,每日一次,口服。
氯苯噗酸和氯苯嚏酸葡胺不能按1 mg 互换。
如果在给药后发生呕吐,且呕吐物中含完整的氯苯噗酸葡胺胶囊,则应尽可能给再口 服一粒氯苯嚏酸葡胺胶囊。
如果呕吐物不含有胶囊,则不需要额外服用药物,第二天 继续照常量服药。
给药方法口服用药。
软胶囊应整粒吞服,不能压碎或切碎,本品在空腹或餐后服用均可。
特殊人群好功点防害初浮功点防害:肾功能损害或轻度和中度肝功能损害患者无需调整剂量。
关于重度肾功能损害(肌酊清除率小于或等于30mL∕min )患者的数据有限。
尚未在 重度肝功能损害患者中研究过氯苯喋酸葡胺,因此建议慎用(见【药代动力学】)。
【不良反应】安全性特征汇总总体临床数据反映了 127名转甲状腺素蛋白淀粉样变性多发性神经病(ATTR-PN )患 者每天服用20mg 氯苯噗酸葡胺,平均538天(范围15・994天)的暴露量。
2018年兴奋剂目录与2017年兴奋剂目录的区别

2018年兴奋剂目录与2017年兴奋剂目录的区别
2018年1月26日,国家体育总局、商务部、卫计委、海关总署及食药监总局发布了2018年兴奋剂目录,新增34个品种,删除了5个品种。
其中新增蛋白同化制剂品种5个,包括:3α-羟基-5α-雄甾-1-烯-17-酮、3β-羟基-5α-雄烷-17-酮、雄诺龙(双氢睾酮)、LGD-4033和RAD140;
新增肽类激素品种22个,包括AOD9604、CJC-1293、地洛瑞林、促红素受体激动剂类、生长激素释放肽类(GHRPs)、生长激素释放肽-1、GHRP-2(普拉莫瑞林(生长激素释放肽-2))、生长激素释放肽-3、生长激素释放肽-4、生长激素释放肽-5、生长激素释放肽-6、戈舍瑞林、生长因子类、生长因子调节剂类、生长激素片段类、生长激素释放因子类、人生长激素176-191、先天修复受体激动剂类、那法瑞林、肽类激素和激素调节剂类、胸腺肽-β 4 及其衍生物(如TB-500)、曲普瑞林;
新增刺激剂(含精神药品)品种1个,为1,3-二甲基正丁胺;
新增其他品种14个,包括:倍他米松、布地纳德、可的松、地夫可特、地塞米松、氟替卡松、氢化可的松、甲泼尼松龙、泼尼松龙、泼尼松、SR9009(3-[[(4-氯苄基)-[(5-硝基噻吩基-2-基)甲基]氨基]甲基]吡咯烷基-1-甲酸乙酯)、他莫瑞林、曲安西龙、妥洛特罗;
删除品种包括蛋白同化制剂品种1个双氢睾酮;肽类激素品种4个,包括促红素衍生物、促红素模拟肽类、缺氧诱导因子(HIF)稳定剂
类、普拉莫瑞林(生长激素释放肽-2)(GHRP-2);。
蓝耳BlueParrott B650-XT S650-XT用户手册说明书

BlueParrottB650-XT/S650-XT User Manual© 2021 GN Audio A/S. All rights reserved. Jabra ® is a trademark of GN Audio A/S. The Bluetooth ® word mark and logos are registered trademarks owned by the Bluetooth SIG, Inc. and any use of such marks by GN Audio A/S is under license.Declaration of Conformity can be found on Made in ChinaMODEL: OTE960Contents1. Welcome (5)2. Headset overview (6)2.1 BlueParrott B650-XT2.2 BlueParrott S650-XT2.3 Included accessories3. How to wear (9)3.1 Adjusting the headband tension3.2 Switch from stereo to mono sound4. How to charge (12)5. How to connect (14)5.1 Power on5.2 Power off5.3 Pair using Bluetooth5.4 Pair using NFC6. How to use (17)6.1 Calls6.2 The BlueParrott Button TM6.3 Voice control TM6.4 Active Noise Cancellation6.5 HearThrough6.6 Multiple call handling6.7 Multipoint mode6.8 How to reset7. BlueParrott app (26)8. Support (27)8.1 FAQ8.2 How to care for your headset8.3 How to clean your headset1. WelcomeThank you for using the BlueParrott B650-XT or S650-XT. We hope you will enjoy it! BlueParrott B650-XT/S650-XT features• Crystal-clear calls anywhere. Ultra-noise-cancelling mic removes 96% of background noise.*• The best of both worlds. Converts easily from stereo to mono sound with a removable second earcup.**• Silence the road. Powerful Active Noise Cancellation (ANC) helps you focus on what you’re listening to.• A battery that runs and runs. Get up to 36 hours of talk time on a single charge.• Built for life on the road. With IP54-rated protection against dust and water, theS650-XT is truly roadworthy.• Personalize your experience. Speed dial, mute, or Push-to-Talk, with the customizable BlueParrott Button™.• Hands on the wheel. Eyes on the road. 100% hands-free voice control lets you direct the headset with just your voice.* Verified by independent labs** Available for S650-XT out of the box. Convert B650-XT to stereo with the second earcup, which is available as accessory.2. Headset overview2.1 BlueParrott B650-XT*The BlueParrott Button is customizable using the BlueParrott appmicrophoneTM *(hold)(hold)(side button)2.2 BlueParrott S650-XT*The BlueParrott Button is customizable using the BlueParrott appmicrophoneTM *(hold)(hold)(side button)NFC Zone2.3 Included accessoriesSecond earcup for stereo sound (included with S650-XT)USB-C cable3.5mm jack cable(included with S650-XT)Car charger(included with B650-XT)MicrophonewindscreensCarry case(included withS650-XT)3. How to wearThe headset can be worn with the microphone on the left or right side. Adjust the headband for best fit, and position the flexible microphone one finger width from the corner of your mouth. Do not wear the headset without the ear cushions.3.1 Adjusting the headband tensionIf the headband feels too tight or too loose, it is possible to adjust the tension of the headband for a more comfortable fit.To adjust the tension of the headband, grip and flex it ONLY as shown in the diagrams below. Flex inward to increase tension. Flex outward to reduce the tension.Use mild to moderate force when flexing the headband. Repeat the process until desired tension comfort level is attained.Only grip and flex theheadband betweenthe two arrows in thisdiagramFlex inward to increase tension3.2 Switch from stereo to mono sound Press both release buttons on the earcup and firmly pull the earcup down to remove it from the headset. After removing the second earcup, the headset is ready to be worn.For the B650-XT headset, a stereo earcup can be purchased at /accessories4. How to chargeTo charge the headset, connect the USB-C charging cable to the headset’s charging port. It is recommended to charge the headset using the supplied charging cable.It takes up to 192 minutes to fully charge the battery.Low batteryChargingFully charged5. How to connect5.3 Pair using Bluetooth1. Ensure the headset is powered off.2. Press and hold the Multi-function button for approx. 6 seconds until you hear “Pair mode” and the LED flashes red and blue. The headset is now ready to pair.5.4 Pair using NFC1. Ensure your headset is powered on and that NFC is enabled on your smartphone.2. Place the NFC zone of your smartphone against the NFC zone of the headset until your smartphone confirms the connection. Please note that not all phones support NFCNFC Zone6.1 CallsAnswer call Press the Multi-functionAssistant (i.e. Siri,Google Assistant)button when not ona call*Transfer audio from headset to smartphone While on a call, press and hold (2 sec) the Volume down button to transfer to the call audio to your paired smartphone. Repeat to transfer the call audio back to your headset. This featue is phone dependant.Redial last number Press and hold (1 sec) the Volume down button until you heara beep.Mute/un-mutemicrophonePress the BlueParrottButton when on a call.Alternatively, pressand hold (2 sec) theVolume up button. Voice controlPress the BlueParrottButton when not on acall and say "What canI say?" for a list of voicecommands.6.2 The BlueParrott Button TMBy default, the BlueParrott Button mutes the microphone. However, the button can be configured for other functions, such as speedTo change the BlueParrott Button functionality, use the BlueParrott app for Android or iOS.6.3 Voice control TMThe headset can be controlled by giving it voice commands, such as "Answer" to answer an incoming call, or "Ignore" to reject an incoming callTo use more voice controls, tap the BlueParrott Button and say "Hello BlueParrott". You can then issue one of the commands below.• Pair Mode• Phone Command• Am I Connected?• Check Battery• Redial• Call Back• Play Music• Cancel• What can I say?6.4 Active Noise CancellationActive Noise Cancellation (ANC) counters noise by detecting and analyzing the pattern of incoming sound and then generating an anti-noise signal to cancel it out. As a result, you experience a drastically reduced level of6.5 HearThroughHearThrough enables you to pay attention to your surroundings and engage in conversation, when not on a call, without needing to remove the headset. The microphones pick up surrounding sounds and transmit them to the speakers.6.6 Multiple call handlingThe headset can accept and handle multiplecalls at the same time.Press the Multi-function button to end the call when on a callthe Multi-function button6.7 Multipoint modeThe headset can pair to 7 mobile devices and connect to two at once (Multipoint mode).To connect to two mobile devices, use the normal pairing process separately for each device (refer to section 5.3).The headset will monitor both mobile devices and enable you to answer a call on either one using the headset.If you are on a call and the other mobile device receives a call, you will hear a notification in the headset.6.8 How to resetResetting the headset clears the list of paired devices.1. Ensure the headset is powered on.2. Press and hold (10 sec) both the Volume up and Volume down buttons until you hear'Pair mode' and the LEDs flash red and blue.3. Re-pair the headset to your smartphone using the normal process (see page 14).7.BlueParrott appBlueParrott app Program your BlueParrott Button TM anywhere, anytime.Push-to-talk to your contacts on Dial2Do’s MySay network.Get the most out of your headsetwith firmware updates via the app.8. Support8.1 FAQView the FAQs at:• /supportpages/BlueParrott-B650-XT• /supportpages/BlueParrott-S650-XT8.2 How to care for your headset• To prevent the depletion of the battery lifetime or capacity, avoid storing the headset in hot or cold environments, such as a closed truck in summer or in winter conditions.• When exposed to water, allow the headset sufficient time to dry.• Do not store the headset for extended periods of time without recharging it (max. three months).8.3 How to clean your headset1. Apply normal dish soap and water to a cloth, making sure to wring out any excess liquid so the cloth is not dripping wet. Use soap and water only; do not use strong cleaning agents.2. Gently wipe the headset with the wet cloth, making sure the headset is clean with no layer of suds on the surface.3. Let the headset rest for at least one minute.4. Wipe the headset clean with a cloth dampened with water only.5. Allow the product to dry.。
Schafgarbe Tropfen酊剂

上市厂商
德国D HU 公 司 。
适应证
消 化 机 能 障 碍 , 食 欲 缺 乏 、 胀 感 和 胃肠 气 胀 。 如 饱
剂 量 与 用 法 硬 胶 囊 : 人 和 1 成 2岁 以 上 儿 童 每 日 2 3次 , ~
1 6 Hy oo i—a t u 7 p tneg sr ⑧R4 e 4滴 剂
警告
1 8 S h f ab r p e  ̄ 剂 7 c ag r eT o fn 酊
成分 1 0g含 洋 蓍 草 ( 5 1 0g 0 1: )0 。提 取 剂 :1 5 乙醇 。 3 .
每 日 3 6次 , 次 1 ~ 2 ~ 每 5 O滴 。
适应证
消 化 不 良( 胃肠 道 轻 度 痉 挛性 不 适 ) 食 欲 不 振 。 如 、
成 分 1 0mL含 山 楂 1mL、 桂 樱 1mL、 洲 夹 竹 桃 1 0 月 欧
mL、 爪 豆 1mL 以及 乙 醇 、 净 水 。 鹰 纯
每 次 2粒 ; 餐 前半 小 时 用 足 量 水 送 服 。 酊 剂 : 人 和 1 正 成 2岁
以 上 儿 童 每 日 3 , 次 2 滴 ; 前 半 小 时 服 。有 效 剂 量 中 次 每 O 餐
上 市厂 商 德 国 S o nn eg n公 司 。 h ee b re
mL、 根 木 3mL、 藜 芦 3 蛇 蒜 2mL、 寄 生 提 取 液 3 2mL。 欧 . 适 应 证 高 血 压 、 脉 硬 化 、 环 障 碍 、 眩 和 平 衡 障 碍 。 动 循 晕 剂量与 用法
服 用 1 次 , 次 5 1 滴 。 性 病 : 日 1 3次 , 次 5 1 2 每 ~ 0 慢 每 ~ 每 ~ 0 滴 。学 龄 儿 童 : 1 从 2岁 起 5 8 , 据上 述 方 案 给 药 。 ~ 滴 根 禁忌证 1 2岁 以下 儿 童禁 用 。
Adcetris (brentuximab vedotin) 商品说明书

Adcetris® (brentuximab vedotin)Document Number: IC-0004 Last Review Date: 2/06/2018Date of Origin: 02/28/2012Dates Reviewed: 06/2012, 09/2012, 09/2012, 12/2012, 03/2013, 06/2013, 09/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 05/2015, 08/2015, 11/2015, 02/2016, 05/2016, 08/2016, 11/2016, 02/2017, 05/2017, 08/2017, 11/2017, 02/2018I.Length of AuthorizationCoverage will be provided for six months and may be renewed.Treatment for cHL post-auto HSCT has a maximum of 16 cycles.II.Dosing LimitsA.Quantity Limit (max daily dose) [Pharmacy Benefit]:∙50 mg vial: 4 vials per 21 daysB.Max Units (per dose and over time) [Medical Benefit]:∙200 billable units per 21 daysIII.Initial Approval CriteriaCoverage is provided in the following conditions:∙Patient is 18 years or older; AND∙Patient is CD30-positive; AND∙Patient must not be receiving concomitant bleomycin; ANDClassical Hodgkin Lymphoma (cHL) †∙Used as single agent therapy; ANDo Patient is at high risk for relapse or progression as post-autologous hematopoietic stem cell transplant consolidation (auto-HSCT)†; ORo Patient with relapsed disease after failure of auto-HSCT or at least 2 prior multi-agent chemotherapy regimens who are not auto-HSCT candidates †; ORo Used as subsequent therapy for relapsed or refractory disease‡; ORo Used as maintenance therapy following high-dose therapy and autologous stem cell rescue (HDT/ASCR) for relapsed or refractory disease with high risk for relapse‡;OR∙Used as subsequent systemic therapy for relapsed or refractory disease in combination with cytotoxic chemotherapyNon-Hodgkin Lymphoma (NHL)∙Used as a single agent for relapsed or refractory disease as subsequent therapy for one of the following:-Systemic Anaplastic Large Cell Lymphoma (sALCL) †-Peripheral T-Cell Lymphoma (PTCL) ‡-Angioimmunoblastic T-cell Lymphoma (ATCL) ‡∙Breast-Implant Associated Anaplastic Large Cell Lymphoma (ALCL) ‡; ANDo Used as adjuvant therapy for localized disease to the capsule or implant or breast; ANDo Patient had incomplete excision or partial capsulectomy with residual or extended disease∙Adult T-Cell Leukemia/Lymphoma ‡o Must be used as a single agent for acute disease or lymphoma; AND▪Used second-line for non-responders to first-line therapy; OR▪Subsequent therapy after high-dose therapy with autologous stem cell rescue (HDT/ASCR)∙Mycosis Fungoides (MF) †/Sezary Syndrome (SS) ‡∙Primary cutaneous CD30+ T-Cell Lymphoproliferative Disorders ‡o Used as a single agent; AND▪Patient has primary cutaneous anaplastic large cell lymphoma (pcALCL) †; OR▪Patient has cutaneous ALCL with regional nodes (excludes systemic ALCL); OR▪Patient has lymphomatoid papulosis (LyP) relapsed or refractory to all treatment options†FDA Approved Indication(s), ‡Compendia Recommended Indication(s)IV.Renewal CriteriaCoverage can be renewed based on the following criteria:∙Patient continues to meet the criteria in section III; AND∙Disease response as defined by lack of disease progression, improvement in tumor size and/or improvement in patient symptoms; AND∙Absence of unacceptable toxicity from the drug. Examples of unacceptable toxicity include: progressive multifocal leukoencephalopathy, peripheral neuropathy, anaphylaxis andinfusion reactions, hematologic toxicities (thrombocytopenia, neutropenia and anemia),serious infections, tumor lysis syndrome increased toxicity in patients with severe renal(CrCl < 30 ml/min) and hepatic impairment (Child-Pugh B or C), hepatotoxicity, pulmonarytoxicity, serious dermatologic reactions, gastrointestinal complications, etc.V.Dosage/AdministrationVI.Billing Code/Availability InformationJcode:J9042 - Injection, brentuximab vedotin, 1 mg; 1 billable unit = 1 mgNDC:Adcetris single-use vial; 50 mg powder for injection: 51144-0050-xxVII.References1.Adcetris [package insert]. Bothell, WA; Seattle Genetics, Inc; November 2017. AccessedDecember 2017.2.Referenced with permission from the NCCN Drugs & Biologics Compendium (NCCNCompendium®) for brentuximab vedotin. National Comprehensive Cancer Network,2017. The NCCN Compendium® is a derivative work of the NCCN Guidelines®.NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, and NCCNGUIDELINES® are trademarks owned by the National Comprehensive Cancer Network,Inc.” To view the most recent and complete version of the Compendium, go online to. Accessed December 2017.3.Referenced with permission from the NCCN Drugs & Biologics Compendium (NCCNCompendium®) T-Cell Lymphomas. Version 2.2018. National Comprehensive CancerNetwork, 2018. The NCCN Compendium® is a derivative work of the NCCN Guidelines®.NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, and NCCNGUIDELINES® are trademarks owned by the National Comprehensive Cancer Network,Inc.” To view the most recent and complete version of the Compendium, go online to. Accessed January 2018.4.Referenced with permission from the NCCN Drugs & Biologics Compendium (NCCNCompendium®) Hodgkin Lymphoma. Version 1.2018. National Comprehensive CancerNetwork, 2017. The NCCN Compendium® is a derivative work of the NCCN Guidelines®.NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, and NCCNGUIDELINES® are trademarks owned by the National Comprehensive Cancer Network,Inc.” To view the most recent and complete version of the Compendium, go online to. Accessed December 2017.Appendix 1 – Covered Diagnosis CodesAppendix 2 – Centers for Medicare and Medicaid Services (CMS)Medicare coverage for outpatient (Part B) drugs is outlined in the Medicare Benefit Policy Manual (Pub. 100-2), Chapter 15, §50 Drugs and Biologicals. In addition, National Coverage Determination (NCD) and Local Coverage Determinations (LCDs) may exist and compliance with these policies is required where applicable. They can be found at: /medicare-coverage-database/search/advanced-search.aspx. Additional indications may be covered at the discretion of the health plan.Medicare Part B Covered Diagnosis Codes (applicable to existing NCD/LCD): N/A。
酊酊食品(北京)有限公司介绍企业发展分析报告

Enterprise Development专业品质权威Analysis Report企业发展分析报告酊酊食品(北京)有限公司免责声明:本报告通过对该企业公开数据进行分析生成,并不完全代表我方对该企业的意见,如有错误请及时联系;本报告出于对企业发展研究目的产生,仅供参考,在任何情况下,使用本报告所引起的一切后果,我方不承担任何责任:本报告不得用于一切商业用途,如需引用或合作,请与我方联系:酊酊食品(北京)有限公司1企业发展分析结果1.1 企业发展指数得分企业发展指数得分酊酊食品(北京)有限公司综合得分说明:企业发展指数根据企业规模、企业创新、企业风险、企业活力四个维度对企业发展情况进行评价。
该企业的综合评价得分需要您得到该公司授权后,我们将协助您分析给出。
1.2 企业画像类别内容行业农副食品加工业-其他农副食品加工资质空产品服务市场主体依法自主选择经营项目,开展经营活1.3 发展历程2工商2.1工商信息2.2工商变更2.3股东结构2.4主要人员2.5分支机构2.6对外投资2.7企业年报2.8股权出质2.9动产抵押2.10司法协助2.11清算2.12注销3投融资3.1融资历史3.2投资事件3.3核心团队3.4企业业务4企业信用4.1企业信用4.2行政许可-工商局4.3行政处罚-信用中国4.4行政处罚-工商局4.5税务评级4.6税务处罚4.7经营异常4.8经营异常-工商局4.9采购不良行为4.10产品抽查4.11产品抽查-工商局4.12欠税公告4.13环保处罚4.14被执行人5司法文书5.1法律诉讼(当事人)5.2法律诉讼(相关人)5.3开庭公告5.4被执行人5.5法院公告5.6破产暂无破产数据6企业资质6.1资质许可6.2人员资质6.3产品许可6.4特殊许可7知识产权7.1商标7.2专利7.3软件著作权7.4作品著作权7.5网站备案7.6应用APP7.7微信公众号8招标中标8.1政府招标8.2政府中标8.3央企招标8.4央企中标9标准9.1国家标准9.2行业标准9.3团体标准9.4地方标准10成果奖励10.1国家奖励10.2省部奖励10.3社会奖励10.4科技成果11土地11.1大块土地出让11.2出让公告11.3土地抵押11.4地块公示11.5大企业购地11.6土地出租11.7土地结果11.8土地转让12基金12.1国家自然基金12.2国家自然基金成果12.3国家社科基金13招聘13.1招聘信息感谢阅读:感谢您耐心地阅读这份企业调查分析报告。
干血斑中34种苯二氮卓类物质的实时直接分析-串联质谱快速筛查研究

第43 卷第 2 期2024 年2 月Vol.43 No.2309~314分析测试学报FENXI CESHI XUEBAO(Journal of Instrumental Analysis)干血斑中34种苯二氮卓类物质的实时直接分析-串联质谱快速筛查研究刘富邦1,张瑛2*,王继芬1*,周沛龙3,侯晓龙1(1.中国人民公安大学侦查学院,北京100038;2.北京市公安司法鉴定中心,法庭毒物分析公安部重点实验室,北京100192;3.中国医学科学院北京协和医学院药物研究所,北京100050)摘要:该文通过优化仪器条件和前处理方法建立了实时直接分析-串联质谱快速筛查干血斑(DBS)中34种苯二氮卓类物质(BZDs)的方法。
首先将50 μL血液沉积在采血卡上形成DBS,DBS经溶剂充分提取后取上层溶液,使用DART 12Dip-It TM模块进样5 μL,在多反应监测模式下检测。
结果表明:选用400 ℃作为载气加热器温度,以乙酸乙酯-水(体积比3∶1)混合溶剂提取DBS样品,所得响应强度较高且建立的筛查方法选择性良好,无延迟效应影响,除阿地唑仑、奥沙西泮和α-羟基三唑仑外,其他物质的检出限均在5~50 ng/mL。
所建方法可在90.3%的阳性检材中成功筛选出BZDs物质,方法快速、简便、有效,适用于服药自杀等类案件中DBS检材的快速筛查检验。
关键词:实时直接分析;干血斑;苯二氮卓类物质;快速筛查中图分类号:O657.6;R917文献标识码:A 文章编号:1004-4957(2024)02-0309-06Rapid Screening for 34 Benzodiazepines in Dried Blood SpotsUsing Direct Analysis in Real Time-Tandem Mass SpectrometryLIU Fu-bang1,ZHANG Ying2*,WANG Ji-fen1*,ZHOU Pei-long3,HOU Xiao-long1(1.School of Criminal Investigation,People’s Public Security University of China,Beijing 100038,China;2.Key Laboratory of Forensic Toxicol,Ministry of Public Security,Forensic Science Service of Beijing PublicSecurity Bureau,Beijing 100192,China;3.Institue of Materia Medica,Peking Union Medical College,Chinese Academy of Medical Science,Beijing 100050,China)Abstract:In this paper,a rapid and effective screening method for 34 benzodiazepines(BZDs) in dried blood spots(DBS)using direct analysis in real time-tandem mass spectrometry(DART-MS/ MS) was established by optimizing the instrumental conditions and pre-treatment methods. 50 μL of blood was deposited on the blood collection card to form DBS,the upper solution was taken after suf⁃ficient solvent extraction and 5 μL was injected using the DART 12Dip-It TM module and assayed in multiple reaction monitoring(scheduled MRM) mode. The results showed that:400 ℃ was chosen as the carrier gas heater temperature,and the DBS samples were extracted using a solvent mixture of ethyl acetate-water(3∶1,by volume),and the upper layer of the solution was injected,which showed a high response intensity and good selectivity without the influence of delayed effect. The lim⁃its of detection were in the range of 5-50 ng/mL for all substances except for adinazolam,oxazepam and α-hydroxytriazolam.BZDs were successfully screened in 90.3%of the positive case samples. The method is fast,simple,effective and suitable for the rapid screening test of DBS samples in cas⁃es such as suicide by drug taking.Key words:direct analysis in real time;dried blood spot;benzodiazepines;rapid screening苯二氮卓类物质(Benzodiazepines,BZDs)是一类功能强大的中枢神经系统抑制剂[1],其种类繁多,使用较为普遍,是法医毒物检验中常见的检验目标。
碘酊

碘酊
【药品名称】
通用名称:碘酊
英文名称:Iodine Tincture
【成份】
碘,乙醇。
【适应症】
用于皮肤感染和消毒。
【用法用量】
外用。
用棉签蘸取少量碘酊,由中心向外涂搽局部,消毒后再用70%酒精脱碘。
【不良反应】
偶见过敏反应和皮炎。
【禁忌】
尚未明确
【注意事项】
1.不宜用于破损皮肤、眼及口腔黏膜的消毒。
2.本品仅供外用,切忌口服。
如误服中毒,应立即用淀粉糊或米汤灌胃,并送医院救治。
3.用药部位如有烧灼感、瘙痒、红肿等情况应停药,并将局部药物洗净,必要时向医师咨询。
4.如果连续使用3日无效,应咨询医师。
5.对本品过敏者禁用,过敏体质者慎用。
6.本品性状发生改变时禁止使用。
7.请将本品放在儿童不能接触的地方。
8.儿童必须在成人监护下使用。
9.如正在使用其他药品,使用本品前请咨询医师或药师。
【药物相互作用】
P> 1.不得与碱、生物碱、水合氯醛、苯酚、硫代硫酸钠、淀粉、鞣酸同用或接触。
2.如与其他药物同时使用可能会发生药物相互作用,详情请咨询医师或药师。
【药理作用】
本品为消毒防腐剂,其作用机制是使菌体蛋白质变性、死亡,对细菌、真菌、病毒均有杀灭作用。
【贮藏】
密封
【有效期】
24个月。
【批准文号】
国药准字H20056262
【生产企业】
企业名称:武汉中联药业集团股份有限公司
生产地址:湖北省武汉市江汉区复兴一村24号。
ZEISS TIVATO 700 清洁与消毒指南说明书

ZEISS TIVATO 7007.1 Safety during cleaning and disinfectionG-30-2032-en - 7.0 - 2019-07-12169 / 2167Cleaning and disinfection7.1Safety during cleaning and disinfectionContamination with pathogenic germs!If the device is cleaned or disinfected using unsuitable methods orcleaning agents, pathogenic germs can accumulate on the deviceand the device may also be damaged.u Make sure that the device is operated only in a sterile mannerand with sterile accessories, Attaching a ZEISS SMARTDRAPE[} 121].u Make sure that the device is operated, cleaned and disinfectedonly by personnel who have been instructed in these proce-dures.uWhen cleaning and disinfecting the device, only use cleaning/disinfection agents that are suitable and approved for the surface in question.7.2Contamination of the systemDust can penetrate into the internal optics of the device or itsindividual components.Action u Always close all openings that are not in use with the coversprovided (e.g. openings for eyepieces, tube outlets or thelateral co-observation outlets).u Always store tubes, eyepieces and accessories in dust-free caseswhen they are not being used.u After use, cover the system with the supplied dust cover toprotect it from dust.uClean used accessories directly after use.7.3Cleaning and disinfecting agent7.3.1Cleaning agents7.4CleaningClean the device before the first use and after every use.ZEISS TIVATO 7007.4 Cleaning 170 / 216G-30-2032-en - 7.0 - 2019-07-127.4.1Cleaning optical surfacesThe multi-layer T* coating of the optical components (e.g.eyepieces, objective lenses) ensures optimal image quality. Imagequality is impaired by even slight contamination of the optics or bya fingerprint. Clean the exterior surfaces of the optical components(eyepieces, objective lenses) only when necessary:Action uDo not use any chemical cleaning agents.u Use a clean and grease-free brush to remove dust.TIP: For the regular cleaning of surgical microscope's objectivelenses and eyepieces, we recommend the optics cleaning setavailable from ZEISSCleaning agents [} 169].7.4.2Cleaning the monitor (touchscreen)To prevent damage, ensure that no moisture or cleaning agents get inside the monitor.Incorrect use of glass cleaner!Damage to the monitor.u Never spray glass cleaner directly onto the monitor.PrerequisiteþSwitch off the device.Action u Clean the monitor with a soft, clean microfiber cloth or withmoist optical lens wipes.u If necessary, moisten the cloth slightly with water or pure glasscleaner.uWipe the monitor with a moist cloth.7.4.3Cleaning mechanical surfacesAll mechanical surfaces of the system can be cleaned by wipingthem with a damp cloth.Action uDo not use any aggressive or abrasive cleaning agents.u Remove any possible residue using a mixture of 50% ethylalcohol and 50% distilled water plus a dash of householddishwashing liquid.ZEISS TIVATO 7007.5 DisinfectionG-30-2032-en - 7.0 - 2019-07-12171 / 2167.4.4Fogging of optical surfacesWe recommend using an anti-fogging agent to prevent fogging ofoptical surfaces. Anti-fogging agents like the ones offered byopticians for applications with eyeglasses are also suitable foroptical surfaces from ZEISS.Action u Observe the Instructions for Use pertaining to the anti-foggingagent concerned.An anti-fogging agent does not ensure fog-free eyepiece optics. Itcleans eyepiece optics and protects them against dirt, grease, dust,lint and fingerprints.7.5Disinfection7.5.1Disinfecting the mechanical surfacesThe maximum application concentrations are:•For alcohol (tested with isopropyl alcohol): 60%•For aldehyde (tested with glutaraldehyde): 2%•For quaternary compounds (tested with DDAC): 0.2%Surface damage caused by wrong disinfectants!Performing disinfection with the wrong disinfectants may result indamage to the surfaces of the device.u Use an aldehyde and/or alcohol-based disinfectant. Theaddition of quaternary compounds is acceptable.u In order to prevent surface tensions, you may use only the disinfecting components specified above.Action uDisinfect all of the required surfaces.。
常用农药作用机理
阿克泰
thiamethoxam
actara
25%、粒
3-(2氯-硫唑-5-基甲 基)-5-甲基-[1,3,5], 蚜、蓟、虱 恶二烷,4-基胺-N-硝基 胺 15%、粒
针对刺吸式口器害虫. 触杀和胃毒,强内吸传导性。模仿乙酰胆 碱,使昆虫一直处于高度兴奋,直到死亡。药后2--3天死亡。持 5000-10000倍 效期达1月左右
炔螨特
propargite
克螨特、除螨 25%73%、乳 净 尼索朗 5%10%乳、粉
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2017年民间固定资产投资增长6.0%
国家统计局2018-01-18 15:00
2017年,民间固定资产投资381510亿元,比上年名义增长6.0%,增速比1-11月份提高0.3个百分点。
民间固定资产投资占全国固定资产投资(不含农户)的比重为60.4%,比1-11月份回落0.1个百分点。
分地区看,东部地区民间固定资产投资175573亿元,比上年增长8.6%,增速比1-11月份提高0.4个百分点;中部地区110938亿元,增长7.4%,增速提高0.3个百分点;西部地区73440亿元,增长3.9%,增速回落0.4个百分点;东北地区21558亿元,增长3.2%,增速提高2.9个百分点。
分产业看,第一产业民间固定资产投资16911亿元,比上年增长13.3%,增速比1-11月份回落0.7个百分点;第二产业186404亿元,增长3.8%,增速提高0.6个百分点;第三产业178194亿元,增长7.7%,增速提高0.1个百分点。
第二产业中,工业民间固定资产投资185090亿元,比上年增长4.0%,增速比1-11月份提高0.6个百分点。
其中,采矿业4935亿元,
比上年下降19%,降幅收窄0.3个百分点;制造业168784亿元,比上年增长4.8%,增速提高0.7个百分点;电力、热力、燃气及水生产和供应业11371亿元,增长4.6%,增速回落1.2个百分点。
2017年民间固定资产投资主要数据
指标
2017年
绝对量
比上年增长
(亿元)
(%)
民间固定资产投资
381510
6.0
分地区
东部地区
175573。
