CE技术文件指南NB-MED2[1].5.1(中文)资料
CE标志产品的管理规定(修改)

caution
注意,参考随附文件
manufacturer
生产厂家
Authorized Representative in the European Community
欧盟授权代表
举例 (Examples)
7
符号 (Symbol)
说明 (Used for)
Sufficient for, counting, contains sufficient for <n> tests
1
负责人提交 CE 检测过程中所需材料,项目负责人在接到通知后应在 2 个工作日内提交材料。 5、法规标准部接到 CE 检测机构的时间安排后当天通知项目负责人,项目负责人应在规定时间内送产品样 机到检测机构进行测试。 6、法规标准部负责与 CE 检测机构的联系工作以及 CE 检测合同的签定,应在 3 个工作日内完成与检测机
由项目负责人与检测机构沟通相关检测事宜,直至完成检测。 8、CE 检测完成取得 CE 证书后,法规标准部应在 1 个工作日内将信息转告国际营销中心或销售公司市场部 (或招标部)。 9、需进入国际市场并在欧盟国家注册的产品,不论是需做 CE 检测的产品还是不需要做 CE 检测的产品, 均由国际营销中心填写“优利特产品 CE 注册确认单”(以下简称“注册确认单”),提交需要办理注册的产 品所在项目负责人,产品项目负责人需在 2 个工作日内将是否接受“注册确认单”的意见及确认后 CE 注 册资料提供负责人名单反馈国际营销中心,国际营销中心留存原件,复印一份转法规标准部,法规标准部 根据确认单上的内容确认部门审核人员和确定递交材料所需要的时间,并反馈产品指定提交材料负责人。 10、原则上一个产品填写一张“检测申请/确认单”(或“注册确认单”),若申请的 2 个或 2 个以上产品均 属同一项目经理负责,则可填在同一张申请单(或确认单)中,CE 技术文档编制时间以经注册产品所在部 门领导批准的时间开始计算。 11、不需要做 CE 检测的产品,项目负责人应在 20 个工作日内提供附录一 Part A 中第三条款的全部内容 至法规标准部,除此之外的其他 CE 技术文档对于单一产品的可延后 10 个工作日提交,对于系列产品原则 上每增加一个型号可在单一产品的完成时间上增加 5 个工作日。 12、需做 CE 检测的产品,项目负责人在接到通知后应在 30 个工作日内提供附录一 Part A 中第三条款 的 全部内容至法规标准部,除此之外的其他 CE 技术文档对于单一产品的可延后 10 个工作日提交,系列产品 原则上每增加一个型号可在单一产品的完成时间上增加 5 个工作日。 13、无需销售到国外的产品,若不希望提供 CE 文档,必须报告申请并得到该产品公司负责人批准,可在。 14、法规标准部在接到 CE 技术文档后,附录一 Part A 中第三条款的全部内容对于单一产品应在 5 个工作 日内完成审核提交文档的规范性,系列产品的审核每增加一个型号可在单一产品的完成时间上增加 2 个工 作日。对不符合规范的文档应退回责任部门,责任部门在接到退回文档后需在 5 个工作日内进行修改并再 次提交。 15、如 CE 技术文档不能按预期完成的,可以申请延期,但需获得国际营销中心经理批准。 16、对提交合格的 CE 技术文档,法规标准部应在 2 个工作日内转国际营销中心,国际营销中心在接到文 档后单一产品应在 10 个工作日完成审核,系列产品的审核每增加一个型号可在单一产品的完成时间上增 加 5 个工作日,若审核中发现提交的 CE 技术文档有错误,应将错误技术文档退回责任部门,责任部门接 到退回文档后应在 3 个工作日内进行修改并再次提交国际营销中心。国际营销中心审核完成后应在 2 个工
临床试验数据管理工作技术指南

临床试验数据管理工作技术指南2021年3月12日目录一、详述............................................................................ ....................................................................11.11.2国内临床试验数据管理现状............................................................................ ......................1国际临床试验数据管理简介............................................................................ . (2)1.2.1ich-gcp对临床试验数据管理的原则性指导...............................................................21.2.221cfrpa rt11对电子记录和电子签名的基本建议.......................................................31.2.3“临床试验中使用计算机系统的指导原则”提供更多计算机系统研发的参考标准.............31.2.4gcdmp(goodclinicaldatamanagementpractice)提供更多全面具体内容的数据管理建议....4二、数据管理有关人员的责任、资质及培训............................................................................ ..........52.1相关人员的责任............................................................................ .. (5)2.1.1主办权者............................................................................ .....................................................52.1.2研究者............................................................................ .....................................................62.1.3监查员............................................................................ .....................................................62.1.4数据管理员............................................................................ .............................................62.1.5合约研究非政府(cro)....................................................................... ..............................72.2数据管理人员的资质及培训............................................................................ . (8)三、临床试验数据管理系统............................................................................ ......................................93.13.2临床试验数据管理系统的重要性............................................................................ .. (9)数据质量管理体系的创建和实行............................................................................ .............93.3临床试验数据管理系统的基本建议............................................................................ ........103.3.1系统可靠性............................................................................ ...........................................103.3.2临床试验数据的可以追溯性(traceability)................................................................ .......113.3.3数据管理系统的权限管理(accesscontrol)............................................................. ..12四、试验数据的标准化............................................................................ ............................................124.14.2临床数据标准化的现状与发展趋势............................................................................ ........12临床试验的数据标准化............................................................................ (13)4.2.1cdisc和hl7........................................................................... .......................................144.2.2医学术语标准............................................................................ .......................................164.2.3临床试验报告的统一标准(consort)................................................................... ...20五、数据管理工作的主要内................................215.1crf的设计与核对............................................................................ .. (21)5.1.1crf的设计............................................................................ ..........................................215.1.2crf填写指南............................................................................ ......................................225.1.3注释crf........................................................................... ...............................................225.1.4crf的填写............................................................................ ..........................................225.25.35.45.5数据库的设计............................................................................ ...........................................22数据发送与打印............................................................................ .......................................23数据核查............................................................................ ...................................................23数据批评表的管理............................................................................ .. (25)5.65.75.85.95.105.11数据修改的档案............................................................................ .......................................25医学编码............................................................................ ...................................................25试验方案增选修正............................................................................ ...................................26实验室及其他外部数据............................................................................ ...........................26数据盲态审查............................................................................ ...........................................28数据库瞄 (28)5.11.1数据库锁定清单............................................................................ ...................................285.11.2数据库锁定后发现数据错误............................................................................ ...............295.125.135.14数据备份与恢复正常............................................................................ .......................................30数据留存............................................................................ ...................................................30数据保密及受试者个人私密性的维护............................................................................ . (33)5.14.1数据保密............................................................................ ...............................................335.14.2受试者个人私密性的保护............................................................................ ...................33六、数据质量的保障及评估............................................................................ ....................................336.1质量确保............................................................................ (33)6.1.1质量控制............................................................................ ...............................................346.1.2质量保证............................................................................ ...............................................376.2质量评估............................................................................ (40)七、安全性数据及严重不良事件报告............................................................................ ....................417.17.27.37.4不当事件的以获取、管理和报告............................................................................ ................42实验室数据............................................................................ ...............................................44其他数据............................................................................ ...................................................44轻微不当事件数据............................................................................ .. (45)八、参考文献............................................................................ .. (46)一、详述临床试验数据质量是评价临床试验结果的基础。
(完整word版)无源医疗器械CE技术文档和CE设计档案材料指南(中英文 也是极好的)

无源医疗器械技术文件和设计文档指南Whereas the term “Technical File“ is used for Medical Devices of class I, class IIa and class IIb, the term “Design Dossier“ is used for the class III products.标题中的“技术文件”适用于I类,IIa类,IIb类医疗器械,“设计文档”适用于III类医疗器械。
Technical Files are retained in the premises of the manufacturer or the Authorized Representative for potential review of Competent Authorities and Notified Body.Part B of the Technical File may be available at the manufacturer only.技术文件是保留在制造商或授权代表单位的主管部门和认证机构。
部分技术文件B部分只保留在制造商处。
Whereas Design Dossiers have to be submitted to the Notified Body for review prior to CE-Marking of the product (use form Application for CE Conformity Assessment (Product)MED_F_03.03). We will assign a project manager who will entrust one or more further experts with the review of particular modules. All experts are at your disposal directly or indirectly through the project manager. After successful review, the Notified Body issues a design examination certificate according to Annex II.4 of the Council Directive certifying compliance with the relevant provisions of Annex I of the MDD.设计档案材料已被提交到公告机构用于需要CE认证前的产品审查(用CE合格评定(产品)MED_F_03.03规定的格式)。
无源医疗器械CE技术文档和CE设计档案材料指南(中英文 也是极好的)

无源医疗器械技术文件和设计文档指南Whereas the term “Technical File“ is used for Medical Devices of class I, class IIa and class IIb, the term “Design Dossier“ is used for the class III products.标题中的“技术文件”适用于I类,IIa类,IIb类医疗器械,“设计文档”适用于III类医疗器械。
Technical Files are retained in the premises of the manufacturer or the Authorized Representative for potential review of Competent Authorities and Notified Body.Part B of the Technical File may be available at the manufacturer only.技术文件是保留在制造商或授权代表单位的主管部门和认证机构。
部分技术文件B部分只保留在制造商处。
Whereas Design Dossiers have to be submitted to the Notified Body for review prior to CE-Marking of the product (use form Application for CE Conformity Assessment (Product)MED_F_03.03). We will assign a project manager who will entrust one or more further experts with the review of particular modules. All experts are at your disposal directly or indirectly through the project manager. After successful review, the Notified Body issues a design examination certificate according to Annex II.4 of the Council Directive certifying compliance with the relevant provisions of Annex I of the MDD.设计档案材料已被提交到公告机构用于需要CE认证前的产品审查(用CE合格评定(产品)MED_F_03.03规定的格式)。
欧盟医疗器械CE认证技术文档编写Technicalfileeditting

Module 3 – CE技术文档编写
技术文档的语言
? 如果欧盟指令未包括对文档语言的特 别规定时,生产者必须对成员国的需 求进行评估。
? 可能不会出现更多状况来影响到相关 的翻译件。 (例如经过验证的)
13
Module 3 – CE技术文档编写
技术文档-第一部分
包括与符合性评价程序有关的,最重要 技术数据: ? 制造商信息 ? 欧洲代表信息 ? 质量系统所涉及的全部制造场所的清
19
示例
6.设计验证
如果生产者能证明这些年来市场验证后是一 种安全设计且其性能按设计要求,则不需要。
否则,按如下步骤提供文件:
-设计合格检验
-关于预期用途的设计预测(如果有关,参 考其他产品)
7.风险分析
-器械的风险评估
20
示例
8.符合基本要求和调查标准
-列出所有使用的调整标准或,如果使用不 完整,详细叙述如何满足基本要求。
物理性能报告
? 不同的产品有不同的物理性能
? 例如:带针注射器
尺寸
针管韧性和刚性
推拉力
器身密合性
残留容量
52
化学性能报告
? 不同的产品有不同的化学性能
? 例如:带针注射器
酸碱度Βιβλιοθήκη 可萃取金属含量易氧化物环氧乙烷残留(生物性能)
53
可用性报告
? EN 62366:2008
54
EMC测试报告 电气安全测试报告
綠色能源 利用自然,開發綠色能源
綠色生活 讓環保融入您生活的各個層面
綠色產品 更低的環境影響, 帶來更高的市占率
綠色循環 負責的回收再利用
始於產品設計
德國萊因 綠色解決方案
ce 医疗器械使用说明书

ce 医疗器械使用说明书
CE认证的医疗器械使用说明书应包括以下内容:
1. 器械的预期用途:明确说明器械的适应症、禁忌症、患者目标群体和预期用户。
2. 临床收益规范:如适用,提供预期的临床收益规范。
3. 器械的性能特征:详细描述器械的性能特点,以便医疗保健专业人员了解器械的特性和功能。
4. 软件和附件选择:如适用,提供信息用于医疗保健专业人员验证器械是否合适,并选择相应的软件和附件。
5. 剩余风险、禁忌症和不良副作用:明确说明任何剩余风险、禁忌症和任何不良副作用,包括传达给患者的关于这方面的信息。
6. 使用者要求:说明使用者要求适当使用该装置的规格,例如,若器械具有测定功能,提供其所要求的准确度。
7. 预处理或处理细节:在准备使用之前或在其使用(例如,灭菌、最终组装、校准等)期间器械的任何预处理或处理的细节,包括确保患者安全所需的消毒水平和实现所需消毒水平所需的所有可用方法。
8. 特定资格要求:说明对特殊设备、特殊培训或设备用户和/或其他人的特
定资格的任何要求。
以上内容仅供参考,具体应基于制造商的风险管理文档的特定部分,详细说明这些特征和技术因素。
CE技术文件指南NB-MED2[1].5.1(中文)资料
![CE技术文件指南NB-MED2[1].5.1(中文)资料](https://img.taocdn.com/s3/m/5a56d39ee518964bce847c82.png)
产品技术文档推荐NB-MED/2.5.1/Rec5 章节: 2.5.1 符合性评估程序;总则关键词:设计档案,技术文档,技术档案1.介绍对技术文档的要求在医疗器械指令的不同附件多有提及,适当时用于符合性评估程序和涉及到的产品。
作为总则,该文档应包括产品的设计、制造和产品的操作。
备注:产品的“操作” 包括安装、使用准备、用前检查和维护、校准以及对特殊医疗器械的服务。
文档所包含的具体细节决定于产品的特性和必要的关注点。
从技术角度来看,就是陈述产品符合对医疗器械指令本质要求。
如果使用了该统一标准,技术文档应该明确这些用于说明符合实质要求的哪里是被该标准覆盖的。
注:该推荐标准特为满足医疗器械和有源植入式医疗器械的技术文档的指导需要而写。
该推荐标准也许也是有用的,然而,涉及到体外诊断医疗器械,但是可能根据体外诊断医疗器械指令的实际实施的经验需要修正。
2.目的一份原理和历史页是需要的,请联系技术秘书处。
该推荐标准的目的是向认证方、主管当局和生产商在需要满足医疗器械指令要求的技术文档方面提供指导。
备注:迫使生产商修改现有的已证明适合和充分的技术文档不是该推荐标准的目的。
3.技术资料3.1综述主题指导(ⅰ)技术文档内该推荐标准不是欲详尽罗列所有特殊情况可能需要的技术文档的列表。
容也就是,一些特殊的资料没有没包括近来,而此处会提供一个正当的说法。
指令要求生产商准备技术文档进行确定和证明什么是适宜的和充分的,而确保他的医疗器械符合相关指令。
这在不同的情况有明显的不用,取决于产品的种类、制造随附的风险、安装使用和服务,以及其在市场上的时期。
比如,所有的已有产品,不管类别都有非常正视的设计验证是不可能的。
但是生产商在使用经验和他如何应对出现的问题上都有丰富的经验,这些资料都应用于验证方面。
ⅱ)其他技术文档应包括3.2-3.5 部分所阐述的资料3.2 产品描述主题ⅰ)器械的概括描述指导包括变化范围(比如,只有长度不同的同一型号的一组导管),和有关器械性能和特性储存的包装描述。
CE 临床数据评估 官方指南
on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECNB-MED/2.7/Rec3Title:Evaluation of clinical dataChapter: 2.7 Clinical investigations, clinical evaluationText: ...Key words: Clinical data, EvaluationA rationale and history sheet is available; please contact Technical Secretariat.Reference to Directives: Article/ Annex: Reference to standards: AIMD Annex 1 (I.1, I.2, I.5), Annex 7 MDD Annex I (I.1, I.3, I.6), Annex X IVDStage proposed by Rev.-Nr. Rev. date accepted amended withdrawn Page 3 task force 5 11.05.99 08.06.99 1/141. Introduction and purposeIt is the primary purpose of this document to provide guidance to Notified Bodies when reviewing the manufacturers evaluation of clinical data as part of the confor-mity assessment procedures required by 90/385/EEC (AIMD) [1] and 93/42/EEC (MDD) [2].This document will also assist manufacturers, by providing guidance on what is ex-pected.It is not the purpose of this document to define the circumstances under which clini-cal data are provided and the extent of clinical data needed in relation to a particular medical device.2. BackgroundThe manufacturer must demonstrate that his intended purpose(s) and claim(s) made in relation to safety and performance are achieved, as referred to in the Directives. As a general rule, such demonstration will require clinical data (see Annex X, 1.1 of MDD).Evaluation of clinical data as described in Annex X of the MDD and Annex 7 of the AIMD is particularly relevant to assessment of conformity with essential requirements given in MDD, Annex I, I General requirements, sections 1 and 3 and AIMD Annex 1,NB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical dataI General requirements, sections 1 and 2. Attention should also be paid to Annex I, I.6 (MDD) and Annex 1, I.5 (AIMD).3. Explanation of termsFor the purpose of this document:3.1. Clinical data is data which is relevant to the various aspects of the clinicalsafety and performance of the device. This may include data from prospective and retrospective clinical investigations of the device concerned as well as market experience of the same or similar devices and medical procedures and information from the scientific literature.3.2. The Evaluation of clinical data is the process by which clinical data from all se-lected sources (literature, results of clinical investigations and other) is as-sessed to establish conformity of the device with the pertinent essential re-quirements of the Directive, and to demonstrate that the device performs as intended by the manufacturer. The outcome of this process is a report which includes a conclusion on the acceptability of risks and side effects when weighed against the intended benefits of the device.4. Clinical data to be provided by the manufacturerAs a general rule, and in particular in the case of implantable devices and devices in Class III, evidence of the clinical performance and safety of a medical device is pro-vided by means of clinical data, which is supplied by the manufacturer in accordance with Annex X (MDD) or Annex 7 (AIMD). All the conformity assessment procedures leading to ”CE” marking address the issue of clinical evaluation by the manufacturer. In the case of Annexes II and III, the Notified Body is involved.Clinical evaluation is based on the assessment of the risks and the benefits, associ-ated with use of the device, through either1. A compilation of relevant scientific literature, that is currently available as wellas, where appropriate, a written report containing a critical evaluation of this compilation (the "literature route") or2. The results of all the clinical investigations made (the "clinical investigationroute"), orNB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical data3. a combination of 1 and 2 above.Where the clinical evaluation is based on such a combination of 1 and 2, it should include an overall assessment. It is important that the manufacturer relates the data to the specific device, having regard to the hazards identified (see 4.1).The manufacturer must decide whether the available data is sufficient to demon-strate conformity with the Directive, having regard to (a) the similarity of the charac-teristics of the device(s) to which the data relates and the device(s) for which con-formity is being assessed, and so the applicability of the findings to the devices being assessed, and (b) the adequacy of the data in addressing the relevant aspects of Directive conformity.NOTE If the available literature is sufficient to demonstrate conformity with the Directive, there will be ethical considerations associated with performing further clinical investigations (e.g. delay in availability of a given device leading to the loss of benefit of this device).4.1. Identification of aspects of safety and performance to be addressedthrough clinical dataThe manufacturer is required by the Directive to perform a risk analysis. A risk analysis is important in helping the manufacturer identify known or reasonably fore-seeable hazards associated with use of the device, and decide how best to estimate the risks associated with each hazard1. From the results of the risk analysis, the manufacturer lays out how each risk is addressed and decides on the acceptability of risks when weighed against the intended benefits.The risk analysis includes technical and clinical aspects relating to the particular device concerned.1The loss/absence of the performance of a given device as claimed by the manufacturer and which could result in the loss of benefit of a treatment may be considered a hazard.NB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical dataIt should distinguish between aspects associated with(i) the medical procedure for which the device is intended,For example, the risks versus benefits associated with extracorporeal lithotripsy as compared with conventional (surgical and not surgical) methods of kidney stone removal.(ii) the technical solutions adopted,For example the risks versus benefits associated with different technologies of extracorporeal lithotripsy such as those involving generating shock-waves with electric sparks (electrohydraulic method), with an electromagnetic generator ora piezoelectric system.(iii) aspects specific to the design and use of the particular device concerned.For example the risks versus benefits associated with the shockwave coupling method, size of the focal zone, the stone localisation and targeting system (X-ray, ultrasound) and the trigger methodThis distinction should be used to identify the type and specificity of clinical data needed. Where the available data is not sufficient to address the identified clinical hazards relating to one or more of the above aspects, a clinical investigation will be needed (see also section 4.3.1). The objectives of the clinical investigation should focus on those aspects not sufficiently addressed by the available data.route4.2. LiteratureIf the manufacturer's clinical evaluation to be submitted to the Notified Body takes the form of a review of the relevant scientific literature, the following requirements should be fulfilled:4.2.1. Compilation of the relevant literaturea) The compilation should be related to the hazards identified in the clinical part ofthe risk analysis (see 4.1.), and support the arguments set out in the report.b) Published literature should be taken from recognized scientific publications in-cluding unfavourable as well as favourable data.NB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical dataFactors which may influence the scientific validity of the published literature in-clude:i) whether or not the author's conclusions are substantiated by the availabledata;ii) the extent to which the published literature is the outcome of a study, fol-lowing scientific principles for example, in having demonstrable and ap-propriate endpoints, inclusion and exclusion criteria and an appropriateand validated number of patients submitted.iii) whether or not the literature still reflects the “generally acknowledged”state of the art;iv) the relevance of the author's background and expertise in relation to the particular device and/or medical procedure involved; andv) lack of impartiality.c) Other scientific data, such as the documented results of the manufacturersbench testing, including in vitro testing and animal studies, and an assessment of compliance with relevant technical standards, may be necessary to(i) alone, or in combination with other data, demonstrate compliance with rele-vant essential requirements of the Directives;(ii) establish the extent of similarity between device(s) and medical procedure(s) covered by the scientific literature and the characteristics of the device being assessed.d) For well-established or simple devices, documented expert opinions with ra-tionale from duly qualified medical practitioner(s) or other expert(s) suitably qualified in the area concerned can also be used to demonstrate safety and performance. In selecting such expert(s), the manufacturer should give due re-gard to their background and expertise in relation to the area concerned and any conflict of interest which may compromise impartiality. Such an expert opinion should be signed and dated by the author.report4.2.2. Writtena) A written report containing a critical evaluation is required, unless a justificationfor its absence is given, e.g. if the particular device itself is the subject of the publication and the critical evaluation of risks and intended benefits is already adequately covered by the publication.b) The report should be written by a person suitably qualified in the relevant fieldand knowledgeable in the state-of-the-art.NB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical datac) The report should be based on the scientific literature considered, and shouldbe accompanied by a listing and copy of the publications quoted.d) The report should be related directly to the device under certification.e) The report should discuss all the referenced scientific literature and should ad-dress unfavourable as well as favourable dataf) The report should contain a short description of the medical device and its in-tended functions, a description of the intended purpose and the application of the device as well as the indications and contraindications for its use.g) The report should clearly establish the extent to which the literature relates tothe specific characteristics and features of the device being assessed. This should take due account of the extent of similarity between the device(s) cov-ered by the literature and the device now being assessed, and therefore rele-vance in the areas of, for example, design, technology, principles of operation, critical performance characteristics, specified conditions for use etc.h) The report should demonstrate that those aspects of use of the device, includ-ing performance, addressed in the clinical part of the risk analysis are met as claimed by the manufacturer, and that the device fulfils its intended purpose asa medical device.i) The identified hazards, the associated risks and the appropriate safety meas-ures for patients, medical staff and third parties should be covered in the report, for example by reference to the manufacturer’s risk analysis.j) A risk/benefit assessment, which justifies the acceptability of each remaining risk when weighed against the intended benefits from use of the device, should be included.k) The report should contain appropriate cross-references to the attached publi-cations.l) Market experience of the same or similar devices can form part of the report.m) Results of laboratory testing, biocompatibility and compliance with technical standards, or reference to these results, if available, can additionally be used in the report to demonstrate compliance with certain essential requirements and the relevance of the scientific literature that is referred to.NB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical datan) Statements concerning the field of use of the device and its indications, con-traindications, effects and side effects should be consistent with the instructions for use.o) The report should give a concluding opinion with rationale.p) The report should be signed and dated by the author.4.3. Clinical investigations route4.3.1. Need for clinical investigationsWhen reviewing the manufacturer’s evaluation of clinical data and whether or not a clinical investigation is needed as part of this, due regard should be paid to NB-MED/2.7/R1 [5].4.3.2. Conduct of clinical investigationsWhere the results of clinical investigations form part of the clinical data, the clinical investigations should comply with the relevant sections of Annex X MDD or Annex 7 AIMD. Compliance with the EN 540 [3] carries the presumption that the design and conduct and monitoring of the clinical investigation conforms with the requirements of these Annexes. Whilst not carrying such a presumption of conformity, other equivalent standards may be used.2[4]4.3.3. The results and final report of the clinical investigationThe documentation on a clinical investigation should include:1. The identification of the medical device which is the subject of the clinical in-vestigation, consisting of short description of the device. This description should be sufficient to address all the aspects relevant to the clinical investigation. It should include in particular:- normal use, intended purpose, indications, contraindications- performance as claimed by the manufacturer2. A clear definition of the objectives of the clinical investigation2Where justified, the Notified Body may require further information to assess the manufacturers clinical investigation data.NB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical data3. Methodology- enrolment of subjects, including inclusion and exclusion criteria, rate of en-rolment, numbers and grouping- study start and completion dates- medical procedures involved- appropriate, objective endpoints which, if achieved, demonstrate the required safety and performances- parameters assessed, with frequency and methods of assessment and data acquisition- statistical methods4. Results and conclusion- details of any deviations from the agreed Clinical Investigation Plan, with the reasons and any resulting amendments to the Clinical Investigation Plan forthe remainder of the Clinical Investigation, together with the implications forinterpretation of results.NOTE In the case of multi-centre investigations, it should be made clearwhether deviations and any subsequent amendments and/or additional ordifferent treatment of results apply to all or only particular centres - critical evaluation of all the data collected during the clinical investigation- appraisal of clinical relevance- demonstration that the objectives of the clinical investigation have been met in the context of the overall assessment of the device's safety and perform-ance.5. Date and signature of the responsible investigator.NB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical data5. The role of the Notified BodyWith regard to the evaluation of clinical data the Notified Body has different roles depending on the conformity assessment procedure followed.As part of the design/type examination under Annexes II.4 or III, the Notified Body assesses the clinical data assembled by the manufacturer and the manufacturer’s evaluation and the validity of the conclusions drawn. (see 5.1)As part of quality system approval under Annex II.3, the Notified Body assesses the manufacturer’s procedure for clinical data evaluation. This may include a review of examples of such evaluations. (see 5.2)5.1. Examination of a design dossier (Annex II.4) or of a type examinationdossier (Annex III)The Notified Body (NB) examines the documentation submitted according to the pre-ceding sections. In order to do so, the NB should possess enough knowledge and experience in clinical evaluation as stated in section 6 of this document.making5.1.1. DecisionIn reviewing the evaluation of clinical data submitted by the manufacturer, the Notified Body decides whether or not the manufacturer has adequately:a) described and verified the intended characteristics and performances related toclinical aspects.b) performed a risk analysis and estimated the undesirable side effects.c) concluded on the basis of documented justification that the risks are acceptablewhen weighed against the intended benefits.The assessment carried out by the Notified Body will typically cover the following aspects of the manufacturer’s clinical data evaluation:1. The listing and characterisation of the clinical performance of the device in-tended by the manufacturer and the expected benefits for the patient2. The use of the list of identified hazards to be addressed through evaluation ofclinical data as described in paragraph 4.1. of this documentNB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical data3. The adequate estimation of the associated risks for each identified hazard by:a) characterising the severity of the hazardb) estimating and characterising the probability of occurrence of the harm (orhealth impairment or loss of benefit of the treatment) (document with ra-tionale)4. The decision on the acceptability of risks in relation to each identified hazard,based on the combination of 1, 3a, and 3b using the ALARP3 philosophy [6,7], and characterisation of the corresponding risk/benefit ratio as:- unacceptable or- broadly acceptable or- acceptable under specified conditions4 (see ISO/IEC Guide 51 [9])5.1.2. The report of the Notified BodyThe Notified Body writes a report on its assessment of the submitted clinical docu-mentation. The report may be a separate report or part of the Notified Body’s overall report. In the latter case the clinical part should be clearly identified.The Notified Body’s report should include:- Identification of the manufacturer- Identification of the medical device- Basis of evaluation (which Directive and which Annex(es))- Submitted documents- Description of the device- Assessment of clinical safety and performance- Conclusion. The NB should justify and document each step of the decision making process referred in 5.1.1. One single “unacceptable risk/benefit ratio” leads to a negative conclusion.53ALARP means "As Low As Reasonably Practicable"4The assessment of a risk/benefit ratio as ”acceptable under specified conditions” implies the determination of those specified conditions under which it can be accepted. At the stage ofassessment, the expected benefit to the patient, as well as the risk, has to take account of thegenerally acknowledged state of the art.5In some cases, the combination of the conditions specified in order to characterise different Risk/benefit ratios as acceptable may be contradictory or impracticable, and so also leads to anegative conclusion.NB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical data- the names of all NB internal assessors and external experts involved in the as-sessment of the manufacturers documentation, together with details of the as-pects assessed by each- Date and signature of the responsible assessor5.2. Evaluation as part of quality system related procedures (Annex II.3)5.2.1. Review of the proceduresWhen the manufacturer selects this procedure, the Notified Body should, as part of the review of the manufacturer’s quality system, assess the establishment, mainte-nance and application of the manufacturer’s procedures for the documented evalua-tion of clinical data. This should cover:a) the responsibility for the evaluation of the clinical data by a suitably qualifiedperson;b) the identification of clinical data, both unpublished (for example contained in themanufacturers files e.g. the complaints history) and published.c) the relevance of the clinical data identified as demonstrating compliance withparticular requirements of the Directive or cited in particular aspects of the risk analysis6.d) assuring that clinical investigations are performed in compliance with the appli-cable regulations and the clinical investigation plan, with a suitable justification for any deviationse) identification and evaluation of undesirable side-effects.This latter point involves identification of known or reasonably foreseeable hazards, qualification of their severity and of their probability of occurrence. It is part of the manufacturer’s documented risk analysis based on both favourable and unfavourable data identified as relevant in order to give a balanced view.5.2.2. Review of samples6The record of this may take the form of relevant entries in the “ER Checklist” or the risk analysis document within the manufacturer’s technical documentation (check with “Explanation of terms”)NB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical dataThe Notified Body, when reviewing samples of the manufacturer’s clinical data evaluation, should pay special attention to the following:(a) whether or not the data is relevant to the device or medical procedure involved;(b) where the manufacturer, in the selected sample, has chosen the “literatureroute” (see 4.2.), whether the criteria defined in 4.2. have been applied;(c) where the manufacturer, in the selected sample, has selected the “clinical in-vestigations route” (see.4.3.), whether the criteria defined in 4.3. have been applied.When performing the assessment on samples of a manufacturers risk/benefit as-sessment, the Notified Body will follow the steps indicated in 5.1.1.,1-4.6. Notified Body Specific Procedures and ExpertiseNotified Bodies should establish and implement internal policies and procedures for the assessment of clinical data in order to:a) Ensure that suitable resources, especially relevant knowledge and competencenecessary for such evaluation, are available within the Notified Body and/or by contracting external experts.Such expertise should be sufficient to identify and estimate the risks and benefits associated with the use of the medical devices. The evaluation team should be able to evaluate a risk analysis and the risk management strategy performed by the manufacturer. The evaluation team should understand the device technology as well as the medical procedure [8].Such an evaluation may require input from a qualified medical practitioner (for example physician, dentist, nurse), as appropriate for the particular device, who has clinical experience.When examining the results of clinical investigations, the evaluation team should have knowledge in planning, conduct and interpretation of clinical investigations.All evaluators should be trained and qualified.Particular attention should be drawn to training of external experts with regard to the conformity assessment procedure. The Notified Body should be responsibleNB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical datafor reviewing the opinion of these experts, taking account of their level of knowledge of the provisions of the Directives.b) review the evaluation of clinical data provided by the manufacturer.c) document the opinion with rationale of all experts involved.d) ensure that any external experts involved are impartial and independent from anyparties involved, having due regard to any conflict of interest which may compro-mise impartiality (see also MedDev 2.10/2 [11]) .e) document the result of their assessment. This is achieved through a specificreport which may be part of, or may be referenced in the overall design / type examination report.f) preserve confidentiality of the information and data received from themanufacturer, especially within the terms for contracting external experts.7. References1. Council Directive 90/385/EEC of 20 June 1990 concerning active implantablemedical devices2. Council Directive 93/42/EEC of 14 June 1993 concerning medical devices3. EN 540: Clinical investigation of medical devices for human subjects, 19934. ISO 14155 Clinical investigation of medical devices5. Guidance on when a clinical investigation is needed for CE marking, NB-MED/2.7/Rec16. Task force on CE marking of existing products, NBM/026/95 rev 2.7. EN 60601-1-4 : Medical electrical equipment Part 1: General requirements forsafety, 4. Collateral Standard: Programmable electrical medical systems8. Committee draft ISO/CD 14971,Medical Devices - Risk Management Dated1998-05-21.NB-MED/2.7/Rec3on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECTitle: Evaluation of clinical data9. MedDev 1/94 Rev 10: Guidelines for Regulatory Auditing of Quality Systems ofMedical Device Manufacturers.10. ISO/IEC Guide 51 Guideline for the inclusion of safety aspects in Standards11. MedDev 2.10/2 Rev 01.03.99: Designation and monitoring of Notified Bodieswithin the framework of EC Directives on medical devices.on Council Directives 90/385/EEC,93/42/EEC and 98/79/ECNB-MED/2.7/Rec3Title: Evaluation of clinical dataRev.-Nr. Rev. date accepted amended withdrawnPage 11.05.99 1/3Rev. 1 Notified Body Meeting, Brussels, November 3 & 4, 1998:The NB-MED task force on …Evaluation of clinical data“ presented the current status of the work (see document NBM/172/98).Revision no: 1 The general structure of this work was accepted on occasion of the last meetingsof NB-MED. The document does not deal with clinical investigation but with evaluation of clinical data. Also the key-terms of "clinical data" and "evaluation of clinical data" are explained. Further work will be done also by merging the tabled document with the NB-MED Recommendations NB-MED/2.7/Rec1 Guidance on clinicals . Also it was tried for reaching consistency to follow the ALARP philosophy as applied by ISO/TC 210 on Risk management in accordance with the ISO/IEC guidance 51. In the current work alternative medicines and miracle products were left out of consideration because it was not so easy to make a guidance; also this is more a task for the member states.The members of NB-MED were asked to send their comments within one monthto the Technical Secretariat especially to literature route/ quality of the scientific data and to decision making process . Next meeting of the task force will be held in January 1999. Further development will be made finally within the NBRG.Rev. 2A new draft document NB-MED Recommendation 2.7/Rec3 …Evaluation of clini-cal data“ was delivered to NBRG for further discussion on its meeting on 01./02.02.99.Revision no: 2 stage 1.Rev. 3: NBRG meeting, Dublin, February 1 & 2, 1999: Previous changes to the document were confirmed, and a few further changeswere made to improve clarity, at the 01-02 February 1999, Dublin meeting of the NBRG. It was also decided to send the draft document, with its "Rationale and history" sheet to all member of NB-MED for commenting before presenting it for approval in the Plenary meeting in March 1999.Revision no: 3 stage 1.Rev. 4: NB-MED task force on “Evaluation of clinical data” meeting, Brussels, April 071999The document was amended to address the comments made by the UK MedicalDevices Agency in relation to Rev 3. The changes include revision of the defini-tion of “Clinical data”. The document now clarifies the role of pre-clinical data, for example, in establishing the extent of similarity between device(s) and medical procedures(s) covered by the scientific literature and the characteristics of the device being assessed. Changes also make clear the need to decide whether。
CE技术文件指南NB-MED2[1].5.1(中文)
![CE技术文件指南NB-MED2[1].5.1(中文)](https://img.taocdn.com/s3/m/fde9a31c79563c1ec5da716d.png)
产品技术文档推荐NB-MED/2.5.1/Rec5 章节:2.5.1符合性评估程序;总则关键词:设计档案,技术文档,技术档案1.介绍对技术文档的要求在医疗器械指令的不同附件多有提及,适当时用于符合性评估程序和涉及到的产品。
作为总则,该文档应包括产品的设计、制造和产品的操作。
备注:产品的“操作”包括安装、使用准备、用前检查和维护、校准以及对特殊医疗器械的服务。
文档所包含的具体细节决定于产品的特性和必要的关注点。
从技术角度来看,就是陈述产品符合对医疗器械指令本质要求。
如果使用了该统一标准,技术文档应该明确这些用于说明符合实质要求的哪里是被该标准覆盖的。
注:该推荐标准特为满足医疗器械和有源植入式医疗器械的技术文档的指导需要而写。
该推荐标准也许也是有用的,然而,涉及到体外诊断医疗器械,但是可能根据体外诊断医疗器械指令的实际实施的经验需要修正。
2.目的一份原理和历史页是需要的,请联系技术秘书处。
该推荐标准的目的是向认证方、主管当局和生产商在需要满足医疗器械指令要求的技术文档方面提供指导。
备注:迫使生产商修改现有的已证明适合和充分的技术文档不是该推荐标准的目的。
3.技术资料3.1综述主题指导(ⅰ)技术文档内容(ⅱ)其他该推荐标准不是欲详尽罗列所有特殊情况可能需要的技术文档的列表。
也就是,一些特殊的资料没有没包括近来,而此处会提供一个正当的说法。
指令要求生产商准备技术文档进行确定和证明什么是适宜的和充分的,而确保他的医疗器械符合相关指令。
这在不同的情况有明显的不用,取决于产品的种类、制造随附的风险、安装使用和服务,以及其在市场上的时期。
比如,所有的已有产品,不管类别都有非常正视的设计验证是不可能的。
但是生产商在使用经验和他如何应对出现的问题上都有丰富的经验,这些资料都应用于验证方面。
技术文档应包括3.2-3.5部分所阐述的资料3.2 产品描述主题指导(ⅰ)器械的概括描述 包括变化范围(比如,只有长度不同的同一型号的一组导,和有关器械性能和特性储存的包装描述。
(完整版)CE认证的全套技术文件
受控状态CE.JS-01CE 文件清单拟制日期 2014 年5 月17 日审核日期 2014 年5 月17 日批准日期 2014 年5 月17 日版号 A 生效日期2014 年8 月1 日XX 有限公司受控状态CE.JS-02企业简介拟制日期 2014 年5 月17 日审核日期 2014 年5 月17 日批准日期 2014 年5 月17 日版号 A 生效日期2014 年8 月1 日XX 有限公司企业概况受控状态CE.JS-03 关于欧洲代表声明拟制日期 2014 年5 月17 日审核日期 2014 年5 月17 日批准日期 2014 年5 月17 日版号 A 生效日期2014 年8 月1 日XX 有限公司关于确定欧洲代表的声明本公司欧洲代表是 XX,地址:XXX,联系方式:XXX。
特此确定声明!职位签名日期受控状态CE.JS-04产品描述拟制 ****** 日期 2014 年5 月17 日审核 ****** 日期 2014 年5 月17 日批准 ****** 日期2014 年5 月17 日版号 A 生效日期 2014 年8 月1 日XX 有限公司产品描述一、产品性能特性:“”(商品名:)是由有限公司根据市场和临床治疗的需要,产品中以无机生物活性材料为主要原料而研发生产的 XXX 医疗产品。
当 XXX 产品与创面组织接触时,其中具有生物活性的无机生物活性材料与组织发生离子交换,提高创面局部的氧分压和 PH 值,在表面形成较强的负电势,并通过一系列生化反应,形成一个羟基磷灰石(HCA)组成的多孔网状结果组织,能吸附大量与组织再生有关的各种物质,使新生组织得以顺利爬移。
从而达到加速创面愈合的作用。
二、产品适用范围:“******”(商品名:******)适用与各种手术及外伤造成的创面,皮肤溃疡及褥疮以及浅Ⅱ度烧、烫伤的创面愈合。
三、产品主要技术性能及参数:1、产品命名:1.1 产品通用名:******1.2 产品商品名:******2、产品成份:2.1 粉状产品由组成2、2 膏状产品由组成3、产品形式与规格:3.1产品形式:膏状、粉状3.2产品规格:1)粉状:2)膏状:,3)4、主要技术性能及参数:4、1 产品外观:粉状产品为。
ce技术主文档

ce技术主文档Technical File一次性使用无菌安全自毁型注射器带针Disposal sterile safety & auto-disable syringe,with needle一次性使用无菌注射器带针Disposal sterile syringe,with needleDrafted by:Approved by:Number:Revision:AStatus:ControlledDate:有限公司Co.,Ltd.地址:Address:电话:传真:E-mail:目录第一章:产品简介1.1 生产商信息单位名称:英文:地址:Address:电话:传真:E-mail:联系人:1.2 公司简介有限公司是一家医疗机械专业生产企业。
公司位于村镇,占地面积近两万平米,以生产注射器、输液器等一次性医用耗材为主。
公司环境优美,拥有雄厚的技术力量和先进的生产设备以及精确的检测仪器。
公司将秉承“专业、务实、创新、奉献”的精神,为国内外用户提供产品和服务。
简介写的有点简单,可以增加点:公司的注册资金、人员情况、部门设置、设备情况、生产的产品情况、销售区域等等)1.3、公司组织机构图:Company Organization Structure1.4 关键原材料、供方及认证证书:(证书见附件1 )1.4.2产品关键件清单注:注射针外购,检验报告见附件1.5 产品描述一次性使用无菌安全自毁型注射器带针Disposal sterile safety & auto-disable syringe,with needle 一次性使用无菌注射器带针Disposal sterile syringe,with needle1.5.1产品主要结构:a)一次性使用无菌安全自毁型注射器带针产品结构:由芯杆、连接座、外套、胶塞、注射针组成。
01—芯杆;02—外套;03—橡胶活塞;04—连接座;05-密封圈;06—注射针;07—注射针护套b)一次性使用无菌注射器带针产品结构:由芯杆、外套、胶塞、注射针组成。
东盟质量指南中文版

人体用药物注册的东盟通用技术文件第二部分:质量目录第一节:质量综述 11.原料药 (1)2.制剂 (4)指南范围 (8)第二节:目录 (8)第三节:数据主体 81.原料药 (8)2.制剂 (14)第四节:主要参考文献 (18)第一节:质量综述(QOS)NCE : New Chemical Entity新生化学实体Biotech : Biotechnological Products生物科技产品MaV : Major Variation主要变异体MiV : Minor Variation次要变异G : Generics泛型本文件旨在对东盟合同技术要求范围内的制剂的注册申请格式提供指南。
此格式适用于新生化学实体,生物科技产品,主要变异,次要变异和泛型。
为确定次格式是否适用于特定类型的产品,申请者应和相应的国家管理部门咨询。
本指南中的“主题数据” 仅表明信息应放在何处。
具体的支持性数据的类型和程度都未发表在本指南中,但他们都应根据各个区域的指南和或接受国际主要参考标准(药典)。
对于新生化学实体和生物科技产品的要求,请参照相关国际协调会指南。
第二节:目录应提供申请文件的目录第三节:主体数据S 原料药S1 一般信息S1.1 命名●国际非专利名称(INN)●相关的药典名●化学文摘服务社登记号(CAS)●实验室代码(如果适用)●化学名S1.2 结构式新化学实体:应提供包括相对的和绝对的立体化学的结构式,分子式和相对的分子量。
新科技产品:如果合适,应提供糖基化部位的化学图解氨基酸顺序,平移后变体和相关分子量。
属性:药典要求和生产商等值信息。
S1.3一般性质应提供原料药的物理化学的或其他相关性质的列表,包括生物技术产品的生物活性。
参考ICH指南:NCE:Q6A, Biotech:Q6B.S2 生产制造S2.1生产商名称和包括活性成分制造商所在城市和国家在内的全地址。
S2.2制造工艺和工艺控制的叙述原料药生产工艺的叙述代表了申请者对原料药制造的承诺。
医疗器械CE认证及MDD指令介绍

深圳市龙德生物科技有限公司版权所有
医疗器械全球咨询 医疗器械全球贸易
欧盟医疗器械指令
第2章 上市及使用
Hlongmed
各成员国必须采取所有必要的措施,以确保器械依其预期的用途进行安装,维护以及使用 时不会危害病人,使用者,适用时,其他人员的安全和健康后方可上市。
深圳市龙德生物科技有限公司版权所有
医疗器械全球咨询
医疗器械全球贸易
欧盟医疗器械指令——分类
Hlongmed
第9条 分类 根据MDD指令附录IX,医疗器械根据 预期用途、接触人体时间与部位等 分为I,IIa, IIb, III类,风险从低到 高。 如果制造商与公告机构在产品分类上有 争议时,则由该公告机构隶属的会 员国主管机关裁决。
医疗器械CE认证及MDD指令
Hlongmed 龙德
欧洲联盟成员国
Hlongmed
欧洲联盟成员国是根据欧洲联盟条约,自愿 加入欧洲联盟的国家,和一般的国际组织不 同,作为欧盟的成员国,要遵守共同指定的 同一法律。 到目前为止,欧盟共有27个成员国,分别是: 奥地利、比利时、保加利亚、塞浦路斯、捷 克、丹麦、爱沙尼亚、芬兰、法国、德国、 希腊、匈牙利、爱尔兰、意大利、拉脱维亚、 立陶宛、卢森堡、马耳他、荷兰、波兰、葡 萄牙、罗马尼亚、斯洛伐克、斯洛文尼亚、 西班牙、瑞典、英国。 克罗地亚于2013年7月1日成为第28个欧盟 成员国。 进入欧盟的产品强制性地要求产品携带CE标 志。
2
深圳市龙德生物科技有限公司版权所有
医疗器械全球咨询 医疗器械全球贸易
什么是CE标志
Hlongmed
进入欧盟的产品强制性地要求产品携带CE标志。 字母CE是法文句子“”的缩写,其意思是“符合欧洲(标准)”。 CE标记是一个特定的标志,可以按一定比例进行放大和缩小。且两 个字母是等高的。CE标记高度不能低于5mm。
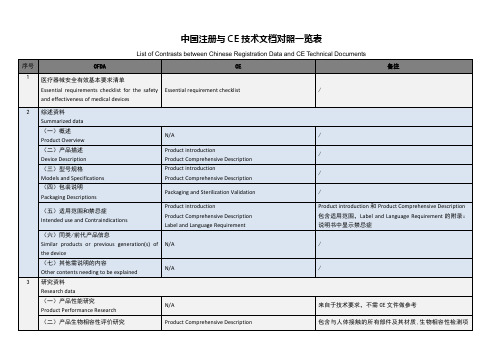
模板 中国注册资料与CE技术资料对照一览表
/
(三)型号规格
Models and Specifications
Product introduction
Product Comprehensive Description
/
(四)包装说明
Packaging Descriptions
Packaging and Sterilization Validation
(七)软件研究
Software Research
Softwarevalidationreport
Software description
/
(八)其他资料
Other Research Data
N/A
/
4ቤተ መጻሕፍቲ ባይዱ
生产制造信息
Manufacturing information
Production Flow Description
List of Applicable Standards and Directives
包含适用的标准及指令清单
8
产品说明书和最小销售单元的标签样稿
Instructions for use & label design drafts for the minimum marketing unit
Label and Language Requirement
Essential requirement checklist
/
2
综述资料
Summarized data
(一)概述
Product Overview
N/A
/
(二)产品描述
Device Description
Product introduction
无源医疗器械CE技术文档和CE设计档案材料指南(中英文 也是极好的)

无源医疗器械技术文件和设计文档指南Whereas the term “Technical File“ is used for Medical Devices of class I, class IIa and class IIb, the term “Design Dossier“ is used for the class III products.标题中的“技术文件”适用于I类,IIa类,IIb类医疗器械,“设计文档”适用于III类医疗器械。
Technical Files are retained in the premises of the manufacturer or the Authorized Representative for potential review of Competent Authorities and Notified Body.Part B of the Technical File may be available at the manufacturer only.技术文件是保留在制造商或授权代表单位的主管部门和认证机构。
部分技术文件B部分只保留在制造商处。
Whereas Design Dossiers have to be submitted to the Notified Body for review prior to CE-Marking of the product (use form Application for CE Conformity Assessment (Product)MED_F_03.03). We will assign a project manager who will entrust one or more further experts with the review of particular modules. All experts are at your disposal directly or indirectly through the project manager. After successful review, the Notified Body issues a design examination certificate according to Annex II.4 of the Council Directive certifying compliance with the relevant provisions of Annex I of the MDD.设计档案材料已被提交到公告机构用于需要CE认证前的产品审查(用CE合格评定(产品)MED_F_03.03规定的格式)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
及为什么特定法规不适用的原因,如果理由不是一目了
然的
话。 对于体外诊断医疗器械,在体外诊断医疗器械指令的附件
Ⅱ中所给出的列表中或器械已标贴 为“自 检” 的情况,特
定器械的分类是一目了然的。
指导
(ⅰ) 技术要求的识别
生产商应明确所关注特定器械应用的指令, 包括所有指令而 不只
是医疗器械指令。 对于不明显的, 生产商应将其分为医 疗器械的
此处,器械因素是关心的主题(比如,可能引起过敏反应的乳 胶的使用), 技术文档中的风险分析应说明这些因素。
( ⅵ) 设想生产方法的描述
几个主要生产期要求有生产方法
的概述(比如,注塑 / 吹塑, 挤塑化学处理组装包装 / 标识) 以及 灭菌方法的概述
挤塑,化学处理, 组装,包装 / 标识)以及灭菌方法的概述, 如有
因为体外诊
断医疗器械不作用于人体。 风险分析应说明包含医药物质附
加风险和受益。技术文档 应包括对该联系所进行试验的数
据。 备注:对于 “副产品” 和“药物 / 器械合并” 的监
管指导见 MedDev 2.1/3 。
对于该类器械,技术文档中的风险分析应说明包含该材料 的附加风险和受益和所采取的措施(比如,动物来源,牲 畜医治和控制和消除 / 组织传染作用物的措施) 备 注 : 对 于 控 制 和 消 除 / 组 织 传 染 作 用 物 的 措 施 指 导 见 MedDev 2.5/5 。 备注:体外诊断器械可能包括动物(人类)源材 料,技术 文档应包括相关详细信息,包括来源和保护人员 的措施以 及储存器械而保护其性能的措施。
标准也许也是有用的,然而,涉及到体外诊断医疗器械,但是可能根据体外诊断医疗器械Fra bibliotek指令的
实际实施的经验需要修正。
2. 目的 一份原理和历史页是需要的,请联系技术秘书处。
该推荐标准的目的是向认证方、主管当局和生产商在需要满足医疗器械指令要求的技术文档方 面提供指导。 备注:迫使生产商修改现有的已证明适合和充分的技术文档不是该推荐标准的目的。
比如,脉冲血氧
计的输出和处理和不同波长的光的吸收有
关。而输出可能包
括血红蛋白的氧饱和度显示和患者脉冲
的图形显示。
对于包含了医药物质的器械, 其“ 技术文 档” 应明确包含
该物质的目的与其在应用中的作用模式。这只适用于该物
质
依赖于由于器械的辅助措施而对身体产生的行为。
备注:出
于这个原因,该部分不涉及体外诊断医疗器械,
作” 包括安装、使用准备、用前检查和维护、校准以及对特殊医疗器械的服务。文档所包含的具
体细节决定于产品的特性和必要的关注点。从技术角度来看,就是陈述产品符合
对医疗
器械指令本质要求。如果使用了该统一标准,技术文档应该明确这些用于说明符合实
质要求的哪
里是被该标准覆盖的。
注:该推荐标准特为满足医疗器械和有源植入式医疗器械的技术文档的指导需要而写。该推荐
产品技术文档
推荐 NB-MED/2.5.1/Rec5 章节: 2.5.1 符合性评估程序;总则
关键词:设计档案,技术文档,技术档案
1. 介绍 对技术文档的要求在医疗器械指令的不同附件多有提及,适当时用于符合性评估程序和
涉及到的产品。作为总则,该文档应包括产品的设计、制造和产品的操作。
备注:产品 的“操
联系。应明确确保所生产器械的欲特性和性能涉及到
的技术和
方法。不需要详尽描写整个生产过程。
(ⅶ) 生产商欲和器械联合 使用 的附件、接头和 其他器械 或设备以及 其他接合部位 的描述
技术文档应包括该器械欲联合使用的其他器械或设备的描
述。比如,生产商已做了关于兼容性的特殊声明。同时也
应
包括联合使用的安全和性能方面的验证和确认数据。
取 决于产品的种类、 制造随附的风险、 安装使用和服务, 以及其在市场上
的时期。 比如, 所有的已有产品, 不管类别都有非常正视的设计验证是
不
可能的。但是生产商在使用经验和他如何应对出现的问题上都有丰富
的经
验,这些资料都应用于验证方面。
(ⅱ) 其他
技术文档应包括 3.2-3.5 部分所阐述的资料
3.2 产品描述 主题
3. 技术资料 3.1 综述
主题
指导
(ⅰ) 技 术 文 档 内 容
该推荐标准不是欲详尽罗列所有特殊情况可能需要的技术文档的列表。
也就是,一些特殊的资料没有没包括近来, 而此处会提供一个正当的说
法。 指令要求生产商准备技术文档进行确定和证明什么是适宜的和充分
的, 而确保他的医疗器械符合相关指令。 这在不同的情况有明显的不用,
(ⅰ) 器械的概括描述
(ⅱ) 器械的预用途和操作描述
(ⅲ) 包含医药物质的器械
(ⅳ) 包含动物源的不能存活材 料的器械
(ⅴ) 需要特殊关注的器械
指导
包括变化范围(比如,只有长度不同的同一型号的一组导
管),和有关器械性能和特性储存的包装描述。所有这些是
需要
的,但只是简介描述到能够充分了解设计、特性,如
描述安全
和正确操作的要求,当和来自其他生产商的器械
或设备共用
时,只简单进行描述,足够了解重要参数或接
口(比如需要
的接头或电压、频率和 / 或电源的稳定性。 技术文档应说明已
知的不兼容,比如在标识或者使用说明
上。
(ⅷ) 该相关指令下的器械分类
3.3 技术要求 主题
技术文档应包括该指令下所应用的法规号码,和分类原理以
适用,
器械的性能和变化之间的区分即可。很多情况,只
有器械名
称就足够了。 如果“ 技术 文档” 是用来认证方评 估的,一
个器械的概括绘图,比如示意图,照片或绘图是
有助审核
的。 备注:对于指导中的产品变化的“范围”
, 见 NB-MED
推荐 NB-MED/2.1/Rec2。
对器械的计划目的 / 应用和 / 或使用方式需要简单的描述。 可
能包括,如适合,该器械欲用患者人群和医用状况的详
细情
况。同时说明欲使用人,尤其是该器械是否是专业人
员使
用。所有以上理所当然都是来自该器械的概括描述。
该信息
可以以参考 “使 用说明 ”或 操作手册的方式给出。 没必
要详细介绍该器械达到预期目的的机械原理。
该描述应明确
有关器械欲操作的重要输入和输出的处理。
原理编制成文件并确定还要应用的企业要求。
每种情况,所应
用的指令(比如,MDD附件 I, AI MD附件 I, IV DD附件 I, )的这些基本
要求( ERs)和这些指令的其他要求均 应被识别。生 产商应界定
