fuel_cell_city_bus_Yang
燃料电池FLUENT仿真
Manifold( cell stack inlet) velocity vector>>> vector
(1) The velocity distribution is uniform (2) Without negative velocity
•Modeling of the flow field
University of Victoria, Canada
2006.10.11
Outline of the Presentation
R & D of fuel cells in WUT Introduction to fuel cell modeling Gas field modeling of PEM fuel cells Water transport modeling of PEM fuel cells Heat transfer modeling of PEM fuel cells Fractal models of GDLs in PEM fuel cells
Work focused on
Flow and diffusion of gases in fuel cells. Phase change of water, two-phase flow in fuel cells. Heat production and transport in stacks, optimization of the cooling manner. Electrochemical reaction process in fuel cells, distribution of potential field, current density and over potential. Mechanical and thermal stress produced during the assembly of stack and service.
车载燃料电池冷却系统去离子树脂吸附性能研究

1 绪论车载燃料电池冷却系统中,冷却液电导率高会导致整车在启动时无法通过自身的高压绝缘检测,导致整车无法接通高压系统并启动[1,2]。
在冷却系统运行过程中,导致冷却液电导率提升的因素包括散热器、管路、阀体及燃料电池电堆本体的冷却管路,主要包括铝合金、不锈钢以及硅胶管等主要材料,随着冷却液的循环,各种材料都会有一定程度的离子析出[3,4]。
为降低冷却液中的电导率,目前通常采用的方式为在冷却回路中增加去离子罐,通过去离子罐中的树脂进行吸附,降低电导率[5]。
但整体吸附效率、使用周期以及使用效果仍很难满足燃料电池汽车的规模化推广,降低了车辆使用过程便捷性。
在树脂选型上,不仅需要考虑树脂本身的吸附性能和要求,同时还要考虑其与冷却液的配合,避免出现相互影响。
不同性能的树脂对于溶液中的导电离子有不同的吸附作用和效果,因此树脂的选择及处理也直接关系到整体的吸附效率和后期的使用。
通过前期研究发现,为了提升整车的散热效率,燃料电池汽车通常采用钎焊式散热器以增加有限空间的散热量,而冷却液中的离子主要来源于散热器在加工过程中残留的助焊剂[6,7]。
车载燃料电池冷却系统去离子树脂吸附性能研究 张少鹏1 申 彤2 韦 瑾2 张 晓2 段伦成1 梁 晨1,3*Study on Adsorption Performance of Ion Exchange Resin for fuel Cell Cooling System on VehiclesAbstract: The high-voltage insulation of the fuel cell vehicle is strongly affected by the electrical conductivity of the coolant in the fuel cell cooling system, which can be effectively reduced by ion exchange resin. But there are also problems such as short service life, replacement and adsorption efficiency when applying this resin. In this paper the ion exchange resin is tested to verify its ion exchange performance, efficiency and impact on the overall cooling system. The research of removal performance for different ions in the coolant is also conducted. It shows that the increase in coolant temperature can improve the ion exchange efficiency, and adding fluorosis to the base resin can strengthen the exchange of fluoride ions in the coolant. In practical applications, different resins can be mixed together to improve the ion exchange efficiency with little increase on overall resin volume.Key words: fuel cell, cooling system, electrical conductivity, ion exchange resin, ion exchange针对助焊剂在冷却液中所析出的阴阳离子,对不同离子交换树脂在不同温度及流量的工况下进行交换吸附的吸附效率、交换容量以及压力损失等比较测试,为整车装车及燃料电池系统长期推广应用提供基础保障。
质子交换膜燃料电池电堆水传输机理综述

174AUTO TIMEAUTO PARTS | 汽车零部件质子交换膜燃料电池电堆水传输机理综述夏增刚上海捷氢科技有限公司 上海市 201800摘 要: 质子交换膜燃料电池是一种直接将储存在H2的化学能经与O2反应转化成电能、热能和水的电化学装置。
它不受卡诺循环的限制,转化效率高,可以长时间连续运行,具有运行温度低、功率密度高、响应快、启动快、稳定性好以及当使用纯氢气时不会造成环境污染等特点,是未来汽车的理想动力装置之一。
合适的湿度条件是燃料电池健康高效运行的必要条件,本文针对水在燃料电池内的传输问题进行综述归纳,为燃料电池内的水传输问题建立研究基础。
关键词:质子交换膜燃料电池 水传输1 研究背景与意义质子交换膜燃料电池技术因具有,启动快,效率高,温度低,功率密度高,运行平稳,使用纯氢时不会造成环境污染等优点。
随着环境污染与全球气候变暖问题的日益严重,其作为一种汽车动力系统解决方案而日益受到关注[1-2]。
对于车载应用,质子交换膜燃料电池系统的耐久性与可靠性的提升是最具有挑战性的问题。
燃料电池电堆的正常工作的需要适中的湿度范围,所以良好水管理是提升质子交换膜燃料电池可靠性与耐久性的重要手段[3-4]。
随着燃料电池技术的发展,对于实用的车载质子交换膜燃料的电池系统,MEA 普遍趋势是越来越薄,对水的传输性能更好,容易发生水淹故障。
燃料电池在高电流密度下,产生更多的水且工作压力更大,气态水更容易液化,堵塞气体扩散层(GDL)与流道,阻碍反应气体传质,造成反应欠气,电堆性能下降同时损害电堆耐久性[5]。
所以燃料电池水状态传输机理是燃料电池系统研究的重要问题。
2 燃料电池内部水传输燃料电池内部的水传输机制如图1所示,主要有TOD (Thermal-osmotic drag),EOD (Electro-osmotic drag),BD(Back Diffusion)和HP(Hydraulic Permeation)[6]。
8.5米氢燃料电池城市客车动力电池匹配方案设计

8.5米氢燃料电池城市客车动力电池匹配方案设计李兵戴蕤睿丁延军(安徽安凯汽车股份有限公司)摘要:根据某款8.5米氢燃料电池城市客车的主要性能参数,计算并设计出了该款车的动力电池匹配方案。
关键词:氢燃料电池城市客车动力电池Design of8.5-meter hydrogen fuel cell city bus power battery matching scheme Abstract:According to the main performance parameters of a8.5-meter hydrogen fuel cell city bus,it calcu⁃lates and designs the power battery matching scheme of the vehicle.Keywords:hydrogen fuel cell,city bus,power battery.0引言氢燃料电池汽车是利用氢气在燃料电池中反应产生的电能,直接驱动电机运转或对动力电池充电,从而驱动车辆行驶的新能源汽车[1]。
氢燃料电池汽车的排放物只有水,真正实现了无污染、零排放[1-2],因此国家加大了对氢燃料电池汽车的财政补贴力,如2018年国家对8-10米燃料电池客车的最高财政补贴高达50万元[3-4]。
同时随着电动汽车电池的能量密度、pack技术、安全性能等技术成熟度的提高[5-6],国家对电动汽车的财政补贴也相应地出现了退坡现象[3-4],因此许多汽车生产企业将发展氢燃料电池汽车提上议事日程,如宇通客车和福田汽车都已经通过了氢燃料电池客车的生产资质审查,同时也有部分新产品(燃料电池客车)在市场上亮相[7]。
安凯客车一直关注着氢燃料电池客车的技术路线与市场走向,经过研究人员的努力,研发出了某款8.5米氢燃料电池客车。
本文针对该款车的主要技术参数,计算并设计出了动力电池的匹配方案。
元胞自动机模型在城市交通流模拟中的应用

元胞自动机模型在城市交通流模拟中的应用第一章:引言随着城市化的不断加速,城市交通流成为了城市运行中至关重要的组成部分。
如何高效地管理和规划城市交通,成为了城市发展的重要课题。
而元胞自动机模型作为一种重要的仿真工具被广泛应用于城市交通流模拟中,能够模拟城市交通的复杂流动。
本文将讨论元胞自动机模型在城市交通流模拟中的应用并分析其优势和不足。
第二章:元胞自动机模型元胞自动机是由冯·诺依曼在1950年代中期提出的,是一种抽象的离散动力学系统,由一些简单的局部规则来描述整个系统的行为。
元胞是一个计算单元,可能处于一些离散的状态之一。
当局部规则被应用于元胞的状态时,整个系统就会发生变化。
元胞自动机可用于模拟复杂的自然或社会现象,如交通流。
第三章:城市交通流模拟城市交通模拟是一种仿真技术,可以模拟城市道路网络流量以及各个交通参与者之间的相互作用。
现代城市交通模拟通常基于计算机建模技术,能够精确地描述城市交通中的各个要素,如车辆、行人等,并计算其在时空上的分布与运动。
通过交通模拟,可以优化交通系统,提高交通效率。
第四章:元胞自动机模型在城市交通流模拟中的应用元胞自动机模型是城市交通模拟中的一种重要的建模技术。
它通过将城市交通网络离散化,将交通系统划分为单个空间单元,从而模拟道路上的交通流量和交通参与者之间的相互作用。
元胞自动机模型能够精确地描述道路上的交通情况,模拟车辆的行驶路径和速度,并考虑车辆之间的相互作用。
同时,元胞自动机模型还可以模拟行人、自行车等不同类型的交通参与者,在交通规划方面具有很大的价值。
第五章:元胞自动机模型的优势与其他建模技术相比,元胞自动机模型具有一些优势。
首先,元胞自动机模型可以模拟非线性关系,能够更好地反映真实的交通场景。
其次,元胞自动机模型可以模拟复杂的交通现象,如拥堵、事故等,可以为交通规划提供较为准确的数据支持。
此外,元胞自动机模型非常适合进行探索性研究和情景分析,可以帮助决策者更好地了解交通系统的运作,并制定更好的交通规划。
Carbon nanotube-polyaniline composite as anode material

Journal of Power Sources170(2007)79–84Short communicationCarbon nanotube/polyaniline composite as anode materialfor microbial fuel cellsYan Qiao a,b,Chang Ming Li a,b,∗,Shu-Juan Bao a,b,Qiao-Liang Bao a,ba School of Chemical and Biomedical Engineering,Nanyang Technological University,70Nanyang Drive,Singapore637457,Singaporeb Center for Advanced Bionanosystems,Nanyang Technological University,70Nanyang Drive,Singapore637457,SingaporeReceived9December2006;accepted7March2007Available online30March2007AbstractA carbon nanotube(CNT)/polyaniline(PANI)composite is evaluated as an anode material for high-power microbial fuel cells(MFCs).Fourier transform infrared spectroscopy(FTIR)and scanning electron microscopy(SEM)are employed to characterize the chemical composition and morphology of plain PANI and the CNT/PANI composite.The electrocatalytic behaviour of the composite anode is investigated by means of electrochemical impedance spectroscopy(EIS)and discharge experiments.The current generation profile and constant current discharge curves of anodes made from plain PANI,1wt.%and20wt.%CNT in CNT–PANI composites reveal that the performance of the composite anodes is superior. The20wt.%CNT composite anode has the highest electrochemical activity and its maximum power density is42mW m−2with Escherichia coli as the microbial catalyst.In comparison with the reported performance of different anodes used in E.coli-based MFCs,the CNT/PANI composite anode is excellent and is promising for MFC applications.©2007Elsevier B.V.All rights reserved.Keywords:Polyaniline;Carbon nanotube;Microbial fuel cell;Anode modification;Power density1.IntroductionMicrobial fuel cells(MFCs)use bacteria as catalysts to oxi-dize organic and inorganic matter for energy generation.A number of factors affect MFC performance,namely,micro-bial inoculums,chemical substrate,proton exchange material (or absence of this material),cell internal and external resis-tance,solution ionic strength,electrode materials,and electrode spacing[1–3].Among these factors,a high-performance elec-trode material is most essential.To improve the power output of MFCs,much research has focused on cathode modification and optimization of bacteria inoculums[4,5].The anode is also an important determinant of MFC performance and is often the limiting factor for a high power output.The anode material and its structure can directly affect the bacteria attachment,elec-tron transfer and substrate oxidation.To date,carbon materials such as carbon cloth and carbon paper are applied in most MFC ∗Corresponding author at:School of Chemical and Biomedical Engineer-ing,Nanyang Technological University,70Nanyang Drive,Singapore637457, Singapore.Tel.:+6567904485;fax:+6567911761.E-mail address:ECMLi@.sg(C.M.Li).anodes due to their stability in a microbial inoculum mixture, high conductivity and high specific surface-area.Nevertheless, they have little electrocatalytic activity for the anode microbial reactions and thus modification of the carbon materials is the main approach for improving their performance.Thus,there is a great need to develop a new type of anode material for MFCs that is more advantageous than regular carbon cloth and carbon papers.Carbon nanotubes(CNTs)have exhibited very promising properties as a catalyst support in fuel cell applications due to their unique electrical and structural properties[6].For exam-ple,CNTs are superior to carbon blacks as catalyst supports for proton exchange membrane fuel cells(PEMFCs)[7,8].They have also served as anode materials for enzymatic biofuel cells [9,10].It has been reported,however,that CNTs have a cellular toxicity that could lead to proliferation inhibition and cell death [11,12].Thus,they are not suitable for MFCs unless modified to reduce the cellular toxicity.Conductive polymers have attracted intensive research in dif-ferent electrochemical devices[13–15].Polyaniline(PANI)an important conducting polymer due to its relatively facile pro-cessability,electrical conductivity,and environmental stability.0378-7753/$–see front matter©2007Elsevier B.V.All rights reserved. doi:10.1016/j.jpowsour.2007.03.04880Y.Qiao et al./Journal of Power Sources170(2007)79–84The electronic properties of PANI can be reversibly controlled by both doping/de-doping and protonation processes,in which its emeralding base and emeraldine salt form can be interchanged [16].The polymer has been used to detect microorganism such as Escherichia coli with an enzyme based method[17,18]. Schroder and Scholz[19]have employed PANI to modify a platinum electrode for use as the anode of MFC and obtained one order of magnitude increase in current outputs.Conductive PANI used in the MFC not only provides a protective function for bacteria,but also directly contributes to electrocatalysis to give a high current density[19,20].On the other hand,the lower con-ductivity and poor electron transfer of PANI limit its application in MFCs.The fabrication of CNT/PANI composites has attracted great interest in recent years because the incorporation of CNTs into PANI can result in new composite materials with enhanced elec-tronic properties.For example,it has been reported[21]that PANIfibres containing CNTs displayed significant improve-ments in both mechanical strength and conductivity[21].Liu et al.[22]constructed CNT/PANI multilayerfilms by means of a layer-by-layer assembly method,and the CNTs inside the multilayerfilm could expand the electroactivity of PANI to a neutral electrolyte.Thus,the enhanced conductive CNT/PANI composite could possibly be used in MFCs for performance improvement.The electrocatalytic behaviour and application of the CNT/PANI composite in MFCs has not been studied.In the work reported here,a CNT/PANI composite is used as an anode of MFCs and its electrocatalytic properties associated with a bacterium biocatalyst are examined.2.Experimental2.1.Chemicals and materialsAniline(≥99.0%),ammonium persulfate(APS,ACS reagent,≥98.0%),polytetrafluoroethylene(PTFE)solution (1wt.%)and2-hydrox-1,4-naphthoquinone(HNQ,97%)were obtained from Sigma–Aldrich.Multi-walled CNTs(95%, 10–20nm)were received from Shenzhen Nanotech Co.Ltd. (Shenzhen,China).All other chemicals were of analytical grade and used as-received,unless stated otherwise.De-ionized(DI) water(resistance over18M cm)from a Millipore Q water purification system was used in all experiments.2.2.Synthesis of PANI and CNT/PANI compositesAniline monomer was distilled under reduced pressure before polymerization.The PANI was chemically synthesized as fol-lows:aniline(1mL)was mixed with HCl(0.3mL)in50mL DI water in an ice bath.An APS solution(2.3g in25mL DI water) was added to the mixture.The polymerization was carried out for6h in the ice bath(0–5◦C).A green solid of proton-doped PANI was obtained after rinsing with DI water for several times. The CNTs were ultrasonically treated using a mixture of3:1 of H2SO4:HNO3at50◦C for24h to produce carboxylic acid groups at the defect sites and thereby improve solubility in HCl solution.The composite of proton-doped PANI/CNT was syn-thesized in situ via chemical oxidation.Different weight ratios of CNT to aniline were used.The CNTs were dissolved in1.0M HCl solution,subjected to ultrasonic treatment for3h,and then transferred to a250mLflask placed in the ice bath.A1.0M HCl aniline monomer solution was added to the prepared CNT–HCl suspension.The APS solution was added to the suspension with constant stirring at0–5◦C for6h.A precipitate was produced. Afterfiltering and rinsing for several times with DI water and methanol,the precipitate was vacuum-dried at60◦C for24h and a powder containing CNT and PANI was obtained,which was ready for preparation of the anode of the MFC.2.3.Characterization of PANI and CNT/PANI compositeInfrared spectra were obtained by means of Fourier trans-form infrared spectroscopy(Nicolet MAGNA-IR560,FTIR, U.S.A.)with an attenuated total reflection(ATR)accessory. The morphology of the plain PANI and the CNT/PANI com-posite powder was studied with a JEOL6700field emission scanning electron microscope(FESEM).Nitrogen adsorption isotherms were measured with an automated gas sorption system (AUTOSORB-1,Quantachrome Instruments)at liquid nitro-gen temperature.The specific surface-area was calculated using Brunauer–Emmett–Teller(BET)method.2.4.Electrode preparationThe plain PANI and its composite powders were mixed with a PTFE solution to prepare pastes.The pastes were coated on the surface of nickel foams(1cm×1cm×0.1cm)to produce uniformfilms that were then pressed to fabricate PANI and CNT/PANI electrodes,respectively.Thefilm covered the whole surface of the foam to prevent exposure of the nickel to the elec-trolyte.After drying at120◦C to remove water,the electrodes were used as anodes for MFCs.2.5.Bacteria growthE.coli K12(ATCC29181)was grown anaerobically at37◦C for12h in a standard glucose medium,which was a mixture of 10g glucose,5g yeast extract,10g NaHCO3and8.5g NaH2PO4 per litre.For chronoamperometric measurements,the overnight culture in the above medium was inoculated into a fresh anaer-obic medium.For impedance and constant-current discharge experiments,bacteria culture was harvested by centrifuging at 4◦C(6000rpm,5min).The produced bacteria were washed for three times and then suspended in a0.1M anaerobic phosphate buffer containing5.5mM glucose.The concentration of E.coli cells was about109cells mL−1.Before every test,nitrogen was purged into the suspension for20min to remove oxygen from the cell.2.6.Electrochemical measurementsAll electrochemical experiments were carried out with PGSTAT30Autolab system(Ecochemie,Netherlands)in a three-electrode cell that consisted of the working electrode,aY.Qiao et al./Journal of Power Sources170(2007)79–8481 saturated calomel(sat.KCl)reference electrode,and a platinumwire counter electrode.2-Hydroxy-1,4-naphthoquinone(HNQ)was chosen as the electron mediator because it can generatehigher coulombic output than commonly used mediators such asresazurin or thionine[23].Except otherwise stated,electrochem-ical impedance measurements for the PNAI and CNT/PANIcomposite electrodes were performed over a frequency rangeof0.5Hz to100KHz in0.1M phosphate buffer at open-circuitpotential and a perturbation signal of10mV.3.Results and discussion3.1.FTIR spectroscopy and morphology characterizationThe FTIR spectra of plain PANI and the CNT/PANI com-posite are shown in Fig.1.The peak at835cm−1is attributedto the N–H out-of-plane bending absorption.The peaks at1500and1600cm−1can be assigned to the stretching vibration ofthe quinoid ring and benzenoid ring,respectively,which arecharacteristic of PANI.The presence and the enhanced relativeintensity of the absorption band with a maximum at1240cm−1for the composites are very prominent.The features are due to theC–N stretching vibration in proton-doped PANI.The fact of theremarkable enhancement of the spectra indicates the formationof C–N coordinate-covalent bonds between the polymer chainand the radical cation CNT fragments[16,24].The broad bandat1730cm−1in PANI(C O vibration)is drastically enhancedfor the CNT/PANI composite and becomes the most promi-nent peak for the20wt.%CNT/PANI powder.The band near3000cm−1is due to the C–H stretching absorption.This signalis broad and strong in the composite samples and relatively weakin the plain PANI.This phenomenon was reported recently and itwas explained that the sp2carbons of the carbon nanotubes per-turbed the H-bonding environment and then increased the N–Hstretch intensity.These results strongly support the formation ofCNT/PANI through the chemical oxidation method.Scanning electron micrographs offilms made from plainPANI and the CNT/PANI composite powders are showninFig.1.FTIR spectra of PANI and composite powders(a:plain PANI;b:1wt.% CNT/PANI composite;c:20wt.%CNT/PANIcomposite).Fig.2.SEM images of PANI and CNT/PANI compositefilms(a:plain PANI; b:20wt.%CNT/PANI composite).Fig.2.The pure PANIfilm is compact andfibrillar,while the CNT/PANI compositefilms have a networked-rod nanostruc-ture,in which the outer layer is PANI and the inner layer is constructed by CNTs.The rough,amorphous outer PANI layer has an average thickness of about several tens of nanometers.To verify the effect of CNT doping on the structure of the polymer, the specific surface-areas of plain PANI and the nanocomposite were measured.The cumulative adsorption surface area(BJH Method)of plain PANI is34.1m2g−1.For the nanocomposite, the value is50.2m2g−1.These values show that the specific surface-area of the composite is much larger than that of the plain polymer.Therefore,it is expected that the difference in the structure of the materials can result in different discharge profiles and impedance spectra of the anodes constructed from these materials.3.2.Electrochemical impedance spectra studiesElectrochemical impedance spectroscopy(EIS)measure-ments were carried out to compare the characteristics of charge82Y.Qiao et al./Journal of Power Sources170(2007)79–84Fig.3.(A)EIS of PANI(a),1wt.%CNT/PANI(b)and20wt.%CNT/PANI(c) composite electrode in0.1M phosphate buffer(pH7)at open-circuit potential and equivalent circuit used tofit the EIS.(B)EIS of20wt.%CNT/PANI in 0.1M phosphate buffer with or without bacteria and glucose at100mV vs. SCE.transfer and ion transport in plain and composite polymers.The measured EIS results(Fig.3)show well-defined single semicir-cles over the high frequency range,followed by short straight lines in the low-frequency region for all samples.Although the impedance spectra have similar shapes,the diameters of the semicircles decline greatly with increasing content of the CNTs in the polymerfilm.The diameter of the semicircle corresponds to the interfacial charge-transfer resistance(R ct),which usu-ally represents the resistance of electrochemical reactions on the electrode and is often called the Faraday resistance[25].In the absence of bacteria and glucose,the R ct of the three electrodes is1317(PANI),827(1wt.%CNT/PANI)and434 (20wt.% CNT/PANI),respectively.Obviously,the R ct of the CNT/PANI composite is much lower than that of the pure polyaniline.Since there are no glucose and bacteria in the electrolyte,the R ct should be ascribed to the doping/de-doping redox reactions of PANI. The impedance plane plots in Fig.3show only a very short part of a straight line region,which is an indication of diffusion con-trol for the doping/de-doping process[25].It is known that the reactants in the doping/de-doping reaction are anions.The nar-row region of the diffusion-controlled process indicates that all the tested anodes have a good micro/nanostructure for the reac-tant to access the reaction centres without a diffusion limit over a wide frequency range.In a conducting polymer/CNT compos-ite,it has been suggested that either the polymer functionalizes the CNTs,or the conductive polymer is doped with CNTs,and a charge transfer occurs between the two constituents[26].It is also considered that the CNTs have an obvious improve-ment effect for a faster charge transfer rate than with plain PANI.The EIS of the20wt.%CNT/PANI electrode in phos-phate buffer in the presence of bacteria with and without5.5mM glucose were also investigated at a dc bias of100mV versus SCE.The results are shown in Fig.3B.When the electrode is tested in the phosphate buffer with both bacteria and glucose, the R ct is about156 ,which is significantly smaller than that in presence of bacteria but without glucose in the electrolyte (400 ).As discussed above,the electrochemical reactions with-out glucose in the solution are due to the doping/de-doping process of PANI.The significant reduction in R ct indicates that the glucose oxidation in such a CNT/PANI/HNQ/E.coli K12 anode system has an even faster reaction rate than that of the doping/de-doping redox reaction.The result reveals that the composite anode not only improves the electrode conductiv-ity and specific surface-area,but also provides unique active centres,possibly due to its specific nanostructure as shown in Fig.2b,to host the bacteria for more efficient electro-catalysis.3.3.Anode discharge performance in MFCIn order to evaluate the discharge performance of different anodes in a MFC,an anode-limiting MFC was designed which had a Pt cathode with a much larger surface area than that of the anodes.Thus,polarization of the cathode was insignificant.The catalytic current from glucose oxidation at a constant potential was measured.As shown in Fig.4,the current increases withthe Fig.4.Chronoamperometric plots of PANI and CNT/PANI composite elec-trodes placed in stirred anaerobic culture of Escherichia coli K12in standard glucose medium.Potential applied to electrode is0.1V.Curve a:plain PANI;b: CNT/PANI composite containing1wt.%CNT;c:CNT/PANI composite con-taining20wt.%CNT.Y.Qiao et al./Journal of Power Sources170(2007)79–8483Fig.5.Potential–time curve of PANI and CNT/PANI composite electrodes placed in a stirred anaerobic culture of E.coli K12in0.1M phosphate buffer with5.5mM glucose.Curve a:plain PANI;b:CNT/PANI composite containing 1wt.%CNT;c:CNT/PANI composite containing20wt.%CNT.growth and proliferation of the bacteria on the electrode surface.The current–time curves of PANI and composite anodes are sig-nificantly different from each other.For the plain PANI anode,the current increases very slowly and the current density is muchlower than that the composites.For the two CNT/PANI com-posite anodes,when the current of the1wt.%CNT/PANI anodereaches a plateau,the current of the20wt.%CNT/PANI anodeis still increasing and the current density is much higher than theformer.This can be explained by the fact that the nanocompos-ite electrodes have more reaction activity sites(larger specificsurface-area)for the bacteria-catalytic oxidation of glucose andthe active sites increase with increasing doping of the CNTs.This result is in agreement with the impedance analysis and theBET results.The constant-current discharge experiments of the MFCswith three different anodes in5.5mM glucose solution wereconducted at50mA m−2.The anode potential versus time data(Fig.5)show different discharge profiles for the three anodes.Within180min,the discharge potentials for plain PANI,1wt.%CNT/PANI and20wt.%CNT/PANI change from−0.01to −0.2,−0.36,and−0.38V,respectively.For a fuel cell system, the more negative the anode discharge potential,the higher is theoperational voltage of the fuel cell.The discharge results showthat the composite anode can provide a higher power density dueto its lower polarization,which further indicates that the nanos-tructured composites have a faster reaction rate.It is also seenthat the composite with the higher CNT content(20wt.%)gives abetter discharge performance than that with a lower CNT content(1wt.%).It is observed that the discharge profile of the bacte-ria anode is fundamentally different from that of conventionalanode behaviour.The bacteria anode has a high polarizationpotential initially and thus becomes lower with the extension ofthe discharge time.This is due to the bacteria growth processsince the discharge process starts immediately after addition ofglucose solution and bacteria.The bacteria require time to growto their maximum level and to distribute into the inner surfaceof the anode,as shown in Fig.4.Fig.6.Power output and polarization curve of MFC with20wt.%CNT/PANI anode.3.4.Power output of20wt.%CNT/PANI MFCAs the20wt.%CNT/PANI MFC exhibits the best perfor-mance,its power output and polarization was examined with the anode-limiting MFC.The results are presented in Fig.6. The polarization curve shows that the cell voltage drops to 250mV at a current density of145mA m−2.The power density of the20wt.%CNT/PANI MFC is calculated from the results of chronopotential measurements under different current den-sities.The plot of power density versus current density has a volcano shape.The power density increases with increase in current density,reaches a maximum value,and then sharply falls with further increase in current density.This is a typical relationship of output power density against the current density. The maximum power density is42mW m−2,which is obtained at a current density of about100mA m−2with a cell voltage of 450mV.It has been reported[27]that woven graphite electrodes in E.coli microbial fuel cells could deliver a maximum power density of0.47–2.6mW m−2with cell voltages of0.6–3.3V;the power density reached91mW m−2with Mn4+modified woven graphite as the anode but the cell voltage was about0.28V. Given that the thickness of the anode used in our work is smaller than1mm in comparison with the1cm thickness of the anodes used in[27],the CNT/PANI anode has superior electrocatalytic activity.4.ConclusionsThis study has shown that a nanostructured CNT/PANI nanocomposite can be used as the anode for a microbial fuel cell. The addition of CNTs to PANI increases the specific surface-area of the electrode and enhances the charge transfer capability so as to cause considerable improvement of the electrochemical activity for the anode reaction in a MFC.A CNT/PANI/E.coli K12/HNQ anode system gives a much higher power output than that of a PANI/E.coli K12/HNQ system.This demonstrates the superior and specific electrocatalytic effect of the nanocompos-ite for MFCs in comparison the existing systems.The composite containing20wt.%CNT gives the best discharge performance and delivers a high power output of42mW m−2with a cell volt-84Y.Qiao et al./Journal of Power Sources170(2007)79–84age of450mV.The CNT-doped PANI nanocomposite therefore offers good prospects for application in MFCs.AcknowledgementThe authors are grateful to the Asian Office of Aerospace Research and Development,Department of The Air Force of USA,forfinancial support under contract of AOARD-05-4073.References[1]B.E.Logan,B.Hamelers,R.Rozendal,et al.,Environ.Sci.Technol.40(2006)5181–5192.[2]R.A.Bullen,T.C.Arnot,keman,et al.,Biosens.Bioelectron.21(2006)2015–2045.[3]K.Rabaey,W.Verstraete,Trends Biotechnol.23(2005)291–298.[4]R.M.Allen,H.P.Bennetto,Appl.Biochem.Biotechnol.39(1993)27–40.[5]S.K.Chaudhuri,D.R.Lovley,Nat.Biotechnol.21(2003)1229–1232.[6]H.S.Liu,C.J.Song,L.Zhang,et al.,J.Power Sources155(2006)95–110.[7]Z.L.Liu,X.H.Lin,J.Y.Lee,et al.,Langmuir18(2002)4054–4060.[8]C.Wang,M.Waje,X.Wang,et al.,Nano Letters4(2004)345–348.[9]D.Ivnitski,B.Branch,P.Atanassov,et al.,mun.8(2006)1204–1210.[10]Y.M.Yan,W.Zheng,L.Su,et al.,Adv.Mater.18(2006)2639.[11]E.Flahaut,M.C.Durrieu,M.Remy-Zolghadri,et al.,J.Mater.Sci.41(2006)2411–2416.[12]A.Magrez,S.Kasas,V.Salicio,et al.,Nano Letters6(2006)1121–1125.[13]T.A.R.L.E.Skotheim,J.R.Reynolds,Handbook of Conducting Polymers,Dekker,New York,1998.[14]C.M.Li,W.Chen,X.Yang,et al.,Front.Biosci.10(2005)2518–2526.[15]H.Dong,C.M.Li,W.Chen,et al.,Anal.Chem.8(2006)7424–7431.[16]W.Z.Huseyin Zengin,J.Jin,R.Czerw,D.W.Smith Jr.,L.Echegoyen,D.L.Carroll,S.H.Foulger,J.Ballato,Adv.Mater.14(2002)1480–1483.[17]Z.Muhammad-Tahir,E.C.Alocilja,IEEE Sens.J.3(2003)345–351.[18]S.C.K.Misra,R.Angelucci,Indian J.Pure Appl.Phys.39(2001)726–730.[19]J.N.Uwe Schroder,F.Scholz,Angewandte Chemie115(2003)2986–2989.[20]U.S.Juliane Niessen,M.Rosenbaum,F.Scholz,mun.6(2004)571–575.[21]V.Mottaghitalab,G.M.Spinks,G.G.Wallace,Synth.Met.152(2005)77–80.[22]J.Y.Liu,S.J.Tian,W.Knoll,Langmuir21(2005)5596–5599.[23]S.A.Lee,Y.Choi,S.H.Jung,et al.,Bioelectrochemistry57(2002)173–178.[24]A.M.B.Raquel Sainz,M.Teresa Martinez,J.F.Galindo,J.Sotres,A.M.Baro,B.Corraze,O.Chauvet,W.K.Master,Adv.Mater.17(2005) 278–281.[25]A.J.Bard,L.R.Faulkner,Electrochemical Methods:Fundamentals andApplications,2nd ed.,Wiley,New York,2001.[26]D.J.Guo,H.L.Li,J.Solid State Electrochem.9(2005)445–449.[27]D.H.Park,J.G.Zeikus,Biotechnol.Bioeng.81(2003)348–355.。
sbdata标准

SBDS – Addendum For Fuel Cell SystemsRelease 1.02April 11, 2007Revision History:Revision Changes Date Release 8/29/06 1.0 Initial1/1907 1.01 Correction to Auto_Soft-OFF(2Bh) function [Section 5.2.7]Release for votingXpress-P x-Data(24h) as a result of review telecon held on1.02 Removed4/11/07 2/13/2007. This is because the existing ManufacturerData(23h) is ableto duplicate the proposed Xpress-P x-Data(24h) function.The command codes for those commands following 24h have allchanged command codes to remove the break in sequence.Table of Contents1. INTRODUCTION 51.1. Scope 52. REFERENCES 53. DEFINITIONS 64. FUEL CELL SYSTEM OVERVIEW 64.1. Smart Battery or Fuel Cell System Software Definition 74.1.1. SMBus Host to Smart Battery or Fuel Cell System 74.1.2. Smart Battery Charger to Smart Battery or Fuel Cell System (or vice versa) 74.1.3. Smart Battery or Fuel Cell system to SMBus Host or Smart Battery Charger 74.2. Smart Battery or Fuel Cell System Characteristics 74.2.1. Initial Conditions 84.2.2. Fuel Cell OFF State 84.2.3. Smart Fuel Soft OFF State 84.2.4. Smart Fuel Start Up Sate84.2.5 Smart Fuel Power ON State 84.2.6. Smart Fuel Hybrid State 94.2.7. SmartFuel Idle State 94.2.8 State Diagram and System Transitions 94.2.9. Safety Signal Hardware Requirements (Smart Battery Charger Interface) 105. Smart Battery or Fuel Cell System Interface 115.1. Standard SBDS for Fuel Cell Systems – SES Subset 115.1.1. BatteryMode() (0x03) 115.1.2. Temperature() (0x08) 115.1.3. Voltage() (0x09) 115.1.4. Current() (0x0a) 115.1.5. RelativeStateOfCharge() (0x0d) 125.1.6. RemainingCapacity() (0x0f) 125.1.7. FullChargeCapacity() (0x10) 125.1.8. AverageTimeToEmpty() (0x12) 145.1.9. BatteryStatus() (0x16) 145.1.10. ChargingCurrent() (0x14) 145.1.11. ChargingVoltage() (0x15) 155.1.12. CycleCount() (0x17) 155.1.13. DesignCapacity() (0x18) 165.1.14. DesignVoltage() (0x19) 165.1.15. ManufactureDate() (0x1b) 165.1.16. SerialNumber() (0x1c) 165.1.17. ManufacturerName() (0x20) 175.1.18. DeviceName() (0x21) 175.1.19. DeviceChemistry() (0x22) 175.1.20. ManufacturerData() (0x23) 185.2. SBDS Addendum – Additional Functions for Fuel Cell Systems 195.2.1. DesignPower() (0x24) 195.2.2. StartTime() (0x25) 195.2.3. TotalRuntime() (0x26) 195.2.4. FCtemp() (0x27) 205.2.5. FCStatus(0x28) 205.2.6 FCMode() (0x29) 215.2.7. Auto_Soft_OFF (0x2a) 225.3. SBDS for Fuel Cell Systems – In Addition to the SES Subset 225.3.1. ManufacturerAccess() (0x00) 225.3.2. RemainingCapacityAlarm() (0x01) 225.3.3. RemainingTimeAlarm() (0x02) 225.3.4. AtRate() (0x04) 225.3.5. AtRateTimeToFull() (0x05) 235.3.6. AtRateTimeToEmpty() (0x06) 235.3.7. AtRateOK() (0x07) 235.3.8. AverageCurrent() (0x0b) 245.3.9. MaxError() (0x0c) 245.3.10. AbsoluteStateOfCharge() (0x0e) 245.3.11. RunTimeToEmpty() (0x11) 255.3.12. AverageTimeToFull() (0x13) 255.3.13. SpecificationInfo() (0x1a) 25 Appendix A. Required functions for Fuel Cell Systems 27 Appendix B. The command set in tabular form 281. IntroductionSmart Battery Data Specifications (SBDS) is an ideal solution for many of the issues related to batteries used in portable electronic equipment (such as laptop computer systems, cellular telephones or video cameras) but SBDS does not take into account Fuel Cell systems as power sources. This addendum is compatible with SBDS while adding new functions that allow greater control of Fuel Cell systems and will result in better performance. Additionally, this addendum specifically defines how Fuel Cell systems can respond to SBDS functions to remain compatible with current SBDS compatible devices.Fuel Cell systems presently have a number of differences compared to traditional batteries. Fuel Cell systems can be turned on and off. They have a startup time during which they might not produce power or might produce only a very limited amount of power. Fuel Cell systems are refueled instead of recharged. Fuel Cell system peak power levels are generally far less than similarly sized battery packs. Fuel Cell systems degrade in a different manner than traditional batteries, becoming less efficient with age. Additionally some Fuel Cell systems may include internal rechargeable batteries.The main differences of using Fuel Cell systems in place of batteries are: turning the Fuel Cell system “On” and the corresponding possible delay in power production, and maximum power limitations.This addendum is not designed to limit innovation amongst battery manufacturers, but rather, provide the user and the SMBus Host with a consistent set of information about any particular Smart Battery or Fuel Cell System. Additionally, although SMBUS and I2C interface are used as the underlying physical layer for this addendum, however these command sets can be implanted using other single or multi-wire interfaces.1.1. ScopeThis document specifies how Fuel Cell systems can return a data set that is compatible with SBDS. This document also defines new functions for Fuel Cell systems for added capabilities. This specification is generic with regard to the type of battery or Fuel Cell system chemistry, the battery or Fuel Cell system voltage, the battery pack or Fuel Cell system fuel cartridge capacity as well as the battery pack or system's physical packaging.2. References•Smart Battery Data Specification, Revision 1.1, SBS-Implementers Forum, December, 1998•Smart Battery Charger Specification, Revision 1.1, SBS-Implementers Forum, December, 1998•Smart Battery Selector Specification, Revision 1.1, SBS-Implementers Forum, December, 1998•Smart Battery System Manager Specification, Revision 1.1, SBS-Implementers Forum, December, 1998•System Management Bus Specification, Revision 1.1, SBS-Implementers Forum, December, 1998•System Management Bus BIOS Interface Specification, Revision 1.0, February 15, 1995•ACPI Specifications, Version 1.0a, Intel Corporation, Microsoft Corporation, Toshiba Corp., July 1998 (/~acpi)•The I²C-bus and how to use it, Philips Semiconductors document #98-8080-575-01.•ACCESS.bus Specifications -- Version 2.2, ACCESS.bus Industry Group, 370 Altair Way Suite 215, Sunnyvale, CA 94086 Tel (408) 991-3517•IEC SC21A - "Alkaline Secondary Cells and Batteries", IEC committee 21, Sub-committee A. (Responsible for development of standard battery pack sizes and electrical specifications.)•IEC SC48B - "Connectors", IEC committee 48, Sub-committee B. (Responsible for development of connector standards for batteries.)3. Definitions•Fuel Cell: An electro-chemical device that converts fuel into electricity.•Fuel Cell System: A system consisting of a Fuel Cell, a fuel cartridge or storage tank, possibly a battery, and hardware that controls the Fuel Cell and provides present state, calculated and predicted information to itsSMBus Host under software control. The content and method are described in this specification.•Fuel Cartridge: A container (either replaceable or refillable) that contains the fuel used by a Fuel Cell system to produce electricity.•Internal Battery: An optional rechargeable battery inside the Fuel Cell system which can be transparent to the host which can be recharged from external sources•I²C-bus: A two-wire bus developed by Phillips, used to transport data between low-speed devices.•Smart Battery: A battery equipped with specialized hardware that provides present state, calculated and predicted information to its SMBus Host under software control.•Smart Battery Charger: A battery charger that periodically communicates with a Smart Battery and alters its charging characteristics in response to information provided by the Smart Battery.•SMBus: The System Management Bus is a specific implementation of an I²C-bus that describes data protocols, device addresses and additional electrical requirements that is designed to physically transport commands and information between the Smart Battery, SMBus Host, Smart Battery Charger and other Smart Devices.•SMBus Host: A piece of portable electronic equipment powered by a Smart Battery. It is able to communicate with the Smart Battery and use information provided by the battery.4. Fuel Cell System OverviewThe Fuel Cell System communicates with other devices (such as the SMBus Host and the Smart Battery Charger) via two separate communication interfaces:- The first uses the SMBus CLOCK and DATA lines and is the primary communication channel between the Fuel Cell System and other SMBus devices. The Fuel Cell System will provide data when requested, send charging information to the Smart Battery Charger, and broadcast critical alarm information when parameters (measured or calculated) exceed predetermined limits within the particular Fuel Cell System.- The other required communication interface is the secondary signaling mechanism or ‘Safety Signal’ (the ‘T-pin’ on a Smart Battery pack connector). This is a variable resistance output from the Smart Battery or Fuel Cell system which indicates when charging is permitted. If this ‘Safety Signal’ pin is left open, it will signal the Smart Battery Charger that charging is not allowed.It is possible in some cases such as a tethered fuel system to include an internal rechargeable smart battery where this battery can be recharged by an external source. In these cases the system has to be able to communicate with this battery for its status as well as its charging status and requirements over the SMBus.4.1. Smart Battery or Fuel Cell System Software Definition4.1.1. SMBus Host to Smart Battery or Fuel Cell SystemSMBus Host to Smart Battery or Fuel Cell System communications are performed:•To allow the user to know the Smart Battery or Fuel Cell System’s remaining runtime•To allow Smart Batteries or Fuel Cell System’s to provide accurate information to their user•To determine the SMBus Host's real-time power requirements•To enable power management based on “real” values supplied by the battery or Fuel Cell system•To enable battery or Fuel Cell system manufacturers to collect information about a Smart Battery or Fuel Cell System’s usage•To allow battery or Fuel Cell system manufacturers to electronically "stamp" batteries or Fuel Cell systems at time of manufacture•To allow the SMBus Host to change Fuel Cell system status (startup, shutdown, idle)4.1.2. Smart Battery Charger to Smart Battery or Fuel Cell System (or vice versa)Smart Battery Charger to Smart Battery or Fuel Cell System communications are performed:•To allow Smart Batteries or Fuel Cell System internal batteries to be charged as rapidly and as safely as possible•To allow access to the "correct" charger algorithm for the battery or Fuel Cell system internal battery.4.1.3. Smart Battery or Fuel Cell system to SMBus Host or Smart Battery ChargerSmart Battery or Fuel Cell System to SMBus Host or Smart Battery Charger communications are performed: •To allow the Smart Battery or Fuel Cell System to warn other system components of potential problems.•To allow the Smart Battery or Fuel Cell System to warn the user about potentially dangerous situations that they can rectify.•To allow the Smart Battery or Fuel Cell System internal battery to instruct the Smart Battery Charger what Charge Current and Charge Voltage it would like to be charged with.•To allow the Fuel Cell system to indicate its status (startup, shutdown, fuel cartridge removed).4.2. Smart Battery or Fuel Cell System CharacteristicsThe Smart Battery or Fuel Cell System may or may not be present in a system. Additionally, it may dynamically be added or removed while the system is powered. Therefore, it must exhibit predictable behaviors when inserted in a system and/or when the system is turned on. The following is a description of the battery or Fuel Cell system’s states and a description of the actions that take place as a result of changes.4.2.1. Initial Conditions;The system must detect when a Smart Battery or Fuel Cell System is present and this is done by using BatteryMode()status bit 10.as indicated by bit10 of BatteryMode() command.Function (Data Value) Initial Value (Smart Battery) Initial Value (Fuel Cell Systems) UnitsRemainingCapacityAlarm() 10%of DesignCapacity() value 0 mAhRemainingTimeAlarm()10 10 minutesBatteryMode() Bit 15:CAPACITY_MODE=0Bit 14:CHARGER_MODE=0Bit 13:ALARM_MODE=0Bit 10 :Fuel_Cell_Mode = 0Bit 9:PRIMARY_BATTERY=0BIT 8:CHARGE_CONTROLLER_ENABLED=0 Bit 15:CAPACITY_MODE=1Bit 14:CHARGER_MODE=1Bit 13:ALARM_MODE=1Bit 10 :Fuel_Cell_Mode = 1Bit 9:PRIMARY_BATTERY=0BIT 8:CHARGE_CONTROLLER_ENABLED=0bit valueBatteryStatus() Bit 7: INITIALIZED=1 Bit 7: INITIALIZED=1 bit value CycleCount() typically less than 5 typically less than 5 decimal4.2.2. Fuel Cell ‘OFF” StateThe Fuel Cell System may enter the “Off State” whenever the SMBus Clock and Data lines both remain low for greater than 2.5 seconds or driven by the host to this state. In this condition the power is removed from the Fuel Cell and no communication will occur. If the Fuel Cell system has a physical “On/Off switch”, it will go into OFF state immediately when the switch is moved to the “Off” position and will stop communicating via SMBus.Fuel Cell must enter this mode automatically in case of any critical alarms and or loss of system communications.4.2.3. Soft-OFF State:In this mode the Fuel Cell can communicate to the host or other system electronics. System may drive the Fuel Cell to this state from idle mode when the load no longer exists and or charging is complete.The Fuel Cell must enter Soft OFF mode automatically after charging the optional internal battery is complete with or without the host interventionThe Fuel Cell enters this state whenever it detects that the SMBus Clock and Data lines go high and will remain in this mode until its operating mode is changed via the SFMode() function call.The Fuel Cell may also automatically transition to Start Up state after entering Soft-Off state in order to prepare to produce power..The Fuel Cell system may not disrupt traffic on the SMBus, however the physical act of inserting a new device onto a live bus may cause an inadvertent communication interruption. The Smart Fuel Cell System may not begin broadcasting messages to either the SMBus Host or Smart Battery Charger for at least 10 seconds after entering the SMBus “On State.” Including the Soft-Off State.4.2.4. STARTUP State:In this state, the Fuel Cell system prepares itself to provide power since the fuel cell system can not deliver the load power immediately and requires a set up time (SetupTime(0x26h). The Fuel Cell should automatically enter this state initially by changing FCMode() upon power up and when ready it should transition to Idle state awaiting command to provide power.4.2.5. Power ON State:In this state, the Fuel Cell system is producing power to the load and could be charging the battery at the same time.4.2.6. Hybrid State:Hybrid state is when the load power requirements exceed the capability of the Fuel Cell. Under this condition the Fuel Cell is working in conjunction (parallel) with battery to produce the required platform power and must be able to toggle to the Power-ON state automatically once the load’s power is with in the Fuel Cell’s capability.4.2.7. Idle StateIn IDLE state the Fuel Cell System could deliver power and is ready to enter the Power On state. This state could also be used as a low power state (standby) where the Fuel Cell System is awaiting for the load to be turned on. The Fuel Cell system may also enter Idel state under alarm conditions4.2.8 State Diagram and System TransitionsTransition TableHost initiated Fuel Cell initiatedPossible Conditions OFF to Soft OFF 000 Æ 001 - - User initiated (On-Off Switch) Soft OFF to OFF 001 Æ 000 √ √ Alarm, System disconnect, Turn Of Soft OFF to Start Up 001 Æ 010 √ - System ON Start Up to Soft OFF 010 Æ 001 √ √ Error Start Up to Idle 010 Æ011 - √ Start-up complete, system interrupts Idle to Soft Off 011 Æ 001 √ √ Error, Alarm, Turn-OFF Idel to Power-ON/Hybrid011 Æ 10x √ - Load ready, Toggle to battery if Pow Load Power to Idle 10x Æ 011 √ √Load off command, Alarm, Error Note: All other Transitions are invalid and ignored.Power removedOr Go-to4.2.9. Safety Signal Hardware Requirements (Smart Battery Charger Interface)The Fuel Cell System, similar to Smart Batteries, must also provide an additional signal to allow for safe charging. This ‘Safety Signal’ is also commonly referenced as the ‘T-pin’ or ‘Thermistor’ on some Smart Battery hardware connectors. The ‘Safety Signal’ is an output from the Smart Battery and may be used by a Smart Battery Charger (or other device) to determine if charging of the Smart Battery is permitted. This signal is a variable resistance output as measured between the ‘Safety Signal’ pin and the negative terminal of the battery.If a Fuel Cell System has an internal rechargeable battery that has the capability of being recharged by the Smart Battery Charger, it must have a Safety Signal Resistance of 2850Ω to 31.5kΩ. Otherwise, the Safety Signal should be open (Rss > 95k Ω). If a Fuel Cell system wants to stop the Smart Battery Charger from charging its internal battery, the Safety Signal should be open (Rss > 95k Ω).The Smart Battery Charger’s capabilities are altered by the value of the Safety Signal. As a required safety feature, the charger must NOT charge a battery when it senses the resistance between the Safety Signal pin and ground to be in the range between 425 and 3150 ohms. The valid ranges of the Safety Signal are summarized below along with the charger’s capabilities for the range. (Please also refer to the Smart Battery Charger Specification.)5. Smart Battery or Fuel Cell System InterfaceThe following functions are used by the Smart Battery or Fuel Cell System to communicate with a SMBus Host, Smart Battery Charger and other devices connected via the SMBus.The SMBus Host, acting in the role of a SMBus master, uses the Read Word and Write Word protocols to communicate numeric data with the Smart Battery or Fuel Cell System. Non-numeric data, such as the ManufacturerName(), is read using the Read Block protocol.The Host Device can obtain data that can then be either presented to a user or applied by the Device’s power management system. Two types of data are available from the Smart Battery: static data and dynamic data. Static data includes items that are not changing, such as chemistry, the original capacity, or the design voltage. Dynamic data includes both measured and calculated information. Measured data is obtained by the monitoring electronics and includes items such as temperature, voltage and current. Calculated information is based on the battery or Fuel Cell system’s present state and the battery or Fuel Cell system’s characteristics, such as the remaining life at the present rate of drain.The functions are described as follows:FunctionName() 0xnn (command code)Description: A brief description of the function.Purpose: The purpose of the function, and an example where appropriate.SMBus Protocol: Refer to Section 6 for details.Required: Is this function required for SES compatible Fuel Cell Systems?Data Type: Dynamic or StaticInput, Output or Input/Output: A description of the data supplied to or returned by the function.The data is described as follows:Data type: The type of data the function conveysUnits: The units the data is presented inRange: The range of valid dataGranularity: described as percentage of a maximum value, determined by least accurate data.Accuracy: How "good" is the data.Fuel Cell system: A description of how a Fuel Cell System will return information to this function to be compatible with SBDS and SES P X.If an optional internal smart battery exists as part of the Fuel Cell, all commands associated with this battery return the same vales as any other smart battery in the system unless noted so.A Fuel Cell System that complies with SBDS v1.1 must support all the command codes contained in this specification. It must support the defaults as specified. Additionally, it must support all modes and functions specified except those which it can explicitly signal the presence or absence thereof (e.g. the presence of an internal charge controller and the ability to enable or disable that controller). Portions of this specification designated ‘optional’ are not required for compliance.To be compatible with SBDS, Fuel Cell Systems must supply values for functions as described in the “Fuel Cell system” headings in sections 5.1.1-5.1.21 and 5.3.1-5.3.13. For extra functionality, Fuel Cell Systems must also supply values for functions as described in the “Fuel Cell system” headings in sections 5.2.1-5.2.6. To be compatible with SES function definitions, Fuel Cell Systems need only supply values for functions as described in the “Fuel Cell system” headings in sections 5.1.1-5.1.21.5.1. Standard SBDS for Fuel Cell Systems – SES SubsetThis section lists SES P x function definitions and the values that Fuel Cell Systems will report for these functions. SES P x functions are a subset of SBDS. For full compatibility with SBDS, Fuel Cell Systems must also return values for thefunctions listed in section 5.3. In a sense, this is a guide to Fuel Cell system manufacturers as to how to make Fuel Cell Systems compatible with devices designed for standard Smart Batteries.5.1.1. BatteryMode(03h)Description: This function reports the battery system’s operational modes and capabilities, and flags minor conditions requiring attention.Purpose: To allow the Host Device to determine the presence of Fuel Cell system and the particular data reporting formats. (See individual bit definitions).SMBus Protocol: Read or Write WordRequired?: YesData Type: DynamicInput/Output: unsigned int – bit mappednotapplicableUnits:0 (1)Range:Fuel Cell system: In SBDS, BatteryMode has eight reserved bits along with eight defined bits (see SBDS spec). The currently reserved bits are 2-6 and 10-12. Fuel Cell systems will use Bit,10, to report presence of Fuel Cells as an alternative source of energy The BatteryMode flag bit 10 is set when the SBDS Primary Exchange electronics are representing a fuel-cell device. This is an indication to the Host Device that there may be load limitations, start-up delays, or other aspects of Fuel Cell operation that may change operational parameters. Additionally, the Host device will recognize the new additional functions such as DesignMaxCurrent(0x25),StartTime(0x26), FuelCellRegister(0x2A) and other optional new functions defined in the SBDS addendum for Fuel Cells.Bit 15 (read only) always is set (1) as Capacity_mode () for current is not supported for Fuel Cells (It only reports capacity in Watts)5.1.2. Temperature(08h)Description: Returns the battery pack’s internal temperature in degrees Kelvin (ºK)Purpose: The Temperature function provides an accurate temperature for use by the battery-powered device’s thermal management system. Since Fuel Cell systems may have a variety of internal temperatures, it is more appropriate that they report ambient temperature or internal battery temperature.SMBus Protocol: Read WordRequired?: YesData Type: DynamicOutput: unsigned int – battery temperature in tenth degree Kelvin increments0.1ºKUnits:Range: 0 to +6553.5 ºKGranularity: 1.0 ºK or betterAccuracy: ± 4 ºKFuel Cell system: This function is used only for reporting internal battery temperature. For reporting internal temperatures associated with Fuel Cell, FCTemp() command must be used.5.1.3. Voltage(09h)Description: Returns the battery pack or Fuel Cell system voltage in milli-Volts (mV).Purpose: The Voltage function provides the battery or Fuel Cell system terminal voltageSMBus Protocol: Read WordRequired?: YesData Type: DynamicOutput: unsigned int – battery or Fuel Cell system terminal voltage in milli-VoltsmVUnits:Range: 0 to 65,535 mVGranularity: 0.5% of DesignVoltageAccuracy: ± 2.0% of DesignVoltageFuel Cell system: returns same.5.1.4. Current(0Ah)Description: Returns the current being supplied through the battery or Fuel Cell system’s terminals in milli-Amps (mA) Purpose: The Current function provides a measurement of the current flowing out of the battery or Fuel Cell system. SMBus Protocol: Read WordRequired?: YesData Type: DynamicOutput: unsigned int – discharge rate in mA increments, negative for dischargeUnits:mARange: 0 to -32,768 mA for dischargeGranularity: 0.5% of DesignCapacityAccuracy: ± 2.0% of DesignCapacityFuel Cell system: returns same.5.1.5. RelativeStateOfCharge(0Dh)Description: Returns the predicted remaining battery capacity or Fuel Cell system fuel cartridge capacity expressed as a percentage of the DesignCapacity (%)Purpose: The RelativeStateOfCharge function is used to estimate the amount of charge remaining in the battery or fuel in the Fuel Cell system fuel cartridge(s).SMBus Protocol: Read WordRequired?: YesData Type: DynamicOutput: unsigned int – percent of remaining capacityUnits:%Range: 0 to 100%2%Granularity:10%Accuracy:Fuel Cell system: Returns the predicted remaining fuel in fuel cartridge expressed as a percentage of the DesignCapacity (%). If no fuel cartridge is installed, return 0.5.1.6. RemainingCapacity(0Fh)Description: Returns the predicted remaining battery capacity or Fuel Cell system internal battery capacity in milli-Amp-hours (mAh)Purpose: The RemainingCapacity function returns the battery or Fuel Cell system fuel cartridge(s) remaining capacity in absolute terms but relative to a specific discharge rate.SMBus Protocol: Read WordRequired?: NoData Type: DynamicOutput: unsigned int – remaining charge in mAhmAhUnits:Range: 0 to 65,535 mAhGranularity: 0.5% of DesignCapacitynotapplicableAccuracy:Fuel Cell system: If the Fuel Cell system has an internal rechargeable battery, this function returns the predicted remaining capacity in that battery in milli-Amp-hours (mAh). If the Fuel Cell system does not have an internal rechargeable battery, return the result of this calculation: RelativeStateOfCharge(0Dh)* DesignCapacity(18h)5.1.7. FullChargeCapacity(10h)Description: Returns the predicted pack capacity or Fuel Cell system internal battery capacity when it is fully charged in milli-Amp-hours (mAh)Purpose: The FullChargeCapacity function provide the user with a means of understanding the “tank size” of their battery or Fuel Cell system fuel cartridge(s).SMBus Protocol: Read WordRequired?: NoData Type: DynamicOutput: unsigned int – estimated full charge capacity in mAhUnits:mAhRange: 0 to 65,535 mAhGranularity: 0.5% of DesignCapacityapplicablenotAccuracy:Fuel Cell system: If the Fuel Cell system has an internal rechargeable battery, this function returns the predicted pack capacity of that battery when it is fully charged in milli-Amp-hours (mAh). If the Fuel Cell system does not have an internal rechargeable battery, return the same value as DesignCapacity(18h)5.1.8. AverageTimeToEmpty(12h)Description: Returns a rolling average of the predicted remaining battery life or Fuel Cell system fuel cartridge remaining runtime in minutes.Purpose: The AverageTimeToEmpty displays state-of-charge information in a more useful way. By averaging the estimations, the remaining time will not appear to “jump” around.SMBus Protocol: Read WordRequired?: YesData Type: DynamicOutput: unsigned int – minutes of operation leftminutesUnits:Range: 0 to 65,535 minminutesGranularity:525minutesAccuracy:Invalid Data Indication: 65,535 indicates battery or Fuel Cell system is not being discharged.Fuel Cell system: Returns a rolling average of the predicted remaining number of minutes of runtime of the Fuel Cell system using the currently installed fuel cartridge(s). If the Fuel Cell system is in PRODUCING POWER MODE, this number is calculated based on power out of the Fuel Cell system. If the Fuel Cell system is not in PRODUCING POWER MODE, return 65,535. If no fuel cartridge is installed, return time until Fuel Cell system enters SHUTDOWN MODE or IDLE MODE from PRODUCING POWER MODE.5.1.9. BatteryStatus(16h)Description: Returns the status word which contains alarm and status bit flags which indicate end-of-discharge, over-temperature, and other conditions.Purpose: The BatteryStatus() function is used by the Host Device to get alarm and status bits, as well as error codes from the Smart Battery.SMBus Protocol: Read WordRequired?: YesData Type: DynamicOutput: unsigned int – Status Register with alarm conditions bit mapped as follows:*****Alarm Bits*****0x8000 OVER_CHARGED_ALARM0x4000 TERMINATE_CHARGE_ALARM0x2000 reserved0x1000 OVER_TEMP_ALARM0x0800 TERMINATE_DISCHARGE_ALARM0x0400 reserved0x0200 REMAINING_CAPACITY_ALARM0x0100 REMAINING_TIME_ALARM*****Status Bits*****0x0080 INITIALIZED0x0040 DISCHARGING0x0020 FULLY_CHARGED0x0010 FULLY_DISCHARGEDFuel Cell system: Returns only internal battery alarms if present. A new command FCStatus has been defined for Fuel Cell Alarm conditions.5.1.10. ChargingCurrent(14h)Description: Returns the Smart Battery’s desired charging rate in milli-Amps (mA)Purpose: The ChargingCurrent function returns the maximum current that a Smart Battery Charger may deliver to the Smart Battery. In combination with the ChargingVoltage these functions permit a Smart Battery Charger to dynamically adjust its charging profile (current/voltage) for optimal charge. The battery can effectively turn off the Smart Battery。
维克顿能源Lynx DC分布系统说明说明书
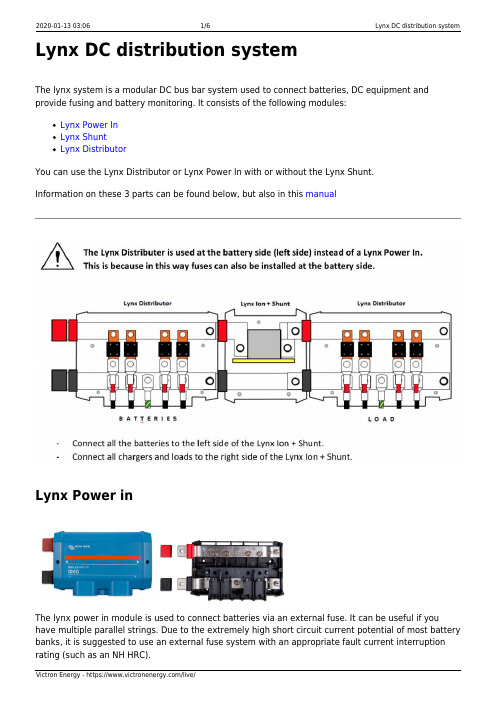
Lynx DC distribution systemThe lynx system is a modular DC bus bar system used to connect batteries, DC equipment and provide fusing and battery monitoring. It consists of the following modules:Lynx Power InLynx ShuntLynx DistributorYou can use the Lynx Distributor or Lynx Power In with or without the Lynx Shunt.Information on these 3 parts can be found below, but also in this manualLynx Power inThe lynx power in module is used to connect batteries via an external fuse. It can be useful if you have multiple parallel strings. Due to the extremely high short circuit current potential of most battery banks, it is suggested to use an external fuse system with an appropriate fault current interruption rating (such as an NH HRC).The Power In contains a negative and positive DC bus-bar with M8 bolts in which to connect batteries cables. It can take cables up to 22 mm in diameter through the bottom entry guides, and no limit via the two side connections.Lynx shuntThe Lynx shunt contains a positive bus-bar with space to mount a CNN fuse and a negative bus-bar with a shunt. It also contains battery monitoring electronics. The Lynx shunt can send via the VE.CAN bus battery monitoring information to a CCGX or VGX or to a third party CANbus monitoring system, such as a NMEA2000. The CCGX, VGX or third party display acts as battery monitor display and is also used to set up the built-in battery monitor.The Lynx shunt is available with a VE.Can connection. A previous model had a connection and is now discontinued.FuseCIP140325000 - Fuse CNN 325A/80V for Lynx shuntOr alternatively a CNN fuses by Littlefuse can be used. The CNN fuse is a 48 Vdc fast blow fuse and is available up to 800A.ShuntRated at 1000 ASetupSetup is like setting up a BMV and this is done via the CCGX or VGX.To find out the meaning of the various settings, please see the BMV manualPlease don’t use the 2-pin terminal block on the Lynx-shunt.FAQQ - Is there a way of setting the battery instance?A - There is a program available to change this but as the default is 0, which is the same as what a CCGX reads we would not advise changing this!!Further Questions and Answers about the Lynx Shunt are available on the Victron Community topic. Lynx DistributorContains a positive and negative bus-bar and provides a connection for 4 individual DC equipment circuits, loads or DC groups. It has a space for individual DC fuses per DC group.There is an optional feature of an LED for each fuse to indicate if fuse is blown.If you wish to use the fuse indication LEDs on the Lynx Distributor, it also requires a connection to a Lynx Shunt. The Lynx Shunt contains the necessary wide input (8 to 70VDC) power supply to output the required 5V (4,5V-5,5V) power requirement of the Lynx Distributor circuit board.RJ11 fly leads are used to connect the Lynx Shunt to the Distributor. This RJ11 cable is supplied with the Distributor in the box. There are two ports on the Lynx Shunt, and it is possible to use either.When all is OK, only the central LED is green; when a fuse has blown (or is missing) the central and blow fuse circuit LEDs turn red. If you are not using all connection points in your DC distribution, it may be necessary to fit dummy fuses to extinguish the red LEDs.A future upgrade is planned to also send info back to the GX device over the VE-can, but is not yet implemented.FusesUses MEGA fuses. Please note that some MEGA fuses are only rated to 36Volt (suitable for 24V systems), You must use 58/64V rated fuses for 48V systems.InstallationConnectors and dip-switches:The R11 connector is to power the Lynx-distributor from the Lynx-shuntThe DIP switch on the Lynx-distributor are for specific manual settings, to do with our 24Vbatteries. Please don’t touch.The 6-pin header block on the Lynx Distributor is for reading out the fuses, but this feature isnot supported.FAQQ - There is a small 4-pin cable shipped with the Distributor - I can only assume that the cable interconnects between the Shunt and Distributor?A - Yes, but this has only to power the LEDs on the distributor, there is no other function for this at the moment.Q - The Distributor has a DIP switch. What are the settings?A - That’s for manual settings, please don’t touch as the system will auto configure (up to 32 battery’s )Q - The Distributor has a 6-pin header block. What is it for?A - In the future, the read out of fuses, but this is not supported yet.Why Fuse the Bus?Batteries are special. They are both a source and destination of energy.Power can flow from them to a load (or a fault). It can also flow to them from a charge source (eg other batteries or solar).It is important that in cases where batteries are combined, and there is the opportunity for current to flow from one connection to another in fault, that there is sufficient and robust circuit protection.For example, the BYD Lithium IP55 cabinets.In this case the 4 module battery cabinets are each protected by individual 85A circuit breakers and also collectively protected by a 125A circuit breaker at the cabinet end.These cabinets are then connected through the Lynx Distributor, and those main battery take offwires are connected at the Bus end by another 125A MEGA fuse.Both the 125A circuit breaker and the 125A fuse are as close as possible to their respective sources of current. The battery cells, where the internal wire is protected inside the cabinet. And the shared bus bar (where current can flow from other batteries or a charge source).This means that if there is a short circuit fault somewhere along the battery cabinet take off cable, there is sufficient protection.Without the 125A bus bar MEGA fuse. There is the possibility of the battery take off cables fault that the 125A cabinet circuit breaker trips, but that the fault is continued by the supply of the other batteries (up to their combined 375A) and this exceeds the current rating of the cable to the fault and starts a fire.This is an unlikely scenario, but consequences of a fire in an energy storage situation are so serious that it is a recommended level of protection.Customer ImagesHere are some images from customer's installations that may help you to understand the installation of the Lynx System.Mark on Victron CommunityDiscovery YachtsYsebaert's Hybrid Back-up System in BelgiumAMSolar's 2015 Redwood 38RL, 42’。
泛马汽车GM AUTOnomy水化燃料单元系统概述说明书

52April 2002GM’s Futuristic ‘Skateboard’General Motors has devel-oped the world’s first vehi-cle designed completely around a hydrogen fuel cell propulsion system. Dubbed AUTOnomy, this futuristic GM concept vehicle so pro-foundly changes the auto-motive industry, says GM,that the company is seeking 24 patents covering related business models, technolo-gies and manufacturing processes. The chassis,which GM nicknamed “skateboard,” would con-tain all of its propulsion and control systems within a 6-inch-thick skateboardlike chassis.All of AUTOnomy’s es-sential systems, including the fuel cell stack and on-board hydrogen storage system, are neatly packaged in the thin chassis, whose unique design simplifies manufacturing and service,and enables a wide variety of vehicles to be built on a small number of platforms,with much shorter product development cycles, as well. AUTOnomy runs on a fuel cell adapted from GM’s existing HydroGen III fuel cell system.The nerve center of AUTOnomy’s electrical sys-tem is the universal “dock-ing port,” or connection, at the center of the chassis. It creates a quick and fool-proof way to connect all the body systems—controls,Tom Nash As shown in the illustration above, GM’s AUTOnomy concept houses the entire hydrogen fuelcell powertrain and all controls in what GM calls a “skateboard” chassis. This allows for avariety of body styles to be used interchangeably. AUTOnomy’s configuration could be changed from a sleek sports sedan (shown below) to a pickup body for hauling, say, fire-wood. Different body styles could be leased from the local GM dealership.The Mercury Marauder Convertible concept vehicle shown at the recent Chicago Auto53April 2002but Mercury says that me-dia and public response has been positive.MAP Revises Its StandardsThe Motorist Assurance Program has released its re-vised 2002 Uniform In-spection & Communication Standards (UICS) for all seven vehicle systems—Brakes, Drivetrain, Engine Maintenance & Perfor-mance, Electrical, Exhaust, HVAC and Steering & Sus-pension. Service providers participating in MAP need to implement the new UICS immediately.“These revisions are made biannually to reflect changes in technology as part of MAP’s commitment to consumers and partici-pating service providers,”explained MAP presidentLawrence Hecker. “We alsoupdate the Uniform In-spection and Communica-tion Standards to continuemaking them as user-friendly as possible fortechnicians and their cus-tomers.”Copies of the UICS canbe downloaded from theMembers Only section ofMAP’s website (located at)using apass code provided by theMAP office. A copy is alsoavailable on CD. Nonmem-bers may purchase copies inprinted form by contactingMAP at 301-634-4955 or****************.Associations Stand UpAgainst Scrappage LawsSeven automotive aftermar-ket associations recentlysigned a letter to U.S. Sen-ate Majority Leader TomDaschle (D-SD) opposingSection 803 of Senate Bill1766, the Energy PolicyAct of 2002. Section 803,“Assistance for State Pro-grams to Retire Fuel-Inef-ficient Motor Vehicles,”would provide states fundsfor vehicle scrappage pro-grams. The program is de-signed to reduce domesticfuel consumption.The letter was written onbehalf of the following after-market groups: AutomotiveService Assn. (ASA), Auto-motive Aftermarket IndustryAssn. (AAIA), AutomotiveEngine Rebuilders Assn.(AERA), Automotive PartsRebuilders Assn. (APRA),Automotive Warehouse Dis-tributors Assn. (AWDA),Specialty Equipment Man-ufacturers Assn. (SEMA)and Tire Assn. of NorthAmerica (TANA).The program in Section803 allows for the purchaseof vehicles that are 15 yearsold or older and requiresthat they be scrapped. Thesection does not provide arepair option or a means todetermine if a vehicle hasvalue as a classic model orcontains salvageable parts.Section 803 does notguarantee a scrapped vehi-cle will be replaced with amore fuel-efficient vehicle;it does not guarantee thatlow-income or fixed-in-come persons will be ableto afford a new vehicle,much less a fuel-efficientone; and it does not recog-nize that older vehicles canbe repaired and maintainedto be fuel-efficient vehicles.RADIATOR FANASSEMBLIESApplication Specific AssembliesHonda Accord 1997-92AC Fan 2.2, 2.7LChrysler Corp. Mini Vans 1995-912.5,3.0, 3.3, 3.8L54April 2002“ASA has consistently tak-en issue with any scrappage legislation, state or federal,that did not, at a minimum,provide a repair option for these older vehicles,” said Bob Redding, ASA’s Wash-ington DC representative.“The few state programs in existence have also eliminat-ed in their policy discussions the hope of a repair option,but none of these programs have produced reduced emis-sions to any large degree.”NAPA/ASE NamesPiraino Tech of the YearNAPA Auto Parts, in con-junction with the National Institute for Automotive Service Excellence (ASE),named Jim Piraino, owner of Camarillo Car Care Cen-ter in Camarillo, California,its 2002 NAPA/ASE Tech-nician of the Year. Piraino was honored for demon-strating superior technical skills and business prac-tices, showing a commit-ment to customer service and technician training,supporting the community in which his business oper-ates and demonstrating outstanding service to the automotive repair and maintenance industry.“In our business plan, we set the goal of being recog-nized as the premier ser-vice facility in our commu-nity,” said Piraino. “Being recognized as best in the nation was beyond my wildest dreams. It’s a tremendous honor, and something we will take a great deal of pride in as we represent NAPA and ASE in 2002.”Jim Piraino, owner of Camarillo Car Care Center in Camarillo,California, was named 2002 NAPA/ASE Technician of the Year (he was a finalist in each of the previous five years). His NAPA AutoCare shop employs six ASE-Certified Master Technicians.FLEXIBLE W AND GETS INTO TIGHT AREAS!NEW! UL TRA-BRIGHT LED TECHNOLOGY!COMFOR T GRIP WITH E-Z ACCESS ON/OFFBUTTONFLEX n GLOW53512 (P A T . PEND.)CORDL ESS 12V/50W UV LIGHT KIT53400UV LEAK DETECTION•This Unique cordless light offers convenience and portability •Light kit includes high intensity light, UV enhancing safety goggles, rechargeable battery pack and battery chargerAdding dye to an A/C systemis FAST & EASY!Simply connect the injector and twist the handle just one turn to the next application line. Replaceable cartridges are available for 10 or 25 applications of Universal Dye that is compatible with R12 and R134a systems. The injectorhose comes complete with a R134a Adapter for 1/4FL systems.“World Class Quality”MASTERCOOL • 2 Aspen Drive • Randolph, NJ • PH: (973) 252-9119 • FAX: (973) 252-9119Circle #24。
基于低频信号注入法的PMSM低速无传感器控制

2 1年 3月 0l
电 力 电子 技 术
P w rE e to is o e l cr n c
Vo -5.No3 l 4 . Ma c 0 1 rh2 1
基于低频信号注入法的 P M 低速无传感器控制 MS
徐 艳 平 ,郜 亚秋 ,钟 彦 儒
( 西安 理工大 学 , 电气工 程系 ,陕西 西安 704 ) 1 0 8
摘 要 : 永磁 同步 电机 ( MS 无速 度传 感器 控制 方法 中通 常 利用 反 电动势 来估 计 电机转 速 , 在 P M) 而反 电动 势在 电 机 低速 时 会过 小 , 从而 导 致低 速 运行 时 无法 实现 P M 的无 速度 传 感器 运 行 , MS 在此 采用 低 频信 号 注入 法 实现
定 义 g轴 反 电 势 为 :q一 e= 3 n 2n为极对数。 ,
系 统 的 运 动 方程 为 :
d tn( ) w/ =。 d 一 / J
式 中 : 为转 动惯量 ; 为负载 转矩 。 t 厂
() 4
图 2 P M 无速 度传 感器矢 量控 制系 统框 图 MS
.
实验 研 究 , 电机 参 数 : 定 电压 2 0V, 定 电流 额 0 额
94 额 定 转 矩 7 1 I. 定 转 速 20 0r m n . . A, .5N・ 额 n 0 ・ i~ 极 对 数 4对 , 子 惯 量 1 3 1 k I . 子 电阻 转 . × 0 g・I 定 2 T
s n o l s o to to s b tt e b c — m s to s l t s ma e mo o p e t lw s e d a d s n o ls o t l e s re s c n rl meh d , u h a k e f i o mal o e t t t r s e d a o p e n e s r s c n r i e o
燃料电池阳极蛇形优于平行流场开孔率50%最优

INTERNATIONAL JOURNAL OF ENERGY RESEARCH Int.J.Energy Res.2009;33:719–727Published online 13January 2009in Wiley InterScience ().DOI:10.1002/er.1507Effect of anode current collector on the performance of passive directmethanol fuel cellsQin-Zhi Lai,Ge-Ping Yin Ã,y ,z and Zhen-Bo WangSchool of Chemical Engineering and Technology,Harbin Institute of Technology,Harbin 150001,ChinaSUMMARYThe effect of anode current collector on the performance of passive direct methanol fuel cell (DMFC)was investigated in this paper.The results revealed that the anode of passive DMFC with perforated current collector was poor at removing the produced CO 2bubbles that blocked the access of fuel to the active sites and thus degraded the cell performance.Moreover,the performances of the passive DMFCs with different parallel current collectors and different methanol concentrations at different temperatures were also tested and compared.The results indicated that the anode parallel current collector with a larger open ratio exhibited the best performance at higher temperatures and lower methanol solution concentrations due to enhanced mass transfer of methanol from the methanol solution reservoir to the gas diffusion layer.However,the passive DMFC with a smaller open ratio of the parallel current collector exhibited the best performance at lower temperatures and higher methanol solution concentrations due to the lower methanol crossover rate.Copyright r 2009John Wiley &Sons,Ltd.KEY WORDS:passive direct methanol fuel cell;current collector;open ratio;methanol concentration;cell temperature1.INTRODUCTIONOver the past decades,the worldwide proliferation of portable electronic devices has created a large and growing demand for energy sources that are compact,lightweight,and powerful.The direct methanol fuel cell (DMFC)has received much attention for its high theoretical energy density,using liquid fuel,employing solid polymer electro-lyte,and working at low temperature,and has presented beneficial opportunities for use as a power source for small mobile devices [1,2].However,these accessorial devices make the fuel cell system complex.Therefore,the passive DMFC with neither liquid pumps nor gas compressors has been proposed and studied.In the passive DMFC,the oxygen diffuses into the cathode side from ambient air without any help of external devices,and the methanol solution stored in the reservoir attaches to the anode current collector and the methanol driven by concentration gradient be-tween the reservoir and anode also diffuses intozProfessor.*Correspondence to:Ge-Ping Yin,School of Chemical Engineering and Technology,Harbin Institute of Technology,Harbin 150001,China.yE-mail:yingphit@ Contract/grant sponsor:Natural Science Foundation of China;contract/grant numbers:20606007,50872027Received 21October 2008Revised 19November 2008Accepted 24November 2008Copyright r 2009John Wiley &Sons,Ltd.the anode catalyst layer.Therefore,the passive DMFC can potentially result in higher reliability, lower cost,higher fuel utilization,and higher energy density,which are in favor of mobile equipments in future electronic devices[3–7].The key component of a passive DMFC system consists of a methanol solution reservoir,anode current collector,membrane electrode assembly (MEA),and cathode current collector.Liu et al.[8] presented that the optimal concentration of methanol solution in passive DMFC was 4.0mol LÀ1.Similar opinions about the effect of methanol concentration on the performance of passive DMFC could also be found in the literature[9–13].The utilizations of highly concentrated fuel of DMFCs with vapor fed had also been reported[14,15].Chen et al.reported a suitable MEA for DMFC[16,17]and optimized theflow-fields[7,18].Lu et al.[19]applied a highly hydrophobic cathode microporous layer(MPL) and a thin membrane to control the water crossover.Thus,the cathodeflooding was avoided by facilitating water backflow to the anode via hydraulic permeation.Yang et al.[20] presented that the micro DMFC with a parallel flow-field at the anode and a perforatedflow-field at the cathode performed the best.Jeong et al.[21] presented the open area of cathode and the relative humidity of the atmosphere on the performance of passive air breathing PEMFCs.Chen et al.[22] found that the cell performance was improved with the increase of cathode and anode open ratios,which resulted in the improvement of mass transport of both methanol and oxygen,although the cell operating temperature decreases slightly with the increase of open ratio.The anode current collector is effective on the PEMFC performance and the methanol crossover flux is very important[23–25].In a passive DMFC, the methanol solution stored in the reservoir attaches to the anode current collector,and the methanol driven by the concentration gradient between the reservoir and the anode diffuses into the anode.The anode current collector is to provide channels for methanol fuel to access the MEA surface.Therefore,the anode current collector had a significant effect on the cell performance.In this paper,we experimentally investigated the effect of the parallel current collector and the perforated current collector on the performance of a passive DMFC.We found that the parallel current collector exhibited a better performance than the perforated current collector. Then we focused on studying the effects of the parallel current collector with different open ratios on the cell performance at different methanol concentrations and cell temperatures.2.EXPERIMENTAL2.1.MEA fabricationAll the electrocatalysts used in this paper were prepared in-house by chemical reduction with formaldehyde of H2PtCl6and RuCl3as precursors [26].The anode catalyst was40wt%PtRu(with an atomic ratio of1:1)/C and the cathode catalyst was 40wt%Pt/C.The gas diffusion layers(GDLs)for the cathode electrodes were wet-proofed Toray carbon papers coated with the MPLs,which comprised Vulcan XC-72carbon black and 10wt%of PTFE.The anode GDLs were the Toray carbon papers coated with the MPLs,which comprised Vulcan XC-72carbon black and 10wt%of Nafion ionomer(DuPont).The loading of carbon black was2mg cmÀ2for both the anode and the cathode.The catalyst powder and5wt% Nafion ionomer solution were ultrasonically mixed in isopropyl alcohol to form a homogeneous catalyst ink.Then the catalyst ink was scraped onto the GDLs,and then the electrodes were dried for2h in the vacuum oven at801C.The Nafion content in both the anode and the cathode was 20wt%and the metal loading(PtRu or Pt)was 2.0mg cmÀ2in each electrode.Nafion117polymer membranes(DuPont) were used to fabricate MEAs.Before being applied to the electrodes,the membranes were pretreated in four steps to eliminate the organic and inorganic contaminants.First,membranes were boiled in3wt%H2O2solution followed by washing in the ultra-pure water.Then,they were boiled in0.5mol LÀ1H2SO4solution.Finally, the membranes were boiled again in the ultra-pure water.Each step took about1h.The pretreated Nafion117membrane was sandwiched betweenI,G.-P.YIN AND Z.-B.WANG 720the anode and the cathode,and then the assembly was hot pressed under a loading of100kg cmÀ2for 90s at1351C.2.2.Single cellfixtureThe prepared MEA was sandwiched between two graphite plates to form an apparent area of approximately9cm2.The graphite plates of the cathode side was machined with many holes for air diffusion and the anode side machined through the graphite plates had channels and large open space for delivering and storing methanol solution, respectively.The oxygen diffused into the cathode side from ambient air without any help of external devices,such as a pump or a fan,and the methanol solution was stored in the reservoir attached to the anode side plate,and the methanol driven by concentration gradient between the reservoir and the anode diffused into the anode.The volume of the methanol reservoir was10cm3.The extension area of the bipolar plates served as a current collector,and a heating tape was attached to the extension area to adjust the operating temperature of passive DMFC to a desired value during the experiments.2.3.Anode current collector designThe geometry areas of current collectors are listed in Table I and Figure 1.As can be seen from Figure1and Table I,the open ratio of the current collector with perforatedflow-fields(perforated current collector)is50%,and the open ratios of current collectors with parallelflow-fields(parallel current collector-1to perforated current collector-3)are from35.5to68.8%.2.4.Electrochemical measurementsThe Fuel Cell Testing System(Arbin Instrument Corp.)connecting with a computer was used to control the conditions of discharge and record the voltage–current curves.For each discharging current point along the I–V curve,a more than 40-s waiting time was used to obtain the stable voltage.A solution of 1.0–8.0mol LÀ1aqueous methanol driven by the concentration gradient between the reservoir and the anode diffused into the anode.The oxygen diffused into the cathode side from ambient air without any help of external devices.Prior to testing the performance of passive DMFC,the MEA was installed in an active cell fixture and activated at801C for about24h. During the activation period,methanol solution of 2.0mol LÀ1was fed at aflow rate of3.0mL minÀ1, while oxygen was supplied under atmospheric pressure at aflow rate of200mL minÀ1.3.RESULTS AND DISCUSSION Figure2shows the performance of passive DMFCs with different anode current collectors. The performance of the passive DMFC with the parallel current collector is better than that with the perforated collector.In the initial discharge process,its performance with the parallel current collector is similar to that with the perforated current collector.However,the performance of the latter evidently decays at higher current densities. This is because the anode of the passive DMFC with the perforated current collector is poor at removing the produced CO2bubbles that block the access of fuel to the active sites and thusTable I.The geometry of the anode current collector.Current collector Perforated currentcollectorParallel currentcollector-1Parallel currentcollector-2Parallel currentcollector-3Channel width(mm)—122Rib width(mm)—221 Aperture(mm)4———Holes–centers distance(mm)5———Depth(mm)2222 Open area(mm2)452319450600 Effective area(mm2)900899900896 Open ratio(%)5035.55068.8 EFFECT OF ANODE CURRENT COLLECTOR ON THE PERFORMANCE OF DMFC721degrade its performance [20,23].On the other hand,the passive DMFC with the parallel current collector exhibits the better performance due to the easier removal of CO 2bubbles from the anode.Figure 3shows that the transient discharging voltage curves of passive DMFCs with different anode current collectors at current density 30mA cm À2at an ambient temperature and a cell temperature of 301C.The passive DMFC with the parallel anode current collector shows the higher transient discharging voltage.In the initial discharge process,the transient discharging voltage of the passive DMFC with the perforated current collector sharply decays to about 0.26V,and then slowly decreases with time.The anode of the passive DMFC with the perforated current collector makes against the removing ofproducedFigure 1.Geometry of the anode current collectors:(a)perforated current collector;(b)parallel current collector-1;(c)parallel current collector-2;and (d)parallel currentcollector-3.Figure parison of the passive DMFC perfor-mance among DMFCs with different anode current collectors (2.0mol L À1methanol solution,cell tempera-ture 301C).I,G.-P.YIN AND Z.-B.WANG722CO 2bubbles.Therefore,its voltage fastly degrades in the initial discharge process.Moreover,we still study the effects of different parallel current collectors as the parallel current collector exhibited a better performance than the perforated current collector.The effects of different open ratios of the parallel current collectors on the cell performance were investigated using collector-1(open ratios:35.5%),collector-2(open ratios:50%),and collector-3(open ratios:68.8%).Figure 4shows the polarization curves and the power density curves of passive DMFCs with the parallel current collector at a methanol concentration of 2.0mol L À1.The maximum power density of the passive DMFC with an open ratio of 50%is the highest.However,three parallel current collectors of passive DMFCs exhibit similar performances.Figure 5shows the polarization curves and the power density curves of passive DMFCs with parallel current collectors at a methanol concentration of 4.0mol L À1.The cell with the parallel current collector with an open ratio of 50%exhibits the best performance.The parallel current collectors with a smaller open ratio (35.5%)and a larger open ratio (68.8%)exhibit the worse cell performances.It is believed that the parallel current collector with a smaller open ratiopresents a smaller effective contact area between the liquid fuel and the MEA,which results in the degradation of the cell performance.On the other hand,the parallel current collector with a larger open ratio presents a larger effective contact area between the liquid fuel and the MEA.However,first of all,the internal resistance of the cell increases with the increase of the open ratio of the parallel current collector [26].Second,the larger methanol permeation area causes a higher methanol crossover rate through the membrane.Figure 6shows the transient discharging voltage curves of passive DMFCs withdifferentFigure 3.Transient discharging voltage–time curves at a current density of 30mA cm À2with a start from the passive DMFC to be fueled with 2.0mol L À1methanol solution (10mL)at passive DMFCs with different anodecurrent collectors (cell temperature 301C).Figure 4.The effect of different open ratios of the parallel current collector on the performance of the cellwith 2.0mol L À1methanol at 301C.Figure 5.The effect of different open ratios of the parallel current collector on the performance of the cellwith 4.0mol L À1methanol at 301C.EFFECT OF ANODE CURRENT COLLECTOR ON THE PERFORMANCE OF DMFC723parallel collectors at a current density of 15mA cm À2.To investigate the fuel utilization,the Faraday efficiency (Z )is adopted for the passive DMFC with different open ratios.The Faradic efficiency is here defined as a ratio of the actual discharge capacity versus the theoretical capacity of fuel used in the fuel cell.It is believed that the Faraday efficiency of the passive DMFC was reduced because of the loss of methanol by crossover and remaining methanol in the reservoir,which cannot be used due to methanol mass transfer limitations.The Faradic efficiency can be calculated with the following equation [8]:Z ¼it6CVFÂ100%ð1Þwhere i (A)is the discharging current,t (s)the time of the discharging process C (mol L À1)the methanol concentration,V (L)the methanol solution volume,F (C)the Faraday constant,and Z the Faradic efficiency.The Faradic efficiencies determined by Equation (1)with different methanol concentrations at a constant current density of 15mA cm À2are given in Table II.The Faradic efficiency decreases from 48.3to 42.0%when the open ratio increased from 35.5to 68.8%.The lower Faradic efficiency is primarily caused by the higher rate of methanol crossover with a higher open ratio.In addition,it can be seen from Figures 4and 5that the highest open-circuit voltage (OCV)is obtained with a parallel current collector-1.It is believed that the gradual decrease of OCVs with the increase of open ratio results from the increase of methanol crossover through the Nafion 117membrane.Figure 7shows that the anode of the passive DMFC with the parallel current collector-3exhibits the best performance with the methanol concentration of 1.0mol L À1.It is believed that the transfer of fuel molecules into the anode catalyst layer is the main factor affecting the cell performance at a lower methanol concentration.Under such a situation,the parallel current collector with a larger open ratio affords a larger effective contact area between the liquid fuel and the GDL,which improves the mass transfer of methanol from the methanol solution reservoir to the catalyst layer.Therefore,the parallel current collector with a larger open ratio tends toyieldFigure 6.Transient discharging voltage at a current density of 15mA cm À2with a start from the passive DMFC to be fueled with 2.0mol L À1methanol solution (10mL)at the passive DMFC with different open ratios (air temperature 201C,the passive DMFCtemperature 301C).Table II.The effect of the open ratio on Faradic efficiencies for the passive DMFC under current density(15.0mA cm À2)discharge.Open ratio (%)i (mA)C (mol L À1)V (ml)Z (%)35.513521048.35013521044.168.813521042.0Figure 7.The effect of different open ratios of the parallel current collector on the performance of the cellwith 1.0mol L À1methanol at 301C.I,G.-P.YIN AND Z.-B.WANG724a better cell performance with a lower methanol solution concentration.Figure 8shows that the parallel current collector-1exhibits the best performance with a methanol concentration of 8.0mol L À1.It is believed that when the cell is operating at a higher methanol solution concentration,the methanol transfer rate becomes faster and the lower performance is primarily caused by the higher rate of methanol crossover and excessive water crossover through the membrane with a higher open ratio [27,28].The experiments were carried out at 20and 401C with the same methanol concentration as in Figures 9and 10,respectively.In Figure 9,the anode of the passive DMFC with the parallel current collector-1exhibits the best performance at a low temperature of 201C.It is believed that there are more methanol molecules in the surface of the anode catalyst layer with a larger open ratio.However,the poor catalytic activity of the anode at a low temperature of 201C leads to a higher methanol crossover rate through the membrane.Therefore,the anode of the passive DMFC with the parallel current collector-1exhibits the best performance.However,as can be seen from Figure 10,at a higher temperature of 401C,the anode of the passive DMFC with the parallel current collector-3exhibits the best performance.It is believed that the catalytic activity of the anode catalyst increases with the increase of temperature,and the higher methanol mass transfer ratebecomes the main factor enhancing the cell performance.On the other hand,the anode of the passive DMFC with smaller open areas means a smaller effective contact area between the liquid fuel and the GDL,which weakens the methanol mass transfer.Therefore,the anode of the passive DMFC with the parallel current collector-3exhibits the best performance.4.CONCLUSIONThe effect of the anode current collector on the performance of the passive DMFCwasFigure 8.The effect of different open ratios of the parallel current collector on the performance of the cellwith 8.0mol L À1methanol at 301C.Figure 9.The effect of different open ratios of the parallel current collector on the performance of the cellwith 2.0mol L À1methanol at 201C.Figure 10.The effect of different open ratios of the parallel current collector on the performance of the cellwith 2.0mol L À1methanol at 401C.EFFECT OF ANODE CURRENT COLLECTOR ON THE PERFORMANCE OF DMFC725investigated.The experimental results show that the passive DMFCs with the perforated current collector are poor at removing the produced CO2 bubbles,which block the access of fuel to the active sites and thus degrade the cell performance, compared with the parallel current collector.Moreover,the effect of different parallel current collectors with various open ratios on performances of the passive DMFCs at different temperatures and different methanol concentrations is also studied.The passive DMFC equipped with a smaller open ratio (35.5%)of the parallel current collector exhibits the best performance at a higher methanol solution concentration and a lower temperature as the methanol mass transfer rate becomes relatively fast,and the best performance is primarily caused by the lowest methanol crossover rate with a smaller open ratio.The experiments also reveal that the passive DMFCs equipped with a larger open ratio(68.8%)of the parallel current collector exhibit the best performance at a lower methanol concentration and a higher temperature.The best performance results primarily from the improvement of the mass transfer of methanol to the GDL due to the larger open ratio.ACKNOWLEDGEMENTSThis work was supported by Natural Science Founda-tion of China(Nos.20606007and50872027).REFERENCES1.Springer TE,Zawodzinski TA,Gottesfeld S.Polymerelectrolyte fuel cell model.Journal of the Electrochemical Society1991;136:2334–2342.2.Fukada S.Analysis of oxygen reduction rate in aprotonexchange membrane fuel cell.Energy Conversion and Management2001;42:1121–1131.3.Heinzel A,Nolte R,Ledjeff-Hey K et al.Membrane fuelcells concepts and system design.Electrochimica Acta1998;43:3817–3820.4.Rice J,Faghri A.A transient,multi-phase and multi-component model of a new passive DMFC.International Journal of Heat and Mass Transfer2006;49:4804–4820. 5.Lu GQ,Lim PC,Liu FQ et al.On mass transport in an air-breathing DMFC stack.International Journal of Energy Research2005;29:1041–1050.6.Yazici MS.Passive air management for cylindrical car-tridge fuel cells.Journal of Power Sources2007;166:137–142.7.Chen R,Zhao TS.Porous current collectors for passivedirect methanol fuel cells.Electrochimica Acta2007;52:4317–4324.8.Liu JG,Zhao TS,Chen R et al.The effect of methanolconcentration on the performance of a passive DMFC.Electrochemistry Communications2005;7:288–294.9.Kim D,Cho EA,Hong SA et al.Recent progress in passivedirect methanol fuel cells at KIST.Journal of Power Sources2004;130:172–177.10.Chu D,Jiang RZ.Effect of operating conditions on energyefficiency for a small passive direct methanol fuel cell.Electrochimica Acta2006;51:5829–5835.11.Kim YJ,Bae B,Scibioh MA et al.Behavioral pattern of amonopolar passive direct methanol fuel cell stack.Journal of Power Sources2006;157:253–259.12.Kho BK,Bae B,Scibioh MA et al.On the consequencesof methanol crossover in passive air-breathing direct methanol fuel cells.Journal of Power Sources2005;142:50–55.13.Hong SA,Ha HY.Performance evaluation of passiveDMFC single cells.Journal of Power Sources2006;158:1256–1261.14.Kim HK.Passive direct methanol fuel cells fed withmethanol vapor.Journal of Power Sources2006;162:1232–1235.15.Guo Z,Faghri A.Vapor feed direct methanol fuel cells withpassive thermal-fluids management system.Journal of Power Sources2007;167:378–390.16.Chen R,Zhao TS.A novel electrode architecture forpassive direct methanol fuel cells.Electrochemistry Com-munications2007;9:718–724.17.Shao ZG,Hsing IM,Zhang HM et al.Influence of anodediffusion layer on the performance of a liquid feed direct methanol fuel cell by AC impedance spectroscopy.International Journal of Energy Research2006;30: 1216–1227.18.Kazim A,Forges P,Liu HT.Effects of cathode operatingconditions on performance of a PEM fuel cell with interdigitatedflowfields.International Journal of Energy Research2003;27:401–414.19.Lu GQ,Lim PC,Liu FQ et al.On mass transport in an air-breathing DMFC stack.International Journal of Energy Research2005;29:1041–1050.20.Yang WM,Choub SK,Shu C.Effect of current-collectorstructure on performance of passive micro direct methanol fuel cell.Journal of Power Sources2007;164:549–554. 21.Jeong SU,Cho EA,Kim HJ et al.Effects of cathode openarea and relative humidity on the performance of air-breathing polymer electrolyte membrane fuel cells.Journal of Power Sources2006;158:348–353.22.Chen R,Zhao TS,Yang WW et al.Two-dimensional two-phase thermal model for passive direct methanol fuel cells.Journal of Power Sources2008;175:276–287.23.Lu GQ,Wang CY.Electrochemical andflow characteriza-tion of a direct methanol fuel cell.Journal of Power Sources 2004;134:33–40.24.Ferng YM,Su A,Lu SM.Experiment and simulationinvestigations for effects offlow channel patterns on the PEMFC performance.International Journal of Energy Research2008;32:12–23.I,G.-P.YIN AND Z.-B.WANG 72625.Su A,Chiu YC,Weng FB.The impact offlowfieldpattern on concentration and performance in PEMFC.International Journal of Energy Research2005;29: 409–425.26.Wang ZB,Yin GP,Shi PF.Effects of ozone treatment ofcarbon support on Pt–Ru/C catalysts performance for direct methanol fuel cell.Carbon2006;44:133–140.27.Lu GQ,Liu FQ,Wang CY.Water transport throughNafion112membrane in DMFCs.Electrochemical and Solid State Letters2005;8:A1–A4.28.Liu FQ,Lu GQ,Wang CY.Low crossover of methanoland water through thin membranes in direct methanol fuel cells.Journal of the Electrochemical Society2006;153:A543–A553.EFFECT OF ANODE CURRENT COLLECTOR ON THE PERFORMANCE OF DMFC727。
generator 产品说明书

Ratings at 0.8 power factor.Please refer to the output ratings technical data section f or specificgenerator set outputs per voltage.Ratings in accordance with ISO 8528, ISO 3046, IEC 60034,BS5000 and NEMA MG-1.22.Generator set pictured may include optional accessories.Prime RatingThese ratings are applicable for supplying continuous electrical power (at variable load) in lieu of commercially purchased power. There is no limitation to the annual hours of operation and this model can supply 10% overload power for 1 hour in 12 hours.Standby RatingThese ratings are applicable for supplying continuous electrical power (at variable load) in the event of a utility power failure. No overload is permitted on these ratings. The alternator on this model is peak continuous rat ed (as defined in ISO 8528-3).Standard Reference ConditionsNote: Standard reference conditions 25°C (77°F) Air Inlet Temp, 100m (328 ft) A.S.L. 30% relative humidity.F uel consumption data at full load with diesel fuel with specific g ravity of 0.85 and conforming to BS2869: 1998, Class A2.FG W ilson off er a range of optional features to allow you to tailor our generator sets to meet your power needs. Options available include:•Upgrade to CE Cer tification• A wide range of Sound Attenuated Enclosures• A variety of generator set control and synchronising panels •Additional alarms and shutdowns •A selection of exhaust silencer noise levelsFor further information on all of the standard and optional features accompanying this product please contact your local Dealer or visit:#DEALER_LOGOP22-6(Skid)#FGWILSON_GENSET_IMAGEDesigned to operate in ambient conditions up to 50°C (122°F).Contact your local FG Wilson Dealer for pow er ratings at specific sit e conditions.Alternator Physical Data1* dependant on voltage code selectedAlternator Operating Data2250Alternator Performance Data 60 Hz*Based on 30% voltage dip at 0.6 power factor.** With optional independant excitation system (PMG / AUX winding)220/110#FGWILSON_GENSET_IMAGE#DEALER_LOGODocumentationOperation and maintenance manual including circuit wiring diagrams.Generator Set StandardsThe equipment meets the following standards: BS5000, ISO 8528, ISO 3046, IEC 60034, NEMA MG-1.22.Warranty6.8 – 750 kVA electric power generation products in prime applications the warranty period is 12 months from date of start-up, unlimited hours (8760). For standby applications the warranty period is 24 months from date of start-up, limited to 500 hours per year.730 – 2500 kVA electric power generation products in prime applications the warranty period is 12 months from date of start-up, unlimited hours (8760 hours) or 24 months from date of start-up, limited to 6000 hours. For standby applications the warranty period is 36 months from date of start-up, limited to 500 hours per year.FG Wilson manufactures product in the following locations:Northern Ireland • Brazil • China • IndiaWith headquarters in Northern Ireland, FG Wilson operates through a Global Dealer Network.To contact your local Sales O ffic e please visit the FG Wilson website at .FG Wilson is a trading name of Caterpillar (NI) Limited.In line with our policy of continuous product development, we reserve the right to change specification without notice.2019-08-14P22-6(Skid)Dealer Contact Details。
Fuel cell

专利名称:Fuel cell
发明人:藤井 洋介,小川 隆行,菊池 英明,鈴木 征治申请号:JP2000316658
申请日:20001017
公开号:JP4969719B2
公开日:
20120704
专利内容由知识产权出版社提供
摘要:A fuel cell is provided wherein the production of a separator is easy and a reactant gas channel thereof can be easily arranged. The fuel cell is constituted by clamping an electrolyte membrane electrode assembly provided with an anode and a cathode at opposite positions on both sides of an electrolyte membrane, by a pair of separators via a sealing member. A part of a reactant gas channel formed between the electrolyte membrane electrode assembly and the cathode side separator is formed seamlessly by extended portions of the sealing member.
申请人:本田技研工業株式会社
地址:東京都港区南青山二丁目1番1号
国籍:JP
代理人:志賀 正武,高橋 詔男,青山 正和,鈴木 三義,西 和哉,村山 靖彦
更多信息请下载全文后查看。
SOLID OXIDE FUEL CELL STACK AND MODULE
专利名称:SOLID OXIDE FUEL CELL STACK ANDMODULE发明人:YAKABE HISATAKA,矢加部 久孝,OGASAWARAKEI,小笠原 慶,SAKURAI TERUJI,桜井 輝治申请号:JP2003109583申请日:20030414公开号:JP2004319187A公开日:20041111专利内容由知识产权出版社提供专利附图:摘要:PROBLEM TO BE SOLVED: To obtain a supported film type solid oxide fuel cell stackand a supported film type solid oxide fuel cell module which can be constituted without passing through complicated and numerous processes, and wherein a gas sealing part is reduced.SOLUTION: This stack is the supported film type solid oxide fuel cell stack composed by wrapping the circumference with an alloy foil in which an opening is installed on an air electrode side, and is composed by wrapping a supported film type cell composed by laminating a fuel electrode, an electrolyte film, and the air electrode one by one, and an elastic member and fuel introducing tubes arranged at both sides of the fuel electrode by the alloy foil in which the opening is installed on the air electrode side. In the supported film type solid oxide fuel cell module, this supported film type solid oxide fuel cell stack is used. Without passing through the complicated and numerous processes as before, a supported film type SOFC stack and a supported film type SOFC module can be constituted wherein the gas sealing part is reduced.COPYRIGHT: (C)2005,JPO&NCIPI申请人:TOKYO GAS CO LTD,東京瓦斯株式会社地址:東京都港区海岸1丁目5番20号国籍:JP代理人:加茂 裕邦更多信息请下载全文后查看。
System and methods for implementing a non-linear e
专利名称:System and methods for implementing anon-linear electrical circuit dynamic fuel cellmodel发明人:Yang Wang,Jian-ping Zheng申请号:US11761077申请日:20070611公开号:US07844434B2公开日:20101130专利内容由知识产权出版社提供专利附图:摘要:A nonlinear electrical circuit dynamic model for different fuel cells is provided.The model provides a nonlinear electrical circuit equivalent, the parameters of whichcorrespond to the particular fuel cell being modeled. The parameters can be theoretically or experimentally derived from the responses of the particular fuel cell. The resulting model can have impedances that are equivalent to that of the particular fuel cell, thereby capturing or providing a good approximation of the transient behavior of the particular fuel cell. More particularly, the resulting model can have impedances in the low frequency range less than 100 Hz that are equivalent to that of the particular fuel cell.申请人:Yang Wang,Jian-ping Zheng地址:Tallahassee FL US,Tallahasssee FL US国籍:US,US代理机构:Novak Druce + Quigg更多信息请下载全文后查看。
Method and system for starting up fuel cell stack
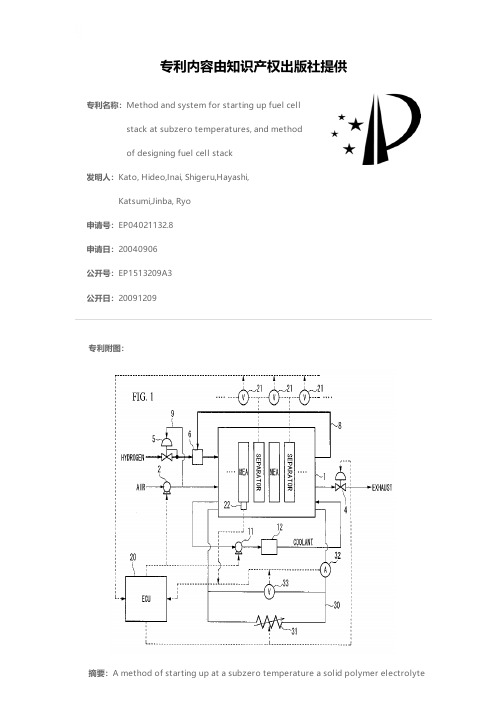
专利名称:Method and system for starting up fuel cellstack at subzero temperatures, and methodof designing fuel cell stack发明人:Kato, Hideo,Inai, Shigeru,Hayashi,Katsumi,Jinba, Ryo申请号:EP04021132.8申请日:20040906公开号:EP1513209A3公开日:20091209专利内容由知识产权出版社提供专利附图:摘要:A method of starting up at a subzero temperature a solid polymer electrolytefuel cell stack that is formed by stacking a plurality of layers of separators and membrane electrode assemblies having a solid polymer electrolyte membrane and electrodes. The method includes a step of using a solid polymer electrolyte fuel cell stack in which the separators are made from metal and have a cross-sectional waveform structure, and a space that is formed between at least a portion of the separators and separators that are placed adjacent to this portion of the separators is used as a coolant flow passage.申请人:HONDA MOTOR CO., LTD.地址:1-1, Minamiaoyama 2-chome, Minato-ku Tokyo JP国籍:JP代理机构:Prechtel, Jörg更多信息请下载全文后查看。
METHOD FOR CONFIGURING SECONDARY CELL, DEVICE AND

专利名称:METHOD FOR CONFIGURING SECONDARYCELL, DEVICE AND COMPUTER STORAGEMEDIUM发明人:YANG, Ning申请号:EP18901084.6申请日:20180119公开号:EP3742809A1公开日:20201125专利内容由知识产权出版社提供专利附图:摘要:Provided are a method for configuring a secondary cell, a device and acomputer storage medium. The method comprises: receiving measurement configuration information, the measurement configuration information comprising measurement frequency point information and determination basis information; measuringmeasurement cells according to the measurement frequency point information; on the basis of identities of the measurement cells and the determination basis information,determining a cell set corresponding to each node and a measurement result of each cell; reporting the cell set corresponding to each node and the measurement result of each cell, the cell set corresponding to each node and the measurement result of each cell being used for a master node (MN), at the same time as configuring a master cell group (MCG) for a UE, to also determine a secondary cell group (SCG) of a secondary node (SN) according to the cell set corresponding to each node and the measurement result of each cell, and configure the secondary cell group (SCG) of the secondary node (SN) for the UE. The present invention thus obviates the need to trigger the MN to independently configure the SCG for the UE, thereby reducing secondary cell configuration delay.申请人:GUANGDONG OPPO MOBILE TELECOMMUNICATIONS CORP., LTD.地址:No. 18 Haibin Road Wusha, Chang'an Dongguan, Guangdong 523860 CN国籍:CN代理机构:Ziebig & Hengelhaupt Patent- und Rechtsanwaltskanzlei PartG mbB更多信息请下载全文后查看。
Cell Signaling Pathways in Development

Cell Signaling Pathways in Development Cell signaling pathways are crucial in the development of organisms. These pathways are complex networks of biochemical reactions that enable cells to communicate with each other, allowing them to coordinate their activities and ultimately form tissues and organs. In this essay, we will explore the importance of cell signaling pathways in development from multiple perspectives.From a biological perspective, cell signaling pathways play a critical role in the development of an organism. During embryonic development, cells must differentiate and specialize into various cell types, such as muscle cells, nerve cells, and skin cells. This differentiation is controlled by signaling pathways that activate specific genes and proteins, leading to the development of specific cell types. For example, the Sonic Hedgehog pathway is essential for the development of the nervous system, while the Wnt pathway is crucial for the development of the digestive system. Without these signaling pathways, the development of an organism would be severely impaired.From a medical perspective, understanding cell signaling pathways is essential for the development of new treatments for various diseases. Many diseases, such as cancer, are caused by abnormalities in cell signaling pathways. By understanding these pathways, researchers can develop drugs that target specific proteins or genes involved in the pathway, leading to more effective treatments. For example, drugs that target the Epidermal Growth Factor Receptor (EGFR) pathway have been developed to treat various types of cancer, including lung cancer and breast cancer.From a philosophical perspective, the study of cell signaling pathways raises questions about the nature of life and the role of genetics in shaping our development. The fact that cells can communicate with each other and coordinate their activities to form complex organisms is a testament to the incredible complexity and beauty of life. Furthermore, the fact that our development is controlled by our genes raises questions about the extent to which we are predetermined by our genetic makeup. While our genes certainly play a significant role in shaping our development, it is also clear that environmental factors can have a profound impact on our development as well.From a social perspective, the study of cell signaling pathways has important implications for issues such as genetic engineering and stem cell research. The ability to manipulate cell signaling pathways raises the possibility of creating organisms with specific traits or curing diseases by replacing damaged or diseased cells with healthy ones. However, these technologies also raise ethical questions about the extent to which we should manipulate the genetic makeup of living organisms. Furthermore, the fact that these technologies are often expensive and only available to those with access to healthcare raises questions about social justice and the distribution of resources.From a personal perspective, the study of cell signaling pathways highlights the incredible complexity and interconnectedness of the human body. It is humbling to consider the vast number of signaling pathways that are involved in our development and the intricate ways in which they interact with each other. Furthermore, the fact that abnormalities in these pathways can lead to serious health problems underscores the importance of taking care of our bodies and seeking medical attention when necessary.In conclusion, cell signaling pathways are essential for the development of organisms and have important implications for medicine, philosophy, society, and personal health. By studying these pathways, we can gain a deeper understanding of the nature of life and the role of genetics in shaping our development. Furthermore, we can develop new treatments for diseases and explore the ethical implications of genetic engineering and stem cell research. Ultimately, the study of cell signaling pathways reminds us of the incredible complexity and interconnectedness of the human body and the importance of taking care of ourselves and each other.。
解散射问题的一种各向异性优化PML方法

解散射问题的一种各向异性优化PML方法
杨孝英
【期刊名称】《吉林大学学报(理学版)》
【年(卷),期】2018(056)005
【摘要】给出解时谐散射问题的一种带小参数的各向异性优化完美匹配层(P M L)方法.利用最短距离的思想,在矩形区域外定义一个连续的向量场,并沿该向量场方向进行复坐标拉伸变换.通过在吸收函数中引入一个小参数ε0,使散射问题优化PM L 方法的计算不再依赖PM L层的厚度.结果表明,只要参数ε0充分小,各向异性的优化PM L解指数就收敛于原散射问题的解.数值实验结果验证了该方法的有效性.【总页数】6页(P1073-1078)
【作者】杨孝英
【作者单位】长春工业大学基础科学学院,长春130012
【正文语种】中文
【中图分类】O241.3
【相关文献】
1.解声波散射问题的一种优化PML方法 [J], 杨孝英;王旖旎
2.计算开洞穴电磁散射问题的一种优化PML方法 [J], 杨孝英;马富明;杜新伟
3.多个散射体散射问题的一种优化PML方法 [J], 杜新伟
4.时谐散射问题的一种各向异性的优化PML方法 [J], 杨孝英
5.洞穴散射问题的一种优化PML方法 [J], 杜新伟;杨孝英
因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Demonstration of fuel cell city busin BeijingFuyuan YangAssociate Professor Tsinghua UniversityState Key Lab of Automotive Safety & Energy31 Aug, 2010, ArgonneFCED_YFY_04_2010US-China Electric Vehicle & Battery TechnologyWorkshopBrief introduction of myself☐Projects in recent 5 years–Plan 863: FCE performance evaluation technology (’07) –Plan 863: the durability control of the FC city BUS (‘07)–Plan 863: R&D of a specific engine control system fordiesel-electric hybrid bus (‘08) –Plan 973: HCCI enginecontrol duration the transient process(‘02-’11)–Plan 863: APU design for a HCNG-electric hybrid bus(06)☐Major research area–Engine Control & system Optimization☐Objectives–Phase 1(’90~’98):LPG/CNG –Phase 2(’99~’04):Diesel engine–Phase 3(04~):FC/Hybrid managementOutline⏹ Background⏹ Key Technologies ⏹ Demonstration⏹ Summaryresource :「Forecasts of Oil and Gas Supply to 2050」 Jean Laherrere, Peterotech 2003 (New Delhi)BackgroundPost oil age is coming……China: a fast growing market of vehicleVehicle Production & its Growth in ChinaP r o d u c t i o n (10k )燃料效率 CO 2排放 CNGDIHybridPure electric Fuel cell vehicleHybrid HomogeneousDICurrentGasoline+ATL o w C O 2 e m i s s i o nLow fuel consumptionNew automobile powertrain technology is the only way to cope withenergy crisis and environmental pollution.Electronic controlled engine Global automobile industrial revolutionR&D of fuel cell city bus technology in Tsinghua 1999 2001 2002 2003 2004 2005 2007 2009 2010R&D processFC:5kWFC:15kWFC:50kWFC:60kWFC:100kWFC:130kWFC:100kWFC:100kWFC:50kWDemonstrated in OlympicGames and Beijing Bus routesShanghaiExpoExport toSingaporeOutline⏹ Background⏹ Key Technologies ⏹ Demonstration⏹ Summary1 Fuel cell Hybrid System ControlSoft-Run ConceptO p e r a t i n g C o n d i t i o n a n a l y s i s To get typicaloperating para :–Load varying speed–Loading numbers–Percent of idle time–Number of startTo get the drivingrequirement para :–Traction Power –FCE power distribution –Continuous Speedup time & power –Dischrg & chrg of Batt. –SOC –……1.1 Soft-Run control of fuel cell systemsoft-run:Higher efficiency;Longer workinglife time1.2 Battery management system1.3 Serial braking energy recycling systemThe entire fuel consumption can beLowered by 15% with serial brakingrecycling system in “China typicalcity bus cycle”.EnergymanagementCommu-nicationElectricControllerSensorCommand inputFuel cell DC/DCBMSElectric motor AMT ABSPowertrainControllerCommunication protocolFuel cellcontrollerDC/DCcontrollerMotorcontrollerAMTcontrollerBatteryABSPower flow Signal flow2 Vehicle control systemElectricCrash-hydrogen-electric coupled safety technology3 Safety technology0ms 40ms 160ms 360ms 760ms1.36s 3.16s 5.76s 15.76s 91.76sBullet0.0s 2.0s 3.0s 4.0s 5.0s10.0s 15.0s 30.0s 45.0s3 Tests and experimentsGunshot and firing testing of a 35MPa hydrogen tank High pressure hydrogen storagesystem crash testingOutline⏹ Background⏹ Key Technologies ⏹ Demonstration⏹ SummaryHydrogen production and refueling station⏹Supply 20 MPa and 35 MParefueling service since 2005⏹Large scale application ofhydrogen as vehicle fuel, morethan 23 tons hydrogen refueledto fuel cell cars and buses up tothe end of 2008⏹Hydrogen fuel price has beenreduced to 4RMB per cubic meter,thus total fuel cost of FC bus per100km is almost equal to that ofdiesel bus.The first comprehensivehydrogen production andrefueling station in ChinaPictures in Olympic Games, Beijing, 2008Aug. 17 Woman Marathon RaceAug. 24 Man Marathon RaceClimate adaptabilitySpring Summer AutumnWinter Highest Air temp. : 40℃ Lowest Air temp. : -10℃ Highest Humidity :>95% Lowest humidity : <10%Mileages month by monthInternational honor2004, the Hydrogen & Fuel CellLetter :“We’ll buy fuel cell busesfrom them 10 years from now.”◆2006, the first IPHE Technical Achievement Award◆2010, IPHE Technical Achievement Award, Hydrogen Fuel Cell City Bus Commercial-Demonstration for 2008 Beijing Olympic/Paralympics GamesOutline⏹ Background⏹ Key Technologies ⏹ demonstration⏹ SummarySummary“Fuel Cell + Battery” hybrid is a good choice for fuel cell bus in the near futureAt present, Fuel cell system is still a “noble” system, it need more cares and much money, so there is still a lot of works to do for the world, especially on its environment adaptability.Thank you!。
