Block 1 IHE Hazard Identification and Risk Estimation 9-18-2009
办公室隐患排查治理台账

办公室隐患排查治理台账英文回答:Office Hazard Identification and Control Register.Purpose:To identify and control potential hazards in the office environment to ensure the health and safety of employees and visitors.Procedure:1. Hazard Identification: Conduct regular inspections of the office to identify potential hazards, such as:Electrical hazards (e.g., exposed wires, overloaded outlets)。
Physical hazards (e.g., slippery floors, sharpobjects)。
Chemical hazards (e.g., cleaning products, solvents)。
Biological hazards (e.g., mold, bacteria)。
2. Hazard Assessment: Evaluate the identified hazards to determine their severity and likelihood of occurrence.3. Hazard Control: Implement appropriate control measures to eliminate or mitigate the identified hazards, such as:Engineering controls (e.g., installing safety guards, improving ventilation)。
Hazard Identification

PPT-072-01
6
Hazard Identification
• HAZID process must be ongoing to ensure existing hazards are known, and • New hazards recognized before they are introduced: - Prior to modification of facility - Prior to change in workforce - Before and during abnormal operations, troubleshooting - Facility early warning signals - Employee feedback - After an incident
PPT-072-01 15
Safety Inspection Checklist
PPTPPT-072-01
17 16
Brainstorming
Advantages • Useful starting point for many HAZID techniques to focus a group’s ideas, especially at the concept phase • Facilitates active participation and input • Allows employees experience to surface • Enables “thinking outside the box” • Very useful at early stages hazard identification Disadvantages • Less rigorous and systematic than other techniques • High risk of missing hazards unless combined with other tools • Relies on experience and competency of facilitator
基于区块链的HACCP质量溯源模型与系统实现

了 HACCP结合区块链溯源的大体框架。将溯源
统建立在
以太
联盟 三台
Hyper—dges Fabric上,利用无线射频识别技术和智
能合 现
入,可 质量保障,在 程度
上实现了
[29*%但结合HACCP质量体系和区
源、
能合约来保障产品质量的研究却
鲜见报道%
本文以生食牡蛎为例,根据生食牡蛎加工
HACCP质量体系[30 *,
Abstract: To improva tho credibility and e—iciency of quality -yca/btty based on blockchain, soma studios wen npo/edand explainedtho ovarall designs of combining hazard analysis and critical control points ( HACCP) and blockchain tochnoCgy. Howovas, then is still a lack of operational tochnical solutions in documented /search on tho combined use of -a too. To mako up for -a abova shoXayo, a quality -aco/biOty model based on HACCP was constructed by using blockchain Wchno—gy. This critical control points ( CCP) in tho HACCP spoc—icatOn was extracted for mw oystos processing as tho monitoOing data points o ethe t aceabi eity system , obtaining the to-be-OecoOded datastuctuOethatcan tuey eeeeectthe peoduct quaeity. A higheyscaeabeestandaed smaetconteactwasdesigned based on thisdata structu/. Smart con—acts for each key con—ol pointwen then de/vad. What this achiovad was tho onchain data monito eing a-we ea-automatic data quaeity .udgment o ethe who ee eaw oy-teepeoce-ing peoce-.Itaeodicu -ed how a high ey eobu-t dep eoyment mode ewa-imp eemented with theeeeeeencetoa peemi-ioned chain peateoem Hype eeedge eFab eic. The who ee p eoce -o edata on-chain wa-expeained eeom a theoretical pexpoc/va. Lastly, -a -ace/biOty system p/totypo of -a model was implemented. Function and peeeoemancete-ton the imp eemented -y-tem we ee conducted. In eunction te-t , the-y-tem ensueed the t eaceabi eity and c eedibi eity o ethe qua eity t eaceabi eity data by the eeading o ep eoduct histo ey /cords. Tho automatic judgment and re/l-Cma con—ol of p/duct quality wem achiovad at -a sama /mo.
危险源识别及风险评价控制程序(中英文)

危险源识别及风险评价控制程序Hazard source identification and risk evaluation control procedure(ISO45001:2017)1.目的Purpose:对本公司能够控制和可望施加影响的危险因素进行辨识和评价,并从中评价出重要危害因素,为建立职业健康安全目标指标﹐实施运行控制和改善安全卫生行为提供依据。
To identify and evaluate the controllable and excepted and influenced hazard aspects, identify the important hazard aspect, provide the reference for the establishment of the occupational health and safety objectives and the implementation of the operational control and the improvement of safety and health behavior.2.适用范围Application scope:本程序适用于公司产品、活动及服务等危害辨识和危险评价。
It is applicable to the hazard identification and evaluation for the company’s products, activities, services and etc..3.定义Definition:无N/A4.职责Responsibility:4.1 各部门:负责辨识和评价本部门的危害因素。
4.1 Each Department: To identify and evaluate the hazard aspect within the department.4.2 人力资源部:负责辨识和评价相关方的危害因素及危险因素的汇总﹑审核﹐组织评价和确定重要危险因素﹑各部门协助。
危险源识别及风险评价控制程序(中英文)

危险源识别及风险评价控制程序Hazard source identification and risk evaluation control procedure(ISO45001:2017)1.目的Purpose:对本公司能够控制和可望施加影响的危险因素进行辨识和评价,并从中评价出重要危害因素,为建立职业健康安全目标指标﹐实施运行控制和改善安全卫生行为提供依据。
To identify and evaluate the controllable and excepted and influenced hazard aspects, identify the important hazard aspect, provide the reference for the establishment of the occupational health and safety objectives and the implementation of the operational control and the improvement of safety and health behavior.2.适用范围Application scope:本程序适用于公司产品、活动及服务等危害辨识和危险评价。
It is applicable to the hazard identification and evaluation for the company’s products, activities, services and etc..3.定义Definition:无N/A4.职责Responsibility:4.1 各部门:负责辨识和评价本部门的危害因素。
4.1 Each Department: To identify and evaluate the hazard aspect within the department.4.2 人力资源部:负责辨识和评价相关方的危害因素及危险因素的汇总﹑审核﹐组织评价和确定重要危险因素﹑各部门协助。
02_Hazard Identification

Page:1 of 12开工前工作计划/风险分析目的本规程的目的是为鉴定危险提供指导方针,并规划出能够使工作安全执行的安全工作规程。
范围本规程简述了鉴定和分析工程现场危险、评估其风险以及将其风险减少或消除到一个可行程度的步骤。
应用本规程适用于项目组的工程和承包人员工所在的所有工作场所。
定义危险危险是有可能造成伤害/疾病或损坏的所有情况。
风险暴露于任何危险中时引起伤害的可能性评估。
工作风险分析 (JRA)有助于确认在执行特殊工作或任务期间可能出现的特殊危险。
工作任务分析 (JTA)一种由个人或工队在着手一项特殊作业前所完成的一种非正式的JRA。
安全任务委派 (STA)一个与每位员工确认和交流执行任务步骤、与任务有关的危险和风险以及用于安全完成任务的安全工作方法的过程。
Page:2 of 12开工前工作计划/风险分析目的 (1)范围 (1)应用 (1)定义 (1)1.0概要 (3)2.0危险鉴定 (3)3.0风险评估、风险管理和危险管理 (3)3.1工程方法 (3)3.2工程危险研究过程 (3)3.3风险管理和评估 (5)3.4规划解决方案/控制 (5)4.0控制危险的系统方法 (6)4.1危险鉴定方法 (6)4.2评估 (7)4.3控制 (7)4.4评估 (7)4.5监控 (7)5.0检查 (8)6.0工作风险分析 (8)6.1JRA小组 (8)6.2要分析的工作选择 (8)A.潜在的危险 (9)B.失败后果 (9)C.事故历史纪录 (9)D.带电机械/设备 (9)6.3限制范围 (9)6.4完成JRA的基本过程 (9)7.0工作任务分析 (10)8.0安全任务委派 (11)8.1责任 (11)8.2规程 (11)8.3后-STA (11)9.0参考 (12)10.0附件 (12)Page:3 of 12开工前工作计划/风险分析1.0概要所有的工程都要实施和记录危险鉴定。
所有的工程都要执行危险管理规程。
hazard classification and identification report

A hazard classification and identification report is a document that identifies potential hazards in a workplace or environment and classifies them based on their severity and likelihood of occurrence. The purpose of this report is to help organizations identify and manage risks associated with their operations, products, or services.The report typically includes the following sections:1. Introduction: This section provides an overview of the report's purpose, scope, and methodology.2. Hazard identification: This section lists all potential hazards identified during the risk assessment process. Each hazard is described in detail, including its causes, effects, and potential consequences.3. Hazard classification: This section categorizes each hazard based on its severity and likelihood of occurrence. The most severe and likely hazards are classified as high-priority risks that require immediate attention.4. Risk assessment: This section evaluates the overall risk level associated with each hazard. It considers factors such as the frequency and severity of incidents, the number of people exposed to the hazard, and the effectiveness of existing controls.5. Control measures: This section recommends specific actions to be taken to mitigate or eliminate each hazard. These may include changes to work processes, the use of personal protective equipment, or the implementation of engineering controls.6. Conclusion: This section summarizes the key findings of the report and provides recommendations for further action.。
ICH术语表

ICH领域专业术语表(质量、安全性)序号英文中文1"relevant" viruses and "model" viruses“相关”病毒和“模型”病毒225-fold AUC radio25倍的AUC比值3 a single 2 generation study单项包括两代(生殖毒性)的研究4abbreviated or abridged application简略申请5abnormal karyology异常核形6abortions流产7absorbed moisture吸附水8absorption吸收9acceptable daily intake可接受的日摄入量10acceptable test加速试验11acceptance criteia认可标准12accuracy准确性13accuracy准确度14acelerated/stress stability studies加速/强力破坏稳定性研究15acentric fragment无着丝点片段16acetylation 乙酰化作用17achiral assay非手性测定18achlorhydric eldderly老年性胃酸缺乏症19acridine orange吖啶橙20action limits内控限值21active components/compound/moiety活性成分22active ingredient活性组分23active metabolite活性代谢产物24adaption to specific culture conditions特定培养条件的适应25additional test 附加实验26additions添加剂27adduct加合物28adequate exposure充分暴露29adjuvant 佐剂30ADME吸收、分布、代谢、排泄31administration period给药期32adventitious agents外源性因子33adventitious contaminants外来污染物34adventitious viral or mycoplasma contamination外源性病毒或支原体污染35adventitious viruses外源病毒36advers effect不良反应37adverse reaction不良反应38aerobic microorganisms需氧微生物39affinity亲和力40affinity chromatography亲和层析41affinity column亲和柱42against humanised proteins serum antibodies抗人源蛋白血清抗体 43agar and broth琼脂和肉汤44aggregates 聚合体45aggregation聚集46aginal smear阴道涂片 47air ighting reflex空中翻正反射48alkylating electrophilic center烷化亲电子中心49allele基因突变产生的遗传因子50allergenic/allergic extracts过敏原抽提物51allergic reactions过敏性反应(变应性反应)52altenative validated test有效替代试验53altered conjugated forms改变的结合物形式54altered growth 生长改变55ambient condition自然条件56amino acid composition氨基酸组成57amino acid sequence氨基酸顺序58amino acids氨基酸59amino sugars氨基糖60amino-terminal amino acids氨基端氨基酸61ammonia production Rates产氨率62ammoniun sulphide staining of the uterus子宫硫化胺染色 63analogue类似物(同系物)64analogue series of substances同系物65analyte 被测物66analytical method 分析方法67analytical procedure分析方法68anaphase分裂后期69aneuploidy非整倍体70aneuploidy inducer非整倍体诱导剂71animal cell lines动物细胞系72animal tissues or organs动物组织或器官73antennary profile 触角形状74antibiotic resistance genes抗生素耐药基因75antibiotics抗生素76antibody抗体77antibody production tests抗体产生试验78antigenic specificity抗原特异性79antisera抗血清80apoptosis凋亡81applicant申报者82art and ethical standards技术和伦理标准83ascites腹水84assay含量测定85assay procedure定量方法86assessment of genotoxicity遗传毒性评价87attainment of full sexual function达到性成熟 88AUC曲线下面积89auditory startle relex惊愕反射(听觉惊跳反射)90autoimmune自身免疫91autoradiographic assessment放射自显影评价92autoradiography放射自显影93avian鸟类94avidity亲和性95background 背景96bacteria细菌97bacterial mutagenicity test细菌致变突试验98bacterial reverse mutation test细菌回复突变试验99bacterial strains菌株100bacterial test organisms微生物试验菌101base pairs碱基对102base set of strains基本菌株103base substitution碱基置换104batches批次105batch-to-batch逐批106between-assay variation试验间变异107binary fission双数分裂108binding assays结合试验109bioanalytical method生物学分析方法110bioavaiability生物利用度111bioburden生长量/生物负荷112biochemical methods生化方法113bioequivalency生物等效性114biohazard enformation生物有害信息115biological activity生物活性116biological products生物制品117biological relevance生物学意义118bioreactor生物反应器119biotechnological products生物技术产品120biotechnological/biological products生物技术/生物制品121biotechnology-derived pharmaceuticals生物技术药物122biphasic curve双相曲线123birth出生124blood plasma factors血浆因子125body burden机体负担126body fluids体液127bone marrow cell骨髓细胞128bouin's fixation包氏液固定129bovine牛130bovine spongiform encephalopathy(BSE)疯牛病131bracketing括号法132breakage of chromatid染色单体断裂133breakage of chromosome染色体断裂134breeding conditions饲养条件135bridging character桥梁作用136by-products副产物137C(time)一定剂量、某一时间的浓度138calibrate标化139canine犬140cap liner瓶帽内垫141capillary electrophoresis毛细管电泳142carbohydrate碳水化合物143carboxy-terminal amino acids羧基端氨基酸144carcinogen致癌物质145carcinogenesis致癌性146carcinogenic hazard致癌性危害147carcinogenicity bioassay致癌性生物检测148carcinogenicity potential of chemical化合物的潜在致癌性149carcinoginicity(oncogenicity)致癌(致瘤)150cardiovascular心血管151carrier载体/担体152case-by-case个例153catalysts催化剂154cell bank 细胞库155cell bank system细胞库系统156cell banking procedures细胞建库过程157cell banking system细胞库系统158cell culture-derived impurities来源于细胞培养基的杂质159cell cultures 细胞培养物160cell cultures 细胞培养161cell expansion细胞扩增162cell fusion细胞融合163cell line细胞系164cell lines 细胞系165cell membrane lipid细胞膜脂质层166cell metabolites细胞代谢物167cell pooling细胞混合168cell proliferation细胞增植169cell replication system细胞复制系统170cell substrate-derived impurities 来源于细胞基质的杂质171cell substrates细胞基质172cell suspension细胞悬液173cell viability细胞活力174cell-derived biological products细胞来源的生物制品175cell-mediated immunity细胞介导的免疫176cellular blood components血细胞成分177cellular therapy细胞治疗178cemadsorbing viruses红细胞吸附病毒179central nervous systems中枢神经系统180cerbral spinal fluid脑脊液181characterization and testing of cell banks细胞库鉴定及检测182charcoal活性炭183charge电荷184chemical actionmertric system化学光化线强度系统185chemical nature化学性质186chemical reactivity 化学反应性187chemical syntheses化学合成188chemically inert化学惰性189chewable tablets咀嚼片190childbeering potential生育可能性191chinese hamster V79 cell中国仓鼠V79细胞192chiral impurities手性杂质193CHL cell中国仓鼠肺细胞194CHO cell中国仓鼠卵巢细胞195chromatide染色单体196chromatograms色谱图197chromatographic behavior色谱行为198chromatographic procedures色谱方法199chromatography columns色谱分离柱200chromosomal aberration染色体畸变201chromosomal damage染色体损伤202chromosomal integrity染色体完整性203chronic toxicity testing 慢性毒性试验204circular dichroism圆二色性205classfical biotransformation studies经典的生物转化试验206clastogen染色体断裂剂207clastogenic致染色体断裂的208clearance studies清除研究209cleavage of the balanopreputial gland 龟头包皮腺裂开210climatic zones气候带211clinical indication临床适应证212clinical research临床研究213clinical trial application 临床试验申请214clisure闭塞物215cloning 克隆216cloning efficiency克隆形成率217closure of hard palate硬腭闭合218C max峰浓度219coat growth毛发生长220code number编号221coding sequence编码序列222coefficient of variance变异系数223collaborative studies协作实验研究224colony isolation菌落分离225colony sizing集落大小226colony-stimulating factors集落刺激因子227combination product复方制剂228comparative trial对比试验229complement binding补体结合230completely novel compound全新化合物231components成分232compound bearing stuctural alerts结构可疑化合物233concentration threshold阈浓度234conception受孕235concomitant toxicokinetics相伴毒代动力学236confidence interval置信区间237confidence limits可信限238confirmatory studies确认研究239conformance to specifcations符合规范240conformation构型241conjugated product连接产物242conjugation连接243consistency一致性244container容器245container/closure容器/闭塞物246container/closure integrity testing 容器/密封完整性试验247contaminants污染物248contaminated cell substrate污染的细胞基质249content uniformity含量均匀度250continuous treatment 连续接触251control methodology控制方法学252controlled released product控释制剂253conventional live virus vaccines传统的活病毒疫苗254conventional vaccines传统疫苗255cool white fluorescent冷白荧光灯256corpora lutea黄体257corpora lutea count黄体数258correction factor校正因子259correlation coefficient相关系数260covalent or noncovalent共价或非共价261creams霜剂262cross-contamination交叉污染263cross-linking agent交联剂264cross-reactivity交叉反应265cryopreservation冷冻保存266cryoprotectants防冻剂267crystals晶体268culture components 培养基成分269culture condiction培养条件270culture confluency培养克隆率271culture confluenty培养融合272culture media/medium培养基273culture medium培养基274cyanogen bromide溴化氰275cytogenetic细胞遗传学的276cytogenetic change细胞遗传学改变277cytogenetic evaluation细胞遗传学评价278cytokines细胞因子279cytopathic细胞病的280cytoplasmic A-and R-type particles细胞浆a型和r型颗粒281cytotoxicity细胞毒282dark control暗度控制283dead offspring at birth 出生时死亡的子代284deamidation去氨基285deaminated去酰胺化的286deamination脱氨基287decision flow chart/tree判断图288definable and measurable biological activity明确和可测定的生物学活性289degradant降解产物290degradation降解291degradation pathway降解途径292degradation product降解产物293degradation profile降解概况294degree of aggregation 凝集度295degree of scatter离散程度296delay of parturition分娩延迟297delayed-release延迟释放298deleterious有害的299deletion缺失300delivery systems给药体系301derivatives衍生物302description 性状303descriptive statistics描述性统计304detection limit检测限度305detection of bacterial mutagen细菌诱变剂检测306detection of clastogen染色体断裂剂检测307determination of metabolites测定代谢产物308development of the offspring 子代发育309developmental toxicity发育毒性310dilivery systems释放系统311dilution ratio释放倍数312dimers二聚体313diminution of the background lawn背景减少314diode array二极管阵列315diploid cells二倍体细胞316direct genetic damage 直接遗传损伤317dissociation解离318dissolution testing溶出试验319dissolution time溶出时间320distribution分布321DNA adduct DNA加合物322DNA damage DNA损伤323DNA repair DNA修复324DNA strand breaks DNA链断裂325dosage form剂型326dose dependence剂量依赖关系327dose escalation剂量递增328dose level剂量水平329dose -liming toxicity剂量限制性毒性330dose-ranging studies剂量范围研究331dose-related剂量相关 332dose-relatived cytotoxicity剂量相关性细胞毒性333dose-relatived genotoxic activity剂量相关性遗传毒性334dose-relatived mutagenicity剂量相关性诱变性335dose-response curve剂量-反应曲线336dosing route给药途径337downstream purification下游纯化338drug product制剂339drug product components制剂组方340drug substances原料药341duration周期342duration of pregnancy妊娠周期343eaning断奶344earlier physical malformation早期身体畸形345early embryonic development早期胚胎发育346early embryonic development to implantation着床早期的胚胎发育347ectromelia virus脱脚病病毒348elastomeric closures橡皮塞349electro ejaculation电射精350electron microscopy(EM)电镜351electrophoresis电泳352electrophoretic pattern电泳图谱353elimination消除354elution profile洗脱方案355embryofetal deaths胚胎和胎仔死亡356embryo-fetal development 胚胎-胎仔发育357embryo-fetal toxicity胚胎-胎仔毒性358embryonated eggs鸡胚359embryonic death胚胎死亡360embryonic development胚胎发育361embryonic period胚胎期362embryos胚胎 363embryotoxicity胚胎毒性364enantiomer对映体365enantiomer对映异构体366enantiomeric镜像异构体367enantioselective对映体选择性368encephalomyocarditis virus(EMC)脑心肌炎病毒369end of pregnancy怀孕终止370endocytic 内吞噬(胞饮)371endocytic activity内吞噬活性372endogenous agents内源性因子373endogenous components内源性物质374endogenous gene内源性基因375endogenous proteins内源性蛋白376endogenous retrovirus内源性逆转录病毒377endonuclease核酸内切酶378endonuclease release form lysosomes溶酶体释放核酸内切酶379endotoxins内毒素380end-point终点381end-product sterility test-ing最终产品的无菌试验382enhancers增强子383enveloped RNA viruses包膜RNA病毒384environmental factors环境因素385enzymatic reaction rates酶反应速率386enzyme酶387epididymal sperm maturation附睾精子成熟性388epitope表位389epitope抗原决定部位390Epstein-Barr virus (EBV)EB病毒391equine马392error prone repair易错性修复393erythropoietins促红细胞生成素394escalation递增395escherichia coli starn大肠杆菌菌株396esscherichia coli 大肠杆菌397ethnic origin种族起源398eukaryotic cell真核细胞399evaluation of test result试验结果评价400ex vivo体外401exaggerated pharmacological response超常增强的药理作用402excipient赋形剂403excipient specifications赋形剂规范404excretion排泄(消除)405expiration date/dating失效日期406exposure assessment 接触剂量评价407exposure level暴露程度408exposure period光照时间409exposure period接触期410expression constract表达构建体411expression system表达系统412expression vector表达载体413extended-release延时释放414extent of the virus test病毒测试的程度415external metabolising system体外代谢系统416extinction coefficient消光系数417extrachromosomal染色体外418extraneous contaminants外源性污染物419extrapolation 外推法420F1-animals子一代动物421false negative result假阴性结果422false positive result假阳性结果423fecundity多产424feed-back反馈425fermentation发酵426fermentation products发酵产品427fertilisation受精428fertility生育力429fertility studies生育力研究430fetal abnormalities胎仔异常431fetal and neonatal parameters胎仔和仔鼠的生长发育参数432fetal development and growth胎仔发育和生长433fetal period 胎仔期434fetotoxicity胎仔毒性435fill volume装量436filter aids 过滤介质437final manufacturing最终生产438finished product成品439first pass testing 一期试验440flanking region侧翼区441fluorescence in situ hybridisation (FISH)原位荧光分子杂文442foetuses胎仔443forced degradation testing强制降解试验444foreign matter异质性物质445formal labeling正式标签446formal stability studies正式的稳定性研究447formulation 处方/配方448formulation 制剂449fragmentation片段化450frameshift mutation移码突变451frameshift point mutation移码点突变452free-standing独立453freeze-dried product冻干产品454fresh dissection technique新鲜切片技术455friability脆碎度456functional deficits功能试验457functional test功能性指标458funetional indices融合蛋白459fungi真菌460fusion partners融合伴侣461fusion protein融合蛋白462fusion proteins配子463gametes动物性别464gel filtration 凝胶过滤465gender of animals性别专一性药物466gender-specific drug基因剔除467gene amplification基因扩增468gene knockout基因治疗469gene mutation基因突变470gene therapy基因疗法471generation of the cell substrate细胞基质的产生472genetic遗传473genetic change 遗传学改变474genetic damage遗传学损伤475genetic endpoint遗传终点476genetic manipulation基因操作477genetic toxicity遗传毒性478genomic dinucleotide repeats基因组双核苷酸重复数479genomic DNA基因组DNA480genomic polymorphism pattern基因组形态类型481genotoxic activity遗传毒性作用482genotoxic carcinogen遗传毒性致癌剂483genotoxic effect 遗传毒性效应484genotoxic hazard遗传毒性危害485genotoxic potential潜在遗传毒性486genotoxic rodent carcinogen啮齿类动物遗传毒性致癌剂487genotoxicity 遗传毒性488genotoxicity evaluation遗传毒性评价489genotoxicity test遗传毒性试验490genotoxicity test battery遗传毒性试验组合491genotypic 基因型492germ cell mutagen生殖细胞诱变剂493germ line mutation生殖系统突变494GLP临床前研究质量管理规范495glucose consumption rates耗糖率496glycoforms糖化形式497glycosylation糖基化498goegrapgical origin 地理起源499gross chromosomal damage 染色体大损伤500gross evaluation of placenta 胎盘的大体评价501growth factors生长因子502growth hormones 生长激素503guanidine胍504haematoxylin staining苏木素染色505half-life半衰期506hamster antibody production(HAP) test仓鼠抗体产生实验507Hantaan virus汉坦病毒508hardness硬度509heavy metals重金属510hematopoietic cells造血细胞511heparins肝素512heptachlor七氯化合物513herbal products草药514heritable遗传515heritable defect遗传缺陷516heritable disease遗传性疾病517heritable effect 遗传效应518herpes virus 疱疹病毒519heterogeneities异质性520heterohybrid cell lines异种杂交细胞系521high concentration高浓度522high-resolution chromatography高分辨色谱523histologic appearance of reproductive organ生殖器官的组织学表现524histopathological chang组织病理学改变525homogeneity均一性526homologous proteins同系蛋白527homologous series同系528host cell 宿主细胞529host cell banks宿主细胞库530host cell DNA宿主细胞DNA531host cell proteins宿主细胞蛋白质532hot-stage microscopy热价显微镜533human carcinogen人类致癌剂534human cell lines人细胞系535human diploid fibroblasts人二倍体成纤维细胞536human lymphoblastoid TK6 cell 人成淋巴TK6细胞537human mutagen人类致突变剂538human polio virus人脊髓灰质炎病毒539human subjects人体540human tropism人向性541humidity湿度542humidity-protecting containers防湿容器543humoral immunity 体液免疫544hybridization techniques杂交技术545hybridoma cell杂交瘤细胞546hybridomas杂交瘤547hydrolysates水解物548hydrolytic enzymes水解酶549hydrophobicity疏水性550hygroscopic吸湿性551identification/identity鉴别552immature erythrocyte未成熟红细胞553immediate and latent effect速发和迟发效应554immediate container/closure直接接触的容器/密闭物555immediate pack内包装556immediate release立即释放557immortalization激活558immune spleen cells免疫脾细胞559immunoassay免疫检测560immunochemical methods免疫化学方法561immunochemical properties免疫化学性质562immunoelectrophoresis免疫电泳563immunogenicity免疫原性564immunological interations免疫相互作用565immunopathological effects免疫病理反应566immunoreactivity免疫反应性567immunotoxicity免疫毒性568implantation着床569implantation sites着床部位570impurity profile杂质概况571in vitro体外572in vitro and in vivo inoculation tests体内和体外接种试验573in vitro assay体外检测574in vitro cell age体外细胞传代期575in vitro lifespan体外生命周期576in vitro test体外试验577in vitro tests体外试验578in vitro/in vivo correlation体内体外相关性579in vivo体内580in vivo assays体内检测581in vivo test体内试验582inactivated vaccine 灭活疫苗583incidence of polyploid cell 多倍体细胞发生率584incisor eruption门齿萌出585independent test独立试验586indicator cell指示细胞587indicator organisms指示菌588individual fetal body weight单个胎仔体重589indoor indirect daylight室内间接日光590induced and spontaneous models of disease诱发或自发的疾病模型591inducer of micronuclei微核诱导剂592inducers 诱导剂593inedntification test鉴别试验594infectious agents感染性因子595influenza virus流感病毒596inhalation吸入597inhalation dosage forms 吸入剂型598inhibitor of DNA metabolism DNA代谢抑制剂599in-house内部的600in-house criterea内控标准601in-house primary reference material内部一级参比物质602in-house reference materials内部参比物质603in-house working reference material内部工作参比物质604initial filing原始文件605initial submission最初申报606initial text最初文本607inoculation接种608inorganic impurities无机杂质609inorganic mineral无机矿物质610inorganic salts无机盐611in-process acceptance criteia生产过程认可标准612in-process controls生产过程中控制613in-process testing生产过程中检测614insect昆虫615insulins胰岛素616intact animals完整动物(整体动物)617intake摄入618intended effect预期效果619intended storage period 预期的贮藏期620intentional degradation人为降解621interactions相互作用622interferon干扰素623interleukins白细胞介素624intermediate中间体625intermediate precision中间精密度626intermediates半成品627internal control内对照628international reference standards国际参比标准品629interphase muclei分裂间期细胞核630intra-and inter-individual个体与个体间631intra-assay precision间隙含量精密度632intracytoplasmic细胞浆内633introduction of virus病毒介入634inverted or horizontal position倒立或水平位置635ion-exchange离子交换636ionic content离子含量637isoelectric focusing/isoelectrofocusing等电聚焦638isoenzyme analysis同工酶分析639isoform pattern异构体类型640isolated organs离体器官641isomerized 异构化的642Jp/Ph.Eur./Usp.日本药局方/欧洲药典/美国药典643juvenile animal studies未成年动物研究644K virus K病毒645karyology胞核学646Kinetic profile动力学特点647Kinetics 动力学648laboratory scale实验室规模649lactate production rates乳糖产生速率650Lactating授乳、哺乳651lactic dehydrogenase virus (LDM)乳酸脱氢酶病毒652Large deletion event大缺失事件653Late embryo loss后期胚胎丢失654leachables沥出物655Level of safety安全水平656Libido性欲657Life threatering危及生命658ligand 配位体/配体659light光照660light resistant packaging避光包装661limit for in vitro cell age 细胞体外传代限度662limit of acceptance可接受的限度663limit of in vitro cell age 体外细胞代次664limit test限度试验665limulus amoebocyte lysate鲎试剂666linear relation ship 线性关系667linearity线性668Lipophilic compound亲脂性化合物669liquid nitrogen 液氮670liquid oral dosage forms 液体口服制剂671Litter size每窝胎仔数目672Live and dead conceptuese活胎和死胎673Live offspring at birth出生时存活的子代674live vaccine 活疫苗675living cells活细胞676Local toxicity局部毒性677Lockl tolerance studies 局部耐受性研究678Locu位点679logarithmic scale:对数级680long term test长期试验681Long-term carcinogenicity study长期致癌性试验682long-time and accelerated stability长期和加速稳定性试验683Loss of the tk gene tk 基因丢失684losses of activity活性丧失685lot release 批签发686low molecular weight subsances低分子量物质687lower-observed effect level (LOEL)能观察到反应的最低量688lymphocytic choriomeningitis virus (LCM)淋巴细胞性脉络丛脑膜炎病毒689lyophilised cakes冻干粉饼690lysate of cells 细胞溶解物691Major organ fomeation主要器官形成692Male fertility雄性生育力693Male fertility assessment雄性生育力评价694mammalian哺乳类695Mammalian cell mutation test哺乳动物细胞致突变试验696Mammalian cells哺乳动物细胞697Mammalian species哺乳类动物698manufacturing scale生产规模699marieting pack 上市包装700marker chromosome 标志染色体701marketing approval批准上市702Marketing approval上市许可703mass 重量704mass balance质量平衡705mass spectrometry质谱706master cell bank (MCB)主细胞库707Matemal animal亲代动物708material balance物质平衡709Mating behaviour交配行为710Mating period交配期711Mating ratio交配比例712Matrices基质713matrix基质、矩阵714matrix system矩阵化设计715matrixing每日最大剂量716maximum daily dose平均动力学温度717Maximum tolerated dese(MTD)最大耐受剂量718mean kinetic temperature后生动物细胞培养719Mechanism of genotoxicity遗传毒性机制720Mechanistic activation代谢活化721Mechanistic activation pathway代谢活化途径722Mechanistic activation system代谢活化系统723Mechanistic investigation机制研究724Metabolism代谢725Metabolites profile代谢物的概况726Metaphase中期727Metaphase analysis分裂中期相分析728Metaphase cell分裂中期细胞729metazoan cell culture微生物细胞培养730microbial cells微生物细胞731microbial contamunation 微生物污染732microbial expression system微生物表达系统733microbial limits微生物限度734microbial metabolites微生物代谢物735microbial proteases微生物蛋白酶736microbial vaccine antigens微生物疫苗抗原737microbiological testing 微生物学试验738Micronucleus微核739Micronucleus formation微核形成740Microtitre微滴定741Microtitre method微滴定法742Mimicking模拟743minimum exposure time最低作用时间744minimum of pilot plant试产规模745minute virus of mice小鼠小病毒746mirror image 镜像747mismached S-S linked错连的S-S键748Mitotic index有丝分裂指数749modified-/modifying release修饰释放750modifying factor修正因子751moisture level水分752molar absorptivity克分子吸收753Molecular characterisation分子特性754molecular characteristics分子特性755molecular confirmation分子构型756molecular entities/entity分子实体757molecular size分子大小758Molecular technique分子技术759Monitor监测760Monoclonal antibodies单克隆抗体761monoclonal antibody单克隆抗体762mork run空白对照试验763morphological analysis形态学分析764mouse antibody production (MAP) test小鼠抗体产生试验765mouse cytomegalovirus (MCMV)小鼠巨细胞病毒766mouse encephalomyelitis virus (GDVII)小鼠脑脊髓炎病毒767mouse hepatitis virus (MHV)小鼠肝炎病毒768Mouse lymphoma tk assay小鼠淋巴瘤tk检测769Mouse lymphoma L5178Y cell小鼠淋巴瘤L5178Y细胞770mouse rotavirus (EDIM)小鼠小轮状病毒771MuLV murine leukemia virus鼠白血病病毒772murine hybridoma cell lines鼠杂交瘤细胞系773Mutagen诱变原774Mutagen carcinogen诱变性致癌剂775Mutagen potential of chemical化合物的潜在致突变性776Mutant colony突变体集落777Mutation突变778Mutation induction in transgenes转基因诱导突变779mutations 突变780mycoplasma支原体781myeloma cell line骨髓瘤细胞系782Naked eye肉眼783national or international reference material国家或国际参比物质784national reference standards国家参比标准品785near ultraviolet lamp近紫外灯786Necropsy(macroscopic examination)解剖(大体检查)787Negative control阴性对照788Negative result阴性结果789Neonate adaptation to extrautenrine life新生仔宫外生活的适应性790neural sugars中性糖791new chemical entity新化学体792new dosage form新剂型793new drug products/produce新药制剂794new drug substance新原料药795new molecular entities新分子体796Newbom新生仔797Newcleated有核798no effect level不产生反应的量799Non rodent非啮齿类800Non-clinical非临床801noncovalent/convalent forces非共价/共价键802non-enveloped viruses非包膜病毒803Non-genotoxic carcinogen非遗传毒性致癌剂804Non-genotoxic mechanism非遗传毒性机制805Non-human primate非人灵长类806Non-linear非线性807non-mammalian animal cell lines非哺乳动物细胞系808non-recombinant cell-cul-ture expression systems非重组细胞培养表达系统809non-recombinant products/vaccines非重组制品/疫苗810non-specific model virus非特异模型病毒811Non-toxic compound无毒化合物812Non-toxic-effect dose level无毒性反应剂量水平813no-observed effect level不能观察到反应的量814N-terminal sequencing N端测序815nuclear magnetic resonance 核磁共振816Nucleated bone marrow cell有核骨髓细胞817nucleic acid核酸818Nucleoside analogue核苷酸同系物819nucleotide sequences 核苷酸序列820Number of live and dead implantation宫内活胎和死胎数821Numerical chromosmal aberration染色体数目畸变822Numerical chromosome changes染色体数目改变823Oestrous cycle动情周期824official procedure正式方法825ointments软膏826oligonucleotide低聚核苷酸827Oligonucleotide grugs寡核苷酸药物828oligosaccharide pattern寡糖类型829One,two,three generation studies一、二、三子代研究830opacity浊度831Organ development器官发育832organic impurities有机杂质833origins of replication复制起点834osmolality摩尔渗透压浓度835outdoor daylight室外日光836Ovulation rate排卵率837oxidation氧化838oxygen consumption rates耗氧量839package包装840Paraffine embedding石蜡包埋841parainfluenza virus副流感病毒842parallel control assays 平行对照分析843Parameter参数844Parent compound母体化合物845parent stability Guideline稳定性试验总指导原则846parental cell line母细胞系847Parenteral非肠道848parenterals非肠道制剂849particle size粒度850Particulate material颗粒物851particulate matter微粒852Parturition分娩延迟853parvoviruses细小病毒854passage history of the cell line细胞系的传代史855pathogenic agents致病因子856pathogenicity致病性857patterns of degradation降解方式858Pediatric populations小儿人群859peptide肽860peptide map 肽图861percent recovery回收率862periodic/skip testing定期检验/抽验863Peripheral blood erythrocyte外周血红细胞864permitted daily exposure允许的日接触量865Perpoductive competence生殖能力866phage typing噬菌体分型867pharcodynamic studies药效学研究868Pharmacodinetic药代动力学869Pharmacodynamic effects药效作用870Pharmacodynamics药效学(药效动力学)871pharmacopoeial药典872pharmacopoeial pharmacoppeial specifications药典规范873pharmacopoeial standards药典标准874phenotypic 表型875Phenylene diamine苯二胺876phosphorylation磷酸化作用877photostability testing光稳定性试验878Physical development身体发育879physicochemical changes理化改变880physicochemical methods物理化学方法881physico-chemical properties物理化学特性882Physiological stress生理应激883Pilot studies 前期研究884pilot-plant scale试生产规模/中试规模885Pinna unfolding耳廓张开886piston release force活塞释放力887piston travel force活塞移动力888pivotal stability studies关键的稳定性研究889plaque assays菌斑测定890plasmid质粒891Plasmid质粒892plasmid banks质粒库893plasminogen activators纤溶酶原激活素894Plasminogen activators纤维蛋白溶解酶原激活因子895Ploidy整倍体896pneumonia virus of mice小鼠肺炎病毒897Point mutation点突变898poisson distribution泊松分布899Polychromatic erythrocyte嗜多染红细胞900polyclonal antibody多克隆抗体901Polycyclic hydrocarbon多环芳烃902Polymer聚合物903polymerase chain reaction (PCR)聚合酶链式反应904polymorphic form多晶性型905polymorphs多晶型906polyoma virus多瘤病毒907polypeptides多肽908Polyploid cell多倍体细胞909Polyploidy多倍体910Polyploidy induction多倍体诱导911pooled havest集中回收912Poorly soluble compound难溶化合物913population doubling细胞数倍增/群体倍增914porcine猪915Positive control阳性对照916Positive result阳性结果917Post meiotic stages减数分裂后期918Post-approval批准后919Postcoital time frame交配后日期920Postimplantation deaths着床后死亡921Postnatal deaths出生后死亡922post-translational modifications批准后923post-translationally modified forms翻译后修饰924Postweaning development and growth断奶后发育和生长925potency效价926potent功效927Potential 潜在性928potential adverse consequences潜在的不良后果929potential excipients准赋形剂930Potential immunogenecity潜在免疫原性931potential impurity潜在杂质932potential new drug products准新药制剂933potential new drug substances准新药原料934Potentialtarget organs for toxicity潜在毒性靶器官935potentiometric titrimetry电位滴定936powders粉剂937power outages and human error断电和人为错误938preamble引言939Pre-and post-natal development study围产期的发育研究940Pre-and postweaning survival and growth断奶前后的存活和生长941pre-approval or pre-liscense stage批准前或发证前阶段942Precipitate沉淀物943precision精密度944preclinical and clinical studies临床前和临床研究945Preclinical safety evaluation临床前安全性评价946precursors前体947Predetermined criteria预定标准948Prediction of carcinogenicity致癌性预测949Pregnant怀孕950Pregnant and lactating animals怀孕与哺乳期动物951Preimplantation development着床前发育952Preimplantation stages of the embryo胚胎着床前期953preliminary assessment初步评估954preliminary cell bank初级细胞库955Preliminary studies预试验956Premating交配前957Premating treatment交配前给药958preparation制剂959Pre-screening预筛选960preservative防腐剂961Prevalence of abnormalities异常情况的普遍程度962Preweaning断奶前963Primary active entity主要活性实体964primary cells原代细胞965primary stability data主要稳定性数据966primary stability study/formal study/formal stability study主要稳定性研究/正式研究/正式稳定性研究967primary structure一级结构968primer引物969priming regimen接种方案970Priority selection优先选择971probability概率972process characterisation studies工艺鉴定研究973process controls工艺控制974process optimisation工艺优化975process parameters工艺参数976process validation工艺确证977process-related impurities工艺相关杂质978Pro-drug前体药物979product-related imputies产品相关杂质980progenitor祖细胞981prokaryotic cell原核细胞982Prolongation of parturition产程延长983promoters启动子984proposed commercial process模拟上市985protected samples避光样品。
巴斯夫验厂资料
101
building?是否明确指出在某一区域/建筑物必须穿戴哪些个人防护装
备?
Does everybody, including visitors and contractors, comply with the
108
regulations for wearing PPE without exception?所有人,包括访客
each shift (as legally required) and are refresher courses
120
conducted regularly?每班是否有足够数量的员工接受过急救培训(法
律要求),并定期进行进修课程?
3.2.4 Hygiene卫生
Yes No NA
Are the required hygiene measures complied with:是否遵守了规定
有危险物质都能根据法律规定得到充分识别(例如,管道/容器/罐的
GHS标签,具有名称和危险象形图)?
3.2.3 Protective measures保护措施
Yes No NA
Comment
Comment
Seite 6 von 20
Are safety showers and eye wash stations installed, where required
3.3 PPE
Yes No NA
Is the selection and use of PPE determined by HIRA (or other
regulatory requirement) and documented in the corresponding
procedures considering the concentration of hazardous
简述隐患排查流程及分级管理

简述隐患排查流程及分级管理英文回答:Hazard Identification and Risk Assessment Process.1. Hazard Identification: Identify potential hazards in the workplace through inspections, risk assessments, and employee involvement.2. Risk Assessment: Determine the likelihood and severity of each hazard to estimate the overall risk level.3. Risk Management: Implement control measures to mitigate risks to an acceptable level, considering elimination, substitution, engineering controls, administrative controls, and personal protective equipment.4. Monitoring and Review: Regularly monitor the effectiveness of control measures and make necessary adjustments based on changes in the workplace or newinformation.Hazard Classification and Prioritization.1. High-Risk Hazards: Hazards with the highest likelihood and severity, requiring immediate attention and robust control measures.2. Medium-Risk Hazards: Hazards with a moderate likelihood and severity, requiring prompt action and effective control measures.3. Low-Risk Hazards: Hazards with a lower likelihood and severity, requiring regular monitoring and occasional control measures.中文回答:隐患排查流程。
hazard control standard

hazard control standardA hazard control standard is a set of guidelines or requirements designed to minimize or eliminate the risks associated with a particular hazard. These standards are typically developed and enforced by government agencies, industry organizations, or other entities to ensure the safety of workers, the public, and the environment.Hazard control standards may cover a wide range of hazards, including chemical exposures, physical hazards, biological hazards, and ergonomic hazards. They may apply to various settings, such as industrial facilities, laboratories, healthcare settings, and construction sites.The main components of a hazard control standard typically include:1. Identification of hazards: The standard should clearly define the hazards that it is intended to address, including the potential risks and consequences associated with those hazards.2. Exposure limits: The standard may establish exposure limits or thresholds for the hazard, specifying the maximum acceptable levels of exposure to the hazard.3. Engineering controls: The standard may recommend or require the use of engineering controls, such as ventilation systems, containment devices, or guards, to prevent or minimize exposure to the hazard.4. Administrative controls: The standard may outline administrative controls, such as training programs, work practices, or procedural guidelines, to ensure safe handling and management of the hazard.5. Personal protective equipment (PPE): If engineering and administrative controls are not sufficient, the standard may specify the use of appropriate PPE to protect workers from exposure to the hazard.6. Monitoring and assessment: The standard may require regular monitoring and assessment of the hazard to ensure that control measures are effective and to identify any necessary modifications or improvements.7. Documentation and record-keeping: The standard may stipulate the keeping ofrecords and documentation related to hazard assessment, control measures, and employee training.Hazard control standards are important tools for promoting workplace safety and protecting the health of workers and the public. Compliance with these standards is typically required by law or regulation, and failure to comply may result in fines, penalties, or other legal consequences.。
进入密闭空间的正确流程

进入密闭空间的正确流程英文回答:Confined Space Entry Procedure.1. Hazard Identification and Assessment.Prior to entering a confined space, it is crucial to identify and assess potential hazards. Conduct a thorough visual inspection and atmospheric testing to determine the presence of hazardous gases, vapors, oxygen deficiency, flammable or explosive atmospheres, or other hazardous conditions.2. Planning and Preparation.Develop a detailed confined space entry plan that outlines specific roles, responsibilities, communication protocols, emergency procedures, and equipment requirements. Ensure adequate equipment, including personal protectiveequipment (PPE), monitoring devices, ventilation systems, lighting, and communication devices, is available and in good working order.3. Isolation and Ventilation.Isolate the confined space from potential energy sources (e.g., electrical, mechanical) by lockingout/tagging out equipment and securing pipelines. Establish adequate ventilation to ensure a breathable atmosphere and minimize the accumulation of hazardous substances.4. Entry Authorization.Obtain written authorization from a designated competent person responsible for authorizing entry into the confined space. The authorized entrant(s) must be trained and qualified for the specific task.5. Entry and Monitoring.Enter the confined space cautiously and follow theestablished safety protocols. Continuously monitor the atmosphere, respiratory protection, and overall health of the entrants. Maintain communication with the outside support team.6. Emergency Response and Rescue.Establish clear emergency response and rescue procedures, including designated rescue personnel, equipment, and communication channels. Ensure safe entry and exit points are available and unobstructed.7. Exit and Decontamination.Upon exiting the confined space, check for any signs of exposure to hazardous substances and decontaminate as necessary. Remove and dispose of PPE properly.中文回答:密闭空间进入程序。
隐患排查各模块介绍

隐患排查各模块介绍### English Answer:1. Hazard Identification.Hazard identification is the process of identifying and evaluating potential hazards that could cause harm to people, property, or the environment. This process involves identifying and assessing the risks associated with a particular operation, process, or activity. Hazard identification can be performed using a variety of methods, including:Job safety analysis (JSA): A JSA is a systematic review of a job or task to identify potential hazards and develop controls to mitigate those hazards.Hazard and operability study (HAZOP): A HAZOP is a structured brainstorming session that is used to identify and evaluate potential hazards in a process or system.What-if analysis: A what-if analysis is a brainstorming session that is used to identify potential hazards by asking "what if" questions about a particular operation or activity.2. Hazard Assessment.Hazard assessment is the process of evaluating the likelihood and severity of potential hazards. This process involves considering the following factors:The probability of the hazard occurring.The severity of the consequences of the hazard.The number of people who could be affected by the hazard.3. Hazard Control.Hazard control is the process of implementing measuresto mitigate or eliminate potential hazards. This process involves the following steps:Identifying the most appropriate hazard control measures.Implementing the hazard control measures.Evaluating the effectiveness of the hazard control measures.4. Hazard Monitoring.Hazard monitoring is the process of monitoring the effectiveness of hazard control measures and identifying any new hazards that may arise. This process involves the following steps:Regularly inspecting hazard control measures.Reviewing incident reports and other data.Conducting hazard audits.### 中文回答:1. 隐患排查。
危险评估hazard identification and risk assessment form
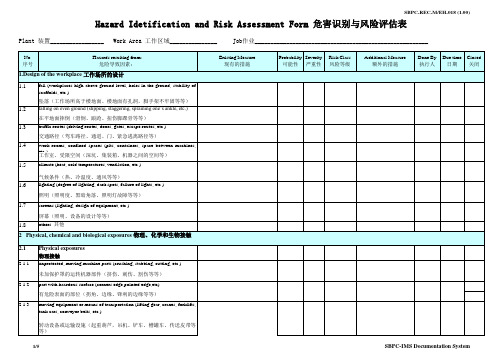
No. 3.1.1 序号
Work Area 工作区域_______________
Job作业_______________________________________________________
Existing Measure 现有的措施 Probability Severity 可能性 严重性 Risk Class 风险等级 Additional Measure 额外的措施 Done By 执行人 Due time 日期 Closed 关闭
hot or cold media and/or surfaces (hot liquids, vapors, gases, Dry Ice, etc.) 接触热或冷媒介和/或表面(热的液体、汽化物、气体、干冰等)
3.2.2
Noise 噪声
3.2.3
Ultrasound 超频率音响
3.2.4
vibrations of the body (pneumatic hammers, drills, etc.) 身体的振动(气锤、气钻等)
SBPC-REC-M/EH-018 (1.00)
Hazard Idetification and Risk Assessment Form 危害识别与风险评估表
Plant 装置_________________
No. 序号
Work Area 工作区域_______________
Job作业_______________________________________________________
Hazards resulting from: 危险导致因素: 由于气体、液体和固体(粉尘)引起的爆炸危险
journal of hazardous materials 的guide for authors

journal of hazardous materials 的guide for authorsThe Journal of Hazardous Materials (JHM) has specific guidelines for authors to follow when submitting their research papers for publication. These guidelines are designed to ensure that all manuscripts are submitted in a consistent format and meet the necessary criteria for publication. Here is an overview of the guide for authors for the JHM:1. Scope and focus: The JHM publishes articles that contribute to the understanding of hazard and risk assessment, management, and mitigation of hazardous materials. Authors should ensure that their research aligns with the scope and focus of the journal.2. Manuscript preparation: Authors should prepare their manuscripts according to the JHM's formatting guidelines. This includes using a clear and concise writing style, following a logical structure (including sections such as Introduction, Methods, Results, Discussion, and Conclusion), and ensuring correct grammar and punctuation.3. Title and abstract: The title of the manuscript should accurately reflect the content of the research. The abstract should provide a brief summary of the study's objectives, methods, results, and conclusions. The abstract should be informative and concise, typically limited to 250 words.4. Keywords: Authors should provide a list of keywords that accurately reflect the main topics and concepts covered in their research. These keywords help index and categorize the manuscript, making it easier for others to find.5. Results and discussion: Authors should present their findings clearly and concisely. The discussion should analyze and interpret the results, provide context, and explain the significance of the findings. Authors should avoid excessive repetition of information presented in tables or figures.6. References: Authors should provide a list of references used in their research. The JHM follows the Vancouver citation style, which requires numbered references in the order they appear in the manuscript. Citations within the text should be marked with superscript Arabic numerals.7. Supplementary information: Authors may provide supplementary information, such as additional data, figures, or tables, to support their research. This information should be included as separate files when submitting the manuscript.8. Ethical considerations: Authors are expected to adhere to ethical guidelines, including obtaining necessary permissions and approvals for human or animal studies, as well as disclosing any potential conflicts of interest.9. Submission process: Manuscripts should be submitted electronically through the JHM's online submission system. The system will guide authors through the submission process and allow them to upload their manuscript, figures, and supplementary information.It is important for authors to carefully review the full guide for authors on the JHM's official website to ensure they comply with all requirements and guidelines before submitting their research papers for publication.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Identifying Hazards
Matching Exercise
•Crushing •Drawing-In •Cutting •Shearing •Puncture •Impact •Entanglement
Identifying Hazards
Matching Exercise
Entanglement –
Risk-evaluation
NO
Machine is safe?
Yes
END + accepted risks described in OM
Identification of Mechanical Hazard
• Mechanical Hazard: A risk created by a moving part, or the potential movement or start-up of a part.
Electrical (motor) energy
Identifying Hazards
Matching Exercise
•Crushing •Drawing-In •Cutting •Shearing •Puncture •Impact •Entanglement
Identifying Hazards
Identifying Hazards
Matching Exercise
•Crushing •Drawing-In •Cutting •Shearing •Puncture •Impact •Entanglement
Identifying Hazards
Matching Exercise
Inertia Energy! A consideration for many machines
• Noise
Documentation of Mechanical Hazards
• Document hazards in RA. • Global RA drop-down menu shown below:
– Choose “mechanical” from hazard category menu – Chose from 7 mechanical hazards listed
PROBABILITY OF OCCURRENCE OF HARM
VERY LIKELY LIKELY
SEVERITY OF HARM
CATASTROPHIC High High SERIOUS High High MODERATE High Medium MINOR Medium Low
UNLIKELY
Identifying Mechanical Hazards
Answer: Gravity Energy
• Crushing hazard
Identifying Hazards
Types of Mechanical Hazards
Identifying Hazards
Types of Mechanical Hazards
Identifying Hazards
Matching Exercise
•Crushing •Drawing-In •Cutting •Shearing •Puncture •Impact •Entanglement
Identifying Hazards
Matching Exercise
Cutting or Shear (knife blades) – Comppressed Air energy
• Movement requires an energy source
Energy Sources
Electrical Motors
Identification of Mechanical Hazards
Examples of Moving Parts
Power transmission: Belts, pulleys … Point of operation: Turning rolls, conveyor belts
Risk-evaluation
NO
Machine is safe?
Yes
END + accepted rΒιβλιοθήκη sks described in OM
Risk Estimation
• Several methods of risk estimation exist
– most common: ANSI and ISO
Inertia Spring
Stopping time following stop command, unclutched hand wheel, flywheel …
Identifying Mechanical Hazards
Can you identify the Hazard and its Energy source?
Course Title: Mechanical Hazard Identification and Risk Estimation
Instructor:
Jim Pittman Corporate Isolation of Hazardous Energy competency owner
Objectives:
Risk Estimation
• Global RA process uses ANSI TR3 risk table. • Risk automatically populated when Severity and Probability is assigned.
Risk Table from ANSI
Compressors
Air or hydraulic cylinders and associated linkages/parts
Identification of Mechanical Hazards
Energy Source Examples of Moving Parts
Gravity Any elevated load. Doors that open vertically, palletizers, cams, vertically mounted air cylinders ..
Machine?
• Where lesson objectives fit into the RA
Machine limits/ characteristics Risk analysis Hazard identification
Risk Estimation Risk Assessment
Risk reduction
Where are we in the RA process?
Machine ? Machine limits/ characteristics Risk analysis Hazard identification
Risk Assessment Risk Estimation
Risk reduction
REMOTE
Medium
Low
Medium
Low
Low
Negligible
Negligible
Negligible
Risk Estimation (cell comment in RA form)
Risk Estimation
• Factors impacting Probability of Occurrence of Harm
– each moving part. – entire range or movement and adjacent objects
Identifying Hazards
Matching Exercise
Instructions: For each picture, identify • mechanical hazard, and • energy source
•Able to identify mechanical hazardous energy
•Able to estimate and evaluate risk level using global Risk Assessment tool.
Risk Assessment (RA) Process
Draw-in (due to rotation of rolls) – Electrical (motor) energy
Crushing (when rolls bump) - Compressed Air Energy
Identifying Hazards
Matching Exercise
•Crushing •Drawing-In •Cutting •Shearing •Puncture •Impact •Entanglement
• level of training/skill/experience, awareness of hazards, and potential for unintentional error. (including the risk of an untrained person/visitor entering the hazard). • Motivation to deviate from established safe practices or to defeat or circumvent guards or safety circuits.
Matching Exercise
Hazard: crushing due to • vertical movement during operation driven by cam rotation •Downward movement when machine stopped due to weight (gravity) of the apparatus causing the cam to shift.
