ImageJ对WesternBlot进行灰度分析
用imagej进行WB定量分析

ImagJ是一款简单的图像处理与分析软件,可以用来进行WB定量分析。
一、利用ImagJ对WB条带进行灰度分析1、File——》open 打开WB片子2、把图片转化成灰度图片image——》type——》8-bit3、消除背景影响process——》subtract background 选择50基本可以4、设置定量参数analyze——》set measurements,点击面积,平均密度和灰度值及Integrated Density 5、设置单位analyze——》set scale ,在“unit of length”的方框里输入“pixels”6、把图片转换成亮带,Edit——》invert7、选择FreehandSelection,尽量把条带圈起来,点击键盘m,出来IntDen灰度值当测定完所有条带,选结果中的“Edit ”的“SelectAll”,然后复制数据“IntDen”到Excel表即可进行分析8、复制数据IntDen进行分析二、利用ImagJ对WB条带进行密度分析1、File——》open 打开WB片子2、如条带不正,需修正image——》transform——》rotate调节angle值,直到条带水平为止3、选中矩形选项,圈中第一个条带,然后 analyze——》gels——》select firstlane(快捷键ctrl+1),然后移动第一个条带上的矩形到第二个条带上,analyze——》gels——》select secondlane(快捷键 ctrl+2),最后analyze——》gels——》plotlanes4、选中直线工具,将开口的波峰关闭5、选中魔棒工具,点击波峰可以显示波峰下面积,即条带的密度值6、以第一个数值为基数,其他数值与第一个数值的比值为相对密度The protocol of imageJ for quantitative analysis of Western blotting:- open an image- transfer the image to 8-bitsimage → type: 8 bits- Process → substract background 50- analyze → set measurements: pick area, mean gray value, inte grated density. - analyze → set scale, fill “unit of length” with “pixels”- switch the image to bright bandsedit → invert- freehand selection, choose the target area on the image . a band), type a “m” for measurement, then we get the result from a new window.- after measuring all of the bands, pick the “edit” of the result window. “select all”, copy the integrated density values to excel to analyze.The integrated density means that the area value multiplies the mean gray value. For Western blotting analysis the integrated density is very important, because the area and gray value both of them have meaning for Western blotting bands.。
ImagJWB定量分析

一、利用ImagJ对WB条带进行灰度分析1、File——》open 打开WB片子2、把图片转化成灰度图片image——》type——》8-bit3、消除背景影响process——》subtract background 选择50基本可以4、设置定量参数analyze——》set measurements,点击面积,平均密度和灰度值及Integrated Density5、设置单位analyze——》set scale ,在“unit of length”的方框里输入“pixels”6、把图片转换成亮带,Edit——》invert7、选择FreehandSelection,尽量把条带圈起来,点击键盘m(小写),出来IntDen灰度值8、复制数据IntDen进行分析二、利用ImagJ对WB条带进行密度分析1、File——》open 打开WB片子2、如条带不正,需修正image——》transform——》rotate调节angle值,直到条带水平为止3、选中矩形选项,圈中第一个条带,然后analyze——》gels——》select firstlane(快捷键ctrl+1),然后移动第一个条带上的矩形到第二个条带上,analyze——》gels——》select second lane(快捷键ctrl+2),最后analyze——》gels——》plotlanes4、选中直线工具,将开口的波峰关闭5、选中魔棒工具,点击波峰可以显示波峰下面积,即条带的密度值6、以第一个数值为基数,其他数值与第一个数值的比值为相对密度*/link?url=iFFzpXWQu3ULvbjcoe0ME-Oi1oVTR3ANKR6xYvQXfMPUjK3Re5lnNI2kRpSadvUdXUPUr9 zJvJau9y3P4ZyoMxZR8ghHwpvUJeqRj1vn5Ty*如果有数据产生,一般是批量多次western blot结果的一个统计数据一般采取方差分析或者t检验分析就可以了*纵坐标我看文献上是用的检测蛋白的灰度值比上内参的灰度值。
image j蛋白灰度分析步骤
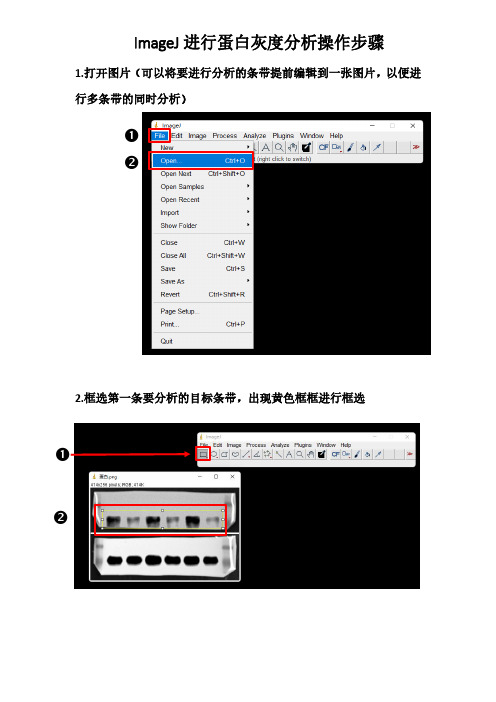
❷ ❷ 1.打开图片(可以将要进行分析的条带提前编辑到一张图片,以便进
行多条带的同时分析)
2.框选第一条要分析的目标条带,出现黄色框框进行框选
❶
❶
ImageJ 进行蛋白灰度分析操作步骤
3.将选出的条带设置为第一条要分析的条带
❶
❷
❸
4.框选下一条蛋白条带区域,操作位直接用鼠标左键移动第一条黄色框至下一条要分析的条带处
5.将框选的第二条条带设置为要进行分析的区域(如果有更多条带分析,则重复第二条带的操作步骤)
❶
❷
❸6.点击Plot Lanes,跳转至下一步
7.选择直线工具,画出各条带永道的封闭面积,然后使用魔术棒点击各条带灰度封闭取峰图,
得到result 框中定量灰度结果。
❶
❷
❸。
10分钟Get!大牛教你用imageJ对Westernblot条带进行灰度分析!
10分钟Get!大牛教你用imageJ对Westernblot条带进行
灰度分析!
科研人员做的western blot实验一般需要对其结果扫描后进行灰度分析,一般使用的软件为Image 。
采用Image J 软件对蛋白质印迹实验结果(Western Blot)进行灰度半定量分析,是分子生物学实验中比较常用的方法,可实现对WB实验结果进行准确、简单和快速的分析。
ImageJ分析Westernblot条带图
Step 1:打开Image J, 导入图片→Image→Type→8 bit
Step 2:选择工具框中第一个矩形框选项,选中所有的条带
Step 3:点击Analyze→Gels→Plot Lanes
Step 4:选用工具框中的直线工具,将开口波峰封闭
Step 5:选用工具框中的魔棒工具,点击相应的波峰,相应area值
ImageJ分析Westernblot条带图
Step 1:打开Image J, 导入图片。
Image→Type→8 bit
Step 2:扣除背景P→rocess→Subtract Background→选择50 pixels和Lightbackground
Step 3:设定参数→Analyze→Set Measures→按照下图勾选参数
Step 4:设定参数→Analyze→Set scale→unit of length选项改为pixels
Step 5:图像分割→Edit→Invert→选用椭圆或自定义工具选中条带
Step 6:Analyze→Measure→得到 IntDen值Step 7:重复Step5、6,得到所有条带的IntDen值
以上实用干货来源于课程
30分钟精通Image J图片处理。
WB灰度值测定

Image J测到了WB条带灰度值关于用Image J量化westernblot条带灰度值,在网上一直盛传着两种操作方法,然鹅~小伙伴们却一直不确定到底哪种才是正确的测量灰度方法,甚至将灰度与光密度弄混淆。
今天咱们一起就这个机会学习下,请先看看你是用以下那种方法测量灰度值?以下是两种方法的具体操作。
方法一:1、打开Image J软件→左上角file→Open 选择自己的条带图片(事先把条带摆正)2、把图片转化成灰度图片:Image→type→8—bit3、第一个矩形工具→选上所有条带4、analyze→Gels→select first lane→Gels→plot lanes(在这第四步也可以分开选取,如:框选第一个条带→analyze→Gels→select firstlane→将第一个框拖移到余下条带→Gels→select nextlane)5、选中直线工具,将开口波峰关闭6、选中魔棒工具(正数第七个),点击波峰,就得出所有area值。
方法二1、打开Image J软件→左上角file→Open 选择自己的条带图片(事先条带弄正)2、把图片转化成灰度图片:Image→type→8—bit3、扣除背景:Process→Subtract Background→50pixels 并勾选Light Background5、设定参数:Analyze→set measurement→勾选Area、Mean gray value、Min & max gray value、Integrated density6、Analyze→set scale→unit of length选项里改为pixels,确定6、图像分割。
Edit→Invert→选中椭圆圈(手残党首选)/不规则圆圈(心灵手巧党义无反顾),手动圈上单个条带7、Analyze→Measurement即得到Intden,重复6、7步就得到所有Intden值小伙伴们,你们是哪种方法呢?首先先把结论说出来,其实两者得出的area和Intden严格来说都不是灰度值,前者是面积而后者是光密度值,但用来量化WB条带都是OK的。
灰度分析和细胞计数神器:ImageJ(附软件下载)
灰度分析和细胞计数神器:ImageJ(附软件下载)作者:解螺旋·叶子如需转载请注明来源:解螺旋·医生科研助手导语Image J是NIH推出的分子生物分析软件,使用方便,简单易学。
与PS比起来,Image J有个强大的优势,就是分析处理的结果比较受到普遍承认,不会被怀疑作假(小保方晴子哭晕在厕所)。
Image J用于生物医学领域最多的就是条带灰度值分析(DNA电泳或者Western Blot条带分析)和细胞计数。
来具体看看怎么操作。
条带灰度值分析打开Image J软件,在File里打开图片,并且转化为8-bit灰度图(Image→Type→8bit)。
方框工具选择并画出条带,然后选择Analyze→Gels→Select First Lane。
最后按Analyze→Gels→plot Lanes,就会出现山峰样图。
但这样是不够的,要把每个山峰分开。
这就用到了直线功能,把下面都封口后点击"魔棒"。
分别点击每个峰下方的区域,在"Results" 里就会出现面积,表示相应条带的灰度值。
如果要测多个条带的灰度,就用在Select First Lane后,点Select Next Lane来复选。
这里注意,每个框的形状大小必须是一样的。
分析图像中的颗粒数(细胞计数)打开Image J软件,在File里打开图片,并且转化为8-bit灰度图(Image→Type→8bit)。
这种黑底的图数起来非常困难,需要反相下变成白底图。
框定区域后,选择Edit→Invert。
比之前清楚多了吧,再设置下阈值(Image→Adjust→Threshold)。
经过调整后对比非常明显。
最后,进入Analyze→Analyze Particles, 键入微粒大小的下限和上限,并且选择显示轮廓(Show outlines)和显示结果(Display Results)。
点击ok,被计数的微粒将显示轮廓和编号。
使用ImageJ分析Western
使用ImageJ 分析Western Blot (2013-04-16 21:40:31)转载▼标签: imagejwesterndnaThe following information is an updated version of a method for using ImageJ to analyze western blots from a now-deprecated older page. Don’t use the alternate methods discussed on the old page, as they are subject to way too much user bias.A pdf copy of this page is available.ImageJ (/ij/index.html ) can be used to compare the density (aka intensity) of bands on an agar gel or western blot. This tutorial assumes that you have carried your gel or blot through the visualization step, so that you have a digital image of your gel in .tif, .jpg, .png or other image formats (.tif would be the preferred format to retain the maximum amount of information in the original image). If you are scanning x-ray film on a flatbed scanner, make sure you use a scanner with the ability to scan transparencies (i.e. film). See the references at the end of this tutorial for a discussion of the various ways that you can screw this step up.The method outlined here uses the Gel Analysis method outlined in the ImageJdocumentation: Gel Analysis. You may prefer to use it instead of the methods I outline below. There should be very little difference between the results obtained from the various methods. This version of the tutorial was created using ImageJ 1.42q on a Windows 7 64-bit install.1. Open the image file using File>Open in ImageJ.2. The gel analysis routine requires the image to be a gray-scale image. The simplest method to convert to grayscale is to go to Image>Type>8-bit. Your image should look like Figure 1.3. Choose the Rectangular Selections tool from the ImageJ toolbar. Draw a rectangle around the first lane. ImageJ assumes that your lanes run vertically (so individual bands arehorizontal), so your rectangle should be tall and narrow to enclose a single lane. If you draw a rectangle that is short and wide, ImageJ will switch to assuming the lanes run horizontally(individual bands are vertical), leading to much confusion.4. After drawing the rectangle over your first lane, press the 1 key or go toAnalyze>Gels>Select First Lane to set the rectangle in place. The 1st lane will now be highlighted and have a 1 in the middle of it.5. Use your mouse to click and hold in the middle of the rectangle on the 1st lane and drag it over to the next lane. You can also use the arrow keys to move the rectangle, though this is slower. Center the rectangle over the lane left-to-right, but don’t worry about lining it up perfectly on the same vertical axis. Image-J will automatically align the rectangle on the same vertical axis as the 1st rectangle in the next step.6. Press 2 or go to Analyze>Gels>Select Next Lane to set the rectangle in place over the 2nd lane. A 2 will appear in the lane when the rectangle is placed.7. Repeat Steps 5 + 6 for each subsequent lane on the gel, pressing 2 each time to set the rectangle in place (Figure 3).8. After you have set the rectangle in place on the last lane (by pressing 2), press 3, or go to Analyze>Gels>Plot Lanes to draw a profile plot of each lane.9. The profile plot represents the relative density of the contents of the rectangle over each lane. The rectangles are arranged top to bottom on the profile plot. In the example western blot image, the peaks in the profile plot (Figure 4) correspond to the dark bands in the original image (Figure 3). Because there were four lanes selected, there are four sections in the profile plot. Higher peaks represent darker bands. Wider peaks represent bands that cover a wider size range on the original gel.10. Images of real gels or western blots will always have some background signal, so the peaks don’t reach down to the baseline of the profile plot. Figure 5 shows a peak from a real blot where there was some background noise, so the peak appears to float above the baseline of the profile plot. It will be necessary to close off the peak so that we can measure its size.11. Choose the Straight Line selection tool from the ImageJ toolbar (Figure 6). For each peak you want to analyze in the profile plot, draw a line across the base of the peak to enclose the peak (Figure 5). This step requires some subjective judgment on your part to decide where the peak ends and the background noise begins.12. Note that if you have many lanes highlighted, the later lanes will be hidden at the bottom of the profile plot window. To see these lanes, press and hold the space bar, and use the mouse to click and drag the profile plot upwards.13. When each peak has been closed off at the base with the Straight Line selection tool, select the Wand tool from the ImageJ toolbar (Figure 8).14. Using the spacebar and mouse, drag the profile plot back down until you are back at the first lane. With the Wand tool, click inside the peak (Figure 9). Repeat this for each peak as you go down the profile plot. For each peak that you highlight, measurements should pop up in the Results window that appears.15. When all of the peaks have been highlighted, go to Analyze>Gels>Label Peaks. This labels each peak with its size, expressed as a percentage of the total size of all of the highlighted peaks.16. The values from the Results window (Figure 10) can be moved to a spreadsheet program by selecting Edit>Copy All in the Results window. Paste the values into a spreadsheet.Note: If you accidentally click in the wrong place with the Wand, the program still records that clicked area as a peak, and it will factor into the total area used to calculate the percentage values. Obviously this will skew your results if you click in areas that aren’t peaks. If you do happen to click in the wrong place, simple go to Analyze>Gel>Label Peaks to plot the current results, which displays the incorrect values, but more importantly resets the counter for theResults window. Go back to the profile plot and begin clicking inside the peaks again, starting with the 1st peak of interest. The Results window should clear and begin showing your new values. When you’re sure you’ve click in all of the correct peaks without accidentally clicking in any wrong areas, you can go back to Analyze>Gels>Label Peaks and get the correct results. Data analysisWith your data pasted into a spreadsheet, you can now calculate the relative density of the peaks. As a reminder, the values calculated by ImageJ are essentially arbitrary numbers, they only have meaning within the context of the set of peaks that you selected on the single gel image you’ve been working on. They do not have units of μg of protein or any other real-world units that you can think of. The normal procedure is to express the density of the selected bands relative to some standard band that you also selected during this process.1. Place your data in a spreadsheet. One of the peaks should be your standard. In this example we’ll use the 1st peak as the standard.2. In a new column next to the Percent column, divide the Percent value for each sample by the Percent value for the standard (the 1st peak in this case, 26.666).3. The resulting column of values is a measure of the relative density of each peak, compared to the standard, which will obviously have a relative density of 1.4. In this example, the 2nd lane has a higher Relative Density (1.86), which corresponds well with the size and darkness of that band in the original image (Figure 1). Recall that these data are for the upper row of bands on the original western blot image.5. If you want to compare the density of samples on multiple gels or blots, you will need to use the same standard sample on every gel to provide a common reference when you calculate Relative Density values. See the sections below for more detailed discussion of these requirements.6. In order to test for significant differences between treatments in an experiment, all of your gels or blots will need to be scanned and quantified using this method, and the values will be expressed in terms of Relative Density, or you can treat Relative Density as a fold-change value (i.e. a Relative Density difference of 2 between a control and treatment wouldindicate a 2-fold change in expression). If you will be using analysis of variance techniques to test your data, you may need to ensure that your Relative Density values are normally distributed and that there is homogeneity of variance among the different treatments.7. It should be noted here that some researchers make the extra effort to include a set of serial dilutions of a known standard on each blot. Using the serial dilution curve and the quantification techniques outlined above, it should be possible to express your sample bands in terms of picograms or nanograms of protein.A more involved example using loading-controls.We’ll use Figure 12 as a representative western blot. On this blot, we will pretend that we loaded four replicate samples of protein (four pipette loads out of the same vial of homogenate), so we expect the densities in each lane to be equivalent. The upper row of bars will represent our protein of interest. The lower set of bars will represent our loading-control protein, which is meant to ensure that an equal amount of total protein was loaded in each lane. This loading-control protein is a protein that is presumably expressed at a constant level regardless of the treatment applied to the original organisms, such as actin (though many people will question the assertion that actin will be expressed equivalently across treatments).Looking at Figure 12, we had hoped to load equivalent amounts of total protein in each lane, but after running the western blot, the size and intensity of the lower bars in each lane varies quite a lot. The two left lanes appear equivalent, but the 3rd lane has half the density (gray value) compared to lanes 1+2, while lane 4 has half the density and half the size compared to lanes 1+2. Because our loading controls are so different, the density values of the upper set of bands may not be directly comparable.We’ll use ImageJ’s gel analysis routine to quantify the density and size of the blots, and use the results from our loading-controls (lower bands) to scale the values for our protein of interest (upper bands).1. Open the western blot image in ImageJ.2. Make sure that the image is in 8-bit mode: go to Image>Type>8-bit.3. Use the rectangle tool to draw a box around the entire 1st lane (both upper and lower bars included.4. Press “1″ to set the rectangle. A “1″ should appear in the middle of the rectangle.5. Click and hold in the middle of the rectangle and drag it over the 2nd lane.6. Press “2″ to set the rectangle for lane 2. A “2″ should appear in the middle of the rectangle.7. Repeat steps 5 + 6 for each subsequent lane, pressing “2″ to set the rectangle over each subsequent lane (see Figure 13).8. When you have placed the last lane (and pressed “2″ to set it in place), you can press “3″ to produce a plot of the selected lanes (see Figure 14).9. The profile plot essentially represents the average density value across a set of horizontal slices of each lane. Darker blots will have higher peaks, and blots that cover a larger size range (kD) will have wider peaks. In our example western blot, the bands are perfect rectangles, but you will notice some slope in the profile plot peaks, as ImageJ is applying a bit of averaging of density values as it moves from top to bottom of each lane. As a result, the sharp transition from perfect white to perfect black on the bands of lane 1 is translated into a slight slope on the profile plot due to the averaging.10. On our idealized western blot used here, there is no background noise, so the peak reaches all the way down to the baseline of the profile plot. In real western blots, there will be some background noise (the background will not be perfectly white), so the peaks won’t reach the baseline of the profile plot (see figure 5 above). As a result, each plot will need to have a line drawn across the base of the peak to close it off.11. Choose the Straight Line selection tool from the ImageJ toolbar. For each peak you want to analyze in the profile plot, draw a line across the base of the peak to enclose the peak (Figure 7). This step requires some subjective judgment on your part to decide where the peak ends and the background noise begins.12. When each peak of interest is closed off with the straight line tool, switch to the Wand tool. We will use the wand tool to highlight each peak of interest so that Image-J can calculate its relative area+density.13. We will start by highlighting the loading-control bands (lower row) on our example western blot. Beginning at the top of the profile plot, use the wand to click inside the 1st peak (Figure 15). The peak should be highlighted after you click on it. Continue clicking on the loading-control peaks for the other lanes. If a lane is not visible at the bottom of the profile plot, hold down the space bar and click-and-drag the profile plot upwards to reveal the remaining lanes.14. When the loading control peak for each lane has been highlighted with the wand, go to Analyze>Gel>Label Peaks. Each highlighted peak will be labeled with its relative size expressed as a percentage of the total area of all the highlighted peaks. You can go to the Results window and choose Edit>Copy All to copy the results for placement in a spreadsheet.15. Repeat steps 13 + 14 for the real sample peaks now. We are selecting these peaks separately from the loading-control peaks so that those areas are not factored into the calculation of the density of our proteins-of-interest. As before, use the Wand tool to click inside the area of the peak in the 1st lane, then continue clicking inside the peaks of the remaining lanes. When finished, go to Analyze>Gel>Label Peaks to show the results. Copy the results to a spreadsheet alongside the data for the loading-control bands (Figure 17).Data Analysis with loading-control bands1. With all of the relative density values now in the spreadsheet, we can calculate the relative amounts of protein on the western blot. Remember that the “Area” and “Percent” values returned by ImageJ are expressed as relative values, based only on the peaks that you highlighted on the gel. Start the analysis by calculating Relative Density values for each of the loading-standard bands. In this case, we’ll pretend that Lane 1 is our contr ol that we want to compare the other 3 lanes to. Divide the Percent value for each lane by the Percent value in the control (Lane 1 here) to get a set of density values that is relative to the amount of protein in Lane 1′s loading-control band (Figure 18).2. Next we’ll calculate the Relative Density values for our sample protein bands (upper row on the example western blot). We carry out a similar calculation as step 1, dividing the Percent value in each row by the Percent value of our control’s protein band (Lane 1 here).Note: Recall that because some of our loading-control bands were wildly different on the original western blot, we can’t simply use the Relative Density values from our Samples calculated in Step 2 as the final results. Now it is necessary to scale the Relative Density values for the Samples by the Relative Density of the corresponding loading-control bands for each lane. We do this based on the assumption that the proportional differences in the Relative Densities of the loading-control bands represent the proportional differences in amounts of total protein we loaded on the gel. In our example western blot, we have evidence of massively different amounts of total protein in each sample (poor pipetting practice, probably).3. The final step is to scale our Sample Relative Densities using the Relative Densities of the loading-controls. On the spreadsheet, divide the Sample Relative Density of each lane by the loading-control Relative Density for that same lane.9. The profile plot essentially represents the average density value across a set of horizontal slices of each lane. Darker blots will have higher peaks, and blots that cover a larger size range (kD) will have wider peaks. In our example western blot, the bands are perfect rectangles, but you will notice some slope in the profile plot peaks, as ImageJ is applying a bit of averaging of density values as it moves from top to bottom of each lane. As a result, the sharp transition from perfect white to perfect black on the bands of lane 1 is translated into a slight slope on the profile plot due to the averaging.10. On our idealized western blot used here, there is no background noise, so the peak reaches all the way down to the baseline of the profile plot. In real western blots, there will be some background noise (the background will not be perfectly white), so the peaks won’t reach the baseline of the profile plot (see figure 5 above). As a result, each plot will need to have a line drawn across the base of the peak to close it off.11. Choose the Straight Line selection tool from the ImageJ toolbar. For each peak you want to analyze in the profile plot, draw a line across the base of the peak to enclose the peak (Figure 7). This step requires some subjective judgment on your part to decide where the peak ends and the background noise begins.12. When each peak of interest is closed off with the straight line tool, switch to the Wand tool. We will use the wand tool to highlight each peak of interest so that Image-J can calculate its relative area+density.13. We will start by highlighting the loading-control bands (lower row) on our example western blot. Beginning at the top of the profile plot, use the wand to click inside the 1st peak (Figure 15). The peak should be highlighted after you click on it. Continue clicking on the loading-control peaks for the other lanes. If a lane is not visible at the bottom of the profile plot, hold down the space bar and click-and-drag the profile plot upwards to reveal theremaining lanes.14. When the loading control peak for each lane has been highlighted with the wand, go to Analyze>Gel>Label Peaks. Each highlighted peak will be labeled with its relative size expressed as a percentage of the total area of all the highlighted peaks. You can go to the Results window and choose Edit>Copy All to copy the results for placement in a spreadsheet.15. Repeat steps 13 + 14 for the real sample peaks now. We are selecting these peaks separately from the loading-control peaks so that those areas are not factored into the calculation of the density of our proteins-of-interest. As before, use the Wand tool to click inside the area of the peak in the 1st lane, then continue clicking inside the peaks of the remaining lanes. When finished, go to Analyze>Gel>Label Peaks to show the results. Copy the results to a spreadsheet alongside the data for the loading-control bands (Figure 17).。
干货▎ImageJ分析WesternBlot蛋白条带灰度值

干货▎ImageJ分析WesternBlot蛋白条带灰度值聊点学术,一键关注“你已经是个成熟的条带了,要学会自己分析!”“连Western blot都没学好,又让我学Image J,我......”This is the dividing line.Image J分析蛋白条带灰度值首先解释一下,为什么要分析灰度值?灰度的概念是使用黑色调表示物体,即用黑色为基准色,不同的饱和度的黑色来显示图像。
采用ECL发光时,蛋白条带发出的荧光会曝光在胶片上,留下的黑色条带。
然而胶片扫描后为蓝色背景、黑色条带,这并不是单纯的黑灰白颜色。
因此,我们首先得在Photoshop 中将整张图片去色,使彩色图片变成黑白图片,形成近灰色背景、黑色条带的图片类型。
此时,条带的深浅和面积综合代表着蛋白的量。
灰度值分析自然就成为我们的首选。
延伸说一下,免疫组织化学染色(IHC)结果本身为近白色背景、棕黄色阳性表达,这时我们就不能直接用灰度反映阳性表达强弱了,而是积分吸光度,也就是咱们常说的平均光密度(IOD)。
如果你真的想用灰度计算IHC结果,显然你需要将图片转为灰度图再计算。
此处不表。
分析图文步骤如下:Image J软件界面↓1. 图片转换为黑白图:首先使用Photoshop打开胶片扫描图片,点击“图像>调整>去色”,将彩色图片转化为黑白图片,并使用裁剪工具将目标条带裁剪至合适大小后另存图片。
2.打开Image J软件:2.1.打开图片文件:File>Open;将图片转化为8bit类型:Image>Type>8-bit。
(此步骤的目的是为了将图片的每个像素用8bit表示,这样的话,整个图片的灰度将分为256个级别,即黑、灰、白的像素模式;若保留原始的16bit格式或RGB格式,图片灰度级别会呈指数增长,并有其它颜色混入,造成计算误差。
)2.2.将整张图片背景灰度均一化,消除图片背景影响:Process>Subtract Background,默认数值为50即可。
用ImageJ对Western DNA和Blot图片灰度分析

用ImageJ对W estern Blot图片灰度分析教程收集于互联网来源中生网The good news is that even if you don't have access t o a photo editing program such as Photoshop, you can now do all the same analyses using free programs. My favorite option is the freely available ImageJ from the National Institut es of Health.The homepage for ImageJ is here: /ij/index.html wherein you can find links t o the download, document ation, additional plugins and so on.Once ImageJ is inst alled, open it up and open your scanned film file. We'll start the ImageJ section by duplicating the method outlined above for Phot oshop.1. Open your file.2. Under Image>Type click on 8-bit t o convert the image to grayscale.3. Go to the menu Process>Subt ract Background. Try a rolling ball radius of 50. This removes some of the background coloration from your image.4. Go to Analyze>Set Measurements, and click the boxes for Area, Mean Gray Value, and Int egrated D ensit y.5. Go to Analyze>Set Scale, and ent er "pixels" in the box next t o Unit of length.6. Go to Edit>Invert (or hit Ct rl+Shift+I) t o invert the colors on the image. Now the dark areas are light, and the light areas are dark. As outlined above, this has the benefit of making the measured values for bands increase with increasing prot ein expression.7. Choose the F reehand Selection tool from the t ool palette.8. Draw a line around the boundary of your first band. As above, you need to use your own judgement aboutwhere t he edges of the band are, and what is simply background noise.9. Hit the m key to take a measurement of the enclose area that you select ed. The Result s window should pop up, and each of the measurements you select ed in step 4 should appear. Not e that the Integrated Density column is simply the Area and Mean Gray Value columns multiplied together.10. Use the Freehand Selection tool to select the next band, and press m to take the measurement. Repeat this for each of you bands, including the st andard.11. When you are finished, you can go to the Edit menu in the Results window, and choose Copy All. You can then paste the results into a spreadsheet for lat er use.教你使用ImageJ分析电泳条带灰度比-ImageJ使用教程ImageJ这套软件可以自动帮你你计算细胞数,也可以定量分析DNA电泳或是Western blot条带。
老谈手把手教:ImageJ灰度扫描、PS组合图、荧光图片处理....
老谈手把手教:ImageJ灰度扫描、PS组合图、荧光图片处理....感觉ImageJ和PS太难?其实不然,看视频操作就行了。
上周《lncRNA研究套路》系列课的第二节内容已通过以下案例文章介绍了lncRNA发挥作用的四种模式,并对文章中数据进行了详细地解读和分析。
本周,老谈将继续以该文章为例,从SCI作图基本原则,Image J和Photoshop三部分讲解文章数据处理和展示方法。
01常言道,无规矩不成方圆。
因而,SCI论文中图片制作和排版也是存在一些约定熟成的“规矩”,只有熟知“规矩”才能练就“火眼金睛”,成功避开图片制作各种坑。
首先,以参考文献中的6张图片为实例,课程通过找茬的方式为大家展示各个小图制作的小错误,并说明如何根据“规矩”进行改正。
虽然这6张图片质量整体不错,没有太多的低级失误,但仍存在一些格式上不协调的微小瑕疵。
以问题最多的Figure4为例,就存在以下问题:图片边界、Y轴标题太长、Y轴刻度线太密集、图片对比度等。
此外课程也重点关注了排版问题,通过修改前和修改后图片对比展示,说明SCI图片排版的基本方法。
同时,也会通过实例探讨小图拼成大图的方法和技巧。
02检测蛋白的Western blot(wb)作为基础科研中的经典分子实验,在大多数情况下,其结果图仅仅是典型的图片展示,但有时也需要对wb结果图进行定量统计。
Image J是最常用的western blot定量方法。
本节课程则会详解Image J软件分析图片灰度状态表达的方法及步骤。
首先,就以黑白为基础的灰度图片而言,其灰度值可表征图片灰度的状态。
以8bit图片为例,全黑状态时灰度定义为0;全白状态时灰度定义为255;从0到255之间有256个级差,可覆盖全黑到全白的整个广谱。
所以实际应用中一般以8bit图片为准。
其次,课程也展示了WB结果定量分析的逻辑思路,以及蛋白WB 图片定量前所需要制备的4个表格。
最后分别选取背景干净图片(文献Figure3a)和背景干扰典型图片(文献Figure5e)为实例,手把手教授Image J如何获得灰度值和数据均一化方法,并可获得以下数据。
老司机带你解锁ImageJ实用技巧(下)

老司机带你解锁ImageJ实用技巧(下)文章转载自公众号科研讲坛,作者半夏今天我们继续来聊一聊ImageJ的高阶使用技巧。
问题三、为什么总是全部圈起来的灰度值,有没有大神指导呢求助!本问题涉及免疫印迹(Western Blot)分析,提问者不能分别得到每个条带的值。
灰度值0为纯黑,255为纯白,灰度值与光密度值(OD值)的关系如下图所示:以灰度来统计WB条带的话,无条带的纯白255左右,条带越黑着色越深灰度值反而越小,这与我们的认知不符。
步骤:1. File -> Open -> 打开需要分析的WB条带。
2. Image –> Type -> 8-bit, 将图像转换为8-bit的灰度图片;Image-> Invert,黑白反转,得到如下图片:3. Analyze -> Calibrate, 校正光密度值Function中选择Uncalibrated OD,左下方Global calibration,勾选表示有多张图片打开时对所有图片进行此操作。
否则只对当前图片进行此操作。
4. 在工具栏中选择矩形工具——Rectangular, 最左边的为矩形工具,选择条带:键盘上按数字1,弹出如下提示框:点Yes,键盘上按数字3,得到如下:5. 工具栏中选择直线工具——Straight,下图最右边的为直线工具,按住Shift键使用直线工具画竖线将步骤4的峰进行分割:6. 选择魔棒工具——Wand tool,分别点击分割好的峰,即可得到结果,保存结果(File -> Save as…)即可:7. WB统计分析一般将对照组标准化为100%或1,上述结果6个条带前3个为对照组。
在Excel中,先计算对照组3个的平均值(AVERAGE(B2:B4)):然后所有B列的数值除以对照组平均值,此时对照组的均值为1:8. 将所得数据放入Graphpad prism绘图即可:拓展:弯曲的WB条带应如何使用ImageJ拉直?1. 使用Segmented line沿着倾斜条带画间断线段:2. 双击Segmented line,设置线段的宽度至包含所有条带:3. 点击菜单栏Edit -> Selection-> Straighten,倾斜的WB条带即可拉直:问题四、如何统计SEM图片小球数量?步骤:1. 打开ImageJ软件,File -> Open打开SEM图片。
image J使用说明(版本1)

Western Blot图片灰度分析-Image JThis method is the Gel Analysis method outlined in the ImageJ documentation:Gel Analysis.You may prefer to use it instead of the methods outlined below. There will likely be very little difference in the results between the various methods.1.Open your file.2.Go to Analyze>Gels>Gel Analyzer Options and click the boxes for Label With Percentages,Outline Lanes and Invert Peaks.3.Choose the Rectangular Selection tool.Draw a rectangle around your first lane.Encompass some area of the lane above and below the band of interest. Edit July2009:Note that for a gel with the lanes oriented vertically as shown here(i.e.the visible bands are horizontal across the image),you want to make your bounding rectangle taller than it is wide.However,if you have the image rotated so that the lanes are running horizontally,you need to make your bounding rectangle at least twice as wide as it is tall,at which point Image-J will recognize that your lanes are horizontal and it will allow you to move the box up or down the image to enclose the neighboring lanes.4.Press the1button(or go to Analyze>Gels>Select First Lane).A new window will pop up with a copy of your image and a label over your first rectangular selection.e the arrow keys to move the rectangle over the next lane.Press2(or go to Analyze>Gels>Select Next Lane)to place a selection around the lane. Repeat this for each lane on the membrane,moving the box and pressing2to place the selection.6.When finished,press3(or go to Analyze>Gels>Plot Lanes),which pops up a new window with a profile plot of each lane.7.Now choose the Straight Line selection tool.At the base of each peak,draw a line from one side of the peak to the other.This encloses the area of the peak.The tails to either side of the peak are the background signal.Note that if you have many lanes,the later lanes will be hidden at the bottom of the profile plot.To see these lanes,press and hold the space bar,and use the mouse to drag the profile plot upwards.8.When each peak has been closed off at the base with the Straight Line tool,choose the Magic Wand(Wand tracing tool)from the tool palette.ing the spacebar and mouse,drag the profile plot back down until you are at the top peak.With the wand,click inside the peak.Repeat this for each peak as you go down the profile plot.10.When each peak has been selected,go to Analyze>Gels>Label Peaks.This labels each peak with its size expressed as a percentange of the total size of all the measured peaks.You can go to the Results window and choose Edit>Copy All to copy the results for placing in a spreadsheet.Note:If you accidentally click in the wrong place with the Magic Wand,the program still records that clicked area,and it will factor into the total area used to calculate the percentages.Obviously this would skew your results if you click in areas that aren't peaks.Therefore,if you should click in the wrong place, simply go to A nalyze>Gels>Label Peaks to plot the current results,which displays these incorrect values,but more importantly resets the counter for the Results window.If you now go back to the Profile Plot and click in the peaks with the Magic Wand,the Results window clears and starts over.When you're sure you've clicked in the correct peaks without accidentally clicking in any wrong areas,you can go back to Analyze>Gels>Label Peaks and get the correct results.教程下载:/files/uv1JN-8*NsvjXs-Lfcz2XiblWXipjZeuSt02X5Nh6CZO1ky1uGEz9J3K0brzHT7WWaM4jhg4JlSmcJGniGt8TQMIrak7s1fQ/imageJ.pdf。
ImageJ分析western步骤

点击file →ope n→任意一张图
点击再点击,左右鼠标键可改变图片大小,以便分析,图片大小不影响数据。
点击再点倒数第二项,减去背景,保存原设置值。
点击再点invert
点击再点 set measurements,勾选如下
Ok确定
Mean Gray Value:平灰度值
Integrated Density:总灰度值
点击,在区域内可改变大小,最好刚刚圈住每个印记, 不改变方框大小,移到旁边黑色区域,计算背景面积
点击,measure
再把方框移到每个组别,仍点击
所有值将会依次出现,
如果中间改变方框大小,那么必须再重新将方框移到黑色区域,计算背景面积。
所有印记的真实值=系统出现值-同样方框下的背景值。
ImageJ对WesternBlot进行灰度分析

ImageJ对WesternBlot进行灰度分析Western Blotting是生物学领域常用的分析蛋白质的实验技术,它可以提供蛋白质定量和免疫印迹图像。
Western Blot的数据分析需要使用图像处理软件,ImageJ是一款免费的开源软件,是生物医学图像处理分析领域的标准工具之一。
本文将介绍ImageJ如何对Western Blot图像进行灰度分析。
背景Western Blotting技术是分析蛋白质表达及功能的一种常用方法,它可以检测蛋白质的存在、分子量、相对表达量、修饰等信息。
通常利用免疫学方法检测Western Blot膜上的蛋白质,检测后得到的结果是一张黑白图像。
这个图像需要进行处理和分析,以得到蛋白质的数量和变化。
ImageJ是一个开源的图像处理和分析软件,它可以用于各种各样的生物医学图像分析,包括Western Blotting图像分析。
ImageJ提供了灰度分析的功能,可以实现对Western Blotting图像的结果定量分析。
灰度分析可以测量Western Blotting图像的灰度值,这个值可以代表蛋白质的含量,从而实现对蛋白质表达量的分析。
安装ImageJ和导入图像在使用ImageJ进行Western Blotting图像分析前,需要先安装并打开ImageJ。
安装完成后,可以通过以下步骤导入Western Blotting图像:1.打开ImageJ并选择“File”菜单中的“Open”选项。
2.找到Western Blotting图像并选择打开。
3.如果需要对导入的图像进行调整,可以使用ImageJ提供的功能或插件进行操作。
灰度分析灰度分析是Western Blotting图像分析的一个重要步骤,旨在定量分析蛋白质的含量。
以下是使用ImageJ进行灰度分析的步骤:1.进入“Analyze”菜单并选择“Gels”选项。
2.在“Gels”菜单中,选择“Lane Profile”选项。
Western Blot图片灰度分析 -Image J

Western Blot图片灰度分析-Image J2010年02月21日星期日08:19This method is the Gel Analysis method outlined in the ImageJ documentation:Gel Analysis.You may prefer to use it instead of the methods outlined below.There will likely be very little difference in the results between the various methods.1.Open your file.2.Go to Analyze>Gels>Gel Analyzer Options and click the boxes for Label With Percentages, Outline Lanes and Invert Peaks.3.Choose the Rectangular Selection tool.Draw a rectangle around your first lane.Encompass some area of the lane above and below the band of interest.Edit July2009:Note that for a gel with the lanes oriented vertically as shown here(i.e.the visible bands are horizontal across the image),you want to make your bounding rectangle taller than it is wide.However,if you have the image rotated so that the lanes are running horizontally,you need to make your bounding rectangle at least twice as wide as it is tall,at which point Image-J will recognize that your lanes are horizontal and it will allow you to move the box up or down the image to enclose the neighboring lanes.4.Press the1button(or go to Analyze>Gels>Select First Lane).A new window will pop up with a copy of your image and a label over your first rectangular selection.e the arrow keys to move the rectangle over the next lane.Press2(or go to Analyze>Gels>Select Next Lane)to place a selection around the lane.Repeat this for each lane on the membrane,moving the box and pressing2to place the selection.6.When finished,press3(or go to Analyze>Gels>Plot Lanes),which pops up a new window witha profile plot of each lane.7.Now choose the Straight Line selection tool.At the base of each peak,draw a line from one side of the peak to the other.This encloses the area of the peak.The tails to either side of the peak are the background signal.Note that if you have many lanes,the later lanes will be hidden at the bottom of the profile plot.To see these lanes,press and hold the space bar,and use the mouse to drag the profile plot upwards.8.When each peak has been closed off at the base with the Straight Line tool,choose the Magic Wand(Wand tracing tool)from the tool palette.ing the spacebar and mouse,drag the profile plot back down until you are at the top peak. With the wand,click inside the peak.Repeat this for each peak as you go down the profile plot.10.When each peak has been selected,go to Analyze>Gels>Label Peaks.This labels each peak with its size expressed as a percentange of the total size of all the measured peaks.You can go to the Results window and choose Edit>Copy All to copy the results for placing in a spreadsheet.Note:If you accidentally click in the wrong place with the Magic Wand,the program still records that clicked area,and it will factor into the total area used to calculate the percentages.Obviously this would skew your results if you click in areas that aren't peaks.Therefore,if you should click in the wrong place,simply go to A nalyze>Gels>Label Peaks to plot the current results,which displays these incorrect values,but more importantly resets the counter for the Results window. If you now go back to the Profile Plot and click in the peaks with the Magic Wand,the Results window clears and starts over.When you're sure you've clicked in the correct peaks without accidentally clicking in any wrong areas,you can go back to Analyze>Gels>Label Peaks and get the correct results.教程下载:/files/uv1JN-8*NsvjXs-Lfcz2XiblWXipjZeuSt02X5Nh6CZO1ky1uGEz9J3K0brzHT 7WWaM4jhg4JlSmcJGniGt8TQMIrak7s1fQ/imageJ.pdf。
imageJ软件分析westernblot灰度值

imageJ软件分析westernblot灰度值ImagJ是一款简单的图像处理与分析软件,可以用来进行WB定量分析。
一、利用ImagJ对WB条带进行灰度分析1、File——》open 打开WB片子2、把图片转化成灰度图片image——》type——》8-bit3、消除背景影响process——》subtract background 选择50基本可以4、设置定量参数analyze——》set measurements,点击面积,平均密度和灰度值及Integrated Density5、设置单位analyze——》set scale ,在“unit of length”的方框里输入“pixels”6、把图片转换成亮带,Edit——》invert7、选择FreehandSelection,尽量把条带圈起来,点击键盘m,出来IntDen灰度值8、复制数据IntDen进行分析二、利用ImagJ对WB条带进行密度分析1、File——》open 打开WB片子2、如条带不正,需修正image——》transform——》rotate调节angle值,直到条带水平为止3、选中矩形选项,圈中第一个条带,然后analyze——》gels——》select firstlane(快捷键ctrl+1),然后移动第一个条带上的矩形到第二个条带上,analyze——》gels——》select secondlane(快捷键ctrl+2),最后analyze——》gels——》plotlanes4、选中直线工具,将开口的波峰关闭5、选中魔棒工具,点击波峰可以显示波峰下面积,即条带的密度值6、以第一个数值为基数,其他数值与第一个数值的比值为相对密度。
Westen blot 灰度测定方法

WB灰度计算image j
1.载入图片:File----open---选中文件
2.图片处理:
(1)改变类型:RGB转化为8-bit(灰度图)
(2)去除背景:(灰背景影响计算)process---subtract background---默认50.0 light background---OK(CTRL加加号放大,加减号缩小)
3.分析方法一:
(1)选择分析范围:矩形工具框选分析泳道
(3)analyze---gels---select first lane---yes
(4)绘图:analyze---plot lanes(几个条带对应几个峰值)
(5)计算每一个峰值的面积:峰的根部分隔开,用直线工具画竖的知县分割成闭合区域
(6)计算面积:魔棒工具,分别点击每一个峰值的区域,得到面积数据(面积对应灰度值,蛋白质量越多则灰度面积越大)
蛋白质跑之前内参调齐肉眼即可判断趋势。
4.分析方法二:
矩形工具单独框选后命名(除了第一个ctrl+1以外都ctrl+2,最后一个ctrl+3可以挪到旁边,框框大小必须一样)
nalyze---plot lanes绘图后,得到单独分割的峰图,直接选择魔棒工具(更加精确也可以直线封闭),选择面积计算即可
#一般需要与目的蛋白的分子量相差5kd以上。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Image J 对Western blot 条带进行灰度分析
Image J软件:Windows 版本
第一步:软件安装
1.下载地址:/ij/download.html
2.下载windows 32位带Java版本,双击软件安装。
第二步:界面介绍
1.软件界面
第三步:图片分析
1.下图为样板图片
2.导入图片:File> Open> Sample1.jpg
图片导入后,Sample1.jpg在新的窗口中打开
3.图片类型设置:Image> Type> 8 bit
4.去除图片背景:Process> Subtract Background> …
4.1Subtract Background 窗口:Rolling ball radius 设为50 pixels,
勾选light background,可选preview,点击“OK”确定
5.工具栏:选择“矩形选框”
6.最大化“Sample1.jpg”窗口,便于矩形选择,矩形选择第一个泳道
7.标记“矩形选框”为1:矩形选择后,调整矩形至合适大小,按下数字键
“1”,或者Analyze> Gels> Select Fist Lane。
8.选择“泳道2”:拖动“1”的矩形框,会出现两个矩形选框,拖动矩形框
至泳道2,调整位置,并按下数字键“2”,Image J 会自动调整大小使“矩形框2”与“矩形框1”保持同一水平。
9.继续拖动,会出项新的“矩形选框”,调增至泳道3,并按下数字键“2”
(注意:是按下数字键“2”),重复9至6个泳道全部选择。
10.所有泳道选择后,按下数字键“3”,出现分析图谱。
11.“直线”封闭峰:工具栏选择“直线”,从起峰处至落峰处。
11.计算峰值:工具栏> Wand(tracing) tool,点下刚才封闭的区域,峰值结
果出现在新的窗口中。
计算结果:
12.其他泳道,以此类推,本文原创,如有疑问或建议,欢迎Email
biowhb@
(注:本资料素材和资料部分来自网络,仅供参考。
请预览后才下载,期待您的好评与关注!)。
