Guidance for Industry Part 11,Electronic Records;Electronic Signatures -Scope and Application
氮等离子体掺杂石墨烯 掺杂位置

英文回答:Our country is vigorously promoting the research and application of nitrogen plasma—mixed graphite technology, which introduces nitrogen atoms into graphite structures through plasma treatment to change their properties and properties. Nitrogen plasma mixing can be achieved by chemical gas deposition, ion injection, etc. Some carbon atoms may be replaced by nitrogen atoms, acting as plasmas, forming nitrogen—blended graphite structures. This technology can effectively change the electronic structure of graphene to provide better conductivity and photoelectric properties, while increasing chemical reaction activity and expanding applications in such areas as catalysts, sensors, etc. This technology is in line with our innovation—driven development strategy and is expected to make an important contribution to the development of our material sciences and technological innovation.我国正在大力推动氮等离子体掺杂石墨烯技术的研究与应用,该技术是通过等离子体处理方法将氮原子引入石墨烯的结构中,以改变其性质和特性。
A国内外焊接标准

DIN德国标准
EN欧洲标准
ISO国标标准化组织
JIS日本工业标准
DIN 1733 铜和铜合金用焊接填加剂成份.应用及交货技术条件
DIN EN ISO 18274 焊接消耗品.镍和镍合金熔焊用实心焊丝、条和棒材.分类DIN EN ISO 14172 焊接消耗品.镍和镍合金的手工金属弧焊接的药皮电焊条DIN 1787 铜,半成品
DIN EN ISO 14731 焊接协作任务及职责
DIN EN 729-3焊接质量要求,金属材料熔焊
其中DIN EN ISO 18274 焊接消耗品.镍和镍合金熔焊用实心焊丝、条和棒材.分类
DIN EN ISO 14172 焊接消耗品.镍和镍合金的手工金属弧焊接的药皮电焊条ISO 3834《焊接的质量要求金属材料的熔化焊》(共5个部分)(GB/T12467) ISO 14731 《焊接管理任务与职责》(GB/T19419)
BS EN 287《熔化焊焊工考试》(共3个分册)
EN 1418 《自动熔化焊及电阻焊焊接操作工考试》
EN ISO 9606《熔化焊焊工考试》(共5个分册)
EN ISO 14732《自动熔化焊及电阻焊焊接操作工考试》
美国标准
AWS D1.1《钢结构焊接规范》(AWS标准)
AWS QC1《美国焊接学会焊接检验师认证标准》(AWS标准)
SNT-TC 1A《无损检测人员资格评定及指南》(ASNT标准)
CP-189《无损检测人员资格评定》(ANSI/ASNT标准)
新上传国内外焊接标准(081107)。
IEC标准及电力系统常用单词_20121008

第一部分序号标准编号1 IEC 60050(151)2 IEC 60050(441)3 IEC 60050(601)4 IEC 600595 IEC 600506 IEC 60071-17 IEC 604178 IEC604179IEC60417M1 0IEC 60417N1 1IEC 6041701 2IEC 60417P13 IEC 60617-114 IEC 60617-215 IEC 60617-316 IEC 60617-4 17 IEC 60617-518 IEC 60617-619 IEC 60617-720 IEC 60617-821 IEC 60617-922 IEC 60617-1023 IEC 60617-11国际通用技术标准IEC 清单标准名称International Electro-technical VocabularyPart 51: Electrical andMagnetic DevicesChapter 441: Switchgear,Control gear and fusesChapter 601:Generation,Transmission anddistribution ofelectrical – GeneralIEC Standard CurrentRatingsInternational Electro-technical VocabularyInsulation Co-ordinationPart 2: Application GuideGraphical Symbols forUse on Equipment,Index, Survey andComplication of SingleSheetEleventh SupplementTwelfth SupplementThirteenth SupplementIEC 417 SupplementFifteenth Supplement Graphical Symbols for Diagrams - Part 1: General Information, General Index Cross-Reference Table Graphical Symbols for Diagrams - Part 2: Symbols Elements and other Symbols having general application Graphical Symbols for Diagrams - Part 2: Conductors and Connecting DevicesGraphical Symbols for Diagrams - Part 4:Passive ComponentsGraphical Symbols for Diagrams - Part 5: Semiconductors and Electron TubesGraphical Symbols for Diagrams - Part 6:Production and Conversion of Electrical Energy Graphical Symbols for Diagrams - Part 7: Switchgear, Control gear and Protective Devices Graphical Symbols for Diagrams – Part 8:Measuring Instruments, Lamps and Signaling Devices Graphical Symbols for Diagrams – Part 9: Telecommunication Switching and Peripheral Equipment Graphical Symbols for Diagrams – Part 10: Telecommunication and TransmissionGraphical Symbols for Diagrams – Part 11: Architectural and Graphical Installation Plans and Diagram24 IEC 60617-12Graphical Symbols for Diagrams – Part 12:Binary Logic Elements25 IEC 60617-13Graphical Symbols for Diagrams – Part 13:Analogue Elements26 Insulation Media27 IEC 60156 Insulation Liquids – Determination of the Breakdown Voltage at Power Frequency – Test Method28 IEC 60247Measurement of Relative Permittivity DielectricDissipation Factor and D.C. Resistivity of Insulation Liquids29 IEC 60296 Specification for Unused mineral Insulation Oils for Transformers and Switchgear30 IEC 60376Specification and Acceptance of NewSulphur Hexafluoride31 IEC 60376A First Supplement: Section Thirteen. Mineral Oil Content32 IEC 60376B Second Supplement: Clause 2633 IEC 60475 Method of Sampling Liquid Dielectrics34 Guide to the Checking of SF6 Taken From Electrical Equipment35 High Voltage Test36 IEC 60052 Recommendations for Voltage measurement by Means of Sphere-Gaps (one sphere earthed)37 IEC 60060-1High Voltage Test Techniques Part-I: GeneralDefinitions and Test Requirements38 IEC 60060-2High Voltage Test Techniques Part-2:Measuring Systems39 IEC 60060-2-aml Amendment No.1 to IEC 60060-240 IEC 60270 Partial Discharge Measurements41 IEC 60298 High Voltage Switchgear42 Power Transformer43 IEC 60076-1 Part 1: General44 IEC 60076-2 Part 2: Temperature Rise45 IEC 60076-3Part 3: Insulation Levels and Dielectric tests andExternal Clearances in Air46 IEC 60076-4 Guide to Lighting, Impulse Switching, Impulse Testing - Power Transformers and Reactors47 IEC 60076-5 Part 5: Ability to Withstand Short-Circuit48 IEC 60076-7 Loading Guide for Oil-immersed Power Transformer49 IEC 60076-8 Power Transformer – Application Guide50 IEC 60076-10 Determination of Sound LevelsPart 14: Design and Applicaion of Liquid Immersed 51 IEC 60076-14 Power Transformers using high temperatureinsulation materials52 IEC 60214On-load Tap changerSwitchgear1 IEC 62271-1 Common Specifications2 IEC 62271-100High Voltage Switchgear and ControlgearPart 100: high Voltage Alternating Current Circuit Breakers3 IEC 62271-102 Part 102: High Voltage Alternating Current Disconnectors and Earthing Switches4 IEC 62271-110 Part 110: Inductive Load SwitchingPart 200: AC metal- enclosed switchgear and controlgearfor rated voltages above 1kV and up to and including 52kVPart 1 01: Synthetic Testing.Part(300): Guide for Seismic qualification ofhigh voltage alternating current circuit breakers.Part(303): Use and handling of SulphurHexafluoride (SF6) in high voltage switchgearand control gear.Part(307): Use of electronic and associated5 IEC 62271-200 technologies in auxiliary equipment ofswitchgear and control gear.Part 308: Guide for asymmetrical short-circuitbreaking test duty T 100aPart 309: TRV parameters of high voltageswitchgear and control gear for rated voltagesabove 1 kV and les than 100kV.Part 310: Electrical endurance testing of circuitbreakers rated 72.5kV and above.*(xxx) Final number to be given by IEC.6 IEC 60947 Low-Voltage Switchgear and ControlgearHV Switches Part 1: HV Switches for Rated VoltagesAbove 1kV and less than 52kV7 IEC 60265-18 IEC 60265-1-aml Amendment No.19 IEC 60265-1-am2Amendment No.2HV Switches Part 2: HV Switches for Rated10 IEC 60265-2Voltages of 52kV and ahove.11 IEC 60265-2-aml Amendment No.1Low-Voltage Switchgear and Controlgear IEC 60439-112 Assemblies 一 Part 1: Type Tested and PartiallyType Tested Assemblies.13 IEC 60439-1-aml Amendment No.114 IEC 60439-1-am2 Amendment No.2Low Voltage Switchgear and Cantralgear-15 IEC 60439-2 Assemblies 一 dart 2: Particular Requirementsfor Busbar Trunking Systems (Busways)16 IEC 60439-2-am117 IEC 60439-318 IEC 60439-3-aml19 IEC 60439-420 IEC 60439-4-aml21 IEC 60439-522 IEC 60943Instrument Transformers:Amendment No.1Low Voltage Switchgear and Controlgear18Assemblies Part 3:Particular RequirementsFo19r Law-Voltage Switchgear and ControlgearAsse20mblies Intended to be Installed in Placeswhere 21Unskilled Persons have Access for theirUse-Dist22ribution Boards_Amendment No.1Low Voltage Switchgear and ControlgearAssemblies Part 4: Particular Requirementsfar Assemblies for Construction Sites (ACS)Amendment No.1Low Voltage Switchgear and Controlgear Assemblies Part 5:Particular Requirements for Assemblies Intended to beInstalled Outdoors in Public Places 一 Cable Distribution Cabinets (CI3Cs} for Power Distribution in Networks.Guidance concerning the permissible temperature rise for parts of electrical equipment, in particular for equipments. Instrument Transformers:1 IEC 60044-1Part 1:Current Transformers2 IEC 60044-2 Part 2:Inductive Voltage Transformers3 IEC 60044-3Part 3:Combined Transformers.Part 6: Requirements for Protection Current 4 IEC 60044-6Transformers for Transient Performance.5 Relays6 IEC 60255 Electrical Relays7 Surge ArrestersSurge Arresters Part 1:Non-linear >Resistor8 IEC 60099-1Type Gapped Arresters far A.G. SystemsSurge Arresters Part 3:Artificial Pollution9 IEC 60099-3Testing of Surge Arresters.Surge Arresters 一 Part 4: Metal Oxide Surge10 IEC 60099-4Arresters Without Gaps far AC. System.Surge Arresters - Part 5: Selection and, Application11 IEC 60099-5Recommendations - Section General12 Telecommunication and Teleprotection:13 IEC 60663 Planning of (SSb) PLC Systems14 IEC 60353 Line Traps for A.C. Power Systems15 IEC 6D481 Coupling devices for PLC Systems16 IEC 60358 Coupling Capacitors anti Capacitor DividerBatteries:1 IEC 60896- Stationary Lead Acid Batteries-General Requirements and2 IEC 60896-1-aml3 IEC 60896-1-am24 IEC 60896-2Insulators and Bushings1 IEC 60 1372 IEC 601683 IEC 60168-aml4 IEC 602735 IEC 60383-16 IEC 60383-27 IEC 604378 IEC 604719 IEC 60471-aml10 IEC 6050611 IEC 7050712 IEC TR 6057513 IEC 60815Power & Control Cables 1 IEC 60183 2 IEC 60183-aml3 IEC 602304 IEC 602875 IEC 60287-1-16 IEC 60287-1-27 IEC 60287-2-1Methods of Test-Part Vented Types. Amendment No.1 Amendment No.2Stationary Lead Acid Batteries-General Requirements and Test Methods-Part 2: Vale Regulated Types.Insulating Bushings for Alternating Voltages above 1000VTest an Indoor and outdoor Past Insulator of Ceramic Material or Glass for System with Nominal Voltages Greater than 1000VAmendment No.1 Characteristics of Indoor and Outdoor Post Insulators Far Systems with Normal Voltages theater than 1000 V.Insulators far Overhead Lines with a Nominal Voltage above 10}}V Part 1:Ceramic or Glass Insulator Units for A.C Systems-Definitions, Test Methods and Acceptance Criteria. Insulators for overhead Lines with a Nominal Voltage above IOQ}V Fart 2: Insulator Strings and Insulator Sets for A.C. Systems- Definitions, Test Methods and Acceptance Criteria. Radio Interference Test on High Voltage Insulators.Din3ensions of Cle}is and Tongue Couplings of String Insulator Units.Amendment No.1 Switching Impulse Tests on High Voltage Insulators Artificial Pollution Tests on High Voltage Insulators to he Used on A.C SystemsThermal-Mechanical, Performance Test and Mechanical Performance Test on String Insulator UnitsGuide far the Selection of Insulators in respect of polluted conditions.Guide to the Selection of High Voltage Cables Amendment No.1Impulse Tests an Cables and their AccessoriesElectric Cables-Calculation of the Current Rating Part 1: Current Rating Equations ( 100% load factor) and Calculation of Losses-Section l: GeneralAmendment No.1Electric Cables-Calculation of the Current Rating-Part 1: Current Rating Equations ( 100% load factor ) and Calculation of Losses-Section 2: Sheath Eddy Current loss Factors for Two Circuits in Flat FormationElectric Cables-Calculation of the Current Rating-Part 2:Thermal Resistance-Section 1:Calculation of ThermalResistanceElectric Cables-Calculation of the Current Rating-Part 2:A Method for Calculating Reduction Factors forGroups of8 IEC 60287-2-2Ca61es in Free Air, Protected from Solar Radiation.Electric Cables-Calculation of the Current Rating-Part3: Sections on Operating Conditions-Section- 1:Reference Operating Conditions and Selection of CableType.Electric Cables-Calculation of the Curret Rating-Part 9 IEC 60287-3-2 3:Sections on Operating Conditions-Section 1:Economic Optimization of Power Cable Size.10 IEC 60287-3-2-aml Amendment No.1 to IEC 287-3-211 IEC 60228 Nominal gross-sectional areas and composition of conductors of insulated cables.12 IEC 60811Common test methods for insulating and sheaths material of electric ( Series) cables.Power Cables with Extruded Insulation and their Accessories13 IEC 60502-1for Rated Voltages from 1 kV (Um=1.2kV) up to 30kV (Um=36kV)-Part l: Cables for Rated Voltages from 1 kV (Um=1.2kV) and 3kV (Um=3.6kV)Power Cables with Extruded Insulation and their Accessories14 IEC 60502-2for Rated Voltages from 1 kV (Um=1.2kV) up to 30kV (Um=36kV)-Part 2: Cables for Rated Voltages from 6 kV (Um=7.2kV) up to 30kV (Um=36kV)Power Cables with Extruded Insulation and their Accessories15 IEC 60502-4 for Rated Voltages from 1 kV (Um=1.2kV) up to 30kV (Um=36kV)-Part 4: Cables for Rated Voltages from 6 kV (Um=7.2kV) up to 30kV (Um=36kV)16 IEC 60529 Degree of protection provided by Enclosures (IP Code).Electromagnetic compatibility (EMC), Part }-3: Tested and17 Measurement Techniques-Radiated, Radio Frequency,electromagnetic Field Immunity Test.18 IEC 61000-4-2 Testing and Measurement Techniques- Electrostatic Discharge Immunity Test.1 234 5Steel StructuresASTM A361/A36M-97aASTM A36/A36M-97aASTM A 143-74(Reapproved 1994)ASTM A153/A153M-98ASTM A325-97Standard Specification for Carbon Structural SteelStandard Specification far Zinc( Hat-Dip Galvanized}Coatings on Iron and Steel ProductsStandard Practice far Safeguarding againstEmbrittlement of Hot-Dip Galvanized Structural SteelProducts and Procedure for Detecting EmbrittlementStandard 5pecifcatian for Zinc Coating (Hot-Dip) on Iran andSteel HardwareStandard Specification far High-strength Bolts far67 8910111213141516171819202122232425262728 29ASTM A387-76(Reapproved 1996)ASTM A394-93ASTMA572/A572M-97cASTM b201ASTM E94 -93ASTM E709-95ISO R898Overhead ConductorsASTMB-231,J1SH-2110. I EC61089(1991-06)IEC61089-aml(1997-05)Civil Works:BSASTMACIISOASTM A615-96aASTM C33-99ASTM C150-98ASTM C260-98ASTM C494-98aASTM D1556-90(Reapproved 1998)ASTM D1557-91(Reapproved 1998)ASTM D2419-95ASTM D2937-97IEC 61850IEC 60255-24 IEC 60255-26 IEC6005 0-444 IEC6005 0-445 IEC6005 0-446Structural Steel JointsStandard Practice forSafeguarding againstWarpage and DistortionDuring Hot-DipGalvanizing of SteelAssembliesStandard Specificationfor Steel TransmissionTower Bolts, ZincCoated and BareStandard Specificationfor High StrengthLow-AlloyColumbium-VanadiumStructural SteelStandard Practice forTesting ChromaticCoatings zinc andCadmium SurfacesStandard Guide forRadiographic TestingStandard Guide forMagnetic ParticleExaminationMechanical Properties ofFastenersRound WireConcentric LayOverhead ElectricalStrandedConductors.Amendment No.1British StandardsAmerican Society For Testing and MaterialsAmerican Concrete InstituteInternational Standard OrganizationStandard Specification for Deformed and PlainBillet-Steel Bars for Concrete ReinforcementStandard Specification for Concrete AggregatesStandard Specification for Portland CementStandard Specification for Air EntrainingAdmixtures for ConcreteStandard Specification for Chemical Admixtures for ConcreteStandard Test Method for Density arid Unit Weight of Soil inPlace by Sand-Cone MethodTest Method for Laboratory Compaction Characteristics ofSoil Using Modified EffortStandard Test Method for Sand Equivalent Value of Soils andFine AggregateStandard Test Method for Density of Soil inPlace by the Drive 一 Cylinder MethodCommunication Networks and Systems in SubstationElectrical relaysMeasuring Relays and Protection EquipmentInternational Electrotechnical Vocabulary:Elementary RelaysInternational Electrotechnical Vocabulary:Specified Time Allor Nothing RelaysInternational Electrotechnical Vocabulary: Electrical relaysIEC60050-448International Electrotechnical Vocabulary: Power SystemProtectionIEC 60839 Alarm SystemsIEC61000-4-6 Electromagnetic Compatibility(EMC)IEEE C37.2 IEEE Standard Electrical Power System Device Function Numbers and Contact DesignationsIEC 61037 Electricity Metering-Tariff and Load Control IEC 62040Uninterruptible Power Systems第二部分电力系统常用单词中文 英文 变电站内电气设备 电力变压器 Power transformer 自耦变压器 Auto-transformer 断路器/短路开关 Circuit-breaker 隔离器/隔离开关 Disconnector /Disconnecting link 隔离开关带接地开关 Disconnector with earthing switch 电流互感器 Current transformer电容式电压互感器 Capacitor voltage transformer带滤波装置的电容式电压 Capacitor voltage transformer with 互感器 carrier frequency facility避雷器 Surge arrester/lightning arrester并联电抗器 Shunt reactor电容器组 Capacitor banks电力电容器 Power capacitor阻波器 Line trap支柱绝缘子 Post insulator 12345678 9 10 11 12 13 14 15 16序号备注23 架空地线/避雷线 Overhead ground wire24 三相联动 Three poles operation25 单相操作 Single pole operation26 开关柜(中压) Switchgear27 配电柜 Switchboard28 套管式电流互感器 Bushing type current transformer29 并联电容器 Shunt capacitor30 高压开关设备 High-voltage switchgear31 零序电流互感器 Zero sequence current transformer(ZCT)二变电站内功能房间1 主控制楼 Control house building2 通讯机房 Telecom room3 工器具间 Store room4 会议室 Conference room5 资料室 Reference room6 休息室 Resting room7 交接班室 Shifting room8 蓄电池室 Battery room9 继电器室 Relay room10 站长室 Station master room11 厨房 Kitchen12 淋浴间 Shower room13 柴油发电机房 Diesel generator room14 站用变 Auxiliary transformer三变电站内其它常用名词1 户外配电装置/开关站 Switchyard2 35KV 高压室 35KV switchgear room3 油池 Oil tank4 轨道 Rail5 架空 Overhead6 避雷线/针 Lightning conductor/wire7 构架 Gantry8 围墙 Boundary wall9 前面/屏前/正面Front10 后面/屏后Rear11 进线Incoming12 出线Outgoing13 电器开关室/配电室Switchboard room /Distributionroom14 开关柜(中高压)Switchgear。
译林版高考英语一轮总复习课后习题 选择性必修第一册Unit 3

选择性必修第一册Unit3Ⅰ.阅读理解A(福建龙岩三模)Ruth Bader Ginsburg spent a lifetime flourishing(茁壮成长) in the face of misfortunes before being appointed a Supreme Court justice.She was born on March 15,1933 in Brooklyn,NewYork.Ginsburg’s mother implanted a love of education in Ginsburg through her devotion to her brother.She graduated first in her class at Columbia Law in 1959.Even her eic record was not enough to shelter her from the gender-based discrimination women faced in the workplace in the 1960s.She had difficulties finding a job until a favorite Columbia professor directly refused to recommend any other graduates before U.S.District Judge Edmund L hired Ginsburg as a clerk for two years.After this,she was offered some jobs at law firms,but always at a much lower salary than her male colleagues.She instead took some time to pursue her other legal passion,civilprocedure,choosing to join the Columbia Project on International Civil Procedure.In 1963,she accepted a job as a professor at Rutgers University Law School.And in 1972,she became the first female professor at Columbia to earn tenure(终身职位).Ginsburg also directed the influential Women’s Rights Project of the American Civil Liberties Union during the 1970s.In this position,she led the fight against gender discrimination and successfully argued six landmark cases before the U.S.Supreme Court.Ginsburg took a broad look at gender discrimination,fighting not just for the women left behind,but for the men who were discriminated against as well.Ginsburg accepted Jimmy Carter’s appointment to the U.S.Court of Appeals for the District of Columbia in 1980.She served on the court for thirteen years until 1993,when Bill Clinton nominated(提名) her to the Supreme Court of the United States.Ginsburg began her career as a justice where she left off as an advocate fighting for women’s rights.Overtime,Justice Ginsburgproved time and again that she was a force to count until her death in .1.What can be learned about Ginsburg from the first paragraph?A.She was a successful lawyer.B.She was a top student of law.C.She got inspired by her brother.D.She studied law due to her mother.2.What is true about women in the workplace in the 1960s?A.They faced racial discrimination.B.They were offered legal guidance.C.They loved to teach at a law school.D.They got less paid than male colleagues.3.Which of the following can best describe Ginsburg?A.Devoted and competent.anized and tolerant.C.Skilled and independent.D.Determined and imaginative.4.What can be the best title for the teising Chief JusticeB.An Influential Legal FigureC.An Inspiring Political PowerD.A Courageous Freedom FighterB(安徽皖南八校三模)What does it take to become an astronaut?It’s a question that’s been asked since the start of the Space Age in the 1960s.In those days,pilots were considered the most well-trained professionals,so military fliers were first in line to go to space.More recently,people from a wide range of professional backgrounds—doctors,scientists,and even teachers—have trained to live and work in near-Earth orbit.Even so,those selected to go to space must meet high standards.People who want to become astronauts must be in top physical condition.Each country’s space program has health requirements for its space travelers.They usually asses s a candidate’s fitness to withstand some pretty tough conditions.For eust have the abilityto endure the rigors of lift-off and to function in weightlessness.All astronauts must have good visual acuity and normal blood pressure.Beyond that,there is no age limit.Most astronaut trainees are between the ages of 25 and 46,although older people have also flown to space later in their careers.People who go to space are usuallyself-confident,risk-takers,adept at stress management and multitasking.They also need to be able to work as part of a team for any given assignment.On Earth,astronauts are usually required to perform various public relations duties,such as speaking to the public,working with other professionals,and sometimes even testifying before government officials.So,astronauts who can relate well to many different kinds of people are seen as valuable team members.Often,astronauts have a background as scientists and many have high-level degrees,like Ph.Ds.Others have military training or space industry expertise.Regardless of their background,once anastronaut is accepted into a country’s space program,he or she goes through rigorous training to actually live and work in space.5.Which of the following is of least importance to an astronaut?A.Normal blood pressure.B.Good eyesight.C.Tough body.D.Young age.6.Why are astronauts asked to perform public duties?A.To make them famous among people.B.To relieve their feeling of tension.C.To raise their awareness of teamwork.D.To promote public interest in the aerospace.7.What could be the best title for the passage?A.Everyone Can Be an AstronautB.The Professional Qualities of an AstronautC.Training Astronauts Is Much Easier NowadaysD.It’s Not Mysterious to Be an AstronautⅡ.完形填空(河北石家庄二模)I started cooking when I was thirteen.Both of my parents worked,so I was usually 1 after school.One day,my parents forgot to 2 dinner before they went to work.As we were short of money,eating out was beyond 3 .So I decided to be the 4 of the day.A few moments later,I 5 to cook fried rice,the best option out of 6 resources,including my cooking knowledge.After 7 a pan on the stove and turning up the heat,it soon began to sizzle.I was so hungry that I 8 and threw everything into the pan all together.In went rice,pieces of meat and vegetables.Little did I know that the 9 of ingredients(食材) was crucial in cooking.I 10 waited for the meat to be thoroughly cooked,but other ingredients were 11 .At first,I mistook it as a steam from cooking,but I soon 12 something went horribly wrong when smoke 13 to fill the kitchen.Later that day,my mother told me,“You cannot rush yourself when cooking.” Rather,I should take steps,turning to thebasics,such as learning about the ingredients and 14 the recipe.I’ve learned that the first 15 seems hard but we should just start and let the journey teach us.1.A.alone B.energeticC.anxiousD.excited2.A.enjoy B.prepareC.serveD.take3.A.expectation B.controlC.descriptionD.budget4.A.owner B.rulerC.chefD.researcher5.A.agreed B.settledC.failedD.switched6.A.various B.deliciousC.priceyD.limited7.A.placing B.coveringC.washingD.breaking8.A.quit B.sleptC.rushedD.collapsed9.A.standard B.orderC.qualityD.amount10.A.proudly B.unconsciouslyC.fearfullyD.eagerly11.A.burning B.eixing12.A.insisted B.declaredC.graspedD.explained13.A.stopped B.reducedC.eerged14.A.working on B.fixing onC.referring toD.contributing to15.A.race B.tryC.routeD.ride参考答案选择性必修第一册Unit3Ⅰ.【语篇导读】本文是一篇记叙文。
中国焊接标准(GB welding standards)

GB/T19867.4-2008
激光焊接工艺规程
Weldingprocedurespecificationforlaserbeamwelding
GB/T19867.5-2008
电阻焊焊接工艺规程
Weldingprocedurespecificationforresistancewelding
火力发电厂锅炉汽包焊接修复技术导则
()
DL/T752-2010
火力发电厂异种钢焊接技术规程
()
DL/T754-2001
铝母线焊接技术规程
()
DL/T768.6-2002
电力金具制造质量焊接件
()
DL/T816-2003
电力工业焊接操作技能教师资格考核规则
()
DL/T819-2002
火力发电厂焊接热处理技术规程
GB20262-2006
焊接、切割及类似工艺用气瓶减压器安全规范
Safetyspecificationsofregulatorsforwelding,cuttingandthesimilarprocesses
GB24159-2009
焊接绝热气瓶
Weldedinsulatedcylinders
GB50128-2005
GB50341-2003
立式圆筒形钢制焊接油罐设计规范
Codefordesignofverticalcylindricalweldedsteeloiltanks
GB5100-1994
钢质焊接气瓶
Weldedsteelgascylinders
GB6653-2008
guidance for industry 指导原则

"Guidance for Industry" 通常指的是由政府监管机构、行业协会或标准化组织发布的一系列建议、指导原则或最佳实践,旨在为特定行业或领域内的企业、从业人员和利益相关者提供方向、解释和规范性建议。
这些指导原则可能涉及产品质量、安全性、合规性、制造工艺、环境保护、标签和包装要求等方面。
发布这些指导原则的目的通常包括:
确保安全和合规:通过提供明确的期望和标准,帮助行业参与者遵守相关法律法规,从而确保产品、服务和运营的安全性和合规性。
促进一致性:在行业内推广统一的标准和做法,有助于减少差异,提高产品和服务的质量和可靠性。
提高效率和创新:通过提供最佳实践和推荐的技术解决方案,帮助企业改进流程、减少浪费、提升效率,并鼓励技术创新和持续改进。
保护消费者权益:确保消费者能够获得准确的信息,并在使用产品或接受服务时享有适当的保护。
促进国际贸易:在全球范围内统一或协调标准和指导原则,有助于减少贸易壁垒,促进国际贸易和合作。
环境保护和可持续发展:提供关于环境保护、资源利用和废物管理的指导,支持行业的可持续发展。
这些指导原则通常不是强制性的法律要求,但它们往往反映了监管机构的期望和行业标准,因此遵守这些指导原则通常被视为良好的业务实践。
在某些情况下,不遵守这些指导原则可能会导致监管机构的审查、处罚或声誉损害。
因此,行业内的企业和个人通常会认真考虑并遵循这些指导原则。
IEC60601系列对应的国标医疗器械通用、专用要求
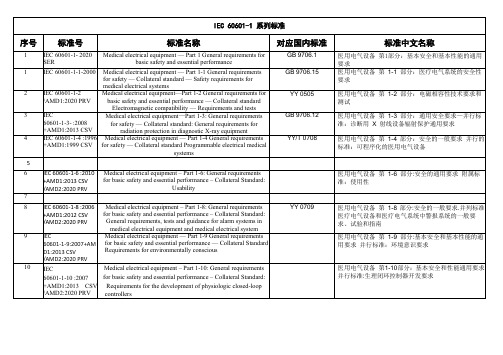
对应国内标准
GB 9706.5
GB 9706.4 YY 91086 YY 91087 GB 9706.8 GB 9706.7
6 IEC 60601-2-6:2012 +AMD1:2016 CSV
标准名称
Medical electrical equipment — Part 2-1 Particular requirements for the safety of electron accelerators in the range 1 MeV to 50
2 IEC 60601-1-2
Medical electrical equipment—Part 1-2 General requirements for
/AMD1:2020 PRV
basic safety and essential performance — Collateral standard
11 IEC 0601-2-11:2013
Medical electrical equipment — Part 2-11 Particular requirements for safety — Section 2.11 Specification for gamma beam therapy equipment
Electromagnetic compatibility — Requirements and tests
3 IEC
Medical electrical equipment—Part 1-3: General requirements
中国焊接标准(GB welding standards)
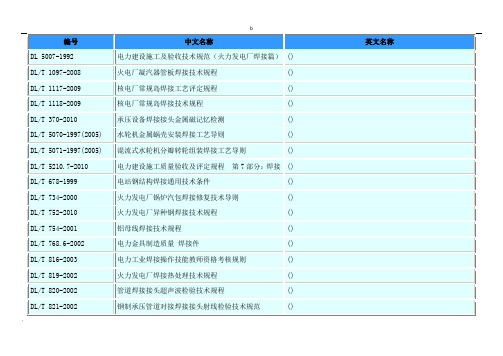
GB/T 15970.8-2005 GB/T 16957-1997 GB/T 18256-2000
GB/T 18591-2001
Welding--Guidance on the measurement of preheating 焊接预热温度、道间温度及预热维持温度的测量指南 temperature,interpass temperature and preheat maintenance temperature 印制板组装第 1 部分:通用规范采用表面安装和相关 组装技术的电子和电气焊接组装的要求 Printed board assemblies--Part 1: Generic specification--Requirements for soldered electrical and electronic assemblies using surface mount and related assembly technologies
英文名称
第 7 部分:焊接 () () () () () () () () () ()
b
DL/T 868-2004 DL/T 869-2004 DL/T 905-2004 FZ 92065-2006 GB 13075-1999 GB 15579.1-2004 GB 15579.12-1998 GB 17268-2009 GB 17673-1999 GB 17878-2009 GB 20262-2006 GB 24159-2009 GB 50128-2005 GB 50236-1998
() () () Stainless steel welding cylinder Periodic inspection and evaluation of welded steel gas cylinders Arc welding equipment--Part 1:Welding power sources Safety requirements for arc welding equipment--Part 12:Coupling devices for welding cables Non-refillable steel welded cylinders for industrial use Welded steel cylinders for liquefied propylene and propane gases Cylinder valve for non-refillable steel welded industry cylinder Safety specifications of regulators for welding, cutting and the similar processes Welded insulated cylinders Code for construction and acceptance of vertical cylindrical steel welded storage tanks Code for construction and acceptance of field
Guidance for Industry Changes to an Approved NDA or ANDA Q&A 中英对照

Guidance forIndustry Changes to an Approved NDA or ANDA Questions and Answers目录REPORTING CATEGORIES报告类别 (1)GENERAL REQUIREMENTS一般要求 (2)MANUFACTURING SITES生产地址 (3)MANUFACTURING PROCESS生产工艺 (8)SPECIFICATIONS规程 (12)PACKAGE包装 (13)MISCELLANEOUS CHANGES多重变更 (15)Guidance for Industry1Changes to an Approved NDA or ANDAQuestions and AnswersThis document provides questions and answers relating to the guidance on Changes to an Approved NDA or ANDA (the guidance).2 The questions are based on those posed to CDER by applicants. The questions and answers are presented using subject headings that correspond to the table of contents in the guidance.本文提供指南文件的常见问题及解答。
常见问题来源于申请人向CDER提出的问题。
常见问题及解答按照指南文件的目录顺序列表。
REPORTING CATEGORIES报告类别Q1: For a change that is reported in a Supplement — Changes Being Effected in 30 Days, will CDER complete the review of the supplement within 30 Days?问1:对于按照30日内生效变更申请提交的报告,CDER是否能在30日内将增补文件审核完毕?A1: Within 30 days CDER will notify the applicant that prior approval isrequired for the change (i.e., CDER has designated the supplement a priorapproval supplement) or that the FDA has determined appropriate information is missing, including information that should have been developed by theapplicant in assessing the effects of the change. Supplement reviews will beperformed consistent with standard procedures. It is unlikely that a substantive review and action letter will be completed within 30 days.答1:30日内,CDER确定增补应该按照批准前增补提交,将通知申请人该项事务;或者FDA判断应有的信息存在缺陷,包括申请人应当开展的变更影响评估报告,也会通知申请人。
中国焊接标准gb welding standards)
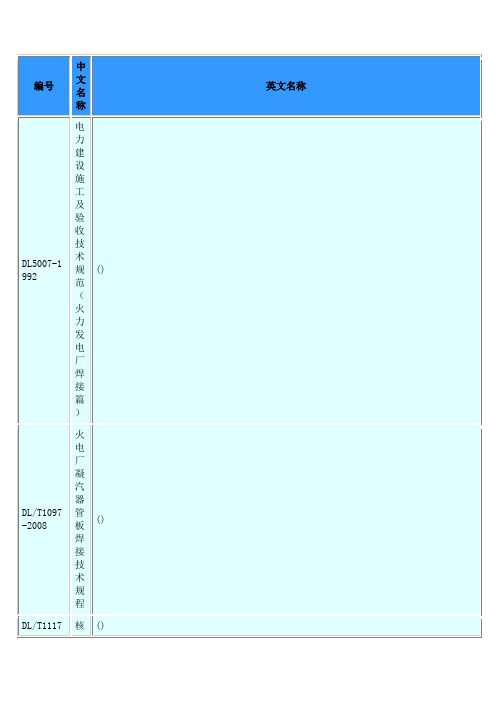
防护服装阻燃防护第2部分:焊接服
Protectiveclothing-Flame-retardantprotection-Part2:Protectiveclothingforwelders
GB9448-1999
焊接与切割安全
Safetyinweldingandcutting
GB/T16957-1997
复合钢板焊接接头力学性能试验方法
Mechanicaltestsonweldedjointsincladplates
GB/T18256-2000
焊接钢管(埋弧焊除外)用于确认水压密实性的超声波检测方法
Weldedsteeltubes(exceptsubmergedarc-welded)--Ultrasonictestingforverificationofhydraulicleak-tightness
火力发电厂锅炉汽包焊接修复技术导则
()
DL/T752-2010
火力发电厂异种钢焊接技术规程
()
DL/T754-2001
铝母线焊接技术规程
()
DL/T768.6-200T816-2003
电力工业焊接操作技能教师资格考核规则
()
DL/T819-2002
火力发电厂焊接热处理技术规程
GB/T19867.1-2005
电弧焊焊接工艺规程
Weldingprocedurespecificationforarcwelding
GB/T19867.2-2008
气焊焊接工艺规程
Weldingprocedurespecificationforgaswelding
GB/T19867.3-2008
电子束焊接工艺规程
热喷涂国标

热喷涂自熔合金涂层(由GB/T 16744-2002代替)
GB/T 13222-91
金属热喷涂层剪切强度的测定
GB/T 12608-90
热喷涂涂层材料命名方法(由GB/T 12608-2003和GB/T 19356-2003代替)
GB/T 12607-90
热喷涂涂层设计命名方法(由GB/T 12607-2003代替)
热喷涂热喷涂结构的质量要求第4部分:基本的质量要求
GB/T 19355-2003
钢铁结构耐腐蚀防护锌和铝覆盖层指南(ISO 14713:1999,MOD)
GB/T 19356-2003
热喷涂粉末成分和供货技术条件(ISO 14232:2000,MOD)(代替GB/T 12608-1990)
GB/T 8642-2002
1991代替gb979388gb979488gb979588gb979688gbt167441997热喷涂自熔合金涂层由gbt167442002代替gbt1322291金属热喷涂层剪切强度的测定gbt1260890热喷涂涂层材料命名方法由gbt126082003和gbt193562003代替gbt1260790热喷涂涂层设计命名方法由gbt126072003代替gb1137489热喷涂涂层厚度的无损测量方法gb1137389热喷涂金属件表面预处理通则gb979688热喷涂铝及铝合金涂层试验方法iso2063由gbt97931997代替gb979488热喷涂锌及锌合金涂层试验方法iso2063由gbt97931997代替gb864288热喷涂层结合强度的测定由gbt86422002代替gb864188热喷涂层抗拉强度的测定gb864088金属热喷涂层表面洛氏硬度试验方法jbt41081999热喷涂设备分类及型号编制方法jbt842596铁基喷涂粉末中铬镍钼和钒的x射线荧光光谱分析标准试验方法jbt842796钢结构腐蚀防护热喷涂锌铝及其合金涂层选择与应用导则jbt770395热喷涂陶瓷涂层技术条件jbt750994热喷涂涂层孔隙率试验方法铁试剂法jbt697393热喷涂操作人员考核要求jbt697493线材喷涂碳钢及不锈钢jbt507091热喷涂常用术语ybt036131992冶金设备制造通用技术条件氧乙炔焰金属粉末喷涂yst5793热喷涂用fecrbsi系合金粉hgt20442003机械密封用喷涂氧化铬密封环技术条件代替hgt20441991yy03041998等离子喷涂羟基磷灰石涂层钛基牙种植体astm标准theamericansocietymaterialsastmb83301astandardspecificationzincalloywirethermalsprayingmetallizingastmb90704standardspecificationzinctincadmiumbasealloysusedthermalsprayingastmc63301standardtestmethodcohesionstrengththermalspraycoatingsastmd4541testmethodpulloffstrengthcoatingusingportableadhesiontestersast
中国接头标准(GB joint standards)
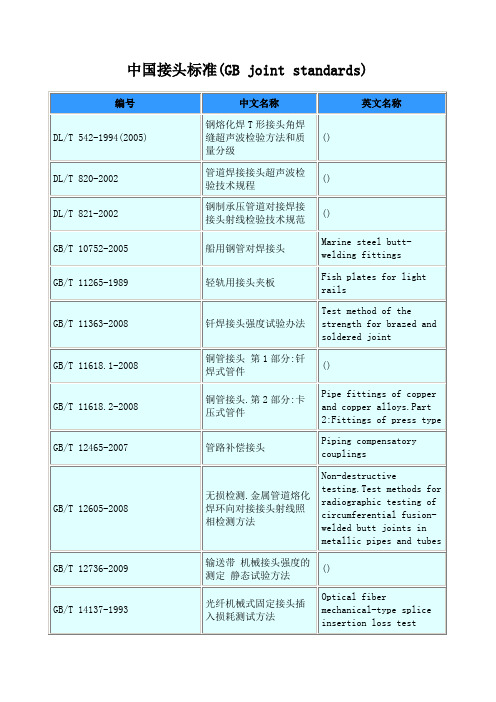
Piping compensatory couplings
GB/T 12605-2008
无损检测.金属管道熔化焊环向对接接头射线照相检测方法
Non-destructive testing.Test methods for radiographic testing of circumferential fusion-welded butt joints in metallic pipes and tubes
GB/T 19674.1-2005
液压管接头用螺纹油口和柱端 螺纹油口
Connections for general use and fluid power Ports and stud ends with thread-Threaded port
GB/T 19674.2-2005
液压管接头用螺纹油口和柱端 填料密封柱端(A型和E型)
GB/T 22087-2008
铝及铝合金的弧焊接头.缺欠质量分级指南
Arc-welded joints in aluminium and its alloys.Guidance on quality levels for imperfection
GB/T 23682-2009
制冷系统和热泵 软管件、隔震管和膨胀接头 要求、设计与安装
GB/T 1962.1-2001
注射器、注射针及其他医疗器械6%(鲁尔)圆锥接头 第1部分;通用要求
The conical fittings with a6%(Luer)taper for syringes,needles andcertain other medical equipment Part 1:General requirement
电化学电容器电极材料的制备及其电容性能研究

电化学电容器电极材料的制备及其电容性能研究一、本文概述Overview of this article随着科技的快速发展和新能源技术的广泛应用,电化学电容器作为一种高效能量储存与转换器件,在现代电子设备和电动汽车等领域中扮演着日益重要的角色。
而电极材料作为电化学电容器的核心组件,其性能直接影响着电容器的整体性能。
因此,研究和开发高性能的电化学电容器电极材料对于提升电容器性能、推动新能源技术的发展具有重要意义。
With the rapid development of technology and the widespread application of new energy technologies, electrochemical capacitors, as an efficient energy storage and converter device, play an increasingly important role in modern electronic devices and electric vehicles. As the core component of electrochemical capacitors, the performance of electrode materials directly affects the overall performance of the capacitor. Therefore, researching and developinghigh-performance electrode materials for electrochemicalcapacitors is of great significance for improving capacitor performance and promoting the development of new energy technologies.本文旨在探讨电化学电容器电极材料的制备方法,并深入研究其电容性能。
最新 21 CFR PART 11 企业指南 中英对照

Guidance for IndustryPart11,Electronic Records;Electronic Signatures —Scope and ApplicationAugust2003Pharmaceutical CGMPsFDA工业指南联邦法规11部分电子记录和电子签名—范围和应用2003年8月药物CGMPsTABLE OF CONTENTS目录I.Introduction (2)II.Background (3)III.Discussion (5)A.Overall Approach to Part11Requirements (5)B.Details of Approach-Scope of Part11 (6)1.Narrow Interpretation of Scope (6)2.Definition of Part11Records (7)C.Approach to Specific Part11Requirements (9)1.Validation (9)2.Audit Trail (9)3.Legacy Systems (10)4.Copies of Records (11)5.Record Retention (12)IV.REFERENCES (13)This guidance represents the Food and Drug Administration's(FDA's)current thinking on this topic.It does not create or confer any rights for or on any person and does not operate to bind FDA or the public.You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations.If you want to discuss an alternative approach,contact the FDA staff responsible for implementing this guidance.If you cannot identify the appropriate FDA staff,call the appropriate number listed on the title page of this guidance.I.IntroductionThis guidance is intended to describe the Food and Drug Administration's(FDA’s)current thinking regarding the scope and application of part11of Title 21of the Code of Federal Regulations;Electronic Records;Electronic Signatures(21CFR Part11).This document provides guidance to persons who,in fulfillment of a requirement in a statute or another part of FDA's regulations to maintain records or submit information to FDA,have chosen to maintain the records or submit designated information electronically and,as a result,have become subject to part11.Part11applies to records in electronic form that are created,modified,maintained,archived, retrieved,or transmitted under any records requirements set forth in Agency regulations.Part11 also applies to electronic records submitted to the Agency under the Federal Food,Drug,and Cosmetic Act(the Act)and the Public Health Service Act(the PHS Act),even if such records are not specifically identified in Agency regulations(§11.1).The underlying requirements set forth in the Act,PHS Act, and FDA regulations(other than part11)are referred to in this guidance document as predicate rules.As an outgrowth of its current good manufacturing practice(CGMP)initiative for human and animal drugs and biologics,FDA is re-examining part11as it applies to all FDA regulated products.We anticipate initiating rulemaking to change part11as a result of that re-examination.This guidance explains that we 本指南代表了FDA在此领域的当前思路,它不赋予任何人任何权利,也并非用于约束FDA或公众。
毒理学缩写词

Toxicological AbbreviationsMay 1998AA activation analysis; atomic absorptionAAF acetylaminofluoreneAAPCC American Association of Poison Control CentersAAS atomic absorption spectrophotometryABS acrylonitrile-butadiene-styreneACE angiotensin-converting enzymeACGIH American Conference of Governmental Industrial Hygienists ACh acetylcholineAChE acetylcholinesteraseAcP acid phosphataseACTH adrenocorticotropic hormoneAD Alzheimer's diseaseADH alcohol dehydrogenase; anti-diuretic hormoneADI acceptable daily intakeADP adenosine diphosphateAEP auditory evoked potentialAES atomic emission spectroscopyAFID alkali flame ionization detectionAh aromatic hydrocarbonAHH aryl hydrocarbon hydroxylaseai active ingredientAIDS acquired immune deficiency syndromeAIN acute interstitial nephritisALA aminolevulinic acidALAD ²-aminolaevulinic acid dehydrataseALAS o-aminolevulinic acid synthetaseALAT alanine aminotransferaseALC approximate lethal concentrationALD approximate lethal doseALMS atomic line molecular spectrometryALT alanine aminotransferaseAM alveolar macrophagesAMP amperometric titrationAMPA aminomethylphosphonic acidANTU alpha-naphthylthioureaAP alkaline phosphataseAPDM aminopyrine-N-demethylaseAPP ammonium polyphosphateAQG Air Quality GuidelinesARF acute renal failureARL acceptable or tolerable residue limitART alternate reproductive testASAT aspartate aminotransferaseASV anodic stripping voltametryATA alimentary toxic aleukiaATH alumina trihydrateATPase adenosine triphosphataseAUC area under the curveAWI acceptable or tolerable weekly intakeB bursa-dependentBA 2-bromoacroleinBAER brainstem auditory-evoked responseBAF bioaccumulation factorBAL bronchoalveolar lavageBBB blood-brain barrierBBPP bis(2,3-dibromopropyl) phosphateBCF bioconcentration factorBEA 2-bromoethylamineBEI biological exposure indexBEN Balkan endemic nephropathyBGG bovine gammaglobulinBHA butylated hydroxyanisoleBHK baby hamster kidneyBIC butyl isocyanateBMAA beta-N-methylamino-L-alanineBMD benchmark dose (US EPA)BMF biomagnification factorBOAA beta-N-oxalylamino-L-alanineBOD biochemical oxygen demandBP benzo[a]pyreneBSA bovine serum albuminBSP bromosulfophthaleinBUB 2-(3-butylureido)benzimidazoleBUN blood urea nitrogenbw body weightCA chrysanthemic acidCALLA common acute lymphoblastic leukaemia antigencAMP cyclic adenosine monophosphateCAS Chemical Abstracts ServiceCBF cerebral blood flowCC critical concentrationCCFA Codex Committee on Food Additives (see definition of Codex AlimentariousCommission)CCPR Codex Committee on Pesticide ResiduesCCTTE Computerized Listing of Chemicals Being Tested for Their Toxicological Effects(IPCS/IRPTC)CD cluster of differentiation; coulometric detectioncDNA complementary DNACE capillary electrophoresisCEC Commission of the European CommunitiesCEFIC European Council of Chemical Industry FederationsCFC chlorofluorocarboncGMP cyclic guanosine monophosphateChE cholinesteraseCHO Chinese hamster ovaryCi curie(s)CI confidence intervalCIN chronic interstitial nephritisCKSCC cystic keratinizing squamous cell carcinomaClCA 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic 2acidCLD chemiluminescence nitrogen detectorCLM chemiluminescence methodCLV ceiling valueCl-Vacid(= CPIA) 2-(4-chlorophenyl)isovaleric acidCMI cell-mediated immunityCML cell-mediated lympholysisCNS central nervous systemCOD chemical oxygen demandCO-Hb carboxyhaemoglobinCOPD chronic obstructive pulmonary diseaseCOT Committee on Toxicity (United Kingdom)cP centipoise(s)CP coproporphyrinsCPA cyclopropane carboxylic acidCPK creatine phosphokinaseCRMs certified reference materialsCSF cerebrospinal fluidCSM Committee on Safety of Medicines (United Kingdom)CT computerized tomographycv coefficient of variation2,4-D 2,4-dichlorophenoxyacetic acidDAB 4-dimethylaminobenzeneDABA L-2,4-diaminobutyric acidDBCP 1,2-dibromo-3-chloropropaneDBP di-n-butyl phthalate; 2,3-dibromopropanolDBT dibutyltinDCB dichlorobenzeneDCVC S-(1,2-dichlorovinyl)-L-cysteineDDC diethyldithiocarbamateDDT dichlorodiphenyltrichloroethaneDEHP diethylhexyl phthalateDEN diethylnitrosamineDES diethylstilbestrolDFB diflubenzuronDFP diisopropylfluorophosphate (a delayed neurotoxic)DHPN N-bis(2-hydroxypropyl) nitrosamineDiBP di-iso-butyl phthalateDIDT 5,6-dihydro-3H-imidazo (2,1-C)-1,2,4-dithiazole-3-thione DIT diiodotyrosineDL detection limitDMA dimethylamineDMBA dimethylbenzathralineDMF dimethylformamideDMP dimethylphenolDMSO dimethyl sulfoxideDNA deoxyribonucleic acidDNCB dinitrochlorobenzeneDNPH 2,4-dinitrophenyl hydrazineDOC dissolved organic carbonDPASV differential pulse anodic stripping voltametryDPTA diaminopropanoltetraacetic aciddegradation time for 50% of a compoundDT50DTPA diethylenetriamine pentaacetic acidEBDC ethylene bisdithiocarbamateEBK ethyl n-butyl ketoneEC effective concentration; electron capture; emulsifiable concentratemedian effective concentrationEC50ECD electron capture detectionECETOC European Chemical Industry Ecology and Toxicology Centre ECG electrocardiogramECMO extracorporeal membrane oxidationECOD 7-ethoxycoumarin-o-deethylaseEDA ethylenediamine; exploratory data analysisEDB 1,2-dibromoethane (ethylene dibromide)EDCF endothelial-derived contracting factorEDI ethylene diisothiocyanateEDL electrode discharge lampEDTA ethylenediaminetetraacetic acid2-EE 2-ethoxyethanol2-EEA 2-ethoxyethyl acetateEEC European Economic Community; electroencephalographEEG electroencephalogramEGF epidermal growth factorEH epoxide hydrataseEHC Environmental Health CriteriaEI electron impactEIFAC European Inland Fisheries Advisory Commission of FAO ELISA enzyme-linked immunosorbent assayEMDI estimated maximum daily intakeEMG electromyographyEMTD estimated maximum tolerated doseENG electroneurographyENL erythema nodosum leprosumENU ethylnitrosoureaEP electrophoresisEPA Environmental Protection Agency (USA)EPDM ethylene propylene rubberEPI exposure/potency indexEPS expandable polystyreneEQC external quality controlEQT environmental quality targetERL extraneous residue limitEROD 7-ethoxyresurofin-o-deethylaseESCA electron spectroscopy for chemical analysisESR erythrocyte sedimentation rateESRD end-stage renal diseaseETA electrothermal atomizationETD ethylene bisthiuram disulfideETG epidermal transglutaminaseETU ethylenethioureaETV electrothermal vaporizationEU ethyleneureaparental generationFFfilial generation, first1filial generation, secondF2FAA flameless atomic absorptionFACS fluorescence activated cell sorterFAD flavin adenine dinucleotideFAO Food and Agriculture OrganizationFCAT Freund's complete adjuvant testf.c. Field capacityFD fluorescence detectionFDA Food and Drug Administration (USA)FEF forced expiratory flowFEP free erythrocyte porphyrinFEV forced expiratory volumefg femtogram (10-15g)FI flame ionizationFID flame ionization detectorFMLP formyl methionyl leucyl phenylalanineFMN flavin mononucleotideFOB functional observational batteryFPD flame photometric detectorFR flame retardantFS fluorescence spectrophotometryFSC Food Safety Council (USA)FSD flame photometric detector selective for sulfur FSH follicle-stimulating hormoneFTIR Fourier transform infraredFVC forced vital capacityGA gibberillic acidGABA gamma-aminobutyric acidGAG glycosaminoglycanGALT gut-associated lymphoid tissueGAP good agricultural practiceGBM glomerular basement membraneGC gas chromatographyGC-ECD gas chromatography with electron capture detectorGC-SIM gas chromatography with selected ion monitoringGDH glutamate dehydrogenaseGDMS glow discharge mass spectrometryGEMS Global Environmental Monitoring SystemGESAMP Group of Experts for the Scientific Aspects of Marine PollutionGFAAS graphite furnace atomic absorption spectrometryGFR glomerular filtration rateGGT gamma-glutamyltranspeptidaseGH growth hormoneGI gastrointestinalGIFAP International Group of National Association of Manufacturers of Agrochemical Products(French Acronym for: Groupement International des Associations Nationales deFabricants de Produits Agrochimiques)GLC gas-liquid chromatographyGLDH glutamate dehydrogenaseGLP good laboratory practicecGMP cyclic guanosine monophosphateGOT glutamic-oxaloacetic transaminaseGPC gel permeation chromatographyGPMT guinea-pig maximization testGPT glutamic-pyruvic transaminaseGS glutamine synthetaseGSH glutathione-SHGST glutathione-S-transferaseGTB glomerular tubular balanceGV guidance valueGWP global-warming potentialh hour(s)ha hectareHb haemoglobinHBCD hexabromocyclododecaneHBDH hydroxybutyric dehydrogenaseH & E haematoxolin and eosinHCB hexachlorobenzeneHCBD hexachloro-1,3-butadieneHCFC hydrochlorofluorocarbonHCFH-22 chlorodifluoromethane (CHClF)2HCG human chorionic gonadotropinHCH hexachlorocyclohexaneHDL high density lipoproteinHDPE high density polyethyleneHEAL Human Exposure Assessment LocationHECD Hall electron capture detectorHEV high endothelial venuleHEX hexachlorocyclopentadieneHGPRT hypoxanthine-guanine phosphoribosyltransferase HIPS high impact polystyreneHIV human immunodeficiency virusHLB hydrophilic-lipophilic balanceHLV hygienic limit valuehnRNA heterogeneous nuclear RNAHPCA human progenitor cell antigenHPI cyclohexane-1,2-dicarboximideHPLC high-performance liquid chromatographyHPTLC high-performance thin-layer chromatographyHQ hydroquinoneHS headspaceHSA heat-stable antigen; human serum albumin HSG Health and Safety GuideIARC International Agency for Research on CancerIC ion chromatographyICAM intercellular adhesion moleculemedian inhibitory concentrationIC50ICD International Classification of DiseasesICG indocyanine greenICP inductively coupled plasmaICST isolated cold stress testingi.d. internal diameterIDMS isotope dilution mass spectrometryIFN interferonIg immunoglobulinIL interleukinILO International Labour OrganisationILSI International Life Science Instituteim intramuscularip intraperitonealIPA isopropylamineIPCS International Programme on Chemical SafetyIQ intelligence quotientIQC internal quality controlIR infraredIRPTC International Register of Potentially Toxic Chemicals ISO International Organization for StandardizationIT isomeric transitionIU international unitIUPAC International Union of Pure and Applied Chemistryiv intravenousJECFA Joint FAO/WHO Expert Committee on Food AdditivesJMPR Joint FAO/WHO Meeting on Pesticide Residueskcal kilocalorie(s)keV kiloelectronvolt(s)Koctanol/water partition coefficientowLAP leucine aminopeptidaseLAQL lowest analytically quantifiable levelLC liquid chromatographyLCmedian lethal concentration50lethal dose for 1%LD01median lethal doseLD50LDH lactate dehydrogenaseLDL low density lipoproteinLDPE low density polyethyleneLDQ lowest detectable quantityLEI lifetime exposure intensityLEV local exhaust ventilationLf limit flocculationLFA lymphocyte function-related antigenLFP lavage fluid proteinLH luteinizing hormoneLI labelling indexLIF laser-induced fluorescence; leukaemia inhibitory factor LLD lowest lethal doseLMS linear multistage modelLMW low molecular weightLOAEL lowest-observed-adverse-effect levelLOD limit of determinationLOEL lowest-observed-effect levelLRNI lower reference nutrient intake (UK)LSC liquid scintillation countermedian lethal timeLT50LTP long-term potentiationMAA methoxyacetic acidMAC maximum allowable concentrationMAD maximum allowable deviationMAFF Ministry of Agriculture, Forestry and Fisheries (Japan) MAK maximum workplace concentration (Maximale Arbeitsplatzkonzentration)MALT mucosa-associated lymphoid tissueMAM methylazoxymethanolMAP mutagenic activity profileMAOI monoamine oxidase inhibitorMARC Monitoring and Assessment Research Centre (UK)MARE monoclonal anti-rat immunoglobulin EMAS molecular absorption spectrometryMAT mean absorption timeMATC maximum acceptable toxicant concentrationMBC minimum bactericidal concentrationMBDE mass balance differential equationMBK methyl n-butyl ketoneMBP monobutyl phthalateMBT monobutyltinMC methyl chloroform3-MC 3-methylcholanthreneMCD microcoulometric detectionMCH mean cell haemoglobinMCHC mean cell haemoglobin concentrationmCi millicurieMCL melanotic cell linesMCPA 4-chloro-o-tolyoxyacetic acidMCV mean cell volumeMDA malondialdehydeMDI methylene-diphenyl diisocyanateMDMA methylenedioxymethamphetamine2-ME 2-methoxyethanol2-MEA 2-methoxyethyl acetateMeBmethylcobalamin12MED minimum effective dose; minimal erythemal doseMEHP monoethylhexyl phthalateMEK methyl ethyl ketonemEq milliequivalentMeV megaelectronvolt(s)MFO mixed-function oxidaseMHC major histocompatibility complexMHW Ministry of Health and Welfare (Japan)MIBK methyl isobutyl ketoneMIC minimal inhibitory concentrationMIT methylisothiocyanate; monoiodotyrosineMLD minimum lethal doseMLR mixed lymphocyte response assayMLSS mixed liquor suspended solidsMMA methoxyacetic acidMMAD mass median aerodynamic diameterMMH monomethylhydrazinemmHg millimetre(s) of mercuryMMMF man-made mineral fibreMNNG N-methyl-N -nitro-N-nitrosoguanidineMNU N-methyl-N-nitrosureamPa millipascal (7.5 x 10-6 mmHg)MPC maximum permissible limitMQL minimum quantifiable limitMRBIS mean running bias index scoreMRL maximum residue limitmRNA messenger RNAMRVIS Mean Running Variance Index ScoreMS mass spectrometryMSD mass selective detectionMSW municipal solid wasteMTBE methyl tertiary butyl etherMTC maximum tolerable or acceptable concentrationMTD maximum tolerated doseMTE mild toxic encephalopathyMTI N-(hydroxymethyl)-3,4,5,6-tetrahydrophthalamideNAA neutron activation analysisNAC N-acetyl cysteineNAD nicotinamide adenine dinucleotideNADP nicotinamide adenine dinucleotide phosphateNADPH reduced nicotinamide adenine dinucleotide phosphate NBU N-nitrosobutylureaNCAM neural cell adhesion moleculeNCI National Cancer Institute (USA); negative ion chemical ionizationNCV nerve conduction velocityND not detectableNDDC sodium diethyldithiocarbamateNDMA nitrosodimethylamineNDMC sodium dimethyldithiocarbamateNEQUAS National External Quality Assessment Scheme (UK)NFT neurofibrillary tangleng nanogram (10-9g)NIOSH National Institute for Occupational Safety and Health (USA) NK natural killernm nanometerNMCL nonmelanolic cell linesNMN N-methylnicotinamideNMOR N-nitrosomorpholineNMR nuclear magnetic resonanceNNM N-nitrosomorpholineNO nitrogen oxideNOAEL no-observed-adverse-effect levelNOEC no-observed-effect concentrationNOEL no-observed-effect levelNOLC no-observed lethal concentrationNPD nitrogen-phosphorus sensitive detectorNPSH non-protein sulfhydrylNSAID non-steroidal anti-inflammatory drugsNSD nitrogen selective detectorNTA nitrilotriacetic acidNTE neuropathy target esteraseNTEL no-toxic-effect levelNTP National Toxicology Program (USA)OCT ornithine carbamoyltransferaseODC ornithine decarboxylaseODP ozone-depletion potentialOECD Organisation for Economic Co-operation and Development OEL occupational exposure limitOER oxygen enhancement ratioOES occupational exposure standard; optical emission spectrometryOET open epicutaneous testOP organophosphateOPIDN organophosphate-induced delayed neuropathyOR odds ratioOSC oil-enhanced suspension concentrateOSHA Occupational Safety and Health Administration (USA)OVA ovalbuminOZT oxazolidinethionePA polyamidesPAA photon activation analysis2-PAMchloride pralidoxine (2-pyridine aldoxime methyl) chloridePAH p-aminohippurate; polycyclic aromatic hydrocarbonPALS periarteriolar lymphocyte sheathPAN peroxyacetyl nitrate; polyacrylonitrilePAS periodic acid Schiff stainPB phenobarbitalPBA phenoxybenzoic acidPBalc 3-phenoxybenzyl alcoholPBald 3-phenoxybenzaldehydePBB polybrominated biphenylPBDD polybrominated dibenzodioxinPBDE polybrominated diphenyl etherPBDF polybrominated dibenzofuranPBI protein-bound iodinePBPK physiologically based pharmacokineticsPBT polybutylene terephthalatePCBD S-(1,2,3,4,4-pentachloro-1,3-butadienyl)PCDD polychlorinated dibenzodioxinPCDF polychlorinated dibenzofuranPCA para-chloroaniline (4-chloroaniline); passive cutaneous anaphylaxisPCB polychlorinated biphenylPCBD S-(1,2,3,4,4-pentachloro-1,3-butadienyl)PCDD polychlorinated dibenzo-p-dioxinPCDF polychlorinated dibenzofuranPCDPE polychlorinated diphenyletherPCE polychromatic erythrocytesPCOM phase contrast optical microscopyPCPY polychlorinated pyrenePCQ polychlorinated quaterphenylPCT porphyria cutanea tardaPCV packed-cell volumePD plasma desorptionPDG phosphate-dependent glutaminasePE polyethylenePEF peak expiratory flowPEG pneumoencephalographyPEL permissible exposure limitPET polyethylene terephthalatePFC plaque-forming cellpg picogram (10-12g)PG prostaglandinpH the negative logarithm of the hydrogen ion concentration PHF paired helical filamentsPHS prostaglandin-H-synthetasePIB piperonyl butoxidePIC picrotoxinPID photo-ionization detectionPIXE proton-induced X-ray emissionpKa the negative logarithm of the dissociation constant PMBA p-methylbenzyl alcoholPMN polymorphonuclear leukocytePMTDI provisional maximum tolerable daily intakePMR proportional mortality ratePMSG pregnant mare serum gonadotrophinPNS peripheral nervous systempO2 plasma partial pressure (concentration) of oxygenPOCP photochemical ozone-creation potentialPoG proteoglycanPOS psycho-organic syndromePP polypropyleneppb parts per billionppm parts per millionppt parts per trillionPSD passive sampling devicePSPS Pesticides Safety Precautions Scheme (United Kingdom) PT prothrombin timePTH parathyroid hormonePTT partial thromboplastin timePTU propylenethioureaPTWI provisional tolerable weekly intakePTZ pentylenetetrazolePVC polyvinyl chloridePYR pyreneQA quality assuranceQAP quality assurance programmeQC quality controlQSAR quantitative structure-activity relationshipRACB reproductive assessment by continuous breedingRAST radioallergosorbent testRBC red blood cellRBP retinal binding proteinRDA recommended dietary allowance (USA)REL recommended exposure limitRER rough endoplasmic reticulumRIA radio-immuno assayRIPT repeat insult patch testRMA reflex modification audiometryRNA ribonucleic acidRNI reference nutrient intake (UK)ROC reactive organic carbonRPN renal papillary necrosisRR relative riskRTECS Registry of Toxic Effects of Chemical Substances RUBISCO ribulose 1,5-biphosphate carboxylaseS9 9000 x g supernatantSAM S-adenosylmethionineSAP serum alkaline phosphataseSAR structure-activity relationshipsc subcutaneousSC suspension concentrateSCE sister chromatid exchangeSCF stem-cell factorSCID severe combined immunodeficiencySCOPE Scientific Committee on Problems of the Environment of the International Council ofScientific UnionsSD standard deviationSDAT senile dementia of Alzheimer typeSE standard errorSEM standard error of the mean; scanning electron microscopy SER smooth endoplasmic reticulumSFC supercritical fluid chromatographySFS subjective facial sensationSGOMSEC Scientific Group on Methodologies for the Safety Evaluation of ChemicalsSGOT serum glutamic-oxaloacetic transaminaseSGPT serum glutamic-pyruvic transaminaseSHE Syrian Hamster embryoSIM selected ion monitoringSIMS secondary ion mass spectrometrySLE systemic lupus erythematosusSMA sequential multiple analyserSMR standardized mortality ratioSOP standard operating procedureSPF specific pathogen freeSPM suspended particulate matterS-PMA S-phenyl-mercapturic acidSRT simple reaction timeSSB single strand breaksSTEL short-term exposure limitttriiodothyronine3thyroxinet42,4,5-T 2,4,5-trichlorophenoxyacetic acidTADI temporary acceptable daily intakeTAN tropical ataxic neuropathyTAP trialkyl/aryl phosphateTBBPA tetrabromobisphenol ATBG thyroxine-binding globulinTBP tributyl phosphateTBPP tris(2,3-dibromopropyl) phosphateTBT tributyltinTBTO tributyltin oxideTCA tricarboxylic acid cycleTCD thermal conductivity detectionTCDD 2,3,7,8-tetrachlorinated dibenzo-p-dioxinTCDF 2,3,7,8-tetrachlorinated dibenzofuranTCE 1,1,1-trichloroethyleneTCP trichlorophenol; tricresyl phosphateTCPP tris(1-chloro-2-propyl)phosphateTCR T-cell receptorTDC thermal conductivity detectionTDI tolerable daily intake; toluene diisocyanateTDLAS tuneable diode laser absorption spectrometryTEA tetraethyl ammonium; thermal energy analyser TEAC tetraethylammonium chlorideTEAM total exposure assessment methodologyTEF toxicity equivalency factorTEPP tetraethyl-pyrophosphateTGA thermogravimetric analysisTH thyroid hormoneTHF tetrahydrofolateTI tolerable intakeTLC thin-layer chromatographyTLV threshold limit valueTMCP tri-meta-cresyl phosphateTMDI theoretical maximum daily intakeTML tetramethylleadTMRL temporary maximum residue limitTMT trimethyltinTNF tumour necrosis factorTOCP tri-ortho-cresyl phosphateTOD total oxygen demandTPA 12-O-tetradecanoylphorbol-13-acetateTPCP tri-para-cresyl phosphateTPI 3,4,5,6-tetrahydrophthalimideTPIA 3,4,5,6-tetrahydrophthalic acidTPN total parenteral nutritionTPO thyroid peroxidaseTPP triphenyl phosphateTPTA triphenyltin acetateTPTH triphenyltin hydroxidetRNA transfer ribonucleic acidTRP tubular reabsorption of phosphateTSH thyroid-stimulating hormone (thyrotropin) TSP total suspended particulateTST temperature sensitivityTT toxicity thresholdTWA time-weighted averageUCM urographic contrast mediumUCL upper confidence limitUDP uridine diphosphateUDPGA UDP-glucuronic acidUDPGT UDP-glucuronosyltransferaseUDS unscheduled DNA synthesisUF uncertainty factorULV ultra-low volumeUNEP United Nations Environment ProgrammeUNICEF United Nations Children's FundUS ATSDR US Agency for Toxic Substance and Disease Registry UV ultravioletUVB ultraviolet BVC vinyl chlorideVCAM vascular cell adhesion moleculeVER visual evoked responseVHH volatile halogenated hydrocarbonVLA very late antigenVLDL very low-density lipoproteinsVOC volatile organic carbon compoundVSD virtually safe doseVTR vibration thresholdv/v volume per volumeWAIS Weschler Adult Intelligence ScaleWBC white blood cellWG water-dispersible granuleWHO World Health OrganizationWISN warfarin-induced skin necrosisWP wettable powderw/v weight per volumeXRF X-ray-generated atomic fluorescenceXRFS X-ray fluorescence spectroscopyZPP zinc protoporphyrin。
光学5版 Hecht 纸质版说明书

For courses in introductory calculus-based physics
The 4th Edition of Physics for Scientists and Engineers builds on strong research-based foundations with fine-tuned and streamlined content, hallmark features, and an even more robust Mastering Physics program. By extending problem- solving guidance to include a greater emphasis on modeling and significantly revised and more challenging problem sets, students gain confidence and skills in problem solving. The addition of advanced topics accommodates different teaching preferences and course structures.
Sustaining market leadership for over twenty years, Optics, 5th Edition demonstrates the range and balance in subject matter. The text is grounded in traditional methodology, while providing an early introduction to the powerful perspective of the Fourier theory, which is crucial to present- day analysis. Electron and neutron diffraction patterns are pictured alongside the customary photon images, and every piece of art has been scrutinized for accuracy and altered where appropriate to improve clarity.
VACUUM ELECTRONIC DEVICE

专利名称:VACUUM ELECTRONIC DEVICE发明人:BACHMANN, Peter, K.,SCHIEBEL, Ulrich,VAN DER VAART, Nijs, C.申请号:IB2002005655申请日:20021220公开号:WO03/054901P1公开日:20030703专利内容由知识产权出版社提供摘要:The invention describes a vacuum electronic device comprising an electron source having at least one cathode for emitting of electrons, at least one electron beam guidance cavity for concentrating electrons emitted from the cathode, said cavity having an entrance aperture and an exit aperture, a portion of an inner side of said cavity around the exit aperture being provided with an insulating material, and a first electrode being connectable to a first power supply means for applying, in operation, an electric field with a first field strength E1 between the cathode and the exit aperture to allow electron transport throught he electron beam guidance cavity, wherein the cathode comprises a carbon-based cold emitter.申请人:BACHMANN, Peter, K.,SCHIEBEL, Ulrich,VAN DER VAART, Nijs, C.地址:NL,DE,NL,NL,NL国籍:NL,DE,NL,NL,NL代理机构:VOLMER, Georg更多信息请下载全文后查看。
eCTD 文件的编写与递交

ADMIN ELEMENTS
Administrative information is contained in the admin element, which is contained in the fda-regional:fdaregional element. The admin element contains two child elements: applicant-info application-set. These elements should be placed in order as listed below.
Application-set Element
Application-information Element
Application-number element <application-number application-type="fdaat1 ">456789</application-number> Cross-reference-application-number element … <application-number application-type="fdaat1">456789</application-number> <cross-reference-application-number application-type="fdaat5">012345</crossreference-application-number>
eCTD 介绍eCTD Template
The ICH Web site (/eCTD) includes an empty eCTD folder template as an example of an eCTD submission folder structure. It shows all of the possible Module 2-5 and can be populated with the applicant data and edited as appropriate (i.e., adding additional subfolders or removing unnecessary folders). The applicant should still add the relevant regional Module 1 folders and content, add the appropriate utility folders and content, and create the XML index files to complete a valid eCTD submission.
GC-ECD法检测甲磺酸伊马替尼中甲磺酸烷基酯

GC-ECD法检测甲磺酸伊马替尼中甲磺酸烷基酯张倩颖;冯芳【摘要】An method of in situ derivatization-headspace gas chromatrograph-electron capture detecter was established to determine methyl methanesulfonate (MMS),ethyl methanesulfonate (EMS) and isopropyl methanesulfonate (IMS),known as potential genotoxic impurities in imatinib mesylate. An INOWAX (30 m×0.25 mm,1.0 μm) capillary column was used to separate and electron capture detecter (ECD) wasused for quantitative determination. Sodium iodide was used as a derivatizing agent. Headspace sampling was conducted with a split ratio of 1:10. Calibration curves showed good linearity over the concentration range of 5~125 ng/mL for three of alkyls methanesulfonate. The results of limit of quantification of MMS,EMS and IMS were 5,2.5 and 2.5ng/mL,respectively. Recoveries of MMS,EMS and IMS were 87.91%, 102.35% and 101.94%, respectively. The method showed satisfactory convenience, accuracy, repeatability and specificity.%建立了同时检测甲磺酸伊马替尼中甲磺酸甲酯、甲磺酸乙酯和甲磺酸异丙酯3种基因毒性杂质的原位衍生化顶空气相色谱法.采用Welch WM-INNOWAX(30 m×0.25 mm, 1 μm)色谱柱,电子捕获检测器(ECD),分流比20:1;碘化钠原位衍生化顶空进样.3种甲磺酸烷基酯在5~125ng/mL的范围内线性关系良好,定量限分别为5、2.5和2.5 ng/mL,平均回收率分别为87.91%、102.35%和101.94%.该方法简便、准确、重现性好且专属性强.【期刊名称】《广州化工》【年(卷),期】2018(046)009【总页数】3页(P66-68)【关键词】甲磺酸伊马替尼;甲磺酸烷基酯;基因毒性杂质;原位衍生化顶空GC-ECD 【作者】张倩颖;冯芳【作者单位】中国药科大学药物分析教研室,江苏南京 210009;中国药科大学药物分析教研室,江苏南京 210009;药物质量与安全预警教育部重点实验室,江苏南京210009【正文语种】中文【中图分类】R917甲磺酸伊马替尼(Imatinib Mesylate)是一种小分子抗肿瘤药物,属于酪氨酸激酶抑制剂,结构见图1,是目前治疗慢性粒细胞白血病地首选药[1]。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Part 11, Electronic Records; Electronic Signatures — Scope and ApplicationU.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Biologics Evaluation and Research (CBER)Center for Devices and Radiological Health (CDRH)Center for Food Safety and Applied Nutrition (CFSAN)Center for Veterinary Medicine (CVM)Office of Regulatory Affairs (ORA)August 2003Pharmaceutical CGMPsPart 11, Electronic Records; Electronic Signatures — Scope and ApplicationDivision of Drug Information, HFD-240Center for Drug Evaluation and Research (CDER)(Tel) 301-827-4573/cder/guidance/index.htmorOffice of Communication, Training andManufacturers Assistance, HFM-40Center for Biologics Evaluation and Research (CBER)/cber/guidelines.htmPhone: the Voice Information System at 800-835-4709 or 301-827-1800orCommunications Staff (HFV-12),Center for Veterinary Medicine (CVM)(Tel) 301-594-1755/cvm/guidance/guidance.htmlorDivision of Small Manufacturers Assistance (HFZ-220)/cdrh/ggpmain.htmlManufacturers Assistance Phone Number: 800.638.2041 or 301.443.6597Internt'l Staff Phone: 301.827.3993orCenter for Food Safety and Applied Nutrition (CFSAN)/~dms/guidance.html.U.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Biologics Evaluation and Research (CBER)Center for Devices and Radiological Health (CDRH)Center for Food Safety and Applied Nutrition (CFSAN)Center for Veterinary Medicine (CVM)Office of Regulatory Affairs (ORA)August 2003Pharmaceutical CGMPsTABLE OF CONTENTSI.INTRODUCTION (1)II.BACKGROUND (2)III.DISCUSSION (3)A.Overall Approach to Part 11 Requirements (3)B.Details of Approach – Scope of Part 11 (4)1. Narrow Interpretation of Scope (4)2. Definition of Part 11 Records (5)C.Approach to Specific Part 11 Requirements (6)1.Validation (6)2.Audit Trail (6)3.Legacy Systems (7)4.Copies of Records (7)5.Record Retention (8)IV.REFERENCES (9)Guidance for Industry 1 1Part 11, Electronic Records; Electronic Signatures — 2Scope and Application 34 56 This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. It7 does not create or confer any rights for or on any person and does not operate to bind FDA or the public.8 You can use an alternative approach if the approach satisfies the requirements of the applicable statutes9 and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for 10 implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate 11 number listed on the title page of this guidance. 12131415I. INTRODUCTION 1617This guidance is intended to describe the Food and Drug Administration's (FDA’s) current 18thinking regarding the scope and application of part 11 of Title 21 of the Code of Federal 19Regulations; Electronic Records; Electronic Signatures (21 CFR Part 11).2 2021This document provides guidance to persons who, in fulfillment of a requirement in a statute or 22another part of FDA's regulations to maintain records or submit information to FDA,3 have 23chosen to maintain the records or submit designated information electronically and, as a result, 24have become subject to part 11. Part 11 applies to records in electronic form that are created, 25modified, maintained, archived, retrieved, or transmitted under any records requirements set 26forth in Agency regulations. Part 11 also applies to electronic records submitted to the Agency 27under the Federal Food, Drug, and Cosmetic Act (the Act) and the Public Health Service Act (the 28PHS Act), even if such records are not specifically identified in Agency regulations (§ 11.1). 29The underlying requirements set forth in the Act, PHS Act, and FDA regulations (other than part 3011) are referred to in this guidance document as predicate rules. 3132 1 This guidance has been prepared by the Office of Compliance in the Center for Drug Evaluation and Research (CDER) in consultation with the other Agency centers and the Office of Regulatory Affairs at the Food and Drug Administration.2 62 FR 134303 These requirements include, for example, certain provisions of the Current Good Manufacturing Practiceregulations (21 CFR Part 211), the Quality System regulation (21 CFR Part 820), and the Good Laboratory Practice for Nonclinical Laboratory Studies regulations (21 CFR Part 58).As an outgrowth of its current good manufacturing practice (CGMP) initiative for human and3334animal drugs and biologics,4 FDA is re-examining part 11 as it applies to all FDA regulated35products. We anticipate initiating rulemaking to change part 11 as a result of that re-examination. This guidance explains that we will narrowly interpret the scope of part 11. While3637the re-examination of part 11 is under way, we intend to exercise enforcement discretion with38respect to certain part 11 requirements. That is, we do not intend to take enforcement action to 39enforce compliance with the validation, audit trail, record retention, and record copying40requirements of part 11 as explained in this guidance. However, records must still be maintained 41or submitted in accordance with the underlying predicate rules, and the Agency can take42regulatory action for noncompliance with such predicate rules.4344In addition, we intend to exercise enforcement discretion and do not intend to take (or45recommend) action to enforce any part 11 requirements with regard to systems that wereoperational before August 20, 1997, the effective date of part 11 (commonly known as legacy4647systems) under the circumstances described in section III.C.3 of this guidance.48Note that part 11 remains in effect and that this exercise of enforcement discretion applies only4950as identified in this guidance.51FDA's guidance documents, including this guidance, do not establish legally enforceable5253responsibilities. Instead, guidances describe the Agency's current thinking on a topic and should 54be viewed only as recommendations, unless specific regulatory or statutory requirements arecited. The use of the word should in Agency guidances means that something is suggested or5556recommended, but not required.575859II. BACKGROUND6061In March of 1997, FDA issued final part 11 regulations that provide criteria for acceptance by FDA, under certain circumstances, of electronic records, electronic signatures, and handwritten6263signatures executed to electronic records as equivalent to paper records and handwritten64signatures executed on paper. These regulations, which apply to all FDA program areas, were intended to permit the widest possible use of electronic technology, compatible with FDA's6566responsibility to protect the public health.6768After part 11 became effective in August 1997, significant discussions ensued among industry, 69contractors, and the Agency concerning the interpretation and implementation of the regulations.70FDA has (1) spoken about part 11 at many conferences and met numerous times with an industry 71coalition and other interested parties in an effort to hear more about potential part 11 issues; (2) 72published a compliance policy guide, CPG 7153.17: Enforcement Policy: 21 CFR Part 11;73Electronic Records; Electronic Signatures; and (3) published numerous draft guidance74documents including the following:4 See Pharmaceutical CGMPs for the 21st Century: A Risk-Based Approach; A Science and Risk-Based Approachto Product Quality Regulation Incorporating an Integrated Quality Systems Approach at/oc/guidance/gmp.html.7576• 21 CFR Part 11; Electronic Records; Electronic Signatures, Validation77• 21 CFR Part 11; Electronic Records; Electronic Signatures, Glossary of Terms78• 21 CFR Part 11; Electronic Records; Electronic Signatures, Time Stamps79• 21 CFR Part 11; Electronic Records; Electronic Signatures, Maintenance of Electronic 80Records81• 21 CFR Part 11; Electronic Records; Electronic Signatures, Electronic Copies of82Electronic Records8384Throughout all of these communications, concerns have been raised that some interpretations of 85the part 11 requirements would (1) unnecessarily restrict the use of electronic technology in a86manner that is inconsistent with FDA's stated intent in issuing the rule, (2) significantly increase 87the costs of compliance to an extent that was not contemplated at the time the rule was drafted, 88and (3) discourage innovation and technological advances without providing a significant public 89health benefit. These concerns have been raised particularly in the areas of part 11 requirements 90for validation, audit trails, record retention, record copying, and legacy systems.9192As a result of these concerns, we decided to review the part 11 documents and related issues,93particularly in light of the Agency's CGMP initiative. In the Federal Register of February 4,942003 (68 FR 5645), we announced the withdrawal of the draft guidance for industry, 21 CFR95Part 11; Electronic Records; Electronic Signatures, Electronic Copies of Electronic Records. 96We had decided we wanted to minimize industry time spent reviewing and commenting on the draft guidance when that draft guidance may no longer represent our approach under the CGMP9798initiative. Then, in the Federal Register of February 25, 2003 (68 FR 8775), we announced the 99withdrawal of the part 11 draft guidance documents on validati on, glossary of terms, timestamps,5 maintenance of electronic records, and CPG 7153.17. We received valuable public 100101comments on these draft guidances, and we plan to use that information to help with future102decision-making with respect to part 11. We do not intend to re-issue these draft guidance103documents or the CPG.104105We are now re-examining part 11, and we anticipate initiating rulemaking to revise provisions of 106that regulation. To avoid unnecessary resource expenditures to comply with part 11107requirements, we are issuing this guidance to describe how we intend to exercise enforcement 108discretion with regard to certain part 11 requirements during the re-examination of part 11. As 109mentioned previously, part 11 remains in effect during this re-examination period.110111112III. DISCUSSION113114A. Overall Approach to Part 11 Requirements1155 Although we withdrew the draft guidance on time stamps, our current thinking has not changed in that when usingtime stamps for systems that span different time zones, we do not expect you to record the signer’s local time. When using time stamps, they should be implemented with a clear understanding of the time zone reference used. In such instances, system documentation should explain time zone references as well as zone acronyms or other namingconventions.As described in more detail below, the approach outlined in this guidance is based on three main 116117elements:118• Part 11 will be interpreted narrowly; we are now clarifying that fewer records will be 119120considered subject to part 11.121• For those records that remain subject to part 11, we intend to exercise enforcement122discretion with regard to part 11 requirements for validation, audit trails, record retention, 123and record copying in the manner described in this guidance and with regard to all part 11 124requirements for systems that were operational before the effective date of part 11 (also 125known as legacy systems).126• We will enforce all predicate rule requirements, including predicate rule record an d127recordkeeping requirements.128It is important to note that FDA's exercise of enforcement discretion as described in this129guidance is limited to specified part 11 requirements (setting aside legacy systems, as to which 130the extent of enforcement discretion, under certain circumstances, will be more broad). We131intend to enforce all other provisions of part 11 including, but not limited to, certain controls for 132closed systems in § 11.10. For example, we intend to enforce provisions related to the following 133controls and requirements:134• limiting system access to authorized individuals135136• use of operational system checks137• use of authority checks• use of device checks138139• determination that persons who develop, maintain, or use electronic systems have the 140education, training, and experience to perform their assigned tasks• establishment of and adherence to written policies that hold individuals accountable for 141142actions initiated under their electronic signatures143• appropriate controls over systems documentation144• controls for open systems corresponding to controls for closed systems bulleted above (§ 14511.30)146• requirements related to electronic signatures (e.g., §§ 11.50, 11.70, 11.100, 11.200, and 14711.300)148149We expect continued compliance with these provisions, and we will continue to enforce them. 150Furthermore, persons must comply with applicable predicate rules, and records that are required to be maintained or submitted must remain secure and reliable in accordance with the predicate 151152rules.153B. Details of Approach – Scope of Part 111541551561. Narrow Interpretation of Scope157158We understand that there is some confusion about the scope of part 11. Some have understood 159the scope of part 11 to be very broad. We believe that some of those broad interpretations couldlead to unnecessary controls and costs and could discourage innovation and technological160161advances without providing added benefit to the public health. As a result, we want to clarify 162that the Agency intends to interpret the scope of part 11 narrowly.163164Under the narrow interpretation of the scope of part 11, with respect to records required to be 165maintained under predicate rules or submitted to FDA, when persons choose to use records inelectronic format in place of paper format, part 11 would apply. On the other hand, when166167persons use computers to generate paper printouts of electronic records, and those paper records 168meet all the requirements of the applicable predicate rules and persons rely on the paper records to perform their regulated activities, FDA would generally not consider persons to be "using 169170electronic records in lieu of paper records" under §§ 11.2(a) and 11.2(b). In these instances, the use of computer systems in the generation of paper records would not trigger part 11.1711721732. Definition of Part 11 Records174175Under this narrow interpretation, FDA considers part 11 to be applicable to the following records 176or signatures in electronic format (part 11 records or signatures):177178• Records that are required to be maintained under predicate rule requirements and that are 179maintained in electronic format in place of paper format. On the other hand, records (andany associated signatures) that are not required to be retained under predicate rules, but 180181that are nonetheless maintained in electronic format, are not part 11 records.182We recommend that you determine, based on the predicate rules, whether specific records 183are part 11 records. We recommend that you document such decisions.184185• Records that are required to be maintained under predicate rules, that are maintained in 186electronic format in addition to paper format, and that are relied on to perform regulated 187activities.188In some cases, actual business practices may dictate whether you are using electronicrecords instead of paper records under § 11.2(a). For example, if a record is required to 189190be maintained under a predicate rule and you use a computer to generate a paper printout 191of the electronic records, but you nonetheless rely on the electronic record to performregulated activities, the Agency may consider you to be using the electronic record192193instead of the paper record. That is, the Agency may take your business practices into 194account in determining whether part 11 applies.195Accordingly, we recommend that, for each record required to be maintained under196predicate rules, you determine in advance whether you plan to rely on the electronic197record or paper record to perform regulated activities. We recommend that you198document this decision (e.g., in a Standard Operating Procedure (SOP), or specification 199document).200• Records submitted to FDA, under predicate rules (even if such records are not201specifically identified in Agency regulations) in electronic format (assuming the recordshave been identified in docket number 92S-0251 as the types of submissions the Agency 202203accepts in electronic format). However, a record that is not itself submitted, but is usedin generating a submission, is not a part 11 record unless it is otherwise required to be 204205maintained under a predicate rule and it is maintained in electronic format.206• Electronic signatures that are intended to be the equivalent of handwritten signatures, initials, and other general signings required by predicate rules. Part 11 signatures include 207208electronic signatures that are used, for example, to document the fact that certain events 209or actions occurred in accordance with the predicate rule (e.g. approved, reviewed, and210verified).211212C. Approach to Specific Part 11 Requirements2132141. Validation215216The Agency intends to exercise enforcement discretion regarding specific part 11 requirements 217for validation of computerized systems (§ 11.10(a) and corresponding requirements in § 11.30).218Although persons must still comply with all applicable predicate rule requirements for validation 219(e.g., 21 CFR 820.70(i)), this guidance should not be read to impose any additional requirements220for validation.221222We suggest that your decision to validate computerized systems, and the extent of the validation,223take into account the impact the systems have on your ability to meet predicate rule224requirements. You should also consider the impact those systems might have on the accuracy,225reliability, integrity, availability, and authenticity of required records and signatures. Even if 226there is no predicate rule requirement to validate a system, in some instances it may still be227important to validate the system.228229We recommend that you base your approach on a justified and documented risk assessment and230a determination of the potential of the system to affect product quality and safety, and record 231integrity. For instance, validation would not be important for a word processor used only to232generate SOPs.233234For further guidance on validation of computerized systems, see FDA’s guidance for industry235and FDA staff General Principles of Software Validation and also industry guidance such as the 236GAMP 4 Guide (See References).2372382. Audit Trail239240The Agency intends to exercise enforcement discretion regarding specific part 11 requirements 241related to computer-generated, time-stamped audit trails (§ 11.10 (e), (k)(2) and any242corresponding requirement in §11.30). Persons must still comply with all applicable predicate243rule requirements related to documentation of, for example, date (e.g., § 58.130(e)), time, or 244sequencing of events, as well as any requirements for ensuring that changes to records do notobscure previous entries.245246247Even if there are no predicate rule requirements to document, for example, date, time, or248sequence of events in a particular instance, it may nonetheless be important to have audit trails or 249other physical, logical, or procedural security measures in place to ensure the trustworthiness andreliability of the records.6 We recommend that you base your decision on whether to apply audit 250251trails, or other appropriate measures, on the need to comply with predicate rule requirements, a 252justified and documented risk assessment, and a determination of the potential effect on product 253quality and safety and record integrity. We suggest that you apply appropriate controls based on 254such an assessment. Audit trails can be particularly appropriate when users are expected to255create, modify, or delete regulated records during normal operation.2562573. Legacy Systems7258259The Agency intends to exercise enforcement discretion with respect to all part 11 requirements 260for systems that otherwise were operational prior to August 20, 1997, the effective date of part 26111, under the circumstances specified below.262263This means that the Agency does not intend to take enforcement action to enforce compliance 264with any part 11 requirements if all the following criteria are met for a specific system:265266• The system was operational before the effective date.267• The system met all applicable predicate rule requirements before the effective date.• The system currently meets all applicable predicate rule requirements.268269• You have documented evidence and justification that the system is fit for its intended use 270(including having an acceptable level of record security and integrity, if applicable).271272If a system has been changed since August 20, 1997, and if the changes would prevent the273system from meeting predicate rule requirements, Part 11 controls should be applied to Part 11 274records and signatures pursuant to the enforcement policy expressed in this guidance.2754. Copies of Records276277278The Agency intends to exercise enforcement discretion with regard to specific part 11279requirements for generating copies of records (§ 11.10 (b) and any corresponding requirement in 280§11.30). You should provide an investigator with reasonable and useful access to records during 281an inspection. All records held by you are subject to inspection in accordance with predicate 282rules (e.g., §§ 211.180(c), (d), and 108.35(c)(3)(ii)).283284We recommend that you supply copies of electronic records by:285286• Producing copies of records held in common portable formats when records are287maintained in these formats• Using established automated conversion or export methods, where available, to make 288289copies in a more common format (examples of such formats include, but are not limited 290to, PDF, XML, or SGML)6 Various guidance documents on information security are available (see References).7 In this guidance document, we use the term legacy system to describe systems already in operation before theeffective date of part 11.291In each case, we recommend that the copying process used produces copies that preserve the 292content and meaning of the record. If you have the ability to search, sort, or trend part 11293records, copies given to the Agency should provide the same capability if it is reasonable and 294technically feasible.You should allow inspection, review, and copying of records in a humanreadable form at your site using your hardware and following your established procedures and 295296techniques for accessing records.2972985. Record Retention299300The Agency intends to exercise enforcement discretion with regard to the part 11 requirements 301for the protection of records to enable their accurate and ready retrieval throughout the records 302retention period (§ 11.10 (c) and any corresponding requirement in §11.30). Persons must still 303comply with all applicable predicate rule requirements for record retention and availability (e.g., 304§§ 211.180(c),(d), 108.25(g), and 108.35(h)).305306We suggest that your decision on how to maintain records be based on predicate rulerequirements and that you base your decision on a justified and documented risk assessment and 307308a determination of the value of the records over time.309310FDA does not intend to object if you decide to archive required records in electronic format to nonelectronic media such as microfilm, microfiche, and paper, or to a standard electronic file 311312format (examples of such formats include, but are not limited to, PDF, XML, or SGML).313Persons must still comply with all predicate rule requirements, and the records themselves and 314any copies of the required records should preserve their content and meaning. As long as315predicate rule requirements are fully satisfied and the content and meaning of the records are 316preserved and archived, you can delete the electronic version of the records.In addition, paper 317and electronic record and signature components can co-exist (i.e., a hybrid8 situation) as long as 318predicate rule requirements are met and the content and meaning of those records are preserved. 3198 Examples of hybrid situations include combinations of paper records (or other nonelectronic media) and electronicrecords, paper records and electronic signatures, or handwritten signatures executed to electronic records.319320IV. REFERENCES321322Food and Drug Administration References3231. Glossary of Computerized System and Software Development Terminolo gy (Division of 324325Field Investigations, Office of Regional Operations, Office of Regulatory Affairs, FDA 3261995) (/ora/inspect_ref/igs/gloss.html)3272. General Principles of Software Validation; Final Guidance for Industry and FDA Staff 328329(FDA, Center for Devices and Radiological Health, Center for Biologics Evaluation and 330Research, 2002) (/cdrh/comp/guidance/938.html)3313. Guidance for Industry, FDA Reviewers, and Compliance on Off-The-Shelf Software Use 332333in Medical Devices (FDA, Center for Devices and Radiological Health, 1999)334(/cdrh/ode/guidance/585.html)3354. Pharmaceutical CGMPs for the 21st Century: A Risk-Based Approach; A Science and 336337Risk-Based Approach to Product Quality Regulation Incorporating an Integrated Quality 338Systems Approach (FDA 2002) (/oc/guidance/gmp.html)339340341Industry References3421. The Good Automated Manufacturing Practice (GAMP) Guide for Validation of343344Automated Systems, GAMP 4 (ISPE/GAMP Forum, 2001) (/gamp/) 3453462. ISO/IEC 17799:2000 (BS 7799:2000) Information technology – Code of practice for 347information security management (ISO/IEC, 2000)3483. ISO 14971:2002 Medical Devices- Application of risk management to medical devices 349350(ISO, 2001)351352。
