雷诺嗪缓释片说明书(英文)
雷诺嗪 药典标准

雷诺嗪药典标准
雷诺嗪(Ranolazine)是一种心血管选择性抗缺血药,其药典标准主要参考美国药典(USP)或者中国药典(Ch.P)中关于雷诺嗪的规定。
以下是雷诺嗪的药典标准概述。
1.品名:雷诺嗪
2.化学名称:5-[2-[[4-[(2-氰基乙基)氨基]苯基]-1,3-二氧杂-2-丙醇]-2-甲基-1,3-二氧杂-2-丙醇
3.分子式:C24H31N3O4
4.分子量:433.51
5.CAS号:95635-55-5
6.外观:白色或类白色结晶性粉末
7.熔点:178-182℃
8.用途:抗心绞痛药
9.制剂:片剂、缓释片剂
10.规格:500mg、1000mg(美国);375mg、500mg、750mg(英国)
需要注意的是,雷诺嗪的药典标准可能会随着不同国家和地区的药典版本而有所差异。
在实际应用中,应遵循医生开具的处方以及药品说明书上的用法用量。
如有疑问,请咨询专业医生。
FDA批准雷诺嗪缓释片

FDA批准雷诺嗪缓释片
佚名
【期刊名称】《世界临床药物》
【年(卷),期】2006(27)5
【摘要】美国FDA批准CV Therapeutics公司的雷诺嗪(ranolazine)500mg 薄膜包衣缓释片(Ranexa)上市,用于治疗慢性心绞痛。
本品可延长心电图QT 间隔,建议保留用于对其它抗心绞痛药物治疗无效者。
本品必需与氨氯地平、β受体阻断剂或硝酸酯类药物联用。
女性心绞痛患者接受本品治疗的疗效及治疗后运动耐量的改善,均低于男性患者。
【总页数】2页(P257-258)
【关键词】FDA批准;缓释片;雷诺嗪;Therapeutics公司;药物治疗;β受体阻断剂;慢性心绞痛;心绞痛患者;薄膜包衣;抗心绞痛
【正文语种】中文
【中图分类】R97;R971.1
【相关文献】
1.FDA拓展批准瑞戈非尼用于治疗肝癌/FDA批准midostaurin和化疗药结合治疗急性髓系白血病/FDA批准丙肝药物Sovaldi和Harvoni用于儿科患者 [J],
2.FDA批准首例用于治疗巨细胞动脉炎药物Actemra/FDA批准首个治疗ADHD 仿制药/FDA批准Ceritinib用于一线治疗ALK阳性的转移性非小细胞肺癌 [J],
3.FDA批准CV Therapeutics公司雷诺嗪缓释片作为一线抗心绞痛用药 [J],
4.拉莫三嗪缓释片获FDA批准 [J],
5.美FDA批准拉莫三嗪缓释片Lamictal XL用作部分癫痫发作附加治疗药物 [J], 马培奇
因版权原因,仅展示原文概要,查看原文内容请购买。
雷诺嗪缓释片可减少心绞痛发作频次和硝酸甘油用量

在该项试 验 中 ,4 % 的受试 5 患者 同时服 用长 效 的硝酸 酯类药
Mahu 博 士 对 此 指 出 ,药 cof
物抗性 的产 生不利于 抗逆 转录病 毒 的治疗 ,但 由于药 物抗 性的产 生同样 亦 能 削 弱 病 毒 的 复 制 能
加拿大的研究人 员在 近期 的
物治疗 ,且雷诺 嗪具有 良好的 耐
他 突变 的产生。
( 杉菜 编 译 物 I 抗性变异的产生可 使这 些病毒株
显示出更强的毒力。
嗪作为数十年来 研发 的最新 制药 技术可适用于心 绞痛 患者的 临床
治疗选择。 目前 ,雷 诺 嗪 已 获 得 美 国 F A批准用作慢性 心绞痛 治疗 的 D 二线药物 ,该 药具有 抗心 绞痛和 局部缺血 治疗作 用 ,且不 依赖 于
受性 。研究人 员迄今 尚未 在雷诺 嗪组与安 慰剂组 间发现严 重 的不
良反应 差异。
力 ,因此 可能具有 双 重性。一 旦 患者对药物产 生抗性 ,没必要立 即更换药物 ,但研 究人 员应密切 观察病毒突变的路径 。
Mahu 最 后 强 调 指 出 ,并 cof
本文第一作 者、来 自于美 国 波士顿 Sm e A Lv e妇女 医 a ul . ei n 院心脏科 的 P t t e医生 报道 er o eS n 说 ,心绞痛发作 可为 患者 及其 家 庭 以及国 家医疗保健 部 门带来 巨 大 的经济 负担 ,而 E I A研究结 RC 果提示雷诺 嗪的治 疗可为 这部 分 患者带 来显著 的益处 ,因为雷 诺
研究结果 显示 ,仅有 3种 逆
维普资讯
绞痛类药物不能达 到有 效治疗 的
患者服用。研究表 明女性 使用 雷
difene_英文说明书

Package: Blister Pack (10 tablets per blister)Ingredients:- Active Ingredient: Ibuprofen (200 mg)- Inactive Ingredients: Magnesium stearate, cellulose, lactose monohydrate, pregelatinized starch, croscarmellose sodium, hypromellose, titanium dioxide, and iron oxide.Indications:Difene Tablets are a nonsteroidal anti-inflammatory drug (NSAID) used to relieve symptoms of various conditions, including:- Pain: such as headache, dental pain, menstrual cramps, arthritis, backache, and muscular pain.- Fever: to reduce feverish symptoms.- Inflammation: to reduce inflammation in conditions such as arthritis, ankylosing spondylitis, and tendinitis.Contraindications:Do not use Difene Tablets if you have any of the following conditions:- Hypersensitivity to ibuprofen or any other NSAID.- Severe kidney disease.- Active peptic ulcer or gastrointestinal bleeding.- Third trimester of pregnancy.- Severe liver disease.- Hemorrhagic diathesis.- Children under 12 years of age (unless directed by a physician).Warnings and Precautions:- Consult a healthcare professional before taking Difene Tablets if you have a history of stomach ulcers, gastrointestinal bleeding, heart disease, high blood pressure, liver or kidney disease, asthma, or if you are taking any other medications.- Do not exceed the recommended dose to avoid potential side effects.- Do not use Difene Tablets for more than 10 days unless directed by a healthcare professional.- Avoid alcohol consumption while taking Difene Tablets.- Use caution when driving or operating machinery as Difene Tablets may cause drowsiness or dizziness in some individuals.- Difene Tablets may interact with certain medications, including anticoagulants, corticosteroids, and diuretics. Consult a healthcare professional if you are taking any of these medications.Dosage:The recommended dosage of Difene Tablets is as follows:- Adults and children over 12 years of age: 1 tablet every 4 to 6 hours as needed, not to exceed 6 tablets in 24 hours.- Elderly patients: Adjust dosage based on renal function and healthcare professional advice.How to Use:- Swallow the tablet whole with a glass of water.- Do not chew or crush the tablet.- Take Difene Tablets with or without food.Side Effects:The following side effects may occur while taking Difene Tablets:- Gastrointestinal: Nausea, vomiting, stomach pain, heartburn, indigestion, diarrhea, constipation, and ulcers.- Hematologic: Increased risk of bleeding and bruising.- Dermatologic: Skin rash, itching, and hives.- Cardiovascular: Increased blood pressure, heart failure, and myocardial infarction.- Central nervous system: Dizziness, headache, and drowsiness.Overdose:If an overdose is suspected, contact a healthcare professional immediately. Symptoms of an overdose may include severe stomach pain, vomiting, bleeding, and kidney damage.Storage:- Store Difene Tablets at room temperature (15°C to 30°C or 59°F to 86°F).- Keep the blister pack tightly closed when not in use.- Protect from light and moisture.- Do not use after the expiration date.Manufactured by:[Manufacturing Company Name][Address][City, State, ZIP Code]Please read this leaflet carefully before taking Difene Tablets. If you have any questions or concerns, consult your healthcare professional.---Note: This is a fictional product and the information provided here is for illustrative purposes only. The actual dosage, contraindications, warnings, and side effects may vary based on the specific product andits labeling. Always consult the product's official labeling and a healthcare professional before use.。
雷诺嗪合成药物的制备

公司目前主营产品包括阿达帕林、氨曲 南、那氟沙星,曲司氯铵,普卢利沙星,消 旋卡多曲,罗沙替丁,以及盐酸去甲托品醇、 盐酸去甲托品酮等托品类中间体等几十种医 药原料药,中间体等新产品。此外,公司还 协助国内医药化工企业开拓国际市场,提供 产品代理和技术服务。热忱欢迎国内外客户 携手合作,共同发展。业务范围:1.自主开 发、生产和销售医药原料和中间体。2.定制 开发和生产医药原料和中间体。3.为跨国公 司和中小企业开展在中国的采购业务。4.提 供医药原料和中间体的资讯和技术服务。
上述文献路线基本上概括了雷诺嗪的合成方法, 其中路线(2)具有较大的优势,但缺点是哌嗪没有保 护,易生成对称二取代产物,使原料的消耗量增大, 成本升高。为此本合成参考合成路线二,设计了如下 合成路线,并对合成工艺进行了系统优化。 本合成路线在合成单取代哌嗪(5)时,采用哌嗪 二盐酸盐与等摩尔哌嗪反应生成的哌嗪单盐前的抗心绞痛药物联合使用 可以显著减少心绞痛的发作次数,而且对患 者的血压只有轻微的影响,可能成为现有抗 心绞痛治疗药物的重要补充。这项试验结果 在2001年11月美国阿纳海姆召开的美国心 脏病学会科学会议上公布。现在该药物正等 待FDA的批准,如果获批,将成为近20年来 第一个新类型的抗心绞痛药物。
2000年12月,CV Therapeutics公司宣布开始 一项治疗NYHA III或IV级充血性心力衰竭的II 期临 床试验。雷诺嗪的作用机制被认为是通过将能量代 谢由脂肪酸氧化转为葡萄糖氧化而改善心肌代谢。 雷诺嗪还有一种缓释剂型,CV Therapeutics公司正 在与Catalytica制药公司进行谈判生产该剂型并进行 临床试验。1995年12月,桔生制药公司与罗氏制药 公司签定了合作协议,桔生公司拥有在亚洲(包括 日本)开发和上市该药物的独家权利。在1996年4 月,CV Therapeutics从罗氏公司手中购买了雷诺嗪 在北美和欧洲的开发和上市权。1999年5月,CV Therapeutics公司与Innovex(Quintiles)公司签定 了合作协议,共同完成雷诺嗪在美国的商业化经营。
盐酸丁咯地尔缓释片

盐酸丁咯地尔缓释片药品名称:【通用名称】盐酸丁咯地尔缓释片【商品名称】步复迈【英文名称】 Buflomedil Hydrochloride Sustained-Release Tablets【汉语拼音】 Yan Suan Ding Luo Di Er Huan Shi Pian所属类别:化药及生物制品>> 神经系统药物>> 脑血管药性状:本品为白色或类白色片。
适应症:1、周围血管疾病:雷诺综合症、血栓闭塞性脉管炎、间歇性跛行。
2、慢性脑血管供血不足引起的症状:眩晕、耳鸣、智力减退、记忆力下降、注意力不集中、定向力障碍等。
规格:0.6/片用法用量:整片吞服,每日一次,每次0.6克(一片)。
不良反应:肠胃不适、头痛、晕眩、肢端灼热刺痛感。
禁忌:下列患者禁用:对本品过敏者、急性心肌梗塞、心绞痛、阵发性心动过速、脑出血、有其他出血倾向或近期内有大量失血者。
注意事项:肝、肾功能损害者及正在服用抗高血压药物者慎用。
本品可引起头晕和思睡,因此司机及操作机器者不宜使用。
孕妇及哺乳期妇女用药:孕妇(尤其是妊娠三个月内的孕妇)及哺乳期妇女慎用。
儿童用药:儿童不宜使用。
老年用药:老年患者在肝脏代谢缓慢,通过肾脏、粪便排泄率减慢,应慎用。
药物相互作用:0. 与降血压药物合用时,可能加重血压的下降,应注意。
药物过量:过量服用会导致严重低血压、心动过速、激动、呕吐、惊厥等症状,应及时采取对症治疗。
药理毒理:本品为α肾上腺受体抑制剂,可松弛血管平滑肌,扩张血管,减少血管阻力,并有较弱的钙拮抗作用。
另外,本品还具有改善红细胞变形性,抑制血小板聚集,改善微循环,增加氧分压的作用。
药代动力学:盐酸丁咯地尔口服给药后,在肠道中容易吸收。
并广泛分布到体液和组织中。
单剂量口服本品600mg达峰时间3~6小时,血峰浓度1000~1600ng/ml,血浆半衰期为4~9小时;多剂量口服后(每日一次,每日600mg),稳态时,血峰浓度1400~2000ng/ml,谷浓度400~1000ng/ml,平均稳态血浓度1000~1500ng/ml,血药浓度波动系数0.7~1.1。
开瑞能(氯雷伪麻缓释片)

开瑞能(氯雷伪麻缓释片)【药品名称】商品名称:开瑞能通用名称:氯雷伪麻缓释片英文名称:Loratadine and Pseudoephedrine Sulfate Sustained Release T ablets【成份】本品为复方制剂,其组分为:对乙酰氨基酚,硫酸伪麻黄碱,氯雷他定。
【适应症】用于感冒引起的打喷嚏、流鼻涕、鼻塞、咽喉不适、乏力、发热、肌肉酸痛、头痛等症状及季节性过敏性鼻炎。
【用法用量】口服。
成人及12岁以上儿童每12小时服用一次,每次1~2片。
【不良反应】氯雷他定:常见与氯雷他定树敌良反应报道包括乏力,头痛、思睡、口干,胃肠功能紊乱如事业心和胃痛,过敏症状如皮疹。
在氯雷他定片的上市期间,罕见脱发、过敏反应和肝功能紊乱。
硫酸伪麻黄碱:对拟交感神经药特别敏感的患者可能有轻微的刺激反应:恶心、头痛、思睡、虚弱、眩晕、心动过速、心悸、失眠、神经过敏、兴奋、不安、瞳孔放大也有报道。
此外对乙酰氨基酚:对乙酰氨基酚推荐剂量的不良反应通常较弱。
曾有报道有血液学反应和皮疹。
有长期服用引起肾毒性作用的报道。
【禁忌】对本品任何成份或肾上腺素能类药物过敏者禁用本品;正在服用或14天内服用过单胺氧化酶抑制剂的患者禁用本品;闭角性青光眼病、尿潴留、严重高血压、严重冠状动脉疾病和甲状腺功能亢进患者亦禁用本品。
【注意事项】1.青光眼、消化性溃疡、幽门十二指肠梗阴、前列腺肥大、心血管疾患、眼内压增加或糖尿病患者以及接受洋地黄类药物治疗的患者应慎用本品。
2.对阿司匹林过敏者一般对本品不发生过敏反应。
但有报告在因阿司匹林过敏发生喘息的病人中,有不到5%的病人可用于服用本品后发生轻度支气管痉挛性反应。
3.当乙醇中毒,肝病或病毒性肝炎时,本品有增加肝脏毒性的危险,应慎用。
4.除非利大于弊,否则高血压、青光眼、肺气肿以及慢性支气管炎患者应避免服用本品。
5.服用本品期间,不宜过量饮酒。
【药物相互作用】氯雷他定:1.有临床试验显示同时服用酮康唑(Ketoconazole),红霉素(erythromycin)或西咪替丁(cimetidine)会使氯雷他定血药浓度增加,但没显著的临床症状(包括心电图);2.除非完成确定的药物相互作用,其它已知对肝代谢具有抵制作用的药物应谨慎同服;3.进行皮肤试验前至少48小时内应停用本品,抗组胺药氯雷他定药物可能抑制或消除皮肤阳性反应症状。
Leqvio(inclisiran)中文说明书

Leqvio(inclisiran)中文说明书2020年12月11日,Novartis Pharma GmbH诺华制药公司宣布欧盟已经正式批准该公司的Leqvio®(inclisiran),作为饮食控制的一种辅助手段,用于治疗成人原发性高胆固醇血症(杂合子家族性和非家族性)或混合型血脂异常,具体为:(1)Leqvio联合他汀类药物或他汀类药物和其他降脂疗法,用于治疗接受最大耐受剂量他汀类药物无法达到LDL-C治疗目标的患者;(2)Leqvio联合其他降脂疗法,用于治疗他汀类药物不耐受或他汀类药物禁忌症的患者。
值得一提的是,随着此次批准,Leqvio成为了全球第一个也是唯一一个小干扰RNA(siRNA)降胆固醇(LDL-C)疗法;是一款全球首批的“first-in-class”的治疗药物,因此具有里程碑意义。
【生产企业】:诺华制药公司【规格】: 284mg 预填充注射器 1支×1.5mL【商标】:Leqvio【英文名称】:inclisiran【性状】:一种透明、无色至淡黄色的注射液,可通过预填充注射器皮下注射。
【贮藏】:本药品不需要任何特殊的储存条件。
不冻结。
【Leqvio(inclisiran)适应症和用途】用于治疗成人原发性高胆固醇血症(杂合子家族性和非家族性)或混合型血脂异常:(1)Leqvio联合他汀类药物或他汀类药物和其他降脂疗法,用于治疗接受最大耐受剂量他汀类药物无法达到LDL-C 治疗目标的患者;(2)Leqvio联合其他降脂疗法,用于治疗他汀类药物不耐受或他汀类药物禁忌症的患者。
【Leqvio(inclisiran)剂量和给药方法】皮下注射给药,在第0、3个月各给药一次后,维持期每6个月给药一次,一年只需注射2次。
给药方法:皮下注射使用:Leqvio(inclisiran)用于腹部皮下注射;替代注射部位包括上臂或大腿。
不应在活动性皮肤病或损伤部位注射,如晒伤、皮疹、炎症或皮肤感染。
myrbetriq(mirabegron)缓释片使用说明书第一版

()缓释片使用说明书年第一版批准日期: 年月日;公司:美国药物评价和研究中心药物评价室主任,说“估计美国有千百万患膀胱过度活动症(),是一种不舒适的,破坏和潜在严重的,”“今天的批准为有这种失能情况患者提供一种新治疗选择“。
处方资料:处方资料重点这些重点不包括安全和有效使用所需所有资料。
请参阅下文为的完整处方资料(),为口服使用美国初次批准:适应证和用途是一种β肾上腺能激动剂适用于有急迫性尿失禁,急迫,和尿频症状膀胱过度活动症()的治疗()剂量和给药方法()推荐起始剂量是每天次,有或无食物。
()()是在周内有效。
根据个体疗效和耐受性,可增加剂量至每天次。
(,)()与水完整吞咽,不要咀嚼,分开或压碎。
()()患者有严重肾受损或有中度肝受损患者,最大剂量为每天次。
(,,,)()有终末肾病()或患者有严重肝受损患者。
不建议使用。
(,,,)剂量和规格缓释片:和()禁忌证无()警告和注意事项()血压增加:可增加血压。
建议定期测定血压,特别在高血压患者。
在严重未控制高血压患者中建议不使用。
().()有膀胱出口阻塞尿潴留患者和对膀胱过度活动症用抗胆碱药物患者:因为尿潴留风险在这些患者中谨慎给药。
().()用被代谢药物的患者:是一种中度抑制剂。
建议适当监视和对治疗指数狭窄的底物可能需要调整剂量。
(,,)不良反应报道的最常见不良反应(> 和> 安慰剂)是高血压,鼻咽炎,尿道感染和头痛。
()报告怀疑不良反应,联系美国,. 公司电话或电话或药物相互作用()被代谢药物(如美托洛尔[]和地昔帕明[]): 是抑制剂和当同时与被代谢药物使用时,特别治疗指数窄药物,适当监视和可能需要调整这些药物的剂量。
(,,).()地高辛[]: 当开始和地高辛联用时,处方用最低剂量的地高辛;监视血清地高辛浓度点滴逐步调整地高辛剂量至需要的临床效应。
(,).特殊人群中使用()妊娠:只有如对母亲的获益胜过对胎儿的潜在风险时才使用。
() •()哺乳母亲: 被预计在人乳汁中排泄和不建议哺乳母亲使用。
新型抗心绞痛药物雷诺嗪的研究进展

Zhao G 等人实验发现雷诺嗪既 不会影响心率,也不调节血液动力状 态或增加冠状动脉血流量。 Antzelevitch C 等人发现,雷诺嗪是
18 中国处方药 2007.10 No.67
新药指南
晚钠离子的选择性抑制剂。在离体的 心肌细胞中,晚钠离子病理性增加, 雷诺嗪可以防止或逆转其导致的机 能失常,改善心室复极化的异常情 况。Gralinski MR 通过研究发现,雷 诺嗪能防止肌原纤维结构紊乱和 Z 带的模糊,并能减轻线粒体嵴及膜损 伤。Zacharowski K 等研究发现雷诺 嗪能显著降低心肌梗死面积和肌钙 蛋白 T 的释放。
目前雷诺嗪的具体作用机制仍 不清楚。起初,人们认为它主要是通 过抑制脂肪酸 β- 氧化,增加丙酮酸 脱 氢 酶 (PDH) 活 性 ,从 而 使 另 一 能 量 来源— ——葡萄糖氧化增加。由于每摩 尔氧耗葡萄糖产生的三磷酸腺苷 (ATP)摩尔数比脂肪酸高 12%,因而 提高了心肌在缺血缺氧时氧的利用 率。然而,在临床试验中发现,只有当 Байду номын сангаас 药 浓 度 超 过 治 疗 浓 度 (>10 μmol/L)才会显示出这种药理作用。 所以以前假设的作用机制似乎不能
临床应用及不良反应
Ranexa(商品名)为薄膜包衣长 方形的缓释片,有两种规格,含雷诺 嗪 500 mg 的 鲜 橙 色 片 及 含 雷 诺 嗪 1000 mg 的淡黄色片。开始应该服用 500 mg/ 次,日两次,再根据临床症状 提高到 1000 mg/ 次。使用雷诺嗪时, 如果同时服用系 HMG 辅酶 A 还原酶
新型抗心绞痛药物雷诺嗪的研究进展
□ 广东药学院药科学院 张 浩 梁 可 曹 蕾 朱尔佳 谭载友 *
心绞痛是冠心病中较为常见的 类型。在我国,随着生活方式的改变 以及生活节奏的加快,心绞痛的发病 率逐年增高。目前仍呈上升趋势,已 接近欧美国家水平。目前广泛应用于 缺血性心脏病的药物包括硝酸酯类、 肾上腺素能受体阻滞剂、钙通道拮抗 剂等,这些药物都是通过减慢心率、 降低血压或削弱心脏泵血功能,从而 使心脏做功减少以缓解心绞痛症状。 然而,这些药物会对已经衰弱的心脏 功能产生进一步损害。因此,研究人 员一直致力于寻找其他更有效而副 作用少的途径来改善心肌缺血。
药物化学
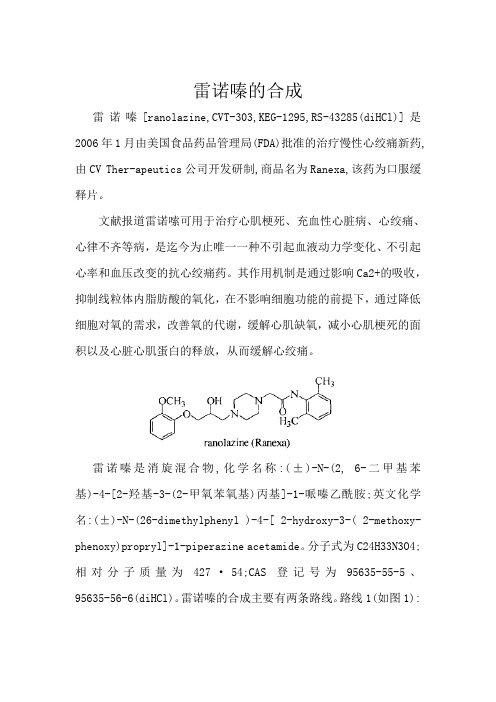
雷诺嗪的合成雷诺嗪[ranolazine,CVT-303,KEG-1295,RS-43285(diHCl)]是2006年1月由美国食品药品管理局(FDA)批准的治疗慢性心绞痛新药,由CV Ther-apeutics公司开发研制,商品名为Ranexa,该药为口服缓释片。
文献报道雷诺嗦可用于治疗心肌梗死、充血性心脏病、心绞痛、心律不齐等病,是迄今为止唯一一种不引起血液动力学变化、不引起心率和血压改变的抗心绞痛药。
其作用机制是通过影响Ca2+的吸收,抑制线粒体内脂肪酸的氧化,在不影响细胞功能的前提下,通过降低细胞对氧的需求,改善氧的代谢,缓解心肌缺氧,减小心肌梗死的面积以及心脏心肌蛋白的释放,从而缓解心绞痛。
雷诺嗪是消旋混合物,化学名称:(±)-N-(2, 6-二甲基苯基)-4-[2-羟基-3-(2-甲氧苯氧基)丙基]-1-哌嗪乙酰胺;英文化学名:(±)-N-(26-dimethylphenyl )-4-[ 2-hydroxy-3-( 2-methoxy-phenoxy)propryl]-1-piperazine acetamide。
分子式为C24H33N3O4;相对分子质量为427·54;CAS登记号为95635-55-5、95635-56-6(diHCl)。
雷诺嗪的合成主要有两条路线。
路线1(如图1):以2, 6-二甲基苯胺与氯乙酰氯发生酰化反应, 得2-氯-N-(2, 6.二甲苯基)乙酰胺;以愈创木酚与环氧氯丙烷缩合反应得1-(2-甲氧基苯氧基)-2, 3-环氧丙烷,再经哌嗪取代,环氧基开环,缩合、成盐,得雷诺嗪。
图1 雷诺嗪的合成路线1路线2(如图2):将化合物1与哌嗪进行缩合,缩合产物[(2, 6-二甲苯基)氨甲酰基甲基]哌嗪2再与1-(2-甲氧基苯氧基)-2, 3一环氧丙烷3进行环加成反应,成盐,得产品雷诺嗪。
通过比较实验,参考文献[2, 3],最终选用路线2作为合成路线,并对关键步骤进行改进,使操作简单,反应条件温和,提高了收率,适合工业化生产。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
______________________________________________________________________________________________________________________________________________________________________________________________________________HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Ranexa safely and effectively. See full prescribing information for Ranexa. Ranexa (ranolazine) extended-release tablets Initial U.S. Approval: 2006------------------------INDICATIONS AND USAGE------------------------- Ranexa is indicated for the treatment of chronic angina. (1) ----------------DOSAGE AND ADMINISTRATION------------------------ 500 mg twice daily and increase to 1000 mg twice daily, based on clinical symptoms (2.1) ----------------DOSAGE FORMS AND STRENGTHS--------------------- Extended-release tablets: 500 mg, 1000 mg (3) -------------------------CONTRAINDICATIONS----------------------------- • Use with strong CYP3A inhibitors (e.g., ketoconazole, clarithromycin, nelfinavir) (4, 7.1) • Use with CYP3A inducers (e.g., rifampin, phenobarbital) (4, 7.1) • Use in patients with clinically significant hepatic impairment (4, 8.6) ----------------WARNINGS AND PRECAUTIONS--------------------• QT interval prolongation: Can occur with ranolazine. Little data available on high doses, long exposure, use with QT interval-prolonging drugs, or potassium channel variants causing prolonged QT interval. (5.1) --------------------------ADVERSE REACTIONS------------------------- Most common adverse reactions (> 4% and more common than with placebo) are dizziness, headache, constipation, nausea. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc., at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or /medwatch. ------------------------DRUG INTERACTIONS--------------------------- • CYP3A inhibitors: Do not use Ranexa with strong CYP3A inhibitors. With moderate 3A inhibitors (e.g., diltiazem, verapamil, erythromycin), limit maximum dose of Ranexa to 500 mg twice daily. (7.1) • CYP3A inducers: Do not use Ranexa with CYP3A inducers. (7.1) • P-gp inhibitors (e.g., cyclosporine): May need to lower Ranexa dose based on clinical response. (7.1) • Drugs transported by P-gp or metabolized by CYP2D6 (e.g., digoxin, tricyclic antidepressants): May need reduced doses of these drugs when used with Ranexa. (7.2) See 17 for PATIENT COUNSELING INFORMATION Revised: 09/2010FULL PRESCRIBING INFORMATION: CONTENTS* 1 INDICATIONS AND USAGE2 DOSAGE AND ADMINISTRATION2.1 Dosing Information 2.2 Dose Modification3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 QT Interval Prolongation 6 A DVERSE REACTIONS6.1 Clinical Trial Experience 7 D RUG INTERACTIONS7.1 Effects of Other Drugs on Ranolazine 7.2 Effects of Ranolazine on Other Drugs 8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy 8.3 Nursing Mothers 8.4 Pediatric Use 8.5 Geriatric Use 8.6 Use in Patients with Hepatic Impairment 8.7 Use in Patients with Renal Impairment 8.8 Use in Patients with Heart Failure 8.9 Use in Patients with Diabetes Mellitus 10 OVERDOSAGE 11 DESCRIPTION 12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action 12.2 Pharmacodynamics 12.3 Pharmacokinetics 13 N ONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility 13.3 Reproductive Toxicology Studies 14 C LINICAL STUDIES14.1 Chronic Stable Angina 14.2 Lack of Benefit in Acute Coronary Syndrome 15 REFERENCES 16 HOW SUPPLIED/STORAGE AND HANDLING 17 PATIENT COUNSELING INFORMATION* Sections or subsections omitted from the full prescribing information are not listed.FULL PRESCRIBING INFORMATION1 INDICATIONS AND USAGERanexa is indicated for the treatment of chronic angina.Ranexa may be used with beta-blockers, nitrates, calcium channel blockers, anti-platelet therapy, lipid-lowering therapy, ACE inhibitors, and angiotensin receptor blockers.2 DOSAGE AND ADMINISTRATION2.1 Dosing InformationInitiate Ranexa dosing at 500 mg twice daily and increase to 1000 mg twice daily, as needed, based on clinical symptoms. Take Ranexa with or without meals. Swallow Ranexa tablets whole; do not crush, break, or chew.The maximum recommended daily dose of Ranexa is 1000 mg twice daily.If a dose of Ranexa is missed, take the prescribed dose at the next scheduled time; do not double the next dose.Modification2.2 DoseDose adjustments may be needed when Ranexa is taken in combination with certain other drugs [see Drug Interactions (7.1)]. Limit the maximum dose of Ranexa to 500 mg twice daily in patients on diltiazem, verapamil, and other moderate CYP3A inhibitors. Down-titrate Ranexa based on clinical response in patients concomitantly treated with P-gp inhibitors, such as cyclosporine.3 DOSAGE FORMS AND STRENGTHSRanexa is supplied as film-coated, oblong-shaped, extended-release tablets in the following strengths:•500 mg tablets are light orange, with GSI500 on one side•1000 mg tablets are pale yellow, with GSI1000 on one side4 CONTRAINDICATIONSRanexa is contraindicated in patients:•Taking strong inhibitors of CYP3A [see Drug Interactions (7.1)]•Taking inducers of CYP3A [see Drug Interactions (7.1)]•With clinically significant hepatic impairment [see Use in Specific Populations (8.6)]5 WARNINGS AND PRECAUTIONS5.1 QT Interval ProlongationRanolazine blocks I Kr and prolongs the QTc interval in a dose-related manner.Clinical experience in an acute coronary syndrome population did not show an increased risk of proarrhythmia or sudden death [see Clinical Studies (14.2)]. However, there is little experience with high doses (> 1000 mg twice daily) or exposure, other QT-prolonging drugs, or potassium channel variants resulting in a long QT interval.6 ADVERSE REACTIONS6.1 Clinical Trial ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.A total of 2,018 patients with chronic angina were treated with ranolazine in controlled clinical trials. Of the patients treated with Ranexa, 1,026 were enrolled in three double-blind, placebo-controlled, randomized studies (CARISA, ERICA, MARISA) of up to 12 weeks duration. In addition, upon study completion, 1,251 patients received treatment with Ranexa in open-label, long-term studies; 1,227 patients were exposed to Ranexa for more than 1 year, 613 patients for more than 2 years, 531 patients for more than 3 years, and 326 patients for more than 4 years. At recommended doses, about 6% of patients discontinued treatment with Ranexa because of an adverse event in controlled studies in angina patients compared to about 3% on placebo. The most common adverse events that led to discontinuation more frequently on Ranexa than placebo were dizziness (1.3% versus 0.1%), nausea (1% versus 0%), asthenia, constipation, and headache (each about 0.5% versus 0%). Doses above 1000 mg twice daily are poorly tolerated.In controlled clinical trials of angina patients, the most frequently reported treatment-emergent adverse reactions (> 4% and more common on Ranexa than on placebo) were dizziness (6.2%), headache (5.5%), constipation (4.5%), and nausea (4.4%). Dizziness may be dose-related. In open-label, long-term treatment studies, a similar adverse reaction profile was observed.The following additional adverse reactions occurred at an incidence of 0.5 to 2.0% in patients treated with Ranexa and were more frequent than the incidence observed in placebo-treated patients:Cardiac Disorders – bradycardia, palpitationsEar and Labyrinth Disorders – tinnitus, vertigoGastrointestinal Disorders – abdominal pain, dry mouth, vomitingGeneral Disorders and Administrative Site Adverse Events – peripheral edemaRespiratory, Thoracic, and Mediastinal Disorders – dyspneaVascular Disorders – hypotension, orthostatic hypotensionOther (< 0.5%) but potentially medically important adverse reactions observed more frequently with Ranexa than placebo treatment in all controlled studies included: angioedema, renal failure, eosinophilia, blurred vision, confusional state, hematuria, hypoesthesia, paresthesia, tremor, pulmonary fibrosis, thrombocytopenia, leukopenia, and pancytopenia.A large clinical trial in acute coronary syndrome patients was unsuccessful in demonstrating a benefit for Ranexa, but there was no apparent proarrhythmic effect in these high-risk patients [see Clinical Trials (14.2)].Laboratory AbnormalitiesRanexa produces small reductions in hemoglobin A1c. Ranexa is not a treatment for diabetes.Ranexa produces elevations of serum creatinine by 0.1 mg/dL, regardless of previous renal function. The elevation has a rapid onset, shows no signs of progression during long-term therapy, is reversible after discontinuation of Ranexa, and is not accompanied by changes in BUN. In healthy volunteers, Ranexa 1000 mg twice daily had no effect upon the glomerular filtration rate. The elevated creatinine levels are likely due to a blockage of creatinine’s tubular secretion by ranolazine or one of its metabolites.7 DRUG INTERACTIONS7.1 Effects of Other Drugs on RanolazineRanolazine is primarily metabolized by CYP3A and is a substrate of P-glycoprotein (P-gp).CYP3A InhibitorsDo not use Ranexa with strong CYP3A inhibitors, including ketoconazole, itraconazole, clarithromycin, nefazodone, nelfinavir, ritonavir, indinavir, and saquinavir. Ketoconazole (200 mg twice daily) increases average steady-state plasma concentrations of ranolazine 3.2-fold [see Contraindications (4)].Limit the dose of Ranexa to 500 mg twice daily in patients on moderate CYP3A inhibitors, including diltiazem, verapamil, aprepitant, erythromycin, fluconazole, and grapefruit juice or grapefruit-containing products. Diltiazem (180–360 mg daily) and verapamil (120 mg three times daily) increase ranolazine steady-state plasma concentrations about 2-fold [see Dosage and Administration (2.2)].Weak CYP3A inhibitors such as simvastatin (20 mg once daily) and cimetidine (400 mg three times daily) do not increase the exposure to ranolazine in healthy volunteers.P-gp InhibitorsDown-titrate Ranexa based on clinical response in patients concomitantly treated with P-gp inhibitors, such as cyclosporine [see Dosage and Administration (2.2)].CYP3A and P-gp InducersAvoid co-administration of Ranexa and CYP3A inducers such as rifampin, rifabutin, rifapentin, phenobarbital, phenytoin, carbamazepine, and St. John’s wort. Rifampin (600 mg once daily) decreases the plasma concentration of ranolazine (1000 mg twice daily) by approximately 95% by induction of CYP3A and, probably, P-gp.7.2 Effects of Ranolazine on Other DrugsIn vitro studies indicate that ranolazine and its O-demethylated metabolite are weak inhibitors of CYP3A, moderate inhibitors of CYP2D6 and moderate P-gp inhibitors.Drugs Transported by P-gpRanexa (1000 mg twice daily) causes a 1.5-fold elevation of digoxin plasma concentrations. The dose of digoxin may have to be adjusted.Drugs Metabolized by CYP2D6Ranexa 750 mg twice daily increased the plasma concentrations of a single dose of immediate-release metoprolol (100 mg), a CYP2D6 substrate, by 1.8-fold. The exposure to other CYP2D6 substrates, such as tricyclic antidepressants and antipsychotics, may be increased during coadministration with Ranexa, and lower doses of these drugs may be required.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyPregnancy Category CIn animal studies, ranolazine at exposures 1.5 (rabbit) to 2 (rat) times the usual human exposure caused maternal toxicity and misshapen sternebrae and reduced ossification in offspring. These doses in rats and rabbits were associated with an increased maternal mortality rate [see Reproductive Toxicology Studies (13.3)]. There are no adequate well-controlled studies in pregnant women. Ranexa should be used during pregnancy only when the potential benefit to the patient justifies the potential risk to the fetus.8.3 Nursing MothersIt is not known whether ranolazine is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions from ranolazine in nursing infants, decide whether to discontinue nursing or to discontinue Ranexa, taking into account the importance of the drug to the mother.8.4 Pediatric UseSafety and effectiveness have not been established in pediatric patients.Use8.5 GeriatricOf the chronic angina patients treated with Ranexa in controlled studies, 496 (48%) were≥ 65 years of age, and 114 (11%) were ≥ 75 years of age. No overall differences in efficacy were observed between older and younger patients. There were no differences in safety for patients ≥ 65 years compared to younger patients, but patients ≥ 75 years of age on ranolazine, compared to placebo, had a higher incidence of adverse events, serious adverse events, and drug discontinuations due to adverse events. In general, dose selection for an elderly patient should usually start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease, or other drug therapy.8.6 Use in Patients with Hepatic ImpairmentRanexa is contraindicated in patients with clinically significant hepatic impairment. Plasma concentrations of ranolazine were increased by 30% in patients with mild (Child-Pugh Class A) and by 60% in patients with moderate (Child-Pugh Class B) hepatic impairment. This was not enough to account for the 3-fold increase in QT prolongation seen in patients with mild to severe hepatic impairment [see Contraindications (4)].8.7 Use in Patients with Renal ImpairmentIn patients with varying degrees of renal impairment, ranolazine plasma levels increased up to 50%. The pharmacokinetics of ranolazine has not been assessed in patients on dialysis.8.8 Use in Patients with Heart FailureHeart failure (NYHA Class I to IV) had no significant effect on ranolazine pharmacokinetics. Ranexa had minimal effects on heart rate and blood pressure in patients with angina and heart failure NYHA Class I to IV. No dose adjustment of Ranexa is required in patients with heart failure.8.9 Use in Patients with Diabetes MellitusA population pharmacokinetic evaluation of data from angina patients and healthy subjects showed no effect of diabetes on ranolazine pharmacokinetics. No dose adjustment is required in patients with diabetes.Ranexa produces small reductions in HbA1c in patients with diabetes, the clinical significance of which is unknown. Ranexa should not be considered a treatment for diabetes.10 OVERDOSAGEHigh oral doses of ranolazine produce dose-related increases in dizziness, nausea, and vomiting. High intravenous exposure also produces diplopia, paresthesia, confusion, and syncope. In addition to general supportive measures, continuous ECG monitoring may be warranted in the event of overdose.Since ranolazine is about 62% bound to plasma proteins, hemodialysis is unlikely to be effective in clearing ranolazine.11 DESCRIPTIONRanexa (ranolazine) is available as a film-coated, non-scored, extended-release tablet for oral administration.Ranolazine is a racemic mixture, chemically described as 1-piperazineacetamide, N-(2,6dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]-, (±)-. It has an empirical formula of C24H33N3O4, a molecular weight of 427.54 g/mole, and the following structural formula:HN N NOHOOMeRanolazine is a white to off-white solid. Ranolazine is soluble in dichloromethane and methanol; sparingly soluble in tetrahydrofuran, ethanol, acetonitrile, and acetone; slightly soluble in ethyl acetate, isopropanol, toluene, and ethyl ether; and very slightly soluble in water. Ranexa tablets contain 500 mg or 1000 mg of ranolazine and the following inactive ingredients: carnauba wax, hypromellose, magnesium stearate, methacrylic acid copolymer (Type C), microcrystalline cellulose, polyethylene glycol, sodium hydroxide, and titanium dioxide. Additional inactive ingredients for the 500 mg tablet include polyvinyl alcohol, talc, Iron Oxide Yellow, and Iron Oxide Red; additional inactive ingredients for the 1000 mg tablet include lactose monohydrate, triacetin, and Iron Oxide Yellow.12 CLINICAL PHARMACOLOGY12.1 Mechanism of ActionThe mechanism of action of ranolazine’s antianginal effects has not been determined. Ranolazine has anti-ischemic and antianginal effects that do not depend upon reductions in heart rate or blood pressure. It does not affect the rate-pressure product, a measure of myocardial work, at maximal exercise. Ranolazine at therapeutic levels can inhibit the cardiac late sodium current (I Na). However, the relationship of this inhibition to angina symptoms is uncertain.The QT prolongation effect of ranolazine on the surface electrocardiogram is the result of inhibition of I Kr, which prolongs the ventricular action potential.12.2 PharmacodynamicsHemodynamic EffectsPatients with chronic angina treated with Ranexa in controlled clinical studies had minimal changes in mean heart rate (< 2 bpm) and systolic blood pressure (< 3 mm Hg). Similar results were observed in subgroups of patients with CHF NYHA Class I or II, diabetes, or reactive airway disease, and in elderly patients.Electrocardiographic EffectsDose and plasma concentration-related increases in the QTc interval [see Warnings and Precautions (5.1)], reductions in T wave amplitude, and, in some cases, notched T waves, have been observed in patients treated with Ranexa. These effects are believed to be caused by ranolazine and not by its metabolites. The relationship between the change in QTc and ranolazine plasma concentrations is linear, with a slope of about 2.6 msec/1000 ng/mL, through exposures corresponding to doses several-fold higher than the maximum recommended dose of 1000 mg twice daily. The variable blood levels attained after a given dose of ranolazine give a wide range of effects on QTc. At T max following repeat dosing at 1000 mg twice daily, the mean change in QTc is about 6 msec, but in the 5% of the population with the highest plasmaconcentrations, the prolongation of QTc is at least 15 msec. In subjects with mild or moderate hepatic impairment, the relationship between plasma level of ranolazine and QTc is much steeper [see Contraindications (4)].Age, weight, gender, race, heart rate, congestive heart failure, diabetes, and renal impairment did not alter the slope of the QTc-concentration relationship of ranolazine.No proarrhythmic effects were observed on 7-day Holter recordings in 3,162 acute coronary syndrome patients treated with Ranexa. There was a significantly lower incidence of arrhythmias (ventricular tachycardia, bradycardia, supraventricular tachycardia, and new atrial fibrillation) in patients treated with Ranexa (80%) versus placebo (87%), including ventricular tachycardia ≥ 3 beats (52% versus 61%). However, this difference in arrhythmias did not lead to a reduction in mortality, a reduction in arrhythmia hospitalization, or a reduction in arrhythmia symptoms.12.3 PharmacokineticsRanolazine is extensively metabolized in the gut and liver and its absorption is highly variable. For example, at a dose of 1000 mg twice daily, the mean steady-state C max was 2600 ng/mL with 95% confidence limits of 400 and 6100 ng/mL. The pharmacokinetics of the (+) R- and(-) S-enantiomers of ranolazine are similar in healthy volunteers. The apparent terminal half-life of ranolazine is 7 hours. Steady state is generally achieved within 3 days of twice-daily dosing with Ranexa. At steady state over the dose range of 500 to 1000 mg twice daily, C max andAUC0-τ increase slightly more than proportionally to dose, 2.2- and 2.4-fold, respectively. With twice-daily dosing, the trough:peak ratio of the ranolazine plasma concentration is 0.3 to 0.6. The pharmacokinetics of ranolazine is unaffected by age, gender, or food.Absorption and DistributionAfter oral administration of Ranexa, peak plasma concentrations of ranolazine are reached between 2 and 5 hours. After oral administration of 14C-ranolazine as a solution, 73% of the dose is systemically available as ranolazine or metabolites. The bioavailability of ranolazine from Ranexa tablets relative to that from a solution of ranolazine is 76%. Because ranolazine is a substrate of P-gp, inhibitors of P-gp may increase the absorption of ranolazine.Food (high-fat breakfast) has no important effect on the C max and AUC of ranolazine. Therefore, Ranexa may be taken without regard to meals. Over the concentration range of 0.25 to10 μg/mL, ranolazine is approximately 62% bound to human plasma proteins.Metabolism and ExcretionRanolazine is metabolized mainly by CYP3A and, to a lesser extent, by CYP2D6. Following a single oral dose of ranolazine solution, approximately 75% of the dose is excreted in urine and 25% in feces. Ranolazine is metabolized rapidly and extensively in the liver and intestine; less than 5% is excreted unchanged in urine and feces. The pharmacologic activity of the metabolites has not been well characterized. After dosing to steady state with 500 mg to 1500 mg twicedaily, the four most abundant metabolites in plasma have AUC values ranging from about 5 to 33% that of ranolazine, and display apparent half-lives ranging from 6 to 22 hours.Drug InteractionsEffect of other drugs on ranolazineCYP2D6 InhibitorsThe potent CYP2D6 inhibitor, paroxetine (20 mg once daily), increases ranolazine concentrations 1.2-fold. No dose adjustment of Ranexa is required in patients treated with CYP2D6 inhibitors.DigoxinDigoxin (0.125 mg) does not significantly alter ranolazine levels.Effect of ranolazine on other drugsRanolazine and its most abundant metabolites are not known to inhibit the metabolism of substrates for CYP 1A2, 2C8, 2C9, 2C19, or 2E1 in human liver microsomes, suggesting that ranolazine is unlikely to alter the pharmacokinetics of drugs metabolized by these enzymes. Drugs Metabolized by CYP3AThe plasma levels of simvastatin, a CYP3A substrate, and its active metabolite are each increased about 2-fold in healthy subjects receiving simvastatin (80 mg once daily) and Ranexa (1000 mg twice daily). Dose adjustments of simvastatin are not required when Ranexa isco-administered with simvastatin.The pharmacokinetics of diltiazem is not affected by ranolazine in healthy volunteers receiving diltiazem 60 mg three times daily and Ranexa 1000 mg twice daily.13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of FertilityRanolazine tested negative for genotoxic potential in the following assays: Ames bacterial mutation assay, Saccharomyces assay for mitotic gene conversion, chromosomal aberrations assay in Chinese hamster ovary (CHO) cells, mammalian CHO/HGPRT gene mutation assay, and mouse and rat bone marrow micronucleus assays.There was no evidence of carcinogenic potential in mice or rats. The highest oral doses used in the carcinogenicity studies were 150 mg/kg/day for 21 months in rats (900 mg/m2/day) and50 mg/kg/day for 24 months in mice (150 mg/m2/day). These maximally tolerated doses are 0.8 and 0.1 times, respectively, the maximum recommended human dose (MRHD) of 2 grams on a surface area basis. A published study reported that ranolazine promoted tumor formation and progression to malignancy when given to transgenic APC (min/+) mice at a dose of 30 mg/kg twice daily [see References (15)]. The clinical significance of this finding is unclear.13.3 Reproductive Toxicology StudiesAnimal reproduction studies with ranolazine were conducted in rats and rabbits.There was an increased incidence of misshapen sternebrae and reduced ossification of pelvic and cranial bones in fetuses of pregnant rats dosed at 400 mg/kg/day (2 times the MRHD on a surface area basis). Reduced ossification of sternebrae was observed in fetuses of pregnant rabbits dosed at 150 mg/kg/day (1.5 times the MRHD on a surface area basis). These doses in rats and rabbits were associated with an increased maternal mortality rate.14 CLINICAL STUDIES14.1 Chronic Stable AnginaCARISA (Combination Assessment of Ranolazine In Stable Angina) was a study in 823 chronic angina patients randomized to receive 12 weeks of treatment with twice-daily Ranexa 750 mg, 1000 mg, or placebo, who also continued on daily doses of atenolol 50 mg, amlodipine 5 mg, or diltiazem CD 180 mg. Sublingual nitrates were used in this study as needed.In this trial, statistically significant (p < 0.05) increases in modified Bruce treadmill exercise duration and time to angina were observed for each Ranexa dose versus placebo, at both trough (12 hours after dosing) and peak (4 hours after dosing) plasma levels, with minimal effects on blood pressure and heart rate. The changes versus placebo in exercise parameters are presented in Table 1. Exercise treadmill results showed no increase in effect on exercise at the 1000 mg dose compared to the 750 mg dose.Table 1 Exercise Treadmill Results (CARISA)Mean Difference from Placebo (sec) Study CARISA (N = 791)Ranexa Twice-daily Dose 750 mg 1000 mgExercise DurationTrough Peak 24*34**24*26*Time to AnginaTrough Peak 30*38**26*38**Time to 1 mm ST-Segment DepressionTrough Peak 2041**2135*** p-value ≤0.05 ** p-value ≤ 0.005The effects of Ranexa on angina frequency and nitroglycerin use are shown in Table 2.Table 2 Angina Frequency and Nitroglycerin Use (CARISA)Placebo Ranexa750 mg aRanexa1000 mg aAngina Frequency (attacks/week) N 258272261 Mean 3.3 2.5 2.1p-value vs placebo — 0.006 <0.001Nitroglycerin Use (doses/week) N 252262244 Mean 3.1 2.1 1.8p-value vs placebo — 0.016 <0.001a Twice dailyTolerance to Ranexa did not develop after 12 weeks of therapy. Rebound increases in angina, as measured by exercise duration, have not been observed following abrupt discontinuation of Ranexa.Ranexa has been evaluated in patients with chronic angina who remained symptomatic despite treatment with the maximum dose of an antianginal agent. In the ERICA (Efficacy of Ranolazine In Chronic Angina) trial, 565 patients were randomized to receive an initial dose of Ranexa 500 mg twice daily or placebo for 1 week, followed by 6 weeks of treatment with Ranexa 1000 mg twice daily or placebo, in addition to concomitant treatment with amlodipine 10 mg once daily. In addition, 45% of the study population also received long-acting nitrates. Sublingual nitrates were used as needed to treat angina episodes. Results are shown in Table 3. Statistically significant decreases in angina attack frequency (p = 0.028) and nitroglycerin use (p = 0.014) were observed with Ranexa compared to placebo. These treatment effects appeared consistent across age and use of long-acting nitrates.Table 3 Angina Frequency and Nitroglycerin Use (ERICA)Placebo Ranexa aAngina Frequency (attacks/week) N 281 277 Mean 4.3 3.3 Median 2.4 2.2Nitroglycerin Use (doses/week) N 281 277 Mean 3.6 2.7 Median 1.7 1.3a 1000 mg twice dailyGenderEffects on angina frequency and exercise tolerance were considerably smaller in women than in men. In CARISA, the improvement in Exercise Tolerance Test (ETT) in females was about 33% of that in males at the 1000 mg twice-daily dose level. In ERICA, where the primary endpoint was angina attack frequency, the mean reduction in weekly angina attacks was 0.3 for females and 1.3 for males.。
