应用化学专业英语
应用化学专业英语

英译汉:1.First, electrons are added one at a time moving from left to right across aperiod……首先,从左向右横跨一个周期时每次增加一个电子。
当这种情况发生时,最外层电子将受到逐渐增强的核引力,所以电子将更接近原子核而受到其更紧密的束缚力。
其次,在周期表中从上向下移动一列,最外层电子受到核的束缚力将变弱。
这是因为主能级数(屏蔽最外层电子受到核的吸引)在每族向下移动时增加。
这些趋势解释了通过观察元素的原子半径、电离能、电子亲和力和电负性而得到的元素性质的周期性规律。
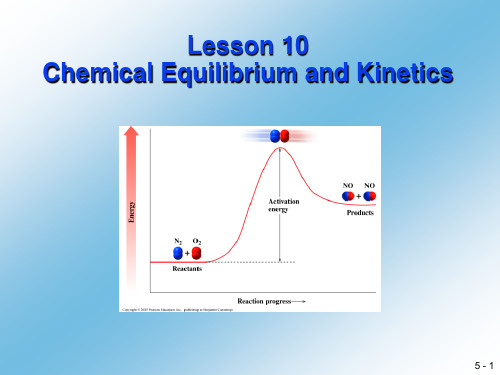
2.It is important to note that at equilibrium the rates of reaction,rate r and rate fare equilibrium mixture are usually not equal……值得注意的是,在化学平衡时的反应速率,正反应速率和你反应速率相等但反应物和生成物的摩尔浓度在平衡混合态时一般不相等。
但是,事实上每种反应物和生成物在平衡时其浓度为定值,因为每种物质在一个反应中的消耗速率与其在相应你反应正的生成速率相等。
在化学平衡提出之前,这种系统被称为动力学平衡状态。
3.This is a mathematical expression of the law of chemical equilibrium which maybe stated as follows: When a reversible…………这是化学平衡定律的数学表达式,它可以通过如下所述:当一个可逆反应在给定温度下达到平衡时,在方程式中箭头右边物质的摩尔浓度的积除以左边物质摩尔浓度的积(每种物质浓度的幂等于反应方程式中每种物质的分子数)为定值,4.Analytical chemistry,or the art of recognizing different substances anddetermining their constituents, takes a prominent position among分析化学或鉴定不同物质并测定其成分的技术,因为可以解决每当化学过程被用于科学的或技术性的目的是产生的问题,而在科学应用领域中占显著地位。
应用化学专业英语lesson10ChemicalEquilibriumandkinetics
NH3
Add more NH3?
Reaction shifts to the left [N2] and [H2] inc
5 - 35
Le Chatelier’s principle
Adding Pressure affects an equilibrium with gases
N2(g) + 3 H2(g)
N2(g) + 3 H2(g)
Keq =
[ NH3 ] 2 [ N2 ] [ H2 ] 3
2 NH3(g)
5 - 33
Le Chatelier’s principle
Stress causes shift in equilibrium Adding or removing reagent
N2(g) + 3 H2(g)
Temperature: 2. Higher Temperature:
Faster cars More collisions
More Energy More collisions
Reacting molecules move faster, providing colliding molecules w/ Eact.
At this point, equilibrium is achieved. Time
5 - 26
Figure 9.8
2SO2(g) + O2(g)
At Equilibium
2SO3(g)
SO2(g)+O2(g)
Initially
SO3(g)
Initially
5 - 27
Figure 9.9
2SO2(g) + O2(g)
应用化学专业英语 -无机化学命名

(3) 基本元素有多种价态 酸:最低氧化态(次酸) 基础元素(前缀 hypo-, 后缀 -ous) +acid 较低氧化态(亚酸) 基础元素加后缀-ous + acid 较高氧化态 (正酸) 基础元素加后缀-ic + acid 最高氧化态(高酸) 基础元素(前缀 per-, 后缀 -ic) +acid 盐:最低氧化态 阳离子元素 + 基础元素(前缀 hypo-, 后缀 -ite) 较低氧化态 阳离子元素 + 基础元素加后缀-ite 较高氧化态 阳离子元素 + 基础元素加后缀-ate 最高氧化态 阳离子元素 + 基础元素(前缀 per-, 后缀 -ate)
16. K4[Fe(CN)6]; 17. CuSO4· 5H2O 18. Cu2(OH)2CO3 19. NaNH4SO4
1. (NH4)2CO3: ammonium carbonate 2. N2O: nitrogen(Ⅰ) oxide; nitrous oxide ; laughing gas 3. H2SO4: sulphuric acid 4. P4O6 diphosphorus trioxide 5. Al2O3 Aluminum oxide 6. SnCl4 tin(Ⅳ) chloride; stannic chloride; tin terachloride 7. KHSO4 Potassium hydrogen sulfate 8. Cu2S copper(I) sulphide; dicopper sulphide 9. HClO4 perchloric acid
含氧酸及其盐:
(1) 基本元素仅有一种氧化态
酸:基本元素加后缀-ic +acid 例:H2CO3 carbonic acid 盐:阳离子元素+基础元素加后缀-ate 例:Na2CO3 sodium carbonate (2) 基本元素有两种氧化态 酸:基础元素加后缀(-ous 低价态,-ic 高价态) + acid HNO2:nitrous acid HNO3: nitric acid 盐:阳离子元素+基础元素加后缀( -ite低价态,-ate 高价态) NaNO2: Co(NO3)2: sodium nitrite cobalt(II) nitrate or cobaltous nitrate
应用化学专业英语复习资料

一单词短语1.Molecule 分子molecular 分子的2.chemical process 化学过程element 元素3.a t o m原子a t t r a c t i o n吸引力4.repulsion 排斥力distillation 蒸馏、n5.distill 蒸馏v rectification 精馏position 构成structure 结构7.property 性质mass 质量8.atomicweight 原子量atomic number 原子序数9.ionization energy 电离能period 周期10.g r o u p族f a m i l y族11.transition group 过渡族main group 主族12.i o n离子s u b s t i t u t i o n取代反应13.el i mi na ti on消除反应nucl eoph i l i c 亲核的14.nucleophilie 亲核试剂electrophilie亲电试剂15.alkyl 烷基的functional group 官能团16.halides 卤素的leaving group 离去基团17.transition state过渡态intermediate 中间体18.r e a c t a n t反应物p r o d u c t生成物19.concentration 浓度rate equation 速率方程20.c o n s t a n t常数e t h e r醚21.endothermic 吸热的substrate 反应底物22.mechanism 机理reagen 试剂23.alkene 烯烃exothermic 放热的24.A n i o n阴离子n i t r o g e n氮气25.Hydrocarbon 碳氢化合物carbonhydrate 碳水化合物26.Alkane 烷烃substituent 取代基27.Isomerism 同分异构现象isomer 同分异构28.V i n y l乙烯基d e r i v a t i v e s衍生物29.acid halides 酰卤acid anhydrides 酸酐30.e s t e r s酯a m i d e酰胺31.ammonia NH3 Acetic anhydride乙酸酐32.phenol 芬acid—base titration 酸碱滴定33.precipitation沉淀analyses 化学分析员34.IR 红外UV紫外MS质谱GC色相色谱HPLC高效液相色谱TLC薄层色谱X—rayX射线衍射二选词填空1、We can now easily account for many things,which were thought to be mysterious by theancients2、the acid acts on the metal and a gas is givenoff.3、you should adapt yourself to new ways oflooking at matters4、electrolytes have more pronounced effect oncolligative properties than do nonelectrolytes. 5、if water in these lakes evaporated at the samerate as fresh water ,both would nearly dryup in a matter of year.6、both laks evaporated very slow compared with afresh lake or even the ocean.7、a property that depends only on the relativeamounts of solute and solvent is know as acolligative property.8、for example ,both NaCl (ionic) and HCl (polarcovalent)are classified as electrolytes becausethey form ions in aqueous solution.9、when compounds such as NaCl and HCl aredissolved in water ,the effect is obvious.10、if the wires is cut ,the light goes out becausethe circuit is broken.11、when wires are attached to a charged batteryand then to a light bulb ,the light shinesbrightly.12、glass and wood as well as pure water areexamples or nonconductors of electricity.13、other substances resist the flow of electricityand are known as nonconductors orinsulators.14、it has long been known that the presence of asolute in water may affect its ability toconduct electricity.15、when the collection of papers was first broughtout,it was well received by the reviewers.16、in the same way the dozen or so mostcommon kinds of kinds of atoms can be put together in many millions of different ways tomake molecules .17、elements are made up of tiny fundamentalparticles called atoms. Fundamental, as it is usedhere ,means that they cannot be furtherdivided by any chemical metheods.18、each element has atoms that is different fromthe atoms of other elements.19、it would not be quite round; on the contraryit would consist of three parts represented byspheres.20、it is not to be summed up in a singleproduct or word ,but in an idea or basicconcept.21、the chemical symbol of an element may standthe element for.22、the rate of a chemical reaction is influencedby several factors such as temperature ,concentration of reagents , particle size ,light ,and catalyst.23、all forms of life in earth are very dependenton chemical reactions or chemical changes.24、a chemical reaction occurs when elements andcompounds react together to produce differentcompounds , or when compounds break down into simpler compounds or elements.三无机物的命名H Hydrogen Li Lithium Na Sodium K Potassium Mg Magnesium Ca CalciumMn manganese Cu copper Zn zinc Fe iron Hg mercury Ag silver Au gold C Carbon Si Silicon Pb Lead Al Aluminium F Fluorine Cl Chlorine Br Bromine I IodineO Oxygen S Sulfur N Nitrogen P Phosphorus1.直呼其名,即读其元素名称+ ion如:Na+ sodium ionK+ potassium ion2.对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous 表示低价,-ic 表示高价如:Cu+ copper (Ⅰ) ion 或cuprous ion Cu2+ copper (Ⅱ) ion 或cupric ionFe2+ iron (Ⅱ) ion 或ferrous ionFe3+ iron (Ⅲ) ion 或ferric ion3.含氢酸根:酸根中的H读做hydrogen,氢原子的个数用希腊前缀表示:mono- di - tri- tetra - penta- hexa-hepta- octa- nona- deca-举例:CO32-carbonate ionHCO3-hydrogen carbonate ionPO43- phosphate ionHPO42hydrogencarbonate ionH2PO4- dihydrogenphosphate ion4.结晶水读做hydrate ,结晶水的个数用希腊前缀表示:mono-di - tri- tetra - penta- hexa- hepta- octa- nona- deca-CuSO4·5H2O copper(Ⅱ) sulfate pentahydrateAlCl3 ·6H2O aluminum chloride hexahydrate5.测试Mg(OH)2magnesium hydroxide AlCl3aluminum chlorideFeBr2 iron(II) bromide CaSO4calcium sulfateZnCO3zinc carbonate HF hydrofluoric acidH3PO4phosphoric acid NO2nitrogen dioxideCuO copper(II) oxide Al2O3aluminum oxideNaHSO3sodium hydrogen sulfiteKMnO4potassium permanganateNaClO sodium hypochloride四有机物的命名1)命名正烷基时,只需把烷烃的词尾“-ane换成“-yl”,加在相应的烷烃的字首后2)字母规则:Butyl>Ethyl>Isopropyl>Methyl>Neopentyl>tert-Pentyl >Propyl3)环烷烃:只需在所对应的烷烃前加上cyclo-即可4)有些结构较复杂的烷基,需添加词头5)烯烃和炔烃命名时将相应的烷烃的词尾“烷”(ane)改为“烯”(ene)或“炔”(yne),后缀前加上不饱和键的编号即可。
应用化学专业英语翻译

Unit1 The Roots ofChemistry 化学的起源1.Chemistry can be broadly defines as the science of molecules and their transf ormations.化学可以被广泛的定义为分子的科学和它们之间的转换。
和数学不同,化学在人类之前。
我们的星球(地球)上的生命和人类的外观很可能是化学进程的具体结果。
化学过程从历史的开端一直到现在都出现在人们的生活中。
最初,这些过程不在我们的掌控之中,例如,果汁的发酵,肉和鱼的腐烂,木头的燃烧。
后来我们学着去控制化学进程使用它来生产不同的产品,比如食物,金属,陶瓷和皮革。
在化学的发展上,主要区分为四个阶段:史前化学,希腊化学,炼金术,科学化学。
2.The early beginnings of chemistry were clearly motivated by practical needs of people .早期的化学显然是出于实际的需要。
火的发现为远古人提供了第一个机会去实现控制化学反应过程。
他们学会制备铜制物品,铜和其它材料是现成的。
.由于化学过程的使用早于人们的书写,因而没有书面记录有关它们的化学技巧。
可以判断他们的化学能力只有从考古的发现的各个手工艺品。
正如早期的数学发展,清楚的预示着实际需求影响着化学的发展。
但化学和数学在这个阶段可能没有互相影响。
如果它们影响了,但是没有记录证明这个。
3. Greek chemistry was based mainly on speculation rather than on experiment . 希腊化学主要基于猜测而不是实验。
这是所有古代希腊化学的一个共同特征。
古代希腊化学家实际是希腊哲学家。
所以不足为奇的是希腊人思考比实验更有兴趣。
实际上他们很少进行实验以外的思维实验。
对于数学来说这是一个好方法,但没有一个人把它推荐在物理、化学或生物科学上。
应用化学专业英语(课后答案和课文翻译)

Unit 1 The Roots of ChemistryI. Comprehension.1.It can be inferred from this article which one of the following items is not mainly based on practical use C. Greek chemistry2. It was B. Empedocless who first introduced the idea that all things are not formed from just one element.3. In the development of Greek chemistry, D. Democritus was the first one definiting the ultimately constituents of matter?4. According to Plato, there are B. 4 ―elements‖ whose faces are constituted by regular polygons.5. In the last paragraph,authors think that experiment DD.can deal with the reactions by which one substance is converted into anotherII. Make a sentence out of each item by rearranging the words in brackets.1.The purification of an organic compound is usually a matter of considerable difficulty, and itis necessary to employ various methods for this purpose.2.Science is an ever-increasing body of accumulated and systematized knowledge and is also anactivity by which knowledge is generated.3.Life, after all, is only chemistry, in fact, a small example of chemistry observed on a singlemundane planet.4.People are made of molecules; some of the molecules in people are rather simple whereasothers are highly complex.5.Chemistry is ever present in our lives from birth to death because without chemistry there isneither life nor death.6.Mathematics appears to be almost as humankind and also permeates all aspects of human life,although many of us are not fully aware of this.III. Translation.1.(a)化学过程;(b)自然科学;(c)蒸馏技术(a) chemical process (b) natural science (c) the technique of distillation2.正是原子构成铁、水、氧等。
应用化学专业英语(课后答案和课文翻译)

Unit 1 The Roots of ChemistryI. Comprehension.1.It can be inferred from this article which one of the following items is not mainly based on practical use C. Greek chemistry2. It was B. Empedocless who first introduced the idea that all things are not formed from just one element.3. In the development of Greek chemistry, D. Democritus was the first one definiting the ultimately constituents of matter?4. According to Plato, there are B. 4 ―elements‖ whose faces are constituted by regular polygons.5. In the last paragraph,authors think that experiment DD.can deal with the reactions by which one substance is converted into anotherII. Make a sentence out of each item by rearranging the words in brackets.1.The purification of an organic compound is usually a matter of considerable difficulty, and itis necessary to employ various methods for this purpose.2.Science is an ever-increasing body of accumulated and systematized knowledge and is also anactivity by which knowledge is generated.3.Life, after all, is only chemistry, in fact, a small example of chemistry observed on a singlemundane planet.4.People are made of molecules; some of the molecules in people are rather simple whereasothers are highly complex.5.Chemistry is ever present in our lives from birth to death because without chemistry there isneither life nor death.6.Mathematics appears to be almost as humankind and also permeates all aspects of human life,although many of us are not fully aware of this.III. Translation.1.(a)化学过程;(b)自然科学;(c)蒸馏技术(a) chemical process (b) natural science (c) the technique of distillation2.正是原子构成铁、水、氧等。
应化专业英语(词汇)

Elements(元素)碳carbon氢hydrogen硫sulfur, sulphur氮nitrogen氧oxygen氟fluorine氯chlorine溴bromine碘iodine砷arsenic硅silicon磷phosphorus金gold银silver铁iron钴cobalt镍nickel钒vanadium铜copper铝aluminum, aluminium 钾potassium钠sodium镁magnesium钙calcium铅lead锌zinc铂platinum非含氧酸(尾缀-ic acid)氢氟酸hydrofluoric acid 氢氯酸,盐酸hydrochloric acid氢溴酸hydrobromic acid氢碘酸hydroiodic acid氢硫酸hydrosulfuric acid含氧某酸(尾缀-ic acid)碳酸carbonic acid硝酸nitric acid硫酸sulfuric acid磷酸phosphoric acid硼酸boric acid硫代硫酸thiosulfuric acid氯酸chloric acid溴酸bromic acid碘酸iodic acid铬酸chromic acid重铬酸dichromic acid含氧亚某酸(尾缀-ous acid)亚硝酸nitrous acid亚硫酸sulfurous acid亚磷酸phosphorous acid亚硼酸borous acid亚氯酸chlorous acid亚溴酸bromous acid含氧次某酸(前缀hypo-, 尾缀-ous acid)次磷酸hypophosphorous acid次氯酸hypochlorous acid次溴酸hypobromous acid次碘酸hypoiodous acid次硝酸nitroxylic acid次硫酸sulfoxylic acid含氧偏某酸(前缀meta-, 尾缀-ic acid)偏硼酸metaboric acid偏磷酸metaphosphoric acid偏硅酸metasilicic acid偏钒酸metavanadic acid含氧高某酸(前缀per-, 尾缀-ic acid)高氯酸perchloric acid高碘酸periodic acid高锰酸permanganic acid高铁酸ferric acid无机酸对应的盐(以-ic acid结尾的,-ate;以-ous acid结尾的,-ite)硫酸钠sodium sulfate亚硫酸钠sodium sulfite次硫酸钠sodium sulfoxylate次磷酸钠sodium hypophosphite偏磷酸钠sodium metaphosphateThe nomenclature of inorganic substances(无机物的命名)某化物(尾缀-ide)氧化物oxide硫化物sulfide氮化物nitride碳化物carbide氢氧化物hydroxide氟化物fluoride氯化物chloride溴化物bromide碘化物iodide氧化物一氧化碳carbon monoxide二氧化碳carbon dioxide二氧化硫sulfur dioxide三氧化硫sulfur trioxide五氧化二氮dinitrogen pentoxide氧化铜copper oxide, cupric oxide, copper monoxide氧化亚铜cuprous oxide, copper(I) oxide, copper hemioxide氧化铁iron oxide, ferric oxide 氧化亚铁ferrous oxide硫化物硫化氢hydrogen sulfide 二硫化碳carbon disulfide硫化钠sodium sulfide氧化亚铁ferrous sulfide氯化物氯化氢hydrogen chloride四氯化碳carbon tetrachloride,tetrachloromethane氯仿,三氯甲烷trichloromethane, chloroform氯化钠sodium chloride氯化铵ammonium chloride氯化铝aluminium chloride,aluminium trichloride氯化银silver chloride氯化亚铁ferrous chloride,iron(II) chloride, irondichloride氢氧化物氢氧化钠sodium hydroxide氢氧化铝aluminium hydroxide氢氧化镁magnesium hydroxide氢氧化铜cupric hydroxide, copperhydroxide, copper(II)hydroxideNomenclature of derivative(命名的衍生)醇(尾缀-ol, ~ alcohol)甲醇methanol, methyl alcohol乙醇ethanol, ethyl alcohol2-丙醇2-propanol1,3-丁二醇1,3-butanediol1,2,3-丁三醇1,3-butanetriol1,2-乙二醇1,2-ethanediol,glycol, ethylene glycol醚(~ ether)甲醚methyl ether乙醚ethyl ether异丙醚isopropyl ether叔丁醚tert-butyl ether甲乙醚methyl ethyl ether二苯醚diphenyl ether醛(~ aldehyde, 尾缀-al)甲醛methanal, formaldehyde乙醛ethanal, acetic aldehyde丙醛propanal, propyl aldehyde丁醛butyl aldehyde, butanal戊醛pentanal, valeraldehyde己醛hexanal, hexaldehyde苯甲醛benaldehyde, benzoicaldehyde酮(~ ketone, 尾缀-one)丙酮propanone, acetone丁酮butanone, methyl ethyl ketone戊酮pentanone, methyl propyl ketone2,4-己二酮2,4-hexanedione 1,4-环己二酮1,4-cyclohexanedione羧酸(尾缀-ic acid)甲酸methanoic acid, formic acid乙酸ethanoic acid, acetic acid 丙酸propanoic acid, propionic acid, ethylformic acid异丁酸i-butanoic acid, isobutyric acid, 2-methyl…戊二酸pentanedioic acid苯甲酸benzoic acid苯乙酸phenylacetic acid苯丙酸benzenepropanoic acid 对氯苯丙酸p-chlorophenyl propionic acid,p-chlorobenzenepropanoic acid 对羟基苯乙酸p-hydroxyphenyl acetic acid羧酸盐(~ ester, 尾缀-ate)甲酸乙酯ethyl formate乙酸异丙酯isopropyl acetate 丙酸钙calcium propionate丁酸丁酯butyl butanate, butylbutyrate苯甲酸乙酯benzoic acid ethylester, ethyl benzoate异丁酸钠sodium isobutyrate酚(尾缀-ol)苯酚phenol1,3-苯二酚1,3-benzenediol,1,3-dihydroxybenzene1,2,4-苯三酚1,2,4-benzenetriol邻、间、对- ortho-, meta-, para-萘酚naphthol醇与酚的盐(尾缀-olate)甲醇钠sodium methanolate乙醇钾potassium ethanolate三异丙醇铝aluminumtriisopropanolate苯酚钠sodium phenolateNomenclature ofHydrocarbon(烃的命名)Meth:1Eth:2Prop:3But:4Pent:5Hex:6Hept:7Oct:8Non:9Dec:10正构normal, n-异构isomery, iso-, i-伯primary, pri-仲secondary, sec-叔tertiary, tert-季quaternary, quat-正丁基normal butyl, n-butyl异丁基isomery butyl, iso-butyl仲丁基secondary butyl,sec-butyl叔丁基tertiary butyl, tert-butyl叔戊基tertiary pentyl,tert-pentyl二甲基dimethyl三甲基trimethyl四甲基tetramethyl五乙基pentaethyl六丙基hexapropyl三叔丁基tri-tertbutyl二异丁基di-isobutyl烯烃(尾缀-ene)乙烯ethene, ethylene丙烯propene, propylene丁烯butene, butylene异丁烯iso-butene2-丁烯2-butene戊烯pentene2,4-二甲基-2-己烯2,4-dimethyl-2-hexene多烯烃(尾缀-adiene, -atriene)1,3-丁二烯1,3-butadiene1,4-戊二烯1,4-pentadiene2-甲基- 1,3-戊二烯2-methyl-1,3-pentadiene2-甲基-1,3,5-庚三烯2-methyl-1,3,5-heptatriene乙炔ethyne丙炔propyne2-丁炔2-butyne4-甲基-2-戊炔4-methyl-2-pentyne环丙烷cyclopropane环己烷cyclohexane甲基环戊烷methyl-cyclopentane3-甲基环己烯3-methyl-cyclohexene1,4-环己二烯1,4-cyclohexadiene2-甲基-1,3-环己二烯2-methyl-1,3-cyclohexadiene Petroleum refining(石油炼制)原油蒸馏petroleum distillation热转化thermal conversion催化裂化catalytic cracking催化加氢catalytichydroprocessing催化重整catalytic reforming气体加工gas processing油品精制oil refining原油蒸馏过程闪蒸flash distillation初馏primary distillation常压蒸馏atmospheric distillation减压蒸馏vacuum distillation闪蒸塔flasher, flashdistillation column, flash(ing)tower初馏塔primary distillationtower, primary fractionator常压塔atmospherictower/column减压塔vacuum tower/column汽提塔stripping tower, stripper再沸器reboiler加热炉heater, heating furnace换热器heat-exchanger热转化过程热裂化thermal cracking减粘裂化visbreaking蒸汽裂解steam pyrolysis焦化/焦炭化coking延迟焦化delayed coking, DC流化焦化fluid coking灵活焦化flexicoking焦化反应器coker, cokingreactor焦化汽油coker gasoline焦化柴油coker diesel焦化蜡油coker gas oil, CGO催化裂化过程流化催化裂化fluid catalyticcracking, FCC提升管反应器riser再生器regenerator沉降器disengager汽提段stripper zone催化裂化装置catalyticcracking unit催化裂化汽油FCC gasoline,catalytic gasoline催化裂化柴油FCC diesel催化裂化油浆FCC slurry裂化催化剂cracking catalyst催化剂失活deactivation ofcatalyst催化剂再生regeneration ofcatalyst待生催化剂spent catalyst再生催化剂regenerated catalyst催化加氢过程加氢处理hydrotreating, hydrotreatment加氢裂化hydrocracking加氢精制hydrofining加氢脱硫hydrodesulfurization, HDS加氢脱硫hydrodenitrogenation, HDN加氢脱氧hydrodeoxygenation, HDO加氢脱金属hydrodementallization, HDM加氢脱芳hydrodearomatization加氢异构hydroisomerization 部分加氢partial hydrogenation 选择性加氢selective hydrogenation循环氢recycle hydrogen补充氢make-up hydrogen新氢fresh hydrogen高压分离器high pressure separator低压分离器low pressure separator循环压缩机recycle compressor 催化重整过程重整装置/器reformer重整汽油reformed gasoline,reformate芳烃抽提aromatic extraction脱氢环化dehydrocyclization重整催化剂reforming catalyst气体加工过程烷基化alkylation碳四烷基化isobutanealkylation烷基化油alkylate醚化etherification聚合polymerization异构化isomerization油品精制过程溶剂精制solventrefining/treating溶剂精制油solvent-refined oil加氢精制hydrofining萃取脱硫desulfurization byextraction溶剂脱蜡solvent dewaxing溶剂脱沥青过程溶剂脱沥青solvent deasphalting脱沥青油deasphalted oil, DAO脱油沥青deoiled asphalt, DOA丙烷脱沥青propane deasphalting超临界溶剂脱沥青supercritical solventdeasphalting加工过程分类脱碳carbon rejection加氢hydrogen additionUnit1. The roots of chemistry(化学的起源)Atomism: 原子学说,原子论Derived from: 由…而来,起源于Be familiar with: 熟悉Be traced back: 追踪Bronze: 青铜Consist of: 由…组成Cube: 立方体Flammable: 易燃物,可燃的Geometry: 几何Mercury: 水银,汞Metric: 公制的Molecule: 分子Particle: 微粒,粒子Speculation: 思索,推测Theorem: 定理,原则Treatise: 论述,论文Benzene: 苯Isomer: 异构体Unit 6. the periodic table(元素周期表)Proton: 质子Electron: 电子Neutron: 中子Isotope: 同位素Element: 元素Indicate: 指出Periodicity: 周期性Tabulate: 制表V ertical: 垂直的Unit 7. acids, bases and salts (酸、碱、盐)Acid 酸Base 碱Salt 盐Citric acid 柠檬酸Lactic acid 乳酸Sour 酸性的Vinegar 醋Hydrochloric HCl Hydrogen 氢Carbon dioxide CO2Ion 离子Cation 阳离子Anion 阴离子Polar极性Hydronium水合氢离子Neutralization reaction 中和反应Alkaline 碱溶液Hydroxide OH-Unit 8. Chemical Bonds(化学键)Compound化合物Bond化学键—Ionic bond离子键—Covalent bond共价键—Polar covalent bond极性共价键Fiber纤维Cation (cat-ion) 阳离子Anion (ann-ion) 阴离子Nucleus原子核toxic有毒的affinity亲和力Unit 9. Chemical Kinetics:Basic concepts化学动力学:基本概念Kinetics 动力学Equilibrium (-bria)平衡Dynamic动态Forward reaction正向反应Reverse reaction逆向反应Reaction mechanism反应机理Activation energy活化能Rate of reaction反应速率Rate equation速率方程Concentration浓度Partial pressure分压Rate-determining step速率控制步骤Combustion燃烧Pre-exponential factor指前因子Reciprocal倒数Free energy自由能Enthalpy焓Entropy熵Parameter参数Differential微分Integrate整合Coefficient系数Unit 17. Crystallisation(晶体)Saturate饱和Supersaturate过饱和Solute溶质Solvent溶剂Solution溶液Solubility溶解度Yield产率Evaporation蒸发Labile不安定的metastable亚稳态Isomorphous同形的Homologues同族体Magma乳浆剂Diluent稀释剂Precipitant沉淀剂Unit 19. Solvent Extraction (溶剂萃取)Funnel 漏斗Chelates 螯合物Association 配位化合物Chromatography 色谱Terms 术语Hydrophilic 亲水性Hydrophobic 憎水性Electrostatic 静电Unit 21. Reactor Types(反应器类型)均相反应homogeneous reaction非均相反应heterogeneous reaction吸热反应endothermic reaction放热反应exothermic reaction 基元反应elementary reaction 宏观反应macroscopic reaction 微观反应microscopic reaction 分子反应molecular reaction 可逆反应reversible reaction不可逆反应irreversible reaction,non-reversible reaction主反应main reaction副反应side reaction理想反应ideal reaction非理想反应non-ideal reaction一次反应primary reaction二次反应secondary reaction简单反应simple reaction复杂反应complex reaction平行反应parallel reaction顺序反应sequential reaction歧化反应disproportionationreaction氧化反应oxidation reaction还原反应reduction reaction加成反应addition reaction重排反应rearrangementreaction裂化反应cracking reaction链/连锁反应chain reaction反应动力学reaction kinetics反应速率reaction rate快速反应quick reaction慢反应slow reaction瞬间反应instant reaction反应级数reaction order一级反应first order reaction二级反应second order reaction准/拟一级反应pseudo-firstorder reaction零级反应zero order reaction活化能activation energy频率因子,指前因子frequencyfactor, pre-exponential factor反应速率常数reaction rateconstant表观活化能apparentactivation energy阿累尼乌斯Arrheniusequation反应温度reaction temperature反应时间reaction time反应压力reaction pressure反应物浓度reactantconcentration原料转化率conversion of feed反应选择性reaction selectivity反应机理reaction mechanism反应途径reaction pathway自由基free radical正碳离子carbonium ion气相反应gas phase reaction气-液反应gas-liquid reaction气-液-固反应gas-liquid-solidreaction液-液反应liquid-liquidreaction平推流/活塞流piston/plugflow reactor全混流perfect mixing flow串联反应器reactors in series 并联反应器reactors in parallel 连续搅拌反应釜/槽式反应器continuous stirred tank reactor, CSTR固定床反应器fixed bed reactor流化床fluidized/fluid bed固定流化床confined fluidized bed移动床moving bed鼓泡床ebullated bed悬浮床/浆态床slurry/suspension bed滴流床trickle bed快速床fast bed输送床transport bed管式反应器tubular reactor塔式反应器column/tower reactorUnit operations(单元操作)传质过程蒸馏distillation吸收absorption吸附adsorption萃取extraction离子交换ion exchange膜分离membrane separation 传热过程换热heat exchange/transfer蒸发evaporation, vaporization传质、传热过程干燥dry, drying, desiccation 结晶crystallization增湿humidification减湿dehumidification动量传递沉降sedimentation, settlement 过滤filtration, filtering搅拌stir, agitate, mixing流态化fluidization塔类型气泡塔bubble column喷雾塔spray tower填料塔packed/filled column 板式塔plate/tray tower。
应用化学专业英语

应用化学专业英语Cu—copper Fe—iron Hg—mercury Na—sodium K—potassium Ag—silverNaOH—sodium hydroxide KOH—potassium hydroxide Fe(OH)2—iron(Ⅱ)hydroxide Fe(OH)3—iron(Ⅲ)hydroxide NH4OH—ammonium hydroxideK3[Fe(CN)6]—potassium hexacyanoferrate(Ⅲ)K4[Fe(CN)6]—potassium hexacyanoferrate(Ⅱ)Ⅰ.Name the following1.(NH4)2CO3Ammonium carbonate2.N2O Nitrous Oxide3.H2SO4Sulfuric acid4.P4O6Phosphorus(Ⅲ)trioxide5.Al2O3Aluminium oxide6.SnCl4Tin(IV)chloride7.KHSO4Potassium hydrogen sulfate8.Cu2S Copper(Ⅰ)sulfide9.HClO4Perchloric acid10.BaCl2Barium chloride11.P4O10Phosphorus(Ⅴ)pentoxide12.NaH Sodium hydride13.Ca(MnO4)2Calcium permanganate14.PF5Phosphorus pentafluoride15.(NH4)2HPO4Diammonium hydrogen phosphateⅡ.Give formulas for the following1.ammonium sulfate(NH4)2SO42.barium iodide BaI23.iron(Ⅱ)sulfate Fe2SO44.potassium permanganate KMnO45.copper(Ⅱ)oxide CuO6.carbonic acid H2CO3Melting point 熔点boiling point 沸点1.Which particles play the most active role in chemical bonding?(a)electrons (b)neutrons (c)protons (d)valence electrons2.An ionic bond is formed when electrons are:(a)completely destroyed (b)compeltely transferred (c)divied (d)equally shared3.Due to the that Ionic compounds have strong intermolecular forces they are at room temperature.(a)bonded covalently (b)gases (c)liquids(d)solids 1-butene trans -2-butenecis -2-butene iso -butene (E )-2-butene (Z )-butene 2-methylpropene1.Draw structure that correspond to the following names.(a)2,2-dimethylpentane (b)4-isobutyl-2,5-dimethylheptane (c)(Z)-3-menthyl-2-octene (d)(2R,3S)-2,3-pentanediol2.Give the IUPAC name for each of the following structures.(e)(f)(E)-1-methyl-4-ethylcyclohexane(g)(h)(S)-2-chloro-butyraldehyde (2R,3R)-2,3-dichlorobutyric acid补充:(E)-2-chloro-3-methyl-2-octene Nucleophile亲核试剂carbocation碳阳离子Compressible可压缩的incompressible不可压缩的1.A chemical system can be studied from either a or a(n)viewpiont.(A)physical...chenical(B)molecual...atomic (C)Microscopic...macroscopic(D)Mechanic...kinetic2.Is a macroscopic science that studies the interrelationships between the various equilibrium properties of a stystem.(A)Kinetics(B)Thermodynamics (C)Statistical mechanics(D)Quantum chenistry3.In,the molecular and macroscopic levels are related to each other.(A)quantum(B)statistical(C)thermodynamics(D)kinetics4.thermodynamics studies.(A)heat,work,energy,and the changes they produce in the states of systems(B)The relationships between the molecules of a system(C)heat,work,temperature,and the energy they produce in the states of systems(D)heat,energy,and work5.For a(n)system,neither matter nor energy can be transferred between system and surroundings.(A)closed(B)open(C)isolated(D)none of the aboveⅠ.Translate the following from English into Chinese.(1)pollution of the atmosphere(2)nondegradable pollutant大气污染不可降解污染物(3)harmless pollutant(4)interacting chemicals无害污染物相互作用的化学物质(5)threshold level(6)sound pressure level限定值,阈值声压水平(7)speech interference(8)transmission path 语音干扰传输途径Translate the following from Chinese into English.(1)定性分析qualitative analysis (2)分析物analyte (3)准确度accuracy (5)反应速率reaction-rate (5)解吸附作用deserption (6)吸附absorption conduction 热传导convection 对流radiation 辐射Balance and classify each of the following chemical equations as a (1)combination reactions ,(2)decomposition reaction ,(3)displacement reaction ,or (4)partner-exchange reaction.(a))()(2243l O H s Fe H O Fe +→+)(4)(342243l O H s Fe H O Fe +→+displacement reaction 置换反应(b))()()(23g O s KCl s KClO +→)(3)(2)(223g O s KCl s KClO +→decomposition reaction 分解反应(c)steam and hot carbon react to form gasecous hyfrogen and gaseous carbon monoxide.)()()()(22g CO g H s C l O H +→+displacement reaction 置换反应(d))()()(4272aq HClO g O H g O Cl →+)(2)()(4272aq HClO g O H g O Cl →+combination reactions 化合反应(e))()()(22aq HBrO aq HBr O H l Br +→+)()()(22aq HBrO aq HBr O H l Br +→+decomposition reaction 分解反应(f))()()()()(43442243aq PO H s CaSO aq SO H s PO Ca +→+)(2)(3)(3)()(43442243aq PO H s CaSO aq SO H s PO Ca +→+partner-exchange reaction 复分解反应(g)Potassium reacts with water to give aqueous potassium hydroxide and gaseous hydroxide.)()(2)(2)(222g H aq KOH l O H s K +→+displacement reaction 置换反应(h)Solid magnesium carbonate decomposes to form solid magnesium oxide and gaseous carbon monoxide.)()()(23g CO s MgO s MgCO +→decomposition reaction 分解反应Abstract 摘要Results and discussion 结果与讨论Experimental实验References参考文献E-factor影响因素Journal of the American Chemical Society美国化学会志Journal of the Chemical Society化学会志Journal of Organic Chemistry有机化学杂志Tetrahedron四面体'\.._/ ( Wb川ache mical reaction?Acherr山al react i on occurs when subs'孟忘"(tlie reactants) collide (碰撞) with enough energy to rearrange to form different compounds (由e produc时. η1e change in energy由at occurs when a reaction take place is described by thermodynamics (热力学) and the rate or speed at which a reaction occ u rs is described by kfaetics (动力学) . Reactions in which the reactants and produc臼coexist are considered to be in equ山brium (处于平衡). A chemical equation consists of the chemical formula (化学式) of the reactants,且目the chemical formula of the products. The two are separated by an 一一- usually read as ”yielas·’and each chemical formula is separated from others by a plus sign (加号) . Sometimes a triangle is drawn over the arrow symbol to denote energy must be added to the substances for the reaction to begin. Each chemical formula may be preceded by a scalar (数量的) coefficient ind i cating the proportion (比例) of that substance necessary to produce the reaction in formula. For instance, the formula for the burning of methane (C比+ 202 →C02 + 2H20) indicates that twice as much 02 as C比is needed, and when they react, twice as much H20 as C02 will be produced.η1is is because during the reaction, each atom of carbon needs exactly two atoms of oxygen to combine with, to produce the C02, and every two atoms of hydrogen need an atom of oxygen to combine with to produce the H20. If the proportions of t he reactants are not respected, when they are forced to react, either not all of the substanc e used will participate in the react i on, or the react i on that will take p l ace will be different from the one noted in the equation.。
最新应用化学专业英语词汇.5

Chemical Literature化学文献;primary literature一次文献;research journal 学术期刊;computer databases计算机数据库;Chemical Abstracts 化学文摘;Science Citation Index科学引文索引;keyword index关键词索引;author index作者索引;chemical substance index化学物质索引;formula index分子式索引,patent index 专利索引;general subject index一般主题索引;collective Index累计索引;Ring Index 环索引;Organic Chemistry有机化学;Organometallic chemistry 有机金属化学;Heterocyclic Chemistry杂环化学;Heterocyclic Compound杂环化合物;Comprehensive Organic Synthesis综合有机合成;titration volumes滴定体积;melting points熔点boiling points沸点;noxious material 有害的(有毒的)材料;contamination污染;abrasive 研磨剂;15 percent trisodium phosphate solution 15%的Na3PO4溶液;chromic acid铬酸;chromium trioxide (CrO3);concentrated sulphuric acid浓硫酸;sodium dichromate重铬酸钠;potassium dichromate重铬酸钾;volumetric analysis容量分析;reducing agent还原剂;oxidizing agent氧化剂;concentrated nitric acid浓硝酸;induction period诱导期;ethanol乙醇;industrial spirit 工业酒精acetone丙酮;distillation蒸馏;filter过滤器;corrosive liquids腐蚀性液体,toxic liquids有毒液体;fire extinguisher灭火器;fire blanket灭火毯;Waste Disposal废弃物处理;inflammable materials易燃材料;organic material有机材料;hazardous chemicals危险化学品;toxic chemicals有毒的化学品;water-insoluble organic solvent水不溶有机溶剂;IR spectroscopy 红外光谱distilled water 蒸馏水deionized water 去离子水X-ray diffraction pattern X-射线衍射图谱gas chromatography 气相色谱electron diffraction 电子衍射scanning electron microscope 扫描电子显微镜scanning tunneling microscope 扫描隧道显微镜thermogravimetry 热重法X-ray photo-electron spectroscopy X射线光电子能谱electron probe microanalysis 电子探针显微分析differential thermal analysis 差热分析differential scanning calorimetry 差示扫描量热分析auger electron spectroscopy 俄歇电子谱atomic force microscopy 原子力显微镜transmission electron microscopy 透射电子显微镜field emission microscopy 场发射显微镜atomic emission spectrometry 原子发射光谱atomic absorption spectrometry 原子吸收光谱nuclear magnetic resonance spectrometry 核磁共振波谱ultraviolet & visible absorption spectrum 紫外、可见吸收光谱photochemistry 光化学transition-metal 过渡金属solution 溶液solvent 溶剂solute 溶质anion 阴离子cation 阳离子substituted derivative 取代衍生物excitation 激发emission 发射catalyst 催化剂drug 药hazard 危险品nitrate 硝酸盐nitric acid 硝酸Carbon-iodine bond C-I 键acetone (propanone)丙酮Ethanol 乙醇methanol 甲醇2-propanol 2-丙醇isopropyl alcohol 异丙醇Benzene ring苯环toluene 甲苯Saturated hydrocarbon 饱和碳氢化合物methyl ether 甲醚Methyl ethyl ether 甲乙醚Aldehydes 醛ketone 酮Formaldehyde (methanal)甲醛Acetic acid (ethanoic acid)乙酸Formic acid (methanoic acid)甲酸Salicylic acid 水杨酸Aspirin 阿司匹林Methyl acetate (methyl ethanoate)乙酸甲酯Methane甲烷;ethane乙烷;propane丙烷;butane丁烷;hexane己烷;Ethene 乙烯;propene丙烯;1-butene1-丁烯;Ethyne乙炔;cyclohexane环己烷;Methyl甲基;ethyl乙基;phenyl苯基;Cis-1,2-dimethylcyclohexane 顺式-1,2-二甲基环己烷Trans-1,2-dimethylcyclohexane反式-1,2-二甲基环己烷Organic Compound有机化合物;carbon碳;hydrocarbon碳氢化合物;cyclic structure环状结构;branched hydrocarbon支化的碳氢化合物;Straight-chain Hydrocarbon直连碳氢化合物;Alkyl Group 烷基基团;Cis and Trans Isomer顺式及反式异构体;chiral carbon手性碳oxygen O nitrogen N magnesium Mgiron Fe copper Cu lead Pbsodium Na potassium K Silver Ag;Aluminium Al;Arsenic As;Barium Ba;Bromine Br;Calcium Ca;Chlorine Cl;Cohalt Co; Chromium Cr; Fluorine F;Hydrogen H; Mercury Hg; Iodine I;Magnesium Mg; Manganese Mn;Nickel Ni;Phosphorus P; Palladium Pd; Platinum Pt;Sulfur S; Silicon Si; Tin Sn;Titanium Ti; Uranium U; Zinc ZnTungsten 钨vanadium-iron alloy 钒铁合金Metal Oxide, 金属氧化物;positive ion 正离子negative ion负离子Base碱;acid酸;Salt盐sodium chloride NaCl, aluminium hydroxide Al(OH)3iron ( II ) bromide 【ferrous bromide】FeBr2; calcium acetate Ca(OAc)2; chromium (III) sulphate or chromic sulphate Cr2(SO4)3;Iron (II) oxide (Ferrous oxide) FeO;Iron( III) oxide (Ferric oxide) Fe2O3;Tin(II) hydroxide Sn(OH)2;Tin(IV) hydroxide Sn(OH)4;Mercury(I) sulphate (Mercurous sulphate) Hg2SO4;Mercury(II) sulphate (Mercuric sulphate) HgSO4;Sodium hypochlorite NaClO;Potassium dichromate K2Cr2O7;Carbon monoxide CO; Carbon dioxide CO2;Sulphur trioxide SO3; Dinitrogen trioxide N2O3;Diphosphorus P2O5Acetic acid CH3COOH; hydrobromic acid HBr;perchloric acid HClO4; hydrocyanic acid HCN;phosphoric acid H3PO4;sulphuric acid H2SO4;disodium hydrogen phosphate Na2HPO4;sodium dihydrogen phosphate NaH2PO4 ;sodium bicarbonate NaHCO3; calcium bisulphite Ca(HSO3)2.bismuth dihydroxynitrate Bi(OH)2NO3;sodium potassium sulphate NaKSO4;Calcium carbide Ca2C; Lithium carbonate Li2CO3;Magnesium phosphate Mg3(PO4)2; Molybdenum trioxide MoO3.Thin Layer Chromatography 薄层色谱;methylene chloride二氯甲烷;acetonitrile 乙腈ethyl acetate乙酸乙酯chloroform 氯仿dichloromethane 二氯甲烷diethyl ether 二乙醚toluene甲苯cyclohexane环己烷, petroleum-ether石油醚,hexane己烷, pentane戊烷High Performance Liquid Chromatography (HPLC)高效液相色谱。
[09953]应用化学专业英语
![[09953]应用化学专业英语](https://img.taocdn.com/s3/m/c649d7fdc8d376eeaeaa3179.png)
应用化学〈〈专业英语》教学大纲一、课程信息二、课程内容1、课程教学目标本课程分为化学化工科技英语和专业英语两部分,通过学习要求充分了解专业文章的特点,掌握一定量的专业词汇,具有较强的英文文献阅读能力和基本的专业论文英文写作技巧。
2、基本教学内容(一)科技英语部分该部分从内容上分为10个单元,包含化学基本知识、无机化学、分析化学、有机化学、物理化学、结构化学基础及化工技术原理。
每一个单元由Reading and Comprehension、Reading and Practice和reading and Translation三部分组成。
对单元第一部分要求学生重点学习,另外课堂上选择第三部分作为快速阅读理解补充材料,扩大学生的专业词汇量,训练学生阅读理解速效,单元第二部分作为学生课后作业。
10个单元的教学内容如下:1.Chemistry a Natural Science2.Acids and Bases3.Introduction to Analytical Chemistry4.Titrimetric Methods of Analysis5.Alkans6.Types of High Polymers7.Refrigeration and the Heat Pump8.Large-Scale Synthetic Nitrogen Fixaiton9.Measurement of Temperature10.Environmental Protection(二)专业英语部分该部分从内容上分为8个单元,包括金属材料、材料的腐蚀与防护技术原理、金属材料的表面处理技术等方面的英语专业文章。
8个单元的教学内容如下:1.Caron Steels and Alloy2.Surface Treatment3.Nature of Electrode Reactions4.Theories of Passivity5.Steady State Potentiostatic and Galvanostatic Measurement6.Thermostatic Water Bath7.Electrolytic Plating8.Effect of Sodium Nitrite Inhibitor on the Corrosion Behavior of Steel In Neutral Chloride Solutions三、学时分配专业英语32学时全部安排课内教学,教学安排如下表:四、考核方式闭卷考试80%,作业20%。
应用化学专业英语(课后答案和课文翻译)

Unit 1 The Rootsof Chemis tryI. Compre hensi on.1.It can be inferred from this articl e whichone of the followi ng itemsi s not mainly basedon practi c al use C. Greekchemis try2. It was B. Empedo cless who firsti ntrod ucedthe idea that all things are not formed from just one elemen t.3. In the develo pment of Greekchemis t ry, D. Democri tus was the first one defini tingthe ultimatelyconsti tuent s of matter?4. Accord i ng to Plato, thereare B. 4 ―elemen ts‖ whosefacesare consti tuted by regula r polygons.5. In the last paragraph,authors thinkthat experi ment DD.can deal with the reacti ons by whichone substa n ce is converted into anothe rII. Make a senten ce out of each item by rearra nging the wordsin bracke ts.1.The purifi catio n of an organi c compou nd is usuall y a matter of consid erabl e diffic ulty, and itis necess ary to employ variou s method s for this purpos e.2.Scienc e is an ever-increa singbody of accumu lated and system atize d knowle dge and is also anactivi ty by whichknowle dge is genera ted.3.Life, afterall, is only chemis try, in fact, a smallexampl e of chemis try observ ed on a si nglemundan e planet.4.People are made of molecul es; some of the molecul es in people are rather simple wherea sothers are highly comple x.5.Chemist ry is ever presen t in our livesfrom birthto deathbecause withou t chemis t ry therei sneithe r life nor death.6.Mathem atics appears to be almost as humanki nd and also permea tes all aspect s of humanlife,althou gh many of us are not fullyawareof this.III. Transl ation.1.(a)化学过程;(b)自然科学;(c)蒸馏技术(a) chemic al proces s (b) natura l science (c) the techni que of distil latio n2.正是原子构成铁、水、氧等。
应用化学专业外语词汇

Aabscissa[æbˈsɪsə] n. 横座标 abundance n. 丰富, 充裕acceptor n. 接受体accumulator n. 储料器 acetic acid n. 醋酸, 乙酸 acknowledge v. &n. 致谢activation n. 活化acylation ['æsil] n. 酰化addition [əˈdiʃən] n. 加成反应adhesive [ædˈhisɪv, -zɪv] n. 粘合剂advancement n. 进展,增长advantageous adj. 有利的aerosol[ˈeərəˌsɔ:l, -ˌsɔl] n. 烟雾affinity [əˈfɪnɪti:] n. 亲合力agent [ˈeidʒənt] n. 试剂aldehyde [ˈældəˌhaɪd]n. 醛aldol[ˈældəul]n. 醛醇aliphatic acid [ˌæliˈfætik]n. 脂肪酸alkaline[ˈælkəlɪn, -ˌlaɪn] adj. 碱的alkaloid[ˈæl kəlɔid] n. 生物碱alkane[ˈælˌken]n. 烷烃alkene[ˈælki:n]n. 烯烃alkylation [ˌælkiˈleiʃ(ə)n]n. 烃化, 烷基化alkyl halide[ˈælkil][ˈhælaid]n. 烷基卤, 卤烷alkyne n. 炔alphabetic adj. 依字母顺序ambiguity n. 模糊, 意义不明确amide n. 酰胺amine n. 胺amino acid n. 氨基酸amorphous adj. 无定形analogue n. 类似物anhydride n. 酸酐aniline n. 苯胺anion n. 阴离子anomaly n. 异常,反常antibiotics n. 抗菌素antifreezing agent n. 抗冻剂antioxidant n. 抗氧剂appreciable adj. 可估计的architect n. 建筑师, 设计师arene n. 芳烃aromatic adj. 芳香的aromatization n. 芳构化asymmetric adj. 不对称的autooxidation n. 自氧化awarenness n, 意识azeotrope n.共沸混合物azo dye n. 偶氮染料Bbackup n. /adj 备用设备base n. 碱, 基, 底beaker n. 烧杯benzene n. 苯biological degradation n. 生物降解biosynthesis vt. 生物合成bleach vt. 漂白bond n. 键branched chain n. 支链budget n. & v. 预算bubble-cap tower n. 泡罩塔buffer n. 缓冲,缓冲剂Ccarbanion n. 负碳离子, 阴碳离子carbene n. 碳烯, 卡宾carbide n. 碳化物, 碳化钙carbocation n. 正碳离子, 阳碳离子carbonyl group n. 羰基carboxy group n. 羧基carboxylic acid n. 羧酸carcinogenic adj. 致癌的β-carotene n. β胡萝卜素carrier n. 载体cartridge n. 软片暗盒catalysis n. 催化(作用) cation n. 阳离子cellulose n. 纤维素ceramic adj/n. 陶瓷(的) chemical shift n. 化学位移chirality n. 手性chlorination n. 氯化作用chlorohydrocarbon n. 氯代烃chromophore n. 发色团cis-trans isomer n. 顺反异构体classic adj. 经典的, 传统的cluster n. 蔟,一串,一束coherent adj. 黏附的,相干的(光学) coil n. 蛇管colorant n. 颜料,着色剂commodity n. 用品compensation n. 补偿competitive n. 竞争的complementary n. 补充的complex n. 络合物complication n. 复杂concerted reaction n. 协同反应condensation n. 缩合反应condiment n. 调味品conformation n. 构象conjugation n. 共轭construction n. 建设, 建筑consultant n. 顾问consumer n. 消耗container n. 容器containment n. 抑制cooler n. 冷却器corporate adj. 共同的correlate n. 相关的事物cosmetic n. 化妆品counteract vt. 抵消,抵抗coupling reaction n. 偶合反应covalent bond n. 共价键critical adj. 临界的cumulative adj. 累积的,累加的customary adj. 通常的, 常例的cycloparaffin n. 环烷烃Ddecolorant n. 脱色剂decolorize v. 脱色degradation n.降解dehydration n. 脱水作用dehydrogenation n. 脱氢作用delocalization n. 离域作用denatured alcohol n. 变性酒精denominator n. 分母derivation n. 衍生,由来derivative n. 衍生物desorption n. 解吸作用destructive distillation 分解蒸馏detergent n. 洗涤剂developer n. 显影剂dextrorotary adj. 右旋的diazonium salt n. 重氮盐diazotization n. 重氮化作用dielectric adj.不导电的,n.电介质dipole n. 偶极directory n. 地址录disclose vt. 揭露, 揭发discrete adj. 离散的,不连续的disposal vt. 排出, 处理director n. 定位基dissolve v.溶解distillation n. 蒸馏dominant adj. 支配的,统治的donor n. 给体drastic n. 激烈的, 猛烈的droplet n. 液滴dyestuff n. 染料Eelectrophilic reagent n. 亲电试剂electrophobic adj 疏电子的electronegative adj 电负性的electron withdrawing group n. 吸电子基electrostatic adj. 静电的elimination n. 消除反应emulsion n. 乳剂endothermic adj. 吸热的enantiomer n. 对映体enzyme n. 酶epoxy adj. 环氧化的essential oil n. (香)精油ester n. 酯esterification n. 酯化作用ethanol n. 乙醇ether n. 醚, 乙醚ethyl n. 乙基ethylene n. 乙烯ethynyl n. 乙炔基evaluation n. 评价,估价evaporation n. 蒸发excitation n. 激发态exothermic adj. 放热的extract vt. 萃取extrapolation n. 推断Ffermentation n. 发酵fiber n. 纤维filament n. 细丝,丝状体filter n.过滤器,滤色片flare v. & n. 闪耀, 闪烁flavoring n. 香剂, 调味剂fluorescent n. 荧光fore adj. 先时的, 前部的formaldehyde n. 甲醛fossil n. 化石fractional distillation n. 分馏free radical n. 自由基fumigant n. 熏蒸(消毒)剂functional group n. 官能团furan n. 呋喃Ggeneralization n. 一般(性), 普遍(性) genetic code n. 遗传密码geological adj. 地质(学)的geomatrical adj. 几何学的glacial acetic acid n. 冰醋酸glucose n. 葡萄糖glycerol n. 甘油, 丙三醇graphics n. 图,制图法Hhabituation n. 习惯作用, 毒瘾halogenation n. 卤化hazardous adj. 危险的, 有危害的herbicide n.除草剂heterocyclic compound n.杂环化合物heterogeneous adj. 非均相的, 多相的hexagon n. 六边形highlight n. 光线明亮处hold-up n. 塔储量, 容纳量homologous series n. 同系列hormone n. 激素humectant n. 润湿剂hybrid n. 杂化hydration n. 水合作用hydrogenation n. 氢化作用hydrolysis n. 水解hydrophobic adj. 疏水的hydroxyl group n. 羟基Iidealize vt. 理想化inasmuch as adv. 因为, 由于indicator n. 指示剂indiscriminate adj. 不加选择的indol n. 吲哚inductive effect n. 诱导效应ineffective adj. 无效的, 低效率的infrared spectroscopy n. 红外光谱ingenious adj. 坦率的, 天真的ingestion n. 吸收, 吸入inlet n. 进口, 入口insecticide n. 杀虫剂insulin n. 胰岛素integrate vt. 积分,使...一体化interchangeable adj. 可互换的intermediate n. 中间体ion n. 离子isoelectric point n. 等电点isomer n. 异构体Jjacket n. 套, 夹套justification n. 认为正当, 正当的理由Kketone n. 酮Llactic acid n. 乳酸leakage n. 泄漏lesser adj. 较小的, 更少的lime n. 石灰lining n. 衬里, 衬料, 衬套link vt. 连接,键合liquefy vt. 液化lubricating grease n. 润滑脂Mmanipulation n. 操作, 操纵manuscript n. 稿子, 手稿mass spectroscopy n. 质谱mechanism n. 机理, 历程medium n. 介质, 培养基metallurgical adj. 冶金(学)的methane n. 甲烷methnol n. 甲醇methodology n. 方法论micelle n. 胶粒microorganism n. 微生物migrate vi. 迁移miscible adj. 可溶混的modification n. 修饰monomer n. 单体monosaccharide n. 单糖multiplet n. 多重峰multiplicity n 多重性Nnaphthalene n. 萘nitration n. 硝化作用nitric acid n. 硝酸nitrile n. 腈noble adj. 贵重的, 惰性的nomenclacture n. 命名法noteworthy adj. 显著的nucleophile n. 亲核试剂nucleic acid n. 核酸neutralization n. 中和numerator n. (数学上) 分子nutrient n. 营养素, 养分Oobservable a. 可观察到的octane number n. 辛烷值olefin n. 烯烃optical activity n. 旋光性optics n. 光学optimum n. 最佳条件orbital n. 轨道organometallic compound 金属有机化合物originate vi./vt. 起源outermost adj. 最外层的,远离中心的overhead n. 塔顶馏出物overheat vt. 过热overlap vt. 重叠oxidation n. 氧化作用ozonide n. 臭氧化合物ozonolysis n. 臭氧分解Pparaffin n. 链烷烃, 石蜡peptide n. 肽perfume n. 香料peroxide n. 过氧化合物persistence n. 坚持, 固执pesticide n. 杀虫剂pharmaceuticals n. 药物phenol n. 苯酚phenoxide n. (苯)酚盐phenylsulfonic acid n. 苯磺酸phosphoric acid n. 磷酸photochemical reaction n. 光化学反应photochromism n. 光致变色photoconductivity n. 光电导性pigment n. 颜料pink n. 粉红色polyamide n. 聚酰胺polarization n. 极化作用polyhydric alcohol n. 多元醇polymerization n. 聚合作用precipitate vi. /n. 沉淀preservative n. 防腐剂prolong vt. 延长, 拖延propellant n. 推进剂prospective adj. 预期的, 有希望的protecting group n. 保护基purity n. 纯度pyridine n. 吡啶pyrolysis n. 热解pyrrole n. 吡咯Qquantify vt. 使量化,确定数量quaternary ammonium salt n. 季铵盐quench vt. 淬灭quinoline n. 喹啉Rracemization n. 外消旋作用reagent n. 试剂realization n. 实现recover vt. 回收recrystallization n. 重结晶rectifier n. 精馏器reduction n. 还原(作用) reflux n. 回流refract vt. 折射refrigerant n. 冷冻剂remainder n. 剩余物, 残余部分的replica n. 复制品,拷贝resolution n. 分辨, 拆开restrictive adj. 限制性的ribonucleic acid n. 核糖核酸(RNA) rigorous adj. 严厉(格)的Ssaccharin n. 糖精saponification n. 皂化(作用) screen n. 筛子, 屏幕seal n. 密封(垫) segment n. 部分, 链段selectivity n. 选择性settle vt. (使)沉淀, 澄清setup vt. 装置, 装配sewage n. 污水silica gel n. 硅胶singlet n. 单重峰skeleton n. 骨架solubility n. 溶解度solvant n. 溶剂化物solvent n. 溶剂, 有溶解力的sophistication n. 复杂spectroscopy n. 光谱spin-spin coupling n. 自旋-自旋偶合stabilization n. 稳定作用stereoisomerism n. 立体异构现象steric factors n. 位阻因素, 空间因素still pot n.蒸馏釜stoichiometric adj. 化学计算的straightforward adj.一直向前, 正直的substituent n. 取代基substitution reaction n. 取代反应sucrose n. 蔗糖sulfa drug n. 磺胺药sulfonation n.磺化作用sulfuric acid n. 硫酸supervisor n. 导师, 监督人, 主管人suspension n. 悬浮液sweetener n. 增甜剂symmetry n. 对称性symposium n. 座谈会syn addition n. 顺式加成Ttar n. 焦油(沥青)tartaric acid n. 酒石酸tautomerism n. 互变异构现象terpene n. 萜烯tertiary adj. 叔的, 第三的tetrahedron n. 四面体thiazole n. 噻唑thiophene n. 噻吩toluene n. 甲苯toxicity n. 毒性transesterification n. 酯交换反应transition state n. 过渡状态tray n. 盘, 分馏塔盘triplet n. 三重峰trivial adj. 轻微的Uultraviolet-visible spectroscopy n. 紫外-可见光谱unify vt. 统一urea n. 尿素Vvalidate vt. 使生效vaporize vt.蒸发versatile adj. 多方面的vice versa adj. 反之也然vinegar n. 醋violate vt. 破坏,侵害Wwhereas conj. 而, 却, 鉴于withdraw vt. 拉, 提取, 取出withdrawal n. 收回,撤回Xxerography n. 静电复印法Yyeast n. 酵母Zzymochemistry n. 酶化学。
应用化学专业英语

Ensure language is precise, objective, and free of grammar and spelling errors Use appropriate chemical termination
Experimental report writing
Literature reading skills
Analyze the structure of the article
Skim through the title, abstract, introduction, methods, results, and discussion sections to get a general understanding of the article
Identify the main points
Pay attention to the main findings, conclusions, and experimental design to understand the significance of the article
Take notes
Special Considerations
Include raw data, tables, figures, and any deviations from the protocol Follow the institutional reporting guidelines
Summary and Introduction Writing
CHAPTER
Academic paper writing
Purpose
To communicate chemical research findings to other disciplines and professionals
应用化学专业英语第二版课后练习题含答案

应用化学专业英语第二版课后练习题含答案Chapter OneMultiple Choice Questions1.The scientific method of problem solving consists of___________. A. observation, experimentation, hypotheses, theory B.experimentation, observation, theory, hypotheses C. observation, hypotheses, theory, experimentation D. hypothesis, theory,experimentation, observationAnswer: A2.Which of the following is NOT one of the key steps involvedin the scientific method? A. Formulating hypotheses B. Building experiments C. Rejecting hypotheses D. Proving hypothesesAnswer: D3.Which of the following is a physical property? A. Density B.Flammability C. Reaction with acid D. RustingAnswer: A4.Which subatomic particle carries a positive charge? A.Proton B. Neutron C. Electron D. AtomAnswer: A5.Which of the following is NOT a chemical change? A. Burningof wood B. Digestion of food C. Melting of ice D. Rusting of ironAnswer: CShort Answer Questions1.What is the difference between a chemical and physicalchange? Answer: A physical change is a change in the physicalproperties of a substance, whereas a chemical change is a change in the chemical properties or composition of a substance.2.What is an atom? Answer: An atom is the smallest particle ofmatter that retns the chemical properties of an element.3.What is a molecule? Answer: A molecule is a group of two ormore atoms that are chemically bonded together.4.What is an element? Answer: An element is a pure substancethat cannot be broken down into simpler substances by chemical means.5.What is a compound? Answer: A compound is a pure substancemade up of two or more elements that are chemically bondedtogether.Essay Questions1.What is the scientific method and how is it used to solveproblems in science? Answer: The scientific method is a logical and systematic approach to problem solving in science. It consists of several key steps including observation, hypotheses,experimentation, and theory. Scientists use the scientific method to answer questions and solve problems by gathering data through observation and experimentation, forming hypotheses based on that data, testing those hypotheses through experimentation, andfinally developing theories to expln the results of those experiments.2.Describe the properties of matter and how they are used to differentiate between substances. Answer: Properties of matter can be either physical or chemical. Physical properties include mass, volume, density, color, melting point, boiling point, and solubility. These properties can be used to differentiate between substances by comparing their physical properties. Chemical properties include reactivity, flammability, and toxicity. These properties are used to differentiate between substances based on their chemical behavior under certn conditions.3.Describe the structure of an atom and the role that subatomic particles play in determining chemical behavior. Answer: Atoms have a central nucleus that contns protons and neutrons. Electrons orbit around the nucleus in shells or orbitals. Protons carry a positive charge, electrons carry a negative charge, and neutrons carry no charge. The number of protons in the nucleus determines the element to which the atom belongs, while the number of electrons in the outermost shell determines the chemical behavior of the atom.4.Expln the difference between a mixture and a pure substance. Give examples of each. Answer: A mixture is a combination of two or more substances that are not chemically bonded together. Mixtures can be either homogeneous or heterogeneous. A homogeneous mixture is a uniform mixture where the composition is the same throughout, such as saltwater. A heterogeneous mixture is a non-uniform mixture where the composition varies, such as oil and water. A pure substance is a substance that cannot be broken down into simpler substances by chemical means. Examples of pure substances include elements such as gold and silver, and compounds such as water and carbon dioxide.5.Expln the difference between a physical change and a chemical change. Give examples of each. Answer: A physical change is a change in the physical properties of a substance, such as shape, size, or state of matter, without changing its chemical composition. Examples of physical changes include melting ice, boiling water, and cutting paper. A chemical change is a change in the chemical properties or composition of a substance, resulting in the formation of a new substance or substances. Examples of chemical changes include burning of wood, digestion of food, and rusting of iron.。
应用化学英语名称

应用化学英语名称Application Chemistry in English is often referred to as "Applied Chemistry." It is a branch of chemistry that focuses on the practical application of chemical theory andprinciples to real-world problems and the development of useful materials, products, and technologies.Applied Chemistry is a diverse field that encompasses a wide range of sub-disciplines, including materials science, chemical engineering, pharmaceutical chemistry, environmental chemistry, and more. It plays a crucial role in the development of new technologies and the improvement of existing ones, contributing significantly to various industries such as healthcare, agriculture, energy, and manufacturing.In the pharmaceutical industry, applied chemists work on the synthesis of new drugs, the development of drug delivery systems, and the improvement of drug efficacy and safety. They are instrumental in the fight against diseases by designing and testing compounds that can target specific biological pathways.Environmental chemistry, another sub-discipline of applied chemistry, deals with the study of chemical processes in the environment and the impact of human activities on the environment. Applied chemists in this field work on solutions to reduce pollution, develop sustainable energy sources, andremediate contaminated sites.Material science is a field where applied chemistry is heavily utilized. Chemists in this area focus on the design and synthesis of new materials with specific properties, such as high strength, light weight, or electrical conductivity. These materials are critical in the advancement of electronics, aerospace, and many other technologies.Furthermore, applied chemistry is essential in the agricultural sector where it is used to develop fertilizers, pesticides, and other chemicals that can enhance crop yields and protect plants from diseases.The field of applied chemistry is constantly evolving with new discoveries and innovations. It requires a solid foundation in theoretical chemistry as well as practical skills in laboratory techniques and an understanding of the broader implications of chemical applications on society and the environment.In conclusion, Applied Chemistry is a dynamic and essential field that bridges the gap between scientific theory and practical use. It is a key driver of technological advancement and plays a significant role in addressing some of the world's most pressing challenges.。
应用化学专业英语-Lesson-2..

• Elements are composed of extremely small particles called atoms. All atoms of a given element are identical. The atoms of one element are different from the atoms of all other elements.
• Compounds are composed of atoms of more than one element.
• Chemical reactions involve only the rearrangement of atoms; atoms are not created or destroyed in chemical reactions.
• Protons and neutrons are located in the nucleus of the atom, which is small. Most of the mass of the atom is due to the nucleus.
• Electrons are located outside of the nucleus. Most of the volume of the atom is due to electrons.
Some Complex Ions
Name Carbonate Nitrate Phosphate Dihydrogen Phosphate Sulfate Sulfite Thiosulfate Perchlorate Chlorite Cyanide Chromate
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
应掌握的词和技术用语Lesson 1.
element composition sample thermometer sodium chloride vapor pressure melting point boiling point structure
Lesson 2
valence electron chemical bond ionic bond covalent bond configuration electronegativity polar solvent noble gas bonding electron positive charge negative charge dipole moment coordinate bond complex intermolecular force volatility viscosity
surface tension dispersion force hydrogen bond polarizability ether
Lesson 3
acid
base
metallic oxide electrolyte chemical formula acid anhydride proton ionization characterize indicator dye neutralization evaporation crystal
Lesson 4
nomenclature
atomic number
Table 4.1 (except for As, Be, Bi, Ga, Ge, Mo, Se, Sn, W)
Table 4.2 anion oxidation state ligand carbonyl
Lesson 5
synthesize
halogen
aliphatic compound alicyclic compound aromatic compound heterocyclic compound hydrocarbon alkane alkene alkyne acetylene ethylene double bond triple bond
olefin
fatty acid
benzene
derivatives
phenyl
conjugated double bond functional group aldehyde ketone carboxylic acid primary amine
Lesson 6
substituent
straight chain branched chain Table 6.1 (C1 ~ C20) side chain
main chain cycloalkane propylene
1,3-butadiene
vinyl allyl
aromatic hydrocarbon toluene
xylene
arene
ortho, meta, para pyridine
pyrrole
furan
thiophene
Lesson 7
hydroxyl group parent compound ethylene glycol glycerol
ester
amide
acyl halide imide hydroperoxide peroxide phenol
alkoxy group tetrahydrofuran formaldehyde
acetone benzophenone carboxyl group benzoic acid
adipic acid
phthalic acid isophthalic acid terephthalic acid maleic anhydride
N,N-dimethylformamide
Lesson 8
organometallic compound reactivity
synthesis
nucleophile reduction reaction Grignard reagent yield
stability
Lesson 9
polymer plastics
fiber macromolecule monomer polymerization mechanism
addition polymerization condensation polymerization initiator
free radical
cation
reactive center catalyst esterification reaction chain polymerization step polymerization cellulose
chemical modification backbone chain thermoplastic polymer thermosetting polymer elastomer
resin
adhesives
sealant
Appendix 1
condenser
beaker
buret chromatography column dropping funnel
one-neck (round) flask separatory funnel pipet
three-neck flask test tube
oven
rotary evaporator water bath
oil bath
Lesson 10
thin layer chromatography (TLC) purity
polarity
flow rate
detector
refractive index
retention time
qualitative analysis quantitative analysis calibration
gas chromatography
adsorption
resolution
mass spectrometry
spectrum
ultraviolet spectroscopy chromophore
nuclear magnetic resonance spectroscopy
Lesson 11
simple distillation fractional distillation steam distillation purification reversible reaction decomposition reflux
Lesson 12
extraction pigment adsorbent side reaction
Lesson 13
crystallization
van der Waals force recrystallization filter
Lesson 14
characterization optical rotation isotope infrared spectroscopy chemical shift wavenumber
transmittance absorbance methylene vibration concentration mass-to-charge ratio sublimate
thesis
Unit 16
thermodynamics entropy enthalpy
free energy endothermic reaction exothermic reaction equilibrium
Unit 18
isomerism molecular formula isomer constitutional isomer stereoisomer enantiomer
cis-trans isomer chiral molecule
Unit 23
natural product biological activity antibacterial additives microorganism total synthesis
科技论文英文摘要的撰写
aldol condensation
surfactant
precursor。
