英语学习应用化学专业英语考试必背
应用化学专业英语

英译汉:1.First, electrons are added one at a time moving from left to right across aperiod……首先,从左向右横跨一个周期时每次增加一个电子。
当这种情况发生时,最外层电子将受到逐渐增强的核引力,所以电子将更接近原子核而受到其更紧密的束缚力。
其次,在周期表中从上向下移动一列,最外层电子受到核的束缚力将变弱。
这是因为主能级数(屏蔽最外层电子受到核的吸引)在每族向下移动时增加。
这些趋势解释了通过观察元素的原子半径、电离能、电子亲和力和电负性而得到的元素性质的周期性规律。
2.It is important to note that at equilibrium the rates of reaction,rate r and rate fare equilibrium mixture are usually not equal……值得注意的是,在化学平衡时的反应速率,正反应速率和你反应速率相等但反应物和生成物的摩尔浓度在平衡混合态时一般不相等。
但是,事实上每种反应物和生成物在平衡时其浓度为定值,因为每种物质在一个反应中的消耗速率与其在相应你反应正的生成速率相等。
在化学平衡提出之前,这种系统被称为动力学平衡状态。
3.This is a mathematical expression of the law of chemical equilibrium which maybe stated as follows: When a reversible…………这是化学平衡定律的数学表达式,它可以通过如下所述:当一个可逆反应在给定温度下达到平衡时,在方程式中箭头右边物质的摩尔浓度的积除以左边物质摩尔浓度的积(每种物质浓度的幂等于反应方程式中每种物质的分子数)为定值,4.Analytical chemistry,or the art of recognizing different substances anddetermining their constituents, takes a prominent position among分析化学或鉴定不同物质并测定其成分的技术,因为可以解决每当化学过程被用于科学的或技术性的目的是产生的问题,而在科学应用领域中占显著地位。
化学专业英语考试词汇表.

Applied Chemistry (1000 specialized English Words)Aaberration n. 偏差ablation n. 脱落,消融abscissa n. 横坐标*absorbance n. 吸收率absorption n. 吸收absorption coefficient n.吸收系数*abstract vt. 提取accommodation n.容纳性*accuracy n. 精度*acetamide n. 乙酰胺acetic acid n.醋酸acetophanone n. 乙酰苯,苯乙酮*acetylene n.乙炔acetyl-salicylic acid n. 乙酰水杨酸achiral a.非手性的actinium n.锕actinide n. 锕系元素*activation n.活化*addition n. 加成反应adiabat n. 绝热线adipic acid n. 己二酸aerobe n. 需氧微生物*affinity n. 亲和力alchemy n. 炼金术*alcohol n. 醇*aldehyde n. 醛, 乙醛algebra n. 代数学*aliphatic a. 脂肪族*alkali n. 碱*alkane n. 烷烃*alkene n. 稀烃*alkoxy n. 烷氧基alkyl n. 烷基alkylation n. 烷基化反应alkylbenzene烷基苯alkyne 炔烃allowance n. 允许(量),许可(量)alphabetical a. 以字母顺序的*aluminum n. 铝aluminosilicate n. 铝硅酸盐amalgam n. 汞齐ambient a. 周围的americium n. 镅amide n. 酰胺,氨基化合物aminoethane n. 乙胺*ammonia n. 氨水*ammonium n. 胺amphiphile n. 两亲物,亲水脂分子amplitude n.广大,充足,振幅amylase n. 淀粉酶anaerobe n. 厌氧微生物analogue n. 类似物angstrom n. 埃anharmonicity n. 非协性anhydride n. 酐*aniline n. 苯胺*anion n. 阴离子anti- 对位交叉,反式antiknock n; a. 防爆(的),抗爆(的)antimony n. 锑antiperspirant n. 抗汗剂apatite n. 磷石灰*apparatus n. 设备appreciable a. 明显的,可察觉的aqueous a. 水的aquchlorochromium n. 水合氯化铬*aromatic a. 芳香烃的argon n. 氩arrangement n.排列方式arsenic n. 砷, a. 含砷的*aryl n. 芳基assimilate vt. 吸收, vi. 被吸收,同化assumption n. 假定,推测astatine n. 砹asymmetric a. 非对称的,不匀称的,不均匀的atomism n. 原子学说axially ad. 轴对称地*azeotrope n.共沸物Bbacteriological a.细菌学的barium n. 钡batch n. 一批,大量batchwise a.成批的*benzene n.苯benzoyl n.苯甲酰berkelium n. 锫bicarbonate n.重碳酸盐bilayer n. 双分子层binary a. 二元的biodiversity n.生物合成法bioenergy n.生物能biogas n.生物气体biomass n.生物量biosphere n.生存范围,生物圈biosythetic a.生物合成的biphenyl n.联苯bismuth n. 铋bleach vt. 漂白,vi.变白blemish vt. 有损…的完美,玷污n.污点,瑕疵bride n. 硼化物*bond v; n. 键(合)bonding n. 键合*boron n.硼botanist n.植物学家bounce n.; v. 反射,发射brine n.盐水bronze n.青铜,青铜色,青铜制品bromine n. 溴brewage n.(酒)酿造,饮料调制butadiene n. 丁二烯butane n.丁烷butene n.丁烯byproduct n.副产物Ccadmium n.镉caesium .n. 铯calandria n. 加热体,加热器calcium n.钙calibrate vt 校准,使标准化caloric n.热(量),热质 a.热(量)的,卡的*capillary n. 毛细管*carbanion n. 碳阴离子carbon tetrachloride n. 四氯化碳*carbonate n.碳酸盐vt. 使变成碳酸盐,使充满二氧化碳*carbonium n. 碳正离子carbonyl n.碳酰基,羰基carboxylate n.羰化物*carboxylic a.羧酸的*carcinogen n.致癌的cascade n.阶式蒸发器catabolism n.分解代谢,降解代谢category n.种类*catalysis n.催化(作用)catalyst n. 催化剂,刺激(或促进)因素catalyze vt.催化catalytic reforming 催化重整*cation n.阳离子caustic a.碱性的caustic potash 苛性钾(KOH)caustic soda 苛性钠(NaOH)*ceramic a.陶瓷的,陶器的,n.陶瓷制品cerium n. 铈cesium n.铯centripetal a. 向心力的chain-initiating 链增长*chelate n.螯合物*chiral a.手性的*chloride n.氯化物*chlorine n.氯chloroacetophenone n.氯苯乙酮chloroform n.氯仿cholesterol n.胆固醇chlorofluoro carbon n. 氯氟烃*chromatography n.色谱法chromium n.铬chromophore n.发色团chronic a. 长期的,慢性的,惯常的,经常的circulating pump 往复泵citric acid n. 柠檬酸clarification n.澄清,净化cleave v.劈开,分裂clinch n.解决,确定v.证明…是对的cobalt n.钴coexistence n.共存,共处*coefficient n. 系数collision n. 碰撞,冲突combust v.燃烧compensate vt.补偿,赔偿complementary a.补充的*complex n. 络合物component n. 组分*concentration n. 浓度conceptualize vt.使概念化*condensation n.冷凝configuration n.轮廓,构成*conformation n. 构象congruent a.和谐的,一致的,全等的consistency n.稠度,一贯,坚固*constituent a.形成的,组成的,n.成分,要素contaminant n.沾染物contemplate v.注视,沉思contemporary a.当代的,同龄的,同时代的contour n.轮廓,外形vt. 画轮廓controversy n.争论,辩论conversely ad. 相反地converter n. 转化器*coordinate n.配位,配价,坐标coplanarity n.共面*copper n. 铜coprecipitate vt.共沉淀coprous a. 亚铜的*corrosion n.腐蚀*cosmetic n.化妆品,a.化妆用的counter ion n.反离子coupling n.联结,结合,偶和*covalent a.共有原子价的,共价的*criteria n.标准cross-reference n. 前后参照,交叉引用crucial a. 至关重要的cryogenic a.低温学的,低温实验法的*crystallinity n.结晶性的,水晶的*cube n.立方体,立方形,立方,三次幂curium n. 锯curvature n. 曲率customary a.习惯的,惯例的cuticle n. 表皮,透明薄膜*cyanide n.氰化物cyano n.氰,氰基cylinder n.圆柱体*cycloalkane n. 环烷烃cytochrome n.细胞色素dative bond 配(价)键*deactivate vt.使不活动decentralize v.使分散,划分decipher v.解开(疑团),破译(密码)decouple n.v.分开,去偶合装置deduce vt.推论,推断deficiency n.缺乏,缺陷*deflect v.歪斜,使偏向*degradation n.降低*delocalization n.离位demolish vt. 拆除,毁坏denote v.指示,表示,意味着depletion n.削减,消耗*deposit vt.(使)沉淀,安置*derivative a.派生的*desolvation n. 去溶剂化作用*desorption n.解吸作用destructive a.破坏(的),危害的*detector n. 检测器*detectable a.能发觉的,detergent a.使干净的*deuterium n. 重氢diagnose v.诊断(疾病),分析diatomic a. 二原子的,二价的,dicarboxylic a.二元羧酸的*dichroism n.二色性,分光特性,两色现象didactic a.教诲的,说教的*diene n.二烯(烃)*diesel n. 柴油机,内燃机*displacement n. 换置,移位,移动,取代*diffraction vt.衍射*diffuse a.散开的,弥散的,v.扩散,散播digestibility n.可消化性,消化率dilemma n.窘境,进退两难*dilute n.a.冲淡(的),稀释*diol n.二醇,二酚*dipole n.双极,偶极discipline n.学科*discriminate v.区别,辨别,有差别地对待disinfection n.消毒*dioxide n.二氧化物disrupt vt.(使)破裂,使中断*dissociate vt.使分离,将…分开*dissociation energy 离解能distaste n.讨厌,嫌恶*distill vt.用蒸馏法提取,蒸馏distillate n.馏出物,馏出液distortion n.扭曲,变形,失真*distribution coefficient 分配系数disubstitute a.二取代的disulfate n.硫酸氢盐,焦硫酸盐divergence n. 分歧dodecahedron n.十二面体donor n.施主downfield n.低磁场*driving force 推动力droplet n. 小滴dynamite n. 炸药dysprosium n. 镝E.*ecological a. 生态学的ecosystem n. 生态系统*effluence n 射出物,流出物effluent a 流出的einsteinium n. 锿*electrode n.电极*electric potential moment 电子偶极矩*electric potential 电势*electrolyse vt. 电解*electromagnetic a. 电磁的,由电磁石产生的*electron acceptor 电子接受体*electron donor 电子给予体electron-capture detector 电子捕获检测器*electronegativity n.电负性*electropositive a.电正性的electronelectrode n. 阴电极electron-withdrawing a. 推电子的*electrophilic a. 亲电的*electroplate vt. 电镀n. 电镀物品,电铸版*electrospray n. 电子喷射elevation n. 升高*elimination n. 除去,剔除,淘汰ellipticity n. 椭圆率elucidation n. 阐明,解释*eluent n. 洗脱液elusive a. 难捉摸的,巧妙地逃避的emanate vi. (气体)发出,(光等)发散,放射embed v. 嵌于emission n. 散发,发射,发射物emitter n. 发射器empirically ad. 经验主义地*emulsifier n. 乳化剂,乳化器*enantiomer n. 对映体*endogenous a. 内存的*endothermic a. 吸热的energetic a. 有精力的,精力旺盛的enhance vt. 提高,增加*enrich vt. 使浓缩,加浓,富集*enthalpy n. 焓,热函entropy n. 熵enzymatic a. 酶的epidemics a. 流行的,n.病equation n. 等式,方程式,相等,均衡equilibrate vt. (使)平衡,(使)均衡equimolar a. 当量克分子的,克分子数相等的ester n. 酯esterification n. 酯化ethane n. 乙烷1,2-ethanediol n. 1,2-乙二醇ethanolamine n. 乙醇胺*ethanoyl n. 乙酰基ethoxyethane n. 乙氧基乙烷,乙醚ethyl 乙基*ethylamine 乙胺ethylene 乙稀ethylenediamine 乙二胺ethyne 乙炔europium n.铕evacuate v. 清除,搬空exactitude n. 精确,正确,精密*exclusively ad. 排外地,专有地excreting n. 排泄,分泌exergonic a. 释出能量的exponent n. 幂,指数expound vt. 阐述,说明exquisite a. 精美的,灵敏的*extraction n. 萃取,提取,摘要*extrapolate n. 推断,外推extravagance n. 浪费,铺张Ffabric n. 编织物facet n. 小平面*facilitate v. 使容易,使便利,推进far-fetched牵强的,勉强的*feasibility n. 可行性featureless a. 无特色的,平凡的*ferment n. 酶,酵酶,*ferric a. 铁的ferrous a. 亚铁的fictitious a. 虚构的,假造的filament n. 丝状体,单纤维filamentous a. 细丝的,单纤维的,细丝状的*fixed-bed 固定床flammabilit n. . 可燃性flash distillation 闪蒸flexibility n. 柔韧性,弹性,折射性,灵活性flip v. 轻击,掷,弹,抽打,迅速翻动*flocculate n. 絮凝物‘*fluorescence n. 萤光,发荧光,荧光性*fluorine n. 氟*fluorocarbon n. 氟碳化合物fluoromethane n. 氟代甲烷fluorspar n. 萤石,氟石flux n. 不断变化的,变迁,流,涨潮,流量focus on 聚焦于,集中(注意力)于forepump n. 前置泵,预抽泵*formaldeehyde n. 甲醛*formic a. 蚁的,蚁酸的*formula n. 公式,程式,处方fossil n. 化石,旧事物;成化石的,陈旧的foul a. 恶臭的,邪恶的;弄脏;犯规fraction n. 小部分,片段,馏分,级分*fractionating column 分馏柱*fragmentation n. 分裂,崩溃,爆破francium n. 钫froth n. 泡沫,起泡,v. 使(啤酒)起泡fructose n.果糖,左旋糖frustrate v.击败,阻拦fungi n.(的形式)真菌fullerence n. C60furniture n.家具,设备,装置fuzzy a. 模糊的,失真的Ggalactose n. 半乳糖gallium n. 镓garnet n. 石榴石*gasifier n. 汽化器,煤气发生器gastric a. 胃的*gene n. 基因,遗传因子germanium n. 锗glucose n.葡萄糖glycerine n 甘油glucerol n. 丙三醇,甘油gold n. 金*graphite n. 石墨grease n.动物脂grid n.格栅,栅极gauche a.偏转gyromagnetic ratio 回转磁比率H.hafnium n. 铪*halide n. 卤化物*halogen n. 卤素*halogenation n. 卤化,卤代hamper vt. 防碍haphazard a. 偶然的,随便的*helium n. 氦heptane n. 庚烷heredity n. 遗传herein ad. 在此处,如此*heterocyclic a. 杂环的,不同环式的*heterogeneous a. 不同的,异类的hexaaqua a. 六合水的hexadiene n.已二烯hexane n.已烷hexafluoride a. 六氟化合物*hexagonal n. 六角型的holmium n. 钬*homogeneity n. 同种,同质homogenize vt.使均匀,变均匀*homologue n. 同系物horizontal a. 水平的*hormone n. 激素*hybrid n.杂种,混血儿*hydrate n. 水合物,含水物vt.水合作用*hydration n. 水合*hydride n. 氢化物*hydrigen n.氢*hydrocarbon n.烃类hydroelectric a.水力电气的hydrogen halide n. 氢卤化物*hydrolyse vt.水解hydronium ion n.水合离子*hydrophilic a.亲水的*hydrophobic a. 憎水的*hydrostatic(al) a.流体静力学的hydrostatics(pl) 流体静力学*hydrothermal a.水热的*hydroxide n.氢氧化物hydroxyl n.氢氧根,羟基*hydroxylation n.羟基化hypochlorite n.次氯酸盐hypothetcal a.假说的,臆说的I.icosahedron n.二十面体identical a.同一的n.一样,相等*ill-defined a.不清楚的,不确切定义的immaterial a.非物质的,无形的,不重要的immaterialism n.非物质论immaterize vt.使无实体,使无形immerse vt.沉浸,使浸入immiscible a.不混溶性impetus n.推动力,动力,推动impurity n.杂质,混杂物,不纯inactivate vt.钝化,使减少活性inactive a.不活动的,停止的incentive n.诱因,刺激 a.刺激的incident a.入射的,伴随而来的incipient a.开始的,起初的insertion n.插入,插入物inclement a.恶险的,严酷的incontrovertible a.无争论余地的,无疑的,明白的incorporate v.结合,合并indices n.index的复数,指数,刻度,索引indivisible a.不可分割的,不可分裂的induced enzyme 诱导酶indigestion n.消化不良inelastic a.无弹性的,无弹力的,无适应性的inert a.惰性的,不活泼的infectious disease 传染病infinitely ad.无限地,无数地infix n.插入词,中缀vt.使…插入infrared a.红外线的,红外区的n.红外线inherently ad.天生的,本质的inhibitor n.禁止剂,抑制剂,抑制因素inhomogeneity n.不同族,不同质initiating n.开始,创始,入门inlet n.进口vt.引进inoculate vt.给…做注射,给…接种inorganic a.无机的,无生物的inroad n.损害insoluble a.不溶的intact a.尚未被人碰过的,原封不动的integer n.整数,完整的东西interface n.界面,分界面,接触面interferogram n.干涉图,干涉照片interhalogen n.杂卤素intermedium n.中间体intermittent a.断续的,间歇的interrelate vt.使相互关联intimacy n.密切,熟悉intriguing a.吸引人的,有趣的intrinsic a.本质的,固有的,内在的,体内的intuitive a.直观的,直觉的invariably ad.不变的,始终如一的invoke vt.行使(法权等),实行iodic a.含碘的iodide n.碘化物iodine n.碘ion n.离子ion-association complex 离子缔合ion-exchange chromatography 离子交换色谱ionic a.离子的ionisation n.电离作用ionization n.离子化irradiate vt.使明亮,照耀irreversible a.不可逆isocratic a.无梯度的,等度的isomer n.同分异构体isomeric a.同分异构的,同质异能的isopropane n.异丙烷isopropanol n. 异丙醇isopentylamine n.异戊胺isothermally ad.等温的isotope n.同位素isotopically labeled 同位素标记的Jjargon n.行话,专业术语joule n.焦耳Kkerosene n.煤油ketone n.酮kinetics n.动力学krypton n.氪Llabile a.不安定的,不稳定的lactic acid n.乳酸lanolin n. 羊毛脂lanthanide n.镧系金属lanthanum n.镧lattice n.格子,点阵,网格,晶格lattice energy 点阵能legislation n.立法,立案ligand n.配位limestone n.石灰石lipid n.类脂(化合物)literacy n.有文化,有读写能力lithium n.锂litmus n.石蕊longitude n.经线,经度liquefaction n.液化lubricant n.润滑剂,润滑的lustrous a.有光泽的lye n.碱液vt.用碱液洗涤Mmacromolecular n.大分子magma n.岩浆magnitude n.大小,数量,巨大,广大magnesium n.镁makeup n.组成,构造,化妆品malonic n.丙二酸maltose n.麦芽糖mamganese n.锰manifestation n.显示,表现manipulation n.改造,操纵masking agent 掩蔽剂mass spectrometry 质谱mass-to-charge 质荷比matrix n.矩阵membrane n.薄膜,细胞膜membranous a.薄(状)的,形成膜的mercury n.水银,汞mesh n.筛眼,每平方米英寸的网孔(筛眼)数mesophase n.中间相meta-间位-metabolic a.变化的,变形的,新陈代谢metabolism n.代谢作用,新陈代谢metabolite n.代谢物metallurgy n.冶金学metastable a.亚稳的methanol n.甲醇microbe n.细菌,微生物microbiological a.微生物学的microenvironment n.小环境micron n.微米microporous a.多孔的microsecond n.微秒mightily ad.强烈的,非常的milligram n.毫克mingle v.使混合,混合起来minimize v.使减少到最小,降到最低misbrand vt贴错(药品,食品)标记,贴假标签于miscibility n.混溶性modulation n.调整molal a.摩尔的molasses n.糖蜜,糖浆molecular a.分子的,摩尔的molten a.熔融的,融化的molybdenum n. 鉬momentum n.运动量,要素,冲力,衡量monatomic a.单原子的monochromatic a.单色的,单色光的,全色盲的monochrome n.单色器,单色仪monograph n.专题论文monomer n.单体monoxide n.一氧化物mucleosid n.核苷mucletide n.核苷酸multidecker n.多层(板)multifarious a.多种的,各式各样的multitude n.众多,大量,大群,大众multivalued a.多值的mutagen n.诱变mutate v.变化,产生变化mutually ad.互相地,nanoampere n.10-9安培nanometer n.纳米,nmnaphthene n.脂环烃nebulization n.喷雾(作用)nematic a.向列相(的),丝状的neon n.氖neopentane n.新戊烷neutralization n.中和neutron n.中子nitrate n.硝酸盐nitric oxide n.氮氧化物nitrite n.亚硝酸盐nitroglycerine n.硝化甘油noble gas n.稀有气体nomenclature n.术语,命名系统nominally ad.名义上地nonaqueous a.非水的nonbonding a.非粘和的nondestructive a.非破坏性的nonmagnetic a.非磁性的n.非磁性物nonprescription n.非处方nonvolatile a.非挥发性的nucleate v.成核 a.有核的nucleophile a.亲核的numerical a.数字的,用数字表示的,数值的nutrient a.营养的,滋养的nylon n. 尼龙Ooctadecyl n.十八(烷)基octahedral a.八面体的octene n.辛烯octet n.八重态,八重峰octyl n.辛基off-the-shelf a.非定制的olefin n.链烯ongoing n.程序,处置,前进opaque a.不透明的,不透光的optically ad.眼睛地,视力地optimization n.最优化,最佳化orbital a.轨道的orientation n.定位,方向orifice n.小开口,小孔originate v.起源,发生,首创ortho- 邻,正,原,直oscillation n.振动,动摇,变动osmium n. 鋨outermost a.最外面的,最远的overhead n.塔顶流出物 a.高出地面的,架空的overlap v.重叠,与…交叠overtone n.倍频oxalic a.草酸的oxidation n.氧化作用oxidation number n.氧化数oxide n.氧化物oxoacid n.酮酸oxoanion n.含氧阴离子oxonium n.氧鎓oxyanion n.氧离子ozone n.臭氧,新鲜空气Ppalladium n.钯para- 对位paradox n.反论paraffin n.石蜡烃parameter n.参(变)数,参(变)量,因素,特征paratope n.抗体结合部位parentacid n.母体酸partition n.瓜分,隔开penetration n.穿入,渗透,洞察力pentachloide n.五氯化物pentane n.戊烷pentoxide n.五氧化物perchlorate n.高氯酸盐perforate v.穿孔,渗透,有孔的,穿孔的periodic a.周期的,定期的高氯酸盐permeable a.可渗透的peroxidation n.过氧化反应peroxide n.过氧化物peroxo- [词头]过氧化pertain n.附属,关于,相配pesticide n.杀虫剂pharmaceutical a.药学的pharmacy n.药房,药学,配药,制药phenol n.酚phenyl n.苯基phosphorus n.磷,磷光体,发光物质photon n.光子potosynthesis n.光合作用planar a.平面的,平坦的,二维的platinum n.铂pneumatic a.风动的,空气的polar a.极性的,两个相反方向的pollutant n.污染物,污染源polycrystalline a.多晶的polyene a.多烯的potassium n.钾pilyfuctional a.多功能的polygon n.平面多边形polyhalogen n.多卤素polyhedra [polyhedron的复数]形式n.多面体polymer n.聚合物polymer nylon 尼龙polymeric a.聚合的polymerization n.聚合作用polyvinyl a.乙烯聚合物的pore n.气孔,毛孔,细孔postulate n.假定,基本条件v.假设potassium n.钾potassium dichromate 重铬酸钾potassium permanganate 高锰酸钾potent a.强有力的p-phenylenediamine n.亚苯基precipitator n.沉淀器,除尘器precipitate n.沉淀predilection n.偏爱predominantly ad.占优势的preferential a.优先的premature a.早熟的,不成熟的prerequisite n.先决条件preservative a.有保护力的,防腐的n.防腐剂,防腐料primary a.最初的,伯-probabilistic a.概率统计的,椭圆的probe n.探针,探测品v.探查,穿刺prominent a.突起的,著名的,突出的promote vt.促进,发扬,提升,发起propanal n.丙醛propanol n. 丙醇propene n.丙稀proportional a.比例的,相称的propyne n.丙炔proton n.质子protonation n.质子化,质子化作用provision v.供以物质n.准备,供应pseudothermodynamic a.假(热)力学purge n.v.清除,吹洗,净化pyridine n.吡啶quadrupole n.a.四极(的)qualitative a.定性的quality parameter 质量参数quantitative a.数量的,定量的quantum n.量子quintessence n.典型Rradius n.半径racemic a.消旋地radialization n.辐射,放射radial a.径向的,半径的,光线的,射线的radioactive a.放射性的,有辐射的radium n.镭radon n.氡random a.随机的,随意的,随机,随意reactant n.反应物reactor n.反应器reagent n.反应试剂rearrange vt.vi重新安排reboiler n. 再沸器recipe n. 配方,食谱,方法rectification n. 精馏,纠正refabrication n. 重组,重新安排reflux n. 回流,逆流,退潮refractive a. 折射的,有折射力的,屈折的regenerability n. 再生能力reoxidation n. 再氧化repulsive a. 排斥的residual a. 残余的,剩余的resonance n. 回响,共鸣resolution n. 分辨率resonance n.共振response n.响应respiration n. 呼吸作用,生物的氧化作用retention n. 保留,保持,保持力retort n. 曲颈瓶,蒸馏器(蒸馏器中加热)提纯reversibility n. 可反转性,可逆性rhodium n.铑rigorous a. 严厉的,严格的rotation n. 转动roundabout a. 迂回的,不直接的的rubble n. 碎石rubidium n. 铷rudimentary a. 根本的,初期的,发育不健全的ruthenium n.钌Ssaline a. 盐的,盐湖samarium n.钐sanitation(环境)n. 卫生,卫生设备saponification n. 皂化scandium n.钪schematically ad. 图解地,扼要地sebum n. 皮脂,脂肪segregate vt. 分凝,分异selectivity n. 选择性selenium n. 硒semipermeable a. 半透性的sensitivity n. 敏感性,灵敏性separation coefficient 分离系数sequester v. 隔绝sewage n. 污水,污物,污水处理sieve-plate column 筛板柱silanol n. 硅醇,硅烷醇silica n. 氧化硅,硅土silicon n. 硅silver n.银spoilage n. 食品等)腐败,损坏,损坏的东西spontaneity n. 自然,自发,自发性spontaneously ad. 自发地spray n. 喷雾,飞沫,v. 喷,喷射sprinkle v. 洒,喷淋stationary a. 不动的,静止的,固定的,停留的stepwise a. 逐步的steric a. 空间的,位的sterilization n. 灭菌,削菌still n. 蒸馏室,蒸馏器,蒸馏stoichiometric a. 化学计量的strontium n.锶substantial a. 物质的,坚固的,大量的substituent n. 取代,替代,交换substitution n. 代理,交换,替换subtract v. 减去,减掉,扣除succcinc n. 琥珀,丁二酸的,琥珀酸的sucrose 蔗糖+suffice v. 足够,是满足,满足需要suffix n. 后缀sulfate n. 硫酸盐用硫酸处理sulfite n. 亚硫酸盐sulfur n. 硫磺,v. 用硫磺处理sulfuric a. 硫磺的,含多量硫磺的sulphinate vt.磺化n. 磺酸盐summarization n. 概括,概述,综述superconductibity n. 超导性supersaturation n. 过饱和(现象)supersaturation zone 过饱和区surfactant n. 表面活性剂sustainable a.足可支撑的,可忍受的swirl vt. 旋动,使打旋,旋涡symmetrically ad. 对称性地,对称地,平衡地synchronize vi. 同步发生,同步vt. 使时间上发生一致,使同步synchrotron n. 同步加速器synthesize v. 合成syringe n. 注射器,洗涤器,vt. 注射,洗涤Ttable salt (餐桌上的)食盐tantalum n.钽taxology n. 分类学tellurium n. 碲‘template n. (切金属、石、木等用的)样板,模板tensile a. 张力的,抗张的,拉力的terminology n. 术语thallium n.铊theorem n. 定理,原则thermal a. 热量的温度的,热的thermal conductivity 热导性thermochemistry n. 热化学thermodynamics n. 热力学thionyl n. 亚硫酰thiophenol n. 硫酚three-dimensional structure 三维结构thulium n.铥tin n.锡titanium n.钛titrant n. 滴定管titration n. 滴定方法toluidine n. 甲苯胺torr n. 托(压力单位)toxic a. 有毒的toxicant n.有毒物toxicological a. 毒理学的transmutation n. 变化,变形,变质transverse a. 横向的,横切的treatise n. 论文,论述trial-and-error 反复试验法tricarboxylic a. 三羧酸的trichloroethylene n. 三氯乙烯triene n. 三烯tube-and-shell heat exchanger 管壳式热交换器tubular reactor 管式反应器turbid a. 浑浊的,不清的typify vt. 代表,象征,为…典型Uultraviolet a. 紫外的unambiguous a. 不含糊的,明确的unconjugate a. 未共轭的underlie vt. 位于…之下unimolecular a. 单分子的univalent a. 一价的upfield n. 高磁场uranyl n. 铀基,铀酸基,铀氧基urea n. 尿素Vvalence n.(化合)价,原子价valve n. 阀,真空管,电子管vanadium n. 钒vaporize vt.(使)蒸发,(使)汽化vent v. 发泄(情绪),开孔n. 孔,口ventilation n. 通风,公开讨论,通风设备versatile a. 通用的,多才多艺的,反复无常的vertices n. 顶点,头顶,顶vibration n. 振动,摆动,颤动vibronic a. 电子振动的vicinity n. 附近,接近,邻近,邻近地区vinyl n. 乙烯基化学专业英语考试词汇表. vinyl chloride n. 氯乙烯viscosity n. 粘度viscosity index n. 黏度系数vitalism n. 活力论,生机说volatile a. 反复无常的,挥发性的volatility n. 挥发度volumetric a. 测容量的,的容量Wwater fitting n. 水管wavelength n. 波长wavelet n. 子波wavenumber n. 波数well-defined a. 定义明确的,清晰的Xxenon n.氙Yyeast n. 酵母,酵母片vi. 发酵,起泡沫yttrium n. 钇Zzeolite n. 沸石zinc n.鋅zirconia n. 氧化锆zirconium n. 锆11 / 11。
应用化学专业英语及答案

黄冈师范学院2009—2010学年度第一学期期末试卷考试课程:专业英语考核类型:考试A卷考试形式:闭卷出卷教师:杨一思考试专业:化学考试班级:应用化学200601 一、Translate the following into English(20 points)1.过滤2.浓缩3.结晶化4.吸附5. 蒸馏6.超临界的7.二氯甲烷8.热力学平衡9.亲电性10.表面张力11.共轭的12.酮13.平衡常数14.丙基15.丁基16.亚甲基18.环己酮19.同位素20.标准熵二、Translate the following into Chinese(20 points)1. methyl propanoate2. rate constant3. ethyl methyl ketone4. free energy5. radical intermediate6. isobutyl methyl ether7. 3-chloropropene8. primary radical9. n-propyl bromide10. bond energy 11. circulating electrons12. local magnetic fields13. tetramethylsilane14. mass to charge ratios15 phenylamine16 amide17. amine18. nucleophile19. perchlorate20. carbocation三、Translation the following into chinese (40 points)A卷【第1页共 3 页】1. We can see why benzene is stable: according to resonance theory, the more resonance forms a substance has, the more stable it is. Benzene, with two resonance forms of equal energy, is therefore more stable and less reactive than a typical alkene.2. Membranes can be defined essentially as barrier, which separates two phases and restricts transport of various chemicals in a selective manner. A membrane can be homogenous or heterogeneous, symmetric or asymmetric in structure, solid or liquid, can carry a positive or negative charge or be neutral or bipolar. Transport through a membrane can be effected by convection or by diffusion of individual molecules, induced by an electric field or concentration, pressure or temperature gradient. The membrane thickness may vary from as small as 100 micron to several mms.3. The most common industrial adsorbents are activated carbon, silica gel, and alumina, because they present enormous surface areas per unit weight.A surface already heavily contaminated by adsorbates is not likely to have much capacity for additional binding, but further heating will drive off these compounds to produce a surface with high adsorptive capacity.Temperature effects on adsorption are profound, and measurements are usually at a constant temperature. Graphs of the data are called isotherms. Most steps using adsorbents have little variation in temperature.A卷【第2页共 3 页】4. In the absence of peroxides, hydrogen bromide adds to peopene via the Markovnikov pathway to yield isopropyl bromide. In the presence of peroxides, however, the order of addition is reversed, and the product is n-propyl bromide; the addition in this case is said to be anti-Markovnikov. This is interpreted in terms of initiation of the addition reaction by bromine atom, rather than by a proton, as is the case for electrophilic addition.四、Translate the following paragraphs into Chinese(20 points)1.Benzene and its derivatives can be nitrated using a mixture of concentrated nitric and sulphuric acid. The temperature must be controlled to prevent more than one nitro-group going in.2. Benzene can be made to react with halogen derivatives using aluminium chloride as a catalyst. This is called a Friedel-Crafts reaction.can be sulphonated by reacting it with fuming sulphuric acid(oleum). The benzene reacts with sulphur trioxide in the oleum.benzene is converted into ethylbenzene by reacting it with ethene. The ethylbenzene (also called styrene) is used to make polystyrene.黄冈师范学院2009—2010学年度第一学期期末试卷参考答案及评分标准考试课程:专业英语考核类型:考试A卷考试形式:闭卷出卷教师:杨一思考试专业:化学考试班级:应用化学200601 一、Translate the following into English(20 points)2. concentrate 4. adsorption chlorideequilibriumtensionconstant14. propylmagneticresonanceentropy二、Translate the following into Chinese(20 points)1. 丙酸甲酯2. 速率常数3. 甲乙酮4. 自有能5. 自由基中间体6. 异丁基甲醚7. 3-氯丙烯8. 伯自由基9. 正丙基溴化10. 键能11.循环电子12. 局部电磁场13. 四甲基硅烷14. 质荷比15.苯胺16.氨基化合物17.胺18亲核试剂19.高氯酸盐20.碳正离子三、Translation the following into chinese (50 points)1.依据共振理论,物质具有的共振式越多就越稳定。
化学英语证书考试(pec)-基础化学常用词汇

化学英语证书考试(PEC)-基础化学常用词汇1. The Ideal-Gas Equation 理想气体状态方程2. Partial Pressures 分压3. Real Gases: Deviation from Ideal Behavior 真实气体:对理想气体行为的偏离4. The van der Waals Equation 范德华方程5. System and Surroundings 系统与环境6. State and State Functions 状态与状态函数7. Process 过程8. Phase 相9. The First Law of Thermodynamics 热力学第一定律10. Heat and Work 热与功11. Endothermic and Exothermic Processes 吸热与发热过程12. Enthalpies of Reactions 反应热13. Hess’s Law 盖斯定律14. Enthalpies of Formation 生成焓15. Reaction Rates 反应速率16. Reaction Order 反应级数17. Rate Constants 速率常数18. Activation Energy 活化能19. The Arrhenius Equation 阿累尼乌斯方程20. Reaction Mechanisms 反应机理21. Homogeneous Catalysis 均相催化剂22. Heterogeneous Catalysis 非均相催化剂23. Enzymes 酶24. The Equilibrium Constant 平衡常数25. the Direction of Reaction 反应方向26. Le Chatelier’s Principle 列·沙特列原理27. Effects of Volume, Pressure, Temperature Changes and Catalystsi. 体积,压力,温度变化以及催化剂的影响28. Spontaneous Processes 自发过程29. Entropy (Standard Entropy) 熵(标准熵)30. The Second Law of Thermodynamics 热力学第二定律31. Entropy Changes 熵变32. Standard Free-Energy Changes 标准自由能变33. Acid-Bases 酸碱34. The Dissociation of Water 水离解35. The Proton in Water 水合质子36. The pH Scales pH值37. Bronsted-Lowry Acids and Bases Bronsted-Lowry 酸和碱38. Proton-Transfer Reactions 质子转移反应39. Conjugate Acid-Base Pairs 共轭酸碱对40. Relative Strength of Acids and Bases 酸碱的相对强度41. Lewis Acids and Bases 路易斯酸碱42. Hydrolysis of Metal Ions 金属离子的水解43. Buffer Solutions 缓冲溶液44. The Common-Ion Effects 同离子效应45. Buffer Capacity 缓冲容量46. Formation of Complex Ions 配离子的形成47. Solubility 溶解度48. The Solubility-Product Constant Ksp 溶度积常数49. Precipitation and separation of Ions 离子的沉淀与分离50. Selective Precipitation of Ions 离子的选择沉淀51. Oxidation-Reduction Reactions 氧化还原反应52. Oxidation Number 氧化数53. Balancing Oxidation-Reduction Equations 氧化还原反应方程的配平54. Half-Reaction 半反应55. Galvani Cell 原电池56. V oltaic Cell 伏特电池57. Cell EMF 电池电动势58. Standard Electrode Potentials 标准电极电势59. Oxidizing and Reducing Agents 氧化剂和还原剂60. The Nernst Equation 能斯特方程61. Electrolysis 电解62. The Wave Behavior of Electrons 电子的波动性63. Bohr’s Model of The Hydrogen Atom 氢原子的波尔模型64. Line Spectra 线光谱65. Quantum Numbers 量子数66. Electron Spin 电子自旋67. Atomic Orbital 原子轨道68. The s (p, d, f) Orbital s(p,d,f)轨道69. Many-Electron Atoms 多电子原子70. Energies of Orbital 轨道能量71. The Pauli Exclusion Principle 泡林不相容原理72. Electron Configurations 电子构型73. The Periodic Table 周期表74. Row 行75. Group 族76. Isotopes, Atomic Numbers, and Mass Numbers 同位素,原子数,质量数77. Periodic Properties of the Elements 元素的周期律78. Radius of Atoms 原子半径79. Ionization Energy 电离能80. Electronegativity 电负性81. Effective Nuclear Charge 有效核电荷82. Electron Affinities 亲电性83. Metals 金属84. Nonmetals 非金属85. Valence Bond Theory 价键理论86. Covalence Bond 共价键87. Orbital Overlap 轨道重叠88. Multiple Bonds 重键89. Hybrid Orbital 杂化轨道90. The VSEPR Model 价层电子对互斥理论91. Molecular Geometries 分子空间构型92. Molecular Orbital 分子轨道93. Diatomic Molecules 双原子分子94. Bond Length 键长95. Bond Order 键级96. Bond Angles 键角97. Bond Enthalpies 键能98. Bond Polarity 键矩99. Dipole Moments 偶极矩100. Polarity Molecules 极性分子101. Polyatomic Molecules 多原子分子102. Crystal Structure 晶体结构103. Non-Crystal 非晶体104. Close Packing of Spheres 球密堆积105. Metallic Solids 金属晶体106. Metallic Bond 金属键107. Alloys 合金108. Ionic Solids 离子晶体109. Ion-Dipole Forces 离子偶极力110. Molecular Forces 分子间力111. Intermolecular Forces 分子间作用力112. Hydrogen Bonding 氢键113. Covalent-Network Solids 原子晶体114. Compounds 化合物115. The Nomenclature, Composition and Structure of Complexes 配合物的命名,组成和结构116. Charges, Coordination Numbers, and Geometries 电荷数、配位数、及几何构型117. Chelates 螯合物118. Isomerism 异构现象119. Structural Isomerism 结构异构120. Stereoisomerism 立体异构121. Magnetism 磁性122. Electron Configurations in Octahedral Complexes 八面体构型配合物的电子分布123. Tetrahedral and Square-planar Complexes 四面体和平面四边形配合物124. General Characteristics 共性125. s-Block Elements s区元素126. Alkali Metals 碱金属127. Alkaline Earth Metals 碱土金属128. Hydrides 氢化物129. Oxides 氧化物130. Peroxides and Superoxides 过氧化物和超氧化物131. Hydroxides 氢氧化物132. Salts 盐133. p-Block Elements p区元素134. Boron Group (Boron, Aluminium, Gallium, Indium, Thallium) 硼族(硼,铝,镓,铟,铊)135. Borane 硼烷136. Carbon Group (Carbon, Silicon, Germanium, Tin, Lead) 碳族(碳,硅,锗,锡,铅)137. Graphite, Carbon Monoxide, Carbon Dioxide 石墨,一氧化碳,二氧化碳138. Carbonic Acid, Carbonates and Carbides 碳酸,碳酸盐,碳化物139. Occurrence and Preparation of Silicon 硅的存在和制备140. Silicic Acid,Silicates 硅酸,硅酸盐141. Nitrogen Group (Phosphorus, Arsenic, Antimony, and Bismuth) 氮族(磷,砷,锑,铋)142. Ammonia, Nitric Acid, Phosphoric Acid 氨,硝酸,磷酸143. Phosphorates, phosphorus Halides 磷酸盐,卤化磷144. Oxygen Group (Oxygen, Sulfur, Selenium, and Tellurium) 氧族元素(氧,硫,硒,碲)145. Ozone, Hydrogen Peroxide 臭氧,过氧化氢146. Sulfides 硫化物147. Halogens (Fluorine, Chlorine, Bromine, Iodine) 卤素(氟,氯,溴,碘)148. Halides, Chloride 卤化物,氯化物149. The Noble Gases 稀有气体150. Noble-Gas Compounds 稀有气体化合物151. d-Block elements d区元素152. Transition Metals 过渡金属153. Potassium Dichromate 重铬酸钾154. Potassium Permanganate 高锰酸钾155. Iron Copper Zinc Mercury 铁,铜,锌,汞156. f-Block Elements f区元素157. Lanthanides 镧系元素158. Radioactivity 放射性159. Nuclear Chemistry 核化学160. Nuclear Fission 核裂变161. Nuclear Fusion 核聚变162. analytical chemistry 分析化学163. qualitative analysis 定性分析164. quantitative analysis 定量分析165. chemical analysis 化学分析166. instrumental analysis 仪器分析167. titrimetry 滴定分析168. gravimetric analysis 重量分析法169. regent 试剂170. chromatographic analysis 色谱分析171. product 产物172. electrochemical analysis 电化学分析173. on-line analysis 在线分析174. macro analysis 常量分析175. characteristic 表征176. micro analysis 微量分析177. deformation analysis 形态分析178. semimicro analysis 半微量分析179. systematical error 系统误差180. routine analysis 常规分析181. random error 偶然误差182. arbitration analysis 仲裁分析183. gross error 过失误差184. normal distribution 正态分布185. accuracy 准确度186. deviation 偏差187. precision 精密度188. relative standard deviation 相对标准偏差(RSD)189. coefficient variation 变异系数(CV)190. confidence level 置信水平191. confidence interval 置信区间192. significant test 显著性检验193. significant figure 有效数字194. standard solution 标准溶液195. titration 滴定196. stoichiometric point 化学计量点197. end point 滴定终点198. titration error 滴定误差199. primary standard 基准物质200. amount of substance 物质的量201. standardization 标定202. chemical reaction 化学反应203. concentration 浓度204. chemical equilibrium 化学平衡205. titer 滴定度206. general equation for a chemical reaction 化学反应的通式207. proton theory of acid-base 酸碱质子理论208. acid-base titration 酸碱滴定法209. dissociation constant 解离常数210. conjugate acid-base pair 共轭酸碱对211. acetic acid 乙酸212. hydronium ion 水合氢离子213. electrolyte 电解质214. ion-product constant of water 水的离子积215. ionization 电离216. proton condition 质子平衡217. zero level 零水准218. buffer solution 缓冲溶液219. methyl orange 甲基橙220. acid-base indicator 酸碱指示剂221. phenolphthalein 酚酞222. coordination compound 配位化合物223. center ion 中心离子224. cumulative stability constant 累积稳定常数225. alpha coefficient 酸效应系数226. overall stability constant 总稳定常数227. ligand 配位体228. ethylenediamine tetraacetic acid 乙二胺四乙酸229. side reaction coefficient 副反应系数230. coordination atom 配位原子231. coordination number 配位数232. lone pair electron 孤对电子233. chelate compound 螯合物234. metal indicator 金属指示剂235. chelating agent 螯合剂236. masking 掩蔽237. demasking 解蔽238. electron 电子239. catalysis 催化240. oxidation 氧化241. catalyst 催化剂242. reduction 还原243. catalytic reaction 催化反应244. reaction rate 反应速率245. electrode potential 电极电势246. activation energy 反应的活化能247. redox couple 氧化还原电对248. potassium permanganate 高锰酸钾249. iodimetry 碘量法250. potassium dichromate 重铬酸钾251. cerimetry 铈量法252. redox indicator 氧化还原指示253. oxygen consuming 耗氧量(OC)254. chemical oxygen demanded 化学需氧量(COD) 255. dissolved oxygen 溶解氧(DO)256. precipitation 沉淀反应257. argentimetry 银量法258. heterogeneous equilibrium of ions 多相离子平衡259. aging 陈化260. postprecipitation 继沉淀261. coprecipitation 共沉淀262. ignition 灼烧263. fitration 过滤264. decantation 倾泻法265. chemical factor 化学因数266. spectrophotometry 分光光度法267. colorimetry 比色分析268. transmittance 透光率269. absorptivity 吸光率270. calibration curve 校正曲线271. standard curve 标准曲线272. monochromator 单色器273. source 光源274. wavelength dispersion 色散275. absorption cell 吸收池276. detector 检测系统277. bathochromic shift 红移278. Molar absorptivity 摩尔吸光系数279. hypochromic shift 紫移280. acetylene 乙炔281. ethylene 乙烯282. acetylating agent 乙酰化剂283. acetic acid 乙酸284. adiethyl ether 乙醚285. ethyl alcohol 乙醇286. acetaldehtde 乙醛287. β-dicarbontl compound β–二羰基化合物288. bimolecular elimination 双分子消除反应289. bimolecular nucleophilic substitution 双分子亲核取代反应290. open chain compound 开链族化合物291. molecular orbital theory 分子轨道理论292. chiral molecule 手性分子293. tautomerism 互变异构现象294. reaction mechanism 反应历程295. chemical shift 化学位移296. Walden inversio 瓦尔登反转n297. Enantiomorph 对映体298. addition rea ction 加成反应299. dextro- 右旋300. levo- 左旋301. stereochemistry 立体化学302. stereo isomer 立体异构体303. Lucas reagent 卢卡斯试剂304. covalent bond 共价键305. conjugated diene 共轭二烯烃306. conjugated double bond 共轭双键307. conjugated system 共轭体系308. conjugated effect 共轭效应309. isomer 同分异构体310. isomerism 同分异构现象311. organic chemistry 有机化学312. hybridization 杂化313. hybrid orbital 杂化轨道314. heterocyclic compound 杂环化合物315. peroxide effect 过氧化物效应t316. valence bond theory 价键理论317. sequence rule 次序规则318. electron-attracting grou p 吸电子基319. Huckel rule 休克尔规则320. Hinsberg test 兴斯堡试验321. infrared spectrum 红外光谱322. Michael reacton 麦克尔反应323. halogenated hydrocarbon 卤代烃324. haloform reaction 卤仿反应325. systematic nomenclatur 系统命名法e326. Newman projection 纽曼投影式327. aromatic compound 芳香族化合物328. aromatic character 芳香性r329. Claisen condensation reaction克莱森酯缩合反应330. Claisen rearrangement 克莱森重排331. Diels-Alder reation 狄尔斯-阿尔得反应332. Clemmensen reduction 克莱门森还原333. Cannizzaro reaction 坎尼扎罗反应334. positional isomers 位置异构体335. unimolecular elimination reaction 单分子消除反应336. unimolecular nucleophilic substitution 单分子亲核取代反应337. benzene 苯338. functional grou 官能团p339. configuration 构型340. conformation 构象341. confomational isome 构象异构体342. electrophilic addition 亲电加成343. electrophilic reagent 亲电试剂344. nucleophilic addition 亲核加成345. nucleophilic reagent 亲核试剂346. nucleophilic substitution reaction亲核取代反应347. active intermediate 活性中间体348. Saytzeff rule 查依采夫规则349. cis-trans isomerism 顺反异构350. inductive effect 诱导效应t351. Fehling’s reagent 费林试剂352. phase transfer catalysis 相转移催化作用353. aliphatic compound 脂肪族化合物354. elimination reaction 消除反应355. Grignard reagent 格利雅试剂356. nuclear magnetic resonance 核磁共振357. alkene 烯烃358. allyl cation 烯丙基正离子359. leaving group 离去基团360. optical activity 旋光性361. boat confomation 船型构象362. silver mirror reaction 银镜反应363. Fischer projection 菲舍尔投影式364. Kekule structure 凯库勒结构式365. Friedel-Crafts reaction 傅列德尔-克拉夫茨反应366. Ketone 酮367. carboxylic acid 羧酸368. carboxylic acid derivative 羧酸衍生物369. hydroboration 硼氢化反应370. bond oength 键长371. bond energy 键能372. bond angle 键角373. carbohydrate 碳水化合物374. carbocation 碳正离子375. carbanion 碳负离子376. alcohol 醇377. Gofmann rule 霍夫曼规则378. Aldehyde 醛379. Ether 醚380. Polymer 聚合物。
应用化学专业英语-Lesson-2..

• Elements are composed of extremely small particles called atoms. All atoms of a given element are identical. The atoms of one element are different from the atoms of all other elements.
• Compounds are composed of atoms of more than one element.
• Chemical reactions involve only the rearrangement of atoms; atoms are not created or destroyed in chemical reactions.
• Protons and neutrons are located in the nucleus of the atom, which is small. Most of the mass of the atom is due to the nucleus.
• Electrons are located outside of the nucleus. Most of the volume of the atom is due to electrons.
Some Complex Ions
Name Carbonate Nitrate Phosphate Dihydrogen Phosphate Sulfate Sulfite Thiosulfate Perchlorate Chlorite Cyanide Chromate
应用化学专业英语lesson10ChemicalEquilibriumandkinetics
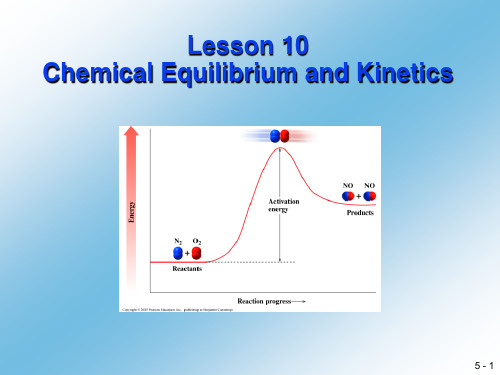
NH3
Add more NH3?
Reaction shifts to the left [N2] and [H2] inc
5 - 35
Le Chatelier’s principle
Adding Pressure affects an equilibrium with gases
N2(g) + 3 H2(g)
N2(g) + 3 H2(g)
Keq =
[ NH3 ] 2 [ N2 ] [ H2 ] 3
2 NH3(g)
5 - 33
Le Chatelier’s principle
Stress causes shift in equilibrium Adding or removing reagent
N2(g) + 3 H2(g)
Temperature: 2. Higher Temperature:
Faster cars More collisions
More Energy More collisions
Reacting molecules move faster, providing colliding molecules w/ Eact.
At this point, equilibrium is achieved. Time
5 - 26
Figure 9.8
2SO2(g) + O2(g)
At Equilibium
2SO3(g)
SO2(g)+O2(g)
Initially
SO3(g)
Initially
5 - 27
Figure 9.9
2SO2(g) + O2(g)
应用化学专业英语复习资料

一单词短语1.Molecule 分子molecular 分子的2.chemical process 化学过程element 元素3.a t o m原子a t t r a c t i o n吸引力4.repulsion 排斥力distillation 蒸馏、n5.distill 蒸馏v rectification 精馏position 构成structure 结构7.property 性质mass 质量8.atomicweight 原子量atomic number 原子序数9.ionization energy 电离能period 周期10.g r o u p族f a m i l y族11.transition group 过渡族main group 主族12.i o n离子s u b s t i t u t i o n取代反应13.el i mi na ti on消除反应nucl eoph i l i c 亲核的14.nucleophilie 亲核试剂electrophilie亲电试剂15.alkyl 烷基的functional group 官能团16.halides 卤素的leaving group 离去基团17.transition state过渡态intermediate 中间体18.r e a c t a n t反应物p r o d u c t生成物19.concentration 浓度rate equation 速率方程20.c o n s t a n t常数e t h e r醚21.endothermic 吸热的substrate 反应底物22.mechanism 机理reagen 试剂23.alkene 烯烃exothermic 放热的24.A n i o n阴离子n i t r o g e n氮气25.Hydrocarbon 碳氢化合物carbonhydrate 碳水化合物26.Alkane 烷烃substituent 取代基27.Isomerism 同分异构现象isomer 同分异构28.V i n y l乙烯基d e r i v a t i v e s衍生物29.acid halides 酰卤acid anhydrides 酸酐30.e s t e r s酯a m i d e酰胺31.ammonia NH3 Acetic anhydride乙酸酐32.phenol 芬acid—base titration 酸碱滴定33.precipitation沉淀analyses 化学分析员34.IR 红外UV紫外MS质谱GC色相色谱HPLC高效液相色谱TLC薄层色谱X—rayX射线衍射二选词填空1、We can now easily account for many things,which were thought to be mysterious by theancients2、the acid acts on the metal and a gas is givenoff.3、you should adapt yourself to new ways oflooking at matters4、electrolytes have more pronounced effect oncolligative properties than do nonelectrolytes. 5、if water in these lakes evaporated at the samerate as fresh water ,both would nearly dryup in a matter of year.6、both laks evaporated very slow compared with afresh lake or even the ocean.7、a property that depends only on the relativeamounts of solute and solvent is know as acolligative property.8、for example ,both NaCl (ionic) and HCl (polarcovalent)are classified as electrolytes becausethey form ions in aqueous solution.9、when compounds such as NaCl and HCl aredissolved in water ,the effect is obvious.10、if the wires is cut ,the light goes out becausethe circuit is broken.11、when wires are attached to a charged batteryand then to a light bulb ,the light shinesbrightly.12、glass and wood as well as pure water areexamples or nonconductors of electricity.13、other substances resist the flow of electricityand are known as nonconductors orinsulators.14、it has long been known that the presence of asolute in water may affect its ability toconduct electricity.15、when the collection of papers was first broughtout,it was well received by the reviewers.16、in the same way the dozen or so mostcommon kinds of kinds of atoms can be put together in many millions of different ways tomake molecules .17、elements are made up of tiny fundamentalparticles called atoms. Fundamental, as it is usedhere ,means that they cannot be furtherdivided by any chemical metheods.18、each element has atoms that is different fromthe atoms of other elements.19、it would not be quite round; on the contraryit would consist of three parts represented byspheres.20、it is not to be summed up in a singleproduct or word ,but in an idea or basicconcept.21、the chemical symbol of an element may standthe element for.22、the rate of a chemical reaction is influencedby several factors such as temperature ,concentration of reagents , particle size ,light ,and catalyst.23、all forms of life in earth are very dependenton chemical reactions or chemical changes.24、a chemical reaction occurs when elements andcompounds react together to produce differentcompounds , or when compounds break down into simpler compounds or elements.三无机物的命名H Hydrogen Li Lithium Na Sodium K Potassium Mg Magnesium Ca CalciumMn manganese Cu copper Zn zinc Fe iron Hg mercury Ag silver Au gold C Carbon Si Silicon Pb Lead Al Aluminium F Fluorine Cl Chlorine Br Bromine I IodineO Oxygen S Sulfur N Nitrogen P Phosphorus1.直呼其名,即读其元素名称+ ion如:Na+ sodium ionK+ potassium ion2.对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous 表示低价,-ic 表示高价如:Cu+ copper (Ⅰ) ion 或cuprous ion Cu2+ copper (Ⅱ) ion 或cupric ionFe2+ iron (Ⅱ) ion 或ferrous ionFe3+ iron (Ⅲ) ion 或ferric ion3.含氢酸根:酸根中的H读做hydrogen,氢原子的个数用希腊前缀表示:mono- di - tri- tetra - penta- hexa-hepta- octa- nona- deca-举例:CO32-carbonate ionHCO3-hydrogen carbonate ionPO43- phosphate ionHPO42hydrogencarbonate ionH2PO4- dihydrogenphosphate ion4.结晶水读做hydrate ,结晶水的个数用希腊前缀表示:mono-di - tri- tetra - penta- hexa- hepta- octa- nona- deca-CuSO4·5H2O copper(Ⅱ) sulfate pentahydrateAlCl3 ·6H2O aluminum chloride hexahydrate5.测试Mg(OH)2magnesium hydroxide AlCl3aluminum chlorideFeBr2 iron(II) bromide CaSO4calcium sulfateZnCO3zinc carbonate HF hydrofluoric acidH3PO4phosphoric acid NO2nitrogen dioxideCuO copper(II) oxide Al2O3aluminum oxideNaHSO3sodium hydrogen sulfiteKMnO4potassium permanganateNaClO sodium hypochloride四有机物的命名1)命名正烷基时,只需把烷烃的词尾“-ane换成“-yl”,加在相应的烷烃的字首后2)字母规则:Butyl>Ethyl>Isopropyl>Methyl>Neopentyl>tert-Pentyl >Propyl3)环烷烃:只需在所对应的烷烃前加上cyclo-即可4)有些结构较复杂的烷基,需添加词头5)烯烃和炔烃命名时将相应的烷烃的词尾“烷”(ane)改为“烯”(ene)或“炔”(yne),后缀前加上不饱和键的编号即可。
化学专业英语词汇记忆法

如关于电化学分析法 吸附伏安法: 吸附伏安法: adsorption voltammetry 溶出伏安法: 溶出伏安法: stripping Voltammetry 富集: accumulate 富集: 线性扫描: 线性扫描: linear scan 检出限: detection limit 检出限: 电化学行为: electrochemical behavior 电化学行为: 工作电极: 工作电极: the working electrode 辅助电极: 辅助电极: the auxiliary electrode 参比电极: 参比电极: the reference electrode 峰电位: 峰电位: peak potential 峰电流: 峰电流: peak current 正电位方向扫描: 正电位方向扫描: positive -going potential scan 负电位方向扫描: 负电位方向扫描: negative -going potential scan
反应物 reactant 生成物 product 共轭酸 conjugate acid 共轭碱 conjugate base 合成 synthesize decompose 分解 晶型的 crystalline 无定形的 amorphous 金属 metal 非金属 non – metal 阳离子 cation 阴离子 anion 阴极 cathode 阳极 anode
导体 conductor 半导体 semiconductor 绝缘体 insulator
孤立体系 isolated system 绝热体系 adiabatic system 敞开体系 open system 分子 原子 离子 质子 电子 中子 光子 molecule atom ion proton electron neutron photon
东华大学应用化学专业英语总结

东华大学应用化学专业英语总结专业英语重点总结单词Toxic chemicals:有毒化学品Chemical pollution:化学污染Physical property :物性 Isolate:分离Determine:测定 Synthesize:合成Fundamental principles:基本原理 Investigation:研究Utilize:利用 Catalyst 催化剂Enzyme 酶Biosphere 生物圈Heterogeneous catalyst 非均相催化剂Nanotechnology 纳米技术Carbon monoxide 一氧化碳Chemical formulas:化学式anion: 阴离子 Oxidation number:氧化值sulphate: 硫酸盐 Hydrides: 氢化物Sodium:钠 cation: 阳离子Covalent bond:共价键electroneutral: 电中性的Electronegative atom:电负性原子 trivial names:俗名Oxidation:氧化Peroxides:过氧化物Superoxide:超氧化物Periodic table:周期表Noble gases: 惰性气vacant orbital:空轨道Coordination (complex) compound: 配位化合物Unshared pair of electrons:未共用电子对oxidation state:氧化态 hydroxides:氢氧化物caustic soda solution:苛性钠溶液vacant orbital:空轨道Formula 分子式 Common name 俗名Derivative 衍生物 Acid salt 酸式盐Hydrate 水合物 Anhydrous 无水的Oxidizing agent 氧化剂Reducing agent 还原剂Oxidation reduction reaction氧化还原反应Electrochemistry 电化学 Electrolysis 电解Strong acid 强酸 Weak base 弱碱Acid-base indicator 酸碱指示剂Distilled water 蒸馏水Buffer solution 缓冲溶液Common ion effect 同离子效应Equivalencepoint 等效点 Neutralization 中和Dissociation 离解度 Anhydride 脱水物Periodic law: 元素周期率 periods (rows):周期group (columns):族 protons:质子Valence electrons:价电子 Halogens: 卤素Atomic radius: 原子半径alkaline earths:碱土金属attractive force: 吸引力electronegativity: 电负性electropositive:正电性univalent ion: 一价离子electron shell: 电子层 bonding force 结合力monatomic 单原子的 Neutrons:中子hydrogen bond 氢键conduct electricity 导电Electrically neutral 电中性的Electrostatic 静电的isomerism :异构现象Reversible :可逆的。
化学专业英语词汇背诵版

第 1 页,共 8 页
52. Oxidation Number 53. Balancing Oxidation-Reduction Equations 54. Half-Reaction 55. Galvani Cell 56. Voltaic Cell 57. Cell EMF 58. Standard Electrode Potentials 59. Oxidizing and Reducing Agents 60. The Nernst Equation 61. Electrolysis 62. The Wave Behavior of Electrons 63. Bohr’s Model of The Hydrogen Atom 64. Line Spectra 65. Quantum Numbers 66. Electron Spin 67. Atomic Orbital 68. The s (p, d, f) Orbital 69. Many-Electron Atoms 70. Energies of Orbital 71. The Pauli Exclusion Principle 72. Electron Configurations 73. The Periodic Table 74. Row 75. Group 76. Isotopes, Atomic Numbers, and Mass Numbers 77. Periodic Properties of the Elements 78. Radius of Atoms 79. Ionization Energy 80. Electronegativity 81. Effective Nuclear Charge 82. Electron Affinities 83. Metals 84. Nonmetals 85. Valence Bond Theory 86. Covalence Bond 87. Orbital Overlap 88. Multiple Bonds 89. Hybrid Orbital 90. The VSEPR Model 91. Molecular Geometries 92. Molecular Orbital 93. Diatomic Molecules 94. Bond Length 95. Bond Order 96. Bond Angles 97. Bond Enthalpies 98. Bond Polarity 99. Dipole Moments 100. Polarity Molecules 101. Polyatomic Molecules 102. Crystal Structure
应用化学专业英语考试指南

化学化工基础部分题型和内容:一、词汇(英译汉)10个词语(10分)二、分子式(16*2分)包括常见非金属氧化物、酸、碱、盐、甲烷、乙烷等的英文表达三、英译汉(20分,4段)课文及课后作业四、化学方程式的英文表达(2个方程式、6分)反应类型:中和反应、氨的合成反应、取代反应五、根据课文内容回答问题(一个问题8分)1. characteristics for an ideal refrigerant. 一个理想的制冷剂的特点2. requirements a reaction must satisfy before it can be used in titrimetric analysis?必须满足要求的反应,才能在滴定分析方法3. reactions which can be used in titrimetric analysis.可在滴定分析中使用的反应专业部分:1.词汇(汉译英)10个词语(10分)2.英译汉(28分,4段)3.汉译英(10分,2句)插座socket 指示灯pilot lamp 甲醇methanol 共价键covalent bond稳态steady state反馈系统feedback system 配体ligand1.Figures 1 and 2 show that electrode potentials after 30 minutes immersion in salt solutionscontaining sodium nitrite at 1N and 0.1N levels of concentration, have all moved in the anodic direction as compared to the potentials just after immersion, while in the case of 10-3N concentration of sodium nitrite, this is so only in the case of salt solution containing 200ppm of sodium chloride.图1和图2表明,30分钟后,电极在盐含有亚硝酸钠在1N的和0.1N的解决方案,浸泡的浓度水平的潜力,都搬到了阳极方向,这一比例仅为浸泡后的潜力,而在10例- 3晚亚硝酸钠的浓度,这是所以只有在盐溶液中的氯化钠200ppm的情况下2.For each element an extended table, a reduced table and tables with notes and bibliographyare given. In the extended tables the different electrode-systems are arranged alphabetically according to the right-hand term of the electrode reaction.对于每个元素扩展表,减少表和注释和参考书目表给出。
应用化学专业英语第一单元The-Roots-Of-Chemistry

The Roots Of ChemistryChemistry can be broadly defines as the science of molecules and their transformations.化学可以广泛地定义为科学的分子和他们的转换。
In contrast to mathematics,chemistry is older than people. 与数学不同,化学比人类更久远。
The appearance of life and people on our planet (earth) is most probably the end result of specific chemical processes .生命的出现和人类生活在我们地球上都最可能是特殊化学过程的结果。
Chemical processes have been present in the lives of people from the dawn of history until the present time .化学过程存从古至今存在人们的生活中。
Initially ,these processes were not under our control 最初,这些过程不受我们的控制,,for instance ,the fermentation of fruit juice,the rotting of meat and fish ,and the burning of wood .例如,果汁的发酵,肉和鱼的腐烂,木材的燃烧。
Later on we learned to control chemical processes and to use them to prepare a variety of different products such as food ,metals ,ceramics and leather .后来,我们学着控制化学过程,用它们来准备一系列不同的产品例如食物。
应用化学专业英语

应用化学专业英语Cu—copper Fe—iron Hg—mercury Na—sodium K—potassium Ag—silverNaOH—sodium hydroxide KOH—potassium hydroxide Fe(OH)2—iron(Ⅱ)hydroxide Fe(OH)3—iron(Ⅲ)hydroxide NH4OH—ammonium hydroxideK3[Fe(CN)6]—potassium hexacyanoferrate(Ⅲ)K4[Fe(CN)6]—potassium hexacyanoferrate(Ⅱ)Ⅰ.Name the following1.(NH4)2CO3Ammonium carbonate2.N2O Nitrous Oxide3.H2SO4Sulfuric acid4.P4O6Phosphorus(Ⅲ)trioxide5.Al2O3Aluminium oxide6.SnCl4Tin(IV)chloride7.KHSO4Potassium hydrogen sulfate8.Cu2S Copper(Ⅰ)sulfide9.HClO4Perchloric acid10.BaCl2Barium chloride11.P4O10Phosphorus(Ⅴ)pentoxide12.NaH Sodium hydride13.Ca(MnO4)2Calcium permanganate14.PF5Phosphorus pentafluoride15.(NH4)2HPO4Diammonium hydrogen phosphateⅡ.Give formulas for the following1.ammonium sulfate(NH4)2SO42.barium iodide BaI23.iron(Ⅱ)sulfate Fe2SO44.potassium permanganate KMnO45.copper(Ⅱ)oxide CuO6.carbonic acid H2CO3Melting point 熔点boiling point 沸点1.Which particles play the most active role in chemical bonding?(a)electrons (b)neutrons (c)protons (d)valence electrons2.An ionic bond is formed when electrons are:(a)completely destroyed (b)compeltely transferred (c)divied (d)equally shared3.Due to the that Ionic compounds have strong intermolecular forces they are at room temperature.(a)bonded covalently (b)gases (c)liquids(d)solids 1-butene trans -2-butenecis -2-butene iso -butene (E )-2-butene (Z )-butene 2-methylpropene1.Draw structure that correspond to the following names.(a)2,2-dimethylpentane (b)4-isobutyl-2,5-dimethylheptane (c)(Z)-3-menthyl-2-octene (d)(2R,3S)-2,3-pentanediol2.Give the IUPAC name for each of the following structures.(e)(f)(E)-1-methyl-4-ethylcyclohexane(g)(h)(S)-2-chloro-butyraldehyde (2R,3R)-2,3-dichlorobutyric acid补充:(E)-2-chloro-3-methyl-2-octene Nucleophile亲核试剂carbocation碳阳离子Compressible可压缩的incompressible不可压缩的1.A chemical system can be studied from either a or a(n)viewpiont.(A)physical...chenical(B)molecual...atomic (C)Microscopic...macroscopic(D)Mechanic...kinetic2.Is a macroscopic science that studies the interrelationships between the various equilibrium properties of a stystem.(A)Kinetics(B)Thermodynamics (C)Statistical mechanics(D)Quantum chenistry3.In,the molecular and macroscopic levels are related to each other.(A)quantum(B)statistical(C)thermodynamics(D)kinetics4.thermodynamics studies.(A)heat,work,energy,and the changes they produce in the states of systems(B)The relationships between the molecules of a system(C)heat,work,temperature,and the energy they produce in the states of systems(D)heat,energy,and work5.For a(n)system,neither matter nor energy can be transferred between system and surroundings.(A)closed(B)open(C)isolated(D)none of the aboveⅠ.Translate the following from English into Chinese.(1)pollution of the atmosphere(2)nondegradable pollutant大气污染不可降解污染物(3)harmless pollutant(4)interacting chemicals无害污染物相互作用的化学物质(5)threshold level(6)sound pressure level限定值,阈值声压水平(7)speech interference(8)transmission path 语音干扰传输途径Translate the following from Chinese into English.(1)定性分析qualitative analysis (2)分析物analyte (3)准确度accuracy (5)反应速率reaction-rate (5)解吸附作用deserption (6)吸附absorption conduction 热传导convection 对流radiation 辐射Balance and classify each of the following chemical equations as a (1)combination reactions ,(2)decomposition reaction ,(3)displacement reaction ,or (4)partner-exchange reaction.(a))()(2243l O H s Fe H O Fe +→+)(4)(342243l O H s Fe H O Fe +→+displacement reaction 置换反应(b))()()(23g O s KCl s KClO +→)(3)(2)(223g O s KCl s KClO +→decomposition reaction 分解反应(c)steam and hot carbon react to form gasecous hyfrogen and gaseous carbon monoxide.)()()()(22g CO g H s C l O H +→+displacement reaction 置换反应(d))()()(4272aq HClO g O H g O Cl →+)(2)()(4272aq HClO g O H g O Cl →+combination reactions 化合反应(e))()()(22aq HBrO aq HBr O H l Br +→+)()()(22aq HBrO aq HBr O H l Br +→+decomposition reaction 分解反应(f))()()()()(43442243aq PO H s CaSO aq SO H s PO Ca +→+)(2)(3)(3)()(43442243aq PO H s CaSO aq SO H s PO Ca +→+partner-exchange reaction 复分解反应(g)Potassium reacts with water to give aqueous potassium hydroxide and gaseous hydroxide.)()(2)(2)(222g H aq KOH l O H s K +→+displacement reaction 置换反应(h)Solid magnesium carbonate decomposes to form solid magnesium oxide and gaseous carbon monoxide.)()()(23g CO s MgO s MgCO +→decomposition reaction 分解反应Abstract 摘要Results and discussion 结果与讨论Experimental实验References参考文献E-factor影响因素Journal of the American Chemical Society美国化学会志Journal of the Chemical Society化学会志Journal of Organic Chemistry有机化学杂志Tetrahedron四面体'\.._/ ( Wb川ache mical reaction?Acherr山al react i on occurs when subs'孟忘"(tlie reactants) collide (碰撞) with enough energy to rearrange to form different compounds (由e produc时. η1e change in energy由at occurs when a reaction take place is described by thermodynamics (热力学) and the rate or speed at which a reaction occ u rs is described by kfaetics (动力学) . Reactions in which the reactants and produc臼coexist are considered to be in equ山brium (处于平衡). A chemical equation consists of the chemical formula (化学式) of the reactants,且目the chemical formula of the products. The two are separated by an 一一- usually read as ”yielas·’and each chemical formula is separated from others by a plus sign (加号) . Sometimes a triangle is drawn over the arrow symbol to denote energy must be added to the substances for the reaction to begin. Each chemical formula may be preceded by a scalar (数量的) coefficient ind i cating the proportion (比例) of that substance necessary to produce the reaction in formula. For instance, the formula for the burning of methane (C比+ 202 →C02 + 2H20) indicates that twice as much 02 as C比is needed, and when they react, twice as much H20 as C02 will be produced.η1is is because during the reaction, each atom of carbon needs exactly two atoms of oxygen to combine with, to produce the C02, and every two atoms of hydrogen need an atom of oxygen to combine with to produce the H20. If the proportions of t he reactants are not respected, when they are forced to react, either not all of the substanc e used will participate in the react i on, or the react i on that will take p l ace will be different from the one noted in the equation.。
应用化学专业英语考试必背

3、 读 法
高温,高压
• 3.1 Nitrogen reacts with hydrogen to form ammonia at high temperature and pressure with the presence of a catalyst.
• 1 mol nitrogen reacts with 3 mol hydrogen to form 2 mol ammonia at high temperature and pressure with the presence of a catalyst.
pale yellow dark brown
2)state
solid
liquid
gas
gaseous
crystalline molten
oily
uncrystalline fused
3)smell
odourless
pungent
penetrating
choking
offensive
sour sweet bitter
CuO: copper(II) oxide或cupric oxide
2.化合物负电荷部分的读法:
2.1二元化合物 2.2 非金属氢化物 2.3 无氧酸 2.4 含氧酸与含氧酸根阴离子 2.5 盐
4.2.1 二元化合物
常见的二元化合物有卤化物,氧化物,硫化物,氮化物,磷化物,碳化 物,金属氢化物等,命名时需要使用后缀-ide, 如 : fluoride , chloride , bromide , iodide , oxide , sulfide , nitride, phosphide, carbide,hydride; OH -的名称也是用后缀-ide:hydroxide, 非金属氢化物不用此后缀,而是将其看成其它二元化合物(见4.2.2), 非最低价的二元化合物还要加前缀, 如O22-: peroxide O2-: superoxide 举例:NaF: sodium fluoride AlCl3: aluminium chloride Mg2N3 : magnesium nitride Ag2S: silver sulfide CaC2: calcium carbide Fe(OH)2:iron(II) hydroxide 有些物质常用俗称,如NO nitric oxide N2O nitrous oxide
应用化学专业英语词汇.5汇编

Chemical Literature化学文献;primary literature一次文献;research journal 学术期刊;computer databases计算机数据库;Chemical Abstracts 化学文摘;Science Citation Index科学引文索引;keyword index关键词索引;author index作者索引;chemical substance index化学物质索引;formula index分子式索引,patent index 专利索引;general subject index一般主题索引;collective Index累计索引;Ring Index 环索引;Organic Chemistry有机化学;Organometallic chemistry 有机金属化学;Heterocyclic Chemistry杂环化学;Heterocyclic Compound杂环化合物;Comprehensive Organic Synthesis综合有机合成;titration volumes滴定体积;melting points熔点boiling points沸点;noxious material 有害的(有毒的)材料;contamination污染;abrasive 研磨剂;15 percent trisodium phosphate solution 15%的Na3PO4溶液;chromic acid铬酸;chromium trioxide (CrO3);concentrated sulphuric acid浓硫酸;sodium dichromate重铬酸钠;potassium dichromate重铬酸钾;volumetric analysis容量分析;reducing agent还原剂;oxidizing agent氧化剂;concentrated nitric acid浓硝酸;induction period诱导期;ethanol乙醇;industrial spirit 工业酒精acetone丙酮;distillation蒸馏;filter过滤器;corrosive liquids腐蚀性液体,toxic liquids有毒液体;fire extinguisher灭火器;fire blanket灭火毯;Waste Disposal废弃物处理;inflammable materials易燃材料;organic material有机材料;hazardous chemicals危险化学品;toxic chemicals有毒的化学品;water-insoluble organic solvent水不溶有机溶剂;IR spectroscopy 红外光谱distilled water 蒸馏水deionized water 去离子水X-ray diffraction pattern X-射线衍射图谱gas chromatography 气相色谱electron diffraction 电子衍射scanning electron microscope 扫描电子显微镜scanning tunneling microscope 扫描隧道显微镜thermogravimetry 热重法X-ray photo-electron spectroscopy X射线光电子能谱electron probe microanalysis 电子探针显微分析differential thermal analysis 差热分析differential scanning calorimetry 差示扫描量热分析auger electron spectroscopy 俄歇电子谱atomic force microscopy 原子力显微镜transmission electron microscopy 透射电子显微镜field emission microscopy 场发射显微镜atomic emission spectrometry 原子发射光谱atomic absorption spectrometry 原子吸收光谱nuclear magnetic resonance spectrometry 核磁共振波谱ultraviolet & visible absorption spectrum 紫外、可见吸收光谱photochemistry 光化学transition-metal 过渡金属solution 溶液solvent 溶剂solute 溶质anion 阴离子cation 阳离子substituted derivative 取代衍生物excitation 激发emission 发射catalyst 催化剂drug 药hazard 危险品nitrate 硝酸盐nitric acid 硝酸Carbon-iodine bond C-I 键acetone (propanone)丙酮Ethanol 乙醇methanol 甲醇2-propanol 2-丙醇isopropyl alcohol 异丙醇Benzene ring苯环toluene 甲苯Saturated hydrocarbon 饱和碳氢化合物methyl ether 甲醚Methyl ethyl ether 甲乙醚Aldehydes 醛ketone 酮Formaldehyde (methanal)甲醛Acetic acid (ethanoic acid)乙酸Formic acid (methanoic acid)甲酸Salicylic acid 水杨酸Aspirin 阿司匹林Methyl acetate (methyl ethanoate)乙酸甲酯Methane甲烷;ethane乙烷;propane丙烷;butane丁烷;hexane己烷;Ethene 乙烯;propene丙烯;1-butene1-丁烯;Ethyne乙炔;cyclohexane环己烷;Methyl甲基;ethyl乙基;phenyl苯基;Cis-1,2-dimethylcyclohexane 顺式-1,2-二甲基环己烷Trans-1,2-dimethylcyclohexane反式-1,2-二甲基环己烷Organic Compound有机化合物;carbon碳;hydrocarbon碳氢化合物;cyclic structure环状结构;branched hydrocarbon支化的碳氢化合物;Straight-chain Hydrocarbon直连碳氢化合物;Alkyl Group 烷基基团;Cis and Trans Isomer顺式及反式异构体;chiral carbon手性碳oxygen O nitrogen N magnesium Mgiron Fe copper Cu lead Pbsodium Na potassium K Silver Ag;Aluminium Al;Arsenic As;Barium Ba;Bromine Br;Calcium Ca;Chlorine Cl;Cohalt Co; Chromium Cr; Fluorine F;Hydrogen H; Mercury Hg; Iodine I;Magnesium Mg; Manganese Mn;Nickel Ni;Phosphorus P; Palladium Pd; Platinum Pt;Sulfur S; Silicon Si; Tin Sn;Titanium Ti; Uranium U; Zinc ZnTungsten 钨vanadium-iron alloy 钒铁合金Metal Oxide, 金属氧化物;positive ion 正离子negative ion负离子Base碱;acid酸;Salt盐sodium chloride NaCl, aluminium hydroxide Al(OH)3iron ( II ) bromide 【ferrous bromide】FeBr2; calcium acetate Ca(OAc)2; chromium (III) sulphate or chromic sulphate Cr2(SO4)3;Iron (II) oxide (Ferrous oxide) FeO;Iron( III) oxide (Ferric oxide) Fe2O3;Tin(II) hydroxide Sn(OH)2;Tin(IV) hydroxide Sn(OH)4;Mercury(I) sulphate (Mercurous sulphate) Hg2SO4;Mercury(II) sulphate (Mercuric sulphate) HgSO4;Sodium hypochlorite NaClO;Potassium dichromate K2Cr2O7;Carbon monoxide CO; Carbon dioxide CO2;Sulphur trioxide SO3; Dinitrogen trioxide N2O3;Diphosphorus P2O5Acetic acid CH3COOH; hydrobromic acid HBr;perchloric acid HClO4; hydrocyanic acid HCN;phosphoric acid H3PO4;sulphuric acid H2SO4;disodium hydrogen phosphate Na2HPO4;sodium dihydrogen phosphate NaH2PO4 ;sodium bicarbonate NaHCO3; calcium bisulphite Ca(HSO3)2.bismuth dihydroxynitrate Bi(OH)2NO3;sodium potassium sulphate NaKSO4;Calcium carbide Ca2C; Lithium carbonate Li2CO3; Magnesium phosphate Mg3(PO4)2; Molybdenum trioxide MoO3.Thin Layer Chromatography 薄层色谱;methylene chloride二氯甲烷;acetonitrile 乙腈ethyl acetate乙酸乙酯chloroform 氯仿dichloromethane 二氯甲烷diethyl ether 二乙醚toluene甲苯cyclohexane环己烷, petroleum-ether石油醚,hexane己烷, pentane戊烷High Performance Liquid Chromatography (HPLC)高效液相色谱。
应用化学专业外语词汇

Aabscissa[æbˈsɪsə] n. 横座标 abundance n. 丰富, 充裕acceptor n. 接受体accumulator n. 储料器 acetic acid n. 醋酸, 乙酸 acknowledge v. &n. 致谢activation n. 活化acylation ['æsil] n. 酰化addition [əˈdiʃən] n. 加成反应adhesive [ædˈhisɪv, -zɪv] n. 粘合剂advancement n. 进展,增长advantageous adj. 有利的aerosol[ˈeərəˌsɔ:l, -ˌsɔl] n. 烟雾affinity [əˈfɪnɪti:] n. 亲合力agent [ˈeidʒənt] n. 试剂aldehyde [ˈældəˌhaɪd]n. 醛aldol[ˈældəul]n. 醛醇aliphatic acid [ˌæliˈfætik]n. 脂肪酸alkaline[ˈælkəlɪn, -ˌlaɪn] adj. 碱的alkaloid[ˈæl kəlɔid] n. 生物碱alkane[ˈælˌken]n. 烷烃alkene[ˈælki:n]n. 烯烃alkylation [ˌælkiˈleiʃ(ə)n]n. 烃化, 烷基化alkyl halide[ˈælkil][ˈhælaid]n. 烷基卤, 卤烷alkyne n. 炔alphabetic adj. 依字母顺序ambiguity n. 模糊, 意义不明确amide n. 酰胺amine n. 胺amino acid n. 氨基酸amorphous adj. 无定形analogue n. 类似物anhydride n. 酸酐aniline n. 苯胺anion n. 阴离子anomaly n. 异常,反常antibiotics n. 抗菌素antifreezing agent n. 抗冻剂antioxidant n. 抗氧剂appreciable adj. 可估计的architect n. 建筑师, 设计师arene n. 芳烃aromatic adj. 芳香的aromatization n. 芳构化asymmetric adj. 不对称的autooxidation n. 自氧化awarenness n, 意识azeotrope n.共沸混合物azo dye n. 偶氮染料Bbackup n. /adj 备用设备base n. 碱, 基, 底beaker n. 烧杯benzene n. 苯biological degradation n. 生物降解biosynthesis vt. 生物合成bleach vt. 漂白bond n. 键branched chain n. 支链budget n. & v. 预算bubble-cap tower n. 泡罩塔buffer n. 缓冲,缓冲剂Ccarbanion n. 负碳离子, 阴碳离子carbene n. 碳烯, 卡宾carbide n. 碳化物, 碳化钙carbocation n. 正碳离子, 阳碳离子carbonyl group n. 羰基carboxy group n. 羧基carboxylic acid n. 羧酸carcinogenic adj. 致癌的β-carotene n. β胡萝卜素carrier n. 载体cartridge n. 软片暗盒catalysis n. 催化(作用) cation n. 阳离子cellulose n. 纤维素ceramic adj/n. 陶瓷(的) chemical shift n. 化学位移chirality n. 手性chlorination n. 氯化作用chlorohydrocarbon n. 氯代烃chromophore n. 发色团cis-trans isomer n. 顺反异构体classic adj. 经典的, 传统的cluster n. 蔟,一串,一束coherent adj. 黏附的,相干的(光学) coil n. 蛇管colorant n. 颜料,着色剂commodity n. 用品compensation n. 补偿competitive n. 竞争的complementary n. 补充的complex n. 络合物complication n. 复杂concerted reaction n. 协同反应condensation n. 缩合反应condiment n. 调味品conformation n. 构象conjugation n. 共轭construction n. 建设, 建筑consultant n. 顾问consumer n. 消耗container n. 容器containment n. 抑制cooler n. 冷却器corporate adj. 共同的correlate n. 相关的事物cosmetic n. 化妆品counteract vt. 抵消,抵抗coupling reaction n. 偶合反应covalent bond n. 共价键critical adj. 临界的cumulative adj. 累积的,累加的customary adj. 通常的, 常例的cycloparaffin n. 环烷烃Ddecolorant n. 脱色剂decolorize v. 脱色degradation n.降解dehydration n. 脱水作用dehydrogenation n. 脱氢作用delocalization n. 离域作用denatured alcohol n. 变性酒精denominator n. 分母derivation n. 衍生,由来derivative n. 衍生物desorption n. 解吸作用destructive distillation 分解蒸馏detergent n. 洗涤剂developer n. 显影剂dextrorotary adj. 右旋的diazonium salt n. 重氮盐diazotization n. 重氮化作用dielectric adj.不导电的,n.电介质dipole n. 偶极directory n. 地址录disclose vt. 揭露, 揭发discrete adj. 离散的,不连续的disposal vt. 排出, 处理director n. 定位基dissolve v.溶解distillation n. 蒸馏dominant adj. 支配的,统治的donor n. 给体drastic n. 激烈的, 猛烈的droplet n. 液滴dyestuff n. 染料Eelectrophilic reagent n. 亲电试剂electrophobic adj 疏电子的electronegative adj 电负性的electron withdrawing group n. 吸电子基electrostatic adj. 静电的elimination n. 消除反应emulsion n. 乳剂endothermic adj. 吸热的enantiomer n. 对映体enzyme n. 酶epoxy adj. 环氧化的essential oil n. (香)精油ester n. 酯esterification n. 酯化作用ethanol n. 乙醇ether n. 醚, 乙醚ethyl n. 乙基ethylene n. 乙烯ethynyl n. 乙炔基evaluation n. 评价,估价evaporation n. 蒸发excitation n. 激发态exothermic adj. 放热的extract vt. 萃取extrapolation n. 推断Ffermentation n. 发酵fiber n. 纤维filament n. 细丝,丝状体filter n.过滤器,滤色片flare v. & n. 闪耀, 闪烁flavoring n. 香剂, 调味剂fluorescent n. 荧光fore adj. 先时的, 前部的formaldehyde n. 甲醛fossil n. 化石fractional distillation n. 分馏free radical n. 自由基fumigant n. 熏蒸(消毒)剂functional group n. 官能团furan n. 呋喃Ggeneralization n. 一般(性), 普遍(性) genetic code n. 遗传密码geological adj. 地质(学)的geomatrical adj. 几何学的glacial acetic acid n. 冰醋酸glucose n. 葡萄糖glycerol n. 甘油, 丙三醇graphics n. 图,制图法Hhabituation n. 习惯作用, 毒瘾halogenation n. 卤化hazardous adj. 危险的, 有危害的herbicide n.除草剂heterocyclic compound n.杂环化合物heterogeneous adj. 非均相的, 多相的hexagon n. 六边形highlight n. 光线明亮处hold-up n. 塔储量, 容纳量homologous series n. 同系列hormone n. 激素humectant n. 润湿剂hybrid n. 杂化hydration n. 水合作用hydrogenation n. 氢化作用hydrolysis n. 水解hydrophobic adj. 疏水的hydroxyl group n. 羟基Iidealize vt. 理想化inasmuch as adv. 因为, 由于indicator n. 指示剂indiscriminate adj. 不加选择的indol n. 吲哚inductive effect n. 诱导效应ineffective adj. 无效的, 低效率的infrared spectroscopy n. 红外光谱ingenious adj. 坦率的, 天真的ingestion n. 吸收, 吸入inlet n. 进口, 入口insecticide n. 杀虫剂insulin n. 胰岛素integrate vt. 积分,使...一体化interchangeable adj. 可互换的intermediate n. 中间体ion n. 离子isoelectric point n. 等电点isomer n. 异构体Jjacket n. 套, 夹套justification n. 认为正当, 正当的理由Kketone n. 酮Llactic acid n. 乳酸leakage n. 泄漏lesser adj. 较小的, 更少的lime n. 石灰lining n. 衬里, 衬料, 衬套link vt. 连接,键合liquefy vt. 液化lubricating grease n. 润滑脂Mmanipulation n. 操作, 操纵manuscript n. 稿子, 手稿mass spectroscopy n. 质谱mechanism n. 机理, 历程medium n. 介质, 培养基metallurgical adj. 冶金(学)的methane n. 甲烷methnol n. 甲醇methodology n. 方法论micelle n. 胶粒microorganism n. 微生物migrate vi. 迁移miscible adj. 可溶混的modification n. 修饰monomer n. 单体monosaccharide n. 单糖multiplet n. 多重峰multiplicity n 多重性Nnaphthalene n. 萘nitration n. 硝化作用nitric acid n. 硝酸nitrile n. 腈noble adj. 贵重的, 惰性的nomenclacture n. 命名法noteworthy adj. 显著的nucleophile n. 亲核试剂nucleic acid n. 核酸neutralization n. 中和numerator n. (数学上) 分子nutrient n. 营养素, 养分Oobservable a. 可观察到的octane number n. 辛烷值olefin n. 烯烃optical activity n. 旋光性optics n. 光学optimum n. 最佳条件orbital n. 轨道organometallic compound 金属有机化合物originate vi./vt. 起源outermost adj. 最外层的,远离中心的overhead n. 塔顶馏出物overheat vt. 过热overlap vt. 重叠oxidation n. 氧化作用ozonide n. 臭氧化合物ozonolysis n. 臭氧分解Pparaffin n. 链烷烃, 石蜡peptide n. 肽perfume n. 香料peroxide n. 过氧化合物persistence n. 坚持, 固执pesticide n. 杀虫剂pharmaceuticals n. 药物phenol n. 苯酚phenoxide n. (苯)酚盐phenylsulfonic acid n. 苯磺酸phosphoric acid n. 磷酸photochemical reaction n. 光化学反应photochromism n. 光致变色photoconductivity n. 光电导性pigment n. 颜料pink n. 粉红色polyamide n. 聚酰胺polarization n. 极化作用polyhydric alcohol n. 多元醇polymerization n. 聚合作用precipitate vi. /n. 沉淀preservative n. 防腐剂prolong vt. 延长, 拖延propellant n. 推进剂prospective adj. 预期的, 有希望的protecting group n. 保护基purity n. 纯度pyridine n. 吡啶pyrolysis n. 热解pyrrole n. 吡咯Qquantify vt. 使量化,确定数量quaternary ammonium salt n. 季铵盐quench vt. 淬灭quinoline n. 喹啉Rracemization n. 外消旋作用reagent n. 试剂realization n. 实现recover vt. 回收recrystallization n. 重结晶rectifier n. 精馏器reduction n. 还原(作用) reflux n. 回流refract vt. 折射refrigerant n. 冷冻剂remainder n. 剩余物, 残余部分的replica n. 复制品,拷贝resolution n. 分辨, 拆开restrictive adj. 限制性的ribonucleic acid n. 核糖核酸(RNA) rigorous adj. 严厉(格)的Ssaccharin n. 糖精saponification n. 皂化(作用) screen n. 筛子, 屏幕seal n. 密封(垫) segment n. 部分, 链段selectivity n. 选择性settle vt. (使)沉淀, 澄清setup vt. 装置, 装配sewage n. 污水silica gel n. 硅胶singlet n. 单重峰skeleton n. 骨架solubility n. 溶解度solvant n. 溶剂化物solvent n. 溶剂, 有溶解力的sophistication n. 复杂spectroscopy n. 光谱spin-spin coupling n. 自旋-自旋偶合stabilization n. 稳定作用stereoisomerism n. 立体异构现象steric factors n. 位阻因素, 空间因素still pot n.蒸馏釜stoichiometric adj. 化学计算的straightforward adj.一直向前, 正直的substituent n. 取代基substitution reaction n. 取代反应sucrose n. 蔗糖sulfa drug n. 磺胺药sulfonation n.磺化作用sulfuric acid n. 硫酸supervisor n. 导师, 监督人, 主管人suspension n. 悬浮液sweetener n. 增甜剂symmetry n. 对称性symposium n. 座谈会syn addition n. 顺式加成Ttar n. 焦油(沥青)tartaric acid n. 酒石酸tautomerism n. 互变异构现象terpene n. 萜烯tertiary adj. 叔的, 第三的tetrahedron n. 四面体thiazole n. 噻唑thiophene n. 噻吩toluene n. 甲苯toxicity n. 毒性transesterification n. 酯交换反应transition state n. 过渡状态tray n. 盘, 分馏塔盘triplet n. 三重峰trivial adj. 轻微的Uultraviolet-visible spectroscopy n. 紫外-可见光谱unify vt. 统一urea n. 尿素Vvalidate vt. 使生效vaporize vt.蒸发versatile adj. 多方面的vice versa adj. 反之也然vinegar n. 醋violate vt. 破坏,侵害Wwhereas conj. 而, 却, 鉴于withdraw vt. 拉, 提取, 取出withdrawal n. 收回,撤回Xxerography n. 静电复印法Yyeast n. 酵母Zzymochemistry n. 酶化学。
应用化学专业英语

Ensure language is precise, objective, and free of grammar and spelling errors Use appropriate chemical termination
Experimental report writing
Literature reading skills
Analyze the structure of the article
Skim through the title, abstract, introduction, methods, results, and discussion sections to get a general understanding of the article
Identify the main points
Pay attention to the main findings, conclusions, and experimental design to understand the significance of the article
Take notes
Special Considerations
Include raw data, tables, figures, and any deviations from the protocol Follow the institutional reporting guidelines
Summary and Introduction Writing
CHAPTER
Academic paper writing
Purpose
To communicate chemical research findings to other disciplines and professionals
应用化学专业英语(课后答案和课文翻译)

应用化学专业英语(课后答案和课文翻译)Unit 1 The Roots of ChemistryI. Comprehension.1.It can be inferred from this article which one of the following items is not mainly based on practical use C. Greek chemistry2. It was B. Empedocless who first introduced the idea that all things are not formed from just one element.3. In the development of Greek chemistry, D. Democritus was the first one definiting the ultimately constituents of matter?4. According to Plato, there are B. 4 ―elements‖ whose faces are constituted by regular polygons.5. In the last paragraph,authors think that experiment DD.can deal with the reactions by which one substance is converted into anotherII. Make a sentence out of each item by rearranging the words in brackets.1.The purification of an organic compound is usually a matter of considerable difficulty, and itis necessary to employ various methods for this purpose.2.Science is an ever-increasing body of accumulated and systematized knowledge and is also anactivity by which knowledge is generated.3.Life, after all, is only chemistry, in fact, a small example of chemistry observed on a singlemundane planet.4.People are made of molecules; some of the molecules in people are rather simple whereasothers are highly complex.5.Chemistry is ever present in our lives from birth to death because without chemistry there isneither life nor death.6.Mathematics appears to be almost as humankind and also permeates all aspects of human life,although many of us are not fully aware of this.III. Translation.1.(a)化学过程;(b)自然科学;(c)蒸馏技术(a) chemical process (b) natural science (c) the technique of distillation2.正是原子构成铁、水、氧等。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Part 1 Physical Properties 物理性质
1)Colour 〖颜色〗 colourless red-brown violet-black purple-black pale yellow dark brown
oxide and carbon dioxide
•
Calcium carbonate decomposes to calcium oxide
and carbon dioxide when it is heated
Part 3 Chemical Calculation(化学计算 )
2、反应条件
• heat ; burn • ignite/ignition (点燃) • electrolyze/electrolysis(电解) • under/at ambient/room temperature • under standard pressure • with/in the prescence of catalyst
3.3
• Reaction between nitrogen and hydrogen at high temperature and pressure with the presence of a catalyst gives ammonia.
• At high temperature and pressure, reaction of nitrogen with hydrogen in the presence of a catalyst takes place.
Teaching Plan on Specialized English Course for Applied Chemistry
Part 1 Physical Properties Part 2 Chemical Equations Part 3 Chemical Calculation Part 4 Nomenclature Of Inorganic Chemicals Part 5 Some Basic Chemical Theories Part6 Translation
3、 读 法
高温,高压
• 3.1 Nitrogen reacts with hydrogen to form ammonia at high temperature and pressure with the presence of a catalyst.
• 1 mol nitrogen reacts with 3 mol hydrogen to form 2 mol ammonia at high temperature and pressure with the presence of a catalyst.
soluble insoluble slightly soluble very soluble
5)observations
brisk effervescence precipitate milky
aqueous solution
6)density
heavy light less dense denser
水解反应)
• exothermic reaction(放热反应) • endothermic reaction(吸热反应) • reversible reaction(可逆反应) • forward reaction(正向反应) • reverse reaction(逆反应) • spontaneous reaction(自发的反应) • nonspontaneous reaction (非自发反应)
3.2
•
ห้องสมุดไป่ตู้
Nitrogen combines with hydrogen to
form ammonia at high temperature and
pressure with the presence of a catalyst.
• Ammonia decomposes to nitrogen and hydrogen at high temperature and pressure with the presence of a catalyst.
greatly denser slightly denser about the same dense
7)hardness
hard soft
ductile malleable
8)toxicity
toxic poisonous
9)melting point boiling point
High low
10)conductivity
electronic conductivity thermal conductivity conductor semiconductor insulator
Part 2 Chemical Equations
1.反应名称:
化学方程式
• Disproportionation(歧化反应) • neutralization; hydrolysis(中和反应,
2
• Zinc treated with hydrochloric acid forms hydrogen and zinc chloride
3.4
Calcium carbonate when heated produces calcium oxide and carbon dioxide
•
Calcium carbonate is heated to yield calcium
2)state
solid liquid gas gaseous oily crystalline uncrystalline molten fused
3)smell
odourless pungent penetrating choking offensive sour sweet bitter
4)solubility
