BCA试剂盒说明书
BCA蛋白浓度测定试剂盒

BCA蛋白浓度测定试剂盒产品编号产品名称包装价格P0012 BCA蛋白浓度测定试剂盒500次258.00 元O 碧云天生产的BCA蛋白浓度测定试剂盒是根据目前世界上最常用的两种蛋白浓度检测方法之一BCA法研制而成,实现了蛋白浓度测定的简单,高稳定性,高灵敏度和高兼容性。
O 灵敏度高,检测浓度下限达到25微克/毫升,最小检测蛋白量达到0.5微克,待测样品体积为1-20微升。
O 在50-2000微克/毫升浓度范围内有较好的线性关系。
O BCA法测定蛋白浓度不受绝大部分样品中的化学物质的影响,可以兼容样品中高达5%的SDS,5%的Triton X-100,5%的Tween20, 60, 80。
但受螯合剂和略高浓度的还原剂的影响,需确保EDTA低于10mM,无EGTA,二硫苏糖醇低于1mM,β-巯基乙醇低于1mM。
不适用BCA法时建议使用碧云天Bradford蛋白浓度测定试剂盒。
O BCA蛋白浓度测定试剂盒对样品中各种物质详细的兼容性表,参见BCA蛋白浓度测定兼容性表。
O 每个试剂盒可以检测500个样品。
包装清单:BCA试剂A 100 mlBCA试剂B 3 ml蛋白标准(5mg/ml BSA) 1 ml说明书 1 份保存条件:BCA试剂A和B室温保存,蛋白标准请-20℃冻存。
本试剂盒自订购之日起一年注意事项:O 需酶标仪一台,测定波长为540-595nm之间,562nm最佳。
需96孔板。
如果没有酶标仪,也可以使用普通的分光光度计测定,但是测定蛋白浓度时,需根据测定吸光度的杯子的体积,按比例调整A液,B液和样品的体积。
使用分光光度计测定蛋白浓度时,每个试剂盒可以测定的样品数量可能会显著减少。
O 如发现样品稀释液或裂解液本身就有较高背景,请试用Bradford蛋白浓度测定试剂盒。
O 为了加快BCA法测定蛋白浓度的速度可以适当用微波炉加热,但是切勿过热。
O EDTA浓度必需小于10mM,不兼容EGTA。
不适用BCA法时,请试用Bradford蛋白浓度测定试剂盒。
P0012S BCA蛋白浓度测定试剂盒说明书

使用说明:
1. 取0.8ml蛋白标准配制液加入到一管蛋白标准(20mg BSA)中,充分溶解后配制成25mg/ml的蛋白标准溶液。配制后可立即 使用,也可以-20℃长期保存。
2. 取适量25mg/ml蛋白标准,稀释至终浓度为0.5mg/ml。例如取20l 25mg/ml蛋白标准,加入980l稀释液即可配制成 0.5mg/ml蛋白标准。蛋白样品在什么溶液中,标准品也宜用什么溶液稀释。但是为了简便起见,也可以用0.9%NaCl或PBS 稀释标准品。稀释后的0.5mg/ml蛋白标准也可以-20℃长期保存。
15. Zong-Chun Yi, Hong Wang, Guang-Yao Zhang, Bing Xia. Downregulation of connexin 43 in nasopharyngeal carcinoma cells is related to promoter methylation. Oral Oncol. 2007 Feb 14;
注: 也可以室温放置2小时,或60℃放置30分钟。BCA法测定蛋白浓度时,颜色会随着时间的延长不断加深。并且显色反 应会因温度升高而加快。如果浓度较低,适合在较高温度孵育,或适当延长孵育时间。 7. 测定A562,540-595nm之间的波长也可接受。根据标准曲线计算出样品的蛋白浓度。
常见问题:
1. 测定标准曲线时发现随着标准品浓度的增加吸光度或颜色没有明显变化。 可能的原因是样品中含有严重干扰BCA法测定蛋白浓度的物质,详细的BCA法的兼容性列表请参考碧云天如下网页: /Compatibility Chart For BCA Kit.pdf
9. Le-Feng Zhang, Shuang-Qing Peng, Sheng Wang. Influence of lead (Pb2+) on the reactions of in vitro cultured rat aorta to 5-hydroxytryptamine. Toxicology Letters 159 (2005) 71–82.
BCA蛋白定量

BCA蛋白定量试剂盒配制及内部操作SOP一、BCA试剂盒配制流程1、标准品配制:配制2mg/ml的BSA标准品,以50ml为例,各成分加入量如下表:原料浓度加入量(mg)厂家货号牛血清白蛋白(BSA)0.2%100北京(二楼生产部提供)YSCW2500氯化钠(NaCl)0.9%450国药集团化学试剂有限公司10019308叠氮化钠0.05%25成都金山化学试剂有限公司/注意:为了确保检测的准确性,应用微量天平精准称量100mg,然后定容至50ml,1ml/管分装,4℃保存。
2、工作液配制由于A液和B液加入比例为50:1,因此配制工作液时A液各成分加入量按照1000ml配制量加入,B液成分按照25ml配制量加入。
原料浓度加入量(g)厂家货号A液(1000ml)二喹啉甲酸(BCA)1%10aladdin B107658无水碳酸钠2%20天津博迪化工股份有限公司/碳酸氢钠0.95%9.5国药集团化学试剂有限公司10018960酒石酸钠0.16% 1.6国药集团化学试剂有限公司30169818氢氧化钠0.4%4国药集团化学试剂有限公司10019762B液(25ml)硫酸铜4%1国药集团化学试剂有限公司81005261备注:A液各成分混合之后,调节PH至11.25。
二、BCA法蛋白定量操作SOP1、标准品稀释按照下表制备一组蛋白质标准品。
将一安瓿的牛血清白蛋白标准品(BSA)稀释到几个干净的小瓶中,最好使用与待测样品相同的缓冲液。
每一个1mL安瓿的2mg/mL牛血清白蛋白标准品足够用于制备下表中所列出的任意一组稀释范围的标准品;每个稀释浓度的标准品的体积足够用于3次重复检测。
用于标准方案的稀释方法(检测范围=20-2,000ug/mL)如下:标准液编号稀释液体积(ul)标准品体积(ul)标准液终浓度(ug/ml)A03002000B125375ul的A1500C325325ul的A1000D175175ul的B750E325325ul的C500F325325ul的E250G325325ul的F125H400100ul的G25I40000用于试管增强方案的稀释方法如下(检测范围=5–250ug/mL):标准液编号稀释液体积(ul)标准品体积(ul)标准液终浓度(ug/ml)A700100250B400400ul的A125C450300ul的B50D400400ul的C25E400100ul的D5F400002.样品稀释:根据需要,将样品用用PBS或者与待测样品缓冲液相同的溶液稀释至线性范围内(所用稀释液与标准品稀释液应保持一致)。
BCA试剂盒操作说明

INSTRUCTIONSWarranty: Pierce products are warranted to meet stated product specifications and to conform to label descriptions when used and stored properly. Unless otherwise stated, this warranty is limited to one year from date of sale for products used, handled and stored according to Pierce instructions. Pierce’s sole liability for the product is limited toNumberDescription23225BCA™ Protein Assay Kit , sufficient reagents for 500 test tube or 5,000 microplate assays 23227BCA™ Protein Assay Kit , sufficient reagents for 250 test tube or 2,500 microplate assays Kit Contents:BCA™ Reagent A , 1,000 ml (in Product No. 23225) or 500 ml (in Product No. 23227), containing sodium carbonate, sodium bicarbonate, bicinchoninic acid and sodium tartrate in 0.1 M sodium hydroxideBCA™ Reagent B , 25 ml, containing 4% cupric sulfateAlbumin Standard Ampules, 2 mg/ml , 10 x 1 ml ampules, containing bovine serum albumin (BSA)at 2.0 mg/ml in 0.9% saline and 0.05% sodium azideStorage: Upon receipt store at room temperature. Product shipped at ambient temperature.Note: If either Reagent A or Reagent B precipitates upon shipping in cold weather or during long-term storage, dissolve precipitates by gently warming and stirring solution. Discard any kit reagent that shows discoloration or evidence of microbial contamination.Table of ContentsIntroduction..................................................................................................................................................................................1Preparation of Standards and Working Reagent (required for both assay procedures)................................................................2Test Tube Procedure (Sample to WR ratio = 1:20).....................................................................................................................3Microplate Procedure (Sample to WR ratio = 1:8)......................................................................................................................3Troubleshooting...........................................................................................................................................................................4Related Pierce Products...............................................................................................................................................................5Additional Information................................................................................................................................................................5Cited References..........................................................................................................................................................................6Product References. (6)IntroductionThe BCA™ Protein Assay is a detergent-compatible formulation based on bicinchoninic acid (BCA) for the colorimetric detection and quantitation of total protein. This method combines the well-known reduction of Cu +2 to Cu +1 by protein in an alkaline medium (the biuret reaction) with the highly sensitive and selective colorimetric detection of the cuprous cation (Cu +1) using a unique reagent containing bicinchoninic acid.1 The purple-colored reaction product of this assay is formed by the chelation of two molecules of BCA with one cuprous ion. This water-soluble complex exhibits a strong absorbance at 562 nm that is nearly linear with increasing protein concentrations over a broad working range (20-2,000 µg/ml). The BCA™method is not a true end-point method; that is, the final color continues to develop. However, following incubation, the rate of continued color development is sufficiently slow to allow large numbers of samples to be assayed together.The macromolecular structure of protein, the number of peptide bonds and the presence of four particular amino acids (cysteine, cystine, tryptophan and tyrosine) are reported to be responsible for color formation with BCA.2 Studies with di-,tri- and tetrapeptides suggest that the extent of color formation caused by more than the mere sum of individual color-producing functional groups.2 Accordingly, protein concentrations generally are determined and reported with reference to standards of a common protein such as bovine serum albumin (BSA). A series of dilutions of known concentration are3747 N. Meridian Road P.O. Box 117Rockford, IL 61105BCA™ Protein Assay Kitprepared from the protein and assayed alongside the unknown(s) before the concentration of each unknown is determined based on the standard curve. If precise quantitation of an unknown protein is required, it is advisable to select a protein standard that is similar in quality to the unknown; for example, a bovine gamma globulin (BGG) standard (see Related Pierce Products) may be used when assaying immunoglobulin samples.Two assay procedures are presented. Of these, the Test Tube Procedure requires a larger volume (0.1 ml) of protein sample; however, because it uses a sample to working reagent ratio of 1:20 (v/v), the effect of interfering substances is minimized. The Microplate Procedure affords the sample handling ease of a microplate and requires a smaller volume (10-25 µl) of protein sample; however, because the sample to working reagent ratio is 1:8 (v/v), it offers less flexibility in overcoming interfering substance concentrations and obtaining low levels of detection.Preparation of Standards and Working Reagent (required for both assay procedures) A.Preparation of Diluted Albumin (BSA) StandardsUse Table 1 as a guide to prepare a set of protein standards. Dilute the contents of one Albumin Standard (BSA) ampule into several clean vials, preferably using the same diluent as the sample(s). Each 1 ml ampule of 2.0 mg/ml Albumin Standard is sufficient to prepare a set of diluted standards for either working range suggested in Table 1. There will be sufficient volume for three replications of each diluted standard.Table 1. Preparation of Diluted Albumin (BSA) StandardsDilution Scheme for Standard Test Tube Protocol and Microplate Procedure (Working Range = 20–2,000 µg/ml) Vial Volume of Diluent Volume and Source of BSA Final BSA ConcentrationA0300 µl of Stock2,000 µg/mlB125 µl375 µl of Stock1,500 µg/mlC325 µl325 µl of Stock1,000 µg/mlD175 µl175 µl of vial B dilution750 µg/mlE325 µl325 µl of vial C dilution500 µg/mlF325 µl325 µl of vial E dilution250 µg/mlG325 µl325 µl of vial F dilution125 µg/mlH400 µl100 µl of vial G dilution25 µg/mlI400 µl00 µg/ml = Blank Dilution Scheme for Enhanced Test Tube Protocol (Working Range = 5–250 µg/ml)Vial Volume of Diluent Volume and Source of BSA Final BSA ConcentrationA700 µl100 µl of Stock250 µg/mlB400 µl400 µl of vial A dilution125 µg/mlC450 µl300 µl of vial B dilution50 µg/mlD400 µl400 µl of vial C dilution25 µg/mlE400 µl100 µl of vial D dilution 5 µg/mlF400 µl00 µg/ml = BlankB.Preparation of the BCA™ Working Reagent (WR)e the following formula to determine the total volume of WR required:(# standards + # unknowns) x (# replicates) x (volume of WR per sample) = total volume WR required Example: for the Standard Test Tube Protocol with 3 unknowns and 2 replicates of each sample:(9 standards + 3 unknowns) x (2 replicates) x (2 ml) = 48 ml WR requiredNote: 2.0 ml of the WR is required for each sample in the Test Tube Procedure, while only 200 µl of WR reagent is required for each sample in the Microplate Procedure.2.Prepare WR by mixing 50 parts of BCA™ Reagent A with 1 part of BCA™ Reagent B (50:1, Reagent A:B). For theabove example, combine 50 ml of Reagent A with 1 ml of Reagent B.Note: When Reagent B is first added to Reagent A, a turbidity is observed that quickly disappears upon mixing to yield a clear, green WR. Prepare sufficient volume of WR based on the number of samples to be assayed. The WR is stable for several days when stored in a closed container at room temperature (RT).Procedure Summary (Test Tube Procedure, Standard Protocol)Test Tube Procedure (Sample to WR ratio = 1:20)1.Pipette 0.1 ml of each standard and unknown sample replicate into an appropriately labeled test tube.2.Add 2.0 ml of the WR to each tube and mix well.3.Cover and incubate tubes at selected temperature and time:•Standard Protocol:37°C for 30 minutes (working range = 20-2,000 µg/ml)•RT Protocol:RT for 2 hours (working range = 20-2,000 µg/ml)•Enhanced Protocol:60°C for 30 minutes (working range = 5-250 µg/ml)Notes:•Increasing the incubation time or temperature increases the net 562 nm absorbance for each test and decreases both the minimum detection level of the reagent and the working range of the protocol.•Use a water bath to heat tubes for either Standard (37°C incubation) or Enhanced (60°C incubation) Protocol. Usinga forced-air incubator can introduce significant error in color development because of uneven heat transfer.4.Cool all tubes to RT.5.With the spectrophotometer set to 562 nm, zero the instrument on a cuvette filled only with water. Subsequently,measure the absorbance of all the samples within 10 minutes.Note: Because the BCA™ Assay does not reach a true end point, color development will continue even after cooling to RT. However, because the rate of color development is low at RT, no significant error will be introduced if the 562 nm absorbance measurements of all tubes are made within 10 minutes of each other.6.Subtract the average 562 nm absorbance measurement of the Blank standard replicates from the 562 nm absorbancemeasurement of all other individual standard and unknown sample replicates.7.Prepare a standard curve by plotting the average Blank-corrected 562 nm measurement for each BSA standard vs. itsconcentration in µg/ml. Use the standard curve to determine the protein concentration of each unknown sample. Microplate Procedure (Sample to WR ratio = 1:8)1.Pipette 25 µl of each standard or unknown sample replicate into a microplate well (working range = 20-2,000 µg/ml).Note: If sample size is limited, 10 µl of each unknown sample and standard can be used (sample to WR ratio = 1:20).However, the working range of the assay in this case will be limited to 125-2,000 µg/ml.2.Add 200 µl of the WR to each well and mix plate thoroughly on a plate shaker for 30 seconds.3.Cover plate and incubate at 37°C for 30 minutes.4.Cool plate to RT.5.Measure the absorbance at or near 562 nm on a plate reader.Notes:•Wavelengths from 540-590 nm have been used successfully with this method.•Because plate readers use a shorter light path length than cuvette spectrophotometers, the Microplate Procedure requires a greater sample to WR ratio to obtain the same sensitivity as the standard Test Tube Procedure. If higher 562 nm measurements are desired, increase the incubation time to 2 hours.•Increasing the incubation time or ratio of sample volume to WR increases the net 562 nm measurement for each well and lowers both the minimum detection level of the reagent and the working range of the assay. As long as all standards and unknowns are treated identically, such modifications may be useful.6. Subtract the average 562 nm absorbance measurement of the Blank standard replicates from the 562 nm measurementsof all other individual standard and unknown sample replicates.7. Prepare a standard curve by plotting the average Blank-corrected 562 nm measurement for each BSA standard vs. itsconcentration in µg/ml. Use the standard curve to determine the protein concentration of each unknown sample.Note: If using curve-fitting algorithms associated with a microplate reader, a four-parameter (quadratic) or best-fit curve will provide more accurate results than a purely linear fit. If plotting results by hand, a point-to-point curve is preferable to a linear fit to the standard points.TroubleshootingProblemPossible CauseSolutionNo color in any tubesSample contains a copper chelating agentDialyze, desalt, or dilute sampleIncrease copper concentration in working reagent (e.g., use 50:2, Reagent A:B)Remove interfering substances from sample using Product No. 23215Strong acid or alkaline buffer, alters working reagent pHDialyze, desalt, or dilute sample Blank absorbance is OK, but standards and samples show less color than expectedColor measured at the wrong wavelengthMeasure the absorbance at 562 nm Protein concentration is too high Dilute sampleColor of samples appears darker than expectedSample contains lipids or lipoproteinsAdd 2% SDS to the sample to eliminate interference from lipids 3Remove interfering substances from sample using Product No. 23215Buffer contains a reducing agent Buffer contains a thiolAll tubes (including blank) are dark purpleBuffer contains biogenic amines (catecholamines)Dialyze or dilute sampleRemove interfering substances from sample using Product No. 23215Need to measure color at a different wavelengthSpectrophotometer or plate reader does not have 562 nm filterColor may be measure at any wavelength between 540 nm and 590 nm, although the slope of standard curve and overall assay sensitivity will be reducedA. Interfering substancesCertain substances are known to interfere with the BCA™ Assay including those with reducing potential, chelating agents,and strong acids or bases. Because they are known to interfere with protein estimation at even minute concentrations, avoid the following substances as components of the sample buffer:Ascorbic Acid EGTAIron Impure Sucrose Catecholamines Impure Glycerol Lipids Tryptophan Creatinine Hydrogen Peroxide Melibiose Tyrosine CysteineHydrazidesPhenol RedUric AcidOther substances interfere to a lesser extent with protein estimation using the BCA™ Assay, and these have only minor (tolerable) effects below a certain concentration in the original sample. Maximum compatible concentrations for many substances in the Standard Test Tube Protocol are listed in Table 2 (see last page of Instructions). Substances werecompatible at the indicated concentration in the Standard Test Tube Protocol if the error in protein concentration estimation caused by the presence of the substance in the sample was less than or equal to 10%. The substances were tested using WR prepared immediately before each experiment. Blank-corrected 562 nm absorbance measurements (for a 1,000 µg/ml BSA standard + substance) were compared to the net 562 nm measurements of the same standard prepared in 0.9% saline. In the Microplate Procedure, where the sample to WR ratio is 1:8 (v/v), maximum compatible concentrations will be lower.B.Strategies for eliminating or minimizing the effects of interfering substancesThe effects of interfering substances in the BCA™ Protein Assay may be eliminated or overcome by one of several methods.•Remove the interfering substance by dialysis or gel filtration.•Dilute the sample until the substance no longer interferes. This strategy is effective only if the starting protein concentration is sufficient to remain in the working range of the assay upon dilution.•Precipitate the proteins in the sample with acetone or trichloroacetic acid (TCA). The liquid containing the substance that interfered is discarded and the protein pellet is easily solubilized in ultrapure water or directly in the alkaline BCA™WR.4 A protocol for performing this on samples to be assayed with BCA™ Protein Assay Reagent is available at the Pierce web site. Alternatively, Product No. 23215 may be used (see Related Pierce Products).•Increase the amount of copper in the WR (prepare WR as 50:2 or 50:3, Reagent A:B), which may eliminate interference by copper chelating agents.Note: For greatest accuracy, the protein standards must be treated identically to the sample(s).Related Pierce Products23209Albumin Standard Ampules, 2 mg/ml, 10 x 1 ml ampules, containing bovine serum albumin (BSA) at 2.0 mg/ml in 0.9% saline and 0.05% sodium azide23208Pre-Diluted Protein Assay Standards: Bovine Serum Albumin (BSA) Set, 7 x 3.5 ml aliquots in the range of 125-2,000 µg/ml23212Bovine Gamma Globulin Standard, 2 mg/ml, 10 x 1 ml ampules23213Pre-Diluted Protein Assay Standards, Bovine Gamma Globulin Fraction II (BGG) Set, 7 x 3.5 ml aliquots in the range of 125-2,000 µg/ml23221BCA™ Reagent A, 1,000 ml23223BCA™ Reagent A, 250 ml23224BCA™ Reagent B, 25 ml23235Micro BCA TM Protein Assay Kit, working range of 0.5-20 µg/ml23236Coomassie Plus™ Protein Assay Kit, working range of 1-1,500 µg/ml23215Compat-Able TM Protein Assay Preparation Reagent Set, sufficient reagents to pre-treat 500 samples to remove interfering substances before total protein quantitationAdditional InformationA.Please visit the Pierce web site for additional information on this product including the following items:•Frequently Asked Questions•Tech Tip protocol: Eliminate interfering substances from samples for BCA™ Protein Assay•Tech Tip protocol: Shorten BCA™ Protein Assay incubation using a microwave ovenB.Response characteristics for different proteinsEach of the commonly used total protein assay methods exhibits some degree of varying response toward different proteins. These differences relate to amino acid sequence, pI, structure and the presence of certain side chains or prosthetic groups that can dramatically alter the protein’s color response. Most protein assay methods utilize BSA or immunoglobulin (IgG) as the standard against which the concentration of protein in the sample is determined (Figure 1). However, if great accuracy is required, the standard curve should be prepared from a pure sample of the target protein to be measured.Table 3 shows typical BCA™ Protein Assay protein-to-protein variation in color response. All proteins were tested at a concentration of 1,000 µg/ml using the 30-minute/37°C Test Tube Protocol. The average net color response for BSA was normalized to 1.00 and the average net color response of the other proteins is expressed as a ratio to the response of BSA.Table 3. Protein-to-Protein Variation. Absorbance ratios (562 nm) for proteins relative to BSA using the Standard Test Tube Protocol.Ratio = (Avg “test” net Abs.) / (avg. BSA net Abs.)Protein Tested Ratio Albumin, bovine serum 1.00Aldolase, rabbit muscle 0.85α-Chymotrypsinogen, bovine 1.14Cytochrome C, horse heart 0.83Gamma globulin, bovine1.11IgG, bovine 1.21IgG, human 1.09IgG, mouse 1.18IgG, rabbit 1.12IgG, sheep1.17Insulin, bovine pancreas 1.08Myoglobin, horse heart0.74Ovalbumin 0.93Transferrin, human0.89Average ratio1.02Figure 1: Typical color response curves for BSA and BGG using the Standard Test Tube Protocol (37°C/30-minute incubation).Standard Deviation 0.15Coefficient of Variation14.7%C. Alternative Total Protein Assay ReagentsIf interference by a reducing substance or metal-chelating substance contained in the sample cannot be overcome, try the Coomassie Plus™ Protein Assay Kit (Product No. 23236), which is less sensitive to such substances.D. Cleaning and Re-using GlasswareExercise care when re-using glassware. All glassware must be cleaned and given a thorough final rinse with ultrapure water.Cited References1. Smith, P.K., et al. (1985). Measurement of protein using bicinchoninic acid. Anal. Biochem . 150:76-85.2. Wiechelman, K., Braun, R. and Fitzpatrick, J. (1988). Investigation of the bicinchoninic acid protein assay: Identification of the groups responsible for color formation. Anal Biochem . 175:231-7.3. Kessler, R. and Fanestil, D. (1986). Interference by lipids in the determination of protein using bicinchoninic acid. Anal. Biochem . 159:138-42.4.Brown, R., Jarvis, K. and Hyland, K. (1989). Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal. Biochem .180:136-9.Product ReferencesAdilakshami, T. and Laine, R.O. (2002). Ribosomal protein S25 mRNA partners with MTF-1 and La to provide a p53-mediated mechanism for survival ordeath. J. Biol. Chem. 277:4147-51.Fischer, T., et al. (1999). Clathrin-coated vesicles bearing GAIP possess GTPase-activating protein activity in vitro. Proc. Nat. Acad. Sci. 96:6722-7.Prozialeck, W.C., et al. (2002). Chlamydia trachomatis disrupts N-cadherin-dependent cell-cell junctions and sequester β-catenin in human cervicalepithelial cells. Infection and Immunity 70:2605-13.Roberts, K.P., Ensrud, K.M. and Hamilton, D.W. (2002). A comparative analysis of expression and processing of the rat epididymal fluid and sperm-boundforms of proteins D and E. Biology of Reproduction 67:525-33.Triton ® is a registered trademark of Rohm & Haas Co.Brij ®, Tween ® and Span ® are registered trademarks of ICI Americas.Zwittergent ® is a registered trademark of American Hoechst Corporation.The BCA™ Protein Assay is protected by U.S. Patent # 4,839,295©Pierce Biotechnology, Inc., 10/2003. Printed in the USA.Table 2. Compatible Substance Concentrations in the BCA™ Protein Assay (see text for details).Substance CompatibleConcentration Salts/BuffersACES, pH 7.825 mM Ammonium sulfate 1.5 M Asparagine 1 mMBicine, pH 8.420 mMBis-Tris, pH 6.533 mMBorate (50 mM), pH 8.5 (# 28384)undilutedB-PER® Reagent (#78248)undiluted Calcium chloride in TBS, pH 7.210 mMNa-Carbonate/Na-Bicarbonate (0.2 M),pH 9.4 (#28382)undilutedCesium bicarbonate100 mM CHES, pH 9.0100 mMNa-Citrate (0.6 M), Na-Carbonate (0.1M), pH 9.0 (#28388)1:8 dilution*Na-Citrate (0.6 M), MOPS (0.1 M), pH 7.5(#28386)1:8 dilution*Cobalt chloride in TBS, pH 7.20.8 mM EPPS, pH 8.0100 mMFerric chloride in TBS, pH 7.210 mM Glycine•HCl, pH 2.8100 mM Guanidine•HCl 4 MHEPES, pH 7.5100 mM Imidazole, pH 7.050 mMMES, pH 6.1100 mMMES (0.1 M), NaCl (0.9%), pH 4.7 (#28390)undiluted MOPS, pH 7.2100 mM Modified Dulbecco’s PBS, pH 7.4 (#28374)undilutedNickel chloride in TBS, pH 7.210 mMPBS; Phosphate (0.1 M), NaCl (0.15 M),pH 7.2 (#28372)undiluted PIPES, pH 6.8100 mMRIPA lysis buffer; 50 mM Tris, 150 mM NaCl,0.5% DOC, 1% NP-40, 0.1% SDS, pH 8.0undiluted Sodium acetate, pH 4.8200 mM Sodium azide0.2%Sodium bicarbonate100 mM Sodium chloride 1 MSodium citrate, pH 4.8 or pH 6.4200 mM Sodium phosphate100 mM Tricine, pH 8.025 mM Triethanolamine, pH 7.825 mMTris250 mMTBS; Tris (25 mM), NaCl (0.15 M), pH 7.6(#28376)undilutedTris (25 mM), Glycine (192 mM), pH 8.0(#28380)1:3 dilution*Tris (25 mM), Glycine (192 mM), SDS(0.1%), pH 8.3 (#28378)undilutedZinc chloride in TBS, pH 7.210 mM Substance CompatibleConcentration Detergents**Brij®-35 5.0%Brij®-56, Brij®-58 1.0%CHAPS, CHAPSO 5.0% Deoxycholic acid 5.0%Octyl β-glucoside 5.0%Nonidet P-40 (NP-40) 5.0%Octyl β-thioglucopyranoside 5.0%SDS 5.0%Span® 20 1.0%Triton® X-100 5.0%Triton® X-114, X-305, X-405 1.0%Tween®-20, Tween®-60, Tween®-80 5.0% Zwittergent® 3-14 1.0%Chelating agentsEDTA10 mMEGTA--------Sodium citrate200 mM Reducing & Thiol-Containing AgentsN-acetylglucosamine in PBS, pH 7.210 mM Ascorbic acid--------Cysteine--------Dithioerythritol (DTE) 1 mM Dithiothreitol (DTT) 1 mMGlucose10 mM Melibiose--------2-Mercaptoethanol0.01% Potassium thiocyanate 3.0 M Thimerosal0.01%Misc. Reagents & SolventsAcetone10% Acetonitrile10%Aprotinin10 mg/LDMF, DMSO10%DMSO10%Ethanol10%Glycerol (Fresh)10% Hydrazides--------Hydrides (Na2BH4 or NaCNBH3)--------Hydrochloric Acid100 mM Leupeptin10 mg/L Methanol10%Phenol Red--------PMSF 1 mMSodium Hydroxide100 mM Sucrose40%TLCK0.1 mg/LTPCK0.1 mg/LUrea 3 Mo-Vanadate (sodium salt), in PBS, pH 7.2 1 mM* Diluted with ultrapure water; ** Detergents were tested using Pierce high-purity Surfact-Amps™ Products, which have low peroxide content; -- Dashed-line entry indicates that the material is incompatible with the assay.。
BCA蛋白定量试剂盒(Thermo)使用指南

INSTRUCTIONSPierce® BCA Protein Assay Kit23225 Pierce BCA Protein Assay Kit, sufficient reagents for 500 test-tube or 5000 microplate assays 23227 Pierce BCA Protein Assay Kit, sufficient reagents for 250 test-tube or 2500 microplate assays Kit Contents:BCA Reagent A, 1000mL (in Product No. 23225) or 500mL (in Product No. 23227), containingsodium carbonate, sodium bicarbonate, bicinchoninic acid and sodium tartrate in 0.1M sodiumhydroxideBCA Reagent B, 25mL, containing 4% cupric sulfateAlbumin Standard Ampules, 2mg/mL, 10 × 1mL ampules, containing bovine serum albumin (BSA)at 2mg/mL in 0.9% saline and 0.05% sodium azideStorage: Upon receipt store at room temperature. Product shipped at ambient temperature.Note: If either Reagent A or Reagent B precipitates upon shipping in cold weather or during long-termstorage, dissolve precipitates by gently warming and stirring solution. Discard any kit reagent thatshows discoloration or evidence of microbial contamination.Table of ContentsIntroduction (1)Preparation of Standards and Working Reagent (required for both assay procedures) (2)Test Tube Procedure (Sample to WR ratio = 1:20) (3)Microplate Procedure (Sample to WR ratio = 1:8) (3)Troubleshooting (4)Related Thermo Scientific Products (5)Additional Information (5)References (6)IntroductionThe Thermo Scientific Pierce BCA Protein Assay is a detergent-compatible formulation based on bicinchoninic acid (BCA) for the colorimetric detection and quantitation of total protein. This method combines the well-known reduction of Cu+2 to Cu+1 by protein in an alkaline medium (the biuret reaction) with the highly sensitive and selective colorimetric detection of the cuprous cation (Cu+1) using a unique reagent containing bicinchoninic acid.1 The purple-colored reaction product of this assay is formed by the chelation of two molecules of BCA with one cuprous ion. This water-soluble complex exhibits a strong absorbance at 562nm that is nearly linear with increasing protein concentrations over a broad working range (20-2000µg/mL). The BCA method is not a true end-point method; that is, the final color continues to develop. However, following incubation, the rate of continued color development is sufficiently slow to allow large numbers of samples to be assayed together.The macromolecular structure of protein, the number of peptide bonds and the presence of four particular amino acids (cysteine, cystine, tryptophan and tyrosine) are reported to be responsible for color formation with BCA.2 Studies with di-, tri- and tetrapeptides suggest that the extent of color formation caused by more than the mere sum of individual color-producing functional groups.2 Accordingly, protein concentrations generally are determined and reported with reference to standards of a common protein such as bovine serum albumin (BSA). A series of dilutions of known concentration are prepared from the protein and assayed alongside the unknown(s) before the concentration of each unknown is determined based on the standard curve. If precise quantitation of an unknown protein is required, it is advisable to select a proteinstandard that is similar in quality to the unknown; for example, a bovine gamma globulin (BGG) standard (see Related Thermo Scientific Products) may be used when assaying immunoglobulin samples.Two assay procedures are presented. Of these, the Test Tube Procedure requires a larger volume (0.1mL) of protein sample; however, because it uses a sample to working reagent ratio of 1:20 (v/v), the effect of interfering substances is minimized. The Microplate Procedure affords the sample handling ease of a microplate and requires a smaller volume (10-25µL) of protein sample; however, because the sample to working reagent ratio is 1:8 (v/v), it offers less flexibility in overcoming interfering substance concentrations and obtaining low levels of detection.Preparation of Standards and Working Reagent (required for both assay procedures) A.Preparation of Diluted Albumin (BSA) StandardsUse Table 1 as a guide to prepare a set of protein standards. Dilute the contents of one Albumin Standard (BSA) ampule into several clean vials, preferably using the same diluent as the sample(s). Each 1mL ampule of 2mg/mL Albumin Standard is sufficient to prepare a set of diluted standards for either working range suggested in Table 1. There will be sufficient volume for three replications of each diluted standard.Table 1. Preparation of Diluted Albumin (BSA) StandardsVial Volume of Diluent(µL)Volume and Source of BSA(µL)Final BSA Concentration(µg/mL)A 0 300 of Stock 2000B 125 375 of Stock 1500C 325 325 of Stock 1000D 175 175 of vial B dilution 750E 325 325 of vial C dilution 500F 325 325 of vial E dilution 250G 325 325 of vial F dilution 125H 400 100 of vial G dilution 25I 400 0 0 = BlankVial Volume of Diluent(µL)Volume and Source of BSA(µL)Final BSA Concentration(µg/mL)A 700 100 of Stock 250B 400 400 of vial A dilution 125C 450 300 of vial B dilution 50D 400 400 of vial C dilution 25E 400 100 of vial D dilution 5F 400 0 0 = BlankB.Preparation of the BCA Working Reagent (WR)e the following formula to determine the total volume of WR required:(# standards + # unknowns) × (# replicates) × (volume of WR per sample) = total volume WR required Example: for the standard test-tube procedure with 3 unknowns and 2 replicates of each sample:(9 standards + 3 unknowns) × (2 replicates) × (2mL) = 48mL WR requiredNote: 2.0mL of the WR is required for each sample in the test-tube procedure, while only 200 µl of WR reagent is required for each sample in the microplate procedure.2.Prepare WR by mixing 50 parts of BCA Reagent A with 1 part of BCA Reagent B (50:1, Reagent A:B). For the aboveexample, combine 50mL of Reagent A with 1mL of Reagent B.Note: When Reagent B is first added to Reagent A, turbidity is observed that quickly disappears upon mixing to yield a clear, green WR. Prepare sufficient volume of WR based on the number of samples to be assayed. The WR is stable for several days when stored in a closed container at room temperature (RT).Procedure Summary (Test-tube Procedure, Standard Protocol)Test-tube Procedure (Sample to WR ratio = 1:20)1.Pipette 0.1mL of each standard and unknown sample replicate into an appropriately labeled test tube.2.Add 2.0mL of the WR to each tube and mix well.3.Cover and incubate tubes at selected temperature and time:•Standard Protocol: 37°C for 30 minutes (working range = 20-2000µg/mL)•RT Protocol: RT for 2 hours (working range = 20-2000µg/mL)•Enhanced Protocol: 60°C for 30 minutes (working range = 5-250µg/mL)Notes:•Increasing the incubation time or temperature increases the net 562nm absorbance for each test and decreases both the minimum detection level of the reagent and the working range of the protocol.•Use a water bath to heat tubes for either Standard (37°C incubation) or Enhanced (60°C incubation) Protocol. Usinga forced-air incubator can introduce significant error in color development because of uneven heat transfer.4.Cool all tubes to RT.5.With the spectrophotometer set to 562nm, zero the instrument on a cuvette filled only with water. Subsequently, measurethe absorbance of all the samples within 10 minutes.Note: Because the BCA assay does not reach a true end point, color development will continue even after cooling to RT.However, because the rate of color development is low at RT, no significant error will be introduced if the 562nm absorbance measurements of all tubes are made within 10 minutes of each other.6.Subtract the average 562nm absorbance measurement of the Blank standard replicates from the 562nm absorbancemeasurement of all other individual standard and unknown sample replicates.7.Prepare a standard curve by plotting the average Blank-corrected 562nm measurement for each BSA standard vs. itsconcentration in µg/mL. Use the standard curve to determine the protein concentration of each unknown sample. Microplate Procedure (Sample to WR ratio = 1:8)1.Pipette 25µL of each standard or unknown sample replicate into a microplate well (working range = 20-2000µg/mL).Note: If sample size is limited, 10µL of each unknown sample and standard can be used (sample to WR ratio = 1:20).However, the working range of the assay in this case will be limited to 125-2000µg/mL.2.Add 200µL of the WR to each well and mix plate thoroughly on a plate shaker for 30 seconds.3.Cover plate and incubate at 37°C for 30 minutes.4.Cool plate to RT. Measure the absorbance at or near 562nm on a plate reader.Notes:•Wavelengths from 540-590nm have been used successfully with this method.•Because plate readers use a shorter light path length than cuvette spectrophotometers, the Microplate Procedure requires a greater sample to WR ratio to obtain the same sensitivity as the standard Test Tube Procedure. If higher 562nm measurements are desired, increase the incubation time to 2 hours.•Increasing the incubation time or ratio of sample volume to WR increases the net 562nm measurement for each well and lowers both the minimum detection level of the reagent and the working range of the assay. As long as allstandards and unknowns are treated identically, such modifications may be useful.5.Subtract the average 562nm absorbance measurement of the Blank standard replicates from the 562nm measurements ofall other individual standard and unknown sample replicates.6.Prepare a standard curve by plotting the average Blank-corrected 562nm measurement for each BSA standard vs. itsconcentration in µg/mL. Use the standard curve to determine the protein concentration of each unknown sample.Note: If using curve-fitting algorithms associated with a microplate reader, a four-parameter (quadratic) or best-fit curve will provide more accurate results than a purely linear fit. If plotting results by hand, a point-to-point curve is preferable to a linear fit to the standard points.A.Interfering substancesCertain substances are known to interfere with the BCA assay including those with reducing potential, chelating agents, and strong acids or bases. Because they are known to interfere with protein estimation at even minute concentrations, avoid the following substances as components of the sample buffer:Ascorbic Acid EGTA Iron Impure SucroseCatecholamines Impure Glycerol Lipids TryptophanCreatinine Hydrogen Peroxide Melibiose TyrosineCysteine Hydrazides Phenol Red Uric AcidOther substances interfere to a lesser extent with protein estimation using the BCA assay, and these have only minor (tolerable) effects below a certain concentration in the original sample. Maximum compatible concentrations for many substances in the Standard Test Tube Protocol are listed in Table 2 (see last page of Instructions). Substances were compatible at the indicated concentration in the Standard Test Tube Protocol if the error in protein concentration estimation caused by the presence of the substance was less than or equal to 10%. The substances were tested using WR prepared immediately before each experiment. Blank-corrected 562nm absorbance measurements (for a 1000µg/mL BSA standard + substance) were compared to the net 562nm measurements of the same standard prepared in 0.9% saline. Maximum compatible concentrations will be lower In the Microplate Procedure where the sample to WR ratio is 1:8 (v/v). Furthermore, it is possible to have a substance additive affect such that even though a single component is present at a concentration below its listed compatibility, a sample buffer containing a combination of substances could interfere with the assay.B.Strategies for eliminating or minimizing the effects of interfering substancesThe effects of interfering substances in the Pierce BCA Protein Assay may be eliminated or overcome by one of several methods. •Remove the interfering substance by dialysis or gel filtration.•Dilute the sample until the substance no longer interferes. This strategy is effective only if the starting protein concentration is sufficient to remain in the working range of the assay upon dilution.•Precipitate the proteins in the sample with acetone or trichloroacetic acid (TCA). The liquid containing the substance that interfered is discarded and the protein pellet is easily solubilized in ultrapure water or directly in the alkaline BCA WR.4A protocol detailing this procedure is available from our website. Alternatively, Product No. 23215 may be used (seeRelated Pierce Products).•Increase the amount of copper in the WR (prepare WR as 50:2 or 50:3, Reagent A:B), which may eliminate interference by copper-chelating agents.Note: For greatest accuracy, the protein standards must be treated identically to the sample(s).Related Thermo Scientific Products15041 Pierce 96-Well Plates, 100/pkg.15075 Reagent Reservoirs, 200/pkg.15036 Sealing Tape for 96-Well Plates, 100/pkg.23209 Albumin Standard Ampules, 2mg/mL, 10 × 1mL ampules, containing bovine serum albumin (BSA) 23208 Pre-Diluted Protein Assay Standards: Bovine Serum Albumin (BSA) Set, 7 × 3.5mL23212 Bovine Gamma Globulin Standard, 2mg/mL, 10 × 1mL ampules23213 Pre-Diluted Protein Assay Standards, (BGG) Set, 7 × 3.5mL aliquots23235 Pierce Micro BCA Protein Assay Kit, working range of 0.5-20µg/mL23236 Coomassie Plus (Bradford) Assay Kit, working range of 1-1500µg/mL23215 Compat-Able™ Protein Assay Preparation Reagent Set23250Pierce BCA Protein Assay Kit−Reducing Agent CompatibleAdditional InformationA.Please visit our website for additional information including the following items:•Frequently Asked Questions•Tech Tip protocol: Eliminate interfering substances from samples for BCA Protein AssayB.Alternative Total Protein Assay ReagentsIf interference by a reducing substance or metal-chelating substance contained in the sample cannot be overcome, try the Thermo Scientific Coomassie Plus (Bradford) Assay Kit (Product No. 23236), which is less sensitive to such substances.C.Cleaning and Re-using GlasswareExercise care when re-using glassware. All glassware must be cleaned and given a thorough final rinse with ultrapure water.D.Response characteristics for different proteinsEach of the commonly used total protein assay methods exhibits some degree of varying response toward different proteins. These differences relate to amino acid sequence, pI, structure and the presence of certain side chains or prosthetic groups that can dramatically alter the protein’s color response. Most protein assay methods use BSA or immunoglobulin (IgG) as the standard against which the concentration of protein in the sample is determined (Figure 1). However, if great accuracy is required, prepare the standard curve from a pure sample of the target protein.Typical protein-to-protein variation in color response is listed in Table 3. All proteins were tested at 1000µg/mL using the 30-minute/37°C Test Tube Protocol. The average net color response for BSA was normalized to 1.00 and the average net color response of the other proteins is expressed as a ratio to the response of BSA.Figure 1: Typical color response curves for BSA and BGG using the Standard Test Tube Protocol (37°C/30-minute incubation). Table 3. Protein-to-protein variation. Absorbance ratios (562nm) for proteins relative to BSA using Protein Tested Ratio Albumin, bovine serum 1.00 Aldolase, rabbit muscle 0.85 α-Chymotrypsinogen, bovine 1.14 Cytochrome C, horse heart 0.83 Gamma globulin, bovine1.11 IgG, bovine 1.21 IgG, human 1.09 IgG, mouse 1.18 IgG, rabbit 1.12 IgG, sheep1.17 Insulin, bovine pancreas 1.08 Myoglobin, horse heart0.74 Ovalbumin 0.93 Transferrin, human 0.891.02 Standard Deviation 0.15Coefficient of Variation14.7%Cited References1. Smith, P.K., et al. (1985). Measurement of protein using bicinchoninic acid. Anal. Biochem . 150:76-85.2. Wiechelman, K., et al. (1988). Investigation of the bicinchoninic acid protein assay: Identification of the groups responsible for color formation. Anal Biochem . 175:231-7.3. Kessler, R. and Fanestil, D. (1986). Interference by lipids in the determination of protein using bicinchoninic acid. Anal. Biochem . 159:138-42.4.Brown, R., et al. (1989). Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal. Biochem . 180:136-9.Product ReferencesAdilakshami, T. and Laine, R.O. (2002). Ribosomal protein S25 mRNA partners with MTF-1 and La to provide a p53-mediated mechanism for survival ordeath. J. Biol. Chem. 277:4147-51.Fischer, T., et al. (1999). Clathrin-coated vesicles bearing GAIP possess GTPase-activating protein activity in vitro. Proc. Nat. Acad. Sci. 96:6722-7. Prozialeck, W.C., et al. (2002). Chlamydia trachomatis disrupts N-cadherin-dependent cell-cell junctions and sequester β-catenin in human cervicalepithelial cells. Infection and Immunity 70:2605-13.Roberts, K.P., et al. (2002). A comparative analysis of expression and processing of the rat epididymal fluid and sperm-bound forms of proteins D and E.Biology of Reproduction 67:525-33.Triton ® is a registered trademark of Rohm & Haas Co.Brij ®, Tween ® and Span ® are registered trademarks of ICI Americas. Zwittergent ® is a registered trademark of American Hoechst Corporation.This product (“Product”) is warranted to operate or perform substantially in conformance with published Product specifications in effect at the time of sale, as set forth in the Product documentation, specifications and/or accompanying package inserts (“Documentation”) and to be free from defects in material and workmanship. Unless otherwise expressly authorized in writing, Products are supplied for research use only. No claim of suitability for use in applications regulated by FDA is made. The warranty provided herein is valid only when used by properly trained individuals. Unless otherwise stated in the Documentation, this warranty is limited to one year from date of shipment when the Product is subjected to normal, proper and intended usage. This warranty does not extend to anyone other than the original purchaser of the Product (“Buyer”).No other warranties, express or implied, are granted, including without limitation, implied warranties of merchantability, fitness for any particular purpose, or non infringement. Buyer’s exclusive remedy for non-conforming Products during the warranty period is limited to replacement of or refund for the non-conforming Product(s).There is no obligation to replace Products as the result of (i) accident, disaster or event of force majeure, (ii) misuse, fault or negligence of or by Buyer, (iii) use of the Products in a manner for which they were not designed, or (iv) improper storage and handling of the Products.Current product instructions are available at /pierce . For a faxed copy, call 800-874-3723 or contact your local distributor. © 2011 Thermo Fisher Scientific Inc. All rights reserved. Unless otherwise indicated, all trademarks are property of Thermo Fisher Scientific Inc. and its subsidiaries. Printed in the USA.Table 2. Compatible substance concentrations in the BCA Protein Assay (see text for details).§* Diluted with ultrapure water.** Detergents were tested using high-purity Thremo Scientific Surfact-Amps Products, which have low peroxide content.-- Dashed-line entry indicates that the material is incompatible with the assay.§ For a more extensive list of substances, download Tech Tip # 68: Protein Assay Compatibility Table from our website. This Tech Tip includes compatible substances for all of our protein assays and enables easy comparisons.。
BCA试剂盒蛋白质测定仪

BCA试剂盒,Eppdorf蛋白质测定仪实验步骤:1. 配制工作液取5mlBCS-A和10ulBCA-B,配制BCA工作液,充分混匀。
BCA工作液室温24小时内稳定。
2. 稀释标准品:取10ul标准品用PBS稀释至100ul(还有一个对照组用透析液稀释),使最终浓度为0.5mg/ml。
加90ul稀释液。
3. 将稀释的标准品按0,1,2,4,8,12,16,20ul加到EP管中,加PBS(透析液)补足到20ul。
4. 加10ul样品到EP管中,补加PBS(透析液)到20ul。
5. 各孔加入200ulBCA工作液,60度保温箱放置30min。
6. 冷却到室温,在波长562蛋白质测定仪上测量。
蛋白定量试剂盒(BCA法)描述:Bicinchoninic acid (BCA )法是近来广为应用的蛋白定量方法。
其原理与Lowery 法蛋白定量相似,即在碱性环境下蛋白质与Cu2+络合并将Cu2+还原成Cu1+。
BCA 与Cu1+结合形成稳定的紫蓝色复合物,在562 nM处有高的光吸收值并与蛋白质浓度成正比,据此可测定蛋白质浓度。
与Lowery法相比,BCA蛋白测定方法灵敏度高,操作简单,试剂及其形成的颜色复合物稳定性俱佳,并且受干扰物质影响小。
与Bradford 法相比,BCA法的显著优点是不受去垢剂的影响。
组成与储存:(1) BCA Reagent 100 ml,室温保存;(2) Cu Reagent 2.5 ml,室温保存;(3) BSA standard 4 mg/ml 1 ml,-20ºC冻存。
1年内有效。
可进行500次微板(microplate)测定或50次2ml比色杯测定。
描述:Bradford法是最常用的蛋白质快速定量方法。
Coomassie brilliant blue G-250与蛋白质结合使染料的最大吸收峰由465 nm变为595 nm,溶液的颜色由棕色变为兰色,595nm波长下吸光度值与蛋白含量成正比。
BCA蛋白定量试剂盒使用说明书

产品使用说明书BCA 蛋白定量试剂盒试剂盒简介以下货号试剂盒可参照此说明书操作:建议同时参考本说明书英文原文(说明书编号TB380)。
BCA Protein Assay Kit :500 / 2500 rxn 货号71285-3BCA 蛋白定量方法是基于双缩脲反应,即在碱性溶液中蛋白将Cu 2+ 还原成Cu 1+,而根据检测到的单价Cu 离子的浓度可以检测体系中对应蛋白的量。
Bicinchoninic acid 是一种显色剂,可以螯合被还原的铜离子,产生一种在562nm 有强吸收的紫色复合物。
Novagen 的BCA 试剂盒可以用于测定浓度在20-2,000µg/ml 范围内的蛋白的浓度,根据样品量分为标准型和微型两种形式进行测定。
试剂盒提供的组分足够用于500次标准型反应(50µl 蛋白样品加上1ml 反应试剂)或2,500次微型测定(25µl 蛋白样品加上200µl 反应试剂,可以在96孔板中进行高通量定量)。
试剂盒中提供的BSA (牛血清白蛋白,2mg/ml )为用户制作标准浓度曲线提供了便利。
Novagen 的BCA 试剂盒准确度高,兼容性好,能与各种化学试剂和表面活性剂兼容,可以方便地配合默克Novagen 的BugBuster ®,PopCulture ®,CytoBuster™,Reportasol™和Insect PopCulture 抽提蛋白的细胞裂解试剂一起使用。
有些化学成分,例如螯合剂,强酸、强碱、还原剂等,可能会干扰BCA 法采用的还原及螯合定量过程,详细情况请看说明书后附列表。
试剂盒提供的组分500ml BCA 反应液(0.1M NaOH 缓冲的bicinchoninic acid ,碳酸钠,酒石酸钠,碳酸氢钠,pH11.25) 15ml 4% 硫酸铜3×1ml BSA 标准品(2mg/ml )储存室温存放。
BCA蛋白浓度测定试剂盒使用说明书

注意事项
1•在低温条件或长期保存出现沉淀时,可搅拌或37C温育使溶解,如发现细
菌污染则应丢弃
2.样品中若含有EDT
A、EGT
A、DTT硫酸铵、脂类会影响检测结果,请试用Bradford蛋白浓度测定试
剂盒;高浓度的去垢剂也影响实验结果,可用TCA沉淀去除干扰物质。
3.要得到更为精确的蛋白浓度结果,每个蛋白梯度和样品均需做复孔,每次
均应做标准曲线。
4•当试剂A和B混合时可能会有浑浊,但混匀后就会消失,工作液在密闭 情况下可保存1周。
5.需准备37C水浴或温箱、酶标仪或普通分光光度计,测定波长为540-
595nm之间,562nm最佳。酶标仪需与96孔酶标板配套使用。使用分光光度计 测定蛋白浓度时,试剂盒测定的样品数量会因此而减少。使用温箱孵育时,应 注意防止因水粉蒸发影响检测结果。
3•加适当体积样品到96孔板的样品孔中,补加PBS到20微升。
4.各孔加入200微升BCA工作液,37C放置30分钟。
5•冷却到室温,用酶标仪测定A562,根据标准曲线计算出蛋白浓度。BCA蛋白浓度测定试剂盒使用说明书
产品简介
蛋白质定量是蛋白质研究的基础工作之一。博奥森开发的BCA蛋白质检测
试剂(bicinchonic acid是理想的蛋白质定量方法;是当前比Lowry法更优越的专用 于检测总蛋白质含量的产品。该方法以快速灵敏、稳定可靠,对不同种类蛋白 质检测的变异系数非常小而倍受专业人士的青睐。BCA法测定蛋白浓度不受绝
BCA蛋白浓度测定试剂盒

BCA蛋白浓度测定试剂盒产品编号产品名称包装价格P0012 BCA蛋白浓度测定试剂盒500次258.00 元O 碧云天生产的BCA蛋白浓度测定试剂盒是根据目前世界上最常用的两种蛋白浓度检测方法之一BCA法研制而成,实现了蛋白浓度测定的简单,高稳定性,高灵敏度和高兼容性。
O 灵敏度高,检测浓度下限达到25微克/毫升,最小检测蛋白量达到0.5微克,待测样品体积为1-20微升。
O 在50-2000微克/毫升浓度范围内有较好的线性关系。
O BCA法测定蛋白浓度不受绝大部分样品中的化学物质的影响,可以兼容样品中高达5%的SDS,5%的Triton X-100,5%的Tween20, 60, 80。
但受螯合剂和略高浓度的还原剂的影响,需确保EDTA低于10mM,无EGTA,二硫苏糖醇低于1mM,β-巯基乙醇低于1mM。
不适用BCA法时建议使用碧云天Bradford蛋白浓度测定试剂盒。
O BCA蛋白浓度测定试剂盒对样品中各种物质详细的兼容性表,参见BCA蛋白浓度测定兼容性表。
O 每个试剂盒可以检测500个样品。
包装清单:BCA试剂A 100 mlBCA试剂B 3 ml蛋白标准(5mg/ml BSA) 1 ml说明书 1 份保存条件:BCA试剂A和B室温保存,蛋白标准请-20℃冻存。
本试剂盒自订购之日起一年注意事项:O 需酶标仪一台,测定波长为540-595nm之间,562nm最佳。
需96孔板。
如果没有酶标仪,也可以使用普通的分光光度计测定,但是测定蛋白浓度时,需根据测定吸光度的杯子的体积,按比例调整A液,B液和样品的体积。
使用分光光度计测定蛋白浓度时,每个试剂盒可以测定的样品数量可能会显著减少。
O 如发现样品稀释液或裂解液本身就有较高背景,请试用Bradford蛋白浓度测定试剂盒。
O 为了加快BCA法测定蛋白浓度的速度可以适当用微波炉加热,但是切勿过热。
O EDTA浓度必需小于10mM,不兼容EGTA。
不适用BCA法时,请试用Bradford蛋白浓度测定试剂盒。
BCA蛋白定量试剂盒(Thermo)使用指南

BCA蛋白定量试剂盒(Thermo)使用指南BCA蛋白定量试剂盒(Thermo)使用指南1.简介BCA蛋白定量试剂盒是一种用于测定蛋白质浓度的试剂盒。
该试剂盒采用双硫键还原法,可以通过比色法快速、准确地测定样品中的蛋白质浓度。
本使用指南将详细介绍BCA蛋白定量试剂盒的使用方法和相关注意事项。
2.实验前准备2.1 试剂准备根据试剂盒的说明书,准备好所需的试剂物品,包括BCA试剂、标准品、还原缓冲液、洗涤缓冲液等。
2.2 样品准备将需要测定的样品进行处理和提取,并根据实验要求进行稀释。
确保样品无杂质和干扰物,以免影响测定结果。
3.样品处理3.1 样品加标准曲线制备将已知浓度的标准品按照一定比例加入到已处理好的样品中,制备出一系列浓度梯度的样品。
3.2 样品处理步骤按照试剂盒说明书的要求,逐步进行样品处理,包括样品还原、洗涤等步骤。
确保每个步骤的操作正确,以获得准确的测定结果。
4.比色测定4.1 样品吸光度测定使用紫外-可见分光光度计测定各个标准品和待测样品的吸光度值。
记录吸光度值,以备后续计算使用。
4.2 绘制标准曲线将各个标准品的浓度与吸光度值进行统计和计算,绘制出标准曲线。
通过标准曲线,可以计算出待测样品的蛋白质浓度。
5.结果分析根据标准曲线计算出待测样品的蛋白质浓度,并进行结果统计和分析。
6.结论根据实验结果得出结论,并对实验过程中出现的问题进行总结和改进。
附件:1.BCA蛋白定量试剂盒说明书2.实验记录表格法律名词及注释:1.BCA蛋白定量试剂盒:BCA全称为Bicinchoninic Acid,是一种常用于蛋白质浓度测定的试剂盒。
2.双硫键还原法:一种用于将蛋白质中的二硫键还原为巯基的方法,常用于蛋白质结构研究和蛋白质定量实验中。
3.比色法:一种通过物质吸收或散射光线的特性,根据吸光度或透过率的变化来测定物质浓度的方法。
全文结束 \。
BCA法蛋白含量测定试剂盒使用说明

BCA法蛋白含量测定试剂盒使用说明微量法注意:正式测定之前选择2-3个预期差异大的样本做预测定,确保蛋白浓度在20-2000μg/ml内。
货号:BC1720规格:100T/96S产品内容:试剂A:液体×1瓶,4℃保存。
试剂B:液体×1支,4℃保存。
标准品:液体×1支,4℃保存。
产品说明:样品可溶性蛋白质含量常常用于酶活性计算。
此外,可溶性蛋白质含量也用于食品等质量分析。
测定原理:碱性条件下,蛋白质中半胱氨酸、胱氨酸、色氨酸、酪氨酸以及肽键,能将Cu2+还原成Cu+;2分子的BCA与Cu+结合,生成紫色络合物,在540-595nm有吸收峰,562nm 处吸收峰最强。
自备仪器和用品:台式离心机、恒温水浴锅、可见分光光度计/酶标仪、微量石英比色皿/96孔板、移液器和蒸馏水。
工作液配制:临用前请根据拟用工作液体积(样本数×0.2mL),将试剂A和B按照50:1的比例混合,盖紧后充分混匀。
操作步骤:一、样品中可溶性蛋白质提取:1.液体样品:澄清液体样品可以直接测定。
2.组织样品:按照组织质量(g):提取液体积(mL)为1:5~10的比例(建议称取约0.1g组织,加入1mL提取液(自备,根据需要选用酶提取缓冲液或者蒸馏水或者生理盐水),冰浴匀浆,10000rpm,4℃离心10min,取上清,即待测液。
(动物样品常常需要稀释)3.细菌、真菌:按照细胞数量(104个):提取液体积(mL)为500~1000:1的比例(建议500万细胞加入1mL提取液),冰浴超声波破碎细胞(功率300w,超声3秒,间隔7秒,总时间3min);然后10000rpm,4℃,离心10min,取上清置于冰上待测。
二、测定操作:1.可见分光光度计/酶标仪预热30min,调节波长到562nm,蒸馏水调零。
2.工作液置于60℃水浴预热30min。
空白管标准管测定管蒸馏水(μL)4标准品(μL)4待测液(μL)4工作液(μL)200200200混匀后置于60℃保温30min,于微量玻璃比色皿/96孔板,于562nm处测定吸光值A,分别记为A空白管、A标准管、A测定管。
BCA蛋白定量试剂盒使用说明书

产品使用说明书BCA 蛋白定量试剂盒试剂盒简介以下货号试剂盒可参照此说明书操作:建议同时参考本说明书英文原文(说明书编号TB380)。
BCA Protein Assay Kit :500 / 2500 rxn 货号71285-3BCA 蛋白定量方法是基于双缩脲反应,即在碱性溶液中蛋白将Cu 2+ 还原成Cu 1+,而根据检测到的单价Cu 离子的浓度可以检测体系中对应蛋白的量。
Bicinchoninic acid 是一种显色剂,可以螯合被还原的铜离子,产生一种在562nm 有强吸收的紫色复合物。
Novagen 的BCA 试剂盒可以用于测定浓度在20-2,000µg/ml 范围内的蛋白的浓度,根据样品量分为标准型和微型两种形式进行测定。
试剂盒提供的组分足够用于500次标准型反应(50µl 蛋白样品加上1ml 反应试剂)或2,500次微型测定(25µl 蛋白样品加上200µl 反应试剂,可以在96孔板中进行高通量定量)。
试剂盒中提供的BSA (牛血清白蛋白,2mg/ml )为用户制作标准浓度曲线提供了便利。
Novagen 的BCA 试剂盒准确度高,兼容性好,能与各种化学试剂和表面活性剂兼容,可以方便地配合默克Novagen 的BugBuster ®,PopCulture ®,CytoBuster™,Reportasol™和Insect PopCulture 抽提蛋白的细胞裂解试剂一起使用。
有些化学成分,例如螯合剂,强酸、强碱、还原剂等,可能会干扰BCA 法采用的还原及螯合定量过程,详细情况请看说明书后附列表。
试剂盒提供的组分500ml BCA 反应液(0.1M NaOH 缓冲的bicinchoninic acid ,碳酸钠,酒石酸钠,碳酸氢钠,pH11.25) 15ml 4% 硫酸铜3×1ml BSA 标准品(2mg/ml )储存室温存放。
BCA蛋白质定量试剂盒说明书

适用范围:
本检测方法可耐受的干扰物质浓度表:
干扰物质 盐/缓冲液 HEPES (pH7.9) PIPES(pH6.8) NaCl HCl NaOH Sodium citrate TRICINE(pH8.0) Sodium Acetate Guanidine.HCl Tris
BCA Protein Assay Kit
BCA 蛋白质定量试剂盒
目录号:PA115 试剂盒内容:
试剂盒组成
BCA 试剂 A BCA 试剂 B BSA 标准品(2mg/ml)
说明书
PA115-01*
100 ml 3 ml
2× 1 ml 1份
PA115-02**
500 ml 15 ml 10 × 1 ml 1份
产品简介:
BCA 蛋白质定量试剂盒(BCA Protein Assay Kit)是根据目前世界上最常用 的两种蛋白浓度检测方法中的 BCA(bicinchoninic acid)法研制而成,实现了 对蛋白质进行快速、稳定、灵敏的浓度测定。本试剂盒的原理是蛋白质分子中 的肽键结构在碱性环境下能与 Cu2+生成络合物,并将 Cu2+还原成 Cu+,而 BCA 试剂可敏感特异地与 Cu+结合,形成稳定的有颜色的复合物,并在 562nm 处 有最大光吸收值,该复合物颜色深浅与蛋白质浓度成正比,可根据吸收值的大 小来测定蛋白质的含量。
1 小时内即可完成蛋白质定量检测。本试剂盒含有牛血清白蛋白(BSA) 溶液作为蛋白质标准溶液,测定范围为 20~2000 ug/ml。
操作步骤:
1. 标准品的稀释:用与样品相同缓冲体系的稀释剂按下表对 BSA 标准品进
行稀释:
BSA 标准浓度配制表
BCA 法蛋白含量测定试剂盒说明书
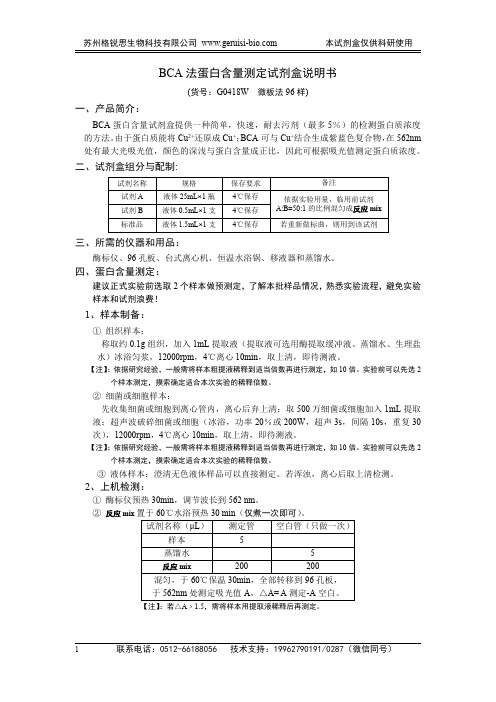
BCA法蛋白含量测定试剂盒说明书(货号:G0418W微板法96样)一、产品简介:BCA蛋白含量试剂盒提供一种简单,快速,耐去污剂(最多5%)的检测蛋白质浓度的方法。
由于蛋白质能将Cu2+还原成Cu+;BCA可与Cu+结合生成紫蓝色复合物,在562nm 处有最大光吸光值,颜色的深浅与蛋白含量成正比,因此可根据吸光值测定蛋白质浓度。
二、试剂盒组分与配制:试剂名称规格保存要求备注试剂A液体25mL×1瓶4℃保存依据实验用量,临用前试剂A:B=50:1的比例混匀成反应mix试剂B液体0.5mL×1支4℃保存标准品液体1.5mL×1支4℃保存若重新做标曲,则用到该试剂三、所需的仪器和用品:酶标仪、96孔板、台式离心机、恒温水浴锅、移液器和蒸馏水。
四、蛋白含量测定:建议正式实验前选取2个样本做预测定,了解本批样品情况,熟悉实验流程,避免实验样本和试剂浪费!1、样本制备:①组织样本:称取约0.1g组织,加入1mL提取液(提取液可选用酶提取缓冲液、蒸馏水、生理盐水)冰浴匀浆,12000rpm,4℃离心10min,取上清,即待测液。
【注】:依据研究经验,一般需将样本粗提液稀释到适当倍数再进行测定,如10倍。
实验前可以先选2个样本测定,摸索确定适合本次实验的稀释倍数。
②细菌或细胞样本:先收集细菌或细胞到离心管内,离心后弃上清;取500万细菌或细胞加入1mL提取液;超声波破碎细菌或细胞(冰浴,功率20%或200W,超声3s,间隔10s,重复30次),12000rpm,4℃离心10min,取上清,即待测液。
【注】:依据研究经验,一般需将样本粗提液稀释到适当倍数再进行测定,如10倍。
实验前可以先选2个样本测定,摸索确定适合本次实验的稀释倍数。
③液体样本:澄清无色液体样品可以直接测定。
若浑浊,离心后取上清检测。
2、上机检测:①酶标仪预热30min,调节波长到562nm。
②反应mix置于60℃水浴预热30min(仅煮一次即可)。
BCA蛋白浓度测定说明书

BCA蛋白浓度测定试剂盒产品编号产品名称包装CHEM001 BCA蛋白浓度测定试剂盒102ml包装清单:产品编号产品名称包装CHEM001A BCA试剂A 100mlCHEM001B BCA试剂B 3mlCHEM001C 蛋白标准(2mg/ml) 1.2ml—说明书1份产品简介:增强型BCA蛋白浓度测定试剂(Enhanced BCA Protein Assay)是常用蛋白浓度测定方法之一。
Viagene的BCA测定试剂测定方法简单,稳定性好,灵敏度高和抗干扰性强。
增强型BCA法测定蛋白浓度不受绝大部分样品中的化学物质影响,可以兼容样品中高达5%的SDS,5%的Triton X-100,5%的Tween 20, 60, 80。
能够耐受低浓度的EDTA、EGTA、二硫苏糖醇,但高浓度螯合剂和还原剂可能会对测定有影响。
增强型BCA蛋白浓度测定试剂适合用于测定Western Blotting样品,EMSA核提取液的蛋白浓度。
检测灵敏度达到10μg/ml。
按照本说明书操作,每套试剂可进行145次比色杯法测定或500次微板法测定。
比色杯法测定的BCA工作液用量较微板法多,但抗干扰性强,结果更准确。
使用说明:A.比色杯法测定1、根据待测定样品数算出所需BCA工作液总体积(每个测定需0.7ml工作液),按50体积BCA试剂A加1体积BCA试剂B(50:1)配制适量BCA工作液,充分混匀。
BCA工作液在室温可稳定24小时。
2、试管编号,按以下操作。
管号S0 S1 S2 S5 S10 S20 S40 样品xH2O (µl)20 19.5 19 17.5 15 10 0 20样品制备液(µl) 4 4 4 4 4 4 4 -蛋白标准液(µl)0 0.5 1 2.5 5 10 20 -样品体积------- 4反应体积24 24 24 24 24 24 24 24 BCA工作液(ml)0.7 0.7 0.7 0.7 0.7 0.7 0.7 0.7蛋白浓度(µg/µl)0 1 2 5 10 20 40 待定注:1)标准曲线一般做4个点加一个空白。
BCA蛋白浓度测定试剂盒完整版.

23235微型BCA蛋白检测试剂盒工作范围为0.5-20 gJ mL
23236考马斯亮蓝检测试剂盒,工作范围的1 - 1500g^mL
23215Compat-Able ?蛋白检测试剂组
23250BCA蛋白检测试剂盒-兼容还原剂
附加信息
A•请访问我们的网站了解更多的信息,包括下列事项:
绍..1
准备标准试剂和工作试
剂..2
准备试
管..3
准备微型
版..3
故障检
修..4
有关美国热电其他产
品..5
附加信
息..5
参考文
献..6
介绍
美国热电(Thermo)公司的BCA蛋白浓度测定试剂盒 是基于二喹啉甲酸(BCA)通过比色检测和定量测定总蛋白的洗涤剂兼容配方。该方法通过碱性介质中的一 种蛋白结合了Cu2使其显著减少转变为Cu1(缩二脲反应)。用一种含二奎琳甲 酸的试剂选择性的比色法高敏感的比色杯中的Cu1.这种测定方法的紫色色反应 产物是通过BCA的两个分子和亚铜离子螯合作用形成的。这种水溶性复合物在562nm处有强吸收峰。在大的活性范围内(20-2000 gMmL)几乎同蛋白浓度增加 呈线性关系。BCA法不是真正的终点的方法;也就是说,最终颜色继续发展。 孵化之后,继续的颜色发展速度是足够慢以允许一起进行测定大量样本。
3)增加培养时间或样品工作试剂的体积比率,增加每个well在562nm的净测量 值,降低实际的最低测量水平和测量的working range。只要每个标准样与未知样 都被同等对待,这样的修改也是有益的。
6、 所有的单个标准品与所测的样品重复在562nm测得的吸光度都要减去在
562nm测得的所有空白标准品的吸光度平均值。
BCA蛋白浓度测定说明书

BCA蛋⽩浓度测定说明书BCA法蛋⽩质浓度测定试剂盒BCA Protein Assay Kit产品编号:SK3021包装规格:500 Assays产品简介BCA法是理想的蛋⽩质定量⽅法,在碱性环境下蛋⽩质分⼦中肽键结构与Cu2+络合并将Cu2+还原成Cu1+。
BCA特异地与Cu1+结合形成稳定的紫蓝⾊复合物,在562 nM处有最⼤的光吸收值并与蛋⽩质浓度成正⽐,颜⾊的深浅与蛋⽩质的含量成正⽐,可以根据吸收值测定蛋⽩质浓度。
该测定⽅法灵敏度⾼,操作简单,试剂及其形成的颜⾊复合物稳定性俱佳,并且受⼲扰物质去垢剂等影响⼩。
产品特点1. 准确灵敏,线性范围⼴,BCA试剂的蛋⽩质测定范围是25-500 µg/ml。
2. 快速:45分钟内完成测定,⽐经典的Lowry法快4倍⽽且更加⽅便。
3. 不受样品中离⼦型和⾮离⼦型去污剂影响。
4. 检测不同蛋⽩质分⼦的变异系数远⼩于考马⽒亮蓝。
5. 可以酶标板法或分光光度计法。
运输和保存条件在常温下运输,收到后,将试剂A、试剂B 4℃保存,BSA蛋⽩标准液-20℃,保质期⼀年。
产品组成本试剂盒由溶液A、溶液B和BSA标准蛋⽩构成,酶标板法可以使⽤500次,分光光度计法100次。
SK3021-1 溶液A 100 mlSK3021-2 溶液B 2 mlBSA标准蛋⽩(5mg/ml) 1 ml使⽤⽅法A. 试剂准备1. 按照以下公式计算所需的BCA⼯作液总体积。
BCA⼯作液总体积=(标准曲线测定点数+样品数)×重复次数×每个样品所需的BCA⼯作液体积2. 根据所需的BCA⼯作液总体积,取50份溶液A和1份溶液B,混匀,制成BCA⼯作液。
3. 取⼀定量的BSA标准溶液,⽤1XPBS溶液稀释为500 µg/ml。
4. 取8⽀1.5 ml离⼼管,按照下表平⾏操作。
B. 分光光度法1. 制作标准曲线(离⼼管法的蛋⽩定量线性范围:25-500µg/ml)1)取8⽀1.5ml离⼼管,各管分别加⼊100µl相应浓度的标准蛋⽩质溶液。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
BCA蛋白浓度测定试剂盒说明书
二. 分光光度计法
如没有酶标仪,可用分光光度计在离心管中混匀后加入比色皿中比色。
步骤如下:
1.配制工作液: 根据标准品和样品数量,按50体积BCA试剂加1体积Cu试剂(50:1)配制成BCA工
作液,充分混匀(混合时可能会有浑浊,但混匀后就会消失)。
BCA工作液室温24小时内稳定。
2.稀释标准品:取100微升BSA标准品用PBS稀释至1ml(样品一般可用PBS稀释),使终浓
度为0.5mg/ml。
3.取八支(或者更多)5ml离心管,标让号,按下表加入试剂。
4.37℃放置15-30分钟。
用分光光度计测562nm处吸光值,根据标准曲线计算出蛋白浓度。
