浙江大学大学物理甲下 chapter 48
2024版大学物理(下)电子工业出版社PPT课件

01大学物理概述与回顾Chapter01掌握物理学基本概念、原理和定律,理解物质的基本结构和基本相互作用。
020304培养科学思维能力和分析解决实际问题的能力。
了解物理学在科学技术发展中的应用和对社会发展的影响。
养成良好的学习习惯和严谨的科学态度。
大学物理课程目标与要求01020304牛顿运动定律、动量守恒定律、能量守恒定律等。
力学热力学第一定律、热力学第二定律、气体动理论等。
热学库仑定律、电场强度、电势差、磁场强度等。
电磁学光的干涉、衍射、偏振等基本概念和原理。
光学上学期知识点回顾01020304振动与波动量子力学基础电磁波的辐射与传播固体物理基础本学期学习内容预览010204学习方法与建议认真听课,做好笔记,及时复习巩固所学知识。
多做习题,加深对物理概念和原理的理解。
积极参加课堂讨论和实验活动,提高分析问题和解决问题的能力。
拓展阅读相关物理书籍和文献,了解物理学前沿动态。
0302电磁学基础Chapter静电场的定义与性质库仑定律电场强度与电势高斯定理静电场及其性质恒定电流与电路分析电流的定义与分类欧姆定律基尔霍夫定律电阻、电容和电感磁场与磁感应强度磁场的定义与性质磁感应强度的定义与计算磁场的高斯定理与安培环路定律磁场对运动电荷的作用力电磁感应定律及应用电磁感应现象与法拉第电磁感应定律描述磁场变化时产生感应电动势的规律。
楞次定律与自感、互感现象描述感应电流的方向以及自感、互感现象中感应电动势的大小和方向。
磁场的能量与磁场力做功描述磁场中储存的能量以及磁场力对电流做功的过程。
电磁感应在日常生活和科技中的应用如交流电的产生、电动机和发电机的原理、电磁炉和微波炉的工作原理等。
03振动与波动Chapter物体在平衡位置附近做周期性的往返运动,称为简谐振动。
简谐振动的定义特征量简谐振动的运动学方程简谐振动的动力学特征振幅、周期(或频率)、相位。
描述简谐振动物体位移随时间变化的规律。
满足F=-kx的回复力特征。
浙江大学《大学物理》课件电磁波

B 0 磁场 D H t
H B
电磁场与电磁波
H E t
E H t
E H E t t t
电磁波除了具有能量,还有动量
w 动量密度为 ,能产生辐射压力 光压 c
分析书上例15.2,重点注意能量是如何进入电容器的!
电磁场与电磁波
电磁波谱
电磁场与电磁波
作业: 15-3 15-5 15-8 15-11 15-14
H y H H x H z i j k t t t t
电磁场与电磁波
电磁波中 E 与 H 方向性关系 H E t
H x Ez Ey 0 y z t H y Ex Ez 0 x t z H z Ey Ex 0 x y t
电磁场与电磁波
有关电场和磁场的规律总结如下:
D dS q0 S 静电场 LE dl 0
B dS 0 S 静磁场 LH dl I
一、位移电流 全电流: 变化的磁场产生涡旋电场 d L Ei dl dt 那么,变化的电场能否产生磁场呢?下面来研究电容器的充 放电过程:
电磁场与电磁波
对S1与S 2的边界L作为积分回路,则安培 环路定理有 对S1 : H dl I 对S 2 : H dl 0
L L
电磁场与电磁波
传导电流在通过电容器时不连续! 但可以发现,两极板的电场是随着传导电流的变化而变化, 而且在数值上与传导电流的大小有重要关系: dq 平行板电容器中D , D D S q,同时电流I , 故有 dt d D dq I dt dt Maxwell将电位移通量的变化看作一种新的等效电流------位 移电流,同时引入全电流的概念,全电流在任何情况下都连 续!
浙江大学大物甲电磁学

S
D dS q0
r 0
D P ' Pn E e 0 E 高斯定理 D E P '
5
四、静电场的能量
1.电容器储能公式
1Q 1 1 2 W QU CU 2 C 2 2
2.求带电体系静电能的三种方法
2
U El l
二、静电场中的导体
1.导体的静电平衡特点
(1).
E内 0
(2).
E表 表面
(3).导体是等势体,表面是等势面 2.导体静电平衡的性质
(1).
q
内
0
(2).
E 0
(3).孤立导体电荷分布与表面曲率有关-----尖端放电现象 3.空腔导体静电平衡的性质 4.静电屏蔽 接地的空腔导体是一个很好的静电屏蔽装置。
3
5.计算有导体存在时,静电场分布的基本依据 (1) 导体静电平衡时的基本性质.
(2) 电荷守恒
(3) 高斯定理 6.电容 电容器 孤立导体的电容: 计算电容的基本步骤: 1.先假设两极板分别带电+q、-q; 2.求两极板间电场强度的分布; 3.求两极板间的电势差; 4.
q C U
q C U A U B
2 E A EB EC
DB DC
DB 0 EB
2 E A EB
DC 0 r EC
EB
EB r EC
r
EA r 1 EB 2 r
14
【例题三】如图所示,一无限大接地导体板的右侧有一无限长 均匀带电直导线垂直与导体板放置,导线的一端距板距离为d, 已知导线上线电荷密度为。求O点处的感应电荷面密度。
浙江大学工学类培养方案

浙江大学Z he jia ngU ni ve r s it y浙江大学Z he ji an g Un iv er si ty浙江大学Z he ji an gUn i2011级工学类培养方案工学类培养方案包括大类培养特色、大类培养面向等内容,课程设置与学分分布主要安排通识课程和大类必修课程的推荐学习计划。
大类课程的专业选修部分和专业课程的推荐学习计划详见各专业培养方案。
专业培养方案包括培养目标、培养要求、专业核心课程、教学特色课程、计划学制、最低毕业学分、学科专业类别、授予学位类别、辅修及双专业/双学位课程修读要求以及课程设置与学分分布等内容。
大类培养特色建有通识、大类、专业三位一体的课程体系,具有基础知识宽厚、交叉和专业知识精深的特点。
通过人文科学、社会科学、自然科学等多学科知识学习,强化工学人才培养的通识性和社会性;通过构建大类课程平台,打好扎实的工程理论基础并有效衔接专业教育;通过专业教育体系的严格训练,培养德、智、体、美全面发展,具有坚实数理基础、富有创新精神、专业知识扎实并具备实践能力,在各工程专业领域具有国际竞争力的高素质本科人才。
大类培养面向学生在入学后两年内确认主修专业,进入专业培养阶段,归属专业所在学院(系)管理。
工学类共有21个专业教育培养通道,能最大限度地满足学生在不同工程专业领域的成才发展需求。
工学类培养主要面向的专业是:1.高分子材料与工程(080204)2.材料科学与工程(080205Y )3.机械设计制造及其自动化(080301)4.过程装备与控制工程(080304)5.机械工程及自动化(080305Y )6.机械电子工程(080307W )7.能源与环境系统工程(080504W )8.新能源科学与工程(080512S )9.电气工程及其自动化(080601) 10.自动化(080602) 11.电子信息工程(080603) 12.土木工程(080703) 13.水资源与海洋工程(080805W ) 14.环境工程(081001) 15.化学工程与工艺(081101) 16.制药工程(081102) 17.海洋工程与技术(081302S ) 18.飞行器设计与工程(081501) 19.工程力学(081701) 20.生物工程(081801)21.工业工程(110103)课程设置与学分分布1.通识课程 47.5+5学分 (1)思政类 11.5+2学分课程号 课程名称 学分 周学时 年级 学期021E0010 思想道德修养与法律基础 2.5 2.0-1.0 一 秋冬 021E0020 中国近现代史纲要 2.5 2.0-1.0 一 秋冬 021E0040 马克思主义基本原理概论 2.5 2.0-1.0 二 秋冬,春夏 031E0031 毛泽东思想和中国特色社会主义理论体系概论 4.0 3.0-2.0 三 秋冬,春夏 02110081 形势与政策 +2.0 2.0-0.0 每学期浙江大学Z he ji a ngU ni ve r s it y浙江大学Z he ji an gU n iv er si ty浙江大学Z he ji an gUni (2)军体类 5.5+3学分体育Ⅰ、Ⅱ、Ⅲ、Ⅳ为必修课程,每门课程1学分,要求在前2年内修读。
浙江大学《大学物理》课件光的衍射1

这是具体的白光单缝夫琅禾费衍射
光的衍射
单缝夫琅禾费衍射图样特征的讨论: ③衍射效应还与缝宽 a、入射光的波长 密切相关。 只有 a~ 才有明显的衍射效应
分析书上P49页例17.1,注意各种物理量单位的统一
【例题】用单色平行光垂直照射到宽度为 a=0.5mm的单缝上, 在缝后放置一个焦距为 f=100cm的透镜,则在焦平面的屏幕 上形成衍射条纹,若在离屏上中央明纹中心距离为1.5mm处 的P点为一亮纹,试求: ①入射光的波长;②P点条纹的级数和该条纹对应的衍射角; ③狭缝处波面可分为几个半波带;④中央明纹的宽度。
②原中央明纹变为3 个小明纹,相当于 插入二条暗纹
光的衍射
2.振幅矢量叠加法:(只须了解其基本原理)
sinu u I A2 sin 2u 2 2 I 0 A0 u A A0
光的衍射
四、光栅衍射:
任何能周期性地分割波阵面的衍射屏------衍射光栅,相邻 两缝(或刻痕)中心间距称为光栅常数-----d
光的衍射
光栅衍射的整个过程是平行光先经各个单缝衍射后,再 进行多光束干涉! 对光栅的每一条缝而言,单缝衍射的结论完全适用,故 光栅的衍射条纹应看作单缝衍射和多光束干涉的综合结果。
光的衍射
多缝衍射的明暗情况:
相邻的两个主 极大之间均有 N 1个极小 N 2个次极大
光的衍射
光的衍射
光栅衍射条纹的明暗条件为: dsin k k 0,1, 2,...主极大 k dsin k 1, 2,..., N 1, N 1,...极小 N
光的衍射
三、单缝夫琅禾费衍射:
原来垂直入射的平行光经过衍射能出射各种角度的平行光, 到达观察屏的光的强度是各个平行衍射光的相干叠加。
大学物理课件PDF版本-2024鲜版

狭义相对论的基 本原理
介绍狭义相对论的两个基本 原理,即相对性原理和光速 不变原理,并解释其物理意 义。
时间膨胀与长度 收缩
阐述狭义相对论中的时间膨 胀和长度收缩效应,以及这 些效应对日常生活的影响。
广义相对论的基 本概念
介绍广义相对论的基本原理, 即等效原理和广义协变原理, 并解释其物理意义。
2024/3/28
2
状态参量
描述系统状态的物理量,如温度、体积、压强等。 对于一定质量的物质,这些参量可以确定系统的 热力学状态。
平衡态与非平衡态
3
在无外界影响的条件下,系统各部分的宏观性质 不随时间变化的状态称为平衡态;否则为非平衡 态。
2024/3/28
13
热力学第一定律及其应用
热力学第一定律
内能
热量可以从一个物体传递到另一个物体,也 可以与机械能或其他能量互相转换,但是在 转换过程中,能量的总值保持不变。
量子力学的基本规律与应用
总结量子力学的基本规律,如态叠加原理、测量假 设和演化方程等,并介绍其在原子分子物理、固体 物理和量子信息等领域的应用。
28
原子核与粒子物理简介
原子核的组成与性质
介绍原子核的组成,包括质子和中 子的性质及其在原子核中的作用, 阐述原子核的稳定性与放射性衰变 等现象。
粒子物理的基本概念与分类
系统内所有微观粒子的动能和势能之和,是 状态参量。
功和热量
应用
在热力学过程中,系统对外界做功或外界对 系统做功,以及系统与外界之间交换的热量。
计算热机效率、制冷系数等,以及解释各种 热力学现象。
2024/3/28
14
热力学第二定律与熵增加原理
热力学第二定律
2024版《大学物理》全套教学课件(共11章完整版)

01课程介绍与教学目标Chapter《大学物理》课程简介0102教学目标与要求教学目标教学要求教材及参考书目教材参考书目《普通物理学教程》(力学、热学、电磁学、光学、近代物理学),高等教育出版社;《费曼物理学讲义》,上海科学技术出版社等。
02力学基础Chapter质点运动学位置矢量与位移运动学方程位置矢量的定义、位移的计算、标量与矢量一维运动学方程、二维运动学方程、三维运动学方程质点的基本概念速度与加速度圆周运动定义、特点、适用条件速度的定义、加速度的定义、速度与加速度的关系圆周运动的描述、角速度、线速度、向心加速度01020304惯性定律、惯性系与非惯性系牛顿第一定律动量定理的推导、质点系的牛顿第二定律牛顿第二定律作用力和反作用力、牛顿第三定律的应用牛顿第三定律万有引力定律的表述、引力常量的测定万有引力定律牛顿运动定律动量定理角动量定理碰撞030201动量定理与角动量定理功和能功的定义及计算动能定理势能机械能守恒定律03热学基础Chapter1 2 3温度的定义和单位热量与内能热力学第零定律温度与热量热力学第一定律的表述功与热量的关系热力学第一定律的应用热力学第二定律的表述01熵的概念02热力学第二定律的应用03熵与熵增原理熵增原理的表述熵与热力学第二定律的关系熵增原理的应用04电磁学基础Chapter静电场电荷与库仑定律电场与电场强度电势与电势差静电场中的导体与电介质01020304电流与电流密度磁场对电流的作用力磁场与磁感应强度磁介质与磁化强度稳恒电流与磁场阐述法拉第电磁感应定律的表达式和应用,分析感应电动势的产生条件和计算方法。
法拉第电磁感应定律楞次定律与自感现象互感与变压器电磁感应的能量守恒与转化解释楞次定律的含义和应用,分析自感现象的产生原因和影响因素。
介绍互感的概念、计算方法以及变压器的工作原理和应用。
分析电磁感应过程中的能量守恒与转化关系,以及焦耳热的计算方法。
电磁感应现象电磁波的产生与传播麦克斯韦方程组电磁波的辐射与散射电磁波谱与光子概念麦克斯韦电磁场理论05光学基础Chapter01光线、光束和波面的概念020304光的直线传播定律光的反射定律和折射定律透镜成像原理及作图方法几何光学基本原理波动光学基础概念01020304干涉现象及其应用薄膜干涉及其应用(如牛顿环、劈尖干涉等)01020304惠更斯-菲涅尔原理单缝衍射和圆孔衍射光栅衍射及其应用X射线衍射及晶体结构分析衍射现象及其应用06量子物理基础Chapter02030401黑体辐射与普朗克量子假设黑体辐射实验与经典物理的矛盾普朗克量子假设的提普朗克公式及其物理意义量子化概念在解决黑体辐射问题中的应用010204光电效应与爱因斯坦光子理论光电效应实验现象与经典理论的矛盾爱因斯坦光子理论的提光电效应方程及其物理意义光子概念在解释光电效应中的应用03康普顿效应及德布罗意波概念康普顿散射实验现象与经德布罗意波概念的提典理论的矛盾测不准关系及量子力学简介测不准关系的提出及其物理量子力学的基本概念与原理意义07相对论基础Chapter狭义相对论基本原理相对性原理光速不变原理质能关系广义相对论简介等效原理在局部区域内,无法区分均匀引力场和加速参照系。
浙江大学电气工程及其自动化专业本科培养方案

浙江大学电气工程及其自动化专业培养方案培养目标培养具有扎实的自然科学基础知识,具有良好的人文社会科学、管理科学基础和外语综合能力,从事电力系统及电气装备的运行与控制、研制开发、自动控制、信息处理、试验分析、以及电力电子技术、机电一体化、经济管理和计算机应用等工作的与国际接轨、并具有知识创新能力的宽口径、复合型高级工程技术人才和管理人才, 具有求是创新精神和国际竞争力的未来领导者。
培养要求本专业学生主要学习电工技术、电子技术、信息控制、计算机等方面的技术基础和专业知识。
本专业主要特点是强弱电结合、电工技术与电子技术结合、软件与硬件结合、元件与系统结合、管理科学与工程技术相结合,学生接受电工、电子、信息、控制及计算机技术方面的基本训练,具备从事电力系统及电气装备的运行、研发及管理的综合能力。
本专业设两个模块课程,学生可任选其一修读。
毕业生应获得以下几个方面的知识和能力:1、具有扎实的数学、物理等自然科学的基础知识,具有较好的人文社会科学、管理科学基础和外语综合能力;2、系统掌握本专业领域必需的技术基础理论知识,主要包括电工理论、电子技术、信息处理、自动控制理论、计算机软硬件基本理论与应用等;3、获得较好的工程实践训练,具有熟练的计算机应用能力;4、具有良好的文献检索与阅读能力,了解本专业学科前沿的发展趋势;5、具备较强的科学研究、科技开发和组织管理能力。
专业核心课程计算方法、工程电磁场与波、信号分析与处理、电机学、控制理论、微机原理与应用、电力电子技术、电器原理与应用模块1:电力系统稳态分析、电力系统暂态分析、发电厂电气系统、高电压技术、继电保护与自动装置、电力技术经济基础、电力信息技术模块2:电气装备CAD技术、电气装备建模与分析、机电运动控制系统、电气装备计算机控制技术、现代驱动技术、自动控制元件教学特色课程双语教学课程:DSP在运动控制系统中的应用、直流输电、电力系统运行于控制、机电一体化技术Matlab与机电系统仿真、现代永磁电机理论与控制、可编程控制器系统原版外文教材课程:机电一体化技术研究型课程:直流输电、直线电机理论与应用、电机计算机控制系统、电力电子技术在电力系统中的应用讨论型课程:自动控制元件计划学制4年毕业最低学分160+4+5授予学位工学学士辅修专业说明辅修专业:30学分,其中必修20.5学分标“**”号的课程,选修9.5学分,在标注“*”号和模块课程中选。
大学物理浙江大学答案

大学物理浙江大学答案【篇一:1992-2016年浙江大学820普通物理考研真题及答案解析汇编】我们是布丁考研网浙大考研团队,是在读学长。
我们亲身经历过浙大考研,录取后把自己当年考研时用过的资料重新整理,从本校的研招办拿到了最新的真题,同时新添加很多高参考价值的内部复习资料,保证资料的真实性,希望能帮助大家成功考入浙大。
此外,我们还提供学长一对一个性化辅导服务,适合二战、在职、基础或本科不好的同学,可在短时间内快速把握重点和考点。
有任何考浙大相关的疑问,也可以咨询我们,学长会提供免费的解答。
更多信息,请关注布丁考研网。
以下为本科目的资料清单(有实物图及预览,货真价实): 2017年浙江大学《普通物理》全套资料包含:一、浙江大学《普通物理》历年考研真题及答案 2016年浙江大学《普通物理》考研真题(含答案解析)2014年浙江大学《普通物理》考研真题 2012年浙江大学《普通物理》考研真题(含答案解析)2011年浙江大学《普通物理》考研真题(含答案解析) 2010年浙江大学《普通物理》考研真题(含答案解析) 2009年浙江大学《普通物理》考研真题(含答案解析) 2008年浙江大学《普通物理》考研真题(含答案解析) 2007年浙江大学《普通物理》考研真题(含答案解析) 2006年浙江大学《普通物理》考研真题(含答案解析)2005年浙江大学《普通物理》考研真题(含答案解析) 2004年浙江大学《普通物理》考研真题(含答案解析)2003年浙江大学《普通物理》考研真题(含答案解析)2002年浙江大学《普通物理》考研真题(含答案解析)2001年浙江大学《普通物理》考研真题(含答案解析)2000年浙江大学《普通物理》考研真题1999年浙江大学《普通物理》考研真题1998年浙江大学《普通物理》考研真题1997年浙江大学《普通物理》考研真题1996年浙江大学《普通物理》考研真题1995年浙江大学《普通物理》考研真题1994年浙江大学《普通物理》考研真题1993年浙江大学《普通物理》考研真题1992年浙江大学《普通物理》考研真题二、浙江大学《大学物理及实验》期中期末试题汇编三、浙江大学《普通物理》复习笔记1、浙江大学《普通物理》考研笔记此笔记是刚考上的2016届研究生在对浙大的普物课本仔细研读和对课后习题以及真题认真分析解答的基础上整理而成的公式定律总结和部分解题技巧。
2024年度2024全新大学物理ppt课件

2024/3/23
03
机械能守恒定律的表达式
在只有重力或系统内弹力做功的物体系统内,动能与势能可以互相转化
,而总的机械能保持不变。
22
05
刚体的转动
Chapter
2024/3/23
23
刚体的基本运动
平动
刚体内任意两点间的连线在运动中始终保持平 行且长度不变。
转动
刚体内任意两点间的连线在运动中不保持平行 。
21
机械能守恒定律
01 02
机械能守恒定律的内容
在只有重力或弹力做功的物体系统内(或者不受其他外力的作用下), 物体系统的动能和势能(包括重力势能和弹性势能)发生相互转化,但 机械能的总能量保持不变。
机械能守恒的条件
只有重力或弹力做功;只受重力作用,其他力不做功;除重力和系统内 弹力外,其他力做功的代数和为零。
热力学第二定律的 数学表达式
对于可逆过程,有dS=(dQ/T) ;对于不可逆过程,有 dS>(dQ/T),其中S为熵,T为 热力学温度。
热力学第二定律的 应用
热力学第二定律揭示了自然界 中宏观过程的方向性,如热传 导、热辐射等过程都是不可逆 的。此外,热力学第二定律还 用于判断热机、制冷机等热力 设备的性能优劣。
加速度与减速度
描述质点速度变化快慢的物理量
2024/3/23
10
质点的曲线运动
2024/3/23
01 曲线运动的描述:位置矢量、位移、速度、加速 度等物理量的矢量性
02 曲线运动的分类:平面曲线运动和空间曲线运动 03 曲线运动的实例分析:圆周运动、抛体运动等
11
相对运动
2024/3/23
参考系与坐标系
1 2
浙江大学大学物理下总复习word精品文档7页

【电磁学】一、电势:1.静电场是保守场、电场力作功与路径无关,仅与起始、终了位置有关0d =⋅⎰Ll E ϖϖ 环流定律移动电荷q 时电场力做的功为 b a b a ab ab W W U U q W A -=-=∆-=)( 2.电势的定义:⎰⋅=零点pp l E U ϖϖd通常取无限远处(或地)为电势零点U =0 电势差(电压) ⎰⋅=-ba b a l E U U ϖϖd电场强度与电势的关系:重点掌握已知U (x,y,z )求电场强度,注意公式中的负号! 3. 电势叠加原理:∑=i U U ,记住下面的常用公式: 在真空中:点电荷的电势分布为rq U 04πε=连续带电体rq U 04d d πε=或⎰=rq U 04d πε均匀带电球面或金属带电球体:⎪⎪⎪⎩⎪⎪⎪⎨⎧>=≤=)( 4 )( 400R r r q U R r R q U πεπε球面内是等势体★ . 求电场中任一点的电势可以用电势叠加的方法,也可以用先求电场强度分布,再从定义来分段积分 ★ . 求解电荷非对称分布电场中的电势时,一定用叠加原理,即⎰=r qU 04d πε★ . 有导体存在时,必须先求感应电荷的分布再求电势分布;求感应电荷时必须以对称中心的电势为参考点。
二. 导体和电介质:要先分清是导体还是电介质,如是导体必须判断是否带电或接地等 (1). 导体:在电场中的导体一定处于静电平衡状态(静电场)计算有导体存在时的电场强度U E ,ϖ分布时★ . 注意导体表面的电荷重新分布,导体接地时是 U =0,两导体相连时是U 1=U 2. ★ . 注意导体附近有点电荷存在时,求感应电荷的方法是以对称中心的电势为参考,叠加各部分电势,通过电势关系求出感应电荷。
(2). 电介质:在电场中电介质处于极化状态,对各向同性的均匀电介质而言,有:电介质中的高斯定理包围的自由电荷)( d ∑⎰=⋅q S D Sϖϖ,灵活使用补偿或叠加原理。
浙江大学建筑学建筑物理历年试题

浙江大学建筑学建筑物理历年试题浙江大学建筑学专业中的建筑物理课程,历来是学生们面临的重要挑战之一。
该课程的试题,旨在检验学生对建筑物理基本理论、应用技能以及相关实际问题的理解和掌握程度。
本文将回顾浙江大学建筑学建筑物理的一些历年经典试题,以期对同学们的学习和备考提供一些参考。
(1)简述建筑物理中“热舒适”的概念及其影响因素。
(2)在建筑光学中,什么是光的反射、折射和漫射?请分别举例说明其在实际中的应用。
(1)一个体育馆的体积为立方米,馆内空气温度为25摄氏度,相对湿度为60%。
假设空气的密度为2千克/立方米,求体育馆内空气的总质量(以千克为单位)。
(1)请结合你所学知识,谈谈你对“绿色建筑”的理解,并列举至少两个具体的绿色建筑案例。
(1)简述建筑传热学中的“热桥”现象及其对建筑能耗的影响。
(2)在建筑声学中,什么是混响时间和吸声系数?请分别举例说明其在实际中的应用。
(1)一栋办公楼的建筑面积为5000平方米,楼层高度为5米。
若该办公楼共需安装50盏功率为30瓦的LED灯,求这些灯的总功率和总电流(以安为单位)。
(1)请结合你所学知识,谈谈你对“可持续城市设计”的理解,并列举至少两个具体的可持续城市设计案例。
(1)简述建筑光学中的“视觉舒适”概念及其影响因素。
(2)在建筑热工学中,什么是热阻和热容?请分别举例说明其在实际中的应用。
(1)一栋住宅楼的南向阳台长度为6米,阳台的透明玻璃窗面积为6平方米。
若阳台内的空气温度为25摄氏度,室外空气温度为-5摄氏度,求通过阳台的热量传递速率(以瓦特为单位)。
(1)请结合你所学知识,谈谈你对“智能建筑”的理解,并列举至少两个具体的智能建筑案例。
物理实验是物理学的基础之一,也是科学研究的重要手段和方法。
在浙江大学的教学体系中,物理实验被视为培养学生科学素养和创新能力的重要环节。
通过物理实验,学生可以深入理解物理概念和规律,掌握实验技能和方法,提高观察、分析和解决问题的能力。
浙江大学大学物理答案

浙江大学大学物理答案【篇一:11-12-2大学物理乙期末试题b】《大学物理乙(上)》课程期末考试试卷 (b)开课分院:基础部,考试形式:闭卷,允许带非存储计算器入场考试日期:2012年月日,考试所需时间: 120 分钟考生姓名学号考生所在分院:专业班级: .一、填空题(每空2分,共50分):1、一个0.1kg的质点做简谐振动,运动方程为x(t)?0.2cos3t m,则该质点的最大加速度amax,质点受到的合力随时间变化的方程f(t。
2、一质点作简谐振动,振幅为a,初始时具有振动能量2.4j。
当质点运动到a/2处时,质点的总能量为 j,其中动能为j。
3、在宁静的池水边,你用手指以2hz的频率轻叩池面,在池面上荡起水波,波速为2m/s,则这些波的波长为 m。
4、两列波在空间相遇时能够产生干涉现象的三个条件为:,振动方向相同,初相位差恒定。
5、如图所示,在均匀介质中,相干波源a和b相距3m,它们所发出的简谐波在ab连线上的振幅均为0.4m,波长均为2m,且a为波峰时b恰好为波谷,那么ab连线中点的振幅为 m,在ba延长线上,a点外侧任一点的振幅为m。
6、已知空气中的声速340m/s,一辆汽车以40m/s的速度驶近一静止的观察者,汽车喇叭的固有频率为555hz,则观察者听到喇叭的音调会更________(填“高”或“低”),其频率为____________ hz。
(请保留三位有效数字)......7、已知800k时某气体分子的方均根速率为500m/s,当该气体降温至200k时,其方均根速率为__________m/s。
8、体积为2?10?3m3的理想气体,气体分子总数为5.4?1022个,其温度为362k,则气体的压强为_________________pa。
9、麦克斯韦速率分布曲线下的面积恒等于_________。
10、一定量氢气在500k的温度下,分子的平均平动动能为______________________j,分子的平均转动动能为________________________j。
浙江大学大学物理甲下chapter

r2
r1
[l(n
1)
2
]
7
2
r2
r1
d D
x
x
D d
(r2
r1)
D d
7
2
2.5mm
r1
x
r2
Example 2: There is an oil film (n2=1.20) on flat grass plate (n1=1.52). A light ray (λ=600nm) illuminates normally and is observed from above by reflected light. Find the thickness of the film at 5th bright fringe from the edge of oil film.
(1) SS2 S2O l nl (SS1 S1O)
(2m 1) , m 3
SS2
2 SS1 l(n 1)
7
2
D
l(n 1) 7 l 4μm
22
(2) SS2 r2 l nl (SS1 r1) 0
d sin m, 2400 sin 30 m 400 m 3
d 3, a 800nm a
Example 9: E43-11
Solution: If the second order spectra overlaps the third order, it is because the 700nm second order line is at a larger angle than the 400nm third order line.
浙江大学大物甲电磁学
dl'
o dl ' '
a
结果
B
o j
2bΒιβλιοθήκη 在无限大均匀平面电流的两侧的磁场都 为均匀磁场,并且大小相等,但方向相反。
j1 j2
B内 0
B外 0 j
B内 0 j
B外 0
B内 B外
0
0
2
2
( j1 j2 ) ( j1 j2 )
12-5 磁场对运动电荷的作用 一、洛仑兹力 实验表明:电量为 q 的带电粒子,以v 在磁场B中运动
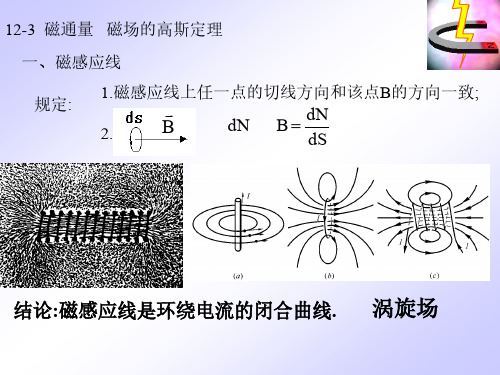
12-3 磁通量 磁场的高斯定理 一、磁感应线 规定: 1.磁感应线上任一点的切线方向和该点B的方向一致; 2.
B
dN
dN B dS
结论:磁感应线是环绕电流的闭合曲线.
涡旋场
二、磁通量 定义:通过任一面积的磁感应线的条数的代数和称 为通过这一面积的磁通量。
d m B dS BcosdS m B dS
VAA' 0 q 0
实验上称 k 为霍耳系数,与材料有关。
*霍耳效应的经典解释
以载流子是负电荷为例,其定向 漂移速度为u与电流反向。
I A
B F q
+
电场力与洛仑兹力平衡时,电子的 漂移达到动态平衡,从而在AA’方向 上形成一恒定电场—— 霍耳电场
h
A' b
横向电势差
qEH quB
L
' dB
r R B dl 2rB 0 L B 0
无限长圆柱面电流外面的磁场与电流 都集中在轴上的直线电流的磁场相同
o I B 2r
'' dB
大学物理ppt课件完整版

THANKS
感谢观看
恒定电流的电场和磁场
恒定电流的产生与性质
由恒定电场产生的电流称为恒定 电流,其大小和方向均不随时间 变化。
01
02
恒定电流的磁场
03
恒定电流周围会产生恒定磁场, 其方向由右手螺旋定则确定。
04
恒定电流的电场
恒定电场是一种无旋场,可以用 电势来描述。
磁感应强度与磁通量
描述恒定磁场的两个重要物理量, 磁感应强度反映磁场力的性质, 磁通量反映磁场在空间中的分布。
匀速直线运动、匀变速直线运动;
曲线运动
抛体运动、圆周运动;
相对运动
参考系的选择、相对速度、相对 加速度。
牛顿运动定律
牛顿第一定律
惯性定律,定义了力和运动的关系;
牛顿第三定律
作用力和反作用力,大小相等、方向 相反。
牛顿第二定律
F=ma,阐述了力、质量和加速度之 间的关系;
动量守恒定律
动量的定义和计算
固体和液体的热性质
固体的热性质
固体具有一定的形状和体积,其 热膨胀系数较小,热传导性能较
好。
液体的热性质
液体没有确定的形状,但有一定的 体积,其热膨胀系数较大,热传导 性能较差。
相变现象
物质从一种相转变为另一种相的过 程,如熔化、凝固、汽化、液化等, 相变过程中伴随着热量的吸收或释 放。
04
电磁学
机械波的产生和传播
机械波的产生
机械波是由振源产生的,振源做周期性振动时,会使周围的介 质产生相应的振动,从而形成机械波。
机械波的传播
机械波在介质中以波的形式传播,传播方向与介质中质点的振 动方向垂直。在传播过程中,机械波会携带能量和信息。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
K : L K K : M K
L shell transit to K shell, leaves a hole in L shell, and fills by further outer electron.
K, L, M…are labeled the principle shells of electrons.
result of experiment
Chapter 48 Atomic Structure
The Continuous X-ray Spectrum (bremsstrahlung) Continuous spectrum is due to the loss of kinetic energy of incident electrons. The kinetic energy transfers to radiation, photons. The minimum λmin corresponds to the loss of all incident energy . It is independent of materials.
1) An energetic electron strikes a target atom and knocks out one of its deep-seated electrons and left a hole.
Chapter 48 Atomic Structure
2) One of the outer electrons moves in to fill this hole and, in the process, the atom emits a characteristic x-ray photon.
18 27 52
Ar39.95 , Co 58.93 , T e127.60 ,
19 28
K
39.10
Ni 58.70 I 126.90
53
Chapter 48 Atomic Structure
In 1913 The British physicist H. G. J. Moseley first introduced the concept of atomic number (symbol Z) and showed that these difficulties were removed if the periodic table was organized in order of increasing Z. In experiments Moseley found that for a given x-ray spectrum line such as Kα, the square root of its frequency against the position of the associated element in the periodic table, a straight line resulted.
min
hcБайду номын сангаас eV
, min hc Ek
Sample problem 48-1
Ek hfmax hc
min
the way of measuring h
Chapter 48 Atomic Structure
The Characteristic X-ray Spectrum Two peaks, together with other peaks, depend on the materials. The reason is:
Chapter 48 Atomic Structure
An electron in outside shell can transit to deep levels in many electron atom.
+Ze
Chapter 48 Atomic Structure
An apparatus of experiment
Chapter 48 Atomic Structure
Chapter 48 48 Atomic Structure Chapter
Chapter 48 Atomic Structure
48-1 The X-Ray Spectrum of Atoms X rays are produced when rapidly moving electrons that have been accelerated through a potential difference of the order of 103 to 105V strike a metal target. The binding energy in the innermost shell is higher than that in outside shell. For example, outside 5eV, innermost 70keV. So the electrons may strike the target with enough energy to knock electrons out of the inner shells of the target atoms.
Chapter 48 Atomic Structure
48-2 X Rays and the Numbering of the Elements From the time of Mendeleev (1834-1907) the elements were listed in the periodic table in the order of increasing atomic weight. However, one difficulty still exists, that is, in several cases, the strict order of increasing atomic weights had to be reversed to maintain the similarity of chemical properties in the vertical columns of the table. For example:
