美国药典 USP36微生物限度检查
美国药典微生物限度检测

61微生物限度检测(MICROBIAL LIMIT TESTS)此章提供方法来检测可能存在的好氧微生物其他制药过程中可能出现的微生物的数量,包括原材料和成品中的。
如果经过验证确认可以得到相同或更好的检测结论,也允许采用自动化的检测方法。
在样品检测过程中须进行无菌操作。
若无特别说明,则“培养(incubate)”一词指在30—35℃的培养箱内培养24至48小时;“生长(growth)”一词用于专门的判定,说明“存在和可能存在活的微生物”。
准备实验 (Preparatory Testing)本章涉及实验结果的有效性取决于:提供的被检测样品本身在实验条件下,被充分证明不会抑制可能存在的微生物的生长。
因此,在准备样品时,需要正规的实验操作和符合要求的实验条件,接种稀释样品到含有以下(微生物)培养物的培养基:金黄色(奥里斯)葡萄球菌(Staphylococcus aureus),大肠埃希氏菌(Escherichia coli), 铜绿假单胞菌(Pseudomonas aeruginosa), 和沙门氏菌(Salmonella)。
方法如下:将用肉汤培养基培养24小时后的(微生物)不小于10-3稀释的微生物培养物,加1 ml(微生物)培养液到磷酸(盐)缓冲液(pH 7.2),液体大豆酪蛋白消化物培养基(Fluid Soybean-Casein Digest Medium),或者液体乳糖培养基(Fluid Lactose Medium)。
相应培养基培养失败则需要采取以下方法更改检测程序:(1)增加稀释液体积,检测样品加入量仍维持不变;或者(2)中和一定数量的干扰因子;或者(3)结合(1)、(2)得出适当条件,使接种物得以生长。
以下是一些物质的成分和浓度,该物质及浓度可用于加入培养基、阻止物质发挥抑菌作用:大豆卵磷脂(soy lecithin, 0.5%)或者聚山梨醇酯20(polysorbate 20, 4.0%)。
奥氮平片美国药典36版质量标准USP36

4562Olanzapine / Official Monographs USP 36C U= concentration of Olanzapine in the Sample C U= concentration of Olanzapine in the Samplesolution (mg/mL)solution (mg/mL)Acceptance criteria:98.0%–102.0% on the anhydrous,F= relative response factor for each impurity from solvent-free basis Table 2Acceptance criteria:See Table 2.IMPURITIES•R ESIDUE ON I GNITION〈281〉:NMT 0.1%Table 2•H EAVY M ETALS, Method II〈231〉:NMT 10ppmRelative Relative AcceptanceRetention Response Criteria, Change to read:Name Time Factor NMT (%)Olanzapine related•O RGANIC I MPURITIES compound Ba0.3 2.30.10 Buffer:Dissolve 13g of sodium dodecyl sulfate inOlanzapine related1500mL of water. Add 5mL of phosphoric acid, andcompound A b0.8v2.3v USP360.10 adjust with a sodium hydroxide solution to a pH of 2.5.Olanzapine 1.0——Solution A:Acetonitrile and Buffer (48:52)Solution B:Acetonitrile and Buffer (70:30)•ChloromethylMobile phase:See Table 1.olanzapiniumchloride c (if pres-0.15•(RB 1-Jun-ent) 1.1 1.02012)Table 1Any individual, un-——Time Solution A Solution B specified impurity0.10(min)(%)(%)Total impurities——0.4 01000a2-Methyl-10H-thieno-[2,3-b][1,5]benzodiazepin-4[5H]-one.101000b5-Methyl-2-((2-nitrophenyl)amino)-3-thiophenecarbonitrile.200100•c1-Chloromethyl-1-methyl-4-(2-methyl-10H-benzo[b]thieno[2,3-e][1,2501004]diazepin-4-yl)piperazin-1-ium chloride.•(RB 1-Jun-2012)271000SPECIFIC TESTS351000•W ATER D ETERMINATION, Method I〈921〉[N OTE—A suitable solvent system for water determina-Edetate disodium solution:37mg/L of edetate diso-tion in ketones and aldehydes (e.g., Hydranal compos-dium in Buffer ite 5K-working medium K or Aquastar composite 5K-Diluent:Acetonitrile and Edetate disodium solution solvent KC or equivalent) is recommended.](40:60)Acceptance criteria:NMT 1.0%System suitability solution:20µg/mL of USPOlanzapine RS and 2µg/mL each of USP Olanzapine ADDITIONAL REQUIREMENTSRelated Compound A RS and USP Olanzapine Related•P ACKAGING AND S TORAGE:Preserve in well-closed contain-Compound B RS in Diluent ers, and store at room temperature.Standard solution:2µg/mL of USP Olanzapine RS in•USP R EFERENCE S TANDARDS〈11〉Diluent USP Olanzapine RSSample solution:0.4mg/mL of Olanzapine in Diluent USP Olanzapine Related Compound A RSChromatographic system5-Methyl-2-((2-nitrophenyl)amino)-(See Chromatography 〈621〉, System Suitability.)3-thiophenecarbonitrile.Mode:LC C12H9N3O2S259.28Detector:UV 220 nm USP Olanzapine Related Compound B RSColumn:4.6-mm × 25-cm; 5-µm packing L72-Methyl-10H-thieno-[2,3-b][1,5]benzodiazepin-4[5H]-Temperatures one.Column:35°C12H10N2OS230.29Sample:5°Flow rate:1.5mL/minInjection volume:20µLSystem suitabilitySample:System suitability solution Olanzapine Tablets[N OTE—Identify the peaks using the Relative RetentionTime values given in Table 2.]DEFINITIONSuitability requirements Olanzapine Tablets contain NLT 90.0% and NMT 110.0% Resolution:NLT 3.0 between olanzapine related com-of the labeled amount of olanzapine (C17H20N4S).pound A and olanzapineTailing factor:NMT 1.5 for the olanzapine peak IDENTIFICATIONRelative standard deviation:NMT 2.0% from fourreplicate injections for the olanzapine peakChange to read:AnalysisSamples:Standard solution and Sample solution•I NFRARED A BSORPTION〈197S〉Calculate the percentage of each impurity in the portionStandard solution:v30v USP36 mg/mL of USP Olanzapine of Olanzapine taken:RS in chloroformResult = (r U/r S) × (C S/C U) × (1/F) × 100Sample solution:Dissolve a quantity of powdered Tab-lets, equivalent to 30mg of olanzapine, in 30mL of r U= peak response of each impurity from the chloroform, and filter. Evaporate completely to dryness Sample solution with the aid of a current of air. Redissolve the residue in r S= peak response of olanzapine from the1mL of chloroform.Standard solutionC S= concentration of USP Olanzapine RS in theStandard solution (mg/mL)USP 36Official Monographs / Olanzapine4563ASSAY Standard solution:An amount, in mg, corresponding tothe Tablet label claim, of USP Olanzapine RS in1000mL of Medium. Transfer 5.0mL of this solution to Change to read: a tube, and add 2.0mL of Mobile phase.Sample solution:Pass a portion of the solution under •P ROCEDURE test through a suitable filter of 0.45-µm pore size.•[N OTE—A few drops of acetonitrile, not to exceed 5%Transfer 5.0mL of the filtrate to a tube, and add 2.0mL of the final volume, may be added to the Standard of Mobile phase.solution and Sample solution before final dilution to Chromatographic systemreduce foaming.]•(RB 1-Jul-2012)(See Chromatography 〈621〉, System Suitability.) Buffer 1:6.9g/L of monobasic sodium phosphate. Ad-Mode:LCjust with phosphoric acid to a pH of 2.5.Detector:UV 260 nmBuffer 2:12g/L of sodium dodecyl sulfate in Buffer 1Column:4.6-mm × 15-cm; 5-µm packing L10Mobile phase:Acetonitrile and Buffer 2 (1:1)Flow rate:1.5mL/minSystem suitability solution:0.1mg/mL of USP Injection volume:50µLOlanzapine RS and 0.01mg/mL of USP Olanzapine Re-System suitabilitylated Compound A RS in Mobile phase Sample:Standard solutionStandard solution:0.1mg/mL of USP Olanzapine RS in Suitability requirementsMobile phase Relative standard deviation:NMT 2.0%Sample solution:Transfer a known quantity of Tablets Analysis•(NLT 5)•(RB 1-Jul-2012), equivalent to NLT 25mg of Calculate the percentage of olanzapine (C17H20N4S) olanzapine, to a suitable volumetric flask. Dilute with dissolved:Mobile phase to volume, mix, and sonicate for 10 min.Centrifuge a portion of this solution, and dilute the Result = (rU/r S) ×C S× (V/L) × 100 clear supernatant with Mobile phase to obtain a solutioncontaining about 0.1mg/mL of olanzapine. [N OTE—Agi-r U= peak response from the Sample solutiontation of the flask may be necessary before sonication r S= peak response from the Standard solution to prevent Tablets from adhering to the flask, making C S= concentration of USP Olanzapine RS in the disintegration and dissolution difficult.]Standard solution (mg/mL)Chromatographic system V= volume of Medium, 900mL(See Chromatography 〈621〉, System Suitability.)L= label claim (mg/Tablet)Mode:LC Tolerances:NLT 80% (Q) of the labeled amount of Detector:UV 260 nm olanzapine (C17H20N4S) is dissolved.Column:4.6-mm × 15-cm; 5-µm packing L7•U NIFORMITY OF D OSAGE U NITS〈905〉:Meet theFlow rate:1.5mL/min requirementsInjection volume:20µLIMPURITIESSystem suitabilitySamples:System suitability solution and Standardsolution Change to read:[N OTE—The relative retention times for olanzapine re-lated compound A and olanzapine are 0.89 and 1.0,•O RGANIC I MPURITIESrespectively.]•[N OTE—A few drops of acetonitrile, not to exceed 5% Suitability requirements of the final volume, may be added to the Standard Resolution:NLT 2.0 between olanzapine and solution and Sample solution before final dilution to olanzapine related compound A, System suitability reduce foaming.]•(RB 1-Jul-2012)solution Buffer 1:3.3mL/L of phosphoric acid. Adjust with 50% Tailing factor:NMT 1.8, Standard solution sodium hydroxide to a pH of 2.5.Relative standard deviation:NMT 2.0%, Standard Buffer 2:8.7g/L of sodium dodecyl sulfate in Buffer 1 solution Buffer 3:18.6mg/L of edetate disodium (EDTA) in Analysis Buffer 2Samples:Standard solution and Sample solution Solution A:Acetonitrile and Buffer 2 (12:13)Calculate the percentage of olanzapine (C17H20N4S) in Solution B:Acetonitrile and Buffer 2 (7:3)the portion of Tablets taken:Diluent:Acetonitrile and Buffer 3 (2:3)System suitability solution:20µg/mL of USP Result = (r U/r S) × (C S/C U) × 100Olanzapine RS, and 2µg/mL each of USP OlanzapineRelated Compound B RS and USP Olanzapine Related r U= peak response from the Sample solutionCompound C RS in Diluentr S= peak response from the Standard solutionStandard solution:2µg/mL of USP Olanzapine RS inC S= concentration of USP Olanzapine RS in theDiluentStandard solution (mg/mL)Sensitivity solution:0.4µg/mL of USP Olanzapine RS inC U= concentration of olanzapine in the SampleDiluent from the Standard solutionsolution (mg/mL)Sample solution:Transfer a known quantity of Tablets Acceptance criteria:90.0%–110.0%to a suitable volumetric flask, and dilute with Diluent to PERFORMANCE TESTS volume to obtain a solution containing either 375 or •D ISSOLUTION〈711〉500µg/mL of olanzapine (based on the label claim).Medium:0.1 N hydrochloric acid; 900mL Centrifuge a portion of this solution, and use the super-Apparatus 2:50 rpm natant. [N OTE—Immediate agitation of the flask may be Time:30 min necessary to prevent Tablets from adhering to the flask, Mobile phase:10g/L of ammonium acetate in a mix-making dissolution and disintegration difficult. [C AU-ture of methanol and water (2:3). Adjust with hydro-TION—Do not sonicate.] The Sample solution is stable for chloric acid to a pH of 4.0.12 h at room temperature and 48 h if refrigerated.]4564Olanzapine / Official MonographsUSP 36Mobile phase:See Table 1.Table 2 (Continued)Relative Relative Acceptance Table 1Retention Response Criteria,NameTimeFactorNMT (%)Time Solution ASolution BAny individual un-(min)(%)(%)specified impuri-—01000ty1.00.20101000Total impurities—— 1.5200100a (Z )-4-(4-Methylpiperazin-1-yl)-3-(2-oxopropylidene)-1H -benzo[b ][1,2501004]diazepin-2(3H )-one.271000b 2-Methyl-10H -thieno-[2,3-b ][1,5] benzodiazepin-4[5H ]-one.c (Z )-1-{4-(4-Methylpiperazin-1-yl)-2-thioxo-1H -benzo[b ][1,4]diazepin-351003(2H )-ylidene}propan-2-one.d 2-Methyl-4-(4-methylpiperazin-1-yl)-10H -benzo[b ]thieno[2,3-e ][1,Chromatographic system4]diazepine 4’-N -oxide.(See Chromatography 〈621〉, System Suitability .)Mode:LCADDITIONAL REQUIREMENTSDetector:UV 220 nm•P ACKAGING AND S TORAGE : Preserve in tight, light-resistant Column:4.6-mm × 25-cm; 5-µm packing L7containers, and store at controlled room temperature.Column temperature:35°•USP R EFERENCE S TANDARDS 〈11〉Flow rate:1.5mL/min USP Olanzapine RSInjection volume:20µL USP Olanzapine Related Compound A RS System suitability5-Methyl-2-((2-nitrophenyl)amino)-Samples:System suitability solution , Standard solution ,3-thiophenecarbonitrile.and Sensitivity solution C 12H 9N 3O 2SSuitability requirementsUSP Olanzapine Related Compound B RSResolution:NLT 3.0 between olanzapine and2-Methyl-10H -thieno-[2,3-b ][1,5]benzodiazepin-4[5H ]-olanzapine related compound C, System suitability one.solutionC 12H 10N 2OSTailing factor:NMT 1.5 for the olanzapine peak, Sys-USP Olanzapine Related Compound C RStem suitability solution2-Methyl-4-(4-methylpiperazin-1-yl)-10H -benzo[b ]thieno Relative standard deviation:NMT 2.0%, Standard [2,3-e ][1,4]diazepine 4′-N -oxide.solutionC 17H 20N 4OS 328.43Signal-to-noise ratio:NLT 10, Sensitivity solution AnalysisSamples:Standard solution and Sample solutionCalculate the percentage of each impurity in the portion of Tablets taken:Olanzapine and Fluoxetine CapsulesResult = (r U /r S ) × (C S /C U ) × (1/F ) × 100DEFINITIONOlanzapine and Fluoxetine Capsules contain an amount of r U= peak response of each impurity from theolanzapine and fluoxetine hydrochloride equivalent to Sample solutionNLT 90.0% and NMT 110.0% each of the labeled r S = peak response from the Standard solution amount of olanzapine (C 17H 20N 4S) and fluoxetine C S = concentration of USP Olanzapine RS in the(C 17H 18F 3NO).Standard solution (µg/mL)C U = concentration of olanzapine in the SampleIDENTIFICATIONsolution (µg/mL)•The retention times of the major peaks in the Sample solu-F = relative response factor for each impurity listedtion correspond to those in the Standard solution , as ob-in Table 2tained in the Assay.Acceptance criteria:See Table 2.ASSAY•P ROCEDURETable 2Buffer:37mg/L of disodium ethylenediaminetetraace-Relative Relative Acceptance tate in water. Add 3.3mL of phosphoric acid, and ad-Retention Response Criteria,just with 50% sodium hydroxide to a pH of 2.5. Dis-Name TimeFactorNMT (%)solve 8.7g of sodium dodecyl sulfate in the resulting Olanzapine solution.lactam a0.26 1.00.50Mobile phase:Acetonitrile and Buffer (1:1)Olanzapine relat-•0.50•(RB 1-Standard solution:0.12mg/mL of USP Olanzapine RS ed compound B b 0.30 2.3Jul-2012)and 0.45mg/mL of USP Fluoxetine Hydrochloride RS in Olanzapine thio-Mobile phaselactam c0.34 1.00.50Sample solution:0.06–0.18mg/mL of olanzapine and 0.25-0.5mg/mL of fluoxetine in Mobile phase from a Olanzapine relat-counted number of Capsules ed compound C d 0.83v0.71v USP360.50Chromatographic systemOlanzapine1.0——(See Chromatography 〈621〉, System Suitability .)a (Z )-4-(4-Methylpiperazin-1-yl)-3-(2-oxopropylidene)-1H -benzo[b ][1,Mode:LC4]diazepin-2(3H )-one.Detector:UV 227 nmb 2-Methyl-10H -thieno-[2,3-b ][1,5] benzodiazepin-4[5H ]-one.Column:4.6-mm × 7.5-cm; 3.5-µm packing L7c (Z )-1-{4-(4-Methylpiperazin-1-yl)-2-thioxo-1H -benzo[b ][1,4]diazepin-Column temperature:40°3(2H )-ylidene}propan-2-one.d 2-Methyl-4-(4-methylpiperazin-1-yl)-10H -benzo[b ]thieno[2,3-e ][1,4]diazepine 4’-N -oxide.。
微生物限度USP36

58〈55〉 Biological Indicators—Resistance Performance Tests / Microbiological TestsUSP 36It is preferable to use the same method as that defined by ENUMERATION METHODSthe biological indicator manufacturer to determine D values.The use of a different method can result in differences that Use the Membrane Filtration method or one of the Plate-are more an artifact of the method than a variation in the Count Methods , as directed. The Most-Probable-Number performance of the biological indicator.(MPN) Method is generally the least accurate method for microbial counts; however, for certain product groups with very low bioburden, it may be the most appropriate Survival Time and Kill Timemethod.The choice of a method is based on factors such as the Take two groups, each consisting of 10 specimens of the nature of the product and the required limit of microorgan-relevant biological indicator, in their original, individual con-isms. The method chosen must allow testing of a sufficient tainers. Place the specimens of a group in suitable specimen sample size to judge compliance with the specification. The holders that permit each specimen to be exposed to the suitability of the chosen method must be established.sterilizing conditions at a specific location in the BIER cham-ber.Expose the specimens for the required survival time, enter GROWTH PROMOTION TEST, SUITABILITY the chamber, and remove the holder(s) containing the 10OF THE COUNTING METHOD AND NEGATIVEspecimens. Repeat the above procedure immediately, or CONTROLSpreheat if a substantial interval has elapsed, so as to subject the second holder(s) containing 10 specimens similarly to the first conditions, but for the required kill time.The Survival time and kill time for all monographed biolog-General Considerationsical indicators is described in the official monograph under the heading for each.The ability of the test to detect microorganisms in the presence of product to be tested must be established.Suitability must be confirmed if a change in testing per-formance or a change in the product that may affect the outcome of the test, is introduced.Preparation of Test Strains〈61〉 MICROBIOLOGICAL Use standardized stable suspensions of test strains or pre-EXAMINATION OF NONSTERILE pare as stated below. Seed-lot culture maintenance tech-niques (seed-lot systems) are used so that the viable micro-PRODUCTS:MICROBIAL organisms used for inoculation are not more than fivepassages removed from the original master seed-lot. Grow ENUMERATION TESTSeach of the bacterial and fungal test strains separately as described in Table 1.Use Buffered Sodium Chloride–Peptone Solution pH 7.0 or Phosphate Buffer Solution pH 7.2 to make test suspensions; to suspend A. brasiliensis spores, 0.05% of polysorbate 80 may INTRODUCTIONbe added to the buffer. Use the suspensions within 2hours,or within 24hours if stored between 2° and 8°. As an alter-The tests described hereafter will allow quantitative enu-native to preparing and then diluting a fresh suspension of meration of mesophilic bacteria and fungi that may grow vegetative cells of A. brasiliensis or B. subtilis, a stable spore under aerobic conditions.suspension is prepared and then an appropriate volume of The tests are designed primarily to determine whether a the spore suspension is used for test inoculation. The stable substance or preparation complies with an established speci-spore suspension may be maintained at 2° to 8° for a vali-fication for microbiological quality. When used for such pur-dated period of time.poses, follow the instructions given below, including the number of samples to be taken, and interpret the results as stated below.Negative ControlThe methods are not applicable to products containing viable microorganisms as active ingredients.To verify testing conditions, a negative control is per-Alternative microbiological procedures, including auto-formed using the chosen diluent in place of the test prepa-mated methods, may be used, provided that their equiva-ration. There must be no growth of microorganisms. A neg-lence to the Pharmacopeial method has been demonstrated.ative control is also performed when testing the products as described under Testing of Products . A failed negative control requires an investigation.GENERAL PROCEDURESGrowth Promotion of the MediaCarry out the determination under conditions designed to avoid extrinsic microbial contamination of the product to be Test each batch of ready-prepared medium and eachexamined. The precautions taken to avoid contamination batch of medium prepared either from dehydrated medium must be such that they do not affect any microorganisms or from the ingredients described.that are to be revealed in the test.Inoculate portions/plates of Soybean–Casein Digest Broth If the product to be examined has antimicrobial activity,and Soybean–Casein Digest Agar with a small number (not this is, insofar as possible, removed or neutralized. If inac-more than 100 cfu) of the microorganisms indicated in Ta-tivators are used for this purpose, their efficacy and their ble 1, using a separate portion/plate of medium for each.absence of toxicity for microorganisms must be Inoculate plates of Sabouraud Dextrose Agar with a small demonstrated.number (not more than 100 cfu) of the microorganisms in-If surface-active substances are used for sample prepara-dicated in Table 1, using a separate plate of medium for tion, their absence of toxicity for microorganisms and their each. Incubate according to the conditions described in Ta-compatibility with any inactivators used must be ble 1.demonstrated.USP 36Microbiological Tests / 〈61〉 Microbiological Examination59Table 1. Preparation and Use of Test MicroorganismsSuitability of Counting Method inGrowth Promotion the Presence of ProductPreparation of Test Total Aerobic Total Yeasts and Total Aerobic Total Yeasts and Microorganism Strain Microbial Count Molds Count Microbial Count Molds Count Staphylococcus aureus Soybean–Casein Digest Soybean–Casein Soybean–Caseinsuch as ATCC 6538,Agar or Soybean–Casein Digest Agar and Digest Agar/MPNNCIMB 9518, CIP 4.83,Digest Broth Soybean–Casein Soybean–Caseinor NBRC 1327630°–35°Digest Broth Digest Broth18–24 hours≤100 cfu≤100 cfu30°–35°30°–35°≤3 days≤3 daysPseudomonas aeruginosa Soybean–Casein Digest Soybean–Casein Soybean–Caseinsuch as ATCC 9027,Agar or Soybean–Casein Digest Agar and Digest Agar/MPNNCIMB 8626, CIP 82.Digest Broth Soybean–Casein Soybean–Casein118, or NBRC 1327530°–35°Digest Broth Digest Broth18–24 hours≤100 cfu≤100 cfu30°–35°30°–35°≤3 days≤3 daysBacillus subtilis such as Soybean–Casein Digest Soybean–Casein Soybean–CaseinATCC 6633, NCIMB Agar or Soybean–Casein Digest Agar and Digest Agar/MPN8054, CIP 52.62, or Digest Broth Soybean–Casein Soybean–CaseinNBRC 313430°–35°Digest Broth Digest Broth18–24 hours≤100 cfu≤100 cfu30°–35°30°–35°≤3 days≤3 daysCandida albicans such as Sabouraud Dextrose Agar Soybean–Casein Sabouraud Soybean–Casein Sabouraud ATCC 10231, NCPF or Sabouraud Dextrose Digest Agar Dextrose Agar Digest Agar Dextrose Agar 3179, IP 48.72, or NBRC Broth≤100 cfu≤100 cfu≤100 cfu≤100 cfu 159420°–25°30°–35°20°–25°30°–35°20°–25°2–3 days≤5 days≤5 days≤5 days≤5 daysMPN: not applica-bleAspergillus brasiliensis such Sabouraud Dextrose Agar Soybean–Casein Sabouraud Soybean–Casein Sabouraud as ATCC 16404, IMI or Potato–Dextrose Agar Digest Agar Dextrose Agar Digest Agar Dextrose Agar 149007, IP 1431.83, or20°–25° 5–7 days, or≤100 cfu≤100 cfu≤100 cfu≤100 cfu NBRC 9455until good sporulation is30°–35°20°–25°30°–35°20°–25°achieved≤5 days≤5 days≤5 days≤5 daysMPN: not applica-bleFor solid media, growth obtained must not differ by a pared) in Buffered Sodium Chloride–Peptone Solution pH 7.0, factor greater than 2 from the calculated value for a stan-Phosphate Buffer Solution pH 7.2, or Soybean–Casein Digest dardized inoculum. For a freshly prepared inoculum, growth Broth. A surface-active agent such as 1g per L of polysor-of the microorganisms comparable to that previously ob-bate 80 may be added to assist the suspension of poorly tained with a previously tested and approved batch of me-wettable substances. If necessary, adjust to a pH of 6 to 8. dium occurs. Liquid media are suitable if clearly visible Further dilutions, where necessary, are prepared with the growth of the microorganisms comparable to that previ-same diluent.ously obtained with a previously tested and approved batch Fatty Products—Dissolve in isopropyl myristate sterilized of medium occurs.by filtration, or mix the product to be examined with theminimum necessary quantity of sterile polysorbate 80 or an-other noninhibitory sterile surface-active reagent heated, if Suitability of the Counting Method in thenecessary, to not more than 40° or, in exceptional cases, to Presence of Product not more than 45°. Mix carefully and if necessary maintainthe temperature in a water bath. Add a sufficient quantity ofthe prewarmed chosen diluent to make a 1 in 10 dilution ofthe original product. Mix carefully, while maintaining the PREPARATION OF THE SAMPLEtemperature for the shortest time necessary for the forma-tion of an emulsion. Further serial 10-fold dilutions may be The method for sample preparation depends on the phys-prepared using the chosen diluent containing a suitableical characteristics of the product to be tested. If none ofconcentration of sterile polysorbate 80 or another noninhib-the procedures described below can be demonstrated to beitory sterile surface-active reagent.satisfactory, a suitable alternative procedure must beFluids or Solids in Aerosol Form—Aseptically transfer developed.the product into a membrane filter apparatus or a sterile Water-Soluble Products—Dissolve or dilute (usually a 1container for further sampling. Use either the total contents in 10 dilution is prepared) the product to be examined inor a defined number of metered doses from each of the Buffered Sodium Chloride–Peptone Solution pH 7.0, Phosphatecontainers tested.Buffer Solution pH 7.2, or Soybean–Casein Digest Broth. If nec-Transdermal Patches—Remove the protective cover essary, adjust to a pH of 6 to 8. Further dilutions, wheresheets (“release liners”) of the transdermal patches and necessary, are prepared with the same diluent.place them, adhesive side upwards, on sterile glass or plastic Nonfatty Products Insoluble in Water—Suspend thetrays. Cover the adhesive surface with a suitable sterile product to be examined (usually a 1 in 10 dilution is pre-60〈61〉 Microbiological Examination / Microbiological Tests USP 36porous material (e.g., sterile gauze) to prevent the patches This information serves to indicate that the article is not from sticking together, and transfer the patches to a suita-likely to be contaminated with the given species of the mi-ble volume of the chosen diluent containing inactivators croorganism. However, it is possible that the product inhib-such as polysorbate 80 and/or lecithin. Shake the prepara-its only some of the microorganisms specified herein, but tion vigorously for at least 30minutes.does not inhibit others not included among the test strainsor those for which the latter are not representative. Then,perform the test with the highest dilution factor compatible INOCULATION AND DILUTION with microbial growth and the specific acceptance criterion.Add to the sample prepared as directed above and to acontrol (with no test material included) a sufficient volume RECOVERY OF MICROORGANISMS IN THE PRESENCE OF of the microbial suspension to obtain an inoculum of not PRODUCTmore than than 100 cfu. The volume of the suspension ofthe inoculum should not exceed 1% of the volume of di-For each of the microorganisms listed, separate tests are luted product.performed. Only microorganisms of the added test strain To demonstrate acceptable microbial recovery from the are counted.product, the lowest possible dilution factor of the prepared Membrane Filtration—Use membrane filters having a sample must be used for the test. Where this is not possible nominal pore size not greater than 0.45 µm. The type of due to antimicrobial activity or poor solubility, further ap-filter material is chosen in such a way that the bacteria-propriate protocols must be developed. If inhibition of retaining efficiency is not affected by the components of the growth by the sample cannot otherwise be avoided, the sample to be investigated. For each of the microorganisms aliquot of the microbial suspension may be added after neu-listed, one membrane filter is used.tralization, dilution, or filtration.Transfer a suitable quantity of the sample prepared as de-scribed under Preparation of the Sample, Inoculation and Dilu-tion, and Neutralization/Removal of Antimicrobial Activity NEUTRALIZATION/REMOVAL OF ANTIMICROBIAL ACTIVITY(preferably representing 1g of the product, or less if largenumbers of cfu are expected) to the membrane filter, filter The number of microorganisms recovered from the pre-immediately, and rinse the membrane filter with an appro-pared sample diluted as described in Inoculation and Dilutionpriate volume of diluent.and incubated following the procedure described in Recov-For the determination of total aerobic microbial countery of Microorganisms in the Presence of Product, is compared(TAMC), transfer the membrane filter to the surface of the to the number of microorganisms recovered from the con-Soybean–Casein Digest Agar. For the determination of total trol preparation.combined yeasts and molds count (TYMC), transfer theIf growth is inhibited (reduction by a factor greater thanmembrane to the surface of the Sabouraud Dextrose Agar. 2), then modify the procedure for the particular enumera-Incubate the plates as indicated in Table 1. Perform thetion test to ensure the validity of the results. Modification ofcounting.the procedure may include, for example,Plate-Count Methods—Perform plate-count methods at (1)An increase in the volume of the diluent or cultureleast in duplicate for each medium, and use the mean count medium;of the result.(2)Incorporation of a specific or general neutralizingagents into the diluent;Pour-Plate Method—For Petri dishes 9cm in diameter,(3)Membrane filtration; or add to the dish 1mL of the sample prepared as described(4)A combination of the above measures.under Preparation of the Sample, Inoculation and Dilution,and Neutralization/Removal of Antimicrobial Activity and 15 to Neutralizing Agents—Neutralizing agents may be used20mL of Soybean–Casein Digest Agar or Sabouraud Dextrose to neutralize the activity of antimicrobial agents (see TableAgar, both media maintained at not more than 45°. If larger 2). They may be added to the chosen diluent or the me-Petri dishes are used, the amount of agar medium is in-dium preferably before sterilization. If used, their efficacycreased accordingly. For each of the microorganisms listed and their absence of toxicity for microorganisms must bein Table 1, at least two Petri dishes are used. demonstrated by carrying out a blank with neutralizer andIncubate the plates as indicated in Table 1. Take the arith-without product.metic mean of the counts per medium, and calculate thenumber of cfu in the original inoculum.Table 2. Common Neutralizing Agents/Methods forSurface-Spread Method—For Petri dishes 9cm in diame- Interfering Substancester, add 15 to 20mL of Soybean–Casein Digest Agar orPotential Neutralizing Sabouraud Dextrose Agar at about 45° to each Petri dish, Interfering Substance Agents/Method and allow to solidify. If larger Petri dishes are used, the vol-Glutaraldehyde, mercurials Sodium hydrogen sulfite ume of the agar is increased accordingly. Dry the plates, for(Sodium bisulfite)example, in a laminar-airflow cabinet or in an incubator. For Phenolics, alcohol, aldehydes,Dilution each of the microorganisms listed in Table 1, at least two sorbate Petri dishes are used. Spread a measured volume of not lessthan 0.1mL of the sample, prepared as directed under Prep-Aldehydes Glycinearation of the Sample, Inoculation and Dilution, and Neutrali-Quaternary ammonium com-Lecithinzation/Removal of Antimicrobial Activity over the surface of pounds (QACs), parahydroxy-the medium. Incubate and count as directed for Pour-Plate benzoates (parabens), bis-Method.biguanidesMost-Probable-Number (MPN) Method—The precision QACs, iodine, parabens Polysorbateand accuracy of the MPN Method is less than that of the Mercurials Thioglycollate Membrane Filtration method or the Plate-Count Method. Un-Mercurials, halogens, aldehydes Thiosulfate reliable results are obtained particularly for the enumeration EDTA (edetate)Mg or Ca ions of molds. For these reasons, the MPN Method is reserved forthe enumeration of TAMC in situations where no othermethod is available. If the use of the method is justified,If no suitable neutralizing method can be found, it can beproceed as follows.assumed that the failure to isolate the inoculated organismPrepare a series of at least three serial 10-fold dilutions of is attributable to the microbicidal activity of the product.the product as described for Preparation of the Sample, Inoc-USP 36Microbiological Tests / 〈61〉 Microbiological Examination61ulation and Dilution, and Neutralization/Removal of Antimicro-RESULTS AND INTERPRETATIONbial Activity. From each level of dilution, three aliquots of1g or 1mL are used to inoculate three tubes with 9 to When verifying the suitability of the Membrane Filtration 10mL of Soybean–Casein Digest Broth. If necessary a surface-method or the Plate-Count Method, a mean count of any of active agent such as polysorbate 80, or an inactivator of the test organisms not differing by a factor greater than 2 antimicrobial agents may be added to the medium. Thus, if from the value of the control defined in Inoculation and Dilu-three levels of dilution are prepared, nine tubes are inocu-tion in the absence of product must be obtained. When lated.verifying the suitability of the MPN Method, the calculated Incubate all tubes at 30° to 35° for not more than 3 days.value from the inoculum must be within 95% confidenceIf reading of the results is difficult or uncertain owing to the limits of the results obtained with the control.nature of the product to be examined, subculture in the If the above criteria cannot be met for one of more of the same broth or in Soybean–Casein Digest Agar for 1 to 2 days organisms tested with any of the described methods, theat the same temperature, and use these results. From Table method and test conditions that come closest to the criteria 3, determine the most probable number of microorganisms are used to test the product.per g or mL of the product to be examined.TESTING OF PRODUCTS Table 3. Most-Probable-Number Values of MicroorganismsObservedCombinations Amount Used for the Test of Numbers of Tubes MPN per g or95%Showing Growth in per mL of ConfidenceUnless otherwise directed, use 10g or 10mL of the prod-Each Set Product Limitsuct to be examined taken with the precautions referred to Number of g or mL of above. For fluids or solids in aerosol form, sample 10 con-Product per Tube tainers. For transdermal patches, sample 10patches.0.10.010.001The amount to be tested may be reduced for active sub-000<30–9.4stances that will be formulated in the following conditions: 00130.1–9.5the amount per dosage unit (e.g., tablet, capsule, injection)is less than or equal to 1mg, or the amount per g or mL 01030.1–10(for preparations not presented in dose units) is less than 011 6.1 1.2–171mg. In these cases, the amount of sample to be tested is 020 6.2 1.2–17not less than the amount present in 10 dosage units or 10g0309.4 3.5–35or 10mL of the product.100 3.60.2–17For materials used as active substances where the sample 1017.2 1.2–17quantity is limited or batch size is extremely small (i.e., lessthan 1000mL or 1000g), the amount tested shall be 1% of 102114–35the batch unless a lesser amount is prescribed or justified 1107.4 1.3–20and authorized.111114–35For products where the total number of entities in a batch 120114–35is less than 200 (e.g., samples used in clinical trials), the 121155–38sample size may be reduced to two units, or one unit if the 130165–38size is less than 100.Select the sample(s) at random from the bulk material or 2009.2 1.5–35from the available containers of the preparation. To obtain 201144–35the required quantity, mix the contents of a sufficient num-202205–38ber of containers to provide the sample.210154–38211205–38Examination of the Product212279–94220215–40221289–94MEMBRANE FILTRATION 222359–94230299–94Use a filtration apparatus designed to allow the transfer of 231369–94the filter to the medium. Prepare the sample using a300235–94method that has been shown to be suitable as described in 301389–104Growth Promotion Test and Suitability of the Counting Method, 3026416–181 transfer the appropriate amount to each of two membranefilters, and filter immediately. Wash each filter following the 310439–181procedure shown to be suitable.3117517–199For the determination of TAMC, transfer one of the mem-31212030–360brane filters to the surface of Soybean–Casein Digest Agar.31316030–380For the determination of TYMC, transfer the other mem-3209318–360brane to the surface of Sabouraud Dextrose Agar. Incubate 32115030–380the plate of Soybean–Casein Digest Agar at 30° to 35° for 3to 5 days and the plate of Sabouraud Dextrose Agar at 20°32221030–400to 25° for 5 to 7 days. Calculate the number of cfu per g or 32329090–990per mL of product.33024040–990When examining transdermal patches, separately filter 33146090–198010% of the volume of the preparation described for Prepara-3321100200–4000tion of the Sample through each of two sterile filter mem-333>1100branes. Transfer one membrane to Soybean–Casein DigestAgar for TAMC and the other membrane to Sabouraud Dex-trose Agar for TYMC.62〈61〉 Microbiological Examination / Microbiological Tests USP 36 PLATE-COUNT METHODS〈62〉 MICROBIOLOGICAL Pour-Plate Method—Prepare the sample using a method EXAMINATION OF NONSTERILE that has been shown to be suitable as described in GrowthPromotion Test and Suitability of the Counting Method. Pre-PRODUCTS:TESTS FORpare for each medium at least two Petri dishes for each levelof dilution. Incubate the plates of Soybean–Casein Digest SPECIFIED MICROORGANISMS Agar at 30° to 35° for 3 to 5 days and the plates ofSabouraud Dextrose Agar at 20° to 25° for 5 to 7 days. Se-lect the plates corresponding to a given dilution and show-ing the highest number of colonies less than 250 for TAMCand 50 for TYMC. Take the arithmetic mean per culture me-INTRODUCTIONdium of the counts, and calculate the number of cfu per gor per mL of product.The tests described hereafter will allow determination of Surface-Spread Method—Prepare the sample using a the absence of, or limited occurrence of, specified microor-method that has been shown to be suitable as described in ganisms that may be detected under the conditions Growth Promotion Test and Suitability of the Counting described.Method. Prepare at least two Petri dishes for each medium The tests are designed primarily to determine whether a and each level of dilution. For incubation and calculation of substance or preparation complies with an established speci-the number of cfu, proceed as directed for the Pour-Plate fication for microbiological quality. When used for such pur-Method.poses, follow the instructions given below, including thenumber of samples to be taken, and interpret the results asstated below.MOST-PROBABLE-NUMBER METHODAlternative microbiological procedures, including auto-mated methods, may be used, provided that their equiva-Prepare and dilute the sample using a method that haslence to the Pharmacopeial method has been demonstrated. been shown to be suitable as decribed in Growth PromotionTest and Suitability of the Counting Method. Incubate alltubes for 3 to 5 days at 30° to 35°. Subculture if necessary,GENERAL PROCEDURESusing the procedure shown to be suitable. Record for eachlevel of dilution the number of tubes showing microbial The preparation of samples is carried out as described in growth. Determine the most probable number of microor-Microbiological Examination of Nonsterile Products: Microbial ganisms per g or mL of the product to be examined from Enumeration Tests 〈61〉.Table 3.If the product to be examined has antimicrobial activity,this is insofar as possible removed or neutralized as de-Interpretation of the Results scribed in Microbiological Examination of Nonsterile Products:Microbial Enumeration Tests 〈61〉.The total aerobic microbial count (TAMC) is considered to If surface-active substances are used for sample prepara-be equal to the number of cfu found using Soybean–Casein tion, their absence of toxicity for microorganisms and their Digest Agar; if colonies of fungi are detected on this me-compatibility with any inactivators used must be demon-dium, they are counted as part of TAMC. The total com-strated as described in Microbiological Examination of Non-bined yeasts and molds count (TYMC) is considered to be sterile Products: Microbial Enumeration Tests 〈61〉.equal to the number of cfu found using Sabouraud DextroseAgar; if colonies of bacteria are detected on this medium,GROWTH-PROMOTING AND INHIBITORY they are counted as part of TYMC. When the TYMC is ex-pected to exceed the acceptance criterion due to the bacte-PROPERTIES OF THE MEDIA, SUITABILITY OF rial growth, Sabouraud Dextrose Agar containing antibiotics THE TEST AND NEGATIVE CONTROLS may be used. If the count is carried out by the MPNMethod, the calculated value is TAMC.The ability of the test to detect microorganisms in the When an acceptance criterion for microbiological quality presence of the product to be tested must be established.is prescribed, it is interpreted as follows:Suitability must be confirmed if a change in testing perfor-—101 cfu: maximum acceptable count = 20;mance or a change in the product that may affect the out-—102 cfu: maximum acceptable count = 200;come of the test is introduced.—103 cfu: maximum acceptable count = 2000;and so forth.Preparation of Test StrainsThe recommended solutions and media are described inTests for Specified Microorganisms 〈62〉.Use standardized stable suspensions of test strains asstated below. Seed-lot culture maintenance techniques(seed-lot systems) are used so that the viable microorgan-isms used for inoculation are not more than five passagesremoved from the original master seed-lot.AEROBIC MICROORGANISMSGrow each of the bacterial test strains separately in con-tainers containing Soybean–Casein Digest Broth or onSoybean–Casein Digest Agar at 30° to 35° for 18 to 24hours.Grow the test strain for Candida albicans separately onSabouraud Dextrose Agar or in Sabouraud Dextrose Broth at20° to 25° for 2 to 3 days.。
无菌USP36

Fluid Thioglycollate Medium (Continued)Interpretation of Resultsor Thioglycolic Acid0.3 mL The product to be examined complies with the test if Resazurin Sodium Solution (1 in 1000),fluorescence typical of Mycoplasmas is not present. The test freshly prepared 1.0 mL is invalid if the positive controls do not show fluorescence Purified Water1000 mLtypical of Mycoplasmas. The test is invalid if the negative controls show fluorescence typical of Mycoplasmas.pH after sterilization: 7.1±0.2.Mix the L -cystine, agar, sodium chloride, dextrose, yeast extract, and pancreatic digest of casein with the purified water, and heat until solution is effected. Dissolve the so-dium thioglycollate or thioglycolic acid in the solution and,if necessary, add 1N sodium hydroxide so that, after sterili-zation, the solution will have a pH of 7.1 ± 0.2. If filtration is 〈71〉 STERILITY TESTSnecessary, heat the solution again without boiling, and filter while hot through moistened filter paper. Add the resazurin sodium solution, mix, and place the medium in suitable ves-sels that provide a ratio of surface to depth of medium such xPortions of this general chapter have been harmonized that not more than the upper half of the medium has un-with the corresponding texts of the European Pharmacopeia dergone a color change indicative of oxygen uptake at the and/or the Japanese Pharmacopeia. Those portions that are end of the incubation period. Sterilize using a validated pro-not harmonized are marked with symbols (x x ) to specify this cess. If the medium is stored, store at a temperature be-fact.xtween 2° and 25° in a sterile, airtight container. If more These Pharmacopeial procedures are not by themselves than the upper one-third of the medium has acquired a designed to ensure that a batch of product is sterile or has pink color, the medium may be restored once by heating been sterilized. This is accomplished primarily by validation the containers in a water-bath or in free-flowing steam until of the sterilization process or of the aseptic processing the pink color disappears and by cooling quickly, taking procedures.care to prevent the introduction of nonsterile air into the The test is applied to substances, preparations, or articles container. Do not use the medium for a longer storage pe-which, according to the Pharmacopeia, are required to be ster-riod than has been validated.ile. However, a satisfactory result only indicates that no con-Fluid Thioglycollate Medium is to be incubated at 30°–35°.taminating microorganism has been found in the sample ex-For products containing a mercurial preservative that cannot amined under the conditions of the test .be tested by the membrane filtration method, Fluid Thiog-lycollate Medium incubated at 20°–25° may be used instead of Soybean–Casein Digest Medium provided that it has been PRECAUTIONS AGAINST MICROBIALvalidated as described in Growth Promotion Test of Aerobes,CONTAMINATIONAnaerobes, and Fungi . Where prescribed or justified and au-thorized, the following alternative thioglycollate medium The test for sterility is carried out under aseptic condi-might be used. Prepare a mixture having the same composi-tions. In order to achieve such conditions, the test environ-tion as that of the Fluid Thioglycollate Medium , but omitting ment has to be adapted to the way in which the sterility the agar and the resazurin sodium solution. Sterilize as di-test is performed. The precautions taken to avoid contami-rected above. The pH after sterilization is 7.1 ± 0.2. Heat in nation are such that they do not affect any microorganisms a water bath prior to use and incubate at 30°–35° under that are to be revealed in the test. The working conditions anaerobic conditions.in which the tests are performed are monitored regularly by appropriate sampling of the working area and by carrying out appropriate controls.Soybean–Casein Digest MediumPancreatic Digest of Casein 17.0 g CULTURE MEDIA AND INCUBATIONPapaic Digest of Soybean Meal 3.0 g TEMPERATURESSodium Chloride5.0 g Media for the test may be prepared as described below or Dibasic Potassium Phosphate2.5 g equivalent commercial media may be used provided that Dextrose Monohydrate/Anhydrous 2.5/2.3 g they comply with the requirements of the Growth Promotion Purified Water1000 mLTest of Aerobes, Anaerobes, and Fungi .The following culture media have been found to be suita-pH after sterilization: 7.3±0.2.ble for the test for sterility. Fluid Thioglycollate Medium is Dissolve the solids in the Purified Water, heating slightly primarily intended for the culture of anaerobic bacteria.to effect a solution. Cool the solution to room temperature,However, it will also detect aerobic bacteria. Soybean–Casein and adjust the pH with 1N sodium hydroxide so that, after Digest Medium is suitable for the culture of both fungi and sterilization, it will have a pH of 7.3 ± 0.2. Filter, if necessary aerobic bacteria.to clarify, dispense into suitable containers, and sterilize us-ing a validated procedure. Store at a temperature between 2° and 25° in a sterile well-closed container, unless it is in-Fluid Thioglycollate Mediumtended for immediate use. Do not use the medium for a longer storage period than has been validated.L -Cystine0.5 g Soybean–Casein Digest Medium is to be incubated at 22.5Sodium Chloride2.5 g ± 2.5°.Dextrose Monohydrate/Anhydrous 5.5/5.0 g Agar0.75 g xMedia for Penicillins or CephalosporinsYeast Extract (water-soluble) 5.0 g Pancreatic Digest of Casein 15.0 g Where sterility test media are to be used in the DirectInoculation of the Culture Medium method under Test for Ste-Sodium Thioglycollate0.5 grility of the Product to be Examined , modify the preparationof Fluid Thioglycollate Medium and the Soybean–Casein Digest cfu) of the following microorganisms, using a separate por-Medium as follows. To the containers of each medium,tion of medium for each of the following species of microor-transfer aseptically a quantity of β-lactamase sufficient to in-ganism: Aspergillus brasiliensis , Bacillus subtilis, and Candida activate the amount of antibiotic in the specimen underalbicans . Incubate for not more than 3 days in the case of test. Determine the quantity of β-lactamase required to inac-bacteria and not more than 5 days in the case of fungi.tivate the antibiotic by using a β-lactamase preparation that Seed lot culture maintenance techniques (seed-lot systems)has been assayed previously for its penicillin- or cephalospo-are used so that the viable microorganisms used for inocula-rin-inactivating power. [N OTE —Supplemented β-lactamase tion are not more than five passages removed from the media can also be used in the membrane filtration test.]original master seed-lot.Alternatively (in an area completely separate from that The media are suitable if a clearly visible growth of the used for sterility testing), confirm that an appropriatemicroorganisms occurs.amount of β-lactamase is incorporated into the medium, fol-lowing either method under Method Suitability Test , using xDILUTING AND RINSING FLUIDS FORless than 100 colony-forming units (cfu) of Staphylococcus aureus (see Table 1) as the challenge. Typical microbial MEMBRANE FILTRATIONgrowth of the inoculated culture must be observed as aconfirmation that the β-lactamase concentration is appropri-ate.xFluid ATable 1. Strains of the Test Microorganisms Suitable for Use inPREPARATIONDissolve 1g of peptic digest of animal tissue in water tomake 1L, filter or centrifuge to clarify, if necessary, and ad-just to a pH of 7.1± 0.2. Dispense into containers, and sterilize using a validated process.PREPARATION FOR PENICILLINS OR CEPHALOSPORINS Aseptically add to the above Preparation , if necessary, a quantity of sterile β-lactamase sufficient to inactivate any re-sidual antibiotic activity on the membranes after the solu-tion of the test specimen has been filtered (see Media for Penicillins or Cephalosporins ).Fluid DTo each L of Fluid A add 1mL of polysorbate 80, adjust to a pH of 7.1 ± 0.2, dispense into containers, and sterilize using a validated process. Use this fluid for articles contain-ing lecithin or oil, or for devices labeled as “sterile x 1pathway.”luteus) ATCC 9341.xx 2An alternative to Clostridium sporogenes, when a nonspore-forming Fluid Kmicroorganism is desired, is Bacteroides vulgatus (ATCC 8482).xThe media used comply with the following tests, carried Dissolve 5.0g of peptic digest of animal tissue, 3.0g of out before, or in parallel, with the test on the product to be beef extract, and 10.0g of polysorbate 80 in water to make examined.1L. Adjust the pH to obtain, after sterilization, a pH of 6.9 ±0.2. Dispense into containers, and sterilize using a validated process.xSterilityIncubate portions of the media for 14 days. No growth of METHOD SUITABILITY TESTmicroorganisms occurs.Carry out a test as described below under Test for Sterility of the Product to be Examined using exactly the same meth-Growth Promotion Test of Aerobes,ods, except for the following modifications.Membrane Filtrationof medium prepared either from dehydrated medium or from ingredients. Suitable strains of microorganisms are in-After transferring the content of the container or contain-dicated in Table 1.ers to be tested to the membrane, add an inoculum of a Inoculate portions of Fluid Thioglycollate Medium with a small number of viable microorganisms (not more than small number (not more than 100 cfu) of the following mi-100 cfu) to the final portion of sterile diluent used to rinse croorganisms, using a separate portion of medium for each of the following species of microorganism: Clostridiumsporogenes , Pseudomonas aeruginosa , and Staphylococcus au-Direct Inoculationreus . x Inoculate portions of alternative thioglycollate me-dium with a small number (not more than 100 cfu) of Clos-After transferring the contents of the container or contain-tridium sporogenes .x Inoculate portions of Soybean–Casein ers to be tested (for catgut and other surgical sutures for Digest Medium with a small number (not more than 100veterinary use: strands) to the culture medium, add an inoc-ulum of a small number of viable microorganisms (not more Table 2. Minimum Quantity to be Used for Eachthan 100 cfu) to the medium.Medium (Continued)In both cases use the same microorganisms as those de-Minimum Quantity to be Used scribed above under Growth Promotion Test of Aerobes, An-(unless otherwise justified andaerobes, and Fungi . Perform a growth promotion test as a Quantity per Container authorized)positive control. Incubate all the containers containing me-xSurgical dressing/cotton/100 mg per package dium for not more than 5 days.gauze (in packages)If clearly visible growth of microorganisms is obtained af-Sutures and other individually The whole deviceter the incubation, visually comparable to that in the control packaged single-use material vessel without product, either the product possesses no anti-Other medical devicesThe whole device, cut into pieces or microbial activity under the conditions of the test or such disassembled xactivity has been satisfactorily eliminated. The test for steril-ity may then be carried out without further modification.If clearly visible growth is not obtained in the presence of the product to be tested, visually comparable to that in the Table 3. Minimum Number of Articles to be Tested in Relationcontrol vessels without product, the product possesses anti-to the Number of Articles in the Batchmicrobial activity that has not been satisfactorily eliminated Minimum Number of Itemsunder the conditions of the test. Modify the conditions in to be Tested for Each order to eliminate the antimicrobial activity, and repeat the Number of Items in the Medium (unless otherwise Method Suitability Test .Batch *justified and authorized)**This method suitability is performed (a) when the test for Parenteral preparationssterility has to be carried out on a new product; and (b)Not more than 100 containers 10% or 4 containers, whichever whenever there is a change in the experimental conditions is the greater of the test. The method suitability may be performed simul-taneously with the Test for Sterility of the Product to be More than 100 but not more 10 containersExamined .than 500 containersMore than 500 containers2% or 20 containers, whichever is lessTEST FOR STERILITY OF THE PRODUCT TOxFor large-volume parenterals2% or 10 containers, whichever BE EXAMINEDis lessAntibiotic solidsPharmacy bulk packages (<5 g)20 containers xNumber of Articles to Be TestedPharmacy bulk packages (≥5 g) 6 containersBulks and blendsSee Bulk solid products xUnless otherwise specified elsewhere in this chapter or in the individual monograph, test the number of articles speci-Ophthalmic and other noninjectable preparations fied in Table 3. If the contents of each article are of suffi-cient quantity (see Table 2), they may be divided so that Not more than 200 containers 5% or 2 containers, whichever isequal appropriate portions are added to each of the speci-the greaterfied media. [N OTE —Perform sterility testing employing two More than 200 containers 10 containers or more of the specified media.] If each article does not If the product is presented in contain sufficient quantities for each medium, use twice the the form of single-dose con-number of articles indicated in Table 3.xtainers,apply the scheme shown above Table 2. Minimum Quantity to be Used for Each Mediumfor preparations for parenteral use.Minimum Quantity to be Used Catgut and other surgical su-2% or 5 packages, whichever is(unless otherwise justified andtures for veterinary use the greater,Quantity per Container authorized)up to a maximum total of 20LiquidspackagesLess than 1 mL The whole contents of each con-x Not more than 100 articles 10% or 4 articles, whichever istainergreater1–40 mLHalf the contents of each container,More than 100, but not more 10 articles but not less than 1 mL than 500 articlesGreater than 40 mL, and not 20 mLMore than 500 articles 2% or 20 articles, whichever isgreater than 100 mL less xGreater than 100 mL 10% of the contents of the contain-Bulk solid products er, but not less than 20 mL Up to 4 containers Each container Antibiotic liquids1 mLMore than 4 containers, but not 20% or 4 containers, whichever Insoluble preparations, creams,Use the contents of each container more than 50 containers is greater and ointments to be suspend-to provide not less than 200 mgMore than 50 containers 2% or 10 containers, whichevered or emulsified is greaterSolids*If the batch size is unknown, use the maximum number of items Less than 50 mgThe whole contents of each con-prescribed.tainer**If the contents of one container are enough to inoculate the two 50 mg or more, but less than Half the contents of each container,media, this column gives the number of containers needed for both 300 mg but not less than 50 mg the media together.300 mg–5 g 150 mg The test may be carried out using the technique of Mem-Greater than 5 g500 mgbrane Filtration or by Direct Inoculation of the Culture Medium Catgut and other surgical su- 3 sections of a strand (each 30-cm with the product to be examined. Appropriate negative tures for veterinary uselong)controls are included. The technique of membrane filtrationis used whenever the nature of the product permits; that is,myristate shown not to have antimicrobial activity in thefor filterable aqueous preparations, for alcoholic or oily prep-conditions of the test. Allow the oil to penetrate the mem-arations, and for preparations miscible with, or soluble in,brane by its own weight, and then filter, applying the pres-aqueous or oily solvents, provided these solvents do not sure or suction gradually. Wash the membrane at least three have an antimicrobial effect in the conditions of the test.times by filtering through it each time about 100mL of asuitable sterile solution such as x Fluid A (see Diluting andRinsing Fluids for Membrane Filtration)x containing a suitable Membrane Filtration emulsifying agent at a concentration shown to be appropri-ate in the Method Suitability Test, for example polysorbate Use membrane filters having a nominal pore size not80 at a concentration of 10g per Lx(Fluid K)x. Transfer the greater than 0.45 µm, in which the effectiveness to retain membrane or membranes to the culture medium or media,microorganisms has been established. Cellulose nitrate fil-or vice versa, as described above for Aqueous Solutions, and ters, for example, are used for aqueous, oily, and weakly incubate at the same temperatures and for the same times. alcoholic solutions; and cellulose acetate filters, for example,are used for strongly alcoholic solutions. Specially adaptedfilters may be needed for certain products (e.g., for OINTMENTS and CREAMS antibiotics).The technique described below assumes that membranes Use for each medium not less than the quantities of the about 50mm in diameter will be used. If filters of a different product prescribed in Tables 2 and 3. Ointments in a fatty diameter are used, the volumes of the dilutions and the base and emulsions of the water-in-oil type may be diluted washings should be adjusted accordingly. The filtration ap-to 1% in isopropyl myristate as described above, by heating, paratus and membrane are sterilized by appropriate means.if necessary, to not more than 40°. In exceptional cases it The apparatus is designed so that the solution to be ex-may be necessary to heat to not more than 44°. Filter as amined can be introduced and filtered under aseptic condi-rapidly as possible, and proceed as described above for Oils tions: it permits the aseptic removal of the membrane for and Oily Solutions.transfer to the medium, or it is suitable for carrying out theincubation after adding the medium to the apparatus itself.x PREFILLED SYRINGESAQUEOUS SOLUTIONS For prefilled syringes without attached sterile needles, ex-pel the contents of each syringe into one or two separateIf appropriate, transfer a small quantity of a suitable, ster-membrane filter funnels or into separate pooling vesselsile diluent such as x Fluid A (see Diluting and Rinsing Fluids for prior to transfer. If a separate sterile needle is attached, di-Membrane Filtration)x onto the membrane in the apparatus rectly expel the syringe contents as indicated above, and and filter. The diluent may contain suitable neutralizing sub-proceed as directed for Aqueous Solutions. Test the sterility of stances and/or appropriate inactivating substances, for ex-the needle, using Direct Inoculation under Method Suitability ample, in the case of antibiotics.Test.Transfer the contents of the container or containers to betested to the membrane or membranes, if necessary, afterdiluting to the volume used in the Method Suitability Test SOLIDS FOR INJECTION OTHER THAN ANTIBIOTICS with the chosen sterile diluent, but using not less than thequantities of the product to be examined prescribed in Ta-Constitute the test articles as directed on the label, and bles 2 and 3. Filter immediately. If the product has antimi-proceed as directed for Aqueous Solutions or Oils and Oily crobial properties, wash the membrane not less than three Solutions, whichever applies. [N OTE—If necessary, excess di-times by filtering through it each time the volume of the luent can be added to aid in the constitution and filtration chosen sterile diluent used in the Method Suitability Test. Do of the constituted test article.]not exceed a washing cycle of five times 100mL per filter,even if during method suitability it has been demonstratedANTIBIOTIC SOLIDS FOR INJECTIONthat such a cycle does not fully eliminate the antimicrobialactivity. Transfer the whole membrane to the culture me-dium or cut it aseptically into two equal parts, and transfer Pharmacy Bulk Packages, <5g—From each of 20 con-one half to each of two suitable media. Use the same vol-tainers, aseptically transfer about 300mg of solids, into a ume of each medium as in the Method Suitability Test. Alter-sterile 500-mL conical flask, dissolve in about 200mL of natively, transfer the medium onto the membrane in the Fluid A (see Diluting and Rinsing Fluids for Membrane Filtra-apparatus. Incubate the media for not less than 14 days.tion), and mix; or constitute, as directed in the labeling,each of 20 containers and transfer a quantity of liquid orsuspension, equivalent to about 300mg of solids, into a SOLUBLE SOLIDS sterile 500-mL conical flask, dissolve in about 200mL ofFluid A, and mix. Proceed as directed for Aqueous Solutions Use for each medium not less than the quantity pre-or Oils and Oily Solutions, whichever applies.scribed in Tables 2 and 3 of the product dissolved in a suita-Pharmacy Bulk Packages, ≥5g—From each of 6 con-ble solvent, such as the solvent provided with the prepara-tainers, aseptically transfer about 1g of solids into a sterile tion, Sterile Water for Injection, sterile saline, or a suitable500-mL conical flask, dissolve in about 200mL of Fluid A, sterile solution such as x Fluid A (Diluting and Rinsing Fluids and mix; or constitute, as directed in the labeling, each of 6 for Membrane Filtration),x and proceed with the test as de-containers and transfer a quantity of liquid, equivalent to scribed above for Aqueous Solutions using a membrane ap-about 1g of solids, into a sterile 500-mL conical flask, dis-propriate to the chosen solvent.solve in about 200mL of Fluid A, and mix. Proceed as di-rected for Aqueous Solutions.OILS and OILY SOLUTIONSANTIBIOTIC SOLIDS, BULKS, and BLENDSUse for each medium not less than the quantity of theproduct prescribed in Tables 2 and 3. Oils and oily solutions Aseptically remove a sufficient quantity of solids from the of sufficiently low viscosity may be filtered without dilution appropriate amount of containers (see Table 2), mix to ob-through a dry membrane. Viscous oils may be diluted as tain a composite, equivalent to about 6g of solids, and necessary with a suitable sterile diluent such as isopropyltransfer to a sterile 500-mL conical flask. Dissolve in about CATGUT and OTHER SURGICAL SUTURES FOR VETERINARIAN 200mL of Fluid A, and mix. Proceed as directed for Aqueous USESolutions.Use for each medium not less than the quantities of theproduct prescribed in Tables 2 and 3. Open the sealed pack-STERILE AEROSOL PRODUCTSage using aseptic precautions, and remove three sections ofthe strand for each culture medium. Carry out the test on For fluid products in pressurized aerosol form, freeze thethree sections, each 30-cm long, which have been cut off containers in an alcohol-dry ice mixture at least at –20° forfrom the beginning, the center, and the end of the strand. about 1hour. If feasible, allow the propellant to escapeUse whole strands from freshly opened cassette packs. before aseptically opening the container, and transfer theTransfer each section of the strand to the selected medium. contents to a sterile pooling vessel. Add 100mL of Fluid DUse sufficient medium to cover adequately the material to to the pooling vessel, and mix gently. Proceed as directedbe tested (20mL to 150mL).for Aqueous Solutions or Oils and Oily Solutions, whicheverapplies.x SOLIDSDEVICES WITH PATHWAYS LABELED STERILETransfer a quantity of the product in the form of a drysolid (or prepare a suspension of the product by adding Aseptically pass not less than 10pathway volumes of Fluidsterile diluent to the immediate container), corresponding to D through each device tested. Collect the fluids in an ap-not less than the quantity indicated in Tables 2 and 3. Trans-propriate sterile vessel, and proceed as directed for Aqueousfer the material so obtained to 200mL of Fluid Thioglycollate Solutions or Oils and Oily Solutions, whichever applies.Medium, and mix. Similarly, transfer the same quantity toIn the case of sterile, empty syringes, draw sterile diluent200mL of Soybean–Casein Digest Medium, and mix. Proceed into the barrel through the sterile needle, if attached, oras directed above.through a sterile needle attached for the purpose of thetest, and express the contents into a sterile pooling vessel.Proceed as directed above.x PURIFIED COTTON, GAUZE, SURGICAL DRESSINGS, andRELATED ARTICLES Direct Inoculation of the Culture MediumFrom each package of cotton, rolled gauze bandage, or Transfer the quantity of the preparation to be examined large surgical dressings being tested, aseptically remove two prescribed in Tables 2 and 3 directly into the culture me-or more portions of 100- to 500-mg each from the inner-dium so that the volume of the product is not more than most part of the sample. From individually packaged, single-10% of the volume of the medium, unless otherwise use materials, aseptically remove the entire article. Immerse prescribed.the portions or article in each medium, and proceed as di-If the product to be examined has antimicrobial activity,rected above.carry out the test after neutralizing this with a suitable neu-tralizing substance or by dilution in a sufficient quantity ofSTERILE DEVICESculture medium. When it is necessary to use a large volumeof the product, it may be preferable to use a concentratedArticles can be immersed intact or disassembled. To en-culture medium prepared in such a way that it takes intosure that device pathways are also in contact with the me-account the subsequent dilution. Where appropriate, thedia, immerse the appropriate number of units per medium concentrated medium may be added directly to the productin a volume of medium sufficient to immerse the devicein its container.completely, and proceed as directed above. For extremelylarge devices, immerse those portions of the device that are OILY LIQUIDS to come into contact with the patient in a volume of me-dium sufficient to achieve complete immersion of those Use media to which have been added a suitable emulsify-portions.ing agent at a concentration shown to be appropriate in the For catheters where the inside lumen and outside are re-Method Suitability Test, for example polysorbate 80 at a con-quired to be sterile, either cut them into pieces such that centration of 10g per L.the medium is in contact with the entire lumen or fill thelumen with medium, and then immerse the intact unit.x OINTMENTS and CREAMSOBSERVATION AND INTERPRETATION OF Prepare by diluting to about 1 in 10 by emulsifying with RESULTSthe chosen emulsifying agent in a suitable sterile diluentsuch as x Fluid A (see Diluting and Rinsing Fluids for Mem-At intervals during the incubation period and at its con-brane Filtration).x Transfer the diluted product to a medium clusion, examine the media for macroscopic evidence of mi-not containing an emulsifying agent.crobial growth. If the material being tested renders the me-Incubate the inoculated media for not less than 14 days.dium turbid so that the presence or absence of microbial Observe the cultures several times during the incubation pe-growth cannot be readily determined by visual examination, riod. Shake cultures containing oily products gently each14 days after the beginning of incubation transfer portions day. However, when Fluid Thioglycollate Medium is used for(each not less than 1mL) of the medium to fresh vessels of the detection of anaerobic microorganisms, keep shaking or the same medium, and then incubate the original and trans-mixing to a minimum in order to maintain anaerobic fer vessels for not less than 4 days.conditions.If no evidence of microbial growth is found, the productto be examined complies with the test for sterility. If evi-dence of microbial growth is found, the product to be ex-amined does not comply with the test for sterility, unless itcan be clearly demonstrated that the test was invalid forcauses unrelated to the product to be examined. The test。
微生物检测美国药典
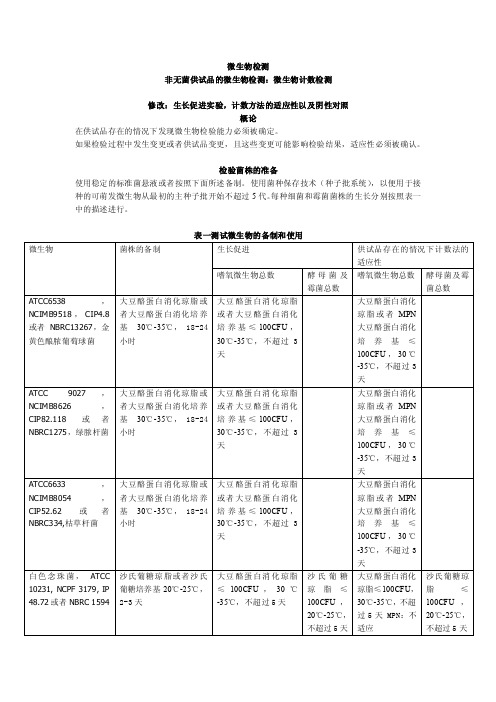
微生物检测非无菌供试品的微生物检测:微生物计数检测修改:生长促进实验,计数方法的适应性以及阴性对照概论在供试品存在的情况下发现微生物检验能力必须被确定。
如果检验过程中发生变更或者供试品变更,且这些变更可能影响检验结果,适应性必须被确认。
检验菌株的准备使用稳定的标准菌悬液或者按照下面所述备制。
使用菌种保存技术(种子批系统),以便用于接种的可萌发微生物从最初的主种子批开始不超过5代。
每种细菌和霉菌菌株的生长分别按照表一中的描述进行。
表一测试微生物的备制和使用使用pH7.0的缓冲氯化钠蛋白胨溶液或者pH7.2的磷酸盐缓冲溶液备制测试菌悬液;备制黑曲霉孢子悬浮液时,0.05%的聚山梨脂80可以被添加到缓冲液中。
测试菌悬液应在两小时内使用,或者在2-8℃的条件下24小时内使用。
也可以通过制备并稀释枯草芽胞杆菌营养细胞的新鲜悬液进行替代,制备稳定的胞子悬液,在接种测试中使用适当体积的胞子菌悬液,稳定的胞子悬液在2-8℃保存,保存期是经过验证的。
阴性对照为了确定检测条件,用选择好的稀释液代替测试备制来进行阴性对照。
必须没有微生物的生长。
当按照供试品检测中的描述进行检验时,也需要进行阴性对照。
如果阴性对照不合格需要进行调查。
培养基的生长促进检测每个批次的已经备制好的培养基以及通过脱水培养基或者描述的配料备制的每个批次的培养基。
在部分/盘大豆酪蛋白消化肉汤培养基以及大豆酪蛋白消化琼脂上接种少量(不超过100CFU)微生物,按表一中所示,每一种微生物应使用单独一部分/一盘培养基。
在沙氏葡萄糖琼脂上接种少量(不超过100CFU)微生物,按表一中所示,每一种微生物应使用单独的一盘培养基。
按照表一中所述的条件进行接种。
固体培养基的生长通过一个不大于2的系数的调节必须不能与标准接种体计算得到的数值有区别。
新鲜备制的接种体微生物的生长应与上一批通过检测的培养基的生长情形相同。
如果肉眼能清晰看到的微生物的生长与上一批通过检测的培养基的生长情形相同的话,液体培养基是适合的。
USP微生物限度及无菌检验方法2

USP微生物限度及无菌检验方法1First supplement, USP-NF 发补1,USP-NFSoybean-casein digest medium (continued)大豆蛋白消化酶(继续)Dibasic Potassium Phosphate二盐基亚磷酸钾……Dextrose Monohydrate/Anhydrous葡萄糖一水/无水……Purified water纯化水……pH after sterilization:7.3±0.2 灭菌后pH值:7.3±0.2Dissolve the solids in the purified water, heating slightly to effect a solution. Cool the solution to room temperature, and adjust the pH within 1N sodium hydroxide so that, after sterilization, it will have a PH of 7.3±0.2. Filter, if necessary to clarify, dispense into suitable containers, and sterilize using a validated procedure. Store at a temperature between 2° and 25° in a sterile well-closed container, unless it is intended for immediate use. Do not use the medium for a longer storage period than has been validated. 将固体物质溶解于纯净水,轻微加热以实现溶解。
美国药典微生物检测精要

美国药典微生物检测精要引言微生物检测在药品生产中起着至关重要的作用。
药品的微生物污染可能对患者的健康造成严重威胁。
为了确保药品的安全性、可靠性和有效性,美国药典(United States Pharmacopeia, USP)制定了一系列用于微生物检测的指南和标准。
本文将概述美国药典微生物检测的精要内容。
检测方法目视法目视法是最简单、最常用的微生物检测方法之一。
该方法通过对样品进行肉眼观察,检验人员可以检测到样品中是否存在明显的微生物污染。
目视法的优点是操作简单,不需要特殊的设备和试剂,适用于多种药品和原料的微生物检测。
然而,目视法存在主观性和局限性,无法检测到微生物的低水平污染。
营养物质法营养物质法是一种常用的培养基法。
该方法以适宜的培养基为基础,通过对样品进行培养,利用微生物的生长特性来检测样品中是否存在微生物污染。
营养物质法可以检测到微生物的低水平污染,并且可以进一步鉴定和计数微生物。
然而,营养物质法需要一定的培养时间,且存在一定的培养基选择性和适应性的局限性。
生物化学法生物化学法是一种通过检测微生物代谢产物来判断样品中是否存在微生物污染的方法。
该方法利用微生物的代谢反应,检测样品中的特定代谢产物的存在与否。
生物化学法可以快速、准确地检测微生物污染,并且不受培养基选择性和适应性的限制。
然而,生物化学法需要特定的试剂和设备,并且对检测人员的技术要求较高。
检测标准美国药典对药品的微生物检测制定了一系列的标准和要求。
这些标准主要包括微生物限度试验、总微生物数试验、鉴别试验和特定微生物试验等。
微生物限度试验微生物限度试验旨在确定样品中存在的微生物数量的限度。
该试验要求将样品接种在适当的培养基中,经过一定的培养时间后,观察并计数生长的微生物数量。
美国药典针对不同类型的药品和原料制定了不同的微生物限度指标,以确保药品的微生物安全性。
总微生物数试验总微生物数试验用于确定样品中所有可培养的微生物的数量。
该试验要求将样品接种在适当的培养基中,经过一定的培养时间后,观察并计数生长的微生物数量。
(最新)美国药典微生物检测精要

美国药典微生物检测精要培训小结一微生物实验室规范重要的控制点:无菌技术、培养基控制、菌种控制、设备操作与控制、数据记录与实验室布局和运作、员工培训等。
1无菌技术:防止和保持无菌物品及无菌区域不被污染的操作方法和管理方法。
可通过无菌环境、灭菌、消毒、卫生要求、无菌操作技术等手段来实现。
2培养基:是微生物实验工作的核心。
包括培养基制备、培养基储存、培养基质量控制。
2.1培养基制备2.1.1根据既定的目的选用正确的培养基2.1.2参考生产商的COA和说明书:关于配制、储存和菌种选用等。
2.1.3所用的水应为去离子水或蒸馏水,应记录用量。
2.1.4成分的称量应用校准过的天平,根据称量量的不同选择合适的天平。
使用清洁的容器和工具(<1051>玻璃器皿清洁)以防外来污染和抑制剂。
应记录称量量。
2.1.5加热帮助溶解时应防止过度加热,通常培养基颜色变深说明加热过度了。
2.1.6灭菌条件:●灭菌参数应验证,验证应同时考虑无菌和培养基生长能力●采用高压蒸汽灭菌或过滤灭菌。
湿热灭菌应考虑装载分布,加热速度过慢会使培养基过度加热,通常为121℃15min,此时间是指培养基温度达到121℃后15min。
●保证最低SAL,在无菌和过度加热之间平衡。
●与污染物分开灭菌●消毒结束后,不应在灭菌柜中储存培养基,过度加热会影响培养基颜色、澄清、pH和凝固能力。
2.2培养基储存2.2.1制备培养基的储存●琼脂培养基易受冷冻影响,低于0℃会破坏凝胶结构。
●应避光避热,密封防止水分蒸发(建议使用螺旋盖的瓶子密封,保存时间长)。
2.2.2培养基融化●不能超过一次,以防过度加热或潜在污染。
●融化可采用水浴、流通蒸汽或微波炉加热。
微波炉加热宜采用少量多次,以防过度。
●培养基融化后在40-50℃水浴中储存不应超过8小时,浇制平皿前应擦干以防污染。
2.2.3培养基标示●培养基名称●批号、制备批号●制备日期、有效期2.2.4有效期确定●根据成分、配方、容器、储存条件等确定●有生长能力试验数据支持2.2.5应在验证过的条件下储存2.2.6重要区域环控用培养基必须●双层包装●最终灭菌,否则进行全数培养及用前检查2.2.7根据当地生物危险品安全程序处置过期培养基2.3培养基质控2.3.1灭菌后培养基检查2.3.1.1生长能力●每批制备的培养基均需进行●测试菌种根据药典,供应商说明书及环境中分离得到●测试不合格应进行调查,如无原因,或无有效纠正措施,不得使用该批号2.3.1.2无菌2.3.1.3pH2.3.1.4容器/平皿的完整性2.3.1.5抑制或指示能力2.3.2定期稳定性检查以确认储存有效期3菌种保藏3.1建立标准程序处理、保存菌种,防止污染和特性变化3.2使用前确认菌种身份和纯度3.3根据生产商说明书复苏菌种,使用接种技术3.4-70℃以下或冷冻干燥保存储备菌种,可延长保存周期3.5开启后不要重新冷冻菌种,剩余部分应弃置3.6传代次数(每接种一次即为一代)不超过5次3.7保藏时间根据具体保藏条件确定4设备维护4.1定期校准、保养4.2定期性能检查4.3定期清洁、消毒5实验室布局和运作5.1防止交叉污染5.2活动分区:无菌、环控样品的处理和培养应在无菌环境中进行5.3当发现微生物生长,后续的工作应转移至“阳性”区5.4环控设备应放置在待取样区域环境中并小心操作5.5应在受控条件下小心取样6样品处理6.1大部分样品、水或环控样品中的微生物对操作、储存环境敏感6.2减少样品,取样与测试间的时间间隔6.3长时间的转移需确认转移条件对测试及样品的影响6.4在无菌条件下,使用无菌技术取样,即使是非无菌样品6.5取样记录:样品信息、取样日期、送样日期、人员/部门、实验室样品接受及确认7培养基培养时间7.1短于3天,用小时表示7.2长于3天,用天表示(上午、下午,相同时间段)8人员培训8.1每个岗位应有相应培训要求,进行上岗前资质确认9实验室文件9.1微生物人员培训和能力确认(上岗资质)9.2设备验证、校准和维护9.3测试中的设备性能(如温度记录)9.4培养基制备、无菌检查、生长能力测试、选择性测试9.5培养基清单与控制9.6根据程序(SOP)进行测试的重要数据9.7数据和计算的确认9.8经QA人员或相应领导审核过的报告9.9超标结果调查10实验室结果维护10.1检测报告至少应包括:●日期●测试样品●微生物检验人员名字●程序编号●测试结果●偏差(如有)●测试参数(设备、菌种、培养基)●管理人员审核签名10.2数据纠错:错误的地方划一横线,签名和日期,必要时说明原因10.3测试结果●应包含平皿计数结果(如有)●数据分析方法应罗列在相关SOP中●对粘贴的图表、数据骑缝签名10.4存档:记录应存档并防止丢失,应有记录留存计划11测试结果解释11.1微生物数据有时不容易解释●与人类相关的微生物群落被广泛的用于许多测试●人员污染是始终被关心的问题●样品或环境中的微生物并非均匀分布●微生物检测可变范围大:可能在+/-0.5log10单位左右11.2不符合的结果可能是由2个原因造成的:实验室错误或产品不符合。
美国药典微生物限度检测

61微生物限度检测(MICROBIAL LIMIT TESTS)此章提供方法来检测可能存在的好氧微生物其他制药过程中可能出现的微生物的数量,包括原材料和成品中的。
如果经过验证确认可以得到相同或更好的检测结论,也允许采用自动化的检测方法。
在样品检测过程中须进行无菌操作。
若无特别说明,则“培养(incubate)”一词指在30—35℃的培养箱内培养24至48小时;“生长(growth)”一词用于专门的判定,说明“存在和可能存在活的微生物”。
准备实验 (Preparatory Testing)本章涉及实验结果的有效性取决于:提供的被检测样品本身在实验条件下,被充分证明不会抑制可能存在的微生物的生长。
因此,在准备样品时,需要正规的实验操作和符合要求的实验条件,接种稀释样品到含有以下(微生物)培养物的培养基:金黄色(奥里斯)葡萄球菌(Staphylococcus aureus),大肠埃希氏菌(Escherichia coli), 铜绿假单胞菌(Pseudomonas aeruginosa), 和沙门氏菌(Salmonella)。
方法如下:将用肉汤培养基培养24小时后的(微生物)不小于10-3稀释的微生物培养物,加1 ml(微生物)培养液到磷酸(盐)缓冲液(pH 7.2),液体大豆酪蛋白消化物培养基(Fluid Soybean-Casein Digest Medium),或者液体乳糖培养基(Fluid Lactose Medium)。
相应培养基培养失败则需要采取以下方法更改检测程序:(1)增加稀释液体积,检测样品加入量仍维持不变;或者(2)中和一定数量的干扰因子;或者(3)结合(1)、(2)得出适当条件,使接种物得以生长。
以下是一些物质的成分和浓度,该物质及浓度可用于加入培养基、阻止物质发挥抑菌作用:大豆卵磷脂(soy lecithin, 0.5%)或者聚山梨醇酯20(polysorbate 20, 4.0%)。
USP微生物限度检查 中文

USP微生物限度检查中文61)微生物限度检测(MICROBIAL LIMIT TESTS)此章提供方法来检测可能存在的好氧微生物其他制药过程中可能出现的微生物的数量,包括原材料和成品中的。
如果经过验证确认可以得到相同或更好的检测结论,也允许采用自动化的检测方法。
在样品检测过程中须进行无菌操作。
若无特别说明,则“培养(incubate)”一词指在30—35℃的培养箱内培养24至48小时;“生长(growth)”一词用于专门的判定,说明“存在和可能存在活的微生物”。
准备实验 (Preparatory Testing)本章涉及实验结果的有效性取决于:提供的被检测样品本身在实验条件下,被充分证明不会抑制可能存在的微生物的生长。
因此,在准备样品时,需要正规的实验操作和符合要求的实验条件,接种稀释样品到含有以下(微生物)培养物的培养基:金黄色(奥里斯)葡萄球菌(Staphylococcus aureus),大肠埃希氏菌(Escherichia coli), 铜绿假单胞菌(Pseudomonas aeruginosa), 和沙门氏菌(Salmonella)。
方法如下:将用肉汤培养基培养24小时后的(微生物)不小于10-3稀释的微生物培养物,加1 ml(微生物)培养液到磷酸(盐)缓冲液(pH 7.2),液体大豆酪蛋白消化物培养基(Fluid Soybean-Casein Digest Medium),或者液体乳糖培养基(Fluid Lactose Medium)。
相应培养基培养失败则需要采取以下方法更改检测程序:(1)增加稀释液体积,检测样品加入量仍维持不变;或者(2)中和一定数量的干扰因子;或者(3)结合(1)、(2)得出适当条件,使接种物得以生长。
以下是一些物质的成分和浓度,该物质及浓度可用于加入培养基、阻止物质发挥抑菌作用:大豆卵磷脂(soy lecithin, 0.5%)或者聚山梨醇酯20(polysorbate 20, 4.0%)。
奥氮平片美国药典36版质量标准USP36

4562Olanzapine / Official Monographs USP 36C U= concentration of Olanzapine in the Sample C U= concentration of Olanzapine in the Samplesolution (mg/mL)solution (mg/mL)Acceptance criteria:98.0%–102.0% on the anhydrous,F= relative response factor for each impurity from solvent-free basis Table 2Acceptance criteria:See Table 2.IMPURITIES•R ESIDUE ON I GNITION〈281〉:NMT 0.1%Table 2•H EAVY M ETALS, Method II〈231〉:NMT 10ppmRelative Relative AcceptanceRetention Response Criteria, Change to read:Name Time Factor NMT (%)Olanzapine related•O RGANIC I MPURITIES compound Ba0.3 2.30.10 Buffer:Dissolve 13g of sodium dodecyl sulfate inOlanzapine related1500mL of water. Add 5mL of phosphoric acid, andcompound A b0.8v2.3v USP360.10 adjust with a sodium hydroxide solution to a pH of 2.5.Olanzapine 1.0——Solution A:Acetonitrile and Buffer (48:52)Solution B:Acetonitrile and Buffer (70:30)•ChloromethylMobile phase:See Table 1.olanzapiniumchloride c (if pres-0.15•(RB 1-Jun-ent) 1.1 1.02012)Table 1Any individual, un-——Time Solution A Solution B specified impurity0.10(min)(%)(%)Total impurities——0.4 01000a2-Methyl-10H-thieno-[2,3-b][1,5]benzodiazepin-4[5H]-one.101000b5-Methyl-2-((2-nitrophenyl)amino)-3-thiophenecarbonitrile.200100•c1-Chloromethyl-1-methyl-4-(2-methyl-10H-benzo[b]thieno[2,3-e][1,2501004]diazepin-4-yl)piperazin-1-ium chloride.•(RB 1-Jun-2012)271000SPECIFIC TESTS351000•W ATER D ETERMINATION, Method I〈921〉[N OTE—A suitable solvent system for water determina-Edetate disodium solution:37mg/L of edetate diso-tion in ketones and aldehydes (e.g., Hydranal compos-dium in Buffer ite 5K-working medium K or Aquastar composite 5K-Diluent:Acetonitrile and Edetate disodium solution solvent KC or equivalent) is recommended.](40:60)Acceptance criteria:NMT 1.0%System suitability solution:20µg/mL of USPOlanzapine RS and 2µg/mL each of USP Olanzapine ADDITIONAL REQUIREMENTSRelated Compound A RS and USP Olanzapine Related•P ACKAGING AND S TORAGE:Preserve in well-closed contain-Compound B RS in Diluent ers, and store at room temperature.Standard solution:2µg/mL of USP Olanzapine RS in•USP R EFERENCE S TANDARDS〈11〉Diluent USP Olanzapine RSSample solution:0.4mg/mL of Olanzapine in Diluent USP Olanzapine Related Compound A RSChromatographic system5-Methyl-2-((2-nitrophenyl)amino)-(See Chromatography 〈621〉, System Suitability.)3-thiophenecarbonitrile.Mode:LC C12H9N3O2S259.28Detector:UV 220 nm USP Olanzapine Related Compound B RSColumn:4.6-mm × 25-cm; 5-µm packing L72-Methyl-10H-thieno-[2,3-b][1,5]benzodiazepin-4[5H]-Temperatures one.Column:35°C12H10N2OS230.29Sample:5°Flow rate:1.5mL/minInjection volume:20µLSystem suitabilitySample:System suitability solution Olanzapine Tablets[N OTE—Identify the peaks using the Relative RetentionTime values given in Table 2.]DEFINITIONSuitability requirements Olanzapine Tablets contain NLT 90.0% and NMT 110.0% Resolution:NLT 3.0 between olanzapine related com-of the labeled amount of olanzapine (C17H20N4S).pound A and olanzapineTailing factor:NMT 1.5 for the olanzapine peak IDENTIFICATIONRelative standard deviation:NMT 2.0% from fourreplicate injections for the olanzapine peakChange to read:AnalysisSamples:Standard solution and Sample solution•I NFRARED A BSORPTION〈197S〉Calculate the percentage of each impurity in the portionStandard solution:v30v USP36 mg/mL of USP Olanzapine of Olanzapine taken:RS in chloroformResult = (r U/r S) × (C S/C U) × (1/F) × 100Sample solution:Dissolve a quantity of powdered Tab-lets, equivalent to 30mg of olanzapine, in 30mL of r U= peak response of each impurity from the chloroform, and filter. Evaporate completely to dryness Sample solution with the aid of a current of air. Redissolve the residue in r S= peak response of olanzapine from the1mL of chloroform.Standard solutionC S= concentration of USP Olanzapine RS in theStandard solution (mg/mL)USP 36Official Monographs / Olanzapine4563ASSAY Standard solution:An amount, in mg, corresponding tothe Tablet label claim, of USP Olanzapine RS in1000mL of Medium. Transfer 5.0mL of this solution to Change to read: a tube, and add 2.0mL of Mobile phase.Sample solution:Pass a portion of the solution under •P ROCEDURE test through a suitable filter of 0.45-µm pore size.•[N OTE—A few drops of acetonitrile, not to exceed 5%Transfer 5.0mL of the filtrate to a tube, and add 2.0mL of the final volume, may be added to the Standard of Mobile phase.solution and Sample solution before final dilution to Chromatographic systemreduce foaming.]•(RB 1-Jul-2012)(See Chromatography 〈621〉, System Suitability.) Buffer 1:6.9g/L of monobasic sodium phosphate. Ad-Mode:LCjust with phosphoric acid to a pH of 2.5.Detector:UV 260 nmBuffer 2:12g/L of sodium dodecyl sulfate in Buffer 1Column:4.6-mm × 15-cm; 5-µm packing L10Mobile phase:Acetonitrile and Buffer 2 (1:1)Flow rate:1.5mL/minSystem suitability solution:0.1mg/mL of USP Injection volume:50µLOlanzapine RS and 0.01mg/mL of USP Olanzapine Re-System suitabilitylated Compound A RS in Mobile phase Sample:Standard solutionStandard solution:0.1mg/mL of USP Olanzapine RS in Suitability requirementsMobile phase Relative standard deviation:NMT 2.0%Sample solution:Transfer a known quantity of Tablets Analysis•(NLT 5)•(RB 1-Jul-2012), equivalent to NLT 25mg of Calculate the percentage of olanzapine (C17H20N4S) olanzapine, to a suitable volumetric flask. Dilute with dissolved:Mobile phase to volume, mix, and sonicate for 10 min.Centrifuge a portion of this solution, and dilute the Result = (rU/r S) ×C S× (V/L) × 100 clear supernatant with Mobile phase to obtain a solutioncontaining about 0.1mg/mL of olanzapine. [N OTE—Agi-r U= peak response from the Sample solutiontation of the flask may be necessary before sonication r S= peak response from the Standard solution to prevent Tablets from adhering to the flask, making C S= concentration of USP Olanzapine RS in the disintegration and dissolution difficult.]Standard solution (mg/mL)Chromatographic system V= volume of Medium, 900mL(See Chromatography 〈621〉, System Suitability.)L= label claim (mg/Tablet)Mode:LC Tolerances:NLT 80% (Q) of the labeled amount of Detector:UV 260 nm olanzapine (C17H20N4S) is dissolved.Column:4.6-mm × 15-cm; 5-µm packing L7•U NIFORMITY OF D OSAGE U NITS〈905〉:Meet theFlow rate:1.5mL/min requirementsInjection volume:20µLIMPURITIESSystem suitabilitySamples:System suitability solution and Standardsolution Change to read:[N OTE—The relative retention times for olanzapine re-lated compound A and olanzapine are 0.89 and 1.0,•O RGANIC I MPURITIESrespectively.]•[N OTE—A few drops of acetonitrile, not to exceed 5% Suitability requirements of the final volume, may be added to the Standard Resolution:NLT 2.0 between olanzapine and solution and Sample solution before final dilution to olanzapine related compound A, System suitability reduce foaming.]•(RB 1-Jul-2012)solution Buffer 1:3.3mL/L of phosphoric acid. Adjust with 50% Tailing factor:NMT 1.8, Standard solution sodium hydroxide to a pH of 2.5.Relative standard deviation:NMT 2.0%, Standard Buffer 2:8.7g/L of sodium dodecyl sulfate in Buffer 1 solution Buffer 3:18.6mg/L of edetate disodium (EDTA) in Analysis Buffer 2Samples:Standard solution and Sample solution Solution A:Acetonitrile and Buffer 2 (12:13)Calculate the percentage of olanzapine (C17H20N4S) in Solution B:Acetonitrile and Buffer 2 (7:3)the portion of Tablets taken:Diluent:Acetonitrile and Buffer 3 (2:3)System suitability solution:20µg/mL of USP Result = (r U/r S) × (C S/C U) × 100Olanzapine RS, and 2µg/mL each of USP OlanzapineRelated Compound B RS and USP Olanzapine Related r U= peak response from the Sample solutionCompound C RS in Diluentr S= peak response from the Standard solutionStandard solution:2µg/mL of USP Olanzapine RS inC S= concentration of USP Olanzapine RS in theDiluentStandard solution (mg/mL)Sensitivity solution:0.4µg/mL of USP Olanzapine RS inC U= concentration of olanzapine in the SampleDiluent from the Standard solutionsolution (mg/mL)Sample solution:Transfer a known quantity of Tablets Acceptance criteria:90.0%–110.0%to a suitable volumetric flask, and dilute with Diluent to PERFORMANCE TESTS volume to obtain a solution containing either 375 or •D ISSOLUTION〈711〉500µg/mL of olanzapine (based on the label claim).Medium:0.1 N hydrochloric acid; 900mL Centrifuge a portion of this solution, and use the super-Apparatus 2:50 rpm natant. [N OTE—Immediate agitation of the flask may be Time:30 min necessary to prevent Tablets from adhering to the flask, Mobile phase:10g/L of ammonium acetate in a mix-making dissolution and disintegration difficult. [C AU-ture of methanol and water (2:3). Adjust with hydro-TION—Do not sonicate.] The Sample solution is stable for chloric acid to a pH of 4.0.12 h at room temperature and 48 h if refrigerated.]4564Olanzapine / Official MonographsUSP 36Mobile phase:See Table 1.Table 2 (Continued)Relative Relative Acceptance Table 1Retention Response Criteria,NameTimeFactorNMT (%)Time Solution ASolution BAny individual un-(min)(%)(%)specified impuri-—01000ty1.00.20101000Total impurities—— 1.5200100a (Z )-4-(4-Methylpiperazin-1-yl)-3-(2-oxopropylidene)-1H -benzo[b ][1,2501004]diazepin-2(3H )-one.271000b 2-Methyl-10H -thieno-[2,3-b ][1,5] benzodiazepin-4[5H ]-one.c (Z )-1-{4-(4-Methylpiperazin-1-yl)-2-thioxo-1H -benzo[b ][1,4]diazepin-351003(2H )-ylidene}propan-2-one.d 2-Methyl-4-(4-methylpiperazin-1-yl)-10H -benzo[b ]thieno[2,3-e ][1,Chromatographic system4]diazepine 4’-N -oxide.(See Chromatography 〈621〉, System Suitability .)Mode:LCADDITIONAL REQUIREMENTSDetector:UV 220 nm•P ACKAGING AND S TORAGE : Preserve in tight, light-resistant Column:4.6-mm × 25-cm; 5-µm packing L7containers, and store at controlled room temperature.Column temperature:35°•USP R EFERENCE S TANDARDS 〈11〉Flow rate:1.5mL/min USP Olanzapine RSInjection volume:20µL USP Olanzapine Related Compound A RS System suitability5-Methyl-2-((2-nitrophenyl)amino)-Samples:System suitability solution , Standard solution ,3-thiophenecarbonitrile.and Sensitivity solution C 12H 9N 3O 2SSuitability requirementsUSP Olanzapine Related Compound B RSResolution:NLT 3.0 between olanzapine and2-Methyl-10H -thieno-[2,3-b ][1,5]benzodiazepin-4[5H ]-olanzapine related compound C, System suitability one.solutionC 12H 10N 2OSTailing factor:NMT 1.5 for the olanzapine peak, Sys-USP Olanzapine Related Compound C RStem suitability solution2-Methyl-4-(4-methylpiperazin-1-yl)-10H -benzo[b ]thieno Relative standard deviation:NMT 2.0%, Standard [2,3-e ][1,4]diazepine 4′-N -oxide.solutionC 17H 20N 4OS 328.43Signal-to-noise ratio:NLT 10, Sensitivity solution AnalysisSamples:Standard solution and Sample solutionCalculate the percentage of each impurity in the portion of Tablets taken:Olanzapine and Fluoxetine CapsulesResult = (r U /r S ) × (C S /C U ) × (1/F ) × 100DEFINITIONOlanzapine and Fluoxetine Capsules contain an amount of r U= peak response of each impurity from theolanzapine and fluoxetine hydrochloride equivalent to Sample solutionNLT 90.0% and NMT 110.0% each of the labeled r S = peak response from the Standard solution amount of olanzapine (C 17H 20N 4S) and fluoxetine C S = concentration of USP Olanzapine RS in the(C 17H 18F 3NO).Standard solution (µg/mL)C U = concentration of olanzapine in the SampleIDENTIFICATIONsolution (µg/mL)•The retention times of the major peaks in the Sample solu-F = relative response factor for each impurity listedtion correspond to those in the Standard solution , as ob-in Table 2tained in the Assay.Acceptance criteria:See Table 2.ASSAY•P ROCEDURETable 2Buffer:37mg/L of disodium ethylenediaminetetraace-Relative Relative Acceptance tate in water. Add 3.3mL of phosphoric acid, and ad-Retention Response Criteria,just with 50% sodium hydroxide to a pH of 2.5. Dis-Name TimeFactorNMT (%)solve 8.7g of sodium dodecyl sulfate in the resulting Olanzapine solution.lactam a0.26 1.00.50Mobile phase:Acetonitrile and Buffer (1:1)Olanzapine relat-•0.50•(RB 1-Standard solution:0.12mg/mL of USP Olanzapine RS ed compound B b 0.30 2.3Jul-2012)and 0.45mg/mL of USP Fluoxetine Hydrochloride RS in Olanzapine thio-Mobile phaselactam c0.34 1.00.50Sample solution:0.06–0.18mg/mL of olanzapine and 0.25-0.5mg/mL of fluoxetine in Mobile phase from a Olanzapine relat-counted number of Capsules ed compound C d 0.83v0.71v USP360.50Chromatographic systemOlanzapine1.0——(See Chromatography 〈621〉, System Suitability .)a (Z )-4-(4-Methylpiperazin-1-yl)-3-(2-oxopropylidene)-1H -benzo[b ][1,Mode:LC4]diazepin-2(3H )-one.Detector:UV 227 nmb 2-Methyl-10H -thieno-[2,3-b ][1,5] benzodiazepin-4[5H ]-one.Column:4.6-mm × 7.5-cm; 3.5-µm packing L7c (Z )-1-{4-(4-Methylpiperazin-1-yl)-2-thioxo-1H -benzo[b ][1,4]diazepin-Column temperature:40°3(2H )-ylidene}propan-2-one.d 2-Methyl-4-(4-methylpiperazin-1-yl)-10H -benzo[b ]thieno[2,3-e ][1,4]diazepine 4’-N -oxide.。
美国药典USP36微生物限度检查

USP36 1117 优良微生物检测规范(中英文1/ 2)2013-08-09 15:30:46| 分类:USP|举报|字号订阅1117 MICROBIOLOGICAL BEST LABORATORY PRACTICES 优良微生物检测规范INTRODUCTION 介绍Good laboratory practices in a microbiology laboratory consist of activities that depend on several principles: aseptic technique, control of media, control of test strains, operation and control of equipment, diligent recording and evaluation of data, and training of the laboratory staff. Because of the inherent risk of variability in microbiology data, reliability and reproducibility are dependent on the use of accepted methods and adherence to good laboratory practices.优良微生物检测规范由一些活动组成,这些活动依赖于几个基本要素:无菌技术、培养基控制、检测用菌株控制、设备操作和控制、完善的记录和数据评估、化验室员工的培训。
由于微生物数据具有天生的不确定性,数据的可靠性和重复性取决于是否使用被接受的方法,以及是否严格遵守化验室规范。
MEDIA PREPARATION AND QUALITY CONTROL 培养基制备和质量控制Media Preparation 培养基制备Culture media are the basis for most microbiological tests. Safeguarding the quality of the media is therefore critical to the success of the microbiology laboratory. Media preparation, proper storage, and quality control testing can ensure a consistent supply of high-quality media.培养基是大多数微生物测试的基础。
纯化水微生物限度检查方法优选_金宝华
广等特点[4]。冯氏等[5]发现腹针治疗落枕 快速有效。笔者采用了薄智云教授治疗落 枕的规范处方,中脘疗头部诸疾,商曲位 于腹全息图的颈部支出处与颈部相应,治 疗头颈部的疾病,商曲还是足少阴肾经的 经穴,肾与膀胱相表里,足太阳膀胱经又 循颈项而至背,故取商曲而治颈项。滑肉 门是腹四关四穴中的两穴,居神阙之上, 为治疗颈、肩、肘、腕等诸关节疾病的要 穴,有滑利关节、肌肉、筋、脉的作用。 且薄氏在临床中发现患侧的商曲疗效好于 健侧,故取患侧的商曲配患侧的滑肉门。 中药熏蒸疗法是一种利用药物加水煮沸产 生的气体熏蒸患处或穴位来治疗疾病的方 法,是以热药蒸汽为治疗因子的化学、物 理综合疗法,具有药力和热力双重作用, 在临床中对痹证导致的关节肿胀、疼痛和 活动受限、风湿和类风湿疾病、产后怕 风、全身关节疼痛以及病程迁移、反复发 作、经久不愈的患者疗效显著[6]。本研究 使用的中药熏蒸处方中肉桂、桂枝温通经 脉,红花、赤芍、丹参、川芎活血化瘀, 羌活、川牛膝、伸筋草通经络,木香行 气,诸药合用共奏温经通络、理气活血之 功,加水煮沸熏蒸,具有药力和热力双重 作用,共奏温通活血之效。运用中药熏蒸
附表3 验证用菌液表
典型代表菌种 大肠埃希菌 铜绿假单胞菌 金黄色葡萄球菌 枯草芽孢杆菌 白色念珠菌
黑曲霉
菌株编号(CMCC) CMCC(B)44102 CMCC(B)10104 CMCC(B)26003 CMCC(B)63501 CMCC(F)98001 CMCC(F)98003
附表4 美国药典、中国药典与欧洲药典纯化水微生物限度检查方法对比
能力之间是否存在显著性差异。 以纯化水微生物检查方法为因子(使
用字母 A 表示),使用不同检查方法(不 同的因子水平,见附表5)获得的微生物计 数结果。 3.3.1.2 分析方法 方差分析(ANOVA): 又称变异数分析或F检验,其目的是推断两
美国药典注射用水检验规程

目的使注射用水的检验操作标准化、规范化,保证注射水的质量,从而保证产品质量。
范围适用于注射用水的检验职责由化验室起草;部门经理及相关人员审核;质量总监批准;化验员执行。
内容1编制依据USP362定义注射用水是通过蒸馏或一个特殊的净化过程的来的水资源,在净化过程中更进一步去除水中的化学物质和微生物。
它的原水是符合美国环境保护署、欧盟、日本国家饮用水条例或世界卫生组织指标的饮用水。
不含任何添加剂。
3执行标准《注射用水质量标准》。
4检查4.1细菌内毒素4.1.1鲎试剂灵敏度试验:鲎试剂用于试验前做4次平行试验标记鲎试剂的灵敏度λ,用EU / ml表示。
4.1.1.1试验内容:根据鲎试剂灵敏度的标示值(λ),将细菌内毒素工作标准品用细菌内毒素检查用水溶解,在室温放置的30-60分钟里,每5-10分钟混匀一次,然后制成2λ、λ、0.5λ和0.25λ四个浓度的内毒素标准溶液,每稀释一步均应在旋涡混合器上混匀30秒钟。
取分装有0.2ml/瓶鲎试剂安瓿16支,分别加入0.2ml不同浓度的内毒素标准溶液,每一个内毒素浓度平行做4管。
将试管中溶液轻轻混匀后,封闭管口,垂直放入37℃±1℃的恒温器中,保温60分钟±2分钟。
4.1.1.2结果判断:将试管从恒温器中轻轻取出,缓缓倒转180°,若管内形成凝胶,并且凝胶不变形、不从管壁滑脱者为阳性。
保温和拿取试管过程应避免受到振动造成假阴性结果。
当所有的重复试验最低浓度0.25λ为阴性,每组试验方为有效。
每组的终点浓度是内毒素产生凝集反应的最低浓度。
按下式计算反应终点浓度的几何平均值,即为鲎试剂灵敏度的测定值(λ c )。
λc =lg-1(∑X /4)X为反应终点内毒素浓度的对数值。
反应终点浓度是指系列递减的内毒素浓度中最后一个呈阳性结果的浓度。
当λc在0.5 λ -2.0λ(包括0.5λ和2.0λ)时,方可以用于细菌内毒素检查并以λ为该鲎试剂的灵敏度。
美国药典36版目录

检测和含量分析的一般要求
<1>
注射剂
<3>
局部和经皮的药物产品-产 品质量检测
<11>
USP对照标准品
检测和含量分析的仪器
<16>
自动分析方法
<17>
处方容器具标签
<21>
测温仪
容量装置,如容量瓶、移
<31>
液管、滴定管,各种规格
的误差限度
<41>
砝码和天平
微生物检测
<51>
抗菌效力检测
药典协调(指欧洲药典、 美国药典、日本药局方三 方机构讨论协调的原则和 方法)
大批量药用辅料的分配原 则
无菌产品包装:完整性评 价
灭菌试验:隔离系统的验 证
灭菌:化学和物理化学的 指示剂与积分仪
药典收载品种的灭菌和灭 菌保证 片剂脆性检查 片剂破碎力 药品灭菌终点的放行参数 微生物替代方法的验证 分析方法的转移 药典方法的验证
营养和饮食增补剂中不允 许存在的微生物(如金葡 菌、沙门氏菌、大肠杆菌 、梭状芽胞杆菌属)检查 法
<2023>
<2030> <2040> <2091> <2750>
非灭菌的营养和饮食增补 剂中的微生物特征
植物来源物品的增补资料 崩解和溶解膳食补充剂 膳食补充剂的称量多样性 膳食补充剂的生产原则
监控装置:时间、温度、 湿度 近红外分光光度法 拉曼(Raman)分光光度 法 药品命名法 核酸类技术—基本 核酸类技术-提取,选择和 排序 核酸类技术-放大 核酸类技术-基因芯片 核酸类技术-基因分型
USP微生物限度检查中文

USP微生物限度检查中文USP微生物限度检查中文61)微生物限度检测(MICROBIAL LIMIT TESTS)此章提供方法来检测可能存在的好氧微生物其他制药过程中可能出现的微生物的数量,包括原材料和成品中的。
如果经过验证确认可以得到相同或更好的检测结论,也允许采用自动化的检测方法。
在样品检测过程中须进行无菌操作。
若无特别说明,则“培养(incubate)”一词指在30—35℃的培养箱内培养24至48小时;“生长(growth)”一词用于专门的判定,说明“存在和可能存在活的微生物”。
准备实验 (Preparatory Testing)本章涉及实验结果的有效性取决于:提供的被检测样品本身在实验条件下,被充分证明不会抑制可能存在的微生物的生长。
因此,在准备样品时,需要正规的实验操作和符合要求的实验条件,接种稀释样品到含有以下(微生物)培养物的培养基:金黄色(奥里斯)葡萄球菌(Staphylococcus aureus),大肠埃希氏菌(Escherichia coli), 铜绿假单胞菌(Pseudomonas aeruginosa), 和沙门氏菌(Salmonella)。
方法如下:将用肉汤培养基培养24小时后的(微生物)不小于10-3稀释的微生物培养物,加1 ml(微生物)培养液到磷酸(盐)缓冲液(pH 7.2),液体大豆酪蛋白消化物培养基(Fluid Soybean-Casein Digest Medium),或者液体乳糖培养基(Fluid Lactose Medium)。
相应培养基培养失败则需要采取以下方法更改检测程序:(1)增加稀释液体积,检测样品加入量仍维持不变;或者(2)中和一定数量的干扰因子;或者(3)结合(1)、(2)得出适当条件,使接种物得以生长。
以下是一些物质的成分和浓度,该物质及浓度可用于加入培养基、阻止物质发挥抑菌作用:大豆卵磷脂(soy lecithin, 0.5%)或者聚山梨醇酯20(polysorbate 20, 4.0%)。
美国微生物检查要求及微生物监控体系

USDA FSIS对出口国肉类微生物检测要求
1、商业无菌产品:这类产品主要应注意避免使用不合格的原 料,防止金黄色葡萄球菌在加工前的繁殖与生长。应对这类 产品进行感官检查。实验室应能够具备检测芽孢和非芽孢菌、 需氧和厌氧菌、嗜温的和耐温的细菌以及罐体的密封性检查 的能力。
2、对即食产品的微生物检测要求:美国对即食产品进行分类: A、加工过程中同时使用后致死处理和抗菌物质或其他杀菌手 段;B、使用上述手段的一种;C、 仅在加工过程中采用卫生 措施。对上述产品的加工过程中使用杀菌手段的效果有一定 的指标要求。
陽性反應者作 進一步証實
生化反應鑒定 血清凝集鑒定 或 EHEC 乳膠凝集試驗
大腸杆菌 O157 : H7 檢測程序
USDA FSIS对实验室质量控制的要求——检测方法
总大肠杆菌
一般由企业自行检测,随机抽样全鸡,并 将整只全鸡放到塑料袋中,加入400ml BPW, 震荡,取缓冲液检测,用计数法; 要求检测13个样品,其中大于100个/g的样 品数不超过3个,大于1000个/g的样品数0个 (n=13,m=3,M=0),如果超过上述标准,则 可判定企业对大肠杆菌污染控制不佳。 罐装食品不做大肠杆菌检测。
Half-Fraser肉汤 Fraser肉汤
SN标准
MBP
EB增菌液
MMA琼脂、OXA琼脂
GB标准
EB增菌液、LB1 肉汤、LB2肉汤
MMA琼脂
USDA FSIS对实验室质量控制的要求——检测方法
大肠杆菌O157:H7
USDA FSIS及企业一般只对绞碎牛肉
检测该项目。
SN/T 0937-2000 肠出血性大肠杆菌 O157
SN0169-92: 25g肉类样品十倍稀释LSTEC EMB营 养琼脂生化试验(色氨酸肉汤、MR-VP试验、Koser氏枸橼酸盐 肉汤、LST肉汤、革兰氏染色)(3管-MPN法) SN/T 1059.2-2002:进出口食品中的大肠菌群、大肠杆菌计数 滤膜MUG法 USDA FSIS推荐方法:全鸡十倍稀释 3管-MPN法(AOAC 17.2.01-17.2.02,AOAC 17.4.01)、3M Platefilm法(AOAC 17.3.04)滤膜计数法(AOAC 17.3.09) 比较: 1、SN方法的取样和制样与USDA FSIS方法不同; 2、3管-MPN法和滤膜计数法基本步骤大同小异; 3、USDA FSIS推荐的方法不仅有传统方法,而且有现代化的 快速检测技术( 3M Platefilm法),但青岛局食品实验室已将 3M Platefilm法制订为实验室的SOP.
USP微生物试验说明

美國藥典微生物試驗說明Essentials of USP Microbiological Testing研討會大綱I.基礎微生物學II.良好微生物實驗室規範III.藥典協同之改變IV.IV.滅菌滅菌滅菌與無菌保證與無菌保證V.微生物方法之確效微生物學研究微小生命形式的生物學分支研究微小生命形式的生物學分支。
典型的微小生命形式是單細胞的生命形式的微小生命形式是單細胞的生命形式,,也包括非常少也包括非常少量量的多細胞生物體 新陳代謝基本需求能量(如光、硫、一氧化碳或氨一氧化碳或氨))碳、氮、鈣、磷、硫、鎂、鉀和鈉 水(即使真菌也需要0.6 Aw 以上的游以上的游離離水)分類學生物環境多樣性嗜低溫菌嗜中溫菌嗜高溫菌生物環境多樣性嗜酸性菌嗜中性菌嗜鹼性菌依DNA在細胞中的情況可將細胞分為兩大類:真核細胞Eukaryotic cells原核細胞Prokaryotic cells原核生物Prokaryotic cells大部分細菌對人體無害如: 乳酸菌但, 也有微生物會致病細菌(分2界) 古細菌 真細菌原核生物革原核生物革蘭蘭氏分類氏分類法法Hans C.J. Gram –丹麥細菌學家(1853-1938).發現發現了了細胞學上的染色和染色技術細胞學上的染色和染色技術((革蘭氏染色染色),),),用於區分細菌的分用於區分細菌的分用於區分細菌的分類類學組群學組群。
脂多糖(相對耐熱)如果進入人體,能導致發燒、白血球減少、腹瀉、休克(熱原Pyrogen)(內毒素Endotoxin)革蘭氏陽性結構革蘭氏陰性結構什麼是芽孢?在惡劣惡劣的環境條件下產生的有再生能的環境條件下產生的有再生能的環境條件下產生的有再生能力力的細胞或細胞群的細胞或細胞群。
特徵特徵::–厚壁–耐惡劣惡劣環境環境–非常緩慢的代謝速非常緩慢的代謝速度度分4界原生動物 動物植物真菌真菌-特徵皆為化學異異營菌皆為化學具強大的酵素 (腐生或寄生腐生或寄生) ) ) 具強大的酵素pH5))酸性的生活環境((pH5 比細菌比細菌更更酸性的生活環境高鹽、、高糖環境中生長高鹽黴菌和蕈是多細胞酵母是單細胞新陳代謝Metabolism 依據分解代謝是否能夠使用O2為什麼了解微生物對新陳代謝的需求很重要?為的是想培養微生物生長更為的是防止微生物生長USP微生物相關務必遵守章節<51> 防腐效力/Antimicrobial Preservatives –Effectiveness耐受力力測試<55> 生物指示劑–耐受Biological Indicators –Resistance Performance Tests微生物計數數試驗非無菌產品的微生物檢驗::微生物計<61> 非無菌產品的微生物檢驗Microbiological Examination of Nonsterile Products:Microbial Enumeration Tests<62> 特定菌的微生物檢查/ Microbiological Proceduresfor Absence of Specified Microorganisms<71> 無菌試驗/ Sterility Tests<85> 細菌內毒素試驗Bacterial Endotoxins TestUSP微生物相關章節建議遵循<1035> 滅菌生物指示劑<1072> 消毒劑與殺菌劑<1111> 非無菌產品的微生物評估<1112> 水活性應用<1116> 清淨室和其他管制環境的微生物評估<1117> 微生物實驗室規範 <1207> 無菌產品包裝–完整性評估 <1209> 滅菌–化學和物理化學指示劑,及綜合指示劑<1211> 滅菌與無菌保證<1222> 最終滅菌藥品–參數放行 <1223> 微生物方法確效<1227> 藥物的微生物回收率確效 <1231> 製藥用水<2021> 微生物計數試驗–營養和膳食補充劑<2022> 特定菌的微生物檢查–營養和膳食補充劑<2023> 非無菌營養和膳食補充劑的微生物評估EP & USP & JP 藥典方法的國際協合International Harmonization of Pharmacopoeia時限–現行的EP 只維持到2008年12月31日–USP 將維持至2009年4月影響行適用性試驗並需重新進行–各藥廠將必需提早因應,並需重新進批產品來來測試其一致性.( Suitability Test),以至少3 批產品此協合後的方法不不僅在產品( non-sterile)的稀釋液此協合後的方法及預試驗,生長試驗上有改變,在部份培養基的組成有所改變,甚至新增一些檢驗項目與培養基,在有些品管菌株及接種量量都有改變品管菌株及接種微生物限量(USP <61> & EP 2.6.12) Microbial Enumeration TestTAMC= Total aerobic microbial count (on TSA, incl. fungi)TYMC= Total combined yeast/mould count (on Sab.4%-Dextrose Agar, incl. bacteria)特定微生物USP <62> & EP 2.6.13 Tests for Specific Microorganisms目前–Escherichia coli–Salmonella–Pseudomonasaeruginosa–Staphylococcusaureus 協調後–Escherichia coli–Salmonella–Pseudomonas aeruginosa–Staphylococcus aureus–Clostridia 產芽孢梭菌–Bile-tolerant Gram-negative bacteria 耐膽鹽革耐膽鹽革蘭蘭氏陰性菌–Candida albicans白色白色念念珠菌Test for Escherichia coli:Presence/Absence (Current USP Method)Test for Escherichia coli:Presence/Absence (Harmonized Method)<1112> 水活性應用水活性是水活性是什什麼?–產品中水的蒸氣壓P 與相同溫與相同溫度度下純水中的蒸氣壓Po 的比的比率率。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
USP36 1117 优良微生物检测规范(中英文1/ 2)2013-08-09 15:30:46| 分类:USP|举报|字号订阅1117 MICROBIOLOGICAL BEST LABORATORY PRACTICES 优良微生物检测规范INTRODUCTION 介绍Good laboratory practices in a microbiology laboratory consist of activities that depend on several principles: aseptic technique, control of media, control of test strains, operation and control of equipment, diligent recording and evaluation of data, and training of the laboratory staff. Because of the inherent risk of variability in microbiology data, reliability and reproducibility are dependent on the use of accepted methods and adherence to good laboratory practices.优良微生物检测规范由一些活动组成,这些活动依赖于几个基本要素:无菌技术、培养基控制、检测用菌株控制、设备操作和控制、完善的记录和数据评估、化验室员工的培训。
由于微生物数据具有天生的不确定性,数据的可靠性和重复性取决于是否使用被接受的方法,以及是否严格遵守化验室规范。
MEDIA PREPARATION AND QUALITY CONTROL 培养基制备和质量控制Media Preparation 培养基制备Culture media are the basis for most microbiological tests. Safeguarding the quality of the media is therefore critical to the success of the microbiology laboratory. Media preparation, proper storage, and quality control testing can ensure a consistent supply of high-quality media.培养基是大多数微生物测试的基础。
保证培养基的质量因而成为微生物实验室成功的关键。
培养基的制备、合适的存贮和质量控制检测可以保证持续高质量培养基供应。
It is important to choose the correct media or components in making media based on the use of accepted sources or references for formulas. The manufacturer's formula and instructions for preparation routinely accompany dehydrated media andready-made media. Because different media types may have different preparation requirements (e.g., heating, additives, and pH adjustment), it is important to follow these instructions to ensure preparation of acceptable media quality. A certificate of analysis describing expiration dating and recommended storage conditions accompanies ready-made media, as well as the quality control organisms used in growth-promotion and selectivity testing of that media.在制备培养基过程中使用已接受的来源或配方标准,选择正确的培养基或成份是非常重要的。
一般生产商在供应培养基干粉和配制好的培养基时,其配方和配制指示都会随货发送。
由于不同的培养基类型可能有不同的配制要求(例如,加热、添加剂,和pH值调节),重要的一点是需要遵守其提供的配制指示以保证配制出的培养基质量。
如果是已制备好的培养基碟/瓶,随货会收到指明有效期和推荐的存贮条件的分析报告,以及促生产试验用微生物种类,和该培养基的选择性试验用微生物种类。
Water is the universal diluent for microbiological media. Purified Water is most often used for media preparation, but in certain cases the use of deionized or distilled water may be appropriate. Water of lesser quality should not be used for microbiological media preparation. The volume of the water used should be recorded.水是通用的微生物培养基稀释剂。
纯化水常用于培养基制备,但在某些情况下,也可以使用去离子水或蒸馏水。
更低品质的水不应用于微生物培养基制备。
水的用量应记录。
Consistent preparation of media requires accurate weighing of dehydrated media or media constituents. A calibrated balance with the appropriate weight range for the ingredients should be used (See Weighing on an Analytical Balance1251). Clean weighing containers and tools (such as spatulas) should be used to prevent foreign substances from entering the formulation. The weight of the components should be recorded.要保持培养基配制的一致性,需要对培养基干粉或培养基组份进行准确称量。
应使用在所需称量范围内经过校正的天平(见1251分析天平称量)。
应对称重容器和工具(例如料勺)进行清洁以保证无异物进入所配物料。
各成份的重量应进行记录。
Dehydrated media should be thoroughly dissolved in water before dispensing and sterilization. If heating is necessary to help dissolve the media, care should be taken not to overheat media, because all culture media, to a greater or lesser extent, areheat-sensitive. Equipment used in the preparation of media should be appropriate to allow for controlled heating, constant agitation, and mixing of the media.培养基干粉应在水中完全溶解,然后进行配制和灭菌。
如果需要加热助溶,要注意不能过热,因为所有的培养基,或多或少,都是对热敏感的。
用于培养基配制的设备应适当,以便控制加热、持续搅拌和培养基混合。
Darkening of media (Maillard-type reaction or nonenzymatic browning) is a general indication of overheating. When adding required supplements to media, adequate mixing of the medium after adding the supplement should be performed.培养基变黑(美拉德类型反应或非酶褐变)一般说明过热。
在向培养基中加入所需要的补充成分时,在加入后需要进行充分搅拌混合。
Preparation of media in poorly cleaned glassware can allow inhibitory substances to enter the media.在清洁不彻底的玻璃器皿中配制培养基会使得抑制性物质带入培养基。
Inhibitory substances can come from detergent residue after cleaning glassware or from prior materials used in the glassware. Be sure that the cleaning process removes debris and foreign matter, and that the detergent is thoroughly rinsed out with Purified Water. See Cleaning Glass Apparatus1051for additional guidance.抑制性物质可能来自于玻璃器皿中的清洁剂残留,或来自于玻璃器皿中上次所盛装的物料。
要保证清洗程序可以去除残渣和外来物质,并且清洁剂可以被纯化水彻底冲洗掉。
参见1051玻璃容器的清洁。
Sterilization of media should be performed within the parameters provided by the manufacturer or validated by the user. Commercially prepared media should provide documentation of the sterilization method used.培养基灭菌应在生产商提供的参数范围,或用户验证的参数范围内实施。
