药物合成反应课后翻译
药物合成反应(第三版)第二章课后翻译

第二章课后翻译Preparation of cyclopropane 1,1- dicarboxylic acid环丙烷1,1-二甲酸的制备(1). To a 1-L solution of aqueous 50% sodium hydroxide(Note 1), mechanically stirred in a 2-L, three-necked flask, was added, at 25°C, 114.0 g (0.5 mol) of triethylbenzylammonium chloride(TEBA三乙基苄基氯化铵)(Note 2).1L的50%氢氧化钠加入到2L的三口烧瓶中,加入TEBA三乙基苄基氯化铵114.0g(0.5mol)在25℃机械搅拌。
To this vigorously stirred suspension was added a mixture of 80.0 g (0.5 mol) of diethyl malonate and 141.0 g (0.75 mol) of 1,2-dibromoethane all at once.充分搅拌至混悬状,一次性加入丙二酸二乙酯80.0g(0.5mol)和1,2-二溴乙烷141.0个(0.75mol)的混合物。
The reaction mixture was vigorously stirred for 2 hr (Note 3).反应混合物强烈搅拌2小时。
The contents of the flask were transferred to a 4-L Erlenmeyer flask by rinsing the flask with three 75-mL portions of water.把烧瓶中的物质转移到4L的锥形瓶中,并用75ml清水洗涤烧瓶三次。
The mixture was magnetically stirred by dropwise addition of 1 L of concentrated hydrochloric acid.混合物在磁力搅拌下缓慢滴加浓盐酸。
药物合成之缩合反应翻译

药物合成反应——缩合反应翻译一、In a 3L round-bottomed flask with a reflux condenser are placed 625mL of 95 percent alcohol, 500mL of water, 500g (476ml, 4.7mol) of pure benzaldehyde, and 50g of sodium cyanide (96-98 percent). The mixture is then heated and kept boiling for one-half hour. In the course of about twenty minutes, crystals begin to separate from the hot solution. At the end of thirty minutes, the solution is cooled, filtered with suction, and washed with a little water. The yield of the dry crude benzoin, which is white or light yellow, is 450-460g (90-92 percent of the theoretical amount). In order to obtain it completely pure, the crude substance is recrystallized from 95 percent alcohol. 90g of crude material being dissolved in about 700ml of boiling alcohol; upon cooling, a yield of 83g of white, pure benzoin which melts at 129℃is obtained.二、In a 1L three-necked round-bottomed flask equipped with mechanical stirrer, short reflux condenser, and bent glass tube reaching below the surface of the liquid for the introduction of hydrogen chloride, are placed 50g(0.36mol) of p-nitrophenol, 650ml of concentrated hydrochloric acid, 5ml concentrated sulfuric acid, and 76g(1mol) of methylal. The mixture is stirred while the temperature is maintained at 70±2℃for 4-5 hours by means of a water bath. During this time hydrogen chloride is bubbled into the reaction mixture through the bent glass tube, and the excess gas is carried away through the refluxcondenser to a hood or gas absorption trap. The 2-hydroxy-5-nitrobenzyl chloride begins to separate as a solid about 1 hour after the beginning of the reaction. At the end of the mixture is cooled in ice for 1 hour whereby more crystals separate, after which the acid liquors are either filtered or decanted from the crystal. The 2-hydroxy-5-nitrobenzyl chloride is purified by recrystallization from 125ml of hot benzene. The yield is 46g (69% based on p-nitrophenol) of a white product melting at 129-130.翻译为:一、在一个3L圆底烧瓶中加入650mL 95%的乙醇,500mL水,476mL苯甲醛,50gNaCN(96-98%)并装上冷凝回流装置.混合物保持沸腾1个半小时,正常情况下大约20分钟有晶体从溶液中析出来。
药物合成反应第五章(翻译题)

药物合成反应第五章作业1)翻译通过Cu r ti u s 重排,从反-2,4-戊二烯酸合成苄基,反-1,3-丁二烯-氨基甲酸一个干燥的,配备磁性搅拌棒和温度计和压力平衡轴承氮入口滴液漏斗的,250毫升三口圆底烧瓶。
烧瓶用氮冲洗后,称取49克(0.50摩尔)的反-2,4 -戊二烯酸、80克(0.62摩尔)的N,N -二异丙基和300毫升的丙酮放于烧瓶中。
这个溶液被搅拌并用冰盐浴冷却至0℃。
55克(0.51m o l)氯甲酸乙酯的150毫升的丙酮溶液加入到烧瓶中,保持温度低于0℃超过30分钟。
把65g (1.0m o l)叠氮化钠的170ml的水冷冻溶液加到烧瓶中,在温度低于0°下保持20分钟,之后在0℃下继续搅拌30分钟。
这个烧瓶被额外在0℃下被搅拌10~15分钟,然后倒倒装有500m l冰水的2L的分液漏斗中。
用6份250m l的甲苯提取酰基叠氮。
结合提取得到的甲苯,用无水硫酸镁干燥,集中到一个体积约300毫升的水浴温度40-50℃旋转蒸发器中。
当甲苯溶液浓缩时,同时组装一个干燥的带有压力平衡滴液漏斗、一个简单的蒸馏头、一个加热地幔的和装有机械搅拌器的2L三口圆底烧瓶和称取43克(0.40摩尔)苯甲醇、250m g4 - 叔丁基邻苯二酚和200m l苯。
大约30m l的甲苯通过蒸馏除去微量的水份,蒸馏头用冷凝和有氮气保护的代替。
搅拌并加热氮气保护下,酰基叠氮的甲苯溶液被添加,超过30分钟。
红外线分析证实了,酰基叠氮化物和异氰酸酯的消失。
通过冰浴迅速把溶液冷却至温,在这之后,在10-30分钟全部转换成氨基甲酸酯类。
甲苯通过40-50℃水浴旋转蒸发仪迅速去除,产生一个黄色的固体残渣,将其溶解在95%的乙醇中,并可以在-25℃冷冻几个小时结晶。
得到的两种淡黄色晶体,熔点69-72℃,减压干燥,得到39-46g。
母液浓缩得到了一个油性残留物,放置在6× 80厘米,装有500克的硅胶和1:9(V/V)的洗脱乙酸乙酯- 正己烷的容器中。
(完整word版)药物合成反应(闻韧_第三版)课后翻译(word文档良心出品)

1、About 216–224 g. (1.62–1.68 moles) of powdered anhydrous aluminum chloride is added to a 1Lthree-necked flask.在1L的三口烧瓶中加入大约216-224g(1.62–1.68 moles)的无水三氯化铝。
While the free-flowing catalyst is stirred (Note 3), 81 g. (0.67 mole) of acetophenone is added from the dropping funnel in a slow stream over a period of 20–30 minutes. 自由流动的催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. 放热反应,假如滴加的酮不能被分散,就会变黑或是碳化。
When about one-third of the acetophenone has been added, the mixture becomes a viscous ball-like mass that is difficult to stir.当三分之一的乙酰苯被滴加,反应混合物变成一个很难搅拌的粘性的球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. 在这时,改用手动搅拌或快速滴加酮是非常必要的。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. 然而,速度不能太快,当反应温度超过180℃时。
闻韧版 药物合成反应 课后翻译

第六章
(1)、二吡啶三氧化铬
在一个干燥的装有密封机械搅拌器,温度计,和干燥管的1L的三颈烧瓶里面装入500毫升无水吡啶,搅拌,用冰浴冷却到大约15°。干燥管是定期拿开,将68克(0.68摩尔)无水三氧化铬在一个30分钟内通过瓶颈分次加入。氧化铬应增加在这样的速度,温度不超过20°,并以这种方式,迅速与吡啶氧化物混合,不粘附瓶内。随着氧化铬的加入,一种深黄色的,絮状沉淀物从吡啶中分离出来,混合物的粘度增加。当添加完后,这混合物
第四章
(1)、 在配有回流冷凝器的3L圆底烧瓶中加入625ml的95%酒精、500ml水、500g(476ml,4,7mol)的苯甲醛和50g 96-98%的氰化钠。混合物加热并保持沸腾1.5小时。在20分钟后晶体开始从热溶液中析出。在最后的30分钟,冷却溶液,抽滤并用少量水洗涤有450-460g白色或亮黄色的干燥的安息香。理论产率90-92%。为了得到纯度高的产品,粗产品要在酒精中重结晶,90g粗品溶解在700ml沸腾的酒精中,冷却, 得到83g熔点为129摄氏度的白色安息香纯品。
合并二氯甲烷溶液_可用稀盐酸,碳酸氢钠溶液和水洗涤,或直接通过助滤剂过滤,或通过色谱柱去除吡啶铬盐_痕迹。去除二氯甲烷获得该产品;少量残余吡啶可通过减少压力下去除。
(3)庚醛
在一个干燥,1L的装有机械搅拌器的三颈烧瓶中加入650ml无水二氯甲烷。开始搅拌,在室温下加入77.5g二吡啶三氧化铬,再一次性加入5.8g 1-庚醇。搅拌20分钟后,倒出上层溶液从这不溶性棕色胶状物中,并用3个100ml乙醚冲洗。乙醚和二氯甲烷的溶液相结合,并先后用300毫升5%氢氧化钠的水,100毫升5%的盐酸(注12),两个100毫升部分饱和碳酸氢钠,并最后用100毫升饱和氯化钠水溶液冲洗。无水硫酸镁干燥有机层,并通过蒸馏去除溶剂。在残余油通过Claisen缩合____减压蒸馏分离4.0-4.8克。 (70-84%)的庚醛,B.P. 80-84°(65毫米),n25D1.4094
药物合成反应闻韧_第三版课后翻译

1、About 216–224 g. (1.62–1.68 moles) of powdered anhydrous aluminum chloride is added to a 1Lthree-necked flask.在1L的三口烧瓶中加入大约216-224g(1.62–1.68 moles)的无水三氯化铝。
While the free-flowing catalyst is stirred (Note 3), 81 g. (0.67 mole) of acetophenone is added from the dropping funnel in a slow stream over a period of 20–30 minutes. 自由流动的催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. 放热反应,假如滴加的酮不能被分散,就会变黑或是碳化。
When about one-third of the acetophenone has been added, the mixture becomes a viscous ball-like mass that is difficult to stir.当三分之一的乙酰苯被滴加,反应混合物变成一个很难搅拌的粘性的球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. 在这时,改用手动搅拌或快速滴加酮是非常必要的。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. 然而,速度不能太快,当反应温度超过180℃时。
药物合成反应(第三版)第一,二 三章课后翻译

第二章课后翻译Preparation of cyclopropane 1,1- dicarboxylic acid环丙烷1,1-二甲酸的制备(1). To a 1-L solution of aqueous 50% sodium hydroxide(Note 1), mechanically stirred in a 2-L, three-necked flask, was added, at 25°C, 114.0 g (0.5 mol) of triethylbenzylammonium chloride(TEBA三乙基苄基氯化铵)(Note 2).1L的50%氢氧化钠加入到2L的三口烧瓶中,加入TEBA三乙基苄基氯化铵114.0g(0.5mol)在25℃机械搅拌。
To this vigorously stirred suspension was added a mixture of 80.0 g (0.5 mol) of diethyl malonate and 141.0 g (0.75 mol) of 1,2-dibromoethane all at once.充分搅拌至混悬状,一次性加入丙二酸二乙酯80.0g(0.5mol)和1,2-二溴乙烷141.0个(0.75mol)的混合物。
The reaction mixture was vigorously stirred for 2 hr (Note 3).反应混合物强烈搅拌2小时。
The contents of the flask were transferred to a 4-L Erlenmeyer flask by rinsing the flask with three 75-mL portions of water.把烧瓶中的物质转移到4L的锥形瓶中,并用75ml清水洗涤烧瓶三次。
The mixture was magnetically stirred by dropwise addition of 1 L of concentrated hydrochloric acid.混合物在磁力搅拌下缓慢滴加浓盐酸。
药物合成反应与设计翻译部分

药物合成反应与设计翻译部分(第三版闻韧主编)第一章翻译:About 216–224 g. (1.62–1.68 moles) of powdered anhydrous aluminum chloride is added to a 1Lthree-necked flask.在1L的三口烧瓶中加入大约216-224g(1.62–1.68 moles)的无水三氯化铝。
While the free-flowing catalyst is stirred (Note 3), 81 g. (0.67 mole) of acetophenone is added from the dropping funnel in a slow stream over a period of 20–30 minutes. 自由流动的催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. 放热反应,假如滴加的酮不能被分散,就会变黑或是碳化。
When about one-third of the acetophenone has been added, the mixture becomes a viscous ball-like mass that is difficult to stir.当三分之一的乙酰苯被滴加,反应混合物变成一个很难搅拌的粘性的球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. 在这时,改用手动搅拌或快速滴加酮是非常必要的。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. 然而,速度不能太快,当反应温度超过180℃时。
药物合成反应闻韧第三版课后翻译

1、About 216–224 g. –moles) of powdered anhydrous is added to a 1Lthree-necked flask.在1L的三口烧瓶中加入大约216-224g– moles)的无水三氯化铝。
While the free-flowing catalyst is stirred , 81 g. mole) of is added from the dropping funnel in a slow stream over a period of 20–30 minutes. 自由流动的催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. 放热反应,假如滴加的酮不能被分散,就会变黑或是碳化。
When about one-third of the has been added, the mixture becomes a viscous ball-like mass that is difficult to stir.当三分之一的乙酰苯被滴加,反应混合物变成一个很难搅拌的粘性的球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. 在这时,改用手动搅拌或快速滴加酮是非常必要的。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. 然而,速度不能太快,当反应温度超过180℃时。
药物合成反应(第三版_闻韧)第一章卤化反应

Organic Reactions for Drug Synthesis
例1.
C6H5 H
CC
H
COOC2H5
Br2 / CCl4
Br
C6H5
C
H C
H Br COOC2H5
C6H5 H CC
H Br COOC2H5
1. 卤素与烯烃的亲电加成反应
(1)反应历程: 第一步:卤正离子向π 键进攻,形成三员环卤正离子 或开放式碳正离子的过渡态。
R1 R3
R2
R4
δ +δ XX
Organic Reactions for Drug Synthesis
R1 R3 CC
R2 X R4
(1)
R1 R3
CC
R2
X R4
(2)
第二步:
反应类型
亲电加成 亲电取代 亲核取代 自由基反应
Organic Reactions for Drug Synthesis
常用的卤化剂 卤素(X2):Cl2、Br2
次卤酸(HOX):HOCl、HOBr
N-卤代酰胺:
如 N-溴(氯)代乙酰胺( NBA,NCA) N-溴(氯)代丁二酰亚胺(NBS,NCS)
Ph H CC
H CH3
NBS / DMSO / H2O
OH
Ph
H
CC
H
Br CH3
NBS / 干燥的DMSO
O H
Ph C C Br CH3
Organic Reactions for Drug Synthesis
五、卤化氢与烯烃的加成
药物合成反应 (第三版 闻韧) 课后答案Chapter 1 Halogenation Reaction
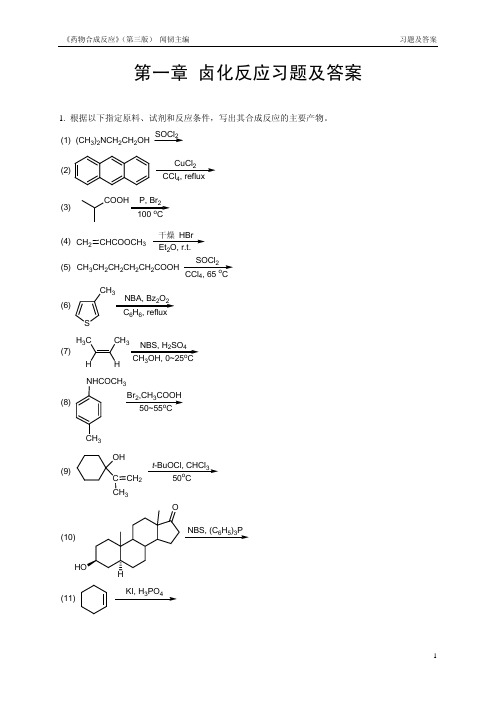
第一章 卤化反应习题及答案1. 根据以下指定原料、试剂和反应条件,写出其合成反应的主要产物。
(1)(CH 3)2NCH 2CH 2OHSOCl 2(2)CuCl 24(3)P, Br 2o(4)CH 2CHCOOCH 3干燥 HBr 2 (5)CH 3CH 2CH 2CH 2CH 2COOHSOCl 24o(6)S CH 32266(7)H 3C CH 3NBS, H 2SO 43o(8)NHCOCH 3CH 3Br 2,CH 3COOH o(9)OHC CH 3CH 2t -BuOCl, CHCl 3o(10)NBS, (C 6H 5)3P(11)KI, H 3PO 4(12)C 6H 5H 3Br 2,Cl 4o (13)CH 3CH CH CH 3232(14)(CH 3)3CCH 2OHHBr(15)OOP2(16)NBS, Et 3N ·3HF 22o(17)OHBr 24o(18)O23o2. 在下列指定原料和产物的反应式中分别填入必需的化学试剂(或反应物)和反应条件。
(1)CH 3CH 2CH 2CH 2CH CHCH 3CH 3CH 2CH 2CHCHCHCH 3Br(2)COOHBr(3)(4)OHBr(5)2CH 2BrBr(6)(7)(CH 3)3CCH 2OH(CH 3)3CCH 2Br(8)OOBocHNO OBocHNBr(9)OOBr OO BrBr2. 在下列指定原料和产物的反应式中分别填入必需的化学试剂(或反应物)和反应条件。
(参考答案)题号答案注释(1) NBS/(PhCO)2O, CCl4, △(2) Br2/HgO/tetrachloroethaneNaNO2, HCl, H2O; 2. HPF6; 3. △ (168℃)(3) 1.(4) Ph3P, Br2, CH3CN, △ (200-340℃)(5) NBS/(PhCO)2O, CCl4refluxing (10min. )acetone,(6) NaI,(7) Bu3P, Br2, DMF(8) NBS/hv, CCl4(9) NBS/(PhCO)2O, CCl4, reflux3. 阅读(翻译)以下有关反应操作的原文,请在理解基础上写出:(1)此反应的完整反应式(原料、试剂和主要反应条件);(2)此反应的反应机理(历程)。
药物合成翻译习题答案

药物合成翻译习题答案In a 3L round-bottomed flask fitted with a reflux condenser are placed 625 mL of 95 percent alcohol, 500 mL of water, 500 g of pure benzaldehyde, and 50 g of sodium cyanide. The mixture is then heated and kept boiling for one-half hour. In the course of about twenty minutes, crystals begin to separate from the hot solution. At the end of the thirty minutes, the solution is cooled, filtered with suction, and washed with a little water. The yield of dry crude benzoin, which is white or light yellow, is 450–460 g. In order to obtain it completely pure, the crude substance is recrystallized from 95 percent alcohol, 90 g of crude material being dissolved in about 700 mL of boiling alcohol; upon cooling, a yield of 83 g of white, pure benzoin which melts at 129° C is obtained.(benzoin:安息香胶)在3L圆底烧瓶装上回流冷凝器置于625毫升百分之95酒精,500毫升水,500克和50克的纯甲醛、氰化钠。
药物合成反应(第三版)第一章课后翻译

About 216–224 g. (1.62–1.68 moles) of powdered anhydrous aluminum chloride is added to a 1Lthree-necked flask.在1L的三口烧瓶中加入大约216-224g(1.62–1.68 moles)的无水三氯化铝。
While the free-flowing catalyst is stirred (Note 3), 81 g. (0.67 mole) of acetophenone is added from the dropping funnel in a slow stream over a period of 20–30 minutes.自由流动的催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. 放热反应,假如滴加的酮不能被分散,就会变黑或是碳化。
When about one-third of the acetophenone has been added, the mixture becomes a viscous ball-like mass that is difficult to stir.当三分之一的乙酰苯被滴加,反应混合物变成一个很难搅拌的粘性的球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. 在这时,改用手动搅拌或快速滴加酮是非常必要的。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. 然而,速度不能太快,当反应温度超过180℃时。
药物合成反应课后翻译

四次把深色的油从混合物中用 150ml 萃取岀来。
The extracts are combined, washedto produce a temperature above 180° .然而,速度不能太快,当反应温度超过either heated or cooled. The molte n mass, in which the acet ophenon e is complexed with aluminum chloride, ranges in color from tan to brown.明苯乙酰已经和三氯化铝混合完全,颜色也逐渐从黄褐色变为棕色。
混合均匀。
Part of the cold aqueous layer is added to the reacti on flask to deco mposewhatever part of the reactionmixture remains there, and the resultingmixture is addedto the beaker.把部分的冰水层加入到烧瓶中洗涤残留物,然后合并到烧杯中。
that settles out is extracted from the mixture with four 150-ml. p orti ons of etherAbout 216 — 224 g. — moles) of po wdered an hydrous aluminum chloride is added to a1Lthree-necked flask.在1L 的三口烧瓶中加入大约 216-224g - moles)的无水三氯化铝。
While the freeflow ing catalyst is stirred (Note 3), 81 g. mole) of acet ophenone is added from the dropping funnel in a slow stream over a p eriod of 20 -30 minutes. 自由流动 的催化剂边搅拌边用滴液漏斗缓慢滴加 81g 苯乙酰。
药物合成反应第三版第四章课后翻译

有回流冷凝器的3L圆底烧瓶中参加625ml的95%酒精、500ml水、500g〔476ml,4,7mol〕的苯甲醛和50g 96-98%的氰化钠。
The mixture is then heated and kept boiling forminutes, crystals begin to separate from the hot solution. 在20分钟后晶体开始从热溶液中析出。
At the end of the thirty minutes, the solution is cooled, filtered with suction, and washed with a little water. 在最后的30分钟,冷却溶液,抽滤并用少量水450-460g白色或亮黄色的枯燥的安息香。
(90–92 per cent of the theoretical amount). 理论产率90-92%。
In order to obtain it pletely pure, the crude substance iswhich melts at 129° is obtained.为了得到纯度高的产品,粗产品要在酒精中重结晶,90g 粗品溶解在700ml沸腾的酒精中,冷却,得到83g熔点为129摄氏度的白色安息香纯品。
In a 1-l. three-necked round-bottomed flask equipped with a mechanical stirrer, short reflux condenser, and bent glass tube reaching below the surface of the liquid for the introduction of hydrogen chloride, are placed 50 g. (0.36 mole) of p-nitrophenol (Note 1), 650 ml. of concentrated hydrochloric acid, 5 ml. of concentrated sulfuric acid (Note 2), and 76 g. (1 mole) of methylal (Note 3). 在配有机械搅拌,短期冷凝回流器和一个为的是深入液面下通氯化氢气体的弯曲的玻璃管三口圆底烧瓶中参加50g〔0.36mol〕对硝基苯酚,650ml的浓盐酸,5ml的浓硫酸和76g〔1mol〕的二甲氧基甲烷。
药物合成反应与设计翻译部分

药物合成反应与设计翻译部分(第三版闻韧主编)第一章翻译:About 216–224 g. (1.62–1.68 moles) of powdered anhydrous aluminum chloride is added to a 1Lthree-necked flask.在1L的三口烧瓶中加入大约216-224g(1.62–1.68 moles)的无水三氯化铝。
While the free-flowing catalyst is stirred (Note 3), 81 g. (0.67 mole) of acetophenone is added from the dropping funnel in a slow stream over a period of 20–30 minutes. 自由流动的催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. 放热反应,假如滴加的酮不能被分散,就会变黑或是碳化。
When about one-third of the acetophenone has been added, the mixture becomes a viscous ball-like mass that is difficult to stir.当三分之一的乙酰苯被滴加,反应混合物变成一个很难搅拌的粘性的球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. 在这时,改用手动搅拌或快速滴加酮是非常必要的。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. 然而,速度不能太快,当反应温度超过180℃时。
药物合成反应与设计翻译部分(优.选)

药物合成反应与设计翻译部分(第三版闻韧主编)第一章翻译:About 216–224 g. (1.62–1.68 moles) of powdered anhydrous aluminum chloride is added to a 1Lthree-necked flask.在1L的三口烧瓶中加入大约216-224g(1.62–1.68 moles)的无水三氯化铝。
While the free-flowing catalyst is stirred (Note 3), 81 g. (0.67 mole) of acetophenone is added from the dropping funnel in a slow stream over a period of 20–30 minutes. 自由流动的催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. 放热反应,假如滴加的酮不能被分散,就会变黑或是碳化。
When about one-third of the acetophenone has been added, the mixture becomes a viscous ball-like mass that is difficult to stir.当三分之一的乙酰苯被滴加,反应混合物变成一个很难搅拌的粘性的球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. 在这时,改用手动搅拌或快速滴加酮是非常必要的。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. 然而,速度不能太快,当反应温度超过180℃时。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1、About 216–224 g. (1.62–1.68 moles) of powdered anhydrous aluminum chloride is added to a 1Lthree-necked flask.在1L的三口烧瓶中加入大约216-224g(1.62–1.68 moles)的无水三氯化铝。
While the free-flowing catalyst is stirred (Note 3), 81 g. (0.67 mole) of acetophenone is added from the dropping funnel in a slow stream over a period of 20–30 minutes. 自由流动的催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. 放热反应,假如滴加的酮不能被分散,就会变黑或是碳化。
When about one-third of the acetophenone has been added, the mixture becomes a viscous ball-like mass that is difficult to stir.当三分之一的乙酰苯被滴加,反应混合物变成一个很难搅拌的粘性的球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. 在这时,改用手动搅拌或快速滴加酮是非常必要的。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. 然而,速度不能太快,当反应温度超过180℃时。
Near the end of the addition, the mass becomes molten and can be stirred easily without being either heated or cooled. The molten mass, in which the acetophenone is complexed with aluminum chloride, ranges in color from tan to brown.当快滴加完时,团块开始融化,表明苯乙酰已经和三氯化铝混合完全,颜色也逐渐从黄褐色变为棕色。
Bromine (128 g., 0.80 mole) is added dropwise to the well-stirred mixture over a period of 40 minutes (Note 4). 在40分钟内在搅拌下把溴缓慢滴加到混合物中。
After all the bromine has been added, the molten mixture is stirred at 80–85° on a steam bath for 1 hour.溴滴加完后,熔融混合物在80-85℃蒸气浴下搅拌1小时。
The complex is added in portions to a well-stirred mixture of 1.3 l. of cracked ice and 100 ml. of concentrated hydrochloric acid in a 2-l. beaker (Note 6).反应物加入到1.3L碎冰和100ml浓盐酸的混合物中在2L的烧杯中混合均匀。
Part of the cold aqueous layer is added to the reaction flask to decompose whatever part of the reaction mixture remains there, and the resulting mixture is added to the beaker.把部分的冰水层加入到烧瓶中洗涤残留物,然后合并到烧杯中。
The dark oil that settles out is extracted from the mixture with four 150-ml. portions of ether 分四次把深色的油从混合物中用150ml萃取出来。
The extracts are combined, washed consecutively with 100 ml. of water and 100 ml. of 5% aqueous sodium bicarbonate solution, dried with anhydrous sodium sulfate, and transferred to a short-necked distillation flask. 合并萃取液,用100ml水和100ml 5%的小苏打洗涤,用无水硫酸钠干燥。
The ether is removed by distillation at atmospheric pressure, and crude 3-bromoacetophenone is stripped from a fewgrams of heavy dark residue by distillation at reduced pressure. 乙醚在常压下蒸馏,微量的溴苯乙酮通过减压蒸馏的方法从大量深色残渣中被分离出来。
The colorless distillate is carefully fractionated to obtain 94–100 g.通过分馏,得到无色的流出液94-100g2、反应式:3、2-Methyl-4-ethoxalylcyclopentane-1,3,5-trione. A solution of sodium ethoxide is prepared in a 2-l. three-necked, round-bottomed flask fitted with amercury-sealed stirrer, a reflux condenser carrying a drying tube, and a stopper by the addition of 69.0 g. (3 moles) of sodium to 950 ml. of absolute ethanol. 69.0g (3mol)钠和950ml无水乙醇在配有干燥回流冷凝管和汞封搅拌器的2L三口圆底烧瓶中制备乙醇钠。
The solution is cooled to 0–5° in an ice bath and stirred.溶液在0-5℃下冰浴搅拌。
The stopper is replaced by a dropping funnel, and a cold mixture (5–15°) of 108 g. (1.50 moles) of freshly distilled 2-butanone and 482 g. (3.30 moles) of diethyl oxalate (Note 1) is added gradually over a period of 30 minutes.瓶塞用分液漏斗取代,108g(1.5mol)的丁二酮和482g(3.3mol)的乙二酸二乙酯在5-15℃下低温混合,在30分钟内逐步滴加到溶液中。
After the addition is complete, the thick, orange-red mixture is allowed to warm with continued stirring to room temperature, heated under reflux for 30 minutes, and cooled again to 0° in an ice bath. 完全加入后,橘红色的粘稠物继续搅拌至室温,加热回流30分钟后在冰浴中冷却至0℃。
The mixture is decomposed by stirring with 165 ml. of sulfuric acid (1:1 by volume) added in portions.将165ml浓硫酸(体积比1:1)在搅拌加入,分解混合物。
The sodium sulfateformed is filtered by suction and washed with ethanol (150–200 ml.) (Note 2). 硫酸钠抽滤后用乙醇(150–200 ml)洗涤。
The washings and filtrate are combined and concentrated by evaporation .合并滤液和洗涤液后蒸发浓缩。
The yellowish brown product which accumulates by slow crystallization is collected by filtration, washed with small quantities of ice-cold water, and dried in air. 过滤缓慢析出的棕黄色产品用小剂量的冰水洗涤后在空气中干燥。
The crude product weighs 140–150 g.粗产品140-150g。
Further evaporative concentration of the mother liquor followed by cooling furnishes an additional 40–50 g. of the keto ester, 此外将母液用冷冻蒸发浓缩后又得到40-50g的酮酯。
bringing the total yield to 180–200 g. (53–59%)产品总共180-200g(产率53-59%)(Note 2). This crude material (m.p. 120–130°) is used in the next step.粗品(熔点120–130℃)用于下一步中A pure sample can be obtained by crystallization from ethyl acetate after treatment with Norit activated carbon, m.p. 160–162°.纯品是经过活性炭处理后在乙酸乙酯中结晶得到,熔点160–162℃。
