特罗凯说明书
特罗凯说明书

本品用于 EGFR 突变人群一线治疗的临床研究正在进行中。建议经治医生根据本品和同 类药物研究进展以及患者自身状况综合考虑适宜的治疗选择。
【规格】 (1)100 毫克 (2)150 毫克
【用法用量】 本品必须在有此类药物使用经验的医生指导下使用。 厄洛替尼单药用于非小细胞肺癌的推荐剂量为 150mg/日,至少在饭前 1 小时或饭后 2
厄洛替尼的安全性评估是基于 1200 多例至少接受过一次 150mg 厄洛替尼单药治疗患者
的数据和 300 多例接受过厄洛替尼 100mg 或 150mg 联合吉西他滨治疗患者的数据,以及 1228
例接受厄洛替尼联合化疗患者的数据。
来自于临床试验中厄洛替尼单药或联合化疗报告的不良反应 (ADR) 总结如下。下
第 4 页 共 33 页
优先术语 皮疹 腹泻 疲劳 厌食 瘙痒 痤疮 痤疮样皮炎 皮肤干燥 体重下降 甲沟炎
所有分级% 49.2 20.3 9.0 9.2 7.4 6.2 4.6 4.4 3.9 3.9
III 级% IV 级%
6.0
0
1.8
0
TORK-LOK 拧紧锁筒和椅子系列说明书

Precision Mated Flats on Both Expander and Arbor BodyPrecision Mated Flats on ColletSafety Stops Contraction ExpansionTork-Lok Arbors and ColletsTORK-LOK ARBORS AND COLLETSThe Tork-Lok arbor design answers the need for a completely versatile workholding device. Precision ground flats on collets and arbors improve long-term accuracy and torque transmission. Standardization of components permits interchangeability and combination possibilities. Locates straight or tapered holes on true center.INSTALLATION AND OPERATING INSTRUCTIONSSafety StopsThe expansion safety stop is fixed in all styles and requires no adjustment. The contraction or loading position safety stop is adjusted to suit the part being chucked.PreloadAll collets are manufactured to include a preload. Collets should not be adjusted below the range minimum for that collet. Preloads are .008 average on sizes from .500-.874 and .015 from .875-4.467.Special Work Stop ConsiderationCombination Work Stop and Retaining Sleeve: Restrictor Type Work Stops are necessary for parts whose gripping length is shorter than the collet. Since the Tork-Lok collet expands at both ends, a retaining sleeve is needed to prevent the free end of the collet from over expanding; a Restrictor Expander is used to reduce the expansion safety stop, preventing collet breakage (in case arbor is operated without a part in position).NOTES:• All Tork-Lok arbor applications require a part stop• If Restrictor Type Stop is used, a special Restrictor Expander is required • Contact us at 1-800-228-BUCK for more informationDRAWBAR MODELDrawbar should be in forward or push position. Adjust forwardexpander with Allen wrench until part is a slip fit on the collet. Collet flats both ends must mate.Collet ChangingDrawbar should be in forward positions. Using Allen wrench, remove forward expander. Collet and rubber sleeve can then be removed. Wash parts in clean solvent. Lightly grease expander flats in contact. Install forward expander. Adjust according to instructions outlined above.AIR-OPERATED MODELAir pressure on the cylinder should be at 70 psi. Adjust forward expander with Allen wrench until part is slip fit on the collet. Collet flats on both ends must mate.Collet ChangingWith air pressure at 70 psi, follow instructions listed in Drawbar Model.To Interchange AssembliesApply 70 psi, remove mounting screws, turn arbor assembly counterclockwise until drawbar disengages from piston assembly.To mount assembly, apply 70 psi. Be sure threads on drawbar are clean and oiled. Turn arbor assembly clockwise until face contacts cylinder. Back off to align tapped holes in cylinder. Insert screws and adjust according to instructions outlined above.Expansion StopForward ExpanderDirt Seal Contraction StopDrawbar ConnectorForward ExpanderExpansion StopContraction StopNote: Max. Drawbar pullstamped hereDirt SealChip ClearanceMachine ID of sleeve to high limit of part +.02 - +.004AIR-OPERATED MODELDRAWBAR MODELTYPICAL APPLICATIONS FOR ARBORSPISTON DIFF. CARRIER TRACK ROTOR BALL JOINT GEAR BLANK PUMP HOUSINGCOMPRESSION PLATEFRONT BRAKE DRUMSpecial arbor combinations are available upon request. Please contact us at 1-800-228-BUCK for more information.Arbor Applications36Tork-Lok Drawbar ModelsLong Series ArborsFEATURES AND BENEFITS:• Answers your need for versatility• Precision-ground flats improve accuracy• Standardization permits interchangeability• Locates straight or tapered holes on true center• Requires a part stopSHIPS COMPLETE WITH:• Flange body• Connector• Expander•Collet sold separately; see pages 40 & 41FEATURES AND BENEFITS:• Bolt circle has versatile three-bolt pattern • Tighter tolerance on size and parallelism for locator mounting• Provisions for air sensing• Precision-ground flats improve accuracy • Standardization permits interchangeability• Locates straight or tapered holes on true center • Requires a part stopTork-Lok Metric Drawbar ModelsLong Series ArborsDimensions denoted in millimeters unless otherwise specified.SHIPS COMPLETE WITH:• Flange body • Connector • Expander• Collet sold separately; see pages 40 & 41- Optional assembly method with male thread38SHIPS COMPLETE WITH:• Flange body • Connector • Expander• Collet sold separately; see pages 40 & 41FEATURES AND BENEFITS:• Answers your need for versatility• Precision-ground flats improve accuracy • Standardization permits interchangeability• Locates straight or tapered holes on true center • Requires a part stopTork-Lok Drawbar ModelsShort Series Arbors1-800-228-BUCK 39Tork-Lok Metric Drawbar ModelsShort Series ArborsFEATURES AND BENEFITS:• Bolt circle has versatile three-bolt pattern • Tighter tolerance on size and parallelism for locator mounting• Provisions for air sensing• Precision-ground flats improve accuracy • Standardization permits interchangeability• Locates straight or tapered holes on true center • Requires a part stopSHIPS COMPLETE WITH:• Flange body • Connector • Expander• Collet sold separately; see pages 40 & 41- Optional assembly method with male threadT o r k -L o k L o n g S e r i e s C o l l e tsF E A T U R E S A N D B E N E F I T S :• P r e c i s i o n g r o u n d fla t s • C o l l e t s i n t e r c h a n g e a b l e o n a l l a r b o r s w i t h i n s p e c i fie d r a n g e • A c c u r a c y o f a r b o r n o t a f f e c t e d b y i n d e x i n g o f c o l l e t s h a f t • H i g h -g r a d e s t e e l s , p r e c i s e l y h e a t t r e a t e d • S i l i c o n e s e a l i n g o f c o l l e t s l o t s f o r s p e c i a l j o b s i s a v a i l a b l e o n r e q u e s t f o r a d d i t i o n a l c h a r g e • C o l l e t s i n o v e r s i z e (o v e r l a p ) r a n g e s a r e a v a i l a b l e – s e e b o l d r a n g esO v e r s i z e c o l l e t s A C 107-110;209-212 a n d 311-318 s h o u l d b e u s e d o n l y i n l i g h t t u r n i n g o r g r i n d i n g o p e r a t i o n s .T o r k -L o k S h o r t S e r i e s C o l l e tsF E A T U R E S A N D B E N E F I T S :• P r e c i s i o n g r o u n d fla t s • C o l l e t s i n t e r c h a n g e a b l e o n a l l a r b o r s w i t h i n s p e c i fie d r a n g e • A c c u r a c y o f a r b o r n o t a f f e c t e d b y i n d e x i n g o f c o l l e t s h a f t • H i g h -g r a d e s t e e l s , p r e c i s e l y h e a t t r e a t e d • S i l i c o n e s e a l i n g o f c o l l e t s l o t s f o r s p e c i a l j o b s i s a v a i l a b l e o n r e q u e s t f o r a d d i t i o n a l c h a r g e • C o l l e t s i n o v e r s i z e (o v e r l a p ) r a n g e s a r e a v a i l a b l e – s e e b o l d r a n g esO v e r s i z e c o l l e t s A C 7110-7115; 7213-7220 a n d 7317-7328 s h o u l d b e u s e d o n l y i n l i g h t t u r n i n g o r g r i n d i n g o p e r a t i o n s .4142Tork-Lok Replacement ComponentsSold in KitsAir-Operated ModelsMeasurements in inches.1-800-228-BUCK 43Manual Tork-Lok FixturesOPERATING INSTRUCTIONS1. Mount fixture on machine.2. Adjust expander with Allen wrench so that part is a slip fit on collet. Be sure collet and arbor flats mate on both ends.3. To clamp part, move handle clockwise.4. To release part, move handle counterclockwise.ORDERING INSTRUCTIONSA complete fixture is ready to mount on your machine. When ordering additional arbor assemblies for fixture use, indicate connector also. When ordering base assemblies, indicate connector(s) desired.TO CHANGE ARBOR ASSEMBLIESFrom one size range to another (see chart above for arbor assembly and connector):1. Remove cap screws and turn arbor assembly off the base clockwise.2. CAUTION Handle must be in unlocked position.3. Turn replacement arbor assembly counterclockwise into base until snug. Continue movement until holes line up. Replace cap screws.ADAPTATION OF DRAWBAR MODELS1. Procure proper fixture base and connectors to accommodate drawbar model you have (see chart above).2. Remove drawbar connector (A) by backing out expander (B) with Allen wrench until connector is free. Remove retaining ring.3. Insert fixture connector (C or D) by turning in expander (B) until flats mate and are firm on both ends. No retainer ring is necessary.4. CAUTION Handle must be in unlocked position.5. Turn assembly counterclockwise into base assembly until snug. Continue movement until holes line up. Replace cap screws.Installation and Operating InstructionsTORK-LOK FIXTUREThe Tork-Lok Fixture is a powerful locking mechanism for use with Tork-Lok Arbors. It features low initial cost and little maintenance for milling and drilling operations.onArbor AssemblyBase Assembly Locked PositionUnlocked PositionOUR BRANDSFORKARDT MAIN OFFICE 2155 Traversefi eld Dr Traverse City, MI 49686Tel: (+1) 800-544-3823Fax: (+1) 231-995-8361E-Mail:*****************FORKARDT CHINABuilding, No.209, Taigu Road Shanghai Waigaoqiao F .T.Z.200131 P .R.CTel: 86-021-********E-mail:****************.comFORKARDT DEUTSCHLAND GMBH Heinrich-Hertz-Str. 7D-40699 ErkrathTel: (+49) 211-25 06-0Fax: (+49) 211-25 06-221E-Mail:*****************FORKARDT FRANCE S.A.R.L.28 Avenue de BobignyF-93135 Noisy le Sec Cédex Tel: (+33) 1-4183 1240Fax: (+33) 1-4840 4759E-Mail:****************************FORKARDT INDIA L.L.P .P No. 39, No. 5-5-35/187Ayyanna Ind Park IE Prashant Nagar Kukatpally, Hyderabad, AP . 500072Tel: 040-40020571Fax: 040-40020576E-mail:**********************。
专家称特罗凯疗效显著.docx

认识认识丙卡巴肼药物名称:丙卡巴肼药物别名:Procarbazine甲苄肼、甲基苄肼,Methylhydrazine,NATULAN,MATULAN,PCB,PCZ,MZH英文名称:Procarbazine说明:片剂:每片50mg。
功用作用:主要用于霍奇金病,有1/3~1/2的病人能获得完全缓解,缓解期为3周~6个月或更长。
对其他恶性淋巴瘤、多发性骨髓瘤和肺癌亦有一定疗效。
用法用量:一般每日150~200mg,分3~4次服用。
一疗程总量可根据血象而定。
注意事项:常见的不良反应有:①胃肠道反应,主要为恶心、呕吐,多数能耐受。
②骨髓抑制,一般出现较晚,多在服药后4~6周出现,主要表现为白细胞和血小板下降,其程度与剂量有关。
停药后2~3周可恢复到正常水平。
③神经系统,部分病人可出现中枢神经系统毒性如眩晕、嗜睡、精神错乱、脑电图不正常等。
亦可出现下肢感觉异常、深反射消失、麻痹等周围神经炎的表现。
④其他,皮炎、脱发。
本品为弱的单胺氧化酶抑制剂,服药期间凡含有高酪胺成分的食物如乳酪和香蕉等均不宜食用。
若同时用拟交感神经药和抗抑郁剂丙咪嗪则应小心。
酒类宜少用。
与苯噻嗪类药物有协同的镇静作用,与巴比妥类、麻醉药、抗组织胺类和利血平等合用亦应谨慎。
本品可引起溶血性贫血,对肝、肾功能或骨髓机能不全的病人应减少剂量。
少数年轻妇女服药后可引起闭经。
:想知道还有哪些抗肿瘤药吗?[详情请看>>>>抗肿瘤药点滴]想了解更多肿瘤的相关知识,请点击>>>>癌症频道网址: 第 1 页,共 1 页。
特洛哥氯液141说明书
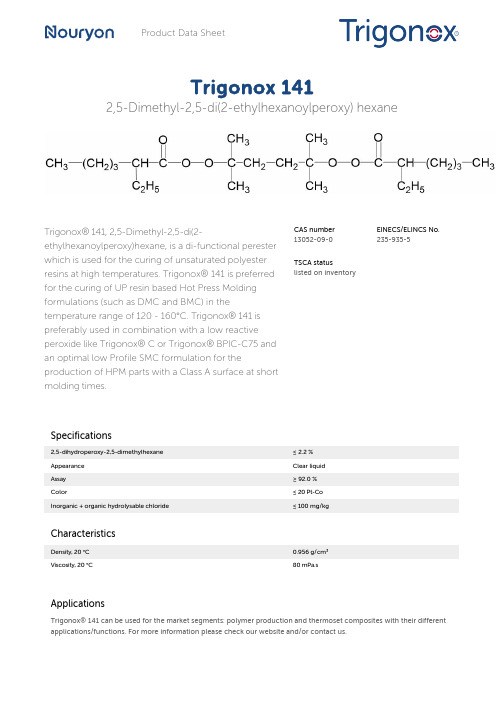
Product Data SheetTrigonox 1412,5-Dimethyl-2,5-di(2-ethylhexanoylperoxy) hexaneTrigonox® 141, 2,5-Dimethyl-2,5-di(2-ethylhexanoylperoxy)hexane, is a di-functional perester which is used for the curing of unsaturated polyester resins at high temperatures. Trigonox® 141 is preferred for the curing of UP resin based Hot Press Molding formulations (such as DMC and BMC) in the temperature range of 120 - 160°C. Trigonox® 141 is preferably used in combination with a low reactive peroxide like Trigonox® C or Trigonox® BPIC-C75 and an optimal low Profile SMC formulation for the production of HPM parts with a Class A surface at short molding times.CAS number13052-09-0EINECS/ELINCS No.235-935-5TSCA statuslisted on inventorySpecifications2,5-dihydroperoxy-2,5-dimethylhexane≤ 2.2 %Appearance Clear liquidAssay≥ 92.0 %Color≤ 20 Pt-CoInorganic + organic hydrolysable chloride≤ 100 mg/kgCharacteristicsDensity, 20 °C0.956 g/cm³Viscosity, 20 °C80 mPa.sApplicationsTrigonox® 141 can be used for the market segments: polymer production and thermoset composites with their different applications/functions. For more information please check our website and/or contact us.Thermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT35°C (95°F)Emergency temperature (Tₑ)25°C (77°F)Control temperature (Tc)20°C (68°F)Method The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts max.15°CNote When stored according to these recommended storage conditions, Trigonox®141 will remain within the Nouryon specifications for a period of at least 3 monthsafter delivery.Packaging and transportThe standard packaging is a 30-liter HDPE can (Nourytainer®) for 25 kg peroxide. Both packaging and transport meet the international regulations. For the availability of other packed quantities consult your Nouryon representative. Trigonox®141 is classified as Organic peroxide type C; liquid, temperature controlled, Division 5. 2; UN 3113.Safety and handlingKeep containers tightly closed. Store and handle Trigonox® 141 in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Trigonox® 141. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsCarbon dioxide, Acetone, 2-Pentanone, Heptane, Heptenes, tert-Amyl alcohol, 2,5-Bis(1-ethylpentoxy)-2,5-dimethylhexane, 2,5-Dimethyl-2,5-hexanediolAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox®, Nourytainer and Perkadox are registered trademarks of Nouryon Functional Chemicals B.V. or affiliates.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2023-1-11© 2023Thermoset composites Trigonox 141。
印度特罗凯中文说明书

印度特罗凯中文说明书【药品名称】商品名:特罗凯[1]通用名:盐酸厄洛替尼片英文商品名:Tavceva英文通用名:Erlotinib HCL Tablets份子结构名:盐酸厄洛替尼片化学名称:N-(3-乙炔苯基)-6,7-双(2-甲氧乙氧基)-4-喹啉胺盐酸盐化学结构式:分子式:C22H23N3O4·HCl分子量:429.90【成份】每片内含150mg厄洛替尼(以盐酸厄洛替尼形式存在)【性状】圆形、双凸、白色包衣片。
【作用机制】Tarceva的抗肿瘤作用机制主要为抑制表皮生长因子(EGFR)酪氨酸激酶胞内磷酸化。
【药代动力学】Tarceva口服后60%吸收,半衰期约36小时,主要由CYP3A4代谢清除。
口服Tarceva150mg的生物利用度约60%,4小时后达血浆峰浓度。
对591例接受单药Tarceva治疗的药代动力学分析显示,达到稳定血药浓度需7-8 天,患者的年龄、体重、性别与药物的清除速率无显著关系,吸烟可使药物清除率增加24%。
【适应症】Tarceva用于两个或两个以上化疗方案失败的局部晚期或转移的非小细胞肺癌的三线治疗。
【禁忌症】对本品及成份过敏者禁用。
【不良反应】最常见的不良反应是皮疹和腹泻,3/4度皮疹和腹泻的发生率分别为9%和6%,皮疹的中位出现时间是8天,腹泻中位出现时间为12天。
发生率大于10%的不良反应有:皮疹、腹泻、食欲减低、疲劳、呼吸困难、咳嗽、恶心、感染、呕吐、口腔炎、瘙痒、皮肤干燥、结膜炎、角膜结膜炎、腹痛。
肺毒性:有较少的报道提示在接受Tarceva治疗的NSCLC患者或其他实体瘤患者中可出现严重的间质性肺病(ILD),甚至导致死亡。
在随机对照研究中,ILD的发生率是0.8%,并且这一发生率在Tarceva治疗组和安慰剂组是相同的。
报道的ILD包括:肺炎、间质性肺炎、间质性肺病、闭塞性细支气管炎、肺纤维化、急性呼吸应激综合征和肺渗出。
症状发生于治疗后5天~超过9个月,中位发生时间为47天。
药物肝损伤

药物性肝损伤风险的新视点肝脏是药物代谢的主要脏器,同时,也是药物损伤的主要靶器官。
药物性肝损伤( drug-induced liver injury,DILI) 是指药物在治疗过程中,由于药物本身或其代谢产物引起的肝细胞毒性损害或肝脏对药物及其代谢产物的过敏反应所致的疾病。
DILI 发病机制的复杂性和疾病的隐匿性,往往导致其在药品在上市前的临床试验中不能完全被发现,而是在药品大量用于临床后才逐渐显现出来。
有统计结果显示,引起DILI 的药物有600 余种。
随着医药的研发,新药不断问世、用于临床,新药上市后的安全性问题越来越引起关注。
笔者查阅了近几年美国食品药品监督管理局( FDA) 、国家食品药品监督管理总局( CFDA) 、欧洲及加拿大等发布的DILI 相关报道,加以归纳、分析,以提示临床医务工作者关注和重视DILI 的风险,全面了解和掌握最新药品不良反应监测信息,降低或避免DILI 对患者带来更多、更严重的危害,保障公众用药安全。
1 修订说明书,警示肝损伤风险1. 1 丙硫氧嘧啶的肝损伤风险2010 年4 月,FDA 在甲状腺功能亢进症治疗药物丙硫氧嘧啶的药品说明书中添加黑框警告,提示其致严重肝损伤和急性肝衰竭的信息,其中包括成人和儿童患者使用该药品导致死亡的病例。
FDA 已发现34 例与丙硫氧嘧啶相关的严重肝损伤报告,23 例成年患者报告中,有13 例死亡和5 例肝移植病例; 11例儿科患者报告中,有2 例患者死亡,7例患者需要接受肝脏移植,1例患者在被列入移植名单时死亡。
另外,结合医学文献的回顾,FDA 得出结论,对于成年患者和儿科患者来说,丙硫氧嘧啶引起临床严重肝损伤甚至致命的风险比甲巯咪唑更高。
1. 2 加拿大修订说明书,警示莫西沙星的严重肝损伤风险2010 年3 月,加拿大卫生部发布修改处方类抗菌药物莫西沙星( moxifloxacin,商品名: Avelox) 药品说明书的通知,包括口服片剂和注射液。
Odor Hunter TM 商品说明书

MATERIAL SAFETY DATA SHEETSostram Corporation In Case of Emergency, Call300 Colonial Center Parkway, Suite 230 Sostram Corporation: 770-587-9807Roswell, GA 30076 CHEMTREC: 800-424-9300I.GENERAL INFORMATION1-Slight Health Hazard 0-Slight Flammability 1-Slight ReactivityAbove: Ratings based on NIOSH “Identification System for Occupationally Hazardous Materials” (1974).II.TRANSPORTATION INFORMATIONThis product is not regulated for transportation purposesThis product is below the maximum VOC limits established by the California Environmental Protection Agency Air Resource Board for this type of product as of last update 2005.III.PRODUCT IDENTIFICATIONProduct Name: Odor Hunter TMIV.HAZARDOUS INGREDIENTSThe substances listed below are those identified as hazardous chemicals under the criteria of the OSHA Hazard Communication Standard (29 CFR 1910.1200).Component CAS No. % AgencyTert-butanol 76-65-0 0.2 N/AV.PHYSICAL DATABoiling Point: 212 °F (100 °C)Freezing Point: Not determinedDensity Approximately 1 (8.34 lbs/gallon)Vapor Pressure at 20 °C: Not determinedWater Solubility: Completely MisciblepH: 8-10Viscosity: Not determined% Volatiles by Weight With the exception of water, none presentAppearance and Odor: Clear colorless liquid; fresh odorVI.FIRE AND EXPLOSION DATAFlash Point: >230 °F, >110 °CFlammability Limits in Air: Not established, this product is completely non-flammableExtinguishing Media: CO2, foam, dry chemical, water spraySpecial Fire Fighting Procedures: Move containers from fire area if you can do it without risk. Do not scatterspilled material with high-pressure water streams. Avoid breathing vapors,keep upwind. Positive pressure self-contained breathing apparatus with fullfacepiece and structural firefighters’ protective clothing will provide limitedprotection (1996 North American Emergency Guidebook- Guide 129).VII.HEALTH HAZARD INFORMATIONOral LD50 (rat): >5,000 mg/kgPrimary Dermal Irritation (rabbit): None irritatingDermal Sensitization – Buehler method: Non-contact sensitizerEmergency and First Aid ProceduresEyes: Flush eyes with plenty of water for at least 15 minutes holding eyelids apart to ensure flushing of the entire eye surface. Get medical attention immediately.Skin: Remove contaminated clothing promptly. Wash affected skin areas with soap and large amounts of water until no evidence of chemical remains (approximately 15 minutes). If irritation or rednessdevelops, get medical attention. Wash clothing before reuseInhalation: Remove subject to fresh air. If breathing is difficult, oxygen should be administered by qualified personnel. If breathing has stopped, perform artificial respiration. Keep person warm and at rest.Treat symptomatically and supportively. Get medical attention.Ingestion: Immediately drink two glasses of milk or gelatin mixture, or if these are not available, a large quantity of water. Avoid alcohol. DO NOT give anything by mouth if the person is unconscious or havingconvulsions. Get medical attention immediately.Possible routes of entry : Inhalation, ingestion, dermalAcute Health Effects:Eyes/Skin: Mild irritation is possibleIngestion: Mild gastrointestinal irritation with nausea, vomiting and/or diarrhea is possible Inhalation: None knownVIII.REACTIVITY DATAStability: Store at ambient temperatures.Incompatibility: Avoid contact with strong bases and ammonia. Stability in orwith other products must be tested on an individual basisHazardous Decomposition Products: None known.Hazardous Polymerization: Cannot occur.IX.SPILL, LEAK AND DISPOSAL PROCEDURESSpill or Leak:Dilute wash away spill by spraying area with plenty of water. When spraying area with water, avoid spraying in direction of any person(s). Avoid getting on clothing. Keep unnecessary people away. Avoid getting spilled material on clothing.Disposal:Disposal is to be performed in compliance with all federal, state and local regulations. Small quantities may be carefully disposed of down sewer drain using plenty of water.X.INDUSTRIAL HYGIENE CONTROL MEASURESSPECIFIC PERSONAL PROTECTIVE EQUIPMENTEYE: No requirement with casual use. Use goggles in an industrial setting.SKIN: No requirement with casual use. Use impervious gloves and impervious clothing to prevent repeated or prolonged skin contact.RESPIRATORY/VENTILATION: No respiratory protection is required for casual use. Local ventilation is notnecessary unless this material is used in an industrial setting. In this situationthe use of a mechanical fume hood or exhaust system will minimize exposureto vapor or mist. Alternatively use respiratory protection. The specificrespirator selected must be based on contamination levels found in the workplace and must be NIOSH/MSHA approved. A respiratory protectionprogram that meets OSHA’s 29 CFR 1910.134 and ANSI Z88.2 requirementsmust be followed whenever workplace conditions warrant a respirator’s use.。
1Tarceva产品简介及不良反应处理

酪氨酸激酶(jīméi)结构域改动 1–3
EGFR 失活
ATP
P
P
细胞内酪氨酸激酶结构域 磷酸化,下游信号通路 (tōnglù)激活,肿瘤细胞
发作增殖,迁移,黏附等
特罗凯 与ATP 竞争性结合在 酪氨酸酶结构域,抑制磷酸化, 从而阻断(zǔ duàn)细胞内信号
通路的传导
1.Cohen S, et al. J Biol Chem 1980;255:4834–42; 2.Soderquist AM, et al. Fed Proc 1983;42:2615–20
3.Chinkers M, et al. Nature 1981;290:516–9; 4.Carey et al. Cancer Res 2006;66:8163–71
5.Wells A. Int J Biochem Cell Biol 1999;31:637–43
第九页,共56页。
特罗凯 概略(gàilüè)
特罗凯 —更强的药代动力学特征(tèzhēng)
Cmax(ng/ml)
AUC0-24 (ng•hour/mL)
特罗凯1
( 150mg/d )
吉非替尼2 吉非替尼2 吉非替尼2
( 225mg/d ) ( 525mg/d ) ( 700mg/d )
2,120
307
903
2,146
38,420
5,041 14,727 36,077
药物抑制EGFR后可影响角质化细胞的增生,分化,迁移 以及黏附,这一实践有助于解释丘疹脓疱及单调病的构 Lacouture M. Nat Rev Cancer 2006;6:803–12 成
第十八页,共56页。
表皮生长因子受体抑制剂惹起的皮肤不良反响 (fǎnxiǎng)
DEMKO 12ATEX1204259X 调节温度保护器说明书

Underwriters Laboratories Inc.Ordinary LocationsHazardous (Classified) Location
3. Date code format on nameplate is “YYWW” for year and week.
4. Zone hazardous locations flameproof gap and joint details. Activation plunger to guide through hole gap joints: 28.1 mm minimum length by 0.08 mm maximum annular gap. Adjustment shaft to shaft through hole gap joints: 27.0 mm minimum length by 0.08 maximum annular gap.
THERMON The Heat Tracing Specialists®
European Headquarters: Boezemweg 25 • PO Box 205 • 2640 AE Pijnacker • The Netherlands • Phone: +31 (0) 15-36 15 37 Corporate Headquarters:100 Thermon Dr • PO Box 609 San Marcos, TX 78667-0609 • Phone: 512-396-5801 • 1-800-820-4328 For the Thermon office nearest you visit us at . . .
特罗凯

特罗凯( 特罗凯(Tarceva) )
——肺癌的治疗药物
药品的简介
通 用 名:盐酸厄洛替尼片 商品名称:特罗凯 英文:Tarceva 英文名称:Erlotinib Hydrochloride Tablets 生产企业:Schwarz Pharma Manufacturing Inc. 处方类型:处方药
药品的简介
结构式:
化学名称: N-(3-乙炔苯基)-6,7-双(2-甲氧乙 氧基)-4-喹啉胺盐酸盐 适应症:厄洛替尼可试用于两个或两 个以上化疗方案失败的局部晚期或转 移的非小细胞肺癌的三线治疗
作用机制
非小细胞肺癌较高的发病率和 病死率,与血管内皮生长因子高 表达密切相关。研究证实在人 体内广泛分布,能够促进肿瘤的 血管新生,肿瘤细胞的血道转移 和淋巴服剂量为150 mg/ d。该药口 服后生物利用度约60 % ,4 h 达到血浆峰浓度,食物可以显著 提高其生物利用度,提高率高达 100 %。
最常见的不良反应为皮疹和腹泻, Ⅲ度以上的皮疹和腹泻的发生率分别为 9 %和6 % ,约1 %的患者因为这两种不 良反应而终止治疗。约4 %的患者有Ⅱ 度以上的谷丙转氨酶升高。
EGFR 是一种跨膜糖蛋白, 由一条多肽链组成, 属于结 构相关的受体酪氨酸激酶家 族, 对许多细胞信号传导通 路至关重要, 能够影响细胞 的分裂、凋亡、迁移和黏附 功能。厄洛替尼就是通过抑 制肿瘤细胞EGFR的信号转导, 达到抑制肿瘤细胞增殖、促 进肿瘤细胞凋亡的目的。
代 谢
厄洛替尼主要通过CYP3A4代谢,少量通 过CYP1A2和肝外同工酶CYP1A1代谢。口 服100mg剂量后,可以回收到91%的药物, 其中在粪便中为83%(1%剂量为原形), 尿液中为8%(0.3%剂量为原形)。
服用特罗凯能活多久?

服用特罗凯能活多久?
特罗凯片是一种酪氨酸激酶抑制剂(TKI)的癌症治疗药物。
可以阻断被称为表皮生长因子受体(EGFR)的蛋白质生长,通常患有这种受体的癌症被称为EGFR阳性。
在开始治疗之前,你的医生会检查你的癌细胞,看看你是否有这种受体突变,只有符合突变的患者在服用后的效果才能有保障。
特罗凯一种口服片剂,患者在用药时必须在医生或药剂师的指导下开始。
每天需要口服一次150mg,直到病情进展或耐药。
要在进食前1小时或进食后2小时服用。
如果您有吞咽困难的情况,可以将药片与50ml的水充分溶解后,通过鼻咽管服下。
在没有医生的建议下,不要停止服用。
只要特罗凯还有效,没有发生耐药,患者就要坚持服用。
根据以往的肺癌临床研究,分析了特罗凯在肺癌患者治疗中的无病生存率。
在研究中有100名非小细胞肺癌患者,其中有些肺癌患者是在手术后接受了常规的放疗和化疗辅助治疗,有些患者是手术后接受了特罗凯辅助治疗。
在5年的随访期间,发现接受印度版特罗凯辅助治疗的患者在两年内无病生存率为88%。
5年无病存活率为56%,五年总存活率为86%。
由此可以看出,采用靶向药物治疗的晚期非小细胞肺癌(NSCLC),患者的无进展生存期明显提高了很多。
另外在服用的过程中,患者需要按时服用,直至耐药为止,除了服用药品,在饮食上也需要多加注意,适当的锻炼,增强对抗疾病的体质。
厄洛替尼(特罗凯)说明书

厄洛替尼(特罗凯)
【规格】
100mg/片;150mg/片
【适应症】
用于晚期或转移性非小细胞肺癌。
【禁忌症】
对本品有严重超敏反应者禁用。
【用法用量】
厄洛替尼单药用于非小细胞肺癌的推荐剂量为150mg/日,至少在饭前1小时或饭后2小时服用。
【不良反应】
最常见的不良反应是皮疹和腹泻。
胃肠道异常:消化器官溃疡出血(胃炎、胃与十二指肠溃疡)、咯血、便血、黑粪症以及可能的结肠炎出血。
肝功能异常,眼疾(角膜炎、结膜炎)
【注意事项】
1.观察大便的次数、性状。
2.加强皮疹和角膜炎的护理。
忌用碱性肥皂液。
Protekt Gazelle 跛脚车用户手册说明书

吐Proacti �medical productsPROTEKT ®GAZELLESteera b le Knee Walker with BasketUSER MANUALModel: KWADCSLifetime Limited WarrantyYour Protekt ® Gazelle Knee Walker is warrantied to be free of defects in materials and workmanship for the lifetime of the product for the original purchaser.The applicable warranty period shall commence from date of shipment to the original purchaser, unless there is an expiration date on the component in which case the warranty shall expire on the earlier of warranty period or the expiration date.This mobility device was manufactured to the highest standards and carefully inspected prior to shipment. This Lifetime Limited Warranty is a statement of our confidence in the materials and workmanship of our products and our assurance to the consumer of years of reliable service.This warranty does not cover device failure due to owner misuse or negligence, or normal wear and tear. The warranty does not extend to non-durable components, such as casters, rubber accessories and grips, which are subject to normal wear and require periodic replacement.If you have a question about your Protekt ® Gazelle Knee Walker mobility device or this Lifetime Limited Warranty, please contact Proactive Medical Products or an authorized Proactive dealer.吐 Proacti �medical productsTips to Reduce the Riskof an Accident!Prior to using the Knee Walker, you should b e properly trained b y a skilled healthcare professional.Engage the lock and practice reaching, bending and transferring o n and off the Knee Walker. D O NOT use the Knee Walker unattended until you have been adequately trained, you have practiced with the aid of someone and you feel confident you can maneuver b y yourself.Use of the Knee Walker i s specific to the individual. Y o u should develop your own techniques for using the Knee Walker based o n your personal level of ability and function. NEVER attempt a maneuver that has not been previously practiced.Pay attention to your surroundings when operating the Knee Walker. Always avoid all dangerous situations.Operating the Knee WalkerThe Protekt® Gazelle Knee Walker i s a mobility device that provides assistance and comfort to an individual who may have a n injury below the knee.It allows a n even distribution of body weight b y supporting half of the weight o n the device and the other half o n the non-injured leg. The Knee Walker has a cushioned platform pad to support the injured leg to ensure that n o weight will b e placedo n the lower leg.Placement of the injured leg on the pad should be centered from side to side and positionedforward to cover the full length of the pad.-With the injured leg on the pad, stand as straight as possible and adjust the height of the Knee Walker to achieve a comfortable position.With the injured foot pointing downward, thepropulsion leg should be positioned as close as possible to the pad.Start out with small steps with the propulsionleg. Concentrate on keeping that leg positioned next to the pad. With minimal practice, youshould be able to move forward without veering to the opposite side.*Maximum Weight Capacity 350 lbs.The Knee Walker is designed for easy steering, stopping and maneuverability. A user friendly brake system is also featured on the Knee Walker forcontrolled movement and user safety.Instructions to Assemble1.Remove Knee Walker contents from outer packaging.2.Straighten the tiller to the upright position. Slidelocking mechanism to the side to allow tiller to reach full upright position. Release locking mechanism to lock tiller i n position.3. Slide locking mechanism into groove on tiller, presslocking handle to secure tiller lock in place.4. Insert pad post into receptacle on frame. Securepad i n place by inserting locking pin into hole. Lock pin i n place by sliding retainer tab into position.5. Lock pad i n position using locking lever.6. Attach basket by sliding basket receptacles downover hooks onthe front of the tiller.Hand Brake InstructionsIf the brake system is not working properly, DO NOT continue to use the Knee Walker!The Knee Walker's brake system is similar to abicycle and includes a locking feature.T o apply the brake, simply pull the lever with your finger tips toward the handle bar.T o lock the brake, pull the brake lever towards the handlebar and push down the spring loaded push button (located on top of brake). When donecorrectly, push button will remain down and brake lever will be locked into position.T o release the brake, simply pull lever towards the handle bar and the push pin will pop upautomatically.Hand Brake Adjustment For minor adjustments, the brake adjuster located on the handbrake can be turned out from the hand brake counter clockwise to tighten the brake, orclockwise to loosen the brake. Position the cable adjuster nut against the hand brake.If further adjustment is required, apply the samemethod of adjustment at the lower cable adjuster.As you adjust the cable adjuster and the adjuster nut away from each other, the brakes will tighten.As you adjust them closer, the brakes will loosen.Handle Height AdjustmentT o adjust the handle height, loosen the adjustment screw on the handle by turning it counter clockwise until it can be removed. Adjust the hand grip to the desired height and reinstall the adjustment screw by turning it clockwise until it is tightened.Pad Height AdjustmentT o adjust the pad height, loosen the locking lever under the pad (shown i n step 5 of instructions), and then remove locking pin (shown i n step 4 of instructions). Adjust pad to the desired height and then reinstall the locking pin and secure it in place with the retainer tab (as shown i n step 4 of instructions). Next tighten the locking lever (as shown i n step 5 of instructions) to lock pad i n place.To Fold TillerT o fold the tiller down, release the tiller locking handle (as shown i n step 3 of instructions) and slide assembly to the side (as shown i n step 2 of instructions) until the tiller is disengaged. Fold the tiller down until it rests on the pad and release the locking mechanism.Precautions!-Do not operate the Knee Walker over large cracks or dangerous surfaces.-Do not travel on loose gravel or in unstable areas.-Stop and move forward cautiously when nearing inclines, declines or gaps in walkway surfaces.-Do not travel over barriers or declines such as curbs. -Do not operate in excess of 3 miles per hour (slow to moderate walking speed).-Do not turn unless one foot is securely on the ground. -Never attempt to make a sharp turn at a high rate of speed.-Do not turn at speeds over 1 mph (very slow walking speed).-Do not operate on inclines above 15 degrees.-Always check the fasteners on the tiller, wheels, brakes, and pad for tightness before each use.-Contact your authorized provider if any parts are lo o se or show excessive wear.-This i s a medical device and should NOT b e treated a sa toy.。
特罗凯副作用及日常处理方法汇总

特罗凯副作用及处理方法汇总感谢这位咚友!冬瓜医生特罗凯作为一种可延长患者生存的一种小分子EGFR-TKI,最常见副作用是腹泻和皮疹,乏力。
服用特罗凯出现皮疹一、皮疹出现的症状(1)首次服用时,皮疹首先大片出现于背部,约7-10天时皮疹严重,布满全身,可以自愈和再现,属可逆的,治疗中止消失;如果对症处理后不见缓解可以考虑减量。
(2)停药若干个月后再启用时,皮疹仍出现,但数量会大减。
治疗方法(1)“百雀羚”止痒润肤露;(2)小儿止痒“宝宝金水”;(3)氯洁霉素、百多邦,每5-6小时一次,二者交替使用;(4)口服四环素类药物50mg,2次/天;(5)局部应用皮质激素类软膏,2次/天;(6)皮疹重时可同时应用润肤剂,乳酸,抗组胺药物。
临床上对服用特罗凯出现皮疹的处理办法1.轻度毒性:(1)患者可能毋需任何形式的干预,亦可局部使用复方醋酸地塞米松软膏(皮炎平)、氢化可的松软膏(1%或2.5%)或氯林可霉素凝胶(10%)以及红霉素软膏。
(2)对皮肤干燥伴瘙痒者,可予薄酚甘油洗剂(每天2次)或苯海拉明软膏涂抹瘙痒局部。
不应因轻度毒性而更改EGFRI的剂量。
(3)2周后再次评估,若情况恶化或无明显改善则按中度毒性处理。
2.中度毒性:(1)局部使用氢化可的松软膏(2.5%)或红霉素软膏,并口服氯雷他定。
(2)对皮肤干燥伴瘙痒者,可予苯海拉明软膏或复方苯甲酸软膏涂抹瘙痒局部。
有自觉症状者应尽早口服米诺环素(美满霉素100 mg Bid)。
(3)2周后再行评估,若情况恶化或无明显改善则按重度毒性处理。
3.重度皮疹:(1)干预措施基本同中度皮疹,但药物剂量可适当增加。
(2)必要时可予冲击剂量的甲泼尼龙(甲强龙),并可减少EGFRI剂量;(3)若合并感染,则选择合适的抗菌素进行治疗,如头孢呋辛(250 mg Bid)。
(4)若2~4周后不良反应仍未充分缓解,则考虑暂停用药或中止治疗注意:无论皮疹消失与否,要继续治疗。
皮疹的严重程度同特罗凯疗效正相关。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
特罗凯说明书
【批准文号】
注册证号H20060108
【中文名称】
盐酸厄洛替尼片
【产品英文名称】
Erlotinib Hydrochloride Tablets
【生产企业】
上海罗氏制药有限公司
【功效主治】
Tarceva用于两个或两个以上化疗方案失败的局部晚期或转移的非小细
胞肺癌的三线治疗。
【化学成分】
每片内含150mg厄洛替尼(以盐酸厄洛替尼形式存在)。
【药理作用】
Tarceva的抗肿瘤作用机制主要为抑制表皮生长因子(EGFR)酪氨酸激酶胞内磷酸化。
【药物相互作用】
【不良反应】
最常见的不良反应是皮疹和腹泻,3/4度皮疹和腹泻的发生率分别为9%
和6%,皮疹的中位出现时间是8天,腹泻中位出现时间为12天。
发生率大于10%的不良反应有:皮疹、腹泻、食欲减低、疲劳、呼吸困难、咳嗽、恶心、感染、呕吐、口腔炎、瘙痒、皮肤干燥、结膜炎、角膜结膜炎、腹痛。
肺毒性:有较少的报道提示在接受Tarceva治疗的NSCLC患者或其他实体瘤患者中可出现严重的间质性肺病(ILD),甚至导致死亡。
在随机对照研究中,ILD的发生率是0.8%,并且这一发生率在Tarceva治疗组和安慰剂组是相同的。
报道的ILD包括:肺炎、间质性肺炎、间质性肺病、闭塞性细支气管炎、肺纤维化、急性呼吸应激综合征和肺渗出。
症状发生于治疗后5天~超过9个月,中位发生时间为47天。
多数患者常有混杂因素导致ILD发生,如:之前有化疗/放疗、原有实质性肺疾病、肺转移或肺部感染。
当有新出现的、难以解释的肺部症状,例如:呼吸困难、咳嗽、发热等,需进行检查评价,一旦诊断ILD,应停止继续使用Tarceva,并采取适当治疗。
肝毒性:Tarceva治疗可引起无症状的肝转氨酶升高,因此,治疗期间应定期复查肝功能,包括:转氨酶、胆红素、碱性磷酸酶等,如果肝功能损害严重应减量或停药。
肝功能损害常为暂时性的或伴有肝转移。
较少报道有胃肠道出血,常发生于同时应用华法林的患者,所以,同时服用华法林或其他抗凝剂的患者应监测凝血酶原时间。
老年患者:安
全性和药代动力学在年轻人和老年患者中无明显差异,因此,应用于老年患者时不建议调整剂量。
【禁忌症】
对本品及成份过敏者禁用。
【产品规格】
150mg
【用法用量】
本品必须在有此类药物使用经验的医生指导下使用,并仅在国家肿瘤药物临床试验基地或三级甲等医院使用。
厄洛替尼单药用于非小细胞肺癌的推荐剂量为150mg/日,至少在进食前1小时或进食后2小时服用。
持续用药直到疾病进展或出现不能耐受的毒性反应。
无证据表明进展后继续治疗能使患者受益。
【贮藏方法】
25℃保存,15℃~30℃之间也可接受。
【注意事项】
本品必须在有此类药物使用经验的医生指导下使用,并仅在国家肿瘤药物临床试验基地或三级甲等医院使用。
特罗凯
【药品名称】
商品名:特罗凯
通用名:盐酸厄洛替尼片
【规格】150mg
【生产厂家】罗氏集团
【适应症】用于两个或两个以上化疗方案失败的局部晚期或转移的非小细胞肺癌的三线治疗。
【用法与用量】本品必须在有此类药物使用经验的医生指导下使用,并仅在国家肿瘤药物临床试验基地或三级甲等医院使用。
厄洛替尼单药用于非小细胞肺癌的推荐剂量为150mg/日,至少在进食前1小时或进食后2小时服用。
持续用药直到疾病进展或出现不能耐受的毒性反应。
无证据表明进展后继续治疗能使患者受益。
【规格】150mg
【生产厂家】罗氏集团
【适应症】用于两个或两个以上化疗方案失败的局部晚期或转移的非小细胞肺癌的三线治疗。
【用法与用量】本品必须在有此类药物使用经验的医生指导下使用,并仅在国家肿瘤药物临床试验基地或三级甲等医院使用。
厄洛替尼单药用于非小细胞肺癌的推荐剂量为150mg/日,至少在进食前1小时或进食后2小时服用。
持续用药直到疾病进展或出现不能耐受的毒性反应。
无证据表明进展后继续治疗能使患者受益。
【成份】每片内含150mg厄洛替尼(以盐酸厄洛替尼形式存在)
【性状】圆形、双凸、白色包衣片,一面印有棕色"T"和"150",另一面空白。
【药代动力学】
Tarceva口服后60%吸收,半衰期约36小时,主要由CYP3A4代谢清除。
口服Tarceva150mg的生物利用度约60%,4小时后达血浆峰浓度。
对591例接受单药Tarceva治疗的药代动力学分析显示,达到稳定血药浓度需7-8天,患者的年龄、体重、性别与药物的清除速率无显著关系,吸烟可使药物清除率增加24%。
【禁忌症】对本品及成份过敏者禁用。
【不良反应】
最常见的不良反应是皮疹和腹泻,3/4度皮疹和腹泻的发生率分别为9%和6%,皮疹的中位出现时间是8天,腹泻中位出现时间为12天。
发生率大于10%的不良反应有:皮疹、腹泻、食欲减低、疲劳、呼吸困难、咳嗽、恶心、感染、呕吐、口腔炎、瘙痒、皮肤干燥、结膜炎、角膜结膜炎、腹痛。
肺毒性:
有较少的报道提示在接受Tarceva治疗的NSCLC患者或其他实体瘤患者中可出现严重的间质性肺病(ILD),甚至导致死亡。
在随机对照研究中,ILD的发生率是0.8%,并且这一发生率在Tarceva治疗组和安慰剂组是相同的。
报道的ILD包括:肺炎、间质性肺炎、间质性肺病、闭塞性细支气管炎、肺纤维化、急性呼吸应激综合征和肺渗出。
症状发生于治疗后5天~超过9个月,中位发生时间为47天。
多数患者常有混杂因素导致ILD发生,如:之前有化疗/放疗、原有实质性肺疾病、肺转移或肺部感染。
当有新出现的、难以解释的肺部症状,例如:呼吸困难、咳嗽、发热等,需进行检查评价,一旦诊断ILD,应停止继续使用Tarceva,并采取适当治疗。
肝毒性:
Tarceva治疗可引起无症状的肝转氨酶升高,因此,治疗期间应定期复查肝功能,包括:转氨酶、胆红素、碱性磷酸酶等,如果肝功能损害严重应减量或停药。
肝功能损害常为暂时性的或伴有肝转移。
较少报道有胃肠道出血,常发生于同时应用华法林的患者,所以,同时服用华法林或其他抗凝剂的患者应监测凝血酶原时间。
老年患者:
安全性和药代动力学在年轻人和老年患者中无明显差异,因此,应用于老年患者时不建议调整剂量。
【注意事项】本品必须在有此类药物使用经验的医生指导下使用,并仅在国家肿瘤药物临床试验基地或三级甲等医院使用。
【贮存】25℃保存,15℃~30℃之间也可接受。
【包装】PVC泡罩包装;30片/盒。
【有效期】36个月
【进口药品注册证号】H20060108
【生产企业】Roche Pharma(Schweiz)Ltd.
本文由特罗凯格列卫整理!。
