厦门大学海洋科学导论课件(水文部分)
合集下载
海洋学导论-第三章(PPT文档)

第三章 海水的物理特性和 世界大洋的层化结构 (4学时)
基本要求:掌握 标准海水、位温、密度超量、盐度(3种定义)、太阳高 度、温(盐)跃层、水团等概念(特性);理解 海面热平衡方程、整个海洋全 热量平衡方程、局部海域的及整个海洋的全水量平衡方程(状态);熟悉 温度、 盐度、密度一般分布规律及三者的水平、铅直分布特征(形式);了解一些物 理海洋学中常用术语及表示方法,包括分布图形(类型)。本章是学习物理海 洋学、化学海洋学、海洋生物学的基础。
KCl溶液的电导比来确定海水的盐度值。 【教材59页】 即电导比K15 = C标海/C标KCl = 1)则
(二)实用盐度的计算公式
∑5Βιβλιοθήκη S=aiK i/2 15
i=0
(3-3)
式中K15是在101325Pa下,温度15℃时,海水样品的电导率与标准所配 KCl溶液的电导率之比。式中a0=0.0080,a1=-0.1692,a2=25.3851, a3=14.0941,a4=-7.0261,a5=2.7081;,适用范围为2≤S≤42。【S定义域2 为淡水最高值,计算结果1×10-3,盐度都是在10-3小数点变化】故此:
S‰=-0.08996+28.29720R15+12.80832R215
-10.67869R315+5.98624R415-1.32311R515
(3-2)
实测的海水的电导率,查《海洋学常用表》可得S‰ 值
三、1978年实用盐度标度(P78)
(一)建立实用盐度的固定参考点 配制浓度为32.435‰KCl溶液代替上述标准海水,用海水相对于此
§3.1 海水的主要热学和力学性质 (1学时)
海水是一种溶解有多种无机盐、有机物质和气体以及含有许多悬浮物质的 混合液体。
基本要求:掌握 标准海水、位温、密度超量、盐度(3种定义)、太阳高 度、温(盐)跃层、水团等概念(特性);理解 海面热平衡方程、整个海洋全 热量平衡方程、局部海域的及整个海洋的全水量平衡方程(状态);熟悉 温度、 盐度、密度一般分布规律及三者的水平、铅直分布特征(形式);了解一些物 理海洋学中常用术语及表示方法,包括分布图形(类型)。本章是学习物理海 洋学、化学海洋学、海洋生物学的基础。
KCl溶液的电导比来确定海水的盐度值。 【教材59页】 即电导比K15 = C标海/C标KCl = 1)则
(二)实用盐度的计算公式
∑5Βιβλιοθήκη S=aiK i/2 15
i=0
(3-3)
式中K15是在101325Pa下,温度15℃时,海水样品的电导率与标准所配 KCl溶液的电导率之比。式中a0=0.0080,a1=-0.1692,a2=25.3851, a3=14.0941,a4=-7.0261,a5=2.7081;,适用范围为2≤S≤42。【S定义域2 为淡水最高值,计算结果1×10-3,盐度都是在10-3小数点变化】故此:
S‰=-0.08996+28.29720R15+12.80832R215
-10.67869R315+5.98624R415-1.32311R515
(3-2)
实测的海水的电导率,查《海洋学常用表》可得S‰ 值
三、1978年实用盐度标度(P78)
(一)建立实用盐度的固定参考点 配制浓度为32.435‰KCl溶液代替上述标准海水,用海水相对于此
§3.1 海水的主要热学和力学性质 (1学时)
海水是一种溶解有多种无机盐、有机物质和气体以及含有许多悬浮物质的 混合液体。
厦大海洋科学导论

·水汽压:湿空气中,由水汽所引起的那一部分压强.
·饱和水汽压:当温度一定时,若从纯水的水表面逸入空气中的水分与从空气中进入水面的水分在数量上相同,此时水汽所造成的那部分压强。温度越高,E越大。
·相对湿度:空气中实际水气压e与同温度下的饱和水气压E之比。RH=e/E*100%。
·风:空气相对于地面作水平运动。
·朔望月:月相完全更替一轮的周期。
·海洋潮汐:海洋潮汐是由天体(主要是月球和太阳)的引潮力产生的。
·月球引潮力:地球绕地月公共质心所产生的惯性离心力与月球引力的合力。
·平衡潮理论:
(1)假设前提:1.地球为一圆球,其表面完全被等深的海水所覆盖,不考虑陆地的存在;2.海水没有粘滞性,也没有惯性,海面随时与等势面重叠;3.海水不受地转偏向力和摩擦力的作用。
·波的波形传播特征:1.水质点只在平衡位置做圆周运动;2.波峰水质点向前,波谷处水质点向后,峰前节点水质点向上,峰后节点水质点向;3..海浪的传播实际上只是波形的传播。
·有限振幅波动(斯托克斯波):1.波面不是简谐曲线,且对于横轴不对称,水质点振动中心高于平均水面;2.c与H有关,δ,c;3.水质点的轨迹很近于圆,但不是封闭的;4.波动的动能大于势能Ek>Ep,而且Ek铅直>E k水平;5.δ大于一定程度波面将破碎,理论上其破碎角为120°或δ≥1/7。
不利的方面:
1.内波引起的等温度面和等密度面的起伏会影响到海洋中声信号的传播速度与方向。从而降低了声纳的功能,增加了水下通讯和目标探测的困难。
2.内波会引起等密度面的快速(流速急)的大振幅上下起伏。如果有潜艇和鱼雷等水下航行物体位于这种等密度面处,它们将随等密度面上下运动或快速上浮下沉,导致鱼雷脱靶,潜艇难以操作。如有海上采油平台,也会受到严重影响和损害。
·饱和水汽压:当温度一定时,若从纯水的水表面逸入空气中的水分与从空气中进入水面的水分在数量上相同,此时水汽所造成的那部分压强。温度越高,E越大。
·相对湿度:空气中实际水气压e与同温度下的饱和水气压E之比。RH=e/E*100%。
·风:空气相对于地面作水平运动。
·朔望月:月相完全更替一轮的周期。
·海洋潮汐:海洋潮汐是由天体(主要是月球和太阳)的引潮力产生的。
·月球引潮力:地球绕地月公共质心所产生的惯性离心力与月球引力的合力。
·平衡潮理论:
(1)假设前提:1.地球为一圆球,其表面完全被等深的海水所覆盖,不考虑陆地的存在;2.海水没有粘滞性,也没有惯性,海面随时与等势面重叠;3.海水不受地转偏向力和摩擦力的作用。
·波的波形传播特征:1.水质点只在平衡位置做圆周运动;2.波峰水质点向前,波谷处水质点向后,峰前节点水质点向上,峰后节点水质点向;3..海浪的传播实际上只是波形的传播。
·有限振幅波动(斯托克斯波):1.波面不是简谐曲线,且对于横轴不对称,水质点振动中心高于平均水面;2.c与H有关,δ,c;3.水质点的轨迹很近于圆,但不是封闭的;4.波动的动能大于势能Ek>Ep,而且Ek铅直>E k水平;5.δ大于一定程度波面将破碎,理论上其破碎角为120°或δ≥1/7。
不利的方面:
1.内波引起的等温度面和等密度面的起伏会影响到海洋中声信号的传播速度与方向。从而降低了声纳的功能,增加了水下通讯和目标探测的困难。
2.内波会引起等密度面的快速(流速急)的大振幅上下起伏。如果有潜艇和鱼雷等水下航行物体位于这种等密度面处,它们将随等密度面上下运动或快速上浮下沉,导致鱼雷脱靶,潜艇难以操作。如有海上采油平台,也会受到严重影响和损害。
海洋学导论海洋生物(精品资料)PPT

海洋学导论海洋生物
海洋生物环境分区
• 海洋生物的栖居环境分水层和底层两局 部
• 水温是决定海洋生物的生存区域、物种 丰度及其变动的主要环境因素
• 盐度对海洋生物的作用主要在于影响渗 透压,因为大多数海洋生物和海水是等 渗的
海洋生物环境分区
• 海水深度对生物最明显的影响是流体静 压力的作用和光照深度
• 海洋生物的生理过程“生物泵〞对全球 起哄的作用
海洋生物生态类群
• 海洋生物生态类群分为浮游生物、游泳 生物和底栖生物三大类群
• 海洋浮游生物〔marine plankton〕 • 这个生态类群的生物缺乏兴旺的运动
器官,没有或仅有微弱的游动能力,悬 浮在水层中随水流移动。又分为 phytoplankton • zooplankton
上升流或铅直方向的海水混合,能把较冷但富有营养物质的深层海水输送到上表层,是指成为富于生产能力的海域。 任何一个生态系统包含生命和非生命两大局部,前者依其在生态系统中的功能分为生产者、消费者和分解者,后者包含无机物质、有
• 全世界底栖植物的平均生产力为海洋浮 机化合物和气候因素
海洋生态系统的反响机制,是生态系统会保持自身的生态平衡 海洋生物为我们提供食物、医药材料、工业材料等
游植物的2%-5% 生物因素:浮游动物的摄食
海洋动物生产力
• 二级生产力:指以植物、细菌等初级生产力为 营养来源的生物生产能力,主要包括大局部浮 游动物、底栖动物和植食性游泳
• 三级生产力:指以浮游动物等二级生产者为营 养来源的生物生产能力;主要包括一些肉食性 的鱼类和大型无脊椎动物
• 终极生产力:指一些自身不再被其他生物所消 耗的生物生产力;主要包括凶猛的鱼类和其他 大型或特大型动物,也可以是任何一级生产者
海洋生物环境分区
• 海洋生物的栖居环境分水层和底层两局 部
• 水温是决定海洋生物的生存区域、物种 丰度及其变动的主要环境因素
• 盐度对海洋生物的作用主要在于影响渗 透压,因为大多数海洋生物和海水是等 渗的
海洋生物环境分区
• 海水深度对生物最明显的影响是流体静 压力的作用和光照深度
• 海洋生物的生理过程“生物泵〞对全球 起哄的作用
海洋生物生态类群
• 海洋生物生态类群分为浮游生物、游泳 生物和底栖生物三大类群
• 海洋浮游生物〔marine plankton〕 • 这个生态类群的生物缺乏兴旺的运动
器官,没有或仅有微弱的游动能力,悬 浮在水层中随水流移动。又分为 phytoplankton • zooplankton
上升流或铅直方向的海水混合,能把较冷但富有营养物质的深层海水输送到上表层,是指成为富于生产能力的海域。 任何一个生态系统包含生命和非生命两大局部,前者依其在生态系统中的功能分为生产者、消费者和分解者,后者包含无机物质、有
• 全世界底栖植物的平均生产力为海洋浮 机化合物和气候因素
海洋生态系统的反响机制,是生态系统会保持自身的生态平衡 海洋生物为我们提供食物、医药材料、工业材料等
游植物的2%-5% 生物因素:浮游动物的摄食
海洋动物生产力
• 二级生产力:指以植物、细菌等初级生产力为 营养来源的生物生产能力,主要包括大局部浮 游动物、底栖动物和植食性游泳
• 三级生产力:指以浮游动物等二级生产者为营 养来源的生物生产能力;主要包括一些肉食性 的鱼类和大型无脊椎动物
• 终极生产力:指一些自身不再被其他生物所消 耗的生物生产力;主要包括凶猛的鱼类和其他 大型或特大型动物,也可以是任何一级生产者
海洋科学导论第五章

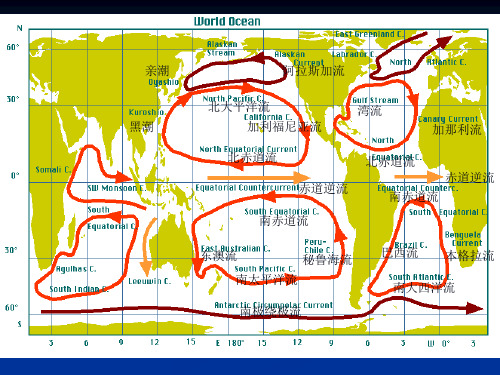
湾流
西边界流 湾流系统: 佛罗里达海流 湾流 北大西洋海流
右侧:温暖低密 左侧:低温高密
年变化 夏强冬弱
非周期性变化 ——弯曲现象
弯曲与主流断离----独立涡旋 左侧暖涡,右侧冷涡
(二)、太平洋的表面环流
亚北极海流 寒流 阿拉斯加海流 暖流
亲潮 寒流
北太平洋流 暖流
黑潮 暖流 北赤道流 暖流
30.7
流速的大小,与等值线倾斜的程度成正比
T
22.5℃ 22.6℃ 22.7℃ 22.8℃ 22.9℃ 23.0℃
S
33.2 33.3 33.4 33.5 33.6 33.7 33.8
三、地转流 海水密度均匀,等压面(海面)---等势面倾斜β角
Fz
Fx
β
fc
g
∵
Fx=gtgβ
fc=2ωvsinф
3、北半球强大的 西边界流;
4、主涡旋北部有小的 气旋式环流;
5、西风漂流绕南极大 陆流动;
6、南极大陆附近东 风漂流。
三、各大洋的表层环流 (一)大西洋
东格陵兰海流 寒流 拉布拉多海流
寒流
北大西洋流 暖流
湾流
暖流
加那利海流 寒流
北赤道流 暖流
南赤道流
暖流
巴西海流
暖流
本格拉海流 寒流 西风漂流 寒流
∴ gtgβ=2ωvsinф
地转流的速率 v g tg 2sin
y x
-z
北半球 顺流而立,右方高
南半球相反
四、地形对海流的影响 隆起地形: 北半球 上坡,向右偏转(顺时针) 下坡,向左偏转(逆时针)
南半球方向相反
第三节、风海流 一、风海流的受力分析
1、风的切应力 2、地转偏向力 3、下层海水阻力
厦门大学海洋科学导论课件(水文部分)lect11_1107

AAIW NADW AABW
AAIW: Antarctic Intermediate Water AABW: Antarctic Bottom Water NADW: North Atlantic Deep Water
沿大西洋南北纵断面上几种水团的分布情形 摘自Stowe, K. (1995) "Exploring Ocean Science", 2th ed.。
所有的生物“沙漠”都在扩大。其增 大的面积达到660万平方公里,即比原 有面积增加了15%。
Polovina J.J., GRL, 2008.
4. Undersurface circulation (大洋表层以下的环流)
(1) Movement and distribution of subsurface water (次表层水) 介于表层水(Surface water)与大洋主温跃层(Main Thermocline)之间;副热带海域表层水下沉而成;高 温高盐;大部分水体流向低纬一侧,沿主温跃层散布, 少部分流向高纬一侧 (2) Movement of intermediate water(中层水) (a) 南极辐聚和西北辐聚区海水下沉形成,约在8001000m,带有源地的低盐特征 (b) 地中海、红海-波斯湾水等溢出的高盐中层水
副热 南北半球反气 带辐 旋式大环流的 聚区 中间海域
a) b)
流速小
世界大洋最高的水色和最大透明度, 也是生产力最低的地方
副热带辐聚区
副热带辐聚区
NP
NP,december SP NA,december NA
SP,August SA
Time series of the monthly mean area (km2) with surface chlorophyll less than or equal to 0.07mg chl/m3 between 5–45 N/S latitude
海洋科学概论优品ppt资料

李永棠、黃國銘
■授課 □分組討論
■授課 □分組討論
□授課 ■分組討論
協同教學「海洋沉積物探索核爆與地震之軌跡」 蘇志杰、黃國銘
由於經濟高度成長,工業發達,人口大量集中都 市,使得未經處裡的各類廢水經由河川進入海洋,使 海域直接、間接承受相當大的汙染量。
各組議題口頭報告:「全球暖化對海洋之影響」 「聖 嬰現象」
課程主軸結構
本課程主旨在培養學生如何在人類繼 續追求向前發展的同時,做到人與海洋環 境的和諧相處,以達永續經營與發展的理 想。本課程內容在介紹海洋和任何在海洋 中所發生的現象。近年來人類對海洋學的 興趣日增,目前相當重視並利用海洋資源, 且積極保育海洋生態。
開課基本資料
• 開課年級:無限制 • 開課教師:黃國銘 • 學分數:2 學分 • 修課人數:女 8 人、男 52 人,共60人 • 師資團隊總人數: 6 人 • 外聘校外師資人數: 5 人
協同教學「我國海洋政策、法制與執行」
台海探輔灣洋討導海西 觀 海 分洋部光洋組沿休油期科海閒污中技地的染書區概主面長念要與久、成期抽台因末方取灣來口地的自頭孟下海大專德水洋氣題灌觀、報溉光自告、休然,養閒漏以能殖、出確工、海、認源甚洋都同業與至觀市學技於光排的環飲休放報術境用閒、告研,的海方研極本域向究究度土油與院依案田內所賴例探容地、勘可下台產以底氯水灣生契泥聯資海、合中苯利源洋油本,觀輪課作P用A帶光及程為H海來發卸主s海作洋當展油旨地因站。域為沈沿素正污海積海 分 常染域物居析情民。況源污岩生漏調染心命出查源探與及人油調討。文輪查污極意、染大外之事牡歷衝件蠣史擊漏中。出,。多
黃國銘 黃國銘
□授課 ■分組討論 各組議題口頭報告:「沿岸海域監測」「海洋油污染」
黃國銘
厦门大学海洋科学导论课件(水文部分)lect12_1114
深水波(短波) 浅水波(长波)
有限振幅波(a≈ λ)
Wave of Finite Amplitude
② 风浪(wind wave)、涌浪(swell)、 近岸浪(coastal wave)
• Deep water waves(深水波): h (water depth)>½ λ(wavelength) • Shallow water waves(浅水波):h <1/20λ
2. 有限水深( 1 h 1 )
20
2
轨迹方程为
( x x0 )2 A2
(z
z0 )2 B2
1
A a ch[k(h z)], B a sh[k(h z)]
sh(kh)
sh(kh)
(1)轨迹为椭圆 (2)椭圆的长短轴皆随z↑而↓,但长轴变化较慢,短
轴变化较快,在海底短轴为0
次表层水(subsurface water): 副热带海域表层 海水下沉。高盐、 相对高温
中层水(intermediate water):南极辐聚和西北辐 聚区海水下沉形成,低盐
深层水(deep water):北大西洋格陵兰南部的上 层海洋形成。贫氧
底层水(bottom water):具有最大的密度,主要 来源于威德尔海、罗斯海,其次是格陵兰海 与挪威海
P ec
c* c gh
(3) 波动所具有的能量相当可观
例:波高为3m,周期为7s的一个波动,跨过10km宽的 海面。求它的功率(波动功率指单位时间内跨过单位 截面的能量)
W P 1 ec 1 1 gH 2 gT
2
28
2
g g H 2 T 32
若H单位为米,周期为秒,取g=9.8m/s2,则
厦门大学海洋科学导论课件(水文部分)lect15_1205

D C1 C
* * 2
1 D
1 2 c0 gh
2
c0 gh
深水波 浅水波
c1
c0 gh
在D的影响下,波浪从深水→ 浅水,波高开始略有降低然后 随着水深的减小而增大
★ 可见波浪传到近岸,波高的变化完全取决于能量的变化。一 般来说,D的作用比折射因子大,但在海岬与海湾处,由于波 向转折,其影响对H的变化往往起着明显的作用。
4. Wave breakers(波浪破碎)
Wave steepness (stability) is a ratio of wave height divided by wave length (= H/L).
H/L is larger than or equals 1/7 (H/L 1/7), the wave becomes unstable. There are three types of breakers:, Spilling breakers溢出型 , Plunging breakers 崩捲型 , and Surging breakers崩塌型 .
Mustang Island regular wave trains
1) 波速和波长的变化
c
2
g 2
tanh(
2 h
)
Shallow-water wave
c
2 2 0
c0
2
g 0 2
Deep water wave
c
0
tanh(
2 h
)
周期保持不变: T=T0
c c0
反射﹕波浪前進遭遇固體邊界時,為滿足水流只能平行於邊界面運動之限制, 於是會產生向相反方向傳播的波浪,這就是反射現象。
* * 2
1 D
1 2 c0 gh
2
c0 gh
深水波 浅水波
c1
c0 gh
在D的影响下,波浪从深水→ 浅水,波高开始略有降低然后 随着水深的减小而增大
★ 可见波浪传到近岸,波高的变化完全取决于能量的变化。一 般来说,D的作用比折射因子大,但在海岬与海湾处,由于波 向转折,其影响对H的变化往往起着明显的作用。
4. Wave breakers(波浪破碎)
Wave steepness (stability) is a ratio of wave height divided by wave length (= H/L).
H/L is larger than or equals 1/7 (H/L 1/7), the wave becomes unstable. There are three types of breakers:, Spilling breakers溢出型 , Plunging breakers 崩捲型 , and Surging breakers崩塌型 .
Mustang Island regular wave trains
1) 波速和波长的变化
c
2
g 2
tanh(
2 h
)
Shallow-water wave
c
2 2 0
c0
2
g 0 2
Deep water wave
c
0
tanh(
2 h
)
周期保持不变: T=T0
c c0
反射﹕波浪前進遭遇固體邊界時,為滿足水流只能平行於邊界面運動之限制, 於是會產生向相反方向傳播的波浪,這就是反射現象。
(海洋科学概论课件)第四章海洋中的热收支和水平衡

A、 太阳高度h:
B、大气透明度 C、天空中的云量、云状、不同纬度和季节 D、真正进入海洋的太阳辐射
2019/1/14
10
海洋中的热收支和水平衡
3、 总辐射能分布(The distribution of total radiant energy):
1)纬度(latitude): A、随纬度升高而减小 B、除赤道地区外,夏半年均 高于冬半年且差值随纬度升 高而增大。 C、经向梯度夏半年小于冬半年。
2019/1/14
7
海洋中的热收支和水平衡
2
辐射定律(law of radiation):
1)斯蒂芬—波尔兹曼定律:
任何温度高于绝对零度的物体都能以辐射的形式向
外释放能量,它与绝对温度Tk的4次方成正比。
F为辐射体的透明系数,
F=0,绝对透明体,F=1,绝对黑体。
2019/1/14 8
海洋中的热收支和水平衡
2019/1/14
11
海洋中的热收支和水平衡
3 总辐射能分布(The distribution of total radiant energy):
2)进入海水中的辐射能: 主要被表层海水吸收,随深度增加指数衰减。
2
辐射定律(law of radiation):
2)维恩定律:
辐射能量的最大波长与辐射体表面的绝对温度成反比。
C=2898(µm·k) 太阳辐射能量最强的波长为??? 0.475µm, 称为短波辐射,
对应于可见光中的青光波段
2019/1/14 9
海洋中的热收支和水平衡
3、影响因素( influencing factors):
海洋科学导论 第四章PPT幻灯片

异常升高(降低)现象。 别名: 气象海啸
与天文大潮结合,水位暴涨
5).内波(interal wave) 流体中密度垂直分布层化时,由外力扰动
产生的波动 。
海洋内波的特点
相同能量产生的内波振幅大于海面波振幅
海面的海水上升需要克服重力 海洋内部海水上升,重力和浮力几乎抵消
海洋内波的利弊
内波引起的混合,尤其是穿过跃层的混合有 利于物质和能量的输送,对海洋环境和生态 保护发挥重要作用。
2)已知λ
C1.25 T0.8
3)已知C T=0.64C λ=0.64C2
2、浅水波
C= gh
C仅决定于h
三、波动随深度而变化 1、深水波
振幅a=a0e-2πz/λ a0:表面水质点的圆半径(振幅), z:水质点所处的深度
H=H0 e-2πz/λ
深度递增,波高指数规律递减
表面波
2、浅水波
C:波速,g:重力加速度,λ:波长,h:水深
波长、波速与水深有关
tanh2h :双曲线正切
定义为:
ex -e-x tanhxex e-x
x很大时,tanhx = 1 x很小时, tanhx = x
1、深水波
C= g 2
C仅1)已知T C=1.56 T λ=1.56T2
正弦曲线→波峰尖,波谷圆的形状
5、按作用力的影响情况
1).自由波(free wave) 外力作用消失,波动继续存在并自由振动
2).强制波(forced wave) 扰动力连续作用产生的波动
第二节、波浪运动的基本特性
小振幅波理论
振幅相对于波长为无限小
有限振幅波理论 振幅是有限的
一、水质点运动与波形传播 波形的传播:
深度增加,波高线性递减,至海底为0, 水质点的水平运动上下一致。
与天文大潮结合,水位暴涨
5).内波(interal wave) 流体中密度垂直分布层化时,由外力扰动
产生的波动 。
海洋内波的特点
相同能量产生的内波振幅大于海面波振幅
海面的海水上升需要克服重力 海洋内部海水上升,重力和浮力几乎抵消
海洋内波的利弊
内波引起的混合,尤其是穿过跃层的混合有 利于物质和能量的输送,对海洋环境和生态 保护发挥重要作用。
2)已知λ
C1.25 T0.8
3)已知C T=0.64C λ=0.64C2
2、浅水波
C= gh
C仅决定于h
三、波动随深度而变化 1、深水波
振幅a=a0e-2πz/λ a0:表面水质点的圆半径(振幅), z:水质点所处的深度
H=H0 e-2πz/λ
深度递增,波高指数规律递减
表面波
2、浅水波
C:波速,g:重力加速度,λ:波长,h:水深
波长、波速与水深有关
tanh2h :双曲线正切
定义为:
ex -e-x tanhxex e-x
x很大时,tanhx = 1 x很小时, tanhx = x
1、深水波
C= g 2
C仅1)已知T C=1.56 T λ=1.56T2
正弦曲线→波峰尖,波谷圆的形状
5、按作用力的影响情况
1).自由波(free wave) 外力作用消失,波动继续存在并自由振动
2).强制波(forced wave) 扰动力连续作用产生的波动
第二节、波浪运动的基本特性
小振幅波理论
振幅相对于波长为无限小
有限振幅波理论 振幅是有限的
一、水质点运动与波形传播 波形的传播:
深度增加,波高线性递减,至海底为0, 水质点的水平运动上下一致。
厦门大学课程之海洋生态学PPT课件

图4.7 引入竞争机制的种群增长过程
第20页/共49页
• 直线方程:
dN dt
·N1
=-Kr
·N+r
• 整ddN理t =得r:N(1-NK )
第21页/共49页
(三)具时滞的种群增长模型
dN dt
= rN〔K-NK(t - T) 〕
3.0
2.0
2.5
种群大小
2.0
1.5
1.4
1.0
1.0
K
0.5
r–选择
K–选择
(机会种,opportunistic species) (平衡种,equilibrium species)
候
多变,难以预测,不确定
稳定,可预测,较确定
成体大小
小
大
生长率
快
慢
性成熟时间
早
迟
繁殖周期
多
少
幼体数量
多
少
扩散能力
高
低
种群大小 竞争能力
可变,常<K 值 低
相对稳定,接近 K 值 高
死亡率
高,非密度制约
低,密度制约
生命周期
短(<1a)
长(>1a)
水层/底栖的比率
高
低
第27页/共49页
(二)生活史模式的多样化
• 两种极端对策之间是一个连续谱。 • 大部分海洋真骨鱼类是偏向于r–选择,很多软骨鱼类
(鲨、鳐)趋向于采取K–选择。 • 浮游植物通常属于r–选择的类别,但如果深入分析,
第12页/共49页
(二)生命表和存活曲线
1、动态生命表 (dynamic life table)或称股群生命 表(cohort life table)
第20页/共49页
• 直线方程:
dN dt
·N1
=-Kr
·N+r
• 整ddN理t =得r:N(1-NK )
第21页/共49页
(三)具时滞的种群增长模型
dN dt
= rN〔K-NK(t - T) 〕
3.0
2.0
2.5
种群大小
2.0
1.5
1.4
1.0
1.0
K
0.5
r–选择
K–选择
(机会种,opportunistic species) (平衡种,equilibrium species)
候
多变,难以预测,不确定
稳定,可预测,较确定
成体大小
小
大
生长率
快
慢
性成熟时间
早
迟
繁殖周期
多
少
幼体数量
多
少
扩散能力
高
低
种群大小 竞争能力
可变,常<K 值 低
相对稳定,接近 K 值 高
死亡率
高,非密度制约
低,密度制约
生命周期
短(<1a)
长(>1a)
水层/底栖的比率
高
低
第27页/共49页
(二)生活史模式的多样化
• 两种极端对策之间是一个连续谱。 • 大部分海洋真骨鱼类是偏向于r–选择,很多软骨鱼类
(鲨、鳐)趋向于采取K–选择。 • 浮游植物通常属于r–选择的类别,但如果深入分析,
第12页/共49页
(二)生命表和存活曲线
1、动态生命表 (dynamic life table)或称股群生命 表(cohort life table)
海洋科学导论精选 课件

14
漫长的西非海岸向南方延 伸下去,一眼望不到头, 使习惯于内海航行的水手 们极不适应。亨利王子每 年都派出船队,要他们沿 着海岸尽力向南探索,然 而一开始却少有成果。他 们遇到的头一个障碍是被 称作诺恩角 (Cape Noun)的地方, 海风卷来内陆的沙尘,把 海水染成红色,使水手们 感到莫名的恐惧,视之为 畏途。事实上它并不是什 么不可逾越的险阻,更多 的是一种心理上的“障 碍”,但却一度成为了葡 萄牙航海的南界。腊时代所认知的世界,公元前450年Herodotus所绘之地图。
5
§1.2 海洋科学的发展史 公元前140年古希腊的Ptolemy所绘之地图
6
Vikings discovered Greenland and America
7
地球探险时代 (15-16世纪):
另一个巨大的险阻, 即著名的博哈多尔 角 (Cape Bojador), 一个令水手们闻之 变色、让船长们束 手无策、使亨利王 子真实的信件(写 于1433年10月22日) 说,为了越过这个 海角,从1421年起 连续进行了14次探 险远征,却一直没 有取得成功。
1434年,埃内斯改变了以往紧紧靠着 海岸航行的方法——他的船直接南下 航行到远离博哈多尔角西边的海面上。 那里风平浪静的景象和海角附近的惊 涛骇浪形成了鲜明的对比,于是这个 曾阻挡了葡萄牙航海家前进的步伐多 年的海角就此轻而易举地被绕过去了。 埃内斯把船靠岸,发现天上的太阳并 没有什么异象,陆地上依然贫瘠荒凉, 稀疏的长着一些植物,极目望去,毫 无人踪。埃内斯采摘了一些玫瑰花, 葡萄牙人称之为“圣玛丽玫瑰”,以 此作为到过此地的证物。
19
20
21
22
The Voyages of Captain James Cook 1768 - 1780
漫长的西非海岸向南方延 伸下去,一眼望不到头, 使习惯于内海航行的水手 们极不适应。亨利王子每 年都派出船队,要他们沿 着海岸尽力向南探索,然 而一开始却少有成果。他 们遇到的头一个障碍是被 称作诺恩角 (Cape Noun)的地方, 海风卷来内陆的沙尘,把 海水染成红色,使水手们 感到莫名的恐惧,视之为 畏途。事实上它并不是什 么不可逾越的险阻,更多 的是一种心理上的“障 碍”,但却一度成为了葡 萄牙航海的南界。腊时代所认知的世界,公元前450年Herodotus所绘之地图。
5
§1.2 海洋科学的发展史 公元前140年古希腊的Ptolemy所绘之地图
6
Vikings discovered Greenland and America
7
地球探险时代 (15-16世纪):
另一个巨大的险阻, 即著名的博哈多尔 角 (Cape Bojador), 一个令水手们闻之 变色、让船长们束 手无策、使亨利王 子真实的信件(写 于1433年10月22日) 说,为了越过这个 海角,从1421年起 连续进行了14次探 险远征,却一直没 有取得成功。
1434年,埃内斯改变了以往紧紧靠着 海岸航行的方法——他的船直接南下 航行到远离博哈多尔角西边的海面上。 那里风平浪静的景象和海角附近的惊 涛骇浪形成了鲜明的对比,于是这个 曾阻挡了葡萄牙航海家前进的步伐多 年的海角就此轻而易举地被绕过去了。 埃内斯把船靠岸,发现天上的太阳并 没有什么异象,陆地上依然贫瘠荒凉, 稀疏的长着一些植物,极目望去,毫 无人踪。埃内斯采摘了一些玫瑰花, 葡萄牙人称之为“圣玛丽玫瑰”,以 此作为到过此地的证物。
19
20
21
22
The Voyages of Captain James Cook 1768 - 1780
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2.2.1 Temperature
Measurement
Absolute Temperature T
Unit: Kelvin (K)
t [℃] = T [K]-273.15
The practical temperature scale (1887, 1927, 1948, 1968, and 1990)
Review
The shape and size of the earth Pear-shaped; average radius(6371km);rotation rate (7.3×105s-1); L(1°) ↔111.13km; L(1′) ↔1.852km; 1 nautical mile(海里)=1.852km; 1kts(节)=1nautical mile/hour=0.51m/s
Distribution of Ocean and Land
Land and water are not uniformly distributed on the surface of the earth
Ocean and Sea
Four Principle Ocean
Hydrological features of ocean, sea, fjord(bay), strait(channel)
Principle of constant proportion states that the absolute amount of salt in sea water varies, but the relative proportions of the ions is constant.
Lehodey,P. et al., 2000. El Nino Southern Oscillation and tuna in the western Pacific. Science
Temperature distribution in California coastal area
长江水的扩展
溶解力强:水分子有很强的极性
密度变化异常
不遵从“热胀冷缩”。度<4,有利于水分子的缔合;冻结为冰时,全部缔合成一个巨大的分子缔合体(分子晶 体).由于晶体结构排列松散,故密度减小.当温度从04度以前,主要过程是较大的缔合分 子逐渐分解成为较小的分子,所以体积收缩,密度增大.>4度时,热运动加强,导致体积膨 胀,密度所温度增高而减小
热性质特殊
沸点(boiling point)和融点(melting point)、比热(specific heat)、蒸发潜热 (latent heat of vaporization)等热性质比氧的同族化合物高
2.2 温度、盐度和密度的概念及关系 Temperature, Salinity and Density
分子结构(Molecular structure):极性,分子缔合力
Hydrogen
Hydrogen
105 deg
Oxygen
Hydrogen Bonds–polar molecules give water a structure that is responsible for a number of unique and important properties
§2 海水的物理性质 Physical Properties of Sea Water
2.1 Water
Most common substance on the earth’s surface Ocean water makes up 98% of water inventory 96.5% is water, 3.5% is salt and dissolved minerals
Because of this principle, it is necessary to test for only one salt ion, usually chlorine, to determine the total amount of salt present.
海水组成恒定性原理
1) 传统的盐度定义(1902): 1kg海水中将(Br-,I-)以氯置换,碳酸盐分
ITS-90: the International Temperature Scale of 1990 ITS-68: the International Temperature Scale of 1968
At 0℃ they are the same, and above ℃ its-90 is slightly
Accumulated on the surface from mass degassing of earth’s interior… still happening, but much less
Only substance that exists naturally in all three states in the normal temperature range of earth Solid, Liquid, and Gas
99% of all the salt ions in the sea are sodium (Na+), chlorine (Cl-), sulfate (SO4-2), Magnesium (Mg+2), calcium (Ca+2) and potassium (K+).
Definition of Salinity
cooler.
t90-t68 = -0.002 at 10 ℃; -0.005 at 20 ℃, -0.007 at 30 ℃ and -0.010 at 40 ℃.
t90=0.99976 t68; t68=1.00024 t90
2.2.2 Salnity
11种主要无机盐,占99.99%;
