有机酸无机酸PKa值表
pka的计算例子

pka的计算例子pKa是表示酸碱强弱的指标,是酸碱性常数的负对数。
在化学中,pKa值的计算是非常重要的,它可以用来预测化合物的酸碱性质,以及在化学反应中的反应速率和平衡位置。
下面将列举一些pKa的计算例子,以帮助读者更好地理解和应用这一概念。
1. 甲酸(HCOOH)和乙酸(CH3COOH)的pKa值分别为3.75和4.76。
这意味着在等浓度下,甲酸的酸性比乙酸强,其pKa值越小,酸性越强。
2. 醋酸(CH3COOH)和水(H2O)的pKa值分别为4.76和15.7。
由于水的pKa值远高于醋酸,可以得出结论,醋酸是一个较强的酸,而水是一个较弱的酸。
3. 胺是一类含有氮原子的有机化合物,它们可以作为碱。
例如,乙胺(CH3CH2NH2)的pKa值为10.6,而二甲基胺((CH3)2NH)的pKa值为10.8。
这表明二甲基胺比乙胺稍微弱碱一些。
4. 碳酸根离子(HCO3-)是碳酸(H2CO3)的共轭碱。
碳酸的pKa值为6.35,而碳酸根离子的pKa值为10.33。
这意味着在碱性条件下,碳酸会脱去一个质子形成碳酸根离子。
5. 氨基酸是一类化合物,它们同时含有酸性和碱性基团。
例如,天冬氨酸的pKa值为2.09和9.47,而丙氨酸的pKa值为2.34和9.88。
这说明天冬氨酸在酸性条件下更容易失去质子,而丙氨酸在碱性条件下更容易得到质子。
6. 苯胺(C6H5NH2)是一种芳香胺,它的pKa值为4.6。
这表明苯胺在酸性条件下可以失去一个质子,形成苯胺阳离子。
7. 氯酸(HClO3)和硝酸(HNO3)是两种常见的无机酸。
它们的pKa 值分别为-1.8和-1.3,这说明它们是非常强的酸。
8. 羟胺(NH2OH)是一种含氮的有机化合物,它的pKa值为9.50。
这表明在碱性条件下,羟胺可以得到一个质子,形成羟胺阳离子。
9. 酚是一类化合物,它们含有一个或多个羟基(-OH)。
对苯二酚(C6H4(OH)2)的pKa值为9.99,这说明在碱性条件下,对苯二酚可以失去一个质子。
各种酸的pKa及pH值-pb的pka

各种酸的pKa及pH值-pb的pka 各种酸的 pKa 及 pH 值 pb 的 pka在化学的世界里,酸的性质和行为是一个非常重要的研究领域。
其中,酸的解离常数(pKa)以及溶液的pH 值是描述酸性质的关键参数。
在这篇文章中,我们将深入探讨各种酸的 pKa 以及它们与 pH 值之间的关系,并特别关注一下 pb 的 pka。
首先,让我们来了解一下什么是 pKa 和 pH 值。
pKa 是酸解离常数(Ka)的负对数。
酸解离常数Ka 表示酸在溶液中解离出氢离子(H⁺)和酸根离子的程度。
Ka 值越大,说明酸的解离程度越大,酸性越强;而 pKa 值越小,酸性越强。
pH 值则是用来衡量溶液中氢离子浓度的指标。
它的定义是氢离子浓度的负对数,即 pH = logH⁺。
pH 值的范围通常在 0 到 14 之间,7为中性,小于 7 为酸性,大于 7 为碱性。
常见的无机酸如盐酸(HCl)、硫酸(H₂SO₄)和硝酸(HNO₃)都是强酸,它们在水溶液中几乎完全解离。
盐酸的 pKa 约为-63,硫酸的第一步解离 pKa 约为-3,硝酸的 pKa 约为-14。
由于它们的解离程度非常高,在计算 pH 值时,通常可以将其视为完全解离,根据其浓度直接计算氢离子浓度。
而弱酸在溶液中的解离则是一个平衡过程。
例如,乙酸(CH₃COOH)是一种常见的弱酸,其 pKa 约为 476。
这意味着在一定条件下,乙酸在溶液中只有一部分会解离出氢离子和乙酸根离子。
当我们知道乙酸的初始浓度和溶液的平衡状态时,可以通过解离平衡常数的表达式来计算溶液中的氢离子浓度,从而得出 pH 值。
再来说说磷酸(H₃PO₄),它是一种多元酸,具有三步解离。
第一步解离的 pKa 约为 212,第二步约为 721,第三步约为 1232。
多元酸的解离过程是逐步进行的,每一步的解离程度都不同,这也使得其溶液的 pH 值计算相对复杂,需要综合考虑各步解离的情况。
接下来谈谈有机羧酸,比如苯甲酸(C₆H₅COOH),其 pKa 约为42。
第五章_有机化合物的酸碱性

第五章 有机化合物的酸碱性酸碱是化学中的重要概念,从广义的角度讲,多数的有机化学反应都可以被看作是酸碱反应。
因此,酸碱的概念在有机化学中有着重要的应用,在学习有机化学的时候,学习与了解有机化合物的酸碱性是十分必要的。
5.1 Brönsted 酸碱理论1923年,为了克服S. A. Arrehenius 依据电离学说,所提出的水溶液中酸碱理论的不足,丹麦的J. N. Brönsted 和英国的J. M. Lowry 分别独立地提出了新的酸碱理论。
该理论给出的酸碱定义为:凡是能给出质子的任何物质(分子或离子),叫做酸;凡是能接受质子的任何物质,叫做碱。
简言之,酸是质子的给予体,碱是质子的接受体。
因此,Brönsted 酸碱理论又称为质子酸碱理论。
依据Brönsted 酸碱理论,酸给出质子后产生的碱,称之为酸的共轭碱;碱接受质子生成的物质就是它的共轭酸。
即:酸碱 +质子CH3CO 2H CH 3CO 2- + H +C2H 5OHC 2H 5O - + H +可以看出,CH 3CO 2H 给出质子是酸,生成的CH 3CO 2―则是碱。
这样的一对酸碱,称为共轭酸碱对。
C 2H 5OH 和C 2H 5O ―也是如此。
酸、碱的电离可以看作是两对酸碱的反应过程。
例如:CH3CO 2H + H 2OCH 3CO 2- + H 3O +酸1 + 碱2碱1 + 酸2H2O + CH 3NH 2OH - + CH 3NH 3+醋酸在水中的电离,CH 3CO 2H 给出一个质子是酸,H 2O 接受一个质子为碱。
这里,CH 3CO 2H/CH 3CO 2―与H 2O/H 3O +分别是两个共轭酸碱对。
但是,甲胺在水中电离时,H 2O 给出一个质子是酸,CH 3NH 2接受一个质子为碱。
H 2O/OH ―与CH 3NH 2/CH 3NH 3+分别是两个共轭酸碱对。
由此可见, Brönsted 理论中的酸碱概念是相对的。
有机、无机化合物酸碱解离常数pKa_pKb数据

pKa ValuesINDEXInorganic2Phenazine24 Phosphates3Pyridine25 Carboxylic acids4, 8Pyrazine26Aliphatic4, 8Aromatic7, 8Quinoline27Phenols9Quinazoline27Alcohols and oxygen acids10, 11Quinoxaline27Amino Acids12Special Nitrogen Compounds28Peptides13Hydroxylamines28Nitrogen Compounds14Hydrazines28Aliphatic amines15, 17, 19Semicarbazones28 Cyanoamines16Amidoximes28Anilines17, 18, 20Thiols29 Nucleosides21Carbon Acids30,31 Special Table Heterocycles22Indicators31Acridine23References32-34 Benzoquinoline24Cinnoline23Hydantoin24Imidazole24For complex chelating agents, see also reference 77.Note. This document was compiled by W.P. Jencks and has been added to by F.H. WestheimerACIDSCompound pK Ref.H3PO2 2.0, 2.23*28H2PO4–7.21*77 AgOH 3.964HPO4_12.32*77Al(OH)311.228H3PO3 2.028As(OH)39.2228H2PO3– 6.58*77H3AsO4 2.22, 7.0, 13.028H4P2O7 1.52*77H2AsO4– 6.98*77H3 P2O7– 2.36*77 HAsO4*11.53*77H2P2O7= 6.60*77As2O304H3AsO39.22*HP2O7=9.25*77H3BO39.23*28HReO4-1.2530H2B4O7 4.0034HSCN 4.0034H2SeO3 2.6, 8.3, 2.62*28 HB4O79.0034HSeO38.3277Be(OH)2 3.74H2SeO4Strong, 2.028 HBr-9.0031HOBr8.728HSeO4 2.0034 HOCl7.53, 7.4628, 33H3SiO310.034 HClO2 2.028H2SO3 1.9, 7.0, 1.76*28, 77 HClO3-1.0028H2SO4-3.0, 1.928 HClO4 (70%)-10.0031HSO37.21*77CH3SO3H-0.631HSO4– 1.99*77 HCN9.4034H2S2O4 1.929H2CO3 6.37, 6.35*, 3.5834, 32H2Se 3.89*77 HCO310.33*HSe–11.00*77H2CrO4-0.9830H2S7.00*77 HCrO4 6.50*2, 30HS–12.92*77 HOCN 3.9234HSbO211.034 HZ 3.17*, 0.59*77HTe 5.0034H2GeO38.59, 12.7234, 78H2Te 2.64, 11.034, 78 Ge(OH)48.68, 12.728H2TeO3 2.7, 8.028HI-10.031Te(OH)6 6.2, 8.828 HOI11.028H2VO4–8.9530 HIO30.828HVO4=14.430H4IO6– 6.0034H2CrO40.7477H5IO6 1.64, 1.55, 8.2734, 28HOCN 3.7377 HMnO4-2.2530HSCN0.8577 NH3OH* 5.98*H3PO2 1.0777 NH4*9.24*77H3PO4 2.12*77 HN3 4.72*77H2S2O30.60*, 1.72*77 HNO2 3.2928H3AuO313.3, 16.078 HNO3-1.328H3GaO310.32, 11.778N2H5+7.99*77H5IO6 3.29, 6.70, 15.078H2N2O27.0534(see above!)H2N2O2–11.034H4V6O17 1.9678H2OsO512.134H2NSO3H 1.080H2O15.7noneH3O+-1.7none* Indicates a thermodynamic value.Pb(OH)2 6.48 (10.92) 4 (78)PHOSPHATES AND PHOSPHONATES CF 3- 1.16, 3.9357CCl 3- 1.63, 4.8157Phosphates NH 3+CH 2- 2.35, 5.957CompoundpKRef.(–OOCCH 2)2NH +CH 2– --, 5.5757Phosphate 1.97, 6.82, 12.555CHCl 2- 1.14, 5.6157Glyceric acid 2-phosphate 3.6, 7.153CH 2CI- 1.40, 6.3057Enolpyruvic acid 3.5, 6.453CH 2Br- 1.14, 6.5257Methyl- 1.54, 6.3155(–OOCCH 22NH +(CH 2)2- --, 6.5457Ethyl- 1.60, 6.6255CH 2I- 1.30, 6.7257n-Propyl- 1.88,6.6755NH 3+CH 2CH 2- 2.45, 7.0057n-Butyl- 1.80, 6.8455Dimethyl- 1.2955C 6H 5CH=CH- 2.00, 7.157Di-n-propyl 1.5955HOCH 2- 1.91, 7.1557Di-n-butyl- 1.7255C 6H 5NH 2+(CH 2)3- 2.1, --57Glucose-3-0.84, 5.6756C 6H 5NH(CH 2)3---, 7.1757Glucose-4-0.84, 5.6756Br(CH 2)2- 2.25, 7.357α-glycero- 1.40, 6.4454CH 3(CH 2)5CH(COO –)---, 7.557β-glycero- 1.37, 6.3454C 6H 5CH 2- 2.3, 7.55573-phosphoglyceric acid 1.42, 3.4254NH 3+(CH 2)4)- 2.55, 7.55572-phosphoglyceric acid 1.42, 3.55, 7.1peroxymonophosphoric acid 4.0569NH 3+(CH 2)5- 2.6, 7.657diphosphoglyceric acid 7.40, 7.9954NH 3+(CH 2)10---, 8.0057glyceraldehyde- 2.10, 6.7554–OOC(CH 2)10---, 8.2557dioxyacetone- 1.77,6.4554(CH 3)3SiCH 2- 3.22, 8.7057hexose di- 1.52, 6.3154fructose-6-0.97, 6.1154C 6H 5CH 2- 3.3, 8.457glucose-6-0.94, 6.1154(C 6H 5)SC- 3.85, 9.0057glucose-1- 1.10, 6.1354adenylic acid 3.8?, 6.2?54Arylphosphonic acids inosinic acid 2.4?, 6.4?542X-RC 6H 3PO 3H 2ADP 2 strong, 6.654X R ATP 3 strong, 6.654Cl 4-O 2N 1.12, 6.1457pyrophosphoric acid 0.9, 2.0, 6.6, 9.454Br 5-O 2N (a), 6.1457phosphopyruvic acid 3.5, 6.3854Cl 5-Cl (a), 6.6357creatine phosphate 2.7, 4.554Cl H 1.63, 6.9857arginine phosphate 2.8, 4.5, 9.6, 11.254Br H 1.64, 7.0057arginine 2.02, 9.0, 12.554Br 5-CH 3 1.81, 7.1557amino phosphate (-0.9), 2.8, 8.254Cl 4-NH 2--, 7.3357trimetaphosphate 2.0577CH 3O 4-O 2N 1.53, 6.9657CH 3O H 2.16, 7.7757PhosphonatesCH 3O 4-O 2N --, 8.2257H 2O 3P(CH 2)4PO 3H 2 <2, 2.75, 7.54, 8.3857HO 4-O 2N 1.22, 5.3957H 2O 3P(CH 2)3PO 3H 2 <2, 2.65, 7.34, 8.3557O 2N H 1.45, 6.7457H 2O 3PCH 2CH(CH 3)PO 3H 2 <2, 2.6, 7.00, 9.2757F H 1.64, 6.8057H 2O 3PCH 2PO 3H 2 <2, 2.57, 6.87, 10.3357I H 1.74, 7.0657Methyl- 2.3557NH 2H --, 7.2957Ethyl- 2.4357CH 3H 2.10, 7.6857n-propyl- 2.4557C 6H 5H (a), 8.1357isopropyl- 2.55, 7.7557HOOC H1.71, 9.1757n-butyl- 2.59, 8.1957isobutyl- 2.70, 8.4357**These values were obtained in 50% ethanol.s-butyl- 2.74, 8.4857(a) The compounds were not sufficiently soluble.t-butyl- 2.79, 8.8857For graphical plots of a large number of substituted phosphorus compounds see 83.neopentyl- 2.84, 8.65571,1 Dimethylpropyl- 2.88, 8.9657n-hexyl- 2.6, 7.957triphosphate8.90, 6.26, 2.3077n-dodecyl---, 8.2557tetrametaphosphate 2.7477CH 3(CH 2)5CH(COOH)- 1, --57fluorophosphate0.55, 4.856Acetic acids, substituted Phosphonates (Ref. 2)H- 4.76*20 X-H-H-NH3+-NH3+O2N- 1.68*20 X(CH2)PO3H2 2.357.1 1.85 5.35(CH3)3N+- 1.83*20 X(CH2)2PO3H2 2.457.85 2.457.00(CH3)2NH+- 1.95*20 X(CH2)4PO3H2 2.557.55CH3NH2+- 2.16*20 X(CH2)5PO3 H2 2.67.65NH3+- 2.31*20 X(CH2)6PO2H2 2.67.9CH3SO2- 2.36*20 X(CH2)10PO2H28.00NC- 2.43*20 Phosphines in acetonitrile, see ref. 89.C6H5SO2- 2.4420HO2C 2.83*20 CARBOXYLIC ACIDSAliphatic C6H5SO- 2.6620 Compound pK Ref.F- 2.6620 Acetoacetic 3.586Cl- 2.86*20 Acetopyruvic 2.61, 7.85 (enol)6Br- 2.8620 Aconitic, trans- 2.80, 4.466Cl2- 1.2920 Betaine 1.846F2- 1.2420 Citric 3.09, 4.75, 5.416Br3-0.6620 Crotonic 4.696Cl3-0.6520 Dihydroxyfumaric 1.146F3-0.23 (-0.26) (2)20 Dethylenediamine- 2.00, 2.676HONC4 3.0120 tetraacetic 6.16, 10.26F3C- 3.07*20 Formic 3.77*2N3- 3.0320 Fumaric 3.03, 4.546I- 3.1220 Glyceric 3.556C6H5O- 3.1220 Glycollic 3.826C2H5O2C- 3.3520 Glyoxylic 3.326C6H5S- 3.52*20 Homogentistic 4.406CH3O- 3.5320α-keto-β-methyl valeric 2.36NCS- 3.5820 Lactic 3.866CH3CO- 3.58*20 Maleic 1.93, 6.586Malic 3.40, 5.26C2H5O- 3.6020 Oxaloacetic (trans-enol) 2.566n-C3H7O 3.6520 +(cis-enol) 2.15, 4.066n-C4H9O 3.6620 Protocatechuic 4.486sec.-C4H9O- 3.6720 Pyruvic 2.506HS- 3.67*20Tartaric + 2.99, 4.406i-C3H7O- 3.69*20 + or - 2.89, 4.406CH3S- 3.72*20 meso 3.22, 4.856i-C3H7S- 3.72*20 Vinylacetic 4.426C6H5CH2S- 3.73*20C2H5S- 3.74*20n-C3H7S- 3.77*20n-C4H9S- 3.81*20HO- 3.83*20–O3S- 4.0520(C6H5)3CS- 4.30*20C6H5- 4.31*20CH2-CH- 4.35*20* Indicates thermodynamic values.Unsaturated acids (25°)CompoundpK poundpK ref.trans-CH 3-CH=CHCO 2H 4.69*20H-CH 2CH 2CO 2H 4.88*2cis-CH 3-CH=CHCO 2H 4.44*2H-CH=CHCO 2H 4.25*2C 6H 5-CH 2CH 2CO 2H4.66*2C 6H 5CH 2CH 2CO 2H 4.66*2trans-C 6H 5-CH=CHCO 2H 4.44*2C 6H 5CH=CHCO 2H** 4.44*2m-CH 3OC 6H 4CH 2CH 2CO 2H4.65*2C 6H 5CH 2CH 2CO 2H 4.66*2C 6H 5CH=CHCO 2H**4.442m-CH 3OC 6H 4CH=CHCO 2H 4.38*2m-ClC 6H 4CH=CHCO 2H**4.29*2m-ClC 6H 4CH 2CH 2CO 2H 4.58*2Unsaturated acids, Cis- and Trans-C C R 2H R 1CO 2H C C R 2R 1HCO 2H Cis-Acid Trans-Acid R 1R 2cis-acid trans-acid Ref.H-H- 4.25*4.25*2CH 3-H- 4.44*4.69*2Cl-H- 3.323.652C 6H 5-H- 3.88*4.44*2ClC 6H 4H- 3.91 4.4126-BrC 6H 4H- 4.02 4.412CH 3-CH 3- 4.305.022C 6H 5-H- 5.26***5.58***22,4,6-(CH 3)3C 6H 2-H-6.12***5.70***2C 6H 5-CH 3- 4.98***5.98***2Dicarboxylic acids, unsaturated*Maleic 1.92, 6.232Alicyclic Dicarboxylic acidsCitraconic (Dimethylmaleic acid)2.29, 6.152cis-Caronic(1,1-dimethylcyclopropane-23-dicarboxylic acid 2.34*, 8.31*2Acetylenedicarboxylic 1.73, 4.402∆1-tetrahydrophthalic 3.01, 5.3421,2-trans-cyclopropanedicarboxylic3.65*, 5.13*2Bromomaleic 1.45, 4.622trans-caronic 3.82*, 5.32*2Bromofumaric 1.46, 3.5721,2-cis-cyclopropane-dicarboxylic3.33*, 6.47*2Chlorofumaric 1.78, 3.812Fumaric 3.02,4.382Mesaconic (Dimethylfumaric acid)**trans3.09,4.752***in 40% acetone Phthalic 2.95, 5.412*thermodynamicItaconic (1-Propene-2-3-dicarboxylic acid)3.85, 5.452Chloromaleic 1.72, 3.862AliphaticAlicyclic Dicarboxylic acidsCompound pK Ref Compound pK Ref 1,2-trans-Cyclopropane-cis-Ethyleneoxide-dicarboxylic 3.65, 5.132dicarboxylic 1.94, 3.922 trans-Ethyleneoxide-1,3-cis-Cyclobutane-dicarboxylic 1.93, 3.252dicarboxylic 4.03, 5.3121,3-trans -Cyclobutanedi-1,2-cis-Cyclopentane-carboxylic 3.81, 5.282dicarboxylic 4.37, 6.5121,2-trans-Cyyclopentane-1,3-cis-Cyclopentanedicarboxylic 3.89, 5.912dicarboxylic 4.23, 5.5321,3-trans-Cyclopentane-1,2-cisCyclohexane-dicarboxylic 4.40, 5.452dicarboxylic 4.34, 6.7621,2-trans-Cyclohexane-1,3 -cis-Cyclohexane-dicarboxylic 4.18, 5.932dicarboxylic 4.10, 5.4621,3-trans-Cyclohexane-1,4-cis-Cyclohexanedicarboxylic 4.31, 5.732di-carboxylic 4.44, 5.7921,4-trans-Cyclohexane-dicarboxylic 4.18, 5.422Dicarboxylic acids*oxalic 1.23, 4.192Succinic 4.19, 5.482 Malonic 2.83, 5.692 O-O’-Dimethyl- 3.77, 5.942 Methyl- 3.05, 5.762 (high melting)Ethyl- 2.99, 5.832 O-O’-Dimethyl- 3.94, 6.202n-propyl 3.00, 5.842 (low melting)i-propyl- 2.94, 5.882 O,O’-Diethyl- 3.63, 6.462 Dimethyl- 3.17, 6.062 (high melting)Methylethyl- 2.86, 6.412 O,O’-Diethyl- 3.51, 6.602Diethyl- 2.21, 7.292 (low melting)Ethyl-n-propyl- 2.15, 7.432Tetramethyl- 3.50, 7.282Di-n-propyl- 2.07, 7.512Glutaric 4.34, 5.422Adipic 4.42, 5.412B-Methyl 4.25, 6.222Pimelic 4.48, 5.422B-Ethyl 4.29, 6.332Suberic 4.52, 5.402B-n-Propyl 4.31, 6.392Azelaic 4.55, 5.412B,B-Dimethyl- 3.70, 6.292DL-1:2-Dichlorosuccinic 1.68, 3.1820 B,B-Methylethyl- 3.62, 6.702meso-1:2-Dichlorosuccinic 1.74, 3.2420 B,B-Diethyl- 3.62, 7.122DL-1:2-Dibromosuccinic 1.48, ----20 B,B-Di-n-propyl 3.69, 7.312meso-1:2-Dibromosuccinic 1.42, 2.9720D-Tartaric 3.03, 4.4520DL-1:2-Dimethylsuccinic 3.93, 6.0020DL-Tartaric 3.03, ----20meso-1:2-Dimethylsuccinic 3.77, 5.3620 meso-Tartaric 3.29, 4.9220*All are thermodynamic valuesAliphatic HO- 6.332Br- 6.082 Bicyclo[2.2.2]octane-1-carboxylic acids, 4-substitutedLysergic acid, etc.H- 6.752ergometrine 6.8, --2 C2H5O2C- 6.312Dihydroergometrine7.4, --2β-dihydrolysergol8.2, --2 NC- 5.902Lysergic acid7.8, 3.32C6H5O- 3.53* 3.95* 4.52*α-dihydrolysergic8.3, 3.62CH3- 3.91* 4.24* 4.34* ergometrinine7.3, --2(CH3)2CH- 4.35*α-dihydrolysergol8.3, --2(CH3)3N+- 1.37 3.45 3.436-methylergoline8.85, --2NC- 3.60* 3.55* isolysergic acid8.4, 3.42HO2C* 2.95* 3.54 3.51γ-dihydrolysergic8.6, 3.62F3C- 3.79HO- 2.98* 4.08* 4.58*I- 2.85* 3.86* Hydroxycyclohexanecarboxylic acids Cl- 2.94* 3.83* 3.99* Cyclohexanecarboxylic 4.902(CH3)3Si- 4.24* 4.27* cis-1,2 4.802C2H5O- 4.21* 4.17* 4.45* cis-1,3 4.602i-C3H7O- 4.24* 4.15* 4.68* cis-1,4 4.842n-C5H11O- 4.55* trans-1,2 4.682C6H5- 3.46*trans-1,3 4.822CH3CH2- 3.77 4.35* trans-1,4 4.682(CH3)3C- 3.46 4.28 4.40*–HO3P- 3.78 4.03 3.95 Aromaticbenzene-CO3H 4.20*2–O3S- 4.15 4.11 Anthracene-1-COOH 3.692H2N- 4.98 4.79 4.92 Anthracene-9-COOH 3.652(CH3)2N-8.42 5.10 5.03 naphthalene-2-COOH 4.172–HO3As- 4.22 Naphthalene-1-COOH 3.692–O2C- 5.41** 4.60 4.82CH3NH- 5.3 5.10 5.04 Substituted benzoic acids (ref. 2)COOH*thermodynamicfor complex chelating agents, see also ref. 84.see also page 9a for more carboxylic acids. Benzoic acid o m pOrtho-substituted benzoic acidsH- 4.20* 4.21*Benzoic acid pK Ref.O2N- 2.17* 3.45* 3.442-CH3- 3.91**2CH3CO-2-t-C4H9- 3.462CH3SO2- 3.64* 3.52*2,6-(CH3)2- 3.212CH3S-2,3,4,6-(CH3)4- 4.002HS-2,3,5,6-(CH3)4- 3.522Br- 2.85* 3.81* 4.00*2-C2H5- 3.772F- 3.27* 3.87* 4.14*CH3O- 4.09* 4.09* 4.47*2-C6H5- 3.46**2n-C3H7O- 4.24* 4.20* 4.46*2,4,6-(CH3)3- 3.432n-C4H9O- 4.25* 4.53*2,3,4,5-(CH3)4- 4.222 Benzene Polycarboxylic acids Ref. 2Acid Position of carboxyl pK I pK II pK III pK IV pK V pK VI Benzoic1 4.17*Phthalic1,2 2.98* 5.28*Isophthalic1,3 3.46* 4.46*Terephthalic1,4 3.51* 4.82*Hemimellitic1,2,3 2.80* 4.20* 5.87*Trimellitic1,2,4 2.52* 3.84* 5.20*8Trimesic 1,3,5 3.12* 3.89* 4.70*Mellophanic 1,2,3,4 2.06* 3.25* 4.73* 6.21*Prehnitic 1,2,3,5 2.38* 3.51* 4.44* 5.81*Pyromellitic1,2,4,5 1.92* 2.87* 4.49* 5.63*Benzenepentacarboxylic 1,2,3,4,5 1.80* 2.73* 3.97* 5.25* 6.46*Mellitic1.2,3,4,5,61.40*2.19*3.31*4.78*5.89*6.96**ionic strength 0.032-Methoxyethyliminodiacetic 2.2, 8.96**thermodynamic2-Methylthioethyliminodiacetic 2.1, 8.91oxalic acid* 1.25, 4.14N-n-propylaminoacetic 2.25, 10.03Carboxylic Acids Ref. 77N-2-sulfoethyliminodiacetic 1.92, 2.28, 8.16Aminomalonic acid* 3.32, 9.83α-Bromobutyric acid 2.97N-Butylaminoacetic acid 2.29, 10.07N-(carbamoylmethyl)-imino-diacetic acid2.30, 6.602-carboxyethyliminodiacetic acid2.06,3.69, 9.66Cyanomethyliminodiacetic 3.06, 4.34β-carboxymethylaminopropionic 3.61, 9.46α,β-diaminopropionic acid 1.23, 6.69α,α-diaminobutyric 1.85, 8.24, 10.44Diethylaminoacetic 2.04, 10.47Di-(carboxymethyl)-aminomethyl phosphonic acid 2.00, 2.25, 5.57, 10.76Dimethylaminoacetic 2.08, 9.80N-ethylaminoacetic 2.30, 10.10α,β-dimercaptosuccinic 2.40, 3.46, 9.44, 11.82Gluconic* 3.86β-hydroxybutyric 4.39Ethylenediamine-N,N-diacetic 5.58, 11.05α-hydroxybutyric 3.65β-hydroxypropionic 3.73N-2-hydroxyethyliminodiacetic 2.2, 8.73Iminodiacetic* 2.98, 9.893-hydroxypropyliminodiacetic 2.06, 9.24β-iodopropionic* 4.04Iminodipropionic 4.11, 9.61N-isopropylaminoacetic 2.36, 10.06Isobutyric* 4.86α-mercaptobutyric 3.53Mandelic acid 3.41N-methylaminoacetic 2.24, 10.012-MercaptoethyliminodiaceticNitrilotriacetic 3.03, 3.07, 10.-2.14, 8.17, 10.792-PhosphonoethyliminodiaceticMethyliminodiacetic 2.81, 10.181.95,2.45, 6.54, 10.46*ThermodynamicPHENOLSCompound pK pound pK Ref. Chromotropic acid 5.36, 15.66Resorcinol--, 9.15 (30o)50o-Methoxyphenol--, 9.9350p-Methoxyphenol--, 10.1650 o-Hydroxybenz-3-Hydroxyanthran-aldehyde7.9550ilic acid10.09, 5.20512-Amino-4,5 dimethyl-2-Aminophenolphenol hydrochloride10.4 5.2851hydrochloride9.99, 4.86514,5-dihydroxybenzene-1,3 disulphonic acid7.6612.6eKojic acid9.4077Phenol o m p Phenol o m pH-9.95*9.94*O2N-7.23*8.35*7.14* (CH3)3N+-7.4288OCH- 6.798.007.66CH3SO2-9.337.83NC-8.61**7.95CH3CO-9.198.05CH3O2C-8.47*C2H5O2C-8.50*n-C4H9O2C-8.47*C3H5CH2O2C-8.41*I-9.17*Br-8.42*9.11*9.34*Cl-8.48*9.02*9.38*F-8.81*9.28*9.95*CH3S-9.539.53HO-9.489.449.96HOCH2-9.92*9.83*9.82*CH3-10.28*10.08 10.19*C2H5-10.29.910.0CH3O-9.939.6510.20H2N-9.719.8710.30-O2C-9.94*9.39*-O3S-9.299.03--O3P-10.29.9--O3As8.37 C6H5-9.939.599.51NO- 6.35**2-Chloro-4-Nitro- 5.42792-Nitro-4-Chloro- 6.4679* Thermodynamic**Reference 52ALCOHOLS and other OXYGEN ACIDSAlcoholsCompound pK pound pK Ref. Choline13.96C3F7•CH(C2F5)•OH 10.4865 Chloral hydrate9.66, 11.061(C3F7)2CH•OH10.5265 Trifluoroethanol12.562Carbonium ionsCF3CH2OH11.4, 12.4363CF3CH(OH)CH311.863Triphenylmethanols in H2SO4 HC1O4 HNO3refCF3CH2(CH3)3OH12.43104,4,4-Trimethoxy.82. .82 .8066C3F7CH2OH11.4**634,4’-Dimethoxy-1.24-1.14-1.1166(C3F7)2CHOH10.6**634-Methoxy-3.40-3.59-3.4166HCCCH2OH13.55644-Methyl-5.41-5.6766C(CH2OH))414.1644-Trideuteriomethyl- 5.43 5.6766HOCH2CHOHCH2OH 4.4643,3’,3”-Trimethyl- 6.35-5.9566HOCH2CH2OH14.7764Unsubstituted triphenyl-CH3CCH2OH14.8264methanol- 6.63-6.89 6.6066CH3OH15.54644,4;,4;-Trichloro- 7.74-8.0166 CH2=CHCH2OH15.52644-Nitro-9.15-9.7666 H2O15.7464CCl3CH2OH 11.8***CH3CH2OH1664CF3CH2OH 11.3***Substituent effects for ionization of RCH2OHRCCl-312.24,11.8064,65CF3-12.3764CHF2CH2-12.7464Hydroxamic acidsCHCl2-12.8964Furo-8.4572CHEC-13.5564Glycine7.4072H2Cl-14.3164Hippuro-8.8072CH3CCH2-14.864iso Nicotin7.8572HOCH215.164p-Methylbenz-8.9072H-15.564Nicotin-8.3072CH2=CH-15.564Nicotin-methiodide 6.4672CH3-(extrap)(15.9)64m-Nitrobenz-8.0772CF3C(CH3)2OH11.664Picolin8.5072HOCH2CF2CH2OH1164Pyrimidine-2-carbox-7.8872Primary alcohols=R•CH2•OH and Salicyl-7.4372Secondary alcohols in 50% alcohol Tropo-9.0972C2F511.3565C4F911.3565C5F1111.3765C7F1511.3565Other oxygen acidsCHF212.0065Trimethylamine-n-oxide 4.618CF2Cl11.6365Dimethylglyoxime12.8477CHF2CF211.3465(50% dioxane)CHF2 • (CF2)211.3565O-methyl ether12.9277CF3 • CH212.765Tropolone12a77CF3 • (CH2)212.965 α-Bromotropolone 6.95a77CF3 • CHMe • OH11.2865Acetald hydrate13.4891C3F7 • CHMe • OH11.3865Formald hydrate13.2991C3F7CHEt • OH11.3765C3F7CHPr • OH11.3765C3F7 • CH(CF3) • OH10.4665a50% dioxane***50 aquaeous ethanolOTHER OXYGEN ACIDSHydroxamic acids Aceto-9.4068Compound pK Ref.n-Butyro-9.4868Pyridine oxidesn-Butyro-9.00684-Aminopyridine 1-oxide 3.6967p-Methoxybenzo-9.19684-Dimethylaminopyridine 1-oxide3.8867N-Hydroxyphthalimide 7.00, 6.1071, 72Salicylo 7.32684-Dimethylaminopyridine 1-oxide3.8867Benzo-8.8868p-Chlorobenzo-9.59684-Dimethylamino-1-methoxypyridinium perchlorate >1167α-Naphtho-~7.768Propiono-9.46682-Methylaminopyridine 1-oxide 2.61672-Amino-1-methoxypyridinium perchlorate12.467Oximes4-Hydroxypyridine 1-oxide 2.4567Benzophenone oxime 11.3184-Methoxypyridine 1-oxide 2.0567Diethyl ketoxime 12.6181-Methoxypyridi-4-one 2.5767Isonitrosoacetylacetone (INAA) 7.4762-Hydroxypyridine 1-oxide -0.8675-Methyl-1,2,3-cyclohexanetrione-1,3-dioxime8.3762-Ethoxypyridine 1-oxide 1.18671-Methoxypyrid-2-one -1.3Acetophenone oxime 11.48184-Methylaminopyridine 1-oxide 3.8567Acetoxime 11.42184-Amino-1-methoxypyridinium perchlorate>1167Isonitrosoacetone (INA) 8.376Salicyclaldoxime (SA)9.2762-Aminopyridine 1-oxide 2.67671,2,3-Cyclohexanetrionetrioxime 8.0762-Dimethylaminopyridine 1-oxide2.27675-Methyl-1,2,3-cyclohexane-trionetrioxime8.0762-Methylamino-1-methoxypyridinium toluene-p-sulphonate >11674-Benzyloxypyridine 1-oxide 1.9967Oxygen acids1-Benzyloxypyrid-4-one 2.5867sulfinic acids 2-Methoxypyridine 1-oxide 1.2367p-Toluene- 1.99731-Benzyloxypyrid-2-one -1.767p-Chlorobenzene-73p-Nitrobenzene-73Pyridine 1-oxides p-Bromobenzene- 1.8973RpK Ref.m-Nitrobenzene- 1.88734-CH 3 1.2947Benzene-1.84,2.16733-CH 3 1.0847Peroxyacids3,4-(CH)4 1.0147Peroxymonosulfuric 9.4693-COOC 4H 90.0347Acetic 8.2704-NO 2-1.747n-Butyric 8.2703-NH 2 1.4747Formic 7.170H0.7947Propionic 8.1703-COOH 0.0947peroxydiphosphoric 5.18, 7.8854-COOH-0.4847peroxymonophosphoric 4.8590Peroxides ROOH (Ref. 70)H CH 3C 2H 5iso-C 3H 7tert-C 4H 9iso-C 4H 911.611.511.812.112.812.8Oximesref. 93Pyridine-2-aldoxime heptiodide 8.00benzoquinoline mon- 6.25Pyridine-4-aldoxime methiodide 8.503-pyridine-1,2-ethanedione-2-oxime methiodide7.20Pyridine-4-aldoxime pentiodide 8.504-Pyridine-1,2-ethanedione-2-oxime methiodide7.1O-Methyltyrosine ethyl ester 7.3122 octopine 13, 1.368.776Pyridine-2-aldoxime methiodide8.0Phenylglyoxald-8.3 2.40Pyridine-4-aldoxime dodeciodide8.5Phenylalanine 1.839.136 Pyridine-3-alkoxime methiodide9.22-Pyrrolidoone-5-carboxylic acid (glucamicacid) 3.32Hydroxamic acids ref. 93Serine 2.219.156 D-Lysine-7.93Threonine 2.6310.436 N-phenylnicotino-8.00N-Trimethyl tyrosine9.7521 Chloroaceto-8.40Tyrosine 10.07, 2.209.11 Formo-8.65Urocanic acid 5.8 3.5p-Chlorophenoxyaceto-8.75Valine 2.329.626 p-Hydroxybenzo-8.93β-Alanine 3.6010.196 p-Methoxybenzo-9.00γ-Aminobutyric acid 4.2310.436 N-Phenylbenzo-9.15Arginine 12.48 2.179.046 o-Aminobenzo-9.17Asparagine 2.028.86 L-Tyrosine9.20Azaserine8.556 L-Lysine7.9Canavanine7.40, 9.2511.50 (?)6 p-Nitrobenzo-8.0Creatine 2.6711.026 p-Aminobenzo-9.3Cysteine 10.78 1.718.336 L-Lacti-9.33,4-DihydroxyphenylalaninePropiono-9.49.88, 2.368.686 Phthalo-9.411.68Indole-3-aceto-9.5Glutamine 2.179.136 Cyclohexano-9.7Histamine 5.09.76 Hexano-9.7β-Hydroxyglutamic 2.099.206acid 4.18Amino Acids Hydroxyproline 1.929.736 Compound pK Ref.Leucine 2.369.606-COOH-NH3Methionine 2.289.21 Alanine 2.359.6961-Methylhistidine 6.48, 1.698.856α-Aminobutyric acid 2.559.60Norleucine 2.399.766α-Aminoisobutyric 2.3610.216Norvaline 2.369.766 Argininosuccinic >12, 1.629.586Ornithine 1.718.6962.70, 4.2610.76 Aspartic acid 2.09,3.869.826Proline 1.9910.606 Canaline10.3, 9.2011.6 (?)6Sarcosine 2.2310.016 Creatinine4.849.26Taurine 1.58.746 Cystine 1.657.856Thiolhistidine <1.5, 11.42.269.856 1.848.476 Diidotyrosine 6.48, 2.127.826Tryptophan 2.389.396 Glutamic acid 2.19, 4.259.676Tyrosine ethyl ester 7.339.8022 Glycine 2.349.66PeptidesHistidine 6.0, 1.829.176Anserine 7.0 2.659.56 Carnosine 6.83--9.516Hydroxylsine 2.138.626Cystinyldiglycine 3.12 6.3669.67 3.12 6.95 Isoleucine 2.369.686Glycylglycine 3.06 8.13 Lysine 2.188.956Gly-gly-gly 3.267.912310.53Glycylproline 2.848.556 O-Methyl tyrosine9.2721Aspartyl histi- 2.457.98dine 6.82 3.02Gly-gly-gly-gly 3.057.7523 Diglycylcystine 2.717.946Lysyl-lysine (L,L) 3.017.536 Glutathione 9.12 2.128.66610.0511.013.53Compound-COOH-NH2-NH2-NH2-NH2Ref. Gly•Ala (L) or (D) 3.178.2327 Ala•Gly (L) or (D) 3.168.2427 Gly•Ala•Ala (LL) 3.388.1027 Gly•Ala•Ala (LD) 3.308.1727 Ala•Ala•OH (DD) 3.308.1427 Ala•Ala•OH (LD) 3.128.3027 H•Ala•Ala•Ala•OH (3L) 3.398.0327 H•Ala•Ala•Ala•OH (LLD) 3.378.0527 H•Ala-Ala-Ala•OH (LDL) 3.318.1327 H•Ala-Ala-Ala•OH (DLL) 3.378.0627 H-Ala-Ala-Ala•OH (3D) 3.398.0627 H•Ala-Ala-Ala-Ala•OH (4L) 3.427.9427 H•Ala-Ala-Ala-Ala•OH (LLDL) 3.247.9327 H•Ala-Ala-Ala-Ala•OH (LDLL) 3.227.9927 H•Ala-Ala-Ala-Ala•OH (DLLL) 3.427.9927 H•Lys-Ala•OH (LL) 3.227.6210.7027 H•Lys-Ala•OH (LD) 3.007.7410.6327 H•Ala-Lys-Ala•OH (3L) 3.157.6510.3027 H•Ala-Lys-Ala•OH (LDL) 3.337.9710.3627 H•Ala-Lys-Ala•OH (LLD) 3.297.8410.4927 H•Ala-Lys-Ala-Ala•OH (4L) 3.588.0110.5827 H•Ala-Lys-Ala•OH (LDLL) 3.328.0110.3727 H•Ala-Lys-Ala-Ala-Ala•OH (5L) 3.537.7510.3527 H•Ala-Lys-Ala-Ala-Ala•OH (LDLLL) 3.307.8510.2927 H•Lys-Lys•OH (LL) 3.017.5310.0511.0127 H•Lys-Lys•OH (LD) 2.857.539.9210.9827 H•Lys-Lys•OH (3L) 3.087.349.8010.5411.3227 H•Lys-Lys-Lys•OH (LDL) 2.917.299.7910.5411.4227 H•Lys-Lys-Lys•OH (LDD) 2.947.149.6010.3811.0927 Compound pK ref.Glutathione 3.59, 8.75, 9.6577Glycylserine8.2377Glycylleucine8.1377Leucylglycine7.9677Glycylisoleucine7.9677Leucylglycylglycine7.6677Glycylphenylalanine8.2877Glycyltyrosine8.2277Benzylglutamic acid 3.49, 4.9977Glycyltryptophane8.0477Glutathione, oxidized 3.15, 4.03, 8.57, 9.5477Alanylalanine (LL) 3.308.1492Alanylalanine (LD) 3.128.3092Lysylalanine (LL) 3.227.6210.7092Lysylalanine (LD) 3.007.7410.6392Leucyltyrosine (LL) 3.467.8410.0992Leucyltyrosine (DL) 3.128.3810.3592Lysyllysine (LD) 2.857.539.9292NITROGEN COMPOUNDSAliphatic Amines pK ref.Ammonia9.211n-Propyl-10.531 Primary Amines Trimethylsilymethyl-10.961β-Alanine ester9.131CH3ONH2 4.6012 Allylamine-9.692Allyl-9.491 Benzyl9.341γ-Amino-n-butyric acid ester 9.711n-Butyl-10.591sec-Butyl-10.561t-Butyl-10.551Cyclohexyl-10.641 Cyclohexylmethyl-10.491β-difluoroethyl-7.521 Ethanol-9.501Ethyl10.631 Ethylenedi-9.98, 7.521, 77Glycine ester7.751 Hydrazine8.101Hydroxyl- 5.971 Isopropyl-10.631Methoxy- 4.601 Methyl-10.621neo-Pentyl-10.211 Phenylamyl-10.492δ-Phenylbutyl10.402β-Phenylethyl-9.831γ-Phenylpropyl-10.201Triethylenedi-8.8*?X XNH3+XCH2NH3+X(CH2)2NH3+X(CH2)3NH3+X(CH2)4NH3+X(CH2)5NH3+ref. H-9.25*10.64*10.67*10.58*10.61*10.63*2 HF2C-7.52RO2C-7.759.139.7110.15*10.372 HO- 5.96*9.50*C6H5- 4.58*9.37*9.83*10.20*10.39*10.49*2 H2N-8.12*9.98*10.65*10.84*11.05*2 H2C=CH-9.69CH3-10.64*10.67*10.58*10.61*10.63*10.64*2 X-H-NH3+-CO2–-SO3–-PO3–2X-NH3+9.25*-.88110.25X(CH2)2NH3+10.649.77 5.7510.8X(CH2)2NH3+10.6710.199.2010.8X(CH2)4NH3+10.619.3110.7710.6510.9X(CH2)5NH3+10.639.7410.7510.9511.0X(CH2)8NH3+10.6510.10X(CH2)10NH3+10.6411.3511.25X(CH2)3NH3+10.588.5910.4310.05Secondary amines Di-n-butyl-11.251 Dimethyl-10.641Diisobutyl-10.501Di-n-propyl-11.001α-Ethylpyrroline7.432 Diisopropyl-11.051α-Benzylpyrroline-7.082t-Butylcyclohexyl-11.2312-Methylpiperidine10.992α-Cyclohexylpyrroline7.952α-Cyclohexylpyrrolidine10.802α-(p-Tolyl)pyrroline7.592α-(p-Tolyl)pyrrolidine10.012α-Ethylpyrrolidine10.432N,O-dimethylhydroxylamine 4.7512α-Benzylpyrrolidine10.362Acetanilide+0.614N-methylhydroxylamine 5.9612*thermodynamic valueDiethyl-10.981Aliphatic Amines Methyl-β-diethylamino-ethyl-sulfide 1,2-Iminoethane 7.9871,2-Dimethyl-∆2-pyrroline 11.942cis-2,3-Iminobutane 8.7271-methyl-2-n-butyl-∆2-pyrroline 11.901,2-Imino-2-methylpropane 8.6171-Ethyl-2-methyl-∆2-pyrroline 11.9221,2-Iminobutane 8.2971-n-Butyl-2-methyl-∆2-pyrroline 11.902trans-2,3-Iminobutane 8.6971,2-Dimethyl-∆2-tetrahydropyridine11.572Secondary Amines N-Ethyl derivative of: 1,2-Imino-ethane7.937Allylmethyl-10.111Benzylethyl-9.681Trans-2,3-Iminobutane 9.477Morpholine 8.361Trimethylhydroxylamine 3.6512N-Benzoylpiperazine 7.781Dimethylethyl-9.991Di-sec-butyl-11.011Triethyl-10.651N-Methylmethoxyamine 4.751Dimethyl-n-butyl-10.021Pyrolidine 11.271Dimethyl-isopropyl-10.3011-Tosylpiperazine 7.39Dimethyl-t-butyl-10.521Benzylmethyl-9.581Tri-n-butyl-10.891Piperidine 11.221Diallylmethyl-8.791N-Carbethoxypiperazin 8.2811-n-Propylpiperidine 10.482Dietrimethylsilylmethyl-11.40110.110.15Diallyl-9.2919.8--5N-Methylhydroxyl- 5.9611,2-Dimethylpyrrolidine 10.262Trimethyleneimine 11.2911-Methyl-2-n-butylpyrrolidin 10.242Cis-2,6-dimethyl-piperidine 10.9231-Ethyl-2-methylpyrrolidine 10.6421-n-Butyl-2-methylpyrrolidine 10.4321-Ethyl-2-methylpyrrolidine 10.7021,2-Iminobutane 8.187Tertiary amines cis-2,3-Iminobutane 8.567Trimethyl-9.761N-dimethylhydroxylamine 5.2012Dimethyldiethyl-10.291Allyldimethyl 8.781Dimethyl-n-propyl-9.9911,2-Dimethylpiperidine 10.262Dimethyl-isobutyl-9.9111-Ethyl-2-methyl-∆2-tetrahydropyridine11.572Dimethyl-sec-butyl-10.401Tri-n-propyl-10.651Triallyl-8.311N-Allylpiperidine 9.6921-Diethylamino-hexane-thiol-(6)Cyanoamines2-Amino-2-cyanopropane 5.39N-piperidine-CH 2CN 4.558β-Isopropylaminopropionitrile 8.09Et 2NCN -2.08β-Diethylaminopropionitrile 7.69Et 2N(CH 2)2CN 7.658Et 2NCH 2CN 4.558Et 2N(CH 2)4CN 10.088Et 2N(CH 2)3CN 9.298Et 2NC(CH 3)2CN 9.138Et 2N(CH 2)5CN 10.468EtN(CH 2CN)2-0.68HN(CH 2CN)20.28EtN(CH 2CH 2CN)2 4.558HN(CH 2CH 2CN)2 5.268H 2NCH 2CN 5.348N(CH 2CH 2CN)3 1.18N-Amphetamine-(CH 2)2-CN 7.238N-piperidine-C(CH 3)2CN 9.228N-Norcodeine-(CH 2)2CN 5.688N-Methamphetamine-(CH 2)2CN 6.958Dimethylcyanimide 1.29Methyl cyanamide 1.29Diethylcyanimide 1.29Ethyl cyanamide 1.29Aminoacetonitrile 5.39Cyanamide 1.19Diethylaminoacetonitrile 4.59Dimethylaminoacetonitrile 4.29β-Aminopropionitrile7.79CF3CH2NHCH3 6.0510β-Dimethylaminopropionitrile7.09Phenylethylaminesβ,β"-Dicyanodiethylamine 5.292-phenylethylamine9.7811 For complex chelating agents of aliphatic amines,see also ref. 77.N-methyl-2-(3,4-dihydroxyphenyl)-ethylamine8.7811N-methyl-2-phenyl10.3111 Fluoro-substituted aminesEpinephrine8.5511 CF3CH2NH2 5.710Arterenol8.5511 CF3CH2N(CH3)2 4.7510R2R1CHCH2NHR4R3ref. 11R1R2R3R4pKH H H H9.78H H OH H8.90H OH OH H8.81OH H OH H8.67H OH H H9.22OH OH H H8.93OH OH OH H8.58H H H CH310.31H H OH CH39.31H OH OH CH28.62OH H OH CH38.89H OH H CH39.36OH OH H CH38.78OH OH OH CH38.55Ring amines and imines (in 80% methyl cellosolve) (ref. 2)Pentamethylene9.99Cyclotridecyl9.63 Hexamethylene10.00Cyclotetradecyl9.54 Heptamethylene9.77Cyclopentadecyl9.54 Octamethylene9.39Cycloheptadecyl9.57 Nonamethylene9.14Cyclooctadecyl9.54 Decamethylene9.04Undecamethylene9.14Amines otherDodecamethylene9.31Dimeoone 5.2318 Tridecamethylene9.35Phthalimide8.3018 Tetradecamethylene9.35Nitrourea 4.5718 Hexadecamethylene9.29Nitrourethane 3.2818 Heptadecamethylene9.27Diphenylthiocarbazone 4.56 Cyclohexyl9.82β,β,β"-Triaminotriethylamine Cycloheptyl9.998.42, 9.44, 10.1387 CyclooctylCyclononyl9.95Anilines Ref. 2Cyclodecyl9.85MonosubstitutedCycloundecyl9.71Substituent o m p Cyclododecyl9.62H- 4.62* 4.64* 4.58*。
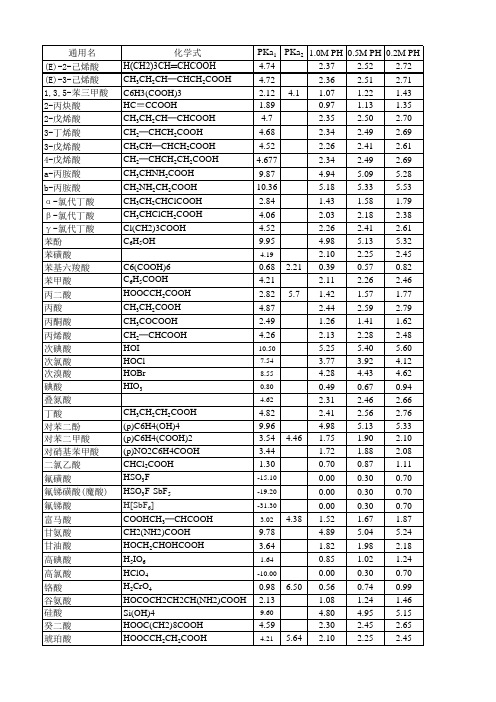
各种酸的pKa及pH值

化学式 H(CH2)3CH═ CHCOOH CH3CH2CH═ CHCH2COOH C6H3(COOH)3 HC≡CCOOH CH3CH2CH═ CCHH2C═OOH CCHH3CCHH2C═OOH CHCH2COOH CH2═ CHCH2CH2COOH CH3CHNH2COOH CH2NH2CH2COOH CH3CH2CHClCOO HCH3CHClCH2COO HCl(CH2)3COOH C6H5OH
9.78
4.89 5.04 5.24 5.39
甘油酸
HOCH2CHOHCOO 3.64 H
1.82 1.98 2.18 2.33
高碘酸 高氯酸 铬酸
H5IO6 HClO4 H2CrO4
1.64
0.85 1.02 1.24 1.42
-10.00
0.00 0.30 0.70 1.00
0.98
6.50 0.56 0.74 0.99 1.20
9.45 2.95 2.18 2.12 -2.00
1.92
12.8 4.72 4.88 5.41 1.48 1.63
1.11 1.27 7.20 1.08 1.24 1.99 -0.04 0.25
6.23 0.98 1.14
5.07 5.22 1.83 1.99 1.48 1.65 1.45 1.62 0.62 0.90
1.44 1.59 1.80 1.96
乙醇酸
CH2(OH)COOH
3.83
1.92 2.07 2.27 2.42
乙二胺四乙酸 EDTA
0.90
1.60 0.53 0.71 0.97 1.18
乙二酸
HOOCCOOH
1.27
0.69 0.86 1.10 1.29
部分酸的酸度系数pKa

部分酸的pKa值砷酸H3AsO4 PKa=2.2 7.00 11.50 亚砷酸HAsO2PKa=9.22硼酸H3BO3 PKa=9.24焦硼酸H2B4O7PKa=4 9碳酸H2CO3 PKa=6.38 10.21氢氰酸HCN PKa=9.21铬酸H2CrO4PKa=0.74 6.50氢氟酸HF PKa=3.18亚硝酸HNO2PKa=3.29过氧化氢H2O2PKa=11.75磷酸H3PO4PKa=2.12 7.2 12.36 焦磷酸H4P2O7 PKa=1.52 2.36 6.60 9.25 亚磷酸H3PO3 PKa=1.3 6.60氢硫酸H2S PKa1=7.05 18.15硫酸H2SO4 PKa2=1.99亚硫酸H2SO3 PKa=1.9 7.20偏硅酸H2SiO3PKa=9.77 11.8甲酸HCOOH PKa=3.74乙酸CH3COOH PKa=4.74一氯乙酸CH2ClCOOH PKa=2.86二氯乙酸CHCl2COOH PKa=1.3三氯乙酸Cl3COOH PKa=0.64氨基乙酸盐+NH3CH2COOH PKa=2.35 9.6 乳酸CH3CHOHCOOH PKa=3.86苯甲酸C6H5COOH PKa=4.21草酸H2C2O4PKa=1.22 4.19d-酒石酸HOOCHOHC-CHOHCOOH PKa=3.04 4.37 邻-苯二甲酸HOOC-Ph-COOH PKa=2.95 5.41 柠檬酸HOOCOHC-(CH2COOH)2PKa=3.13 4.76 6.4 苯酚C6H5OH PKa=9.95乙二胺四乙酸H6-EDTA2+ PKa=0.9 1.6 2.0 2.67 6.16 10.26 铵离子NH4+PKa=9.26联铵离子+H3NNH3+PKa=8.48羟铵离子NH3+OH PKa=5.96甲胺离子CH3NH3+ PKa=10.62乙胺离子C2H5NH3+PKa=10.75二甲胺离子(CH3)2NH2+PKa=10.07二乙胺离子(C2H5)2NH2+PKa=11.11乙醇胺离子HOCH2CH2NH3+PKa=9.50三乙醇胺离子(HOCH2CH2)3NH+ PKa=7.76六亚甲基四胺离子(CH2)6NH+ PKa=5.15乙二胺离子+H3NCH2CH2NH3+PKa1=6.85 PKa2=9.93部分有机酸甲酸HCOOH PKa=3.77 乙酸CH3COOH PKa=4.74 丙酸CH3CH2COOH PKa=4.87 丁酸CH3CH2CH2COOH PKa=4.82阿尔法氯代丁酸CH3CH2CHClCOOH PKa=2.84 贝塔氯代丁酸CH3CHClCH2COOH PKa=4.06 伽马氯代丁酸ClCH2CH2CH2COOH PKa=4.52 氯乙酸ClCH2COOH PKa=2.86 二氯乙酸(Cl)2CHCOOH PKa=1.26 三氯乙酸(Cl)3CCOOH PKa=0.64 氟乙酸FCH2COOH PKa=2.66 氯乙酸ClCH2COOH PKa=2.86 溴乙酸BrCH2COOH PKa=2.86 碘乙酸ICH2COOH PKa=3.21。
有机、无机化合物酸碱解离常数pKa_pKb数据

pKa ValuesINDEXInorganic2Phenazine24 Phosphates3Pyridine25 Carboxylic acids4, 8Pyrazine26Aliphatic4, 8Aromatic7, 8Quinoline27Phenols9Quinazoline27Alcohols and oxygen acids10, 11Quinoxaline27Amino Acids12Special Nitrogen Compounds28Peptides13Hydroxylamines28Nitrogen Compounds14Hydrazines28Aliphatic amines15, 17, 19Semicarbazones28 Cyanoamines16Amidoximes28Anilines17, 18, 20Thiols29 Nucleosides21Carbon Acids30,31 Special Table Heterocycles22Indicators31Acridine23References32-34 Benzoquinoline24Cinnoline23Hydantoin24Imidazole24For complex chelating agents, see also reference 77.Note. This document was compiled by W.P. Jencks and has been added to by F.H. WestheimerACIDSCompound pK Ref.H3PO2 2.0, 2.23*28H2PO4–7.21*77 AgOH 3.964HPO4_12.32*77Al(OH)311.228H3PO3 2.028As(OH)39.2228H2PO3– 6.58*77H3AsO4 2.22, 7.0, 13.028H4P2O7 1.52*77H2AsO4– 6.98*77H3 P2O7– 2.36*77 HAsO4*11.53*77H2P2O7= 6.60*77As2O304H3AsO39.22*HP2O7=9.25*77H3BO39.23*28HReO4-1.2530H2B4O7 4.0034HSCN 4.0034H2SeO3 2.6, 8.3, 2.62*28 HB4O79.0034HSeO38.3277Be(OH)2 3.74H2SeO4Strong, 2.028 HBr-9.0031HOBr8.728HSeO4 2.0034 HOCl7.53, 7.4628, 33H3SiO310.034 HClO2 2.028H2SO3 1.9, 7.0, 1.76*28, 77 HClO3-1.0028H2SO4-3.0, 1.928 HClO4 (70%)-10.0031HSO37.21*77CH3SO3H-0.631HSO4– 1.99*77 HCN9.4034H2S2O4 1.929H2CO3 6.37, 6.35*, 3.5834, 32H2Se 3.89*77 HCO310.33*HSe–11.00*77H2CrO4-0.9830H2S7.00*77 HCrO4 6.50*2, 30HS–12.92*77 HOCN 3.9234HSbO211.034 HZ 3.17*, 0.59*77HTe 5.0034H2GeO38.59, 12.7234, 78H2Te 2.64, 11.034, 78 Ge(OH)48.68, 12.728H2TeO3 2.7, 8.028HI-10.031Te(OH)6 6.2, 8.828 HOI11.028H2VO4–8.9530 HIO30.828HVO4=14.430H4IO6– 6.0034H2CrO40.7477H5IO6 1.64, 1.55, 8.2734, 28HOCN 3.7377 HMnO4-2.2530HSCN0.8577 NH3OH* 5.98*H3PO2 1.0777 NH4*9.24*77H3PO4 2.12*77 HN3 4.72*77H2S2O30.60*, 1.72*77 HNO2 3.2928H3AuO313.3, 16.078 HNO3-1.328H3GaO310.32, 11.778N2H5+7.99*77H5IO6 3.29, 6.70, 15.078H2N2O27.0534(see above!)H2N2O2–11.034H4V6O17 1.9678H2OsO512.134H2NSO3H 1.080H2O15.7noneH3O+-1.7none* Indicates a thermodynamic value.Pb(OH)2 6.48 (10.92) 4 (78)PHOSPHATES AND PHOSPHONATES CF 3- 1.16, 3.9357CCl 3- 1.63, 4.8157Phosphates NH 3+CH 2- 2.35, 5.957CompoundpKRef.(–OOCCH 2)2NH +CH 2– --, 5.5757Phosphate 1.97, 6.82, 12.555CHCl 2- 1.14, 5.6157Glyceric acid 2-phosphate 3.6, 7.153CH 2CI- 1.40, 6.3057Enolpyruvic acid 3.5, 6.453CH 2Br- 1.14, 6.5257Methyl- 1.54, 6.3155(–OOCCH 22NH +(CH 2)2- --, 6.5457Ethyl- 1.60, 6.6255CH 2I- 1.30, 6.7257n-Propyl- 1.88,6.6755NH 3+CH 2CH 2- 2.45, 7.0057n-Butyl- 1.80, 6.8455Dimethyl- 1.2955C 6H 5CH=CH- 2.00, 7.157Di-n-propyl 1.5955HOCH 2- 1.91, 7.1557Di-n-butyl- 1.7255C 6H 5NH 2+(CH 2)3- 2.1, --57Glucose-3-0.84, 5.6756C 6H 5NH(CH 2)3---, 7.1757Glucose-4-0.84, 5.6756Br(CH 2)2- 2.25, 7.357α-glycero- 1.40, 6.4454CH 3(CH 2)5CH(COO –)---, 7.557β-glycero- 1.37, 6.3454C 6H 5CH 2- 2.3, 7.55573-phosphoglyceric acid 1.42, 3.4254NH 3+(CH 2)4)- 2.55, 7.55572-phosphoglyceric acid 1.42, 3.55, 7.1peroxymonophosphoric acid 4.0569NH 3+(CH 2)5- 2.6, 7.657diphosphoglyceric acid 7.40, 7.9954NH 3+(CH 2)10---, 8.0057glyceraldehyde- 2.10, 6.7554–OOC(CH 2)10---, 8.2557dioxyacetone- 1.77,6.4554(CH 3)3SiCH 2- 3.22, 8.7057hexose di- 1.52, 6.3154fructose-6-0.97, 6.1154C 6H 5CH 2- 3.3, 8.457glucose-6-0.94, 6.1154(C 6H 5)SC- 3.85, 9.0057glucose-1- 1.10, 6.1354adenylic acid 3.8?, 6.2?54Arylphosphonic acids inosinic acid 2.4?, 6.4?542X-RC 6H 3PO 3H 2ADP 2 strong, 6.654X R ATP 3 strong, 6.654Cl 4-O 2N 1.12, 6.1457pyrophosphoric acid 0.9, 2.0, 6.6, 9.454Br 5-O 2N (a), 6.1457phosphopyruvic acid 3.5, 6.3854Cl 5-Cl (a), 6.6357creatine phosphate 2.7, 4.554Cl H 1.63, 6.9857arginine phosphate 2.8, 4.5, 9.6, 11.254Br H 1.64, 7.0057arginine 2.02, 9.0, 12.554Br 5-CH 3 1.81, 7.1557amino phosphate (-0.9), 2.8, 8.254Cl 4-NH 2--, 7.3357trimetaphosphate 2.0577CH 3O 4-O 2N 1.53, 6.9657CH 3O H 2.16, 7.7757PhosphonatesCH 3O 4-O 2N --, 8.2257H 2O 3P(CH 2)4PO 3H 2 <2, 2.75, 7.54, 8.3857HO 4-O 2N 1.22, 5.3957H 2O 3P(CH 2)3PO 3H 2 <2, 2.65, 7.34, 8.3557O 2N H 1.45, 6.7457H 2O 3PCH 2CH(CH 3)PO 3H 2 <2, 2.6, 7.00, 9.2757F H 1.64, 6.8057H 2O 3PCH 2PO 3H 2 <2, 2.57, 6.87, 10.3357I H 1.74, 7.0657Methyl- 2.3557NH 2H --, 7.2957Ethyl- 2.4357CH 3H 2.10, 7.6857n-propyl- 2.4557C 6H 5H (a), 8.1357isopropyl- 2.55, 7.7557HOOC H1.71, 9.1757n-butyl- 2.59, 8.1957isobutyl- 2.70, 8.4357**These values were obtained in 50% ethanol.s-butyl- 2.74, 8.4857(a) The compounds were not sufficiently soluble.t-butyl- 2.79, 8.8857For graphical plots of a large number of substituted phosphorus compounds see 83.neopentyl- 2.84, 8.65571,1 Dimethylpropyl- 2.88, 8.9657n-hexyl- 2.6, 7.957triphosphate8.90, 6.26, 2.3077n-dodecyl---, 8.2557tetrametaphosphate 2.7477CH 3(CH 2)5CH(COOH)- 1, --57fluorophosphate0.55, 4.856Acetic acids, substituted Phosphonates (Ref. 2)H- 4.76*20 X-H-H-NH3+-NH3+O2N- 1.68*20 X(CH2)PO3H2 2.357.1 1.85 5.35(CH3)3N+- 1.83*20 X(CH2)2PO3H2 2.457.85 2.457.00(CH3)2NH+- 1.95*20 X(CH2)4PO3H2 2.557.55CH3NH2+- 2.16*20 X(CH2)5PO3 H2 2.67.65NH3+- 2.31*20 X(CH2)6PO2H2 2.67.9CH3SO2- 2.36*20 X(CH2)10PO2H28.00NC- 2.43*20 Phosphines in acetonitrile, see ref. 89.C6H5SO2- 2.4420HO2C 2.83*20 CARBOXYLIC ACIDSAliphatic C6H5SO- 2.6620 Compound pK Ref.F- 2.6620 Acetoacetic 3.586Cl- 2.86*20 Acetopyruvic 2.61, 7.85 (enol)6Br- 2.8620 Aconitic, trans- 2.80, 4.466Cl2- 1.2920 Betaine 1.846F2- 1.2420 Citric 3.09, 4.75, 5.416Br3-0.6620 Crotonic 4.696Cl3-0.6520 Dihydroxyfumaric 1.146F3-0.23 (-0.26) (2)20 Dethylenediamine- 2.00, 2.676HONC4 3.0120 tetraacetic 6.16, 10.26F3C- 3.07*20 Formic 3.77*2N3- 3.0320 Fumaric 3.03, 4.546I- 3.1220 Glyceric 3.556C6H5O- 3.1220 Glycollic 3.826C2H5O2C- 3.3520 Glyoxylic 3.326C6H5S- 3.52*20 Homogentistic 4.406CH3O- 3.5320α-keto-β-methyl valeric 2.36NCS- 3.5820 Lactic 3.866CH3CO- 3.58*20 Maleic 1.93, 6.586Malic 3.40, 5.26C2H5O- 3.6020 Oxaloacetic (trans-enol) 2.566n-C3H7O 3.6520 +(cis-enol) 2.15, 4.066n-C4H9O 3.6620 Protocatechuic 4.486sec.-C4H9O- 3.6720 Pyruvic 2.506HS- 3.67*20Tartaric + 2.99, 4.406i-C3H7O- 3.69*20 + or - 2.89, 4.406CH3S- 3.72*20 meso 3.22, 4.856i-C3H7S- 3.72*20 Vinylacetic 4.426C6H5CH2S- 3.73*20C2H5S- 3.74*20n-C3H7S- 3.77*20n-C4H9S- 3.81*20HO- 3.83*20–O3S- 4.0520(C6H5)3CS- 4.30*20C6H5- 4.31*20CH2-CH- 4.35*20* Indicates thermodynamic values.Unsaturated acids (25°)CompoundpK poundpK ref.trans-CH 3-CH=CHCO 2H 4.69*20H-CH 2CH 2CO 2H 4.88*2cis-CH 3-CH=CHCO 2H 4.44*2H-CH=CHCO 2H 4.25*2C 6H 5-CH 2CH 2CO 2H4.66*2C 6H 5CH 2CH 2CO 2H 4.66*2trans-C 6H 5-CH=CHCO 2H 4.44*2C 6H 5CH=CHCO 2H** 4.44*2m-CH 3OC 6H 4CH 2CH 2CO 2H4.65*2C 6H 5CH 2CH 2CO 2H 4.66*2C 6H 5CH=CHCO 2H**4.442m-CH 3OC 6H 4CH=CHCO 2H 4.38*2m-ClC 6H 4CH=CHCO 2H**4.29*2m-ClC 6H 4CH 2CH 2CO 2H 4.58*2Unsaturated acids, Cis- and Trans-C C R 2H R 1CO 2H C C R 2R 1HCO 2H Cis-Acid Trans-Acid R 1R 2cis-acid trans-acid Ref.H-H- 4.25*4.25*2CH 3-H- 4.44*4.69*2Cl-H- 3.323.652C 6H 5-H- 3.88*4.44*2ClC 6H 4H- 3.91 4.4126-BrC 6H 4H- 4.02 4.412CH 3-CH 3- 4.305.022C 6H 5-H- 5.26***5.58***22,4,6-(CH 3)3C 6H 2-H-6.12***5.70***2C 6H 5-CH 3- 4.98***5.98***2Dicarboxylic acids, unsaturated*Maleic 1.92, 6.232Alicyclic Dicarboxylic acidsCitraconic (Dimethylmaleic acid)2.29, 6.152cis-Caronic(1,1-dimethylcyclopropane-23-dicarboxylic acid 2.34*, 8.31*2Acetylenedicarboxylic 1.73, 4.402∆1-tetrahydrophthalic 3.01, 5.3421,2-trans-cyclopropanedicarboxylic3.65*, 5.13*2Bromomaleic 1.45, 4.622trans-caronic 3.82*, 5.32*2Bromofumaric 1.46, 3.5721,2-cis-cyclopropane-dicarboxylic3.33*, 6.47*2Chlorofumaric 1.78, 3.812Fumaric 3.02,4.382Mesaconic (Dimethylfumaric acid)**trans3.09,4.752***in 40% acetone Phthalic 2.95, 5.412*thermodynamicItaconic (1-Propene-2-3-dicarboxylic acid)3.85, 5.452Chloromaleic 1.72, 3.862AliphaticAlicyclic Dicarboxylic acidsCompound pK Ref Compound pK Ref 1,2-trans-Cyclopropane-cis-Ethyleneoxide-dicarboxylic 3.65, 5.132dicarboxylic 1.94, 3.922 trans-Ethyleneoxide-1,3-cis-Cyclobutane-dicarboxylic 1.93, 3.252dicarboxylic 4.03, 5.3121,3-trans -Cyclobutanedi-1,2-cis-Cyclopentane-carboxylic 3.81, 5.282dicarboxylic 4.37, 6.5121,2-trans-Cyyclopentane-1,3-cis-Cyclopentanedicarboxylic 3.89, 5.912dicarboxylic 4.23, 5.5321,3-trans-Cyclopentane-1,2-cisCyclohexane-dicarboxylic 4.40, 5.452dicarboxylic 4.34, 6.7621,2-trans-Cyclohexane-1,3 -cis-Cyclohexane-dicarboxylic 4.18, 5.932dicarboxylic 4.10, 5.4621,3-trans-Cyclohexane-1,4-cis-Cyclohexanedicarboxylic 4.31, 5.732di-carboxylic 4.44, 5.7921,4-trans-Cyclohexane-dicarboxylic 4.18, 5.422Dicarboxylic acids*oxalic 1.23, 4.192Succinic 4.19, 5.482 Malonic 2.83, 5.692 O-O’-Dimethyl- 3.77, 5.942 Methyl- 3.05, 5.762 (high melting)Ethyl- 2.99, 5.832 O-O’-Dimethyl- 3.94, 6.202n-propyl 3.00, 5.842 (low melting)i-propyl- 2.94, 5.882 O,O’-Diethyl- 3.63, 6.462 Dimethyl- 3.17, 6.062 (high melting)Methylethyl- 2.86, 6.412 O,O’-Diethyl- 3.51, 6.602Diethyl- 2.21, 7.292 (low melting)Ethyl-n-propyl- 2.15, 7.432Tetramethyl- 3.50, 7.282Di-n-propyl- 2.07, 7.512Glutaric 4.34, 5.422Adipic 4.42, 5.412B-Methyl 4.25, 6.222Pimelic 4.48, 5.422B-Ethyl 4.29, 6.332Suberic 4.52, 5.402B-n-Propyl 4.31, 6.392Azelaic 4.55, 5.412B,B-Dimethyl- 3.70, 6.292DL-1:2-Dichlorosuccinic 1.68, 3.1820 B,B-Methylethyl- 3.62, 6.702meso-1:2-Dichlorosuccinic 1.74, 3.2420 B,B-Diethyl- 3.62, 7.122DL-1:2-Dibromosuccinic 1.48, ----20 B,B-Di-n-propyl 3.69, 7.312meso-1:2-Dibromosuccinic 1.42, 2.9720D-Tartaric 3.03, 4.4520DL-1:2-Dimethylsuccinic 3.93, 6.0020DL-Tartaric 3.03, ----20meso-1:2-Dimethylsuccinic 3.77, 5.3620 meso-Tartaric 3.29, 4.9220*All are thermodynamic valuesAliphatic HO- 6.332Br- 6.082 Bicyclo[2.2.2]octane-1-carboxylic acids, 4-substitutedLysergic acid, etc.H- 6.752ergometrine 6.8, --2 C2H5O2C- 6.312Dihydroergometrine7.4, --2β-dihydrolysergol8.2, --2 NC- 5.902Lysergic acid7.8, 3.32C6H5O- 3.53* 3.95* 4.52*α-dihydrolysergic8.3, 3.62CH3- 3.91* 4.24* 4.34* ergometrinine7.3, --2(CH3)2CH- 4.35*α-dihydrolysergol8.3, --2(CH3)3N+- 1.37 3.45 3.436-methylergoline8.85, --2NC- 3.60* 3.55* isolysergic acid8.4, 3.42HO2C* 2.95* 3.54 3.51γ-dihydrolysergic8.6, 3.62F3C- 3.79HO- 2.98* 4.08* 4.58*I- 2.85* 3.86* Hydroxycyclohexanecarboxylic acids Cl- 2.94* 3.83* 3.99* Cyclohexanecarboxylic 4.902(CH3)3Si- 4.24* 4.27* cis-1,2 4.802C2H5O- 4.21* 4.17* 4.45* cis-1,3 4.602i-C3H7O- 4.24* 4.15* 4.68* cis-1,4 4.842n-C5H11O- 4.55* trans-1,2 4.682C6H5- 3.46*trans-1,3 4.822CH3CH2- 3.77 4.35* trans-1,4 4.682(CH3)3C- 3.46 4.28 4.40*–HO3P- 3.78 4.03 3.95 Aromaticbenzene-CO3H 4.20*2–O3S- 4.15 4.11 Anthracene-1-COOH 3.692H2N- 4.98 4.79 4.92 Anthracene-9-COOH 3.652(CH3)2N-8.42 5.10 5.03 naphthalene-2-COOH 4.172–HO3As- 4.22 Naphthalene-1-COOH 3.692–O2C- 5.41** 4.60 4.82CH3NH- 5.3 5.10 5.04 Substituted benzoic acids (ref. 2)COOH*thermodynamicfor complex chelating agents, see also ref. 84.see also page 9a for more carboxylic acids. Benzoic acid o m pOrtho-substituted benzoic acidsH- 4.20* 4.21*Benzoic acid pK Ref.O2N- 2.17* 3.45* 3.442-CH3- 3.91**2CH3CO-2-t-C4H9- 3.462CH3SO2- 3.64* 3.52*2,6-(CH3)2- 3.212CH3S-2,3,4,6-(CH3)4- 4.002HS-2,3,5,6-(CH3)4- 3.522Br- 2.85* 3.81* 4.00*2-C2H5- 3.772F- 3.27* 3.87* 4.14*CH3O- 4.09* 4.09* 4.47*2-C6H5- 3.46**2n-C3H7O- 4.24* 4.20* 4.46*2,4,6-(CH3)3- 3.432n-C4H9O- 4.25* 4.53*2,3,4,5-(CH3)4- 4.222 Benzene Polycarboxylic acids Ref. 2Acid Position of carboxyl pK I pK II pK III pK IV pK V pK VI Benzoic1 4.17*Phthalic1,2 2.98* 5.28*Isophthalic1,3 3.46* 4.46*Terephthalic1,4 3.51* 4.82*Hemimellitic1,2,3 2.80* 4.20* 5.87*Trimellitic1,2,4 2.52* 3.84* 5.20*8Trimesic 1,3,5 3.12* 3.89* 4.70*Mellophanic 1,2,3,4 2.06* 3.25* 4.73* 6.21*Prehnitic 1,2,3,5 2.38* 3.51* 4.44* 5.81*Pyromellitic1,2,4,5 1.92* 2.87* 4.49* 5.63*Benzenepentacarboxylic 1,2,3,4,5 1.80* 2.73* 3.97* 5.25* 6.46*Mellitic1.2,3,4,5,61.40*2.19*3.31*4.78*5.89*6.96**ionic strength 0.032-Methoxyethyliminodiacetic 2.2, 8.96**thermodynamic2-Methylthioethyliminodiacetic 2.1, 8.91oxalic acid* 1.25, 4.14N-n-propylaminoacetic 2.25, 10.03Carboxylic Acids Ref. 77N-2-sulfoethyliminodiacetic 1.92, 2.28, 8.16Aminomalonic acid* 3.32, 9.83α-Bromobutyric acid 2.97N-Butylaminoacetic acid 2.29, 10.07N-(carbamoylmethyl)-imino-diacetic acid2.30, 6.602-carboxyethyliminodiacetic acid2.06,3.69, 9.66Cyanomethyliminodiacetic 3.06, 4.34β-carboxymethylaminopropionic 3.61, 9.46α,β-diaminopropionic acid 1.23, 6.69α,α-diaminobutyric 1.85, 8.24, 10.44Diethylaminoacetic 2.04, 10.47Di-(carboxymethyl)-aminomethyl phosphonic acid 2.00, 2.25, 5.57, 10.76Dimethylaminoacetic 2.08, 9.80N-ethylaminoacetic 2.30, 10.10α,β-dimercaptosuccinic 2.40, 3.46, 9.44, 11.82Gluconic* 3.86β-hydroxybutyric 4.39Ethylenediamine-N,N-diacetic 5.58, 11.05α-hydroxybutyric 3.65β-hydroxypropionic 3.73N-2-hydroxyethyliminodiacetic 2.2, 8.73Iminodiacetic* 2.98, 9.893-hydroxypropyliminodiacetic 2.06, 9.24β-iodopropionic* 4.04Iminodipropionic 4.11, 9.61N-isopropylaminoacetic 2.36, 10.06Isobutyric* 4.86α-mercaptobutyric 3.53Mandelic acid 3.41N-methylaminoacetic 2.24, 10.012-MercaptoethyliminodiaceticNitrilotriacetic 3.03, 3.07, 10.-2.14, 8.17, 10.792-PhosphonoethyliminodiaceticMethyliminodiacetic 2.81, 10.181.95,2.45, 6.54, 10.46*ThermodynamicPHENOLSCompound pK pound pK Ref. Chromotropic acid 5.36, 15.66Resorcinol--, 9.15 (30o)50o-Methoxyphenol--, 9.9350p-Methoxyphenol--, 10.1650 o-Hydroxybenz-3-Hydroxyanthran-aldehyde7.9550ilic acid10.09, 5.20512-Amino-4,5 dimethyl-2-Aminophenolphenol hydrochloride10.4 5.2851hydrochloride9.99, 4.86514,5-dihydroxybenzene-1,3 disulphonic acid7.6612.6eKojic acid9.4077Phenol o m p Phenol o m pH-9.95*9.94*O2N-7.23*8.35*7.14* (CH3)3N+-7.4288OCH- 6.798.007.66CH3SO2-9.337.83NC-8.61**7.95CH3CO-9.198.05CH3O2C-8.47*C2H5O2C-8.50*n-C4H9O2C-8.47*C3H5CH2O2C-8.41*I-9.17*Br-8.42*9.11*9.34*Cl-8.48*9.02*9.38*F-8.81*9.28*9.95*CH3S-9.539.53HO-9.489.449.96HOCH2-9.92*9.83*9.82*CH3-10.28*10.08 10.19*C2H5-10.29.910.0CH3O-9.939.6510.20H2N-9.719.8710.30-O2C-9.94*9.39*-O3S-9.299.03--O3P-10.29.9--O3As8.37 C6H5-9.939.599.51NO- 6.35**2-Chloro-4-Nitro- 5.42792-Nitro-4-Chloro- 6.4679* Thermodynamic**Reference 52ALCOHOLS and other OXYGEN ACIDSAlcoholsCompound pK pound pK Ref. Choline13.96C3F7•CH(C2F5)•OH 10.4865 Chloral hydrate9.66, 11.061(C3F7)2CH•OH10.5265 Trifluoroethanol12.562Carbonium ionsCF3CH2OH11.4, 12.4363CF3CH(OH)CH311.863Triphenylmethanols in H2SO4 HC1O4 HNO3refCF3CH2(CH3)3OH12.43104,4,4-Trimethoxy.82. .82 .8066C3F7CH2OH11.4**634,4’-Dimethoxy-1.24-1.14-1.1166(C3F7)2CHOH10.6**634-Methoxy-3.40-3.59-3.4166HCCCH2OH13.55644-Methyl-5.41-5.6766C(CH2OH))414.1644-Trideuteriomethyl- 5.43 5.6766HOCH2CHOHCH2OH 4.4643,3’,3”-Trimethyl- 6.35-5.9566HOCH2CH2OH14.7764Unsubstituted triphenyl-CH3CCH2OH14.8264methanol- 6.63-6.89 6.6066CH3OH15.54644,4;,4;-Trichloro- 7.74-8.0166 CH2=CHCH2OH15.52644-Nitro-9.15-9.7666 H2O15.7464CCl3CH2OH 11.8***CH3CH2OH1664CF3CH2OH 11.3***Substituent effects for ionization of RCH2OHRCCl-312.24,11.8064,65CF3-12.3764CHF2CH2-12.7464Hydroxamic acidsCHCl2-12.8964Furo-8.4572CHEC-13.5564Glycine7.4072H2Cl-14.3164Hippuro-8.8072CH3CCH2-14.864iso Nicotin7.8572HOCH215.164p-Methylbenz-8.9072H-15.564Nicotin-8.3072CH2=CH-15.564Nicotin-methiodide 6.4672CH3-(extrap)(15.9)64m-Nitrobenz-8.0772CF3C(CH3)2OH11.664Picolin8.5072HOCH2CF2CH2OH1164Pyrimidine-2-carbox-7.8872Primary alcohols=R•CH2•OH and Salicyl-7.4372Secondary alcohols in 50% alcohol Tropo-9.0972C2F511.3565C4F911.3565C5F1111.3765C7F1511.3565Other oxygen acidsCHF212.0065Trimethylamine-n-oxide 4.618CF2Cl11.6365Dimethylglyoxime12.8477CHF2CF211.3465(50% dioxane)CHF2 • (CF2)211.3565O-methyl ether12.9277CF3 • CH212.765Tropolone12a77CF3 • (CH2)212.965 α-Bromotropolone 6.95a77CF3 • CHMe • OH11.2865Acetald hydrate13.4891C3F7 • CHMe • OH11.3865Formald hydrate13.2991C3F7CHEt • OH11.3765C3F7CHPr • OH11.3765C3F7 • CH(CF3) • OH10.4665a50% dioxane***50 aquaeous ethanolOTHER OXYGEN ACIDSHydroxamic acids Aceto-9.4068Compound pK Ref.n-Butyro-9.4868Pyridine oxidesn-Butyro-9.00684-Aminopyridine 1-oxide 3.6967p-Methoxybenzo-9.19684-Dimethylaminopyridine 1-oxide3.8867N-Hydroxyphthalimide 7.00, 6.1071, 72Salicylo 7.32684-Dimethylaminopyridine 1-oxide3.8867Benzo-8.8868p-Chlorobenzo-9.59684-Dimethylamino-1-methoxypyridinium perchlorate >1167α-Naphtho-~7.768Propiono-9.46682-Methylaminopyridine 1-oxide 2.61672-Amino-1-methoxypyridinium perchlorate12.467Oximes4-Hydroxypyridine 1-oxide 2.4567Benzophenone oxime 11.3184-Methoxypyridine 1-oxide 2.0567Diethyl ketoxime 12.6181-Methoxypyridi-4-one 2.5767Isonitrosoacetylacetone (INAA) 7.4762-Hydroxypyridine 1-oxide -0.8675-Methyl-1,2,3-cyclohexanetrione-1,3-dioxime8.3762-Ethoxypyridine 1-oxide 1.18671-Methoxypyrid-2-one -1.3Acetophenone oxime 11.48184-Methylaminopyridine 1-oxide 3.8567Acetoxime 11.42184-Amino-1-methoxypyridinium perchlorate>1167Isonitrosoacetone (INA) 8.376Salicyclaldoxime (SA)9.2762-Aminopyridine 1-oxide 2.67671,2,3-Cyclohexanetrionetrioxime 8.0762-Dimethylaminopyridine 1-oxide2.27675-Methyl-1,2,3-cyclohexane-trionetrioxime8.0762-Methylamino-1-methoxypyridinium toluene-p-sulphonate >11674-Benzyloxypyridine 1-oxide 1.9967Oxygen acids1-Benzyloxypyrid-4-one 2.5867sulfinic acids 2-Methoxypyridine 1-oxide 1.2367p-Toluene- 1.99731-Benzyloxypyrid-2-one -1.767p-Chlorobenzene-73p-Nitrobenzene-73Pyridine 1-oxides p-Bromobenzene- 1.8973RpK Ref.m-Nitrobenzene- 1.88734-CH 3 1.2947Benzene-1.84,2.16733-CH 3 1.0847Peroxyacids3,4-(CH)4 1.0147Peroxymonosulfuric 9.4693-COOC 4H 90.0347Acetic 8.2704-NO 2-1.747n-Butyric 8.2703-NH 2 1.4747Formic 7.170H0.7947Propionic 8.1703-COOH 0.0947peroxydiphosphoric 5.18, 7.8854-COOH-0.4847peroxymonophosphoric 4.8590Peroxides ROOH (Ref. 70)H CH 3C 2H 5iso-C 3H 7tert-C 4H 9iso-C 4H 911.611.511.812.112.812.8Oximesref. 93Pyridine-2-aldoxime heptiodide 8.00benzoquinoline mon- 6.25Pyridine-4-aldoxime methiodide 8.503-pyridine-1,2-ethanedione-2-oxime methiodide7.20Pyridine-4-aldoxime pentiodide 8.504-Pyridine-1,2-ethanedione-2-oxime methiodide7.1O-Methyltyrosine ethyl ester 7.3122 octopine 13, 1.368.776Pyridine-2-aldoxime methiodide8.0Phenylglyoxald-8.3 2.40Pyridine-4-aldoxime dodeciodide8.5Phenylalanine 1.839.136 Pyridine-3-alkoxime methiodide9.22-Pyrrolidoone-5-carboxylic acid (glucamicacid) 3.32Hydroxamic acids ref. 93Serine 2.219.156 D-Lysine-7.93Threonine 2.6310.436 N-phenylnicotino-8.00N-Trimethyl tyrosine9.7521 Chloroaceto-8.40Tyrosine 10.07, 2.209.11 Formo-8.65Urocanic acid 5.8 3.5p-Chlorophenoxyaceto-8.75Valine 2.329.626 p-Hydroxybenzo-8.93β-Alanine 3.6010.196 p-Methoxybenzo-9.00γ-Aminobutyric acid 4.2310.436 N-Phenylbenzo-9.15Arginine 12.48 2.179.046 o-Aminobenzo-9.17Asparagine 2.028.86 L-Tyrosine9.20Azaserine8.556 L-Lysine7.9Canavanine7.40, 9.2511.50 (?)6 p-Nitrobenzo-8.0Creatine 2.6711.026 p-Aminobenzo-9.3Cysteine 10.78 1.718.336 L-Lacti-9.33,4-DihydroxyphenylalaninePropiono-9.49.88, 2.368.686 Phthalo-9.411.68Indole-3-aceto-9.5Glutamine 2.179.136 Cyclohexano-9.7Histamine 5.09.76 Hexano-9.7β-Hydroxyglutamic 2.099.206acid 4.18Amino Acids Hydroxyproline 1.929.736 Compound pK Ref.Leucine 2.369.606-COOH-NH3Methionine 2.289.21 Alanine 2.359.6961-Methylhistidine 6.48, 1.698.856α-Aminobutyric acid 2.559.60Norleucine 2.399.766α-Aminoisobutyric 2.3610.216Norvaline 2.369.766 Argininosuccinic >12, 1.629.586Ornithine 1.718.6962.70, 4.2610.76 Aspartic acid 2.09,3.869.826Proline 1.9910.606 Canaline10.3, 9.2011.6 (?)6Sarcosine 2.2310.016 Creatinine4.849.26Taurine 1.58.746 Cystine 1.657.856Thiolhistidine <1.5, 11.42.269.856 1.848.476 Diidotyrosine 6.48, 2.127.826Tryptophan 2.389.396 Glutamic acid 2.19, 4.259.676Tyrosine ethyl ester 7.339.8022 Glycine 2.349.66PeptidesHistidine 6.0, 1.829.176Anserine 7.0 2.659.56 Carnosine 6.83--9.516Hydroxylsine 2.138.626Cystinyldiglycine 3.12 6.3669.67 3.12 6.95 Isoleucine 2.369.686Glycylglycine 3.06 8.13 Lysine 2.188.956Gly-gly-gly 3.267.912310.53Glycylproline 2.848.556 O-Methyl tyrosine9.2721Aspartyl histi- 2.457.98dine 6.82 3.02Gly-gly-gly-gly 3.057.7523 Diglycylcystine 2.717.946Lysyl-lysine (L,L) 3.017.536 Glutathione 9.12 2.128.66610.0511.013.53Compound-COOH-NH2-NH2-NH2-NH2Ref. Gly•Ala (L) or (D) 3.178.2327 Ala•Gly (L) or (D) 3.168.2427 Gly•Ala•Ala (LL) 3.388.1027 Gly•Ala•Ala (LD) 3.308.1727 Ala•Ala•OH (DD) 3.308.1427 Ala•Ala•OH (LD) 3.128.3027 H•Ala•Ala•Ala•OH (3L) 3.398.0327 H•Ala•Ala•Ala•OH (LLD) 3.378.0527 H•Ala-Ala-Ala•OH (LDL) 3.318.1327 H•Ala-Ala-Ala•OH (DLL) 3.378.0627 H-Ala-Ala-Ala•OH (3D) 3.398.0627 H•Ala-Ala-Ala-Ala•OH (4L) 3.427.9427 H•Ala-Ala-Ala-Ala•OH (LLDL) 3.247.9327 H•Ala-Ala-Ala-Ala•OH (LDLL) 3.227.9927 H•Ala-Ala-Ala-Ala•OH (DLLL) 3.427.9927 H•Lys-Ala•OH (LL) 3.227.6210.7027 H•Lys-Ala•OH (LD) 3.007.7410.6327 H•Ala-Lys-Ala•OH (3L) 3.157.6510.3027 H•Ala-Lys-Ala•OH (LDL) 3.337.9710.3627 H•Ala-Lys-Ala•OH (LLD) 3.297.8410.4927 H•Ala-Lys-Ala-Ala•OH (4L) 3.588.0110.5827 H•Ala-Lys-Ala•OH (LDLL) 3.328.0110.3727 H•Ala-Lys-Ala-Ala-Ala•OH (5L) 3.537.7510.3527 H•Ala-Lys-Ala-Ala-Ala•OH (LDLLL) 3.307.8510.2927 H•Lys-Lys•OH (LL) 3.017.5310.0511.0127 H•Lys-Lys•OH (LD) 2.857.539.9210.9827 H•Lys-Lys•OH (3L) 3.087.349.8010.5411.3227 H•Lys-Lys-Lys•OH (LDL) 2.917.299.7910.5411.4227 H•Lys-Lys-Lys•OH (LDD) 2.947.149.6010.3811.0927 Compound pK ref.Glutathione 3.59, 8.75, 9.6577Glycylserine8.2377Glycylleucine8.1377Leucylglycine7.9677Glycylisoleucine7.9677Leucylglycylglycine7.6677Glycylphenylalanine8.2877Glycyltyrosine8.2277Benzylglutamic acid 3.49, 4.9977Glycyltryptophane8.0477Glutathione, oxidized 3.15, 4.03, 8.57, 9.5477Alanylalanine (LL) 3.308.1492Alanylalanine (LD) 3.128.3092Lysylalanine (LL) 3.227.6210.7092Lysylalanine (LD) 3.007.7410.6392Leucyltyrosine (LL) 3.467.8410.0992Leucyltyrosine (DL) 3.128.3810.3592Lysyllysine (LD) 2.857.539.9292NITROGEN COMPOUNDSAliphatic Amines pK ref.Ammonia9.211n-Propyl-10.531 Primary Amines Trimethylsilymethyl-10.961β-Alanine ester9.131CH3ONH2 4.6012 Allylamine-9.692Allyl-9.491 Benzyl9.341γ-Amino-n-butyric acid ester 9.711n-Butyl-10.591sec-Butyl-10.561t-Butyl-10.551Cyclohexyl-10.641 Cyclohexylmethyl-10.491β-difluoroethyl-7.521 Ethanol-9.501Ethyl10.631 Ethylenedi-9.98, 7.521, 77Glycine ester7.751 Hydrazine8.101Hydroxyl- 5.971 Isopropyl-10.631Methoxy- 4.601 Methyl-10.621neo-Pentyl-10.211 Phenylamyl-10.492δ-Phenylbutyl10.402β-Phenylethyl-9.831γ-Phenylpropyl-10.201Triethylenedi-8.8*?X XNH3+XCH2NH3+X(CH2)2NH3+X(CH2)3NH3+X(CH2)4NH3+X(CH2)5NH3+ref. H-9.25*10.64*10.67*10.58*10.61*10.63*2 HF2C-7.52RO2C-7.759.139.7110.15*10.372 HO- 5.96*9.50*C6H5- 4.58*9.37*9.83*10.20*10.39*10.49*2 H2N-8.12*9.98*10.65*10.84*11.05*2 H2C=CH-9.69CH3-10.64*10.67*10.58*10.61*10.63*10.64*2 X-H-NH3+-CO2–-SO3–-PO3–2X-NH3+9.25*-.88110.25X(CH2)2NH3+10.649.77 5.7510.8X(CH2)2NH3+10.6710.199.2010.8X(CH2)4NH3+10.619.3110.7710.6510.9X(CH2)5NH3+10.639.7410.7510.9511.0X(CH2)8NH3+10.6510.10X(CH2)10NH3+10.6411.3511.25X(CH2)3NH3+10.588.5910.4310.05Secondary amines Di-n-butyl-11.251 Dimethyl-10.641Diisobutyl-10.501Di-n-propyl-11.001α-Ethylpyrroline7.432 Diisopropyl-11.051α-Benzylpyrroline-7.082t-Butylcyclohexyl-11.2312-Methylpiperidine10.992α-Cyclohexylpyrroline7.952α-Cyclohexylpyrrolidine10.802α-(p-Tolyl)pyrroline7.592α-(p-Tolyl)pyrrolidine10.012α-Ethylpyrrolidine10.432N,O-dimethylhydroxylamine 4.7512α-Benzylpyrrolidine10.362Acetanilide+0.614N-methylhydroxylamine 5.9612*thermodynamic valueDiethyl-10.981Aliphatic Amines Methyl-β-diethylamino-ethyl-sulfide 1,2-Iminoethane 7.9871,2-Dimethyl-∆2-pyrroline 11.942cis-2,3-Iminobutane 8.7271-methyl-2-n-butyl-∆2-pyrroline 11.901,2-Imino-2-methylpropane 8.6171-Ethyl-2-methyl-∆2-pyrroline 11.9221,2-Iminobutane 8.2971-n-Butyl-2-methyl-∆2-pyrroline 11.902trans-2,3-Iminobutane 8.6971,2-Dimethyl-∆2-tetrahydropyridine11.572Secondary Amines N-Ethyl derivative of: 1,2-Imino-ethane7.937Allylmethyl-10.111Benzylethyl-9.681Trans-2,3-Iminobutane 9.477Morpholine 8.361Trimethylhydroxylamine 3.6512N-Benzoylpiperazine 7.781Dimethylethyl-9.991Di-sec-butyl-11.011Triethyl-10.651N-Methylmethoxyamine 4.751Dimethyl-n-butyl-10.021Pyrolidine 11.271Dimethyl-isopropyl-10.3011-Tosylpiperazine 7.39Dimethyl-t-butyl-10.521Benzylmethyl-9.581Tri-n-butyl-10.891Piperidine 11.221Diallylmethyl-8.791N-Carbethoxypiperazin 8.2811-n-Propylpiperidine 10.482Dietrimethylsilylmethyl-11.40110.110.15Diallyl-9.2919.8--5N-Methylhydroxyl- 5.9611,2-Dimethylpyrrolidine 10.262Trimethyleneimine 11.2911-Methyl-2-n-butylpyrrolidin 10.242Cis-2,6-dimethyl-piperidine 10.9231-Ethyl-2-methylpyrrolidine 10.6421-n-Butyl-2-methylpyrrolidine 10.4321-Ethyl-2-methylpyrrolidine 10.7021,2-Iminobutane 8.187Tertiary amines cis-2,3-Iminobutane 8.567Trimethyl-9.761N-dimethylhydroxylamine 5.2012Dimethyldiethyl-10.291Allyldimethyl 8.781Dimethyl-n-propyl-9.9911,2-Dimethylpiperidine 10.262Dimethyl-isobutyl-9.9111-Ethyl-2-methyl-∆2-tetrahydropyridine11.572Dimethyl-sec-butyl-10.401Tri-n-propyl-10.651Triallyl-8.311N-Allylpiperidine 9.6921-Diethylamino-hexane-thiol-(6)Cyanoamines2-Amino-2-cyanopropane 5.39N-piperidine-CH 2CN 4.558β-Isopropylaminopropionitrile 8.09Et 2NCN -2.08β-Diethylaminopropionitrile 7.69Et 2N(CH 2)2CN 7.658Et 2NCH 2CN 4.558Et 2N(CH 2)4CN 10.088Et 2N(CH 2)3CN 9.298Et 2NC(CH 3)2CN 9.138Et 2N(CH 2)5CN 10.468EtN(CH 2CN)2-0.68HN(CH 2CN)20.28EtN(CH 2CH 2CN)2 4.558HN(CH 2CH 2CN)2 5.268H 2NCH 2CN 5.348N(CH 2CH 2CN)3 1.18N-Amphetamine-(CH 2)2-CN 7.238N-piperidine-C(CH 3)2CN 9.228N-Norcodeine-(CH 2)2CN 5.688N-Methamphetamine-(CH 2)2CN 6.958Dimethylcyanimide 1.29Methyl cyanamide 1.29Diethylcyanimide 1.29Ethyl cyanamide 1.29Aminoacetonitrile 5.39Cyanamide 1.19Diethylaminoacetonitrile 4.59Dimethylaminoacetonitrile 4.29β-Aminopropionitrile7.79CF3CH2NHCH3 6.0510β-Dimethylaminopropionitrile7.09Phenylethylaminesβ,β"-Dicyanodiethylamine 5.292-phenylethylamine9.7811 For complex chelating agents of aliphatic amines,see also ref. 77.N-methyl-2-(3,4-dihydroxyphenyl)-ethylamine8.7811N-methyl-2-phenyl10.3111 Fluoro-substituted aminesEpinephrine8.5511 CF3CH2NH2 5.710Arterenol8.5511 CF3CH2N(CH3)2 4.7510R2R1CHCH2NHR4R3ref. 11R1R2R3R4pKH H H H9.78H H OH H8.90H OH OH H8.81OH H OH H8.67H OH H H9.22OH OH H H8.93OH OH OH H8.58H H H CH310.31H H OH CH39.31H OH OH CH28.62OH H OH CH38.89H OH H CH39.36OH OH H CH38.78OH OH OH CH38.55Ring amines and imines (in 80% methyl cellosolve) (ref. 2)Pentamethylene9.99Cyclotridecyl9.63 Hexamethylene10.00Cyclotetradecyl9.54 Heptamethylene9.77Cyclopentadecyl9.54 Octamethylene9.39Cycloheptadecyl9.57 Nonamethylene9.14Cyclooctadecyl9.54 Decamethylene9.04Undecamethylene9.14Amines otherDodecamethylene9.31Dimeoone 5.2318 Tridecamethylene9.35Phthalimide8.3018 Tetradecamethylene9.35Nitrourea 4.5718 Hexadecamethylene9.29Nitrourethane 3.2818 Heptadecamethylene9.27Diphenylthiocarbazone 4.56 Cyclohexyl9.82β,β,β"-Triaminotriethylamine Cycloheptyl9.998.42, 9.44, 10.1387 CyclooctylCyclononyl9.95Anilines Ref. 2Cyclodecyl9.85MonosubstitutedCycloundecyl9.71Substituent o m p Cyclododecyl9.62H- 4.62* 4.64* 4.58*。
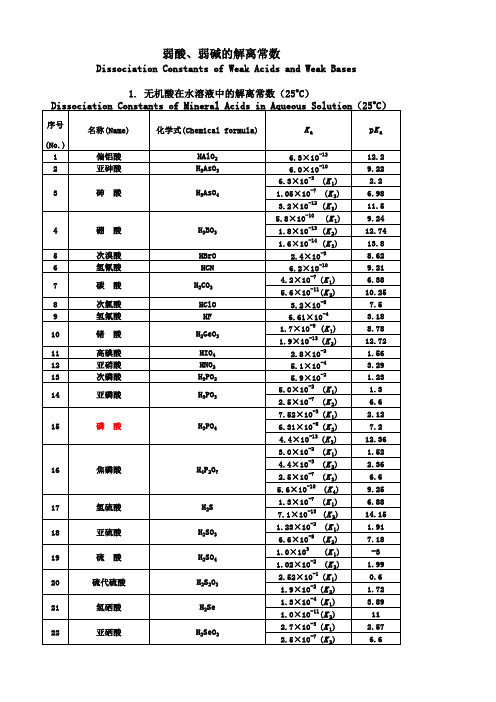
有机酸无机酸PKa值表

2.6×10 (K 1) 2.6×10 (K 2) 1.0×10-2(K 1) 2.14×10 (K 2) 6.92×10 (K 3) 5.5×10
-11 -7 -3
55
乙二胺四乙酸(EDTA)
∣ CH2—N(CH2COOH)2
(K 4)
3. 无机碱在水溶液中的解离常数(25oC) Dissociation Constants of Mineral Bases in Aqueous Solution (25oC)
1.7×10-5(K 2) 4.0×10 (K 3)
-7
邻苯二酚 间苯二酚 对苯二酚 2,4,6-三硝基苯酚 葡萄糖酸 苯甲酸 水杨酸 邻硝基苯甲酸 间硝基苯甲酸 对硝基苯甲酸 邻苯二甲酸 间苯二甲酸 对苯二甲酸
1.1×10 3.6×10-10 1.6×10-13 3.6×10-10(K 1) 8.71×10-12(K 2) 1.1×10-10 5.1×10-1 1.4×10-4 6.3×10-5 1.05×10-3(K 1) 4.17×10-13(K 2) 6.6×10 3.5×10-4 3.6×10-4 1.1×10-3(K 1) 4.0×10-6(K 2) 2.4×10-4(K 1) 2.5×10-5(K 2) 2.9×10 (K 1) 3.5×10 (K 2) 7.6×10-3(K 1) 7.9×10-5(K 2) 6.6×10 (K 3) 2.1×10-1(K 1) 6.2×10 (K 2)
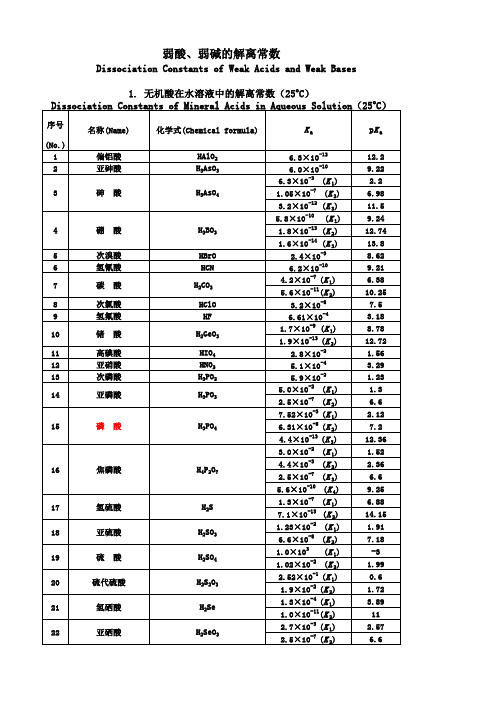
弱酸、弱碱的解离常数
Dissociation Constants of Weak Acids and Weak Bases 1. 无机酸在水溶液中的解离常数(25oC) Dissociation Constants of Mineral Acids in Aqueous Solution(25oC)
有机酸无机酸PKa值表
38
苯酚
39
邻苯二酚
C6H5OH (o )C6H4(OH)2
40
间苯二酚
41
对苯二酚
42 2,4,6-三硝基苯酚
43
葡萄糖酸
44
苯甲酸
45
水杨酸
46
邻硝基苯甲酸
47
间硝基苯甲酸
48
对硝基苯甲酸
49
邻苯二甲酸
(m )C6H4(OH)2
(p )C6H4(OH)2 2,4,6-(NO2)3C6H2OH CH2OH(CHOH)4COOH
2 2.67 6.16 10.26
3. 无机碱在水溶液中的解离常数(25oC) Dissociation Constants of Mineral Bases in Aqueous Solution (25oC)
序号 (No.)
名称(Name)
化学式 (Chemical formula)
Kb
pK b
14
三乙醇胺
(HOCH2CH2)3N
5.75×10-7
6.24
15
丁胺
C4H9NH2
4.37×10-4
3.36
16
异丁胺
C4H9NH2
2.57×10-4
3.59
17
叔丁胺
C4H9NH2
4.84×10-4
3.315
18
己胺
H(CH2)6NH2
序号 (No.)
名称(Name)
化学式(Chemical formula)
Kb
pK b
1
甲胺
CH3NH2
4.17×10-4
3.38
2
尿素(脲)
CO(NH2)2
各种酸的pKa及pH值-pb的pka

6.10 6.25 6.75 7.00 7.00
7.00
7.00
4.42
4.62 4.77 5.27 5.77 6.28
6.81
7.00
4.93
5.12 5.28 5.78 6.28 6.78
7.00
7.00
1.40
1.75 2.03 3.00 4.00 5.00
6.00
7.00
2.97
3.17 3.32 3.84 4.42 5.12
6.00
7.00
2.92
3.12
3.27
3.80
4.38
5.10
6.01
7.00
5.63
5.82
5.98
6.48
6.98
7.00
7.00
7.00
2.75
2.96
3.11
3.65
4.27
5.06
6.01
7.00
1.26
1.58
1.83
2.73
3.70
4.70
5.70
6.70
2.76
2.97
3.12
3.66
焦硼酸
H2B4O7
4.90
2.45 2.60 2.80 2.95
酒石酸
HOOCHOHCCHOHCOOH
3.04
4.37 1.51 1.66 1.86 2.01
邻苯二酚 邻苯二甲酸 邻硝基苯甲酸 磷酸 硫酸
马来酸
(o)C6H4(OH)2 HOOC-Ph-COOH (o)NO2C6H4COO H H3PO4 H2SO4 COOHCH2═ CHCOOH
己二酸
HOCOCH2CH2CH 2CH2COOH
PKa值

部分酸的PKa值砷酸H3AsO4 PKa=2.2 7.00 11.50 亚砷酸HAsO2PKa=9.22硼酸H3BO3 PKa=9.24焦硼酸H2B4O7PKa=4 9碳酸H2CO3 PKa=6.38 10.21氢氰酸HCN PKa=9.21铬酸H2CrO4PKa=0.74 6.50氢氟酸HF PKa=3.18亚硝酸HNO2PKa=3.29过氧化氢H2O2PKa=11.75磷酸H3PO4PKa=2.12 7.2 12.36 焦磷酸H4P2O7 PKa=1.52 2.36 6.60 9.25 亚磷酸H3PO3 PKa=1.3 6.60氢硫酸H2S PKa1=7.05 18.15硫酸H2SO4 PKa2=1.99亚硫酸H2SO3 PKa=1.9 7.20偏硅酸H2SiO3PKa=9.77 11.8甲酸HCOOH PKa=3.74乙酸CH3COOH PKa=4.74一氯乙酸CH2ClCOOH PKa=2.86二氯乙酸CHCl2COOH PKa=1.3三氯乙酸Cl3COOH PKa=0.64氨基乙酸盐+NH3CH2COOH PKa=2.35 9.6 乳酸CH3CHOHCOOH PKa=3.86苯甲酸C6H5COOH PKa=4.21草酸H2C2O4PKa=1.22 4.19d-酒石酸HOOCHOHC-CHOHCOOH PKa=3.04 4.37 邻-苯二甲酸HOOC-Ph-COOH PKa=2.95 5.41 柠檬酸HOOCOHC-(CH2COOH)2PKa=3.13 4.76 6.4 苯酚C6H5OH PKa=9.95乙二胺四乙酸H6-EDTA2+ PKa=0.9 1.6 2.0 2.67 6.16 10.26 铵离子NH4+PKa=9.26联铵离子+H3NNH3+PKa=8.48羟铵离子NH3+OH PKa=5.96甲胺离子CH3NH3+ PKa=10.62乙胺离子C2H5NH3+PKa=10.75二甲胺离子(CH3)2NH2+PKa=10.07二乙胺离子(C2H5)2NH2+PKa=11.11乙醇胺离子HOCH2CH2NH3+PKa=9.50三乙醇胺离子(HOCH2CH2)3NH+ PKa=7.76六亚甲基四胺离子(CH2)6NH+ PKa=5.15乙二胺离子+H3NCH2CH2NH3+PKa1=6.85 PKa2=9.93部分有机酸甲酸HCOOH PKa=3.77 乙酸CH3COOH PKa=4.74 丙酸CH3CH2COOH PKa=4.87 丁酸CH3CH2CH2COOH PKa=4.82阿尔法氯代丁酸CH3CH2CHClCOOH PKa=2.84 贝塔氯代丁酸CH3CHClCH2COOH PKa=4.06 伽马氯代丁酸ClCH2CH2CH2COOH PKa=4.52 氯乙酸ClCH2COOH PKa=2.86 二氯乙酸(Cl)2CHCOOH PKa=1.26 三氯乙酸(Cl)3CCOOH PKa=0.64 氟乙酸FCH2COOH PKa=2.66 氯乙酸ClCH2COOH PKa=2.86 溴乙酸BrCH2COOH PKa=2.86 碘乙酸ICH2COOH PKa=3.21。
各种酸的pKa及pH值
H2B4O7
4.90
2.45 2.60 2.80
HOOCHOHC-CHOHCOOH
3.04 4.37 1.51 1.66 1.86
(o)C6H4(OH)2
9.45 12.8 4.72 4.88 5.07
HOOC-Ph-COOH
2.95 5.41 1.48 1.63 1.83
(o)NO2C6H4COOH
4.09
5.01
6.00
7.00
1.30
1.51
1.81
2.07
3.01
4.00
5.00
6.00
7.00
1.00
1.30
1.70
2.00
3.00
4.00
5.00
6.00
7.00
1.00
1.30
1.70
2.00
3.00
4.00
5.00
6.00
7.00
1.00
1.30
1.70
2.00
3.00
4.00
5.00
PKa1 PKa2 1.0M PH 0.5M PH 0.2M PH
4.74
2.37 2.52 2.72
4.72
2.36 2.51 2.71
2.12 4.1 1.07 1.22 1.43
1.89
0.97 1.13 1.35
4.7
2.35 2.50 2.70
4.68
2.34 2.49 2.69
4.52
2.26 2.41 2.61
2.13
1.08 1.24 1.46
9.60
4.80 4.95 5.15
4.59
2.30 2.45 2.65
有机酸无机酸PKa值表
Dissociation Constants of Weak Acids and Weak Bases
1. 无机酸在水溶液中的解离常数(25oC) Dissociation Constants of Mineral Acids in Aqueous Solution(25oC)
序号
32
正己酸
33
异己酸
34
(E )-2-己烯酸
35
(E )-3-己烯酸
36
己二酸
CH3(CH2)4COOH (CH3)2CH(CH2)3—COOH
H(CH2)3CH═CHCOOH CH3CH2CH═CHCH2COOH
HOCOCH2CH2CH2CH2COOH
37
柠檬酸
HOCOCH2C(OH)(COOH)CH2COOH
Ka
pK a
1
甲酸
HCOOH
1.8×10-4
3.75
2
乙酸
CH3COOH
1.74×10-5
4.76
3
乙醇酸
CH2(OH)COOH
1.48×10-4
3.83
4
草酸
5
甘氨酸
(COOH)2 CH2(NH2)COOH
5.4×10-2(K 1) 5.4×10-5(K 2)
1.7×10-10
1.27 4.27 9.78
H3BO3 HBrO HCN H2CO3 HClO
HF H2GeO3 HIO4 HNO2 H3PO2 H3PO3
H3PO4
H4P2O7
H2S H2SO3 H2SO4 H2S2O3 H2Se H2SeO3
6.3×10-13
6.0×10-10 6.3×10-3 (K 1) 1.05×10-7 (K 2) 3.2×10-12 (K 3) 5.8×10-10 (K 1) 1.8×10-13 (K 2) 1.6×10-14 (K 3)
部分酸的酸度系数pKa

部分酸的pKa值砷酸H3AsO4 PKa=2.2 7.00 11.50 亚砷酸HAsO2PKa=9.22硼酸H3BO3 PKa=9.24焦硼酸H2B4O7PKa=4 9碳酸H2CO3 PKa=6.38 10.21氢氰酸HCN PKa=9.21铬酸H2CrO4PKa=0.74 6.50氢氟酸HF PKa=3.18亚硝酸HNO2PKa=3.29过氧化氢H2O2PKa=11.75磷酸H3PO4PKa=2.12 7.2 12.36 焦磷酸H4P2O7 PKa=1.52 2.36 6.60 9.25 亚磷酸H3PO3 PKa=1.3 6.60氢硫酸H2S PKa1=7.05 18.15硫酸H2SO4 PKa2=1.99亚硫酸H2SO3 PKa=1.9 7.20偏硅酸H2SiO3PKa=9.77 11.8甲酸HCOOH PKa=3.74乙酸CH3COOH PKa=4.74一氯乙酸CH2ClCOOH PKa=2.86二氯乙酸CHCl2COOH PKa=1.3三氯乙酸Cl3COOH PKa=0.64氨基乙酸盐+NH3CH2COOH PKa=2.35 9.6 乳酸CH3CHOHCOOH PKa=3.86苯甲酸C6H5COOH PKa=4.21草酸H2C2O4PKa=1.22 4.19d-酒石酸HOOCHOHC-CHOHCOOH PKa=3.04 4.37 邻-苯二甲酸HOOC-Ph-COOH PKa=2.95 5.41 柠檬酸HOOCOHC-(CH2COOH)2PKa=3.13 4.76 6.4 苯酚C6H5OH PKa=9.95乙二胺四乙酸H6-EDTA2+ PKa=0.9 1.6 2.0 2.67 6.16 10.26 铵离子NH4+PKa=9.26联铵离子+H3NNH3+PKa=8.48羟铵离子NH3+OH PKa=5.96甲胺离子CH3NH3+ PKa=10.62乙胺离子C2H5NH3+PKa=10.75二甲胺离子(CH3)2NH2+PKa=10.07二乙胺离子(C2H5)2NH2+PKa=11.11乙醇胺离子HOCH2CH2NH3+PKa=9.50三乙醇胺离子(HOCH2CH2)3NH+ PKa=7.76六亚甲基四胺离子(CH2)6NH+ PKa=5.15乙二胺离子+H3NCH2CH2NH3+PKa1=6.85 PKa2=9.93部分有机酸甲酸HCOOH PKa=3.77 乙酸CH3COOH PKa=4.74 丙酸CH3CH2COOH PKa=4.87 丁酸CH3CH2CH2COOH PKa=4.82阿尔法氯代丁酸CH3CH2CHClCOOH PKa=2.84 贝塔氯代丁酸CH3CHClCH2COOH PKa=4.06 伽马氯代丁酸ClCH2CH2CH2COOH PKa=4.52 氯乙酸ClCH2COOH PKa=2.86 二氯乙酸(Cl)2CHCOOH PKa=1.26 三氯乙酸(Cl)3CCOOH PKa=0.64 氟乙酸FCH2COOH PKa=2.66 氯乙酸ClCH2COOH PKa=2.86 溴乙酸BrCH2COOH PKa=2.86 碘乙酸ICH2COOH PKa=3.21。
常见酸碱的pka

常见酸碱的pka
除了那些p K a值低于-1.76的物质,以下列出一般物质在25℃水下量度的p K a值:
- 31.30:氟锑酸
- 19.20:魔酸
- 18.00:碳硼烷酸
- 15.10:氟磺酸
- 10.00:过氯酸
- 10.00:氢碘酸
- 9.00:氢溴酸
- 8.00:盐酸
- 3.00、1.99:硫酸
- 2.00:硝酸
- 1.76:水合氢离子
3.15:氢氟酸
3.60:碳酸
3.75:甲酸
4.04:抗坏血酸(维生素C)
4.19:琥珀酸
4.20:苯甲酸
4.63:苯胺*
4.74:醋酸
4.76:柠檬酸二氢根离子
5.21:吡啶*
6.40:柠檬酸一氢根离子
6.99:乙二胺*
7.00:硫化氢、咪唑*(作为酸)
7.50:次氯酸
9.25:氨*
9.33:苯甲胺*
9.81:三甲胺*
9.99:酚
10.08:乙二胺*
10.66:甲胺*
10.73:二甲胺*
10.81:乙胺*
11.01:三乙胺*
11.09:二乙胺*
11.65:过氧化氢
12.50:胍*
12.67:磷酸一氢根离子(磷酸盐)
14.58:咪唑(作为碱)
- 19.00(pKb):氨基化钠
26.00 六甲基二硅基胺基钾(KHMDS)
*氨和胺基的数值是相应的氨离子的p K a值。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
肼(联氨) 羟 氨
8.86 3.96 2.43 1.4 4.75 6.02 14.9 8.04 3.02 7.52 3.02
氢氧化铅 氢氧化锌
4. 有机碱在水溶液中的解离常数(25oC) Dissociation Constants of Organic Bases in Aqueous Solution (25oC)
-3 -8
亚碲酸
2. 有机酸在水溶液中的解离常数(25oC) Dissociation Constants of Organic Acids in Aqueous Solution(25oC)
序号 (No.) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 名称(Name) 甲 酸 乙 酸 乙醇酸 草 酸 化学式 (Chemical formula) HCOOH CH3COOH CH2(OH)COOH (COOH)2 CH2(NH2)COOH CH2ClCOOH CHCl2COOH CCl3COOH CH3CH2COOH CH2═CHCOOH CH3CHOHCOOH HOCOCH2COOH HC≡CCOOH HOCH2CHOHCOOH CH3COCOOH CH3CHNH2COOH CH2NH2CH2COOH CH3(CH2)2COOH (CH3)2CHCOOH CH2═CHCH2COOH CH2═C(CH2)COOH HOCOCH═CHCOOH HOCOCH═CHCOOH HOCOCH(OH)CH(OH)COOH CH3(CH2)3COOH (CH3)2CHCH2COOH CH3CH2CH═CHCOOH CH3CH═CHCH2COOH CH2═CHCH2CH2COOH HOCO(CH2)3COOH
序号 (No.) 1 2 3 名称(Name) 偏铝酸 亚砷酸 砷 酸 化学式(Chemical formula) HAlO2 H3AsO3 H3AsO4
Ka
6.3×10-13 6.0×10-10 6.3×10-3 (K 1) 1.05×10-7 (K 2) 3.2×10-12 (K 3) 5.8×10
2.6×10 (K 1) 2.6×10 (K 2) 1.0×10-2(K 1) 2.14×10 (K 2) 6.92×10 (K 3) 5.5×10
-11 -7 -3
55
乙二胺四乙酸(EDTA)
∣ CH2—N(CH2COOH)2
(K 4)
3. 无机碱在水溶液中的解离常数(25oC) Dissociation Constants of Mineral Bases in Aqueous Solution (25oC)
甘氨酸 一氯乙酸 二氯乙酸 三氯乙酸 丙 酸 丙烯酸 乳酸(丙醇酸) 丙二酸 2-丙炔酸 甘油酸 丙酮酸 a -丙胺酸 b -丙胺酸 正丁酸 异丁酸 3-丁烯酸 异丁烯酸 反丁烯二酸(富马酸) 顺丁烯二酸(马来酸) 酒石酸 正戊酸 异戊酸 2-戊烯酸 3-戊烯酸 4-戊烯酸 戊二酸
31
谷氨酸
HOCOCH2CH2CH(NH2)COOH
(K 1) (K 2) (K 3)
4 5 6 7 8 9 10 11 12 13 14
硼
酸
H3BO3 HBrO HCN H2CO3 HClO HF H2GeO3 HIO4 HNO2 H3PO2 H3PO3
1.8×10 1.6×10
次溴酸 氢氰酸 碳 酸
2.4×10-9 6.2×10-10 4.2×10-7 (K 1) 5.6×10-11(K 2) 3.2×10 6.61×10-4 1.7×10-9 (K 1) 1.9×10-13 (K 2) 2.8×10 5.1×10-4 5.9×10-2 5.0×10-2 (K 1) 2.5×10-7 (K 2) 7.52×10
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51
柠檬酸 苯 酚
HOCOCH2C(OH)(COOH)CH2COOH C6H5OH (o )C6H4(OH)2 (m )C6H4(OH)2 (p )C6H4(OH)2 2,4,6-(NO2)3C6H2OH CH2OH(CHOH)4COOH C6H5COOH C6H4(OH)COOH (o )NO2C6H4COOH (m )NO2C6H4COOH (p )NO2C6H4COOH (o )C6H4(COOH)2 (m )C6H4(COOH)2 (p )C6H4(COOH)2
-3 -8 -2 -8
次氯酸 氢氟酸 锗 酸
高碘酸 亚硝酸 次磷酸 亚磷酸
(K 1) (K 2)
15
磷
酸
H3PO4
6.31×10
4.4×10-13 (K 3) 3.0×10-2 (K 1) 16 焦磷酸 H4P2O7 4.4×10 2.5×10
-3 -7
(K 2) (K 3)
5.6×10-10 (K 4) 17 18 19 20 21 22 氢硫酸 亚硫酸 硫 酸 H2S H2SO3 H2SO4 H2S2O3 H2Se H2SeO3 1.3×10-7 (K 1) 7.1×10
-3 -4 -6 -6 -5 -4 -3
-10
52
1,3,5-苯三甲酸
C6H3(COOH)3
53
苯基六羧酸
C6(COOH)6
3.0×10 (K 3) 8.1×10 (K 4) 4.8×10-7(K 5) 3.2×10 (K 6)
-8 -5 -6
54
癸二酸
HOOC(CH2)8COOH CH2—N(CH2COOH)2
Ka
1.8×10-4 1.74×10-5 1.48×10-4 5.4×10-2(K 1) 5.4×10-5(K 2) 1.7×10 1.4×10-3 5.0×10-2 2.0×10-1 1.35×10-5 5.5×10-5 1.4×10-4 1.4×10-3(K 1) 2.2×10-6(K 2) 1.29×10 2.29×10-4 3.2×10-3 1.35×10-10 4.4×10-11 1.52×10-5 1.41×10-5 2.1×10-5 2.2×10-5 9.3×10-4(K 1) 3.6×10-5(K 2) 1.2×10 (K 1) 5.9×10 (K 2) 1.04×10 (K 1) 4.55×10 (K 2) 1.4×10 1.67×10-5 2.0×10-5 3.0×10-5 2.10×10-5 1.7×10-4(K 1) 8.3×10-7(K 2) 7.4×10 (K 1)
弱酸、弱碱的解离常数
Dissociation Constants of Weak Acids and Weak Bases 1. 无机酸在水溶液中的解离常数(25oC) Dissociation Constants of Mineral Acids in Aqueous Solution(25oC)
-3 -5 -5 -3 -7 -2 -2 -10
pK a 3.75 4.76 3.83 1.27 4.27 9.78 2.86 1.3 0.7 4.87 4.26 3.86 2.85 5.66 1.89 3.64 2.49 9.87 10.36 4.82 4.85 4.68 4.66 3.03 4.44 1.92 6.23 2.98 4.34 4.86 4.78 4.7 4.52 4.677 3.77 6.08 2.13 4.31 9.358
序号 (No.) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 名称(Name) 甲胺 尿素(脲) 乙胺 乙醇胺 乙二胺 二甲胺 三甲胺 三乙胺 丙胺 异丙胺 1,3-丙二胺 1,2-丙二胺 三丙胺 三乙醇胺 丁胺 异丁胺 叔丁胺 己胺 辛胺 苯胺 苄胺 环己胺 吡啶 六亚甲基四胺 2-氯酚 3-氯酚 4-氯酚 邻氨基苯酚 间氨基苯酚 化学式(Chemical formula) CH3NH2 CO(NH2)2 CH3CH2NH2 H2N(CH2)2OH H2N(CH2)2NH2 (CH3)2NH (CH3)3N (C2H5)3N C3H7NH2 i -C3H7NH2 NH2(CH2)3NH2 CH3CH(NH2)CH2NH2 (CH3CH2CH2)3N (HOCH2CH2)3N C4H9NH2 C4H9NH2 C4H9NH2 H(CH2)6NH2 H(CH2)8NH2 C6H5NH2 C7H9N C6H11NH2 C5H5N (CH2)6N4 C6H5ClO C6H5ClO C6H5ClO (o )H2NC6H4OH (m )H2NC6H4OH
序号 (No.) 名称(Name) 化学式 (Chemical formula)
Kb
pK b
1 2 3 4 5 6 7 8
氢氧化铝 氢氧化银 氢氧化钙 氨 水
Al(OH)3 AgOH Ca(OH)2 NH3+H2O N2H4+H2O NH2OH+H2O Pb(OH)2 Zn(OH)2
1.38×10-9(K 3) 1.10×10 3.72×10-3 3.98×10-2 1.78×10-5 9.55×10-7(K 1) 1.26×10-15(K 2) 9.12×10 9.55×10-4(K 1) 3.0×10-8(K 2) 9.55×10
1.39×10-5 1.43×10-5 1.8×10-5 1.9×10-5 3.8×10-5(K 1) 3.9×10-6(K 2) 7.4×10 (K 1)
-4
4.86 4.85 4.74 4.72 4.42 5.41 3.13 4.76 6.4 9.96 9.45 12.8 9.3 11.06 9.96 0.29 3.86 4.2 2.98 12.38 2.18 3.46 3.44 2.96 5.4 3.62 4.6 3.54 4.46 2.12 4.1 5.18 0.68 2.21 3.52 5.09 6.32 7.49 4.59 5.59 2 2.67 6.16 10.26
