诺贝尔化学奖简介原文及翻译
诺贝尔化学奖

诺贝尔化学奖诺贝尔化学奖是世界上最负盛名的化学科学奖项,它每年为化学领域做出卓越贡献的科学家颁发该奖项,以表彰他们的杰出成就、引领未来的探索及创新。
自1901年诺贝尔化学奖设立以来,共有183位化学家获得了这一殊荣。
化学是自然科学的一个重要分支,主要研究物质的组成、性质、结构、变化规律与反应。
多年来,化学家们通过不断创新,推动了许多重要发现和科技进步,如:发现新元素、合成新化合物、掌握新的分析测定方法、解析原子分子构造和化学反应机制,以及为人类提供新药物、化工品和材料等等。
早在19世纪末,瑞典化学家阿尔弗雷德·诺贝尔就开始筹备创建化学奖项,以激励各国的化学家们积极开展研究工作。
1901年,诺贝尔化学奖隆重设立,匡列奖项的宗旨是“授予那些在物质结构、化学反应、化学合成等领域做出杰出贡献的人”。
1902年,首届诺贝尔化学奖颁发给德国化学家赫曼·冯·亥姆霍兹和约翰·雅各布·贝尔萨里乌斯,表彰他们在生物和无机化学方面的重要成就。
诺贝尔化学奖得主的评选是由瑞典皇家科学院负责的,其评选过程严谨、公平,评审委员会由瑞典皇家科学院会员组成,每年都会公布一份关于入围候选人的报告。
该奖项评选的标准主要包括科学家的研究贡献、成就和影响。
多年来,诺贝尔化学奖已经颁发给了很多杰出的化学家,他们在各自的领域取得了重大成就,例如:研究DNA分子结构的詹姆斯·沃森、弗朗西斯·克里克和毛罗·威尔金,制造化学合成物的罗伯特·克姆、理解有机反应的里查德·希尔、制备金属有机化合物和研究电荷转移反应的理查德·萨蒂、研究新型催化剂的杨振宁等等。
诺贝尔化学奖对于化学界的发展做出了巨大贡献,它极大地鼓舞了化学界的研究工作,推动了科学研究的向前发展。
它的创立充分说明了人类对于科学研究的高度重视,并鼓励人们投入更多的精力和资源来努力探索自然界的奥秘,这也将继续激励今后的科学家不断追求化学科学领域的进步和创新。
1999年诺贝尔化学奖简介

新型超强超短脉冲激光的出现与迅猛发 展,为人类提供了前所未有的全新的实 验手段与极端的物理条件。就时间尺度 而言,可以说人类已由飞秒(10的负15 次方秒)时代稳步迈进亚飞秒甚至阿秒 (10的负18次方秒)时代。所有这一切, 都对自然科学和人类社会的进步产生重 要的影响。
飞秒科学的发展
飞秒科学技术的发展已有近20年历史,80年代末 泽维尔教授做了一系列试验,他用可能是世界上速 度最快的激光闪光照相机拍摄到一百万亿分之一秒 瞬间处于化学反应中的原子的化学键断裂和新形成 的过程。这种照相机用激光以几十万亿分之一秒的 速度闪光,可以拍摄到反应中一次原子振荡的图像。 他创立的这种物理化学被称为飞秒化学,飞秒即毫 微微秒(是一秒的千万亿分之一),即用高速照相 机拍摄化学反应过程中的分子,记录其在反应状态 下的图像,以研究化学反应。常规状态下,人们是 看不见原子和分子的化学反应过程的,现在则可以 通过泽维尔教授在80年代末开创的飞秒化学技术研 究单个原子的运动过程
艾哈迈德· 泽维尔 (1946-)
艾哈迈德· 泽维尔1946年2月26日生于埃及。后在美国 亚历山德里亚大学获得理工学士和硕士学位;又在宾西法 尼亚大学获得博士学位。1976年起在加州理工学院任教。 1990年成为加州理工化学系主任。他目前是美国科学院、 美国哲学院、第三世界科学院、欧洲艺术科学和人类学院 等多家科学机构的会员。 1998年埃及还发行了一枚印有他本人肖像的邮票以表 彰他在科学上取得的成就。 1999年诺贝尔化学奖授予埃及出生的科学家艾哈迈 德· 泽维尔(Ahmed H.Zewail),以表彰他应用超短激光闪 光成照技术观看到分子中的原子在化学反应中如何运动, 从而有助于人们理解和预期重要的化学反应.
物质在高强度飞秒激光的作用下会出现非常奇特的现 象:气态、液态、固态的物质瞬息间变成了等离子体。 这种等离子体可以辐射出各种波长的射线的激光。高功 率飞秒激光与电子束碰撞能够产生硬X射线飞秒激光, 产生β射线激光,产生正负电子对。 高功率飞秒激光在医学、超精细微加工、高密度信 息储存和记录方面都有着很好的发展前景。高功率飞秒 激光还可以将大气击穿,从而制造放电通道,实现人工 引雷,避免飞机、火箭、发电厂因天然雷击而造成的灾 难性破坏。利用飞秒激光能够非常有效地加速电子,使 加速器的规模得到上千倍的压缩。高功率飞秒激光与物 质相互作用,能够产生足够数量的中子,实现激光受控 核聚变的快速点火。从而为人类实现新一代能源开辟一 条崭新的途径。
2019诺贝尔化学奖英文介绍

2019诺贝尔化学奖英文介绍The 2019 Nobel Prize in Chemistry was awarded jointly to John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino for their contributions towards the development of lithium-ion batteries. This revolutionary technology has transformed the way we use and store energy, impacting various sectors including mobile devices, electric vehicles, and renewable energy sources.Lithium-ion batteries are rechargeable and have significantly higher energy density compared to traditional batteries. This breakthrough was initiated by M. Stanley Whittingham in the 1970s, who began exploring the concept of using lithium as the battery's anode. He discovered that lithium ions could intercalate into layered titanium disulfide, leading to reversible lithium ions being transferred during charging and discharging. This work laid the foundation for the development of lithium-ion batteries.Further advancements were made by John B. Goodenough, who in the 1980s sought to increase the overall voltage and energy density of the batteries. Goodenough replaced the titanium disulfide in Whittingham's design with cobalt oxide,enabling a four-volt battery. This increased the battery's potential and made it a more suitable choice for commercial applications.Akira Yoshino, building upon the previous contributions, made a crucial breakthrough by eliminating the use ofreactive lithium metal in the battery anode. Instead, he utilized petroleum coke, a carbon-rich material, thus creating the first practical lithium-ion battery in 1985. Yoshino's design not only improved the stability and safety of the battery but also enhanced its overall performance.These advancements led to the commercialization oflithium-ion batteries, paving the way for their widespread use across industries. Today, these batteries power our smartphones, laptops, e-bikes, and even electric vehicles. They also play a vital role in storing energy generated from renewable sources like solar and wind power, making the dream of a sustainable energy future more attainable.The impact of lithium-ion batteries goes beyond convenience; it significantly contributes to the reduction of greenhouse gas emissions and dependence on fossil fuels. Electric vehicles, powered by these batteries, offer a sustainable alternative to traditional vehicles, helping combat air pollution and climate change. The ability to storerenewable energy efficiently also supports the integration of more renewable sources into the grid, promoting a greener and more sustainable energy system.The Nobel Prize awarded to Goodenough, Whittingham, and Yoshino recognizes the transformative nature of their work. Their dedication and innovative breakthroughs have revolutionized the energy storage industry, improving our quality of life and providing a path towards a greener future. Their achievements serve as an inspiration for young researchers and scientists worldwide, highlighting the importance of perseverance and curiosity in pushing the boundaries of scientific knowledge to benefit humanity.In conclusion, the 2019 Nobel Prize in Chemistrycelebrates the immense contributions of Goodenough, Whittingham, and Yoshino towards the development of lithium-ion batteries. Their pioneering work has revolutionizedvarious sectors and has the potential to fundamentally change our energy landscape. By recognizing their achievements, we acknowledge the significance of sustainable energy storage solutions and their impact on shaping a brighter and more sustainable future for all.。
第二章诺贝尔化学奖简介

第二章诺贝尔化学奖简介诺贝尔化学奖总表从化学诺贝尔奖看化学学科的发展2004年诺贝尔化学奖诺贝尔化学奖总表1901-19101901年荷兰雅克布斯·范特霍夫o发现了化学动力学法则和溶液渗透压德国赫尔曼·费歇尔o合成了糖类和嘌呤衍生物瑞典阿累尼乌斯o提出了电离理论,促进了化学的发展。
英国威廉·拉姆齐爵士o发现了空气中的稀有气体元素并确定他们在周期表里的位置。
德国阿道夫·拜耳o对有机染料以及氢化芳香族化合物的研究促进了有机化学与化学工业的发展。
法国穆瓦桑o研究并分离了氟元素,并且使用了后来以他名字命名的电炉。
德国爱德华·毕希纳o对酶及无细胞发酵等生化反应的研究。
新西兰欧内斯特·卢瑟福爵士o对元素的蜕变以及放射化学的研究。
德国威廉·奥斯特瓦尔德o对催化作用、化学平衡以及化学反应速率的研究。
德国奥托·瓦拉赫:o在脂环类化合物领域的开创性工作促进了有机化学和化学工业的发展的研究。
1911-19201911年法国玛丽亚·居里o发现了镭和钋,提纯镭并研究镭的性质。
法国格利雅o发明了格氏试剂,促进了有机化学的发展。
法国保罗·萨巴蒂埃o发明了有机化合物的催化加氢的方法,促进了有机化学的发展。
瑞士阿尔弗雷德·沃纳o对分子内原子成键的研究,开创了无机化学研究的新领域。
美国西奥多·理查兹o精确测量了大量元素的原子量。
德国理查德·威尔施泰特o对植物色素的研究,特别是对叶绿素的研究。
德国弗里茨·哈伯o对单质合成氨的研究。
德国沃尔特·能斯特o对热力学的研究。
1921-19301921年英国弗雷德里克·索迪o对放射性物质以及同位素的研究。
英国弗朗西斯·阿斯顿o使用质谱仪发现了非放射性元素的同位素,并且阐明了整数法则。
奥地利弗里茨·普雷格尔o创立了有机化合物微量分析法。
2008诺贝尔化学奖

北京时间2008年10月8日下午5点45分,2008年诺贝尔化学奖揭晓,三位美 国科学家,美国Woods Hole海洋生物学实验室的Osamu Shimomura (下村修)、哥伦比亚大学的Martin Chalfie和加州大学圣地亚哥分校的 Roger Y. Tsien(钱永健,钱学森的堂侄)因发现并发展了绿色荧光蛋白 (GFP)而获得该奖项。帮助他们获奖的是绿色荧光蛋白。这种蛋白为生 物与医学实验带来革命,它发出的荧光像一盏明灯,帮助研究人员照亮生 命体在分子层面和细胞层面的诸多反应。
1968年,即以金属如何与硫氰 酸盐结合为题获美国西屋科学 天才讲 (The Westinghouse Science Talent) 1972年,拿了美国国家优等生 奖学金进入哈佛大学获学士 (化学和物理,Witha National Merit Scholarship) 1977年,获得剑桥大学博士及 博士后(生理学)。 1981年,钱永健来到加州大学 伯克利分校,并在这里工作8 年,成为大学教授。 1989年,钱永健将他的实验室 搬到加州大学圣迭戈分校,现 在他是该校的药理学教授以及 化学与生物化学教授。 1995年,当选美国医学研究院院士 1998年,当选美国国家科学院 院士和美国艺术与科学院院士。 2009年,获香港中文大学颁授 荣誉理学博士学位,获香港大 学颁授荣誉科学博士学位。
前人的每一次成功都是对 我们的启迪和鼓励,就像获得 诺贝尔奖的这些伟大优秀的科 学家们,他们的努力和对科学 执着追求的精神是我们应该学 习的,我们相信只要努力并坚 持,我们每个人都会赢得属于 自己的成功……
1001-诺贝尔化学奖-2

他有一个响亮的外号: 他有一个响亮的外号 合成氨之父 他有一个同样响亮的外号: 他有一个同样响亮的外号 化学战之父 最重要的是他的一生留下了一 个沉重话题: 个沉重话题 科学家应该承担什么样的社会 责任? 责任?
Fritz Haber
• Haber于1933年4月30日声明:“40多年来,我一直是以知识和品德为标 于 日声明: 多年来, 年 月 日声明 多年来 准去选择我的合作者,而不是考虑他们的国籍和民族,在我的余生, 准去选择我的合作者,而不是考虑他们的国籍和民族,在我的余生,要 我改变认为是如此完好的方法,则是我无法做到的。 随后, 我改变认为是如此完好的方法,则是我无法做到的。”随后,Haber被迫 被迫 离开了祖国,流落他乡。首先他应英国剑桥大学的邀请, 离开了祖国,流落他乡。首先他应英国剑桥大学的邀请,到鲍伯实验室 工作。 个月后 个月后, 工作。4个月后,以色列的希夫研究所聘任他到那里领导物理化学的研究 工作。但是在去希夫研究所的途中, 的心脏病发作, 工作。但是在去希夫研究所的途中,Haber的心脏病发作,1934年1月29 的心脏病发作 年 月 日在瑞士逝世。 日在瑞士逝世。 • Haber被迫离开了德国,在他逝世一周年的那天,德国的许多学会和学者 被迫离开了德国,在他逝世一周年的那天, 被迫离开了德国 不顾当局的阻挠,纷纷组织集会,缅怀这位伟大的科学家。 ,不顾当局的阻挠,纷纷组织集会,缅怀这位伟大的科学家。他是天使 为人类带来丰收和喜悦,是用空气制造面包的圣人;他是魔鬼, ,为人类带来丰收和喜悦,是用空气制造面包的圣人;他是魔鬼,给人 类带来灾难、痛苦和死亡。 类带来灾难、痛苦和死亡。他走过了辉煌而又坎坷以至于毁誉参半的一 德国在20世纪初物理化学走在世界前列 世纪初物理化学走在世界前列, 年的Willstatter、1920 生。德国在 世纪初物理化学走在世界前列,1915年的 年的 、 年的Nernst都获得了诺贝尔化学奖,但在他们中间,Haber永远是最闪亮 都获得了诺贝尔化学奖, 年的 都获得了诺贝尔化学奖 但在他们中间, 永远是最闪亮 他的诺贝尔化学奖当之无愧。 的。他的诺贝尔化学奖当之无愧。
2019诺贝尔化学奖英文介绍

2019诺贝尔化学奖英文介绍
(原创实用版)
目录
1.2019 年诺贝尔化学奖的背景和获奖者
2.锂离子电池的研究及其在现代科技中的应用
3.诺贝尔化学奖的历史和评选标准
4.2019 年诺贝尔化学奖的预测和意义
正文
2019 年诺贝尔化学奖于 10 月 9 日揭晓,授予了约翰·古迪纳夫(John B.Goodenough)、斯坦利·威廷汉(M.Stanley Whittingham)和吉野彰,以表彰他们在锂离子电池领域的杰出贡献。
锂离子电池是一种可充电电池,为手机、笔记本电脑等无线电子产品提供了基础,同时也为电动汽车、储存可再生能源等领域带来了革命性的变革。
锂离子电池的研究始于 20 世纪 70 年代,当时斯坦利·威廷汉首次提出了嵌入脱出型的正极材料 TiS2,这标志着从早期的锂 - 锰氧化物一次电池向现代二次锂电池的转变。
随后,约翰·古迪纳夫在 1980 年代研究出了锂离子电池的正极材料,进一步提高了电池的性能。
吉野彰则在1990 年代成功地开发出了第一个商业化的锂离子电池,为电池的实际应用奠定了基础。
诺贝尔化学奖是瑞典著名化学家阿尔弗雷德·诺贝尔(1833-1896)的部分遗产设立的,旨在表彰在化学领域做出杰出贡献的科学家。
诺贝尔化学奖自 1901 年设立以来,已经表彰了众多杰出的化学家,他们的研究成果对人类的生活产生了深远的影响。
对于 2019 年诺贝尔化学奖的预测,许多人认为锂离子电池的研究将会是获奖的热门领域。
果不其然,这一预测最终成为了现实。
espanol 课文翻译

Leccion 4这是一年的最后一个晚上。
新年前夕,天气特别冷,雪下个不停,夜幕就要降临。
一个可怜的小女孩赤脚走在街上。
实际上,走出家门时他穿着鞋,但那双鞋没用。
对小女孩来说,鞋太大了。
他一着急,在跑过一条很宽的大街时就把鞋丢了。
小女孩在街上走着,脚冻成了青紫色。
破旧的围裙兜里装着许多火柴。
手里还拿着一盒。
这一天她过得很糟糕。
没有人买他的火柴,哪怕是一根。
他一分钱都没有赚到。
他饥寒交迫,看上去虚弱极了。
可怜的小女孩呀!房屋每扇窗都可以看到明亮的灯光,但他却只想着在晚上挣些钱才能回家。
小女孩在一个角落里坐下,想在两栋房之间取暖。
他觉得越来越冷,却不能回家,因为一根火柴也没有卖出去。
他爸爸会打他的。
他的一双小手几乎被冻僵了。
一根点亮的火柴至少可以暖暖手。
于是,他取出一根火柴。
咔嚓!看那火花呀!那燃烧的火柴呀!火光微弱,一点点的温暖着他的双手。
小女孩觉得他坐在一个厨房中,里面有一个大大的火炉,他把脚放到炉子前想暖暖,可就在这是火柴熄灭了,厨房消失了。
他坐在那,手中只有一小截然过的火柴。
他又点了一根,随后看见一张大桌子上放着一个烤熟的火鸡,两旁还有刀叉。
他点燃了第三根,发现自己坐在一棵特别漂亮的圣诞树下。
这可圣诞树比他在去年平安夜透过全城最富的那户人家的大窗户看到的那颗还要高,还要漂亮。
小女孩举起他瘦小的双臂,可就在这时,火光熄灭了。
所有的光明都离他远去。
他点燃一只新的火柴。
这次,小女孩年迈的奶奶出现了。
他面貌和蔼可亲。
“亲爱的奶奶!”小女孩哭着叫道,“你带我走吧。
我知道你和火炉,烤火鸡,圣诞树一样,火柴一灭就会消失的。
”他赶紧点燃剩下的火柴,希望留住奶奶。
火柴的光芒比白昼还要明亮。
奶奶从来没有如此高大美丽,他拉着女孩的胳膊,一起升上天空。
可怜的女孩不再感到寒冷,饥饿和恐惧。
寒冷的清晨,人们在房子的一角发现了那个小女孩,双颊泛红,嘴角上挂着微笑…他死了,冻死在除夕之夜。
Leccion 5诺贝尔奖是一项每年颁发给上一年度在文学,化学,物理,生理学和医学,和平及经济学五个领域有杰出工作成就的个人或者机构的一个奖项。
1990年诺贝尔化学奖

1990年诺贝尔化学奖伊利亚斯·詹姆士·科里1990年10月17日,瑞典皇家科学院授予美国哈佛大学的有机化学家伊利亚斯·詹姆士·科里(Elias James Corey)以1990年的诺贝尔化学奖,表彰他在有机合成的理论和方法学方面的贡献。
科里从50年代后期开始进行有机合成的研究工作,30多年来他和他的同事们合成了几百个重要的天然产物。
这些化合物的结构都比较复杂,而且越往后,他合成的目标化合物越复杂,合成的难度也越大。
按照科里和他的学生成学敏在1989年出版的一本名为《化学合成的逻辑》的书分类,他的合成工作主要涉及(1)大环结构:主要是一些大环内酯和大环内酰胺类的抗菌化合物;(2)杂环结构:主要是一些生物碱和维生素等;(3)倍半萜类化合物:由3个异戊二烯结构单位组成分子碳架的各种天然的烃类和其衍生物;(4)多环异戊二烯类化合物:含有更多异戊二烯结构单位的天然多环化合物;(5)前列腺素类化合物:一类激素;(6)白三烯类化合物:一类具有很强生物活性的多烯和其衍生物。
下面列出科里首先合成的有代表性的几个化合物:从这几个例子就足以看出,即使他最早期的合成工作(如长叶烯的合成)也已经能够显示出他的巨大天才。
但是,科里最大的功绩并不在于他的那些艰巨的合成工作,而是在1967年他提出具有严格逻辑性的“逆合成分析原理”,以及有关在合成过程中,各种功能团的转变、加入和消去的一系列系统地修饰分子的原则和方法。
逆合成分析原理,简单地说,就是确定如何将要合成的目标分子按可再结合的原则在合适的键上进行分割,使其成为合理的、较简单的和较易得的较小起始反应物分子;然后,再反过来将找到的这些小分子或等价物按一定的顺序和立体方式,逐个地通过合成反应再结合起来,并经过必要的修饰,而得到所要合成的目标化合物。
所以逆合成分析是决定整个合成路线的关键,关系到整个合成的策略、成败和评价。
例如,科里选用的长叶烯逆合成是:在a键处分割长叶烯是可取的逆合成分析方式之一。
2019诺贝尔化学奖英文介绍

2019诺贝尔化学奖英文介绍
(实用版)
目录
1.2019 年诺贝尔化学奖的获奖者
2.获奖者们的主要贡献
3.锂离子电池的发展及其应用领域
4.诺贝尔化学奖的历史和评选标准
正文
2019 年诺贝尔化学奖授予了三位杰出的科学家,他们分别是约翰·古迪纳夫、斯坦利·威廷汉和吉野彰,以表彰他们在锂离子电池领域的重要贡献。
约翰·古迪纳夫是一位美国科学家,他因为在锂电池领域的研究而广受赞誉。
斯坦利·威廷汉是英国科学家,他首次提出了锂离子电池的概念,并成功研制出了第一个可充电的锂离子电池原型。
而来自日本的吉野彰,则是将锂离子电池技术推向实际应用的关键人物,他成功地研发出了世界上第一个商业化的锂离子电池。
锂离子电池的发明,为无线电子产品和电动汽车等设备提供了强大的能源支持,同时也为可再生能源的储存和利用开辟了新的途径。
锂离子电池的广泛应用,不仅推动了电子科技的发展,也为环境保护做出了重要贡献。
诺贝尔化学奖是瑞典著名化学家阿尔弗雷德·诺贝尔设立的奖项之一,旨在表彰在化学领域做出卓越贡献的科学家。
诺贝尔化学奖的历史可以追溯到 1901 年,每年都会在全球范围内评选出一些杰出的化学家,以表彰他们在化学领域的重要发现和贡献。
诺贝尔化学奖的评选标准非常严格,要求获奖者在化学领域做出具有
重要意义的发现或贡献。
这些发现或贡献,不仅需要具有科学价值,还需要在实际应用中产生广泛的影响。
《诺贝尔化学奖》(中文)-2014年
墨西哥
美国
荷兰
年份
成就
获奖者
国籍
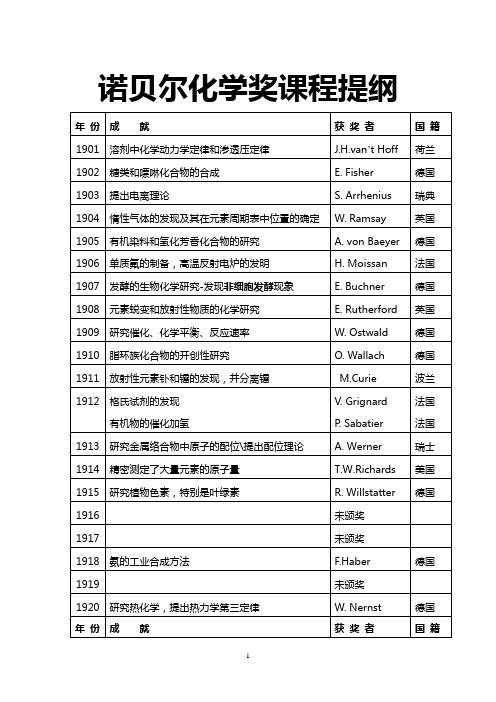
1996
发现C60(富勒氏球C-60)
R. F. Curl
R. E. Smalley
H. W. Kroto
美国
美国
英国
1997
发现了能量分子三磷酸腺苷(ATP)的形成过程
发现了维持细胞中钠离子和钾离子浓度平衡的酶,并阐明其作用机理
P. Boyer
J. Skou
白川英树
美国
美国
日本
2001
不对称催化氧化反应(催化剂)的研究
不对称催化氢化反应(催化剂)的研究
发现和制造不对称手性催化剂
夏普莱斯(美)
诺尔斯(美)
野依良治(日本)
2002
核磁共振技术测定生物大分子三维结构的方法
发明了对生物大分子进行确认和结构分析方法
发明了对生物大分子的质谱分析法
维特里希(1/2)芬恩(合1/2)
肖万;格拉布;
施罗克
法国
美国
2006
基因信息如何从DNA被转录至信使RNA.子承父业
对真核细胞转录的分子基础所作的研究
罗杰科恩伯格
美国
德国
德国
德国
1989
发现核糖核酸(Ribozyme)催化作用的研究
T. Cech切赫
S. Altman
美国
美国
1990
有机合成特别是发展了逆合成分析理论和方法
E. J. Corey
美国
1991
发展付立叶变换二维核磁共振波谱
R. R. Ernst
瑞士
1992
创立和发展了电子转移反应理论
R. A. Marcus
诺贝尔化学奖简介原文及翻译

1. Phase-Switching CatalysisBy simply adding or removing carbon dioxide,chemists in Scotland devised a neat trick forreversibly shuttling a homogeneous catalystbetween the organic and aqueous phases in abiphasic solvent system (C&EN, Jan. 26, page 11;Angew. Chem. Int. Ed. 2009, 48, 1472). Thephase-switchable catalyst designed by Simon L.Desset and David J. Cole-Hamilton of theUniversity of St. Andrews adds flexibility to theoften complicated techniques required to isolateproducts and recycle catalysts duringhomogeneous reactions. The secret to theswitchability is a weakly basic amidine group,–N=C(CH 3)N(CH 3)2, that the researchers added tothe phenyl rings of triphenylphosphine. Therhodium catalyst made with the modifiedphosphine ligand is soluble in organic solvent. On bubbling CO 2 into an aqueous-organic reaction system containing the catalyst, the CO 2 reacts with water to form carbonic acid (H 2CO 3). The transient acid protonates the amidine groups and renders the catalyst water-soluble. Subsequently bubbling N 2 into the biphasic system drives off CO 2 and shifts the equilibrium of the catalyst-carbonic acid complex, leading thecatalyst to deprotonate and making it water-insoluble again. After a reaction iscompleted in either organic solvent or water, the researchers separate the product and catalyst into different phases, remove the product, and then shuttle the catalyst back into the original phase for the next reaction cycle. Building switchability into basic chemicals in this manner could facilitate greener and less-energy-intensive industrial chemical processes.能够转相的催化反应通过简单的添加或除去二氧化碳,苏格兰的科学家发明了一种在两相系统中来回转运匀相催化剂的灵巧的把戏。
诺贝尔化学奖

约翰·沃克詹斯·斯科保罗·博耶三磷酸腺苷 (ATP) 是世间所有生命体的能最载体。
在细胞中,ATP分子在形成之后1分钟内就消耗掉了。
ATP 的转换率惊人之高:处在休息状态的人,42 小时就消耗相当于自身重盘一半的A T P ; 在激烈运动时,1 天能转化多达自身重盈2 0倍的 A仰。
运动、主动转运、信号放大和生物合成等,只有当AT P 不断地由二磷酸腺苷 (APD)再生时才能发生。
光能营养生物即植物,靠捕获光中的自由能以形成 ATP;而化学能营养生物即动物,则靠燃料分子的氧化以形成 APT。
因为有了A T P的存在,才有了生物体内的能盆转换,一切生灵才得以生存和繁衍。
鉴此,生物学家们形象地将AT P誉为“能量货币”。
生命是大自然造就成的精灵之物,造化出的 ATP ,具有神妙的基因转移优势之结构基础。
随着科学家们的不屈探索,AT P 的隐秘逐一被揭开。
笔者在兴奋之余,感慨戏言道:“能量货币不贬值。
”1997年诺贝尔化学奖,就颁发给探索“能量货币不贬值”真谛的3位生物化学家:奖金的一半由美国加利福尼亚大学教授保罗·博耶和英国剑桥大学教授约翰·沃克共享,另一半由丹麦奥尔胡斯大学教授廷斯·斯科获得。
瑞典皇家科学院的公报指出,博耶和沃克揭开了AT P合成酶的隐秘,从而探明“能量货币”A T P 的形成过程。
斯科发现了离子传输酶,这种酶即AT P合成酶的一种存在形式,起着离子泵的作用。
他们共同说明了“能量货币不贬值”的道理。
1997年度诺贝尔化学奖一半授予美国洛杉矶加利福尼亚大学的保罗·波耶尔(Paul D. Boyer)和英国剑桥医学研究委员会分子生物学实验室的约翰·沃克(John E. Walker),因为他们阐明了腺三磷(ATP)合成的基本酶学机制;另一半授予丹麦奥尔胡斯大学的因斯·斯寇(Jens C. Skou),因为他首先发现了一种转运离子的酶,钠离子、钾离子-腺三磷酶(Na+, K+-ATP)。
诺贝尔化学奖简介

1904年诺贝尔化学奖1904年诺贝尔化学奖授予英国化学家威廉·拉姆塞,以表彰他发现六种稀有气体,并确定了它们的化学性质和在元素周期表上的位置。
1892年,英国物理学家瑞利在精密测量不同来源的氮气质量,发现由氨制得的氮总比由空气制得的氮轻千分之一,反复研究不得其解。
拉姆塞征得瑞利的慷慨同意,开始采用新方法研究大气中氮的成分。
1894年,以两人名义宣布了一种惰性气体元素的发现,并命名为氩(即懒惰的气体)。
拉姆塞在开发的领域继续深入研究,把沥青铀矿经无机酸处理之后,制得一种新气体,经分析研究确定惰性气体氦(太阳),他成为世界上第一个拿到太阳元素的化学家。
1898年拉姆塞在分馏液态空气时发现了3种新的稀有气体元素,分别命名为氖(意为新奇)、氪(隐藏)、氙(陌生人)。
1908年他又分离出放射性稀有气体氡。
拉姆塞将所发现六种稀有气体气体作为一族,完整安插到元素周期表的零族位置,这样化学元素周期表更加完善。
稀有气体广泛应用到光学、冶金和医学等领域中,由于特有的化学“惰性”被常用作保护气,可制作电光源如五光十色的霓虹灯,还有液氦成为超低温技术领域的无价之宝,氙气在医学上作麻醉剂等。
1996年诺贝尔化学奖美国和英国的科学家柯尔、斯莫利、克鲁托,因发现碳元素的第三种存在形式-C60(富勒烯)而获1996年诺贝尔化学奖。
克鲁托对含碳丰富的红巨星的特殊兴趣,导致了富勒烯的发现。
三位科学家用一个激光束将物质蒸发并加以分析,最后十分意外地发现碳元素也可以非常稳定地以球的形状存在。
他们称这些新的碳球为富勒烯。
富勒烯是石墨在惰性气体中蒸发时形成的,通常含有60或70个碳原子。
“C60”包含有12个五边形和20个六边形,每个角上有一个碳原子,这样的碳簇球与足球的形状相同。
围绕富勒烯,一门新型的碳化学发展起来了。
化学家们可以在碳球中嵌入金属和稀有惰性气体,制成新的超导材料或新的有机化合物、新的高分子材料。
在富勒烯的制备方法中略加以改进后可制造出世界上最小的管-纳米碳管,这种管直径非常小,大约1毫微米。
诺贝尔奖中英文简介

“诺贝尔奖”知多少很多人都知道诺贝尔奖是瑞典著名化学家、硝化甘油炸药发明人阿尔弗雷德•诺贝尔用遗产作为基金创立的,那么诺贝尔奖共有几类,每类奖项又有什么特别之处呢?让我们一起来了解一下吧。
遵照诺贝尔遗嘱,物理奖和化学奖由瑞典皇家科学院评定,生理或医学奖由瑞典皇家卡罗林医学院评定,文学奖由瑞典文学院评定,和平奖由挪威议会选出。
经济奖委托瑞典皇家科学院评定。
每个授奖单位设有一个由5人组成的诺贝尔委员会负责评选工作,该委员会三年一届。
诺贝尔基金会于1900年成立,1901年首次颁发奖项。
Nobel Literature Prize / Nobel Prize in literature诺贝尔文学奖The Nobel Prize in literature has been awarded annually to an author from any country who has, in the words from the will of Alfred Nobel, produced "in the field of literature the most outstanding work in an ideal direction". The Swedish Academy decides who, if anyone, will receive the prize in any given year.根据创立者的遗嘱,诺贝尔文学奖金授予“最近一年来”“在文学方面创作出具有理想倾向的最佳作品的人”。
1900年经国王批准的基本章程中改为“近年来创作的”或“近年来才显示出其意义的”作品,“文学作品”的概念扩展为“具有文学价值的作品”,即包括历史和哲学著作。
Nobel Peace Prize诺贝尔和平奖According to Nobel's will, the Peace Prize should be awarded to the person who " ...shall have done the most or the best work for fraternity between nations, for the abolition or reduction of standing armies and for the holding and promotion of peace congresses".奖给为促进民族团结友好、取消或裁减常备军队以及为和平会议的组织和宣传尽到最大努力或作出最大贡献的人。
———2000年诺贝尔化学奖简介

第16卷 第2期 大学化学 2001年4月塑料,导体和有机光电信息材料———2000年诺贝尔化学奖简介裴 坚(北京大学化学学院 北京100871) 自然科学在各个领域的迅速发展改变着我们日常生活的许多习惯思维,这种改变对人类社会的进步发挥着越来越大的作用。
在过去的一个世纪里,人们也许已经习惯了自然科学的研究成果对思维方式的巨大冲击。
2000年的诺贝尔化学奖也毫不例外。
它再次使我们对自然科学的发展有了一个新的思考角度。
瑞典皇家科学会在2000年10月9日将今年的诺贝尔化学奖授予了在导电高分子研究领域作出突出贡献的3位科学家,他们分别是美国加利福尼亚大学圣巴巴拉分校高分子和有机固体研究所所长、材料科学系和物理系教授Alan J. Heeger;美国费城宾夕法尼亚大学化学系教授Alan G.MacDiarmid和日本筑波大学材料科学学院教授Hideki Shirakawa。
虽然这项发明发生在20多年以前,但是诺贝尔化学奖评审委员会认为这项奠基性和开创性的科学成果使导电高分子材料和有机半导体材料发展成为了材料科学基础研究中的一个重要的研究领域。
同时,这项发明对于我们的日常生活具有积极的意义,产生了许多极具价值的应用成果,如新的导电材料的发现,将半导体材料和各种分子器件所具有的独特优势应用在抗静电,抗腐蚀,化学和生物传感器方面;为新的发光器件提供各种不同颜色的光源;利用新材料可以制造各种更加轻便的彩色显示器以及用于计算机识别的商业用塑料电子标签等;同时更让科学家们感兴趣的是这类新材料可能使人类在不久的将来拥有便携的、可卷曲的、柔韧性强的电视机和其他大屏幕显示器。
那么,塑料是怎么变成导体的呢?正如我们所知,根据物质的导电性,材料可以分为绝缘体(电导率σ≤10-8S/m),半导体(σ=10-8~100S/m),导体(σ=100~108S/m)和超导体(σ→∞)。
而高分子材料,如我们日常生活中广泛使用的塑料,以前一直被认为是很好的绝缘体,是不导电的。
2020年诺贝尔化学奖简介

信息直通车2020年诺贝尔化学奖简介2020年10月7日瑞典皇家科学院宣布ꎬ将2020年诺贝尔化学奖授予法国科学家埃马纽埃尔 沙尔庞捷(EmmanuelleCharpentier)和美国科学家詹妮弗 杜德纳(JenniferA.Doudna)ꎬ以表彰她们在新一代基因编辑技术CRISPR/Cas9研究领域作出的贡献ꎮ这是诺贝尔奖史上首次由两位女性双双获得同一奖项ꎬ为诺贝尔奖增添了一抹绚丽的色彩!CRISPR/Cas系统的发现30年前ꎬ一位西班牙年轻人FranciscoMojica在当地的一所大学开始攻读博士学位ꎬ在分析圣波拉海滩上古细菌(H.mediterranei)的DNA序列时ꎬMojica观察到了一个有趣的现象 这些微生物的基因组里ꎬ存在许多奇怪的 回文 片段ꎮ对于这种具有规律性的重复ꎬMojica称之为 成簇规律间隔短回文重复序列 (clusteredregularlyinterspacedshortpalin ̄dromicrepeats)ꎬ缩写为CRISPRꎮMojica推断:如果两种有着巨大差异的微生物细胞中都有这种奇怪的序列ꎬ这就说明它肯定有着某种特殊的功能ꎮ在成立了自己的实验室后ꎬ他又发现大约另外20多种微生物中ꎬ同样具有CRISPR序列ꎮ然而ꎬ这种奇怪序列的功能ꎬ却迟迟未能得到解答ꎮ事实上ꎬCRISPR的首次报道是在1987年ꎬ日本学者石野良纯(YoshizumiIshino)小组在分析大肠杆菌基因iap及周边序列时偶然发现了一段位于该基因3ᶄ端的重复序列ꎬ其中含5个长29个碱基对(bp)的高度同源序列ꎬ它们与一段不保守但等长的32bp序列间隔排列ꎮ然而ꎬIshino当时并没有对CRISPR序列进行深入的研究ꎮ2002年ꎬ荷兰学者RuudJansen等通过生物信息学分析发现了Cas(CRISPR ̄associated)蛋白ꎮCas为核酸相关蛋白ꎬ具有螺旋酶(helicase)和核酸酶(nuclease)结构域(其酶活性HNH结构域剪切crRNA互补链ꎬ而RuvC剪切非互补链)ꎬ存在于含有CRISPR结构的原核基因组中ꎬ总是位于CRISPRs的邻近位置ꎮJansen与Mojica将该系统命名为CRISPR/Cas系统(CRISPR ̄Cassystem)ꎮ结构决定功能ꎬ这个重复结构到底有什么功能?当时并不为人们所知ꎮCRISPR/Cas是功能上的获得性防御系统细菌和古细菌如何辨识到入侵病毒是一种威胁的?2005年ꎬMojica等3个独立的小组通过生物学信息分析证明CRISPR的间隔(spacer)序列是来自外源的DNAꎬ他们推测这种spacer可能对外来DNA具有防御作用ꎮ2010年ꎬMoineau小组对嗜热链球菌(S.thermophilus)Ⅱ型CRISPR系统进行研究ꎬ确定了该系统在外源双链质粒DNA上精确的切割位点ꎬ确认Cas9是介导靶序列切割所需的唯一蛋白ꎮ2011年ꎬCharpentier在利用化脓性链球菌(S.pyogenes)的研究中发现了一种未知分子:反式编码的小RNA(trans ̄encodedsmallRNAꎬtracrRNA)ꎮ研究表明ꎬtracrRNA是细菌免疫防御系统CRISPR/Cas的一部分ꎬ该系统通过切割噬菌体的DNA而解除其武装ꎬ从而抵抗噬菌体入侵细菌ꎮ至此ꎬ对CRISPR/Cas获得性防御性功能的作用机制已有了确切的认识ꎬ这为以后利用该系统发展基因编辑技术奠定了基础ꎮ整个过程大体分为3个步骤(图1)ꎮ步骤①:CRISPR/Cas系统识别出入侵病毒的 名字 原间隔序列邻近基序(protospaceradjacentmotifꎬPAM)ꎻ登记它的 身份证 原间隔序列(protospac ̄er)ꎻ把入侵者身份信息间隔(spacer)序列作为 档案 ꎬ记录到 名单 (CRISPR)序列中ꎮ完成外源DNA俘获ꎮ步骤②:由crRNA㊁Cas(包括Cas9)和tracrRNA组成的复合物形成最终的防御系统ꎮ此复合物将根据入侵病毒的类型ꎬ选取对应的间隔(spacer)序列RNAꎬ并在RNaseⅢ的协助下对这段序列进行剪切ꎮ最终产生一段短小的成熟crRNA(CRISPR ̄derivedRNAꎬ包含单一种类的间隔序列RNA和部分重复序列区)ꎮ步骤③:这个复合物将扫描整个外源DNA序列ꎬ并识别出与crRNA互补的原间隔序列ꎮ这时ꎬCas9蛋白发挥作用ꎮ最终ꎬCas9使DNA双链断裂ꎬ外源DNA的表达被沉默ꎬ破坏入侵病毒ꎬ完成靶向干扰ꎮCRISPR/Cas9系统作为一种基因编辑工具的发现CRISPR/Cas系统广泛存在于原核生物并可作为获得性防御系统抗击入侵的噬菌体和外源质粒ꎮCas蛋白的功能体现在三个不同的层次:1)新的DNA间隔序列与CRISPR基因座整合ꎻ2)crRNAs生物合成ꎻ3)沉默入侵DNAꎮⅢ㊀㊀分为三个阶段:适应:来自病毒或质粒的双链DNA短片段被纳入宿主DNA上的CRISPR阵列ꎻcrRNA成熟:pre ̄crRNA通过转录产生ꎬ然后进一步加工成较小的crRNAꎬ每个crRNA包含单个间隔和部分重复ꎻ干扰:当crRNA识别并特异性地与进入的质粒或病毒DNA上某一区域的碱基配对时ꎬ裂解就开始了图1㊀CRISPR ̄Cas适应性(获得性)㊀㊀免疫系统功能图解CRISPR/Cas9相关实验证明了其可能作为基因编辑工具的曙光ꎮ经过一系列的改进ꎬCRISPR/Cas9已经是目前比较精确㊁切割效率较高的一种基因剪刀(geneticscissors)ꎮ利用这把剪刀可以对动物㊁植物和微生物的DNA进行有目标性的编辑加工(剪切㊁删除㊁位移和替换等)ꎬ从而治疗疾病ꎬ尤其是遗传病ꎬ并且获得人们想要的作物和生物产品ꎮtracrRNA的发现及其在crRNA成熟过程中的作用2011年ꎬCharpentier团队报道了在化脓性链球菌(S.pyogenes)中crRNA成熟的机制ꎮ他们根据前crRNA(pre ̄crRNA)和成熟crRNA分子的表达ꎬ确定了一个活性CRISPR位点ꎬ并且在其上游210bp处意外发现一个高表达RNA分子ꎬ称为 tracrRNA ꎮtracrRNA分子内含25个核苷酸(nt)ꎬ与CRISPR位点的重复区域几乎完全互补ꎬ预测可与pre ̄crRNA的碱基配对ꎬ将形成包括tracrRNA/pre ̄crRNA加工位点的两个RNA协同加工ꎮ如果tracrRNA基因座缺失将阻止pre ̄crRNA双链RNA的加工ꎬ反之亦然ꎮCharpentier团队发现ꎬ当tracrRNA/pre ̄crRNA双链共同加工时将得到短的3ᶄ突出端ꎬ并证明内切核糖核酸酶Ⅲ(RNaseⅢ)负责tracrRNA/pre ̄RNA双链的加工ꎮ这一加工过程也需要Cas9蛋白的存在ꎬ缺失Cas9将破坏tracrRNA/pre ̄crRNA的加工过程ꎮCas9蛋白相当于一个分子锚ꎬ促进了tracrRNA/pre ̄crRNA之间的碱基配对ꎬ进而被宿主RNaseⅢ蛋白识别和裂解ꎮCharpentier和Doudna随即的合作研究发现:在纯化的Cas9中添加crRNA并不能刺激Cas9催化靶DNA裂解ꎻ在体外反应中如果加入tracrRNA则触发Cas9对靶DNA的裂解ꎮ这说明tracrRNA至少具有2个关键功能:1)触发RNaseⅢ对pre ̄crRNA的加工ꎻ2)随后激活由Cas9对crRNA引导的DNA裂解ꎮ在Cas9催化的原间隔裂解中ꎬ裂解的特异性由crRNA序列决定ꎮ那么ꎬtracrRNA对目标DNA序列特异性切割是否有同样重要的作用呢?Charpentier和Doudna证明了Cas9催化过程中所必需的tracrRNA和crRNA区域ꎮ她们率先在tracrRNA中鉴定出了1个活性识别区域ꎬ并确认在目标链PAM近端区域(约为10nt)对于目标识别尤为重要的 种子区(seedregion) ꎮ尤为重要的是ꎬ她们的实验证明Cas9复合体的2个RNA成分(crRNA和tracrRNA)可以嵌合在一起ꎬ形成有活性的单导RNA(single ̄guideRNAꎬsgRNA)分子ꎮ嵌合sgRNA的序列可以改变ꎬ使CRISPR/Cas9能够靶向目标DNA序列ꎬ唯一的限制是在目标DNA附近的PAM序列ꎮ至此ꎬ她们巧妙地创造了一个简单的包含sgRNA和Cas9的双组分内切酶系统ꎬ通过编程实现任意切割DNA序列ꎮCRISPR/Cas9技术在高等细胞中的应用正如Charpentier和Doudna在体外实验所观察到的ꎬ该系统可以在体内进一步简化:一个嵌合的sgRNA分子与Cas9足以裂解靶DNAꎮ迄今为止ꎬ该系统还被用于在许多其他真核生物系统中引入基因组修饰ꎬ包括酿酒酵母㊁黑腹果蝇㊁秀丽隐线虫㊁斑马鱼和拟南芥ꎬ显示了其广泛的适用性ꎮ目前ꎬ科学家们正试图扩大CRISPR/Cas系统在基因组编辑中的作用ꎮ除了来自S.pyogenes的Cas9蛋白ꎬ许多其他Cas同源物被用于基因组编辑和相关目的ꎮ天然的CRISPR系统有PAM需求和限制ꎬ然而Cas9新变体的设计将改变PAM的兼容性ꎬ改变其酶切活性ꎬ用于更精确的同源重组和内源基因表达㊁激活和抑制ꎮ除了DNAꎬCRISPR/Cas系统也可用于靶向RNAꎮ重写生命密码的CRISPR/Cas9技术将给肿瘤㊁基因缺陷疾病带来希望ꎬ并使治愈遗传疾病的梦想成为现实ꎮ如此强大的CRISPR/Cas9技术也随之引发了伦理和社会问题ꎮ需要强调的是ꎬ这项技术要以负责任的方式进行科学的管理和使用ꎮ王㊀欣㊀编译(中国医学科学院基础医学研究所)Ⅳ。
2013诺贝化学奖--文字版

2013诺贝化学奖――将实验带入网络空间迈克尔·莱维特:美国斯坦福大学医学院教授。
他1947年生于南非比勒陀利亚,1971年在英国剑桥大学获得博士学位。
年生于奥地利维也纳,1953年在美国加州理工学院获得博士学位。
以色列,1969年在以色列魏茨曼科学研究所获得博士学位。
2013年诺贝尔化学奖颁给了3位美国科学家马丁·卡普拉斯、迈克尔·莱维特和阿里耶·瓦谢尔,以表彰他们在电脑模拟化学反应领域作出的开创性贡献。
3位科学家结合经典和量子物理学,设计出多尺度模型,将传统的化学实验搬到了网络世界。
得奖理由为复杂化学系统创立多尺度模型瑞典皇家科学院说,在上世纪70年代,3位科学家为借助电脑程序理解和预测化学过程及其结果奠定了基础。
化学反应速度极快,传统方法几乎不可能弄清过程中的每一步。
借助3人的研究成果,科学家能够用电脑揭示化学反应的奥秘,比如催化剂净化废气和光合作用。
先前,化学家们只能在经典物理和量子物理之间作出选择。
前者计算简便,但只能用于大分子,无法模拟化学反应;后者则只能用于小分子并且计算量庞大。
3位获奖者做出“开创性工作”,融合经典物理和量子物理,创造出新方法。
比如,模拟一种药物如何作用于人体中特定蛋白质时,电脑用量子物理计算蛋白质内的原子与药物之间的相互作用,用经典物理模拟蛋白质剩余部分。
详细解析图1:现在的化学家在电脑上做实验几乎与在实验室做实验一样频繁,从电脑上得到的计算结果经由真实的实验得到结果证实后,让我们对原子世界如何运作会得到新的线索。
此可谓理论与实践相辅相成。
■化学反应以闪电的速度进行着,电子在原子核间跳跃,躲避着化学家们虎视眈眈的双眼。
2013年的诺贝尔化学奖得主们利用电脑,让化学反应的神祕路径得以现形。
对于化学运作的细部了解,使得催化剂、药物以及太阳能电池的最佳化变得更有效率。
全世界的许多化学家几乎每天在电脑上设计以及执行实验,透过马丁·卡普拉斯(Martin Karplus)、迈克尔·莱维特(Michael Levitt)以及阿里耶·瓦谢尔(Arieh Warshel)于70年代所发展的方法,化学家们检视着用肉眼无法看到的复杂化学过程中每一个小小的步骤。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1. Phase-Switching Catalysis
By simply adding or removing carbon dioxide,
chemists in Scotland devised a neat trick for
reversibly shuttling a homogeneous catalyst
between the organic and aqueous phases in a
biphasic solvent system (C&EN, Jan. 26, page 11;
Angew. Chem. Int. Ed. 2009, 48, 1472). The
phase-switchable catalyst designed by Simon L.
Desset and David J. Cole-Hamilton of the
University of St. Andrews adds flexibility to the
often complicated techniques required to isolate
products and recycle catalysts during
homogeneous reactions. The secret to the
switchability is a weakly basic amidine group,
–N=C(CH 3)N(CH 3)2, that the researchers added to
the phenyl rings of triphenylphosphine. The
rhodium catalyst made with the modified
phosphine ligand is soluble in organic solvent. On bubbling CO 2 into an aqueous-organic reaction system containing the catalyst, the CO 2 reacts with water to form carbonic acid (H 2CO 3). The transient acid protonates the amidine groups and renders the catalyst water-soluble. Subsequently bubbling N 2 into the biphasic system drives off CO 2 and shifts the equilibrium of the catalyst-carbonic acid complex, leading the
catalyst to deprotonate and making it water-insoluble again. After a reaction is
completed in either organic solvent or water, the researchers separate the product and catalyst into different phases, remove the product, and then shuttle the catalyst back into the original phase for the next reaction cycle. Building switchability into basic chemicals in this manner could facilitate greener and less-energy-intensive industrial chemical processes.
能够转相的催化反应
通过简单的添加或除去二氧化碳,苏格兰的科学家发明了一种在两相系统中来回转运匀相催化剂的灵巧的把戏。
St. Andrews 大学的Simon L. Desset and David J. Cole-Hamilton 发明的这种可以转相的催化剂使得通常需要复杂的技术来分离产品和重复利
Angew. Chem. Int. Ed. Switchphos Bubbling CO 2 and then N 2 into a reaction tube modifies the rhodium catalyst’s phosphine ligands, switching the catalyst (yellow) from the organic reaction phase to the aqueous phase while the organic product is removed, and then back to a fresh organic phase.
用催化剂的均相反应的灵活性增强了。
催化剂的这种转相的能力的秘密是科学家在三苯基膦中的苯环上加上的弱碱性的脒基团,–N=C(CH3)N(CH3)2。
用这种修改过的配位体配位的催化剂铑可溶于有机溶剂。
当往水-有机含有催化剂的两相反应体系中通入CO2时,二氧化碳就和水反应生成碳酸。
碳酸电离的氢离子与脒基团结合,使得催化剂变成水溶性的。
之后,向两相体系通入氮气,赶走里面的二氧化碳,改变原有的平衡,从而使得催化剂再次变成疏水性的。
通过这样,科学家们就可以在有机相内的反应结束后,把催化剂转移到水中,然后将产物取走,之后,又把催化剂转到有机溶剂中,开始催化新的反应。
通过这种方式在基础化学上建立这种转换能力能够促进更环保和低能耗的化学工业。
W ater On The Moon
This year,
space
scientists were
finally able to
answer one of
the biggest
questions in
lunar science:
Is there water
on the moon?
The answer is
yes. NASA
announced last month that debris kicked up
during the deliberate crash of the Lunar Crater
Observation & Sensing Satellite (LCROSS)
spacecraft did contain a sprinkling of water and
possibly some organic compounds (C&EN, Nov.
23, page 31). LCROSS launched on June 18
together with a long-term mapping spacecraft, the Lunar Reconnaissance Orbiter. On Oct. 9, LCROSS sent a spent booster rocket crashing into a crater near the moon’s south pole and then hurtled itself into the crater in a planned self-destruction. Scientists had suspected water ice might exist in the permanently darkened crater, named Cabeus, and others like it. After several weeks of analysis, the team reported that both infrared and ultraviolet spectrometers had indeed found evidence of water in the plumes of debris created by the impacts—the tons of ejected material contained about 100 kg of water. Possible sources of the water include comets or hydrogen ions from the solar wind interacting with mineral oxides on the lunar surface.
月球上的水
今年,太空科学家终于可以回答月球科学中的大问题:月亮上有水吗?答案是,有。
NASA在上个月声明,精心准备的LCROSS飞行器撞击月球激起的废墟中确实有稀稀落落的水,可能还有一些有机化合物。
LCROSS在六月十八日和一个长期观测航天器,Lunar Reconnaissance Orbiter.(大致是一种绕月飞行的飞行器,我也不知道该叫它什么好了)一起发射升空。
十月九号,LCROSS发射了一个失效的火箭推进器撞向靠近月球南极的一个火山口,然后依照计划好的自毁计划,撞向了同一个火山口。
科学家们猜测在死火山的火山口处或者其他类似的地方存在冰。
这种死火山的火山口,称作Cabeus。
通过数周的分析,研究小组报告说,红外线和紫外线光谱仪都确实在撞击后的废墟喷发出来的烟柱中找到了水存在的证据——喷发出来的数吨物质中大约有100kg的水。
这些水可能的来源包括彗星或者来自太阳风的氢离子与月球表面的矿物中的氧的反应。
姐姐啊,这种文章真的不难,你真该拿本字典好好地自己翻译一下。
都快要读研的人了,这点英语都看不过来,还怎么去搞研究啊。
我翻
译得也不咋地,你自己看着办吧。
