MSDS奥美拉唑镁
埃索美拉唑镁肠溶片-耐信说明书

【通用名称】埃索美拉唑镁肠溶片【商品名称】耐信 Nexium【英文名称】Esomeprazole Magnesium Enteric-coated Tablets【成份】本品活性成份及其化学名称、化学结构式、分子式、分子量为:活性成份:埃索美拉唑镁化学名称为:双-S-5-甲氧基-2-{[(4-甲氧基-3,5-二甲基-2-吡啶基)甲基]亚磺酰基}-1H -苯并咪唑镁三水合物化学结构式:分子式: C34H36MgN6O6S2·3H2O分子量:767.15主要成份相关链接:埃索美拉唑镁【性状】本药为肠溶片剂。
20 mg为浅粉红色,40 mg为粉红色,均为长椭圆双凸形。
赋形剂:单硬脂酸甘油酯40-55,羟丙纤维素,羟丙甲纤维素,氧化铁(红棕色,黄色)(E172),硬脂酸镁,甲基丙烯酸及丙烯酸乙酯共聚物(1:1),30%分散剂。
微晶纤维素,人工石蜡,聚乙二醇,聚山梨酯80,交聚维酮,硬脂酰富马酸钠,糖球(蔗糖和黄色淀粉),滑石粉,二氧化钛(E171),枸橼酸三乙酯。
【适应症】胃食管反流性疾病(GERD)-糜烂性反流性食管炎的治疗-已经治愈的食管炎患者防止复发的长期维持治疗-胃食管反流性疾病(GERD)的症状控制与适当的抗菌疗法联合用药根除幽门螺杆菌,并且-愈合与幽门螺杆菌感染相关的十二指肠溃疡-防止与幽门螺杆菌相关的消化性溃疡复发【规格】按C17H19N3O3S计(1)20mg(2)40mg【用法用量】药片应和液体一起整片吞服,而不应当咀嚼或压碎。
对于存在吞咽困难的患者,可将片剂溶于半杯不含碳酸盐的水中(不应使用其他液体,因肠溶包衣可能被溶解),搅拌,直至片剂完全崩解,立即或在30分钟内服用,再加入半杯水漂洗后饮用。
微丸决不应被嚼碎或压破。
对于不能吞咽的患者,可将片剂溶于不含碳酸盐的水中,并通过胃管给药。
重要的是应仔细检查选择的注射器和胃管的合适程度。
准备工作及使用指导如下:通过胃管给药:1. 将片剂放入合适的注射器,并加入约25 mL水及5 mL空气。
美国药典USP-1478549奥美拉唑镁的msds

Material Safety Data Sheet USP Reference Standards are sold for chemical test and assay purposes only, and NOT for human consumption. The information contained herein is applicable solely to the chemical substance when used as a USP Reference Standard and does not necessarily relate to any other use of the substance described, (i.e. at different concentrations, in drug dosage forms, or in bulk quantities). USP Reference Standards are intended for use by persons having technical skill and at their own discretion and risk. This information has been developed by USP staff from sources considered reliable but has not beenindependently verified by the USP. Therefore, the USP Convention cannot guarantee the accuracy of the information in these sources nor should the statements contained herein be considered an official expression. NO REPRESENTATION OR WARRANTY, EXPRESS OR IMPLIED, INCLUDING THE WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE is made with respect to the information contained herein.ATTENTION !12601 Twinbrook Parkway, Rockville, MD 20852 USA Phone Calls:301-816-81298 a.m. to 5 p.m. EST Mon. - Fri.OMEPRAZOLE MAGNESIUM Catalog Number:1478549Revision Date:March 20, 2007SECTION 2 - HAZARD INFORMATIONCommon Name:Omeprazole Magnesium SECTION 1 - PRODUCT AND COMPANY IDENTIFICATIONSECTION 3 - COMPOSITION/INFORMATION ON INGREDIENTSEMERGENCY OVERVIEW - Allergen.Adverse Effects:Adverse effects of omeprazole may include cough; nasal or ear congestion; chills, fever, or sore throat; loss of voice;runny nose; difficulty breathing; body aches or pains; heart burn, diarrhea, gas, skin rash or itching, unusual tiredness,dizziness, constipation, headache, and nausea or vomiting. Possible allergic reaction to material if inhaled, ingestedor in contact with skin.Overdose Effects:Symptoms of omeprazole overdose may include blurred vision, confusion, increased sweating, drowsiness, drymouth, flushing, headache, severe stomach pain, nausea or vomiting, and fast or irregular heartbeat.Acute:Possible eye, skin, gastrointestinal and/or respiratory tract irritation.Chronic:Possible hypersensitization.Medical Conditions Aggravated by Exposure:Hypersensitivity to the material and chronic liver disease (current or history of).Cross Sensitivity:n/fTarget Organs:Gastrointestinal tractFor additional information on toxicity, see Section 11.Common Name:Omeprazole MagnesiumFormula:C34H36MgN6O6S2Manufacturer:U. S. PharmacopeiaResponsible Party:Reference Standards Technical ServicesMailing Address:12601 Twinbrook Parkway, Rockville, MD 20852 USAPhone:301-816-8129Hours:8 a.m. to 5 p.m. EST Mon. - Fri.Product Use:USP Reference Standards and Authentic Substances are used for chemical tests and assays in analytical,clinical, pharmaceutical, and research laboratories.Synonym:n/fChemical Name:5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, magnesium salt (2:1) CAS:95382-33-5RTECS Number:n/fChemical Family:Substituted benzimidazoleTherapeutic Category:Gastric acid secretory depressantComposition:Pure MaterialSECTION 4 - FIRST AID MEASURESInhalation:May cause irritation. Avoid inhalation. Remove to fresh air.Eye:May cause irritation. Avoid contact. Flush with copious quantities of water for at least 15 minutes.Skin:May cause irritation and sensitization. Avoid contact. Flush with copious quantities of soap and water.Ingestion:May cause irritation. Flush out mouth with water.General First Aid Procedures:Remove from exposure. Remove contaminated clothing. Persons developing serious hypersensitivity(anaphylactic) reactions must receive immediate medical attention. If person is not breathing giveartificial respiration. If breathing is difficult give oxygen. Obtain medical attention.Note to PhysiciansOverdose Treatment:Treatment of omeprazole overdose should be symptomatic and supportive and may include the following:1. Administer activated charcoal as a slurry.2. Sinus tachydysrhythmias do not need to be routinely treated unless patient is hemodynamically unstable.3. Omeprazole is not readily dialyzable. [Meditext 2007 & USP DI 2007]SECTION 5 - FIREFIGHTING MEASURESExtinguisher Media:Water spray, dry chemical, carbon dioxide or foam as appropriate for surrounding fire and materials.Fire and Explosion Hazards:This material is assumed to be combustible. As with all dry powders it is advisable to ground mechanical equipment in contact with dry material to dissipate the potential buildup of static electricity.Firefighting Procedures:As with all fires, evacuate personnel to a safe area. Firefighters should use self-contained breathingequipment and protective clothing.SECTION 6 - ACCIDENTAL RELEASE MEASURESSpill Response:Wear approved respiratory protection, chemically compatible gloves and protective clothing. Wipe up spillage or collect spillage using a high efficiency vacuum cleaner. Avoid breathing dust. Place spillage in appropriately labeledcontainer for disposal. Wash spill site.SECTION 7 - HANDLING AND STORAGEHandling:As a general rule, when handling USP Reference Standards avoid all contact and inhalation of dust, mists, and/or vapors associated with the material. Wash thoroughly after handling.Storage:Store in tight, light-resistant container as defined in the USP-NF. This material should be handled and stored per label instructions to ensure product integrity. Store in a refrigerator.SECTION 8 - EXPOSURE CONTROL / PERSONAL PROTECTIONEngineering Controls:Engineering controls such as exhaust ventilation are recommended.Respiratory Protection:Use a NIOSH-approved respirator, if it is determined to be necessary by an industrial hygiene surveyinvolving air monitoring. In the event that a respirator is not required, an approved dust mask should be used. Gloves:Chemically compatibleEye Protection:Safety glasses or gogglesProtective Clothing:Protect exposed skin.Exposure Limits:Industry: 0.5 mg/m3 (omeprazole)SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIESProperties as indicated on the MSDS are general and not necessarily specific to the USP Reference Standard Lot provided. Appearance and Odor:White to almost white crystalline powderOdor Threshold:n/fpH:Approximately 10.5 (aqueous suspension)Melting Range:n/fBoiling Point:n/fFlash Point:n/fAutoignition Temperature:n/fEvaporation Rate:n/fUpper Flammability Limit:n/fLower Flammability Limit:n/fVapor Pressure:n/fVapor Density:n/fSpecific Gravity:n/fSolubility in Water:Very slightly solubleFat Solubility:n/fOther Solubility:Soluble in methanolPartition Coefficient: n-octanol/water:n/fPercent Volatile:n/fReactivity in Water:n/fExplosive Properties:n/fOxidizing Properties:n/fFormula:C34H36MgN6O6S2Molecular Weight:713.12Oral Rat:LD50: >2000 mg/kgOral Mouse:n/fNTP:No IARC:No OSHA:NoListed as a Carcinogen by: Irritancy Data:n/f SECTION 11 - TOXICOLOGICAL PROPERTIESOther Toxicity Data:n/fCorrosivity:n/f Sensitization Data:Omeprazole is a strong sensitizer in guinea pigs. (Guinea pig maximization test)Other Carcinogenicity Data:No evidence of carcinogenicity was found in mice orally administered up to 140 mg/kg/day omeprazolefor 66 weeks. In two 2-year studies in rats, omeprazole given in doses up to 140.8 mg/kg/day causedgastric carcinoid tumors and enterochromaffin-like (ECL) cell hyperplasia in a dose-dependent manner in both males and females.Mutagenicity Data:Omeprazole produced clastogenic effects in an in vitro human lymphocyte chromosomal aberration assay, in oneof two in vivo mouse micronucleus tests, and in an in vivo bone marrow cell chromosomal aberration assay. Omeprazole was negative in the in vitro Ames Salmonella typhimurium assay, an in vitro mouse lymphoma cell forward mutation assay, and an in vivo rat liver DNA damage assay.Reproductive and Developmental Effects:Sporadic instances of developmental abnormalities in infants born to women who receivedomeprazole during pregnancy have been reported. Epidemiological studies have not foundan association between the use of omeprazole during pregnancy and an increased risk ofbirth defects.Omeprazole did not impair fertility in rats at parenteral doses of up to 138 mg/kg/day. Nobirth defects were seen in pregnant rabbits and rats administered omeprazole in doses up to69 mg/kg/day and 138 mg/kg/day, respectively.In rabbits, 6.9 - 69.1 mg/kg/day omeprazole produced dose-related increases in embryo-lethality, fetal resorptions, and pregnancy disruption. In rats, dose-related embryo/fetaltoxicity and post natal developmental toxicity were observed in the rat offspring of parentstreated with 13.8 - 138 mg/kg/day.SECTION 12 - ECOLOGICAL INFORMATIONEcological Information:Omeprazole sodium, a related compound, may cause long-term adverse effects in the aquatic environment andis not readily biodegradable.SECTION 13 - DISPOSAL CONSIDERATIONSDisposal:Dispose of waste in accordance with all applicable Federal, State and local laws.SECTION 14 -TRANSPORT INFORMATIONShipping Name:n/fClass:n/f Stable?Yes Conditions to Avoid:Avoid exposure to light.Incompatibilities:n/fDecomposition Products:When heated to decomposition material emits toxic fumes of SOx and NOx. Emits toxic fumes under fireconditions.Hazardous Polymerization?No SECTION 10 - STABILITY AND REACTIVITYUN Number:n/fPacking Group:n/fAdditional Transport Information:n/fSECTION 15 - REGULATORY INFORMATION U.S. Regulatory Information:n/fInternational Regulatory Information:Risk Phrases: R43, R52/53Safety Phrases: S37, S61SECTION 16 - OTHER INFORMATIONRevision:20-Mar-07Previous Revision Date:05-Feb-07。
奥美拉唑制剂的调查 (2)

对奥美拉唑药物的调查奥美拉唑,就是一种H+,K+-ATP酶抑制剂,可以抑制胃酸的分泌,主要用于十二指肠溃疡与卓-艾综合征,也可用于胃溃疡与反流性食管炎,静脉注射可用于消化性溃疡急性出血的治疗。
同时与阿莫西林与克林霉素或与甲硝唑与克拉霉素合用,可以用于杀灭幽门螺杆菌。
奥美拉唑,分子式为C17H19N3O3S,分子量为345,理化性质包括:为白色或类白色结晶性粉末;无臭;遇光易变色。
在二氯甲烷中易溶,在甲醇或乙醇中略溶,在丙酮中微溶,在水中不溶;在0、lmol/L氢氧化钠溶液中溶解。
结构式如下:奥美拉唑结构式一、奥美拉唑的制剂种类:现在市场上的奥美拉唑,制剂种类主要有两大种类:一种就是奥美拉唑肠溶剂,包括片剂与胶囊剂;另一种就是注射用无菌粉末(注射用奥美拉唑钠)。
二、奥美拉唑的生产企业(部分):通过检索,整理出不同剂型的部分生产企业,如下: 1、奥美拉唑肠溶剂生产企业:2、奥美拉唑注射用剂:三、奥美拉唑的申报情况:通过“药智网”()对奥美拉唑进行检索,药品的注册与受理总计862条,其中多数药品属于补充申请,并且已进入审批流程,大部分药品属于审批完毕;中国新药批准信息(1978~2003)有41条,大多属于第二类与第四类新药;药品转让信息11条,原料药、针粉剂、胶囊剂均有转让;中国临床试验记录41条,多数处于进行中,少数已完成。
通过专利信息平台,对奥美拉唑相关专利进行检索,共检索出353件专利,包括发明专利305件,实用新型4件,外观设计44件。
发明专利多与奥美拉唑的制备方法与制备工艺以及其她新应用有关,实用新型多与片剂类型改进有关,实用新型多与药品的外包装有关。
通过检索,我们可以发现,许多企业不光单纯的研发奥美拉唑药物,而且还与其她药物联合用药,用于预防或者更好的治疗胃溃疡,例如:北京韩美药品有限公司申报的奥美拉唑阿司匹林肠溶胶囊,主要用于预防心血管疾病患者减少阿司匹林诱发的胃溃疡风险;吉林益民堂制药有限公司申报的奥美拉唑碳酸氢钠干混悬剂,可更好地治疗十二指肠溃疡、胃溃疡、糜烂性食管炎等。
奥美拉唑镁肠溶片说明书

奥美拉唑血浆消除半衰期约为 40 分钟(30-90 分钟)。大约 80%的代谢物从尿中排出,其余从 粪便排出。
患者因素
奥美拉唑的生物利用度在老年患者或肾功能低下的患者中无明显改变,在肝功能损害的患者 中升高,但这些患者的清除率都明显下降。 贮藏: 密封,25℃以下保存。 包装: 双铝塑复合膜泡包装。 (1)10 毫克:3 片/板/盒;7 片/板/盒;7 片/板×2 板/盒。 (2)20 毫克:7 片/板/盒;7 片/板×2 板/盒。 有效期: 36 个月 执行标准:
预防非甾体类抗炎药相关的胃溃疡,胃糜烂或消化不良症状:常用剂量 20 毫克,一日一次。
反流性食管炎:剂量可依疾病的严重程度进行个体化调整。本品常用剂量 20 毫克,一日一 次。通常四周内可治愈,若初始疗程疗效不肯定,应再治疗四周。其它治疗无效的反流性食 管炎患者,可给予 40 毫克,一日一次,通常八周内可以治愈。一旦复发,应重复治疗。
幽门螺杆菌的根除:
三联疗法:本品 20 毫克,阿莫西林 1000 毫克和克拉霉素 500 毫克,均为一日 2 次,持续 一周,或本品 20 毫克,克拉霉素 250 毫克和甲硝唑 400 毫克,均为一日 2 次,持续一周。
二联疗法:本品 40 毫克,一日一次,和克拉霉素 500 毫克,一日 3 次,持续二周。或本品 20 毫克,阿莫西林 750-1000 毫克,均为一日 2 次,持续二周。为确保治愈,可参考十二指肠 溃疡的推荐剂量。
目前国内尚无儿童使用本品的经验。 老年用药: 老年患者无需调整剂量。 药物相互作用: 0. 1.由于本品对胃内 pH 有影响而可能影响其他药物的吸收。因此在用奥美拉唑或其它抑制 剂或抗酸剂治疗时,酮康唑和伊曲康唑的吸收会下降。 2.由于本品在肝脏中通过 CYP2C19 酶代谢,因此会增加其他通过该酶代谢药物的血浆浓度, 如安定、苯妥英、华法林(R-华法林,低活性)。对于正在接受苯妥英、华法林或其他维生 素 K-拮抗剂治疗的患者,开始或停用奥美拉唑时应进行监测。 3.本品(每天 40mg)使伏立康唑(CYP2C19 底物)的 Cmax 和 AUC 分别增加 15%和 41%。伏立 康唑使奥美拉唑的 AUCτ增加 280%,在进行联合使用和长期治疗时,对肝功能损伤严重的患 者应考虑调整奥美拉唑的剂量。 4.当本品与克拉霉素或红霉素合用时,奥美拉唑的血药浓度会增加。但与甲硝唑或阿莫西林 合用时,无相互作用。 5.本品与抑制 CYP2C19 或 CYP3A 酶的药物(HIV 蛋白酶抑制剂,酮康唑,伊曲康唑)合用可 能会使奥美拉唑的血药浓度升高。 6.研究表明,每日口服本品 20~40mg 并不影响其他相关的 CYP 同功酶,与下列酶底物无代 谢性相互作用,CYP1A2(咖啡因、非那西丁、茶碱)、CYP2C9(S-华法林、吡罗昔康、双氯 芬酸和萘普生)、CYP2D6(美托洛尔、普萘洛尔)、CYP2E1(乙醇)和 CYP3A(环孢菌素、 利多卡因、奎尼丁、雌二醇、红霉素、布地奈德)。 7.包括奥美拉唑在内的质子泵抑制剂不应与阿扎那韦合用。奥美拉唑(40mg,每天一次)与 阿扎那韦 300mg/利托那韦 100mg 合用会降低健康人群阿扎那韦的暴露量(AUC,Cmax 和 Cmin 约降低 75%)。阿扎那韦剂量增加至 400mg 不能补偿奥美拉唑对阿扎那韦暴露量的影响。 8.奥美拉唑与他克莫司合用会增加后者血清浓度。推荐当开始合用和终止奥美拉唑时,监测 他克莫司的血浆浓度。 药物过量: 过量使用本品有可能发生急性毒性,成人使用 320-800mg 会导致低、中度的中毒。其症状: 头晕,情感淡漠,头痛,意识错乱,血管扩张,心动过速,恶心,呕吐,腹胀,腹泻。
镁MSDS 新版 化学品安全技术说明书

化学品安全技术说明书
第一部分化学品及企业标识
第二部分危险性概述
危险
第三部分成分/组成信息
第四部分急救措施
第五部分消防措施
第六部分泄露应急处理
第七部分操作处置与储存
第八部分接触控制和个体防护
第九部分理化特性
第十部分稳定性和反应性
第十一部分毒理学信息
第十二部分生态学信息
第十三部分废弃处理
第十四部分运输信息
包装类别
I
包装方法
第十五部分法规信息
第十六部分其他信息
免责声明
本安全技术说明书格式符合我国GB/T16483和GB/T17519要求,数据来源于国际权威数据库和企业提交的数据,其它的信息是基于公司目前所掌握的知识。
我们尽量保证其中所有信息的正确性,但由于信息来源的多样性以及本公司所掌握知识的局限性,本文件仅供使用者参考。
安全技术说明书的使用者应根据使用目的,对相关信息的合理性做出判断。
我们对该产品操作、存储、使用或处置等环节产生的任何损害,不承担任何责任。
奥美拉唑镁肠溶片说明书

核准日期:修改日期:奥美拉唑镁肠溶片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:奥美拉唑镁肠溶片商品名称:洛赛克MUPS英文名称:Omeprazole Magnesium Enteric-coated Tablets汉语拼音:Aomeilazuomei Changrongpian【成份】本品主要成份为奥美拉唑镁。
其化学名称为:双-5-甲氧基-2-{[(4-甲氧基-3,5-二甲基-2-吡啶基)甲基]亚磺酰基}-1H-苯并咪唑镁化学结构式为:分子式:C34H36N6MgO6S2分子量:713.21【性状】本品为淡粉红色(10mg)或粉红色(20mg)薄膜衣片。
【适应症】治疗十二指肠溃疡、胃溃疡和反流性食管炎;与抗生素联合用药,治疗幽门螺杆菌引起的十二指肠溃疡;治疗非甾体类抗炎药相关的消化性溃疡或胃十二指肠糜烂;预防非甾体类抗炎药引起的消化性溃疡、胃十二指肠糜烂或消化不良症状;亦用于慢性复发性消化性溃疡和反流性食管炎的长期治疗;用于胃食管反流病的烧心感和反流的对症治疗;溃疡样症状的对症治疗及酸相关性消化不良;用于卓-艾氏综合征的治疗。
【规格】以C17H19N3O3S计(1)10 毫克;(2)20 毫克【用法用量】必须整片吞服,至少用半杯液体送服。
药片不可咀嚼或压碎,可将其分散于水或微酸液体中(如:果汁),分散液必须在30分钟内服用。
十二指肠溃疡:本品常用剂量20毫克,一日一次,通常溃疡可在二周内治愈。
如果初始疗程疗效不肯定,应再治疗二周。
对用其它药物治疗无效的十二指肠溃疡,用40毫克,一日一次,通常四周内可治愈。
对复发患者,可重复予以治疗。
幽门螺杆菌的根除:三联疗法:本品20毫克,阿莫西林1000毫克和克拉霉素500毫克,均为一日2次,持续一周。
或本品20毫克,克拉霉素250毫克和甲硝唑400毫克,均为一日2次,持续一周。
二联疗法:本品40毫克,一日一次,和克拉霉素500毫克,一日3次,持续二周。
袋装奥美拉唑说明书

注射用奥美拉唑说明书【药品名称】注射用奥美拉唑钠【英文名称】 omeprazole sodium for injection【药品别名】奥克?本品主要成份为:奥美拉唑钠,其化学名称为:5-甲氧基-2-{[(4-甲氧基-3, 5-二甲基-2-吡啶基)-甲基]-亚磺酰基}-1h-苯并咪唑钠盐一水合物。
【性状】本品为白色疏松块状物或粉末,专用溶剂为无色的透明液体。
【药理毒理】本品为胃壁细胞质子泵抑制剂,能特异性地抑制壁细胞顶端膜构成的分泌性微管和胞浆内的管状泡上的h+、k+-atp酶,从而有效地抑制胃酸的分泌。
由于h+、k+-atp酶是壁细胞泌酸的最后一个过程,故本品抑酸能力强大。
它不仅能非竞争性抑制促胃液素、组胺、胆碱及食物、刺激迷走神经等引起的胃酸分泌,而且能抑制不受胆碱或h2受体阻断剂影响的部分基础胃酸分泌,对h2受体拮抗剂不能抑制的由二丁基环腺苷酸(dcamp)刺激引起的胃酸分泌也有强而持久的抑制作用。
本品对胃蛋白酶分泌也有抑制作用,对胃黏膜血流量改变不明显,也不影响体温、胃腔温度、动脉血压、静脉血红蛋白、动脉氧分压、二氧化碳分压及动脉血ph。
【药代动力学】静脉注射本品后,体内分布在肝、肾、胃、十二指肠、甲状腺等组织,分布容积为0.19~0.48l/kg,与细胞外液体积相当。
t1/2为0.5~1小时,慢性肝病患者为3小时。
本品主要在肝脏中经细胞色素p450代谢,代谢产物主要为硫醚、砜和羟基衍生物。
对胃酸的分泌无作用,代谢完全,仅少数以原形排泄。
约有80%的代谢物经肾排出,部分(18~23%)随粪便排出。
有肠肝循环过程,血浆蛋白结合率高,达95%左右。
肾衰患者对本品的清除无明显变化,肝功能受损者清除半衰期可有延长。
【适应症】主要用于:(1)消化性溃疡出血、吻合口溃疡出血。
(2)应激状态时并发的急性胃黏膜损害,和非甾体类抗炎药引起的急性胃黏膜损伤;(3)亦常用于预防重症疾病(如脑出血、严重创伤等)胃手术后预防再出血等;(4)全身麻醉或大手术后以及衰弱昏迷患者防止胃酸反流合并吸入性肺炎。
奥美拉唑镁肠溶片说明书

预防非甾体类抗炎药相关的十二指肠溃疡,十二指肠糜烂或消化不良症状:正常剂量为 20 毫克,一日一次。
为预防幽门螺杆菌根除治疗无效的反复发作的十二指肠溃疡的复发,剂量可依疾病的严重程 度进行个体化调整。疗效呈剂量依赖性。本品常用剂量 20 毫克,一日一次。一些患者每日 10 毫克可能已足够;若该剂量无效,可增至 40 毫克。
处理:必要时洗胃或使用活性炭,对症治疗。 药理毒理: 奥美拉唑是一种取代的苯并咪唑化合物,是一对活性旋光对映体的消旋物,奥美拉唑通过特 殊机制作用于壁细胞中的质子泵而减少胃酸分泌,此作用是可逆的。奥美拉唑是一种弱碱, 在壁细胞的酸性环境中被浓缩并转化为活性形式,抑制胃液中产生盐酸的最后环节:H+、 K+ -ATP 酶,该抑制作用呈剂量依赖性,对基础的及刺激后的胃酸分泌都有作用,而与刺激 物类型无关。奥美拉唑对胆碱能及组胺受体无作用。和 H2 受体阻滞剂相似,奥美拉唑降低 胃内酸度,从而使胃泌素呈与酸度降低成比例的增加,胃沁素的增加是可逆的。有报道发现, 在长期治疗中,胃腺囊肿的发生增加。这些变化均为胃酸分泌受抑制的生理学结果,是良性 且可逆的。质子汞抑制剂或其他酸抑制剂引起的胃酸减少会使胃肠道中正常细菌的数量增加, 因而治疗会导致胃肠道感染(如沙门氏菌和弯曲杆菌)的风险轻微增大。
不能口服药物的患者,可用奥美拉唑的非肠道给药剂型,见洛赛克针剂或粉针剂 40 毫克的 说明书。 不良反应:
临床试验表明,最常见的是头痛和胃肠道症状如:腹泻、恶心、便秘、发生率均在 1-3%。
有报道发现个别病例有 Stevens-Johnson 综合征及中毒性表皮坏死松懈症,但未确定有因果 关系。 临床试验提示,奥美拉唑与克拉霉素联合用药可增加中枢神经系统(主要是头痛)及胃肠道不 良反应的发生率。 禁忌: 1.对奥美拉唑过敏者。 2.与其它质子汞抑制剂一样,奥美拉唑不应与阿扎那韦合用。 注意事项: 1.当怀疑有消化性溃疡时,应尽早通过 X 线、内境检查确诊,以免治疗不当。 2.治疗胃溃疡时,必须排除恶性肿瘤。。因用本品治疗可掩盖其症状,从而延误诊断。 3.本品对胃肠道的运动紊乱无效。 4.对经内镜确诊为食管炎而长期服用奥美拉唑的患者,每天 10 毫克治疗较每天 20 毫克治疗 的缓解率低,因此每天服用 10 毫克者应定期进行内境监测。 孕妇及哺乳期妇女用药: 流行病学研究结果表明,奥美拉唑对孕妇或胎儿/新生儿的健康无不良影响。孕妇可以使用 奥美拉唑。 奥美拉唑可被分泌入乳汁,尚不知对婴儿的影响。哺乳期妇女慎用。 儿童用药:
奥美拉唑说明书

_______________________________________________________________________________________________________________________________________ _______________________________________________________________________________________________________________________________________ HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to useOmeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets safely and effectively. See full prescribing information for Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets. Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets Initial U.S. Approval: 2006----------------------------INDICATIONS AND USAGE--------------------------- Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets is a proton pump inhibitor indicated for: • Treatment of duodenal ulcer (1.1) • Treatment of gastric ulcer (1.2) • Treatment of gastroesophageal reflux disease (GERD) (1.3) • Maintenance of healing of erosive esophagitis (1.4)----------------------DOSAGE AND ADMINISTRATION----------------------- • Short-Term Treatment of Active Duodenal Ulcer: 20 mg once daily for 4 weeks (some patients may require an additional 4 weeks of therapy (14.1)) (2.2) • Gastric Ulcer: 40 mg once daily for 4-8 weeks (2.3) • Gastroesophageal Reflux Disease (GERD) (2.4) -Symptomatic GERD (with no esophageal erosions): 20 mg once daily for up to 4 weeks- Erosive Esophagitis: 20 mg once daily for 4-8 weeks • Maintenance of Healing of Erosive Esophagitis: 20 mg once daily (2.5) ---------------------DOSAGE FORMS AND STRENGTHS---------------------- • Tablets 20 mg omeprazole, 750 mg sodium bicarbonate, and 343 mg magnesium hydroxide (3) • Tablets 40 mg omeprazole, 750 mg sodium bicarbonate, and 343 mg magnesium hydroxide (3) -------------------------------CONTRAINDICATIONS----------------------------- • Known hypersensitivity to Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets or any components in the formulation (4) • Patients who cannot take magnesium (4)-----------------------WARNINGS AND PRECAUTIONS-----------------------• Concomitant Gastric Malignancy: Symptomatic response to therapy withOmeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets does not preclude the presence of gastric malignancy (5.1)• Atrophic Gastritis: Has been observed in gastric corpus biopsies from patients treated long-term with omeprazole (5.2) • Buffer Content: Sodium content should be taken into consideration when administering to patients on a sodium-restricted diet or at risk of developing congestive heart failure (CHF). (5.3)• Buffer Content: Magnesium content increases risk of hypermagnesemia andmagnesium toxicity in the elderly and in patients with renal impairment or renal disease (5.3) • Buffer Content: Use with caution in patients with Bartter’s syndrome, hypokalemia, respiratory alkalosis, and problems with acid-base balance because of its sodium bicarbonate content; long-term administration of bicarbonate with calcium or milk can cause milk-alkali syndrome (5.3) ------------------------------ADVERSE REACTIONS------------------------------- Most common adverse reactions (incidence ≥ 2%) are:Headache, abdominal pain, nausea, diarrhea, vomiting, and flatulence (6)To report SUSPECTED ADVERSE REACTIONS, contact Santarus Inc. at 1-888-778-0887 or FDA at 1-800-FDA-1088 or /medwatch . ------------------------------DRUG INTERACTIONS------------------------------- • Drugs metabolized by cytochrome P450 (e.g., diazepam, warfarin,phenytoin, cyclosporine, disulfiram, benzodiazepines): Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets can prolong their elimination. Monitor to determine the need for possible dose adjustments when taken with Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets (7) • Patients treated with proton pump inhibitors and warfarin concomitantly may need to be monitored for increases in INR and prothrombin time (7) • Drugs for which gastric pH can affect bioavailability (e.g., ketoconazole,ampicillin esters, iron salts): Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets may interfere with absorption due to inhibition of gastric acid secretion (7) • Voriconazole: May increase plasma levels of omeprazole (7) • Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets mayreduce plasma levels of atazanavir and nelfinavir (7) • Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets mayincrease serum levels of tacrolimus, voriconazole, saquinavir, and clarithromycin (7) -----------------------USE IN SPECIFIC POPULATIONS----------------------- • Pregnancy: Based upon animal data, may cause fetal harm (8.1) • The safety and effectiveness of Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets in pediatric patients less than 18 years of age have not been established. (8.4) • Hepatic Impairment: Consider dose reduction, particularly for maintenance of healing of erosive esophagitis (8.6)See 17 for PATIENT COUNSELING INFORMATION.Revised: 12/2009 FULL PRESCRIBING INFORMATION: CONTENTS*1 INDICATIONS AND USAGE1.1 Duodenal Ulcer 1.2 Gastric Ulcer1.3 Treatment of Gastroesophageal Reflux Disease (GERD)1.4 Maintenance of Healing of Erosive Esophagitis 2 DOSAGE AND ADMINISTRATION2.1 Instructions for Use 2.2 Short-Term Treatment of Active Duodenal Ulcer 2.3 Gastric Ulcer2.4 Gastroesophageal Reflux Disease (GERD) 2.5 Maintenance of Healing of Erosive Esophagitis 3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 Concomitant Gastric Malignancy 5.2 Atrophic Gastritis5.3 Buffer Content 6 ADVERSE REACTIONS6.1 Clinical Trials Experience 6.2 Post-marketing Experience 7 DRUG INTERACTIONS 8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy 8.3 Nursing Mothers8.4 Pediatric Use 8.5 Geriatric Use 8.6 Hepatic Impairment 8.7 Renal Impairment 8.8 Asian Population 10 OVERDOSAGE 11 DESCRIPTION 12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action 12.2 Pharmacodynamics 12.3 Pharmacokinetics 13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility 13.2 Animal Toxicology and/or Pharmacology 14 CLINICAL STUDIES 14.1 Duodenal Ulcer Disease 14.2 Gastric Ulcer 14.3 Gastroesophageal Reflux Disease (GERD) 14.4 Long Term Maintenance Treatment of Erosive Esophagitis 15 REFERENCES 16 HOW SUPPLIED/STORAGE AND HANDLING 17PATIENT COUNSELING INFORMATION * Sections or subsections omitted from the full prescribing information are not listed.FULL PRESCRIBING INFORMATION:1 INDICATIONS AND USAGE1.1 Duodenal UlcerOmeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets is indicated for short-term treatment of active duodenal ulcer. Most patients heal within four weeks. Some patients may require an additional four weeks of therapy. [See Clinical Studies (14.1)]1.2 Gastric UlcerOmeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets is indicated for short-term treatment (4-8 weeks) of active benign gastric ulcer. [See Clinical Studies (14.2)]1.3 Treatment of Gastroesophageal Reflux Disease (GERD)Symptomatic GERDOmeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets is indicated for the treatment of heartburn and other symptoms associated with GERD. [See Clinical Studies (14.3)]Erosive EsophagitisOmeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets is indicated for the short-term treatment (4-8 weeks) of erosive esophagitis that has been diagnosed by endoscopy.The efficacy of Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets used for longer than 8 weeks in these patients has not been established. If a patient does not respond to 8 weeks of treatment, it may be helpful to give up to an additional 4 weeks of treatment. If there is recurrence of erosive esophagitis or GERD symptoms (e.g., heartburn), additional 4-8 week courses of omeprazole may be considered. [See Clinical Studies (14.3)]1.4 Maintenance of Healing of Erosive EsophagitisOmeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets is indicated to maintain healing of erosive esophagitis. Controlled studies do not extend beyond 12 months. [See Clinical Studies (14.4)]2 DOSAGE AND ADMINISTRATION2.1 Instructions for UseOmeprazole / Sodium Bicarbonate / Magnesium Hydroxide is available as tablets in 20 mg and 40 mg strengths of omeprazole for oral administration in adult patients 18 years and older.All recommended doses throughout the labeling are based upon omeprazole. Since both the 20 mg and 40 mg tablets contain the same amount of sodium bicarbonate (750 mg) and magnesium hydroxide (343 mg), two 20 mg tablets are not equivalent to one 40 mg tablet;therefore, two 20 mg tablets should not be substituted for one 40 mg tablet.Because Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets contain magnesium hydroxide, the tablets should not be substituted for ZEGERID products (e.g., ZEGERID Powder for Oral Suspension or ZEGERID Capsules).Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets should be taken on an empty stomach with water at least one hour before a meal.Do not use other liquids.2.2 Short-Term Treatment of Active Duodenal UlcerThe recommended adult oral dose of Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets is 20 mg once daily. Most patients heal within 4 weeks. Some patients may require an additional 4 weeks of therapy.2.3 Benign Gastric UlcerThe recommended adult oral dose of Omeprazole / Sodium Bicarbonate /Magnesium Hydroxide Tablets is 40 mg once daily for 4-8 weeks.2.4 Gastroesophageal Reflux Disease (GERD)The recommended adult oral dose for the treatment of patients withsymptomatic GERD and no esophageal erosions is 20 mg once daily for up to 4 weeks. The recommended adult oral dose for the treatment of patients with erosive esophagitis is 20 mg once daily for 4-8 weeks.2.5 Maintenance of Healing of Erosive EsophagitisThe recommended adult oral dose of Omeprazole / Sodium Bicarbonate /Magnesium Hydroxide Tablets is 20 mg once daily. 3 DOSAGE FORMS AND STRENGTHSOmeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets, 20 mg, are white oval-shaped tablets. One side of each tablet is embossed with “ZM 20.” Each tablet contains 20 mg omeprazole and 750 mg sodium bicarbonate plus 343 mg magnesium hydroxide.Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets, 40 mg, are white oval-shaped tablets. One side of each tablet is embossed with “ZM 40.” Each tablet contains 40 mg omeprazole and 750 mg sodium bicarbonate plus 343 mg magnesium hydroxide.4 CONTRAINDICATIONSOmeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets is contraindicated in patients with known hypersensitivity to any components of the formulation. Hypersensitivity reactions may include anaphylaxis, anaphylactic shock, angioedema, bronchospasm, interstitial nephritis, and urticaria.Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets is contraindicated in patients who cannot take magnesium. [See Warnings and Precautions (5.3)]5 WARNINGS AND PRECAUTIONS5.1 Concomitant Gastric MalignancySymptomatic response to therapy with omeprazole does not preclude the presence of gastric malignancy.5.2 Atrophic GastritisAtrophic gastritis has been noted occasionally in gastric corpus biopsies from patients treated long-term with omeprazole.5.3 Buffer ContentEach 20 mg and 40 mg Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablet contains 750 mg (9 mEq) of sodium bicarbonate (equivalent to 209 mg of Na+) and 343 mg (12 mEq) of magnesium hydroxide (equivalent to 143 mg of Mg2+).Sodium BicarbonateBecause Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets contains sodium bicarbonate, it should be used with caution in patients with Bartter’s syndrome, hypokalemia, hypocalcemia, respiratory and metabolic alkalosis, and problems with acid-base balance. Long-term administration of bicarbonate with calcium or milk can cause milk-alkali syndrome.The sodium content of this product should be taken into consideration when administering to patients on a sodium-restricted diet or at risk of developing congestive heart failure (CHF).Chronic use of sodium bicarbonate may lead to systemic alkalosis and increased sodium intake can produce edema and weight increase.Magnesium HydroxideBecause Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets contains magnesium hydroxide, it should be used with caution in elderly and in patients with renal impairment or renal disease due to increased risk of developing hypermagnesemia and magnesium toxicity.Magnesium hydroxide should not be used in patients with renal failure unless serum magnesium levels are being closely monitored.Hypermagnesemia has been reported in infants whose mothers were using magnesium-containing antacid products chronically in high doses.6 ADVERSE REACTIONS6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.In the U.S clinical trial population, of 465 patients, the adverse reactions summarized in Table 1 were reported to occur in 1% or more of patients on therapy with omeprazole. Numbers in parentheses indicate percentages of the adverse reactions considered by investigators as possibly, probably, or definitely related to the drug.Table 1: Adverse Reactions Occurring in 1% or More of Patients on Omeprazole Therapy from U.S. Studies Omeprazole Placebo Ranitidine(n = 465) (n = 64) (n = 195) Headache 6.9 (2.4) 6.3 7.7 (2.6) Diarrhea 3.0 (1.9) 3.1 2.1 (0.5) Abdominal Pain 2.4 (0.4) 3.1 2.1 Nausea 2.2 (0.9) 3.1 4.1 (0.5) URI 1.9 1.6 2.6 Dizziness 1.5 (0.6) 0.0 2.6 (1.0) Vomiting 1.5 (0.4) 4.7 1.5 (0.5) Rash 1.5 (1.1) 0.0 0.0 Constipation 1.1 (0.9) 0.0 0.0Cough 1.1 0.0 1.5 Asthenia 1.1 (0.2) 1.6 (1.6) 1.5 (1.0) Back Pain 1.1 0.0 0.5 The international clinical trials were double-blind and open-label in design. Table 2: Incidence of Adverse Reactions ≥ 1% Causal Relationship Not Assessed from International Studies Omeprazole Placebo (n = 2631) (n = 120) Body as a whole, site unspecified Abdominal Pain 5.2 3.3Asthenia 1.3 0.8Digestive System Constipation 1.5 0.8Diarrhea 3.7 2.5Flatulence 2.7 5.8Nausea 4.0 6.7Vomiting 3.2 10.0Acid Regurgitation 1.9 3.3Nervous System / Psychiatric Headache 2.9 2.5The most common adverse reactions reported (i.e., with an incidence rate ≥ 2%) from omeprazole-treated patients enrolled in these studies includedheadache (6.9%), abdominal pain (5.2%), nausea (4.0%), diarrhea (3.7%), vomiting (3.2%), and flatulence (2.7%). Additional adverse reactions that were reported with an incidence of ≥ 1% included acid regurgitation (1.9%), upper respiratory infection (1.9%), constipation (1.5%), dizziness (1.5%), rash (1.5%), asthenia (1.3%), back pain (1.1%), and cough (1.1%). The clinical trial safety profile in patients greater than 65 years of age was similar to that in patients 65 years of age or less. 6.2 Post-marketing ExperienceThe following adverse reactions have been identified during post-approvaluse of omeprazole. Because these reactions are voluntarily reported from a population of uncertain size, it is not always possible to reliably estimatetheir actual frequency or establish a causal relationship to drug exposure. Body As a Whole: Hypersensitivity reactions including anaphylaxis, anaphylactic shock, angioedema, bronchospasm, interstitial nephritis, urticaria (see also Skin below); fever; pain; fatigue; malaise Cardiovascular: Chest pain or angina, tachycardia, bradycardia,palpitations, elevated blood pressure, peripheral edemaEndocrine: Gynecomastia Gastrointestinal : Pancreatitis (some fatal), anorexia, irritable colon, fecal discoloration, esophageal candidiasis, mucosal atrophy of the tongue, stomatitis, abdominal swelling, dry mouth. During treatment with omeprazole, gastric fundic gland polyps have been noted rarely. These polyps are benign and appear to be reversible when treatment is discontinued. Gastroduodenal carcinoids have been reported in patients with Zollinger-Ellison syndrome on long-term treatment with omeprazole. This finding is believed to be a manifestation of the underlying condition, which is known to be associated with such tumors. Hepatic: Liver disease including hepatic failure (some fatal), liver necrosis (some fatal), hepatic encephalopathy, hepatocellular disease, cholestatic disease, mixed hepatitis, jaundice, and elevations of liver function tests (ALT, AST, GGT, alkaline phosphatase, and bilirubin) Metabolic/Nutritional: Hypoglycemia, hyponatremia, weight gainMusculoskeletal: Muscle weakness, myalgia, muscle cramps, joint pain, leg painNervous System/Psychiatric: Psychiatric and sleep disturbances including depression, agitation, aggression, hallucinations, confusion, insomnia, nervousness, apathy, somnolence, anxiety, and dream abnormalities; tremors, paresthesia, vertigoRespiratory: Epistaxis, pharyngeal pain Skin: Severe generalized skin reactions including toxic epidermal necrolysis (some fatal). Stevens-Johnson syndrome, and erythema multiforme; photosensitivity; urticaria; rash; skin inflammation; pruritus; petechiae; purpura; alopecia; dry skin; hyperhidrosis Special Senses: Tinnitus, taste perversionOcular: Optic atrophy, anterior ischemic optic neuropathy, optic neuritis, dry eye syndrome, ocular irritation, blurred vision, double vision Urogenital: Interstitial nephritis, hematuria, proteinuria, elevated serum creatinine, microscopic pyuria, urinary tract infection, glycosuria, urinary frequency, testicular pain Hematologic: Agranulocytosis (some fatal), hemolytic anemia, pancytopenia, neutropenia, anemia, thromobocytopenia, leukopenia,leucocytosis 7 DRUG INTERACTIONSDrugs metabolized by cytochrome P450 (CYP) Omeprazole can prolong the elimination of diazepam, warfarin and phenytoin, drugs that are metabolized by oxidation in the liver. There have been reports of increased INR and prothrombin time in patients receivingproton pump inhibitors, including omeprazole, and warfarinconcomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with proton pump inhibitors and warfarin may need to be monitored for increases in INR and prothrombin time.Although in normal subjects no interaction with theophylline or propranolol was found, there have been clinical reports of interaction with other drugs metabolized via the cytochrome P450 system (e.g., cyclosporine, disulfiram, benzodiazepines). Patients should be monitored to determine if it is necessary to adjust the dosage of these drugs when taken concomitantly with Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets. Drugs for which gastric pH can affect bioavailability Because of its inhibition of gastric acid secretion, it is theoreticallypossible that omeprazole may interfere with absorption of drugs where gastric pH is an important determinant of their bioavailability (e.g., ketoconazole, ampicillin esters, and iron salts). In the clinical efficacy trials antacids were used concomitantly with the administration ofomeprazole. Concomitant administration of omeprazole and voriconazole (a combinedinhibitor of CYP2C19 and CYP3A4) resulted in more than doubling of the omeprazole exposure. Dose adjustment of omeprazole is not normally required.. When voriconazole (400 mg every 12 hours for one day, then 200 mg for 6 days) was given with omeprazole (40 mg once daily for 7 days) to healthy subjects, it significantly increased the steady-state Cmax and AUC0-24 of omeprazole, an average of 2 times (90% CI: 1.8, 2.6) and4 times (90% CI: 3.3, 4.4) respectively as compared to when omeprazole was given without voriconazole. Antiretroviral Agents Concomitant use of atazanavir and proton pump inhibitors is not recommended. Co-administration of atazanavir with proton pump inhibitors is expected to substantially decrease atazanavir plasma concentrations and thereby reduce its therapeutic effect. Omeprazole has been reported to interact with some antiretroviral drugs. The clinical importance and the mechanisms behind these interactions are not always known. Increased gastric pH during omeprazole treatment may change the absorption of the antiretroviral drug. Other possible interaction mechanisms are via CYP2C19. For some antiretroviral drugs, such as atazanavir and nelfinavir, decreased serum levels have been reported when given together with omeprazole. Following multiple doses of nelfinavir (1250 mg, twice daily) and omeprazole (40 mg, daily), AUC was decreased by 36% and 92%, Cmax by 37% and 89% and Cmin by 39% and 75% respectively for nelfinavir and M8. Following multiple doses of atazanavir (400 mg, daily) and omeprazole (40 mg, daily, 2 hours before atazanavir), AUC was decreased by 94%, Cmax by 96%, and Cmin by 95%. Concomitant administration with omeprazole and drugs such as atazanavir and nelfinavir is therefore not recommended. For otherantiretroviral drugs, such as saquinavir, elevated serum levels have beenreported with an increase in AUC by 82%, in Cmax by 75% and in Cminby 106% following multiple dosing of saquinavir/ritonavir (1000/100 mg)twice daily for 15 days with omeprazole 40 mg daily co-administered days11 to 15. Dose reduction of saquinavir should be considered from thesafety perspective for individual patients. There are also someantiretroviral drugs of which unchanged serum levels have been reportedwhen given with omeprazole.AntimicrobialsOmeprazole 40 mg daily was given in combination with clarithromycin500 mg every 8 hours to healthy adult male subjects. The steady stateplasma concentrations of omeprazole were increased (Cmax, AUC0-24,and T1/2 increases of 30%, 89% and 34% respectively) by the concomitantadministration of clarithromycin. The observed increases in omeprazoleplasma concentration were associated with the following pharmacologicaleffects. The mean 24-hour gastric pH value was 5.2 when omeprazole wasadministered alone and 5.7 when co-administered with clarithromycin.The plasma levels of clarithromycin and 14-hydroxyclarithromycin wereincreased by the concomitant administration of omeprazole. Forclarithromycin, the mean Cmax was 10% greater, the mean Cmin was 27%greater, and the mean AUC0-8 was 15% greater when clarithromycin wasadministered with omeprazole than when clarithromycin was administeredalone. Similar results were seen for 14-hydroxyclarithromycin, the meanCmax was 45% greater, the mean Cmin was 57% greater, and the meanAUC0-8 was 45% greater. Clarithromycin concentrations in the gastrictissue and mucus were also increased by concomitant administration ofomeprazole.Table 3: Clarithromycin Tissue Concentrations2 hours after Dose1Tissue Clarithromycin Clarithromycin+OmeprazoleAntrum 10.48 ± 2.01 (n = 5) 19.96 ± 4.71 (n = 5)Fundus 20.81 ± 7.64 (n= 5) 24.25 ± 6.37 (n = 5)Mucus 4.15 ± 7.74 (n = 4) 39.29 ± 32.79 (n = 4)Mean ± (µg/g)TacrolimusConcomitant administration of omeprazole and tacrolimus may increasethe serum levels of tacrolimus.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyPregnancy Category CThere are no adequate and well-controlled studies on the use ofomeprazole in pregnant women. The vast majority of reported experiencewith omeprazole during human pregnancy is first trimester exposure andthe duration of use is rarely specified, e.g., intermittent versus chronic. Anexpert review of published data on experiences with omeprazole useduring pregnancy by TERIS – the Teratogen Information System –concluded that therapeutic doses during pregnancy are unlikely to pose asubstantial teratogenic risk (the quantity and quality of data were assessedas fair).1Three epidemiological studies compared the frequency of congenitalabnormalities among infants born to women who used omeprazole duringpregnancy to the frequency of abnormalities among infants of womenexposed to H2-receptor antagonists or other controls. A population-basedprospective cohort epidemiological study from the Swedish Medical BirthRegistry, covering approximately 99% of pregnancies, reported on 955infants (824 exposed during the first trimester with 39 of these exposedbeyond first trimester, and 131 exposed after the first trimester) whosemothers used omeprazole during pregnancy.2 In utero exposure toomeprazole was not associated with increased risk of any malformation(odds ratio 0.82, 95% CI 0.50-1.34), low birth weight or low Apgar score.The number of infants born with ventricular septal defects and the numberof stillborn infants was slightly higher in the omeprazole exposed infantsthan the expected number in the normal population. The author concludedthat both effects may be random.A retrospective cohort study reported on 689 pregnant women exposed toeither H2-blockers or omeprazole in the first trimester (134 exposed toomeprazole).3 The overall malformation rate was 4.4% (95% CI 3.6-5.3)and the malformation rate for first trimester exposure to omeprazole was3.6% (95% CI 1.5-8.1). The relative risk of malformations associated withfirst trimester exposure to omeprazole compared with nonexposed womenwas 0.9 (95% CI 0.3-2.2). The study could effectively rule out a relative risk greater than 2.5 for all malformations. Rates of preterm delivery or growth retardation did not differ between the groups.A controlled prospective observational study followed 113 women exposed to omeprazole during pregnancy (89% first trimester exposures).4 The reported rates of major congenital malformations was 4% for the omeprazole group, 2% for controls exposed to nonteratogens, and 2.8% in disease-paired controls (background incidence of major malformations 1-5%). Rates of spontaneous and elective abortions, preterm deliveries, gestational age at delivery, and mean birth weight did not differ between the groups. The sample size in this study has 80% power to detect a 5-fold increase in the rate of major malformation.Several studies have reported no apparent adverse short term effects on the infant when single dose oral or intravenous omeprazole was administered to over 200 pregnant women as premedication for cesarean section under general anesthesia.Hypermagnesemia has been reported in infants whose mothers were using magnesium-containing antacid products chronically in high doses. Reproduction studies conducted with omeprazole in rats at oral doses up to 28 times the human dose of 40 mg/day (based on body surface area) and in rabbits at doses up to 28 times the human dose (based on body surface area) did not show any evidence of teratogenicity. In pregnant rabbits, omeprazole at doses about 2.8 to 28 times the human dose of 40 mg /day (based on body surface area) produced dose-related increases in embryo-lethality, fetal resorptions, and pregnancy loss. In rats treated with omeprazole at doses about 2.8 to 28 times the human dose (based on body surface area), dose-related embryo/fetal toxicity and postnatal developmental toxicity occurred in offspring. [See Nonclinical Toxicology (13.2)]There are no adequate and well-controlled studies in pregnant women. Because animal studies and studies in humans cannot rule out the possibility of harm, Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets should be used during pregnancy only if the potential benefit to pregnant women justifies the potential risk to the fetus.8.3 Nursing MothersOmeprazole concentrations have been measured in breast milk of a woman following oral administration of 20 mg. The peak concentration of omeprazole in breast milk was less than 7% of the peak serum concentration. The concentration will correspond to 0.004 mg of omeprazole in 200 mL of milk. Because omeprazole is excreted in human milk, because of the potential for serious adverse reactions in nursing infants from omeprazole, and because of the potential for tumorigenicity shown for omeprazole in rat carcinogenicity studies, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. In addition, sodium bicarbonate and magnesium hydroxide should be used with caution in nursing mothers.8.4 Pediatric UseThe safety and effectiveness of Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets in pediatric patients less than 18 years of age have not been established.8.5 Geriatric UseOmeprazole was administered to over 2000 elderly individuals (≥ 65 years of age) in clinical trials in the U.S. and Europe. There were no differences in safety and effectiveness between the elderly and younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.Pharmacokinetic studies with buffered omeprazole have shown the elimination rate was somewhat decreased in the elderly and bioavailability was increased. The plasma clearance of omeprazole was 250 mL/min (about half that of young subjects). The plasma half-life averaged one hour, about twice that in nonelderly, healthy subjects taking Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets. However, no dosage adjustment is necessary in the elderly. [See Clinical Pharmacology (12.3)] 8.6 Hepatic ImpairmentConsider dose reduction, particularly for maintenance of healing of erosive esophagitis. [See Clinical Pharmacology (12.3)]8.7 Renal ImpairmentNo dose reduction is necessary. However, Omeprazole / Sodium Bicarbonate / Magnesium Hydroxide Tablets contains magnesium hydroxide (143 mg of Mg2+); therefore, magnesium levels should be closely monitored when using this product in patients with renal impairment. [See Clinical Pharmacology (12.3)]。
奥美拉唑

3.代谢/内分泌系统:长期应用奥美拉唑可导致维生素B12缺乏。
4.致癌性:动物实验表明奥美拉唑可引起胃底部和胃体部主要内分泌细胞-肠嗜铬细胞增生,长期用药还可 发生胃部类癌。
5.其他:可有皮疹、男性乳腺发育、溶血性贫血等。
1.对奥美拉唑过敏者。 2.严重肾功能不全者。 3.婴幼儿。 4.孕妇、哺乳妇女禁用。
奥美拉唑
目录
01 化合物简介
03 药物说明
02 药典标准 04 专家点评
奥美拉唑,主要用于十二指肠溃疡和卓-艾综合征,也可用于胃溃疡和反流性食管炎;静脉注射可用于消化性 溃疡急性出血的治疗。与阿莫西林和克林霉素或与甲硝唑与克拉霉素合用,以杀灭幽门螺杆菌。
化合物简介
物化性质
基本信息
安全信息ቤተ መጻሕፍቲ ባይዱ
中文名称:奥美拉唑 中文别名:埃索美拉唑镁杂质F;奥克;奥咪拉唑;奥西康;福尔丁奥美拉唑;洛赛克;欧麦亚砜;沃必唑; 渥咪哌唑;涯米哌唑;亚砜咪唑;安胃哌唑;甲氧磺唑 英文名称:(R)-omeprazole 英文别名:LOSEC;MEPRAL;GASTROGARD;OMEPRAZOLE;Loec;Moprial;Omeprazolum CAS号:-89-8 分子式:C17H19N3O3S 结构式: 分子量:345. 精确质量:345. PSA:96.
【检查】二氯甲烷溶液的澄清度与颜色取本品0.5g,加二氯甲垸25ml溶解,溶液应澄清无色;如显色,立即 照紫外-可见分光光度法(附录IV A),在440nm的波长处测定吸光度,不得过0.10。有关物质避光操作。
药物说明
01
分类
02
性状
03
剂型
奥美拉唑杂质分析

奥美拉唑杂质奥美拉唑( omeprazole),化学名为5-甲氧基-2 - [ [ ( 4-甲氧基-3,5-二甲基-2-吡啶基)甲基]亚硫酰基]-1-苯并咪唑,是英国AstraZeneca公司研发的质子泵抑制剂类抗溃疡药,临床主要用于治疗消化道溃疡和反食性胃炎。
欧洲药典(European Pharmacopeia ,缩写为EP)共列出其杂质9个。
奥美拉唑及其九由于合成方法和步骤的不同,所产生的杂质也有所不同。
根据本实验所采用的方法及所使用的原料情况,现分析其可能产生杂质如下:1. Omeprazole Imp. A (EP):化学名5-Methoxy-1H-benzimidazole-2-thiol ,是合成奥美拉唑中间体奥美拉唑硫醚的一个原料。
该方法的具体步骤为:500ml 三颈圆底烧瓶1只,安装搅拌轮,100。
C 温度计和加液管置于恒温水槽中。
烧瓶内加入苯并咪唑物(5-Methoxy-1H-benzimidazole-2-thiol )26.8g (0.15mol )、去离子水30ml 、NaOH6.0g(0.15mol)和乙醇200ml ,搅拌溶解后加入氯甲基物(2-Chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride )33.3g (0.15mol ),加热搅拌回流3h 。
反应完毕,冷却,过滤,滤液用乙酸调至pH=7,加入去离子水适量,用乙酸乙酯萃取4次,合并有机层,水洗,无水Na 2SO 4干燥。
过滤,滤液减压蒸馏,剩余物用乙酸乙酯重结晶得米黄色固体,即奥美拉唑硫醚。
(李鸿运.奥美拉唑合成工艺研究.哈尔滨商业大学学报(自然科学版),2006).本实验所用方法与其所用方法与其相似,但有所改进,将氢氧化钠得使用量加大3倍,用乙酸调PH=8~9。
所以反应中有该杂质。
由于其为原料,所以较易获得。
2. Omeprazole Imp. B (EP):化学名2-[(R ,S)-[(3,5-Dimethylpyridin-2-yl)methyl]sulphinyl]-5-methoxy-1H-benzimi dazole ,是另一种方法所产生的副产物,该方法使用2-氯甲基-3,5-二甲基-4-硝基吡啶-N-氧化物与苯并咪唑物进行反应,在甲醇钠进行取代时未反应完全会产生该杂质,(颜国和,王飞武.奥美拉唑合成路线图解[J].中国医药工业杂志,1991,2 2( 6 ) :2 83-28)本反应与其使用方法不同,反应历程也不相同,最终结果未检测到该杂质,所以不对该杂质进行研究。
奥美拉唑溶出度
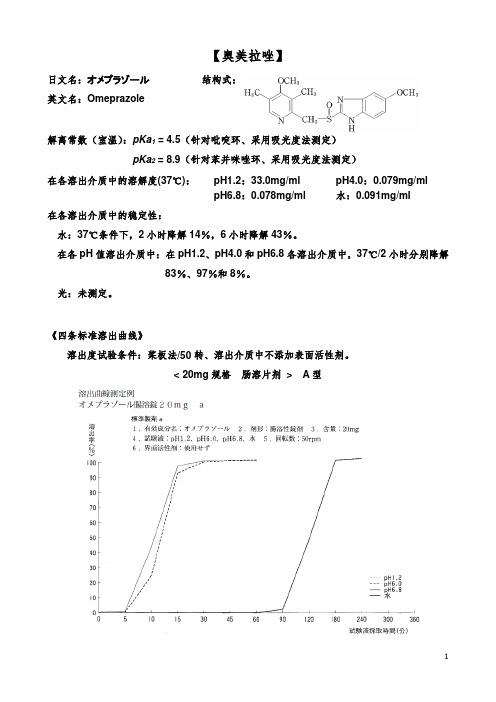
【奥美拉唑】日文名:オメプラゾール英文名:Omeprazole结构式:解离常数(室温):pKa1 = 4.5(针对吡啶环、采用吸光度法测定)pKa2 = 8.9(针对苯并咪唑环、采用吸光度法测定)在各溶出介质中的溶解度(37℃):pH1.2:33.0mg/ml pH4.0:0.079mg/mlpH6.8:0.078mg/ml 水:0.091mg/ml在各溶出介质中的稳定性:水:37℃条件下,2小时降解14%,6小时降解43%。
在各pH值溶出介质中:在pH1.2、pH4.0和pH6.8各溶出介质中,37℃/2小时分别降解83%、97%和8%。
光:未测定。
《四条标准溶出曲线》溶出度试验条件:桨板法/50转、溶出介质中不添加表面活性剂。
< 20mg规格肠溶片剂> A型< 20mg规格肠溶片剂> B型《质量标准》【在pH1.2介质中】取本品,照溶出度测定法(桨板法),以盐酸溶液(氯化钠2.0g溶于盐酸7.0ml中,加水稀释至1000ml,即得)900ml为溶剂,转速为每分钟50转,依法操作,经120分钟时,取溶液适量滤过,弃去至少10ml初滤液,精密量取续滤液适量,加溶出介质稀释制成每1ml中含22μg的溶液,作为供试品溶液。
另精密称取奥美拉唑对照品0.022g,置50ml量瓶中,加乙醇溶解并稀释至刻度,摇匀,精密量取5ml,置100ml 量瓶中,加溶出介质稀释至刻度,摇匀,作为对照品溶液。
取上述两种溶液照紫外-可见分光光度法,分别在323nm波长处测定吸光度,计算每片溶出量,不得过标示量的5%,应符合规定。
【在pH6.8介质中】取本品,照溶出度测定法(桨板法),以磷酸盐缓冲液(pH6.8) 900ml 为溶剂,转速为每分钟50转,依法操作,经15分钟时,取溶液适量滤过,弃去至少10ml 初滤液,精密量取续滤液适量,加溶出介质稀释制成每1ml中含22μg的溶液,作为供试品溶液。
另精密称取奥美拉唑对照品0.022g,置50ml量瓶中,加乙醇溶解并稀释至刻度,摇匀,精密量取5ml,置100ml量瓶中,加溶出介质稀释至刻度,摇匀,作为对照品溶液。
美国药典USP-1478549奥美拉唑镁的msds

Material Safety Data Sheet USP Reference Standards are sold for chemical test and assay purposes only, and NOT for human consumption. The information contained herein is applicable solely to the chemical substance when used as a USP Reference Standard and does not necessarily relate to any other use of the substance described, (i.e. at different concentrations, in drug dosage forms, or in bulk quantities). USP Reference Standards are intended for use by persons having technical skill and at their own discretion and risk. This information has been developed by USP staff from sources considered reliable but has not beenindependently verified by the USP. Therefore, the USP Convention cannot guarantee the accuracy of the information in these sources nor should the statements contained herein be considered an official expression. NO REPRESENTATION OR WARRANTY, EXPRESS OR IMPLIED, INCLUDING THE WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE is made with respect to the information contained herein.ATTENTION !12601 Twinbrook Parkway, Rockville, MD 20852 USA Phone Calls:301-816-81298 a.m. to 5 p.m. EST Mon. - Fri.OMEPRAZOLE MAGNESIUM Catalog Number:1478549Revision Date:March 20, 2007SECTION 2 - HAZARD INFORMATIONCommon Name:Omeprazole Magnesium SECTION 1 - PRODUCT AND COMPANY IDENTIFICATIONSECTION 3 - COMPOSITION/INFORMATION ON INGREDIENTSEMERGENCY OVERVIEW - Allergen.Adverse Effects:Adverse effects of omeprazole may include cough; nasal or ear congestion; chills, fever, or sore throat; loss of voice;runny nose; difficulty breathing; body aches or pains; heart burn, diarrhea, gas, skin rash or itching, unusual tiredness,dizziness, constipation, headache, and nausea or vomiting. Possible allergic reaction to material if inhaled, ingestedor in contact with skin.Overdose Effects:Symptoms of omeprazole overdose may include blurred vision, confusion, increased sweating, drowsiness, drymouth, flushing, headache, severe stomach pain, nausea or vomiting, and fast or irregular heartbeat.Acute:Possible eye, skin, gastrointestinal and/or respiratory tract irritation.Chronic:Possible hypersensitization.Medical Conditions Aggravated by Exposure:Hypersensitivity to the material and chronic liver disease (current or history of).Cross Sensitivity:n/fTarget Organs:Gastrointestinal tractFor additional information on toxicity, see Section 11.Common Name:Omeprazole MagnesiumFormula:C34H36MgN6O6S2Manufacturer:U. S. PharmacopeiaResponsible Party:Reference Standards Technical ServicesMailing Address:12601 Twinbrook Parkway, Rockville, MD 20852 USAPhone:301-816-8129Hours:8 a.m. to 5 p.m. EST Mon. - Fri.Product Use:USP Reference Standards and Authentic Substances are used for chemical tests and assays in analytical,clinical, pharmaceutical, and research laboratories.Synonym:n/fChemical Name:5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, magnesium salt (2:1) CAS:95382-33-5RTECS Number:n/fChemical Family:Substituted benzimidazoleTherapeutic Category:Gastric acid secretory depressantComposition:Pure MaterialSECTION 4 - FIRST AID MEASURESInhalation:May cause irritation. Avoid inhalation. Remove to fresh air.Eye:May cause irritation. Avoid contact. Flush with copious quantities of water for at least 15 minutes.Skin:May cause irritation and sensitization. Avoid contact. Flush with copious quantities of soap and water.Ingestion:May cause irritation. Flush out mouth with water.General First Aid Procedures:Remove from exposure. Remove contaminated clothing. Persons developing serious hypersensitivity(anaphylactic) reactions must receive immediate medical attention. If person is not breathing giveartificial respiration. If breathing is difficult give oxygen. Obtain medical attention.Note to PhysiciansOverdose Treatment:Treatment of omeprazole overdose should be symptomatic and supportive and may include the following:1. Administer activated charcoal as a slurry.2. Sinus tachydysrhythmias do not need to be routinely treated unless patient is hemodynamically unstable.3. Omeprazole is not readily dialyzable. [Meditext 2007 & USP DI 2007]SECTION 5 - FIREFIGHTING MEASURESExtinguisher Media:Water spray, dry chemical, carbon dioxide or foam as appropriate for surrounding fire and materials.Fire and Explosion Hazards:This material is assumed to be combustible. As with all dry powders it is advisable to ground mechanical equipment in contact with dry material to dissipate the potential buildup of static electricity.Firefighting Procedures:As with all fires, evacuate personnel to a safe area. Firefighters should use self-contained breathingequipment and protective clothing.SECTION 6 - ACCIDENTAL RELEASE MEASURESSpill Response:Wear approved respiratory protection, chemically compatible gloves and protective clothing. Wipe up spillage or collect spillage using a high efficiency vacuum cleaner. Avoid breathing dust. Place spillage in appropriately labeledcontainer for disposal. Wash spill site.SECTION 7 - HANDLING AND STORAGEHandling:As a general rule, when handling USP Reference Standards avoid all contact and inhalation of dust, mists, and/or vapors associated with the material. Wash thoroughly after handling.Storage:Store in tight, light-resistant container as defined in the USP-NF. This material should be handled and stored per label instructions to ensure product integrity. Store in a refrigerator.SECTION 8 - EXPOSURE CONTROL / PERSONAL PROTECTIONEngineering Controls:Engineering controls such as exhaust ventilation are recommended.Respiratory Protection:Use a NIOSH-approved respirator, if it is determined to be necessary by an industrial hygiene surveyinvolving air monitoring. In the event that a respirator is not required, an approved dust mask should be used. Gloves:Chemically compatibleEye Protection:Safety glasses or gogglesProtective Clothing:Protect exposed skin.Exposure Limits:Industry: 0.5 mg/m3 (omeprazole)SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIESProperties as indicated on the MSDS are general and not necessarily specific to the USP Reference Standard Lot provided. Appearance and Odor:White to almost white crystalline powderOdor Threshold:n/fpH:Approximately 10.5 (aqueous suspension)Melting Range:n/fBoiling Point:n/fFlash Point:n/fAutoignition Temperature:n/fEvaporation Rate:n/fUpper Flammability Limit:n/fLower Flammability Limit:n/fVapor Pressure:n/fVapor Density:n/fSpecific Gravity:n/fSolubility in Water:Very slightly solubleFat Solubility:n/fOther Solubility:Soluble in methanolPartition Coefficient: n-octanol/water:n/fPercent Volatile:n/fReactivity in Water:n/fExplosive Properties:n/fOxidizing Properties:n/fFormula:C34H36MgN6O6S2Molecular Weight:713.12Oral Rat:LD50: >2000 mg/kgOral Mouse:n/fNTP:No IARC:No OSHA:NoListed as a Carcinogen by: Irritancy Data:n/f SECTION 11 - TOXICOLOGICAL PROPERTIESOther Toxicity Data:n/fCorrosivity:n/f Sensitization Data:Omeprazole is a strong sensitizer in guinea pigs. (Guinea pig maximization test)Other Carcinogenicity Data:No evidence of carcinogenicity was found in mice orally administered up to 140 mg/kg/day omeprazolefor 66 weeks. In two 2-year studies in rats, omeprazole given in doses up to 140.8 mg/kg/day causedgastric carcinoid tumors and enterochromaffin-like (ECL) cell hyperplasia in a dose-dependent manner in both males and females.Mutagenicity Data:Omeprazole produced clastogenic effects in an in vitro human lymphocyte chromosomal aberration assay, in oneof two in vivo mouse micronucleus tests, and in an in vivo bone marrow cell chromosomal aberration assay. Omeprazole was negative in the in vitro Ames Salmonella typhimurium assay, an in vitro mouse lymphoma cell forward mutation assay, and an in vivo rat liver DNA damage assay.Reproductive and Developmental Effects:Sporadic instances of developmental abnormalities in infants born to women who receivedomeprazole during pregnancy have been reported. Epidemiological studies have not foundan association between the use of omeprazole during pregnancy and an increased risk ofbirth defects.Omeprazole did not impair fertility in rats at parenteral doses of up to 138 mg/kg/day. Nobirth defects were seen in pregnant rabbits and rats administered omeprazole in doses up to69 mg/kg/day and 138 mg/kg/day, respectively.In rabbits, 6.9 - 69.1 mg/kg/day omeprazole produced dose-related increases in embryo-lethality, fetal resorptions, and pregnancy disruption. In rats, dose-related embryo/fetaltoxicity and post natal developmental toxicity were observed in the rat offspring of parentstreated with 13.8 - 138 mg/kg/day.SECTION 12 - ECOLOGICAL INFORMATIONEcological Information:Omeprazole sodium, a related compound, may cause long-term adverse effects in the aquatic environment andis not readily biodegradable.SECTION 13 - DISPOSAL CONSIDERATIONSDisposal:Dispose of waste in accordance with all applicable Federal, State and local laws.SECTION 14 -TRANSPORT INFORMATIONShipping Name:n/fClass:n/f Stable?Yes Conditions to Avoid:Avoid exposure to light.Incompatibilities:n/fDecomposition Products:When heated to decomposition material emits toxic fumes of SOx and NOx. Emits toxic fumes under fireconditions.Hazardous Polymerization?No SECTION 10 - STABILITY AND REACTIVITYUN Number:n/fPacking Group:n/fAdditional Transport Information:n/fSECTION 15 - REGULATORY INFORMATION U.S. Regulatory Information:n/fInternational Regulatory Information:Risk Phrases: R43, R52/53Safety Phrases: S37, S61SECTION 16 - OTHER INFORMATIONRevision:20-Mar-07Previous Revision Date:05-Feb-07。
硫糖铝混悬凝胶奥美拉唑镁肠溶片(洛赛克)

硫糖铝混悬凝胶奥美拉唑镁肠溶片(洛赛克)硫糖铝混悬凝胶硫糖铝混悬凝胶,适应症为胃溃疡、十二指肠溃疡、急性及有症状的慢性胃炎、FANS胃病、食管溃疡。
药品名称硫糖铝混悬凝胶。
药品类型处方药。
用途分类粘膜保护药。
成份:本品主要成份及其化学名称:硫糖铝。
分子式:C12H54Al16O75S8分子量:2086.75性状:本品为白色或类白色的黏稠混悬液。
适应症:胃溃疡、十二指肠溃疡、急性及有症状的慢性胃炎、FANS胃病、食管溃疡。
规格:5ml:1g。
用法用量:本品为特殊的混悬凝胶剂,具有很强的生物粘附性,每日服用两次即可保证其临床疗效。
1)[u]一般用量[/u]:每日两次,每次一袋(1g),晨起饭前1小时及晚间休息前空腹服用。
2)[u]维持及巩固用量[/u]:可酌情减半,每次服用量不变,服药次数可减少。
如每日服用一次,最好在晚间服用。
每次服用后可服用饮料一杯。
不良反应:长期服用偶见便秘。
少见或偶见口干、皮疹、瘙痒、头晕及失眠等。
禁忌:对本品过敏者禁用。
注意事项1)如服用本品过量或出现严重不良反应,请立即就医。
2)当药品性状发生改变时禁止使用。
3)本品入口会产生一种独特的涩味,若想消除这种感觉,可服用少量清水或其它饮料。
4)肝肾功能不全者慎用本品。
孕妇及哺乳期妇女用药妊娠前三个月、习惯性便秘者慎用,其它时期孕妇及哺乳期妇女必需服用时,请遵医嘱。
儿童用药儿童用量请咨询医师或药师。
儿童必须在成人监护下使用。
请将药品放在儿童接触不到的地方。
老年用药:无特殊规定,见用法用量的具体描述。
药物相互作用1)本品与四环素类抗生素可以在体内形成复杂的盐,因此可降低此类化合物的吸收和利用。
2)由于本品可影响某些药物的生物利用度,如果在服用本品的同时需服用其它药品,请至少间隔两小时使用,或遵医嘱。
药物过量:按照本品推荐量使用,尚未发现药物过量现象,如出现药物过量请立即就医。
药理毒理:硫糖铝是含有氢氧化铝的硫酸蔗糖复合物,在酸性条件下可离解为带负电荷的八硫酸蔗糖,能聚合成胶体直接在溃疡面或炎症处形成一层薄膜,保护溃疡或炎症粘膜,抵御胃酸的侵袭。
奥美拉唑镁EP

EUROPEAN PHARMACOPOEIA 7.0OmeprazolemagnesiumG.9-methoxy-1,3-dimethyl-12-thioxopyrido[1′,2′:3,4]imidazo-[1,2-a ]benzimidazol-2(12H)-one,H.2-[(RS )-[(4-chloro-3,5-dimethylpyridin-2-yl)methyl]sulfinyl]-5-methoxy-1H-benzimidazole,I.4-methoxy-2-[[(5-methoxy-1H -benzimidazol-2-yl)sulfonyl]methyl]-3,5-dimethylpyridine 1-oxide.01/2009:2374corrected 6.7OMEPRAZOLE MAGNESIUM Omeprazolummagnesicum C 34H 36MgN 6O 6S 2M r 713[95382-33-5]DEFINITION Magnesium bis[5-methoxy-2-[(RS )-[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl]-1H -benzimidazol-1-ide].It contains a variable quantity of water.Content :97.5per cent to 102.0per cent (anhydrous substance).CHARACTERS Appearance :white or almost white,hygroscopic powder.Solubility :very slightly soluble in water,sparingly soluble in methanol,practically insoluble in heptane.IDENTIFICATION Carry out either tests A,B,C or tests A,B,D.A.Optical rotation (2.2.7):−0.10°to +0.10°.Dissolve 0.250g in methanol R and dilute to 25.0mL with the same solvent.B.Infrared absorption spectrophotometry (2.2.24).Comparison :omeprazole magnesium CRS .C.Atomic absorption spectrometry (2.2.23)as described in the test for magnesium.The test solution shows the absorption maximum at 285.2nm.D.Ignite about 0.5g of the substance to be examined according to the procedure for the sulfated ash test (2.4.14).Dissolve the residue in 10mL of water R .2mL of this solution gives the reaction of magnesium (2.3.1).TESTSAbsorbance (2.2.25):maximum 0.10at 440nm.Dissolve 0.500gin methanol R and dilute to 25.0mL with thesame solvent.Filter the solution through a membrane filter(nominal pore size 0.45μm).Related substances .Liquid chromatography (2.2.29):use thenormalisation procedure.Prepare the solutions immediately before use.Test solution .Dissolve 3.5mg of the substance to be examined in the mobile phase and dilute to 25.0mL with the mobile phase.Reference solution (a).Dissolve1mgof omeprazole CRS and1mg of omeprazole impurity D CRS in the mobile phase and dilute to 10.0mL with the mobile phase.Reference solution (b).Dissolve 3mg of omeprazole for peakidentification CRS (containing impurity E)in the mobile phaseand dilute to 20.0mL with the mobile phase.Reference solution(c).Dilute1.0mL of the test solution to100.0mL with the mobile phase.Dilute 1.0mL of this solution to 10.0mL with the mobile phase.Column :—size :l =0.125m,Ø=4.6mm;—stationary phase :octylsilyl silica gel for chromatography R (5μm).Mobilephase :mix 27volumes of acetonitrile R and 73volumes of a 1.4g/L solution of disodium hydrogen phosphate Rpreviously adjusted to pH 7.6with phosphoric acid R .Flow rate :1mL/min.Detection :spectrophotometer at 280nm.Injection :40μL.Run time :5times the retention time of omeprazole.Identification of impurities :—use the chromatogram supplied with omeprazole for peak identification CRS and the chromatogram obtained with reference solution (b)to identify the peak due to impurity E;—use the chromatogram obtained with reference solution (a)to identify the peak due to impurity D.Relativeretention with reference to omeprazole (retention time =about 9min):impurity E =about 0.6,impurity D =about0.8.System suitability :reference solution (a):—resolution :minimum 3.0between the peaks due to impurityD and omeprazole;if necessary,adjust the pHofthe aqueous part of the mobile phase or its proportion of acetonitrile;an increase in the pH will improve the resolution.Limits :—impurities D,E :for each impurity,maximum 0.1per cent;—unspecifiedimpurities :for each impurity,maximum 0.10percent;—total :maximum 0.5per cent;—disregardlimit :half the area of the principal peak in thechromatogram obtained with reference solution (c)(0.05per cent).Magnesium:3.30per cent to 3.55per cent (anhydroussubstance).Atomic absorption spectrometry (2.2.23,Method I ).Testsolution .Dissolve 0.250g in 20.0mL of a 103g/L solution of hydrochloric acid R by slow addition of the acid and dilute to100.0mL with water R .Dilute10.0mL of the solution to200.0mL with water R .To 10.0mL of this solution add 4mL of lanthanum chloride solution R and dilute to 100.0mL with water R .Reference solutions.Prepare the reference solutions using magnesium standard solution (1000ppm Mg)R ,diluting with a mixture of 1mL of a 103g/L solution of hydrochloric acid R and 1000.0mL of water R .General Notices (1)apply to all monographs and other texts 2623Omeprazole sodium EUROPEAN PHARMACOPOEIA7.0Wavelength :285.2nm.Water (2.5.12):7.0per cent to 10.0per cent,determined on0.200g.ASSAYLiquid chromatography (2.2.29).Buffer pH 11.0.Mix 11mLof a 95.0g/L solution of trisodiumphosphate dodecahydrate R and 22mL of a 179.1g/L solution of disodium hydrogen phosphate R .Dilute to 100.0mL with water R .Test solution .Dissolve 10.0mg of the substance to be examined in about 10mL of methanol R .Add 10mL of buffer pH 11.0and dilute to 200.0mL with water R .Reference solution .Dissolve10.0mg of omeprazole CRS inabout 10mL of methanol R .Add 10mL of buffer pH 11.0and dilute to 200.0mL with water R .Column :—size :l =0.125m,Ø=4mm;—stationary phase :octylsilyl silica gel for chromatography R(5μm).Mobile phase :mix 35volumes of acetonitrile R and 65volumesof a 1.4g/L solution of disodium hydrogen phosphate R previously adjusted to pH 7.6with phosphoric acid R .Flow rate :1mL/min.Detection :spectrophotometer at 280nm.Injection :20μL.Run time :1.5times the retention time of omeprazole.Retention time :omeprazole =about 4min.Calculate the percentage content of C 34H 36MgN 6O 6S 2from the declared content of omeprazole CRS .1g of omeprazole is equivalent to 1.032g of omeprazole magnesium.STORAGE In an airtight container,protected from light.IMPURITIES Specified impurities :D,E .Other detectable impurities (the following substances would,if present at a sufficient level,be detected by one or other of the tests in the monograph.They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034).It is therefore not necessary to identify these impurities for demonstration of compliance.See also 5.10.Control of impurities in substances for pharmaceutical use ):A,B,C.A.5-methoxy-1H-benzimidazole-2-thiol,B.R =H,X =SO:2-[(RS )-[(3,5-dimethylpyridin-2-yl)methyl]sulfinyl]-5-methoxy-1H -benzimidazole,C.R =OCH 3,X =S:5-methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfanyl]-1H -benzimidazole,D.R =OCH 3,X =SO 2:5-methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfonyl]-1H-benzimidazole, E.4-methoxy-2-[[(RS )-(5-methoxy-1H -benzimidazol-2-yl)sulfinyl]methyl]-3,5-dimethylpyridine 1-oxide.01/2011:1032OMEPRAZOLE SODIUM Omeprazolumnatricum C 17H 18N 3NaO 3S,H 2O Mr 385.4[95510-70-6]DEFINITIONSodium5-methoxy-2-[(RS )-[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl]-1H -benzimidazole monohydrate.Content :98.0per cent to 101.0per cent (anhydrous substance).CHARACTERSAppearance :white or almost white,hygroscopic powder.Solubility:freely soluble in water and in ethanol (96per cent),soluble in propylene glycol,very slightly soluble in methylenechloride.IDENTIFICATIONA.Opticalrotation (2.2.7):−0.10°to +0.10°,determined on solution S.B.Infrared absorption spectrophotometry (2.2.24).Preparation :dissolve 0.50g of the substance to be examinedin1.50mL of water R ,add 3.0mL of methanol R andstir;while stirring,adjust to pH 8-9by adding,dropwise,diluteacetic acid R (about 0.4mL);continuestirringuntil crystallisation and isolate the crystalline precipitate by filtration;wash with 5mL of water R ,then 2mL ofmethanol R ,and dry in vacuo at 40°C for parison :omeprazole CRS .Ifthe spectra obtained in the solid state show differences,dissolve the crystalline precipitate and the reference substance separately in methanol R ,evaporate to dryness and record new spectra using the residues.C.Ignite 1g and cool.Add 1mL of water R to the residue andneutralise with hydrochloric acid R .Filter and dilute the filtrate to 4mL with water R .0.1mL of the solution givesreaction (b)of sodium (2.3.1).TESTSSolution S .Dissolve 0.50g in carbon dioxide-free water R anddilute to 25mL with the same solvent.Appearance of solution .Solution S is clear (2.2.1)and not moreintensely coloured than reference solution B 6(2.2.2,Method II ).pH (2.2.3):10.3to 11.3for solution S.Related substances .Liquid chromatography (2.2.29).Preparesolutions immediately before use.Test solution .Dissolve 3mg of the substance tobe examined inthe mobile phase and dilute to 25.0mL with the mobile phase.Referencesolution (a).Dissolve 1mg of omeprazole CRSand1mg of omeprazole impurity D CRS in the mobile phase and dilute to 10.0mL with the mobile phase.2624See the information section on general monographs (cover pages)。
奥美拉唑制剂的调查

对奥美拉唑药物的调查奥美拉唑,是一种H+,K+—ATP酶抑制剂,可以抑制胃酸的分泌,主要用于十二指肠溃疡和卓—艾综合征,也可用于胃溃疡和反流性食管炎,静脉注射可用于消化性溃疡急性出血的治疗。
同时与阿莫西林和克林霉素或与甲硝唑与克拉霉素合用,可以用于杀灭幽门螺杆菌。
奥美拉唑,分子式为C17H19N3O3S,分子量为345,理化性质包括:为白色或类白色结晶性粉末;无臭;遇光易变色。
在二氯甲烷中易溶,在甲醇或乙醇中略溶,在丙酮中微溶,在水中不溶;在0.lmol/L氢氧化钠溶液中溶解。
结构式如下:奥美拉唑结构式一、奥美拉唑的制剂种类:现在市场上的奥美拉唑,制剂种类主要有两大种类:一种是奥美拉唑肠溶剂,包括片剂和胶囊剂;另一种是注射用无菌粉末(注射用奥美拉唑钠)。
二、奥美拉唑的生产企业(部分):通过检索,整理出不同剂型的部分生产企业,如下:1、奥美拉唑肠溶剂生产企业:2、奥美拉唑注射用剂:三、奥美拉唑的申报情况:通过“药智网”(http://www。
/)对奥美拉唑进行检索,药品的注册和受理总计862条,其中多数药品属于补充申请,并且已进入审批流程,大部分药品属于审批完毕;中国新药批准信息(1978~2003)有41条,大多属于第二类和第四类新药;药品转让信息11条,原料药、针粉剂、胶囊剂均有转让;中国临床试验记录41条,多数处于进行中,少数已完成.通过专利信息平台,对奥美拉唑相关专利进行检索,共检索出353件专利,包括发明专利305件,实用新型4件,外观设计44件。
发明专利多与奥美拉唑的制备方法和制备工艺以及其他新应用有关,实用新型多与片剂类型改进有关,实用新型多与药品的外包装有关.通过检索,我们可以发现,许多企业不光单纯的研发奥美拉唑药物,而且还与其他药物联合用药,用于预防或者更好的治疗胃溃疡,例如:北京韩美药品有限公司申报的奥美拉唑阿司匹林肠溶胶囊,主要用于预防心血管疾病患者减少阿司匹林诱发的胃溃疡风险;吉林益民堂制药有限公司申报的奥美拉唑碳酸氢钠干混悬剂,可更好地治疗十二指肠溃疡、胃溃疡、糜烂性食管炎等。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
OMEPRAZOLE MAGNESIUM CRSêX Xi : IrritantTrade name:OMEPRAZOLE MAGNESIUM CRSUse:For laboratory tests and assays only, as described in the EuropeanPharmacopoeia.Directions for use:For any questions: www.edqm.eu/hd (HelpDesk)Company identification:EDQM7, Allée Kastner CS 30026F-67081 Strasbourg FRANCETel. +33 (0)3 88 41 20 35Fax. + 33 (0)3 88 41 27 71Risk Phrases:Irritating to eyes, respiratory system and skin. - May cause sensitization by skincontact. - Harmful to aquatic organisms, may cause long-term adverse effects inthe aquatic environment.Adverse human health effects:Metabolism and nutrition disorders.Breathing difficulties. Gastrointestinal disorders. Fever. Sore throat. Nausea.Vomiting. Exposure may produce an allergic reaction.Components:This product is hazardous.Substance name Contents CAS No EC No Annex No ClassificationOmeprazole magnesium:95382-33-5---------------Xi; R36/37/38R43R52-53First aid measures- Inhalation:Assure fresh air breathing. Rest. If you feel unwell, seek medical advice.- Skin contact:Remove affected clothing and wash all exposed skin area with mild soap andwater, followed by warm water rinse.- Eye contact:Rinse immediately with plenty of water. Obtain medical attention if pain, blinking,tears or redness persist.- Ingestion:Rinse mouth. If swallowed, seek medical advice immediately and show thiscontainer or label.In case of reactions described in hazards identification or other severe, immediate or persisting symptoms seek medical advice and call the nearest poison centre. Show the label and this safety data sheet.Extinguishing mediaOMEPRAZOLE MAGNESIUM CRS- Suitable extinguishing media:Water spray. Carbon dioxide. Dry powder.- Unsuitable extinguishing media:Do not use a heavy water stream.Surrounding fires:Use water spray or fog for cooling exposed containers.Protection against fire:Do not enter fire area without proper protective equipment, including respiratoryprotection.Special procedures:Avoid (reject) fire-fighting water to enter environment. Prevent entry to sewers andpublic waters.Hazardous combustion products:Incomplete combustion will generate poisonous carbon monoxide, carbon dioxideand other toxic gases.General precautions:Remove ignition sources. Evacuate area.Personal precautions:Spill should be handled by trained cleaning personnel properly equipped withrespiratory and eye protection.Clean up methods:To clean the floor and all objects contaminated by this material, use : Water. /Detergent. Avoid dust production. Ensure adequate ventilation.Environmental precautions:Prevent entry to sewers and public waters. Notify authorities if product enterssewers or public waters.Personal protection:Avoid all unnecessary exposure. Ensure prompt removal from eyes, skin andclothing.Technical protective measures:Material should be handled in a laboratory hood whenever possible.Handling:Handle in accordance with good industrial hygiene and safety procedures. Storage:OMEPRAZOLE MAGNESIUM CRS is not intended for long-term storage. Keepcontainer tightly closed in a cool, well ventilated place.Storage - away from:All heat sources, including direct sunlight. Open flame. Sources of ignition. Sparks.Incompatible materials, see §10Personal protection= 8 :- Respiratory protection:Wear approved mask. (P2)In case of insufficient ventilation, wear suitable respiratory equipment.- Hand protection:Wear suitable gloves resistant to chemical penetration.- Skin protection:Wear suitable protective clothing.- Eye protection:Chemical goggles or safety glasses.Industrial hygiene:Provide local exhaust or general room ventilation.Chemical formula:C34H36N6O6S2MgMolecular weight:713OMEPRAZOLE MAGNESIUM CRSPhysical state at 20 °C:Powder.Colour:White.pH value:10.5Melting point [°C]:150-160Boiling point [°C]:No data available.Solubility in water:Insoluble.Solubility:Methylene chloride.Flash point [°C]:No data available.Log P octanol / water at 20°C:2.23Stability and reactivity:Stable under normal conditions.Materials to avoid:Acids. Strong oxidizers.Conditions to avoid:Light.Hazardous decomposition products:Carbon monoxide. Carbon dioxide. Sulfur compounds. Nitrogen oxides.When heated to decomposition, emits dangerous fumes.Hazardous reactions:None under normal conditions.Hazardous polymerization:Will not occur.RTECS nr:DD9087000 (omeprazole) ( See actual entry in RTECS for complete information. ) Rat oral LD50 [mg/kg]:2210Rabbit dermal LD50 [mg/kg]:No data available.Rat inhalation LC50 [mg/L/4h]:No data available.Acute toxicity:Metabolism and nutrition disorders. Gastrointestinal disorders.Chronic toxicity:No data available.Sensitization:May cause sensitization by skin contact.Ecological effects information:Harmful to aquatic organisms, may cause long-term adverse effects in the aquaticenvironment.LC50-96 Hour - fish [mg/L]:42EC50-48 Hour-Daphnia magna [mg/L]:>100IC50-72h-Algae [mg/L]:30Biodegradation [%]:No data available.Persistence - degradability:Partially biodegradable.Log P octanol / water at 20°C:2.23Bioaccumulative potential:No data available.Environmental precautions:Avoid release to the environment.OMEPRAZOLE MAGNESIUM CRSGeneral:Dispose of this material and its container at hazardous or special waste collectionpoint.Dispose in a safe manner in accordance with local/national regulations.General information:Not classified.Symbol(s):Xi : IrritantR Phrase(s):R36/37/38 : Irritating to eyes, respiratory system and skin.R43 : May cause sensitization by skin contact.R52/53 : Harmful to aquatic organisms, may cause long-term adverse effects in theaquatic environment.S Phrase(s):S22 : Do not breathe dust.S24 : Avoid contact with skin.S26 : In case of contact with eyes, rinse immediately with plenty of water and seekmedical advice.S36/37 : Wear suitable protective clothing and gloves.S51 : Use only in well-ventilated areas.S61 : Avoid release to the environment. Refer to special instructions/Safety datasheets.Further information:Revision - See : *List of relevant R phrases:R36/37/38 : Irritating to eyes, respiratory system and skin.R43 : May cause sensitization by skin contact.R52/53 : Harmful to aquatic organisms, may cause long-term adverse effects in theaquatic environment.The contents and format of this SDS are in accordance with REGULATION (EC) No 1907/2006 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL, as amended.DISCLAIMER OF LIABILITY The information in this SDS was obtained from sources which we believe are reliable. However, the information is provided without any warranty, express or implied, regarding its correctness. The conditions or methods of handling, storage, use or disposal of the product are beyond our control and may be beyond our knowledge. For this and other reasons, we do not assume responsibility and expressly disclaim liability for loss, damage or expense arising out of or in any way connected with the handling, storage, use or disposal of the product. This MSDS was prepared and is to be used only for this product. If the product is used as a component in another product, this MSDS information may not be applicable.End of document。
