聚焦DNA双链断裂(DSBs)
DNA 同源重组修复与乳腺癌的研究进展

DNA 同源重组修复与乳腺癌的研究进展邱宇凡;胡蕴慧;张瑾【摘要】Double-strand DNA breaks ( DSBs) are the most dele-terious events in eukaryotic cells , and there are two major path-ways for repairing them: homologous recombination ( HR ) and non-homologous DNA end joining ( NHEJ ) .BRCA1/2 proteins are key factors in HR repair pathway for DSBs and play an es-sential role in BRCA tumor suppressor network , which maintains genomic integrity and stability .Pathogenic mutations of core genes in this network may damage the DNA repair process and increase the risk of breast cancer .This review summarizesthe current reports on the relationship between breast cancer suscep-tibility and mutations of the key factors in DNA HR repair asso-ciated tumor suppressor network , and aims at promoting the pre-vention, molecular diagnosis and precision treatment of the breast cancer patients with aforementioned mutations .%双链断裂是真核细胞最严重的DNA损伤类型,主要依赖同源重组途径进行修复。
分子遗传学试题

一、名词解释1.NHEJ:非同源末端连接,一种双链断裂的修复形式,修复时将断头直接连接起来,不需要进行同源重组。
2.焦磷酸化编辑:RNA聚合酶利用活性位点在一个简单的逆反应中,通过加入PPi,去除错误插入的核糖核苷酸。
3.交叉端化:交叉端化是在减数分裂终变期中,染色体更粗更短,此时可见到交叉二价体的两端移动,且逐渐近于末端的现象。
4.差别基因活性:某些特定奢侈基因表达的结果生成一种类型的分化细胞,另一组奢侈基因表达的结果导致出现另一类型的分化细胞。
其本质是开放某些基因,关闭某些基因,导致细胞的分化。
5.超级摆动假说:密码子与反密码子之间互相识别的时候,前两对碱基严格遵守标准的碱基配对规则,即A与U配对,C与G配对,最后一对碱基具有一定的自由度。
6.弱化子(attenuator):弱化子是指原核生物操纵子中能显著减弱甚至终止转录作用的一段核苷酸序列,该区域能形成不同的二级结构,利用原核微生物转录与翻译的偶联机制对转录进行调节。
7.TALENs:TALENs即转录激活因子样效应物核酸酶,TALENs是一种可靶向修饰特异DNA序列的酶,它借助于TAL效应子一种由植物细菌分泌的天然蛋白来识别特异性DNA碱基对。
TAL效应子可被设计识别和结合所有的目的DNA序列。
对TAL效应子附加一个核酸酶就生成了TALENs。
TAL效应核酸酶可与DNA结合并在特异位点对DNA链进行切割,从而导入新的遗传物质。
8.母体基因:母体基因是在卵母细胞成熟过程中,在看护细胞中转录,然后将合成的mRNA运送到卵母细胞的基因。
由于这些mRNA翻译合成的蛋白质在早期胚胎发育中调节合子基因的转录,故将它们称为母体基因。
根据它们的作用,可分为四群,每一群在胚胎发育过程中控制不同区域的分化。
9.反式剪接:反式剪接指的是两条不同的pre-mRNA的外显子连接到一起。
与正常的顺式剪接不同,这里的两段外显子是来自不同的pre-mRNA的,但却可能来自同一基因。
γh2ax、53bp1及rad51焦点用于分析dna双链断裂损伤

第 37 卷第 1 期
2020 年 2 月
生 物 学 杂 志
Vol 37 No 1
Feb 2020
JOURNAL OF BIOLOGY
doi∶10 3969 / j issn 2095 - 1736 2020 01 016
DNA损伤修复

DNA damage response (DDR)——损伤太强导致的细胞周期停滞(cell-cycle arrest)DNA 分子在进行一系列DNA 的复制、包装、分离和转录等复杂的动态过程中伴随着大量的DNA 损伤及复制错误。
基因组还会受到各种存在于环境中的诱变剂攻击,例如电离辐射和化学试剂。
各式各样的基因毒性压力伴随着许多类型的基因突变,包括双螺旋断裂(DSBs),单链的DNA 断裂,DNA 交叉互换及插入,核苷酸碱基修饰、插入、缺失及染色体易位。
其中危害最大的是DNA 双链断裂。
DNA 的损伤可以进一步破坏机体细胞、组织及器官。
细胞进化出了一种DNA 损伤应激反应可以修复DNA 损伤,高度保守的DNA 损伤修复应答机制可以维持基因组的完整性[1]。
DNA损伤应答(DNA Damage Response)是细胞对DNA损伤后的一种应激反应。
正常细胞具有复杂的DNA损伤应激反应通路,其激活会引起细胞周期停滞、细胞凋亡或者细胞衰老,并抑制细胞癌变的发生[1]。
当高水平的DNA 损伤发生时,细胞周期检查点被激活,使细胞周期停滞,待细胞修复系统修复这些损伤后,细胞周期恢复运转,其中,细胞周期检查点的激活需要DNA 损伤达到一个阈值[2]。
细胞周期检查点的激活需要DNA 损伤达到一个阈值DNA 损伤能够激活细胞周期检查点,但是,事实上在增殖的哺乳动物细胞中,每小时每个细胞DNA 大约发生一万次修饰。
并不是任何程度的DNA 损伤细胞都会发生激活检查点,在低水平的DNA 损伤情况下,细胞周期检查点不会被激活,单个DNA 双链断裂足以引起检查点的激活。
DNA 损伤程度依赖于处理时间的长短,细胞周期检查点的激活需要DNA 损伤达到一个阈值。
DNA 损伤激活细胞周期检查点[2]自然界各种物理、化学因子如射线或化学药物可以引起细胞内DNA 损伤。
细胞增殖过程中,当DNA 损伤发生时,细胞激活一种调节机制,使细胞周期停滞,待细胞修复系统修复这些损伤后,细胞周期恢复运转。
Nature发表基于选择性非同源末端连接修复的基因治疗方法

Nature发表基于选择性非同源末端连接修复的基因治疗方法DNA双链断裂(Double-strand breaks, DSBs)的修复通路主要有非同源末端连接修复(Non-homologous end-joining, NHEJ)和同源重组修复(Homologous recombination, HR)两种。
除此之外,还存在不常用的选择性非同源末端连接修复(Microhomology-mediated end joining,MMEJ,也叫Alternative-NHEJ, Alt-NHEJ),它主要利用损伤区域的微同源片段(2-25 bp)发生退火反应,切除非同源ssDNA(single-strand DNA),最后将断裂的DNA 重新连接在一起【2】。
MMEJ修复断裂的DNA常会造成连接处发生微同源片段的缺失甚至导致基因重排,因此是一种易错的修复方式。
研究表明,MMEJ途径存在于植物、细菌、人类等多种类型细胞中,同时MMEJ途径也被应用在对哺乳动物细胞、斑马鱼和青蛙胚胎中定向插入外源性供体DNA的研究中【3-5】。
那么,基于HR途径进行的基因编辑所带来的困难能否通过利用MMEJ途径得到改善?2019年4月4日,来自于美国马萨诸塞大学医学院的Scot A. Wolfe团队和Charles P. Emerson Jr团队合作在Nature在线发表了一篇题为Precise therapeutic gene correction by a simple nuclease-induced double-stranded break的文章,描述了一种简单有效的基因纠正疗法,即利用Streptococcus pyogenes Cas9(SpCas9)核酸酶系统对基因组上致病性微重复中心附近区域通过MMEJ通路进行修复编辑,以实现精准回复野生型基因序列的目的。
在本文中,研究人员选择了两种均为常染色体隐性遗传但是由不同长度的致病性微重复造成的疾病模型:肢带型肌营养不良2G型( limb-girdle muscular dystrophy type 2G, LGMD2G)和白化病亚型Hermansky-Pudlak综合征1型(Hermansky–Pudlak syndrome type 1, HPS1),设计试验以评估基于MMEJ的修正方式的有效性【6,7】。
拟南芥NBS1_互作蛋白的筛选和鉴定

引文格式:吴钒漳, 孙旭东, 徐慧妮. 拟南芥NBS1互作蛋白的筛选和鉴定[J]. 云南农业大学学报(自然科学), 2023, 38(4):558−565. DOI: 10.12101/j.issn.1004-390X(n).202202008拟南芥NBS1互作蛋白的筛选和鉴定*吴钒漳1, 孙旭东2, 徐慧妮1 **(1.昆明理工大学 生命科学与技术学院,云南 昆明 650500;2. 中国科学院 昆明植物研究所,云南 昆明 650201)摘要: 【目的】筛选拟南芥中NBS1的互作蛋白,探究NBS1蛋白的新功能,为后续研究奠定基础。
【方法】利用酵母双杂交技术筛选拟南芥cDNA 文库,对得到的序列进行BLAST 比对、亚细胞定位分析和基因本体注释。
【结果】共有221个阳性克隆,测序后通过BLAST 比对得到97个与NBS1互作的蛋白,包括膜蛋白、转运蛋白和折叠蛋白等,它们主要定位于细胞质、细胞核和叶绿体等,主要富集于4个分子功能、5个细胞组分和13个生物过程。
【结论】拟南芥中与NBS1互作的蛋白在刺激响应、信号转导和细胞代谢等方面发挥着重要作用,也证明NBS1是一种多功能蛋白,但其功能及分子机制还需进一步研究。
关键词: 拟南芥;NBS1;蛋白互作;酵母双杂交;基因本体注释中图分类号: Q949.748.306 文献标志码: A 文章编号: 1004–390X (2023) 04−0558−08Screening and Identifying Interaction Proteins ofNBS1 in Arabidopsis thalianaWU Fanzhang 1,SUN Xudong 2,XU Huini 1(1. Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming 650500,China; 2. Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China)Abstract: [Purpose ]To screen the interaction protein and explore the new function of NBS1 pro-tein in Arabidopsis thaliana , laying the foundation for further research. [Methods ]The yeast two-hybrid technology was used to screen the interaction proteins of NBS1 from A. thaliana cDNA lib-rary. BLAST alignment, subcellular localization analysis and gene ontology annotations were per-formed on the obtained sequences. [Results ]There were 97 proteins interacted with NBS1 in 221positive clones by BLAST after sequencing, including membrane proteins, transporters and folding proteins. These proteins were mainly localized in cytoplasm, nucleus and chloroplast, which were mainly enriched in four molecular functions, five cellular components and 13 biological processes.[Conclusion ]The proteins interacting with NBS1 play an important role in stimulus response, sig-nal transduction and cell metabolism. It is also confirmed that NBS1 is a multifunctional protein, but its function and molecular mechanism need further studies.Keywords: Arabidopsis thaliana ; NBS1; protein interaction; yeast two-hybrid; gene ontology云南农业大学学报(自然科学),2023,38(4):558−565Journal of Yunnan Agricultural University (Natural Science)E-mail: ********************收稿日期:2022-02-16 修回日期:2022-03-02 网络首发日期:2023-09-06*基金项目:国家自然科学基金项目(31760582)。
p53_及EGFR_与头颈部鳞癌放疗抵抗的关系

第 44卷第4期2023 年7月Vol.44 No.4July 2023中山大学学报(医学科学版)JOURNAL OF SUN YAT⁃SEN UNIVERSITY(MEDICAL SCIENCES)p53及EGFR与头颈部鳞癌放疗抵抗的关系代永杰,徐钢(暨南大学第二临床医学院肿瘤放疗科,广东深圳 518000)摘要:头颈部恶性肿瘤是世界上第七大常见的癌症类型。
超过90%的头颈部恶性肿瘤是鳞状细胞癌(HN⁃SCC)。
放疗是HNSCC的重要治疗方法之一,肿瘤细胞对放疗的敏感性是放疗有效性的一个关键因素。
p53是HN⁃SCC中最常见的突变基因之一,表皮生长因子受体(EGFR)在许多的HNSCC中过度表达,这两者都能增强细胞DNA的修复,可能与HNSCC的放疗抵抗有关。
本文就肿瘤细胞通过p53和EGFR介导的DNA修复来逃避辐射介导的凋亡机制作一综述。
关键词:p53;表皮生长因子受体;放疗抵抗中图分类号:R739.91 文献标志码:A 文章编号:1672-3554(2023)04-0596-05DOI:10.13471/ki.j.sun.yat-sen.univ(med.sci).2023.0408Relationship Between p53,EGFR and Radiotherapy Resistance of Head andNeck Squamous Cell CarcinomaDAI Yong-jie, XU Gang(Department of Oncology and Radiotherapy, The Second School of Clinical Medicine, Jinan University, Shenzhen518000,China)Correspondence to: XU Gang; E-mail:**************Abstract:Head and neck cancers are the seventh most common type of cancer in the world, among which more than 90% are squamous cell carcinomas(HNSCC). Radiotherapy is one of the important treatments for HNSCC, and the sensi⁃tivity of tumor cells to the therapy is a key factor influencing the efficacy of treatment. p53 is one of the most common mutat⁃ed genes in HNSCC, and epidermal growth factor receptor(EGFR) is overexpressed in many HNSCC. Both of these genes could enhance cellular DNA repair, which may be related to the radiotherapy resistance of HNSCC. This review focuses on the mechanisms by which tumor cells escape radiation-mediated apoptosis through p53 and EGFR-mediated DNA repair.Key words:p53; epidermal growth factor receptor; radiotherapy resistance[J SUN Yat⁃sen Univ(Med Sci),2023,44(4):596-600]头颈部恶性肿瘤是世界上第七大常见的癌症类型,全球每年超过65万例患者被确诊为头颈部恶性肿瘤,其中约50%的患者最终可能死于该病[1]。
文献翻译

小RNA:在DNA损伤应答中扮演新的角色这DNA损伤应答是一个信号转导途径,决定着细胞的命运,是进入细胞修复或者在严重受损时进入细胞凋亡程序。
在DDR途径中,通过转录后修饰调节蛋白复合体的组装和活性。
小RNA正在成为一个内源基因调节器控制蛋白质水平,从而在DDR中,增加了一个新的监测管理。
在这篇文章里,我们描绘了小RNA的一个新的角色在调节当DNA损伤时的细胞应答。
我们这次的焦点在DNA双链的断裂损伤。
同时我们讨论了小RNA潜在的角色在DDR 到干细胞,包括胚胎干细胞和肿瘤干细胞,预示着一个潜在的应用即可被用于作为敏化剂在癌症的放疗和化疗中。
关键词:小RNA、DNA损伤应答、放射性敏感、干细胞引言:DDR是细胞感觉到DNA的受损,转换这些信号并且促进他们的修复的一个分子机制。
(Harper and Elledge, 2007).这DDR一个必不可少的细胞作用是阻止细胞的周期,让损伤的DNA进行修复或者在严重受损的情况下,进入到细胞的凋亡程序。
因为,DDR可被看成是肿瘤发生的一个防御机制。
(Bartek et al.,2007). 除了这一重要角色,DDR还参与许多其他的生理过程,如减数分裂,染色体端粒的内稳定和病毒感染。
(Jackson and Bartek, 2009).人体中的每个细胞都会经历DNA损伤,因为环境因素的不断的刺激,比如电离辐射,或者从内在因素如活性氧。
由此产生的DNA损害,包括碱基,DNA错配, 插入/删除, O6烷基,DNA交联, 单链断裂(SSBs)和双链断裂(DSBs),可以被修复通过细胞使用不同的修复机制。
(Jackson and Bartek, 2009). DSBs,尽管他们是很难发生的,是最难修复的也是极为不利的对细胞来说。
细胞利用两个主要的途径去修复DSBs,非同源末端连接途径(NHEJ),它是快速的但是也是极易出错的在细胞的G0/G1阶段,同源重组修复(HRR)是很慢的,并且在S/G2阶段很容易出错。
SCGE法检测DNA损伤(包括DNA单链断裂和DNA交联)

SCGE法检测DNA损伤SCGE的原理(中性电泳液,高盐和变性剂检测双链断裂;碱性检测单链,双链断裂和碱不稳定性)SCGE技术是一种在单细胞水平上检测有核细胞DNA损伤和修复的方法。
该技术的原理是基于有核细胞的DNA分子量很大,DNA超螺旋结构附着在核基质中,用琼脂糖凝胶将细胞包埋在载玻片上,在细胞裂解液作用下,细胞膜、核膜及其它生物膜破坏,使细胞内的RNA、蛋白质及其它成分进入凝胶,继而扩散到裂解液中,唯独核DNA仍保持缠绕的环区(Loop)附着在剩余的核骨架上,并留在原位。
如果细胞未受损伤,电泳中核DNA因其分子量大停留在核基质中,经荧光染色后呈现圆形的荧光团,无拖尾现象。
若细胞受损,在中性电泳液(pH8)中,核DNA仍保持双螺旋结构,偶有单链断裂(SSBs)并不影响DNA双螺旋大分子的连续性。
只有当DNA双链断裂(DSBs)时,其断片进入凝胶中,电泳时断片向阳极迁移,形成荧光拖尾现象,形似彗星。
如果在碱性电泳液(pH>13)中,先是DNA双链解螺旋且碱变性为单链,单链断裂的碎片分子量小即可进入凝胶中,在电泳时断链或碎片离开核DNA向阳极迁移,形成拖尾。
细胞核DNA受损愈重,产生的断链或碱易变性断片就愈多,其断链或断片也就愈小,在电场作用下迁移的DNA量多,迁移的距离长,表现为尾长增加和尾部荧光强度增强。
因此,通过测定DNA迁移部分的光密度或迁移长度就可定量测定单个细胞DNA损伤程度。
1. 仪器及试剂冰箱,载玻片,水平电泳槽,荧光显微镜。
不含Ca2+、Mg2+的PBS (PH=7.4);正常溶点琼脂糖(NMA)及低溶点琼脂糖(LMA)(0.6%溶于PBS);细胞裂解液(2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris-HCl, 1%肌氨酸钠, PH=10, 用前加1%TritonX-100, 10% 二甲亚砜(DMSO));电泳缓冲液(0.3 M NaOH, 1 mM Na2EDTA, PH=13);0.4 M Tris-HCl(PH=7.5);无水乙醇;20-30μg/mL 溴化乙锭(EB)。
DNA损伤修复通路因子53BP1在骨髓干细胞自我更新和分化发育中的作用

·19·维持基因组序列信息的完整性对于生物体的存在至关重要,细胞应对各种类型损伤因子所导致的DNA损伤主要是通过激活复杂而精细的DNA 损伤应答 (DNA damage response,DDR ) 通路[1]。
具有细胞毒性的双链断裂损伤 (double strand breaks,DSBs ) 是最为严重的DNA损伤形式之一,未得到正确处理的DSBs除可增加细胞的致死率外,还会增加· 论著 ·DNA损伤修复通路因子53BP1在骨髓干细胞自我更新和分化发育中的作用尤放,王美莲(中国医科大学基础医学院病原生物学教研室,沈阳 110122) 摘要 目的 探讨DNA 损伤修复通路因子53BP1在骨髓干细胞自我更新及定向分化过程中发挥的作用。
方法 构建53BP1全身敲除C57/6小鼠模型,免疫荧光染色检测53BP1在细胞中的定位,Western blotting 对53BP1蛋白表达水平进行检测及鉴定;流式细胞术进行细胞分选和计数、细胞周期检测、以及细胞表面分子marker 分析,对比WT 组和Mut 组细胞生长速率以及定向分化过程,Co -IP 检测分析表观遗传学磷酸化修饰蛋白,SPSS 22.0对数据进行统计学分析。
结果 体内和体外实验证实伴随KSL 细胞分化发育进行,53BP1表达含量显著增加,53BP1基因敲除可影响KSL 细胞的自我更新和定向分化以及表面分子标记的表达;53BP1基因敲除与否并不显著影响处于细胞周期G 1/G 0的KSL 细胞百分含量;53BP1可影响磷酸化修饰蛋白的表达水平,53BP1基因敲除后磷酸化修饰蛋白p -H4 (k20) 和p -H3 (k79) 表达水平明显增加,但p -ATM 和p -53-Ser -20含量显著减少。
结论 53BP1参与并影响骨髓干细胞的自我更新及定向分化过程,53BP1基因敲除可显著影响磷酸化蛋白p -H4 (k20)、p -H3 (k79)、p -ATM 和p -53-Ser -20的表达水平。
DNA同源重组修复的分子机制

・综述・作者单位:300192 天津,中国医学科学院放射医学研究所DNA 同源重组修复的分子机制王勇 樊飞跃 电离辐射直接造成生物靶分子细胞DNA 的损伤,DNA 的损伤类型很多,其中以DNA 双链断裂(double strand break ,DS B )最为严重。
DNA DS B 的修复较其他类型的DNA 损伤更加困难,不修复则可能导致染色体断裂和细胞死亡,而修复不当则可能导致染色体缺失、重排、转位和倒置等,从而易于形成肿瘤等疾病[1]。
DNA 损伤的不完全修复可导致基因组不稳定,机体细胞为了对抗损伤,发展出多个修复系统来保证基因组的完整性,同源重组修复(hom olog ous recombinationrepair ,HRR )是DNA DS B 损伤修复的主要方式,对于保持哺乳动物细胞的基因组完整性十分重要[2]。
重组即遗传物质的重排,同源重组是指发生在同源DNA 序列间的重组,主要是利用DNA 序列间的同源性来识别,而负责配对和重组的蛋白质因子并无碱基序列特异性。
本文综述了国外近来对HRR 分子机制的研究进展,包括引发HRR 的DNA 损伤机制;HRR 的基本修复过程和多条修复通路;HRR 关键分子重要功能的实现机制;HRR 与细胞周期调控等其他事件的相互关系;辐射和HRR 及其与肿瘤之间的关系。
11DNA 损伤的感受识别:HRR 参与的蛋白质有RAD51,RAD51b ,c ,d ,RAD52,RAD54,BRC A1,BRC A2,XRCC2,XRCC3和MRN 复合物等,另外还需大量起始和损伤应激感受分子,包括AT M ,ATR 和DNA 2PK cs [3]。
细胞在受到电离辐射照射后,一系列重组或修复蛋白及复合物重新定位形成核焦点,以应答DNA 损伤。
这些蛋白有γ2H2AX ,AT M ,RAD51,BRC A1,BRC A2,NBS1,RPA 和MRN ,它们相互作用,完成DNA 损伤信号的接受功能并转导信号给其他蛋白质,介导并协调包括周期检控点、凋亡、修复的损伤应答。
γH2AX:DNA双链断裂的标志

军1
( 浙江大学医学院 1 病理生理学教研室,2 机能中心,浙江 杭州 . .
摘要: H 2 A X是目前国内外研究细胞 D N A损伤应激反应的 γ 热点之一。细胞在电离辐射或其他因素作用下直接诱导或 是通过复制压力诱导形成的双链断裂( ) , 以及细胞自身 D S B s 和逆转录病毒转染细胞过程中产生的 调控的程序性 D S B s 均可诱导 H ) 和 簇 集。本 文 对 D S B s 2 A X的 磷 酸 化 ( H 2 A X γ 以及 γ 之间的关系及 H 2 A X及其组蛋白家族, H 2 A X与 D S B s 可能作为一个探测 D N A损伤的新分子探针来利用作一简要 综述。 关键词: ;D H 2 A X N A损伤;诱变剂 γ 中图分类号:R 3 9 4 . 6 文献标识码:A 文 章 编 号:1 ( ) 0 0 0 3 0 0 2 2 0 0 5 0 3 0 2 3 7 0 4 许多环境理化因素可导致癌症的发生, 比如黄曲霉素致 肝癌, 胺基染料致膀胱癌, 苯致白血病, 吸烟致肺癌, 以及电 , ) 致白血病等。大多数的已知环境 离辐射( i o n i z i n g r a d i a t i o n I R 致癌物都是遗传毒物, 即它们与细胞的 D N A发生反应而诱
的 I 2 . 1 直接诱导细胞 D S B s R或 其 他 遗 传 毒 物 可 诱 导 H 2 A X焦点的形成 γ 细胞在 I 位点簇 R作用后, H 2 A X迅速磷酸化并在 D S B s [ ] 6 并且 γ 集形成焦点, 这一过程可发生在几分钟之内 , H 2 A X 所形成的焦点的数量与 I R造成的 D N A双链断裂数量存在一
] 2 。 D N A的修复[ 目前 研 究 发 现, 组蛋白 H 2 A 家 族 中 共 有 7个 成 员: , , , , , H 2 A 1H 2 A 2H 2 A XH 2 A Zm a c r o H 2 A X 1 m a c r o H 2 A X 2和 H 2 A
基因编辑技术的概念和原理

基因编辑的 研究背景
传统的动物育种方法受到种源的限制,其程 需要耗费大量的人力、物力和财力,经历漫 长的培育过程。而且不同种间的杂交很困难, 育种成果很难取得突破性进展。
基因编辑的研 究背景
01
现代动物分子育种中,分子 标记技术能够定位与经济性 状相关的分子标记,锁定基 因与性状的对应关系,从而 快捷可靠地对动物后代进行 筛选。但是,利用分子标记 技术辅助筛选,改良的程度 依然受限于品种自身已有的 基因。
基因编辑技 术的展望
随着越来越多物种基因组测序的完成,基 因功能的研究成为后基因时代的重点。基 因编辑技术既可以用正常基因替代突变基 因进行性状改良和基因治疗,也可以用突 变基因代替正常基因进行基因功能的研究。
基因编辑技 术的展望
与传统的基因打靶技术相比,基因编辑技术 摆脱了对 ES 细胞的限制,可以应用于更多 的物种,且定向修饰更加精确,效率更高, 所需时间更短,得到的突变可以通过种系遗传. 其中,CRISPR 系统可以同时进行多靶点的 切割,易于得到纯合子突变体。
ZFN 由锌指蛋白(ZFP)和 FokⅠ核酸内切 酶组成。其中,由 ZFP 构成的 DNA 识别 域能识别特异位点并与之结合,而由 FokⅠ构成的切割域能执行剪切功能,两 者结合可使靶位点的双链DNA 断裂(DSB)。 于是, 细胞可以通过同源重组(HR)修复机 制和非同源末端连接(NHEJ)修复机制来修 复 DNA。HR 修复有可能会对靶标位点进 行恢复修饰或者插入修饰,而 NHEJ 修复 极易发生插入突变或缺失突变。两者都可 造成移码突变,因此达到基因敲除的目的。
基因编辑的 研究背景
近年来,随着高特异性及更具操作性的人工 核酸酶的出现和技术体系的完善,基因组编 辑技术获得了飞速发展,并将靶向基因操作 推向高潮,使得定点基因敲除、敲入变得更 为简单且高效。
SCGE法检测DNA损伤(包括DNA单链断裂和DNA交联)

SCGE法检测DNA损伤SCGE的原理(中性电泳液,高盐和变性剂检测双链断裂;碱性检测单链,双链断裂和碱不稳定性)SCGE技术是一种在单细胞水平上检测有核细胞DNA损伤和修复的方法。
该技术的原理是基于有核细胞的DN A分子量很大,DNA超螺旋结构附着在核基质中,用琼脂糖凝胶将细胞包埋在载玻片上,在细胞裂解液作用下,细胞膜、核膜及其它生物膜破坏,使细胞内的R NA、蛋白质及其它成分进入凝胶,继而扩散到裂解液中,唯独核DNA仍保持缠绕的环区(Loop)附着在剩余的核骨架上,并留在原位。
如果细胞未受损伤,电泳中核DN A因其分子量大停留在核基质中,经荧光染色后呈现圆形的荧光团,无拖尾现象。
若细胞受损,在中性电泳液(pH8)中,核DNA仍保持双螺旋结构,偶有单链断裂(SSBs)并不影响DN A双螺旋大分子的连续性。
只有当DNA双链断裂(DSBs)时,其断片进入凝胶中,电泳时断片向阳极迁移,形成荧光拖尾现象,形似彗星。
如果在碱性电泳液(pH>13)中,先是DNA双链解螺旋且碱变性为单链,单链断裂的碎片分子量小即可进入凝胶中,在电泳时断链或碎片离开核DNA向阳极迁移,形成拖尾。
细胞核DNA受损愈重,产生的断链或碱易变性断片就愈多,其断链或断片也就愈小,在电场作用下迁移的DN A量多,迁移的距离长,表现为尾长增加和尾部荧光强度增强。
因此,通过测定DN A迁移部分的光密度或迁移长度就可定量测定单个细胞D NA损伤程度。
1. 仪器及试剂冰箱,载玻片,水平电泳槽,荧光显微镜。
不含Ca2+、Mg2+的PBS (PH=7.4);正常溶点琼脂糖(NMA)及低溶点琼脂糖(LMA)(0.6%溶于PBS);细胞裂解液(2.5 M NaCl, 100 mM Na2EDT A, 10 mM Tris-HCl, 1%肌氨酸钠, PH=10, 用前加1%Triton X-100, 10% 二甲亚砜(DMSO));电泳缓冲液(0.3 M NaOH, 1 mM Na2EDT A, PH=13);0.4 M Tris-HCl (PH=7.5);无水乙醇;20-30μg/mL 溴化乙锭(EB)。
DNA损伤断裂分析试剂盒(IFFMHCS法)
DNA 损伤断裂分析试剂盒(IF FM HCS 法)说明书修订日期:2016.07.14Cat number :KGAF010Store at -20℃for 12months ,避光For Research Use Only(科研专用)一、产品描述哺乳动物细胞中,双链断裂(DSB )的基因组DNA 是一种潜在的致命病变。
现已确知DSB 的形成为导致H2A 组蛋白的磷酸化。
具体来说,在DNS 双链断裂处形成DNA 灶的过程中,DNA 损伤诱导剂可以诱导组蛋白变体H2AX 的在Ser139位点上的磷酸化。
磷酸化的H2AX 有利于募集蛋白质从而帮助DSB 部位进行修复。
在哺乳动物细胞中,磷脂酰肌醇3-激酶样蛋白激酶,如ATM ,ATR 、DNA-PKCs 蛋白,以及磷酸化的组蛋白变体,H2AX 。
凯基DNA 损伤试剂盒通过高内涵分析可以对于同一细胞的健康参数、遗传毒性和细胞毒性进行同时定量分析。
通过以磷酸化的H2AX (Ser139)抗体检测DNA 损伤,可以对遗传毒性进行分析,进而反映DNA 损伤情况。
而细胞毒性的分析是通过试剂盒提供的死活细胞核染料进行染色区分鉴定的。
二、产品包装客户自备试剂:磷酸盐缓冲液(PBS )、细胞培养基、多聚甲醛16%水溶液、Triton®X-100、牛血清白蛋白(BSA)三、操作说明1.染色液制备:以下染液请在使用前新鲜配制,以下方法针对96孔板培养细胞设计。
1.1试剂A 工作液(死细胞核染料):将1.8μL 的试剂A 加入到6mL 的完全细胞培养基中。
1.2制备固定液:1.5mL 的16%多聚甲醛水溶液加入到4.5mL PBS 中,得到4%的多聚甲醛固定液。
1.3制备促渗液:加15μL 的Triton®X-100到6mL 的PBS 中。
1.4制备封闭液:溶解0.25g 的BSA 到25mL 的PBS 中,混匀。
1.5试剂B 工作液(一抗工作液):加6μL 的pH2AX 抗体溶液(试剂B )到6mL 的封闭液中。
γh2ax和53bp1 foci dsb的指标

文章题目:γH2AX和53BP1 Foci DSB的指标:深度解析在细胞和分子生物学研究领域,γH2AX和53BP1 Foci DSB(双链断裂)的指标一直是焦点话题。
这两种蛋白质在DNA修复过程中扮演着重要的角色,同时也是衡量DNA损伤和修复程度的重要标志。
本文将深入探讨γH2AX和53BP1 Foci DSB的指标,从其基本概念到应用研究中的最新进展,带您全面了解这一领域的重要内容。
一、γH2AX和53BP1 Foci DSB的基本概念1. γH2AX和53BP1的定义和作用γH2AX和53BP1均是在细胞DNA双链断裂修复过程中发挥作用的蛋白质。
γH2AX是由H2AX磷酸化形成的一种变体,主要在DNA损伤发生后迅速富集于断裂位点,发挥着促进DNA修复的作用。
而53BP1则是在DNA损伤后与γH2AX相互作用,形成聚焦体(foci),并参与调控DNA修复途径的选择性。
2. Foci DSB的形成和检测方法Foci DSB是指在DNA双链断裂部位形成的一种可见的光学结构,可通过荧光显微镜或其他成像技术进行直接观察。
研究者可利用特定的抗体标记γH2AX或53BP1,并通过免疫荧光染色或免疫组织化学等技术来检测Foci DSB的形成情况,从而评估DNA双链断裂的程度和DNA修复的效率。
二、γH2AX和53BP1 Foci DSB的应用研究1. DNA双链断裂与肿瘤发生和治疗在肿瘤细胞中,由于癌基因的活化和抑癌基因的失活,DNA双链断裂的频率通常较高。
通过检测γH2AX和53BP1 Foci DSB的形成情况,可以评估肿瘤细胞的DNA损伤程度,为肿瘤的诊断、预后判断和治疗反应监测提供重要依据。
2. 放射线和化疗的辐射效应评估放射线和化疗是常用的抗肿瘤治疗手段,其治疗效果与诱导肿瘤细胞的DNA双链断裂有关。
通过检测γH2AX和53BP1 Foci DSB的形成情况,可评估肿瘤细胞对放射线和化疗的敏感性,为临床治疗方案的选择和调整提供重要参考。
Biomics CRISPR Cas9 基因敲除技术
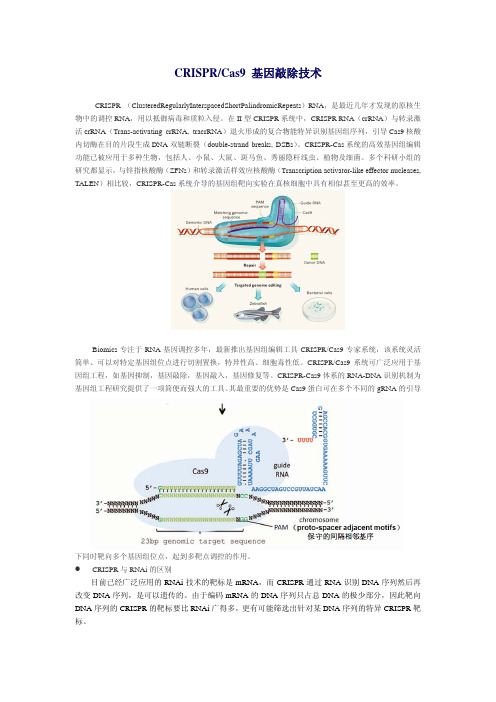
CRISPR/Cas9 基因敲除技术CRISPR (ClusteredRegularlyInterspacedShortPalindromicRepeats)RNA,是最近几年才发现的原核生物中的调控RNA,用以抵御病毒和质粒入侵。
在II型CRISPR系统中,CRISPR RNA(crRNA)与转录激活crRNA(Trans-activating crRNA, tracrRNA)退火形成的复合物能特异识别基因组序列,引导Cas9核酸内切酶在目的片段生成DNA双链断裂(double-strand breaks, DSBs)。
CRISPR-Cas系统的高效基因组编辑功能已被应用于多种生物,包括人、小鼠、大鼠、斑马鱼、秀丽隐杆线虫、植物及细菌。
多个科研小组的研究都显示,与锌指核酸酶(ZFNs)和转录激活样效应核酸酶(Transcription activator-like effector nucleases, TALEN)相比较,CRISPR-Cas系统介导的基因组靶向实验在真核细胞中具有相似甚至更高的效率。
Biomics专注于RNA基因调控多年,最新推出基因组编辑工具CRISPR/Cas9专家系统,该系统灵活简单、可以对特定基因组位点进行切割置换,特异性高、细胞毒性低。
CRISPR/Cas9系统可广泛应用于基因组工程,如基因抑制,基因敲除,基因敲入,基因修复等。
CRISPR-Cas9体系的RNA-DNA识别机制为基因组工程研究提供了一项简便而强大的工具。
其最重要的优势是Cas9蛋白可在多个不同的gRNA的引导下同时靶向多个基因组位点,起到多靶点调控的作用。
z CRISPR与RNAi的区别目前已经广泛应用的RNAi技术的靶标是mRNA,而CRISPR通过RNA识别DNA序列然后再改变DNA序列,是可以遗传的。
由于编码mRNA的DNA序列只占总DNA的极少部分,因此靶向DNA序列的CRISPR的靶标要比RNAi广得多,更有可能筛选出针对某DNA序列的特异CRISPR靶标。
大黄素通过抑制ATP和拓扑异构酶II的结合导致DNA产生双链断裂

大黄素通过抑制ATP与拓扑异构酶II的结合导致DNA产生双链断裂李妍,栾洋,任进(药物安全评价研究中心,上海药物研究所,中国科学院,201203)摘要:目的:大黄素作为泻药中常见的一种成分,广泛应用于临床,近期研究表明它能够引起遗传毒性,但是机制尚不清楚。
为了探讨大黄素引起遗传毒性的机制,我们开展了一系列的研究。
方法:采用Ames试验,TK基因突变试验以及体内和体外的微核试验来评价大黄素的遗传毒性。
在研究大黄素与拓扑异构酶的相互作用时,进行了一系列的分子和细胞水平的实验,研究了大黄素对拓扑异构酶II (topoisomerase II, Topo II) 活性以及Topo II α-DNA可切割复合物的影响。
另外,我们还应用了计算机模拟分子对接技术来预测大黄素与Topo II的相互作用位点。
最后,通过同位素标记技术和核磁共振技术(Nuclear Magnetic Resonance, NMR)验证模拟结果并检测了大黄素对Topo II介导的ATP水解的影响。
结果:TK基因突变试验和体外微核试验表明,在非代谢活化的条件下,80 µg/mL 的大黄素具有弱的遗传毒性。
但是中性彗星试验和磷酸化的H2AX的表达上调表明,大黄素能够引起DNA的双链断裂。
而且,在非细胞体系中,大黄素能够抑制Topo II α介导的pBR322的解螺旋和kDNA的去连环,表明大黄素能够抑制Topo II的活性。
Topo II催化抑制剂阿克拉霉素能够保护大黄素对TK6细胞引起的DNA双链断裂的损伤作用,同样的结果也显现在Topo II缺陷型HL-60/MX2细胞中。
实验表明大黄素能够通过与ATP竞争结合至Topo II的ATP结构域,并能抑制ATP的水解来影响Topo II的活性。
结论:试验结果表明,大黄素能够抑制ATP结合至Topo II,从而影响Topo II的活性,导致DNA双链断裂。
关键词:大黄素、DNA双链断裂、拓扑异构酶、ATPEmodin inhibited ATP binding to Topoisomerase II toinduce DNA double-strand breaksLi Yan, Luan Yang, Ren Jin(Center for Drug Safety Evaluation and Research, Shanghai Institute of Materia Medica,Chinese Academy of Sciences)AbstractOBJECTIVE: Emodin has been widely used as a component of laxatives, whereas it can lead to genotoxicity. However, the mechanism underlining its genotoxicity is not entirely clear. We applied different assays to investigate the mechanisms by which emodin induces genotoxicity.METHODS: Ames assay, thymidine kinase (TK) gene mutation assay and in vitro and in vivo micronucleus (MN) test were used to evaluate genotoxicity caused by emodin. A series of standard biochemical and cellular methods were applied to investigate the inhibition of emodin on topoisomerases. Nuclear Magnetic Resonance (NMR) was used to investigate the effects of emodin on ATP hydrolysis. Finally, molecular docking analyses were used to demonstrate the direct interaction between emodin and Topo II.RESULTS: Without metabolic activation, emodin at 80 µg/mL was mildly genotoxic as indicated in thymidine kinase (TK) gene mutation assay and micronucleus (MN) test. But in the neutral comet assay and the detection of γ-H2AX, emodin at 80 µg/mL induced DNA double-strand breaks (DSBs). Moreover, results obtained from inhibitions of kDNA decatenation and relaxation of supercoiled pBR322 induced by topoisomerase II (Topo II) showed that emodin inhibited Topo II activity. Further, using both aclarubicin, a Topo II catalytic inhibitor, and HL-60/MX2 cells deficient in Topo II, we showed that emodin-triggered DSBs s were in a Topo II –dependent manner. However, emodin did not intercalate into DNA. In contrast, emodin interacted with Topo II by competing with ATP for binding to the ATPase domain of human Topo II α and inhibited ATP hydrolysis.CONCLUSION: Taken together, these results suggested that emodin inhibited ATP binding to Topo II to induce DSBs.Key words: emodin, DNA double-strand breaks, topoisomerase II α, ATP一、前言大黄素,化学名称是6-甲基-1,3,8-三羟基蒽醌,是从大黄属、蓼属、鼠李属和番泻叶等中药中分离得到的活性成分,目前广泛用于中药为基础的泻药制备中。
DNA双链断裂
ห้องสมุดไป่ตู้
研究人员利用人类和小鼠细胞以及不同的DNA 双链断裂(DSBs)诱导因子和测序平台对 BLESS法进行验证。BLESS能够识别端粒末 端、SCE核酸内切酶诱导的DNA双链断裂以及 复杂的DSB全基因组。
作为一种原理证明,我们让人类细胞复制压以 敏感性基因组为特点,确定了多于2000非均 匀分布的阿非迪霉素敏感区(ASRS),它们中 基因过多,富含微卫星重复序列。人类癌症重 排区也富含ASRS,许多癌症相关的基因对复 制压具有极高的敏感性。
带着期望了解更多有关DNA损伤,Mucke的团 队将研究焦点放在双链DNA断裂上,这是神经 科学家认为最严重的损伤形式。此研究小组揭 示了一种自然行为,如探索一种新环境,能引 起野生型年轻成年小鼠DNA双链断裂。DNA 双链断裂发生在多个脑区域,但在海马齿状回 神经最丰富。海马齿状回神经参与学习和记忆 ,能在24小时内修复。
罗格斯大学的神经遗传学家Karl Herrup表示 ,DNA损伤对神经元的伤害极其危险。它无可 更换,细胞必须从这些断裂中获取某些有价值 的物质。加州大学神经科医生Lennart Mucke ,在他研究改变阿尔茨海默氏病(AD)基因组稳 定性,偶然发现这种逮捕概念:切断和修复 DNA是正常大脑活动的一部分。
?作为一种原理证明我们让人类细胞复制压以敏感性基因组为特点确定了多于2000非均匀分布的阿非迪霉素敏感区asrs它们中基因过多富含微卫星重复序列
顶级期刊聚焦DNA双链断裂(DSBs)
最近,有关DNA双链断裂(DSBs)研究成果相继 发表在顶级期刊Nature杂志子刊上。首先科学家提 出一种利用全基因组技术进行DNA双链断裂( DSBs)作图新方法,相关研究成果荣登 Nature Method杂志。于此同时,Nature杂志另一子刊 Nature Neuroscience也刊登了一项成果,讲述了引 发DNA双链断裂的因素。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
最近,有关DNA双链断裂(DSBs)研究成果相继发表在顶级期刊Nature杂志子刊上。
首先科学家提出一种利用全基因组技术进行DNA双链断裂(DSBs)作图新方法,相关研究成果荣登Nature Method杂志。
于此同时,Nature杂志另一子刊Nature Neuroscience也刊登了一项成果,讲述了引发DNA双链断裂的因素。
Nature.Med:全基因组DSB作图新方法
近日,科学家提出一种利用全基因组技术进行DNA双链断裂(DSBs)作图新方法,这种作图法以单核苷酸为基本单位,称之为BLESS(direct in situ breaks labeling, enrichment on streptavidin and next-generation sequencing),相关研究论文于2013年3月20日在线发表在Nature Method杂志上。
此项研究由德国法兰克福歌德大学医学院生物化学研究所 II、美国得克萨斯医学科大学转化科学研究院等处研究人员共同完成。
研究人员利用人类和小鼠细胞以及不同的DNA双链断裂(DSBs)诱导因子和测序平台对BLESS法进行验证。
BLESS能够识别端粒末端、SCE核酸内切酶诱导的DNA双链断裂以及复杂的DSB全基因组。
作为一种原理证明,我们让人类细胞复制压以敏感性基因组为特点,确定了> 2,000非均匀分布的阿非迪霉素敏感区(ASRS),它们中基因过多,富含微卫星重复序列。
人类癌症重排区也富含ASRS,许多癌症相关的基因对复制压具有极高的敏感性。
研究人员称,这种新方法适合各种细胞核实验条件下DSBs全基因组作图,其特异性和分辨率是现在的技术无法实现的。
罗格斯大学的神经遗传学家 Karl Herrup表示,这项工作令人惊叹。
DNA
损伤对神经元的伤害极其危险。
它无可更换,细胞必须从这些断裂中获取某些有价值的物质。
Herrup并未参与此项研究。
加州大学神经科医生 Lennart Mucke,在他研究改变阿尔茨海默氏病(AD)基因组稳定性,偶然发现这种逮捕概念:切断和修复DNA是正常大脑活动的一部分。
AD特征ß是淀粉样蛋白和受损的神经元DNA纠结成块。
带着期望了解更多有关DNA损伤及其相关高水平的淀粉样蛋白,Mucke的团队将研究焦点放在双链DNA断裂上,这是神经科学家认为最严重的损伤形式。
此研究小组揭示了一种自然行为,如探索一种新环境,能引起野生型年轻成年小鼠DNA双链断裂(DSBs)。
DNA双链断裂(DSBs)发生在多个脑区域,但在海马齿状回神经最丰富。
海马齿状回神经参与学习和记忆,能在24小时内修复。
感受器或光刺激下增加的神经元活动,增加了相关神经元DNA双链断裂(DSBs),而非不相关的神经网络。
人淀粉样前体蛋白(hAPP)转基因小鼠增加了基线神经元DNA双链断裂(DSBs),且在探索新环境后出现严重和长期的DSBs。
其中hAPP可刺激AD关键方面。
hAPP 小鼠中,抑制神经元异常活动的中断以及学习和记忆提高,使小鼠中DNA双链断裂(DSBs)水平正常化。
神经细胞培养中,阻断突触外NMDA型谷氨酸
受体能阻止β-淀粉样蛋白(Aβ)诱导的DNA双链断裂(DSBs)。
因此,神经细胞DNA双链断裂(DSBs)的明显增加,是由于大脑生理活动引发的,且β-淀粉样蛋白(Aβ)加剧DNA损伤极有可能是由于突触功能障碍引发的。
原文摘要:
Nucleotide-resolution DNA double-strand break mapping by
next-generation sequencing
We present a genome-wide approach to map DNA double-strand breaks (DSBs) at nucleotide resolution by a method we termed BLESS (direct in situ breaks labeling, enrichment on streptavidin and next-generation sequencing). We validated and tested BLESS using human and mouse cells and different DSBs-inducing agents and sequencing platforms. BLESS was able to detect telomere ends, Sce endonuclease–induced DSBs and complex genome-wide DSB landscapes. As a proof of principle, we characterized the genomic landscape of sensitivity to replication stress in human cells, and we identified >2,000 nonuniformly distributed aphidicolin-sensitive regions (ASRs) overrepresented in genes and enriched in satellite repeats. ASRs were also enriched in regions rearranged in human cancers, with many cancer-associated genes exhibiting high sensitivity to replication stress. Our method is suitable for genome-wide mapping of DSBs in various cells and experimental conditions, with a specificity and resolution unachievable by current techniques.
Physiologic neuron activity causes DNA double-strand breaks in neurons,
with exacerbation by amyloid-ß
We show that a natural behavior, exploration of a novel environment, causes DNA double-strand breaks (DSBs) in neurons of young adult
wild-type mice. DSBs occurred in multiple brain regions, were most abundant in the dentate gyrus, which is involved in learning and memory, and were repaired within 24 h. Increasing neuronal activity by sensory or optogenetic stimulation increased neuronal DSBs in relevant but not irrelevant networks. Mice transgenic for human amyloid precursor protein (hAPP), which simulate key aspects of Alzheimer's disease, had increased neuronal DSBs at baseline and more severe and prolonged DSBs after exploration. Interventions that suppress aberrant neuronal activity and improve learning and memory in hAPP mice normalized their levels of DSBs. Blocking extrasynaptic NMDA-type glutamate receptors prevented amyloid-β (Aβ)-induced DSBs in neuronal cultures. Thus, transient increases in neuronal DSBs occur as a result of physiological brain
activity, and Aβ exacerbates DNA damage, most likely by eliciting synaptic dysfunction.。
