高分子材料专外翻译
高分子材料工程专业英语课文翻译 (2)

高分子材料工程专业英语课文翻译Polymer Science and Polymer Engineering are closely related andoften used interchangeably. Polymer Science is concerned with the chemistry and physics of polymers, while Polymer Engineering teaches students how to design and manufacture polymer products. No matter which field you choose, there is constant innovation and new developments in the field of Polymer Science and Engineering.高分子科学和高分子工程密切相关,常常互换使用。
高分子科学研究聚合物的化学和物理学,而高分子工程则教授学生如何设计和制造聚合物产品。
无论您选择哪个领域,高分子科学和工程的领域中都不断有创新和新发展。
Polymers are large molecules that are made up of repeating units called monomers. These molecules are characterized by their high molecular weight, which gives them unique properties such as strength, elasticity, and durability. There are many types of polymers, including plastics, rubbers, and fibers.聚合物是由称为单体的重复单位组成的大分子。
高分子材料工程专业英语课文翻译9页word

A高分子化学和高分子物理UNIT 1 What ar e Polym er?第一单元什么是高聚物?什么是高聚物?首先,他们是合成物和大分子,而且不同于低分子化合物,譬如说普通的盐。
与低分子化合物不同的是,普通盐的分子量仅仅是58.5,而高聚物的分子量高于105,甚至大于106。
这些大分子或“高分子”由许多小分子组成。
小分子相互结合形成大分子,大分子能够是一种或多种化合物。
举例说明,想象一组大小相同并由相同的材料制成的环。
当这些环相互连接起来,可以把形成的链看成是具有同种分子量化合物组成的高聚物。
另一方面,独特的环可以大小不同、材料不同,相连接后形成具有不同分子量化合物组成的聚合物。
许多单元相连接给予了聚合物一个名称,poly意味着“多、聚、重复”,mer意味着“链节、基体”(希腊语中)。
例如:称为丁二烯的气态化合物,分子量为54,化合将近4000次,得到分子量大约为200000被称作聚丁二烯(合成橡胶)的高聚物。
形成高聚物的低分子化合物称为单体。
下面简单地描述一下形成过程:丁二烯+丁二烯+…+丁二烯——→聚丁二烯(4000次)因而能够看到分子量仅为54的小分子物质(单体)如何逐渐形成分子量为200000的大分子(高聚物)。
实质上,正是由于聚合物的巨大的分子尺寸才使其性能不同于象苯这样的一般化合物。
例如,固态苯,在5.5℃熔融成液态苯,进一步加热,煮沸成气态苯。
与这类简单化合物明确的行为相比,像聚乙烯这样的聚合物不能在某一特定的温度快速地熔融成纯净的液体。
而聚合物变得越来越软,最终,变成十分粘稠的聚合物熔融体。
将这种热而粘稠的聚合物熔融体进一步加热,不会转变成各种气体,但它不再是聚乙烯(如图1.1)。
固态苯——→液态苯——→气态苯加热,5.5℃加热,80℃固体聚乙烯——→熔化的聚乙烯——→各种分解产物-但不是聚乙烯加热加热图1.1 低分子量化合物(苯)和聚合物(聚乙烯)受热后的不同行为发现另一种不同的聚合物行为和低分子量化合物行为是关于溶解过程。
高分子材料工程专业英语课文翻译

Polymer Materials Engineering Professional EnglishText TranslationIntroductionAs an interdisciplinary field incorporating elements of both chemistry and engineering, Polymer Materials Engineering seeks to synthesize, process, and analyze polymers and polymer-based materialsfor a variety of industrial applications. Materials in this field can range from thermoplastics to thermosets, from elastomers to composites, and from gels to liquid crystals. The study of Polymer Materials Engineering is crucial for industries such as manufacturing, automotive, aerospace, healthcare, and electronics.To master Polymer Materials Engineering, one must not only have a solid foundation in engineering, chemistry, and physics, but also be proficient in technical English. Therefore, reading and translating English texts related to Polymer Materials Engineering is a vital skill for students and professionals in this field.In this article, we will provide a translation of an English text related to Polymer Materials Engineering, with the m of improving readers’ understanding and usage of specialized vocabulary in this field.Text TranslationOriginal English text:Rightly or wrongly, a connection often is made between themechanical performance of a polymeric material and its degree of crystallinity. The inference, however, can be incorrect as many other factors affect the mechanical response of polymer materials. Simply stated, crystalline regions are usually stronger and stiffer than amorphous regions. Generally, the degree of crystallinity that yields optimum properties depends on the polymer type and on the application.Translated text:通常我们会认为高分子材料的力学性能与其结晶度相关联,这种推论并不总是正确的。
高分子专业英语答案

高分子专业英语答案【篇一:高分子材料工程专业英语课文翻译(曹同玉,冯连芳)主编】txt>unit 1 what are polymer?第一单元什么是高聚物?what are polymers? for one thing, they are complex and giant molecules and are different from low molecular weight compounds like, say, common salt. to contrast the difference, the molecular weight of common salt is only 58.5, while that of a polymer can be as high as several hundred thousand, even more than thousand thousands. these big molecules or‘macro-molecules’ are made up of much smaller molecules, can be of one or more chemical compounds. to illustrate, imagine that a set of rings has the same size and is made of the same material. when these things are interlinked, the chain formed can be considered as representing a polymer from molecules of the same compound. alternatively, individual rings could be of different sizes and materials, and interlinked to represent a polymer from molecules of different compounds.什么是高聚物?首先,他们是合成物和大分子,而且不同于低分子化合物,譬如说普通的盐。
高分子材料与工程专业英语答案

高分子材料与工程专业英语答案【篇一:高分子材料专业英语第二版部分答案2】t all polymers are built up from bonding together a single kindof repeating unit. at the other extreme ,protein molecules are polyamides in which n amino acide repeat units are bonded together. although we might still call n the degree of polymerization in this case, it is less usefull,since an aminoacid unit might be any one of some 20-odd molecules that are found in proteins. in this case the molecular weightitself,rather than the degree of the polymerization ,is generally used to describe the molecule. when the actual content of individual amino acids is known,it is their sequence that is of special interest to biochemists and molecular biologists.并不是所有的聚合物都是由一个重复单元链接在一起而形成的。
在另一个极端的情形中,蛋白质分子是由n个氨基酸重复单元链接在一起形成的聚酰胺。
尽管在这个例子中,我们也许仍然把n称为聚合度,但是没有意义,因为一个氨基酸单元也许是在蛋白质中找到的20多个分子中的任意一个。
高分子英语课文翻译

unit11.Not all polymers are built up from bonding together a single kind of repeating unit. At the other extreme ,protein molecules are polyamides in which n amino acide repeat units are bonded together. Although we might still call n the degree of polymerization in this case, it is less usefull,since an amino acid unit might be any one of some 20-odd molecules that are found in proteins. In this case the molecular weight itself,rather than the degree of the polymerization ,is generally used to describe the molecule. When the actual content of individual amino acids is known,it is their sequence that is of special interest to biochemists and molecular biologists.并不是所有的聚合物都是由一个重复单元链接在一起而形成的。
在另一个极端的情形中,蛋白质分子是由n个氨基酸重复单元链接在一起形成的聚酰胺。
尽管在这个例子中,我们也许仍然把n称为聚合度,但是没有意义,因为一个氨基酸单元也许是在蛋白质中找到的20多个分子中的任意一个。
在这种情况下,一般是分子量本身而不是聚合度被用来描述这个分子。
高分子材料工程专业英语词汇及部分课文翻译

专业英语词汇accordion 手风琴activation 活化(作用)addition polymer 加成聚合物,加聚物aggravate 加重,恶化agitation 搅拌agrochemical 农药,化肥Alfin catalyst 醇(碱金属)烯催化剂align 排列成行aliphatic 脂肪(族)的alkali metal 碱金属allyl 烯丙基aluminum alkyl 烷基铝amidation 酰胺化(作用)amino 氨基,氨基的amorphous 无定型的,非晶体的anionic 阴(负)离子的antioxidant 抗氧剂antistatic agent 抗静电剂aromatic 芳香(族)的arrangement (空间)排布,排列atactic 无规立构的attraction 引力,吸引backbone 主链,骨干behavior 性能,行为biological 生物(学)的biomedical 生物医学的bond dissociation energy 键断裂能boundary 界限,范围brittle 脆的,易碎的butadiene 丁二烯butyllithium 丁基锂calendering 压延成型calendering 压延carboxyl 羧基carrier 载体catalyst 催化剂,触媒categorization 分类(法)category 种类,类型cation 正[阳]离子cationic 阳(正)离子的centrifuge 离心chain reaction 连锁反应chain termination 链终止char 炭characterize 表征成为…的特征chilled water 冷冻水chlorine 氯(气)coating 涂覆cocatalyst 助催化剂coil 线团coiling 线团状的colligative 依数性colloid 胶体commence 开始,着手common salt 食盐complex 络合物compliance 柔量condensation polymer 缩合聚合物,缩聚物conductive material 导电材料conformation 构象consistency 稠度,粘稠度contaminant 污物contour 外形,轮廓controlled release 控制释放controversy 争论,争议conversion 转化率conversion 转化copolymer 共聚物copolymerization 共聚(合)corrosion inhibitor 缓释剂countercurrent 逆流crosslinking 交联crystal 基体,结晶crystalline 晶体,晶态,结晶的,晶态的crystalline 结晶的crystallinity 结晶性,结晶度crystallite 微晶decomposition 分解defect 缺陷deformability 变形性,变形能力deformation 形变deformation 变形degree of polymerization 聚合度dehydrogenate 使脱氢density 密度depolymerization 解聚deposit 堆积物,沉积depropagation 降解dewater 脱水diacid 二(元)酸diamine 二(元)胺dibasic 二元的dieforming 口模成型diffraction 衍射diffuse 扩散dimension 尺寸dimensional stability 尺寸稳定性dimer 二聚物(体)diol 二(元)醇diolefin 二烯烃disintegrate 分解,分散,分离dislocation 错位,位错dispersant 分散剂dissociate 离解dissolution 溶解dissolve 使…溶解distort 使…变形,扭曲double bond 双键dough (生)面团,揉好的面drug 药品,药物elastic modulus 弹性模量elastomer 弹性体eliminate 消除,打开,除去elongation 伸长率,延伸率entanglement 缠结,纠缠entropy 熵equilibrium 平衡esterification 酯化(作用)evacuate 撤出extrusion 注射成型extrusion 挤出fiber 纤维flame retardant 阻燃剂flexible 柔软的flocculating agent 絮凝剂folded-chain lamella theory 折叠链片晶理论formulation 配方fractionation 分级fragment 碎屑,碎片fringed-micelle theory 缨状微束理论functional group 官能团functional polymer 功能聚合物functionalized polymer 功能聚合物gel 凝胶glass transition temperature 玻璃化温度glassy 玻璃(态)的glassy 玻璃态的glassy state 玻璃态globule 小球,液滴,颗粒growing chain 生长链,活性链gyration 旋转,回旋hardness 硬度heat transfer 热传递heterogeneous 不均匀的,非均匀的hydocy acid 羧基酸hydrogen 氢(气)hydrogen bonding 氢键hydrostatic 流体静力学hydroxyl 烃基hypothetical 假定的,理想的,有前提的ideal 理想的,概念的imagine 想象,推测imbed 嵌入,埋入,包埋imperfect 不完全的improve 增进,改善impurity 杂质indispensable 不了或缺的infrared spectroscopy 红外光谱法ingredient 成分initiation (链)引发initiator 引发剂inorganic polymer 无机聚合物interaction 相互作用interchain 链间的interlink 把…相互连接起来连接intermittent 间歇式的intermolecular (作用于)分子间的intrinsic 固有的ion 离子ion exchange resin 离子交换树脂ionic 离子的ionic polymerization 离子型聚合irradiation 照射,辐射irregularity 不规则性,不均匀的isobutylene 异丁烯isocyanate 异氰酸酯isopropylate 异丙醇金属,异丙氧化金属isotactic 等规立构的isotropic 各项同性的kinetic chain length 动力学链长kinetics 动力学latent 潜在的light scattering 光散射line 衬里,贴面liquid crystal 液晶macromelecule 大分子,高分子matrix 基体,母体,基质,矩阵mean-aquare end-to-end distance 均方末端距mechanical property 力学性能,机械性能mechanism 机理medium 介质中等的,中间的minimise 最小化minimum 最小值,最小的mo(u)lding 模型mobility 流动性mobilize 运动,流动model 模型modify 改性molecular weight 分子量molecular weight distribution 分子量分布molten 熔化的monofunctional 单官能度的monomer 单体morphology 形态(学)moulding 模塑成型neutral 中性的nonelastic 非弹性的nuclear magnetic resonance 核磁共振nuclear track detector 核径迹探测器number average molecular weight 数均分子量occluded 夹杂(带)的olefinic 烯烃的optimum 最佳的,最佳值[点,状态] orient 定向,取向orientation 定向oxonium 氧鎓羊packing 堆砌parameter 参数parison 型柸pattern 花纹,图样式样peculiarity 特性pendant group 侧基performance 性能,特征permeability 渗透性pharmaceutical 药品,药物,药物的,医药的phenyl sodium 苯基钠phenyllithium 苯基锂phosgene 光气,碳酰氯photosensitizer 光敏剂plastics 塑料platelet 片晶polyamide 聚酰胺polybutene 聚丁烯polycondensation 缩(合)聚(合)polydisperse 多分散的polydispersity 多分散性polyesterification 聚酯化(作用)polyethylene 聚乙烯polyfunctional 多官能度的polymer 聚合物【体】,高聚物polymeric 聚合(物)的polypropylene 聚苯烯polystyrene 聚苯乙烯polyvinyl alcohol 聚乙烯醇polyvinylchloride 聚氯乙烯porosity 多孔性,孔隙率positive 正的,阳(性)的powdery 粉状的processing 加工,成型purity 纯度pyrolysis 热解radical 自由基radical polymerization 自由基聚合radius 半径random coil 无规线团random decomposition 无规降解reactent 反应物,试剂reactive 反应性的,活性的reactivity 反应性,活性reactivity ratio 竞聚率real 真是的release 解除,松开repeating unit 重复单元retract 收缩rubber 橡胶rubbery 橡胶态的rupture 断裂saturation 饱和scalp 筛子,筛分seal 密封secondary shaping operation 二次成型sedimentation 沉降(法)segment 链段segment 链段semicrystalline 半晶settle 沉淀,澄清shaping 成型side reaction 副作用simultaneously 同时,同步single bond 单键slastic parameter 弹性指数slurry 淤浆solar energy 太阳能solubility 溶解度solvent 溶剂spacer group 隔离基团sprinkle 喷洒squeeze 挤压srereoregularity 立构规整性【度】stability 稳定性stabilizer 稳定剂statistical 统计的step-growth polymerization 逐步聚合stereoregular 有规立构的,立构规整性的stoichiometric 当量的,化学计算量的strength 强度stretch 拉直,拉长stripping tower 脱单塔subdivide 细分区分substitution 取代,代替surfactant 表面活性剂swell 溶胀swollen 溶胀的synthesis 合成synthesize 合成synthetic 合成的tacky (表面)发粘的,粘连性tanker 油轮,槽车tensile strength 抗张强度terminate (链)终止tertiary 三元的,叔(特)的tetrahydrofuran 四氢呋喃texture 结构,组织thermoforming 热成型thermondynamically 热力学地thermoplastic 热塑性的thermoset 热固性的three-dimensionally ordered 三维有序的titanium tetrachloride 四氯化钛titanium trichloride 三氯化铁torsion 转矩transfer (链)转移,(热)传递triethyloxonium-borofluoride 三乙基硼氟酸羊trimer 三聚物(体)triphenylenthyl potassium 三苯甲基钾ultracentrifugation 超速离心(分离)ultrasonic 超声波uncross-linked 非交联的uniaxial 单轴的unsaturated 不饱和的unzippering 开链urethane 氨基甲酸酯variation 变化,改变vinyl 乙烯基(的)vinyl chloride 氯乙烯vinyl ether 乙烯基醚viscoelastic 黏弹性的viscoelastic state 黏弹态viscofluid state 黏流态viscosity 黏度viscosity average molecular weight 黏均分子量viscous 粘稠的vulcanization 硫化weight average molecular weight 重均分子量X-ray x射线x光yield 产率Young's modulus 杨氏模量课文翻译第一单元什么是高聚物?什么是高聚物?首先,他们是合成物和大分子,而且不同于低分子化合物,譬如说普通的盐。
高分子材料工程专业英语翻译

Unit 1 What are polymers?What are polymers? For one thing, they are complex and giant molecules and are different from low molecular weight compounds like, say, common salt.什么是高聚物?首先,他们是合成物和大分子,而且不同于低分子化合物,譬如说普通的盐。
To contrast the difference, the molecular weight of common salt is only 58.5, while that of a polymer can be as high as several hundred thousand, even more than thousand thousands.与低分子化合物不同的是,普通盐的分子量仅仅是58.5,而高聚物的分子量高于105,甚至大于106。
These big molecules or ‘macro-molecules’ are made up of much sma ller molecules, can be of one or more chemical compounds.这些大分子或“高分子”由许多小分子组成。
小分子相互结合形成大分子,大分子能够是一种或多种化合物。
To illustrate, imagine that a set of rings has the same size and is made of the same material. When these things are interlinked, the chain formed can be considered as representing a polymer from molecules of the same compound.举例说明,想象一组大小相同并由相同的材料制成的环。
高分子材料专业英语第二版(曹同玉,冯连芳,张菊华)课后例句翻译

Unit11.The essentially the ‘giantness’of the size of the polymer molecule that makes its behavior different from that of a commonly known chemical compound such as benzene.实质上,正是由于聚乙烯的巨大的分子尺寸才使其性能不同于像苯这样的一般化合物(的性能)。
2.The globules of polyvinyl alcohol firstly absorb water,swell and get distorbed in shape and aftera long time go into solution.聚乙烯醇颗粒首先吸水溶胀,发生变形,经过很长的时间以后,(聚乙烯醇分子)进入到溶液中。
3.Another peculiarity is that ,in water,polyvinyl alcohol never retains its original powdery nature as the excess sodium chloride does in a saturated salt solution.另一个特点是,在水中聚乙烯醇不会像过量的氯化钠在饱和盐溶液中那样能保持其初始的粉末状态。
UNIT21.The initiation of the chain reaction can be observed most clearly with radical or ionic initiators.用自由基型引发剂或离子型引发剂引发连锁反应可以很清楚的进行观察。
2.Such reactions occur through the initial addition of a monomer molecule to an initiator radical or an initiator ion,by which the active state is transferred from the added monomer.这样的反应是通过单体分子首先加成到引发剂自由基或引发剂离子上而进行的,靠这些活性中心由引发剂转移到被加成的单体上。
高分子材料与工程专业英语11单元课后作业

4.Put the following words into Chinese
合成橡胶 synthetic rubber
不饱和单体 unsaturated monomer 双键 double bond 二元酸 diacid 偶合终止 coupling termination 平衡反应 balanced reaction 异丁烯和苯乙烯的共聚物 copolymers of isobutylene and
纤维的初始弹性模量较橡胶和塑料两者 都大。(√)
3. Put the following words into Chinese fragment 碎片 hydroxy acid 羟基酸 positive ion 阳离子 reactivity ratio 竞聚率 irregularity 不规则性,不均匀性 viscosity-average molecular weight 粘均分子量 thermodynamically 热力学地 random coil 无规线团 dislocation 错位,位错 vulcanization 硫化
Unit 11 (Page 61) Exercises 之2(并翻译)、3、4
2. Please mark “yes” or “no” in the brankets (1) Polyethylene is a typical vinyl polymers.
聚乙烯是一种典型的乙烯基聚合物。(×)
(2) The term nylon has been accepted as a generic commercial name for po力intermolecular forces 微晶 crystallite 取向 orientation
术语“尼龙”作为聚酰胺的一个通用的商业名称已经被接 受。(√)
高分子材料纳米二氧化硅外文文献翻译

纳米二氧化硅对成核、结晶和热塑性能的影响外文文献翻译(含:英文原文及中文译文)文献出处:Laoutid F, Estrada E, Michell R M, et al. The influence of nanosilica on the nucleation, crystallization andtensile properties of PP–PC and PP–PA blends[J]. Polymer, 2013, 54(15):3982-3993.英文原文The influence of nanosilica on the nucleation, crystallization andtensileproperties of PP–PC and PP–PA blendsLaoutid F, Estrada E, Michell R M, et alAbstractImmiscible blends of 80 wt% polypropylene (PP) with 20 wt% polyamide (PA) or polycarbonate (PC) were prepared by melt mixing with or without the addition of 5% nanosilica. The nanosilica produced a strong reduction of the disperse phase droplet size, because of its preferential placement at the interface, as demonstrated by TEM. Polarized Light Optical microscopy (PLOM) showed that adding PA, PC or combinations of PA-SiO2 or PC-SiO2 affected the nucleation density of PP. PA droplets can nucleate PP under isothermal conditions producing a higher nucleation density than the addition of PC or PC-SiO2. PLOM was found to be more sensitive to determine differences in nucleation than non-isothermal DSC. PP developed spherulites, whose growth was unaffected by blending, while its overall isothermal crystallizationkinetics was strongly influenced by nucleation effects caused by blending. Addition of nanosilica resulted in an enhancement of the strain at break of PP-PC blends whereas it was observed to weaken PP-PA blends. Keywords:Nanosilica,Nucleation,PP blends1 OverviewImmiscible polymer blends have attracted attention for decades because of their potential application as a simple route to tailor polymer properties. The tension is in two immiscible polymerization stages. This effect usually produces a transfer phase between the pressures that may allow the size of the dispersed phase to be allowed, leading to improved mixing performance.Block copolymers and graft copolymers, as well as some functional polymers. For example, maleic anhydride grafted polyolefins act as compatibilizers in both chemical affinities. They can reduce the droplet volume at the interface by preventing the two polymers from coalescing. In recent years, various studies have emphasized that nanofillers, such as clay carbon nanotubes and silica, can be used as a substitute for organic solubilizers for incompatible polymer morphology-stabilized blends. In addition, in some cases, nanoparticles in combination with other solubilizers promote nanoparticle interface position.The use of solid particle-stabilized emulsions was first discovered in 1907 by Pickering in the case of oil/emulsion containing colloidalparticles. In the production of so-called "Pickling emulsions", solid nanoparticles can be trapped in the interfacial tension between the two immiscible liquids.Some studies have attempted to infer the results of blending with colloidal emulsion polymer blends. Wellman et al. showed that nanosilica particles can be used to inhibit coalescence in poly(dimethylsiloxane)/polyisobutylene polymers. mix. Elias et al. reported that high-temperature silicon nanoparticles can migrate under certain conditions. The polypropylene/polystyrene and PP/polyvinyl acetate blend interfaces form a mechanical barrier to prevent coalescence and reduce the size of the disperse phase.In contrast to the above copolymers and functionalized polymers, the nanoparticles are stable at the interface due to their dual chemical nature. For example, silica can affect nanoparticle-polymer affinities locally, minimizing the total free energy that develops toward the system.The nanofiller is preferentially placed in equilibrium and the wetting parameters can be predicted and calculated. The difference in the interfacial tension between the polymer and the nanoparticles depends on the situation. The free-diffusion of the nanoparticle, which induces the nanoparticles and the dispersed polymer, occurs during the high shear process and shows that the limitation of the viscosity of the polymer hardly affects the Brownian motion.As a result, nanoparticles will exhibit strong affinity at the local interface due to viscosity and diffusion issues. Block copolymers need to chemically target a particular polymer to the nanoparticle may provide a "more generic" way to stabilize the two-phase system.Incorporation of nanosilica may also affect the performance of other blends. To improve the distribution and dispersion of the second stage, mixing can produce rheological and material mechanical properties. Silica particles can also act as nucleating agents to influence the crystallization behavior. One studies the effect of crystalline silica on crystalline polystyrene filled with polybutylene terephthalate (polybutylene terephthalate) fibers. They found a stable fibril crystallization rate by increasing the content of polybutylene terephthalate and silica. On the other hand, no significant change in the melt crystallization temperature of the PA was found in the PA/ABS/SiO2 nanocomposites.The blending of PP with engineering plastics, such as polyesters, polyamides, and polycarbonates, may be a useful way to improve PP properties. That is, improving thermal stability, increasing stiffness, improving processability, surface finish, and dyeability. The surface-integrated nano-silica heat-generating morphologies require hybrid compatibilization for the 80/20 weight ratio of the thermal and tensile properties of the blended polyamide and polypropylene (increasedperformance). Before this work, some studies [22] that is, PA is the main component). This indicates that the interfacially constrained hydrophobic silica nanoparticles obstruct the dispersed phase; from the polymer and allowing a refinement of morphology, reducing the mixing scale can improve the tensile properties of the mixture.The main objective of the present study was to investigate the effect of nanosilica alone on the morphological, crystalline, and tensile properties of mixtures of nanosilica alone (for mixed phases with polypropylene as a matrix and ester as a filler. In particular, PA/PC or PA/nano The effect of SiO 2 and PC/nanosilica on the nucleation and crystallization effects of PP as the main component.We were able to study the determination of the nucleation kinetics of PP and the growth kinetics of the particles by means of polarization optical microscopy. DSC measures the overall crystallization kinetics.Therefore, a more detailed assessment of the nucleation and spherulite growth of PP was performed, however, the effect of nanosilica added in the second stage was not determined. The result was Akemi and Hoffman. And Huffman's crystal theory is reasonable.2 test phase2.1 Raw materialsThe polymer used in this study was a commercial product: isotactic polypropylene came from a homopolymer of polypropylene. The Frenchformula (B10FB melt flow index 2.16Kg = 15.6g / 10min at 240 °C) nylon 6 from DSM engineering plastics, Netherlands (Agulon Fahrenheit temperature 136 °C, melt flow index 240 °C 2.16kg = 5.75g / 10min ) Polycarbonate used the production waste of automotive headlamps, its melt flow index = 5g / 10min at 240 °C and 2.16kg.The silica powder TS530 is from Cabot, Belgium (about 225 m/g average particle (bone grain) about 200-300 nm in length, later called silica is a hydrophobic silica synthesis of hexamethyldisilane by gas phase synthesis. Reacts with silanols on the surface of the particles.2.2 ProcessingPP_PA and PP-PC blends and nanocomposites were hot melt mixed in a rotating twin screw extruder. Extrusion temperatures range from 180 to 240 °C. The surfaces of PP, PA, and PC were vacuumized at 80°C and the polymer powder was mixed into the silica particles. The formed particles were injected into a standard tensile specimen forming machine at 240C (3 mm thickness of D638 in the American Society for Testing Materials). Prior to injection molding, all the spherulites were in a dehumidified vacuum furnace (at a temperature of 80°C overnight). The molding temperature was 30°C. The mold was cooled by water circulation. The mixture of this combination is shown in the table.2.3 Feature Description2.31 Temperature Performance TestA PerkineElmer DSC diamond volume thermal analysis of nanocomposites. The weight of the sample is approximately 5 mg and the scanning speed is 20 °C/min during cooling and heating. The heating history was eliminated, keeping the sample at high temperature (20°C above the melting point) for three minutes. Study the sample's ultra-high purity nitrogen and calibrate the instrument with indium and tin standards.For high temperature crystallization experiments, the sample cooling rate is 60°C/min from the melt directly to the crystal reaching the temperature. The sample is still three times longer than the half-crystallization time of Tc. The procedure was deduced by Lorenzo et al. [24] afterwards.2.3.2 Structural CharacterizationScanning electron microscopy (SEM) was performed at 10 kV using a JEOL JSM 6100 device. Samples were prepared by gold plating after fracture at low temperature. Transmission electron microscopy (TEM) micrographs with a Philips cm100 device using 100 kV accelerating voltage. Ultra-low cut resection of the sample was prepared for cutting (Leica Orma).Wide-Angle X-Ray Diffraction Analysis The single-line, Fourier-type, line-type, refinement analysis data were collected using a BRUKER D8 diffractometer with copper Kα radiation (λ = 1.5405A).Scatter angles range from 10o to 25°. With a rotary step sweep 0.01° 2θ and the step time is 0.07s. Measurements are performed on the injection molded disc.This superstructure morphology and observation of spherulite growth was observed using a Leica DM2500P polarized light optical microscope (PLOM) equipped with a Linkam, TP91 thermal stage sample melted in order to eliminate thermal history after; temperature reduction of TC allowed isothermal crystallization to occur from the melt. The form is recorded with a Leica DFC280 digital camera. A sensitive red plate can also be used to enhance contrast and determine the birefringence of the symbol.2.3.3 Mechanical AnalysisTensile tests were carried out to measure the stretch rate at 10 mm/min through a Lloyd LR 10 K stretch bench press. All specimens were subjected to mechanical tests for 20 ± 2 °C and 50 ± 3% relative humidity for at least 48 hours before use. Measurements are averaged over six times.3 results3.1 Characterization by Electron MicroscopyIt is expected that PP will not be mixed with PC, PA because of their different chemical properties (polar PP and polar PC, PA) blends with 80 wt% of PP, and the droplets and matrix of PA and PC are expectedmorphologies [ 1-4] The mixture actually observed through the SEM (see Figures 1 a and b).In fact, because the two components have different polar mixtures that result in the formation of an unstable morphology, it tends to macroscopic phase separation, which allows the system to reduce its total free energy. During shearing during melting, PA or PP is slightly mixed, deformed and elongated to produce unstable slender structures that decompose into smaller spherical nodules and coalesce to form larger droplets (droplets are neat in total The size of the blend is 1 ~ 4mm.) Scanning electron microscopy pictures and PP-PC hybrid PP-PA neat and clean display left through the particle removal at cryogenic temperatures showing typical lack of interfacial adhesion of the immiscible polymer blend.The addition of 5% by weight of hydrophobic silica to the LED is a powerful blend of reduced size of the disperse phase, as can be observed in Figures 1c and D. It is worth noting that most of the dispersed phase droplets are within the submicron range of internal size. The addition of nano-SiO 2 to PA or PC produces finer dispersion in the PP matrix.From the positional morphology results, we can see this dramatic change and the preferential accumulation at the interface of silica nanoparticles, which can be clearly seen in FIG. 2 . PP, PA part of the silicon is also dispersed in the PP matrix. It can be speculated that thisformation of interphase nanoparticles accumulates around the barrier of the secondary phase of the LED, thus mainly forming smaller particles [13, 14, 19, 22]. According to fenouillot et al. [19] Nanoparticles are mixed in a polymer like an emulsifier; in the end they will stably mix. In addition, the preferential location in the interval is due to two dynamic and thermodynamic factors. Nanoparticles are transferred to the preferential phase, and then they will accumulate in the interphase and the final migration process will be completed. Another option is that there isn't a single phase of optimization and the nanoparticles will be set permanently in phase. In the current situation, according to Figure 2, the page is a preferential phase and is expected to have polar properties in it.3.2 Wide-angle x-ray diffractionThe polymer and silica incorporate a small amount of nanoparticles to modify some of the macroscopic properties of the material and the triggered crystal structure of PP. The WAXD experiment was performed to evaluate the effect of the incorporation of silica on the crystalline structure of the mixed PP.Isotactic polypropylene (PP) has three crystalline forms: monoclinic, hexagonal, and orthorhombic [25], and the nature of the mechanical polymer depends on the presence of these crystalline forms. The metastable B form is attractive because of its unusual performance characteristics, including improved impact strength and elongation atbreak.The figure shows a common form of injection molding of the original PP crystal, reflecting the appearance at 2θ = 14.0, 16.6, 18.3, 21.0 and 21.7 corresponding to (110), (040), (130), (111) and (131) The face is an α-ipp.20% of the PA incorporation into PP affects the recrystallization of the crystal structure appearing at 2θ = 15.9 °. The corresponding (300) surface of the β-iPP crystal appears a certain number of β-phases that can be triggered by the nucleation activity of the PA phase in PP (see evidence The following nucleation) is the first in the crystalline blend of PA6 due to its higher crystallization temperature. In fact, Garbarczyk et al. [26] The proposed surface solidification caused by local shear melts the surface of PA6 and PP and forms during the injection process, promoting the formation of β_iPP. According to quantitative parameters, KX (Equation (1)), which is commonly used to evaluate the amount of B-crystallites in PP including one and B, the crystal structure of β-PP has 20% PP_PA (110), H(040) and Blends of H (130) heights (110), (040) and (130). The height at H (300) (300) for type A peaks.However, the B characteristic of 5 wt% silica nanoparticles incorporated into the same hybrid LED eliminates reflection and reflection a-ipp retention characteristics. As will be seen below, the combination of PA and nanosilica induces the most effective nucleatingeffect of PP, and according to towaxd, this crystal formation corresponds to one PP structure completely.The strong reductive fracture strain observations when incorporated into polypropylene and silica nanoparticles (see below) cannot be correlated to the PP crystal structure. In fact, the two original PP and PP_PA_SiO2 hybrids contain α_PP but the original PP has a very high form of failure when the strain value.On the other hand, PP-PC and PP-PC-Sio 2 blends, through their WAXD model, can be proven to contain only one -PP form, which is a ductile material.3.3 Polarized Optical Microscopy (PLOM)To further investigate the effect of the addition of two PAs, the crystallization behavior of PC and silica nanoparticles on PP, the X-ray diffraction analysis of its crystalline structure of PP supplements the study of quantitative blends by using isothermal kinetic conditions under a polarizing microscope. The effect of the composition on the nucleation activity of PP spherulite growth._Polypropylene nucleation activityThe nucleation activity of a polymer sample depends on the heterogeneity in the number and nature of the samples. The second stage is usually a factor in the increase in nucleation density.Figure 4 shows two isothermal crystallization temperatures for thePP nucleation kinetics data. This assumes that each PP spherulite nucleates in a central heterogeneity. Therefore, the number of nascent spherulites is equal to the number of active isomerous nuclear pages, only the nucleus, PP-generated spherulites can be counted, and PP spherulites are easily detected. To, while the PA or PC phases are easily identifiable because they are secondary phases that are dispersed into droplets.At higher temperatures (Fig. 4a), only the PP blend inside is crystallized, although the crystals are still neat PP amorphous at the observed time. This fact indicates that the second stage of the increase has been able to produce PP 144 °C. It is impossible to repeat the porous experiment in the time of some non-homogeneous nucleation events and neat PP exploration.The mixed PP-PC and PP-PC-SiO 2 exhibited relatively low core densities at 144 °C, (3 105 and 3 106 nuc/cm 3) suggesting that either PC nanosilica can also be considered as good shape Nuclear agent is used here for PP.On the other hand, PA, himself, has produced a sporadic increase in the number of nucleating events in PP compared to pure PP, especially in the longer crystallization time (>1000 seconds). In the case of the PP-PA _Sio 2 blend, the heterogeneous nucleation of PP is by far the largest of all sample inspections. All the two stages of the nucleating agent combined with PA and silica are best employed in this work.In order to observe the nucleation of pure PP, a lower crystallization temperature was used. In this case, observations at higher temperatures found a trend that was roughly similar. The neat PP and PP-PC blends have small nucleation densities in the PP-PC-SiO 2 nanocomposite and the increase also adds further PP-PA blends. The very large number of PP isoforms was rapidly activated at 135°C in the PP-PA nanoparticle nanometer SiO 2 composites to make any quantification of their numbers impossible, so this mixed data does not exist from Figure 4b.The nucleation activity of the PC phase of PP is small. The nucleation of any PC in PP can be attributed to impurities that affect the more complex nature of the PA from the PC phase. It is able to crystallize at higher temperatures than PP, fractional crystallization may occur and the T temperature is shifted to much lower values (see References [29-39]. However, as DSC experiments show that in the current case The phase of the PA is capable of crystallizing (fashion before fractionation) the PP matrix, and the nucleation of PP may have epitaxy origin.The material shown in the figure represents a PLOAM micrograph. Pure PP has typical α-phase negative spherulites (Fig. 5A) in the case of PP-PA blends (Fig. 5B), and the PA phase is dispersed with droplets of size greater than one micron (see SEM micrograph, Fig. 1) . We could not observe the spherulites of the B-phase type in PP-PA blends. Even according to WAXD, 20% of them can be formed in injection moldedspecimens. It must be borne in mind that the samples taken using the PLOAM test were cut off from the injection molded specimens but their thermal history (direction) was removed by melting prior to melting for isothermal crystallization nucleation experiments.The PA droplets are markedly enhanced by the nucleation of polypropylene and the number of spherulites is greatly increased (see Figures 4 and 5). Simultaneously with the PP-PA blend of silica nanoparticles, the sharp increase in nucleation density and Fig. 5C indicate that the size of the spherulites is very small and difficult to identify.The PP-PC blends showed signs of sample formation during the PC phase, which was judged by large, irregularly shaped graphs. Significant effects: (a) No coalesced PC phase, now occurring finely dispersed small droplets and (B) increased nucleation density. As shown in the figure above, nano-SiO 2 tends to accumulate at the interface between the two components and prevent coalescence while promoting small disperse phase sizes.From the nucleation point of view, it is interesting to note that it is combined with nanosilica and as a better nucleating agent for PP. Combining PCs with nanosilica does not produce the same increase in nucleation density.Independent experiments (not shown here) PP _ SiO 2 samplesindicate that the number of active cores at 135 °C is almost the same as that of PP-PC-SiO2 intermixing. Therefore, silica cannot be regarded as a PP nucleating agent. Therefore, the most likely explanation for the results obtained is that PA is the most important reason for all the materials used between polypropylene nucleating agents. The increase in nucleation activity to a large extent may be due to the fact that these nanoparticles reduce the size of the PA droplets and improve its dispersion in the PP matrix, improving the PP and PA in the interfacial blend system. Between the regions. DSC results show that nano-SiO 2 is added here without a nuclear PA phase.4 Conclusion5% weight of polypropylene/hydrophobic nanosilica blended polyamide and polypropylene/polycarbonate (80E20 wt/wt) blends form a powerful LED to reduce the size of dispersed droplets. This small fraction of reduced droplet size is due to the preferential migration of silica nanoparticles between the phases PP and PA and PC, resulting in an anti-aggregation and blocking the formation of droplets of the dispersed phase.The use of optical microscopy shows that the addition of PA, the influence of PC's PA-Sio 2 or PC-Sio 2 combination on nucleation, the nucleation density of PP polypropylene under isothermal conditions is in the following approximate order: PP <PP-PC <PP -PC-SiO 2<<PP-PA<<< PP-PA-SiO 2. PA Drip Nucleation PP Production of nucleation densities at isothermal temperatures is higher than with PC or PC Sio 2D. When nanosilica is also added to the PP-PA blend, the dispersion-enhanced mixing of the enhanced nanocomposites yields an intrinsic factor PP-PA-Sio2 blend that represents a PA that is identified as having a high nucleation rate, due to nanoseconds Silicon oxide did not produce any significant nucleation PP. PLOAM was found to be a more sensitive tool than traditional cooling DSC scans to determine differences in nucleation behavior. The isothermal DSC crystallization kinetics measurements also revealed how the differences in nucleation kinetics were compared to the growth kinetic measurements.Blends (and nanocomposites of immiscible blends) and matrix PP spherulite assemblies can grow and their growth kinetics are independent. The presence of a secondary phase of density causes differences in the (PA or PC) and nanosilica nuclei. On the other hand, the overall isothermal crystallization kinetics, including nucleation and growth, strongly influence the nucleation kinetics by PLOAM. Both the spherulite growth kinetics and the overall crystallization kinetics were successfully modeled by Laurie and Huffman theory.Although various similarities in the morphological structure of these two filled and unfilled blends were observed, their mechanical properties are different, and the reason for this effect is currently being investigated.The addition of 5% by weight of hydrophobic nano-SiO 2 resulted in breaking the strain-enhancement of the PP-PC blend and further weakening the PP-PA blend.中文译文纳米二氧化硅对PP-PC和PP-PA共混物的成核,结晶和热塑性能的影响Laoutid F, Estrada E, Michell R M, et al摘要80(wt%)聚丙烯与20(wt %)聚酰胺和聚碳酸酯有或没有添加5%纳米二氧化硅通过熔融混合制备不混溶的共聚物。
高分子材料工程专业英语翻译全

第一单元什么是高聚物?什么是高聚物?首先,他们是合成物和大分子,而且不同于低分子化合物,譬如说普通的盐。
与低分子化合物不同的是,普通盐的分子量仅仅是58.5,而高聚物的分子量高于105,甚至大于106。
这些大分子或“高分子〞由许多小分子组成。
小分子互相结合形成大分子,大分子可以是一种或多种化合物。
举例说明,想象一组大小一样并由一样的材料制成的环。
当这些环互相连接起来,可以把形成的链看成是具有同种分子量化合物组成的高聚物。
另一方面,独特的环可以大小不同、材料不同,相连接后形成具有不同分子量化合物组成的聚合物。
许多单元相连接给予了聚合物一个名称,poly意味着“多、聚、重复〞,mer意味着“链节、基体〞〔希腊语中〕。
例如:称为丁二烯的气态化合物,分子量为54,化合将近4000次,得到分子量大约为200000被称作聚丁二烯〔合成橡胶〕的高聚物。
形成高聚物的低分子化合物称为单体。
下面简单地描绘一下形成过程:丁二烯+丁二烯+…+丁二烯——→聚丁二烯〔4000次〕因此可以看到分子量仅为54的小分子物质〔单体〕如何逐渐形成分子量为200000的大分子〔高聚物〕。
本质上,正是由于聚合物的宏大的分子尺寸才使其性能不同于象苯这样的一般化合物。
例如,固态苯,在5.5℃熔融成液态苯,进一步加热,煮沸成气态苯。
与这类简单化合物明确的行为相比,像聚乙烯这样的聚合物不能在某一特定的温度快速地熔融成纯洁的液体。
而聚合物变得越来越软,最终,变成非常粘稠的聚合物熔融体。
将这种热而粘稠的聚合物熔融体进一步加热,不会转变成各种气体,但它不再是聚乙烯〔如图1.1〕。
固态苯——→液态苯——→气态苯加热,5.5℃加热,80℃固体聚乙烯——→熔化的聚乙烯——→各种分解产物-但不是聚乙烯加热加热图1.1 低分子量化合物〔苯〕和聚合物〔聚乙烯〕受热后的不同行为发现另一种不同的聚合物行为和低分子量化合物行为是关于溶解过程。
例如,让我们研究一下,将氯化钠渐渐地添加到固定量的水中。
高分子专业英语翻译[最新]
![高分子专业英语翻译[最新]](https://img.taocdn.com/s3/m/a82cc3a6f78a6529657d5380.png)
高分子专业英语翻译[最新]第五课乳液聚合大部分的乳液聚合都是由自由基引发的并且表现出其他自由基体系的很多特点,最主要的反应机理的不同源自小体积元中自由基增长的场所不同。
乳液聚合不仅允许在高反应速率下获得较高分子量,这在本体聚合中是无法实现或效率低下的,,同时还有其他重要的实用优点。
水吸收了大部分聚合热且有利于反应控制,产物在低粘度体系中获得,容易处理,可直接使用或是在凝聚,水洗,干燥之后很快转化成固体聚合物。
在共聚中,尽管共聚原理适用于乳液体系,单体在水相中溶解能力的不同也可能导致其与本体聚合行为不同,从而有重要的实际意义。
乳液聚合的变化很大,从包含单一单体,乳化剂,水和单一引发剂的简单体系到这些包含有2,3个单体,一次或分批添加,,混合乳化剂和助稳定剂以及包括链转移剂的复合引发体系。
单体和水相的比例允许变化范围很大,但是在技术做法上通常限制在30/70到60/40。
单体和水相比更高时则达到了直接聚合允许的极限,只有通过分批添加单体方法来排除聚合产生的大量的热。
更复杂的是随着胶体数的增加粘度也大大增加,尤其是当水溶性的单体和聚合物易容时,反应结束胶乳浓度降低。
这一阶段常常伴随着通过聚集作用或是在热力学不稳定时凝结作用而使胶粒尺寸增大。
第十课高分子的构型和构象本课中我们将使用根据经典有机化学术语而来的构型和构象这两个词。
构型异构是由于分子中存在一个或多个不对称中心,以最简单的C原子为例,每一碳原子的绝对构型为R型和S型,当存在双键时会有顺式和反式几何异构。
以合成聚合物为例,构型异构的典型问题和R.S型不对称碳原子在主链上的排布有关。
这些不对称碳原子要么来自不对称单体,如环氧丙烷,要么来自对称单体,如乙烯单体,,这些物质的聚合,在每个单体单元中形成至少一个不对称碳原子。
大分子中的构型异构源于侧链上存在不对称的碳原子,例如不对称乙烯单体的聚合,也是可能的,现今已经被广泛研究。
和经典有机化学术语一致,构象,旋转体,旋转异构体,构象异构体,指的是由于分子单键的内旋转而形成的空间排布的不同。
高分子材料工程专业英语翻译

高分子材料工程专业英语翻译UNIT 1 What are Polymer 第一单元什么是高聚物What are polymers For one thing they are complex and giant molecules and are different from low molecular weight compounds like say common salt. To contrast the difference the molecular weight of common salt is only 58.5 while that of a polymer can be as high as several hundred thousand even more than thousand thousands. These big molecules or‘macro-molecules’ are made up of much smaller molecules can be of one or more chemical compounds. To illustrate imagine that a set of rings has the same size and is made of the same material. When these things are interlinked the chain formed can be considered as representing a polymer from molecules of the same compound. Alternatively individual rings could be of different sizes and materials and interlinked to represent a polymer from molecules of different compounds. 什么是高聚物首先他们是合成物和大分子而且不同于低分子化合物譬如说普通的盐。
高分子英语课文翻译

unit1all polymers are built up from bonding together a single kind of repeating unit. At the other extreme ,protein molecules are polyamides in which n amino acide repeat units are bonded together. Although we might still call n the degree of polymerization in this case, it is less usefull,since an amino acid unit might be any one of some 20-odd molecules that are found in proteins. In this case the molecular weight itself,rather than the degree of the polymerization ,is generally used to describe the molecule. When the actual content of individual amino acids is known,it is their sequence that is of special interest to biochemists and molecular biologists.并不是所有的聚合物都是由一个重复单元链接在一起而形成的;在另一个极端的情形中,蛋白质分子是由n个氨基酸重复单元链接在一起形成的聚酰胺;尽管在这个例子中,我们也许仍然把n称为聚合度,但是没有意义,因为一个氨基酸单元也许是在蛋白质中找到的20多个分子中的任意一个;在这种情况下,一般是分子量本身而不是聚合度被用来描述这个分子;当知道了特定的氨基酸分子的实际含量,它们的序列正是生物化学家和分子生物学家特别感兴趣的地方;1,题目:Another striking ...答案:.that quantity low saturation bottom much absorb 2. 乙烯分子带有一个双键,为一种烯烃,它可以通过连锁聚合大量地制造聚乙烯,目前,聚乙烯已经广泛应用于许多技术领域和人们的日常生活中,成为一种不可缺少的材料;Ethylene molecule with a double bond, as a kind of olefins, it can make chain polymerization polyethylene, at present, polyethylene has been widely used in many fields of technology and People's Daily life, become a kind of indispensable materials.Unit31 The polymerization rate may be experimentally followed by measuring the changes in any of several properties of the system such as density,refractive index,viscosity, or light absorption. Density measurements are among the most accurate and sensitive of the techniques. The density increases by 20-25 percent on polymerization for many monomers. In actual practice the volume of the polymerizing system is measured by carrying out the reaction in a dilatometer. This is specially constructed vessel with a capillary tube which allows a highly accurate measurement of small volume changes. It is not uncommon to be able to detect a few hundredths of a percent polymerization by the dilatometer technique. 聚合速率在实验上可以通过测定体系的任一性质的变化而确定,如密度、折射率、黏度、或者吸光性能;密度的测量是这些技术中最准确最敏感的;对许多单体的聚合来说,密度增加了20%-25%;在实际操作中,聚合体系的体积是通过在膨胀计中进行反应测定的;它被专门设计构造了毛细导管,在里面可以对微小体积变化进行高精确度测量;通过膨胀计技术探测聚合过程中万分之几的变化是很常见的;Unti42 合成聚合物在各个领域中起着与日俱增的重要作用,聚合物通常是由单体通过加成聚合与缩合聚合制成的;就世界上的消耗量而论,聚烯烃和乙烯基聚合物居领先地位,聚乙烯、聚丙烯等属聚烯烃,而聚氯乙烯、聚苯乙烯等则为乙烯基聚合物;聚合物可广泛地用作塑料、橡胶、纤维、涂料、粘合剂等The synthetic polymers play an increasingly important role on a range of domains, which are synthesized by monomers through addition polymerization or condensation polymerization. Polyolefin and vinyl polymer have taken the lead in terms of the world consumption. PE, PP, etc. belong to the polyolefin, while PS, PVC etc. belong to the vinyl polymer. Polymers can be widely applied in plastics, rubbers, fibers, coatings, glues and so on.Unit7Ring-opening polymerizations proceed only by ionic mechanisms, the polymerization of cyclic ethers mainly by cationic mechanisms, and the polymerization of lactones andlactones by either a cationic or anionic mechanism. Important initiators for cyclic ethers and lactone polymerization are those derived from aluminum alkyl and zinc alkyl/water systems. It should be pointed out that substitution near the reactive group of the monomer is essential for the individual mechanism that operates effectively in specific cases; for example, epoxides polymerize readily with cationic and anionic initiators, while fluorocarbon epoxides polymerize exclusively by anionic mechanisms.开环聚合反应只能通过离子机理进行,环醚的开环聚合主要通过阳离子机理,而内酯和内酰胺的聚合物是通过阳离子或阴离子机理;对于环醚和内酯型聚合物很重要的引发剂是那些来自于烷基铝和烷基锌/水的体系;应该指出的是对于在活性基团附近有取代的单体,只能由单一机理,这一机理是在特定条件下的有效;1 Polymers can be classified into two main groups, addition polymers and ___condensation__ polymers. This classification is based on whether or not the repeating unit of the polymer contains the same atoms __as____ the monomer. The repeating unit of an addition polymer is identical _with/to____ the monomer, while condensation polymers contain __different/less___ because of formation of __compound/byproduct___ during the polymerization process. The corresponding polymerization processed would then be called addition polymerization and condensation polymerization. As was mentioned earlier, this classification can result ___in__ confusion, since it has been shown in later years that many important types of polymers can be _prepared by both addition and condensation processes. For example, polyesters, polyamides and polyurethanes are usually considered to be _condensation____ polymers, but they can be prepared by addition as well as by condensation reaction. Similarly, polyethylene normally considered an _addition_ polymer, can also be prepared by _condensation_ reaction.2. Answer the following questions in English1 What is chain polymerization Manyolefinicandvinylunsaturatedcompoundsareabletoformchain-likemacromoleculesthrougheliminationofdoublebond.2 Which kinds of monomers can carry out step-growth polymerization processThere are two kinds of monomers could carry out step-growth polymerization process. One ispolyfunctionalmonomers and the other isasinglemonomercontainingbothtypesoffunctional groups.3 What properties of polymers can be based on for measuring the molecular weightThe molecular weight of polymer could be measured based on colligativeproperties, lightscattering, viscosity, ultracentrifugation sedimentation.3. Please write out at least 10 kinds of polymers both in English and in Chinesethe corresponging chemical structure5 In general,head-to-tail addition is considered to be the predominant mode of propagation in all polymerizations;However,when the substitutes on the monomer are small and do not offer appreciable steric hindrance to the approaching radical or do not have a large resonance stabilizing effect,as in the case of fluorine atoms,sizable amounts of head-to-head propagation may occur. The effect of increasing polymerization temperature is to increase the amount of head-to-head placement;Increased temperature leads to less selective more random propagation but the effect is not large. Thus,the head-to-head content in poly vinyl acetate only increases from to percent when the polymerization temperature in increased from 30 to 90 ℃.通常在所有聚合物的链增长中,头-尾加成是主要方式;然而,当单体中的取代基很小对接近的自由基没有空间阻碍或没有较大的共振稳定作用,如氟原子,则有相当量的头头增长发生;提高聚合温度的影响是提高头-头排列的量;温度的提高导致较少的选择更多的无规增长,但影响不大;因而,在聚乙酸乙烯酯中,当聚合温度由30C提高到90C,头-头含量仅由%提高到%;2.Write out an abstract in English for the text in this unitPolymers with different structures present various properties. Usually, polymers are divided into three categories, . plastic, elastomer, fiber with different initial modulus range respectively. Polymers show quite different behaviors due to the different interchain forces in elastomer and fiber. However, with the advent of new techniques and mechanisms to improve the structure of polymers, polymers may be classified and named according to the mechanism, and their properties will largely depend on the structure. 3.Put the following words into Chineseentanglement 纠缠 irregularity 无规 sodium isopropylate异丙醇钠 permeability渗透性crystallite 微晶stoichiomertric balance 当量平衡fractionation分馏法light scattering光散射 matrix 基体 diffraction衍射4.Put the following words into English形态 morphology 酯化 esterification 异氰酸酯isocyanate杂质impurity 二元胺 diamine 转化率change ratio 多分散性polydispersity 力学性能mechanical property 构象conformation 红外光谱法infrared spectroscopy常见聚合物命名1常见杂链和元素有机聚合物类型Polyamide ----聚酰胺. Polyester----聚酯 Poly‘urethane ------聚氨酯 Polysiloxane -------聚硅氧烷Phenol-formaldehyde----酚醛.Urea-formaldehyde-----脲醛Polyureas------聚脲 Polysulfide -----聚硫Polyacetal-------聚缩醛 Polysulfone polysulphone------聚砜 Polyether---------聚醚第五单元Traditional methods of living polymerization are based on ionic, coordination or group transfer mechanisms.活性聚合的传统方法是基于离子,配位或基团转移机理;Ideally, the mechanism of living polymerization involves only initiation and propagation steps.理论上活性聚合的机理只包括引发和增长反应步骤;All chains are initiated at the commencement of polymerization and propagation continues until all monomer is consumed.在聚合反应初期所有的链都被引发,然后增长反应继续下去直到所有的单体都被消耗殆尽;A type of novel techniques for living polymerization, known as living possibly use “controlled” or “mediated” radical polymerization, is developed recently. 最近开发了一种叫做活性自由基聚合的活性聚合新技术;The first demonstration of living radical polymerization and the current definition of the processes can be attributed to Szwarc.第一个活性自由基聚合的证实及目前对这一过程的解释或定义,应该归功于Szwarc;Up to now, several living radical polymerization processes, including atom transfer radical polymerization ATRP, reversible addition-fragmentation chain transfer polymerization RAFT, nitroxide-mediated polymerization NMP, etc., have been reported one after another.到目前为止,一些活性自由基聚合过程,包括原子转移自由基聚合,可逆加成-断裂链转移聚合,硝基氧介导聚合等聚合过程一个接一个被报道;The mechanism of living radical polymerization is quite different not only from that of common radical polymerization but also from that of traditional living polymerization. 活性自由基聚合的机理不仅完全不同于普通自由基聚合机理,也不同于传统的活性聚合机理;It relies on the introduction of a reagent that undergoes reversible termination with the propagating radicals thereby converting them to a following dormant form:活性自由基聚合依赖于向体系中引入一种可以和增长自由基进行可逆终止的试剂,形成休眠种:The specificity in the reversible initiation-termination step is of critical importance in achieving living characteristics.这种特殊的可逆引发-终止反应对于获得分子链活性来说具有决定性的重要意义;This enables the active species concentration to be controlled and thus allows such a condition to be chosen that all chains are able to grow at a similar rate if not simultaneously throughout the polymrization.可逆引发终止使活性中心的浓度能够得以控制;这样就可以来选择适宜的反应条件,使得在整个聚合反应过程中只要没有平行反应所有的分子链都能够以相同的速度增长;This has, in turn, enabled the synthesis of polymers with controlled composition, architecture and molecular weight distribution.这样就可以合成具有可控组成,结构和分子量分布的聚合物;They also provide routes to narrow dispersity end-functional polymers, to high purity block copolymers, and to stars and other more complex architecture.这些还可以提供获得狭窄分布末端功能化聚合物,高纯嵌段共聚物,星型及更复杂结构高分子的合成方法;The first step towards living radical polymerization was taken by Ostu and his colleagues in 1982.活性自由基聚合是Ostu和他的同事于1982年率先开展的;In 1985, this was taken one step further with the development by Solomon et al. of nitroxide-mediated polymerization NMP.1985年,Solomon等对氮氧化物稳定自由基聚合的研究使活性自由基聚合进一步发展;This work was first reported in the patent literature and in conference papers but was not widely recognized until 1993 when Georges et al. applied the method in the synthesis of narrow polydispersity polystyrene.这种方法首先在专利文献和会议论文中报道,但是直到1993年Georges等把这种方法应用在窄分子量分布聚苯乙烯之后,才得以广泛认知;The scope of NMP has been greatly expended and new, more versatile, methods have appeared. NMP的领域已经得到很大的延展,出现了新的更多样化的方法;The most notable methods are atom transfer radical polymerization ATRP and polymerization with reversible addition fragmentation RAFT.最引人注目的方法是原子转移自由基聚合和可逆加成断裂聚合;到2000年,这个领域的论文已经占所有自由基聚合领域论文的三分之一;如图所示;Naturally, the rapid growth of the number of the papers in the field since 1995 ought to be almost totally attributable to development in this area. 、自然地,纸的数量的迅速增长在领域,因为1995在这个区域应该是几乎完全可归属的到发展;UNIT9 Structure and Properties of Polymers 聚合物的结构和性质Most conveniently, polymers are generally subdivided in three categories, namelyviz., plastics, rubbers and fibers. 很方便地,聚合物一般细分为三种类型,就是塑料,橡胶和纤维; In terms of initial elastic modules, rubbers ranging generally between 106 to 107dynes/cm2, represent the lower end of the scale, while fibers with high initial modjulai, of 1010 to 1011dynes/cm2 are situated on the upper end of the scale; plastics, having generally an initial elastic modulus of 108 to 109dynes/cm2, lie in-between. 就初始弹性模量而言,橡胶一般在 6到107达因平方厘米,在尺度的低端, 10到1011达因平方厘米,尺度的高端,而纤维具有高的初始模量, 达到10到1011达因平方厘米,尺度的高端,塑料的弹性模量一般在 8到109达因平方厘米,在尺度的中间As is found in all phases of polymer chemistry, there are many exceptions to this categorization. 正如高分子化学的各个部分都可以看到的那样,在高分子化学的所有阶段,我们都可以发现,这种分类方法有许多例外的情况;An elastomer or rubber results from a polymer having relatively weak interchain forces and high molecular weights. 弹性体是具有相对弱的链之间作用力和高分子量的聚合物; When the molecular chains are “straightened out” or stretched by a process of extension, they do not have sufficient attraction for each other to maintain the oriented state and will retract once the force is released. This is the basis of elastic behavior. 当通过一个拉伸过程将分子链拉直的时候,分子链彼此之间没有足够的相互吸引力来保持其取向状态,作用力一旦解除,将发生收缩;这是弹性行为的基础;However, if the interchain forces are very great, a polymer will make a good fiber. 然而,如果分子链之间的力非常大,聚合物可以用做纤维;Therefore, when the polymer is highly stretched, the oriented chain will come under the influence of the powerful attractive forces and will “crystallize” permanently in a more or less oriented matrix. 因此,当聚合物被高度拉直的时候,取向分子链在不同程度取向的母体中将受强引力的影响而“永久地结晶;These crystallization forces will then act virtually as crosslinks, resulting in a material of high tensile strength and high initial modulus, ., a fiber. 而后,这些结晶力实际上以交联方式作用,产生高拉伸强度和高初始模量的材料,如纤维;Therefore, a potential fiber polymer will not become a fiber unless subjected to a “drawing” process, ., a process resulting in a high degree of intermolecular orientation. 因此,一个可能的潜在的纤维高分子不会变成纤维,除非经历一个拉伸过程, 即, 这导致分子间高度取向的拉伸过程;Crosslinked species are found in all three categories and the process of crosslinking may change the cited characteristics of the categories. 交联的种类在所有三种类型塑料,橡胶,纤维中找到,而交联过程可以改变分类的引用特征;Thus, plastics are known to possesspzes a marked range of deformability in the order of 100 to 200%; they do not exhibit this property when crosslinked, however. 因此,我们熟知塑料具有的形变能力大约在100-200%范围内,然而当交联发生时塑料不能展示这个性能; Rubber, on vulcanization, changes its properties from low modulus, low tensile strength, low hardness, and high elongation to high modulus, high tensile strength, high hardness, and low elongation. 对橡胶而言,硫化可以改变其性质,从低模量,低拉伸强度,低硬度及高拉伸率到高模量,高拉伸强度,高硬度及低拉伸率;Thus, polymers may be classified as noncrosslinked and crosslinked, and this definition agrees generally with the subclassification in thermoplastic and thermoset polymers. 这样,聚合物可以分为非交联和交联的,这个定义与把聚合物细分为热塑性和热固性聚合物相一致; From the mechanistic point of view, however, polymers are properly divided into addition polymers and condensation polymers. Both of these species are found in rubbers, plastics, and fibers. 然而,从反应机理的观点看,聚合物可以分成加聚物和缩聚物;这些种类聚合物在塑料,橡胶和纤维中都可以找得到;In many cases polymers are considered from the mechanistic point of view. Also, the polymer will be named according to its source whenever it is derived from a specific hypothetical monomer, or when it is derived from two or more components which are built randomly into the polymer. 在许多情况下,聚合物可以从反应机理的角度考虑分类; 每当聚合物来自于一个假象单体,或来自于两个或两个以上组成物无规则构建聚合物时,也可以根据聚合物的来源来命名; This classification agrees well with the presently used general practice. 这种分类方法与目前实际情况相符合;When the repeating unit is composed of several monomeric components following each other in a regular fashion, the polymer is commonly named according to its structure. 当重复单元由几个单体组成物规则排布,聚合物通常根据它的结构来命名;It must be borne in mind that, with the advent of Ziegler-Natta mechanisms and new techniques to improve and extend crystallinity, and the closeness of packing of chains, many older data given should be critically considered in relation to the stereoregular and crystalline structure. 必须记住,随着Ziegler-Natta机理,以及提高结晶度和链堆砌紧密度新技术的出现,对许多过去已经得到的关于空间结构和晶体结构旧的资料,应当批判地接受;The properties of polymers are largely dependent on the type and extent of both stereoregularity and crystallinity. As an example, the densities and melting points of atactic and isotactic species are presented in Table . 聚合物的性质主要依靠立体规整性和结晶度的类型和程度;如,无规立构和全同立构物质的密度和熔点展示在表中 ;UNIT11 Functional PolymersFunctional polymers are macromolecules to which chemically functional groups are attached; they have the potential advantages of small molecules with the same functional groups. 功能聚合物是具有化学功能基团的大分子,这些聚合物与具有功能聚合物是具有化学功能基团的大分子, 相同功能基团的小分子一样具有潜在的优点;Their usefulness is related both to the functional groups and to the nature of the polymers whose characteristic properties depend mainly on the extraordinarily large size of the molecules.它们的实用性不仅与功能基团有关,而且与巨大分子尺寸带来的聚合物特性有关;The attachment of functional groups to a polymer is frequently the first step towards the preparation of a functional polymer for a specific use. 把功能基团连接到聚合物上常常是制备特殊用途功能高分子的第一步;However, the proper choice of the polymer is an important factor for successful application. 然而,对成功应用而言,选择适当的聚合物是的一个重要因素;In addition to the synthetic aliphatic and aromatic polymers, a wide range of natural polymers have also been functionalized and used as reactive materials. 除了合成的脂肪组和芳香组聚合物之外,许多天然高分子也被功能化,被用做反应性材料;Inorganic polymers have also been modified with reactive functional groups and used in processes requiring severesi’vi service conditions. 无机聚合物也已经用反应功能基团改性,被用于要求耐用条件的场合;In principle, the active groups may be part of the polymer backbone or linked to a side chain as a pendant group either directly or viavai a space rs’peis group. 理论上讲,活性基团可以是聚合物主链上的一部分,或者直接连接到侧链或通过一个中间基团的侧基;A required active functional group can be introduced onto a polymeric support chain 1 by incorporation during the synthesis of the support itself through polymerization or copolymerization of monomers containing the desired functional groups, 2 by chemical modification of a nonfunctionalized performed support matrix and 3 by a combination of 1 and 2. 所需的活性功能基团可以通过几种方法引入到聚合物主链上, 1在主链的合成过程中,通过聚合或共聚合含有理想功能基团的单体来获得,2通过对已有的非功能化主链进行化学改性的方法,3通过结合1和2来获得;Each of the two approaches has its own advantages and disadvantages, and one approach may be preferred for the preparation of a particular functional polymer when the other would be totally impractical.两种途径中的每一种都有自身的优点和缺点,对特殊功能聚合物的制备而言,当其他方法都无法实现时,所选的方法或许是更合适的;The choice between the two ways to the synthesis of functionalized polymers depends mainly on the required chemical and physical properties of the support for a specific application. 功能聚合物合成的两种方法中,如何选择主要取决于特殊应用要求的主链聚合物的化学和物理性质;Usually the requirements of the individual system must be thoroughly examined in order to take full advantage of each of the preparative techniques. 为了充分利用每种制备方法,必须全面地考察独立体系的要求;Rapid progress in the utilization of functionalized polymeric materials has been noted in the recent past. 近年来,功能化聚合物材料的使用方面有了飞速的发展;Interest in the field is being enhanced due to the possibility of creating systems that combine the unique properties of conventional active moieties and those of high molecular weight polymers. 由于能够制造出来兼有活性官能团特性和高分子量聚合物性能的功能聚合物,所以,人们对功能聚合物这个领域的兴趣与日俱增;The successful utilization of these polymers are based on the physical form, solution behavior, porosity, chemical reactivity and stability of the polymers. 这些聚合物的成功利用,基于功能聚合物的物理形态,溶液行为,空隙率,化学活性及稳定性;The various types of functionalized polymers cover a broad range of chemical applications, including the polymeric reactants, catalysts, carriers, surfactants, stabilizers,ionexchange resins, etc.各种功能化聚合物类型覆盖化学应用的宽广领域,包括聚合物试剂,催化剂, 载体,表面活性剂,稳定剂,离子交换树脂等;In a variety of biological and biomedical fields, such as the pharmaceutical, agriculture, food industry and the like, they have become indispensable materials, especially in controlled release formulation of drugs and agrochemicals. 在生物学及生物医学领域中,如药物,农业,食品工业等, 在生物学及生物医学领域中,如药物,农业,食品工业等,功能聚合物是不可缺少的材料,尤其在药物和农药的控制释放配方上;Besides, these polymers are extensively used as the antioxidants, flame retardants, corrosion inhibitors, flocculating agents, antistatic agents and the other technological applications. 此外,这些聚合物被广泛地用做抗氧化剂,阻燃剂,缓蚀剂, 絮凝剂,抗静电剂及其他技术应用;In addition, the functional polymers possessp’zes broad application prospects in the high technology area as conductive materials, photosensitizers, nuclear track detectors, liquid crystals, the working substances for storage and conversion of solar energy, etc. 另外,功能化聚合物在高科技领域具有广阔的应用前景; 如导电材料,光敏剂,核径迹探测器,液晶,用于太阳能等的转化与储存的工作物质;第十二单元实验室制备氨基树脂氨基树脂是由氨基衍生物和醛在酸性或碱性条件下反应生产得到的其中最重要最具代表性的物质是脲醛树脂和蜜胺树脂; 药品:尿素,福尔马林37%,乙醇,2N NaOH, NaOH溶液,1N标准NaOH溶液,1N标准HCl溶液,冰醋酸,糠醇,三乙醇胺,木粉,磷酸钙,氯化铵, H2SO4溶液,Na 2SO3,1%乙醇百里酚酞指示剂溶液,三聚氰胺,甘油和单羟甲基脲; 装置:烧瓶和烧杯,500ml的三口烧瓶,加热套,机械搅拌器,冷凝器,迪安—斯达克塔分水器,烘箱,广泛试纸,试管,250mL的容量烧瓶,冰浴,10ml 的移液管,滴管,油浴和广口瓶; 酸性条件下制备脲醛树脂:为了证明尿素和甲醛在酸性条件下的迅速反应,将5 g尿素和6 mL福尔马林在试管中混合,振荡试管直到尿素全部溶解;滴加4滴 N H2SO4以调节溶液pH到4,观察析出沉淀所需要的时间,取出部分沉淀并比较此沉淀以及单羟甲基脲样品在水中的溶解性;制备脲醛树脂粘合剂:将600g1mole尿素和137g福尔马林放入500ml三口烧瓶中,并安装好机械搅拌器和回流冷凝器,通过用广泛试纸测定用2NNaOH溶液把混合物PH值调至7~~8,然后将混合物回流2小时;1每隔半小时用下面的方法测定一次混合物中的自由甲醛含量,直到水完全脱除为止;2 当混合物回流2小时后,将迪安—斯达克塔分水器安装在烧瓶和回流冷凝器之间 ;大约有40ml水被蒸馏,用5滴冰醋酸将溶液酸化;将44g糠醇和的三乙醇胺加入到反应混合液中,加热此溶液到90℃并恒温15分钟;将混合物冷却到室温;取出15g的树脂样品和由1g木粉,磷酸钙和氯化铵组成的硬化剂混合 ;将混合物进行室温固化;3将剩下的没有加工硬化剂的树脂放入广口瓶中并提交给实验导师;自由甲醛含量的测定:自由甲醛含量的测定:准备250mL 1N Na2SO3溶液,并中和该溶液,从而使其产生淡蓝色的百里酚酞指示剂溶液;在250ml锥形瓶中加入重为2到3克的树脂样品到100mL的水中,摇晃锥形瓶使锥形瓶内的溶液充分溶解;如果树脂不能溶解,加入乙醇可以帮助溶解;在冰浴中使溶液的温度下降到4℃,加25mL的1M Na2SO3溶液在100mL的烧瓶中,用移液管移取10ml标准的1N HCl溶液到烧瓶中,降温至4℃;加10-15滴百里酚酞指示剂溶剂到样品烧瓶中,调整溶液的颜色至淡蓝色;用冷水冷却以后迅速地转移酸式亚硫酸盐溶液到样品烧瓶中;4滴定溶液到百里酚酞的终点标准1N NaOH 溶液;CH2O+Na2SO3+H2O →CH2OHSO3-Na++NaOH通过中和树脂溶液的HCl溶液的量来测定自由甲醛的百分含量;三聚氰胺甲醛树脂的制备:在一个500ml的配置有机械搅拌器和一个冷凝器的反应器中加入63g 的三聚氰胺和122g的福尔马林37%;混合物回流40分钟;%自由甲醛需要每隔十分钟测定一次;自由甲醛的测定步骤如上所述;样品经过20分钟加热后,在烧瓶和冷凝器间插入一个迪安—斯达克分水器,从而有10mL的水被蒸馏掉;把未固化的样品放入螺丝帽的坛子中,连同固化的树脂一起交给实验指导老师;15单元到目前为止大多数的PVC生产通过悬浮聚合;在这个过程中,氯乙烯单体悬浮液体滴,在连续水相剧烈的搅拌和保护胶体的悬浮剂;使用单体溶自由基引发剂polymeri等自下而上发生在悬浮液滴内,通过一个机制,已被证明相当于本体聚合;商业植物是基于批量反应堆,这增加了支持的大小,多年来;原来的工厂建于1940年代通常由IOOO 加仑反应堆;在1960年代和1950年代这t0 3000一5000加仑和增加随后,在1970年代初,29000加仑反应堆系统开发的胫完全②,t0 44000加仑200立方米的德国公司Huls;目前一些新的工厂正在建造的反应堆由不到isooo加仑容量,有一个批处理大小约25吨单体;小型反应堆通常衬玻璃给光洁度,抵制存款的搁置在墙上;~大反应堆通常的抛光不锈钢;氯乙烯的聚合反应是一个放热反应的能力,移走热量通常试图减少反应时间的限制因素;随着规模的反应堆已经增加了表面积体积比,因此加重这一问题;内部冷却线圈通常不用作吸引存款和很难清洁,从而对产品性能有不利影响;问题通常是克服使用冷冻水或回流冷凝器的装置,通过氯乙烯单体的连续回流;利用其潜热冷却的目的;一个简单的悬浮聚合配方可能包含以下成分:冷水通常是首先向反应堆虽然有时预热;然后添加pH值调节剂紧随其后的是分散剂的形式解决方案;发起者年代立即撒到水相的表面密封反应堆然后撤离前去除氧,因为这可以增加聚合时间,影响产品性能;当引发反应完成乙烯氯化物被指控和加热反应堆的内容开始;反应但真正的,产品分子量的主要控制因素;通常是在50——70 'c导致反应堆压力范围100 - 165 psi;趋势是朝着大的操作只打开关闭反应堆维护或可能偶尔打扫道;”:在这种情况下所有的原料都是负责解决方案或分散体,一般不需要疏散的一步;当达到所需的转换了,通常75%一95%,反应可以如果需要化学short-stopped和剩余的大部分单体恢复;他产品泥浆然后剥下来非常低的残留氯乙烯治疗-水平表示“状态”姆温度升高,在反应堆或类似容器,或接触蒸汽在逆流多平台汽提塔;然后脱水离心法和由此产生的泥浆湿饼乾,多级闪蒸干燥机一般,虽然各种不同的干燥类型使用不同的生产;干燥后,产品是通过某种剥皮屏幕去除无关的大颗粒装袋之前或装载散装油轮;—T 16 Styrene-Butadiene Copolymer第十六单元丁二烯-苯乙烯共聚物合成橡胶工业,以自由基乳液过程为基础,在第二次世界大战期间几乎很快地形成;那时,丁苯橡胶制造的轮胎性能相当优越,使天然橡胶在市场黯然失色;丁苯橡胶的标准制法是组分重量分数组分重量分数丁二烯72 过硫酸钾苯乙烯25 肥皂片十二烷基硫醇水180 混合物在搅拌下50℃加热,每小时转化5%~6%,在转化率达70%~75%时通过加入“终止剂”聚合反应终止,例如对苯二酚大约的重量百分含量,抑制自由基并避免过量支化和微凝胶形成;未反应的丁二烯通过闪蒸去除,苯乙烯在萃取塔中通过蒸汽萃取剥离;在加入抗氧剂后,例如N-甲基-β-萘胺的重量百分含量,加入盐水,其次加入稀释的硫酸或硫酸铝后乳液凝胶;凝胶碎片被洗涤、干燥。
高分子材料专业英语配翻译

As against this well-defined behavior of a simple chemical compound, a polymer like polyethylene does not melt sharply at one particular temperature into clean liquid. 与这类简单化合物明确的行为相比,像聚乙烯这样的聚合物不能在某一特定的温度快速地熔融成纯净的液体。
Also, we can add a very large quantity of the polymer to the same quantity of water without the saturation point ever being reached. 同样地,我们可以将大量的聚合物加入到同样量的水中,不存在饱和点。
While linear polymers are important, they are not the only type of molecules possible. 线状聚合物是很重要的,他们不是唯一可能类型的分子。
Substituent groups such as methyl or phenyl groups on the repeat units are not considered branches. Branching is generally introduced into a molecule by intentionally adding some monomer with the capability of serving as a branch. Let us consider the formation of a polyester. The presence of difunctional acids and difunctional alcohols allows the polymer chain to grow. These difunctional molecules are incorporated into the chain with ester linkages at both ends of each. Trifunctional acids or alcohols, on the other hand, produce a linear molecule by reacting two of their functional groups. If the third reacts and the resulting chain continues to grow, a branch has been introduced into the original chain. Adventitious branching sometimes occurs as a result of an atom being abstracted from the original linear molecule, with chain growth occurring from the resulting active site. Molecules with this kind of accidental branching are generally still called linear, although the presence of significant branching has profound effects on some properties of the polymer, most notably the tendency to undergo crystallization.The polymerization is a chain reaction in two ways: because of the reaction kinetic and because as a reaction product one obtains a chain molecule. 聚合反应是链式反应的原因有两种:因为反应动力学和因为作为反应产物它是一种链式分子。
高分子材料工程专业英语翻译完整版本

第一单元什么是高聚物?什么是高聚物?首先,他们是合成物和大分子,而且不同于低分子化合物,譬如说普通的盐。
与低分子化合物不同的是,普通盐的分子量仅仅是58.5,而高聚物的分子量高于105,甚至大于106。
这些大分子或“高分子”由许多小分子组成。
小分子相互结合形成大分子,大分子能够是一种或多种化合物。
举例说明,想象一组大小相同并由相同的材料制成的环。
当这些环相互连接起来,可以把形成的链看成是具有同种分子量化合物组成的高聚物。
另一方面,独特的环可以大小不同、材料不同,相连接后形成具有不同分子量化合物组成的聚合物。
许多单元相连接给予了聚合物一个名称,poly意味着“多、聚、重复”,mer意味着“链节、基体”(希腊语中)。
例如:称为丁二烯的气态化合物,分子量为54,化合将近4000次,得到分子量大约为200000被称作聚丁二烯(合成橡胶)的高聚物。
形成高聚物的低分子化合物称为单体。
下面简单地描述一下形成过程:丁二烯+丁二烯+…+丁二烯——→聚丁二烯(4000次)因而能够看到分子量仅为54的小分子物质(单体)如何逐渐形成分子量为200000的大分子(高聚物)。
实质上,正是由于聚合物的巨大的分子尺寸才使其性能不同于象苯这样的一般化合物。
例如,固态苯,在5.5℃熔融成液态苯,进一步加热,煮沸成气态苯。
与这类简单化合物明确的行为相比,像聚乙烯这样的聚合物不能在某一特定的温度快速地熔融成纯净的液体。
而聚合物变得越来越软,最终,变成十分粘稠的聚合物熔融体。
将这种热而粘稠的聚合物熔融体进一步加热,不会转变成各种气体,但它不再是聚乙烯(如图1.1)。
固态苯——→液态苯——→气态苯加热,5.5℃加热,80℃固体聚乙烯——→熔化的聚乙烯——→各种分解产物-但不是聚乙烯加热加热图1.1 低分子量化合物(苯)和聚合物(聚乙烯)受热后的不同行为发现另一种不同的聚合物行为和低分子量化合物行为是关于溶解过程。
例如,让我们研究一下,将氯化钠慢慢地添加到固定量的水中。
高分子专业英语
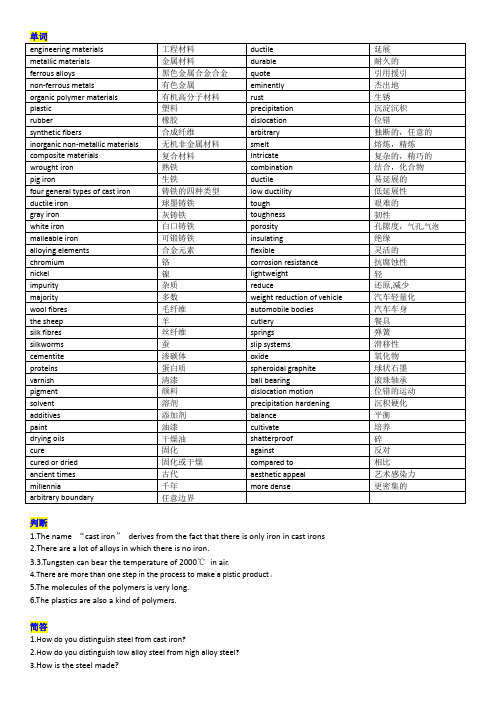
单词engineeringmaterials 工程材料ductile 延展metallic materials 金属材料durable 耐久的ferrous alloys 黑色金属合金合金quote 引用援引non-ferrous metals 有色金属eminently 杰出地organic polymer materials 有机高分子材料rust 生锈plastic 塑料precipitation 沉淀沉积rubber 橡胶dislocation 位错synthetic fibers 合成纤维arbitrary 独断的,任意的inorganic non-metallic materials 无机非金属材料smelt 熔炼,精炼composite materials 复合材料Intricate 复杂的,精巧的wrought iron 熟铁combination 结合,化合物pig iron 生铁ductile 易延展的four general types of cast iron 铸铁的四种类型low ductility 低延展性ductile iron 球墨铸铁tough 艰难的gray iron 灰铸铁toughness 韧性white iron 白口铸铁porosity 孔隙度,气孔,气泡malleable iron 可锻铸铁insulating 绝缘alloying elements 合金元素flexible 灵活的chromium 铬corrosion resistance 抗腐蚀性nickel 镍lightweight 轻impurity 杂质reduce 还原,减少majority 多数weight reduction of vehicle 汽车轻量化wool fibres 毛纤维automobile bodies 汽车车身the sheep 羊cutlery 餐具silk fibres 丝纤维springs 弹簧silkworms 蚕slip systems 滑移性cementite 渗碳体oxide 氧化物proteins 蛋白质spheroidal graphite 球状石墨varnish 清漆ballbearing 滚珠轴承pigment 颜料dislocation motion 位错的运动solvent 溶剂precipitation hardening 沉积硬化additives 添加剂balance 平衡paint 油漆cultivate 培养drying oils 干燥油shatterproof 碎cure 固化against 反对cured or dried 固化或干燥compared to 相比ancient times 古代aesthetic appeal 艺术感染力millennia 千年more dense 更密集的arbitrary boundary 任意边界判断1.The name “cast iron”derives from the fact that there is only iron in cast irons2.There are a lot of alloys in which there is no iron.3.3.Tungsten can bear the temperature of 2000℃in air.4.There are more than one step in the process to make a plstic product。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
第一单元什么是高聚物?什么是高聚物?首先,他们是合成物和大分子,而且不同于低分子化合物,譬如说普通的盐。
与低分子化合物不同的是,普通盐的分子量仅仅是58.5,而高聚物的分子量高于105,甚至大于106。
这些大分子或“高分子”由许多小分子组成。
小分子相互结合形成大分子,大分子能够是一种或多种化合物。
举例说明,想象一组大小相同并由相同的材料制成的环。
当这些环相互连接起来,可以把形成的链看成是具有同种分子量化合物组成的高聚物。
另一方面,独特的环可以大小不同、材料不同,相连接后形成具有不同分子量化合物组成的聚合物。
许多单元相连接给予了聚合物一个名称,poly意味着“多、聚、重复”,mer意味着“链节、基体”(希腊语中)。
例如:称为丁二烯的气态化合物,分子量为54,化合将近4000次,得到分子量大约为200000被称作聚丁二烯(合成橡胶)的高聚物。
形成高聚物的低分子化合物称为单体。
下面简单地描述一下形成过程:丁二烯+丁二烯+…+丁二烯——→聚丁二烯(4000次)因而能够看到分子量仅为54的小分子物质(单体)如何逐渐形成分子量为200000的大分子(高聚物)。
实质上,正是由于聚合物的巨大的分子尺寸才使其性能不同于象苯这样的一般化合物。
例如,固态苯,在5.5℃熔融成液态苯,进一步加热,煮沸成气态苯。
与这类简单化合物明确的行为相比,像聚乙烯这样的聚合物不能在某一特定的温度快速地熔融成纯净的液体。
而聚合物变得越来越软,最终,变成十分粘稠的聚合物熔融体。
将这种热而粘稠的聚合物熔融体进一步加热,不会转变成各种气体,但它不再是聚乙烯(如图1.1)。
固态苯——→液态苯——→气态苯加热,5.5℃加热,80℃固体聚乙烯——→熔化的聚乙烯——→各种分解产物-但不是聚乙烯加热加热图1.1 低分子量化合物(苯)和聚合物(聚乙烯)受热后的不同行为发现另一种不同的聚合物行为和低分子量化合物行为是关于溶解过程。
例如,让我们研究一下,将氯化钠慢慢地添加到固定量的水中。
盐,代表一种低分子量化合物,在水中达到点(叫饱和点)溶解,但,此后,进一步添加盐不进入溶液中却沉到底部而保持原有的固体状态。
饱和盐溶液的粘度与水的粘度不是十分不同,但是,如果我们用聚合物替代,譬如说,将聚乙烯醇添加到固定量的水中,聚合物不是马上进入到溶液中。
聚乙烯醇颗粒首先吸水溶胀,发生形变,经过很长的时间以后进入到溶液中。
同样地,我们可以将大量的聚合物加入到同样量的水中,不存在饱和点。
将越来越多的聚合物加入水中,认为聚合物溶解的时间明显地增加,最终呈现柔软像面团一样粘稠的混合物。
另一个特点是,在水中聚乙烯醇不会像过量的氯化钠在饱和盐溶液中那样能保持其初始的粉末状态。
总之,我们可以讲(1)聚乙烯醇的溶解需要很长时间,(2)不存在饱和点,(3)粘度的增加是典型聚合物溶于溶液中的特性,这些特性主要归因于聚合物大分子的尺寸。
如图1.2说明了低分子量化合物和聚合物的溶解行为。
氯化钠晶体加入到水中——→晶体进入到溶液中.溶液的粘度不是十分不同于充分搅拌水的粘度——→形成饱和溶液.剩余的晶体维持不溶解状态.加入更多的晶体并搅拌氯化钠的溶解聚乙烯醇碎片加入到水中——→碎片开始溶胀——→碎片慢慢地进入到溶液中允许维持现状充分搅拌——→形成粘稠的聚合物溶液.溶液粘度十分高于水的粘度继续搅拌聚合物的溶解图1.2 低分子量化合物(氯化钠)和聚合物(聚乙烯醇)不同的溶解行为第二单元链式聚合反应Staudinger第一个发现一例现象,许多烯烃和不饱和烯烃通过打开双键可以形成链式大分子。
二烯烃以同样的方式聚合,然而,仅限于两个双键中的一个。
这类反应是通过单体分子首先加成到引发剂自由基或引发剂离子上而进行的,靠这些反应活性中心由引发剂转移到被加成的单体上。
以同样的方式,借助于链式反应,单体分子一个接一个地被加成(每秒2000~20000个单体)直到活性中心通过不同的反应类型而终止。
聚合反应是链式反应的原因有两种:因为反应动力学和因为作为反应产物它是一种链式分子。
链分子的长度与动力学链长成正比。
链式反应可以概括为以下过程(R•相当与引发剂自由基):略因而通过上述过程由氯乙烯得到聚氯乙烯,或由苯乙烯获得聚苯乙烯,或乙烯获得聚乙烯,等等。
借助于聚合度估算的分子链长,在一个大范围内可以通过选择适宜的反应条件被改变。
通常,通过大量地制备和利用聚合物,聚合度在1000~5000范围内,但在许多情况下可低于500、高于10000。
这不应该把所有聚合物材料的分子量理解为由500,或1000,或5000个单体单元组成。
在几乎所有的事例中,聚合物材料由不同聚合度的聚合物分子的混合物组成。
聚合反应,链式反应,依照与众所周知的氯(气)-氢(气)反应和光气的分解机理进行。
双键活化过程的引发剂反应,可以通过热、辐射、超声波或引发剂产生。
用自由基型或离子型引发剂引发链式反应可以很清楚地进行观察。
这些是高能态的化合物,它们能够加成不饱和化合物(单体)并保持自由基或离子活性中心以致单体可以以同样的方式进一步加成。
对于增长反应的各个步骤,每一步仅需要相当少的活化能,因此通过一步简单的活化反应(即引发反应)即可将许多烯类单体分子转化成聚合物,这正如连锁反应这个术语的内涵那样。
因为少量的引发剂引发形成大量的聚合物原料(1:1000~1:10000),从表面上看聚合反应很可能是催化反应。
由于这个原因,通常把聚合反应的引发剂看作是聚合反应的引发剂,但是,严格地讲它们不是真正意义上的催化剂,因为聚合反应的催化剂进入到反应内部而成为一部分,同时可以在反应产物,既聚合物的末端发现。
此外离子引发剂和自由基引发剂有的是金属络合物引发剂(例如,通过四氯化钛或三氯化钛与烷基铝的反应可以得到),Z引发剂在聚合反应中起到了重要作用,它们催化活动的机理还不是十分清楚。
第三单元逐步聚合许多不同的化学反应通过逐步聚合可用于合成聚合材料。
这些反应包括酯化、酰胺化、氨基甲酸酯、芳香族取代物的形成等。
通过反应聚合反应在两种不同的官能团,如,羟基和羧基,或异氰酸酯和羟基之间。
所有的逐步聚合反应根据所使用单体的类型可分为两类。
第一类涉及两种不同的官能团单体,每一种单体仅具有一种官能团。
一种多官能团单体每个分子有两个或多个官能团。
第二类涉及含有两类官能团的单种单体。
聚酰胺的合成说明了聚合反应的两个官能团。
因此聚酰胺可以由二元胺和二元酸的反应或氨基酸之间的反应得到。
两种官能团之间的反应一般来说可以通过下列反应式表示反应(3.1)说明前一种形式,而反应(3.2)具有后一种形式。
图3.1 逐步聚合的示意图(a)未反应单体;(b)50%已反应;(c)83.3%已反应;(d) 100%已反应(虚线表示反应种类)聚酯化,是否在二元酸和二元醇或羟基酸分子间进行,是逐步聚合反应过程的一个例子。
酯化反应出现在单体本体中两个单体分子相碰撞的位置,且酯一旦形成,依靠酯上仍有活性的羟基或羧基还可以进一步进行反应。
酯化的结果是单体分子很快地被消耗掉,而分子量却没有多少增加。
图3.1说明了这个现象。
例如,假定图3.1中的每一个方格代表一个羟基酸分子。
(b)中的二聚体分子,消耗二分之一的单体分子聚合物种类的聚合度(DP)是2。
(c)中当三聚体和更多的二聚体形成,大于80%的单体分子已反应,但DP仅仅还是2.5。
(d)中当所有的单体反应完,DP是4。
但形成的每一种聚合物分子还有反应活性的端基;因此,聚合反应将以逐步的方式继续进行,其每一步酯化反应的反应速率和反应机理均与初始单体的酯化作用相同。
因此,分子量缓慢增加直至高水平的单体转化率,而且分子量将继续增加直到粘度的增加使其难以除去酯化反应的水或难以找到相互反应的端基。
在A-A+B-B的聚合反应中也可以看到,精确的当量平衡是获得高分子量所必需的。
假如存在一些但官能团杂质,由于链的端基失活,反应将使分子量减少。
同样,在A-B类的缩聚反应中高纯度的单体是必要的,而且可以归结高收率的反应仅是形成聚合物的实际反应,因为副反应会破坏当量平衡。
第四单元离子聚合反应离子聚合反应,与自由基聚合反应相似,也有链反应的机理。
但是,离子聚合的动力学明显地不同于自由基聚合反应。
(1)离子聚合的引发反应仅需要很小的活化能。
因此,聚合反应的速率仅对温度有较少的依赖性。
在许多情况下离子聚合猛烈地发生甚至低于50℃(例如,苯乙烯的阴离子聚合反应在-70℃在四氢呋喃中,或异丁烯的阳离子聚合在-100℃在液态乙烯中)。
(2)对于离子聚合来说,不存在通过再结合反应而进行的强迫链终止,因为生长链之间不能发生链终止。
链终止反应仅仅通过杂质而发生,或者说通过和某些像水、醇、酸、胺或氧这样的化合物进行加成而发生,且一般来说(链终止反应)可通过这样的化合物来进行,这种化合物在中性聚合物或没有聚合活性的离子型聚合物生成的过程中可以和活性聚合物离子进行反应。
如果引发剂仅仅部分地离解,引发反应即为一个平衡反应,在出现平衡反应的场合,在一个方向上进行链引发反应,而在另一个方向上则发生链终止反应。
通常离子聚合反应能通过酸性或碱性化合物被引发。
对于阳离子聚合反应来说,BF3,AlCl3,TiCl4和SnCl4与水、或乙醇,或叔烊盐的络合物提供了部分活性。
正离子是产生链引发的化合物。
例如:(反应略)三乙基硼氟酸烊然而,BF3也可以与HCl、H2SO4和KHSO4引发阳离子聚合反应。
阴离子聚合反应的引发剂是碱金属和它们的有机金属化合物,例如苯基锂、丁基锂和三苯甲基锂,它们在不同的溶剂中或多或少地强烈分解。
所谓的Alfin催化剂就是属于这一类,这类催化剂是异丙醇钠、烯丙基钠和氯化钠的混合物。
BF3为引发剂(异丁烯为单体),证明仅在痕量水或乙醇的存在下聚合反应是可以进行的。
如果消除痕量的水,单纯的BF3不会引发聚合反应。
按照上述反应为了能形成BF3-络合物和引发剂离子水或乙醇是必需的。
但是不应将水或乙醇描述成“助催化剂”。
正与自由基聚合反应一样,通过离子聚合反应也能制备共聚物,例如,苯乙烯-丁二烯阴离子共聚物,或异丁烯-苯乙烯阳离子共聚物,或异丁烯-乙烯基醚共聚物,等等。
正如对自由基型聚合已经详细描述过那样,人们可以用所谓的竞聚率r1和r2来表征每单体对。
然而,这两个参数的实际意义不同于那些用于自由基共聚合反应的参数。
第十四单元丁二烯-苯乙烯共聚物合成橡胶工业,以自由基乳液过程为基础,在第二次世界大战期间几乎很快地形成。
那时,丁苯橡胶制造的轮胎性能相当优越,使天然橡胶在市场黯然失色。
丁苯橡胶的标准制法是混合物在搅拌下50℃加热,每小时转化5%~6%,在转化率达70%~75%时通过加入“终止剂”聚合反应终止,例如对苯二酚(大约0.1的重量百分含量),抑制自由基并避免过量支化和微凝胶形成。
