佐匹克隆说明书
佐匹克隆片的用法

佐匹克隆片的用法佐匹克隆片是一种抗生素药物,可以治疗多种细菌感染疾病。
该药物的主要成分是氯霉素,它可以通过干扰细菌的蛋白质合成来杀死和控制细菌。
佐匹克隆片广泛应用于治疗上呼吸道感染、皮肤感染、泌尿生殖系统感染等疾病。
佐匹克隆片的使用方法佐匹克隆片通常口服使用,一般是每日三次,每次1-2片,具体剂量应根据病情和医生的建议来确定。
患者在服用时应该按照医生的嘱咐来使用,并按时完成疗程。
一般而言,佐匹克隆片治疗轻度疾病需要7-10天,而严重疾病需要2-4周的治疗时间。
注意事项出现下列情况应及时告知医生:过敏反应(如荨麻疹、皮疹、呼吸困难等)、恶心、呕吐、腹泻、腹部不适、口干、口苦等不适症状。
同时,患者在使用佐匹克隆片时,也需要注意以下几点:1.不要过量服用,一定按照医生的指示或者药物说明书的用法用量进行使用。
2.具有肝肾功能异常或疾病、贫血等患者在使用该药物时应该特别小心,以免加重病情。
3.佐匹克隆片可能会影响对机动车的驾驶技能,因此在使用这些药物时,遵循医生的建议,避免开车或机器操作。
4.佐匹克隆片是一种处方药,只能在医生的处方下购买和使用。
5.不要将佐匹克隆片与其他药物一起使用,应避免饮酒或吸烟。
佐匹克隆片的优缺点优点1.佐匹克隆片可以快速并且有效地杀死和控制细菌,用于治疗多种细菌感染性疾病。
2.它能够带来症状快速缓解,让患者在疗程中减轻不适症状。
3.佐匹克隆片副作用较少,可以使用于多种患者,并且不会对人体产生过大的刺激。
缺点1.该药物会影响到身体的自身免疫系统,因而可能增加一些副作用,如口干、口臭等。
2.部分患者在使用佐匹克隆片时,可能会出现过敏反应(如荨麻疹、皮疹、呼吸困难等)。
3.佐匹克隆片不能同时与某些药物一起使用,会产生不利影响,从而增加副作用。
结语佐匹克隆片是一种广泛使用的抗生素药物,能够有效治疗多种细菌感染疾病。
然而,为了确保药物的使用效果,患者需要按照医生的建议使用,并遵守一些注意事项,如不过量使用,不与其他药物混用等。
佐匹克隆

佐匹克隆一概述佐匹克隆(Zopiclone)为环吡咯酮的第三代催眠药。
系抑制性神经递质-氨基丁酸受体激动剂,其结构与苯二氮类不同,为环吡酮化合物,与苯二氮类结合于相同的受体和部位,但作用于不同区域。
本品作用迅速,与苯二氮类相比作用更强。
本品除具有催眠、镇静作用外,还具有抗焦虑、肌松和抗惊厥作用。
口服吸收迅速,用药后1.5~2小时后可达血药浓度峰值。
在组织中分布广,通过肝脏代谢,主要代谢产物为无药理活性的N-甲基佐匹克隆,N-氧化物有一定的药理活性,大多数药物以代谢物的形式由肾脏排泄。
二适应证用于各种原因引起的失眠症,尤其适用于不耐受次晨残余作用的患者。
三临床应用口服:7.5mg睡前服用。
老年人初临睡时服3.75mg,必要时服7.5mg;肝功能不全者,服3.75mg为宜。
四不良反应不良反应可见困倦、口苦、口干、肌无力、头痛;长期服用药后突然停药可出现反跳性失眠、噩梦、恶心、呕吐、焦虑、肌痛、震颤。
罕见有痉挛、肌肉颤抖、意识模糊。
五注意事项1.肌无力患者用药时需注意医疗监护,呼吸功能不全者和肝肾功能不全者应适当调整剂量。
2.使用本品时应绝对禁止摄入酒精饮料。
3.连续用药时间不宜过长,一般不超过4周,突然停药可引起停药综合征应谨慎,服药后不宜操作机械及驾车。
4.孕期妇女慎用。
因为本品在乳汁中浓度较高。
六用药禁忌1.对本品过敏者禁用。
2.失代偿的呼吸功能不全患者,重症肌无力、重症睡眠呼吸暂停综合征患者禁用。
3.妊娠期妇女、哺乳期妇女及15岁以下儿童不宜使用。
七药物相互作用1.与神经肌肉阻滞药或其他中枢神经抑制药同服可增强镇静作用。
2.与苯二氮类抗焦虑药和催眠药同服,戒断综合征的出现几率可增加。
说明:上述内容仅作为介绍,药物使用必须经正规医院在医生指导下进行。
佐匹克隆说明书

佐匹克隆说明书佐匹克隆说明书第一章:产品介绍佐匹克隆是一种全新的辅助生育技术,旨在帮助那些不同种族、不同年龄、不同性别或身体状况下的个人实现生育愿望。
佐匹克隆是一项经过精心研发的技术,通过科学的方法,使得个体能够再生体细胞,并在实验室中通过特定操作产生复制的胚胎。
这些复制的胚胎最终可以用于助孕或生殖研究。
第二章:使用方法1.准备工作:在使用佐匹克隆之前,用户需要进行全面的体检,并与医生进行咨询确认适用程度。
2.提取体细胞:通过医学操作,从用户体内提取出一部分体细胞。
这些细胞可以来自皮肤、血液等不同组织。
3.克隆过程:提取的体细胞将被送往实验室,进行克隆过程。
该过程是在严格的实验室环境下进行的,并符合伦理道德规范。
4.胚胎培养:在克隆过程完成后,经过一段时间的培养,胚胎会发育到一定程度,并达到适合助孕或生殖研究的要求。
5.助孕或生殖研究:经过医生判断,如果用户适合助孕,则将胚胎植入母体进行孕育。
如果用户选择参与生殖研究,则胚胎将用于科学实验。
6.后续咨询:佐匹克隆过程结束后,用户可与医生进行咨询,并获得恰当的建议和支持。
第三章:注意事项1.佐匹克隆是一项相对复杂的技术,需要密切配合医生进行操作和监控。
2.佐匹克隆的成功率和效果因个体差异而异,不同情况下可能会有不同的结果。
3.佐匹克隆需要遵守伦理道德规范,并受到法律法规的限制。
4.在使用佐匹克隆前,请确保已充分了解该技术的原理、效果、安全性等方面。
5.佐匹克隆的使用可能会涉及一定的费用,用户需要根据自身情况做出决策。
6.佐匹克隆结果并不保证100%的成功率,用户应做好心理准备。
第四章:风险与副作用使用佐匹克隆技术存在一定的风险和副作用,包括但不限于以下几个方面:1.体细胞提取过程可能会对用户身体造成一定创伤和不适。
2.克隆过程中可能会出现技术失败、胚胎发育异常等情况。
3.胚胎植入过程可能会引发一些并发症,如感染、出血等。
4.使用佐匹克隆技术进行生殖研究时,可能会带来一些未知的道德、伦理等问题。
佐匹克隆说明书

佐匹克隆说明书关于《佐匹克隆说明书》,是我们特意为大家整理的,希望对大家有所帮助。
在如今的社会髙速发展趋势,生活的节奏变的越来越快,经常熬夜加班加点,休息不好,它是经常出现的事儿,日常生活不规律性,精神压力过大,压力较为大,生活压力也较为大,非常容易造成人体的各类病症产生。
在其中失眠便是在其中一项,接下去为大伙儿详解佐匹克隆这一药品,期待会对有要求的小伙伴们有协助。
通用名:佐匹克隆胶襄英文名:Zopiclone Capsules拼音字母:Zuopikelong Jiaonang生产成份疫苗佐匹克隆化学名称:6-(5-氯吡啶-2-基)-7-[(4-羟基哌嗪-1-基)羰氧基]-5,6-二氢吡咯[3,4-b]吡嗪-5-酮。
其化学结构式为:化学式:C17H17ClN6O3相对分子质量:388.81生产性状疫苗本产品为胶囊剂,內容物为乳白色或类乳白色颗粒物或粉末状。
生产适应症疫苗失眠抑郁症。
特别是在适用不可以承受次晨残留功效的病人。
生产规格疫苗7.5mg生产使用方法使用量疫苗内服,7.5mg,临睡时服。
老人最开始使用量为3.75mg,临睡时服,仅在必要时服食7.5mg。
生产副作用疫苗与使用量及病人的敏感度相关。
少许思睡、口干舌燥、肌肉无力、忘却、醉态,易过度紧张或精神错乱、易激惹,头痛、困乏。
长期性吃药后忽然断药会出現戒断症状。
可能有比较轻的兴奋、焦虑情绪、肌疼、震颠、反跳性失眠及噩梦、恶心想吐及呕吐,少见偏重的筋挛、肌肉发抖、神智不清模糊不清。
生产忌讳疫苗禁止使用于对本产品过敏症状,失偿还的吸气作用不全病人,重症肌无力、危重症睡眠质量睡眠呼吸暂停综合征病人。
生产常见问题疫苗肌肉无力病人服药时特别注意诊疗监测,吸气作用不全者和肝、肾功能不全者适度调节使用量。
应用本产品时要肯定严禁摄取酒精饮料。
服药時间不适合太长,忽然断药应当心监测,吃药后不适合实际操作机械设备或开车。
生产孕妇及哺乳期间服药疫苗怀孕期间女性谨慎使用。
右佐匹克隆片

特殊人群:严重肝脏损伤患者应慎重使用本品,初始剂量为1mg。
合用CYP抑制剂:与CYP3A4强抑制剂合用,本品初始剂量不应大于1mg,必要时可增加至2mg。
不良反应
根据国外临床试验的报道:
儿童用药
有关18岁以下儿童用药的安全性、有效性尚未确立,不推荐服用此药。
老年用药
用药时,可先从小剂量开始逐渐增量,以便得到适合于患者的剂量。参见[用法用量]项。
药物相互作用
1.具CNS活性药物 酒精:右佐匹克隆与0.70g/kg酒精合用可对神经运动功能产生相加作用影响,可持续4小时。 帕罗西汀:每天合用3mg右佐匹克隆及2mg帕罗西汀,共7天,无药代动力学及药效学间的相互作用。 劳拉西泮:合用3mg右佐匹克隆及2mg劳拉西泮无临床相关性的药效学及药代动力学的影响。 奥氮平:合用3mg右佐匹克隆及10mg奥氮平使DSST评分降低。相互作用为药效学的改变而非药代动力学的改 变。 2.抑制CYP3A4的药物(酮康唑) CYP3A4是右佐匹克隆消除的主要代谢通道。与400mg酮康唑(一种CYP3A4的强抑制剂)合用5天可使右佐匹克 隆AUC增加2.2倍。Cmax和t1/2分别增加1.4倍和1.3倍。其他CYP3A4的强抑制剂可能产生相似的作用(例如:伊曲 康唑、克拉霉素、奈法唑酮、竹桃霉素、利托那韦、奈非那韦)。 3.诱导CYP3A4的药物(利福平) 与CYP3A4的强诱导剂利福平合用可使消旋佐匹克隆的暴露率降低80%。右佐匹克隆可能产生相似的作用。
消除
口服吸收后,右佐匹克隆消除半衰期大约为6小时。
贮藏
密封,在干燥处保存。
包装
铝塑板,7片/盒。
右佐匹克隆片Eszopiclone-详细说明书与重点

右佐匹克隆片Eszopiclone英文名称: Eszopiclone T ablets【成分】本品的活性成份为右佐匹克隆。
化学名称:(+)-(7S)-6-(5-氯-2-吡啶基)-7-[(4-甲基哌嗪-1-基)甲酰氧基]-5,6-二氢吡咯并[3,4-b]吡嗪-5-酮,化学结构式:【性状】本品为薄膜衣片,除去薄膜衣后显白色或类白色。
【适应症】用于治疗失眠。
【规格】3mg【用法用量】本品应个体化给药,成人推荐起始剂量为1mg。
如有临床需求,剂量可增至2mg或3mg。
某些患者服用2mg或3mg剂量后导致的晨起高血药浓度将会增加次晨宿醉现象发生的风险,即对驾驶或需要精神锐敏的活动的功能的损害(参见【注意事项】)主诉入睡困难的老年患者推荐起始剂量为睡前1mg,必要时可增加到2mg。
睡眠维持障碍的老年患者推荐剂量为入睡前2mg(见注意事项)。
如高脂肪饮食后立刻服用右佐匹克隆有可能会引起药物吸收缓慢,导致右佐匹克隆对睡眠潜伏期的作用降低(见药代动力学)。
特殊人群:严重肝损患者应慎重使用本品,初始剂量为1mg。
合用CYP抑制剂:与CYP3A4强抑制剂合用,本品初始剂量不应大于1mg,必要时可增加至2mg。
考虑到潜在的中枢神经系统抑制协同作用,当与其它中枢神经系统抑制剂合并用药时,可能需要适当调整右佐匹克隆的剂量。
【不良反应】鉴于临床试验是在不同条件下进行的,临床试验中观察到的不良反应发生率不能直接与其他药物临床试验中的发生率比较,也无法直接反映药物实际临床使用中不良反应的发生率。
右佐匹克隆上市前研究中,大约有400名正常受试者参加了临床药理/药代动力学研究,大约1500名患者参加了安慰剂对照的临床有效性研究(相当于大约263例患者暴露年限)。
上市前研究中,右佐匹克隆治疗的条件及疗程差异较大,包括开放试验和双盲试验(类别)、住院病人和门诊病人、长期和短期试验,不良反应是通过收集不良事件,评估体格检查、生命体征、体重、实验室检测,心电图等结果来进行评价的。
右佐匹克隆-处方指南

用法用量
其它警告/注意事项
失眠本身可能是原发性障碍的一项症状,而不是原发性障 碍本身
与其他CNS抑制剂一样,某些患者可能出现异常的思维或 行为的变化(即:抑制作用或脱抑制作用)
某些抑郁患者可能加重自杀观念 仅慎用于呼吸功能受损或阻塞性睡眠呼吸暂停的患者 仅在就寝时服用右佐匹克隆
THANKs
检测
• 对于健康个体,无需检测
副作用
药物怎样引起副作用?
作用于苯二氮卓类受体,次日会产生宿醉反应,引起日间 镇静、遗忘和共济失调
♣右佐匹克隆的长期研究提示:随着时间的延长,未发生明显 的耐药性或依赖性
副作用
特别的副作用?
♣不愉快的味觉 镇静 眩晕 剂量依赖性的遗忘 紧张不安 口干、头痛
用法用量
禁用
已知对右佐匹克隆或佐匹克隆过敏者 使用镇静安眠药物可能发生罕见的血管性水肿,如果涉及
咽喉、声门或喉,可能引起致命的呼吸道阻塞;因此,如 果发生血管性水肿,应停止治疗 服用镇静安眠药物的患者曾报道有:睡眠驾驶和其它的复 杂行为(如:进食、做饭和打电话)
特殊人群
肾功能受损
一般无需调整剂量
用法用量
给药的重要提醒(Biblioteka ) 但剂量>3mg可能有宿醉反应、幻觉或其他CNS不良反应 为了避免记忆问题或宿醉性镇静作用,仅在需要整晚睡眠
时服用右佐匹克隆 最明显的副作用可能是不愉快的味道 其他副作用可能包括:镇静、眩晕、剂量依赖性的遗忘、
紧张不安、口干和头痛
用法用量
过量
较少右佐匹克隆过量的报道,但可能与佐匹克隆过量相似 在佐匹克隆的过量中,报告有罕见的致命性 与佐匹克隆过量相关的症状包括:笨拙、情绪变化、镇静
右佐匹克隆和佐匹克隆

右佐匹克隆和佐匹克隆,傻傻分不清楚?中国有45.4%的被调查者在过去1个月中曾经历过不同程度的失眠。
成人中符合失眠症诊断标准者高达10%~15%。
右佐匹克隆(艾司佐匹克隆)和佐匹克隆是临床常用的镇静催眠药,这两者有何区别呢?一、临床疗效佐匹克隆是消旋体,由S-佐匹克隆(右佐匹克隆,艾司佐匹克隆)和R-佐匹克隆(左旋佐匹克隆)组成。
佐匹克隆属于非苯二氮卓类镇静催眠药,主要通过选择性激动γ-氨基丁酸受体上的的α1亚基,发挥镇静和催眠作用。
右佐匹克隆与γ-氨基丁酸受体的亲和力是左旋佐匹克隆的50倍。
3mg右佐匹克隆的催眠作用与7.5mg佐匹克隆相当。
二、毒性大小动物实验结果显示:右旋佐匹克隆的半数致死量(LD50)为1500mg/kg,左旋佐匹克隆的LD50为300mg/kg,消旋体的LD50为850mg/kg。
3mg右旋佐匹克隆的毒性小于7.5mg佐匹克隆。
三、药代动力学右佐匹克隆吸收更快和半衰期更长(见下表)。
因为佐匹克隆中含有50%的右佐匹克隆,所以右佐匹克隆和佐匹克隆的起效时间和维持时间没有区别。
需要注意的是:老年人(≥65岁)的吸收总量比成年人增加41%,而且半衰期更长,因此老年患者应从小剂量开始。
右佐匹克隆药品说明书:入睡困难的老年患者:推荐起始剂量1mg,必要时可增加到2mg;睡眠维持障碍的老年患者:推荐剂量为入睡前2mg。
四、不良反应佐匹克隆可经唾液排泄。
右佐匹克隆和佐匹克隆最常见不良反应是味觉异常(口苦、金属味),而且与剂量相关,停药后可以消失。
味觉异常是一种什么感觉?举例说明如下:患者,女,87岁,睡前口服2mg右佐匹克隆,第二天晨起后口腔感觉异常,舌体麻木,无疼痛及肿胀,对辣、甜、苦、咸味均减弱。
用药第3天,晨起后上述症状明显加重,自觉舌体麻木,对味觉缺失,对冷热反应表现正常。
诊断为右佐匹克隆所致不良反应,停用右佐匹克隆,3天后味觉逐渐恢复正常。
特别提醒:非苯二氮卓类药物(右佐匹克隆、扎来普隆、唑吡坦),可引起梦游、睡眠驾驶等。
佐匹克隆注意事项:

佐匹克隆注意事项:
各种因素引起的失眠症,包括时差、工作导致失眠及手术前焦虑导致失眠等。
佐匹克隆用法及用量:
口服:成人常用7.5mg(1片),睡前服用。
老年人开始治疗时,每次3.75mg (半片)睡前服用。
必要时,遵医嘱增加剂量到7.5mg(1片)睡前服用。
肝脏机能不全者:每次 3.75mg(半片)睡前服用。
人如果超大量服用催眠药可使睡眠时间延长,但大多仍可自动苏醒。
不良反应和注意:
可能有白天瞌睡,口苦,口干,肌张力减低,酒醉感。
怎么摆脱佐匹克隆:
我得病近十年,以前吃二片佐匹克隆能睡四五个小时,但佐匹克隆副作用太大依赖性太强,需要不断加大药量才能维持效果,药越吃越多睡眠是越来越差,后来听说中药效果很好,而且还能逐渐减停西药,我就试了一下,现在是药越吃越少睡眠越来越好,一共三个来月吧,不仅把吃了几年的佐匹克隆逐渐都停掉了,而且睡眠一直维持在近七个小时的深睡眠,感觉很好,早上起床后神清气爽,精力体力充沛。
佐匹克隆注意事项:
本品不推荐用于孕妇及哺乳期妇女;肌无力症,需在医学监护下使用本品;服用本品时,应避免饮酒;肝脏机能不全者,使用本品需适量。
可能有白天瞌睡,口苦,口干,肌张力减低,酒醉感。
对本品过敏者;呼吸代偿机能不全者;
幼儿病人禁用。
佐匹克隆片说明书

核准日期:2004年8月27日修改日期:2009年6月17日2009年12月2日2010年1月28日2012年8月28日 2013 年10月8 日2014年11月03日佐匹克隆片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:佐匹克隆片商品名称:忆孟返®/Imovane ®英文名称: ZOPICLONE TABLETS汉语拼音:ZUOPIKELONG PIAN【成份】活性成份:佐匹克隆化学名称: 6-(5-氯吡啶-2-基)-7-[(4-甲基哌嗪-1-基)羰氧基]-5,6-二氢吡咯[3,4-b]吡嗪-5-酮。
化学结构式:分子式:C 17H 17O 3N 6Cl分子量:388.8【性状】本品为白色,椭圆形,有刻痕的片剂。
【适应症】 NNNOON N C H 3NClO本品仅限应用在以下严重睡眠障碍的治疗中:- 短暂性失眠症- 短期失眠症【规格】 7.5mg【用法用量】用法: 口服剂量应该始终在最低有效剂量下开始治疗,而且不应该超过最高给药剂量。
本品应该在晚上临睡前服药。
通常的给药剂量如下所示:∙年龄低于65岁的成年人:每日7.5 mg。
∙年龄大于65岁的老年患者:推荐的给药剂量为每日3.75 mg;7.5 mg的给药剂量仅应该在例外的情况下应用。
∙肝脏或呼吸功能损害的患者:推荐剂量是每天1/2片。
(参见【药代动力学】)。
∙肾脏功能不全的患者:推荐起始剂量是每天1/2片。
(参见【药代动力学】)。
在任何情况下,本品的每日给药剂量均不应该超过7.5 mg。
治疗持续时间治疗持续时间应该尽可能短,从数天到4周,包括减药期(参见【注意事项】)。
必须将治疗持续时间告知患者:∙对于短暂性失眠症而言,治疗持续2 -5 天(比如,由旅行导致的失眠症)。
∙对于短期失眠症而言,治疗持续2 - 3 周(比如,由于发生严重事件而导致的失眠症)在一些情况下,可能有必要延长治疗持续时间,以至于超过推荐的治疗时间。
艾司佐匹克隆FDA说明书原版
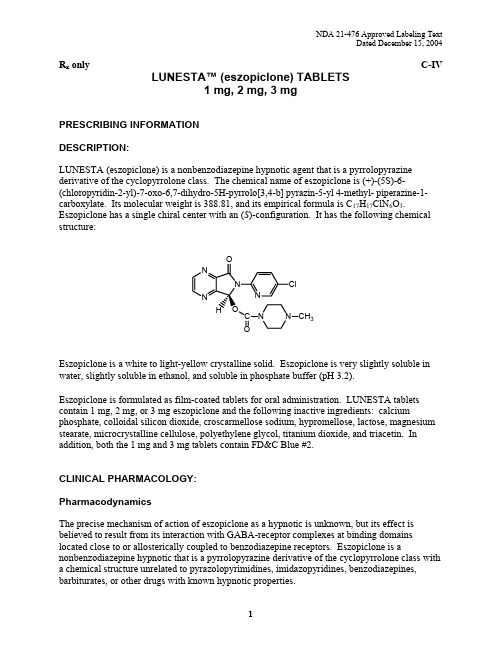
R x only C-IV LUNESTA™ (eszopiclone) TABLETS1 mg,2 mg,3 mgPRESCRIBING INFORMATIONDESCRIPTION:LUNESTA (eszopiclone) is a nonbenzodiazepine hypnotic agent that is a pyrrolopyrazine derivative of the cyclopyrrolone class. The chemical name of eszopiclone is (+)-(5S)-6-(chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b] pyrazin-5-yl 4-methyl- piperazine-1-carboxylate. Its molecular weight is 388.81, and its empirical formula is C17H17ClN6O3. Eszopiclone has a single chiral center with an (S)-configuration. It has the following chemical structure:3Eszopiclone is a white to light-yellow crystalline solid. Eszopiclone is very slightly soluble in water, slightly soluble in ethanol, and soluble in phosphate buffer (pH 3.2).Eszopiclone is formulated as film-coated tablets for oral administration. LUNESTA tablets contain 1 mg, 2 mg, or 3 mg eszopiclone and the following inactive ingredients: calcium phosphate, colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, titanium dioxide, and triacetin. In addition, both the 1 mg and 3 mg tablets contain FD&C Blue #2.CLINICAL PHARMACOLOGY:PharmacodynamicsThe precise mechanism of action of eszopiclone as a hypnotic is unknown, but its effect is believed to result from its interaction with GABA-receptor complexes at binding domains located close to or allosterically coupled to benzodiazepine receptors. Eszopiclone is a nonbenzodiazepine hypnotic that is a pyrrolopyrazine derivative of the cyclopyrrolone class with a chemical structure unrelated to pyrazolopyrimidines, imidazopyridines, benzodiazepines, barbiturates, or other drugs with known hypnotic properties.PharmacokineticsThe pharmacokinetics of eszopiclone have been investigated in healthy subjects (adult and elderly) and in patients with hepatic disease or renal disease. In healthy subjects, the pharmacokinetic profile was examined after single doses of up to 7.5 mg and after once-daily administration of 1, 3, and 6 mg for 7 days. Eszopiclone is rapidly absorbed, with a time to peak concentration (t max) of approximately 1 hour and a terminal-phase elimination half-life (t1/2) of approximately 6 hours. In healthy adults, LUNESTA does not accumulate with once-daily administration, and its exposure is dose-proportional over the range of 1 to 6 mg.Absorption And DistributionEszopiclone is rapidly absorbed following oral administration. Peak plasma concentrations are achieved within approximately 1 hour after oral administration. Eszopiclone is weakly bound to plasma protein (52-59%). The large free fraction suggests that eszopiclone disposition should not be affected by drug-drug interactions caused by protein binding. The blood-to-plasma ratio for eszopiclone is less than one, indicating no selective uptake by red blood cells.MetabolismFollowing oral administration, eszopiclone is extensively metabolized by oxidation and demethylation. The primary plasma metabolites are (S)-zopiclone-N-oxide and (S)-N-desmethyl zopiclone; the latter compound binds to GABA receptors with substantially lower potency than eszopiclone, and the former compound shows no significant binding to this receptor. In vitro studies have shown that CYP3A4 and CYP2E1 enzymes are involved in the metabolism of eszopiclone. Eszopiclone did not show any inhibitory potential on CYP450 1A2, 2A6, 2C9,2C19, 2D6, 2E1, and 3A4 in cryopreserved human hepatocytes.EliminationAfter oral administration, eszopiclone is eliminated with a mean t1/2 of approximately 6 hours. Up to 75% of an oral dose of racemic zopiclone is excreted in the urine, primarily as metabolites.A similar excretion profile would be expected for eszopiclone, the S-isomer of racemic zopiclone. Less than 10% of the orally administered eszopiclone dose is excreted in the urine as parent drug.Effect Of FoodIn healthy adults, administration of a 3 mg dose of eszopiclone after a high-fat meal resulted in no change in AUC, a reduction in mean C max of 21%, and delayed t max by approximately 1 hour. The half-life remained unchanged, approximately 6 hours. The effects of LUNESTA on sleep onset may be reduced if it is taken with or immediately after a high-fat/heavy meal.Special PopulationsAgeCompared with non-elderly adults, subjects 65 years and older had an increase of 41% in total exposure (AUC) and a slightly prolonged elimination of eszopiclone (t1/2 approximately 9 hours).C max was unchanged. Therefore, in elderly patients the starting dose of LUNESTA should be decreased to 1 mg and the dose should not exceed 2 mg.GenderThe pharmacokinetics of eszopiclone in men and women are similar.RaceIn an analysis of data on all subjects participating in Phase 1 studies of eszopiclone, the pharmacokinetics for all races studied appeared similar.Hepatic ImpairmentPharmacokinetics of a 2 mg eszopiclone dose were assessed in 16 healthy volunteers and in8 subjects with mild, moderate, and severe liver disease. Exposure was increased 2-fold in severely impaired patients compared with the healthy volunteers. C max and t max were unchanged. The dose of LUNESTA should not be increased above 2 mg in patients with severe hepatic impairment. No dose adjustment is necessary for patients with mild-to-moderate hepatic impairment. LUNESTA should be used with caution in patients with hepatic impairment. (See DOSAGE AND ADMINISTRATION.)Renal ImpairmentThe pharmacokinetics of eszopiclone were studied in 24 patients with mild, moderate, or severe renal impairment. AUC and C max were similar in the patients compared with demographically matched healthy control subjects. No dose adjustment is necessary in patients with renal impairment, since less than 10% of the orally administered eszopiclone dose is excreted in the urine as parent drug.Drug InteractionsEszopiclone is metabolized by CYP3A4 and CYP2E1 via demethylation and oxidation. There were no pharmacokinetic or pharmacodynamic interactions between eszopiclone and paroxetine, digoxin, or warfarin. When eszopiclone was coadministered with olanzapine, no pharmacokinetic interaction was detected in levels of eszopiclone or olanzapine, but a pharmacodynamic interaction was seen on a measure of psychomotor function. Eszopiclone and lorazepam decreased each other’s C max by 22%. Coadministration of eszopiclone 3 mg to subjects receiving ketoconazole 400 mg, a potent inhibitor of CYP3A4, resulted in a 2.2-foldincrease in exposure to eszopiclone. LUNESTA would not be expected to alter the clearance of drugs metabolized by common CYP450 enzymes. (See PRECAUTIONS.)CLINICAL TRIALS:The effect of LUNESTA on reducing sleep latency and improving sleep maintenance was established in studies with 2100 subjects (ages 18-86) with chronic and transient insomnia in six placebo-controlled trials of up to 6 months’ duration. Two of these trials were in elderly patients (n=523). Overall, at the recommended adult dose (2-3 mg) and elderly dose (1-2 mg), LUNESTA significantly decreased sleep latency and improved measures of sleep maintenance (objectively measured as wake time after sleep onset [WASO] and subjectively measured as total sleep time).Transient InsomniaHealthy adults were evaluated in a model of transient insomnia (n=436) in a sleep laboratory in a double-blind, parallel-group, single-night trial comparing two doses of eszopiclone and placebo. LUNESTA 3 mg was superior to placebo on measures of sleep latency and sleep maintenance, including polysomnographic (PSG) parameters of latency to persistent sleep (LPS) and WASO.Chronic Insomnia (Adults And Elderly)The effectiveness of LUNESTA was established in five controlled studies in chronic insomnia. Three controlled studies were in adult subjects, and two controlled studies were in elderly subjects with chronic insomnia.AdultsIn the first study, adults with chronic insomnia (n=308) were evaluated in a double-blind, parallel-group trial of 6 weeks’ duration comparing LUNESTA 2 mg and 3 mg with placebo. Objective endpoints were measured for 4 weeks. Both 2 mg and 3 mg were superior to placebo on LPS at 4 weeks. The 3 mg dose was superior to placebo on WASO.In the second study, adults with chronic insomnia (n=788) were evaluated using subjective measures in a double-blind, parallel-group trial comparing the safety and efficacy of LUNESTA 3 mg with placebo administered nightly for 6 months. LUNESTA was superior to placebo on subjective measures of sleep latency, total sleep time, and WASO.In addition, a 6-period cross-over PSG study evaluating eszopiclone doses of 1 to 3 mg, each given over a 2-day period, demonstrated effectiveness of all doses on LPS, and of 3 mg on WASO. In this trial, the response was dose-related.ElderlyElderly subjects (ages 65-86) with chronic insomnia were evaluated in two double-blind, parallel-group trials of 2 weeks’ duration. One study (n=231) compared the effects of LUNESTA with placebo on subjective outcome measures, and the other (n=292) on objective and subjective outcome measures. The first study compared 1 mg and 2 mg of LUNESTA with placebo, while the second study compared 2 mg of LUNESTA with placebo. All doses were superior to placebo on measures of sleep latency. In both studies, 2 mg of LUNESTA was superior to placebo on measures of sleep maintenance.Studies Pertinent To Safety Concerns For Sedative/Hypnotic DrugsCognitive, Memory, Sedative, and Psychomotor EffectsIn two double-blind, placebo-controlled, single-dose cross-over studies of 12 patients each (one study in patients with insomnia; one in normal volunteers), the effects of LUNESTA 2 and 3 mg were assessed on 20 measures of cognitive function and memory at 9.5 and 12 hours after a nighttime dose. Although results suggested that patients receiving LUNESTA 3 mg performed more poorly than patients receiving placebo on a very small number of these measures at 9.5 hours post-dose, no consistent pattern of abnormalities was seen.In a 6-month double-blind, placebo-controlled trial of nightly administered LUNESTA 3 mg,8/593 subjects treated with LUNESTA 3 mg (1.3%) and 0/195 subjects treated with placebo (0%) spontaneously reported memory impairment. The majority of these events were mild in nature (5/8), and none were reported as severe. Four of these events occurred within the first 7 days of treatment and did not recur. The incidence of spontaneously reported confusion in this 6-month study was 0.5% in both treatment arms. In a 6-week adult study of nightly administered LUNESTA 2 mg or 3 mg or placebo, the spontaneous reporting rates for confusion were 0%, 3.0%, and 0%, respectively, and for memory impairment were 1%, 1%, and 0%, respectively. In a 2-week study of 264 elderly insomniacs randomized to either nightly LUNESTA 2 mg or placebo, spontaneous reporting rates of confusion and memory impairment were 0% vs. 0.8% and 1.5% vs. 0%, respectively. In another 2-week study of 231 elderly insomniacs, the spontaneous reporting rates for the 1 mg, 2 mg, and placebo groups for confusion were 0%,2.5%, and 0%, respectively, and for memory impairment were 1.4%, 0%, and 0%, respectively.A study of normal subjects exposed to single fixed doses of LUNESTA from 1 to 7.5 mg using the DSST to assess sedation and psychomotor function at fixed times after dosing (hourly up to 16 hours) found the expected sedation and reduction in psychomotor function. This was maximal at 1 hour and present up to 4 hours, but was no longer present by 5 hours.In another study, patients with insomnia were given 2 or 3 mg doses of LUNESTA nightly, with DSST assessed on the mornings following days 1, 15, and 29 of treatment. While both the placebo and LUNESTA 3 mg groups showed an improvement in DSST scores relative to baseline the following morning (presumably due to a learning effect), the improvement in theplacebo group was greater and reached statistical significance on night 1, although not on nights 15 and 29. For the LUNESTA 2 mg group, DSST change scores were not significantly different from placebo at any time point.Withdrawal-Emergent Anxiety And InsomniaDuring nightly use for an extended period, pharmacodynamic tolerance or adaptation has been observed with other hypnotics. If a drug has a short elimination half-life, it is possible that a relative deficiency of the drug or its active metabolites (i.e., in relationship to the receptor site) may occur at some point in the interval between each night’s use. This is believed to be responsible for two clinical findings reported to occur after several weeks of nightly use of other rapidly eliminated hypnotics: increased wakefulness during the last quarter of the night and the appearance of increased signs of daytime anxiety.In a 6-month double-blind, placebo-controlled study of nightly administration of LUNESTA3 mg, rates of anxiety reported as an adverse event were 2.1% in the placebo arm and 3.7% in the LUNESTA arm. In a 6-week adult study of nightly administration, anxiety was reported as an adverse event in 0%, 2.9%, and 1.0% of the placebo, 2 mg, and 3 mg treatment arms, respectively. In this study, single-blind placebo was administered on nights 45 and 46, the first and second days of withdrawal from study drug. New adverse events were recorded during the withdrawal period, beginning with day 45, up to 14 days after discontinuation. During this withdrawal period, 105 subjects previously taking nightly LUNESTA 3 mg for 44 nights spontaneously reported anxiety (1%), abnormal dreams (1.9%), hyperesthesia (1%), and neurosis (1%), while none of 99 subjects previously taking placebo reported any of these adverse events during the withdrawal period.Rebound insomnia, defined as a dose-dependent temporary worsening in sleep parameters (latency, sleep efficiency, and number of awakenings) compared with baseline following discontinuation of treatment, is observed with short- and intermediate-acting hypnotics. Rebound insomnia following discontinuation of LUNESTA relative to placebo and baseline was examined objectively in a 6-week adult study on the first 2 nights of discontinuation (nights 45 and 46) following 44 nights of active treatment with 2 mg or 3 mg. In the LUNESTA 2 mg group, compared with baseline, there was a significant increase in WASO and a decrease in sleep efficiency, both occurring only on the first night after discontinuation of treatment. No changes from baseline were noted in the LUNESTA 3 mg group on the first night after discontinuation, and there was a significant improvement in LPS and sleep efficiency compared with baseline following the second night of discontinuation. Comparisons of changes from baseline between LUNESTA and placebo were also performed. On the first night after discontinuation of LUNESTA 2 mg, LPS and WASO were significantly increased and sleep efficiency was reduced; there were no significant differences on the second night. On the first night following discontinuation of LUNESTA 3 mg, sleep efficiency was significantly reduced. No other differences from placebo were noted in any other sleep parameter on either the first or second night following discontinuation. For both doses, the discontinuation-emergent effect was mild, had the characteristics of the return of the symptoms of chronic insomnia, and appeared to resolve by the second night after LUNESTA discontinuation.INDICATIONS AND USAGE:LUNESTA is indicated for the treatment of insomnia. In controlled outpatient and sleep laboratory studies, LUNESTA administered at bedtime decreased sleep latency and improved sleep maintenance.CONTRAINDICATIONS:None known.WARNINGS:Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative/hypnotic drugs, including LUNESTA. Because some of the important adverse effects of LUNESTA appear to be dose-related, it is important to use the lowest possible effective dose, especially in the elderly (see DOSAGE AND ADMINISTRATION).A variety of abnormal thinking and behavior changes have been reported to occur in association with the use of sedative/hypnotics. Some of these changes may be characterized by decreased inhibition (e.g., aggressiveness and extroversion that seem out of character), similar to effects produced by alcohol and other CNS depressants. Other reported behavioral changes have included bizarre behavior, agitation, hallucinations, and depersonalization. Amnesia and other neuropsychiatric symptoms may occur unpredictably. In primarily depressed patients, worsening of depression, including suicidal thinking, has been reported in association with the use of sedative/hypnotics.It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above are drug-induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.Following rapid dose decrease or abrupt discontinuation of the use of sedative/hypnotics, there have been reports of signs and symptoms similar to those associated with withdrawal from other CNS-depressant drugs (see DRUG ABUSE AND DEPENDENCE).LUNESTA, like other hypnotics, has CNS-depressant effects. Because of the rapid onset of action, LUNESTA should only be ingested immediately prior to going to bed or after the patient has gone to bed and has experienced difficulty falling asleep. Patients receiving LUNESTAshould be cautioned against engaging in hazardous occupations requiring complete mental alertness or motor coordination (e.g., operating machinery or driving a motor vehicle) after ingesting the drug, and be cautioned about potential impairment of the performance of such activities on the day following ingestion of LUNESTA. LUNESTA, like other hypnotics, may produce additive CNS-depressant effects when coadministered with other psychotropic medications, anticonvulsants, antihistamines, ethanol, and other drugs that themselves produce CNS depression. LUNESTA should not be taken with alcohol. Dose adjustment may be necessary when LUNESTA is administered with other CNS-depressant agents, because of the potentially additive effects.PRECAUTIONS:GeneralTiming Of Drug AdministrationLUNESTA should be taken immediately before bedtime. Taking a sedative/hypnotic while still up and about may result in short-term memory impairment, hallucinations, impaired coordination, dizziness, and lightheadedness.Use In The Elderly And/Or Debilitated PatientsImpaired motor and/or cognitive performance after repeated exposure or unusual sensitivity to sedative/hypnotic drugs is a concern in the treatment of elderly and/or debilitated patients. The recommended starting dose of LUNESTA for these patients is 1 mg. (See DOSAGE AND ADMINISTRATION.)Use In Patients With Concomitant IllnessClinical experience with eszopiclone in patients with concomitant illness is limited. Eszopiclone should be used with caution in patients with diseases or conditions that could affect metabolism or hemodynamic responses.A study in healthy volunteers did not reveal respiratory-depressant effects at doses 2.5-fold higher (7 mg) than the recommended dose of eszopiclone. Caution is advised, however, if LUNESTA is prescribed to patients with compromised respiratory function.The dose of LUNESTA should be reduced to 1 mg in patients with severe hepatic impairment, because systemic exposure is doubled in such subjects. No dose adjustment appears necessary for subjects with mild or moderate hepatic impairment. No dose adjustment appears necessary in subjects with any degree of renal impairment, since less than 10% of eszopiclone is excreted unchanged in the urine.The dose of LUNESTA should be reduced in patients who are administered potent inhibitors of CYP3A4, such as ketoconazole, while taking LUNESTA. Downward dose adjustment is alsorecommended when LUNESTA is administered with agents having known CNS-depressant effects.Use In Patients With DepressionSedative/hypnotic drugs should be administered with caution to patients exhibiting signs and symptoms of depression. Suicidal tendencies may be present in such patients, and protective measures may be required. Intentional overdose is more common in this group of patients; therefore, the least amount of drug that is feasible should be prescribed for the patient at any one time.Information For PatientsPatient information is printed at the end of this insert. To assure safe and effective use of LUNESTA, this information and the instructions provided in the patient information section should be discussed with patients.Laboratory TestsThere are no specific laboratory tests recommended.Drug InteractionsCNS-Active DrugsEthanolAn additive effect on psychomotor performance was seen with coadministration of eszopiclone and ethanol 0.70 g/kg for up to 4 hours after ethanol administration.ParoxetineCoadministration of single doses of eszopiclone 3 mg and paroxetine 20 mg daily for 7 days produced no pharmacokinetic or pharmacodynamic interaction.LorazepamCoadministration of single doses of eszopiclone 3 mg and lorazepam 2 mg did not have clinically relevant effects on the pharmacodynamics or pharmacokinetics of either drug. OlanzapineCoadministration of eszopiclone 3 mg and olanzapine 10 mg produced a decrease in DSST scores. The interaction was pharmacodynamic; there was no alteration in the pharmacokinetics of either drug.Drugs That Inhibit CYP3A4 (Ketoconazole)CYP3A4 is a major metabolic pathway for elimination of eszopiclone. The AUC of eszopiclone was increased 2.2-fold by coadministration of ketoconazole, a potent inhibitor of CYP3A4,400 mg daily for 5 days. C max and t1/2 were increased 1.4-fold and 1.3-fold, respectively. Other strong inhibitors of CYP3A4 (e.g., itraconazole, clarithromycin, nefazodone, troleandomycin, ritonavir, nelfinavir) would be expected to behave similarly.Drugs That Induce CYP3A4 (Rifampicin)Racemic zopiclone exposure was decreased 80% by concomitant useof rifampicin, a potent inducer of CYP3A4. A similar effect would be expected with eszopiclone.Drugs Highly Bound To Plasma ProteinEszopiclone is not highly bound to plasma proteins (52-59% bound); therefore, the disposition of eszopiclone is not expected to be sensitive to alterations in protein binding. Administration of eszopiclone 3 mg to a patient taking another drug that is highly protein-bound would not be expected to cause an alteration in the free concentration of either drug.Drugs With A Narrow Therapeutic IndexDigoxinA single dose of eszopiclone 3 mg did not affect the pharmacokinetics of digoxin measured at steady state following dosing of 0.5 mg twice daily for one day and 0.25 mg daily for the next 6 days.WarfarinEszopiclone 3 mg administered daily for 5 days did not affect the pharmacokinetics of (R)- or (S)-warfarin, nor were there any changes in the pharmacodynamic profile (prothrombin time) following a single 25 mg oral dose of warfarin.Carcinogenesis, Mutagenesis, Impairment Of FertilityCarcinogenesisIn a carcinogenicity study in Sprague-Dawley rats in which eszopiclone was given by oral gavage, no increases in tumors were seen; plasma levels (AUC) of eszopiclone at the highest dose used in this study (16 mg/kg/day) are estimated to be 80 (females) and 20 (males) times those in humans receiving the maximum recommended human dose (MRHD). However, in a carcinogenicity study in Sprague-Dawley rats in which racemic zopiclone was given in the diet, and in which plasma levels of eszopiclone were reached that were greater than those reached in the above study of eszopiclone, an increase in mammary gland adenocarcinomas in females andan increase in thyroid gland follicular cell adenomas and carcinomas in males were seen at the highest dose of 100 mg/kg/day. Plasma levels of eszopiclone at this dose are estimated to be 150 (females) and 70 (males) times those in humans receiving the MRHD. The mechanism for the increase in mammary adenocarcinomas is unknown. The increase in thyroid tumors is thought to be due to increased levels of TSH secondary to increased metabolism of circulating thyroid hormones, a mechanism that is not considered to be relevant to humans.In a carcinogenicity study in B6C3F1 mice in which racemic zopiclone was given in the diet, an increase in pulmonary carcinomas and carcinomas plus adenomas in females and an increase in skin fibromas and sarcomas in males were seen at the highest dose of 100 mg/kg/day. Plasma levels of eszopiclone at this dose are estimated to be 8 (females) and 20 (males) times those in humans receiving the MRHD. The skin tumors were due to skin lesions induced by aggressive behavior, a mechanism that is not relevant to humans. A carcinogenicity study was also performed in which CD-1 mice were given eszopiclone at doses up to 100 mg/kg/day by oral gavage; although this study did not reach a maximum tolerated dose, and was thus inadequate for overall assessment of carcinogenic potential, no increases in either pulmonary or skin tumors were seen at doses producing plasma levels of eszopiclone estimated to be 90 times those in humans receiving the MRHD — i.e., 12 times the exposure in the racemate study. Eszopiclone did not increase tumors in a p53 transgenic mouse bioassay at oral doses up to300 mg/kg/day.MutagenesisEszopiclone was positive in the mouse lymphoma chromosomal aberration assay and produced an equivocal response in the Chinese hamster ovary cell chromosomal aberration assay. It was not mutagenic or clastogenic in the bacterial Ames gene mutation assay, in an unscheduled DNA synthesis assay, or in an in vivo mouse bone marrow micronucleus assay.(S)-N-desmethyl zopiclone, a metabolite of eszopiclone, was positive in the Chinese hamster ovary cell and human lymphocyte chromosomal aberration assays. It was negative in the bacterial Ames mutation assay, in an in vitro32P-postlabeling DNA adduct assay, and in an in vivo mouse bone marrow chromosomal aberration and micronucleus assay.Impairment Of FertilityEszopiclone was given by oral gavage to male rats at doses up to 45 mg/kg/day from 4 weeks premating through mating and to female rats at doses up to 180 mg/kg/day from 2 weeks premating through day 7 of pregnancy. An additional study was performed in which only females were treated, up to 180 mg/kg/day. Eszopiclone decreased fertility, probably because of effects in both males and females, with no females becoming pregnant when both males and females were treated with the highest dose; the no-effect dose in both sexes was 5 mg/kg (16 times the MRHD on a mg/m2 basis). Other effects included increased pre-implantation loss (no-effect dose 25 mg/kg), abnormal estrus cycles (no-effect dose 25 mg/kg), and decreases in sperm number and motility and increases in morphologically abnormal sperm (no-effect dose 5 mg/kg).PregnancyPregnancy Category CEszopiclone administered by oral gavage to pregnant rats and rabbits during the period of organogenesis showed no evidence of teratogenicity up to the highest doses tested (250 and 16 mg/kg/day in rats and rabbits, respectively; these doses are 800 and 100 times, respectively, the maximum recommended human dose [MRHD] on a mg/m2 basis). In the rat, slight reductions in fetal weight and evidence of developmental delay were seen at maternally toxic doses of 125 and 150 mg/kg/day, but not at 62.5 mg/kg/day (200 times the MRHD on a mg/m2 basis). Eszopiclone was also administered by oral gavage to pregnant rats throughout the pregnancy and lactation periods at doses of up to 180 mg/kg/day. Increased post-implantation loss, decreased postnatal pup weights and survival, and increased pup startle response were seen at all doses; the lowest dose tested, 60 mg/kg/day, is 200 times the MRHD on a mg/m2 basis. These doses did not produce significant maternal toxicity. Eszopiclone had no effects on other behavioral measures or reproductive function in the offspring.There are no adequate and well-controlled studies of eszopiclone in pregnant women. Eszopiclone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.Labor And DeliveryLUNESTA has no established use in labor and delivery.Nursing MothersIt is not known whether LUNESTA is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when LUNESTA is administered to a nursing woman.Pediatric UseSafety and effectiveness of eszopiclone in children below the age of 18 have not been established.Geriatric UseA total of 287 subjects in double-blind, parallel-group, placebo-controlled clinical trials who received eszopiclone were 65 to 86 years of age. The overall pattern of adverse events for elderly subjects (median age = 71 years) in 2-week studies with nighttime dosing of 2 mg eszopiclone was not different from that seen in younger adults (see ADVERSE REACTIONS, Table 2). LUNESTA 2 mg exhibited significant reduction in sleep latency and improvement in sleep maintenance in the elderly population.。
佐匹克隆的用法

佐匹克隆的用法佐匹克隆,也被称为左旋酒石酸环丙孕酮,是一种合成雌激素类药物,常用于医疗和研究领域。
它主要应用于人类的垂体功能减退症、停经、多囊卵巢综合症以及促排卵等治疗中。
佐匹克隆也可用于动物实验中模拟雌激素的作用,以及在植物组织培养和生长调节中也有广泛的应用。
本文将为您介绍佐匹克隆的用法和相关信息。
一、佐匹克隆的用法佐匹克隆的用法主要有以下几个方面:1. 医疗用途佐匹克隆在医疗领域主要用于垂体功能减退症、停经、多囊卵巢综合症、促排卵等治疗中。
这些疾病或症状往往与雌激素的水平不平衡有关,佐匹克隆通过模拟雌激素的作用来调节患者体内的激素水平,从而达到治疗效果。
2. 动物实验在动物实验中,佐匹克隆常被用来模拟雌激素的作用。
科研人员可以通过给实验动物注射佐匹克隆,来研究雌激素对生理和病理的影响,以及相关疾病的发病机制。
3. 植物生长调节佐匹克隆也被用于植物组织培养和生长调节中。
通过外源添加佐匹克隆,可以促进植物的生长、增加植株的茎节和叶片数量,从而实现对植物生长过程的调控。
二、佐匹克隆的剂型和剂量1. 剂型佐匹克隆通常以片剂或注射剂的形式供应,片剂用于口服给药,而注射剂则用于肌肉或静脉注射。
2. 剂量根据患者的具体病情和医生的建议,佐匹克隆的剂量会有所不同。
一般来说,医生会根据患者的年龄、体重、病情严重程度等因素来确定合适的剂量。
患者在使用佐匹克隆之前应该仔细阅读药品说明书,遵医嘱使用,严格控制剂量,切勿擅自增减用药。
三、佐匹克隆的不良反应和注意事项1. 不良反应佐匹克隆在使用过程中可能会出现一些不良反应,包括头痛、恶心、疲乏、乳房胀痛等。
少数患者可能会出现过敏反应或严重不良反应,如出血、肝功能异常等。
患者在使用佐匹克隆时应密切关注身体状况,如有不适应立即停药并就医。
2. 注意事项患者在使用佐匹克隆前应告知医生自己的过敏史、疾病史、用药史等相关信息。
女性患者在怀孕或哺乳期间应避免使用佐匹克隆。
对于老年患者和有肝肾功能障碍的患者,使用佐匹克隆时需要格外谨慎。
右佐匹克隆片详细说明书

右佐匹克隆片详细说明书右佐匹克隆片说明书通用名称:右佐匹克隆片主要成份:右佐匹克隆。
用法用量:本品应个体化给药,成年人推荐起始剂量为入睡前2mg,由于3mg可以更有效的延长睡眠时间,可根据临床需要起始剂量为或增加到3mg。
主诉入睡困难的老年患者推荐起始剂量为睡前1mg,必要时可增加到2mg、睡眠维持障碍的老年患者推荐剂量为入睡前2mg(见注意事项)低收用致高有隆期睡后。
食会隆起对克导,缓伏克的匹右眠脂可立引物能肪匹用佐潜佐如作饮吸服右药慢刻降(见药代动力学)。
特殊人群:严重肝损患者应慎重使用本品,初始剂量为1mg。
合用CYP抑制剂:与CYP3A4强抑制剂合用,本品初始剂量不应大于1mg,必要时可增加至2mg。
右佐匹克隆片不良反应:主要不良反应为口苦和头晕,其他如瞌睡、乏力、恶心和呕吐等轻度消化系统和中枢神经系统的不良反应一般持续时间短,症状轻微,不会影响受试者的生活和功能,可自行缓解,停药后症状即可消失。
右佐匹克隆片禁忌:对本品及其成分过敏者,失代偿的呼吸功能不全患者,重症肌无力、重症睡眠呼吸暂停综合症患者。
如何摆脱右佐匹克隆片毒副作用我失眠七八年,佐匹克隆从半片加到二片,大概二三个小时才能入睡,整晚能睡三四个小时,西药的副作用特别大,但想停还停不下来,后来听说百艺舒中药可以逐渐减停西药,我就试了一下,先共同吃段时间,当睡眠达到七个小时,睡眠质量满意后,再将佐匹克隆一次停1/4片,半个月为一个周期逐渐减停,方法很科学,很轻松的就将吃了几年的佐匹克隆停掉了,睡眠也一直很好。
右佐匹克隆片注意事项:右佐匹克隆应在临睡前服用。
服用镇静/催眠药物有可能产生短期记忆损伤、幻觉、协调障碍、眩晕和头晕眼花。
孕妇及哺乳期妇女用药本品由于具有适当的亲脂性,容易进入大脑,右佐匹克隆及其代谢产物可部分通过胎盘屏障,同时本品在乳汁中浓度可能较高,因此妊娠妇女及哺乳期妇女慎用此药。
儿童用药有关18岁以下儿童用药的安全性、有效性尚未确立,不推荐服用此药。
佐匹克隆片的英文说明书

佐匹克隆片的英文说明书Zopiclone TabletsEnglish Instruction Manual1. IntroductionThank you for choosing Zopiclone Tablets as your preferred medication for the treatment of insomnia. This instruction manual is designed to provide you with all the necessary information regarding the usage, dosage, precautions, and possible side effects of Zopiclone tablets. Please read this manual carefully before using the product to ensure safe and effective use.2. UsageZopiclone tablets are intended for the short-term treatment of insomnia in adults. It belongs to a class of medications called hypnotics, which work by acting on the brain to produce a calming effect, helping individuals fall asleep faster and stay asleep longer. Zopiclone tablets should be taken orally with water, and it is recommended to take it just before bed.3. DosageThe recommended dosage for Zopiclone tablets may vary depending on individual circumstances and response to treatment. The usual starting dose for adults is 7.5mg, taken shortly before bedtime. However, your doctor may prescribe a lower dose (e.g., 3.75mg) for elderly patients or in cases of liver or kidney impairment. It is essential to follow the prescribed dosage and never exceed the recommended amount to avoid potential health risks or side effects.4. Precautions4.1 Medical ConditionsBefore using Zopiclone tablets, inform your doctor or pharmacist if you have any of the following medical conditions:- Allergies or sensitivities to medications- Liver or kidney disease- Breathing difficulties or lung problems- Mental health disorders such as depression or anxiety- History of drug or alcohol addiction4.2 Pregnancy and BreastfeedingZopiclone tablets should not be used during pregnancy or breastfeeding as its effects on unborn babies or infants are not yet fully understood. If you are pregnant, planning to become pregnant, or breastfeeding, consult your doctor for alternative treatment options.4.3 Driving and Operating MachineryZopiclone may cause drowsiness or dizziness, which can impair your ability to drive or operate machinery safely. It is advised to avoid such activities until you know how you react to the medication. If you experience excessive drowsiness or feel unable to concentrate, avoid driving or operating machinery until these effects subside.4.4 Other MedicationsInform your doctor or pharmacist about any other medications you are currently taking, including prescription drugs, over-the-counter medications, herbal supplements, or vitamins. Some medications may interact with Zopiclone tablets, leading to increased side effects or reduced effectiveness.5. Side EffectsWhile Zopiclone tablets are generally well-tolerated, some individuals may experience side effects. Common side effects include:- Dry mouth- Unpleasant taste in mouth- Drowsiness or dizziness- Headache- Nausea or stomach upsetContact your doctor if these side effects persist or worsen. In rare cases, serious allergic reactions or severe mood changes may occur. Seek immediate medical attention if you experience symptoms such as difficulty breathing, swelling of the face or limbs, or sudden changes in mood or behavior.6. StorageStore Zopiclone tablets in a cool, dry place away from direct sunlight and moisture. Keep it out of reach of children and pets. Do not use the medication if it has expired or shows signs of deterioration. Dispose of unused or expired medication properly according to local regulations.7. ConclusionZopiclone tablets are an effective short-term treatment for insomnia. By following the dosage recommendations, precautions, and understanding possible side effects, you can ensure safe and effective use of this medication. If you have any further questions or concerns, please consult your doctor or pharmacist. Rest well with Zopiclone Tablets and enjoy a good night's sleep.Note: This instruction manual is intended for informational purposes only and should not replace professional medical advice. Always consult your healthcare provider for personalized guidance and recommendations.。
右佐匹克隆片说明书?

右佐匹克隆片说明书?
一、成份性状
右佐匹克隆片是化学片剂,其主要成份是右佐匹克隆,常见的包装规格是铝塑泡罩包装,3毫克*6片*1板*1盒。
二、用法与用量
右佐匹克隆片是个体化给药,具体来说,成年人初始用药的剂量应为睡前2毫克,后期可以加量,但不可超过3毫克。
对于难入睡的老年人来说,初始的用
药剂量应为1毫克,必要的时候可以加至2毫克。
而严重肝病患者需慎重服用本
药,参考初始剂量为1毫克,或咨询医生。
三、怎么减停右佐匹克隆片依赖和副作用
我得病近十年,以前吃二片右佐匹克隆勉强能睡四五个小时,用百艺舒中药先调养睡眠再逐渐减停西药,当睡眠调整到七个小时,睡眠质量自己满意后,再
将右佐匹克隆一次停1/4片,半个月为一个周期逐渐减停,方法很科学很有效,
以前是药越吃越多睡眠越来越差,现在是药越吃越少睡眠越来越好,坚持了四五
个月不仅把吃了几年的二片右佐匹克隆都停掉了,而且睡眠一直维持在近七个小
时的深睡眠,睡得很香甜很舒服。
四、用药禁忌
由于右佐匹克隆片治疗的是失眠,会对神经系统造成影响,因此,大家在服用此药期间应注意一些禁忌。
首先,右佐匹克隆片需在睡前服用。
然后,此药和
其他催眠药物一样,可能会给患者带来协调障碍、眩晕、头晕、眼花等不适症状。
PDF 文件使用 "pdfFactory Pro" 试用版本创建w 。
西药丨每日一药--佐匹克隆

西药丨每日一药--佐匹克隆
大家好,新新老师带大家学习化学结构式了。
今日西药--佐匹克隆
【化学结构】
【结构特点】
✦吡咯酮类。
✦艾司佐匹克隆为S-右旋体有短效催眠作用。
✦左旋体无活性且有毒性。
【适应证】
用于失眠。
【典型不良反应】
常见嗜睡、精神错乱、酒醉感、戒断现象。
【禁忌证】
重症肌无力、失代偿呼吸功能不全、严重睡眠呼吸暂停综合征及对佐匹克隆过敏者禁用。
【药物相互作用】
(1)与肌松药或其他中枢神经抑制剂合用可增强镇静作用。
(2)与苯二氮䓬类抗焦虑药或催眠药合用,可增加戒断症状的出现。
【注意事项】
(1)本品可由乳汁分泌,哺乳期妇女不宜使用。
(2)大量长期用药突然停药可引起戒断症状。
(3)连续用药时间不宜过长,一般不应超过4周。
(4)15岁以下儿童不宜应用。
【用法用量】
口服:睡前服用,成人一次7.5mg,老年、体弱或肝功能不全者一次3.75mg。
【制剂与规格】
片剂:7.5mg。
【习题训练】下列关于佐匹克隆说法,错误的是( )。
A.艾司佐匹克隆是其异构体
B.作用于γ-氨基丁酸(GABA)受体
C.仅具有抗焦虑、抗惊厥作用
D.属于环吡咯烷酮类镇静催眠药
E.属于非苯二氮䓬类药物
点击下方空白区域查看答案
▼
参考答案:C
解析:佐匹克隆具有镇静催眠、抗焦虑、肌肉松弛和抗惊厥等作用。
佐匹克隆片说明书

核准日期:2004年8月27日修改日期:2009年6月17日2009年12月2日2010年1月28日2012年8月28日2013年10月8日2014年11月3日2015年5月21日2016年3月25日2017年5月16日2018年8月8日2020年11月16日2021年11月18日2022年5月31日2022年10月21日佐匹克隆片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:佐匹克隆片商品名称:忆孟返®/Imovane®英文名称:ZOPICLONE TABLETS汉语拼音:ZUOPIKELONG PIAN【成份】活性成份:佐匹克隆化学名称:6-(5-氯吡啶-2-基)-7-[(4-甲基哌嗪-1-基)羰氧基]-5,6-二氢吡咯[3,4-b]吡嗪-5-酮化学结构式:分子式:C 17H 17O 3N 6Cl分子量:388.8【性状】 本品为白色,椭圆形,有刻痕的片剂。
【适应症】本品仅限应用在成人以下严重睡眠障碍的短期治疗中:- 短暂性失眠症- 短期失眠症【规格】 7.5mg【用法用量】用法: 口服剂量:• 年龄低于65岁的成年人:每日7.5 mg 。
• 年龄高于65岁的老年人:推荐剂量为每日3.75 mg ,经评估必要时可以增加至7.5mg 。
• 肝脏或呼吸功能损害的患者:推荐剂量为每日3.75mg 。
• 肾脏功能不全的患者:推荐起始剂量为每日3.75mg 。
• 超过65岁的人群及高风险人群的最佳剂量为3.75mg 。
NN N OONN C H 3NClO应该始终在最低有效剂量下开始治疗,每日给药剂量不应超过7.5 mg。
应在晚上临睡前服药,按单次摄入剂量服用,同一晚不得再次服用。
本品不推荐用于18岁以下儿童和青少年。
治疗持续时间:与所有催眠药一样,不建议长期使用佐匹克隆。
治疗持续时间应该尽可能短,从数天到4周,包括减药期。
由于滥用和依赖风险会随治疗持续时间的增加而升高,因此在未对患者状况进行重新评估的情况下,不应在超出最长治疗期后延长治疗时间(见【注意事项】)。
佐匹克隆的用法

佐匹克隆的用法佐匹克隆(Zopiclone)是一种用于治疗失眠的药物,属于非苯二氮䓬类药物。
它可以帮助患者入睡并保持睡眠,通常用于短期治疗失眠问题。
佐匹克隆在一些国家被广泛使用,但也存在着一些潜在的风险和副作用。
在使用佐匹克隆之前,患者需要了解其用法、剂量和注意事项,以确保安全使用。
一、佐匹克隆的用途:佐匹克隆主要用于治疗短期的失眠问题,有助于改善入睡困难、夜间醒来或睡眠质量不佳的症状。
它是一种安眠药,通过作用于大脑中的GABA受体来产生镇静和催眠效果,从而帮助患者放松并入睡。
二、佐匹克隆的剂量和用法:1. 剂量:一般情况下,成人的起始剂量为每日3.75毫克至7.5毫克,可根据个体需要和医生建议适当调整。
老年人或肝功能受损患者,剂量需要相应减少。
2. 用法:佐匹克隆通常口服,建议于睡前30分钟内服用。
患者在服用佐匹克隆期间应尽量避免饮用酒精或同时服用其他镇静药物,以免增加镇静剂的效应。
三、使用佐匹克隆的注意事项:1. 不宜长期连续使用:佐匹克隆主要适用于短期治疗失眠问题,长期连续使用可能导致对药物的耐受性增加,或者出现依赖和戒断症状。
2. 不宜与其他药物同时使用:在使用佐匹克隆期间,患者应避免同时使用其他镇静药物、酒精或麻醉药物,以免增加镇静和催眠的效应,导致意外和危险。
3. 不适用于部分人群:对佐匹克隆过敏、严重呼吸衰竭、肌无力症、严重肝功能受损和严重睡眠呼吸暂停等疾病患者,一般不宜使用佐匹克隆。
四、佐匹克隆的潜在风险和副作用:使用佐匹克隆可能出现一些副作用,包括但不限于头痛、恶心、口干、肌肉疼痛、焦虑、失眠等。
在使用过程中,如果出现严重的过敏反应、呼吸困难、肌肉无力、精神异常等严重副作用,应立即停药并就医。
佐匹克隆是一种用于治疗短期失眠问题的药物,但患者在使用前应充分了解其用法、剂量和注意事项,避免滥用或不当使用,以确保安全有效地治疗失眠问题。
在使用佐匹克隆期间,患者应注意观察自身身体状况,及时就医处理任何不适或异常反应。
佐匹克隆的用法

佐匹克隆的用法
佐匹克隆是一种用于治疗失眠症的处方药物,其用法如下:
1. 适应症:主要用于短期治疗失眠,特别是对于入睡困难和/或夜间频繁醒来而无法继续睡眠的患者。
2. 剂量:
1)成人常用剂量为口服7.5mg,通常在临睡前服用。
2)老年人初始剂量应减半,即3.75mg,同样在临睡时服,并且仅在必要时才
增至7.5mg。
3. 注意事项:
1)不推荐长期连续使用佐匹克隆,因为它可能引起依赖性。
2)用药期间不可随意增减剂量,也不可突然停药,以免出现戒断症状或反弹性
失眠。
3)对于肝、肾功能不全者或呼吸功能受限(如呼吸暂停综合症)以及肌无力患
者,需谨慎使用并在医生指导下调整剂量。
4)使用佐匹克隆后可能会有思睡、口干、遗忘等不良反应,因此服药后不应驾
驶车辆或操作重型机械。
4. 禁忌症:
1)对佐匹克隆成分过敏的患者禁用。
2)在失代偿的呼吸功能不全、重症肌无力、重症睡眠呼吸暂停综合症患者中禁
止使用。
5. 药物相互作用:
避免与增强中枢抑制作用的其他药物同时使用,如神经肌肉阻滞剂、苯二氮卓类抗焦虑药和催眠药等。
6. 过量处理:
如果意外过量服用,可能出现深度睡眠甚至昏迷,需要立即就医,根据情况采取相应的解毒和支持疗法。
总之,在使用佐匹克隆之前,务必遵医嘱,了解详细的用药指导,并告知医生您的病史和正在使用的其他药物。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
佐匹克隆胶囊说明书
请仔细阅读说明书并在医师指导下使用
【药品名称】
通用名:佐匹克隆胶囊
英文名:Zopiclone Capsules
汉语拼音:Zuopikelong Jiaonang
【成份】佐匹克隆
化学名称:6-(5-氯吡啶-2-基)-7-[(4-甲基哌嗪-1-基)羰氧基]-5,6-二氢吡咯[3,4-b]吡嗪-5-酮。
其结构式为:
分子式:C17H17ClN6O3
分子量:388.81
【性状】本品为胶囊剂,内容物为白色或类白色颗粒或粉末。
【适应症】失眠症。
尤其适用于不能耐受次晨残余作用的患者。
【规格】7.5mg
【用法用量】口服,7.5mg,临睡时服。
老年人最初用量为 3.75mg,临睡时服,仅在必要时服用7.5mg。
【不良反应】与剂量及患者的敏感性有关。
偶见思睡、口干、肌无力、遗忘、醉态,易受刺激或精神混乱、易激惹,头疼、乏力。
长期服药后突然停药会出现戒断症状。
可能有较轻的激动、焦虑、肌痛、震颤、反跳性失眠及恶梦、恶心及呕吐,罕见较重的痉挛、肌肉颤抖、神志模糊。
【禁忌】禁用于对本品过敏者,失代偿的呼吸功能不全患者,重症肌无力、重症睡眠呼吸暂停综合症患者。
【注意事项】肌无力患者用药时需注意医疗监护,呼吸功能不全者和肝、肾功能不全者适当调整剂量。
使用本品时应绝对禁止摄入酒精饮料。
用药时间不宜过长,突然停药应小心监护,服药后不宜操作机械或驾车。
【孕妇及哺乳期妇女用药】孕期妇女慎用。
因本品在乳汁中浓度高,哺乳期妇女不宜应用。
【儿童用药】15岁以下儿童不宜使用本品。
【老年用药】老年人最初用量为3.75mg,临睡时服,必要时服用7.5mg。
【药物相互作用】与神经肌肉阻滞药(筒箭毒,肌松药)或其他中枢神经抑制药同服可增强中枢抑制作用,与苯二氮卓类抗焦虑药和催眠药同服,戒断综合症的出现可增加。
【药物过量】服用过量的药物可出现熟睡甚至昏迷,应对症治疗。
【药理毒理】本品为环咯酮类催眠药,本品作用于苯二氮卓受体,但结合方式不同于苯二氮卓类药物。
【药代动力学】口服吸收迅速,1.5~2.0小时后可达血药浓度峰值,血浆蛋白结合率均为45%,消除半衰期约5小时。
连续多次给药无蓄积作用。
本品在肝脏代谢,主要代谢产物为N-氧化物(有活性)和N-脱甲基物(无活性)。
代谢物主要经肺脏排出约占50%,其余由尿液排出。
4~5%以原药随尿排出。
老年人半衰期约7小时。
肝硬化患者因脱甲基作用减慢,血浆消除能力明显降低,应遵医嘱调整剂量。
【贮藏】遮光,密封,在凉暗处保存。
【有效期】24个月。
