ALK阳性非小细胞肺癌全程管理
非小细胞肺癌中EML4-ALK 融合基因的研究现状

非小细胞肺癌中EML4-ALK 融合基因的研究现状发表时间:2015-05-07T16:07:30.757Z 来源:《世界复合医学》2015年第4期供稿作者:魏琼英[导读] 该基因可以编码一个蛋白质,由于该蛋白的氨基酸组成特点,推测这一基因是由EML4 基因和ALK 基因融合而成。
魏琼英福建医科大学附属协和医院呼吸内科【摘要】肺癌已是全球发病率和死亡率最高的恶性肿瘤[1]。
其中NSCLC 约占85%。
针对其异常基因的靶向治疗是目前研究热点。
2007年发现的EML4-ALK 融合基因,是肺癌特异性较高的分子标记物,已开始引领新的临床靶向治疗。
本文就非小细胞肺癌中EML4-ALK 融合基因的研究现状进行综述。
【关键词】非小细胞肺癌;EML4-ALK 融合基因;靶向治疗【中图分类号】R817.5【文献标识码】A【文章编号】1276-7808(2015)-04-372-01一、EML4-ALK 融合基因的发现及结构特征2007 年Soda 等[2]从一名男性吸烟肺腺癌患者手术切除的标本中扩增出一个DNA 片段,该基因可以编码一个蛋白质,由于该蛋白的氨基酸组成特点,推测这一基因是由EML4 基因和ALK 基因融合而成。
进一步研究证实,这基因是由分别位于人类2 号染色体的p21 基因片段EML4 和p23 基因片段ALK 倒位融合重排而成,导致异常酪氨酸激酶表达。
2008 年Soda 建立了肺泡上皮细胞特异性表达EML4-ALK 融合蛋白的转基因小鼠模型[3],证实了EML4-ALK 融合基因在肺腺癌的发生中起到关键作用。
EML4 属于棘皮动物微管相关蛋白样蛋白质家族成员[4],ALK 为跨膜受体,均与细胞增殖、存活、迁移密切相关,其异常可导致肿瘤形成[5]。
目前已发现20 多种EML4-ALK 融合基因的变异体[6]。
Soda等[4]的体外转化实验表明,不同的变异体均具有恶性转化和致瘤能力,不同变异体其酪氨酸激酶活性程度有明显差异,猜测可能对临床用药剂量具有一定的指导价值,但还有待进一步的研究。
一线化疗方案

一线化疗方案简介化疗是指使用化学药物治疗癌症的方法。
一线化疗方案是指初次诊断为癌症的患者首次接受的化疗治疗方案。
一线化疗方案的选择对于患者的预后非常重要,不同类型的癌症和临床分期需要制定不同的一线化疗方案。
本文将重点介绍几种常见癌症的一线化疗方案。
乳腺癌一线化疗方案乳腺癌是女性最常见的恶性肿瘤之一,早期乳腺癌的一线化疗方案通常包括以下药物组合:•顺铂•帕妥珠单抗•卡培他滨这种三药联合方案被广泛应用于乳腺癌的一线化疗,能够有效降低肿瘤大小,并减少淋巴结转移的风险。
肺癌一线化疗方案肺癌是全球最常见的恶性肿瘤之一,其一线化疗方案通常根据患者的基因型和分期来选择。
常见的一线化疗方案包括:1.EGFR突变阳性:•非小细胞肺癌患者中的常见突变类型之一。
对于EGFR突变阳性的非小细胞肺癌患者,可以选择以下方案之一:–吉非替尼–奥希替尼–伊马替尼2.ALK融合阳性:•ALK融合阳性为另一个非小细胞肺癌的常见突变类型。
对于ALK融合阳性的非小细胞肺癌患者,可以选择以下方案之一:–克唑替尼–利妥昔单抗加克唑替尼3.PD-L1表达阳性:•近年来,免疫治疗成为肺癌一线化疗的重要选择。
对于PD-L1表达阳性的晚期非小细胞肺癌患者,可以选择以下方案之一:–雷珠单抗–佩姆布鲁珠单抗结肠癌一线化疗方案结肠癌是一种常见的消化系统肿瘤,常规剖腹手术是结肠癌的一线治疗方法,但术后患者仍然需要接受化疗。
结肠癌的一线化疗方案通常包括以下药物:•氟尿嘧啶•双氟尿嘧啶•亚叶酸钙•安托替尼这种联合化疗方案能够有效控制结肠癌的进展,降低复发和转移的风险。
总结一线化疗方案的选择对于癌症患者的治疗效果和预后至关重要。
不同类型的癌症需要制定不同的一线化疗方案。
针对乳腺癌、肺癌和结肠癌这几种常见癌症,本文详细介绍了它们的一线化疗方案。
未来,随着医学的进步和个体化治疗的发展,一线化疗方案将更加精确和个性化,为癌症患者带来更好的治疗效果和生存率。
1.ALK阳性非小细胞肺癌全程管理

首要研究终点ORR (研究者评估): • 46% vs. 55%
15.6 (11.1–19.4)
HR = 0.64 (0.45–0.91)
0 0 6 12 18 Time (months) 24 30 36
ALTA-1L研究(III期,ALK初治 vs 克唑替尼)进行中
Ahn, et al. WCLC 2017
Driver oncogenes
Sacher et al., JAMA oncol, 2, 313-20 (2016)
ALK抑制剂问世前,化疗方案效果不好 ALK+患者的OS较短
Response rate to SOC
Chemo (Pt doublet) EGFR-TKI ALK+ (n=15) 25% 0% EGFRmt (n=25) 50% 70% WT/WT (n=49) 35% 13%
RET INSR KDR ROS1 ABL EGFR EGFR2 HER2 IGF1R JAK1 KIT MET PDFGRβ RON SRC AKT1 AuroraA CDK1 CDK2 MEK1 PKA PKCα PKCβ1 PKCβ2 Raf-1
ALK
RET INSR KDR ROS1 ABL EGFR EGFR2 HER2 IGF1R JAK1 KIT MET PDFGRβ RON SRC AKT1 AuroraA CDK1 CDK2 MEK1 PKA PKCα PKCβ1 PKCβ2 Raf-1
10.9
8.1
Ceritinib (ASCEND-5)2
5.4
16.6
Median PFS (months)
塞瑞替尼为ALK+NSCLC提供了另一个一线治疗的可选方案 塞瑞替尼在与化疗的对比的临床研究中体现出了更长的PFS值
EGFR-TKI全程管理

合计
0.79 (0.48– 1.30)
0.94 (0.57– 1.54)
1.52 (0.91– 2.52)
1.04 (0.71– 1.51)
0.01 0.1 有利于厄洛替尼
1
10
有利于化疗
1.05 (0.60–1.84)
1.00 (0.56–1.79)
100
0.01 0.1
1
10 100
0.92 (0.55–1.54) 有利于厄洛替尼 有利于化疗
0.28
LUX-Lung 6
0.20
汇总
0.24
95% CI
外显子19Del
0.12–0.33 0.17–0.43 0.15–0.38 0.07–0.24 0.26–0.66 0.18–0.44 0.13–0.32 0.20–0.29
HR
95% CI
外显子21 L858R
0.54 0.32–0.91
厄洛替尼
吉非替尼*
阿法替尼 奥希替尼
含铂类化疗
8
* 对于仅含常见突变的患者,未报告PFS;EGFR,表皮生长因子受体;NSCLC,非小细胞肺癌;PFS,无进展生存期;TKI,酪氨酸激酶抑制剂。 Chen G, et al. Ann Oncol 2013;24:1615–22; Gefitinib Summary of Product Characteristics 2010; Han JY, et al. J Clin Oncol.2012;30:1122–8; Maemondo M, et al. N Engl J Med.2010;362:2380–8; Mok T, et al. N Engl J Med.2009; 361:947–57; Mitsudomi T, et al. Lancet Oncol.2010;11:121–8; Rosell R, et al. Lancet Oncol.2012;13:239–46; Sequist LV, et al. J Clin Oncol.2013;31:3327–34; Wu YL, et al. Lancet Oncol.2014;15:213–22; Wu YL, et al. Ann Onc.2015; Ann Oncol.2015; 26:1883-9; Zhou C, et al. Lancet Oncol.2011; 8:735–42.
肺癌常见突变基因EGFR与ALK的认知

肺癌常见突变基因EGFR与ALK的认知肺癌是我国发病率最高,也是我国死亡率最高的癌症,而幸运的是在我国大约有40-50%的肺癌具有敏感基因突变,最常见的是EGFR突变及ALK融合突变(欧美10%),可以应用靶向药物治疗,EGFR/ALK靶点的突变应用靶向药物有效率高达70%,明显提高患者生存质量,提高生存期。
有效率虽然很高但总有一个跨不过去的坎那就是耐药。
一、EGFR(表皮生长因子受体)突变EGFR大家已经非常熟悉了,是非小细胞肺癌最常见的致癌基因,是目前肺癌靶向药物对应的主要驱动基因,常见的突变位点发生在18、19、20和21号外显子上。
最常见的有两种,一种是19号外显子的缺失(45%),另外一种是21号外显子L858R(40-45%)的突变。
针对EGFR突变的肺癌患者,比如19外显子缺失和L858突变,常用的药物一代EGFR抑制剂厄洛替尼、吉非替尼、埃克替尼和二代EGFR抑制剂阿法替尼,三代奥西替尼(9291),这些药物对EGFR 突变的非小细胞肺癌患者不错的药物。
但多数病人在使用第一代靶向药物1-2年时间内,就会出现耐药,肿瘤进展。
其中原因有四:1,60%的患者是由于出现继发耐药突变——T790M突变,一旦T790M突变,可以使用三代靶向药物奧希替尼(9291);2,20%的患者耐药是因为旁路激活,比如c-MET扩增,也就是说肿瘤细胞的增殖绕开了EGFR,走了另外一条路。
如果基因检测显示MET扩增或突变,可以应用克唑替尼;3,表型改变也是一代靶向药物产生耐药的一种情况,比如腺癌会向小细胞肺癌转化;4,EGFR驱动基因的下游信号通路激活,也会导致的原发耐药或者获得性耐药。
这种情况就要考虑化疗。
奧希替尼作为一代靶向药耐药后的选择,仍然会产生耐药。
比如继发C797S的共生突变,其他旁路激活等。
在EGFR突变的患者中,除了常见的19/21基因突变外,还有3种罕见突变:G719X(18外显子)、S768I(20外显子)和L861Q (21外显子)。
ALK在非小细胞肺癌中的表达

E 6 ] S a l i d o M, P  ̄ u a n L, Ma r t i n e z — Av i l e s L e t a 1 . I n c r e a s e d AL K g e n e
( 1 . 吉林省肿瘤医院 , 吉林 长 春 1 3 0 0 1 2 ; 2 . 长春市妇产 医院)
间变 性淋 巴瘤 激 酶 ( AL K) 于 1 9 9 4年首 先 发 现
于 间变性 大 细胞淋 巴瘤 中 ; E ML 4 - AL K融 合基 因于 2 0 0 7年 首 次 发 现 , 是 新 兴 的 生 物 标 记 物 和 治 疗 靶 标 ] 。我们 检测 了 1 0 6 例 经手 术切 除 的非 小细 胞肺 癌组 织标 本 , 观 察 并 分 析 AL K 融 合 基 因 的表 达 情 况 以及 阳性病 例 的临 床病理 特 征 。 1 材料 与 e ma l A, S i e g e l R, Wa r d E, e t a 1 . C a n c e r s t a t i s t i c s , 2 0 0 8 E J 3 . C A
Ca n c e r J Cl i n, 2 0 08, 5 8: 71 .
AL K 探针 试剂 盒说 明操 作 , 荧 光显微 镜 下观察 。 1 . 3 . 4 结 果判 读 ①组 织学 诊 断标 准 : 参照 2 0 0 4版
世 界 卫生组 织 编写 的《 肺、 胸膜、 胸 腺 及 心 脏 肿瘤 病
理 学 和遗传 学 》 诊 断标 准 以及 国际肺 癌研 究学会 / 美
中国实验诊断学
2 0 1 5年 5月 第 1 9卷
第 5期
一
8 3 3 一
文章编号 : 1 0 0 7 —4 2 8 7 ( 2 0 1 5 ) 0 5 —0 8 3 3 —0 3
ALK新抗体D5F3在非小细胞肺癌患者中的临床应用价值

ALK新抗体D5F3在非小细胞肺癌患者中的临床应用价值发表时间:2016-03-01T14:43:45.590Z 来源:《中国综合临床》2015年12月供稿作者:林清华1吴联平1陈丽珠1谢强2刘加夫1林玉琼1[导读] 福建省福州市肺科医院 EML4-ALK基因是NSCLC的一个潜在治疗靶点,目前已有了ALK 抑制剂-克唑替尼。
林清华1吴联平1陈丽珠1谢强2刘加夫1林玉琼1黄小红1福建省福州市肺科医院1病理科;2肿瘤科福建福州350008基金项目:福州市卫生系统科技计划青年科研项目(2013-S-wq24) 作者简介:林清华,女,主治医师.【摘要】目的探究ALK新抗体D5F3在非小细胞肺癌患者中的临床应用价值.方法选择经RT-PCR检测的200例非小细胞肺癌患者的石蜡包埋标本(包括EML4-ALK突变型和野生型),应用ALK新型抗体D5F3(CST公司生产)检测该基因蛋白表达情况,分别从阴性、阳性(+~3+)进行对比其敏感性和特异性.结果检测结果显示,(+)符合率为15.78%,(++)符合率为23.07%,(+++)符合率为100%,差异有统计学意义(P<0.05).结论D5F3新抗体在NSCLC患者高度的敏感性及特异性,具有筛选价值,节约社会资源,为广大肺癌患者服务. 【关键词】D5F3; 非小细胞肺癌; 临床应用价值; EML4-ALK; EffectoftheantibodyD5F3innonsmallcelllungcancerpatients 【Abstract】objective:ToinvestigatetheeffectoftheantibodyD5F3innonsmallcelllungcancerpatient.Method200paraffinembeddedspecimensofpatientswithnonsmallcelllungcancertestedbyRT-PCR(includingEML4-ALKmutantandwildtype)wereselected.ThegeneproteinexpressionweretestedbyALKnewantibodyD5F3andthesensitivityandspecificitywerecomparedbynegativeandpositive(+~3+).ResultTestresultsshow (+)thecoincidencerateis15.78%,(++)thecoincidencerateis23.07%and(+++)complianceratewas100%,(P<0.05).Conclusion:D5F3newantibodyinNSCLCpatientswithahighdegre【eKoefysweonrsidtsiv】ityandspecificity,withthescreeningvalue,savesocialresources,forthemajorityoflungcancerpatients. D5F 3;nonsmallcelllungcancer;clinicalapplicationvalue;EML4-ALK; 【中图分类号】R734.2【文献标识码】B 【文章编号】1008-6315(2015)12-0056-02前言肺癌是全世界死亡率最高的恶性肿瘤,每年新增肺癌人群超过65万人, 其中80-85%为非小细胞肺癌(non-smallcelllungcancer,NSCLC)患者,治疗以铂类药物化疗为主,疗效不到15%[1].随着基因组学的不断进步发展,分子靶细胞已成为治疗非小细胞肺癌(non-smallcelllungcancer,NSCLC)的研究热点,其中棘皮动物微管相关蛋白样4-间变性淋巴瘤激酶(echinoderm miGcrotubule -associatedprotein-like4-anaplasticlymphomakinase,EML4-ALK)继EGFR肺癌基因之后发现的新的肺癌分子靶点,临床已寻找到显效的靶向药物应对[2-3].临床上筛选ALK 融合阳性的患者成为主要关注的问题[4],因此本研究入组经RT-PCR检测的200例非小细胞肺癌患者的石蜡包埋标本,应用ALK新型抗体D5F3(CST公司生产)检测该基因蛋白表达情况, 从阴性,阳性(+,2+,3+)等方面分析其敏感性和特异性,进而评估其临床应用价值.1资料与方法1.1临床资料收集2013年6月~ 2015年3月福州肺科医院病理科经RT-PCR检测的200例非小细胞肺癌(NSCLC)患者的石蜡标本,其中包括经支气管镜活检、CT 引导经皮肺穿刺、内科胸腔镜胸膜活检、闭式胸膜活检标、淋巴结活检、手术切除等标本.男性112例,女性88例,年龄为30~86岁,平均年龄(60.24±3.75)岁,两组患者的一般资料相仿,差异无统计学意义(P>0.05). 1.2纳入标准所有患者均符合以下条件:?非小细胞肺癌指南?[5]具有以下部分临床特点:胸部胀痛、痰血、低热、咳嗽等症状;晚期可出现疲乏、体重减轻、食欲下降以及呼吸困难等症状;X 线胸片显示肺内异常,组织活检病理确诊为非小细胞肺癌,并且经RT-PCR检测EML4-ALK 融合基因突变情况. 所有患者在检测前均未接受过放疗以及化疗治疗. 1.3方法1.3.1检测方法采用免疫组化法.首选优化实验条件,每批染色设阳性对照.将已知阳性片作为阳性对照,正常肺组织为阴性内对照.免疫组化染色采用EliVision两步法,一抗ALK(D5F3)XP兔单抗试剂购自美国CST 中国分公司,EliVision试剂盒购自福州迈新公司.ALK(D5F3)抗体按1:250稀释,新鲜配制DAB 显色剂显色.染色步骤按照试剂盒说明书进行操作(手工操作).1.3.2结果判读[6-8] 由两名有经验的诊断医生进行结果判读.判读标准:肿瘤细胞明确无着色为(—);>5%肿瘤细胞呈微弱或模糊的胞质着色为(+);>5%肿瘤细胞呈中等强度胞质着色为(2+);>5%肿瘤细胞呈颗粒状胞质强着色为(3+).排除已知染色因素,具体分为以下几种:(1)巨噬细胞内颜色较淡的胞浆点状染色;神经来源细胞,如神经细胞以及神经节细胞;(3)排除出现正常黏膜内以及坏死组织区域的背景染色. 1.4统计学分析计量数据以均数±标准差(X±s)表示,计 €€€数资料采用%表示,采用SPSS17.0统计软件进行单因素方差分析法,即one-wayANOVA 进行差异性统计,使用Turkey检验进行组间检验、校正.所有数据比较,P<0.05认为有统计学意义. 2结果2.1EML4-ALK 蛋白在NSCLC 组织中表达情况EML4-ALK 蛋白在NSCLC组织中阳性表达主要存在于细胞浆,表现为强的颗粒状胞浆染色.检测200例肺癌组织中腺癌151例,鳞癌40例,腺磷癌2例,大细胞癌1例,复合型大细胞癌1例,类癌2例,巨细胞癌1例,难分型非小细胞癌2例,46例阳性表达, 总阳性表达率为23%(图1、2).所有患者EML4-ALK蛋白表达与年龄、病理分期以及分化程度均无显著差异,差异无统计学意义(P>0.05)见表1.3讨论EML4-ALK基因是NSCLC的一个潜在治疗靶点,目前已有了ALK 抑制剂-克唑替尼[9,10],目前ALK常用的检测方法有荧光原位杂交(FISH)、聚合酶链反应法(PCR)以及免疫组织化学法(Immunohistochemistry,IHC).由于FISH 、PCR检测法的技术、设备、结果判读需求及条件较高,不易在我国得到广泛普及.本文通过对RT-PCR检测的200例非小细胞肺癌患者的石蜡包埋标本进行ALK新型抗体D5F3免疫组化检测的研究,发现IHC对NSCLC病例进行ALK筛查,就能对阴性及IHC3+病例做出初步判断,再对IHC1+以上病例进行分子病理验证,能够快速、准确对融合基因突变位点做出判断.从而证实了D5F3在NSCLC患者中有高度的敏感性及特异性,具有筛选价值,能节约社会资源,为广大肺癌患者服务.同时由于免疫组化表达的不确定性,其最终结果还需要以分子表达为依据.本研究我们还发现ALK突变在腺癌患者中的比例远高于鳞癌及其他类型的NSCLC患者,差异具有统计学意义(P<0.05). 以往在肺癌检测相应的驱动基因中通常认为EGFR与EML 4-ALK融合基因存在排斥性,不能同时出现其EGFR 与EML4-ALK 融合基因的同时突变[11].我们研究中发现了一例EGFR与ALK 同时突变的患者.同时王旭洲等[12]也报道了六例两者同时发生突变的病例.此种现象值得我们进一步的研究,以进一步为临床广大患者服务.参考文献[1] 徐风华,郭荣荣,李欣,等.非小细胞肺癌患者ERCC1表达与铂类药物化疗敏感性的相关性的系统分析[J].中国临床药理学与治疗学,2013,18 (1):45-50. [2] ZhuJ,CaiL,YangH,etal.Echinodermmicrotubule‐associatedproteinlike4‐anaplasticlymphomakinaserearrangementandepidermalgrowthfactor receptor mutation coexisting in Chinese patients with lung[ adenocarcinoma[J].ThoracicCancer,2014,5(5):411-416.3] 石远凯,孙燕.非小细胞肺癌分子靶向治疗的发展趋势[J].中华肿瘤杂志,[ 2014,36(7):481-484.4] 印永祥,时姗姗,马恒辉,等.非小细胞肺癌EML4-ALK 融合基因检测结果的初步分析[J].临床肿瘤学杂志,2013,9(3):003. [5] 屈若祎,周宝森.2004-2010年中国肺癌死亡分布及趋势分析[J].中国卫生统计,2014,31(6):932-935. [6] ZhangXC,LuS,ZhangL,etal.Anaplasticlymphomakinase (ALK)-positivenon-smallcelllungcancerexpertconsensus(2013edition)ofChina.ZhonghuaBingLiXueZaZhi,2013,6(42):402-406.[张旭超,陆舜,张力,等.中国间变性淋巴瘤激酶(ALK)阳性非小细胞肺癌诊断专家共识(2013版).中华病理学杂志,2013,6(42):402-406.][7] ZhangNN,LiuYT,MaL,etal.ThemoleculardetectionandclinicalsignifiGcanceofALKrearrangementinselectedadvancednon-smallcelllungcancer:ALKexpressionprovidesinsightsintoALKtargetedtherapy.PloSOne,2014,[ 9(1):e84501.8] ParkHS,LeeJK,Kim DW,etal.ImmunohistochemicalscreeningforanaGplasticlymphomakinase(ALK)rearrangementinadvancednon-smallcell[ lungcancerpatients.LungCancer,2012,77(2):288-292.9] TianHX,WuYL,ZhangXC,etal.NonsmallcelllungcancerEML4-ALKfusionmethodinthetumorsamplesdiversity.ZhonghuaJianYanYiXueZaZhi,2012,35(7):593-597.[田红霞,吴一龙,张绪超,等.非小细胞肺癌肿瘤样本中EML4-ALK融合方式的多样性.中华检验医学杂志,2012,35(7):593-597.][10] TakaH,SonobeMJ,KobayashiM,etal.ClinicopatholgicfeaturesofnonsGmall-celllungcancerwithEML4-ALKfusiongene.AnnSurgOncol,[ 2010,17(3):889-897.11] KatayamaR,KhanTM,BenesC,etal.Therapeuticstrategiestoovercomecrizotinibresistanceinnon-smallcelllungcancersharboringthefusiononcoGgeneEML4-ALK[J].ProceedingsoftheNationalAcademyofSciences,[ 2011,108(18):7535-7540.12] 王旭洲,陈炜生,余英豪.非小细胞肺癌患者EML4-ALK融合基因突变研究.中国肺癌杂志,2015,18(2):80-84.。
非小细胞肺癌有望迎来新疗法Lorbrena,死亡风险降72%

近日,美国FDA宣布接受第三代ALK抑制剂劳拉替尼(Lorlatinib,商品名Lorbrena)的补充新药申请(sNDA),用于一线治疗ALK阳性转移性非小细胞肺癌(NSCLC)患者。
同时,FDA还授予其优先审查资格,预计今年4月做出答复。
▌拥有“钻石靶点”的肺癌肺癌是全球癌症死亡的主要病因之一,每年有180万人确诊肺癌。
非小细胞肺癌(NSCLC)是最常见的肺癌类型,约占所有肺癌的85%。
其中,约3%~5%的NSCLC患者会出现间变性淋巴瘤激酶(ALK)重排。
ALK基因重排多发生在年轻患者和不吸烟或少量吸烟人群中,而且绝大多数表现为肺腺癌。
ALK突变可以说是肺癌中的“钻石突变”,其靶向药物的有效率远远高于EGFR突变的患者。
截至目前,针对ALK突变的靶向药物有五种。
第一代:克唑替尼;第二代:色瑞替尼、阿来替尼、布加替尼;第三代:劳拉替尼。
这些药物大大提高了这类肺癌患者人群的生存率。
▌第三代ALK抑制剂:劳拉替尼劳拉替尼是第三代ALK抑制剂,对目前已知的几乎所有ALK耐药突变均有效,具有较强的血脑屏障穿透能力,可用于治疗脑转移。
高达40%的ALK阳性肺癌患者出现脑转移。
2018年11月,劳拉替尼获批二线治疗ALK阳性转移性NSCLC患者。
作为全新一代的ALK抑制剂,劳拉替尼的优势在于可以针对不同类型的ALK+患者,有效率最高;而且,对于三种ALK抑制剂都耐药的患者,也有很好的疗效。
▌死亡风险降低72%!临床试验数据喜人这一sNDA是来自关键的3期CROWN临床试验的结果。
CROWN是一项全球、随机、3期临床试验,旨在评估劳拉替尼和第一代ALK抑制剂克唑替尼(Crizotinib)在ALK+ NSCLC患者中的疗效和安全性。
研究入组296例初治晚期ALK阳性NSCLC患者,按1:1的比例随机分配,其中约1/4的患者(26.4%)在基线时已经发生脑转移。
去年11月发表在《新英格兰医学杂志》的研究结果显示:1、与现有一线标准疗法克唑替尼相比,劳拉替尼将疾病进展或死亡风险降低72%。
EML4-ALK在非小细胞肺癌中的研究进展

tep st ert fE A- K b u % 一8 i ainswi o ・malcl ln a c r( CL .Mu il ML - K ains h oiv aeo MI AL i a o t i s 3 % np te t t n n s ]—el u gc n e NS C) h hpeE 4AL v r t a
E A-L o - l clln a cr MI A K i n ns l e gc n e n ma — lu
ZHAO ng rve n Mi e iwig,S ONG ng c ek n Yo h c i g
( eat et fR si t yDiae Me i lSho o N ni nvrt D p r n e r o s s , dc col m o par e a aj g U i sy/N ni e e l o i l N ni f n ei aj g Gnr s t aj g n a H pa o f n Mitr o a d P , aj g2 0 0 , i gu hn ) layC mm n , N ni 10 2 J n s ,C i i n a a
3种变 异体 占大多数 , 其他变异体相对较少。 目前 尚无标准 的 、 快速简便 的检 测 E 4A K的方 法 , ML -L 使其 临床应用推 广受到 限
制 , 待进一步研究 。新近 的临床研究发现 ,M 4 L 有 E L - K阳性率 和患者是 否吸烟高度 相关 , A 也与 年龄 、 腺癌 以及 表皮 生长 因子
BRAF V600E突变非小细胞肺癌患者全程管理的研究进展

BRAF V600E突变非小细胞肺癌患者全程管理的研究进展杨淋;王玉波
【期刊名称】《重庆医学》
【年(卷),期】2024(53)5
【摘要】肺癌是全球发病及死亡人数最多的恶性肿瘤,其中以非小细胞肺癌(NSCLC)占比最多。
鼠类肉瘤病毒癌基因同源物B1(BRAF)是一种原癌基因,与NSCLC的不良预后有关,其中以BRAF V600E突变为代表。
近年来,关于BRAF V600E突变NSCLC患者的诊治已成为肺癌精准诊疗研究的焦点,特别是其全程管理备受关注。
关于BRAF V600E突变患者的靶向、免疫及化疗等的相关研究层出不穷,该文就国内外相关的研究进展进行综述。
【总页数】5页(P782-786)
【作者】杨淋;王玉波
【作者单位】重庆大学附属江津医院呼吸与危重症医学科
【正文语种】中文
【中图分类】R734.2
【相关文献】
1.非小细胞肺癌阳性胸水 BRAF V600E基因突变的研究
2.BRAF基因V600E突变在恶性肿瘤中作用的研究进展
3.BRAF基因V600E突变与甲状腺乳头状癌关系的研究进展
4.非小细胞肺癌患者ALK、ROS1及BRAF⁃V600E基因突变与临床特征的相关性
5.BRAF V600E基因突变在结直肠癌治疗中的研究进展
因版权原因,仅展示原文概要,查看原文内容请购买。
ALK阳性晚期肺癌的靶向治疗
ALK阳性晚期肺癌的靶向治疗今天讲讲ALK阳性晚期肺癌的靶向治疗相关知识。
EML4-ALK融合基因是非小细胞肺癌的治疗靶点之一,它是通过细胞外配体结合区与胞内的酪氨酸激酶区结合,导致酪氨酸激酶激活并表达,从而获得致癌作用。
ALK融合基因阳性肺癌占非小细胞肺癌总体的3%~5%,对ALK 酪氨酸激酶抑制剂治疗有效。
克唑替尼作为第一个用于治疗晚期ALK重排肺癌的药物,耐受良好,与传统化疗相比受益更大,提高客观缓解率,且改善患者的生活质量。
二三代药物包括赛瑞替尼、艾乐替尼、布格替尼、恩莎替尼和劳拉替尼也取得可观的临床获益,与一代药物相比更具优势。
随之而来的问题是ALK酪氨酸激酶抑制剂的耐药,包括固有耐药和获得性耐药,目前最常见的耐药机制是ALK基因的二次突变,L1196M突变和G1202R突变分别是克唑替尼和二代ALK抑制剂最常见的耐药突变。
目前针对耐药发生后,首选的治疗措施是下一代ALK 抑制剂,其他还包括与热休克蛋白90抑制剂或培美曲塞的联合治疗。
ALK基因重排在NSCLC的总体发生率约为4%,常见于年轻、不吸烟/轻度吸烟、其他致癌基因驱动突变缺乏的肺腺癌(尤其是印戒细胞癌)。
目前ALK融合突变检测方法包括免疫组织化学(IHC)、荧光原位杂交(FISH)、RT-PCR、NGS。
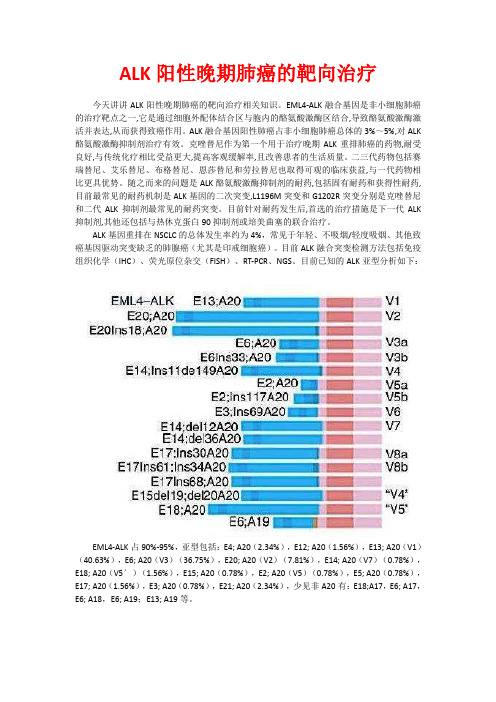
目前已知的ALK亚型分析如下:EML4-ALK占90%-95%,亚型包括:E4; A20(2.34%),E12; A20(1.56%),E13; A20(V1)(40.63%),E6; A20(V3)(36.75%),E20; A20(V2)(7.81%),E14; A20(V7)(0.78%),E18; A20(V5′)(1.56%),E15; A20(0.78%),E2; A20(V5)(0.78%),E5; A20(0.78%),E17; A20(1.56%),E3; A20(0.78%),E21; A20(2.34%),少见非A20有:E18;A17,E6; A17,E6; A18,E6; A19;E13; A19等。
非小细胞肺癌中ALK阳性病例临床病理特征分析

[文章编号] 1006-2440[2019]06-0568-04非小细胞肺癌中ALK阳性病例临床病理特征分析*王 勇1**,冯 佳2,钱 粒2,章建国2(1海门市中医院病理科,江苏226100;2南通大学附属医院病理科)[摘 要] 目的:旨在通过免疫组化Ventana法检测间变性淋巴瘤激酶(anaplastic lymphoma kinase,ALK)蛋白表达的情况,同时探讨阳性病例的临床病理学特征,以及免疫组化筛查ALK的诊断意义。
方法:运用免疫组化 Ventana 法对非小细胞肺癌(non-small cell lung cancer, NSCLC)患者726例标本,其中组织学标本591例,胸腔积液细胞学标本135例,分别进行ALK(D5F3)检测,并分析其表达情况。
结果:NSCLC标本726例中有26例ALK(D5F3)阳性,阳性率为3.58%,其中肺组织学标本的阳性率为3.21%(19/591),胸腔积液细胞学标本的阳性率为5.18%(7/135)。
ALK阳性患者组年龄明显小于阴性组(P<0.05)。
组织学类型上,ALK阳性患者中以实性型生长为主的低分化肺腺癌占比显著,而腺泡型为主与贴壁型为主的高中分化肺腺癌占比较少,且与阴性组对比差异具有统计学意义(P<0.05)。
结论:免疫组化Ventana法用于ALK(D5F3)蛋白检测是一种可行的筛查方法,可显著提高肺腺癌中ALK的阳性检出率。
相比年纪大患者,年轻患者ALK阳性率显著增高,组织学亚型以实性型患者的肺腺癌患者为主,且ALK阳性率显著高于其它在组织学亚型。
[关键词] 肺腺癌;间变性淋巴瘤激酶;免疫组化Ventana法[中图分类号] R734.2 [文献标志码] A [DOI] 10.19767/ki.32-1412.2019.06.008Analysis of clinicopathological features of ALK positive cases ofnon-small cell lung cancerWANG Yong1, FENG Jia2, QIAN Li2, ZHANG Jianguo2(1Department of Pathology, Haimen Traditional Medicine Hospital, Jiangsu 226100;2Department of Pathology,the Affiliated Hospital of Nantong University)[Abstract] Objective: To detect the expression of ALK (D5F3) protein by immunohistochemical Ventana method, and to explore the clinicopathological features of positive cases and the diagnostic significance of immunohistochemical screening for ALK. Methods: ALK (D5F3) was performed on 726 patients with NSCLC by using immunohistochemical Ventana method, including 591 histological specimens and 135 pleural effusion cytology specimens, and their expression was analyzed. Results: Of the 726 NSCLC specimens, 26 were positive for ALK (D5F3), with a positive rate of 3.58%. The positive rate of lung histology was 3.33% (19/591), and the positive rate of pleural effusion cytology was 5.19%. (7/135). The age of ALK positive patients was significantly lower than that of the negative group (P<0.05). Among the histological types, the proportion of poorly differentiated lung adenocarcinoma with solid growth was significant in the ALK-positive patients, while the high-centralized lung adenocarcinoma with predominantly acinar and adherent type accounted for less. What's more, the difference in the negative group was statistically significant (P<0.05). Conclusion: The immunohistochemical Ventana method is a feasible screening method for detecting ALK (D5F3) protein expression, which can significantly improve the positive detection rate of ALK in NSCLC.[Key words] pulmonary adenocarcinoma; anaplastic lymphoma kinase(ALK); IHC Ventana method* [基金项目] 南通市社会民生科技重点项目(MS22018001)。
(参考课件)ALK全程管理

赵琼教授在CSCO分享了ALK阳性NSCLC 患者长期生存的挑战
克唑替尼耐药后的治疗结局分析。治疗耐药后的治 疗策略包括以下3种:
继续克唑替尼或加局部治疗;换二代TKI;改为化 疗。赵教授对三种模型PFS分析,首次进展后, 使用化疗的PFS约为5.5-7.3个月。二代ALK抑制 剂约为6.9个月。而继续克唑替尼±局部治疗的 PFS为4.5个月,二次进展后,再使用二代ALK抑 制剂或化疗,可使患者预估的PFS达到20.9-22.7 个月,
目前针对ALK融合基因检测常用的方法主要有3种:荧光原位杂交 (FISH)、基于聚合酶链反应(PCR)扩增基础上的技术和针对融 合蛋白表达的免疫组织化学法(IHC)。2015年06月12日FDA批准 Ventana ALK(克隆号:D5F3)伴随诊断(Ventana IHC)检测可作为 ALK诊断的重要方法之一。
ceritinib的获得性耐药机制,目前认为包括基因突变、致癌旁路及药 代动力学逃逸等,这与克唑替尼和Alectinib获得性耐药机制较为相似。
13
Alectinib
Alectinib,二代ALK-TKI,可透过血脑屏障,拥有极好的的CNS渗透性,其对 ALK的抑制作用高于克唑替尼约5倍,且可抑制大多数克唑替尼耐药的ALK突 变。2016年JCO的II期,经一线TKI治疗的ALK重排患者应用Alectinib, ORR 为50%,中位缓解持续时间是11.2月。备受关注的是Alectinib在CNS作用: 在35例基线可测量的CNS转移灶患者中,CNS ORR为57%。在23例基线存 在CNS转移灶且前期未经放疗的患者中,10例(43%)达到了CNS CR。在 治疗的第12个月,33名患者(24.8%)CNS进展,43名患者(33.2%)非 CNS进展,随着时间的推移,非CNS较CNS更早出现进展发生率的升高,而 死亡累计发生率升高速度显著低于其他事件。提示Alectinib在治疗ALK基因重 排且对克唑替尼耐药的晚期非小细胞肺癌(包括存在脑转移)效果显著且耐 受性良好,有望为该类患者提供更优的治疗选择。
肺癌全程治疗之ALK阳性非小细胞肺癌

诊断标准
01
病理学诊断是确诊ALK阳性非小 细胞肺癌的金标准,需要找到 ALK基因融合或重排的证据。
02
根据病理学诊断结果,结合影像 学检查结果和临床表现,综合评 估病情,制定治疗方案。
03
ALK阳性非小细胞肺癌的治 疗
早期治疗
手术切除
对于早期ALK阳性非小细胞肺癌,手 术切除是首选的治疗方法,可以彻底 清除病灶,提高治愈率。
通过痰液细胞学检查、支气管镜 活检或肺穿刺等方法获取肺部组 织样本,进行病理学诊断,确定
肺癌类型和ALK基因状态。
诊断方法
01
02
03
痰液细胞学检查
通过收集痰液并检查其中 的癌细胞,初步判断是否 存在肺癌。
支气管镜活检
通过支气管镜进入肺部, 直接观察并采集肺部组织 样本进行病理学诊断。
肺穿刺
通过CT引导下对肺部肿块 进行穿刺,获取组织样本 进行病理学诊断。
营养支持
为患者提供个性化的营养支持方案,保证其营养 需求得到满足。
预防与生活方式调整
戒烟限酒
01
劝诫患者戒烟、限制饮酒,以降低肺癌复发的风险。
健康饮食
02
建议患者保持均衡的饮食结构,增加蔬菜、水果等富含维生素
和纤维素的食物摄入。
适量运动
03
鼓励患者进行适量的有氧运动,如散步、慢跑等,以增强体质
和免疫力。
监测肿瘤标志物
通过监测血液中的肿瘤标志物水平,如CEA、CA19-9等,有助于 早期发现肿瘤进展或复发。
密切观察症状
关注患者是否有咳嗽、胸痛、呼吸困难等症状,一旦出现异常应及 时就医。
心理支持与康复指导
心理疏导
对患者进行心理疏导,缓解其焦虑、抑郁等情绪, 提高治疗依从性和生活质量。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
PFS probability % of patients with AEs
Efficacy
1.0
Crizotinib(n=172)
0.8
Chemotherapy(n=171)
HR=0.45(95%CI: 0.35–0.60)
0.6
p<0.000
5
10
15
20
25
30
35
crizotinib
2014 (Jul)
Alectinib
approved in Japan
2016 (Sep)
FDA granted
Alecensa 2nd BTD for 1L
ALK+ NSCLC
2017 (Apr)
Brigatinib FDA Accelarate
approval for ALKpositive NSCLC
Ceritinib
Alectinib
ASCEND 4
ALEX、J-ALEX
Ceritinib优于化疗
Alectinib优于克唑替尼
2018年6月1日(二线) 2018年8月17日(全线)
首个在头对头III期研究中 证实优于另一种TKI药物
的靶向治疗药物
PROFILE 1014:克唑替尼与化疗的比较
KIT MET
PDFGRβ RON SRC AKT1
AuroraA CDK1 CDK2 MEK1 PKA PKCα PKCβ1 PKCβ2 Raf-1
10
1
ROS1
MET
Ceritinib
IC50 (nM) 10,000
ALK RET
INSR KDR ROS1 ABL EGFR EGFR2 HER2 IGF1R JAK1
MET PDFGRβ
RON SRC AKT1 AKT2 AKT3 AuroraA CDK1 CDK2 MEK1 PKA PKCα PKCβ1 PKCβ2 Raf-1
100
10
1
高效选择性ALK抑制剂
III期研究 CFDA适应症
Crizotinib
PROFILE 1014、1029 克唑替尼优于化疗 2013年1月(全线)
ALK+非小细胞肺癌患者全程管理
ALK阳性晚期NSCLC
Kinasedomain
Sodaet al., Nature, 448,561-6(2007)
Driver oncogenes
ALK抑制剂问世前,传统治疗方案 ALK+患者的OS较短
Geneticalterationsin NSCLC
(DFCI: 2002~2014)
2007
EML4–ALK fusion
discovered in NSCLC
2011 (Aug)
Crizotinib, approved
for advanced
ALK+ NSCLC
2013 (Jun)
FDA granted Alectinib BTD
for ALK+ NSCLC patients
whohave progressedon
EGFR
Others
KRAS
ALK(5% ROS1 )
BRAF V600E
Sacheret al., JAMA oncol, 2, 313-20(2016)
Leeet al. Cancer,118, 3579-86(2012)
JAMA. 2014May 21;311(19):1998-2006.
ALK通路药物发展是精准医学的完美演绎
2018 (Jun)
Ceritinib
CFDA 2Lapproval
2018 (Nov)
Lorlatinib FDA 2L approval
1.Dearden, et al. Ann Oncol2013; 2. Gridelli, et al. CancerTreat Rev 2014 3. Hallberg, et al. Nat Rev Cancer2013; 4. Rikova, et al. Cell 2007; 5. Soda, et al. Nature 2007; 6. American Cancer Society 2013 7. Torre, et al. CA CancerJ Clin 2015; 8. Perez, et al. Lung Cancer; 9/Lancet. 2016 ;388(10048):1012-24.
progressingon/or intolerantto crizitinib
2018 (Aug)
Alectinib CFDA
approval
Crizotinib Alectinib
Certinib Brigatinib
2013 (Jan)
Crizotinib ,
approved in China
to crizitinib
2017 (Feb)
Alectinib approved
in EU (Crizotinib
failure)
2017 (May)
Ceritinib FDA 1L approval
2017 (Nov)
AlectinibFDA 1Lapproval
(Dec)
AlectinibEMA 1Lapproval
Time (months)
Safety
100
80
60
KIT MET
1,000
PDFGRβ RON SRC AKT1
AuroraA CDK1 CDK2 MEK1 PKA PKCα PKCβ1 PKCβ2 Raf-1
100
10
1
ROS1 IGF1R
Alectinib
IC50 (nM) 10,000 1,000
ALK RET
INSR KDR ROS1 ABL EGFR FGFR2 HER2 IGF1R JAK1 KIT
2014 (Apr)
Certinib FDA
approved for ALKpositive, crizotinib resistant NSCLC
2015
(Dec)
AlectinibFDA approval for
ALK-positive NSCLC
progressing on/or intolerant
1
ALK+患者一线治疗的优化
2
一线耐药后的治疗现状
3
ALK-TKI的耐药机制及后续治疗
指南推荐的一线治疗药物(NCCN 2018 v3)
细胞信号激酶
Crizotinib
IC50 (nM) 10,000 1,000 100
ALK RET
INSR KDR ROS1 ABL EGFR EGFR2 HER2 IGF1R JAK1
