逆转录试剂盒
天根 一步法逆转录PCR试剂盒说明书

通用型一步法RT-PCR试剂盒说明书前言本试剂盒适用于对各种动、植物、病毒RNA进行PCR 检测。
用户可根据被分析基因,配合一对引物,或同时采用Taqman 荧光探针,先将提取的RNA反转录(reverse transcription).成cDNA,再经过聚合酶链式反应(Polymerase Chain Reaction)技术对特异性片段进行扩增,进行电泳或实时荧光分析。
一步法RT-PCR可使RT及PCR过程在单管中完成,比分开进行RT及PCR更方便。
由于不需要在RT完成后打开反应管,尽可能地避免了样品间的相互污染。
规格20人份适用仪器适用于各种普通PCR仪器和实时荧光PCR仪器。
试剂盒组成试剂准备根据下表配制反应液:振荡混匀后,按每管 40 μl(可根据需要调整)分装,备用。
PCR扩增将均一化后的RNA提取样本10 μl加入各反应管,混匀、离心后,置普通PCR仪器或实时荧光PCR仪器上进行扩增及实时检测。
结果判断扩增产物可直接进行电泳检测;实时荧光检测可根据相应仪器的配套软件进行结果分析。
保存及有效期-20℃保存,有效期为6个月。
注意事项1. 开始检测前请仔细阅读本说明书全文。
2. 整个检测过程中,反应体系的配制、样本处理及加样、PCR扩增(荧光检测)应分区进行以避免污染。
3. 操作人员应戴口罩,经常更换一次性手套,以避免RNA酶的污染;实验中所用器具均应经过除RNA酶处理。
4. 试剂盒组成中的试剂使用前应充分融化并混匀。
5. 进行实时荧光分析时,应使用透光性能较好的一次性薄壁离心管;.荧光探针应避光保存,加入缓冲液中后,应尽快进行扩增。
6. 注意适当处理检测中遗留的样品、扩增产物及可能被污染的试剂。
生产企业:上海蓝创生物科技发展有限公司。
逆转录试剂盒( transcriptor first strand cdna synthesis kit ) 说明书

3
04 379 012 001
Transcriptor First Strand cDNA Synthesis Kit y Version 6.0
1. What this Product Does, continued
Vial/ Cap
Label
Store the kit at ؊15 to ؊25°C N Store Control RNA (vial 7 in
Cat. No 04 379 012 001) at —70°C or below.
y Version 6.0
Content version: September 2010
9 (7 for b,c) colorless
Water, PCR-grade
Content
a) Cat. No. 04 379 012 001 b) Cat. No. 04 896 866 001 c) Cat. No. 04 897 030 001
a) 1 vial, 1 ml b) 2 vials, each 1ml c) 3 vials, each 1 ml
L In Cat. No. 04 896 866 001 and Cat. No. 04 897 030 001 the control reagents (vial 7 and 8) are not included. Therefore, in these kits vial 7 is Water, PCR Grade.
Random Hexamer Primer
a) 1 vial, 100 l (600 M) b) 1 vial, 200 l (600 M) c) 2 vials, each 200 l (600 M)
BioRT逆转录扩增(RT-PCR)试剂盒说明书(一步法)

b、按以下条件进行PCR反应94℃3m i n94℃30s25-35循环***37℃-65℃**30s72℃****45s-7min72℃5min注:*RT产物可增加至5μl 。
**退火温度根据引物Tm值调整,一般为Tm-5℃。
Control引物退火温度为55℃。
*** 当实验样品RNA特别稀少时,可将循环数增加至40-45循环。
**** 延伸时间根据PCR产物大小确定,一般1kb/min 。
Control引物延伸时间45s。
c、反应结束后,取3-5 μl PCR产物进行琼脂糖凝胶电泳,确认PCR反应产物。
如果此PCR产物需用于以后实验,应将PCR产物冷冻保存。
RNA control反应时,退火温度55度,30个循环,扩增片段大小为500bp。
使用提示1. RNA模板可以采用总RNA或mRNA,建议使用Biozol(BSC51M1)制备高质量RNA;2. RT实验应避免RNase污染,可采用以下措施:1)因人的皮肤表面和唾液都有RNase,因此实验中应戴一次性手套和口罩;2) RT实验应使用专门的仪器和耗材,建议在专门区域操作RNA;3) RT实验相关耗材应使用干热灭菌(180℃,60分钟)或用0.1% DEPC(焦碳酸二乙酯)水溶液在37℃处理12小时后在121℃高压灭菌30分钟;3. AMV逆转录酶,DNA聚合酶和RNase抑制剂在取用之前应离心后再吸取,吸取时动作要慢,使用后应尽快放回-20℃;4. dNTP应避免反复冻融以免失效;5. 引物的选择可根据具体情况,Oligo-dT适用于具有PolyA尾的RNA(一般是真核生物的mRNA),Random Hexamer Primer适用于所有RNA(包括mRNA,rRNA,tRNA等),尤其适用于有复杂二级结构的RNA,特异性引物适用于已知模板序列的RNA;6. PCR反应中MgCl2浓度可依据不同条件进行调整,当目的片段长度大于2kb时,我们建议增加MgCl2浓度,以0.5mM间隔梯度增加。
东洋纺反转录试剂盒

本产品为科研用试剂。请勿作为诊断、临床试剂使用。此外,对于本产品的 有害性调查还不十分全面,因此,在使用过程中,请严格遵守实验室作业的一般 注意事项,适当使用保护用品,安全操作。
组分中的5×RT Buffer在溶解时,可能会出现白色沉淀现象,但不影响其品 质。此时,请使用振荡器等仪器使其混合均匀,等完全溶解后再使用。
G3PDH mRNA
Primer F 559
intron
Size(bases)
1634
1237
1110 Primer R
90 129 90 92 k-193 104
图 2. Control Primer F,R 的 location
[Positive Control RNA]
本 试 剂 盒 作 为 Positive Control 用 RNA , 添 附 有 Human G3PDH (Glyceraldehyde 3-Phosphate Dehydrogenase)遗传基因的 in vitro 转录产物。 (在 3’末端添加了 22mer 的 Poly(A)tail)(请参照图 3)。
G3PDH 基因是在各种哺乳类组织上表达的“管家基因”。其 mRNA 表达水 平不受一部分细胞因子和含有 Tumor-promoting Phorbol Esters 等的诱导物质 的影响,并且,在几乎所有组织上都是恒定的。因此,使用从各种组织中抽提出 来的 RNA 样品的时候,可以作为最合适的对照而使用。
cDNA
5’
Synthesis of the Second Strand cDN A
DN A Polym erase
Am plification
PCR
图1. RT-PCR法的原理
A3500 逆转录标准操作规程
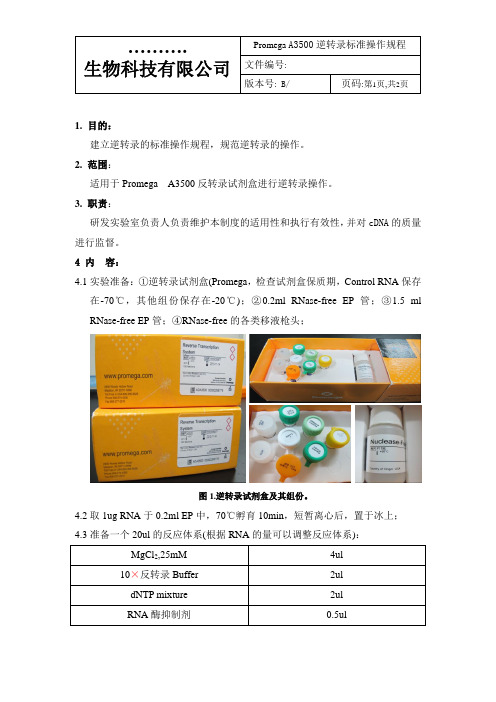
版本号: B/页码:第1页,共2页1. 目的:建立逆转录的标准操作规程,规范逆转录的操作。
2. 范围:适用于Promega A3500反转录试剂盒进行逆转录操作。
3. 职责:研发实验室负责人负责维护本制度的适用性和执行有效性,并对cDNA的质量进行监督。
4 内容:4.1实验准备:①逆转录试剂盒(Promega,检查试剂盒保质期,Control RNA保存在-70℃,其他组份保存在-20℃);②0.2ml RNase-free EP管;③1.5 ml RNase-free EP管;④RNase-free的各类移液枪头;图1.逆转录试剂盒及其组份。
4.2取1ug RNA于0.2ml EP中,70℃孵育10min,短暂离心后,置于冰上;4.3准备一个20ul的反应体系(根据RNA的量可以调整反应体系):MgCl2,25mM 4ul10×反转录Buffer 2uldNTP mixture 2ulRNA酶抑制剂0.5ul版本号: B/页码:第2页,共2页AMV反转录酶15UOligo(dT)15引物or随机引物0.5ugRNA 1ugNuclease-free water Up to 20ul4.4使用Oligo(dT)15引物时,42℃孵育15min。
当使用随机引物时,室温放置10min,然后转入42℃孵育15min(额外的室温孵育是为了随机引物的延伸,从而温度上升至42℃时引物与RNA保持结合状态);4.5 95℃,5min,然后转入0-5℃放置5min。
这一过程是为了阻止AMV反转录酶结合到合成的cDNA上并使其失活。
合成的cDNA可以直接用于第二条链的合成或者琼脂糖凝胶电泳分析,参照“琼脂糖凝胶电泳标准操作规程”;图1.逆转录反应程序设置。
4.6 在管壁和管盖上再次标清样本唯一性编号,并用透明胶带缠绕保护;4.7 将cDNA保存在冻存在-20℃;4.8 及时做好纸质和电子文档记录。
编制: 审核: 批准:日期: 日期: 日期:。
miRNA加尾法逆转录试剂盒特点

miRNA加尾法逆转录试剂盒特点
BIOG miRNA加尾法逆转录试剂盒采用基因工程改造的Poly A聚合酶,可为miRNA高效添加Poly A尾,以便于后续的RT反应。逆转录引物为特别设计的含有Oligo d(T)的引物,高度亲和含有Poly A尾的miRNA,使RT反应能够高效进行。BIOG miRNA加尾法逆转录试剂盒的RT效率优于多家同类产品,特别地,与百代生物BIOG miRNA加尾染料法荧光定量PCR试剂配套使用,可免费获得配套的qPCR下游引物和更加理想的实验结果。
3750
5
40T,货号 31029600Tຫໍສະໝຸດ 2个货号 410584620
6
40T,货号 31029
800T,2个货号 41044
5380
产品特点
1.基因工程优化的Poly A聚合酶,miRNA Poly A聚合高效快速;
2.特别设计的含Oligo d(T)的引物,能够与miRNA Poly A+高效结合,RT效率高于多家同类试剂;
3.同一RNA一次RT可获得多个miRNA的RT产物,后续可进行多个miRNA的检测,节省样本用量;
4.可配套BIOG miRNA加尾染料法荧光定量PCR试剂使用,获得优化的实验结果并可免费获得配套的下游引物。
配套qPCR
序号
加尾法逆转录试剂
qPCR试剂(用量推荐)
逆转录+PCR
目录价(元)
1
20T,货号 31028
200T,货号 41043
1960
cDNA反转录试剂盒
1
将试剂盒组分在冰上解冻
2
下列表格,计算各组分的的体积来准备反应体的数量。
注意:准备RT混合液必须在冰上
成分
试剂盒反应体积(每微升)
含有RNase抑制剂
不含RNase抑制剂
10X RT Buffer
2.0
2.0
25X dNTP Mix(mM)
0.8
0.8
10X RT Random Primers
2.0
2.0
MultiScribeTMReverse Transcriptase(反转录酶)
1.0
1.0
RNase inhibitor(RNA酶抑制剂)
1.0
--
Nuclease-free H2O
3.2
4.2
总量
10.0
10.0
3
将2X RT混合液放在冰上,并轻轻混匀
1、2X RT混合液的准备(每20微升反应)
注意:这些程序对于使用高容量cDNA反转录试剂盒已经优化
Step1
Step2
Step3
ep4
温度
25℃
37℃
85℃
4℃
时间
10min
120min
5min
∞
2、cDNA反转录反应
1、吸取10微升的2X RT混合液到96孔板的每个孔,或者单个的一个试管
2、吸取10微升的RNA样液加到每个孔中,用吸管上下吸两次混匀。
3、密封平板或试管
4、短暂离心平板或试管,沉淀物质,消除气泡
5、将平板或试管放于冰上,直到你准备好负载人循环仪
3、设计热循环仪程序
1、
热循环仪的的使用程序如下
invitrog逆转录试剂盒说明书

ThermoScript ™ RT-PCR SystemCatalog nos. (25 reactions): Catalog nos. (100 reactions): 11146-02411146-01611146-057 (w/ Platinum ® Taq DNA polymerase) 11146-032 (w/ Platinum ® Taq DNA polymerase)11146-040 (w/ Platinum ® Taq DNA polymerase High Fidelity)Store at -20°C (stability can be extended by storing at -70°C)DescriptionThe ThermoScript ™ RT-PCR System is designed for the sensitive and reproducible detection and analysis of RNA molecules in a two-step process. ThermoScript ™ RT, an avian reverse transcriptase with reduced RNase H activity, is engineered to have higher thermal stability,produce higher yields of cDNA, and produce more full-length cDNA transcripts than AMV RT. cDNA synthesis is performed in the first step using either total RNA or poly(A)+-selected RNA primed with oligo(dT), random primers or a gene-specific primer, at 50-65°C. In the second step, PCR is performed in a separate tube using primers specific for the gene of interest. RNA targets from 100 bp to >12 kb can be detected with this system, using 10 pg to 5 Yg of total RNA. PCR is carried out with Platinum ® Taq DNA Polymerase or Platinum ® Taq DNA Polymerase High Fidelity. Platinum ® Taq DNA Polymerase High Fidelity is suitable for templates from 100 bp to >12 kb. Platinum ® Taq DNA polymerase (1) provides automatic hot-start conditions for increased specificity up to 3 kb.Reagents are provided for 25 or 100 cDNA synthesis reactions of 20 μl each and 25 or 100 amplification reactions of 50 μl each.Component 25 rxn kit 100 rxn kit ThermoScript ™ RT (15 U/Yl) 25 μl 100 μl 5X cDNA Synthesis Buffer* 500 μl 500 μl 0.1 M DTT 250 μl 250 μl 10 mM dNTP Mix 100 μl 2 × 250 μlRNaseOUT ™ (40 U/Yl) 25 μl 100 μlOligo (dT)20 (50 YM) 25 μl 100 μlRandom Hexamers (50 ng/Yl) 50 μl 250 μlDEPC-Treated Water 1.25 ml 1.25 mlE . coli RNase H (2 U/Yl) 50 μl 2 × 50 μl*250 mM Tris acetate (pH 8.4), 375 mM potassium acetate, 40 mMmagnesium acetate, stabilizerCatalog numbers 11146-057 (25 rxns) and 11146-032 (100 rxns) include the following, in addition to the components to the left:Component 25 rxn kit 100 rxn kitPlatinum ® Taq DNA polymerase (5 U/Yl) 100 units 250 units10X PCR buffer Minus Mg 1.0 ml 1.0 ml 50 mM MgCl 2 1.0 ml 1.0 ml Catalog number 11146-040 (100 rxns) includes the following, in addition to the components to the left: Component 100 rxn kit Platinum ®Taq DNA Polymerase High Fidelity (5 U/μl) 100 units 10X High Fidelity PCR Buffer 1.0 ml 50 mM MgSO 4 1.0 ml Quality ControlThe Certificate of Analysis (CofA) provides detailed quality control information for each product. The CofA is available on our website at/cofa, and is searchable by product lot number, which is printed on each box .Summary of ProcedurePart no. 11146.pps MAN0000941 Rev. date: 11 Jun 201020 GSPr andom hexame r C, 30-60 min 50-65°C, 30-60 min 25°C, 10 min ↓ 50-60°C, 20-50 min↓85°C, 5 min↓ Add 1 μl RNase H 37°C, 20 min ↓ Remove 2 μl aliquot for PCRWater 1 *If less than 1 ng of RNA is used, reduce the amount of ThermoScript RT in the reaction to 0.5 μl. For technical support, email tech_support@. For country-specific contact information, visit .Page 2 of 4 Important Parameters to ConsiderRNA•High quality intact RNA is essential for successful full-length cDNA synthesis and successful long RT-PCR.•RNA should be devoid of any RNase contamination and aseptic conditions should be maintained. •Recommended methods of total RNA isolation include the Micro-to-Midi Total RNA PurificationSystem (Catalog no. 12183-018) and TRIzol® Reagent(Catalog no. 15596-026) (2, 3). Oligo(dT)-selection forpoly(A)+ RNA is typically not necessary, althoughincorporating this step may improve the yield ofspecific cDNAs.cDNA Synthesis Primers•Oligo(dT)20 (50 pmoles/reaction) is recommended for priming polyadenylated RNA. Use of Oligo(dT)20allows the detection of multiple transcripts from asingle first-strand reaction.•Random hexamers (50-250 ng/reaction) are efficient primers for the detection of multiple short RT-PCRtargets. Use of more than 50-100 ng primer/Yg ofRNA can increase the yield of short products but may inhibit detection of long targets (>3kb) or raretranscripts. If random hexamers are used, the first-strand reaction must be incubated at 25°C for 10 minto extend the primers prior to increasing the reactiontemperature for synthesis.•Gene-specific primers (GSP) should be used at 10 to20 pmol/reaction. Specificity of priming may beimproved by optimizing annealing/reactiontemperature.•Treatment of cDNA with RNase H to remove the complementary RNA prior to PCR is optional. RNaseH digestion will improve the RT-PCR signal of manytargets and is required for the efficient and consistentamplification of long RT-PCR templates.cDNA Synthesis Reaction•Denaturation of the RNA template and primer by incubating at 65°C for 5 min is optional. Most targetscan be reverse transcribed efficiently without this step.However, heating the RNA in the absence of reactionbuffer and enzyme prior to cDNA synthesis canremove secondary structure that may impede full-length cDNA synthesis.•ThermoScript™ RT can be used at 50-65°C. We recommend incubation at 50-55°C for most RT-PCRtargets. However, incubation at 50-60°C for oligo(dT)and 50-65°C for gene-specific primers can beemployed to reduce secondary structure or to improve specificity.•Most targets can be amplified after only a 30-min incubation for the first-strand reaction. Rare RNAs,long transcripts, or targets at the 5′ end of longtranscripts benefit from longer incubation times (50-60 min).PCR Primers• A final primer concentration of 0.2 - 0.4 μM for each primer is generally optimal; however, a primertitration is recommended for best results.•Design primers that anneal to sequence in exons on both sides of an intron or exon/exon boundary of the mRNA to allow differentiation between amplification of cDNA and potential contaminating genomic DNA. •Primers should not be self-complementary or complementary to each other at the 3′ ends.PCR Reactions•Most targets will be efficiently amplified using 2 Yl or less of the cDNA synthesis reaction.•The optimum magnesium concentration varies from1.5 to 3 mM. Generally, 1.82 mM magnesium chloridefor Platinum® Taq DNA polymerase and 2.32 mMmagnesium sulfate for Platinum® Taq DNAPolymerase High Fidelity is effective for most primer sets. However, titration of the magnesiumconcentration is recommended for the best result.Each Yl of the cDNA synthesis reaction adds 0.16 mM to the final magnesium concentration in a 50-μl PCRreaction.•Assemble the PCR reactions on ice, transfer them to a pre-heated thermal cycler (85-95°C) and immediately start the PCR amplification program.•The annealing temperature should be 10°C below the melting temperature of the primers used.•The optimum extension temperature for Platinum®Taq DNA Polymerase High Fidelity is 68°C. Theextension time varies with the size of the amplicon(approximately 1 min per 1 kb of amplicon).Page 3 of 4cDNA Synthesis1. In a 0.2- or 0.5-ml tube, combine primer (50 μM Oligo(dT)20,50 ng/μl random primer or 10 μM gene-specific primer), RNA, and dNTP mix and adjust volume to 12 Yl withDEPC-treated water.Component AmountPrimer 1 YlRNA (10 pg -5 Yg) x Yl10 mM dNTP Mix 2 YlDEPC-treated water to 12 Yl2. Denature RNA and primer by incubating at 65°C for 5 minand then place on ice (optional).3. Vortex the 5X cDNA Synthesis Buffer for 5 s just prior touse.4. Prepare a master reaction mix on ice and vortex gently.Component 1 Reaction 10 Reactions5x cDNA Synthesis Buffer 4 Yl 40 Yl0.1 M DTT 1 Yl 10 YlRNaseOUT™ (40 U/Yl) 1 Yl 10 YlDEPC-treated water 1 Yl 10 YlThermoScript™ RT (15 units/Yl)1 Yl* 10 Yl**NOTE: If less than 1 ng of template RNA is used, reduce the amount of ThermoScript™ RT in the reaction to 0.5 μl/reaction(5 μl/10 reactions). Increase the amount of DEPC-treatedwater in the master reaction mix to 1.5 μl/reaction (15 μl/10 reactions).5. Pipet 8 Yl of master reaction mix into each reaction tube on ice.6. Transfer the sample to a thermal cycler preheated to the appropriate cDNA synthesis temperature and incubate as follows.Oligo(dT)20 primed: 30-60 min at 50°C (or 50-60°C)Gene-specific primed 30-60 min at 50°C (or 50-65°C) Random-hexamer primed: 25°C for 10 min, followed by 20-50 min at 50°C (or 50-65°C)7. Terminate the reaction by incubating at 85°C for 5 min.8. Add 1 Yl of RNase H and incubate at 37°C for 20 min (optional).9. cDNA synthesis reactions can be stored at -20°C or used for PCR immediately.PCR with Platinum®Taq DNA Polymerase High FidelityUse only 10% of the cDNA synthesis reaction (2 Yl) for PCR.Use of 2 μl of 50 mM MgSO4 and 2 Yl of cDNA (0.32 mM magnesium in a 50-Yl PCR) results in a final concentration of2.32 mM magnesium, which is effective for most primer sets. However, titration of the magnesium concentration with the provided 50 mM MgSO4 is recommended for best results.1. Add the following to a 0.2- or 0.5-ml, thin-walled, PCR tube: Component 1 Reaction 10 Reactions10X High Fidelity PCR Buffer 5 Yl 50 Yl50 mM MgSO4 2μl 20μl10 mM dNTP Mix 1 Yl 10 Yl10 YM sense primer 1 Yl 10 Yl10 YM antisense primer 1 Yl 10 YlPlatinum®Taq High Fidelity 0.2 Yl 2 YlcDNA (from cDNA synthesis reaction) 2 Yl 20 YlDEPC-treated water 37.8 Yl 378 YlFinal volume 50 Yl 500 μl 2. Mix gently and overlay with silicone oil or mineral oil if thethermal cycler lacks a heated lid.3. Incubate at 94°C for 2 min, then perform 20 to 40 cycles ofPCR with optimized conditions for your sample (1 min/kb extension time at 68°C).4. Analyze 10 Yl of the amplified sample by agarose gelelectrophoresis.PCR with Platinum® Taq DNA PolymeraseUse only 10% of the cDNA synthesis reaction (2 Yl) for PCR. Use of 50 mM MgCl2 and 2 Yl of cDNA will result in a final magnesium concentration of 1.82 mM, which is adequate for most primers and targets. However, titration of magnesium concentration is recommended for best results.1. Add the following to a 0.2- or 0.5-ml, thin-walled, PCR tube: Component 1 Reaction 10 Reactions 10X PCR Buffer Minus Mg 5 Yl 50 Yl50 mM MgCl2 1.5 Yl 15 Yl10 mM dNTP Mix 1 Yl 10 Yl10 YM sense primer 1 Yl 10 Yl10 YM antisense primer 1 Yl 10 YlPlatinum®Taq DNA polymerase 0.4 Yl 4 Yl(5 U /Yl)cDNA (from cDNA synthesis reaction) 2 Yl 20 Yl DEPC-treated water 38.1 Yl 381 Yl Final volume 50 Yl 500 μl 2. Mix gently and overlay with silicone oil or mineral oil if thethermal cycler lacks a heated lid.3. Incubate at 94°C for 2 min, then perform 20 to 40 cycles ofPCR with optimized conditions for your sample (1 min/kb extension time at 68-72°C)4. Analyze 10 Yl of the amplified sample by agarose gelelectrophoresis.Control ReactionsAn RT-PCR Primer and Control Set is available separately for monitoring the performance of the system (Cat. Number 10929-016).1. Use 1 ng of the Control RNA in the cDNA SynthesisReaction.2. Perform the PCR using Platinum® Taq DNA Polymerase, as described above.Page 4 of 4 Troubleshooting GuideProblem Possible cause Possible solutionNo RT-PCR product No cDNA synthesis (temperature too high) For the cDNA synthesis step, incubate at 45-50°C.Incomplete synthesis of target cDNA (secondary structure of RNA blocks synthesis) For the cDNA synthesis step, incubate at 50-70°C. For long mRNAs, increase cDNA synthesis incubation time (up to 50 min)RNase contamination Maintain aseptic conditions; add RNaseOUT™ (RNaseinhibitor).Concentration of template RNA in reaction is too low Increase the concentration of template RNA; use 1-5 μg of total RNA or reduce the volume of ThermoScript™ RT used in the reaction.RNA has been damaged or degraded Replace RNA.RT inhibitors are present in RNA Remove inhibitors in the RNA preparation by anadditional 70% ethanol wash after ethanolprecipation.Note: Inhibitors of RT include SDS, EDTA,guanidinium chloride, formamide, sodiumphosphate and spermidine (4).Cycle number is too low Increase cycle number.Low yield/low specificity in PCR Reaction conditions not optimal Optimize magnesium concentration.Optimize the primer concentrationOptimize the annealing temperature and extensiontime.Increase temperature of RT reaction to 50-60°C.Unexpected bands after electrophoresis RNA contamination with genomic DNA Pre-treat RNA with DNase I.Redesign PCR primers to anneal to sequence in exonson both sides of an intron in the target gene.References1.Westfall, B., Sitaraman, K., Solus, J., Hughes, J., and Rashtchian, A. (1997) Focus®19, 46.2.Chomczynski, P and Sacchi, N. (1987) Anal. Biochem. 162, 156.3.Chirgwin, J.M., Przybyla, A.E., MacDonald, R.J., and Rutter, W.J. (1979) Biochemistry 18, 5294.4.Gerard, G.F (1994). Focus®16, 102.Limited Use Label License No. 1: Thermostable Polymerases (applies to 11146-057, 11146-032, and 11146-040)Use of this product is covered by one or more of the following US patents and corresponding patent claims outside the US: 5,789,224, 5,618,711, and 6,127,155. The purchase of this product includes a limited, non-transferable immunity from suit under the foregoing patent claims for using only this amount of product for the purchaser’s own internal research. No right under any other patent claim, no right to perform any patented method, and no right to perform commercial services of any kind, including without limitation reporting the results of purchaser's activities for a fee or other commercial consideration, is conveyed expressly, by implication, or by estoppel. This product is for research use only. Diagnostic uses under Roche patents require a separate license from Roche. Further information on purchasinglicenses may be obtained by contacting the Director of Licensing, Applied Biosystems, 850 Lincoln Centre Drive, Foster City, California 94404, USA.Limited Use Label License No. 4: Products for PCR that include no rights to perform PCR (applies to 11146-016 and 11146-024)This product is compatible for use in the Polymerase Chain Reaction (PCR) process claimed in patents owned by Roche Molecular Systems, Inc. and F. Hoffmann-La Roche Ltd. No license under these patents is conveyed expressly, by implication, by estoppel or otherwise to the purchaser by the purchase of this product. A license to use these patented processes for certain research and development activities accompanies the purchase of certain reagents of Applied Biosystems and other licensed suppliers when used in conjunction with an authorized thermal cycler, or is available from Applied Biosystems. Further information on purchasing licenses may be obtained by contacting the Director of Licensing, Applied Biosystems, 850 Lincoln Centre Drive, Foster City, California 94404, USA.Limited Use Label License No: 5: Invitrogen Technology (applies to all)The purchase of this product conveys to the buyer the non-transferable right to use the purchased amount of the product and components of the product in research conducted by the buyer (whether the buyer is an academic or for-profit entity). The buyer cannot sell or otherwise transfer (a) this product (b) its components or (c) materials made using this product or its components to a third party or otherwise use this product or its components or materials made using this product or its components for Commercial Purposes. The buyer may transfer information or materials made through the use of this product to a scientific collaborator, provided that such transfer is not for any Commercial Purpose, and that such collaborator agrees in writing (a) not to transfer such materials to any third party, and (b) to use such transferred materials and/or information solely for research and not for Commercial Purposes. Commercial Purposes means any activity by a party for consideration and may include, but is not limited to: (1) use of the product or its components in manufacturing; (2) use of the product or its components to provide a service, information, or data; (3) use of the product or its components for therapeutic, diagnostic or prophylactic purposes; or (4) resale of the product or its components, whether or not such product or its components are resold for use in research. For products that are subject to multiple limited use label licenses, the terms of the most restrictive limited use label license shall control. Life Technologies Corporation will not assert a claim against the buyer of infringement of patents owned or controlled by Life Technologies Corporation which cover this product based upon the manufacture, use or sale of a therapeutic, clinical diagnostic, vaccine or prophylactic product developed in research by the buyer in which this product or its components was employed, provided that neither this product nor any of its components was used in the manufacture of such product. If the purchaser is not willing to accept the limitations of this limited use statement, Life Technologies is willing to accept return of the product with a full refund. For information about purchasing a license to use this product or the technology embedded in it for any use other than for research use please contact Out Licensing, Life Technologies, 5791 Van Allen Way, Carlsbad, California 92008; Phone (760) 603-7200 or e-mail:outlicensing@.Limited Use Label License No: 14: Direct Inhibition by Anti-Polymerase Antibodies(applies to 11146-057, 11146-032, and 11146-040)Licensed to Life Technologies Corporation, under U.S. Patent Nos. 5,338,671; 5,587,287; and foreign equivalents for use in research onlyLimited Use Label License No. 17: AFLP® Products (applies to 11146-040)The AFLP® technique is covered by patents or patented applications owned by Keygene n.v and this product is sold under license from Keygene n.v. This kit may be used for research purposes only. For use of this kit in plant breeding, contact the Licensing Department, Life Technologies Corporation, 5791 Van Allen Way, Carlsbad, California 92008. Phone (760) 603-7200. Fax (760) 602-6500. The use of this kit for any other purpose, including but not limited to the use for clinical, diagnostic, and/or therapeutic purposes; or for providing services to third parties, requires a license from Keygene n.v., P.O. Box 216, 6700 AE Wageningen, The Netherlands.©2010 Life Technologies Corporation. All rights reserved.For research use only. Not intended for any animal or human therapeutic or diagnostic use.The trademarks mentioned herein are the property of Life Technologies Corporation or their respective owners.。
PROMEGA逆转录试剂盒说明书

4. Composition of Buffers and Solutions .........................................................5 5. References ...........................................................................................................5 6. Related Products ................................................................................................5 1. Description
PRINTED IN USA. Revised 3/09
Promega Corporation · 2800 Woods Hollow Road · Madison, WI 53711-5399 USA Toll Free in USA 800-356-9526 · Phone 608-274-4330 · Fax 608-277-2516 · Part# TB099
For Laboratory Use. Each system contains sufficient reagents for 100 reactions, processing 1μg of RNA per reaction. Includes:
• • • • • • • • •
1,500u 2,500u 50μg 50μg 5μg 320μl 1.4ml 1.2ml 13ml
Reverse Transcription System
RevertAid 逆转录试剂盒说明书

Kit is designed for preparation of full-length first strand cDNA from RNA templates. The RevertAid™ first strand cDNA synthesis kit relies on a genetically engineered version of the Moloney Murine Leukemia Virus reverse transcriptase (RevertAid™ M-MuLV RT) with low RNase H activity. This allows the synthesis of full-length cDNA from long templates (up to 13 kb). RevertAid™ M-MuLV RT synthesizes first strand cDNA at sites determined by the type of primer used: • random hexamer primer: at non-specific points along an RNA template. In this case, all RNA’s in a population are templates for cDNA synthesis. • oligo(dT)18 : at the 3’-end of poly(A)+ mRNA. In this case, only mRNA’s with 3’-poly(A) tails are templates for cDNA synthesis. • sequence specific primer: at a primer-binding site. First strand cDNA synthesized with this system can be used as a template in the polymerase chain reaction (PCR)*. As the reaction conditions of cDNA synthesis and PCR are compatible, cDNA reaction mixture can be added directly to a PCR mixture. The first strand cDNA synthesized can also be used as a template for second strand synthesis. Radiolabeled DNA can be used as a probe in hybridization experiments. 1.1 kb RNA with 3’-poly(A) tail is provided 3
东洋纺 逆转录试剂盒

(1)反应液的配制和反应 反应液组成(例)
Nuclease-free Water
up to 10μl
Total RNA(<10μg)
Xμl
10×DNase I Buffer
(10mM Tris-Cl, pH7.6, 2.5mM MgCl2, 0.5mM CaCl2)
RNase-free DNase I(10U /μl)
※ 本产品的试剂盒内没有添附 Realtime PCR 试剂。关于 Realtime PCR 产品,推荐使 用本公司的 Realtime PCR 用试剂 Realtime PCR Master Mix 系列。(详情请参考 [7] 相关产品的内容)。
◆本产品特征◆ 1.对应各种不同长度目标片段的高效率逆转录
关仪器。
• Nuclease-free Water 本产品已添附可使用 200 次的 Nuclease-free Water,此外请再准备一些
Nuclease-free Water,以便在稀释模板 RNA 时使用。推荐使用未经过 DEPC 处 理 的 Nuclease-free Water。也可以使 用经 DEPC 处 理 过 的 水,但 残 留的 DEPC 会抑制反应的进行,因此请使用高压灭菌器彻底清除 DEPC 之后再使 用。此外,为防止核酸的混入,用于逆转录反应和 PCR 反应的 Nuclease-free Water,建议和用于其它实验的 Nuclease-free Water 分开保存,避免共用。 • Total RNA
【注意】
本试剂盒中所含的试剂均为科研用试剂。请勿作为诊断、临床试 剂用。在使用时,请严格遵守实验室的一般注意事项,适当使用保护 用品,安全操作。
【保存】
所有组分请均保存于 -20℃条件下。
东洋纺反转录试剂盒

ReverTra Ace -α-(Code No.:FSK-100,FSK-101)是以ReverTra Ace为核 心酶的First strand cDNA合成试剂盒。由于本试剂盒包含了RT用引物及Positive Control,所以能够简单地进行逆转录反应。也可以应用于手头上各种DNA聚合 酶的PCR反应模板的制备。例如,通过和High fidelity PCR用酶,Long PCR用 酶等的结合,能够进行目的性更强的RT-PCR。
【 保存 】
•除Positive Control RNA以外,其他组分请均保存于-20℃条件下。 •Positive Control RNA请保存于-80℃条件下。 ※ Positive Control RNA由于运输过程中不能一直保证-80℃条件,在运输过
程中可能降解。建议用户选择人、鼠来源的Total RNA 作为Control RNA。 •为了保证实验效果,请在收到产品的一年之内使用完毕。
G3PDH 基因是在各种哺乳类组织上表达的“管家基因”。其 mRNA 表达水 平不受一部分细胞因子和含有 Tumor-promoting Phorbol Esters 等的诱导物质 的影响,并且,在几乎所有组织上都是恒定的。因此,使用从各种组织中抽提出 来的 RNA 样品的时候,可以作为最合适的对照而使用。
↓
99℃、5 min. *****
↓
4℃、5 min.
↓
瞬间离心
5
TaKaRa逆转录试剂盒

*1 Random 6 mers 和 Oligo dT Primer 同时使用,可有效地将全长 mRNA 反转录成
cDNA。使用单引物进行反转录时,使用量分别如下:
Random 6 mers(100 μM) 2.0 μl(200 pmol)
Oligo dT Primer(50 μM)
0.5 μl(25 pmol)
热循环仪(或 37℃、42℃水浴和 85℃加热块) 反转录反应所用 0.2 ml 和 1.5 ml 的微量反应管 微量移液器和枪头(高压灭菌)
● 保 存: -20℃。
●特 长
1. 可以快速、高效合成 Real Time PCR 用 cDNA,是进行 2 Step Real Time RT-PCR 反应的最佳试 剂。
2. 含有 Oligo dT Primer 和 Random 6 mers 两种反转录引物,可根据实际情况区别使用。反转录反应 可以使用 Random 6 mers 或 Oligo dT Primer,也可以 Oligo dT Primer 和 Random 6 mers 同时 使用。只扩增一种目的基因时,也可以使用 Specific Primer 作为反转录引物。
● 使用注意
以下为使用本试剂盒时的注意事项,使用前一定认真阅读。 1. 当同时需要进行数次反应时,应先配制各种试剂的混合液(Master Mix;其中包括 RNase Free dH2O、
Buffer、酶等),然后再分装到每个反应管中。这样可使所取的试剂体积更准确,减少试剂损失,避免 重复分取同一试剂。同时也可以减少实验操作或实验之间产生的误差。 2. PrimeScript RT Enzyme Mix I 使用前要小心地离心收集到反应管底部。由于酶保存液中含有 50%的 甘油,粘度高,分取时应慢慢吸取。同时,要使用精确、量程适合的移液枪,并且不要使 Tip 插入液 面过深,否则会因 Tip 壁粘着造成损失。 3. 分装试剂时务必使用新的枪头(Tip),以防止样品间污染。
invitrog逆转录试剂盒说明书

ThermoScript ™ RT-PCR SystemCatalog nos. (25 reactions): Catalog nos. (100 reactions): 11146-02411146-01611146-057 (w/ Platinum ® Taq DNA polymerase) 11146-032 (w/ Platinum ® Taq DNA polymerase)11146-040 (w/ Platinum ® Taq DNA polymerase High Fidelity)Store at -20°C (stability can be extended by storing at -70°C)DescriptionThe ThermoScript ™ RT-PCR System is designed for the sensitive and reproducible detection and analysis of RNA molecules in a two-step process. ThermoScript ™ RT, an avian reverse transcriptase with reduced RNase H activity, is engineered to have higher thermal stability,produce higher yields of cDNA, and produce more full-length cDNA transcripts than AMV RT. cDNA synthesis is performed in the first step using either total RNA or poly(A)+-selected RNA primed with oligo(dT), random primers or a gene-specific primer, at 50-65°C. In the second step, PCR is performed in a separate tube using primers specific for the gene of interest. RNA targets from 100 bp to >12 kb can be detected with this system, using 10 pg to 5 Yg of total RNA. PCR is carried out with Platinum ® Taq DNA Polymerase or Platinum ® Taq DNA Polymerase High Fidelity. Platinum ® Taq DNA Polymerase High Fidelity is suitable for templates from 100 bp to >12 kb. Platinum ® Taq DNA polymerase (1) provides automatic hot-start conditions for increased specificity up to 3 kb.Reagents are provided for 25 or 100 cDNA synthesis reactions of 20 μl each and 25 or 100 amplification reactions of 50 μl each.Component 25 rxn kit 100 rxn kit ThermoScript ™ RT (15 U/Yl) 25 μl 100 μl 5X cDNA Synthesis Buffer* 500 μl 500 μl 0.1 M DTT 250 μl 250 μl 10 mM dNTP Mix 100 μl 2 × 250 μlRNaseOUT ™ (40 U/Yl) 25 μl 100 μlOligo (dT)20 (50 YM) 25 μl 100 μlRandom Hexamers (50 ng/Yl) 50 μl 250 μlDEPC-Treated Water 1.25 ml 1.25 mlE . coli RNase H (2 U/Yl) 50 μl 2 × 50 μl*250 mM Tris acetate (pH 8.4), 375 mM potassium acetate, 40 mMmagnesium acetate, stabilizerCatalog numbers 11146-057 (25 rxns) and 11146-032 (100 rxns) include the following, in addition to the components to the left:Component 25 rxn kit 100 rxn kitPlatinum ® Taq DNA polymerase (5 U/Yl) 100 units 250 units10X PCR buffer Minus Mg 1.0 ml 1.0 ml 50 mM MgCl 2 1.0 ml 1.0 ml Catalog number 11146-040 (100 rxns) includes the following, in addition to the components to the left: Component 100 rxn kit Platinum ®Taq DNA Polymerase High Fidelity (5 U/μl) 100 units 10X High Fidelity PCR Buffer 1.0 ml 50 mM MgSO 4 1.0 ml Quality ControlThe Certificate of Analysis (CofA) provides detailed quality control information for each product. The CofA is available on our website at/cofa, and is searchable by product lot number, which is printed on each box .Summary of ProcedurePart no. 11146.pps MAN0000941 Rev. date: 11 Jun 201020 GSPr andom hexame r C, 30-60 min 50-65°C, 30-60 min 25°C, 10 min ↓ 50-60°C, 20-50 min↓85°C, 5 min↓ Add 1 μl RNase H 37°C, 20 min ↓ Remove 2 μl aliquot for PCRWater 1 *If less than 1 ng of RNA is used, reduce the amount of ThermoScript RT in the reaction to 0.5 μl. For technical support, email tech_support@. For country-specific contact information, visit .Page 2 of 4 Important Parameters to ConsiderRNA•High quality intact RNA is essential for successful full-length cDNA synthesis and successful long RT-PCR.•RNA should be devoid of any RNase contamination and aseptic conditions should be maintained. •Recommended methods of total RNA isolation include the Micro-to-Midi Total RNA PurificationSystem (Catalog no. 12183-018) and TRIzol® Reagent(Catalog no. 15596-026) (2, 3). Oligo(dT)-selection forpoly(A)+ RNA is typically not necessary, althoughincorporating this step may improve the yield ofspecific cDNAs.cDNA Synthesis Primers•Oligo(dT)20 (50 pmoles/reaction) is recommended for priming polyadenylated RNA. Use of Oligo(dT)20allows the detection of multiple transcripts from asingle first-strand reaction.•Random hexamers (50-250 ng/reaction) are efficient primers for the detection of multiple short RT-PCRtargets. Use of more than 50-100 ng primer/Yg ofRNA can increase the yield of short products but may inhibit detection of long targets (>3kb) or raretranscripts. If random hexamers are used, the first-strand reaction must be incubated at 25°C for 10 minto extend the primers prior to increasing the reactiontemperature for synthesis.•Gene-specific primers (GSP) should be used at 10 to20 pmol/reaction. Specificity of priming may beimproved by optimizing annealing/reactiontemperature.•Treatment of cDNA with RNase H to remove the complementary RNA prior to PCR is optional. RNaseH digestion will improve the RT-PCR signal of manytargets and is required for the efficient and consistentamplification of long RT-PCR templates.cDNA Synthesis Reaction•Denaturation of the RNA template and primer by incubating at 65°C for 5 min is optional. Most targetscan be reverse transcribed efficiently without this step.However, heating the RNA in the absence of reactionbuffer and enzyme prior to cDNA synthesis canremove secondary structure that may impede full-length cDNA synthesis.•ThermoScript™ RT can be used at 50-65°C. We recommend incubation at 50-55°C for most RT-PCRtargets. However, incubation at 50-60°C for oligo(dT)and 50-65°C for gene-specific primers can beemployed to reduce secondary structure or to improve specificity.•Most targets can be amplified after only a 30-min incubation for the first-strand reaction. Rare RNAs,long transcripts, or targets at the 5′ end of longtranscripts benefit from longer incubation times (50-60 min).PCR Primers• A final primer concentration of 0.2 - 0.4 μM for each primer is generally optimal; however, a primertitration is recommended for best results.•Design primers that anneal to sequence in exons on both sides of an intron or exon/exon boundary of the mRNA to allow differentiation between amplification of cDNA and potential contaminating genomic DNA. •Primers should not be self-complementary or complementary to each other at the 3′ ends.PCR Reactions•Most targets will be efficiently amplified using 2 Yl or less of the cDNA synthesis reaction.•The optimum magnesium concentration varies from1.5 to 3 mM. Generally, 1.82 mM magnesium chloridefor Platinum® Taq DNA polymerase and 2.32 mMmagnesium sulfate for Platinum® Taq DNAPolymerase High Fidelity is effective for most primer sets. However, titration of the magnesiumconcentration is recommended for the best result.Each Yl of the cDNA synthesis reaction adds 0.16 mM to the final magnesium concentration in a 50-μl PCRreaction.•Assemble the PCR reactions on ice, transfer them to a pre-heated thermal cycler (85-95°C) and immediately start the PCR amplification program.•The annealing temperature should be 10°C below the melting temperature of the primers used.•The optimum extension temperature for Platinum®Taq DNA Polymerase High Fidelity is 68°C. Theextension time varies with the size of the amplicon(approximately 1 min per 1 kb of amplicon).Page 3 of 4cDNA Synthesis1. In a 0.2- or 0.5-ml tube, combine primer (50 μM Oligo(dT)20,50 ng/μl random primer or 10 μM gene-specific primer), RNA, and dNTP mix and adjust volume to 12 Yl withDEPC-treated water.Component AmountPrimer 1 YlRNA (10 pg -5 Yg) x Yl10 mM dNTP Mix 2 YlDEPC-treated water to 12 Yl2. Denature RNA and primer by incubating at 65°C for 5 minand then place on ice (optional).3. Vortex the 5X cDNA Synthesis Buffer for 5 s just prior touse.4. Prepare a master reaction mix on ice and vortex gently.Component 1 Reaction 10 Reactions5x cDNA Synthesis Buffer 4 Yl 40 Yl0.1 M DTT 1 Yl 10 YlRNaseOUT™ (40 U/Yl) 1 Yl 10 YlDEPC-treated water 1 Yl 10 YlThermoScript™ RT (15 units/Yl)1 Yl* 10 Yl**NOTE: If less than 1 ng of template RNA is used, reduce the amount of ThermoScript™ RT in the reaction to 0.5 μl/reaction(5 μl/10 reactions). Increase the amount of DEPC-treatedwater in the master reaction mix to 1.5 μl/reaction (15 μl/10 reactions).5. Pipet 8 Yl of master reaction mix into each reaction tube on ice.6. Transfer the sample to a thermal cycler preheated to the appropriate cDNA synthesis temperature and incubate as follows.Oligo(dT)20 primed: 30-60 min at 50°C (or 50-60°C)Gene-specific primed 30-60 min at 50°C (or 50-65°C) Random-hexamer primed: 25°C for 10 min, followed by 20-50 min at 50°C (or 50-65°C)7. Terminate the reaction by incubating at 85°C for 5 min.8. Add 1 Yl of RNase H and incubate at 37°C for 20 min (optional).9. cDNA synthesis reactions can be stored at -20°C or used for PCR immediately.PCR with Platinum®Taq DNA Polymerase High FidelityUse only 10% of the cDNA synthesis reaction (2 Yl) for PCR.Use of 2 μl of 50 mM MgSO4 and 2 Yl of cDNA (0.32 mM magnesium in a 50-Yl PCR) results in a final concentration of2.32 mM magnesium, which is effective for most primer sets. However, titration of the magnesium concentration with the provided 50 mM MgSO4 is recommended for best results.1. Add the following to a 0.2- or 0.5-ml, thin-walled, PCR tube: Component 1 Reaction 10 Reactions10X High Fidelity PCR Buffer 5 Yl 50 Yl50 mM MgSO4 2μl 20μl10 mM dNTP Mix 1 Yl 10 Yl10 YM sense primer 1 Yl 10 Yl10 YM antisense primer 1 Yl 10 YlPlatinum®Taq High Fidelity 0.2 Yl 2 YlcDNA (from cDNA synthesis reaction) 2 Yl 20 YlDEPC-treated water 37.8 Yl 378 YlFinal volume 50 Yl 500 μl 2. Mix gently and overlay with silicone oil or mineral oil if thethermal cycler lacks a heated lid.3. Incubate at 94°C for 2 min, then perform 20 to 40 cycles ofPCR with optimized conditions for your sample (1 min/kb extension time at 68°C).4. Analyze 10 Yl of the amplified sample by agarose gelelectrophoresis.PCR with Platinum® Taq DNA PolymeraseUse only 10% of the cDNA synthesis reaction (2 Yl) for PCR. Use of 50 mM MgCl2 and 2 Yl of cDNA will result in a final magnesium concentration of 1.82 mM, which is adequate for most primers and targets. However, titration of magnesium concentration is recommended for best results.1. Add the following to a 0.2- or 0.5-ml, thin-walled, PCR tube: Component 1 Reaction 10 Reactions 10X PCR Buffer Minus Mg 5 Yl 50 Yl50 mM MgCl2 1.5 Yl 15 Yl10 mM dNTP Mix 1 Yl 10 Yl10 YM sense primer 1 Yl 10 Yl10 YM antisense primer 1 Yl 10 YlPlatinum®Taq DNA polymerase 0.4 Yl 4 Yl(5 U /Yl)cDNA (from cDNA synthesis reaction) 2 Yl 20 Yl DEPC-treated water 38.1 Yl 381 Yl Final volume 50 Yl 500 μl 2. Mix gently and overlay with silicone oil or mineral oil if thethermal cycler lacks a heated lid.3. Incubate at 94°C for 2 min, then perform 20 to 40 cycles ofPCR with optimized conditions for your sample (1 min/kb extension time at 68-72°C)4. Analyze 10 Yl of the amplified sample by agarose gelelectrophoresis.Control ReactionsAn RT-PCR Primer and Control Set is available separately for monitoring the performance of the system (Cat. Number 10929-016).1. Use 1 ng of the Control RNA in the cDNA SynthesisReaction.2. Perform the PCR using Platinum® Taq DNA Polymerase, as described above.Page 4 of 4 Troubleshooting GuideProblem Possible cause Possible solutionNo RT-PCR product No cDNA synthesis (temperature too high) For the cDNA synthesis step, incubate at 45-50°C.Incomplete synthesis of target cDNA (secondary structure of RNA blocks synthesis) For the cDNA synthesis step, incubate at 50-70°C. For long mRNAs, increase cDNA synthesis incubation time (up to 50 min)RNase contamination Maintain aseptic conditions; add RNaseOUT™ (RNaseinhibitor).Concentration of template RNA in reaction is too low Increase the concentration of template RNA; use 1-5 μg of total RNA or reduce the volume of ThermoScript™ RT used in the reaction.RNA has been damaged or degraded Replace RNA.RT inhibitors are present in RNA Remove inhibitors in the RNA preparation by anadditional 70% ethanol wash after ethanolprecipation.Note: Inhibitors of RT include SDS, EDTA,guanidinium chloride, formamide, sodiumphosphate and spermidine (4).Cycle number is too low Increase cycle number.Low yield/low specificity in PCR Reaction conditions not optimal Optimize magnesium concentration.Optimize the primer concentrationOptimize the annealing temperature and extensiontime.Increase temperature of RT reaction to 50-60°C.Unexpected bands after electrophoresis RNA contamination with genomic DNA Pre-treat RNA with DNase I.Redesign PCR primers to anneal to sequence in exonson both sides of an intron in the target gene.References1.Westfall, B., Sitaraman, K., Solus, J., Hughes, J., and Rashtchian, A. (1997) Focus®19, 46.2.Chomczynski, P and Sacchi, N. (1987) Anal. Biochem. 162, 156.3.Chirgwin, J.M., Przybyla, A.E., MacDonald, R.J., and Rutter, W.J. (1979) Biochemistry 18, 5294.4.Gerard, G.F (1994). Focus®16, 102.Limited Use Label License No. 1: Thermostable Polymerases (applies to 11146-057, 11146-032, and 11146-040)Use of this product is covered by one or more of the following US patents and corresponding patent claims outside the US: 5,789,224, 5,618,711, and 6,127,155. The purchase of this product includes a limited, non-transferable immunity from suit under the foregoing patent claims for using only this amount of product for the purchaser’s own internal research. No right under any other patent claim, no right to perform any patented method, and no right to perform commercial services of any kind, including without limitation reporting the results of purchaser's activities for a fee or other commercial consideration, is conveyed expressly, by implication, or by estoppel. This product is for research use only. Diagnostic uses under Roche patents require a separate license from Roche. Further information on purchasinglicenses may be obtained by contacting the Director of Licensing, Applied Biosystems, 850 Lincoln Centre Drive, Foster City, California 94404, USA.Limited Use Label License No. 4: Products for PCR that include no rights to perform PCR (applies to 11146-016 and 11146-024)This product is compatible for use in the Polymerase Chain Reaction (PCR) process claimed in patents owned by Roche Molecular Systems, Inc. and F. Hoffmann-La Roche Ltd. No license under these patents is conveyed expressly, by implication, by estoppel or otherwise to the purchaser by the purchase of this product. A license to use these patented processes for certain research and development activities accompanies the purchase of certain reagents of Applied Biosystems and other licensed suppliers when used in conjunction with an authorized thermal cycler, or is available from Applied Biosystems. Further information on purchasing licenses may be obtained by contacting the Director of Licensing, Applied Biosystems, 850 Lincoln Centre Drive, Foster City, California 94404, USA.Limited Use Label License No: 5: Invitrogen Technology (applies to all)The purchase of this product conveys to the buyer the non-transferable right to use the purchased amount of the product and components of the product in research conducted by the buyer (whether the buyer is an academic or for-profit entity). The buyer cannot sell or otherwise transfer (a) this product (b) its components or (c) materials made using this product or its components to a third party or otherwise use this product or its components or materials made using this product or its components for Commercial Purposes. The buyer may transfer information or materials made through the use of this product to a scientific collaborator, provided that such transfer is not for any Commercial Purpose, and that such collaborator agrees in writing (a) not to transfer such materials to any third party, and (b) to use such transferred materials and/or information solely for research and not for Commercial Purposes. Commercial Purposes means any activity by a party for consideration and may include, but is not limited to: (1) use of the product or its components in manufacturing; (2) use of the product or its components to provide a service, information, or data; (3) use of the product or its components for therapeutic, diagnostic or prophylactic purposes; or (4) resale of the product or its components, whether or not such product or its components are resold for use in research. For products that are subject to multiple limited use label licenses, the terms of the most restrictive limited use label license shall control. Life Technologies Corporation will not assert a claim against the buyer of infringement of patents owned or controlled by Life Technologies Corporation which cover this product based upon the manufacture, use or sale of a therapeutic, clinical diagnostic, vaccine or prophylactic product developed in research by the buyer in which this product or its components was employed, provided that neither this product nor any of its components was used in the manufacture of such product. If the purchaser is not willing to accept the limitations of this limited use statement, Life Technologies is willing to accept return of the product with a full refund. For information about purchasing a license to use this product or the technology embedded in it for any use other than for research use please contact Out Licensing, Life Technologies, 5791 Van Allen Way, Carlsbad, California 92008; Phone (760) 603-7200 or e-mail:outlicensing@.Limited Use Label License No: 14: Direct Inhibition by Anti-Polymerase Antibodies(applies to 11146-057, 11146-032, and 11146-040)Licensed to Life Technologies Corporation, under U.S. Patent Nos. 5,338,671; 5,587,287; and foreign equivalents for use in research onlyLimited Use Label License No. 17: AFLP® Products (applies to 11146-040)The AFLP® technique is covered by patents or patented applications owned by Keygene n.v and this product is sold under license from Keygene n.v. This kit may be used for research purposes only. For use of this kit in plant breeding, contact the Licensing Department, Life Technologies Corporation, 5791 Van Allen Way, Carlsbad, California 92008. Phone (760) 603-7200. Fax (760) 602-6500. The use of this kit for any other purpose, including but not limited to the use for clinical, diagnostic, and/or therapeutic purposes; or for providing services to third parties, requires a license from Keygene n.v., P.O. Box 216, 6700 AE Wageningen, The Netherlands.©2010 Life Technologies Corporation. All rights reserved.For research use only. Not intended for any animal or human therapeutic or diagnostic use.The trademarks mentioned herein are the property of Life Technologies Corporation or their respective owners.。
Taka逆转录试剂盒

2.Real Time PCR 相关试剂。 SYBR Green I方法检测试剂:SYBR® Premix Ex TaqTM(Perfect Real Time)
SYBR® Premix Ex TaqTM II(Perfect Real Time) SYBR® Premix DimerEraserTM TaqMan探针方法检测试剂:Premix Ex TaqTM(Perfect Real Time)
●实验操作方法
① 在 Microtube 中配制下列混合液。
Oligo dT Primer(50 μM)[or Random 6 mers(50 μM)]
dNTP Mixture(10 mM each) Total RNA(or Poly(A)+ RNA) RNase free dH2O
l μl*1 1 μl X μg*2 up to 10 μl
*1 ① 2 kb 以上的长片段 cDNA 合成时,Random 6 mers 引物的使用量为 0.4 μ(l 20 pmol)。 ② Real Time PCR 反应时,Random 6 mers 引物使用 2 μl(100 pmol)可以得到较好的 实验结果。 ③ 也可以使用 Gene Specific Primer,此时 Primer 终浓度为 0.1 μM。
-2-
*2 ① Total RNA的使用量小于 5 μg;Poly(A)+ RNA的使用量小于 1 μg。 ② Real Time RT-PCR 反应时,Total RNA 的最大使用量为 1 μg。
逆转录试剂盒(transcrip...

逆转录试剂盒(transcrip...Transcriptor First Strand cDNA Synthesis Kit 逆转录步骤1,使用前解冻结冰的试剂2,将试剂短暂离心3,冰上操作:(操作RNA实验需要戴手套)将模板与引物在PCR管上混合试剂体积最终浓度细胞总RNA 或 1 ug 总RNA 或10ng多聚尾(A)+m RNA 多聚尾(A)+ mRNA任选其中一个引物:Anchored-oligo(dT)18 Primer, 1 ul 2.5 uM50 pmol/ul (vial 5)或Random Hexamer Primer, 2ul 60uM600 pmol/ul (vial 6)或 Sequence-Specific Primer 可变0.5-2.5 uM1%DEPC水, (vials 7 or 9) 可变使总体积为 13 ul总体积13ul注:以上为初次实验的推荐浓度。
总RNA的适用浓度为10ug到5ng,以及mRNA的适用浓度为1到100ng,当RNA模板浓度较低时添加10ug/ml 的MS2 RNA* 来稳定模板RNA。
4,(可选)65度水浴上述混合物10分钟,使模板引物混合物变性(确保失活RNA二级结构),水浴后立即冰浴至少一分钟5,往上述混合物继续加入以下试剂(冰上操作)试剂体积最终浓度.Transcriptor Reverse Transcriptase 4 ul 1×(8 mM MgCl2) Reaction Buffer, 5× conc. (vial 2)Protector RNase Inhibitor, 0.5 ul40 U/ul (vial 3)20 U Deoxynucleotide Mix, 2 ul 每个1 mM 10 mM each (vial 4)Transcriptor Reverse 0.5 ul 10U Transcriptase, 20 U/ul (vial 1) 最终体积20 ul注:小心混合上述试剂,不要振荡,短暂离心使试剂沉在管底6,将PCR管放在PCR仪上孵育,孵育条件如下:使用的引物目标mRNA长度孵育温度与时间Anchored-oligo(dT)18 不大于4kb 55度30分钟大于4kb 50度60分钟primer, 50 pmol/ul 或Sequence–specific primerRandom hexamer primers, 不大于4kb 25度10分钟,然后55度30分钟600pmol/ul 大于4kB 25度10分钟,然后50度60分钟6,85℃水浴5分钟灭活逆转录酶8、-20℃保存,或立即进行PCR。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------广州东盛生物科技有限公司 电话:020-87791356 传真:020-87791381 网址:
●R1011 可进行
产品说明 R1011 20 μl 12 μl 20 μl 20 μl 80 μl 1 ml 50 μl 1份 R1012 100 μl 60 μl 100 μl 100 μl 400 μl 1 ml×5 250 μl 1份 cDNA 第一链合成试剂盒是专为两步法 RT-PCR 第一步实验配制的、 具有高灵敏度的 RT-PCR 反应系统。该系统合理配备了与cDNA 第一 链合成反应相关的各种组分,优化体系的保证了 M-MLV 具有高效的 逆转录酶活性, 所得 cDNA 对后续的 PCR 或定量 PCR 实验兼容性好, 可用于检测稀有基因的表达、 从极少数细胞中定量检测特定mRNA 的 表达水平、克隆特定基因的 cDNA 片段等。
成功的 cDNA 合成来自高质量的 RNA。高质量的 RNA 至少应保证全长并且不含逆转录酶的抑制剂,如 EDTA 或 SDS。用于
cDNA 合成反应的溶液试剂尽可能用 DEPC 进行处理,并在高压灭菌后使用。有些试剂不能高压灭菌时,首先用经为了增加贮存 RNA 样品的稳定性,可 以将 RNA 溶解在去离子的甲酰胺中,存 于-70℃。用 于保存 RNA 的甲酰胺一定不能含有降解 RNA 的杂物。 来源于胰脏的 RNA 至少可以在甲酰胺中保存一年。当准备使用 RNA 时,可以使用下列方法沉淀 RNA:加入 NaCl 至 0.2 M 及 4 倍体积的乙醇,室温放置 3-5 min,10,000 rpm 离心 5 min。
●
在逆转录反应中经常加入 RNase 抑制剂以增加 cDNA 合成的长度和产量。RNase 抑制剂要在第一链合成反应中,在缓冲
液和还原剂(如 DTT)存在的条件下加入,因为 cDNA 合成前的过程会使抑制剂变性,从而释放结合的可以降解 RNA 的 RNase。蛋白 RNase 抑制剂仅防止 RNase A,B,C 对 RNA 的降解,并不能防止皮肤上的 RNase,因此尽管使用了这些抑 制剂,也要小心不要从手指上引入 RNase。
PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿ
逆转录系列
注意事项-----------------------------------------------------------------------------------------------------------------------------------------------------------●
20 次逆转录反应,R1012 可进行 100 次逆转
录反应(20 μl 标准 PCR 反应体系,每次使用 M-MLV 1 μl)。
M-MLV 储存液 20 mM Tris-HCl (pH 7.5) 200 mM NaCl 0.1 mM EDTA 1 mM DTT 0.01% NP-40 50% glycerol 5xfirst-strand buffer 成分 250 mM Tris-HCl (pH 8.3 at 25 ℃) 375 mM KCl 15 mM MgCl2 50 mM DTT 保存条件 各组分均-20℃保存,避免反复冻融。
----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------广州东盛生物科技有限公司 电话:020-87791356 传真:020-87791381 网址:
菌的器具、水等配制溶液后,再将溶液进行过滤除菌处理。
●
法)制备的高纯度 RNA。
● ●
PCR 反应条件。
● ●
----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------广州东盛生物科技有限公司 电话:020-87791356 传真:020-87791381 网址:
东盛生物出产的 M-MLV 是由一个 71kDa 的单亚基组成的重组型 DNA 逆转 录聚合酶。可以催化以 RNA 或 DNA:RNA 杂交链为模板的互补 DNA 的聚 合反应。本酶经修饰 RNaseH 活性比普通的逆转录酶要弱很多,因此在合成第 一链 cDNA 的过程中,可保证 RNA 的降解程度较低,从而使得率提高。
PDF 文件使用 "pdfFactory Pro" 试用版本创建
逆转录系列
Q&A-----------------------------------------------------------------------------------------------------------------------------------------------------------------问:反转录怎样选择使用哪种引物? 答:反转录引物的选择,应结合实验的具体情况,选择以下三种引物,即:Random 6 mers、Oligo dT Primer 或特异性下游引物。 对没有发夹结构的短链 mRNA,上述三种引物都可以使用;一般情况下的反转录引物的选择请参照以下说明。 Random primer :适用于长的或具有 Hairpin 构造的 RNA。包括 rRNA、mRNA、tRNA 等在内的所有 RNA 的反转录反应 都可 使用本引物。 Oligo(dT)15primer :适用于具有 Poly (A)+ Tail 的 RNA。( 注意:原核生物的 RNA、真核生物的 rRNA、tRNA 以及某些 种类的真 核生物的 mRNA 等不具有 Poly (A)+ Tail 。 ) 特异性下游引物(PCR 时的下游引物): 必须与模板序列互补,需了解 Target序列。 问:反转录引物的用量怎样? 答:普通情况下 Oligo(dT)15 和 Random primer 均用 1 μl 2 kb 以上的长片段 cDNA 合成时,Random primer 的使用量为 0.4 μl (20pmol) ;Real Time PCR反应时,Random primer 引物使用 2 μl (100 pmol)可以得到较好的实验结果; 也可以使用 Gene Specific Primer, 此时引物终浓度为 0.1μl。 问:Random primer 的使用与 Oligo(dT)15primer 有何异同? 答:以 Random primer 反转录时,应先 25 ℃反应 10 min 再 42 ℃反应 1 h。 问:试剂盒及反转录用的 RNA 应如何保存? 答:试剂盒及 cDNA 产物应在-20 ℃保存,RNA 应避免反复冻融,使 RNA 在冰浴中保持融化状态。
0.6 μl RNasin , M-MLV ; 按下列条件进行反转录反应 42℃ 温浴 60 min(随即引物 37℃ 温浴 60 min) ; 5 终止反应 70℃ 温浴 5 min 终止反应,置冰上进行后续实验或-20℃ 保存。 6 用 RNase-free ddH2O 将反应体系稀释到 50 μl ,取 2-5 μl 进行 PCR 扩增反应。
逆转录系列
RT-PCR Kit(逆转录试剂盒)
产品组分 组分 M-MLV(200 U/μl) RNase(40 U/μl) Oligo(dT)15(50 μM) Random primer(50 μM) 5×first-strand buffer RNase-free ddH2O dNTPs(10 mM each) 说明书
● ●
较高的保温温度有助于 RNA 二级结构的打开,增加了反应的产量。 用于 cDNA 合成反应的溶液试剂尽可能用 DEPC 进行处理,并在高压灭菌后使用。有些试剂不能高压灭菌时,首先用经过灭 使用简单的 RNA 纯化方法即可获得满足于 RT-PCR反应的 RNA,但为了保证实验的成功率,建议使用 GTC 法(异硫氰酸胍 为防止 RNA 降解,应尽量避免反复冻融,有条件的实验室最好保存于-70℃。 最佳的 PCR 反应条件,因 PCR 扩增仪的不同而不同,所以在使用您的样品之前最好先试做一下 control 反应,以确定最佳的 cDNA 产物应置于-20℃保存。 当以 cDNA 为模板进行 PCR 之前,使用 RNaseH 处理 cDNA,可以提高 PCR 反应的灵敏度。
PDF 文件使用 "pdfFactory Pro" 试用版本创建
逆转录系列ห้องสมุดไป่ตู้
操作步骤-----------------------------------------------------------------------------------------------------------------------------------------------------------1 在冰浴的无菌离心管中配制下列混合物 1-5 μg RNA 1 μl Oligo(dT)15 或 Random primer, 补 RNase-free ddH2O 至 12.4 μl ; 2 进行变性退火反应 70℃ 温浴 5 min, 简短离心后冰浴 5 min; 3 在上述离心管中配制反转录反应液 4 μl 1 μl 1 μl 4 5 ×first-strand buffer , (10 mM each) , dNTPs
