片仔癀新英文版产品说明书(1-16)
英文药品说明书

英文药品说明书5. Interactions或drug Interactions(相互作用,或药物的交互作用):本项内容主要是介绍药物合用时的注意事项。
例11 Temaril tablets should not be administered with 4 hours of medications containing mgnesium,aluminium or iron salts as interference with absorption may occur.在使用了含镁、铝或铁盐的药物之后的4小时内不应使用环丙沙星片剂,因为可能影响吸收。
例12 Simultaneous consumption of alcohol can impair reaction time.e.g. in traffic or during operation of machines. At higher dosage of Elantan 20 wlth simultaneous administration of blood-pressure-lowering medicaments the effect of the latter can be potentiated.同时饮酒可能损害反应时间,例如驾驶车辆或操纵机器期间。
大量服用单硝酸异山梨醇的同时服用降血压药物,可能会增强后者的药效。
例13 Dormicum can enhance the central sedative effect of neuroleptics, tranquitizers, antidepressants, sleep-inducing drugs, analgesics and anesthetics.速眠安能增强神经抑制剂、安定剂、抗抑郁剂、催眠、镇静剂和麻醉剂的中枢神经镇静作用。
例14 Concomitant treatment with other vasodilators, calcium antagonists, betablockers, diuretics, antihypertensives, tricyclic antidepressants, major tranquilizers, and dihydroergotamine, as well asthe consumption of alcohol, may potentiate the blood pressure lowering effect of Nitroderm TTS.与其他药物,例如:血管扩张药、钙桔抗剂、B-受体阻断剂、利尿剂、抗高血压药、三环抗抑郁药、强镇静剂及二氢麦角胺合用,以及饮酒等,可加强硝酸甘油护心贴膏的降血压作用。
《英文药品说明书》课件模板

solution 溶液
tablets 片剂
derivative 衍生物
colo(u)rless 无色的 tasteless 无味的 liquid 液体
sterile 无菌的
powder 粉末
solid 固体
soluble 可溶的 molecular weight 分子量
常见的句型
例1.Folic acid is a yellowish to orange, crystalline powder; odourless or almost odourless. 叶酸是淡黄色至橙色结晶粉末,无臭或几乎无臭。
例 4 Nebcin is indicated for the treatment of the following infections caused by susceptible microorganisms. 乃柏欣适用于治疗下列由敏感细菌引 起的感染:
课后作业
丙磺舒(Benemid)被推荐用于治疗痛风及 在抗感染治疗时增加并延长青霉素类 (penicillins)的血浆浓度;
常见句型举例
例3. 已发现制霉菌素(Nystain)在肠道内可 抑制酵母菌(yeast)的生长。 Nystain has been found to inhibit the growth of yeast in the intestinal tract.
例4. 熊去氧胆酸的生物半衰期时3.5-5.8天 the biological half-life of ursodeoxycholic
be associated with in association with be combined with in combination with be compatible with in conjunction with concomitant with together with
复方片仔癀软膏(片仔癀)的说明书

复方片仔癀软膏(片仔癀)的说明书皮肤病的传染性是很强的,对于自身的健康也不利,许多皮肤病是由于过敏引起的,经常洗澡也不一定能保证皮肤健康。
因此皮肤病的药物就至关重要了,复方片仔癀软膏(片仔癀)就是目前治疗皮肤病最好的药物,它的功效有哪些您知道吗?它的具体用药原则和效果我们在如下内容为您介绍。
【药品名称】通用名称:复方片仔癀软膏商品名称:复方片仔癀软膏(片仔癀)拼音全码:FuFangPianZihuangRuanGao(PianZihuang)【主要成份】片仔癀粉、蛇药片。
【性状】本品为浅棕黄色的软膏,具特殊的油腻气。
【适应症/功能主治】清热,解毒,止痛。
用于带状疱疹、单纯疱疹、脓疱疮、毛囊炎、痤疮。
【规格型号】10g*1支【用法用量】外用,涂于患处,一日2~3次。
【不良反应】尚不明确【禁忌】尚不明确【注意事项】1.忌食烟、酒及辛辣、油腻食物。
2.孕妇慎用。
3.本品为外用药,禁止内服。
4.用药后局部出现皮疹等过敏现者停用。
5.热毒较重伴有恶寒发热者应去医院就诊。
6.对局部病变不宜挑破,切忌挤压。
7.对本品过敏者禁用,过敏体质者慎用。
8.本品性状发生改变时禁止使用。
9.儿童必须在成人监护下使用。
10.请将本品放在儿童不能接触的地方。
11.如正在使用其他药品,使用本品前请咨询医师或药师。
【药物相互作用】如与其他药物同时使用可能会发生药物相互作用,详情其咨询医师或药师。
【包装】药用铝管,10克×1支/盒。
【有效期】24 月【批准文号】国药准字Z35020234【生产企业】漳州片仔癀药业股份有限公司看完上述对于复方片仔癀软膏(片仔癀)的介绍,您现在对于复方片仔癀软膏(片仔癀)这种药物有了一定的了解了吧?皮肤病的传染性比较强,日常生活中大家一定要勤洗澡常换衣,毕竟预防大于治疗。
并且治疗皮肤病一定要选好药,盲目治疗只会雪上加霜。
中成药片仔癀详细说明

5、片仔癀治疗痈疽疔疮
痈、疽、疔、疮
痈:分内痈、外痈。外痈即西医所称的生于体表部皮肉之间的急性化脓性炎症,局部光软无头,红肿热痛,易溃,易敛;内痈指生于脏腑的脓肿,如肝痈、肺痈、肠痈。
疽:分有头疽和无头疽。有头疽:初起有粟米状脓头,红肿热痛,易向深部及周围扩散。溃破后,状如蜂窝,项背部多见,常见于成年人及老年人。无头疽:发于骨骼及关节间,患部漫肿皮色不变,疼痛彻骨,难消难溃难敛,溃后多损伤筋骨,是一种骨与关节间的急性化脓性疾病。
【片仔癀使用方法】
急性肝炎
0.6g/次,3次/天 2周为一个疗程
胆囊炎
0.6g/次,3次/天 2周为一个疗程
慢性肝炎
0.6g/次,3次/天 3个月为一个疗程,黄疸指数(血清胆红素值)、谷丙转氨酶或谷草转氨酶、血蛋白超过正常范围者继续服用
脂肪肝
0.6g/次,2次/天 2周为一个疗程,服用两个疗程以上
酒精肝
2、片仔癀早期说明书有“无病每旬可服一、二次,养气行血,百病不侵”,当然由于当今的压力和环境污染比以前严重得多,因此旬服一次会偏少以致起不到作用,建议三或五天服一次;
3、也可如现在有些人的服用片仔癀的方法――春秋各一粒,保你全年健康,因春是木,对应肝,若春天养肝可收到事半功倍的效果,而秋是金,金克木,因此秋天养肝可使肝木免受到肺金的伤害。
片仔癀详细说明
【片仔癀药业战略部署】
片仔癀药业秉承“顺应新常态、打造新产品、拓展新市场”的理念,建设以传统中药生产为核心,以保健药品、保健食品、功能饮料和特色功效化妆品、日化产品为两翼,以药品流通,电子商务为补充的大健康产业集团,把片仔癀打造成拥有厚重中医药文化价值,国内一流的健康养生品牌。
片仔癀新英文版产品说明书(116)

英文版产品说明书片仔癀Pien Tze Huang[Medicine name]Proprietary name: Pien Tze HuangChinese pronunciation:Pianzaihuang[Ingredients] Calculus Bovis, Moschus, Radix Notoginsing, Snake’s gall,etc. [Description]It’s an oblate-like mass, with an oblate circle on the surface. The surface is brownish-yellow or greyish-brown, with thin serried striation and mould speckles. Texture is hard, uneasily broken. Section is slightly rough, with even brown color and a small amount of mycelia occasionally. Powder is brownish-yellow or thin brownish-yellow. It smells a bit fragrant and tastes bitter and a bit sweet.[Main functions and actions] Relieving internal heat and deleting toxin, cooling blood and reducing stasis, relieving swelling and stopping pain. Used for treating acute, chronic or viral hepatitis resulting from internal heat or blood stasis, ulcer and pyogenic infections, unknown galls, injuries from falls, fractures, contusions and strains as well as all kinds of inflammations. [Specification] 3 grams per piece[Usage and Dosage]Orally, 0.6 grams a time. for children aged under 8 ,take 0.15-0.3 gram each time. To be taken 2-3 times a day. For external use, grind into powder and mixed it unfiformity with boiled water or vinegar , take suitable quantity and apply to the affected part for several times one day ,keep wetness. or adhere to the instructions of the physician.[Adverse Reactions] Not known[Points for attention] Not to be applied by pregnant women[Precautions] Not known[Storage] Preserve in tightly closed containers, protected from moisture. [Packing]Aluminium and plastic composite- film,3 grams×1 piece/box[Period of Validity] Five years[Approval number] State medical permitment number. Z[Manufactured] By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.片仔癀胶囊Pien Tze Huang Capsule[Medicine Name]Proprietary name : Pien Tze Huang CapsuleChines pronunciation:Pianzaihuang Jiaonang[Ingredients] Pien Tze Huang[Description]Capsule containing brownish-yellow grain and powder. It smellsfragrant and tastes bitter and a bit sweet.[Main functions and actions]Clearing away heat and toxic materials, relieving inflammation and arresting pain, promoting blood circulation to dispel blood stasis. Used in virus hepatitis, boils, inflammatory process of tissue of unknown origin, injury and kinds of inflammatory lesions.[Specification] 0.3 gram per capsule[Usage and Dosage] Orally, 2 pieces a time, one piece a time for children aged 1-5, 3 times a day or under doctor advice[Adverse Reactions] Not known[Points for attention] Not to be applied by pregnant women[Precautions] Not known[Storage] Preserve in tightly closed containers[Packing]Aluminium and plastic packing ,0.3 gram×6 pieces /box[Period of Validity] Three years[Approval number] State medical permitment number. Z.[Manufactured] By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.茵胆平肝胶囊Yindan Pinggan Capsule[Medicine name]Proprietary name: Yindan Pinggan CapsuleChinese pronunciation:Yindan Pinggan Jiaonang[Ingredients] Herba Aryemisiae Scopariae,Radix Gentianae, Pulvis Fellis Suis, Fructus Gardeniae,Radix Scutellariae,Radix Angelicae Sinensis,Radix Paeoniae Alba, Radix Glycyrrhizae[Description] Capsule containing brownish-yellow grain .It tastes very bitter [Functions]Relieving internal heat and deleting moisture, It has significant effectiveness for acute jaundice hepatitis and chronic hepatitis.[Specification] 0.5 gram per capsule[Usage and Dosage] Orally, 2 capsules a time, 3 times a day .[Adverse Reactions] Not known[Contraindications]This medicine is not for the patient whose gallbladder vessel was choked completely .[Precautions] Not known[Storage] Preserve in tightly closed containers, protected from moisture, stored in a cool place.[ Packing]Aluminium and plastic composite packing,0.5 gram×10 pieces×2 sheets /box or medicine plastic bottle , 0.5 gram×36 pieces /bottle[Period of Validity] Three years[Approval number] State medical permitment number. Z[Manufactured] By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.复方片仔癀软膏说明书Unguentum Pien Tze Huang compositum OintmentPlease read the instruction carefully and use it according to the direction or buy and use it under chemist’s suggestion.[Medicine name]Proprietary name: Unguentum Pien Tze Huang compositum OintmentChinese pronunciation:Fufang Pianzaihuang Ruangao[Ingredients] The power of Pien Tze Huang ,Antivenom tablet[Description] Light brown-yellow ointment, it has special oleaginous taste.[Main action] Relieving internal heat, deleting toxin and stopping pain. Used for viral or bacillary skin disease, such as zosters, herpes simplex, impetigo, folliculitis and acne etc.[Specification] 10 gram per tube/box .[Usage and Dosage]External use only,daub on the affected part,2~3 times daily. [Adverse Reactions] Not known[Contraindications] Not known[Precautions]1.Smoke,alcohol,piguancy and fat food are forbidden.2.Pregnant women should use with caution.3.This medicine is for external use only ant it should not be taken orally.4.Stop using if part anaphylactoid reaction such as rash occur after used this medicine.5. Go to see a doctor if toxic heat strictness with chill fever happens.6.Don’t stave or extrusion when part pathological changes occur.7.It should not be used for patients who have anaphylatoid reaction with this medicine, and irritability corporeity should used with caution.8.It should not be used when the character of this medicine has changed.9.Children administere under the supervision by adults.10.It should be kept out of reach of children.11.If in process of using other medicine, please consult with a physician or chemist before use this medicine.[Medicine reaction] If used other medicines at the same time, the medicine reaction may occur, please consult with physician or chemist the correlative detail. [Storage] Preserve in tightly closed containers, stored in a cool and dry place [Packing] Aluminum tube, 10 gram per tube.[Period of Validity] Two years[Approval number] State medical permitment number. Z[Manufactured] By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.复方片仔癀含片Compound Pien Tze Huang Buccal Tablet。
Pepperl+Fuchs NCB5-18GM40-N0-V1 产品说明书

12R e l e a s e da t e : 2 0 1 7 -01 -2 4 1 5 :34 D a t e o f i s s u e : 2 0 1 7 -0 1 -2 4 1 8 1 1 0 7 _ e n g . x m lPinoutL+L-13421 BN2 BUWire colors in accordance with EN 60947-5-6(brown)(blue)3R e l e a s e d a t e : 2017-01-24 15:34D a t e o f i s s u e : 2017-01-24181107_e n g .x m lInstructionManual electrical apparatus for hazardous areas Device category 1Gfor use in hazardous areas with gas, vapour and mist EC-T ype Examination CertificateCE marking ATEX marking ¬ II 1G Ex ia IIC T6…T1 G a The Ex-related marking can also be printed on the enclosed label.Standards EN 60079-0:2012+A11:2013 EN 60079-11:2012 Ignition protection "Intrinsic safety" Use is restricted to the following stated conditions Appropriate typeNCB5-18GM...-N0...Effective internal inductivity C i≤ 95 nF ; a cable length of 10 m is considered.Effective internal inductance L i ≤ 100 µH ; a cable length of 10 m is considered.G eneralThe apparatus has to be operated according to the appropriate data in the data sheet and in this instruction manual.The EU-type examination certificate has to be observed. The special conditions must be adhered to!The ATEX directive and therefore the EU-type examination certificates apply in gen-eral only to the use of electrical apparatus under atmospheric conditions.The use in ambient temperatures of > 60 °C was tested with regard to hot surfaces by the mentioned certification authority.If the equipment is not used under atmospheric conditions, a reduction of the permis-sible minimum ignition energies may have to be taken into consideration.Ambient temperatureDetails of the correlation between the type of circuit connected, the maximum per-missible ambient temperature, the temperature class, and the effective internal reac-tance values can be found on the EC-type examination certificate. Note: Use the temperature table for category 1 The 20 % reduction in accordance with EN 1127-1 has already been applied to the temperature table for category 1.Installation, commissioningLaws and/or regulations and standards governing the use or intended usage goal must be observed.The intrinsic safety is only assured in connection with an appropriate related appara-tus and according to the proof of intrinsic safety.The associated apparatus must satisfy the requirements of category ia.Due to the possible danger of ignition, which can arise due to faults and/or transient currents in the equipotential bonding system, galvanic isolation of the power supply and signal circuit is preferable. Associated apparatus without electrical isolation must only be used if the appropriate requirements of IEC 60079-14 are met. If the Ex-related marking is printed only on the supplied label, then this must be attached in the immediate vicinity of the sensor. The sticking surface for the label must be clean and free from grease. The attached label must be legible and indelible, including in the event of possible chemical corrosion.Maintenance No changes can be made to apparatus, which are operated in hazardous areas.Repairs to these apparatus are not possible.Special conditionsThe connecting parts of the sensor must be set up in such a way that degree of pro-tection IP20, in accordance with lEC 60529, is achieved as a minimum.Protection from mechanical dangerWhen using the device in a temperature range of -60 °C to -20 °C, protect the sensor against the effects of impact by installing an additional enclosure.The information regarding the minimum ambient temperature for the sensor as pro-vided in the datasheet must also be observed.Electrostatic chargeElectrostatic charges must be avoided on the mechanical housing components. Dangerous electrostatic charges on the mechanical housing components can be avoided by incorporating these in the equipotential bonding.4Releasedate:217-1-2415:34Dateofissue:217-1-2418117_eng.xml Instruction Manual electrical apparatus for hazardous areasDevice category 2G for use in hazardous areas with gas, vapour and mistEC-T ype Examination CertificateCE markingATEX marking ¬ II 1G Ex ia IIC T6…T1 G aThe Ex-significant identification is on the enclosed adhesive labelStandards EN 60079-0:2012+A11:2013 EN 60079-11:2012 Ignition protection "Intrinsic safety"Use is restricted to the following stated conditionsAppropriate type NCB5-18GM...-N0...Effective internal inductivity C i≤ 95 nF ; a cable length of 10 m is considered.Effective internal inductance L i≤ 100 µH ; a cable length of 10 m is considered.G eneral The apparatus has to be operated according to the appropriate data in the data sheetand in this instruction manual. The EU-type examination certificate has to beobserved. The special conditions must be adhered to!The ATEX directive and therefore the EU-type examination certificates apply in gen-eral only to the use of electrical apparatus under atmospheric conditions.The use in ambient temperatures of > 60 °C was tested with regard to hot surfacesby the mentioned certification authority.If the equipment is not used under atmospheric conditions, a reduction of the permis-sible minimum ignition energies may have to be taken into consideration.Maximum permissible ambient temperature T amb Details of the correlation between the type of circuit connected, the maximum per-missible ambient temperature, the temperature class, and the effective internal reac-tance values can be found on the EC-type examination certificate.Installation, commissioning Laws and/or regulations and standards governing the use or intended usage goalmust be observed. The intrinsic safety is only assured in connection with an appro-priate related apparatus and according to the proof of intrinsic safety.If the Ex-related marking is printed only on the supplied label, then this must beattached in the immediate vicinity of the sensor. The sticking surface for the labelmust be clean and free from grease. The attached label must be legible and indeli-ble, including in the event of possible chemical corrosion.Maintenance No changes can be made to apparatus, which are operated in hazardous areas.Repairs to these apparatus are not possible.Special conditions The connecting parts of the sensor must be set up in such a way that degree of pro-tection IP20, in accordance with lEC 60529, is achieved as a minimum.Protection from mechanical danger When using the device in a temperature range of -60 °C to -20 °C, protect the sensoragainst the effects of impact by installing an additional enclosure. The informationregarding the minimum ambient temperature for the sensor as provided in thedatasheet must also be observed.Electrostatic charge Electrostatic charges must be avoided on the mechanical housing components.Dangerous electrostatic charges on the mechanical housing components can beavoided by incorporating these in the equipotential bonding.5R e l e a s e d a t e : 2017-01-24 15:34D a t e o f i s s u e : 2017-01-24181107_e n g .x m lInstructionManual electrical apparatus for hazardous areas Device category 3G (ic) for use in hazardous areas with gas, vapour and mist CertificateCE marking ATEX marking ¬ II 3G Ex ic IIC T6…T1 GcThe Ex-significant identification is on the enclosed adhesive labelStandardsEN 60079-0:2012+A11:2013 EN 60079-11:2012 Ignition protection category "ic" Use is restricted to the following stated conditions Effective internal inductivity C i≤ 95 nF ; a cable length of 10 m is considered.Effective internal inductance L i≤ 100 µH ; A cable length of 10 m is considered.G eneralThe apparatus has to be operated according to the appropriate data in the data sheet and in this instruction manual. The data stated in the data sheet are restricted by this operating instruction!The special conditions must be observed!The ATEX Directive applies only to the use of apparatus under atmospheric condi-tions.If you use the device outside atmospheric conditions, consider that the permissible safety parameters should be reduced.Installation, commissioningLaws and/or regulations and standards governing the use or intended usage goal must be observed. The sensor must only be operated with energy-limited circuits, which satisfy the requirements of IEC 60079-11. The explosion group complies with the connected, supplying, power limiting circuit. If the Ex-relevant identification is printed exclusively on the adhesive label provided, this label must be affixed in the immediate vicinity of the sensor! The background surface to which the adhesivelabel is to be applied must be clean and free from grease! The applied label must be dura-ble and remain legible, with due consideration of the possibility of chemical corro-sion!Maintenance No changes can be made to apparatus, which are operated in hazardous areas.Repairs to these apparatus are not possible.Special conditionsfor Pi=34 mW, Ii=25 mA, T6 55 °C (131 °F) for Pi=34 mW, Ii=25 mA, T5 55 °C (131 °F) for Pi=34 mW, Ii=25 mA, T4-T1 55 °C (131 °F) for Pi=64 mW, Ii=25 mA, T6 55 °C (131 °F) for Pi=64 mW, Ii=25 mA, T5 55 °C (131 °F) for Pi=64 mW, Ii=25 mA, T4-T1 55 °C (131 °F) for Pi=169 mW, Ii=52 mA, T6 52 °C (125.6 °F) for Pi=169 mW, Ii=52 mA, T5 52 °C (125.6 °F) for Pi=169 mW, Ii=52 mA, T4-T1 52 °C (125.6 °F) for Pi=242 mW, Ii=76 mA, T6 44 °C (111.2 °F) for Pi=242 mW, Ii=76 mA, T5 44 °C (111.2 °F) for Pi=242 mW, Ii=76 mA, T4-T1 44 °C (111.2 °F)Protection from mechanical dangerThe sensor must not be mechanically damaged.When used in the temperature range below -20 °C the sensor should be protected from knocks by the provision of an additional housing.Electrostatic charge Electrostatic charges must be avoided on the mechanical housing components. Dangerous electrostatic charges on the mechanical housing components can be avoided by incorporating these in the equipotential bonding.Connection partsThe connection parts are to be installed, such that a minimum protection class of IP20 is achieved, in accordance with IEC 60529.6Releasedate:217-1-2415:34Dateofissue:217-1-2418117_eng.xml Instruction Manual electrical apparatus for hazardous areasDevice category 1D for use in hazardous areas with combustible dustEC-T ype Examination CertificateCE markingATEX marking ¬ II 1D Ex ia IIIC T135°C Da The Ex-related marking can also be printed on theenclosed label.Standards EN 60079-0:2012+A11:2013 EN 60079-11:2012 Ignition protection "Intrinsic safety"Use is restricted to the following stated conditionsAppropriate type NCB5-18GM...-N0...Effective internal inductivity C i≤ 95 nF ; a cable length of 10 m is considered.Effective internal inductance L i≤ 100 µH ; a cable length of 10 m is considered.G eneral The apparatus has to be operated according to the appropriate data in the data sheetand in this instruction manual.The EU-type examination certificate has to be observed.The ATEX directive and therefore the EU-type examination certificates apply in gen-eral only to the use of electrical apparatus under atmospheric conditions.The use in ambient temperatures of > 60 °C was tested with regard to hot surfacesby the mentioned certification authority.If the equipment is not used under atmospheric conditions, a reduction of the permis-sible minimum ignition energies may have to be taken into consideration.Maximum permissible ambient temperature T amb Details of the correlation between the type of circuit connected, the maximum per-missible ambient temperature, the surface temperature, and the effective internalreactance values can be found on the EC-type-examination certificate.The maximum permissible ambient temperature of the data sheet must benoted, in addition, the lower of the two values must be maintained. Installation, commissioning Laws and/or regulations and standards governing the use or intended usage goalmust be observed.The intrinsic safety is only assured in connection with an appropriate related appara-tus and according to the proof of intrinsic safety.If the Ex-related marking is printed only on the supplied label, then this must beattached in the immediate vicinity of the sensor. The sticking surface for the labelmust be clean and free from grease. The attached label must be legible and indeli-ble, including in the event of possible chemical corrosion.Maintenance No changes can be made to apparatus, which are operated in hazardous areas.Repairs to these apparatus are not possible.Special conditions The connecting parts of the sensor must be set up in such a way that degree of pro-tection IP20, in accordance with lEC 60529, is achieved as a minimum.Protection from mechanical danger When using the device in a temperature range of -60 °C to -20 °C, protect the sensoragainst the effects of impact by installing an additional enclosure. The informationregarding the minimum ambient temperature for the sensor as provided in thedatasheet must also be observed.Electrostatic charge Electrostatic charges must be avoided on the mechanical housing components.Dangerous electrostatic charges on the mechanical housing components can beavoided by incorporating these in the equipotential bonding.Do not attach the nameplate provided in areas where electrostatic charge can buildup.。
少林正骨精(片仔癀)的说明书

少林正骨精(片仔癀)的说明书到了一定的岁数就容易患上风湿跌打方面的疾病,这是无法避免的。
中老年人很容易患上这种疾病,主要的原因是由于年轻的时候过于操劳所致。
因此,及时治疗风湿疾病是刻不容缓的。
药物治疗风湿疾病的效果很显著,关键要选对药选好药,只有这样才能更好的治愈风湿疾病,服用少林正骨精(片仔癀)就能帮助您很好的治愈风湿疾病。
【药品名称】通用名称:少林正骨精商品名称:少林正骨精(片仔癀)拼音全码:ShaoLinZhengGuJing(PianZaiHuang)【主要成份】接骨仙桃草、当归、五加皮、独活、羌活、三棱(醋制)、莪术(醋制)、土鳖虫、艾叶、延胡索(醋制)、花椒、寻骨风、血竭、乳香、伸筋草、活络草、苏木、薄荷脑、樟脑、冰片。
【性状】本品为红棕色澄清液体;具特殊芳香气。
【适应症/功能主治】活血祛瘀,消肿止痛、祛风散寒。
用于跌打损伤、积瘀肿痛、腰肢麻木、风湿骨痛。
【规格型号】60ml【用法用量】外用。
取本品擦于患处,亦可沐浴时用。
每日数次。
【不良反应】尚不明确。
【禁忌】尚不明确。
【注意事项】孕妇慎用。
【药物相互作用】如与其他药物同时使用可能会发生药物相互作用,详情请咨询医师或药师。
【贮藏】密封,置阴凉干燥处。
【包装】药用塑料瓶包装,60ml×1瓶/盒。
【有效期】36 月【批准文号】国药准字Z35020235【生产企业】漳州片仔癀药业股份有限公司看完上面对于少林正骨精(片仔癀)的介绍,您对于该药物一定有了一个清晰的了解了吧?风湿疾病不像其他疾病那么恶劣,但是也是属于慢性疾病的范畴的,对生活影响大,因此及时治疗才是对身体负责的表现。
片仔癀

片仔癀处方来源闽卫药准字(86)25537。
剂型胶囊;丸剂药物组成麝香、牛黄、蛇胆、三七等。
功效:清热解毒,消肿止痛。
主治:治疗胁痛,黄疸,牙龈肿痛,咽喉肿痛,烫伤,灼伤,跌打损伤,蜂蛇咬伤,癥积,疔疮及无名肿毒等及急慢性病毒性肝炎,癌症等。
用法用量:丸剂:每丸重3g;胶囊剂:每粒0.3g,每瓶10粒。
内服,1-8岁1次0.5-0.3g,8岁以上1次0.6g,日2-3次。
外用,切成薄片冷开水调化,涂敷患处,1日数次,保持湿润。
如有伤口,应在患处周围敷之。
用药禁忌孕妇忌用。
创口上忌涂。
临床应用经观察的25例患者均是经院内外确诊的晚期大肠癌,其中男性17例,女性8例;年龄最大73岁,最小44岁,平均年龄58.5岁。
病变部位:直肠9例,乙状结肠6例,横结肠2例,回盲部4例,降结肠3例,结肠肝曲1例。
病理检查诊断:其中腺癌10例,高分化腺癌2例,中分化腺癌5例,低分化腺癌4例,粘液腺癌2例,乳头状腺癌2例。
25例患者中,已经手术者18例,未经手术者7例,患者在服用本方前,均因化疗毒副反应难以忍受而被迫中止者。
经用本方1疗程后作为统计对象,同时辅以一般西医支持疗法。
结果:发现本方对治疗大肠癌具有一定的疗效,并有待于在临床中进一步观察研究。
药理作用:主要有镇痛,抗炎,止血,抗应激,增强免疫功能,抗癌等作用。
1.镇痛:片仔癀0.6、1.2、2.4g/Kg灌胃,明显抑制冰醋酸引起的小鼠扭体反应,延长热板引起的小鼠痛反应潜伏期。
2.抗炎:片仔癀0.6、1.2、2.4g/kg灌胃,明显抑制二甲苯引起的小鼠耳肿胀,角叉菜胶引起的大鼠足肿胀和冰醋酸性小鼠腹膜炎性渗出。
对片仔癀中药物炮炙与否,其药理作用有别,炮炙者片仔癀的镇痛及抗炎作用强于未炮炙药物的片仔癀,外层显著优于核心。
3.镇静:片仔癀2.4g/kg灌胃明显抑制小鼠自主活动次数。
4.抗应激:片仔癀 0.6、1.2、2.4g/kg灌胃,明显延长小鼠常压耐缺氧时间,并有一定的抗疲劳和耐低温作用,2.4g/kg灌胃有明显的抗高温作用。
心舒宝片(片仔癀)的说明书

心舒宝片(片仔癀)的说明书
药物治疗心脑血管是一种比较普遍的方式,还有通过输液来疏通血管的方式,这些都是比较好的治疗方法,药店和医院里也有各种各样治疗心脑血管的药物,到底哪种治疗效果好呢,是广大患者都想知道的答案。
心舒宝片(片仔癀)是目前治疗心脑血管疾病效果最显著的药物,很多患者用过之后病情都有所减轻。
【药品名称】
通用名称:心舒宝片
商品名称:心舒宝片(片仔癀)
拼音全码:XinShuBaoPian(PianZiHuang)
【主要成份】山楂、丹参、郁金、白芍、刺五。
【性状】本品为褐色的片;味酸、微苦。
【适应症/功能主治】活血化瘀,益气止痛。
用于冠心病,气虚
血瘀引起的胸闷,心绞痛,以及高血压、高血脂、动脉硬化等。
【规格型号】0.5s*12s*2板
【用法用量】口服,一次1-2片,一日2次或遵医嘱,饭后服。
【不良反应】尚不明确。
【禁忌】尚不明确。
【注意事项】尚不明确。
【药物相互作用】如与其它药物同时使用可能会发生药物相互作用,详情请咨询药师或医师。
【贮藏】密封,置阴凉干燥处。
【包装】12片/盒*2板。
【有效期】36 月
【批准文号】国药准字Z35020241
【生产企业】漳州片仔癀药业股份有限公司
综上所述,就是关于心舒宝片(片仔癀)的各种药效和药性医生所做出的介绍,大家都了解了吗?如果您现在还在为心脑血管的治疗而烦恼,心舒宝片(片仔癀)就可以很好的帮您解决这个问题,最后祝所有的老年人都身体健康,生活开心!。
片仔癀简介

片仔癀简介目录•1拼音•2概述•3片仔癀的别名•4组成•5片仔癀的用法用量•6功能主治•7片仔癀药典标准o7.1品名o7.2性状o7.3鉴别o7.4检查▪7.4.1重量差异▪7.4.2干燥失重▪7.4.3其他o7.5含量测定▪7.5.1色谱条件与系统适用性试验▪7.5.2校正因子测定▪7.5.3测定法o7.6功能与主治o7.7用法与用量o7.8注意o7.9规格o7.10贮藏o7.11版本•8片仔癀中药部颁标准o8.1拼音名o8.2标准编号o8.3性状o8.4鉴别o8.5检查o8.6功能与主治o8.7用法与用量o8.8注意o8.9规格o8.10贮藏•9片仔癀说明书o9.1药品类型o9.2药品名称o9.3药品汉语拼音o9.4成份o9.5性状o9.6片仔癀的功能主治o9.7规格o9.8片仔癀的用法用量o9.9片仔癀的禁忌o9.10注意事项o9.11片仔癀与其它药物的相互作用o9.12备注•10参考资料•附:o1古籍中的片仔癀o*片仔癀相关药品说明书其它版本1拼音piàn zǎi huáng2概述片仔癀为明末京都太医秘方,出自《新编中成药手册》[1]。
《中华人民共和国药典》(2010年版)记载有此中成药的药典标准。
3片仔癀的别名八宝片仔癀[1]。
4组成麝香、牛黄、蛇胆、三七等[1]。
5片仔癀的用法用量丸剂,每丸3g;胶囊剂,每粒0.3g[1]。
一至八岁每服0.15~0.3g,八岁以上每服0.6g,一日2~3次[1]。
外用,冷开水调化,涂敷患处(创口上忌涂)[1]。
6功能主治功能清热解毒,消肿止痛[1]。
治急慢性肝炎,耳炎,眼炎,牙龈肿痛,咽喉肿痛,乳蛾,烫伤烧伤,金疮伤痛,挫伤扭伤,蜂蛇咬伤,疔疮,无名肿毒等[1]。
7片仔癀药典标准7.1品名片仔癀Pianzaihuang本品为牛黄、麝香、三七、蛇胆等药味经加工制成的锭剂。
7.2性状本品为类扁椭圆形块状,块上有一椭圆环。
表面棕黄色或灰褐色,有密细纹,可见霉斑。
Crafter’s Choice Peppermint Candy 香料油商品说明书
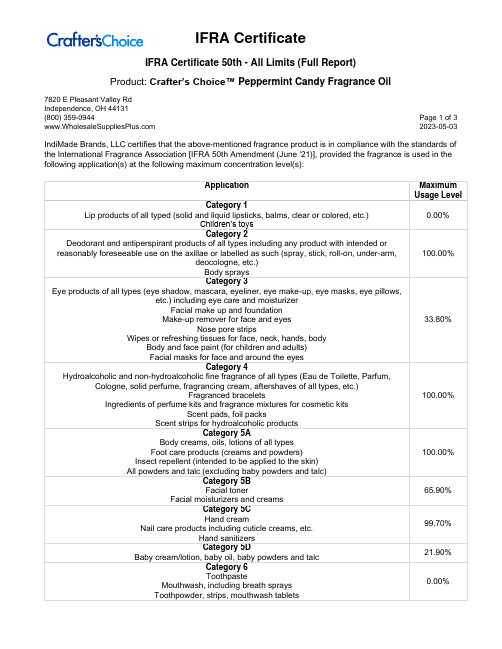
Product: Crafter’s Choice™ Peppermint Candy Fragrance Oil7820 E Pleasant Valley RdIndependence, OH 44131(800) 359-0944 Page 1 of 3 2023-05-03 IndiMade Brands, LLC certifies that the above-mentioned fragrance product is in compliance with the standards of the International Fragrance Association [IFRA 50th Amendment (June '21)], provided the fragrance is used in the following application(s) at the following maximum concentration level(s):Product: Crafter’s Choice™ Peppermint Candy Fragrance Oil7820 E Pleasant Valley RdIndependence, OH 44131(800) 359-0944 Page 2 of 3 2023-05-03Product: Crafter’s Choice™ Peppermint Candy Fragrance Oil7820 E Pleasant Valley RdIndependence, OH 44131(800) 359-0944 Page 3 of 3 2023-05-03For all other applications, or use at higher concentration levels, a new evaluation will be required.The IFRA standards regarding use restrictions are based on safety assessments by the Research Institute for Fragrance Materials (RIFM) Expert Panel (REXPAN) and are enforced by the IFRA Scientific Committee. Evaluation of individual fragrance materials is made according to the safety standards contained in the relevant section of the IFRA Code of Practice.It is the ultimate responsibility of the customer to ensure the safety of the final product containing this fragrance, by further testing, if necessary.The above-mentioned fragrance product contains ingredients which are NOT considered GRAS, Generally Regarded as Safe as a Flavor Ingredient.。
片仔癀胶囊(片仔癀)的说明书

片仔癀胶囊(片仔癀)的说明书随着皮肤病的发病率越来越高,也出现了很多治疗皮肤病的药物,所以很多患者在如何选择药物上比较头疼。
如果药物选择不当,不仅没有效果,甚至会使病情加重。
但是自从出现了片仔癀胶囊(片仔癀)这种治疗皮肤病的药物,那么皮肤病的治疗就不是问题了。
【药品名称】通用名称:片仔癀胶囊商品名称:片仔癀胶囊(片仔癀)拼音全码:PianZiHuangJiaoNang(PianZiHuang)【主要成份】片仔癀。
【性状】本品为胶囊剂,内容物为棕黄色的颗粒及细粉;气香,味苦、微甘。
【适应症/功能主治】清热解毒,消炎止痛,活血化瘀。
用于急、慢性病毒性肝炎,痈疽疔疮,无名肿毒,跌打损伤及各种炎症。
【规格型号】0.3g*6s【用法用量】口服,一次2粒,一至五岁儿童一次1粒;一日3次,或遵医嘱。
【不良反应】尚不明确。
【禁忌】孕妇忌服。
【注意事项】孕妇忌服。
【药物相互作用】如与其他药物同时使用可能会发生药物相互作用,详情请咨询医师或药师。
【药理毒理】从片仔癀的合理组方,综合起来能驱除湿热、热毒、湿毒、瘀血等多种邪气,使邪去则正安;淤去则血脉通畅,诸病皆去。
【贮藏】密封。
【包装】0.3g*6s/盒。
【有效期】36 月【批准文号】国药准字Z35020242【生产企业】漳州片仔癀药业股份有限公司以上是医生针对片仔癀胶囊(片仔癀)的各种药效和药性做出的详细介绍,现在患有皮肤病的患者们是不是如同找到了救星一般高兴呢?其实皮肤病并不可怕,可怕的是不重视,不治疗,最后愈演愈烈。
选择片仔癀胶囊(片仔癀),远离皮肤疾病!。
片仔癀药品说明书

片仔癀
药品名称:
通用名称:片仔癀汉语拼音:PIANZAIHUANG。
成份:
枇杷叶、苦杏仁、川贝母、麦冬、地黄、甘草、桔梗、薄荷。
辅料为蔗糖、防腐剂。
性状:
本品为类扁椭圆形块状,块上有一椭圆环。
表面棕黄色或灰褐色,有密细纹,可见霉斑。
质坚硬,难折断。
折断面微粗糙,呈棕褐色,色泽均匀,偶见少量菌丝体。
粉末呈棕黄色或淡棕黄色,气微香,味苦、微甘。
功能主治:
清热解毒,凉血化瘀,消肿止痛。
用于痈疽疔疮,无名肿毒,跌打损伤。
规格:
每粒重3克。
用法用量:
口服,每次0.6克,8岁以下儿童每次0.15克~0.3克,每日2~3次;外用研末用冷开水或食醋少许调匀涂在患处,每日数次,常保持湿润。
禁忌:
孕妇忌服。
注意事项:
1.忌食辛辣、油腻食物。
2.服用3天后症状无改善,或服药期间伴有恶寒发热等全身症状者,应到医院就诊。
3.对局部病变切忌碰撞、挤压。
4.局部病灶红肿热痛反应剧烈,初起疮顶即有多个脓头者均应到医院就诊。
5.对本品过敏者禁用,过敏体质者慎用。
6.本品性状发生改变时禁止使用。
7.儿童必须在成人监护下使用。
8.请将本品放在儿童不能接触的地方。
9.如正在使用其他药品,使用本品前请咨询医师或药师。
药物相互作用:
如与其他药物同时使用可能会发生药物相互作用,详情请咨询医师或药师。
执行标准:
部标十八册。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
英文版产品说明书片仔癀Pien Tze Huang[Medicine name]Proprietary name: Pien Tze HuangChinese pronunciation:Pianzaihuang[Ingredients] Calculus Bovis, Moschus, Radix Notoginsing, Snake’s gall,etc. [Description]It’s an oblate-like mass, with an oblate circle on the surface. The surface is brownish-yellow or greyish-brown, with thin serried striation and mould speckles. Texture is hard, uneasily broken. Section is slightly rough, with even brown color and a small amount of mycelia occasionally. Powder is brownish-yellow or thin brownish-yellow. It smells a bit fragrant and tastes bitter and a bit sweet.[Main functions and actions] Relieving internal heat and deleting toxin, cooling blood and reducing stasis, relieving swelling and stopping pain. Used for treating acute, chronic or viral hepatitis resulting from internal heat or blood stasis, ulcer and pyogenic infections, unknown galls, injuries from falls, fractures, contusions and strains as well as all kinds of inflammations. [Specification] 3 grams per piece[Usage and Dosage]Orally, 0.6 grams a time. for children aged under 8 ,take 0.15-0.3 gram each time. To be taken 2-3 times a day. For external use, grind into powder and mixed it unfiformity with boiled water or vinegar , take suitable quantity and apply to the affected part for several times one day ,keep wetness. or adhere to the instructions of the physician.[Adverse Reactions] Not known[Points for attention] Not to be applied by pregnant women[Precautions] Not known[Storage] Preserve in tightly closed containers, protected from moisture. [Packing]Aluminium and plastic composite- film,3 grams×1 piece/box[Period of Validity] Five years[Approval number] State medical permitment number. Z35020243 [Manufactured] By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.片仔癀胶囊Pien Tze Huang Capsule[Medicine Name]Proprietary name : Pien Tze Huang CapsuleChines pronunciation:Pianzaihuang Jiaonang[Ingredients] Pien Tze Huang[Description]Capsule containing brownish-yellow grain and powder. It smells fragrant and tastes bitter and a bit sweet.[Main functions and actions]Clearing away heat and toxic materials, relieving inflammation and arresting pain, promoting blood circulation to dispel blood stasis. Used in virus hepatitis, boils, inflammatory process of tissue of unknown origin, injury and kinds of inflammatory lesions.[Specification] 0.3 gram per capsule[Usage and Dosage] Orally, 2 pieces a time, one piece a time for children aged 1-5, 3 times a day or under doctor advice[Adverse Reactions] Not known[Points for attention] Not to be applied by pregnant women[Precautions] Not known[Storage] Preserve in tightly closed containers[Packing]Aluminium and plastic packing ,0.3 gram×6 pieces /box[Period of Validity] Three years[Approval number] State medical permitment number. Z35020242. [Manufactured] By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.茵胆平肝胶囊Yindan Pinggan Capsule[Medicine name]Proprietary name: Yindan Pinggan CapsuleChinese pronunciation:Yindan Pinggan Jiaonang[Ingredients] Herba Aryemisiae Scopariae,Radix Gentianae, Pulvis Fellis Suis, Fructus Gardeniae,Radix Scutellariae,Radix Angelicae Sinensis,Radix Paeoniae Alba, Radix Glycyrrhizae[Description] Capsule containing brownish-yellow grain .It tastes very bitter [Functions]Relieving internal heat and deleting moisture, It has significant effectiveness for acute jaundice hepatitis and chronic hepatitis.[Specification] 0.5 gram per capsule[Usage and Dosage] Orally, 2 capsules a time, 3 times a day .[Adverse Reactions] Not known[Contraindications]This medicine is not for the patient whose gallbladder vessel was choked completely .[Precautions] Not known[Storage] Preserve in tightly closed containers, protected from moisture, stored in a cool place.[ Packing]Aluminium and plastic composite packing,0.5 gram×10 pieces×2 sheets /box or medicine plastic bottle , 0.5 gram×36 pieces /bottle[Period of Validity] Three years[Approval number] State medical permitment number. Z35020240 [Manufactured] By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.复方片仔癀软膏说明书Unguentum Pien Tze Huang compositum OintmentPlease read the instruction carefully and use it according to the direction or buy and use it under chemist’s suggestion.[Medicine name]Proprietary name: Unguentum Pien Tze Huang compositum OintmentChinese pronunciation:Fufang Pianzaihuang Ruangao[Ingredients] The power of Pien Tze Huang ,Antivenom tablet[Description] Light brown-yellow ointment, it has special oleaginous taste.[Main action] Relieving internal heat, deleting toxin and stopping pain. Used for viral or bacillary skin disease, such as zosters, herpes simplex, impetigo, folliculitis and acne etc.[Specification] 10 gram per tube/box .[Usage and Dosage]External use only,daub on the affected part,2~3 times daily. [Adverse Reactions] Not known[Contraindications] Not known[Precautions]1.Smoke,alcohol,piguancy and fat food are forbidden.2.Pregnant women should use with caution.3.This medicine is for external use only ant it should not be taken orally.4.Stop using if part anaphylactoid reaction such as rash occur after used this medicine.5. Go to see a doctor if toxic heat strictness with chill fever happens.6.Don’t stave or extrusion when part pathological changes occur.7.It should not be used for patients who have anaphylatoid reaction with this medicine, and irritability corporeity should used with caution.8.It should not be used when the character of this medicine has changed.9.Children administere under the supervision by adults.10.It should be kept out of reach of children.11.If in process of using other medicine, please consult with a physician or chemist before use this medicine.[Medicine reaction] If used other medicines at the same time, the medicine reaction may occur, please consult with physician or chemist the correlative detail. [Storage] Preserve in tightly closed containers, stored in a cool and dry place [Packing] Aluminum tube, 10 gram per tube.[Period of Validity] Two years[Approval number] State medical permitment number. Z35020234 [Manufactured] By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.复方片仔癀含片Compound Pien Tze Huang Buccal Tablet[Medicine name]Proprietary name:Compound Pien Tze Huang Buccal TabletChinese pronunciation:Fufang Pianzaihuang Hanpian[Ingredients]Herba Wedeliae,Herba Sarcandrae,Radix Scrophulariae,Radix Ophiopogonis, Radix Glycyrrhizae, The power of Pien Tze Huang, Mentholum. [Description]It is film-coated tablets with slightly yellow-brown inside, it smells aroma, tastes sweet and cold.[Functions and actions] Relieving internal heat and deleting toxin, benefiting throat and stopping pain, producing fluid and moistening throat. Used for acute and chronic faucitis resulted from upward invasion of wind-fire and excessive heat in lung and stomach.[Specification] 0.5 gram per tablet[Usage and Dosage] For buccal use, 2 pieces a time, 5 times daily.[Adverse Reactions] Not known[Contraindications]Not be applied by pregnant women[Precautions] Not known[Storage] Preserve in tightly closed containers.[ Packing] Aluminium and plastic composite packing , 0.5 gram×12 pieces×2 sheets / boxes.[Period of Validity] Two years[Administer standard] National medicine standard WS-5047(B-0047)-2005 [Approval number] State medical permitment number. Z20050066 [Manufactured] By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.复方片仔癀痔疮软膏Pien Tze Huang Hemorrhoids Ointment Compositum [Medicine Name]Proprietary name : Pien Tze Huang Hemorrhoids Ointment CompositumChinese pronunciation: Fufang Pianzaihuang Zhichuang Ruangao[Ingredients]The power of Pien Tze Huang, The power of Margarita, Succinum, Borneolum Syntheticum[Description] Light brown-yellow ointment, it smells fragrant, and feel cool . [Functions and actions]Relieving internal heat and deleting toxin, dispelling stasis and easing pain, stopping bleeding and eliminating hemorrhoid. Used for internal, external or mixed hemorrhoid .[Specification]10 grams per piece.[Dosage and usage]External use only.Take suitable quantity and apply to anus or the affected part, 2~3 times a day.[Adverse Reactions] Not known[Points for attention] Not to be applied by pregnant women[Precautions] Not known[Storage] Preserve in tightly closed containers, stored in a cool place.[Packing]Aluminium tube,10 grams/tube[Period of Validity][Administer standard] National medicine standard WS-5002(B-0002)-2006 [Approval number] State medical permitment number. Z20060001 [Manufactured] By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.小柴胡颗粒Xiaochaihu GranulePlease read the instruction carefully and use it according to the direction or buy and use it under chemist’s suggestion.[Medicine name]Proprietary name: Xiaochaihu GranuleChinese pronunciation:Xiaochaihu Keli[Ingredients]Radix Bupleuri,Radix Scutellariae, Rhizoma Pinelliae(processed with ginger), Radix Codonopsis, Rhizoma Zingiberis Recens, Radix Glycyrrhizae, Fructus Jujubae[Description] Brown-yellow granule, it tastes tasteless and slightly pungent. [Functions and actions]Diaphoresis, dredging liver and regulating stomach . it used for alternative chill and heat, chest tightness, being perturbed and sick ,bitter mouth and non-spittle.[Specification] 4 gram each bag[Usage and Dosage]Take after it is dissolved in boiled water, three times a day and 1~2 bags a time.[Adverse Reactions] Not known[Contraindications]Not known[Precautions]1.Smoke, alcohol, piquancy ,cold, crude, fat foods are forbidden.2.Do not used the restorative patent medicine at one time when use this medicine.3.It’s not suitable for patients with chill.4.Patients with high blood pressure, cardiopathy, nephropathy, edema should use under physician’s suggestion.5.Children, pregnant women, women in lactation, oldness and infirmness should use under physician’s suggestion.6. Fever heat patient whose body temperature exceeds 38.5℃should go to see a doctor.7. The patient whose symptom haven’t been catabatic after used this medicine for 3 days should go to see a doctor.8.It should not be used in patient who has anaphylatoid reaction with this medicine, and irritability corporeity should be used with caution.9. It should not be used when the character of this medicine has changed.10. Children should be administered under the supervision of adults.11. It should be kept out of reach of children.12.If in process of using other medicine, please consult with physician or chemist before use this medicine.[Medicine reaction] If used other medicines at the same time, the medicine reaction may occur, please consult physician or chemist the correlative detail.[Storage] Preserve in tightly closed containers[Packing]10 grams per bag(with sugar),4 gram s×6 bags/box[Period of Validity] Two years[Administer standard] PHARMACOPOEIA OF THE PEOPLES’S REPUBLIC OF CHINA (2005 V olume I )[Approval number] State medical permitment number. Z35020728 [Manufactured] By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.小青龙颗粒Xiaoqinglong GranuleDo please read the instruction carefully and use it according to the direction or buy and use it by physician’s suggestion.[Medicine name]Proprietary name: Xiaoqinglong GranuleChinese pronunciation: Xiaoqinglong Keli[Ingredients]Herab Ephedrae, Ramulus Cinnamomi, Rhizoma Zingiberis, Radix Asari , Fructus Schisandrae , Radix Paeoniae Alba[Description]Light-brown to brown granule, or grey to light-brown, it smells slightly fragrant tastes sweet and slightly pungent.[Functions and actions]To release the exterior to resolve retained fluid, and relieve coughing, subside asthma. It’s used for foul chill, fever, adiaphoresis, asthma or difficulty in breathing and coughing with expectoration of thin phlegm due to wind-cold and retention of fluid.[Specification]5 gram each bag (No saccharose)[Usage and Dosage]Taken after dissolved in boiled water, 5 grams a time, 3 times a day.(No saccharose)[Adverse Reactions] Not known[Contraindications]Not known[Precautions ]1. Avoid Smoke, alcohol, piquancy, crude and fatness .2. Do not used the restorative patent medicine at one time when use this medicine.3 .This medicine is not suitable for the patient who cough and dyspnea, calor internus or dyspnea due to deficiency4. The patient who has bronchiectasia, lung abcess, corpulmonale or phthisic arises cough should go to see a doctor.5. High blood pressure and cardiopathy should use with cautious. Hepatitis, nephropathy, edema and other chronic patient should use under physician’s suggestion.6. Children, pregnant women, women in lactation, oldness and infirmness should use under physician’s suggestion.7.Fever heat patient whose body temperature exceeds38.5℃or hurried broken wind, get more phlegm should go to see a doctor .8. Use according to Usage and Dosage strictly, this medicine is not suitable for long-term used.9. If the symptom haven’t catabatic after used this medicine for 3 days, should go to see a doctor.10.It should not be used in patients who has anaphylatoid reaction with this medicine, and irritability corporeity should use with caution.11. It should not be used when the character of this medicine has changed.12. Children should be administered under the supervision of adult.13. It should be kept out of reach of children.14. If in process of using other medicine, please consult with physician or chemist before use this medicine.[Medicine reaction] If used other medicines at the same time, the medicine reaction may occur, please consult physician or chemist the correlative detail.[Storage] Preserve in tightly closed containers.[Packing] Aluminium and plastic composite film ,5 grams×3 bags /box[Period of Validity]: Two years[Administer standard]: PHARMACOPOEIA OF THE PEOPLES’S REPUBLIC OF CHINA (2005 V olume I )[Approval number]: State medical permitment number. Z35020729 [Manufactured]: By Zhangzhou Pien Tze Huang Pharmaceutical co ., Ltd.蜂乳胶囊Royal Jelly Capsule[Medicine name]Proprietary name: Royal Jelly CapsuleChinese pronunciation: Fengru Jiaonang[Main ingredients]Royal Jelly, amylum[Usage and Dosage] Take in 2 capsules each time ,5 times daily [Specification]0.5g×10 capsules×5 bags , 0.5g×48 capsules×3 bottles . [Storage] Avoid sun’s rays, preserve in tightly closed containers and in a cool place。
