简单重复序列区间(ISSR)引物反应条件优化与筛选-已看
中国兰ISSR—PCR反应体系优化及引物筛选

中国兰ISSR—PCR反应体系优化及引物筛选作者:黄晓慧巫伟峰陈春张毅智汪长水徐建球陈发兴陈孝丑来源:《南方农业学报》2018年第07期摘要:【目的】优化中国兰的ISSR-PCR反应体系,并筛选适用于中国兰ISSR分析的候选引物,为ISSR分子标记在中国兰的辅助育种及亲缘关系和遗传多样性分析等提供技术参考。
【方法】以6个中国兰品种为材料,采集其叶片样品,分别用研钵法和研磨仪法进行破碎研磨,比较两种方法提取DNA的效果,利用L25(53)正交试验和单因素试验对DNA模板量、引物浓度、2×Taq Master Mix添加量、循环数和退火温度进行优化,建立最佳ISSR-PCR 反应体系,并从ISSR分子标记通用引物中筛选适用于中国兰的ISSR分析候选引物。
【结果】研磨仪法提取的DNA浓度明显高于研钵研磨法,但二者提取的DNA质量均较好(OD260/OD280为1.7~2.0)。
对ISSR-PCR反应体系扩增结果的影响程度排序为DNA模板量>引物浓度>2×Taq Master Mix添加量。
综合考虑成本和DNA模板量,最佳ISSR-PCR反应体系(20.0 μL):DNA模板10.0 ng、引物0.8 μmol/L和2×Taq Master Mix 9.0 μL。
最佳循环数为35,最佳退火温度为49.6 ℃。
基于上述优化结果,从100条引物中共筛选出42条适用于中国兰的ISSR候选引物。
【结论】研磨仪法可有效提高中国兰基因组DNA的提取率和质量,且利用优化后的ISSR-PCR反应体系和扩增程序及筛选出的引物,扩增获得的条带清晰、稳定,多样性好,可用于中国兰的遗传多样性和亲缘关系等分析研究。
关键词:中国兰;ISSR;分子标记;反应;引物筛选中图分类号: S682.310.36 文献标志码:A 文章编号:2095-1191(2018)07-1282-070 引言【研究意义】中国兰又称国兰,是中国传统兰花的统称,为兰科(Orchidaceae)兰属(Cymbidium)植物,其味幽香,花色淡雅,素有花中君子和天下第一香之美称,且具有独特的内涵和意境,观赏和经济价值很高(陈心启,2011)。
细胞遗传学课后题答案

细胞遗传学课后题答案《细胞遗传学》复习题第⼀章染⾊体的结构与功能+第三章染⾊体识别1.什么是花粉直感?花粉直感是怎样发⽣的?作物种⼦的哪些部分会发⽣花粉直感?花粉直感⼜叫胚乳直感,植物在双受精后,在3n胚乳上由于精核的影响⽽直接表现⽗本的某些性状。
由雄配⼦供应的⼀份显性基因能够超过由母本卵核或两个极核隐形基因的作⽤,杂交授粉当代母本植株所结的种⼦表现显性性状。
胚乳和胚性状均具有花粉直感的现象。
2.什么叫基因等位性测验?如何进⾏基因等位性测验?确定两个基因是否为等位基因的测验为基因的等位性测验。
将突变性状个体与已知性状的突变种进⾏杂交,凡是F1表现为已知性状,说明两对基因间发⽣了互补,属于⾮等位基因。
若F1表现为新性状,表明被测突变基因与已知突变基因属于等位基因。
3.原位杂交的原理是什么?原位杂交所确定的基因位置与遗传学上三点测验所确定的基因位置有何本质的不同?根据核酸碱基互补配对原则,将放射性或⾮放射性标记的外源核酸探针,与染⾊体经过变性的单链DNA互补配对,探针与染⾊体上的同源序列杂交在⼀起,由此确定染⾊体特定部位的DNA序列的性质;可将特定的基因在染⾊体上定位。
第⼀步,制备⽤来进⾏原位杂交的染⾊体制⽚;第⼆步,对染⾊体DNA进⾏变性处理;第三步,进⾏杂交;第四步,信号检出和对染⾊体进⾏染⾊;第五步,显微镜检查。
原位杂交是⼀种物理图谱绘制的⽅法,它所确定是特定基因在染⾊体上的物理位置;三点测验是绘制连锁图谱的实验⽅法,它是利⽤三对连锁基因杂合体,通过⼀次杂交和⼀次测交,确定三对基因在同⼀染⾊体上排列顺序以及各个基因的相对距离。
4.什么叫端粒酶(telomerase)?它有什么作⽤?端粒酶是参与真核⽣物染⾊体末端的端粒DNA复制的⼀种核糖核蛋⽩酶,由RNA和蛋⽩质组成,其本质是⼀种逆转录酶。
作⽤:它以⾃⾝的RNA作为端粒DNA复制的模版,合成出富含G的DNA序列后添加到染⾊体的末端并与端粒蛋⽩质结合,从⽽稳定了染⾊体的结构。
分子生药学

分子生药学研究策略
分子遗传标记技术
通过直接分析遗传物质的多态性来诊断生物内在基因排布规律及其 外在性状表现规律的技术。任何生物种或个体都具有特定的DNA 多态性,通过直接诊断分析DNA 的多态性,便能避开遗传特性表 现过程中的环境因素、数量性状遗传或部分与完全显性的干扰,快
速准确地鉴定药材真伪。
分子生药学研究方法
PCR反应的结果。①循环参数
变性 退火 延伸
②反应成分
(3)PCR反应引物的设计 引物的设计在整个PCR扩增中占有十分重要的地位 特异性,扩增性 ①引物的序列应位于基因组DNA的高度保守区,且与非扩增区无同源序列。这样 可以减少引物与基因组的非特异性结合,提高反应的特异性 ②引物长度:15-30nt为宜。引物过短或过长均可使反应的特异性下降。 ③引物的碱基尽可能随机发布,避免出现数个嘌呤或嘧啶的连续排列,G+C碱基
dNTP:一般为50-200μmol/L
Mg2+ 模板:PCR对模板的要求不高,单、双链DNA均可,但
样品中不能混有蛋白酶、核酸酶、DNA聚合酶抑制剂以
及能与 DNA结合的蛋白质。 添加剂:DMSO(二甲基亚枫),提高扩增效率及特异性
(1) 理论上PCR合成产物的数量经过每轮循环都将增加一倍,应按2n-2n 的指数方式递增,PCR反应30轮循环后,PCR扩增应达到230个拷贝,约109个拷 贝。但由于DNA聚合酶的质量、待扩增片段的序列及反应系统的条件等各种因素 的影响,实际扩增效率比预期的要低,一般可达106-107个拷贝。 “平台效应”:PCR反应中,当引物-模板与DNA聚合酶达到一定比值时, DNA聚合酶催化反应趋于饱和,即PCR反应不再增加。 平台效应在PCR反应中是不可避免的,但一般在平台效应出现前,PCR产物 的数量足以满足实验的需要。 (2)PCR反应条件的优化 PCR方法操作简便,但影响因素颇多,因此需要根据不同的DNA模板,摸索最 适条件。主要从: 反应的特异性、敏感性、忠实性、扩增效率等四个方面衡量
简单序列重复(ISSR)多态性及其在植物育种中的应用

Euphytica128:9–17,2002.©2002Kluwer Academic Publishers.Printed in the Netherlands.9Inter simple sequence repeat(ISSR)polymorphism and its application in plant breedingM.Pradeep Reddy,N.Sarla∗&E.A.SiddiqDirectorate of Rice Research,Rajendranagar,Hyderabad–500030,India;(∗author for correspondence,e-mail: nsarla@)Received3July2001;accepted6March2002Key words:anchored primer,DNA marker,genome mapping,gene tagging,genetic diversity,ISSR-PCR SummaryInter simple sequence repeat(ISSR)-PCR is a technique,which involves the use of microsatellite sequences as primers in a polymerase chain reaction to generate multilocus markers.It is a simple and quick method that combines most of the advantages of microsatellites(SSRs)and amplified fragment length polymorphism(AFLP) to the universality of random amplified polymorphic DNA(RAPD).ISSR markers are highly polymorphic and are useful in studies on genetic diversity,phylogeny,gene tagging,genome mapping and evolutionary biology.This review provides an overview of the details of the technique and its application in genetics and plant breeding in a wide range of crop plants.IntroductionDNA markers have proved valuable in crop breed-ing,especially in studies on genetic diversity and gene mapping.The commonly used polymerase chain reaction(PCR)-based DNA marker systems are ran-dom amplified polymorphic DNA(RAPD),amplified fragment length polymorphism(AFLP)and more re-cently simple sequence repeats(SSRs)or microsatel-lites(Staub et al.,1996;Gupta&Varshney,2000).The major limitations of these methods are low reprodu-cibility of RAPD,high cost of AFLP and the need to know theflanking sequences to develop species spe-cific primers for SSR polymorphism.ISSR-PCR is a technique that overcomes most of these limitations (Zietkiewicz et al.,1994;Gupta et al.,1994;Wu et al.,1994;Meyer et al.,1993).It is rapidly being used by the research community in variousfields of plant improvement(Godwin et al.,1997).The technique is useful in areas of genetic diversity,phylogenetic stud-ies,gene tagging,genome mapping and evolutionary biology in a wide range of crop species.In this method SSRs are used as primers to amplify mainly the inter-SSR regions.SSRs or microsatellites are short tandem repeats(STRs)or variable number of tandem repeats (VNTRs)of1–4bases of DNA ubiquitously present in eukaryote genomes(Tautz&Renz,1984).They are dispersed throughout the genome and vary in the number of repeat units.The details of the technique and its major applications are discussed in this review. The techniqueInter simple sequence repeat(ISSR)technique is a PCR based method,which involves amplification of DNA segment present at an amplifiable distance in between two identical microsatellite repeat regions oriented in opposite direction.The technique uses mi-crosatellites,usually16–25bp long,as primers in a single primer PCR reaction targeting multiple gen-omic loci to amplify mainly the inter-SSR sequences of different sizes.The microsatellite repeats used as primers can be di-nucleotide,tri-nucleotide,tetra-nucleotide or penta-nucleotide.The primers used can be either unanchored(Gupta et al.,1994;Meyer et al., 1993;Wu et al.,1994)or more usually anchored at3’or5’end with1to4degenerate bases extended into theflanking sequences(Zietkiewicz et al.,1994)(Fig-ure1).The technique combines most of the benefits of10Figure1.ISSR-PCR:A schematic representation of a single primer(AG)8,unanchored(a),3’-anchored(b)and5’-anchored(c)targeting a (TC)n repeat used to amplify inter simple sequence repeat regionflanked by two inversely oriented(TC)n sequences.(a)Unanchored(AG)n primer can anneal anywhere in the(TC)n repeat region on the template DNA leading to slippage and ultimately smear formation(b)(AG)n primer anchored with2nucleotides(NN)at the3’end anneals at specific regions on the template DNA and produces clear bands(c)(AG)n primer anchored with2nucleotides(NN)at the5’end anneals at specific regions and amplifies part of the repeat region also leading to larger bands.11AFLP and microsatellite analysis with the universality of RAPD.ISSRs have high reproducibility possibly due to the use of longer primers(16–25mers)as com-pared to RAPD primers(10-mers)which permits the subsequent use of high annealing temperature(45–60◦C)leading to higher stringency.The studies on reproducibility show that it is only the faintest bands that are not reproducible.About92–95%of the scored fragments could be repeated across DNA samples of the same cultivar and across separate PCR runs when detected using polyacrylamide(Fang&Roose,1997; Moreno et al.,1998).10ng template DNA yielded the same amplification products as did25or50ng per20µl PCR reaction.The annealing temperature depends on the GC content of the primer used and usually ranges from45to65◦C.ISSRs segregate mostly as dominant markers fol-lowing simple Mendelian inheritance(Gupta et al., 1994;Tsumura et al.,1996;Ratnaparkhe et al.,1998; Wang et al.,1998).However,they have also been shown to segregate as co-dominant markers in some cases thus enabling distinction between homozygotes and heterozygotes(Wu et al.,1994;Akagi et al.,1996; Wang et al.,1998;Sankar&Moore,2001).Source of variability/polymorphismThe evolutionary rate of change within microsatellites is considerably higher than most other types of DNA, so the likelihood of polymorphism in these sequences is greater.The source of variability in the ISSRs can be attributed to any one of the following reasons or any combination of these.(a)Template DNASlippage of DNA polymerase during DNA replica-tion and failure to repair mismatches is considered as a mechanism for creation and hypervariability of SSRs(Levinson&Gutman,1987).Mutations at the priming site i.e.SSR could prevent amplifica-tion of a fragment,as also in RAPD markers and thus give a presence/absence polymorphism.An in-sertion/deletion event within the SSR region or the amplified region would result in the absence of a product or length polymorphism,depending on the amplifiability of the resulting fragment size.Variab-ility in number of nucleotides within a microsatellite repeat would result in length polymorphisms when using a5’-anchored primer.(b)Nature of primer usedThe extent of polymorphism also varies with the nature(unachored,3’-anchored,or5’-anchored)and sequence of the repeats(motif)in the primer em-ployed.When unanchored i.e only the SSRs are used as primers,the primer tends to slip within the repeat units during amplification leading to smears instead of clear bands(Figure1a).Extending the primer(an-choring)with1to4degenerate nucleotides at the3’end(Figure1b)or5’end(Figure1c)assures anneal-ing only to the ends of a microsatellite in template DNA thus obviating internal priming and smear form-ation.Secondly,the anchor allows only a subset of the microsatellites to serve as priming sites.When 5’anchored primers are used,the amplified products include the microsatellite sequences and their length variations across a genome and therefore give more number of bands and a higher degree of ually di-nucleotide repeats,anchored either at3’or5’end reveal high polymorphism(Blair et al.,1999;Joshi et al.,2000;Nagaoka&Ogihara, 1997).The primers anchored at3’end(Figure1b) give clearer banding pattern as compared to those anchored at5’end(Tsumura et al.,1996;Blair et al., 1999;Nagaoka&Ogihara,1997).Since the primer is a SSR motif the frequency and distribution of the microsatellite repeat motifs in different species also influence the generation of bands.There is a difference of abundance of SSRs between nuclear and organ-elle DNA sequences.Taking di-and tri-nucleotides together,one SSR was found every33Kb in nuclear DNA compared to every423-Kb of organelle DNA sequence(Wang et al.,1994).In general,primers with(AG),(GA),(CT),(TC),(AC),(CA)repeats show higher polymorphism than primers with other di-,tri-or tetra-nucleotide repeats.(AT)repeats are the most abundant di-nucleotides in plants but the primers based on(AT)would self-anneal and not amplify.Tri-and tetra-nucleotides are less frequent and their use in ISSRs is lesser than the di-nucleotides.The(AG)and (GA)based primers have been shown to amplify clear bands in rice(Blair et al.,1999;Joshi et al.,2000; Reddy et al.,2000;Sarla et al.,2000),trifoliate orange (Fang et al.,1997),Douglasfir and sugi(Tsumura et al.,1996)and chickpea(Ratnaparkhe et al.,1998), whereas primers based on(AC)di-nucleotide repeats were found more useful in wheat(Nagaoka&Ogihara, 1997;Kojima et al.,1998)and potato(McGregor et al.,2000).Resolving power Rp is an index developed to compare the value of different primers in terms12of the informative bands obtained in a given set of germplasm(Prevost&Wilkinson,1999).(c)Detection methodThe level of polymorphism detected has been shown to vary with the detection method used.Polyacrylamide gel electrophoresis(PAGE)in combination with ra-dioactivity(labelled nucleotide in PCR reaction)was shown to be most sensitive,followed by PAGE with silver staining and then agarose-ethidium bromide sys-tem of detection.Markedly higher number of bands were resolved per primer when polyacrylamide was used compared to agarose(Moreno et al.,1998).In a study on trifoliate orange germplasm,silver stain-ing using high quality chemicals could detect all the bands detected by autoradiography(Fang et al.,1997). However,high levels of polymorphism have been detected even when products of ISSR amplification are resolved on agarose gels without radiolabelling (Tsumura et al.,1996;Arcade et al.,2000;Kojima et al.,1998;Wolff&Morgan-Richards,1998;Sankar &Moore,2001)Thus,the need for radioactivity can be avoided when many samples have to be screened as in germplasm characterization.ISSR-PCR is a simple,quick,and efficient tech-nique.It has high reproducibility.The use of radio-activity is not essential.The primers are not proprietary (as in SSR-PCR)and can be synthesized by any-one.Variations in primer length,motif and anchor are possible.The primers are long(16–25bp)resulting in higher stringency.The amplified products(ISSR markers)are usually200–2000bp long and amenable to detection by both agarose and polyacrylamide gel electrophoresis.In the literature this technique and its variations have been referred to by different names (Table1).ApplicationThe potential for integrating ISSR-PCR into programs of plant improvement is enormous(Table2).The ma-jor areas of the application of ISSR-PCR in different crops are discussed below.GenomicfingerprintingDNAfingerprinting is an important tool for character-ization of germplasm and establishment of the iden-tity of varieties/hybrids/parental sources etc.in plant breeding and germplasm management.Di-nucleotide based ISSR primers anchored at5’or3’end have been used infingerprinting studies with high reprodu-cibility for maintenance of cocoa collection(Charters &Wilkinson,2000).ISSRs showed sufficient poly-morphism to distinguish between various cultivars of chrysanthemum(Wolff et al.,1995).Microspore de-rived plants could be distinguished from those derived from somatic tissues in anther culture offlax at an early seedling stage(Chen et al.,1998).Genetic diversity and phylogenetic analysisISSRs have been successfully used to estimate the extent of genetic diversity at inter-and intra-specific level in a wide range of crop species which include rice (Joshi et al.,2000),wheat(Nagaoka&Ogihara,1997),fingermillet(Salimath et al.,1995),Vigna(Ajibade et al.,2000),sweet potato(Huang&Sun,2000)and Plantago(Wolff&Morgan-Richards,1998).Superi-ority of ISSR-PCR over other marker techniques has been brought out in such investigations by various workers.Anchored SSR primers for instance,have been found to be more useful and reproducible than isozymes,RFLPs and RAPDs in the diversity analysis of trifoliate orange germplasm(Fang et al.,1997). ISSRs were more useful for the analysis of diversity in the genus Eleusine in terms of quality and quant-ity of data output as compared to RFLP and RAPD (Salimath et al.,1995).Significantly,the efficiency of the technique was evident in characterization even at the varietal level of a species.For instance,three5’anchored primers together could distinguish20cul-tivars of Brassica napus(Charters et al.,1996).ISSR is the marker of choice for assessment of genetic diversity in cocoa(Charters&Wilkinson,2000),gym-nosperms such as Douglasfir and sugi(Tsumura et al., 1996)and even fungi(Hantula et al.,1996).In a study on white lupin it has been demonstrated that among 10primers used any two were sufficient to distinguish all the37accessions studied(Gilbert et al.,1999). Similarly,4primers were sufficient to distinguish34 cultivars of potato(Prevost&Wilkinson,1999)and3 primers could distinguish16genotypes of redcurrant (Lanham&Brennan,1998).The use of such highly informative primers lowers the cost,time and labour for diversity analysis.Various marker techniques have been used in phylogenetic investigations based on relative simil-arity.Inspite of their higher efficiency and repro-ducibility ISSR markers have as yet not been used extensively.It has however been found effective in13 Table1.Synonyms of the ISSR-PCR technique and its variantsS.No Terms used Reference1MP-PCR,Microsatellite primed PCR(refers to unanchored primer)Meyer et al.(1993)2SSR-anchored PCR,Inter-SSR amplification Zietkiewicz et al.(1994)3SPAR(single primer amplification reaction)Gupta et al.(1994)4RAMPs(random amplified microsatellite polymorphisms)Wu et al.(1994)5RAMs(randomly amplified microsatellites)Hantula et al.(1996)6AMP-PCR(anchored microsatellite primed PCR)Weising et al.(1998)7ASSR(anchored simple sequence repeats)Wang et al.(1998)resolving problems relating to the phylogeny of Asian cultivated rice Oryza sativa(Joshi et al.,2000),wheat (Nagaoka&Ogihara,1997),finger millet(Salimath et al.,1995),Vigna(Ajibade et al.,2000)and Dip-lotaxis species(Martin&Sanchez-Yelamo,2000). There is immense scope to use this powerful tech-nique in resolving species/inter-species status in many a genus and in deciding the distinctness of different genera within a family.Significantly,genome/species specific ISSR markers have been reported in four gen-era Oryza(Joshi et al.,2000),Lolium and Festuca (Pasakinskiene et al.,2000)and Diplotaxis(Mar-tin&Sanchez-Yelamo,2000)which are useful in delineating species.Genome mappingISSR markers are unmapped but can be used to sat-urate RFLP and SSR linkage maps.The RFLP map of barley was saturated with60ISSRs(referred as RAMPs in the study)which mapped to all chromo-somes(Becker&Heun,1995).Many of these markers are mapped in between clustered RFLPs,flanking RFLP clusters,at the tips of chromosomes and more importantly in areas of low RFLP marker density. In Einkorn wheats,however,the nine ISSR mark-ers mapped at or close to the RFLP marker positions (Kojima et al.,1998).ISSRs have also been used along with AFLP and RAPD markers in the mapping of Ja-panese and European larch genomes(Arcade et al., 2000).The genetic linkage map of Citrus was fur-ther saturated using75ISSR markers,which were dispersed among all the linkage groups(Sankar& Moore,2001).Also it was shown that the level of segregation distortion of ISSRs is lower compared to RAPDs.In soybean,58ISSR markers were mapped onto18RAPD/RFLP linkage groups(Wang et al.,1998).CA polymorphisms had a biased distribution and GA polymorphisms were randomly dispersed. Gene tagging and marker assisted selectionDNA markers closely linked to important agronomic traits greatly contribute to practical crop improvement programs.In rice,an ISSR marker generated by primer (AG)8YC was converted to a sequence tagged site (STS)marker to identify the fertility restoration gene, Rf-1(Akagi et al.,1996).This co-dominant marker can be used in management of genetic purity of hy-brid seed.In chickpea,ISSR markers UBC855500 generated by primer(AG)8YT and UBC8251200us-ing primer(AG)8T were linked to the gene conferring resistance to race4of Fusarium wilt(Ratnaparkhe et al.,1998).Markers closer to a given gene are gener-ated by altering5’or3’anchors.Recently,ISSR-PCR was used in identifying two allelic dominant DNA markers,one linked in coupling and the other in re-pulsion phase to a major locus Fgr,which modulates fructose to glucose ratio in tomatoes(Levin et al., 2000).These PCR products were obtained from two ISSR-PCR reactions using(TC)8CC and(TC)8CG as primers.Another trait of value in hybrid seed produc-tion viz.,temperature-sensitive genic male sterility has been tagged with an ISSR marker UBC8551060in rice (Hussain et al.,2000).ISSRs have also been used to generate species spe-cific,gene specific and trait specific markers.While delineating the phylogenetic relationship among dif-ferent species of the genus Oryza,87putative gen-ome/species specific markers were identified(Joshi et al.,2000).The582bp inter-SSR Festuca specific sequence and1350bp F.arundinacea specific se-quence have potential as markers to confirm presence of closely linked Festuca genes(Pasakinskiene et al., 2000).Likewise,race specific markers have been de-14Table2.Applications of ISSR-PCR techniqueS.No Application Reference1GenomicfingerprintingCocoa germplasm Charters&Wilkinson,2000Potato cultivars Prevost&Wilkinson,1999Chrysanthemum cultivars Wolff et al.,1995 2Genetic diversity and phylogenetic analysisRice cultivars Virk et al.,2000Oryza granulata Qian et al.,2001Wheat(Triticum sp.)Nagaoka&Ogihara,1997Barley(Hordeum vulgare)Sanchez et al.,1996Maize inbred lines(Zea mays)Kantety et al.,1995Fingermillet(Eleusine sp)Salimath et al.,1995Sorghum(Chinese)(Sorghum bicolor)Yang et al.,1996White lupin germplasm(Lupinus albus)Gilbert et al.,1999Vigna sp Ajibade et al.,2000Pea germplasm(Pisum sativum)Lu et al.,1996Soybean(Glycine max)Wang et al.,1998Oilseed rape cultivars(Brassica napus)Charters et al.,1996Sweet potato,wild relatives(Ipomoea sp)Huang&Sun,2000Potato cultivars(Solanum tuberosum)McGregor et al.,2000Redcurrant germplasm(Ribes sp)Lanham&Brennan,1998Grapevine germplasm(Vitis vinifera)Moreno et al.,1998Citrus cultivars(Citrus sp)Fang&Roose,1997Trifoliate orange germplasm(Poncirus trifoliata)Fang et al.,1997Plantago major subspecies Wolff&Morgan-Richards,1998Gymnosperms,Douglasfir and sugi Tsumura et al.,1996 3Genome mappingSaturating RFLP linkage map in barley Becker&Heun,1995Construction of a genetic linkage map in Einkorn wheat Kojima et al.,1998Genetic mapping of Japanese and European types of larch Arcade et al.,2000Saturating genetic linkage map in citrus Sankar&Moore,2001Saturating RFLP/RAPD linkage map in soybean Wang et al.,1998 4Determining SSR motif frequencyRecovery of microsatellite sequences in the mustard genome Varghese et al.,2000Distribution pattern of microsatellites across eukaryotic genomes Gupta et al.,1994Analysis of microsatellite frequency in rice cultivars Blair et al.,19995Gene tagging and use in marker assisted selectionRf-1gene for fertility restoration in rice Akagi et al.,1996Gene for resistance to Fusarium wilt Race4in chickpea Ratnaparkhe et al.,1998Temperature sensitive genic male sterility in rice Hussain et al.,2000Fgr gene for modulating fructose to glucose ratio in tomato Levin et al.,2000Genome/species specific markers in Lolium and Festuca Pasakinskiene et al.,2000Putative genome/species specific markers in Oryza.Joshi et al.,2000Race specific markers in fungi Hantula et al.,1996 6Evolutionary biologyDiplotaxis species Martin&Sanchez-Yelamo,2000Diploid hybrid speciation in Penstemon Wolfe et al.,199815veloped in various fungi groups using ISSRs(Hantula et al.,1996).Determining SSR motif frequencyISSR analysis provides insights into the organization (clustered or not),frequency and levels of polymorph-ism of different simple sequence repeats in a genome. In rice and wheat,di-nucleotide simple sequence re-peats used as primers gave the maximum number of bands and are,therefore,more common than any SSRs with larger units(Blair et al.,1999;Nagaoka&Ogi-hara,1997).Poly(GA)based3’-anchored primers pro-duced5times as many bands as those with poly(GT) motif indicating low frequency or lack of cluster-ing of(GT)motif(Blair et al.,1999).Using ISSRs it has been shown that tetra-nucleotide repeats were abundant across eukaryotic genomes(Gupta et al., 1994)and that tetramers of tetra-nucleotides AGAC and GACA are scattered within the genome of grasses (Pasakinskiene et al.,2000).It has been demonstrated in Brassica that enhanced recovery of microsatellite markers is possible using ISSR primers(Varghese et al.,2000).Studies on natural populations/speciationThe hypervariable nuclear ISSR markers have proved useful in testing hypotheses of speciation,introgres-sion and systematics(Wolfe et al.,1998).The hybrid origin of Penstemon clevelandi was clearly brought out by the use of just8ISSR markers.Population of P. clevelandi has been found to have an additive profile of bands of the two proposed progenitor species viz.P. centranthifolius and P.spectabilis.On the other hand the population of P.spectabilis lacked the additive profile of bands of its proposed putative parents.The hybrid origin of P.spectabilis was thus negated and its origin was attributed instead to introgression of genes and not the genome of a related species.The util-ity of the technique has been demonstrated in a wide range of applications in molecular ecology in plant families which include Asteraceae,Brassicaceae, Hippocastanaceae,Orchidaceae,Poaceae,Scro-phulariaceae and Violaceae(/∼awolfe/issri.issr.html).Variation within and between populations can be compared using dis-persed multilocus markers such as ISSR.It was shown that the amount of variation between O.granulata populations from different regions(49.2%)was higher than that between populations within a region(38%)or within a population(12%)using ISSR markers (Qian et al.,2001).PerspectivesAs the need to protect proprietary germplasm is likely to increase in the future,ISSRs will have an import-ant role in securing plant variety rights by virtue of its unique efficiency in distinguishing even closely re-lated germplasm.To date,more polymorphism has been detected with the use of ISSRs than with any other assay procedure(Gupta et al.,1994;Salimath et al.,1995;Virk et al.,2000).In many of the studies for determining the extent of polymorphism or com-paring marker systems only one family of SSRs,eg. tri-nucleotides or tetra-nucleotides had been used as primers.Such repeats are infrequent as compared to di-nucleotides and their use may not help arrive at precise classification.As more data on the occurrence and distribution of SSR motifs becomes available,it should be possible to use primers that give more ac-curate span of the whole genome.Also,different com-binations of the motif,anchor and length of primers can be used.Strategies to detect additonal polymorph-ism could include use of ISSRs in combination with RAPD(Joshi et al.,2000;Becker&Heun,1995;Wu et al.,1994)or AFLP primers in the same reaction or restriction digestion of ISSR products(Becker& Heun,1995).Unlimited combinations of motif and length of both primers and use of different restriction enzymes are thus possible.Well chosen primers can provide reasonably accuratefingerprinting and thereby quick estimate of genetic diversity especially in large sized accessions to identify core sets and the pattern of geographical distribution.The technique is not without limitations.For in-stance,there is the possibility as in RAPD,that fragments with the same mobility originate from non-homologous regions,which can contribute to some distortion in the estimates of genetic similarities (Sanchez et al.,1996).The molecular nature of the polymorphisms can be known only if the fragments extracted from the gel are sequenced.ISSR mark-ers linked to the traits of agronomic importance have been sequenced and used as STS markers in marker aided selection.An attractive possibility is thus the use of ISSRs as probes for in-situ hybridization for physical mapping of homologous chromosome sites (Pasakinskiene et al.,2000).Another advantage in the use of ISSR markers lies in their linkage to SSR loci.16Although microsatellites themselves are probably non-functional and selectively neutral,they are known to be linked to coding regions,so that ISSRs are likely to mark gene rich regions(Kojima et al.,1998). ReferencesAjibade,S.R.,N.F.Weeden&S.M.Chite,2000.Inter-simple sequence repeat analysis of genetic relationships in the genus Vigna.Euphytica111:47–55.Akagi,H.,Y.Yokozeki,A.Inagaki,A.Nakamura&T.Fujimura, 1996.A co-dominant DNA marker closely linked to the rice nuc-lear restorer gene,Rf-1,identified with inter-SSRfingerprinting.Genome39:1205–1209.Arcade,A.,F.Anselin,P.F.Rampant,M.C.Lesage,L.E.Paques&D.Prat,2000.Application of AFLP,RAPD and ISSR markersto genetic mapping of European and Japanese larch.Theor Appl Genet100:299–307.Becker,J.&M.Heun,1995.Mapping of digested and undiges-ted random amplified microsatellite polymorphisms in barley.Genome38:991–998.Blair,M.W.,O.Panaud&S.R.McCouch,1999.Inter-simple se-quence repeat(ISSR)amplification for analysis of microsatellite motif frequency andfingerprinting in rice(Oryza sativa L).Theor Appl Genet98:780–792.Charters,Y.M., A.Robertson,M.J.Wilkinson&G.Ramsay, 1996.PCR analysis of oilseed rape cultivars(Brassica napus L.ssp.oleifera)using5’-anchored simple sequence repeat(SSR) primers.Theor Appl Genet92:442–447.Charters,Y.M.&M.J.Wilkinson,2000.The use of self-pollinated progenies as‘in-groups’for the genetic characterization of cocoa germplasm.Theor Appl Genet100:160–166.Chen,Y.,G.Hausner,E.Kenaschuk,D.Procunier,P.Dribnenki&G.Penner,1998.Identification of microspore-derived plants inanther culture offlax(Linum usitatissimum L.)using molecular markers.Plant Cell Reports18:44–48.Fang, D.Q.,M.L.Roose,R.R.Krueger&C.T.Federici,1997.Fingerprinting trifoliate orange germplasm accessions with isozymes,RFLPs and inter-simple sequence repeat markers.Theor Appl Genet95:211–219.Fang,D.Q&M.L.Roose,1997.Identification of closely related citrus cultivars with inter-simple sequence repeat markers.Theor Appl Genet95:408–417.Gilbert,J.E.,R.V.Lewis,M.J.Wilkinson&P.D.S.Caligari,1999.Developing an appropriate strategy to assess genetic variab-ility in plant germplasm collections.Theor Appl Genet98: 1125–1131.Godwin,I.D.,E.A.B.Aitken&L.W.Smith,1997.Application of inter-simple sequence repeat(ISSR)markers to plant genetics.Electrophoresis18:1524–1528.Gupta,M.,Y-S.Chyi,J.Romero-Severson&J.L.Owen,1994.Amplification of DNA markers from evolutionarily diverse gen-omes using single primers of simple-sequence repeats.Theor Appl Genet89:998–1006.Gupta,P.K.&R.K.Varshney,2000.The development and use of microsatellite markers for genetic analysis and plant breeding with emphasis on bread wheat.Euphytica113:163–185. Hantula,J.,M.Dusabenyagasani&R.C.Hamelin,1996.Random amplified microsatellites(RAMS)-a novel method for char-acterizing genetic variation within fungi.Eur J for Path26: 159–166.Huang,J.&S.M.Sun,2000.Genetic diversity and relationships of sweet potato and its wild relatives in Ipomoea series Batatas (Convolvulaceae)as revealed by inter-simple sequence repeat (ISSR)and restriction analysis of chloroplast DNA.Theor Appl Genet100:1050–1060.Hussain,A.J.,V.Gupta,J.Ali,P.K.Ranjekar&E.A.Siddiq,2000.Physiological characterization,genetics and molecular mapping of a new source of temperature sensitive genetic male sterility in rice.Fourth International Rice Genetics Symposium,22–27 October2000,IRRI,Philippines,Abstracts p.95.Joshi,S.P.,V.S.Gupta,R.K.Aggarwal,P.K.Ranjekar&D.S.Brar, 2000.Genetic diversity and phylogenetic relationship as revealed by inter-simple sequence repeat(ISSR)polymorphism in the genus Oryza.Theor Appl Genet100:1311–1320.Kantety,R.V.,X.P.Zeng,J.L.Bennetzen&B.E.Zehr,1995.As-sessment of genetic diversity in dent and popcorn(Zea mays L.) inbred lines using inter-simple sequence repeat(ISSR)amplific-ation.Molecular Breeding1:365–373.Kojima,T.,T.Nagaoka,K.Noda&Y.Ogihara,1998.Genetic link-age map of ISSR and RAPD markers in Einkorn wheat in relation to that of RFLP markers.Theor Appl Genet96:37–45. Lanham,P.G.&R.M.Brennan,1998.Characterization of the ge-netic resources of redcurrant(Ribes rubrum:subg.Ribesia)using anchored microsatellite markers.Theor Appl Genet96:917–921. Levin,I.N.,E.Gilboa,S.Yeselson,Shen&A..A.Schaffer,2000.Fgr,a major locus that modulates the fructose to glucose ratio in mature tomato fruits.Theor Appl Genet100:256–262. Levinson,G.&G.A.Gutman,1987.Slipped strand mispairing:a major mechanism for DNA sequence evolution.Mol Biol Evol 4:203–221.Lu,J.,M.R.Knox,M.J.Ambrose,J.K.M.Brown&T.H.N.Ellis,parative analysis of genetic diversity in pea as-sessed by RFLP-and PCR-based methods.Theor Appl Genet93: 1103–1111.Martin,J.P.&M.D.Sanchez-Yelamo,2000.Genetic relationships among species of the genus Diplotaxis(Brassicaceae)using inter-simple sequence repeat markers.Theor Appl Genet101: 1234–1241McGregor,C.E.,mbert,M.M.Greyling,J.H.Louw&L.Warnich,2000.A comparative assessment of DNAfingerprinting techniques(RAPD,ISSR,AFLP and SSR)in tetraploid potato (Solanum tuberosum L)germplasm.Euphytica113:135–144. Meyer,W.,T.G.Mitchell, E.Z.Freedman&R.Vilgays,1993.Hybridization probes for conventional DNAfingerprinting used as single primers in the polymerase chain reaction to distin-guish strains of Cryptococcus neoformans.J Clin Microbiol31: 2274–2280.Moreno,S.,J.P.Martin&J.M.Ortiz,1998.Inter-simple sequence repeats PCR for characterization of closely related grapevine germplasm.Euphytica101:117–125.Nagaoka,T.&Y.Ogihara,1997.Applicability of inter-simple se-quence repeat polymorphisms in wheat for use as DNA markers in comparison to RFLP and RAPD markers.Theor Apppl Genet 94:597–602.Pasakinskiene,I.,C.M.Griffiths,A.J.E.Bettany,V.Paplauskiene, M.W.Humphreys,2000.Anchored simple-sequence repeats as primers to generate species-specific DNA markers in Lolium and Festuca grasses.Theor Appl Genet100:384–390.Prevost,A.&M.J.Wilkinson,1999.A new system of comparing PCR primers applied to ISSRfingerprinting of potato cultivars.Theor Appl Genet98:107–112.Qian,W.,S.Ge&D.Y.Hong,2001.Genetic variation within and among populations of a wild rice Oryza granulata from China。
三角梅ISSR反应体系的建立和优化

三角梅ISSR反应体系的建立和优化李房英;黄彦晶;吴少华【摘要】对影响三角梅ISSR-PCR扩增反应的各个参数进行优化,建立适合三角梅的ISSR反应体系:PCR反应体积为20μL,其中10×buffer(含Mg2+)2.0μL,dNTP 250 μmol/L,Taq酶1.0U,引物0.3μmol/L,模板DNA20ng.扩增程序为:94℃预变性5min;94℃变性1min,51.6℃退火1min,72℃延伸2min,34个循环;最后72℃延伸7min.该反应体系标记点位清晰、稳定、重复性好,适宜三角梅ISSR分析,为应用ISSR技术鉴定三角梅种质资源、分子标记辅助选择育种及其遗传多样性研究奠定了基础.【期刊名称】《海峡科学》【年(卷),期】2010(000)010【总页数】4页(P216-219)【关键词】三角梅;ISSR;体系建立;优化【作者】李房英;黄彦晶;吴少华【作者单位】福建农林大学园林学院;福建农林大学园艺学院;福建农林大学园艺学院【正文语种】中文三角梅(Bougainvillea spectabilis Willd)又名九重葛、毛宝巾、勒杜鹃,在我国已有100多年的栽培历史,品种丰富。
我国部分研究者在同工酶水平上对三角梅品种进行亲缘关系分析但存在难以解释之处[1],至今仍有分类标准不统一、分类手段有一定的局限性,所得分类结果不一致以及同名异物、同物异名等问题,从而给生产、园林应用、交流等方面带来诸多困难。
而三角梅有关分子标记方面的研究仍是空白,因此利用分子标记技术进行三角梅的分类、品种鉴定等研究显得尤为重要。
ISSR(简单重复序列间隔区,Inter-Simple Sequence Repeat)是由Zietkiewicz等于1994年创建的一种简单序列重复区间扩增多态性的分子标记[2]。
ISSR具有操作简单,标记重复性好,稳定程度高,多态性丰富,DNA用量少,成本低等优点。
麻栗坡兜兰ISSR引物筛选及反应体系的优化

麻栗坡兜兰ISSR引物筛选及反应体系的优化高丽霞【摘要】[目的]对麻栗坡兜兰ISSR引物进行筛选,并建立一个稳定性高、重现性好、适合麻栗坡兜兰ISSR反应体系.[方法]以麻栗坡兜兰DNA为模板,分别对Mg2+、dNTP、引物、Taq DNA聚合酶、DNA等PCR反应成分进行优化,并用优化的体系对25条兰科中报道的ISSR引物进行筛选.[结果]确立了麻栗坡兜兰最适ISSR-PCR反应体系:在25μl反应体系中,Mg2+2 mmol/L、引物0.4 mmoL/L、dNTP 0.20 mmol/L、DNA 1.5μl、Taq酶1.4U.利用该体系,共筛选得到10条ISSR引物用于麻栗坡兜兰分析,并对21份麻栗坡兜兰材料进行扩增,获得了较好的扩增效果.[结论]该体系稳定可靠,为今后ISSR标记在兜兰属植物的种质鉴定、遗传多样性等方面的广泛应用奠定了重要基础.【期刊名称】《安徽农业科学》【年(卷),期】2014(000)020【总页数】3页(P6553-6555)【关键词】麻栗坡兜兰;ISSR-PCR;体系优化【作者】高丽霞【作者单位】河池学院化学与生物工程学院,广西宜州546300【正文语种】中文【中图分类】S188麻栗坡兜兰(Paphiopedilum malipoense)属兰科(Orchidaceae)兜兰属(Paphiopedilum)地生兰或半附生兰。
麻栗坡兜兰是兜兰属现存种类中最原始的类型,代表了由杓兰属向兜兰属过渡的种类[1],主要分布于云南东南部、广西西部、贵州西北部,以及越南北部。
一方面由于近年来的过渡采摘,另一方面由于其自身的生物学原因,它没有像硬叶兜兰和杏黄兜兰那样延长的地下根状茎[2],是当前最需要保护的濒临物种之一。
ISSR分子标记技术已在兰科中得以广泛应用。
赵谦等[3]用14条ISSR引物分析了14个蝴蝶兰品种间的遗传关系,其多态百分比为82%,表明蝴蝶兰品种间存在丰富的遗传多样性,并构建了ISSR遗传图谱;吴振兴等[4]用15条ISSR引物对兰属植物进行遗传多样性分析,其多态百分比为27.2%,并构建了ISSR遗传图谱;孙小琴等[5]用12条ISSR引物对江西省寒兰进行了遗传多样性分析,其多态百分比为78.9%;马佳梅等[6]用12条ISSR引物对西双版纳地区流苏石斛进行遗传多样性分析,其多态百分比为89.74%,表明流苏石斛品种间存在丰富的遗传多样性;严华等[7]采用ISSR分子标记技术对38种国兰亲缘关系进行分析;沈颖等[8]用ISSR分子标记技术对9种石斛属植物进行品种鉴定分析。
虉草ISSR-PCR反应体系优化与引物筛选

虉草ISSR-PCR反应体系优化与引物筛选张永亮;刘鹏;骆秀梅;刘杨【摘要】22个虉草基因组DNA为ISSR-PCR扩增模板,采用单因素试验方法,对影响PCR扩增体系中dNTP、引物浓度、Taq酶和模板DNA用量4个因素及引物退火温度进行梯度试验,优化得到最佳的ISSR-PCR反应体系,即20μL反应体系中分别加入0.3μL Taq DNA聚合酶(5 U/μL),2μL 10×PCRBuffer(mg2+ plus),1.5 μL dNTP (2.5 mmol/L),1.5 μL引物(10 pmol/μL),50 ng模板DNA,ddH2O补足体积.以此体系对24条引物进行筛选,最终获得了多态性高,重复性好的引物12条.引物UBC808、809、811、815、818、820、826的适宜退火温度为55℃,引物835,841和842的适宜退火温度为56℃,而引物810和834的适且退火温度分别为52℃和54℃.12条引物共扩增总条带数192条,其中,多态性条带数173条,多态位点百分率89.81%.【期刊名称】《草原与草坪》【年(卷),期】2016(036)003【总页数】7页(P1-6,11)【关键词】虉草;ISSR-PCR反应体系;引物筛选【作者】张永亮;刘鹏;骆秀梅;刘杨【作者单位】内蒙古民族大学,内蒙古通辽028042;内蒙古民族大学,内蒙古通辽028042;内蒙古民族大学,内蒙古通辽028042;内蒙古民族大学,内蒙古通辽028042【正文语种】中文【中图分类】S543;Q786虉草(Phalaris arundinacea),属于禾本科(Gramineae)、虉草属植物,别名草芦、园草芦。
主要分布于欧洲、北美和亚洲,在我国主要分布于东北、华北、华中、华东等地区。
虉草耐盐碱、耐湿涝,又耐旱,生长快,营养繁殖能力强,产草量高、叶量丰富,蛋白质含量高,被广泛用于饲草、人工湿地植物、生物能源材料或造纸原料等[1-2]。
李种质资源ISSR反应体系引物筛选

安佰义,王 迦,王 ?,等.李种质资源ISSR反应体系引物筛选[J].江苏农业科学,2019,47(10):63-65.doi:10.15889/j.issn.1002-1302.2019.10.014李种质资源ISSR反应体系引物筛选安佰义1,王 迦1,王 ?1,于慧颖1,范爱淇1,张艳波2,张 艳3,李 锋2(1.吉林农业大学园艺学院,吉林长春130118;2.吉林省农业科学院果树研究所,吉林长春130033;3.吉林省农业科学院,吉林长春130124) 摘要:以李18个品种种质资源为试验材料,对其开展ISSR反应体系引物筛选,结果表明,从41个随机引物中筛选出25个多态性引物用于PCR扩增,每个多态性引物扩增出的条带数在8~23条之间,扩增出的DNA片段长度大多在150~2400bp之间;共扩增出条带数为385条,其中,样品间相同的条带数有53条;所选引物的多态位点百分率为86.23%。
关键词:李;种质资源;ISSR;引物;多态位点 中图分类号:S662.302.4 文献标志码:A 文章编号:1002-1302(2019)10-0063-03收稿日期:2018-01-31基金项目:吉林省重点科技攻关项目(编号:20160204031NY)。
作者简介:安佰义(1978—),男,山东日照人,博士,副教授,从事园林植物种质资源及栽培生理研究。
E-mail:lli10437@126.com。
区间简单重复序列标记(inter-simplesequencerepeat,ISSR)是一种微卫星基础上的第2代分子标记技术,其基本原理是用锚定的微卫星DNA为引物,在简单重复序列(SSR)的3′端或5′端各加2~4个非重复随机核苷酸序列作为引物,在聚合酶链式反应(PCR)中锚定引物可引起特定位点退火,并对重复序列间的DNA片段进行PCR扩增[1-3]。
ISSR标记结合了随机扩增多态性DNA标记(RAPD)、SSR技术的优点,具有模板DNA用量少、引物设计简单、成本低、PCR扩增产物多态性丰富、产物重复性和稳定性好、试验安全性较高及技术难度较低等特点[4],现已在植物遗传作图、基因定位、品种鉴定、遗传多样性及亲缘关系分析等方面被广泛应用[5-9]。
ISSR(inter-simple sequence repeat)分子标记的实验原理及操作流程

ISSR(inter-simple sequence repeat)分子标记的实验原理及操作流程ISSR(inter-simple sequence repeat)标记是一种类似RAPD,但利用包含重复序列并在3’或5’锚定的单寡聚核酸引物对基因组进行扩增的标记系统,即用SSR引物来扩增重复序列之间的区域。
其原理具体是,ISSR 标记根据生物广泛存在 SSR的特点,利用在生物基因组常出现的SSR本身设计引物,无需预先克隆和测序。
关键词:标记分子ISSRinter-simplesequencerepeatSSR引物RAPD分子标记单寡聚核酸ISSR标记ISSR(inter-simple sequence repeat)标记是一种类似RAPD,但利用包含重复序列并在3’或5’锚定的单寡聚核酸引物对基因组进行扩增的标记系统,即用SSR引物来扩增重复序列之间的区域。
其原理具体是,ISSR标记根据生物广泛存在SSR的特点,利用在生物基因组常出现的SSR本身设计引物,无需预先克隆和测序。
用于扩增的引物一般为16-18个碱基序列,由1-4个碱基组成的串联重复和几个非重复的锚定碱基组成,从而保证了引物与基因组DNA 中SSR的5’或3’末端结合,导致位于反向排列、间隔不太大的重复序列间的基因组节段进行PCR扩增。
一、实验材料不同来源的DNA(30-50ng/ul)。
二、实验设备PCR仪,PCR管或硅化的0.5ml eppendorf管,电泳装置,凝胶成像仪。
三、试剂1、ISSR引物:购买成品或根据加拿大British Columbia大学设计的ISSR引物序列(见附录)自己合成。
2、Taq酶3、10xPCR 缓冲液4、MgCl2:25mmol/L。
5、dNTP:2.5mmol/L。
四、操作步骤:1. 在25ul反应体系中,加入模板DNA 1ul (30-50ng)ISSR引物1ul (约5pmol)10xPCR Buffer 2.5ulMgCl22uldNTP 2ulTaq酶1单位(U)加ddH2O 至25ul混匀稍离心, 加一滴(约20 ul)矿物油。
风信子ISSR-PCR体系的优化及引物筛选

2 t x L 1 0 x B u f e r , 1 . 4 L M ( 2 5 mmo l / L ) , 1 . 5 t x L d NT P s ( 2 . 5 mmo l / L ) , 1 . 5 I x L p r i me r ( 1 0 p mo l / I x L ) , 0 . 2 l x L p o l y me r a s e( 5 U / L , 1 . 2 t x L t e mp l a t e ( 3 o n g / t x L ) , a n d d d H 2 O t o 2 0 L a t l a s t . A c c o r d i n g t o t h i s P C R s y s t e m, 1 1 0
1 . 5 L引物( 1 0 p m o l / t x L ) , 0 . 2 t x L 酶( 5 U / p . L ) , 1 . 2 L模板( 3 0 n g / I x L ) , d d H O补足体积。 并以此体系对 1 1 0 条 引物 进行 筛选 , 最 终获 得 了多态 性高 , 重复 性好 的 引物 1 2条 。
Hy a c i n t h
Hu F e n g r o n g 。 ’ Wa n g F e i Wa n g Z h i q i a n g Re n Cu i Ba o Re n l e i
1 B e i j i n g F o r e s t r y U n i v e r s i t y , B e i j i n g , 1 0 0 0 8 3 ; 2 N a n j i n g F o r e s t y r U n i v e r s i t y , N a m i n g , 2 1 0 0 3 7
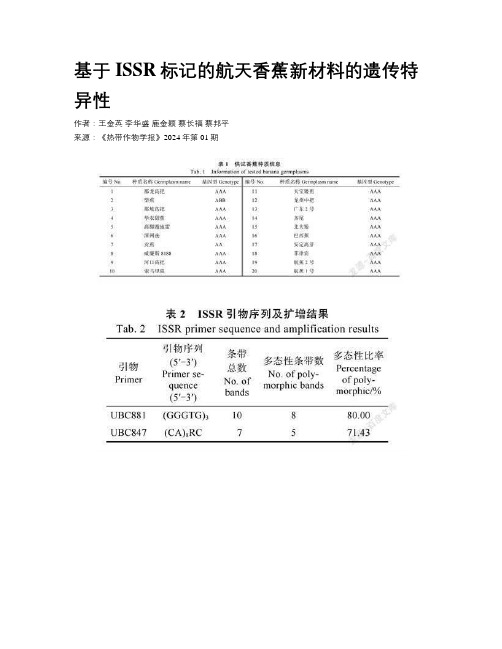
基于ISSR标记的航天香蕉新材料的遗传特异性
基于ISSR标记的航天香蕉新材料的遗传特异性作者:王金英李华盛鹿金颖蔡长福蔡邦平来源:《热带作物学报》2024年第01期关键词:航天育种;香蕉;ISSR;遗传特异性根据APGⅢ分类系统,香蕉(MusananaLour.)是单子叶植物分支下的姜目(Zingiberales)植物,属于芭蕉科(Musaceae)芭蕉属(Musa)。
香蕉主要产于热带及亚热带地区,是世界上非常重要的一种热带水果和粮食作物,也是我国南部地区的重要经济作物之一。
福建省香蕉栽培历史悠久,但是福建地处我国香蕉栽培适区的北缘,具有一定的局限性[1]。
现今福建的香蕉产业受到病害、寒害或风害的严重威胁,以及外来或进口香蕉的冲击,正面临着规模萎缩的处境。
因此,选育抗病、耐寒、优质、高产的香蕉新品种,已成为福建香蕉研究亟需解决的重要课题。
航天育种是利用太空环境中的极端条件(微重力、强辐射、高真空等因素),诱发植物的遗传物质发生改变,从中选择优良变异,培育优质、高产、多抗的植物新品种的育种方法[2]。
将传统的育种技术与航天诱变技术相结合实现育种目标的方法,已经得到国内外育种专家的广泛认可。
我国自1987年首次开展返回式卫星航天搭载水稻等农作物种子,距今已有30多年[3]。
随着我国航天事业的发展,通过航天育种方式已选育出一大批优质、高产、多抗的新品种,正服务并助推着相关产业的发展。
航天育种为植物新品种的选育打开了新世界的大门,也为香蕉新品种的选育开拓了新的途径。
简单重复序列区间(inter-simplesequencerepeat,ISSR)是由ZIEDEWIEZ等[4]提出的、基于SSR的基础上发展起来的一种DNA扩增多态性检测技术。
因其具有DNA模板用量少、引物设计简单、操作快速高效、多态性好、稳定性好、可重复性高等优势,常在植物遗传多样性、种质资源鉴定、分类及亲缘关系分析等方面有广泛的应用[5-8]。
本研究以2份航天香蕉种质和18份其他香蕉种质为材料,通过ISSR标记技术,旨在构建航天香蕉新材料的分子指纹图谱,以期为航天香蕉新品种的鉴定和分类提供参考。
鸡血藤ISSR体系优化及引物筛选

收稿日期:2022-10-19基金项目:广西中医药大学博士启动基金项目(2017BS029);广西中医药大学青年创新研究团队项目(2018QT001)作者简介:李金梅(1998-),女,广西灵山人,在读硕士研究生,研究方向为分子生药学,(电话)178****8953(电子信箱)*****************;通信作者,谭勇(1976-),男,重庆人,教授,主要从事中药资源研究,(电子信箱)***************。
李金梅,王一婷,潘丽梅,等.鸡血藤ISSR 体系优化及引物筛选[J ].湖北农业科学,2024,63(1):206-211.鸡血藤为豆科植物密花豆(Spatholobus suberec⁃tus Dunn.)的干燥藤茎,具有活血补血、调经止痛、舒经活络的功能,广泛用于月经不调、痛经、经闭、风湿痹痛、麻木瘫痪、血虚萎黄等症[1]。
鸡血藤是多年生木质藤本,野生于灌丛中、山地林间,主要分布于中国广西壮族自治区、云南省及越南、老挝、缅甸等国家[2,3]。
现代研究表明,鸡血藤具改善造血系统、免疫调节、降血脂、抗血栓、抗肿瘤等多种作用[4]。
目前该药材主要来源于野生资源,因其生长周期长,过度采伐,植被破坏,导致其野生资源逐年减少。
ISSR (Inter-simple sequence repeat )即简单重复序列区间扩增,由Zietkiewicz 等[5]于1994年提出,是基于PCR 的一种分子标记技术。
广泛应用于植物种质资源鉴定、遗传多样性、亲缘关系研究等方面[6,7]。
关于优化ISSR-PCR 反应体系的研究也越来越多,如淫羊藿[8]、藜芦[9]等。
因此,本研究采用L 16(45)正交试验和单因素试验对ISSR-PCR 体系中的主要成分进行优化,建立鸡血藤最佳ISSR-PCR 反应体系,并利用该体系筛选100条ISSR 引物,选出扩增条带清晰、稳定,适合鸡血藤的ISSR 引物。
为鸡血藤的遗传多样性及亲缘关系等方面研究提供科学依据。
龙头鱼ISSR-PCR多态性引物最佳退火温度的筛选

龙头鱼ISSR-PCR多态性引物最佳退火温度的筛选1. 引言1.1 研究背景龙头鱼(Siniperca chuatsi)是一种重要的淡水经济鱼类,具有重要的经济价值和科研意义。
随着人们对龙头鱼遗传资源研究的深入,分子生物学技术在龙头鱼研究中得到了广泛应用。
ISSR-PCR技术是一种基于PCR扩增的分子生物学技术,可以用于鱼类遗传多样性研究。
通过设计特定的引物,可以扩增鱼类基因组中的多态性DNA序列。
而引物的退火温度是影响ISSR-PCR扩增效果的重要因素之一。
对龙头鱼ISSR-PCR多态性引物最佳退火温度的筛选具有重要的理论和实践意义。
本研究旨在通过对龙头鱼ISSR-PCR多态性引物的最佳退火温度进行筛选,为进一步研究龙头鱼遗传多样性提供可靠的技术支持。
通过对龙头鱼不同退火温度下的ISSR-PCR扩增效果进行比较分析,可以为龙头鱼的遗传多样性研究提供重要参考。
1.2 研究意义龙头鱼是一种重要的商业性淡水鱼类,具有很高的经济价值和研究意义。
随着生物技术的不断发展,ISSR-PCR技术在遗传多态性研究中得到了广泛应用。
通过对龙头鱼ISSR-PCR多态性引物最佳退火温度的筛选,可以揭示龙头鱼遗传多样性的分布和差异,为种质资源的保护和利用提供重要理论依据。
研究龙头鱼ISSR-PCR多态性引物最佳退火温度也有助于深入了解龙头鱼的遗传背景和进化历史,为龙头鱼的种质改良和遗传育种提供参考。
本研究的意义在于为龙头鱼遗传多样性研究和种质资源保护提供理论支持,同时也为龙头鱼的遗传育种提供重要的参考信息,推动龙头鱼产业的可持续发展。
2. 正文2.1 实验材料与方法龙头鱼样品:从实验室保存的龙头鱼个体中随机选择10个个体作为实验样本。
ISSR引物:选择了5个ISSR引物作为实验引物进行多态性分析。
PCR试剂盒:包括Taq DNA聚合酶、引物、dNTPs、PCR缓冲液等。
琼脂糖电泳试剂:包括琼脂糖、TAE缓冲液等。
1. DNA提取:采用CTAB法从龙头鱼尾鳍组织中提取总DNA。
真藓(Bryum argenteum)ISSR-PCR反应体系优化及引物筛选

真藓(Bryum argenteum)ISSR-PCR反应体系优化及引物筛选马晓英;韦伟;臧程;于晶【摘要】In order to determine optimum ISSR-PCR reaction system for moss Bryum argenteum,the concentrations of template DNAprimers,dNTPs,Mg2 + and Taq DNA polymerase were optimized in four levels by PCR orthogonal experimental method.The appropriate primers were screened from 100 primers by temperature gradient PCR,and the optimal anneal temperature of the screened primers were determined.The results showed that the optimized 20 μL ISSR-PCR reaction system was as follows:template DNA 20 ng/20 μL,primers 0.45 μmol/L,Mg2 + 2.65 mmol/L,Taq DNA polymerase 0.4 U/20 μL,dNTPs 0.45 mmol/ing this system,50 primers with clear bands,repeatability well and polymorphism highly were selected from 100 primers.The establishment of this system,the screened primers and the annealing temperature could provide a theoretical basis for further research on the genetic diversity of bryophytes using ISSR molecular markers.%为了确定真藓最佳ISSR-PCR反应体系,采用PCR正交实验设计方法,对影响ISSR-PCR试验的模板DNA、引物、dNTPs、Mg2+、和TaqDNA聚合酶5个因素在4个水平上进行优化,并对100条引物逐一进行温度梯度PCR,筛选合适的引物并确定每个引物的最佳退火温度.结果显示优化的20 μL ISSR-PCR反应体系中包括20 ng /20 μL DNA模板、0.45 μmol/L引物、2.65 mmol/L Mg2+、0.4 U/20 μL Taq DNA聚合酶、0.45 mmol/LdNTPs.利用该体系最终筛选出50条扩增条带清晰,重复性好,多态性高的引物并确定其退火温度.这一体系的建立、引物的筛选及退火温度的确立为进一步利用ISSR分子标记技术对苔藓遗传多样性的研究提供理论基础.【期刊名称】《上海师范大学学报(自然科学版)》【年(卷),期】2017(046)005【总页数】7页(P668-674)【关键词】真藓;ISSR;正交设计;引物筛选;退火温度【作者】马晓英;韦伟;臧程;于晶【作者单位】上海师范大学生命与环境科学学院植物种质资源开发协同创新中心,上海200234;上海师范大学生命与环境科学学院植物种质资源开发协同创新中心,上海200234;上海师范大学生命与环境科学学院植物种质资源开发协同创新中心,上海200234;上海师范大学生命与环境科学学院植物种质资源开发协同创新中心,上海200234【正文语种】中文【中图分类】Q949真藓(Bryum argenteum Hedw.)为真藓科真藓属植物,属世界广布种,中国各地均有分布,在薄土岩面,石缝,屋顶,以及阴沟边缘等多种生境下均可生长[1].具有清热解毒,止血,治疗鼻窦炎、细菌性痢疾、灼伤、心绞痛等功效[2-4].目前,国内外用于苔藓植物遗传关系的分子标记技术,主要有inter-simple sequence repeat(ISSR)和random amplified polymorphic DNA(RAPD)两种.ISSR是一种简单序列重复区间扩增多态性DNA分子标记技术,是1994年由加拿大蒙特利尔大学的Zietkiewicz等[5-6]基于微卫星技术上创建起来的.该技术具有操作简单,成本低,有较好的重复性和稳定性的优点[7-10],但其结果易受反应体系中各个因素的影响[11-13],因此要建立适合研究材料,条带清晰,重复性好的反应体系.不同种植物适用不同的ISSR引物,且相同引物应用于不同种植物退火温度也会有差异,ISSR引物退火温度对扩增结果影响较大,且与其退火温度(Tm)值没有明显的规律性[14],根据目前国内已发表的相关报道可知,苔藓植物相对于其他很多植物筛得的ISSR引物数目较少,且退火温度多集中于48~55 ℃之间[15-20].本研究采用正交试验法对反应体系中的5种因素进行优化以得到最优的ISSR-PCR 反应体系,并利用最佳PCR扩增体系对哥伦比亚大学公布的100条引物进行筛选,对已筛得引物的退火温度进行总结,旨在为进一步利用ISSR分子标记技术对苔藓遗传多样性研究提供理论基础.1.1 实验材料所用的10×LA PCR Buffer II(Mg2+ free)、MgCl2、dNTP Mixture、LA Taq聚合酶购自宝生物工程(大连)有限公司;所用的哥伦比亚大学公布的100条引物由上海生工生物工程技术服务有限公司合成.1.2 实验方法1.2.1 DNA提取采用改良的十六烷基三甲基溴化铵(CTAB)法提取真藓基因组DNA.采用岛津Uv1800微量分光光度计测定所提DNA的浓度和纯度,OD260/OD280比值皆在1.9左右,说明DNA样品纯度较高,适合进一步PCR扩增反应.样品DNA存于-20 ℃作为后续的PCR扩增反应模板.1.2.2 ISSR-PCR反应体系优化用L16(45)正交试验设计方法,选择引物UBC825,在20 μL的反应体系中,参照汪琛颖等[20]的研究报道,对影响ISSR-PCR试验的模板DNA质量浓度,引物、dNTPs、Mg2+的物质的量浓度,和Taq DNA聚合酶生物量浓度5个因素在4个水平上进行优化(表1),共16个处理(表2)每个处理包含2 μL10×LA PCR Buffer II(Mg2+ free),用ddH2O补足.扩增反应程序94 ℃预变性4 min;94 ℃变性1 min,退火2 min,72 ℃延伸1 min,40个循环;最后72 ℃延伸7 min;4 ℃保存.PCR扩增产物用含有溴化乙锭(EB)核酸染料的2%琼脂糖凝胶电泳分离,电压100 V,60 min.并用全自动数码凝胶成像分析系统(Tanon 2500)进行观察和拍照.1.2.3 细调性正交试验为了最终获得清晰,可重复性好,亮度适宜,条带多态性丰富的扩增条带,在L16(45)正交试验结果的基础上,缩小各个因素的浓度梯度进行细调性正交试验.对影响ISSR-PCR试验的引物、Mg2+、dNTPs和Taq DNA聚合酶4个因素在3个水平上进行筛选(表3),共9个处理(表4).1.2.4 引物及其最佳退火温度筛选试验所用的哥伦比亚大学公布的100条引物,扩增条带稳定性且多态性各有不同,所以为选取背景清晰,条带稳定性好且多态性丰富的引物,对100条引物进行筛选.退火温度对扩增结果有十分关键的影响[21],在最佳反应体系基础上,利用PCR仪自动生成10个温度梯度,对所有引物逐一进行温度梯度PCR,以确定每个引物的最佳退火温度.2.1 L16(45)ISSR-PCR反应体系的正交试验结果分析图1为L16(45)正交试验电泳结果.由图1可知,16个处理的扩增产物凝胶电泳结果具有一定的差异性.其中6、8、11、12、15、16无扩增条带,1、2、5有微弱条带,9、10、13背景较亮条带模糊,7、14条带清晰但条带数较少,3、4条带清晰丰富且4较3扩增结果更好.说明最优组合偏差不大,为得到最适扩增体系,以此为基点,缩小各因素的浓度梯度进行细调正交试验.2.2 L9(34)细调性正交试验电泳结果图2为L9(34)细调性正交试验电泳结果.由图2可知,由于在L16(45)正交试验的基础上进行细调,所以各条带之间差异不大,除了1号处理之外均有明显条带,其中6号处理条带数较其他处理少,8、9号处理虽然条带数较多但条带模糊,亮度较弱,其余2、3、4、5、7扩增效果差异不大,综合条带数、亮度、清晰度、扩增特异性等方面,确定组合3为最优反应体系.即20 μL最优反应体系中,五因素最佳水平为:20ng/20 μLDNA 模板,0.45 μmol/L引物,2.65 mmol/L Mg2+,0.4 U/20 μL Taq DNA 聚合酶,0.45 mmol/L dNTPs.2.3 引物的筛选和退火温度的确立利用梯度PCR仪自动生成10个温度梯度,根据不同退火温度下各引物的扩增效果确定退火温度,从而对100条ISSR引物进行筛选和各引物最佳退火温度的确立.图3为引物840的退火温度梯度优化结果.图4为引物888、890、834退火温度梯度优化结果.由图3、4可知,4、16、23、32泳道的PCR扩增条带数量多且清晰.因此引物840、888、890、834的最佳退火温度分别为49.1、53.9、51.6、51.0 ℃.已筛得ISSR引物及其退火温度见表5.由表5可知,不同引物的最适退火温度差异较大,为验证所筛得引物可以区分不同地区真藓的差异性以及引物的有效性,选择来自云南省大理州剑川县(N26°32′29.784″,E99°53′12.905″)、云南省丽江市玉龙县白沙镇文海村(N27°00′48.24″,E100°09′23.92″)和浙江省杭州市龙岗镇仙人塘村(N30°17′32″,E119°2′47″)、舟山市岱山蓬莱公园(N30°14′40.465″,E122°12′17.238″)4个地点的8份真藓标本,提取DNA,对已筛得的引物进行PCR扩增(图5),不同地点的真藓DNA扩增结果有明显差异,且条带清晰明亮.说明所筛得的引物和优化的体系可用于不同地区真藓的遗传多样性的研究. ISSR-PCR扩增体系中的模板DNA、引物、dNTPs、Mg2+、和Taq DNA聚合酶的浓度均会对扩增结果产生影响,引物的浓度与条带清晰度和背景亮度有关,引物浓度过高会导致背景明亮条带模糊,引物浓度过低则会导致扩增不充分[20].Mg2+的浓度会影响聚合酶的活性,从而影响扩增效果[21],dNTPs浓度过高会影响聚合酶活性,过低会使扩增量降低,Taq DNA聚合酶与扩增的条带数目有关[22],模板DNA 对扩增结果影响较小,一般仅需微量的模板DNA,模板DNA浓度过大会使条带变得弥散[20].所以通过正交实验法检测各个因素的同时找到最优反应体系,正交试验结果表明,在20 μL ISSR-PCR反应体系中包括20 ng/20 μL DNA模板、0.45μmol/L引物、2.65 mmol/L Mg2+、0.4 U /20 μLTaq DNA聚合酶、0.45 mmol/L dNTPs.对于不同植物,适用的ISSR引物不同,同一ISSR引物应用于不同植物,其退火温度不同,退火温度对扩增效果起着至关重要的作用,退火温度会影响扩增条带的清晰度[22-23].所以本实验对引物设置温度梯度进行最适退火温度的筛选,从100条ISSR 引物中筛选出50条条带清晰,多态性丰富,稳定性好的ISSR引物.说明真藓具有丰富的遗传背景,可为今后真藓科植物遗传多样性及分类学研究提供科学依据.【相关文献】[1] 黎兴江.中国苔藓志(第四卷) [M].北京:科学出版社,2006:72.Li X J.Flora bryophytorum sinicorum (Vol.4) [M].Beijing:Science Press,2006:72.[2] 衣艳君.中国药用苔藓植物资源 [J].中草药,2000,31(8):624-628.Yi Y J.Resource of medicinal bryophytes in China [J].Chinese Traditional and Herbal Drugs,2000,31(8):624-628.[3] 袁志良,叶永忠,李孝伟.河南省药用苔藓植物的初步研究 [J].河南科学,2003,21(2):176-178. Yuan Z L,Ye Y Z,Li X W.The study of medicinal bryophytes in Henan Province [J].Henan Science,2003,21(2):176-178.[4] 吴玉环,杨海英,罗昊,等.东北地区药用苔藓植物资源及其开发利用前景 [J].生态学杂志,2004,23(5):218-223.Wu Y H,Yang H Y,Luo H,et al.Resources of medicinal bryophytes in north-eastern China and their exploitation [J].Chinese Journal of Ecology,2004,23(5):218-223.[5] Vos P,Hogers R,Bleeker M,et al.AFLP:A new technique for DNA finger-printing [J].Nucleic Acids Research,1995,23(21):4407-4417.[6] Zietkiewicz E,Rafalski A,Labuda D.Genome finger-printing by simple sequencerepeat(SSR)-anchored polymerase chain reaction amplification[J].Genomics,1994,20(2):176-183.[7] Lian C L,Zhou Z H,Hogetsu T.A simple method for developing microsatellite markers using amplified fragments of inter-simple sequence repeat(ISSR) [J].Journal of Plant Research,2001,114(1115):381-385.[8] Zhou Y Q,Du P L,FU P,et al.Optimization of ISSR-PCR system for Nerviliae fordii (Hance) Schltr.and its primer screening [J].Medicinal Plant,2015(6):33-39.[9] 王明明,宋振巧,王建华.ISSR标记技术及其在药用植物遗传育种中的应用 [J].中草药,2007,38(1):134-137.Wang M M,Song Z Q,Wang J H.Inter-simple sequence repeat and its application in medicinal plant genetic breeding [J].Chinese Traditional and Herbal Drugs,2007,38(1):134-137.[10] 欧立军,颜旺,廖亚西,等.天门冬ISSR分子标记技术的建立与体系优化 [J].中草药,2011,42( 2):353-357.Ou L J,Yan W,Liao Y X,et al.Establishment and optimization of ISSR-PCR reaction system in asparagus cochinchinensis [J].Chinese Traditional and Herbal Drugs,2011,42(2):353-357. [11] 代培红,周贵玲,苏秀娟,等.大赖草ISSR-PCR反应体系的优化及引物筛选 [J].分子植物育种,2016,14(10):2732-2738.Dai P H,Zhou G L,Su X J,et al.Optimization of ISSR-PCR system and primers screening of Leymus racemosus [J].Molecular Plant Breeding,2016,14(10):2732-2738.[12] 李硕,李敏,卢道会,等.当归ISSR-PCR反应体系的建立优化及引物筛选 [J].李时珍国医国药.2015,26(2):373-376.Li S,Li M,Lu D H,et al.Optimization of ISSR-PCR system and primers screening of angelica sinensis [J].Lishizhen Medicine and Materia Medica Research,2015,26(2):373-376.[13] 狄魁颖,白志军,杨书祥,等.杠柳ISSR引物的筛选及PCR反应体系的优化 [J].河北林果研究.2016,31(2):140-145.Di K Y,Bai Z J,Yang S X,et al.Optimization of ISSR primer and PCR reaction system for Periploca sepium [J].Hebei Journal of Forestry and Orchard Research,2016,31(2):140-145.[14] 郭秀霞,李灵芝,马太光,等.西葫芦ISSR-PCR反应体系优化及引物筛选 [J].山西农业科学,2017,45(3):325-327,331.Guo X X,Li L Z,Ma T G,et al.Optimization of ISSR-PCR reaction system and selection of primers in Cucurbita pepo [J].Journal of Shaanxi Agricultural Sciences,2017,45(3):325-327,331.[15] 王莹莹.浙江千岛湖生境片断化对苔藓植物物种及遗传多样性的影响 [D].上海:华东师范大学,2011.Wang Y Y.Effects of habitat fragmentation on bryophytes richness and genetic diversity inThousand-Island Lake region,Zhejiang [D].Shanghai:East China Normal University,2011. [16] 石蕾.R语言在藓类形态与遗传变异研究中的应用 [D].上海:上海师范大学,2015.Shi L.Application of R in studies of morphological and genetic variation of plant population:A case of Hypnum plumaeforme Wilson [D].Shanghai:Shanghai Normal University,2015.[17] Hassel K,Gunnarsson U.The use of inter simple sequence repeats (ISSR) in bryophyte population studies [J].Lindbergia,2003,28(3):152-157.[18] 魏青永,郭水良,曹同,等.基于ISSR数据探讨卷叶凤尾藓(Fissidens dubius P.Beauv.)遗传多样[J].植物科学学报,2016,34(2):238-245.Wei Q Y,Guo S L,Cao T,et al.Diversity and geographical distribution of Fissidens in eastern subtropical region,China [J].Plant Science Journal,2016,34(2):238-245.[19] 刘小慧.三种蓑藓属植物的形态变异与遗传结构研究 [D].上海:上海师范大学,2016.Liu X H.Research on morphological variations and genetic structure of three macromitrium species [D].Shanghai:Shanghai Normal University,2016.[20] 汪琛颖,赵建成.真藓科植物ISSR-PCR反应体系的优化及ISSR指纹图谱的初步构建 [J].安徽农业科学,2011,39(27):16490-16493.Wang C Y,Zhao J C.Optimization of ISSR-PCR reaction system and preliminary construction of ISSR fingerprinting of some species in Bryaceae [J].Journal of Anhui Agricultural Sciences,2011,39(27):16490-16493.[21] 刘德好,陆永良,娄玉霞.正交试验优化稗属植物ISSR-PCR反应体系 [J].上海农业学报,2016,32(4):17-21.Liu D H,Lu Y L,Lou Y X.Optimization of ISSR-PCR reaction system of Echinochloa by orthogonal design [J].Acta Agriculturae Shanghai,2016,32(4):17-21.[22] 林萍,张含国,谢运海.正交设计优化落叶松ISSR-PCR反应体系 [J].生物技术,2005,15(5):34-37. Lin P,Zhang H G,Xie Y H.Study on optimization for ISSR reaction system of larix using orthogonal design [J].Biotechnology,2005,15(5):34-37.[23] 刘威生,冯晨静,杨建民,等.杏ISSR反应体系的优化和指纹图谱的构建 [J].果树学报,2005,22(6):30-33.Liu W S,Feng C J,Yang J M,et al.Optimization of ISSR reaction system and construction of cultivar finger-print in Apricot [J].Journal of Fruit Science,2005,22(6):30-33.。
一种利用ISSR开发SSR引物的方法

植物生理学通讯 第42卷 第2期,2006年4月268一种利用ISSR开发SSR引物的方法郭大龙 罗正荣*华中农业大学园艺植物生物学教育部重点实验室,武汉430070A Method for Development of SSR Primers from ISSRGUO Da-Long, LUO Zheng-Rong *Key Laboratory of MOE for Horticultural Plant Biology, Huazhong Agricultural University, Wuhan 430070, China提要 介绍从简单序列重复间区(ISSR)开发微卫星(SSR)引物的方法。
该方法分两步: (1)正向引物的分离,即试材ISSR扩增,产物纯化回收并克隆至T载体,测序后设计正向引物(引物1)和巢式引物(引物2); (2)反向引物的分离,即基因组DNA酶切并连接接头,结合抑制PCR技术,对产物克隆测序后设计反向引物。
应用该方法前人已在多个物种中开发出多对SSR引物。
关键词 引物开发;ISSR;SSR微卫星(simple sequence repeat, SSR)以1 ̄6 bp短核苷酸为基本单位,呈串联重复状广布于整个基因组,具有多态性丰富、易于检测、进化所受选择压小、以孟德尔方式分离、共显性遗传等特点。
在遗传多样性检测、种质鉴定及系谱分析、遗传连锁图谱构建、基因定位与标记辅助育种等领域得到广泛应用。
SSR标记检测的位点多态性水平明显高于RFLP(限制性酶切片段长度多态性),而且重复性优于RAPD(随机扩增多态性DNA), 与AFLP(扩增片段长度多态性)相比,不同试材所获结论有差异(Powell等1996)。
但AFLP是一种显性标记,不能获得相关位点的详细信息。
由于SSR标记在图谱上的丰富性,它已表现出更大的优势(高志红等2002)。
但SSR分析时引物的获得需要预知核酸序列,其广泛应用常受限于从特定物种中分离SSR位点的难度和耗费(Hakki和Akkaya 2000)。
湿地植物小叶章SSR引物筛选及反应体系优化

湿地植物小叶章SSR引物筛选及反应体系优化徐明怡;王丽媛;冷海楠;张玉;倪红伟【摘要】开引物发合适的小叶章PCR-SSR引物并建立优化的反应体系是开展小叶章遗传多样性研究的基础。
利用正价设计方法对小叶章SSR反应体系中的DNA 模板和引物2个反应因素进行了9个水平的优化,并通过温度梯度优化引物退火温度。
自主开发了51对小叶章SSR引物,筛选出12对扩增条带清晰、多态性丰富的SSR引物,为小叶章遗传多样性研究提供方法和理论依据。
%A orthogonal design was used to optimize SSR amplification for Deyeuxia angustifolia in 9 levels of two factors (DNA template and primer). 51 pairs of SSR primers were developed independently. The screened 12 pairs of polymorphic SSR primers were suitable for studying on genetic diversity of Deyeuxia angustifolia.【期刊名称】《国土与自然资源研究》【年(卷),期】2016(000)003【总页数】5页(P88-92)【关键词】小叶章;SSR分子标记;引物筛选【作者】徐明怡;王丽媛;冷海楠;张玉;倪红伟【作者单位】湿地与生态保育国家地方联合工程实验室,哈尔滨150040;湿地与生态保育国家地方联合工程实验室,哈尔滨150040;湿地与生态保育国家地方联合工程实验室,哈尔滨150040;湿地与生态保育国家地方联合工程实验室,哈尔滨150040;湿地与生态保育国家地方联合工程实验室,哈尔滨150040【正文语种】中文【中图分类】S722小叶章(Deyeuxia angustifolia)是禾本科野青茅属植物。
SSR分析简单序列重复SSR又称...

摘要梨属于蔷薇科的梨亚科,品种很多,长期以来在分类上存在很多的问题。
本论文的目的是研究主要梨品种细胞质遗传多态性。
采用PCR-RFLP方法,对提取出的总DNA用10对叶绿体通用引物进行扩增,对PCR产物用限制性内切酶AluI,HaeIII,HinfI,Hin6I,RsaI,MvaI 和TaqI进行酶切,对19种梨(包括新疆梨系统、白梨系统、西洋梨系统、秋子梨系统、杜梨、沙梨系统)的叶绿体基因组trnS-trnfM非编码区进行克隆、测序。
应用DPS v7.05和DNAMAN、DNAStar、ClustalX-1.83、PHYLIP -3.68软件进行分析。
通过序列比对,再进行聚类分析,最后依据所得结果确定所测分子序列的亲缘关系,构建系统进化树。
结果显示:10对引物中只有7对(cp01,cp 02,cp 03,cp 04,cp 06,cp 09,cp 10)能在梨属植物上扩增出一条特异性谱带,这说明梨属植物叶绿体基因组序列十分保守,3个引物对(cp05,cp07,cp08)不能在梨属植物上扩增出谱带。
931份引物对/酶切组合中,cp09/MvaI,cp03/Hin6I 的酶切位点有显著差异。
对梨属植物的cpDNA trnS-trnfM区域进行克隆、测序,所得的序列长度为:库尔勒香梨和鸭梨的序列最长(1642bp),苹果梨、早酥梨、慈梨、象牙、翠伏的序列最短(1272bp)。
用DNAMAN软件对序列进行比对分析:库尔勒香梨与白梨系统的同源性为:85.01%,与新疆梨系统的同源性为:78.60%,与西洋梨系统的同源性为:78.28%,与沙梨系统的同源性为:77.47%,与秋子梨系统的同源性为:77.91%。
根据ClustalX软件完全比对的结果,用PHYLIP -3.68软件的邻接法对cpDNA trnS-trnfM区域序列变异位点构建系统进化树。
黑酸梨和京白聚为一类,伏茄和身不知聚为一类,冬巴和新世纪聚为一类。
