原子核的基本性质
第24章 原子核物理和粒子物理简介

M (Z , A) m(Z , A) Zme Be (Z ) / c
例如,对于氢原子,我们有
2
Be (Z ) 13.6eV
mHc 13.6eV mpc mec
2 2
2
二、原子核的模型
卢瑟福用粒子轰击金箔的散射实验
1 1 (2 e )(79 e ) 2 mα v 2 4π 0 2R
I I0et
国际单位:贝克勒尔(Bq) 1Bq表示每秒发生一次核衰变的放射源的活度。 常用单位:居里( Ci)
1Ci 3.7 10 Bq
10
二、原子核的三种衰变方式
1、 粒子衰变
是不稳定核自发地放出氦核的过程。 射线是粒子流,是带正电的氦核。
一般的过程可以表示为:
A Z
X
A4 Z 2
T1
2
ln 2
0.693
平均寿命
每个原子核衰变前存在的时间的平均值。
原子核的寿命:
L t (dN ) t Ndt t N 0 e
0
t
0
dt
N0
平均寿命:
L 1 N0
平均寿命与半衰期的关系:
T1 2 ln 2
几种放射性同位素的半衰期 同位素 衰变方式 半衰期
原子核的自旋和磁矩
原子核
2 1
6 3 7 3
自旋量 子数
1 1 3/2 1
磁矩
0.8565p
自旋量 原子核 子数
16 8
磁矩
——
1.16p
H
O
0 3/2 3/2 9/2
Li Li
0.8213p
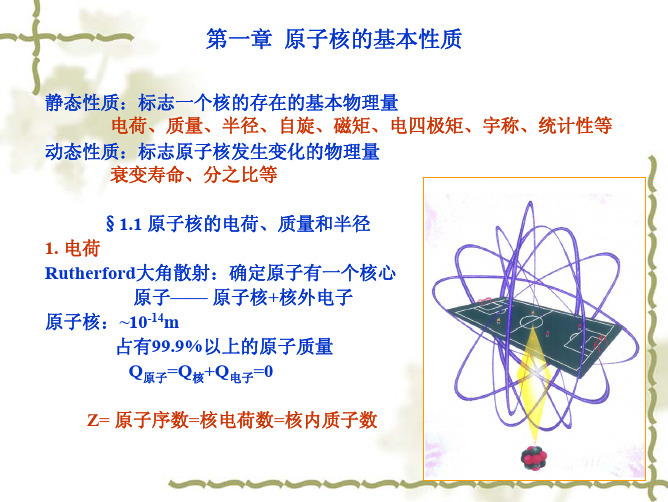
第一章 原子核的基本性质

式中
r0 (1.4 1.5) 10 cm (1.4 1.5) fm
1fm=10-13cm = 10-15m
R r0 A
1/ 3
(1.1 8)
13
(2) 电荷分布半径
测量方法:利用高能电子在原子核上的散射,电 子波长小于核半径
E E m c 2 k 0 1 2 2 4 2 E (c p m0 c ) h p hc 解之得 1 2 [ Ek ( Ek 2m0c )] 2
3. 测量方法---质谱仪
三部分: 离子源、电磁场、探测器
质量为M的离子通过加速电极后所具有的速度v,满足下列关系:
M 2 qV 2
(1.1 2)
被加速的离子在磁场B的作用下,将在垂直磁场的平面内以半 径R作圆弧运动,最后通过狭缝 S 2到达接收电极。于是有
由以上两个式子消去v可得
Mv 2 qvB R
e e l Pl gl P l 2me 2me
(1.3-2)
s g s B Ps
(1.3-3)
和
l g l B Pl
(1.3-4)
e 式中 B 9.2740 10 24 A · m 2,称之为玻尔磁子。 2me
qB 2 R 2 M 2V
3作用半径
中子、质子等粒子的散射 快中子---核散射
2 ( R )
2
散射截面等于单位时间的散射粒子数除以入射 粒子通量[表示一个入射粒子被单位面积靶上 一个靶核散射的几率]
测得R
实验表明:核半径与质量数A有关。它们之间的关系可近似地表 示作下面的经验公式:
设F=I+j,I+j-1,…时的相互作用能E分别为E1, E2,…,由(1.2-8)式就容易算得两相邻能级的间距
2.核物理与粒子物理讲义-第一章原子核的基本性质1
与此同时,天体物理的许多重要问题如能量和元素的来源,中子星 的结构和冷却,超新星的爆发,都涉及到基本的核物理问题,尤其是弱 束缚核的结构和反应。另一方面,天体中的核过程与核聚变等装置中的 核过程相似,通过相关研究可以为核能源开发应用等提供重要信息和参 考资料。核物理与天体物理的交叉不仅是人类认识天体及宇宙演化过程 及规律的重要方式,并且与能源开发和利用、国防安全建设等密切相 关。放射性核束物理涉及众多新的核样本和核数据,将在超重核合成合 成、新型核材料、新型核能装置等方面产生难以估量的重大影响。
1、259Db合成:首次进入超重核区
测量结果: Eα = 9.47MeV,
22Ne+241Am→259Db
探测器面对产物样品测得的α谱
T1/2 = 0.47 s, Qα=9.70MeV 我国新核素合成首次进入超重区!
A new alpha-emitting isotope 259Db Euro. Phys. J.,A10, (2001) 21-25 产物样品移去后测得的α谱
(197 Au, 10 B, 16 C, 10 He, 11Li, 11Be) 79 5 6 2 3 4 2 3 235 U, 238 U) (1 1H, 1H, 1H 92 92 3 4 (2 1H, 2 He, 3 Li) 40Ar , 40K , 40Ca ) (18 19 20 60m 60 * 同核异能素(Isomer):有确定的质子数和中子数但能量不同的核素 ( 27 Co或27 Co )
■
未来5年— 超重元素探索和新核素的合成
关键科学问题:超重核合成的新机制和技术
1)截面1 pb以下;2)现有融合体系中子数缺10个左右;3)长寿命核无法利 用现有在束 α-α 级联衰变的方法进行单个事件鉴别
原子核的基本性质

1 12
12
1 1 =1.6605655×10 27kg = NA 12 6.022045×1023
8
第一章 原子核的基本性质
测质量的质谱仪方法(电磁方法):
首先让原子电离,然后在电场中加速以获得一定动能,接着在磁场中偏 转,由偏转的曲率半径的大小可求得离子的质量。 D 为一扁平的真空盒,放于磁铁间隙内;
实际仪器中,B和R都已固定,q也已知,只要改变加速电势差V就可测得 不同的粒子质量M。
例:设离子带一个单位电荷,B=0.3580T,R=0.05m,实验测得V=672V
时,离子电流有一极大值,则由公式可以算出所测离子质量
19 ×(0.358)2 ×(0.05)2 qB2R2 1.6×10 Kg = 3.81×10 26kg = 2×672 2V
13
第一章 原子核的X :元素符号
Z :核电荷数 N :中子数 A :核子数(A=Z+N)
Li4
A
元素符号X与Z具有唯一的确定关系,
Z可省略, N=A-Z 也可省略。
X
7
Li
14
第一章 原子核的基本性质
§1.3.核的半径
(1)核力作用半径:核力有一作用半径,在半径之外,核力为零。这
7
第一章 原子核的基本性质
§1.2.核的质量
原子的质量是原子核质量与核外电子质量之和,同时考虑结合能时:
MA=MN+Me-We
一般不必推算原子核质量,对于核的变化(核反应),变化前后的
电子数目不变,电子的质量可以自动相消 一个原子质量单位定义如下:
1u=12C原子质量的
原子质量单位与kg的关系为:
种半径叫做核半径,这样定义的核半径是核力作用的半径
原子核的基本性质

四、 质量和结合能
原子核的液滴模型
1.质量:核质量=原子质量-核外电子总质量
实际中,常近似用原子质量。 原子质量单位:
1u
12 1 1.6605387 1027 kg N A 12
由质能关系: E
mc 2
1uc 2 931.494MeV
电子静止质量:
me c 2 0.511MeV
R 1.1 A1/ 3 fm
高能电子
3.改进公式:
R rp z1/ 3 , rp 1.64 fm
4.实验表明:对中质比大的原子核,中子的分布半径比质子的大, 出现“中子皮”,“中子晕”。
6 2
He, 48 Be
11 3
Li
5.估计核的密度
4 4 V R 3 r03 A A 3 3
不能直接测量,通过原子核与其它粒子相互作用间接测量.
1.核力作用半径
通过中子、质子或者其它原子核与核作用,得到经验公式:
R r0 A1/ 3 , r0 (1.4 1.5) fm
n, p 原子核
2.电荷分布半径:
用高能电子在原子核上的散射,要求:电子的波长必须小于核的半径, 即要求电子的能量高
第一节
一、 组成
原子核的电荷、质量和半径
原子核=质子+中子 核子
A Z
X A Z
同位素(Isotope):
Z相同
同中子素(Isotone):
同量异位素(Isobar): 同量异能素(Isomer):
A-Z相同
A相同 能量状态不同
60
Co, 60 mCo
7 3 7 Li4 , 4 Be3
镜像核(mirror nuclei): A相同,质子数和中子数互换
原子核的性质

1920年J.Chadwick用已知Z的金属箔进行α粒子散射实验,直 接确定出核电荷。
3、核电荷数守恒
电荷具有两种功能:
1)表示电磁作用的强度 a e2 / c
2)服从相应的守恒定律
在核物理所研究的所有相互作用中,电荷均守恒。
电荷守恒定律已为实验所证实。实验的基本思想是试图记录相 应于原子内电子向K层跃迁的电磁辐射,而K层中的空穴是由违反 电荷守恒定律的电子衰变所形成。
M 4.81010 (3.58103 )2 52 3.821023 g 2 672 ( 1300) (31010 )2
A
M 1.66 1024
23
实验测得的接收电极的离子电流I随加速电势差V的变化关系 的典型曲线如图2所示。根据曲线峰值电压之比V1:V2,可求得 质量之比M2:M1。因此如果利用已知质量的峰值电压,可以得出 待测离子的质量。
M Dc2 (M A M B MC )c2 TA TB TC TD
(M A M B MC )c2 Q
D的质量为:
M D M A M B M C M
M
Q 931.50
Q TC TD TA TB
Q称为反应能
4、核子的质量
为了分析原子核的性质,非常重要的是要知道构成原子核的成
3、核质量的测量方法
原子核质量的测定常用以下方法 1)质谱测定法 2)核反应能量平衡分析 3)α衰变平衡 4)β衰变平衡 5)微波放射光谱测定法 1)、质谱仪测定法 基本原理:首先让原子电离,然后在电场中加速以获得一定 动能,接着在磁场中偏转,由偏转的曲率半径的大小可求得离 子的质量。
图1是早期所用的一种质谱仪的原理图。D为一扁平的真空 盒,它放置在一磁铁间隙内。磁铁产生的均匀磁场,其磁场强度 H垂直于真空盒平面。真空盒内主要有离子源K,加速电极E1, E2和接收电极A。由离子源产生的被测离子,通过加速电极的狭 缝S1后,获得动能:
原子物理第九章原子核

三. 衰变
是核电荷数改变而核子数不变的核衰变。主要有: -衰变,+衰变,K俘获
1.- 衰变能谱与中微子假设
-衰变中,放出负电子,原子核变为原子序数增加1的核。
衰变面临的难题
衰变连续谱导致了下列无法解释的难题:
1)连续谱的出现与能量守恒以及核能级量子 化相矛盾 由 衰变知核能级是量子化的, 而衰变能 是一定的,等于 E, 一定的衰变能在核与 粒 子之间分配时, 若 粒子分得的能量是连续 的,那就意味着核能级也是连续的,如果核能 级不连续,那么在没有核能级的地方, 系统能 量不守恒;
它的两侧,构成稳定核素区。
②、稳定核素中质子数与中子数之比:轻核
为1;最重的核 N / Z 1.6
③、Z<84的核素有一个或几个稳定的同位素;
Z>84的以及质子数或中子数过多的核都
是不稳定的放射性的同位素。
4.原子核的大小和形状
原子核的形状一般为近似椭球,其长短半轴之比一般不大于 5/4,可近似看作球形。核电四极矩是核偏离球形的量度。
1930年,泡利针对上述矛盾,大胆地提出 了中微子假说。他预言,在 衰变的同时,还 发射一个自旋为 1 2 ,不带电, 静质量几乎为0 的粒子。 称其为中微子 ( ) , 引入中微子之 后,上述矛盾迎刃而解。并且人们在1956年从 实验中找到了中微子。 中微子特性 中微子 的静质量几乎为0--不大于 10eV ; 穿透本领极大,在原子密度为 10 (个 cm ) 的 物质中,其平均自由程约为 1016 km ;即使在 核物质中,平均自由程也达 1km,因此,它 穿越地球被俘获的几率是 1012 ,它的自 旋为 2
µ ´I(核磁子) -1.91280
+2.79255 +0.857348 0 +0.82189 +3.25586 -1.1774 表6.2续
原子核的基本性质

ρ = nmn =1.66×1014 g / cm3
即,一个火柴盒那样大体积的核物质的重量为10亿吨。
四、 质量和结合能
原子核的液滴模型
1.质量:核质量=原子质量-核外电子总质量
实际中,常近似用原子质量。 原子质量单位:
u 1 =
12 1 ⋅ 1.6605387×10−27 kg NA 12
由质能关系: E = mc2
一、原子核的比结合能几乎为常量, B ∝ A 说明核子之间的相互作用力具有饱和性,与液体分子力的饱和性类似。 二、体积近似正比于核子数,即核物质密度几乎是常量,不可压缩性,与液体类似。 因此,把原子核看成带电的液滴。
(2)魏扎克(Weizsacker)公式
1935年,结合能半经验公式: (2).8页
2I −1 Q 0 2(I +1 )
第五节 原子核的宇称
宇称:微观物理领域中特有的概念,描述微观体系状态
波函数的一种空间反演
宇 算 :ˆ 称 符 P
ˆ PΨ(x) =Ψ(−x) ˆ PΨ(x) = kΨ(x), Ψ P 本 态 k 本 值 ( (x)是ˆ的 征 , 是 征 )
ˆ P2Ψ(x) = k2Ψ(x) =Ψ(x)
原子核的磁矩
µs =−
µl =−
r
r
e r e r ps = gs ( ) ps (gs =−2) m 2m e e
e r e r pl = gs ( ) pl (gs =−1 ) 2m 2m e e
二.核子的磁矩 质子自旋的磁矩: 中子自旋的磁矩:
µp = gp (
e r ) ps 2mN e r r µn = gn ( ) ps 2mN
(1) 当 ≤ j时 有 I , 2I+1个 , 值能级分裂成2I+1个 级 能 ,
原子物理-原子核物理

二同号点电荷及其 等效电荷分布
图7.1.2Biblioteka 旋转椭球所以旋转椭球式的电荷分布等效于一个单电荷和一个四极
子的迭合。令Q=2a3/e,称为电四极矩。可以证明原子核的电 四极矩可以用下式表示:
2.原子核的自旋 在§4.8节已经讲过原子核的自旋与磁矩的内容。这里我们给
出由实验测得原子核基态时的自旋I有如下规律:
第七章
§7.1
原子核物理学
原子核的基本性质
7.1.1
原子核的电荷、质量和密度
1.原子核的电荷和电荷数
2.原子核的质量和质量数
3.原子核的大小和密度
核半径与A 1/3成正比,这说明以下两点: (1)原子核的体积V正比于核内核子数A,即
也就是说,在不同的原子核内,每个核子所占的体
例题7.2.3:已知 235U原子的质量为235.043
944u,试计算其结
合能和比结合能。
解:由(7.2.1)式和(7.2.2)式知235U的结合能为
EB(235,92)=(92×1.007 825+143×1.008 665
-235.043
944)×931.5 MeV≈1783.87MeV 783.87MeV/235≈7.59MeV
(2)核力的电荷无关性
(3)核力是具有饱和性的交换力
(4)非有心力的存在
3.核力的介子理论
P=n+π+
n= p+π -
p=p±π0
n=n±π0
图7.2.2π介子作为核力的传播子 §7.3
原子核的结构模型
:(1)原子核的结合能近似地正比于核中的核子 数A,即比结合能近似为常数,这说明核子间相互作用力具有 饱和性,这与液体分子间相互作用力的饱和性类似。
原子核的电荷和质量

2、原子核电荷的测量
核电荷的测量方法有多种,比较精确的方法是在1913年 由莫塞莱(H.G.J.Moseley)提出的。他发现元素所放出的特 征X射线的频率ν与原子序数Z之间有下列关系
AZ B
式中A,B是常数,对于一定范围内的元素,它们不随Z改变。
例如:由元素钇(39Y)到银(47Ag)的K 线的频率可定出:
E=M0c2+T 运动粒子的质量增加为:ΔΜ=T/c2 其质量为:
M M 0 T c2 M 0 M 0 1 2
其中:
1 1 2
v c
所以:
E M0c2 T M0c2
Mc2
1 2
这里:
MEMM00c2 T
1 2
是总能量 是相对论质量
Ek qV
其中:q是离子的电荷;V是加速 电极E1和E2之间的电势差。
则质量为M的离子通过加速电极 后所具有的速度v满足下列关系:
1 Mv2 qV 2
被加速的离子在磁场H的作用下,将在垂直磁场的平面内以半径R 作圆弧运动,最后通过狭缝S2到达接收电极,有:
qvH Mv2
cR
消去v则有:
分析实验结果得出:原子中存在一个带正电的核心,叫做原 子核。原子核的大小约为10-12cm数量级,只有原子大小的万分 之一。原子核的质量却占整个原子质量的99.9%以上。
由于原子是电中性的,因而原子核带的电量必定等于核外电 子的总电量,但两者符号相反。
任何原子的核外电子数就是该原子的原子序数Z。因此原子序 数为Z的原子核的电量为Ze。
q M
2Vc 2 H 2R2
M
qH 2R2 2Vc 2
20-1原子核的基本性质

表20-2 原子核的自旋量子数和磁矩
自旋 量子数 自旋 量子数
核
2 1
磁矩
核
7 3
磁矩
D
N
1 1 1 0
0.86 μ p
0.82 μ p
Li
3/ 2 3/ 2 3/ 2
9/2
3.25μ p
6 3
Li
O
23 11
Na
K
14 7 16 8
0.40 μ p
39 19 113 49
In
2.22 μ p 1.14 μ p 5.49 μ p
4 3 πR ∝ A 3
R ∝ A R ∝ A3
3
fm为飞米,则上式可写为:
R = R0 A
1 3
1 3
( R0 = 1.2 × 1015 m = 1.2fm)
R = 1.2 A fm
原子核的大小和形状
根据式(20-1)可以算得 C、O、 Ag 和 半径分别为:
12 6C : R 16 8O : R 1 ≈ 1.2 × 123 1 ≈ 1.2 × 163
核力和介子
π +介子:质子放出一个 π + (c)质子与中子间交换
介子被中子吸收,同时质子转化为中子,中子转 化为质子;
π -介子:中子放出一个 π (d)中子与质子间交换
介子被质子吸收,同时中子转化为质子,质子 转化为中子。
核力和介子
p p p
π
0
p
n
π0
p n
n n
质子与质子间和中子与中子间的相互作用示意图
核磁共振
核磁矩在磁场 B中受到作用,具有能量 E,而 该能量是量子化的:
v v E1 = M p B cosθ = M p B (θ = 0) E = M p B E2 = M p B cosθ = M p B (θ = π )
原子核的基本性质和结构

原子核的基本性质和结构原子核是原子的中心部分,它由质子和中子组成,它们被称为核子。
在原子核中,质子和中子被强相互作用力所约束,并保持着基本稳定的结构。
质子是带有正电荷的粒子,它们的质量约为1.67×10^-27千克。
中子是没有电荷的粒子,它们的质量与质子相近。
原子核的质量可以通过质子和中子的质量之和来计算。
原子核的直径约为1到10费米,而整个原子的直径则约为0.1到1纳米,因此原子核相对于整个原子来说非常小。
原子核的密度非常大,约为10^17千克/立方米,比普通物质的密度高了几个数量级。
原子核中质子和中子的数目决定了元素的化学性质和同位素的存在。
质子数目决定了元素的原子序数,即元素在周期表中的位置。
原子核中的中子数目可以有所不同,这就导致了同一元素的不同同位素。
质子和中子是由夸克组成的。
质子由两个上夸克和一个下夸克组成,而中子由一个上夸克和两个下夸克组成。
夸克是一种基本粒子,它们具有分数的电荷。
夸克通过强相互作用力相互绑在一起,形成质子和中子。
原子核内部的夸克之间通过交换胶子来保持稳定。
胶子是一种传递强相互作用力的粒子。
这种相互作用力非常强大,能够克服质子和中子之间的静电排斥力,使原子核保持相对稳定。
原子核的能级结构与电子的能级结构有所不同。
原子核中的质子和中子也具有能级,但是这些能级非常密集,因此它们表现为连续的能带而不是离散的能级。
原子核的能级结构对于核反应和放射性衰变等核物理过程非常重要。
原子核的稳定性受到核力和库伦排斥力的竞争影响。
核力是一种短程强相互作用力,它能够克服库伦排斥力,使原子核保持相对稳定。
当原子核中的质子数目太多时,库伦排斥力开始支配,原子核变得不稳定,这导致了放射性衰变的发生。
总之,原子核是原子的中心部分,由质子和中子组成。
它具有基本稳定的结构,其中质子和中子通过强相互作用力相互绑在一起。
原子核的能级结构与电子的能级结构有所不同,并且原子核的稳定性受到核力和库伦排斥力的竞争影响。
-原子核的基本性质

原子核物理基础概论原子核是原子的中心体。
研究这个中心体的性质、特征、结构和变化等问题的一门学科称为原子核物理学。
一、原子核物理的发展简史1.1886年 Bequenel发现天然放射性。
进一步研究表明,放射性衰变具有统计性质;放射性元素经过衰变(α,β, );一种元素会变成另一种元素,从而突破了人们头脑中元素不可改变的观点。
2.1911年 Rutherford α粒子散射实验,由α粒子的大角度散射确定了原子的核式结构模型。
3.1919年α粒子实验首次观察到人工核反应(人工核蜕变)。
使人们意识到用原子核轰击另外的原子核可以实现核反应,就象化学反应一样。
4.1932年查德威克中子的发现表明原子核由质子和中子构成,中子不带电荷,易进入原子核引起核反应。
在这件大事中,实际上有我国物理学家的贡献。
根据杨振宁先生的一篇文章介绍,我国物理学家赵忠尧在1931年发表了一篇文章,文中预言了中子的存在,但查德威克看了之后未引用,故失去了获得诺贝尔奖的机会。
5.20世纪40年代核物理进入大发展阶段(引用科学史材料):(1)1939年Hahn发现核裂变现象;(2)1942年Fermi建立第一座链式反应堆,这是人类利用原子能的开端;(3)加速器的发展,为核物理理论和核技术提供了各种各样的粒子流,便于进行各种各样的研究;(4)射线探测器技术的提高和核电子学的发展,改变了人类获取实验数据的能力;(5)计算机技术的发展和应用,一方面进一步改进了人们获取数据,处理核数据的能力,另一方面提供了在理论上模拟各种核物理过程的工具。
例如模拟反应堆中中子的减速、慢化过程等物理过程。
二、核物理的主要研究内容核物理学可以分为理论和应用两个方面。
理论方面是对原子核的结构、核力及核反应等问题的研究。
同其它基础研究一样,是为了了解自然、掌握自然规律,为更好地改造自然而开辟道路的。
另一方面是原子能和各种核技术的应用,包括民用与军用。
这两方面的研究相互联系,相互促进,相互推动向前发展。
(完整版)原子核物理知识点归纳详解

原子核物理重点知识点第一章 原子核的基本性质1、对核素、同位素、同位素丰度、同量异位素、同质异能素、镜像核等概念的理解。
(P2)核素:核内具有一定质子数和中子数以及特定能态的一种原子核或原子。
(P2)同位素:具有相同质子数、不同质量数的核素所对应的原子。
(P2)同位素丰度:某元素中各同位素天然含量的原子数百分比。
(P83)同质异能素:原子核的激发态寿命相当短暂,但一些激发态寿命较长,一般把寿命长于0.1s 激发态的核素称为同质异能素。
(P75)镜像核:质量数、核自旋、宇称均相等,而质子数和中子数互为相反的两个核。
2、影响原子核稳定性的因素有哪些。
(P3~5)核内质子数和中子数之间的比例;质子数和中子数的奇偶性。
3、关于原子核半径的计算及单核子体积。
(P6)R =r 0A 1/3 fm r 0=1.20 fm 电荷半径:R =(1.20±0.30)A 1/3 fm 核力半径:R =(1.40±0.10)A 1/3 fm 通常 核力半径>电荷半径单核子体积:A r R V 3033434ππ==4、核力的特点。
(P14)1.核力是短程强相互作用力;2.核力与核子电荷数无关;3.核力具有饱和性;4.核力在极短程内具有排斥芯;5.核力还与自旋有关。
5、关于原子核结合能、比结合能物理意义的理解。
(P8)结合能:),()1,0()()1,1(),(),(2A Z Z Z A Z c A Z m A ZB ∆-∆-+∆=∆= 表明核子结合成原子核时会释放的能量。
比结合能(平均结合能):A A Z B A Z /),(),(=ε原子核拆散成自由核子时外界对每个核子所做的最小平均功,或者核子结合成原子核时平均每一个核子所释放的能量。
6、关于库仑势垒的理解和计算。
(P17)1.r>R ,核力为0,仅库仑斥力,入射粒子对于靶核势能V (r ),r →∞,V (r ) →0,粒子靠近靶核,r →R ,V (r )上升,靠近靶核边缘V (r )max ,势能曲线呈双曲线形,在靶核外围隆起,称为库仑势垒。
1.2 原子核的基本性质

质子数Z
A ZA
X Z N
XN
中子数N
实际上核素符号X和质子数Z具有唯一、确定的关 A 系,所以用符号 X足以表示一个特定的核素。
III. 原子核物理常用术语及意义
1、核素(nuclide)
具有一定数目的中子和质子以及特定能态的一种 原子核或原子称为核素。 A 12 12 12 Z N 6 6 6 核子数、中子数、质子数和能态只要有一个不同, 就是不同的核素。 208 208 两种核素,A同,Z、N不同。 86Tl 82 Pb 90 91 Sr 两种核素,N同,A、Z不同。 38 39Y
Cl18 奇质子核。
镜像核:中子数N、质子数Z互换的核素。
7 3
Li4
7 4
Be3
IV. 核素图及β 稳定曲线 核素图
β稳定曲线
核素图及β稳定曲线的特点:
1).核素图包括300多个天然存在的核素 (其中稳定核素280多个,放射性核素30 多个)及1600多个人工放射性核素。
2).稳定同位素几乎全落在一条光滑的 曲线,稳定曲线在轻核靠近 Z=N 线, 而对重核则 N > Z. 3).偏离稳定曲线上方的核素为丰中子核 素,易发生β-衰变;下方的核素为缺中 子核素,易发生β+衰变。
释放核能的两个途径:
1)重核裂变 分裂成较小的原子核
2)轻核聚变 形成较重的原子核
•重核裂变 —— 中子引起
235U
裂变
原子核能
1)1903年卢瑟福研究了射线能 量后指出,"这些需要加以思考 的事实都只相同一个结论,即 潜在原子里面的能量必须是巨 大无比的。”由此,人们把放 射能称作原子能。
2 0 0 MeV !
H 71%
生的能量不再能维持聚变反应继续进行(包括较重元
原子核物理学

原子核物理学是研究原子核的结构、性质、形成以及相互作用的物理分支。
这一领域涉及从基本粒子到宇宙尺度的广泛现象,是现代物理学中极为重要的组成部分。
原子核物理学是研究原子核内部结构、性质以及相互作用的科学。
自从1932年詹姆斯·查德威克发现中子以来,原子核物理学得到了迅速的发展。
这一领域的研究不仅对基础科学具有重要意义,而且对核能、核技术以及核医学等应用领域有着深远的影响。
一、原子核的基本性质1. 组成与结构原子核由质子和中子组成,这两种粒子统称为核子。
质子带有正电荷,中子不带电。
原子核的大小约为10^15米,远小于原子的大小。
2. 质量与结合能原子核的质量小于组成它的核子的质量之和,这种质量的亏损称为质量亏损。
根据爱因斯坦的质能方程E=mc^2,质量亏损对应着原子核的结合能,即核子结合在一起所释放的能量。
3. 电荷与自旋原子核带有正电荷,其大小等于核内质子的数目。
原子核具有自旋角动量,其大小取决于核子数和核子的排列方式。
二、原子核的稳定性与放射性1. 稳定性条件原子核的稳定性取决于其质子与中子的比例。
在轻核区域,质子与中子的比例接近1:1,而在重核区域,中子的数目多于质子。
原子核的稳定性还受到其自旋和形状的影响。
2. 放射性衰变不稳定的原子核会自发地发生放射性衰变,释放出粒子或电磁辐射。
常见的放射性衰变类型有α衰变、β衰变、γ衰变等。
α衰变:原子核释放出一个α粒子(两个质子和两个中子组成的粒子),转变为一个新的原子核。
β衰变:原子核中的一个中子转变为一个质子,同时释放出一个电子和一个反中微子,或者一个质子转变为一个中子,同时释放出一个正电子和一个中微子。
γ衰变:原子核从激发态跃迁到基态时,释放出γ射线。
三、原子核反应与核能1. 核反应核反应是指原子核之间或原子核与粒子之间的相互作用。
核反应可以是自然的,也可以是人工引发的。
常见的核反应有核裂变、核聚变等。
核裂变:重核在中子的轰击下分裂成两个或多个轻核,同时释放出大量能量。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
原子核的半径
r r0 A
• 原子核密度的量级:
1
3
• 原子核半径参数r0:1.1(1.2)fm(核电荷分 布), 1.4~1.5fm(核力作用) 1014 g/cm3
讨论和提问
Werner Heisenberg
• born Dec. 5, 1901, Wurzburg, Ger. died Feb. 1, 1976, Munich • German physicist and philosopher who discovered a way to formulate quantum mechanics in terms of matrices (1925). For that discovery, he was awarded the Nobel Prize for Physics for 1932. • In 1927 he published his indeterminacy, or uncertainty, principle, upon which he built his philosophy and for which he is best known.
中子(neutron)
• 电荷、质量 • 存在方式 • 发现: 9Be(α,n)12C
• 用途 • 稳定性
James Chadwick
• born Oct. 20, 1891, Manchester, Eng. died July 24, 1974, Cambridge, Cambridgeshire
Proton
• The discovery of the proton dates to the earliest investigations of atomic structure. • While studying streams of ionized gaseous atoms and molecules from which electrons had been stripped, Wilhelm Wien (1898) and J.J. Thomson (1910) identified a positive particle equal in mass to the hydrogen atom. • Ernest Rutherford showed (1919) that nitrogen under alpha-particle bombardment ejects what appear to be hydrogen nuclei. • By 1920 he had accepted the hydrogen nucleus as an elementary particle, naming it proton.
Nuclide(EB)
• also called nuclear species, species of atom as characterized by the number of protons, the number of neutrons, and the energy state of the nucleus.
同质异能素(isomer)
• 激发态寿命较长的原子核称为基态原子核的同质 异能素
• 质量数和质子数均相同,而能量状态不同的核素 称为同核异能素(卢希庭) • One of two or more atomic nuclei that have the same atomic number and the same mass number but different energy states (Concise Oxford)
同质异能素(isomer)
• Isomers are the nuclides wi and neutrons, but having different energy states. • The excited states are called the isomeric states, which can have a lifetime varying from picoseconds to years.
原子核的组成、电荷、质量及半径
质子(proton)
• 电荷、质量 • 存在方式 • 发现、命名 • 用途
Proton
• stable subatomic particle that has a positive charge equal in magnitude to a unit of electron charge and a rest mass of 1.67262×10-27 kg, which is 1,836 times the mass of an electron.
• When the isomeric states are long-lived, they are referred to as metastable states. These states are denoted by “m” as in 99mTc. (Saha)
核素术语小结
• 同位素、同量异位素、同质异能素等术语表达的 是核素之间的一种关系,而非某个具体的核素 • 核素是包含特定原子核(质子数与中子数一定) 的一类原子的总称,否则,讲“同位素具有相同 的化学性质”便没有意义
• Every chemical element has one or more isotopes.
同位素丰度(abundance)
• 同位素中各核素天然含量的百分比
例如,天然存在的氧有三种稳定同位素: 16O,17O和18O,它们的同位素丰度分别为 99.757%, 0.038%, 0.205% (IUPAC 2003)
第二章 原子核的基本性质
Basic Characteristics of the Atomic Nucleus
目的
• 熟悉原子核的组成
• 了解原子核稳定性的相关因素 • 了解核力的主要性质 • 掌握原子核结合能的概念与计算
内容
• 原子核的组成
• 原子核的稳定性
• 核力的主要性质 • 原子核的结合能
• A nuclide is thus characterized by the mass number (A) and the atomic number (Z).
同位素(isotope)
• 质子数相同而中子数不同的各核素统称为某元素 的同位素(同位素是指各核素在元素周期表中处 于同一个位置,具有相同的化学性质) • 质子数相同,中子数不同的核素称为同位素(卢 希庭) • Chem. One of two or more forms of an element differing from each other in relative atomic mass, and in nuclear but not chemical properties
Isotope(EB)
• one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behaviour but with different atomic masses and physical properties.
(Concise Oxford)
• any of two or more species of atoms of a chemical element with the same atomic number and nearly identical chemical behavior but with differing atomic mass or mass number and different physical properties (Merriam-Webster)
• English physicist who received the Nobel Prize for Physics in 1935 for the discovery of the neutron
原子核的中子-质子模型
• 原子序数为Z质量数为A的原子核的组成
• 核子(nucleon)
• 原子核的质子数,中子数与核子数 • 注:原子核的质子-电子模型
Isotopic abundance
• Isotopic abundances refer to the relative proportions of the stable isotopes of each element. • They are most often quoted as atom percentages
• 同质异能素这个术语的出现表明,核素还与能量 相关
讨论和提问
(1~3分钟)
原子核的电荷
qZ e
• 人类已发现元素的核电荷数
• 超铀元素 • 核电荷数测量方法
原子核的质量
mN M A Zme
• 在一般工作中,如果采用原子质量来代替 原子核的质量进行计算,对计算结果往往 并无多大影响
同量异位素(isobar)
• 质量数相同而质子数不同的核素
例如
40Ar, 40K ,40Ca
• One of two or more isotopes of different elements, with the same atomic weight (Concise Oxford)
• one of two or more atoms or elements having the same atomic weights or mass numbers but different atomic numbers (Merriam-Webster)
