NMI N-甲基咪唑
N-甲基咪唑合成工艺研究

N-甲基咪唑合成工艺研究张平;汤琳;张婷;宋常春【摘要】Objective:Different influential factors were optimized during the synthetic technology of N-methy-limidazole in synthesizing process. Method:N-methy-limidazole was synthesized by a one-pot reaction with using glyoxal,methylamine,ammonia and paraformaldehyde as raw materials. The influential factor including materials adding order,reaction temperature and reaction time on the conversion rate of N-methy-limidazole were optimized. The content of N-methy-limidazole in synthesizing process was also accurately and rapidly de-tected through the standard curve of refractive index for N-methy-limidazole. The chemical structure of the fi-nal product was characterized by 1H and 13C NMR spectroscopy and( GC-MS)mass spectrometry. Result:The results show that the conversion of the reaction is over 98% using the materials adding order of paraformalde-hyde,ammonia methylamine and glyoxal,reaction temperature of 60℃ and reaction time of 4 h. The water was removed under vacuum and then using cyclohexane as azeotrope,the product was finally obtained with a purity of 99. 5%. Conclusion:The synthetic technology has the advantages of high conversion,low sewage treatment, rapid and efficient detection method,which will be suitable for the industrial production.%目的:优化N-甲基咪唑合成工艺。
NMI N-甲基咪唑

N-甲基咪唑(NMI)N-甲基咪唑试剂级价格N-甲基咪唑CAS号: 616-47-7英文名称: 1-Methylimidazole英文同义词: MIM;DY 070;NSC 88064;LUPRAGEN(R) NMI;Araldite DY 070;methyl imidazole;1-Methylimdazole;1-methyl-imidazo;1-methylmidazole;1-METHYLIMIDAZOLE中文名称: N-甲基咪唑中文同义词: 甲基咪唑;1-甲基咪;1-甲咪唑;甲基引咪唑;1-甲基咪唑;N-甲基咪唑;1-甲基甘噁啉;56-37-1%;1-甲基咪唑溶液;1-甲基-1H-咪唑CBNumber: CB1316726分子式: C4H6N2分子量: 82.1MOL File: 616-47-7.molN-甲基咪唑化学性质熔点: −60 °C(lit.)沸点: 198 °C(lit.)密度: 1.03 g/mL at 25 °C(lit.)蒸气压: 0.4 mm Hg ( 20 °C)折射率: n20/D 1.495(lit.)闪点: 198 °F储存条件: Store at RT.敏感性: HygroscopicBRN : 105197稳定性: Stable, but moisture sensitive. Incompatiblewith acids, acid anhydrides, strong oxidizingagents, moisture, carbon dioxide, acidchlorides.CAS 数据库: 616-47-7(CAS DataBase Reference)NIST化学物质信息: 1H-Imidazole, 1-methyl-(616-47-7)EPA化学物质信息: 1H-Imidazole, 1-methyl-(616-47-7)安全信息危险品标志: C,Xn,F,T危险类别码: 21/22-34-19-11-20/21/22-52/53-24-22-40-37-21安全说明: 26-36-45-1/2-36/37/39-16-33-29-61危险品运输编号: UN 3267 8/PG 2WGK Germany : 1RTECS号: NI7000000F : 3-10HazardClass : 8PackingGroup : III海关编码: 29332990N-甲基咪唑 MSDS1-Methyl-1H-imidazoleN-甲基咪唑性质、用途与生产工艺化学性质外观:无色透明液体,熔点:-60℃,沸点:198℃,密度d20/4=1.036g/ml。
化学品安全技术说明书(MSDS):1-甲基咪唑

h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 爆炸上限: 15.7 %(V)
爆炸下限: 2.7 %(V)
k) 蒸气压
0.5 hPa 在 20 °C
l) 蒸汽密度
无数据资料
m) 密度/相对密度
1.03 g/mL 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
6. 泄露应急处理 6.1 作业人员防护措施、防护装备和应急处置程序
戴呼吸罩。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 移去所有火源。 人员疏散到安全区域。 谨防蒸气积累达到可爆炸的浓度。蒸气能在低洼处积聚。 6.2 环境保护措施 如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。 一定要避免排放到周围环境中。 6.3 泄漏化学品的收容、清除方法及所使用的处置材料 围堵溢出,用防电真空清洁器或湿刷子将溢出物收集起来,并放置到容器中去,根据当地规定处理(见第13部 分)。 放入合适的封闭的容器中待处理。 6.4 参考其他部分 丢弃处理请参阅第13节。
附加说明 化学物质毒性作用登记: NI7000000
12. 生态学资料
1-乙基-3-甲基咪唑醋酸盐的制备

学年论文题目:1-乙基-3-甲基咪唑醋酸盐的制备及其用于纤维素溶解纺丝的研究进展学院:化学化工学院专业:化学学生姓名:王昱周学号:2011730104381-乙基-3-甲基咪唑醋酸盐的制备及其用于纤维素溶解纺丝的研究进展摘要离子液体1-乙基-3-甲基咪唑醋酸盐([ Emim]Ac) 可以溶解天然高分子等许多聚合物,尤其对于纤维素具有较强的溶解能力,且溶解过程基本不造成纤维素降解,故可以作为纤维素的有效溶剂,用于纤维素的溶解加工。
与其它溶剂相比,[Emim]Ac具有使用安全、不污染环境、易回收循环利用等优势,故在纤维素溶解、纺丝方面具有广阔的应用前景。
本文主要介绍了以N-甲基咪唑为原料,采用两步法对离子液体[Emim]Ac 进行制备;并概述了[Emim] Ac在纤维素溶解、纺丝等方面的应用研究进展。
关键词离子液体;[Emim]Ac;制备;纤维素;溶解;纺丝Abstract Ionic liquid 1 - ethyl - 3 - methyl imidazole acetate (Ac) [Emim] can dissolve natural polymer and many other polymers, especially for cellulose has strong dissolving ability, basic cause no cellulose degradation and dissolving process, therefore, can be a effective cellulose solvent, used for processing the dissolution of cellulose. Compared with other solvents, [Emim] Ac with the use of safe, no pollution, easy to recycle use of advantages, so in cellulose dissolution, spinning has broad application prospects. In N - methyl imidazole is mainly introduced in this paper as a raw material, adopts the two-step preparation for ionic liquids [Emim] Ac; And [Emim] Ac in cellulose dissolution, spinning and so on application research. Keywords:Ionic liquids; [Emim]Ac; Preparation; Cellulose; Dissolve; spinning目录1.实验 (3)1.1试剂和仪器 (3)1.1.1试剂 (3)1.1.2仪器 (3)1.2离子液体[Emim]Ac的制备过程 (3)1.2.1 N-甲基咪唑的预处理 (4)1.2.2离子液体中间产物[Emim]Br的制备 (4)1.2.3 离子液体[Emim]Ac的得到 (4)1.2.4 中间体合成机理探讨 (4)2. 1-乙基-3-甲基咪唑醋酸盐[Emim]Ac在纤维素溶解及纺丝方面的研究进展 (5)2.1对纤维素的溶解机理 (5)2.2 离子液体对纤维素的溶解特性 (6)2.2.1 溶解速度 (6)2.2.2 溶解浓度 (6)2.2.3 溶液粘度 (6)2.2.4 溶液稳定性 (7)2.3 再生纤维素纤维的制备和性能 (7)3. 1-乙基-3-甲基咪唑醋酸盐的其它用途 (7)4. 展望 (8)参考文献 (9)致谢 (10)离子液体[1]( Ionic Liquids,简称IL) 是指在室温或接近室温下呈现液态的,完全由有机阳离子和无机阴离子或有机阴离子所组成的盐,也被称为低温熔融盐或室温离子液体。
气相色谱法测定离子液体中间体中N-甲基咪唑残留量

气相色谱法测定离子液体中间体中N-甲基咪唑残留量李继民;王彦吉;赵彦军;邹宁【摘要】采用气相色谱法对离子液体中间体中N-甲基咪唑残留量进行测定.以N,N-二甲基苯胺作为内标,采用DB-FFAP石英毛细管色谱柱(30 m×0.32mm,0.25 μm)分离,用氮磷检测器测定.N-甲基咪唑的质量浓度在0.02~0.140 g·L-1范围内与其峰面积呈线性关系,检出限(3S/N)为6.52 mg·L-1,测定下限(10S/N)为21.73 mg·L-1.在两个浓度水平作回收试验,平均回收率(n=5)分别为96.4%,96.2%,测定结果的相对标准偏差(n=5)为0.88%.【期刊名称】《理化检验-化学分册》【年(卷),期】2010(046)009【总页数】3页(P1052-1054)【关键词】气相色谱法;N-甲基咪唑;离子液体中间体【作者】李继民;王彦吉;赵彦军;邹宁【作者单位】中国刑警学院,法医系,沈阳,110035;中国人民公安大学,北京,100038;中国刑警学院,法医系,沈阳,110035;中国刑警学院,法医系,沈阳,110035【正文语种】中文【中图分类】O657.7N甲基咪唑是合成咪唑类离子液体的重要中间体[13]。
离子液体作为一种清洁的溶剂和新型催化剂体系得到了各国催化界和石化企业的青睐。
绿色化学是化学发展的必然趋势[4]。
离子液体是由含氮杂环的有机阳离子和无机阴离子组成,具有优异的化学和热力学稳定性,有较宽的温度范围,对有机和无机化合物有很好的溶解性。
室温下几乎没有蒸气压,可用于高真空条件下的反应,具有良好的导电性,较高的离子迁移和扩散速度,不燃烧,无味,是一种强极性、低配位能力的溶剂[5]。
目前有很多重要工业应用价值的反应,如 FriedelCrafts反应,Beck mann重排,烯烃氢化,选择性烯烃氧化,Heck反应,Suzuki反应等都在离子液体催化剂体系中取得了很好的结果[6]。
N-甲基咪唑MSDS

名称:N-甲基咪唑CAS:616-47-7第一部分化学品标识MSDS名称:N-甲基咪唑别名:1-甲基咪唑第二部分组成及成分信息危险信号:C风险短语:34第三部分危害识别危险综述可燃,腐蚀性物质潜在的健康影响眼睛:引起眼烧伤。
可能引起化学性结膜炎、角膜损伤。
皮肤:导致皮肤灼伤。
影响可能会有延迟。
可能导致肢端发绀。
可能会导致皮疹(在温和的情况下),皮肤发绀或苍白颜色。
摄入:可能会导致严重的和永久的消化道损伤。
引起消化道灼伤。
可能导致消化道穿孔。
这种物质的毒理学性质没有得到充分的调查。
可能会导致系统的影响。
吸入:影响可能会有延迟。
引起呼吸道化学烧伤。
这种物质的毒理学性质没有得到充分的调查。
吸入可导致肺水肿。
可能会导致系统的影响。
在高浓度吸入可引起中枢神经系统抑制。
慢性的:影响可能会有延迟。
第四部分急救措施眼睛:就医,不要让受害者擦眼或保持闭上眼睛。
用水冲洗至少30分钟。
皮肤:就医或用大量肥皂水冲洗皮肤至少15分钟,脱去被污染的衣物和鞋子。
摄入:不要诱导呕吐。
如果伤者清醒,给2-4杯牛奶或水。
若伤者昏迷,不能喂任何食物。
及时就医。
吸入:立即移至空气新鲜处。
如果呼吸停止,进行人工呼吸。
如呼吸困难,输氧,就医。
如果呼吸已经停止,使用氧气和适当的机械装置。
第五部分消防措施一般信息:灭火时,要求戴自给式呼吸器和全套保护装置。
火灾中,由于热分解或燃烧可能产生刺激性和有毒气体。
喷水保持容器冷却。
该物质属于易燃液体,蒸气比空气重,蒸汽可沿地面和低或受限的区域富集。
可发生聚合,参与火灾,产生爆炸。
接触金属时,可能生成易燃的氢气。
加热时,容器可能会爆炸。
灭火介质:用水喷洒,冷却容器。
小火灾,使用干粉,二氧化碳,或水喷雾。
大火灾,使用干粉,二氧化碳,醇耐泡,或水喷雾。
第六部分泄露应急处理一般信息:使用适当的个人防护设备,具体内容参见第八部分。
泄漏/渗漏:用惰性物质(如蛭石,砂或土)吸收,然后放在合适的容器。
避免流入下水道、沟渠。
4甲基咪唑的合成方法简介

4甲基咪唑的合成方法简介总述与回顾:在本文中,我将为您介绍4甲基咪唑的合成方法。
4甲基咪唑是一种重要的有机化合物,广泛用于药物合成、催化剂和材料科学等领域。
通过深入探讨其合成方法的多个方面,我将帮助您更全面、深入地理解这一主题。
1. 工业合成方法1.1 一锅法合成一锅法合成是最常用的工业生产方法之一。
该方法基于咪唑的胺类前体和甲醇反应生成4甲基咪唑,反应一般在高温和高压下进行。
由于反应条件较为严苛,产率较低,但适用于大规模生产。
1.2 溶剂法合成溶剂法合成是另一种常见的工业制备方法。
该方法使用N-甲基咪唑作为原料,在溶剂中和甲醛发生反应,生成4甲基咪唑。
溶剂法合成相对于一锅法合成更加温和,产率较高且方法操作简单。
2. 实验室合成方法2.1 非传统方法在实验室合成4甲基咪唑时,还有一些非传统方法可以选择。
可以利用咪唑与甲醛在碱性条件下反应生成4甲基咪唑。
还可以通过在微波加热下进行咪唑和甲醛的反应来合成4甲基咪唑,这种方法具有反应时间短、产率高的优势。
2.2 传统方法——碱性条件传统方法中,咪唑和甲醛在碱性条件下进行反应是最常见的合成路线之一。
将咪唑溶于碱性溶液中,然后逐渐加入甲醛,控制反应温度和反应时间,最终得到4甲基咪唑。
2.3 传统方法——非碱性条件咪唑和甲醛在非碱性条件下也可以进行反应。
通常,在溶剂中加入一定的溶剂助剂,利用咪唑与甲醛之间的酸碱中和反应进行合成,得到4甲基咪唑。
3. 我的观点和理解在4甲基咪唑的合成过程中,选择适合的合成方法非常重要。
根据实际需求和合成条件,可以选择不同的方法进行合成。
工业合成方法通常适用于大规模生产,而实验室合成方法则更加注重反应条件的控制和产物纯度的提高。
对于实验室合成方法,需要注意反应条件的选择和优化,以提高产率和纯度。
探索非传统方法的合成路线,可以提高合成效率和反应速率。
4甲基咪唑的合成方法因其应用广泛而备受关注。
工业合成方法和实验室合成方法各具特点,可以根据需要进行选择。
聚氨酯催化剂大全

聚氨酯催化剂大全rrchem_dou 2楼DABCO BDMA BDMA 减低于高水份配方中产生之脆性及表面固化。
DABCO BL-11 A-1 70双二甲胺基乙基醚的二丙二醇溶液「发泡」型催化剂。
DABCO BL-17 BL-17 具有延迟反应效果的双二甲胺基乙基醚衍生物。
DABCO BL-22 BL-22 复合胺具有强烈「发泡」效果可取代BL-11。
DABCO Crystalline 固体胺固体三乙烯二胺工业标准产品。
DABCO CS-90 CS-90 复合胺具有强烈「发泡」作用改善泡沫密度梯度及开孔效果可减少箱泡角落破裂。
DABCO DC-2R DC-2 特殊复合胺适用于硬质喷涂加速固化优良储存稳定性。
DABCO DMAEE DMAEE 低气味表面固化催化剂与33LV等主要基础催化剂共用。
DABCO DMDEE DMDEE 「发泡」催化剂尤其适用于单组份密封泡沫与MDI相溶而不反应。
2010-3-7 02:17 回复rrchem_dou 3楼DABCO DMEA DMEA 中文名称: 二甲基乙醇胺温和平衡性催化剂乳白时间较短。
DABCO EG EG 凝胶催化剂33三乙烯二胺的乙二醇溶液用于鞋材乙二醇系统。
DABCO NE200新NE200 特殊低雾化反应型「发泡」催化剂。
DABCO NE400新NE400 低气味特殊低雾化反应型催化剂用于聚酯泡沫。
DABCO NE500新NE500 特殊低雾化反应型「胶化」催化剂能大大减低气味及雾化。
DABCO NE600新NE600 特殊低雾化反应型「发泡」催化剂能大大减低气味及雾化。
DABCO NE1060新NE1060 特殊低雾化反应型「胶化」催化剂。
DABCO S-25 S-25 凝胶催化剂25三乙烯二胺75 14丁二醇混合物。
DABCO T T 发泡型催化剂有低雾化效果用于包装材料。
2010-3-7 02:17 回复rrchem_dou 4楼DABCO TMR TMR 用于聚异氰脲酸酯PIR加速末段固化而不影响乳白时间。
N-甲基咪唑MSDS

名称:N-甲基咪唑CAS:616-47-7第一部分化学品标识MSDS名称:N-甲基咪唑别名:1-甲基咪唑第二部分组成及成分信息危险信号:C风险短语:34第三部分危害识别危险综述可燃,腐蚀性物质潜在的健康影响眼睛:引起眼烧伤。
可能引起化学性结膜炎、角膜损伤。
皮肤:导致皮肤灼伤。
影响可能会有延迟。
可能导致肢端发绀。
可能会导致皮疹(在温和的情况下),皮肤发绀或苍白颜色。
摄入:可能会导致严重的和永久的消化道损伤。
引起消化道灼伤。
可能导致消化道穿孔。
这种物质的毒理学性质没有得到充分的调查。
可能会导致系统的影响。
吸入:影响可能会有延迟。
引起呼吸道化学烧伤。
这种物质的毒理学性质没有得到充分的调查。
吸入可导致肺水肿。
可能会导致系统的影响。
在高浓度吸入可引起中枢神经系统抑制。
慢性的:影响可能会有延迟。
第四部分急救措施眼睛:就医,不要让受害者擦眼或保持闭上眼睛。
用水冲洗至少30分钟。
皮肤:就医或用大量肥皂水冲洗皮肤至少15分钟,脱去被污染的衣物和鞋子。
摄入:不要诱导呕吐。
如果伤者清醒,给2-4杯牛奶或水。
若伤者昏迷,不能喂任何食物。
及时就医。
吸入:立即移至空气新鲜处。
如果呼吸停止,进行人工呼吸。
如呼吸困难,输氧,就医。
如果呼吸已经停止,使用氧气和适当的机械装置。
第五部分消防措施一般信息:灭火时,要求戴自给式呼吸器和全套保护装置。
火灾中,由于热分解或燃烧可能产生刺激性和有毒气体。
喷水保持容器冷却。
该物质属于易燃液体,蒸气比空气重,蒸汽可沿地面和低或受限的区域富集。
可发生聚合,参与火灾,产生爆炸。
接触金属时,可能生成易燃的氢气。
加热时,容器可能会爆炸。
灭火介质:用水喷洒,冷却容器。
小火灾,使用干粉,二氧化碳,或水喷雾。
大火灾,使用干粉,二氧化碳,醇耐泡,或水喷雾。
第六部分泄露应急处理一般信息:使用适当的个人防护设备,具体内容参见第八部分。
泄漏/渗漏:用惰性物质(如蛭石,砂或土)吸收,然后放在合适的容器。
避免流入下水道、沟渠。
N-甲基咪唑

N-甲基咪唑中文名:N-甲基咪唑英文名:N-methylimidazole分子式(Formula):C4H6N2分子量(Molecular Weight):82.10CAS编号:616-47-7物化性质N-甲基咪唑为无色透明液体。
凝固点:-2~-1℃沸点:72~73℃(1.3kPa)产品应用N-甲基咪唑可用作聚氨酯催化剂。
供应商新典化学材料(上海)有限公司本公司还供应下列聚氨酯催化剂:二甲基环己胺(DMCHA):聚氨酯硬泡催化剂N,N-二甲基苄胺(BDMA):在聚氨酯行业是聚酯型聚氨酯块状软泡、聚氨酯硬泡及胶黏剂涂料的催化剂,主要用于硬泡三乙烯二胺(TEDA):聚氨酯高效催化剂,用于软泡双(二甲氨基乙基)醚:高催化活性的聚氨酯催化剂,多用于聚氨酯软泡N,N-二甲基乙醇胺:聚氨酯反应型催化剂五甲基二乙烯三胺(PMDETA):聚氨酯凝胶发泡催化剂,广泛用于聚氨酯硬泡2,4,6-三(二甲氨基甲基)苯酚(DMP-30):聚氨酯三聚催化剂,也可作环氧促进剂双吗啉二乙基醚(DMDEE):聚氨酯强发泡催化剂二甲氨基乙氧基乙醇(DMAEE):用于硬质包装泡沫的低气味反应性催化剂二月桂酸二丁基锡(T-12):聚氨酯强凝胶性催化剂三(二甲氨基丙基)六氢三嗪(PC-41):具有优异发泡能力的高活性三聚共催化剂四甲基乙二胺(TEMED):中等活性发泡催化剂,发泡/凝胶平衡性催化剂四甲基丙二胺(TMPDA):可用于泡沫塑料微孔弹性体的催化剂,也可作环氧促进剂四甲基己二胺(TMHDA):特别用于聚氨酯硬泡,是发泡/凝胶平衡性催化剂三甲基羟乙基丙二胺(Polycat 17):反应性低烟雾平衡性叔胺催化剂三甲基羟乙基乙二胺(Dabco T):反应性发泡催化剂,具有低雾化性新典化学。
2024年N-甲基咪唑市场调查报告

N-甲基咪唑市场调查报告1. 引言N-甲基咪唑(N-Methylimidazole)是一种重要的有机化合物,广泛应用于医药、化工和农药等领域。
本报告旨在通过市场调查,全面了解N-甲基咪唑的市场现状、发展趋势以及竞争格局。
2. 市场概述N-甲基咪唑是一种无色透明液体,具有较高的熔点和沸点。
其主要用途包括医药合成中的原料、化学试剂以及农药中的活性成分。
由于其独特的化学性质和广泛的应用范围,N-甲基咪唑市场需求持续增长。
3. 市场规模及趋势根据市场调查数据显示,2019年N-甲基咪唑市场规模达到XXX万美元,预计到2025年将增长至XXX万美元。
市场规模的增长主要受益于医药和化工行业的快速发展,以及对高品质化合物的需求不断增加。
此外,N-甲基咪唑在农药领域也有广泛的应用。
随着农业现代化的推进和对农产品质量的要求提高,农药市场对N-甲基咪唑的需求也在增加。
4. 市场竞争格局目前,全球N-甲基咪唑市场存在着多家重要的制造商和供应商。
其中,主要的竞争企业包括ABC化工、XYZ药业、123农药公司等。
这些企业凭借其技术实力、产品质量和市场拓展能力取得了一定的市场份额。
此外,N-甲基咪唑市场还存在着一些中小型企业。
这些企业注重产品差异化和定制化,通过技术创新和服务提升来满足特定客户的需求。
他们在一些细分市场中表现出较强的竞争力,并有望进一步扩大市场份额。
5. 市场前景分析未来几年,N-甲基咪唑市场有望继续保持稳定增长。
主要驱动因素包括医药、化工和农药行业的快速发展,以及对高品质化合物的需求不断增加。
此外,随着可持续发展和环保意识的提升,对绿色、环保型N-甲基咪唑的需求也将逐渐增加。
然而,市场竞争将会更加激烈。
制造商需要不断提高产品质量和技术创新能力,以保持竞争优势。
同时,灵活的市场定位和多元化的市场拓展策略也将成为企业获得市场份额的关键因素。
6. 结论N-甲基咪唑市场是一个具有广阔前景的市场。
随着医药、化工和农药行业的发展,对N-甲基咪唑的需求将持续增加。
咪唑衍生物及其应用
咪唑衍生物及其应用摘要:本文介绍咪唑衍生物的分类、性能特点及其作为环氧树脂固化剂/促进剂的应用。
该研究为选择使用咪唑衍生物提供了一定的依据。
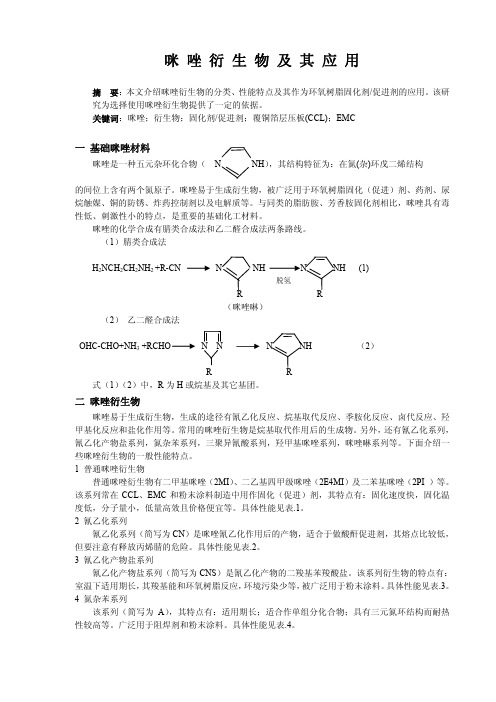
关键词:咪唑;衍生物;固化剂/促进剂;覆铜箔层压板(CCL);EMC一基础咪唑材料咪唑是一种五元杂环化合物(N NH),其结构特征为:在氮(杂)环戊二烯结构的间位上含有两个氮原子。
咪唑易于生成衍生物,被广泛用于环氧树脂固化(促进)剂、药剂、尿烷触媒、铜的防锈、炸药控制剂以及电解质等。
与同类的脂肪胺、芳香胺固化剂相比,咪唑具有毒性低、刺激性小的特点,是重要的基础化工材料。
咪唑的化学合成有腈类合成法和乙二醛合成法两条路线。
(1)腈类合成法H2NCH2CH2NH2 +R-CN N NH N NH (1)脱氢R(咪唑啉)(2)乙二醛合成法OHC-CHO+NH3+RCHO N N N NH (2)R式(1)(2)中,R为H或烷基及其它基团。
二咪唑衍生物咪唑易于生成衍生物,生成的途径有氰乙化反应、烷基取代反应、季胺化反应、卤代反应、羟甲基化反应和盐化作用等。
常用的咪唑衍生物是烷基取代作用后的生成物。
另外,还有氰乙化系列,氰乙化产物盐系列,氮杂苯系列,三聚异氰酸系列,羟甲基咪唑系列,咪唑啉系列等。
下面介绍一些咪唑衍生物的一般性能特点。
1 普通咪唑衍生物普通咪唑衍生物有二甲基咪唑(2MI)、二乙基四甲级咪唑(2E4MI)及二苯基咪唑(2PI )等。
该系列常在CCL、EMC和粉末涂料制造中用作固化(促进)剂,其特点有:固化速度快,固化温度低,分子量小,低量高效且价格便宜等。
具体性能见表.1。
2 氰乙化系列氰乙化系列(简写为CN)是咪唑氰乙化作用后的产物,适合于做酸酐促进剂,其熔点比较低,但要注意有释放丙烯腈的危险。
具体性能见表.2。
3 氰乙化产物盐系列氰乙化产物盐系列(简写为CNS)是氰乙化产物的二羧基苯羧酸盐。
该系列衍生物的特点有:室温下适用期长,其羧基能和环氧树脂反应,环境污染少等,被广泛用于粉末涂料。
nmi化学结构

nmi化学结构全文共四篇示例,供读者参考第一篇示例:N-甲基卬胺酸(N-methylimidazole,NMI)是一种重要的有机化合物,也是一种重要的中间体,广泛应用于药物合成和材料科学等领域。
NMI的化学结构简单,但却具有许多独特的化学性质,使其在各种领域都有重要的应用价值。
NMI的化学结构如下图所示:NMI的分子式为C4H6N2,分子量为82.1 g/mol。
它是一种白色固体,能溶解于水和许多有机溶剂中。
NMI具有较强的碱性,能够和酸反应生成盐,也能够和醇、醛、酮等化合物发生亲核加成反应。
NMI作为催化剂在有机合成中有着重要的应用。
它可以促进亲核取代反应、醚化反应、酰胺合成等反应。
NMI还可作为弱碱参与弱碱碱催化的加成反应中。
NMI和二甲基甲酰胺在298K恩加烷基芳酐(和甲烷)和邻苯二苯醌反应中可产生1-(2-甲基苄氨基)-1-氧二甲苯。
在药物合成中,NMI也被广泛应用。
NMI可以用来合成阿昔洛韦(Acyclovir),一种常用的抗病毒药物。
NMI在这个合成过程中扮演着催化剂的重要角色,使得合成过程更加高效、绿色。
NMI还可以用来合成氮杂环化合物、樟脑酚等药用活性物质。
在材料科学领域,NMI也有着广泛的应用。
NMI可以被用来合成金属有机骨架材料(MOFs),这是一类具有多孔结构的晶体材料,具有很好的气体吸附性能。
NMI在MOFs的合成中可以起到孔内配体的作用,从而调控材料的孔径和表面积。
第二篇示例:nmi(N-脱甲基甲硅烷-3-甲基-恩伯丁胺)是一种常用的溶剂,具有广泛的应用范围。
它的化学结构为N-脱甲基甲硅烷-3-甲基-恩伯丁胺,化学式为C6H16N2Si。
nmi是一种无色透明液体,具有类似于醇类的性质,是一种优秀的极性溶剂,在有机合成领域被广泛应用。
nmi的分子结构中含有一根硅烷基链和一个含氮的三元环,硅烷基链具有亲水性,使得nmi能够在有机和水相中都具有良好的溶解性,适用于溶解多种有机物。
n-甲基咪唑质量标准

N-甲基咪唑是一种重要的有机化工原料,广泛应用于医药、农药、染料、表面活性剂等领域。
其质量标准对保证产品质量、保护用户利益具有重要意义。
1. 外观:N-甲基咪唑应为无色或淡黄色液体,无悬浮物和沉淀。
2. 纯度:N-甲基咪唑的纯度是其最重要的质量指标之一。
一般要求纯度在98%以上,最高可达99.5%。
纯度的测定通常采用气相色谱法。
3. 水分:N-甲基咪唑中的水分会影响其化学稳定性和反应性,因此对其含量有严格的限制。
一般要求水分含量在0.1%以下。
4. 酸度:N-甲基咪唑的酸度反映了其酸性杂质的含量。
一般要求酸度在0.01%以下。
5. 金属离子:N-甲基咪唑中的金属离子会影响其化学稳定性和反应性,因此对其含量有严格的限制。
一般要求金属离子含量在1ppm以下。
6. 蒸发残渣:蒸发残渣反映了N-甲基咪唑中不挥发性杂质的含量。
一般要求蒸发残渣在0.1%以下。
7. 包装:N-甲基咪唑应用清洁、干燥的镀锌铁桶或铝桶包装,每桶净重200kg或25kg。
桶盖应严密,不得渗漏。
8. 储存:N-甲基咪唑应储存在阴凉、干燥、通风良好的仓库中,远离火源和热源,避免阳光直射。
容器应垂直放置,不得倒置。
以上就是N-甲基咪唑的质量标准,只有严格按照这些标准生产和检验,才能保证N-甲基咪唑的质量和性能,满足用户的需求。
tcfh_nmi反应机理

tcfh_nmi反应机理
TCFH_NMI(Triethylchlorosilane-N-Methylimidazole)是一种常用的有机化学试剂,常用于有机合成中的氯硅烷化反应。
下面我将从多个角度来解释TCFH_NMI的反应机理。
1. 反应组成:
TCFH_NMI由两个主要的成分组成,三乙基氯硅烷(TCES)和N-甲基咪唑(NMI)。
TCES是一种氯硅烷化合物,它具有亲电性,可以与亲核试剂发生反应。
NMI是一种碱性试剂,可以提供亲核性。
2. 反应步骤:
TCFH_NMI的反应机理可以分为以下几个步骤:
步骤1,NMI与TCES发生质子转移反应,形成NMI的共轭酸和TCES的共轭碱。
步骤2,NMI的共轭酸与亲核试剂发生亲核加成反应,生成中间体。
步骤3,中间体经过质子转移反应,生成最终产物和NMI。
3. 反应条件:
TCFH_NMI反应通常在室温下进行,反应条件温和。
常见的溶剂
包括氯代烃类(如氯仿、二氯甲烷)和芳香烃类(如苯、二甲苯)。
4. 反应应用:
TCFH_NMI反应在有机合成中有广泛的应用。
它可以用于氯硅烷
化反应,将氯硅烷引入有机分子中,从而引入硅基官能团。
这对于
合成有机硅化合物、有机金属配合物以及有机合成中的其他重要中
间体具有重要意义。
总结起来,TCFH_NMI反应机理涉及了质子转移反应和亲核加成
反应。
TCES作为亲电试剂与NMI发生质子转移反应,生成中间体,
然后中间体与亲核试剂发生亲核加成反应,最终生成产物。
这种反
应在有机合成中具有重要的应用价值。
n-甲基咪唑结构式

n-甲基咪唑结构式
N-甲基咪唑是一种有机化合物,化学式为C4H6N2CH3,也被称为
N-甲基-1H-咪唑。
它是一种无色至淡黄色的液体,具有类似于鱼腥味
的气味。
N-甲基咪唑在生化实验室中被广泛用作缓冲剂,其缓冲范围
在PH7.0-8.5之间。
此外,它还用于医药、有机合成和染料等领域。
N-甲基咪唑分子由一个五元杂环和一个甲基基团组成。
这个五元
杂环由一个氮原子和四个碳原子组成,氮原子位于其中心位置。
它的
化学性质稳定,可以与酸和碱相互作用,表现出缓冲性质。
N-甲基咪
唑比其他缓冲剂更稳定,在高温和高PH值条件下仍能保持其缓冲功能。
N-甲基咪唑广泛应用于蛋白质组学、基因组学、药物开发、组织
工程等领域。
它可以用作PCR反应的缓冲剂,在DNA电泳实验中也可
以用作染料。
此外,N-甲基咪唑还是一种很好的溶剂,可以用于表征
和分离蛋白质。
在环保领域中,N-甲基咪唑还被用于废物的处理,因
为它可以与污染物结合并协同促进其降解。
然而,在N-甲基咪唑的使用过程中,需要注意安全问题。
它对皮肤和眼睛有刺激性和腐蚀性,需要戴手套和防护眼镜。
同时,储存和
使用时也要避免其接触火源,以免引起火灾和爆炸。
综上所述,N-甲基咪唑是一种广泛应用于生命科学研究中的有机
化合物。
它具有缓冲性质,可以用作PCR反应的缓冲剂和DNA电泳实
验的染料,同时还可以用于蛋白质组学、基因组学、药物开发等领域。
在使用时需要注意安全问题,避免皮肤接触和火源。
关于n-甲基咪唑质量标准的文章

关于n-甲基咪唑质量标准的文章n-甲基咪唑是一种重要的有机化合物,广泛应用于医药、农药、染料、涂料等领域。
为了确保其质量和安全性,制定了一系列的质量标准。
首先,n-甲基咪唑的外观应为白色结晶或结晶性粉末,无杂质。
通过目测和显微镜观察,应该没有明显的颗粒、结块或异物。
其次,n-甲基咪唑的纯度要求较高。
一般来说,其纯度应在99%以上。
可以通过高效液相色谱(HPLC)等方法进行检测。
同时,还需要检测有机杂质和无机杂质的含量。
有机杂质包括其他同系物或降解产物,而无机杂质包括金属离子等。
此外,n-甲基咪唑还需要符合一定的物化性质要求。
例如,其熔点应在148-152℃之间;溶解度应在水中可溶解;相对密度应在1.04-1.06之间;粒径分布应符合规定范围等。
对于n-甲基咪唑的微生物限度,也有一定的要求。
微生物限度测试包括总菌落计数、大肠菌群、霉菌和酵母菌等指标。
这些测试可以确保n-甲基咪唑在使用过程中不会引起细菌感染或其他微生物污染。
最后,n-甲基咪唑的包装和储存也需要符合一定的标准。
一般来说,它应该被密封在无菌、干燥、避光的容器中,并存放在干燥、阴凉的地方。
同时,还需要标明生产日期、批号和有效期等信息。
总之,n-甲基咪唑作为一种重要的有机化合物,在应用过程中需要符合一系列的质量标准。
这些标准涵盖了外观、纯度、物化性质、微生物限度以及包装和储存等方面。
只有确保了n-甲基咪唑的质量和安全性,才能更好地发挥其在各个领域的应用价值。
1-烷基-3-甲基咪唑牛磺酸离子液体[Cnmim][Tau](n=3,4)
![1-烷基-3-甲基咪唑牛磺酸离子液体[Cnmim][Tau](n=3,4)](https://img.taocdn.com/s3/m/586d874300f69e3143323968011ca300a6c3f6cc.png)
1-烷基-3-甲基咪唑牛磺酸离子液体[Cnmim][Tau](n=3,4)
1-烷基-3-甲基咪唑牛磺酸离子液体[Cnmim][Tau](n=3,4)
中文名称:1-烷基-3-甲基咪唑牛磺酸离子液体[Cnmim][Tau](n=3,4)
纯度:95%+
外观与形状:液体/固体,
储存:存放于惰性气体之中
应避免湿气 (吸湿)
包装规格(Packing):50g、100g、500g
保存方法:密闭,阴凉,通风干燥处
稳定性:避氧化物
水溶解性:水溶性:不溶;可溶于:甲醇,二氯甲烷;不溶:甲苯
产品:
OTf-三氟甲磺酸盐离子液体
OH-氢氧盐离子液体
OA-油酸离子液体
N-甲基咪唑硫酸氢盐([Hmim]HSO4)
N-甲基-N-丁基吗啉三氮唑盐([Bmmo]tr)
N-甲基-2-吡咯烷酮硫酸氢盐([Hnmp]HSO4)
N-丁基吡啶溴(BPyBr)离子液体
N-丁基吡啶三氮唑盐([Bpy]tr)
N-丙基-吡啶二氰胺[C3py][DCA]
NTf2-双三氟甲磺酰亚胺盐离子液体
以上资料来自小编axc,2022.11.14。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
N-甲基咪唑(NMI)N-甲基咪唑试剂级价格N-甲基咪唑CAS号: 616-47-7英文名称: 1-Methylimidazole英文同义词: MIM;DY 070;NSC 88064;LUPRAGEN(R) NMI;Araldite DY 070;methyl imidazole;1-Methylimdazole;1-methyl-imidazo;1-methylmidazole;1-METHYLIMIDAZOLE中文名称: N-甲基咪唑中文同义词: 甲基咪唑;1-甲基咪;1-甲咪唑;甲基引咪唑;1-甲基咪唑;N-甲基咪唑;1-甲基甘噁啉;56-37-1%;1-甲基咪唑溶液;1-甲基-1H-咪唑CBNumber: CB1316726分子式: C4H6N2分子量: 82.1MOL File: 616-47-7.molN-甲基咪唑化学性质熔点: −60 °C(lit.)沸点: 198 °C(lit.)密度: 1.03 g/mL at 25 °C(lit.)蒸气压: 0.4 mm Hg ( 20 °C)折射率: n20/D 1.495(lit.)闪点: 198 °F储存条件: Store at RT.敏感性: HygroscopicBRN : 105197稳定性: Stable, but moisture sensitive. Incompatiblewith acids, acid anhydrides, strong oxidizingagents, moisture, carbon dioxide, acidchlorides.CAS 数据库: 616-47-7(CAS DataBase Reference)NIST化学物质信息: 1H-Imidazole, 1-methyl-(616-47-7)EPA化学物质信息: 1H-Imidazole, 1-methyl-(616-47-7)安全信息危险品标志: C,Xn,F,T危险类别码: 21/22-34-19-11-20/21/22-52/53-24-22-40-37-21安全说明: 26-36-45-1/2-36/37/39-16-33-29-61危险品运输编号: UN 3267 8/PG 2WGK Germany : 1RTECS号: NI7000000F : 3-10HazardClass : 8PackingGroup : III海关编码: 29332990N-甲基咪唑 MSDS1-Methyl-1H-imidazoleN-甲基咪唑性质、用途与生产工艺化学性质外观:无色透明液体,熔点:-60℃,沸点:198℃,密度d20/4=1.036g/ml。
含量:≥99% 。
用途主要用于环氧树脂和其它树脂的固化剂;用于浇注、粘接和玻璃钢等领域。
用途有机合成中间体和树脂固化剂、粘合剂等。
可用于浇注、粘接和玻璃钢等领域。
用途用作有机合成中间体和树脂固化剂、粘合剂等N-甲基咪唑上下游产品信息上游原料咪唑4-氯-3-硝基苯甲酸碳酸二甲酯下游产品1-乙基-3-甲基咪唑四氟硼酸盐1-甲基-1H-咪唑-2-甲醛1-甲基-1H-咪唑-2-羰酰氯1-丁基-3-甲基咪唑四氟硼酸盐盐酸萘甲唑啉1-甲基-1H-咪唑-2-甲酸乙酯1-乙基-3-甲基咪唑二氨腈,98%毒死蜱硝唑芬酮1-丁基-3-甲基咪唑溴盐1-丁基-3-甲基咪唑六氟磷酸盐1-甲基-1H-咪唑-2-羧酸1-甲基咪唑-5-甲酰氯盐酸盐1-乙基-3-甲基咪唑啉双(三氟甲基磺酰基)亚胺洛沙坦甲基毒死蜱2-(1-甲基-1H-咪唑-2-基)-2-羰基乙酸乙酯1-MethylimidazoleFrom Wikipedia, the free encyclopedia616-47-7CHEBI:113454ChEMBL5431348verify (what is ?)1-Methylimidazole or N-methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. It is a colourless liquid that is used as a specialty solvent, a base, and as a precursor to some ionic liquids. It is a fundamental nitrogen heterocycle and as such mimics for various nucleoside bases as well as histidine and histamine,Contents[hide]∙1Basicity∙2Synthesis∙3Applicationso 3.1Ionic liquid precursor∙4See also∙5ReferencesBasicity[edit]With the N-methyl group, this particular derivative of imidazole cannot tautomerize. It is slightly more basic than imidazole, as indicated by the pKa's of the conjugate acids of 7.0 and 7.4.[1] Methylation also provides a significantly lower melting point, which makes 1-methylimidazole a useful solvent.Synthesis[edit]1-Methylimidazole is prepared mainly by two routes industrially. The main one is acid-catalysed methylation of imidazole by methanol. The second method involves the Radziszewski reaction from glyoxal, formaldehyde, and a mixture of ammonia and methylamine.[2][3](CHO)2 + CH2O + CH3NH2 + NH3→ H2C2N(NCH3)CH + 3 H2OThe compound can be synthesized on a laboratory scale by methylation of imidazole at the pyridine-like nitrogen and subsequent deprotonation.[4] Similarly, 1-methylimidazole may be synthesized by first deprotonating imidazole to form a sodium salt followed by methylation.[5][6]H2C2N(NH)CH + CH3I → [H2C2(NH)(NCH3)CH]IH2C2(NH)(NCH3)CH + NaOH → H2C2N(NCH3)CH + H2O + NaI Applications[edit]In the research laboratory, 1-methylimidazole and related derivatives have been used as mimic aspects of diverse imidazole-based biomolecules.1-Methylimidazole is also the precursor for the synthesis of the methylimidazole monomer of pyrrole-imidazole polyamides. These polymers can selectively bind specific sequences of double-stranded DNA by intercalating in a sequence dependent manner.[7]Ionic liquid precursor[edit]1-Methylimidazole alkylates to form dialkyl imidazolium salts. Depending on the alkylating agent and the counteranion, various ionic liquids result, e.g. 1-butyl-3-methylimidazolium hexafluorophosphate ("BMIMPF6"):[8][9]BASF has used 1-methylimidazole as a means to remove acid duringtheir industrial-scale production of diethoxyphenylphosphine. In this biphasic acid scavenging using ionic liquids (BASIL) process, 1-methylimidazole reacts with HCl to produce 1-methylimidazolium hydrochloride, a salt that is easily separated and deprotonated to regenerate 1-methylimidazole.[8]2 MeC3N2H3 + C6H5PCl2 + 2 C2H5OH → 2 [MeC3N2H4]Cl + C6H5P(OC2H5)2See also[edit]4-Methylimidazole。
