匹伐他汀钙片(力清之)的说明书
他汀类药物

还原酶抑制药
01 药理作用
目录
02 适用范围
03 不良反应
04 注意事项
05
他汀类药品说明书修 订
他汀类)还原酶抑制药,不仅能强效地降低总胆固醇(TC) 和低密度脂蛋白(LDL),而且能一定程度上降低三酰甘油(TG),还能升高高密度脂蛋白(HDL),所以他汀类药物 也可以称为较全面的调脂药。他汀类药物的作用机制是通过竞争性抑制内源性胆固醇合成限速酶 HMG-CoA还原酶, 阻断细胞内羟甲戊酸代谢途径,使细胞内胆固醇合成减少,从而反馈性刺激细胞膜表面低密度脂蛋白(LDL)受 体数量和活性增加,使血清胆固醇清除增加、水平降低临床上主要用于降低胆固醇尤其是低密度脂蛋白-胆固醇 (LDL-C),治疗动脉粥样硬化,现已成为冠心病预防和治疗的最有效药物。近年来,研究发现他汀类药物具有多 方面非降脂作用,其中包括抑制动脉粥样硬化与血栓形成,还具有缓解器官移植后的排异反应、治疗骨质疏松症、 抗肿瘤、抗老年痴呆等多种作用。
药理作用
他汀类药物主要以降血清、肝脏、主动脉中的胆固醇及降低极低密度脂蛋白胆固醇、低密度脂蛋白胆固醇水 平为主。
1.调血脂作用:具有明显的调血脂作用,降低LDL-C的作用最强,TC次之,降TG作用很小,而HDL-C略有升高。 他汀类与HMc-coA具有相似的化学结构,且和HMG-coA还原酶的亲和力高出HMG-CoA数千倍,因而对羟甲基戊二酸 单酰辅酶A还原酶发生竞争性抑制,使胆固醇合成受阻。
2.非调血脂作用:改善血管内皮功能、抑制血管平滑肌细胞的增殖和迁移、抗氧化作用、抗炎作用、抑制血 小板聚集和抗血栓作用等,有利于防止动脉硬化的形成或稳定和缩小动脉粥样硬化斑块。
适用范围
适于高胆固醇血症和以胆固醇升高为主的混合型高脂血症。防治冠心病、心肌梗死、脑卒中,延缓动脉粥样 硬化。
hhb匹伐他汀

山西省心血管病医院心内四病区韩 会宾
病史简介
• 基本资料: • 主诉: • 现病史: • 既往史: • 体格检查: • 辅助检查: • 化验: • 心电图:
病史简介
• 心脏彩超: • 冠脉造影及PCI:
诊断
治疗:
最新中国血脂指南提示:中等强度他汀适合于我国 大多数血脂异常患者
力清之说明书
主要 粪便排泄
反复肠肝循环
肾 脏
2%通过肾脏排泄
小结
• 力清之不断获得指南和共识推荐,是临床优选强效他汀之 一
• 力清之药代动力学特点独特,安全性高,更适合长期使用
部分他汀类药物增加新发糖尿病风险
13项他汀类大型研究的 Meta 分析
Prof.Naveed Sattar 格拉斯哥大学
T. Teramoto et al, Expert Opin. Pharmacother. 2010; 11(5): 817-28
J-PREDICT临床研究
研究人群 主要终点 次要终点 研究药物 目标患者数 研究时间 主要研究者
开放, 随机, 对照组比较研究
血脂异常伴糖耐量降低 糖尿病累积发病率
任何心血管等疾病的发病率 匹伐他汀 1-2mg/天 和对照药
证据等级
A B
B
部分极高危患者LDL基线值已在基本目标值以内,这时可以将其LDL-C从 A
基线值降低30%左右
中国2型糖尿病防治指南(2017年版)
匹伐他汀(力清之)是指南中糖尿病患者推荐的 调脂药物之一
中国2型糖尿病防治指南(2017年版)
主要内容
• 力清之不断获得最新指南和共识推荐一览表 • “指南与共识”对力清之的临床应用阐述
匹伐他汀钙片说明书

亲爱的朋友,很高兴能在此相遇!欢迎您阅读文档匹伐他汀钙片说明书,这篇文档是由我们精心收集整理的新文档。
相信您通过阅读这篇文档,一定会有所收获。
假若亲能将此文档收藏或者转发,将是我们莫大的荣幸,更是我们继续前行的动力。
匹伐他汀钙片说明书匹伐他汀钙片(力清之)用于高胆固醇血症和家族性高胆固醇血症。
下面是我们整理的,欢迎阅读。
匹伐他汀钙片商品介绍通用名:匹伐他汀钙片生产厂家:KowaCompany,Ltd,NagoyaFactory(日本)批准文号:Hxx0553药品规格:2mg*7片药品价格:¥63元【通用名称】匹伐他汀钙片【商品名称】匹伐他汀钙片(力清之)【英文名称】PitavastatinCalciumTablets【拼音全码】PiFaTaTingGaiPian(LiQingZhi)【主要成份】匹伐他汀钙。
【性状】匹伐他汀钙片(力清之)为淡粉色薄膜衣片,除去薄膜衣片后显橙色。
【适应症/功能主治】力清之(匹伐他汀钙片)用于高胆固醇血症和家族性高胆固醇血症。
【规格型号】2mg*7s【用法用量】成人晚餐后口服力清之(匹伐他汀钙片)一日1~2毫克,根据年龄和症状调整剂量,若降LDL-C的疗效不理想,可增加剂量,大日剂量为4毫克。
【不良反应】主要不良反应有腹痛、皮疹、抑郁、搔痒以及r-谷氧酰胺转肽酶、肌酸激酶(CK1、谷丙转氯酶和谷草转氨酶升高。
严重不良反应有横纹肌溶解和肌病。
【禁忌】对力清之(匹伐他汀钙片)过敏者禁用,孕妇禁用。
【注意事项】力清之(匹伐他汀钙片)慎用于过度饮酒及有肝病史者若有不明原因肌痛、肌无力发生时,特别是伴有发热者应立即向医生报告,这些患者应检测CK水平,若CK水平高于正常或临床怀疑有肌病时应立即停用匹伐他汀钙片(力清之)。
【儿童用药】尚不明确。
【老年患者用药】尚不明确。
【孕妇及哺乳期妇女用药】尚不明确。
【药物相互作用】如与其他药物同时使用可能会发生药物相互作用,详情其咨询医师或药师。
氨氯地平阿托伐他汀钙片使用说明书

通用名称:氨氯地平阿托伐他汀钙片商品名称:氨氯地平阿托伐他汀钙片汉语拼音:anlvdipingatuofatatinggaipian批准文号:国药准字J20080048药品分类:化学药品生产企业:Goedecke GmbH(进口)药品性质:处方药相关疾病:原发性高血压,慢性稳定性心绞痛及变异性心绞痛,杂合子家族性或非家族性高胆固醇血症和混合性高脂血症,纯合子高胆固醇血症。
,性状:白色或类白色片剂。
主要成份:本品为复方制剂,其组分为苯磺酸氨氯地平和阿托伐他汀钙。
适应症:本品适用于高血压或心绞痛患者合并高胆固醇血症或混合型高脂血症的治疗,可用于下列情况: 1.高血压或心绞痛患者合并高胆固醇血症或混合型高脂血症的初始治疗。
2.该类患者的治疗剂量调整。
如可以先给予患者含有两种成分常规起始治疗剂量的氨氯地平阿托伐他汀钙片,然后根据其抗心绞痛?降压或降脂效果增加氨氯地平或阿托伐他汀的剂量。
3.用于原来使用其中一种单药成分需要增加另一种药物的患者。
规格:5mg/10mg*7片不良反应:1.本品(氨氯地平阿托伐他汀钙)的安全性在一项入选了1092例高血压合并高脂血症患者的双盲、安慰剂对照研究中得到评估。
2.本品在治疗中有良好的耐受性。
对大多数患者,不良反应为轻到中度。
在本品的临床研究中,未见与该复方制剂相关的特殊不良反应。
3.不良反应的性质,程度和发生频率同氨氯地平和阿托伐他汀已有的报告相似。
用法用量:口服,根据病人两种疾病病情差异设计有多种不同剂量规格,减少了病人为控制血压和血胆固醇浓度所需服药数量。
可以在任何时间服药,饭前饭后均可。
禁忌:1.本品包含阿托伐他汀成分,因此对于伴有活动性肝脏疾病或伴有原因不明的血清转氨酶持续升高的患者应禁用。
2.已知对本品中任何成分过敏的患者应禁用。
3.孕妇与哺乳期妇女。
4.动脉粥样硬化是一个慢性过程,在妊娠期间中断降脂药物对原发性高胆固醇血症的长期治疗结果影响甚微。
胆固醇和胆固醇生物合成的其它产物是胎儿发育(包括类固醇和细胞膜的合成)的重要组成成分。
匹伐他汀钙

结 论
匹伐他汀钙片2mg/d口服可以明显降低LDL-C 、TC、甘油三酯,其降脂幅度 与10mg/d阿托伐他汀钙片相当。 匹伐他汀钙片1mg/d组与阿托伐他汀钙片10mg/d组降低动脉硬化指数幅度相 当,匹伐他汀钙片2mg/d降低动脉硬化指数幅度大于阿托伐他汀钙片10mg/d 组。 匹伐他汀钙片2mg/d口服的安全性与10mg/d阿托伐他汀钙片相当,匹伐他汀 钙片组CK升高发生率低于阿托伐他汀钙片组。
4S - Pl
25
LDL降低值
4S - Rx
二级预防
20
事件率降低值
15
LIPID - Pl
10
CARE - Pl LIPID - Rx CARE - Rx IDEAL-Sim HPS - Pl HPS - Rx TNT – Atv10 PROVE-IT - Pra TNT – Atv80 IDEAL-Atv PROVE-IT – Atv AFCAPS - Pl AFCAPS - Rx ASCOT - Rx 40 (1.0) 60 (1.6) 80 (2.1) 100 (2.6) ASCOT-PL 120 (3.1)
匹伐他汀临床结果
——降低动脉硬化指数优于阿托伐他汀
1mg匹伐他汀钙使用4周后,可降低动脉硬化指数23.44%;使用剂量 增加到2mg后,12周时可降低LDL-C 26.36%。
匹伐他汀临床结果——显著升高HDL-C
1mg匹伐他汀钙使用4周后,可使HDL-C基线在1.2~1.5mmol/L患者升 高3%;使用剂量增加到2mg后,8周时可升高11.28% (p<0.01)
活性成分:本品活性成份为匹伐他汀钙,其化学名称为 (+)-双 {(3R,5S,6E)-7-[2-环丙基-4-(4-氟苯基)-3–喹啉基]-3,5-二羟基-6-庚酸} 单钙盐。 性状:本品为白色薄膜衣片,除去薄膜衣后显白色或类白色。 规格: (1)1mg (2)2mg 用法用量:
各种他汀类药物如何选择

各种他汀类药物如何选择?他汀类药物因能安全有效地降低胆固醇、稳定逆转斑块,目前已成为抗动脉粥样硬化,降低心血管疾病风险的基石。
临床常用的他汀类药物有洛伐他汀(美降之)、辛伐他汀(舒降之),普伐他汀(普拉固)、氟伐他汀(来适可)、阿托伐他汀(立普妥)、瑞舒代他汀(可定)以及匹伐他汀(力清之)。
这么多他汀有什么不同,哪一种适合自己呢?他汀类药物有相同的功能团一羟基戊二酸,都具有相同的共性,如均可竞争性抑制胆固醇的合成。
但因具有不同的取代基,故每个他汀类药又具有独特的异质性(个性): 如药效学的强度(降脂效价)、药动学的参数、适应证、肌肾毒性的大小、相互作用的多少以及药物基因组学的影响等均不相同。
下面从不同的几个方面对他汀类药物做一比较。
他汀类药物分为天然化合物和完全人工合成化合物1天然化合物洛伐他丁、辛伐他汀、普伐他汀2人工合成化合物氟伐他汀、阿托伐他汀、匹伐他汀、瑞舒伐他汀亲脂性对于他汀类药的肝选择性十分重要,而更高亲脂性的则可更多的分布于非肝组织如肌肉等(包括与受体结合力强、作用持续久、风险更大等),也就可能存在更多的肌肉安全性问题。
他汀类药物是水溶性还是脂溶性,是由它们的油水分配系数或辛醇/水分配系数,他汀类药的油水分配系数与其吸收、分布、代谢、以及排泄强烈相关。
无论亲水性他汀类药还是亲脂性他汀类药均有利有弊,而理想的分配系数应是中性,即不太疏水性也不太亲水性。
他汀类药物亲脂性顺序依次为西立伐他汀>辛伐他汀>洛伐他汀>氟伐他汀>阿托伐他汀>瑞舒伐他汀>普伐他汀两类他汀类药分布方式的区别在于亲脂性他汀类药是通过被动转运和非选择性扩散进入肝细胞和非肝组织;而亲水性他汀类药则更多的是依靠主动转运进入到肝脏与非肝组织如肌肉等。
匹伐他汀属于亲脂性的1半衰期阿托伐他汀钙、瑞舒伐他汀、匹伐他汀半衰期大于10小时,属于长效他汀,可以在一天任何时间服用。
其他半衰期较短,需要晚上睡前服用。
2药物代谢除了普伐他汀不需要经过肝脏代谢外,其他或多或少都要通过肝药酶代谢。
匹伐他汀钙药品说明书(英文)

HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LIVALO® safely and effectively. See full prescribing information for LIVALO.LIVALO (pitavastatin) Tablet, Film Coated for Oral useInitial U.S. Approval: 2009RECENT MAJOR CHANGESDosage and AdministrationDosage in Patients with Renal Impairment (2.2) 8/2011INDICATIONS AND USAGELIVALO is a HMG-CoA reductase inhibitor indicated for:•Patients with primary hyperlipidemia or mixed dyslipidemia as an adjunctive therapy to diet to reduce elevated total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), triglycerides (TG), and to increase high-density lipoprotein cholesterol (HDL-C) (1.1)Limitations of Use (1.2):•Doses of LIVALO greater than 4 mg once daily were associated with an increased risk for severe myopathy in premarketing clinical studies. Do not exceed 4 mg once daily dosing of LIVALO.•The effect of LIVALO on cardiovascular morbidity and mortality has not been determined.•LIVALO has not been studied in Fredrickson Type I, III, and V dyslipidemias.DOSAGE AND ADMINISTRATION•LIVALO can be taken with or without food, at any time of day (2.1) Dose Range: 1 mg to 4 mg once daily (2.1)•Primary hyperlipidemia and mixed dyslipidemia: Starting dose 2 mg. When lowering of LDL-C is insufficient, the dosage may be increased to a maximum of 4 mg per day. (2.1)•Moderate and severe renal impairment (glomerular filtration rate 30 –59 and 15 - 29 mL/min/1.73 m2, respectively) as well as end-stage renal disease on hemodialysis: Starting dose of 1 mg once daily and maximum dose of 2 mg once daily (2.2)DOSAGE FORMS AND STRENGTHS•Tablets: 1 mg, 2 mg, and 4 mg (3)CONTRAINDICATIONS•Known hypersensitivity to product components (4)•Active liver disease, which may include unexplained persistent elevations in hepatic transaminase levels (4)•Women who are pregnant or may become pregnant (4, 8.1)•Nursing mothers (4, 8.3)•Co-administration with cyclosporine (4, 7.1, 12.3)WARNINGS AND PRECAUTIONS•Skeletal muscle effects (e.g., myopathy and rhabdomyolysis): Risks increase in a dose-dependent manner, with advanced age (≥65), renal impairment, and inadequately treated hypothyroidism. Advise patientsto promptly report report unexplained and/or persistent muscle pain, tenderness, or weakness, and discontinue LIVALO (5.1)•Liver enzyme abnormalities : Persistent elevations in hepatic transaminases can occur. Check liver enzyme tests before initiating therapy and as clinically indicated thereafter (5.2)ADVERSE REACTIONSThe most frequent adverse reactions (rate ≥2.0% in at least one marketed dose) were myalgia, back pain, diarrhea, constipation and pain in extremity. (6)To report SUSPECTED ADVERSE REACTIONS, contact Kowa Pharmaceuticals America, Inc. at 1-877-334-3464 or FDA at 1-800-FDA-1088 or /medwatch.To report SUSPECTED ADVERSE REACTIONS, contact at or FDA at 1-800-FDA-1088 or /medwatchDRUG INTERACTIONS•Erythromycin: Combination increases pitavastatin exposure. Limit LIVALO to 1 mg once daily (2.3, 7.2)•Rifampin: Combination increases pitavastatin exposure. Limit LIVALO to 2 mg once daily (2.4, 7.3)•Concomitant lipid-lowering therapies : Use with fibrates or lipid-modifying doses (≥1 g/day) of niacin increases the risk of adverse skeletal muscle effects. Caution should be used when prescribing with LIVALO. (5.1, 7.4, 7.5)USE IN SPECIFIC POPULATIONS•Pediatric use: Safety and effectiveness have not been established. (8.4)•Renal impairment: Limitation of a starting dose of LIVALO 1 mg once daily and a maximum dose of LIVALO 2 mg once daily for patientswith moderate and severe renal impairment as well as patients receiving hemodialysis (2.2, 8.6)See 17 for PATIENT COUNSELING INFORMATIONRevised: 11/2012FULL PRESCRIBING INFORMATION: CONTENTS *1 INDICATIONS AND USAGE1.1 Primary Hyperlipidemia and Mixed Dyslipidemia1.2 Limitations of Use2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information2.2 Dosage in Patients with Renal Impairment2.3 Use with Erythromycin2.4 Use with Rifampin3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 Skeletal Muscle Effects5.2 Liver Enzyme Abnormalities5.3 Endocrine Function6 ADVERSE REACTIONS6.1 Clinical Studies Experience6.2 Postmarketing Experience:7 DRUG INTERACTIONS7.1 Cyclosporine7.2 Erythromycin7.3 Rifampin7.4 Gemfibrozil7.5 Other Fibrates7.6 Niacin7.7 Warfarin8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use8.6 Renal Impairment8.7 Hepatic Impairment10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacodynamics12.3 Pharmacokinetics13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility13.2 Animal Toxicology and/or Pharmacology14 CLINICAL STUDIES14.1 Primary Hyperlipidemia or Mixed Dyslipidemia16 HOW SUPPLIED/STORAGE AND HANDLING17 PATIENT COUNSELING INFORMATION17.1 Dosing Time17.2 Muscle Pain17.3 Pregnancy17.4 Breastfeeding17.5 Liver Enzymes* Sections or subsections omitted from the full prescribing information are not listedFULL PRESCRIBING INFORMATION1 INDICATIONS AND USAGEDrug therapy should be one component of multiple-risk-factor intervention in individuals who require modifications of their lipid profile. Lipid-altering agents should be used in addition to a diet restricted in saturated fat and cholesterol only when the response to diet and other nonpharmacological measures has been inadequate.1.1 Primary Hyperlipidemia and Mixed DyslipidemiaLIVALO® is indicated as an adjunctive therapy to diet to reduce elevated total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), triglycerides (TG), and to increase HDL-C in adult patients with primary hyperlipidemia or mixed dyslipidemia.1.2 Limitations of UseDoses of LIVALO greater than 4 mg once daily were associated with an increased risk for severe myopathy in premarketing clinical studies. Do not exceed 4 mg once daily dosing of LIVALO.The effect of LIVALO on cardiovascular morbidity and mortality has not been determined.LIVALO has not been studied in Fredrickson Type I, III, and V dyslipidemias.2 DOSAGE AND ADMINISTRATION2.1 General Dosing InformationThe dose range for LIVALO is 1 to 4 mg orally once daily at any time of the day with or without food. The recommended starting dose is 2 mg and the maximum dose is 4 mg. The starting dose and maintenance doses of LIVALO should be individualized according to patient characteristics, such as goal of therapy and response.After initiation or upon titration of LIVALO, lipid levels should be analyzed after 4 weeks and the dosage adjusted accordingly.2.2 Dosage in Patients with Renal ImpairmentPatients with moderate and severe renal impairment (glomerular filtration rate 30 – 59 mL/min/1.73 m2 and 15 – 29 mL/min/1.73 m2 not receiving hemodialysis, respectively) as well as end-stage renal disease receiving hemodialysis should receive a starting dose of LIVALO 1 mg once daily and a maximum dose of LIVALO 2 mg once daily.2.3 Use with ErythromycinIn patients taking erythromycin, a dose of LIVALO 1 mg once daily should not be exceeded [see Drug Interactions (7.2)].2.4 Use with RifampinIn patients taking rifampin, a dose of LIVALO 2 mg once daily should not be exceeded [see Drug Interactions (7.3)].3 DOSAGE FORMS AND STRENGTHS1 mg: Round white film-coated tablet. Debossed “KC” on one side and “1” on the other side of the tablet.2 mg: Round white film-coated tablet. Debossed “KC” on one side and “2” on the other side of the tablet.4 mg: Round white film-coated tablet. Debossed “KC” on one side and “4” on the other side of the tablet.4 CONTRAINDICATIONSThe use of LIVALO is contraindicated in the following conditions:•Patients with a known hypersensitivity to any component of this product. Hypersensitivity reactions including rash, pruritus, and urticaria have been reported with LIVALO [see Adverse Reactions (6.1)].•Patients with active liver disease which may include unexplained persistent elevations of hepatic transaminase levels [see Warnings and Precautions (5.2), Use in Specific Populations (8.7)].•Women who are pregnant or may become pregnant. Because HMG-CoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, LIVALO may cause fetal harm when administered to pregnant women. Additionally, there is no apparent benefit to therapy during pregnancy, and safety in pregnant women has not been established. If the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus and the lack of known clinical benefit with continued use during pregnancy [see Use in Specific Populations (8.1) and Nonclinical Toxicology (13.2)].•Nursing mothers. Animal studies have shown that LIVALO passes into breast milk. Since HMG-CoA reductase inhibitorshave the potential to cause serious adverse reactions in nursing infants, LIVALO, like other HMG-CoA reductase inhibitors, is contraindicated in pregnant or nursing mothers [see Use in Specific Populations (8.3) and Nonclinical Toxicology (13.2)].•Co-administration with cyclosporine [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].5 WARNINGS AND PRECAUTIONS5.1 Skeletal Muscle EffectsCases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including LIVALO. These risks can occur at any dose level, but increase in a dose-dependent manner. LIVALO should be prescribed with caution in patients with predisposing factors for myopathy. These factors include advanced age (≥65 years), renal impairment, and inadequately treated hypothyroidism. The risk of myopathy may also be increased with concurrent administration of fibrates or lipid-modifying doses of niacin. LIVALO should be administered with caution in patients with impaired renal function, in elderly patients, or when used concomitantly with fibrates or lipid-modifying doses of niacin [see Drug Interactions (7.6), Use in Specific Populations (8.5, 8.6) and Clinical Pharmacology (12.3)].There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated withstatin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation; improvement with immunosuppressive agents.LIVALO therapy should be discontinued if markedly elevated creatine kinase (CK) levels occur or myopathy is diagnosed or suspected. LIVALO therapy should also be temporarily withheld in any patient with an acute, serious condition suggestive of myopathy or predisposing to the development of renal failure secondary to rhabdomyolysis (e.g., sepsis, hypotension, dehydration, major surgery, trauma, severe metabolic, endocrine, and electrolyte disorders, or uncontrolled seizures). All patients should be advised to promptly report unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing LIVALO.5.2 Liver Enzyme AbnormalitiesIncreases in serum transaminases (aspartate aminotransferase [AST]/serum glutamic-oxaloacetic transaminase, or alanine aminotransferase [ALT]/serum glutamic-pyruvic transaminase) have been reported with HMG-CoA reductase inhibitors, including LIVALO. In most cases, the elevations were transient and resolved or improved on continued therapy or after a brief interruption in therapy.In placebo-controlled Phase 2 studies, ALT >3 times the upper limit of normal was not observed in the placebo, LIVALO 1 mg, or LIVALO 2 mg groups. One out of 202 patients (0.5%) administered LIVALO 4 mg had ALT >3 times the upper limit of normal.It is recommended that liver enzyme tests be performed before the initiation of LIVALO and if signs or symptoms of liver injury occur.There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including pitavastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with LIVALO, promptly interrupt therapy. If an alternate etiology is not found do not restart LIVALO.As with other HMG-CoA reductase inhibitors, LIVALO should be used with caution in patients who consume substantial quantities of alcohol. Active liver disease, which may include unexplained persistent transaminase elevations, is a contraindication to the use of LIVALO [see Contraindications (4)].5.3 Endocrine FunctionIncreases in HbA1c and fasting serum glucose levels have been reported with HMG-CoA reductase inhibitors, including LIVALO.6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in greater detail in other sections of the label:•Rhabdomyolysis with myoglobinuria and acute renal failure and myopathy (including myositis) [see Warnings and Precautions (5.1)].•Liver Enzyme Abnormalities [see Warning and Precautions (5.2)].Of 4,798 patients enrolled in 10 controlled clinical studies and 4 subsequent open-label extension studies, 3,291 patients were administered pitavastatin 1 mg to 4 mg daily. The mean continuous exposure of pitavastatin (1 mg to 4 mg) was 36.7 weeks (median 51.1 weeks). The mean age of the patients was 60.9 years (range; 18 years – 89 years) and the gender distribution was 48% males and 52% females. Approximately 93% of the patients were Caucasian, 7% were Asian/Indian, 0.2% were African American and 0.3% were Hispanic and other.6.1 Clinical Studies ExperienceBecause clinical studies on LIVALO are conducted in varying study populations and study designs, the frequency of adverse reactions observed in the clinical studies of LIVALO cannot be directly compared with that in the clinical studies of other HMG-CoA reductase inhibitors and may not reflect the frequency of adverse reactions observed in clinical practice.Adverse reactions reported in ≥ 2% of patients in controlled clinical studies and at a rate greater than or equal to placebo are shown in Table 1. These studies had treatment duration of up to 12 weeks.Table 1. Adverse Reactions* Reported by ≥2.0% of Patients Treated with LIVALO and > Placebo in Short-Term Controlled StudiesAdverse Reactions* PlaceboN= 208LIVALO1 mgN=309LIVALO2 mgN=951LIVALO4 mgN=1540Back Pain 2.9% 3.9% 1.8% 1.4% Constipation 1.9% 3.6% 1.5% 2.2% Diarrhea 1.9% 2.6% 1.5% 1.9%Myalgia 1.4% 1.9% 2.8% 3.1%Pain in extremity 1.9% 2.3% 0.6% 0.9%* Adverse reactions by MedDRA preferred term.Other adverse reactions reported from clinical studies were arthralgia, headache, influenza, and nasopharyngitis.The following laboratory abnormalities have also been reported: elevated creatine phosphokinase, transaminases, alkaline phosphatase, bilirubin, and glucose.In controlled clinical studies and their open-label extensions, 3.9% (1 mg), 3.3% (2 mg), and 3.7% (4 mg) of pitavastatin-treated patients were discontinued due to adverse reactions. The most common adverse reactions that led to treatment discontinuation were: elevated creatine phosphokinase (0.6% on 4 mg) and myalgia (0.5% on 4 mg).Hypersensitivity reactions including rash, pruritus, and urticaria have been reported with LIVALO.6.2 Postmarketing Experience:The following adverse reactions have been identified during postapproval use of LIVALO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.Adverse reactions associated with LIVALO therapy reported since market introduction, regardless of causality assessment, include the following: abdominal discomfort, abdominal pain, dyspepsia, nausea, asthenia, fatigue, malaise, hepatitis, jaundice, fatal and non-fatal hepatic failure, dizziness, hypoesthesia, insomnia, depression, interstitial lung disease, erectile dysfunction and muscle spasms. There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).There have been rare reports of immune-mediated necrotizing myopathy associated with statin use [see Warnings and Precautions (5.1)].7 DRUG INTERACTIONS7.1 CyclosporineCyclosporine significantly increased pitavastatin exposure. Co-administration of cyclosporine with LIVALO is contraindicated [see Contraindications (4), and Clinical Pharmacology (12.3)].7.2 ErythromycinErythromycin significantly increased pitavastatin exposure. In patients taking erythromycin, a dose of LIVALO 1 mg once daily should not be exceeded [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].7.3 RifampinRifampin significantly increased pitavastatin exposure. In patients taking rifampin, a dose of LIVALO 2 mg once daily should not be exceeded [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].7.4 GemfibrozilDue to an increased risk of myopathy/rhabdomyolysis when HMG-CoA reductase inhibitors are coadministered with gemfibrozil, concomitant administration of LIVALO with gemfibrozil should be avoided.7.5 Other FibratesBecause it is known that the risk of myopathy during treatment with HMG-CoA reductase inhibitors is increased with concurrent administration of other fibrates, LIVALO should be administered with caution when used concomitantly with other fibrates [see Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].7.6 NiacinThe risk of skeletal muscle effects may be enhanced when LIVALO is used in combination with niacin; a reduction in LIVALO dosage should be considered in this setting [see Warnings and Precautions (5.1)].7.7 WarfarinLIVALO had no significant pharmacokinetic interaction with R- and S- warfarin. LIVALO had no significant effect on prothrombin time (PT) and international normalized ratio (INR) when administered to patients receiving chronic warfarin treatment [see Clinical Pharmacology (12.3)]. However, patients receiving warfarin should have their PT and INR monitored when pitavastatin is added to their therapy.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyTeratogenic effects: Pregnancy Category XLIVALO is contraindicated in women who are or may become pregnant. Serum cholesterol and TG increase during normal pregnancy, and cholesterol products are essential for fetal development. Atherosclerosis is a chronic process and discontinuationof lipid-lowering drugs during pregnancy should have little impact on long-term outcomes of primary hyperlipidemia therapy [see Contraindications (4)].There are no adequate and well-controlled studies of LIVALO in pregnant women, although, there have been rare reports of congenital anomalies following intrauterine exposure to HMG-CoA reductase inhibitors. In a review of about 100 prospectively followed pregnancies in women exposed to other HMG-CoA reductase inhibitors, the incidences of congenital anomalies, spontaneous abortions, and fetal deaths/stillbirths did not exceed the rate expected in the general population. However, this study was only able to exclude a three-to-four-fold increased risk of congenital anomalies over background incidence. In 89% of these cases, drug treatment started before pregnancy and stopped during the first trimester when pregnancy was identified.Reproductive toxicity studies have shown that pitavastatin crosses the placenta in rats and is found in fetal tissues at ≤36% of maternal plasma concentrations following a single dose of 1 mg/kg/day during gestation.Embryo-fetal developmental studies were conducted in pregnant rats treated with 3, 10, 30 mg/kg/day pitavastatin by oral gavage during organogenesis. No adverse effects were observed at 3 mg/kg/day, systemic exposures 22 times human systemic exposure at 4 mg/day based on AUC.Embryo-fetal developmental studies were conducted in pregnant rabbits treated with 0.1, 0.3, 1 mg/kg/day pitavastatin by oral gavage during the period of fetal organogenesis. Maternal toxicity consisting of reduced body weight and abortion was observed at all doses tested (4 times human systemic exposure at 4 mg/day based on AUC).In perinatal/postnatal studies in pregnant rats given oral gavage doses of pitavastatin at 0.1, 0.3, 1, 3, 10, 30 mg/kg/day from organogenesis through weaning, maternal toxicity consisting of mortality at ≥0.3 mg/kg/day and impaired lactation at all doses contributed to the decreased survival of neonates in all dose groups (0.1 mg/kg/day represents approximately 1 time human systemic exposure at 4 mg/day dose based on AUC).LIVALO may cause fetal harm when administered to a pregnant woman. If the patient becomes pregnant while taking LIVALO,the patient should be apprised of the potential risks to the fetus and the lack of known clinical benefit with continued use during pregnancy.8.3 Nursing MothersIt is not known whether pitavastatin is excreted in human milk, however, it has been shown that a small amount of another drug in this class passes into human milk. Rat studies have shown that pitavastatin is excreted into breast milk. Because another drug in this class passes into human milk and HMG-CoA reductase inhibitors have a potential to cause serious adverse reactions in nursing infants, women who require LIVALO treatment should be advised not to nurse their infants or to discontinue LIVALO [see Contraindications (4)].8.4 Pediatric UseSafety and effectiveness of LIVALO in pediatric patients have not been established.8.5 Geriatric UseOf the 2,800 patients randomized to LIVALO 1 mg to 4 mg in controlled clinical studies, 1,209 (43%) were 65 years and older. No significant differences in efficacy or safety were observed between elderly patients and younger patients. However, greater sensitivity of some older individuals cannot be ruled out.8.6 Renal ImpairmentPatients with moderate and severe renal impairment (glomerular filtration rate 30 – 59 mL/min/1.73 m2 and 15 – 29 mL/min/1.73m2 not receiving hemodialysis, respectively) as well as end-stage renal disease receiving hemodialysis should receive a starting dose of LIVALO 1 mg once daily and a maximum dose of LIVALO 2 mg once daily [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].8.7 Hepatic ImpairmentLIVALO is contraindicated in patients with active liver disease which may include unexplained persistent elevations of hepatic transaminase levels.10 OVERDOSAGEThere is no known specific treatment in the event of overdose of pitavastatin. In the event of overdose, the patient should be treated symptomatically and supportive measures instituted as required. Hemodialysis is unlikely to be of benefit due to high protein binding ratio of pitavastatin.11 DESCRIPTIONLIVALO (pitavastatin) is an inhibitor of HMG-CoA reductase. It is a synthetic lipid-lowering agent for oral administration.The chemical name for pitavastatin is (+)monocalcium bis{(3R, 5S, 6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-3,5-dihydroxy-6-heptenoate}. The structural formula is:The empirical formula for pitavastatin is C50H46CaF2N2O8 and the molecular weight is 880.98. Pitavastatin is odorless and occursas white to pale-yellow powder. It is freely soluble in pyridine, chloroform, dilute hydrochloric acid, and tetrahydrofuran, soluble in ethylene glycol, sparingly soluble in octanol, slightly soluble in methanol, very slightly soluble in water or ethanol, and practically insoluble in acetonitrile or diethyl ether. Pitavastatin is hygroscopic and slightly unstable in light.Each film-coated tablet of LIVALO contains 1.045 mg, 2.09 mg, or 4.18 mg of pitavastatin calcium, which is equivalent to1 mg,2 mg, or 4 mg, respectively of free base and the following inactive ingredients: lactose monohydrate, low substituted hydroxypropylcellulose, hypromellose, magnesium aluminometasilicate, magnesium stearate, and film coating containing the following inactive ingredients: hypromellose, titanium dioxide, triethyl citrate, and colloidal anhydrous silica.12 CLINICAL PHARMACOLOGY12.1 Mechanism of ActionPitavastatin competitively inhibits HMG-CoA reductase, which is a rate-determining enzyme involved with biosynthesis of cholesterol, in a manner of competition with the substrate so that it inhibits cholesterol synthesis in the liver. As a result, the expression of LDL-receptors followed by the uptake of LDL from blood to liver is accelerated and then the plasma TC decreases. Further, the sustained inhibition of cholesterol synthesis in the liver decreases levels of very low density lipoproteins.12.2 PharmacodynamicsIn a randomized, double-blind, placebo-controlled, 4-way parallel, active-comparator study with moxifloxacin in 174 healthy participants, LIVALO was not associated with clinically meaningful prolongation of the QTc interval or heart rate at daily doses up to 16 mg (4 times the recommended maximum daily dose).12.3 PharmacokineticsAbsorption: Pitavastatin peak plasma concentrations are achieved about 1 hour after oral administration. Both C max and AUC0-inf increased in an approximately dose-proportional manner for single LIVALO doses from 1 to 24 mg once daily. The absolute bioavailability of pitavastatin oral solution is 51%. Administration of LIVALO with a high fat meal (50% fat content) decreases pitavastatin C max by 43% but does not significantly reduce pitavastatin AUC. The C max and AUC of pitavastatin did not differ following evening or morning drug administration. In healthy volunteers receiving 4 mg pitavastatin, the percent change from baseline for LDL-C following evening dosing was slightly greater than that following morning dosing. Pitavastatin was absorbed in the small intestine but very little in the colon.Distribution: Pitavastatin is more than 99% protein bound in human plasma, mainly to albumin and alpha 1-acid glycoprotein, and the mean volume of distribution is approximately 148 L. Association of pitavastatin and/or its metabolites with the blood cells is minimal.Metabolism: Pitavastatin is marginally metabolized by CYP2C9 and to a lesser extent by CYP2C8. The major metabolite inhuman plasma is the lactone which is formed via an ester-type pitavastatin glucuronide conjugate by uridine 5'-diphosphate (UDP) glucuronosyltransferase (UGT1A3 and UGT2B7).Excretion: A mean of 15% of radioactivity of orally administered, single 32 mg 14C-labeled pitavastatin dose was excreted in urine, whereas a mean of 79% of the dose was excreted in feces within 7 days. The mean plasma elimination half-life is approximately 12 hours.Race: In pharmacokinetic studies pitavastatin C max and AUC were 21 and 5% lower, respectively in Black or African American healthy volunteers compared with those of Caucasian healthy volunteers. In pharmacokinetic comparison between Caucasian volunteers and Japanese volunteers, there were no significant differences in C max and AUC.Gender: In a pharmacokinetic study which compared healthy male and female volunteers, pitavastatin C max and AUC were 60 and 54% higher, respectively in females. This had no effect on the efficacy or safety of LIVALO in women in clinical studies. Geriatric: In a pharmacokinetic study which compared healthy young and elderly (≥65 years) volunteers, pitavastatin C max and AUC were 10 and 30% higher, respectively, in the elderly. This had no effect on the efficacy or safety of LIVALO in elderly subjects in clinical studies.Renal Impairment: In patients with moderate renal impairment (glomerular filtration rate of 30 – 59 mL/min/1.73 m2) and end stage renal disease receiving hemodialysis, pitavastatin AUC0-inf is 102 and 86% higher than those of healthy volunteers, respectively, while pitavastatin C max is 60 and 40% higher than those of healthy volunteers, respectively. Patients received hemodialysis immediately before pitavastatin dosing and did not undergo hemodialysis during the pharmacokinetic study. Hemodialysis patients have 33 and 36% increases in the mean unbound fraction of pitavastatin as compared to healthy volunteers and patients with moderate renal impairment, respectively.。
瑞舒伐他汀钙片说明书
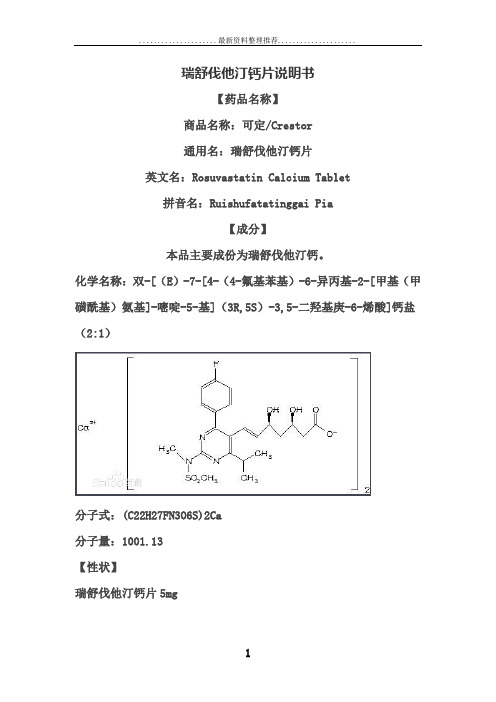
瑞舒伐他汀钙片说明书【药品名称】商品名称:可定/Crestor通用名:瑞舒伐他汀钙片英文名:Rosuvastatin Calcium Tablet拼音名:Ruishufatatinggai Pia【成分】本品主要成份为瑞舒伐他汀钙。
化学名称:双-[(E)-7-[4-(4-氟基苯基)-6-异丙基-2-[甲基(甲磺酰基)氨基]-嘧啶-5-基](3R,5S)-3,5-二羟基庚-6-烯酸]钙盐(2:1)分子式:(C22H27FN3O6S)2Ca分子量:1001.13【性状】瑞舒伐他汀钙片5mg圆形,黄色薄膜衣片,一面压印有“ZD4522”字样以及“5”,另一面平滑。
瑞舒伐他汀钙片10mg圆形,粉红色薄膜衣片,一面压印有“ZD4522”字样以及“10”,另一面平滑。
瑞舒伐他汀钙片20mg圆形,粉红色薄膜衣片,一面压印有“ZD4522”字样以及“20”,另一面平滑。
【适应症】本品适用于经饮食控制和其它非药物治疗(如:运动治疗、减轻体重)仍不能适当控制血脂异常的原发性高胆固醇血症(Ⅱa型,包括杂合子家族性高胆固醇血症)或混合型血脂异常症(Ⅱb型)。
本品也适用于纯合子家族性高胆固醇血症的患者,作为饮食控制和其它降脂措施(如LDL去除疗法)的辅助治疗,或在这些方法不适用时使用。
【规格】按瑞舒伐他汀计(1)5mg(2)10mg(3)20mg【用法用量】在治疗开始前,应给予患者标准的降胆固醇饮食控制,并在治疗期间保持饮食控制。
本品的使用应遵循个体化原则,综合考虑患者个体的胆固醇水平、预期的心血管危险性以及发生不良反应的潜在危险性。
口服。
本品常用起始剂量为5mg,一日一次。
起始剂量的选择应综合考虑患者个体的胆固醇水平、预期的心血管危险性以及发生不良反应的潜在危险性。
对于那些需要更强效地降低低密度脂蛋白胆固醇(LDL-C)的患者可以考虑10mg一日一次作为起始剂量,该剂量能控制大多数患者的血脂水平。
如有必要,可在治疗4周后调整剂量至高一级的剂量水平。
匹伐他汀钙原料药的性状研究

基 ). 3一 喹啉基] . 3 ,5 . 二 羟基 . 6 . 庚 酸} 单 钙 盐 ,系 日本 化 学 工业株 式会社 与兴和株 式会社 共 同开 发 的第 三代他 汀类药 物[ f 】 , 匹 伐 他 汀 对 HMG . C o A 还 原 酶 抑 制 作 用 强 , 呈 肝 细 胞
表 1 溶解度测定结果 ( 温度 :2 5 。 G)
2 方 法 与结果
临床研究用药 品质 量标准 中的检查 项中 。在实验所用 的8 种 2 . 1 外 观 、色 泽 、嗅 本 品 为 白 色 或 类 白 色 无 定 形 粉 末 , 无 嗅 , 对 光 不 稳 溶剂 中氯仿, n o . 1 mo l / L H C 1 是最合适的溶剂。下一步将 对本
选 择性 ,同时具 有作用 时间长 、耐受 性好 、安全性 高等特 点,被制药界誉为 “ 超级他汀 ”口 ] 。通过 查阅相关文献 ,发 现 匹伐他汀钙 与 同类药 品相 比具有 如下特 点[ 3 - 5 ] :①在 降低 低密度脂 蛋 白 ( L DL —c)的 同时 ,还可 显著 升高高密度脂 蛋白 ( HDL—C)。② 对遗 传性 高血脂 症 以及 伴有 糖尿 病 的高脂 血症有 显著疗 效。③很 少通过细 胞色素P 4 5 0 途径 代 谢 ,因而不 像 其 他他 汀 类 药物 那 样 易 受 可 改变 细 胞 色 素 P 4 5 0活性 药物 的影 响 。 由于 具备 上述 优 点 ,匹伐他 汀钙
被 药 学 界 颇 为 看 好 ,对 匹 伐 他 汀 钙 的 相 关 研 究 就 相 当 的有
意 义。
1 仪器 和试 药
通 过 上 述 溶 解 度 试 验 , 可 见 本 品 在 氯 仿 ,0 . 1 mo l / L HC 1
阿托伐他汀钙片说明书

阿托伐他汀钙片说明书【药品名称】通用名称:阿托伐他汀钙片商品名称:阿托伐他汀钙片(立普妥)英文名称:Atorvastatin Calcium Tablets拼音全码:ATuoFaTaTingGaiPian(LiPuTuo)【主要成份】本品的主要成分是阿托伐他汀钙,化学名称为:EP,-(R’,R’)]-2-(4-氟苯基)一B·6一二羟基一5一(1-甲基乙基)一3-苯基-4-[(苯胺)基关]-1-氢一吡咯-1-庚酸钙三水台物。
【性状】本品为白色椭圆形薄膜衣片。
【适应症/功能主治】高胆固醇血症原发性高胆固醇血症患者。
包括家族性高胆固醇血症(杂合子型)或混合型高脂血症(相当于Fredrickson分类法的IIa和IIb型)患者,如果饮食治疗和其他非药物治疗疗效不满意,应用本品可治疗其总胆固醇(TC)升高、低密度脂蛋白胆固醇(LDL-C)升高、载脂蛋白B(Apo B)升高和甘油三酯(TG)升高。
在纯合子家族性高胆固醇血症患者,阿托伐他汀可与其他降脂疗法(如LDL血浆透析法)合用或单独使用(当无其他治疗手段时),以降低TC和LDL-C。
冠心病冠心病或冠心病等危症(如:糖尿病、症状性动脉粥样硬化疾病等)合并高胆固醇血症或混合型血脂异常的患者,本品适用于:降低非致死性心肌梗死的风险,降低致死性和非致死性卒中的风险、降低血管重建术的风险,降低因充血性心力衰竭而住院的风险,降低心绞痛的风险。
【规格型号】 20mg*7片【用法用量】病人在开始本品治疗前,应进行标准的低胆固醇饮食控制,在整个治疗期间也应维持合理膳食。
应根据低密度脂蛋白胆固醇基线水平、治疗目标和患者的治疗效果进行剂量的个体化调整。
常用的起始剂量为10mg,每日一次。
剂量调整时间间隔应为4周或更长。
本品最大剂量为每天一次80mg。
可在一天内的任何时间服用,并不受进餐影响。
对于心血管事件的低危患者治疗目标是LDL-C<4.14mmol/L(或<160mg/dL)和总胆固醇<6.62mmol/L(或<240mg/dL)。
临床常见不合理用药实例解析16

不合理用药实例分析
解析
1.用药与诊断不相符(1)匹伐他汀钙片用药与诊断不相符解析:匹伐他汀无用药指征。匹伐他汀用于高胆固醇血症、家族性高胆固醇血症,无指征应用匹伐他汀,或应完善相关诊断。
2.剂量、用法不正确,单次处方总量不符合规定(1)匹伐他汀钙片剂量、用法不正确,单次处方总量不符合规定解析:匹伐他汀给药总剂量不适宜。匹伐他汀每日最大给药量为4mg,本处方日剂量6mg过大,建议根据患者病情,将单次剂量改为4mg或2mg。
2.有用药禁忌(1)盐酸莫西沙星片有用药禁忌解析:莫西沙星遴选药物不适宜。氟喹诺酮类抗菌药物不良反应包括心动过速高血压、心悸、QT 间期延长等,原有心脏病患者慎用氟喹诺酮类。患者不应使用莫西沙星治疗,可选用头孢菌素类。
感谢聆听,批评指导
2024
临床常见不合理用药实例解析
背景
处方评析,是药剂科开展的对用药的适宜性和合理性进行审核,是临床药学工作中的关键环节。处方评析工作能够改进医疗质量,提高药品临床管理和临床药物治疗水平,促进医院的医疗管理制度优化,降低患者的医药负担。今天,咱们就分享临床上常见的不合理用药解析。
不合理用药实例分析
一、不合理用药实例一
患者信息:女 ,50 周岁临床诊断:慢性支气管炎急性发作注释:无1.阿莫西林舒巴坦匹酯片(0.5g*12)用法:口服qd(1日1次)1次0.5g
2.盐酸氨溴索缓释胶囊(75mg*10)用法:口服bid(1日2次)1次1粒
不合理用药实例分析
解析
1.无注明过敏试验及结果的判定(1)阿莫西林舒巴坦匹酯片无注明过敏试验及结果的判定解析:未标明皮试结果阿莫西林舒巴坦钠未注明过敏试验结果。依据说明书用药前需做青霉素皮内敏感试验。
2.盐酸普罗帕酮片(50mg*100片)用法:口服tid(1日3次)1次150mg3.复方可待因口服溶液(120ml/瓶*1瓶)用法:口服tid(1日3次)1次15ml
阿托伐他汀钙片说明书

阿托伐他汀钙片说明书【药品名称】通用名称:阿托伐他汀钙片【性状】本品为白色薄膜衣片,除去薄膜衣后显白色,【适应症】高胆固醇血症原发性高胆固醇血定患者,包括家族性高胆固醇血定(杂合子型)或混合性高脂血症(相当于Fredrickson分类法的Ⅱa和Ⅱb型)患者,如果饮食治疗和其他非药物治疗疗效不满意,应用本品可治疗其总胆固醇(TC)升高、低密度脂蛋白胆固醇(LDL-C)升高、载脂蛋白B (Apo B)升高和甘油三脂(TG)升高.在纯合子家族性高胆固醇血症患者,阿托伐他汀钙可与其他降脂疗法合用或单独使用(当无其他治疗手段时).以降低总胆固醇(TC)和低密度脂蛋白胆固醇(LDL-C).冠心病冠心病或冠心病等危症(如:糖尿病,症状性动脉粥样硬化性疾病等)合并高胆固醇血症或混合性血脂异常患者,本品适用于:降低非致死性心肌梗死的风险、降低致死性和非致死性卒中的风险、降低血管重建术的风险、降低因充血性心力衰竭而住院的风险、降低心绞痛的风险。
【规格】(1) lOmg, (2) 20mg.(3)40mg【用法用量】病人在开始本品治疗前,应进行标准的低胆固醇饮食控制,在整个治疗期问也应维持合理膳食.应根据低密度脂蛋白胆固醇基线水平,治疗目标和患者的治疗效果进行剂量的个体化调整.常用的起始剂量为lOmg每日一次.剂量调整时间为4周或更长.本品最大剂量为80mg 每日一次.阿托伐他汀每日用量可在一日内的任何时间一次服用,并不受进餐影响。
对于确诊的冠心病患者或缺血事件危险性增加的其他患者治疗目标足低密度脂蛋白胆固醇<3mmol/L或115mg/dL和总胆固醇<5mmol/L或190mg/dL(摘自-动脉粥样硬化杂志1998年第140期199-270页“在临床实践中冠心病的防治:欧洲冠脉疾病预防第二次联合建议”).原发性高胆固醇血症和混合性寅高血症的治疗大多数患者服用阿托伐他汀每日一次lOmg.其血脂水平可得到控制。
匹伐他汀钙片说明书

匹伐他汀钙片说明书匹伐他汀钙片商品介绍通用名:匹伐他汀钙片生产厂家: Kowa Company,Ltd, Nagoya Factory(日本)批准文号:H药品规格:2mg某7片药品价格:¥63元匹伐他汀钙片说明书【通用名称】匹伐他汀钙片【商品名称】匹伐他汀钙片(力清之)【英文名称】PitavastatinCalciumTablets【拼音全码】PiFaTaTingGaiPian(LiQingZhi)【主要成份】匹伐他汀钙。
【性状】匹伐他汀钙片(力清之)为淡粉色薄膜衣片,除去薄膜衣片后显橙色。
【适应症/功能主治】力清之(匹伐他汀钙片)用于高胆固醇血症和家族性高胆固醇血症。
【规格型号】2mg某7s【用法用量】成人晚餐后口服力清之(匹伐他汀钙片)一日1~2毫克,根据年龄和症状调整剂量,若降LDL-C的疗效不理想,可增加剂量,大日剂量为4毫克。
【不良反应】主要不良反应有腹痛、皮疹、抑郁、搔痒以及r-谷氧酰胺转肽酶、肌酸激酶(CK1、谷丙转氯酶和谷草转氨酶升高。
严重不良反应有横纹肌溶解和肌病。
【禁忌】对力清之(匹伐他汀钙片)过敏者禁用,孕妇禁用。
【注意事项】力清之(匹伐他汀钙片)慎用于过度饮酒及有肝病史者若有不明原因肌痛、肌无力发生时,特别是伴有发热者应立即向医生报告,这些患者应检测CK水平,若CK水平高于正常或临床怀疑有肌病时应立即停用匹伐他汀钙片(力清之)。
【儿童用药】尚不明确。
【老年患者用药】尚不明确。
【孕妇及哺乳期妇女用药】尚不明确。
【药物相互作用】如与其他药物同时使用可能会发生药物相互作用,详情其咨询医师或药师。
【药物过量】尚不明确。
【药理毒理】匹伐他汀通过竞争性抑制羟甲戊二酰辅A(H毫克-CoA)还原酶,减少胆固醇的生物合成。
血管内胆固醇浓度的降低可使肝脏内低密度脂蛋白一胆固醇(LDL—C)受体下调,使LDL—C从血中的清除加快。
【药代动力学】尚不明确。
【贮藏】密封。
【包装】2mg某7s/盒。
瑞舒伐他汀钙片说明书

瑞舒伐他汀钙片说明书【药品名称】商品名称:可定/Crestor通用名:瑞舒伐他汀钙片英文名:Rosuvastatin Calcium Tablet拼音名:Ruishufatatinggai Pia【成分】本品主要成份为瑞舒伐他汀钙。
化学名称:双-[(E)-7-[4-(4-氟基苯基)-6-异丙基-2-[甲基(甲磺酰基)氨基]-嘧啶-5-基](3R,5S)-3,5-二羟基庚-6-烯酸]钙盐(2:1)分子式:(C22H27FN3O6S)2Ca分子量:1001.13【性状】瑞舒伐他汀钙片5mg ?圆形,黄色薄膜衣片,一面压印有“ZD4522”字样以及“5”,另一面平滑。
瑞舒伐他汀钙片10mg圆形,粉红色薄膜衣片,一面压印有“ZD4522”字样以及“10”,另一面平滑。
瑞舒伐他汀钙片20mg圆形,粉红色薄膜衣片,一面压印有“ZD4522”字样以及“20”,另一面平滑。
【适应症】本品适用于经饮食控制和其它非药物治疗(如:运动治疗、减轻体重)仍不能适当控制血脂异常的原发性高胆固醇血症(Ⅱa型,包括杂合子家族性高胆固醇血症)或混合型血脂异常症(Ⅱb型)。
本品也适用于纯合子家族性高胆固醇血症的患者,作为饮食控制和其它降脂措施(如LDL去除疗法)的辅助治疗,或在这些方法不适用时使用。
【规格】按瑞舒伐他汀计(1)5mg ?(2)10mg ?(3)20mg【用法用量】在治疗开始前,应给予患者标准的降胆固醇饮食控制,并在治疗期间保持饮食控制。
本品的使用应遵循个体化原则,综合考虑患者个体的胆固醇水平、预期的心血管危险性以及发生不良反应的潜在危险性。
口服。
本品常用起始剂量为5mg,一日一次。
起始剂量的选择应综合考虑患者个体的胆固醇水平、预期的心血管危险性以及发生不良反应的潜在危险性。
对于那些需要更强效地降低低密度脂蛋白胆固醇(LDL-C)的患者可以考虑10mg一日一次作为起始剂量,该剂量能控制大多数患者的血脂水平。
如有必要,可在治疗4周后调整剂量至高一级的剂量水平。
瑞舒伐他汀钙片说明书

瑞舒伐他汀钙片说明书瑞舒伐他汀钙片说明书【药品名称】商品名称:可定/Crestor通用名:瑞舒伐他汀钙片英文名:Rosuvastatin Calcium Tablet拼音名:Ruishufatatinggai Pia【成分】本品主要成份为瑞舒伐他汀钙。
化学名称:双-[(E)-7-[4-(4-氟基苯基)-6-异丙基-2-[甲基(甲磺酰基)氨基]-嘧啶-5-基](3R,5S)-3,5-二羟基庚-6-烯酸]钙盐(2:1)分子式:(C22H27FN3O6S)2Ca分子量:1001.13【性状】瑞舒伐他汀钙片5mg圆形,黄色薄膜衣片,一面压印有“ZD4522”字样以及“5”,另一面平滑。
瑞舒伐他汀钙片10mg圆形,粉红色薄膜衣片,一面压印有“ZD4522”字样以及“10”,另一面平滑。
瑞舒伐他汀钙片20mg圆形,粉红色薄膜衣片,一面压印有“ZD4522”字样以及“20”,另一面平滑。
【适应症】本品适用于经饮食控制和其它非药物治疗(如:运动治疗、减轻体重)仍不能适当控制血脂异常的原发性高胆固醇血症(Ⅱa型,包括杂合子家族性高胆固醇血症)或混合型血脂异常症(Ⅱb型)。
本品也适用于纯合子家族性高胆固醇血症的患者,作为饮食控制和其它降脂措施(如LDL去除疗法)的辅助治疗,或在这些方法不适用时使用。
【规格】按瑞舒伐他汀计(1)5mg(2)10mg(3)20mg【用法用量】在治疗开始前,应给予患者标准的降胆固醇饮食控制,并在治疗期间保持饮食控制。
本品的使用应遵循个体化原则,综合考虑患者个体的胆固醇水平、预期的心血管危险性以及发生不良反应的潜在危险性。
口服。
本品常用起始剂量为5mg,一日一次。
起始剂量的选择应综合考虑患者个体的胆固醇水平、预期的心血管危险性以及发生不良反应的潜在危险性。
对于那些需要更强效地降低低密度脂蛋白胆固醇(LDL-C)的患者可以考虑10mg一日一次作为起始剂量,该剂量能控制大多数患者的血脂水平。
如有必要,可在治疗4周后调整剂量至高一级的剂量水平。
阿托伐他汀钙片况明书

阿托伐他汀钙片说明书立普妥®请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:阿托伐他汀钙片商品名称:立普妥®英文名称:Atorvastatin Calcium Tablets汉语拼音:Atuofatatinggai Pian【成份】本品主要成份为阿托伐他汀钙。
【性状】本品为白色椭圆形薄膜衣片。
【适应症】高胆固醇血症原发性高胆固醇血症患者,包括家族性高胆固醇血症(杂合子型)或混合性高脂血症(相当于Fredrickson分类法的Ila和llb型)患者,如果饮食治疗和其它非药物治疗疗效不满意,应用本品可治疗其总胆固醇(Total cholesterol,TC)升高、低密度脂蛋白胆固醇(Low-density lipoprotein cholesterol,LDL-C)升高、载脂蛋白B(Apolipoprotein B,Apo B)升高和甘油三酯(Triglycerides,TG)升高。
在纯合子家族性高胆固醇血症患者,阿托伐他汀钙可与其它降脂疗法(如低密度脂蛋白血浆透析法)合用或单独使用(当无其它治疗手段时),以降低总胆固醇(TC)和低密度脂蛋白胆固醇(LDL-C)。
冠心病冠心病或冠心病等危症(如:糖尿病,症状性动脉粥样硬化性疾病等)合并高胆固醇血症或混合型血脂异常的患者,本品适用于:降低非致死性心肌梗死的风险、降低致死性和非致死性卒中的风险、降低血管重建术的风险、降低因充血性心力衰竭而住院的风险、降低心绞痛的风险。
【规格】(1)l0mg; (2) 20mg; (3) 40mg【用法用量】病人在开始本品治疗前,应进行标准的低胆固醇饮食控制,在整个治疗期间也应维持合理膳食。
应根据低密度脂蛋白胆固醇基线水平、治疗目标和患者的治疗效果进行剂量的个体化调整。
常用的起始剂量为l0mg每日一次。
剂量调整时间间隔应为4周或更长。
本品最大剂量为80mg每日一次。
阿托伐他汀每日用量可在一天内的任何时间一次服用,并不受进餐影响。
阿托伐他汀钙片说明书请仔细阅读说明书并在医师指导下使用
强 化 降 脂 进 一 步 减 少 临 床 终 点 事 件 研 究 ( Incremental Decrease in Endpoints Through
Aggressive Lipid Lowering Study, IDEAL) 强化降脂进一步减少临床终点事件研究(IDEAL) (见【临床试验】)涉及了8888名患者(年 龄范围26~80岁,19%女性;99.3%高加索白人,0.4%亚洲人,0.3%黑人,0.04%其他人种) , 每日接受立普妥80 mg (n=4439) 或辛伐他汀20~40 mg (n=4449) 治疗, 在随访中位值年限为4.8 年期间,两个治疗组不良事件或严重不良事件的总发生率没有差异。
核准日期:2007 年 03 月 09 日 修改日期: 2007 年 12 月 11 日; 2009 年 03 月 11 日; 2011 年 04 月 20 日; 2012 年 10 月 08 日; 2013 年 09 月 10 日;2013 年 11 月 07 日;2014 年 04 月 11 日;2014 年 09 月 12 日;2015 年 07 月 31 日;2016 年 02 月 24 日;2016 年 05 月 05 日;2017 年 09 月 18 日;2017 年 09 月 27 日
【不良反应】 下列严重不良反应在本说明书其它部分另有详细描述: 横纹肌溶解与肌病(见【注意事项】 ) 肝酶异常(见【注意事项】 ) 临床不良反应 临床试验实施过程中受试者病情复杂,因此两种不同药物在临床研究中获得的不良反应发 生率不能直接进行比较,同时可能不能反映临床实践中不良反应的发生率。 立普妥安慰剂对照临床试验共纳入 16066 名患者(立普妥 n=8755,安慰剂 n=7311,年龄从 10 岁到 93 岁,39%为女性;91%为高加索白人,3%为黑人,2%为亚洲人,4%为其他人种) , 中位治疗期为 53 周;在不考虑因果关系的情况下,立普妥组和安慰剂组分别有 9.7%和 9.5%患 者因不良反应停药。导致患者停药且立普妥组发生率高于安慰剂组最常见的 5 种不良反应分别 是:肌痛(0.7%) 、腹泻(0.5%) 、恶心(0.4%) 、丙氨酸氨基转移酶(Alanine aminotransferase, ALT)升高(0.4%)和其他肝酶升高(0.4%) 。 在不考虑因果关系的情况下,立普妥安慰剂对照试验(n=8755)中最常见(≥2%)且发生 率高于安慰剂的不良反应依次为: 鼻咽炎 (8.3%) 、 关节痛 (6.9%) 、 腹泻 (6.8%) 、 四肢痛 (6.0%) 和泌尿道感染(5.7%) 。表 1 总结了 17 项安慰剂对照试验中 8755 名接受立普妥治疗的患者发 生率≥2%且高于安慰剂组的不良反应(不考虑因果关系) 。
选择他汀需慎

0项
匹伐他汀
4S: Lancet. 1994;344(8934):1383-9 HPS: Lancet. 2002;360:7-22 PACT: Thompson PL, et al. Am Heart J. 2004;148(1):e2 LIPID: N Engl J Med. 1998;339(19):1349-57 CARE: Sacks FM, et al. N Engl Med. 1996;335(14):1001-9 ALLHAT-LLT: JAMA. 2002;288_2998-3007 FLORIDA: Liem AH, et al. Eur Heart J. 2002;23(24):1931-7 LIPS: Serruys PW, et al. JAMA. 2002;287(24):3215-22
CHD GREACE3(+) ALLIANCE4(+) TNT5(+) IDEAL6(+) 4S8(+) LIPID10(+) CARE11(+) LIPS13(+) 有效期:2018-4-11 PP-LIP-CHN-0194
立普妥®
辛伐他汀 普伐他汀 氟伐他汀 瑞舒伐他汀 匹伐他汀
*:指的是被国际权威指南引用的高质量RCT证据
循证证据
立普妥®六大里程碑研究涵盖 5大高危/极高危人群,为指南提供坚实循证基础
研究名称 PROVE IT1 SPARCL2 GREACE3 TNT4 CARDS5 ASCOT-LLA6 主要终点 死亡或主要心血管事件* 致死性或非致死性卒中 冠脉死亡 主要心血管事件** 主要心血管事件*** 非致死性心梗和 致死性冠心病 风险降低
CHD
CHD
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
匹伐他汀钙片(力清之)的说明书
由于现在很多老年人都会有高血压,高血脂等疾病,尤其是50岁以上的中老年人,所以很容易就会出现心血管疾病,脑血管疾病等,那么老年人应该如何选择用药呢?现在有一款叫做匹伐他汀钙片(力清之)的治疗心脑血管疾病的药物在众多药物中效果突出,那么它到底好在哪呢?
【药品名称】
通用名称:匹伐他汀钙片
商品名称:匹伐他汀钙片(力清之)
英文名称:Pitavastatin Calcium Tablets
拼音全码:PiFaTaTingGaiPian(LiQingZhi)
【主要成份】匹伐他汀钙。
【性状】本品为淡粉色薄膜衣片,除去薄膜衣片后显橙色。
【适应症/功能主治】力清之(匹伐他汀钙片)用于高胆固醇血症和家族性高胆固醇血症。
【规格型号】2mg*7s
【用法用量】成人晚餐后口服力清之(匹伐他汀钙片)一日1~2毫克,根据年龄和症状调整剂量,若降LDL-C的疗效不理想,可增加剂量,最大日剂量为4毫克。
【不良反应】主要不良反应有腹痛、皮疹、抑郁、搔痒以及r-谷氧酰胺转肽酶、肌酸激酶(CK1、谷丙转氯酶和谷草转氨酶升高。
严重不良反应有横纹肌溶解和肌病。
【禁忌】对力清之(匹伐他汀钙片)过敏者禁用,孕妇禁用。
【注意事项】力清之(匹伐他汀钙片)慎用于过度饮酒及有肝病史者若有不明原因肌痛、肌无力发生时,特别是伴有发热者应立即向医生报告,这些患者应检测CK水平,若CK水平高于正常或临床怀疑有肌病时应立即停用本品。
【儿童用药】尚不明确。
【老年患者用药】尚不明确。
【孕妇及哺乳期妇女用药】尚不明确。
【药物相互作用】如与其他药物同时使用可能会发生药物相互作用,详情其咨询医师或药师。
【药物过量】尚不明确。
【药理毒理】匹伐他汀通过竞争性抑制羟甲戊二酰辅A(H毫克-CoA)还原酶,减少胆固醇的生物合成。
血管内胆固醇浓度的降低可使肝脏内低密度脂蛋白一胆固醇(LDL—C)受体下调,使LDL —C从血中的清除加快。
【药代动力学】尚不明确。
【贮藏】密封。
【包装】2mg*7s/盒。
【有效期】24 月
【批准文号】H20080553
【生产企业】Kowa Company,Ltd, Nagoya Factory(日本)
到底心脑血管疾病会给老年人的身体健康带来多大的威胁,我们看过上边介绍后相信都清楚了解了,也知道了匹伐他汀钙片(力清之)是治疗心脑血管疾病药物的不二之选。
老年人健康了,子女的负担也轻了,关心老年人健康也是每个子女的义务和责任。
