3配合物命名
[co(nh3)6]br3配合物的名称
![[co(nh3)6]br3配合物的名称](https://img.taocdn.com/s3/m/c89ff2ef2dc58bd63186bceb19e8b8f67d1cef70.png)
[co(nh3)6]br3配合物的名称配位化合物的命名配体的种类很多,必须有统一的名称。
最常见的配体列在下面F-氟Cl-氯OH-羟CN-氰O2-氧 O22-过氧根SO42-硫酸根 N3-叠氮NO2-硝基ONO-亚硝酸根-SCN- 硫氰根-NCS-异硫氰根C6H5 -苯基py ()吡啶en 乙二胺Ph3P 三苯基膦NO 亚硝酰CO 羰基H2O 水NH3 氨(O2)双氧1.配位化合物内外界之间的连缀词在配位化合物中,先阴离子,后阳离子。
[ Co(NH3)5 H2O ] Cl3三氯化五氨•水合钴(III)Cu2 [ SiF6 ]六氟合硅(IV)酸亚铜阴阳离子之间加”化” 字或“ 酸”字,配阴离子看成是酸根。
[ Co(NH3)5 H2O ] Cl3三氯化五氨•水合钴(III)Cu2 [ SiF6 ]六氟合硅(IV)酸亚铜1.配位单元在配位单元中,先配体后中心,配体与中心之间加”合”字。
[ Co(NH3)5 H2O ] Cl3三氯化五氨•水合钴(III)Cu2 [ SiF6 ]六氟合硅(IV)酸亚铜配体前面用二、三、四· · ·[ Co(NH3)5 H2O ] Cl3三氯化五氨•水合钴(III)Cu2 [ SiF6 ]六氟合硅(IV)酸亚铜[ Co(NH3)5 H2O ] Cl3三氯化五氨•水合钴(III)Cu2 [ SiF6 ]六氟合硅(IV)酸亚铜几种不同的配体之间加“ • ”隔开。
[ Co(NH3)5 H2O ] Cl3三氯化五氨•水合钴(III)Cu2 [ SiF6 ]六氟合硅(IV)酸亚铜中心后面加(),内写罗马数字表示中心的化合价。
1.配体的先后顺序在配位单元中,可能涉及多种配体,所以要明确规定命名时配体的次序。
下述的每条规定均以其前一条规定为基础。
① 先无机配体后有机配体。
[ PtCl(2 Ph3P)2 ]二氯•二(三苯基膦)合铂(II)② 先阴离子类配体,后阳离子类配体,最后分子类配体。
高中化学竞赛-配合物,络合物,配位化学,配体,配位数,中心体

高中化学奥林匹克竞赛辅导配合物(配位化合物)化学基础【竞赛要求】配位键。
常见的配合物的中心离子(原子)和常见的配体(水、羟离子、卤离子、拟卤离子、氨分子、酸根离子、不饱和烃等)。
螯合物及螯合效应。
常见的络合剂及常见的配合反应。
定性说明配合反应与酸碱反应、沉淀反应、氧化还原反应的联系。
配合物几何构型和异构现象的基本概念。
配合物的杂化轨道理论。
八面体配合物的晶体场理论。
Ti(H2O)6的颜色。
路易斯酸碱的概念。
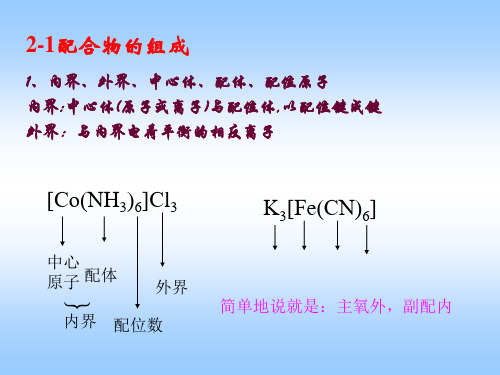
1.配合物:由中心离子(或原子)和几个配体分子(或离子)以配位键相结合而形成的复杂分子络合物。
如[Co(NH3)6]3+、[Cr(CN)6]3–、Ni(CO)4都是配位单元,分别称作配阳离子、配阴离子、配分子。
判断物质是配合物的关键在于物质是否含有配位单元。
配合物和复盐的区别:前者一定含有配位键,后者没有配位键,如KCl·MgCl2·6H2O是复盐,不是配合物。
2.配合物的组成:为外界,内外界(1)配合物的内界和外界:以[Cu(NH3)4]SO4为例,[Cu(NH3)4]2+为内界,SO-24之间是完全电离的。
内界是配位单元,外界是简单离子。
又如K3[Cr(CN)6]之中,内界是[Cr(CN)6]3–,外界是K+。
配合物可以无外界,但不能没有内界,如Ni(CO)4。
(2)中心离子(原子)和配位体:a.中心离子(原子):又称配合物的形成体或中心体,多为过渡金属离子,如Fe3+、Fe2+、Co2+、Ni2+、Cu2+,也有电中性的原子为配合物的中心原子,如Ni(CO)4、Fe(CO)5中的Ni和Fe都是电中性的原子。
只要能提供接纳孤对电子的空轨道的离子或原子均可作中心体。
b.配位体:含有孤对电子的阴离子或分子。
如NH3、Cl—、CN—等。
配位体中直接同中心原子配合的原子,叫做配位原子。
如[Cu(NH3)4]2+配阳离子中,NH3是配位体,其中N原于是配位原子。
配位原子经常是含有孤对电子的原子。
无机化学-配位化学基础-配合物的命名

Br-
bromo
NH3
ammine
F-
fluoro
H2O
aqua
O2-
oxo
NO
Nitrosyl
OH-
Hydroxo CO
Carbonyl
CN-
cyano
O2
dioxygen
C2O42-
oxalato N2
dinitrogen
CO32-
carbonato C5H5N
pyridine
CH3COO- acetato H2NCH2CH2NH2 ethylenediamine
[Co(en)3]2: tris(ethylenediamine)cobalt(II)
Ligands that bridge two metal centres are denoted by a prefix μ (mu) added to the name of the relevant ligand. If the number of centres bridged is greater than two, a subscript is used to indicate the number.
[(H3N)5CoOCo(NH3)5]4+ μ-oxido-bis(pentamminecobalt(III))
Square brackets are used to indicate which groups are bound to a metal atom, and should be used whether the complex is charged or not; however, in casual usage, neutral complexes and oxoanions are often written without brackets
三配位配合物

Cl Cu Cl Cu
Cl Cu Cl
四配位配合物, 常有平面正方形 D4h Td 和四面体两种构型。 一般非过渡元素的四配位化合物都是四面体构型。这是因为 采取四面体空间排列, 配体间能尽量远离, 静电排斥作用最小能 量最低。如FeCl4-及第Ⅱ副族的配合物。 过渡金属的四配位化合物究竟采用哪种构型需考虑下列两 种因素的影响。 (1) 配体之间的相互静电排斥作用; (2) 配位场稳定化能的影响(见后)。
五配位有两种基本构型 , 三角 双锥和四方锥, 它们分别属于D3h和 C4v对称群。此外,还存在变形的三 角双锥和变形的四方锥构型, 这种五配位配合物d1~d5组态的离 子较少,而组态为d8的粒子数为数最多。 这两种构型易于互相转化, 热力 学稳定性相近, 例如在Ni(CN)53-的结 晶化合物中, 两种构型共存。这是两 种构型具有相近能量的有力证明。 D3h C4v
2 三配位配合物(ML3) 这种配位数的金属配合物是比较少的。 已经确认的如 KCu(CN)2, 它是一 个聚合的阴离子, 其中每个Cu (I)原子 与两个C原子和一个N原子键合。 [Cu(Me3PS)3]Cl 中的Cu也是三配 位的。 在所有三配位的情况下, 金属原 子与三个直接配位的配位原子都是共 平面的, 有平面三角形的结构。 ◆并非化学式为MX3都是三配位的。 如, CuCl3是链状的, 为四配位, 其中 含有氯桥键, AuCl3也是四配位的, 确切的分子式为 Au2Cl6。
Oh群
变形的另一种型式是三方形畸 变, 包括了八面体沿三重对称轴的 缩短或伸长, 形成三方反棱柱体。
3.2 配位化合物的异构现象
配位化合物有两种类型的异构现象: 立体异构 化学结构异构
立体异构是化学式和原子排列次序都相同, 仅原子在空间的排 列不同的异构体。包括几何异构和光学异构。 化学结构异构是化学式相同, 原子排列次序不同的异构体。包 括电离异构、键合异构、配位异构、配位体异构、构型异构、 溶剂合异构和聚合异构;
第4章 配合物 4-1 配合物的基本概念4-2 配合物的异构现象与立体结构4-3 配合物的价键理论4-4 配

MA4B2 反式- (trans-)
顺式- (cis-)
MA3B3 经式- (mer-)
面式- (fac-)
MA2B2
反式-
顺式-
PtCl2 (NH3)2
顺式 cis-
顺式 cis-
顺式 cis-
Cl
NH3
Pt
H2O
Cl
NH3
反式 trans-
Cl
NH3 H2O
Pt
H3N Cl
H3N OH Pt
1. 内界: [AB n ]
配位数-配体名-合-中心原子(氧化数) (离子)
n
B
A (罗马数字)
例: [Cu(NH3)4]SO4 [Co(NH3)6]Cl3 [Ni(CO)4]
K3[Fe(CN)6] [Pt(NH3)6] [PtCl4]
2. 多种配体: 先无后有;先负后中;先A后B ;先简后繁。
NH3, en; Br-, NH3; NH3, H2O; NH3, NH2OH
Fe(CN)63-
价键理论
研究 M L 之间的化学键 Fe CN
价键理论的要点:
晶体场理论 配位场理论
配合物的空间构型(磁矩,内轨、外轨) 价键理论的应用和局限性
1. 价键理论的基本要点:
杂化轨道(配离子的空间构型) +配位键
①中心原子采用杂化轨道成键,决定空间构型
例: Fe(CN)63- Fe(III) 3d54s0 配位数: 6Cl[Fra bibliotek化 ]
OH
(酸) SO4
NO3
[Co(NH3)6]Cl3 [Cu(NH3)4]SO4
[Pt(NH3)6] [PtCl4]
4-2 配合物的异构现象与立体结构
高中选修三配合物

离子位于中心,六个氨分子作为配体通过配位键与铁离子相连。
02 03
铜(II)与乙二胺的配合物
铜(II)离子可以与乙二胺形成四乙二胺合铜(II)配合物([Cu(en)2]2+),其 中铜离子位于中心,四个乙二胺分子作为配体通过配位键与铜离子相连 。
钴(II)与氯离子的配合物
钴(II)离子可以与氯离子形成四氯合钴(II)配合物([CoCl4]2-),其中钴离 子位于中心,四个氯离子作为配体通过配位键与钴离子相连。
B
溶液的性质
溶液的pH值、离子强度、溶剂性质等也会 影响配合物的稳定性。例如,在强酸性溶液 中,一些配合物可能会发生解离;在强碱性
D
溶液中,一些配合物可能会发生沉淀。
03 配合物在化学反应中作用
氧化还原反应中作用
配合物作为氧化剂
01
某些配合物中的中心原子可以接受电子,从而作为氧化剂参与
氧化还原反应。
以中心原子的元素符号表示,其氧化态用 罗马数字标明。
配合物结构与性质
02
配合物空间构型
01
直线型
配合物中心原子与配体以直线方式排列,如 [Cu(NH3)2]+。
02
平面型
配合物中心原子与配体在同一平面上,形成平面构型, 如[PtCl4]2-。
03
四面体型
配合物中心原子位于四面体中心,四个配体位于四面体 的顶点,如[Ni(CO)4]。
谢谢聆听
稀土金属离子
如镧系和锕系元素,具有 特殊的电子构型和化学性 质,能与多种配体形成稳 定的配合物。
常见配体类型及其性质
氨和胺类配体
具有孤对电子的氮原子可以与 金属离子形成配位键,如氨 (NH3)、乙二胺(en)等。
配合物的立体结构

R=正丙基 平面正方形
Ni2+ + 水杨醛来胺衍生物—— R=叔丁基 四面体构型
R=异丁基 在顺式平面
CHNR
和四面体间转换
OH
4、离子半径比和溶剂化效应
5、配位数为5的配合物 ——三角双锥、四方锥
三角双锥(trigonal bipyramid, TBP) 中心原子以dsp3杂化 [Fe(CO)5] 、 [Cu(dipy)2I]+
根据2个金属离子之间的连接方式可将双 核配合物分为: 单齿桥联、双齿桥联、无桥联
根据2个金属离子的种类又可分为: 同双核、异双核
3、三核配合物(trinuclear complex)、 四核配合物(tetranuclear complex)等
原子簇化合物——簇合物(cluster)
Fe—S金属簇合物立方烷单元 双立方烷Mo(W)-Fe-S簇合物的主要结构类型
原子数的配体排在前面,较多原子数的配体
列后。 [Pt(NO2)(NH3)(NH2OH)py]Cl 氯化硝基·氨·羟氨·吡啶合铂(II)
(5) 配位原子相同,配体中所含的原子数目也 相同时,按结构式中与配原子相连的原子的元 素符号的英文顺序排列。
[Pt(NH2-)(NO2-)(NH3)2] 氨基·硝基·二氨合铂(II)
PPh 3
Cl
Cu
Cu
PPh 3
PPh 3
Cl
(2)三角锥形:中心原子具有非键
Sn
电子对,并占据角锥顶点 [SnCl3]- [AsO3]3-
Cl
Cl Cl
4、配位数为4的配合物
非过渡金属——四面体 [BeCl4]- [AlF4]- [SnCl4] 过渡金属——四面体、平面正方形
配合物eu(no3)3(phen)2的晶体结构和性质

配合物eu(no3)3(phen)2的晶体结构和性质
EU(NO3)3(PHEN)2是一种均一的配合物,它是铕(Eu)三氧化物和酚(PHEN)二酸盐的组合。
颜色介于深紫色和棕色之间,熔点为350℃。
它的晶体结构主要是由Eu3+和NO3-组成的网格结构,而这些网格结构内部嵌入了phend伴随物质。
此外,EU(NO3)3(PHEN)2有三个主要的物理性质:板条状,磁性和荧光。
由于PHEN和Eu3+之间存在强烈的依赖性,对于PHEN伴随物质而言,EU(NO3)3(PHEN)2具有板条状的晶体结构,它的晶胞的孔径较大,从而可以将PHEN伴随物质固定住,从而使PHEN伴随物质较小。
此外,EU(NO3)3(PHEN)2具有可以被外部磁场诱导的磁性,它的磁性特征主要由Eu3+元素贡献,但是当较低的外部磁场磁化作用下,PHEN伴随部分也受到磁场的影响而形成磁性结构。
最后, EU(NO3)3(PHEN)2也具有荧光性质,它在紫外线照射下发出微弱的绿色荧光,这是Eu3+元素受到电子跃迁而产生的绿色荧光。
总之,EU(NO3)3(PHEN)2是一种晶体结构稳定且性质多样的配合物,具有板条性,磁性,以及荧光性等卓越的特征,因此,它被广泛用于化学分析,催化,催化剂的研究和应用,荧光成像等领域。
苏教版高中化学选修三4.2《配合物的形成》参考教案

教专题专题 4 分子空间结构与物质性质学单元第二单元配合物的形成和应用课节题第一课时配合物的形成题知识与技能〔1〕了解人类对配合物结构认识的历史〔2〕知道简单配合物的根本组成和形成条件教〔3〕了解配合物的结构与性质及其应用学过程与方法通过配位键作为配离子化学构型,构筑配合物结构平台的方目法逐渐深入地理解配合物的结构与性质之间的关系标情感态度通过学生认识配合物在生产生活和科学研究方面的广泛应与价值观用体会配位化学在现代科学中的重要地位,从而激发学生进一步深入地研究化学。
教学重点配合物结构和性质,配合物形成条件和过程实验解释教学难点配合物结构和性质,配合物形成条件和过程实验解释教学方法探究讲练结合教学准备教师主导活动学生主体活动[复习 ]教 1.以下微粒中同时有离子键和配位键的是4B、NaOH 3+D、MgOA、NH Cl C、H O学3+是H2O和+结合而成的微粒,其化学键属于P692. H O HA、配位键B、离子键C、氢键D、范德华力讨论后口答过[知识回忆 ]1.配位键2.杂化和杂化轨道类型程[导入 ]实验 1:硫酸铜中逐滴参加浓氨水实验 2:氯化铜、硝酸铜中逐滴参加浓氨水实验分析:[知识梳理 ]一、配合物的形成1、配合物: 由提供孤 子 的配体与接受孤 子 的中 察心原子以配位 合形成的化合物称 配位化合物 称 配合物。
理解 教 主 活 学生主体活2、配合物的 成从溶液中析出配合物 , 配离子 常与 有相反 荷的其他离子合成 , 称 配 。
配 的 成可以划分 内界和外界。
配离子属于内界, 配离子以外的其他离子属于外界。
内、外界之 以离子 合。
外界离子所 荷 数等于配离子的 荷数。
〔1〕中心原子:通常是 渡金属元素〔离子和原子〕 ,少数是非金属元素,例如: Cu 2+,Ag +,Fe 3+,Fe ,Ni ,B Ⅲ,P Ⅴ⋯⋯〔2〕配位体: 含孤 子 的分子和离子。
如:- ,OH -, CN -,H 2 ,3 , CO ⋯⋯ I O NH配位原子: 配位体中具有孤 子 的原子。
宋天佑版无机化学 第11章配位化学基础

一个环上有几个原子称 为几原子环。 五或六元环最稳定。
螯合剂:能与中心离子形成螯合物的配体叫 螯合剂。
一般常见螯合剂是含有N、O、S、P等配位原 子的有机化合物。en,EDTA,C2O42-,NH2CH2COO-. 螯合剂必须具备的条件:
(1) 含2个或2个以上的配位原子;
(2) 任两个配位原子间最好间隔2-3个其它原子.
§11-1 配合物的空间构型、命名和磁性
§11-2 配位化合物的化学键理论 §11-3 配位化合物的稳定性
前 言
实验: 1.CuSO4+NaOH→Cu(OH)2→ CuSO4+BaCl2→BaSO4→
2.CuSO4
NH3H2O
C u 2( OH ) 2S O 4
NH 3H 2O
深蓝色溶液
深蓝 溶液
外界为其它阳离子 K4[Fe(CN)6]
2.配离子的命名
“某酸某”
配体数→配体名称→合→中心离子(氧化数)
汉字大写
罗马数字
⑴先阴离子(先无机后有机,先简后繁)后中性 分子(先无机后有机)。 ⑵同类配体,按配位原子元素符号的英文字母顺 序排列。NH3、H2O。
⑶同类配体中,若配位原子相同,含原子数少的 配体在前。NH3、NH2OH。
2.配位化合物的组成
配位键 离子键
[Cu( NH3 )4 ] SO4
中心 离子 配位 原子
配 位 体
配 体 数
外 界
内界(配阳离子)
K3[Fe(CN)6]
配(阴) 离子 中心 原子
Ni(CO)
4
中性配合物 (无外界)
一般配合物图示如下:
[中心离子(配位体)配体数]外界离子
内界(配离子)
配合物命名

之后用酸字结尾,如H2[PtCl6]称六氯合铂(IV)酸。若
外界为氢氧根离子则称氢氧化某,如[Cu(NH3)4](OH)2
称为氢氧化四氨合铜(II)。
配合物内界即配离子,其命名方法一般按照如下顺序:
配体数--配体名称(不同配体名称之间以中圆点(· )
分开)—“合”字——中心离子名称—中心离子氧化态
分子或离子 CO OH- F- PH3 NO2- N2 H- 游离态 一氧化碳 氢氧根 氟离子 磷化氢 亚硝酸根 氮 负氢离子 作配体 羰基 羟基 氟 膦 硝基(氮为配位原子时) 双氮 氢
⑵若配离子中的配体不止一种,在命名时配体的顺序:
①先无机后有机:配离子中如既有无机配体又有
有机配体时,则先无机配体排列后有机配体列。书写
四氯化六氨合铂(Ⅳ)
三氯化五氨•一水合钴(Ⅲ)
[CrCl2(H2O)4]Cl•2H2O 二水合一氯化二氯• 四水 合铬(Ⅲ)
3. 中性配合物 例如:[PtCl2(NH3)2] 二氯•二氨合铂(Ⅱ)
[CoCl3(NH3)3]
(Ⅲ)
三氯•三氨合钴
[Ni(CO)4]
四羰基合镍
注意: ⑴ 许多配体的名称与游离态的名称不同。
(用带圆括号的罗马数字表示)。
配合物内界即配离子,其命名方法一般按照如下顺序: 配体数--配体名称(不同配体名称之间以中圆点(· ) 分开)—“合”字——中心离子名称—中心离子氧化态 (用带圆括号的罗马数字表示)。
1. 配阴离子配合物
氢配酸的命名次序是:⑴阴配离子⑵中性分子配
体 ⑶中心离子 ⑷词尾用氢酸,氢字通常略去。例如, H[PtCl3(NH3)]称三氯一氨合铂(II)氢酸,或略去氢字 称三氯一氨合铂(II)酸。 氢配酸盐的命名次序同上,但词尾用酸而不用氢
选修三第二章第二节分子的立体结构3配合物理论简介 - 副本

K3[Fe(CN)6]
六氰合铁酸钾
络盐
[Ag(NH3)2]OH 氢氧化二氨合银
K[Pt(NH3)Cl3] 三氯一氨合铂酸钾
[Cu(NH3)4] SO4 硫酸四氨合铜
络盐 络盐
思考与 交流3
除水外,是否有其他电子给予体?
实验探究[2—2] (取实验[2-1]所得硫酸铜溶 液1/3实验)根据现象分析溶液成分的变化并说 明你的推断依据,写出相关的离子方程式
①复盐:由两种或两种以上的阳离子与一种酸根离子 组成的盐叫做复盐。 如: KAl(SO4)2· 12H2O ②混盐:是指一种金属离子与多种酸根离子所构成的盐。 如氯化硝酸钙[Ca(NO3)Cl] CaOCl2 ③配合物盐(络盐):是在配合物的溶液或晶体中,十分明 确地存在着含有配位键的、能独立存在的复杂组成的离 子: [Cu(NH3)4]SO4 [Cu(NH3)4]2+ + SO42-
(5) 配合物的应用
a 叶绿素 在生命体中的应用 血红蛋白 酶 含锌的配合物 含锌酶有80多种 维生素B12 钴配合物 在医药中的应用 抗癌药物 配合物与生物固氮 固氮酶 王水溶金 H[AuCl4] 照相技术的定影 在生产生活中的应用 电解氧化铝的助熔剂 Na3[AlF6] 镀银工业
b c d
(6)复盐、混盐、配合物盐
第二节 分子的立体构型 3
——配合物理论简介
实验2-1
四、配合物理论简介:
CuSO4 CuCl2.2H2O CuBr2 NaCl 无色 K2SO4 KBr
固体颜色 白色
溶液颜色 蓝色
绿色 蓝色
深褐色 白色
蓝色
白色
无色
白色
3.配合物命名(ppt)

A coordination complex is a substance in which a metal atom or ion accepts electrons from (and thus associates with) a group of neutral molecules or anions called ligands.A complex can be an anion, a cation ion, or a neutral molecule. Coordination compounds are neutral substances (i.e. uncharged) in which at least one ion is present as a complex.The coordination compounds are named in the following way.A. When naming coordination compounds, always name the cation before the anion. This rule holds regardless of whether the complex ion is the cation or the anion. (This is just like naming an ionic compound.)B. In naming the complex ion:1. Name the ligands first, in alphabetical order, and then name the central metal. Note: In the chemical formula the central metal is written before the ligands.2. The names of some common ligands are listed in Table 1.·Anionic ligands end in "-o." For anions that end in "-ide"(e.g. chloride, hydroxide), "-ate" (e.g. sulfate, nitrate), and "-ite" (e.g. nirite), change the endings as follows:-ide → -o; e.g., chloride → chloro and hydroxide → hydroxo-ate → -ato; e.g., sulfate → sulfato and nitrate → nitrato-ite → -ito; e.g., nitrite → nitrito·For neutral ligands, the common name of the molecule is used (e.g. H2NCH2CH2NH2 (ethylenediamine)). Important exceptions: water is called ‘aqua’, ammonia is called ‘ammine’, carbon monoxide is called ‘carbonyl’, and the N2 and O2 molecules are called ‘dinitrogen’ and ‘dioxygen’.Table 1. Names of Some Common Ligands3. The Greek prefixes di-, tri-, tetra-, etc. are used to designate the number of each type of ligand in the complex ion. If the ligand already contains a Greek prefix (e.g. ethylene di amine) or if it is a polydentate ligand (i.e. it can attach at more than one coordination site), the prefixes bis-, tris-, tetrakis-, and pentakis- are used instead. (See examples 3 and4.) The numerical prefixes are listed in Table 2.Table 2. Numerical Prefixes4. After naming the ligands, name the central metal. If the complex ion isa cation, the metal is named same as the element. For example, Co in acomplex cation is called cobalt and Pt is called platinum. (See examples 1-4.) If the complex ion is an anion, the name of the metal ends with the suffix -ate. (See examples 5 and 6.) For example, Co in a complex anion is called cobaltate and Pt is called platinate. For some metals, the Latin names are used in the complex anions (e.g. Fe is called ferrate and not ironate).Table 3: Name of Metals in Anionic Complexes5. Following the name of the metal, the oxidation state of the metal in the complex is given as a Roman numeral in parentheses.C. To name a neutral complex molecule, follow the rules of naming a complex cation. Remember: Name the (possibly complex) cation BEFORE the (possibly complex) anion. See examples 7 and 8.For historic reasons, some coordination compounds are called by their common names. For example: Fe(CN)63- and Fe(CN)64- are named ferricyanide and ferrocyanide respectively, and Fe(CO)5 is called iron carbonyl.Examples:Give the systematic names for the following coordination compounds: 1. [Cr(NH3)3(H2O)3]Cl3Answer: triamminetriaquachromium(III) chlorideSolution:· The complex ion is found inside the parentheses. In this case, the complex ion is a cation.· The ammine ligands are named first because alphabetically(按字母顺序地), "ammine" comes before "aqua."· The compound is electrically neutral and thus has an overall charge of zero. Since there are three chlorides associated with one complex ion and each chloride has a –1 charge, the charge on the complex ion must be +3.· From the charge on the complex ion and the charge on the ligands, we can calculate the oxidation number of the metal. In this example, all the ligands are neutral molecules. Therefore, the oxidation number of chromium must be the same as the charge of the complex ion, +3.2. [Pt(NH3)5Cl]Br3Answer: pentaamminechloroplatinum(IV) bromideSolution:· The complex ion is a cation, and the counter anions are the 3 bromides.· The charge of the complex ion must be +3 since it is associated with 3 bromides.· The NH3 molecules are neutral while the chloride carries a - 1 charge.· Therefore, the oxidation number of platinum must be +4.3. [Pt(H2NCH2CH2NH2)2Cl2]Cl2Answer: dichlorobis(ethylenediamine)platinum(IV) chloride Solution:· Since Ethylenediamine is a bidentate ligand, the prefix bis- is used instead of the prefix di-.4. [Co(H2NCH2CH2NH2)3]2(SO4)3Answer: tris(ethylenediamine)cobalt(III) sulfateSolution:· The sulfate has a charge of –2 and is the counter anion in this molecule.· Since it takes 3 sulfates to bond with two complex cations, the charge on each complex cation must be +3.· Since ethylenediamine is a neutral molecule, the oxidation number of cobalt in the complex ion must be +3.· Again, remember that you never have to indicate the number of cations and anions in the name of an ionic compound.5. K4[Fe(CN)6]Answer: potassium hexacyanoferrate(II)Solution:· Potassium is the cation, and the complex ion is the anion.· Since there are 4 K+ associated with the complex ion (each K+ having a +1 charge), the charge on the complex ion must be - 4.·Since each ligand carries –1 charge, the oxidation number of Fe must be +2.·The common name of this compound is potassium ferrocyanide.6. Na2[NiCl4]Answer: sodium tetrachloronickelate(II)Solution:· The complex ion is the anion so we have to add the suffix –ate to the name of the metal.7. Pt(NH3)2Cl4Answer: diamminetetrachloroplatinum(IV)Solution:· This is a neutral molecule because the charge on Pt+4 equals the negative charges on the four chloro ligands.· If the compound is [Pt(NH3)2Cl2]Cl2, even though the number of ions and atoms in the molecule are identical to the example, it should be named: diamminedichloroplatinum(IV) chloride because the platinum in the latter compound is only four coordinated instead of six coordinated.8. Fe(CO)5Answer: pentacarbonyliron(0)Solution:· Since it is a neutral complex, it is named in the same way as a complex cation. The common name of this compound, iron carbonyl, is used more often.9. (NH4)2[Ni(C2O4)2(H2O)2]Answer: ammonium diaquabis(oxalato)nickelate(II)Solution: The oxalate ion is a bidentate ligand.10. [Ag(NH3)2][Ag(CN)2]Answer: diamminesilver(I) dicyanoargentate(I)You can have a compound where both the cation and the anion are complex ions. Notice how the name of the metal differs even though they are the same metal ions.Can you give the molecular formulas of the following coordination compounds?1. hexaammineiron(III) nitrate2. ammonium tetrachlorocuprate(II)3. sodium monochloropentacyanoferrate(III)4. potassium hexafluorocobaltate(III)Can you give the name of the following coordination compounds?5. [CoBr(NH3)5]SO46. [Fe(NH3)6][Cr(CN)6]7. [Co(SO4)(NH3)5]+8. [Fe(OH)(H2O)5]2+Answers:1. [Fe(NH3)6](NO3)32. (NH4)2[CuCl4]3. Na3[FeCl(CN)5]4. K3[CoF6]5. pentaamminebromocobalt(III) sulfate6. hexaammineiron(III) hexacyanochromate (III)7. pentaamminesulfatocobalt(III) ion8. pentaaquahydroxoiron(III) ion。
配合物的总结大全

(1)在配合物中,以(n-1)d、ns、np等轨道 杂化形成的配合物称内轨型配合物,由ns、np 或ns、np、nd等轨道杂化形成的配合物称外 轨型配合物。
(2)内、外轨型配合物的稳定性比较:
内轨型配合物比外轨型配合物更稳定。(?)
(3) 一个配合物是内轨型还是外轨型,取决 于中心形成体的价层电子排布方式、空余价层 电子轨道的数目及类型、配体的强弱和配位数 等因素。可分下面几种情形:
M←NO2 硝基 (NO2) 来自HO - NMO←2ONO 亚硝酸根 (ONO) 来自H - ONO
NO 亚硝酰基 CO 羰基 M←CN 氰根 M ←NC 异氰根
3、配位数
(1)定义 中心原子(或离子)所接受的配位原子的数
目,称为配位数。
(2)求算方法 若单基配体,则配位数 = 配体数;若多基配
Co(CO)4
17e (unstable)
c.可以判断中性羰基配合物是否双聚
Mn(CO)5 17e,2Mn(CO)5 → CMon(2C(COO)4)1017e,2Co(CO)4 → Co2(CO)8
d.判断双核配合物中金属原子之间是否存在金 属键(式中数字为配体提供的电子数以及中心体 的价电子数)
若形成内轨型配合物,必须使部分未配对的d电子 先行配对,空出足够的内层轨道需要消耗能量即电子 配对能。在形成配合物时释放出的键能,将有一部分 被电子配对能抵消。
Cl(OC)4W
Cl
x
1 2 4 6 1 2 x 18
W(CO)4Cl
Cl
∴ x = 0 无金属键
O C
(C5H5)(OC)Mn
x Mn(CO)(C5H5)
C O
5 2 7 11 x 18
配合物命名

①先无机后有机:配离子中如既有无机配体又有
有机配体时,则先无机配体排列后有机配体列。书写
时把有机配体置于圆括号中。
例如:[Pt(en)Cl2] [Co(en)2Cl2]Cl
钴
二氯一(乙二胺)合铂 氯化二氯二(乙二胺)合
K[Co(en)Cl4] 钾
四氯一(乙二胺)合钴酸
②先阴离子后中性分子:有多种无机配体和有机配体
时,先阴离子配体,后中性分子配体。
例如:[Pt(NH3)2Cl2] K[PtCl3NH3]
钾
二氯二氨合铂 三氯一氨合铂(Ⅱ)酸
[Co(N3)(NH3)5]SO4 硫酸叠氮五氨合钴 (Ⅲ)
感谢观看
二硫酸根合钴(II)酸钾
分子或离子
游离态
作配体
1. 配阴离子配合物
氢配酸的命名次序是:⑴阴配离子⑵中性分子配
体 ⑶中心离子 ⑷词尾用氢酸,氢字通常略去。例如,
H[PtCl3(NH3)]称三氯一氨合铂(II)氢酸,或略去氢字 称三氯一氨合铂(II)酸。
氢配酸盐的命名次序同上,但词尾用酸而不用氢
酸,酸字后面在附上金属名称。例如,
游离态
作配体
四氯化六氨合铂(Ⅳ)
[Co(NH ) (H O)]Cl 三氯化五氨•一水合钴(Ⅲ) K2[Co(SO4)2]
二硫酸根合钴(II)酸钾
例如:[Pt(en)Cl2]
二氯一(乙二胺)合铂
若外界为氢氧根离子则称氢氧化某3,如5[Cu(N2H3)4](OH)2称为3氢氧化四氨合铜(II)。
CO
一氧化碳
配合物命名
4-1-4 配合物的命名
如果配合物中的酸根是一个简单的阴离子,称某化 某,如[Co(NH6)]Cl3称三氯化六氨合钴(III)。如果是一 个复杂的阴离子,则称某酸某,如[Cu(NH3)4]SO4称 硫酸四氨合铜(II)。若外界为氢离子,配阴离子的名称 之后用酸字结尾,如H2[PtCl6]称六氯合铂(IV)酸。若 外界为氢氧根离子则称氢氧化某,如[Cu(NH3)4](OH)2 称为氢氧化四氨合铜(II)。
配合物的基本概念与命名
(5) 配位原子相同,配体中所含的原子数目也
相同时,按结构式中与配原子相连的原子的元素符
号的英文顺序排列。
[Pt (NH2)(NO2)(NH3)2] 氨基·硝基·二氨合铂(II)
(6)配体化学式相同但配位原子不同,(- SCN, -NCS)
时,则按配位原子元素符号的字母顺序排列。 (7)配位原子的标记:若一个配体上有几种可能的配
CH2 H2N
CH2 NH2
乙二胺(en)
N
N
1,10-二氮菲(邻菲咯啉)
NN
联吡啶(bpy)
R'
C
O
R"C
C R
_
O
-双酮
多齿配体
- OOC -OOC
NCH2CH2N
COOCOO-
六齿配体 EDTA
L
N
N
Co
O
O
四齿配体
二水杨醛缩乙二 胺合钴Co(Salen)
EDTA配合物的结构
H2N
NH2
H
NH3
H2N
N
NH2
HN
NH
HN
NH
H
H2N
N
N
NH2
H
HN
NH NH
HN
NH NH
单齿配位 螯合作用
大环作用
穴合作用
N
N N
N
N N
N
N
酞菁 大环配体
N OO O
O O
O
N
穴2,醚2,2[-2,2,2]
穴状配体
B、根据键合电子的特征分为三种:
σ-配体:凡能提供孤对电子对与中心原子形成σ-配键 的配体。如:X-,NH3,OH-
配合物的中文命名法

若外界为OH-离子,则称氢氧化某;
[Cu(NH3)4](OH)2
氢氧化四氨合铜
配合物内界
按下列顺序依次命名:
配位体数→配体名称→合→中心离子(氧化数)
Cu(NH3)42+ [Mn(H2O)6]2+
四氨合铜(II)离子 六水合锰(II)离子
①配体名称置于中心原子之前
例1:[Fe(CN)6]4-
六氰合铁(III)酸钾
阴离子配体→中性分子配体→合→中心离子→酸。
如:K[PtCl3NH3] 三氯·一氨合铂(II)酸钾
含配阳离子的配合物
阴离子在前,配阳离子在后 例:[Cr(en)3](ClO4)3 高氯酸三(乙二胺)合铬(III) • 阳离子命名顺序: • 外界阴离子→化→酸性原子团→中性分子配体→中
普鲁士蓝染色肝细胞
普鲁士蓝除了可以作为染料,还作为铊中毒的药物。 在医疗上铊可置换普鲁士蓝上的钾后形成不溶性物质随 粪便排出,对治疗经口急慢性铊中毒有一定疗效。用量一般 为每日250mg/kg,分4次,溶于50ml 15%甘露醇中口服。 (适量补充氯化钾,高钾能增加肾对铊的清除能力,可能与 钾竞争性阻断肾小管对铊的吸收有关,同时钾可动员细胞内 的铊到细胞外,使血铊含量增加,可使临床病情加重,因此 要慎用)
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
A coordination complex is a substance in which a metal atom or ion accepts electrons from (and thus associates with) a group of neutral molecules or anions called ligands.A complex can be an anion, a cation ion, or a neutral molecule. Coordination compounds are neutral substances (i.e. uncharged) in which at least one ion is present as a complex. You will learn more about coordination compounds in the lab lectures for experiment 5 in this course.The coordination compounds are named in the following way.A. When naming coordination compounds, always name the cation before the anion. This rule holds regardless of whether the complex ion is the cation or the anion. (This is just like naming an ionic compound.)B. In naming the complex ion:1. Name the ligands first, in alphabetical order, and then name the central metal. Note: In the chemical formula the central metal is written before the ligands.2. The names of some common ligands are listed in Table 1.·Anionic ligands end in "-o." For anions that end in "-ide"(e.g. chloride, hydroxide), "-ate" (e.g. sulfate, nitrate), and "-ite" (e.g. nirite), change the endings as follows:-ide → -o; e.g., chloride → chloro and hydroxide → hydroxo-ate → -ato; e.g., sulfate → sulfato and nitrate → nitrato-ite → -ito; e.g., nitrite → nitrito·For neutral ligands, the common name of the molecule is used (e.g.H2NCH2CH2NH2 (ethylenediamine)). Important exceptions: water is called ‘aqua’, ammonia is called ‘ammine’, carbon monoxide is called‘carbonyl’, and the N2 and O2 m olecules are called ‘dinitrogen’ and‘dioxygen’.Table 1. Names of Some Common Ligands3. The Greek prefixes di-, tri-, tetra-, etc. are used to designate the number of each type of ligand in the complex ion. If the ligand already contains a Greek prefix (e.g. ethylene di amine) or if it is a polydentate ligand (i.e. it can attach at more than one coordination site), the prefixes bis-, tris-, tetrakis-, and pentakis- are used instead. (See examples 3 and4.) The numerical prefixes are listed in Table 2.Table 2. Numerical Prefixes4. After naming the ligands, name the central metal. If the complex ion isa cation, the metal is named same as the element. For example, Co in a complex cation is called cobalt and Pt is called platinum. (See examples 1-4.) If the complex ion is an anion, the name of the metal ends with the suffix -ate. (See examples 5 and 6.) For example, Co in a complex anion is called cobaltate and Pt is called platinate. For some metals, the Latin names are used in the complex anions (e.g. Fe is called ferrate and not ironate).Table 3: Name of Metals in Anionic Complexes5. Following the name of the metal, the oxidation state of the metal in the complex is given as a Roman numeral in parentheses.C. To name a neutral complex molecule, follow the rules of naming a complex cation. Remember: Name the (possibly complex) cation BEFORE the (possibly complex) anion. See examples 7 and 8.For historic reasons, some coordination compounds are called by their common names. For example: Fe(CN)63- and Fe(CN)64- are named ferricyanide and ferrocyanide respectively, and Fe(CO)5 is called iron carbonyl.Examples:Give the systematic names for the following coordination compounds: 1. [Cr(NH3)3(H2O)3]Cl3Answer: triamminetriaquachromium(III) chlorideSolution:· The complex ion is found inside the parentheses. In this case, the complex ion is a cation.· The ammine ligands are named first because alphabetically, "ammine" comes before "aqua."· The compound is electrically neutral and thus has an overall charge of zero. Since there are three chlorides associated with one complex ion and each chloride has a –1 charge, the charge on the complex ion must be +3.· From the charge on the complex ion and the charge on the ligands, we can calculate the oxidation number of the metal. In this example, all the ligands are neutral molecules. Therefore, the oxidation number of chromium must be the same as the charge of the complex ion, +3.2. [Pt(NH3)5Cl]Br3Answer: pentaamminechloroplatinum(IV) bromideSolution:· The complex ion is a cation, and the counter anions are the 3 bromides.· The charge of the complex ion must be +3 since it is associated with 3 bromides.· The NH3 molecules are neutral while the chloride carries a - 1 charge.· Therefore, the oxidation number of platinum must be +4.3. [Pt(H2NCH2CH2NH2)2Cl2]Cl2Answer: dichlorobis(ethylenediamine)platinum(IV) chloride Solution:· Since Ethylenediamine is a bidentate ligand, the prefix bis- is used instead of the prefix di-.4. [Co(H2NCH2CH2NH2)3]2(SO4)3Answer: tris(ethylenediamine)cobalt(III) sulfateSolution:· The sulfate has a charge of –2 and is the counter anion in this molecule.· Since it takes 3 sulfates to bond with two complex cations, the charge on each complex cation must be +3.· Since ethylenediamine is a neutral molecule, the oxidation number of cobalt in the complex ion must be +3.· Again, remember that you never have to indicate the number of cations and anions in the name of an ionic compound.5. K4[Fe(CN)6]Answer: potassium hexacyanoferrate(II)Solution:· Potassium is the cation, and the complex ion is the anion.· Since there are 4 K+ associated with the complex ion (each K+ having a +1 charge), the charge on the complex ion must be - 4.·Since each ligand carries –1 charge, the oxidation number of Fe must be +2.·The common name of this compound is potassium ferrocyanide.6. Na2[NiCl4]Answer: sodium tetrachloronickelate(II)Solution:· The complex ion is the anion so we have to add the suffix –ate to the name of the metal.7. Pt(NH3)2Cl4Answer: diamminetetrachloroplatinum(IV)Solution:· This is a neutral molecule because the charge on Pt+4 equals the negative charges on the four chloro ligands.· If the compound is [Pt(NH3)2Cl2]Cl2, even though the number of ions and atoms in the molecule are identical to the example, it should be named: diamminedichloroplatinum(IV) chloride because the platinum in the latter compound is only four coordinated instead of six coordinated.8. Fe(CO)5Answer: pentacarbonyliron(0)Solution:· Since it is a neutral complex, it is named in the same way as a complex cation. The common name of this compound, iron carbonyl, is used more often.9. (NH4)2[Ni(C2O4)2(H2O)2]Answer: ammonium diaquabis(oxalato)nickelate(II)Solution: The oxalate ion is a bidentate ligand.10. [Ag(NH3)2][Ag(CN)2]Answer: diamminesilver(I) dicyanoargentate(I)You can have a compound where both the cation and the anion are complex ions. Notice how the name of the metal differs even though they are the same metal ions.Can you give the molecular formulas of the following coordination compounds?1. hexaammineiron(III) nitrate2. ammonium tetrachlorocuprate(II)3. sodium monochloropentacyanoferrate(III)4. potassium hexafluorocobaltate(III)Can you give the name of the following coordination compounds?5. [CoBr(NH3)5]SO46. [Fe(NH3)6][Cr(CN)6]7. [Co(SO4)(NH3)5]+8. [Fe(OH)(H2O)5]2+Answers:1. [Fe(NH3)6](NO3)32. (NH4)2[CuCl4]3. Na3[FeCl(CN)5]4. K3[CoF6]5. pentaamminebromocobalt(III) sulfate6. hexaammineiron(III) hexacyanochromate (III)7. pentaamminesulfatocobalt(III) ion8. pentaaquahydroxoiron(III) ion。
