GSI Bovine TNFα ELISA kit
碧云天 Human IgE ELISA Kit 产品说明书
Human IgE ELISA Kit产品编号 产品名称包装 PI479Human IgE ELISA Kit96次产品简介:碧云天的Human IgE ELISA Kit (Human IgE Enzyme-Linked ImmunoSorbent Assay Kit),即人总免疫球蛋白E 酶联免疫吸附检测试剂盒,是一种用于特异性地高灵敏地定量检测人血清、血浆中的IgE 的ELISA 试剂盒。
本产品检测灵敏度高,特异性强,重复性好。
多次重复检测结果表明,最小检出量为14.3ng/ml ,本试剂盒采用特异性抗体识别人IgE ,与IgG 、IgA 没有交叉反应性,板内、板间变异系数均小于10%。
免疫球蛋白E (IgE)只能在哺乳动物身上发现。
IgE 存在于血液中,是免疫系统抵御细菌和病毒等外来物质入侵的重要工具。
IgE 是一类具有δ链的亲同种细胞抗体,是参与过敏性鼻炎、过敏性哮喘和湿疹等发病机制调节的主要抗体。
IgE 是组胺和白三烯等肥大细胞释放抗炎剂水平的主要调剂者。
尽管只有少量IgE 在血液中,但它还负责触发严重过敏反应。
IgE 在I 型超敏反应中起作用,I 型超敏反应表现为各种过敏性疾病,如过敏性哮喘、大多数类型的鼻窦炎、过敏性鼻炎、食物过敏,以及某些类型的慢性荨麻疹和特应性皮炎。
此外,有足够证据证明IgE 还参与免疫系统对寄生虫入侵的反应,并增加与癌症发病有关的白血细胞数量。
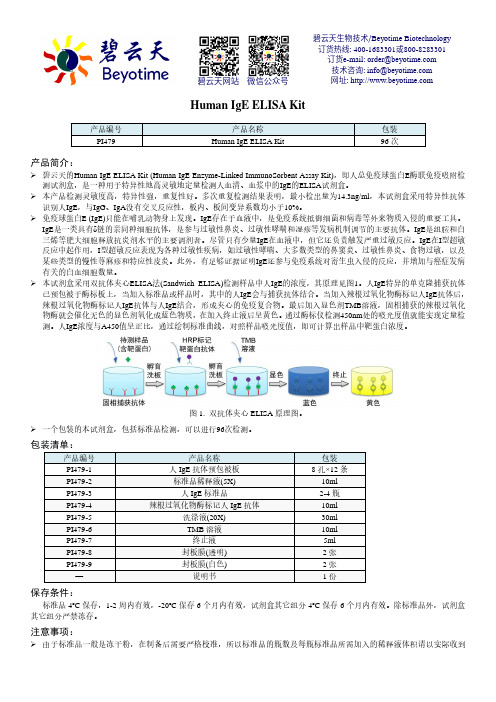
本试剂盒采用双抗体夹心ELISA 法(Sandwich ELISA)检测样品中人IgE 的浓度,其原理见图1。
人IgE 特异的单克隆捕获抗体已预包被于酶标板上,当加入标准品或样品时,其中的人IgE 会与捕获抗体结合。
当加入辣根过氧化物酶标记人IgE 抗体后,辣根过氧化物酶标记人IgE 抗体与人IgE 结合,形成夹心的免疫复合物。
最后加入显色剂TMB 溶液,固相捕获的辣根过氧化物酶就会催化无色的显色剂氧化成蓝色物质,在加入终止液后呈黄色。
免疫印迹常用试剂(除一抗外)

200µg/0.2ml 1000µg/1ml 浓缩液 亲和纯化抗体
产品应用
WB=1:200-500 Elisa =1:1000-2000 IP=1:20-100 IHC=1:200-500
2.酶标二抗
产品编号
bse-0295G
英文名称
Goat Anti-Rabbit IgG/HRP
中文名称
辣根过氧化物酶标记羊抗兔IgG
产品标准
1L(100ml浓缩液)
产品应用
蛋白印迹(Western Blot,WB)
13.WB洗涤液
产品编号
C-0043
英文名称
WesternBlotWashBuffer
中文名称
WB洗涤液
生产产地
北京博奥森生物技术有限公司
产品类别
分子生物学试剂
产品价格
30ml/48元 70ml/78元
产品标准
10X浓缩液
免疫印迹常用试剂(除一抗外)
1.内参抗体
产品编号
bs-0061R
英文名称
β-Actin(内参抗体)
中文名称
β-肌动蛋白(抗体)(浓缩液)
生产产地
北京博奥森生物技术有限公司
产品类别
兔抗人、羊、狗、猪、牛、兔、鸡、猴和大、小鼠β-Actin多克隆抗体
产品价格
0.1ml/400元 0.2ml/650元 1ml/1860元
产品编号
C-0075
中文名称
KODAK胶片
生产产地
KODAK原装
产品类别
曝光胶片
产品价格
100张/盒/240元
产品标准
5X7英寸
产品应用
转膜曝光
17.硝酸纤维素膜
牛结节性皮肤病ELISA检测试剂盒验证试验

牛结节性皮肤病ELISA检测试剂盒验证试验
牛结节性皮肤病(bovine nodular dermatitis,BND),也称为“木眼病”,是一种
由病毒引起的牛皮肤病。
这种病毒属于大痘病毒(Orthopoxvirus)属,是一种DNA病毒。
在病发地区,BND对牛业造成了很大的损失。
一种用于检测病毒存在的方法是酶联免疫吸附实验(enzyme-linked immunosorbent assay,ELISA)。
ELISA是一种定量分析技术,主要用于检测、诊断、研究和监测各种病原体的存在和反应。
ELISA技术已广泛应用于病原体和相关蛋白的检测中,因其灵敏度高、
稳定性好、操作简便和费用低等方面的优点而受到越来越多的关注。
为了验证牛结节性皮肤病ELISA检测试剂盒的灵敏度和特异性,进行了一系列的实验。
首先,用经过纯化的BND病毒蛋白质对测试剂盒进行评估。
评估结果表明,该测试剂盒具
有很高的灵敏度和特异性,可以准确地检测到BND病毒蛋白质的存在。
其次,采集30头罹患木眼病的牛的血清样品,以及30头健康牛的血清样品,并使用BNS ELISA测试剂盒进行检测。
结果显示,在30头罹患木眼病的牛中,有29头(97%)血清样品检测出BND病毒蛋白质。
而在30头健康牛中,所有血清样品均未检测出BND病毒蛋白质,这表明该测试剂盒具有很高的特异性。
综上所述,本研究验证了牛结节性皮肤病ELISA检测试剂盒的灵敏度和特异性。
在日
常的病原体检测中,该测试剂盒可以起到重要的作用,有利于疾病的快速及时发现和治疗,从而降低生产成本,提高养殖效益。
牛结节性皮肤病ELISA检测试剂盒验证试验

牛结节性皮肤病ELISA检测试剂盒验证试验
牛结节性皮肤病(Bovine Nodular Dermatosis)是一种常见的牛类皮肤病,主要由多义鼠天著病毒(Duboisia Species Poxvirus)引起。
该病在全球范围内都有报道,对养殖业造成了严重的经济损失。
目前,依靠临床症状来进行诊断的方法并不准确,快速、灵敏、特异性的实验方法是关键。
ELISA(酶联免疫吸附试验)是一种常用的生物学实验技术,能够在较短的时间内检测到血清中的抗体或抗原。
对于牛结节性皮肤病的检测,ELISA可以用于检测牛体内特异性
的抗体,以确诊和追踪流行病学数据。
为了验证牛结节性皮肤病ELISA检测试剂盒的准确性和可靠性,我们进行了一系列的
试验。
我们收集了不同地区患有牛结节性皮肤病的牛的血清样本,并对其进行了处理和稀释。
然后,将处理后的血清样本添加到已经涂有特异性抗原的微孔板中,使抗原与抗体结合。
随后,将酶标记的二抗加入孔中,与特异性抗体结合。
经过洗涤,添加底物后,酶活
性会产生颜色反应,反应的强度与样本中抗体的浓度成正比。
通过对一系列样本的检测,我们发现牛结节性皮肤病ELISA检测试剂盒具有较高的敏
感性和特异性。
我们的结果表明,该试剂盒可以可靠地检测到牛结节性皮肤病抗体的存在,并可用于流行病学调查和疾病诊断。
在验证试验中,我们还比较了该试剂盒与其他相关试剂盒的结果。
结果表明,牛结节
性皮肤病ELISA检测试剂盒的结果与其他试剂盒的结果一致,且具有较高的一致性和可重
复性。
这进一步验证了该试剂盒的准确性和可靠性。
华大基因 牛结核病(TB)酶联免疫分析(ELISA) 试剂盒 使用说明书

牛结核病(TB)酶联免疫分析(ELISA)试剂盒使用说明书本试剂仅供研究使用目的:本试剂盒用于测定牛血清,血浆及相关液体样本中结核病(TB)的含量。
实验原理:本试剂盒应用双抗体夹心法测定标本中牛结核病(TB)水平。
用纯化的牛结核(TB)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入结核病(TB),再与HRP 标记的结核(TB)抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB 显色。
TMB在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的结核病(TB)呈正相关。
用酶标仪在450nm波长下测定吸光度(OD值),通过标准曲线计算样品中牛结核病(TB)浓度。
试剂盒组成:试剂盒组成48孔配置96孔配置保存说明书1份1份封板膜2片(48)2片(96)密封袋1个1个酶标包被板1×481×962-8℃保存标准品:27U/ml0.5ml×1瓶0.5ml×1瓶2-8℃保存标准品稀释液 1.5ml×1瓶 1.5ml×1瓶2-8℃保存酶标试剂 3 ml×1瓶 6 ml×1瓶2-8℃保存样品稀释液 3 ml×1瓶 6 ml×1瓶2-8℃保存显色剂A液 3 ml×1瓶 6 ml×1瓶2-8℃保存显色剂B液 3 ml×1瓶 6 ml×1瓶2-8℃保存终止液3ml×1瓶6ml×1瓶2-8℃保存浓缩洗涤液(20ml×20倍)×1瓶(20ml×30倍)×1瓶2-8℃保存样本处理及要求:1.血清:室温血液自然凝固10-20分钟,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如出现沉淀,应再次离心。
2.血浆:应根据标本的要求选择EDTA或柠檬酸钠作为抗凝剂,混合10-20分钟后,离心20分钟左右(2000-3000转/分)。
(20111104)ELISA试剂盒的选择

6、经济性:试剂在同等质量条件下通过大规模生产或技术进步降低成本而市场价格比较合理。
7、安全性:指试剂对操作者和ቤተ መጻሕፍቲ ባይዱ境安全无害传染性。
ELISA试剂盒的选择
拜力生物编辑整理:
ELISA试剂盒选择应注意以下几点:
1、卫生部规定乙肝,丙肝,艾滋病,梅毒试剂及血型试剂必须使用经卫生部生物制品检定所检定合格,并贴有防伪标签,试剂应从灵敏度,精密度,稳定性,简便性,特异性,安全性及经济性作出全面的评价。指试剂正确检定不存在的被检物质的能力(无假阳性),取决于包被抗原(体)及标记抗原(体)的纯度及特异性。
2、特异性:灵敏度:包括1)为试剂检出被检物质的最低量的能力;2)为试剂对人群或大量样品中阳性检出的能力(假阴性越少越好),取决于包被物的全面性。
3、精密度:ELISA试剂一般指其批内CV,其值应小于15%;定量试剂应同时考察线性范围
4、简便性:指在不影响试剂的前三项指标的前题下,实验和测定步骤越少越好,在定性试验中结果判断简单明了,)定量试验结果计算也应简单
8、试剂评价需要有权威的血清考核盘(Panel)进行检测,一般实验室不易从生检所或临检中心取得,每进一次试剂评价一次也很麻烦。可以通过间接的信息对试剂进行选择。
9、参考室间质评评价报告中对试剂的评价结果,这比较客观公正,因为统计数字均来自各参评医院,反映了试剂在某一地区的使用情况;
10、通过询问试剂包被物的组成,如原料来源(基因工程或合成多肽),片段的组合(按比例混合或化学合成),片段的长短等判断试剂的优劣;
牛结节性皮肤病ELISA检测试剂盒验证试验

牛结节性皮肤病ELISA检测试剂盒验证试验牛结节性皮肤病是一种常见的皮肤疾病,主要由牛结节性皮肤病病毒(Bovine Papular Stomatitis Virus,BPSV)引起。
该疾病主要通过接触感染传播,造成牛群中的经济损失。
早期诊断和有效的防控策略对于牛群的健康和生产至关重要。
目前,牛结节性皮肤病的诊断方法主要包括临床症状观察、病原学检测和免疫学检测等。
ELISA检测方法因其高灵敏度和高特异性而广泛应用于牛结节性皮肤病的诊断。
为了保证ELISA检测试剂盒的准确性和可靠性,需要进行验证试验。
验证试验需要准备阳性和阴性样品。
阳性样品是已确诊感染牛结节性皮肤病的牛血清,阴性样品是未感染牛血清。
这些样品可以通过实验室收集或者通过与兽医机构合作获得。
确保样品的来源可靠和可追溯。
验证试验需要按照说明书中的操作步骤进行,确保操作的准确性和规范性。
将阳性和阴性样品稀释成相同浓度。
然后,取一定量的稀释样品加入试验板中,进行一系列的洗涤、染色和显色操作。
根据试验板中反应产生的颜色强度,判断样品中是否存在BPSV抗体。
验证试验的结果需要进行数据分析和统计学处理。
可以使用标准曲线法将样品的OD值与阳性和阴性对照品的OD值进行比较,计算出相对OD值或者抗体滴度。
通过比较阳性和阴性样品的结果,可以确定ELISA检测试剂盒的准确性和可靠性。
验证试验的结果需要验证机构进行评估和批准。
评估机构将根据验证试验的结果和数据分析,评估ELISA检测试剂盒是否符合标准要求。
只有经过评估和批准的ELISA检测试剂盒才能在临床实验室中使用。
牛结节性皮肤病ELISA检测试剂盒的验证试验是确保检测结果的准确性和可靠性的重要步骤。
通过准备样品、规范操作、数据分析和评估等步骤,可以保证ELISA检测试剂盒在诊断牛结节性皮肤病中的应用价值和有效性。
牛结节性皮肤病ELISA检测试剂盒验证试验

牛结节性皮肤病ELISA检测试剂盒验证试验牛结节性皮肤病是一种常见的牛类皮肤疾病,由牛结节性皮肤病病毒引起。
该疾病主要通过接触传播,对牛群的健康和生产都会造成严重影响。
对于牛结节性皮肤病的检测和诊断是非常重要的。
ELISA检测试剂盒是目前常用的一种诊断方法,然而对于其准确性和稳定性的验证试验是至关重要的。
本文就牛结节性皮肤病ELISA检测试剂盒的验证试验进行介绍和讨论。
一、试验目的本次试验的主要目的是对牛结节性皮肤病ELISA检测试剂盒进行验证,评估其准确性、灵敏度和特异性,为该检测试剂盒的临床应用提供科学依据。
二、试验样品本次试验选取了来自不同地区的50份牛血清样品,其中包括25份阳性样品和25份阴性样品。
阳性样品经过病毒分离鉴定,确诊为牛结节性皮肤病,而阴性样品则经过临床检测及相关实验,排除了患有该疾病的可能。
三、试验方法1. ELISA检测方法将阳性和阴性样品进行ELISA检测,按照试剂盒说明书进行操作。
根据检测结果,评估该检测试剂盒的准确性和稳定性。
2. PCR检测方法对所有样品进行牛结节性皮肤病病毒的PCR检测,作为对ELISA检测结果的对照和验证。
四、试验结果经过ELISA检测后,阳性样品的OD值明显高于阴性样品,且阳性样品出现阳性反应,而阴性样品未出现阳性反应。
通过比对ELISA检测结果和PCR检测结果,发现两种方法的检测结果一致,验证了ELISA检测试剂盒的准确性和稳定性。
本次验证试验结果表明,牛结节性皮肤病ELISA检测试剂盒具有较高的准确性和稳定性,能够准确检测牛结节性皮肤病的阳性和阴性样品。
该检测试剂盒可以作为牛结节性皮肤病的临床诊断工具,为防控该疾病提供了重要的技术支持。
六、试验注意事项在进行本次验证试验时,我们需要特别注意以下几点:1. 严格按照试剂盒说明书进行操作,确保操作的准确性和一致性。
2. 样品的选择要具有代表性,能够充分反映实际检测的情况,以保证试验结果的可靠性。
Human β-NGF ELISA Kit 说明书

Humanβ-NGF ELISA Kit检测试剂盒(酶联免疫吸附法)Catalog NumberEK1141-48EK1141-96定量检测血清、血浆和细胞培养上清中的人β神经生长因子(β-NGF)浓度。
本产品仅用于科学研究,非诊断试剂,不能用于临床诊断。
一、产品介绍1.背景介绍神经生长因子(NGF)是一种神经肽,主要参与调节某些靶神经元的生长、增殖和存活。
当表达时,NGF最初是以7S、分子量为130kDa的复合物存在,这个复合物由α-NGF、β-NGF和γ-NGF(2:1:2比例)三个蛋白组成。
“神经生长因子”通常指的是2.5S、分子量为26kDa的β亚基蛋白。
β亚基是7S NGF复合物中唯一一个有生物活性的成分(如作为信号分子)。
NGF在先天性和获得性免疫中都起着重要作用。
研究表明NGF通过血浆在机体中循环,对整体的稳态维持很重要。
NGF可促进髓鞘的修复,亦可参与多种精神疾病,如老年痴呆症、抑郁症、精神分裂症、自闭症、雷特综合征、神经性厌食症,神经性贪食症。
NGF信号失调与阿尔茨海默病有关。
同样,NGF在许多心血管疾病中发挥作用,如冠状动脉粥样硬化、肥胖、2型糖尿病、代谢综合征。
NGF也可促进伤口愈合。
2.检测原理本试剂盒采用双抗体夹心酶联免疫吸附检测技术。
特异性抗人β-NGF抗体预包被在高亲和力的酶标板上。
酶标板孔中加入标准品、待测样本和生物素化的检测抗体,经过孵育,样本中存在的β-NGF与固相抗体和检测抗体结合。
洗涤去除未结合的物质后,加入辣根过氧化物酶标记的链霉亲和素(Streptavidin-HRP)。
洗涤后,加入显色底物TMB,避光显色。
颜色反应的深浅与样本中β-NGF的浓度成正比。
加入终止液终止反应,在450nm波长(参考波长570-630nm)测定吸光度值。
3.试剂盒检测的局限1)请在本试剂盒标示的有效期内使用。
2)试剂盒的试剂不能与其他批号的试剂或其他来源的试剂混合使用。
3)任何标准品稀释、操作人员、移液技术、洗涤技术、孵育温度、试剂盒保存时间的改变,都将影响结合反应。
ELISA试验基本步骤及材料和试剂

ELISA试验基本步骤及材料和试剂ELISA(酶联免疫吸附测定)是一种常用于检测蛋白质或抗体的定性和定量方法。
它的基本原理是利用酶-抗体偶联物来检测特定目标物质的存在,通过酶催化底物的变化来实现信号的放大和检测。
下面将介绍ELISA试验的基本步骤以及相关的材料和试剂。
1.涂板:首先,将微孔板(96孔板或其他规格的板)用目标蛋白质或抗体溶液涂覆在表面上,使其吸附固定。
这一步通常称为“涂板”。
2.阻断:将蛋白质或抗体涂覆后的微孔板用阻断剂(例如牛血清蛋白、BSA)进行阻断处理,以防止非特异性结合。
3.样品处理:将待检测的样品加入到微孔板中,并与目标蛋白质或抗体结合(如果存在)。
样品通常需要经过一系列稀释步骤,以便在量化检测时得出准确的结果。
4.洗涤:使用缓冲液反复洗涤微孔板,以去除未结合的物质。
5.二抗处理:加入与待检测物种类对应的二抗荧光素,使其与待检测物结合。
这一步通常称为“二级标记”。
6.洗涤:再次使用缓冲液反复洗涤微孔板,以去除未结合的二抗。
7.底物结合:加入底物(如TMB或ABTS)并等待一定的反应时间,使酶催化底物的变化产生可观察的颜色变化。
8.反应停止:加入停止液(如硫酸、盐酸)停止底物的反应。
9.测量:利用酶催化反应产生的颜色变化通过酶标仪或光谱仪进行测量,得出待测量物的浓度或定性。
1.微孔板:一般使用96孔的微孔板,也可根据需要选择其他规格的板。
2.涂板物质:目标蛋白质或抗体等。
3.阻断剂:常用的阻断剂有牛血清蛋白(BSA)等。
4.样品:待检测的生物样品,可以是血清、细胞提取物或其他组织液。
5.二抗:与待检测物种类对应的二抗,通常是抗体。
6.底物:用于酶催化反应并产生颜色变化的化学底物,如TMB(3,3',5,5'-四甲基苯基二胺)、ABTS等。
7.停止液:停止底物的反应,如硫酸、盐酸等。
8. 缓冲液:用于洗涤和稀释的缓冲液,如PBS(磷酸盐缓冲液)、TBST(含Tween-20的洗涤缓冲液)等。
牛(Bovine)病毒性腹泻病毒抗体(BVDV-Ab)-定性ELISA试剂盒说明书

4. 组织匀浆:将组织加入适量生理盐水捣碎。3000 转离心 10 分钟
本试剂盒只能用于科学研究,不得用于医学诊断
取上清。 5. 保存:如果样本收集后不及时检测,请按一次用量分装,冻存
牛(Bovine)病毒性腹泻病毒抗体(BVDV-Ab)
all Standards and Samples be added in duplicate to the Microelisa Stripplate. 2. Separately add Positive control and Negative control 50μl to the Positive and Negative well; Add testing sample 10μl then add Sample Diluent 40μl to testing sample well. 3. Add 100μl of HRP-conjugate reagent to each well, cover with an adhesive strip and incubate for 60 minutes at 37°C. 4. Aspirate each well and wash, repeating the process four times for a total of five washes. Wash by filling each well with Wash Solution (400μl) using a squirt bottle, manifold dispenser or autowasher. Complete removal of liquid at each step is essential to good performance. After the last wash, remove any remaining Wash Solution by aspirating or decanting. Invert the plate and blot it against clean paper towels. 5. Add chromogen solution A 50μl and chromogen solution B 50μl to each well. Gently mix and incubate for 15 minutes at 37°C. Protect from light. 6. Add 50μl Stop Solution to each well. The color in the wells should change from blue to yellow. If the color in the wells is green or the color change does not appear uniform, gently tap the plate to ensure thorough mixing. 7. Read the Optical Density (O.D.) at 450 nm using a microtiter plate reader within 15 minutes.
小鼠tnf-a ELISA kit说明书

钟。
3
7. 洗板4次。 8. 加入显色剂100ul/孔,避光37℃孵箱孵育10--20分钟。 9. 加入终止液100ul/孔,混匀后即刻测量OD450值(5分钟内)。
结果判断: 1. 每个标准品和标本的OD值应减去零孔的OD值。 2. 手工绘制标准曲线。以标准品浓度作横坐标,OD值作纵坐标,以平滑线连接各标准品的坐标
4℃ 4℃ 4℃ 4℃ 4℃(避光) 4℃ 4℃ 4℃(避光) 4℃ 4℃
*:[96/48 Tests]
所需物品(不提供,但可协助购买) : 1. 酶标仪(450nm) 2. 高精度加液器及吸头:0.5-10,2-20,20-200,200-1000ul 3. 37℃温箱, 双蒸水或去离子水,坐标纸。
4
参考文献: Vet Immunol Immunopathol. 1995 Aug;47(3-4):187-201. Immunobiology. 1995 Jul;193(2-4):186-92. Annu Rev Med. 1994;45:491-503. Int J Cardiol. 1993 Dec 31;42(3):231-8. J Periodontol. 1993 May;64(5 Suppl):445-9.
点。通过标本的OD值可在标准曲线上查出其浓度。 3. 若标本OD值高于标准曲线上限,应适当稀释后重测,计算浓度时应乘以稀释倍数
OD
2.5 2
1.5 1
0.5 0
0
m TNF-a
500
1000
pg/ml
1500
注意:本图仅供参考,应以同次试验标准品所绘标准曲线计算标本含量。
Human TNF-α 预包被 ELISA kit说明书

产品信息和操作指南Human TNF-α预包被 ELISA kit Cat# : DKW12-1720-048 / DKW12-1720-096本试剂盒专用于科研,而非用于诊断Human TNF-αDKW12-1720目录产品简介 (1)知识背景 (1)试剂盒提供的试剂 (2)需要实验者自行准备的试剂与仪器 (2)注意事项 (3)试剂的配制 (5)操作过程 (7)结果分析 (9)试剂盒的保存 (9)操作步骤一览表 (10)参考文献 (11)ELISA测定中可能会出现的问题及解决方法 (12)预包被ELISA 试剂盒系列产品 (15)1、产品简介:达优®人TNF-α ELISA试剂盒是通过酶联免疫吸附技术,体外定量检测人血清、血浆、缓冲液或细胞培养液中的TNF-α,可同时检测天然的和重组的TNF-α。
本试剂盒为预包被板,“夹心一步”完成,整个过程孵育时间不超过4小时,洗涤6次,操作时间大大减少。
本试剂盒专用于科研,而非用于诊断。
使用前请仔细阅读说明书并检查试剂盒组分,若有任何疑问请与达科为生物工程有限公司联系,E-mail:*************.检测范围:800-25 pg/mL灵敏度:8 pg/mL重复性:板内、板间变异系数均<10%。
2、知识背景:肿瘤坏死因子-α(Tumor necrosis factorα,TNF-α)亦称为恶质素,由活化的巨噬细胞和其它类型的细胞分泌,包括T细胞、B 细胞、NK细胞、LAK细胞、星形胶质细胞、内皮细胞、平滑肌细胞和一些肿瘤细胞(1-4),在正常宿主对抗感染和恶性肿瘤的生长过程中起重要的作用。
其过量表达跟一系列的病理状态有关,包括恶病质、败血性休克和自身免疫失调。
3、试剂盒提供的试剂:试剂规格配制Cytokine standard 2/1瓶* 干粉状,按瓶上说明操作Biotinylated antibody 2/1瓶* 1:50用Dilution buffer R(1×)稀释Streptavidin-HRP 2/1瓶* 1:100用Dilution buffer R(1×)稀释Dilution buffer R(1×) 3/2瓶* 即用型Washing buffer(50×)1瓶 150∶用蒸馏水稀释TMB 1瓶即用型Stop solution 1瓶即用型Precoated ELISA plate 8×12或8×6*即用型封板膜 2/1张* 即用型说明书1份*:96/48 Tests4、需要实验者自行准备的试剂与仪器:1.酶标仪(建议参考仪器使用说明提前预热)2.微量加液器及吸头:P10,P50,P100,P200,P1000 3.蒸馏水或去离子水4.全新滤纸5.旋涡振荡器和磁力搅拌器5、注意事项:1.试剂应按瓶上标签说明储存,使用前室温平衡20-30分钟。
elisa的检测原理及步骤

Elisa Kit使用方法检测原理:本实验采取双抗体夹心ELISA法。
抗人IL-4单抗包被于酶标板上,标本和尺度品中的IL-4会与单抗结合,游离的成分被洗去。
加入生物素化的抗人IL-4抗体和辣根过氧化物酶标识表记标帜的亲和素。
生物素与亲和素特异性结合;抗人IL-4抗体与结合在单抗上的人IL-4结合而形成免疫复合物,游离的成分被洗去。
加入显色底物,若反应孔中有IL-4,辣根过氧化物酶会使无色的显色剂现蓝色,加终止液变黄。
在450nm处测OD值,IL-4浓度与OD450值之间呈正比,可通过绘制尺度曲线求出标本中IL-4的浓度。
试剂盒组成:1.抗体预包被酶标板(8×12 或 8×6)2.冻干尺度品(2 / 1 支;0.5ng/支)3.尺度品和标本稀释液(橙盖瓶)(1 瓶;20ml/12ml)4.浓缩生物素化抗体(紫盖瓶)(1 支)5.生物素化抗体稀释液(蓝盖瓶)(1 瓶;16ml/10ml)6.浓缩酶结合物(棕盖瓶)(1 支)7.酶结合物稀释液(紫盖瓶)(1 瓶;16ml/10ml)8.浓缩洗涤液 20×(白盖瓶)(1 瓶;50ml/25ml)9.显色底物(TMB)(棕盖瓶)(1 瓶;12ml/6ml)10.反应终止液(红盖瓶)(1 瓶;12ml/6ml)11.封板胶纸(6/3 张)12.产品说明书(1 份)标本收集:1. 收集血液的试管应为一次性的无热原,无内毒素试管。
2. 血浆抗凝剂推荐使用EDTA。
防止使用溶血,高血脂标本。
3. 标本应清澈透明,悬浮物应离心去除。
4. 标本收集后若不及时检测,请按一次使用量分装,冻存于-20℃,-70℃电冰箱内,防止反复冻融,3-6月内检测。
5. 如果您的样本中检测物浓度高于尺度品最高值,请根据实际情况,做适当倍数稀释(建议做预实验,以确定稀释倍数)。
注意事项:1. 试剂盒使用前请保管在2-8℃。
复溶后的尺度品应分装后,将其放在-20~-70℃贮存。
牛肿瘤坏死因子β(TNFβ)ELISA kit试剂盒说明书

牛肿瘤坏死因子β(TNFβ)ELISA kit试剂盒说明书试剂盒:ELISA试剂盒、检测试剂盒、免疫组化试剂盒、放免试剂盒、分子生物学试剂盒、金标检测试剂盒、Omega试剂盒、细胞凋亡试剂盒、生化试剂盒。
各种动物血清:内皮细胞专用血清、Hyclone、GIBCO胎牛血清。
抗体:进口原装抗体、进口分装抗体、单克隆抗体、多克隆抗体、单标抗体、双标抗体、多标抗体、一抗、二抗;免疫组化抗体、免疫荧光抗体、流式细胞抗体、免疫细胞化学抗体。
细胞:原装进口细胞、肿瘤细胞、正常细胞、肿瘤耐药细胞。
耗材:细胞培养耗材、普通实验耗材。
生化试剂:原装进口生化试剂、进口分装生化试剂。
牛肿瘤坏死因子β(TNFβ)ELISA kit试剂盒说明书规格型号:96T/48T,来源:elisa酶联免疫试剂盒(进口分装),产品别名:人内分泌腺来源的血管内皮生长因子酶联免疫试剂盒、人内分泌腺来源的血管内皮生长因子elisa酶联免疫试剂盒用途:用于测定兔血清、血浆、组织及相关液体样本中的含量或活性。
种属:人、大鼠、小鼠、豚鼠、兔子、猪犬、牛羊、鸡鸭、植物ELISA试剂盒等试剂盒操作:牛肿瘤坏死因子β(TNFβ)ELISA kit试剂盒说明书测定的血清标本宜为新鲜采集;如不能立即测定,5天内测定的血清标本可存放于4℃,1周后测定的血清标本应低温冻存;冻存后融解的标本,蛋白质局部浓缩,分布不均,应充分混合后再测定,但混匀时应轻柔,不可强烈振荡。
标本凝集不全在没有促凝剂和抗凝剂存在的情况下,正常血液采集后1/2~2h开始凝固,18~24h完全凝固。
临床检验工作中,有时为了争取时间快速检测,常在血液还未开始凝固时即强行离心分离血清,此时的血清中仍残留部分纤维蛋白原,在牛肿瘤坏死因子β(TNFβ)ELISA kit试剂盒说明书测定过程中可以形成肉眼可见的纤维蛋白块,易造成假阳性结果;这类情况于次日复查时因血凝已完全,血清中不再有纤维蛋白原存在,故复查结果变为阴性。
检测TNF的 elisa试剂盒说明书.

QuantikineÒRat TNF-a ImmunoassayCatalog Number RTA00SRTA00PRTA00For the quantitative determination of rat tumor necrosis factor alpha (TNF-a concentrations in cell culture supernates, rat serum, and plasma.This package insert must be read in its entirety before using this product.FOR RESEARCH USE ONLY.NOT FOR USE IN DIAGNOSTIC PROCEDURES.TABLE OF CONTENTSContents PageINTRODUCTION2 PRINCIPLE OF THEASSAY. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3 LIMITATIONS OF THE PROCEDURE3 PRECAUTION. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3 TECHNICAL HINTS3 MATERIALSPROVIDED. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4 STORAGE5 OTHER SUPPLIES REQUIRED. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 SAMPLE COLLECTION AND STORAGE6 SAMPLEPREPARATION. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6 REAGENT PREPARATION7 ASSAYPROCEDURE. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 PROCEDURESUMMARY AND CHECKLIST9 CALCULATION OFRESULTS. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10 TYPICAL DATA10 PRECISION. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11 RECOVERY11 LINEARITY. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12 SENSITIVITY12 CALIBRATION. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13 SAMPLE VALUES13 SPECIFICITY. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13 REFERENCES14 PLATE LAYOUT. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15MANUFACTURED AND DISTRIBUTED BY:R&D Systems, Inc.TELEPHONE:(800 343-7475614 McKinley Place NE(612 379-2956Minneapolis, MN 55413FAX:(612 656-4400United States of America E-MAIL:info@DISTRIBUTED BY:R&D Systems Europe, Ltd.19 Barton Lane TELEPHONE:+44 (01235 529449Abingdon Science Park FAX:+44 (01235 533420Abingdon,OX143NB E-MAIL:info@United KingdomR&D Systems China Co. Ltd.24A1 Hua Min Empire Plaza TELEPHONE:+86 (21 52380373726 West Yan An Road FAX:+86 (21 52371001Shanghai PRC 200050E-MAIL:info@INTRODUCTIONTumor necrosis factor alpha (TNF-a, also known as cachectin; and tumor necrosis factor beta (TNF-b, also known as lymphotoxin, are two closely related proteins (approximately 34% amino acid sequence identity that bind to the same cell surface receptors and show many common biological functions. TNF-a and -b play critical roles in normal host resistance to infection and to the growth of malignant tumors, serving as immunostimulants and as mediators of the inflammatory response. Over-production of TNFs, however, has been implicated as playing a role in a number of pathological conditions, including cachexia, septic shock, and autoimmune disorders. TNF-a is produced by activated macrophages and other cell types including T and B cells, NK cells, LAK cells, astrocytes, endothelial cells, smooth muscle cells and some tumor cells (1 - 4.Rat TNF-a cDNA encodes a 235 amino acid (aa residue type II membrane protein (5. The 156 aa residue soluble TNF-a is released from the C-terminus of themembrane-anchored TNF-a by TNF-a-converting enzyme (TACE, a matrix metalloprotease (6, 7. The membrane-anchored form of TNF-a has been shown to have lytic activity and may also play an important role in intercellular communication (8. The biologically active TNF-a has been shown to exist as a trimer (9, 10.Two distinct TNF receptors, referred to as type I (or type B or p55 and type II (or type A or p75, that specifically bind TNF-a and TNF-b with equal affinity have been identified (11, 12. The two TNF receptors transduce signals independently of one another. The amino acid sequence of the extracellular domains of the two receptors are homologous and both receptors are members of the TNF receptor family which also include the NGF receptor, fas antigen, CD27, CD30, and CD40. The intracellular domains of the two receptors are apparently unrelated, suggesting that the two receptorsemploy different signal transduction pathways. Soluble forms of both types of receptors have been found in human serum and urine (13 - 15. These soluble receptors are capable of neutralizing the biological activities of the TNFs and may serve to modulate the activities of TNF.The Quantikine Rat TNF-a Immunoassay is a 4.5 hour solid phase ELISA designed to measure rat TNF-a levels in cell culture supernates, serum, and plasma. It containsE. coli-expressed recombinant rat TNF-a and antibodies raised against the recombinant factor. This immunoassay has been shown to quantitate the recombinant rat TNF-a accurately. Results obtained using natural rat TNF-a showed dose response curves that were parallel to the standard curves obtained using the recombinant kit standards. These results indicate that the Quantikine Rat TNF-a Immunoassay kit can be used to determine relative mass values for natural rat TNF-a.PRINCIPLE OF THE ASSAYThis assay employs the quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for rat TNF-a has been pre-coated onto a microplate. Standards, Control, and samples are pipetted into the wells and any rat TNF-a present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for rat TNF-a is added to the wells. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution is added to the wells. The enzyme reaction yields a blue product that turns yellow when the Stop Solution is added. The intensity of the color measured is in proportion to the amount of rat TNF-a bound in the initial step. The sample values are then read off the standard curve.LIMITATIONS OF THE PROCEDURE·FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES.·The kit should not be used beyond the expiration date on the kit label.·Do not mix or substitute reagents with those from other lots or sources.·If samples generate values higher than the highest standard, further dilute the samples with Calibrator Diluent and repeat the assay.·Any variation in operator, pipetting technique, washing technique, incubation time ortemperature, and kit age can cause variation in binding.·This assay is designed to eliminate interference by soluble receptors, binding proteins, and other factors present in biological samples. Until all factors have been tested in the Quantikine Immunoassay, the possibility of interference cannot be excluded.PRECAUTIONThe Stop Solution provided with this kit is an acid solution. Wear eye, hand, face, and clothing protection when using this material.TECHNICAL HINTS·When mixing or reconstituting protein solutions, always avoid foaming.·To avoid cross-contamination, change pipette tips between additions of each standard level, between sample additions, and between reagent additions. Also, use separatereservoirs for each reagent.·When using an automated plate washer, adding a 30 second soak period following the addition of wash buffer, and/or rotating the plate 180 degrees between wash steps may improve assay precision.·For best results, pipette reagents and samples into the center of each well.·It is recommended that the samples be pipetted within 15 minutes.·To ensure accurate results, proper adhesion of plate sealers during incubation steps is necessary.·Substrate Solution should remain colorless until added to the plate. Keep SubstrateSolution protected from light. Substrate Solution should change from colorless togradations of blue.·Stop Solution should be added to the plate in the same order as the Substrate Solution.The color developed in the wells will turn from blue to yellow upon addition of the Stop Solution.MATERIALS PROVIDEDDescription Part #Cat. #RTA00Cat. #SRTA00Rat TNF-a Microplates- 96 well polystyrene microplates(12 strips of 8 wells coated with a monoclonal antibody specificfor rat TNF-a.890682 2 plates 6 platesRat TNF-a Conjugate- 23 mL/vial of a polyclonal antibodyagainst rat TNF-a conjugated to horseradish peroxidase withpreservatives.892668 1 vial 3 vialsRat TNF-a Standard- 1.6 ng/vial of recombinant rat TNF-a in abuffered protein base with preservatives; lyophilized.890684 3 vials9 vials Rat TNF-a Control- Recombinant rat TNF-a in a bufferedprotein base with preservatives; lyophilized. The concentrationrange of rat TNF-a after reconstitution is shown on the vial label.The assay value of the Control should be within the rangespecified on the label.890685 3 vials9 vialsAssay Diluent RD1-41- 12.5 mL/vial of a buffered protein basewith preservatives.895514 1 vial 3 vials Calibrator Diluent RD5-17- 21 mL/vial of a buffered proteinbase with preservatives.895512 2 vials 6 vials Wash Buffer Concentrate- 50 mL/vial of a 25-fold concentratedsolution of a buffered surfactant with preservative.895024 1 vial 3 vials Color Reagent A- 12.5 mL/vial of stabilized hydrogen peroxide.895000 1 vial 3 vials Color Reagent B- 12.5 mL/vial of stabilized chromogen(tetramethylbenzidine.895001 1 vial 3 vials Stop Solution- 23 mL/vial of a diluted hydrochloric acid solution.895174 1 vial 3 vials Plate Covers- Adhesive strips.___8 strips24 strips RTA00 contains sufficient materials to run ELISAs on two 96 well plates.SRTA00 (SixPak contains sufficient materials to run ELISAs on six 96 well plates.This kit is also available in a PharmPak (R&D Systems, Catalog # PRTA00. PharmPaks contain sufficient materials to run ELISAs on 50 microplates. Specific vial counts of each component may vary. Please refer to the literature accompanying your order for specific vial counts.*Provided this is within the expiration date of the kit.OTHER SUPPLIES REQUIRED·Microplate reader capable of measuring absorbance at 450 nm, with the correction wavelength set at 540 nm or 570 nm.·Pipettes and pipette tips.·Deionized or distilled water.·Squirt bottle, manifold dispenser, or automated microplate washer.·100 mL and 1000 mL graduated cylinders.·Polypropylene test tubes for dilution.SAMPLE COLLECTION AND STORAGECell Culture Supernates- Remove particulates by centrifugation and assay immediately or aliquot and store samples at£-20° C. Avoid repeated freeze-thaw cycles.Serum- Allow blood samples to clot for 2 hours at room temperature before centrifuging for 20 minutes at 1000 x g. Remove serum and assay immediately or aliquot and store samples at£-20° C. Avoid repeated freeze-thaw cycles.Plasma- Collect plasma using EDTA or heparin as an anticoagulant. Centrifuge for20 minutes at 1000 x g within 30 minutes of collection. Assay immediately or aliquot and store samples at£-20° C. Avoid repeated freeze-thaw cycles.Note:Grossly hemolyzed or lipemic samples may not be suitable for measurement of rat TNF-a with this assay.SAMPLE PREPARATIONRat serum and plasma samples require a 2-fold dilution into Calibrator Diluent RD5-17 prior to assay. A suggested 2-fold dilution is 75m L sample + 75m L Calibrator Diluent RD5-17. Mix well.Rat cell culture supernate samples require a 3-fold dilution into Calibrator Diluent RD5-17 prior to assay. A suggested 3-fold dilution is 50m L sample + 100m L CalibratorDiluent RD5-17. Mix well.REAGENT PREPARATIONBring all reagents to room temperature before use.Rat TNF-a Kit Control- Reconstitute the Kit Control with 1.0 mL deionized or distilled water. Assay the Control undiluted.Wash Buffer- If crystals have formed in the concentrate, warm to room temperature and mix gently until the crystals have completely dissolved. To prepare enough Wash Buffer for one plate, add 25 mL Wash Buffer Concentrate into deionized or distilled water to prepare 625 mL of Wash Buffer.Substrate Solution- Color Reagents A and B should be mixed together in equal volumes within 15 minutes of use. Protect from light. 100m L of the resultant mixture is required per well. Rat TNF-a Standard- Reconstitute the rat TNF-a Standard with 2.0 mL of CalibratorDiluent RD5-17. Do not substitute other diluents. This reconstitution produces a stock solution of 800 pg/mL. Allow the standard to sit for a minimum of 5 minutes with gentle mixing prior to making dilutions.Use polypropylene tubes.Pipette 200m L of Calibrator Diluent RD5-17 into each tube. Use the stock solution to produce a dilution series (below. Mix each tubethoroughly before the next transfer. The undiluted rat TNF-a Standard serves as the high standard (800 pg/mL. Calibrator Diluent RD5-17 serves as the zero standard (0 pg/mL.ASSAY PROCEDUREBring all reagents and samples to room temperature before use. It isrecommended that all samples, standards, and control be assayed in duplicate.1.Prepare reagents, working standards, control, and samples as directed in the previoussections.2.Remove excess microplate strips from the plate frame, return them to the foil pouchcontaining the desiccant pack, and reseal.3.Add 50m L of Assay Diluent RD1-41 to each well.4.Add 50m L of Standard, Control, or sample* to each well. Mix by gently tapping the plateframe for 1 minute. Cover with the adhesive strip provided. Incubate for 2 hours at room temperature. A plate layout is provided to record standards and samples assayed.5.Aspirate each well and wash, repeating the process four times for a total of five washes.Wash by filling each well with Wash Buffer (400m L using a squirt bottle, manifolddispenser, or autowasher. Complete removal of liquid at each step is essential to good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.6.Add 100m L of Rat TNF-a Conjugate to each well. Cover with a new adhesive strip.Incubate for 2 hours at room temperature.7.Repeat the aspiration/wash as in step 5.8.Add 100m L of Substrate Solution to each well. Incubate for 30 minutes at roomtemperature.Protect from light.9.Add 100m L of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.10.Determine the optical density of each well within 30 minutes, using a microplate readerset to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. Ifwavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate.Readings made directly at 450 nm without correction may be higher and less accurate. *Samples require dilution as directed in the Sample Preparation section.PROCEDURE SUMMARY AND CHECKLISTCALCULATION OF RESULTSAverage the duplicate readings for each standard, control, and sample and subtract the average zero standard optical density.Create a standard curve by reducing the data using computer software capable ofgenerating a four parameter logistic (4-PL curve-fit. As an alternative, construct a standard curve by plotting the mean absorbance for each standard on the y-axis against theconcentration on the x-axis and draw a best fit curve through the points on the graph. The data may be linearized by plotting the log of the rat TNF-a concentrations versus the log of the O.D. and the best fit line can be determined by regression analysis. This procedure will produce an adequate but less precise fit of the data.Because samples have been diluted, the concentration read from the standard curve must be multiplied by the dilution factor.TYPICAL DATAThis standard curve is provided for demonstration only. A standard curve should be generated for each set of samples assayed.(pg/mL012.52550100200400800O.D.0.0340.0340.0850.0800.1280.1270.2140.2160.3830.3 720.6920.6981.2181.2222.0231.988Average 0.0340.0820.1280.2150.3780.6951.2202.006Corrected___0.0480.0940.1810.3440.6611.1861.972PRECISIONIntra-assay Precision(Precision within an assayThree samples of known concentration were tested twenty times on one plate to assess intra-assay precision.Inter-assay Precision(Precision between assaysThree samples of known concentration were tested in twenty assays to assess inter-assay precision.Intra-assay Precision Inter-assay precisionSample123123n202020202020Mean (pg/mL6523259363246656Standard3.3 5.112.5 6.123.657.6deviationCV (% 5.1 2.2 2.19.79.68.8RECOVERYThe recovery of rat TNF-a spiked to three levels throughout the range of the assay in various matrices was evaluated.*Samples were diluted as directed in the Sample Preparation section.LINEARITYTo assess the linearity of the assay, samples spiked with various concentrations of rat TNF-a were diluted with Calibrator Diluent RD5-17 and then assayed. Results from typical sample dilutions are shown.*Samples were diluted prior to assay, as directed in the Sample Preparationsection.SENSITIVITYThe minimum detectable dose (MDD of rat TNF-a is typically less than 5 pg/mL.The MDD was determined by adding two standard deviations to the mean optical density value of 20 zero standard replicates and calculating the corresponding concentration.CALIBRATIONThis immunoassay is calibrated against a highly purified E. coli -expressed recombinant rat TNF-a produced at R&D Systems. The recombinant N-methionyl form of rat TNF-a contains 157 amino acid residues and has a predicted molecular mass of 17 kDa.Based on total amino acid analysis, the absorbance of a 1 mg/mL solution of the E. coli-expressed recombinant rat TNF-a at 280 nm was determined to be 1.33 A.U.SAMPLE VALUESSerum/Plasma - Forty individual rat serum samples and thirteen individual rat plasma samples were evaluated for detectable levels of rat TNF-a in this assay. All samples measured less than the lowest rat TNF-a Standard, 12.5 pg/mL.Cell Culture Supernates - Rat splenocytes (1 x 107cells/mL were cultured for 2 days in DMEM supplemented with 10% fetal calf serum and stimulated with 5m g/mL Conconavalin A.The cell culture supernate was removed, assayed for levels of rat TNF-a and measured 900 pg/mL.SPECIFICITYThis assay recognizes both recombinant and natural rat TNF-a . The factors listed below were prepared at 50 ng/mL in Calibrator Diluent RD5-17 and assayed for cross-reactivity.Preparations of the following factors prepared at 50 ng/mL in a mid-range rat TNF-a control were assayed for interference. No significant cross-reactivity or interference was observed.Some cross-reactivity was observed with the following:Recombinant rat:CINC-1GDNF IFN-g IL-1b IL-2IL-4b -NGF PDGF-BBRecombinant mouse:TNF sRI TNF sRIIRecombinant human:TNF-a TNF-b TNF sRI TNF sRIIREFERENCES1. Vilcek J. and T.H. Lee (1991 J. Biol. Chem.266:7313.2. Ware, C.F.et al.(1996 J. Cellular Biochemistry60:47.3.Tumor Necrosis Factor: Structure, Function and Mechanism of Action,Aggarwal,B.B. andJ. Vilcek eds. (1991 Marcel Dekker, Inc., New York.4. Beutler, B. and A. Cerami (1989 Annu. Rev. Immunol.7:625.5. Kwon, J.et al.(1993 Gene132:227.6. Gearing, A.J.H.et al.(1994 Nature370:555.7. Maskos, K.et al.(1998 Biochem.95:3408.8. Perez, C.et al.(1990 Cell63:251.9. Jones, E.Y.et al.(1989 Nature338:225.10. Eck, M.J. and S.R. Sprang (1989 J. Biol. Chem.264:17595.11. Tartaglia, L.A. and D.V. Goeddel (1992 Immunol. Today13:151.12. Aggarwal, B. and S. Reddy (1994 in Guidebook to Cytokines and their Receptors, N.A. Nicolaed., Oxford University Press, New York, p. 110.13. Seckinger, P.et al.(1989 J. Biol. Chem.264:11966.14. Olsson, K.et al.(1989 Eur. J. Haematol.42:270.15. Engelmann, H.et al.(1990 J. Biol. Chem.265:1531.PLATE LAYOUT Use this plate layout to record standards and samples assayed. © 2010 R&D Systems, Inc. 11.98 751180.3 15 6/10。
Elisa Kit定义及应用

Elisa Kits
1 Elisa Kit原理
Elisa(酶联免疫):是免疫酶技术的一种,是将原抗体反应的特异性与酶反应的敏感性相
结合而建立的一种新技术。
Elisa的技术原理是:将酶分子与抗体(或抗原)结合,形成稳定的酶标抗体(或抗原)结合物,当酶标抗体(或抗原)与固相载体上的抗原(或抗体)
结合时,即可在底物溶液参与下,产生肉眼可见的颜色反应,颜色的深浅与抗原或抗体的
量成比例关系,使用Elisa检测仪,即酶标仪,测定其吸收值可作出定量分析。
此技术具有特异、敏感、结果判断客观、简便和安全等优点。
用来测定生物样品中蛋白质或其他(潜在)抗原物质的浓度。
针对待测物的特异性抗体被吸附在一个不溶性的载体表明,如聚氯乙烯板。
添加已知量的物品。
这样待测物品被
抗体结合。
清洗载体,针对这一待测物质的第二个位点添加二抗,这一份子携带了一个酶,它会使下一个试剂出现颜色变化。
颜色变化的强度能被光度计测定,并同待测物质的标准
溶液进行对比。
ELSA广泛应用于临床医学和兽医学的诊断及科研当中。
2 Elisa Kit配置
(1)酶标板
(2)标准溶液
(3)底物
(4)单抗和二抗
(5)PBS-磷酸缓冲液作为稀释液
(6)终止溶液。
1、ELISA 试剂盒使用说明书(5 孔板格式)

ELISA kitInstruction Manual5-plate formatJuly, 2006For research use only.Not for use in diagnostic or therapeutic procedures.ContentsAbbreviations 2 Introduction 3 Contents of the kit 4 Hazard information 4 Materials and reagents required but not provided 4 Working solutions 4 General procedure 5 Coating antibodies 5 Blocking 5 Test samples and standards 5 Biotinylated detector antibodies 5 SPP conjugate 5 Substrate 5 Cytokine standards 6 Storage kit reagents 6 Directions for washing 7 Trouble shooting 7 References 8AbbreviationsAPC Antigen presenting cellsBSA Bovine serum albuminCD Cluster of differentiationCSB Cytokine stabilization bufferDMSO Dimethyl sulfoxideELISA Enzyme linked immunosorbent assayGM-CSF Granulocyte-Macrophage Colony Stimulating Factor IFN InterferonIL InterleukinMHC Major histocompatibility complexOD Optical densityPB Phosphate bufferPBS Phosphate buffered salinePBST PBS containing 0.05% Tween-20PBST-B PBST containing 0.5% bovine serum albuminSPP Streptavidin-HRP polymerT h T helper subsetTMB TetramethylbenzidineTNF Tumor necrosis factorIntroductionCytokines are a group of regulatory proteins critically involved in many physiological processes such as immune recognition, cell differentiation and cell proliferation. They have been identified in many vertebrate species and are produced by a variety of different cell types. Cytokines are usually produced transiently and locally, acting in a paracrine or autocrine manner. They interact with high affinity cell surface receptors specific for each cytokine or cytokine group and are active at very low concentrations mostly in the picogram range.It is well known now that the type of an antigen-specific immune response largely depends on the selection or preferential activation of defined CD4+T cell subsets (i.e. T h1 and T h2). Activation of these subsets is characterized by the secretion of distinct patterns of cytokines. T h1, but not T h2 cells, primarily secrete IL-2 and IFN-γ while T h2, but not T h1 cells, produceIL-4, IL-5, IL-6, IL-10 and IL-13. Other cytokines, such as TNF-α and GM-CSF are produced by both T h subsets. In addition, the production of IL-12 and IL-10, produced by antigen presenting cells (APC) such as macrophages and dendritic cells, critically contributes to the preferential expansion of T h1- or T h2-type of cells. For instance, early production of IL-12 is considered essential for the development of T h1 cells. On the other hand, the absence or low concentrations of IL-12 and IFN-γ in the early phase of an immune response and concomitant production of IL-4 by cells of the mastcell/basophil lineage or T cells themselves is known to favor the development of T h2 cells. In addition to their regulatory effects on T h subset differentiation, the cytokines released by the two types of T h cells also produce distinct effector functions. For instance, IL-4 and IFN-γhave differential or antagonistic activities on immunoglobulin isotype selection or MHC class II expression. Therefore, the properties of an immune response can be best studied by determining the amounts of cytokines produced by the responding T cells and APC.Contents of the kitItemsQuantity(5-plate format)StorageconditionsCoating antibodies 1 vial 4ºC (39ºF)Cytokine standard 5 vials 4ºC (39ºF)Biotinylated detector antibodies 1 vial 4ºC (39ºF)SPP conjugate (Streptavidin-HRP polymer) 1 vial ≤ -20ºC (-4°F)TMB substrate tablets 5 4ºC (39ºF)Substrate buffer capsules 5 Rt*BSA stock solution (10%) 2 vials (24 ml) 4ºC (39ºF)Cytokine stabilization buffer (CSB)** 1 vial (5 ml) 4ºC (39ºF)Tween-20 1 vial (5 ml) Rt*ELISA plates8 Rt*Adhesive cover slips 10 Rt** Room temperature** For serum and plasma samples only; see under “Test samples and standards”Materials and reagents required but not provided•PB stock: dissolve 96.0 g Na2HPO4.2H2O plus 17.5 g KH2PO4in 1.0 L distilled water and adjust pH to 7.4•Sterile distilled water•H2SO4•Dimethyl sulfoxide (DMSO)•Pipetting devices for the accurate delivery of volume required for the assay performance •Plate washer: automated or manual (squirt bottle, manifold dispenser, etc)•Reading device for microtiter-plate set to 370, 450 and/or 655 nmWorking solutions•PBS: add 10 ml PB stock and 8.8 g NaCl to 1 L distilled water. Adjust pH to 7.4.Alternatively, use commercially available liquid PBS from Invitrogen or other suppliers.Do not use commercially available PBS tablets for the preparation of the coating solution (the filler in the tablets interferes with the coating process).•PBST: 0.5 ml Tween-20 dissolved in 1 L PBS.•PBST-B: 2 ml BSA stock solution (10%) added to 38 ml PBST.•Blocking buffer: 2 ml BSA stock solution (10%) added to 18 ml PBS (for 1 ELISA plate). •Substrate buffer: the contents of one capsule is dissolved in 100 ml distilled water (takes approximately 5 minutes). For optimal performance, the buffer solution should be used within60 minutes.•Stopping solution: 2 M H2SO4TMB (tetramethylbenzidine) and sodium perborate (in substrate buffer)General procedureCoating antibodies•Reconstitute the lyophilized antibodies by injecting 250 µl of sterile distilled water into the vial. Mix the solution gently for approximately 15 seconds and allow it to stand for 2 minutes at room temperature. Avoid vigorous shaking. To coat 96 wells of an ELISA plate 50 µl is pipetted out of the vial (or use a frozen aliquot of 50 µl; see "Storage kit reagents") and added to 5 ml PBS. Mix gently.•Add 50 µl of diluted antibody solution to each well of the ELISA plate and fill up to 100 µl with PBS.•Seal the plate to prevent evaporation.Incubate overnight at 4ºC or alternatively 1 to 2 hours at 37ºC.Blocking•Remove the coating antibody solution and wash the wells at least six times with PBST. •Add 200 µl of blocking buffer.•Seal the plate and incubate at 37ºC for 1 hour.Test samples and standards•Remove the blocking buffer but do not wash.•Add 1/20 volume of CSB to serum or plasma samples but not to other samples such as cell culture supernatants; CSB inhibits the degradation of cytokines in pure serum or plasma. •Dilute standards and test samples in an appropriate diluent (see “Cytokine standards”). •Add 100 µl to each well.•Seal the plate and incubate at 37ºC for 2 hours or overnight at 4ºC.Biotinylated detector antibodies•Remove test samples/standards and wash the wells at least six times with PBST. •Reconstitute the lyophilized antibodies by injecting 0.5 ml of sterile distilled water into the vial. Mix the solution gently for approximately 15 seconds and allow it to stand for 2 minutes at room temperature. Avoid vigorous shaking. Hundred microliter is pipetted out of the vial (or use a frozen aliquot of 100 µl; see "Storage kit reagents") and added to 10 ml PBST-B.Mix gently.•Add 100 µl of diluted antibody solution to each well.•Seal the plate and incubate at 37ºC for 1 hour.SPP conjugate•Remove detector antibody solution and wash the wells at least six times with PBST. •Reconstitute the contents of the vial by injecting 0.5 ml of sterile distilled water into the vial.Mix the solution gently for approximately 15 seconds and allow it to stand for 1 minute at room temperature. Avoid vigorous shaking. Hundred microliter is pipetted out of the vial (or use a frozen aliquot of 100 µl; see "Storage kit reagents") and added to 10 ml PBST-B. Mix gently.•Add 100 µl to each well.•Seal the plate and incubate at 37ºC for 1 hour.Substrate•Remove SPP conjugate and wash the wells at least six times with PBST.•Dissolve one TMB tablet in 1.0 ml DMSO (vortex at high speed for 5 minutes for complete dissolution)and than add 10 ml substrate buffer.•Mix thoroughly and immediately dispense 100 µl into each well. Leave the plate on the laboratory bench at room temperature (color development between 10 and 30 minutes).The substrate produces a soluble end-product that is blue in color and can be read spectrophotometrically at 370 or 655 nm. The reaction can be stopped by adding 50 µl of2 M H2SO4 (resulting in a yellow solution which can be read at 450 nm).Cytokine standardsFor maximum recovery, the vial with lyophilized cytokine standard should be reconstituted in 0.5 ml distilled water and allowed to stand for 1 minute at room temperature. Thereafter, the reconstituted cytokine standard (stock solution) is placed on melting ice and is immediately diluted as indicated below (preferentially within one hour). Use vials with cytokine standards only once.Please note that temperature of buffers and standard solution(s) should now be kept at 0-4ºC until use in the ELISA.The total amount of cytokine standard is indicated on the label of the vial (ng/vial). After reconstitution in 0.5 ml water, the concentration (ng/ml) will become twice the amount on the label [e.g. amount on label is 4.8 ng/vial; after reconstitution, the concentration becomes9.6 ng/ml = 9600 pg/ml].The standard stock solution is diluted to 320 pg/ml in PBST-B (highest concentration cytokine to be used in the standard range).The linear region of the cytokine standard curve is now obtainable in a series of two-fold dilutions in PBST-B ranging from 320 to 5 pg/ml. Always include a blank control (PBST-B only) in the standard range.Before establishing the standard curve, the OD value of the blank control (OD.bl) is subtracted from the measured OD values of the different standard solutions. The standard curve is now plotted as the standard cytokine concentration versus the corresponding (measured) OD value minus OD.bl. In addition, the actual OD values of the test samples are determined by subtracting OD.bl from the measured OD values.The concentration of the cytokine in the test sample can then be interpolated from the standard curve. It is useful to prepare a series of dilutions of the unknown test sample to assure that the OD will fall in the linear portion of the standard curve.Note 1: The OD value measured for the blank control (OD.bl) must be below 0.2.Note 2: for measuring cytokines in cell culture supernatant, samples should be diluted inPBST-B. However, when measuring cytokines in pure serum or plasma, the diluent for the standard and blank control should preferentially be control serum or plasma originating from the same species.Storage kit reagentsThe vials with lyophilized coating antibodies and biotinylated detector antibodies can be safely stored in a refrigerator for a defined length of time (expiry date indicated on the vial). After reconstitution, the antibodies remain fully active for minimal 6 months at 4ºC (39ºF) when kept sterile. However, it is strongly recommended to divide the reconstituted antibody solutions into small aliquots for single use. These aliquots should be stored at ≤-20ºC. Under these conditions the antibodies are stable for at least one year.Upon arrival, the vial with lyophilized SPP conjugate should be stored at ≤ -20°C. Storage of the vial at room temperature or at 4ºC for several months may lead to lower OD readings in the ELISA. After reconstitution, the SPP solution is stable for 2 months at 4°C but rapidly looses activity when stored at room temperature. It is strongly recommended that after reconstitution, the solution is immediately divided into small aliquots for single use and stored at ≤-20°C. Under these conditions SPP is stable for minimal 12 months.Directions for washing•Incomplete washing will adversely affect the assay. All washing must be performed with wash buffer (PBST).•Washing can be performed manually as follows: completely aspirate the liquid from all wells by gently lowering an aspiration tip (aspiration device) into each well. After aspiration, fill the wells with at least 300 µl wash buffer. Let soak for 10 to 20 seconds, then aspirate the liquid. Repeat as directed under "General procedure". After washing, the plate is inverted and tapped dry on absorbent paper.•Alternatively, the wash buffer may be put into a squirt bottle. If a squirt bottle is used, flood the plate with wash buffer, completely filling all wells. After washing, the plate is inverted and tapped dry on absorbent paper.•If using an automated washing device, the operating instructions should carefully be followed.Trouble shooting•Poor consistency of replicates can be overcome by increasing the stringency of washes particularly after the incubation step with detector antibody.•High values of the blank control (optical density > 0.2) can be overcome by shortening the incubation time with the substrate solution or is caused by improper washing procedures. •Inconsistent replicates may be due to cross-contamination of wells by improper pipetting procedures.•If no signal is observed in the wells with the standards•try a new vial with cytokine standard•check the pH of the substrate solution (between 5.0 and 5.5)•verify whether the antibody, SPP conjugate and standardpreparations were properly diluted•Avoid sodium azide in wash buffers and diluents, as this is an inhibitor of peroxidase activity.•Storage of reconstituted SPP at room temperature for several days can lead to a significant loss of SPP activity and consequently low OD readings.ReferencesBooks:•Practice and theory of enzyme immunoassays 1985In: Laboratory techniques in biochemistry and molecular biology, Vol.15 (eds R.H.Burdon and P.H. van Knippenberg)Science Publishers bv, Amsterdam, The Netherlands•ELISA and other Solid Phase Immunoassays.Theoretical and Practical Aspects 1988(eds D.M.Kemeny and S.J.Challacombe)John Wiley & Sons Ltd, Chichester, UK• A practical guide to ELISA 1991(ed D.M.Kemeny) Pergamon Press, Oxford, UKReview of U-CyTech ELISA references:Human cytokines:•Arend, S.M. et al. 2000 J. Infect. Diseases 181: 1850-1854 •Demirkiran, A. et al. 2006 Liver Transpl. 12: 277-284 •Hoogendoorn, M. et al. 2005 Clin. Cancer Res. 11: 5310-5318 •Tang, Y-M. et al. 2006 World J. Gastroenterol. 11: 4575-4578•de Waal, L. et al. 2004 J. Virol. 78: 1775-1781Monkey cytokines:•Fallon, P.G. et al. 2003 J. Infect. Dis. 187: 939-945•Hartman, G. et al. 2005 Vaccine 23: 3310-3317•Kornfeld, C. et al. 2005 J. Clin. Invest. 115: 1082-1091 •Mascarell, L. et al. 2006 Vaccine 24: 3490-3499•Polakos, N.K. et al. 2001 J. Immunol. 166: 3589-3598•de Swart, R.L. et al. 2002 J. Virol. 76: 11561-11569Mouse cytokines:•Eijkelkamp, N. et al. 2004 J. Neuroimmun. 150: 3-9•Kavelaars, A. et al. 2005 J. Neuroimmun. 161: 162-168•Vroon, A. et al. 2005 J. Immunol. 174: 4400-4406Rat cytokines:•Dieleman, J.M. et al. 2006 Life Sci. 79: 551-558•Pacheco-López, G. et al. 2005 J. Neurosci. 25: 2330-2337•Sajti, E. et al. 2004 Brain Behav. Immun. 18: 505-514•Teunis, M.A.T. et al. 2002 J. Neuroimmun. 13: 30-38。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
KEY WORDS: Histophilus somni, calves, adverse effect, sexABSTRACTTo analyze the factors associated with an appearance of adverse events in calves fol-lowing Histophilus somni vaccination, we investigated changes in rectal temperature, tumor necrosis factor-alpha (TNF-a) levels, and clinical signs in vaccinated Japanese Black calves (n=19) . The average rectal temperature 24 hr post-vaccination was significantly higher in male calves compared with pre-vaccination. In contrast, the rectal temperature of female calves remained constant during the observation period. Febrile male calves aged over 9 months had inappetence, while the appetite of calves aged less than 8 months was normal. These results suggest that sex and age are associ-ated with adverse effects elicited by H. somni vaccination. The amount of TNF-a in the peripheral blood derived from male calves was significantly lower than that derived from steer and female calves. SexFactors Associated with Adverse Events Following Administration of Histophilus somni Vaccine to CalvesSaiki Imamura aHirohisa Mekata bYumi Kirino bMari Nakamizo aFumiya Hirano aMichiko Kawanishi aTsukasa Yamamoto aHidetaka Nagai aMayumi Kijima aIkuo Kobayashi ca National Veterinary Assay Laboratory, Ministry of Agriculture,Forestry and Fisheries, 1-15-1 Tokura, Kokubunji, Tokyo 185-8511, Japanb Project for Zoonoses Education and Research, Faculty of Agriculture,University of Miyazaki, 1-1 Gakuen-Kibanadai-Nishi, Miyazaki 889-2192, Japanc Sumiyoshi Livestock Science Station, Field Science Center,University of Miyazaki, Shimanouchi 10100-1, Miyazaki 880-0121, JapanCorresponding author: Saiki Imamura; National Veterinary Assay Laboratory,Ministry of Agriculture, Forestry and Fisheries,1-15-1 Tokura, Kokubunji, Tokyo 185-8511, JapanE-mail: saiki_imamura@nval.maff.go.jpTel: +81-42-321-1841; Fax: +81-42-321-1769and/or age-related effects were not identified in reported cases of adverse events. Acute shock was the most common presentation in reported cases, typically when the vaccine was administered with another multivalent vaccine. Therefore, H. somni vaccination is considered safe when administered accord-ing to the manufacturer’s instructions. INTRODUCTIONSevere adverse events following adminis-tration of vaccines containing inactivated Gram-negative bacteria have been reported in Japan (http://www.maff.go.jp/nval/). In particular, most cases have been relatedto cattle vaccines containing inactivated Histophilus somni. It has been speculated that this is due to endotoxins in the vaccines (Usui et al., 2013). As part of general risk management for vaccination, the manufac-turer’s directions instruct veterinarians to avoid vaccinating sick cattle. Administration of the vaccine is also prohibited in combina-tion with other vaccines, and observation of animals post-vaccine for adverse effects is required. Although there have been concerns about the safety of the H. somni vaccine, risk factors associated with adverse events have not been determined, making predic-tion of adverse events difficult.Generally, immunological responses to endotoxin, a common component of Gram-negative bacteria, activate leukocytes and produce proinflammatory cytokines (Van Amersfoort et al., 2003). Proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-a), produced by many types of cells are responsible for early immunologi-cal responses, including a thermostatic set point increase at the level of the hypothala-mus via prostaglandin E2 (Van Deventer et al., 1990; Suffredini et al., 1999; Günther et al., 2011). Proinflammatory cytokine signals are further transmitted to the brain, where complex thermoregulatory mecha-nisms are triggered to increase the body temperature (Dinarello et al., 1984; Saper and Breder, 1994; Luheshi and Rothwell, 1996; Dinarello, 1999). The inflammatory cascade triggered by proinflammatory cyto-kines can eventually cause toxic shock and death (Günther et al., 2011). Measurement of rectal temperature and quantification of proinflammatory cytokines are therefore useful to monitor progression of inflamma-tory responses in cattle.In this study, we therefore measured rectal temperatures and TNF-a levels in the peripheral blood of Japanese Black calves following vaccination, as well as hemato-logical and biochemical changes and clinical signs to investigate any adverse events. MATERIALS AND METHODSFarm and AnimalsThis study was conducted between June 3rd and 7th 2013 on the Sumiyoshi Livestock Science Station of Miyazaki University, Miyazaki, Japan. A total of 19 clinically healthy Japanese Black calves (5 male calves, 6 steers, and 8 female calves) aged 5–11 months (average: 8.34 months) were included in the study, as adverse effects had been reported in this age group. Calves were fed twice daily (9am and 4pm) with 2.0 kg of concentrate diet, with access to fresh water, orchard grass hay, and mineral salt blocks ad libitum during the observation period. For male calves, the concentrate diet contained 68–70 % total digestible nutrients (TDN) and 15–15.5 % crude protein (CP) on a dry matter (DM) basis. For female calves, the diet contained 72–73 % TDN and 12–13 % CP on a DM basis. All protocols were approved by the Institutional Review Board for Animal Experiments of the University of Miyazaki (Approval number: 2013-015). VaccinationA commercial inactivated H. somni vaccine was purchased from KyotoBiken Laborato-ries, Inc. (Kyoto, Japan). The vaccine was administered intramuscularly and needles were changed after each injection. Animal MonitoringRectal temperatures were measured using the Matsuda veterinary thermometer (Asahi Glass Co., Ltd., Chiba, Japan). Animals were examined by three veterinarians at 0, 1, 3, 24, and 72 h post-vaccination (hpv).Quantification of TNF-α, Biochemical, and Hematological AnalysesBlood from the jugular vein of each calf was collected into 4-ml Vacuette tubes without an anticoagulant (Greiner Bio-one GmbHy, Kremsmunster, Austria). Serum was harvest-ed after centrifugation at 1,400 g for 5 min. TNF-α serum levels were determined using the GSI Bovine TNF-α ELISA kit (Genorise Scientific, Inc., Paoli, USA) as per the man-ufacturer’s instructions. Total protein (TP), total cholesterol (T. chol.), creatinine (Cre), triglyceride (TG), total bilirubin (T. bil.), uric acid (UA), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lac-tate dehydrogenase (LDH), gamma-glutamyl transpeptidase (g-GTP), and cholinesterase (ChoE) in sera were measured using an auto-analyzer (Hitachi 7600-110S, Tokyo, Japan) at 0, 1, 3, 24, and 72 hpv.Blood from the jugular vein of each calf was collected into 4-ml Vacuette EDTA tubes (Greiner Bio-one GmbHy). The total white blood cells (WBCs), total red blood cells (RBCs), hemoglobin level (Hb), mean corpuscular volume (MCV), mean corpus-cular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and blood platelet count were measured by us-ing an autoanalyzer (IDEXX laboratories, Tokyo, Japan) at 0, 1, 3, 24, and 72 hpv. Previously Reported Adverse Events with H. somni VaccinationReports of previous adverse events were obtained from the Japanese online adverse effect database (http://www.nval.go.jp/asp/ se_search.asp). The name of the vaccine was used as a search key to determine the num-ber of previously reported adverse events. StatisticsStatistically significant differences were determined using the Mann-Whitney U test. RESULTSClinical Findings in Vaccinated Calves The changes in rectal temperatures in vaccinated calves are shown in Table1. The rectal temperature in vaccinated male and steer calves at 24 hpv was sig-nificantly increased compared with pre-vaccination. In contrast, the rectal tem-perature in female calves remained stableHourspost-vaccination0132472 Groups Avg(+SD)Avg(+SD)Avg(+SD)Avg(+SD)Avg(+SD) Calves (n=19)39.28+0.3339.18+0.3139.22+0.3239.86**+0.7638.94+0.23 Females (n=8)39.31+0.4339.28+0.3639.28+0.4239.27+0.4738.98+0.15 Intact males (n=5)39.38+0.1539.20+0.2439.22+0.2240.16*+0.6538.73+0.22 Steers (n=6)39.15+0.2638.98+0.2039.12+0.2340.58**+0.4038.73+0.18 Table 1. Rectal temperatures following vaccinations. aa Annotations denote p-values less than 0.01 (**) and 0.05 (*) obtained via Mann-Whitney U-testHourspost-vaccination0132472 Groups Avg(+SD)Avg(+SD)Avg(+SD)Avg(+SD)Avg(+SD) Calves (n=19)9.55+7.6311.20+9.619.41+8.7310.11+11.1310.24+8.60 Females (n=8)11.01+7.1113.44+11.3811.09+9.6013.12+14.4312.83+10.56 Intact males (n=5) 5.39+1.57 4.71+2.65 3.82+2.32 4.52+2.11 5.82+2.91 Steers (n=6)10.79+11.8213.19+8.8213.07+10.3211.07+9.5910.61+8.53 Table 2. TNF-a (ng/mL) levels following vaccinationthroughout the observation period. Febrile male and steer calves aged over 9 months had inappetence once febrile, while no ap-petite changes were detected in calves aged less than 8 months and female calves (data not shown). All calves showing clinical signs recovered within 72 hpv.TNF-a Levels in Calves Following VaccinationThe amount of TNF-a in peripheral blood derived from male, steer and female calves remained constant during the observation period (Table 2). No significant differences were observed during the pre- and post-vaccination. However the average of TNF-a in peripheral blood differed in each group (male, steer and female calves: 4.9, 11.7 and 12.3 ng/mL, on average, respectively); in addition, the average of TNF-a in peripheral blood derived from male calves were signifi-cantly lower than that derived from steer and female calves (p<0.01). Hematological and Biochemical Changes in Vaccinated CalvesTotal WBC count was significantly in-creased at 24 hpv compared with that of pre-vaccination in all calves (0 and 24 hpv: 8980 and 9908 WBC/m L, on average, respectively, p<0.05). However, there was no significant difference between male and female calves. No significant differences were detected in total RBC count, Hb level, MCV, MCH, MCHC, and platelet count (data not shown). The amount of Cre in male calves was significantly increased at 24 hpv compared with pre-vaccination (0 and 24 hpv: 0.792 and 0.87 mg/dL, in average, re-spectively, p<0.01). No significant changes were detected in the other parameters (TP, T. chol., TG, T. bil., UA, AST, ALT, LDH, g-GTP, and ChoE).Analyses of Previously Reported Adverse EventsAccording to the National Veterinary Assay Laboratory (NV AL) database, 46 adverse effects have been previously reported (Table 3). Twenty-three of these died because of shock. However, specific factors associated with appearance of adverse effects in cattle following administration of H. somni vac-cine were not determined. DISCUSSIONGender-based differences in immune sys-tem function have long been observed in humans. Several factors contribute to this immunological dimorphism, including sex hormones, genetic makeup, environmental causes, and, more recently microchimerism (Ghazeeri et al., 2011). Thus, lack of fever in female calves may be explained by hormon-al differences (Mouihate et al., 2003). While the mechanism behind sexual dimorphism is still unclear, the risk of the adverse effect associated with the vaccination seems tobe higher in male calves, particularly those aged over 9 months. However, hematologi-cal and biochemical changes in vaccinated calves reveal that the early systemic effects of the vaccination are minor.Detection of a vaccine-induced inflam-matory response using peripheral blood examination was difficult. In humans, men generally have higher blood levels of several proinflammatory cytokines (such as inter-leukin-6 and TNF-a) typically produced by monocytes and macrophages, suggesting sex-based differences in innate immunity (Asai et al., 2001; Bouman et al., 2004; Bouman et al., 2005). One manifestationof this is that women are more resistant to bacterial infections (Bouman et al., 2005). These reports also suggest that sex mustbe considered in examination of adverse effects following vaccination. In the pres-ent study, TNF-a levels in the peripheral blood from male calves were lower thanJapanese black calves39Females23Steers16Dairy calves7Females6Males1Total46 Table 3. Numbers of adverse events reportedthose from steer and female calves. On the other hand, the rectal temperature at 24 hpv was higher in male and steer calves than in female calves as compared with that at pre-vaccination period. These findings suggest that only TNF-a in the peripheral blood do not appear to directly correlate to increased rectal temperature. However, when severer adverse effects such as shock once devel-oped, TNF-a) levels may be increased in lo-cal site as well as systemic site including the peripheral blood, resulting in aggravation of inflammation.Field cases of fatal shock were re-ported in both steers and female calves. The discrepancy between these historical cases and the results of the current study is likely multifactorial. In the historical cases, 31 out of 46 calves had other vaccines administered simultaneously with the H. somni vaccine. Furthermore, these vaccinations were often administered on the farm without direct examination of cattle by veterinarians. These situations may increase the risk of severe shock following vaccination. CONCLUSIONFurther studies are necessary to determine factors that will ensure vaccines are used safely. While sex and age are associated with mild adverse effects of H. somni vac-cination, the vaccine is generally safe when administered according to the manufac-turer’s directions. ACKNOWLEDGEMENTSWe thank Dr. Shigeyuki Nakamura (NV AL) for technical advice.REFERENCES1. Asai K, Hiki N, Mimura Y, Ogawa T, Unou K,Kaminishi M. Gender differences in cytokinesecretion by human peripheral blood mononuclearcells: role of estrogen in modulating LPS-inducedcytokine secretion in an ex vivo septic model.Shock. 2001 Nov;16(5):340-343.2. Bouman A, Heineman MJ, Faas MM. Sex hormonesand the immune response in humans. Hum ReprodUpdate. 2005 Jul-Aug;11(4):411-423.3. Bouman A, Schipper M, Heineman MJ, Faas MM.Gender difference in the non-specific and specificimmune response in humans. Am J Reprod Immu-nol. 2004 Jul;52(1):19-26.4. Dinarello CA. Cytokines as endogenous pyrogens. JInfect Dis. 1999 Mar;179 Suppl 2:S294-304.5. Dinarello CA, O’Connor JV, LoPreste G, Swift RL.Human leukocytic pyrogen test for detection ofpyrogenic material in growth hormone producedby recombinant Escherichia coli. J Clin Microbiol.1984 Sep;20(3):323-329.6. Ghazeeri G, Abdullah L, Abbas O. Immunologi-cal differences in women compared with men:overview and contributing factors. Am J ReprodImmunol. 2011 Sep;66(3):163-169.7. Günther J, Esch K, Poschadel N, Petzl W, Zerbe H,Mitterhuemer S, Blum H, Seyfert parative kinetics of Escherichia coli- and Staphylococ-cus aureus-specific activation of key immunepathways in mammary epithelial cells demon-strates that S. aureus elicits a delayed responsedominated by interleukin-6 (IL-6) but not by IL-1A or tumor necrosis factor alpha. Infect Immun.2011 Feb;79(2):695-707.Luheshi G, Rothwell N.Cytokines and fever. Int Arch Allergy Immunol.1996 Apr;109(4):301-307. Mouihate A, PittmanQJ..Neuroimmune response to endogenous and ex-ogenous pyrogens is differently modulated by sexsteroids. Endocrinology. 2003 Jun;144(6):2454-2460.8. Noreen M, Shah MA, Mall SM, Choudhary S,Hussain T, Ahmed I, Jalil SF, Raza MI. TLR4polymorphisms and disease susceptibility. Inflamm Res. 2012 Mar;61(3):177-188.9. Saper CB, Breder CD. The neurologic basis of fever.N Engl J Med. 1994 Jun 30;330(26):1880-1886. 10. Suffredini AF, Hochstein HD, McMahon FG.Dose-related inflammatory effects of intravenousendotoxin in humans: evaluation of a new clinicallot of Escherichia coli O:113 endotoxin. J InfectDis. 1999 May;179(5):1278-1282.11. Usui M, Nagai H, Tamura Y. An in vitro method forevaluating endotoxic activity using prostaglandinE(2) induction in bovine peripheral blood. Biologi-cals. 2013 May;41(3):158-161.12. Van Amersfoort ES, Van Berkel TJ, Kuiper J.Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin MicrobiolRev. 2003 Jul;16(3):379-414.13. Van Deventer SJ, Büller HR, ten Cate JW, AardenLA, Hack CE, Sturk A. Experimental endotoxemia in humans: analysis of cytokine release and co-agulation, fibrinolytic, and complement pathways.Blood. 1990 Dec 15;76(12):2520-2526.。
