2002 Coating of Methyltriethoxysilane—Modified Colloidal Silica on Polymer substrates for abrasion
纳米二氧化硅的改性与应用及聚倍半硅氧烷结构和性能研究

复旦大学碾士学位论文1.4.6复合材料中纳米二氧化硅的形貌表征图1—11和1-12是纳米二氧化硅SPl和A200分散在丙烯酸树脂中的透射电镜照片。
与纳米二氧化硅在醋酸丁酯中的分散性一样,用MAPTS改性的二氧化硅相对未改性的二氧化硅来说,具有较好的分散性,这点对于SPl来说尤为明显(见图1—1la和1.1lb)。
另外,通过原位聚合制备的纳米复合材料中,二氧化硅的分散性优于通过共混法制各的(见图1-llb和】.1lc),这是由于改性的二氧化硅中含有可与丙烯酸酯单体反应的基团,在原位聚合中,与丙烯酸酯链段有较强作用,有利其分散。
然而这些对于纳米二氧化硅A200来说都不是那么明显(见图1-12),无论是否改性,无论使用原位或者共混得方法,对于A200在丙烯酸树脂中的分散性没有很大影响。
这可能是纳米二氧化硅A200相对SPl而言,本身就具有较小的比表面积以及较低的羟基含量,使其在丙烯酸树脂中具有比较好的分散性,所以通过MAPTS对其改性,欲使其更易分散并没有在A200中体现出来。
(a)复旦大学硕士学位论文(c)图1-ll含有SPl的复合涂层的TEM照片(a)含有共混的未改性的二氧化硅(b)含有共混的改性的二氧化硅(c)含有原位生成改性的二氧化硅Figure1-11TEMpicturesofcompositescontainingSPIpreparedby【a)blendingwithunmodifiednano-silica,(b)blendingwithmodifiednano·silicaand(c)in—situmethodwithmodifiednano-silica(a)(b)复旦大学硕士学位论文(c)图1-12含有A200的复合涂层的TEM照片(a)含有共混的未改性的二氧化硅(b)含有共混的改性的二氧化硅(c)古有原位生成改性的二氧化硅Figure1-12TEMpicturesofcompositescontainingA200preparedby(a)blendingwithunmodifiednano-silica,(b)blendingwithmodifiedriano-silicaand(c)in-situmethodwithmodifiednano.silica1.4.7改性对复合树脂Tg的影响图1.13至图1.15为纳米复合树脂的DMA损耗曲线。
分子量 202 二乙基二硫代氨基甲酸

分子量 202 二乙基二硫代氨基甲酸1.概述分子量202的二乙基二硫代氨基甲酸是一种有机硫化合物,化学式为C10H20N2S4,也被称为ETDTA。
它是一种白色固体,可以溶解在有机溶剂中,具有较好的化学稳定性和储存稳定性。
常用于金属离子的络合剂,有着广泛的应用领域。
2.物化性质2.1 外观二乙基二硫代氨基甲酸是白色固体,常见的形式是粉末状或结晶状。
2.2 溶解性二乙基二硫代氨基甲酸具有较好的溶解性,可以溶解在多种有机溶剂中,如乙腈、二甲基甲酰胺等。
2.3 化学稳定性ETDTA具有较好的化学稳定性,不易发生分解或氧化反应,在常规储存条件下可以长时间保存。
3.应用领域3.1 金属离子的络合剂二乙基二硫代氨基甲酸可以与金属离子形成络合物,在化学分析和处理金属离子的过程中具有重要的应用价值。
它可以作为萃取剂、浮选剂等,用于金属离子的富集和分离。
ETDTA还广泛应用于电镀、清洗剂等领域。
3.2 医药领域ETDTA具有较强的螯合作用,被广泛应用于医药领域。
如用于治疗金属中毒、放射性核素污染等。
3.3 其他领域除了以上两个主要领域,ETDTA还被用作溶剂、稳定剂等。
由于其良好的化学稳定性和溶解性,常被用于一些工业领域,如涂料、油墨、合成树脂等。
4.生产工艺ETDTA的生产工艺主要包括合成反应和纯化分离两个部分。
4.1 合成反应ETDTA的合成反应主要是通过两步反应完成的。
首先是通过硫化氢与N,N-二乙基乙二胺在适当的条件下反应得到二乙基二硫代氨基乙基硫酸盐,然后经过进一步的还原反应,得到最终的产物二乙基二硫代氨基甲酸。
4.2 纯化分离得到的产物需要经过纯化分离工艺,通常采用结晶、萃取等方法进行纯化,得到产品后进行干燥处理即可。
5.安全性与环境友好性ETDTA的安全性较好,但由于其具有螯合作用,对于人体和环境仍具有一定的毒性。
在使用和生产过程中,应当严格遵守相关的安全操作规程,做好防护措施。
在废弃物处理和排放管理方面,也应当根据相关法律法规进行规范管理,避免对环境造成污染。
二丁基二硫代氨基甲酸盐

二丁基二硫代氨基甲酸盐是一种化学物质,也被称为二乙基二硫代氨基甲酸钠或橡胶促进剂TP。
这种物质在常温下呈现出橙黄至橙红色的粘性透明液体形态。
它的分子量为227,相对密度在1.075~1.14之间。
此外,该物质能与水和醇混溶,但不溶于烃和氯代烃类溶剂。
在化学工业中,二丁基二硫代氨基甲酸盐被用作天然橡胶、丁苯橡胶、丁腈橡胶、氯丁橡胶的促进剂。
当与二乙基二硫代氨基甲酸二乙铵并用时,它能在室温下实现硫化。
此外,提纯二乙基二硫代氨基甲酸钠的一种方法是:在5000ml的玻璃烧瓶(或烧杯)中加入1500ml的分析纯无水乙醇,并开启搅拌及加热。
然后,分批次加入2kg的工业二乙基二硫代氨基甲酸钠,并控制温度在50~60℃使其溶解。
待全溶后,加入1~2%的针剂活性炭(767型针剂活性炭),保温30分钟后进行过滤。
接着,在室温下冷却12小时使其结晶,然后分离结晶,并在25~30℃的室温下避光风干,即可得到提纯的产品。
涂料用高醚化三聚氰胺甲醛树脂的合成
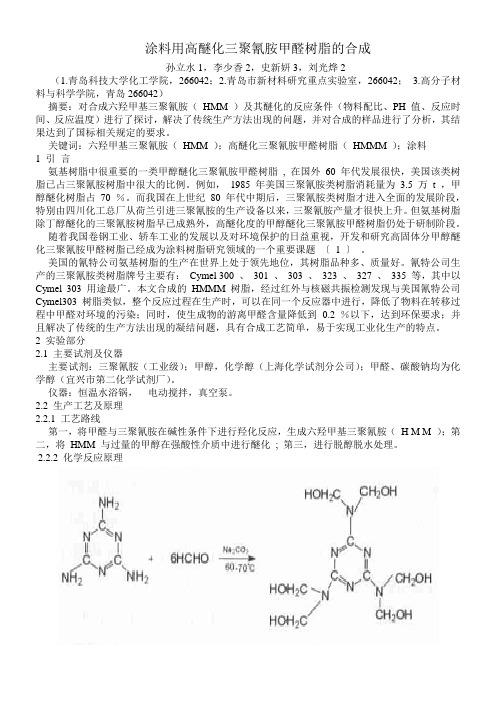
涂料用高醚化三聚氰胺甲醛树脂的合成孙立水1,李少香2,史新妍3,刘光烨2(1.青岛科技大学化工学院,266042;2.青岛市新材料研究重点实验室,266042; 3.高分子材料与科学学院,青岛266042)摘要:对合成六羟甲基三聚氰胺(HMM )及其醚化的反应条件(物料配比、PH 值、反应时间、反应温度)进行了探讨,解决了传统生产方法出现的问题,并对合成的样品进行了分析,其结果达到了国标相关规定的要求。
关键词:六羟甲基三聚氰胺(HMM );高醚化三聚氰胺甲醛树脂(HMMM );涂料1 引言氨基树脂中很重要的一类甲醇醚化三聚氰胺甲醛树脂, 在国外60 年代发展很快,美国该类树脂已占三聚氰胺树脂中很大的比例。
例如,1985 年美国三聚氰胺类树脂消耗量为3.5 万t ,甲醇醚化树脂占70 %。
而我国在上世纪80 年代中期后,三聚氰胺类树脂才进入全面的发展阶段,特别由四川化工总厂从荷兰引进三聚氰胺的生产设备以来,三聚氰胺产量才很快上升。
但氨基树脂除丁醇醚化的三聚氰胺树脂早已成熟外,高醚化度的甲醇醚化三聚氰胺甲醛树脂仍处于研制阶段。
随着我国卷钢工业、轿车工业的发展以及对环境保护的日益重视,开发和研究高固体分甲醇醚化三聚氰胺甲醛树脂已经成为涂料树脂研究领域的一个重要课题〔 1 〕。
美国的氰特公司氨基树脂的生产在世界上处于领先地位,其树脂品种多、质量好。
氰特公司生产的三聚氰胺类树脂牌号主要有:Cymel 300 、301 、303 、323 、327 、335 等,其中以Cymel 303 用途最广。
本文合成的HMMM 树脂,经过红外与核磁共振检测发现与美国氰特公司Cymel303 树脂类似,整个反应过程在生产时,可以在同一个反应器中进行,降低了物料在转移过程中甲醛对环境的污染;同时,使生成物的游离甲醛含量降低到0.2 %以下,达到环保要求;并且解决了传统的生产方法出现的凝结问题,具有合成工艺简单,易于实现工业化生产的特点。
甲醚化三聚氰胺甲醛树脂的研究进展

甲醚化三聚氰胺甲醛树脂的研究进展摘要:氨基化合物与醛类、醇类经羟化和醚化反应制得的氨基树脂作为交联剂多用于涂料行业,其性能受甲醛品质、甲醇量、PH值、反应时间、温度及则自身酸碱性等因素的影响,甲醚化三聚氰胺甲醛树脂因其高固含、低粘度、低游离醛及水溶性等特性而应用广泛。
关键词:三聚氰胺甲醛树脂,甲醚化一、前言三聚氰胺甲醛树脂作为涂料交联剂,用于涂料行业已有60多年历史,其发展与应用同工业涂料的发展休戚相关。
其因含有大量强极性的羟甲基而不溶于有机溶剂,需用醇类改性后方可用于涂料,此用醇类改性的过程即醚化。
30年代发明的丁醇改性的三聚氰胺甲醛树脂是最早使用的氨基树脂,因其漆膜具有优良的耐久性和耐磨性而被广泛应用于家电、厨具和金属家具。
60年代发明的六甲氧基甲基三聚氰胺是首次发明的单体型甲醚化三聚氰胺甲醛树脂,其因高固体份、低挥发性和水溶性的特性而开启并确立了在氨基树脂交联剂领域的发展及地位。
二、甲醚化三聚氰胺甲醛树脂特性含有氨基官能团的化合物(尿素、三聚氰胺等),与醛类(甲醛)和醇类(甲醇、丁醇等)经缩聚反应制得的具有热固性树脂即氨基树脂。
三聚氰胺甲醛树脂是氨基树脂的一种,因其具有较好的反应性和交联性而应用广泛。
经醇改性后的三聚氰胺甲醛树脂含有一定的活性基团(主要是亚氨基、羟甲基和甲氧基),相溶于有机溶剂,可作为环氧树脂、醇酸树脂及丙烯酸树脂等基体树脂的交联剂,其活性基团和基体树脂中的羟基、羧基和酰胺基等具有良好的反应性,可提高基体树脂的光泽、硬度、耐化性以及烘干速度,同时改善附着力,克服其自身的脆性。
依据所使用的醚化剂(甲醇、丁醇等)不同,三聚氰胺甲醛树脂可分为丁醚化、甲醚化及混醚化三聚氰胺甲醛树脂。
丁醚化三聚氰胺甲醛树脂与溶剂型基体树脂混溶性好,大多不具有水溶性。
随着人们环保意识的增强,甲醚化三聚氰胺甲醛树脂因其优异的高固含、低游离醛及良好的水溶性而受到人们的青睐,同时其反应活性大、高硬度、快干等特性均助力其发展快于并优于丁醚化三聚氰胺甲醛树脂。
甲基三甲氧基硅烷MSDS

2 合 成/ 成 分 方 面 的 数 据
化 学 特 性: CAS 号 码 描 述 Methyltrimethoxysilane (CAS# 1185-55-3): 100% 鉴别编号︰ EINECS 编 号: 214-685-0
3 危 险 识 别 号:
GHS卷标元素
危险
2.6/1 - 极度易燃液体及蒸汽。
页 4/6 在 2009.06.20 审 核
(在 3 页 继 续)
(在 5 页 继 续)
RC
DR
打 印 日 期 2009.06.20
材料安全数据单张
根 据 EEC91/ 155
商 品 名 称: Methyltrimethoxysilane
页 5/6 在 2009.06.20 审 核
在 眼 睛 上 面: 刺 激 的 影 响. 致 敏 作 用: 没 有 已 知 的 敏 化 影 响.
11 毒 物 资 料:
急 性 毒 性:
与 分 类 相 关 的 LD/ LC50 值:
口腔
LD50 12300 µL/kg (rat)
皮肤
LD >10 mL/kg (rbt)
刺 激 皮 肤 mild 500 mg (rbt)
刺 激 眼 睛 mild 100 µL/24H (rbt)
主 要 的 刺 激 性 影 响: 在 皮 肤 上 面: 刺 激 皮 肤 和 粘 膜.
13 丢 弃 考 虑
产 品: 建 议: 请 参 考 州、 地 方 和 国 家 有 关 法 规 正 确 进 行 处 理。 将 该 产 品 交 给 危 险 废 物 处 置 者. 必 须 遵 照 政 府 的 规 例 来 特 别 处 理.
未 清 理 的 包 装: 建 议: 必 须 根 据 官 方 的 规 章 来 丢 弃.
离子色谱法测定甲磺酸伊马替尼中残留的二甲胺

离子色谱法测定甲磺酸伊马替尼中残留的二甲胺嵇海澄;李孝壁;李琴;姜涛;胡春勇【摘要】Objective To establish a method the determination of dimethylamine in imatinib mesylate by ion chromatography. Methods The determination was carried out on a Dionex IonPacCS12A(250mm×4.6mm)column with 20mmol/L methanesulfonic acid solution as eluent by electrical conductivity detector.The flow rate was1.0mL/min,The column temperature was 30℃,and temperature of conductivity detection pool was 35℃. Results There was a good linearity over the range of 0.10-9.84μg/mL with a the correlation coefficient r higher than 1.0000(n=6).The RSD for repeatition was 0.11%(n=6),The average recovery was 97.5%with RSD of accuracy at 1.8%. Conclusion The Methods was simple,accurate and good reproducibility,which can be used for the determination of dimethylamine for imatinib mesylate.%目的:建立了一种离子色谱法测定伊马替尼中二甲胺的方法。
六羟甲基三聚氰胺树脂

潍坊金水源化工有限公司资料信息:六羟甲基三聚氰胺树脂汽车工业三滤纸--改性六羟树脂-乙醚级,JSY(潍坊)环保型、水溶性、热固型滤纸浸渍树脂胶作为:汽车三滤纸----滤清器纸、燃油滤纸、空气滤纸、机油滤纸、的浸渍树脂胶料.干燥-固化后的成品滤纸可获得优良的物理性能指标,及下游加工的良好成型性能:不影响透气性能、固化后挺度:>12mN.m、耐破度:>700Kpa、抗水性:>160min 适用浸渍机型:浸渍涂布机、浸渍定型一体机、浸渍烘干一体机、醇溶性-热固型-酚醛树脂浸渍涂布机,醇溶固化纸.性能:水溶性树脂依靠原纸微孔吸附和树脂扩散作用均匀地渗透和分散到原纸中,然后加热干燥使树脂交联固化在原纸中纤维表面和交叉点,但不影响透气度,形成稳定耐水、耐溶剂、具有高挺度和综合强度的高分子树脂膜,达到树脂涂布目的;树脂干燥固化之后,树脂变硬并在滤纸中形成一种三维结构,提高了滤纸的物理强度、结构强度,从而使制成的滤芯的刚度大大提高,坚挺结实;同时赋予滤纸具有高度的抗高低温、耐老化、抗化学的性能,从而使滤纸具有良好的加工性能和使用性能。
指标:1、外观:无色透明粘稠液体2、固含量:75±2%3、游离醛:<0.3%4、PH值:7.8至8.25、粘度:600±200cps6、理化特性:无毒、不燃、不爆、不挥发、不腐蚀7、水溶分散性:可溶于冷清水,分散均匀,不分层无沉淀(1:7水溶液)8、保质期:30度6个月(25度以下可储存8个月)包装、运输、储存:1.250公斤或1.2吨包装2.按普通商品运输即可;皮肤接触-包装破裂时,用清水冲洗15分钟并马上就医。
3.储存于阴凉、通风仓库。
汽车工业三滤纸浸渍树脂胶料---应用:适用于:不同品种、档次、质量要求的空气滤纸、燃油滤纸、机油滤纸、醇溶固化滤纸.浸渍胶料成份组成/吨纸用法:使用时,先将六羟树脂兑冷水1倍稀释搅拌、均匀后再加入胶料中—搅拌均匀即可.高档、中档、低档滤纸浸渍胶料配比:1、(纯淀粉胶)=175至200kg淀粉+25kg六羟树脂+10kgPVA.可兼顾滤纸挺度及抗水性2、(半胶)淀粉+苯丙乳液=100kg淀粉+200kg苯丙乳液(含量40%)+15kg 六羟树脂+10kgPVA3、(全胶)苯丙乳液=700至800苯丙乳液(含量40%)+10%六羟树脂(相对苯丙的折干量)4、(全胶)丙烯酸树脂=500至600kg水性丙烯酸树脂(含量45%)+7至8%六羟树脂(相对丙烯酸的折干量)5、(醇溶固化滤纸)=(2:7:1)醇溶性酚醛树脂2+苯丙乳液7+乙醚级六羟树脂1、上胶量20%6、以六羟树脂为主胶的浸渍胶料应用:六羟树脂1+冷水3至6倍+催化剂+其他树脂.。
氟伐他汀钠

主要用途
医药原料药
储存条件
干燥时-20°C
谢谢观看
基本信息
中文名称:氟伐他汀钠 中文别名:3R﹡,5S﹡-(E)]-(±)-7-[3-(4-氟苯基)-1-异丙基-1H-吲哚 -2-基]-3,5-二羟基-6-庚烯酸钠 英文名称:FLUVASTATIN SODIUM 英文别名:Sodium(+-)(E)-3,5-dihydroxy-7-[3'-(4”-fluorophenyl)-1'-methylethyl-indol-2'yl]hept-6-enoate CAS NO.:93957-55-2 分子式:C24H25FNNaO4 分子量:433.4478
方法2:间苯三酚经催化氢化得化合物(Ⅳ)。与叔丁基二苯基氯硅烷反应得化合物(V),再氧化为(Ⅵ)。经间 氯过苯甲酸氧化为(Ⅶ),开环得(Ⅷ)。再氧化为(Ⅸ)。(Ⅸ)和中间体x缩合得(Ⅺ),脱保护基再水解,即得氟伐 他汀钠。
中间体(X)可从化合物(I)出发,经甲酰化、还原、氯化,再和三苯膦作用而得 。
物化信息
沸点:681.8°C at 760 mmHg 闪点:366.1°C 熔点 :194-197°C 蒸汽压:1.6E-19mmHg at 25°C 性状:本品为类白色结晶粉末 主要用途:医药原料药
适应症
高血脂症
用途
氯乙酰基)氟苯和N-异丙基苯胺缩合,得化合物(I)。然后在乙腈中,三氯氧磷存在下,和N,N二甲基氨基丙烯醛反应,得化合物(Ⅱ)。化合物(Ⅱ)在强碱作用下,和乙酰乙酸甲酯缩合,再经拆分,得化合物 (Ⅲ)。在-77~-74℃,将化合物(Ⅲ)滴加到硼氢化钠、甲氧基二乙基硼、四氢呋喃和甲醇的混合液中,搅拌 30min;得到的环状硼酸酯在乙酸乙酯中,用30%双氧水处理;再水解得氟伐他汀钠。
硅酸三甲基甲硅基酯

硅酸三甲基甲硅基酯
硅酸三甲基甲硅基酯,也称为Methyl Trimethoxysilane,是一种有机硅化合物。
它的分子式为C4H12O3Si,分子量为136.22。
硅酸三甲基甲硅基酯具有优异的水解性,能够在潮湿的环境中迅速水解成为甲醇和硅酸三甲基。
它还具有较低的粘度、低表面张力、良好的渗透性和耐高温性,被广泛应用于硅橡胶、涂料、密封材料、树脂、配合剂等行业。
此外,硅酸三甲基甲硅基酯还可以用于制备特种硅酸酯材料,如硅酸三乙酯基甲基硅基酯、硅酸三甲基苯基硅基酯等。
它的广泛应用使得它成为有机硅化学中的重要一员。
- 1 -。
二甲基二硫代氨基甲酸钠

编号系统
CAS号:128-04-1 MDL号:MFCD RTECS号:FD BRN号: PubChem号:
物性数据
性状:白色或近似白色结晶。对湿敏感。商品还有40%溶液,呈琥珀色至亮绿色。相对密度:1.17~1.20溶解 性:P):无 2.氢键供体数量:0 3.氢键受体数量:2 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积36.3 7.重原子数量:7 8.表面电荷:0 9.复杂度:64 10.同位素原子数量:0 11.确定原子立构中心数量:0
贮存方法
密封干燥保存。
用途
有机合成中间体。聚合反应速止剂。橡胶促进剂。杀菌剂。腐蚀抑制剂。
安全信息
危险品标志:有害 安全标识:S26S36 危险标识:R22R36
急救措施
急救: 吸入:如果吸入,请将患者移到新鲜空气处。 皮肤接触:脱去污染的衣着,用肥皂水和清水彻底冲洗皮肤。如有不适感,就医。 眼晴接触:分开眼睑,用流动清水或生理盐水冲洗。立即就医。 食入:漱口,禁止催吐。立即就医。 对保护施救者的忠告: 将患者转移到安全的场所。咨询医生。出示此化学品安全技术说明书给到现场的医生看。
操作处置与储存
操作注意事项: 操作人员应经过专门培训,严格遵守操作规程。 操作处置应在具备局部通风或全面通风换气设施的场所进行。 避免眼和皮肤的接触,避免吸入蒸汽。 个体防护措施参见第8部分。 远离火种、热源,工作场所严禁吸烟。 使用防爆型的通风系统和设备。 如需罐装,应控制流速,且有接地装置,防止静电积聚。 避免与氧化剂等禁配物接触。 搬运时要轻装轻卸,防止包装及容器损坏。 倒空的容器可能残留有害物。
二甲基二硫代氨基甲酸钠
化学物质
01 编号系统
目录
02 物性数据
第八章氨基树脂

7
聚合型部分甲醚化三聚氰胺树脂可溶于醇类,也具有水溶性,可用于水性 涂料。树脂中的反应基团主要是甲氧基甲基和羟甲基。它与醇酸树脂、环氧树 脂、聚酯树脂、热固性丙烯酸树脂配合作交联剂时,易于基体树脂的羟基进行 缩聚反应,同时也进行自缩聚反应,产生性能优良的涂膜。基体树脂的酸值可 有效地催化固化反应,增加配方中的氨基树脂的用量,涂膜的硬度增加,但柔 韧性下降。与丁醚化三聚氰胺树脂相比,它具有快固性,有较好的耐化学性, 可代替丁醚化三聚氰胺树脂应用于通用型磁漆及卷材涂料中。 聚合型高亚氨基高甲醚化三聚氰胺树脂的相对分子质量比部分甲醚化的三 聚氰胺树脂低,易溶于芳烃溶剂、醇和水,适于作高固体涂料,以及需要高温 快固的卷材涂料交联剂。与聚合型部分甲醚化三聚氰胺树脂不同之处在于树脂 中保留了一定量的未反应的活性氢原子。由于醚化反应较完全,经缩聚反应后 树脂中残余的羟甲基较少,但它能像部分烷基化的氨基树脂一样在固化时能进 行交联反应,也能进行自缩聚反应。增加涂料配方中氨基树脂的用量可得到较 硬的涂膜。这类树脂与含羟基、羧基、酰胺基的基体树脂反应时,基体树脂的 酸值可有效地催化交联反应,外加弱酸催化剂如苯酐、烷基磷酸脂等可加速固 化反应。由于树脂中亚氨基含量较高,使它有较快的固化性。在低温(120℃ 以下)固化时,其自缩聚反应速率快于交联反应而使涂膜过分硬脆,性能下降。 在较高温度(150℃以上)固化时,由于进行自缩聚的同时进行了有效的交联 反应,故能得到有优良性能的涂膜。以它交联的涂料固化时释放甲醛较少,厚 涂层施工时不易产生缩孔,并且在烘烤后涂料的保重性也较好。
返回
10
第三节 氨基树脂的合成原料
用于生产氨基树脂的原料主要有氨基化合物、醛类、醇类。 一、氨基化合物 氨基化合物主要有尿素、三聚氰胺和苯代三聚氰胺。 (1) 尿素 尿素(urea)又称碳酰二胺,其分子式为CO(NH2)2,相对分子质量为60.06, 结构式为
中国药典2000版二部高三尖杉酯碱

中国药典2000版二部:高三尖杉酯碱PDF转换可能丢失图片或格式,建议阅读原文/html/493/s_493442_c67.htm:.:%药品名称%高三尖杉酯碱%拼音名%%英文名%%来源(分子式)与标准%本品为从粗榧科植物三尖杉..或其同属植物提取得到的一种生物碱。
按干燥品计算,含应为.~.%。
%性状% 本品为类白色或微黄色结晶性粉末或无定形疏松固体;有引湿性;遇光色变深。
本品在甲醇、乙醇或氯仿中易溶,在水或乙醚中微溶。
熔点 本品的熔点(附录Ⅵ)为~℃。
%检查% 溶液的澄清度 取本品,加.%酒石酸溶液溶解后,溶液应澄清。
有关物质 取本品,加甲醇制成每中含的溶液,作为供试品溶液;精密称取适量,加甲醇稀释成每中含.的溶液作为对照溶液。
照薄层色谱法(附录Ⅴ)试验,吸取上述两种溶液各μ,分别点于经水蒸汽饱和小时以上的同一硅胶G薄层板上,点样后再置水蒸汽饱和槽中小时以上,取出,以氯仿-丙酮-甲醇(.:.:.)为展开剂,展开后,晾干,以碘蒸气显色。
供试品溶液如显杂质斑点,不得多于个,其颜色与对照溶液的主斑点比较,均不得更深。
干燥失重 取本品,以五氧化二磷为干燥剂,减压干燥至恒重,减失重量不得过.%(附录Ⅷ)。
%鉴别% ()取本品约.,加水溶解后,加碘化铋钾试液滴,即生成桔红色沉淀。
()取本品约,加变色酸约,加硫酸~滴,在~℃的水浴中加热数分钟,即显紫红色。
()取本品约,加无水乙醇溶解后,加盐酸羟胺的饱和乙醇溶液数滴与氢氧化钾的饱和乙醇溶液数滴,微热至有气泡发生,放冷,加稀盐酸使成酸性,加三氯化铁试液滴,即显棕红色。
()取含量测定项下的溶液,照分光光度法(附录Ⅳ)测定,在与的波长处有最大吸收。
()本品的红外光吸收图谱应与对照的图谱(光谱集图)一致。
%含量测定% 取干燥失重项下的干燥品适量,精密称定,加无水乙醇溶解并定量稀释制成每中约含μ的溶液,照分光光度法(附录Ⅳ),在的波长处测定吸收度,按的吸收系数(E%)为计算,即得。
二甲基甲硅烷基化硅石相关文献

二甲基甲硅烷基化硅石相关文献
【实用版】
目录
1.二甲基甲硅烷基化硅石的概述
2.二甲基甲硅烷基化硅石的用途
3.二甲基甲硅烷基化硅石的安全性
4.二甲基甲硅烷基化硅石在化妆品中的应用
5.二甲基甲硅烷基化硅石与硅油的区别
正文
二甲基甲硅烷基化硅石是一种有机硅化合物,具有优良的柔软性和滑爽感,常用于化妆品、护肤品中作为吸附剂和控油抗脂溢剂。
其英文名称为 silica, dimethyl silylate,分子量为 154.29。
二甲基甲硅烷基化硅石的主要用途有:
1.作为化妆品和护肤品中的吸附剂,可以有效吸附皮肤表面的油脂和污垢,使肌肤保持清爽不油腻。
2.作为控油抗脂溢剂,可以控制皮肤油脂分泌,减少毛孔堵塞和痘痘的产生。
3.在洗发水中作为硅油替代品,可以提供头发光滑度和柔软度,同时减少对头皮的刺激。
二甲基甲硅烷基化硅石的安全性较高,风险系数为 1,对皮肤和眼睛基本无刺激,且对孕妇一般没有影响。
然而,它不可用于临床用途。
二甲基甲硅烷基化硅石与硅油的区别在于,硅油是一种商品名,化学名为聚二甲基硅氧烷,也可以是硅橡胶及其它有机硅材料的化学名。
而二甲基甲硅烷基化硅石是用硅油处理过的二氧化硅,具有更优良的柔软性和
滑爽感。
总之,二甲基甲硅烷基化硅石是一种安全、有效的化妆品和护肤品成分,可以提供良好的护肤效果,同时与硅油有明显的区别。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Journal of Sol-Gel Science and Technology24,175–180,2002c 2002Kluwer Academic Publishers.Manufactured in The Netherlands. Coating of Methyltriethoxysilane—Modified Colloidal Silica on PolymerSubstrates for Abrasion ResistanceMAN SUNG LEEDepartment of Chemical Industry,Dong-eui Institute of Technology,Pusan614-715,KoreaNAM JU JODepartment of Polymer Science and Engineering,Pusan National University,Pusan609-735,KoreaReceived January29,2001;Accepted November27,2001anic-inorganic hybrid composites were prepared by the sol-gel method for the hard coating agent of transparent plastic,and their abrasion resistance,optical and surface characteristics were evaluated.Methyltriethoxy-silane(MTES)and colloidal silica were used as starting materials.The addition of MTES to colloidal silica enabled the formation of dense thinfilms with very smooth surface on the substrates.The thinfilms were strongly adhered to the substrates without primer treatment.The abrasion resistance increased with the increase in the ratio of MTES to the colloidal silica.Optimal amount of MTES for the hard coating agent was100wt%to the colloidal silica.The addition of curing catalyst,tetramethylammonium formate was found to be very effective to enhance the adhesion strength of coating agent to the substrates and reduced curing time.Keywords:abrasion resistance,sol-gel method,hard coating agent,methyltriethoxysilane,colloidal silica1.IntroductionRecently transparent polymer materials have been utilized as windows in aircraft,buildings,and opti-cal lens.However,while transparent polymer materials have excellent optical transparency and many benefi-cial mechanical properties,they show poor abrasion re-sistance and weatherability.To solve these problems, many kinds of hard coating agents such as melamin, acrylic and urethane resin based hard coating agents were developed[1–4].However they were not satisfied with abrasion-resistance and weatherability. Organic-inorganic hybrid composites have created very effective hard coating agents possessing advan-tages of both organic and inorganic materials.These are based on the use of metal alkoxide and organosiloxane by the sol-gel method.The sol-gel method is a novel procedure among solution reactions,which is based on the preparation of macromolecular network through the typical hydrolysis of alkoxide groups and the conden-sation reaction in following Eq.(1):R -Si-(OR)3+3H2O→R -Si-(OH)3+3ROH(hydrolysis)R -Si-(OH)3+R -Si(OH)3→R (OH)2-Si-O-Si-(OH)2R +H2O(condensation)(1) where R and R are the alkyl group and short alkyl hydrocarbon,respectively.Hydrolysis of the metal-lic alkoxide groups provides unstable and highly ac-tive metallic hydroxides under acid or base condition in alcohol solution.And metallic hydroxides become macromolecules subsequently due to the condensation reaction of themselves.Moreover,the degree of poly-merization can be controlled according to the reac-tion condition such as concentration,temperature,and176Lee and Jocatalyst [5].Then,sol-gel method is one of the effective methods for the coating of colloidal silica at relatively low temperature on various organic polymer substrates such as polymethylmetacrylate (PMMA),polycarbon-ate (PC),polyethyleneterephthalate (PET),Nylon,and so on [6–8].With the development of synthesis technology for silicone compounds,E.I.Dupont made silicone based hard coating agents by using hydrolysis of tetraalkoxysilane and vinyl acetate at the first time in 1943[9].Owenes-Illinosis applied condensed mate-rials of methyltrialkoxysilane and phenyltrialkoxysi-lane to coatings [9].Dow-Corning established the basic technology of hard coating according to the con-densed materials prepared by cohydrolysis of colloidal silica and methyltrialkoxysilane in 1974.However,the traditional organic-inorganic hybrid hard coat-ing agent has shrinkage problem during curing pro-cess,and the abrasion resistance was not so good.And the traditional coating agent is very poor econ-omy,because the primer must be used to enhance the interfacial strength between substrate and coating layer.In this study,nano-sized colloidal silica was mixed with MTES,which was hydrolyzed and condensed with the silanol groups on the surface of silica.Coat-ing films were made from these solutions on PMMA substrates.After curing,the abrasion resistance was measured and compared with an uncoated substrate.To improve the adhesion of the coating solutions with substrates,curing catalyst was introduced to the coating solution without the primer.In this report,the effects of MTES contents on abrasion resistance were exten-sively examined.2.Experimental2.1.MaterialsColloidal silica (LUDOX RLS,solid contents 30wt%,water dispersion solution)and MTES were purchased from Aldrich and used as received.PMMA sheets(GREENGLAS R,Bayer-Sewon Ltd.)with a thick-ness of 2mm were cleaned with isopropanol before use.Tetramethyl ammonium formate as a curing cat-alyst was purchased from Aldrich and used as re-ceived.Various compositions of the coating solutions were prepared and represented as RX,where X shows the wt%of MTES to colloidal silica in the startingsolution.Figure 1.The procedure for preparing the hybrid thin film.2.2.SynthesisThe procedure for preparing the hybrid thin films is schematically shown in Fig. 1.Colloidal silica and MTES were used as starting materials.Proper amounts of MTES and colloidal silica were poured into 4-necked flask and stirred slowly.0.2wt%of acetic acid was added to control the acidity of the solution and to increase hydrolysis reaction rate.The solution was controlled at pH 4.7and stirred for 24hours at 30◦C for enough hydrolysis reaction.The acid catalyst was added slowly into the alkoxide solution with stirring.After being stirred for about 24hours,partially condensed and hydrolyzed solution was obtained.To eliminate excess water and ethanol,additional ethanol was added for azeotropic elimination into the solution.It was stirred and heated at 40◦C under reduced pres-sure.To minimize additional condensation reaction,the coating solutions were kept at below 0◦C in the refrigerator.To make coating agents,isobuthylalcohol (IBA)and ethyleneglycolmonoethylether (EE)were added as diluent solvents.Also,0.2wt%of curing catalyst,tetramethylammonium formate,was added.Finally,the coating solution with 40wt%of solid content was obtained.The coating was carried out on the substrates in flowing or dipping-withdrawing manner at the atmo-spheric temperature.The obtained coating films were heat-treated at 85◦C for one hour.2.3.CharacterizationFT-IR spectra were obtained using a Nicolet Impact 400D FT-IR Spectrometer.Adhesion was measured byCoating of MTES —Modi fied Colloidal Silica 177tape test using ASTM D3359.A lattice pattern with eleven cuts in each direction is made in the film to the substrate,pressure-sensitive tape is applied over the lattice and then removed,and adhesion is evaluated by comparison with descriptions.Abrasion resistance was measured with a Teledyne Taber type abrader with CS-10F abrasion wheel under 500g loading.Surface roughness was measured with a Digital Instrument,NanoScope RE atomic force microscope (AFM)by tapping mode.Light transmittance was measured with a Toyoseiki Direct Reading Hazemeter.Refractive index was also measured with a spectroscopic Ellipsometer (V ASE,J.A.Woollam,USA).3.Results and Discussion 3.1.FT-IR SpectroscopyFigure 2shows the time dependence of infrared spec-tra for the coating solution,R100.The Si CH 3group in the MTES is easily recognized by a strong and sharp band at about 1260cm −1together with one or more strong bands in the range of 865–750cm −1.The Si O C 2H 5group is recognized by a small band at about 1170–1160cm −1together with small bands in the range 1100,1075(s),and 970–940cm −1.Siloxane shows one or more very strong infrared bands in the region 1130–1100cm −1.Disiloxane and small-ring siloxane show a single Si O Si band.As the silox-ane chain become longer or branched,the Si OSiFigure 2.Time dependence of FT-IR spectra of the coating solutions prepared from R100.absorption become broader and more complex,show-ing two or more overlapping band.The H-bonded Si OH group is recognized by a broad band at about 3400–3200cm −1with single broad band,in the range 950–810cm −1.Also,free Si OH group is recognized at 3690cm −1[10].Analysis of reaction medium by FT-IR indicated that firstly,hydrolysis reaction occurred and conden-sation reaction was observed at about 3hr spectrum.The peak intensities of Si O(H)stretching mode at about 900cm −1and the (Si)O H stretching mode at 3400cm −1corresponding to the hydrolysis of ethoxy group in MTES were increased with an in-crease in reaction time.Asymmetric stretching modes of Si O C 2H 5at 1160,970,1075and 1100cm −1were disappeared in 3hr spectrum.It has been known that the partial condensation was occurred by the Si O Si asymmetric stretching mode at 1130–1100cm −1,the Si O Si symmetric stretching vibration at 1120cm −1,the symmetric Si O Si stretching mode at 870cm −1.Finally,partially condensed silane sol with free silanol groups and H-bonded silanol groups was obtained dur-ing 24hr reaction time.3.2.The Formation of Coating FilmsTable 1shows the state of the coating films on PMMA sheets in the RX system.When colloidal silica alone was used,the thin film formed on PMMA sheet was easily cracked and the appearance was looks like white powder.The addition of MTES to the colloidal silica178Lee and JoTable 1.The state of the coating films on PMMA sheet in the R x system (compositions are represented as Rx,where x shows the wt%of MTES to colloidal silica in the starting solution).Colloidal MTES Formation of Composition silica (wt%)(wt%)coating film R0100–None R1010010None R2010020Haze R3010030Transparent R5010050Transparent R7010070Transparent R100100100Transparentyielded crack free and transparent films.Transparent thin films without crack and haze were obtained in the composition range from R30to R100.Measured thick-ness of coating film after curing was 8micrometer in average.It should be noted that the films with good transparency and low haze could be obtained when proper amounts of MTES were used.3.3.Adhesion and the Effects of Curing CatalystFigure 3represents the reaction of tetramethyl ammo-nium formate with silanol in the coating solution and formation of ester linkages.Formed ester groups have chemical attraction with other ester groups on the PMMA substrates.Thus curing catalyst was linked ef-fectively between PMMA substrate and coated film and reduced curing time.Thin coating films were not peeled off from the tape peel off test after cross cutting.Also,curing catalyst showed good adhesion for polycarbon-ate substrate without primer treatment.Table 2shows the results of adhesion test of coating film on PMMA sheet coated with R100.Maximum curing temperature on PMMA substrate was 90◦C.PMMA substrates were thermal-transformed at above 90◦C.When curing catalyst was not used,coating filmsFigure 3.Reaction mechanism of tetramethylammoniumformate as curing catalyst with silanol group.Table 2.The result of adhesion test of coating film on PMMA sheet coated with R100on curing temperature and time.Curing time (hr)Amount of curing catalysts Curing tempera-ture (◦C)0.51235None80–0/1000/1000/1000/10085–0/1000/1000/1000/10090–0/1000/1000/1000/1000.2wt%8080/100100/10085100/100were easily peeled off from the substrate having no concerned with curing time and temperature.There-fore,optimum curing time was 30min at 85◦C with 0.2wt%of curing catalyst to the coating solu-tions.When the amounts of curing catalyst were over 0.2wt%,curing time was reduced.But rough film sur-face was obtained.3.4.Light TransmittanceFigure 4shows total light transmittance in the range of visible light.In the case of coated PMMA sheet with R100,light transmittance (about 92%)increased over 2%in all visible light range (400–780nm)compared with uncoated PMMA sheet (about 90%).In general,the change of refractive index (RI)in fluences on the light transmittance.RI of uncoated PMMA sheet with 2mm thickness is 1.40and PMMA sheet coated with R100was 1.38.Higher light transmittance of coating film was probably due to lower RI because coating films exhibited lower RI than that of the PMMA sheet.ItisFigure 4.Light transmittance of PMMA sheets uncoated and coated with R100.Coating of MTES —Modi fied Colloidal Silica179Figure 5.AFM photographs of PMMA sheets uncoated and coated with R70or R100.also considered that the very smooth surface of coating agents composed of MTES and colloidal silica reduced light scattering on the film surface.3.5.Surface Roughness and Abrasion ResistanceIt is obvious that surface roughness affects abrasion resistance,light scattering and adhesion of coating film.Figure 5shows AFM images of the coating film prepared from R70,R100compalable with un-coated PMMA sheet.The value of surface roughness of the PMMA sheets,uncoated and coated with R70or R100was summarized in Table 3.The surface rough-ness substantially decreased with increasing MTESTable 3.Surface roughness of PMMA sheets uncoated and coated with R70or R100.Roughness Uncoated (nm)PMMA R70R100R max 40.24523.44314.773R mean6.2630.8340.421content.(Figure 5and Table 3)Smooth surface with unevenness of less than 1nm was obtained in coated PMMA sheets with R70and R100,while rough surface larger than a few nanometer was obtained in uncoated PMMA sheets.The abrasion resistances of PMMA sheets uncoated and coated with R100or AS4000that is one of the cur-rent top commercial abrasion resistant silicone resin solution was compared in Fig.6.Taber type abrader with CS-10F abrasion wheel under 500g loading was used.The haze of scratched surface in PMMA sheet coated with R100was considerably decreased in com-parison with uncoated PMMA sheet.At higher num-ber of cycles R100had more effective abrasion resis-tance compared with AS4000.The addition of MTES to the inorganic particle effectively improved the coat-ing toughness by formation of siloxane chemical bonds between MTES and inorganic particle.It is also con-sidered that the enhancement of abrasion resistance is caused by that silane treatment lowers the surface energy of the colloidal silica,which reduces capil-lary stresses during drying.On the other hands,coat-ing solution with excess MTES had worse abrasion180Lee andJoFigure 6.Haze (%)as a function of number of wear cycles for uncoated and coated PMMA sheets.CS-10F abrasion wheel was used under 500g loading.resistance than R100.Therefore,it could be concluded that the organic-inorganic hybrid coating film consist-ing of MTES and colloidal silica formed high dense siloxane network between particles,resulting in good abrasion resistance.4.ConclusionCoatings were prepared from suspensions containing colloidal silica and various amounts of MTES.Light transmittance of coated films increased over 2%com-pared with uncoated PMMA sheet.And it was origi-nated from the decrease of refractive index and surface roughness.Surface roughness substantially decreased by the increase of MTES contents.Abrasion resistance of PMMA sheet coated with silica coatings increasedwith the addition of MTES and the optimum amount of MTES was 100wt%to colloidal silica.The MTES adsorbed on the silica surface and formed a strong siloxane bonding between particles by the condensa-tion reaction.The curing catalyst,tetramethyl ammo-nium formate,was very effective material to enhance the adhesion strength of coating agent to the substrates.These hard coating agents could have ef ficient adhe-sion and durability under sunlight,which leads to good productivity and economic ef ficiency.AcknowledgmentThis work was supported by grant No.2000-2-30800-001-2from the Basic Research Program of the Korea Science &Engineering Foundation.References1.J.D.Blizzard and L.J.Cottington,U.S.Patent 5,403,535,1995.2.S.H.Schroeter and D.R.Olson,U.S.Patent 4,210,699,1980.3.M.Ogawa and Y .Hara,U.S.Patent 4,764,576,1988.4.R.E.Martison et al.,U.S.Patent 5,403,535,1995.5.C.J.Brinker and G.W.Scherer,Sol-Gel Science:The Physics and Chemistry of Sol-Gel Processing (Academic Press,New York,1990).6.R.Nass,E.Arpac,W.Glaubbit,and H.Schmit,J.Non-Cryst.Solids 121,370(1990).7.B.Wang and G.L.Wilkes,J.Macromol.Sci.Pure Appl.Chem.A 31,249(1994).8.K.Tadanaga,K.Iwashita,and T.Minami,J.Sol-Gel Sci.and Tech.6,107(1996).9.W.A.Finzel and H.L.Vincent,Silicones in Coatings ,New Federation Series on Coating Technology,(Federation of Societies for Coatings Technology,Blue Bell,PA,1996).10.B.Arkles,Silanes,Silicones,and Metal-Organics ,2nd ed.,(Gelest,PA,1998).。
