Intersil product training-2010
ISO-34-1-2010

ii
© ISO 2010 – All rights reserved
ISO 34-1:2010(E)
Contents
பைடு நூலகம்
Page
Foreword ............................................................................................................................................................iv
5.2 Nick cutter ..............................................................................................................................................3
Annex B (normative) Calibration schedule ....................................................................................................11
Bibliography ...................................................................................................................................................... 13
© ISO 2010 – All rights reserved
企业培训-sieble培训材料1 精品

11
A Unified Database
Siebel eBusiness uses a single database and integrates with the back office to ensure consistent customer interactions
Customer
Web & E-mail Call Center
Section 1: Introduction Module i: Siebel eBusiness Essentials
Training
1
Module Objectives
This module provides an introduction to the
➢ Training facilities ➢ Siebel eBusiness Essentials Training
• Siebel ePublic Sector
• Siebel eFinance
• Siebel eAutomotive • Siebel eConsumer Goods
• Siebel eInsurance
• Siebel eEnergy
• Siebel eApparel & Footwear
• Siebel eCommunications • Siebel ePharma
Contains Siebel technical publications (manuals)
➢ Name cards
3
Training Site Information
Bathrooms
Phones
Class Duration and Breaks
Incoterms 2010-presentation

Seller Seller Seller Seller
Mainly difference for common terms CIP vs CIF
• Same: both of the price included the general freight, agreed the insurance terms; both are shipment contract; • Difference:
Origin Terminal Charges Buyer Buyer Seller Seller Seller Seller Seller Seller Seller Seller Seller Loading on Carriage Carriage Charges Buyer Buyer Buyer Seller Seller Seller Seller Seller Seller Seller Seller Buyer Buyer Buyer Buyer Seller Seller Seller Seller Seller Seller Seller
Training Objective
• Provide an overview of Incoterms 2010 • Describe the more common terms referred the various categories
Changes at a glance
13 terms have been reduced to 11 • 4 terms have been deleted: 1. DAF(Delivered at Frontier ) 2. DES (Delivered Ex Ship ) 3. DDU (Delivered Duty Unpaid) 4. DEQ (Delivered Ex Quay) DAT • Replaced by: 1. DAP ( Delivered at Place) 2. DAT (Delivered at Terminal)
simetrix_simplis仿真软件入门简介

2010-08-18 22:232010-08-18 22:301旅长不过本人也是只懂皮毛,就发个贴做一些简单的介绍。
希望有高手能来补充。
2旅长对非线性器件采用分段线性建模,将一个完整的系统定义为线性电路拓扑的循环序列,以描述开关电源系统中半导体器件的开关特性。
因此可以取得很高的速度,同样硬件配置下,其仿真速度比3旅长放大字体 缩小字体 默认4旅长5旅长6旅长34连长我都不会画地的符号,每次出来都是系统默认的那种,原副边地不能一样的哈。
这个怎么办?回复35旅长在仿真软件里面,原副边的地,可以一样的。
37连长谢谢,我又看了一下原理图发现原副边是用的一个地。
7旅长8旅长然后会出现下面对话框。
9旅长12营长 13旅长 吃完中饭回来慢慢看14旅长 多谢17旅长 请版主继续讲解怎么建库,我现在想做变压器,不知如何下手回复10旅长 要是能搞到完整版的软件就好了。
11营长不错的帖啊,顶一下15旅长 顶!16旅长18旅长19工兵 学习回复20工兵 真的不错,浅显易懂,希望继续深入,我跟着老师学习了。
谢谢!21营长 不错,学习一下22旅长 23旅长24旅长25工兵 不知可否上传你上面仿真的这个例子的文件,谢谢26旅长 Intersil_iSimPE_ISL6741回复27营长 老师上传的这个是安装文件吗?还是例题呢?28旅长 是例题,安装文件看最前面几贴。
29营长 太好了,呵呵……30班长 只会用,没太搞懂。
31班长 电脑慢,回覆重了32旅长会出现如下对话33旅长36连长不知道斑竹是否有postmenow01@38旅长 这个我也没有呢39连长 非常感谢回复40工兵 我一直在为41旅长 个人感觉42排长 报到先回复43帖 旅长44工兵学习了,谢谢回复。
“1+X”背景下产品艺术设计专业数字创意建模课证融通培养模式研究

广东轻工职业技术学院 易显钦“1+X”背景下产品艺术设计专业数字创意建模课证融通培养模式研究RESEARCH ON THE TEACHING STRATEGY OF PRODUCT ART DESIGN MAJOR: BASE ON METRA ANALY OF DIGITAL MODELLING 1+X SKILLS CERTIFICATE AND COURSE引言数字创意建模(产品艺术设计方向)1+X 证书是教育部于2020年1月22日发布的第三批74个试点证书之一。
该证书旨在满足我国数字经济快速发展的需求,专注于产品艺术设计及相关专业的职业技能认证。
该证书基于产品设计建模相关岗位日益细分的职业发展需要,是职业技能水平的重要凭证。
实施1+X 证书与专业建设、课程设计、师资队伍建设等紧密融合,有效提升本专业的人才培养效果。
1+X 数字创意建模课证融通人才培养模式研究可以推进“1”和“X”的有机衔接,提升职业教育质量和学生专业技能,更契合产业经济发展要求,更贴合企业岗位需求。
一、“1+X”数字创意建模职业技能证书根据《国务院关于印发国家职业教育改革实施方案的通知》(国发〔2019〕4号)和《教育部等四部门印发〈关于在院校实施“学历证书+若干职业技能等级证书”制度试点方案〉的通知》(教职成〔2019〕6号),积极稳妥推进1+X 证书制度试点工作。
各省级教育行政部门应将该制度视为深化职业教育改革、提高人才培养质量和拓展就业本领的重要举措,落实相关文件精神。
具体而言,职业院校将实施“学历证书+若干职业技能等级证书”制度试点工作。
1+X 职业技能等级证书是职业教育改革的一项重大制度设计,是《国家职业教育改革实施方案》(职教20条)提出的职业教育与普通教育是两种不同教育类型,具有同等重要地位的职业教育顶层政策设计。
(一)面向开设专业。
数字创意建模职业技能等级考试产品艺术设计(工业设计)方向,是面向本科的产品设计、工业设计、艺术设计学、公共艺术、工艺美术等专业[1];面向高职的产品艺术设计、工业设计、家具艺术设计、陶瓷设计与工艺、首饰设计与工艺、玉器设计与工艺、公共艺术设计等专业[2];面向中等职业学校的美术设计与制作、工艺美术、珠宝首饰设计与制作、皮革制品造型设计等专业[3];面向技工院校的工业设计、工艺美术、3D 打印技术应用、计算机辅助设计与制造、玩具设计与制造、家具设计与制造等专业[4]。
2010版GMP培训课件

新版药品GMP的主要变化 ---GMP规范正文章节
2010版GMP 第一章 总则 98版GMP 第一章 总则 第十四章 附则 第十章 质量管 理 第二章 机构与 人员 第六章 卫生 对比结果 比原规范增加2条,有关附则中关 于GMP基本原则与通则的内容 调整到总则中 比原规范增加9条,明确了质量控 制和质量保证的职责。提出了 质量风险概念 将原规范有关第六章人员卫生管 理条款调整到本章节中
新版GMP质量系统建立
欧盟和新版中国GMP都建立了质量系统要求, 包括了确立相应质量目标,应用从药品注册到整 个药品生产工艺的安全,成效和质量控制。欧盟 和新版中国GMP引入了相同的质量保证概念。美 国GMP并没有建立质量系统的需求,但是通过对 21CFR Parts 210和211的解读能够体现质量系统概 念。 欧盟和中国新版GMP又再次重申了了由ICH Q9引 出的质量管理方法。相对陈旧的美国GMP 21CFR 没有涉及到这些最新概念。
厂房与设施
2606
2575
6.6
1.5
新版药品GMP的主要变化---条款内容
5 6 7 8 9 10 设备 物料 卫生 验证 文件 生产管理 493 747 575 255 803 963 设备 物料与产品 -确认与验证 文件管理 生产管理 1885 2870 -1032 3828 2352 3.8 3.8 分别归到人员、厂房 与设施、设备各章中 4.0 4.8 2.4
新版药品GMP的主要变化---条款内容
11 12 13 质量管理 -398 -质量控制与 8086 质量保证 委托生产与 1111 委托检验 产品发运与 680 召回 20.3 新增 2.5
产品销售与回 270 收
14
15 16 总 计
伟创力年度培训计划--课程
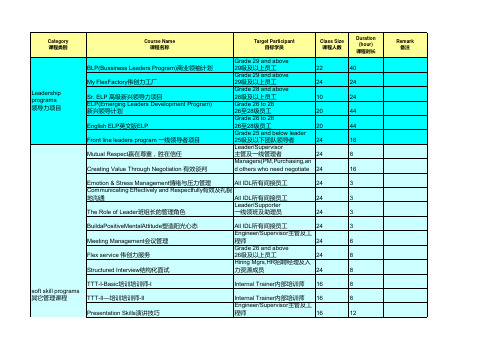
Excel Skill Training-ChartEXCEL 培训-图表 All所有员工 Excel Skill Training-Data AnalysisEXCEL 培训-数据分 析 All所有员工 Word Skill Training-AdvanceWORD高级培训 Outlook Skill TrainingOUTLOOK培训 All所有员工 All所有员工
CAP SMT Intermediate 表面贴装中级课程 CAP-Quality Fundamental品管基础课程 CAP-Quality Intermediate 品管中级课程 CAP-Quality both level TTT品管课程讲师认证课程 Technical(CAP) CAP-Plastics Fundamental 注塑基础课程 技术类课程(能力 加速计划) CAP-Plastics Intermediate 注塑中级课程 CAP-IE Fundamental 工业工程初级课程 CAP-IE Intermediate 工业工程中级课程
Engineer/Supervisor主管及工 程师 24 Clerk文员 24 Manager/Leader/HR经理/领班 /人力资源部员工 24 All IDL所有间接员工 Driver司机 Project trainer 项目培训师 All所有员工 Internal Coach 内部教练 All所有员工 IDL间接员工 IDL间接员工 IDL间接员工 Clerk文员 24 30 24 24 16 60 20 20 30 24 30 30 30 30 30 30 30
Engineer工程师 Technician技术员 Engineer工程师 Engineer & Manager 工程师及经理 Technician技术员 Engineer工程师 Technician技术员 Engineer工程师 Engineer & Manager 工程师及经理
1.LG_WAY_text-2010revised

学习目标
1. LG Way必要性理解 2. 正确理解LG Way的概念并能背诵 3.值经营的重要性 III. LG Way IV. LG Way电影研究
V. 结尾
I. 导入
我们的过去、现在、未来
LG集团70年代为止是韩国国内市场的Pioneer, 80年代以后是追赶先进企业的 Follower,目前是向至高点前进的企业。
超越利润追求…
愿景企业是超出利润为了人类社会和顾客提供真正价值为目标而努力, 以其结果来享受成功, 利润还有成长。
目的(使命) “我们是谁?”
“以应用先进科学的电子产品贡献社会服务于人类福利。” “帮助人类与疾病抗衡, 从痛苦中解脱。” “通过技术和革新使生活丰富多彩。” “如同与朋友在一起, 给出家在外人们提供方便。” “总动员我们的想象力, 使世上的人们享受幸福。” “适当的价格提供优秀和产品和服务,造福社会。”
Vision
LG追求的志向是在市场上被认可 并引领市场的企业
行动方式
伦理经营为基础, 努力培养实力,正大光明地获胜 是LG独有的行动方式 企业活动的目的 也是企业运营的原则
经营理念
LG任职员思考和行动的基础
LG Way 构成体系
为了正确的理解LG Way,把定义和构成要素书面化,提示了成为组织成员决策时的判断基准 和实践指南的行动原则。
一等LG
以为顾客创造价值、尊重人的经营、正道经营 追求世界最佳
正道经营
对顾客正直 公正的交易/对待 正大光明的竞争 正直报告 公正的评价/报偿 培养实力 顾客价值极大化 员工价值最大化
为顾客创造 价值
尊重人的 经营
构成要素及行动原则 构成要素 重视人 尊重创造/自律 成果主义 能力开发/发挥极大化 行动原则 给顾客创造价值的组织成员给与最高的重视。 尊重新的想法,创造尽心尽力工作的条件。 针对长短期成果公正的评价、差别化报偿。 通过有挑战性的业务,自主的学习和开发能力。 给与组织成员发挥无穷潜能、发挥最大能量的机会。
AMESim简介

12 copyright LMS International - 2010
Braking
Suspension
Power steering
Our Vision
As a supplier: to be able to simulate and validate components as soon as possible, to provide models for their final customer environment As a manufacturer: to be able to simulate the integration of all suppliers components and systems in order to match the product functions specifications and validate design choice
Engine
Engine Control, Hybrid Combustion, Air Path
Energy
Fuel Cell, Battery, Power Generation
Electromechanical
Electromechanical Components & Networks
16 copyright LMS International - 2010
Usual design issues :
Is the electric motor powerful enough? What is the time response of the system? What maximum pressure can be reach? Is there any risk of vibration? How to optimize the control design?
IPS2010目录-中文

40 37 27 13 34 43 45 44
工程设计(E)
e-pr-460 e-pr-551 e-pr-700 e-pr-750 e-pr-771 e-pr-810 e-pr-850
安全、防火、环境污染控制(SF) 二级目录 标准号 英文
中文
页数
施工与安装(C)
c-sf-550
safety boundary limit 土建(CE)
工程设计(E) e-pi-221 e-pi-240 材料与设备(M) m-pi-150
Piping material selection(on plot 管材选择(平面管线) piping)part one general Plant piping systems Flanges and fittings
工程设计(E)
e-pr-170 e-pr-190
工程设计(E)
e-pr-200 e-pr-230 e-pr-308 e-pr-360 e-pr-440
Basic engineering design data Piping & instrumentation diagram Numbering system Process design of liquid & gas transfer and storage Process design of piping systems(process piping and pipline size) Process design of pressure relieving systems inclusive saftey relief valves Process design of flare blowdown systems Process design of gas treating units Process design of crude oil electrostatic desalters Process design of compressors Process requirements of heat exchanging equipment Process design of furnaces Process requirements of vessels,reactors and separators
中国GMP2010中英文对照ISPE翻译版

)》(2010年修订年修订)《药品生产质量管理规范药品生产质量管理规范(《Good Manufacturing Practice (2010 revision) 》Reviewed by ISPEMa Yiling, Zhang Jianye, Yang YalanInitial Translation from NNE Pharmaplan目录Table of Contents第一章 总则 (5)第一章Chapter1 General Provisions (5)第二章质量管理 (6)Chapter 2 Quality management (6).第一节原 则 (6).Section 1 Principle (6).第二节 质量保证 (6).Section 2 Quality Assurance (6).第三节 质量控制 (8).Section 3 Quality Control (8)第三章 机构与人员 (10)Chapter 3 Organization and personnel (10).第一节 原 则 (10).Section 1 principle (10).第二节 关键人员 (10).Section 2 Key Personnel (10).第三节 培 训 (14).Section 3 training (14)第四章 厂房与设施 (16)Chapter 4 Premises and facilities (16).第一节 原 则 (16).Section 1 principle (16).第二节 生产区 (17).Section 2 Production Area (17).第三节 仓储区 (20).Section 3 Storage Areas (20).第四节 质量控制区 (21).Section 4 Quality Control Areas (21).第五节 辅助区 (21).Section 5 Ancillary Areas (21)第五章 设 备 (22)Chapter 5 Equipment (22).第一节 原 则 (22).Section 1 principle (22).第二节 设计和安装 (22).Section 2 Design and Installation (22).第三节 维护和维修 (23).Section 3 Maintenance and Repair (23).第四节 使用和清洁 (23).Section 4 Usage and Clean (23).第五节 校 准 (24).Section 5 Calibration (24).第六节 制药用水 (25).Section 6 Water for Pharmaceutical Process (25)第六章物料与产品 (26)Chapter 6 Material and products (26).第一节原 则 (26).Section 1 Principle (26).第二节 原辅料 (27).Section 2 Raw materials and Excipients (27).第三节中间产品和待包装产品 (28).Section 3 Intermediate and Bulk products (28).第四节 包装材料 (29).Section 4 Packaging material (29).第五节成 品 (30).Section 5 Finished products (30).第六节特殊管理的物料和产品 (30).Section 6 Particular management for materials and products (30).第七节其 他 (30).Section 7 others (30)第七章确认与验证 (32)Chapter 7 Qualification and validation (32)第八章文件管理 (34)Chapter 8 Documentation (34).第一节原 则 (34).Section 1 Principle (34).第二节质量标准 (36).Section 2 Specification (36).第三节工艺规程 (37).Section 3 Process procedures (37).第四节批生产记录 (39).Section 4 Batch Production Records (39).第五节批包装记录 (40).Section 5 Batch Packaging Records (40).第六节操作规程和记录 (41).Section 6 Operation Procedures and Records (41)第九章 生产管理 (42)Chapter 9 Production Section (42).第一节 原 则 (42).Section 1 Principle (42).第二节防止生产过程中的污染和交叉污染 (44).Section 2 Prevention of Contamination and cross contamination (44).第三节生产操作 (45).Section 3 Production Operations (45).第四节包装操作 (45).Section 4 Packaging Operations (45)第十章质量控制与质量保证 (47)Chapter 10Quality Control and Quality Assurance (47).第一节质量控制实验室管理 (47).Section 1 Good Quality Control Laboratory Practice (47).第二节物料和产品放行 (55).Section 2 Release for Materials and Products (55).第三节持续稳定性考察 (56).Section 3 On-going stability programme (56).第四节变更控制 (58).Section 4 Change control (58).第五节偏差处理 (59).Section 5 Deviation Treatment (59).第六节纠正措施和预防措施 (60).Section 6 Corrective action and preventive action (CAPA) (60).第七节供应商的评估和批准 (61).Section 7 Audit and approal of suppliers (61).第八节产品质量回顾分析 (63).Section 8 Product Quality Review (63).第九节 投诉与不良反应报告 (65).Section 9 Complaints and Adverse Reactions Report (65)第十一章 委托生产与委托检验 (66)Chapter 11 Contract manufacture and analysis (66).第一节原 则 (66).Section 1 Principle (66).第二节委托方 (66).Section 2 The Contract Giver (66).第三节受托方 (66).Section 3 The Contract Acceptor (66).第四节合 同 (67).Section 4 The Contract (67)第十二章 产品发运与召回 (68)Chapter 12 Product distribution and recall (68).第一节原 则 (68).Section 1 Principle (68).第二节发 运 (68).Section 2 Distribution (68).第三节召 回 (68).Section 3 Recalls (68)第十三章 自 检 (69)Chapter 13Self inspection (69).第一节 原 则 (69).Section 1Principle (69).第二节 自 检 (69).Section 2Self inspection (69)第十四章 附 则 (70)Chapter 14 Glossary (70)Note:Highlight (Yellow) is the differences between EU GMP and SFDA GMP (new version). The first difference is that Chinese GMP combines all the requirements for both API and medicinal products, while EU GMP divides them to two parts.No highlight: is the similarity between the two guidelines.第一章 总则Chapter1 General Provisions第一条为规范药品生产质量管理,根据《中华人民共和国药品管理法》、《中华人民共和国药品管理法实施条例》,制定本规范。
iso8536-42010医用输液器具-重力输液式一次性使用输液器

Reference number ISO 8536-4:2010(E)© ISO 2010INTERNATIONAL STANDARD ISO 8536-4Fifth edition 2010-10-01Infusion equipment for medical use — Part 4:Infusion sets for single use, gravity feedMatériel de perfusion à usage médical —Partie 4: Appareils de perfusion non réutilisables, à alimentation par gravitéISO 8536-4:2010(E)PDF disclaimerThis PDF file may contain embedded typefaces. In accordance with Adobe's licensing policy, this file may be printed or viewed but shall not be edited unless the typefaces which are embedded are licensed to and installed on the computer performing the editing. In downloading this file, parties accept therein the responsibility of not infringing Adobe's licensing policy. The ISO Central Secretariat accepts no liability in this area.Adobe is a trademark of Adobe Systems Incorporated.Details of the software products used to create this PDF file can be found in the General Info relative to the file; the PDF-creation parameters were optimized for printing. Every care has been taken to ensure that the file is suitable for use by ISO member bodies. In the unlikely event that a problem relating to it is found, please inform the Central Secretariat at the address given below.COPYRIGHT PROTECTED DOCUMENT© ISO 2010All rights reserved. Unless otherwise specified, no part of this publication may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying and microfilm, without permission in writing from either ISO at the address below or ISO's member body in the country of the requester. ISO copyright officeCase postale 56 • CH-1211 Geneva 20 Tel. + 41 22 749 01 11 Fax + 41 22 749 09 47 E-mail copyright@ Web Published in Switzerlandii © ISO 2010 – All rights reservedISO 8536-4:2010(E)Contents PageForeword (iv)1Scope (1)2Normative references (1)3General requirements (1)4Designation (4)4.1Infusion set (4)4.2Air-inlet device (4)5Materials (4)6Physical requirements (5)6.1Particulate contamination (5)6.2Leakage (5)6.3Tensile strength (5)6.4Closure-piercing device (5)6.5Air-inlet device (5)6.6Tubing (6)6.7Fluid filter (6)6.8Drip chamber and drip tube (6)6.9Flow regulator (6)6.10Flow rate of infusion fluid (6)6.11Injection site (6)6.12Male conical fitting (6)6.13Protective caps (6)7Chemical requirements (7)7.1Reducing (oxidizable) matter (7)7.2Metal ions (7)7.3Titration acidity or alkalinity (7)7.4Residue on evaporation (7)7.5UV absorption of extract solution (7)8Biological requirements (7)8.1General (7)8.2Sterility (7)8.3Pyrogenicity (7)8.4Haemolysis (7)8.5Toxicity (8)9Labelling (8)9.1Unit container (8)9.2Shelf or multi-unit container (8)10Packaging (9)Annex A (normative) Physical tests (10)Annex B (normative) Chemical tests (14)Annex C (normative) Biological tests (16)Bibliography (17)© ISO 2010 – All rights reserved iiiISO 8536-4:2010(E)ForewordISO (the International Organization for Standardization) is a worldwide federation of national standards bodies (ISO member bodies). The work of preparing International Standards is normally carried out through ISO technical committees. Each member body interested in a subject for which a technical committee has been established has the right to be represented on that committee. International organizations, governmental and non-governmental, in liaison with ISO, also take part in the work. ISO collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization.International Standards are drafted in accordance with the rules given in the ISO/IEC Directives, Part 2.The main task of technical committees is to prepare International Standards. Draft International Standards adopted by the technical committees are circulated to the member bodies for voting. Publication as an International Standard requires approval by at least 75 % of the member bodies casting a vote.Attention is drawn to the possibility that some of the elements of this document may be the subject of patent rights. ISO shall not be held responsible for identifying any or all such patent rights.ISO 8536-4 was prepared by Technical Committee ISO/TC 76, Transfusion, infusion and injection equipment for medical and pharmaceutical use.This fifth edition cancels and replaces the fourth edition (ISO 8536-4:2007), of which it constitutes a minor revision. In detail, 7.1 was more clarified in alignment with B.2, and A.2.2 was changed in order to go back with the leakage test pressure to 20 kPa and to restrict the leakage test for (40 ± 1) °C.ISO 8536 consists of the following parts, under the general title Infusion equipment for medical use:⎯Part 1: Infusion glass bottles⎯Part 2: Closures for infusion bottles⎯Part 3: Aluminium caps for infusion bottles⎯Part 4: Infusion sets for single use, gravity feed⎯Part 5: Burette infusion sets for single use, gravity feed⎯Part 6: Freeze drying closures for infusion bottles⎯Part 7: Caps made of aluminium-plastics combinations for infusion bottles⎯Part 8: Infusion equipment for use with pressure infusion apparatus⎯Part 9: Fluid lines for use with pressure infusion equipment⎯Part 10: Accessories for fluid lines for use with pressure infusion equipment⎯Part 11: Infusion filters for use with pressure infusion equipment⎯Part 12: Check valvesiv © ISO 2010 – All rights reservedINTERNATIONAL STANDARD ISO 8536-4:2010(E)Infusion equipment for medical use —Part 4:Infusion sets for single use, gravity feed1 ScopeThis part of ISO 8536 specifies requirements for single use, gravity feed infusion sets for medical use in order to ensure their compatibility with containers for infusion solutions and intravenous equipment.Secondary aims of this part of ISO 8536 are to provide guidance on specifications relating to the quality and performance of materials used in infusion sets and to present designations for infusion set components.In some countries, the national pharmacopoeia or other national regulations are legally binding and take precedence over this part of ISO 8536.2 Normative referencesThe following referenced documents are indispensable for the application of this document. For dated references, only the edition cited applies. For undated references, the latest edition of the referenced document (including any amendments) applies.ISO 594-1, Conical fittings with a 6 % (Luer) taper for syringes, needles and certain other medical equipment — Part 1: General requirementsISO 594-2, Conical fittings with 6 % (Luer) taper for syringes, needles and certain other medical equipment — Part 2: Lock fittingsISO 3696, Water for analytical laboratory use — Specification and test methodsISO 7864, Sterile hypodermic needles for single useISO 14644-1, Cleanrooms and associated controlled environments — Part 1: Classification of air cleanliness1) ISO 15223-1, Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements2)3 General requirements3.1 The nomenclature to be used for components of infusion sets and of a separate air-inlet device is given in Figures 1, 2 and 3. These figures illustrate examples of the configuration of infusion sets and air-inlet devices; other configurations may be used provided they lead to the same results. Infusion sets as illustrated in Figure 2 should only be used for collapsible plastic containers. Infusion sets as illustrated in Figure 2 used1) Under preparation. (Revision of ISO 14644-1:1999)2) To be published. (Revision of ISO 15223-1:2007)© ISO 2010 – All rights reserved1ISO 8536-4:2010(E)with separate air-inlet devices as illustrated in Figure 3, or infusion sets as illustrated in Figure 1, shall be used for rigid containers.3.2 The infusion set shall be provided with protective caps to maintain sterility of the internal parts of the set until the set is used. The air-inlet device shall be provided with a protective cap over the closure-piercing device or needle.Key1 protective cap of closure-piercing device 7 fluid filter2 closure-piercing device 8 tubing3 air inlet with air filter and closure 9 flow regulator4 fluid channel 10 injection site5 drip tube 11 male conical fitting6 drip chamber 12 protective cap of male conical fittinga Closure of the air inlet is optional.b The fluid filter may be positioned at other sites, preferably near the patient access. Generally, the fluid filter used has anominal pore size of 15 µm.c The injection site is optional.Figure 1 — Example of a vented infusion set2 © ISO 2010 – All rights reservedISO 8536-4:2010(E)Key1 protective cap of closure-piercing device 7 tubing2 closure-piercing device 8 flow regulator3 fluid channel 9 injection site4 drip tube 10 male conical fitting5 drip chamber 11 protective cap of the male conical fitting6 fluid filtera The fluid filter may be positioned at other sites, preferably near the patient access. Generally, the fluid filter used has anominal pore size of 15 µm.b The injection site is optional.Figure 2 — Example of a non-vented infusion set© ISO 2010 – All rights reserved3ISO 8536-4:2010(E)Key1 protective cap 4 clamp2 closure-piercing device or needle 5 air-inlet with air filter3 tubinga Other designs are acceptable if the same safety aspects are ensured.Figure 3 — Example of an air-inlet device4 Designation4.1 Infusion setInfusion sets complying with the requirements specified in this part of ISO 8536 shall be designated by the descriptor words, followed by a reference to this part of ISO 8536, followed by the letters IS, followed by the letter G:Infusion set ISO 8536-4 - IS - G4.2 Air-inlet deviceAir-inlet devices complying with the requirements specified in this part of ISO 8536 shall be designated by the descriptor words, followed by a reference to this part of ISO 8536, followed by the letters IS, followed by the letters AD:Air-inlet device ISO 8536-4 - IS - AD5 MaterialsThe materials from which the infusion set and its components are manufactured (as described in Clause 3) shall comply with the requirements specified in Clause 6. Where components of the infusion set come into contact with solutions, the materials shall also comply with the requirements specified in Clauses 7 and 8.4 © ISO 2010 – All rights reservedISO 8536-4:2010(E)6 Physical requirements6.1 Particulate contaminationThe infusion sets shall be manufactured under conditions that minimize particulate contamination. All parts shall be smooth and clean at the fluid pathway surfaces. When tested as specified in A.1, the number of particles shall not exceed the contamination index limit.6.2 LeakageThe infusion set, when tested in accordance with A.2, shall show no signs of air leakage.6.3 Tensile strengthWhen tested as specified in A.3, the infusion set, excluding protective caps, shall withstand a static tensile force of not less than 15 N for 15 s.6.4 Closure-piercing deviceThe dimensions of the closure-piercing device shall conform to the dimensions shown in Figure 4.NOTE The dimension of 15 mm in Figure 4 is a reference measurement. The cross-section of the piercing device at this site is a circle.The closure-piercing device shall be capable of piercing and penetrating the closure of a fluid container without pre-piercing. No coring should occur during this procedure.Dimensions in millimetresFigure 4 — Dimensions of the closure-piercing device6.5 Air-inlet deviceThe air-inlet device shall conform to 3.2 and 8.2.The air-inlet device shall be provided with an air filter to prevent the ingress of microorganisms into the container into which the device is to be inserted.The air-inlet device shall be separate from, or integral with, the closure-piercing device.When the air-inlet device is inserted into a rigid infusion container, the air admitted into the container shall not become entrained in the liquid outflow.The air filter shall be fitted such that all air entering the rigid container passes through it, and such that the flow of fluid is not reduced by more than 20 % of that from a freely ventilated container when tested in accordance with A.4.© ISO 2010 – All rights reserved5ISO 8536-4:2010(E)6.6 TubingThe tubing, made of flexible material, shall be transparent or sufficiently translucent that the interface of air and water during the passage of air bubbles can be observed with normal or corrected vision.The tubing from the distal end to the drip chamber shall be not less than 1 500 mm in length, including the injection site, when provided, and the male conical fitting.6.7 Fluid filterThe infusion set shall be provided with a fluid filter.When tested in accordance with A.5, the retention of latex particles on the filter shall be not less than 80 %. 6.8 Drip chamber and drip tubeThe drip chamber shall permit continuous observation of the fall of drops. The liquid shall enter the drip chamber through a tube that projects into the chamber. There shall be a distance of not less than 40 mm between the end of the drip tube and the outlet of the chamber, or a distance of not less than 20 mm between the drip tube and the fluid filter. The wall of the drip chamber shall not be closer than 5 mm to the end of the drip tube. The drip tube shall be such that 20 drops of distilled water or 60 drops of distilled water at (23 ± 2) °C at a flow rate of (50 ± 10) drops/min deliver a volume of (1 ± 0,1) ml or a mass of (1 ± 0,1) g. The drip chamber should permit and facilitate the priming procedure.6.9 Flow regulatorThe flow regulator shall adjust the flow of the infusion solution between zero and the maximum. The flow regulator should be capable of continuous use throughout an infusion without the tubing being damaged. There should be no deleterious reaction between the flow regulator and the tubing when they are stored in such a way that there is contact.6.10 Flow rate of infusion fluidThe infusion set shall deliver not less than 1 000 ml of a sodium chloride solution [mass concentration ρ(NaCl) = 9 g/l] in 10 min under a static head of 1 m.6.11 Injection siteWhen provided, the self-sealing injection site shall reseal when tested in accordance with A.6, and there shall be no leakage of more than one falling drop of water. The injection site should be located near the male conical fitting.6.12 Male conical fittingThe distal end of the tubing shall terminate in a male conical fitting in accordance with ISO 594-1 or ISO 594-2. Luer lock fittings in accordance with ISO 594-2 should preferably be used.6.13 Protective capsThe protective caps at the end of the infusion set shall maintain the sterility of the closure-piercing device, the male conical fitting and the interior of the infusion set. Protective caps should be secure but easily removable.6 © ISO 2010 – All rights reserved7 Chemical requirements7.1 Reducing (oxidizable) matterWhen tested in accordance with B.2, the difference of volume of Na2S2O3 solution [c(Na2S2O3) = 0,005 mol/l] for the extract solution S1 and of volume of Na2S2O3 solution for blank solution S0 shall not exceed 2,0 ml. 7.2 Metal ionsThe extract shall not contain in total more than 1 µg/ml of barium, chromium, copper, lead and tin, and not more than 0,1 µg/ml of cadmium, when determined by atomic absorption spectroscopy (AAS) or an equivalent method.When tested in accordance with B.3, the intensity of the colour produced in the test solution shall not exceed that of the standard matching solution with a mass concentration ρ(Pb2+) = 1 µg/ml.7.3 Titration acidity or alkalinityWhen tested in accordance with B.4, not more than 1 ml of either standard volumetric solution shall be required for the indicator to change to the colour grey.7.4 Residue on evaporationWhen tested in accordance with B.5, the total amount of dry residue shall not exceed 5 mg.7.5 UV absorption of extract solutionWhen tested in accordance with B.6, the extract solution S1 shall not show absorption greater than 0,1.8 Biological requirements8.1 GeneralThe infusion set shall be assessed for biological compatibility according to the guidelines given in C.2.8.2 SterilityThe infusion set or the air-inlet device, or both, in its unit container shall have been subjected to a validated sterilization process (see ISO 11135, ISO 11137 and ISO 17665).8.3 PyrogenicityThe infusion set and/or the air-inlet device shall be assessed for freedom from pyrogens by using a suitable test, and the results shall indicate that the infusion set is free from pyrogens. Guidance on testing for pyrogenicity is given in C.1.8.4 HaemolysisThe infusion set shall be assessed for freedom from haemolytic constituents and the result shall indicate that the infusion set is free from haemolytic reactions. Guidance on testing for haemolytic constituents is given in ISO 10993-4.© ISO 2010 – All rights reserved78.5 ToxicityMaterials shall be assessed for toxicity by carrying out suitable tests, and the results of the tests shall indicate freedom from toxicity. Guidance on testing for toxicity is given in ISO 10993-1.9 Labelling9.1 Unit containerThe unit container shall be labelled with at least the following information:a) a textual description of the contents, including the words “Gravity feed only”;b) indication that the infusion set is sterile, using the graphical symbol as given in ISO 15223-1;c) indication that the infusion set is free from pyrogens, or that the infusion set is free from bacterialendotoxins;d) indication that the infusion set is for single use only, or equivalent wording, or using the graphical symbolin accordance with ISO 15223-1;e) instructions for use, including warnings, e.g. about detached protective caps;NOTE Instructions for use can also take the form of an insert.f) the lot (batch) designation, prefixed by the word LOT, or using the graphical symbol in accordance withISO 15223-1;g) year and month of expiry, accompanied by appropriate wording or the graphical symbol in accordancewith ISO 15223-1;h) the manufacturer's or supplier's name and address, or both;i) a statement that 20 drops of distilled water or 60 drops of distilled water delivered by the drip tube areequivalent to a volume of (1 ± 0,1) ml or a mass of (1 ± 0,1) g;j) the nominal dimensions of the intravenous needle, if included.9.2 Shelf or multi-unit containerThe shelf or multi-unit container, when used, shall be labelled with at least the following information:a) a textual description of the contents, including the words “Gravity feed only”;b) the number of infusion sets;c) indication that the infusion sets are sterile, using the graphical symbol as given in ISO 15223-1;d) the lot (batch) designation, prefixed by the word LOT, or using the graphical symbol in accordance withISO 15223-1;e) year and month of expiry, accompanied by appropriate wording or the graphical symbol in accordancewith ISO 15223-1;f) the manufacturer's and/or supplier's name and address;g) the recommended storage conditions, if any.10 Packaging10.1 The infusion set and/or the air-inlet device shall be individually packed so that they remain sterile during storage. The unit container shall be sealed in a tamper-evident manner.10.2 The infusion sets and/or the air-inlet devices shall be packed and sterilized in such a way that there are no flattened portions or kinks when they are ready for use.© ISO 2010 – All rights reserved9Annex A(normative)Physical testsA.1 Test for particulate contaminationA.1.1 PrincipleThe particles are rinsed from the inner fluid pathway surfaces of the infusion set, collected on a membrane filter and microscopically counted.A.1.2 Reagents and materialsA.1.2.1 Distilled water,filtered through a membrane of pore size 0,2 µm.A.1.2.2 Non-powdered gloves.A.1.2.3 Vacuum filter, single membrane filter of pore size 0,45 µm.A.1.3 ProcedureThe filter unit, filter and all other equipment shall be thoroughly cleaned before the test using distilled water (A.1.2.1).Flush through 10 ready-to-use infusion appliances, under laminar flow conditions (clean-air work station class N5 in accordance with ISO 14644-1), with 500 ml of distilled water (A.1.2.1). The total volume is subsequently vacuum filtered (A.1.2.3). Place the particles on the membrane screen filter under a microscope at ×50 magnification using diagonally incident illumination, and measure and count in accordance with the size categories given in Table A.1.A.1.4 Determination of resultsA.1.4.1 GeneralAn appropriate number of single infusion sets (minimum of 10) are tested. The number of particles per10 infusion sets tested in each of the three size categories is the assay result.A.1.4.2 Particle countsThe values obtained from a blank control sample shall be recorded in a test report and taken into account when calculating the contamination index limit.The blank control sample is the number and size of particles obtained from 10 equivalent 500 ml water samples classified in accordance with the three size categories set out in Table A.1, using the same test equipment but not passed through the appliances under test.The number of particles in the blank, N b, shall not exceed the value of 9. Otherwise, the test apparatus shall be disassembled, re-cleaned, and the background test performed again. Values of the blank determination shall be noted in the test report.Table A.1 — Evaluation of contamination by particlesSize categoryParticle parameters1 2 3 Particle size in µm 25 to 50 51 to 100 over 100Number of particles in 10 infusion appliances n a1n a2n a3Number of particles in the blank control sample n b1n b2n b3Evaluation coefficient 0,1 0,2 5The contamination index limit is calculated as follows.For each of the three size categories, multiply the number of particles in 10 infusion appliances by the evaluation coefficients, and add the results in order to obtain the number of particles in the infusion appliances(test pieces), N a. Then, for each of the size categories, multiply the number of particles in the blank control sample by the evaluation coefficients and add the results to obtain the number of particles in the blank sample,N b.Subtract N b from N a to obtain the contamination index limit.Number of particles in the infusion appliances (test pieces):N a=n a1× 0,1 +n a2× 0,2 +n a3× 5Number of particles in the blank sample:N b=n b1× 0,1 +n b2× 0,2 +n b3× 5Contamination index limit:N=N a−N b u 90A.2 Test for leakageA.2.1 At the beginning of the test, condition the whole system at the test temperature.A.2.2 Immerse the infusion set, with one end blocked, in water at (40 ±1) °C and apply an internal air pressure of 20 kPa for 15 s. Examine the infusion set for air leakage.A.2.3 Fill the infusion set with degassed, distilled water, connect it with its openings sealed to a vacuumdevice and subject it to an internal excess pressure of −20 kPa at (40 ± 1) °C for 15 s. Atmospheric pressureshall be the reference pressure. Excess pressure, in accordance with ISO 80000-4, can assume positive or negative values. Ascertain whether air enters the infusion set.A.3 Test for tensile strengthExpose the infusion set to be tested to a static tensile force of 15 N applied along the longitudinal axis for 15 s. Inspect whether the infusion set withstands the test force applied.© ISO 2010 – All rights reserved11A.4 Determination of flow rate when using an air-inlet deviceA.4.1 Fill an infusion container with distilled water at (23 ± 2) °C and insert its closure. Insert the air-inlet device through the closure into the container and then insert the infusion set with the flow regulator set, such that no liquid flows. Arrange the container to give the equivalent of a pressure of 1 m head of water throughout the test. Open the flow regulator of the infusion set to maximum and measure the rate of flow of water from the set. Repeat the procedure with the filter removed from the air-inlet device.A.4.2 For air-inlet devices integral with the closure-piercing device of the infusion set, follow the procedure given in A.4.1 but omit the insertion of the separate air-inlet device.A.5 Test for efficiency of the fluid filterA.5.1 Preparation of the test fluidAs a test liquid, use an aqueous suspension of latex particles with a diameter of (20 ± 1) µm and a concentration of approximately 1 000 particles per 100 ml.A.5.2 ProcedureAssemble the fluid filter and position it so that it is equivalent to that of actual use in a suitable test apparatus in accordance with Figure A.1. Cut the tubing of the infusion set approximately 100 mm below the fluid filter. Flush the fluid filter with 5 ml of the test fluid from the storage bottle and discard the filtrate. Pass 100 ml of the test fluid through the fluid filter and collect the effluent under vacuum after passing it through a black gridded membrane filter with a pore size of 5 µm to 8 µm and 47 mm diameter. Mount the membrane with any retained latex particles on a suitable microscope slide or holder and count the latex particles in a minimum of 50 % of the grid squares under a magnification of ×50 to ×100. Disregard any particles which are obviously non-latex.Carry out the test in duplicate.Repeat the test if the required limit value of 80 % retention rate is not met.All procedures involved in this test should be conducted in a clean environment, if possible under laminar flow.A.5.3 Expression of results The retention rate of the filter, expressed as a percentage, is given by101100n n ⎛⎞−×⎜⎟⎝⎠ (A.1)wheren 1 is the number of particles retained on the filter;n 0 is the number of particles in the test fluid used.Dimensions in millimetresKey1 storage bottle 5 piercing device2 transfer tube 6 fluid filter3 flow regulator 7 membrane filter4 connecting pieceFigure A.1 — Apparatus for testing the efficiency of the fluid filterA.6 Test of the injection sitePlace the injection site in a horizontal, stress-free position. Fill the infusion set with water in such a manner that no air bubbles are trapped and apply a pressure of 50 kPa above the atmospheric air pressure. Perforate the injection site at the foreseen area using a hypodermic needle with an outside diameter of 0,8 mm and which conforms to ISO 7864. Keep the needle in position for 15 s. Remove the needle and immediately dry the perforated site. Over a period of 1 min, observe whether there is any leakage from the injection site. In the case of an alternative injection site design, the test should be performed by injection into the site in accordance with the instructions provided by the manufacturer.© ISO 2010 – All rights reserved13。
要求员工产品知识培训通关计划

要求员工产品知识培训通关计划I believe that providing product knowledge training to employees is essential in ensuring they have a comprehensive understanding of the products they are selling. By equipping employees with in-depth product knowledge, they are better able to answer customer inquiries, address concerns, and ultimately drive sales. 产品知识培训对员工非常重要,可以帮助员工深入了解他们销售的产品。
通过为员工提供深入的产品知识培训,他们能更好地回答客户的询问,解决顾客的顾虑,从而增加销量。
One of the key benefits of product knowledge training is that it helps to build employee confidence. When employees are well-versed in product features, benefits, and specifications, they feel more confident in their ability to effectively communicate with customers. This can lead to improved customer satisfaction and loyalty. 产品知识培训的一个主要好处是有助于建立员工的自信心。
当员工熟知产品的特点、优势和规格时,他们会更有信心与客户有效沟通。
这可以提高客户满意度和忠诚度。
离散数据测量系统分析专题培训课件

评估 者A 的第一次测量
优
劣
0.5
0.1
0.6
0.05
0.35
0.4
0.55
0.45
行和列的总数
Copyright © 2010 宝钢股份 版权所有
26
六西格玛精益运营黑带训练教材
计算评估者A的Kappa 值:
评估者A的第一次测量
优
劣
优 评估者A的第
二次测量 劣
0.5
0.1
0.6
0.05
这些评估等级也具备精确的可能性。 • 如果评估者之间存在分歧的话,那么这些评估等级的实用性是非常
有限的。
不良的离散数据测量系统通常可以被追溯到不良的 运作定义。
Copyright © 2010 宝钢股份 版权所有
12
六西格玛精益运营黑带训练教材
Kappa 是什么? “K”
K Pob1serPvcehdPacnhcaence
• 你可以把其中一些劣质等级归结为“其他”一类。
• 这种分类标准必须是互相排斥的,如果不是,那么这些分类应该 被合并。
Copyright © 2010 宝钢股份 版权所有
16
六西格玛精益运营黑带训练教材
Kappa 范例#1
• 黑带想改进一个具有极高返工率上漆流程 • 在这个项目的早期过程中,由于评估者与评估者之间以及评估者
Copyright © 2010 宝钢股份 版权所有
11
六西格玛精益运营黑带训练教材
运作定义
• 有一些质量特性很难定义或是需要花费一定的时间来定义。 • 为了保证评估等级的一致性,被评估的部件必须进行分类,同时针
对一位评估者来说将尽量多。 • 如果评估者之间的意见真正一致的话,即使没有百分之百的保证,
Chinese version CEDIA Sales Training March 25 2010

© Gassel Group 2010
奢侈品的名字
© Gassel Group 2010
品牌的名字
当你想着香奈儿或路易威登或苹果电脑名字的时候,他们会带给你什么 想法?每个品牌的符号都会给你一种特别的感觉 他们的名字都有各自很高的识别度,而且不会撞车。因为这些品牌公司 投入了大量的时间和金钱来获取这样的结果 但在知名品牌和奢侈品牌之间还是有很大的区别的。这就是为何在麦当 劳里喝一杯可乐远比比喝一杯Dom Perignon香槟要便宜的多的多?
© Gassel Group 2010
卖奢侈品
© Gassel Group 2010
奢侈品的定义
奢侈品有着特殊的地位,能给购买它的人们带来声望和独有的气质。 这个定义通常适用于流行商品、时装、珠宝和配件,但是知名的名牌并不是都 在出售昂贵的奢侈商品。
© Gassel Group 2010
1. Chanel •香奈儿的品牌表达出了永恒的信誉并使它在经济低迷期一直保持盈利 2. Louis Vuitton •LV的简单字母组合是众多知名品牌中最古老的一个 Rolex •他们的手表以自己的方式散发出自己独特的优雅,可靠性,耐用性和极度的 豪华 Hermès •时尚与历史有着完美的协调 •有所有业界最高的利润 Gucci •Gucci的 经典地位帮助它继续保持良好的利润,不会因它的创意总监的离开 而有所改变
© Gassel Group 2010
你的公司品牌
品牌整合一切,让您的企业与众不同。 这些要素结合了您的名称,标识,以及 可识别的目的为整体。 品牌是一个挂钩,让您挂上您的帽子,把你自己充分展示在人们面前 您的品牌应该是您的广告、价格、标识等,有一致性的主题。 1. 了解你擅长做什么,然后重复着做 2. 提供出众的客户服务 - 它会帮助顾客确认了您品牌的承诺 3. 可识别的品牌不同于一个可行的商业活动
office ergonomics training 办公室人机工程学培训PPT课件

support by chair.
Office Ergo Risk Factors: Posture
HandsExtension, flexion
Arms
Forward extension, elevated elbows, Elbows not at 90 degrees.
Shoulders Raising or “hunching”
Proper Posture and Workstation Setup
Proper Posture and Workstation Setup
What is our site Ergonomic Reporting Process and Procedures?
• Report to your supervisor or ergo team member (refer to the last page).
twisted • Elbows approximately 90
degrees • Forearms parallel to floor • Keyboard / input device at
elbow height • Thighs parallel to floor • Knees approximately 90
(Arm, Wrist, Hands)
• Sore/stiff arms or shoulders • Stiff wrists/hands • Hand ache • Wrist pain • Elbow discomfort • Numbness
Signs & Symptoms of MSDs
华丽君
Employee
张小敏
MFG
simetrix_simplis仿真软件入门简介

2010-08-18 22:232010-08-18 22:301旅长不过本人也是只懂皮毛,就发个贴做一些简单的介绍。
希望有高手能来补充。
2旅长对非线性器件采用分段线性建模,将一个完整的系统定义为线性电路拓扑的循环序列,以描述开关电源系统中半导体器件的开关特性。
因此可以取得很高的速度,同样硬件配置下,其仿真速度比3旅长放大字体 缩小字体 默认4旅长5旅长6旅长34连长我都不会画地的符号,每次出来都是系统默认的那种,原副边地不能一样的哈。
这个怎么办?回复35旅长在仿真软件里面,原副边的地,可以一样的。
37连长谢谢,我又看了一下原理图发现原副边是用的一个地。
7旅长8旅长然后会出现下面对话框。
9旅长12营长 13旅长 吃完中饭回来慢慢看14旅长 多谢17旅长 请版主继续讲解怎么建库,我现在想做变压器,不知如何下手回复10旅长 要是能搞到完整版的软件就好了。
11营长不错的帖啊,顶一下15旅长 顶!16旅长18旅长19工兵 学习回复20工兵 真的不错,浅显易懂,希望继续深入,我跟着老师学习了。
谢谢!21营长 不错,学习一下22旅长 23旅长24旅长25工兵 不知可否上传你上面仿真的这个例子的文件,谢谢26旅长 Intersil_iSimPE_ISL6741回复27营长 老师上传的这个是安装文件吗?还是例题呢?28旅长 是例题,安装文件看最前面几贴。
29营长 太好了,呵呵……30班长 只会用,没太搞懂。
31班长 电脑慢,回覆重了32旅长会出现如下对话33旅长36连长不知道斑竹是否有postmenow01@38旅长 这个我也没有呢39连长 非常感谢回复40工兵 我一直在为41旅长 个人感觉42排长 报到先回复43帖 旅长44工兵学习了,谢谢回复。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
4A MOSFET ISL97656
干电池升压 ISL9111
干电池升压 ISL98012
17
2014-4-27
升压电路
ISL98012
18
2014-4-27
电压基准
19
2014-4-27
20
2014-4-27
21
2014-4-27
视频(Surveillance)-矩阵
16X8 CD22M3494
2014-4-27
30
接口(Interface)
RS-485
5V/ESD
3.3V
3.3V/ESD
ISL3152E ISL81487L ISL8483E/5/8
ISL83483/5/8 ISL83490/1
31
ISL83072E/5/7 ISL83082E/3/5
2014-4-27
新一代485接口芯片ISL3152E的特点
Intersil acquires Elantec
2004
Intersil –a pure-play analog company
1956
RCA Solid State begins operations
2
2014-4-27
Intersil 重点推广产品
1. 2. 3. 4. 5. 6. 电源管理(Power Management) 视频(Surveillance) 接口(Interface) 实时时钟(RTC 运放(Op Amp) Class D 功放
16X16 ISL59530
32X16 ISL59534
32X32 ISL59532
22
2014-4-27
视频(Surveillance)-运放
单路 EL5160
双路 EL5205
三路 EL5364
四路 EL2480
23
2014-4-27
视频(Surveillance)-双绞线传输
<1200m EL5171+EL5172
4.5~24V ISL6440 MOSFET (0.8~0.93Vin)/10A (0.8~0.93Vin)/10A
MOSFET
7
2014-4-27
PWM 控制器
三路输出PWM 控制器
4.5~24V
ISL6441/3
MOSFET
MOSFET MOSFET
(0.8~0.93Vin)/10A (0.8~0.93Vin)/10A (0.8~0.93Vin)/10A (0.8~0.93Vin)/10A (0.8~0.93Vin)/10A (0.8~0.93Vin)/10A 3.3V/0.5A
o
Intersil ISL12022M
20-Ld SOIC 10.65 mm x 13.00 mm Yes Yes ±2 C ± 5 ppm o o TA = -40 C to +85 C 2.7 to 5.5V 1.8 to 5.5V 1 μA @ VBAT = 3V Yes Yes Yes 1 1 No 128 Bytes Yes Yes Yes Yes Yes
8-ld DIP, SOIC $1.50
SOIC.TSSOP Expensive than ISL1208
34
2014-4-27
ISL12022M—内置晶振和温度传感器高精度RTC
Intersil ISL12020M
Package/ Size 20-Ld DFN 4.0 x 5.5 mm Yes Yes ±2 C ± 5 ppm o o TA = -40 C to +85 C 2.7 to 5.5V 1.8 to 5.5V 1 μA @ VBAT = 3V Yes Yes Yes 1 1 No 128 Bytes Yes Yes Yes Yes Yes
32 2014-4-27
实时时钟(RTC)
Basic ISL1208
4Kb EEPROM ISL12026/7/8
EEPROM&ID ISL12024/5
内置晶振 ISL12022M
33
2014-4-27
Parameter Time Keeping Current (IBAT) Supply Voltage (VDD) Battery Voltage (VBAT) Accuracy Crystal Frequency Trimming Trickle Charger Battery Reseal Alarms Memory Selectable Frequency Output Packages Available Web Price (1 Ku)
1
2014-4-27
Intersil公司历史
1980
2006
Pure Play Analog
1950
Radiation Inc. founded GE Solid State acquires Intersil
1999
1986
GE Solid State merges with RCA Solid State Intersil becomes independent company
2003 2001
Intersil sells discrete power business to Fairchild
Intersil sells wireless network products group
2004
Intersil acquires Xicor
1967
Harris merges with Radiation Inc.
PCF8563 250 nA (VDD = 3.0V) 1.0 to 5.5V 1.8 to 5.5V NA No
No No 1 2 Bytes SRAM 15 Outputs
Yes No 2 128 Bytes SRAM 7 Outputs
No Yes 1 128 Bytes SRAM 15 Outputs
ISL8033/6
ISL65426
(0.6~Vin)/3.0A (0.6~Vin)/3.0A (1.0~4.0)/3.0A (1.0~4.0)/3.0A
6
2014-4-27
PWM 控制器
单路输出PWM 控制器
5.0~28V
ISL6420A
MOSFET
(0.6~0.93Vin)/20A
双路输出PWM 控制器
1. RX端为高电流(28mA)输出,可以直接驱动光藕,符合电表中RS485接 口隔离传输的要求 2.支持热插拔,在上下电的过程中,输入/输出端保持高阻态 3. ESD 保护支持IEC61000标准,提高到+/—16.5KV, 人体模式由一般的 2KV提高到7KV 4.支持电流限制,热保护及驱动器过负载保护,保护功能更加完善了 5.支持星型或其他非标准拓扑接线方式,宽摆幅,最小2.4V的输出摆幅,大 大降低了工程安装的要求,提高了通讯的可靠性 综上所述,ISL3152E已经是全新一代485接口芯片,比目前市面上一般 MAX485E,SP485E的综合性能都要高,非常适合用在电表行业,以及其 他工业应用环境 高端性能 低端价格
3
2014-4-27
电源管理(Power Management)
MOSFET 内置单路输出DC/DC
4.5~5.5V 2.7~5.5V ISL6410 (1.2/1.8/3.3)/0.6A
ISL8010
ISL8011
(0.8~Vin)/0.6A
(0.8~Vin)/1.2A (0.8~Vin)/1.5A (0.8~Vin)/2.0A (0.8~Vin)/2.0A (0.8~Vin)/3.0A (0.8~Vin)/4.0A
2014-4-27
视频(Surveillance)-KVM
300m
EL4543+EL9111+ISL59920
150m EL4543+ISL5991 0
<100m EL4543+EL5375+EL5205
25
2014-4-27
视频(Surveillance)-Other
ISL1208 (Reduced Cost) 400 nA (VBAT = 3.0V) 2.7 to 5.5V 1.8 to 5.5V ±10 ppm Yes
ISL12032 (Feature-Rich) 800 nA (VBAT = 2.7V) 2.7 to 5.5V 1.8 to 5.5V NA Yes
28
2014-4-27
EL5171+EL5172 应用方案
29
2014-4-27
接口(Interface)
RS-232
5V HIN238CBZ HIN232CPZ
5V/ESD HIN202E HIN213E
3.3V/ESD ICL3221E ICL3232E ICL3243E ICL3238E ICL3222E
双节 ISL9220
三节 ISL6251
单节/USD ISL9219
11
2014-4-27
ISL9219
12
2014-4-27
ISL9219
13
2014-4-27
ISL9220
14
2014-4-27
LDO
15
2014-4-27
16
2014-4-27
升压电路
2A MOSFET ISL97516
4 2014-4-27
ISL8009
EL7532
ISL8012
ISL8013 ISL8014
ISL9016
MOSFET 内置单路输出
3.0~6.0V EL7554
