氟马西尼注射液
关于氟马西尼的描述

关于氟马西尼的描述氟马西尼(Flumazenil)是一种广泛应用于临床的镇静药物拮抗剂,常用于对苯二氮䓬类药物的过量或过度使用引起的镇静状态进行逆转。
下面将从引言概述、正文内容和总结三个方面,详细描述氟马西尼的相关内容。
引言概述:氟马西尼是一种受体拮抗剂,作用于苯二氮䓬类药物的中枢神经系统受体。
它具有高度选择性和亲和力,可以迅速逆转苯二氮䓬类药物引起的镇静状态,恢复患者的清醒和意识。
正文内容:1. 氟马西尼的作用机制1.1 氟马西尼与受体的结合氟马西尼通过与中枢神经系统的苯二氮䓬类药物受体结合,阻断受体的作用,从而逆转药物的镇静效应。
1.2 氟马西尼的药代动力学氟马西尼经过静脉注射后迅速吸收,达到峰值浓度的时间约为1-3分钟。
它主要通过肝脏代谢,半衰期约为1小时。
由于其短效特点,可能需要多次给药来维持逆转效果。
2. 氟马西尼的临床应用2.1 苯二氮䓬类药物过量的逆转氟马西尼是治疗苯二氮䓬类药物过量引起的镇静状态的首选药物。
它可以迅速恢复患者的清醒和意识,减少中枢神经系统的抑制效应。
2.2 镇静药物的拮抗除了逆转苯二氮䓬类药物外,氟马西尼还可以用于拮抗其他镇静药物,如苯妥英钠和异戊巴比妥等,以减少药物的不良反应和副作用。
2.3 麻醉药物的拮抗氟马西尼也可以用于拮抗麻醉药物,如地西泮和丙泊酚等。
它可以迅速逆转麻醉药物的效应,帮助患者恢复清醒。
总结:综上所述,氟马西尼是一种广泛应用于临床的镇静药物拮抗剂。
它通过与苯二氮䓬类药物受体结合,逆转药物的镇静状态,恢复患者的清醒和意识。
氟马西尼的临床应用包括逆转药物过量、拮抗镇静药物和麻醉药物。
在临床实践中,合理使用氟马西尼可以有效减少药物的不良反应和副作用,提高患者的治疗效果。
国家卫生应急救援队伍基本装备目录(常用应急药物储备目录)

13.强心药 14.抗心律失常药 15.抗心绞痛药 16.呼吸中枢兴奋药 17.止咳药 18.平喘药
19.消化系统药
20.利尿剂 21.脱水药 22.促凝血药 23.抗凝血药
24.激素药
25.内分泌药 26.维生素类
27.水电酸碱平衡药
28.血浆及血浆代用 品
毒毛花苷 K 注射液、毛花苷丙注射液、地高辛片 利多卡因注射液、普罗帕酮注射液、维拉帕米注射液、美托洛尔片、胺碘酮注射液 硝酸甘油片、硝酸甘油注射液、速效救心丸、硝苯地平片、消心痛片 尼可刹米注射液、盐酸山梗碱注射剂 磷酸可待因片、复方甘草片、咳必清片 氨茶碱注射液、氨茶碱片、硫酸纱丁醇气雾剂 阿托品注射液、氢溴酸山莨菪碱注射液、硫糖铝胶囊、雷尼替丁胶囊、注射用奥美拉唑、丁溴东莨菪碱片、多 潘立酮片、胃复安片、胃复安注射液、果导片、思密达 双氢克尿噻片、呋塞米注射液 甘露醇注射液 氨甲苯酸注射液、注射用立止血冻干粉、注射用凝血酶冻干粉 注射用枸橼酸钠、肝素钠注射液 地塞米松注射液、地塞米松片、甲基强的松龙注射液、氢化可的松注射液、地塞米松注射液、脑垂体后叶注射 液、缩宫素注射液 普通胰岛素注射液、胸腺五肽注射液 维生素 B1 片、维生素 B2 片、维生素 B6 片、维生素 C 片、维生素 C 注射液 5%葡萄糖注射液、10%葡萄糖注射液、50%葡萄糖注射液、葡萄糖氯化钠注射液、0.9%氯化钠注射液、10%氯化 钠注射液、氯化钾注射液、葡萄糖酸钙注射液、碳酸氢钠注射液、注射用水、口服补液盐、复方氯化钠注射液、 乳酸钠注射液
制品
病疫苗、口服霍乱灭活疫苗、肾综合征出血热灭活疫苗、肉毒抗毒素、抗狂犬病血清、各种抗蛇毒血清
33.中成药
柴胡注射液、速效救心丸、板蓝根冲剂、通便灵胶囊、云南白药、麝香壮骨膏、精万红烫伤膏、清凉油
氟马西尼注射液-详细说明书与重点

氟马西尼注射液英文名称: Flumazenil Injection【成分】本品主要成份为氟马西尼。
化学名称:8-氟-5,6-二氢-5甲基-6-氧代-4H-咪唑并-[1,5-a][1,4]-苯并二氮䓬-3-甲酸乙酯。
本品辅料为:依地酸二钠、醋酸、氯化钠、盐酸或氢氧化钠、注射用水。
【性状】本品为无色的澄明液体。
【适应症】用于逆转苯二氮䓬类药物所致的中枢镇静作用:1.终止用苯二氮䓬类药物诱导及维持的全身麻醉。
2.作为苯二氮䓬类药物过量时中枢作用的特效逆转剂。
3.用于鉴别诊断苯二氮䓬类、其它药物或脑损伤所致的不明原因的昏迷。
【规格】5ml:0.5mg【用法用量】可用5%的葡萄糖水、乳酸林格氏液或普通生理盐水稀释后注射,稀释后应在24小时内使用。
1.终止用苯二氮䓬类药物诱导及维持的全身麻醉:推荐的初始剂量为15秒内静脉注射0.2毫克。
如果首次注射后60秒内清醒程度未达到要求,则追加给药0.1毫克,必要时可间隔60秒后再追加给药一次,直至最大总量1毫克,通常剂量为0.3-0.6毫克。
2.作为苯二氮䓬类药物过量时中枢作用的特效逆转剂:推荐的首次静脉注射剂量为0.3毫克。
如果在60秒内未达到所需的清醒程度,可重复使用直至患者清醒或达总量2毫克。
如果再度出现昏睡,可以每小时静脉滴注0.1-0.4毫克药物,滴注的速度应根据所要求的清醒程度进行个体调整。
在重症监护情况下,对大剂量和/或长时间使用苯二氮䓬类药物的病人只要缓慢给药并根据个体情况调整剂量并不会引起戒断症状。
如果出现意外的过度兴奋体征,可静脉注射5毫克安定或5毫克咪达唑仑并根据患者的反应小心调整用量。
3.用于鉴别诊断苯二氮䓬类、其它药物或脑损伤所致的不明原因的昏迷:如果重复使用本品后,清醒程度及呼吸功能尚未显著改善,必须考虑到苯二氮䓬类药物以外的其他原因。
【不良反应】1.少数患者在麻醉时用药,会出现面色潮红、恶心和/或呕吐。
在快速注射氟马西尼后,偶尔会有焦虑、心悸、恐惧等不适感。
镇静催眠药物中毒

镇静催眠药物中毒1 .镇静催眠药物中毒(sedative-hypnotic poisoning)诊断原则包括3个方面:有明确或可疑的过量摄入镇静催眠药物的病史;出现意识障碍、呼吸抑制、血压下降等临床表现;胃液、血液或尿液中检出镇静催眠药物或其代谢产物。
2.疑诊患者进行血常规、血生化、血气分析、血尿毒物定性检测,如条件允许常规行床旁胸部X线片、头CT及心电图检查。
3.重度镇静催眠药物中毒患者可出现呼吸抑制或呼吸衰竭可血压下降,此类患者应立即进入抢救区,进行有效的支持治疗。
4.中毒患者尽早洗胃、催吐、导泻;巴比妥类和吩嚏嗪类无特效解毒剂;氟马西尼是苯二氮革类拮抗剂。
5.存在意识障碍、瞳孔缩小患者,应与有机磷、阿片类及其他药物中毒鉴别,同时应除外急性脑血管病等内科系统疾病。
病历摘要女性,23岁,主因“意识不清4小时”入院。
患者于4小时前被人发现意识不清,无发热,无抽搐,无二便失禁。
分诊台初步体格检查:T 36.2°C , P 60次/分,R 10次/分,BP100/60mmHg,SpO2 96%,神志不清,双瞳等大等圆,直径约2mm,对光反射存在,心肺腹查体未见异常,病理征阴性。
既往体健。
于床旁发现一空地西泮(安定)药瓶。
【问题1】患者目前有无生命危险?是否需要进入抢救室诊治?患者青年女性,突发意识不清,查体无明显异常,应急查血糖、血气分析并完善血常规、血生化、血液毒物检测、头CT等。
患者目前生命体征相对平稳,但其血压、心率、呼吸偏慢,意识不清,可能发生误吸、窒息等,应进入抢救室进一步诊治。
知识点镇静催眠药物分类1 .苯二氮草类长效类(半衰期>30小时):氯氮、地西泮、氟西泮;中效类(半衰期6〜30小时):阿普哩仑、奥沙西泮、替马西泮;短效类(半衰期V6小时):三哩仑。
2.巴比妥类长效类(作用时间6~8小时):巴比妥和苯巴比妥;中效类(作用时间3~6小时):戊巴比妥、异戊巴比妥、布他比妥;短效类(作用时间2〜3小时):司可巴比妥、硫喷妥钠。
2009年新批准药品筛选

国药准字H20093461 空 氟马西尼注射液 Flumazenil Injection 空 广东世信药业有限公司 2009-04-15 化学药品 2ml:0.2mg 注射剂 空
国药准字H20090148 空 法罗培南钠 Faropenem Sodium 空 山东鲁抗辰欣药业有限公司 2009-03-23 化学药品 ---- ---- 空
国药准字H20090149 空 法罗培南钠片 Faropenem Sodium Tablets 迪哌 山东鲁抗辰欣药业有限公司 2009-03-23 化学药品 0.1g 片剂 空
国药准字H20093386 空 安吖啶 Amsacrine 空 海南中化联合制药工业有限公司 2009-04-09 化学药品 ---- ---- 空
国药准字H20093037 空 胞磷胆碱钠 Citicoline Sodium 空 开平牵牛生化制药有限公司 2009-01-04 化学药品 ---- ---- 空
国药准字H20093650 空 氟马西尼注射液 Flumazenil Injection 空 宁波市天衡制药有限公司 2009-05-22 化学药品 10ml:1.0mg 注射剂 空
国药准字H20093176 空 复方a-酮酸片 Compound α-Ketoacid Tablets 空 北京万生药业有限责任公司 2009-02-10 化学药品 0.63g 片剂 空
国药准字H20093462 空 氟马西尼注射液 Flumazenil Injection 空 广东世信药业有限公司 2009-04-15 化学药品 5ml:0.5mg 注射剂 空
氟马西尼的使用说明

药代动力学: 据文献资料,氟马西尼为一种亲脂性药物,血浆蛋白结合
率约为50%,所结合的血浆蛋白中2/3为白蛋白。氟马西尼广泛 分布于血管外,稳态时的平均分布容积(Vss)为0.95升/千克。 氟马西尼主要在肝脏代谢。在血浆和尿中的主要代谢物为羧酸 代谢物,该主要代谢物没有苯二氮䓬类受体激动剂或拮抗剂的 活性。氟马西尼几乎完全(99%)通过非肾脏途径消除。药物 消除半衰期为50~60分钟
症状。如果出现意外的过度兴奋体征,可静脉注射5mg安定或5mg咪达唑仑并根据
患者的反应小心调整用量
用于鉴别诊断苯二氮䓬类、其它药物或脑损伤所致的不明原因的昏迷:如果重复
4
使用本品后,清醒程度及呼吸功能尚未显著改善,必须考虑到苯二氮䓬类药物以
外的其他原因
不良 反应
不良反应
少数患者在麻醉时用药,会出现面色潮红、恶心和/或呕吐。在
适应症
用于逆转苯二氮䓬类药物所致的中枢镇静作用
01 终止用苯二氮类药物诱导及维持的全身麻醉
适应症
02 作为苯二氮类药物过量时中枢作用的特效逆转剂
03
用于鉴别诊断苯二氮䓬类、其它药物或脑损伤所致的 不明原因的昏迷
用法用量
可用5%的葡萄糖水、乳酸林格氏液或普通生理盐水稀释后注射,稀释后应在24小
1
时内使用
氟马西尼注射液
目录
CONTENT
01 药品名称、成份 02 适应症 03 用法用量 04 不良反应及禁忌症 05 注意事项 06 药物相互作用
药品名称 氟马西尼注射液(Flumazenil Injection)
成份
本品主要成份为氟马西尼。本品辅料为:依地酸二钠
氟马西尼
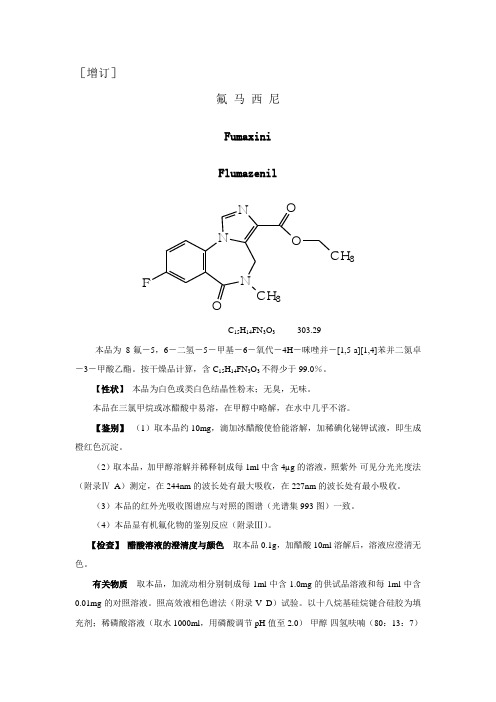
[增订]氟 马 西 尼FumaxiniFlumazenil NN FCH 3NO O CH 3C 15H 14FN 3O 3 303.29本品为8-氟-5,6-二氢-5-甲基-6-氧代-4H -咪唑并-[1,5-a][1,4]苯并二氮卓-3-甲酸乙酯。
按干燥品计算,含C 15H 14FN 3O 3不得少于99.0%。
【性状】 本品为白色或类白色结晶性粉末;无臭,无味。
本品在三氯甲烷或冰醋酸中易溶,在甲醇中略解,在水中几乎不溶。
【鉴别】 (1)取本品约10mg ,滴加冰醋酸使恰能溶解,加稀碘化铋钾试液,即生成橙红色沉淀。
(2)取本品,加甲醇溶解并稀释制成每1ml 中含4µg 的溶液,照紫外-可见分光光度法(附录Ⅳ A )测定,在244nm 的波长处有最大吸收,在227nm 的波长处有最小吸收。
(3)本品的红外光吸收图谱应与对照的图谱(光谱集993图)一致。
(4)本品显有机氟化物的鉴别反应(附录Ⅲ)。
【检查】 醋酸溶液的澄清度与颜色 取本品0.1g ,加醋酸10ml 溶解后,溶液应澄清无色。
有关物质 取本品,加流动相分别制成每1ml 中含1.0mg 的供试品溶液和每1ml 中含0.01mg 的对照溶液。
照高效液相色谱法(附录V D )试验。
以十八烷基硅烷键合硅胶为填充剂;稀磷酸溶液(取水1000ml ,用磷酸调节pH 值至2.0)-甲醇-四氢呋喃(80:13:7)为流动相;检测波长为230nm。
理论板数按氟马西尼峰计算不低于3000。
取对照溶液20µl 注入液相色谱仪,调节仪器灵敏度,使主成分峰的峰高约为满量程的10%;再精密量取供试品溶液与对照溶液各20µl分别进样,记录色谱图至主成分峰保留时间的3倍,供试品溶液的色谱图中,单个杂质峰的峰面积不得大于对照溶液主峰面积的1/5(0.2%),各杂质峰面积的和不得大于对照溶液主峰面积的1/2(0.5%)。
干燥失重取本品,在105℃干燥至恒重,减失重量不得过0.2%(附录ⅧL)。
氟马西尼注射液过敏性、溶血性和局部刺激性实验研究

由上海 长 征 富 民药 业 华 中 有 限 公 司生 产 , 批 号 :
【 收稿 日期】 0 80 .0 20 .53
【 者简 介】 丽瑛 (9 7)女, 作 徐 17 一 , 硕士 , 理研 究员 , 要 从事 新 助 主
处剪 毛 ,每侧 1点 ,按 顺序 皮 内注入 各 组相 应 抗
血清 ( l: 、右 l: ) 0 1 左 2 8各 . ml 8 h后进 行 ,4
药的药理毒理工作, — i u t @yh o o c Ema :xa a o . m. l r t n 【 通讯作者】 华 饶
抗 原攻击 ,各组静脉 注射与致敏剂 量相 同的激发抗 原加 等 量 的 05 伊 文 思蓝染 Байду номын сангаас 共 1 ml .% ,进 行激
随机 分为 生理盐 水组, 阳性 对照( 蛋清 02ml g 鸡 . / ) k 组和 氟马西尼 注射 液 01 . mgk .、02 /g剂量组 , 组 每 6只 。各 组动物 隔 日腹腔注 射相应 药物 ( 2ml g 按 / k
体 重) ,共 5次 。末 次注 射 后 1 ,各 组 豚 鼠分 4d 别静脉注 射 2倍剂量相 应受试药物 进行激 发 ,并进
1 材 料 与方 法
11 实验动 物 .
行观察 ,如 变态反应级 数分级超过 2 即判 断变态 级,
反应 为阳性 。 132 被 动皮肤 过敏试验 取大 鼠 8只 ,分 为 4组 , ..
普通级 雄性 豚 鼠 2 4只, 体重 2 0 3 0g 购 自 2 ~ 0 ,
上 海 市 松江 区 松 联 实验 动 物场 [ CXK( ) 0 2 S 沪 2 0—
氟马西尼注射液临床给药途径为静脉注射为进一步探讨该产品临床使用的安全性按照sfda所颁发的编号茭jhgtp41化学药物刺激性过敏性和溶血性研究技术指导原则技术要求我们进行如下安全性试验
瑞马唑仑联合氟马西尼在老年患者胃镜检查中的应用

depression and injection pain as well as a shorter pause time and awakening time ( P<0 05) .Neither group had complications occurred.
Conclusion Both propofol and remazolam used in painless gastroscopy in elderly patients have satisfactory effects. Compared to
propofol + flumazenilꎬremazolam + flumazenil have significantly greater cardiovascular safety and less adverse reactionsꎬwhich may be
推注至患者熟睡) ꎬ之后使用 1 ml / ( kgh) ( 稀释为 1
45 ~ 55ꎮ C 组使用丙泊酚进行诱导( 缓慢推注至患
者熟睡即停止) ꎬ之后使用 1 ml / ( kgh) 进行维持
(稀释成 6 mg / ml) ꎮ 同样维持 BIS 45 ~ 55ꎮ 所有患
者在发生呼吸抑制等不良反应时对症处理ꎮ 手术
表 3 两组不良反应比较 ( n)
组别
心动过速
心动过缓
高血压
低血压
呼吸抑制
氟马西尼在全身麻醉术后高龄患者催醒方面的作用分析

㊃论著㊃D O I:10.3969/j.i s s n.1672-9455.2024.07.008氟马西尼在全身麻醉术后高龄患者催醒方面的作用分析*吴祥,陈世新,伍青青,桂江华江西省南昌市第一医院麻醉科,江西南昌330006摘要:目的分析氟马西尼在全身麻醉(简称全麻)术后高龄患者催醒方面的作用㊂方法选取2020年10月至2021年12月该院收治的60例全麻手术患者作为研究对象,采用抽签法将其分为对照组和观察组,每组30例㊂对照组患者催醒采用生理盐水,观察组患者催醒采用氟马西尼,比较两组患者注射后神经敏感程度㊁清醒程度㊁不良反应㊁催醒效果㊂结果两组注射后0~<10m i n的神经敏感程度及清醒程度比较,差异无统计学意义(P>0.05),观察组患者注射10㊁20㊁30m i n后神经功能敏感程度与清醒程度优于对照组,差异有统计学意义(P<0.05)㊂两组患者的不良反应发生率比较,差异无统计学意义(P>0.05)㊂观察组的催醒效果优于对照组,差异有统计学意义(P<0.05)㊂结论氟马西尼在高龄患者全麻术后的应用,可以提高其神经功能敏感程度,使其更快清醒,未增加不良反应,具有显著的催醒效果㊂关键词:全身麻醉;术后;氟马西尼;高龄;催醒中图法分类号:R971+.2文献标志码:A文章编号:1672-9455(2024)07-0900-04A n a l y s i s o n r o l e o f f l u m a z e n i l i n a w a k e n i n g a f t e r g e n e r a l a n e s t h e s i a i n a d v a n c e d a g e p a t i e n t s*WU X i a n g,C H E N S h i x i n,WU Q i n g q i n g,G U I J i a n g h u aD e p a r t m e n t o f A n e s t h e s i o l o g y,N a n c h a n g M u n i c i p a l F i r s t H o s p i t a l,N a n c h a n g,J i a n g x i330006,C h i n aA b s t r a c t:O b j e c t i v e T o a n a l y z e t h e r o l e o f f l u m a z e n i l i n a w a k e n i n g a f t e r g e n e r a l a n e s t h e s i a i n t h e a d-v a n c e d a g e p a t i e n t s.M e t h o d s S i x t y p a t i e n t s w i t h g e n e r a l a n e s t h e s i a a d m i t t e d i n t h i s h o s p i t a l f r o m O c t o b e r2020t o D e c e m b e r2021w e r e s e l e c t e d a s t h e r e s e a r c h s u b j e c t s a n d d i v i d e d i n t o t h e c o n t r o l g r o u p a n d o b s e r v a t i o n g r o u p b y t h e l o t t e r y m e t h o d,30c a s e s i n e a c h g r o u p.T h e p a t i e n t s i n t h e c o n t r o l g r o u p a d o p t e d n o r m a l s a l i n e f o r a w a k e n i n g,a n d t h e p a t i e n t s i n t h e o b s e r v a t i o n g r o u p a d o p t e d f l u m a z e n i l f o r a w a k e n i n g.T h e n e u r a l s e n s i t i v i t y d e g r e e,l u c i d i t y d e g r e e,a d-v e r s e r e a c t i o n s a n d w a k e-u p e f f e c t s a f t e r i n j e c t i o n w e r e c o m p a r e d b e t w e e n t h e t w o g r o u p s.R e s u l t s T h e r e w a s n o s t a t i s t i c a l l y s i g n i f i c a n t d i f f e r e n c e i n n e r v e s e n s i t i v e d e g r e e a n d l u c i d i t y d e g r e e a t0-10m i n a f t e r i n j e c t i o n b e-t w e e n t h e t w o g r o u p s(P>0.05).T h e n e r v e f u n c t i o n s e n s i t i v i t y d e g r e e a n d l u c i d i t y d e g r e e a t10,20,30m i n a f t e r i n j e c t i o n i n t h e o b s e r v a t i o n g r o u p w e r e s u p e r i o r t o t h e c o n t r o l g r o u p,a n d t h e d i f f e r e n c e w a s s t a t i s t i c a l l y s i g n i f i c a n t(P<0.05).T h e r e w a s n o s t a t i s t i c a l l y s i g n i f i c a n t d i f f e r e n c e i n t h e i n c i d e n c e r a t e o f a d v e r s e r e a c-t i o n s b e t w e e n t h e t w o g r o u p s(P>0.05).T h e w a k e-u p e f f e c t o f t h e o b s e r v a t i o n g r o u p w a s s u p e r i o r t h a n t h a t o f t h e c o n t r o l g r o u p,t h e d i f f e r e n c e w a s s t a t i s t i c a l l y s i g n i f i c a n t(P<0.05).C o n c l u s i o n T h e a p p l i c a t i o n o f f l u m a z e n i l a f t e r g e n e r a l a n e s t h e s i a i n a d v a n c e d a g e p a t i e n t s c o u l d i n c r e a s e t h e i r n e r v o u s f u n c t i o n s e n s i t i v i t y, m a k e t h e m w a k e u p f a s t e r w i t h o u t i n c r e a s i n g a d v e r s e r e a c t i o n s,a n d h a s a s i g n i f i c a n t w a k e-u p e f f e c t.K e y w o r d s:g e n e r a l a n e s t h e s i a;a f t e r o p e r a t i o n;f l u m a z e n i l;a d v a n c e d a g e; w a k e u p随着医疗卫生行业及社会的不断发展,接受全身麻醉(简称全麻)手术的高龄患者逐渐增多㊂因高龄患者特殊的生理特征,术后经常出现各种并发症㊂若要在术后尽早逆转全麻手术对中枢神经的镇静作用,使患者在短时间内苏醒,应对患者开展催醒治疗[1]㊂氟马西尼是一种常用的催醒药物,可拮抗苯二氮 类药物的镇静作用[2]㊂本研究将南昌市第一医院2020年10月至2021年12月收治的60例全麻手术患者作为研究对象,对其分别使用生理盐水及氟马西尼,观察氟马西尼在高龄患者全麻术后的催醒作用,现报道㊃009㊃检验医学与临床2024年4月第21卷第7期 L a b M e d C l i n,A p r i l2024,V o l.21,N o.7*基金项目:江西省卫生和计划生育委员会科技计划项目(20197012)㊂作者简介:吴祥,男,主治医师,主要从事临床麻醉㊁呼吸管理㊁麻醉药品的临床药理分析研究㊂如下㊂1 资料与方法1.1 一般资料 选取2020年10月至2021年12月本院收治的60例全麻手术患者作为研究对象,采用抽签法将其分为对照组和观察组,每组30例㊂对照组男18例,女12例;年龄60~87岁,平均(73.50ʃ2.30)岁㊂观察组男17例,女13例;年龄60~85岁,平均(72.59ʃ2.88)岁㊂两组一般资料比较,差异无统计学意义(P >0.05),具有可比性㊂纳入标准:(1)配合度较高患者;(2)符合手术指征患者㊂排除标准:(1)有精神疾病病史患者;(2)中途退出患者㊂本研究通过本院医学伦理委员会批准(审批号:20197012),参与研究者均知情同意㊂1.2 方法 对照组:催醒采用生理盐水㊂全麻使用咪达唑仑0.5m g /k g +丙泊酚1.8~2.0m g /k g +舒芬太尼5~10μg㊂手术过程中使用瑞芬太尼及丙泊酚维持麻醉,泵注持续时间控制在3h 左右㊂手术结束后,待患者的吞咽及咳嗽动作出现后,静脉注射5m g 生理盐水㊂观察组:催醒采用氟马西尼(厂家:浙江仙琚制药股份有限公司,规格5m L ʒ0.5m g)㊂手术使用麻醉方案与对照组相同㊂手术结束后,待患者的吞咽及咳嗽动作出现后,静脉注射5m L 氟马西尼㊂1.3 观察指标与评价标准 观察注射0~<10㊁10㊁20㊁30m i n 后神经敏感程度以及清醒程度㊁不良反应㊁催醒效果㊂1.3.1 两组患者的神经功能敏感程度及清醒程度分级 神经功能敏感程度的评估以医院自制量表为依据,分为3级:1级主要是指医务人员对患者进行轻微触摸,患者有反应;2级主要是指医务人员对患者进行轻微触摸,患者无应答,轻拍患者的身体,患者有反应;3级主要指医务人员对患者进行轻拍,患者无反应,医务人员对患者实施伤害性的刺激才有应答反应[3]㊂清醒程度的评估以医院自制量表为依据,主要对患者的清醒程度进行观察,并统计结果,分为3级:1级为患者完全清醒,医务人员在与其交流时,患者可以正常回答;2级为与医务人员之间的应答反应较迟钝;3级为医疗后,医务人员与患者轻声交谈,患者无反应,大声呼叫才有轻微反应[4]㊂1.3.2 两组不良反应 不良反应发生率为皮疹㊁头痛㊁烦躁㊁恶心发生率之和㊂1.3.3 两组催醒效果 催醒效果分为优㊁良及差㊂优:主要指该病患者在全麻手术之后的催醒效果超过医生预期,且不损害患者的健康,患者在短时间内苏醒;良:主要是指对该病患者实施全麻手术之后,催醒效果一般,患者苏醒时间与预期对比,有一定延迟;无效:主要是指患者在实施全麻手术之后,催醒效果较差㊂催醒效果为优良率之和[5]㊂1.4 统计学处理 采用S P S S 20.0统计软件分析数据㊂计量资料以x ʃs 表示,组间比较采用t 检验;计数资料以例数或百分率表示,组间比较采用χ2检验;等级资料比较采用秩和检验㊂以P <0.05为差异有统计学意义㊂2 结 果2.1 两组患者的神经功能敏感程度以及清醒程度比较 两组注射后0~<10m i n 的神经功能敏感程度及清醒程度比较,差异无统计学意义(P >0.05),但是,观察组患者注射10㊁20㊁30m i n 后神经功能敏感程度与清醒程度优于对照组,差异有统计学意义(P <0.05)㊂见表1㊂表1 两组患者的神经功能敏感程度以及清醒程度比较[n (%)]组别n0~<10m i n 神经功能敏感程度1级2级3级10m i n 神经功能敏感程度1级2级3级20m i n 神经功能敏感程度1级2级3级30m i n 神经功能敏感程度1级2级3级观察组306(20.00)8(26.67)16(53.33)18(60.00)10(33.33)2(6.67)22(73.33)6(20.00)2(6.67)28(93.33)1(3.33)1(3.33)对照组307(23.33)10(33.33)13(43.33)8(26.67)11(36.67)11(36.67)13(43.33)10(33.33)7(23.33)18(60.00)5(16.67)7(23.33)Z10.1942.98812.0255.022P0.7540.0010.0010.001组别n0~<10m i n 清醒程度1级2级3级10m i n 清醒程度1级2级3级20m i n 清醒程度1级2级3级30m i n 清醒程度1级2级3级观察组306(20.00)16(53.33)8(26.67)19(63.33)10(33.33)1(3.33)22(73.33)6(20.00)2(6.67)27(90.00)2(6.67)1(3.33)对照组307(23.33)10(33.33)13(43.33)8(26.67)11(36.67)11(36.67)13(43.33)10(33.33)7(23.33)18(60.00)5(16.67)7(23.33)Z5.66910.5248.6935.624P0.7540.006<0.0010.007㊃109㊃检验医学与临床2024年4月第21卷第7期 L a b M e d C l i n ,A pr i l 2024,V o l .21,N o .72.2两组不良反应比较两组患者的不良反应发生率比较,差异无统计学意义(P>0.05)㊂见表2㊂表2两组患者的不良反应发生率比较[n(%)]组别n皮疹头痛烦躁恶心总发生观察组300(0.0)1(3.33)1(3.33)0(0.0)2(6.67)对照组300(0.0)1(3.33)0(0.0)0(0.0)1(3.33)χ27.680 P0.152 2.3两组患者的催醒效果比较观察组的催醒效果优于对照组,差异有统计学意义(P<0.05)㊂见表3㊂表3两组手术患者的催醒效果比较[n(%)]组别n优良无效催醒效果观察组3025(83.33)3(10.00)2(6.67)28(93.33)对照组305(16.67)15(50.00)10(33.33)20(66.67)χ22.451P0.0013讨论全麻手术要保证患者的安全,可以实施催醒[6]㊂有研究报道,生理盐水在全麻手术后高龄患者中应用,可以在一定程度上促进患者的尽快苏醒,但是效果一般[7]㊂氟马西尼起效快㊁毒性低,其静脉注射可以在几分钟内起作用,消除半衰期为50m i n,拮抗效应的维持时间为2h左右,最后通过肝脏代谢[8]㊂其是一种中枢神经系统受体,亲和性较好[9]㊂氟马西尼的半衰期短于咪达唑仑,拮抗作用持续时间相对较短[10]㊂为了避免患者在催醒之后重新出现镇静的情况,在将患者的气管导管拔出之后,需要对患者的病情实施严密观测,保证其呼吸道通畅[11]㊂两组在注射后0~<10m i n的神经功能敏感程度以及清醒程度比较,差异无统计学意义(P>0.05),但是,注射10㊁20㊁30m i n后,观察组的神经功能敏感程度较对照组有明显提高,差异有统计学意义(P< 0.05),可以看出全麻术后氟马西尼在高龄患者中应用,对患者术后神经功能敏感程度有一定改善作用[12]㊂观察组的不良反应发生率与对照组比较,差异无统计学意义(P>0.05),提示全麻术后氟马西尼在高龄患者中应用,对患者的术后不良反应控制有一定作用,其可以在一定范围内保障患者的麻醉及手术安全性,且引起的不良反应与对照组基本保持一致㊂注射10㊁20㊁30m i n后,观察组的清醒程度与对照组比较,差异有统计学意义(P<0.05),可以看出全麻术后对高龄患者注射适量氟马西尼可促进患者术后在短时间内清醒㊂观察组的催醒效果明显优于对照组,差异有统计学意义(P<0.05),说明氟马西尼能够从整体上保证患者的催醒效果㊂主要是因为氟马西尼能够与高龄患者神经中枢的苯二氮 (B D Z)受体有效结合[13],竞争性抑制γ-氨基丁酸-苯二氮 复合物(G A B A-B D Z)识别B D Z受体药物,保证患者的γ-氨基丁酸(G A B A)的释放量增加[14-15],也可促进G A B A 释放带来的中枢抑制逆转效果的进一步提高[16-17]㊂该药物主要用于拮抗B D Z受体激动产生的各类症状的治疗,进而促进术后患者的神经功能敏感性增强,使其可在短时间内清醒,促进催醒效果[18-19]㊂本研究与邝昆合等[20]研究有一定相似之处,主要体现在不良反应上㊂氟马西尼不仅可以改善患者术后清醒程度,还可以改善患者神经功能敏感程度,促进患者术后清醒㊂本次研究也有一定局限性,主要体现为样本量过少,可能会影响研究的准确性,应在后期进行相关研究时,选择恰当的样本量,为研究提供有效支持综上所述,氟马西尼在全麻术后高龄患者中的应用,可以提高其神经功能敏感程度及清醒程度,未增加不良反应,使整体催醒效果增强㊂参考文献[1]李文瑶,陶国才,牛洋,等.瑞马唑仑联合氟马西尼在老年患者胃镜检查中的应用[J].实用医院临床杂志,2021,18(4):89-91.[2]尹柏双,刘铮,林佳琦,等.阿替美唑-纳洛酮复合氟马西尼催醒X T Q麻醉鹿的效果观察[J].中国兽医杂志, 2021,57(6):89-92.[3]吴剑华.氟马西尼对全凭静脉麻醉术后患者的催醒效果观察[J].当代医学,2021,27(3):19-21.[4]王惠军,王珊珊,杜英杰,等.老年眼科手术患者全身麻醉中瑞马唑仑应用效果观察[J].山东医药,2023,63(23): 79-82.[5]黄青云,刘佩蓉.氟马西尼对高龄全身麻醉患者术后认知功能障碍和脑电双频指数的影响[J].西北药学杂志, 2018,33(5):665-668.[6]孙秋红.全凭静脉麻醉术后催醒采用氟马西尼的临床分析[J].中国医药指南,2019,17(25):51-52.[7]单雨.氟马西尼用于全凭静脉麻醉术后催醒的临床研究[J].中外女性健康研究,2018,26(10):49.[8]代玲杰,袁清霞.氟马西尼在术后催醒中的研究进展[J].现代医院,2019,19(8):1214-1217.[9]周玉,李姝霈,李涛,等.瑞马唑仑对全身麻醉下腹腔镜胆囊切除术安全性及有效性评价[J/C D].中国医学前沿杂志(电子版),2022,14(7):41-44.[10]喻僖秦.七氟醚在超高龄手术患者的全麻维持中的应用[J].中国现代医生,2018,56(36):107-109.(下转第906页)㊃209㊃检验医学与临床2024年4月第21卷第7期 L a b M e d C l i n,A p r i l2024,V o l.21,N o.7临床特点及淋巴细胞亚群变化的意义[J].河北医药, 2022,44(6):838-842.[2]李慧,刘晶,杨金英.传染性单核细胞增多症患儿外周血T淋巴细胞亚群变化及其临床意义[J].海南医学,2023, 34(9):1295-1299.[3]张春艳,于风岭.E B病毒抗体检测在儿童传染性单核细胞增多症诊断中的应用[J].中国保健营养,2020,30(1): 265-266.[4]舒畅,刘小乖,李瑞娜.E B病毒感染患儿免疫学㊁肝功能指标及其相关性研究[J].陕西医学杂志,2020,49(12): 1608-1611.[5]戴星星.I L-1β㊁S A A㊁E B V-D N A载量对传染性单核细胞增多症肝功能损害的诊断价值研究[D].大连:大连医科大学,2022.[6]胡杰,李文博,张帆.血清A D A㊁L D H及E B V-D N A对儿童传染性单核细胞增多症的诊断意义[J].分子诊断与治疗杂志,2022,14(1):28-31.[7]全国儿童E B病毒感染协作组,中华实验和临床病毒学杂志编辑委员会.E B病毒感染实验室诊断及临床应用专家共识[J].中华实验和临床病毒学杂志,2018,32(1):2-8.[8]林珊,郭三平,江心怡,等.异型淋巴细胞㊁E B病毒及T L R7联合检测在儿童传染性单核细胞增多症中的诊断价值[J].检验医学与临床,2023,20(1):32-35. [9]钟志辉,蒙国煌,张岳汉,等.E B病毒抗体联合E B V-D N A载量检测诊断儿童传染性单核细胞增多症的临床价值[J].检验医学与临床,2022,19(18):1672-9455. [10]郭红仙,胡玉杰,尹凤蕊,等.传染性单核细胞增多症270例临床和实验室检查分析[J].中华实用儿科临床杂志, 2022,37(19):1478-1481.[11]操晓莉,陈梅俐,胡祥松,等.V C A-I g M与E B-D N A对儿童传染性单核细胞增多症的诊断效能[J].中华医院感染学杂志,2023,33(11):1741-1745.[12]储开东,邵志莉,袁伯稳.重组人干扰素α1b联合更昔洛韦对传染性单核细胞增多症合并肝功能异常患儿炎症免疫和肝功能的影响[J].中国医院用药评价与分析,2023, 23(4):443-446.[13]苏丽丽,储开东,崔蕾,等.N L R㊁S A A和C D19+水平检测对传染性单核细胞增多症肝损害患儿病情及预后的评估价值[J].疑难病杂志,2022,21(8):804-808. [14]刘文田,唐芳华,刘玉花,等.血清T N F-α㊁S A A㊁A D A对儿童E B V相关传染性单核细胞增多症的诊断价值研究[J].河北医科大学学报,2021,42(4):470-473. [15]张静静,贾媛媛,胡苗苗,等.传染性单核细胞增多症肝损害患儿血清A D A㊁L D H及E B V-D N A水平对评估病情及预后的临床价值[J].川北医学院学报,2023,38(8): 1090-1093.[16]张格格,李莲,张有为.外周血淋巴细胞百分率与L D H㊁H B D H联合检测对传染性单核细胞增多症早期筛检价值的探讨[J].湖北医药学院学报,2022,41(5):499-501.[17]肖霄,张城.血清h s-C R P㊁P C T㊁L D H水平变化与急性白血病合并细菌感染患者预后的关联性分析[J].临床血液学杂志,2021,34(4):237-241.[18]计晓兰,胡春霞,孔令军,等.E B V-I M患儿外周血E B V-D N A载量与T h1/T h2相关细胞因子及临床特征的关系[J].中华医院感染学杂志,2023,33(16):2503-2507.(收稿日期:2023-08-25修回日期:2023-11-23)(上接第902页)[11]周颖婷,杨佳.氟马西尼术后催醒对甲状腺手术女性患者术后睡眠质量的影响[J].重庆医学,2022,51(10):1757-1761.[12]许卫平.氟马西尼用于全凭静脉麻醉术后催醒的临床研究[J].首都食品与医药,2018,25(16):66. [13]张新影,刘少霞.呼吸机在妇产科术后恢复室的应用研究[J].中国医疗器械信息,2020,26(6):172-174. [14]吴秋云,陈民辉,曹玲.电感耦合等离子体质谱法测定氟马西尼注射液中金属元素的含量[J].中南药学,2023,21(9):2439-2441.[15]陈聪,夏哲灏,范林平.氟马西尼联合醒脑静用于急性苯二氮卓类药物中毒治疗的效果观察[J].北方药学,2023, 20(4):146-148.[16]李继荣,王志坚,李三清,等.氟马西尼及纳洛酮治疗肝昏迷的疗效比较[J].医学信息,2023,36(9):120-123. [17]李玉娟,肖宁,贾文丹,等.氟马西尼联合肾上腺素对钩吻中毒的解救作用[J].畜牧兽医学报,2022,53(3):938-946.[18]安璐,刘颖,崔秀花,等.氟马西尼拮抗瑞马唑仑全身麻醉术后残余镇静作用的临床观察[J].中国医药,2022,17(12):1846-1849.[19]欧锋,井立说.咪达唑仑联合氟马西尼用于悬雍垂腭咽成形术麻醉苏醒期的有效性与安全性观察[J].检验医学与临床,2022,19(24):3437-3440.[20]邝昆合,郑德志,江进红,等.氟马西尼用于静吸复合全麻术后催醒的临床效果观察[J].中国实用医药,2023,18(9):116-119.(收稿日期:2023-09-10修回日期:2023-11-18)㊃609㊃检验医学与临床2024年4月第21卷第7期 L a b M e d C l i n,A p r i l2024,V o l.21,N o.7。
多种药物中毒患者的药学监护

多种药物中毒患者的药学监护钮佳丽;丁云龙;卢通;居峰;薛婷;赵广玉【摘要】目的通过临床药师对1例地西泮、氨氯地平、水银中毒、割腕患者的药学监护,小结临床药师参与多种药物中毒救治中的工作.方法临床药师参与查房,查阅中毒、感染相关文献,参与制定治疗方案,提出药物调整建议,实施药学监护.结果患者住院8d,病情显著好转,未发生明显药物不良反应.结论临床药师参与患者诊疗过程,从药学的角度提供治疗建议,能够提高药物治疗疗效与安全性.【期刊名称】《医药导报》【年(卷),期】2019(038)004【总页数】4页(P522-525)【关键词】地西泮;氨氯地平;汞;药物中毒;药学监护【作者】钮佳丽;丁云龙;卢通;居峰;薛婷;赵广玉【作者单位】江苏省靖江市人民医院临床药学科,靖江214500;江苏省靖江市人民医院神经内科,靖江214500;江苏省靖江市人民医院临床药学科,靖江214500;江苏省靖江市人民医院临床药学科,靖江214500;江苏省靖江市人民医院临床药学科,靖江214500;江苏省靖江市人民医院临床药学科,靖江214500【正文语种】中文【中图分类】R979.3;R969.3药物中毒是临床常见急重症,部分患者常因病情危重或抢救不及时而危及生命。
社会经济的发展带来更大的工作与生活的压力,焦虑与抑郁的人群增多,自杀的患者呈上升趋势[1-2]。
临床常见服用过量药物自杀的患者。
单一药物中毒的治疗经验,临床多有报道。
然而,少数患者在自杀时会服用多种药物或毒物,对于多种药物中毒的重症患者,临床救治仍需要积累经验。
我院于2016年11月18日收治1例地西泮、氨氯地平、水银中毒,割腕自杀患者并抢救成功,临床药师参与该患者治疗过程,进行药学监护,现汇报如下。
1 病例概况患者,男,47岁。
因发现神志不清3 h于2016年11月18日入院。
患者入院3 h前被发现神志不清,跌倒在地,呕吐一次,为胃内容物,非喷射性,无咖啡样液体,无抽搐,伴大小便失禁,身边可见药品包装盒,送至当地卫生院,予以洗胃等处理,具体治疗过程不详。
氟马西尼对异氟烷麻醉后苏醒影响分析论文

氟马西尼对异氟烷麻醉后苏醒的影响分析【摘要】目的分析氟马西尼对异氟烷麻醉后苏醒的影响。
方法选用我院79例吸入式全麻醉手术患者,随机分成两组,a组在术后使用氟马西尼催醒,根据监测记录并进行geller和freye清醒评估。
结果证明使用氟马西尼的患者比使用生理盐水的患者能更快地清醒。
结论氟马西尼能促进异氟烷麻醉后苏醒和神经功能恢复,提高患者的苏醒质量。
【关键词】氟马西尼;异氟烷;麻醉;苏醒吸入麻醉是指经呼吸道吸入进入人体内并产生全身麻醉作用的药物。
一般吸入麻醉药中,以异氟烷较为安全,氟烷起效最快。
在医学技术不断发展的时代,各种临床手术中广泛使用吸入式来进行麻醉,从而减少患者的痛苦,但是吸入麻醉的苏醒期神经功能恢复效果并不好,苏醒后存在定向障碍,有术后患者的记忆有遗忘并可能使患者出现烦躁感,不能很快地恢复神经反射。
根据国内的研究报告,氟马西尼可以用于吸入异氟烷麻醉后的催醒[1]本研究分析探讨氟马西尼对异氟烷麻醉患者是否有催醒作用。
现分析报道如下:1 资料与方法1.1 一般资料选用我院2011年3月-2012年3月79例吸入式全麻醉手术患者,其中男47例,女32例,年龄30-45岁,手术时间为2-3h,随机将患者分为两组,氟马西尼组40例(a组)和对照组39例(b组)。
1.2 排除标准所有患者均无严重心脏病史,无长期服用精神疾病药物,无视觉听觉障碍等影响比对的因素。
术前不服用任何药物,并进行术前常规检查。
1.3 麻醉镇痛方法患者进入手术室,首先静脉注射咪唑安定0.04mg/kg,随后注射芬太尼2μg/kg和丙泊酚1.0-2.0mg/kg,进行2mg/kg琥珀胆碱的诱导后气管插管,行间歇正压通气维持petco2为30-40mmhg。
根据手术要求用0.1芬太尼来维持麻醉。
手术后期将异氟烷下降至0.4mac并观察,患者出现4各成串刺激和2个以上肌颤反射后停用异氟烷,并使用0.01mg/kg阿托品0.04普洛色林拮抗肌松。
氟马西尼注射液说明书(美国,FDA,英文)

ROMAZICON®(flumazenil)INJECTIONR x onlyDESCRIPTIONROMAZICON® (flumazenil) is a benzodiazepine receptor antagonist. Chemically, flumazenil is ethyl8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a](1,4) benzodiazepine-3-carboxylate. Flumazenil has an imidazobenzodiazepine structure, a calculated molecular weight of 303.3, and the following structural formula:Flumazenil is a white to off-white crystalline compound with an octanol:buffer partition coefficient of14 to 1 at pH 7.4. It is insoluble in water but slightly soluble in acidic aqueous solutions. ROMAZICON is available as a sterile parenteral dosage form for intravenous administration. Each mL contains 0.1 mg of flumazenil compounded with 1.8 mg of methylparaben, 0.2 mg of propylparaben,0.9% sodium chloride, 0.01% edetate disodium, and 0.01% acetic acid; the pH is adjusted to approximately 4 with hydrochloric acid and/or, if necessary, sodium hydroxide.CLINICAL PHARMACOLOGYFlumazenil, an imidazobenzodiazepine derivative, antagonizes the actions of benzodiazepines on thecentral nervous system. Flumazenil competitively inhibits the activity at the benzodiazepine recognition site on the GABA/benzodiazepine receptor complex. Flumazenil is a weak partial agonistin some animal models of activity, but has little or no agonist activity in man.Flumazenil does not antagonize the central nervous system effects of drugs affecting GABA-ergic neurons by means other than the benzodiazepine receptor (including ethanol, barbiturates, or general anesthetics) and does not reverse the effects of opioids.In animals pretreated with high doses of benzodiazepines over several weeks, ROMAZICON elicited symptoms of benzodiazepine withdrawal, including seizures. A similar effect was seen in adult human subjects.PharmacodynamicsIntravenous ROMAZICON has been shown to antagonize sedation, impairment of recall, psychomotor impairment and ventilatory depression produced by benzodiazepines in healthy human volunteers.The duration and degree of reversal of sedative benzodiazepine effects are related to the dose and plasma concentrations of flumazenil as shown in the following data from a study in normal volunteers.Generally, doses of approximately 0.1 mg to 0.2 mg (corresponding to peak plasma levels of 3 to 6 ng/mL) produce partial antagonism, whereas higher doses of 0.4 to 1 mg (peak plasma levels of 12 to 28 ng/mL) usually produce complete antagonism in patients who have received the usual sedating doses of benzodiazepines. The onset of reversal is usually evident within 1 to 2 minutes after the injection is completed. Eighty percent response will be reached within 3 minutes, with the peak effect occurring at 6 to 10 minutes. The duration and degree of reversal are related to the plasma concentration of the sedating benzodiazepine as well as the dose of ROMAZICON given.In healthy volunteers, ROMAZICON did not alter intraocular pressure when given alone and reversed the decrease in intraocular pressure seen after administration of midazolam.PharmacokineticsAfter IV administration, plasma concentrations of flumazenil follow a two-exponential decay model. The pharmacokinetics of flumazenil are dose-proportional up to 100 mg.DistributionFlumazenil is extensively distributed in the extravascular space with an initial distribution half-life of 4 to 11 minutes and a terminal half-life of 40 to 80 minutes. Peak concentrations of flumazenil are proportional to dose, with an apparent initial volume of distribution of 0.5 L/kg. The volume of distribution at steady-state is 0.9 to 1.1 L/kg. Flumazenil is a weak lipophilic base. Protein binding is approximately 50% and the drug shows no preferential partitioning into red blood cells. Albumin accounts for two thirds of plasma protein binding.MetabolismFlumazenil is completely (99%) metabolized. Very little unchanged flumazenil (<1%) is found in the urine. The major metabolites of flumazenil identified in urine are the de-ethylated free acid and its glucuronide conjugate. In preclinical studies there was no evidence of pharmacologic activity exhibited by the de-ethylated free acid.EliminationElimination of radiolabeled drug is essentially complete within 72 hours, with 90% to 95% of the radioactivity appearing in urine and 5% to 10% in the feces. Clearance of flumazenil occurs primarilyby hepatic metabolism and is dependent on hepatic blood flow. In pharmacokinetic studies of normal volunteers, total clearance ranged from 0.8 to 1.0 L/hr/kg.Pharmacokinetic parameters following a 5-minute infusion of a total of 1 mg of ROMAZICON mean (coefficient of variation, range):C max (ng/mL) 24 (38%, 11-43)AUC (ng·hr/mL) 15 (22%, 10-22)0.8-1.6)V ss (L/kg) 1 (24%,Cl (L/hr/kg) 1 (20%, 0.7-1.4)Half-life (min) 54 (21%, 41-79)Food Effects:Ingestion of food during an intravenous infusion of the drug results in a 50% increase in clearance, most likely due to the increased hepatic blood flow that accompanies a meal.Special PopulationsThe ElderlyThe pharmacokinetics of flumazenil are not significantly altered in the elderly.GenderThe pharmacokinetics of flumazenil are not different in male and female subjects.Renal Failure (creatinine clearance <10 mL/min) and HemodialysisThe pharmacokinetics of flumazenil are not significantly affected.Patients With Liver DysfunctionFor patients with moderate liver dysfunction, their mean total clearance is decreased to 40% to 60% and in patients with severe liver dysfunction, it is decreased to 25% of normal value, compared with age-matched healthy subjects. This results in a prolongation of the half-life to 1.3 hours in patients with moderate hepatic impairment and 2.4 hours in severely impaired patients. Caution should be exercised with initial and/or repeated dosing to patients with liver disease.Drug-Drug Interaction:The pharmacokinetic profile of flumazenil is unaltered in the presence of benzodiazepine agonists and the kinetic profiles of those benzodiazepines studied (ie, diazepam, flunitrazepam, lormetazepam, and midazolam) are unaltered by flumazenil. During the 4-hour steady-state and post infusion of ethanol, there were no pharmacokinetic interactions on ethanol mean plasma levels as compared to placebo when flumazenil doses were given intravenously (at 2.5 hours and 6 hours) nor were interactions of ethanol on the flumazenil elimination half-life found.Pharmacokinetics in Pediatric PatientsThe pharmacokinetics of flumazenil have been evaluated in 29 pediatric patients ranging in age from 1 to 17 years who had undergone minor surgical procedures. The average doses administered were 0.53 mg (0.044 mg/kg) in patients aged 1 to 5 years, 0.63 mg (0.020 mg/kg) in patients aged 6 to 12 years, and 0.8 mg (0.014 mg/kg) in patients aged 13 to 17 years. Compared to adults, the elimination half-life in pediatric patients was more variable, averaging 40 minutes (range: 20 to 75 minutes). Clearance and volume of distribution, normalized for body weight, were in the same range as those seen in adults, although more variability was seen in the pediatric patients.CLINICAL TRIALSROMAZICON has been administered in adults to reverse the effects of benzodiazepines in conscious sedation, general anesthesia, and the management of suspected benzodiazepine overdose. Limited information from uncontrolled studies in pediatric patients is available regarding the use of ROMAZICON to reverse the effects of benzodiazepines in conscious sedation only.Conscious Sedation in AdultsROMAZICON was studied in four trials in 970 patients who received an average of 30 mg diazepam or 10 mg midazolam for sedation (with or without a narcotic) in conjunction with both inpatient and outpatient diagnostic or surgical procedures. ROMAZICON was effective in reversing the sedating and psychomotor effects of the benzodiazepine; however, amnesia was less completely and less consistently reversed. In these studies, ROMAZICON was administered as an initial dose of 0.4 mg IV (two doses of 0.2 mg) with additional 0.2 mg doses as needed to achieve complete awakening, up to a maximum total dose of 1 mg.Seventy-eight percent of patients receiving flumazenil responded by becoming completely alert. Of those patients, approximately half responded to doses of 0.4 mg to 0.6 mg, while the other half responded to doses of 0.8 mg to 1 mg. Adverse effects were infrequent in patients who received 1 mg of ROMAZICON or less, although injection site pain, agitation, and anxiety did occur. Reversal of sedation was not associated with any increase in the frequency of inadequate analgesia or increase in narcotic demand in these studies. While most patients remained alert throughout the 3-hour postprocedure observation period, resedation was observed to occur in 3% to 9% of the patients, and was most common in patients who had received high doses of benzodiazepines (see PRECAUTIONS).General Anesthesia in AdultsROMAZICON was studied in four trials in 644 patients who received midazolam as an induction and/or maintenance agent in both balanced and inhalational anesthesia. Midazolam was generally administered in doses ranging from 5 mg to 80 mg, alone and/or in conjunction with muscle relaxants, nitrous oxide, regional or local anesthetics, narcotics and/or inhalational anesthetics. Flumazenil was given as an initial dose of 0.2 mg IV, with additional 0.2 mg doses as needed to reach a complete response, up to a maximum total dose of 1 mg. These doses were effective in reversing sedation and restoring psychomotor function, but did not completely restore memory as tested by picture recall. ROMAZICON was not as effective in the reversal of sedation in patients who had received multiple anesthetic agents in addition to benzodiazepines.Eighty-one percent of patients sedated with midazolam responded to flumazenil by becoming completely alert or just slightly drowsy. Of those patients, 36% responded to doses of 0.4 mg to 0.6 mg, while 64% responded to doses of 0.8 mg to 1 mg.Resedation in patients who responded to ROMAZICON occurred in 10% to 15% of patients studied and was more common with larger doses of midazolam (>20 mg), long procedures (>60 minutes) and use of neuromuscular blocking agents (see PRECAUTIONS).Management of Suspected Benzodiazepine Overdose in AdultsROMAZICON was studied in two trials in 497 patients who were presumed to have taken an overdose of a benzodiazepine, either alone or in combination with a variety of other agents. In these trials, 299 patients were proven to have taken a benzodiazepine as part of the overdose, and 80% of the 148 who received ROMAZICON responded by an improvement in level of consciousness. Of the patients who responded to flumazenil, 75% responded to a total dose of 1 mg to 3 mg.Reversal of sedation was associated with an increased frequency of symptoms of CNS excitation. Of the patients treated with flumazenil, 1% to 3% were treated for agitation or anxiety. Serious side effects were uncommon, but six seizures were observed in 446 patients treated with flumazenil in these studies. Four of these 6 patients had ingested a large dose of cyclic antidepressants, which increased the risk of seizures (see WARNINGS).INDIVIDUALIZATION OF DOSAGEGeneral PrinciplesThe serious adverse effects of ROMAZICON are related to the reversal of benzodiazepine effects. Using more than the minimally effective dose of ROMAZICON is tolerated by most patients but may complicate the management of patients who are physically dependent on benzodiazepines or patients who are depending on benzodiazepines for therapeutic effect (such as suppression of seizures in cyclic antidepressant overdose).In high-risk patients, it is important to administer the smallest amount of ROMAZICON that is effective. The 1-minute wait between individual doses in the dose-titration recommended for general clinical populations may be too short for high-risk patients. This is because it takes 6 to 10 minutes for any single dose of flumazenil to reach full effects. Practitioners should slow the rate of administration of ROMAZICON administered to high-risk patients as recommended below.Anesthesia and Conscious Sedation in Adult PatientsROMAZICON is well tolerated at the recommended doses in individuals who have no tolerance to (or dependence on) benzodiazepines. The recommended doses and titration rates in anesthesia and conscious sedation (0.2 mg to 1 mg given at 0.2 mg/min) are well tolerated in patients receiving the drug for reversal of a single benzodiazepine exposure in most clinical settings (see ADVERSE REACTIONS). The major risk will be resedation because the duration of effect of a long-acting (or large dose of a short-acting) benzodiazepine may exceed that of ROMAZICON. Resedation may be treated by giving a repeat dose at no less than 20-minute intervals. For repeat treatment, no more than 1 mg (at 0.2 mg/min doses) should be given at any one time and no more than 3 mg should be given in any one hour.Benzodiazepine Overdose in Adult PatientsThe risk of confusion, agitation, emotional lability, and perceptual distortion with the doses recommended in patients with benzodiazepine overdose (3 mg to 5 mg administered as 0.5 mg/min) may be greater than that expected with lower doses and slower administration. The recommended doses represent a compromise between a desirable slow awakening and the need for prompt response and a persistent effect in the overdose situation. If circumstances permit, the physician may elect to use the 0.2 mg/minute titration rate to slowly awaken the patient over 5 to 10 minutes, which may help to reduce signs and symptoms on emergence.ROMAZICON has no effect in cases where benzodiazepines are not responsible for sedation. Once doses of 3 mg to 5 mg have been reached without clinical response, additional ROMAZICON is likely to have no effect.Patients Tolerant to BenzodiazepinesROMAZICON may cause benzodiazepine withdrawal symptoms in individuals who have been taking benzodiazepines long enough to have some degree of tolerance. Patients who had been taking benzodiazepines prior to entry into the ROMAZICON trials, who were given flumazenil in doses over 1 mg, experienced withdrawal-like events 2 to 5 times more frequently than patients who received less than 1 mg.In patients who may have tolerance to benzodiazepines, as indicated by clinical history or by the need for larger than usual doses of benzodiazepines, slower titration rates of 0.1 mg/min and lower total doses may help reduce the frequency of emergent confusion and agitation. In such cases, special care must be taken to monitor the patients for resedation because of the lower doses of ROMAZICON used. Patients Physically Dependent on BenzodiazepinesROMAZICON is known to precipitate withdrawal seizures in patients who are physically dependent on benzodiazepines, even if such dependence was established in a relatively few days of high-dose sedation in Intensive Care Unit (ICU) environments. The risk of either seizures or resedation in such cases is high and patients have experienced seizures before regaining consciousness. ROMAZICON should be used in such settings with extreme caution, since the use of flumazenil in this situation has not been studied and no information as to dose and rate of titration is available. ROMAZICON should be used in such patients only if the potential benefits of using the drug outweigh the risks of precipitated seizures. Physicians are directed to the scientific literature for the most current information in this area.INDICATIONS AND USAGEAdult PatientsROMAZICON is indicated for the complete or partial reversal of the sedative effects of benzodiazepines in cases where general anesthesia has been induced and/or maintained with benzodiazepines, where sedation has been produced with benzodiazepines for diagnostic and therapeutic procedures, and for the management of benzodiazepine overdose.Pediatric Patients (aged 1 to 17)ROMAZICON is indicated for the reversal of conscious sedation induced with benzodiazepines (see PRECAUTIONS: Pediatric Use).CONTRAINDICATIONSROMAZICON is contraindicated:•in patients with a known hypersensitivity to flumazenil or benzodiazepines.•in patients who have been given a benzodiazepine for control of a potentially life-threatening condition (eg, control of intracranial pressure or status epilepticus).•in patients who are showing signs of serious cyclic antidepressant overdose (see WARNINGS). WARNINGSTHE USE OF ROMAZICON HAS BEEN ASSOCIATED WITH THE OCCURRENCE OF SEIZURES.THESE ARE MOST FREQUENT IN PATIENTS WHO HAVE BEEN ON BENZODIAZEPINES FOR LONG-TERM SEDATION OR IN OVERDOSE CASES WHERE PATIENTS ARE SHOWING SIGNS OF SERIOUS CYCLIC ANTIDEPRESSANT OVERDOSE.PRACTITIONERS SHOULD INDIVIDUALIZE THE DOSAGE OF ROMAZICON AND BE PREPARED TO MANAGE SEIZURES.Risk of SeizuresThe reversal of benzodiazepine effects may be associated with the onset of seizures in certain high-risk populations. Possible risk factors for seizures include: concurrent major sedative-hypnotic drug withdrawal, recent therapy with repeated doses of parenteral benzodiazepines, myoclonic jerking or seizure activity prior to flumazenil administration in overdose cases, or concurrent cyclic antidepressant poisoning.ROMAZICON is not recommended in cases of serious cyclic antidepressant poisoning, as manifested by motor abnormalities (twitching, rigidity, focal seizure), dysrhythmia (wide QRS, ventricular dysrhythmia, heart block), anticholinergic signs (mydriasis, dry mucosa, hypoperistalsis), and cardiovascular collapse at presentation. In such cases ROMAZICON should be withheld and the patient should be allowed to remain sedated (with ventilatory and circulatory support as needed) until the signs of antidepressant toxicity have subsided. Treatment with ROMAZICON has no known benefit to the seriously ill mixed-overdose patient other than reversing sedation and should not be used in cases where seizures (from any cause) are likely.Most convulsions associated with flumazenil administration require treatment and have been successfully managed with benzodiazepines, phenytoin or barbiturates. Because of the presence of flumazenil, higher than usual doses of benzodiazepines may be required.HypoventilationPatients who have received ROMAZICON for the reversal of benzodiazepine effects (after conscious sedation or general anesthesia) should be monitored for resedation, respiratory depression, or other residual benzodiazepine effects for an appropriate period (up to 120 minutes) based on the dose and duration of effect of the benzodiazepine employed.This is because ROMAZICON has not been established in patients as an effective treatment for hypoventilation due to benzodiazepine administration. In healthy male volunteers, ROMAZICON is capable of reversing benzodiazepine-induced depression of the ventilatory responses to hypercapnia and hypoxia after a benzodiazepine alone. However, such depression may recur because the ventilatory effects of typical doses of ROMAZICON (1 mg or less) may wear off before the effects of many benzodiazepines. The effects of ROMAZICON on ventilatory response following sedation with a benzodiazepine in combination with an opioid are inconsistent and have not been adequately studied. The availability of flumazenil does not diminish the need for prompt detection of hypoventilation and the ability to effectively intervene by establishing an airway and assisting ventilation.Overdose cases should always be monitored for resedation until the patients are stable and resedation is unlikely.PRECAUTIONSReturn of SedationROMAZICON may be expected to improve the alertness of patients recovering from a procedure involving sedation or anesthesia with benzodiazepines, but should not be substituted for an adequate period of postprocedure monitoring. The availability of ROMAZICON does not reduce the risks associated with the use of large doses of benzodiazepines for sedation.Patients should be monitored for resedation, respiratory depression (see WARNINGS) or other persistent or recurrent agonist effects for an adequate period of time after administration of ROMAZICON.Resedation is least likely in cases where ROMAZICON is administered to reverse a low dose of a short-acting benzodiazepine (<10 mg midazolam). It is most likely in cases where a large single or cumulative dose of a benzodiazepine has been given in the course of a long procedure along with neuromuscular blocking agents and multiple anesthetic agents.Profound resedation was observed in 1% to 3% of adult patients in the clinical studies. In clinical situations where resedation must be prevented in adult patients, physicians may wish to repeat the initial dose (up to 1 mg of ROMAZICON given at 0.2 mg/min) at 30 minutes and possibly again at 60 minutes. This dosage schedule, although not studied in clinical trials, was effective in preventing resedation in a pharmacologic study in normal volunteers.The use of ROMAZICON to reverse the effects of benzodiazepines used for conscious sedation has been evaluated in one open-label clinical trial involving 107 pediatric patients between the ages of 1 and 17 years. This study suggested that pediatric patients who have become fully awake following treatment with flumazenil may experience a recurrence of sedation, especially younger patients (ages 1 to 5). Resedation was experienced in 7 of 60 patients who were fully alert 10 minutes after the start of ROMAZICON administration. No patient experienced a return to the baseline level of sedation. Mean time to resedation was 25 minutes (range: 19 to 50 minutes) (see PRECAUTIONS: Pediatric Use).The safety and effectiveness of repeated flumazenil administration in pediatric patients experiencing resedation have not been established.Use in the ICUROMAZICON should be used with caution in the ICU because of the increased risk of unrecognized benzodiazepine dependence in such settings. ROMAZICON may produce convulsions in patients physically dependent on benzodiazepines (see INDIVIDUALIZATION OF DOSAGE and WARNINGS).Administration of ROMAZICON to diagnose benzodiazepine-induced sedation in the ICU is not recommended due to the risk of adverse events as described above. In addition, the prognostic significance of a patient’s failure to respond to flumazenil in cases confounded by metabolic disorder, traumatic injury, drugs other than benzodiazepines, or any other reasons not associated with benzodiazepine receptor occupancy is unknown.Use in Benzodiazepine OverdosageROMAZICON is intended as an adjunct to, not as a substitute for, proper management of airway, assisted breathing, circulatory access and support, internal decontamination by lavage and charcoal, and adequate clinical evaluation.Necessary measures should be instituted to secure airway, ventilation and intravenous access prior to administering flumazenil. Upon arousal, patients may attempt to withdraw endotracheal tubes and/or intravenous lines as the result of confusion and agitation following awakening.Head InjuryROMAZICON should be used with caution in patients with head injury as it may be capable of precipitating convulsions or altering cerebral blood flow in patients receiving benzodiazepines. It should be used only by practitioners prepared to manage such complications should they occur.Use With Neuromuscular Blocking AgentsROMAZICON should not be used until the effects of neuromuscular blockade have been fully reversed.Use in Psychiatric PatientsROMAZICON has been reported to provoke panic attacks in patients with a history of panic disorder.Pain on InjectionTo minimize the likelihood of pain or inflammation at the injection site, ROMAZICON should be administered through a freely flowing intravenous infusion into a large vein. Local irritation may occur following extravasation into perivascular tissues.Use in Respiratory DiseaseThe primary treatment of patients with serious lung disease who experience serious respiratory depression due to benzodiazepines should be appropriate ventilatory support (see PRECAUTIONS)rather than the administration of ROMAZICON. Flumazenil is capable of partially reversing benzodiazepine-induced alterations in ventilatory drive in healthy volunteers, but has not been shown to be clinically effective.Use in Cardiovascular DiseaseROMAZICON did not increase the work of the heart when used to reverse benzodiazepines in cardiac patients when given at a rate of 0.1 mg/min in total doses of less than 0.5 mg in studies reported in the clinical literature. Flumazenil alone had no significant effects on cardiovascular parameters when administered to patients with stable ischemic heart disease.Use in Liver DiseaseThe clearance of ROMAZICON is reduced to 40% to 60% of normal in patients with mild to moderate hepatic disease and to 25% of normal in patients with severe hepatic dysfunction (see CLINICAL PHARMACOLOGY: Pharmacokinetics). While the dose of flumazenil used for initial reversal of benzodiazepine effects is not affected, repeat doses of the drug in liver disease should be reduced in size or frequency.Use in Drug- and Alcohol-Dependent PatientsROMAZICON should be used with caution in patients with alcoholism and other drug dependencies due to the increased frequency of benzodiazepine tolerance and dependence observed in these patient populations.ROMAZICON is not recommended either as a treatment for benzodiazepine dependence or for the management of protracted benzodiazepine abstinence syndromes, as such use has not been studied. The administration of flumazenil can precipitate benzodiazepine withdrawal in animals and man. This has been seen in healthy volunteers treated with therapeutic doses of oral lorazepam for up to 2 weeks who exhibited effects such as hot flushes, agitation and tremor when treated with cumulative doses of up to 3 mg doses of flumazenil.Similar adverse experiences suggestive of flumazenil precipitation of benzodiazepine withdrawal have occurred in some adult patients in clinical trials. Such patients had a short-lived syndrome characterized by dizziness, mild confusion, emotional lability, agitation (with signs and symptoms of anxiety), and mild sensory distortions. This response was dose-related, most common at doses above 1 mg, rarely required treatment other than reassurance and was usually short lived. When required, these patients (5 to 10 cases) were successfully treated with usual doses of a barbiturate, a benzodiazepine, or other sedative drug.Practitioners should assume that flumazenil administration may trigger dose-dependent withdrawal syndromes in patients with established physical dependence on benzodiazepines and may complicate the management of withdrawal syndromes for alcohol, barbiturates and cross-tolerant sedatives.Drug InteractionsInteraction with central nervous system depressants other than benzodiazepines has not been specifically studied; however, no deleterious interactions were seen when ROMAZICON was administered after narcotics, inhalational anesthetics, muscle relaxants and muscle relaxant antagonists administered in conjunction with sedation or anesthesia.Particular caution is necessary when using ROMAZICON in cases of mixed drug overdosage since the toxic effects (such as convulsions and cardiac dysrhythmias) of other drugs taken in overdose (especially cyclic antidepressants) may emerge with the reversal of the benzodiazepine effect by flumazenil (see WARNINGS).The use of ROMAZICON is not recommended in epileptic patients who have been receiving benzodiazepine treatment for a prolonged period. Although ROMAZICON exerts a slight intrinsic anticonvulsant effect, its abrupt suppression of the protective effect of a benzodiazepine agonist can give rise to convulsions in epileptic patients.ROMAZICON blocks the central effects of benzodiazepines by competitive interaction at the receptor level. The effects of nonbenzodiazepine agonists at benzodiazepine receptors, such as zopiclone, triazolopyridazines and others, are also blocked by ROMAZICON.The pharmacokinetics of benzodiazepines are unaltered in the presence of flumazenil and vice versa. There is no pharmacokinetic interaction between ethanol and flumazenil.Use in Ambulatory PatientsThe effects of ROMAZICON may wear off before a long-acting benzodiazepine is completely cleared from the body. In general, if a patient shows no signs of sedation within 2 hours after a 1-mg dose of flumazenil, serious resedation at a later time is unlikely. An adequate period of observation must be provided for any patient in whom either long-acting benzodiazepines (such as diazepam) or large doses of short-acting benzodiazepines (such as >10 mg of midazolam) have been used (see INDIVIDUALIZATION OF DOSAGE).Because of the increased risk of adverse reactions in patients who have been taking benzodiazepines on a regular basis, it is particularly important that physicians query patients or their guardians carefully about benzodiazepine, alcohol and sedative use as part of the history prior to any procedure in which the use of ROMAZICON is planned (see PRECAUTIONS: Use in Drug- and Alcohol-Dependent Patients).Information for PatientsROMAZICON does not consistently reverse amnesia. Patients cannot be expected to remember information told to them in the postprocedure period and instructions given to patients should be reinforced in writing or given to a responsible family member. Physicians are advised to discuss with patients or their guardians, both before surgery and at discharge, that although the patient may feel alert at the time of discharge, the effects of the benzodiazepine (eg, sedation) may recur. As a result, the patient should be instructed, preferably in writing, that their memory and judgment may be impaired and specifically advised:1.Not to engage in any activities requiring complete alertness, and not to operate hazardousmachinery or a motor vehicle during the first 24 hours after discharge, and it is certain no residual sedative effects of the benzodiazepine remain.2.Not to take any alcohol or non-prescription drugs during the first 24 hours after flumazeniladministration or if the effects of the benzodiazepine persist.。
高龄患者全麻术后氟马西尼对呼吸和认知功能的影响

病多见, 且女『略多于男性, 生 这就是玫瑰糠疹, 一种春季多发的 皮肤病。病因尚不明确 , 病程有 自限陛,以对症治疗为主。 发疹 前, 部分患者有全身不适 、 低热 、 头痛、恶心、食欲
分布时相 t 为 1 mi O n,消 除时相 t 为 1 5 . h . ~2 5 I。氟 马西尼的半衰期 比咪哒 唑仑短 ,拮抗作用 的持 续时间也短。 因此 , 防止患者催醒后再 出现镇静作用 , 除气管 内导管 为 拔 后, 要严 密监测患者 病情 , 确保其 呼吸道通 畅 , 给予常规 吸 氧 ,以免发生意 外。因老年人 的生 理特点 ,在麻醉过 程 中, 麻醉 药物可 酌情减 量 ,防止 药物 的蓄积产 生 中毒 。 本文结果显示 ,与使用安慰剂 的对照组 比较 , 观察组患
2 1年 2 02 月第 1 卷 第 2 9 期
高龄患者全麻术后氟马西尼对呼吸和认知功能的影响
付 梅 苏 ( 磐安县中医 3 30 浙江 院 2 0 2 )
氟马 西尼是苯二氮蕈类( Dz 选择性 拮抗药 ,常用于全 B )
麻 手术后 的催 醒。我们将 氟马西尼用于高 龄患者全麻术后 ,
2 8
斑量 , 2 每 ~3天 1 , 次 可缩短病程。④外用药物。常用炉甘
石洗剂 、5 硫磺 乳剂、氢化可 的松霜等 。 %
( : 01 0— 7 收稿 21— 9 0) ( 发稿编辑 :白兰芳)
4 二期梅毒疹。玫瑰糠疹与二期梅毒的玫瑰糠疹样发疹 .
极其相似。后者皮疹呈铜红或暗红色 , 全身分布 ,以手掌及足
趾有铜红色 圆形脱屑性斑疹为特征;患者可有性乱史 , 外阴部
静脉用药输注装置安全规范专家共识

静脉用药输注装置安全规范专家共识(广东省药学会年月日印发)静脉输液作为现代临床重症治疗中的重要方法,尤其在国内,已成为临床常用的治疗手段之一。
药物与输注装置(输液器)的相容性是药物静脉输液安全的重要影响因素,根据药物的理化特性正确选择输液器是药物有效和安全的重要保障。
临床中,以需要用非(聚氯乙烯)而没有用非材质的输液器;需使用避光用输液器而没有使用;以及没有按药物对输液器的过滤孔径要求选择精密输液器等不合理使用情况最为常见。
为规范输液器的使用,保证患者用药安全有效,广东省药学会有关专家达成以下静脉用药输注装置安全规范共识。
1.临床药物输注中选择输液器材质(和非)应注意的问题传统输液器多以为原料制作,是有氯乙烯在引发剂作用下聚合而成的热塑性树脂。
普通的树脂粉没有应用价值,必须加入增塑剂、稳定剂、润滑剂等方可使用。
临床上使用的输液器具有价格便宜、体积小、重量轻、临床应用方便等优点而得到广泛应用,但在实际应用中也存在诸多严重的问题,主要体现在以下方面。
()对某些药物产生吸附[](或与药物反应);()输液器在生产过程中为增加其柔软性和回弹性,需要加入的增塑剂邻苯二甲酸二(乙基己基)酯(,),而塑化剂的对人体多个系统具有毒性作用。
含有吐温、聚氧乙基蓖麻油、环糊精衍生物、丙二醇、乙醇或苯甲醇作为增溶剂的药物可以加速溶出,从而诱发毒性反应。
国家食品药品监督管理总局《一次性使用输注器具产品注册技术审查指导原则》中明确注明:“聚氯乙烯()常用的增塑剂与脂溶性溶液接触后容易浸出;以增塑的聚氯乙烯()作为原料的产品不宜贮存和输注脂肪乳等脂溶性液体和药物”。
从药物的输注安全性、有效性出发,本共识结合现有的药物说明书、国内外文献报道以及或药物剂型特征,对建议使用非材质输液器输注的药物进行了总结,具体内容详见表。
2.临床药物输注中是否需采用避光输液器的相关要求临床上许多药物如:硝普钠、硝酸甘油、氟罗沙星等等,输注过程中如果受到光照、可加速药物的氧化,引起药物光化降解,不仅降低了药物的效价,而且可产生变色和沉淀,严重影响药物的质量,甚至增加了药物的毒性[]。
氟马西尼

氟吗西尼注射液说明书【药品名称】通用名:氟吗西尼注射液英文名:Flumazenil Injection【成份】本品主要成份为氟吗西尼【性状】本品为无色的澄明液体。
【药理毒理】氟吗西尼是苯二氮卓类药物的拮抗剂,它能竞争性抑制苯二氮卓类药物与受体结合,以阻断其中枢作用。
【药代动力学】氟吗西尼为亲脂性药物,据报对静脉注射此药后约50%与血浆蛋白结合,其消除半衰期为41-79分钟,分别以90-95%和5-10%随尿、粪便排出。
【适应症】逆转全身麻醉手术后因使用苯二氮卓类药物所致的中枢镇静和催眠。
【用法用量】首次剂量:在15秒内将氟吗西尼0.2mg(2ml)静脉注射,60秒后唤醒病人,如达到目的,可不再用药。
追加剂量:首次剂量后60秒钟,如不能唤醒病人,可追加0.1mg(1ml),再等60秒,再唤醒。
每次可追加0.1mg(1ml),总量不超过1mg。
【不良反应】少数病人在应用时,会出现恶心或呕吐,在快速注射氟吗西尼后,会有焦虑、心悸、恐惧等不适感,一过性血压增高及心率增加。
癫痫患者可出现抽搐发作,这些不良反应通常不需要特殊处理。
【禁忌】1、对此药及安定类过敏者,有严重抗抑郁剂中毒症状者。
2、正应用苯二氮卓类药控制癫痫持续状态或颅内压者。
3、有严重抗抑郁剂中毒者。
【注意事项】手术结束时,麻醉师请勿在周围肌肉松弛消退前注射氟吗西尼。
【孕妇及哺乳期妇女用药】尚不明确。
【药物相互作用】氟吗西尼可阻滞佐匹克隆的镇静催眠作用,目前尚未发现其他中枢神经系统抑制剂有相互作用。
在应用氟吗西尼时苯二氮蕈类药物的药代动力学不变。
【规格】5ml:0.5mg【贮藏】遮光,密闭保存。
热疗临床常用麻醉药品

钟 , 用药总量最高达6.3mg.
• 【药理及应用】 • 本品具有典型的苯二氮草类药理活性 , 可产生抗焦 虑、镇静、催眠、抗惊厥及肌肉松弛作用。肌内注射或静
脉注射后, 可产生短暂的顺行性记忆缺失, 使患者不能回忆
起在药物高峰期间所发生的事情。本品作用特点为起效快 而持续时间短。服药后可缩短入睡时间( 一般自服药到入
用。
【药代动力学】
据文献资料 , 氟马西尼为一种亲脂性药物 , 血浆 蛋白结合率约为 50%, 所结合的血浆蛋白中 2/3 为白蛋 白。氟马西尼广泛分布于血管外 , 稳态时的平均分布 容积 (Vss) 为 0.95 升/千克。氟马西尼主要在肝脏代谢。 在血浆和尿中的主要代谢物为羧酸代谢物 , 该主要代 谢物没有苯二氮 类受体激动剂或拮抗剂的活性。氟马 西尼几乎完全 (99%) 通过非肾脏途径消除。药物消除 半衰期为 50~60 分钟。
• 【注意】 • (1)常见的不良反应有低血压、谵妄、幻觉、心悸、皮 疹、过度换气 , 少见不良反应有视物模糊、头痛、头晕、 手脚无力、麻刺感。 • (2) 麻醉或外科手术时 10.8%~23.3% 的病人可有呼吸容量 和呼吸频率降低, 静脉注射可有 15% 的患者发生呼吸抑制。 老年人和长期用药者易出现严 重的呼吸抑制。对呼吸功 能的影响多半由于剂量过高或静脉注射过快所致, 因此静 脉注射时速度勿过快。慎用于注射用药, 器质性脑损伤、 严重呼吸功能不全者、老年人或循环系统疾病患者, 用药 后 3 小时内留院观察。
• 【用法】 • 口服 : 治疗失眠症 , 每次 15 mg , 睡前服。 • 肌内注射 : 术前 20~30 分钟注射 , 成人一般为 10~15mg( 每公斤体重 0.10~0.15mg) 。可单用亦可与镇痛药合用。儿童剂量可稍高, 每公斤 体重 0.15~ 0.2mg 。作儿童诱导麻醉时 , 用本品 5~10mg( 每公斤体重 0.15~0.2mg) 与氯胺酮 50~100mg( 每公斤体重 8mg) 合用。 • 静脉注射: 术前准备 , 术前 5~10分钟注射 2.5~5mg( 每公斤体重 0.05~0.1mg),可单用或与抗胆碱药合用。用于诱导麻醉 , 成人为 1015mg( 每公斤体重 0.15~0.2mg), 儿童为每公斤体 重 0.2 mg。用于维持 麻醉, 小剂量静脉注射, 剂量和时间间隔视病人个体差异而定。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
氟马西尼注射液
【药品名称】
通用名称:氟马西尼注射液
英文名称:Flumazenil Injection
【成份】
活性成份:氟马西尼; 辅料:乙二胺四乙酸二钠、乙酸、氯化钠、注射用水。
氟马西尼化学名为8-氟-5,6-二氢-5-甲基-6-氧代-4H-咪唑并-[1,5-a][1,4]苯并二氮卓-3-甲酸乙酯。
【适应症】
临床可用于麻醉后苯并二氮杂过量或中毒及癫痫发作等。
【用法用量】
静注:每次0.3mg~0.6mg。
【不良反应】
1.少数患者在麻醉时用药,会出现面色潮红、恶心和/或呕吐。
在快速注射氟马西尼后,偶尔会有焦虑、心悸、恐惧等不适感。
这些副作用通常不需要特殊处理。
2.有癫痫病史或严重肝功能不全的人群中,尤其是在有苯二氮卓类长期用药史或在有混合药物过量的情况下,使用该药有癫痫发作的报道。
3.在混合药物过量的情况下,特别是环类抗抑郁药过量,使用本品来逆转苯二氮卓类的作用可能引起不良反应(例如惊厥和心率失常。
)
4.有报道此类药物对有惊恐病史的患者可能诱发惊恐发作。
5.对长期应用苯二氮卓类药物并【禁忌】
1.对此药及安定类过敏者,有严重抗抑郁剂中毒症状者禁用;
2.正应用苯二氮类药控制癫痫持续状态或颅内压者禁用;
3.有严重抗抑郁剂中毒者禁用。
【注意事项】
长期服用苯并二氮杂者慎用。
【药物相互作用】
氟马西尼可阻断经由苯二氮卓类受体作用的非苯二氮卓类药物如佐匹克隆和三唑并哒嗪的作用。
苯二氮卓类受体激动剂的药代动力学不受氟马西尼影响,反之亦然。
酒精与氟马西尼无相互作用。
【药理作用】
氟马西尼是苯二氮卓类受体拮抗剂,它通过竞争性抑制苯二氮卓类与其受体反应从而特异性阻断其中枢神经作用。
【贮藏】
遮光,密闭保存。
【有效期】
24个月
【批准文号】
国药准字H20056593
【生产企业】
企业名称:海南灵康制药有限公司
生产地址:海口金盘工业开发区美国工业村第3-6号厂房。
