fugene 6转染试剂
fugene 4k 转染试剂 中文说明书

2022版 CTM694原英文技术手册TM694中 文 说 明 书适用产品目录号:E5911和E5912FuGENE ®4K TransfectionReagent普洛麦格(北京)生物技术有限公司Promega (Beijing) Biotech Co., Ltd 地址:北京市东城区北三环东路36号环球贸易中心B座907-909电话:************网址:技术支持电话:400 810 8133技术支持邮箱:*************************CTM 6942022制作1所有技术文献的英文原版均可在/ protocols 获得。
请访问该网址以确定您使用的说明书是否为最新版本。
如果您在使用该试剂盒时有任何问题,请与Promega 北京技术服务部联系。
电子邮箱:*************************1. 描述 (2)2. 产品组分和储存条件 (2)3. 一般注意事项 (2)3. A. 转染试剂与DNA的比例 (3)3. B. DNA (3)3. C. 时间 (3)3. D. 血清 (3)3. E. 细胞培养条件 (3)3. F. 稳定转染 (3)4. 推荐操作步骤 (4)4. A. 细胞铺板 (5)4. B. FuGENE® 4K Transfection Reagent准备 (5)4. C. 一般转染操作步骤 (6)4. D. 稳定转染操作步骤 (8)4. E. 转染优化 (9)4. F. 报告基因活性和细胞健康的多重检测方案 (11)5. 疑难解答 (12)FuGENE® 4K Transfection Reagent普洛麦格(北京)生物技术有限公司Promega (Beijing) Biotech Co., Ltd 地址:北京市东城区北三环东路36号环球贸易中心B座907-909电话:************网址:技术支持电话:400 810 8133技术支持邮箱:*************************CTM 6942022制作21. 描述FuGENE® 4K Transfection Reagent是一个多组分,非脂质体试剂,用于将DNA高效、低毒地转染至多种哺乳动物细胞系中,无需在加入试剂-DNA复合物后更换培养基。
各种转染试剂中文说明

FuGENE6(Roche)转染步骤:转染前一天将细胞分至培养板,转染当天细胞应50-80%融合。
将细胞以1-3×105/2ml接种于6孔板后孵育过夜将达到如此密度。
将FuGENE6 Reagent在室温孵育10-15分钟。
使用之前将FuGENE6颠倒混匀一下。
1.在PCR管中加入不含血清和双抗的营养液以稀释FuGENE6,直至总体积到100ul。
2.将3-6ul FuGENE6 Reagent直接加入营养液,轻弹管壁混合。
3.加入1-2ug的DNA溶液(0.02-2.0ug/ul),轻弹管壁混合。
4.室温孵育20分钟。
5.将6孔板中的旧营养液吸出,加入约1ml不含血清和双抗的营养液洗涤一次,再加入2ml不含血清和双抗的营养液。
6.将转染复合物加入细胞,混匀使之均匀分布。
7.3-8小时后,加入血清或换成含血清的营养液。
Lipofectamine 2000(Invitrogen)转染试剂转染步骤(6孔板):1.转染前一天,胰酶消化细胞并计数,细胞铺板,使其在转染日密度为90-95%。
细胞铺板在2ml含血清,不含抗生素的正常生长的培养基中。
2.对于每孔细胞,使用250ul无血清培养基(如OPTI-MEM I培养基)稀释4.0ugDNA,轻轻混匀。
3.使用前将Lipofectamine 2000转染试剂轻轻混匀,用250ul无血清培养基(如OPTI-MEM I培养基)稀释10ul Lipofectamine 2000转染试剂,轻轻混匀。
Lipofectamine 2000稀释后,在5分钟内同稀释的DNA混合(<30分钟)。
NOTE:若使用DMEM培养基,则需在5分钟内同稀释的DNA混合。
4.混合稀释的DNA(第二步)和稀释的Lipofectamine 2000(第三步)。
室温放置20分钟。
5.(optional)将6孔板中的旧营养液吸出,用无血清培养基清洗两次。
加入2ml无血清配养基。
各生物公司介绍-中文

但其拳头产品主要是:激酶和常规抗体
CST
• Cell Signaling Technology(CST)是最知名的和老牌的信号转导公司之一,专 注于信号转导产品的提供和研究。产品具有种类多、质量好、价格低、引用
文献多等优点,是您进行信号转导研究不可多得的帮手!
• 主要产品: 抗体类:提供超过1300种磷酸化和非磷酸化抗体,具有最多的磷酸化抗体, 同时提供sample kit和phosphoPlus Ab kit ELISA试剂盒:PathScan Sandwich ELISA Kit系列产品是检测蛋白磷酸化水 平的ELISA试剂盒,灵敏度高,操作简单 WB用蛋白Marker:CST的Biotinylated Protein Ladder Detection Pack产品 包括有10个条带、大小在10-200Kda之间的生物素标记蛋白标准和HRP藕联 的抗生物素二抗(anti-biotin antibody, HRP-linked),有大小两种包装可选 kinase assay kit:试剂盒组成合理全面,非同位素操作,应用起来简单方便。 buffer类:共有6个buffer类,是进行普通WB或磷酸化WB检测的必须辅助试 剂,用于标本蛋白的制备。 激酶类:多种形式的蛋白激酶,可以用于药物筛
•
转染试剂和报告基因分析 转染试剂Fugene 6,升级版本Fugene HD和报告基因分析产品联合使用是您进行基因调控研究的最 佳搭档。
蛋白酶抑制剂 提供多种蛋白酶抑制剂和详尽的实验操作方法,更有新推出的EasyPack包装,操作更方便,降低 污染风险。
快速翻译系统RTS:蛋白表达 采用独特的持续交换能量的Cell-Free蛋白表达系统,可以持续高表达各种类型的蛋白。
TaKaRa
FuGENE HD转染试剂说明书

FuGENE ®HD Transfection Reagent1.What this Product DoesNumber of Transfection ExperimentsIn a typical experiment using HeLa or COS-1 cells, 1 ml of FuGENE ®HD Transfection Reagent can be used to perform up to three hundred transfections in 35-mm tissue-culture dishes, using 3 l of reagent combined with 1 – 2 g DNA per well. This is equivalent to over 6,000wells in a 96-well plate or 1,000 wells in a 24-well plate.L Optimal expression depends upon experimental conditions includ-ing cell type, passage history, confluence, seeding protocol, com-plex incubation time, serum batch, etc. The above amounts of reagents work well with HeLa or COS-1 cells. In other test systems,two- to three-fold higher amounts of reagent yield optimal levels of expression.FormulationFuGENE ® HD Transfection Reagent is a proprietary blend of lipids and other components supplied in 80% ethanol, sterile-filtered, and pack-aged in glass vials. It does not contain any ingredients of human or animal origin.Storage and Stability•FuGENE ® HD Transfection Reagent is shipped at +15 to +25°C.•Store FuGENE ® HD Transfection Reagent at +2 to +8°C, with the lid very tightly closed. The reagent is stable through the expiration date printed on the label when stored under these conditions.L FuGENE ® HD Transfection Reagent remains fully functional evenafter repeatedly opening the vial (at least five times over a two-month period) as long as the vial is tightly recapped and stored at +2 to +8°C between uses.L Do not store FuGENE ® HD Transfection Reagent below 0°C.Components may precipitate and alter results. If you accidently place the reagent at Ϫ20°C, briefly warm it to 37°C to dissolve any precipitate. It should function normally; however, do not return it to the freezer.Special HandlingN Always bring to room temperature and mix FuGENE ® HD Transfec-tion Reagent prior to use (vortex for one second or use inversion).N Do not aliquot FuGENE ® HD Transfection Reagent from the originalglass vials. Chemical residues in plastic vials can significantly decrease the biological activity of the reagent. Minimize the contact of undiluted FuGENE ® HD Transfection Reagent with plastic sur-faces.N Always dilute the reagent by pipetting directlyinto serum-free medium. Do not allow the FuGENE ® HD Transfection Reagent to contact the plastic walls of the tube containing the serum-free medium during the dilution step.N Do not use siliconized pipette tips or tubes.Additional Equipment and Reagents RequiredAdditional reagents and equipment required to perform transfection assays using FuGENE ® HD Transfection Reagent, but not provided,include:General Laboratory Equipment•standard cell culture equipment (e.g., biohazard hoods, incubators,microscope)•standard pipetters and micropipetters •vortex mixerFor Plasmid Preparation•purified plasmid stock (0.1 g/l – 2.0 g/l) in sterile TE (10 mM Tris, 1 mM EDTA, pH 8.0) buffer or sterile water•Genopure Plasmid Midi Kit*, Genopure Plasmid Maxi Kit*, or High Pure Plasmid Isolation Kit* can be used to prepare plasmid.For Verification of Vector Function •assay appropriate for transfected gene•G-418* or Hygromycin B* (optional; for stable transfection experi-ments)For Transfection-Complex Formation•Opti-MEM I Reduced Serum Medium, water, or serum-free medium •24-well plate to serve as test tube rack for FuGENE ® HD Transfec-tion Reagent vial•sterile polystyrene tubes or round-bottom 96-well platesCells Growing in Log Phase•select subconfluent cultures in log phase for preparation of the cell cultures for transfection•method to quantify cell number to reproducibly plate the same number of cellsApplicationFuGENE ® HD Transfection Reagent is a multi-component reagent that forms a complex with DNA, then transports the complex into animal or insect cells. Benefits of FuGENE ® HD Transfection Reagent include:•High transfection efficiency in many common cell types, including HeLa, NIH/3T3, COS-1, COS-7, CHO-K1, Hep G2, HEK-293, MCF7,and some insect cell lines. In addition, you will achieve excellent transfection efficiency in some cell lines (e.g., RAW) that are not transfected well by other reagents. Detailed transfection protocols and sample results are available at .•Demonstrates minimal cytotoxicity or changes in morphology when adequate numbers of cells are transfected, and eliminates the requirement to change media after the addition of transfection complex.•Suitable for transient and stable transfection.•Functions exceptionally well in the presence or absence of serum;eliminates the need to change media.To ensure the quality of cells to be transfected, Roche recommends using freshly-obtained, low-passage cell sines form ATCC ®. For more information please visit and bookmark .For the transient and stable transfection of animal and insect cellsCat. No. 04 709 691 0010.4 ml (up to 120 transfections)Version November 2007Cat. No. 04 709 705 001 1 ml (up to 300 transfections)Store at +2 to +8°CCat. No. 04 709 713 001 1)Mega-pack 5 × 1 ml (up to 1,500 transfections)Cat. No. 05 061 369 00110 ml (up to 3,000 transfections)Cat. No. 04 883 560 001Trial-pack1)The five vials are packaged together in one box with one pack insertFor life science research only. Not for use in diagnostic procedures. FOR IN VITRO USE ONLY.2.How to Use this Product2.1Before you BeginRequired Amount of FuGENE® HD Transfection ReagentFor initial optimization experiments, transfect a monolayer of cells that is 80 – 90% confluent in a six-well culture dish, using 3:2, 4:2, 5:2, 6:2, 7:2, and 8:2 ratios of FuGENE® HD Transfection Reagent (l) to DNA (g), respectively. For most cell types, these FuGENE® HD Transfection Reagent:DNA ratios provide excellent transfection levels.L Subsequent optimization may further increase efficiency in your particular application. In addition to varying the volume of FuGENE® HD Transfection Reagent, other parameters may be evaluated (see section 2.6, Parameters for Optimization, and sec-tion 3, Troubleshooting).Plasmid DNA•It is critical to accurately determine the plasmid DNA concentration using 260-nm absorption (estimates of DNA content based on the intensity of gel bands are not sufficiently accurate). Determine the DNA purity using a 260 nm/280 nm ratio; the optimal ratio is 1.8.•Prepare the plasmid DNA solution in sterile TE (Tris/EDTA) buffer or sterile water at a concentration between 0.1 g/l and 2.0 g/l. Cell Culture ConditionsMinimize both intra- and inter-experimental variance in transfection efficiency by using cells that are regularly passaged, proliferating well (best when in a log-growth phase), and plated at a consistent den-sity. FuGENE® HD Transfection Reagent is different from FuGENE® 6 Transfection Reagent regarding the optimal density of cells required for maximal expression with minimal negative effect; FuGENE®6 Transfection Reagent is formulated to work at low cell densities, whereas FuGENE® HD Transfection Reagent is formulated to work at higher cell densities. Cells must be healthy and free of mycoplasma and other contaminants.L If you have used FuGENE® 6 Transfection Reagent in the past, we suggest that you increase the plating density for initial tests using FuGENE® HD Transfection Reagent. For most cell lines, use the reagent at cell-plating densities at least twice that used with FuGENE® 6 Transfection Reagent to yield maximum protein expression. For most cell lines, cultures should be 80 – 90% con-fluent at the time of transfection. For contact-inhibited cell lines such as NIH/3T3, optimal results are obtained when cells are plated at lower densities.Other Media AdditivesIn some cell types, antimicrobial agents (e.g., antibiotics and fungicides) that are commonly included in cell-culture media may adversely affect the transfection efficiency of FuGENE® HD Transfection Reagent. If possible, exclude additives for initial experi-ments. Once high-efficiency conditions have been established, these components can be added back while monitoring your transfection results. Cell growth and/or transfection efficiency may be affected by variations in sera quality or media formulations.Verification of Vector FunctionOptimize transfection conditions with a known positive-control reporter gene construct prior to transfecting cells with a new vector construct:•Determine transfection efficiency with a reporter gene assay (CAT*,-Gal*, Luciferase*, SEAP*, or hGH*).•Sequence across the flanking vector insert regions to verify the integrity of your new construct.2.2Preparation of Cells for Transfection2.3Overview of Initial Transfection ExperimentAdherent and Suspension Cells in a Six-well Plate or 35-mm Culture DishFor initial optimization, test FuGENE® HD Transfection Reagent:DNA ratios of 3:2, 4:2, 5:2, 6:2, 7:2, and 8:2 (l for FuGENE® HD Transfection Reagent, and g for DNA, respectively). The preparation of the com-plex for a single well of a six-well plate, or a 35-mm culture dish, is described in section 2.4. These ratios will function very well for com-monly used adherent cells and suspension cells. For your particular cell line and culture conditions you may find that ratios of 9:2, 10:2, 11:2, or 12:2 result in even greater expression. Try these ratios if you find the highest expression levels in the 8:2 ratio well.N Prepare the transfection complex in diluent that does not contain serum (e.g., Opti-MEM I Reduced Serum Medium), even if the cells are transfected in the presence of serum. For some cell lines, the complex may be formed in DMEM or sterile water.L For additional optimization tips, see section 2.6 and visit /fugene/hdRatio OverviewPreparation of a transfection complex that is sufficient for a 35-mm culture dish, or one well of a six-well plate, at six different ratios: Tab. 1: Preparation of transfection complex for a 35-mm culture dish.Cell Type ProcedureAdherentcellsOne day before the transfection experiment, trypsinizethe monolayer, adjust cell concentration, and plate thecells in the chosen cell-culture vessel. For most celltypes, plating 3 – 6 × 105 cells in 2 ml of medium in a35-mm culture dish (or six-well plate) overnight willachieve the desired density of Ͼ80% confluency atthe time of transfection. For cell lines with specialcharacteristics, such as contact-inhibited NIH/3T3cells, a lower plating density should be used. If usingculture plates of a different size, adjust the total num-ber of cells, starting volume of FuGENE® HD Transfec-tion Reagent, and the starting mass of DNA inproportion to the relative surface area (Table 2). SuspensioncellsUse freshly passaged cells at a concentration of 5×105/ml to 1 × 106/ml in 2 ml of medium in a 35-mmculture dish (or six-well plate). If using culture platesof a different size, adjust the total number of cells,starting volume of FuGENE® HD TransfectionReagent, and the starting mass of DNA in proportionto the relative volume (Table 2).Tubelabel(ratio)Diluent(l)FuGENE® HDTransfectionReagent (l)DNA(g)Comments3:210032Add the entire volume toa 35-mm culture dish oreach well of a six-wellplate, or 2 – 15 l to eachwell of a 96-well plate.Suggested volumes fordifferent culture vesselsare included in Table 2. 4:2100425:2100526:2100627:2100728:2100822.4Transfection ProcedureNotes:L As with any experiment, include appropriate controls. Preparewells with cells that remain untransfected, cells with transfection reagent alone, and cells with DNA alone.L For stable transfection experiments, the complex-containingmedium should be left unchanged until the cells need to be pas-saged. At that time, include the appropriate selection antibiotics (G 418* or Hygromycin B*).L To prepare transfection complexes for different-sized containers orparallel experiments, proportionally change the quantity of all components according to the total surface area of the cell culture vessel being used (Table 2).L For ease-of-use when transfecting small volumes, as in 96-wellplates containing 0.1 ml culture medium per well, prepare 100 l of transfection complex and add 2 – 15 l to each well depending upon the cell type.L The optimal ratio of transfection reagent:DNA and the optimaltotal amount of complex may vary with cell line, cell density, day of assay, and gene expressed.L After performing the optimization experiment where several ratioswere tested, select a ratio in the middle of the plateau for future experiments.2.5Cotransfection ExperimentsSuggestionsFor cotransfection experiments with FuGENE ® HD Transfection Reagent, maintain the same total reagent:total DNA ratio as that used for a single plasmid in your system. Thus, the total amount of the plas-mid DNA should be equal to the amount of plasmid used in a single plasmid transfection.ᕡAllow FuGENE ® HD Transfection Reagent, DNA, anddiluent to adjust to +15 to +25°C. Vortex for one second or invert the FuGENE ® HD Transfection Reagent vial to mix.ᕢDilute DNA with appropriate diluent, for example, Opti-MEM IReduced Serum Medium, serum-free medium (without anti-biotics or fungicides), or sterile water to a concentration of 2g plasmid DNA/100 l Opti-MEM (0.02g/l).L For insect cells, use sterile water as diluent. For other celllines, try sterile water or serum-free medium as an alterna-tive diluent.ᕣPlace 100 l diluent, containing 2 g DNA into each of six ster-ile tubes labeled 3:2, 4:2, 5:2, 6:2, 7:2, and 8:2.Recommendation : Use sterile polystyrene tubes or round-bottom, 96-well plates to form the transfection complex.L Due to manufacturer variability with release agents for96well plates, we suggest using tissue culture treated 96well plates to reduce variablity.ᕤForm the transfection complex by adding FuGENE ® HDTransfection Reagent to tubes containing diluted DNA :Pipet the FuGENE ® HD Transfection Reagent (3, 4, 5, 6, 7, or 8l) directly into the medium containing the diluted DNA with-out allowing contact with the walls of the plastic tubes.N To avoid adversely affecting transfection efficiency, do notallow undiluted FuGENE ® HD Transfection Reagent to come into contact with plastic surfaces (such as the walls of the tube that contains the serum-free medium) other than pipette tips. Do not use siliconized pipette tips or tubes.ᕥMix and incubate the transfection complex :Vigorously tap the tube or vortex for one to two seconds to mix the contents. If using a 96-well plate, place the plate on a rotat-ing shaker for 5 – 10 seconds. Incubate the transfection reagent:DNA complex for 15 minutes at room temperature.For some ratios and cell types, incubation is not necessary for optimal complex formation, while a longer incubation time is better for other cell types. Determine this for your particular cell line and the ratio you use.ᕦAdd the transfection complex to cell s:Remove culture vessel from the incubator. Removal of growth medium is not necessary. Add the transfection complex to the cells in a drop-wise manner or add below the surface of the medium. Swirl the wells or flasks to ensure distribution over the entire plate surface. Use of a rotating platform shaker for 30 seconds at low speed provides adequate mixing for 96-well plates.Once the FuGENE ® HD Transfection Reagent:DNA complex has been added to the cells, there is no need to remove and replace with fresh medium (as is necessary with some other transfection reagents).L In our experience, the exposure of most common laboratorycell types (COS-1, CHO-K1, HEK-293, HeLa, Hep G2, MCF-7) to the transfection complex until performance of the gene expression assay (24–48 hours later) does not affect the results. If you desire to transfect cells that are in serum-free medium during the transfection process, then replace the medium with serum-containing medium 3 – 8 hours after transfection, unless the cells normally grow in serum-free medium. ᕧIncubate cells and assay the results :Following transfection, incubate the cells for 18 – 72 hours prior to measuring protein expression. The length of incubation depends upon the transfected vector construct, the cell type being transfected, the cell medium, cell density, and the type of protein being expressed. After this incubation period, measure protein expression using an assay that is appropriate for your system.L If you observe low transfection levels or more than10 – 30% cell death, refer to section 3, Troubleshooting and /fugene/hd2.6Parameters for Optimization2.7Transfection of Adherent Cells Adapted for Suspension Growth•In some cases, adherent cells may be adapted for suspension growth, thus enabling the production of transiently transfected cells on a very large scale.•HEK-293 cells grown in suspension in serum-free medium that did not contain heparin or dextran sulfate produced significant amounts of protein following transfection.2.8Guidelines for Preparing FuGENE® HD Transfection Reagent:DNA Complex for Various Culture Vessel SizesThe starting volume and mass to add to the different culture vessels is based upon preparing a 100-l transfection complex as described in sec-tions 2.3 and 2.4. For best results, prepare a 100-l complex at different ratios and add varying amounts of each ratio when optimizing. The amounts below are based on the 100-l complex as prepared in sections 2.3 and 2.4.Suggested seeding density for adherent cells = 30,000 – 70,000 cells per cm2Suggested seeding density for suspension cells = 250,000 – 500,000 cells per mlTab. 2: Refer to the table below when setting up your transfection reac-tions. T hese are suggested seeding densities and are media, passage level, laboratory, and cell-line dependent. It is critical that log phase cultures are selected for subculture for the transfection experiments, and that cultures are seeded at the proper density for the transfection experiment. Observe cultures and plate them so that the monolayer is 80–90% confluent at the time of trans-fection. This must be determined empirically. For some cell lines, 60–80% con-fluency is sufficient. However, a contact-inhibited cell line, such as NIH/3T3, should be plated at lower confluence due to its growth characteristics.1) Scale up total volume for larger vessels.3.TroubleshootingParameter to beoptimizedProcedureFuGENE® HD Transfection Reagent:DNA ratio Form the transfection complex at several ratios: 3:2, 4:2, 5:2, 6:2, 7:2, 8:2, 10:2, and 12:2 (l FuGENE® HD Transfec-tion Reagent: g DNA).In some systems, altering the ratio of FuGENE® HD Transfection Reagent to DNA can increase the level of protein expression.L It has been reported that for some plasmid preparations, a ratio of 2:2 yielded optimal results. This is unusual and may reflect some property of the plasmid preparation rather than a characteristic of the FuGENE® HD Transfec-tion Reagent.Amount of transfectioncomplex addedTry adding 200%, 150%, 75%, 50%, and 25% of the amount of 100-l transfection complex suggested in Table 2.Number of cells plated Plating more cells will overcome negative growth effects of excess transfection complex. For cells with special growth characteristics, such as NIH/3T3 cells, do not use this as the first parameter for optimization.Incubation time for the transfection complex to form Vary the length of incubation time for transfection-complex formation: add the complex to the cells immediately after the components are combined and mixed, and then at several intervals up to 40 minutes (i.e., 0, 15, 25, and 40 min-utes). We have observed that in some cell lines, the transfection-complex incubation time tends to have no effect on results when using higher ratios; however, results using lower-ratio-complexes varied depending on the incubation time for complex formation.Special tips for sensitive cell lines •Reduce the time of exposure to the transfection complex (2–3 hours maximum), then replace the medium.•Use the lower ratios, and allow the complex to form for a longer period of time (determine empirically for your cell line), then add lower amounts of the complex (50% or less of what was originally tested).Culture vessel Surfacearea(cm2)TotalvolumeofmediumSuggested seeding densitySuggested amount ofthe 100-l trans-fection complex toadd to each well (l)Final amount of FuGENE® HDTransfection Reagent (l) ineach well following addition ofsuggested amount of 100-ltransfection complexCells/wellAdherent cellsCells/wellSmall or suspensioncellsUsing the3:2 ratioUsing the8:2 ratio totalvolumevolume forlargerlow high low high96-well plate(1 well)0.30.110,00020,00025,00050,00050.150.424-well plate(1 well)1.90.550,000125,000250,000500,000250.752.012-well plate(1 well)3.8 1.0100,000250,000375,000750,00050 1.54.0 35-mm dish82200,000500,000500,0001,000,000100 3.08.06-well plate(1 well)9.42200,000600,000500,0001,000,000100 3.08.0 60-mm dish215500,0001,400,0001,250,0002,500,000250 1)7.520.0 10-cm dish55101,500,0003,500,0002,500,0005,000,000500 1)15.040.0 T-25 flask256700,0001,700,0001,500,0003,000,000300 1)9.024.0 T-75 flask75202,000,0005,000,0005,000,00010,000,000900 1)27.072.0Low trans-fection efficiency Poor quality orinsufficient quantityof nucleic acidsVerify the amount, purity, and sequence of nucleic acid.Perform a control transfection experiment with a commercially available transfection-grade plasmidpreparation.Chemical contaminants may be in the plasmid preparation. Avoid phosphate buffers until you havetested them in your system.L Endotoxins are reported to be cytotoxic to some very sensitive cell lines.Insufficient numberof cellsUse adherent cells that are at least 80% confluent. Low cell density results in fewer cells available totake up transfection complex, and excess complex may be cytotoxic; in addition, fewer cells yieldless protein.Too many cells orcells post log phaseWhen confluent cultures are subcultured, or cells are plated at too high a density, the cells fail to dividein the culture being transfected. This results in suboptimal expression.Suboptimal FuGENE®HD TransfectionReagent:DNA ratio,complex incubationtime, total amount oftransfection complexadded, or cell densityOptimize the FuGENE® HD Transfection Reagent:DNA ratio, complex incubation time, amount ofcomplex added to cells, and cell density, according to the following procedure:Day before transfection:Prepare two 96-well plates of cells at high and low seeding densities (see Table 2 for suggestions).Day of transfection:•Form 200 l of transfection complex at ratios of 2:2, 3:2, 4:2, 5:2, 6:2. 7:2, and 8:2 (l transfectionreagent:g DNA) following the protocol in this pack insert (sections 2.3, 2.4) and doubling theamounts of all components.•As soon as the complexes are combined and mixed, add 10, 5, or 2.5 l of each complex to one of3columns of cells in each 96-well plate (i.e., columns 2, 3, and 4). Leave all outer wells empty ascontrols.•Continue to incubate the complexes at room temperature. After an additional 10 – 15 minutes, add 10,5, or 2.5 l of each complex to the next 3 columns (5, 6, and 7) of cells in each 96-well plate.•Continue to incubate the complexes at room temperature. After an additional 10 – 15 minutes, add 10,5, or 2.5 l of each complex to the next 3 columns (8, 9, and 10) of cells in each 96-well plate.•Assay the plates 1–2 days later. Select the ratio, amount of complex, and time of transfection-complexincubation that resulted in optimal expression.•If optimal transfection occurs at the higher ratios, repeat this process using ratios of 6:2, 7:2, 8:2, 10:2,12:2, and 14:2. Add 5, 10, and 15 l of complex. We have never successfully transfected cells using theratio of 2:2, but it has been reported that some plasmid preparations transfect at this ratio.See section 2.6, Optimization of FuGENE® HD Transfection Reagent:DNA ratio, for more information andvisit /fugene/hdFuGENE® HD Trans-fection Reagent wasaliquotedCheck that FuGENE® HD Transfection Reagent is stored in the original container. If the reagent wasaliquoted into plastic containers, there is a high chance of inactivation. Make sure the reagent isimmediately mixed with the dilute DNA either by vortexing or pipetting up to 10 – 15 times.FuGENE® HD Trans-fection Reagent cameinto contact withplastic or wasinadequately mixedRepeat transfection, carefully pipetting FuGENE® HD Transfection Reagent directly into the serum-freemedium, being careful not to touch the sides of the container while adding the FuGENE® HD Transfec-tion Reagent to the diluted DNA. If the FuGENE® HD Transfection Reagent is added too gently, it maylayer on top of the medium, thus making contact with the plastic.Transfection complexwas formed in serum-containing mediumCheck original bottle of medium used for complex formation. Repeat experiment using new bottle ofOpti-MEM that does not contain any additives (e.g., serum, antibiotics, growth enhancers, heparin,dextran sulfate, etc.). Try forming the complex in sterile water or plain DMEM.Media and mediacomponentsDifferent media and media components may influence the level of transfection efficiency andsubsequent growth of the transfected cells, as well as expression of the recombinant protein. Some lotsof sera have been reported to interfere with optimal transfection.Quality and/or lot-to-lot differences that affect transfection experiments have been noted in both seraand media. Check that the medium and/or serum is from the same lot that worked previously. Try newlots or a different vendor.Culture may becontaminated withmycoplasmaCultures contaminated with mycoplasma have been shown to have decreased transfection efficacy.Determine if culture is contaminated with mycoplasma; use the Mycoplasma Detection Kit* orMycoplasma PCR ELISA* to assess contamination.Inconsistent results Ratio or amount oftransfection complexis at the edge ofperformance plateauInitial experiments should be completed to determine the ratios, amount of complex to be added, andlength of time for complex formation for optimal performance. In our experience, we have found the pla-teau to be relatively broad. We recommend that future experiments be performed with ratios, incubationtime, and amounts of complex that were in the middle of the plateau. If conditions are selected at theedge of the plateau, very small procedural differences may cause large differences in the resulting pro-tein expression. Increased consistency may be achieved by shifting parameters away from the edge ofthe plateau to the middle of the plateau.Transfection complexformation:timing,amounts, and ratioFormation of the complex involves a multifaceted interaction between the transfection reagent and DNAas well as biological parameters. Differences in any of the components or techniques may result ininconsistencies. If results do not meet your expectations, then repeat the optimization experimentselecting areas near the plateau found in previous experiments. For current experiments, determine ifyou should use a different ratio, length of time, or amount of complex for more consistent transfectionresults.Extensive testing of the FuGENE® HD Transfection Reagent is performed on two cell lines: one easy totransfect and one very difficult to transfect. All reagent lots must pass this rigorous testing before wemake it available to you. However, we cannot test all cell lines, media, sera, and vectors; in your labora-tory, you may find slight differences in the optimal ratio, amount of complex, or time for complex forma-tion for some lots of FuGENE® HD Transfection Reagent.Cells For consistent results, cells must be properly maintained. Cells change with passage level, passage conditions, media, and sera. For some cell lines, these changes have little to no effect on transfectionexperiments, but for other cell lines, these changes have profound effects. Each cell type may have a dif-ferent optimal transfection condition. Optimal values for a single cell type may also change slightly withvector construct and type of protein expressed.Observation Possible cause RecommendationSigns of cytotoxicity Transfected protein iscytotoxic or isproduced at highlevelsReduced viability or slow growth rates may be the result of high levels of protein expression, as the cell’smetabolic resources are directed toward production of the heterologous protein. The expressed proteinmay also be toxic to the cell at the level expressed.To analyze cytotoxicity, prepare experimental controls as described below.Prepare extra control wells containing:ቢ Cells that are not transfectedባ Cells treated with DNA alone (e.g., without FuGENE® HD Transfection Reagent)ቤ Cells treated with FuGENE® HD Transfection Reagent alone (no DNA added)ብ Cells transfected with a non-toxic or secreted protein.Compare experimental transfected cells to cells in the control wells (described above). Considerrepeating the experiment with a secreted reporter gene such as SEAP, hGH, or a standard -gal controlvector. Cells expressing SEAP should show little to no evidence of cytotoxicity.Too much transfec-tion complex fornumber of cellsIncrease the number of cells plated, and/or decrease the total amount of complex added to the cells. Trydifferent ratios and allow the complexes to form for different time intervals. Add different amounts ofcomplex; for example, make the complex as usual but add 75%, 50%, or 25% of the usual amounts toeach well. See Suboptimal FuGENE® HD:DNA Ratio in "Low transfection efficiency" section of this tablefor details or optimization protocol.Culture may becontaminated withmycoplasmaDetermine if culture is contaminated with mycoplasma; use the Mycoplasma Detection Kit* orMycoplasma PCR ELISA* to assess contamination.Cells may not behealthyAssess physiological state of cells and the incubation conditions (e.g., check incubator CO2, humidity,and temperature levels). Observe cells prior to each passage for morphology and absence of contami-nants. Make sure cells do not overgrow. Routinely passage cells prior to reaching confluency. Make surethat culture media and additives are within expiration date and have been stored properly.Diluent is toxic to thecellsDMEM is toxic to some insect cell lines. For these cells, prepare the transfection complex in sterilewater. You may also try forming the complex in the medium in which the cells are growing, providingthat the medium does not contain serum, heparin, or dextran sulfate.Plasmid preparationcontaminated withendotoxinEndotoxin is reported to be cytotoxic to sensitive cell lines.If above tests provenegative, FuGENE®HD TransfectionReagent may not beoptimal for your cells.Try FuGENE® 6 Transfection Reagent*, DOTAP Liposomal Transfection Reagent*, DOSPER LiposomalTransfection Reagent*, or X-tremeGENE Q2 Transfection Reagent*.High protein-expression levelsHigh expression levels of certain intracellular proteins (e.g., Green Fluorescent Protein [GFP]) may becytotoxic to some cell types. Cell proliferation, toxicity, and cell death may be monitored using Apoptosisand Cell Proliferation products from Roche Applied Science (visit /apoptosis for more information).Media and mediacomponentsTest different media and optimize the level of each medium component for these cytotoxic effects.Although it is not usually necessary to remove the transfection complex following the transfection step,it may be necessary to feed your cells with fresh media for extended growth periods. This is particularlyimportant if the transfected cells are allowed to continue to grow for 3 – 7 days to provide maximalprotein expression.。
高效真核转染试剂

高效真核转染试剂( VigoFect )产品说明:本试剂采用一类阳离子非脂性物质为主的配方,可以与DNA形成稳定的复合物,透过细胞膜进入细胞内,并保护DNA免受核酸酶的降解。
该试剂对细胞毒性很小,可在含血清与抗生素的完全培养液中充分发挥作用。
对多数培养细胞种类都有较高的转染效率(不同种类细胞的转染效率可有明显差异)。
此类试剂是目前非病毒介导方法中效率最高的转染试剂。
产品内容与储存方法:名称数量保存条件mlVigoFect 0.44℃可进行200次转染(6孔板或35 mm平皿)。
常温运输,4℃保存。
有效期6个月。
所需其它试剂:使用者需准备150 mM NaCl (超纯水配制,高压或过滤灭菌)或注射用生理盐水作为VigoFect及DNA的稀释液,和要转染的DNA溶液(高纯度,浓度0.1~2 μg/μl)。
操作方法:准备培养细胞:1)转染前24小时,接种适量细胞(接种数量可参考附表)。
至转染时细胞密度以40~60%为宜(80~90%亦可)。
2)转染前1小时,更换新鲜的完全培养液(体积可参考附表),置37℃,5% CO2 培养。
配制转染工作液:(6孔板或35 mm平皿,2 ml培养液)3)取5~8 μg DNA(起始用量5 μg),加入稀释液中至总体积为100 μl,轻轻混匀,室温放置。
4)取VigoFect 1~4μl(起始用量2 μl),加入稀释液中至总体积为100 μl,轻轻混匀,室温放置5分钟。
5)将稀释的VigoFect逐滴加入稀释的DNA溶液中,轻轻混匀,所得的转染工作液在室温放置15分钟。
6)将转染工作液轻轻混匀,逐滴加入2 ml培养液中,轻轻混匀培养液,置37℃,5% CO2培养。
细胞后续处理:7)24~48小时后,观察或收取细胞。
8)稳定转染时,于转染后24~48小时消化细胞分至3~5个培养皿中,加适当浓度的相应抗生素(如G418)筛选。
注意事项:1) 少量使用Vigofect 时,可取精确量的VigoFect 用无菌超纯水稀释一定倍数,以便精确取样量。
FuGENE 转染流程

FuGENE HD真核细胞转染流程FuGENE HD Transfection Reagent Quick Protocol.pdfFuGENE HD Transfection Reagent.pdf1.细胞和质粒的准备①将约5-10×105A549细胞接种于6孔细胞板中,用含10%小牛血清的F-12k培养过夜,约60%~80%铺满时用于转染。
注:一般荧光试验细胞稀一点为宜;WB试验细胞密一点为宜②转染时所用质粒均用转染专用质粒抽提试剂盒无菌提取,抽提后测定其浓度和纯度,浓度在0.1μg/μL以上且纯度在1.8±0.5 (OD260/OD280)的质粒用于转染,质粒提取的具体步骤按说明书提供的方法进行。
(注:质粒浓度测定未必可行,需同时进行酶切和PCR鉴定方可。
)③按照转染剂量,计算质粒使用浓度2.转染流程注:由于不同转染试剂对质粒大小、性质、转染量、细胞的种类有很强的特异性,必须谨慎选择转染试剂。
以多次转染成功使用的转染试剂为佳。
①将培养板中的上清弃去,用细胞用无抗无血清F12K洗涤三次后,每孔加入800ul 无抗无血清F12K。
②取出FuGENE HD转染试剂,使其温度上升到室温。
③计算好质粒、转染试剂、以及无抗无血清F12K的量,最终液体总量为100 ul。
准备好无菌指形管,按计算结果加入90-98ul的无抗无血清F12K,加入2ug 质粒,振荡器混匀;随后加入6ul FuGENE HD转染试剂,立即震荡混匀(转染试剂加入时不能沾到管壁上!)④室温静置7-12分钟后,将质粒:转染试剂混合液均匀滴加在细胞平板孔中,吹打混匀或者震荡混匀。
放入37℃培养箱孵育。
⑤转染后2 h后加入含有血清的F12K,使血清终浓度达到1-4%,上清总量达到1.5-2 ml。
细胞平板放入37℃培养箱培养24-48h。
⑥转染后特定时间点,用4℃预冷的PBS漂洗细胞3次,加入含有PMSF的Western细胞裂解液冰上裂解15min。
FuGENE HD 转染试剂简明操作指导说明书
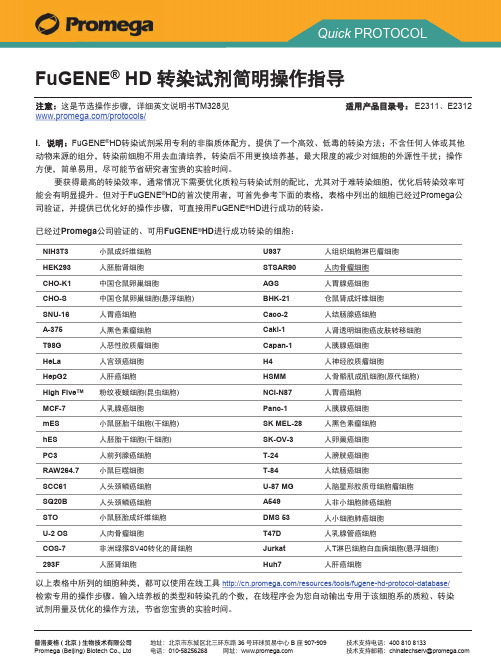
FuGENE®HD转对于表格中未列出的细胞,采用以下步骤进行实验后,可寻找到适用于您所研究细胞的最佳质粒与转染试剂的配比:FuGENE®HD 转染试剂简明操作步骤II. 准备实验所需的细胞、试剂及耗材:•细胞:在合适的培养条件下(建议采用无抗生素培养基,可以含任意比例的血清),实验前细胞系培养至长满60%-80%,原代细胞培养至适当的时间;•转染试剂:使用前,将转染试剂放至室温,颠倒混匀(FuGENE®HD转染试剂储存于4℃,如不慎将FuGENE®HD冰冻,融化后可能会看到不溶物。
这时可将试剂短暂升温至37℃,颠倒混匀后不溶物消失);•质粒:携带报告基因或荧光蛋白的质粒,用于计算转染效率;•无菌、无血清培养基:用于配制质粒与转染试剂的混合物;•无菌的枪头、离心管,超净工作台等。
简易流程图培养细胞至60%-80%融合度配制质粒与转染试剂的混合物混合物加入培养的细胞中检测,计算转染效率I I I.操作步骤1.将无血清培养基预热至室温,按下表的比例配制质粒与转染试剂的混合物:FuGENE®HD与质粒DNA的比例4:1 3.5:13:1 2.5:12:1 1.5:1培养基100μl100μl100μl100μl100μl100μl质粒2μg2μg2μg2μg2μg2μg FuGENE®HD8μl7μl6μl5μl4μl3μl注意:FuGENE®HD应直接加入培养基中,不用沾到离心管的管壁上2.混合物室温静置10-15 min。
3.将混合物滴加至培养的细胞中,96孔板每孔加入5µl,其他孔板的加入量可以按照右侧的表格进行计算。
摇晃或吹打混匀。
继续培养24-48 hr。
4.检测转染效率。
进行报告基因检测或计数表达绿色荧光蛋白的细胞数目,确定最佳的质粒与转染试剂的比例。
注:FuGENE®HD转染试剂对细胞几乎没有毒性,遇到特别难转染的细胞,希望提高转染效率时,可以将混合物的加入量加倍至10μl/孔或15μl/孔(96孔板,其他培养板按培养面积放大)。
Fugene 6

serum-free medium. Do not allow the FuGENE® 6 Transfection Reagent to contact the plastic walls of the tube containing the serum-free medium during the dilution step.
FuGENE® 6 Transfection Reagent remains fully functional even
Application FuGENE® 6 Transfection Reagent is a multi-component reagent perfect for experiments involving cellular analysis. Due to its simple methodology, minimal optimization, low cytotoxicity, and ability to provide high transfection efficiency even in the presence of serum, it is the standard transfection reagent in many different laboratories around the world.
Do not use siliconized pipette tips or tubes.
0909.04977637001➉
www.roche-applied-scienceห้องสมุดไป่ตู้com
Other Media Additives In some cell types, antimicrobial agents (e.g., antibiotics and fungicides) that are commonly included in cell-culture media may adversely affect the transfection efficiency of FuGENE® 6 Transfection Reagent. Up to a 25% decrease in efficiency has been observed. If possible, exclude additives for initial experiments. Once high-efficiency conditions have been established, these components can be added back while monitoring your transfection results. Verification of Vector Function Optimize transfection conditions with a known positive-control reporter-gene construct prior to transfecting cells with a new vector construct: • Determine transfection efficiency with a reporter-gene assay (CAT, -Gal, Luciferase, SEAP, or hGH [see “Ordering Information” section]). • Sequence across the flanking vector insert regions to verify the integrity of your new construct. High Protein- Expression Levels High expression levels of certain intracellular proteins (e.g., GFP) may be cytotoxic to some cell types. Cell proliferation, toxicity, and cell death may be monitored using Roche Apoptosis and Cell Death products (visit /sis/apoptosis for more information). Incubation Time Incubate the cells for 4 – 72 hours. The length of incubation depends upon the transfected vector construct, the cell type being transfected, and the type of protein being expressed. After this incubation period, measure protein expression using an assay that is appropriate for your system. Additional Required Reagents and Supplies Sterile, serum-free culture medium without additives or supplements (optional: add 12.5 mM HEPES buffer to serum-free medium) Plasmid DNA solution (between 0.02 g/l and 2.0 g/l) in sterile TE (Tris/EDTA) buffer or sterile water. To prevent spillage, use a 24-well plate as a test tube rack for the FuGENE® 6 Transfection Reagent. 2.2 Procedures
E6全血基因组DNA及线粒体DNA提取试剂说明书

一步裂解法全血基因组DNA/线粒体DNA提取试剂使用说明本试剂盒由复合裂解液、蛋白沉淀液以及DNA溶解液组成。
可从全血中提取人基因组DNA/线粒体DNA(mtDNA)。
整个提取过程不超过45分钟。
提取过程包括:1裂解红细胞/白细胞核/线粒体。
2盐析沉淀蛋白,分离DNA。
3异丙醇沉淀脱盐并浓缩。
4 70%乙醇清洗,晾干并用DNA溶解液溶解。
本试剂盒采用复合裂解液,省去了第一代提取试剂的红细胞裂解的步骤,其中含有抑制DNA酶的成分,在加热(65°C)状态下,可保证完整的人基因组DNA、线粒体DNA(mtDNA)的充分裂解释放,异丙醇沉淀时,罕见浑浊的蛋白质杂质的共沉淀。
提取的基因组DNA长度大于20kb, 可用于PCR扩增、内切酶消化以及膜杂交等。
一、试剂盒组成(本试剂盒提供的组份, 至少可提取100份100ul全血样本的DNA,每种组份均有足够的富余量)1.复合裂解液:30ml×1瓶。
2.蛋白沉淀液:25ml×1瓶。
3.DNA溶解液:20ml×1瓶。
4.操作说明书一份。
二、本试剂盒未提供,须用户自备的试剂为:异丙醇,70%乙醇(国产分析纯)。
三、全血基因组/线粒体DNA提取操作准备:1.样本要求:用于DNA提取的全血应为抗凝血(EDTA, 肝素或柠檬酸钠抗凝),抗凝血在-20°C存放一个月以内,避免反复冻融。
4°C存放7天以内,均能纯化出高质量的DNA。
-80°C条件下,样本至少可存放2年。
2.全血需求量:根据需要,本试剂盒的复合裂解液,每300ul可以一次提取20-100ul全血。
如果按照每份提取全血/血清100ul来计算,总共可以提取100份全血/血清,提取试剂各组份用量与全血/血清提取量的对应关系见下面表格。
四、提取操作:以下按照提取100ul全血DNA来说明:1.将复合裂解液置65°C水浴加热,使结晶成分溶解,准备1.5-2ml离心管,每个离心管中分装加入300ul的复合裂解液。
qiagen转染试剂说明书中文版

Effectene®Transfection Reagent转染试剂中文说明书●Notes:①细胞处于最佳生理状态,原代细胞以低代数较好;②DNA(质粒)的质量直接影响转染的效率,纯化后的DNA(质粒)转染效果更好;③DNA与Effectene Transfection Reagent的用量比例可能根据具体细胞稍有变动,但DNA与Enhancer 用量比例(1:8)最好别改变。
●protocol :1.对于24孔板,接种细胞密度为2-8X104个/孔,加500ul 无抗生素培养基;2.370C,5%/CO2培养,转染当日细胞密度达到40%-80%;3.稀释溶解在TE buffer里的0.2ug DNA(质粒),加Buffer EC直至体积为60ul,再加入1.6ul Enhancer涡旋混匀1s,低速离心甩净管壁上液滴;Table 14.室温(15–250C)孵育2–5 min ;5.吸5ul Effectene Transfection Reagent到DNA-Enhancer mixture中,小心吹打5次混匀(注:Effectene Transfection Reagent 用完后要及时放回冰上,10-15min室温不会影响);6.室温静置5–10 min;7.当静置的同时,吸走原有培养液,用1ml PBS漂洗一次,再加入350ul 新鲜培养基;8.吸350ul培养液到转染混合液中,小心吹打两次混匀后,立即滴加到24孔板中,最后轻轻打旋混匀;9.培养箱中正常培养24小时,48小时后拍照,一般情况下可以不换液,若发现对细胞有毒性的,可在6-18小时后换液。
优化实验设计方案:a.6孔板Tableb.60mm板流程图。
各种转染试剂中文说明

FuGENE6(Roche)转染步骤:转染前一天将细胞分至培养板,转染当天细胞应50-80%融合。
将细胞以1-3×105/2ml接种于6孔板后孵育过夜将达到如此密度。
将FuGENE6 Reagent在室温孵育10-15分钟。
使用之前将FuGENE6颠倒混匀一下。
1.在PCR管中加入不含血清和双抗的营养液以稀释FuGENE6,直至总体积到100ul。
2.将3-6ul FuGENE6 Reagent直接加入营养液,轻弹管壁混合。
3.加入1-2ug的DNA溶液(0.02-2.0ug/ul),轻弹管壁混合。
4.室温孵育20分钟。
5.将6孔板中的旧营养液吸出,加入约1ml不含血清和双抗的营养液洗涤一次,再加入2ml不含血清和双抗的营养液。
6.将转染复合物加入细胞,混匀使之均匀分布。
7.3-8小时后,加入血清或换成含血清的营养液。
Lipofectamine 2000(Invitrogen)转染试剂转染步骤(6孔板):1.转染前一天,胰酶消化细胞并计数,细胞铺板,使其在转染日密度为90-95%。
细胞铺板在2ml含血清,不含抗生素的正常生长的培养基中。
2.对于每孔细胞,使用250ul无血清培养基(如OPTI-MEM I培养基)稀释4.0ugDNA,轻轻混匀。
3.使用前将Lipofectamine 2000转染试剂轻轻混匀,用250ul无血清培养基(如OPTI-MEM I培养基)稀释10ul Lipofectamine 2000转染试剂,轻轻混匀。
Lipofectamine 2000稀释后,在5分钟内同稀释的DNA混合(<30分钟)。
NOTE:若使用DMEM培养基,则需在5分钟内同稀释的DNA混合。
4.混合稀释的DNA(第二步)和稀释的Lipofectamine 2000(第三步)。
室温放置20分钟。
5.(optional)将6孔板中的旧营养液吸出,用无血清培养基清洗两次。
加入2ml无血清配养基。
两种常用转染试剂转染siRNA至HL-60细胞转染效率的比较

两种常用转染试剂转染siRNA至HL-60细胞转染效率的比较王巍;张晓希;刘新光【摘要】目的比较两种常用转染试剂转染小干扰RNA(siRNA)至悬浮细胞的转染效率及对细胞毒性的影响.方法以羧基荧光素(FAM)标记的siRNA为报告基因,以lipofectamine 2000和siPORT NeoFX为转染试剂,用流式细胞仪检测转染效率,倒置显微镜观察细胞形态,MTT法检测细胞存活率.结果 siRNA>100 nmol/L 时,lipofectamine 2000的转染效率高于siPORT NeoFX(P<0.05);siRNA<100 nmol/L时,前者低于后者(P<0.05).siRNA终浓度及转染试剂用量相同时,lipofectamine 2000组HL-60细胞存活率与SiPORT NeoFX组比较,差异无统计学意义(P>0.05).结论在使用高浓度siRNA时,lipofectamine 2000对HL-60细胞有较高的转染效率和较小细胞毒性.%Objective To compare the transfection efficiency and cytotoxicity between two cationic transfection reagents. Methods Small interfering RNA(siRNA) marked with FAM as a report gene,lipofectamine 2000 and siPORT NeoFX were used as the transfection reagents. Flow cytometer,microscope and MTT assay were used to detect transfection efficiency and cytotoxicity. Results The transfection efficiency of lipofectamine 2000 was higher than siPORT NeoFX when siRNA was over 100 nmol/L(P<0.05) ,otherwise with siRNA under 100 nmol/L(P<0. 05). At the same siRNA concentration and transfection reagent volume, there was no significant difference of vial cells ratios between two groups(P>0.05). Conclusion lipofectamine 2000is an effective and safe transfection reagent to HL-60 cells when siRNA is over 100 nmol/L.【期刊名称】《重庆医学》【年(卷),期】2011(040)004【总页数】4页(P313-314,318,封2)【关键词】脂质体;转染;RNA,小分子干扰【作者】王巍;张晓希;刘新光【作者单位】广东医学院临床血液检验学教研室,东莞,523808;中国人民解放军第四二二医院,广东湛江,524023;广东医学院检验医学研究所,东莞,523808【正文语种】中文RNA干扰(RNA interference,RNAi)是指在进化过程中高度保守的、由双链RNA 诱发的、同源mRNA高效特异性降解的现象[1-3]。
FuGENE6转染试剂中文操作说明

FuGENE6转染试剂中文操作说明FuGENE®6原是Roche公司的旗舰转染产品,如今已归为Promega公司旗下。
它是一种非脂质体转染试剂,广泛适用于高效转染各种细胞系,且细胞毒性非常低。
由于使用该转染试剂转染时不要求去除血清或培养液,并且转染后也不要求换液去除,因而使用起来非常方便。
配方和包装:FuGENE® 6是由脂质与其他组分按照合适比例混合,溶于80%乙醇而成。
试剂通过0.1μm的滤器过滤并分装至小玻璃瓶。
注意事项:转染前须保证FuGENE® 6温度已经达到室温,使用前须上下颠倒数次进行简单混匀。
建议使用标准的24孔板作为FuGENE®6转染试剂的支架。
请不要将原瓶中的FuGENE®6进行分装。
尽可能减少FuGENE®6原液与塑料制品的表面接触。
不要使用硅化的枪头或离心管。
在稀释FuGENE® 6时,请务必保证将FuGENE® 6直接混入培养基,而不要接触到离心管。
(研小弟注:还要防挥发,毕竟溶于80%乙醇,所以也不宜分装)抗生素的使用:在细胞系的常规培养时可以使用抗生素。
但在转染时抗生素的存在可能会影响转染的效率以及转染细胞的整体健康。
除非之前已经在转染细胞中测试过,否则我们不推荐在转染培养液中添加抗生素。
转染步骤:以下是一个简易的中文转染步骤示意图具体的转染步骤包括:•细胞的接种在转染前一天接种细胞,保证在转染时细胞的汇合度在50-80%,悬浮细胞可以在转染当天接种。
通常96孔板中每个孔适宜接种100μl 含1-2×104的贴壁细胞或2×104到1×105的悬浮细胞。
对于其他规格的培养板的推荐细胞数,可参考下表(数据针对Corning公司生产的培养板)。
96-well 0.32 1×24-well 1.88 5×12-well 3.83 10×6-well 9.4 30×35mm 8 25×60mm 21 65×100mm 55 170וFuGENE® 6转染试剂的准备1.转染前保证FuGENE® 6温度已经达到室温2.使用前上下颠倒数次进行简单混匀。
转染试剂使用说明书

2. 3).
配制转染工作液: ( 6 孔板或 35 mm 平皿, 2 ml 培养液) 取 5~8μ g DNA (起始用量 5μ g) ,加入稀释液中至总体积为 100μ l,轻轻混匀,室 温放置。
4).
先将 GenFectin TM 涡旋振荡混匀。取 GenFectinTM 1~4μl(起始用量 2μl,) ,加入稀 释液中至总体积为 100μl,轻轻混匀,室温放置 5 分钟。
5).
将稀释的 GenFectinTM 逐滴加入稀释的 DNA 溶液中,轻轻混匀,所得的转染工作 液在室温放置 15 分钟。
6).
将转染工作液轻轻混匀,逐滴加入 2 ml 培养液中,轻轻混匀培养液,置 37℃,5% CO 2 培养。
3. 细胞后续处理: 7). 24~48 小时后,观察或收取细胞。
-4-
8).
稳定转染时,于转染后 24~48 小时消化细胞分至 3~5 个培养皿中,加适当浓度的 相应抗生素(如 G418)筛选。
建议的起始转染条件 : 培养容器
96 孔板 24 孔板 6 孔板 35mm 培养皿 60mm 培养皿
转染前一天 接种细胞数
1-1.510 个 0.5-1 10 个 2-4 10 个 2-4 10 个 4-6 105 个
5 5 5 4
转染时 培养液体积
0.1 ml 0.5 ml 2 ml 2 ml 4 ml
DNA 用量 与稀释后体积
0.25 μg 1.25 μg 5 μg 5 μg 10 μg 5 μl 25 μl 100 μl 100 μl 200 μl
GenFectin 量 与稀释后体积
0.1 μl 0.5 μl 2 μl 2 μl 4 μl 5 μl 25 μl 100 μl 100 μl 200 μl
转染试剂的中文详解

各种转染试剂的中文转染方法2014-05-06丁香园FuGENE6(Roche)转染步骤:转染前一天将细胞分至培养板,转染当天细胞应50-80%融合。
将细胞以1-3×105/2 ml接种于6孔板后孵育过夜将达到如此密度。
将FuGENE6 Reagent在室温静置10-15分钟以平衡到室温。
使用之前将FuGENE6颠倒混匀一下。
1.在PCR管中加入不含血清和双抗的营养液以稀释FuGENE6,直至总体积到100 ul。
2.将3-6 ul FuGENE6 Reagent直接加入营养液,轻弹管壁混合。
3.加入1-2 ug的DNA溶液(0.02-2.0 ug/ul),轻弹管壁混合。
4.室温孵育20分钟。
5.将6孔板中的旧营养液吸出,加入约1 ml不含血清和双抗的营养液洗涤一次,再加入2 ml不含血清和双抗的营养液。
6.将转染复合物加入细胞,混匀使之均匀分布。
7.3-8小时后,加入血清或换成含血清的营养液。
Lipofectamine 2000(Invitrogen)转染试剂转染步骤(6孔板):1.转染前一天,胰酶消化细胞并计数,细胞铺板,使其在转染日密度为90-95%。
细胞铺板在2 ml含血清,不含抗生素的正常生长的培养基中。
2.对于每孔细胞,使用250 ul无血清培养基(如OPTI-MEM I培养基)稀释4.0 ugDNA,轻轻混匀。
3.使用前将Lipofectamine 2000转染试剂轻轻混匀,用250 ul无血清培养基(如OPTI-MEM I培养基)稀释10 ul Lipofectamine 2000转染试剂,轻轻混匀。
Lipofectamine 2000稀释后,在5分钟内同稀释的DNA混合(<30分钟)。
NOTE:若使用DMEM培养基,则需在5分钟内同稀释的DNA混合。
4.混合稀释的DNA(第二步)和稀释的Lipofectamine 2000(第三步)。
室温放置20分钟。
5.(optional)将6孔板中的旧营养液吸出,用无血清培养基清洗两次。
IPS技术手册

人类诱导性多能干细胞(iPS细胞)技术指导手册目录:1.前言 (1)2.人类胚胎成纤维细胞培养 (2)3.重编程载体构建 (3)4.病毒包装 (4)5.人类iPS细胞的诱导 (6)6.iPS细胞鉴定 (8)6.1碱性磷酸酶活性检测 (9)6.2干细胞表面marker的免疫染色检测 (9)6.3干性因子的去甲基化程度分析 (11)6.4干细胞内源基因的表达分析 (13)6.5端粒酶活性检测 (14)6.6核型检测 (15)6.7拟胚体形成 (15)6.8畸胎瘤形成实验 (15)7.干细胞技术培训及服务一览表 (15)8.附录 (16)1.前言iPS细胞最初从成纤维细胞重编程而来,因为它们准备和操作相对简单。
其他细胞类型,包括来自外胚层、中胚层和内胚层的细胞也可以用于产生iPS细胞(Eminili et al2008)。
2006年Yamanaka和Takahashi利用逆转录病毒系统在成鼠的成纤维细胞导入四个转录因子(Oct3/4,Sox2,c-Myc,和Klf4,Yamanaka因子),将其重编程为iPS细胞,它具有跟小鼠ES十分相似的特性,尤其重要的是,iPS细胞也能产生后代。
2007年,iPS技术在人类体细胞中得以应用,人类iPS 细胞的产生对退行性疾病的治疗产生巨大的影响(Takahashi et al;Yu et al,2007)。
由于iPS细胞具有和ES类似的功能,却绕开了胚胎干细胞研究一直面临的伦理和法律等诸多瓶颈,因此在医疗领域的应用前景非常广阔,成为干细胞研究的热点,《自然》和《科学》杂志分别将其评为2007年第一和第二大科学进展。
随后,iPS细胞的研究日新月异。
.近几年来,iPS研究方面取得的一系列的重大成果让我们欣喜不已,然而,这才刚刚开始,iPS细胞能真正用于临床惠及大众还面临着很多技术上的问题。
一方面iPS产生的方法有待改进;另一方面iPS细胞的定向分化手段需继续探索。
斯丹赛干细胞生物技术公司拥有成熟稳定的胚胎干细胞/iPS细胞技术平台,将竭力为全国各类进行干细胞/iPS研究的科研机构、高校、相关医疗机构和制药企业等提供高品质产品和优质的服务。
FuGENE 6 转染步骤

配方和包装:FuGENE® 6是由脂质与其他组分按照合适比例混合,溶于80%乙醇而成。
试剂通过0.1μm的滤器过滤并分装至小玻璃瓶。
注意事项:转染前须保证FuGENE®6温度已经达到室温,使用前须上下颠倒数次进行简单混匀。
建议使用标准的24孔板作为FuGENE® 6转染试剂的支架。
请不要将原瓶中的FuGENE®6进行分装。
尽可能减少FuGENE®6原液与塑料制品的表面接触。
不要使用硅化的枪头或离心管。
在稀释FuGENE® 6时,请务必保证将FuGENE® 6直接混入培养基,而不要接触到离心管。
(研小弟注:还要防挥发,毕竟溶于80%乙醇,所以也不宜分装)抗生素的使用:在细胞系的常规培养时可以使用抗生素。
但在转染时抗生素的存在可能会影响转染的效率以及转染细胞的整体健康。
除非之前已经在转染细胞中测试过,否则我们不推荐在转染培养液中添加抗生素。
转染步骤:以下是一个简易的中文转染步骤示意图具体的转染步骤包括:•细胞的接种在转染前一天接种细胞,保证在转染时细胞的汇合度在50-80%,悬浮细胞可以在转染当天接种。
通常96孔板中每个孔适宜接种100μl含1-2×104的贴壁细胞或2×104到1×105的悬浮细胞。
对于其他规格的培养板的推荐细胞数,可参考下表(数据针对Corning公司生产的培养板)。
96-well 0.32 1×24-well 1.88 5×12-well 3.83 10×6-well 9.4 30×35mm 8 25×60mm 21 65×100mm 55 170וFuGENE® 6转染试剂的准备1.转染前保证FuGENE® 6温度已经达到室温2.使用前上下颠倒数次进行简单混匀。
•常规转染步骤我们强烈推荐您针对每个细胞系建立优化转染条件。
结构层

酪氨酸为基础的信号介导LRP6的内吞作用和Wnt基因/β-catenin信号脱敏背景:LRP6是一个重要的Wnt/β-catenin共受体信号,控制各种生物途径。
结果:LRP6酪氨酸突变体显示出更长的半衰期和升高的脂质筏分布,磷酸化,和信令。
结论:LRP6的网格蛋白介导的内吞作用介导了它的降解,而小窝依赖的途径促进的Wnt/β-catenin信号。
意义:了解基本的LRP6内吞作用和Wnt基因/β-catenin信号通路的分子机制将有助于这一目标的治疗方法的途径的发展。
摘要Wnt/β-catenin信号编排的一些关键事件,包括发育过程中细胞的生长,分化和细胞存活。
该途径的错误调节导致多种人类疾病,尤其是癌症。
内吞作用和磷酸化低密度脂蛋白受体相关蛋白6(LRP6),一个重要的共受体的Wnt/β-catenin信号通路,在介导的Wnt/β-连环蛋白信号转导中发挥至关重要的作用。
然而,其调控机制尚不完全清楚。
在这项研究中,我们定义了它的LRP6内吞交易调节的Wnt/β-catenin信号通路的激活机制。
我们表明LRP6突变体有缺陷基于酪氨酸的信号在其胞质尾区具有增加细胞表面的分布和内吞作用速率的降低。
这些变化在LRP6内吞作用与对小窝的分配增加,磷酸化增加,以及Wnt/β-catenin 信号增强一致。
我们还表明,治疗的Wnt3a配体或阻塞的LRP6引导的网格蛋白介导的内吞作用,导致对受体脂筏以野生型的再分配。
相比野生型LRP6信号激活,Wnt3a刺激LRP6酪氨酸突变体也表现出增加的响应,并且当胞膜窖介导的胞吞作用被阻止这种激活就被抑制。
我们的研究结果揭示了其中的LRP6内吞途径调节其磷酸化的Wnt/β-catenin信号强度的分子机制,并就如何实现这一途径能在人类疾病中被调制的影响。
Wnt信号/β-catenin信号通路调控胚胎发育过程中的各种活动,包括细胞增殖,存活,迁移和极性,规范细胞的命运,和自我更新(1-3)。
进行转染实验时需要注意的因素

转染——让克隆的核酸进入真核细胞中,已经成为研究和控制真核细胞基因表达的重要手段。
比如表达纯化特定的蛋白;鉴定一个基因的生物学特性;突变分析;研究基因表达对细胞生长的影响,研究基因表达的调控机制等等,等等。
如今广义的转染不单包括了DNA、RNA,还有蛋白质等生物大分子。
很多因素会影响转染效率,细胞株本身啦,细胞培养环境啦,转染的DNA、RNA或者蛋白的质量和特性啦,转染方法啦,等等。
每个转染高手也必然有自己的独门心经,只可惜大家都为实验或者生活而疲于奔命,没有几个人愿意静下心情来仔细总结经验汇集成文字——那些曾经用无数失败的痛苦烦恼换回来的宝贵经验,那些曾经以为会永远铭刻于脑海的教训,随时间的流逝而终于渐渐褪色,到模糊。
即使偶尔有珠玉掩埋于浩瀚的口水中,也难以汇串成珠。
试剂盒的盛行,让实验更快,更简单,也更机械化,相信更多人更愿意先抓起Protocol 123地往下做,直到碰壁再苦思原因。
其实等碰得遍体鳞伤回头一看就会醒悟,很多坑,只要事前注意了就不会陷进去的。
DNA转染后,转入基因的表达可以在1-4天内检测到——仅有一部分转入细胞的DNA被转运到细胞核内进行转录并最终输出mRNA到细胞质进行蛋白合成。
几天内,大部分外源DNA 会被核酸酶降解或随细胞分裂而稀释;一周后就检测不到其存在了。
因此转染也可以分为瞬时转染和稳定转染。
瞬时转染(transient transfection)指的是转染的核酸不整合到染色体上,结果是短暂的高水平表达,可在24—96小时内检测表达效果,表达水平与位置无关,不会受到周围染色体元件的影响。
瞬时表达分析所需的人力和时间比稳定表达少,但因为DNA摄入效率和表达水平在不同实验中差异较大,不长久也不稳定。
超螺旋质粒转染更倾向于瞬时表达。
瞬时表达适合研究启动子等调控元件——不过,诱导型的启动子除外。
稳定转染,就是转染的质粒DNA整合到染色体上,或者相当于附加子(episome)可持续存在,使得转染的细胞可长期表达。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1814443.FM Page 1 Tuesday, August 21, 2001 10:58 AMFor life science research only. Not for use in diagnostic procedures. FuGENE 6Transfection ReagentMulti-component formulation for the transfection of eukaryotic cells Cat. No. 1 815 0910.4 ml(80–120 transfections) Cat. No. 1 814 443 1 ml(200–300 transfections) Cat. No. 1 815 075Multi-pack 5 x 1 ml(1000–1500 transfections) Cat. No. 1 988 484Custom pack Inquire(10 ml or 50 ml glass vials) Store FuGENE 6 Transfection Reagent at –15 to –25ºC.Instruction ManualVersion 5, September 20001. Preface1.1 Table of contents1.Preface (2)1.1Table of contents (2)2.Product characteristics (3)3.Introduction (3)3.1Product overview (3)3.2Background information (4)4.Procedures and required materials (5)4.1Before you begin (5)4.2Factors for successful transfection (5)4.3Standard protocol for transient and stable transfection (8)4.3.1Preparation of cells for transfection (8)4.3.2Preparation of FuGENE 6 Reagent:DNA complex (9)4.3.3Scale-up for large transfection experiments (9)4.3.4Transfection of cells (10)4.3.5Cotransfection experiments (10)4.3.6Optimization of transfection efficiency and protein expression levels (10)4.3.7Measurement of protein expression (11)4.4 Large-scale transfection using FuGENE 6 Reagent fortransient protein expression (11)4.4.1 Optimization in high through-put screening (HTS) applications andprotein production (12)5.Appendix (13)5.1Troubleshooting table (13)5.2How to contact Roche Molecular Biochemicals (16)5.3 References (17)5.4Related products...............................................................................................................................18P R O C E D U R E S1814443.FM Page 2 Tuesday, August 21, 2001 10:58 AMRoche Molecular Biochemicals22. Product characteristicsFormulation FuGENE 6 Transfection Reagent is a proprietary blend of lipids andother components supplied in 80% ethanol, sterile-filtered, andpackaged in polypropylene tubes. Warm FuGENE 6 TransfectionReagent to ambient temperature (approximately 10 to 15 minutes atroom temperature) prior to use. Always mix FuGENE 6 Reagent prior touse (vortex or inversion). After long periods of storage, FuGENE 6Transfection Reagent may be slightly turbid due to the precipitation ofsome components. In most systems, the turbidity has no effect on thebiological activity of the product.NOTE: The new Custom-pack of FuGENE 6 Transfection Reagentis supplied in glass vials.Storage and stability FuGENE 6 Transfection Reagent is stabilized for shipping at room temperature and for extended storage at –20°C through the expiration date printed on the label (two years from date of manufacture). FuGENE 6 Reagent is shipped to you at room temperature.Special handling Do not aliquot FuGENE 6 Reagent from the original polypropylene tubes. Chemical residues in new plastic vials can significantly decrease the biological activity of the reagent.NOTE: Evaluations have shown that FuGENE 6 Transfection Reagent remains fully functional even when vials are repeatedly opened (at least 6 times over a 3 month period), as long as the vials are recapped tightly and stored at –20°C between use. A 20% loss of ethanol due to evaporation does not substantially affect biological activity in COS-1 cells.3. Introduction3.1 Product overviewApplication FuGENE 6 Transfection Reagent efficiently transfects a wide variety of mammalian cells and other cell types with high efficiency. Visit theFuGENE 6 Reagent web page for a current list of >250 successfullytransfected cells lines: http://biochem. /techserv/fugene.htm Number of tests One milliliter of FuGENE 6 Reagent transfects approximately 200–300,35 mm tissue culture dishes (total media volume of 2 ml each) withone of the following cell lines (HeLa, NIH 3T3, COS-1, COS-7,CHO-K1).NOTE: The levels of expression of the gene of interest will varywith the cell line.continued on next page 1814443.FM Page 3 Tuesday, August 21, 2001 10:58 AMRoche Molecular Biochemicals33.1 Product overview, continuedQuality control Functional analysis Three microliters of FuGENE 6 Transfection Reagent is combined with 1–2 µg of a reporter gene vector DNA, and used to transfect COS-1 cells in a monolayer (50–80% confluent) in the presence of 10% fetal bovine serum (FBS). Following transfection, the percentage of cells transfected is analyzed, and typically, 50%–70% of COS-1 cells express reporter gene protein.Cytotoxicity analysis COS-1 cells continuously exposed for 26 hours to FuGENE 6 Reagent,with or without DNA, in the presence of serum, and without a change of medium, are >90% viable by flow cytometric analysis based onpropidium iodide-staining.3.2 Background informationGeneral transfection methods Transfection is the general process of bringing foreign DNA into cells and monitoring protein expression. DNA transfection is essential for the study of gene function and gene regulation.•Common transfection techniques include calcium phosphate coprecipitation (1), electroporation (2,3), and the use of viral vectors(4). These methods have produced variable results in a variety ofcell types (5–9).•Cationic liposome-mediated transfection methods (lipofection, cytofection) were an important addition to the previous methods(10). Additional classes of compounds known to mediatetransfection include lipopolyamines (11) and dendrimers (12).FuGENE 6 Transfection Reagent FuGENE 6 Transfection Reagent is a multi-component lipid-based transfection reagent that complexes with and transports DNA into the cell during transfection. Benefits of FuGENE 6 Reagent include:•Provides very high transfection efficiency in many common cell types.•Demonstrates virtually no cytotoxicity even in many primary cell types.•Functions exceptionally well in the presence or absence of serum.•Requires minimal optimization.1814443.FM Page 4 Tuesday, August 21, 2001 10:58 AMRoche Molecular Biochemicals4Roche Molecular Biochemicals54. Procedures and required materials4.1 Before you begin FuGENE 6 Reagent handlingNOTE: FuGENE 6 Transfection Reagent is unique as compared to other liposomal transfection reagents, and requires special handling. Review the following steps before proceeding further.4.2 Factors for successful transfection OverviewThe following factors affect transfection reactions:•FuGENE 6 Reagent working concentration •Concentration and purity of nucleic acids •Cell culture conditions•Transfection in serum-containing or serum-free media •Other media additives•Verification of vector function •High protein expression levelscontinued on next pageSpecial handling steps1Always dilute FuGENE 6 Reagent by pipetting directly intoserum-free medium. Do not allow the undiluted reagent to come into contact with any plastic surface. Refer to section 4.3.2, Preparation of FuGENE 6 Reagent:DNA complex.2Always use more FuGENE 6 Reagent (µl volume) than DNA (µg mass). For details, see section 4.2.3Always store in the original polypropylene tubes. Do not aliquot. Chemical residues in new plastic vials can significantly decrease the biological activity of the reagent.4FuGENE 6 Reagent is very robust, and unlike other reagents, transfects most cell types equally well in the presence or absence of serum, reducing the number of handling steps.5For most cell types: All additional handling steps after theaddition of the FuGENE 6 Reagent:DNA complex to the cells can be eliminated.Since FuGENE 6 Reagent is virtually nontoxic and transfects in the presence of serum, the transfection complex can be added to cells in serum-containing growth medium without washing (section 4.2). Additionally, the complex can be left on the cells until the time of the protein expression assay.P R O C E D U R E S1814443.FM Page 5 Tuesday, August 21, 2001 10:58 AMRoche Molecular Biochemicals64.2 Factors for successful transfection , continuedFuGENE 6 Reagent working concentration For an initial experiment in a 35 mm culture dish, containing a mono-layer of cells that is 50–80% confluent, transfect cells using FuGENE 6 Transfection Reagent (µl) to DNA (µg) amounts of 3:2, 3:1, and 6:1,respectively (refer to section 4.3.2 for important details of FuGENE 6:DNA complex preparation). For most cell types, these FuGENE 6 Reagent:DNA amounts provide excellent levels of transfection.NOTE: Subsequent optimization may further increase efficiency in your particular application. Hold the amount of DNA constant while increasing the volume of FuGENE 6 Reagent (as illustrated in Figure 2). Transfection efficiency is greatly reduced when the amount of DNA exceeds a ratio of 3:2 (volume FuGENE [µl]): mass DNA [µg], see Figures 1 and 2). Concentration and purity of nucleic acidsIt is critical to accurately determine the concentration of your DNA using 260 nm absorbance. For initial experiments, avoid possible cytotoxic effects by using highly purified nucleic acids (e.g., cesium chloride gradient or column purified), free of traces of residual cesium chloride. Once transfection conditions are established, plasmid prepa-ration with higher endotoxin levels can be tested. NOTE: Use a plasmid concentration between 0.02 and 2.0 µg/µl.Cell culture conditionsUse cells that are healthy, proliferating well, and plated at a consistent density to minimize both intra- and interexperimental variance in transfection efficiency.NOTE: Use only regularly passaged cells in a log-growth phase.P R O C E D U R E SFigure 1. Effect of DNA amount on transfection efficiency using FuGENE 6 Transfection Reagent.COS-1 cells (1.3 x 105) were plated in 35 mm dishes 18 hours prior to transfection. Cells were transfected with one of three fixed volumes of FuGENE 6 Reagent and various quantities of pCMV-SEAP plasmid.Expression of the SEAP reporter gene was deter-mined with the chemiluminescent SEAP Reporter Gene Assay (Cat. No. 1779842).Figure 2. Effect of the presence or absence of serum on transfection efficiency with FuGENE 6Reagent. COS-1 cells (1.3 x 105) were plated in 35mm dishes. The next day, different volumes of FuGENE 6 Reagent were used to transfect cells with 2 µg pCMV-b Gal in the presence or absence of serum. Cells were lysed using 0.25 ml of lysis buffer,and β-galactosidase expression was determined by analyzing 5µl cell lysate with the chemiluminescent β-Gal Reporter Gene Assay (Cat. No. 1758241).1814443.FM Page 6 Tuesday, August 21, 2001 10:58 AM4.2 Factors for successful transfection, continuedTransfection in serum-containing or serum-free media FuGENE 6 Reagent produces highly efficient transfection both in the presence and absence of serum (Figure 2). In some cell types, transfection efficiency may improve in the presence of serum, and in other cell types, higher transfection efficiency may be achieved in reduced-serum medium, or under serum-free conditions. The same type of cell culture medium routinely used for culturing cells can also be used during transfection.If low or reduced serum conditions are used for transfection, return cells to normal serum concentrations by adding additional serum or changing the medium 3–8 hours post transfection.NOTE: Prepare the FuGENE 6 Reagent:DNA complex in medium that does not contain serum.Other media additives In some cell types, antimicrobial agents (e.g., antibiotics and fungicides) commonly included in cell culture media, have been observed to adversely affect the transfection efficiency of FuGENE 6 Transfection Reagent. Exclude additives for initial experiments if possible. Once high-efficiency conditions have been established, these components can be added, however, you will need to monitor your transfection results.Verification of vector function Prior to transfecting cells with a new vector construct, verify and (if necessary) optimize transfection conditions with a known positive control reporter gene construct.•Use the same vector backbone as your construct, where a reporter gene was previously inserted.•Determine the transfection efficiency by measuring the level of reporter protein expression with a reporter gene assay (CAT, β-Gal, luciferase, SEAP, or hGH, see section 5.4, Related products).•Confirm insert size by performing PCR with the Intelli-Search Bacterial Colony Screen Kits (section 5.4, Related products).•Sequence across the flanking vector insert regions to verify the integrity of your new construct.High protein expression levels High levels of expression of certain proteins (e.g., GFP) may becytotoxic for some cell types. Refer to section 5.1, Troubleshootingguide.PROCEDURES1814443.FM Page 7 Tuesday, August 21, 2001 10:58 AMRoche Molecular Biochemicals7Roche Molecular Biochemicals84.3 Standard protocol for transient and stable transfection Additional required reagents•Sterile, serum-free culture medium, (optional: add 12.5 mM HEPES buffer to serum-free medium).•DNA stock solution in 1x sterile TE buffer or sterile water with a concentration determined by 260 nm absorption.4.3.1 Preparation of cells for transfection Adherent cellsPlate the cells one-day before the transfection experiment. The appropriate plating density will depend on the growth rate and the condition of the cells. Use cells that are 50–80% confluent on the day of the experiment. Plating most cell lines at 1–3 x 105 cells in 2 ml in a 35 mm culture dish (or 6-well plate) will achieve this density after overnight incubation.NOTE: Adjust the number of cells accordingly if using culture plates of different sizes (see Table 1 below).Suspension cellsUse freshly passaged cells at a concentration between 5 x 104/ml to 1x 106/ml (2ml in a 35 mm culture dish or 6-well plate). Determine cell number based on your needs and cell type to be transfected. NOTE: It is usually not necessary to wash the cells prior to the addition of the transfection reagent:DNA complex becauseperformance is independent of the presence or absence of fetal bovine serum in the cell culture medium (Figure 2).Table 1Refer to the following table when setting up your transfection reac-tions. This table is based on a transfection reagent:DNA ratio of 3:1. Refer to section 4.3.2 to use other ratios.Type of dish or plate Surface area per well orplate (cm 2)Total media volume per well or plate (ml)Starting volume of FuGENE 6 Reagent (µl/well or plate)Starting mass of DNA (µg/well or plate)60 mm 214 6.0 2.035 mm 82 3.0 1.06-well 9.42 3.0 1.012-well 3.81 1.50.524-well 1.90.50.60.296-well0.30.10.150.05P R O C E D U R E S1814443.FM Page 8 Tuesday, August 21, 2001 10:58 AM4.3.2 Preparation of FuGENE 6 Reagent:DNA complexAdherent and suspension cells in a 35 mm culture dish The following ratios have been optimized for adherent cells. These ratios also work with suspension cells.Use FuGENE 6 Reagent:DNA amounts of 3:2, 3:1, and 6:1 (µl and µg, respectively) in each well of a 6-well plate or 35 mm culture dish (described below).NOTE: To further optimize conditions, test a broader range(2–15 µl) of FuGENE 6 Transfection Reagent, while keeping the amount of DNA constant at 1–2 µg. Refer to section 5.1, Troubleshooting.4.3.3 Scale-up for large transfection experimentsPreparation of master mix complex To prepare transfection complexes using the same DNA for manyparallel experiments, proportionally increase the quantity of all components, including the serum-free medium, FuGENE 6Transfection Reagent, and the vector DNA.Step Preparation of complex1In a small sterile tube, add sufficient serum-free medium asdiluent for FuGENE 6 Transfection Reagent, to a total volume of100 µl. Add 3 to 6 µl of FuGENE 6 Reagent directly into thismedium. The order of addition is critical. The serum-freemedium must be pipetted into the tube first. Tap gently to mix.NOTE: To avoid adversely affecting transfection efficiency,do not allow undiluted FuGENE 6 Reagent to come incontact with plastic surfaces other than the pipette tip.2Add 1–2 µg DNA solution (0.02–2.0 µg/µl) to the predilutedFuGENE 6 Reagent from Step 1. Use a total volume of DNAsolution between 0.5–50µl.3Gently tap the tube to mix the contents. DO NOT VORTEX.Incubate for a minimum of 15 minutes at room temperature.Continued incubation for up to 45 minutes (for some cell linesup to 2 hours) will not affect the transfection efficiency in mostcell types. Go to section 4.3.4, Transfection of cells.FuGENE 6Reagent:DNA ratioFuGENE 6 Reagentvolume (µl)DNA volume(µg)3:2 3 23:1316:1 61PROCEDURES1814443.FM Page 9 Tuesday, August 21, 2001 10:58 AMRoche Molecular Biochemicals9Roche Molecular Biochemicals104.3.4 Transfection of cells Transfection procedureThe following procedure has been optimized for adherent cells (Step 1). Suspension cells can be transiently transfected using the same procedure after transfer to a new plate or flask. 4.3.5 Cotransfection experiments SuggestionsCotransfection experiments with FuGENE 6 Reagent have been performed simultaneously using up to seven different plasmids. Increase the amount of transfection reagent in proportion to theamount of total µg DNA when performing cotransfection experiments. NOTE: Always use excess volume of FuGENE 6 Reagent over the total final mass of DNA (section 4.2).4.3.6 Optimization of transfection efficiency and protein expression levels Optimization factorsConsider the following factors when optimizing your transfection reaction.•FuGENE 6 Reagent:DNA ratio •Cell density and growth phase •Cell passage history•Number of hours to measurement of reporter gene activityStep Transfection procedure1Dropwise, add the complex mixture from Step 3 (section 4.3.2) to your cells, distributing it around the well. Swirl the wells or flasks to ensure even dispersal.2Return the cells to the incubator until the time of the reporter gene assay.NOTE: There is no need to remove the reagent:DNA complex from the cells prior to the reporter gene assay. In our experience, exposure of most common laboratory cell types (COS-1, CHO-K1, HEK-293, HeLa) to the reagent: DNA complex until the time of the reporter gene assay (24–48 hours later), hasproduced no adverse effects, however, this may need to be determined for your particular cell type. If you observe cytotoxicity with the FuGENE 6:DNA complex, refer to section 5.1, Troubleshooting. For stable transfection experiments, the complexcontaining medium can be left unchanged until the cells need to be fed.3 (optional)Use serum-free medium during the transfection procedure, and replace the medium with serum-containing medium 3–8 hours after transfection, or add serum directly to wells.P R O C E D U R E S1814443.FM Page 10 Tuesday, August 21, 2001 10:58 AM4.3.7 Measurement of protein expressionVariables Incubate the cells 4–72 hours. The length of incubation depends upon the transfected vector construct, the cell type being transfected, andthe protein being expressed. After this incubation period, measureprotein expression using an assay appropriate for your system.4.4 Large-scale transfection using FuGENE 6 Reagent fortransient protein expressionOverview•FuGENE 6 Reagent has been successfully used to greatly increasethe speed of drug discovery in pharmaceutical drug screeningprograms. This is accomplished by eliminating the need to establishstable cell lines expressing receptors or ligands of interest.•Large-scale transient transfection experiments using FuGENE 6Reagent yield cells expressing molecules of interest at high levels.The exceptionally low toxicity of the reagent is especially beneficial.After transfection, cells retain most normal physiological functions,and can therefore serve as targets for various screening applica-tions. In addition, several cumbersome steps required with firstgeneration transfection reagents can be eliminated with the use ofFuGENE 6 Reagent (see Table 2). As a result, you can easily screenthousands of small molecules within a very short time period usingtransiently transfected cells.•FuGENE 6 Reagent has also been used to express pharmacologi-cally interesting receptors at high density in cells, followed by thepreparation of membrane fractions for classical drug bindingstudies (unpublished data). In more complex studies, cells can betransfected with molecules of interest, and then the whole cells canbe used in screening assays while monitoring physiological activitywith specific indicators.Table 2.Step FuGENE 6TransfectionReagent First generation transfectionreagentsCount and plate cells in FBS medium÷÷Incubate overnight÷Remove FBS-containing media÷Replace media without FBS÷Add transfection reagent:DNAcomplex÷÷Remove transfection reagent:DNAcomplex after 4–6 h÷Replace with fresh serum-containingmedia÷Total transfection steps27P R O C E D U R E S1814443.FM Page 11 Tuesday, August 21, 2001 10:58 AMRoche Molecular Biochemicals11Roche Molecular Biochemicals124.4.1Optimization in high through-put screening (HTS) applications and protein productionOptimization oflarge-scaletransfectionsRefer to the following table for a list of factors that can shorten the transfection procedure, and help attain maximal levels of protein expression. Optimization of FuGENE 6 Reagent/DNA ratio •In some systems, increasing the amount of both FuGENE 6 Reagent and DNA (more than ten fold higher than the recommended amounts), can continue to increase the level of protein expression. •The very low cytotoxicity of FuGENE 6 Reagent permits both the FuGENE 6 Reagent and DNA levels to be tested at these high levels without adversely affecting cell viability.Transfection of cells immedi-ately following trypsinization •Use FuGENE 6 Reagent to transfect some cell lines immediately following trypsinization and just prior to or after plating. •This will substantially reduce set-up time by eliminating the need to wait 24 hours before transfection.Transfection of adherent cells adapted for suspension growth •In some cases, adherent cells may be adapted for suspension growth, reducing requirements for expensive sterile plastic tissue culture vessels, and enable the production of transiently transfected cells on a very large scale. •HEK-293 cells have been transfected while in suspension growth using FuGENE 6 Reagent (unpublished data).Effect of media and media components, including sera •Different media and media components may influence the level of transfection efficiency and the subsequent growth of the transfected cells, as well as expression of the recombinant protein.•Test different media and optimize the level of each medium component for these effects. Although it is not usually necessary to remove the transfection reagent:DNA complex following the transfection step, it is necessary to feed your cells with fresh media for extended growth periods. This is especially important if the transfected cells are allowed to continue to grow for 3–7 days, allowing for maximal protein expression.P ROCEDURES1814443.FM Page 12 Tuesday, August 21, 2001 10:58 AMRoche Molecular Biochemicals135. Appendix5.1 Troubleshooting tableLow transfection efficiency Refer to the following table if you observe a low transfection efficiency.continued on next page Problem Possible cause RecommendationLow transfection efficiency Nucleic acids of poor quality or insufficient quantity Verify the amount or quality of nucleic acid:•Use only high-quality plasmid preparations, see section 4.2.•Use DNA at a concentration of 0.02–2.0 µg/µl.•Verify that the transfected plasmid construct contains appropriate promoters and other sequences required for protein expression in the cell line being transfected.•Perform a control transfection experiment with a commercially available transfection-grade plasmid preparation (e.g., the β-gal control vectors supplied with the Mammalian Expression Vectors for Epitope Tagging (Cat. No. 1 814 664).NOTE: Endotoxins are reported to be cytotoxic to some very sensitive cell lines (e.g., Huh-7) and primary cultures (13). When using FuGENE 6 Reagent for many common cell types, it may be possible to use DNA containing higher endotoxin levels.Insufficient number of cells were usedUse adherent cells at 50–80% confluency. FuGENE 6 Reagent was aliquoted and stored in a new containerCheck that FuGENE 6 Reagent is stored in the original container.FuGENE 6 Reagent came into contact with plastic Repeat transfection, carefully pipetting FuGENE 6 Reagent directly into the serum-free plex was formed in serum-containing medium Check original bottle of medium used for complex formation. Repeat experiment using new bottle of medium that does not contain any additives (e.g., serum, antibiotics, growth enhancers, etc.)1814443.FM Page 13 Tuesday, August 21, 2001 10:58 AMRoche Molecular Biochemicals145.1 Troubleshooting table , continued continued on next pageProblem Possible cause RecommendationLow transfection efficiency A suboptimal FuGENE 6:DNA ratio was used •Optimize the FuGENE 6 Reagent:DNA ratio according to the following procedure. Note: Always use more FuGENE 6 Reagent (µl) than DNA (µg). For example, combine 3 µl FuGENE 6 Reagent with 1–2 µg DNA for a 35 mm culture dish (6-well plate).•Prepare FuGENE 6:DNA mixtures according to the following table. Do not allow FuGENE 6 Transfection Reagent to come in contact with the plastic tube before dilution with serum-free medium.1Tap the tubes gently. Mix thoroughly, but do notvortex.2Incubate at room temperature for 15-45 minutes.3Add each FuGENE 6 Reagent:DNA mixture to a35mm culture dish or one-well of a 6-well plate. Swirlthe plates.4If you raise the DNA concentration (e.g., in a cotrans-fection experiment), proportionally increase the amount of FuGENE 6 Transfection Reagent.•If your cell line is not easily transfected by theabove FuGENE 6 Reagent:DNA ratios, test a wider range of ratios, including 2–15 µl FuGENE 6Transfection Reagent per 1–2 µg DNA, per 35 mmculture dish.•If no transfection is observed, repeat theexperiments with DOTAP or DOSPER Liposomal Transfection Reagent (see section 5.4, Related products).Label sixtubes 123456Add serum-free media (µl)979797949494Add FuGENE 6 Reagent (µl)333666Add DNA (µg)0.5121231814443.FM Page 14 Tuesday, August 21, 2001 10:58 AMRoche Molecular Biochemicals155.1 Troubleshooting table , continuedSigns of cytotoxicityRefer to the following table if you observe signs of cytotoxicity.continued on next page ProblemPossible cause Recommendation Signs of cytotoxicity NOTE: FuGENE 6 Transfection Reagent has proven to be virtually non-toxic to most cell types. Selection anti-biotic added too soon Repeat transfection and wait an additional 24 to 48 h before adding the selection antibiotic, to allow for sufficient protein production.Selection anti-biotic at too high a concentration Repeat transfection using several lower concentrations of selection antibiotic.Transfected protein is cyto-toxic or is pro-duced at high levels Reduced viability or slow growth rates may be the result of high levels of protein expression, as the cells ’ meta-bolic resources are directed toward production of the heterologous protein. The expressed protein may also be toxic to the cell at the level expressed.To analyze cytotoxicity, prepare experimental controls as described.Prepare extra wells containing:a. Cells that are not transfected.b. Cells transfected with DNA alone (e.g., without FuGENE 6 Transfection Reagent)c. Cells treated with FuGENE 6 Reagent alone (no DNA added).•Compare transfected cells with the experimentalconstruct, to the wells containing these experimentalcontrols.•Consider repeating the experiment with a secretedreporter gene assay such as SEAP, hGH, or a standardβ-gal control vector (see low transfection efficiency above). Cells secreting SEAP should show little to noevidence of cytotoxicity.The culture may be contaminated with mycoplasma •Use the Mycoplasma Detection Kit or Mycoplasma PCRELISA (see section 5.4) to determine if the culture is contaminated.•Treat the cells with BM Cyclin to eliminate the myco-plasma. Alternatively, start the transfections over witha fresh uncontaminated culture.Cells may not be healthy (e.g., malfunctioning incubator, media problems)Assess physiological state of cells and the incubation conditions (e.g., CO 2 and temperature levels). Perform the same controls as suggested above (for cytotoxicity), to eliminate influence of transfection reagent or nucleic acid.1814443.FM Page 15 Tuesday, August 21, 2001 10:58 AM。
