中东呼吸综合征病例流行病学个案调查表
12、世界卫生组织关于中东呼吸综合征病例调查指引
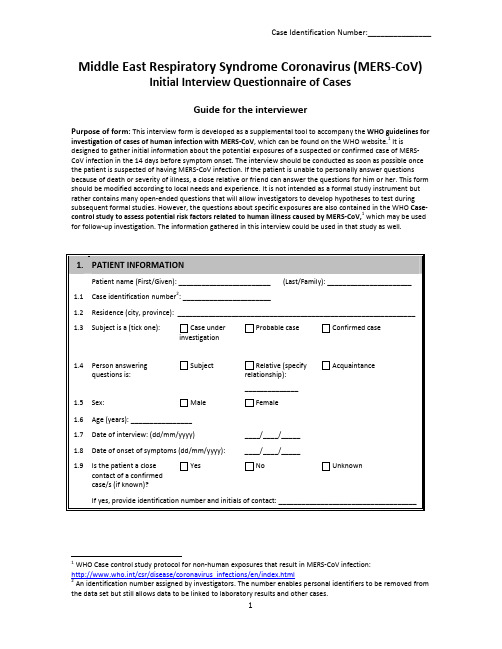
Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Initial Interview Questionnaire of CasesGuide for the interviewerPurpose of form:This interview form is developed as a supplemental tool to accompany the WHO guidelines for investigation of cases of human infection with MERS-CoV, which can be found on the WHO website.1 It is designed to gather initial information about the potential exposures of a suspected or confirmed case of MERS-CoV infection in the 14 days before symptom onset. The interview should be conducted as soon as possible once the patient is suspected of having MERS-CoV infection. If the patient is unable to personally answer questions because of death or severity of illness, a close relative or friend can answer the questions for him or her. This form should be modified according to local needs and experience. It is not intended as a formal study instrument but rather contains many open-ended questions that will allow investigators to develop hypotheses to test during subsequent formal studies. However, the questions about specific exposures are also contained in the WHO Case-control study to assess potential risk factors related to human illness caused by MERS-CoV,1which may be used for follow-up investigation. The information gathered in this interview could be used in that study as well.1 WHO Case control study protocol for non-human exposures that result in MERS-CoV infection:http://www.who.int/csr/disease/coronavirus_infections/en/index.html2 An identification number assigned by investigators. The number enables personal identifiers to be removed from the data set but still allows data to be linked to laboratory results and other cases.。
中东呼吸综合征

(3) 确诊病例 目前,具备下述4项之一者可确诊为中东呼吸综合征实验室确诊病例: 1)至少双靶标PCR检测阳性。 2)单个靶标PCR阳性产物,经基因测序确认。 3)从呼吸道标本中分离出中东呼吸综合征冠状病毒。 4)恢复期血清中东呼吸综合征冠状病毒抗体较急性期血清抗体水平呈4 倍及以上升高。
中东呼吸综合征病例诊断程序
• 影像学表现: 发生肺炎者影像学检查根据病情的不同阶段可表现为单侧至双侧的肺部影像 学改变,主要特点为胸膜下和基底部分布,磨玻璃影为主,可出现实变影。
• 一般实验室检查: 血常规:白细胞总数一般不高,可伴有淋巴细胞减少。 血生化检查:部分患者肌酸激酶、天门冬氨酸氨基转移酶、丙氨酸氨基转移 酶、乳酸脱氢酶、肌酐等升高。
• 9月7日,被收入卡塔尔多哈的一所医院的ICU进行治疗,随后出现 肾功能衰竭; • 9月11日,乘救护飞机从卡塔尔转至英国接受治疗; • 9月21日,该病例呼吸道标本经冠状病毒通用引物检测为阳性,通过 序列比对,怀疑为一种新型冠状病毒感染。
疫情发现
• 英国科学家对两例病例体内的新型冠状病毒聚合酶基因进行了序列比 对(针对250个碱基对),发现同源性高达99.5%;
在MERS冠状病毒中和抗体,且最早追溯到1992年沙特阿拉
伯和肯尼亚的单峰骆驼。
• 在多个国家(沙特阿拉伯、阿曼、埃及、卡塔尔)的单峰骆驼鼻
拭子和排泄物标本中,MERS冠状病毒核酸检测阳性,且幼年骆驼
的病毒载量较高(表明幼年骆驼更为易感);检出阳性标本的骆 驼表现为无症状或轻微呼峰骆驼中分离的MERS冠状病毒与病
(一)病例发现
2. 加强严重急性呼吸道感染(SARI)和不明原因肺炎监测。 1)流行病学史
2)对于缺乏流行病学史,在14天内发生的病因不明的SARI/不明原因 肺炎聚集性病例,医务人员中发生(尤其是在重症监护室)的SARI/ 不明原因肺炎病例,应当开展MERS实验室检测。
中东呼吸综合征

• 病毒性肺炎:起病缓慢,病情一般较轻,病程多 在2周左右。正常人群在受到病毒感染后,并不一 定发生肺炎,只有在全身或呼吸道局部免疫功能 低下时才容易发生本病。特点:通过飞沫与接触 传染,一年四季均可发生,但多见于冬春病毒疾 病流行季节。本病主要影响儿童,成人偶散发, 但老年人、免疫抑制患者易受感染产生肺炎。胸 闷X线征象常与症状不对称,往往症状严重而无 明显的X线表现,一般以间质性12年9月以来,全球共向世卫组织通报 了44例感染新型冠状病毒实验室确诊病例, 其中23例死亡。
• 发现实验室确诊病例的国家包括约旦、卡塔尔、沙特阿拉 伯、阿联酋、法国、德国、突尼斯和英国等。 • 世卫官员称,多数确诊病例为男性,患者年龄分布介于24 岁到94岁,平均年龄为56岁。其中30多名患者来自沙特。 • 世卫总干事陈冯富珍此前表示,此次爆发的新型冠状病毒 感染与“严重急性呼吸道综合征”(SARS)同科。她警告, 该病已发生有限的人际传播,卫生保健工作者已被感染。 • 世卫组织指出,医疗部门应对从病毒感染地区归来并出现 严重急性呼吸道感染的旅客保持警惕,在可能情况下,应 当获取病人呼吸道样本用于疾病诊断。
• 截至2013年6月2日,全球共有8个国家累计 报告病例51例,死亡33例。但并不像非典 病毒那样会在短时间内在人际间大面积传 播。
• 前往中东、欧洲有报告此病例国家旅游的 人员回国后,如出现发热、咳嗽、气短及 呼吸困难等呼吸道疾病,应及时就医。
中东呼吸综合征 • 症状: 感染中东新冠状病毒后的症状和非典有些相 似,感染者会出现急性、严重呼吸道疾病, 伴有发热、咳嗽、气短及呼吸困难,严重 的病例会出现肾功能衰竭和死亡。
病毒性肺炎治疗: 1.一般治疗及支持治疗为主,包括保暖,保持呼吸道通畅,防止水、电 解质和酸碱失衡,必要时氧疗、使用支气管扩张药物等,甚至给予机 械通气,改善患者一般状况,维持生命体征平稳。 2.是否包括病毒感染在内的所有CAP患者都需要接受抗菌素治疗?检测 到病毒病原体是否需要抗病毒治疗?迄今为止,对于这些问题尚无明 确的一致性认识。中国、美国和英国的CAP诊断和治疗指南都指出: 门诊治疗的轻、中度CAP患者直接给予经验性抗菌素治疗[5-7],而不 必普遍进行病原学检查,只有当初始经验性抗菌素治疗无效时才需进 行病原学检查。英国胸科协会还提出对怀疑感染流感病毒的患者一般 并不推荐联合应用经验性抗病毒治疗,只有对于有典型流感症状(发 热、肌痛、全身不适和呼吸道症状)、发病时间<2 d的高危患者及处 于流感流行期时,才考虑联合应用抗病毒治疗。
中东呼吸综合征疫情处理及实验室检测

中东呼吸综合征冠状病毒 MERS-CoV
人类冠状病毒
冠状病毒属 采用RNA依赖RNA聚合酶(RdRp)上1,23bp片段进行分析
MERS-CoV分类地位
• 冠状病毒科,冠状病毒属,β亚属,C种系 • 其它冠状病毒
– 与HKU4(扁颅蝠冠状病毒)和HKU5 (伏翼蝙蝠冠状病毒) 亲缘较近 – 与SARS-CoV、OC43和HKU1分属不同种系 – 与229E、NL63分属不同亚属
呼吸内科病房
科室
感染性疾病科病房
重症医学科病房
儿科病房
重症监护室(ICU)
疑似病例定义
• 患者同时符合临床表现和流行病学史,但尚无实验室确认依据。 临床表现 – 难以用其他病原感染解释的急性呼吸道感染:体温≥38 ℃ 、咳嗽,有胸部影像学改变等。 流行病学史 – 发病前14天内在中东呼吸综合征病例报告或流行地区旅游或居住;或与疑似/临床诊断/确诊病例有密切 接触史。 – 阿拉伯半岛中或附近的国家和地区:巴林、伊拉克、伊朗、以色列、约旦河西岸和加沙地带、约旦、科 威特、黎巴嫩、阿曼、卡塔尔、沙特阿拉伯、叙利亚、阿联酋、也门
病毒基因组
• 单股正链RNA,基因组=30,106bp,分11个开放读取框 • 合成5种独特的辅助蛋白(3, 4a, 4b, 5, 8b)及2种复制酶和4种结构蛋白
病毒结构
• RNA包膜病毒 – 病毒外壳:棘突蛋白、包膜蛋白、膜蛋白 – 病毒核心:单股正链RNA、核衣壳蛋白
膜蛋白 M
核衣壳蛋白 N
疫情发现
• 患者:男性,49岁,卡塔尔籍 • 发病时间:9月3日 • 入院时间:9月7日,多哈某医院;9月11日转 • 主要症状:呼吸道症状、肾功能衰竭 • 流行病学史:发病前曾到沙特阿拉伯 • 实验室检测:呼吸道样本中冠状病毒通用引物
流行病学个案调查表

流行病学个案调查表编码□□□□□□□□□□□□□(编码规则附后)1 一般情况1.1姓名:(14岁以下同时填写家长姓名)1.2性别:①男②女1.3民族:①汉族②其他1.4出生日期:年月日(若无详细日期,填写实足年龄岁)1.5职业:(1)幼托儿童(2)散居儿童(3)学生(4)教师(5)保育员/保姆(6)餐饮食品业(7)公共场所服务业(8)商业服务(9)旅游服务业(10)医务人员(11)干部职员(12)工人(13)民工(14)农民(15)林业(16)采茶(17)牧民(18)狩猎(19)销售/加工野生动物(20)离退人员(21)家务待业(22)不详(23)其他1.6现住址:省市(地、州)县(市、区)乡(镇、街道)村(居委会)组(门牌)1.7联系电话:联系人:与患者关系:1.8 身份证号:2 发病情况2.1发病时间:年月日2.2就诊情况就诊次数就诊日期就诊医疗机构医疗机构级别诊断门诊/住院病例第1次第2次第3次第4次注: 医疗机构级别:(1)村卫生室(2)乡镇级(3)县区级(4)地市级及以上2.3 现住医院入院时间:年月日2.4住院号:2.5入院诊断:2.6是否出院:①是②否□如已出院:2.6.1出院诊断:2.6.2出院时间:年月日2.7本次调查时病人情况:①痊愈②好转③恶化④死亡□2.8最后转归:①痊愈②死亡③其他3 临床表现3.1首发症状:3.2全身症状、体征:3.2.1发热①有②无最高:℃□3.2.2畏寒①有②无□3.2.3头痛①有②无□3.2.4乏力①有②无□3.2.5全身酸痛①有②无□3.2.6眼结膜充血①有②无□3.2.7皮肤瘀点或瘀斑①有②无□3.2.8牙龈出血①有②无□3.2.9食欲减退①轻度②厌食③无□3.2.10恶心①有②无□3.2.11呕吐①有②无□3.2.12呕血①有②无□3.2.13腹痛①有②无□3.2.14腹胀①有②无□3.2.15腹泻①有,次/天②无□3.2.16大便性状①血便②黑便③水样便④其他□3.2.17肾区疼痛①有②无□3.2.18淋巴结肿大①有②无□3.2.18.1若有,肿大部位及大小、是否压痛:3.3其他:4血常规检查序次检查日期(年/月/日)白细胞(109/L)血小板(109/L)中性粒细胞计数(109/L)淋巴细胞计数(109/L)检测单位5流行病学调查5.1发病前1个月居住地类型(可多选):①丘陵或山区②平原③其他________□5.2若5.1选②或③,则发病前1个月是否去过丘陵或山区?①是,具体地点(越细越好)_______________②否③不记得□5.3发病前两周户外活动史:5.3.1种地①是②否□5.3.2割草①是②否□5.3.3打猎①是②否□5.3.4采茶①是②否□5.3.5放牧①是②否□5.3.6采伐①是②否□5.3.6旅游①是_______________②否□5.3.7其它主要活动_______________________________________________ 5.4发病前1个月居住地是否有蜱:①有②无③不知道□5.5发病前1个月内是否见过蜱:①是②否③不认识□5.6发病前2周内是否被蜱叮咬过:①是②否③不知道□5.6.1若被叮咬过,时间及次数:①次数②首次被咬时间:年月日③末次被咬时间:年月日5.6.2叮咬部位(可多选):①脚②腿③腹部④背部⑤颈部⑥其他□5.7发病前2周内有无皮肤破损:①有②无□5.8发病前是否听说过同村有类似病人(未接触):①是②否(跳至5.9)□5.8.1听说类似病人情况姓名性别年龄现住址联系方式5.9发病前是否接触过类似病人:①是②否(跳至5.10)□5.9.1所接触病人情况姓名性别年龄现住址关系诊断接触方式联系方式注:接触方式(可多选):①直接接触病人血液②直接接触病人分泌物、排泄物③救治/护理④同处一室⑤其他(在表中注明)□5.10家中饲养动物情况:①是(填下表)②否③不知道□饲养动物种类发病前2周内是否与饲养动物接触动物身上是否有蜱附着注:动物身上是否有蜱附着:①是②否③不知道□5.11 发病前两周野生动物接触情况:①是(填下表)②否③不知道□动物种类动物身上是否有蜱附着备注5.12 病前1个月内家中是否发现过老鼠?①有②无③不知道□6调查小结:7标本编号(编码规则附后):7.1血清标本:7.1.1急性期血清编号:;7.1.2恢复期血清编号:;8实验室检验结果8.1病毒分离结果:①阳性②阴性③未检测/未收到标本□8.2核酸检测结果:①阳性②阴性③疑似④未检测/未收到标本□8.3血清学检测结果ELASA 间接免疫荧光法(IFA)IgG IgM IgG IgM 急性期血清恢复期血清注:请在空格中填写:①阳性②阴性③未检测/未收到标本编码规则:“年份(2位)-乡镇级地区编码(8位)-流水号(3位)”。
中东呼吸综合征(MiddleEastRespiratorySyndrome,MERS)

疑似病例
符合流行病学史和临床表现,但尚无实验室确认依据。 流行病学史:发病前14天内有中东地区旅游或居住史
;或与疑似/临床诊断/确诊病例有密切接触史。 临床表现:难以用其它病原感染解释的发热,(体温
≥38℃)伴呼吸道症状。
中东呼吸综合征病例诊疗方案(2014年版)
10
防范中东呼吸综合征(MERS)建议
中东呼吸综合征
(Middle East Respiratory Syndrome,MERS)
中国苏丹医疗队 2015年6月
概况
2012年6月13日,沙特诊断首例中东呼吸综合征。追溯 调查,2012年3月该病就已经在中东存在。
截止2015年5月16日,全球报告1142例确诊病例,其中 465例(41.6%)死亡。
沙特一起医院感染暴发 共23例感染 密切接触者: 217 例家庭成员(5个家庭成员中3例确诊)
超过200 名医护人员(2名感染)。
6
临床表现
潜伏期:2-14天
以急性呼吸道感染为主要表现,起病急,体温高达39-40 度,可伴有畏寒、寒战、咳嗽、咳痰、胸痛、头痛、全身 肌肉关节酸痛、乏力、食欲减退等症状。
部分病例以腹泻等非典型临床表现为首发症状。 在肺炎基础上,临床病变进展迅速,很快发展为呼吸衰竭
、或者多器官衰竭,特别是肾衰竭。 少数病例病情较轻。
7
病程第7天(Βιβλιοθήκη 院时)发病第9天存在下列危险因素时易出现严重并发症
男性。 年龄大于60岁。 合并其它疾病,如糖尿病、高血压、哮喘、缺
血性心脏病、免疫系统缺陷和终末期肾脏病。 维持性血液透析患者。
国内关于MERS 的报告制度
发现中东呼吸综合征疑似病例、临床诊断病例、 确诊病例及无症状感染者时,具备网络直报条件 的医疗机构应当于2小时内进行网络直报(“无症 状感染者”选择“隐性感染者”类别);不具备 网络直报条件的,应当于2小时内以最快的通讯方 式(电话、传真)向当地县区级疾控机构报告, 并于2小时内寄送出传染病报告卡,县区级疾控机 构在接到报告后立即进行网络直报。
WHO-中东呼吸综合征(MERS)流行病学调查指引

WHO:中东呼吸综合征(MERS) 流行病学调查指引(2013年7月)世界卫生组织目录1.概述 (3)2.本指引目的与范围 (3)3.调查的关键步骤 (4)3.1调查准备 (4)3.2调查目标 (4)3.3病例确定与面访 (5)3.4病例搜索 (7)3.5生物标本采集与实验室检测 (10)4.数据分析 (11)5.研究与专题调查 (12)5.1病例对照研究 (12)5.2卫生服务暴露研究 (12)5.3近期呼吸道疾病趋势研究 (13)5.4血清流行病学研究 (13)5.5动物卫生与环境调查 (14)5.6临床管理研究 (14)6.感染控制 (15)7.结果报告 (15)8.参考文献 (16)1.概述冠状病毒是一大类能导致人类疾病的病毒,其临床表现涵盖了从普通感冒到严重急性呼吸综合征(SARS)的一个很大的范围。
该类病毒也能在许多种动物中引起疾病。
2012年底,在中东居民身上发现了一种新的冠状病毒,这种冠状病毒以往从未在人类中发现过。
该病毒被命名为中东呼吸综合征冠状病毒(MERS-CoV),现已发现至少50多例实验室确诊的人类感染病例。
目前,所有感染MERS-CoV的病例都和中东地区有直接或间接的联系,但是在其他国家近期前往中东旅行的人群中已发现局部的非持续的人际传播发生。
所有MERS-CoV感染病例主要表现为呼吸道疾病,也有一些后续并发症报道,如急性肾功能衰竭,多器官功能衰竭,急性呼吸窘迫综合征(ARDS)和消耗性凝血病等。
此外,许多病例出现胃肠道症状,如腹泻。
超过一半的病例死亡。
大多数病例至少存在一种合并的疾病,但许多病例既往身体健康。
很小一部分病例合并感染有其他病原体,如流感病毒,副流感病毒,单纯疱疹病毒和肺炎球菌等。
截至2013年6月6日,实验室确诊病例的平均年龄为56岁(范围2–94岁),且多数是男性(72%)。
MERS-CoV被认为是一种动物病毒,偶尔感染人类并导致有限的人际传播。
病毒的动物来源的证据是间接的。
中东呼吸综合征月度风险评估

医院内其他患者陪同家属 16人,救护车有关工作人员12人。
WHO风险评估-1(2015-7-7)
MERS-CoV的传播模式已经发生变化了吗?
没有证据表明社区存在持续的人间传播,也没有证据表明出现 了空气传播
传播模式未发生变化
临床特征未发生变化;没有基础性疾病的续发病例比原发病
例症状常更轻 除韩国外,近期报告的输出病例,均未发生续发病例 密接强化监测表明,家庭内传播少见,航空器上未发生传播 家庭聚集性病例的数量和规模未见增加 MERS-CoV的R0仍小于1,但在医疗机构中可能大于1。沙特 、阿联酋、中国和泰国的经验表明,早期隔离病例和采取完 善的感控措施可使R 小于1
中国病例来自于韩国,与韩国首例输入性病例有流行病学关联
全球MERS病例发病曲线(截至2015年10月30日)
2015/11/30
2015/11/30
2015/11/30
韩国病例随访信息(WHO,10月25日)
10月12日,韩国向WHO通报了一例既往MERS病例随访信息。
既往感染情况:该病例 35岁,男性,有基础性疾病,5月27日与确
工作建议
继续追踪MERS疫情进展,动态开展风险评估 医务人员接诊发热呼吸道病例时,应注意询问病例或其密切接触 的其他类似病例中病前14日内有无中东地区旅行史,尤其是医 疗机构就诊史
继续加强实验室检测和应急技术准备
谢 谢!
MERS全球疫情概况(截至2015年10月29日)
全球累计报告MERS确诊病例1611例,其中575人死亡(病死率 36%) 病例分布在26个国家 中东地区(10个):沙特阿拉伯、阿联酋、卡塔尔、约旦、阿 曼、科威特、也门、埃及、伊朗、黎巴嫩 欧洲(8个):意大利、法国、德国、英国、希腊、荷兰、奥 地利、土耳其 亚洲(5个):马来西亚、菲律宾、韩国、中国、泰国 非洲(2个):突尼斯、阿尔及利亚 北美洲(1个):美国 86%的病例发生在中东地区国家,78%的病例发生在沙特,韩国 病例占11%
13、世界卫生组织关于中东呼吸综合征病例的个案调查表

1WHO guidelines for investigation of casesof human infection with Middle EastRespiratory Syndrome Coronavirus(MERS-CoV)(July 2013)ContentsContents (2)1.Introduction (3)2.Purpose and scope of the document (4)3. Key steps for an investigation (5)3.1Preparation (5)3.2Objectives (5)3.3Case identification and interview (6)3.4Case finding (9)3.5Biological specimen collection and laboratory testing (12)4. Data Analysis (14)5.Studies and specific investigations (15)5.1Case-control studies (15)5.2Health care exposures (15)5.3Recent respiratory disease trends (16)5.4Seroprevalence studies (17)5.5Animal health and environmental investigations (17)5.6Clinical management studies (18)6.Infection control (18)7.Reporting results (19)8.References (21)21.IntroductionCoronaviruses are a large family of viruses that can cause a range of illnesses in humans, from the common cold to severe acute respiratory syndrome (SARS). These viruses also cause disease in a wide variety of animal species.In late 2012, a novel coronavirus that had not previously been seen in humans was identified for the first time in a resident of the Middle East. The virus, now known as the Middle East Respiratory Syndrome Coronavirus (MERS-CoV),1has caused more than 50 laboratory-confirmed cases of human infection. Thus far, all patients infected with MERS-CoV have had a direct or indirect link to the Middle East, however, local non-sustained human-to-human transmission has occurred in other countries, in people who had recently travelled to the Middle East.All MERS-CoV patients have primarily had respiratory disease, although a number of secondary complications have also been reported, including acute renal failure, multi-organ failure, acute respiratory distress syndrome (ARDS), and consumptive coagulopathy. In addition, many patients have also reported gastrointestinal symptoms, including diarrhoea. More than half of infected patients have died. The majority has had at least one comorbid condition, but many have also been in previous good health. A small number of cases had had co-infection with other viruses including influenza A, parainfluenza, herpes simplex, and pneumococcus. As of 6 June, the median age of reported laboratory-confirmed cases is 56 years (Range 2–94 years) and majority (72%) are males.2 A current update of the cases can be found at WHO’s Coronavirus website.The MERS-CoV virus is thought to be an animal virus that has sporadically resulted in human infections, with subsequent limited transmission between humans. The evidence for the animal origin of the virus is circumstantial. Nevertheless, the alternative explanation to explain the sporadic appearance of severe human cases with long periods of time between them, and the wide geographical area over which the virus was apparently distributed, is unrecognized ongoing transmission in people. Surveillance efforts since the discovery of the virus and retrospective testing of stored respiratory specimens suggest this is not the case.The virus has been demonstrated to grow well in cell lines that in the past have commonly been used for diagnostic viral cultures. Finally, early comparisons with other known coronaviruses suggest a genetic similarity to viruses previously described in bats. However, even if an animal reservoir is identified, it is critical to identify the types of exposures that result in infection and the mode of transmission. It is1 http://www.who.int/csr/disease/coronavirus_infections/NamingCoV_28May13.pdf2 WHO Coronavirus website: http://www.who.int/csr/disease/coronavirus_infections/en/.3unlikely that transmission occurs directly from animals to humans and the route of transmission may be complex requiring intermediary hosts, or through contaminated food or drink.A considerable proportion of MERS-CoV cases have been part of clusters in which limited non-sustained human-to-human transmission has occurred. Human-to-human transmission has occurred in health care settings, among close family contacts, and in the work place. Sustained transmission in the community beyond these clusters has not been observed and would represent a major change in the epidemiology of MERS-CoV.A number of unanswered questions remain on the virus reservoir, how seemingly sporadic infections are being acquired, the mode of transmission from animals to humans and between humans, the clinical spectrum of infection, and the incubation period.2.Purpose and scope of the documentThis document provides a standardized approach for public health authorities and investigators at all levels to plan for and conduct investigations around confirmed and probable cases of MERS-CoV infection. It should be read in conjunction with other detailed guidance referenced throughout the text, such as current laboratory testing guidelines and study protocols. It will be updated as necessary to reflect increased understanding of MERS-CoV transmission and control.Most of the advice given in this document will apply primarily to countries in which infection is presumed to have originated from an animal or environmental source, and the exposures that result in infection remain the critical questions. In countries that have secondary transmission related to imported cases, however, the recommendations for finding secondary cases and observing subsequent community transmission are still valid, though on a more limited scale. Similarly, the case-control study recommended as a high priority in the second part of the document is not applicable to countries with imported cases, since the purpose of the study is to uncover the non-human exposures leading to infection. However, other studies on health care facility transmission and clinical management are still recommended.As with nearly all recent emerging novel pathogens, most early cases of MERS-CoV infection will likely be detected by astute clinicians rather than through established indicator or sentinel surveillance systems. Therefore, the most effective tool in detection will be awareness among the health care providers. An effective detection system will also need to include a readily available channel by which4clinicians can report suspect cases, and an effective response mechanism. The Western Pacific Regional Office of WHO (WPRO) has published a guide for event surveillance.3This document addresses two general categories of activities that need to be undertaken to deal with newly identified cases. The first involves further case finding, case description, and surveillance enhancements in the area where the case is discovered. The primary purpose of these activities is to fully describe the epidemiology of the cases, identify and monitor close contacts of the cases and determine the extent of spread of the virus in the area (sections 3 and 4). The second group of activities is a number of discrete studies aimed at answering critical questions related to MERS-CoV (section 5). 3. Key steps for an investigation3.1 PreparationA multi-disciplinary team should be assembled. Team members should have experience in field epidemiology, clinical assessment, laboratory specimen collection, infection control, and social mobilization and risk communication. Animal health specialists are also a critical part of the team. Additional team members may include logisticians, laboratory experts, data managers, and environmental health specialists. The size and composition of the initial investigation team may vary depending in part on the size and complexity of the anticipated investigation. Designation of a team leader and attribution of roles and responsibilities is critical to the success of the investigation.Before deploying, the team should gather preliminary background information, assemble the necessary materials and supplies (e.g. personal protective equipment, specimen collection and transport materials) and inform relevant local public health and animal health authorities.3.2 ObjectivesWhen setting up an investigation, it is critical to clearly define the objectives and use a standardized approach that addresses each of these. These objectives might be to:Public Health Objectives•Identify other cases and quickly detect any human-to-human transmission.•Reduce onward transmission, morbidity and mortality through rapid identification, isolation, treatment and clinical management of cases and follow-up of contacts.3http://www.wpro.who.int/emerging_diseases/documents/eventbasedsurv/en/5•Prevent future cases through identification of potential human, animal, and/or environmental sources of exposure, risk factors for infection, and implementation of appropriate preventionand control measures.Knowledge Objectives•Determine the size of geographic area where the virus is transmitting.•Determine key epidemiological, clinical, and virological characteristics for cases including clinical presentation and natural history, the mode(s) of transmission and disease diagnosis, incubation period, period of transmissibility, and best practices for treatment.•Determine if the efficiency of human-to-human transmission of the virus has changed or increased.3.3 Case identification and interviewLaboratory-confirmation of a MERS-CoV case is an immediate trigger to launch a thorough investigation. However, because collection, shipment, and testing of specimens often require several days or longer, the investigation may need to begin before laboratory test results are available for suspected cases. Even if laboratory-confirmation is not possible, an investigation should still be launched if a patient is strongly suspected to have MERS-CoV infection (e.g. patient with severe acute respiratory infection [SARI] who has a history of travel to Middle East or has been in contact with cases who have died). The patient and/or family members (if the patient is too ill to be interviewed or has died) should be interviewed within the first 24–48 hours of the investigation to collect basic demographic, clinical, and epidemiological information. A sample questionnaire for the initial interview can be found on the WHO Coronavirus website2 but should be adapted and augmented with questions about practices and exposures in the local community.3.3.1 Essential basic informationThe following basic information should be collected, including:•Patient ID number/cluster number (if applicable).•Relationship between the person answering questions on behalf of the case patient (in the case that the patient is too ill for interview or has died).•Date of symptom onset (by symptom, if possible).•Date of initial admission/ visit to health care facility.•Date of initial WHO notification.•Patient contact details (e.g. name, home address, home/mobile telephone numbers).•Demographic information (e.g. date of birth/age, sex).•Occupation (including specific classification such as healthcare worker, laboratory worker, and farm worker etc.).6•Date of sample collection, laboratory testing and specimen type (e.g. nasopharyngeal swab, sputum, etc.).3.3.2 Exposure Information and travel historyPossible exposures in the 14 days4 before the onset of symptoms should be thoroughly explored and described, with special focus on:•Animal exposureso Presence of animals in or around household area where the case patient lives or works(e.g. pets, rats, other rodents, bats, camels, birds, etc.).o Activities that result in animal exposures and type of animals exposed to (e.g. keeping livestock, visiting farms, visiting live animal markets racetracks, or practicing falconry,participating in the slaughter or sacrifice of animals etc.).o Exposures to animal products or products potentially contaminated by animal excreta or body fluids.•Human exposures:o Recent contact with individuals with respiratory illness and/or gastrointestinalsymptoms, including people who have been severely ill or have died (indicate the type(s)of contact, frequency, and duration of exposure, and location).o Recent admission in hospital.o Recent visit to outpatient treatment facility.o Recent visit to traditional healer.•Food exposures:o Recent consumption of unprocessed, raw foods or drinks.o Recent consumption of raw or undercooked meat, or uncooked blood products.o Recent preparation of fresh meat for consumption.o Use of smoking apparatus such as hookah or shisha.•Travel history:o Dates, destinations and details mode of transport for recent travel (local andinternational).o Activities during the period of travel (including information on animal, human and food exposures as listed above).4 Although the incubation period of infection is unknown, given what is known about other coronaviruses and the MERS-CoV cases in which exposures are recognised, it is reasonable to focus on exposures occurring in the 14 days before onset of illness.73.3.3 Clinical InformationData on the presentation of illness, pre-existing medical conditions, clinical course of illness, and occurrence of complications are critical for refining case definitions and informing clinical management recommendations. As such, detailed clinical data should be collected on each confirmed case and systematically summarized. A clinical collection form has been developed by WHO/ISARIC5; see section 5.6 for more information).•Clinical datao Date of illness onset.o Signs and symptoms at initial presentation.o Time course of illness including time from illness onset to: care-seeking, first hospital admission, deterioration requiring advanced clinical management, and final outcome.o Presence of pneumonia and progression to respiratory failure, development of the acute respiratory distress syndrome (ARDS).o Occurrence of other complications such as renal failure or other organ systemcompromise, coagulopathies, secondary infections, sepsis, etc.o Presence of pre-existing chronic conditions (immunosuppression, cancer, renal insufficiency, hemoglobinopathies, liver disease, neurological disease, endocrine andmetabolic disorders, etc.).o Dates and results of any ancillary tests performed (X-Ray, CT scan, etc.).o Use of respiratory support (supplemental oxygen and FiO2; non-invasive and invasive mechanical ventilation, prone positioning, use of inhaled nitric oxide, oscillatoryventilation, Extra Corporeal Membrane Oxygenation [ECMO]).o Use of other organ support modalities (renal replacement therapy, vasopressors, etc.).o Use of antibiotics, corticosteroids, other medical therapies.o Documentation of co-infections (viral, bacterial, fungal).o Clinical outcomes (recovered, ill, critically ill, duration of intensive care unit admission, duration of hospitalization, deceased).o Virological outcomes (if available), including duration of MER-CoV shedding inrespiratory tract specimens, and extrapulmonary clinical specimens.•Infection control relatedo Where patient was located in health care facility. Which other places (e.g. radiology) may have been visited.o Infection control precautions that were used in relation to patient including type of masks, etc.5/isaric/documents/WHO_SARI_NewOutbreak_case_record_form_v2.pdf8•Laboratory data (haematology, biochemistry, and virology)o Date specimen taken.o Type of test, type of specimen.o Test results and date of results.o Name of laboratory performing test.o Name of national laboratory.o Name of reference laboratory (if applicable).3.4 Case finding3.4.1 Develop a case definitionAn additional first step in the investigation is to identify other cases among contacts of the known case and in the community. To do this, the investigation team must first identify the types of clinical presentations or syndromes that will be sought as part of the case finding activity. WHO has developed surveillance case definitions for classification and reporting of human cases6 globally but these are not designed for case finding around newly discovered cases. Definitions for additional case finding must be developed locally and may be shaped by information obtained from the interview with the first case. These definitions are used to identify patients in the community who should be tested for MERS-CoV infection and should incorporate time periods, localities, illness characteristics, exposure and other information. The criteria used should those that clinicians will find simple, easily understandable, and memorable. The case definition should be sensitive enough during the initial stages of the investigation to capture the majority of cases. An example of a case definition to use for this purpose might include features such as:•Location: the local community where the case occurred. This will be defined according to the local situation but should include an area that incorporates other individuals who may haveexposures to the same source of virus to which the patient was exposed. As the relevantexposures are currently unknown, they should include the population area that generallyincludes local markets, places of worship, and health care facilities that the case may haverecently visited.•Time frame: some retrospective case finding should be conducted and therefore the time period should cover at least two weeks before the onset of symptoms of the case.•Patients’ characteristics may include the following, but should be modified according to the latest clinical data on cases:6http://www.who.int/csr/disease/coronavirus_infections/case_definition/en/index.html9o A patient with SARI7 who presents with fever and cough, requires admission to hospital, and whose disease is not completely explained by another pathogen.o A patient with SARI whose clinical course is unexpectedly severe even if another pathogen was initially identified and the patient did not respond to appropriatetreatment.o A patient with SARI with recent exposure to animals.o An immunocompromised patient who presents with an acute illness that is not fully explained by another pathogen.3.4.2 Contact monitoringClose contacts of confirmed or probable cases should be identified and monitored for the appearance of respiratory symptoms for 14 days after last exposure to the confirmed or suspected case, while the case was symptomatic. Any contact that becomes ill in that period of time should be tested for MERS-CoV.A line-listing of all contacts and co-exposed persons that records demographic information, date of first and last common exposure or date of contact with the confirmed or probable case, and date of onset if fever or respiratory symptoms develop should be maintained. The common exposures and type of contact with the confirmed or suspected should be thoroughly documented for any contacts that become infected with MERS-CoV.Initiate active monitoring (e.g. daily visits or telephone calls) for the development of fever and acute respiratory illness or any other symptoms in close contacts for 14 days after the last exposure to the initial case. Contacts should also be advised to contact health care workers as soon as they develop above symptoms. If any of the contacts are confirmed to have MERS-CoV infection, their close contacts should also be monitored.Collect appropriate clinical specimens (see section 4.4.1) on any close contacts with an acute respiratory illness regardless of severity, and test for MERS-CoV. While under investigation, symptomatic contacts should limit their contact with well individuals and practice good respiratory hygiene to prevent onward transmission. Current advice on preventing transmission both in the household and in health care facilities can be found on the WHO Coronavirus website. The decision of whether to admit symptomatic cases or contacts should be based on clinical judgment and concerns about further transmission. If symptomatic individuals are managed at home, they should be monitored closely for progression of their illness. Currently, it is not possible to predict the course of illness of an individual patient.7 SARI definition: An acute respiratory infection with history of fever or measured fever of ≥ 38 C⁰ and cough, with onset within the last seven days, that requires hospitalisation.10Serological investigation of contacts: In addition to monitoring close contacts for the appearance of acute illness and testing with PCR, it is strongly advised that sera be collected on all close contacts, including health care workers. This will assist in demonstrating the presence of mild and asymptomatic MERS-CoV infections and help in defining common exposures in the environment or exposures to the case that might result in infection. Investigators should collect acute sera on all close contacts immediately after the confirmed or suspected case is identified. Sera collection should be repeated in close contacts 3–4 weeks later, regardless of whether contacts have developed symptoms. Symptomatic contacts should also have appropriate respiratory specimens collected for PCR testing (see section 3.5). If the index case was ill more than 3–4 weeks before the investigation is undertaken, only a single serum specimen needs to be collected from contacts. In addition to the second serum specimen, for each contact collect information regarding:•Any illness that may have occurred during the intervening time period, including all signs and symptoms, and their severity.•Specific exposures to the confirmed or suspected case including providing care, exposure to body fluids and other physical contact, duration and proximity of exposure, eating with andsleeping in the same room as the case.•Exposures to animals, unprocessed food and beverages, and other social and environmental contacts.A protocol for contact investigation is available at: /articles/novel-coronavirus-ncov/(Seroepidemiological Investigation of Close Contacts of Novel Coronavirus (nCoV) Patients by the CONSISE network).3.4.3 Active search for additional casesEfforts to identify additional cases beyond close contacts are critical for prevention and control of infection, and to determine the total extent of transmission in the community. Active case finding in the area under investigation should focus on:•Patients currently admitted to health care facilities in the community where the confirmed MERS-CoV case was discovered. Any patients currently in the hospital with unexplained SARIshould be considered for testing for MERS-CoV.•Health care providers in the community; health workers should be interviewed about recent cases of unexplained pneumonia and notified to immediately report any patients who havesigns and symptoms that meet the case definition developed for the investigation as described above in section 3.4.1. Patients meeting the case definition should be tested for MERS-CoV.•Patients who recently died of an unexplained illness consistent with the case definition developed for the investigation should be tested for MERS-CoV infection if appropriate clinical specimens are available.113.4.4 Initiate enhanced surveillanceIn addition to case finding activities, surveillance in the area under investigation should be enhanced to detect cases that might arise subsequent to the discovery of the index case. The geographical area targeted will need to be assessed on a case-by-case basis and is defined by the suspected exposures of the case under investigation. The duration of the enhanced surveillance will depend on the findings of the investigation and whether there is evidence indicating that sustained transmission may be occurring in the area. A minimum of one month of enhanced surveillance is a reasonable starting point. Enhancements include:•Introduction of laboratory capacity for MERS-CoV testing in the local health care facility, if feasible, or establishment of mechanisms for rapid transfer of specimens to a capable laboratory.•Inform clinicians in the community of the need for vigilance and the case definition for case finding (section 3.4.1).•If SARI surveillance is in place, expand to other facilities in the area. If it is not, initiate SARI surveillance at health care facilities in the community of the case. Standards and guidance forSARI surveillance in the WHO Global Interim Epidemiological Surveillance Standards for Influenza document on the WHO influenza surveillance website.8•Increase the testing for MERS-CoV of SARI cases at local health care facilities in the area under investigation.•If resources allow, consider some testing of milder cases of influenza-like illness presenting to surveillance sites.3.5 Biological specimen collection and laboratory testing3.5.1 Specimen CollectionTo confirm the presence of MERS-CoV in suspect cases, collect appropriate clinical specimens for testing:o Available evidence suggests that lower respiratory tract specimens contain higher virus titres than upper respiratory tract specimens and are more sensitive for detecting the presence of the virus. Lower respiratory tract specimens include:•Sputum, induced or non-induced.•Endotracheal aspirate for patients on mechanical ventilation.•Bronchial alveolar lavage for those in whom it is indicated for patient management.8 WHO influenza surveillance website: http://www.who.int/influenza/surveillance_monitoring/en/12o Upper respiratory tract specimens such as nasopharyngeal and oropharyngeal swabs should be collected if lower respiratory tract specimens cannot be collected. If initial testing of an upperrespiratory specimen is negative in a patient suspected of having MERS-CoV infection, repeattesting should be performed.o Collect blood for serological testing. For recent cases, an initial blood specimen should be collected and a repeat specimen taken after a period of at least 3 weeks. For cases that hadsymptom onset more than 3 weeks prior to being investigated, a single blood sample issufficient (note: results of single sera will need to be interpreted with caution as the extent ofcross reactivity of currently available serological assays is unknown).o MERS-CoV has been identified in other body fluids including blood, urine, and stool of infected patients. However, titres of virus in these body fluids are quite low and they may not be useful for diagnostic testing. The presence of virus in these body fluids could have public healthimplications and could be part of an ancillary study of a case.Health care workers collecting clinical specimens should exercise appropriate infection control measures including use of personal protective equipment. Current guidelines for infection control and prevention can be found in the Technical guidance - infection prevention and control document on the WHO Coronavirus website along with guidance on specimen storage and shipment.93.5.2 Molecular diagnosticsPCR is the most widely used method for detecting the presence of the virus. At least three sites in the virus genome have been identified as suitable targets for such assays, including upE, ORF 1A and ORF 1B, and sequences of the necessary primers have been published. To perform these assays, laboratories should order the primers from their usual suppliers. Positive controls for the UpE screening and the ORF 1A confirmation assays are also available. Further details are available in the Technical guidance – laboratory section of the WHO Coronavirus website.2A confirmed case should either have positive test results for at least two different sites in the virus genome, or a positive result for a single site plus sequencing of a different, appropriate site that shows close similarity to known sequences of the virus. Testing should be carried out in laboratories that are experienced in performing these procedures. Specimens should be sent to a reference laboratory for confirmation.9 Novel Coronavirus: Interim Recommendations for Laboratory Biorisk Management.http://www.who.int/csr/disease/coronavirus_infections/NovelCoronavirus_InterimRecommendationsLaboratoryB iorisk_190213/en/index.html13。
中东呼吸综合征概况6.30

其他国家
沙特于6月9日至12日,向WHO报告了3例确诊莫斯病例,其中1例死 亡。第一例为77岁难,5月26日发病,27日住院,31日出院。6月10 日再次出现症状而住院,11日确诊,当日死亡;第二例为60岁女性 ,6月2日发病,8日住院,9日确诊。在5月26日和6月7日,她探视 了在住院的女儿。目前病情稳定。第三例为73岁男性,5月23日发 病,6月1日住院,8日确诊。目前在ICU治疗。 6月15日,阿联酋向WHO报告了1例确诊莫斯病例。患者为65岁男性 ,5月31日发病,6月6日住院,14日确诊。 自从2012年9月以来,全球报告莫斯病例1292例,其中458人死亡。
27例死亡病例均有各种各样的基础疾病(癌心
肺疾病、肾病、糖尿病和免疫功能不全等)
死亡病例: ~性别分布: 男性20例(74.1%) 女性7例(25.9%) ~年龄分布: 60-:9例(33.3%)
70-:8例(29.6%)
80-:5例(18.5%) 50-:4例(14.8%) 40-:1例(3.7%)
二代病例往往比原发病例症状轻,很多二代病例为轻症、无症状感染。
传播途径
空气传播是指微生物通过一个人的说话、咳嗽或打喷嚏等方式漂浮 在空气中,然后飘落入另一个人的眼中、口腔或鼻子中引起感染。 如果一种微生物能够通过空气传播,则一个人无需直接接触到病人 ,就能被传染而罹患疾病。空气传播的疾病如水痘、结核病。 飞沫传播是当微生物以飞沫核的形式通过病人咳嗽或打喷嚏的方式 进入到另一个人的眼、鼻腔或口腔中。飞沫传播距离短,一般小于 3英尺(1米)。
最新韩国进展6.24
其中93号确诊的中国籍患者(女,64岁)近期治愈出院,本月10日, 中国驻韩大使馆证实这名中国女性5月底曾在京畿道东滩圣心医院 工作,有可能是在此期间与第15例确诊患者有过接触而被传染。 新确诊病例现状:
WHO-中东呼吸综合征(MERS)流行病学调查指引

WHO:中东呼吸综合征(MERS) 流行病学调查指引(2013年7月)世界卫生组织目录1.概述 (3)2.本指引目的与范围 (3)3.调查的关键步骤 (4)3.1调查准备 (4)3.2调查目标 (4)3.3病例确定与面访 (5)3.4病例搜索 (7)3.5生物标本采集与实验室检测 (10)4.数据分析 (11)5.研究与专题调查 (12)5.1病例对照研究 (12)5.2卫生服务暴露研究 (12)5.3近期呼吸道疾病趋势研究 (13)5.4血清流行病学研究 (13)5.5动物卫生与环境调查 (14)5.6临床管理研究 (14)6.感染控制 (15)7.结果报告 (15)8.参考文献 (16)1.概述冠状病毒是一大类能导致人类疾病的病毒,其临床表现涵盖了从普通感冒到严重急性呼吸综合征(SARS)的一个很大的范围。
该类病毒也能在许多种动物中引起疾病。
2012年底,在中东居民身上发现了一种新的冠状病毒,这种冠状病毒以往从未在人类中发现过。
该病毒被命名为中东呼吸综合征冠状病毒(MERS-CoV),现已发现至少50多例实验室确诊的人类感染病例。
目前,所有感染MERS-CoV的病例都和中东地区有直接或间接的联系,但是在其他国家近期前往中东旅行的人群中已发现局部的非持续的人际传播发生。
所有MERS-CoV感染病例主要表现为呼吸道疾病,也有一些后续并发症报道,如急性肾功能衰竭,多器官功能衰竭,急性呼吸窘迫综合征(ARDS)和消耗性凝血病等。
此外,许多病例出现胃肠道症状,如腹泻。
超过一半的病例死亡。
大多数病例至少存在一种合并的疾病,但许多病例既往身体健康。
很小一部分病例合并感染有其他病原体,如流感病毒,副流感病毒,单纯疱疹病毒和肺炎球菌等。
截至2013年6月6日,实验室确诊病例的平均年龄为56岁(范围2–94岁),且多数是男性(72%)。
MERS-CoV被认为是一种动物病毒,偶尔感染人类并导致有限的人际传播。
病毒的动物来源的证据是间接的。
中东综合征(2014版)

三、防控措施
• 各级各类医疗机构负责病例的发现与报告、 诊断、救治和临床管理,开展标本采集工 作,并对本机构的医务人员开展培训。
第28页,共59页。
三、防控措施
• (二)加强中东呼吸综合征病例的监测。 各级各类医疗机构、各级疾控机构负责开 展中东呼吸综合征病例的发现和报告工作
第29页,共59页。
三、防控措施
• 1.病例发现。
• (1)建立健全中东呼吸综合征病例的监测体系。各级
各类医疗机构的医务人员在日常诊疗活动中,应提高对 中东呼吸综合征病例的诊断和报告意识,对于不明原因 发热病例,应注意询问发病前14天内的旅行史或可疑的 暴露史,了解本人或其密切接触的类似病人近期有无赴沙 特、阿联酋、卡塔尔、约旦等中东国家以及韩国等其他近 期有中东呼吸综合征病例国家的旅行史,或可疑动物(如
第11页,共59页。
临床诊断
无症状感染者: • 无临床症状,但具备实验室确诊依据4项之一者。
第12页,共59页。
治疗
基本原则: 根据病情严重程度评估,确定治疗场所: • 疑似、临床诊断和确诊病例应在具备有效
隔离和防护条件的医院隔离治疗。 • 危重病例尽早入ICU治疗。 • 转运过程中采取隔离和防护措施。
染时应用。
第14页,共59页。
中医中药治疗
• 依据中医学“温病、外感热病、风温肺热 病”等病证辨证论治。
第15页,共59页。
中医中药治疗
• 1、邪犯肺卫证。 • 主症:咽痛、鼻塞、头身痛,或伴发热恶
寒,咳喘等。 • 治法:解表宣肺,清热利烟。 • 推荐方剂:柴葛解肌汤合银翘散。常用药
物柴胡、葛根、荆芥、赤芍、银花、连翘 、牛蒡子、桔梗、黄芩等。
第31页,共59页。
中东呼吸综合征-黑龙江疾病预防控制中心

分类
•HCoV-2012
MERS与SARS的比较
MERS-CoV病原学
➢ β类冠状病毒的2c亚群,是一种具有包膜、基因组为线性 非节段单股正链的RNA病毒。病毒粒子呈球形,直径为 120-160 nm。基因组全长约30kb。目前已经完成多株 MERS-CoV的全基因组序列测定,从基因组序列分析, MERS-CoV与SARS基因组相似性为55%左右。
2例患者确认为新型冠状病毒感染
➢ 两例病例标本的检测机构 • 荷兰的伊拉兹马斯大学医学中心 • 英国的卫生防护署
➢ 两例病例经实验室确认均为新型冠状病毒感染 ➢ 两株病毒一个250bp的PCR片段同源性达99.5%
• 英国:和蝙蝠冠状病毒有80%的同源性 • 与SARS-CoV-tor2相应区段的同源性为73.6% • 与香港Bat-CoV相应区段的同源性为98%
MERS-CoV核酸检测结果判读
➢ 在实验室要确认一个样本为阳性,需要满足以下 两个条件的其中一个:
一、至少两种中东呼吸综合征冠状病毒特异性 PCR结果阳性;
二、一种PCR结果为阳性,另外一种PCR产物序 列测序,与已知序列相符。
当针对中东呼吸综合征冠状病毒的两种不同的检 测方法结果不一致的时候,应当采用反转录扩增 ,对扩增产物进行测序以确认检测结果。
MERS输出病例
• 有输出病例的国家或地区
– 沙特、阿联酋、卡塔尔、约旦、韩国
• 在中东地区外有输入病例的国家中
– 目前英国、法国、突尼斯报告发生了二代病例 – 韩国报告发生了三代病例。
发病特点
➢病例主要集中在中东地区,也输入到中东 外多个国家,通过朝觐发生的病例有增加 趋势;
➢少数输入病例导致了二代病例发生,二代 病例多为轻症和无症状感染;
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
中东呼吸综合征病例流行病学个案调查表
国标码□□□□□□病例编码□□□□
病例类型:(1)疑似病例(2)临床诊断病例(3)确诊病例
信息提供者:(1)本人(2)家属或知情人(关系)
1.一般情况
1.1 姓名:
1.2 性别:(1)男(2)女
1.2.1如为女性,是否怀孕:(1)是(孕周)(2)否
1.2.2如为女性,是否曾生产:(1)是(最近一次分娩时间:年月日)(2)否
1.3 年龄:岁
1.4 职业:
1.4.1 医务人员:⑴医生⑵护士⑶护工⑷检验⑸行政管理人员⑹其他
1.4.2非医务人员:⑴幼托儿童⑵散居儿童⑶学生⑷教师⑸保育保姆
⑹餐饮业⑺商业服务⑻工人⑼民工⑽农民⑾牧民
⑿渔(船)民⒀干部职员⒁离退人员⒂家务待业⒃其他
1.5工作单位:
1.6现居住地(详填):省市县(区)乡(街道)村1.7户口所在地(详填):省市县(区)乡(街道)村1.8 国籍:(1)中国(2)其他
1.9身份证或护照号码:□□□□□□□□□□□□□□□□□□
1.10 联系电话:
2.临床信息
2.1 发病时间:年月日
2.2 发病地点:
(1)中国境内:省市县(区)
(2)中国境外:
(3)交通工具上:□飞机□火车□轮船□汽车□其他
2.3 临床症状、体征和并发症:
6.细菌培养;
7.其他(需详述)
2.5 住院治疗情况
2.5.1是否住院治疗:(1)是(2)否(跳转至“
3.流行病学信息”部分)
2.5.2入院日期:年月日
2.5.3入住医院名称:
2.5.4 住院号: □□□□□□□□
2.5.5入院诊断:(1)疑似病例(2)临床诊断病例(3)确诊病例(4)其他临床诊断
2.5.6 治疗情况:
2.5.6.1 药物治疗:(1)抗生素(2)激素(3)抗病毒药物(4)其他
2.5.6.2 是否入住ICU:(1)是(入住日期年月日)(2)否
)是(隔离日期年月日)2)否2.5.7 是否存在呼吸系统合并感染:
(1)是(感染病原体名称:)(2)否
3.2 发病前14天内中东呼吸综合征病例接触史:
(1)有(2)无(跳至3.3)
2.接触方式: ⑴与病人同进餐 ⑵与病人同处一室 ⑶与病人同一病区 ⑷与病人共用食具、茶具、毛巾、玩具等 ⑸接触病人分泌物、排泄物等 ⑹诊治、护理 ⑺探视病人 ⑻共用交通工具 ⑼其他接触
3.接触频率描述: ⑴经常 ⑵有时 ⑶偶尔
4.可能的接触地点:⑴家 ⑵工作单位 ⑶学校 ⑷集体宿舍 ⑸医院 ⑹室内公共场所 ⑺其他 3.3 发病前14天内中东地区的单峰骆驼、蝙蝠及其他动物接触情况: ⑴有 ⑵无(跳至3.4)
* (1)饲养(2)交易(3)屠宰 (4)烹饪(5)运输 (6)食用 (7)清理动物饲养场所(8)接触动物排泄物/分泌物(9)其他
3.4
发病前14天内境外旅行史: (1)有 (2)无(跳至3.5) 2.1-有(填写动物名称),2-无 3.1-有(病人姓名),2-无
4.有上述暴露者,需详细记录暴露情况。
3.4.2回国入境时间: 年 月 日 3.4.3 入境口岸: 3.4.4 入境航班号:。
