Immunofluorescence Staining
免疫荧光 原始数据

免疫荧光原始数据English Answer:Immunofluorescence (IF) staining is a technique used to detect and visualize the localization of specific proteins or molecules within cells or tissues. It involves labeling the target molecules with fluorescent dyes, which can then be visualized using a microscope equipped with a fluorescent light source. IF staining is commonly used in biological research to study the expression, localization, and dynamics of proteins within cells.The principle of IF staining relies on the specific binding of antibodies to their target antigens. Primary antibodies, which are specific for the target protein, are applied to the sample and allowed to bind to their targets. These primary antibodies are then labeled with a secondary antibody conjugated to a fluorescent dye. The secondary antibody allows for the visualization of the primary antibody binding sites and, thus, the localization of thetarget protein.IF staining allows for the detection and visualization of specific proteins or molecules within cells or tissues. It provides detailed information about the spatial distribution and expression levels of proteins, which can help researchers understand the cellular and molecular mechanisms underlying various biological processes. IF staining is a powerful tool for studying protein localization, protein-protein interactions, and cellular dynamics.In summary, immunofluorescence staining is a technique widely used in biological research to visualize the localization of specific proteins or molecules within cells or tissues. It involves labeling the target molecules with fluorescent dyes, enabling the detection and visualization of their distribution and expression levels. IF staining provides valuable insights into the molecular mechanisms and cellular processes underlying various biological phenomena.Chinese Answer:免疫荧光原始数据。
信号转导研究方法

信号转导研究方法信号转导是现代生物学中的一个重要研究领域。
通过研究细胞内外分子信号的传递和转导机制,可以深入了解生命现象的本质和生物体的生理状况。
以下将介绍一些常用的信号转导研究方法。
1.免疫共沉淀法免疫共沉淀法主要用于研究蛋白质相互作用和信号转导的分子机制。
实验步骤如下:首先,使用抗体对感兴趣的蛋白质进行免疫化学标记;接着,将其与待研究的混合物进行混合;最后,利用抗体对混合物进行沉淀,将与待研究蛋白质相互作用的蛋白质随着待研究蛋白质一起沉淀下来。
免疫共沉淀法可以识别相互作用的蛋白质并进一步分析它们在信号转导过程中的作用。
但是,该方法存在一些限制,例如需要有足够的特异性和灵敏性以准确识别蛋白质相互作用,而且分析结果的可重复性和可靠性也需要经过严格的验证。
2. Western blottingWestern blotting可以检测蛋白质的表达量和蛋白质的修饰状态等信息,通常用于分析信号转导过程中的途径和机制。
实验步骤如下:首先,通过细胞裂解和离心等步骤获得蛋白质样本;接着,将样本进行SDS-PAGE凝胶电泳分离;最后,通过蛋白质转印到膜上、膜上鉴定和蛋白质定量等步骤,获得感兴趣的蛋白质信息。
Western blotting具有高度的特异性和灵敏性,并且可以在不同的样本之间进行比较和分析。
然而,该方法需要以通量为基础进行分析,且对蛋白质中性化处理的要求比较严格。
3. Immunofluorescence staining免疫荧光染色是用来研究细胞内分子位置和蛋白质相互作用等的有效方法。
实验步骤如下:首先,将荧光标记的抗体与待研究的蛋白质特异性结合;接着,将样本加入到载玻片中并进行固定和渗透化处理;最后,加入荧光染料,进行显微镜观察。
免疫荧光染色技术可以研究蛋白质的亚细胞定位及蛋白质分布情况,促进对信号转导生物学过程的了解。
然而,该方法仅对荧光信号相互作用的精确控制有高要求,并且在荧光信号转移方面也存在诸多困难。
免疫荧光
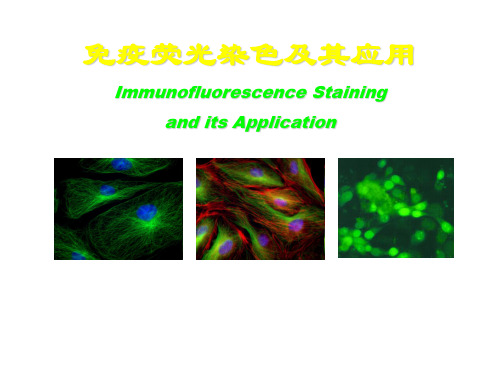
FITC-细胞桨;DAPI-细胞核
SYBR Green I 溶液染色: 病毒(小点)、细菌(大亮 点)、硅藻(细长的细胞) SYBR Green I 凝胶染色
染色体原位杂交检测
免疫荧光染色,多色叠加
实验内容
小鼠B细胞膜表面Ig(mIgG)测定——直接免疫荧光染色法
细胞膜或细胞内的抗原分子与相应的荧光素直 接标记的mAb结合后,形成带有荧光色素的抗原抗 体复合物。 经激发光激发后发出与荧光素相对应的特定波 长的荧光,其荧光强度与被测抗原分子表达密度成 正比例关系,由此可检测细胞与标记抗体对应抗原 的表达量和阳性细胞百分比。 活细胞表面保留有较完整的抗原或受体,根据 所测定的荧光强度和阳性细胞百分率即可知相应抗 原的密度和分布的比例。
3、用眼科剪将脾脏一端剪一小口后,将含5ml Hank’s液的注射器 从另一端刺入脾脏,缓缓注入Hank’s液,即可见脾细胞流入平皿, 在注射Hank’s液同时不断转动针头方向,直至脾脏变苍白为止。
4、将脾细胞悬液吸入试管,静置10分钟,以去除较大块未冲散 的脾组织块,然后将沉淀以上部分移入干净试管。
荧光
指一个分子或原子吸收了外界给予的能量后, 即刻引起发光;停止能量供给,发光亦瞬即停 止。
受到短波激发光照射,发射出波长较长的荧光。 若激发光停止照射,荧光立即熄灭。 荧光的衰减:取决于激发光强度和照射时间。
荧光发生机理
量子理论:光波短,光子能量强;光波长,光子能量小 某些物质接收紫外线和较短波照射------能量增高,处于 不稳定状态-----以光的形式向外释放多余的能量。
在碱性条件下,FITC的异硫氰酸基在水溶液中与 免疫球蛋白的自由氨基经碳酰胺化而形成硫碳氨基键, 成为标记荧光免疫球蛋白,即荧光抗体。 一个Ig分子上最多能标记15~20个FITC分子。
Immunofluorescence Staining Protocol

Immunofluorescence Staining Protocol1.Preparation of SlidesA.Cell Lines∙Grow cultured cells on sterile glass cover slips or slides overnight at37ºC ∙Wash briefly with PBS∙Fix as desired.Possible procedures include:10minutes with10%formalin in PBS(keep wet)5minutes with ice cold methanol,allow to air dry5minutes with ice cold acetone,allow to air dry∙Wash in PBSB.Frozen Sections∙Snap frozen fresh tissues in liquid nitrogen or isopentane pre-cooled in liquid nitrogen,embedded in OCT compound in cryomolds.Store frozen blocks at-80ºC.∙Cut4-8um thick cryostat sections and mount on superfrost plus slides or gelatin coated slides.Store slides at-80ºC until needed.∙Before staining,warm slides at room temperature for30minutes and fix in ice cold acetone for5minutes.Air dry for30minutes.∙Wash in PBSC.Paraffin Sections∙Deparaffinize sections in xylene,2x5min.∙Hydrate with100%ethanol,2x3min.∙Hydrate with95%ethanol,1min.∙Rinse in distilled water.∙Follow procedure for pretreatment as required.2.Pretreatments of Tissue SectionsAntigenic determinants masked by formalin-fixation and paraffin-embedding often may be exposed by epitope umasking,enzymatic digestion or saponin,etc.Do not use this pretreatment with frozen sections or cultured cells that are not paraffin-embedded.3.Procedure1.Rinse sections in PBS-Tween20for2x2min2.Serum Blocking:incubate sections with normal serum block–species sameas secondary antibody,for30minutes to block non-specific binding ofimmunoglobulin.Note:since this protocol uses avidin-biotin detection system, avidin/biotin block may be needed based on tissue type.If you do,theavidin/biotin block should be done after normal serum block.3.Primary Antibody:incubate sections with primary antibody at appropriatedilution in primary antibody dilution buffer for1hour at room temperature or overnight at4 C.4.Rinse in PBS-Tween20.5.Secondary Antibody:incubate sections with biotinylated secondary antibodyat appropriate dilution in PBS for30minutes at room temperature.6.Rinse in PBS-Tween20for3x2min.7.Detection:incubate sections in FITC-Avidin D in PBS for30minutes at roomtemperature.Protecting slides from light starting from this step to the end by covering slides with aluminum foil or black box.8.Rinse in PBS-Tween20for3x2min.9.Counterstain with PI or DAPI if desire.10.Rinse in PBS-Tween20.11.Dehydrate through95%ethanol for2min,100%ethanol for2x3min.12.Coverslip with anti-fade mounting medium.。
常用免疫组织化学染色方法

常用免疫组织化学染色方法免疫组织化学染色是通过将抗原与抗体结合来检测组织中特定蛋白质的染色方法。
它是现代生物医学中常用的一种技术,可以用于研究分子生物学、细胞生物学、病理学等领域。
以下是一些常用的免疫组织化学染色方法介绍:1. 免疫组化染色(Immunohistochemistry, IHC):免疫组化染色是免疫组织化学中最常用的方法之一、其基本原理是使用一种与目标抗原特异性结合的抗体,将抗原与抗体结合,然后通过染色方法将表达该抗原的细胞或组织可视化。
常用的染色剂包括多聚酶、酶标、荧光素和放射性同位素。
IHC可以用于检测细胞表面抗原、细胞器中的抗原以及胞内蛋白质的定位。
2. 免疫荧光染色(Immunofluorescence, IF):免疫荧光染色利用荧光标记的抗体来检测特定抗原。
它可以提供高度特异性和灵敏度的探测,可以用于研究蛋白质的亚细胞定位、蛋白质相互作用等。
利用该方法可以用于检测多种类型的标记(单标记、双标记、复合标记等),从而实现多重染色或共定位染色。
3. 免疫电镜染色(Immunoelectron microscopy, IEM):免疫电镜染色是一种将金粒标记的抗体用于电子显微镜下观察并定位特定抗原的方法。
通过将金粒结合到抗原-抗体复合物上,可以在电子显微镜下清晰地观察到抗原的位置和分布。
这种方法具有高分辨率和高特异性的优点,广泛应用于超微结构的研究。
4. 免疫酶标染色(Immunoenzyme staining, IES):免疫酶标染色是使用酶作为标记物,通过化学反应将抗原与酶标记的抗体结合,从而显示出特定抗原的位置和分布。
常用的标记物包括辣根过氧化物酶(HRP)、碱性磷酸酶(ALP)等。
在检测抗原时,标记物可以与染色底物产生反应生成可见色素,形成染色,以显示抗原的位置和分布。
5. 免疫组织化学原位杂交(Immunohistochemical in situ hybridization, IHC-IS):免疫组化原位杂交技术是一种结合了原位杂交和免疫组化技术的方法。
免疫荧光和几种特殊染色在肾活检病理诊断中的应用

ʌ文章编号ɔ1006-6233(2017)06-0898-04免疫荧光和几种特殊染色在肾活检病理诊断中的应用罗教秀ꎬ㊀储㊀兵ꎬ㊀曹晓珊ꎬ㊀陈应智ꎬ㊀吴师珍(中山大学附属中山医院病理科ꎬ㊀广东㊀中山㊀528403)ʌ摘㊀要ɔ目的:分析免疫荧光染色及其他几种特殊染色法在肾活检病理诊断中的应用效果ꎮ方法:临床纳入58例我院2014年9月至2016年9月期间收治的慢性肾脏疾病患者作为研究对象ꎬ所有患者均进行肾穿刺活检ꎬ穿刺取得组织采用石蜡制片ꎬ分别采用免疫荧光法㊁六胺银染色法㊁甲醇刚果红染色法㊁黏蛋白染色法(PAS)以及Masson三色染色ꎬ对比不同方法染色的结果ꎮ结果:不同类型肾病患者中ꎬ免疫荧光染色中狼疮性肾病中IgA㊁IgM㊁IgG㊁C3以及Clq诊断阳性率明显高于IgA肾病和膜性肾病患者ꎬP<0.05ꎮ在不同染色方法的对比中发现ꎬ免疫荧光染色法在肾病检测IgA㊁IgM㊁IgG㊁C3以及Clq诊断阳性率明显低于其他染色方法的诊断率ꎬP<0.05ꎮ其他染色方法诊断率均无差异ꎬP>0.05ꎮ结论:免疫荧光染色相对于其他染色方式对肾活检病理诊断率稍低ꎬ临床上推荐采用其他特殊染色方法对肾组织进行病理诊断ꎮʌ关键词ɔ㊀免疫荧光ꎻ㊀六胺银ꎻ㊀甲醇刚果红ꎻ㊀黏蛋白染色ꎻ㊀Masson三色染色诊断ʌ文献标识码ɔ㊀A㊀㊀㊀㊀㊀ʌdoiɔ10.3969/j.issn.1006-6233.2017.06.06ApplicationofImmunofluorescenceandotherSpecialStaininginPathologicalDiagnosisofRenalBiopsyLUOJiaoxiuꎬ㊀CHUBingꎬ㊀CAOXiaoshanꎬ㊀etal(ZhongshanAffiliatedHospitalꎬSunYat-senUniversityꎬGuangdongZhongshan528403ꎬChina)ʌAbstractɔObjective:Toanalyzetheeffectofimmunofluorescenceandotherspecialstainingmethodsinthepathologicaldiagnosisofrenalbiopsy.Methods:58patientswithchronickidneydiseaseadmittedinourhospitalfromSeptember2014toSeptember2016wereincludedintheobjectofstudy.AllpatientsunderwentrenalbiopsypuncturetoobtaintissueusingparaffinꎬrespectivelybyimmunofluorescenceꎬsixstainingofmethanolmethodꎬCongoredstainingandmucin(PAS)andthreeMassondyestainingꎬstainingresultsindif ̄ferentways.Results:ThepositiveratesofIgAꎬIgMꎬIgGꎬC3andClqinthepatientswithdifferenttypesofnephropathyweresignificantlyhigherthanthoseofIgAnephropathyandmembranousnephropathyinpatientsbyimmunofluorescencestaining.InthecomparisonofdifferentstainingmethodsꎬitwasfoundthatthepositiverateofIgAꎬIgMꎬIgGꎬC3andClqdetectedbyimmunofluorescencestainingwassignificantlylowerthanthatofotherstainingmethodsꎬP<0.05.TherewasnodifferenceinthediagnosticrateofotherstainingmethodsꎬP>0.05.Conclusion:Comparingwithothermethodsꎬtherateofpathologicaldiagnosisofrenalbiopsyislowerthanthatofothermethods.Itisrecommendedtouseotherspecialstainingmethodstodiagnoserenaltissue.ʌKeywordsɔ㊀Immunofluorescenceꎻ㊀Sixsilveramineꎻ㊀Methanolcongoredꎻ㊀Mucinꎻ㊀Massonstainingsolution㊀㊀肾活检组织病理检查包括免疫病理㊁光学显微镜㊁电子显微镜等ꎬ其中免疫病理检查常采用石蜡切片进行免疫组化染色[1]ꎮ虽然临床研究显示冰冻切片相对于石蜡切片来说免疫组化染色敏感性㊁特异性更高ꎬ但由于冰冻切片对温度要求高ꎬ且运送条件苛刻ꎬ组织不容易保存[2]ꎬ本文采用石蜡切片进行免疫荧光染色以及其他几种特殊染色ꎬ观察不同染色方法对肾活检组织病理的诊断作用ꎬ现报道如下ꎮ1㊀资料与方法1.1㊀一般资料:本次选取58例我院2014年9月至898 ʌ基金项目ɔ广东省科技计划项目ꎬ(编号:2013B031804)ʌ通讯作者ɔ储㊀兵2016年9月期间肾内科收治的慢性肾脏疾病患者作为研究对象ꎬ其中男性患者34例ꎬ女性患者24例ꎬ年龄28~76岁ꎬ平均年龄(51.2ʃ4.3)岁ꎮ病理类型:IgA肾病患者12例ꎬ膜性肾病患者9例ꎬ狼疮性肾病患者37例ꎮ1.2㊀方㊀法1.2.1㊀准备工作:所有患者均采用肾活检进行检查ꎬ取肾活检标本脱水㊁浸蜡等制成石蜡切片ꎬ采用10%福尔马林于4ħ冰箱中固定24hꎮ①玻片:重酪酸清洁液浸泡载玻片24hꎬ自来水洗净后采用蒸馏水浸泡24hꎬ再使用无水酒精浸泡24hꎬ最后捞出烘干ꎬ43ħ水浴展片ꎬ57ħ下干燥24hꎬ备用ꎮ②配备PBS缓冲液ꎬ将PBS粉剂溶于1000mL蒸馏水中ꎬ充分搅拌并静置ꎬ制成PBS缓冲液ꎮ③稀释抗体:5uL抗体加入45uLPBS缓冲液稀释ꎬ对抗体IgA㊁IgM㊁IgG㊁C3以及Clq进行检测ꎮ④组织脱水包埋切片ꎬ免疫荧光1μm厚切片ꎬ10张用防脱片剂(多聚赖氨酸)处理过载玻片进行捞片ꎬ置60ħ烤箱烤片㊁备用ꎮ几种特殊染色2~3μmꎬ5张用上述处理过的玻片捞片ꎬ置60ħ烤箱烤片㊁备用ꎮ1.2.2㊀免疫荧光染色法:①脱蜡至水:二甲苯进行脱蜡处理ꎬ20min/次ꎬ进行2次ꎮ梯度酒精至水ꎬ每个步骤进行5minꎮ②抗原修复:采用由武汉博士德公司生产的柠檬酸(PH为6.0)进行修复ꎬ微波热修复ꎬ3min中火ꎬ停2minꎬ再3min低火ꎮ取出切片后自然冷却至室温ꎬ采用北京中杉公司生产的胰酶消化ꎬ在37ħ水浴中恒温放置15minꎮ③滴加抗体:用洁净吸水纸擦去切片组织外蒸馏水ꎬ马克笔在载玻片背面圈出组织部分ꎬ将50uLFITC标记兔抗人免疫球蛋白抗IgA㊁IgM㊁IgG以及补体抗体C3㊁Clq稀释液滴加至组织上ꎬ使抗体稀释液完全覆盖标本组织ꎮ④湿盒孵育:滴加了抗体稀释液的切片平放于湿盒中ꎬ避光后置于7ħ冰箱中过夜孵育ꎬ时间为24hꎮ⑤冲洗:孵育后采用PBS冲洗3次ꎬ5min/次ꎮ甘油封片在荧光显微镜下观察结果ꎮ1.2.3㊀六胺银染色法:工作液配置:50mL硼砂溶液㊁六次甲基四胺银粉剂ꎮ将硼砂溶液倒入六次甲基四胺银粉剂瓶内ꎬ摇匀2minꎬ再将六次甲基四胺银溶液倒入硼砂溶液瓶ꎬ重复三次上述步骤ꎬ保证粉剂充分溶解ꎬ得到工作液ꎮ染色步骤:①将工作液放入62ħ恒温水浴箱预热ꎮ②切片脱蜡至水ꎮ③高碘酸溶液氧化标本15minꎬ流水冲洗6遍ꎬ5min/遍ꎮ④切片放入62ħ六胺银工作液中30minꎬ保证温度为62ħ恒温ꎬ至黄棕色切片出现黑色反应时取出ꎬ蒸馏水洗涤后镜下检查ꎮ⑤流水冲洗ꎮ⑥硫代硫酸钠溶液处理3minꎬ流水冲洗ꎮ⑦Mayer苏木素染色细胞核5minꎮ⑧流动自来水冲洗3min并甩干ꎮ⑨伊红染液复染1minꎮ⑩梯度酒精脱水ꎬ二甲苯透明ꎬ中性树胶封固ꎮ1.2.4㊀甲醇刚果红染色法:①石蜡切片常规脱蜡至水ꎮ②甲醇刚果红染液染色20minꎮ③碱性酒精分化液分化标本30sꎮ④水洗5minꎮ⑤Mayer苏木素染色细胞核2minꎬ水洗5minꎮ⑥常规脱水透明ꎬ中性树胶封固ꎮ1.2.5㊀黏蛋白染色法(PAS):①切片脱蜡至水ꎮ②蒸馏水洗2minꎮ③高碘酸溶液氧化10minꎮ④充分蒸馏水洗涤ꎬ吸水纸吸干ꎮ⑤雪夫试剂染色15minꎮ⑥流动自来水冲洗10minꎮ⑦Mayer苏木素染色细胞核5minꎮ⑧流动自来水冲洗3min并甩干ꎮ⑨95%乙醇和无水乙醇脱水ꎬ二甲苯透明ꎬ中性树胶封固ꎮ1.2.6㊀Masson三色染色法:①将切片脱蜡至水后置入Bouin氏固定液中室温过夜(18~24h)ꎮ②流水冲洗至切片上的黄色消失ꎮ③Weigert铁苏木素染色10minꎬ流水稍洗ꎮ④1%盐酸酒精分化ꎬ流水冲洗5minꎮ⑤丽春红酸性品红染5minꎬ流水销冲洗ꎮ⑥磷钼酸溶液5minꎬ苯胺蓝染液复染5minꎮ⑦1%冰醋酸1minꎬ95%酒精㊁无水酒精脱水ꎬ二甲苯透明ꎬ中性树胶封固ꎮ1.3㊀观察指标:观察直接免疫荧光法㊁六胺银染色法㊁甲醇刚果红染色法㊁PAS以及Masson三色染色液染色的结果ꎬ判断阳性率情况ꎮ阳性判断情况[3]ꎬ①免疫荧光法ꎬ分为-㊁ʃ㊁+㊁++㊁+++㊁++++ꎬ-:阴性ꎬʃ:低倍镜下不显ꎬ高倍镜下似乎可见ꎻ+:低倍镜下似乎可见ꎬ高倍镜下可见ꎻ++:低倍镜下可见ꎬ高倍镜下较清晰ꎻ+++:低倍镜下较清晰ꎬ高倍镜下耀眼ꎻ++++:低倍镜下耀眼ꎬ高倍镜下刺眼ꎮ++及以上为阳性ꎮ②六胺银染色:肾小球囊基底膜和肾毛细血管球基底膜呈黑色ꎬ细胞核呈蓝色ꎬ背景粉红色ꎮ③刚果红染色:细胞核呈蓝色ꎬ基底膜蓝色ꎬ淀粉样物质㊁红细胞红色ꎬ背景淡黄粉色ꎮ④PAS染色:细胞核呈蓝色ꎬ基底膜呈红色ꎬ肾小球系膜基质呈红色ꎮ⑤Masson染色:细胞核呈蓝黑色ꎬ基底膜ꎬ胶原纤维㊁呈蓝色ꎬ免疫复合物呈红色ꎮ1.4㊀统计学处理:采用SPSS18.0统计软件ꎬ计数资料用百分比表示ꎬ采用χ2检验ꎬP<0.05为差异有统计学意义ꎮ2㊀结㊀果2.1㊀染色结果:不同类型肾病患者中ꎬ免疫荧光染色中狼疮性肾病中IgA㊁IgM㊁IgG㊁C3以及Clq诊断阳性率明显高于IgA肾病和膜性肾病患者ꎬP<0.05ꎮ在不同染色方法的对比中发现ꎬ免疫荧光染色法在肾病检998测IgA㊁IgM㊁IgG㊁C3以及Clq诊断阳性率明显低于其他染色方法的诊断率ꎬP<0.05ꎮ其他染色方法诊断率均无差异ꎬP>0.05ꎮ见表1ꎮ表1㊀不同染色方法对肾活检病理诊断情况对比n(%)染色方法IgAIgMIgGC3Clq免疫荧光染色IgA肾病(n=12)10(83.33)#9(75.00)#9(75.00)#8(66.67)#8(66.67)#膜性肾病(n=9)7(77.78)#7(77.78)#6(66.67)#6(66.67)#7(77.78)#狼疮性肾病(n=37)34(91.89)∗#34(91.89)∗#33(89.19)∗#33(89.19)∗#32(86.49)∗#六胺银染色IgA肾病(n=12)12(100.00)11(91.67)11(91.67)11(91.67)11(91.67)膜性肾病(n=9)9(100.00)9(100.00)9(100.00)8(88.89)9(100.00)狼疮性肾病(n=37)37(100.00)36(97.30)37(100.00)36(97.30)36(97.30)刚果红染色IgA肾病(n=12)12(100.00)12(100.00)11(91.67)11(91.67)11(91.67)膜性肾病(n=9)9(100.00)8(88.89)8(88.89)9(100.00)9(100.00)狼疮性肾病(n=37)37(100.00)36(97.30)36(97.30)37(100.00)36(97.30)PAS染色IgA肾病(n=12)12(100.00)11(91.67)11(91.67)11(91.67)11(91.67)膜性肾病(n=9)9(100.00)9(100.00)9(100.00)9(100.00)9(100.00)狼疮性肾病(n=37)37(100.00)37(100.00)37(100.00)37(100.00)37(100.00)Masson染色IgA肾病(n=12)12(100.00)12(100.00)12(100.00)11(91.67)11(91.67)膜性肾病(n=9)9(100.00)9(100.00)8(88.89)9(100.00)9(100.00)狼疮性肾病(n=37)37(100.00)37(100.00)36(97.30)36(97.30)36(97.30)㊀㊀注:相同组间对比ꎬ∗P<0.05ꎻ不同染色法对比ꎬ#P<0.052.2㊀镜下染色情况图1㊀免疫荧光染色99.9mmꎮ78.2mm(+52.9mmꎬ4.6mm)ə图2㊀免疫荧光染色图3㊀六胺银染色图4㊀刚果红染色009图5㊀Masson染色图6㊀PAS染色图1:IgA沉积在肾小球系膜区ꎬ为颗粒状发光物ꎮ图2:IgM沉积在肾小球系膜区ꎬ为团块状发光物ꎮ图3:六胺银染色显示膜性肾病基膜广泛空泡ꎮ图4:刚果红染色显示肾小球淀粉样物质阳性ꎬ呈砖红色ꎮ图5:Masson染色显示玫瑰红色沉淀物沿毛细血管壁上皮细胞侧分布ꎮ图6:PAS染色显示肾小球基质㊁肾小管基膜等呈玫瑰红色ꎬ细胞核呈蓝色ꎮ3㊀讨㊀论肾组织活检是临床检查肾脏疾病的金标准ꎬ而肾活检组织免疫病理检查在肾组织病理诊断中起到重要价值[4]ꎮ免疫病理检查包括冰冻切片和石蜡切片ꎬ但冰冻切片相对于石蜡切片较厚ꎬ免疫荧光染色组织细胞结构重叠或不清ꎬ因此部分医院采用石蜡切片进行免疫荧光染色[5]ꎮ但石蜡切片虽然能避免冰冻切片较厚的劣势ꎬ但染色背景高ꎬ特异性差ꎬ在观察切片同一部位的不同抗原时存在一定局限性[6]ꎮ因此有学者采用其他免疫组织染色方式对石蜡切片进行染色ꎬ企图提高免疫组化染色的诊断率[7]ꎮ本文对我院慢性肾疾病患者进行肾活检病理诊断ꎬ采用免疫荧光染色㊁六胺银染色法㊁甲醇刚果红染色法㊁PAS染色以及Masson染色对我院患者肾活检组织进行病理诊断ꎬ结果显示ꎬ免疫荧光染色相对于其他几种特殊染色方法ꎬ对IgA肾病㊁膜性肾病以及狼疮性肾病的诊断率均较低ꎮ而相对于不同类型肾疾病的肾组织活检时ꎬ免疫荧光染色法对狼疮性肾病诊断率高于IgA肾病以及膜性肾病ꎮ结果提示ꎬ相对于其他染色法ꎬ免疫荧光染色对肾活检组织石蜡切片的诊断率明显较低ꎮ这可能是由于石蜡切片采用福尔马林进行浸泡ꎬ在浸泡固定过程中ꎬCa2+和其他二价键离子形成紧密复合物封闭抗原ꎬ石蜡切片还会出现脱蜡不充分ꎬ导致抗原暴露不充分ꎬ引起特异性荧光减弱ꎬ影响诊断结果ꎮ六胺银染色法采用高碘酸氧化组织ꎬ使基底膜内枯多糖暴露出醛基ꎬ醛基将六胺银还原为黑色金属银ꎬ硫代硫酸钠固定显色银盐ꎬ去除未反应的银离子ꎬ起到诊断作用ꎮ甲醇刚果红染色法采用对刚果红选择性亲和力的淀粉样物质进行着色ꎬ刚果红与淀粉样物质的羟基以及胺基结合ꎬ平行附着在淀粉样物质的纤维上呈红色[8]ꎮPAS染色法采用氧化剂高碘酸ꎬ破坏多糖类结构的碳键ꎬ使组织中多糖分子的乙二醇基火氨羟基的碳键打开ꎬ生成醛类化合物ꎬ暴露游离的醛基与雪夫试剂作用ꎬ生成新的红至紫红色复合物从而得到定位ꎮMasson染色法利用两种或三种阴离子染料混合完成染色ꎬ阴离子染色分子大小和组织的渗透性有关ꎬ根据不同组织渗透性不同ꎬ选择不同大小阴离子染色可将不同组织显示出来ꎮʌ参考文献ɔ[1]㊀李昌水ꎬ张英杰ꎬ郑江江ꎬ等.蛋白酶K修复石蜡切片免疫荧光染色在肾活检病理诊断中的应用[J].中华病理学杂志ꎬ2014ꎬ43(1):38~41.[2]㊀张翠薇ꎬ刘勇ꎬ张旭ꎬ等.肾活检标本冰冻切片与石蜡切片免疫荧光染色结果的比较[J].泸州医学院学报ꎬ2013ꎬ36(1):31~34.[3]㊀ShiSꎬChengQꎬZhangPꎬetal.Immunofluorescencewithdualmicrowaveretrievalofparaffin-embeddedsectionsintheas ̄sessmentofhumanrenalbiopsyspecimens[J].AmericanJour ̄nalofClinicalPathology[J].OfficialPublicationofAmericanSocietyofClinicalPathologistsꎬ2013ꎬ139(1):71~78.[4]㊀姚伦ꎬ刘树军ꎬ高丹ꎬ等.探讨变色酸2R-亮绿在肾活检组织染色中的应用[J].中国实验诊断学ꎬ2015ꎬ19(10):1777~1779.[5]㊀赵秀芬ꎬ钱军ꎬ曾鸣ꎬ等.免疫荧光病理在乙型肝炎病毒相关性肾小球肾炎诊断中的临床意义[J].江苏医药ꎬ2015ꎬ41(12):1406~1408.[6]㊀邢昌赢ꎬ沈冬云.肾小球疾病病理诊断及其临床意义[J].中华全科医学ꎬ2015ꎬ13(9):1388~1389.[7]㊀董鸿瑞ꎬ王艳艳ꎬ王国勤ꎬ等.免疫组织化学和免疫荧光染色在肾活检组织石蜡切片磷脂酶A2受体检测中的应用[J].中国医学科学院学报ꎬ2015ꎬ37(5):562~566.[8]㊀王弦ꎬ江冬瑞ꎬ秦蓉ꎬ等.肾穿刺活检标本制片体会[J].临床与实验病理学杂志ꎬ2016ꎬ32(6):706~707.109。
组织cd14抗体免疫荧光染色

组织cd14抗体免疫荧光染色免疫荧光染色(immunofluorescence staining)是一种用于检测特定蛋白在细胞或组织中分布情况的方法。
它利用荧光标记的抗体与特定的抗原结合,然后通过荧光显微镜观察抗原在样本中的分布情况。
在免疫学研究中,免疫荧光染色被广泛应用于检测细胞表面和内部蛋白的表达情况,从而了解细胞的功能和分子机制。
CD14是人类和许多其他哺乳动物细胞表面上的一种受体蛋白,它在免疫系统中扮演着重要的角色。
CD14主要存在于单核细胞和巨噬细胞表面,它是LPS(细菌内毒素)的受体,可以介导炎症反应和免疫应答。
因此,在免疫学研究中,对CD14的检测和定位具有重要的意义。
通过免疫荧光染色,可以直观地观察CD14在细胞或组织中的分布情况,从而深入了解其在免疫反应中的作用。
进行CD14抗体免疫荧光染色的步骤一般包括样本制备、样本固定、透化处理、抗原回复、抗体染色和显微镜观察等步骤。
下面将详细介绍CD14抗体免疫荧光染色的步骤。
首先,样本制备是进行免疫荧光染色的第一步。
样本可以是细胞培养物、组织切片或细胞悬液。
对于组织切片,可以选择冰冻切片或石蜡切片,需要根据实验的具体要求来选择。
对于细胞培养物,可以直接在培养皿中进行染色。
第二步是样本固定。
样本固定的目的是保持细胞或组织的形态和结构,防止在染色过程中变性或破坏。
传统的样本固定方法包括用甲醛或甲醛乙酸乙酯进行固定,也可以选择其他适合特定样本的固定方法。
第三步是透化处理。
透化处理可以增强抗体对细胞内分子的穿透力,提高染色效果。
常用的透化剂包括Tween-20、Triton X-100等。
第四步是抗原回复。
抗原回复是在高温和/或酶处理条件下,恢复组织样本中被固定和脱水的蛋白质结构,以增加抗体对抗原的识别和结合能力。
抗原回复的方法包括热处理、酶处理等。
第五步是抗体染色。
在进行CD14抗体免疫荧光染色时,需要选择特异性较好的CD14抗体,并且对抗体进行荧光标记。
Immunofluorescence staining of cells 免疫荧光染色Protocol

Immunofluorescence staining of cells1) Wash cells twice with 1 mL of 1X PBS (aspirate off the medium from each well, add 1mL 1X PBS using p1000 pipetman, then remove it – i.e. don’t need to wait, just repeat it one more time).2) Fix cells in 4% paraformaldehyde (1mL/well) for 15 min.3) Wash cells twice for 5 min with 1 mL 1X PBS (1mL each).4) Blocking & permeabilizing with 3% BSA, 1% Triton X-100 in 1X PBS (Blocking medium) for 20 min.5) Incubate with primary antibody for 1hr. anti-myosin heavy chain; anti-MHC (1:15 dilution, 1mL X 1/15 = 66.7 uL; 66.7uL antibody + 933.3uL in Blocking medium as in 4). Make a master mix for 6.1X66.7 uL X 6.1 = 406.87uL antibody933.3 uL X 6.1 = 5693.13uL blocking medium (3% BSA, 1% Triton X-100 in 1X PBS).6) Wash cells twice for 5 min with 1mL 1XPBS.7) Incubate cells with secondary antibody for 40 min.!!! this step should be done without light, either cover the slides with the aluminum foil or do it with the lights off !!!Use goat anti-mouse Alexa 594 (1:500 dilution, per well, 1000uL X 1/500 = 2uL antibody + 998uL Blocking medium as in step 4), also make a master mix for 6.1 X )2uL X 6.1 = 12.2uL antibody998uL X 6.1 = 6087.8uL Blocking mediumIncubate cells with secondary antibody for 40 min.8) Wash cells twice for 5 min with 1mL 1X PBS, remove the walls of the chamberslide, then dry any residual solution before the next step (mounting).9) Mounting!! Add couple drops of mounting medium (with DAPI, tube labeled DAPI covered with aluminum foil) using p100. Then, place coverglass on top…give a little bit of pressure and make sure the mounting solution covers the whole area without air bubbles.。
抗体免疫荧光染色 pcr

抗体免疫荧光染色 pcr
抗体免疫荧光染色(Antibody Immunofluorescence Staining)和聚合酶链式反应(PCR)是两种不同的生物学技术,它们在研究和诊断领域中都有广泛的应用。
抗体免疫荧光染色是一种用于检测和定位细胞或组织中特定蛋白质的技术。
该技术利用特异性抗体与目标蛋白质结合,然后通过荧光染料标记的二抗来可视化抗体-蛋白质结合的位置。
这种技术可以用于研究蛋白质的表达、分布和功能,以及在细胞和组织水平上进行诊断。
PCR 则是一种用于扩增特定核酸序列的技术。
它通过在体外进行一系列的温度循环,使目标核酸序列在短时间内大量复制。
PCR 可以用于基因克隆、基因分型、基因表达分析等领域,也在分子诊断和遗传学研究中发挥着重要作用。
抗体免疫荧光染色和 PCR 可以结合使用,以实现更全面的分析。
例如,在细胞或组织中进行抗体免疫荧光染色后,可以通过 PCR 对同一样本中的特定核酸序列进行分析,从而提供有关细胞或组织的分子信息。
这两种技术在生物学研究和临床诊断中都具有重要的应用价值。
它们各自具有独特的优势,可以为研究人员提供不同层面的信息,帮助我们更好地理解生物过程和疾病机制。
免疫荧光染色原理

免疫荧光染色原理免疫荧光染色(immunofluorescence staining)是一种用于检测细胞或组织中特定蛋白质的方法,通过荧光显微镜观察,能够提供高分辨率的成像结果。
这种技术在生物医学研究和临床诊断中得到了广泛的应用,特别是在免疫学、细胞生物学和病理学领域。
本文将介绍免疫荧光染色的原理及其在科研和临床中的应用。
免疫荧光染色的原理基于抗体的特异性结合。
首先,需要准备一种特异性抗体,该抗体能够与待检测的蛋白质结合。
然后,将这种特异性抗体与荧光染料结合,形成荧光标记的抗体。
接下来,将荧光标记的抗体加入到待检测的细胞或组织样品中,使其与目标蛋白结合。
最后,利用荧光显微镜观察样品,荧光标记的抗体会发出特定颜色的荧光信号,从而可以定位目标蛋白在细胞或组织中的位置。
免疫荧光染色具有高度的特异性和敏感性,能够在细胞水平上检测特定蛋白质的表达和定位。
在科研领域,研究人员常常利用免疫荧光染色来研究细胞信号转导、蛋白质亚细胞定位、细胞凋亡等生物学过程。
在临床诊断中,免疫荧光染色也被广泛应用于肿瘤标志物检测、自身免疫性疾病诊断、感染病原体鉴定等方面。
除了单色荧光染色,还可以利用多种荧光染料进行多色免疫荧光染色,通过同时检测多个蛋白质的表达和定位,可以提供更加全面的信息。
此外,还可以结合免疫组化染色、原位杂交等技术,实现多种检测手段的组合应用,从而更加全面地了解细胞或组织的生物学特性。
总之,免疫荧光染色作为一种高度特异性和敏感性的检测方法,在生物医学研究和临床诊断中发挥着重要作用。
随着荧光显微镜和荧光探针的不断发展,免疫荧光染色技术将会更加灵活和高效,为科研人员和临床医生提供更多有力的工具,推动生命科学领域的进步和临床诊断的精准化。
泛素化蛋白检测方法

泛素化蛋白检测方法泛素化是一种重要的蛋白质修饰方式,通过共价连接泛素蛋白到靶蛋白上,调控靶蛋白的稳定性、活性和亚细胞定位。
因此,泛素化蛋白的检测对于研究细胞信号传导、蛋白质降解和细胞周期调控具有重要意义。
本文将介绍几种常用的泛素化蛋白检测方法,希望能够为相关研究工作者提供一些参考和帮助。
1. 免疫印迹(Western blotting)。
免疫印迹是一种常用的蛋白质检测方法,可以用于检测泛素化蛋白。
首先,将待检测样品进行SDS-PAGE凝胶电泳分离,然后转膜到聚丙烯膜上,接着用抗泛素抗体进行孵育,最后通过化学发光或者显色底物进行检测。
免疫印迹方法具有高灵敏度和高特异性,可以检测低水平的泛素化蛋白。
2. 免疫荧光染色(Immunofluorescence staining)。
免疫荧光染色是一种用于检测细胞内蛋白质的方法,也可以用于检测泛素化蛋白。
将待检测细胞固定后,用抗泛素抗体进行孵育,然后再与荧光标记的二抗结合,最后通过荧光显微镜观察。
免疫荧光染色方法可以直观地显示泛素化蛋白的亚细胞定位和表达水平。
3. 免疫共沉淀(Co-immunoprecipitation)。
免疫共沉淀是一种用于检测蛋白质相互作用的方法,也可以用于检测泛素化蛋白和其底物蛋白的相互作用。
首先,将待检测样品与抗泛素抗体进行孵育,然后加入蛋白A/G琼脂糖糖珠进行共沉淀,最后用免疫印迹或质谱等方法进行检测。
免疫共沉淀方法可以帮助研究者确定泛素化蛋白与其底物蛋白的相互作用关系。
4. 质谱分析(Mass spectrometry)。
质谱分析是一种高通量的蛋白质检测方法,也可以用于检测泛素化蛋白。
通过将待检测样品进行蛋白水解和质谱分析,可以确定泛素化位点和泛素化蛋白的亚型。
质谱分析方法具有高灵敏度和高分辨率,可以全面地分析泛素化蛋白的修饰情况。
综上所述,泛素化蛋白的检测方法多种多样,研究者可以根据实际需要选择合适的方法进行检测。
希望本文介绍的方法能够为相关研究工作者提供一些参考和帮助,推动泛素化蛋白的研究进展。
荧光抗体稀释液

北京索莱宝科技有限公司
荧光抗体稀释液
货号:A1840
规格:10ml/100ml
保存:-20℃保存,有效期为2年。
短期可2-8℃保存。
产品说明:
本免疫荧光抗体稀释液(Immunofluorescence Staining Antibody Dilution Buffer)可以用于免疫荧光实验时荧光标记抗体的稀释和配制。
产品添加了抗体保护剂、稳定剂等成分,有效增加了稀释后荧光抗体的稳定性。
用此稀释液稀释后的抗体可在2-8℃环境下稳定保存半年。
使用方法:
按照荧光抗体推荐效价(或个人优化条件),将适量抗体加入抗体稀释液即可。
例如,效价为1:1000时,可将1ul抗体加入到1ml抗体稀释液中,充分混匀后即可使用。
抗体最好现用现稀释,稀释后的抗体可2-8℃保存。
注意事项:
如需抗体回收再利用,建议在2-8℃进行抗体的结合反应,以减缓抗体效价的衰减速度。
为了您的安全和健康,请穿实验服并戴一次性手套操作。
第1页共1页。
免疫荧光封闭的原理

免疫荧光封闭的原理免疫荧光封闭(Immunofluorescence staining)是一种通过特定的抗体对标本中的分子进行标记并检测为主的分子检测技术。
它是一种非常重要且广泛应用的分析手段,尤其在免疫学、细胞学和分子生物学等领域得到了广泛应用。
它所展现的优势有很多,如高灵敏度、高特异性和非常直观的结果等。
免疫荧光技术通常分为三个部分:概括的免疫原准备、样本制备和荧光成像。
1、免疫原制备的原理免疫原是指可以引起动物的免疫应答的物质。
一般来说免疫原包括蛋白、多肽、纳米颗粒等。
通过合适的制备工艺免疫原可实现能够特异性识别目标分子的能力。
免疫原的制备通常包括以下几个步骤:① 分离纯化目标蛋白(apoprotein)或病毒等微生物抗原;② 通过合适的方法氨基或羟基化处理免疫原,使其具有高免疫原性和高保真性; ③ 将免疫原通过合适的方法使之与载体偶联起来,用于免疫动物制备抗体。
2、样本制备的原理免疫荧光技术的样本制备要求标本必需处于固态(组织、细胞切片)或液态(细胞涂片、液体控制)等样品形式。
从样品获得的细胞或标本(如细胞切片、染色体、组织切片)必须固定在载玻片上,便于分析。
同时,当前的免疫荧光技术还不支持活体标本的实时观察,因为免疫成像需要在细胞固定后才能进行。
3、荧光成像的原理荧光显微镜用来观察标本中可见的荧光信号,和普通光学显微镜的原理大致相同,通过目镜透射样品,并利用物镜的倍率放大样品图像,最终将图像投射到目视器或数字设备上。
免疫荧光技术所利用的荧光染料有许多种,其中最常用的是荧光素(FITC)和罗丹明-119(Rhodamine-119)。
免疫荧光技术在细胞学和分子生物学方面有重要的应用。
它被广泛用于研究生物界大量的基本问题,如DNA修复、细胞分子的定位、组织学诊断和生物分子的互动等。
此外,因其高灵敏度、高特异性和非常直观的结果,该技术广泛应用于诊断各种疾病(如肿瘤、自身免疫性疾病和感染性疾病)。
免疫荧光染色原理

免疫荧光染色原理
免疫荧光染色(Immunofluorescence Staining,IFS)是一种细胞或组织分子学的常见
技术,可用于鉴定和定位一种特定的蛋白片段。
它是一种同时从细胞中定量和定性检测蛋
白质的技术,给予了研究者高分辨率的观察细胞功能和分子量变化的工具。
IFS的基本原理是先将细胞中的特异性抗体与细胞内的抗原分子结合。
于是,抗体可
比较精确地指出细胞内特异性抗原分子的位置和形态。
之后,紧贴在细胞表面的另一种荧
光染料如荧光素抗体介导将特异性抗体标记,以及这些荧光素抗体附着于细胞表面。
这样,当光子照射到标记的细胞时,有互补配对的荧光染料会发出荧光现象,可以被摄影机记录
和观察。
免疫荧光染色的步骤如下:细胞注射免疫球蛋白,制备荧光抗体,为每个蛋白挑选一
种荧光抗体,各自标记荧光;用抗原抗体纳米颗粒或体外结合抗体进行细胞定位;根据细
胞切片并拍照。
IFS是一种非常有效且简单的技术,可以更有效地检测细胞内特定蛋白质的分布和量。
它使研究者可以观察细胞内分子对对细胞表型的贡献,研究细胞及其通路中的信号传导过程。
此外,该方法还可以用于检测细胞内抗原的空间分布,检测DNA的聚集,以及测定抗
原的数量。
免疫荧光的染色工作流程和原理

免疫荧光的染色工作流程和原理1.免疫荧光染色是一种用于检测特定蛋白质在组织或细胞中分布情况的实验方法。
Immunofluorescence staining is a method used to detect the distribution of specific proteins in tissues or cells.2.工作流程首先需要固定样本,一般是用乙醛或乙醇固定。
The workflow first requires fixing the sample, usually with formaldehyde or ethanol.3.固定后,样本需要被穿切成薄片,并被载玻片上。
After fixation, the sample needs to be sectioned and mounted on slides.4.下一步是渗透处理,用于使抗体能够进入样本内部。
The next step is permeabilization, which allows antibodies to enter the sample.5.样本接下来会被暴露于特定蛋白质的抗体。
The sample is then exposed to antibodies against the specific protein.6.抗体结合后,样本需要被洗涤以去除未结合的抗体。
After antibody binding, the sample needs to be washed to remove unbound antibodies.7.最后,样本被观察在荧光显微镜下,检测特定蛋白质的分布情况。
Finally, the sample is observed under a fluorescent microscope to detect the distribution of specific proteins.8.免疫荧光染色的原理是利用特定抗体与蛋白质结合,然后利用荧光标记的二抗来标记特定蛋白质的位置。
immunofluorescence词根

immunofluorescence词根《immunofluorescence词根》1. 单词概述单词:immunofluorescence含义:免疫荧光。
这是一个在生物学和医学领域非常重要的术语。
它是一种利用抗原与抗体特异性结合的原理,通过荧光标记来检测和定位细胞或组织中的特定抗原的技术。
比如说在研究疾病的时候,科学家们想看看某种特定的蛋白质(抗原)在细胞中的分布情况,就会用到immunofluorescence这种技术,就像拿着一个带荧光的小标签去找到那些隐藏在细胞里的“小目标”一样。
2. 词根词缀解析词根:“immune”:来源于拉丁语,它表示“免除、免疫”的意思。
就像我们说一个人对某种疾病有免疫力,就不会被那种疾病轻易侵袭,就像穿了一层防护铠甲一样。
词缀:“fluorescence”:“fluoro -”表示“荧光”的意思,“ -escence”这个后缀常用来表示某种状态或者性质。
合成逻辑:“immune”(免疫)+“fluorescence”(荧光) = “immunofluorescence”(免疫荧光),也就是把免疫和荧光这两个概念结合起来,利用免疫反应来标记目标,然后用荧光的特性去观察它。
3. 应用短文与场景应用短文1:I'm a biology student, and let me tell you about my firstencounter with immunofluorescence. I was in the lab, and my supervisor, Dr. Smith, was explaining this amazing technique to me. "You see," he said, "immunofluorescence is like a detective in the microscopic world." He held up a sample of tissue. "This tissue is like a big, mysterious city, and we're looking for specific bad guys - the antigens. But how do we find them? Well, that's where immunofluorescence comes in." He then showed me the antibodies with the fluorescent tags. "These antibodies are our search dogs. They're trained to sniff out only the antigens we're interested in. Once they find them, the fluorescent tag lights up like a little beacon, and we can see exactly where the antigens are in the tissue." I was completely fascinated. I thought to myself, "This is like magic! We can actually see things that are so tiny and hidden before." And from that day on, I was hooked on immunofluorescence.中文翻译:我是一名生物学学生,让我来给你讲讲我第一次接触免疫荧光的经历吧。
Immunofluorescence Staining Protocol

Immunofluorescence Staining Protocol1. Preparation of SlidesA. Cell Lines∙Grow cultured cells on sterile glass cover slips or slides overnight at 37 º C ∙Wash briefly with PBS∙Fix as desired. Possible procedures include:10 minutes with 10% formalin in PBS (keep wet)5 minutes with ice cold methanol, allow to air dry5 minutes with ice cold acetone, allow to air dry∙Wash in PBSB. Frozen Sections∙Snap frozen fresh tissues in liquid nitrogen or isopentane pre-cooled in liquid nitrogen, embedded in OCT compound in cryomolds. Store frozen blocks at -80 ºC.∙Cut 4-8 um thick cryostat sections and mount on superfrost plus slides or gelatin coated slides. Store slides at - 80 ºC until needed.∙Before staining, warm slides at room temperature for 30 minutes and fix in ice cold acetone for 5 minutes. Air dry for 30 minutes.∙Wash in PBSC. Paraffin Sections∙Deparaffinize sections in xylene, 2x5min.∙Hydrate with 100% ethanol, 2x3min.∙Hydrate with 95% ethanol, 1min.∙Rinse in distilled water.∙Follow procedure for pretreatment as required.2. Pretreatments of Tissue SectionsAntigenic determinants masked by formalin-fixation and paraffin-embedding often may be exposed by epitope umasking, enzymatic digestion or saponin, etc. Do not use this pretreatment with frozen sections or cultured cells that are notparaffin-embedded.3. Procedure1. Rinse sections in PBS-Tween 20 for 2x2min2. Serum Blocking: incubate sections with normal serum block – species sameas secondary antibody, for 30 minutes to block non-specific binding ofimmunoglobulin. Note: since this protocol uses avidin-biotin detection system, avidin/biotin block may be needed based on tissue type. If you do, theavidin/biotin block should be done after normal serum block.3. Primary Antibody: incubate sections with primary antibody at appropriatedilution in primary antibody dilution buffer for 1 hour at room temperature or overnight at 4 C.4. Rinse in PBS-Tween 20.5. Secondary Antibody: incubate sections with biotinylated secondary antibodyat appropriate dilution in PBS for 30 minutes at room temperature.6. Rinse in PBS-Tween 20 for 3x2min.7. Detection: incubate sections in FITC-Avidin D in PBS for 30 minutes at roomtemperature. Protecting slides from light starting from this step to the end by covering slides with aluminum foil or black box.8. Rinse in PBS-Tween 20 for 3x2min.9 . Counterstain with PI or DAPI if desire.10. Rinse in PBS-Tween 20.11. Dehydrate through 95% ethanol for 2 min, 100% ethanol for 2x3min.12. Coverslip with anti-fade mounting medium.。
细胞膜表面检测方法

细胞膜表面检测方法细胞膜表面检测方法如下:一、流式细胞术(Flow Cytometry)流式细胞术是一种常用的检测细胞膜表面的技术,通过测量细胞的大小、颗粒性、荧光等参数,可以对细胞表面进行分析。
通过使用不同的抗体标记细胞表面抗原,可以检测细胞表面分子的表达情况,进而了解细胞的特征和功能。
二、免疫荧光染色(Immunofluorescence staining)免疫荧光染色是一种用于检测细胞表面分子的技术,通过将荧光标记的抗体与细胞表面分子结合,在显微镜下观察细胞的荧光信号,可以确定细胞表面分子的定位和表达情况。
该技术具有高灵敏度和高特异性,适用于各种组织和细胞类型的检测。
三、酶联免疫吸附法(Enzyme-linked Immunosorbent Assay,ELISA)酶联免疫吸附法是一种基于抗原-抗体反应的检测方法,通过将抗原或抗体固定在固相载体上,加入酶标记的抗原或抗体,再加入底物显色,可以检测细胞表面分子的浓度。
该方法具有较高的灵敏度和特异性,常用于大批量样品的检测。
四、表面等离子共振(Surface Plasmon Resonance,SPR)表面等离子共振是一种光学检测技术,通过测量金属表面反射光的相位变化,可以确定生物分子间的相互作用。
该技术可以用于检测细胞表面分子间的相互作用,提供分子间相互作用的实时监测和动力学信息。
五、质谱分析(Mass Spectrometry)质谱分析是一种用于检测细胞表面分子的技术,通过将细胞表面分子电离并测量其质量,可以确定分子的结构和组成。
该方法具有高灵敏度和高分辨率,可以用于蛋白质、糖类等大分子的检测。
六、微阵列芯片(Microarray)微阵列芯片是一种高通量的检测技术,通过将大量探针固定在固相载体上,与细胞样品进行杂交反应,可以同时检测多个基因或蛋白质的表达情况。
该技术具有高灵敏度、高特异性和高通量等特点,适用于大规模的基因或蛋白质表达分析。
七、表面增强拉曼散射(Surface Enhanced Raman Scattering,SERS)表面增强拉曼散射是一种用于检测细胞表面分子的技术,通过将拉曼散射活性分子与金属表面结合,可以显著增强拉曼散射信号。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Immunofluorescence Staining of
Frozen Sections
1. Samples and Materials
4%Paraformaldehyde; PBS/NS; fresh mammalian tissues/cells; OCT; sucrose; H2O2;
TritonX-100; blocking solution; the first, second (or third) antibodies; mounting medium;
cryotome; travenol infuser; tinfoil; -80 freezer; coverslips/microscopic slides;
37℃incubator; microtome blades;
2. Protocol
1). Perfusion: perfuse the mice models with cold PBS/NS via the systemic blood (or
pulmonary) circulation, depending on the purpose of your experiment, till the effluent liquid appears clear
2). Isolate target tissues or organs and put them into 4%Paraformaldehyde at 4℃
overnight; (Well fixation is very important!)followed by10, 20, 30% sucrose density-gradient dehydration (or simply 30% only) and ready for the next step when samples sink to the bottom
3). Dry the residual sucrose liquid on samples with paper and put them into tin foil
boxes; embed samples in the OCT compound; mark their side(s) and then put into -80 freezer
4). Cut the tissues/organs (turn on the machine in advance)
5). Incubate the slides for half to one hour at 37℃; followed by rinses with PBS/NS, 5
min * 3 (Very important: the samples can NOT be dried after this step!!!)
6). (optional step) Permeabilization; rinse or incubate the sections with PBS/NS, 5 min * 3
7). (optional step) 3%H2O2 for 10min or 0.3% H2O2 for 30min, at RT, depending on tissues or organs (e.g. liver and kidney are rich in endogenous peroxidase and
avidin/biotin…); r inse or incubate the sections with PBS/NS, 5 min * 3
8). Blocking (it would be the best to use the second antibody and blocking solution from the same species); 30min, RT; remove the blocking buffer but NO any buffer
wash/rinse! (Very important!!!)
9). Incubate the samples in the first/primary antibodies, at 4℃, overnight
10). Decant the first/primary antibody solutions and rinse/wash the slides, 5 min * 3
11). Incubate the samples in the secondary antibodies for 2hs, at RT
12). Decant the secondary antibody solutions and rinse/wash the slides, 5 min * 3
13). (optional step) DAPI to dye nuclei for 5min at RT
14). Decant the DAPI solution and rinse/wash the slides, 5 min * 3
15). Mounting: glycerol or other mounting media (if the mounting medium includes DAPI, ignore the step 14 and 15)
16). (optional step) Seal coverslips with nail polish to prevent drying and movement of the samples/sections
17). Ready for imaging (the slides could be stored in dark at 4℃ temporarily)
Note: 1. Please always make sure all the solutions cover the entire samples/sections!!!
2. Please keep the slides in dark when necessary!
3. Be sure to use the first/primary antibodies, the secondary antibodies (or the third…) and blocking solutions APPROPRIATELY!!! (Multicolor staining can be performed either simultaneously, or sequentially)
4. This protocol is flexible and only for a reference. Optimal methods should be determined by the users.
11-12-2014。
