有机人名反应(4)PPT课件
有机化学人名反应

有机化学人名反应
1. Friedel-Crafts反应:由Charles Friedel和James Crafts于1877年首次报道的一种重要的有机化学反应。
2. Grignard反应:法国化学家Victor Grignard于1900年发现的一种有机合成反应。
3. Wolff-Kishner还原反应:德国化学家Kurt Heinrich Wolff和美国化学家Morris Kishner于1913年和1919年分别发现的一种有机还原反应。
4. Birch还原反应:澳大利亚化学家Arthur John Birch于1944年发现的一种有机化学反应。
5. Cannizzaro反应:意大利化学家Stanislao Cannizzaro于1853年发现的一种有机化学反应。
6. Gabriel重氮化反应:德国化学家Siegmund Gabriel于1887年发现的一种有机化学反应。
7. Wurtz反应:法国化学家Charles Adolphe Wurtz于1855年发现的一种有机化学反应。
8. Fries重排反应:德国化学家Karl Fries于1887年发现的一种有机化学反应。
9. Hofmann消去反应:德国化学家August Wilhelm von
Hofmann于1865年发现的一种有机化学反应。
10. Robinson环加成反应:英国化学家Robert Robinson于1925年发现的一种有机化学反应。
常见的人名反应PowerPoint演示文稿

190oC
O CH3 C
C CH3 O
Criegee 邻二醇四醋酸铅氧化裂介
R1 R2
C C
R4 R3
Pb(OAc)4
R1 R2
C O +
R4 R3
C O
D: Darzቤተ መጻሕፍቲ ባይዱn 醛酮与α卤代酯的缩合反应
ClCH2COOEt NaOEt Cl R C - CHCOOEt R' O -
O CH COOEt + R C R' Cl R C - CHCOOEt R' O
O O R' + NH R C CH3 + H C H R'' H2O O R' RC CH2CH2 N R''
Meerwein-Ponndorf反应 醛或酮与异丙醇铝在异丙醇溶液中加热,还原成相 应的醇,而异丙醇则氧化为丙酮,将生成的丙酮由 平衡物中慢慢蒸出来,使反应朝产物方向进行。这 个反应相当于的逆向反应。
1
R
O Cl 3 C C-R R
2
R O-
RO
O R1 3 C C-R R
2
Br O CH3 C C CH3 C2H5ONa CH3 EtOEt
O C CH3 C CH3 CH2
C2H5O
O C OC2H5 CH3 C CH CH3 3
O
Cl CH3ONa
O CH3O
O C OCH3
Friedel-Crafts 芳环烷基化
Hinsberg反应
伯胺、仲胺分别与对甲苯磺酰氯作用生成相应的对甲苯磺酰胺 沉淀,其中伯胺生成的沉淀能溶于碱(如氢氧化钠)溶液,仲胺 生成的沉淀则不溶,叔胺与对甲苯磺酰氯不反应。此反应可用 于伯仲叔胺的分离与鉴定。
《有机化学人名反应》课件
人名反应的定义与重要性
定义
人名反应是指以科学家或化学家的名字命名的有机化学反应。这些反应通常具 有独特性、重要性或实用性。
重要性
人名反应是化学领域中的重要知识,掌握这些反应有助于理解有机化学的基本 原理,提高解决实际问题的能力。同时,人名反应也是化学领域中科学研究的 成果,体现了人类对化学反应的深入认识和探索。
一种重要的烷基化反应
详细描述
Friedel-Crafts反应是一种在芳香化合 物中引入烷基的亲电取代反应。通常 在路易斯酸(如AlCl3)催化下进行, 该反应广泛应用于有机合成中。
Wittig 反应
总结词
制备烯烃的经典方法
详细描述
Wittig反应是一种通过磷酸酯和醛之间的反应制备烯烃的方法。该反应涉及一个五元环 过渡态,生成具有特定立体化学特征的烯烃。
ቤተ መጻሕፍቲ ባይዱ
在材料科学中的应用
材料科学是一个跨学科的领域,涉及材料的设计、制备、性 能和应用。人名反应在材料科学中的应用主要涉及新型材料 的合成和改性。
通过人名反应,可以合成出具有优异性能和功能的新型材料 ,如高分子材料、陶瓷材料、复合材料等。这些材料在能源 、环境、信息等领域具有广泛的应用前景,为科技进步和社 会发展提供重要支持。
亲电取代反应
总结词
亲电取代反应是一种有机化学反应,其中亲电试剂进攻并取代反应物分子中的某 个基团。
详细描述
这类反应通常发生在苯环、芳香烃和杂环化合物的反应中,其中亲电试剂具有正 电性,能够进攻富电子的碳原子。常见的亲电取代反应包括:EAS reaction、 Elimination reaction等。
详细描述
这类反应通常发生在烯烃、炔烃和芳香烃的反应中,其中加成试剂能够与不饱和键结合形成新的键。 常见的加成反应包括:Diels-Alder reaction、Addition reaction等。
有机化学人名反应机理报告204页PPT

36、自己的鞋子,自己知道紧在哪里。——西班牙
37、我们唯一不会改正的缺点是软弱。——拉罗什福科
xiexie! 38、我这个人走得很慢,但是我从不后退。——亚伯拉罕·林肯
39、勿问成功的秘诀为何,且尽全力做你应该做的事吧。——美华纳
Hale Waihona Puke 40、学而不思则罔,思而不学则殆。——孔子
有机化学人名反应机理报告
56、极端的法规,就是极端的不公。 ——西 塞罗 57、法律一旦成为人们的需要,人们 就不再 配享受 自由了 。—— 毕达哥 拉斯 58、法律规定的惩罚不是为了私人的 利益, 而是为 了公共 的利益 ;一部 分靠有 害的强 制,一 部分靠 榜样的 效力。 ——格 老秀斯 59、假如没有法律他们会更快乐的话 ,那么 法律作 为一件 无用之 物自己 就会消 灭。— —洛克
60、人民的幸福是至高无个的法。— —西塞 罗
谢谢!
有机化学人名反应机理ppt课件

这个反响极易进展并且反响速度快,运用范围极广泛, 是合成环状化合物的一个非常重要的方法。 带有吸电子取代基的亲双烯体和带有给电子取代基的双烯 体对反响有利。常用的亲双烯体有:
以下基团也能作为亲双烯体发生反 响:
• 常用的双烯体有
反响机理 这是一个协同反响,反响时,双烯体和亲双烯体彼 此接近,相互作用,构成一个环状过渡态,然后逐 渐转化为产物分子:
• 反响是按顺式加成方式进展的,反响物原来的构型关 系仍保管在环加成产物中。例如:
正常的Diels-Alder反响主要是由双烯体的HOMO(最高已占轨 道)与亲双烯体的LUMO(最低未占轨道)发生作用。反响过程 中,电子从双烯体的HOMO“流入〞亲双烯体的LUMO。也 有由双烯体的LUMO与亲双烯体的HOMO作用发生反响的。
吡啶类化合物不易进展硝化,用硝基复原法制备氨基 吡啶甚为困难。本反响是在杂环上引入氨基的简便有效 的方法,广泛适用于各种氮杂芳环,如苯并咪唑、异喹 啉、吖啶和菲啶类化合物均能发生本反响。
Claisen重排〔克莱森〕
• 烯丙基芳基醚在高温(200°C)下可以重排, 生成烯丙基酚。
当烯丙基芳基醚的两个邻位未被取代基占满时,重 排主要得到邻位产物,两个邻位均被取代基占据时, 重排得到对位产物。对位、邻位均被占满时不发生此 类重排反响。
有些情况下水解很困难,可以用肼解来替代:
反响机理 邻苯二甲酰亚胺盐和卤代烷的反响是亲核取代反
响,取代反响产物的水解过程与酰胺的水解类似。
• 反响实例
Gattermann 反响
• 重氮盐用新制的铜粉替代亚铜盐(见Sandmeyer 反响)作催化剂,与浓盐酸或氢溴酸发生置换反 响得到氯代或溴代芳烃:
有机化学人名反应
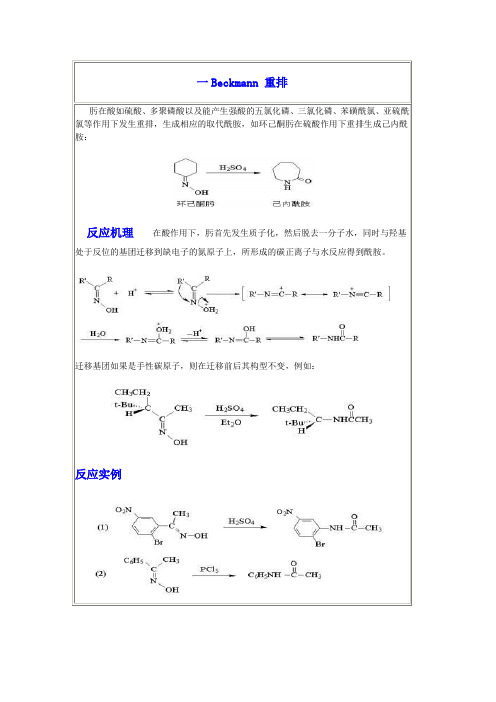
肟在酸如硫酸、多聚磷酸以及能产生强酸的五氯化磷、三氯化磷、苯磺酰氯、亚硫酰氯等作用下发生重排,生成相应的取代酰胺,如环己酮肟在硫酸作用下重排生成己内酰胺:反应机理在酸作用下,肟首先发生质子化,然后脱去一分子水,同时与羟基处于反位的基团迁移到缺电子的氮原子上,所形成的碳正离子与水反应得到酰胺。
迁移基团如果是手性碳原子,则在迁移前后其构型不变,例如:反应实例反应机理反应实例反应机理反应实例反应机理反应实例吡啶类化合物不易进行硝化,用硝基还原法制备氨基吡啶甚为困难。
本反应是在杂环上引入氨基的简便有效的方法,广泛适用于各种氮杂芳环,如苯并咪唑、异喹啉、吖啶和菲啶类化合物均能发生本反应。
二元羧酸酯的分子内酯缩合见Dieckmann 缩合反应。
反应机理反应实例烯丙基芳基醚在高温(200°C)下可以重排,生成烯丙基酚。
当烯丙基芳基醚的两个邻位未被取代基占满时,重排主要得到邻位产物,两个邻位均被取代基占据时,重排得到对位产物。
对位、邻位均被占满时不发生此类重排反应。
交叉反应实验证明:Claisen重排是分子内的重排。
采用 -碳 14C 标记的烯丙基醚进行重排,重排后 -碳原子与苯环相连,碳碳双键发生位移。
两个邻位都被取代的芳基烯丙基酚,重排后则仍是 碳原子与苯环相连。
反应机理Claisen 重排是个协同反应,中间经过一个环状过渡态,所以芳环上取代基的电子效应对重排无影响。
从烯丙基芳基醚重排为邻烯丙基酚经过一次[3,3] 迁移和一次由酮式到烯醇式的互变异构;两个邻位都被取代基占据的烯丙基芳基酚重排时先经过一次[3,3] 迁移到邻位(Claisen 重排),由于邻位已被取代基占据,无法发生互变异构,接着又发生一次[3,3] 迁移(Cope 重排)到对位,然后经互变异构得到对位烯丙基酚。
取代的烯丙基芳基醚重排时,无论原来的烯丙基双键是Z-构型还是E-构型,重排后的新双键的构型都是E-型,这是因为重排反应所经过的六员环状过渡态具有稳定椅式构象的缘故。
有机化学人名反应

有机人名反应Organic Name Reactions目录....................................... 错误!未定义书签。
Bouveault-Blanc还原 .. (1)Cannizzaro 反应 (3)Chichibabin 反应 (4)Claisen重排 (5)Claisen 酯缩合反应 (7)Claisen-Schmidt 反应 (9)Clemmensen 还原 (10)Combes 合成法 .............. 错误!未定义书签。
Cope 重排. (11)Cope 消除反应 (13)Dieckmann 缩合反应 (14)Diels-Alder 反应 (15)Friedel-Crafts烷基化反应 (18)Friedel-Crafts酰基化反应 (19)Gabriel 合成法 (20)Gattermann 反应 (22)Gattermann-Koch 反应...............................23 Gomberg-Bachmann 反应 (24)Hell-Volhard-Zelinski 反应 (25)Hinsberg 反应 (26)Hofmann 烷基化 (27)Hofmann 消除反应 (28)Hofmann 重排(降解) (29)Koble 反应 ......................错误!未定义书签。
Koble-Schmitt 反应 .. (31)Mannich 反应 (32)Michael 加成反应 (33)Oppenauer 氧化 (35)Reformatsky 反应 (36)Rosenmund 还原 (37)Sandmeyer 反应 (38)Schmidt反应 (39)Skraup 合成法 (40)Williamson 合成法 (41)Wolff-Kishner-黄鸣龙反应 (43)反应机理反应实例反应机理反应实例反应机理反应实例吡啶类化合物不易进行硝化,用硝基还原法制备氨基吡啶甚为困难。
基础有机化学人名反应

基础有机化学人名反应第四章狄尔斯-阿尔德反应(Diels - Alder reaction)( 140)1921年,狄尔斯和其研究生巴克(Back)研究偶氮二羧酸二乙酯(半个世纪后因光延反应而在有机合成中大放光芒的试剂)与胺发生的酯变胺的反应,当他们用2-萘胺做反应的时候,根据元素分析,得到的产物是一个加成物而不是期待的取代物。
狄尔斯敏锐地意识到这个反应与十几年前阿尔布莱希特做过的古怪反应的共同之处。
这使他开始以为产物是类似阿尔布莱希特提出的双键加成产物。
狄尔斯很自然地仿造阿尔布莱希特用环戊二烯替代萘胺与偶氮二羧酸乙酯作用,结果又得到第三种加成物。
通过计量加氢实验,狄尔斯发现加成物中只含有一个双键。
如果产物的结构是如阿尔布莱希特提出的,那么势必要有两个双键才对。
这个现象深深地吸引了狄尔斯,他与另一个研究生阿尔德一起提出了正确的双烯加成物的结构。
1928年他们将结果发表。
这标志着狄尔斯-阿德尔反应的正式发现。
他们也因此获得1950年的诺贝尔化学奖。
含有一个活泼的双键或叁键的化合物(亲双烯体)与共轭二烯类化合物(双烯体)发生1,4-加成,生成六员环状化合物:这个反应极易进行并且反应速度快,应用范围极广泛,是合成环状化合物的一个非常重要的方法。
带有吸电子取代基的亲双烯体和带有给电子取代基的双烯体对反应有利。
常 用的亲双烯体有:对苯醍下列基团也能作为亲双烯体发生反应:二C=N —,一N=0 ? CT 七二0 , Q 二o ’常用的双烯体有:a 反应机理这是一个协同反应,反应时,双烯体和亲双烯体彼此靠近,互相作用,形成一 个环状过渡态,然后逐渐转化为产物分子:顺丁烯二酸軒OHN笔P-不饱和硝基化合物C —— CMCICOHCCHO、肉桂醛双晞休亲戏晞休玳股渡态产韌反应是按顺式加成方式进行的,反应物原来的构型关系仍保留在环加成产 物中。
例如:正常的Diels-Alder 反应主要是由双烯体的 HOMO (最高已占轨道)与亲双烯 体的LUMO (最低未占轨道)发生作用。
有机人名反应经典总结——超经典PPT课件

• 肟在酸如硫酸、多聚磷酸以及能产生强酸的五氯化磷相应的取代酰胺,如环己酮肟在硫酸作用下重排生成己内酰胺:
•
• 反应机理 • • 在酸作用下,肟首先发生质子化,然后脱去一分子水,同时与羟基处于反位的基团迁移
到缺电子的氮原子上,所形成的碳正离子与水反应得到酰胺。
第66页/共185页
第67页/共185页
• 卤代烃反应的活泼性顺序为:RF > RCl > RBr > RI ; 当烃基超过3个碳原子时,反应过程中易发生重排。 • 反应机理 • 首先是卤代烃、醇或烯烃与催化剂如三氯化铝作用形成碳正离子:
第68页/共185页
第69页/共185页
所形成的碳正离子可能发生重排,得到较稳定 的碳正离子:
第112页/共185页
第113页/共185页
Fries 重排 • 酚酯在Lewis酸存在下加热,可发生酰基重排反应,生成邻羟基和对羟基芳酮
的混合物。重排可以在硝基苯、硝基甲烷等溶剂中进行,也可以不用溶剂 直接加热进行。 • 邻、对位产物的比例取决于酚酯的结构、反应条件和催化剂等。例如,用 多聚磷酸催化时主要生成对位重排产物,而用四氯化钛催化时则主要生成 邻位重排产物。反应温度对邻、对位产物比例的影响比较大,一般来讲, 较低温度(如室温)下重排有利于形成对位异构产物(动力学控制),较高温 度下重排有利于形成邻位异构产物(热力学控制)。
第9页/共185页
第10页/共185页
• 这个反应极易进行并且反应速度快,应用范围极广泛,是合成环状化合物的一个非常重要的方法。 带有吸电子取代基的亲双烯体和带有给电子取代基的双烯体对反应有利。常用的亲双烯体有:
第11页/共185页
第12页/共185页
有机化学人名反应

亚磷酸三烷基酯作为亲核试剂与卤代烷作用,生成烷基膦酸二烷基酯和一个新的卤代烷:卤代烷反应时,其活性次序为:R'I >R'Br >R'Cl。
除了卤代烷外,烯丙型或炔丙型卤化物、α-卤代醚、α-或β-卤代酸酯、对甲苯磺酸酯等也可以进行反应。
当亚磷酸三烷基酯中三个烷基各不相同时,总是先脱除含碳原子数最少的基团。
本反应是由醇制备卤代烷的很好方法,因为亚磷酸三烷基酯可以由醇与三氯化磷反应制得:如果反应所用的卤代烷R'X 的烷基和亚磷酸三烷基酯(RO)3P 的烷基相同(即R' = R),则Arbuzov 反应如下:这是制备烷基膦酸酯的常用方法。
除了亚磷酸三烷基酯外,亚膦酸酯RP(OR')2和次亚膦酸酯R2POR'也能发生该类反应,例如:反应机理一般认为是按S N2进行的分子内重排反应:反应实例Arndt-Eister反应反应机理反应实例Baeyer-Villiger氧化反应机理反应实例Beckmann重排肟在酸如硫酸、多聚磷酸以及能产生强酸的五氯化磷、三氯化磷、苯磺酰氯、亚硫酰氯等作用下发生重排,生成相应的取代酰胺,如环己酮肟在硫酸作用下重排生成己内酰胺:反应机理在酸作用下,肟首先发生质子化,然后脱去一分子水,同时与羟基处于反位的基团迁移到缺电子的氮原子上,所形成的碳正离子与水反应得到酰胺。
迁移基团如果是手性碳原子,则在迁移前后其构型不变,例如:反应实例Birch还原反应机理反应实例Bischler-Napieralski合成法反应机理反应实例Bouveault-Blanc还原反应机理反应实例Bucherer反应反应机理Cannizzaro反应反应机理Chichibabin反应反应机理反应实例吡啶类化合物不易进行硝化,用硝基还原法制备氨基吡啶甚为困难。
本反应是在杂环上引入氨基的简便有效的方法,广泛适用于各种氮杂芳环,如苯并咪唑、异喹啉、吖啶和菲啶类化合物均能发生本反应。
《有机人名反应》课件

4. Heck反应
概念和原理,反应机理,应用和实例。 • 介绍Heck反应的概念和原理 • 解释反应机理 • 探讨反应在有机合成中的应用和实例
5. Sonogashira反应
概念和原理,反应机理,应用和实例。 • 介绍Sonogashira反应的概念和原理 • 解释反应机理 • 探讨反应在有机合成中的应用和实例
总结
通过学习这些有机人名反应,可以更好地了解化学反应的原理和应用,有助 于提高对化学知识的理解和掌握。
《有机人名反应》PPT课 件有 Nhomakorabea人名反应
本课程将介绍一些以有机人名命名的反应。这些反应是化学家们在研究中发 现和发展出来的,具有一定的实用价值。
1. Friedel-Crafts反应
概念和原理,反应机理,应用和实例。 • 介绍Friedel-Crafts反应的概念和原理 • 解释反应机理 • 探讨反应在各个领域中的应用和实例
2. Grignard反应
概念和原理,反应机理,应用和实例。 • 介绍Grignard反应的概念和原理 • 解释反应机理 • 探讨反应在有机合成中的应用和实例
3. Wittig反应
概念和原理,反应机理,应用和实例。 • 介绍Wittig反应的概念和原理 • 解释反应机理 • 探讨反应在有机合成中的应用和实例
