各种多溴联苯醚的HPLC检测
高分辨气相色谱_高分辨质谱测定活性污泥中的多溴联苯醚

2005年9月Sep te mber 2005色谱C h inese J ou rna l of C h rom a tog raphyVo l .23N o.5492~495谨以此文庆贺卢佩章院士80华诞!收稿日期:2005207217作者简介:王亚伟,男,博士研究生,E 2mail:wangy w_123@.通讯联系人:江桂斌,男,研究员,博士生导师,Tel:(010)62849334,E 2mail:gbjiang@mail .rcees .ac .cn .基金项目:国家重点基础研究发展规划项目(国家“973”项目)(No .2003CB415001).高分辨气相色谱2高分辨质谱测定活性污泥中的多溴联苯醚王亚伟, 张庆华, 刘汉霞, 江桂斌(中国科学院生态环境研究中心环境化学与生态毒理学国家重点实验室,北京100085)摘要:建立了利用高分辨气相色谱2高分辨质谱定量测定多溴联苯醚(PBD Es )的方法,参与了测定PBD Es 的国际比对实验,实验结果表明该法是可行的。
对不同地区(北京、山东临沂、上海)的3个污水处理厂的活性污泥进行了索氏提取、多层复合硅胶柱分离,然后用所建立的方法测定了19种多溴联苯醚单体的含量。
结果表明,北京某污水处理厂的活性污泥中PBD Es 的总量高于其他两者。
关键词:高分辨气相色谱2高分辨质谱;多溴联苯醚;污泥中图分类号:O 658 文献标识码:A 文章编号:100028713(2005)0520492204D e te rm in a t io n o f P o lyb rom in a te d D ip h e n y lE th e rs in S ew a ge S lu d ge b y H igh R e s o lu t io n G a s C h rom a to g rap h y C o up le dw ith H igh R e o lu t io n M a s s S p e c t rom e t ryWAN G Yaw e i,ZHAN G Q inghua,L I U H anx ia,J I AN G G u ib in(S ta te Key L a bora tory of En vi ronm en ta l Chem is try a n d Ecotox icology,Resea rch Cen ter for Eco 2En vi ronm en ta l Scien ces,The Ch in ese A ca dem y of Scien ces,B ei jin g 100085,Ch in a )A b s t ra c t:Po lyb rom ina ted d ip henyl e the rs (PBD Es )a re a g roup of b rom ina ted flam e re ta rd 2an ts,w h ich a re m anufac tu red in la rge quan tities and w ide ly used in a va rie ty of consum e r goods.R ecen tly they sp read ub iqu itous ly as environm en ta l con tam inan ts.In o rde r to inves ti 2ga te the p o llu tion leve l of PBD Es in d iffe ren t env ironm en ta l sam p les,a m e thod has been es tab 2lished by us ing h igh reso lu tion gas ch rom a tograp hy coup led w ith h igh reso lu tion m ass sp ec 2trom e try (HRGC 2HRM S ).The m e thod w as used to ana lyze b iosam p les ob ta ined from the in te r 2na tiona l in te r 2ca lib ra tion.The resu lts w e re sa tisfac to ry by ana lyzing z 2sco re figu res.Then,PBD E con ten ts in th ree sam p les of sew age s ludge w e re inves tiga ted by the m e thod afte r Soxh le t ex trac tion and m u lti 2laye r s ilica ge l ch rom a tograp h ic sep a ra tion.The num be r of PBD Es de te r 2m ined w as 19.F rom the resu lts,w e can see tha t the con tam ina tion leve l of PBD Es in the sam 2p le from B e ijing w as h ighe r than those from t w o o the r a reas.The m e thod w as va lida ted and app lied fo r the ana lys is of environm en ta l sam p les.Ke y w o rd s:h igh reso lu tion gas ch rom a tograp hy 2h igh reso lu tion m ass sp ec trom e try;p o lyb rom 2ina ted d i p henyl e the rs;sew age s ludge 多溴联苯醚(p o lyb rom ina ted d ip henyl e the rs,简称PBD Es )属于溴代阻燃剂(b rom ina ted flam ere ta rdan ts,B FR s )类化合物。
《塑料制品中多溴联苯和多溴二苯醚的测定 气相色谱-质谱法》 GB T 37639-2019 多溴联苯 多溴二苯醚

方法学确认报告方法名称:《塑料制品中多溴联苯和多溴二苯醚的测定气相色谱-质谱法》GB/T 37639-2019 一、基本情况仪器设备型号、名称及编号:气相色谱质谱仪,SB2692验证内容:1)标准曲线;2)检出限;3)加标回收率;4)精密度。
二、标准曲线按照标准建立塑料制品中多溴联苯和多溴二苯醚的测定方法。
配制浓度为0.2 µg/mL、0.4 µg/mL、1.0 µg/mL、2.0 µg/mL、5.0 µg/mL系列标准溶液。
根据检测结果,绘制标准曲线,观察线性效果。
标准曲线见附件1,PBB-1的线性方程为Y = 295900x+98820,相关系数R为0.99367,PBB-2的线性方程为Y = 136300x+14130,相关系数R为0.996665,PBB-3的线性方程为Y = 208500x+41220,相关系数R为0.993898,PBB-4的线性方程为Y = 4487x+292.3,相关系数R为0.996155,PBB-5的线性方程为Y = 22370x+10220,相关系数R为0.997625, PBB-6的线性方程为Y = 151200x+14130,相关系数R为0.996665,PBB-9的线性方程为Y = 510400x+41220,相关系数R为0.993898,PBB-10的线性方程为Y = 43388.701659x+38951.354727,相关系数R为0.9954,BDE-028的线性方程为Y = 12380.561334x+0.953079,相关系数R为0.9997,BDE-047的线性方程为Y = 18979.055658x+0.723268,相关系数R为0.9997,BDE-099的线性方程为Y = 100900x+57941,相关系数R为0.99367,BDE-100的线性方程为Y = 321700x+90147,相关系数R为0.99367,BDE-153的线性方程为Y = 464100x+92780,相关系数R为0.99367,BDE-154的线性方程为Y = 164500x+2140,相关系数R为0.99367,BDE-183的线性方程为Y = 15400x+9010,相关系数R为0.99367,BDE-198的线性方程为Y = 614500x+10120,相关系数R为0.99367,BDE-206的线性方程为Y = 201500x+98820,相关系数R为0.99367,BDE-209的线性方程为Y = 102300x+98820,相关系数R为0.99367。
高压液相色谱紫外(HPLCUV)法测定聚合物中多溴联苯

高压液相色谱/紫外(HPLC/UV)法测定聚合物中多溴联苯(PBB)与多溴联苯醚(PBDE)范围,应用及方法概述此方法提供了聚合物中多溴联苯(PBB)与多溴联苯醚(PBDE)的测试程序。
该文件描述了一种用来测定聚合物和印刷电路板中的阻燃剂PBB/PBDE类型的材料检测方法。
此测试方法使用配有紫外检测器的高压液相色谱仪(HPLC/UV)对聚合物中的多溴联苯(PBB)与多溴联苯醚(PBDE)进行分析。
被分析物是多溴联苯(PBB)与多溴联苯醚(PBDE)。
多溴联苯(PBB)系列中常见的是八溴联苯(OBB)和十溴联苯(DBB)。
多溴联苯醚(PBDE)系列则常见的是八溴联苯醚(OBDE)和十溴联苯醚(DBDE)。
参考书目,参考标准,参考方法及参考材料a) M. Riess 和R. van Eldik,通过倒相液体套色版和紫外线检测议对聚合物材料中溴化的阻燃剂进行鉴定,套色版A杂志,827 (1998) 65-71术语及定义文章中所涉及的关键术语定义如下:a) 均质材料:整个材料成分统一,不能被机械拆解成不同材料。
b) 应用材料:不能被进一步机械拆解为单一材料的单元,例如多层材料和小于1克的电子元器件。
仪器/设备和材料仪器/设备a) 提取单元: 拜耳SMA 12 加热块b) 低压力梯度泵的高压液相色谱系统,自动取样器,柱式加热炉和紫外线扫描仪,(例如,P 580的泵,ASI 100 的自动化取样器,STH 585 的柱式加热炉和德国DIONEX 公司生产的PDA 100 的光电二极管阵列检测仪)c) 先进的典型实验工具和设备是必要的d) 使用的仪器可以用一些功能相近的其它仪器代替材料a) 容量瓶b) 可调整的吸液管c) 12 x 32mm的玻璃瓶d) 过滤圆盘e) 固定相:改性的C18色谱柱,(例如Macherey-Nagel Nucleosil CC 125/4 100-5C18 "Nautilus" with pre-column CC 8/4 Nucleosil 100-5 C18 "Nautilus"(Macherey Nagel))试剂a) 甲醇(HPLC 级)b) 水(HPLC 级)c) KH2PO4 分析纯d) NaHPO4 分析纯e) DE-USC 902 十溴联苯醚(DE-83R-Great Lakes)(例如,LGC-Promochem)f) U-RBF-074 八溴联苯(技术)(FR250 BA, Dow Chemicals)(例如,LGCPromochem)g) DE-USC 910 八溴联苯醚(技术)(DE-79-Great Lakes) (例如,LGCPromochem)(U-RBF-102 十溴联苯) (e.g LGC-Promochem)标样准备(配制)/储备液的配制a) 流动相:97%的甲醇和3%缓冲水。
多溴联苯

电子电气产品中多溴联苯和多溴联苯醚的测定—(高效液相色谱仪)1.范围本部分规定了电子电气产品中多溴联苯和多溴联苯醚高效液相色谱仪测定方法。
本部分适用于电子电气产品中多溴联苯和多溴联苯醚的测定。
2.术语和定义下列术语和定义适用于本部分。
2.1多溴联苯polybromobiphenyls,PBBS多溴联苯的结构式见图一。
图1 多溴联苯的结构根据苯环上的溴原子的个数和位置的不同,多溴联苯总共有209种异构体。
2.2 多溴联苯醚polybromobiphenyls ethers,PBDES多溴联苯醚的结构式见图2.图2 多溴联苯醚的结构根据苯环上的溴原子的个数和位置的不同,多溴联苯总共有209种异构体。
3.方法提要样品采用甲苯作为提取溶剂进行索氏提取,提取液经浓缩处理,用液相色谱进行定量分析。
4.试剂和材料除非另有说明,在分析中仅使用确认为分析纯的试剂盒蒸馏水货去离子水或相当纯度的水。
4.1甲醇:色谱纯4.2磷酸二氢钾4.3磷酸氢二钠4.4甲苯4.5液氮:工业级4.6缓冲溶液(PH=7):将0.1509g磷酸二氢钾(4.3)和0.247.7g磷酸氢二钠(4.2)溶于100ml水中。
4.7PBBs:标准品。
4.8PBBs标准溶液:准确称取10.0mgPBBs标准品(4.7),分别置于100ml容量瓶中,用甲苯(4.4)稀释至刻度,混匀。
该溶液的浓度为100mg/L。
4.9PBDEs标准溶液:50mg/L异辛烷溶液。
4.10标准溶液的配制:分别移动浓度为100mg/L的PBBs(4.8)和50mg/L的PBDEs(4.9)标准溶液适量体积,用甲苯(4.4)稀释,配制成所需浓度的标准溶液。
5仪器和设备5.1高效液相色谱仪:配紫外---可见检测器。
5.2索氏提取装置5.3旋转蒸发器5.4粉碎机或类似设备。
5.5有机相过滤膜:0.45um过滤膜6 样品制备将电子电气产品中拆分的样品破碎成小月1c m×1cm的小块,液氮冷冻后用粉碎机(5.4)破碎成粒径小于1mm的颗粒7分析步骤7.1提取称取上述(0.5-5.0)g样品,精确到0.0001g,放入纤维素套管中,然后将其放至安装好的索氏提取装置(5.2)中,加入1.5倍虹吸管提及的甲苯(4.4)到接收瓶中,抽取3h以上。
气相色谱——质谱联用技术测定多溴联苯醚

气相色谱—质谱联用技术测定多溴联苯醚高春艳刘岩岩唐山三友硅业有限责任公司河北省063305液化空气(中国)投资有限公司上海市200233摘要:多溴联苯醚(PBDEs)是全球性的环境污染物,对其环境问题的研究已成为当前环境科学的一大热点,其检测技术也多种多样。
其中气相色谱-质谱法灵敏度高,选择性强,是最有应用前景的一种方法。
本文介绍了多溴联苯醚的性质、应用、环境行为等,重点介绍其前处理技术,样品的测试与分析,讨论了目前存在的问题,为以后开展PBDEs的研究提供参考。
关键词:多溴联苯醚,气相色谱-质谱法,检测Abstract:Polybrominated diphenyl ethers(PBDEs)is a global environmental pollutants,Its environmental problems has become a hot topic of environmental science,and it also has diverse detection technology.Gas chromatography-mass spectrometry is the most promising method,who has the high sensitivity and selectivity.This article describes the nature of PBDEs,applicationand environmental behavior,focuses on the pre-treatment technology,sample testing and analysis,,and discuss the problems of existing,which to provide reference to carry out research on PBDEs in the future. Keywords:Polybrominated diphenyl ethers(PBDEs),Gas Chromatograph-Mass Spectrometer(GC—MS),Detect中图分类号:O643.13+1文献标识码:A文章编号:1.引言多溴联苯醚(PBDEs)属于溴系阻燃剂(BFRs)的一种,由于其阻燃效率高,热稳定性好,添加量少,对材料性能影响小,价格便宜,因而作为一种添加型阻燃剂被广泛地应用在电子、电器、化工、交通、建材、纺织、石油、采矿等领域中。
某市区内索氏提取-高效液相色谱法测定土壤中多溴联苯醚课件

某地区土壤中多溴联苯醚的测定摘要:多溴联苯醚(PBDEs)作为阻燃剂被广泛应用与工业生产中,其危害也正逐渐扩大。
实验选取潮州市的两家鞋厂以及一处垃圾焚烧地中的土壤样品作为研究对象,采用索氏提取法提取土壤中有机物,加以浓硫酸净化再经过中性氧化铝柱进行纯化和分离,最后采用高效液相色谱法测定其中PBDEs的组成以及含量水平。
结果表明:PBDEs同系物的仪器检出限介于0.68-1.02 mg/L之间,环境样品中浓度较低的PBDEs不能被检出;鞋厂一和鞋厂二附近的土壤样本共有四种该同系物被检出,分别是2,4,4’-三溴联苯醚、2,2',4,4'-四溴联苯醚、2,2',4,4',5-五溴联苯醚、2,2',4,4',5,6'-六溴联苯醚,其中鞋厂二土壤样本中2,2',4,4'-四溴联苯醚浓度最高,达到0.72mg/Kg;垃圾焚烧地的土壤样本中PBDEs同系物检出率及浓度均较高。
其中2,2',4,4'-四溴联苯醚的检出浓度达到33.52mg/Kg。
关键词:多溴联苯醚;土壤;索氏提取;高效液相色谱法Abstract:Polybrominated diphenyl ethers (PBDEs), as a flame retardants are widely used in industrial production, the harm is also gradually expanding. We selected the soil of two shoe factory in chaozhou and a waste incineration as the research object, using the soxhlet extraction method to extract the soil organic matter, concentrated sulphuric acid for purificating neutral alumina column, then using high performance liquid chromatography (HPLC) method to determine the composition and their content of PBDEs level. The results showed that PBDEs homologue of instrument detection limit between 0.68 1.02 mg/L, low concentration in the environmental samples PBDEs cannot be checked out; Shoe factories 1 and shoe factory 2 soil samples near a total of four kinds of the homologue was detected, respectively is2,4,4 '- three bromine biphenyl ether, 2, 2', 4, 4 '- four bromine biphenyl ether, 2, 2', 4, 4 ', 5 - bromine biphenyl ether, 2, 2 ', 4, 4 ', 5, 6 '- bromine biphenyl ether, shoe factory in two soil samples of 2, 2', 4, 4 '- four bromine biphenyl ether concentration is highest, 0.72 mg/Kg; Waste incineration in soil samples of PBDEs homologue were higher detection rate and concentration. 2, 2 ', 4, 4 '- detection of four bromine biphenyl ether concentration is 33.52 mg/Kg.Key words:Polybrominated diphenyl ether; Soil; Soxhlet extraction. High performance liquid chromatography目录1.绪论 (1)1.1多溴联苯醚的简介 (1)1.2PBDEs的应用 (2)1.3土壤中PBDEs的来源 (2)1.4PBDEs的毒性及污染 (3)1.5PBDEs的控制措施 (3)1.6PBDEs的分析方法 (3)2.实验部分 (4)2.1仪器与试剂 (4)2.2样品前处理 (5)2.3仪器分析条件 (6)3结果与讨论 (6)3.1PBDEs的定性 (6)3.2PBDEs标准曲线 (7)3.3检出限 (7)3.4方法空白 (8)3.5实际土壤的测定 (8)4结论 (9)参考文献 (10)致谢 (11)索氏提取-高效液相色谱法测定土壤中多溴联苯醚1.绪论1.1 多溴联苯醚简介多溴联苯醚(PBDEs)是一类溴代芳香化合物, 作为一种嗅代阻燃剂,由于其优异的理化性质被广泛使用于各种工业产品中。
气相色谱-负化学电离源质谱法测定土壤中8种多溴联苯醚

气相色谱-负化学电离源质谱法测定土壤中8种多溴联苯醚1王林1,周友亚*1,杨进2,欧冬妮2,张超艳1,唐艳冬3,韩得满4,颜增光1,2李发生131(中国环境科学研究院环境基准与风险评估国家重点实验室,北京100012)42(通标标准技术服务(上海)有限公司,上海200233)53(环境保护部环境保护对外合作中心,北京100035)64(浙江省台州学院,台州318000)78摘要9建立了气相色谱-负化学电离源-质谱法测定土壤中8种多溴联苯醚的分析方10法。
利用V(二氯甲烷):V(丙酮)=1:1混合溶液提取土壤中多溴联苯醚,采用11气相色谱-负化学源-质谱法进行检测分析。
结果表明,土壤中各PBDEs单体的检12出限为0.05~10.00 ng/g,加标回收率为75%~135%,相对标准偏差为6.3%~24.4%。
13方法用于浙江台州12个实际土壤样品PBDEs的检测,结果令人满意。
14关键词15气相色谱-负化学离子源-质谱法;多溴联苯醚;土壤样品161 引言17多溴联苯醚(PBDEs)是一种常见的溴代阻燃剂(BFRs),因其阻燃效果高,18热稳定性好,价格便宜,对材料性能影响小等优点,而被广泛应用于塑料、纺织19品、油漆及电子产品中[1,2]。
目前,PBDEs已经在底泥、沉积物[3,4]、鱼类[5,6]、人20体[7,8]和土壤[9,10]等基质中被不同程度的检出,环境介质中痕量的PBDEs可通过21食物链对人类和高级生物的健康造成危害,也可通过“蚱蜢跳效应”广域迁移,22导致全球污染。
23目前,国内尚缺少环境样品中PBDEs的检测标准,美国环保署在2003年的24本文系国家自然科学基金(21075114)和环保公益性行业科研专项(201009015)资助* E-mail:zhouyy@EPA1614草案中推荐使用高分辨气相色谱(HRGC)-高分辨质谱(HRMS)检测25环境样品中的PBDEs[11]。
塑料颗粒样品中多溴联苯和多溴联苯醚(upload)
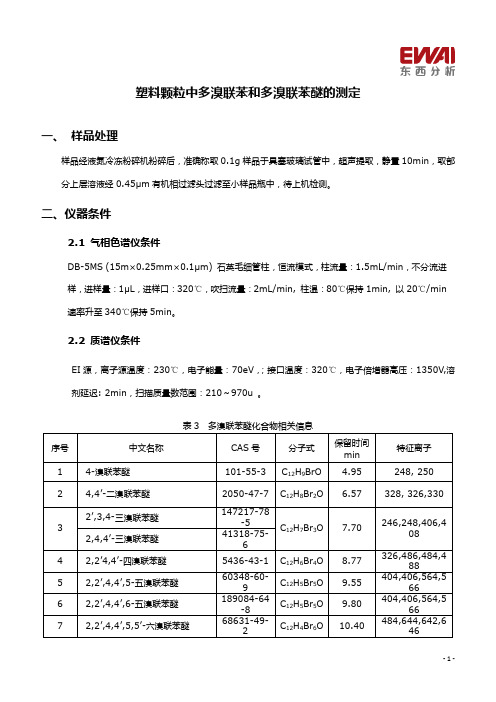
塑料颗粒中多溴联苯和多溴联苯醚的测定一、样品处理样品经液氮冷冻粉碎机粉碎后,准确称取0.1g 样品于具塞玻璃试管中,超声提取,静置10min ,取部分上层溶液经0.45μm 有机相过滤头过滤至小样品瓶中,待上机检测。
二、仪器条件2.1气相色谱仪条件DB-5MS (15m×0.25mm×0.1μm)石英毛细管柱,恒流模式,柱流量:1.5mL/min ,不分流进样,进样量:1μL ,进样口:320℃,吹扫流量:2mL/min,柱温:80℃保持1min,以20℃/min 速率升至340℃保持5min 。
2.2质谱仪条件EI 源,离子源温度:230℃,电子能量:70eV ,;接口温度:320℃,电子倍增器高压:1350V ,溶剂延迟:2min ,扫描质量数范围:210~970u 。
表3多溴联苯醚化合物相关信息序号中文名称CAS 号分子式保留时间min 特征离子14-溴联苯醚101-55-3C 12H 9BrO 4.95248,25024,4’-二溴联苯醚2050-47-7C 12H 8Br 2O6.57328,326,33032’,3,4-三溴联苯醚147217-78-5C 12H 7Br 3O 7.70246,248,406,4082,4,4’-三溴联苯醚41318-75-642,2’4,4’-四溴联苯醚5436-43-1C 12H 6Br 4O 8.77326,486,484,48852,2’,4,4’,5-五溴联苯醚60348-60-9C 12H 5Br 5O 9.55404,406,564,56662,2’,4,4’,6-五溴联苯醚189084-64-8C 12H 5Br 5O 9.80404,406,564,56672,2’,4,4’,5,5’-六溴联苯醚68631-49-2C 12H 4Br 6O10.40484,644,642,646表4多溴联苯化合物相关信息特征离子序号中文名称分子式保留时间min12-溴联苯C12H9Br 4.27232,234 22,5-二溴联苯C12H8Br2 5.75312,310,31432,4,6-三溴联苯C12H7Br3 6.67230,232,390,39242,2’,5,5’-四溴联苯C12H6Br47.98310,389,470,39152,2’,4,5’,6-五溴联苯C12H5Br58.75388,390,469,22862,2’,4,4’,6,6’-六溴联苯C12H4Br69.50627,625,468,5477八溴联苯C12H2Br812.85784,782,704,7068九溴联苯C12H2Br913.52862,864,782,7849十溴联苯C12Br1014.16941,943,862,783三、标样谱图图1多溴联苯醚标样色谱图图2多溴联苯标样色谱图图3塑料颗粒样品色谱图四、标准曲线分别准确移取一定量的多溴联苯醚或多溴联苯的标准储备溶液于容量瓶中,用合适的溶剂分别稀释成合适浓度的标准系列溶液,取1μL 进GC-MS 测定,以组分相应的峰面积为横坐标,浓度为纵坐标绘制标准曲线。
多溴联苯醚PBDE(PBDBE)的GC-MS分析

痕量多溴二苯醚的GC/MS/MS 分析方法冯爽美国瓦里安技术中国有限公司广州办事处1.背景介绍多溴二苯醚(PBDEs)结构式如下,是一系列含溴原子的芳香族化合物(见图1)。
根据苯环上溴原子的个数和位置的不同,多溴二苯醚总共有209种同分异构体。
因其独特的结构性质,多溴二苯醚最大的用途是作为阻燃剂,在制造过程中被人为添加到复合材料中去,使其不易燃烧。
目前,PBDE已被广泛用于电子电器设备、自动控制设备,建筑材料和纺织品等商品化产品。
1999年,全球多溴二苯醚的总使用量达到了80多万吨。
在这些产品的制造,使用,循环回收、或是抛弃的过程中,多溴二苯醚进入到空气,水,土壤的循环系统中,成为日常环境中到处扩散的持久性有机污染物,对环境和人类的威胁日益升高。
图1:2,2’,4,4’,5 –五溴二苯醚(PBDE-99) 的结构式多溴二苯醚具有很强的脂溶性,可以沉积生物体的脂肪组织并进行累积。
研究表明,多溴二苯醚具有和PCB(多溴联苯)类似的神经毒性,会对肝和神经系统的发育造成毒害,同时干扰甲状腺内分泌,可能致癌或引起生物性别错乱。
因此,越来越多国家开始关注起了多溴二苯醚物质的使用。
美国加州州政府已于2003年8月第一個宣布2007年后禁止制造和使用PBDEs。
欧盟2004年8月13日正式出台了《电子垃圾处理法》,要求2006年7月1日以后投放欧盟市场的电气和电子产品不得含有多溴二苯醚。
多溴联苯醚目前的检测方法主要气相色谱-FID检测法和气质联用仪检测法。
本文介绍的是一种高灵敏度,高选择性的方法分析环境和食品样品中的多溴二苯醚。
该方法主要有两大特点:1. 方法中Varian 最新的VF-5HT超低流失高温色谱柱,最高使用温度达到了400度,保证了多溴二苯醚化合物的出峰具有很好的色谱峰形。
2.方法中使用MS/MS串联质谱技术,使方法对八种多溴二苯醚的检出限均小于5ppb。
2. 实验方法:Technique:GC/MS/MSColumn:VF-5HT fused silica,30m x 0.25mm,df=0.10um,Part No:CP9046 Temperature:40℃开始,2℃/min升至230℃,6℃/min升至285℃,25℃/min升至340℃,340℃保持7minCarrier Gas:Helium, 1.3mL/minInjector:Splitless,T=340℃Detector:MSSample Size:1.0 µLConcentration range:八种多溴二苯醚的混标浓度:5μg/mL (PPBDE-47, PBDE-99, PBDE-100, PBDE-153,PBDE-154, PBDE-183, PBDE-205)10μg/mL (PBDE-209)表1:标样中8种多溴二苯醚的分子式、缩写和分子量标样化学名分子式缩写分子量2.2’,4,4’-Tetrabromodiphenylether C12H6OBr4PBDE-47485.812,2’,4,4’,5-Pentabromodiphenylether C12H5OBr5PBDE-99564.712,2’,4,4’,6-Pentabromodiphenylether C12H5OBr5PBDE-100564.712,2’,4,4’,5,5’-Hexabromodiphenylether C12H4OBr6PBDE-153643.612,2’,4,4’,5,6’-Hexabromodiphenylether C12H4OBr6PBDE-154643.612,2’,3,4,4’,5’,6-Heptabromodiphenylether C12H3OBr7PBDE-183722.512,3,3’,4,4’,5,5’,6-Octabromodiphenylether C12H2Obr8PBDE-205801.41Decabromodiphenylether C12OBr10PBDE-209959.213. 一级质谱结果图2:100ng/mL 的8种多溴二苯醚的一级质谱总离子流图4. 二级质谱结果通过对8种多溴二苯醚进行一级质谱全扫描的分析,分别选择其分子离子碎片作为二级质谱分析的母离子,经过CID (Collision-inducted dissociation )碰撞诱导解离后,得到子离子的碎片。
高效液相色谱法测定沉积物中的多溴联苯醚_张向荣

第26卷,第4期光 谱 实 验 室Vol.26,No.4 2009年7月Chinese J ournal of Sp ectroscop y L abor atory July,2009高效液相色谱法测定沉积物中的多溴联苯醚张向荣 薛科社(西北大学南校区99号信箱城市与资源学系段东平收转 西安市长安区新校学府大道1号 710069)摘 要 以二氯甲烷/正己烷为提取溶剂,采用Hy persil OD S柱(150×4.6m m,5 m),流动相为乙腈/水(90∶10),流速为1mL/min,检测波长为226nm,高效液相色谱法测定沉积物中多溴联苯醚.该方法平均回收率为98.87%,RSD小于2%,能够满足分析需求。
关键词 高效液相色谱法;沉积物;多溴联苯醚中图分类号:O657.7+2 文献标识码:A 文章编号:1004-8138(2009)04-0986-041 前言多溴联苯醚(Poly brominated diphenyl ethers,简称PBDEs)是一类目前使用最广泛的溴代阻燃剂,主要添加于塑料、电子以及涂料等产品中。
这种非反应性添加型的阻燃剂,易于通过各种途径进入环境,如PBDEs的生产添加过程、含PBDEs阻燃剂产品的使用和废弃期间以及其他一些途径[1]。
PBDEs具有疏水性、持久性和生物富集性,易于在颗粒物和沉积物中吸附以及在生物体中富集并可以在环境中长距离迁移[2],成为环境中到处扩散的持久性有机污染物。
最近的研究证实, PBDEs这类溴化物会干扰甲状腺激素,妨碍人类和动物脑部与中枢神经系统的正常发育。
目前,国内对环境样品中PBDEs检测的主要方法有气相色谱-负化学电离源质谱法(GC-NCI-MS)[3,4]、气相色谱-电子捕获检测法(GC-ECD)[5]、高分辨气相色谱-高分辨质谱法(HRGC-HRMS)[6]等,但使用高效液相色谱法对其进行检测的非常少[7]。
因此,本研究使用超声波辅助萃取和高效液相紫外检测法,对沉积物中PBDEs进行检测,建立了一种沉积物中PBDEs的分析方法。
气相色谱法同时测定水产品中12种多溴联苯醚

气相色谱法同时测定水产品中12种多溴联苯醚陈洁文柯常亮刘奇莫梦松(农业农村部渔业生态环境重点开放实验室,广东省渔业生态环境重点实验室,中国水产科学研究院南海水产研究所,广州510300)摘要:多溴联苯醚在水产品中的残留会危害人类健康。
本文建立了水产品中12种多溴联苯醚的气相色谱测定方法。
样品用30mL 正己烷︰丙酮(1︰1)超声提取20min ,60%硫酸脱脂,弗罗里硅土和中性氧化铝层析柱净化,5mL 正己烷+5mL 二氯甲烷按顺序洗脱,然后用配电子捕获检测器的气相色谱仪测定多溴联苯醚含量。
多溴联苯醚在1~200μg/L 浓度范围内线性关系良好,相关系数(r 2)>0.9972,检出限和定量限分别为0.2μg/kg 和1.0μg/kg ,样品的平均加标回收率为73.3%~98.4%,相对标准偏差<8.4%。
本方法操作简便快速、灵敏、可靠,适用于水产样品中12种多溴联苯醚的测定。
关键词:水产品;多溴联苯醚;化学物残留;气相色谱法基金项目:中央级公益性科研院所基本科研业务费专项(2016TS23);国家农产品质量安全风险评估项目计划(GJFP201800901)。
作者简介:陈洁文(1984-),助理研究员,从事渔业环境及水产品质量安全风险评估研究。
E -mail :xiaoa312690297@ 。
柯常亮(1980-),副研究员,从事渔业环境及水产品质量安全风险评估研究。
E -mail :kechangliang@ (通讯作者)。
多溴联苯醚(Polybrominated diphenyl ethers ,PBDEs )是一类添加型溴系阻燃剂,因其阻燃效率高,热稳定性好,对材料性能影响小,被大量生产且广泛用于电子电器、自动控制设备、建筑材料和纺织品中[1~3]。
PBDEs 具有很强的脂溶性和低水溶性,在环境中不易分解,易于和颗粒物质结合在沉积物中蓄积。
PBDEs 经水生生物从水体或沉积物中的吸收后,通过食物链逐级传递给顶级消费者人类,从而危害人类健康。
气相色谱质谱联用法检测多溴联苯、多溴联苯醚

气相色谱质谱联用法检测多溴联苯、多溴联苯醚摘 要:塑料等电子电器样品经甲苯索氏提取、硅胶柱净化、浓缩定容后,用气相色谱质谱联用法对其中的多溴联苯、多溴联苯醚进行定性和定量分析。
关键词:塑料等电子电器样品索氏提取气相色谱质谱联用法多溴联苯多溴联苯醚前言多溴联苯(PBBs)、多溴联苯醚(PBDEs)是一种广泛使用的溴代阻燃剂,被广泛应用于纺织、建材、塑料和电子等产品中。
该阻燃剂在自然环境中很难分解,可通过食物链在动物和人体内积聚,对环境和人体造成严重影响。
欧盟已对塑料业的环保标准进行了修改,在(RoHS)中规定全面禁止多溴联苯、多溴联苯醚含溴阻燃剂的使用。
本文利用岛津公司的GCMS-QP2010 SE对多溴联苯、多溴联苯醚进行分析,分离度、线性关系及重现性好。
1 实验部分1.1仪器日本岛津GCMS-QP2010 SE气相色谱-质谱联用仪1.2色谱条件色谱柱:MXT-1 15m x 0.28mm x 0.1µm进样口温度:280℃色谱柱温度: 110℃(2min) 40℃/min250℃10℃/min_300℃(2min) 40℃/min_325℃(5min) 恒线速度:76 cm/s进样方式:不分流进样进样量: 1µL离子源温度:230℃色谱-质谱接口温度:290℃采用SCAN全扫描模式进行定性分析,SIM选择离子模式进行定量分析,选择离子见表1。
表1 多溴联苯、多溴联苯醚选择离子表序号化合物名称参考离子1 一溴联苯monobromobiphenyl 234、232、1522 二溴联苯 dibromobiphenyl 312、310、1523 三溴联苯 tribromobiphenyl 392、390、2304 四溴联苯 tetrabromobiphenyl 470、310、3085 五溴联苯 pentabromobiphenyl 550、390、3886 六溴联苯 hexabromobiphenyl 628、468、4667 七溴联苯 heptabromobiphenyl 705、546、5448 八溴联苯 octabromobiphenyl 785、546、5449 九溴联苯 nonabromobiphenyl 864、705、70310 十溴联苯 decabromobiphenyl 944、783、78111 一溴联苯醚 monobromobiphenyl ether 250、248、14112 二溴联苯醚 dibromobiphenyl ether 328、326、16813 三溴联苯醚 tribromobiphenyl ether 408、406、24814 四溴联苯醚 tetrabromobiphenyl ether 488、486、32615 五溴联苯醚 pentabromobiphenyl ether 564、406、40416 六溴联苯醚 hexabromobiphenyl ether 643、484、48217 七溴联苯醚 heptabromobiphenyl ether 722、562、45618 八溴联苯醚 octabromobiphenyl ether 801、642、63919 九溴联苯醚 nonabromobiphenyl ether 881、721、71920 十溴联苯醚 decabromobiphenyl ether 959、799、7972 样品的制备3结果与讨论3.1多溴联苯、多溴联苯醚色谱图图1 多溴联苯标液TIC图( 1. 1-PBB,2. 2-PBB,3. 3-PBB, 4. 4-PBB,5. 5-PBB,6. 6-PBB,7. 7-PBB,8. 8-PBB,9. 9-PBB,10 10-PBB)图2 多溴联苯醚标液TIC图(1. 1-PBDE,2. 2-PBDE,3. 3-PBDE, 4. 4-PBDE,5. 5-PBDE,6. 6-PBDE,7. 7-PBDE,8. 8-PBDE,9. 9-PBDE,10 10-PBDE)3.2标准曲线将10种多溴联苯用甲苯稀释,配制成0.5、1.0、5.0µg/mL的多溴联苯混合标准溶液,将10种多溴联苯醚用甲苯稀释,配制成0.5、1.0、5.0µg/mL的多溴联苯醚混合标准溶液,以SIM 方式采集,得到各组分的标准曲线如下:1-PBB(R=0.9999842)2-PBB(R=0.9999559)3-PBB(R=0.999892)4-PBB(R=0.9998607)5-PBB(R=0.9999889)6-PBB(R=0.9999889)7-PBB(R=0.999956)8-PBB(R=0.9991274)9-PBB(R=0.9995069)10-PBB(R=0.9995668)1-PBDE(R=0.999938) 2-PBDE(R=0.9997509)3-PBDE(R=0.9983856)4-PBDE(R=0.9989115) 5-PBDE(R=0.999589)6-PBDE(R=0.9999837)7-PBDE(R=0.9999905)8-PBDE(R=0.9987804)9-PBDE(R=0.999632)10-PBDE(R=0.9990846)图3 多溴联苯、多溴联苯醚标准曲线3.3 重现性测试分别取1.0µg/mL的多溴联苯混合标准溶液和多溴联苯醚混合标准溶液进行重现性测试,结果见表2、表3、表4、表5。
高效液相色谱法测定电子电气产品中多溴联苯和多溴联苯醚

高效液相色谱法测定电子电气产品中多溴联苯和多溴联苯醚赖莺;王鸿辉;蔡鹭欣;董清木;林睿;葛秀秀;吴俊杰【摘要】Contents of polybromo biphenyls (PBB's) and polybromo diphenyl ether (PBI)E' s) in electronic and electrical products were determined by HPLC. Samples of common plastics were extracted in a micro-wave extractor with a mixture of n-hexane and acetone (1+1), and samples of plastics containing metals were extracted ultrasonically with ethyl acetate. The extract was separated on NUCLEODUR Sphinx RP C18 column by gradienl elution with water and acetonitrile mixed solutions of different ratio, and determined at the wavelength of 226 nm. Linear relationships between values of peak area and mass concentration ot PBB's and PBI)E's were kept in the same range of 0. 2--50 mg·L^-1 with lower limits of determination (10S/N) in the range of 9. 3--5.0 mg ·kg^-1.%提出了高效液相色谱法测定电子电气产品中多溴联苯和多溴联苯醚含量的方法。
气相色谱质谱联用法检测多溴联苯、多溴联苯醚

气相色谱质谱联用法检测多溴联苯、多溴联苯醚摘 要:塑料等电子电器样品经甲苯索氏提取、硅胶柱净化、浓缩定容后,用气相色谱质谱联用法对其中的多溴联苯、多溴联苯醚进行定性和定量分析。
关键词:塑料等电子电器样品索氏提取气相色谱质谱联用法多溴联苯多溴联苯醚前言多溴联苯(PBBs)、多溴联苯醚(PBDEs)是一种广泛使用的溴代阻燃剂,被广泛应用于纺织、建材、塑料和电子等产品中。
该阻燃剂在自然环境中很难分解,可通过食物链在动物和人体内积聚,对环境和人体造成严重影响。
欧盟已对塑料业的环保标准进行了修改,在(RoHS)中规定全面禁止多溴联苯、多溴联苯醚含溴阻燃剂的使用。
本文利用岛津公司的GCMS-QP2010 SE对多溴联苯、多溴联苯醚进行分析,分离度、线性关系及重现性好。
1 实验部分1.1仪器日本岛津GCMS-QP2010 SE气相色谱-质谱联用仪1.2色谱条件色谱柱:MXT-1 15m x 0.28mm x 0.1µm进样口温度:280℃色谱柱温度: 110℃(2min) 40℃/min250℃10℃/min_300℃(2min) 40℃/min_325℃(5min) 恒线速度:76 cm/s进样方式:不分流进样进样量: 1µL离子源温度:230℃色谱-质谱接口温度:290℃采用SCAN全扫描模式进行定性分析,SIM选择离子模式进行定量分析,选择离子见表1。
表1 多溴联苯、多溴联苯醚选择离子表序号化合物名称参考离子1 一溴联苯monobromobiphenyl 234、232、1522 二溴联苯 dibromobiphenyl 312、310、1523 三溴联苯 tribromobiphenyl 392、390、2304 四溴联苯 tetrabromobiphenyl 470、310、3085 五溴联苯 pentabromobiphenyl 550、390、3886 六溴联苯 hexabromobiphenyl 628、468、4667 七溴联苯 heptabromobiphenyl 705、546、5448 八溴联苯 octabromobiphenyl 785、546、5449 九溴联苯 nonabromobiphenyl 864、705、70310 十溴联苯 decabromobiphenyl 944、783、78111 一溴联苯醚 monobromobiphenyl ether 250、248、14112 二溴联苯醚 dibromobiphenyl ether 328、326、16813 三溴联苯醚 tribromobiphenyl ether 408、406、24814 四溴联苯醚 tetrabromobiphenyl ether 488、486、32615 五溴联苯醚 pentabromobiphenyl ether 564、406、40416 六溴联苯醚 hexabromobiphenyl ether 643、484、48217 七溴联苯醚 heptabromobiphenyl ether 722、562、45618 八溴联苯醚 octabromobiphenyl ether 801、642、63919 九溴联苯醚 nonabromobiphenyl ether 881、721、71920 十溴联苯醚 decabromobiphenyl ether 959、799、7972 样品的制备3结果与讨论3.1多溴联苯、多溴联苯醚色谱图图1 多溴联苯标液TIC图( 1. 1-PBB,2. 2-PBB,3. 3-PBB, 4. 4-PBB,5. 5-PBB,6. 6-PBB,7. 7-PBB,8. 8-PBB,9. 9-PBB,10 10-PBB)图2 多溴联苯醚标液TIC图(1. 1-PBDE,2. 2-PBDE,3. 3-PBDE, 4. 4-PBDE,5. 5-PBDE,6. 6-PBDE,7. 7-PBDE,8. 8-PBDE,9. 9-PBDE,10 10-PBDE)3.2标准曲线将10种多溴联苯用甲苯稀释,配制成0.5、1.0、5.0µg/mL的多溴联苯混合标准溶液,将10种多溴联苯醚用甲苯稀释,配制成0.5、1.0、5.0µg/mL的多溴联苯醚混合标准溶液,以SIM 方式采集,得到各组分的标准曲线如下:1-PBB(R=0.9999842)2-PBB(R=0.9999559)3-PBB(R=0.999892)4-PBB(R=0.9998607)5-PBB(R=0.9999889)6-PBB(R=0.9999889)7-PBB(R=0.999956)8-PBB(R=0.9991274)9-PBB(R=0.9995069)10-PBB(R=0.9995668)1-PBDE(R=0.999938) 2-PBDE(R=0.9997509)3-PBDE(R=0.9983856)4-PBDE(R=0.9989115) 5-PBDE(R=0.999589)6-PBDE(R=0.9999837)7-PBDE(R=0.9999905)8-PBDE(R=0.9987804)9-PBDE(R=0.999632)10-PBDE(R=0.9990846)图3 多溴联苯、多溴联苯醚标准曲线3.3 重现性测试分别取1.0µg/mL的多溴联苯混合标准溶液和多溴联苯醚混合标准溶液进行重现性测试,结果见表2、表3、表4、表5。
高分辨气相色谱-高分辨质谱测定活性污泥中的多溴联苯醚

$ 标 记添 加 内
再加入 (" > 无 水 硫 酸 钠 混 匀, 用 &") 6) 标 &) ! ) , 正己烷 ! 二氯甲烷 混 合 液 ( 体积比为 &. &) 索氏提取 (* = 。 ! ! 将提取液转入 洁 净 的 烧 瓶 中, 加入一定量的酸 性硅胶, 磁力搅拌约 )+ " = , 以去除脂肪等大分子杂 质; 再将提取液过无水硫酸钠小柱, 之后用盐酸处理 过的铜粉进行处理 以 去 除 提 取 液 中 的 硫; 再将提取 液过无水硫酸钠小 柱; 最后采用旋转蒸发法将提取 液浓缩至 ( - , 6) 以便做进一步的处理。 ! ! 将浓缩好的提 取 液 用 复 合 硅 胶 柱 进 行 纯 化, 先 用约 $) 6) 正 己 烷 洗 涤 复 合 硅 胶 柱, 然 后 上 样, 用 $) 6) 正己烷预淋洗, 然后用 $) 6) 二氯甲烷 ! 正己 烷 ( 体积比为 &. & ) 混合液洗脱并接取。 ! ! 将 接 取 的 洗 脱 液 旋 转 蒸 发 至 & - ( 6) , 然后转 入 G!& 管中, 用高 纯 氮 气 吹 至 ()) ! ) , 转入样品管 中吹至 &) ! ) , 再 加 入 &, $ 标 记 #%&’ 回 收 率 内 标, 准备进样。 ! ! $" 色谱和质谱条件 ! ! 色 谱 条 件 ! 色 谱 柱 -#!" ( ,) 6 / )+ (" 66 /+ L+ / )+ (" ! 6 ) ; 柱 温 程 序 升 温: 初 始 温 度 ’) J , 保持 ( 6/2 , 以 (" J K 6/2 的 速 度 升 至 (&) J , 保持 & 6/2 , 然后以 &) J K 6/2 的 速 度 升 至 ($" J , 保持 &) 6/2 , 再以 (" J K 6/2 升至 ,,) J 并保持 &) 6/2 ;
国家测定环境中多溴联苯醚的标准

国家测定环境中多溴联苯醚的标准随着工业化进程的不断加快和生产方式的不断更新,人类对环境的破坏程度也越来越严重。
环境污染对人类健康和生态平衡造成了严重的威胁,因此环境监测和标准制定变得至关重要。
多溴联苯醚是一种常见的污染物,其在环境中的浓度直接影响着人类的生活质量和健康状况。
为了保护环境和人类健康,国家不断加强对多溴联苯醚的监测和标准制定工作。
多溴联苯醚是一类广泛应用于电子产品、建筑材料和家具等领域的化学物质。
由于其在生产和使用过程中很容易泄漏到环境中,因此可能造成环境污染和健康风险。
多溴联苯醚对人体的影响主要包括内分泌干扰、神经毒性和致癌性等,对环境的影响主要表现为生物富集和生态系统破坏。
对多溴联苯醚的监测和标准制定显得尤为重要。
国家对环境中多溴联苯醚的监测工作主要包括以下几个方面:1. 监测点的设置:国家在重点城市、工业区和人口密集地区设置多溴联苯醚监测点,通过对空气、水体和土壤等不同环境介质中多溴联苯醚的监测,全面了解污染物的分布和变化规律。
2. 监测指标:国家针对多溴联苯醚的监测指标进行了明确定义,包括主要成分和代表性衍生物的监测要求,以及对不同环境介质中多溴联苯醚浓度的限值要求。
通过对监测指标的严格要求,确保了监测数据的准确性和可比性。
3. 监测方法:国家制定了多溴联苯醚的监测方法,包括采样、样品处理和分析检测等技术要求。
通过使用标准化和统一的监测方法,保证监测数据的科学性和可靠性。
除了对多溴联苯醚的监测工作,国家还在不断加强对该污染物的标准制定工作。
多溴联苯醚的标准制定主要包括以下几个方面:1. 标准的制定依据:国家依据相关法律法规,借鉴国际标准和先进经验,结合国内实际情况,制定了多溴联苯醚的环境标准。
标准的制定依据科学权威,具有显著的可操作性和推广性。
2. 标准的内容:国家对多溴联苯醚的环境标准进行了明确定义,包括对不同环境介质中多溴联苯醚的限值要求、监测方法和质量控制要求。
通过对标准内容的规范性和权威性,确保了多溴联苯醚标准的科学性和严谨性。
超高效液相色谱法测定纺织品中多溴联苯(醚)类阻燃剂

超高效液相色谱法测定纺织品中多溴联苯(醚)类阻燃剂曾立平【摘要】建立了纺织品中12种多溴联苯(醚)类阻燃剂的超高效液相色谱法,该方法以正己烷-丙酮(7+3)为溶剂,二次超声前处理,采用PDA检测器,流动相为乙腈/5 mmol/L乙酸胺溶液,在反相BEH C18色谱柱上进行梯度淋洗.实验结果表明该方法能将12种PBBs和PBDEs快速有效分离,且具有较好的线性范围与线性关系,检出限为0.007~0.172 mg/L.选取3个浓度添加水平得到的平均回收率为84.2%~ 102.5%,每个水平平行测定7次,相对标准偏差(RSD)为0.98%~6.02%,该方法前处理简单、结果准确,适用于在实际工作中对纺织品中PBBs和PBDEs的快速检测.【期刊名称】《毛纺科技》【年(卷),期】2016(044)007【总页数】6页(P37-42)【关键词】多溴联苯(醚);超声萃取;超高效液相色谱;检测【作者】曾立平【作者单位】国家纺织服装产品质量监督检验中心(浙江桐乡),浙江桐乡 314500【正文语种】中文【中图分类】TS197纺织品中的溴系阻燃剂是目前使用量最大的阻燃剂,因具有优异的阻燃性能和较低廉的价格,广泛应用于纺织、家具、建材和电子等领域[1-2],包括多溴联苯(PBBs )及多溴联苯醚(PBDEs)。
PBDEs(化学通式为C12H(0~9)Br(1~10)O。
依据苯环上溴原子取代数目和位置的不同,共有209种同系物,是一类持久性的有机污染物。
而PBBs与PBDEs类似,根据苯环上溴原子的个数和位置的不同,多溴联苯总共有209种异构体。
PBBs和PBDEs 在环境中难降解,滞留时间长,具有较强的亲脂性,其健康危害已成为全球性的环境污染问题。
研究表明,PBBs和PBDEs对实验动物有致癌性、生殖毒性,可能对人的大脑、肝脏等器官以及神经系统、内分泌系统、生殖系统产生较大的危害,在普通自然环境中难降解,且燃烧时会产生毒性更强的多溴联苯二嗯英和多溴联苯并呋喃 [3-4]。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Journal of Chromatography A,827(1998)65–71Identification of brominated flame retardants in polymeric materials by reversed-phase liquid chromatography with ultraviolet detection*Michael Riess,Rudi van Eldik ¨Institute for Inorganic Chemistry ,University of Erlangen -Nurnberg,Egerlandstr .1,91058Erlangen ,Germany Received 10April 1998;received in revised form 4June 1998;accepted 8September 1998AbstractA fast and simple method for the qualitative identification of technical flame retardants in polymeric materials isdescribed.The LC separation was achieved on a reversed-phase C column.The identification of a large variety of flame 18retardants in a single analytical LC run was achieved using a mixture of aqueous phosphate buffer and methanol with ultraviolet detection in the scanning mode.The method was used for the analysis of flame retardants in a number of polymeric waste materials from TV sets and personal computers,on the basis of a comparison with standards.©1998Elsevier Science B.V .All rights reserved.Keywords :Brominated flame retardants;Polymers1.Introductioncarbons that were mostly used in recent years,such as tetrabromobisphenol A and its derivatives,tri-Organic polymer materials are widely used in bromophenol,tetrabromophthalic anhydride,bistri-various industries [1].If flame retardancy is required,bromophenoxyethane,polybrominated biphenyls and e.g.in electrotechnical applications such as TV biphenyloxides (see Table 1).Due to different struc-cabinets or personal computer housings,flame re-tures and degrees of bromination,their physical and tardant additives containing bromine,chlorine or chemical properties are typical for environmentally phosphorus are often employed [2].Each flame critical substances [4,5].Noticeable concentrations of retardant has its own specific application in a number some of these compounds were detected in various of polymers (see Table 1).biotic samples [6,7].Brominated dioxins and furans In waste plastics,especially in scrap from elec-may be formed during synthesis,polymer com-tronic applications,a large variety of polymers and pounding and combustion,as well as during the use flame retardants can be found.Due to the recent of electrical appliances [8,9].increase in recycling activities,fast universal meth-Various chromatographic techniques have been ods for the identification of flame retardants,in-employed for the analysis of polymer additives [10–dependent of the polymer matrix,are needed [3].13].As a result of the good separation performance,This is especially true for the polybrominated hydro-gas chromatography is often preferred [14].How-ever,for compounds with high molecular weight,*high separation temperatures,that can induce thermal Corresponding author.Tel.:1499131857350;fax:1499131857387reactions,are required.High-performance liquid0021-9673/98/$–see front matter ©1998Elsevier Science B.V .All rights reserved.PII:S0021-9673(98)00748-166M.Riess,R.van Eldik/J.Chromatogr.A827(1998)65–71Table1Flame retardant reference samplesaNo.Chemical name Abbreviation Trade name Molecular Br content Maincweight(%)applicationsI Tetrabromobisphenol A(TBBPA)BA-59BP543.959EPR,PC,ABS II TBBPA-bis-2-(hydroxyethylether)(TBBPA-EO)BA-50632.051ABSIII TBBPA-bis-(2,3-dibromopropylether)(TBBPA-PE)PE-68943.668PE,PPIV TBBPA-bisallylether(TBBPA-AE)BE-SI624.051PS foamsV2,4,6-Tribromophenol(TBP)PH-73330.873EPR,PhenolsVI1,2-Bis(tribromophenoxy)ethane(TBPE)FF-680687.751ABS.PCVII Tetrabromophthalic anhydride(TBPSA)PHT-4463.768UPEVIII Decabromodiphenyloxide(DECA)DE-83959.283general purpose IX Octabromodiphenyloxide(OCTA)DE-79801.480ABS.SB,PS,PC X Pentabromodiphenyloxide(PENTA)DE-71564.771PURbXI Decabromobiphenyl(DBB)-943.385general purposebXII Octabromobiphenyl(OBB)FR250BA785.481PS,SBbXIII Hexabromobiphenyl(HBB)Firemaster BP-6627.676SBa Unless otherwise indicated all substances were obtained from Great Lakes Europe,Frauenfeld,Switzerlandb Substances were obtained from Mallinckrot–Baker Chemicals,Griesheim,Germany.FR250BA is a product of Dow Chemical;Firemaster BP-6was produced by Michigan Chemical.c Abbreviations used:ABS Acrylonitrile butadiene styrene,EPR Epoxyresin,PC Polycarbonate,PE Polyethylene,PP Polypropylene,PS Polystyrene,UPE Unsaturated polyesters,SB Stryrene butadiene,PUR Polyurethane.chromatography(HPLC)circumvents these prob- 2.Experimentallems.There are no thermally induced reactions,andmolecules of high molecular weight and a wide 2.1.Materialsrange of polarity can be analysed.The time ofanalysis is relatively short and ultraviolet(UV) 2.1.1.Reference sample preparationdetection results in good detection limits.Reversed-All solvents used were of HPLC-grade(Aldrich). phase[15]and normal-phase HPLC–UV methods Samples offlame retardants were obtained as a gift [13,23]have already been applied in the analysis of from Great Lakes Europe,Frauenfeld,Switzerland. polymer additives.However,the analysis offlame Some of them are also commercially available(No.I retardants by HPLC was confined to only a few and IX Aldrich,No.V and VII Lancaster).Samples specific compounds[1,16,17,23].No.XII,XIII and XIV were obtained from Mallinck-The identification of brominatedflame retardants rodt–Baker Chemicals.The investigated substances present in real samples,is based on a comparison of are listed in Table1.All reference samples werethe peak retention times t,and the corresponding homogenized before use.Reference sample solutions RUV absorption spectra with those of known stan-were prepared in n-propanol.In order to prevent dards.A characteristic sequence of peaks(peak photodegradation processes,samples in the solid pattern recognition)is also useful for the identifica-state were stored in the dark.Dissolved samples and tion of certain technicalflame retardants.The pur-polymer extracts were stored in brown glass bottles pose of this work was to develop a fast RP-HPLC/at58C.UV method,suitable for the analysis of extractableflame retardants from polymers.With the aid of the 2.1.2.Polymer sample preparation and extraction optimized HPLC parameters,UV spectral and re-Seventy-eight used TV and34personal computer tention time data for the reference substances were housings were collected from a local recycling determined and collected in an analytical data pany.Polymer samples were grinded to a size of The feasibility of this method for the identification of1000m m by the combination of cutting and centrifu-brominatedflame retardants in polymer samples is gal mills(Model SM2000and ZM100,Retsch, demonstrated.Germany)under cooling with liquid nitrogen prior toM.Riess,R.van Eldik/J.Chromatogr.A827(1998)65–7167 extraction.0.50g of the plastic shavings were mixed methanol,ethanol or n-propanol are able to dissolve with0.20gfibrous cellulose in a soxhlet thimble to a relatively polar phenol such as tetrabromobisphenol avoid agglomeration.The samples were extracted A(No.I),as well as the rather non-polar decabro-with50ml methanol and n-propanol in a soxhlet modiphenyloxide(No.VIII).Due to the relative low apparatus for three h.After cooling to room tempera-viscosity of methanol,it was chosen as the main tures,the extraction solutions werefiltered through a component of the mobile phase.n-propanol turned membrane diskfilter(0.45m m).out to be the best sample solvent.Atfirst several stationary phases were tested.A 2.2.Instrumentation reversed-phase(RP)-C18,a RP-CN and a RP-phenylwere compared.Some components were absorbed on Liquid chromatographic separations were per-the CN-phase whereas for the phenyl phase an formed with a Spectra Physics HPLC model SP8800insufficient separation into two groups(one and two LC-Pump system.Sample injections were performed aromatic systems)was observed.The separation with a Spectra Physics SP8780Autosampler,experiments were therefore started with a10m m equipped with a50m l sample loop,allowing the particle size C-18RP-column and100%methanol as variation of the injection ually10m l of the mobile phase.The retention time of tetrabromo-the sample were injected after an equilibration time bisphenol A itself was not reproducible under these of one minute.The ultraviolet detection was done conditions.Modification of the mobile phase with with the aid of a Thermo Separation Products3%of buffered water changed the retention time Spectra FOCUS Detector in the scanning mode.from1.60to1.45min,although an extension of the Control over the pump and autosampler,as well as retention time was expected on the basis of the data acquisition and handling,were performed with a weaker solvent strength.The degree of hydration at Spectra-Physics PC1000software package.The anal-the phenolic function,which depends on the pH ysis was carried out at a temperature of23628C.value of the mobile phase,seems to be responsible Chemical separations were achieved on a4mm ID,for the change in retention times.All other sub-250mm long column(Nucleosil ET250/4/8,stances showed the expected behaviour:their re-Macherey–Nagel,Germany).C18(ODS)reversed-tention times increased on using a weaker eluent.In phase and a particle size of7m m was used as order to increase the separation performance,several stationary phase.97%methanol and3%buffered solvent gradients(e.g.start:A95%,B5%,gradient water of pH57were used as mobile phase at a(t55mm)to A100%;A575%methanol/20%n-21flow-rate of1.0ml min.The water was buffered propanol/5%buffered water;B5buffered water)and21by dissolving0.1509g(M5136.09g mol)a change in particle size to7m m were also tested.21KH PO and0.2477g(M5141.96g mol)The gradient method enhances the selectivity but not 24Na HPO of p.a.quality in100ml water[18].the resolution.Due to polymer wax in the extracts 24[19],7m m is the smallest particle size suitable for amoderate pressure in the system.Under these con-3.Results and Discussion ditions(C18(ODS)stationary phase,7m m particlesize and a mobile phase of97%methanol and3% 3.1.Preliminary studies buffered water(pH57),at a complete run time of18min)all the components of theflame retardants are Thefirst objective of our work was tofind suitable sufficiently resolved.The retention times as well as conditions for the RPLC separation of theflame the UV absorption maxima and detection limits for retardants listed in Table1.These separation con-the latter chromatographic conditions are given in ditions could then be used for the identification of Table2.theflame retardants following extraction from the According to Table2,substances I to VIII can be waste polymers.Theflame retardants investigated,identified by one characteristic peak,with reproduc-show a large difference in polarity.Preliminary ible retention times,if buffered water(pH57)is experiments showed that only alcohols such as used in the mobile phase.The other substances IX to68M.Riess,R.van Eldik/J.Chromatogr.A827(1998)65–71Table2XIII show sequences of peaks(see Table3)under Chromatographic data obtained for reference substances these chromatographic conditions,which can be aNo.Retention Detection limit UV maxima(nm)accounted for in the following way.time t(S/N53)Ret From the literature[16,22,23]it is well known thatc(min)(ppm)commercialflame retardants,based on polybromi-I 1.4514206,291nated biphenyls(PBBs)or biphenylethers(PBDEs), II 2.8080203,280consist of several components of varying degree of III10.3247206,280bromination,although the product itself shows con-IV 6.1631206,280sistent quality and physicochemical properties.This V 1.5524214,252,319VI10.6734209is a result of different production methods or con-VII 1.5223222ditions,depending on the producer[16,22,23].In VIII16.5648226contrast to the decabrominated compounds,penta-bIX9.5640218b and octabromodiphenyloxides,as well as hexa-X 6.2628204b bromo-and octabromodiphenyl,show characteristic XI9.1847226bXII9.3139223sequences of peaks that are also useful for the bXIII7.5120215identification of the substances.The retention times, a area percentage at220nm and ultraviolet maxima of See Table1.b Data are given for the main component of these technical these samples are reported in Table3. mixtures.For instance,in the case of the main band of thec R.S.D.:0.05to0.81%(n55).biphenyls,a barthochromic shift of ca.5to13nmTable3Characteristic sequence of peaks for technicalflame retardantsNo.Abbreviation Retention time Area%UV-maxima[nm]t[min]at l5220nmRIX OCTA7.28 1.12127.860.62188.59 6.62119.56442199.941622010.62 1.821911.809.922312.371522615.00 1.022615.83 1.4228X PENTA 4.91302015.42 2.32045.72112046.26472057.75 4.62028.44 3.9207XII OBB7.61 2.02198.51 3.22259.31632259.6031222XIII HBB 5.90 3.12146.499.32147.51642168.52172219.58 5.2224M.Riess,R.van Eldik/J.Chromatogr.A827(1998)65–7169 occurs for the more highly substituted compounds.characteristicfingerprints or sequence of peaks to-The shift in the ultraviolet maxima is observed[21],gether with the ultraviolet spectra were used(for but it is not linearly correlated with the retention examples see Fig.1).All these data were collected in times.This was already found and reported by de an analytical database.Qualitative detection limits Kok[23].In a normal-phase HPLC system,retention between13and47ppm at a signal to noise ratio of times decrease with an increasing number of bromine3,referring to the identification of theflame re-atoms in the molecules.Ortho substituted com-tardants in polymer samples,are also included in pounds show promoted retention[23].In our case of Table2.a reversed-phase system,retention times behave viceversa.The retention time tends to increase for the 3.2.Identification offlame retardants in polymer higher substituted compounds due to their more non-samples from polymer waste materialspolar character,although this rule is not strictlyadhered to in all cases.In order to demonstrate the feasibility of the For the identification of theflame retardants,developed analytical method for the identification of Fig.1.Chromatogram of an extract(top)of aflame retarded computer housing and the corresponding standard as three dimensional chromatogram(bottom).70M.Riess,R.van Eldik/J.Chromatogr.A827(1998)65–71flame retardants in single polymer samples,a total of78TV housings and34personal computer housingswere analysed as received from the recycling com-pany.The average age of the housings was between17and22years for the TV sets and between two andten years for the personal computers.All polymerswere styrene-based polymers.Following the de-scribed analytical procedure,the leachable additivesin the polymer samples were analysed.From acomparison of the data in the database with those ofthe samples,theflame retardants could be identified.Octabromobiphenyl(No.XII)was identified in17%,decabromobiphenyl(No.XI)in4%and1,2-bis(trib-romophenoxy)ethane(No.VI)in4%of the TV Fig. 2.Differentiation of UV-spectra,UV-absorption of octa-bromodiphenyl oxide at9.56min‘A’;first order differentiation housings.Decabromodiphenyloxide(No.XI)(51%)with respect to l‘A9’second order differentiation with respect to and octabromodiphenyloxide(No.IX)(26%)oftenl‘A0’.occur together in the TV housing samples.The sameis observed for the personal computer housingswhere decabromodiphenyloxide(No.XI)was iden-3,the same effect as described was observed.The of tified in55%and octabromodiphenyloxide(No.IX)the retention time for these samples is influenced by in18%of the samples.Moreover tetrabromobis-matrix effects and a R.S.D.,e.g.for a sequence of phenol A(No.I)was identified in18%and1,2-five extracts containing polybrominated bi-bis(tribromophenoxy)ethane(No.VI)in9%of these phenyloxides,of1.04%(n55)is found.This is due samples.to the large variety and high amount of additives in Fig.1shows a typical three dimensional chro-the polymer extracts.matogram for an extract of aflame retarded ABS-In a specific class of main polymer only certain polymer,as well as the three dimensional chromato-flame retardants occur(see Table1).For recycling gram of the corresponding standard.Octab-purposes,samples are usually sorted by polymer and romodiphenyloxide can be identified,as indicated byflame retardant prior to the material recycling step. the characteristic sequence of peaks and by compar-This circumvents the limitation thatflame retardant I, ing UV spectra with those in the database.V and VII are not sufficiently resolved by the The identification of the UV spectra can be described method.improved by comparing differentiated UV spectra22(d I/d l or d I/(d l)).Fig.2presents an example forthe differentiation of the UV spectrum obtained for 4.Conclusionsthe main component of octabromodiphenyloxide at9.56min.A simple and reliable method for the identification For all investigated samples containing octab-offlame retardant species in polymer samples in a romodiphenyloxide,the sequence of peaks is the single liquid chromatographic step has been de-same as far as retention times and the UV spectra are veloped.This method makes it possible to identify concerned.They are also the same as for the polybrominatedflame retardants in styrene based reference samples(see Fig.1).However,there are polymer samples.The application to other sample slight differences in the composition of theflame matrices has to be investigated in future work.The retardants depending on the manufacturer as already method is characterized by a short analysis time, pointed out,such that the relative peak areas of the which is only influenced by the sample preparation peaks at a certain wavelength do vary.But this does procedures.Modern extraction techniques[20]will not reduce the value of the described identification assist in shortening this time.In future,attention will method.For all otherflame retardants listed in Table be focused on the identification of other leachableM.Riess,R.van Eldik/J.Chromatogr.A827(1998)65–7171[7]P.S.Haglund,D.R.Zook,H.-R.Buser,J.Hu,Environ.Sci. substances of lower molecular weight in the extracts.Technol.31(1997)3281.For this purpose the analytical database has to be[8]H.Thoma,S.Rist,G.Hauschulz,O.Hutzinger,Chemo-extended by including more reference substances forsphere9(1996)2111.this method.The use of the described method for[9]K.S.Brenner,H.Knies,Organohalogen Compounds2 quantitative analysis in comparison to other methods(1990)319.[10]Environmental Health Criteria152:Polybrominated Bi-will be the scope of future work.phenyls,World Health Organisation,Geneva,1994.[11]Environmental Health Criteria162:Brominated BiphenylEthers,World Health Organisation,Geneva,1994; Acknowledgements[12]Environmental Health Criteria172:Tetrabromobisphenol Aand Derivatives,World Health Organisation,Geneva,1995.[13]J.F.Schabron,L.E.Fenska,Anal.Chem.52(1980)1411. We gratefully acknowledgefinancial support for[14]J.Zulaikca,G.Guiochon,Anal.Chem.35(1963)1725. this work from the Bavarian Network for Waste[15]M.A.Haney,W.A.Dark,J.Chromatogr.Sci.18(1980)655. Research and Residual Reuse(BayFORREST)and[16]J.J.de Kok,A.de Kok,J.Chromatogr.171(1979)269. kindly appreciate the support from Great Lakes[17]F.Hoefler,H.Meker,H.-J.Mockel,L.V.Robertson, E. Europe for providing a number offlame retardants.Anklam,J.Agric.Food Chem.36(1988)961.[18]A.Rauen,Biochemical Handbook,Vol.2,Springer,Berlin,1964.[19]T.R.Crompton,Chemical Analysis of Additives in Plastics, ReferencesPergamon Press,Oxford,1977.[20]X.Lou,H.-G.Janssen,C.A.Cramers,J.Microcolumn Sep.7¨¨[1]R.Gachter and H.Muller,Plastic Additives Handbook,(1995)303.Hanser,Munich,1993.[21]H.G.O.Becker,Einfuhrung in die Photochemie,Deutscher[2]M.S.Reisch,Chem.Eng.News24(1997)10.Verlag der Wissenschaften,Berlin,1991.[3]B.Danzer,M.Riess,H.Thoma,O.Vierle,R.van Eldik,[22]Techno-Economic Study on the Reduction of IndustrialOrganohalogen Compounds31(1997)108.Emissions to Air,Discarges to Water and the General[4]A.Blum,B.N.Ames,Science195(1977)17.Production of Wastes from the Production,Processing,and[5]U.Orn,L.Eriksson, E.Jakobsson, A.Bergmann,Acta Destruction of Brominated Flame Retardants,Office forChem.Scand.50(1996)802.Official Publications of the European Communities,Lux-[6]B.G.Longnathan,K.Kannan,I.Watanabe,M.Kawano,K.emburg,1996.Irvine,S.Kumar,H.C.Sikka,Environ.Sci.Technol.29[23]J.J.de Kok,A.de Kok,U.A.Th.Brinkman,R.M.Kok,J.(1995)1832.Chromatogr.142(1977)367.。
