新一代降糖药gosogliptin
Gosogliptin (PF-00734200) 869490-23-3 GlpBio
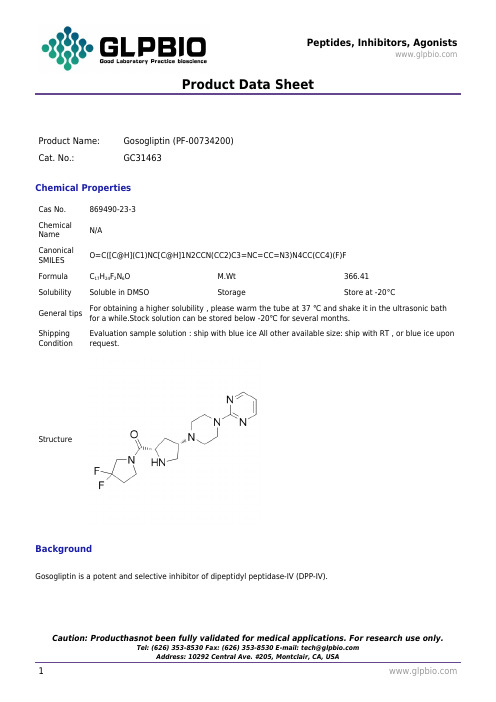
Product Data SheetProduct Name:Gosogliptin (PF-00734200)Cat. No.:GC31463Chemical PropertiesCas No.869490-23-3ChemicalNameN/ACanonicalSMILESO=C([C@H](C1)NC[C@H]1N2CCN(CC2)C3=NC=CC=N3)N4CC(CC4)(F)FFormula C17H24F2N6O M.Wt366.41 Solubility Soluble in DMSO Storage Store at -20°CGeneral tips For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months.Shipping Condition Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request.StructureBackgroundGosogliptin is a potent and selective inhibitor of dipeptidyl peptidase-IV (DPP-IV).Product Data SheetGosogliptin (PF-00734200) is a potent, orally active, selective, and competitive inhibitor of DPP-IV, the enzyme mainly responsible for the degradation of the incretin peptides GLP-1 and glucose-dependent insulinotropic polypeptide. Gosogliptin demonstrates greater than 200-fold selectivity over other members of the DPP family (DPP-2, DPP-3, DPP-8, and DPP-9) and the related serine proteases, fibroblast activation protein, aminopeptidase P, and propyl oligopeptidase, enzymes that possess similar catalytic activities. Gosogliptin demonstrates rapid and reversible inhibition of plasma DPP-4 activity when administered orally to rats, dogs, and monkeys. In various nonclinical models, Gosogliptin stimulates insulin secretion and improves glucose tolerance[2].The objectives of the present study are to characterize the metabolism, pharmacokinetics, and excretion of [14C] Gosogliptin in Sprague-Dawley (SD) rats, beagle dogs, and humans. A single dose of [14C] Gosogliptin is administered orally to intact SD rats (5 mg/kg), beagle dogs (5 mg/kg), and humans (20 mg). After a single oral dose of [14C] Gosogliptin to SD rats, an overall mean of 97.1% of the administered radioactivity is recovered in the urine, feces, and cage wash over a period of 168 h postdose. The mean cumulative dose recovered in feces is 66.0%. The mean cumulative excretion in the urine is 30.8%. Approximately 95% of the excreted radioactivity recovery occurred in the first 48 h. Mean total recoveries of dosed radioactivity from bile duct-cannulated rats are 29.5% in urine and 62.0% in bile. No gender-related differences are observed in the excretion pattern of radioactivity[2].[1]. Dai H, et al. The pharmacokinetics of PF-734200, a DPP-IV inhibitor, in subjects with renal insufficiency. Br J Clin Pharmacol. 2011 Jul;72(1):85-91. [2]. Sharma R, et al. Metabolism, excretion, and pharmacokinetics of ((3,3-difluoropyrrolidin-1-yl)((2S,4S)-4-(4-(pyrimidin-2-yl)piperazin-1-yl)pyrrolidin-2-yl)methanone, a dipeptidyl peptidase inhibitor, in rat, dog and human. Drug Metab Dispos. 2012 Nov;40(11):2143-61.。
全球首个周制剂降糖药物曲格列汀,服用时要谨记这五大注意事项

全球首个周制剂降糖药物曲格列汀,服用时要谨记这五大注意事项对于糖尿病的治疗药物,想必大家比较熟悉的时二甲双胍,阿卡波糖,达格列净,瑞格列奈,胰岛素等传统降糖药物,而对于新型降糖药物曲格列汀了解甚少。
曲格列汀,由日本制药巨头武田药厂研发,于2015年5月上市,属于周制剂,即每周只需要服用一次,主要是通过抑制DPP-4来发挥降糖作用。
那么,长期服用该药物的患者,要谨记这五大注意事项,否则效果减半。
一、用药时机该药物为周制剂,每周只需要服用一次,每次100mg,但是应该在每周的同一天服用。
如果忘记服用时,在发现漏服的当天按照规定剂量服用,此后在每周的同一天时间服用。
对于中度肾功能损害的患者,仍然按照每周一次给药,但给药剂量应减为原来的一半。
二、不良反应患者在服用该药物是,可能会出现低血糖、脂肪酶升高、胰腺炎、肠梗阻、皮疹、肝功能受损等。
但需要注意的是,并非所有的不良反应都会发生,也并非每个人都会发生不良反应。
还有一部分不良反应会在用药过程中消失。
三、换用药物如果患者之前在服用其他的降糖药物,想要换成曲格列汀,是不能直接将原来的降糖药物直接停掉的,需要先将空腹血糖降到6mmol/l以下,连续观察2-4周的血糖,血糖稳定的情况下,才可逐渐停用原来服用的降糖药物。
四、联合用药根据日本的用药经验,餐后血糖不超过15mmol/l的糖友,只服用曲格列汀即可。
餐后血糖超过15mmol/l的糖友,需要配合胰岛素或者二甲双胍使用。
餐后血糖超过20mmol/l的糖友,还需要配合长效胰岛素才能把血糖控制在正常水平。
尤其强调一点,曲格列汀对于餐前血糖控制较好,对于餐后血糖的控制较弱。
五、禁用人群严重酮症、糖尿病昏迷或昏迷前期、1型糖尿病患者、严重感染、严重创伤、肾功能不全的患者禁止使用本品。
新一代口服降糖药

龙源期刊网 新一代口服降糖药作者:来源:《大众健康》2007年第01期糖尿病是一种与环境、免疫、遗传等因素有关的疾病,也是威胁人们健康的常见病。
老龄化社会的形成,不健康的饮食习惯,更加紧张的生活,越来越多的久坐少动的生活模式……这些危险因素均导致了糖尿病的发病率迅猛增长。
无论在发达国家还是发展中国家,糖尿病的发病率都在急剧增加。
据世界卫生组织公布的资料,1985年全世界有3000万糖尿病患者,到1997年已增加至1.35亿人;2000年全球2型糖尿病患病数为1.5亿。
我国糖尿病患者在6000万人以上,且发病年龄日益年轻化。
在数量急剧增加的糖尿病患者中,主要是2型糖尿病即非胰岛素依赖性糖尿病,占患者总数的95%以上。
糖尿病的患病率、致残率和死亡率以及总健康危害程度已居非传染性疾病的第三位,它在我国是仅次于心脑血管疾病和肿瘤的第三大杀手。
糖尿病患者患缺血性心脑血管疾病、肢坏死、肾功能衰竭和双目失明的危险性比正常人高出几倍甚至20多倍。
此病急慢性并发症危害大、范围广。
2型糖尿病的治疗除控制饮食和适当增强体育锻炼外,目前临床治疗糖尿病的药物有口服降糖药、胰岛素、免疫抑制剂及其它药物,其中口服降糖药和胰岛素是最主要的。
目前有多种口服降血糖药,但都有可能导致低血糖症和肥胖症等。
应根据病情选择合适的抗高血糖处方药。
2型糖尿病的治疗应坚持长期、全面等原则。
近期流行病学资料显示,糖尿病心血管病变的发生率和死亡率与餐后高血糖关系密切。
有效地控制餐后高血糖,不但可以提高整体血糖控制水平,而且可减少大血管病的合并症,改善糖尿病的预后。
传统治疗糖尿病药物通过刺激B细胞合成和分泌胰岛素,发挥降血糖的作用,对2型糖尿病的血糖控制发挥了重要作用。
但这类药物降血糖作用起效较慢,落后于餐后血糖吸收速度,导致餐后血糖控制不满意,而药效持续时间较长,肠道糖吸收停止后易发生低血糖,限制了该类药的临床应用。
那格列奈最初由日本味之素公司开发,于1999年在日本上市。
新型降糖药GLP简介

新型降糖药GLP简介背景随着生活水平的提高和生活方式的改变,糖尿病已经成为世界上一种常见的代谢性疾病。
根据统计,在全球范围内,已经有约4.24亿人患有糖尿病,而这个数字还在不断增长。
糖尿病的治疗一直是医学界的焦点,近年来,新型降糖药GLP引起了越来越多的关注。
GLP的定义GLP全称为胰高血糖素样肽-1,是一种肠道激素,在食物摄入后被小肠壁分泌,能够刺激胰岛素分泌,抑制胰高血糖素的分泌,从而降低血糖浓度。
GLP的作用机制GLP可通过以下机制降低血糖浓度:1.促进胰岛素分泌:GLP能够刺激胰岛细胞分泌胰岛素,从而降低血糖浓度。
2.抑制胰高血糖素分泌:胰高血糖素是一种升高血糖浓度的荷尔蒙,在血糖浓度升高时分泌。
GLP通过抑制胰高血糖素的分泌,从而降低血糖浓度。
3.减缓胃部排空:GLP能够减缓胃部的排空速度,这样食物就可以慢慢地转化为血糖,从而降低血糖浓度。
4.抑制胃酸分泌:GLP还能够抑制胃酸的分泌,从而减少食道的酸性刺激,降低糖尿病患者的胃肠反应。
GLP的药物制剂目前,市场上已经出现了多种以GLP为主要成分的药物制剂。
这些药物制剂可以分为以下几类:1.GLP-1受体激动剂:通过模拟自然GLP-1的作用机制,刺激胰岛素分泌和抑制胰高血糖素的分泌,从而降低血糖浓度。
比如埃塞格列汀(exenatide)、利拉鲁肽(liraglutide)等。
2.DPP-4抑制剂:DPP-4是一种酶,可以把自然GLP-1分解成无活性的肽段。
DPP-4抑制剂能够抑制DPP-4的活性,从而延长GLP-1的降糖作用时间。
比如沙格列汀(sitagliptin)、伊格列汀(alogliptin)等。
3.双重激动剂:同时包含GLP-1受体激动剂和DPP-4抑制剂的药物制剂。
比如苑舒酯(lixisenatide)等。
GLP的临床应用GLP制剂在临床上主要用于治疗2型糖尿病。
与传统降糖药物相比,GLP制剂具有以下优点:1.降低血糖浓度:GLP制剂能够有效地降低血糖浓度,而且作用时间较长。
磷酸西格列汀的功能主治

磷酸西格列汀的功能主治磷酸西格列汀简介磷酸西格列汀(SGLT2抑制剂)是一种用于治疗2型糖尿病的药物,它通过抑制肾脏从尿液中吸收葡萄糖的能力,从而降低血糖水平。
下面是磷酸西格列汀的功能主治。
1. 控制血糖水平磷酸西格列汀通过抑制肾小管对葡萄糖的重吸收,通过尿液排出大量的葡萄糖,从而降低血糖水平。
它可以帮助2型糖尿病患者控制高血糖,改善胰岛素抵抗,减少血糖和胰岛素的峰值。
2. 减轻体重磷酸西格列汀的使用还可以帮助患者减轻体重。
它通过增加尿液中的糖分排出量,减少了身体对糖分的吸收,从而减少了卡路里的摄入。
这对于那些需要减少体重的2型糖尿病患者来说非常有益。
3. 降低高血压风险磷酸西格列汀的使用还可以降低2型糖尿病患者的高血压风险。
该药物通过减少葡萄糖和盐分的重吸收,可以降低血容量,从而减少对血管的压力。
这有助于降低患者的血压,减少心血管疾病的风险。
4. 预防心脏疾病多项研究表明,磷酸西格列汀的使用可以降低心血管疾病的发生风险。
该药物通过改善2型糖尿病患者的代谢状况,降低血糖和胰岛素的峰值,减少动脉粥样硬化的发生。
它还可以降低冠心病、心肌梗死和心力衰竭的风险。
5. 保护肾脏磷酸西格列汀的使用可以保护肾脏功能。
它通过减少肾小管对葡萄糖的重吸收,减轻肾脏的负担,延缓肾功能的恶化。
此外,它还可以减少蛋白尿,降低肾脏疾病的风险。
6. 缓解胰岛功能衰竭磷酸西格列汀的使用还可以缓解2型糖尿病患者的胰岛功能衰竭。
它通过降低血糖和胰岛素的峰值,减轻胰岛细胞的负担,有助于保护胰岛细胞的功能,延缓胰岛功能的进一步受损。
7. 防止尿路感染磷酸西格列汀的使用可以减少葡萄糖在尿液中的积聚,从而降低尿液中细菌的生长和繁殖。
这可以减少尿路感染的发生,对于经常患有尿路感染的2型糖尿病患者来说尤为重要。
8. 改善生活质量磷酸西格列汀的使用可以改善2型糖尿病患者的生活质量。
它通过控制血糖、减轻体重、预防心脏疾病等多种作用,可以减少疾病的并发症和不适症状,提高患者的整体感受和健康状况。
司来帕格作用机制

司来帕格作用机制司来帕格(Sitagliptin)是一种口服胰岛素增敏药物,属于二肽基肽酶-4(DPP-4)抑制剂。
它能通过增加胰岛素的分泌和减少胰高血糖素的分泌来降低血糖水平,从而改善糖尿病患者的血糖控制。
接下来我们将深入探讨司来帕格的作用机制。
首先,司来帕格通过抑制DPP-4的活性来增加胰岛素的分泌。
DPP-4是一种酶,它能够降解胰岛素释放激素GLP-1(胰高血糖素样肽-1)和GIP(胰高血糖素样肽-1),这两种激素能够刺激胰岛素的分泌。
当DPP-4受到抑制时,GLP-1和GIP的降解减少,其浓度得以维持或增加,从而促进胰岛素的分泌,帮助降低血糖水平。
其次,司来帕格还可以减少胰高血糖素的分泌。
胰高血糖素是一种激素,它在胰岛素释放后能够抑制胰岛素和促进糖异生。
司来帕格通过抑制DPP-4的活性,减少胰高血糖素的分泌,从而减少其对胰岛素的抑制作用,有利于提高胰岛素的效果,降低血糖水平。
此外,司来帕格还可以减少肝脏的葡萄糖产生。
在糖尿病患者中,肝脏过度产生葡萄糖是导致高血糖的一个重要原因。
司来帕格能够通过减少胰高血糖素的分泌,抑制肝脏的葡萄糖产生,减少外源性葡萄糖的释放,有助于降低血糖水平。
最后,司来帕格还可以通过改善胰岛素的敏感性来降低血糖水平。
胰岛素敏感性是指细胞对胰岛素的反应程度,司来帕格能够通过增加胰岛素的分泌和减少胰高血糖素的分泌,改善胰岛素的效果,提高细胞对胰岛素的敏感性,从而降低血糖水平。
综上所述,司来帕格通过抑制DPP-4的活性,增加胰岛素的分泌,减少胰高血糖素的分泌,抑制肝脏的葡萄糖产生,改善胰岛素的敏感性等方式,能够有效降低血糖水平,改善糖尿病患者的血糖控制。
在使用司来帕格时,患者应当注意遵医嘱,按时按量服用,同时结合合理的饮食和运动,以达到最佳的治疗效果。
新型口服降糖药_二肽基肽酶_抑制剂_蔡乐

年 10 月,西格列汀(sitagliptin,捷诺维)获得 FDA 批准,为第 1 个上市的 DPP-Ⅳ抑制剂 ,近年 来又 有 维 格 列 汀 (vildagliptin,Galvus)、 沙 格 列 汀 (saxagliptin,Onglyza)和阿格 列汀 (alogliptin)相 继 上市,其中西格列汀已于 2009 年 11 月在中国获 得批准。 已上市和进入临床试验阶段的 DDP-Ⅳ 抑制剂的研发情况见表 1[10]。
为克服 GLP-1 易代谢失活的缺点,学者们开 发了 不易被 DPP-Ⅳ代谢 的 降 糖 药 物 (如 GLP-1 受体激动剂、GLP-1 类似物) 以及 DPP-Ⅳ抑制剂 等 。 艾 塞 那 肽 (exenatide, 百 泌 达 ) 即 为 具 有 与 GLP-1 相似的生物学作用的 GLP-1 受体激动剂 , 于 2005 年 4 月获 FDA 批准, 是第 1 个上市的拟 肠降血糖素类降糖药[7]。 自从 1996 年 Pauly 等报 道,在 大 鼠 体 内 DPP-Ⅳ抑 制 剂 (异 亮 氨 酰 基 噻 唑 烷) 可抑制 GLP-1 降解而促进胰岛素分泌以来, 该类药物的研发十分迅速,主要的化学结构类型 包 括 ,氰 基 吡 咯 烷 类 、β-苯 乙 胺 类 、 黄 嘌 呤 及 其 衍 生物、氨基苯喹唑啉类和苯并咪唑类等 。 [8-9] 2006
2 DPP-Ⅳ抑制剂的研发概况 DPP-Ⅳ是 一 种 丝 氨 酸 蛋 白 酶 , 广 泛 存 在 于
脑、肺、肾脏、胰脏、肠道、肝细胞、血管内皮细胞 以及淋巴细胞中,可将肽链 N 端第 2 位为脯氨酸 或丙氨酸的蛋白质裂解。 DPP-Ⅳ广泛表达于肠道 血管内皮细胞中, 因此 GLP-1 在肠道分泌后,迅 速被 DPP-Ⅳ降解失活,t1/2 <2 min, 不适于临床应 用[6]。
进口降糖药有哪些品牌

进口降糖药有哪些品牌随着人们生活水平的提高和饮食结构的改变,糖尿病这种慢性疾病在全球范围内呈现出高发趋势。
针对糖尿病患者来说,药物治疗是控制血糖水平的重要手段之一。
本文将介绍一些国外进口的降糖药品牌,为您提供更多选择。
1. 甲磺酸二甲双胍(Metformin Hydrochloride)甲磺酸二甲双胍是最常见的口服降糖药物之一,在全球范围内得到广泛应用。
它通过减少肝葡萄糖合成和增加组织对葡萄糖的摄取,帮助降低血糖水平。
有很多国外药企生产和销售甲磺酸二甲双胍,如荷兰Merck Sharp & Dohme(MSD)的“Glucophage”、美国罗氏制药的“Avandia”等。
2. 格列美脲(Gliclazide)格列美脲是一种磺脲类口服降糖药物,通过刺激胰岛素分泌和增加胰岛素敏感性来控制血糖水平。
意大利制药公司Servier Laboratories生产的“Diamicron”是其中一个知名品牌。
3. 瑞格列奈(Repaglinide)瑞格列奈属于胰岛素促泌剂,通过刺激胰岛素释放来帮助调节血糖水平。
它对胰岛素敏感性较高,作用时间短暂,适用于餐后高血糖的患者。
瑞士罗氏制药的“Prandin”是最常见的瑞格列奈品牌。
4. 匹格列酮(Pioglitazone)匹格列酮是一种胰岛素增敏剂,通过提高组织对胰岛素的敏感性来降低血糖浓度。
它能够促进脂肪酸的摄取和利用,减少胰岛素抵抗,适用于2型糖尿病患者。
德国波特制药公司的“Actos”是匹格列酮的知名品牌。
5. 苏雷班(Sitagliptin)苏雷班是一种口服的二肽基肽酶-4(DPP-4)抑制剂,通过抑制体内的糖尿病相关酶DPP-4,增加胰岛素的分泌和降低胰岛素的分解,进而减少血糖升高。
丹麦诺和韦必治制药的“Januvia”是苏雷班的常用品牌。
6. 沃格列汀(Voglibose)沃格列汀是一种α-葡萄糖苷酶抑制剂,通过抑制肠道内的α-葡萄糖苷酶,延缓葡萄糖的吸收,降低血糖浓度。
医学知识之格列喹酮

格列喹酮1概述格列喹酮(Gliquidone)系第2代口服磺酰脲类促胰岛素分泌剂,为高活性亲胰岛β细胞剂,与胰岛β细胞膜上的特异性磺酰脲类受体结合,可诱导和刺激胰岛β细胞分泌胰岛素,以降低血糖浓度,同时也增加外周组织(肌肉、肝脏、脂肪)对胰岛素的敏感性。
在胃肠道吸收迅速,在肝脏很快通过羟基化和去甲基化代谢消除,纵使在肝脏功能受损时亦如此,血浆半衰期为1.5小时,全部从血液中消除为16~24小时。
2适应证2型糖尿病合并轻至中度肾病者,但严重肾功能不全时,则应改用胰岛素治疗。
3临床应用口服:开始30mg,早餐前及午餐前(或晚餐前)各1次,也可15mg,一日3次,三餐前服。
1周后按需调整,必要时逐步加量。
一般90~120mg/日,最大剂量不超过180mg/日。
4不良反应1.消化系统(1)胃肠道症状发生率为1%,主要表现为恶心、上腹胀满及胃灼热感。
(2)极少见肝功能异常,可能出现胆汁淤积性黄疸。
2.代谢/内分泌系统低血糖是最常见的不良反应,可表现为头痛、嗜睡、震颤、大汗、心动过速、面色苍白、焦虑、感觉迟钝、疲倦、注意力不集中、饥饿感、恶心、视物模糊等。
严重低血糖可导致癫痫发作、偏瘫和昏迷。
当存在下列一些危险因素时,发生更频繁:如不良饮食习惯、老年患者、剧烈或持久的运动、肝肾功能不全、虚弱或饮酒等。
3.血液少见白细胞减少、粒细胞缺乏、贫血、血小板减少等。
这些反应通常是可逆的,但仍应注意。
4.中枢神经系统很少见头痛。
5.皮肤可引起皮肤过敏(0.35%),出现皮肤瘙痒、红斑、荨麻疹、麻疹样皮疹、斑丘疹等。
5注意事项本品可以减弱病人对酒精的耐受力,而酒精亦可能加强药物的降血糖作用,治疗期间宜戒酒。
同磺酰脲类。
糖尿病合并轻至中度肾功减退者宜用本品。
6用药禁忌1.1型糖尿病患者禁用。
2.糖尿病昏迷或昏迷前期禁用。
3.糖尿病合并酸中毒或酮症患者禁用。
4.对本品或磺胺类、磺酰脲类药物过敏者禁用。
5.妊娠、哺乳期及晚期尿毒症患者禁用。
口服降血糖药物

口服降血糖藥物楊智慧99/06彙整一.黃基尿素Sulfonylureas1.Glibenclamide / Euglucon / Gliben (5mg)2.Gliclazide / Syncon / Diamicron (MR 30mg 80mg)3.Glimepiride / Grumed / Amaryl/Normin (2mg)4.Glipizide / Glix / Minidiab (5mg)藥理作用:刺激胰臟的胰島素分泌.降低血糖.可降2%A1C副作用低血糖(尤以老人及腎功能差的病患).體重增加餐前服用效果佳禁忌:CHF.Liver cirrosis.(此藥大部分由肝臟代謝).Cr>1.5..Glipizide腎功能不全尚可使用.且空腹效果佳二.異構物類Meglitinide (glinides)1.Repaglinide / Rovonorm2.Nateglinide / Starlix藥理作用:刺激胰臟分泌胰島素.較不易有低血糖(可迅速刺激胰島素分泌.之後胰島素濃度又恢復正常)建議: Repaglinide餐前15分鐘先吃或隨餐服用.作用時間在用餐前後30分鐘適用於.三餐不定時又易於兩餐中間低血糖的病患(若當餐沒吃.藥不用吃) Repaglinide-腎功能不全者尚可使用.Nateglinide可控制餐後血糖.若與Sulfonylureas併用(藥理機轉相同.易導致嚴重低血糖)與雙胍類併用效果佳或改用胰島素副作用頭痛.暈眩.關節痛.禁忌:Cr>1.4 DC三.胰島素增敏劑Thiazolidinediones(TZDs)1.Rosiglitazone / Avandia (0.5mg.1mg)2.Pioglitazone / Actos藥理作用胰島素增敏劑抗高血糖藥物;非刺激胰島素分泌.故不會增加低血糖危險性刺激PPAR-r 降低胰島素組抗.在肝臟及周邊組織.增加胰島素在受體與又體後的作用須要使用三個月的時間.藥效才會出來禁忌肝功能異常.有研究顯示Rosiglitazone會使心臟病發作的機率升高43%.CHF人勿使用副作用體重增加.輕.中度的水腫.部分腸胃不適四.雙胍類Biguanides1.Metaformin / Loditon / Glucophage (500mg ER.850mg)藥理作用增加胰島素的敏感性.不會刺激分泌.故無增加低血糖的危險性抑制糖原分解而減少肝臟葡萄糖的生成.可降低空腹血糖.會引起輕微的體重減輕(2-5KG)在脂肪及肌肉組織增加胰島素所刺激的葡萄糖運送(改善胰島素的阻抗)刺要作用: 減少小腸對葡萄糖的吸收禁忌因經由腎臟排出故腎功能不全者禁用(M Cr>1.5 / F Cr>1.4)ㄧ日最大劑量<3000mg副作用:腹脹.噁心.絞痛.腹瀉(常是自限性或暫時性7-14天)建議:剛開始服用Metformin的病人.容易有腸胃症狀(噁心.想吐.拉肚子) 可衞教病患隨餐吃.適應藥物需要3週左右的時間.因為Metformin是降血糖及好的藥物.鼓勵病人給予時間適應~五.甲型葡萄糖分解媒抑制劑α-Glucosidase inhabitor1.Acarbose / Glucobay2.Miglitol / Glyset藥理作用抑制小腸絨毛周邊之α 葡萄糖解甘脢及胰臟α澱粉脢.個可降低醣類所引起之血糖上升.抑制這些酵素系統可延緩消化及葡萄糖吸收α 葡萄糖解甘脢----可在小腸絨毛周邊將寡醣.雙醣及三醣水解成葡萄糖及其他單醣α澱粉脢-------------可在小腸腸道將店粉水解成寡醣禁忌:因用於腸道.有腸疾的病患不適用.肝硬化禁用.Cr>2mg/dl不要使用副作用大劑量時肝指數可能異常.大劑量時有腸胃道(腹瀉.腹脹)症狀(自限性).可慢慢增加劑量減少腸胃道的副作用.合併刺激胰島素分泌劑會有低血糖的危險性.此時治療方式(只適合口服葡萄糖.乳糖及牛奶).其他醣類食物較不易迅速治療低血糖其他未使用之口服藥物一.二肽基肽酶-(DPP-IV)抑制劑1.sitagliptin(Januvia:佳糖維).默沙東(2007.7台灣核準)2.vitagliptin(Galvus).諾華(2007.9歐盟上市.台灣尚無)藥理作用:口服葡萄糖刺激胰島素分泌(腸道荷爾蒙劇加強葡萄糖引發的胰島素分泌)此荷爾蒙稱之為incretin 腸泌素腸泌素的作用:抑制升糖素分泌.促進胰島貝它細胞增生.減少凋亡.增加胰島素敏感度.降低胃排空速度.增加飽足感雖然腸泌素在血糖調解功效顯著.但type2DM病患的腸泌素分泌卻大量減低.導致作用不良.(雖然有一種天然腸泌素製劑可施打.但在血液中卻會被一種叫(DPP4)二肽基肽酶4所分解)於是發展出2種藥物來改善這樣的情況1.長效且可抵抗DPP4分解的類胰高血糖激素胜肽-1(2005.美國exenatide(Byetta))2.DPP4抑制劑-來延長患者體內類胰高血糖激素胜肽-1的血清半衰期及作用好處:2.腸降糖素類似物(INCRETINE MIMETICS)3.Amylin(台灣未上市)Fresh case BS<200 單獨使用Metformin 500mg(低劑量開始.降低副作用) BS>200 合併2種用藥Sulfonylureas+MetforminHbA1C>9% 就需使用胰島素.為了保護β-cell 的功能最好使用胰島素BS>150mg/dl 就有糖毒性(破壞力)TZD與Insulin最好不要合併使用(增加心臟疾病的風險)短效Insulin與Sulfroylureas/glinide 併用(低血糖)。
糖尿病新药IinagIiptin临床试验新数据显示出安全性和疗效结果

糖尿病新药IinagIiptin临床试验新数据显示出安全性和疗效
结果
佚名
【期刊名称】《中国医药导刊》
【年(卷),期】2009(11)6
【摘要】从美国糖尿病学会(ADA)2009年年会(6月5-9日美国新奥尔良)上的首次学术会议上传来消息,德国勃林格殷格翰公司进行的一项临床研究结果表明:糖尿病新药linagliptin(二肽基肽酶4[DPP-4]抑制剂)作为联合治疗药物,用于经二甲双胍治疗血糖控制不佳2型糖尿病患者时,出现了具有临床相关性与统计学显著性的血红蛋白A1c(HbA1c)和空腹血糖(FPG)水平下降。
【总页数】1页(P1036-1036)
【关键词】美国糖尿病学会;数据显示;临床试验;新药;安全性;疗效;血糖控制不佳;二甲双胍治疗
【正文语种】中文
【中图分类】R587.1;R445
【相关文献】
1.勃林格殷格翰糖尿病新药linagliptin临床试验新数据显示出值得期待的安全性和疗效结果 [J],
2.利格列汀治疗≥60岁老年2型糖尿病患者的疗效及安全性:一项来自8个临床试验数据的汇总分析 [J], 郭晓蕙;冯志凯;徐林华
3.替卡格雷用于稳定型冠心病和糖尿病患者的疗效与安全性:一项随机对照临床试验 [J], 罗红敏(编译)
因版权原因,仅展示原文概要,查看原文内容请购买。
几款值得期待的新型降糖药

几款值得期待的新型降糖药导读有关糖尿病最新的六个消息,引起了广大“糖友”的广泛关注,其中,几款值得期待的新型降糖药名列“糖友关心榜”第一。
几款值得期待的新型降糖药吸入型胰岛素:吸入胰岛素呈粉末状,装入一个口哨大小的吸入器中。
吸入器里有卡壳进行剂量分割,每一卡壳包是一次吸入量。
吸入胰岛素在体内存留时间短,2~3个小时后被清除掉,低血糖风险少,体重增加少。
餐前20分钟吸入,初次从4单位开始,而后根据血糖进行调整。
这种吸入型胰岛素在15~20分钟即达到作用峰值,快于目前应用的速效胰岛素,是降低餐后血糖的最佳选择。
可使2型糖尿病患者糖化血红蛋白下降0.4%。
更适用于恐惧胰岛素注射,外出进餐的患者。
但酮症酸中毒、吸烟、哮喘、慢阻肺患者不能用。
不良反应有低血糖、咳嗽、喉痛等。
2014年美国食品药品监督管理局批准上市,未来有望得到大范围应用。
利拉鲁肽/德谷胰岛素复合制剂:由德谷胰岛素与利拉鲁肽配对组成的一种可注射复方制剂,目前在瑞士上市。
德谷胰岛素是一种作用时间长达40小时的新型超长效胰岛素类似物,是外源补充的胰岛素;而利拉鲁肽是肠促胰岛激素(GLP-1)类似物,它可激活胰腺B细胞,依据血糖浓度自然产生需要量的胰岛素。
两者联合,利拉鲁肽刺激了内源胰岛素的分泌,德谷胰岛素补充了自身产生胰岛素的不足。
临床试验显示,该药可平均降低糖化血红蛋白达1.8%。
周效利拉鲁肽长效制剂:利拉鲁肽周效制剂,将注射针头隐藏在笔型胰岛素装置内,每次按下按钮后即输出一周剂量,注射完成5秒后针头会自动退缩回笔腔,每周注射1次,使用方便。
高浓度甘精胰岛素U300:即每0.01ml 的高浓度甘精胰岛素U300含有3U的甘精胰岛素,而目前的甘精胰岛素每0.01ml 仅含1U甘精胰岛素。
高浓度甘精胰岛素U300已在美国和欧洲上市,与目前使用的甘精胰岛素U100相比,降糖幅度大,低血糖少,尤其夜间低血糖少。
对于应用常规剂量甘精胰岛素降低空腹血糖疗效不佳的患者,甘精胰岛素U300可获得更好效果。
GLP-1受体激动剂应用

GLP-1受体激动剂应用简介GLP-1受体激动剂(GLP-1 receptor agonists)是一类新型的药物,用于治疗2型糖尿病。
GLP-1受体激动剂通过模拟肽类激素促胰岛素样肽-1(GLP-1)的作用,能够提高胰岛素分泌和抑制胰高血糖素分泌,从而降低血糖水平。
此外,GLP-1受体激动剂还有其他一些作用,例如减少食欲和延缓胃肠蠕动。
作用机制GLP-1受体激动剂通过与胰岛素样生长因子-1(GLP-1)受体结合,刺激GLP-1信号通路的活化。
GLP-1信号通路的活化会增加胰岛素的分泌,并抑制胰高血糖素的分泌。
这些效应可以帮助降低血糖水平,并改善2型糖尿病患者的胰岛素敏感性。
另外,GLP-1受体激动剂还能够减少食欲,并延缓胃肠蠕动。
这些作用通过中枢神经系统的机制发挥作用,有助于控制体重,减轻胰岛素耐受和支持降低血糖。
主要药物GLP-1受体激动剂是一类药物,常见的药物有:1.Exenatide(延长型胰岛素样生长因子1)2.Liraglutide(利拉鲁肽)3.Dulaglutide(度拉鲁肽)这些药物的使用方法包括注射和皮下注射,具体剂量和频率应根据患者的具体情况和医生的建议确定。
临床应用GLP-1受体激动剂在临床上被广泛应用于2型糖尿病的治疗。
它们可以单独应用,也可以与其他药物(如二甲双胍)联合使用,用以控制血糖。
GLP-1受体激动剂的应用有以下几个方面的优势:•有效性:研究表明,GLP-1受体激动剂能够显著降低血糖水平,并帮助患者达到血糖控制目标。
•胰岛素非依赖性:GLP-1受体激动剂可以增加胰岛素的分泌,但与胰岛素不同,它们不会引起低血糖的风险。
•体重控制:GLP-1受体激动剂可以抑制食欲并延迟胃肠蠕动,有助于减轻体重。
•心血管保护:某些GLP-1受体激动剂还具有心血管保护的效果,可以降低心血管事件的风险。
虽然GLP-1受体激动剂在糖尿病治疗中显示出许多潜在的益处,但仍然存在一些不良反应和限制。
新型降糖药

.
GLP-1受体激动剂
.
GLP-1受体激动剂
半衰期10h-数天 半衰期2-4h
.
药 适应症与禁 用法用量 不良反应 半衰 联用药 肝功能损 肾功能
艾塞那肽
人GLP-1类似物 利拉鲁肽
• 同源性高(约97%) • 半衰期长(11-15h)
.
肠促胰岛素适应症
适用于: • 适用于单用二甲双胍、磺酰脲类等药物,以及二甲
双胍合用磺酰脲类等药物血糖仍控制不佳的患者。 • 肥胖或因降糖治疗体重增加过多的患者。 • ICU中重症或手术、创伤等造成血糖波动大,不易
平稳控制的患者。
.
肠促胰素降糖药物的地位
• 2010年我国公布的《糖尿病治疗指南》,推荐本品为二 线选择药物,建议二甲双胍治疗不能达标,或不适合使 用其他制剂时,可考虑应用DPP-4抑制剂治疗,单用或 与二甲双胍联用。
• 2013年美国临床内分泌医师协会(AACE) 颁布的糖尿病综 合治疗方案指南,其中指出对于 HbA1c≥7.5%的患者, 推荐使用二甲双胍联合 DPP-4抑制剂等进行综合治疗, 显示 DPP-4抑制剂已经成为仅次于二甲双胍推荐地位的 一线用药.同时在老年人中,2013年<中国糖尿病防治指南 >推荐 DPP-4抑制剂为二甲双胍之后的二线用药。
.
DPP-4抑制剂
.
DPP-4抑制剂
.
DPP-4抑制剂
DPP-4抑制剂
非底物类似:西格列汀、阿格列汀
底物类似:维格列汀、沙格列汀
2024年格列吡嗪市场前景分析

2024年格列吡嗪市场前景分析格列吡嗪(Glibenclamide)是一种用于治疗糖尿病的口服降糖药物。
它通过抑制胰岛素的释放以及增加胰岛素敏感性来帮助控制血糖水平。
格列吡嗪市场前景的分析对于投资者、制药公司和糖尿病患者都具有重要意义。
本文将对格列吡嗪市场前景进行分析,并提供一些相关观点。
1. 市场规模和增长趋势格列吡嗪市场目前的规模较大,并且预计在未来几年内将继续保持稳定增长。
糖尿病患者数量的不断增加是格列吡嗪市场增长的主要驱动力之一。
随着人口老龄化和生活方式改变,糖尿病的患病率在全球范围内呈增长趋势。
此外,对于口服药物的需求也在增加,这进一步促使了格列吡嗪市场的发展。
2. 竞争对手分析格列吡嗪市场面临着激烈的竞争。
目前,市场上存在着多个格列吡嗪的替代药物,这些替代药物在降糖效果、安全性和使用便捷性等方面与格列吡嗪具有竞争优势。
此外,其他治疗糖尿病的药物也对格列吡嗪市场构成了竞争压力。
因此,制药公司需要不断改进产品并提供更好的解决方案,以在竞争激烈的市场中保持竞争力。
3. 市场机会和挑战尽管面临着竞争,格列吡嗪市场仍然存在一些机会。
首先,全球范围内的糖尿病患者数量仍在增加,这为格列吡嗪市场提供了持续的需求。
其次,新的研发技术和创新可以改进格列吡嗪的疗效和安全性,同时降低副作用的风险。
然而,格列吡嗪市场也面临着一些挑战,包括严格的监管要求、副作用的风险以及市场价格下降可能导致的利润压力。
4. 市场趋势和未来发展格列吡嗪市场的一些重要趋势和未来发展可以预见。
首先,随着科技的进步,治疗糖尿病的新技术和药物将不断涌现,这可能对格列吡嗪市场产生影响。
其次,个性化治疗在糖尿病管理中的应用不断增加,这可能改变格列吡嗪市场的需求和定位。
此外,格列吡嗪的研发和创新方向值得关注,以提高药物疗效并减少不良反应。
5. 总结格列吡嗪市场具有稳定增长的趋势,但面临激烈的竞争和一些挑战。
制药公司需要不断创新和改进产品,以满足不断增长的糖尿病患者需求。
优格列汀说明

优格列汀说明
“DPP-4”抑制剂以其安全性和有效性,已是老年糖尿病患者考虑首选的药物之一,这一类药物具有调节胰岛功能,在低糖时不刺激胰岛素分泌,单独使用时不增加低血糖发生风险,因其良好的降糖效果和卓越的安全性已被临床医生和患者接受,而优格列汀II期临床研究数据表明,除安全性高外,优格列汀还有其独特的优势:1,优格列汀一周长效控糖,提高患者依从性。
优格列汀超长的消除半衰期及给药后168 h DPP-4抑制率均在80%以上,有力地支撑了优格列汀周制剂的特性。
因此,优格列汀一周服用一次,即可达到平稳控制血糖的目的。
2,优格列汀安全性更高,降糖更平稳。
在降低患者糖化血红蛋白方面效果显著,同时也可降低患者空腹及餐后血糖,且在目前的各项研究中均表现出良好的安全性。
3,优格列汀肾排泄率低,是糖尿病合并肾病患者的福音。
优格列汀经肾排泄率约8~25%,未来在糖尿病伴肾功能损伤的患者治疗中无需调整剂量,显著提高糖尿病肾病患者的顺应性。
与会的三位专家对优格列汀未来在糖尿病全程管理中的应用展开了精彩的讨论,一致认为:优格列汀高安全性、肾排泄率低以及强效降糖力等特性,期待在III期临床研究中得到确证,为糖尿病临床治疗提供更优的解决方案。
最后,优格列汀II期临床研究结果喜人。
相比目前临床常用降糖药物,优格列汀具有高安全性、肾排泄率低以及强效降糖力等特性,希望早日启动III期临床,早日上市、早日让糖尿病患者受益。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
.
2012 THOMSON REUTERS. For more information go to /copyright/
.
2007, and the study was expected to complete in June 2008 [ 868416]. In June 2009, data from the trial were presented at the 69th ADA scientific sessions in New Orleans, LA. A total of 289 patients were treated with 20 or 30 mg of gosogliptin qd or placebo. At week 12, both doses significantly reduced HbA1c compared with placebo, with 55.8 and 52.2% of patients in the 20 and 30 mg dose groups, respectively, achieving HbA1c less than 7% compared with only 23.8% of the placebo group. The drug was well tolerated with the most common adverse events being pain in extremity, pharyngolaryngeal pain, headache and dyspepsia [1013516 ].
Target
Dipeptidyl peptidase IV
Update date
17-JUL-2012
Reason for update
One or more development status entries have been updated, Indexing updated
. OVERVIEW
. . DRUG NAME .
Names associated with this drug
Name
Type
gosogliptin
INN,USAN
PF-00734200
Research Code
PF-734200
Research Code
Satyor
Trade Name
.
SatRx, a subsidiary of ChemRar Hi-Tech, under license from Pfizer, is developing gosogliptin (Satyor; PF-00734200; structure shown), a dipeptidyl peptidase IV (DPP-IV) inhibitor, for the potential treatment of type 2 diabetes (T2D) [1306668]. By July 2012, phase II studies were ongoing in RuJune 2007, a 12 week placebo-controlled, randomized, parallel group, multiple-dose phase II trial (; A7941005) was initiated in subjects (estimated n = 320) with type 2 diabetes on stable treatment with metformin in the US. Subjects were expected to receive placebo or one of four doses of gosogliptin (2, 5, 10, or 20 mg, po qd). The primary endpoints were change from baseline to 12 weeks of HbA1c levels and evaluation of dose response in subjects on a stable dose of metformin hydrochloride. Enrollment was ongoing in December
.
Pfizer was previously developing gosogliptin for T2D. By September 2008, phase II/III trials had commenced in the US and EU [950369]; however, by January 2010, the compound was no longer listed on Pfizer's pipeline [1071193].
.
Phase I In June 2009, pharmacokinetic data from patients with mild and severe renal failure and end-stage renal disease were presented at the 69th ADA Scientific Sessions in New Orleans, LA.Patients experiencing moderate to severe renal impairment had 2- to 3-fold higher AUC values for the drug, compared with individuals with normal renal function, indicating that gosogliptin exposure increases as renal function decreases. Cmax and Tmax values were not altered significantly. In patients requiring chronic hemodialysis, only 26% of the drug was removed following 4 h of hemodialysis. Gosogliptin was found to be well tolerated in all patients [1013807], [1016176].
Actions
DPP IV inhibitor antidiabetic product; Hypoglycemic agent; Dipeptidyl peptidase IV inhibitor
Technologies Oral formulation; Small molecule therapeutic
.
In June 2007, data were presentedat the 67th ADA Scientific Sessions in Chicago, IL. In a randomized, placebo controlled, single oral dose study in healthy adults (n = 27), DPP-IV inhibition by gosogliptin resulted in a nonlinear increase in plasma GLP-1 levels. A maximal 10 mg dose of the compound resulted in a 2.3-fold increase in GLP-1, compared to placebo. However, the nonlinear results suggested another pathway for GLP-1 elimination other than DPP-IV enzymatic breakdown [844506].
DRUG REPORT : gosogliptin
. SUMMARY .
Drug Name
gosogliptin
Company
Pfizer Inc
Highest Dev Status
Phase 2 Clinical
Therapy Areas Non-insulin dependent diabetes
.
PREMARKETING STUDIES Phase II By July 2012, SatRx had commenced phase II studies in Russia [1306669], [1306668].
.
By September 2008, phase II/III EU and US trials had begun [950369]; however, by January 2010, the compound was no longer listed on Pfizer's pipeline [1071193].
.
In February 2008, a phase II study was initiated (; A7941006) worldwide. Subjects (estimated n = 225) were to receive placebo, or gosogliptin (20 or 30 mg qd), for 12 weeks. The primary endpoint was HbA1c at 12 weeks. This study was expected to complete in September 2008 [878568].
.
PRECLINICAL STUDIES In September 2007, preclinical data were presented on the identification of gosogliptin at the 14th RCA-SCI Medicinal Chemistry Conference in Cambridge, UK. Rapid optimization of a prolyl pyrrolidine led to compounds with nM potencies from which gosogliptin was selected based on potency, cellular permeability and low HERG activity. The eutomer had an IC50 value of 14 nM and a Ki value of 2 nM. It was highly bioavailable (greater than 70%) , with a long half-life (7.6 h) in monkeys and was effective in mice at a dose of 1 mg/kg following OGTTs [837272].
