化学专业英语
(完整版)化学类专业英语词汇.doc

专业英语词汇Unit 1TEXT A : Chemical Reactions and Group Reactionscustomary a. 通常的,惯例的handle n.柄vt.触摸handling n.处理,管理derive vt.取得,得到,衍生oxidate vt.使氧化oxidation n.satisfactory a.令人满意的,符合要求的rapid a.快的,迅速的,动作快的combustion n.燃烧somewhat pron. ad. 一点点,几分,有点effort n.努力commercial a.商业的,商务的undesirable a不.合需要的,不受欢迎的,讨厌的retard vt.延迟,放慢,使停滞transformer n.变压器transform vt.改变,转变automotive a.自动的,机动的,汽车的cracked裂化的sluge n.软泥,淤泥stiff a.硬的,强烈的extent 广度,程度distillation n.蒸馏distill vt.vi.unrefined a.未精致,未提炼的acidity n.酸味,酸性acidify vt. Vi.Involve vt. 包缠,卷缠Fell=followingIndividual a.个人的,个体的Presumable a可.假定的,可推测的Destruction n.破坏,毁灭Overall n。
a.全面的,综合的Exceed 超过,胜过Isolate vt.隔离,孤立,使离析iso—构词成分“均匀”“异构”“苯”Analyse vt. 分析,分解Carbonyl 羰基Carboxyl羧基Hydroxyl羟基Decomposition分解Alkyl烷基,烃基Ketone 酮Aldehyde n.醛Yield vt. 出产,产出Explosive a. 爆炸Vapor n.蒸汽, vi.蒸发Propagation 繁殖,增殖;传播Dehydrate vt.使脱水Acet 构词成分Acetaldehyde乙醛Resin n.树脂Resinous a.树脂的Carboxylic a.羟基的Substantial a.物质的,实质的Susceptible a易.受感动的,敏感的Analogous a.类似的,相似的( to)Response n.作答,回答,响应,反应Readily ad.乐意地,很快地Readiness n准.备就绪,愿意Extent n.广度长度Steric 空间的,位的Likewise ad.同样的,照样地;也,又Suffer vt.遭受,经历Progressive a进.步的,长进的,渐次的Adjacent a.邻近的,紧挨着的Terminal a.末端的,终点的MethyleneBromide n.溴化物Substitute n.代替物(人),代用品substitution n.代替,替换Remote a相.隔较远的Acetone n.丙酮Ether n.醚,乙醚Correspond vi.符合,一致;相当,相应Reservation n保.留,预定Tend vi.走向,趋向。
化学专业英语词汇表
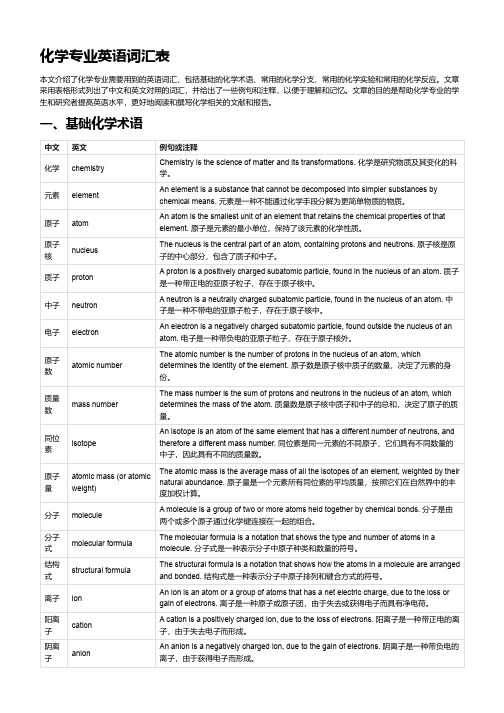
化学专业英语词汇表本文介绍了化学专业需要用到的英语词汇,包括基础的化学术语、常用的化学分支、常用的化学实验和常用的化学反应。
文章采用表格形式列出了中文和英文对照的词汇,并给出了一些例句和注释,以便于理解和记忆。
文章的目的是帮助化学专业的学生和研究者提高英语水平,更好地阅读和撰写化学相关的文献和报告。
一、基础化学术语中文英文例句或注释化学chemistry Chemistry is the science of matter and its transformations. 化学是研究物质及其变化的科学。
元素element An element is a substance that cannot be decomposed into simpler substances bychemical means. 元素是一种不能通过化学手段分解为更简单物质的物质。
原子atom An atom is the smallest unit of an element that retains the chemical properties of thatelement. 原子是元素的最小单位,保持了该元素的化学性质。
原子核nucleus The nucleus is the central part of an atom, containing protons and neutrons. 原子核是原子的中心部分,包含了质子和中子。
质子proton A proton is a positively charged subatomic particle, found in the nucleus of an atom. 质子是一种带正电的亚原子粒子,存在于原子核中。
中子neutron A neutron is a neutrally charged subatomic particle, found in the nucleus of an atom. 中子是一种不带电的亚原子粒子,存在于原子核中。
(完整版)化学专业英语常用词汇

☆常用: ppm: parts per millionppb: parts per billion pH: potential of hydrogen1. 化合物的命名:规则:金属(或某些非金属)元素+阴离子名称(1)MgCl2 magnesium [mæɡ’ni:zj əm] chloride (2)NaNO2 sodium nitrite [‘naitrait](3)KNO3 potassium[p ə’tæsi əm] nitrate [‘naitreit] (4)硝酸 nitric acid(5)NaHCO3 sodium hydrogen carbonate练习:▪ FeBr2 ▪ (NH4)2SO4 ▪ NH4H2PO4▪KMnO4▪亚硫酸▪sulfurous acid▪H2S▪NO2 有机物命名▪Hydrocarbon▪{Aliphatic hydrocarbon; Aromatic Hydrocarbon}▪Aliphatic hydrocarbon (脂肪烃)▪{Alkane (烷); Alkene(烯); Alkyne(炔)}▪Alcohol 醇▪Aldehyde 醛▪Ketone [‘ki:təun] 酮▪Carboxylic acid 羧酸▪Aromatic hydrocarbon(芳香烃)▪{benzene (苯) hydroxybenzene(酚) quinone(醌)无机物中关于数字的写法mono-, di-, tri-, tetra-, penta- hexa-, hepta-, octa-, nona-, deca-一,二,三,四,五,六,七,八,九,十有机物中关于数字的写法meth-, eth-, prop-, but-, pent-, hex-,甲乙丙丁戊已hept-, oct-, non-, dec-, cyclo-, poly-庚辛壬葵环聚练习▪甲烷乙炔▪丙酮丁醇▪戊烷己烯▪庚醛辛烷▪2-甲基壬酸 3,5-二乙基癸醇Lithium [‘liθiəm] n.锂Beryllium [bə’riljəm] n.铍(Be)Sodium [‘səudiəm] n.钠Potassium [pə’tæsiəm] 钾Rubidium [ru:’bidiəm] 铷Caesium [‘si:ziəm] 铯Nucleus[‘nju:kli s] 原子核,是nuclear的复数Halogen[‘hælədʒən] 卤素general chemistry 普通化学positive[‘pƆzətiv] ion 阳离子orbital electron 轨道电子effective nuclear charge 有效核电荷atomic radius 原子半径,raddi的复数ionic radius 离子半径negative ion 阴离子electron cloud 电子云Van der Waals non-bounded radius单质分子晶体中相邻分子间两个非键合原子核间距离的一半称为范德华半径metallic [mi’tælik] character[‘kæriktə] 金属特性electropositive [I’lektrəu’pɔzətiv] a.带正电的Ionization [‘aiənai’zeiʃən] energy 电离能carbon 碳 germanium[dʒə:’meiniəm] 锗tin [tin] 锡 lead [led] 铅sodium[‘səudiəm] 钠 magnesium[mæɡ’ni:zjəm] 镁silicon [‘silikən] 硅 chlorine [’klɔ:ri:n] 氯nonmetallic [‘nɔnmi’tælik]adj.n.非金属的,非金属Electronegativity 电负性Metallic oxide 金属氧化物Metallic hydroxide [hai’drɔksaid] 金属氢氧化物Hydroxyl [hai‘drɔksil] ions 氢氧根离子insoluble[in’sɔljubl] 不溶解的Ionic [ai‘ɔnik] adj. 离子的Transition element 过渡元素Basicity [bə’sisiti] n. 碱性,碱度Oxyacid [,ɔksi’æsid] 含氧酸Carbonate [‘kɑ:bəneit] 碳酸盐Nitrate [‘naitreit] 硝酸盐Sulphate [‘sʌlfeit] 硫酸盐 = sulfateAmphoteric [,æmfə’terik] adj.两性的Acid [‘æsid] n. adj.alkali [‘ælkəlai] n.adj.Hydration [hai’dreiʃən] 水合作用Hydrolyze [‘haidrəlaiz] vi. 水解Oxysalt [‘ɔksisɔ:lt] 含氧酸盐Complex 络合物,复合物句子理解1) Metals are electropositive and have a tendency to loss electrons, if suppliedwith energy: M M+ + e. 金属是电正性的,如果供给能量,有失去电子的趋势。
化学专业英语(化学专业名词)

10
• 3) Anions containing hydrogen(含氢 阴离子):hydrogen + 去掉氢的离子名 称 • • 例:HCO3-:hydrogen carbonate ion
11
3. Names of Acids(酸的命名):酸根 离子中非氧元素名称的词干 + -ic acid
4
2) Polyatomic Cations(多原子阳离子): 原子团名称 + ion
例: Na+:sodium ion;Ag+:silver ion; Ca2+:calcium ion;Al3+:aluminum ion; Fe+:iron(I)ion;Fe2+:iron(II)ion;Fe3+: iron(III)ion; 例:
* 如果某元素能形成一种以上的含氧酸,则按以 下规则:
a. 高(过)* 酸:per- + 酸根离子中非氧元素名 称的词干 + -ic acid
b. * 酸:酸根离子中非氧元素名称的词干 + - ic acid
c. 亚 * 酸:酸根离子中非氧元素名称的词干 + ous acid d. 次 * 酸:hypo- + 酸根离子中非氧元素名称的 词干 + -ous acid
14
5. Names of Salts(盐的命名):
不带“ion”的阳离子名称 + 不带“ion” 的阴离子名称
* 阳 离 子 的 电 荷 数 用 斯 托 克 数 字 ( Stock number)来表示(只形成一种阳离子的元素 不必用). 例:CuCl:copper(I)chloride; CuCl2:copper(II)chloride; CuSO4:copper(II)sulfate; KClO4:potassium perchlorate
化学专业英语电子版

Chapter 1 Matter and MeasurementChemistry is the science of matter and the changes it undergoes. Chemists study the composition, structure, and properties of matter. They observe the changes that matter undergoes and measure the energy that is produced or consumed during these changes. Chemistry provides an understanding of many natural events and has led to the synthesis of new forms of matter that have greatly affected the way we live.Disciplines within chemistry are traditionally grouped by the type of matter being studied or the kind of study. These include inorganic chemistry, organic chemistry, physical chemistry, analytical chemistry, polymer chemistry, biochemistry, and many more specialized disciplines, e.g. radiochemistry, theoretical chemistry.Chemistry is often called "the central science" because it connects the other natural sciences such as astronomy, physics, material science, biology and geology.1.1. Classification of MatterMatter is usually defined as anything that has mass and occupies space. Mass is the amount of matter in an object. The mass of an object does not change. The volume of an object is how much space the object takes up.All the different forms of matter in our world fall into two principal categories: (1) pure substances and (2) mixtures. A pure substance can also be defined as a form of matter that has both definite composition and distinct properties. Pure substances are subdivided into two groups: elements and compounds. An element is the simplest kind of material with unique physical and chemical properties; it can not be broken down into anything simpler by either physical or chemical means. A compound is a pure substance that consists of two or more elements linked together in characteristic and definite proportions; it can be decomposed by a chemical change into simpler substances with a fixedmass ratio. Mixtures contain two or more chemical substances in variable proportions in which the pure substances retain their chemical identities. In principle, they can be separated into the component substances by physical means, involving physical changes. A sample is homogeneous if it always has the same composition, no matter what part of the sample is examined. Pure elements and pure chemical compounds are homogeneous. Mixtures can be homogeneous, too; in a homogeneous mixture the constituents are distributed uniformly and the composition and appearance of the mixture are uniform throughout. A solutions is a special type of homogeneous mixture. A heterogeneous mixture has physically distinct parts with different properties. The classification of matter is summarized in the diagram below:Matter can also be categorized into four distinct phases: solid, liquid, gas, and plasma. The solid phase of matter has the atoms packed closely together. An object that is solid has a definite shape and volume that cannot be changed easily. The liquid phase of matter has the atoms packed closely together, but they flow freely around each other. Matter that is liquid has a definite volume but changes shape quite easily. Solids and liquids are termed condensed phases because of their well-defined volumes. The gas phase of matter has the atoms loosely arranged so they can travel in and out easily. A gas has neither specific shape nor constant volume. The plasma phase of matter has the atoms existing in an excited state.1.2. Properties of MatterAll substances have properties, the characteristics that give each substance its unique identity. We learn about matter by observing its properties. To identify a substance, chemists observe two distinct types of properties, physical and chemical, which are closely related to two types of change that matter undergoes.Physical properties are those that a substance shows by itself, without changing into or interacting with another substance. Some physical properties are color, smell, temperature, boiling point, electrical conductivity, and density. A physical change is a change that does not alter the chemical identity of the matter. A physical change results in different physical properties. For example, when ice melts, several physical properties have changed, such as hardness, density, and ability to flow. But the sample has not changed its composition: it is still water.Chemical properties are those that do change the chemical nature of matter. A chemical change, also called a chemical reaction, is a change that does alter the chemical identity of the substance. It occurs when a substance (or substances) is converted into a different substance (or substances). For example, when hydrogen burns in air, it undergoes a chemical change because it combines with oxygen to form water.Separation of MixturesThe separation of mixtures into its constituents in a pure state is an important process in chemistry. The constituents of any mixture can be separated on the basis of their differences in their physical and chemical properties, e.g., particle size, solubility, effect of heat, acidity or basicity etc.Some of the methods for separation of mixtures are:(1)Sedimentation or decantation. To separatethe mixture of coarse particles of a solidfrom a liquid e.g., muddy river water.(2)Filtration. To separate the insoluble solidcomponent of a mixture from the liquidcompletely i.e. separating the precipitate(solid phase) from any solution.(3)Evaporation. To separate a non-volatilesoluble salt from a liquid or recover thesoluble solid solute from the solution.(4)Crystallization. To separate a solidcompound in pure and geometrical form.(5)Sublimation. To separate volatile solids,from a non-volatile solid.(6)Distillation. To separate the constituents of aliquid mixture, which differ in their boilingpoints.(7)Solvent extraction method. Organiccompounds, which are easily soluble inorganic solvents but insoluble or immisciblewith water forming two separate layers canbe easily separated.1.3 Atoms, Molecules and CompoundsThe fundamental unit of a chemical substance is called an atom. The word is derived from the Greek atomos, meaning “undivisible”or “uncuttable”.An atom is the smallest possible particle of a substance.Molecule is the smallest particle of a substance that retains the chemical and physical properties of the substance and is composed of two or more atoms;a group of like or different atoms held together by chemical forces. A molecule may consist of atoms of a single chemical element, as with oxygen (O2), or of different elements, as with water (H2O).A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. The term is also used to refer to a pure chemical substance composed of atoms with the same number of protons. Until March 2010, 118 elements have been observed. 94 elements occur naturally on earth, either as the pure element or more commonly as a component in compounds. 80 elements have stable isotopes, namely all elements with atomic numbers 1 to 82, except elements 43 and 61 (technetium and promethium). Elements with atomic numbers 83 or higher (bismuth and above) are inherently unstable, and undergo radioactive decay. The elements from atomic number 83 to 94 have no stable nuclei, but are nevertheless found in nature, either surviving as remnants of the primordial stellar nucleosynthesisthat produced the elements in the solar system, or else produced as short-lived daughter-isotopes through the natural decay of uranium and thorium. The remaining 24 elements so are artificial, or synthetic, elements, which are products of man-induced processes. These synthetic elements are all characteristically unstable. Although they have not been found in nature, it is conceivable that in the early history of the earth, these and possibly other unknown elements may have been present. Their unstable nature could have resulted in their disappearance from the natural components of the earth, however.The naturally occurring elements were not all discovered at the same time. Some, such as gold, silver, iron, lead, and copper, have been known since the days of earliest civilizations. Others, such as helium, radium, aluminium, and bromine, were discovered in the nineteenth century. The most abundant elements found in the earth’s crust, in order of decreasing percentage, are oxygen, silicon, aluminium, and iron. Others present in amounts of 1% or more are calcium, sodium, potassium, and magnesium. Together, these represent about 98.5% of the earth’s crust.The nomenclature and their origins of all known elements will be described in Chapter 2.A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together in a defined spatial arrangement by chemical bonds. Compounds that exist as molecules are called molecular compounds. An ionic compound is a chemical compound in which ions are held together in a lattice structure by ionic bonds. Usually, the positively charged portion consists of metal cations and the negatively charged portion is an anion or polyatomic ion.The relative amounts of the elements in a particular compound do not change: Every molecule of a particular chemical substance contains acharacteristic number of atoms of its constituent elements. For example, every water molecule contains two hydrogen atoms and one oxygen atom. To describe this atomic composition, chemists write the chemical formula for water as H2O.The chemical formula for water shows how formulas are constructed. The formula lists the symbols of all elements found in the compound, in this case H (hydrogen) and O (oxygen). A subscript number after an element's symbol denotes how many atoms of that element are present in the molecule. The subscript 2 in the formula for water indicates that each molecule contains two hydrogen atoms. No subscript is used when only one atom is present, as is the case for the oxygen atom in a water molecule. Atoms are indivisible, so molecules always contain whole numbers of atoms. Consequently, the subscripts in chemical formulas of molecular substances are always integers. We explore chemical formulas in greater detail in Chapter 2.The simple formula that gives the simplest whole number ratio between the atoms of the various elements present in the compound is called its empirical formula. The simplest formula that gives the actual number of atoms of the various elements present in a molecule of any compound is called its molecular formula. Elemental analysis is an experiment that determines the amount (typically a weight percent) of an element in a compound. The elemental analysis permits determination of the empirical formula, and the molecular weight and elemental analysis permit determination of the molecular formula.1.4. Numbers in Physical Quantities1.4.1. Measurement1.Physical QuantitiesPhysical properties such as height, volume, and temperature that can be measured are called physical quantity. A number and a unit of defined size are required to describe physical quantity, for example, 10 meters, 9 kilograms.2.Exact NumbersExact Numbers are numbers known withcertainty. They have unlimited number of significant figures. They arise by directly counting numbers, for example, the number of sides on a square, or by definition:1 m = 100 cm, 1 kg = 1000 g1 L = 1000 mL, 1 minute = 60seconds3.Uncertainty in MeasurementNumbers that result from measurements are never exact. Every experimental measurement, no matter how precise, has a degree of uncertainty to it because there is a limit to the number of digits that can be determined. There is always some degree of uncertainty due to experimental errors: limitations of the measuring instrument, variations in how each individual makes measurements, or other conditions of the experiment.Precision and AccuracyIn the fields of engineering, industry and statistics, the accuracy of a measurement system is the degree of closeness of measurements results to its actual (true) value. The precision of a measurement system, also called reproducibility or repeatability, is the degree to which repeated measurements under unchanged conditions show the same results. Although the two words can be synonymous in colloquial use, they are deliberately contrasted in the context of the scientific method.A measurement system can be accurate but not precise, precise but not accurate, neither, or both. A measurement system is called valid if it is both accurate and precise. Related terms are bias (non-random or directed effects caused by a factor or factors unrelated by the independent variable) and error(random variability), respectively. Random errors result from uncontrolled variables in an experiment and affect precision; systematic errors can be assigned to definite causes and affect accuracy. For example, if an experiment contains a systematic error, then increasing the sample size generally increases precision but does not improve accuracy. Eliminating the systematic error improves accuracy but does not change precision.1.4.2 Significant FiguresThe number of digits reported in a measurement reflects the accuracy of the measurement and the precision of the measuring device. Significant figures in a number include all of the digits that are known with certainty, plus the first digit to the right that has an uncertain value. For example, the uncertainty in the mass of a powder sample, i.e., 3.1267g as read from an “analytical balance” is 0.0001g.In any calculation, the results are reported to the fewest significant figures (for multiplication and division) or fewest decimal places (addition and subtraction).1.Rules for deciding the number of significantfigures in a measured quantity:The number of significant figures is found by counting from left to right, beginning with the first nonzero digit and ending with the digit that has the uncertain value, e.g.,459 (3) 0.206 (3) 2.17(3) 0.00693 (3) 25.6 (3) 7390 (3) 7390. (4)(1)All nonzero digits are significant, e.g., 1.234g has 4 significant figures, 1.2 g has 2significant figures.(2)Zeroes between nonzero digits aresignificant: e.g., 1002 kg has 4 significantfigures, 3.07 mL has 3 significant figures.(3)Leading zeros to the left of the first nonzerodigits are not significant; such zeroes merelyindicate the position of the decimal point:e.g., 0.001 m has only 1 significant figure,0.012 g has 2 significant figures.(4)Trailing zeroes that are also to the right of adecimal point in a number are significant:e.g., 0.0230 mL has 3 significant figures,0.20 g has 2 significant figures.(5)When a number ends in zeroes that are notto the right of a decimal point, the zeroes arenot necessarily significant: e.g., 190 milesmay be 2 or 3 significant figures, 50,600calories may be 3, 4, or 5 significant figures.The potential ambiguity in the last rule can be avoided by the use of standard exponential, or "scientific" notation. For example, depending onwhether the number of significant figures is 3, 4, or 5, we would write 50,600 calories as:5.06 × 104 calories (3 significant figures)5.060 ×104calories (4 significant figures), or5.0600 × 104 calories (5 significant figures).2.Rules for rounding off numbers(1)If the digit to be dropped is greater than 5,the last retained digit is increased by one.For example, 12.6 is rounded to 13.(2)If the digit to be dropped is less than 5, thelast remaining digit is left as it is. Forexample, 12.4 is rounded to 12.(3)If the digit to be dropped is 5, and if anydigit following it is not zero, the lastremaining digit is increased by one. Forexample, 12.51 is rounded to 13.(4)If the digit to be dropped is 5 and isfollowed only by zeroes, the last remainingdigit is increased by one if it is odd, but leftas it is if even. For example, 11.5 is roundedto 12, 12.5 is rounded to 12.This rule means that if the digit to be dropped is 5 followed only by zeroes, the result is always rounded to the even digit. The rationale is to avoid bias in rounding: half of the time we round up, half the time we round down.3.Arithmetic using significant figuresIn carrying out calculations, the general rule is that the accuracy of a calculated result is limited by the least accurate measurement involved in the calculation.(1) In addition and subtraction, the result is rounded off to the last common digit occurring furthest to the right in all components. Another way to state this rules, is that, in addition and subtraction, the result is rounded off so that it has the same number of decimal places as the measurement having the fewest decimal places. For example,100 (assume 3 significant figures) + 23.643 (5 significant figures) = 123.643,which should be rounded to 124 (3 significant figures).(2) In multiplication and division, the resultshould be rounded off so as to have the same number of significant figures as in the component with the least number of significant figures. For example,3.0 (2 significant figures ) ×12.60 (4 significant figures) = 37.8000which should be rounded off to 38 (2 significant figures).1.4.3 Scientific NotationScientific notation, also known as standard form or as exponential notation, is a way of writing numbers that accommodates values too large or small to be conveniently written in standard decimal notation.In scientific notation all numbers are written like this:a × 10b("a times ten to the power of b"), where the exponent b is an integer, and the coefficient a is any real number, called the significant or mantissa (though the term "mantissa" may cause confusion as it can also refer to the fractional part of the common logarithm). If the number is negative then a minus sign precedes a (as in ordinary decimal notation).In standard scientific notation the significant figures of a number are retained in a factor between 1 and 10 and the location of the decimal point is indicated by a power of 10. For example:An electron's mass is about 0.00000000000000000000000000000091093822 kg. In scientific notation, this is written 9.1093822×10−31 kg.The Earth's mass is about 5973600000000000000000000 kg. In scientific notation, this is written 5.9736×1024 kg.1.5 Units of Measurement1.5.1 Systems of Measurement1.United States Customary System (USCS)The United States customary system (also called American system) is the most commonly used system of measurement in the United States. It is similar but not identical to the British Imperial units. The U.S. is the only industrialized nation that does not mainly use the metric system in its commercial and standards activities. Base units are defined butseem arbitrary (e.g. there are 12 inches in 1 foot)2.MetricThe metric system is an international decimalized system of measurement, first adopted by France in 1791, that is the common system of measuring units used by most of the world. It exists in several variations, with different choices of fundamental units, though the choice of base units does not affect its day-to-day use. Over the last two centuries, different variants have been considered the metric system. Metric units are universally used in scientific work, and widely used around the world for personal and commercial purposes. A standard set of prefixes in powers of ten may be used to derive larger and smaller units from the base units.3.SISI system (for Système International) was adopted by the International Bureau of Weights and Measures in 1960, it is a revision and extension of the metric system. Scientists and engineers throughout the world in all disciplines are now being urged to use only the SI system of units.1.5.2 SI base unitsThe SI is founded on seven SI base units for seven base quantities assumed to be mutually independent, as given in Table 1.1.Table 1.1 SI Base Physical Quantities and UnitsU n i tN a m e UnitSymbolBaseQuantityQuantitySymbolDimensionSymbolm m l l Le t e r e n g t hk i lo g r a m kgmassm Ms ec o nd stimet Ta mp e r e AelectriccurrentI Ik el v i n KthermodynTΘm i ct e m p e r a t u r em o l e molamountofsubstancen Nc an d e l a cdluminousIvJntensity1.5.3 SI derived unitsOther quantities, called derived quantities, aredefined in terms of the seven base quantities via asystem of quantity equations. The SI derived unitsfor these derived quantities are obtained from theseequations and the seven SI base units. Examples ofsuch SI derived units are given in Table 1.2, where itshould be noted that the symbol 1 for quantities ofdimension 1 such as mass fraction is generallyomitted.Table 1.2 SI Derived Physical Quantities and(symbol) Unit(symbol)UArea (A) squaremeterm V olume (V) cubicmeterm Density (ρ) kilogramper cubicmeterkVelocity (u) meterpersecondmPressure (p) pascal(Pa)kEnergy (E) joule (J) (k Frequency (ν) hertz(Hz)1Quantity of electricity (Q) coulomb(C)AElectromotive force (E) volt (V) (kmsForce (F) newton(N)kFor ease of understanding and convenience, 22SI derived units have been given special names andsymbols, as shown in Table 1.3.Table 1.3 SI Derived Units with special names andsymbolsD e r i v e dq u a n t i t y SpecialnameSpecialSymbolExpressionintermsofotherSIunitsSIbaseunitsp r r ml a n ea n g l e adianad·m-1=1s o l i da n g l e steradiansrm2·m-2=1f r e q u e n c y hertzHzs-1f o r c e newtonN m·kg·s-2p p P N mr e s s u r e ,s t r e s s ascala/m21·kg·s-2e n e r g y ,w o r k ,q u a n t i t yo fh e a jouleJ N·mm2·kg·s-2p o w e r ,r a d i a n tf l u x wattW J/sm2·kg·s-3e l e c t r i cc h a r g e q u a n t i t y coulombC s·Afe l e c t r i c i t ye l e c t r i cp o t e n t i a l ,p o t e n t i a l voltV W/Am2·kg·s-3·A-1i f f e r e n c e ,e l e c t r o m o t i v ef o r c ec a p a c i t a n c e faradF C/Vm-2·kg-1·s 4·A 2e l e c t r i cr e s i s t a n c e ohmΩV/Am2·kg·s-3·A-2e l e c t r i cc o nd u c t a n c siemensS A/Vm-2·kg-1·s2·Aem a g n e t i cf l u x weberWbV·sm2·kg·s-2·A-1m a g n e t i cf l u xd e n s i t y teslaT Wb/m2kg·s-2·A-1i n d henH Wb/m2u c t a n c e ryA ·kg·s-2·A-2C e l s i u st e m p e r a t u r e degreeCelsius°CKl u m i n o u s lumenlmcd·srcd·srl u xi l l u m i n a n c e luxlxlm/m2m-2·cd·sra c t i v i t y( o far a d i o n u c l i d e becquerelBqs-1a b s o r b e dd o se ,s p e c i f i ce n e r g y( i m p a r t e d ) ,grayGyJ/kgm2·s-2e r m ad o s ee q u i v a l e n t ,e ta l .sievertSvJ/kgm2·s-2c a t a l y t i ca c t i v i katalkats-1·molyCertain units that are not part of the SI are essential and used so widely that they are accepted by the CIPM (Commission Internationale des Poids Et Mesures) for use with the SI. Some commonly used units are given in Table 1.4.Table 1.4 Non-SI units accepted for use with theSIN a m e SymbolQuantityEquivalentSIunitmi n u t e mintime1min=6sho u r htime1h6min=36s da y dtime1d=24h=144min=864sdegreeo fa r c °planeangle1°=(π/18)radm i n u t eo fa r c ′planeangle1′=(1/6)°=(π/18radsecondo fa r c ″planeangle1″=(1/6)′=(1/36)°=(π/648)rdhect a r e haarea1ha=1a=1m²l i t r e lorLvolume1l=1dm3=.1m3ton n e tmass1t=13kg=1MgThe 20 SI prefixes used to form decimal multiples and submultiples of SI units are given in Table 1.5.Table 1.5 SI PrefixesF a c t o r NameSymbolFactorNameSymbol1 0 24yottaY 1-1decid1 0 21zettZ 1-2centc。
化学专业英语

unite 1. Inorganic chemistry1.1 what is chemistry(1). 重点专业词汇讲解:Chemical: adj . 化学的、化学药品Transformation: 变化,化学转变,转化Dye: n. 染料染色,或者vt. 染Charcoal: ['t ? ck??l] 木炭Cellulose : 纤维素细胞的['selj?l??z; ] Fat:n. 脂肪肥肉adj . 肥大的alkalis :碱adj . 碱性的glycerin: 甘油丙三醇alkalis: n. 碱金属alloy: 合金使成合金bronze:青铜色的n. 青铜(铜和锡的合金)brass:[br a s] n.黄铜(铜和锌)要求学生会区别黄铜及青铜的不同翻译Poison:毒物毒药t.毒害放毒下毒Proton:n. 质子Nulei: n.核(nucleus 的复数形式)['njukl ??s]Identical : adj . 同一的Chirality n.手性手征和Handeness的区别Amino acid :n. 氨基酸Ala nine: n.丙氨酸2. 课文中重点词组(phrase)Chemical change:化学变化physical change:物理变化Explore: 探险研究research investigate studyIsolate: 分离chemical bonds 化学键chemical reaction :化学反应Natural substance 天然物质Coke : 焦炭carbon monoxide 一氧化碳Carbon Dioxide 二氧化碳Chemical bond 化学键fundamental principle 基本原理The periodic table of elements :元素周期表numbers of protons 质子数atomic number 原子序数covalent bonds 共价键positive 正阳性negative 负阴性3. 课文中重点句子The first and most important principle is that chemical substances are made up of molecules in which atoms of various elements are linked in well-defined ways. 需要着重给学生讲解第一条也是最重要的原理是化学物质是有分子组成的,分子中的不同元素的原子是以一定的方式连接在一起的。
化学专业英语

化学专业英语1、化学专业英语:一、无机化学术语1、periodic table 元素周期表2、electronic structure电子构型3、wavelength波长4、frequency频率5、wave number波数6、diffraction衍射7、quantum量子8、quantized量子化9、quantum theory量子理论10、photoelectric effect光电效应11、photon光子12、quantum mechanics量子力学13、Heisenberg uncertainty principle海森堡测不准原理14、momentum动量15、angular momentum角动量16、ground state基态17、excited states激发态18、quantum number量子数19、atomic orbital原子轨道20、the four quantum numbers四个量子数21、electron configuration电子构型22、Pauli exclusion principle泡利不相容原理23、Hund’s principle洪特规则24、paramagnetism顺磁性25、diamagnetism反磁性26、period周期27、noble gas惰性气体28、Representative elements代表性元素29、Transition elements过渡元素30、Metals金属31、nonmetals非金属32、semiconducting elements半导体元素33、chemical bond化学键34、valence electrons价电子35、Lewis symbol路易斯符号36、Chemical stability化学稳定性37、octet rule八隅体规则38、chemical reactivity化学反应性39、metallic bonding金属键40、ionic bonding 离子键41、Lewis structures路易斯结构42、nonbonding electron pairs(lone pairs)非成键电子对43、covalent bonding共价键44、single单键45、multiple(double,triple) and coordinate(donor atom and acceptor atom) covalent bond配位键46、resonance共振47、resonance hybrid共振杂化48、nonpolar and polar covalent bond非极性和极性共价键49、dipole偶极50、network covalent substances51、bond dissociation energy键解离能52、lattice energy点阵能,晶格能53、atomic radii原子半径54、effective nuclear charge有效核电荷55、screening effect屏蔽效应56、Scanning 扫描57、Lanthanide contraction镧系收缩58、isoelectronic ions等电子离子59、ionization energy电离能60、noble gas configuration惰性气体构型61、electron affinity电子亲和能62、pseudo-noble gas configuration稀有气体原子实63、polarization of an ion离子极化64、electronegativity电负性65、electronegative atom电正性原子66、electropositive atom电负性原子67、Oxidation numbers氧化值68、Oxidation state氧化态69、molecular geometry分子几何70、bond axis键轴71、valence bond theory价键理论72、hybridization杂化73、isomers异构体74、structural isomers结构异构75、delocalized electrons离域电子76、dipole moment偶极矩77、London bond色散力78、nuclide核素79、nucleons核子80、mass defect质量缺陷81、nuclear binding energy核结合能82、nuclear fusion核聚变83、nuclear fission核裂变84、radioactivity放射性85、radionuclides放射性核素86、magic number幻数87、bombardment reaction轰击反应88、antineutrino反中微子89、neutrino中微子90、positron正电子(阳电子)91、electron capture电子捕获92、chain reaction链式反应93、crtical mass临界质量94、nuclear reaction 核反应95、thermonuclear reactions热核反应96、breeder reactor增殖反应97、hydration水合98、solvation溶剂化99、chemical equilibrium化学平衡100、hydrolysis水解101、hydrates水合物102、efflorescence风化物103、hygroscopic 吸湿104、deliquescence潮解105、electrolytes电解质106、strong(weak)electrolytes强电解质107、nonelectrolytes非电解质108、acidic(alkaline)aqueous solution109、polyprotic acids多元酸110、neutralization中和反应111、complex ion络合离子112、ligands配体113、hard water 硬水114、carbonate hardness碳酸盐硬度115、water softening水软化116、permanent hardness永久硬度117、ion exchange离子交换118、fossil fuels化石燃料119、oxidation氧化120、reduction还原121、oxidation-reduction(redox)reactions氧化还原反应122、oxidizing agent氧化剂123、heavy water重水124、absorption吸附125、acidic anhydride(oxide)酸性酸酐126、basic anhydride(oxide)碱性酸酐127、amphoteric两性128、allotropes同素异形体129、acid salt酸式盐130、oxidizing anion氧化性阴离子131、disproportionation reaction歧化反应132、oxidizing acids氧化性酸。
(完整版)化学类专业英语词汇

专业英语词汇Unit 1 TEXT A:Chemical Reactions and Group Reactions customary a. 通常的,惯例的handle n.柄vt.触摸handling n.处理,管理derive vt.取得,得到,衍生oxidate vt.使氧化oxidation n.satisfactory a.令人满意的,符合要求的rapid a.快的,迅速的,动作快的combustion n.燃烧somewhat pron. ad. 一点点,几分,有点effort n.努力commercial a.商业的,商务的undesirable a.不合需要的,不受欢迎的,讨厌的retard vt.延迟,放慢,使停滞transformer n.变压器transform vt.改变,转变automotive a.自动的,机动的,汽车的cracked 裂化的sluge n.软泥,淤泥stiff a.硬的,强烈的extent 广度,程度distillation n.蒸馏distill vt.vi.unrefined a.未精致,未提炼的acidity n.酸味,酸性acidify vt. Vi.Involve vt.包缠,卷缠Fell=followingIndividual a.个人的,个体的Presumable a.可假定的,可推测的Destruction n.破坏,毁灭Overall n。
a.全面的,综合的Exceed 超过,胜过Isolate vt.隔离,孤立,使离析iso—构词成分“均匀”“异构”“苯”Analyse vt. 分析,分解Carbonyl 羰基Carboxyl 羧基Hydroxyl 羟基Decomposition 分解Alkyl 烷基,烃基Aldehyde n.醛Yield vt. 出产,产出Explosive a. 爆炸Vapor n.蒸汽,vi.蒸发Propagation 繁殖,增殖;传播Dehydrate vt.使脱水Acet 构词成分Acetaldehyde 乙醛Resin n.树脂Resinous a.树脂的Carboxylic a.羟基的Substantial a.物质的,实质的Susceptible a.易受感动的,敏感的Analogous a.类似的,相似的(to)Response n.作答,回答,响应,反应Readily ad.乐意地,很快地Readiness n.准备就绪,愿意Extent n.广度长度Steric 空间的,位的Likewise ad.同样的,照样地;也,又Suffer vt.遭受,经历Progressive a.进步的,长进的,渐次的Adjacent a.邻近的,紧挨着的Terminal a.末端的,终点的MethyleneBromide n.溴化物Substitute n.代替物(人),代用品substitution n.代替,替换Remote a.相隔较远的Acetone n.丙酮Ether n.醚,乙醚Correspond vi.符合,一致;相当,相应Reservation n.保留,预定Tend vi.走向,趋向。
化学专业英语常用词8

Inorganic chemistry 无机化学Organic chemistry 有机化学Physical chemistry 物理化学Analytical chemistry 分析化学Polymer chemistry 高分子化学Biochemistry 生物化学Radiochemistry 放射化学Theoretical chemistry 理论化学Matter ['mætə] n 物质Mass [mæs] n 质量V olume ['vɔljuːm] n 体积Pure substance 纯净物Element ['elim(ə)nt] n 元素Compound ['kɔmpaund] n 化合物Mixture ['mikstʃə] n 混合物Homogeneous [,hɔmə(u)'dʒiːniəs; -'dʒen-] 均匀的Homogeneous mixture 均匀混合物Solution [sə'luːʃ(ə)n] n 溶液Heterogeneous mixture 非均匀混合物Solid ['sɔlɪd] n 固体Liquid ['likwid] n 液体Gas [gæs] n 气体Plasma ['plæzmə] n 等离子体Excited state 激发态Physical property 物理性质Physical change 物理变化Chemical property 化学性质Chemical change 化学变化Separation of mixture 混合物分离Molecule ['mɔlikjuːl] n分子Chemical element 化学元素Atomic number 原子序数Technetium [tek'niːʃɪəm] n 锝Promethium [prə'miːθɪəm] n 钷Bismuth ['bizməθ] n 铋Radioactive decay 放射性衰变Nucleosynthesis [,njuːkliəʊ'si nθisis] n 核合成Artificial [ɑːtifiʃ(ə)l] adj 人工的Synthetic [si n'θet ik] adj 合成的Molecular compound 分子化合物Ionic compound 离子化合物Ionic bond 离子键Cation 阳离子Anion 阴离子Polyatomic ion 多原子离子Chemical formula 化学式Empirical formula 实验式Molecular formula 分子式Elemental analysis 元素分析Physical quantity 物理量Accuracy 准确度Precision 精确度Reproducibility [riprə,dju:sə'biliti] 再现性Repeatability [ri'pi:tə'biliti] 重复性Valid ['vælid] n 有效Bias ['baiəs] n 偏差Error ['erə] n 错误Random error 随机误差Systematic error 系统误差Significant figure有效数字Addition [ə'diʃ(ə)n] n 加法Subtraction [səb'trækʃn] n减法Multiplication [,mʌltipli'keiʃ(ə)n] n 乘法Division [di'viʒ(ə)n] n 除法Scientific notation/ Standard form / Exponential notation 科学计数法Coefficient [,kəui'fiʃ(ə)nt] n 系数Significant [sig'nifɪk(ə)nt] P10Mantissa [mæn'tisə] P10Minus sign 减号United States Customary System 美国习惯体系British Imperial Units P10Metric ['metrik] adj 公制的Decimalized system 十进制系统SI system 国际单位制Derived quantities 导出量Length [leŋθ] n 长度Density ['densiti] n 密度Temperature ['temp(ə)rətʃə] n 温度Potential energy 势能Kinetic energy 动能Heat [hiːt] n 热量Specific heat 比热Force [fɔːs] n 压力Pressure ['preʃə] n 压强Factor-lable/united-factor method量纲分析Dimensional analysis 量纲分析Conversion factor 转换因子Numerator ['njuːməreitə] n 分子Denominator [di'nɔmineitə] n 分母。
(完整版)化学专业英语

(完整版)化学专业英语一、基础词汇篇1. 原子与分子Atom(原子):物质的基本单位,由质子、中子和电子组成。
2. 化学反应Reactant(反应物):参与化学反应的物质。
Product(物):化学反应后的物质。
Catalyst(催化剂):能改变化学反应速率而本身不发生永久变化的物质。
3. 物质状态Solid(固体):具有一定形状和体积的物质。
Liquid(液体):具有一定体积,无固定形状的物质。
Gas(气体):无固定形状和体积的物质。
4. 酸碱盐Acid(酸):在水溶液中能电离出氢离子的物质。
Base(碱):在水溶液中能电离出氢氧根离子的物质。
Salt(盐):由酸的阴离子和碱的阳离子组成的化合物。
5. 溶液与浓度Solution(溶液):由溶剂和溶质组成的均匀混合物。
Solvent(溶剂):能溶解其他物质的物质。
Solute(溶质):被溶解的物质。
Concentration(浓度):溶液中溶质含量的度量。
二、专业术语篇1. 有机化学Organic Chemistry(有机化学):研究碳化合物及其衍生物的化学分支。
Functional Group(官能团):决定有机化合物化学性质的原子或原子团。
Polymer(聚合物):由许多重复单元组成的大分子化合物。
2. 无机化学Inorganic Chemistry(无机化学):研究不含碳的化合物及其性质的化学分支。
Crystal(晶体):具有规则排列的原子、离子或分子的固体。
OxidationReduction Reaction(氧化还原反应):涉及电子转移的化学反应。
3. 物理化学Physical Chemistry(物理化学):研究化学现象与物理现象之间关系的化学分支。
Chemical Bond(化学键):原子间相互作用力,使原子结合成分子。
Thermodynamics(热力学):研究能量转换和物质性质的科学。
4. 分析化学Analytical Chemistry(分析化学):研究物质的组成、结构和性质的科学。
有机化学专业英语

1, oxygen ['ɔksidʒən] n. 氧气,氧2, alkenes n. 烯属烃3, methyl ['mi:θail, 'miθil] n. 甲基;木精4, alkanes ['ælkeinz] n. 烷类,链烷烃;烷属烃(alkane的复数)5, turpentine ['tə:pəntain] n. 松节油;松脂 vt. 涂松节油 vi. 提取松节油6, benzene ['benzi:n]n. [化]苯7, chlorine ['klɔ:ri:n]n. 氯(17号化学元素)8, nickle alloy nickle alloy: 而非不含金之镍合金 | 而非不含金的镍合金9, brominate ['brəumineit]vt. 用溴处理,溴化10, haloalkane n. [有化] 卤代烷11, decolourise [di:'kʌləraiz]vt. 使…脱色;将…漂白 vi. 漂白(等于decolorize)12, copper ['kɔpə]n. 铜;铜币;[俚]警察 adj. 铜的 vt. 镀铜于13, hydrolysis [hai'drɔlisis]n. [化]水解作用14, derived adj. 导出的;衍生的,派生的 v. 得到;由…而来;推断(derive的过去分词)15, detergent [di'tə:dʒənt]n. 清洁剂;去垢剂16, sheet [ʃi:t]n. 薄片,纸张;床单;薄板 vt. 盖上被单;覆盖;使成大片 vi. 成片流动;大片落下adj. 片状的17, glycol ['ɡlaikɔl]n. 乙二醇;甘醇;二羟基醇18, bromide ['brəumaid]n. 溴化物;庸俗的人;陈词滥调19, styrene ['stairi:n, 'sti-]n. [有化] 苯乙烯,苏合香烯20, insulation [,insju'leiʃən, 'insə-]n. 绝缘;隔离,孤立21, antiseptics [,ænti'septiks]n. 防腐剂(antiseptic的复数)22, hydration [hai'dreiʃən]n. [化学] 水合作用23, halogenation [,hælədʒi'neiʃən]n. 卤化,加卤作用24, adhesive [əd'hi:siv]n. 粘合剂;胶黏剂 adj. 带粘性的;粘着的25, clay [klei]n. 泥土;粘土;肉体;似黏土的东西 vt. 用黏土处理26, sugarcane ['ʃugə,kein]n. [作物] 甘蔗;糖蔗27, fermentation [,fə:men'teiʃən]n. 发酵28, molasses [mə'læsiz]n. 糖蜜,糖浆29, intermediate [,intə'mi:djət, -dieit]vi. 起媒介作用 adj. 中间的,中级的 n. 中间物;媒介30, volatile ['vɔlətail]adj. 爆炸性的;不稳定的;挥发性的;反覆无常的 n. 挥发物;有翅的动物31, carbonisation n. 碳化,碳化作用32, liquefaction [,likwi'fækʃən]n. 液化;熔解33, synthesis ['sinθisis]n. 综合,合成;综合体34, optimum ['ɔptiməm]adj. 最适宜的 n. 最佳效果;最适宜条件35, usage ['ju:zidʒ]n. 用法;使用;惯例36, empirical [em'pirikəl]adj. 经验主义的,完全根据经验的37, endothermic [,endəu'θə:mik,-məl]adj. [热] 吸热的;温血的38, radical ['rædikəl]adj. 根本的;激进的;彻底的 n. 激进分子;原子团;根数;基础39, prefix [,pri:'fiks, 'pri:fiks]n. 前缀vt. 加前缀;将某事物加在前面40, initiator [i'niʃieitə]n. 发起人,创始者;教导者;启动程序;引爆器41, dimer ['daimə]n. [有化] 二聚物;二量体42, activation [,ækti'veiʃən]n. 激活;活化作用43, propagation [,prɔpə'ɡeiʃən]n. 传播;繁殖;增殖44, rigid ['ridʒid]adj. 严格的;僵硬的,死板的;坚硬的;精确的45, amorphous [ə'mɔ:fəs]adj. 无定形的;无组织的;非晶形的46, viscous ['viskəs]adj. 粘性的;黏的47, bracket ['brækit]n. 支架;括号;墙上凸出的托架 vt. 括在一起;把…归入同一类;排除48, caustic ['kɔ:stik]adj. [化]腐蚀性的;苛性的;刻薄的;[物]焦散的n. [医]腐蚀剂;[化]苛性钠;[物]焦散曲线49, moderate ['mɔdərət, 'mɔdəreit]adj. 适度的,中等的;有节制的;稳健的,温和的vi. 变缓和,变弱 vt. 节制;减轻50, brittle ['britl]adj. 易碎的,脆弱的;易生气的51, stiffiness n. 硬度52, gutter ['ɡʌtə]n. 排水沟;槽;贫民区 vi. 流;形成沟vt. 开沟于…;弄熄 adj. 贫贱的;粗俗的;耸人听闻的53, upholstery [ʌp'həulstəri]n. 家具装饰用品业;垫衬物54, curtain ['kə:tən]n. 窗帘;幕 vt. 遮蔽;装上门帘55, ultraviolet [,ʌltrə'vaiələt]adj. 紫外的;紫外线的 n. 紫外线辐射,紫外光56, decomposition [,di:kɔmpə'ziʃən]n. 分解,腐烂;变质57, cassettes n. 盒式录音带(cassette的复数)58, cassette [kæ'set]n. 盒式磁带;暗盒;珠宝箱;片匣59, disposable [dis'pəuzəbl]adj. 可任意处理的;可自由使用的;用完即可丢弃的60, autoclave ['ɔ:təkleiv]n. 高压灭菌器;高压锅 vt. 用高压锅烹饪 vi. 用高压锅烹饪。
四大化学专业英语词汇

四大化学专业英语词汇专业英语复习词汇习武的书生版无机化学1.periodic table(周期表):根据原子序数从小至大排序的化学元素列表,使特性相近的元素归在同一族中。
2.electronic structure(电子结构):电子在原子中的排列方式。
3.Wavelength(波长):波在一个振动周期内传播的距离,或沿着波的传播方向,两个相邻的同相位质点间的距离。
4.Frequency(频率):单位时间内完成周期性变化的次数,是描述周期运动频繁程度的量。
5.wave number(波数):波长(λ)的倒数,或在光的传播方向上每单位长度内的光波数。
6.diffraction(衍射):指波遇到障碍物时偏离原来直线传播的物理现象。
7.Quantum(量子):能表现出某物质或物理量特性的最小单元。
在微观领域中,某些物理量的变化是以最小的单位跳跃式进行的,而不是连续的,这个最小的单位叫做量子。
8.Quantized(量子化):物理量只能为基本单位(量子)的整数倍的数量。
9.quantum theory(量子理论):对能量进行量化的概念及其后果的总称。
10.p hotoelectric effect(光电效应):在高于某特定频率的电磁波照射下,某些物质(Cs、碱金属:Li,Na,K和Rb)内部的电子会被光子激发出来而形成电流,即光生电。
11.P hoton(光量子):传递电磁相互作用的基本粒子,是一种规范玻色子。
一种辐射能力的量子。
12.q uantum mechanics(量子力学):研究实体(微观粒子)的运动,其足够小、移动得足够快,同时具有可观察的波状和粒子性质,提供能量与物质相互作用的数学描述。
13.H eisenberg uncertainty principle(不确定性原理):不可能同时知道一个粒子的位置和它的速度(动量)。
14.M omentum(动量):动量是质量倍速度。
它不仅表示运动物体保持运动的趋势,而且还表示速度是一个方向性的量,以保持运动的方向。
化学专业英语词汇

专业英语 Professional English现代分析化学 Modern analytical chemistry 生物分析技术 Bioanalytical techniques高分子进展 Advances in polymers功能高分子进展 Advances in functional polym ers有机硅高分子研究进展 Progresses in organosi licon polymers高分子科学实验方法 Scientific experimental methods of polymers高分子设计与合成 The design and synthesis o f polymers反应性高分子专论 Instructions to reactive p olymers网络化学与化工信息检索 Internet Searching f or Chemistry & Chemical Engineering information有序分子组合体概论 Introduction to Organize d Molecular Assembilies两亲分子聚集体化学 Chemistry of amphiphilic aggregates表面活性剂体系研究新方法 New Method for stu dying Surfactant System微纳米材料化学 Chemistry of Micro-NanoMater ials分散体系研究新方法 New Method for studying dispersion 分散体系相行为 The Phase Behavior of Aqueou s Dispersions溶液-凝胶材料 Sol-Gel Materials高等量子化学 Advanced Quantum Chemistry 分子反应动力学 Molecular Reaction Dynamic 计算量子化学 Computational Quantum Chemistr y群论 Group Theory分子模拟理论及软件应用 Theory and Software of Molecular Modelling & Application价键理论方法 Valence Bond Theory量子化学软件及其应用 Software of Quantum Ch emistry & its Application分子光谱学 Molecular Spectrum算法语言 Computational Languange高分子化学 Polymer Chemistry高分子物理 Polymer Physics药物化学 Medicinal Chemistry统计热力学 Statistic Thermodynamics液-液体系专论 Discussion on Liquid-Liquid S ystem配位化学进展 Progress in Coordination Chemi stry无机材料及物理性质 Inorganic Materials and Their Physical Properties物理无机化学 Physical Inorganic Chemistry 相平衡 Phase Equilibrium现代无机化学 Today's Inorganic Chemistry 无机化学前沿领域导论 Introduction to Forwar d Field in Inorganic Chemistry量子化学 Quantum Chemistry分子材料 Molecular Material固体酸碱理论 Solid Acid-Base Theory萃取过程物理化学 Physical Chemistry in Extr action表面电化学 Surface Electrochemistry电化学进展 Advances on Electrochemistry 现代电化学实验技术 Modern Experimental Tech niques of Electrochemistry金属-碳多重键化合物及其应用 Compounds with Metal-Carbon multiple bonds and Their Applications叶立德化学:理论和应用 Ylides Chemistry: The ory and Application立体化学与手性合成 Stereochemistry and Chir al Synthesis杂环化学 Heterocyclic Chemistry有机硅化学 Organosilicon Chemistry药物设计及合成 Pharmaceutical Design and Sy nthesis超分子化学 Supramolecular Chemistry分子设计与组合化学 Molecular Design and Com binatorial Chemistry纳米材料化学前沿领域导论 Introduction to Na no-materials Chemistry纳米材料控制合成与自组装 Controlled-synthes is and Self-assembly of Nano-materials前沿讲座 Leading Front Forum专业英语 Professional English超分子化学基础 Basics of Supramolecular Che mistry液晶材料基础 Basics of Liquid Crystal Mater ials现代实验技术 Modern analytical testing tech niques色谱及联用技术 Chromatography and Technolog y of tandem发光分析及其研究法 Luminescence analysis an d Research methods胶束酶学 Micellar Enzymology分析化学中的配位化合物 Complex in Analytica l Chemistry电分析化学 Electroanalytical chemistry生物分析化学 Bioanalytical chemistry分析化学 Analytical chemistry仪器分析 Instrument analysis高分子合成化学 Polymers synthetic chemistry高聚物结构与性能 Structures and properties of polymers有机硅化学 Organosilicon chemistry功能高分子 Functional polymers有机硅高分子 Organosilicon polymers高分子现代实验技术 Advanced experimental te chnology of polymers高分子合成新方法 New synthetic methods of p olymers液晶与液晶高分子 Liquid crystals and liquid crystal polymers大分子反应 Macromolecules reaction水溶性高分子 Water-soluble polymers聚合物加工基础 The basic process of polymer s聚合物复合材料 Composite materials高等化工与热力学 Advanced Chemical Engineer ing and Thermodynamics高等反应工程学 Advanced Reaction Engineerin g高等有机化学 Advanced Organic Chemistry 高等有机合成 Advanced Organic synthesis 有机化学中光谱分析 Spectrum Analysis in Org anic Chemistry催化作用原理 Principle of Catalysis染料化学 Dye Chemistry中间体化学与工艺学 Intermediate Chemistry a nd Technology化学动力学 Chemical Kinetics表面活性剂合成与工艺 Synthesis and Technolo gy of Surfactants环境化学 Environmental Chemistry化工企业清洁生产 Chemical Enterprise Clean Production化工污染及防治 Chemical Pollution and Contr ol动量热量质量传递 Momentum, Heat and Mass Tr ansmission化工分离工程专题 Separation Engineering耐蚀材料 Corrosion Resisting Material网络化学与化工信息检索 Internet Searching f or Chemistry & Chemical Engineering informa tion新型功能材料的模板组装 Templated Assembly o f Novel Advanced Materials胶体与界面 Colloid and Interface纳米材料的胶体化学制备方法 Colloid Chemical Methods for Preparing Nano-materials脂质体化学 Chemistry of liposome表面活性剂物理化学 Physico-chemistry of sur factants高分子溶液与微乳液 Polymer Solutions and Mi croemulsions两亲分子的溶液化学 Chemistry of Amphiphilic Molecules in solution介孔材料化学 Mesoporous Chemistry超细颗粒化学 Chemistry of ultrafine powder分散体系流变学 The Rheolgy of Aqueous Dispe rsions量子化学 Quantum Chemistry统计热力学 Statistic Thermodynamics群论 Group Theory分子模拟 Molecular Modelling高等量子化学 Advanced Quantum Chemistry价键理论方法 Valence Bond Theory量子化学软件及其应用 Software of Quantum Ch emistry & its Application计算量子化学 Computational Quantum Chemistr y分子模拟软件及其应用 Software of Molecular Modelling & its Application分子反应动力学 Molecular Reaction Dynamic 分子光谱学 Molecular Spectrum算法语言 Computational Languange高分子化学 Polymer Chemistry高分子物理 Polymer Physics腐蚀电化学 Corrosion Electrochemistry物理化学 Physical Chemistry结构化学 structural Chemistry现代分析与测试技术(试验为主) Modern Analysi s and Testing Technology(experimetally)高等无机化学 Advanced Inorganic Chemistry 近代无机物研究方法 Modern Research Methods for Inorganic Compounds萃取化学研究方法 Research Methods for Extra ction Chemistry单晶培养 Crystal Culture固态化学 Chemistry of Solid Substance液-液体系专论 Discussion on Liquid-Liquid S ystem配位化学进展 Progress in Coordination Chemi stry卟啉酞箐化学 Chemistry of Porphyrine and Ph thalocyanine无机材料及物理性质 Inorganic Materials and Their Physical Properties物理无机化学 Physical Inorganic Chemistry 相平衡 Phase Equilibrium生物化学的应用 Application of Biologic Chem istry生物无机化学 Bio-Inorganic Chemistry绿色化学 Green Chemistry金属有机化合物在均相催化中的应用 Applied Ho mogeneous Catalysis with Organometallic Compounds功能性食品化学 Functionalized Food Chemistr y无机药物化学 Inorganic Pharmaceutical Chemi stry电极过程动力学 Kinetics on Electrode Process电化学研究方法 Electrochemical Research Met hods生物物理化学 Biological Physical Chemistry 波谱与现代检测技术 Spectroscopy and Modern Testing Technology理论有机化学 theoretical Organic Chemistry 合成化学 Synthesis Chemistry有机合成新方法 New Methods for Organic Synt hesis生物有机化学 Bio-organic Chemistry药物化学 Pharmaceutical Chemistry金属有机化学 Organometallic Chemistry金属-碳多重键化合物及其应用 Compounds with Metal-Carbon multiple bonds and Their Applications分子构效与模拟 Molecular Structure-Activity and Simulation过程装置数值计算 Data Calculation of Proces s Devices石油化工典型设备 Common Equipment of Petroc hemical Industry化工流态化工程 Fluidization in Chemical Ind ustry化工装置模拟与优化 Analogue and Optimizatio n of Chemical Devices化工分离工程 Separation Engineering 化工系统与优化 Chemical System and Optimiza tion高等化工热力学 Advanced Chemical Engineerin g and Thermodynamics超临界流体技术及应用 Super Cratical Liguid Technegues and Applications膜分离技术 Membrane Separation Technegues溶剂萃取原理和应用 Theory and Application o f Solvent Extraction树脂吸附理论 Theory of Resin Adsorption 中药材化学 Chemistry of Chinese Medicine 生物资源有效成分分析与鉴定 Analysis and Det ection of Bio-materials相平衡理论与应用 Theory and Application o f Phase Equilibrium计算机在化学工程中的应用 Application of Com puter in Chemical Engineering微乳液和高分子溶液 Micro-emulsion and High Molecular Solution传递过程 Transmision Process反应工程分析 Reaction Engineering Analysis 腐蚀电化学原理与应用 Principle and Applicat ion of Corrosion Electrochemistry腐蚀电化学测试方法与应用 Measurement Method and Application of Corrosion Elect rochemistry耐蚀表面工程 Surface Techniques of Anti-cor rosion缓蚀剂技术 Inhabitor Techniques腐蚀失效分析 Analysis of Corrosion Destroy 材料表面研究方法 Method of Studying Materia l Surfacc分离与纯化技术 Separation and Purification Technology现代精细有机合成 Modern Fine Organic Synthe sis化学工艺与设备 Chemical Technology and Appa ratuas功能材料概论 Functional Materials Conspectu s油田化学 Oilfield Chemistry精细化学品研究 Study of Fine Chemicals催化剂合成与应用 Synthesis and Application of Catalyzer低维材料制备 Preparation of Low-Dimension M aterials手性药物化学 Symmetrical Pharmaceutical Che mistry光敏高分子材料化学 Photosensitive Polymer M aterials Chemistry纳米材料制备与表征 Preparation and Characte rization of Nanostructured materials溶胶凝胶化学 Sol-gel Chemistry 纳米材料化学进展 Proceeding of Nano-materia ls Chemistry●化学常用词汇汉英对照表1●氨 ammonia氨基酸 amino acid铵盐 ammonium salt饱和链烃 saturated aliphatic hydrocarbon苯 benzene变性 denaturation不饱和烃unsaturated hydrocarbon超导材料superconductive material臭氧 ozone醇 alcohol次氯酸钾potassium hypochlorite醋酸钠 sodium acetate蛋白质 protein氮族元素nitrogen group element碘化钾potassium iodide碘化钠 sodium iodide电化学腐蚀electrochemicalcorrosion电解质 electrolyte电离平衡ionization equilibrium电子云 electron cloud淀粉 starch淀粉碘化钾试纸starch potassium iodide paper二氧化氮nitrogen dioxide二氧化硅silicon dioxide二氧化硫sulphur dioxide二氧化锰manganese dioxide芳香烃 arene放热反应exothermic reaction非极性分子non-polar molecule非极性键non-polar bond肥皂 soap分馏fractional distillation酚 phenol复合材料 composite干电池 dry cell干馏 dry distillation甘油 glycerol高分子化合物 polymer共价键 covalent bond官能团functional group光化学烟雾photochemical fog过氧化氢hydrogen peroxide合成材料synthetic material合成纤维synthetic fiber合成橡胶synthetic rubber核电荷数nuclear charge number核素 nuclide化学电源chemical power source化学反应速率chemical reaction rate化学键 chemical bond化学平衡chemicalequilibrium还原剂 reducing agent磺化反应sulfonation reaction霍尔槽 Hull Cell极性分子polar molecule极性键 polar bond加成反应addition reaction加聚反应addition polymerization甲烷 methane碱金属 alkali metal碱石灰 soda lime结构式structural formula聚合反应 po1ymerization可逆反应reversible reaction空气污染指数air pollution index勒夏特列原理 Le Chatelier's principle离子反应ionic reaction离子方程式ionic equation离子键 ionic bond锂电池 lithium cell两性氢氧化物amphoteric hydroxide两性氧化物amphoteric oxide裂化 cracking裂解 pyrolysis硫氰化钾potassium thiocyanate硫酸钠sodium sulphide氯化铵ammonium chloride氯化钡barium chloride氯化钾potassium chloride氯化铝aluminium chloride氯化镁magnesium chloride氯化氢hydrogen chloride氯化铁iron (III)chloride氯水 chlorine water麦芽糖 maltose煤 coal酶 enzyme摩尔 mole摩尔质量 molar mass品红magenta或fuchsine葡萄糖 glucose气体摩尔体积 molar volume of gas铅蓄电池lead storage battery强电解质strong electrolyte氢氟酸hydrogen chloride氢氧化铝aluminium hydroxide取代反应substitution reaction醛 aldehyde炔烃 alkyne燃料电池 fuel cell弱电解质weak electrolyte石油 Petroleum水解反应hydrolysis reaction四氯化碳carbon tetrachloride塑料 plastic塑料的降解plastic degradation塑料的老化plastic ageing酸碱中和滴定acid-base neutralization titration酸雨 acid rain羧酸 carboxylic acid碳酸钠 sodium carbonate碳酸氢铵 ammonium bicarbonate碳酸氢钠 sodium bicarbonate糖类 carbohydrate烃 hydrocarbon烃的衍生物 derivative of hydrocarbon烃基 hydrocarbonyl同分异构体 isomer同素异形体 allotrope同位素 isotope同系物 homo1og涂料 coating烷烃 alkane物质的量 amount of substance物质的量浓度amount-of-substance concentration of B烯烃 alkene洗涤剂 detergent纤维素 cellulose相对分子质量 relative molecular mass相对原子质量 relative atomic mass消去反应 elimination reaction硝化反应 nitratlon reaction硝酸钡 barium nitrate硝酸银 silver nitrate溴的四氯化碳溶液 solution of bromine in carbon tetrachloride溴化钠 sodium bromide溴水 bromine water溴水 bromine water盐类的水解 hydrolysis of salts盐析 salting-out焰色反应 flame test氧化剂 oxidizing agent氧化铝 aluminium oxide氧化铁 iron (III) oxide乙醇 ethanol乙醛 ethana1乙炔 ethyne乙酸 ethanoic acid乙酸乙酯 ethyl acetate乙烯 ethene银镜反应silver mirror reaction硬脂酸 stearic acid油脂 oils and fats有机化合物 organic compound元素周期表 periodic table of elements元素周期律 periodic law of elements原电池 primary battery原子序数 atomic number皂化反应 saponification粘合剂 adhesive蔗糖 sucrose指示剂 Indicator酯 ester酯化反应 esterification周期 period族 group(主族:main group)Bunsen burner 本生灯product 化学反应产物flask 烧瓶apparatus 设备PH indicator PH值指示剂,氢离子(浓度的)负指数指示剂matrass 卵形瓶litmus 石蕊litmus paper 石蕊试纸graduate, graduated flask 量筒,量杯 reagent 试剂test tube 试管burette 滴定管retort 曲颈甑still 蒸馏釜cupel 烤钵crucible pot, melting pot 坩埚pipette 吸液管filter 滤管stirring rod 搅拌棒element 元素body 物体compound 化合物atom 原子gram atom 克原子atomic weight 原子量atomic number 原子数atomic mass 原子质量molecule 分子electrolyte 电解质ion 离子anion 阴离子cation 阳离子electron 电子isotope 同位素isomer 同分异物现象polymer 聚合物symbol 复合radical 基structural formula 分子式valence, valency 价monovalent 单价bivalent 二价halogen 成盐元素bond 原子的聚合mixture 混合combination 合成作用compound 合成物alloy 合金organic chemistry 有机化学inorganic chemistry 无机化学derivative 衍生物series 系列acid 酸hydrochloric acid 盐酸sulphuric acid 硫酸nitric acid 硝酸aqua fortis 王水fatty acid 脂肪酸organic acid 有机酸hydrosulphuric acid 氢硫酸hydrogen sulfide 氢化硫alkali 碱,强碱ammonia 氨base 碱hydrate 水合物hydroxide 氢氧化物,羟化物hydracid 氢酸hydrocarbon 碳氢化合物,羟anhydride 酐alkaloid 生物碱aldehyde 醛oxide 氧化物phosphate 磷酸盐acetate 醋酸盐methane 甲烷,沼气butane 丁烷salt 盐potassium carbonate 碳酸钾soda 苏打sodium carbonate 碳酸钠caustic potash 苛性钾caustic soda 苛性钠ester 酯gel 凝胶体analysis 分解fractionation 分馏endothermic reaction 吸热反应 exothermic reaction 放热反应 precipitation 沉淀to precipitate 沉淀to distil, to distill 蒸馏distillation 蒸馏to calcine 煅烧to oxidize 氧化alkalinization 碱化to oxygenate, to oxidize 脱氧,氧化 to neutralize 中和to hydrogenate 氢化to hydrate 水合,水化to dehydrate 脱水fermentation 发酵solution 溶解combustion 燃烧fusion, melting 熔解alkalinity 碱性isomerism, isomery 同分异物现象hydrolysis 水解electrolysis 电解electrode 电极anode 阳极,正极cathode 阴极,负极catalyst 催化剂catalysis 催化作用oxidization, oxidation 氧化reducer 还原剂dissolution 分解synthesis 合成reversible 可逆的1. The Ideal-Gas Equation 理想气体状态方程2. Partial Pressures 分压3. Real Gases: Deviation from Ideal Behavior 真实气体:对理想气体行为的偏离4. The van der Waals Equation 范德华方程5. System and Surroundings 系统与环境6. State and State Functions 状态与状态函数7. Process 过程8. Phase 相9. The First Law of Thermodynamics 热力学第一定律10. Heat and Work 热与功11. Endothermic and Exothermic Processes 吸热与发热过程12. Enthalpies of Reactions 反应热13. Hess’s Law 盖斯定律14. Enthalpies of Formation 生成焓15. Reaction Rates 反应速率16. Reaction Order 反应级数17. Rate Constants 速率常数18. Activation Energy 活化能19. The Arrhenius Equation 阿累尼乌斯方程20. Reaction Mechanisms 反应机理21. Homogeneous Catalysis 均相催化剂22. Heterogeneous Catalysis 非均相催化剂23. Enzymes 酶24. The Equilibrium Constant 平衡常数25. the Direction of Reaction 反应方向26. Le Chatelier’s Principle 列·沙特列原理27. Effects of Volume, Pressure, Temperature Changes and Catalystsi. 体积,压力,温度变化以及催化剂的影响28. Spontaneous Processes 自发过程29. Entropy (Standard Entropy) 熵(标准熵)30. The Second Law of Thermodynamics 热力学第二定律31. Entropy Changes 熵变32. Standard Free-Energy Changes 标准自由能变33. Acid-Bases 酸碱34. The Dissociation of Water 水离解35. The Proton in Water 水合质子36. The pH Scales pH值37. Bronsted-Lowry Acids and BasesBronsted-Lowry 酸和碱38. Proton-Transfer Reactions 质子转移反应39. Conjugate Acid-Base Pairs 共轭酸碱对40. Relative Strength of Acids and Bases 酸碱的相对强度41. Lewis Acids and Bases 路易斯酸碱42. Hydrolysis of Metal Ions 金属离子的水解43. Buffer Solutions 缓冲溶液44. The Common-Ion Effects 同离子效应45. Buffer Capacity 缓冲容量46. Formation of Complex Ions 配离子的形成47. Solubility 溶解度48. The Solubility-Product Constant Ksp 溶度积常数49. Precipitation and separation of Ions 离子的沉淀与分离50. Selective Precipitation of Ions 离子的选择沉淀51. Oxidation-Reduction Reactions 氧化还原反应52. Oxidation Number 氧化数53. Balancing Oxidation-Reduction Equations 氧化还原反应方程的配平54. Half-Reaction 半反应55. Galvani Cell 原电池56. Voltaic Cell 伏特电池57. Cell EMF 电池电动势58. Standard Electrode Potentials 标准电极电势59. Oxidizing and Reducing Agents 氧化剂和还原剂60. The Nernst Equation 能斯特方程61. Electrolysis 电解62. The Wave Behavior of Electrons 电子的波动性63. Bohr’s Model of The Hydrogen Atom 氢原子的波尔模型64. Line Spectra 线光谱65. Quantum Numbers 量子数66. Electron Spin 电子自旋67. Atomic Orbital 原子轨道68. The s (p, d, f) Orbital s(p,d,f)轨道69. Many-Electron Atoms 多电子原子70. Energies of Orbital 轨道能量71. The Pauli Exclusion Principle 泡林不相容原理72. Electron Configurations 电子构型73. The Periodic Table 周期表74. Row 行75. Group 族76. Isotopes, Atomic Numbers, and Mass Numbers 同位素,原子数,质量数77. Periodic Properties of the Elements 元素的周期律78. Radius of Atoms 原子半径79. Ionization Energy 电离能80. Electronegativity 电负性81. Effective Nuclear Charge 有效核电荷82. Electron Affinities 亲电性83. Metals 金属84. Nonmetals 非金属85. Valence Bond Theory 价键理论86. Covalence Bond 共价键87. Orbital Overlap 轨道重叠88. Multiple Bonds 重键89. Hybrid Orbital 杂化轨道90. The VSEPR Model 价层电子对互斥理论91. Molecular Geometries 分子空间构型92. Molecular Orbital 分子轨道93. Diatomic Molecules 双原子分子94. Bond Length 键长95. Bond Order 键级96. Bond Angles 键角97. Bond Enthalpies 键能98. Bond Polarity 键矩99. Dipole Moments 偶极矩100. Polarity Molecules 极性分子101. Polyatomic Molecules 多原子分子102. Crystal Structure 晶体结构103. Non-Crystal 非晶体104. Close Packing of Spheres 球密堆积105. Metallic Solids 金属晶体106. Metallic Bond 金属键107. Alloys 合金108. Ionic Solids 离子晶体109. Ion-Dipole Forces 离子偶极力110. Molecular Forces 分子间力111. Intermolecular Forces 分子间作用力112. Hydrogen Bonding 氢键113. Covalent-Network Solids 原子晶体114. Compounds 化合物115. The Nomenclature, Composition and Structure of Complexes 配合物的命名,组成和结构116. Charges, Coordination Numbers, and Geometries 电荷数、配位数、及几何构型117. Chelates 螯合物118. Isomerism 异构现象119. Structural Isomerism 结构异构120. Stereoisomerism 立体异构121. Magnetism 磁性122. Electron Configurations in Octahedral Complexes 八面体构型配合物的电子分布123. Tetrahedral and Square-planar Complexes 四面体和平面四边形配合物124. General Characteristics 共性125. s-Block Elements s区元素126. Alkali Metals 碱金属127. Alkaline Earth Metals 碱土金属128. Hydrides 氢化物129. Oxides 氧化物130. Peroxides and Superoxides 过氧化物和超氧化物131. Hydroxides 氢氧化物132. Salts 盐133. p-Block Elements p区元素134. Boron Group (Boron, Aluminium, Gallium, Indium, Thallium) 硼族(硼,铝,镓,铟,铊)135. Borane 硼烷136. Carbon Group (Carbon, Silicon, Germanium, Tin, Lead) 碳族(碳,硅,锗,锡,铅)137. Graphite, Carbon Monoxide, Carbon Dioxide 石墨,一氧化碳,二氧化碳138. Carbonic Acid, Carbonates and Carbides 碳酸,碳酸盐,碳化物139. Occurrence and Preparation of Silicon 硅的存在和制备140. Silicic Acid,Silicates 硅酸,硅酸盐141. Nitrogen Group (Phosphorus, Arsenic, Antimony, and Bismuth) 氮族(磷,砷,锑,铋)142. Ammonia, Nitric Acid, Phosphoric Acid 氨,硝酸,磷酸143. Phosphorates, phosphorus Halides 磷酸盐,卤化磷144. Oxygen Group (Oxygen, Sulfur, Selenium, and Tellurium) 氧族元素(氧,硫,硒,碲)145. Ozone, Hydrogen Peroxide 臭氧,过氧化氢146. Sulfides 硫化物147. Halogens (Fluorine, Chlorine, Bromine, Iodine) 卤素(氟,氯,溴,碘)148. Halides, Chloride 卤化物,氯化物149. The Noble Gases 稀有气体150. Noble-Gas Compounds 稀有气体化合物151. d-Block elements d区元素152. Transition Metals 过渡金属153. Potassium Dichromate 重铬酸钾154. Potassium Permanganate 高锰酸钾155. Iron Copper Zinc Mercury 铁,铜,锌,汞156. f-Block Elements f区元素157. Lanthanides 镧系元素158. Radioactivity 放射性159. Nuclear Chemistry 核化学160. Nuclear Fission 核裂变161. Nuclear Fusion 核聚变162. analytical chemistry 分析化学163. qualitative analysis 定性分析164. quantitative analysis 定量分析165. chemical analysis 化学分析166. instrumental analysis 仪器分析167. titrimetry 滴定分析168. gravimetric analysis 重量分析法169. regent 试剂170. chromatographic analysis 色谱分析171. product 产物172. electrochemical analysis 电化学分析173. on-line analysis 在线分析174. macro analysis 常量分析175. characteristic 表征176. micro analysis 微量分析177. deformation analysis 形态分析178. semimicro analysis 半微量分析179. systematical error 系统误差180. routine analysis 常规分析181. random error 偶然误差182. arbitration analysis 仲裁分析183. gross error 过失误差184. normal distribution 正态分布185. accuracy 准确度186. deviation 偏差187. precision 精密度188. relative standard deviation 相对标准偏差(RSD)189. coefficient variation 变异系数(CV)190. confidence level 置信水平191. confidence interval置信区间192. significant test显著性检验193. significant figure 有效数字194. standard solution 标准溶液195. titration 滴定196. stoichiometric point 化学计量点197. end point 滴定终点198. titration error滴定误差199. primary standard 基准物质200. amount of substance 物质的量201. standardization 标定202. chemical reaction 化学反应203. concentration 浓度204. chemical equilibrium 化学平衡205. titer 滴定度206. general equation for a chemical reaction 化学反应的通式207. proton theory of acid-base酸碱质子理论208. acid-base titration酸碱滴定法209. dissociation constant解离常数210. conjugate acid-base pair共轭酸碱对211. acetic acid 乙酸212. hydronium ion 水合氢离子213. electrolyte 电解质214. ion-product constant of water水的离子积215. ionization电离216. proton condition 质子平衡217. zero level 零水准218. buffer solution缓冲溶液219. methyl orange甲基橙220. acid-base indicator酸碱指示剂221. phenolphthalein 酚酞222. coordination compound配位化合物223. center ion中心离子224. cumulative stability constant累积稳定常数225. alpha coefficient酸效应系数226. overall stability constant总稳定常数227. ligand 配位体228. ethylenediamine tetraacetic acid乙二胺四乙酸229. side reaction coefficient副反应系数230. coordination atom配位原子231. coordination number 配位数232. lone pair electron孤对电子233. chelate compound 螯合物234. metal indicator 金属指示剂235. chelating agent 螯合剂236. masking 掩蔽237. demasking 解蔽238. electron 电子239. catalysis催化240. oxidation 氧化241. catalyst 催化剂242. reduction还原243. catalytic reaction催化反应244. reaction rate反应速率245. electrode potential电极电势246. activation energy 反应的活化能247. redox couple 氧化还原电对248. potassium permanganate 高锰酸钾249. iodimetry 碘量法250. potassium dichromate 重铬酸钾251. cerimetry 铈量法252. redox indicator 氧化还原指示253. oxygen consuming 耗氧量(OC)254. chemical oxygen demanded 化学需氧量(COD) 255. dissolved oxygen 溶解氧(DO) 256. precipitation 沉淀反应257. argentimetry 银量法258. heterogeneous equilibrium of ions 多相离子平衡259. aging 陈化260. postprecipitation 继沉淀261. coprecipitation 共沉淀262. ignition 灼烧263. fitration 过滤264. decantation 倾泻法265. chemical factor 化学因数266. spectrophotometry 分光光度法267. colorimetry 比色分析268. transmittance 透光率269. absorptivity 吸光率270. calibration curve 校正曲线271. standard curve 标准曲线272. monochromator 单色器273. source 光源274. wavelength dispersion 色散275. absorption cell 吸收池276. detector 检测系统277. bathochromic shift 红移278. Molar absorptivity 摩尔吸光系数279. hypochromic shift 紫移280. acetylene 乙炔281. ethylene 乙烯282. acetylating agent 乙酰化剂283. acetic acid 乙酸284. adiethyl ether 乙醚285. ethyl alcohol 乙醇286. acetaldehtde 乙醛287. β-dicarbontl compound β–二羰基化合物288. bimolecular elimination 双分子消除反应289. bimolecular nucleophilic substitution 双分子亲核取代反应290. open chain compound 开链族化合物291. molecular orbital theory 分子轨道理论292. chiral molecule 手性分子293. tautomerism 互变异构现象294. reaction mechanism 反应历程295. chemical shift 化学位移296. Walden inversio 瓦尔登反转n297. Enantiomorph 对映体298. addition rea ction 加成反应299. dextro- 右旋300. levo- 左旋301. stereochemistry 立体化学302. stereo isomer 立体异构体303. Lucas reagent 卢卡斯试剂304. covalent bond 共价键305. conjugated diene 共轭二烯烃306. conjugated double bond 共轭双键307. conjugated system 共轭体系308. conjugated effect 共轭效应309. isomer 同分异构体310. isomerism 同分异构现象311. organic chemistry 有机化学312. hybridization 杂化313. hybrid orbital 杂化轨道314. heterocyclic compound 杂环化合物315. peroxide effect 过氧化物效应t316. valence bond theory 价键理论317. sequence rule 次序规则318. electron-attracting grou p 吸电子基319. Huckel rule 休克尔规则320. Hinsberg test 兴斯堡试验321. infrared spectrum 红外光谱322. Michael reacton 麦克尔反应323. halogenated hydrocarbon 卤代烃324. haloform reaction 卤仿反应325. systematic nomenclatur 系统命名法e 326. Newman projection 纽曼投影式327. aromatic compound 芳香族化合物328. aromatic character 芳香性r329. Claisen condensation reaction克莱森酯缩合反应330. Claisen rearrangement 克莱森重排331. Diels-Alder reation 狄尔斯-阿尔得反应332. Clemmensen reduction 克莱门森还原333. Cannizzaro reaction 坎尼扎罗反应334. positional isomers 位置异构体335. unimolecular elimination reaction 单分子消除反应336. unimolecular nucleophilic substitution 单分子亲核取代反应337. benzene 苯338. functional grou 官能团p339. configuration 构型340. conformation 构象341. confomational isome 构象异构体342. electrophilic addition 亲电加成343. electrophilic reagent 亲电试剂344. nucleophilic addition 亲核加成345. nucleophilic reagent 亲核试剂346. nucleophilic substitution reaction亲核取代反应347. active intermediate 活性中间体348. Saytzeff rule 查依采夫规则349. cis-trans isomerism 顺反异构350. inductive effect 诱导效应 t351. Fehling’s reagent 费林试剂352. phase transfer catalysis 相转移催化作用353. aliphatic compound 脂肪族化合物354. elimination reaction 消除反应355. Grignard reagent 格利雅试剂356. nuclear magnetic resonance 核磁共振357. alkene 烯烃358. allyl cation 烯丙基正离子359. leaving group 离去基团360. optical activity 旋光性361. boat confomation 船型构象362. silver mirror reaction 银镜反应363. Fischer projection 菲舍尔投影式364. Kekule structure 凯库勒结构式365. Friedel-Crafts reaction 傅列德尔-克拉夫茨反应366. Ketone 酮367. carboxylic acid 羧酸368. carboxylic acid derivative 羧酸衍生物369. hydroboration 硼氢化反应370. bond oength 键长371. bond energy 键能372. bond angle 键角373. carbohydrate 碳水化合物374. carbocation 碳正离子375. carbanion 碳负离子376. alcohol 醇377. Gofmann rule 霍夫曼规则378. Aldehyde 醛379. Ether 醚380. Polymer 聚合物。
化学专业英语词汇

Lecture 21.atmospheric [,ætməs'ferik,-kəl] adj 与大气相关的大气的大气层的atmospheric pressure[气象] 大气压力,大气压强2. pascal ['pæskəl] n. 帕斯卡(压力的单位)3. torr [tɔ:] n. [物]托(真空压强单位)4.mercury ['mə:kjuri] n. 水银;水银柱;精神5. standard pressure:标准压力;标准气压;标准压standard atmospheric pressure:标准大气压;标准大气压强;标准大气压力6. Maxwell-Boltzmann distribution:玻耳兹曼分布学;麦克斯韦玻耳兹曼分布学;波兹曼分布7. flexible ['fleksibl] adj. 灵活的;柔韧的;易弯曲的8. proportional [prəu'pɔ:ʃənəl] adj. 比例的,成比例的;相称的,均衡n. 比例项9. Charles's law:查理士定律Charles [tʃɑ:lz] n. 查尔斯(人名)10. intersect [,intə'sekt] vi. 相交,交叉vt. 横断,横切;贯穿11. inversely ['invə:sli] adv. 倒转地;相反地inversely proportional 反比的;成反比例12. Avogadro's law:阿伏伽德罗定律Avogadro [avɔ'gadrɔ] n. 阿佛加德罗(意大利科学家)13. multiply ['mʌltiplai] vt. 乘;使相乘;使增加;使繁殖vi. 繁殖;增加;乘adj. 多样的;多层的14. manipulate [mə'nipjuleit] vt. 操作;操纵;巧妙地处理;篡改15. vapor ['veipə] n. 蒸汽;烟雾vt. 使……蒸发;使……汽化vi. 蒸发;吹牛;沮丧Water vapor 水蒸气16. effusion [i'fju:ʒən] n. 泻出;渗出;[医]渗漏物diffusion [di'fju:ʒən] n. 扩散,传播;[物]漫射17. evacuate [i'vækjueit] vt. 排泄;疏散,撤退vi. 撤退;疏散;排泄18. deflate [di'fleit] vt. 放气;使缩小;紧缩通货;打击;使泄气vi. 缩小;物价下降19. Graham's law 格雷姆定律Lecture 3-4notion ['nəuʃən] n. 概念;见解;打算postulate ['pɔstjuleit] vt. 要求;假定;视…为理所当然n. 假定;基本条件laureate ['lɔ:riət] adj. 戴桂冠的;荣誉的n. 桂冠诗人;得奖者vt. 使戴桂冠Lorentz force ['lɔ:rənts] [fɔ:s] 洛伦兹力fundamental [,fʌndə'mentəl] adj. 基本的,根本的n. 基本原理;基本原则apparatus [,æpə'reitəs] n. 装置,设备;器官;仪器friction ['frikʃən] n. 摩擦,[力] 摩擦力spherical ['sferikəl] adj. 球形的,球面的;天体的isotope ['aisəutəup] n. [化]同位素proton ['prəutɔn] n. [物]质子neutron ['nju:trɔn] n. 中子semblance ['sembləns] n. 外貌;假装;类似resemblance [ri'zembləns] n. 相似;相似之处;相似物;肖像neutron ['nju:trɔn] ] 中子Lecture5momentum [məu'mentəm] n. 动力;动量;冲力;势头electrostatic [i,lektrə'stætik] adj. 静电的;静电学的simulation [,simju'leiʃən] n. 仿真;模拟;模仿;假装exceed [ik'si:d] vt. 胜过;超过vi. 超过其他threshold ['θreʃhəuld] n. 极限;门槛;入口;开始;临界值kinetic [ki'netik, kai-] adj. 运动的;活跃ejectedadj. 射出的;被放出的;被驱逐的v. 喷射;驱逐(eject的过去分impinge [im'pindʒ] vi. 撞击;侵犯vt. 撞击diffraction [di'frækʃən] n. (光,声等的)衍射,绕射pattern ['pætən] n. 模式;样品;图案vt. 以图案装饰;模仿vi. 形成图案interference [,intə'fiərəns] n. 干涉;干扰,冲突scattered ['skætəd] adj. 分散的;散乱adjacent [ə'dʒeisənt] adj. 邻近的,毗连的Lecture 6Periodic trends :元素周期律octet rule :八位规则correctness :正确性probe [prəub] n.探索, 调查X-ray Photoelectron Spectrometer:[fəutəui'lektrɔn] [spek'trɔmitə] X射线光电子分光仪ionization [,aiənai'zeiʃən] n.离子化,电离spectrum ['spektrəm] n.光谱simulated ['sɪmjə,leɪtɪd]adj.假装的;冒充的;仿造的;模仿的incident ['insidənt]n.发生的事, 小插曲;敌对行动, 军事冲突;骚乱, 事故, 暴力事件intensity [in'tensiti]n.强烈, 剧烈The pain increased in intensity.疼痛越来越剧烈。
化学类专业英语词汇

专业英语词汇Unit 1 TEXT A:Chemical Reactions and Group Reactions customary a. 通常的,惯例的handle n.柄vt.触摸handling n.处理,管理derive vt.取得,得到,衍生oxidate vt.使氧化oxidation n.satisfactory a.令人满意的,符合要求的rapid a.快的,迅速的,动作快的combustion n.燃烧somewhat pron. ad. 一点点,几分,有点effort n.努力commercial a.商业的,商务的undesirable a.不合需要的,不受欢迎的,讨厌的retard vt.延迟,放慢,使停滞transformer n.变压器transform vt.改变,转变automotive a.自动的,机动的,汽车的cracked 裂化的sluge n.软泥,淤泥stiff a.硬的,强烈的extent 广度,程度distillation n.蒸馏distill vt.vi.unrefined a.未精致,未提炼的acidity n.酸味,酸性acidify vt. Vi.Involve vt.包缠,卷缠Fell=followingIndividual a.个人的,个体的Presumable a.可假定的,可推测的Destruction n.破坏,毁灭Overall n。
a.全面的,综合的Exceed 超过,胜过Isolate vt.隔离,孤立,使离析iso—构词成分“均匀”“异构”“苯”Analyse vt. 分析,分解Carbonyl 羰基Carboxyl 羧基Hydroxyl 羟基Decomposition 分解Alkyl 烷基,烃基Aldehyde n.醛Yield vt. 出产,产出Explosive a. 爆炸Vapor n.蒸汽,vi.蒸发Propagation 繁殖,增殖;传播Dehydrate vt.使脱水Acet 构词成分Acetaldehyde 乙醛Resin n.树脂Resinous a.树脂的Carboxylic a.羟基的Substantial a.物质的,实质的Susceptible a.易受感动的,敏感的Analogous a.类似的,相似的(to)Response n.作答,回答,响应,反应Readily ad.乐意地,很快地Readiness n.准备就绪,愿意Extent n.广度长度Steric 空间的,位的Likewise ad.同样的,照样地;也,又Suffer vt.遭受,经历Progressive a.进步的,长进的,渐次的Adjacent a.邻近的,紧挨着的Terminal a.末端的,终点的MethyleneBromide n.溴化物Substitute n.代替物(人),代用品substitution n.代替,替换Remote a.相隔较远的Acetone n.丙酮Ether n.醚,乙醚Correspond vi.符合,一致;相当,相应Reservation n.保留,预定Tend vi.走向,趋向。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
社,2000.
科技英语翻译方法概论
科技英语要求其客观性、准确性 及严密性,注意叙事逻辑上的连贯及表达上 的明晰、畅达,避免行文晦涩。科技英语力 求平易和精确,避免使用旨在加强语言感染 力和宣传效果的各种修饰词,以免使读者产 生行文浮华,内容虚饰之感。
例句:
李商隐:“春蚕到死丝方尽” • 科学翻译:Spring silkworm spins silk till
its death. • 文学翻译:Spring silkworm till its death
spins silk from lovesick heart.
科技英语的特点
(一)语法特点(有四多)
(二)词汇特点
• 1. 词义专一
文学英语中,经常出现一词多义或一义多词的 现象,科技英语中也不例外,但在表达同一个科 学概念或含义时,一般采用单一词汇。 如: hexachlorocyclohexane 六氯环己烷
• 2. 科技词汇来源于希腊语和拉丁语
据统计,1万个普通英语词汇中,约有46%源于 拉丁语,7.2%来源于希腊语,专业性越强,比率 就越高。
• 2. 描述科学的语言注重事实和逻辑,所以 往往是以图表、公式、数字来表达科学概 念,使用文学上的修饰手法于科技作品, 将会弄巧成拙,破坏科学的严肃性。
• 3. 逻辑语法词使用普遍
表原因的词:because of, due to, owing to 表转折的词:but, however, nevertheless, yet 表示逻辑顺序连接的词:so, thus, therefore, moreover, in addition to,furthermore 表限制的词:if only, except, besides, unless 表假设的词:suppose, assuming, provided
• 研究生阶段学习与工作--
➢ 英语文献检索、阅读、理解 ➢ 英语报告的汇报、听说 ➢ 英语讨论会、多媒体汇报 ➢ 英语论文撰写、投稿、发表 ➢ GRE考试出国留学
教学内容:
1. 大多数化学元素的名称; 2. 无机化学、有机化学、分析化学、物理化学、以
及高分子化学的基础词汇; 3. 氧化物、酸、碱、盐、烷、烯、炔、醇、酚、酮、
化学专业英语
Special English for Chemistry
Jin Jun-Ling Ph.D
Why do we learn Special English ?
➢ 研究生复试--专外(笔试、口试) ➢ 毕业论文--英语文献阅读、翻译
Why do we learn Special English ?
• 3. 广泛使用ቤተ መጻሕፍቲ ባይዱ写词,并且缩写词的词义专
一,使用频率高。
• 4. 前后缀出现频率高
英语的构词法主要有:合成、转化和派生,其 中派生法的核心是依靠添加前缀或后缀来构成新词, 这就导致了前后缀使用频率高。
例如:
• bio-
biochemistry(生物化学); biotechnology(生物科 技); biocatalyst(生物催化); biodegradable(可 生物降解的); bioengineering(生物工程), etc.
1.词类转换多 在翻译过程中将英文中的某种词类
译成汉语中的另一种词类,如名词→ 动词,形容词→动词,动词→名词等 等。
例如: The operation of a machine needs some
knowledge of its performance.
无词类转换的译文: 机器的操作需要机器性能的知识。
羧酸、胺、醚、酯,以及聚合物的IUPAC命名方 法以及部分俗名。 4. 四大谱:紫外、红外、核磁、质谱的基本术语和 表达方式。
学习目的:
通过本课程的学习,掌握化学专业词汇的形 成规律、基本特点及构词方法,进而较轻松自如 地掌握专业词汇、并做到译文准确通顺,符合汉 语习惯。
基本词汇和基本的翻译写作技能
People use mathematics in many different fields.
3. 后置定语多
• 即位于其所修饰名词之后的定语。科技英语由于语 言习惯与汉语的差异,还有为了强调所修饰的名词, 都将定语后置,定语越长,越易后置。
例如: Besides, isomerization [aɪsɒməraɪ‘zeɪʃən] processes may also take place which in turn leads to other fairly complicated reaction.
正确译文: 操作机器需要懂得机器的性能。
(n.→v.)
2. 被动语态多
这是因为科技人员最关心的是行为、活动、事 实本身,至于谁做的,无关紧要。运用被动语 态显得文章所描述的内容更客观,可减少一些 主观印象,意思表达简洁明了。 例如:
Mathematics is used in many different fields.
学时:32学时
➢ 考核方式: 以最终考试为主,占40%;平时占60%
➢ 教材: 《化学专业英语》,兰州大学出版社, 马永祥等主编
➢ 主要参考书目: 化学专业英语 应用化学专业英语 科技英语论文写作
北京师范大学出版社 化学工业出版社 西安交通大学出版社
推荐工具书
1. 英汉化学化工词汇,科学出版社,1991 2. 汉英化学化工词汇,中国地质大学出版社,
此外,还会发生异构化过程,从而相继导致其他复杂反应的 发生。 (注:此句中which作关系代词,修饰process,同时process也
做定语从句的主语。)
4. 复杂长句多
• 科技文章要求叙述准确,推理严谨。为表 达清楚,科技英语句子较长,需认真分析 方能明确句子中各成分的关系,译成汉语 时必须按照汉语习惯破译成若干个简单句。
• chemi(o)-
chemisorb
化学吸收;
chemiluminescence 化学发光;
chemoceptor
化学受体,化学感应器;
chemolysis[ke`mɔləsis] 化学分析
(三)科技英语在修饰上的特点
1. 时态应用有限
叙述过去的研究常用过去时(与现在不发 生联系),也用现在完成时(与现在有直 接联系,并对目前有重要的影响);讨论 推导的理论及结果用将来时;论述理论部 分用现在时。
