认识质粒图谱
质粒图谱大全之欧阳美创编

(转载)一.九种表达载体Pllp-OmpA,pllp-STII,pMBP-P,pMBP-C,pET-GST,pET-Trx,pET-His,pET-CKS,pET-DsbA 二.克隆载体pTZ19RDNApUC57DNAPMD18TPQE30pUC18pUC19pTrcHisApTrxFuspRSET-ApRSET-BpVAX1PBR322pbv220pBluescriptIIKS( )L4440pCAMBIA-1301pMAL-p2XpGD926三.PET系列表达载体ProteinExpression?ProkaryoticExpression?pETDsbFusio nSystems39band40bProteinExpression?ProkaryoticExpression?pETExpressi onSystem33bProteinExpression?ProkaryoticExpression?pETExpressi onSystemsProteinExpression?ProkaryoticExpression?pETExpressi onSystemsplusCompetentCellsProteinExpression?ProkaryoticExpression?pETGSTFusio nSystems41and42ProteinExpression?ProkaryoticExpression?pETNusAFusi onSystems43.1and44ProteinExpression?ProkaryoticExpression?pETVectorDN AProteinPurification?PurificationSystems?Strep?Tacti nResinsandPurificationKits四.PGEX系列表达载体TEcoR?pGEX-1I/BAP pGEX-2T pGEX-2TKpGEX-3XpGEX-4T-1pGEX-4T-2pGEX-4T-3pGEX-5X-1pGEX-5X-2pGEX-5X-3pGEX-6P-1pGEX-6P-2pGEX-6P-3五.PTYBsystemPTYB1PTYB2PTYB11PTYB12六.真核表达载体pCDNA3.1(-)pCDNA3.1( )pPICZalphaApGAPZαAPYES2.0pBI121pEGFP-N1pEGFP-C1pPIC9KpPIC3.5K如何阅读分析质粒图谱载体主要有病毒和非病毒两大类,其中质粒DNA是一种新的非病毒转基因载体。
☆如何阅读质粒图谱

如何阅读质粒图谱最近由于实验需要,需要查阅载体图谱,到园子里搜罗一番,发现虽然有人问载体图谱阅读的问题,也有前辈回答,但都不详细,借自己也在琢磨这个问题的机会,将我学到的东西整理一下,于大家分享。
载体主要有病毒和非病毒两大类,其中质粒DNA是一种新的非病毒转基因载体。
一、一个合格质粒的组成要素#复制起始位点Oril 即控制复制起始的位点。
原核生物DNA分子中只有一个复制起始点。
而真核生物DNA分子有多个复制起始位点。
#抗生素抗性基因可以便于加以检测,如Amp+l ,Kan+#多克隆位点MCS 克隆携带外源基因片段l#P/E 启动子/增强子l#Termsl 终止信号#加poly(A)信号l 可以起到稳定mRNA作用二、如何阅读质粒图谱第一步:首先看Ori的位置,了解质粒的类型(原核/真核/穿梭质粒)第二步:再看筛选标记,如抗性,决定使用什么筛选标记。
(1)Ampr 水解β-内酰胺环,解除氨苄的毒性。
(2)tetr 可以阻止四环素进入细胞。
(3)camr 生成氯霉素羟乙酰基衍生物,使之失去毒性。
(4)neor(kanr)氨基糖苷磷酸转移酶使G418(卡那霉素衍生物)失活(5)hygr 使潮霉素β失活。
第三步:看多克隆位点(MCS)。
它具有多个限制酶的单一切点。
便于外源基因的插入。
如果在这些位点外有外源基因的插入,会导致某种标志基因的失活,而便于筛选。
决定能不能放目的基因以及如何放置目的基因。
第四步:再看外源DNA插入片段大小。
质粒一般只能容纳小于10Kb的外源DNA片段。
一般来说,外源DNA片段越长,越难插入,越不稳定,转化效率越低。
第五步:是否含有表达系统元件,即启动子-核糖体结合位点-克隆位点-转录终止信号。
这是用来区别克隆载体与表达载体。
克隆载体中加入一些与表达调控有关的元件即成为表达载体。
选用那种载体,还是要以实验目的为准绳。
启动子-核糖体结合位点-克隆位点-转录终止信号#启动子-促进DNA转录的DNA顺序,这个DNA区域常在基因或操纵子编码顺序的上游,是DNA分子上可以与RNApol特异性结合并使之开始转录的部位,但启动子本身不被转录。
质粒图谱

)质粒图谱登记号:00012)质粒名称:pIRES3)来源:BD Co4)用途:真核双表达5)是否可以提供更详细资料:可以6)是否可以共享:7)联系方式:PM)质粒图谱登记号:00022)质粒名称:pECFP-C13)来源:BD Co4)用途:检测真核表达5)是否可以提供更详细资料:可以6)是否可以共享:7)联系方式:pm1)质粒图谱登记号:00032)质粒名称:pShuttle3)来源:4)用途:5)是否可以提供更详细资料:6)是否可以共享:7)联系方式:2)pShuttle MCS很多人用pEGFP-C1,我也来发一个)质粒图谱登记号:00042)质粒名称:pSBR322、pUC183)来源:4)用途:5)是否可以提供更详细资料:6)是否可以共享:7)联系方式:1)质粒图谱登记号:00052)质粒名称:pcDNA3.1(+)/CAT3)来源:invitrogen4)用途:真核表达5)是否可以提供更详细资料:可以6)是否可以共享:无偿7)联系方式:PM1)质粒图谱登记号:00062)质粒名称:pQEx3)来源:Qiagen4)用途:原核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM2)1)质粒图谱登记号:00072)质粒名称:pIVEX2.33)来源:Rocho4)用途:体外转录翻译5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM质粒图谱登记号:0008质粒名称:pIRES-EGFP来源:用途:是否可以提供更详细资料:是否可以共享:否联系方式:1)质粒图谱登记号:00092)质粒名称:pET-28a(+)3)来源:Novagen4)用途:原核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM1)质粒图谱登记号:00112)质粒名称:pET-32a(+)3)来源:novagen4)用途:原核表达5)是否可以提供更详细资料:6)是否可以共享:7)联系方式:pm1)质粒图谱登记号:00132)质粒名称:pcDNA3.1/Zeo (+)3)来源:invitrogen4)用途:原核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM)质粒图谱登记号:00132)质粒名称:pEGFP-N33)来源:clontech4)用途:真核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM)质粒图谱登记号:00142)质粒名称:pcDNA33)来源:invitrogen4)用途:真核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM)质粒图谱登记号:00152)质粒名称:pfastbac13)来源:invitrogene4)用途:昆虫表达5)是否可以提供更详细资料:不可以6)是否可以共享:不可以7)联系方式:PM)质粒图谱登记号:00162)质粒名称:pEGFP-C33)来源:clontech4)用途:真核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PMwangjun2002274 edited on 2004-06-22 00:041)质粒图谱登记号:00172)质粒名称:pSecTag23)来源:Invitrogen4)用途:真核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM)1质粒图谱登记号:00192)质粒名称:pET20b3)来源:NOVAGEN4)用途:原核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM1)质粒图谱登记号:00222)质粒名称:pThioHisA3)来源:invitrogen4)用途:原核表达5)是否可以提供更详细资料:4.365kb , HP-thioredoxin fusion proteinexpressionvector, trc promoter, Ampr, a EK cleavage site lies between HP-thioredoxin and MCS宿主菌TOP10(基因型为:F-mcrA △(mrr-hsd RMS-mcrBC)Ф80 lacZ M15 △lacX74 deoR recAl araD139 △(ara-leu)7697 galU galK rpsL endAl nupG6)是否可以共享:交换或其它7)联系方式:PM2)3)1)质粒图谱登记号:00242)质粒名称:pcDNA3.1-Myc-His-A-3)来源:invitrogen4)用途:真核核表达4))质粒图谱登记号:00252)质粒名称:pSUPER.neo3)来源:4)用途:siRNA5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM)质粒图谱登记号:00312)质粒名称:pSilencer1.0-siRNA3)来源:Ambion4)用途:RNAi5)是否可以提供更详细资料:/techlib/Documents.html?fkResSxn=7&fkSub Sxn=236)是否可以共享:实验结束后,可提供含shRNA模板的质粒7)联系方式:pm5) )质粒图谱登记号:00322)质粒名称:pSilencer2.0-U6siRNA 3)来源:Ambion4)用途:RNAi ,与1.0相比,可以建立稳转株5)更详细资料:/techlib/prot/fm_7209.pdf 6)是否可以共享:交换 7)联系方式:pm6)会员名:mlluoE-mail:*************可提供试验资源名称和简要介绍:pSilencer 3.1-H1 neo Vector,是Ambion公司目前最高版本的shRNA 载体,为扩增此载体我已插入目的片段,如有战友需要,将此片段双酶切再连上自己的片段即可。
质粒图谱大全

(转载)一. 九种表达载体Pllp-OmpA, pllp-STII, pMBP-P, pMBP-C,pET-GST, pET-Trx, pET-His, pET-CKS, pET-DsbA二. 克隆载体pTZ19RDNApUC57DNAPMD18TPQE30pUC18pUC19pTrcHisApTrxFuspRSET-ApRSET-BpVAX1PBR322pbv220pBluescriptIIKS( )L4440pCAMBIA-1301pMAL-p2XpGD926三.PET 系列表达载体ProteinExpression?ProkaryoticExpression?pETDsbFusionSystems39band40b ProteinExpression?ProkaryoticExpression?pETExpressionSystem33b ProteinExpression?ProkaryoticExpression?pETExpressionSystems ProteinExpression?ProkaryoticExpression?pETExpressionSystemsplusCompetentCells ProteinExpression?ProkaryoticExpression?pETGSTFusionSystems41and42 ProteinExpression?ProkaryoticExpression?pETNusAFusionSystems43.1and44 ProteinExpression?ProkaryoticExpression?pETVectorDNAProteinPurification?PurificationSystems?Strep?TactinResinsandPurificationKits四.PGEX 系列表达载体TEcoR?pGEX-1I/BAPpGEX-2TpGEX-2TKpGEX-3XpGEX-4T-1pGEX-4T-2pGEX-4T-3pGEX-5X-1pGEX-5X-2pGEX-5X-3pGEX-6P-1pGEX-6P-2pGEX-6P-3五.PTYBsystemPTYB1PTYB2PTYB11PTYB12六. 真核表达载体pCDNA3.1(-)pCDNA3.1( )pPICZalphaApGAPZα APYES2.0pBI121pEGFP-N1pEGFP-C1pPIC9KpPIC3.5K如何阅读分析质粒图谱载体主要有病毒和非病毒两大类, 其中质粒DNA是一种新的非病毒转基因载体。
如何阅读分析质粒图谱[1]
![如何阅读分析质粒图谱[1]](https://img.taocdn.com/s3/m/6e3cdcfe941ea76e58fa04ce.png)
基因酷质粒图谱/bbs/forum-38-1.html,收藏了将近800种质粒的图谱及相关信息特向大家推荐,介绍及使用方法见:/bbs/thread-417-1-1.html质粒图谱信息一.九种表达载体Pllp-OmpA, pllp-STII, pMBP-P, pMBP-C,pET-GST, pET-Trx, pET-His, pET-CKS, pET-DsbA二.克隆载体pTZ19R DNApUC57 DNAPMD18TPQE30pUC18pUC19pTrcHisApTrxFuspRSET-ApRSET-BpVAX1PBR322pbv220pBluescript II KS (+)L4440pCAMBIA-1301pMAL-p2XpGD926三.PET系列表达载体Protein Expression » Prokaryotic Expression » pET Dsb Fusion Systems 39b and 40bProtein Expression » Prokaryotic Expression » pET Expression System 33b Protein Expression » Prokaryotic Expression » pET Expression Systems Protein Expression » Prokaryotic Expression » pET Expression Systems plus Competent CellsProtein Expression » Prokaryotic Expression » pET GST Fusion Systems 41 and 42Protein Expression » Prokaryotic Expression » pET NusA Fusion Systems 43.1 and 44Protein Expression » Prokaryotic Expression » pET Vector DNAProtein Purification » Purification Systems » Strep•Tactin Resins and Purification Kits四.PGEX系列表达载体T EcoR pGEX-1 I/BAPpGEX-2TpGEX-2TKpGEX-3XpGEX-4T-1pGEX-4T-2pGEX-4T-3pGEX-5X-1pGEX-5X-2pGEX-5X-3pGEX-6P-1pGEX-6P-2pGEX-6P-3五.PTYB systemPTYB1PTYB2PTYB11PTYB12六.真核表达载体pCDNA3.1(-)pCDNA3.1(+)pPICZ alpha ApGAPZαAPYES2.0pBI121pEGFP-N1pEGFP-C1pPIC9KpPIC3.5K如何阅读分析质粒图谱载体主要有病毒和非病毒两大类,其中质粒DNA是一种新的非病毒转基因载体。
pUC18 和 pUC19质粒图谱
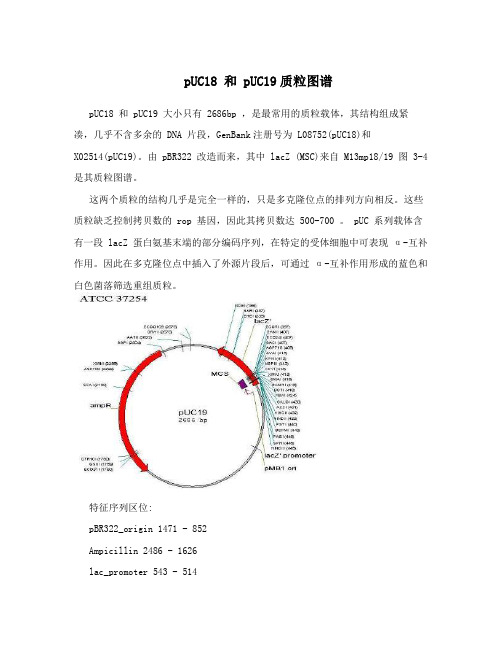
pUC18 和 pUC19质粒图谱pUC18 和 pUC19 大小只有 2686bp ,是最常用的质粒载体,其结构组成紧凑,几乎不含多余的 DNA 片段,GenBank注册号为 L08752(pUC18)和X02514(pUC19)。
由 pBR322 改造而来,其中 lacZ (MSC)来自 M13mp18/19 图 3-4 是其质粒图谱。
这两个质粒的结构几乎是完全一样的,只是多克隆位点的排列方向相反。
这些质粒缺乏控制拷贝数的 rop 基因,因此其拷贝数达 500-700 。
pUC 系列载体含有一段 lacZ 蛋白氨基末端的部分编码序列,在特定的受体细胞中可表现α-互补作用。
因此在多克隆位点中插入了外源片段后,可通过α-互补作用形成的蓝色和白色菌落筛选重组质粒。
特征序列区位:pBR322_origin 1471 - 852Ampicillin 2486 - 1626lac_promoter 543 - 514AmpR_promoter 2556 - 2528M13_pUC_rev_primer 500 - 478M13_reverse_primer 479 - 461M13_forward20_primer 379 - 395M13_pUC_fwd_primer 364 - 386lacZ_a 398 - 250pGEX_3_primer 51 - 29Orf2 2486 - 1626NOS terminator sequence:ggatccatcgttcaaacatttggcaataaagtttcttaagattgaatcctgttgccggtcttgcgatg attatcatataatttctgttgaattacgttaagcatgtaataattaacatgtaatgcatgacgttgtttatgagatgggtttt tatgattagagtcccgcaattatacatttaatacacgatagaaaacaaaatatagcgcgcaaactaggataaattatcgcgcg cggtgtcatctatgttactagtaatcgat设计引物:Forward primer: 5’ ggaGGATCCggatccatcgttcaaac 3’ (含BamH I 位点GGATCC及三个保护碱基)Reverse pri mer: 5’ atcGAATTCATCGATTACTAGTAACATAG 3’ (含EcoR I 位点GAATTC及三个保护碱基)请查一下NOS终止子序列内有没有这两个酶切点。
pcDNA3.1质粒图谱

pcDNA3.1(+)pcDNA3.1(-)Catalog nos. V790-20 and V795-20, respectivelyVersion I08140128-0104tech_service@iiTable of ContentsTable of Contents (iii)Important Information (v)Purchaser Notification (vi)Methods (1)Overview (1)Cloning into pcDNA3.1 (2)Transfection (6)Creation of Stable Cell Lines (7)Appendix (10)pcDNA3.1 Vectors (10)pcDNA3.1/CAT (12)Technical Service (13)References (15)iiiivImportant InformationContents pcDNA3.1 is supplied as follows:Catalog no.ContentsV790-2020 µg pcDNA3.1(+), lyophilized in TE, pH 8.020 µg pcDNA3.1/CAT, lyophilized in TE, pH 8.0V795-2020 µg pcDNA3.1(-), lyophilized in TE, pH 8.020 µg pcDNA3.1/CAT, lyophilized in TE, pH 8.0 Shipping/Storage Lyophilized plasmids are shipped at room temperature and should be stored at -20°C.Product Qualification Each of the pcDNA3.1 vectors is qualified by restriction enzyme digestion with specific restriction enzymes as listed below. Restriction digests must demonstrate the correct banding pattern when electrophoresed on an agarose gel. The table below lists the restriction enzymes and the expected fragments.Vector Restriction Enzyme Expected Fragments (bp) pcDNA3.1(+)Nhe IPst ISac I54281356, 4072109, 5319pcDNA3.1(-)Nhe IPst ISac I54271363, 4064169, 5258pcDNA3.1/CAT Nhe IPst ISac I62172145, 4072109, 6008vPurchaser NotificationIntroduction Use of pcDNA3.1 is covered under a number of different licenses as described below.CMV Promoter Use of the CMV promoter is covered under U.S. Patent Nos. 5,168,062 and 5,385,839owned and licensed by the University of Iowa Research Foundation and may be used forresearch purposes only. Commercial users must obtain a license to these patents directlyfrom the University of Iowa Research Foundation. Inquiries for commercial use should bedirected to:Brenda AkinsUniversity of Iowa Research Foundation (UIRF)214 Technology Innovation CenterIowa City, IA 52242Phone:319-335-4549BGH Polyadenylation Signal The bovine growth hormone (BGH) polyadenylation sequence is licensed under U.S. Patent No. 5,122,458 for research purposes only. “Research purposes” means uses directed to the identification of useful recombinant proteins and the investigation of the recombinant expression of proteins, which uses shall in no event include any of the following:a.any use in humans of a CLAIMED DNA or CLAIMED CELL;b.any use in human of protein or other substance expressed or made at any stage of itsproduction with the use of a CLAIMED DNA or a CLAIMED CELL;c.any use in which a CLAIMED DNA or CLAIMED CELL would be sold ortransferred to another party other than Invitrogen, its AFFILIATE, or itsSUBLICENSEE;d.any use in connection with the expression or production of a product intended forsale or commercial use; ore.any use for drug screening or drug development.Inquiries for commercial use should be directed to:Bennett Cohen, Ph.D.Research Corporation Technologies101 North Wilmot Road, Suite 600Tucson, AZ 85711-3335Tel: 1-520-748-4400Fax: 1-520-748-0025viMethodsOverviewIntroduction pcDNA3.1(+) and pcDNA3.1(-) are 5.4 kb vectors derived from pcDNA3 and designed for high-level stable and transient expression in mammalian hosts. High-level stable andnon-replicative transient expression can be carried out in most mammalian cells. Thevectors contain the following elements:•Human cytomegalovirus immediate-early (CMV) promoter for high-level expressionin a wide range of mammalian cells•Multiple cloning sites in the forward (+) and reverse (-) orientations to facilitatecloning•Neomycin resistance gene for selection of stable cell lines•Episomal replication in cells lines that are latently infected with SV40 or that expressthe SV40 large T antigen (e.g. COS-1, COS-7)The control plasmid, pcDNA3.1/CAT, is included for use as a positive control fortransfection and expression in the cell line of choice.Experimental Outline Use the following outline to clone and express your gene of interest in pcDNA3.1.1.Consult the multiple cloning sites described on pages 3-4 to design a strategy to cloneyour gene into pcDNA3.1.2.Ligate your insert into the appropriate vector and transform into E. coli. Selecttransformants on LB plates containing 50 to 100 µg/ml ampicillin.3.Analyze your transformants for the presence of insert by restriction digestion.4.Select a transformant with the correct restriction pattern and use sequencing toconfirm that your gene is cloned in the proper orientation.5.Transfect your construct into the mammalian cell line of interest using your ownmethod of choice. Generate a stable cell line, if desired.6.Test for expression of your recombinant gene by western blot analysis or functionalassay.1Cloning into pcDNA3.1Introduction Diagrams are provided on pages 3-4 to help you design a cloning strategy for ligating your gene of interest into pcDNA3.1. General considerations for cloning and transformation arelisted below.General Molecular Biology Techniques For help with DNA ligations, E. coli transformations, restriction enzyme analysis, purification of single-stranded DNA, DNA sequencing, and DNA biochemistry, please refer to Molecular Cloning: A Laboratory Manual (Sambrook et al., 1989) or Current Protocols in Molecular Biology (Ausubel et al., 1994).E. coli Strain Many E. coli strains are suitable for the propagation of this vector including TOP10F´,DH5α™-T1R, and TOP10. We recommend that you propagate vectors containing inserts inE. coli strains that are recombination deficient (rec A) and endonuclease A-deficient(end A).For your convenience, TOP10F´ is available as chemically competent or electrocompetentcells from Invitrogen.Item Quantity Catalog no.One Shot® TOP10F´ (chemically competent cells)21 x 50 µl C3030-03Electrocomp™ TOP10F´ 5 x 80 µl C665-55Ultracomp™ TOP10F´ (chemically competent cells) 5 x 300 µl C665-03Transformation Method You may use any method of your choice for transformation. Chemical transformation is the most convenient for most researchers. Electroporation is the most efficient and the method of choice for large plasmids.Maintenance of pcDNA3.1To propagate and maintain pcDNA3.1, we recommend resuspending the vector in 20 µl sterile water to make a 1 µg/µl stock solution. Store the stock solution at -20°C.Use this stock solution to transform a rec A, end A E. coli strain like TOP10F´, DH5α™-T1R, TOP10, or equivalent. Select transformants on LB plates containing 50 to100 µg/ml ampicillin. Be sure to prepare a glycerol stock of your plasmid-containing E. coli strain for long-term storage (see page 5).Cloning Considerations pcDNA3.1(+) and pcDNA3.1(-) are nonfusion vectors. Your insert must contain a Kozak translation initiation sequence and an ATG start codon for proper initiation of translation (Kozak, 1987; Kozak, 1991; Kozak, 1990). An example of a Kozak consensus sequence is provided below. Please note that other sequences are possible (see references above), but the G or A at position -3 and the G at position +4 are the most critical for function (shown in bold). The ATG initiation codon is shown underlined.(G/A)NNATG GYour insert must also contain a stop codon for proper termination of your gene. Please note that the Xba I site contains an internal stop codon (TCTAGA).continued on next page23Multiple Cloning Site of pcDNA3.1(+)Below is the multiple cloning site for pcDNA3.1(+). Restriction sites are labeled to indicate the cleavage site. The Xba I site contains an internal stop codon (TCTAGA). The multiple cloning site has been confirmed by sequencing and functional testing. Thecomplete sequence of pcDNA3.1(+) is available for downloading from our web site( ) or from Technical Service (see page 13). For a map and adescription of the features of pcDNA3.1(+), please refer to the Appendix , pages 10-11.1109TCCTTTCCTA ATAAAATGAG GAAATTGCAT CAAT TA TABGH poly (A) siteCATTGACGTC AATGGGAGTT TGTTTTGGCA CCAAAATCAA CGGGACTTTC CAAAATGTCGTAACAACTCC GCCCCATTGA CGCAAATGGG CGGTAGGCGT GTACGGTGGG AGGTCTATATAGTCTAGAGG GCCCGTTTAA ACCCGCTGAT CAGCCTCGAC TGTGCCTTCT AGTTGCCAGC CATCTGTTGT TTGCCCCTCC CCCGTGCCTT CCTTGACCCT GGAAGGTGCC ACTCCCACTG6897498098699299891049enhancer region (3´ end)*Please note that there are two Bst X I sites in the polylinker.continued on next page4Multiple CloningSite ofpcDNA3.1(-)Below is the multiple cloning site for pcDNA3.1(-). Restriction sites are labeled to indicate the cleavage site. The Xba I site contains an internal stop codon (TCTAGA). The multiple cloning site has been confirmed by sequencing and functional testing. Thecomplete sequence of pcDNA3.1(-) is available for downloading from our web site ( ) or from Technical Service (see page 13). For a map and a description of the features of pcDNA3.1(-), please see the Appendix , pages 10-11.Hin d III CAAT TA TA Bam H I Bst X I*Eco R I Eco R V Bst X I*BGH poly (A) site Kpn I Afl II Pme I Asp 718 I pcDNA3.1/BGH reverse priming site CCTTTCCTAA TAAAATGAGG AAATTGCATC ATCTGTTGTT TGCCCCTCCC CCGTGCCTTC CTTGACCCTG GAAGGTGCCA CTCCCACTGT GGTACCAAGC TTAAGTTTAA ACCGCTGATC AGCCTCGACT GTGCCTTCTA GTTGCCAGCC GCCGCCACTG TGCTGGATAT CTGCAGAATT CCACCACACT GGACTAGTGG ATCCGAGCTC TAACAACTCC GCCCCATTGA CGCAAATGGG CGGTAGGCGT GTACGGTGGG AGGTCTATAT CATTGACGTC AATGGGAGTT TGTTTTGGCA CCAAAATCAA CGGGACTTTC CAAAATGTCG 11091049989929869809749689enhancer region (3´ end)*Please note that there are two Bst X I sites in the polylinker.continued on next pageCloning into pcDNA3.1, continuedE. coli Transformation Transform your ligation mixtures into a competent rec A, end A E. coli strain (e.g. TOP10F´, DH5α™-T1R, TOP10) and select transformants on LB plates containing 50 to 100 µg/ml ampicillin. Select 10-20 clones and analyze for the presence and orientation of your insert.We recommend that you sequence your construct with the T7 Promoter and BGH Reverseprimers (Catalog nos. N560-02 and N575-02, respectively) to confirm that your gene is in thecorrect orientation for expression and contains an ATG and a stop codon. Please refer to thediagrams on pages 3-4 for the sequences and location of the priming sites. The primers areavailable separately from Invitrogen in 2 µg aliquots.Preparing aGlycerol StockOnce you have identified the correct clone, purify the colony and make a glycerol stock forlong-term storage. You should keep a DNA stock of your plasmid at -20°C.•Streak the original colony out on an LB plate containing 50 µg/ml ampicillin. Incubatethe plate at 37°C overnight.•Isolate a single colony and inoculate into 1-2 ml of LB containing 50 µg/ml ampicillin.•Grow the culture to mid-log phase (OD600 = 0.5-0.7).•Mix 0.85 ml of culture with 0.15 ml of sterile glycerol and transfer to a cryovial.•Store at -80°C.TransfectionIntroduction Once you have verified that your gene is cloned in the correct orientation and contains an initiation ATG and a stop codon, you are ready to transfect your cell line of choice. Werecommend that you include the positive control vector and a mock transfection (negativecontrol) to evaluate your results.Plasmid Preparation Plasmid DNA for transfection into eukaryotic cells must be clean and free from phenol and sodium chloride. Contaminants will kill the cells, and salt will interfere with lipids decreasing transfection efficiency. We recommend isolating plasmid DNA using the S.N.A.P.™ MiniPrep Kit (10-15 µg DNA, Catalog no. K1900-01), the S.N.A.P. ™MidiPrep Kit (10-200 µg DNA, Catalog no. K1910-01), or CsCl gradient centrifugation.Methods of Transfection For established cell lines (e.g. HeLa), please consult original references or the supplier of your cell line for the optimal method of transfection. We recommend that you follow exactly the protocol for your cell line. Pay particular attention to medium requirements, when to pass the cells, and at what dilution to split the cells. Further information is provided in Current Protocols in Molecular Biology (Ausubel et al., 1994).Methods for transfection include calcium phosphate (Chen and Okayama, 1987; Wigler et al., 1977), lipid-mediated (Felgner et al., 1989; Felgner and Ringold, 1989) and electroporation (Chu et al., 1987; Shigekawa and Dower, 1988). Invitrogen offers the Calcium Phosphate Transfection Kit (Catalog no. K2780-01) and a large selection of reagents for transfection. For more information, please refer to our World Wide Web site () or call Technical Service (see page 13).Positive Control pcDNA3.1/CAT is provided as a positive control vector for mammalian transfection and expression (see page 12) and may be used to optimize transfection conditions for yourcell line. The gene encoding chloramphenicol acetyl transferase (CAT) is expressed inmammalian cells under the control of the CMV promoter. A successful transfection willresult in CAT expression that can be easily assayed (see below).Assay for CAT Protein You may assay for CAT expression by ELISA assay, western blot analysis, fluorometric assay, or radioactive assay (Ausubel et al., 1994; Neumann et al., 1987). If you wish to detect CAT protein using western blot analysis, you may use the Anti-CAT Antiserum (Catalog no. R902-25) available from Invitrogen. Other kits to assay for CAT protein using ELISA assay are available from Roche Molecular Biochemicals (Catalog no. 1 363 727) and Molecular Probes (Catalog no. F-2900).Creation of Stable Cell LinesIntroduction The pcDNA3.1(+) and pcDNA3.1(-) vectors contain the neomycin resistance gene forselection of stable cell lines using neomycin (Geneticin®). We recommend that you testthe sensitivity of your mammalian host cell to Geneticin® as natural resistance variesamong cell lines. General information and guidelines are provided in this section for yourconvenience.Geneticin®Selective Antibiotic Geneticin® Selective Antibiotic blocks protein synthesis in mammalian cells by interfering with ribosomal function. It is an aminoglycoside, similar in structure to neomycin, gentamycin, and kanamycin. Expression of the bacterial aminoglycoside phosphotransferase gene (APH), derived from Tn5, in mammalian cells results in detoxification of Geneticin®(Southern and Berg, 1982).Geneticin®Selection Guidelines Geneticin® Selective Antibiotic is available from Invitrogen (Catalog no. 10486-025). Use as follows:•Prepare Geneticin® in a buffered solution (e.g. 100 mM HEPES, pH 7.3).•Use 100 to 800 µg/ml of Geneticin® in complete medium.•Calculate concentration based on the amount of active drug (check the lot label).•Test varying concentrations of Geneticin® on your cell line to determine theconcentration that kills your cells (see below). Cells differ in their susceptibility to Geneticin®.Cells will divide once or twice in the presence of lethal doses of Geneticin®, so the effects of the drug take several days to become apparent. Complete selection can take up to 3 weeks of growth in selective media.Determination of Antibiotic Sensitivity To successfully generate a stable cell line expressing your gene of interest frompcDNA3.1, you need to determine the minimum concentration of Geneticin® required to kill your untransfected host cell line. We recommend that you test a range of concentrations to ensure that you determine the minimum concentration necessary for your host cell line.1.Plate or split a confluent plate so the cells will be approximately 25% confluent.Prepare a set of 7 plates. Allow cells to adhere overnight.2.The next day, substitute culture medium with medium containing varyingconcentrations of Geneticin® (0, 50, 100, 200, 400, 600, 800 µg/ml Geneticin®).3.Replenish the selective media every 3-4 days, and observe the percentage of survivingcells.4.Count the number of viable cells at regular intervals to determine the appropriateconcentration of Geneticin® that prevents growth within 2-3 weeks after addition of Geneticin®.continued on next pagePossible Sites for Linearization of pcDNA3.1(+)Prior to transfection, we recommend that you linearize the pcDNA3.1(+) vector. Linearizing pcDNA3.1(+) will decrease the likelihood of the vector integrating into the genome in a way that disrupts the gene of interest or other elements required for expression in mammalian cells. The table below lists unique restriction sites that may be used to linearize your construct prior to transfection. Other unique restriction sites are possible. Be sure that your insert does not contain the restriction enzyme site you wish to use to linearize your vector.Enzyme Restriction Site (bp)Location SupplierBgl II12Upstream of CMV promoter Invitrogen, Catalog no. 15213-028 Mfe I161Upstream of CMV promoter New England BiolabsBst1107 I3236End of SV40 polyA AGS*, Fermentas, Takara, RocheMol. BiochemicalsEam1105 I4505Ampicillin gene AGS*, Fermentas, TakaraPvu I4875Ampicillin gene Invitrogen, Catalog no. 25420-019 Sca I4985Ampicillin gene Invitrogen, Catalog no. 15436-017 Ssp I5309bla promoter Invitrogen, Catalog no. 15458-011 *Angewandte Gentechnologie SystemePossible Sites for Linearization of pcDNA3.1(-)The table below lists unique restriction sites that may be used to linearize yourpcDNA3.1(-) construct prior to transfection. Other unique restriction sites are possible. Be sure that your insert does not contain the restriction enzyme site you wish to use to linearize your vector.Enzyme Restriction Site (bp)Location SupplierBgl II12Upstream of CMV promoter Invitrogen, Catalog no. 15213-028 Mfe I161Upstream of CMV promoter New England BiolabsBst1107 I3235End of SV40 polyA AGS*, Fermentas, Takara, RocheMol. BiochemicalsEam1105 I4504Ampicillin gene AGS*, Fermentas, TakaraPvu I4874Ampicillin gene Invitrogen, Catalog no. 25420-019 Sca I4984Ampicillin gene Invitrogen, Catalog no. 15436-017 Ssp I5308bla promoter Invitrogen, Catalog no. 15458-011 *Angewandte Gentechnologie Systemecontinued on next pageSelection of Stable Integrants Once you have determined the appropriate Geneticin® concentration to use for selection in your host cell line, you can generate a stable cell line expressing your gene of interest.1.Transfect your mammalian host cell line with your pcDNA3.1 construct using thedesired protocol. Remember to include a plate of untransfected cells as a negativecontrol and the pcDNA3.1/CAT plasmid as a positive control.2.24 hours after transfection, wash the cells and add fresh medium to the cells.3.48 hours after transfection, split the cells into fresh medium containing Geneticin® atthe pre-determined concentration required for your cell line. Split the cells such that they are no more than 25% confluent.4.Feed the cells with selective medium every 3-4 days until Geneticin®-resistant foci canbe identified.5.Pick and expand colonies in 96- or 48-well plates.AppendixpcDNA3.1 VectorsMap ofpcDNA3.1(+) and pcDNA3.1(-)The figure below summarizes the features of the pcDNA3.1(+) and pcDNA3.1(-) vectors.The complete sequences for pcDNA3.1(+) and pcDNA3.1(-) are available for down-loading from our World Wide Web site ( ) or from Technical Service (see page 13). Details of the multiple cloning sites are shown on page 3 for pcDNA3.1(+) and page 4 for pcDNA3.1(-).Comments for pcDNA3.1 (+) 5428 nucleotidesCMV promoter: bases 232-819T7 promoter/priming site: bases 863-882Multiple cloning site: bases 895-1010BGH polyadenylation sequence: bases 1028-1252f1 origin: bases 1298-1726SV40 early promoter and origin: bases 1731-2074Neomycin resistance gene (ORF): bases 2136-2930SV40 early polyadenylation signal: bases 3104-3234pUC origin: bases 3617-4287 (complementary strand)Ampicillin resistance gene (bla ): bases 4432-5428 (complementary strand) ORF: bases 4432-5292 (complementary strand)Ribosome binding site: bases 5300-5304 (complementary strand) bla promoter (P3): bases 5327-5333 (complementary strand)I I (+)( )continued on next pagepcDNA3.1 Vectors, continuedFeatures of pcDNA3.1(+) and pcDNA3.1(-)pcDNA3.1(+) (5428 bp) and pcDNA3.1(-) (5427 bp) contain the following elements. All features have been functionally tested.Feature BenefitHuman cytomegalovirus (CMV)immediate-early promoter/enhancerPermits efficient, high-level expression ofyour recombinant protein (Andersson et al.,1989; Boshart et al., 1985; Nelson et al.,1987)T7 promoter/priming site Allows for in vitro transcription in the senseorientation and sequencing through theinsertMultiple cloning site in forward orreverse orientationAllows insertion of your gene andfacilitates cloningBovine growth hormone (BGH)polyadenylation signalEfficient transcription termination andpolyadenylation of mRNA (Goodwin andRottman, 1992)f1 origin Allows rescue of single-stranded DNASV40 early promoter and origin Allows efficient, high-level expression ofthe neomycin resistance gene and episomalreplication in cells expressing SV40 large TantigenNeomycin resistance gene Selection of stable transfectants inmammalian cells (Southern and Berg,1982)SV40 early polyadenylation signal Efficient transcription termination andpolyadenylation of mRNApUC origin High-copy number replication and growthin E. coliAmpicillin resistance gene (β-lactamase)Selection of vector in E. colipcDNA3.1/CATDescription pcDNA3.1/CAT is a 6217 bp control vector containing the gene for CAT. It wasconstructed by digesting pcDNA3.1(+) with Xho I and Xba I and treating with Klenow.An 800 bp Hin d III fragment containing the CAT gene was treated with Klenow and thenligated into pcDNA3.1(+).Map of Control Vector The figure below summarizes the features of the pcDNA3.1/CAT vector. The complete nucleotide sequence for pcDNA3.1/CAT is available for downloading from our World Wide Web site () or by contacting Technical Service (see page 13).Comments for pcDNA3.1(+)/CAT6217 nucleotidesCMV promoter: bases 232-819CAT ORF: bases 1027-1686pcDNA3.1/BGH reverse priming site: bases 1811-1828BGH polyadenylation sequence: bases 1817-2041f1 origin: bases 2087-2515SV40 early promoter and origin: bases 2520-2863Neomycin resistance gene (ORF): bases 2925-3719SV40 early polyadenylation sequence: bases 3893-4023pUC origin: bases 4406-5076 (complementary strand)Ampicillin resistance gene (ORF): bases 5221-6081 (complementary strand)Technical ServiceVisit the Invitrogen Web Resource using your World Wide Web browser. At the site, youcan:•Get the scoop on our hot new products and special product offers•View and download vector maps and sequences•Download manuals in Adobe® Acrobat® (PDF) format•Explore our catalog with full color graphics•Obtain citations for Invitrogen products•Request catalog and product literatureOnce connected to the Internet, launch your web browser (Internet Explorer 5.0 or neweror Netscape 4.0 or newer), then enter the following location (or URL):...and the program will connect directly. Click on underlined text or outlined graphics toexplore. Don't forget to put a bookmark at our site for easy reference!Contact us For more information or technical assistance, please call, write, fax, or email. Additionalinternational offices are listed on our web page ().United States Headquarters:Japanese Headquarters European Headquarters:Invitrogen Corporation Invitrogen Japan K.K.Invitrogen Ltd1600 Faraday Avenue Nihonbashi Hama-Cho Park Bldg. 4F 3 Fountain DriveCarlsbad, CA 92008 USA2-35-4, Hama-Cho, Nihonbashi Inchinnan Business ParkTel: 1 760 603 7200Tel: 81 3 3663 7972Paisley PA4 9RF, UKTel (Toll Free): 1 800 955 6288Fax: 81 3 3663 8242Tel (Free Phone Orders): 0800 269 210 Fax: 1 760 602 6500E-mail: jpinfo@ Tel (General Enquiries): 0800 5345 5345 E-mail:Fax: +44 (0) 141 814 6287tech_service@ E-mail: eurotech@MSDS Requests To request an MSDS, please visit our web site () and follow theinstructions below.1.On the home page, go to the left-hand column under ‘Technical Resources’ andselect ‘MSDS Requests’.2.Follow instructions on the page and fill out all the required fields.3.To request additional MSDSs, click the ‘Add Another’ button.4.All requests will be faxed unless another method is selected.5.When you are finished entering information, click the ‘Submit’ button. Your MSDSwill be sent within 24 hours.continued on next pageTechnical Service, continuedEmergency Information In the event of an emergency, customers of Invitrogen can call the 3E Company, 24 hours a day, 7 days a week for disposal or spill information. The 3E Company can also connect the customer with poison control or with the University of California at San Diego Medical Center doctors.3E CompanyVoice: 1-760-602-8700Limited Warranty Invitrogen is committed to providing our customers with high-quality goods and services. Our goal is to ensure that every customer is 100% satisfied with our products and our service. If you shouldhave any questions or concerns about an Invitrogen product or service, please contact ourTechnical Service Representatives.Invitrogen warrants that all of its products will perform according to the specifications stated on thecertificate of analysis. The company will replace, free of charge, any product that does not meetthose specifications. This warranty limits Invitrogen Corporation’s liability only to the cost of theproduct. No warranty is granted for products beyond their listed expiration date. No warranty isapplicable unless all product components are stored in accordance with instructions. Invitrogenreserves the right to select the method(s) used to analyze a product unless Invitrogen agrees to aspecified method in writing prior to acceptance of the order.Invitrogen makes every effort to ensure the accuracy of its publications, but realizes that theoccasional typographical or other error is inevitable. Therefore Invitrogen makes no warranty ofany kind regarding the contents of any publications or documentation. If you discover an error inany of our publications, please report it to our Technical Service Representatives.Invitrogen assumes no responsibility or liability for any special, incidental, indirect orconsequential loss or damage whatsoever. The above limited warranty is sole and exclusive.No other warranty is made, whether expressed or implied, including any warranty ofmerchantability or fitness for a particular purpose.ReferencesAndersson, S., Davis, D. L., Dahlbäck, H., Jörnvall, H., and Russell, D. W. (1989). Cloning, Structure, andExpression of the Mitochondrial Cytochrome P-450 Sterol 26-Hydroxylase, a Bile Acid Biosynthetic Enzyme. J.Biol. Chem. 264, 8222-8229.Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A., and Struhl, K. (1994).Current Protocols in Molecular Biology (New York: Greene Publishing Associates and Wiley-Interscience).Boshart, M., Weber, F., Jahn, G., Dorsch-Häsler, K., Fleckenstein, B., and Schaffner, W. (1985). A Very Strong Enhancer is Located Upstream of an Immediate Early Gene of Human Cytomegalovirus. Cell 41, 521-530.Chen, C., and Okayama, H. (1987). High-Efficiency Transformation of Mammalian Cells by Plasmid DNA. Mol.Cell. Biol. 7, 2745-2752.Chu, G., Hayakawa, H., and Berg, P. (1987). Electroporation for the Efficient Transfection of Mammalian Cells with DNA. Nuc. Acids Res. 15, 1311-1326.Felgner, P. L., Holm, M., and Chan, H. (1989). Cationic Liposome Mediated Transfection. Proc. West. Pharmacol.Soc. 32, 115-121.Felgner, P. L., and Ringold, G. M. (1989). Cationic Liposome-Mediated Transfection. Nature 337, 387-388.Goodwin, E. C., and Rottman, F. M. (1992). The 3´-Flanking Sequence of the Bovine Growth Hormone GeneContains Novel Elements Required for Efficient and Accurate Polyadenylation. J. Biol. Chem. 267, 16330-16334. Kozak, M. (1987). An Analysis of 5´-Noncoding Sequences from 699 Vertebrate Messenger RNAs. Nuc. Acids Res.15, 8125-8148.Kozak, M. (1991). An Analysis of Vertebrate mRNA Sequences: Intimations of Translational Control. J. Cell Biol.115, 887-903.Kozak, M. (1990). Downstream Secondary Structure Facilitates Recognition of Initiator Codons by Eukaryotic Ribosomes. Proc. Natl. Acad. Sci. USA 87, 8301-8305.Nelson, J. A., Reynolds-Kohler, C., and Smith, B. A. (1987). Negative and Positive Regulation by a Short Segmentin the 5´-Flanking Region of the Human Cytomegalovirus Major Immediate-Early Gene. Mol. Cell. Biol. 7, 4125-4129.Neumann, J. R., Morency, C. A., and Russian, K. O. (1987). A Novel Rapid Assay for Chloramphenicol Acetyltransferase Gene Expression. BioTechniques 5, 444-447.Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, Second Edition (Plainview, New York: Cold Spring Harbor Laboratory Press).Shigekawa, K., and Dower, W. J. (1988). Electroporation of Eukaryotes and Prokaryotes: A General Approach tothe Introduction of Macromolecules into Cells. BioTechniques 6, 742-751.Southern, P. J., and Berg, P. (1982). Transformation of Mammalian Cells to Antibiotic Resistance with a BacterialGene Under Control of the SV40 Early Region Promoter. J. Molec. Appl. Gen. 1, 327-339.Wigler, M., Silverstein, S., Lee, L.-S., Pellicer, A., Cheng, Y.-C., and Axel, R. (1977). Transfer of Purified HerpesVirus Thymidine Kinase Gene to Cultured Mouse Cells. Cell 11, 223-232.©1997-2001 Invitrogen Corporation. All rights reserved.15。
pcDNA3.1质粒图谱

pcDNA3.1(+)pcDNA3.1(-)Catalog nos. V790-20 and V795-20, respectivelyVersion I08140128-0104tech_service@iiTable of ContentsTable of Contents (iii)Important Information (v)Purchaser Notification (vi)Methods (1)Overview (1)Cloning into pcDNA3.1 (2)Transfection (6)Creation of Stable Cell Lines (7)Appendix (10)pcDNA3.1 Vectors (10)pcDNA3.1/CAT (12)Technical Service (13)References (15)iiiivImportant InformationContents pcDNA3.1 is supplied as follows:Catalog no.ContentsV790-2020 µg pcDNA3.1(+), lyophilized in TE, pH 8.020 µg pcDNA3.1/CAT, lyophilized in TE, pH 8.0V795-2020 µg pcDNA3.1(-), lyophilized in TE, pH 8.020 µg pcDNA3.1/CAT, lyophilized in TE, pH 8.0 Shipping/Storage Lyophilized plasmids are shipped at room temperature and should be stored at -20°C.Product Qualification Each of the pcDNA3.1 vectors is qualified by restriction enzyme digestion with specific restriction enzymes as listed below. Restriction digests must demonstrate the correct banding pattern when electrophoresed on an agarose gel. The table below lists the restriction enzymes and the expected fragments.Vector Restriction Enzyme Expected Fragments (bp) pcDNA3.1(+)Nhe IPst ISac I54281356, 4072109, 5319pcDNA3.1(-)Nhe IPst ISac I54271363, 4064169, 5258pcDNA3.1/CAT Nhe IPst ISac I62172145, 4072109, 6008vPurchaser NotificationIntroduction Use of pcDNA3.1 is covered under a number of different licenses as described below.CMV Promoter Use of the CMV promoter is covered under U.S. Patent Nos. 5,168,062 and 5,385,839owned and licensed by the University of Iowa Research Foundation and may be used forresearch purposes only. Commercial users must obtain a license to these patents directlyfrom the University of Iowa Research Foundation. Inquiries for commercial use should bedirected to:Brenda AkinsUniversity of Iowa Research Foundation (UIRF)214 Technology Innovation CenterIowa City, IA 52242Phone:319-335-4549BGH Polyadenylation Signal The bovine growth hormone (BGH) polyadenylation sequence is licensed under U.S. Patent No. 5,122,458 for research purposes only. “Research purposes” means uses directed to the identification of useful recombinant proteins and the investigation of the recombinant expression of proteins, which uses shall in no event include any of the following:a.any use in humans of a CLAIMED DNA or CLAIMED CELL;b.any use in human of protein or other substance expressed or made at any stage of itsproduction with the use of a CLAIMED DNA or a CLAIMED CELL;c.any use in which a CLAIMED DNA or CLAIMED CELL would be sold ortransferred to another party other than Invitrogen, its AFFILIATE, or itsSUBLICENSEE;d.any use in connection with the expression or production of a product intended forsale or commercial use; ore.any use for drug screening or drug development.Inquiries for commercial use should be directed to:Bennett Cohen, Ph.D.Research Corporation Technologies101 North Wilmot Road, Suite 600Tucson, AZ 85711-3335Tel: 1-520-748-4400Fax: 1-520-748-0025viMethodsOverviewIntroduction pcDNA3.1(+) and pcDNA3.1(-) are 5.4 kb vectors derived from pcDNA3 and designed for high-level stable and transient expression in mammalian hosts. High-level stable andnon-replicative transient expression can be carried out in most mammalian cells. Thevectors contain the following elements:•Human cytomegalovirus immediate-early (CMV) promoter for high-level expressionin a wide range of mammalian cells•Multiple cloning sites in the forward (+) and reverse (-) orientations to facilitatecloning•Neomycin resistance gene for selection of stable cell lines•Episomal replication in cells lines that are latently infected with SV40 or that expressthe SV40 large T antigen (e.g. COS-1, COS-7)The control plasmid, pcDNA3.1/CAT, is included for use as a positive control fortransfection and expression in the cell line of choice.Experimental Outline Use the following outline to clone and express your gene of interest in pcDNA3.1.1.Consult the multiple cloning sites described on pages 3-4 to design a strategy to cloneyour gene into pcDNA3.1.2.Ligate your insert into the appropriate vector and transform into E. coli. Selecttransformants on LB plates containing 50 to 100 µg/ml ampicillin.3.Analyze your transformants for the presence of insert by restriction digestion.4.Select a transformant with the correct restriction pattern and use sequencing toconfirm that your gene is cloned in the proper orientation.5.Transfect your construct into the mammalian cell line of interest using your ownmethod of choice. Generate a stable cell line, if desired.6.Test for expression of your recombinant gene by western blot analysis or functionalassay.1Cloning into pcDNA3.1Introduction Diagrams are provided on pages 3-4 to help you design a cloning strategy for ligating your gene of interest into pcDNA3.1. General considerations for cloning and transformation arelisted below.General Molecular Biology Techniques For help with DNA ligations, E. coli transformations, restriction enzyme analysis, purification of single-stranded DNA, DNA sequencing, and DNA biochemistry, please refer to Molecular Cloning: A Laboratory Manual (Sambrook et al., 1989) or Current Protocols in Molecular Biology (Ausubel et al., 1994).E. coli Strain Many E. coli strains are suitable for the propagation of this vector including TOP10F´,DH5α™-T1R, and TOP10. We recommend that you propagate vectors containing inserts inE. coli strains that are recombination deficient (rec A) and endonuclease A-deficient(end A).For your convenience, TOP10F´ is available as chemically competent or electrocompetentcells from Invitrogen.Item Quantity Catalog no.One Shot® TOP10F´ (chemically competent cells)21 x 50 µl C3030-03Electrocomp™ TOP10F´ 5 x 80 µl C665-55Ultracomp™ TOP10F´ (chemically competent cells) 5 x 300 µl C665-03Transformation Method You may use any method of your choice for transformation. Chemical transformation is the most convenient for most researchers. Electroporation is the most efficient and the method of choice for large plasmids.Maintenance of pcDNA3.1To propagate and maintain pcDNA3.1, we recommend resuspending the vector in 20 µl sterile water to make a 1 µg/µl stock solution. Store the stock solution at -20°C.Use this stock solution to transform a rec A, end A E. coli strain like TOP10F´, DH5α™-T1R, TOP10, or equivalent. Select transformants on LB plates containing 50 to100 µg/ml ampicillin. Be sure to prepare a glycerol stock of your plasmid-containing E. coli strain for long-term storage (see page 5).Cloning Considerations pcDNA3.1(+) and pcDNA3.1(-) are nonfusion vectors. Your insert must contain a Kozak translation initiation sequence and an ATG start codon for proper initiation of translation (Kozak, 1987; Kozak, 1991; Kozak, 1990). An example of a Kozak consensus sequence is provided below. Please note that other sequences are possible (see references above), but the G or A at position -3 and the G at position +4 are the most critical for function (shown in bold). The ATG initiation codon is shown underlined.(G/A)NNATG GYour insert must also contain a stop codon for proper termination of your gene. Please note that the Xba I site contains an internal stop codon (TCTAGA).continued on next page23Multiple Cloning Site of pcDNA3.1(+)Below is the multiple cloning site for pcDNA3.1(+). Restriction sites are labeled to indicate the cleavage site. The Xba I site contains an internal stop codon (TCTAGA). The multiple cloning site has been confirmed by sequencing and functional testing. Thecomplete sequence of pcDNA3.1(+) is available for downloading from our web site( ) or from Technical Service (see page 13). For a map and adescription of the features of pcDNA3.1(+), please refer to the Appendix , pages 10-11.1109TCCTTTCCTA ATAAAATGAG GAAATTGCAT CAAT TA TABGH poly (A) siteCATTGACGTC AATGGGAGTT TGTTTTGGCA CCAAAATCAA CGGGACTTTC CAAAATGTCGTAACAACTCC GCCCCATTGA CGCAAATGGG CGGTAGGCGT GTACGGTGGG AGGTCTATATAGTCTAGAGG GCCCGTTTAA ACCCGCTGAT CAGCCTCGAC TGTGCCTTCT AGTTGCCAGC CATCTGTTGT TTGCCCCTCC CCCGTGCCTT CCTTGACCCT GGAAGGTGCC ACTCCCACTG6897498098699299891049enhancer region (3´ end)*Please note that there are two Bst X I sites in the polylinker.continued on next page4Multiple CloningSite ofpcDNA3.1(-)Below is the multiple cloning site for pcDNA3.1(-). Restriction sites are labeled to indicate the cleavage site. The Xba I site contains an internal stop codon (TCTAGA). The multiple cloning site has been confirmed by sequencing and functional testing. Thecomplete sequence of pcDNA3.1(-) is available for downloading from our web site ( ) or from Technical Service (see page 13). For a map and a description of the features of pcDNA3.1(-), please see the Appendix , pages 10-11.Hin d III CAAT TA TA Bam H I Bst X I*Eco R I Eco R V Bst X I*BGH poly (A) site Kpn I Afl II Pme I Asp 718 I pcDNA3.1/BGH reverse priming site CCTTTCCTAA TAAAATGAGG AAATTGCATC ATCTGTTGTT TGCCCCTCCC CCGTGCCTTC CTTGACCCTG GAAGGTGCCA CTCCCACTGT GGTACCAAGC TTAAGTTTAA ACCGCTGATC AGCCTCGACT GTGCCTTCTA GTTGCCAGCC GCCGCCACTG TGCTGGATAT CTGCAGAATT CCACCACACT GGACTAGTGG ATCCGAGCTC TAACAACTCC GCCCCATTGA CGCAAATGGG CGGTAGGCGT GTACGGTGGG AGGTCTATAT CATTGACGTC AATGGGAGTT TGTTTTGGCA CCAAAATCAA CGGGACTTTC CAAAATGTCG 11091049989929869809749689enhancer region (3´ end)*Please note that there are two Bst X I sites in the polylinker.continued on next pageCloning into pcDNA3.1, continuedE. coli Transformation Transform your ligation mixtures into a competent rec A, end A E. coli strain (e.g. TOP10F´, DH5α™-T1R, TOP10) and select transformants on LB plates containing 50 to 100 µg/ml ampicillin. Select 10-20 clones and analyze for the presence and orientation of your insert.We recommend that you sequence your construct with the T7 Promoter and BGH Reverseprimers (Catalog nos. N560-02 and N575-02, respectively) to confirm that your gene is in thecorrect orientation for expression and contains an ATG and a stop codon. Please refer to thediagrams on pages 3-4 for the sequences and location of the priming sites. The primers areavailable separately from Invitrogen in 2 µg aliquots.Preparing aGlycerol StockOnce you have identified the correct clone, purify the colony and make a glycerol stock forlong-term storage. You should keep a DNA stock of your plasmid at -20°C.•Streak the original colony out on an LB plate containing 50 µg/ml ampicillin. Incubatethe plate at 37°C overnight.•Isolate a single colony and inoculate into 1-2 ml of LB containing 50 µg/ml ampicillin.•Grow the culture to mid-log phase (OD600 = 0.5-0.7).•Mix 0.85 ml of culture with 0.15 ml of sterile glycerol and transfer to a cryovial.•Store at -80°C.TransfectionIntroduction Once you have verified that your gene is cloned in the correct orientation and contains an initiation ATG and a stop codon, you are ready to transfect your cell line of choice. Werecommend that you include the positive control vector and a mock transfection (negativecontrol) to evaluate your results.Plasmid Preparation Plasmid DNA for transfection into eukaryotic cells must be clean and free from phenol and sodium chloride. Contaminants will kill the cells, and salt will interfere with lipids decreasing transfection efficiency. We recommend isolating plasmid DNA using the S.N.A.P.™ MiniPrep Kit (10-15 µg DNA, Catalog no. K1900-01), the S.N.A.P. ™MidiPrep Kit (10-200 µg DNA, Catalog no. K1910-01), or CsCl gradient centrifugation.Methods of Transfection For established cell lines (e.g. HeLa), please consult original references or the supplier of your cell line for the optimal method of transfection. We recommend that you follow exactly the protocol for your cell line. Pay particular attention to medium requirements, when to pass the cells, and at what dilution to split the cells. Further information is provided in Current Protocols in Molecular Biology (Ausubel et al., 1994).Methods for transfection include calcium phosphate (Chen and Okayama, 1987; Wigler et al., 1977), lipid-mediated (Felgner et al., 1989; Felgner and Ringold, 1989) and electroporation (Chu et al., 1987; Shigekawa and Dower, 1988). Invitrogen offers the Calcium Phosphate Transfection Kit (Catalog no. K2780-01) and a large selection of reagents for transfection. For more information, please refer to our World Wide Web site () or call Technical Service (see page 13).Positive Control pcDNA3.1/CAT is provided as a positive control vector for mammalian transfection and expression (see page 12) and may be used to optimize transfection conditions for yourcell line. The gene encoding chloramphenicol acetyl transferase (CAT) is expressed inmammalian cells under the control of the CMV promoter. A successful transfection willresult in CAT expression that can be easily assayed (see below).Assay for CAT Protein You may assay for CAT expression by ELISA assay, western blot analysis, fluorometric assay, or radioactive assay (Ausubel et al., 1994; Neumann et al., 1987). If you wish to detect CAT protein using western blot analysis, you may use the Anti-CAT Antiserum (Catalog no. R902-25) available from Invitrogen. Other kits to assay for CAT protein using ELISA assay are available from Roche Molecular Biochemicals (Catalog no. 1 363 727) and Molecular Probes (Catalog no. F-2900).Creation of Stable Cell LinesIntroduction The pcDNA3.1(+) and pcDNA3.1(-) vectors contain the neomycin resistance gene forselection of stable cell lines using neomycin (Geneticin®). We recommend that you testthe sensitivity of your mammalian host cell to Geneticin® as natural resistance variesamong cell lines. General information and guidelines are provided in this section for yourconvenience.Geneticin®Selective Antibiotic Geneticin® Selective Antibiotic blocks protein synthesis in mammalian cells by interfering with ribosomal function. It is an aminoglycoside, similar in structure to neomycin, gentamycin, and kanamycin. Expression of the bacterial aminoglycoside phosphotransferase gene (APH), derived from Tn5, in mammalian cells results in detoxification of Geneticin®(Southern and Berg, 1982).Geneticin®Selection Guidelines Geneticin® Selective Antibiotic is available from Invitrogen (Catalog no. 10486-025). Use as follows:•Prepare Geneticin® in a buffered solution (e.g. 100 mM HEPES, pH 7.3).•Use 100 to 800 µg/ml of Geneticin® in complete medium.•Calculate concentration based on the amount of active drug (check the lot label).•Test varying concentrations of Geneticin® on your cell line to determine theconcentration that kills your cells (see below). Cells differ in their susceptibility to Geneticin®.Cells will divide once or twice in the presence of lethal doses of Geneticin®, so the effects of the drug take several days to become apparent. Complete selection can take up to 3 weeks of growth in selective media.Determination of Antibiotic Sensitivity To successfully generate a stable cell line expressing your gene of interest frompcDNA3.1, you need to determine the minimum concentration of Geneticin® required to kill your untransfected host cell line. We recommend that you test a range of concentrations to ensure that you determine the minimum concentration necessary for your host cell line.1.Plate or split a confluent plate so the cells will be approximately 25% confluent.Prepare a set of 7 plates. Allow cells to adhere overnight.2.The next day, substitute culture medium with medium containing varyingconcentrations of Geneticin® (0, 50, 100, 200, 400, 600, 800 µg/ml Geneticin®).3.Replenish the selective media every 3-4 days, and observe the percentage of survivingcells.4.Count the number of viable cells at regular intervals to determine the appropriateconcentration of Geneticin® that prevents growth within 2-3 weeks after addition of Geneticin®.continued on next pagePossible Sites for Linearization of pcDNA3.1(+)Prior to transfection, we recommend that you linearize the pcDNA3.1(+) vector. Linearizing pcDNA3.1(+) will decrease the likelihood of the vector integrating into the genome in a way that disrupts the gene of interest or other elements required for expression in mammalian cells. The table below lists unique restriction sites that may be used to linearize your construct prior to transfection. Other unique restriction sites are possible. Be sure that your insert does not contain the restriction enzyme site you wish to use to linearize your vector.Enzyme Restriction Site (bp)Location SupplierBgl II12Upstream of CMV promoter Invitrogen, Catalog no. 15213-028 Mfe I161Upstream of CMV promoter New England BiolabsBst1107 I3236End of SV40 polyA AGS*, Fermentas, Takara, RocheMol. BiochemicalsEam1105 I4505Ampicillin gene AGS*, Fermentas, TakaraPvu I4875Ampicillin gene Invitrogen, Catalog no. 25420-019 Sca I4985Ampicillin gene Invitrogen, Catalog no. 15436-017 Ssp I5309bla promoter Invitrogen, Catalog no. 15458-011 *Angewandte Gentechnologie SystemePossible Sites for Linearization of pcDNA3.1(-)The table below lists unique restriction sites that may be used to linearize yourpcDNA3.1(-) construct prior to transfection. Other unique restriction sites are possible. Be sure that your insert does not contain the restriction enzyme site you wish to use to linearize your vector.Enzyme Restriction Site (bp)Location SupplierBgl II12Upstream of CMV promoter Invitrogen, Catalog no. 15213-028 Mfe I161Upstream of CMV promoter New England BiolabsBst1107 I3235End of SV40 polyA AGS*, Fermentas, Takara, RocheMol. BiochemicalsEam1105 I4504Ampicillin gene AGS*, Fermentas, TakaraPvu I4874Ampicillin gene Invitrogen, Catalog no. 25420-019 Sca I4984Ampicillin gene Invitrogen, Catalog no. 15436-017 Ssp I5308bla promoter Invitrogen, Catalog no. 15458-011 *Angewandte Gentechnologie Systemecontinued on next pageSelection of Stable Integrants Once you have determined the appropriate Geneticin® concentration to use for selection in your host cell line, you can generate a stable cell line expressing your gene of interest.1.Transfect your mammalian host cell line with your pcDNA3.1 construct using thedesired protocol. Remember to include a plate of untransfected cells as a negativecontrol and the pcDNA3.1/CAT plasmid as a positive control.2.24 hours after transfection, wash the cells and add fresh medium to the cells.3.48 hours after transfection, split the cells into fresh medium containing Geneticin® atthe pre-determined concentration required for your cell line. Split the cells such that they are no more than 25% confluent.4.Feed the cells with selective medium every 3-4 days until Geneticin®-resistant foci canbe identified.5.Pick and expand colonies in 96- or 48-well plates.AppendixpcDNA3.1 VectorsMap ofpcDNA3.1(+) and pcDNA3.1(-)The figure below summarizes the features of the pcDNA3.1(+) and pcDNA3.1(-) vectors.The complete sequences for pcDNA3.1(+) and pcDNA3.1(-) are available for down-loading from our World Wide Web site ( ) or from Technical Service (see page 13). Details of the multiple cloning sites are shown on page 3 for pcDNA3.1(+) and page 4 for pcDNA3.1(-).Comments for pcDNA3.1 (+) 5428 nucleotidesCMV promoter: bases 232-819T7 promoter/priming site: bases 863-882Multiple cloning site: bases 895-1010BGH polyadenylation sequence: bases 1028-1252f1 origin: bases 1298-1726SV40 early promoter and origin: bases 1731-2074Neomycin resistance gene (ORF): bases 2136-2930SV40 early polyadenylation signal: bases 3104-3234pUC origin: bases 3617-4287 (complementary strand)Ampicillin resistance gene (bla ): bases 4432-5428 (complementary strand) ORF: bases 4432-5292 (complementary strand)Ribosome binding site: bases 5300-5304 (complementary strand) bla promoter (P3): bases 5327-5333 (complementary strand)I I (+)( )continued on next pagepcDNA3.1 Vectors, continuedFeatures of pcDNA3.1(+) and pcDNA3.1(-)pcDNA3.1(+) (5428 bp) and pcDNA3.1(-) (5427 bp) contain the following elements. All features have been functionally tested.Feature BenefitHuman cytomegalovirus (CMV)immediate-early promoter/enhancerPermits efficient, high-level expression ofyour recombinant protein (Andersson et al.,1989; Boshart et al., 1985; Nelson et al.,1987)T7 promoter/priming site Allows for in vitro transcription in the senseorientation and sequencing through theinsertMultiple cloning site in forward orreverse orientationAllows insertion of your gene andfacilitates cloningBovine growth hormone (BGH)polyadenylation signalEfficient transcription termination andpolyadenylation of mRNA (Goodwin andRottman, 1992)f1 origin Allows rescue of single-stranded DNASV40 early promoter and origin Allows efficient, high-level expression ofthe neomycin resistance gene and episomalreplication in cells expressing SV40 large TantigenNeomycin resistance gene Selection of stable transfectants inmammalian cells (Southern and Berg,1982)SV40 early polyadenylation signal Efficient transcription termination andpolyadenylation of mRNApUC origin High-copy number replication and growthin E. coliAmpicillin resistance gene (β-lactamase)Selection of vector in E. colipcDNA3.1/CATDescription pcDNA3.1/CAT is a 6217 bp control vector containing the gene for CAT. It wasconstructed by digesting pcDNA3.1(+) with Xho I and Xba I and treating with Klenow.An 800 bp Hin d III fragment containing the CAT gene was treated with Klenow and thenligated into pcDNA3.1(+).Map of Control Vector The figure below summarizes the features of the pcDNA3.1/CAT vector. The complete nucleotide sequence for pcDNA3.1/CAT is available for downloading from our World Wide Web site () or by contacting Technical Service (see page 13).Comments for pcDNA3.1(+)/CAT6217 nucleotidesCMV promoter: bases 232-819CAT ORF: bases 1027-1686pcDNA3.1/BGH reverse priming site: bases 1811-1828BGH polyadenylation sequence: bases 1817-2041f1 origin: bases 2087-2515SV40 early promoter and origin: bases 2520-2863Neomycin resistance gene (ORF): bases 2925-3719SV40 early polyadenylation sequence: bases 3893-4023pUC origin: bases 4406-5076 (complementary strand)Ampicillin resistance gene (ORF): bases 5221-6081 (complementary strand)Technical ServiceVisit the Invitrogen Web Resource using your World Wide Web browser. At the site, youcan:•Get the scoop on our hot new products and special product offers•View and download vector maps and sequences•Download manuals in Adobe® Acrobat® (PDF) format•Explore our catalog with full color graphics•Obtain citations for Invitrogen products•Request catalog and product literatureOnce connected to the Internet, launch your web browser (Internet Explorer 5.0 or neweror Netscape 4.0 or newer), then enter the following location (or URL):...and the program will connect directly. Click on underlined text or outlined graphics toexplore. Don't forget to put a bookmark at our site for easy reference!Contact us For more information or technical assistance, please call, write, fax, or email. Additionalinternational offices are listed on our web page ().United States Headquarters:Japanese Headquarters European Headquarters:Invitrogen Corporation Invitrogen Japan K.K.Invitrogen Ltd1600 Faraday Avenue Nihonbashi Hama-Cho Park Bldg. 4F 3 Fountain DriveCarlsbad, CA 92008 USA2-35-4, Hama-Cho, Nihonbashi Inchinnan Business ParkTel: 1 760 603 7200Tel: 81 3 3663 7972Paisley PA4 9RF, UKTel (Toll Free): 1 800 955 6288Fax: 81 3 3663 8242Tel (Free Phone Orders): 0800 269 210 Fax: 1 760 602 6500E-mail: jpinfo@ Tel (General Enquiries): 0800 5345 5345 E-mail:Fax: +44 (0) 141 814 6287tech_service@ E-mail: eurotech@MSDS Requests To request an MSDS, please visit our web site () and follow theinstructions below.1.On the home page, go to the left-hand column under ‘Technical Resources’ andselect ‘MSDS Requests’.2.Follow instructions on the page and fill out all the required fields.3.To request additional MSDSs, click the ‘Add Another’ button.4.All requests will be faxed unless another method is selected.5.When you are finished entering information, click the ‘Submit’ button. Your MSDSwill be sent within 24 hours.continued on next pageTechnical Service, continuedEmergency Information In the event of an emergency, customers of Invitrogen can call the 3E Company, 24 hours a day, 7 days a week for disposal or spill information. The 3E Company can also connect the customer with poison control or with the University of California at San Diego Medical Center doctors.3E CompanyVoice: 1-760-602-8700Limited Warranty Invitrogen is committed to providing our customers with high-quality goods and services. Our goal is to ensure that every customer is 100% satisfied with our products and our service. If you shouldhave any questions or concerns about an Invitrogen product or service, please contact ourTechnical Service Representatives.Invitrogen warrants that all of its products will perform according to the specifications stated on thecertificate of analysis. The company will replace, free of charge, any product that does not meetthose specifications. This warranty limits Invitrogen Corporation’s liability only to the cost of theproduct. No warranty is granted for products beyond their listed expiration date. No warranty isapplicable unless all product components are stored in accordance with instructions. Invitrogenreserves the right to select the method(s) used to analyze a product unless Invitrogen agrees to aspecified method in writing prior to acceptance of the order.Invitrogen makes every effort to ensure the accuracy of its publications, but realizes that theoccasional typographical or other error is inevitable. Therefore Invitrogen makes no warranty ofany kind regarding the contents of any publications or documentation. If you discover an error inany of our publications, please report it to our Technical Service Representatives.Invitrogen assumes no responsibility or liability for any special, incidental, indirect orconsequential loss or damage whatsoever. The above limited warranty is sole and exclusive.No other warranty is made, whether expressed or implied, including any warranty ofmerchantability or fitness for a particular purpose.ReferencesAndersson, S., Davis, D. L., Dahlbäck, H., Jörnvall, H., and Russell, D. W. (1989). Cloning, Structure, andExpression of the Mitochondrial Cytochrome P-450 Sterol 26-Hydroxylase, a Bile Acid Biosynthetic Enzyme. J.Biol. Chem. 264, 8222-8229.Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A., and Struhl, K. (1994).Current Protocols in Molecular Biology (New York: Greene Publishing Associates and Wiley-Interscience).Boshart, M., Weber, F., Jahn, G., Dorsch-Häsler, K., Fleckenstein, B., and Schaffner, W. (1985). A Very Strong Enhancer is Located Upstream of an Immediate Early Gene of Human Cytomegalovirus. Cell 41, 521-530.Chen, C., and Okayama, H. (1987). High-Efficiency Transformation of Mammalian Cells by Plasmid DNA. Mol.Cell. Biol. 7, 2745-2752.Chu, G., Hayakawa, H., and Berg, P. (1987). Electroporation for the Efficient Transfection of Mammalian Cells with DNA. Nuc. Acids Res. 15, 1311-1326.Felgner, P. L., Holm, M., and Chan, H. (1989). Cationic Liposome Mediated Transfection. Proc. West. Pharmacol.Soc. 32, 115-121.Felgner, P. L., and Ringold, G. M. (1989). Cationic Liposome-Mediated Transfection. Nature 337, 387-388.Goodwin, E. C., and Rottman, F. M. (1992). The 3´-Flanking Sequence of the Bovine Growth Hormone GeneContains Novel Elements Required for Efficient and Accurate Polyadenylation. J. Biol. Chem. 267, 16330-16334. Kozak, M. (1987). An Analysis of 5´-Noncoding Sequences from 699 Vertebrate Messenger RNAs. Nuc. Acids Res.15, 8125-8148.Kozak, M. (1991). An Analysis of Vertebrate mRNA Sequences: Intimations of Translational Control. J. Cell Biol.115, 887-903.Kozak, M. (1990). Downstream Secondary Structure Facilitates Recognition of Initiator Codons by Eukaryotic Ribosomes. Proc. Natl. Acad. Sci. USA 87, 8301-8305.Nelson, J. A., Reynolds-Kohler, C., and Smith, B. A. (1987). Negative and Positive Regulation by a Short Segmentin the 5´-Flanking Region of the Human Cytomegalovirus Major Immediate-Early Gene. Mol. Cell. Biol. 7, 4125-4129.Neumann, J. R., Morency, C. A., and Russian, K. O. (1987). A Novel Rapid Assay for Chloramphenicol Acetyltransferase Gene Expression. BioTechniques 5, 444-447.Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, Second Edition (Plainview, New York: Cold Spring Harbor Laboratory Press).Shigekawa, K., and Dower, W. J. (1988). Electroporation of Eukaryotes and Prokaryotes: A General Approach tothe Introduction of Macromolecules into Cells. BioTechniques 6, 742-751.Southern, P. J., and Berg, P. (1982). Transformation of Mammalian Cells to Antibiotic Resistance with a BacterialGene Under Control of the SV40 Early Region Promoter. J. Molec. Appl. Gen. 1, 327-339.Wigler, M., Silverstein, S., Lee, L.-S., Pellicer, A., Cheng, Y.-C., and Axel, R. (1977). Transfer of Purified HerpesVirus Thymidine Kinase Gene to Cultured Mouse Cells. Cell 11, 223-232.©1997-2001 Invitrogen Corporation. All rights reserved.15。
质粒图谱的阅读PPT课件

CHENLI
51
2021/3/7
CHENLI
52
2021/3/7
CHENLI
53
2021/3/7
CHENLI
54
2021/3/7
CHENLI
55
免疫法筛选
• 外源基因在细胞内表达出的蛋白质,可 与特异性的抗体结合。
2021/3/7
CHENLI
56
利用抗体筛选
原理 目的基因编码的蛋白质作为抗原,用特异
2021/3/7
CHENLI
33
氨苄青霉素 抗性基因
2021/3/7
CHENLI
复制起点
34
2021/3/7
CHENLI
35
2021/3/7
CHENLI
36
2.利用颜色筛选
插入失活现象
X-gal :5-溴-4-氯-3-吲哚-B-D-半乳糖苷
2021/3/7
CHENLI
37
多 克 隆 位 点
CHENLI
7
一.目的基因与载体的连接
Link of cohesive end
2021/3/7
粘末端连接
CHENLI
8
Link of blunt end
平末端连接
2021/3/7
CHENLI
9
平末端连接
vector
1
T4 DNA ligase
Recombinant DNA
2021/3/7
CHENLI
2021/3/7
CHENLI
40
2021/3/7
CHENLI
表示外源 基因插入 编码半乳 糖苷酶的 基因区段
41
Target gene
质粒图谱大全

(转载)一.九种表达载体Pllp-OmpA, pllp-STII, pMBP-P, pMBP-C,pET-GST, pET-Trx, pET-His, pET-CKS, pET-DsbA二.克隆载体pTZ19RDNApUC57DNAPMD18TPQE30pUC18pUC19pTrcHisApTrxFuspRSET-ApRSET-BpVAX1PBR322pbv220pBluescriptIIKS( )L4440pCAMBIA-1301pMAL-p2XpGD926三.PET系列表达载体ProteinExpression?ProkaryoticExpression?pETDsbFusionSystems39band40b ProteinExpression?ProkaryoticExpression?pETExpressionSystem33b ProteinExpression?ProkaryoticExpression?pETExpressionSystems ProteinExpression?ProkaryoticExpression?pETExpressionSystemsplusCompetentCells ProteinExpression?ProkaryoticExpression?pETGSTFusionSystems41and42 ProteinExpression?ProkaryoticExpression?pETNusAFusionSystems43.1and44 ProteinExpression?ProkaryoticExpression?pETVectorDNAProteinPurification?PurificationSystems?Strep?TactinResinsandPurificationKits四.PGEX系列表达载体TEcoR?pGEX-1I/BAPpGEX-2TpGEX-2TKpGEX-3XpGEX-4T-1pGEX-4T-2pGEX-4T-3pGEX-5X-1pGEX-5X-2pGEX-5X-3pGEX-6P-1pGEX-6P-2pGEX-6P-3五.PTYBsystemPTYB1PTYB2PTYB11PTYB12六.真核表达载体pCDNA3.1(-)pCDNA3.1( )pPICZalphaApGAPZαAPYES2.0pBI121pEGFP-N1pEGFP-C1pPIC9KpPIC3.5K如何阅读分析质粒图谱载体主要有病毒和非病毒两大类,其中质粒DNA是一种新的非病毒转基因载体。
实验新人必读(七):一文学会读懂和查找质粒图谱

实验新⼈必读(七):⼀⽂学会读懂和查找质粒图谱作者:解螺旋.⼦⾮鱼如需转载请注明来源:解螺旋·医⽣科研助⼿导语质粒图谱即为质粒DNA序列的物理图谱,包含了质粒⼤⼩、筛选标记、克隆位点、转录及翻译元件等信息。
尽管它为我们选择质粒、了解质粒特点及应⽤提供了重要依据,然⽽我们常常要为图谱中丰富信息所困扰。
那么,本⽂就为你拨云见⽇,让你快速掌握质粒图谱。
三步法看懂质粒图谱Step 1:先了解质粒的基本组成元素。
1)复制起始点Ori。
该位点决定了质粒的宿主及质粒的拷贝数,它是质粒中⼀段特定序列,富含AT和重复序列。
Tips:图谱上只有⼀个Ori,表⽰质粒是原核克隆表达质粒;有两个Ori,则表⽰该质粒是,穿梭质粒,即可在原核也可在真核中复制。
2)抗性筛选基因。
图谱中Kan/tet就是抗⽣素抗性基因,⽅便后续通过抗⽣素筛选阳性克隆。
特点就是单词最后会以⼤写R或上标r结束。
Tips:⼀般克隆载体只有⼀种抗性筛选标记,部分表达载体及穿梭质粒具有两种抗性筛选标记。
3)多克隆位点(MCS),即⼀系列限制性内切酶酶切位点,是外源DNA插⼊位点,⼀般可通过酶切/连接⽅式将外源DNA插⼊质粒,外源DNA⼀般⼩于10kb,⽽⽚段越长,转化效率越低。
Tips: ⼀般位于转录启动和转录终⽌信号之间;所包含的限制性内切酶位点数量和组成因载体不同会有所差异,且其中的酶切位点在质粒中为单⼀的酶切位点;同时在使⽤时需注意质粒载体与外源DNA酶切位点的兼容性问题4)荧光标记或蛋⽩标签序列。
蛋⽩纯化标签蛋⽩:His-Tag,GST-Tag等;蛋⽩检测标签蛋⽩:Myc-Tag,Flag-Tag,HA-Tag等;荧光蛋⽩表达标签:GFP,mCherry等。
Step 2:看质粒是否是表达载体,如果是,那就必定有这些原件:启动⼦-核糖体结合位点-克隆位点-转录终⽌信号。
1、启动⼦:促进DNA转录的DNA序列,可与RNApol特异性结合。
2、增强⼦/沉默⼦:前者是真核基因组中⼀种增强邻近基因转录过程的调控顺序,其作⽤与增强⼦所在位置或⽅向⽆关。
质粒图谱大全

转载一.九种表达载体Pllp-OmpA, pllp-STII, pMBP-P, pMBP-C,pET-GST, pET-Trx, pET-His, pET-CKS, pET-DsbA 二.克隆载体pTZ19RDNApUC57DNAPMD18TPQE30pUC18pUC19pTrcHisApTrxFuspRSET-ApRSET-BpVAX1PBR322pbv220pBluescriptIIKSL4440pCAMBIA-1301pGD926三.PET系列表达载体ProteinExpressionProkaryoticExpressionpETDsbFusionSystems39band40b ProteinExpressionProkaryoticExpressionpETExpressionSystem33b ProteinExpressionProkaryoticExpressionpETExpressionSystems ProteinExpressionProkaryoticExpressionpETExpressionSystemsplusCompetentCells ProteinExpressionProkaryoticExpressionpETGSTFusionSystems41and42 ProteinExpressionProkaryoticExpression ProteinExpressionProkaryoticExpressionpETVectorDNA ProteinPurificationPurificationSystemsStrepTactinResinsandPurificationKits四.PGEX系列表达载体TEcoR pGEX-1I/BAPpGEX-2TpGEX-2TKpGEX-3XpGEX-4T-1pGEX-4T-2pGEX-4T-3pGEX-5X-1pGEX-5X-3 pGEX-6P-1 pGEX-6P-2 pGEX-6P-3五.PTYBsystem PTYB1PTYB2PTYB11PTYB12六.真核表达载体-pPICZalphaA pGAPZαApBI121pEGFP-N1 pEGFP-C1pPIC9K如何阅读分析质粒图谱载体主要有病毒和非病毒两大类,其中质粒DNA是一种新的非病毒转基因载体;一、一个合格质粒的组成要素复制起始位点Ori 即控制复制起始的位点;原核生物DNA分子中只有一个复制起始点;而真核生物DNA分子有多个复制起始位点;抗生素抗性基因可以便于加以检测,如Amp ,Kan多克隆位点MCS克隆携带外源基因片段P/E 启动子/增强子Terms终止信号加polyA信号可以起到稳定mRNA作用二、如何阅读质粒图谱第一步:首先看Ori的位置,了解质粒的类型原核/真核/穿梭质粒第二步:再看筛选标记,如抗性,决定使用什么筛选标记;1Ampr水解β-内酰胺环,解除氨苄的毒性;2tetr可以阻止四环素进入细胞;3camr生成氯霉素羟乙酰基衍生物,使之失去毒性;4neorkanr氨基糖苷磷酸转移酶使G418长那霉素衍生物失活5hygr使潮霉素β失活;第三步:看多克隆位点MCS;它具有多个限制酶的单一切点;便于外源基因的插入;如果在这些位点外有外源基因的插入,会导致某种标志基因的失活,而便于筛选;决定能不能放目的基因以及如何放置目的基因;第四步:再看外源DNA插入片段大小;质粒一般只能容纳小于10Kb的外源DNA片段;一般来说,外源DNA片段越长,越难插入,越不稳定,转化效率越低;第五步:是否含有表达系统元件,即启动子-核糖体结合位点-克隆位点-转录终止信号;这是用来区别克隆载体与表达载体;克隆载体中加入一些与表达调控有关的元件即成为表达载体;选用那种载体,还是要以实验目的为准绳;启动子-核糖体结合位点-克隆位点-转录终止信号启动子-促进DNA转录的DNA顺序,这个DNA区域常在基因或操纵子编码顺序的上游,是DNA 分子上可以与RNApol特异性结合并使之开始转录的部位,但启动子本身不被转录;增强子/沉默子-为真核基因组包括真核病毒基因组中的一种具有增强邻近基因转录过程的调控顺序;其作用与增强子所在的位置或方向无关;即在所调控基因上游或下游均可发挥作用;/沉默子-负增强子,负调控序列;核糖体结合位点/起始密码/SD序列Rbs/AGU/SDs:mRNA有核糖体的两个结合位点,对于原核而言是AUG起始密码和SD序列;转录终止顺序终止子/翻译终止密码子:结构基因的最后一个外显子中有一个AATAAA的保守序列,此位点down-stream有一段GT或T富丰区,这2部分共同构成polyA加尾信号;结构基因的最后一个外显子中有一个AATAAA的保守序列,此位点down-stream有一段GT或T 富丰区,这2部分共同构成polyA加尾信号;回答有人之前提出的一个问题:为什么质粒图谱上有的箭头顺时针有的箭头逆时针,那其实是代表两条DNA链,即质粒是环状双链DNA,它的启动子等在其中一条链上,而它的抗性基因在另一条链上.三、介绍一下关于载体的知识虽然课本上都有写1. 什么是载体即要把一个有用的基因目的基因——研究或应用基因通过基因工程手段送到生物细胞受体细胞,需要运载工具交通工具携带外源基因进入受体细胞,这种运载工具就叫做载体vector;.基因工程所用的vector实际上是DNA分子,是用来携带目的基因片段进入受体细胞的DNA2. 载体的分类―――按功能分成:1克隆载体都有一个松弛的复制子,能带动外源基因,在宿主细胞中复制扩增;它是用来克隆和扩增DNA片段基因的载体;所以有时实验时扩增效率低下,要注意是不是使用的严谨行载体2表达载体具有克隆载体的基本元件ori,Ampr,Mcs等还具有转录/翻译所必需的DNA顺序的载体;―――按进入受体细胞类型分:1原核载体2真核载体3穿梭载体sbuttlevector指在两种宿主生物体内复制的载体分子,因而可以运载目的基因穿梭往返两种生物之间..穿梭质粒含原核和真核生物2个复制子,以确保两类细胞中都能扩增;3. 基因工程载体的3个特点:一都能独立自主的复制:载体DNA分子中有一段不影响它们扩增的非必需区域,如MCS,插在其中的外源DNA片段,能被动的跟着载体一起复制/扩增,就像载体的正常成分一样;二都能便利的加以检测:如载体的药物抗性基因,多是抗生素抗性基因,将受体细胞放在含有该抗生素培养板上培养生长时,只有携带这些抗性基因的载体分子的受体细胞才能存活;三都能容易进入宿主细胞中去,也易从宿主细胞中分离纯化出来;4. 载体的选择和制备:选择载体主要依据构建的目的,同时要考虑载体中应有合适的限制酶切位点;如果构建的目的是要表达一个特定的基因,则要选择合适的表达载体;载体选择主要考虑下述3点:1构建DNA重组体的目的,克隆扩增/表达表达,选择合适的克隆载体/表达载体;2.载体的类型:1克隆载体的克隆能力-据克隆片段大小大选大,小选小;如<10kb选质粒;2表达载体据受体细胞类型-原核/真核/穿梭,哺乳类细胞表达载体;3对原核表达载体应该注意3点:①选择合适的启动子及相应的受体菌;②用于表达真核蛋白质时注意克服4个困难和阅读框错位;③表达天然蛋白质或融合蛋白作为相应载体的参考;3载体MCS中的酶切位点数与组成方向因载体不同而异,适应目的基因与载体易于链接,不产生阅读框架错位;选用质粒最常用做载体的4点要求:①选分子量小的质粒,即小载体1-→不易损坏,在细菌里面拷贝数也多也有大载体;②一般使用松弛型质粒在细菌里扩增不受约束,一般10个以上的拷贝,而严谨型质粒<10个;③必需具备一个以上的酶切位点,有选择的余地;④必需有易检测的标记,多是抗生素的抗性基因,不特指多位Ampr试一试;无论选用哪种载体,首先都要获得载体分子,然后采用适当的限制酶将载体DNA进行切割,获得分子,以便于与目的基因片段进行连接。
质粒图谱怎么看

一、质粒(plasmid):存在于许多细菌以及酵母菌等生物中,是染色体外能够自主复制的很小的环状DNA分子。
载体(Vector):简单的来说就是把一个有用的目的DNA片段通过重组DNA技术,送进受体细胞中去进行繁殖和表达的工具叫载体,分为病毒类和非病毒类两种。
我们平时常说的载体是在天然质粒的基础上为适应实验室操作而人工构建的,通常带有一个或一个以上的选择性标记基因(如抗生素抗性基因)和一个人工合成的含有多个限制性内切酶识别位点的多克隆位点序列,并去掉了大部分非必需序列,使分子量尽可能减少,以便于基因工程操作,同时加入一些多用途的辅助序列。
二、如何阅读质粒图谱看懂一个质粒图谱我们需要关注以下几点:1)弄清楚质粒的方向看质粒图谱的时候我们会发现大多数质粒都会有顺时针和逆时针两个方向的箭头,箭头的方向:一个方向是复制起始位点的方向,即该质粒在细菌或真菌中DNA复制的一个方向,有时图谱中还会有f1 ori,这个代表的是噬菌体的复制起始方向,只能复制出单链的DNA,但是可以用来测序。
另一个就是转录方向(一般是正向),主要是从启动子开始,即启动子-核糖体结合位点-克隆位点-转录终止信号。
看懂转录的方向,这样就方便设计插入片段的位置和方向性。
2)复制子复制子又称复制起始区,它控制着质粒DNA的复制,并决定了质粒的宿主和拷贝数。
复制子可分为严谨型复制子与松弛型复制子,分别对应低拷贝数的严紧型质粒和高拷贝数的松弛型质粒。
3)筛选标记:了解筛选标记类型(如抗生素抗性标记),方便后续确定筛选重组质粒载体。
常见抗菌素抗性标记有氨苄青霉素抗性(Amp)、卡那霉素抗性(Kan)、四环素抗性(Tet)、链霉素抗性(Str)、氯霉素(Cmr, 某些酵母表达质粒)、潮霉素(Hyg, 农杆菌里常用的)。
4)多克隆位点MCS区:既外源基因的插入位点。
具有多个限制酶酶切位点,外源性的DNA一般可通过酶切/连接的方式插入质粒。
)其他元件,如表达系统元件和蛋白标签等常见的启动子有:CMV、EF1(常规表达,一般启动长片段的),H1、U6(启动短片段的,比如shRNA),CAMV35S、Ubi(植物表达常用),T7(常用原核系统表达启动子)。
质粒图谱——精选推荐

1)质粒图谱登记号:00012)质粒名称:pIRES3)来源:BD Co4)用途:真核双表达5)是否可以提供更详细资料:可以6)是否可以共享:7)联系方式:PM)质粒图谱登记号:00022)质粒名称:pECFP-C13)来源:BD Co4)用途:检测真核表达5)是否可以提供更详细资料:可以6)是否可以共享:7)联系方式:pm1)质粒图谱登记号:00032)质粒名称:pShuttle3)来源:4)用途:5)是否可以提供更详细资料:6)是否可以共享:7)联系方式:2)pShuttle MCS很多人用pEGFP-C1,我也来发一个)质粒图谱登记号:00042)质粒名称:pS BR322、pUC183)来源:4)用途:5)是否可以提供更详细资料:6)是否可以共享:7)联系方式:1)质粒图谱登记号:00052)质粒名称:pcDNA3.1(+)/CAT3)来源:invitrogen4)用途:真核表达5)是否可以提供更详细资料:可以6)是否可以共享:无偿7)联系方式:PM1)质粒图谱登记号:00062)质粒名称:pQEx3)来源:Qiagen4)用途:原核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM2)1)质粒图谱登记号:00072)质粒名称:pIVEX2.33)来源:Rocho4)用途:体外转录翻译5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM质粒图谱登记号:0008质粒名称:pIRES-EGFP来源:用途:是否可以提供更详细资料:是否可以共享:否联系方式:1)质粒图谱登记号:00092)质粒名称:pET-28a(+)3)来源:Novagen4)用途:原核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM1)质粒图谱登记号:00112)质粒名称:pET-32a(+)3)来源:novagen4)用途:原核表达5)是否可以提供更详细资料:6)是否可以共享:7)联系方式:pm1)质粒图谱登记号:00132)质粒名称:pcDNA3.1/Zeo (+)3)来源:invitrogen4)用途:原核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM)质粒图谱登记号:00132)质粒名称:pEGFP-N33)来源:clontech4)用途:真核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM)质粒图谱登记号:00142)质粒名称:pcDNA33)来源:invitrogen4)用途:真核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM)质粒图谱登记号:00152)质粒名称:pfastbac13)来源:invitrogene4)用途:昆虫表达5)是否可以提供更详细资料:不可以6)是否可以共享:不可以7)联系方式:PM)质粒图谱登记号:00162)质粒名称:pEGFP-C33)来源:clontech4)用途:真核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PMwangjun2002274 edited on 2004-06-22 00:041)质粒图谱登记号:00172)质粒名称:pSecTag23)来源:Invitrogen4)用途:真核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM)1质粒图谱登记号:00192)质粒名称:pET20b3)来源:NOVAGEN4)用途:原核表达5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM1)质粒图谱登记号:00222)质粒名称:pThioHisA3)来源:invitrogen4)用途:原核表达5)是否可以提供更详细资料:4.365kb , HP-thioredoxin fusion proteinexpressionvector, trc promoter, Ampr, a EK cleavage site lies between HP-thioredoxin and MCS宿主菌TOP10(基因型为:F-mcrA △(mrr-hsd RMS-mcrBC)Ф80 lacZ M15 △lacX74 deoR recAl araD139 △(ara-leu)7697 galU galK rpsL endAl nupG6)是否可以共享:交换或其它7)联系方式:PM2)3)1)质粒图谱登记号:00242)质粒名称:pcDNA3.1-Myc-His-A-3)来源:invitrogen4)用途:真核核表达4))质粒图谱登记号:00252)质粒名称:pS UPER.neo3)来源:4)用途:siRNA5)是否可以提供更详细资料:可以6)是否可以共享:交换7)联系方式:PM)质粒图谱登记号:00312)质粒名称:pSilencer1.0-siRNA3)来源:Ambion4)用途:RNAi5)是否可以提供更详细资料:/techlib/Documents.html?fkResSxn=7&fkSub Sxn=236)是否可以共享:实验结束后,可提供含shRNA模板的质粒7)联系方式:pm5))质粒图谱登记号:00322)质粒名称:pSilencer2.0-U6siRNA3)来源:Ambion4)用途:RNAi,与1.0相比,可以建立稳转株5)更详细资料:/techlib/prot/fm_7209.pdf6)是否可以共享:交换7)联系方式:pm6)会员名:mlluoE-mail:*************可提供试验资源名称和简要介绍:pSilencer 3.1-H1 neo Vector,是Ambion公司目前最高版本的shRNA 载体,为扩增此载体我已插入目的片段,如有战友需要,将此片段双酶切再连上自己的片段即可。
质粒图谱大全之欧阳索引创编

(转载)欧阳家百(2021.03.07)一.九种表达载体Pllp-OmpA,pllp-STII,pMBP-P,pMBP-C,pET-GST,pET-Trx,pET-His,pET-CKS,pET-DsbA 二.克隆载体pTZ19RDNApUC57DNAPMD18TPQE30pUC18pUC19pTrcHisApTrxFuspRSET-ApRSET-BpVAX1PBR322pbv220pBluescriptIIKS( )L4440pCAMBIA-1301pMAL-p2XpGD926三.PET系列表达载体ProteinExpression?ProkaryoticExpression?pETDsbFusionSystems39 band40bProteinExpression?ProkaryoticExpression?pETExpressionSystem33b ProteinExpression?ProkaryoticExpression?pETExpressionSystems ProteinExpression?ProkaryoticExpression?pETExpressionSystemsplu sCompetentCellsProteinExpression?ProkaryoticExpression?pETGSTFusionSystems41 and42ProteinExpression?ProkaryoticExpression?pETNusAFusionSystems4 3.1and44ProteinExpression?ProkaryoticExpression?pETVectorDNA ProteinPurification?PurificationSystems?Strep?TactinResinsandPurifi cationKits四.PGEX系列表达载体TEcoR?pGEX-1I/BAP pGEX-2TpGEX-2TKpGEX-4T-1 pGEX-4T-2 pGEX-4T-3 pGEX-5X-1 pGEX-5X-2 pGEX-5X-3 pGEX-6P-1 pGEX-6P-2 pGEX-6P-3五.PTYBsystem PTYB1PTYB2PTYB11PTYB12六.真核表达载体pCDNA3.1(-) pCDNA3.1( ) pPICZalphaA pGAPZαA PYES2.0pBI121pEGFP-C1pPIC9KpPIC3.5K如何阅读分析质粒图谱载体主要有病毒和非病毒两大类,其中质粒DNA是一种新的非病毒转基因载体。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
一、如何阅读质粒图谱载体主要有病毒和非病毒两大类,其中质粒DNA是一种新的非病毒转基因载体。
一、一个合格质粒的组成要素复制起始位点Ori,即控制复制起始的位点。
原核生物DNA分子中只有一个复制起始点。
而真核生物DNA分子有多个复制起始位点。
抗生素抗性基因:可以便于加以检测,如Amp+ ,Kan+多l克隆位点:MCS克隆携带外源基因片段P/E:启动子/增强子Terms:终止信号加poly(A)信号:可以起到稳定mRNA作用二、如何阅读质粒图谱第一步:首先看Ori的位置,了解质粒的类型(原核/真核/穿梭质粒)Ori的箭头指复制方向,其他元件标注的箭头多指转录方向(正向)。
第二步:再看筛选标记,如抗性,决定使用什么筛选标记:(1)Ampr:水解β-内酰胺环,解除氨苄的毒性。
(2)tetr :可以阻止四环素进入细胞。
(3)camr:生成氯霉素羟乙酰基衍生物,使之失去毒性。
(4)neor(kanr):氨基糖苷磷酸转移酶,使G418(卡那霉素衍生物)失活。
(5)hygr:使潮霉素β失活。
第三步:看多克隆位点(MCS)。
它具有多个限制酶的单一切点,便于外源基因的插入。
如果在这些位点外有外源基因的插入,会导致某种标志基因的失活,而便于筛选。
决定能不能放目的基因以及如何放置目的基因。
第四步:再看外源DNA插入片段大小。
质粒一般只能容纳小于10Kb的外源DNA片段。
一般来说,外源DNA片段越长,越难插入,越不稳定,转化效率越低。
第五步:是否含有表达系统元件,即启动子-核糖体结合位点-克隆位点-转录终止信号。
这是用来区别克隆载体与表达载体。
克隆载体中加入一些与表达调控有关的元件即成为表达载体。
选用那种载体,还是要以实验目的为准绳。
二、相关概念:启动子-核糖体结合位点-克隆位点-转录终止信号启动子-促进DNA转录的DNA顺序,这个DNA区域常在基因或操纵子编码顺序的上游,是DNA分子上可以与RNApol特异性结合并使之开始转录的部位,但启动子本身不被转录。
增强子/沉默子-为真核l基因组(包括真核病毒基因组)中的一种具有增强邻近基因转录过程的调控顺序。
其作用与增强子所在的位置或方向无关。
即在所调控基因上游或下游均可发挥作用。
沉默子-负增强子,负调控序列。
核糖体结合位点/起始密码/SD序列(Rbs/AGU/SDs):mRNA有核糖体的两个结合位点,对于原核而言是AUG(起始密码)和SD序列。
l转录终止顺序(终止子)/翻译终止密码子:结构基因的最后一个外显子中有一个AATAAA 的保守序列,此位点down-stream有一段GT或T富丰区,这2部分共同构成poly(A)加尾信号。
结构基因的最后一个外显子中有一个AATAAA的保守序列,此位点down-stream有一段GT或T富丰区,这2部分共同构成poly(A)加尾信号。
三、载体及其分类载体:即要把一个有用的基因(目的基因——研究或应用基因)通过基因工程手段送到生物细胞(受体细胞),需要运载工具(交通工具)携带外源基因进入受体细胞,这种运载工具就叫做载体(vector)。
P.S.基因工程所用的vector实际上是DNA分子,是用来携带目的基因片段进入受体细胞的DNA。
载体的分类按功能分成:(1)克隆载体:都有一个松弛的复制子,能带动外源基因,在宿主细胞中复制扩增。
它是用来克隆和扩增DNA片段(基因)的载体。
(所以有时实验时扩增效率低下,要注意是不是使用的严谨型载体)(2)表达载体:具有克隆载体的基本元件(ori,Ampr,Mcs 等)还具有转录/翻译所必需的DNA顺序的载体。
按进入受体细胞类型分:(1)原核载体(2)真核载体(3)穿梭载体(sbuttle vector)指在两种宿主生物体内复制的载体分子,因而可以运载目的基因(穿梭往返两种生物之间)。
P.S. 穿梭质粒含原核和真核生物2个复制子,以确保两类细胞中都能扩增。
基因工程载体的3个特点:(一)都能独立自主的复制:载体DNA分子中有一段不影响它们扩增的非必需区域,如MCS,插在其中的外源DNA片段,能被动的跟着载体一起复制/扩增,就像载体的正常成分一样。
(二)都能便利的加以检测:如载体的药物抗性基因,多是抗生素抗性基因,将受体细胞放在含有该抗生素培养板上培养生长时,只有携带这些抗性基因的载体分子的受体细胞才能存活。
(三)都能容易进入宿主细胞中去,也易从宿主细胞中分离纯化出来。
四、载体的选择和制备选择载体主要依据构建的目的,同时要考虑载体中应有合适的限制酶切位点。
如果构建的目的是要表达一个特定的基因,则要选择合适的表达载体。
载体选择主要考虑下述3点:1、构建DNA重组体的目的,克隆扩增/表达表达,选择合适的克隆载体/表达载体。
2、载体的类型:(1)克隆载体的克隆能力-据克隆片段大小(大选大,小选小)。
如小于10kb选质粒。
(2)表达载体据受体细胞类型-原核/真核/穿梭,E.coli/哺乳类细胞表达载体。
(3)对原核表达载体应该注意3点:①选择合适的启动子及相应的受体菌;②用于表达真核蛋白质时注意克服4个困难和阅读框错位;③表达天然蛋白质或融合蛋白作为相应载体的参考。
3、载体MCS中的酶切位点数与组成方向因载体不同而异,适应目的基因与载体易于链接,不产生阅读框架错位。
选用质粒(最常用)做载体的4点要求:①选分子量小的质粒,即小载体(1-1.5kb)→不易损坏,在细菌里面拷贝数也多(也有大载体);②一般使用松弛型质粒在细菌里扩增不受约束,一般10个以上的拷贝,而严谨型质粒小于10个。
③必需具备一个以上的酶切位点,有选择的余地;④必需有易检测的标记,多是抗生素的抗性基因,不特指多位Ampr(试一试)。
无论选用哪种载体,首先都要获得载体分子,然后采用适当的限制酶将载体DNA进行切割,获得分子,以便于与目的基因片段进行连接。
ori是复制起始点,细的黑箭头是几个不同的转录区,其箭头方向不同,说明每个表达产物(如kan抗性基因、LacI等)都有独立的promoter,有时与T7 promoter方向相反。
粗的黑箭头是MCS,用于目的基因的插入,箭头方向表明目的基因的转录方向,它的转录方向可以与其它几个不同的转录区相同,也可以不同,如Kan抗性基因、LacI的方向是一样的,可能与调控相关,不同的载体是不一样的。
一个载体可只看它的启动子到终止子那一段,其它的可以考虑少些。
五、pET表达菌株的相关信息(DE3 )指宿主为λ DE3 溶原菌,其染色体上带有一拷贝由lacUV5 启动子控制的T7 RNA 聚合酶基因。
这类菌株适用于从克隆到pET载体的目标基因生产蛋白。
命名为pLysS 和pLysE 的宿主菌带有编码T7 溶菌酶(为T7 RNA 聚合酶的天然抑制物)的pET 相容性质粒。
带有pLysS 的细胞产生少量溶菌酶,而pLysE 宿主菌产生更大量酶。
这些菌株用于在诱导前抑制T7 RNA 聚合酶的基础表达,这样可以稳定编码影响细胞生长和活力的目标蛋白的pET 重组体。
带有pLacI 的宿主菌产生额外的抑制pETBlue 和pTriEx 载体基础表达的lac 阻遏蛋白。
λ DE3 溶原化试剂盒用于制备其它遗传背景的新表达宿主菌。
AD494 菌株为硫氧还蛋白还原酶( trxB ) 突变菌株,能够在胞浆内形成二硫键,提供了生产正确折迭的活性蛋白的潜力。
TrxB 突变可用卡那霉素选择,因此该菌株建议用于带氨苄抗性标记bla 的质粒。
B834 为BL21 的亲本菌株。
这些蛋白酶缺陷宿主菌为甲硫氨酸营养缺陷型,可用35 S- 甲硫氨酸和硒代甲硫氨酸对目标蛋白进行高特异活性标记,从而用于结晶学研究。
BL21 应用最广的宿主菌来源,具有lon 和ompT 蛋白酶缺陷的优点。
BL21 TrxB 菌株在蛋白酶缺陷BL21 背景上具有与AD494 菌株相同的硫氧还蛋白还原酶突变( trxB ) 。
由于trxB 宿主有利于胞浆内二硫键形成,它们的使用可增加正确折迭的蛋白组分。
TrxB 突变可用卡那霉素选择,因此该菌株建议用于带氨苄抗性标记bla 的质粒。
BLR 为BL21 的recA - 衍生菌株,能够改善质粒单体产量,有助于稳定含有重复序列或其产物能够引起DE3 噬菌体丢失的目标质粒。
HMS174 菌株在K-12 背景上提供了recA 突变。
与BLR 一样,这些菌株能够稳定其产物能够引起DE3 噬菌体丢失的某些目标基因。
NovaBlue 适合用作初始克隆宿主菌的K-12 菌株,具有高转化效率、蓝/ 白斑筛选能力(与合适质粒)和导致优质质粒DNA 高产的recA endA 突变。
由于存在 F 附加体编码的lacI q 阻遏蛋白,NovaBlue 的DE3 溶原菌是一个非常有用的严紧型宿主菌。
Origami 为K-12 衍生的宿主菌,硫氧还蛋白还原酶突变( trxB ) 和谷胱甘肽还原酶( gor ) 基因均为突变,能够大大增强胞浆内二硫键的形成。
研究表明即使总体表达水平相似,Origami (DE3 )表达的活性蛋白比其它宿主菌高10 倍以上。
Origami 宿主菌与氨苄抗性质粒相容,可用于pET-32 载体,硫氧还蛋白标签能够进一步增强在胞浆内形成二硫键。
TrxB 和gor 突变可分别用卡那霉素和四环素选择,因此该菌株建议用于带氨苄抗性标记bla 的pET 质粒。
Origami B 宿主菌来源于BL21 lacZY 突变株,还带有与原始Origami 菌株相同的TrxB / gor 突变。
Origami B 菌株集BL21 、Tuner 和Origami 宿主菌的优点于一体。
TrxB 和gor 突变可分别用卡那霉素和四环素选择,因此该菌株建议用于带氨苄抗性标记bla 的pET 质粒。
Rosetta 宿主菌从BL21 衍生而来,可增强带有大肠杆菌稀有密码子的真核蛋白的表达。
该菌株通过一个相容性氯霉素抗性质粒补充密码子AUA 、AGG 、AGA 、CUA 、CCC 和GGA 的tRNAs 。
这样Rosetta 菌株提供了“万能”的翻译,从而避免因大肠杆菌密码子使用频率导致的表达限制。
tRNA 基因由它们的天然启动子驱动。
在Rosetta (DE3 )pLysS 和Rosetta (DE3 )pLacI 中,稀有tRNA 基因存在于分别带有T7 溶菌酶和lac 阻遏基因的同一质粒上。
Tuner 菌株为BL21 的lacZY 缺失突变株,能够调整培养物中所有细胞的蛋白表达水平。
lac 通透酶(lacY )突变使得IPTG 均匀进入群体所有细胞,从而具有浓度依赖、水平均一的诱导表达。
通过调整IPTG 浓度,表达可从极低水平调节到极强、完全诱导的表达水平(通常与pET 载体相关)。
低水平表达有时可能增强难表达蛋白的溶解性和活性。
