GP-12早期生产遏制程序
GP12产品质量先期遏制控制程序

过程所有者:GP12 多功能小组
输入: 顾客技术要求、顾 客图纸、项目计 划、技术指标
GP12 控制过程
输出: 技术规范 、控制计 划、检验规范、标准 化作业指导书、 产 品样件
过程衡量指标: GP12 计划遵守率
3.3 发运不合格产品结果: 1) 执行 GP12 失败将会得到受控发运等级 2 和其他可能的结果; 2) 发运不合格产品将会得到受控等级 2;
4 职责 GP12 检验人员执行本程序,质量经理必须监视其实施并决定是否满足退出原则。
5 工作程序: 5.1.1 项目工程师定期召集 APQP 多功能小组对已知的不合格品和废品、PRR 或在产品开发中出现的问题, 确定在 GP12 控制看板中,要求附加检验、测试等检验项目,以确保最新的不合格现象和 PRR 及时列入 GP12 检验。 5.1.2 项目工程师对已确定的 GP12 检测项目建立 GP12 检测项目清单和 GP12 检测项目反应措施。 5.2 GP12 检验: 5.2.1GP12 检验人员对应 GP12 检测项目清单对 GP12 待检区的成品进行 100%检验,并将检验结果填入 GP12 检测检验记录表中; 5.2.2 若检验的产品合格,应及时将合格产品转入 GP12 合格品区; 5.2.3 若检验的产品不合格,检验员按反应措施采取行动,检验出的问题应及时解决,不能及时解决的, 项目工程师应召集多功能小组分析问题的根本原因并采用“5Why”法报告形式解决问题;针对重复发生 的不合格项要采用“8D”报告形式解决;必要时,重新修改 PFMEA 和控制计划,以反映问题解决措施的 结果。 5.2.4 质量检验员每天的检测结果汇总并将结果张贴于 GP12 控制看板中。
早期生产遏制程序

早期生产遏制程序文件编号:KG/ZXGP12-01修订号:00受控状态:版本1早期生产遏制程序编制审核批准:会签2013年11月,发布2013年12月,实施该程序的目的是确保向客户交付的产品在早期生产阶段得到有效的控制和标识。
适用范围:所有生产件批准程序(PPAP)要求的新产品以及严重质量问题的量产产品,直到供方满足下面所定义的退出原则。
职责:1.所有早期遏制的检验人员应严格执行本规程。
2.质量经理必须监视早期遏制实施并且决定是否满足退出原则。
3.技术部项目工程师、品管负责质量问题的快速处理。
工作程序:流程如下:1.新产品试生产;2.新产品量产;3.有严重质量问题的产品。
小组成员:技术部的项目工程师、品管等组成早期生产遏制小组。
技术部项目工程师制定早期生产遏制控制计划,包括:1.功能检测、防错措施、测试和尺寸检查、附加检验项目等;2.检具、工装的制作、送检;3.检验报警准则。
技术部品管:前期准备:1.制定早期生产遏制;2.全检场地的布置(与生产线区分开来,单向流动;质量看板;现场培训设施);3.测量设备、量具的准备;4.检具的检定。
培训:1.技术部项目工程师对早期生产遏制全检人员进行检测项目的培训;2.技术部品管对全检人员进行量具、检具、记录填写的培训。
考核:技术部项目工程师、品管负责对全检人员进行实际操作考核。
全检人员按照《早期生产遏制全检作业指导书》对所检产品实施检验,并填写《全检记录表》:1.全检合格的产品贴早期生产遏制标签(视作业指导书要求在产品或产品外包装上贴标签),办理入库手续;2.全检不合格应立即进行标识、隔离;过程中,达到或超出报警准则时,通知品管部品管进行处理。
特别说明:1.现场不允许返工返修;2.标签:100%全检标贴。
销售部按照产品交付要求实施产品交付发运。
发运不合格产品结果:1.早期生产遏制执行失败将会得到受控发运等级2和其他可能的结果;2.发运不合格材料将会得到受控发运等级2.1.技术部品管针对不合格品进行处理,组织项目工程师对不合格品进行分析和解决。
APQP GP-12早期生产遏制程序

SUPPLIER RESPONSIBILITY: The supplier shall:供应商职责:供应商必须
•在重要时期防止我们的装配和制造中心及服务件仓库出现质量问 题
•Document the supplier‘s efforts to verify control of its processes during start-up, acceleration, after revisions to the manufacturing process, or when manufacturing runs are separated by 3 months or more记 录 供 应 商 在 开 始 生 产 、 加 速 生 产过 程 、生产过程 变更后或生产停产三个月或以上时 , 为 控 制 其 过程 所 做 的努 力
1 Increased frequency/sample size as stated in the Production Control Plan. 1在产品控制计划中增加的频次和样品容量
2 Verification of packaging and label requirements – including service and accessory part requirements, which may include country of origin labels on parts. 2确认包装和标签的要求包括服务件和附件的要求,可能包括产品原产国标签 3 Verification of the effectiveness of error proofing. 确认防错的有效性
GP12早期生产遏制操作指示

GP12早期生产遏制操作指示背景GP12是一种常见的早期生产遏制工具,用于防止产品在生产的早期阶段出现大规模的问题或缺陷。
本操作指示旨在提供关于如何正确使用GP12的指导,以确保生产过程中的高质量和有效性。
操作步骤1. 确定GP12的合适使用时机:在生产的早期阶段,即使用GP12之前,首先需要确定何时使用该工具。
通常情况下,GP12适用于以下情况:- 生产过程中出现异常情况或不明确的问题。
- 需要遏制可能会导致产品质量下降的风险。
2. 设定目标:在使用GP12之前,请确保明确设定操作的目标。
这些目标可以是如下之一:- 防止产品质量下降,确保产品符合质量标准。
- 尽早发现和解决生产过程中的问题,以减少后续修复的工作量和成本。
3. 准备GP12工具:确保GP12工具在使用前已经准备好,并处于可用状态。
按照相关指导手册的要求,检查工具的状态和功能。
4. 针对异常情况进行分析:在使用GP12之前,需要对生产过程中出现的异常情况进行分析。
在分析过程中,请思考以下问题:- 异常情况的根本原因是什么?- 异常情况对产品质量有何影响?- 异常情况是否会持续发展或扩大?5. 制定生产遏制措施:基于分析的结果,制定必要的生产遏制措施。
这些措施可能包括以下内容:- 设计和实施必要的检查和测试程序,以确保问题得到有效遏制。
- 调整生产过程中的参数或设置,以减少异常情况的发生。
- 提供员工培训和指导,以增强他们对异常情况的识别和应对能力。
6. 实施生产遏制措施:根据制定的生产遏制措施,及时实施必要的措施。
确保遵循相关流程和标准,以保证操作的准确性和一致性。
7. 监控和评估:在实施生产遏制措施后,密切监控生产过程,并定期评估措施的有效性。
如果需要,进行必要的调整和改进。
注意事项- 本操作指示仅为一般性指导,具体情况下请遵循相关的法律法规和公司政策。
- 在记录和报告异常情况时,请使用准确的术语和描述,以便后续的分析和理解。
- GP12工具的使用应由受过培训和具备相关技能的员工完成。
早期生产遏制程序

确保进行早期生产遏制的交付给客户的产品得到有效的控制和标识。
合用于所有生产件批准程序(PPAP)要求的新产品以及严重质量问题的量产产品,直到供方满足下面所定义的退出原则。
3.1 所有早期遏制的检验人员应严格执行本规程。
3.2 质量经理必须监视早期遏制实施并且决定是否满足退出原则。
3.3 技术部项目工程师、品管负责质量问题的快速处理。
①新产品试生产;②新产品量产;③ 有严重质量问题的产品。
动态的 GP12 产品清单技质部的项目工程师、技质部的品管等组成早期生产遏制小组。
技质部项目工程师制定早期生产遏制控制计划,包括:① 功能检测、防错措施、测试和尺寸检查、附加检验项目等;②检具、工装的制作、送检;③检验报警准则。
技质部品管:① 制定早期生产遏制;②全检场地的布置(与生产线区分开来,单向流动;质量看板;现场培训设施);③ 测量设备、量具的准备;④检具的检定。
技质部项目工程师对早期生产遏制全检人员进行检测项目的培训;技质部品管对全检人员进行量具、检具、记录填写的培训。
技质部项目工程师、品管负责对全检人员进行实际操作考核。
1.全检人员按照《早期生产遏制全检作业指导书》对所检产品实施检验,并填写《全检记录表》:① 全检合格的产品贴早期生产遏制标签(视作业指导书要求在产品或者产品外包装上贴标签),办理入库手续;②全检不合格应即将进行标识、隔离;2.过程中,达到或者超出报警准则时,通知品管部品管进行处理。
① 现场不允许返工返修;② 标签: 100%全检标贴。
早期生产遏制全检作业指导书全检记录表检具检定报告培训签到表培训记录全检记录表早期生产遏制标签销售部按照产品交付要求实施产品交付发运。
发运不合格产品结果:① 早期生产遏制执行失败将会得到受控发运等级 2 和其他可能的结果;② 发运不合格材料将会得到受控发运等级 2。
○1技质部品管针对挑出的不合格品,组织项目工程师对不合格品和报警问题即将解决,不能即将解决的,质量工程师应召项目工程师分析问题的根本原因并制定纠正措施来解决问题;针对重复发生的不合格项采用“8D”报告形式解决。
GP12早期生产遏制管理办法

1 目的本程序参照SGM GP-12管理标准,结合公司实际情况,规定产品在新开发、工程变更、过程存在风险情况下,在发货前,对产品进行独立于生产的100%全检,识别产品缺陷及过程风险。
遏制缺陷产品流入客户端造成不良影响。
2 适用范围适用于本公司所有试生产之后产品。
3 职责3.1 体系部门是本标准的归口管理部门,质量部负责本程序的编制、更新及维护,负责缺陷数据的收集整理及整改进度的跟踪和效果验证。
3.2 前期质量工程师、客户质量工程师、过程质量工程师负责GP12的通知及退出确认、GP12检验标准制定、GP12检验员的专项培训。
3.3 制造部负责产品的送检、完工品入库;负责对量产项目问题的分析改进。
3.4 项目部负责组织新项目问题的分析改进。
3.5 采购中心负责外协、外购件问题改进的推动。
4 作业标准4. 1 GP12的启动及退出条件4.1.1 GP12启动:新产品试生产后或客户发PRR 、一级/二级受控、客户特殊要求时,以及内部过程发现高风险时,客户/前期/过程质量工程师下发《GP12启动通知单》,通知制造、物流、采购、质量(包括GP12组和制程检验、进料检验)等相关部门启动GP12。
4.1.2 GP12退出1)新产品:3000件产品或SOP+三个月期限完成,且开口问题点全部关闭;2)客户PRR、一级受控、二级受控:PRR/受控已退出、客户要求期限完成,且开口问题点全部关闭。
3)其他:过程受控,且开口问题全部关闭。
客户提出的GP12,退出条件满足内部判定可以退出GP12时,由对应质量工程师向客户提出申请,得到客户认可后,下发《GP12退出通知单》,通知相关部门退出GP12;内部提出的GP12,退出条件满足,由GP12主管向启动人提出,启动人三天内下发退出通知单。
启动人负责推动GP12发现问题的整改,推动按期退出。
4.2 GP12检验标准制定4.2.1 GP12检验标准包括:《GP12检验指导书》、《缺陷警示卡》、《包装作业指导书》、《GP12检验记录表》、标准样件、边界样件、缺陷样件等。
GP12-早期遏制计划

反应计划实施 ↓
PFMEA,Control plan,work instructions to be upated 更新相关PFMEA,控制计划,作业指导书 ↓ Performance follow up (I Chart)
失效跟踪
七、GP12的标识 (Identification for GP12)
在每个发运标签上贴一个直径约25mm的绿色圆点,该圆点应签有GP12区域负责人的 签名。
Attach to each shipping label a gree circular, sticker,approximatelu 25mm in diameter ,signed by the staff person accountable to insure proper implementation of GP-12.
二.目的(purpose)
1.验证生产控制计划 To validate the production control plan
2. 提供持续改进的数据 Provide data for continuous improvement
3.在产品生产过程和质量稳定前,保护客户 Protect the customer before the process and quality are stable. 4.确保质量问题能迅速地鉴别、遏制和解决 Make sure issues are identified and contained and quickly solved 5. 让高层管理层知道相关问题及其解决方法
General Procedure 12 活动简介
一.什么是GP12? (What is GP-12?)
早期质量遏制流程(新)

1.目的
确保进行早期生产遏制(GP-12)的交付给客户的产品得到有效的控 制和标识。
2.适用范围
适用于所有生产件批准程序(PPAP)要求的新产品以及严重质量问题 的量产产品,直到供方满足下面所定义的退出原则。
3.职责
3.1所有GP12早期遏制的检验人员应严格执行本规程。 3.2质检部主任必须监视早期遏制实施并且决定是否满足退出原则。 3.3总工程师、质检部主任负责质量问题的快速处理
质量问题处理 清单 纠正措施记录 单
质检部主任对确定的改进措施进行跟踪,并 质量问题处理
形成跟踪记录。
清单
流程 GP12
流程执行说明
GP12退出原则 1 正式生产满6个月或生产量达到50吨或满
足顾客特殊要求,在不合格品汇总表及缺 陷记录表上未发现不合格现象和顾客工厂 PRR,可以向顾客申请退出GP12,顾客同 意后,方可退出; 2 没有指定时间或数量,GP12 应保持整个 加速期或至少两周(较长的一个); 3 如果一个问题在GP12 或被顾客发现,则
4.工作程序
流程如下
流程
流程执行说明
流程执行必须 形成的记录
早期遏 制项目 确认
GP12小 组成员
1 新产品试生产; 2 新产品量产; 3 有严重质量问题的产品。
总工程师、质检部的人员组成GP12小组。
GP12产品清单
GP12前 期准备
总工程师制定GP-12控制计划,包括: 1 功能检测、防错措施、测试和尺寸检查、
2.过程中,达到或超出报警准则时,通知质
检部主任进行处理。
特别说明:
1 GP12现场不允许返工返修;
2 GP12合格证标签必须由质检部主任亲自检
GP12早期生产遏制培训

供应商的职责
每周对有效性进行评价,更新检查清单 使用信息板明确任务、张贴作业指导书、
质量数据及反应计划,对发生的所有问题, 记录、分析并制定遏制措施,必要时更新 作业指导书、控制计划、PFMEA
早期生产遏制问题清单
序 阶段 问题描述 发现日期 累积件数 等级 采取措施 号 1 2 3 4 5 6 7 8 9 10
目的
➢ 在早期生产阶段使问题自然暴露,平稳进入正常生 产阶段。
➢ 在早期生产阶段,实施额外的检验控制手段,以保 证在供应商现场发现并解决质量问题。保证任何发 生的质量问题能够快速得到确认,围堵并且在供应 商处得到纠正。
➢ 验证供应商的生产控制计划。 ➢ 保护顾客的装配和制造中心以及售后仓库在危险的
备注:累积件数的前5种缺陷需在等级栏中标注符合☆
验证关闭
早期生产遏制退出准则
经一段时间运行后,若满足以下条件,供应商可 退出早期生产遏制: ➢ 采购部门指定的零件数量或持续一段时间,没有不 符合项,若已发现了不符合项客户关闭了PR&R (GM的自我退出条件) ➢ 控制计划得到验证 ➢ 零件数量或生产时间符合要求:GP12必须执行一段 时间或一定数量的产品,具体情况由顾客定义或一 直到生产控制计划被确认有效,这两个条件中时间 长者为标准。如果时间或数量没有被定义,GP12将 贯穿整个产品加速期或最少两个星期这两个条件中 时间长者为标准。 ➢ SQE批准(GM有自我退出的规定)
供应商车间
信息板
生产区域
早期生产控制审 核区域
GM
供应商的职责
➢ 制定并执行GP-12(试生产)控制计划: 和生产控制计划相比增加检验频率/抽样数量 加强对供方的早期遏制 附加检验/控制项目 通过对已知缺陷的分析确认防错的有效性 确认包装和标识(标签)的要求—包括售后件
GP12-早期生产遏制程序

(详见GM1920标准) 详见GM1920标准) GM1920标准 • 与SGM有关的所有新项目开发,新产品早 有关的所有新项目开发, 有关的所有新项目开发 期生产时,供应商必须做GP12(包括一级、 期生产时,供应商必须做 (包括一级、 二级零件)。 二级零件)。 • GP12即我们通常所说的 即我们通常所说的100%全检。全检 全检。 即我们通常所说的 全检 介入点:正常检验结束后,在产品出厂前, 介入点:正常检验结束后,在产品出厂前, 临时增加的一道额外检查。 临时增加的一道额外检查。
•
D 持续时间:GP12必须执行一段时间或一定数量的产品, 具体情况由顾客定义或一直到生产控制计划被确认有效这 两个条件中时间长者为标准。如果时间或数量没有被定义, GP12将贯穿整个产品加速期或最 少两个星期这两个条件 中时间长者为标准。
在GP12阶段100%GP12检验是强制性的要求。基于在GP12检验时没有发现问题或顾 客没有发现问题,这样经过顾客/SQE确认生产过程后可以批准减少100%检验,但 这必须文件化并得到顾客/SQE的批准
• •
额外的测量和测试要求必须得到供应商和/或顾客/SQE 的确认并得到顾客/SQE的批准 E 证明:在运输标签上粘贴由指定管理层代表签名的一 个直径大约25 mm的绿色圆点,来表示遵循了GP12的 要求。
退出标准
• 退出标准:确认过程控制计划和满足以下要求后,供应商将可以退出 GP12。如果供应商不能满足退出条件或供应商的GP12中继续发现有 不合格品,供应商将进行执行围堵政策直到质量问题全部解决得到自 身和顾客的认可,生产控制计划是有效的。
职责
• A 确认过程:建立一个确认的过程包含以下要素:
早期质量遏制流程(新)

早期生产遏制流程
1.目的
确保进行早期生产遏制(GP-12)的交付给客户的产品得到有效的控制和标识。
2.适用范围
适用于所有生产件批准程序(PPAP)要求的新产品以及严重质量问题的量产产品,直到供方满足下面所定义的退出原则。
3.职责
所有GP12早期遏制的检验人员应严格执行本规程。
质检部主任必须监视早期遏制实施并且决定是否满足退出原则。
总工程师、质检部主任负责质量问题的快速处理
4.工作程序
5.相关文件
无
6.相关记录
《GP12产品清单》
《GP12全检作业指导书》
《全检记录表》
《质量问题处理清单》
《GP12退出申请单》
GP12产品清单
GP12全检作业指导书
编号:HYWL QR SOP12-11产品图号:图纸版本号:产品名称:
GP12全检记录表
编号:HYWL QR SOP12-12产品图号:图纸版本号:产品名称:
装配线:全检日期:
GP12质量问题清单
GP12退出申请单
编号:HYWL QR SOP12-14 ******客户:
您好!
以下无固定格式。
早期生产遏制程序
检验员协助确认改进实施效果。
7、场地要求及看板管理
7.1GP12检验区域必须是单独的场地并建立检验信息看板;
7.1.1GP12检验区域必须与生产现场隔离,须划分待检区域、不合格品区域、合格品区域,区域内有充足的光线照明。
4.3.2(其他产品)如果进入时没有指定控制时间或数量,GP12应保持三个月,并且数量到达2000件,如果一个质量问题在GP12或被客户发现,则在纠正措施执行后,则GP12检验必须保留,直至过程过程控制和能力证明有效,生产控制能力得到验证方可退出,如果客户在产品进入GP12时有特殊要求,则按照客户的特殊要求执行控制。
3.1 GP12质检员:负责GP12阶段的产品检验和GP12标识粘贴工作,负责GP12检验信息及时反馈。
3.2技术部:对于需要设立GP12验证岗位的新零件或工程更改后的新零件,编制GP12遏制零件清单,并及时下发通知。
3.3质检部;负责编制《GP12检查表》和《缺陷统计表》,同时定期收集GP12数据,统计及质量问题处理追踪,负责GP12的进入及退出申请;对于批量生产后需临时进入GP12遏制阶段的零件,编制GP12遏制零件清单,并及时下发通知。
5.4经GP12检验的合格产品按照GP12产品检验指导书粘贴绿色圆形标签,注:标签必须由质检部主管或指定人员签字。
6、问题的分析与改进
6.1质检部负责对每日缺陷进行分析,制作缺陷排列图,将重点问题(按实际问题情况、缺陷排列图前5个问题或前3个问题)在每日的QRQC现场会中进行反馈。
6.2质检部负责召开QRQC质量问题解决会议,在会议中针对重点质量问题,确定问题的责任人,制定纠正预防措施及完成的时间。
早期生产遏制程序

早期生产遏制程序
1.目的
确保进行早期生产遏制的交付给客户的产品得到有效的控制和标识。
2.适用范围
适用于所有生产件批准程序(PPAP)要求的新产品以及严重质量问题的量产产品,直到供方满足下面所定义的退出原则。
3.职责
3.1所有早期遏制的检验人员应严格执行本规程。
3.2质量经理必须监视早期遏制实施并且决定是否满足退出原则。
3.3技术部项目工程师、品管负责质量问题的快速处理。
4.工作程序
5.相关文件
无
6.相关记录
早期生产遏制产品清单
早期生产遏制全检作业指导书
全检记录表
全检标签
质量问题处理清单
早期生产遏制退出确认报告
早期生产遏制产品清单
早期生产遏制全检记录表
编号:产品图号:产品名称:
图纸版本号:全检日期:
GP12标签:
100%全检
早期生产遏制质量问题清单。
GP12早期生产遏制操作手册

GP12早期生产遏制操作手册目录1. 引言2. 遏制操作前的准备工作3. 遏制操作步骤4. 遏制操作后的处理5. 总结1. 引言本操作手册旨在为GP12早期生产遏制操作提供指导,以确保操作的顺利进行并最小化安全风险。
遏制操作是一项复杂的任务,需要遵循严格的步骤和安全措施。
本手册将介绍在遏制操作前的准备工作、遏制操作步骤和遏制操作后的处理。
2. 遏制操作前的准备工作在进行遏制操作之前,进行以下准备工作是至关重要的:- 准备必要的个人防护装备,包括护目镜、手套、防护服等。
- 确保所有工具和设备处于良好工作状态,并进行必要的维护保养。
- 安排一个专门的工作区域,并确保其整洁和易于操作。
- 与相关人员进行沟通,确保大家理解遏制操作的重要性和具体步骤。
3. 遏制操作步骤遏制操作需要遵循以下步骤:1. 确定遏制操作的目标和范围,并评估安全风险。
2. 准备并携带必要的遏制工具和材料。
3. 在遏制操作区域进行必要的隔离和标识。
4. 关闭与被遏制物质相关的设备,确保安全停机。
5. 使用适当的方式遏制被遏制物质的泄漏,如使用阀门关闭、封堵管道等。
6. 定期检查遏制操作区域的状态,确保没有新的泄漏。
7. 将被遏制物质转移至安全,确保安全存储和处置。
4. 遏制操作后的处理遏制操作完成后,需要进行以下处理工作:- 清理遏制操作区域,确保其安全和干净。
- 对遏制工具和材料进行清洁和维修。
- 对遏制操作过程进行评估和总结,提出改进建议。
- 将遏制操作的结果和所采取的措施进行记录和报告。
5. 总结本操作手册介绍了GP12早期生产遏制操作的基本步骤和要点。
在进行遏制操作时,务必遵循所有的安全措施,并根据实际情况进行调整和改进。
及时进行遏制操作可以有效降低安全风险,保障生产安全。
早期质量遏制流程(新)
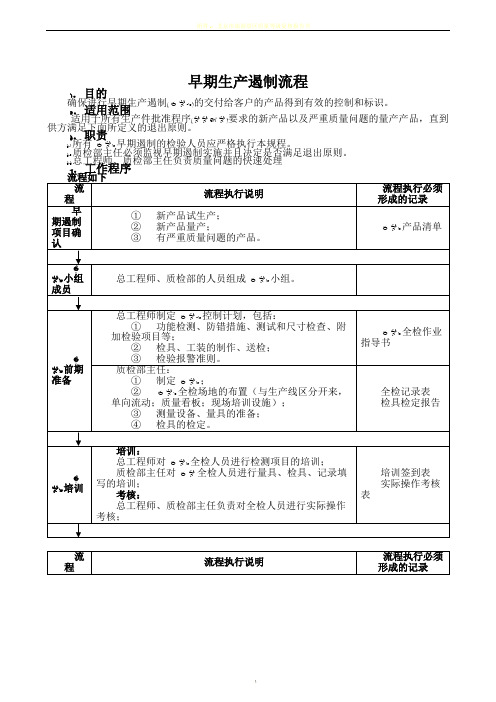
早期生产遏制流程
1.目的
确保进行早期生产遏制(GP-12)的交付给客户的产品得到有效的控制和标识。
2.适用范围
适用于所有生产件批准程序(PPAP)要求的新产品以及严重质量问题的量产产品,直到供方满足下面所定义的退出原则。
3.职责
3.1所有GP12早期遏制的检验人员应严格执行本规程。
3.2质检部主任必须监视早期遏制实施并且决定是否满足退出原则。
3.3总工程师、质检部主任负责质量问题的快速处理
4.工作程序
无
6.相关记录
《GP12产品清单》
《
GP12全检作业指导书》 《全检记录表》
《质量问题处理清单》 《GP12退出申请单》
GP12产品清单
GP12全检作业指导书
编号:HYWL Q R S OP12-11
编号:HYWL Q R S OP12-12产品图号:图纸版本号:产品名称:
GP12退出申请单
编号:HYWL Q R S OP12-14 ******客户:
您好!
以下无固定格式。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
GP-12适用于下面要求的所有的试生产、生产、服务件和配件
Require Production Part Approval Process (PPAP) 要求提交PPAP的 Represent significant risk to the customer facility as mandated by GM GM管理 的对客户的生产厂有重要影响
建立包含以下要素的确认过程 Identify the staff person responsible for ensuring the development and implementation of the verification process. 明确保证开发和执行过程确认的责任人
2
2 3
SUPPLIER RESPONSIBILITY: The supplier shall: C. Documentation: 文件Document the Pre-Launch Control Plan using the Control Plan format referenced in the AIAG Advanced Product Quality Planning and Control Plan Reference Manual or other customer approved Advanced Quality Planning reference manuals. The Pre-Launch Control Plan is not a substitute for the Production Control Plan but, is an addition to the Production Control Plan and is used to validate it 根据AIAG 的 APQP手册中控制计划表格和控制计划参考手册或其它顾客批准的APQP参考手册制定试生产控制计 划,试生产控制计划不能替代量产控制计划,它是控制计划的附加可以用来验证控制计划
1 Increased frequency/sample size as stated in the Production Control Plan.
1在产品控制计划中增加的频次和样品容量
2 Verification of packaging and label requirements – including service and accessory part requirements, which may include country of origin labels on parts. 2确认包装和标签的要求包括服务件和附件的要求,可能包括产品原产国标签 3 Verification of the effectiveness of error proofing. 确认防错的有效性 4 Immediate implementation of containment and irreversible corrective action when nonconformances are discovered in the GP-12 containment area or at the receiving location. 4如果在接受场所或在GP-12 执行的范围内发现不符合立即执行遏制措施和纠正措
1 Document additional inspections, functional testing, and dimensional checks required at the GP-12 containment station or in process check stations on the Control Plan Special Characteristics form referenced in the AIAG APQP Manual – Supplement L and reference said document in the Pre-launch Control Plan as a specific operation. GP-12 遏制站要求的额外检查、功能试验和尺寸检查要文件化,控制计划过程检查项 目特殊特性参考AIAG供应商 APQP 手册附录L,上述文件检查项目在试生产控制计划中作为特定的项 目 2 Document inspection work instruction for the GP-12 containment station to insure standardized work.GP-12 检查站要有书面的保证标准作业的检查作业指导书
3 Document evidence of execution and validation of the control plan utilizing the I-chart (GM1927-66) or other format agreed upon by the customer. The data must be readily available for review by the customer/SQE. 3要有文件证明执行和确认的控制计划运用了I-chart GM1927-66) 或顾客同意的其它格式,这些资料在 顾客或SQE评审时要易于获得 4 Document problem solving for both internal and customer quality concerns utilizing customer acceptable format; including problem description, root cause, irreversible corrective action with break points and update FMEAS and Control Plans as appropriate. The 3 x 5 Why Analysis (GM1927-84) for root cause and Read Across (GM1927-69) to apply lessons learned are to be utilized.内部和涉及到解决顾客质量问题的文件包括 问题描述、根本原因、纠正措施、FMEA和控制计划的修改要用顾客接受的格式,适时利用根本原因3 x 5 Why 分析,进行Read Across培训
The purpose of GP-12 is to: GP-12 的目的 验证供应商的量产控制计划
Validate the supplier’s production control plan
Protect our assembly and manufacturing centers and service part warehouses from quality non-conformances during critical periods 在重要时期防止我们的装配和制造中心及服务件仓库出现质量问题 Document the supplier‘s efforts to verify control of its processes during start-up, acceleration, after revisions to the manufacturing process, or when manufacturing runs are separated by 3 months or more记 录 供 应 商 在 开 始 生 产 、 加 速 生 产过 程 、生产过程变更后或生产停产三个月或以上时 , 为 控 制 其 过程 所 做 的努力 Ensure that any quality issues that may arise are quickly identified, contained, and corrected at the supplier's location 确保任何可能产生的质量问题在供应商的生产场所尽快的得到发现、控制和 纠正 Increase involvement and visibility of supplier’s top management
增加最高管理层的参与和远见性
SUPPLIER RESPONSIBILITY: The supplier shall:供应商职责:供应商必须
A.
A. 1 1
Validation Process: Establish a validation process that contains the following elements:
5 6 7
பைடு நூலகம்
8
SUPPLIER RESPONSIBILITY: The supplier shall:
B. Plan Development: 开发计划 Development of a Pre-Launch Control Plan which is a significant enhancement to the production control plan and also consisting of additional controls, inspections, audits, and testing to insure conformance and capability of the manufacturing process. The plan needs to consider; 试生产控制计划开发对量产控制计划有很重要得作用而且确保产品符合性和过程 制造能力附加的控制、检查、审核和试验也是根据试生产控制计划而来。试生产 控制计划必须考虑以下几个方面:
3
4
设立GP-12 遏制站,遏制站必须设在生产末端而且不在生产线上的、单独和正常的制造过程分开的,另外的 或更有效的或可能用到过程检查站必须文件化并且有顾客或SQE批准
Identify additional inspections, testing, and dimensional checks required at the GP-12 containment station based on Key Product Characteristics (KPCs), Part Quality Characteristics (PQCs), high RPN and/or issues identified during product and process development.在GP-12检查站要识别附加的检查、试验和尺寸检验,这些检查项目来源于产 品的关键特性和产品的质量特性、高RPN值以及在产品和过程开发阶段识别的问题。 Train personnel relative to the standardized work performed at the GP-12 containment stations.培训相关人员使其满 足在GP-12 检查站岗位职责 Establish a reaction plan for single defect.建立一个针对每个缺陷反应计划 Implement an audit process of the GP-12 containment utilizing levels of management (layered audit), including site leadership, to insure conformance to the Pre-Launch Control Plan.实施GP-12审核过程,利用管理层包括现场管理 人员进行分层审核,确保符合试生产控制计划 Include subcontractor (Tier 2) in the validation process. 包括分承包方的过程确认
